Patents
Literature
70 results about "Influenza virus A neuraminidase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
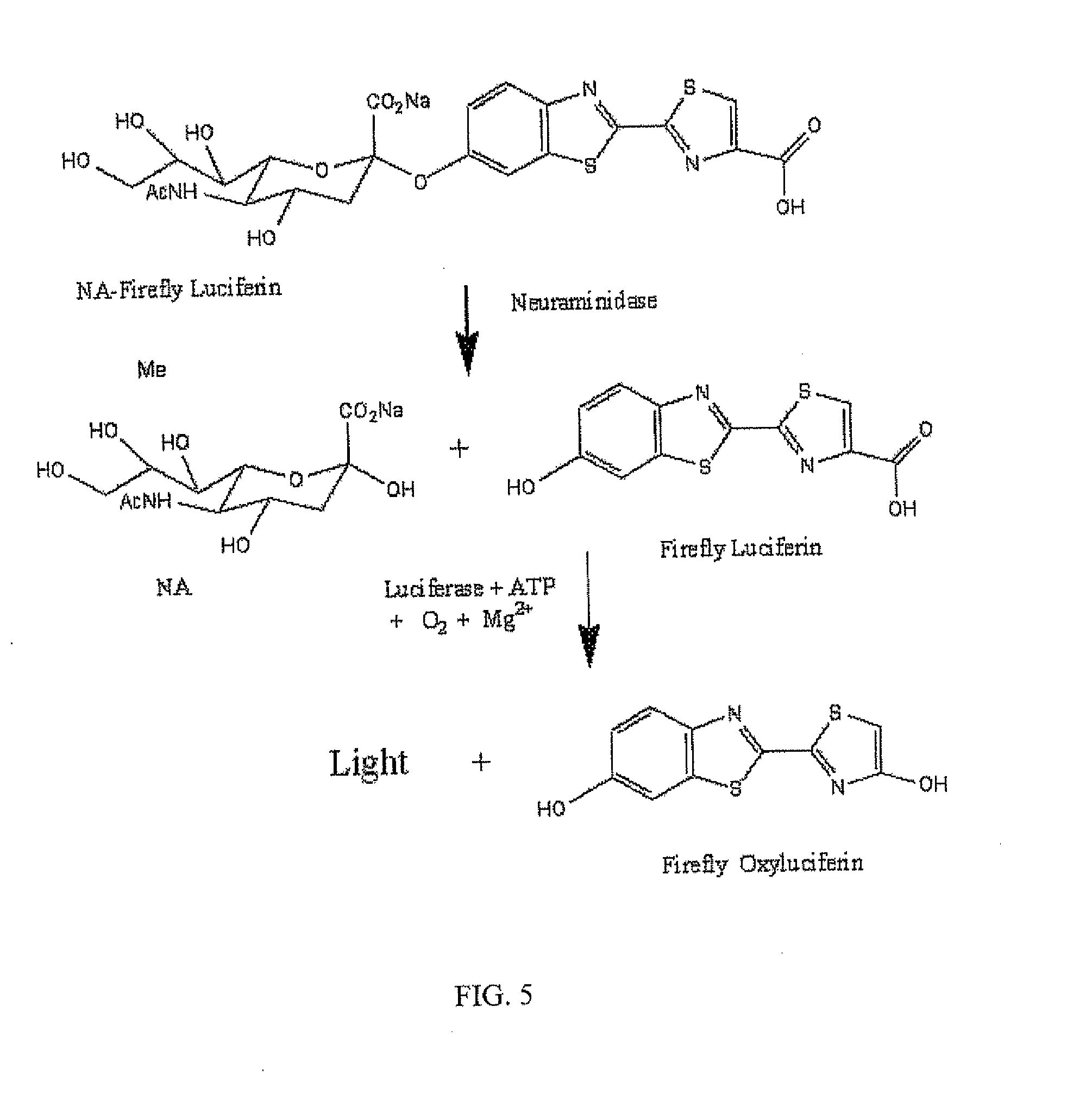
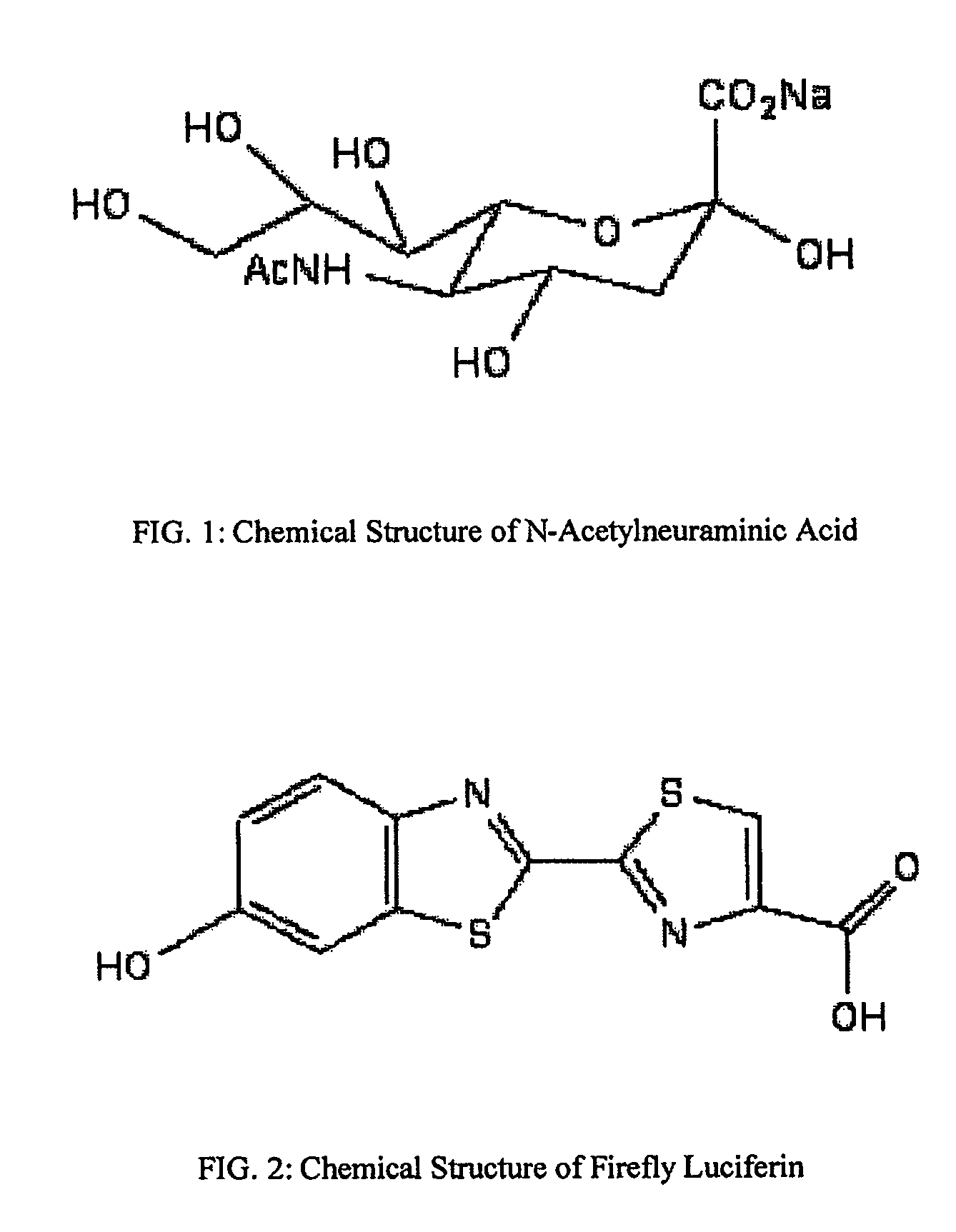
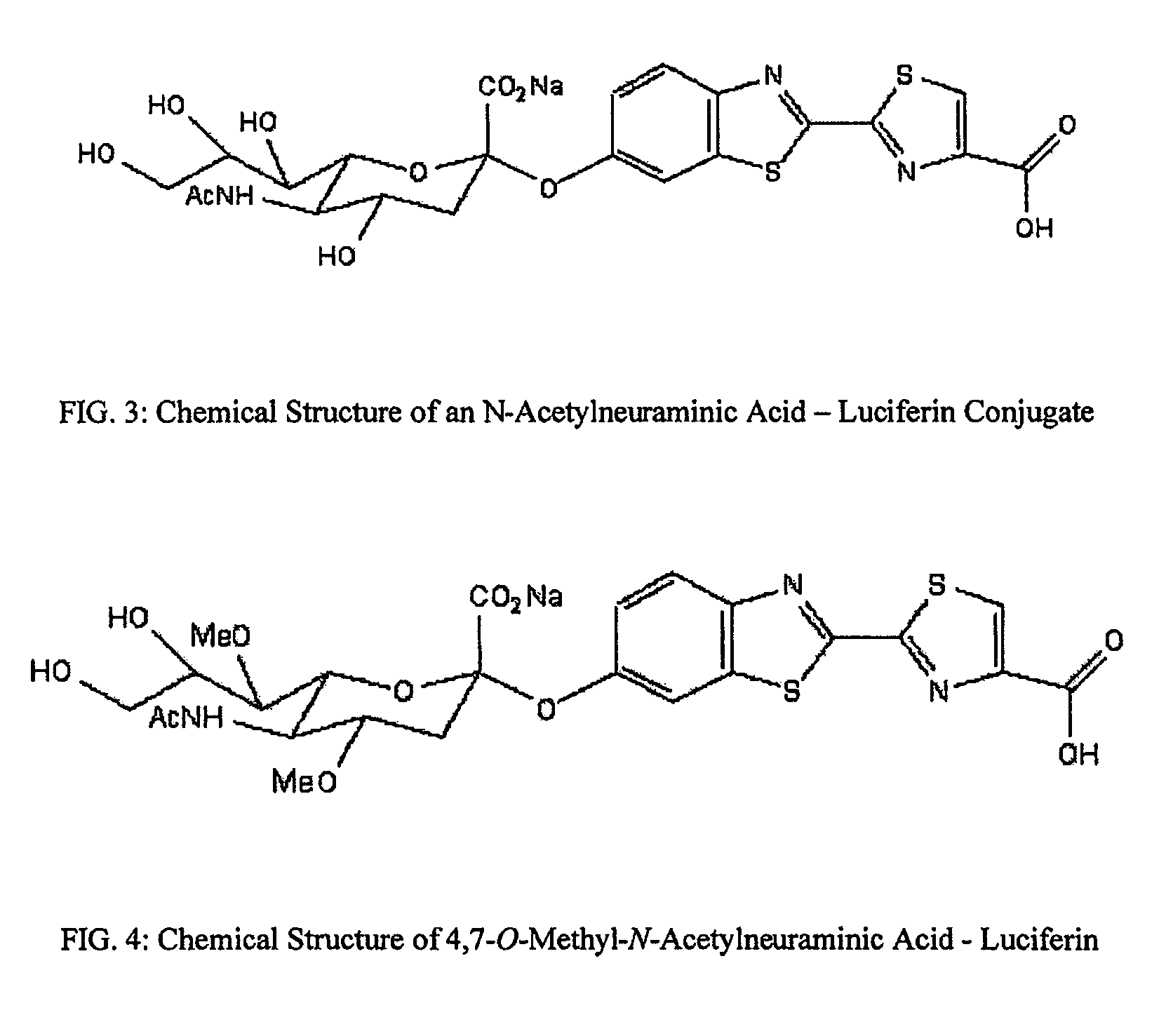
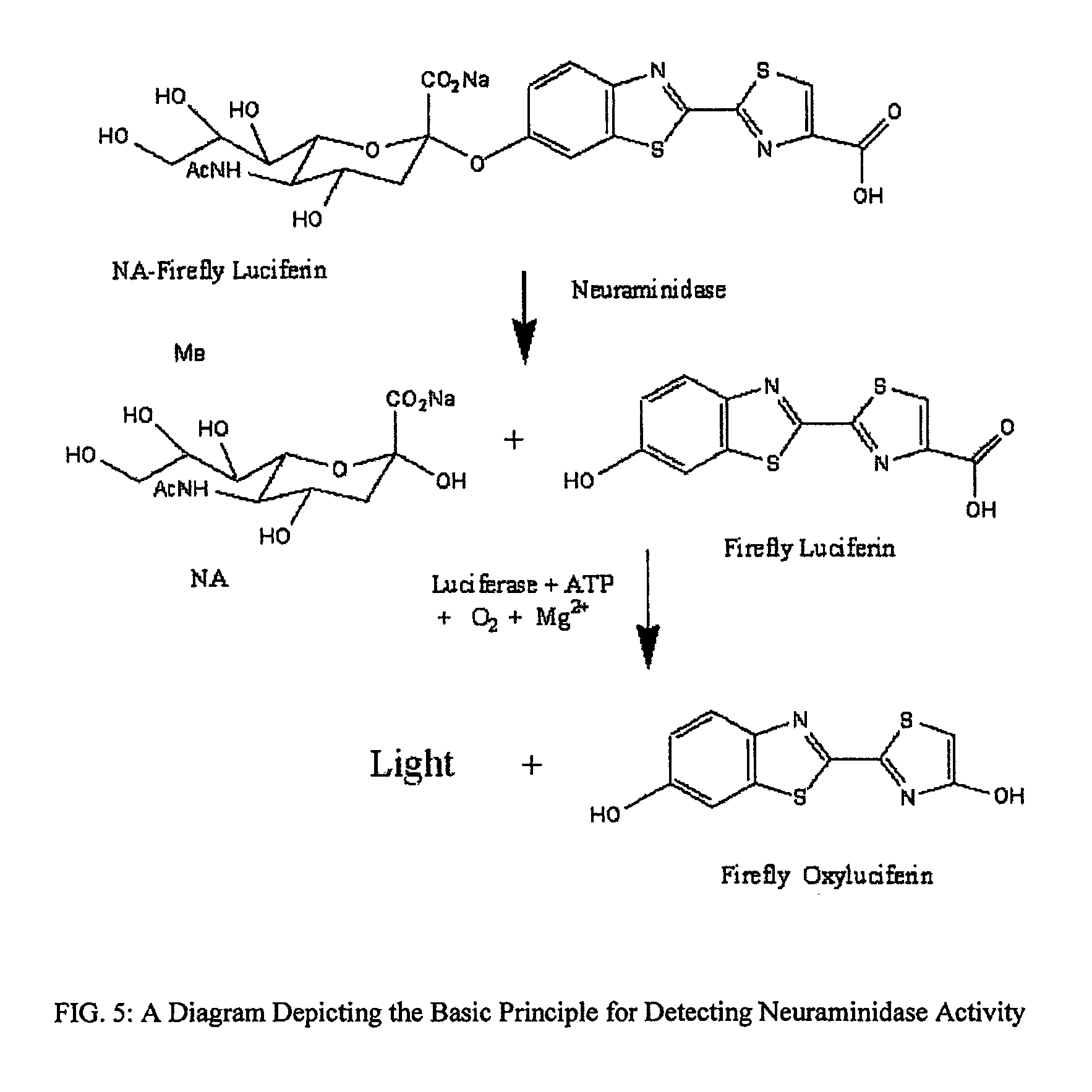
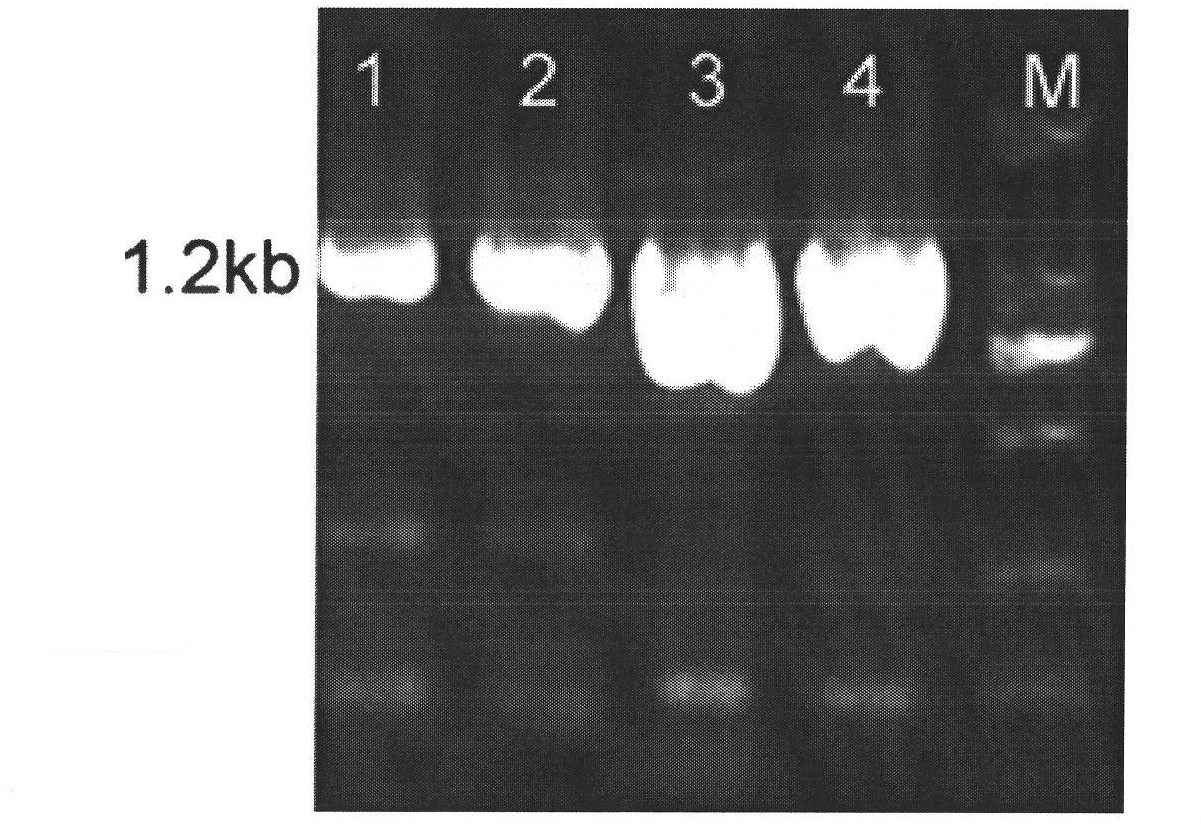
Reagents and kits for detection of influenza virus and the like
ActiveUS20080286758A1Simple and rapid and specific and sensitive detectionHigh detection sensitivityOrganic chemistryMicrobiological testing/measurementNeuraminidaseFluorescein
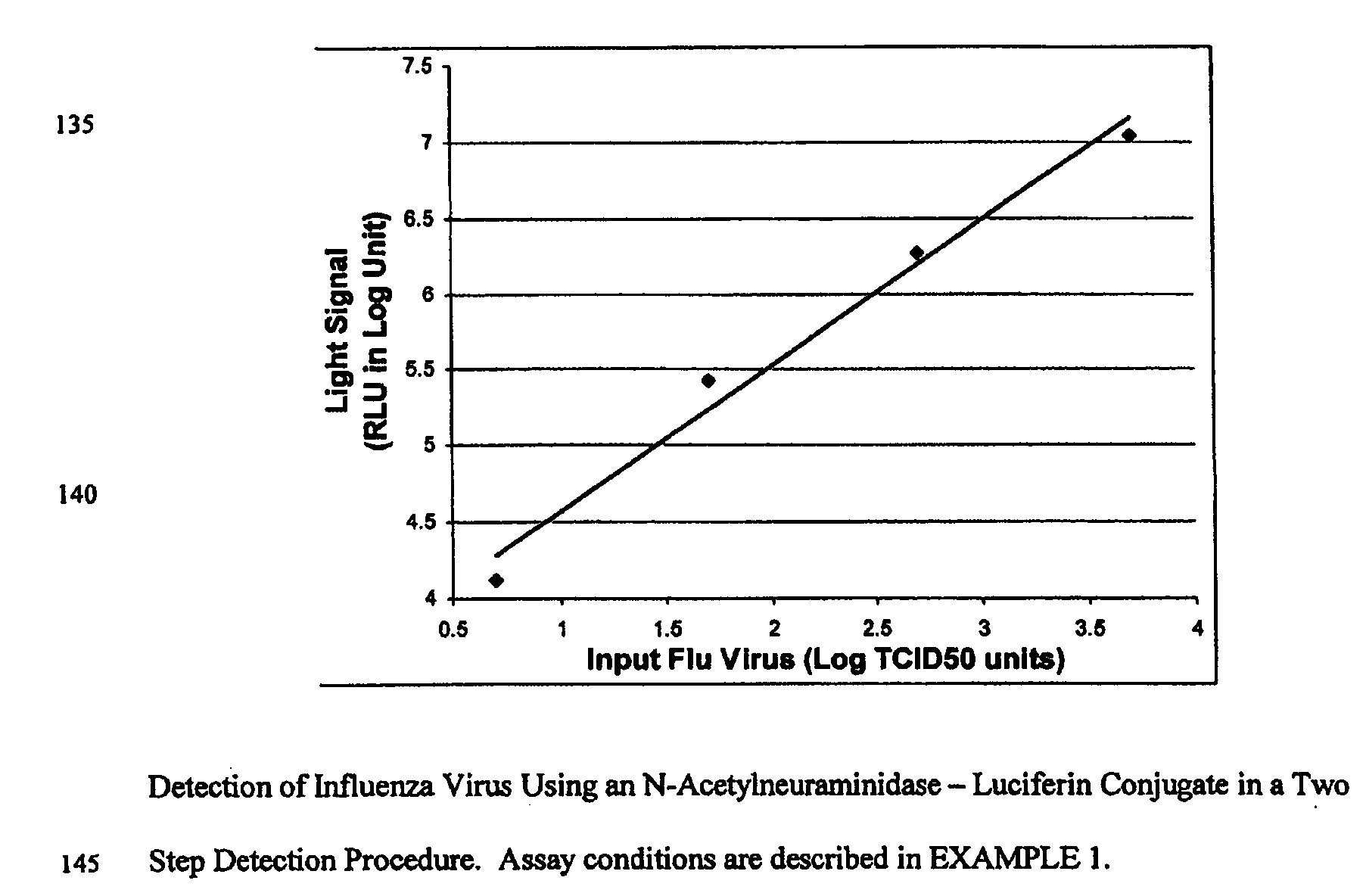
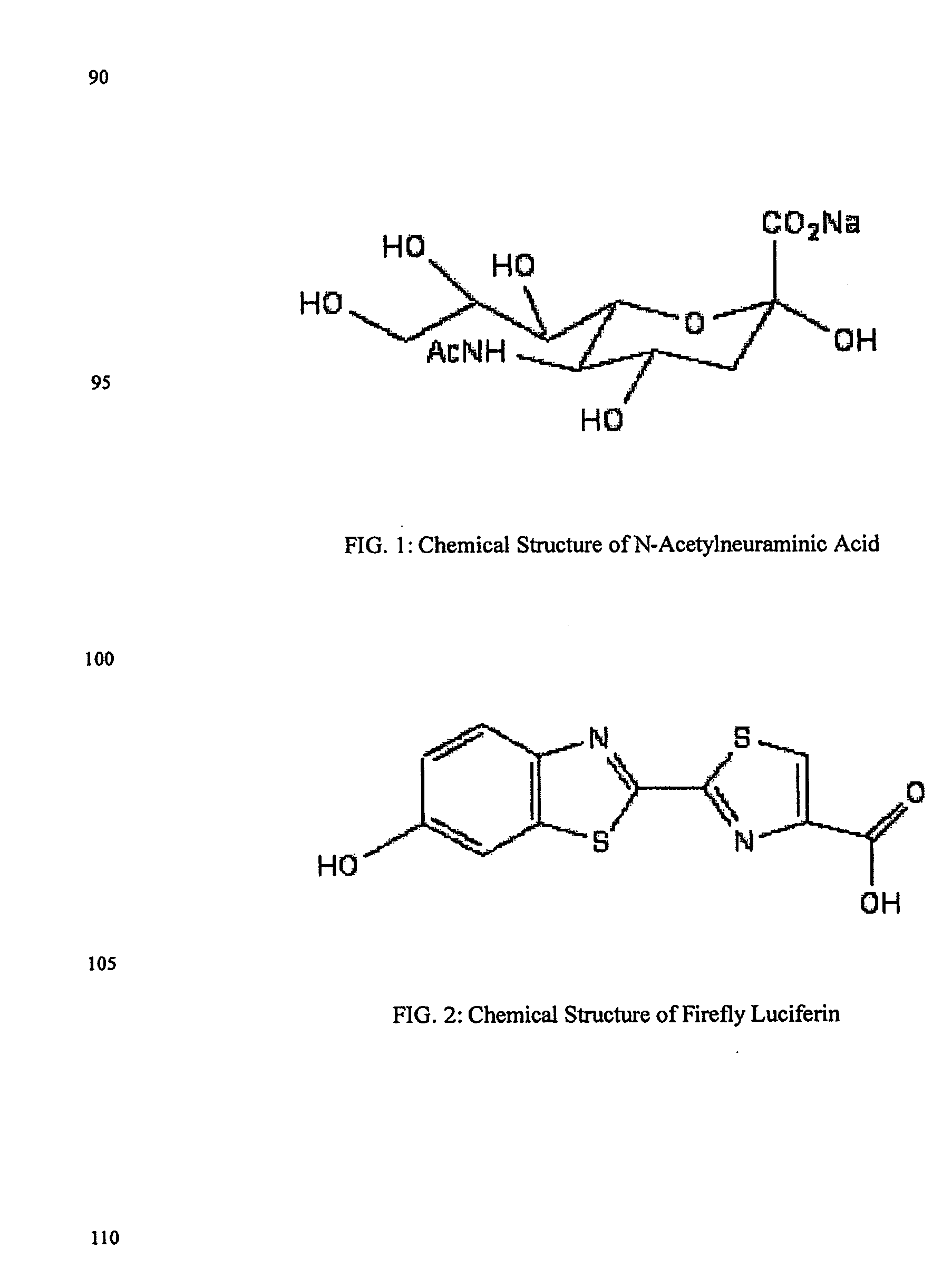
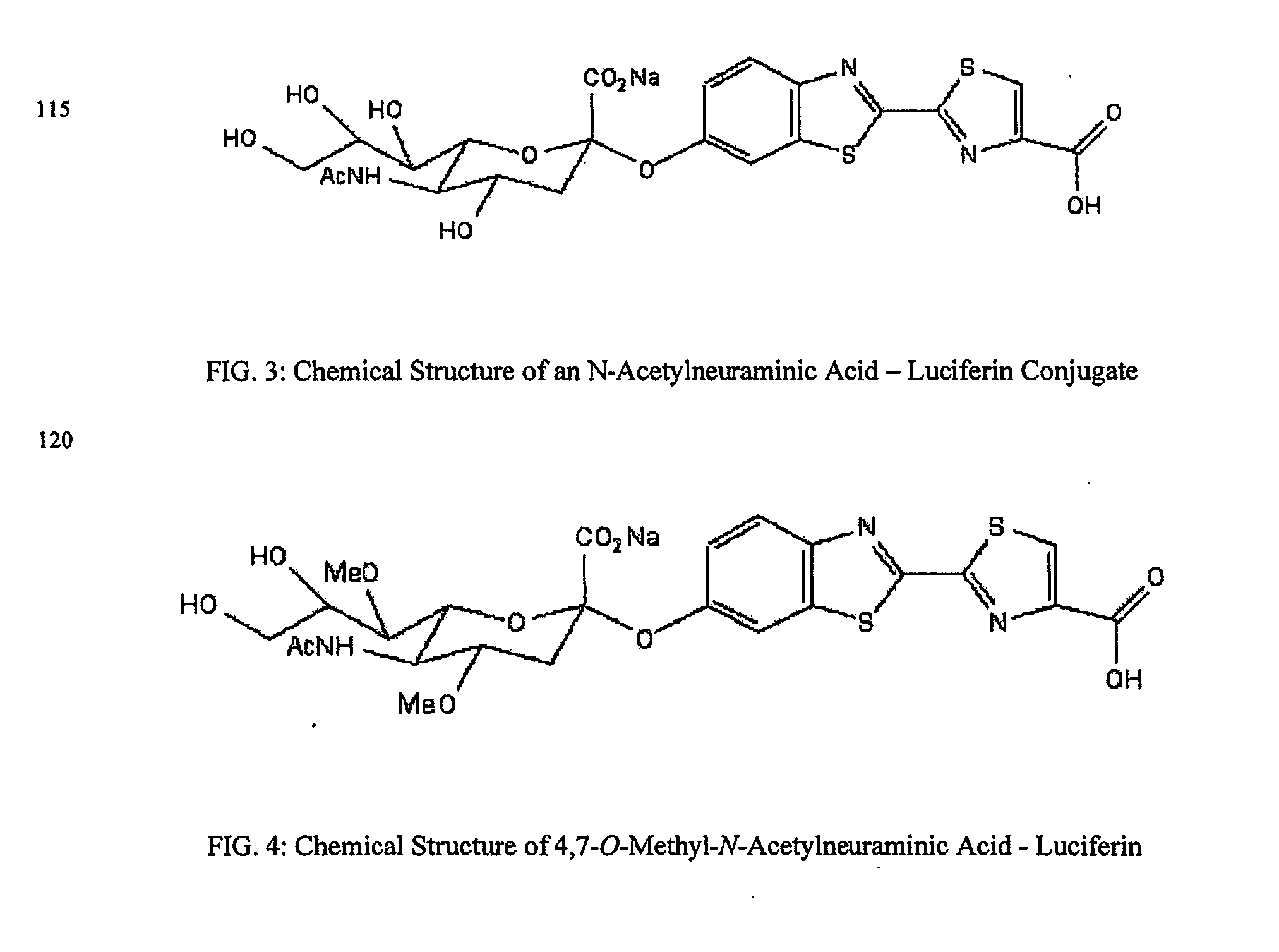
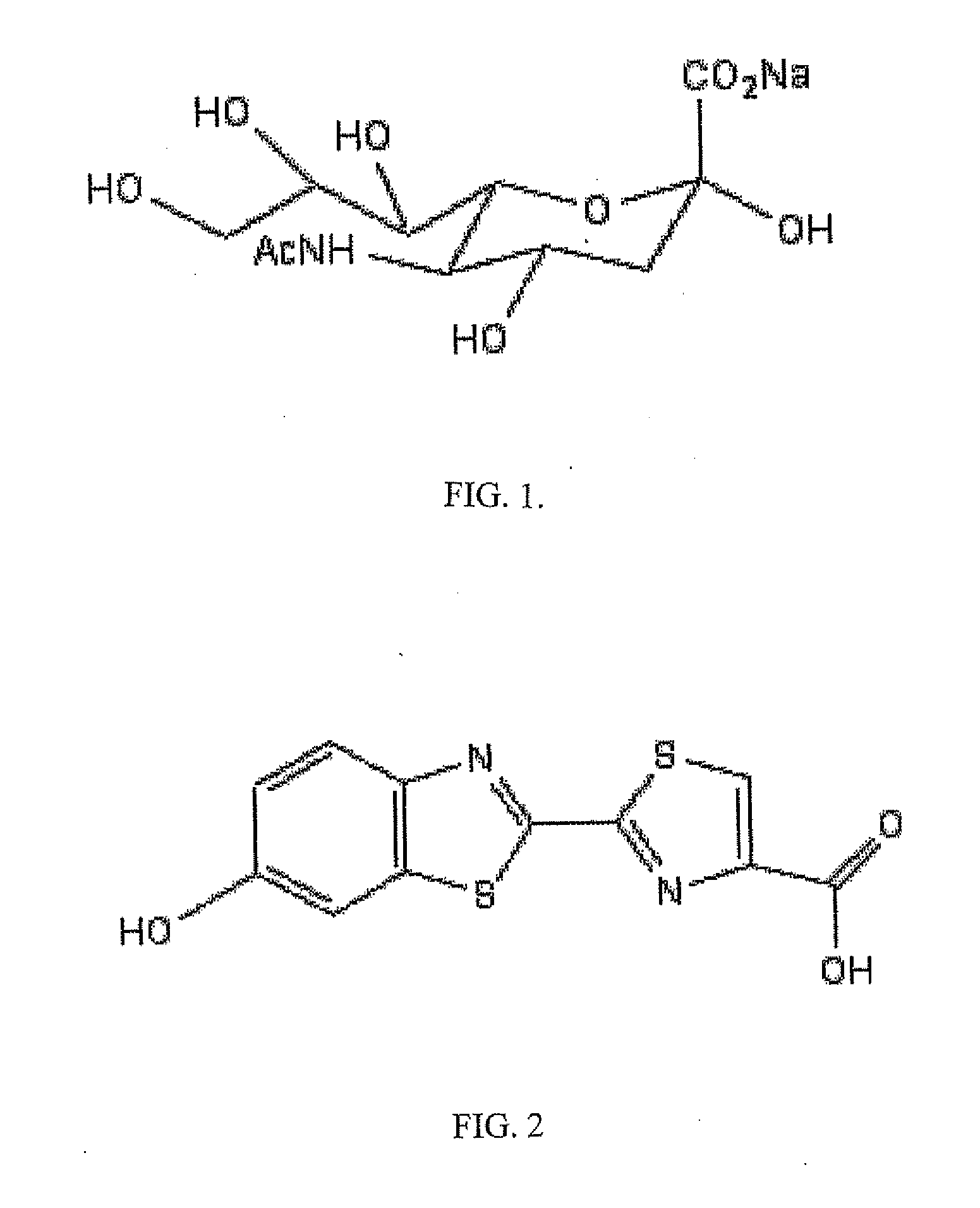
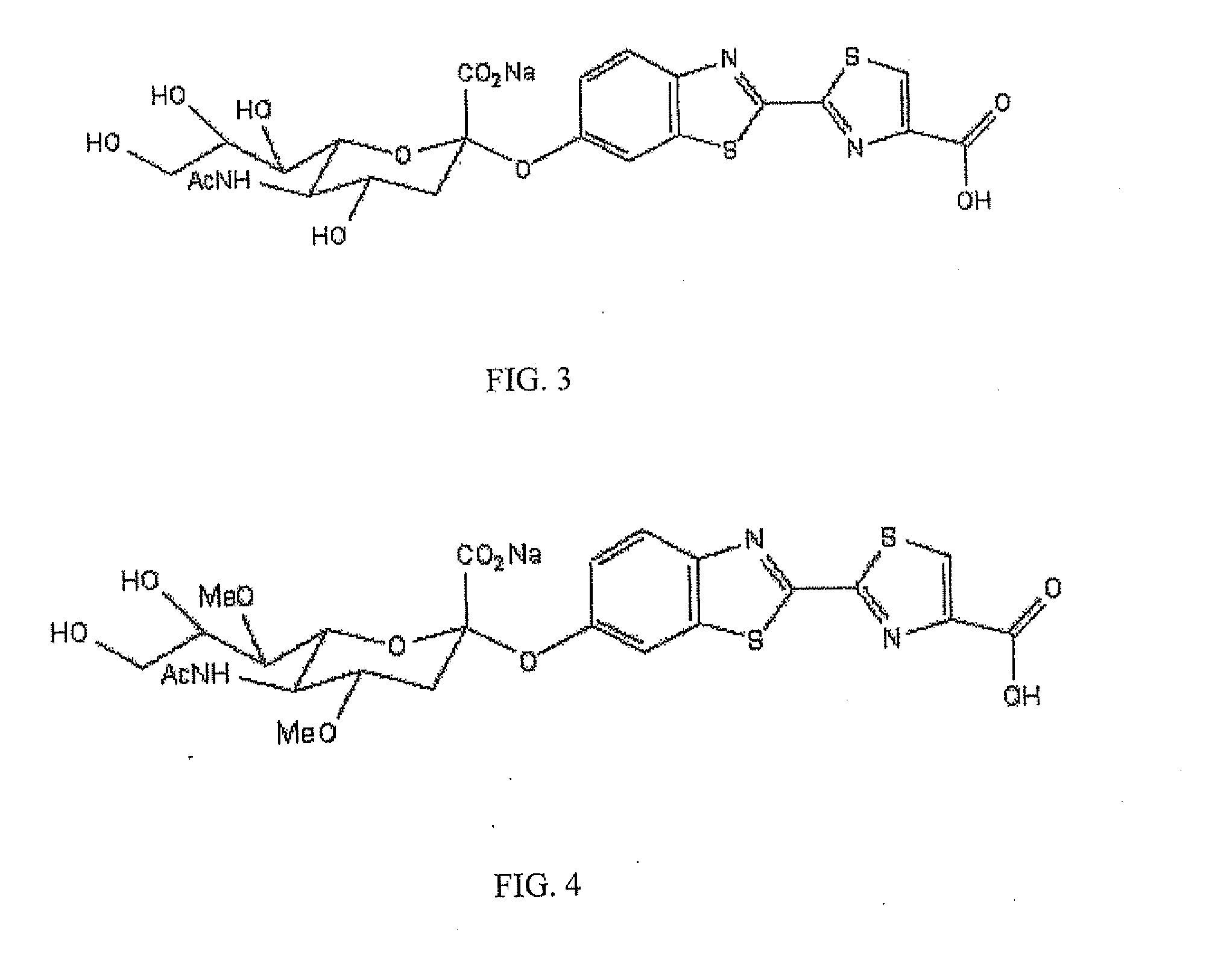
The present invention relates to reagents and methods for influenza virus detection. These reagents and methods disclosed in the present invention enable simple, rapid, specific and sensitive detection of influenza virus types A and B. These reagents are N-acetylneuraminic acid-firefly luciferin conjugates which can be cleaved by influenza virus neuraminidase.
Owner:CELLEX BIOLOGICAL TECH SUZHOU CO LTD
Reagents and kits for detection of influenza virus and the like
ActiveUS20110189655A1Simple and rapid and specific and sensitive detectionHigh detection sensitivitySugar derivativesMicrobiological testing/measurementNeuraminidaseFirefly luciferin
The present invention relates to reagents and methods for influenza virus detection. These reagents and methods disclosed in the present invention enable simple, rapid, specific and sensitive detection of influenza virus types A and B. These reagents are N-acetylneuraminic acid-firefly luciferin conjugates which can be cleaved by influenza virus neuraminidase.
Owner:CELLEX INC
3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof
InactiveCN103755697AHas anti-influenza drug neuraminidase activityOrganic chemistryAntiviralsThiazoleHydrogen
The invention relates to 3-[[2-(benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as shown in chemical structural formulas I and II or a salt thereof. In the chemical structural formulas, R, X<1> and X<2> are selected from hydrogen, deuterium, C1-C2 alkyl, C3-C4 linear-chain alkyl or branched-chain alkyl; X<3> is selected from hydroxyl, methoxyl and ethyoxyl; is selected from hydrogen, deuterium, C1-C2 alkyl, fluorine, fluorine or bromine; X4 and X6 are selected from hydrogen, deuterium, C1-C2 alkyl, fluorine, fluorine, bromine or nitryl; X5 and X7 are selected from hydrogen, deuterium and C1-C2 alkyl. The invention also provides an application of the 3-[[2-(benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone in preparation of an influenza virus neuraminidase inhibitor.
