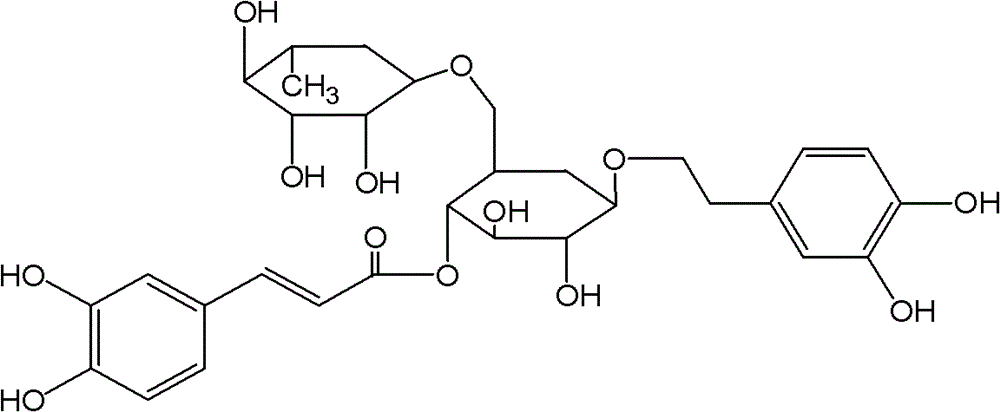
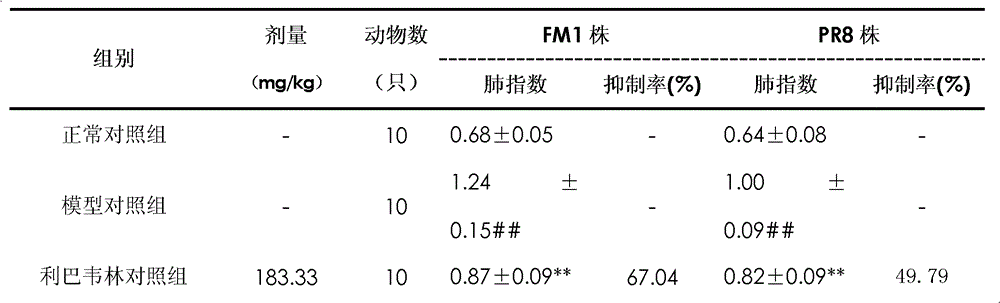
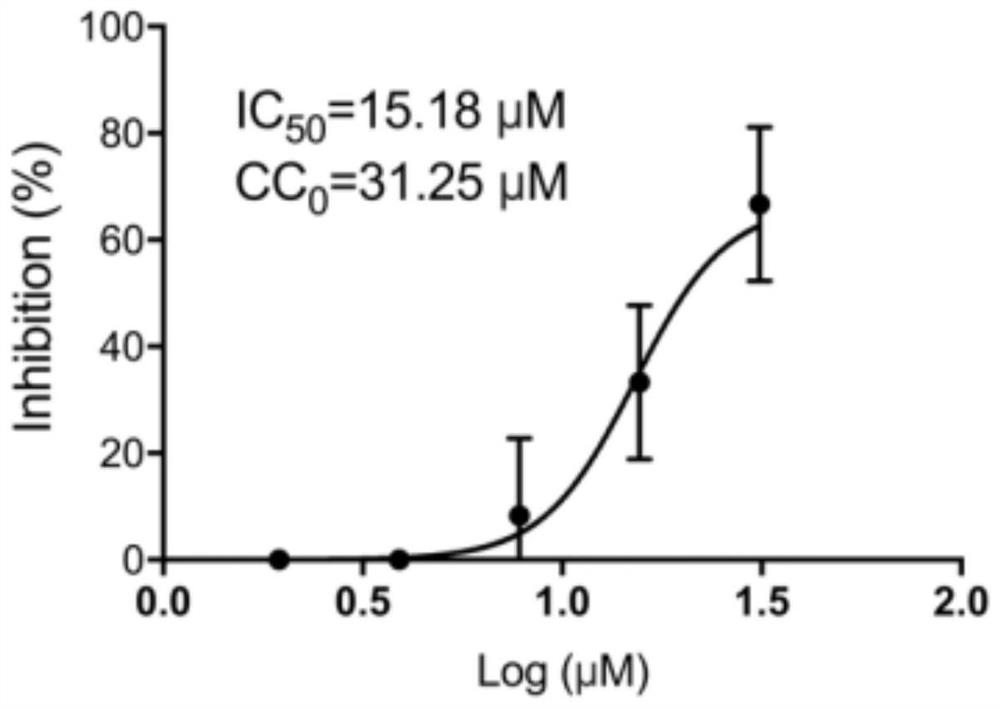
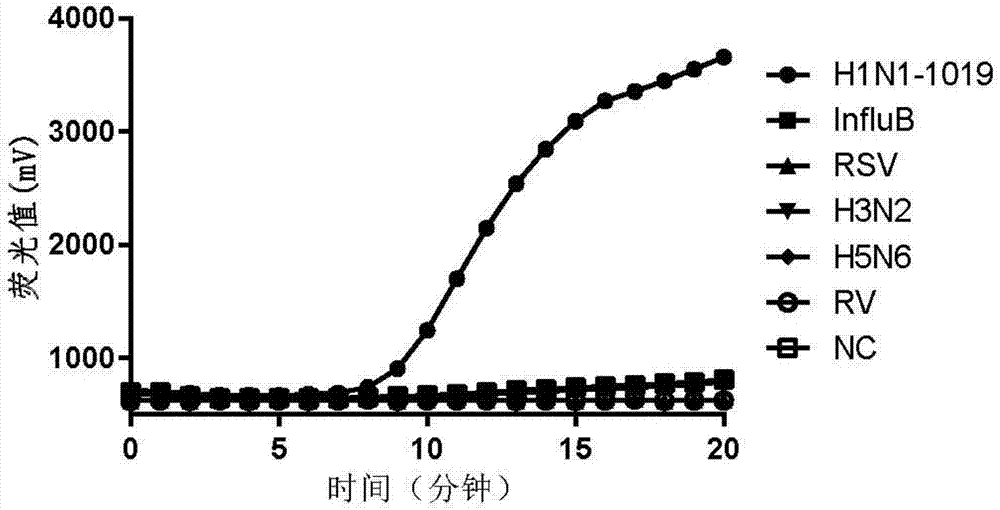
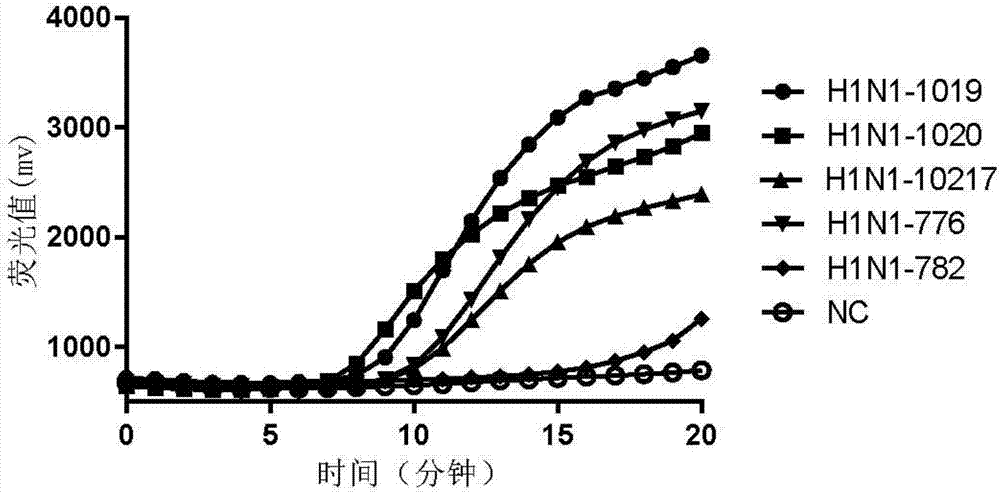
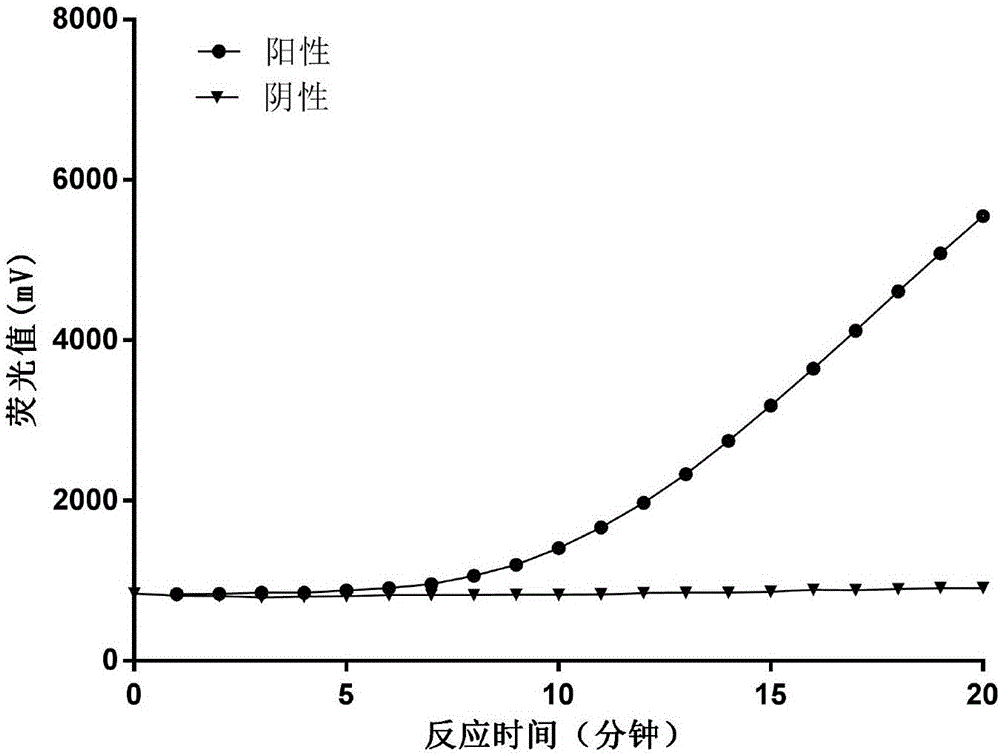
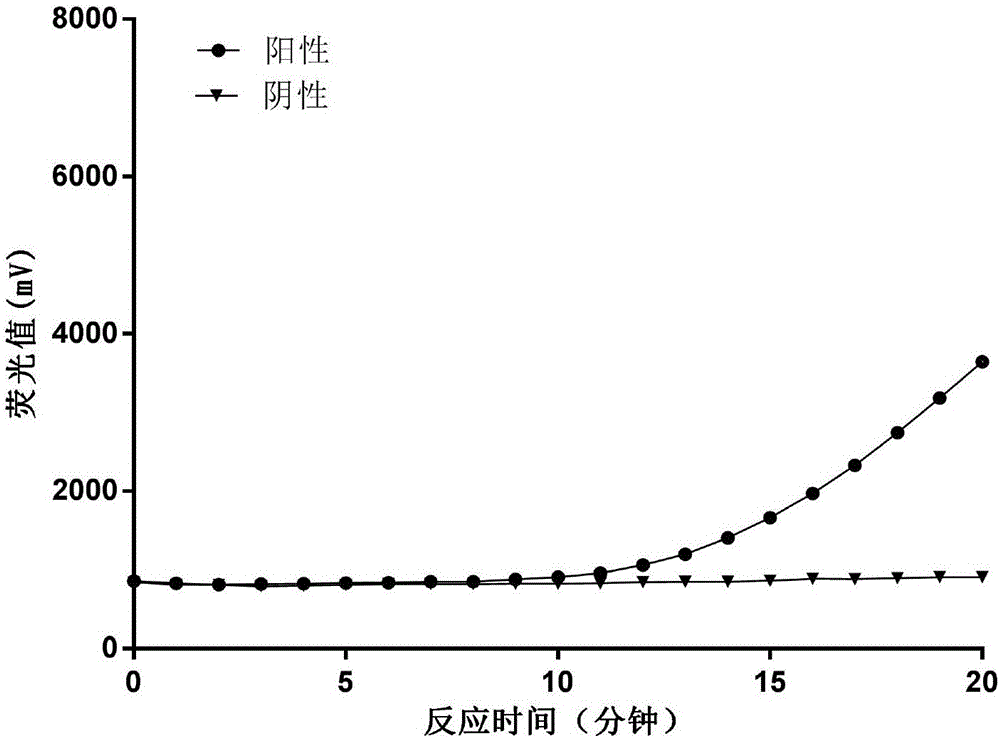
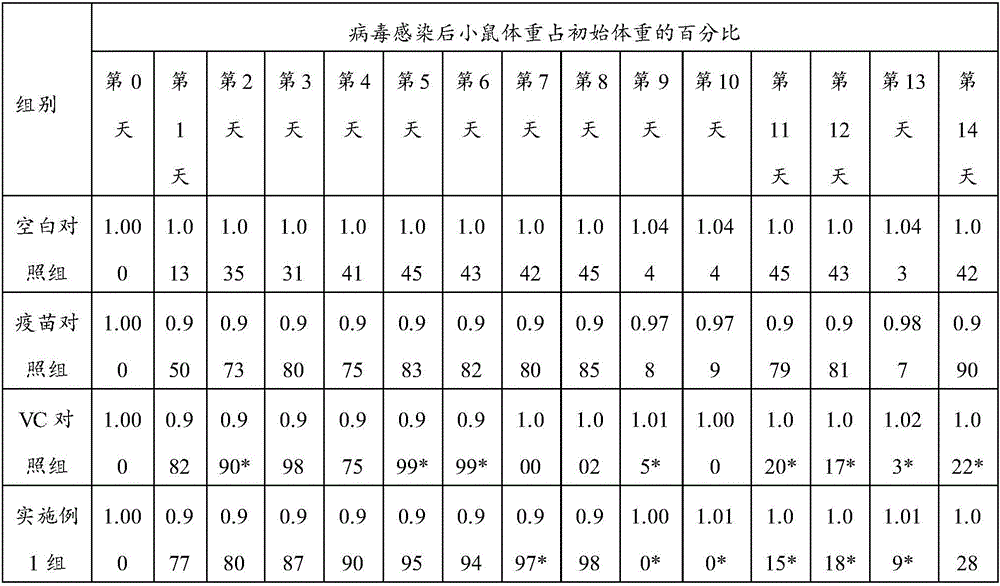
Patents
Literature
76 results about "H1n1 virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
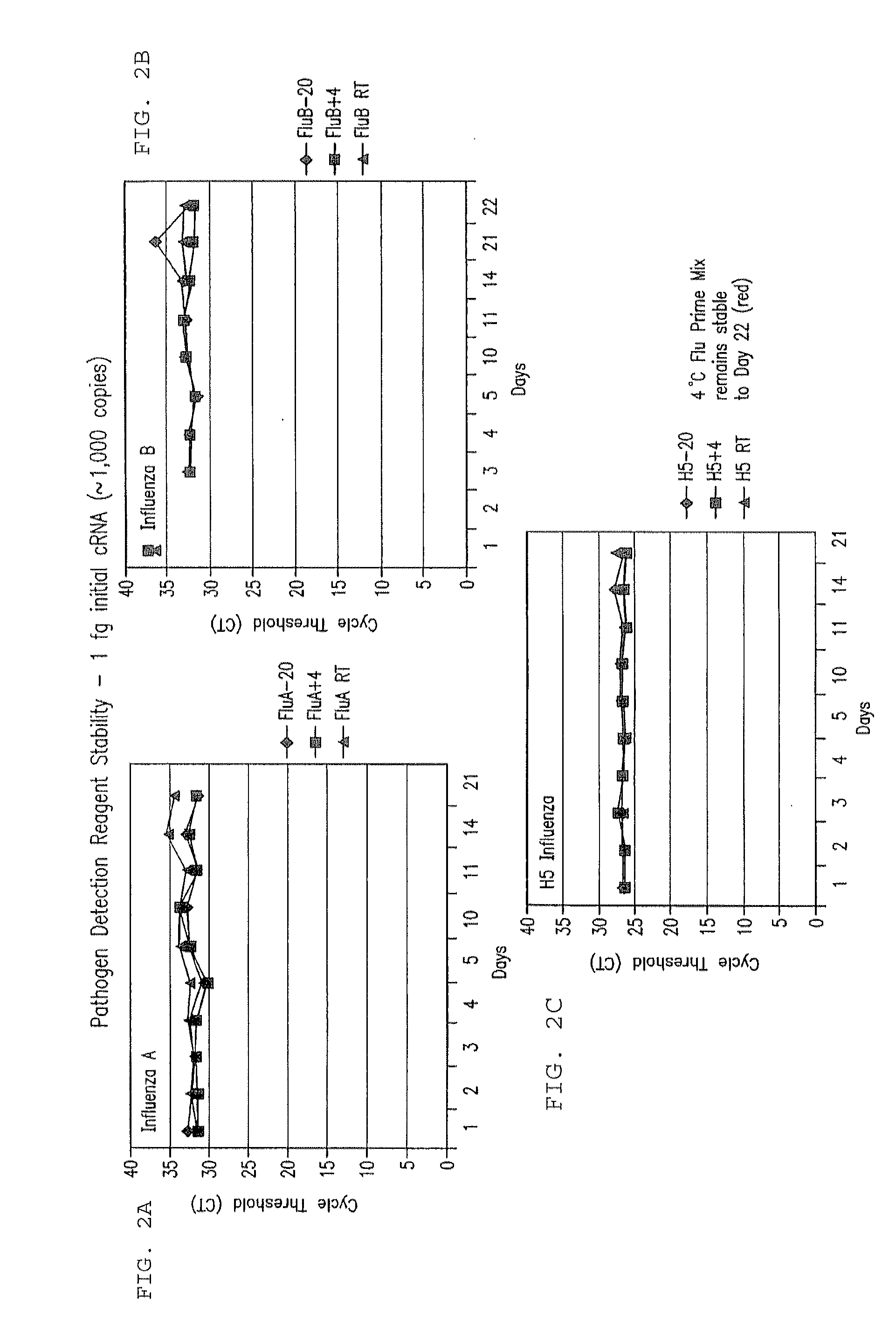
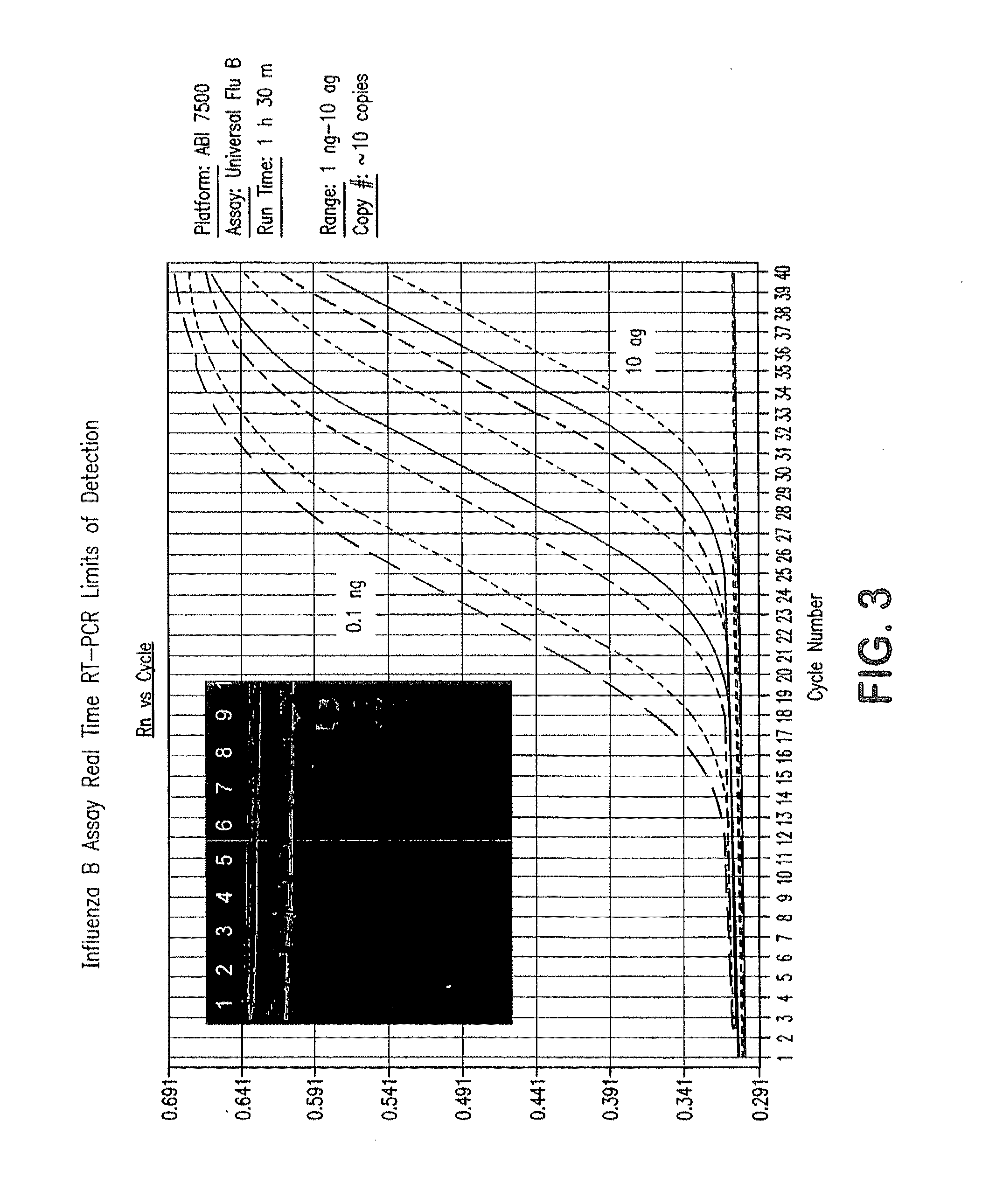
Compositions and method for rapid, real-time detection of influenza A virus (H1N1) swine 2009
ActiveUS8097419B2Rapid detection and identificationMinimize and eliminate contaminationBioreactor/fermenter combinationsBiological substance pretreatmentsH1n1 virusNucleic Acid Probes
Disclosed are oligonucleotide amplification primers and detection probes specific for the amplification and detection of pathogenic organisms, including for example, specific Influenza A H1N1 viral isolates. Also disclosed is a biological organism identification kit including the disclosed nucleic acid probes and primers, as well as thermal cycling reagents that is both portable and durable, and may also be self-contained for remote, or in-field analysis and identification of particular influenza isolates from a variety of biological specimen types.
