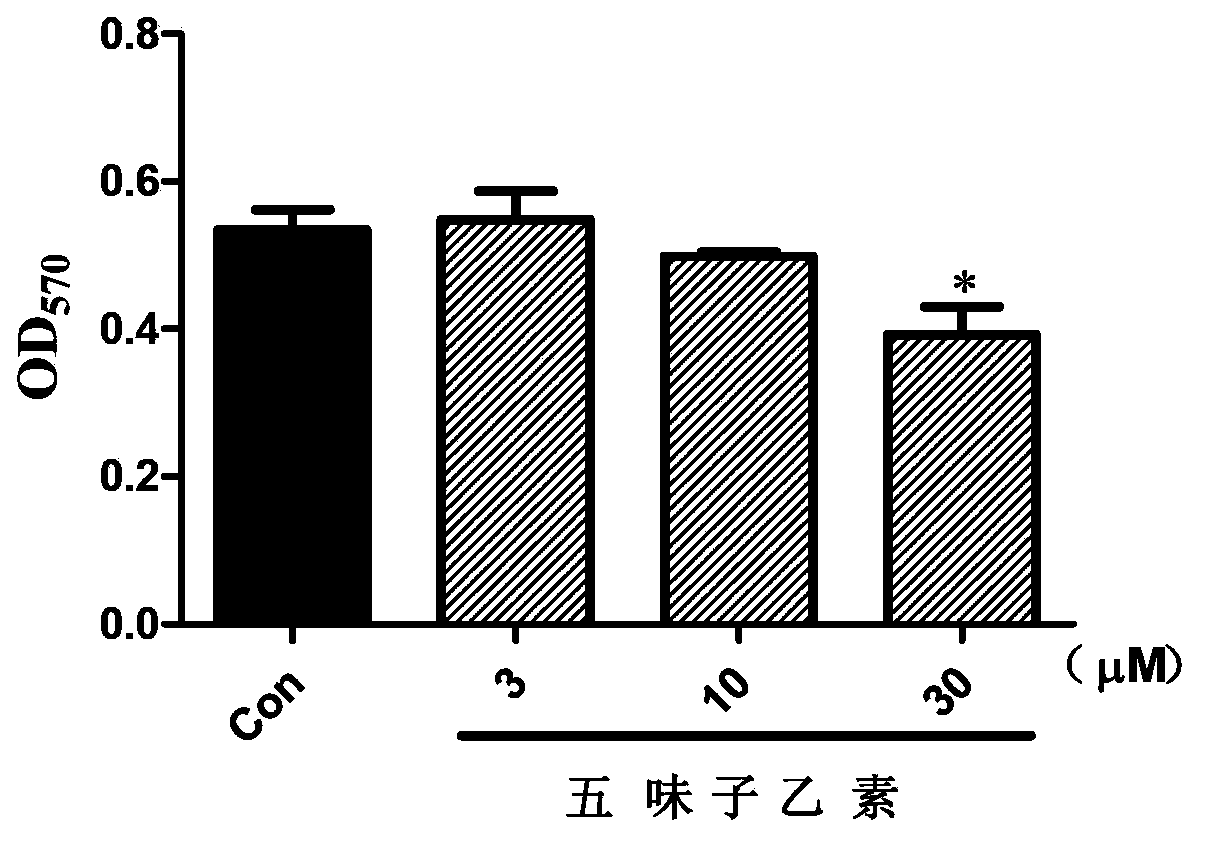
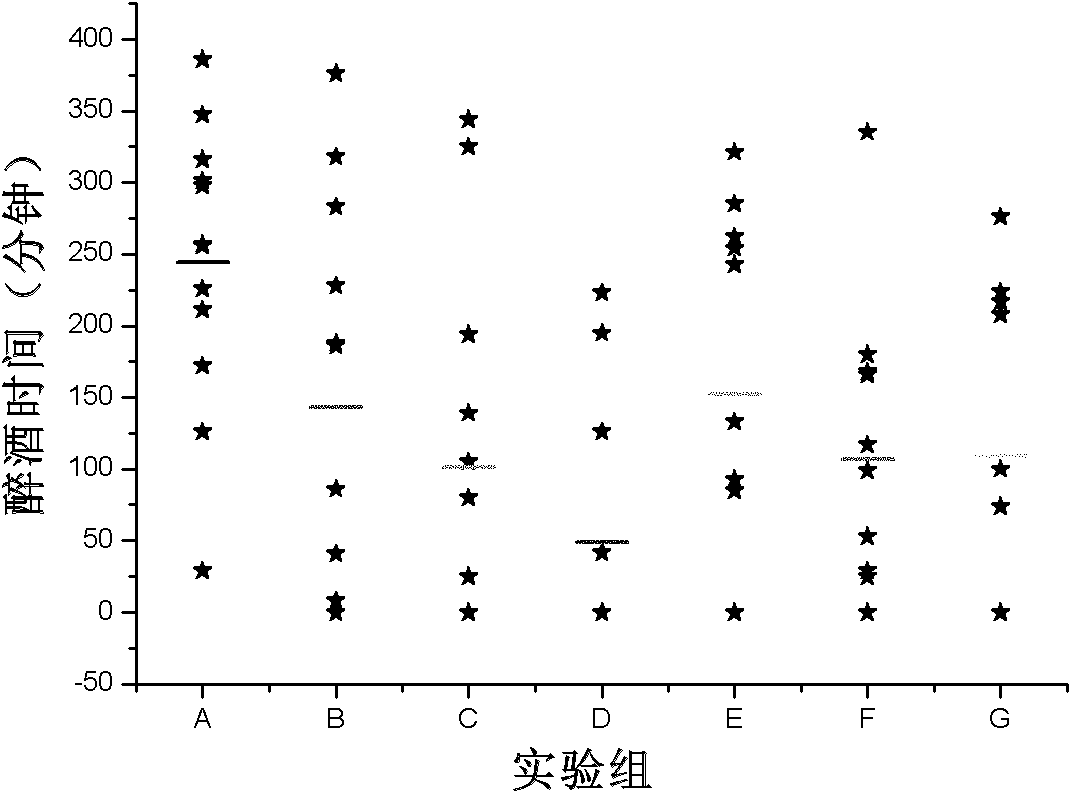
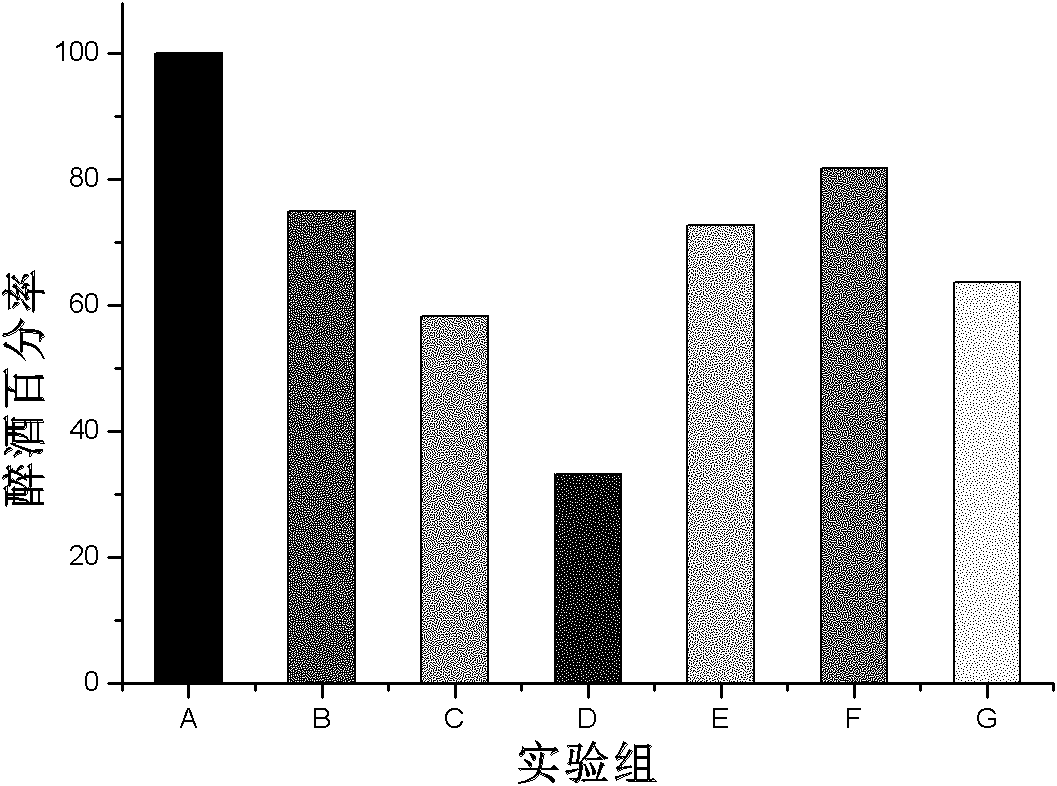
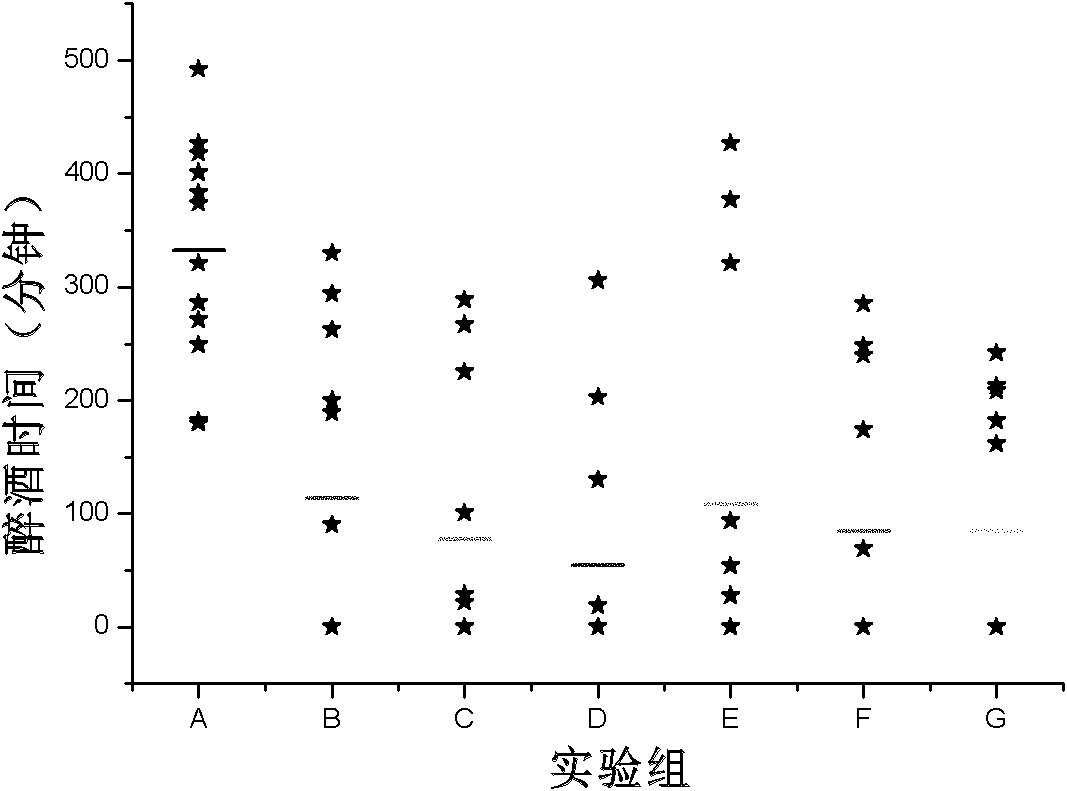
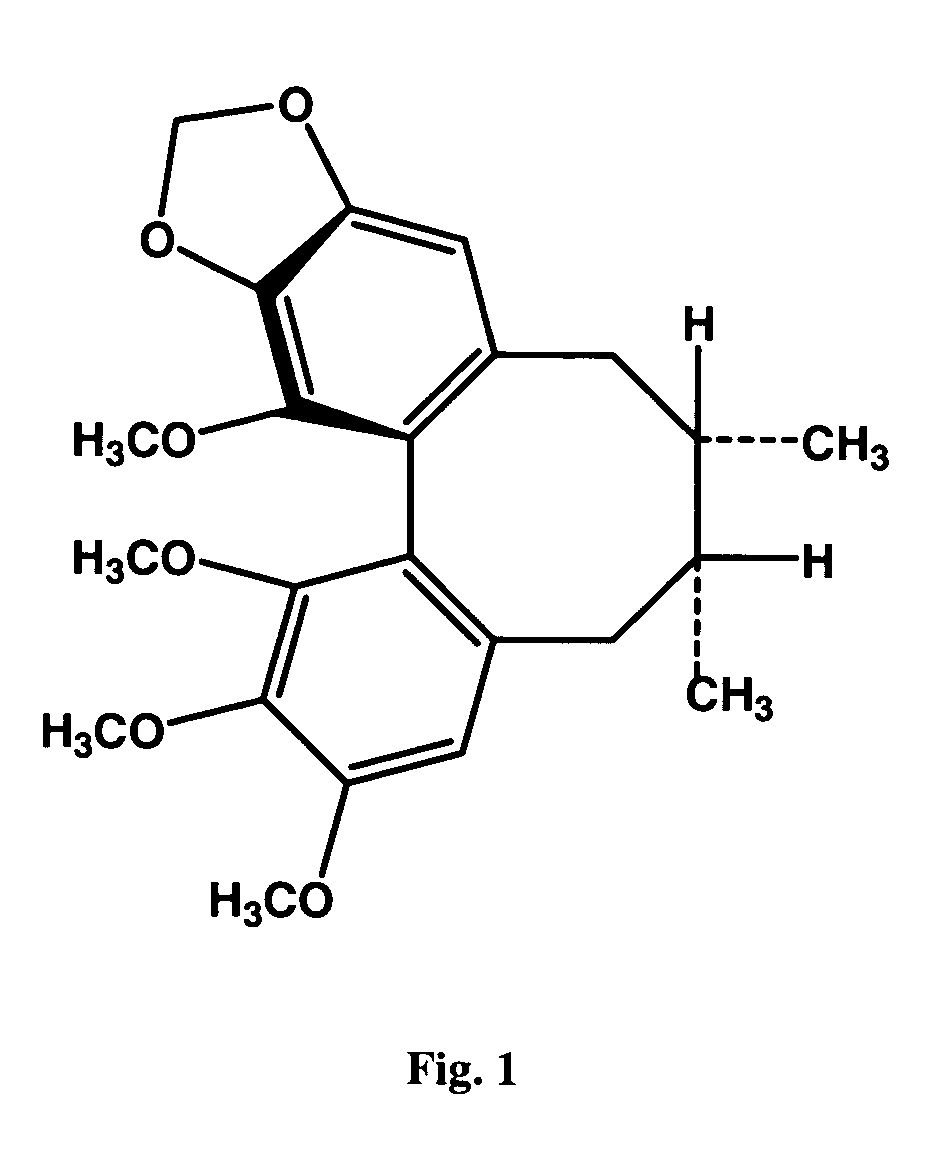
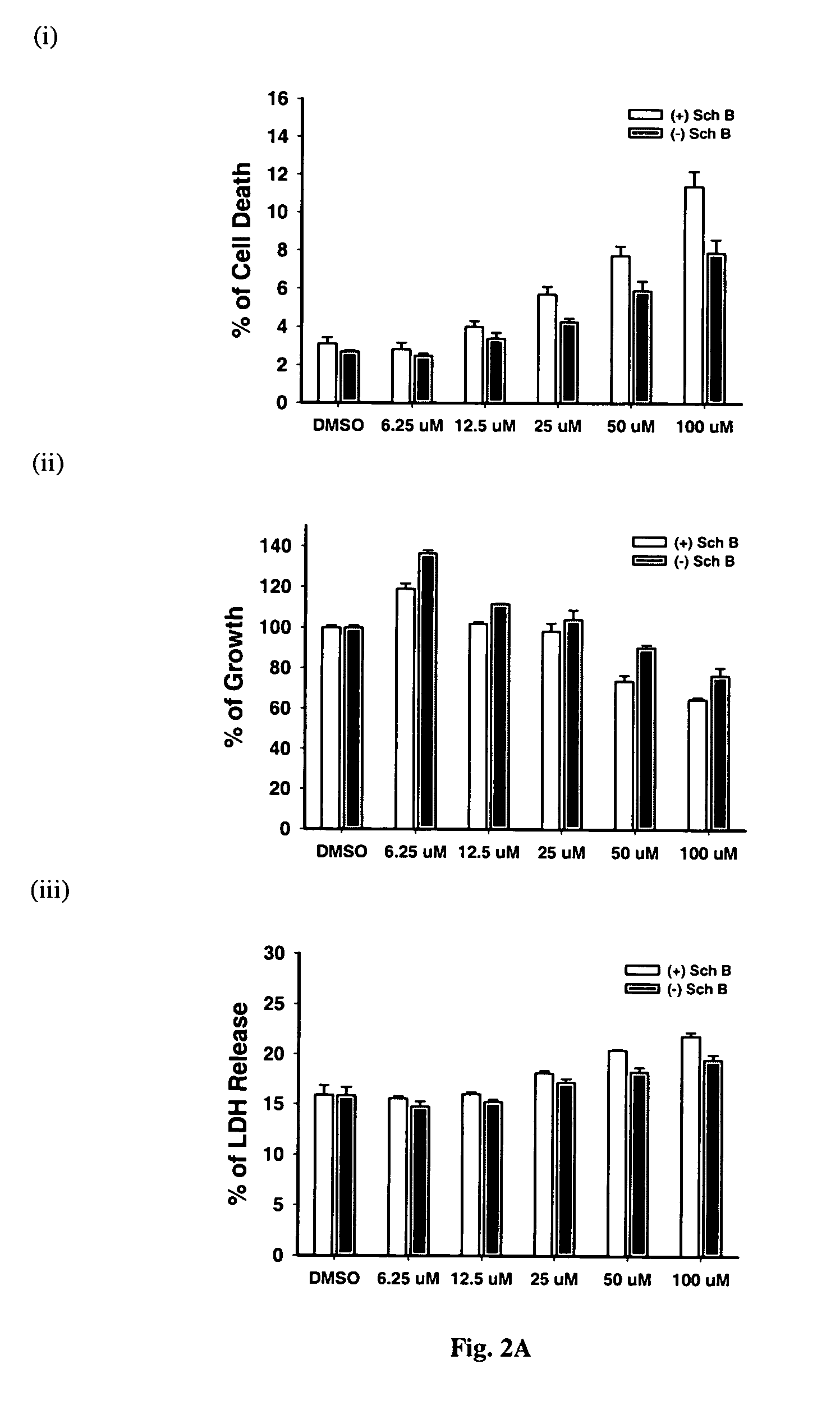
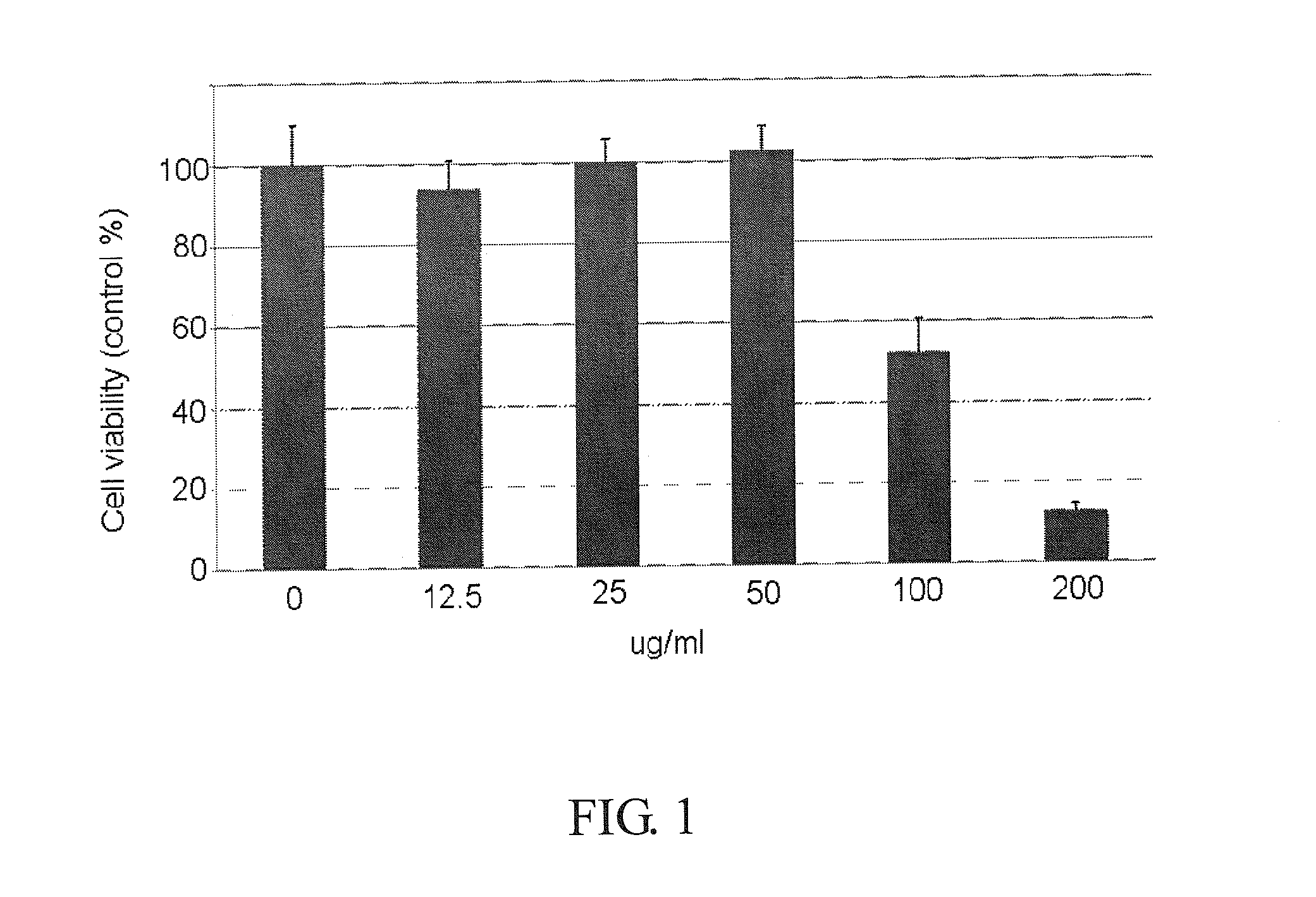
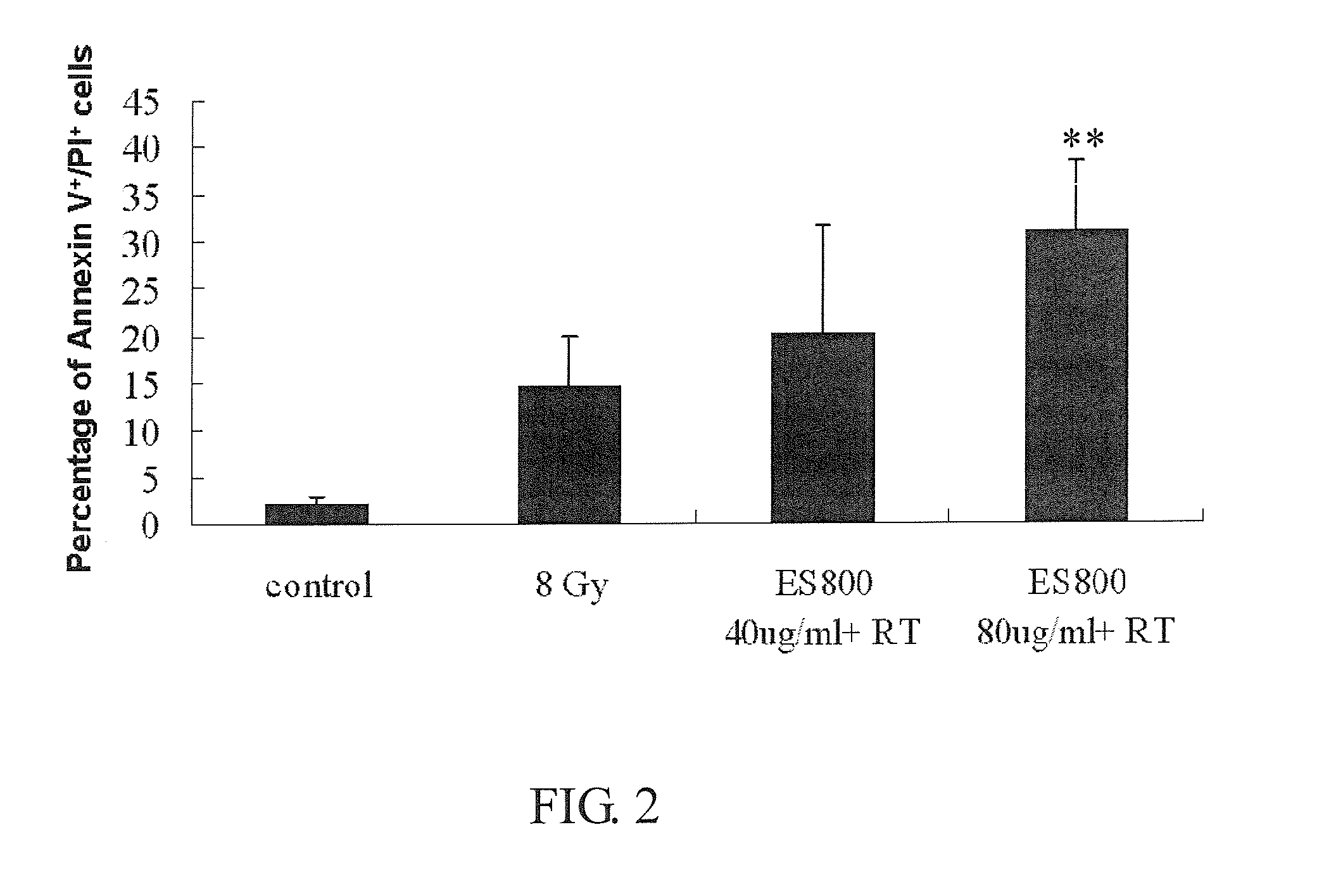
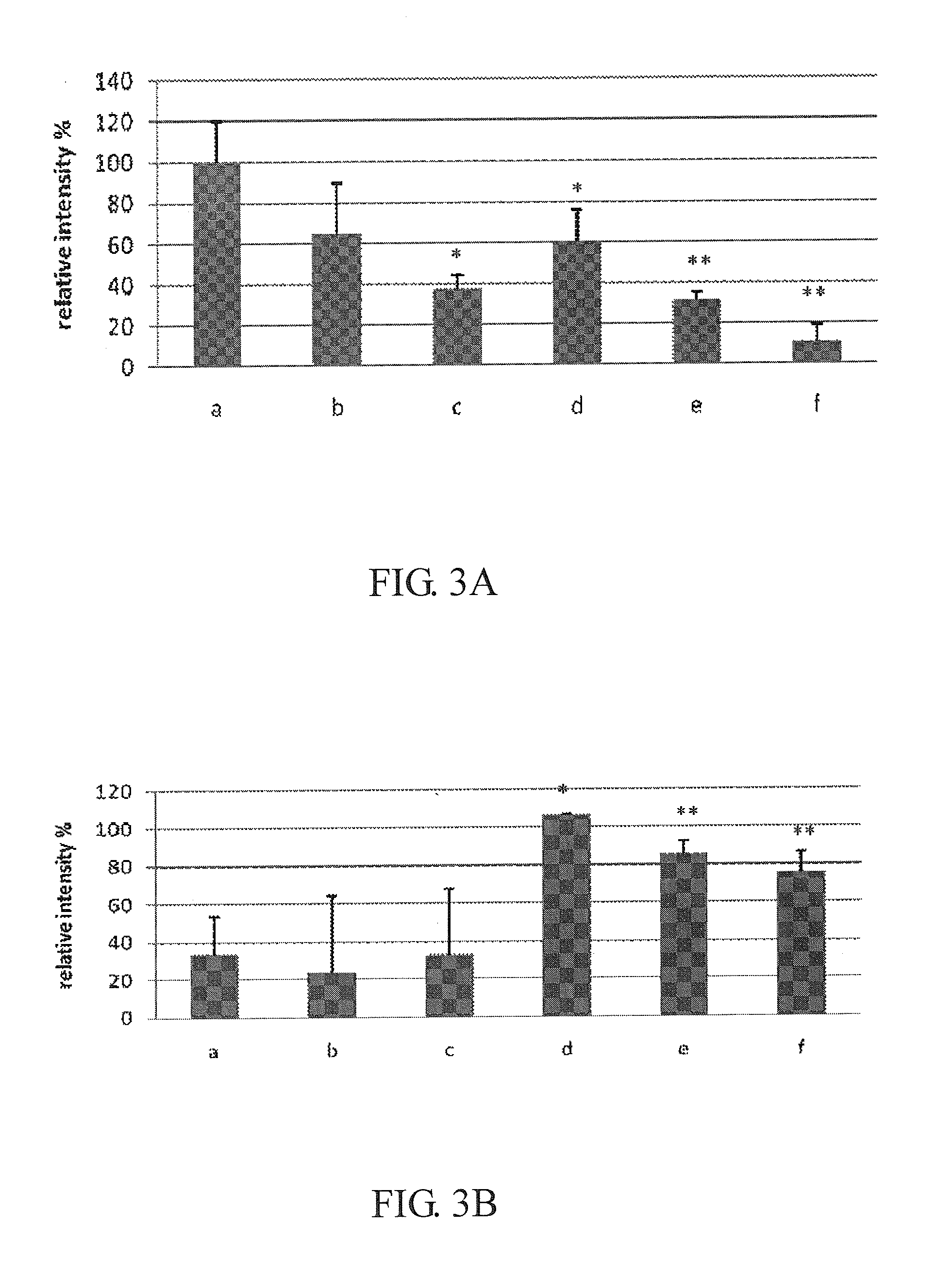
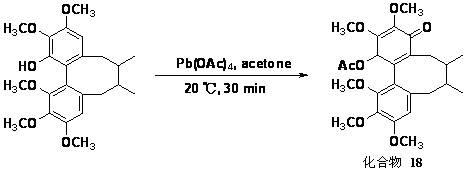
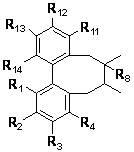
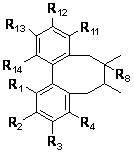
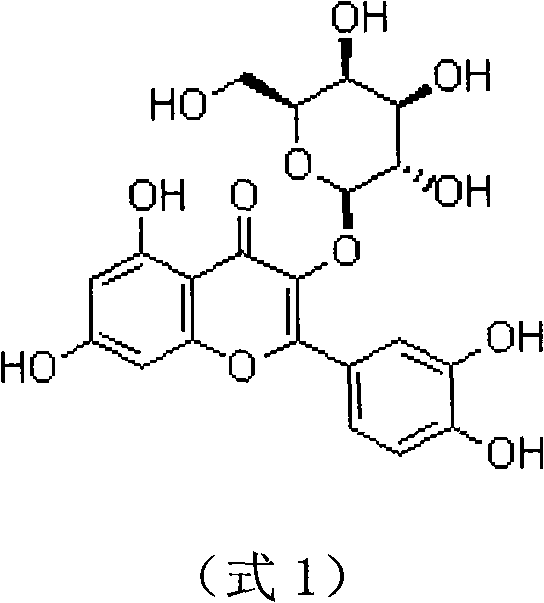
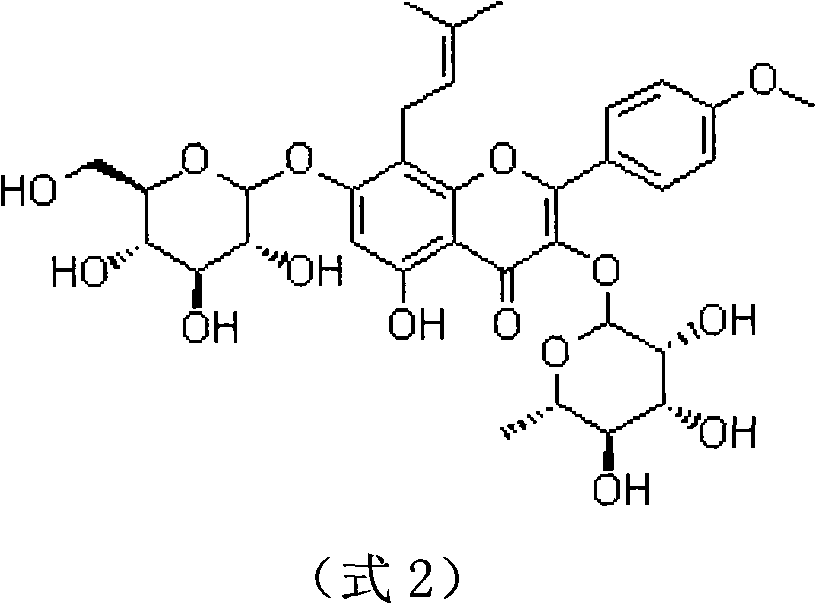
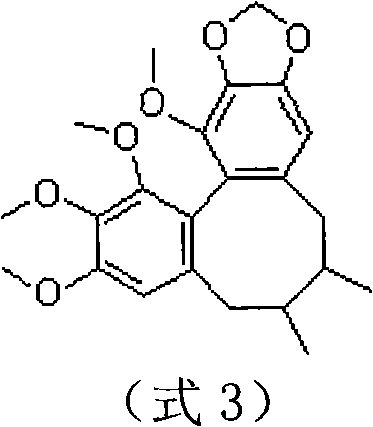
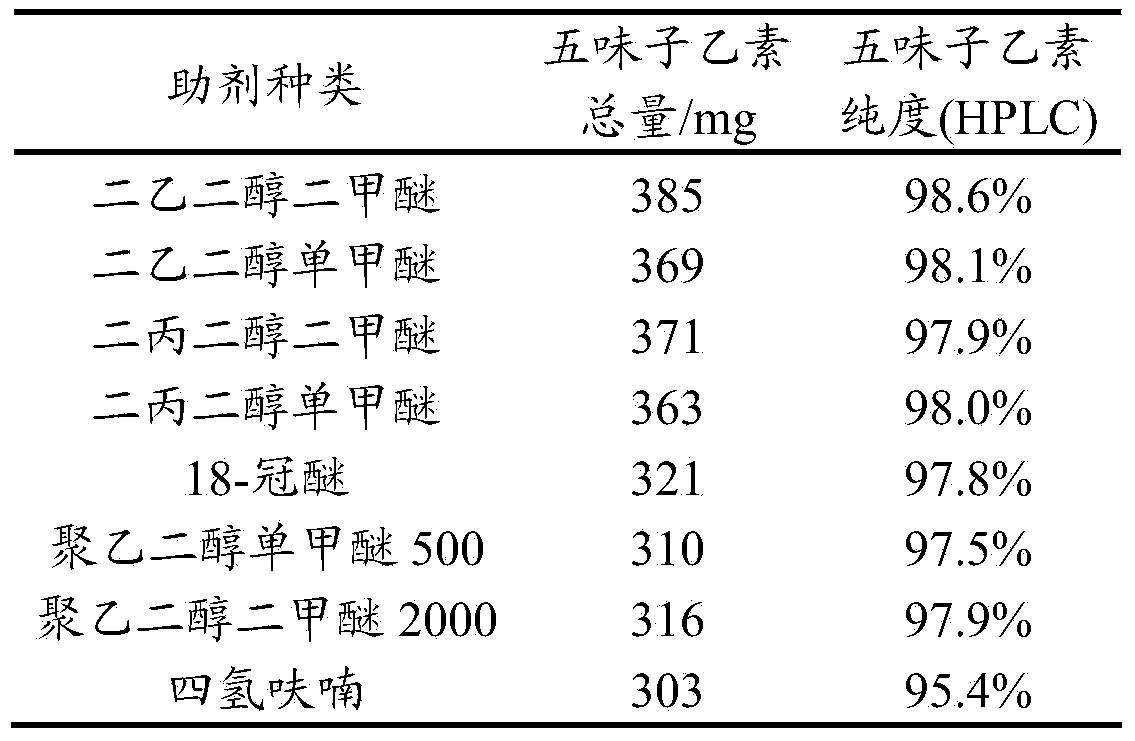
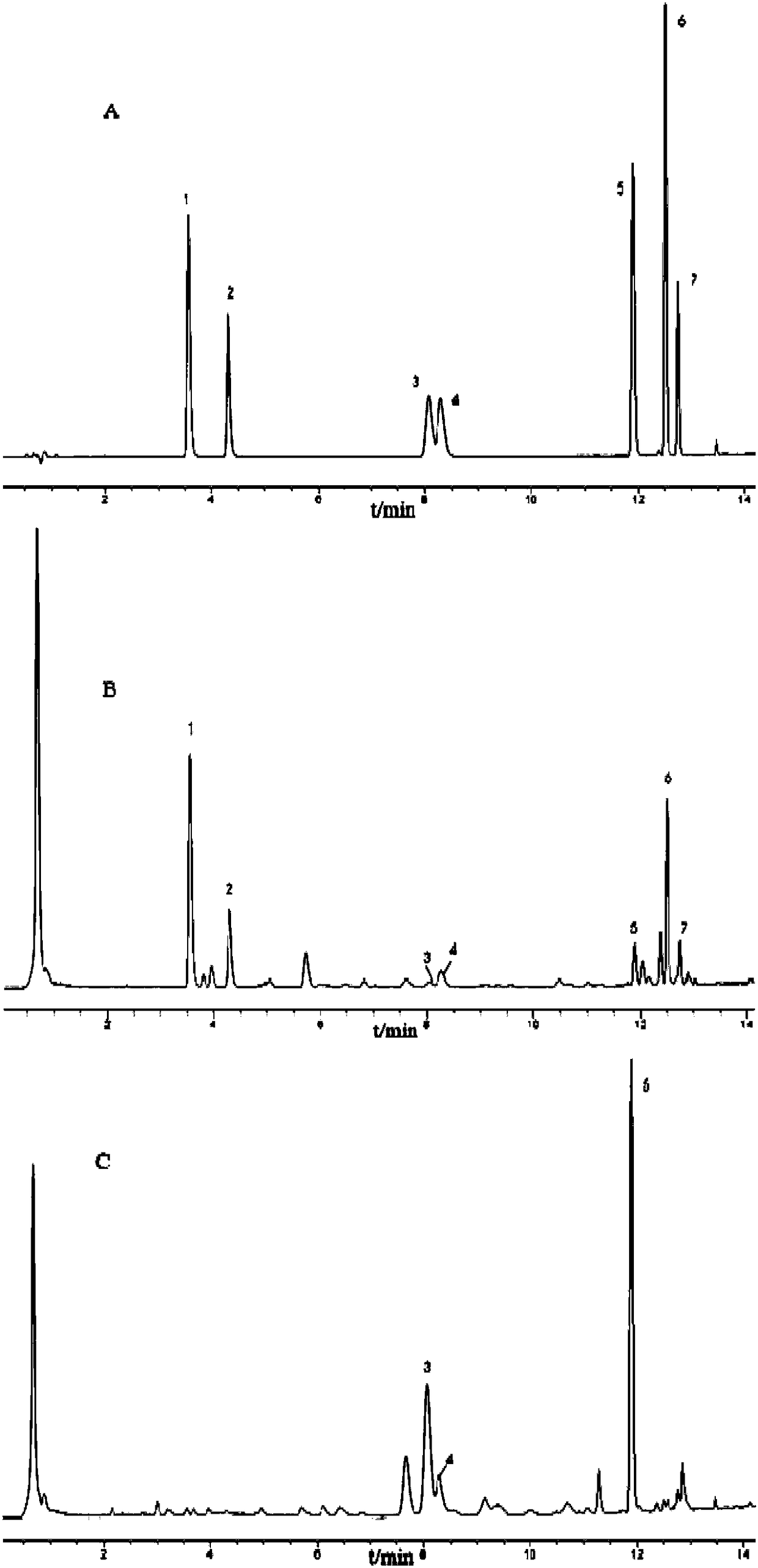
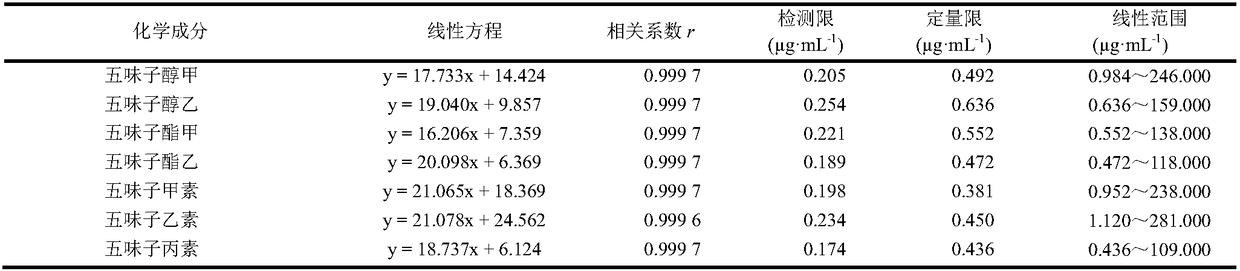
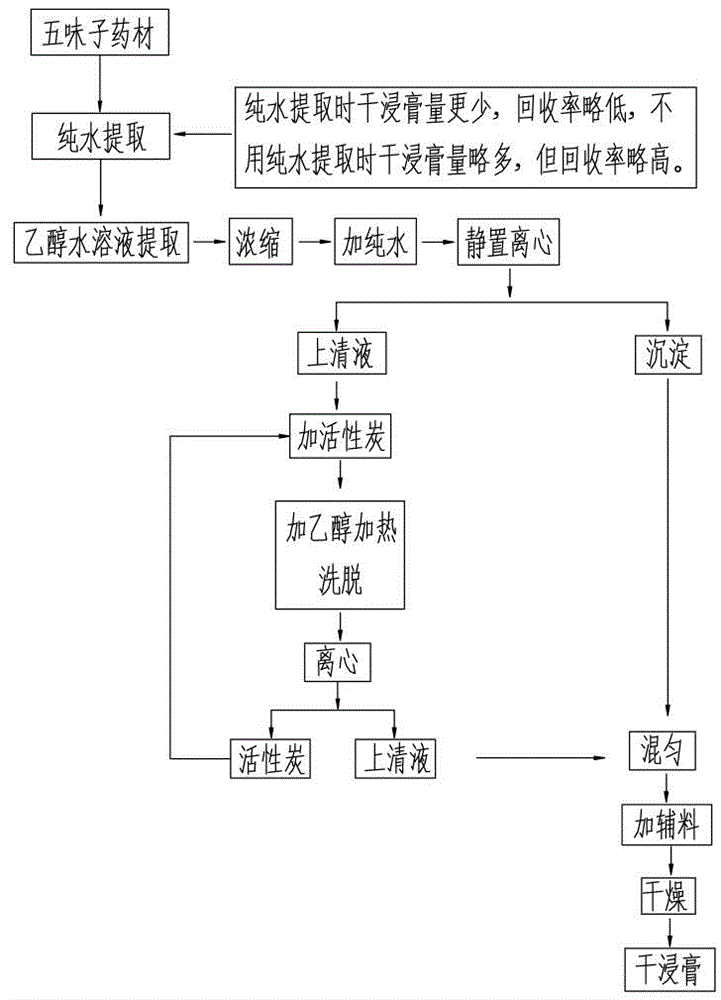
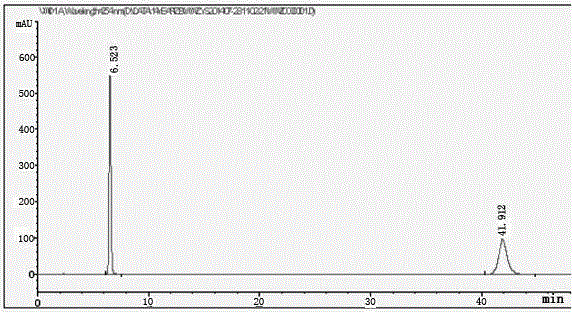
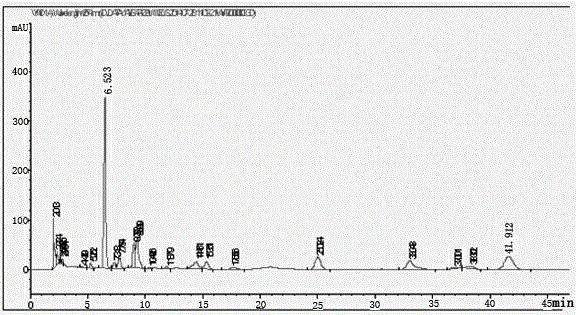
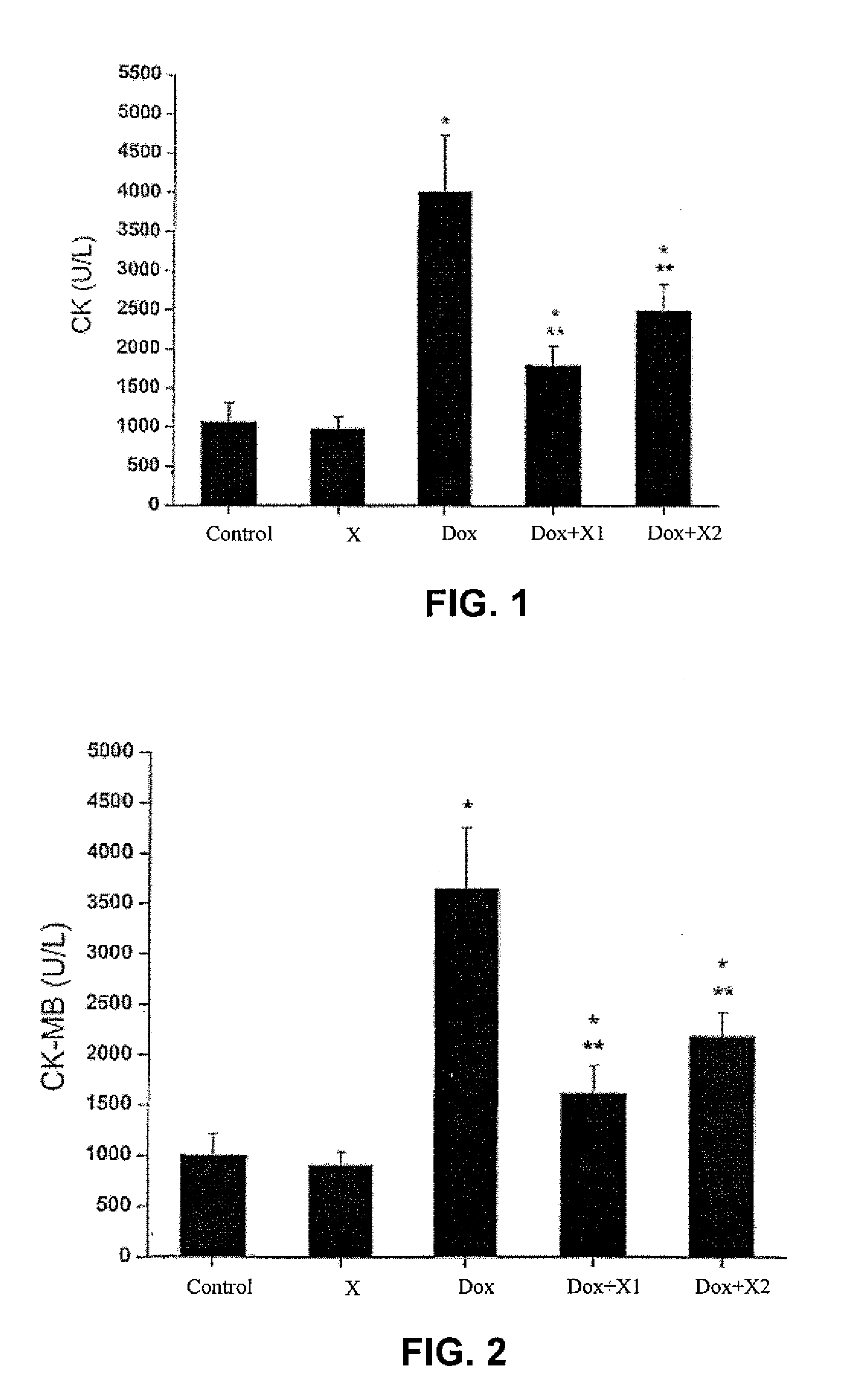
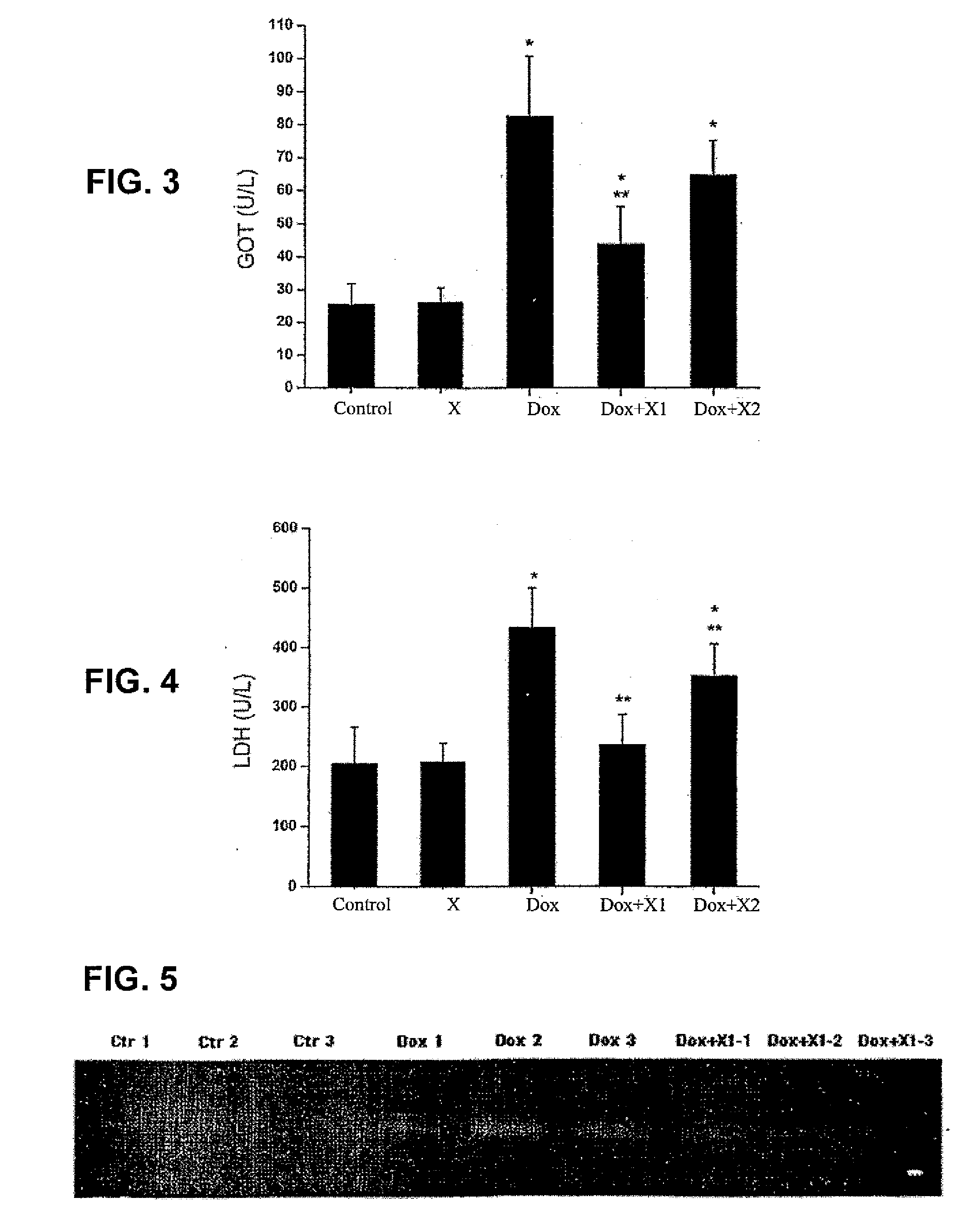
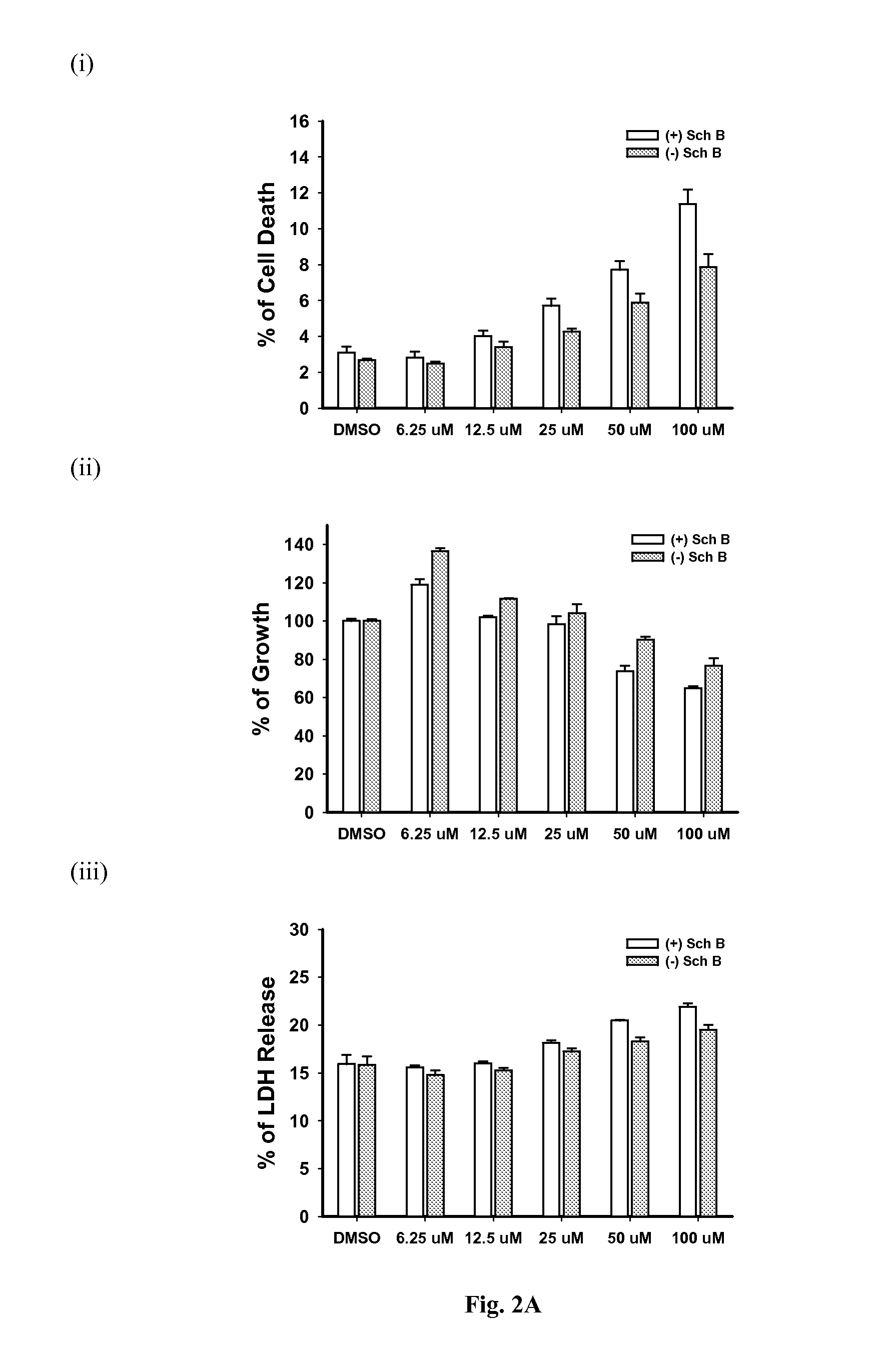
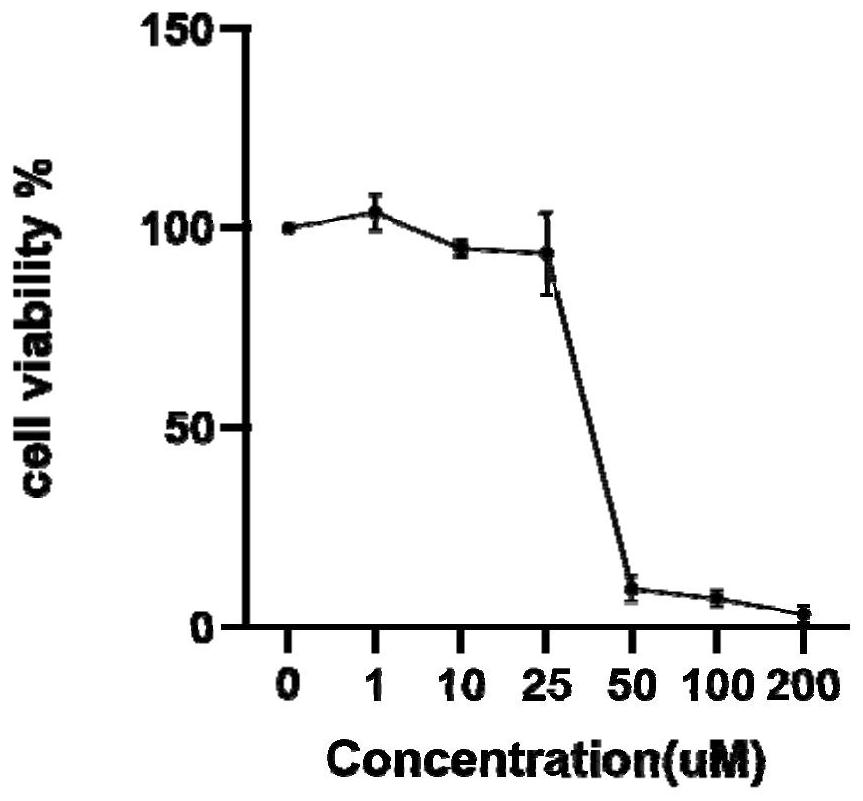
