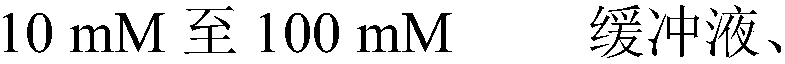Patents
Literature
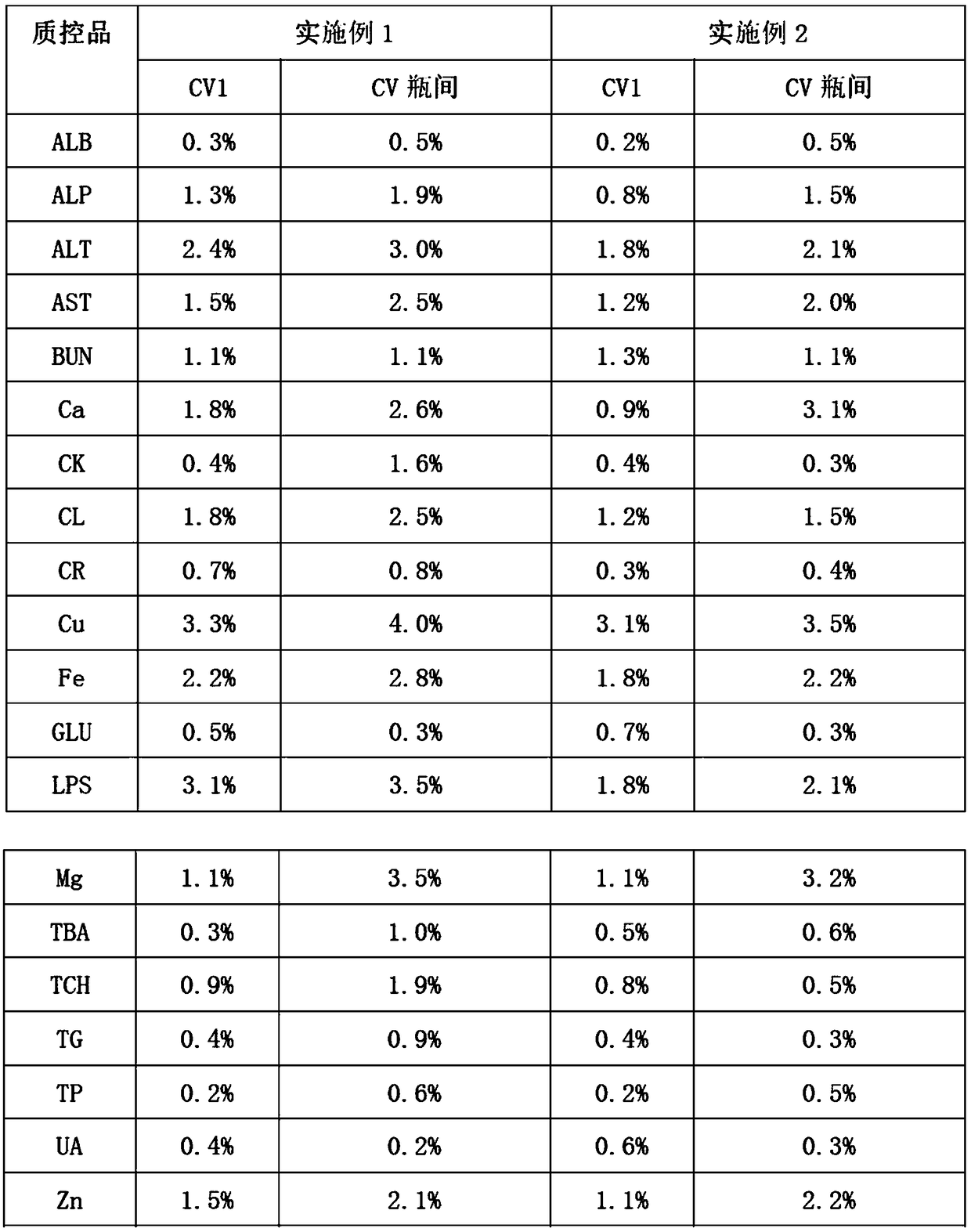
179 results about "Creatine kinase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
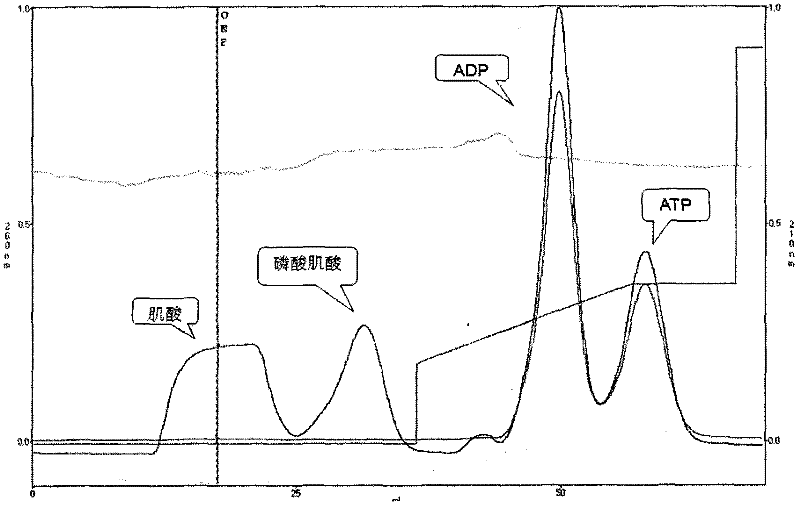
Creatine kinase (CK), also known as creatine phosphokinase (CPK) or phosphocreatine kinase, is an enzyme (EC 2.7.3.2) expressed by various tissues and cell types. CK catalyses the conversion of creatine and uses adenosine triphosphate (ATP) to create phosphocreatine (PCr) and adenosine diphosphate (ADP). This CK enzyme reaction is reversible and thus ATP can be generated from PCr and ADP.
Methods and compositions for the diagnosis of diseases of the aorta
InactiveUS20070224643A1Facilitate patient treatmentConvenient treatmentDiagnosticsSurgeryAortic dissectionSmooth Muscle Myosins
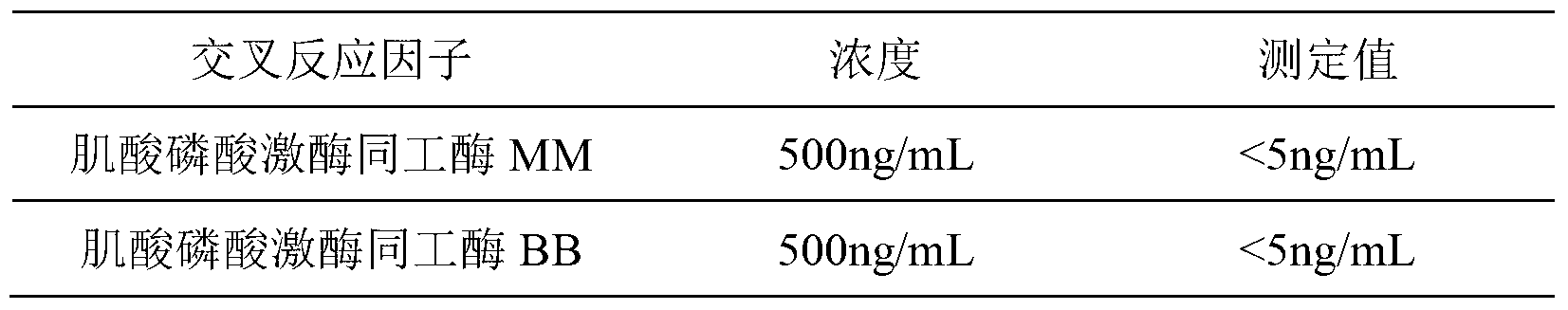
The present invention relates to methods and compositions for symptom-based differential diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to the use of biomarkers, either individually or in combinations with one another to rule in or out diseases of the aorta and its branches, most particularly aortic aneurysm and / or aortic dissection, and for risk stratification in such conditions. Preferred markers include one or more of creatine kinase-BB (CK-BB), creatine kinase-MB (CK-MB), acidic calponin, basic calponin, B-type natriuretic peptide (BNP), NT-proBNP, proBNP, BNP79-108, BNP3-108, caldesmon, caspase-3, D-dimer, soluble elastin fragments, endothelial cell-selective adhesion molecule (ESAM), fibrillin-1, heart-type fatty acid binding protein, MMP-9, myeloperoxidase, myoglobin, smooth muscle myosin, smooth muscle myosin heavy chain, TIMP-1, free cardiac troponin I, complexed cardiac troponin I, free and complexed cardiac troponin I, free cardiac troponin T, complexed cardiac troponin T, and free and complexed cardiac troponin T, and preferred assays are configured to detect these markers.
Owner:BIOSITE INC
Compositions containing a combination of a creatine compound and a second agent
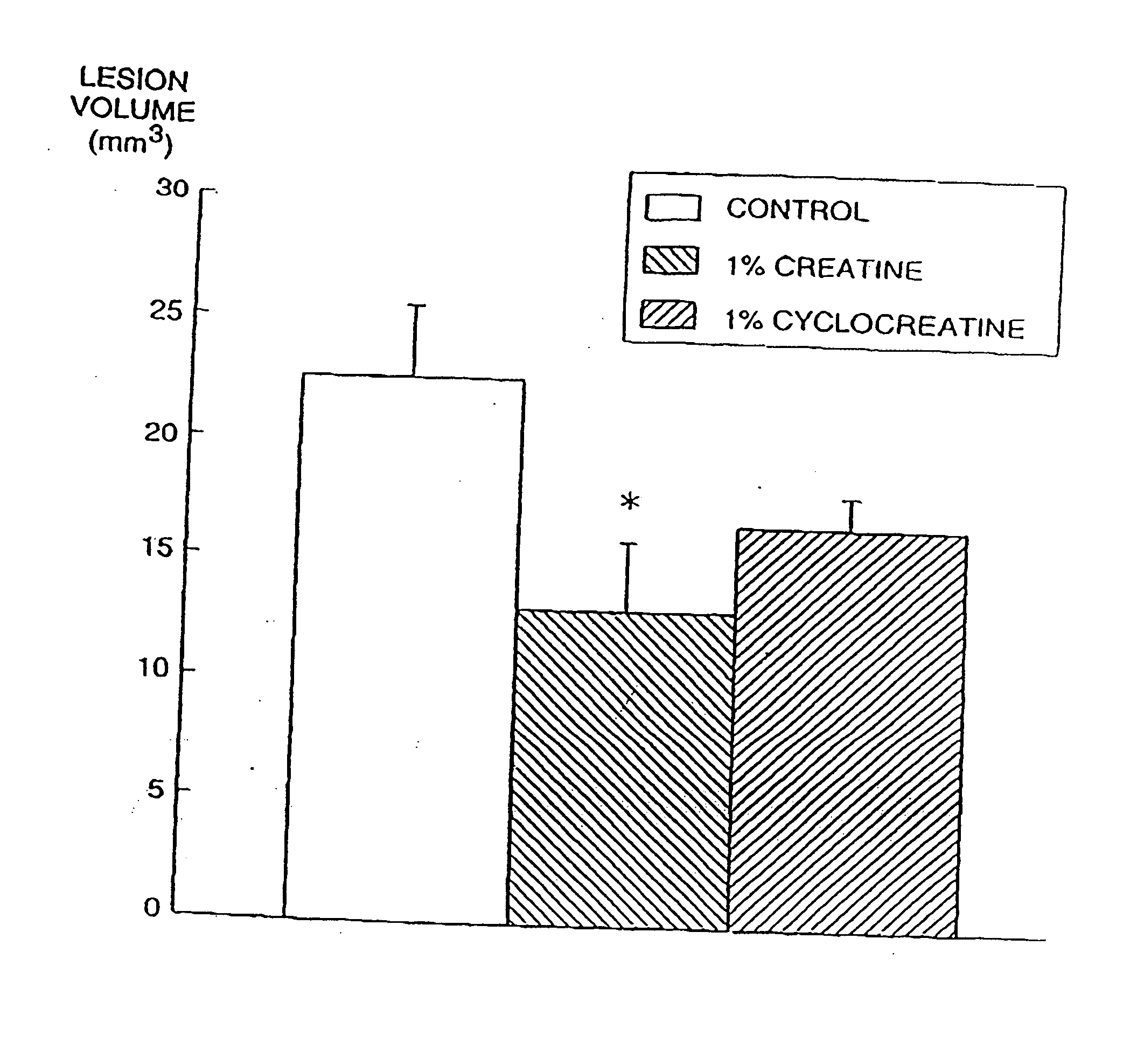
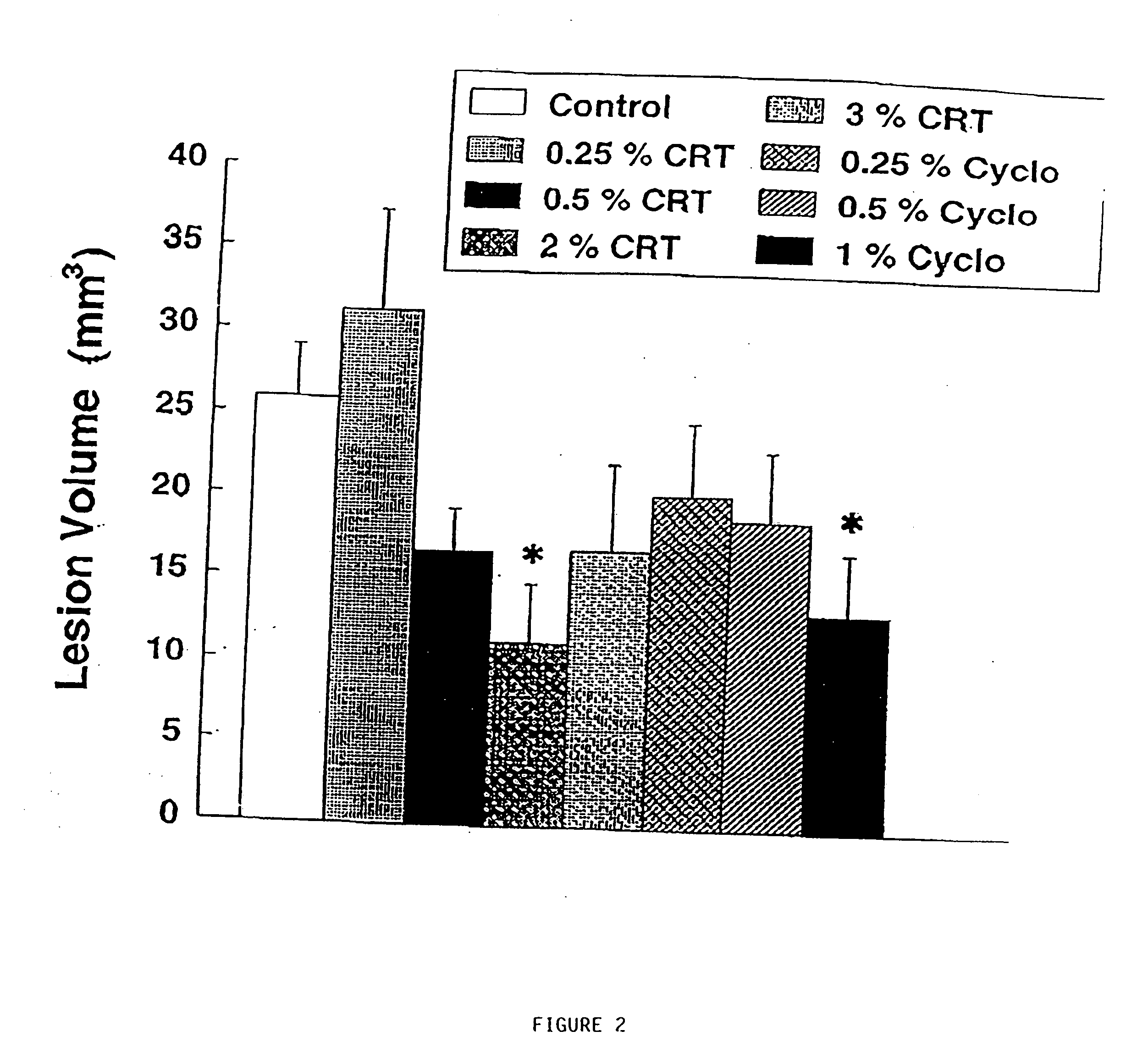
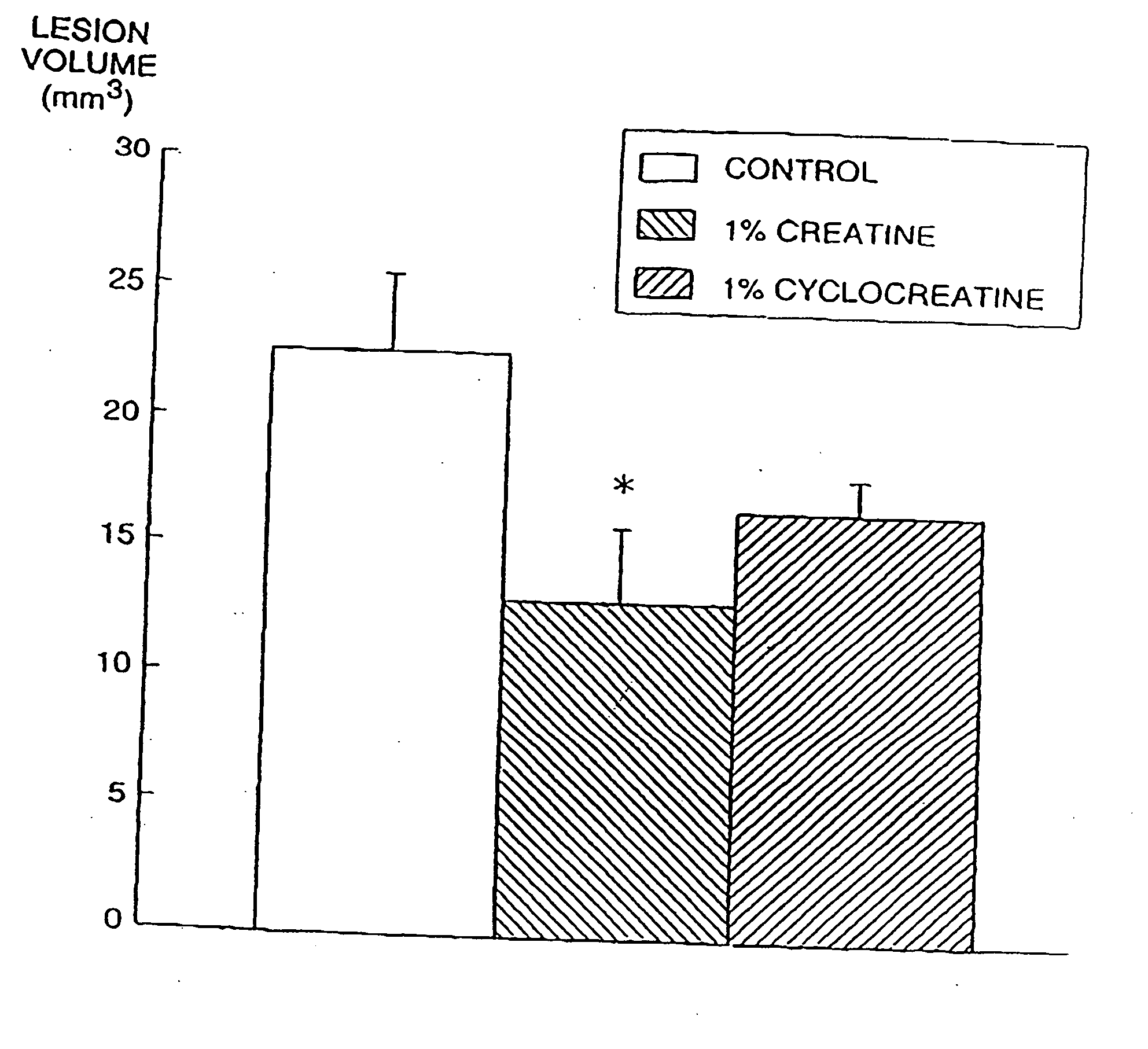
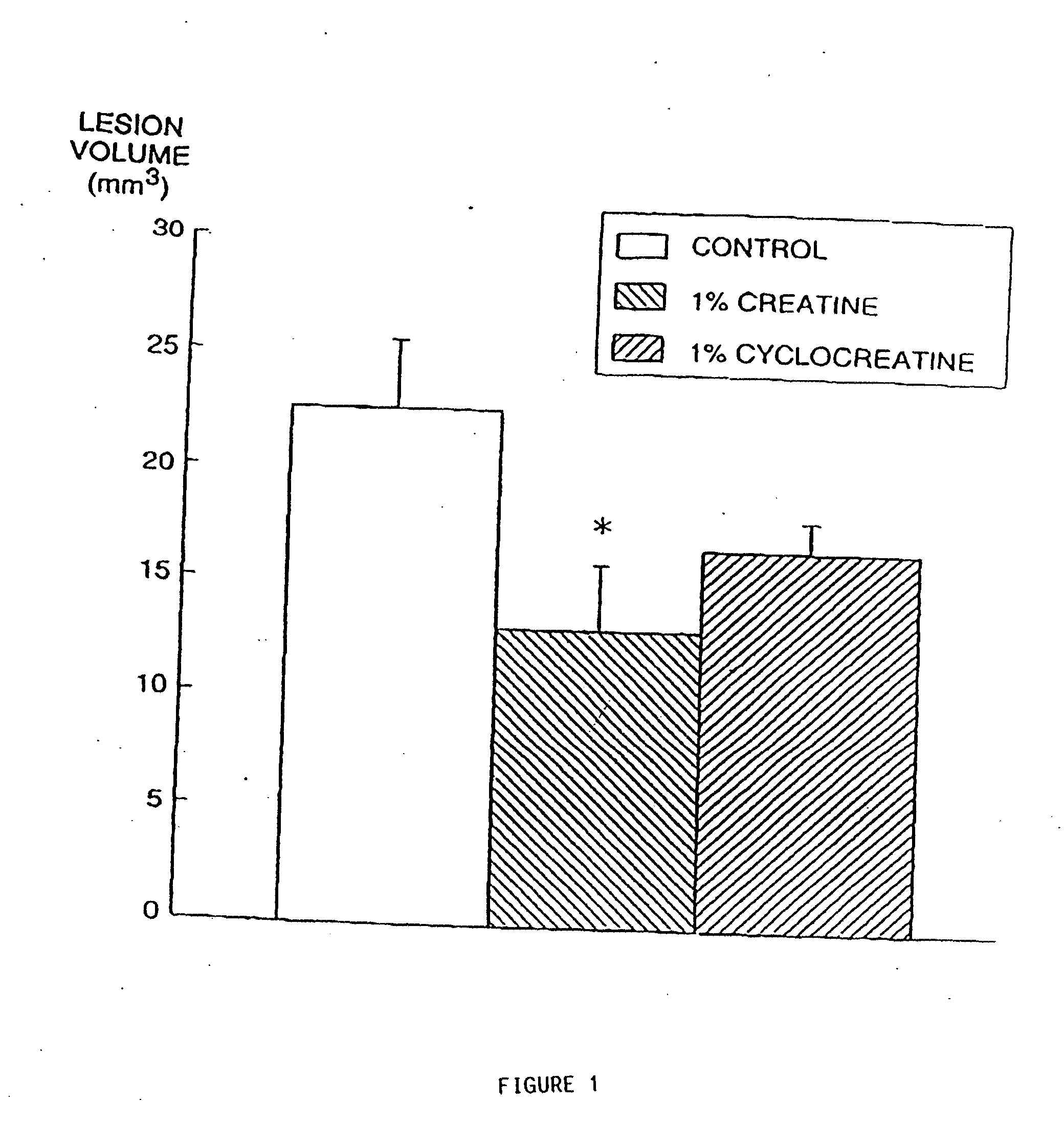
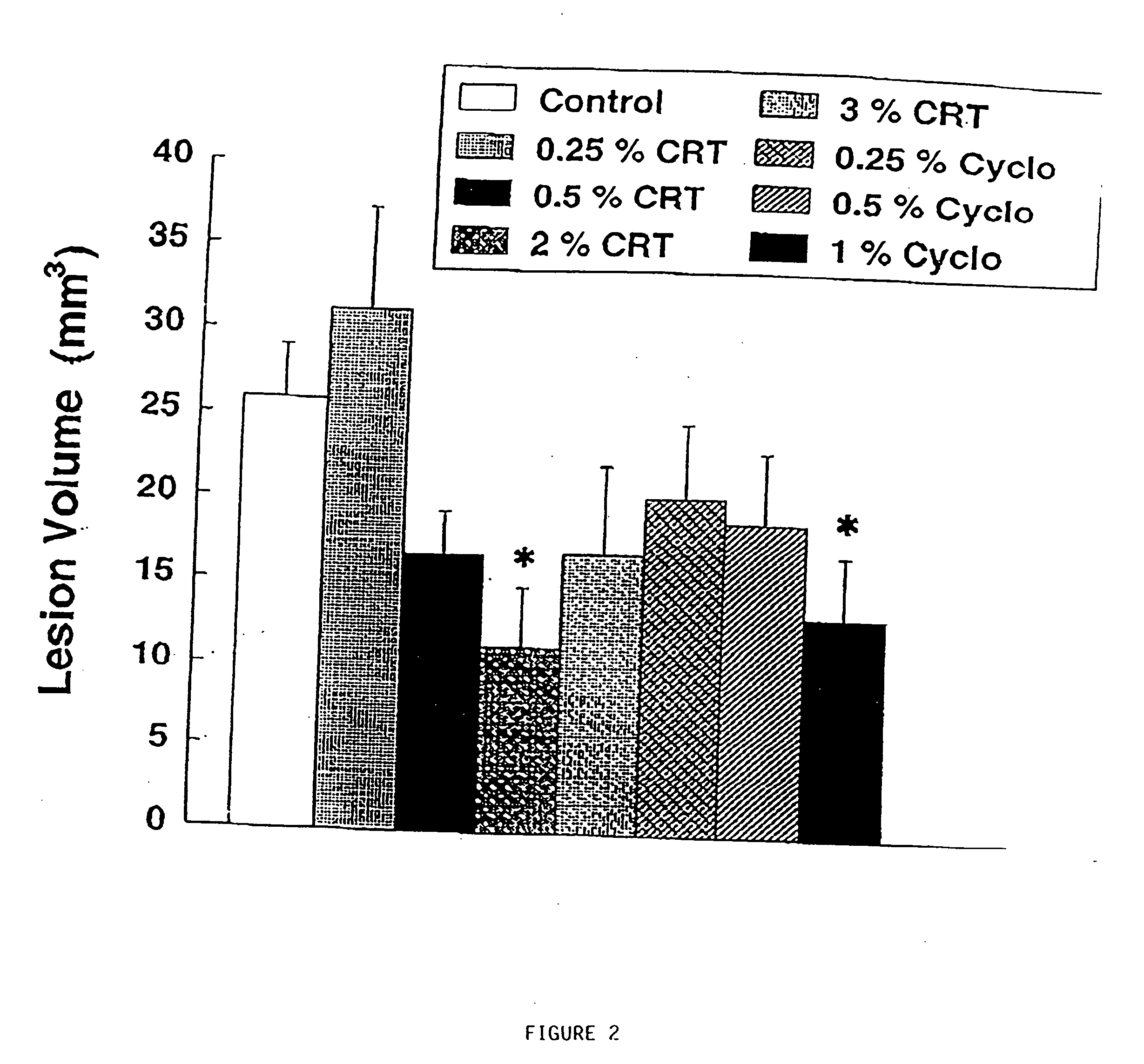
The present invention relates to the use of creatine compound and neuroprotective combinations including creatine, creatine phosphate or analogs of creatine, such as cyclocreatine, for treating diseases of the nervous system. Creatine compounds in combination with neuroprotective agents can be used as therapeutically effective compositions against a variety of diseases of the nervous system such as