Owner:HUNAN UNIV
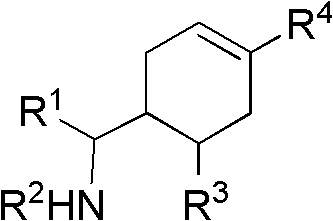
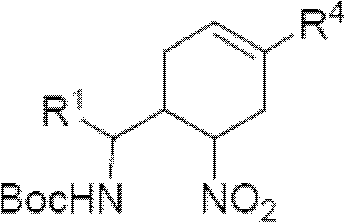
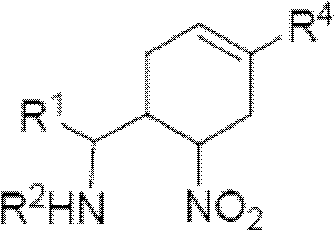
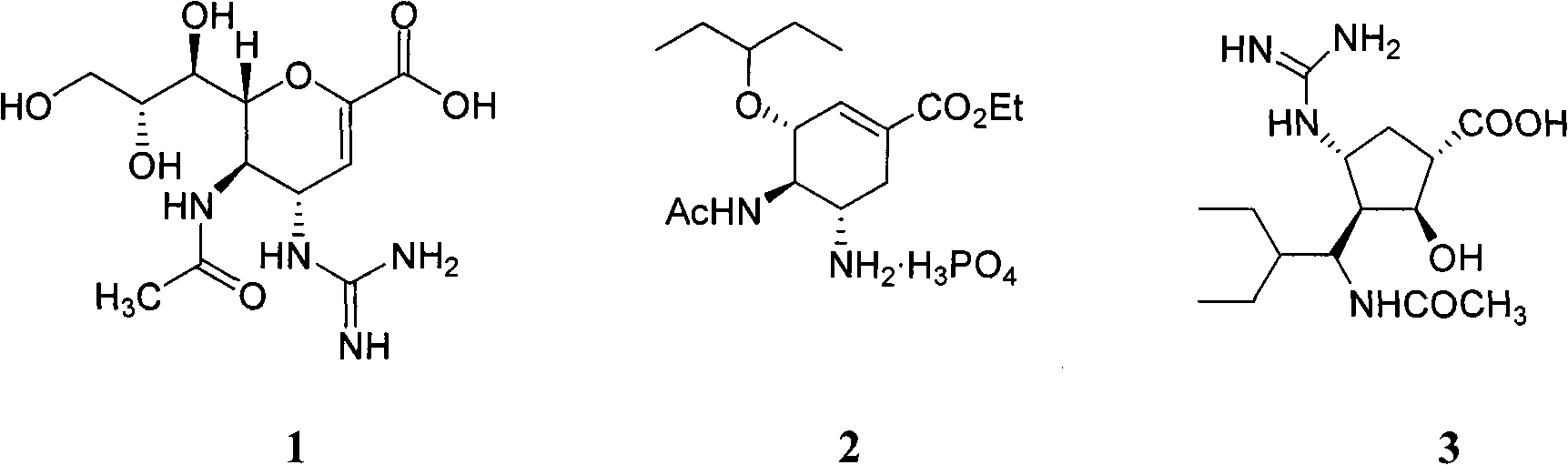
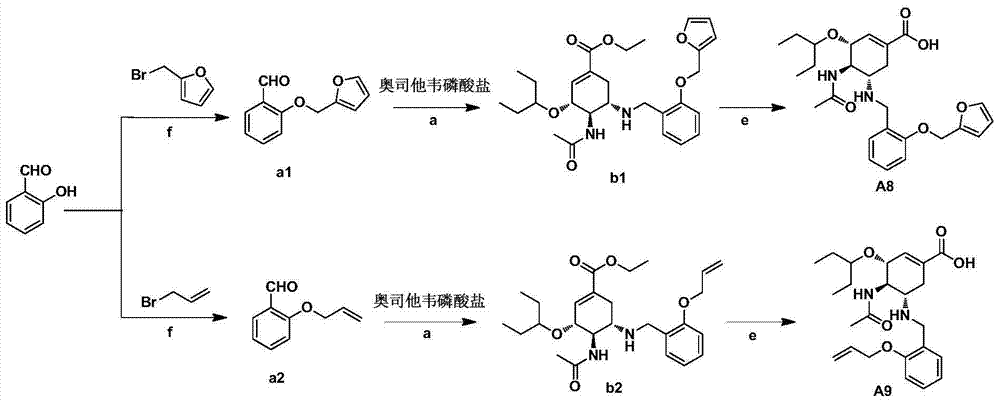
Cyclohexene compound having influenza virus neuraminidase inhibition activity, preparation method and application
InactiveCN102964267AHas neuraminidase inhibitory activityOvercoming drug resistanceCarbamic acid derivatives preparationCarboxylic acid nitrile preparationCyclohexeneEnantiomer
The invention provides a compound expressed by a general formula I, or its pharmaceutically acceptable salt, and an analyzed enantiomer and a purified diastereomer, wherein R<1>is H or C1-12 alkyl groups; R<2> is H, -C(O)R<1a>, -C(O)OR<1a> or -S(O)2R<1b>, wherein R<1a> is H or C1-6 alkyl groups, R<1b> is selected from H or C1-6 alkyl groups halogenated hydrocarbon, phenyl or aryl group; R<3> is selected from C1-4 alkyl groups substituted amino, halogen, hydroxyl, sulfydryl, guanidyl, nitryl or cyano group; and R<4> is -(CH2)nCO2H, -(CH2)nP(O)(OH)2, -(CH2)nCO2R<1a> and -(CH2)nP(O)(OR<1a>)2, wherein R<1a> is H or C1-6 alkyl groups, n is an integer between 0 and 4, or a salt of the above groups. The invention also provides a preparation method of the cyclohexene compound expressed by the general formula I, and an application of the cyclohexene compound taken as an influenza virus neuraminidase inhibitor for preparing the medicines to prevent or treat the influenza diseases.
Owner:SUN YAT SEN UNIV
Reagents and kits for detection of influenza virus and the like
ActiveUS7893272B2Simple and rapid and specific and sensitive detectionHigh detection sensitivityOrganic active ingredientsBiocideNeuraminidaseFluorescein
The present invention relates to reagents and methods for influenza virus detection. These reagents and methods disclosed in the present invention enable simple, rapid, specific and sensitive detection of influenza virus types A and B. These reagents are N-acetylneuraminic acid-firefly luciferin conjugates which can be cleaved by influenza virus neuraminidase.
Owner:CELLEX BIOLOGICAL TECH SUZHOU CO LTD
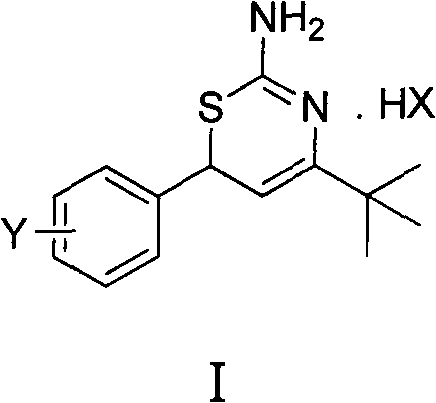
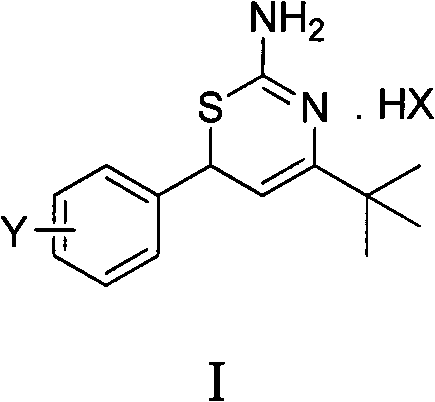
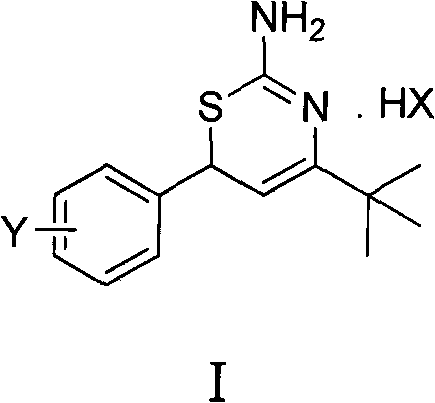
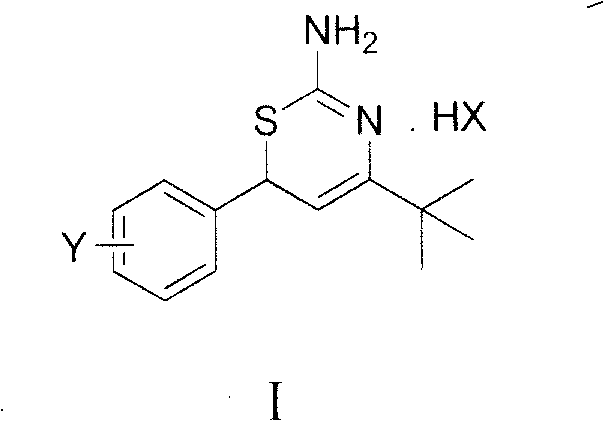
Preparation method and medical application of 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt
The invention discloses a preparation method and medical application of 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt. The preparation method of the 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt comprises the steps of: stirring 0.010 mol of 1-phenyl-4,4-dimethyl pentene-3-ketone, 0.012 mol of thiourea and 0.011 mol of acid in an organic solvent at a temperature of between 30 and 80 DEG C, performing TLC detection on reaction process, completing reaction, filtering, washing and drying the obtained product and obtaining the 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt (I). The 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt (I) can be medically used for preparing influenza virus neuraminidase inhibitors. In a formula, HX is hydrochloric acid, phosphoric acid, hydrobromic acid, sulphuric acid, trifluoroacetic acid or methanesulfonic acid, and Y is Cl, Br, CH3, Et, CF3, OCH3 or OEt.
Owner:HUNAN UNIV
Oseltamivir derivative as well as preparation method and application thereof
InactiveCN103923060AHigh selectivityHigh activityOrganic active ingredientsOrganic compound preparationHigh selectivityInfluenza a
The invention relates to an oseltamivir derivative as well as a preparation method and application thereof. The compound has the structure shown in a formula I. The invention provides an efficient influenza virus neuraminidase inhibitor with high selectivity, which is used for preparing drugs for preventing or curing flu, particularly the diseases caused by N1 influenza virus. The invention also relates to a drug composition comprising the compound shown in the formula I.
Owner:SHANDONG UNIV
3-[[2-(2-benzyl hydrazono) thiazole-5-base] methyl] quinoline-2 (1H)-ketone and preparation and applications thereof
InactiveCN103739599AHas anti-influenza drug neuraminidase activityOrganic active ingredientsOrganic chemistryThiazoleHydrogen
The invention relates to a 3-[[2-(2-benzyl hydrazono) thiazole-5-base] methyl] quinoline-2 (1H)-ketone shown in a chemical structural formula I or a salt therefore as shown in description, wherein R, X1 and X2 are selected from hydrogen, deuterium, C1-C2 alkyls, and C3-C4 linear alkyls or branched alkyls; X3 is selected from hydrogen, deuterium, C1-C2 alkyls, hydroxyl, methoxyl and oxethyl; X4 is selected from hydrogen, deuterium, C1-C2 alkyls, fluorine, chlorine or bromine; X5 and X6 are selected from hydrogen, deuterium, C1-C2 alkyls, fluorine, chlorine, bromine or nitrocellulose; X7 is selected from hydrogen, deuterium, and C1-C2 alkyls; the invention also discloses application of the 3-[[2-(2-benzyl hydrazono) thiazole-5-base] methyl] quinoline-2 (1H)-ketone in preparing an influenza virus neuraminidase inhibitor.
Owner:HUNAN UNIV
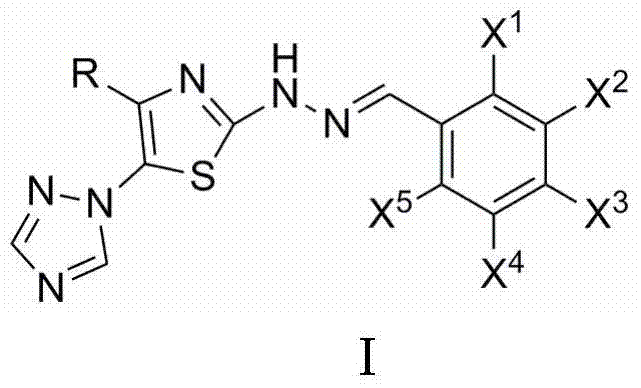
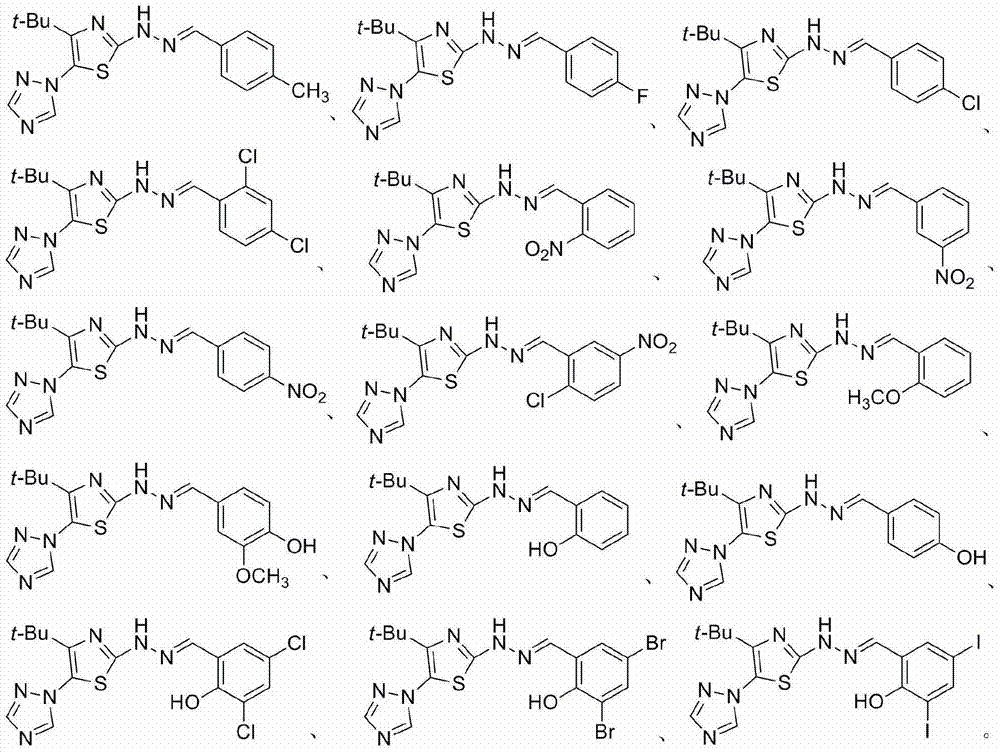
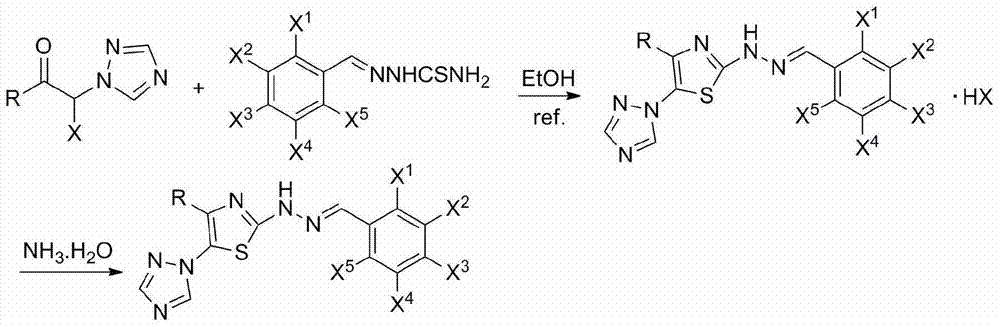
2-(2-benzylidene hydrazino)-5-(1,2,4-triazole-1-yl)thiazole, and preparation and applications thereof
The invention relates to 2-(2-benzylidene hydrazino)-5-(1,2,4-triazole-1-yl)thiazole represented by chemical structural formula I, or salts thereof. According to the chemical structural formula I, R is selected from hydrogen, deuterium, C1-C2 alkyl, and C3-C4 linear alkyl or branched alkyl; and X1-X5 are selected from hydrogen, deuterium, C1-C2 alkyl, hydroxyl, methoxyl, ethyoxyl, fluorine, chlorine, bromine, and nitryl. The invention also discloses applications of 2-(2-benzylidene hydrazino)-5-(1,2,4-triazole-1-yl)thiazole in preparing influenza neuraminidase inhibitors.