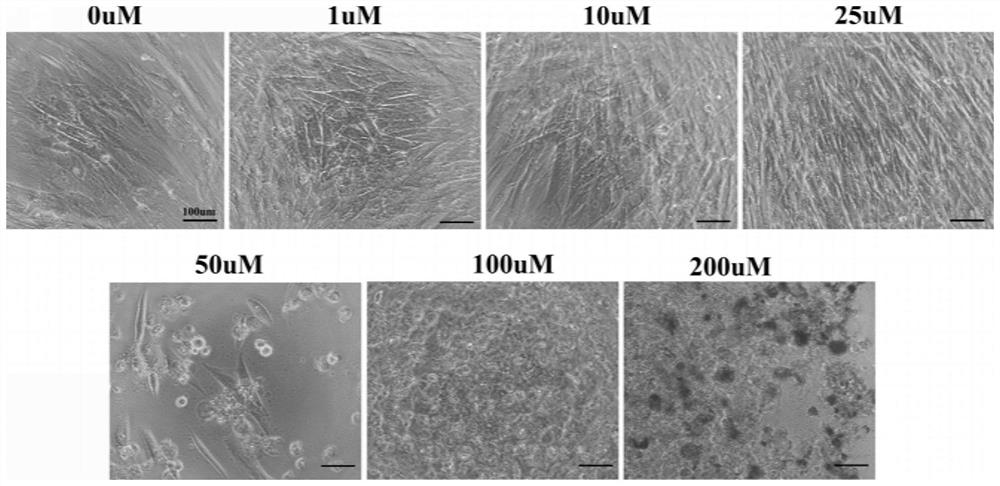
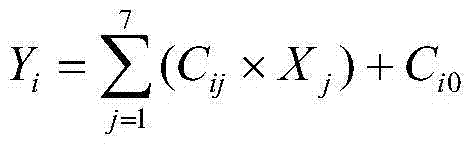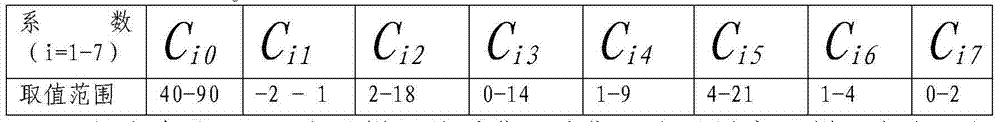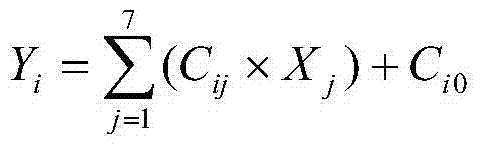Patents
Literature
60 results about "Schisandrin B" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of application of Schisandrin B in the preparation of anticancer medications
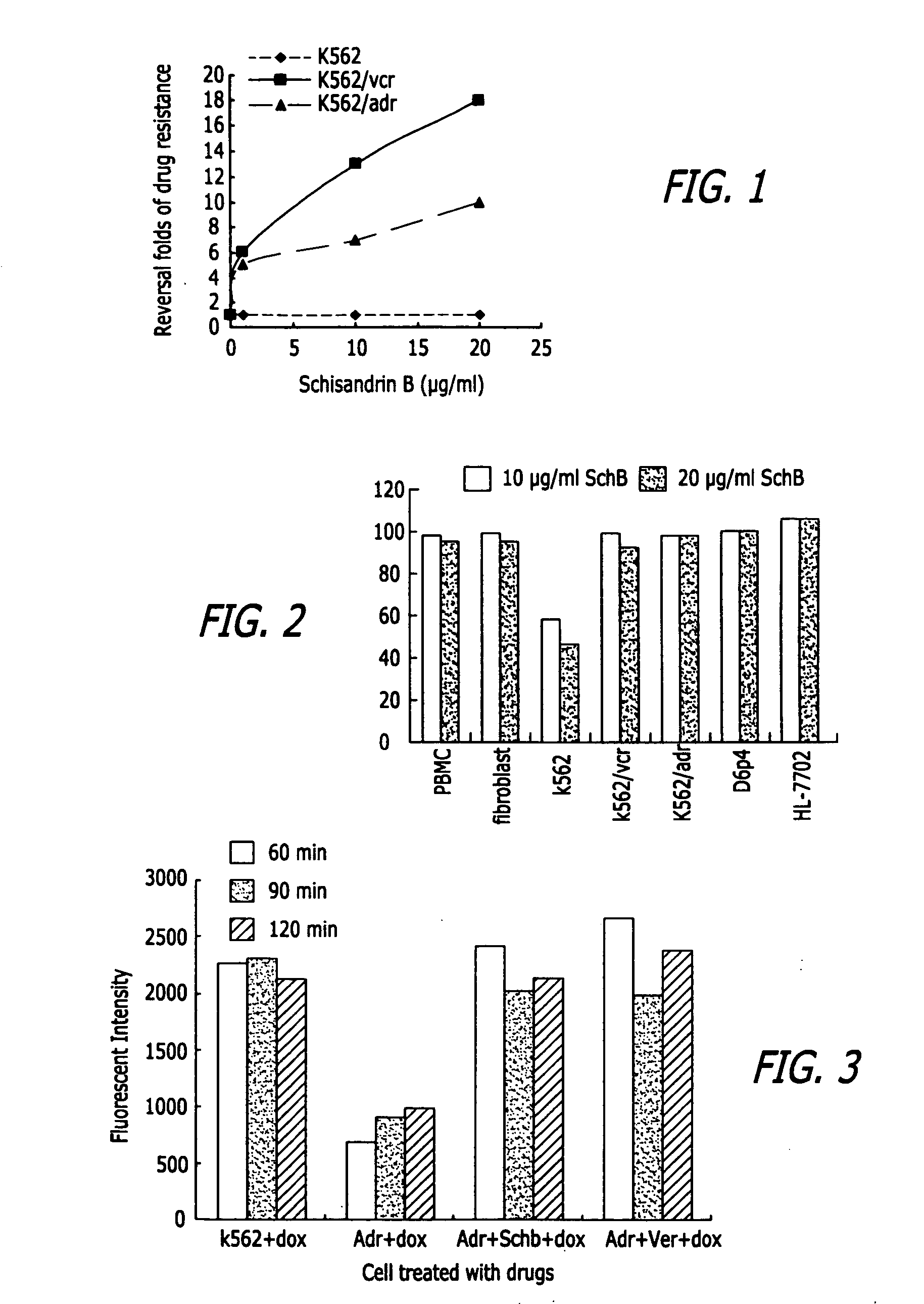
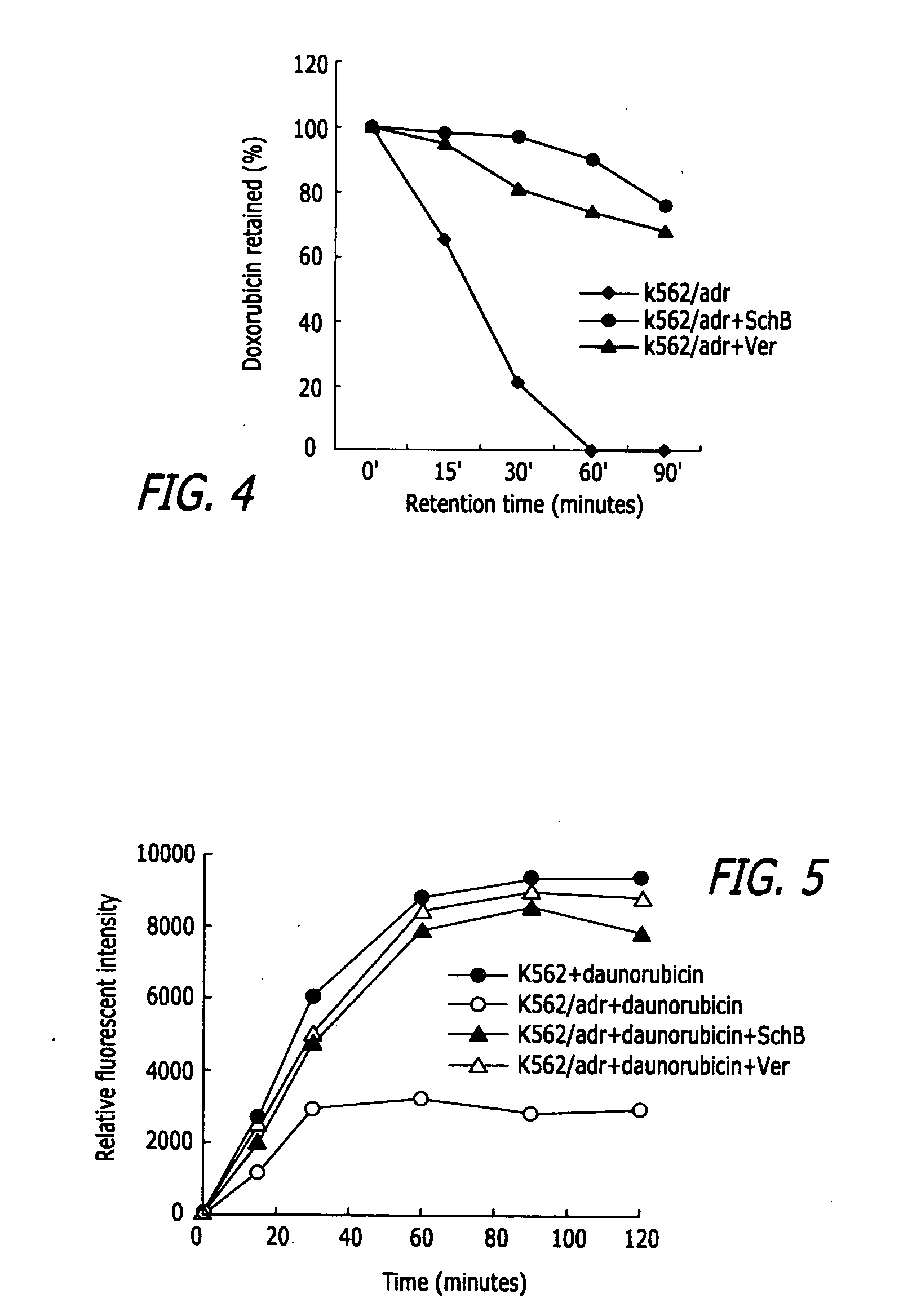
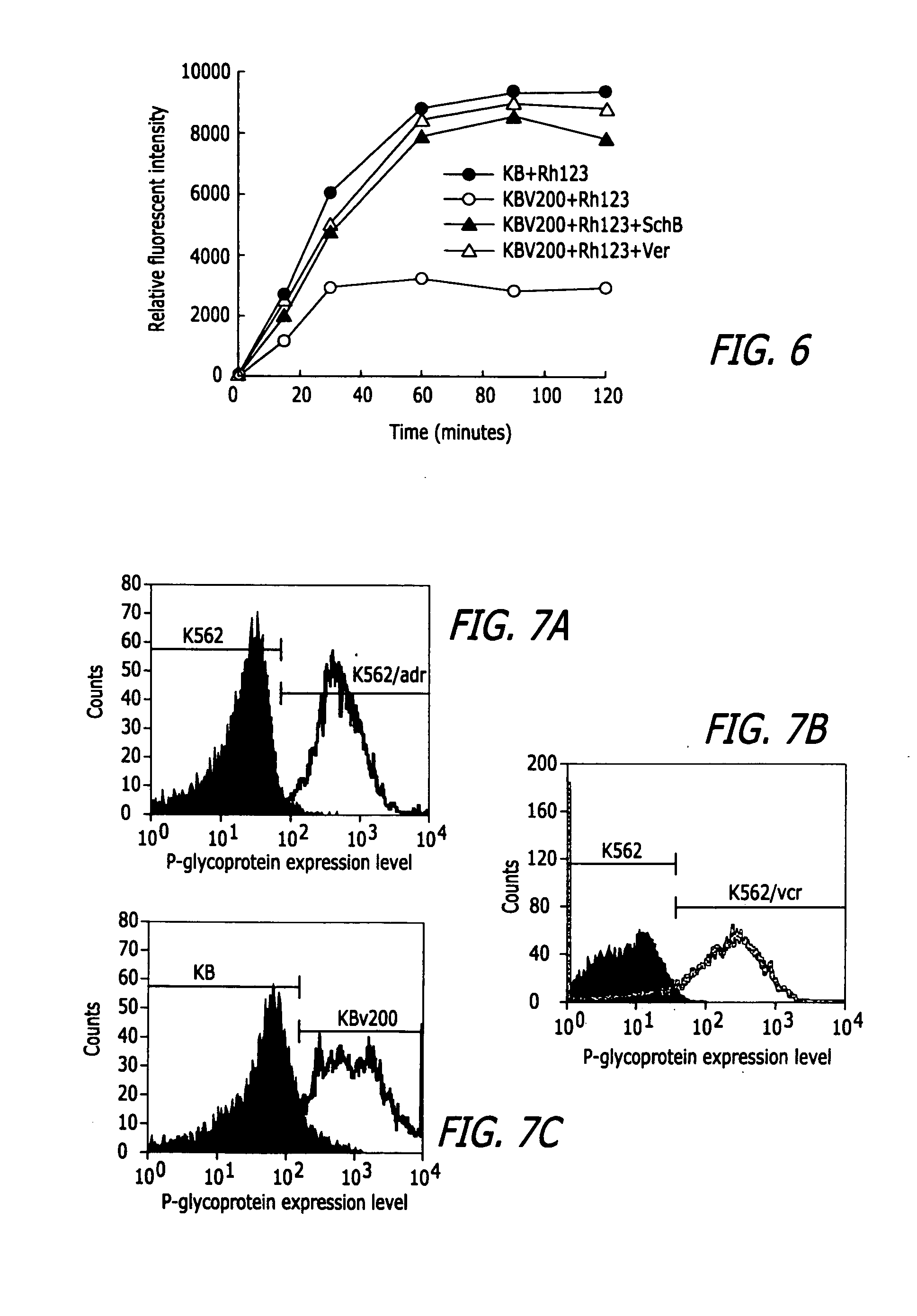
InactiveUS20050119337A1Inhibits drug transport function of P-glycoproteinHigh activityBiocideHeavy metal active ingredientsHuman cancerCancer cell
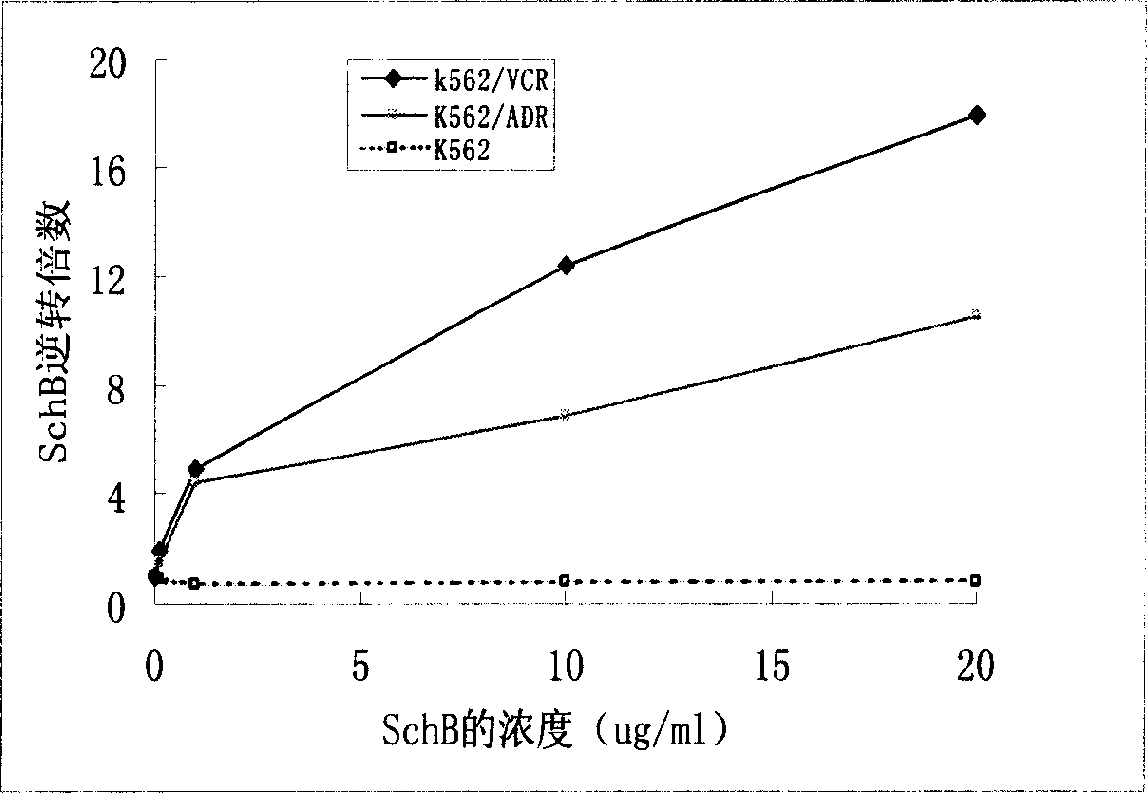
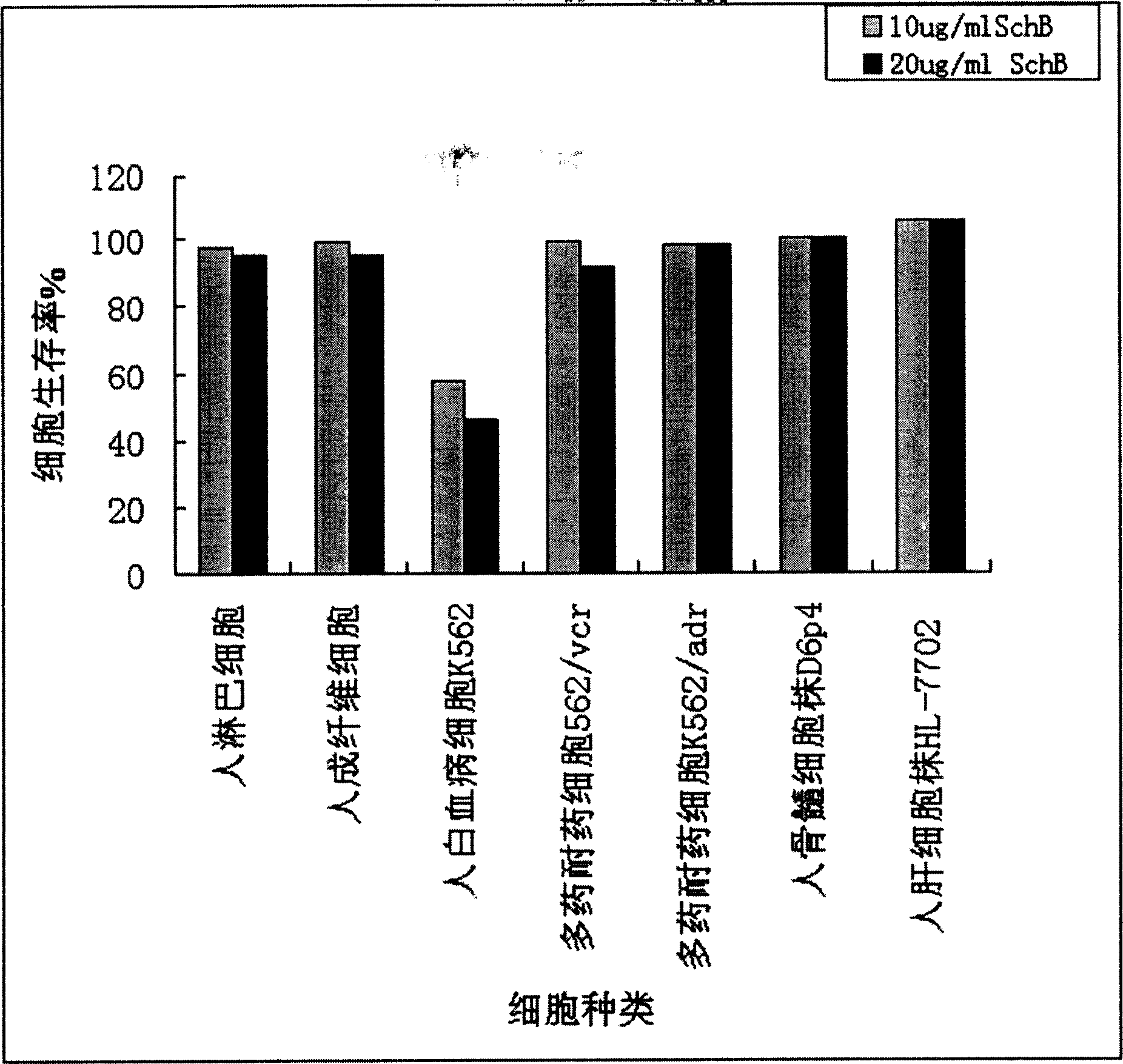
Methods of application of Schisandrin B in the preparation of anticancer medications, and particularly for the preparation of medications for the treatment of multidrug resistant (MDR) cancer. The compound of Schisandrin B effectively reverses MDR cancer in combination with other anticancer chemotherapeutic