diabetic and toxic neuropathies, peripheral nervous system diseases, Alzheimer disease, Parkinson's disease, stroke, Huntington's disease, amyotropic lateral sclerosis, motor neuron disease, traumatic nerve injury, multiple sclerosis, dysmyelination and demyelination disorders, and mitochondrial diseases. The creatine compounds which can be used in the present method include (1) creatine, creatine phosphate and analogs of these compounds which can act as substrates or substrate analogs for creatine kinase; (2) bisubstrate inhibitors of creatine kinase comprising covalently linked structural analogs of adenosine triphosphate (ATP) and creatine; (3) creatine analogs which can act as reversible or irreversible inhibitors of creatine kinase; and (4) N-phosphorocreatine analogs bearing non-transferable moieties which mimic the N-phosphoryl group.
Owner:THE GENERAL HOSPITAL CORP
Quantitative creatine kinase-myoglobin (CK-MB) determination kit and assay method thereof
InactiveCN102435738AHigh detection sensitivityImprove featuresChemiluminescene/bioluminescenceCreatine kinaseQuality control
The invention discloses a quantitative creatine kinase-myoglobin (CK-MB) determination kit. The kit is characterized in that the kit contains CK-MB magnetic separation reagent, enzymatic reactant, reaction enhancer, diluent, CK-MB calibrator, CK-MB quality control material, cleaner concentrate and substrate solution. The invention also discloses a preparation method for the kit. The kit integrates the chemiluminescence technology with immunomagnetic beads to provide a homogeneous phase-approximating reaction system. Compared with the prior art, the kit has higher assay sensitivity and specificity, and achieves better performance parameters, and mover, the product cost is greatly reduced.
Owner:INNER MONGOLIA KEHUI BIOLOGICAL TECH
Creatine jubase MB isozyme activity detection reagent and preparation method thereof
ActiveCN102154443AHigh precisionImprove accuracyMicrobiological testing/measurementAntiendomysial antibodiesCreatine kinase
The invention discloses a creatine jubase MB isozyme activity detection reagent which comprises sulfide-oxidizing coenzyme, 6-phosphaogluconate dehydrogenase, sulfhydryl reagent, magnesium salt, glucokinase or hexoxinase, anti-CK-M antibody, phosphocreatine, adenosine diphosphate, glucose and glucose-6-phosphate dehydrogenase. The preparation method of the reagent is as follows: dissolving all components in a buffer solution with pH of 5.0 to 9.5. The creatine jubase MB isozyme activity detection reagent can enlarge detection sensitivity several times; and the determination result has high precision and degree of accuracy.
