H1n1 flu virus neutralizing antibodies
An influenza virus and antibody technology, applied in antiviral agents, antiviral immunoglobulins, antibodies, etc., can solve problems such as poor results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
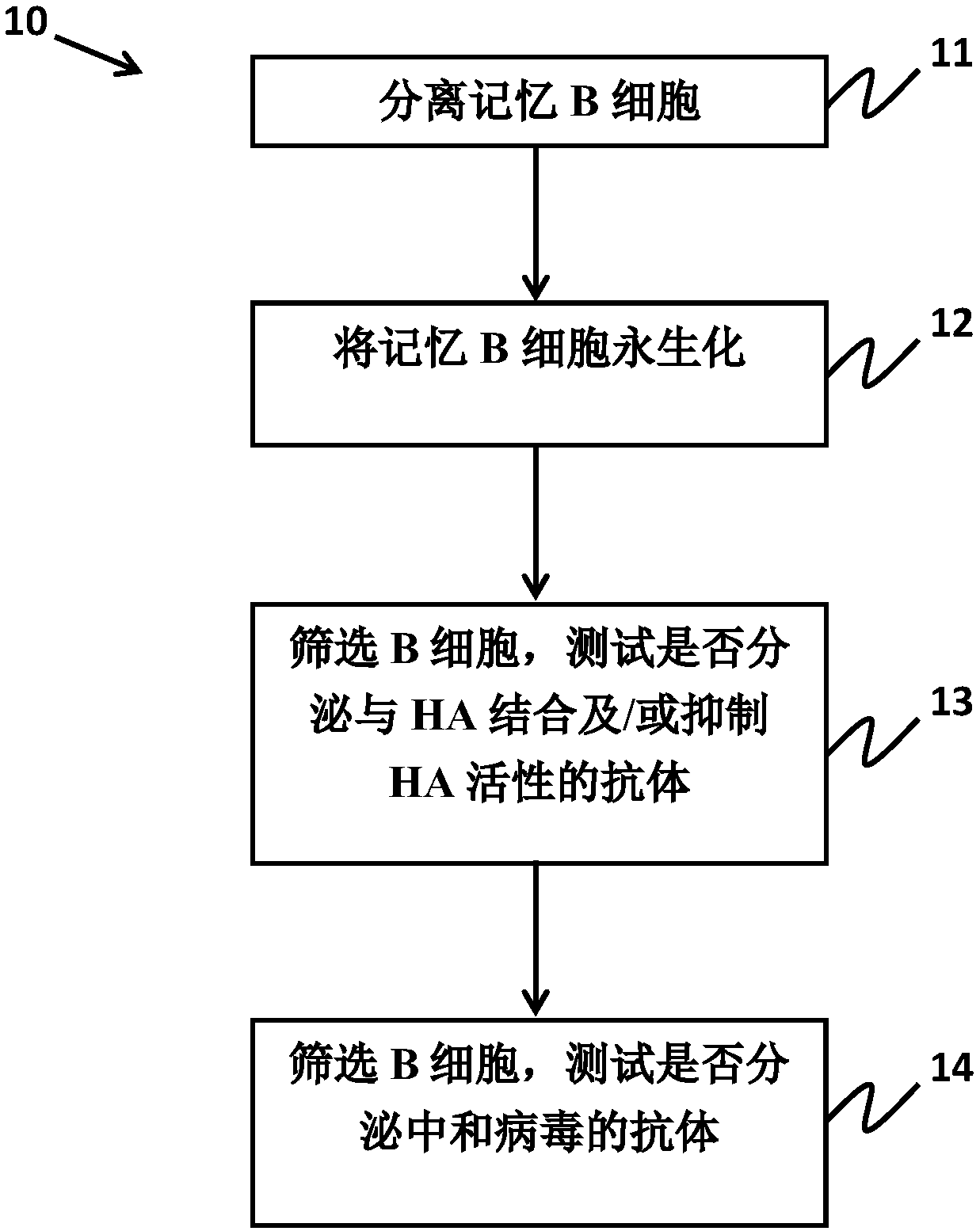
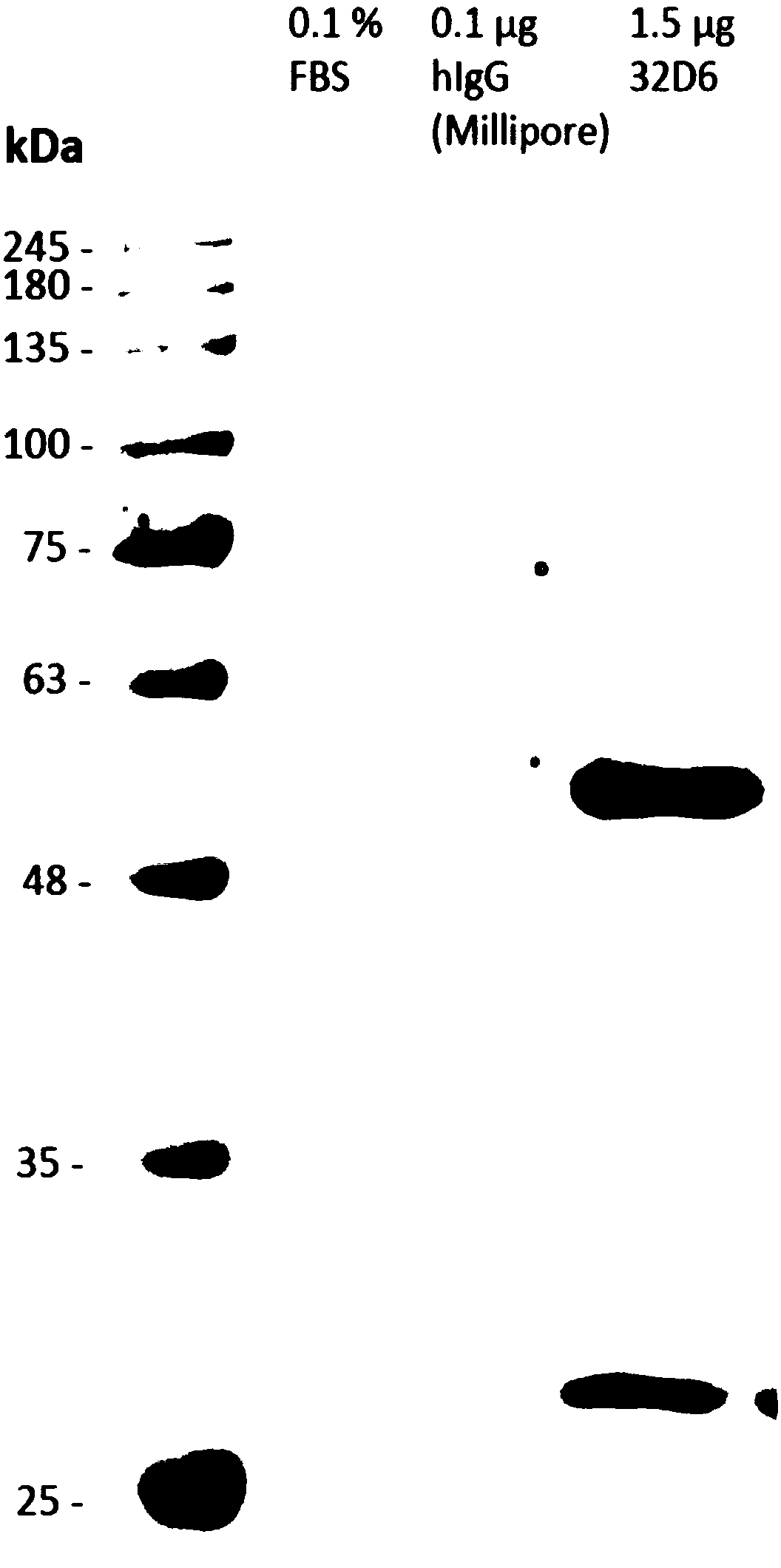
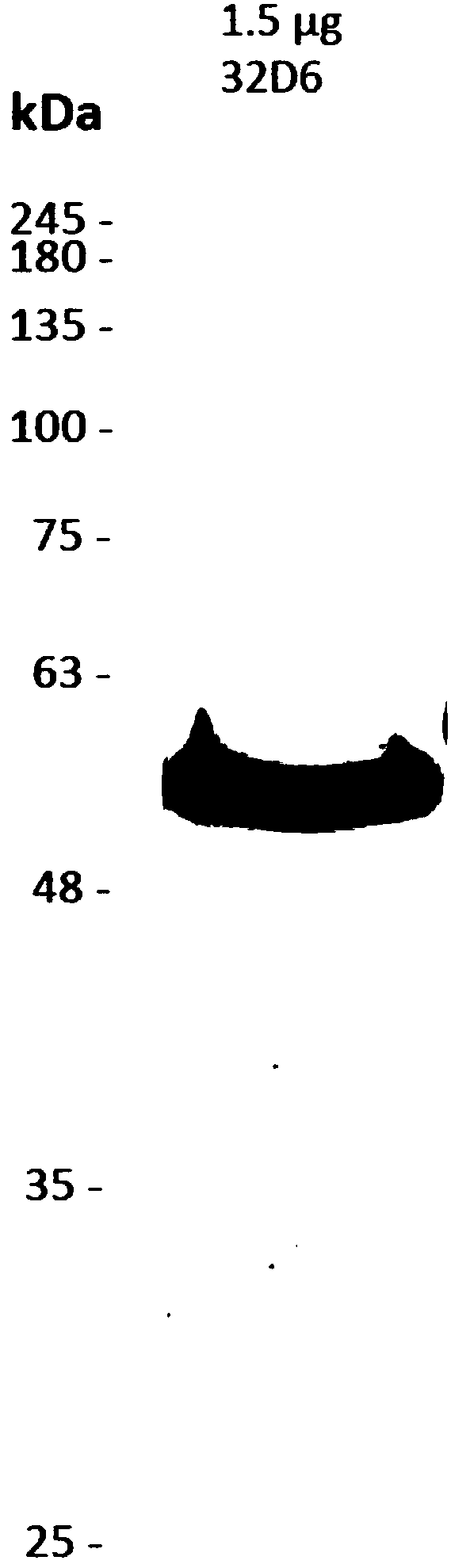
Examples
Embodiment
[0052] Cell culture and virus preparation
[0053] In RPMI1640 medium containing L-glutamine (Invitrogen, Carlsbad, CA, USA), 10% calf serum (FetalCalf Serum, FCS) and penicillin / streptomycin (Caisson) at 37 ° C and 5%CO 2 The marmoset B-lymphoblastoid B95-8 and 293T cells were cultured in a humidified environment. Bobtail hound kidney cells (Mardin-Darby canine kidney, MDCK) were subcultured in DMEM medium (Invitrogen) supplemented with 10% FCS. Spodoptera frugiperda (Sf9) cells were cultured in Sf-900II SFM medium (Invitrogen).
[0054] Influenza A H1N1 virus was prepared by Medigen Vaccine Biologics Co. (MVC, Taiwan). Influenza A virus A / Carlifornia / 7 / 2009NYMCX-179A (H1N1) is the proposed outbreak vaccine strain for vaccine development. Other clinical influenza A virus strains were obtained from Taiwan's Center for Disease Control (CDC) for the neutralization activity test described in this patent specification, 2013-80813 (A / Taiwan / 80813 / 2013(H1N1)), 2013-INF -152 (20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com