agents. Schisandrin B reverses MDR cancer by inhibiting the drug efflux activity of P-glycoprotein, indicating its significance in clinical applications. Although it is of low toxicity, Schisandrin B is cytotoxic to human cancer cells, revealing its application in cancer chemotherapy. It is emphasized that this abstract is provided to comply with the rules requiring an abstract that will allow a searcher or other reader to quickly ascertain the subject matter of the technical disclosure. It is submitted with the understanding that it will not be used to interpret or limit the scope or meaning of the claims.
Owner:NINGBO INNOPHARMA TECH
Method for controlling the quality of schisandra raw material fingerprint in the plant medicine for improving hemorheology
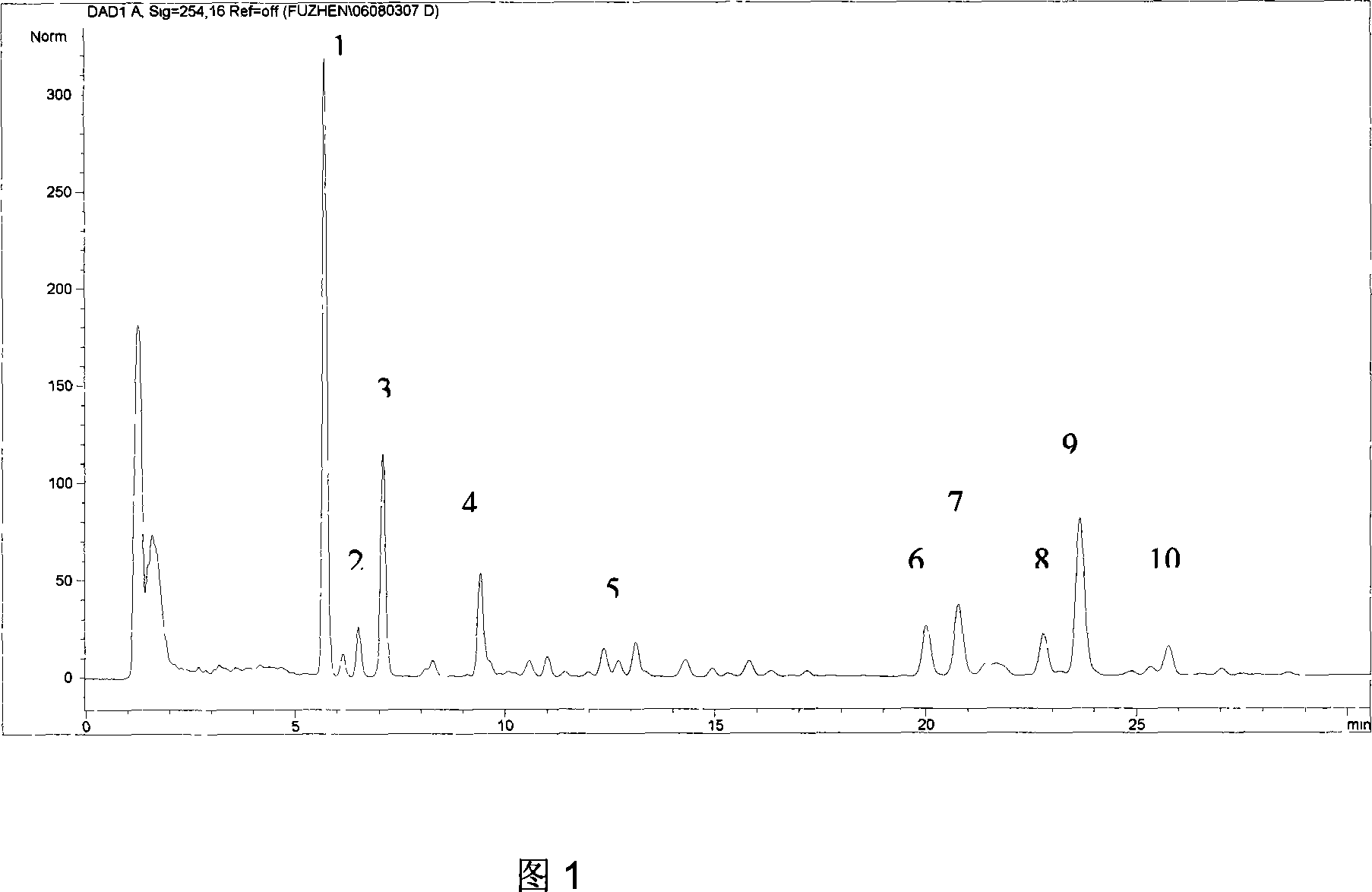
ActiveCN101040909AGuarantee normal implementationMeet the identificationComponent separationPlant ingredientsColumn temperaturePhase gradient
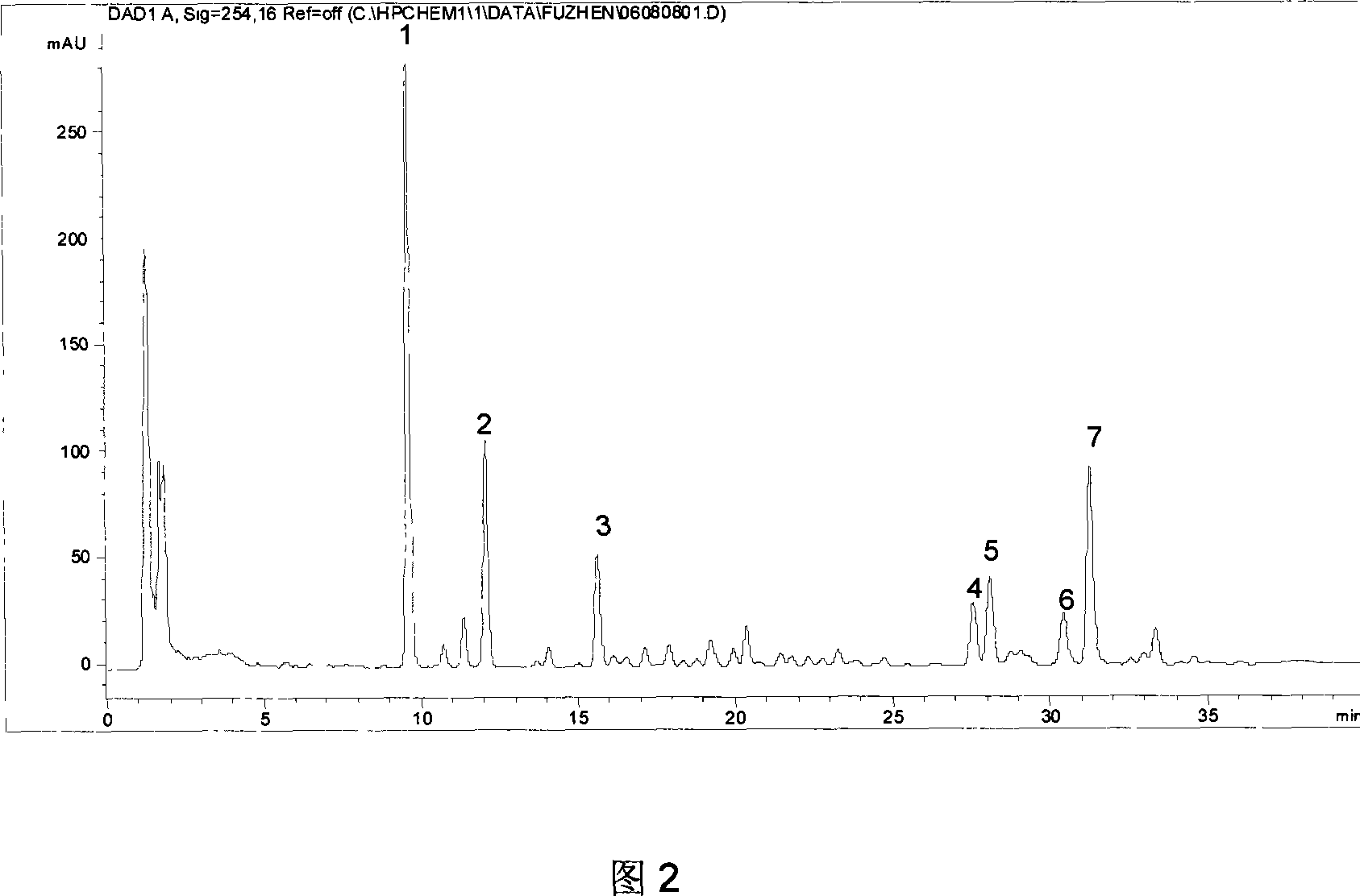
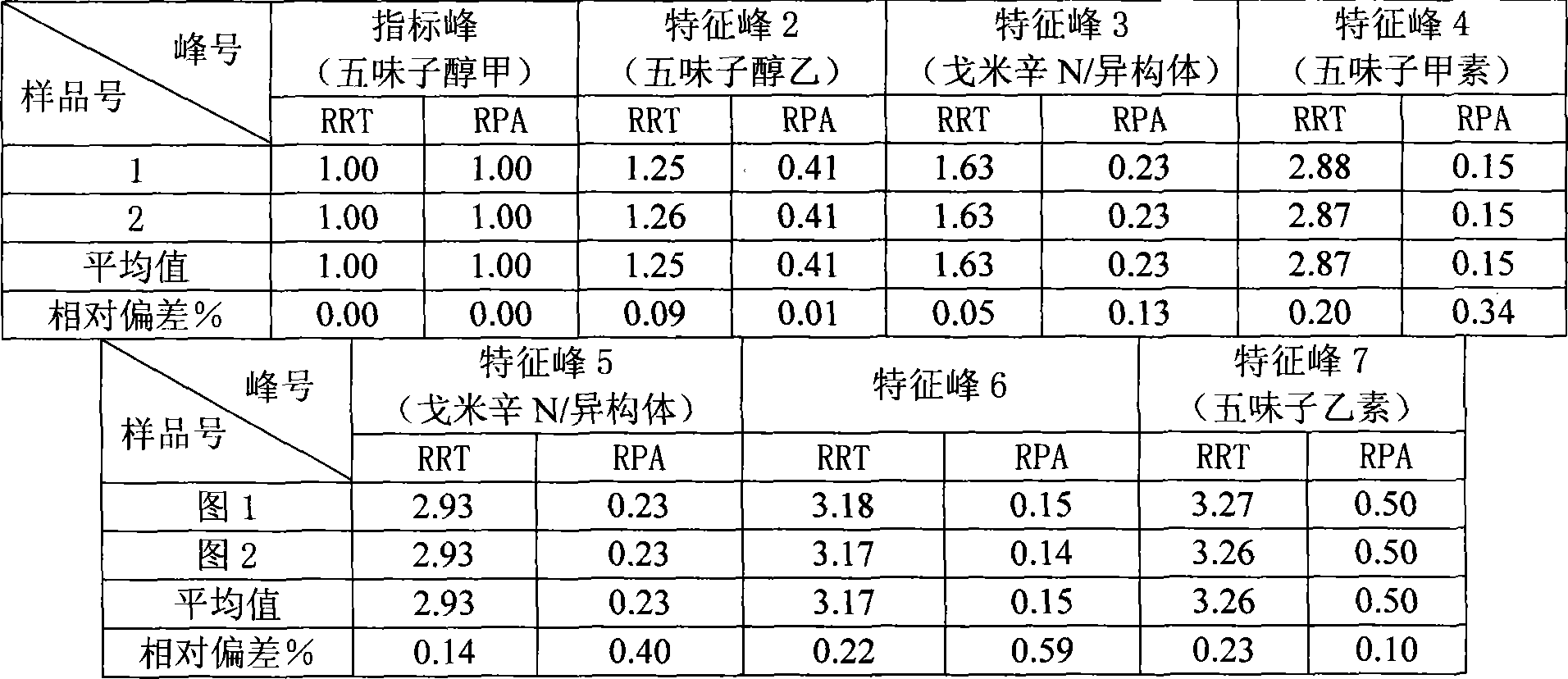