Owner:浙江东瓯诊断产品有限公司
Detection kit for quickly identifying donkey skin, horse skin and mule skin
ActiveCN104046700AIncrease productionHigh purityMicrobiological testing/measurementCreatine kinaseFluorescence
The invention discloses a detection kit for quickly identifying donkey skin, horse skin and mule skin, which is used for accurately identifying donkeys, horses, mules and jennets by creatively applying 16SrRNA genes of CKM (creatine kinase) karyogene (nDNA) and mitochondrial genome DNA (mtDNA) at the same time from genetic background of the horses, the donkeys and the mules. The kit adopts an molecular beacon probe (MB) method to carry out a quintuple multicolor fluorescence quantitative PCR (polymerase chain reaction) detection technology, and also can detect donkey, horse, mule and jennet components; and moreover, the kit is added with exogenous internal reference as an interior label, can detect and avoid false negative results produced by PCR inhibitor contained in a sample. The quintuple multicolor fluorescence quantitative PCR is used for detecting in the same tube without opening a cover, so that the detection kit is not easy to pollute, and is accurate and stable, simple to operate, extremely high in sensitivity, strong in specificity, and the like, and therefore, a novel way is explored for identifying the donkey skin, horse skin and mule skin.
Owner:VEGETABLE RES INST OF SHANDONG ACADEMY OF AGRI SCI
Multi-index protein chip inspection reagent unit of cardiovascular disease diagnosis and prediction
InactiveCN1854737ALuminescent compositionsMaterial analysisCreatine kinaseGlycogen phosphorylase isoenzyme BB
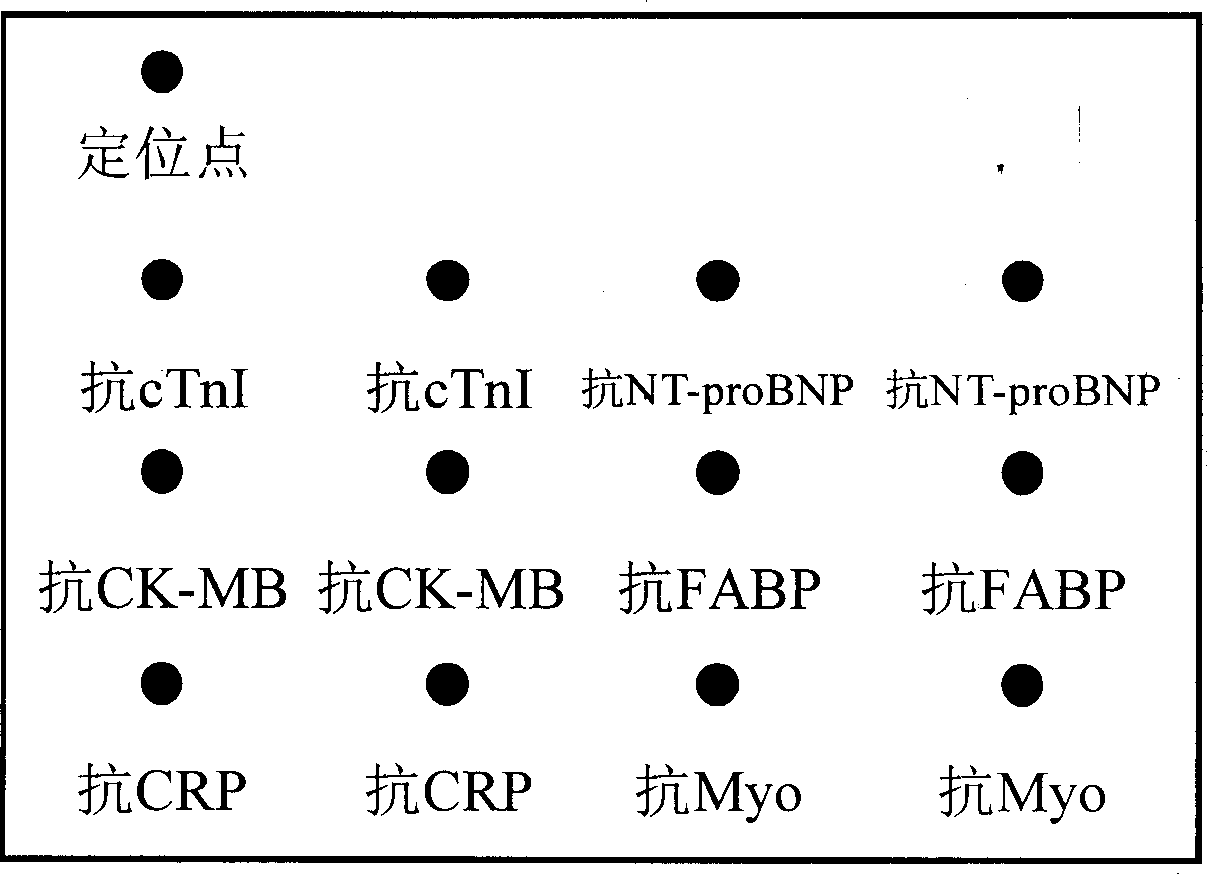
A detection kit of multiindex protein for diagnosing cardiovascular disease is prepared as having base plate and reaction holes on it set on reaction hole plate; including 12-200 sample holes and 5-8 standard piece holes in said reaction holes as each reaction hole bottom being set with solid phase carrier; enveloping micro lattice of eight antibody as anti-CRP, anti-Myoglobin, anti-cTnI, anti-cTnT, anti-NT-proBNP, anti-CK-MB, anti-H-FABP and anti-GPBB on said carrier for accurately diagnose said disease in multiple man-share.
Owner:穆海东
Compositions containing a combination of a creatine compound and a second agent
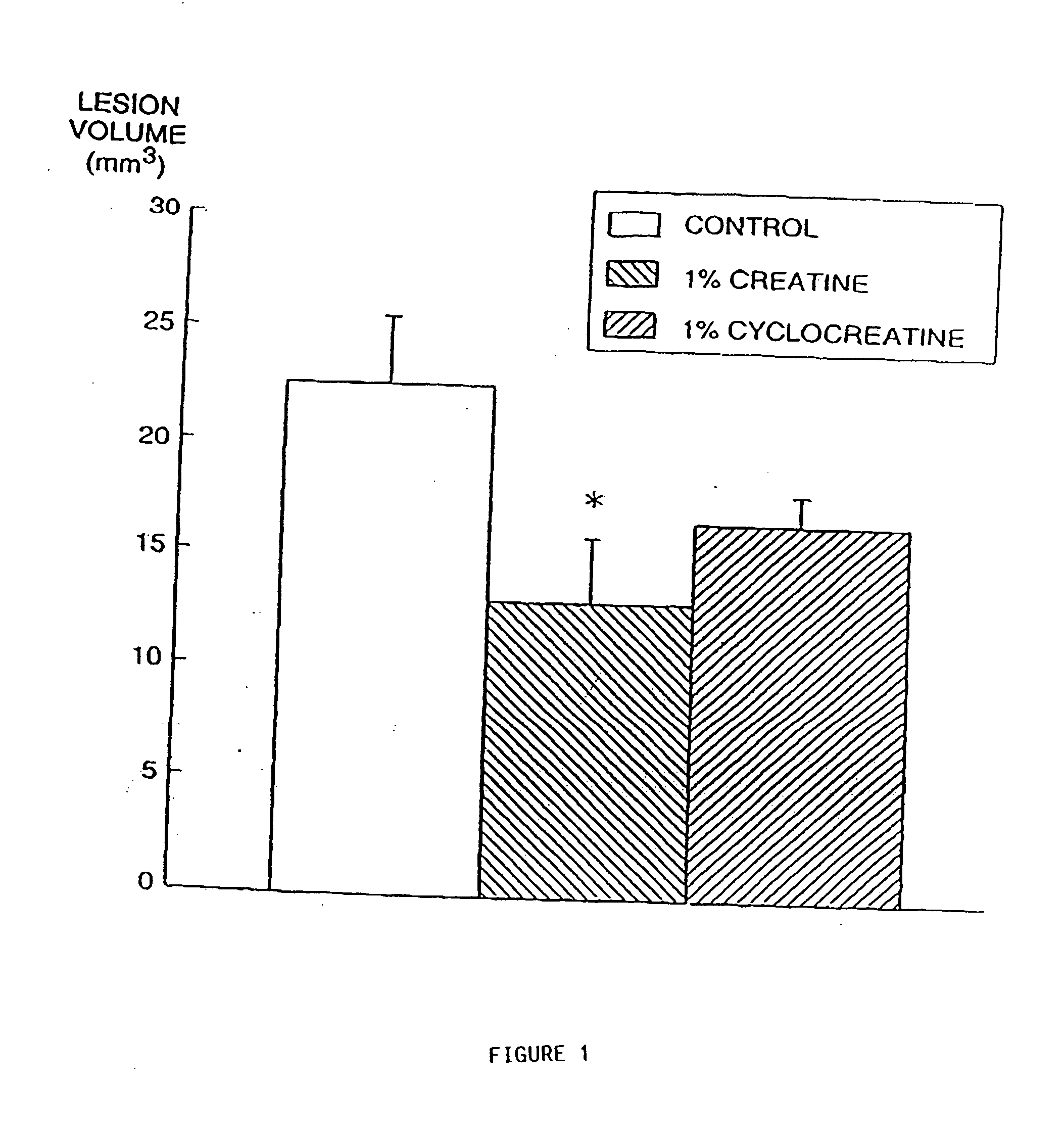
The present invention relates to the use of creatine compound and neuroprotective combinations including creatine, creatine phosphate or analogs of creatine, such as cyclocreatine, for treating diseases of the nervous system. Creatine compounds in combination with neuroprotective agents can be used as therapeutically effective compositions against a variety of diseases of the nervous system such as diabetic and toxic neuropathies, peripheral nervous system diseases, Alzheimer disease, Parkinson's disease, stroke, Huntington's disease, amyotropic lateral sclerosis, motor neuron disease, traumatic nerve injury, multiple sclerosis, dysmyelination and demyelination disorders, and mitochondrial diseases. The creatine compounds which can be used in the present method include (1) creatine, creatine phosphate and analogs of these compounds which can act as substrates or substrate analogs for creatine kinase; (2) bisubstrate inhibitors of creatine kinase comprising covalently linked structural analogs of adenosine triphosphate (ATP) and creatine; (3) creatine analogs which can act as reversible or irreversible inhibitors of creatine kinase; and (4) N-phosphorocreatine analogs bearing non-transferable moieties which mimic the N-phosphoryl group.
Owner:THE GENERAL HOSPITAL CORP
Method of treating insulin resistance, adult onset diabetes and metabolic syndrome x
InactiveUS20050287197A1Little riskReduce absorptionNervous disorderPeptide/protein ingredientsCreatine kinaseCreatine kinase.MB
A method of treating insulin resistance, adult onset diabetes, and metabolic syndrome X and its related complications, in mammalian subject is accomplished by intravenously administering to a mammalian subject, a therapeutically effective amount of a liposomal suspension of lipoprotein small unilamellar vesicles (SUVs) comprising predominantly phospholipids. The liposomal suspension is administered over a period of time, whereby in the levels of some or all of blood glucose, insulin, total cholesterol, LDL cholesterol, triglyceride, creatine kinase (CK), creatine kinase-MB (CK-MB), Hb-A1c, lipoprotein (a), SGOT and SGPT fall back within the normal range or are significantly reduced.
Owner:KURTZ SEYMOUR J
Physical fatigue alleviating beverage
ActiveCN103918965ARelieve physical fatigueReduce decompositionSugar food ingredientsNatural extract food ingredientsCreatine kinaseDecomposition
The invention relates to a physical fatigue alleviating composition containing active ingredients. The active ingredients contain: a Maca extract, oligopeptide and saccharides. The composition provided by the invention can alleviate fatigue, and the mechanism of the composition is related to effective reduction of protein decomposition, avoidance of after-exercise creatine kinase increase and decrease of exercise metabolite lactic acid, has better effects than the prior art, and has a synergistic effect.