Owner:LONGHORN VACCINES & DIAGNOSTICS LLC
Compositions and Methods for Rapid, Real-Time Detection of Influenza A Virus (H1N1) Swine 2009
ActiveUS20120115126A1Rapid detection and identificationMinimize and eliminate contaminationBioreactor/fermenter combinationsBiological substance pretreatmentsH1n1 virusNucleic Acid Probes
Disclosed are oligonucleotide amplification primers and detection probes specific for the amplification and detection of pathogenic organisms, including for example, specific Influenza A H1N1 viral isolates. Also disclosed is a biological organism identification kit including the disclosed nucleic acid probes and primers, as well as thermal cycling reagents that is both portable and durable, and may also be self-contained for remote, or in-field analysis and identification of particular influenza isolates from a variety of biological specimen types.
Owner:LONGHORN VACCINES & DIAGNOSTICS LLC
Computationally optimized broadly reactive antigens for h1n1 influenza
Described herein is the generation of optimized H1N1 influenza HA polypeptides for eliciting a broadly reactive immune response to H1N1 influenza virus isolates. The optimized HA polypeptides were developed through a series of HA protein alignments, and subsequent generation of consensus sequences, based on selected H1N1 viruses isolated from 1918-2012. Provided herein are optimized H1N1 HA polypeptides, and compositions, fusion proteins and VLPs comprising the HA polypeptides. Further provided are codon-optimized nucleic acid sequences encoding the HA polypeptides. Methods of eliciting an immune response against influenza virus in a subject are also provided by the present disclosure.
Owner:UNIVERSITY OF PITTSBURGH
Novel Kit for fluorescence quantitative PCR detection of influenza A H1N1 viruses and detecting method therefor
InactiveCN101665842AAccurate detectionHigh detection specificityMicrobiological testing/measurementMicroorganism based processesH1n1 virusInfluenza A (H1N1) virus
The invention discloses a novel kit for fluorescence quantitative PCR detection of influenza A H1N1 viruses, containing a primer, a probe, Taqman 2*universal PRC master mix, 40*multi scribe<TM> and RNase inhibitor mix and nuclease-free water. The primer and the probe have the advantages of good detecting specificity and high sensitivity so that the kit is particularly suitable for the influenza AH1N1 which breaks out at present and has no cross reaction with the Avian flu, swine flu and common seasonal flu and has lower cost.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Monoclonal antibodies to influenza h1n1 virus uses thereof
InactiveUS20120100142A1Prevention and reduction of severitySugar derivativesMicrobiological testing/measurementH1n1 virusMonoclonal antibody
The present invention is directed to particular monoclonal antibodies and fragments thereof that find use in the detection, prevention and treatment of influenza virus infections. In particular, these antibodies may neutralize or limit the replication of H1N1 influenza virus. Also disclosed are improved methods for producing such monoclonal antibodies.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Application of poly mannuronic acid propyl ester sulfate in preparing anti- H1N1 influenza A virus medication
InactiveCN102743409AAvoid infectionPrevent proliferationOrganic active ingredientsAntiviralsCanine kidneyPolymannuronic acid
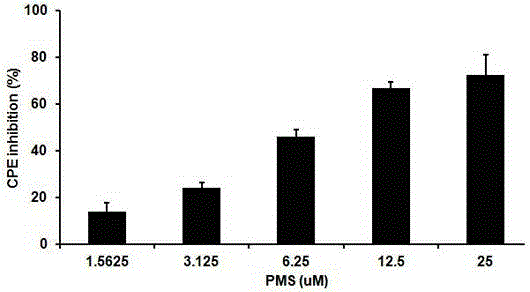
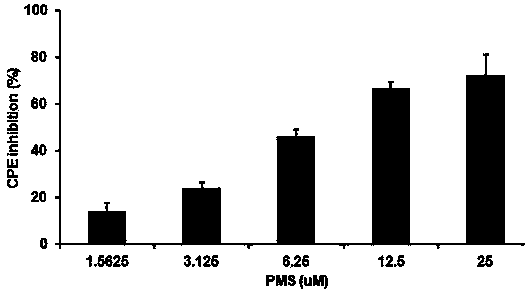
The invention provides applications of poly mannuronic acid propyl ester sulfate (PMS) in preparing anti- H1N1 influenza A virus medication. Experiments of the invention prove that PMS not only has great inhibition effect on the neuraminidase of the influenza A virus, but also has relatively good protection effect on canine kidney epithelial cells infected with the H1N1 influenza A virus, and can reduce the replication of the H1N1 virus with an effect that equal to the positive control medication ribavirin. Additionally, the PMS can effectively reduce the death rate of mice infected with the H1N1 influenza A virus and the survival time of the mice are prolonged. The poly mannuronic acid propyl ester sulfate provided by the invention has significant activity of inhibiting the neuraminidase of the H1N1 influenza A virus, and is proved to have good anti- H1N1 influenza A virus activity both in vivo and in vitro experiments, which shows the wide application prospect of the poly mannuronic acid propyl ester sulfate in preparing anti-H1N1 influenza A virus medication.
Owner:OCEAN UNIV OF CHINA
H1n1 flu virus neutralizing antibodies
ActiveCN107922481ABiological material analysisImmunoglobulins against virusesH1n1 virusComplementarity determining region
An antibody, or a binding fragment of the antibody, against H1N1 virus, includes a heavy chain variable region and a light chain variable region, wherein the heavy chain variable region contains complementarity determining regions (CDR) that have the amino acid sequences of SEQ ID NO: 5, SEQ ID NO: 6, and SEQ ID NO: 7; and wherein the light chain variable region contains complementarity determining regions that have the amino acid sequences of SEQ ID NO: 8, SEQ ID NO: 9, and SEQ ID NO: 10. A method for treating or preventing H1N1 infection in a subject includes administering to the subject theantibody or the binding fragment of the antibody.
