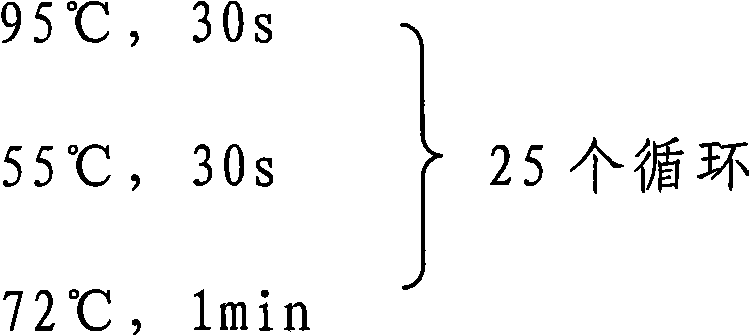Bioengineering method for synthesizing sodium phosphocreatine
A creatine phosphate and bioengineering technology is applied in the field of preparation of creatine kinase to achieve the effects of low process cost and improved controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
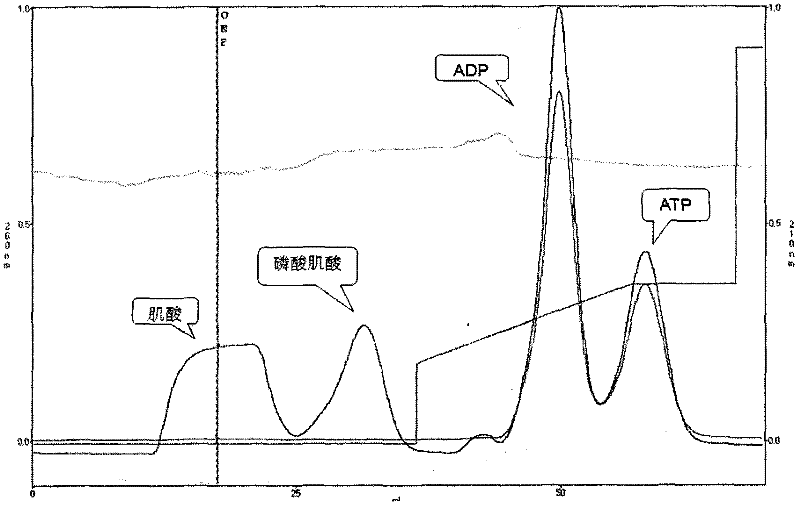
Image
Examples
Embodiment 1
[0012] RNA was extracted from human brain samples by conventional methods, and human brain cDNA was obtained by reverse transcription. Using the primers described in claim 6 as primers, the CKBB gene was fished from human brain cDNA by PCR method. The specific PCR conditions are
[0013] 95℃, 5min;
[0014]
[0015] 72℃, 10min
[0016] The product was detected by agarose gel electrophoresis, and the size was 1109bp, which was consistent with the prediction.
[0017] The above CKBB gene was digested with restriction endonucleases NdeI and XhoI, and after recovery, it was ligated with the vector pET28a digested with NdeI and XhoI, transformed into Escherichia coli, and the transformant with kanamycin resistance was screened. After plasmid extraction and enzyme digestion identification, it was proved that the recombinant protein CKBB gene had been cloned into pET28a.
[0018] The pET28a-CKBB was transformed into BL21(DE3) by CaCl2 method, the transformants were screened on...
Embodiment 2
[0020] The recombinant engineered bacterium gained in embodiment 1 inserts culture medium according to 2% inoculum (containing tryptone 10g / L, yeast extract 10g / L, K 2 HPO 4 3H 2 O 15g / L, NaH 2 PO 4 2H 2 O 10g / L, 1ml defoamer. Sterilize at 121°C for 20 minutes, add kanamycin to a final concentration of 50 mg / L before inoculation, and sterile MgSO 4 To a final concentration of 0.8g / L, sterile glucose to a final concentration of 2g / L, a sterile trace element concentrate 10ml / L, and the pH value adjusted to 6.55) in a 5L fermenter for fed-batch high-density fermentation, the pH value Controlled at 6.55±0.05, after 5 hours of incubation at 37°C, the working concentration of IPTG induction was 0.1mM. After 20 hours of induction, the bacteria were collected by centrifugation and stored in a -20°C refrigerator for later use.
Embodiment 3
[0022] Weigh 10 g of the bacterium obtained in Example 2, dilute to 100 g / L with phosphate buffer, place in an ice-water bath, use an ultrasonic cell disruptor, set the ultrasonic power to 30%, work for 4 seconds and stop for 6 seconds, and circulate for 1 hour. Obtain 100ml of bacteriostasis solution. It was centrifuged at 4° C. and 12000 rpm for 1 hour, and the centrifuged supernatant was collected. Load the sample to a nickel affinity chromatography column (2.6 / 10cm) that has been equilibrated with 50mM phosphate buffer, equilibrate the column with the same buffer, and elute the impurity on the column with a buffer containing 20mM imidazole , and finally the target protein on the column was eluted with a buffer containing 300 mM imidazole, the eluate was collected, dialyzed with 20 mM phosphate buffer, and freeze-dried to obtain a recombinant creatine kinase preparation. Measure the concentration and specific activity of the enzyme preparation, and store it in a -20°C refr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com