Owner:BEIJING TONGRENTANG HEALTH PHARMA
Kit for chemiluminescence immunity quantitative detection of CK-MB (creatine kinase- isoenzyme) nano magnetic particle and preparation method of kit
ActiveCN103278623ANo cross-reactivityStrong specificityBiological testingCreatine kinaseHorse radish peroxidase
The invention discloses a kit for chemiluminescence immunity quantitative detection of a CK-MB nano magnetic particle. The kit comprises a CK-MB calibrator; a nano magnetic particle suspension liquid coupled with streptavidin, bioti-labeled CK-MB antibodies, CK-MB abzyme conjugate, a CK-MB quality control product, a chemiluminescence liquid A and a chemiluminescence liquid B, a 20-time concentrated washing liquor and a reaction tube, wherein for the CK-MB abzyme conjugate, used enzyme adopts horse radish peroxidase with purity RZ larger than or equal to 3.0 and activity larger than or equal to 250 U / mL. Besides, the invention further discloses a preparation method of the kit. Compared with the conventional kit, the kit provided by the invention has the advantages of high sensibility and test automation, wide measurable concentration range, long validity of a reagent, simple operation and the like.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
Kit for detecting creatine kinase isoenzyme and preparation and use methods thereof
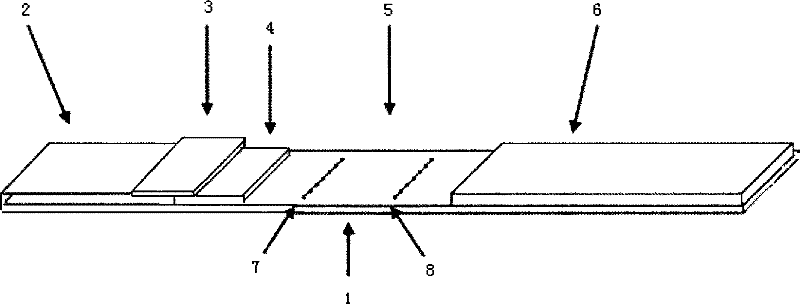
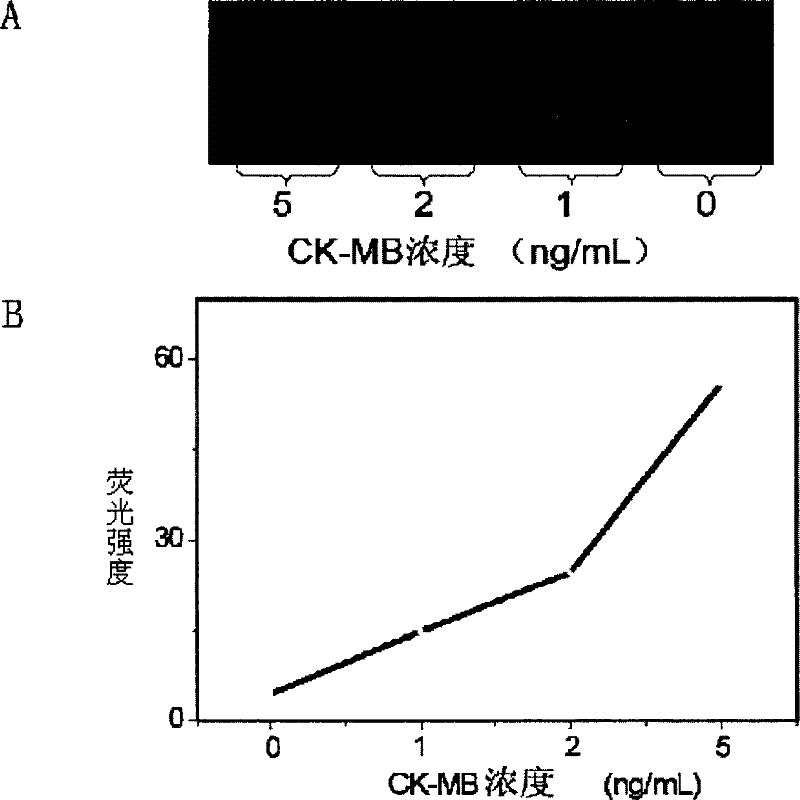
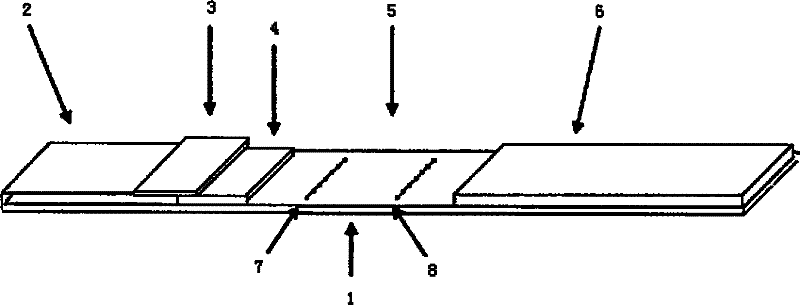
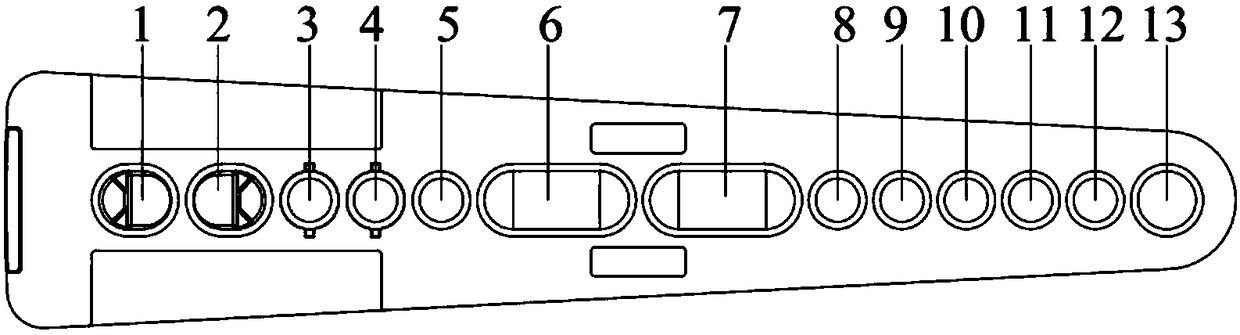
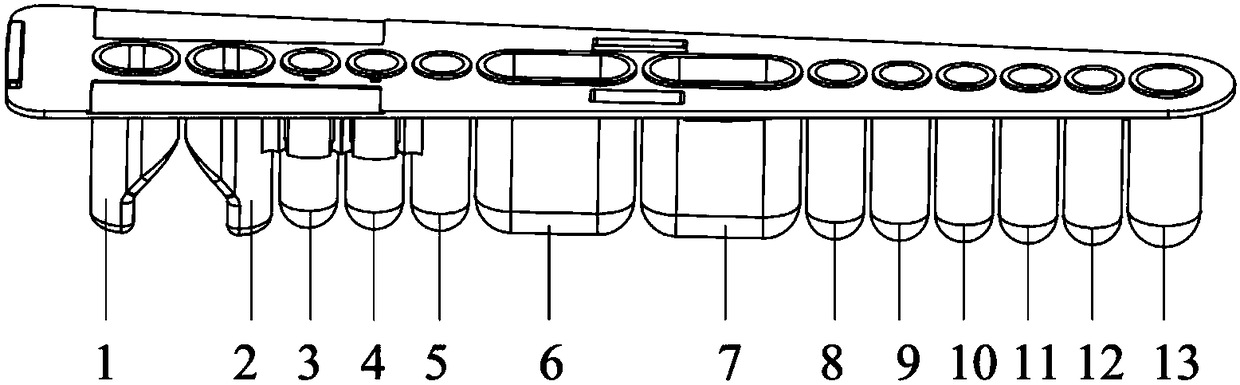
The invention relates to a kit for detecting creatine kinase isoenzymes and preparation and use methods thereof. The kit for detecting creatine kinase isoenzymes comprises a substrate, as well as a sample pad, a conjugate pad, a coupling pad, a nitrocellulose membrane and water absorbent paper, which are arranged from one end to the other end on the substrate, wherein the conjugate pad is coated with a conjugate of fluorescence silicon dioxide nanoparticle-anti-creatine kinase isoenzyme MB monoclonal antibody, the nitrocellulose membrane is provided with a test line and a quality control line, the test line is arranged on the side close to the coupling pad and contains an anti-creatine kinase isoenzyme MB monoclonal antibody, and the quality control line is arranged on the side away from the coupling pad and contains goat anti-mouse IgG (Immunoglobulin G). The kit is low in price and has simple preparation process, and works with a high detection speed to provide the detection result within 15 minutes. The portable fluorescence detection device is adopted to achieve the purpose of accurate quantification of a target analyte. The kit is convenient to operate, can be applied to on-site detection, and is suitable for operators who do not need training. The kit has the advantages of high sensitivity, good specificity and accurate result, and is convenient for generalization and application.
Owner:王迎峰
Creatine kinase isoenzyme detection kit
ActiveCN108548926AMicrobiological testing/measurementBiological material analysisCreatine kinaseCreatine kinase isoenzyme
The invention relates to a creatine kinase isoenzyme detection kit. The kit includes a first reagent and a second reagent. The first reagent comprises a buffer solution, a preservative, a coagulant, asurfactant, a protective agent and a blocker; and the second reagent comprises the buffer solution, a stabilizer, the preservative, the protective agent, polystyrene latex particles and a creatine kinase isozyme antibody. The kit has the advantages of good stability and strong specificity, can be applied to biochemical analyzers in order to carry out large-batch detection, and can replace chemiluminescence products to reduce the detection cost in hospitals.
Owner:BEIJING STRONG BIOTECH INC
High-stability creatine kinase detection kit
InactiveCN104357544AImprove stabilizerNo effect on activityMicrobiological testing/measurementCreatine kinasePreservative
The invention relates to the field of in-vitro diagnosis biochemical reagents, particularly a high-stability creatine kinase detection kit. The high-stability creatine kinase detection kit is composed of a reagent R1 and a reagent R2 which are mutually independent, wherein the reagent R1 is composed of a biological buffer solution, an enzyme protective agent, a reaction substrate, a complex, a surfactant, a preservative and an activator; the reagent R2 is composed of a biological buffer solution, a reaction substrate and a preservative; and the volume ratio of the reagent R1 to the reagent R2 is 4:1. After adding the enzyme protective agent into the detection kit, the enzyme protective agent can be adopted to protect the enzyme on the premise of enhancing the stability of the reagent. After adding the enzyme protective agent, the enzyme can keep stable in the water solution for a long time, and the activity of the enzyme is not influenced.
Owner:CHONGQING ZHONGYUAN BIOLOGICAL TECH
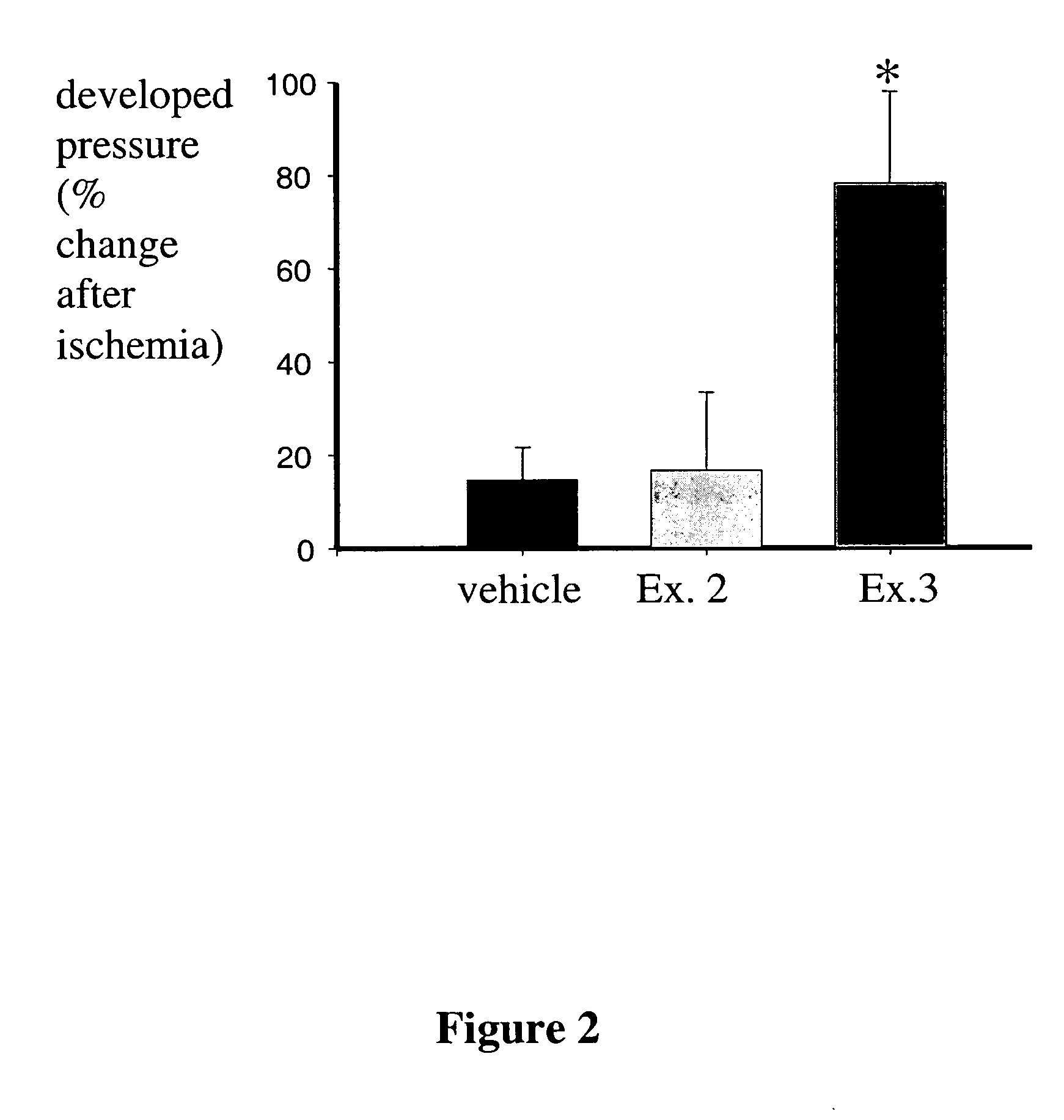
Method of treating or preventing myocardial ischemia-reperfusion injury using NF-kB inhibitors
The present invention concerns a method of treatment or prevention of myocardial ischemia-reperfusion injury by diagnosing that a person is in need of treatment or prevention of myocardial ischemia-reperfusion injury and administering a therapeutically effective amount of a ligand which modulates NF-kB transcription factor by interaction with estrogen receptor ER-α, estrogen receptor ER-β, or both ER-α and ER-β estrogen receptors with a substantial absence of creatine kinase stimulation. In certain preferred embodiments, the administration is substantially without uterotropic activity.