Owner:HUNAN UNIV
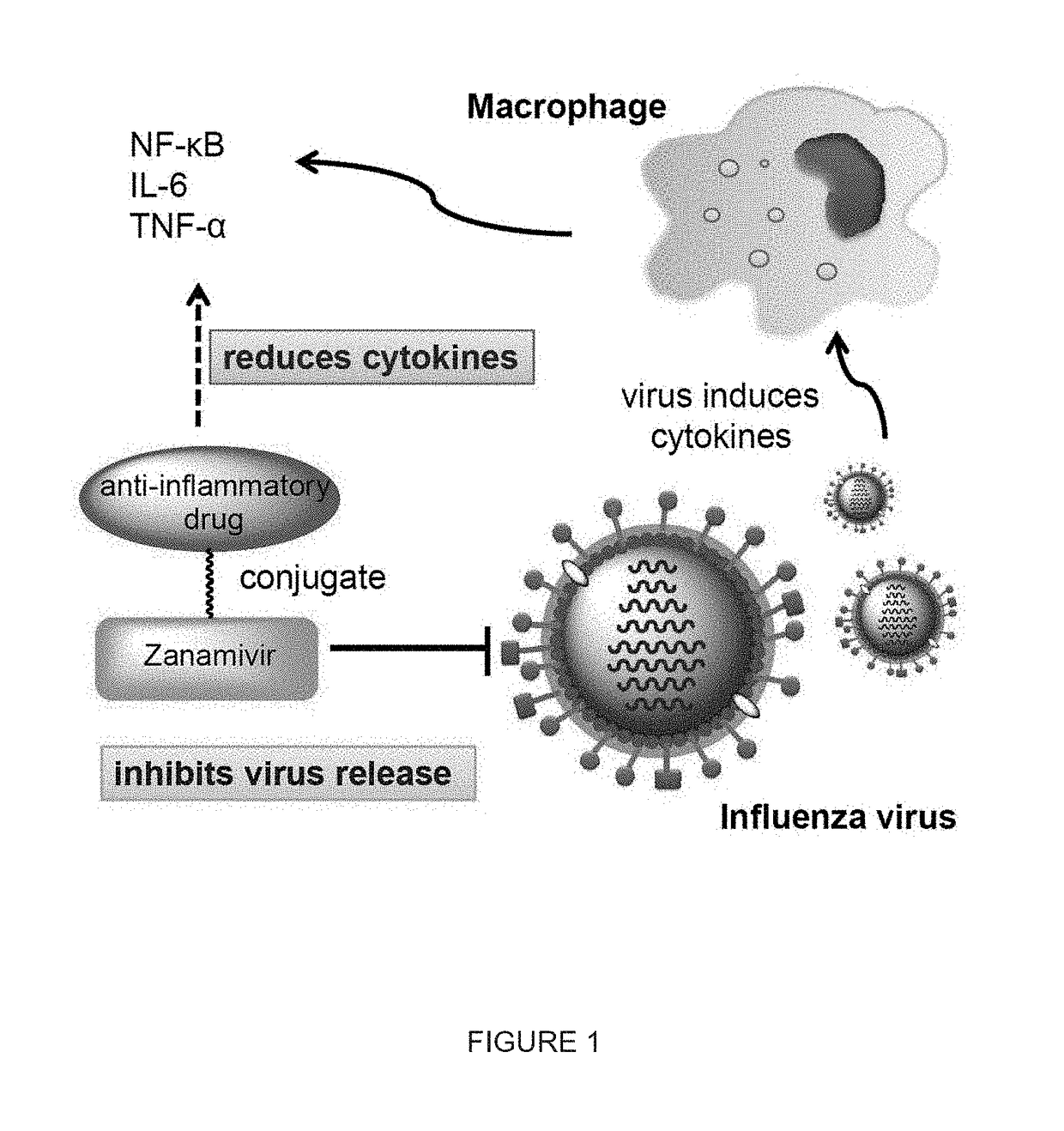
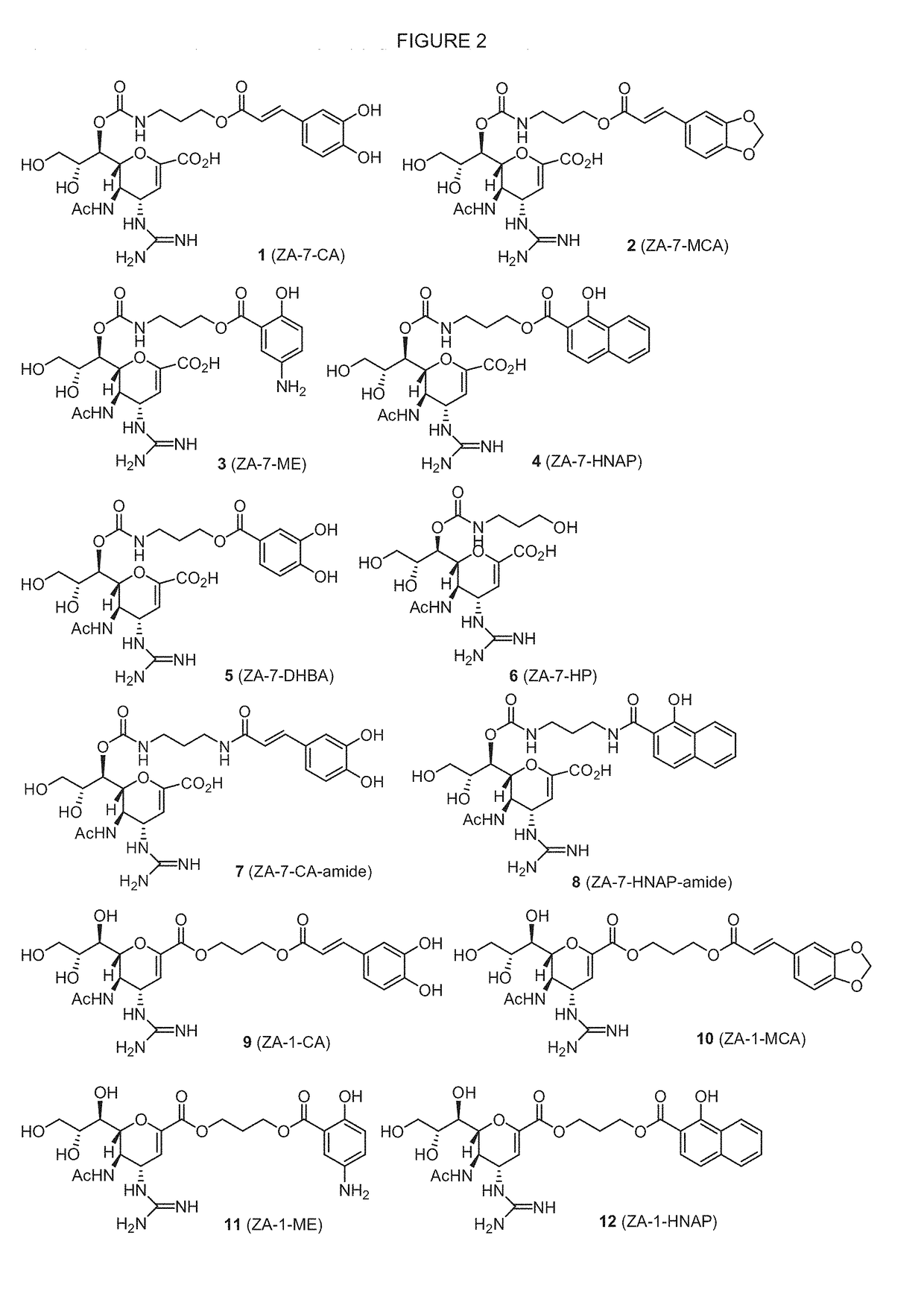
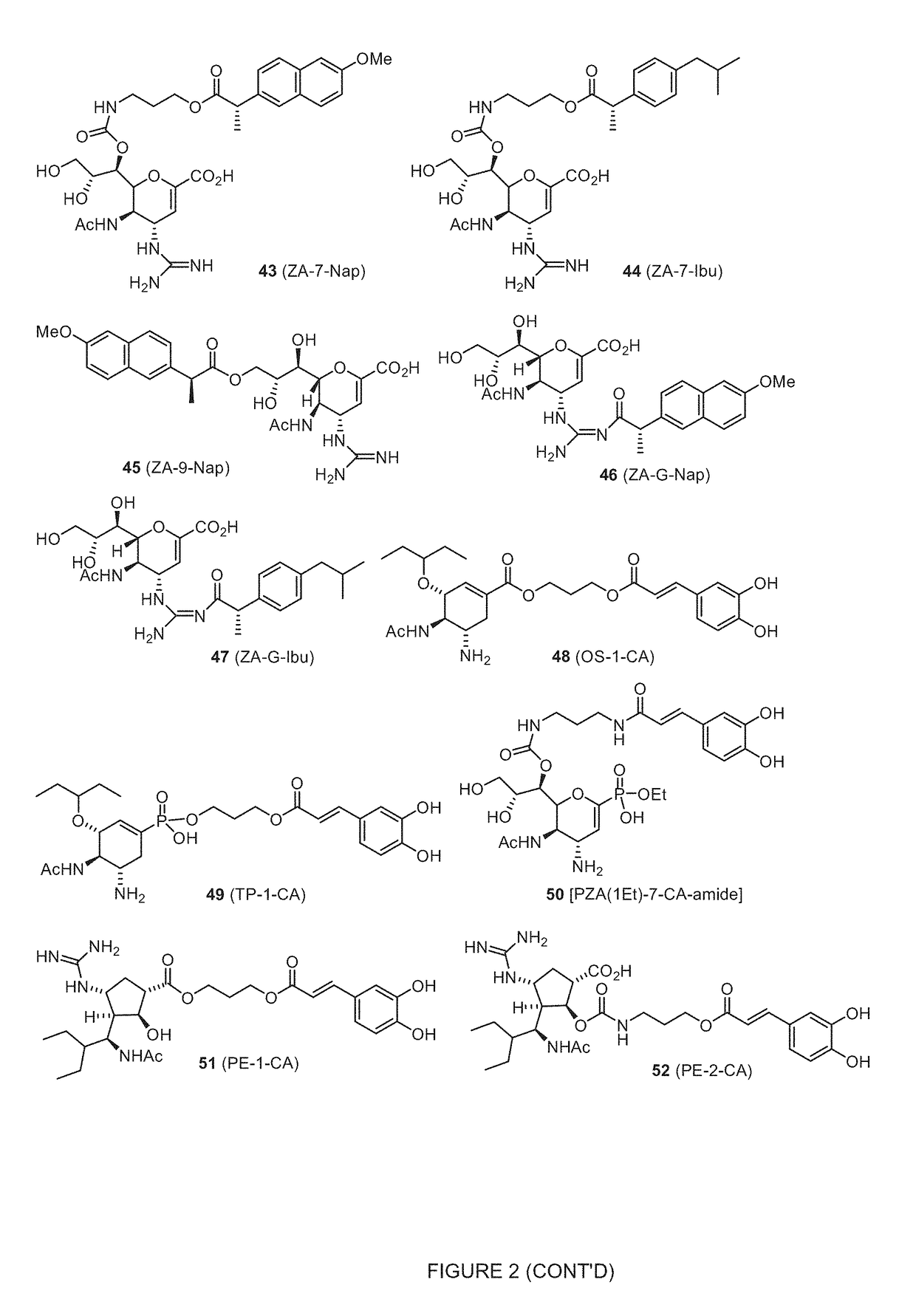
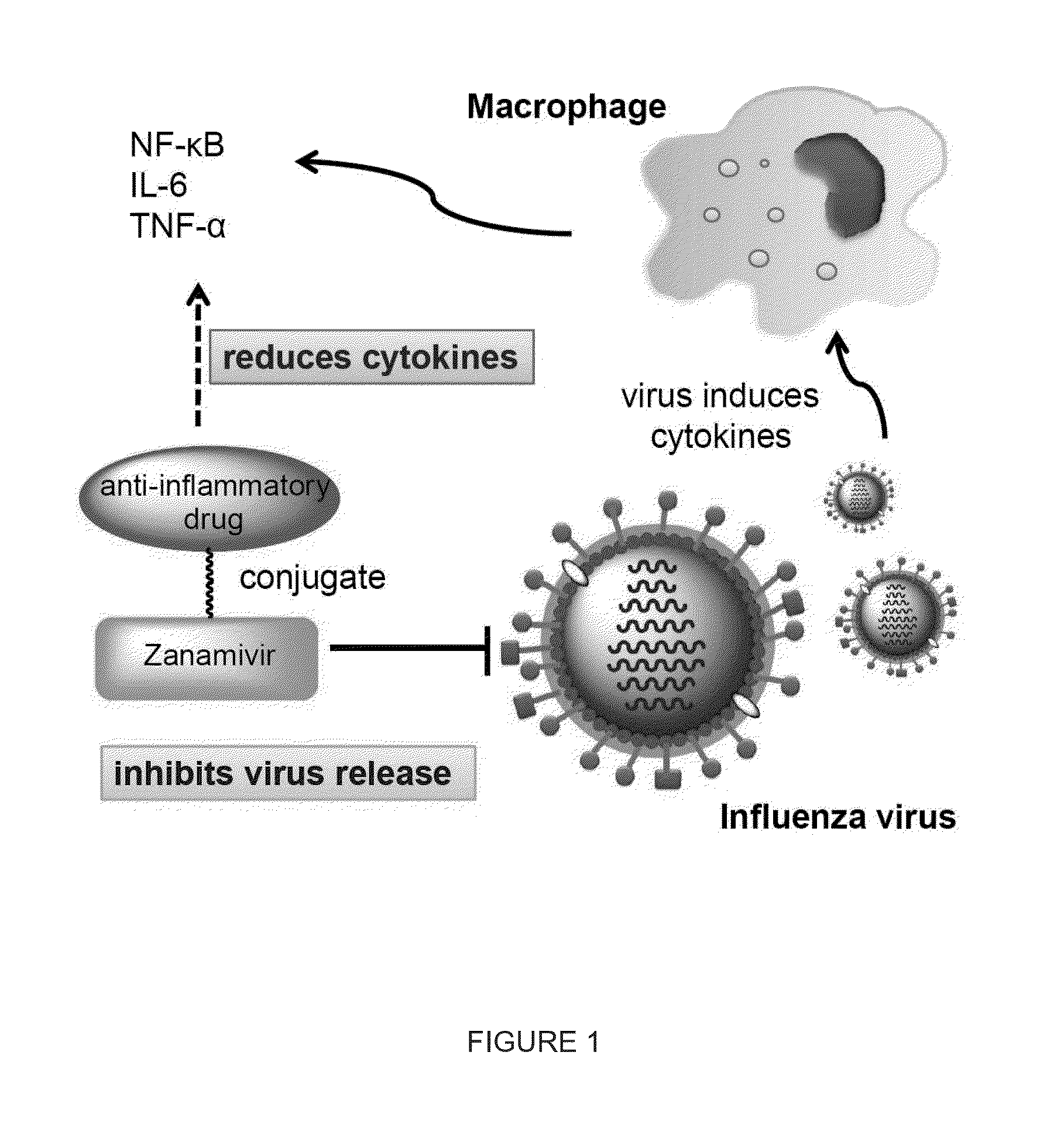
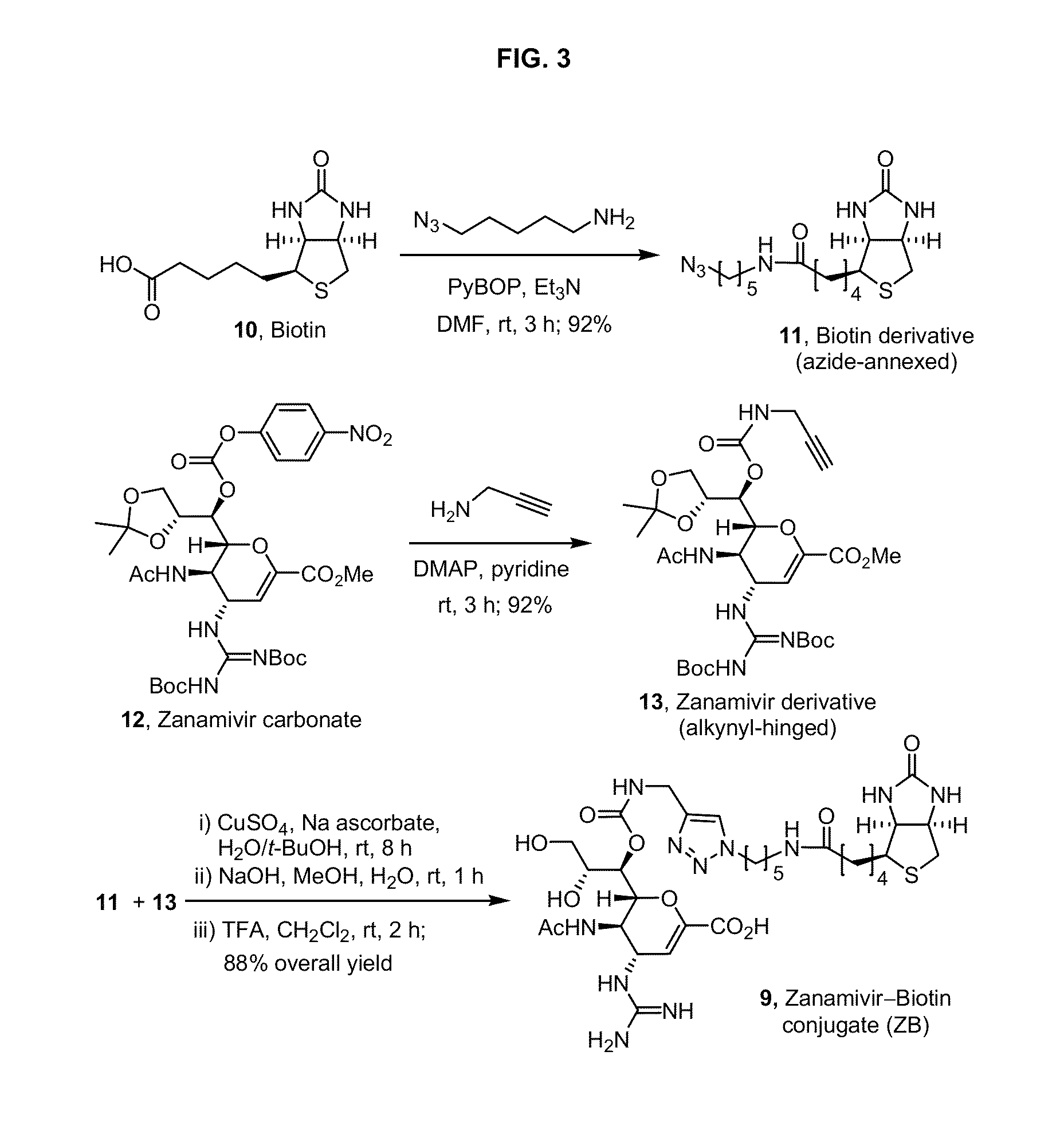
Enhanced anti-influenza agents conjugated with anti-inflammatory activity
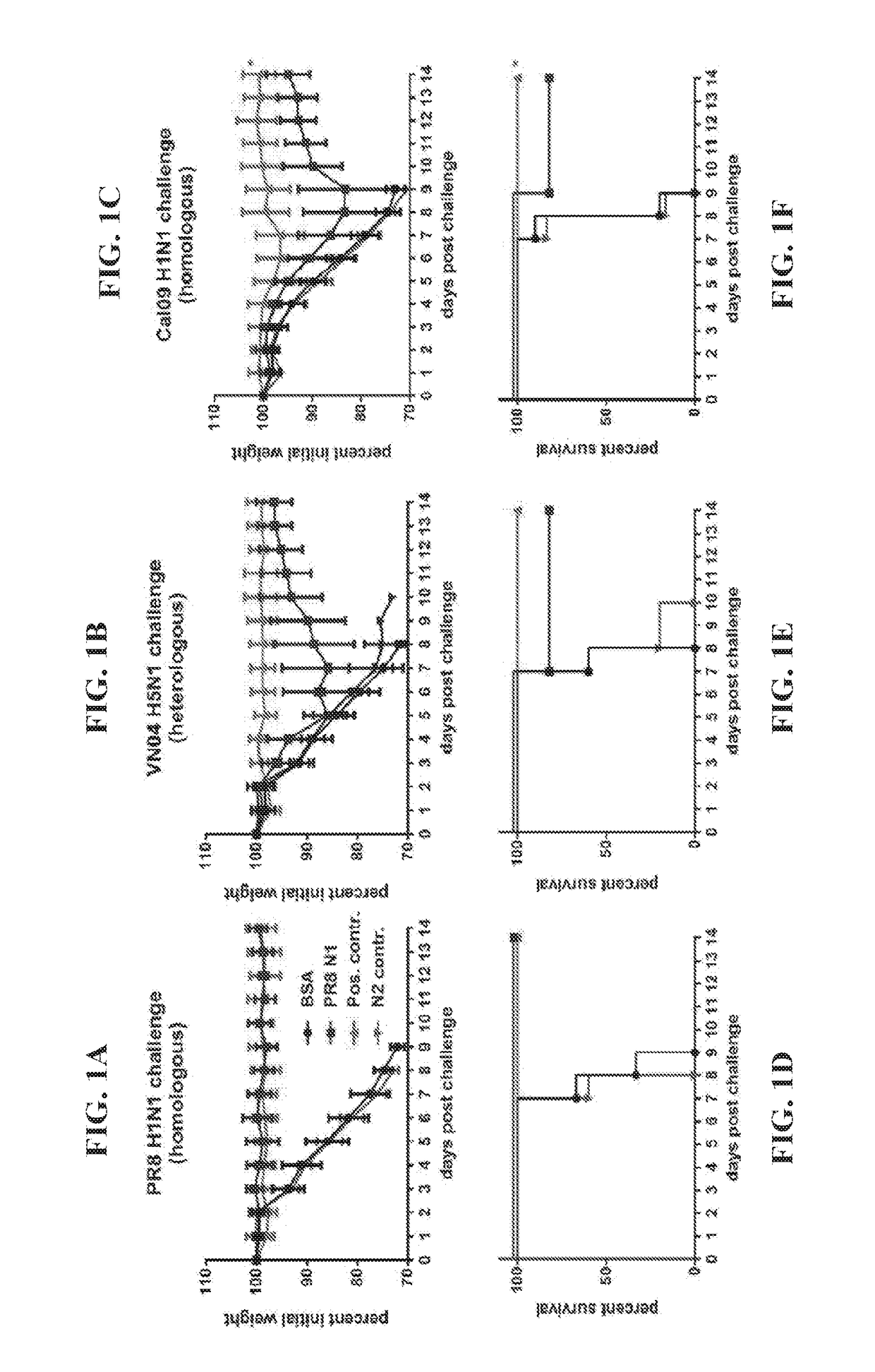
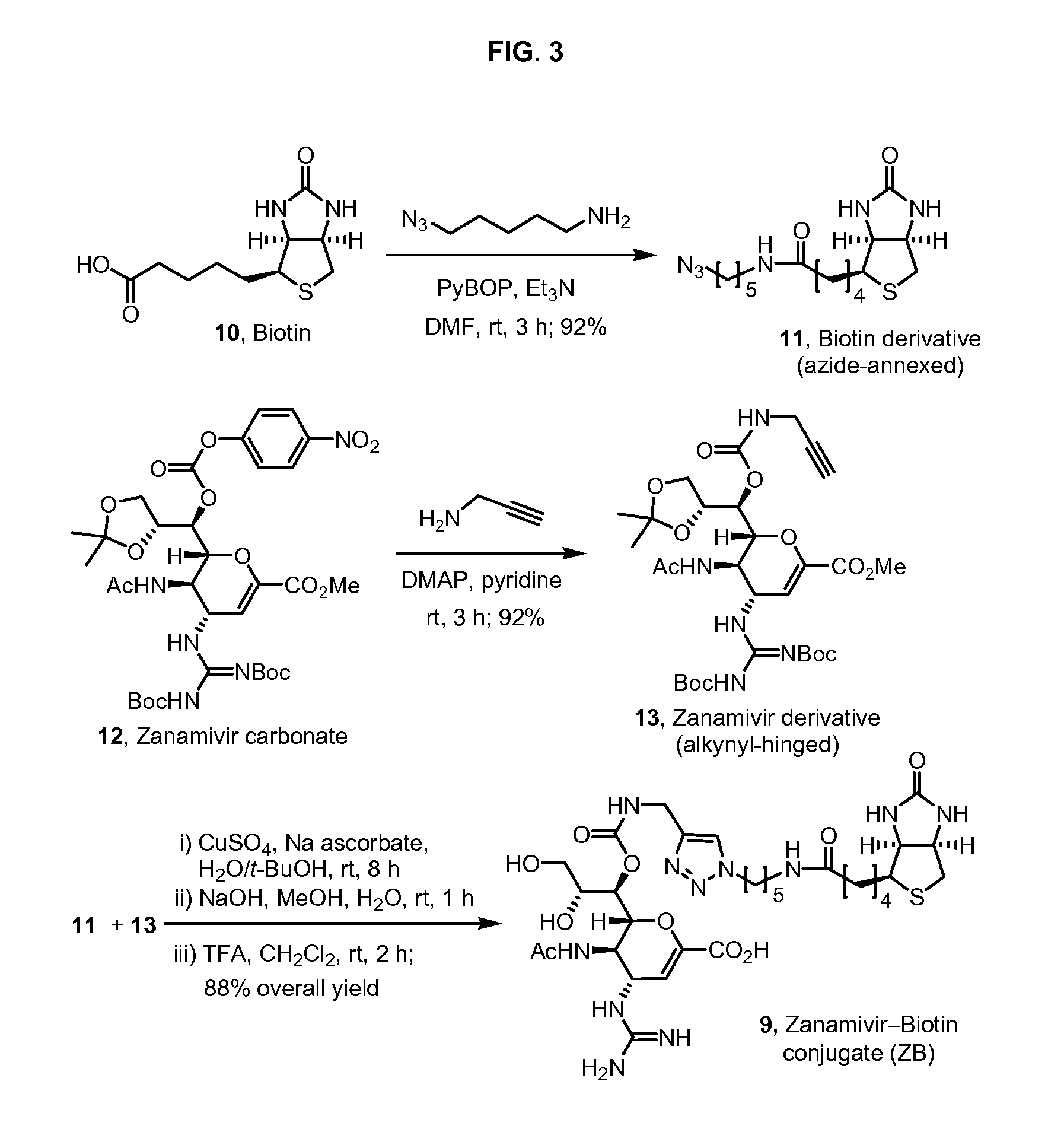
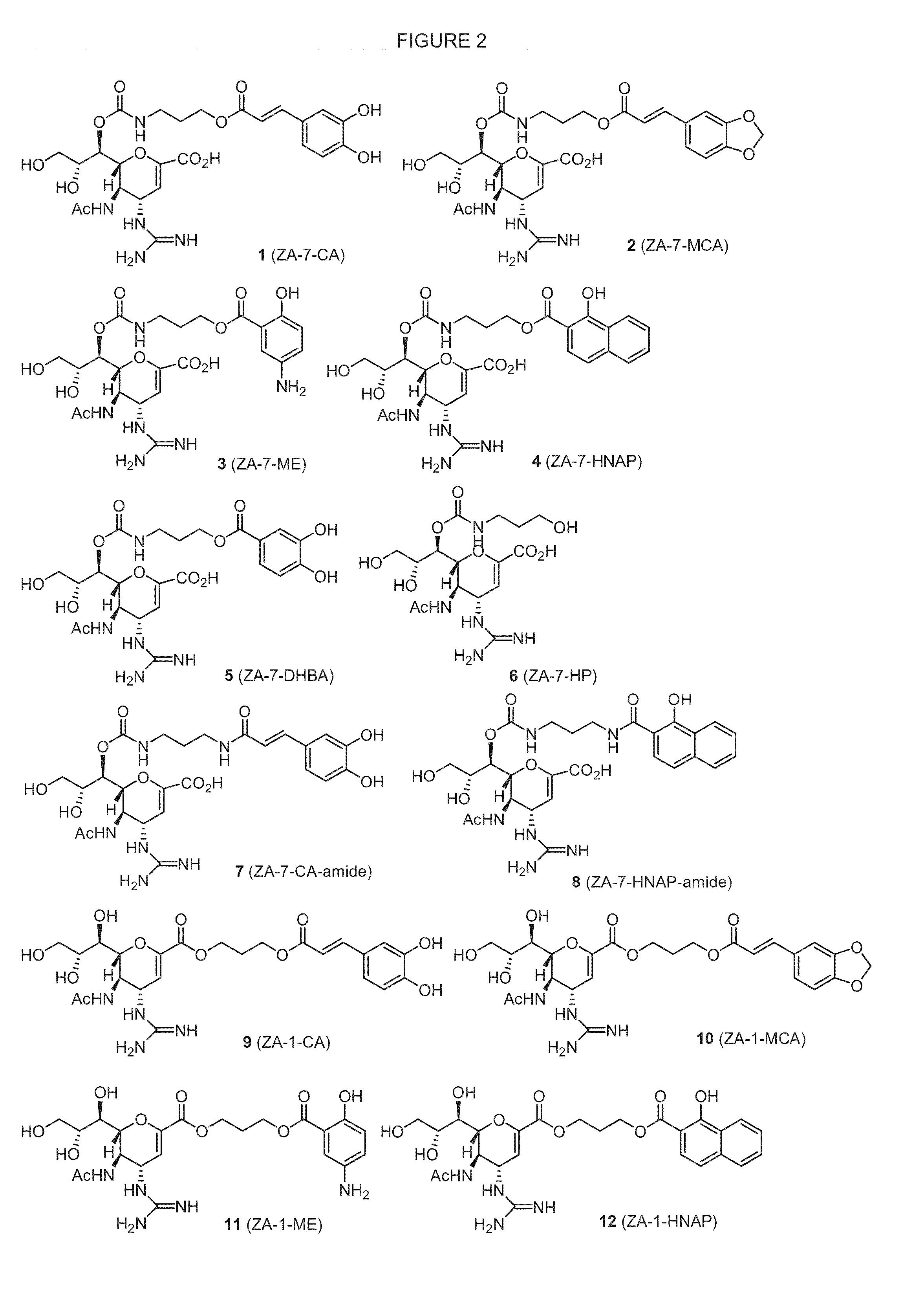
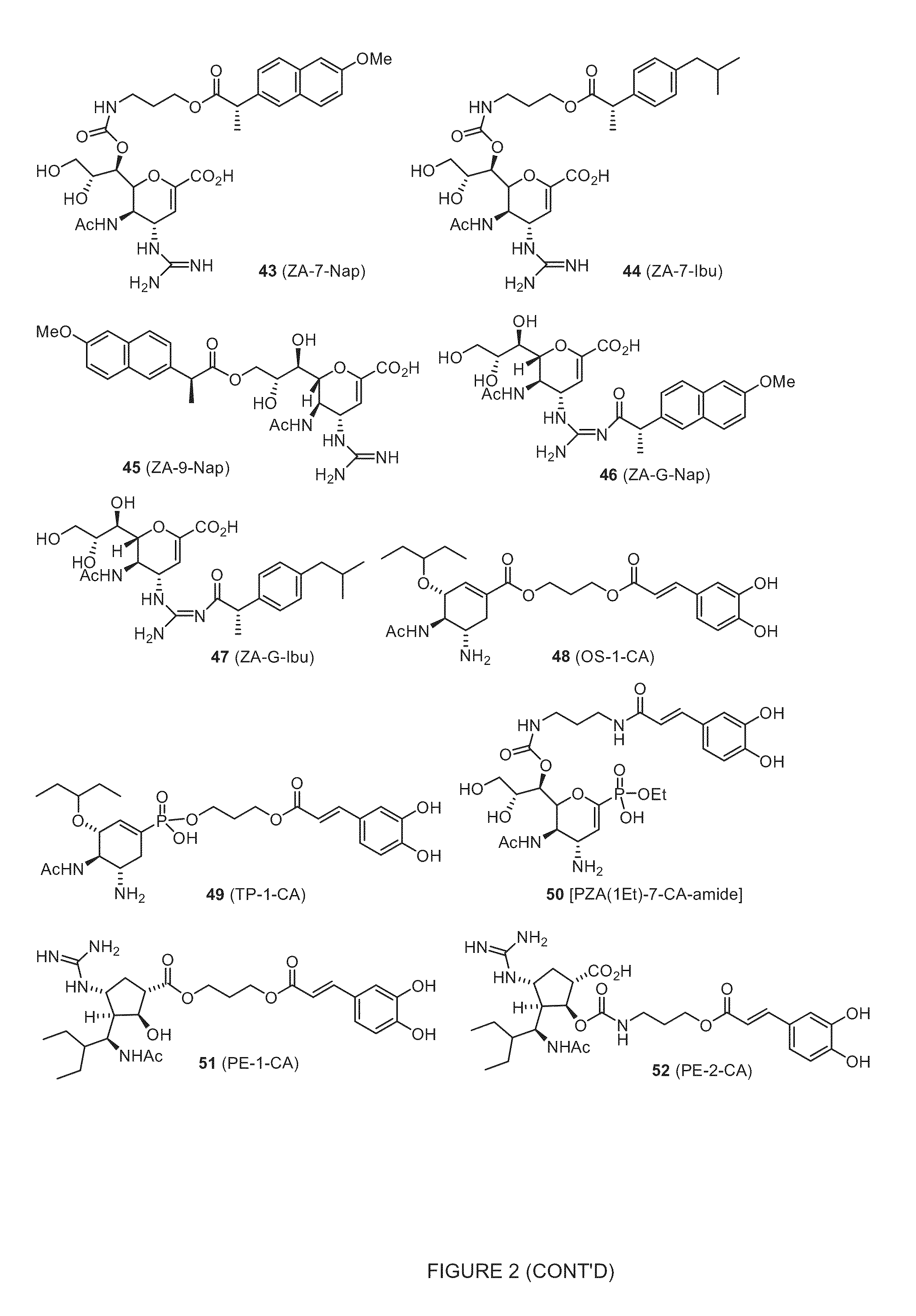
Novel dual-targeted, bifunctional anti-influenza drugs formed by conjugation with anti-inflammatory agents are disclosed. Exemplary drugs according to the invention include caffeic acid (CA)-bearing zanamivir (ZA) conjugates ZA-7-CA (1), ZA-7-CA-amide (7) and ZA-7-Nap (43) for simultaneous inhibition of influenza virus neuraminidase and suppression of proinflammatory cytokines. Synthetic methods for preparation of these enhanced anti-influenza conjugate drugs are provided. The synthetic bifunctional ZA conjugates act synergistically towards protection of mice lethally infected by H1N1 or H5N1 influenza viruses. The efficacy of ZA-7-CA, ZA-7-CA-amide and ZA-7-Nap conjugates is much greater than the combination therapy of ZA with anti-inflammatory agents.
Owner:ACAD SINIC
Medical application of 5-(1, 2, 4-triazole-1-yl)-2-phenylacetyl aminothiazole
InactiveCN103830233AHas anti-influenza virus neuraminidase activityOrganic active ingredientsAntiviralsHydrogenStructural formula
The invention relates to an application of 5-(1, 2, 4-triazole-1-yl)-2-phenylacetyl aminothiazole shown as a chemical structural formula I (img file='597988dest_path_image001. TIF' wi='279' he='152' / ) in preparing an influenza virus neuraminidase inhibitor, wherein R is selected from C1-C2 alkyl, C3-C4 straight chain or branch chain alkyl, and X1-X5 are selected from hydrogen, deuterium, C1-C2 alkyl and C3-C4 straight chain or branch chain alkyl.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
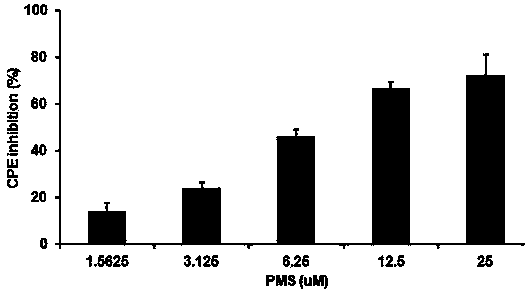
Application of poly mannuronic acid propyl ester sulfate in preparing anti- H1N1 influenza A virus medication
InactiveCN102743409AAvoid infectionPrevent proliferationOrganic active ingredientsAntiviralsCanine kidneyPolymannuronic acid
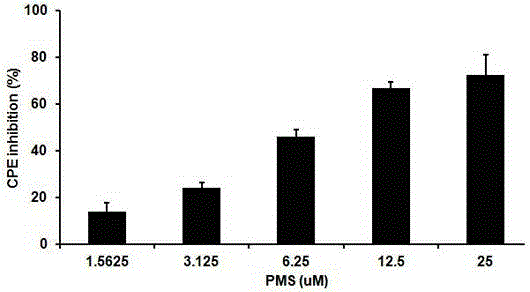
The invention provides applications of poly mannuronic acid propyl ester sulfate (PMS) in preparing anti- H1N1 influenza A virus medication. Experiments of the invention prove that PMS not only has great inhibition effect on the neuraminidase of the influenza A virus, but also has relatively good protection effect on canine kidney epithelial cells infected with the H1N1 influenza A virus, and can reduce the replication of the H1N1 virus with an effect that equal to the positive control medication ribavirin. Additionally, the PMS can effectively reduce the death rate of mice infected with the H1N1 influenza A virus and the survival time of the mice are prolonged. The poly mannuronic acid propyl ester sulfate provided by the invention has significant activity of inhibiting the neuraminidase of the H1N1 influenza A virus, and is proved to have good anti- H1N1 influenza A virus activity both in vivo and in vitro experiments, which shows the wide application prospect of the poly mannuronic acid propyl ester sulfate in preparing anti-H1N1 influenza A virus medication.
Owner:OCEAN UNIV OF CHINA
Rapid detection method of influenza virus neuraminidase antibody
