Phenol AB ring structure compound and preparation method and application thereof
A compound and phenol technology, applied in the field of phenol AB ring structure compounds and their preparation, can solve the problems of few types of polyphenol compounds, low activity and the like, and achieves simple process, easy realization, good anti-influenza virus neuraminic acid The effect of enzymatic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
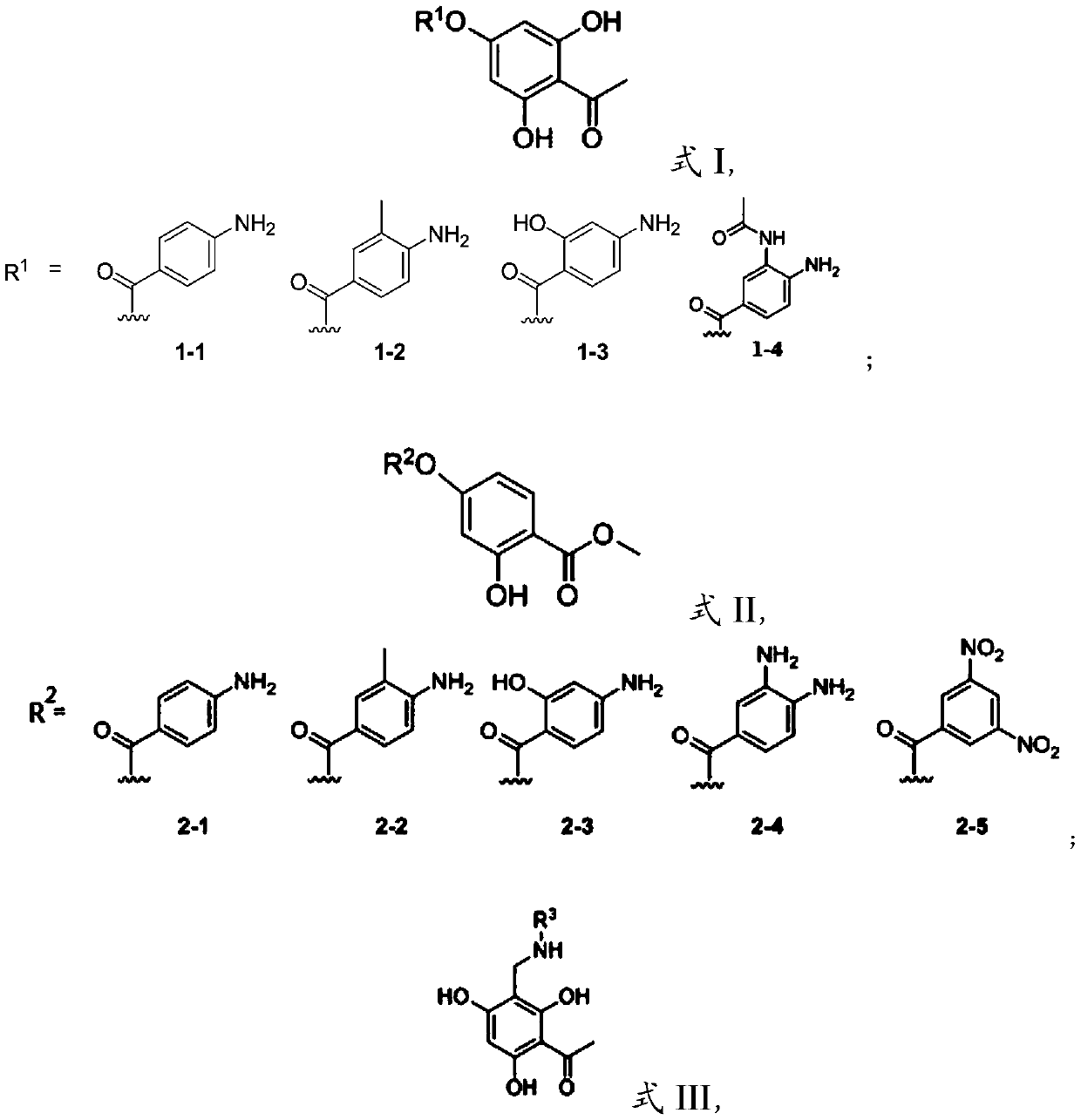
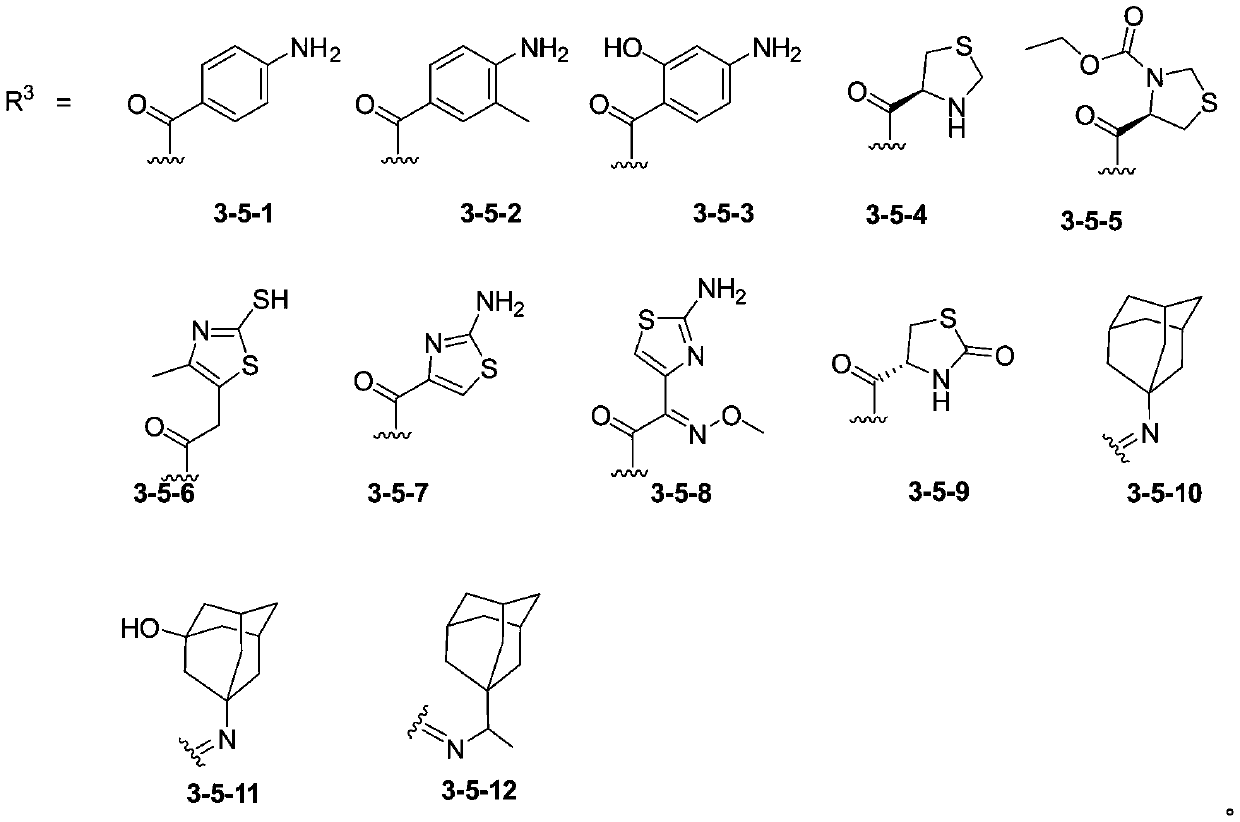
[0034] The present invention also provides that the phenolic AB ring structure compound has the structure shown in formula I and R 1 The preparation method when being 1-1, 1-2 or 1-3, comprises the following steps:
[0035] 2,4,6-trihydroxyacetophenone is subjected to condensation reaction with compound 1 to obtain 1 It is a phenolic AB ring structure compound of 1-1, 1-2 or 1-3, and the compound 1 is p-aminobenzoic acid, 2-hydroxyl-4-aminobenzoic acid or 3-methyl-4-aminobenzoic acid . In the present invention, the condensation reaction is preferably carried out under the conditions of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide) and 4-dimethylaminopyridine.
[0036] In a specific embodiment of the present invention, it is preferred to mix 1.2 equivalents of p-aminobenzoic acid (4.28 mmol, 587.22 mg), 1.5 equivalents of EDAC (1-(3-dimethylaminopropyl)-3-ethylcarbodi Imine) (5.35mmol, 830.94mg), 0.15 equivalents of DMAP (4-dimethylaminopyridine) (535.24mol, 65.39mg) were d...
Embodiment 1
