Amplifying system and method for detecting H1N1 virus RNA
An amplification system and virus technology, applied in DNA/RNA fragments, recombinant DNA technology, biochemical equipment and methods, etc., can solve problems such as being unsuitable for grassroots units and on-site detection, false positives, etc., to achieve real-time monitoring, methods Simple, easy to collect effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
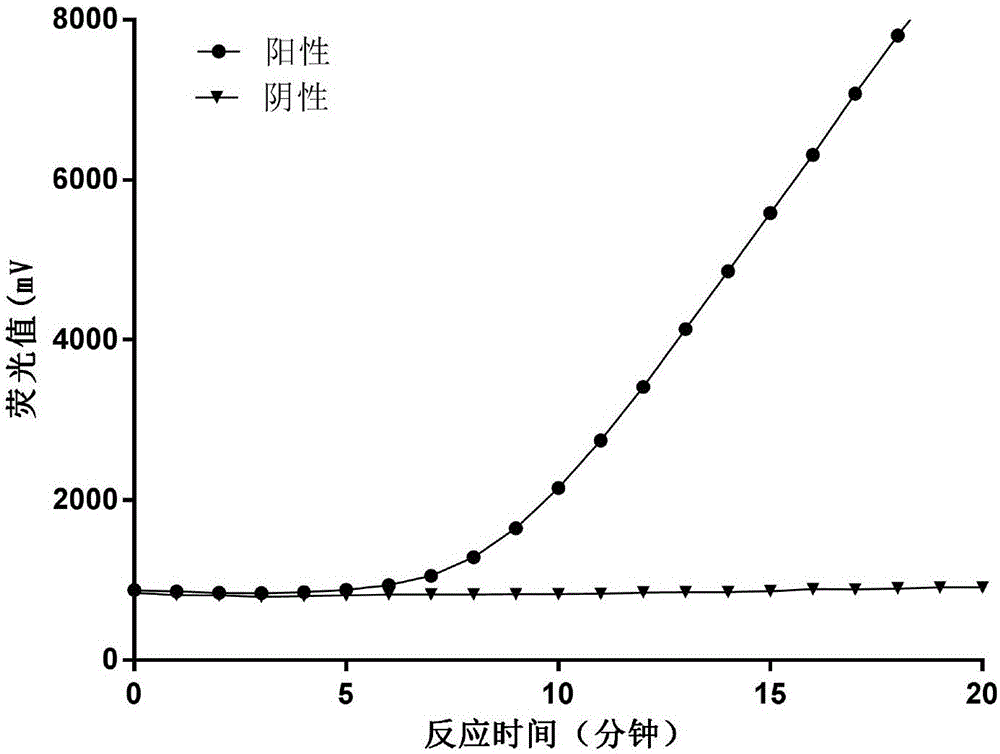
Examples
preparation example Construction
[0057] The preparation method of the amplification system is not particularly limited in the present invention, and it is prepared by a preparation method well known to those skilled in the art. If the amplification system is a freeze-dried powder, the present invention preferably adopts a negative pressure freeze-drying method to prepare the amplification system powder. In the present invention, the preferred freeze-drying pressure is 0.005-0.035KPa, more preferably 0.01-0.02KPa; the freeze-drying temperature is preferably -50-20°C, more preferably -35-10°C; the freeze-drying time is preferably It is 6.5~20h, more preferably 11~18h.
[0058] In the present invention, when the amplification system is used, the raw materials of the amplification system components in freeze-dried powder state are dissolved, and the solvent used for dissolving the raw materials of the amplification system is a reaction buffer. Preferably, the reaction buffer is polyethylene glycol aqueous soluti...
Embodiment 1
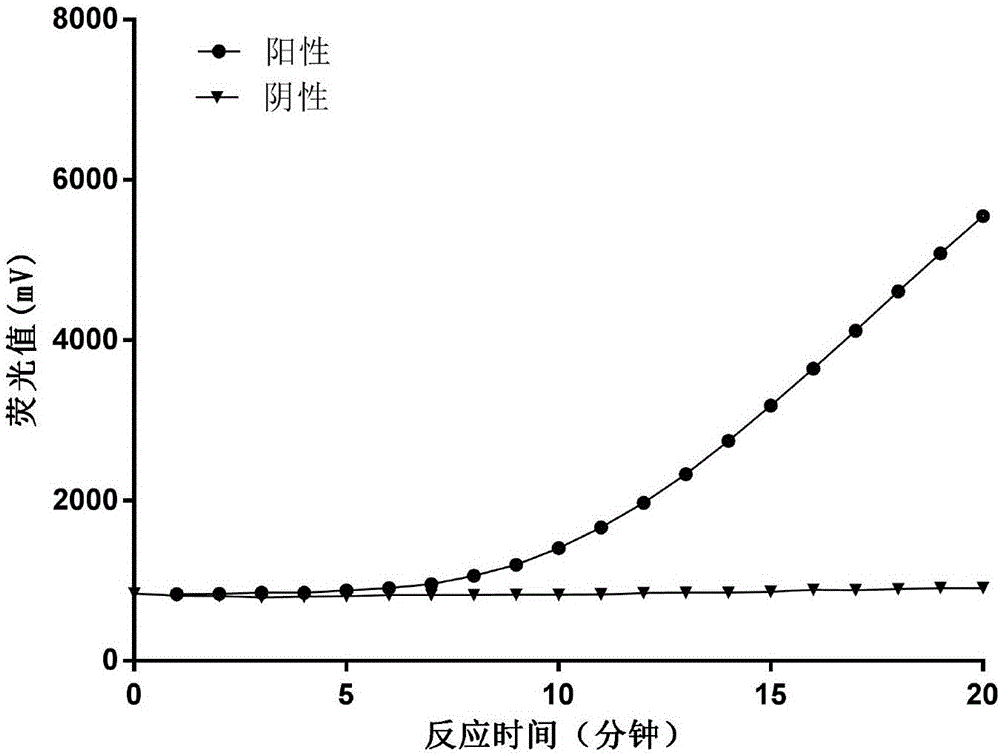
[0068]Preparation of Amplification System for Detection of H1N1 Virus RNA
[0069] Prepare an isothermal nucleic acid amplification system in a 200 μL centrifuge tube according to the following ratio, with a volume of 50 μL.