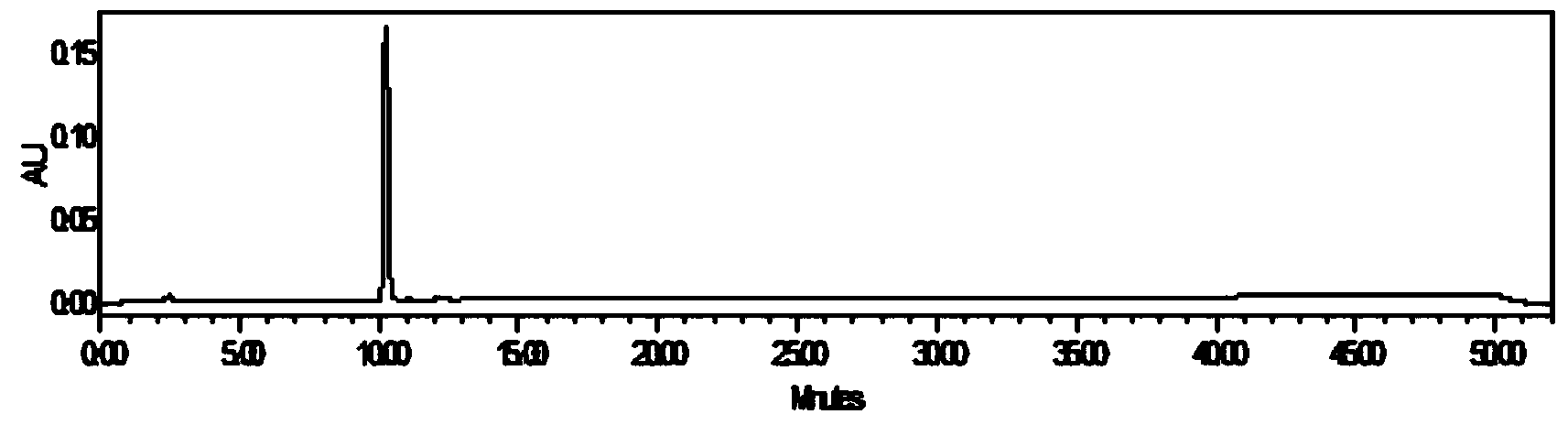
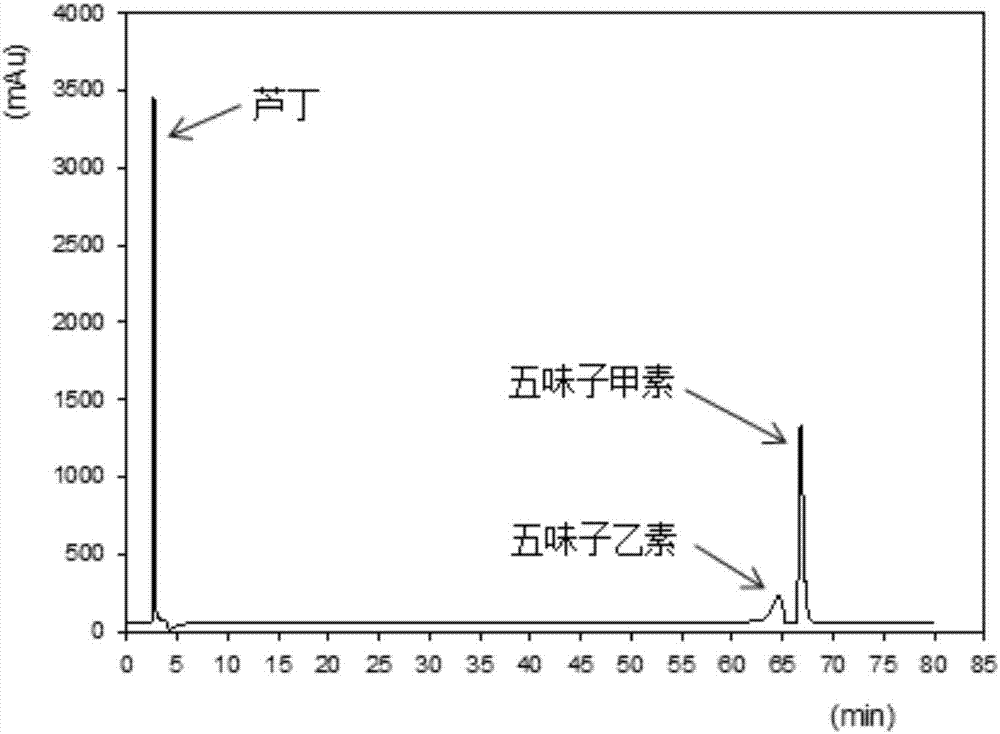
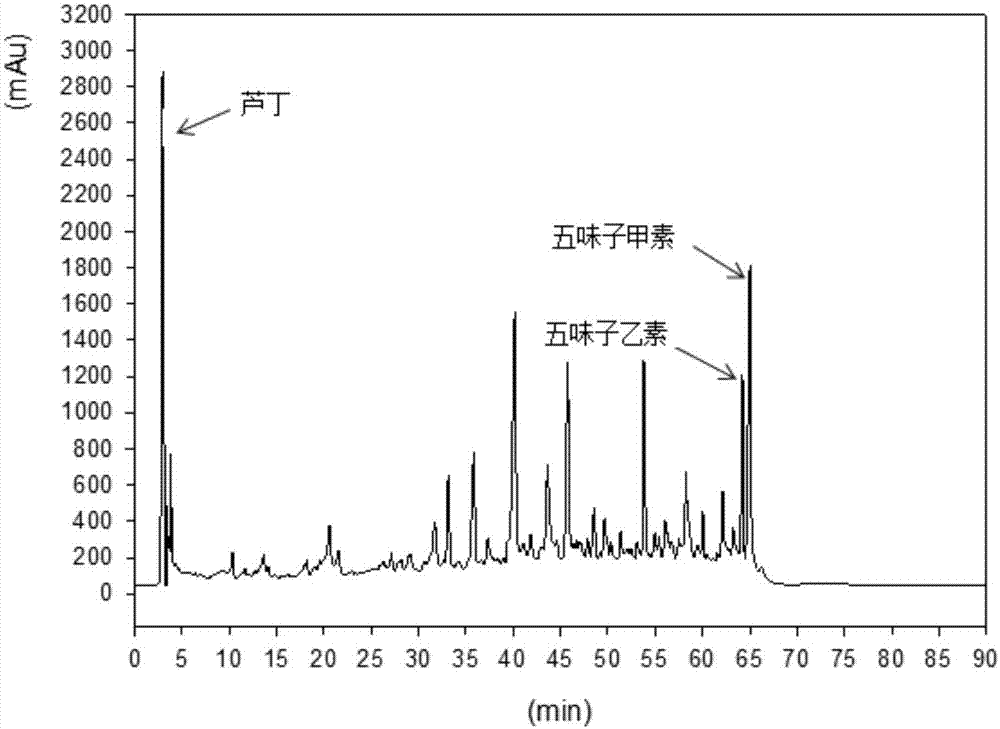
The invention relates to a schisandra fruit fingerprint spectrum quality control method, comprising that (1), adding 0.5g schisandra fruit to extract via microwave for 50min, filtering, (2), washing flow phase gradient that the spectrum column is Eclipse, as 0-40min, 60-20% A, 40-80% B, flow speed is 0.8-1.2ml / min, the wavelength is 210-280nm, the column temperature is 20-40Deg. C, and the sample amount is 10-20ul, (3), building standard fingerprint spectrum that the first peak is schisandra fruit alcohol 1, the third peak is schisandra fruit alcohol 2, the fourth peak is gemixin N / isomer, the sixth peak is schisandra fruit I element, the seventh and eighth peaks are gemixin N / isomer, the ninth peak is schisandra fruit II element, (4), controlling the quality of fingerprint spectrum that the check peak relative holding times are 1.00, 1.25, 1.63, 1.88, 