Owner:MEDIGEN BIOTECH
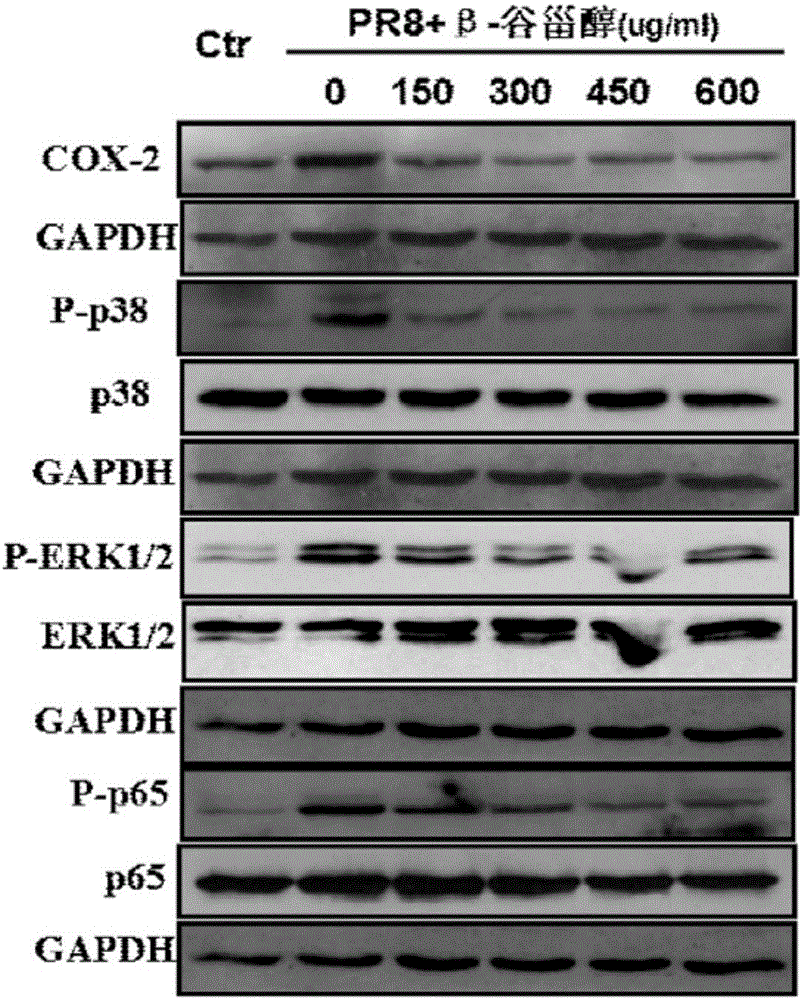
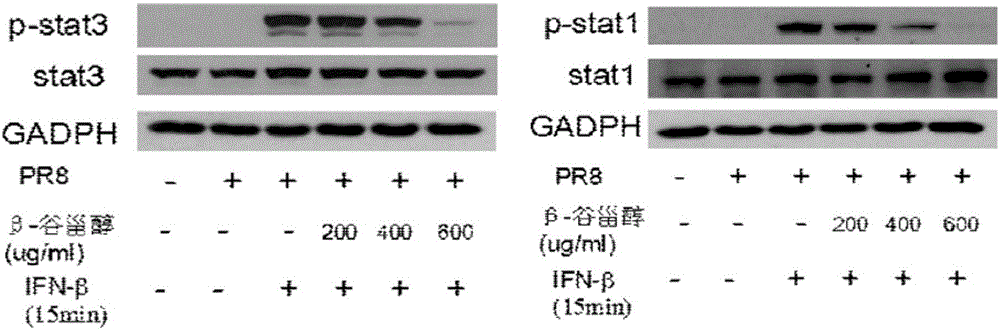
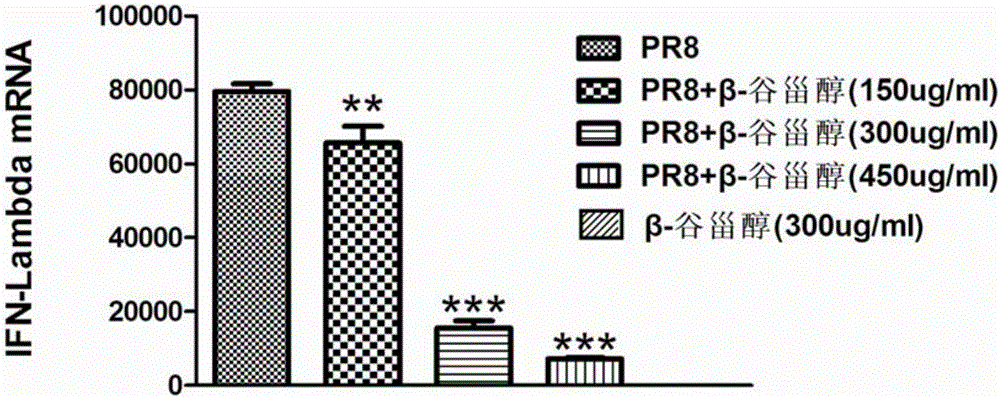
Application of beta-sitosterol to preparation of medicine for treating or preventing influenza A
ActiveCN105769877ANo obvious side effectsClear structureOrganic active ingredientsAntiviralsBiological activationCytokine
The invention provides a new use of beta-sitosterol in preparation of a medicine for treating or preventing influenza A, and further provides the medicine for treating or preventing the influenza A. The medicine comprises an active component of the beta-sitosterol. The inventor of the application finds that the beta-sitosterol has a significant inhibitory activity for an inflammatory reaction mediated by influenza A virus. The fluorescence real-time quantitative polymerase chain reaction (PCR) is used for confirming that the beta-sitosterol can obviously inhibit abnormal expressions of A549 inflammatory cytokines infected by an H1N1 virus and show a dose-dependent relationship; the Western blotting is adopted to confirm that the beta-sitosterol can inhibit activation with host inflammation related signaling pathways and show the dose-dependent relationship. Animal experiments show that the beta-sitosterol can significantly inhibit lung injuries and inflammations induced by influenza virus A (H1N1).
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT) +3
Constant-temperature amplification detection kit of A(H1N1) virus and detection method thereof
InactiveCN101864494AComparableGood repeatabilityMicrobiological testing/measurementSingle sampleH1n1 virus
The invention relates to a constant-temperature amplification detection kit of an A(H1N1) virus and a detection method thereof. The kit comprises an RNA (Ribonucleic Acid) extracting solution, an A(H1N1) virus nucleic acid constant-temperature amplification reaction solution, A(H1N1) virus positive control and A(H1N1) virus negative control. The detection kit has high specificity, high sensitivity and high reaction speed, i.e. only two hours are needed from sample processing to detection finishing of a single sample; the sample detection of high flux and low flux can be simultaneously satisfied, and complicated instruments are not needed in the whole reaction process.
Owner:上海国际旅行卫生保健中心 +1
Kit and special primer for detecting H1N1 influenza A virus and target sequence
InactiveCN101591715AIdentification method is simpleShort detection timeMicrobiological testing/measurementMicroorganism based processesH1n1 virusElectrophoresis
The invention discloses a special primer for detecting H1N1 influenza A virus, a kit comprising the primer for detecting H1N1 influenza A virus and a target sequence corresponding to the primer, and belongs to the field of quick diagnosis of pathogen genes. The base sequence of the target sequence corresponding to the special primer is shown as SEQ ID No.1; and the kit utilizes the technical principles of genetic reverse transcription and isothermal amplification to quickly detect H1N1 influenza A virus nucleic acid, and the detection result can be judged by naked eyes and also can be analyzed by using electrophoresis. The kit has the characteristics of quickness, sensitivity, specificity and convenient operation.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Establishment of method for preparing recombinant protein vaccine of type A H1N1 influenza virus
InactiveCN101716340AEngineering antibody technology is simpleLow costAntiviralsAntibody medical ingredientsEscherichia coliAntigenic analysis
The invention belongs to the field of zoonosis researches, and relates to a method for preparing a vaccine of zoonosis. In the method, an HA gene in a cDNA sequence of a type A H1N1 California virus strain (A / California / 08 / 2009(H1N1)) is selected as a research content; the method comprises the following steps of: firstly, analyzing a sequence of the part of an HA protein leaked outside an envelope according to structural biology software; secondly, selecting a part with high immunogenicity according to an antigenic analysis, and constructing a prokaryotic expression vector pGEX-6P-1-HA by using a genetic fragment obtained through a gene synthesis method; and thirdly, converting a masculine recombinant plasmid into Escherichia coli to obtain a recombinant strain (Escherichia coli BL21 rosseta / pGEX-6P-1-HA truncated protein). Through a plurality of chromatography methods, a purified HA truncated protein of the H1N1 is obtained; and by utilizing the expression vector and an adjuvant together to immune organisms, the protective effect on the H1N1 virus is achieved.