ActiveCN102023212AExtensive testingShort reaction timeFermentationVector-based foreign material introductionAgglutinationViral neuraminidase
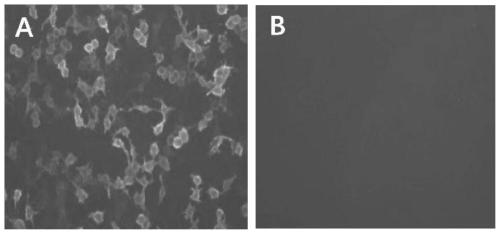
The invention belongs to the technical field of biology and particularly relates to a rapid detection method of an influenza virus neuraminidase antibody. The detection method comprises the following steps of: coning the influenza virus neuraminidase gene and then transfecting eukaryotic cells so that influenza virus neuraminidase protein is in recombination expression on the surface of eukaryotic cells, combining with the influenza virus neuraminidase antibody in a sample to be detected, performing agglutination reaction and judging whether the influenza virus neuraminidase antibody exists in the sample to be detected according to whether the agglutination reaction happens or not. The method is simple, rapid, stable and highly sensitive, and has wide application value.
Owner:SHANTOU UNIV MEDICAL COLLEGE
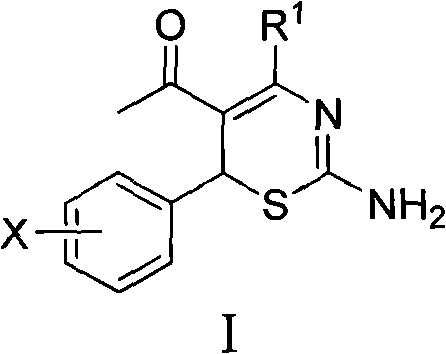
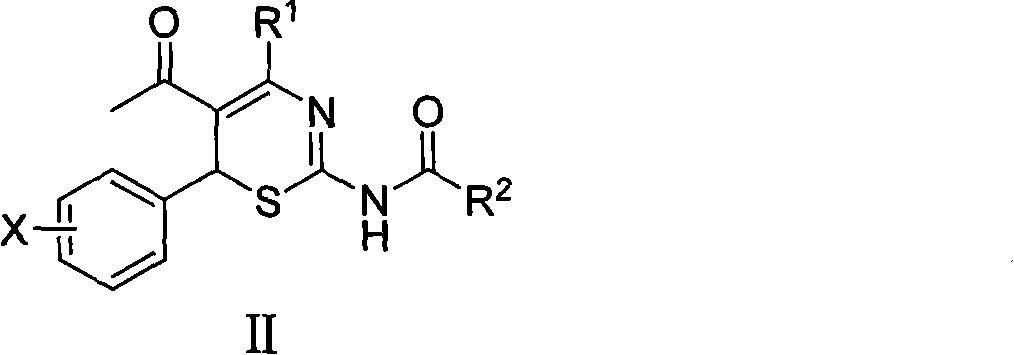
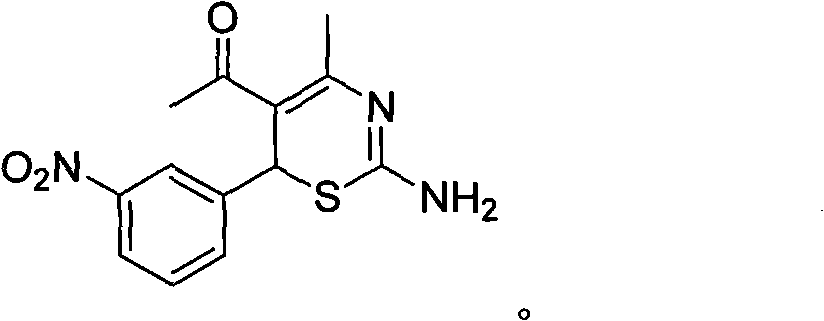
Use of 4-alkyl-6-aryl-5-acetyl-1,3-thiazine for preparing neuraminidase inhibitor
The invention discloses use of 4-alkyl-6-aryl-5-acetyl-1,3-thiazine represented by a chemical structural formula I and 4-alkyl-6-aryl-5-acetyl-2-amino-1,3-thiazine salts, or 4-alkyl-6-aryl-5-acetyl-2-amido-1,3-thiazine represented by a chemical structural formula II and 4-alkyl-6-aryl-5-acetyl-2-amido-1,3-thiazine salts for preparing a neuraminidase inhibitor of influenza viruses.
Owner:HUNAN UNIV
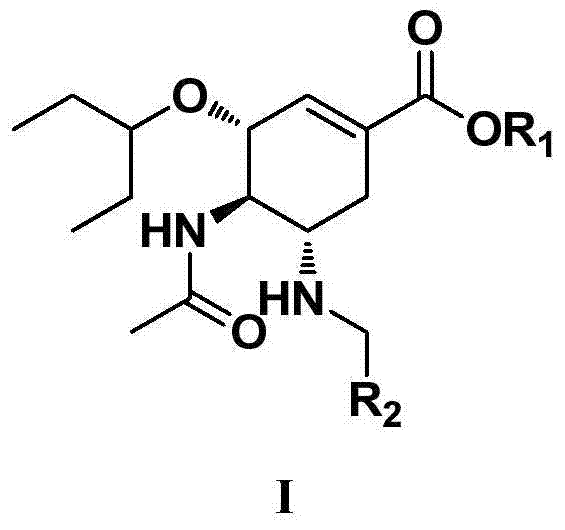
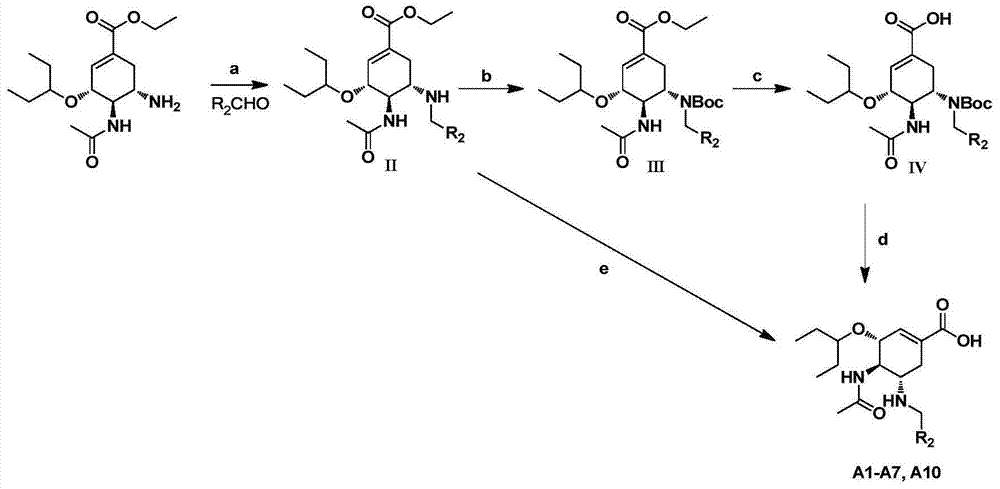
Cyclohexene compounds and application thereof
ActiveCN106496100AStrong inhibitory activityEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryEster prodrugCyclohexene
The invention discloses cyclohexene compounds and an application thereof, and belongs to the field of medicinal chemistry. The cyclohexene compounds are cyclohexene compounds having a structure characteristic represented by the formula I and pharmaceutically acceptable salts and ester prodrugs or stereoisomers thereof; the cyclohexene compounds are subjected to influenza virus neuraminidase activity detection and are confirmed to have good inhibition activity on both wild type and drug-resistant mutant type influenza viruses; a part of the compounds have the inhibition activity IC50 on the wild type and drug-resistant mutant type influenza virus neuraminidase of less than 5 nM, and have the effect for significantly inhibiting the influenza viruses; the cyclohexene compounds or the pharmaceutically acceptable salts or the stereoisomers thereof are showed to have a good application prospect in preparation of anti-influenza virus drugs, thereby providing a new drug choice for clinic treatment of influenza.
Owner:GUANGZHOU HENOVCOM BIOSCI CO LTD
Influenza virus vaccination regimens
ActiveUS20180008696A1Reduce severityImprove survivalSsRNA viruses negative-senseViral antigen ingredientsHemagglutininRegimen
Provided herein are immunization regimens for inducing an immune response (e.g., an antibody response) against influenza virus. In specific aspects, the immunization regimens involve the administration of a chimeric hemagglutinin (HA), a headless HA or another influenza virus stem domain based construct (e.g., the HA stem domain or a fragment thereof) to a subject. In certain aspects, the immunization regimens also involve the administration of an influenza virus neuraminidase immunogen.