3.18 and 3.27, (5), the schisandra fruit planting collecting method. The invention can control the quality of materials to assure the stable quality of product.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV
Method for extracting, separating and preparing lignin monomers from schisandra chinensis
ActiveCN103467438AEasy to separate and purifySimple extraction stepsOrganic compounds purification/separation/stabilisationEther separation/purificationChromatographic separationEthyl acetate
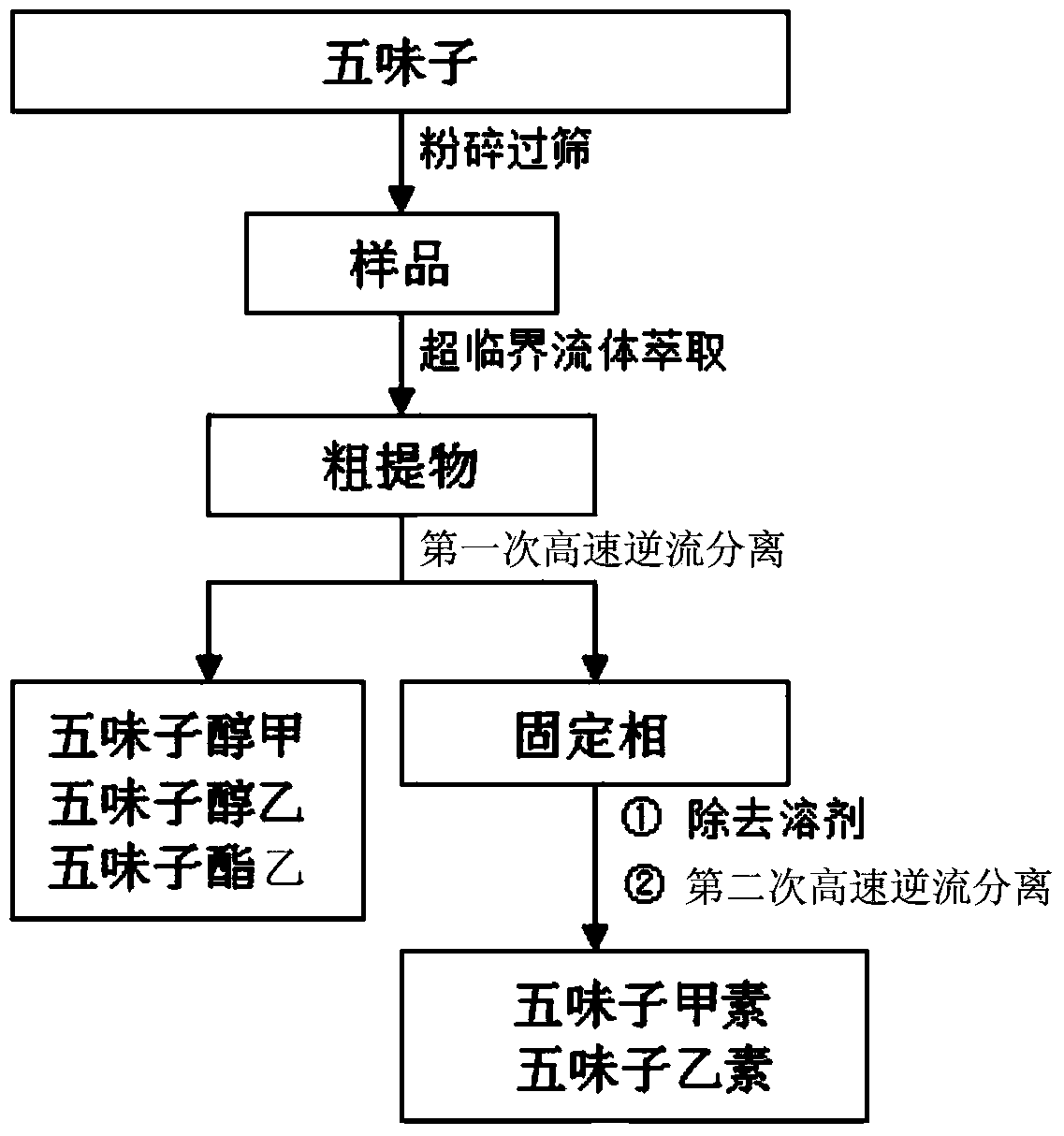
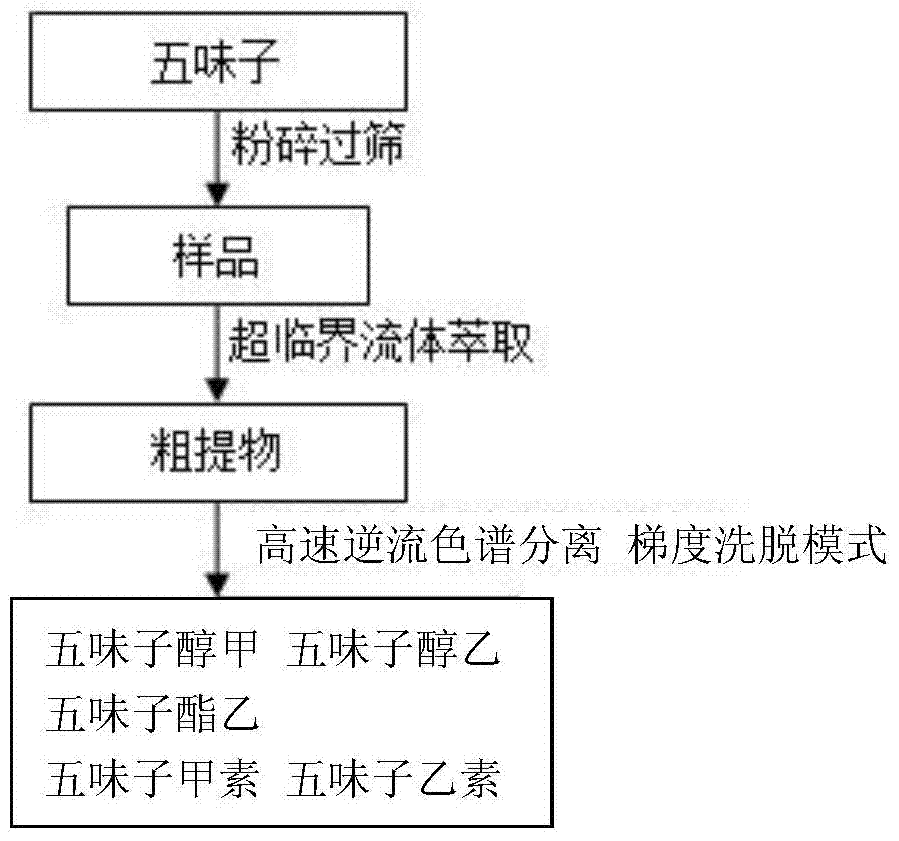
The invention discloses a method for extracting, separating and preparing lignin monomers from schisandra chinensis. The method comprises the following steps: performing supercritical extraction on schisandra chinensis medicinal powder by using CO2 to obtain a crude extract; performing primary high-speed counter-current chromatographic separation on the crude extract; forming a solvent system A by using hexane, ethyl acetate, methyl alcohol and water in the volume ratio of (2-10):(0-10):(0-10):5, wherein the upper phase A is a stationary phase A and the lower phase A is a mobile phase A; preparing a first schizandrol, a second schizandrol and a third schizandrol; recovering the separated stationary A to serve as a sample to be tested; performing secondary high-speed counter-current chromatographic separation; forming a solvent system B by using hexane, ethyl acetate, methyl alcohol and water in the volume ratio of (1-10):(0-4):(0-9):1, wherein the upper phase B is a stationary phase B, and the lower phase B is a mobile phase B; preparing deoxyschizandrin and schisandrin b. By adopting the method which integrates supercritical extraction and high-speed counter-current chromatographic separation, the deoxyschizandrin and the schisandrin b can be directly used for separating and purifying subsequent monomers without complex sample treatment after the extraction; the steps of extracting and separating are simple and efficient.
