Application of schisandrin b in preparing NADPH oxidase inhibitor
An oxidase inhibitor, schisandrin B technology, applied in the directions of active ingredients of heterocyclic compounds, drug combinations, cardiovascular system diseases, etc., can solve the problem of no schisandrin B inhibiting NADPH oxidase tumor metastasis and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
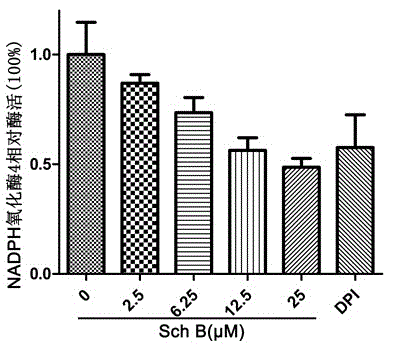
[0019] Example 1: Schizandrin B has a concentration-dependent inhibitory effect on NADPH oxidase 4
[0020] Experimental materials: Siha cell line was purchased from American Type Culture Collection (ATCC); Siha cell lysate was used as the source of NAPDH oxidase 4; β-NADPH was purchased from Roche Company; Jing (Lucigenin), dimethyl sulfoxide (DMSO) were purchased from Sigma.
[0021] Experimental method: Different concentrations of schisandrin B were mixed with 100 μM NADPH and 5 μM lucigeinin in the reaction buffer, and the reaction activity of NADPH oxidase 4 was detected. The relative enzyme activity is relative to the enzyme reaction activity of the control group without adding Schisandrin B.
[0022] Experimental results: see the experimental results figure 1 , it can be seen that the inhibitory effect of schisandrin on NADPH oxidase 4 increases with the increase of drug concentration.
Embodiment 2
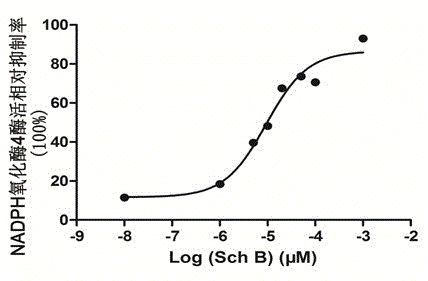
[0023] Example 2: Inhibition of Schizandrin B to NADPH Oxidase 4
[0024] Experimental materials: 4T1 mouse breast cancer cell line was purchased from American Type Culture Collection (ATCC); 4T1 cells were homogenized by a Dunes homogenizer, and 9000 g fraction was separated by differential centrifugation, and used as NADPH oxidase 4 Sources: β-NADPH was purchased from Roche; Schizandrin B was purchased from China Institute for the Control of Pharmaceutical and Biological Products; Lucigenin and DMSO were purchased from Sigma.
[0025] Experimental method: Different concentrations of schisandrin B were mixed with 100 μM NADPH and 5 μM lucigeinin in the reaction buffer, and the reaction activity of NADPH oxidase 4 was detected. The relative enzyme activity is relative to the enzyme reaction activity of the control group without adding Schisandrin B.
[0026] Experimental results: see the experimental results figure 2 , the experimental results proved again that the inhibito...
Embodiment 3
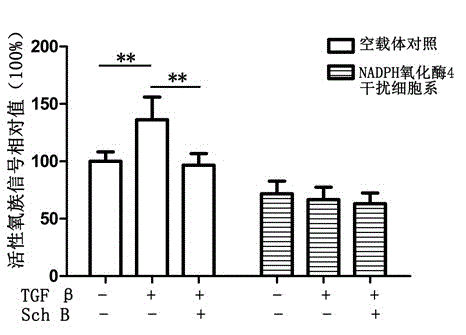
[0027] Example 3: Inhibitory relationship of Schizandrin B to the increase of reactive oxygen species mediated by NADPH oxidase 4 caused by TGF β factor
[0028] Experimental materials: 4T1 mouse breast cancer cell line was purchased from American Type Culture Collection (ATCC); TGF β factor was purchased from Peprotech; Schizandrin B was purchased from China Institute for the Control of Pharmaceutical and Biological Products; DA) was purchased from Sigma Company.
[0029] Experimental method: 10 μM Schizandrin B pretreated empty vector cells and NADPH oxidase 4 interference cells for 2 hours, and then added 5 ng / ml TGF β factor for 8 hours. DCFH-DA was used to detect the signal value of cellular reactive oxygen species. Relative signal is the ratio relative to the signal of untreated empty vector cells.
[0030] Experimental results: see the experimental results image 3 , the experimental results proved that the increase of ROS signal value caused by TGF β was mediated by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com