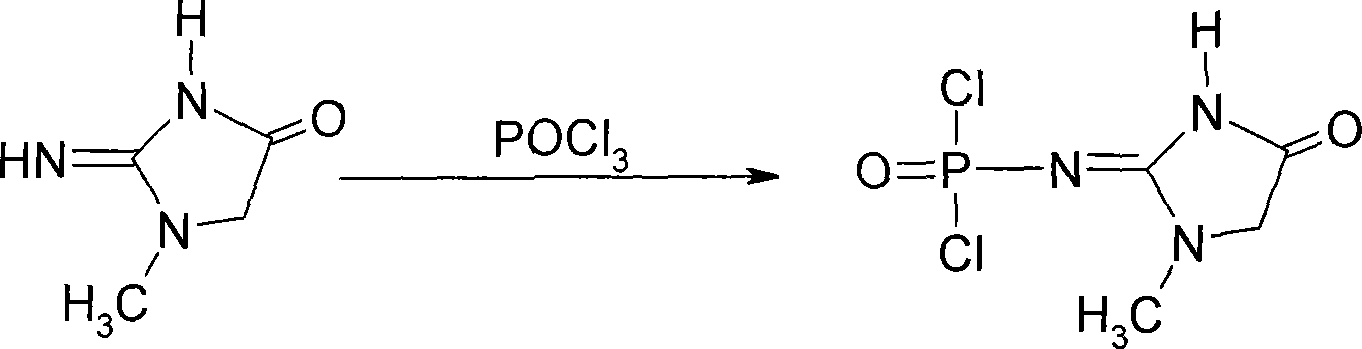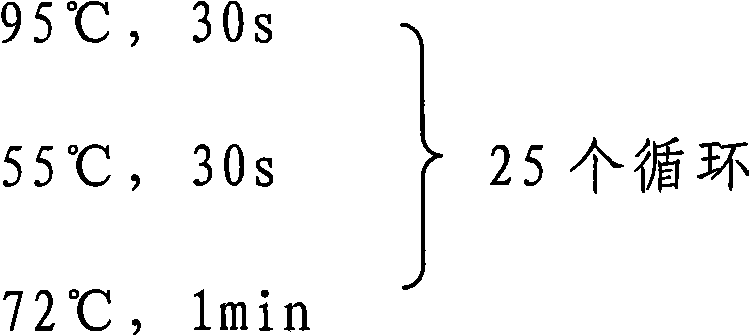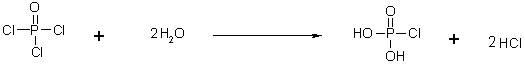Patents
Literature
165 results about "Phosphocreatine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
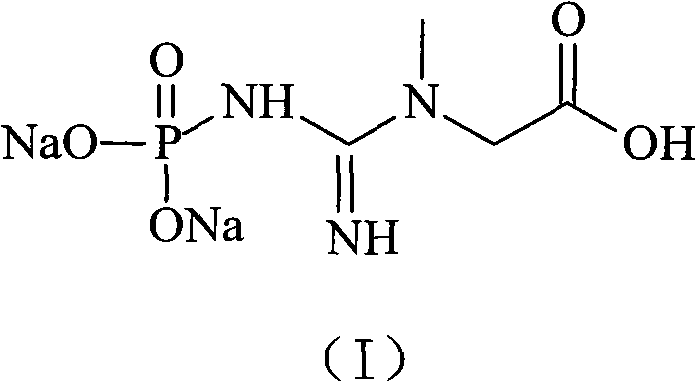
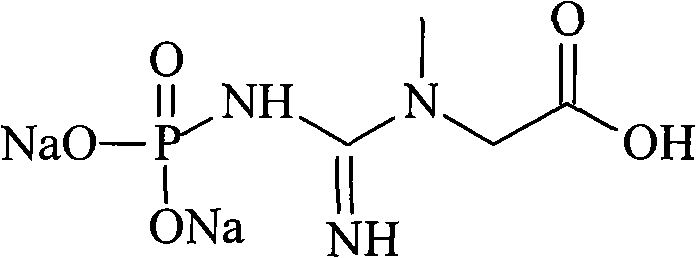
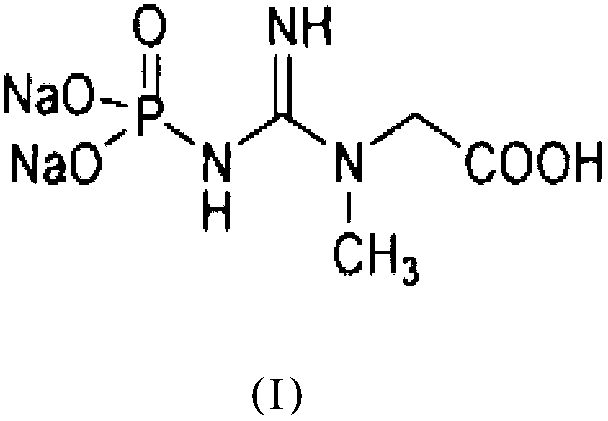
Phosphocreatine, also known as creatine phosphate (CP) or PCr (Pcr), is a phosphorylated creatine molecule that serves as a rapidly mobilizable reserve of high-energy phosphates in skeletal muscle and the brain to recycle adenosine triphosphate, the energy currency of the cell.
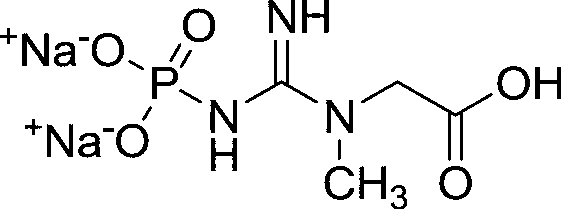
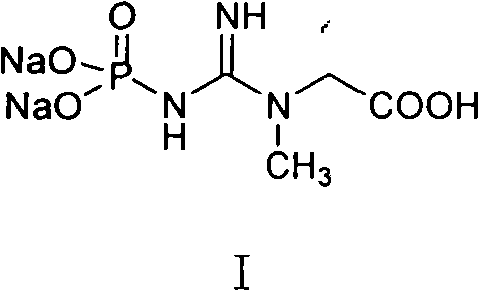
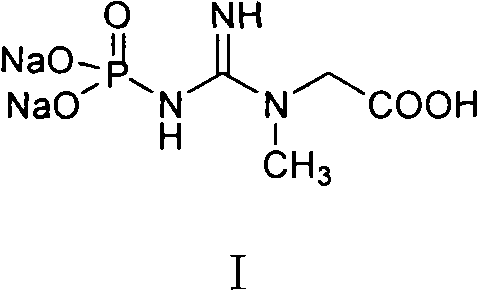
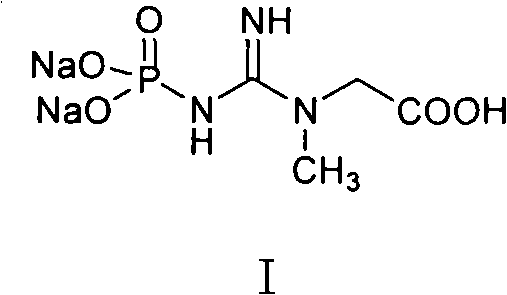
Medicinal disodium creatine phosphate hexahydrate and preparing method thereof
ActiveCN101033237AHigh purityLow drying temperatureGroup 5/15 element organic compoundsCreatinine riseDistillation
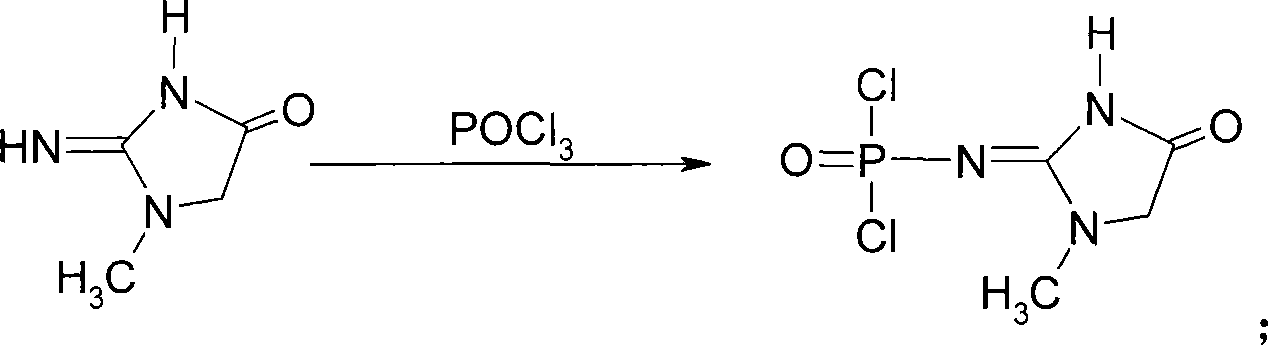
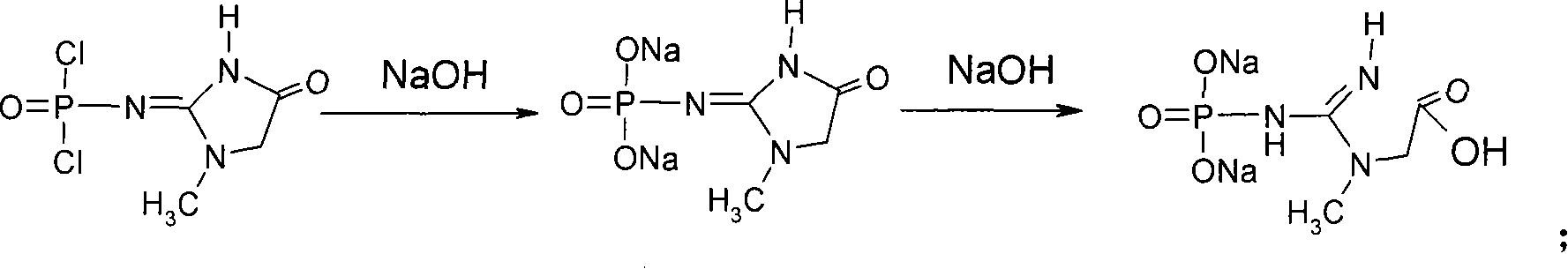
This invention discloses a phosphocreatine disodium salt six-hydrate and its preparation method. The compound is phosphocreatine disodium salt with high purity and six-crystal water, and the preparation method includes the following steps: (1) Kondens of creatinine and phosphorus oxychloride prepares creatinine phosphorus oxychloride, (2) creatinine phosphorus oxychloride is taken the ring opening reaction to get crude phosphorus disodium, (3) purification and crystallization gets the target product. This invention has the advantages of high purity suitable for the manufacture of injection powder, and it avoids the large use of solvents with high yield of products. The excessive phosphorus oxychloride is reused with distillation, and the product has high purity through intermediate and resin purification. It has no potential safety problem about heavy metal residues, and can effectively improve the clarification to reach the intravenous requirement.
Owner:QIDONG HUATUO PHARMA
Phytase-containing animal food and method
InactiveUS7320876B2Lower requirementReduce environmental pollutionSugar derivativesBacteriaBiotechnologyYeast
A method is described for improving the nutritional value of a foodstuff comprising a source of myo-inositol hexakisphosphate by feeding the foodstuff in combination with a phytase expressed in yeast. The method comprises the step of feeding the animal the foodstuff in combination with a phytase expressed in yeast wherein the phytase can be selected from the group consisting of AppA1, AppA2 and a site-directed mutant of AppA. The invention also enables reduction of the feed to weight gain ratio and an increase bone mass and mineral content of an animal. A foodstuff and a feed additive comprising AppA2 or a site-directed mutant of AppA are also described.