Owner:WYETH
Immunological detecting kit and preparation method and using method thereof
InactiveCN102121938AQuick and time-saving detectionShorten detection timeBiological testingHuman bodyCreatine kinase
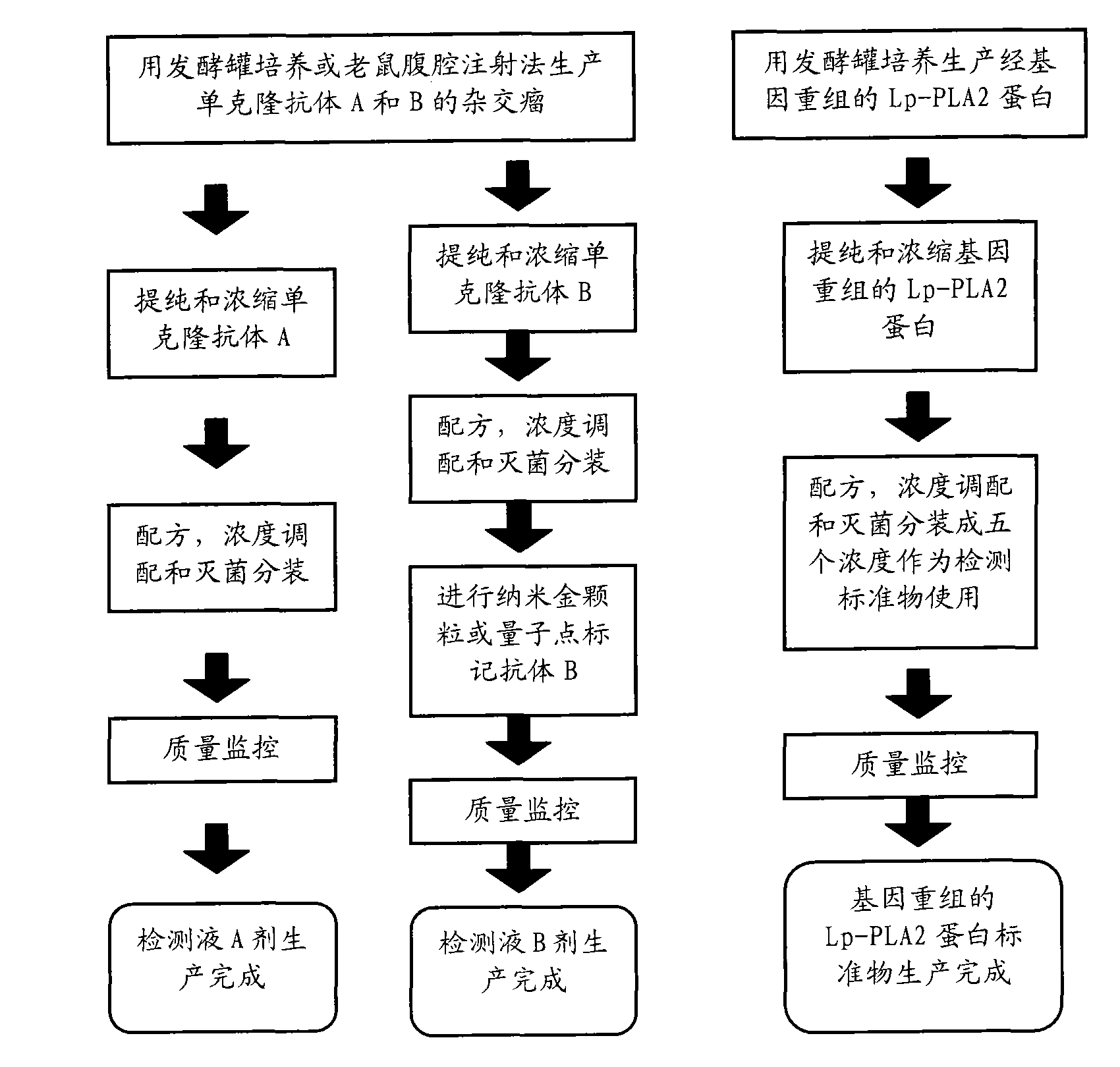
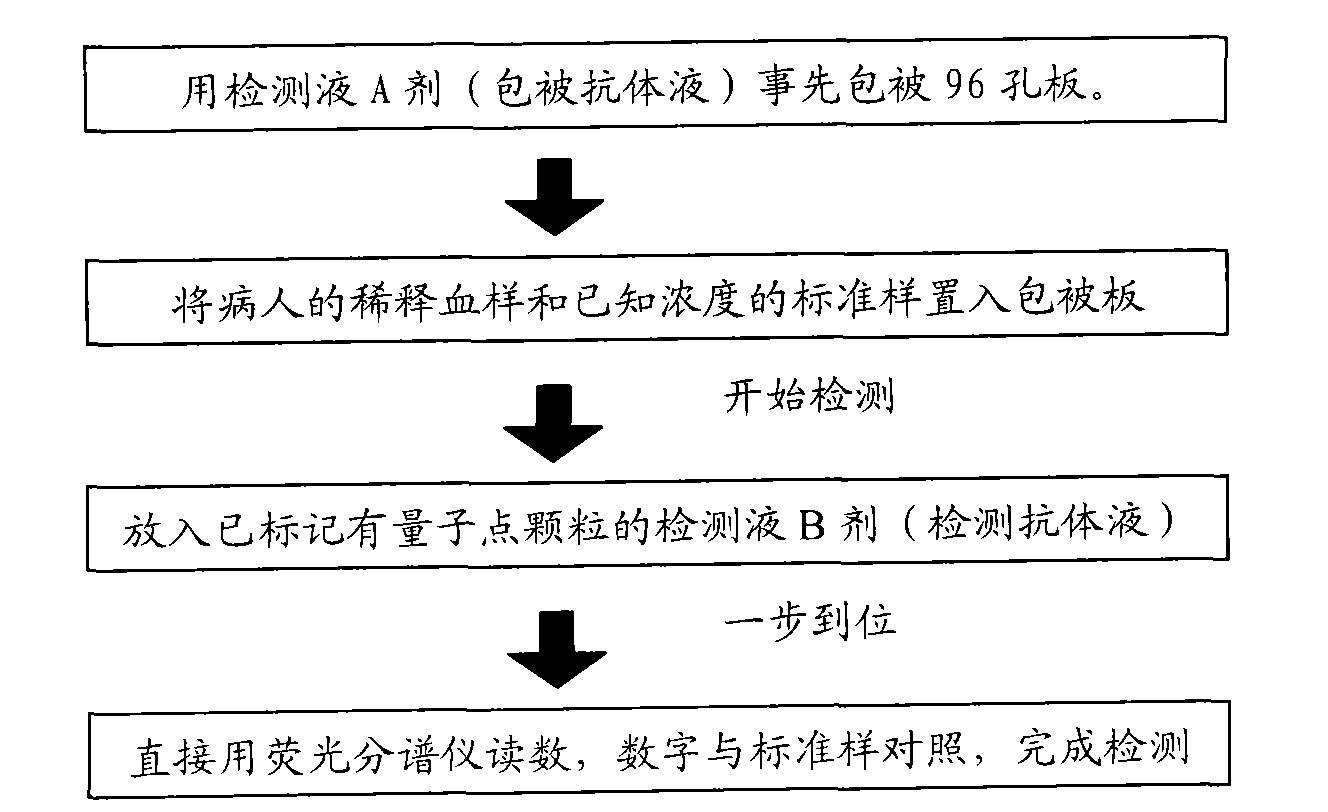
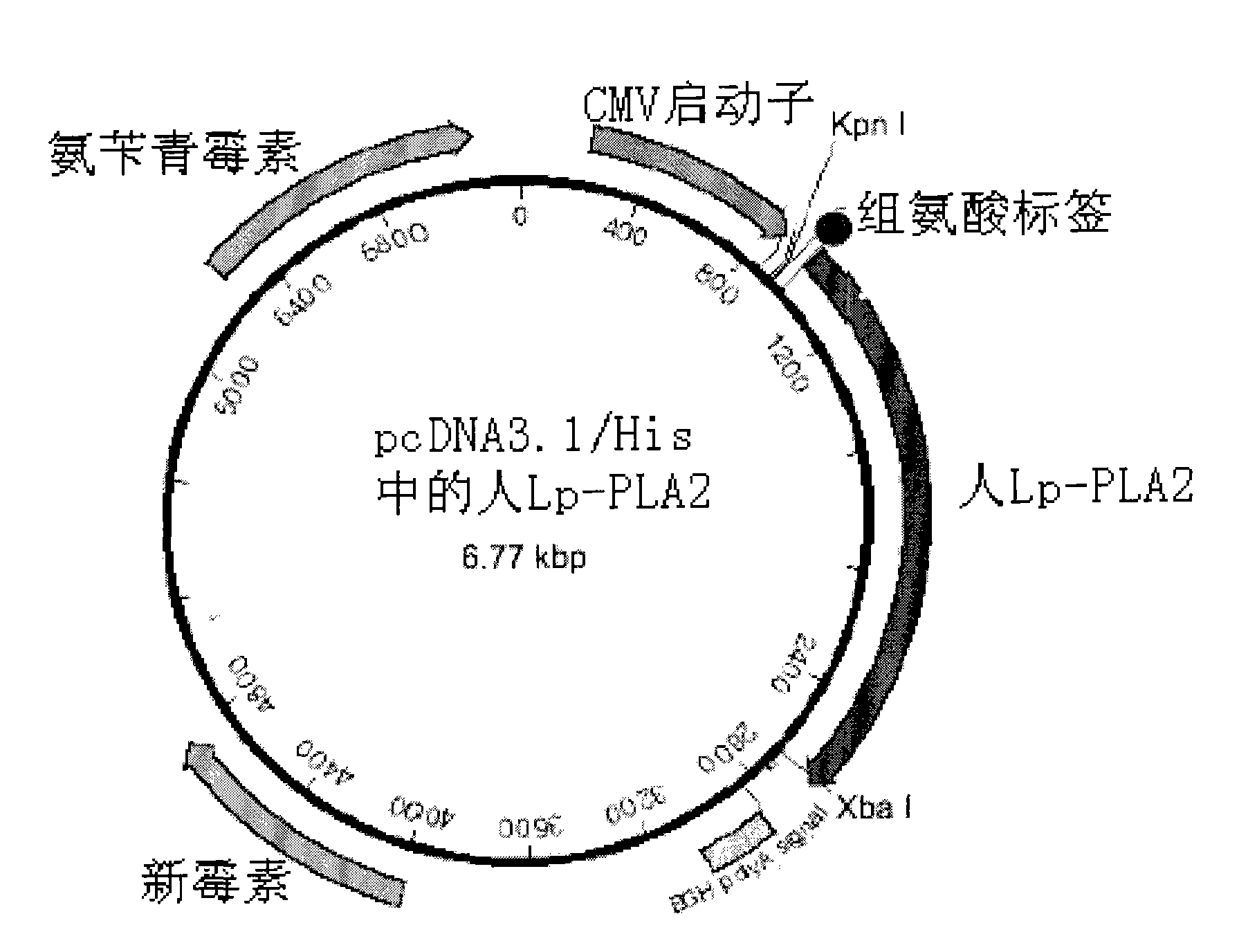
The invention provides an immunological detecting kit, a preparation method and a using method thereof. The kit comprises a protein antibody which is to be detected and is marked by a marker capable of directly detecting, wherein the protein to be detected is the protein related to heart cerebrovascular disease examination. The protein to be detected comprises one or more of fibrinogen, C-reactive protein, thrombus precussor protein, creatine kinase and human body lipoprotein related phospholipase A2. The abundance or the concentration of the protein to be detected in the sample to be detected can be detected in one step by using the kit, so the step and the time are saved; and compared with the traditional method requiring signal cascade amplification such as enzyme-linked immuno sorbent assay (ELISA) and the like, the accuracy is improved.
Owner:TIANJIN KANGERKE BIOSCI
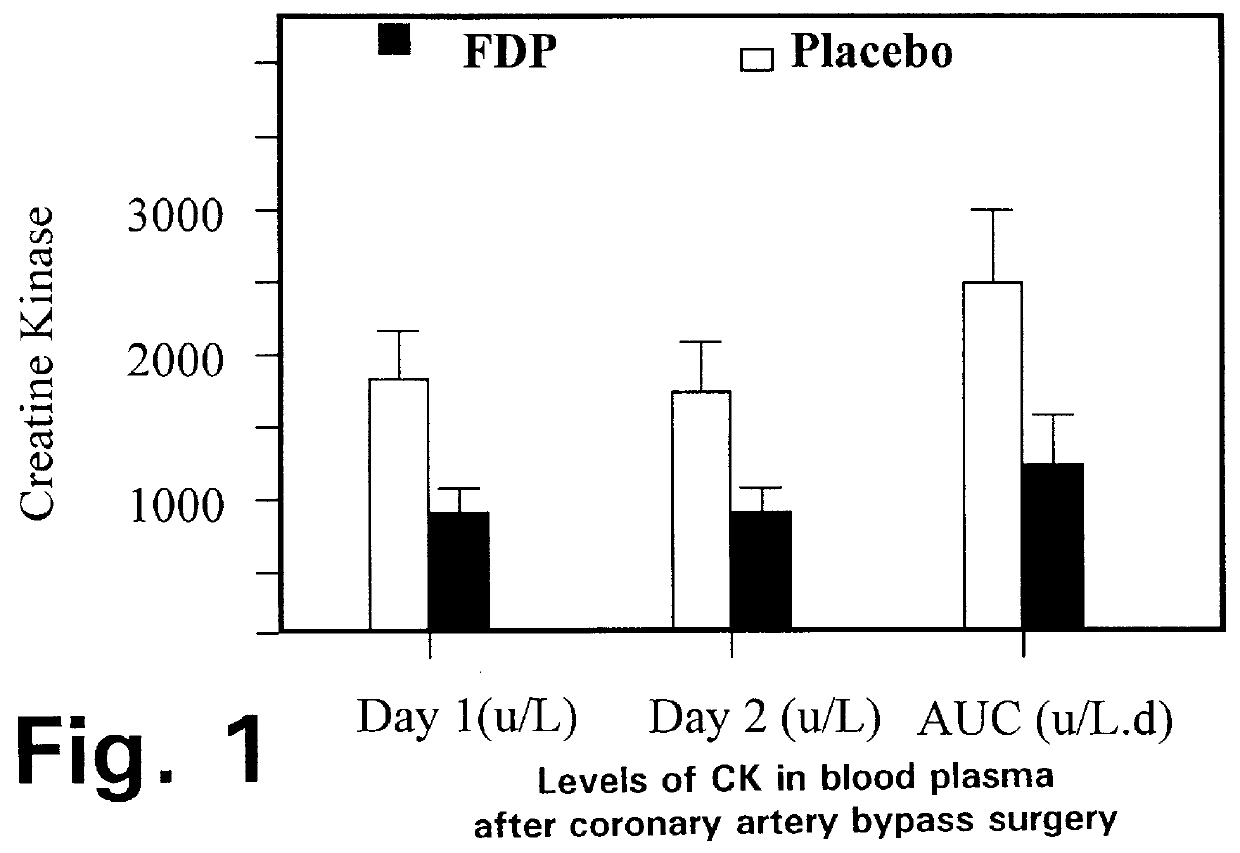
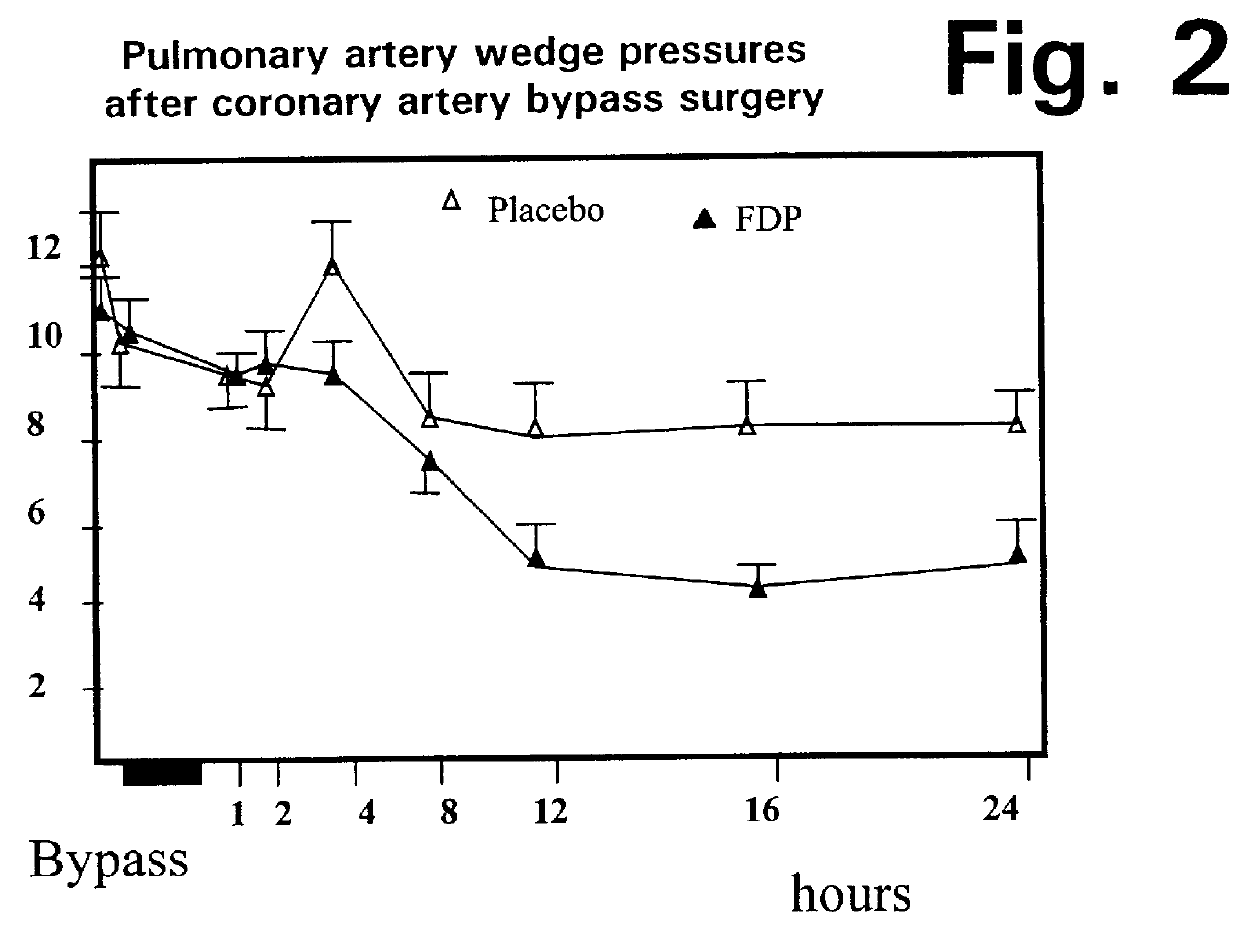
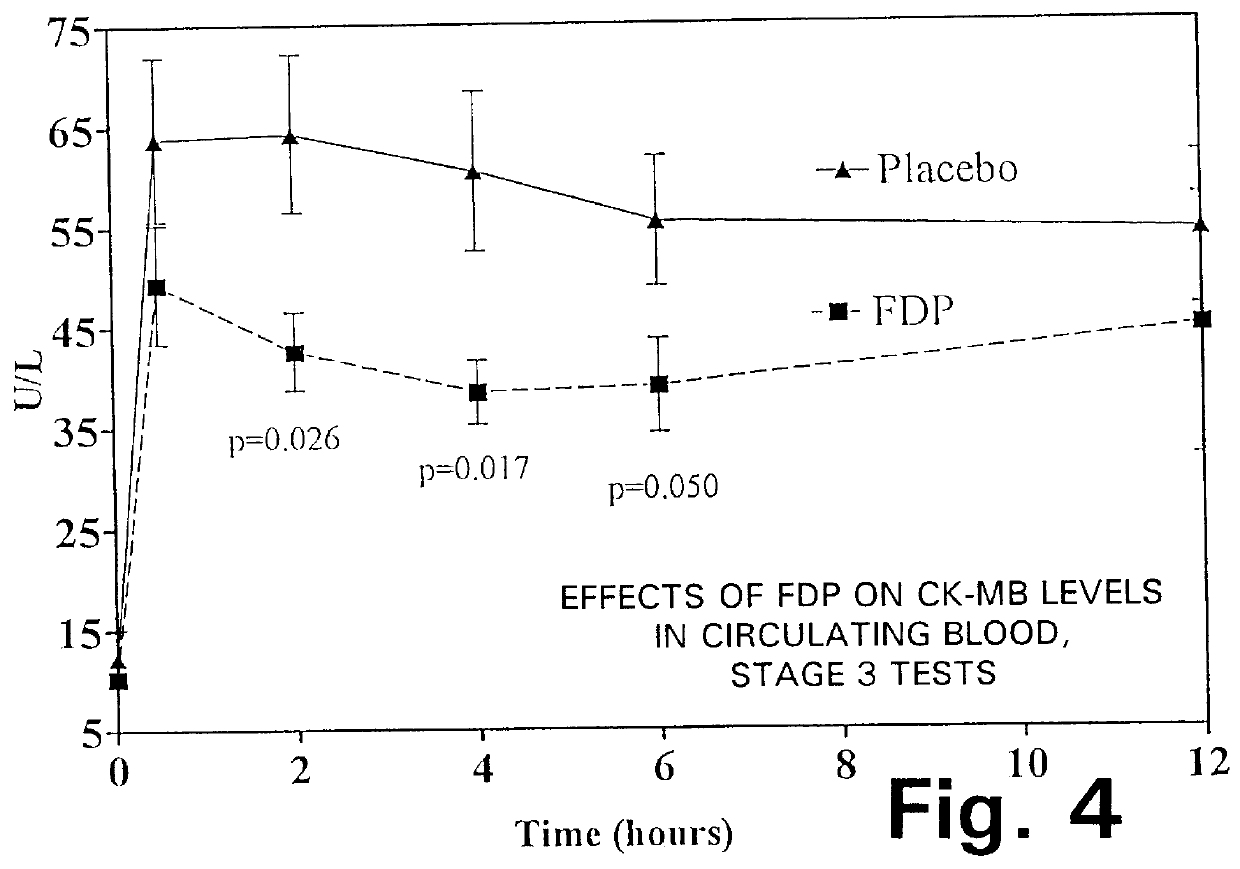
Injection of fructose-1,6-diphosphate (FDP) prior to coronary artery bypass grafting surgery
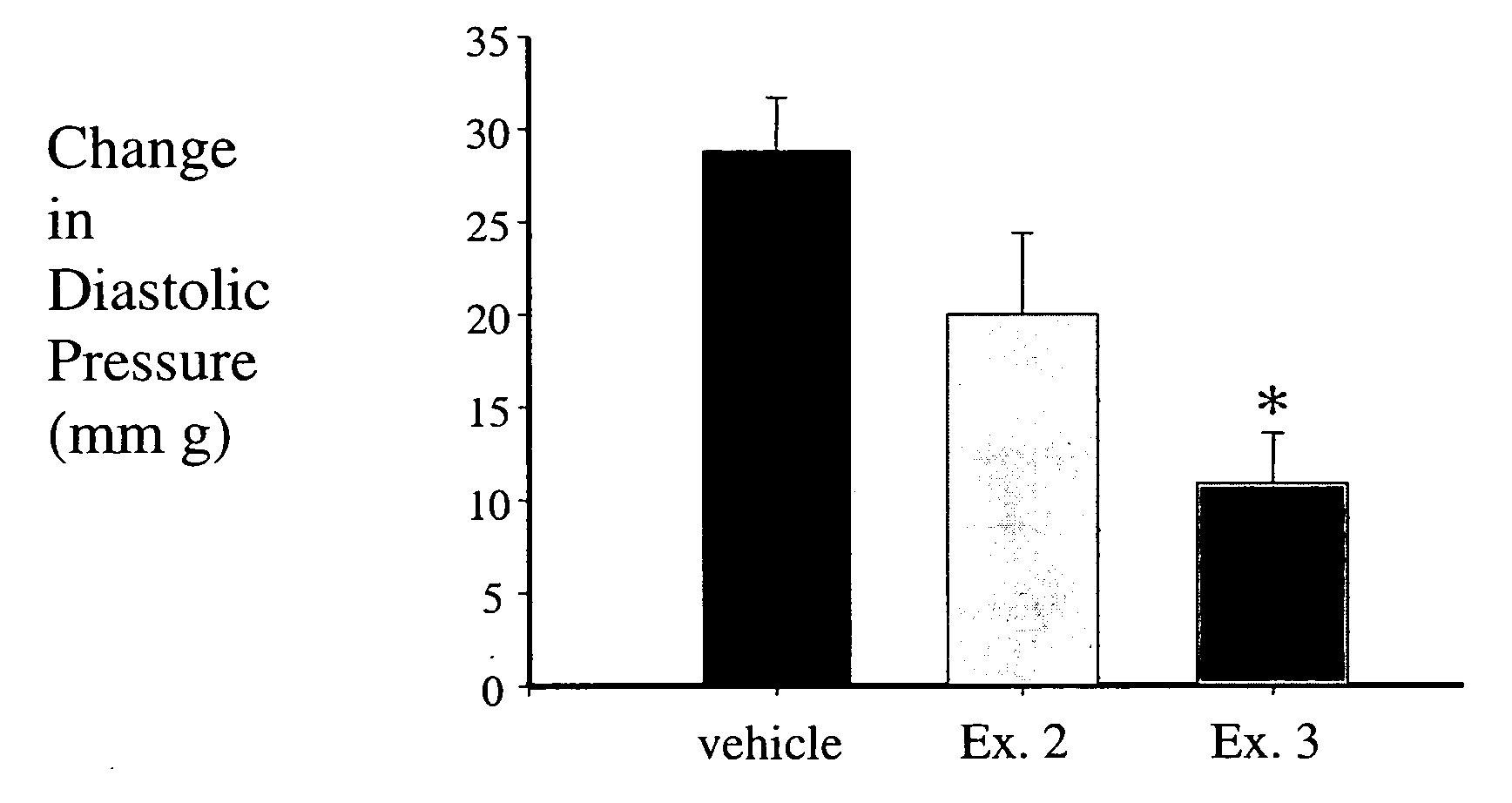
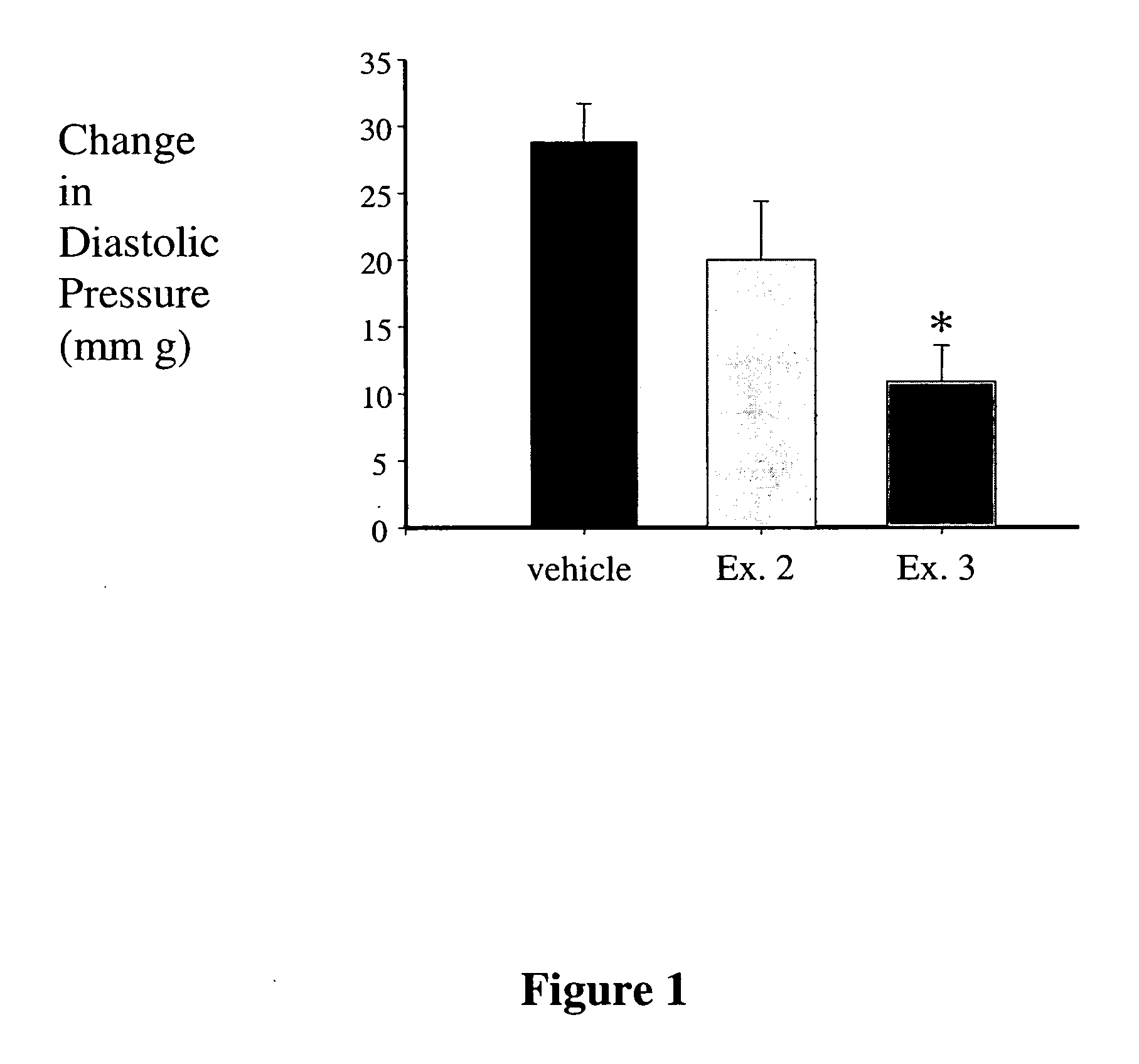