Owner:CUSABIO TECH LLC
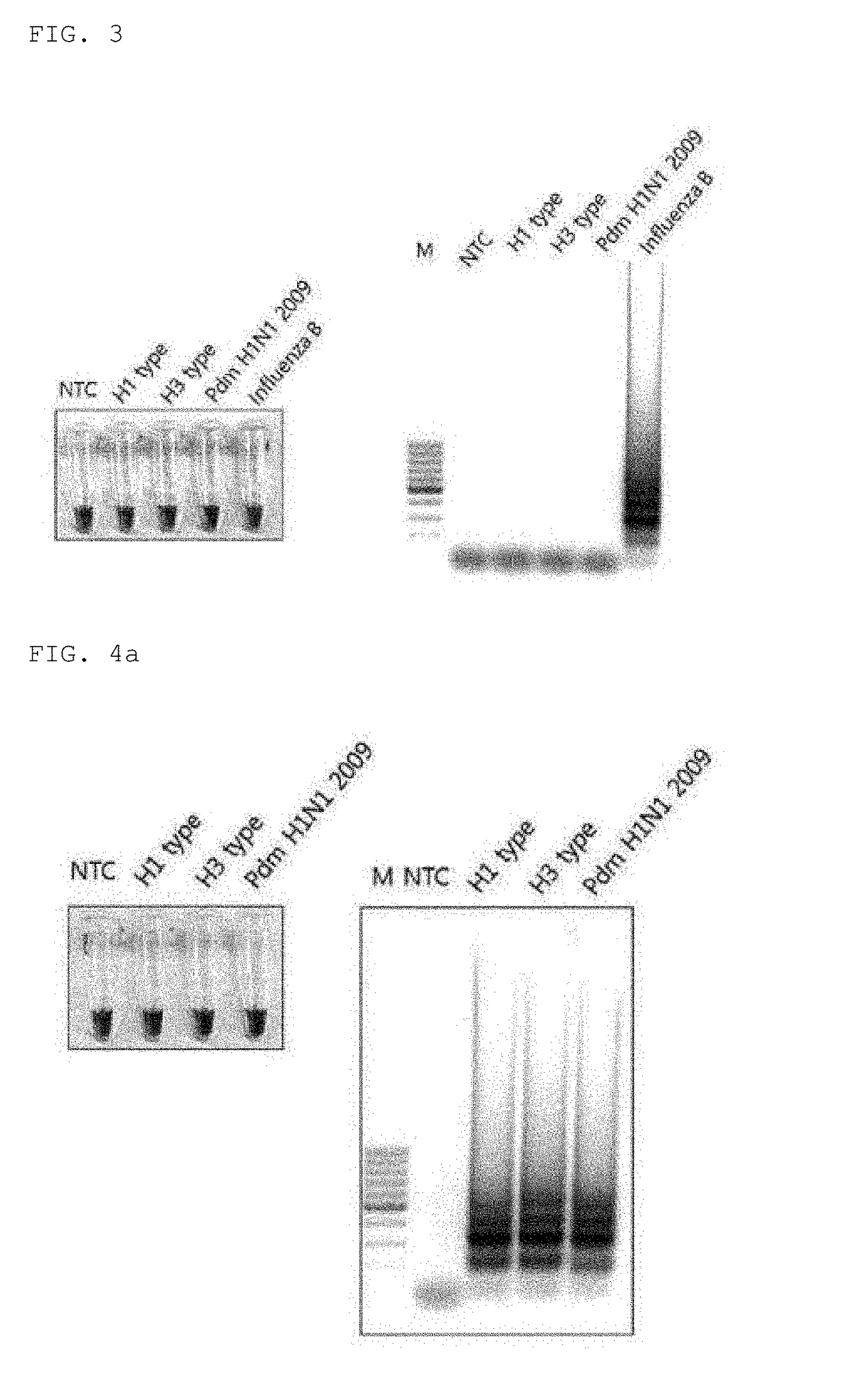
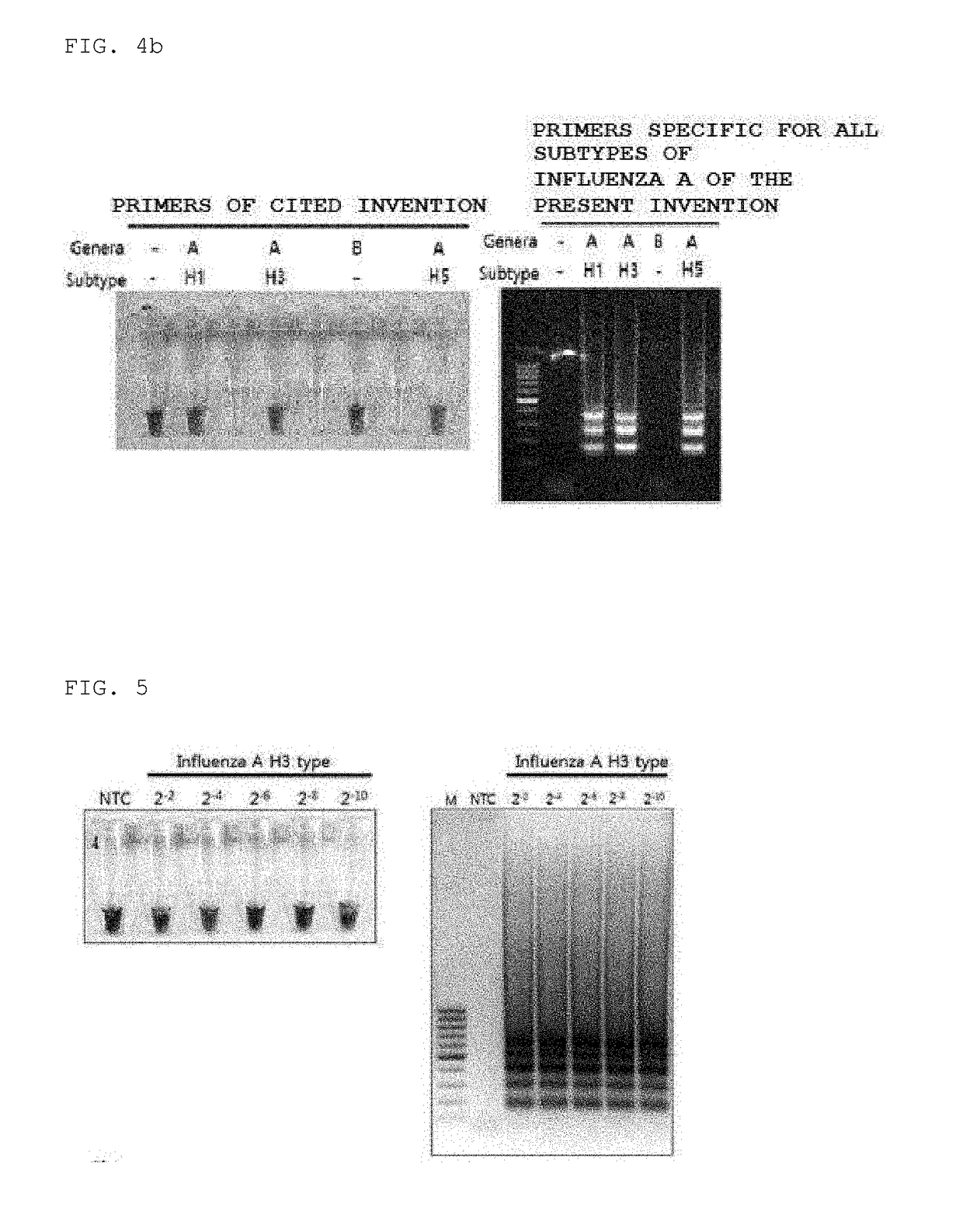
Primers for detecting influenza by using lamp, and use thereof
ActiveUS20190002994A1High sensitivityStrong specificityMicrobiological testing/measurementH1n1 virusSpecific detection
Disclosed are: a primer set enabling the specific detection, by an isothermal amplification method, of an influenza A virus, an influenza A subtype H3 virus, an influenza A subtype pdm H1N1 virus, and an influenza B virus; a composition or a kit comprising the same; and a method for detecting influenza viruses by using the same. The primers and the method, according to the present application, can detect, in a rapid manner and with high sensitivity and specificity, whether influenza virus infection occurs, and enable detection without separate treatments after the completion of the amplification, thereby improving convenience.
Owner:MMONITOR INC
Swine influenza A H1N1 virus and use thereof
The invention belongs to the field of microbial virology and provides a swine influenza A H1N1 virus. The virus contains eight fragments, namely HA, NA, NP, PB1, PB2, PA, NS and M, wherein the nucleotide sequences of the eight fragments are shown by the sequences from No.1 to No.8 in a nucleotide sequence table in the description in turn. In the invention, by separate culture of a nasopharyngeal swab sample of a healthy pig, differential item functioning (DIF) and reverse transcription-polymerase chain reaction (RT-PCR) identification and the comparison of full sequences of 8 genes of the virus, a virus is obtained and verified to be a swine influenza A H1N1 virus strain. The swine influenza A H1N1 virus strain is named A / swine / Zhejiang / 26 / 2009(H1N1), with a collection number of CCTCC No.V201016. The strain obtained by the invention supplies valuable genetic information of swine influenza A virus in China, has a great significance for completing a system for monitoring the infection caused by the pathogen and can be used for preparing a reagent for quickly diagnosing infection with the influenza A H1N1 virus.
Owner:FUDAN UNIV
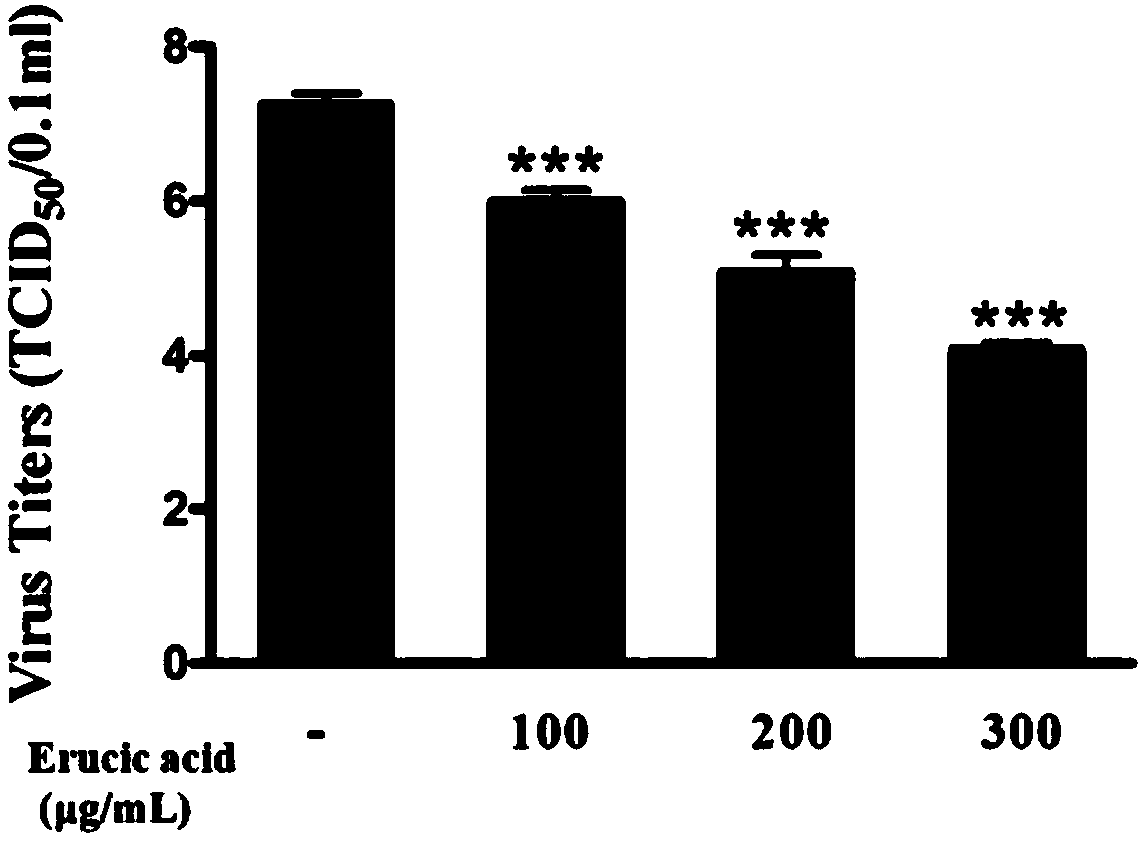
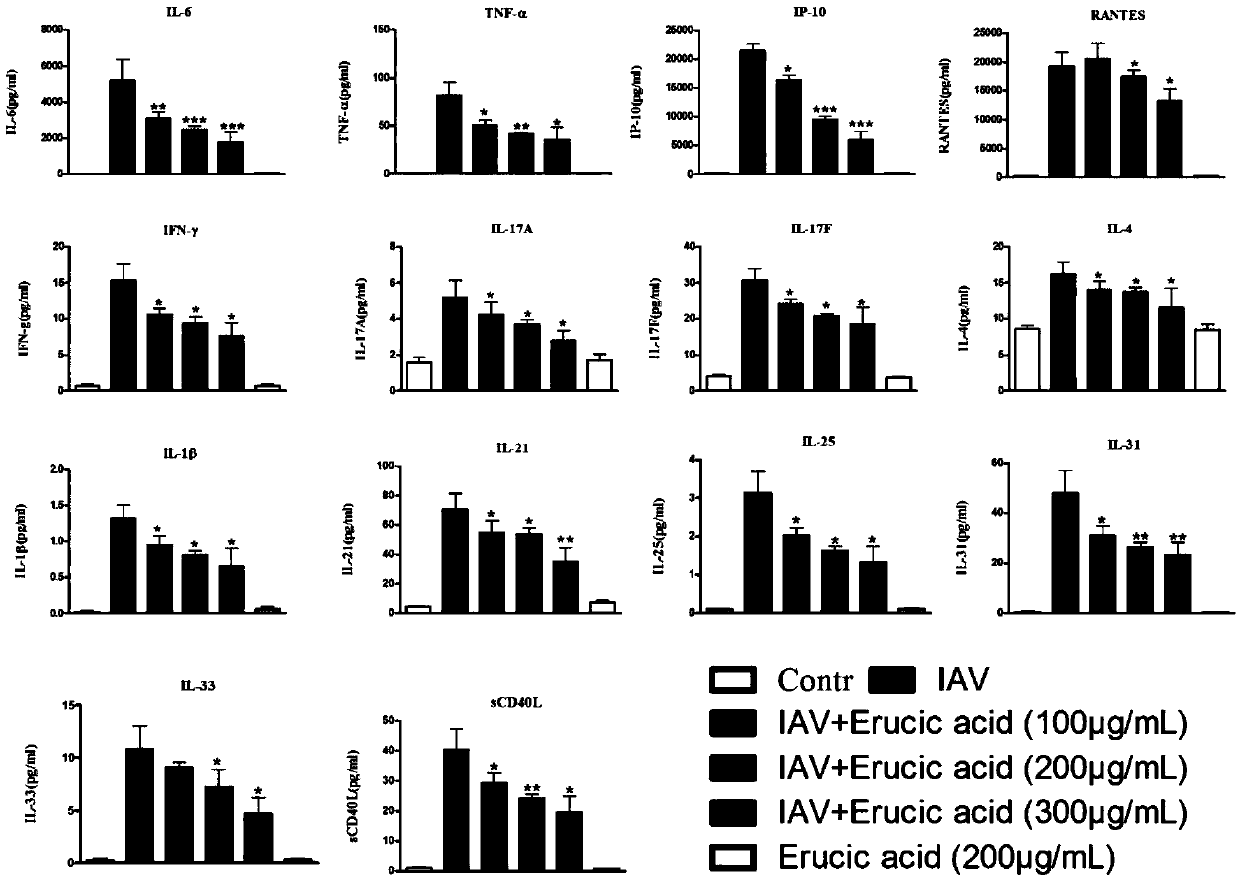
Application of erucic acid in preparing medicine for preventing or treating vaccinum influenzae vivum
ActiveCN108014101ANo obvious side effectsClear structureOrganic active ingredientsAntiviralsInflammatory factorsBiological activation
The invention provides new application of erucic acid in preparing a medicine for preventing or treating vaccinum influenzae vivum. The invention also provides the medicine for preventing or treatingvaccinum influenzae vivum. The medicine is prepared from the active ingredient erucic acid. The inventor finds that the erucic acid has remarkable inhibitory activity on vaccinum influenzae vivum replicated and mediated inflammatory reaction. A liquid chip technology is adopted to prove that the erucic acid can remarkably inhibit the abnormal expression of an H1N1 virus infected A549 cell inflammatory factor and is in a dose-dependent relationship; an immunoblotting method is adopted to prove that the erucic acid