Application of poly mannuronic acid propyl ester sulfate in preparing anti- H1N1 influenza A virus medication
A technology of propyl uronic acid and influenza virus, which can be used in antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., and can solve problems such as drug resistance, strong toxic side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
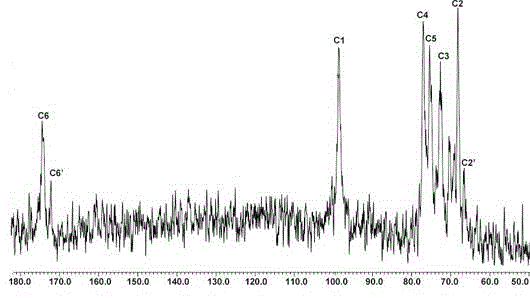
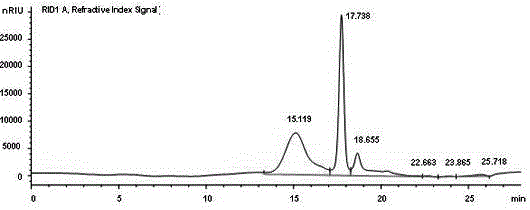
[0025] Embodiment 1, the physicochemical property analysis of polymannuronate propyl ester sulfate
[0026] The present invention prepares and separates polymannuronic acid from seaweed polysaccharide alginate by acid degradation method, and then obtains polymannuronic acid propyl ester sulfate (in patent ZL88109698. The preparation method of polymannuronate propyl sulfate PMS is described).
[0027] The main structural general formula of described polymannuronate propyl sulfate is as follows:
[0028]
[0029] Where R=Na or CH 2 CH(OH)CH 3 , R'=H or SO 3 Na, at least one R'=SO in each sugar ring 3 Na, n≤50, more than 85% of the molecular skeleton is β-1,4-D-mannuronic acid, and the rest is α-1,4-L-guluronic acid, of which 40-60% of carboxyl groups are Esterification of propanol, the part C in the sugar residue 2 and C 3 The hydroxyl group on the position is replaced by a sulfate group, and the content of organic sulfur is 9-13%. The weight-average molecular weight o...
Embodiment 2
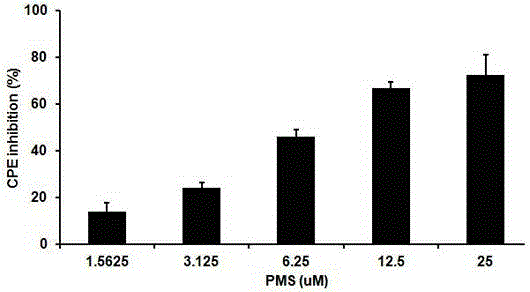
[0036] Embodiment 2, the effect of polymannuronic acid propyl ester sulfate PMS anti-influenza A H1N1 influenza virus proliferation in vitro
[0037] 1) Evaluation of the anti-infection effect of PMS at the cellular level against influenza A (H1N1) virus
[0038] Infection of dog kidney epithelial cells (MDCK) with influenza A virus mouse-adapted strain H1N1 (A / PR / 8 / 34, provided by Wuhan Institute of Virology, Chinese Academy of Sciences) was used to establish a cell model, and this cell model was combined with cytopathic (CPE) inhibition test The inhibitory activity and cytotoxicity of polymannuronate propyl sulfate PMS on IAV were detected by colorimetric method with thiazole basket (MTT), and the half inhibitory concentration IC was calculated 50 and the half cytotoxic concentration CC 50 . Ribavirin was selected as the positive control drug.
[0039] The experimental results are shown in Table 2 and image 3 shown. image 3 Among them, at the concentration of 12.5uM, ...
Embodiment 3
[0046] Embodiment 3, PMS anti-infection effect of influenza A (H1N1) virus in vivo
[0047] 1) Effects of PMS on lung lesions and body weight of mice infected with influenza A (H1N1) virus
[0048] SPF BALB / C mice were infected with nasal drops of mouse lung-adapted strain H1N1 (A / PR / 8 / 34) to establish an animal model, and this mouse model was used to test the effect of polymannuronate propyl sulfate PMS in vivo Effects against IAV infection.
[0049] Divide 80 female BALB / C mice with SPF grade of 14-16g into virus control group (model group), normal control group, positive drug ribavirin group, positive drug oseltamivir group according to body weight, PMS injection and PMS gavage group, 10 rats in each group. Except the normal group, all other groups were treated with 10LD under light ether anesthesia. 50 Infect the nose with IAV virus liquid drops, 40 μL per mouse. Ribavirin group and PMS injection group started intraperitoneal injection 1 day before virus infection, ose...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com