Fructose-1,6-diphosphate (FDP) is used to treat patients who are undergoing coronary artery bypass grafting (CABG) surgery. Before cardiopulmonary bypass begins, a liquid that contains FDP is intravenously infused in the patient, preferably for about 10 to 30 minutes, to allow the FDP to enter the heart and lung tissue while the heart is still beating. FDP can also be added to cardioplegia solution; in addition, FDP can be injected after bypass is terminated, but if post-bypass injection is used, steps should be taken to avoid excess lactic acid accumulation, which appears to increase the risk of atrial fibrillation. To prevent or control lactic acidosis, a buffering or alkalizing agent, such as sodium bicarbonate, or an agent which reduces lactic acid formation, such as dichloroacetate, can be used. In double-blinded trials, this use of FDP substantially reduced heart damage and improved overall outcomes, as shown by lower levels of creatine kinase in blood, improvements in pumping performance, reduced requirements for vasodilator and inotropic drugs, and shorter stays in intensive care units. Certain dosages also reduced the likelihood of atrial fibrillation; however, FDP at high dosages increased the likelihood of A-fib. FDP also helped reduce pulmonary vascular resistance (PVR); this is an important finding, since pulmonary hypertension following cardiopulmonary bypass is a very difficult and often intractable problem, and is a contributing factor in nearly all deaths following CPB surgery.