Owner:CORNELL RES FOUNDATION INC +1
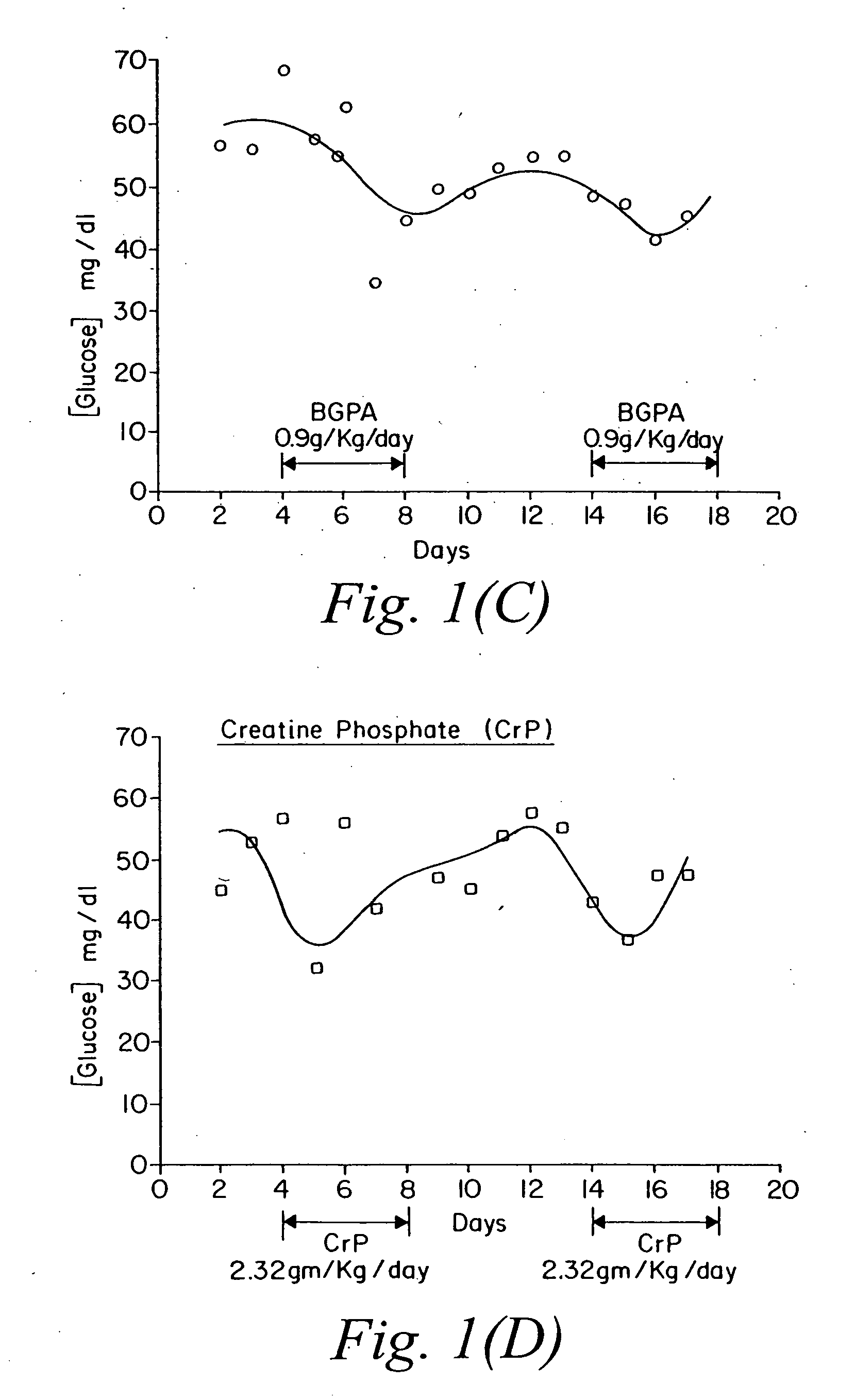
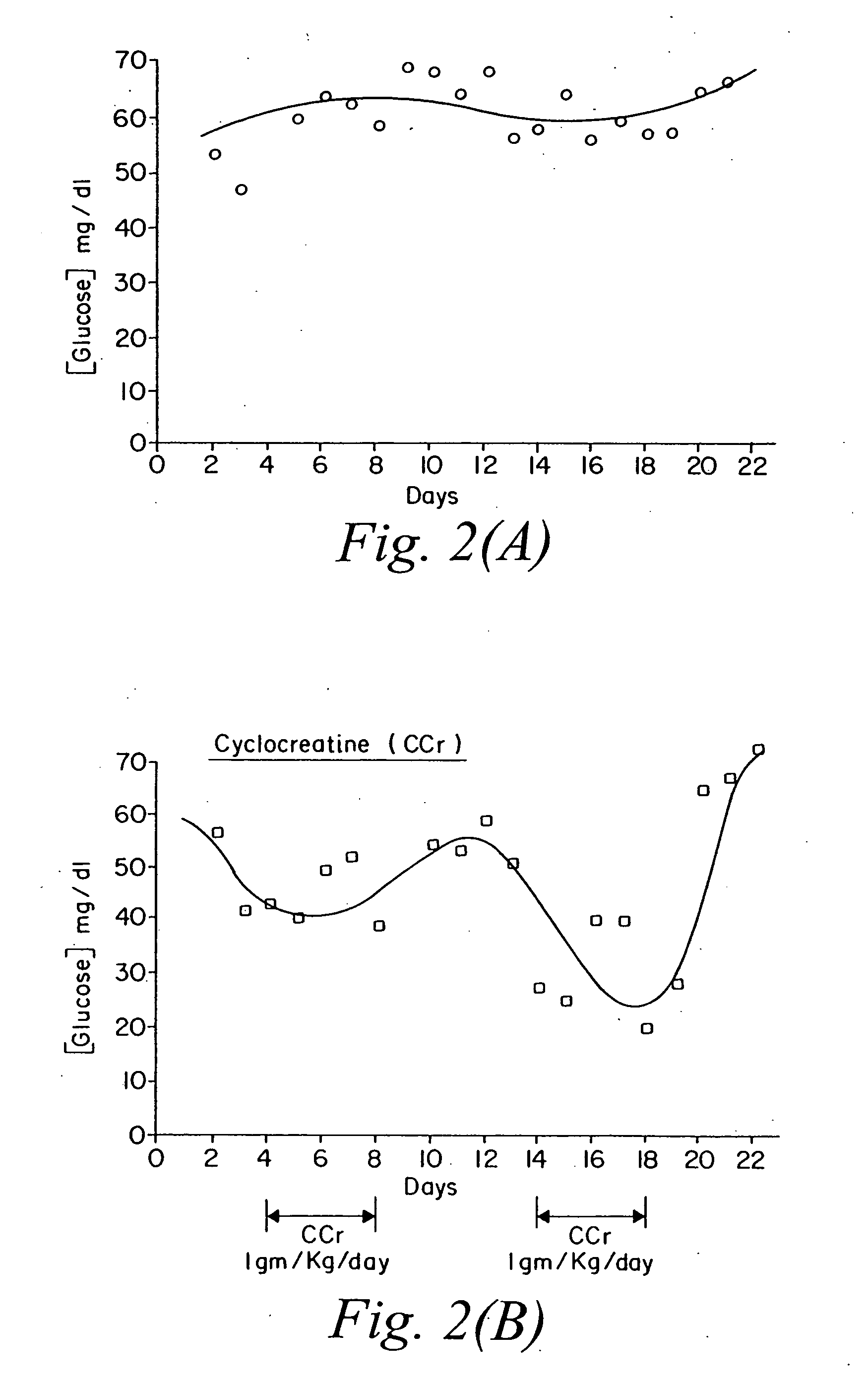
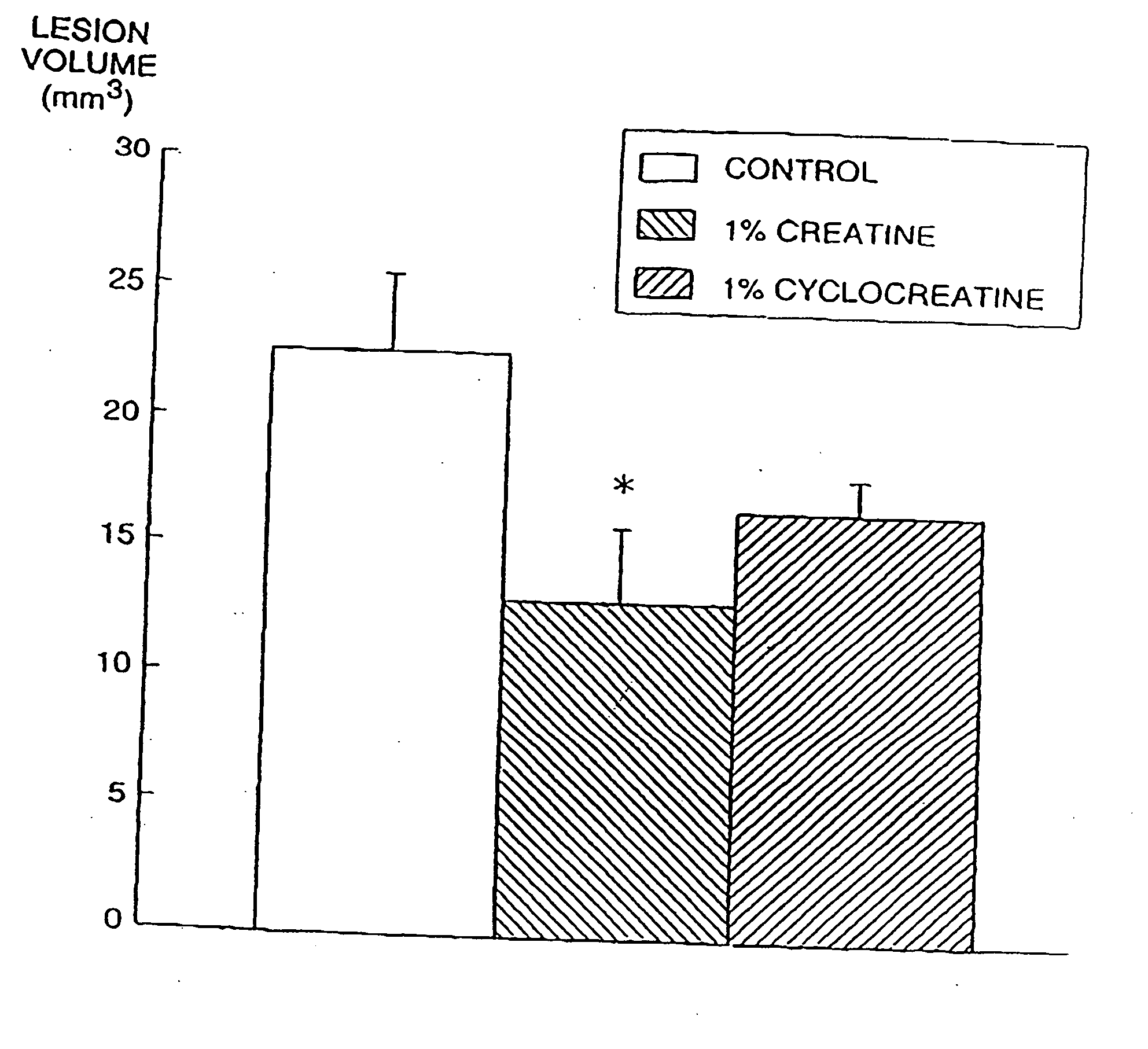
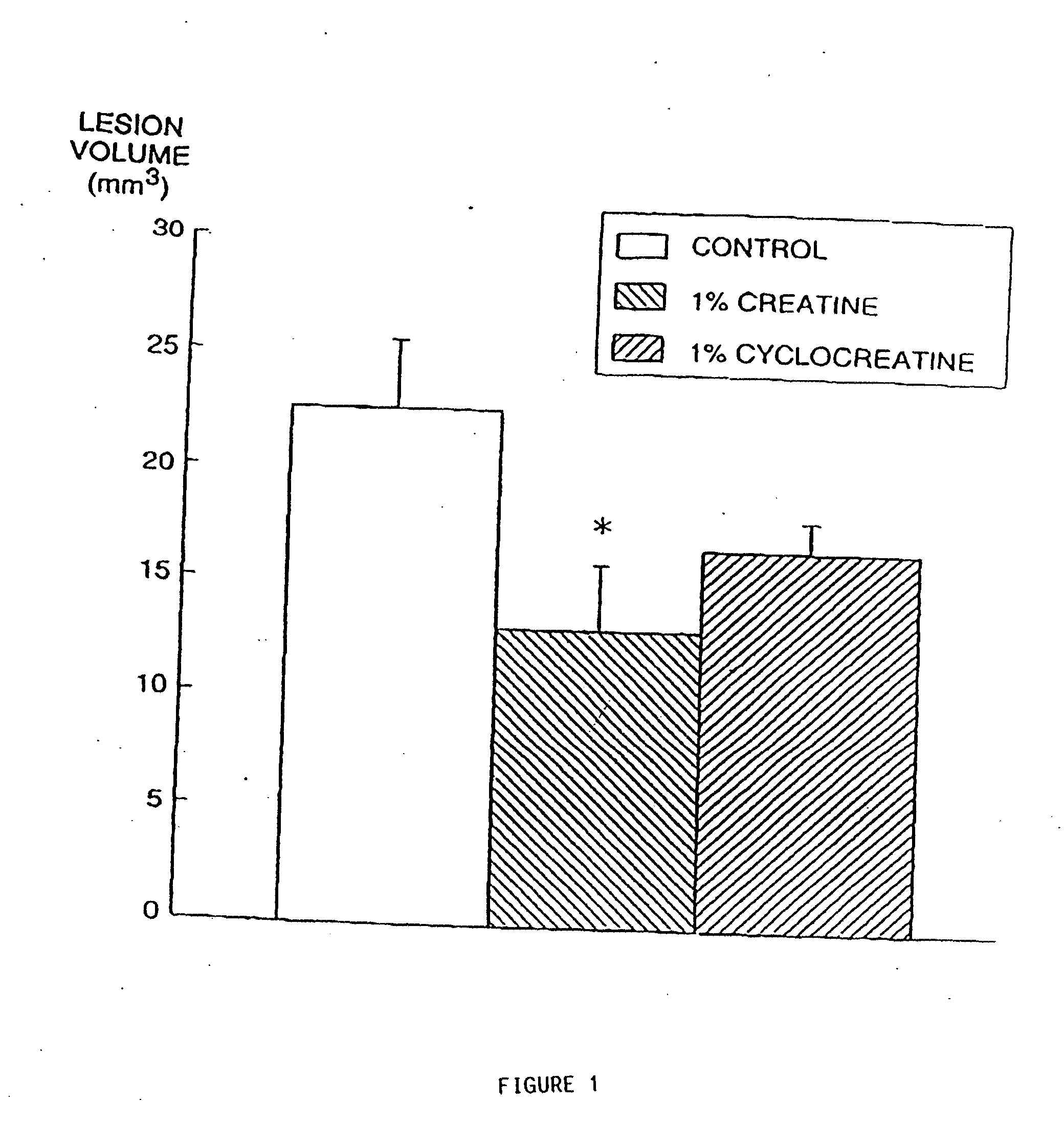
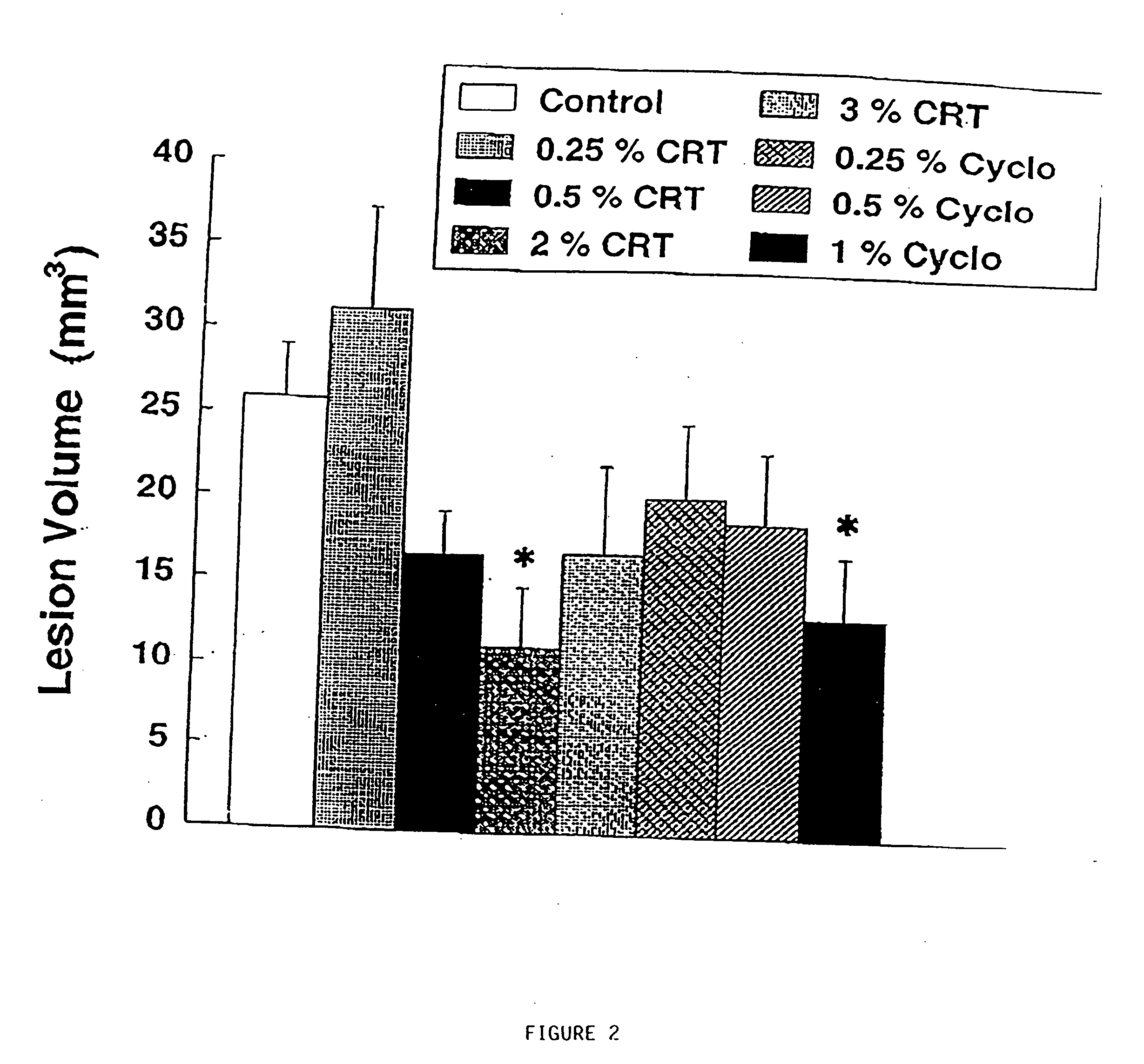
Use of creatine analogues and creatine kinase modulators for the prevention and treatment of glucose metabolic disorders
InactiveUS20050256134A1Affect glucose levelAlleviate and prevent symptomBiocidePeptide/protein ingredientsDiseaseAcute hyperglycaemia
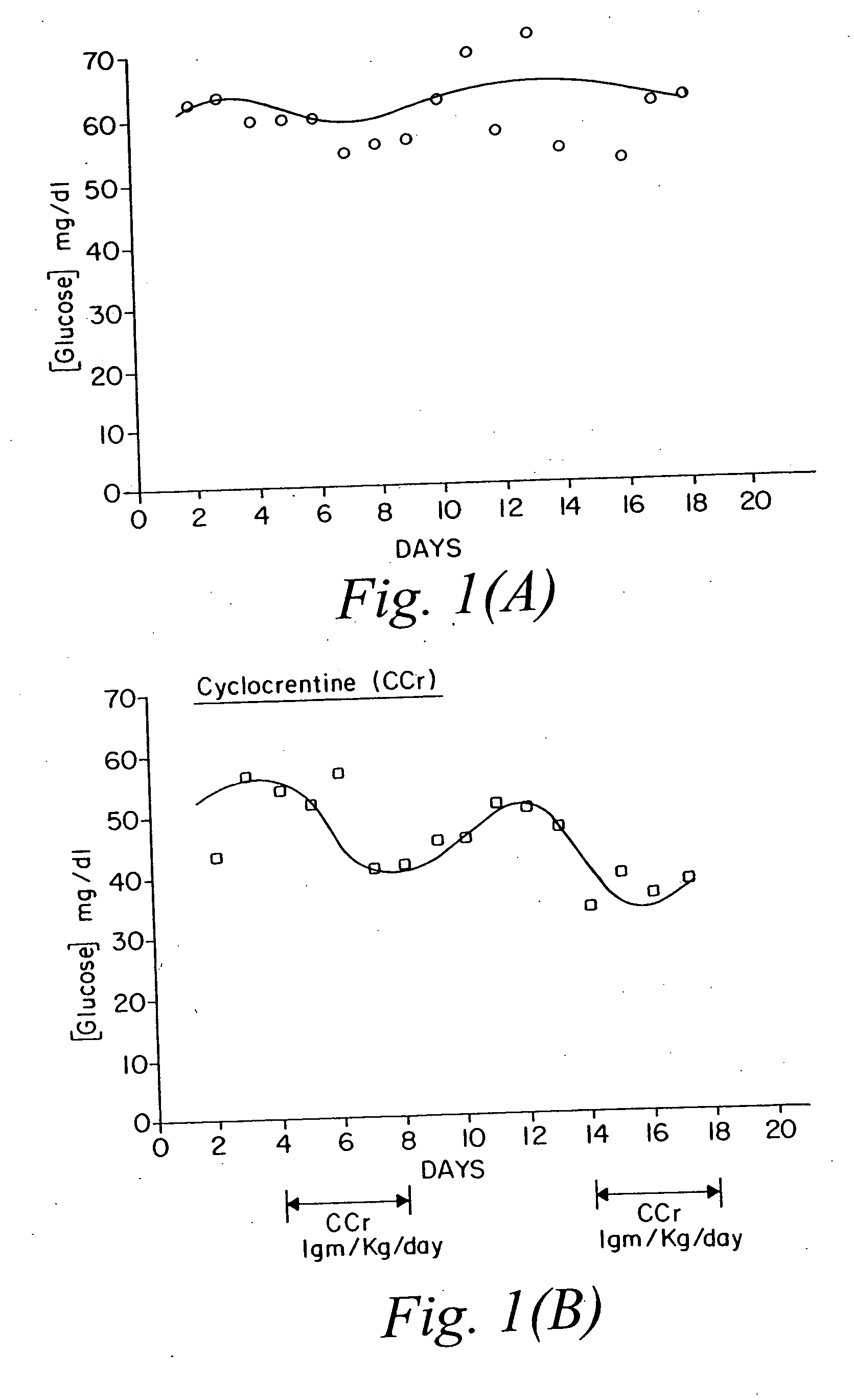
The present invention relates to the use of creatine compounds including cyclocreatine and creatine phosphate for treating or preventing a metabolic disorder consisting of hyperglycemia, insulin dependent diabetes mellitus, impaired glucose tolerance, hyperinsulinemia, insulin insensitivity, diabetes related diseases in a patient experiencing said disorder. The creatine compounds which can be used in the present method include (1) analogues of creatine which can act as substrates or substrate analogues for the enzyme creatine kinase; (2) compounds which can act as activators or inhibitors of creatine kinase; (3) compounds which can modulate the creatine transporter (4) N-phosphocreatine analogues bearing transferable or non-transferable moieties which mimic the N-phosphoryl group. (5) compounds which modify the association of creatine kinase with other cellular components.
Owner:AVICENA GROUP +1
Creatine jubase MB isozyme activity detection reagent and preparation method thereof
ActiveCN102154443AHigh precisionImprove accuracyMicrobiological testing/measurementAntiendomysial antibodiesCreatine kinase
The invention discloses a creatine jubase MB isozyme activity detection reagent which comprises sulfide-oxidizing coenzyme, 6-phosphaogluconate dehydrogenase, sulfhydryl reagent, magnesium salt, glucokinase or hexoxinase, anti-CK-M antibody, phosphocreatine, adenosine diphosphate, glucose and glucose-6-phosphate dehydrogenase. The preparation method of the reagent is as follows: dissolving all components in a buffer solution with pH of 5.0 to 9.5. The creatine jubase MB isozyme activity detection reagent can enlarge detection sensitivity several times; and the determination result has high precision and degree of accuracy.