Owner:MT SINAI SCHOOL OF MEDICINE
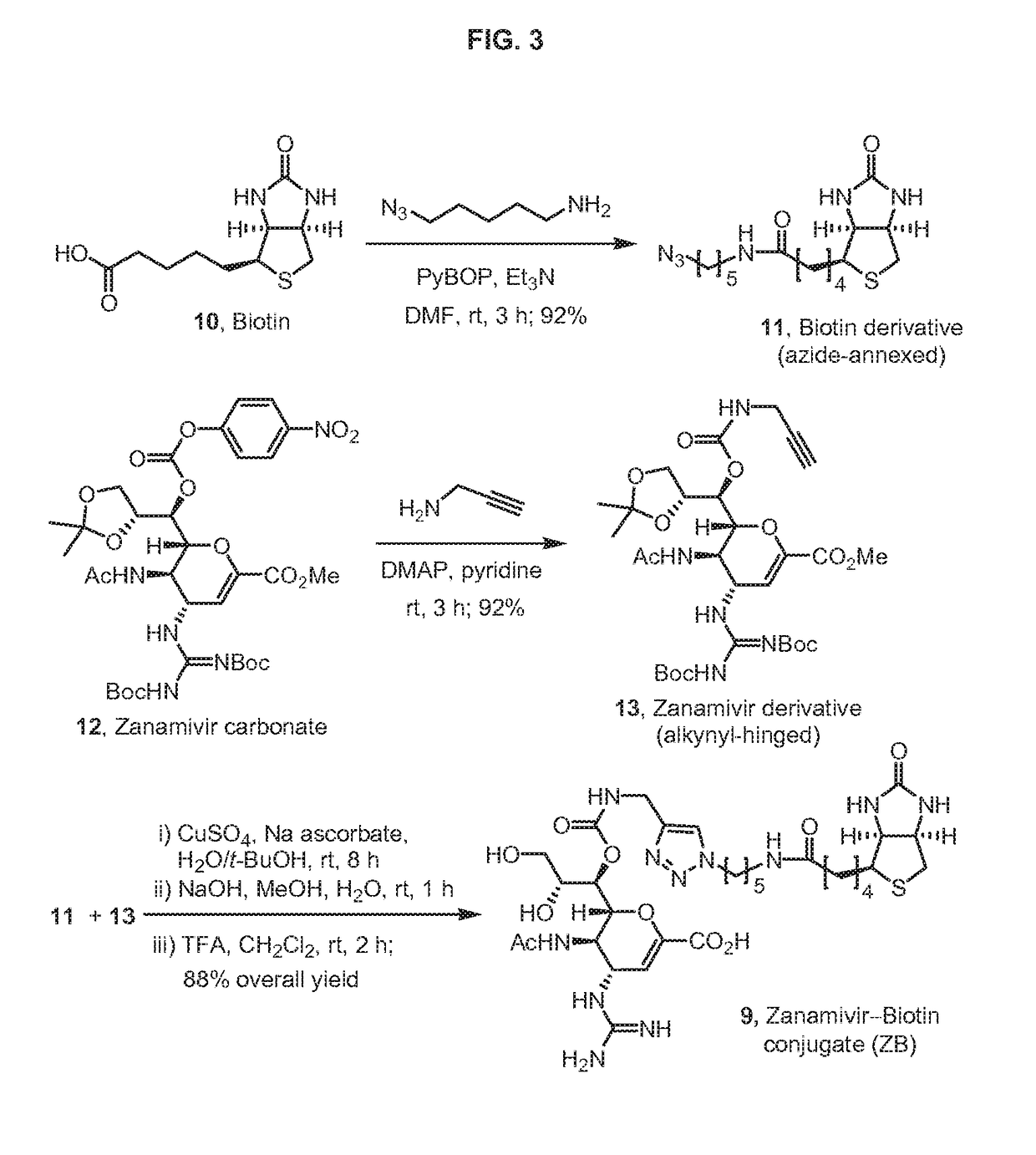
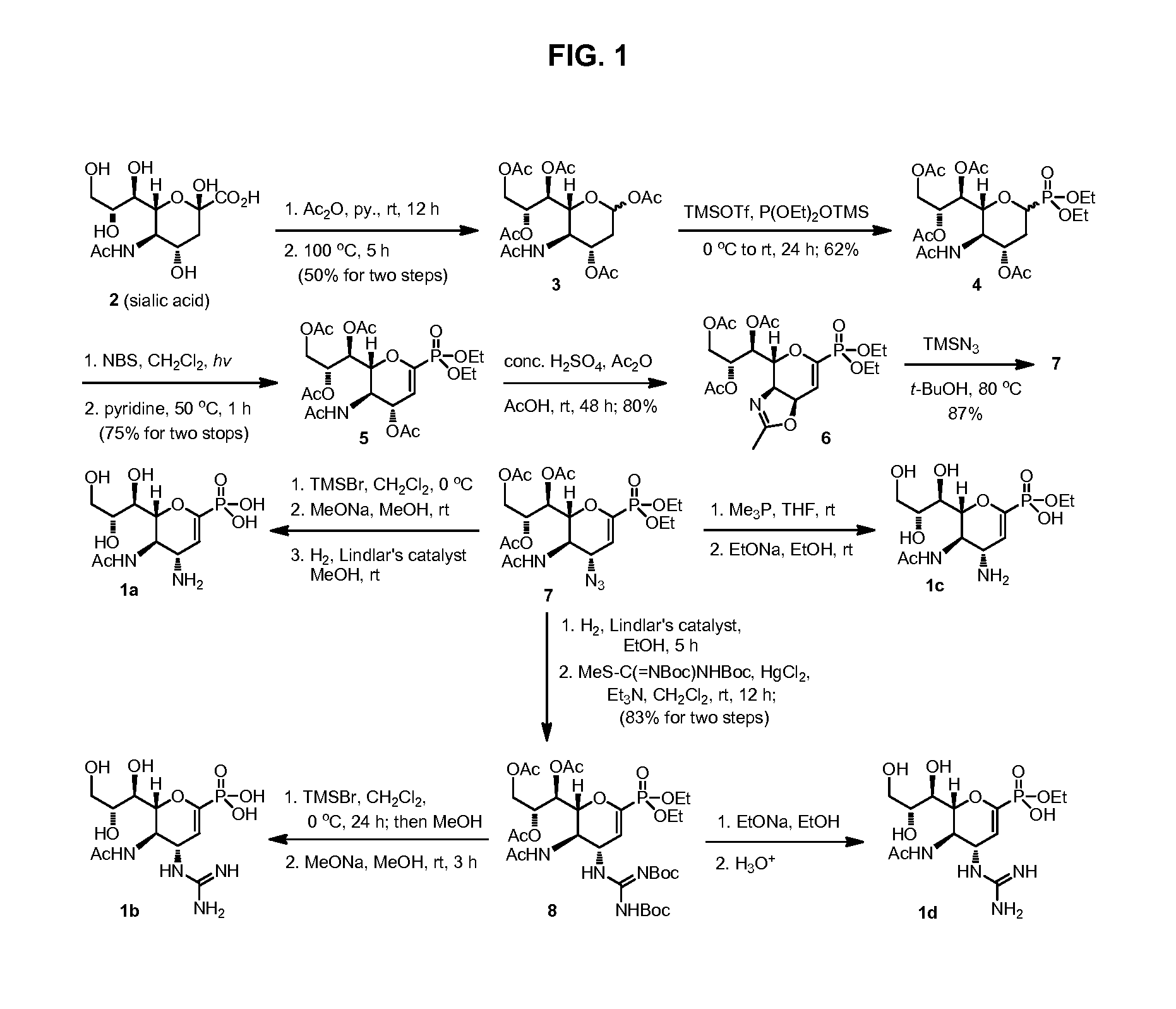
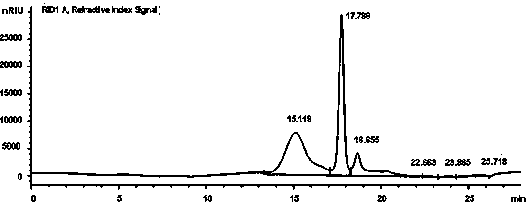
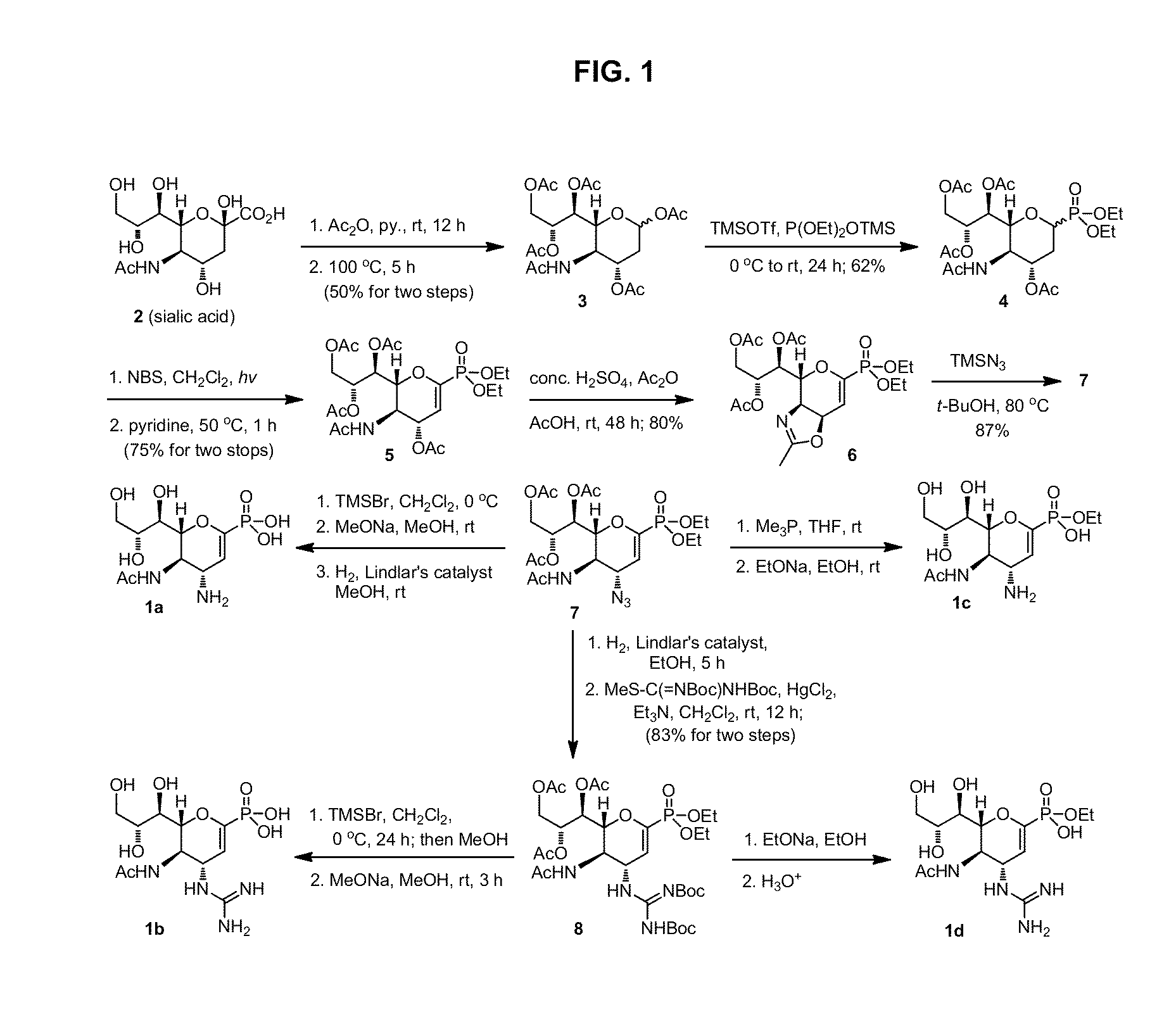
Zanamivir phosphonate congeners with Anti-influenza activity and determining oseltamivir susceptibility of influenza viruses
ActiveUS20170102387A1Organic active ingredientsGroup 5/15 element organic compoundsWild typeDrug resistance
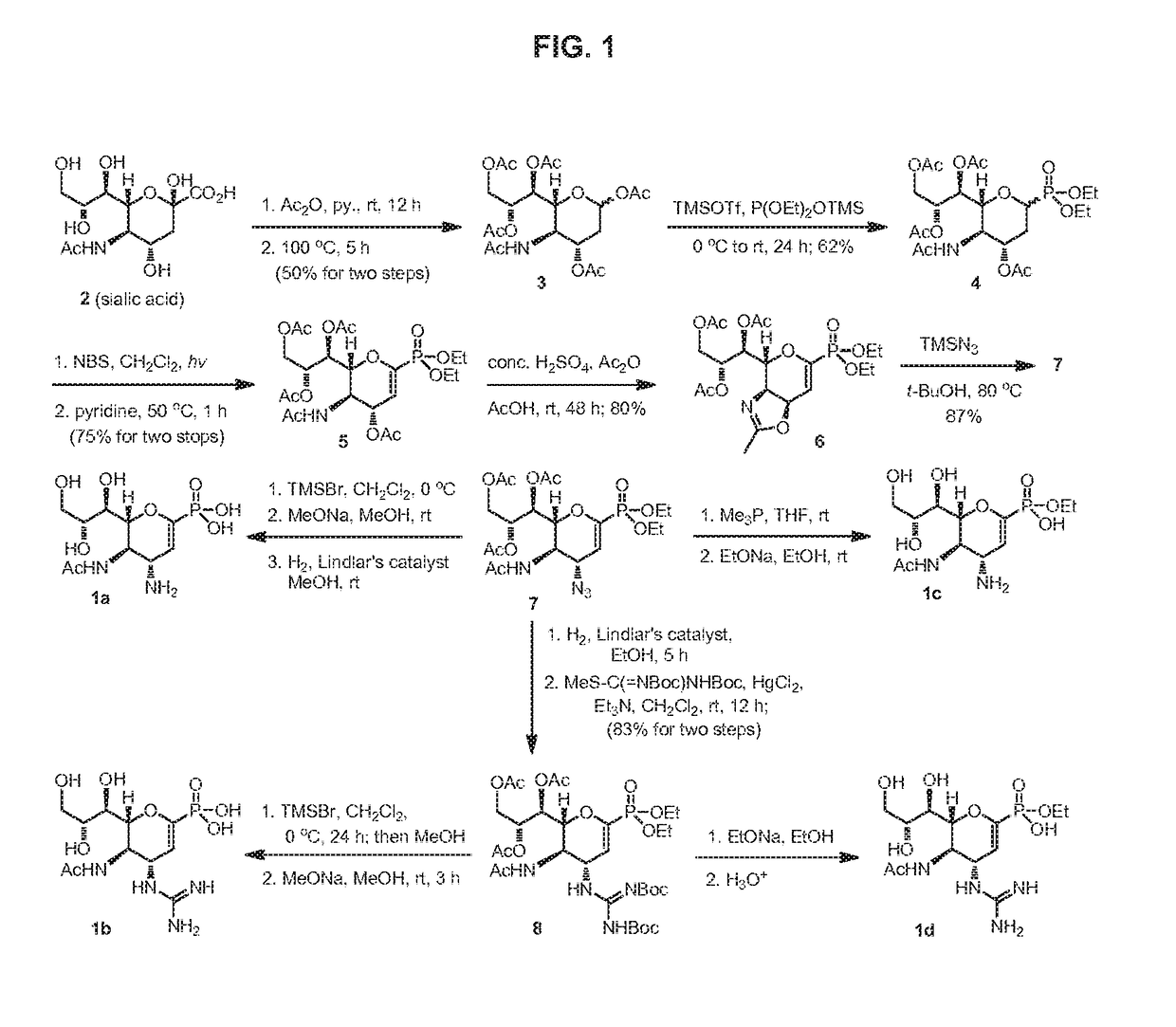
Methods and compositions for detection of drug resistant pathogens and treatment against infections thereof are provided. Methods for detection of oseltamivir-resistant influenza viruses by competitive binding assays utilizing non-oseltamivir influenza virus neuraminidase inhibitors and oseltamivir carboxylate are provided. Influenza virus neuraminidase inhibitors coupled to sensors and useful for employment in the methods of the invention are disclosed. Novel phosphonate compounds active as neuraminidase inhibitors against wild-type and oseltamivir-resistant influenza strains of H1N1, H5N1 and H3N2 viruses are disclosed. An enantioselective synthetic route to preparation of these phosphonate compounds via sialic acid is provided.
Owner:ACAD SINICA
Zanamivir phosphonate congeners with Anti-influenza activity and determining oseltamivir susceptibility of influenza viruses
ActiveUS20130225532A1Reduce the binding effectBiocideOrganic active ingredientsCompetitive bindingWild type
Methods and compositions for detection of drug resistant pathogens and treatment against infections thereof are provided. Methods for detection of oseltamivir-resistant influenza viruses by competitive binding assays utilizing non-oseltamivir influenza virus neuraminidase inhibitors and oseltamivir carboxylate are provided. Influenza virus neuraminidase inhibitors coupled to sensors and useful for employment in the methods of the invention are disclosed. Novel phosphonate compounds active as neuraminidase inhibitors against wild-type and oseltamivir-resistant influenza strains of H1N1, H5N1 and H3N2 viruses are disclosed. An enantioselective synthetic route to preparation of these phosphonate compounds via sialic acid is provided.
Owner:ACAD SINIC
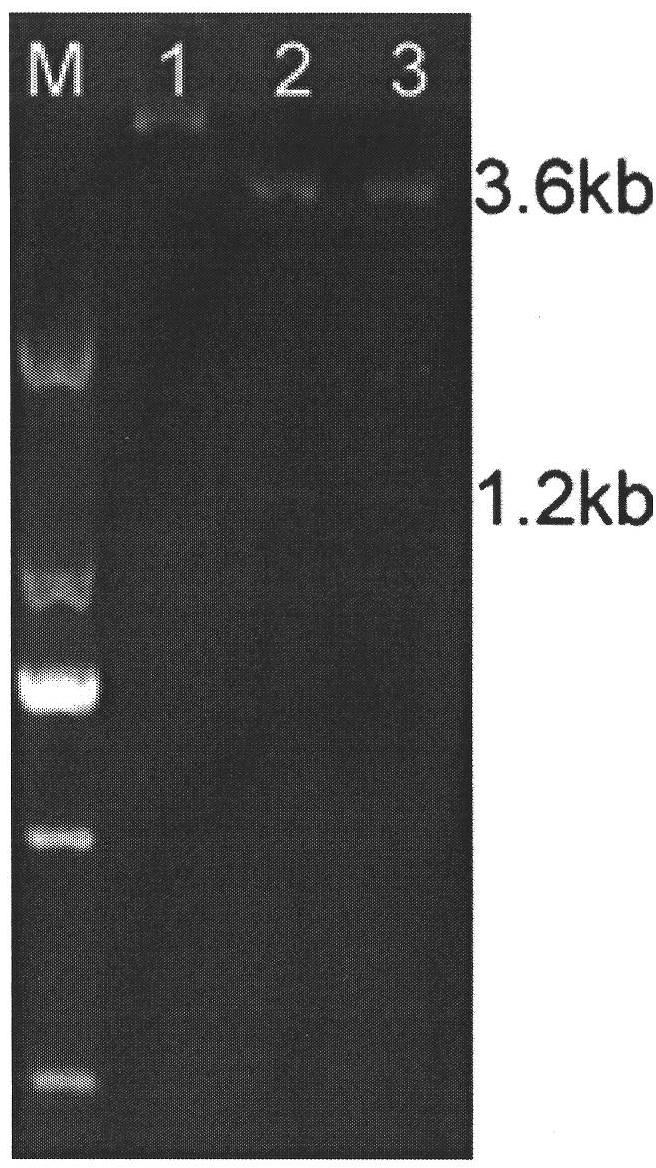
Influenza virus H1N1 subtype neuraminidase as well as gene, inhibitor screening model and application thereof
InactiveCN102586295ABiologically activeHigh secretion and expression efficiencyFungiHydrolasesPichia pastorisHigh-Throughput Screening Methods
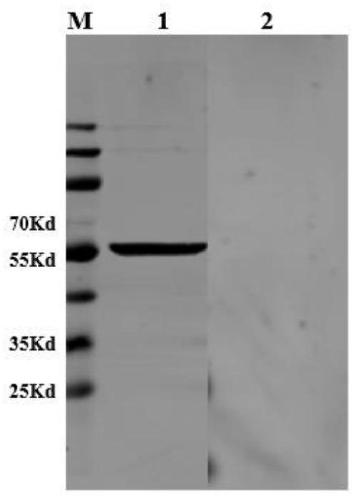
The invention provides influenza A-type influenza virus neuraminidase (NA), a gene and a preparation method thereof as well as a construction method of a screening model of an influenza virus neuraminidase inhibitor. Particularly, influenza A-type influenza virus H1N1 subtype neuraminidase is secretly expressed by pichia pastoris to obtain the neuraminidase with biological activity, and particularly, the truncated neuraminidases of 82 amino acids at N end of an NA full-length sequence is removed, so that an operation process of the NA prepared by totivirus, which is complicated, consumes time and has a safety problem, is avoided. According to the invention, the A-type influenza virus H1N1 subtype neuraminidase inhibitor screening model is also constructed by utilizing the neuraminidase; and the influenza virus H1N1 subtype neuraminidase has the advantages of definite mechanism of drug action, less material consumption, quick screening speed, high sensitivity, easy realization of high throughput screening and the like.