can inhibit activation of a host inflammation-associated signal path and is in adose-dependent relationship; Annexin V-FITC / PI double-tagging flow cytometry is adopted to prove that the erucic acid can remarkably inhibit apoptotic lesion of a flue H1N1 induced host cell and is ina dose-dependent relationship. Animal experiments prove that the erucic acid can remarkably inhibit lung inflammation and apoptotic lesion induced by vaccinum influenzae vivum H1N1, and the survivaltime of rats is effectively prolonged.
Owner:GUANGZHOU INST OF RESPIRATORY DISEASE +2
Application of influenza treating medicine composition in preparation of antiviral medicine
InactiveCN105343481AInhibition of reproductionNo inhibitionGranular deliveryRespiratory disorderFreeze-dryingSemen
The invention discloses application of an influenza treating medicine composition in preparation of antiviral medicine. The medicine composition is prepared from, by weight, 1-9 parts of ephedra, 1-9 parts of mint, 1-9 parts of periostracum cicada, 1-20 parts of honeysuckles, 2-20 parts of scutellaria baicalensis, 2-18 parts of semen armeniacae amarae, 1-9 parts of thunberg fritillary bulb, 1-9 parts of platycodon grandiflorum and 1-9 parts of liquorice. The raw materials are taken, conventional auxiliary materials are added, and clinically-acceptable tablets, granules, pills, capsules, dropping pills, soft capsules, slow-release preparations, oral liquid preparations or freeze-dried powder injections are prepared according to the conventional process; new application is developed, and new medicine is provided for treating infection of viruses RSV, EV71, COX-B3 and H1N1.
Owner:SHANGHAI PHARMA GRP QINGDAO GROWFUL PHARMA CO LTD
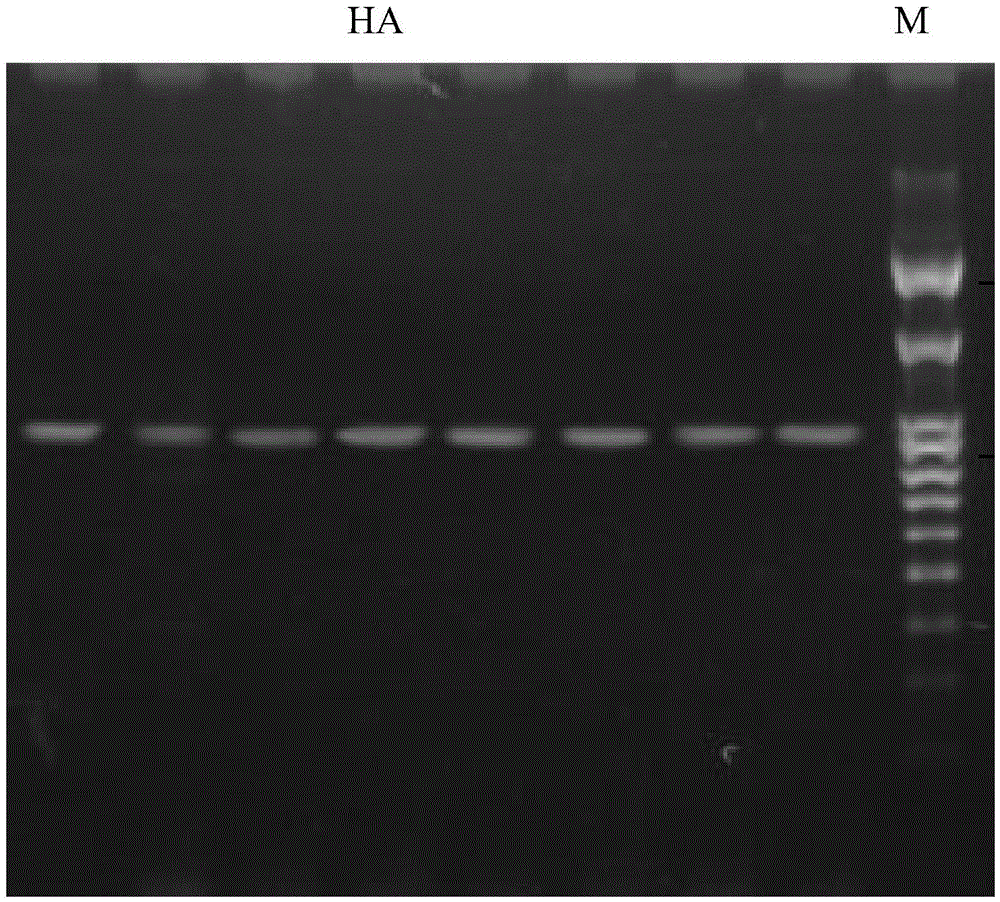
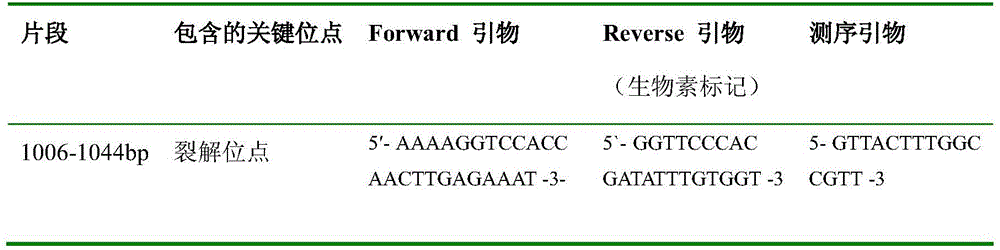
Method for detecting pathogenicity of influenza A (H1N1) virus based on pyrosequencing
The invention relates to a method for detecting the pathogenicity of an influenza A (H1N1) virus based on pyrosequencing. The method comprises the steps of carrying out RT-PCR (Reverse Transcription-Polymerase Chain Reaction) amplification on a hemagglutinin (HA) gene of an H1N1 virus; carrying out pyrosequencing on a PCR amplification product to judge whether the cleavage site of the HA gene of the influenza A (H1N1) virus has a mutation, wherein the pyrosequencing is carried out in an SQA (Sequence Analysis) mode, and a nucleotide sampling sequence is shown as AGCT. The mutation of the cleavage site of the HA gene of the influenza A (H1N1) virus can be detected at rather high accuracy through comparing a detected result with the sequence of the cleavage site of the HA gene of a standard strain of the influenza A / H1N1 virus. The invention provides a simple and rapid experimental scheme for determining the virulence, pathogenicity and host range of the virus, the complex experiment steps related to complete genome sequencing are omitted, the variation direction of the virus can also be accurately mastered, the virus is conveniently monitored, an infection source is isolated, a transmission route is cut off, and the further development of an epidemic situation is stopped.