Owner:浙江东瓯诊断产品有限公司
Compositions containing a combination of a creatine compound and a second agent
The present invention relates to the use of creatine compound and neuroprotective combinations including creatine, creatine phosphate or analogs of creatine, such as cyclocreatine, for treating diseases of the nervous system. Creatine compounds in combination with neuroprotective agents can be used as therapeutically effective compositions against a variety of diseases of the nervous system such as diabetic and toxic neuropathies, peripheral nervous system diseases, Alzheimer disease, Parkinson's disease, stroke, Huntington's disease, amyotropic lateral sclerosis, motor neuron disease, traumatic nerve injury, multiple sclerosis, dysmyelination and demyelination disorders, and mitochondrial diseases. The creatine compounds which can be used in the present method include (1) creatine, creatine phosphate and analogs of these compounds which can act as substrates or substrate analogs for creatine kinase; (2) bisubstrate inhibitors of creatine kinase comprising covalently linked structural analogs of adenosine triphosphate (ATP) and creatine; (3) creatine analogs which can act as reversible or irreversible inhibitors of creatine kinase; and (4) N-phosphorocreatine analogs bearing non-transferable moieties which mimic the N-phosphoryl group.
Owner:THE GENERAL HOSPITAL CORP
Medicine for treating muscular dystrophy and myasthenia gravis, and its prepn. method
ActiveCN1785223ASignificant effectMuscular disorderNeuromuscular disorderPrimary motor neuronPharmacology
A Chinese medicine for treating the myophagism and myasthenia gravis caused by motor neuron diseases, prograssive myodystrophy and congenital myopathy is prepared from ginseng and epimedium. Its preparing process is also disclosed.
Owner:SHIJIAZHUANG YILING PHARMA
A kind of refining method of creatine phosphate disodium salt
ActiveCN102295658ASimple processing methodMild and easy to controlGroup 5/15 element organic compoundsPhosphatePhysical chemistry
The invention discloses a refining method of disodium phosphocreatine. The method comprises steps that: a sodium phosphocreatine crude product is dissolved in water, the solution is filtered, and a micro-molecular solvent is dropped into the filtrate while stirring, such that a large amount of oily matters are precipitated; the oily matters are settled and precipitated, a supernatant is removed, methanol is dropped into the oily matters while stirring, a large amount of crystals are precipitated, and the crystals are beaten and filtered; the filter cake is transferred into a reaction bottle, washed by using a micro-molecular solvent while stirring, and is filtered; the filter cake is dried with reduced pressure at a temperature of 40 to 45 DEG C, such that disodium phosphocreatine tetrahydrate with a high quality is obtained. The refining method provided by the invention has advantages of mild technical condition, simple and easy-to-control method, high yield, and stable product quality. The method satisfies the requirement of large-scale productions, and brings in good social and economic benefits.
Owner:重庆莱美隆宇药业有限公司
Phytase-containing animal food and method
InactiveUS20090074909A1Lower requirementReduce environmental pollutionMilk preparationBacteriaWeight gainingBiotechnology
A method is described for improving the nutritional value of a foodstuff comprising a source of myo-inositol hexakisphosphate by feeding the foodstuff in combination with a phytase expressed in yeast. The method comprises the step of feeding the animal the foodstuff in combination with a phytase expressed in yeast wherein the phytase can be selected from the group consisting of AppA1, AppA2 and a site-directed mutant of AppA. The invention also enables reduction of the feed to weight gain ratio and an increase bone mass and mineral content of an animal. A foodstuff and a feed additive comprising AppA2 or a site-directed mutant of AppA are also described.
Owner:CORNELL RES FOUNDATION INC +1
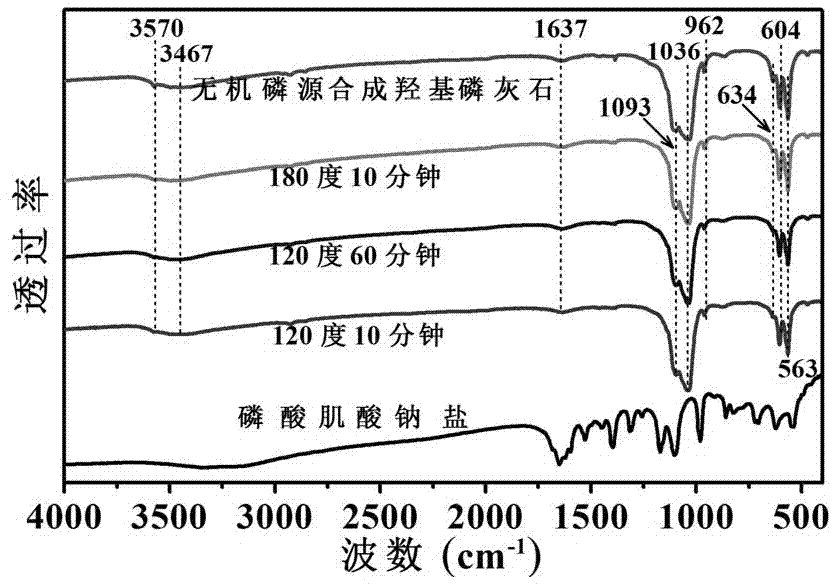
Microwave assisted preparation of hydroxyapatite hollow sphere
ActiveCN102897735APromote degradationShape is easy to controlMaterial nanotechnologyPhosphorus compoundsPhosphateApatite
The invention relates to a microwave assisted preparation of hydroxyapatite hollow sphere. The hydroxyapatite hollow sphere structure is prepared from water-soluble calcium salt serving as a calcium source and phosphorous biomolecules serving as a phosphor source through a microwave assisted hydrothermal reaction, wherein the phosphorous biomolecules are phosphocreatine or creatine phosphate. In the hydroxyapatite hollow sphere, the phosphorous biomolecules have good biodegradability and environmental friendliness. The hydroxyapatite hollow sphere structure prepared according to the preparation method provided by the invention can be widely applied to biomedicine, tissue engineering and other fields. The method has important scientific meaning and application value in expanding preparation of calcium phosphate biomaterials.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
In-vitro cell-free protein synthesis system and application thereof
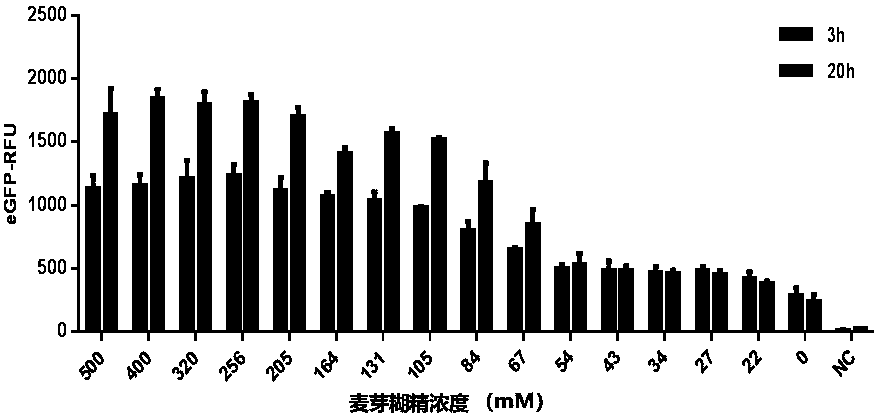
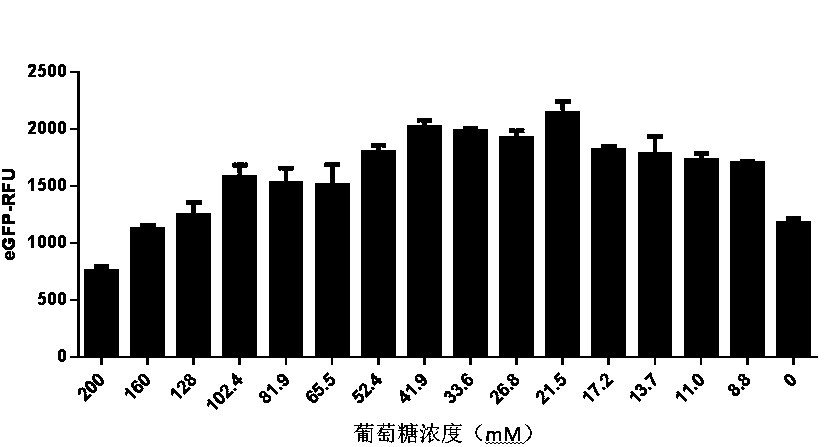
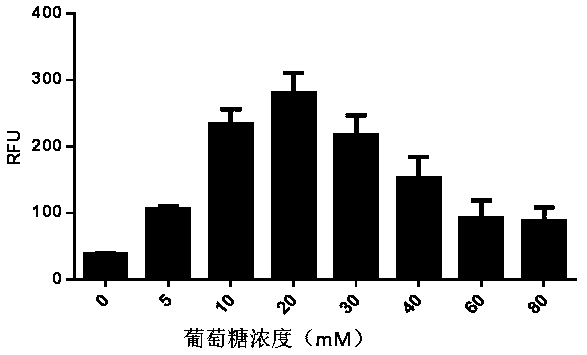
The present invention discloses an in-vitro cell-free protein synthesis system. The system comprises cell extract, carbohydrate and a phosphate compound, wherein the carbohydrate is maltodextrin, lactose, or a combination of the maltodextrin and glucose, or a combination of the lactose and the glucose, or a combination of the maltodextrin, the lactose and the glucose. ATP is provided for an in-vitro reaction by low-cost substances such as the glucose, the maltodextrin and the lactose instead of energy sources such as phosphoenolpyruvic acid, phosphocreatine and acetyl phosphate, so that whilethe cost is reduced, a mode of providing energy by slow release prolongs the reaction time and increases the yield of target protein.