Owner:ZHEJIANG UNIV OF TECH
Method for extracting deoxyschisandrin and schisandrin B from schisandra
InactiveCN102050707APromote dissolutionHigh extraction rateEther separation/purificationPlant ingredientsDeoxyschisandrinVolume concentration
The invention discloses a method for extracting deoxyschisandrin and schisandrin B from schisandra, comprising the following steps: 1) crushing the schisandra, extracting with 80-95% ethanol by volume concentration, concentrating extracting solution to obtain concentrated solution; 2) adding ethanol in the concentrated solution and ensuring that the concentration of ethanol is 60-65% by weight, centrifuging and collecting supernate; 3) passing the supernate through a macroporous resin column, eluting with 55-65% ethanol by volume concentration, wherein the weight of ethanol is 1.5-2.5 times of that of the resin, collecting eluent A, and drying to obtain deoxyschisandrin extract; and 4) eluting with 85-95 % ethanol by volume concentration, wherein the weight of ethanol is 2.5-3.5 times of that of the resin, collecting eluent B, and drying to obtain schisandrin B extract. By using the method in the invention, the deoxyschisandrin and schisandrin B are eluted in turn, so that the deoxyschisandrin and schisandrin B are separated, wherein the content of the deoxyschisandrin is 0.8-1.2%, and the content of the schisandrin B is 3-4%.
Owner:GUILIN NATURAL INGREDIENTS CORP
Application of Schisandrin-B in preparing medicine for treating tumor
InactiveCN1621037ALow toxicityInhibitory functionAntineoplastic agentsHeterocyclic compound active ingredientsTumor chemotherapyNeoplasm
The invention discloses the application of schisandrin B in the preparation of tumor treating drugs, especially in the preparation of tumor cell multidrug resistance reversal agents and tumor chemotherapy drugs. The compound can also be used in combination with other drugs to further improve the reversal effect. Schizandrin B can effectively reverse the multidrug resistance of tumors. The compound acts on P-glycoprotein and can completely inhibit the function of P-glycoprotein, so it has clinical application significance; in addition, Schizandrin B has low toxicity, but It is toxic to certain tumor cells, so Schizandrin B has good application prospects in clinical tumor chemotherapy, especially as a drug for reversing multidrug resistance of tumor cells.
Owner:高陈勇
Quality detection method for liver protection dropping pill of traditional Chinese medicine preparation
ActiveCN103808842AQuality improvementEfficient detectionComponent separationChlorogenic acidMedicine
The invention relates to a quality detection method for a liver protection dropping pill of traditional Chinese medicine preparation. The quality detection method comprises the following steps: measuring the content of effective components of the liver protection dropping pill, including saikoside a and schizandrin, and identifying schisandra chinensis, pulvis fellis suis and artemisia capillaris in the liver protection dropping pill. The quality detection method also comprises the following detection steps: (1) with the saikoside a as a reference substance, measuring whether a radix bupleuri component is contained in a liver protection dropping pill recipe by adopting a high efficiency liquid chromatography method; (2) with the schizandrin as the reference substance, measuring whether a schizandrin component is contained in the liver protection dropping pill by adopting the high efficiency liquid chromatography method; (3) with the schisandrin b as the reference substance, identifying whether a schisandra chinensis component is contained in the liver protection dropping pill by adopting a thin-layer chromatography method; (4) with hyodeoxycholic acid as the reference substance, identifying whether a pulvis fellis suis component is contained in the liver protection dropping pill by adopting the thin-layer chromatography method; and (5) with chlorogenic acid as the reference substance, identifying whether an artemisia capillaris component is contained in the liver protection dropping pill by adopting the thin-layer chromatography method. The quality detection method disclosed by the invention can be used for effectively and reliably controlling the quality of the liver protection dropping pill, and the method is scientific, feasible and reliable.
Owner:HEILONGJIANG KUIHUA PHARMA
Use of schisandrin B in anti-liver fibrosis
InactiveCN103860544ANo obvious side effectsImprove convenienceDigestive systemHeterocyclic compound active ingredientsDiseaseLiver fibrosis
The invention belongs to the field of traditional Chinese medicines or the pharmaceutical chemistry, and relates to a use of schisandrin B or a Chinese magnoliavine extract in the preparation of medicines for treating and / or adjunctively treating and / or preventing liver fibrosis or liver cirrhosis, medicines for treating or adjunctively treating diseases caused by the liver fibrosis or liver cirrhosis, or medicines for inhibiting liver stellate cell proliferation or DNA synthesis. The invention also relates to a method for inhibiting the liver stellate cell proliferation or inhibiting the liver stellate cell DNA synthesis in vitro / in vivo, and a medicinal composition containing the schisandrin B or the Chinese magnoliavine extract. It is proved that the schisandrin B can obviously improve the liver fibrosis or the liver cirrhosis degree, has no obvious hepatotoxicity and has a good application prospect.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Composition for protecting lever and achieving sobering function
ActiveCN102579641AAlcoholic liver damageGood effectNervous disorderDigestive systemMedicineVitamin B12
The invention discloses a composition for protecting lever and achieving a sobering function as well as a preparation method and application of the composition. The composition contains 30-180 parts by weight of sesamin, 1-6 parts by weight of schisandrin B, 150-900 parts by weight of semen hoveniae extract, 150-900 parts by weight of pueraria lobata extract, 0.0012-0.0072 parts by weight of vitamin B12 and 14-84 parts by weight of vitamin E.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Compound type natural traditional Chinese medicine preservative composition as well as preparation method and application thereof
ActiveCN106821779AReduce usageGood antibacterial broad spectrumCosmetic preparationsToilet preparationsBactericidal effectBroad spectrum
The invention discloses a compound type natural traditional Chinese medicine preservative composition as well as a preparation method and application thereof. The compound type natural traditional Chinese medicine preservative composition provided by the invention comprises effective components including schisandrin, baicalein and resveratrol. Preferably, the weight ratio of the schisandrin to the baicalein to the resveratrol is (1-3) to (50-100) to (25-50); more preferably 1 to 50 to 25. An experiment process that the compound type natural traditional Chinese medicine preservative composition has a synergetic bactericidal effect on escherichia coli, staphylococcus aureus and Candida albicans; the dosage of a single drug is reduced; and meanwhile, the compound type natural traditional Chinese medicine preservative composition has a good broad-spectrum antibacterial property, can be used for inhibiting growth of common bacteria of cosmetics and cannot be used for completely killing staphylococcus epidermidis. The compound plant-derived preservative disclosed by the invention has the characteristics of safety and high efficiency, can be used in the fields including the cosmetics, medicines, foods and the like, especially skin-care cosmetics, and has a good application prospect.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
Method for extracting Schisandrin B from Schisandra chinensis
The invention provides a method for extracting Schisandrin B from Schisandra chinensis. The method adopts macroporous adsorption resin column chromatography, silica gel column chromatography and recrystallization to extract and separate Schisandrin B from the Schisandra chinensis medicine in order to obtain high-purity Schisandrin B, and accords with the technological characteristics of industrial production. The method has the following advantages: the purity of the prepared Schisandrin B can reach 90-100%, and the method can be applied to the industrial large-scale preparation of the Schisandrin B.
Owner:梦阳药业上海有限公司
Schisandrin B preparation
The present invention provides a preparation for treatment or prevention of a condition in a patient, said preparation comprising Schisandrin B. The concentration of Schisandrin B in the preparation may be between about 0.01 and about 0.1%, or it may be between about 20 and about 40% w / w or w / v. The preparation may additionally comprise one or more components selected from the group consisting of herbal extracts, fluids, solvents, antioxidants, preservatives, electrolytes, salts and pH control agents.
Owner:THE HONG KONG UNIV OF SCI & TECH
Radiosensitizer compositions comprising schisandra chinensis(turcz.)baill and methods for use
InactiveUS20110124741A1Good effectBiocideEther/acetal active ingredientsRadiation sensitizersBULK ACTIVE INGREDIENT
The present invention provides a method of potentiating radiation therapy for treatment of a cancer or tumor comprising administrating to a subject in need thereof a therapeutically effective amount of a radiosensitizer in combination of a radiation therapy to a locus of the cancer or tumor, wherein the radiosensitizer is an extract of Schisandra chinensis (Turcz.) Baill, or the active ingredient isolated therefrom, particularly Schisandrin B.
Owner:ETEN BIOTECH
Schizandrin, schisanhenol and schisandrin-b derivates and application thereof
InactiveCN102627625ALow price screeningThe curative effect is sure and does not reboundOrganic compound preparationQuinone preparation by oxidationSchisanhenolCurative effect
The invention discloses schizandrin, schisanhenol and schisandrin-b derivates and an application thereof in the preparation of drugs for treating liver cell injury. Compared with the prior art, the schizandrin, the schisanhenol and the schisandrin-b are subjected to structural modification and transformation, 4 substitution positions and / or 11 substitution positions are respectively halogenated and / or oxidized to generate derivates with a structure of a general formula (1), and the derivates have a remarkable protection effect on CC14 human liver HL-7702 cell injury and can serve as candidate compounds of novel anti-liver injury drugs with low screening price and definite and relapse-free treatment effect, particularly anti-chemical injury drugs.
Owner:THE KEY LAB OF CHEM FOR NATURAL PROD OF GUIZHOU PROVINCE & CHINESE ACADEMY OF SCI
Medical composite for preventing and curing Alzheimer's disease
ActiveCN101797267AImprove spatial learning and memoryImprove cognitive impairmentNervous disorderHeterocyclic compound active ingredientsDiseaseMedicine
The invention discloses a medical composite for preventing and curing Alzheimer's disease (AD). The composite comprises the following raw materials in parts by weight: 10-40 parts of hyperin, 20-80 parts of icariin and 5-20 parts of Schisandrin B. Experiments of rat place navigation and space exploration prove that the medical composite of the invention can increase the ability of spatial learning and memory of AD rat model, namely reduce the escape latency and scouting distance and obviously increase the percent of scouting time in quadrant of original platform to total time and the percent of scouting distance in quadrant of original platform to total distance in space exploration experiments. Therefore, the extract composite can be used for preventing and curing AD.
Owner:PEKING UNIV FIRST HOSPITAL
Extraction separation preparation method of lignin monomers in schisandra chinensis
ActiveCN103694213AEasy to separate and purifySimplified extraction stepsEther separation/purificationBulk chemical productionChromatographic separationGradient elution
The invention discloses an extraction separation preparation method of lignin monomers in schisandra chinensis, which comprises the following steps: performing CO2 supercritical extraction of schisandra chinensis medicinal material powder, performing separation of crude extract by high-speed countercurrent chromatography, performing gradient elution, wherein the high-speed countercurrent solvent system A comprises n-hexane, ethyl acetate, methanol and water, fully mixing, standing, performing elution with the upper phase A as a stationary phase A and the bottom phase A as a mobile phase A to obtain schisandrol A and schisandrol B, changing the solvent system, increasing the volume ratio of methanol to water, performing elution with the bottom phase B as a mobile phase B to obtain schisantherin B; changing the solvent system, further increasing the volume ratio of methanol to water, performing elution with the bottom phase C as a mobile phase C to obtain schisandrin B. According to the invention, the method combines supercritical fluid extraction with high-speed countercurrent chromatography separation; the product can be used directly for subsequent monomer separation and purification without complicated sample treatment after extraction; 5 lignin monomers can be obtained by only separation once; the method is simple and high-efficient.
Owner:ZHEJIANG UNIV OF TECH
Medicinal composition for treating diabetes hepatic fibrosis
ActiveCN103860565AGood inhibitory effectEnhanced inhibitory effectDigestive systemHeterocyclic compound active ingredientsHepatic stellate cell proliferationTraditional medicine
The invention belongs to the field of traditional Chinese medicines or the pharmaceutical chemistry, and relates to a medicinal composition for resisting hepatic fibrosis and / or hepatic cirrhosis. The medicinal composition includes ursolic acid and schisandrin B. The invention also relates to a use of the medicinal composition in the preparation of medicines for treating and / or preventing and / or adjunctively treating hepatic fibrosis and hepatic cirrhosis, or medicines for inhibiting hepatic stellate cell proliferation or inhibiting hepatic stellate cell DNA synthesis, and a method for inhibiting hepatic stellate cell proliferation or inhibiting hepatic stellate cell DNA synthesis. The medicinal composition can obviously improve the hepatic fibrosis degree, has the advantages of few components, strong effect, simple preparation, no obvious hepatotoxicity, and good market prospect, and is of certain practical significance for promoting traditional Chinese medicinal modernization.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
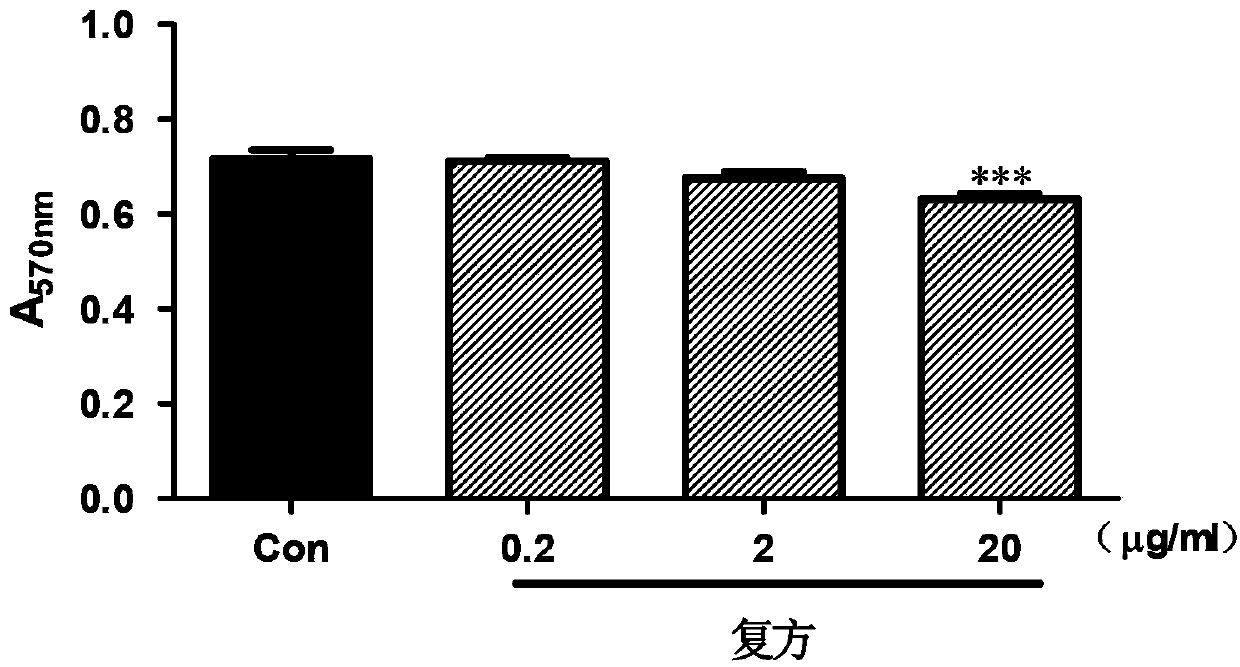
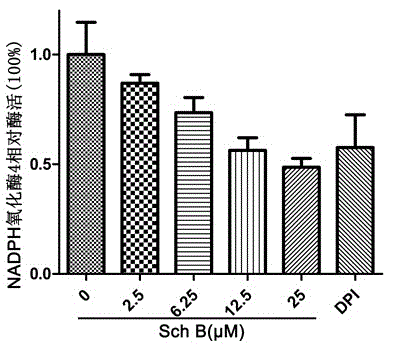
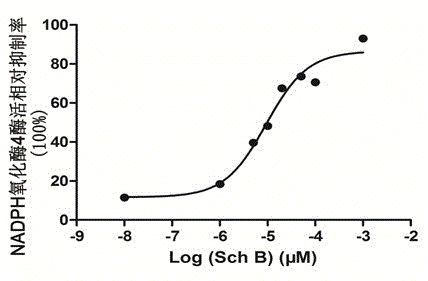
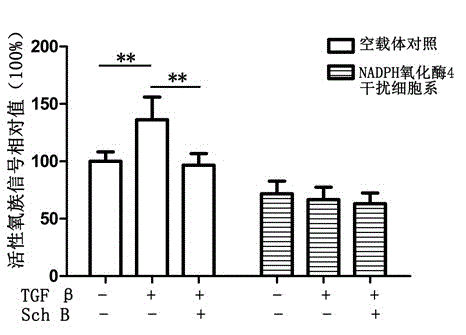
Application of schisandrin b in preparing NADPH oxidase inhibitor
InactiveCN104147001AModerate inhibitory activityGood biological low toxicityNervous disorderRespiratory disorderLymphatic SpreadOxygen ions
The invention provides an application of schisandrin b in preparing an NADPH oxidase inhibitor. The NADPH oxidase comprises various sub-types of oxidase. In in-vitro enzymatic experiments, the schisandrin b is capable of inhibiting super-oxygen ion generation reaction catalyzed by NADPH oxidase and reactive oxygen generation related to NADPH oxidase in cells, and does not have the function of scavenging free radicals, thus being an inhibitor of the NADPH oxidase. Furthermore, the NADPH oxidase is capable of changing the level of a plurality of signal transduction pathways and inhibiting generation of tumor metastasis, and can be applied to preparation of medicaments for treating tumor metastasis.