Owner:SHANGHAI INST OF PHARMA IND
Enhanced Anti-influenza agents conjugated with Anti-inflammatory activity
Novel dual-targeted, bifunctional anti-influenza drugs formed by conjugation with anti-inflammatory agents are disclosed. Exemplary drugs according to the invention include caffeic acid (CA)-bearing zanamivir (ZA) conjugates ZA-7-CA (1), ZA-7-CA-amide (7) and ZA-7-Nap (43) for simultaneous inhibition of influenza virus neuraminidase and suppression of proinflammatory cytokines. Synthetic methods for preparation of these enhanced anti-influenza conjugate drugs are provided. The synthetic bifunctional ZA conjugates act synergistically towards protection of mice lethally infected by H1N1 or H5N1 influenza viruses. The efficacy of ZA-7-CA, ZA-7-CA-amide and ZA-7-Nap conjugates is much greater than the combination therapy of ZA with anti-inflammatory agents.
Owner:ACAD SINIC
MASA (multi-analyte suspension array) detection kit for influenza virus H5N1 subtype and H7N9 subtype
ActiveCN104388599AStrong specificityHigh sensitivityMicrobiological testing/measurementHemagglutininMulti analyte
The invention relates to the field of biological medicines, particularly to an MASA (multi-analyte suspension array) detection kit for influenza virus H5N1 and H7N9 subtypes, and provides a primer pair and a probe for detecting influenza virus surface hemagglutinin antigen H5 and H7 subtypes, a primer pair and a probe for detecting influenza virus neuraminidase antigen N1 and N9 subtypes as well as a kit comprising the primer pairs and the probes and a usage method of the kit. The kit is good in sensitivity, accuracy and specificity and simple, convenient and quick to use, and detection of to-be-detected samples can be finished within four hours. Tests prove that no false positive result exists when the kit is used for detecting negative samples; the detection rate of 100% is realized when the kit is used for detecting samples containing H7N9 subtype viruses and H5N1 subtype viruses only; the H7N9 and H5N1 subtype viruses contained in samples can be completely detected when the kit is used for detecting the samples containing both the H5N1 subtype viruses and the H7N9 subtype viruses. Besides, the kit can be used for effectively detecting to-be-detected samples containing more than 10 copies.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Preparation method of recombinant baculovirus for expressing human transferrin receptor
PendingCN112680424AHigh expressionMaintain antigenicityMicroorganism based processesViruses/bacteriophagesEnzyme digestionShuttle vector
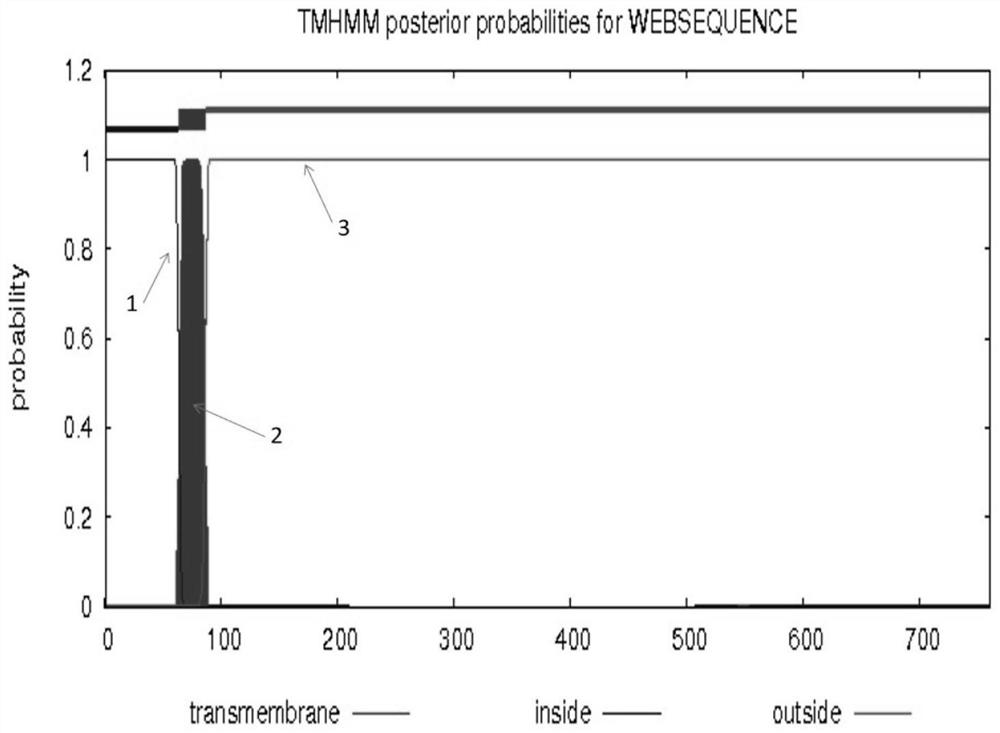
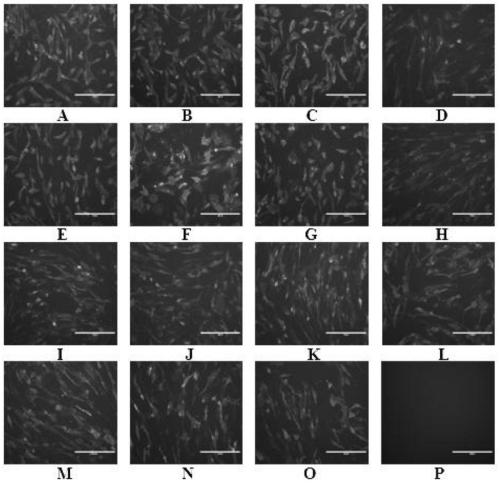
The invention discloses a preparation method of a recombinant baculovirus for expressing a human transferrin receptor. The method comprises the following steps: replacing a transmembrane region of a human transferrin receptor with a signal peptide and a transmembrane region amino acid sequence of influenza A virus neuraminidase, and optimizing a target gene to synthesize a gene sequence; the gene sequence is digested with EcoRI and NotI to obtain a target fragment with enzyme digestion sites, the insect cell expression vector pFastBac1 is digested with EcoRI and NotI, the digested target fragment and linearized vector are to purified and recovered, and a ligation reaction is performed through T4DNA ligase to obtain a ligation product; the ligation product is transformed into DH10Bac competent cells, and a recombinant baculovirus shuttle vector reBacmid-hTfR is obtained through resistant blue-white spot screening and separation; and transfecting the reBacmid-hTfR into an Sf9 insect cell, and carrying out cell subculture for three times, so as to obtain the recombinant baculovirus. The recombinant baculovirus can express hTfR on the surface of a cell membrane in the replication process, the antigenicity and solubility are maintained, and can be used for subsequent development of antibodies and biological functions.
Owner:SHENYANG AGRI UNIV
Application of poly mannuronic acid propyl ester sulfate in preparing anti- H1N1 influenza A virus medication
InactiveCN102743409BAvoid infectionPrevent proliferationOrganic active ingredientsAntiviralsCanine kidneyPolymannuronic acid
The invention provides applications of poly mannuronic acid propyl ester sulfate (PMS) in preparing anti- H1N1 influenza A virus medication. Experiments of the invention prove that PMS not only has great inhibition effect on the neuraminidase of the influenza A virus, but also has relatively good protection effect on canine kidney epithelial cells infected with the H1N1 influenza A virus, and can reduce the replication of the H1N1 virus with an effect that equal to the positive control medication ribavirin. Additionally, the PMS can effectively reduce the death rate of mice infected with the H1N1 influenza A virus and the survival time of the mice are prolonged. The poly mannuronic acid propyl ester sulfate provided by the invention has significant activity of inhibiting the neuraminidase of the H1N1 influenza A virus, and is proved to have good anti- H1N1 influenza A virus activity both in vivo and in vitro experiments, which shows the wide application prospect of the poly mannuronic acid propyl ester sulfate in preparing anti-H1N1 influenza A virus medication.
Owner:OCEAN UNIV OF CHINA
Zanamivir phosphonate congeners with anti-influenza activity and determining oseltamivir susceptibility of influenza viruses
Methods and compositions for detection of drug resistant pathogens and treatment against infections thereof are provided. Methods for detection of oseltamivir-resistant influenza viruses by competitive binding assays utilizing non-oseltamivir influenza virus neuraminidase inhibitors and oseltamivir carboxylate are provided. Influenza virus neuraminidase inhibitors coupled to sensors and useful for employment in the methods of the invention are disclosed. Novel phosphonate compounds active as neuraminidase inhibitors against wild-type and oseltamivir-resistant influenza strains of H1N1, H5N1 and H3N2 viruses are disclosed. An enantioselective synthetic route to preparation of these phosphonate compounds via sialic acid is provided.
Owner:ACAD SINIC
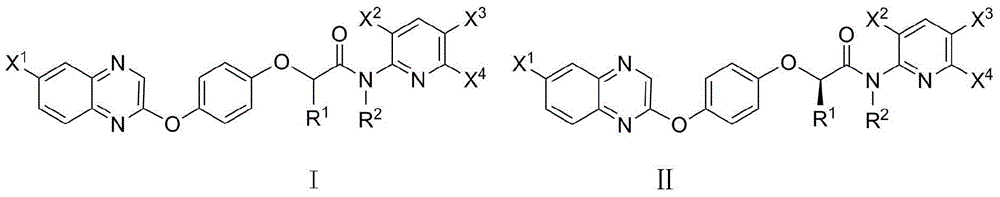
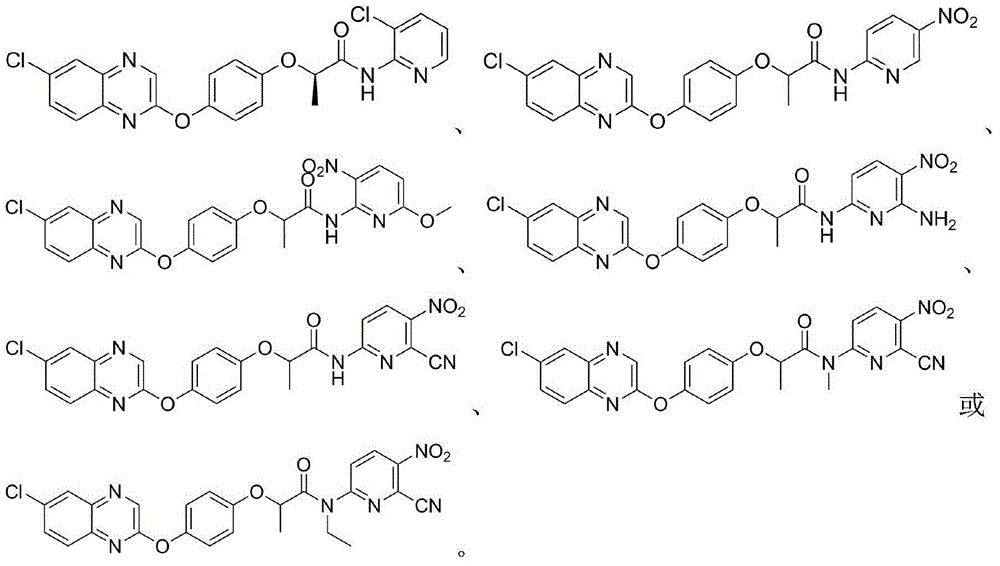
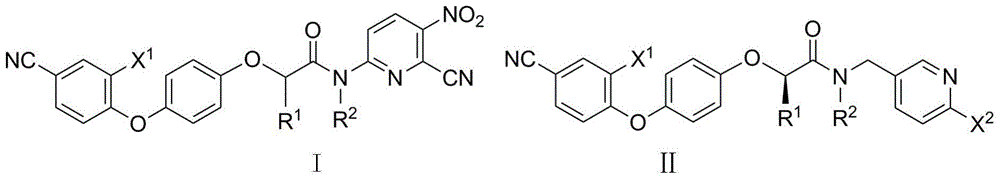
2-phenoxy quinoxaline derivative and pharmaceutical use thereof
ActiveCN104817539ASynthetic raw materials are readily availableEasy to prepareOrganic active ingredientsOrganic chemistryQuinoxalineHydrogen
The invention discloses a 2-phenoxy quinoxaline derivative and pharmaceutical use thereof. The chemical structural formula of the 2-phenoxy quinoxaline derivative is shown as formula I or II in the specification, wherein R1 and R2 are selected from hydrogen and C1-C2 alkyl; X1 is selected from fluorine, chlorine, bromine or iodine; X2 is selected from hydrogen, C1-C2 alkyl, fluorine, chlorine, bromine, iodine or nitro; X3 is selected from hydrogen, C1-C2 alkyl or nitro; and X4 is selected from hydrogen, C1-C2 alkyl, methoxy, cyano or amino. The invention also relates to a pharmaceutical composition containing the compound and application of the 2-phenoxy quinoxaline derivative in preparation of anti-influenza virus neuraminidase inhibitors.
Owner:CHINA THREE GORGES UNIV
Monoclonal antibody for identifying N6 subtype avian influenza virus neuraminidase protein and application of monoclonal antibody
ActiveCN111117970AIncreased sensitivityGood repeatabilityMicroorganism based processesImmunoglobulins against virusesAntibody levelAvian virus
The invention discloses a monoclonal antibody for identifying a N6 subtype avian influenza virus neuraminidase protein and an application of the monoclonal antibody. The monoclonal antibody is secreted by a hybridoma cell strain 3H7 with a preservation number of CGMCC No.18900. Experiments show that the monoclonal antibody 3H7 obtained by the invention can specifically bind to N6 subtype avian influenza viruses and can bind to 15 strains of N6 subtype avian influenza viruses with different evolutionary branches, thereby indicating that the 3H7 has a good broad spectrum; an ELISA detection kitfor N6 subtype avian influenza virus neuraminidase protein antibody blocking and a detection method are established by using the better broad spectrum and specificity of the monoclonal antibody; and experiments show that the method has high sensitivity and good reproducibility, can be used for detection of the antibody level of the N6 subtype avian influenza virus neuraminidase protein in a clinical serum sample, and has good development prospects.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation method and medical application of 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt
The invention discloses a preparation method and medical application of 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt. The preparation method of the 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt comprises the steps of: stirring 0.010 mol of 1-phenyl-4,4-dimethyl pentene-3-ketone, 0.012 mol of thiourea and 0.011 mol of acid in an organic solvent at a temperature of between 30 and 80 DEG C, performing TLC detection on reaction process, completing reaction, filtering, washing and drying the obtained product and obtaining the 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt (I). The 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt (I) can be medically used for preparing influenza virus neuraminidase inhibitors. In a formula, HX is hydrochloric acid, phosphoric acid, hydrobromic acid, sulphuric acid, trifluoroacetic acid or methanesulfonic acid, and Y is Cl, Br, CH3, Et, CF3, OCH3 or OEt.
Owner:HUNAN UNIV
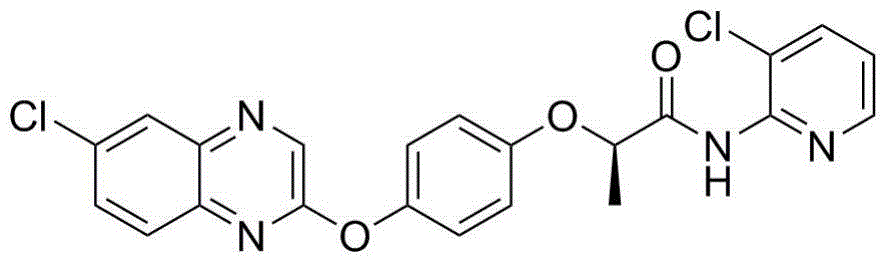
N-pyridyl-2-phenoxy fatty amide and medical application thereof
ActiveCN104817491ASynthetic raw materials are readily availableEasy to prepareOrganic chemistryAntiviralsHydrogenNeuraminidase
The invention discloses N-pyridyl-2-phenoxy fatty amide and medical application thereof. The chemical structural formula of N-pyridyl-2-phenoxy fatty amide is shown as formula I or II in the specification, wherein R1 and R2 are selected from hydrogen and C1-C12 alkyl, X1 is selected from fluorine, chlorine, bromine or iodine, and X2 is selected from fluorine, chlorine, bromine or iodine. The invention also relates to a pharmaceutical composition containing the compound and application of N-pyridyl-2-phenoxy fatty amide in preparation of anti-influenza virus neuraminidase inhibitors.