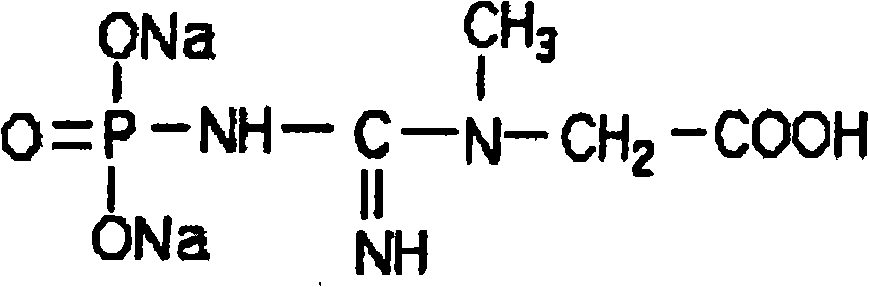
Synthesis and pharmaceutical application of sulfamide derivative
ActiveCN104341450AGood water solubilityImprove stabilityOrganic active ingredientsSenses disorderSolubilitySodium phosphates
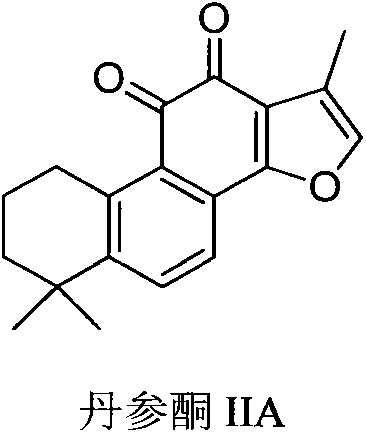
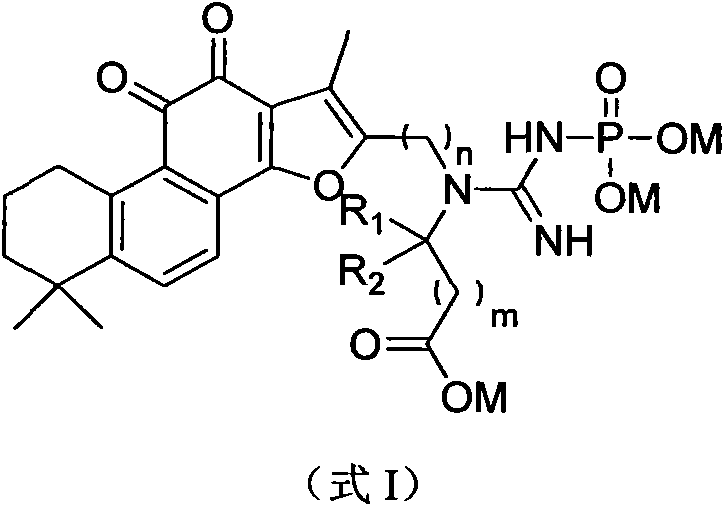
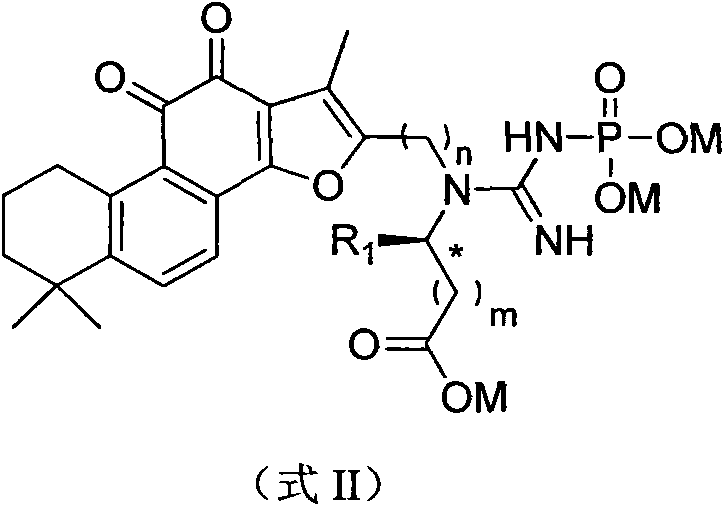
The invention discloses a tanshinone IIA derivative. A connecting arm, phosphocreatine and an analogue of the phosphocreatine form a conjugate of which the structure is as shown in formula (I) in the specification, wherein n is equal to 1-3, m is equal to 0-3, R1 and R2 respectively independently represent hydrogen atoms, C1-C5 alkyl, cycloalkyl, vinyl and alkynyl, the R1 and the R2 also can form ternary to hexatomic rings, and M represents metal ions, like sodium ions. The compound can be used for improving the water solubility of the whole molecule through sodium phosphate in the phosphocreatine on the premise of maintaining the activity of the tanshinone IIA; moreover, a phosphocreatine fragment in the molecule can play a dual protection effect on myocardial muscles.
Owner:北京桦冠医药科技有限公司
Crystal preparation method of creatine phosphate sodium
ActiveCN103012472AHigh crystallinityCrystal habit completeGroup 5/15 element organic compoundsPhosphateKetone
The invention discloses a crystallizing method for preparing high-purity creatine phosphate sodium. The crystallizing method comprises the following steps of: dissolving a creatine phosphate sodium crude product in water to prepare a 0.5-1.1 g / ml creatine phosphate sodium aqueous solution, continuously stirring for 30-60 minutes to decolor; after filtering, transferring a filtrate into a crystallizer, controlling a system temperature to be within 5-45 DEG C, and adding an alcohol or ketone organic solvent to perform solvent-out crystallization; after crystallizing, and separating through filtering, washing with a solvent and drying to obtain a creatine phosphate sodium product. The creatine phosphate sodium crystal provided by the invention is high in crystallinity degree, complete in product crystal habit, and uniform in particle size distribution; and a primary particle size is 67.1-70.2 mu m. The product purity reaches more than 99.5%, and a single molar yield during the crystallizing process is more than 98.0%; and the method is simple in process, low in cost and suitable for industrial production.
Owner:TIANJIN UNIV
Creatine kinase mutant applicable to phosphocreatine enzymic method production process
InactiveCN104357420AImprove catalytic reaction efficiencyReduce manufacturing costTransferasesFermentationPhosphoric acidMutant
The invention relates to a method for improving the property of creatine kinase as a reaction catalytic enzyme in phosphokinase production by mutating and transforming rabbit muscle creatine kinase by using a gene engineering technique. According to the method, the gene engineering technique is adopted to perform multi-point mutation on rabbit muscle creatine kinase, so that the properties, such as the enzymatic property, the substrate affinity, the acid / alkali resistance and the thermal stability, of creatine kinase in phosphokinase production are improved. The method has the advantages that the catalysis property of creatine kinase in a phosphokinase enzymic method production process environment with the pH value of 8-10 and the temperature of 25-45 DEG C is greatly improved through enzyme mutation transformation, the production efficiency is improved, the production cost is lowered, and great significance is achieved for developing the enzymic method phosphocreatine industry.
Owner:杭州清科生物科技有限公司
Synthesis of phosphocreatine disodium salt
InactiveCN101492470AIncrease the number of purificationsIncrease costGroup 5/15 element organic compoundsCardiovascular disorderIce waterFiltration
The invention relates to a synthetic method of disodium creatine phosphate, which is characterized in that a barium-free salt operation method is adopted. In the method, disodium creatinine phosphate is dissolved in water, activated carbon is used in ice-water bath for decolorizing, the filtering is carried out, ethanol is added to filtering liquid, and after the air pump filtration, refined products of the disodium creatinine phosphate are obtained; the refined products of the disodium creatinine phosphate are dissolved in water, the activated carbon is added, and then the refined products are stirred and filtered in the ice-water bath, sodium hydroxide regulating solution with pH value of 13-14 is added to the filtrate and stirred, hydrochloric acid is used in the ice-water bath to regulate the pH value to 7-10, the activated carbon is used for decolorizing at the room temperature, the filtering is carried out, ethanol is added to the filtrate and stirred continuously, and the disodium creatine phosphate is obtained after the air pump filtration; the obtained disodium creatine phosphate is dissolved in water, activated carbon is added and stirred, the filtering is carried out, ethanol is added to the filtrate and stirred continuously, and the refined products of the disodium creatine phosphate are obtained after the air pump filtration. The synthetic method of the invention has the advantage of removing impurities produced during the preparing process of the disodium creatine phosphate to the utmost extent.
Owner:上海慈瑞医药科技股份有限公司
Method for cell-free expression of signal protein and expression system
ActiveCN106011163ASolve the problem of low expressionHigh protein expressionNucleic acid vectorVector-based foreign material introductionEscherichia coliCreatine kinase
The invention relates to a method for cell-free expression of signal protein and an expression system. The method includes joining a target gene of the signal protein on a pIX3.0 vector to obtain expression plasmids; extracting escherichia coli Rosetta (DE3) by S30 buffer solution to obtain escherichia coli extract; preparing an energy supply system comprising PEP (phosphoenolpyruvic acid) / PK (pyruvate kinase), CP (phosphocreatine) / CK (creatine kinase) and glucose; preparing an amino acid mixture and a saline solution; preparing lecithin / cholesterol liposome; adding the expression plasmids into a cell-free protein expression system comprising the escherichia coli extract, the energy supply system, the amino acid mixture, the saline solution and the lecithin / cholesterol liposome for expression. The method is capable of solving the problem of small signal protein expression quantity.
Owner:CUSABIO TECH LLC
Phytase-containing animal food and method
InactiveUS7833743B2Lower requirementReduce environmental pollutionMilk preparationSugar derivativesBiotechnologyAnimal food
A method is described for improving the nutritional value of a foodstuff comprising a source of myo-inositol hexakisphosphate by feeding the foodstuff in combination with a phytase expressed in yeast. The method comprises the step of feeding the animal the foodstuff in combination with a phytase expressed in yeast wherein the phytase can be selected from the group consisting of AppA1, AppA2 and a site-directed mutant of AppA. The invention also enables reduction of the feed to weight gain ratio and an increase bone mass and mineral content of an animal. A foodstuff and a feed additive comprising AppA2 or a site-directed mutant of AppA are also described.
Owner:CORNELL RES FOUNDATION INC +1
Bioengineering method for synthesizing sodium phosphocreatine
InactiveCN102533880AReduce processing costsNo biological toxicityBacteriaTransferasesEscherichia coliCreatine kinase
The invention provides a bioengineering method for synthesizing sodium phosphocreatine. The bioengineering method comprises the following steps of: recombining an escherichia coli engineering strain containing creatine kinase by using a genetic engineering method; carrying out large-scale high-density culture and separate purification by using the escherichia coli engineering strain to obtain high-activity creatine kinase; and carrying out enzyme-method catalytic synthesis by using the creatine kinase under the appropriate condition to obtain the phosphocreatine. The bioengineering method disclosed by the invention has the advantages of high substrate utilization rate, high product recovery rate, mild reaction conditions, short reaction time, little pollution to environment, low cost and the like and is suitable for industrial large-scale production.
Owner:BIOTRAND
Fluorescent constant-temperature amplification technique
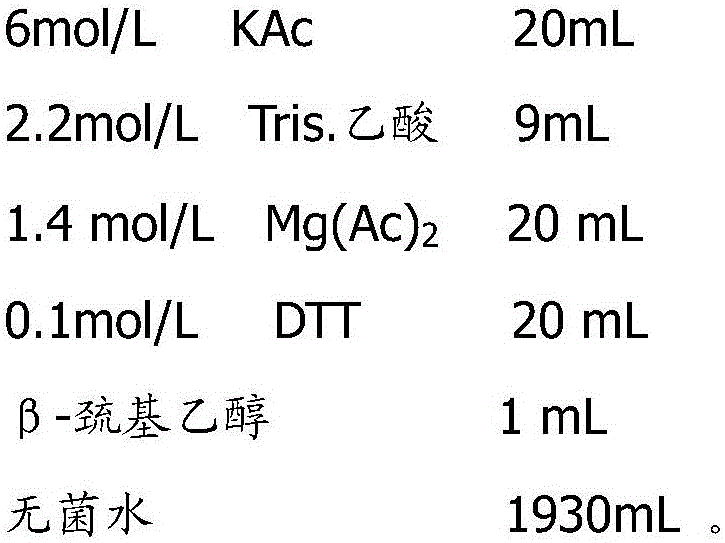
The invention discloses a fluorescent constant-temperature amplification technique. In water used as solvent for a fluorescent constant-temperature amplification reagent used in the technique, Tris-buffer solution, magnesium acetate, dithiothreitol, betaine, Tween 20, DMSO, mycose, PEG, BSA, dNTPs, and proper amounts of fluorescent dye, polymerase, single strand DNA-binding protein and recombinase are added. A constant-temperature amplification system of the invention is dependent of high-energy molecules such as ATP and creatine phosphate; compared with existing constant-temperature amplification systems, the constant-temperature amplification system is simpler in composition, lower in cost and more convenient to use. The constant-temperature amplification system is capable of amplifying DNA and RNA sequences, is applicable to amplification of complex templates stably and quickly, capable of finishing an amplification reaction within 30min, has high amplification sensitivity and accuracy without rare occurrence of mispairing, and has good amplification effect. The constant-temperature amplification system is applicable to real-time quantification and has a wider range of application.
Owner:GUANGZHOU HEAS BIOTECH CO LTD
Creatine phosphate sodium compound and method for synthesizing the same
InactiveCN101486730AReduce dosageReduce pollutionOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsPhosphatePhosphoric acid
The invention relates to a synthetic method of a creatine phosphoric acid sodium salt compound, which comprises the following steps: (1) creatine and acetonitrile are mixed and stirred until the solution is cleaned; after the solution is cooled down, N, N- diisopropylethylamine is added; (2) a mixture obtained in step (1) is cooled down again and then phosphorus oxychloride is added to the mixture and stirred for 60 hours at room temperature; (3) under the condition that pressure is reduced and temperature is not higher than 50 DEG C, most solvent of the mixture obtained in step (2) is distilled; and then the solvent is cooled down to room temperature and a water solution of sodium hydroxide is added to the solvent and stirred for 1 hour; and (4) ethanol of 90 percent is added to the mixture obtained in step (3) and maintained for 6 hours; and then white crystals is separated out and the mixture stands for 10 hours to 20 hours; the mixture is filtered and rough products are obtained; the products are washed with ethanol of 90 percent and dried under vacuum; and then white creatine phosphoric acid sodium salt compounds are obtained. The method has the advantages of low cost, strong operability, high yields, easily controlled conditions during production process and little environmental pollution.