Owner:山东国际旅行卫生保健中心
Sclerotiorin derivatives and their preparation methods and their application as anti-h1n1 influenza virus agents
Owner:OCEAN UNIV OF CHINA
Effective parts of willow herb and preparing method and application tehreof
InactiveCN106344631AInhibitory activityGood anti-HBV effectDigestive systemAntiviralsEthyl acetateSolvent
The invention discloses the effective parts of willow herb and the preparing method and application thereof. The invention belongs to the technical field of plant extract. The method comprises of the steps of selecting the mixture of one or two extracts from the ethyl acetate part and normal butyl alcohol part of willow herb at any weight ratio. Crushing the willow herb and adding 65%-95% (volume) ethanol solution for extraction, concentrating and drying the extracting solution to obtain the extract; adding distilled water for dispersion to obtain extract dispersion, then respectively using petroleum ether, ethyl acetate and normal butyl alcohol as the solvent to extract the extract dispersion, making the solvent volatilize to obtain the ethyl acetate part and normal butyl alcohol part of willow herb. Effective parts of willow herb can be used to prepare anti-HBV, anti-H1N1 A virus drugs, with good effect to resist hepatitis B and influenza A virus.
Owner:QINGHAI UNIV FOR NATITIES
Preparation method of antibacterial and antiviral environment-friendly interior wall modification coating and product of antibacterial and antiviral environment-friendly interior wall modification coating
InactiveCN113652166AHigh broad spectrumSolve the problem of black spots caused by oxidation and discolorationAntifouling/underwater paintsPaints with biocidesH1n1 virusLarge intestine
The invention relates to a preparation method of an antibacterial and antiviral environment-friendly interior wall modification coating and a product thereof. The preparation method comprises the steps of preparation of nano inorganic antibacterial and antiviral powder, preparation of pure water-based polyurethane acrylate nano narrow-band emulsion and preparation of pure water-based nano antibacterial and antiviral coating. The pure water-based nano-scale dendritic copolyester is used as a base material to prepare the zero-VOC pure water-based antibacterial and antiviral interior wall modification coating, and the pure water-based nano-antibacterial and antiviral coating has the advantages of high coating fullness, good film coating effect, excellent water resistance, air permeability, water impermeability, excellent mildew-proof effect, dust is not prone to falling on the wall facing, stains are not prone to being attached, cleaning is easy, lasting antibacterial and antiviral capacity is achieved, the antibacterial rate of large intestines, golden yellow and the like reaches 99%, and the H1N1 virus killing rate reaches up to 92.3%.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
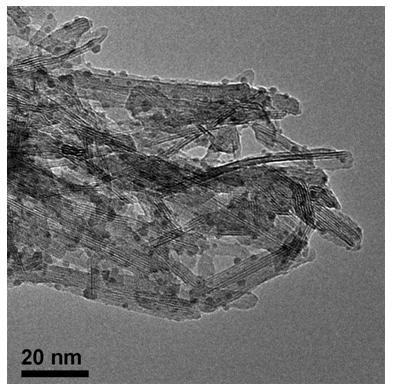
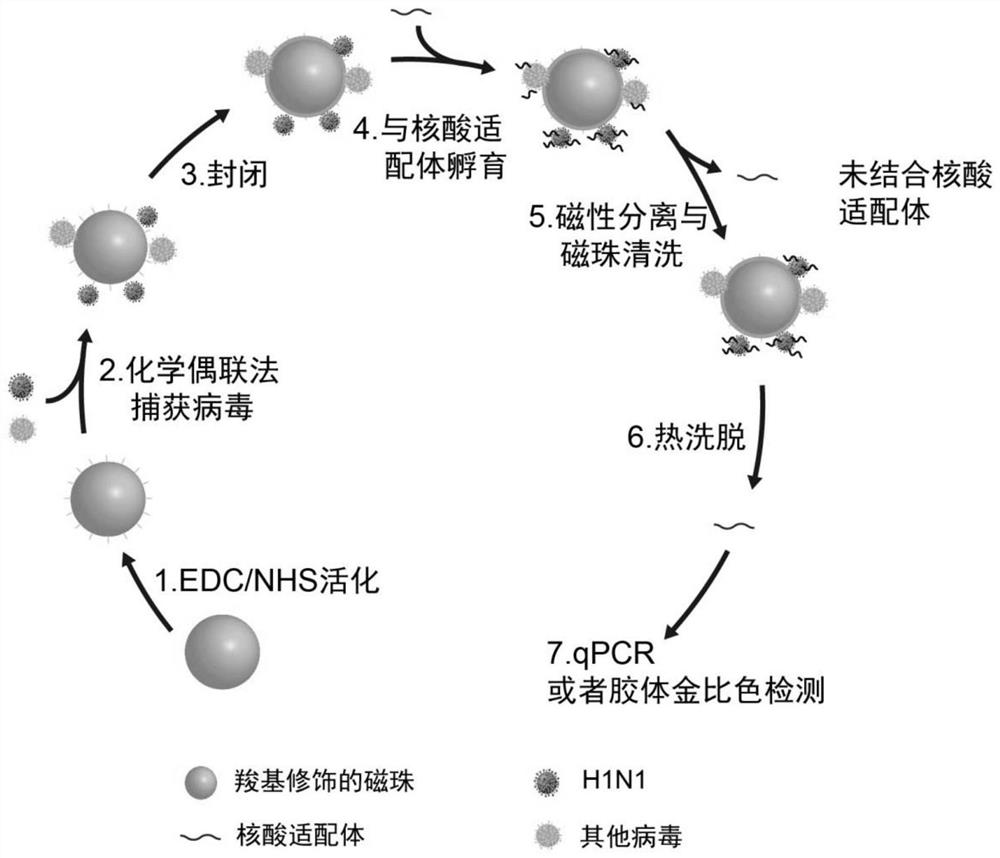
H1N1 influenza virus detection method and kit thereof
The invention relates to an H1N1 influenza virus detection method and a kit thereof. According to the invention, a simple and rapid chemical coupling method is utilized to realize efficient capture ofan inactivated complete H1N1 influenza virus; and a DNA aptamer capable of specifically recognizing the inactivated A-type H1N1 virus is used as a recognition probe to achieve specific recognition ofthe virus, and finally quantitative detection of the inactivated complete H1N1 influenza virus is achieved through real-time quantitative PCR. A detection limit is 1ng / L, and the kit has no responseto other viruses and has good specificity. Colorimetric detection of the H1N1 virus can be achieved by combining with nanogold, and the detection limit is 1 ng / L. According to the method, the completeinactivated virus serves as a target, the low-cost DNA aptamer serves as a recognition molecule, and advantages of good safety and low cost are possessed. Moreover, the method and the kit are high insensitivity and good in specificity, are successfully used for detecting the H1N1 in a throat swab sample, and have a very good clinical application value.
Owner:CAPITAL NORMAL UNIVERSITY
Application of poly mannuronic acid propyl ester sulfate in preparing anti- H1N1 influenza A virus medication
InactiveCN102743409BAvoid infectionPrevent proliferationOrganic active ingredientsAntiviralsCanine kidneyPolymannuronic acid
The invention provides applications of poly mannuronic acid propyl ester sulfate (PMS) in preparing anti- H1N1 influenza A virus medication. Experiments of the invention prove that PMS not only has great inhibition effect on the neuraminidase of the influenza A virus, but also has relatively good protection effect on canine kidney epithelial cells infected with the H1N1 influenza A virus, and can reduce the replication of the H1N1 virus with an effect that equal to the positive control medication ribavirin. Additionally, the PMS can effectively reduce the death rate of mice infected with the H1N1 influenza A virus and the survival time of the mice are prolonged. The poly mannuronic acid propyl ester sulfate provided by the invention has significant activity of inhibiting the neuraminidase of the H1N1 influenza A virus, and is proved to have good anti- H1N1 influenza A virus activity both in vivo and in vitro experiments, which shows the wide application prospect of the poly mannuronic acid propyl ester sulfate in preparing anti-H1N1 influenza A virus medication.
Owner:OCEAN UNIV OF CHINA
Traditional Chinese medicine combination for treating grippe and preparation method thereof
The invention discloses a traditional Chinese medicine combination for curing influenza and a preparation method thereof. The combination is prepared from rhizome paridis and hypericum perforatum or from the extract products of rhizome paridis and hypericum perforatum. The traditional Chinese medicine combination is a medicine for resisting H1N1 virus and H3N2 virus with high efficacy and low toxin, and has excellent effect in curing influenza.
Owner:张文芯
Application of Forsythoside A in preparation of anti-influenza A H1N1 virus medicine and preparation thereof
Belonging to the field of traditional Chinese medicines, the invention discloses application of Forsythoside A in preparation of an anti-influenza A H1N1 virus medicine and a preparation thereof. In vivo pharmacodynamic experimental study on the Forsythoside A provided in the invention in treatment of respiratory virus infection and in vitro anti-respiratory virus pharmacodynamic experimental study on the Forsythoside A show that the Forsythoside A has an obvious inhibiting effect on influenza A H1N1 viruses.