Owner:QUESTCOR PHARMA
Cardiac muscle control material, preparation method therefor, detection kit and detection device for cardiac muscle
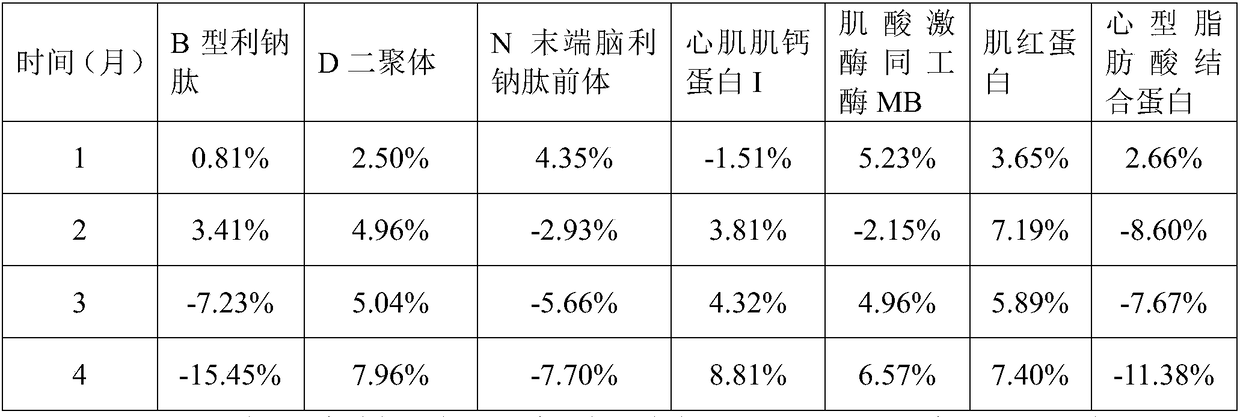
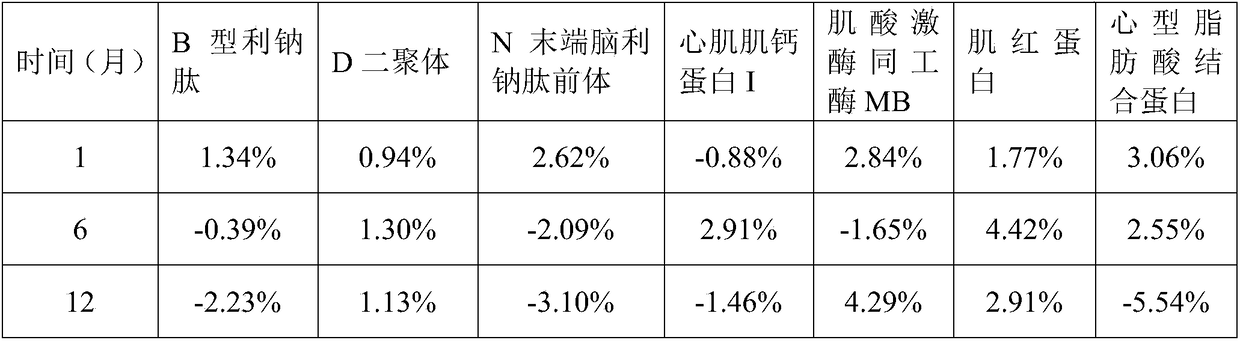
The invention relates to a cardiac muscle control material, a preparation method therefor, a detection kit and a detection device for cardiac muscle. The cardiac muscle control material comprises quality control component and protection component, the quality control component comprises at least one of B-type natriuretic peptide, D-dimer, NT-proBNP, cardiac troponin I, myoglobin, creatine kinase isozyme and cardioid fatty acid binding protein and heart fatty acid binding protein. The protective component comprises 5 mM to 100 mM of a buffer agent, 0.5 % to 5% by weight of an excipient, 0.05 mMto 5 mM of an antioxidant, 0.05 mM to 15 mM of a protease inhibitor, 0.5% to 10% by weight of a stabilizer and 0.01% to 2% by weight of a surfactant. The cardiac muscle control material disclosed bythe invention is good in stability.
Owner:深圳天深医疗器械有限公司
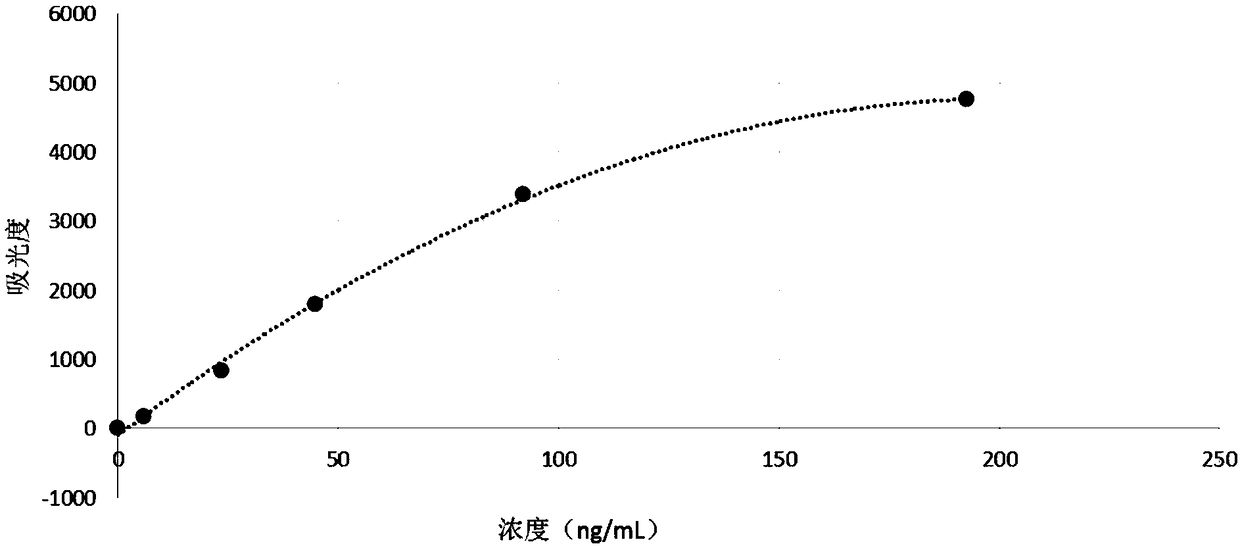
Magnetic bead time resolution fluorescence immunoassay quantitative determination CK-MB (creatine kinase-MB) kit
The invention discloses a magnetic bead time resolution fluorescence immunoassay quantitative determination CK-MB kit. The CK-MB kit comprises an immunomagnetic bead coating a CK-MB monoclonal antibody, a CK-MB standardized product solution, a europium-marked CK-MB monoclonal antibody solution, washing liquid and enhancement liquid. The immunomagnetic bead coating the CK-MB monoclonal antibody isa covalent conjugate of a superpara magnetic bead modified by a functional group and with the diameter being 1-3 microns and the CK-MB monoclonal antibody. The kit has the high sensibility, the sensibility of CK-MB is 1ng / mL, and a blood serum (plasma) does not need to be diluted; the determination time is short, and a report can be resulted within 30 minutes; the demanding amount of the sample isless, and only 50 microliters are needed for one-time sample loading; and the kit is equipped with a full-automatic time resolution immune analysis meter, operation is easy, no artificial error exists, and labor is saved. The kit reasonably utilizes the space of a reagent strip, the structure of the reagent strip is more compact, the reagent strip can be transported more easily, and used conveniently, the operation is simple, and the stability is good.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Creatine kinase isozyme detection kit and preparation thereof
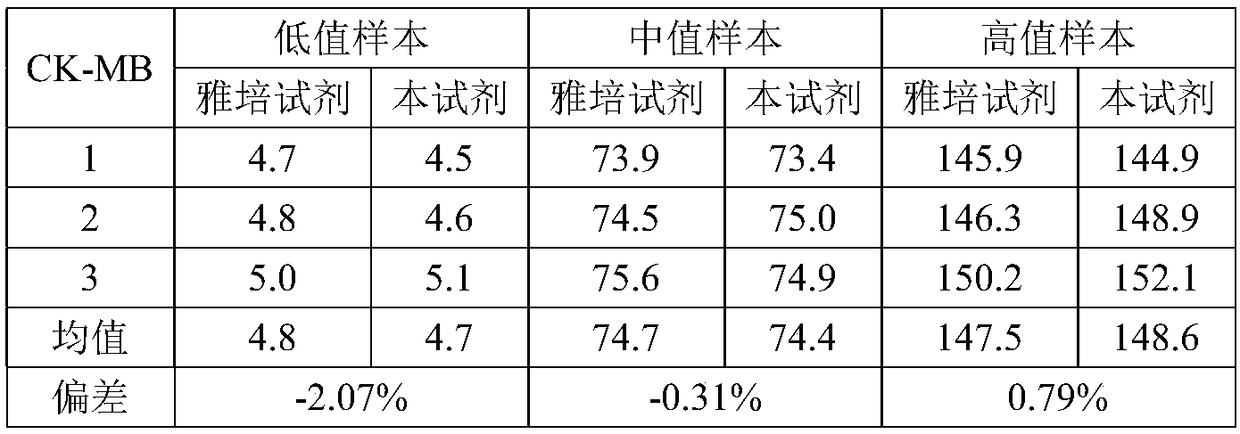
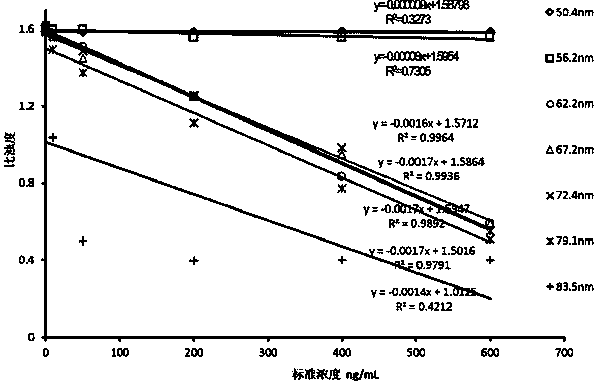
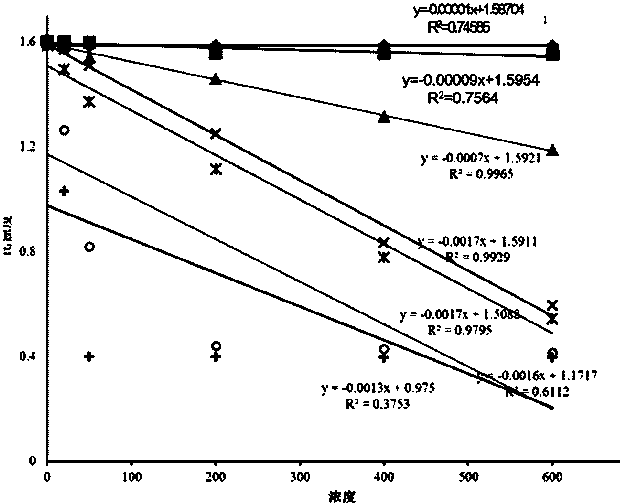
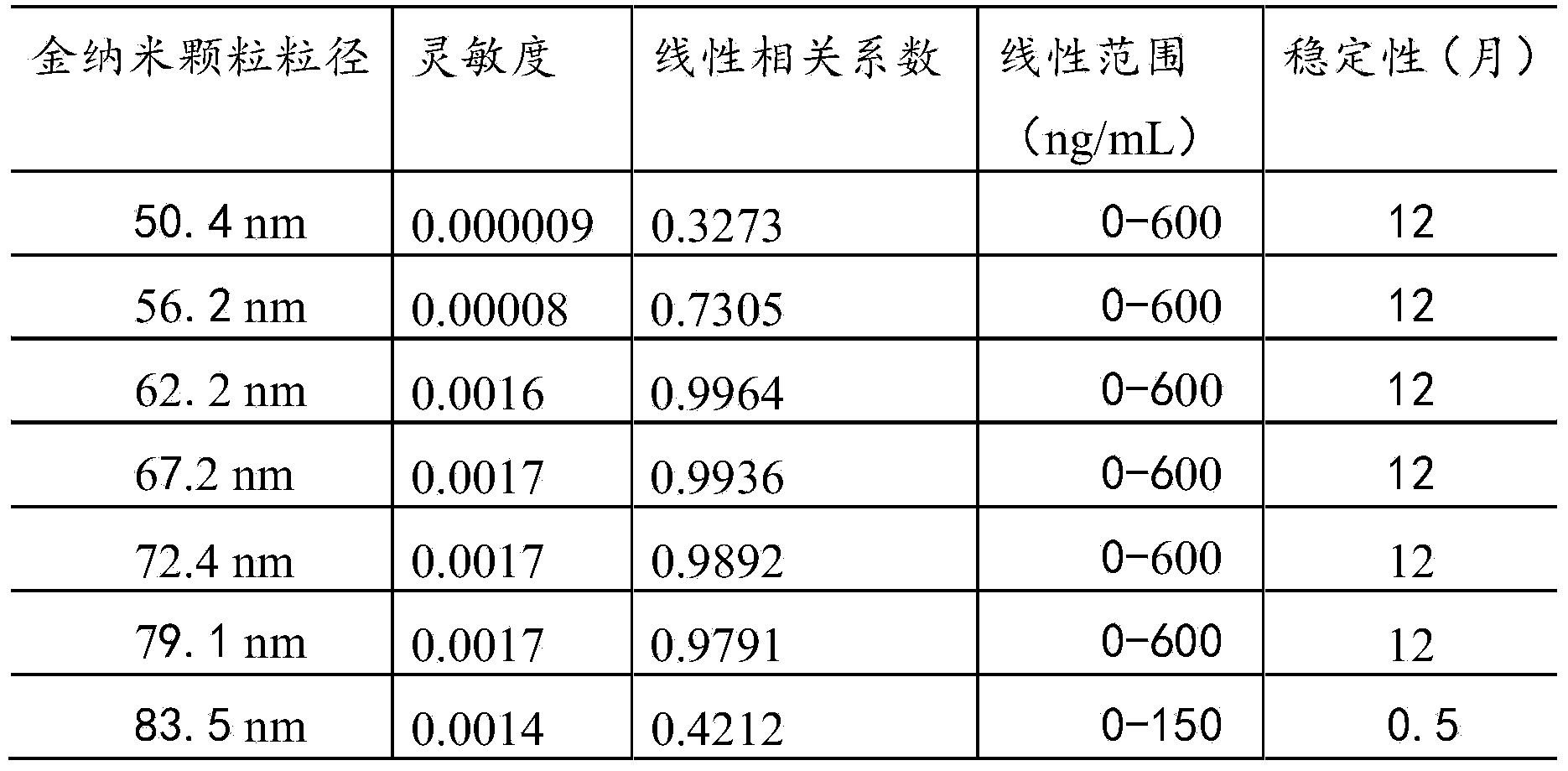
The invention provides a creatine kinase isozyme detection kit. The kit is based on colloidal gold immunoturbidimetry, and comprises reagents R2, and the reagents R2 are solutions of gold nanoparticles marked with creatine kinase isozyme antibodies. The kit is characterized in that the particle diameter of the gold nanoparticles is 62.2 nm to 79.1 nm, and the mass ratio of the gold nanoparticles and the antibodies is 50:20-60. The invention further provides a preparation method of the kit. The kit has the advantages of being high in sensitivity, strong in specificity, fast in response and good in stability, no sediment is generated after a reaction, a biochemical analyzer is convenient to clean, and the service life of the biochemical analyzer is prolonged.