Owner:郑仙锋
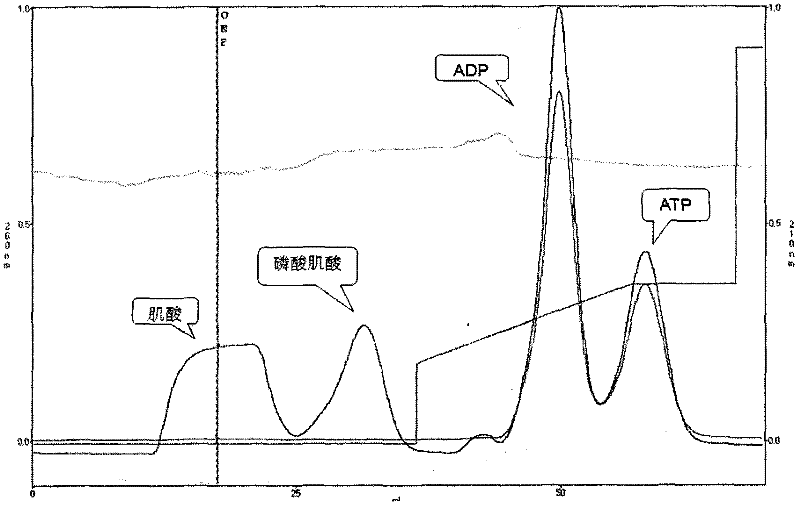
ADP detection using an enzyme-coupled reaction
ActiveUS20060199238A1Minimize contaminationMicrobiological testing/measurementImmunoglobulinsFluorescencePeroxidase
Methods and compositions are provided for determining ADP in the presence of ATP. These comprise including among the assay reagents at least one of the correcting components creatine phosphokinase and phosphocreatine, pyruvate kinase and phosphoenolpyruvate, peroxidase and a non-interfering peroxidase substrate, and catalase. One aspect of the method employs formation of hydrogen peroxide from the ADP by pyruvate kinase, phosphoenolpyruvate and pyruvate oxidase. The hydrogen peroxide is then determined. A combined reagent having all of the reagents may optionally include a peroxidase when the hydrogen peroxide is to be enzymatically determined. A peroxidase substrate is added to the sample in conjunction with the peroxidase substrate reagent, the mixture incubated and depending on whether the peroxidase substrate is a fluorescer or chemiluminescer, the mixture may be illuminated with excitation light and the emitted light determined as a measure of the ADP in the sample.
Owner:DISCOVERYX CORP
High-purity creatine phosphate sodium compound
InactiveCN101812088AHigh purityIncrease contentGroup 5/15 element organic compoundsActivated carbonChromatographic separation
The invention relates to a high-purity creatine phosphate sodium compound; by the acid-base reaction, the activated carbon adsorption and the preparation of chromatographic separation and purification, the purity and content of the creatine phosphate sodium is greatly increased, the product quality of the preparation is optimized and the safety of the clinical medication is guaranteed; and the method has simple technique, low cost and high yield and is suitable for industrialization production.