[0072] Preparation of compound 1-1: There are two methods for the preparation of this compound. The first method is to obtain the product by one-step condensation with common condensing agents (DMAP, EDAC); the second method is to use p-aminobenzoic acid to form p- Aminobenzoyl chloride, the amino group generally does not participate in the reaction because the amino hydrochloride is formed, and then the reaction product is drained for use, and 2,4,6-trihydroxyacetophenone is dissolved in boron trifluoride ether, and the The target product can be obtained by adding the product dropwise into the reaction liquid. Due to the harsh experimental conditions required by the reaction and environmental pollution, the first method is used in this embodiment.
[0073] 1.2 equivalents of p-aminobenzoic acid (4.28mmol, 587.22mg), 1.5 equivalents of EDAC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide) (5.35mmol, 830.94mg), 0.15 equivalent of DMAP (4-dimethylaminopyridine) (535.24mol, 65.39m...
Embodiment 2
[0077] Preparation of compound 2-1: see the method of compound 1-1, the only difference is that the 2,4,6-trihydroxyacetophenone in Example 1 is replaced by 2,4-dihydroxybenzoic acid to obtain a white solid, the yield About 51%. 1 H NMR (DMSO-d 6 ,400MHz,25℃)δ10.69(s,1H),7.82(d,J=8.7,1H),7.79-7.76(m,2H),6.86-6.85(d,J=2.3,1H),6.81- 6.79(dd, J=8.7,2.3,1H),6.66-6.62(d,J=8.7,2H),6.22(s,1H),3.88(s,3H); 13 C NMR (DMSO-d 6 ,101MHz,25℃)δ168.8,163.9,161.2,156.5,154.6,132.2,131.2,113.9,113.8,112.9,110.7,110.5,52.5.
[0078] Preparation of compound 2-2: see the method of compound 1-2, the only difference is that the 2,4,6-trihydroxyacetophenone in Example 1 is replaced by 2,4-dihydroxybenzoic acid, white solid, the yield is about was 61%. 1 H NMR (DMSO-d 6 ,400MHz,25℃)δ10.68(s,1H),7.82(d,J=8.7Hz,1H),7.68(s,1H),7.66(d,J=2.1Hz,1H),6.85(d, J=2.2Hz, 1H), 6.80(dd, J=8.7, 2.3Hz, 1H), 6.66(dd, J=8.7, 2.3Hz, 1H), 5.98(br-s, 2H), 3.88(s, 3H ),2.10(s,3H); 13 C NMR (DMSO-d 6 101 MHz, 25°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com