Owner:ZHEJIANG UNIV
Detection method of functional effective components of radix seu caulis schisandrae propinquae and application
ActiveCN107976498ARealize quality controlFully reflect the efficacyComponent separationTest sampleElution
The invention belongs to the technical field of the detection of components of traditional Chinese medicines, and discloses a detection method of effective components of radix seu caulis schisandrae propinquae and application. The detection method comprises steps of preparation of standard substance solutions of rutin, schizandrin A and schisandrin B respectively, preparation of a test sample solution, HPLC (High Performance Liquid Chromatography) detection and the like, wherein chromatographic conditions of the HPLC detection are as follows: a methanol-1 percent glacial acetic acid solution is used as mobile phase eluent; the gradient elution is carried out for 90min; volume fractions of methanol in the eluent corresponding to each time interval of the elution are respectively: 20 percentduring 0min to 20min, 40 percent during 20min to 40min, 60 percent during 40min to 60min, 80 percent during 60min to 80min and 100 percent during 80min to 90min; a flow speed is 1mL / min; a detectionwavelength is 257nm. The detection method uses the HPLC detection with the gradient elution, can be used for measuring three functional effective components in the radix seu caulis schisandrae propinquae at the same time, and can be used for more completely reflecting a pharmacodynamic effect of the radix seu caulis schisandrae propinquae.
Owner:HUAIHUA UNIV
Application of schisandrin B in preparation of diluted liquid for preserving boar semen
ActiveCN104770362AQuality improvementWell preserved and reliableDead animal preservationSodium bicarbonateRoom temperature
The invention relates to an application of schisandrin B in the preparation of a diluted liquid for preserving boar semen. The invention also relates to a diluted liquid for preserving boar semen. The diluted liquid comprises glucose, sodium bicarbonate, sodium citrate, EDTA, critic acid, potassium chloride, gentamycin, and schisandrin B. The effect of the provided diluted liquid for preserving boar semen is good and reliable. The high quality semen stored at a room temperature can be provided for pig artificial insemination. After the boar semen is preserved in the diluted liquid at a temperature lower than 17 DEG C for five days, the semen activity is still above 0.6.
Owner:NORTHWEST A & F UNIV
Combined type method for extracting schisandrin b from fructus schisandrae
The invention relates to a combined type method for extracting schisandrin b from fructus schisandrae. The method comprises the steps of pretreatment of raw materials, preparation of compound extractant, extraction and separation and purification; the compound extractant formed by appropriate ionic liquid, assistant and hexadecyl trimethyl ammonium bromide is adopted, solid sodium carbonate is added for auxiliary extraction, and besides, microwaves and expanded solvent are adopted for complementary extraction, therefore, the method for extracting the schisandrin b from the fructus schisandrae with high yield is achieved finally, the material utilization rage is improved, waste discharge and environment pollution, caused by a great amount of organic solvents, are avoided, and wide industrial application prospect is achieved.
Owner:闵令杰
Method for simultaneously testing contents of seven lignans in schisandra chinensis
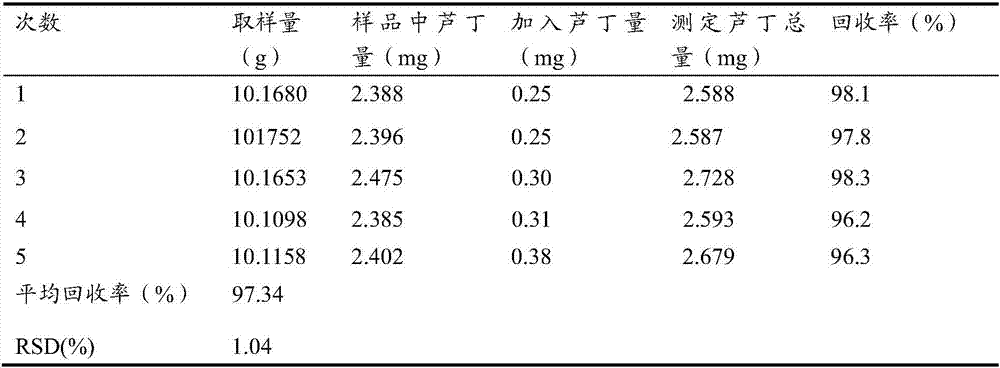
The invention provides a method for simultaneously testing contents of seven lignans in schisandra chinensis. According to the method, a chromatographic column ZORBAX Eclipse Plus C18 column (1.8 [mu]m, 100 mm*2.1 mm) is used; a mobile phase, namely acetonitrile-water, is adopted for gradient elution; the flowing speed is 0.3 mL*minute-1; the column temperature is 40 DEG C; the detection wavelength is 220 nm; and the sample feeding volumn is 1 [mu]L. Results show that components, namely schisandrol A, schisandrol B, schisantherin A, schisantherin B, schizandrin A, schisandrin B and schisandrinC, generally meet linear separation and are in good linear relationship, the average sampling recycling rate is within 98.29-102.5%, and the RSD (Relative Standard Deviation) is within 1.4-1.9%. Testresults show that the method is rapid, stable and reliable and applicable to testing on contents of lignans in southern and northern schisandra chinensis in different production areas, and identification on southern and northern schisandra chinensis.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for purifying schizandrin and schisandrin b from schisandra extract
ActiveCN104926624AEasy to operateReduce consumptionEther separation/purificationSchisandrin BPrecipitation
The invention relates to a method for purifying schizandrin and schisandrin b from a schisandra extract. The method comprises the following steps: extracting a schisandra crude drug, collecting an extracting solution, concentrating the extracting solution to 0.3-0.8 time of the weight of the crude drug, adding purified water with the weight being 2-4 times of that of the crude drug in a concentrated solution, cooling under 0-4 DEG C and standing for 4-6 h for water precipitation, performing first high-speed centrifugation separation, and precipitating to wait treatment; adding 10% to 20% active carbon with the weight of the crude drug to a supernate, stirring for adsorption for 0.5-3 h, performing second high-speed centrifugation separation, and centrifuging the supernate for rejection; adding 60% to 95% (v / v) ethanol solution with the weight being 4-20 times of that of the crude drug to active carbon, heating to 50-80 DEG C for elution for 1-3 h, performing third high-speed centrifugation separation, and collecting the supernate; and adding the supernate for sedimentation after first centrifugation, stirring, homogenizing, adding auxiliary materials with the weight being 3% to 8% of the weight of the crude drug, and drying to obtain a dry extract containing the schizandrin and the schisandrin b. The method is simple in operation, safe, non-toxic, low in cost and high in recovery rate, can recycle the schizandrin and the schisandrin b simultaneously, and has good development prospects of medicine and health food.
Owner:劲牌持正堂药业有限公司
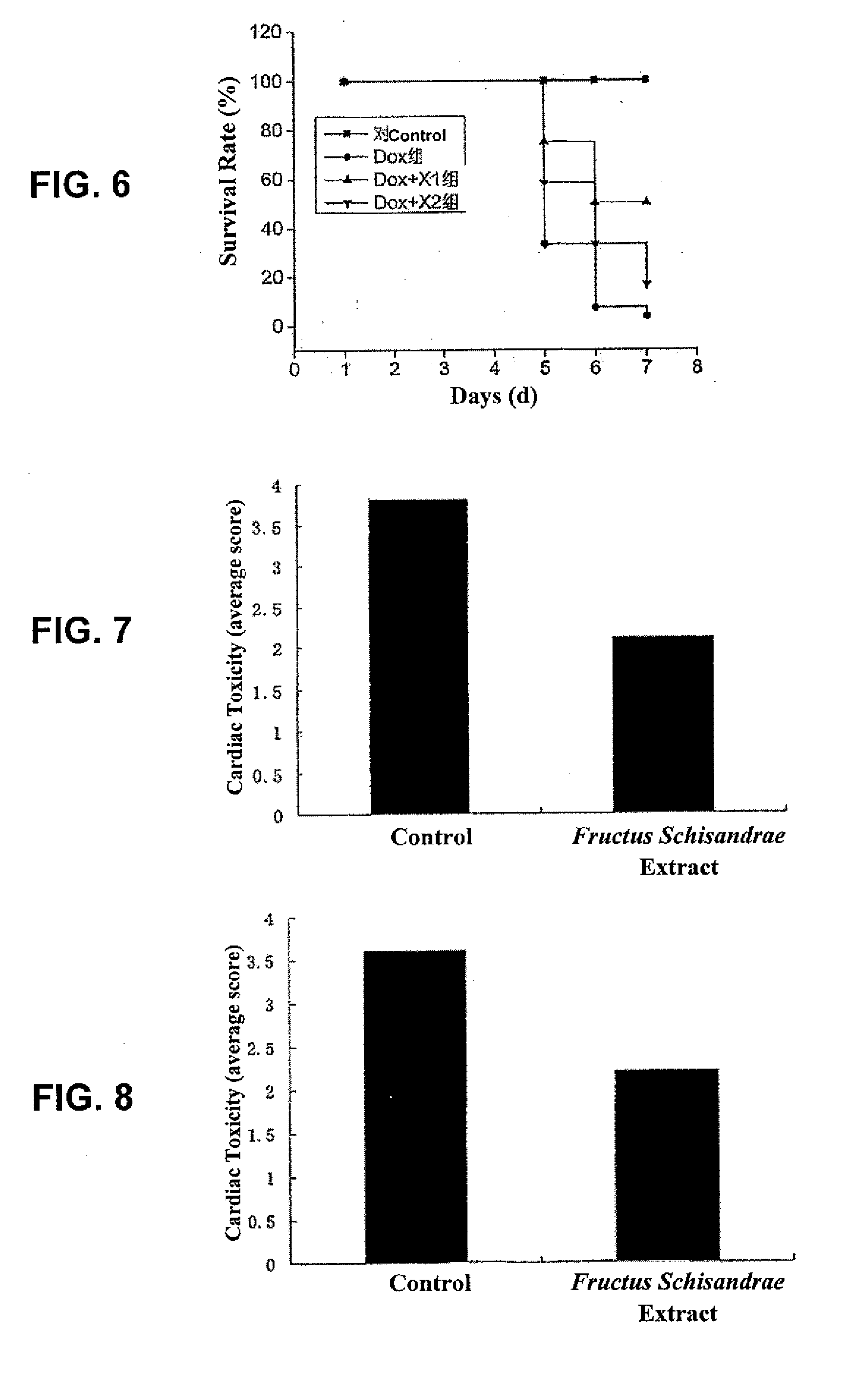
Use of fructus schisandrae and extracts thereof in preventing and decreasing toxic and side effects of antineoplastic drugs
ActiveUS20090022831A1Preventing and reducing toxicityPreventing and reducing and side effectAntibacterial agentsBiocideSide effectNephrotoxicity
Use of Fructus schisandrae in preparation of medicaments for preventing and reducing toxicity and side effects of antineoplastic agents. The toxicity and side effects of antineoplastic agents are cardiovascular toxicity, hepatotoxicity, nephrotoxicity, suppression of bone marrow, immunosuppression, or alopecia etc induced by antineoplastic agents. Fructus schisandrae and extracts thereof, especially ethanol extracts, schisandrin B, are effective in reducing antineoplastic agent's toxicity and side effects.
Owner:ZHE JIANG DINGHUI PHARM
Traditional Chinese medicine formula for treating lung and kidney qi deficiency syndrome of chronic obstructive pulmonary disease and application thereof
ActiveCN107582585AAbundant raw materialsMethod scienceKetone active ingredientsRespiratory disorderAstragalosideGINSENG EXTRACT
The invention relates to a traditional Chinese medicine formula for treating lung and kidney qi deficiency syndrome of chronic obstructive pulmonary disease (COPD). The problem of the medicine for treating the lung and kidney qi deficiency patient in the stable period of COPD is effectively solved. The traditional Chinese medicine formula comprises 6-20mg ginseng extract, 10-100mg icariin, 1-8mg paeonol, 0.5-6mg nobiletin, 5-20mg paeoniflorin, 3-12mg schisandrin b, 1-8mg peimine, 5-20mg hesperidin, 3-12mg astragalus polysaccharide and 1-8mg astragaloside. The traditional Chinese medicine formula provided by the invention can be effectively applied to the treatment for the lung and kidney qi deficiency syndrome of chronic obstructive pulmonary disease and the preparation of the preparationthereof. Repeated test proves that the traditional Chinese medicine formula is capable of improving COPD lung function, relieving lung tissue pathological injury and inflammatory cell infiltration andimproving inflammatory response and oxidative stress and can be used as long-term used medicine in the stable period for treating COPD. The traditional Chinese medicine formula has the advantages ofreasonable composition, scientific method, high innovation, accurate curative effect and obvious economic and social benefits.
Owner:HENAN UNIV OF CHINESE MEDICINE
Agricultural product production place identification method
ActiveCN104764864AGuarantee the safety of medicine and foodRealize the protection of originComponent separationMaterial analysis by electric/magnetic meansAgricultural scienceFood safety
The present invention relates to a discrimination method, particularly to an agricultural product production place identification method, which is mainly used for schisandra chinensis production place identification. According to the agricultural product production place identification method, the independent variable factors in the crushed schisandra chinensis sample is qualitatively and / or quantitatively determined, and is used to carry out model regression, and the belonging production place of the sample is determined according to the dependent variable level, wherein the independent variable factors (Xj) are [delta]<15>N, the schisandrin content, the schisantherin A content, the schizandrin A content, the schisandrin B content, the B content and the Sr content. According to the present invention, the schisandra chinensis is adopted as the demonstration to develop the production place traceability research to screen the geographic labels for effectively indicating the schisandra chinensis production place source, such that the drug food safety of the consumer can be easily ensured, the original production place protection of the authentic herbs can be achieved, and the theoretical basis is provided for the optimized planting of the schisandra chinensis. In addition, the technology can further be promoted and applied for the agricultural product quality safety and control, and has broad application prospects.
Owner:SHENYANG INST OF APPLIED ECOLOGY - CHINESE ACAD OF SCI
Method for carrying out simultaneous quantitative analysis on four lignan components in Chinese magnoliavine raw material and Chinese magnoliavine extract
InactiveCN102419350ARealize simultaneous measurementSolve the problem that the raw materials of Schisandra chinensis cannot be measured at the same timeComponent separationLignanColumn temperature
The invention discloses a method for carrying out simultaneous determination on contents of schisandrin, schisantherin, deoxyschizandrin and schisandrin B in a Chinese magnoliavine raw material or a Chinese magnoliavine extract. The method employs means of crushing, sieving, methanol ultrasonic extraction, calibrating volume and filtering to treat schisandra chinensis or a Chinese magnoliavine extract to obtain a sample solution for testing; a high performance liquid chromatography is utilized for simultaneous determination on contents of four lignan components. The method utilizes a reverse direction distribution chromatographic theory to carry out analysis on the sample by gradient elution, under conditions of a column temperature of 25-45 DEG C, mobile phases of methanol and 0.05-0.2% trifluoroacetic acid aqueous solution (methanol and trifluoroacetic acid aqueous solution in a volume ratio of 50:50-100:0), an elution time of 30-60 min, a flow velocity of 0.7-1.5 ml / min and a detection wavelength of 210-280 nm. The quality detection method is simple, rapid, sensitive and accurate.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Schisandrin b preparation
ActiveUS20070191477A1Reducing aging-related mitochondrial antioxidant status changeReduce sensitivityBiocideNervous disorderReperfusion injurySchisandrin B
Owner:THE HONG KONG UNIV OF SCI & TECH
Chinese magnoliavine active extract
InactiveCN107513008AHigh purityHigh adsorption rateEther separation/purificationReflux extractionDeoxyschizandrin
The invention discloses a Chinese magnoliavine active extract, which comprises schisandrin, deoxyschizandrin, and schisandrin B. The preparation method comprises reflux extraction, concentration and water precipitation, adsorption, elution, and drying. Ethanol (85-95%) is used to extract the Chinese magnoliavine active substances through reflux extraction; then the active substances are purified by water precipitation, adsorption, macroporous resin elution, and reversed-phase high-performance liquid chromatography to obtain schisandrin, deoxyschizandrin, and schisandrin B, and the product quality is high. The production technology is simple, each operation has accurate data, the repeatability is good, the product quality is stable, ethanol is low in toxicity, and the extracted Chinese magnoliavine active substances are very safe and green.
Owner:浦江县美泽生物科技有限公司
Method for inducing human placental mesenchymal stem cells to differentiate into hepatocytes in vitro and composition containing schisandrin B
ActiveCN113604420AImprove expression levelHigh expression of cytochrome enzymesCulture processArtificial cell constructsMesenchymal stem cellSchisandrin B
The invention discloses a method for inducing human placenta mesenchymal stem cells to differentiate into hepatocytes in vitro. Schisandrin B is added in a hepatocellular-like induction stage, the effect of inducing the human placenta mesenchymal stem cells to differentiate into the hepatocytes with functions can be effectively improved, the used stem cells are the human placenta mesenchymal stem cells, and are rich in source, convenient to extract, clear in induction composition component and simple in induction operation, and the method has industrialization and commercialization prospects.
Owner:ZHUJIANG HOSPITAL SOUTHERN MEDICAL UNIV
Preparation process for schisandrol extract
InactiveCN102716187AHigh purityImprove qualityDigestive systemPlant ingredientsReflux extractionAlcohol
The invention discloses a preparation process for a traditional Chinese medicine extract and particularly relates to a preparation process for a schisandrol extract. The preparation process for the schisandrol extract comprises the following steps of pretreatment, extraction, concentration, reflux extraction, ethanol precipitation, reconcentration and drying. The schisandrol extract prepared by the preparation process has a medicinal function of lowering serum glutamic pyruvic transaminase and is used for treating chronic hepatitis. Verified by experiments, schisandrin b and total lignans only can be fully extracted in 85 percent of alcohol. When the extraction is performed by adopting the alcohol with other concentrations, active constituents cannot be completely extracted, and then, the curative effect is influenced. Meanwhile, by adopting the ethanol precipitation process in the preparation process disclosed by the invention, impurities, such as grease, can be removed, so that the purity is greatly increased, the quality is higher, and the curative effect is comparatively obvious.
Owner:安徽海神寿春药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com