Owner:HAINAN MEIDA PHARMA
Method of preparing creatine phosphate sodium
The invention relates to a method of preparing creatine phosphate sodium by utilizing a gene engineering technology. The method comprises the steps: respectively constructing creatine kinase and acetate kinase expression strains, removing the limit of creatine kinase-containing animal source, specially purifying the creatine kinase and acetate kinase by utilizing an affinity purification technology, co-immobilizing the creatine kinase and acetate kinase onto vector particles to form co-immobilized enzyme, continuously regenerating ATP from the acetate kinase for sustained reaction while the creatine kinase catalyzes and generates creatine phosphate sodium, thereby finally providing low-cost, green and pollution-free creatine phosphate sodium.
Owner:HUNAN BAOLISHI BIOTECH
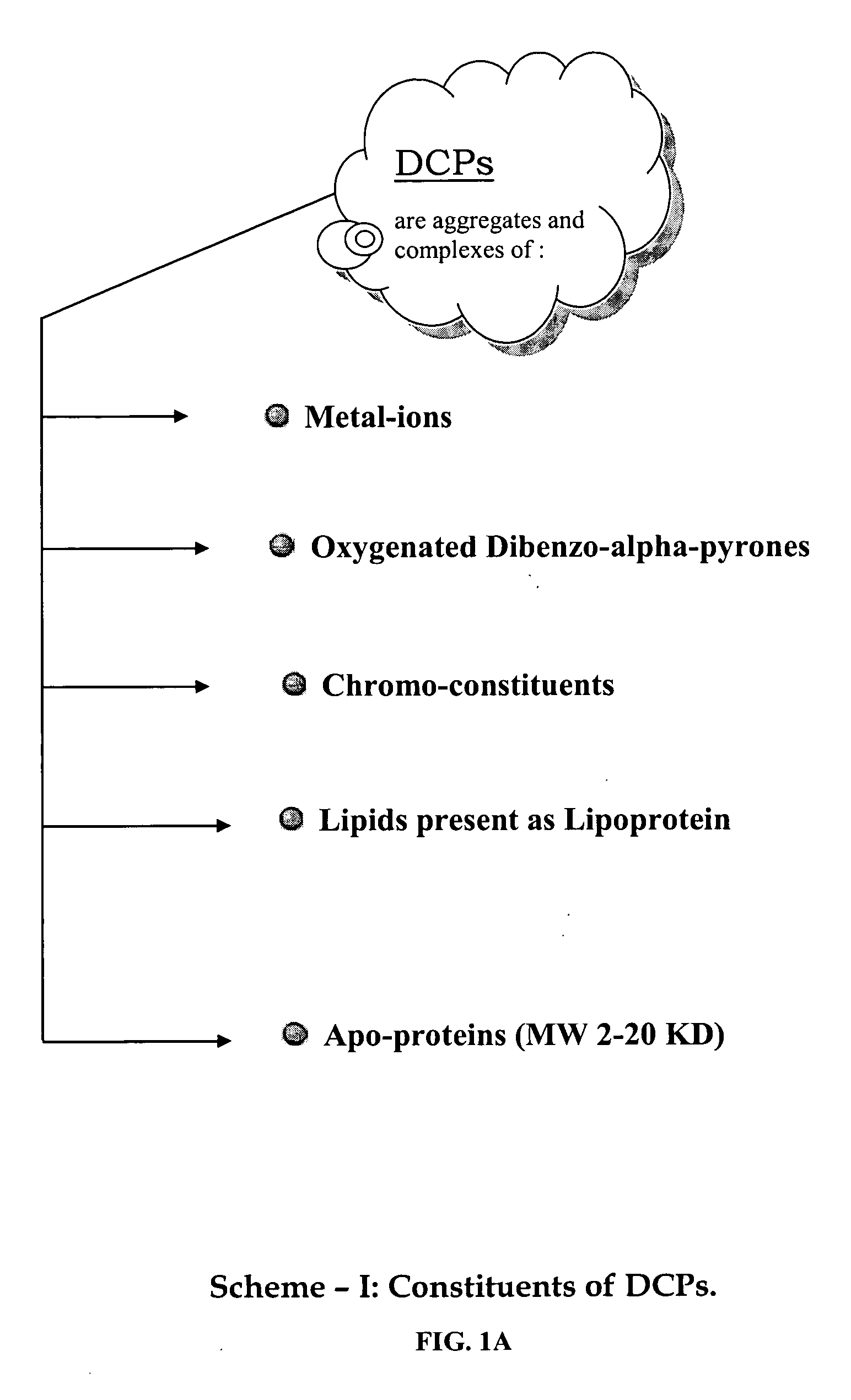
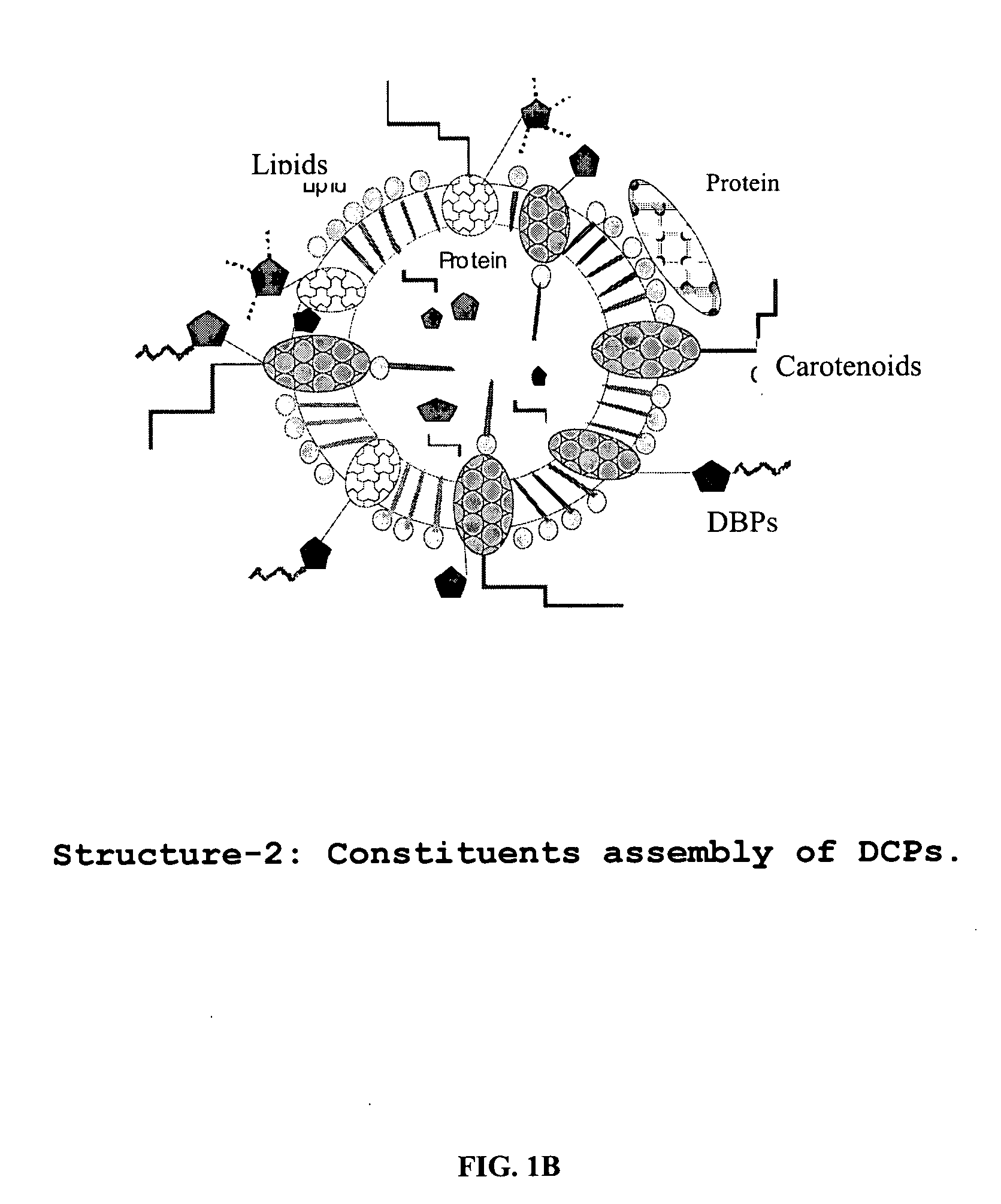
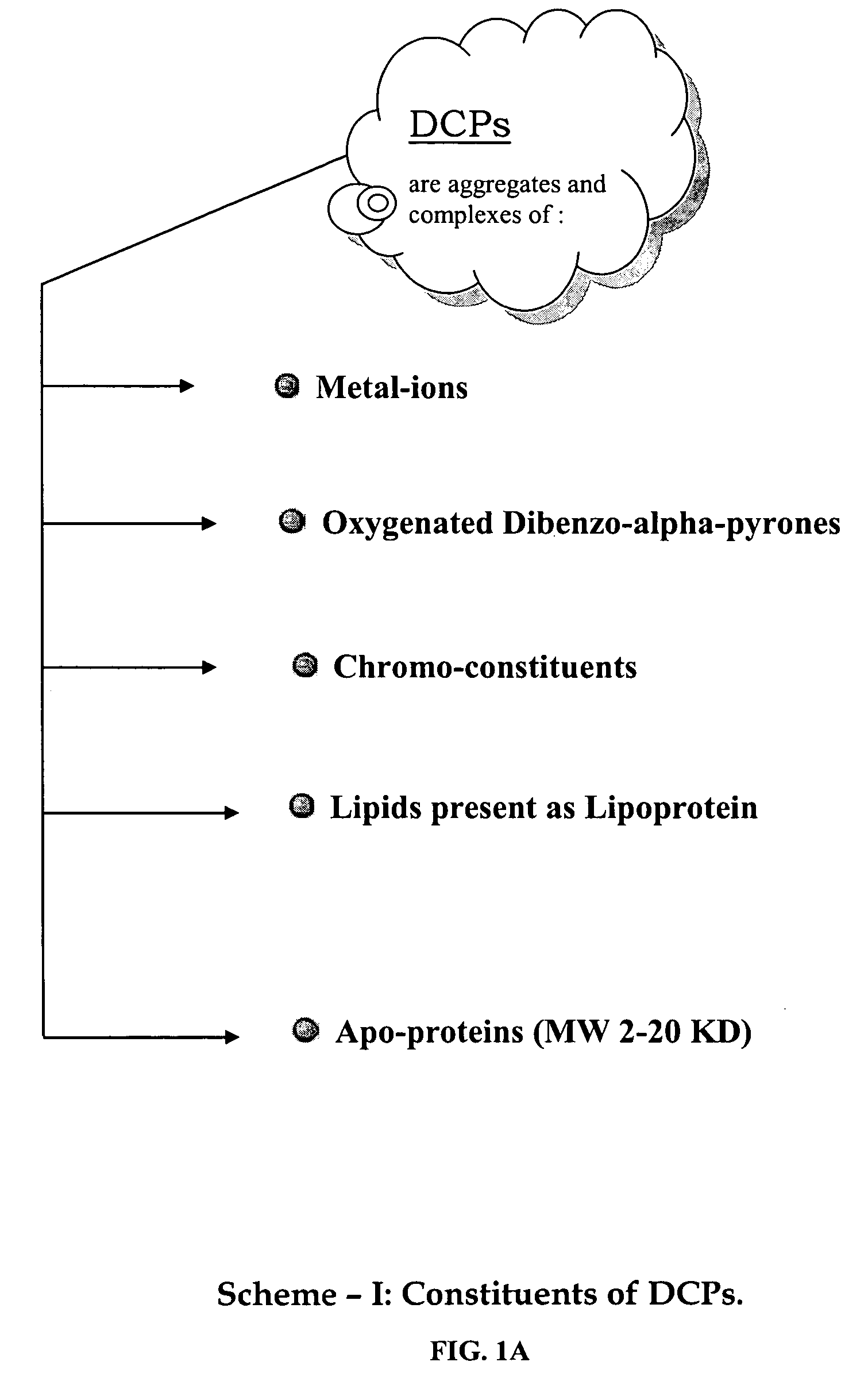
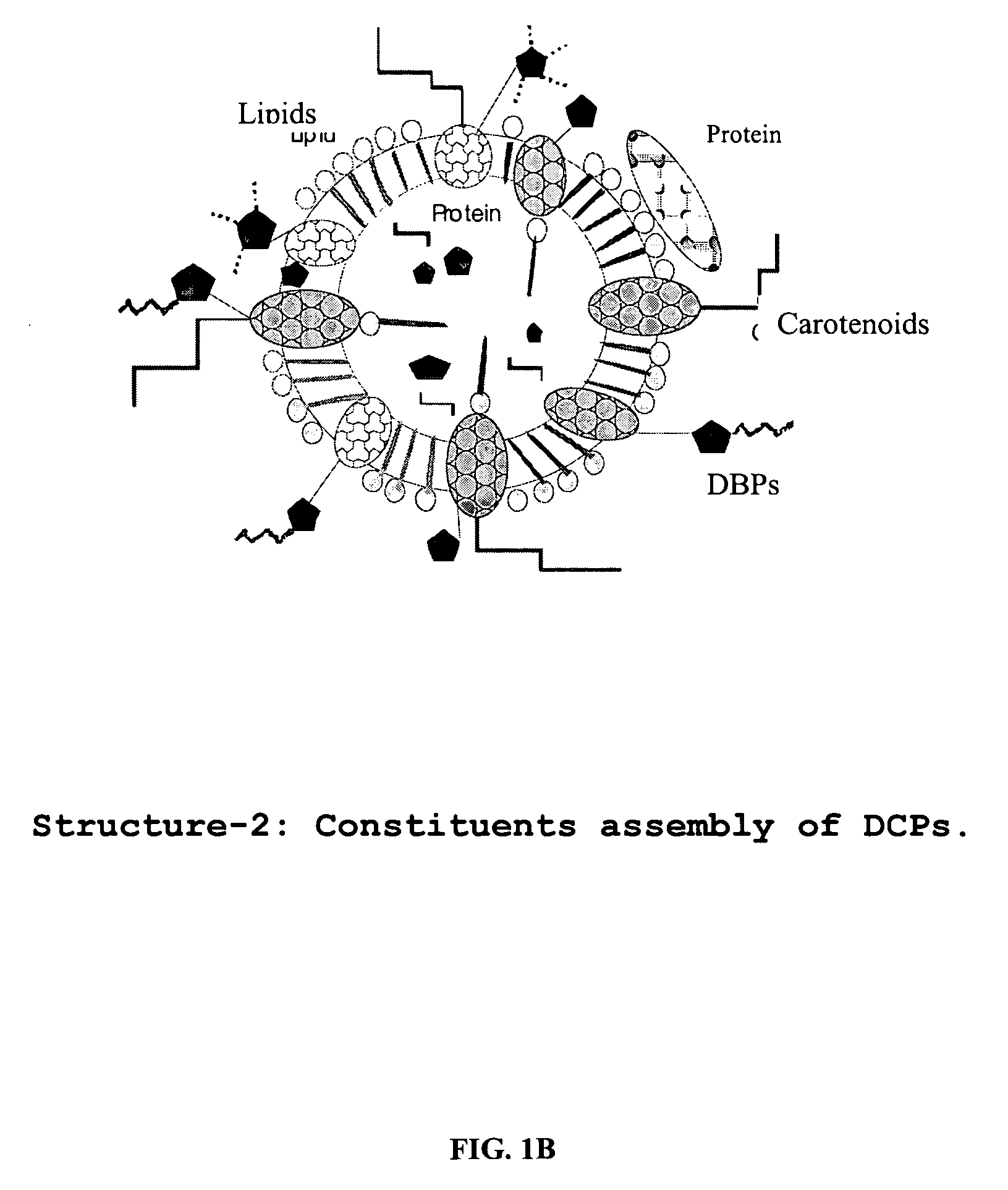
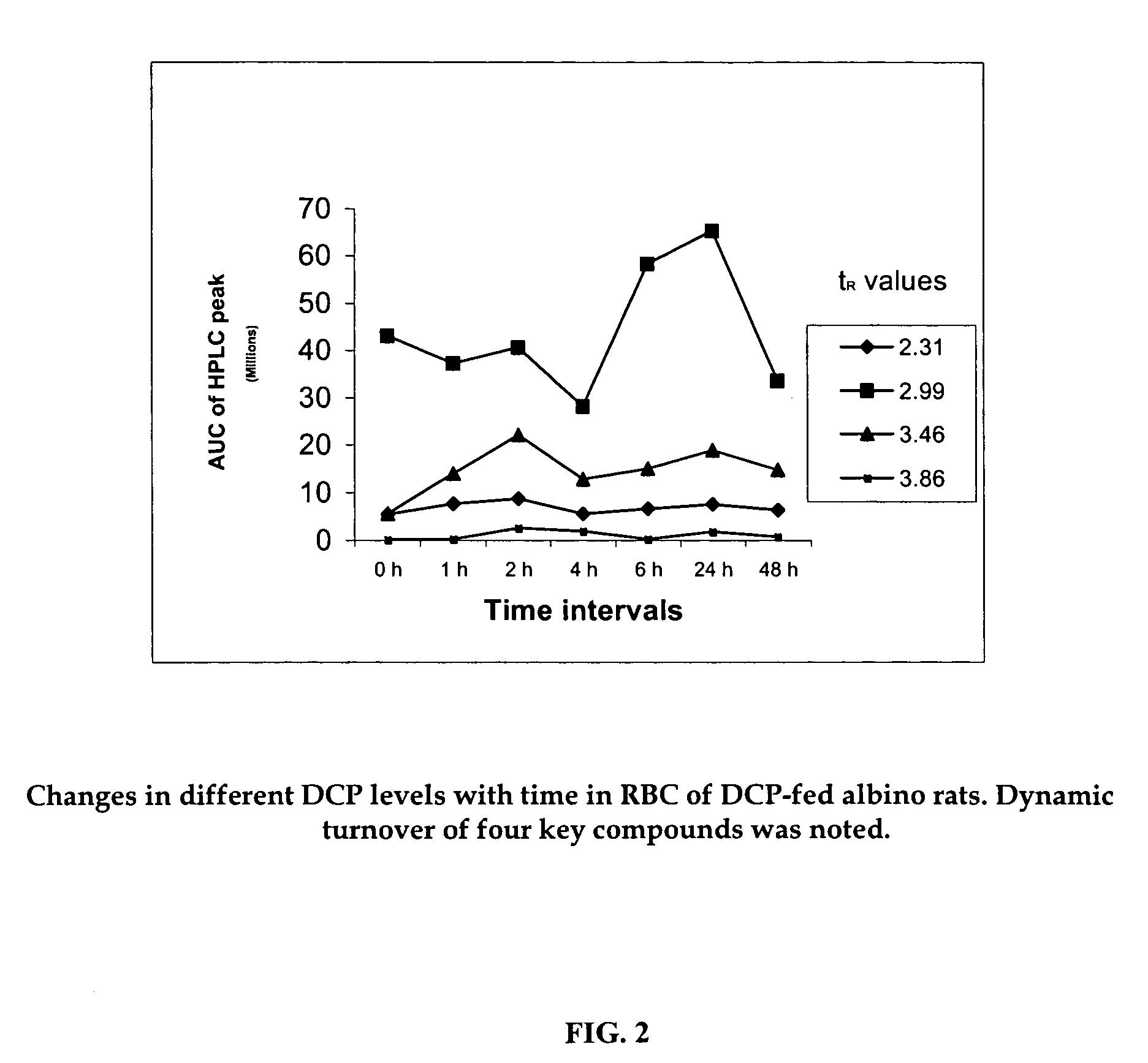
Oxygenated dibenzo-alpha-pyrone chromoproteins
InactiveUS20050245434A1Increasing cognition learningIncrease awarenessCosmetic preparationsHair cosmeticsPersonal careSterol
A composition of oxygenated dibenzo-alpha-pyrone chromoproteins (DCP) and their isolation from shilajit, fossils of ammonites, corals and other invertebrates. More particularly, to the description of DCP-composition comprising oxygenated dibenzo-alpha-pyrone or its conjugates, phosphocreatine, proteins, fatty acyl esters of glycerol and other small ligands, e.g., carotenoids, sterols and aromatic acids, as core structural fragments, and their biological functions. Pharmaceutical, nutritional, skin care and personal care formulations are also described. These findings establish DCPs as the major bioactives of shilajit.