Owner:CHINA THREE GORGES UNIV
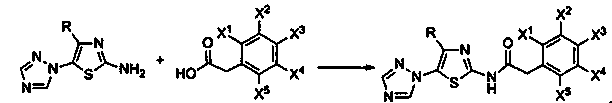
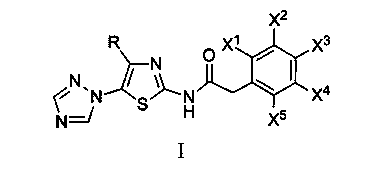
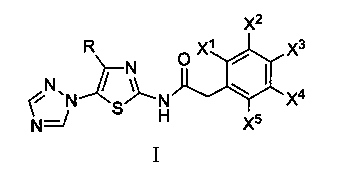
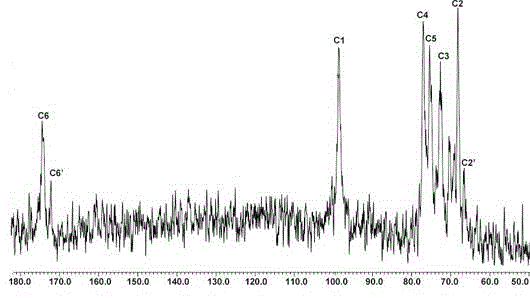
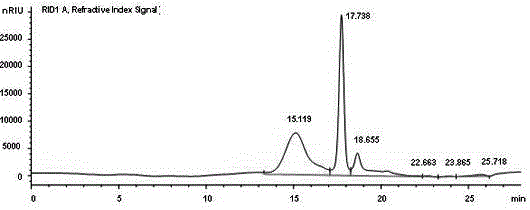
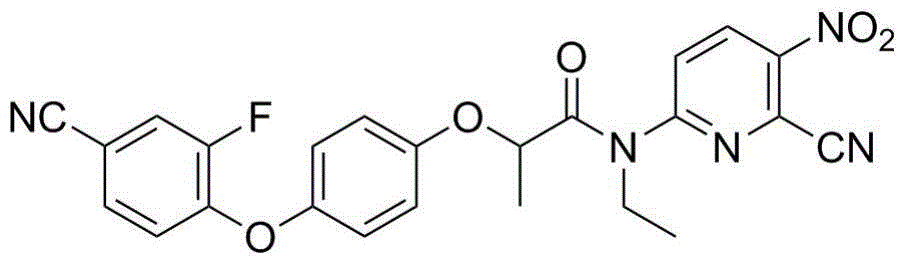
N-[5-(1,2,4-triazole-1-yl)thiazole-2-yl]aromatic amide, and pharmaceutical applications thereof
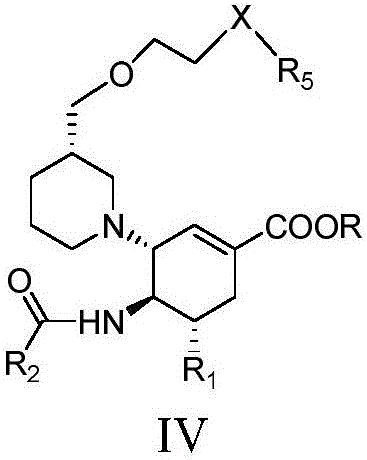
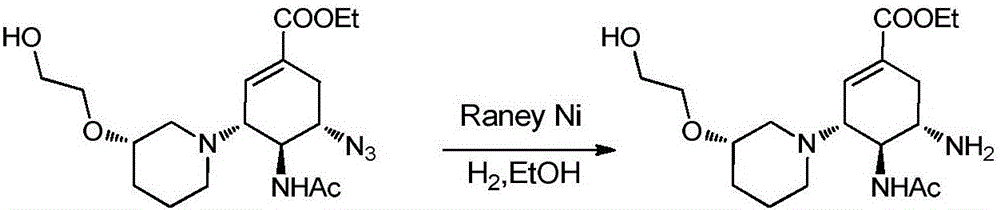
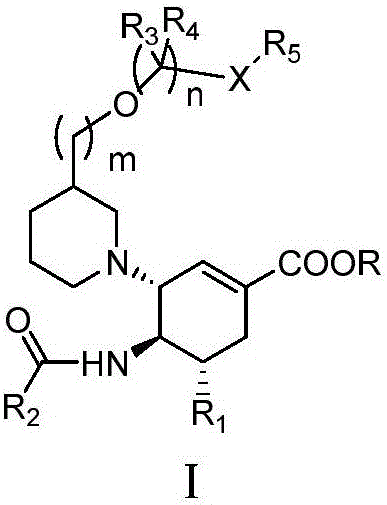
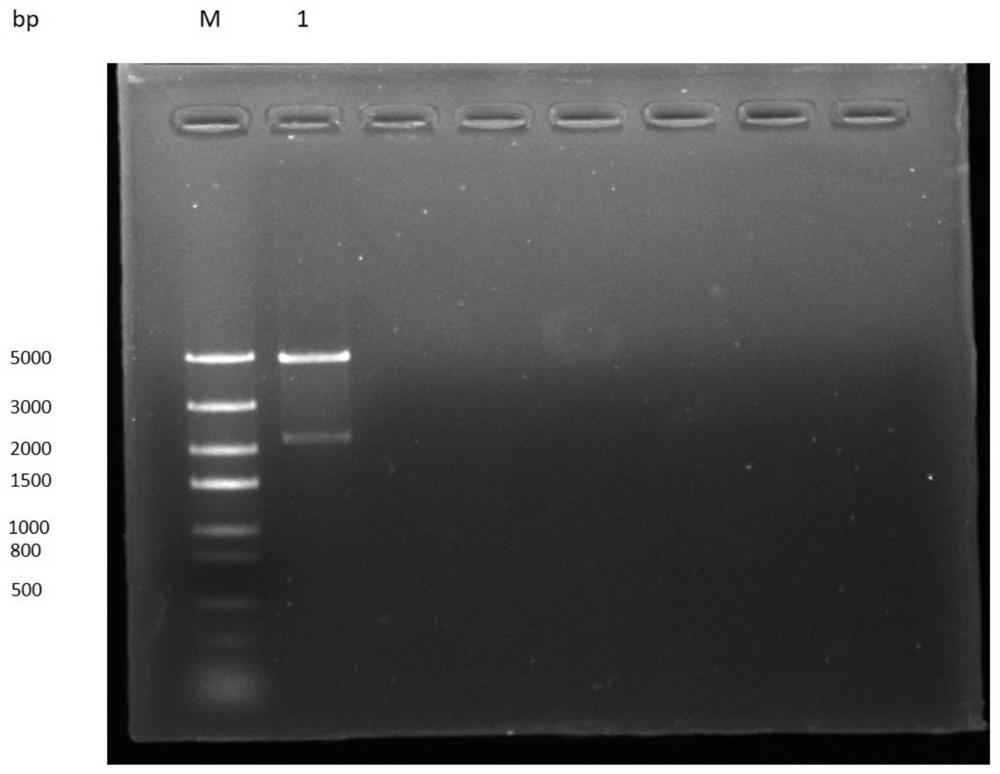
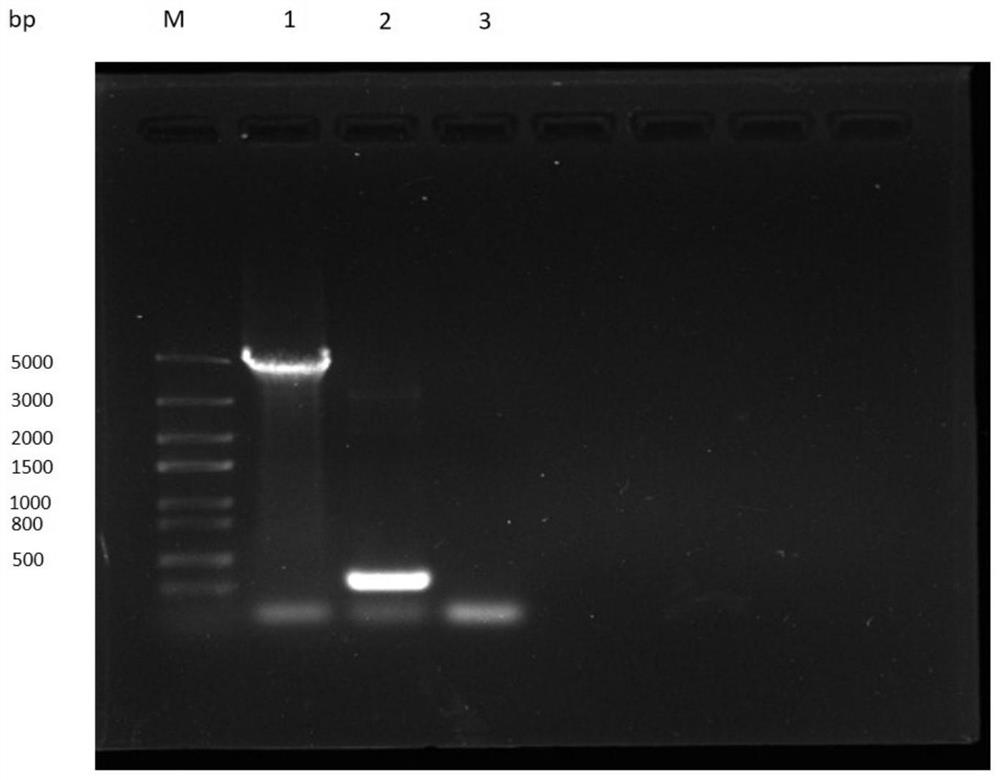
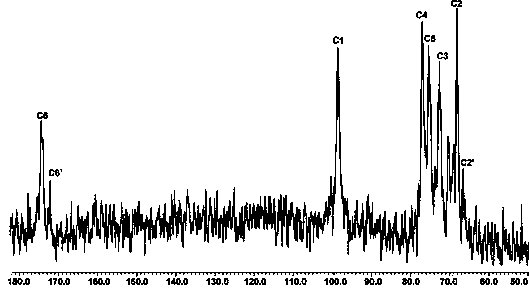
The invention relates to applications of N-[5-(1,2,4-triazole-1-yl)thiazole-2-yl]benzamide represented by chemical structural formula I in preparing influenza neuraminidase inhibitors. According to the chemical structural formula I, R is selected from C1-C2 alkyl, and C3-C4 linear or branched alkyl; R1 and R5 are selected from hydrogen, deuterium, methyl, ethyl, hydroxyl, methoxyl, ethyoxyl, fluorine, chlorine, bromine, nitryl, or trifluoromethyl; R2 and R4 are selected from hydrogen, deuterium, methyl, ethyl, hydroxyl, methoxyl, ethyoxyl, fluorine, chlorine, bromine, nitryl, trifluoromethyl, amino, or acetamido; and R3 is selected from hydrogen, deuterium, methyl, ethyl, hydroxyl, methoxyl, ethyoxyl, fluorine, chlorine, bromine, nitryl, trifluoromethyl, cyanogroup, amino, or acetamido.
Owner:HUNAN UNIV
Reagents and methods for detecting influenza virus proteins
ActiveUS20120141519A1SsRNA viruses negative-senseViral antigen ingredientsSequence analysisConserved sequence
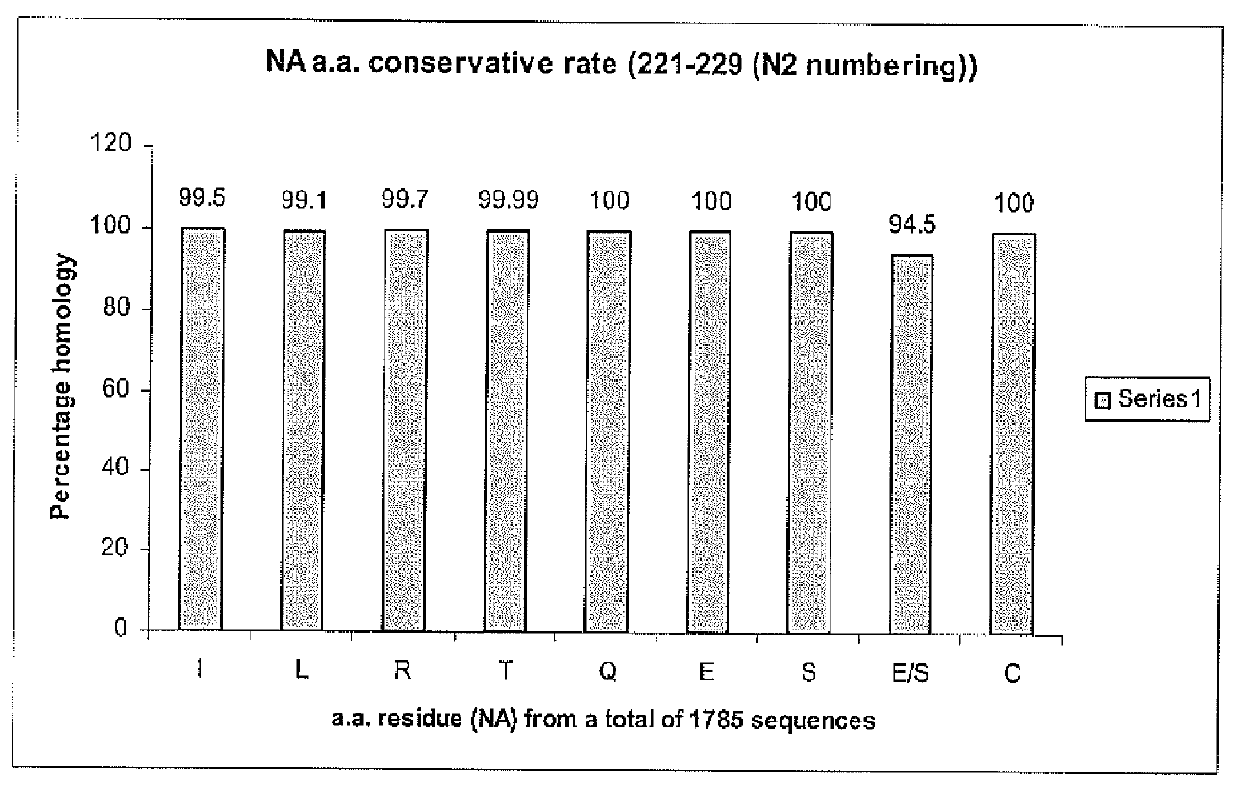
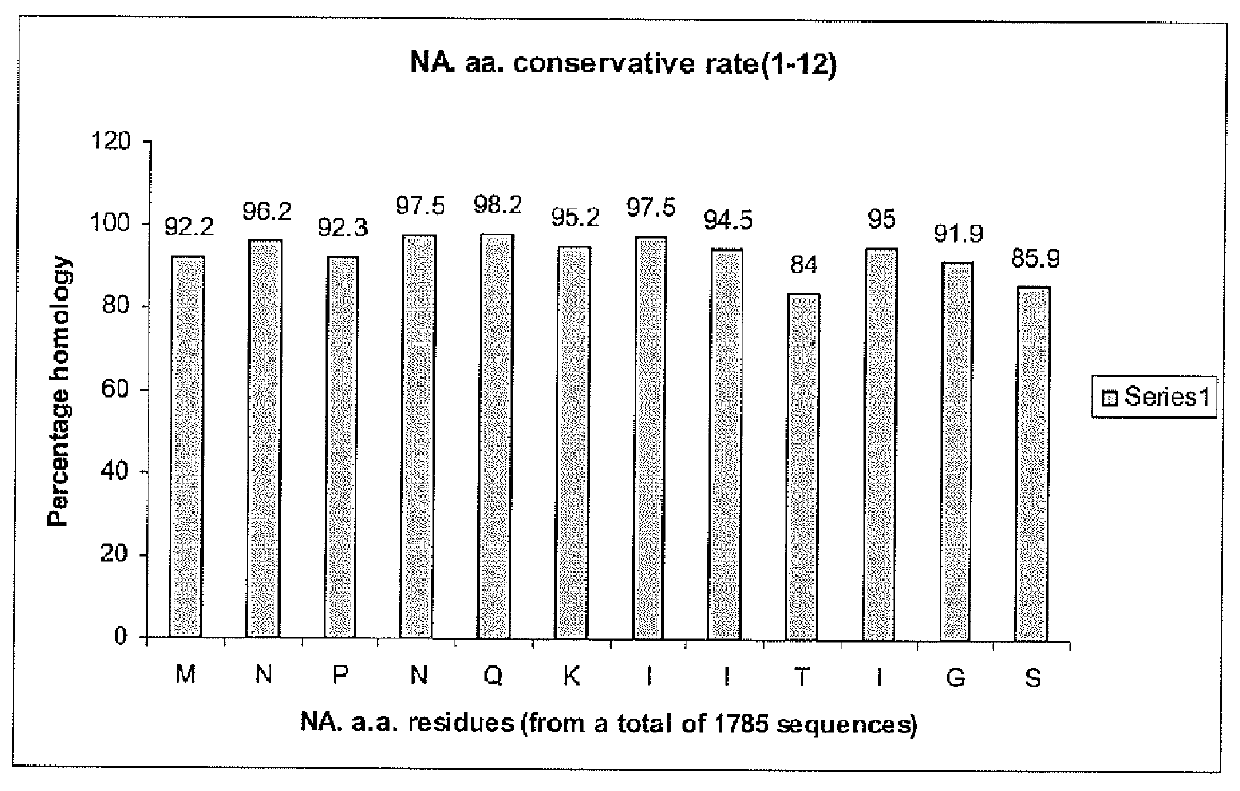
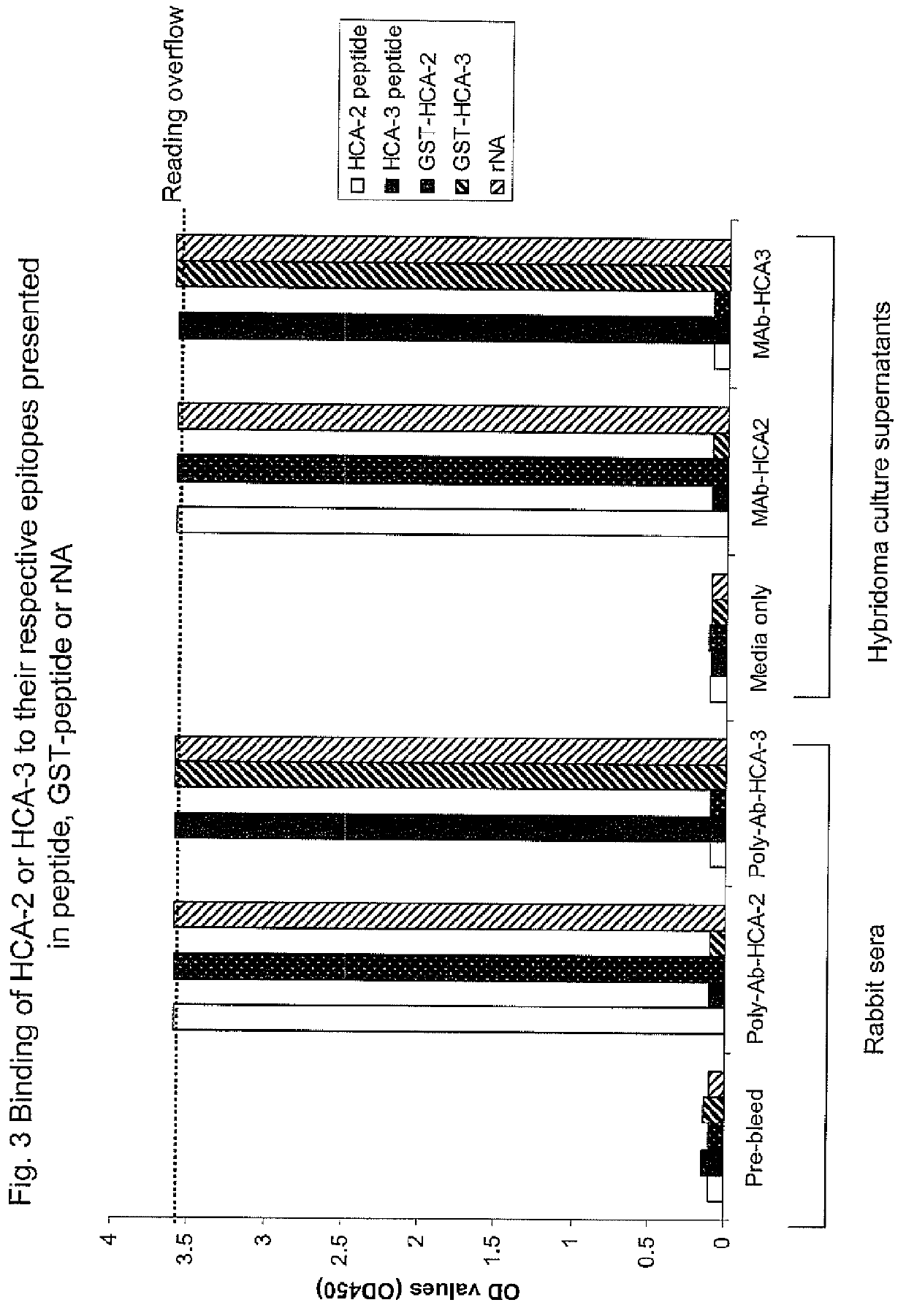
Two universally conserved sequences from influenza type A neuraminidases were identified by large scale sequence analysis then chemically modified and conjugated to carrier proteins to generate mono-specific and monoclonal antibodies. The two antibodies, one targeting the N-terminus of the type A neuraminidase and the other sequence close to enzymatic active site, were capable of binding to all 9 subtypes of neuraminidase while demonstrating remarkable specificity against the viral neuraminidase sequences since no cross-reactivity against allantoic proteins was observed. Quantitative analyses of NA using slot blot suggest that the antibodies can be used for NA antigen quantitation in vaccines. These represent the first time the antibody-based immunoassay can be used for NA quantitative determination.
Owner:LI XUGUANG +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof 3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/1ec64ec5-fc1c-433f-bd19-00bb4ff43c61/BDA0000456427430000021.PNG)
![3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof 3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/1ec64ec5-fc1c-433f-bd19-00bb4ff43c61/BDA0000456427430000022.PNG)
![3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof 3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/1ec64ec5-fc1c-433f-bd19-00bb4ff43c61/BDA0000456427430000023.PNG)
![3-[[2-(2-benzyl hydrazono) thiazole-5-base] methyl] quinoline-2 (1H)-ketone and preparation and applications thereof 3-[[2-(2-benzyl hydrazono) thiazole-5-base] methyl] quinoline-2 (1H)-ketone and preparation and applications thereof](https://images-eureka.patsnap.com/patent_img/4c0ff670-457a-4c5e-b5e8-1ae95f539a5b/FDA0000457085920000011.png)
![3-[[2-(2-benzyl hydrazono) thiazole-5-base] methyl] quinoline-2 (1H)-ketone and preparation and applications thereof 3-[[2-(2-benzyl hydrazono) thiazole-5-base] methyl] quinoline-2 (1H)-ketone and preparation and applications thereof](https://images-eureka.patsnap.com/patent_img/4c0ff670-457a-4c5e-b5e8-1ae95f539a5b/FDA0000457085920000012.png)
![3-[[2-(2-benzyl hydrazono) thiazole-5-base] methyl] quinoline-2 (1H)-ketone and preparation and applications thereof 3-[[2-(2-benzyl hydrazono) thiazole-5-base] methyl] quinoline-2 (1H)-ketone and preparation and applications thereof](https://images-eureka.patsnap.com/patent_img/4c0ff670-457a-4c5e-b5e8-1ae95f539a5b/BDA0000457085930000021.png)

![N-[5-(1,2,4-triazole-1-yl)thiazole-2-yl]aromatic amide, and pharmaceutical applications thereof N-[5-(1,2,4-triazole-1-yl)thiazole-2-yl]aromatic amide, and pharmaceutical applications thereof](https://images-eureka.patsnap.com/patent_img/747eb220-8a6d-4331-9d88-57994c72d1d3/BDA0000454795420000021.PNG)
![N-[5-(1,2,4-triazole-1-yl)thiazole-2-yl]aromatic amide, and pharmaceutical applications thereof N-[5-(1,2,4-triazole-1-yl)thiazole-2-yl]aromatic amide, and pharmaceutical applications thereof](https://images-eureka.patsnap.com/patent_img/747eb220-8a6d-4331-9d88-57994c72d1d3/BDA0000454795420000022.PNG)
![N-[5-(1,2,4-triazole-1-yl)thiazole-2-yl]aromatic amide, and pharmaceutical applications thereof N-[5-(1,2,4-triazole-1-yl)thiazole-2-yl]aromatic amide, and pharmaceutical applications thereof](https://images-eureka.patsnap.com/patent_img/747eb220-8a6d-4331-9d88-57994c72d1d3/BDA0000454795420000031.PNG)