Owner:LUNAN PHARMA GROUP CORPORATION
Application of myricetin in preparing medicine for preventing or treating coronavirus and influenza virus
PendingCN112546038AReduce inflammation in the bodyHigh protein affinityCosmetic preparationsOrganic active ingredientsH1n1 virusReceptor
The invention belongs to the technical field of medicines, and discloses an application of myricetin in preparation of medicines for preventing or treating coronavirus and influenza virus, and administration dosage of myricetin or pharmaceutically acceptable salt thereof is 0.1-1000 mg / kg. The myricetin has high protein affinity to a target point of interaction of SARS-CoV-2, an RBD protein of a Spike protein binding domain of the SARS-CoV-2 and a receptor protein ACE2. In addition, the myricetin can also inhibit activity of SARS-CoV-2, HCoV-229E and H1N1 viruses, and can relieve in-vivo inflammatory reaction of an inflammatory animal model. According to the invention, it is determined for the first time that myricetin has an inhibition effect on SARS-CoV-2 and an action target thereof, and no toxic reaction occurs.
Owner:MACAU UNIV OF SCI & TECH
Pediatric Chinese medicinal oral liquid for preventing and treating H1N1 virus and preparation method thereof
InactiveCN101711831AWith asthmaAntitussivePharmaceutical delivery mechanismAntiviralsSide effectAllergy
The invention discloses a pediatric Chinese medicinal oral liquid for preventing and treating H1N1 virus and a preparation method thereof, relates to a pediatric medicament for preventing and treating the H1N1 virus and a preparation method thereof, and solves the problem that the conventional medicaments for preventing and treating the H1N1 virus are not used for children and have poor treatment effect and side effect. The pediatric Chinese medicinal oral liquid of the invention is prepared by adding water into ephedra herb, bitter apricot seed, gypsum, licorice root, honeysuckle flower, weeping forsythiae capsule, common anemarrhena rhizome, baikal skullcap root, indigowoad root, dwarf lilyturf turber, heartleaf houttuynia herb, preservative, and flavoring agent. The preparation method comprises the following steps: (1) preparing mixed decoction solution; (2) preparing mixed solution A; and (3) filtering the mixed solution A, concentrating the filtrate and adding the water into dilute the filtrate, stirring the mixture, performing cold storage treatment on the obtained product, then refiltering the obtained product, adding the preservative and the flavoring agent into the filtrate, and then adding the water, stirring the product and making volume constant to obtain the pediatric Chinese medicinal oral liquid for preventing and treating the H1N1 virus. The Chinese medicinal oral liquid of the invention has the effects of allaying asthma and relieving cough, relieving fever, eliminating phlegm, resisting allergy and resisting the H1N1 flu; and the product of the invention is safe and has no side or toxic effect.
Owner:HEILONGJIANG KUIHUA PHARMA
Wormwood pill containing 95% of wormwood ethyl alcohol extract that has inactivation efficacy of the h1n1 virus and the h9n2 avian influenza virus
A mugwort pill containing a 95% ethanol extract of wormwood having an inactivation power with respect to a novel swine-origin influenza virus and an avian influenza virus is disclosed. In more details, the conventional mugwort pill is prepared by a hot water extraction method and has 0.8% of crude fat component; however the ethanol extract mugwort crude fat of the present invention is 11.6%, in particular the crude fat component is 14.5 times higher, and the novel swine-origin influenza virus (H1N1) can be inactivated 99.99%, and it has a good disinfection effect to an avian influenza virus (H9N2). When a mugwort pill containing a 95% ethanol extract of wormwood having an inactivation power with respect to a novel swine-origin influenza virus and an avian influenza virus is administrated to people or fowls, the people or fowls can have immunity with respect to a novel swine-origin influenza virus and an avian influenza virus.
Owner:JO BONGKWAN +1
Fluorescent RT-RAA primer for detecting A(H1N1) virus, and probe and detection method
InactiveCN107574262AShorten detection timeHigh sensitivityMicrobiological testing/measurementMicroorganism based processesNucleic acid amplification techniqueH1n1 virus
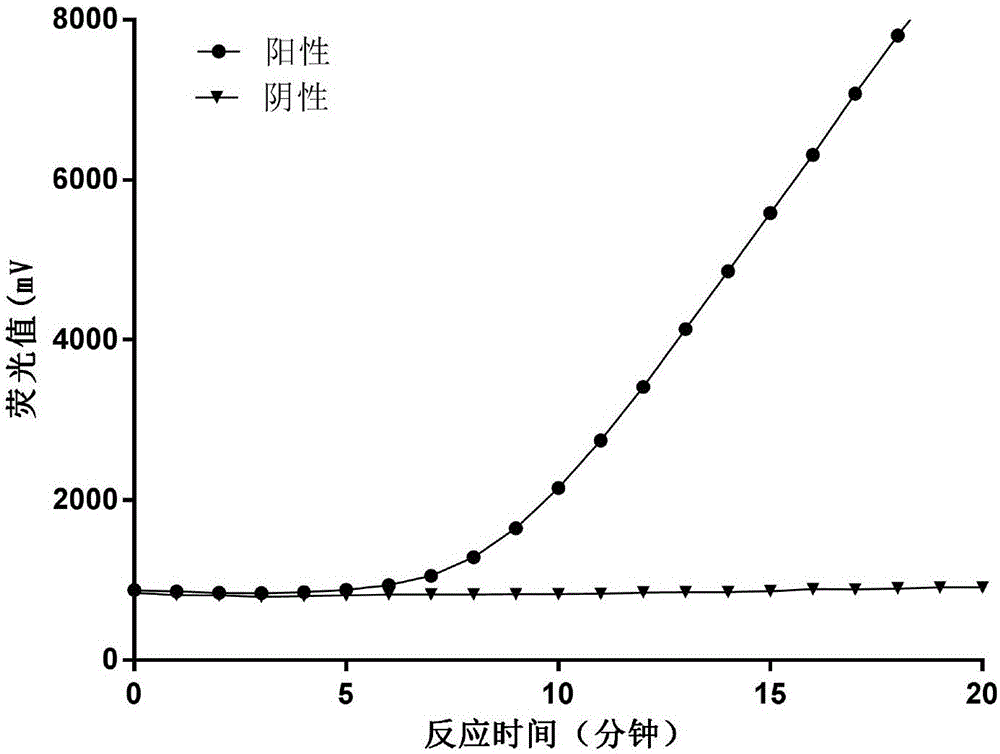
The invention provides a fluorescent RT-RAA primer for detecting an A(H1N1) virus by using a reverse transcription recombinase mediated nucleic acid amplification technique, a fluorescent probe, and an application of the fluorescent RT-RAA primer and the fluorescent probe in detecting the A(H1N1) influenza virus. By adopting the fluorescent RT-RAA primer and the fluorescent probe, the A(H1N1) influenza virus in a throat swab sample can be rapidly and quantitatively detected, detection can be implemented at normal temperature of 39 DEG C, diagnosis results can be obtained within 20 minutes, andthe detection time is greatly shortened; the fluorescent RT-RAA primer and the fluorescent probe are respectively good in specificity and relatively high in sensitivity, rapid diagnosis and whole monitoring on epidemic situations can be met, and time for diagnosing and treating epidemic situations at an early stage, reducing the case fatality rate and controlling the epidemic situations is achieved.
Owner:宁波国际旅行卫生保健中心
Amplifying system and method for detecting H1N1 virus RNA
InactiveCN107523651AEasy to collectSimple methodMicrobiological testing/measurementDNA/RNA fragmentationReverse transcriptaseFluorescence
The invention relates to the field of molecular biological detection and discloses an amplifying system and method for detecting H1N1 virus RNA. The amplifying system comprises Tris buffer liquid, magnesium acetate, potassium chloride, dithiothreitol, polyethylene glycol, ATP, dNTPs, phosphocreatine, recombinase, singe-strand binding protein, UvsY protein, exonuclease, fluorescent probe, DNA polymerase, revertase, RNA enzyme inhibitor and H1N1 virus specificity primer. The method includes: mixing the amplifying system with H1N1 virus RNA, performing equal-temperature amplifying, and using a fluorescent probe to detect an amplifying product in real time. The method is simple to operate, instruments are minimized and convenient to carry, and the amplifying system is short in amplifying time, high in specificity and sensitivity and suitable for basic-level and on-site detection.
Owner:JIANGSU QITIAN GENE BIOTECHNOLOGY CO LTD +1
A method for detecting the pathogenicity of influenza A h1n1 virus based on pyrosequencing
The invention relates to a method for detecting the pathogenicity of an influenza A (H1N1) virus based on pyrosequencing. The method comprises the steps of carrying out RT-PCR (Reverse Transcription-Polymerase Chain Reaction) amplification on a hemagglutinin (HA) gene of an H1N1 virus; carrying out pyrosequencing on a PCR amplification product to judge whether the cleavage site of the HA gene of the influenza A (H1N1) virus has a mutation, wherein the pyrosequencing is carried out in an SQA (Sequence Analysis) mode, and a nucleotide sampling sequence is shown as AGCT. The mutation of the cleavage site of the HA gene of the influenza A (H1N1) virus can be detected at rather high accuracy through comparing a detected result with the sequence of the cleavage site of the HA gene of a standard strain of the influenza A / H1N1 virus. The invention provides a simple and rapid experimental scheme for determining the virulence, pathogenicity and host range of the virus, the complex experiment steps related to complete genome sequencing are omitted, the variation direction of the virus can also be accurately mastered, the virus is conveniently monitored, an infection source is isolated, a transmission route is cut off, and the further development of an epidemic situation is stopped.
Owner:山东国际旅行卫生保健中心
Traditional Chinese medicine composition and application thereof
InactiveCN106421722AHigh activityImprove the quality of lifeMetabolism disorderAntiviralsH1n1 virusTangerine Peel
The invention discloses a prescription, which contains aqueous extracts of shiitake mushrooms, poria cocos, zingiber officinale and dried tangerine peels and plays a role in resisting H1N1 viruses, and application thereof. The prescription is mainly made up of the shiitake mushrooms, the poria cocos, the zingiber officinale and the dried tangerine peels. Proven by antiviral tests, the prescription disclosed by the invention can be used for remarkably improving the immunologic function of H1N1 virus infected mice and improving the pneumonia pathological change degree of the H1N1 virus infected mice and can be used for remarkably improving the specific antibody level in blood serum of the H1N1 virus infected mice, remarkably lowering the content of viruses in lungs of the H1N1 virus infected mice and improving the level of lung cell factors IL-2, so that the prescription has a good H1N1 influenza virus resisting action.
Owner:INFINITUS (CHINA) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com