Owner:NATREON INC
Creatine phosphate sodium preparation method
ActiveCN102633833AReduce dosageReduce pollutionGroup 5/15 element organic compoundsSodium methoxidePhosphate
The invention relates to a creatine phosphate sodium preparation method, which includes the following steps of a), subjecting phosphorus oxychloride and water to be reacted at the temperature ranging from 0 DEG C to 10 DEG C for 2-3 hours to generate chlorophosphate; b), subjecting S-methyl isothiourea sulfate and chlorophosphate to react in dipole non-proton solvent to generate phosphorylated S-methyl isothiourea; c), dissolving the phosphorylated S-methyl isothiourea by adding water to be reacted with sarcosine to generate phosphocreatine; and d), adding sodium hydroxide orsodium methylate to the phosphocreatine to adjust the pH (potential of hydrogen) value to be 8-9, adding absolute ethyl alcohol, crystallizing, filtering and drying in vacuum to obtain creatine phosphate sodium. Phosphate group is introduced by preparing active chlorophosphate by phosphorus oxychloride, so that usage of phosphorus oxychloride can be reduced greatly, and environment pollution is alleviated correspondingly. The creatine phosphate sodium preparation method is a barium-free process, has no requirement for introducing heavy metal, safe, simple in post-treatment, high in yield and low in production cost, and byproducts are gases.
Owner:NANJING CHENGONG PHARM CO LTD
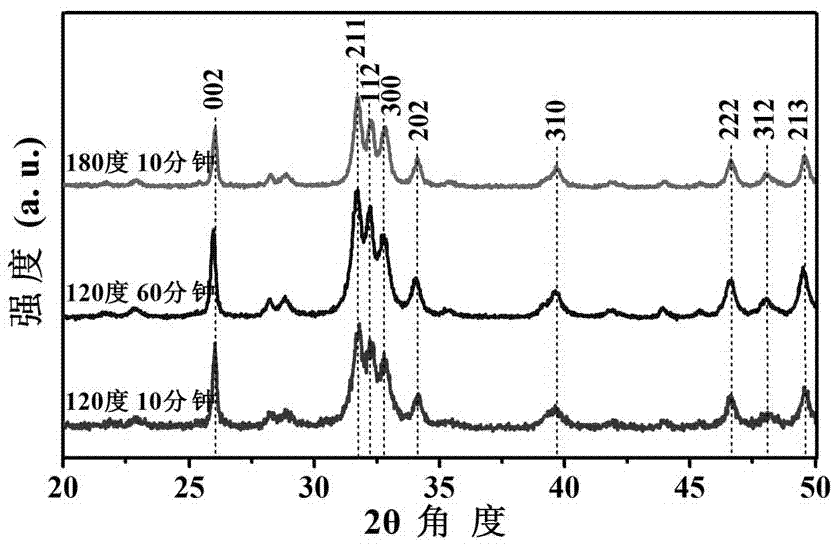
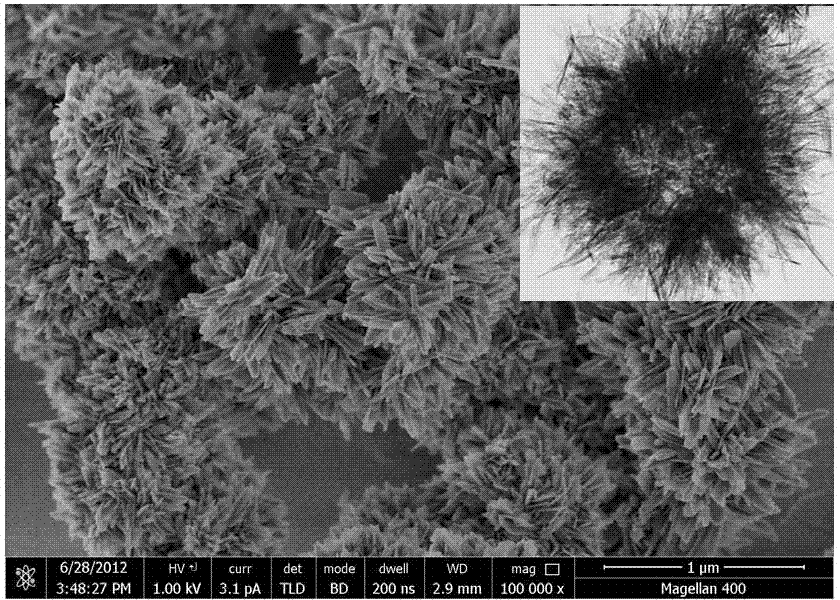
Nano-rod-like structure hydroxyapatite and preparation method thereof
ActiveCN106966375AControl phaseSmall sizeMaterial nanotechnologyPhosphorus compoundsPhosphateControllability
The present invention relates to the field of biomaterial preparation, and provides nano-rod-like structure hydroxyapatite and a preparation method thereof. According to the preparation method, calcium lactate is adopted as a calcium source, creatine phosphate is adopted as a phosphorus source, and a hydrothermal reaction is performed to prepare the nano-rod-like structure hydroxyapatite. According to the present invention, the nano-rod-like structure hydroxyapatite has controlled appearance and controlled size, can be used as the biomedical material in the fields of drug delivery, bone defect repair and the like, and has good application prospect; the nano-rod-like structure hydroxyapatite is prepared by using the hydrothermal method, such that the reaction controllability is good; and the preparation process is simple, the operation is convenient, the complicated and expensive equipment is not needed, and the method is expected to achieve the industrial production.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Preparation technique of sodium phosphocreatine powder and injection preparation
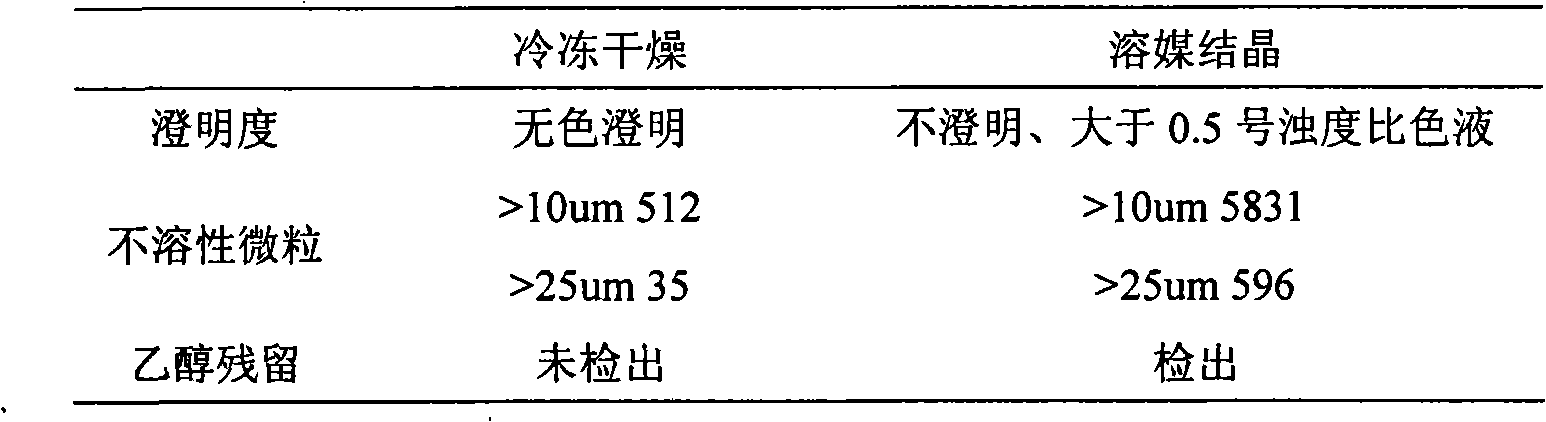
InactiveCN101288649AHigh claritySimple and fast operationOrganic active ingredientsPowder deliveryActivated carbonVacuum pumping
The invention relates to a preparation process of a creatine phosphate sodium powder injection, which is characterized in that, the freeze-drying process is adopted for preparation, the process is that: 1 part of the formulated amount of creatine phosphate sodium is taken, 3 to 6 parts of water for injection is added, the creatine phosphate sodium is dissolved by stirring, 0.1 percent of the solution amount of activated carbon with needle use is added, the filtration for carbon removal and sterilization is carried out, thus forming clarified liquid, the water for injection is supplemented till the sufficient amount, a micro-porous membrane of 0.22Mum is used for filtration, and the filling is carried out after the content is 90 percent to 110 percent by measurement; the freeze-drying and the cover pressing are carried out; the well filled injection is arranged in a freeze-drying machine, the pre-freezing is firstly carried out till minus 50 DEG C to minus 35 DEG C, the temperature is kept for 1 to 5 hours, the vacuum pumping is carried out, the temperature raises to minus 10 DEG C to minus 3 DEG C within 15 to 30 hours, the temperature further raises to 15 to 50 DEG C within 2 to 10 hours, and the temperature is continuously kept for 3 to 10 hours, thus obtaining the creatine phosphate sodium powder injection. The preparation process has the advantages that the preparation process does not adopt ethanol, the clarity of the injection liquid is good, the operation is simple and the preparation process is applicable to mass production.
Owner:上海慈瑞医药科技股份有限公司
Oxygenated dibenzo-alpha-pyrone chromoproteins
InactiveUS20050233942A1Increasing cognition learningIncrease awarenessBiocideNervous disorderPersonal careSterol
A composition of oxygenated dibenzo-alpha-pyrone chromoproteins (DCP) and their isolation from shilajit, fossils of ammonites, corals and other invertebrates. More particularly, to the description of DCP-composition comprising oxygenated dibenzo-alpha-pyrone or its conjugates, phosphocreatine, proteins, fatty acyl esters of glycerol and other small ligands, e.g., carotenoids, sterols and aromatic acids, as core structural fragments, and their biological functions. Pharmaceutical, nutritional, skin care and personal care formulations are also described. These findings establish DCPs as the major bioactives of shilajit.
Owner:NATREON INC
Method for preparing high-content creatine phosphate disodium salt
ActiveCN102702253AImprove securityImprove effectivenessGroup 5/15 element organic compoundsHalohydrocarbonAqueous sodium hydroxide
The invention discloses a method for preparing high-content creatine phosphate disodium salt, comprising the steps as follows: (1) conducting a condensation reaction on creatinine and phosphorus oxytrichloride to obtain crude creatinine phosphorus oxychloride; (2) dissolving the crude creatinine phosphorus oxychloride in a solvent A, then stirring and filtering, adding a solvent B to the filtrate to separate out the crystal and obtain fine creatinine phosphorus oxychloride; (3) hydrolyzing fine creatinine phosphorus oxychloride in sodium hydroxide aqueous solution to obtain the high-content creatine phosphate disodium salt, wherein the solvent A is halohydrocarbon and the solvent B is aliphatic hydrocarbon.
Owner:SHANGHAI LONGXIANG BIO MEDICINE DEV CO LTD
Novel eukaryotic cell-free protein expression system that does not require an artificial energy regeneration system
ActiveUS20180245087A1Improved in vitro synthesisOptimize the reaction systemFermentationVector-based foreign material introductionCreatine kinasePhosphate
This disclosure concerns the systems, methods, and kits for the in vitro synthesis of biological macromolecules in a reaction utilizing cell lysates containing plastids, mitochondria and / or chloroplasts, wherein creatine phosphate and creatine kinase are not added to the reaction to provide artificial energy regeneration.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV +1
Angiotensin receptor antagonist and creatine phosphate sodium complex and uses thereof
ActiveCN106474479AGood treatment effectStable in natureMetabolism disorderGroup 5/15 element organic compoundsTasosartanValsartan
The present invention provides a an angiotensin receptor antagonist and creatine phosphate sodium complex and uses thereof, wherein the complex comprises an angiotensin receptor antagonist and creatine phosphate sodium, a molar ratio of the angiotensin receptor antagonist to the creatine phosphate sodium is 1:1-2, and the angiotensin receptor antagonist is selected from valsartan, losartan, irbesartan, telmisartan, eprosartan, candesartan, olmesartan, saprisartan, tasosartan, and elisartan. According to the present invention, the complex is formed by compounding the angiotensin receptor antagonist and the creatine phosphate sodium, and provides the unexpected double effect and the synergistic effect for treatment of heart failure and high blood pressure, the cocrystallization salt hydrate formed by linking the hydrogen bond has the stable characteristic, the pharmacokinetic property is significantly provided, and the positive application prospects are provided in the fields of anti-high blood pressure treatment and anti-heart failure treatment.
Owner:珠海赛隆药业股份有限公司(长沙)医药研发中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com