[0070]
[0071]
[0072] Wherein, the sequences of forward primer, reverse primer and fluorescent probe are as follows:
[0073] Forward primer sequence:
[0074] 5'-ATGCGAACAATTCAACAGACACTGTAGACACAG-3',
[0075] Reverse primer sequence:
[0076] 5'-CCTGGGTAACACGTTCCATTGTCTGAACTAGGTG-3';
[0077] The fluorescent probe sequence is:
[0078] 5'-AGAATGTAACAGTAACACTCTGTTAACCTTCTAGAAGACAAGCATAACG-3';
[0079] The above primers and probe sequences were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0080] The amplification system prepared above was freeze-dried in a freeze dryer under negative pressure to form a powder amplification system. The vacuum freeze-drying pressure was 0.02KPa, the freeze-drying temperature was -35°C, and the ...
Embodiment 2
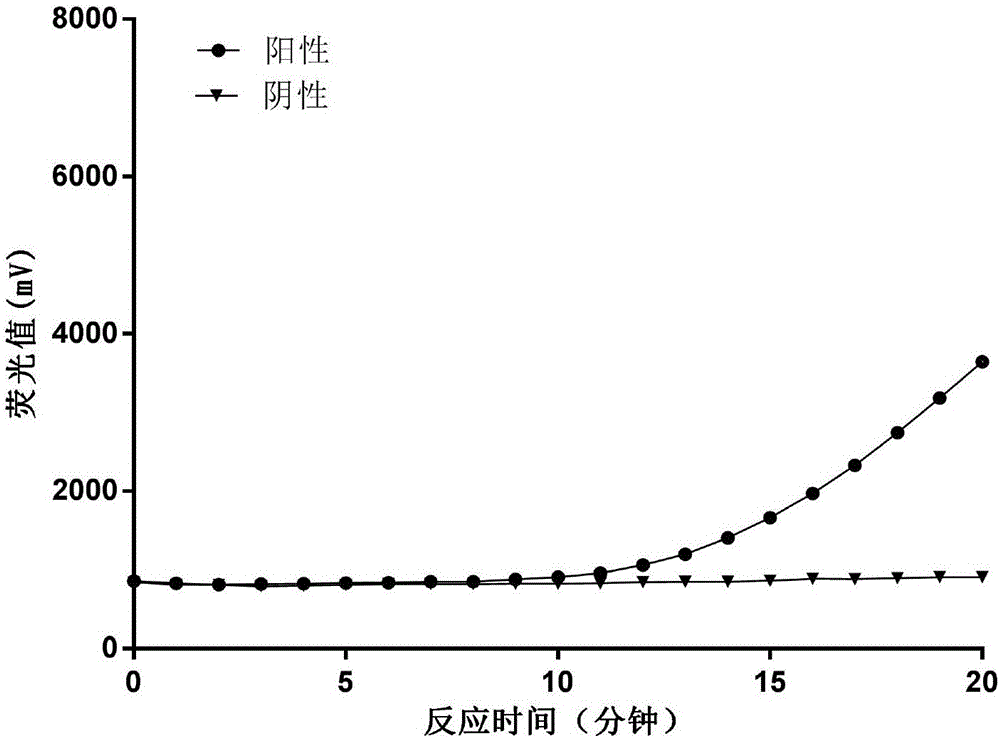
[0082] Preparation of Amplification System for Detection of H1N1 Virus RNA
[0083] Prepare an isothermal nucleic acid amplification system in a 200 μL centrifuge tube according to the following ratio, with a volume of 100 μL.
[0084]
[0085]
[0086] Wherein, the sequences of forward primer, reverse primer and fluorescent probe are as follows:
[0087] Forward primer sequence:
[0088] 5'-ATGCGAACAATTCAACAGACACTGTAGACACAG-3',
[0089] Reverse primer sequence:
[0090] 5'-CCTGGGTAACACGTTCCATTGTCTGAACTAGGTG-3';
[0091] The fluorescent probe sequence is:
[0092] 5'-AGAATGTAACAGTAACACTCTGTTAACCTTCTAGAAGACAAGCATAACG-3';
[0093] The above primers and probe sequences were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0094] The above-prepared amplification system was freeze-dried in a freeze dryer under negative pressure to form a powder amplification system. The vacuum freeze-drying pressure was 0.015KPa, the freeze-drying temperature was -18°C, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com