Owner:BEIJING JIUJIAYI TECH
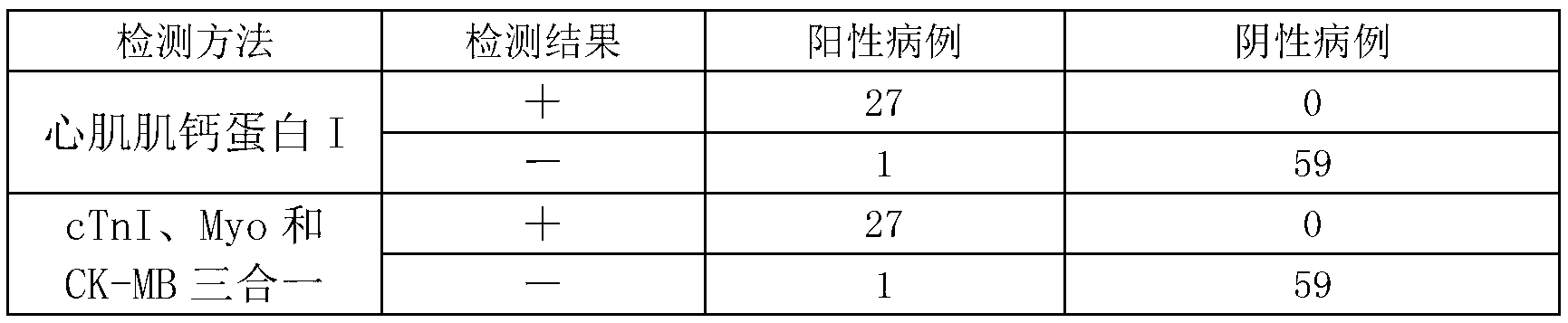
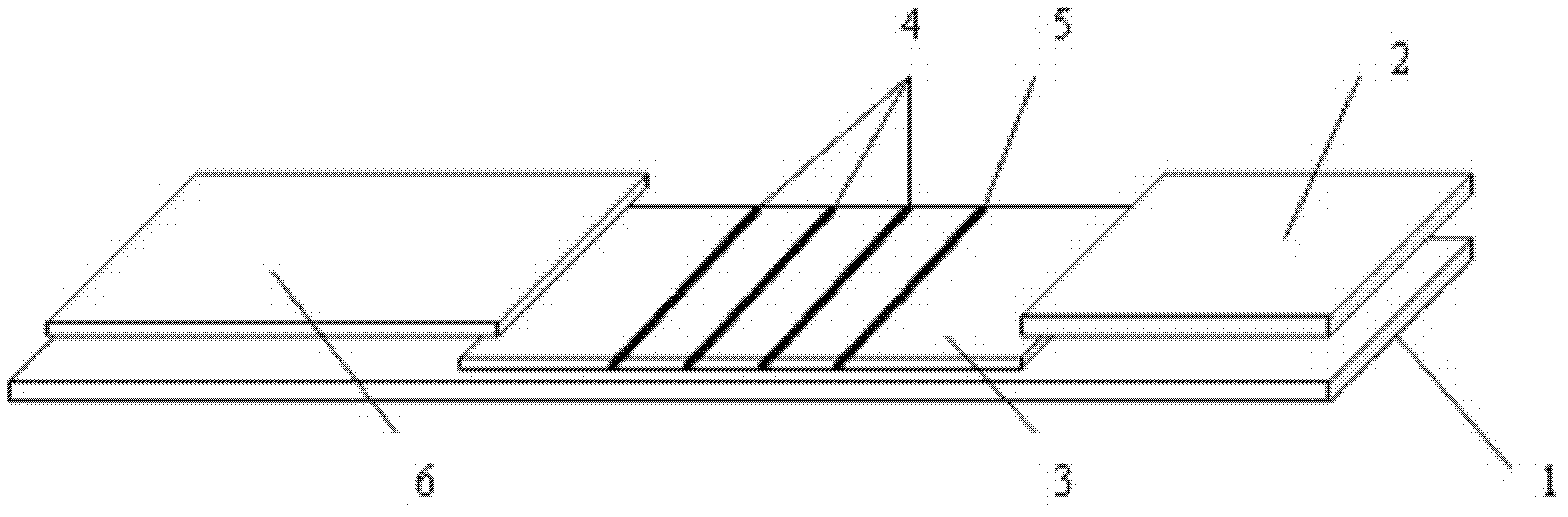
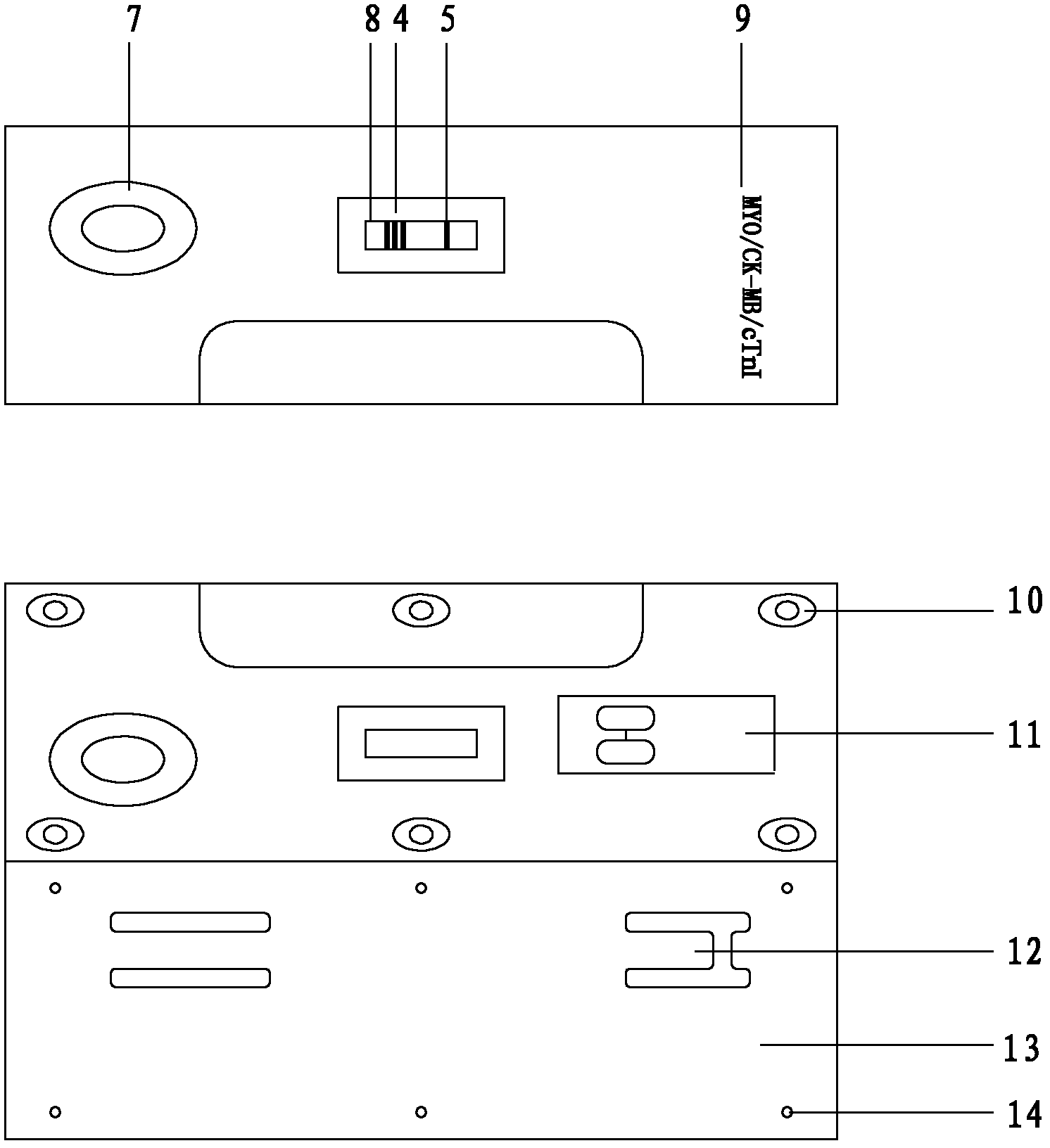
CTnI, Myo and CK-MB three-in-one colloidal gold test strip, kit thereof, and making methods of test strip and kit
The invention discloses a cardiac troponin (cTnI), myoglobin (Myo) and creatine kinase-MB (CK-MB) three-in-one colloidal gold test strip, a kit thereof and making methods of the test strip and the kit. The test strip and the kit are made through adopting a chromatographic technique and a double antibody sandwich principle, and the human cTnI, Myo and CK-MB levels in a clinic specimen (whole blood / serum / blood plasma) can be detected through one-time operation, so the operation process is simplified.
Owner:JIANGSU KEYGEN BIOTECH CORP LTD
Method for cell-free expression of signal protein and expression system
ActiveCN106011163ASolve the problem of low expressionHigh protein expressionNucleic acid vectorVector-based foreign material introductionEscherichia coliCreatine kinase
The invention relates to a method for cell-free expression of signal protein and an expression system. The method includes joining a target gene of the signal protein on a pIX3.0 vector to obtain expression plasmids; extracting escherichia coli Rosetta (DE3) by S30 buffer solution to obtain escherichia coli extract; preparing an energy supply system comprising PEP (phosphoenolpyruvic acid) / PK (pyruvate kinase), CP (phosphocreatine) / CK (creatine kinase) and glucose; preparing an amino acid mixture and a saline solution; preparing lecithin / cholesterol liposome; adding the expression plasmids into a cell-free protein expression system comprising the escherichia coli extract, the energy supply system, the amino acid mixture, the saline solution and the lecithin / cholesterol liposome for expression. The method is capable of solving the problem of small signal protein expression quantity.
Owner:CUSABIO TECH LLC
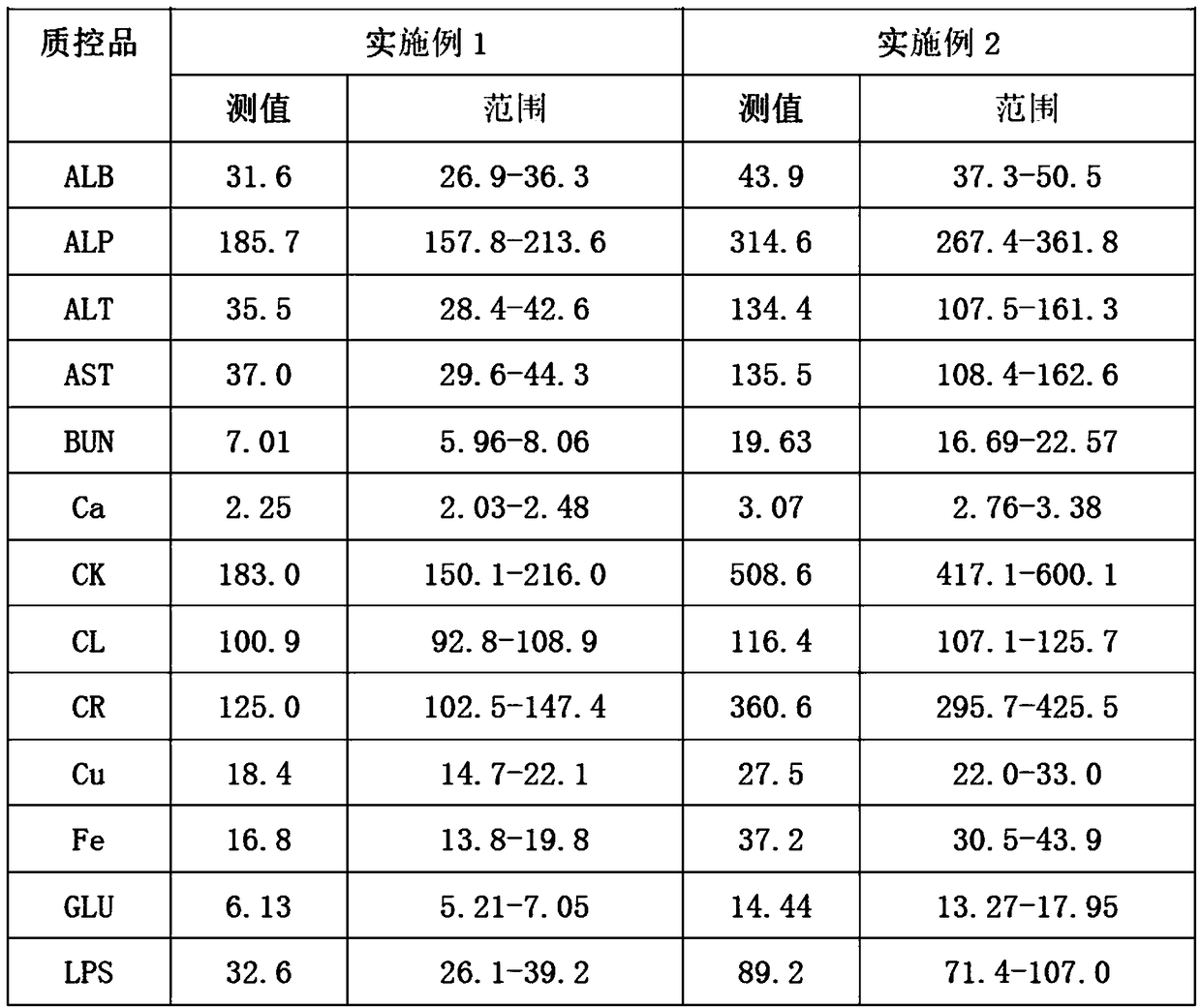
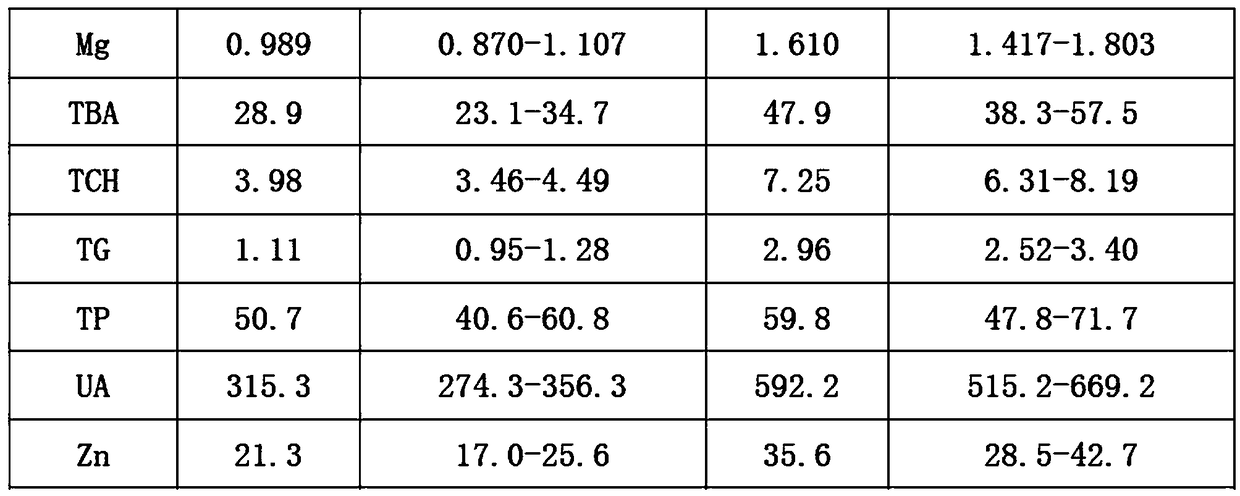
Preparation method of quality control serum for quality control of centrifugal microfluidic chips
ActiveCN108152519ASufficient sourceEasy to getBiological testingFreeze-dryingMonopotassium phosphate
The invention discloses a preparation method of quality control serum for quality control of centrifugal microfluidic chips. The method comprises the following steps: adding 0.6 to 1.0 percent of cholesteryl sodium sulfate into bovine plasma, and adding 0.02 to 0.06 percent of monopotassium phosphate; sequentially adding 0.04 to 0. 08 percent of ammonium ferric sulfate dodecahydrate, 0.5 to 0.9 percent of calcium chloride, 0.2 to 0.6 percent of bitter salt, 0.6 to 1 percent of urea, 9 to 13 percent of sodium chloride, 0.01 to 0.03 percent of zinc vitriol, 0.01 to 0.03 percent of chalcanthite,0.06 to 0.2 percent of glycocholic acid, 2 to 4 percent of glucose, 0.03 to 0.06 percent of creatinine, 0.08 to 0.2 percent of uric acid, and 0.07 to 0.11 percent of triolein into water; uniformly mixing obtained solution; adding glycol, saccharose and triton X-100, and uniformly mixing; sequentially adding albumin bovine serum, sodium azide, alanine aminotransferase, aspartic transaminase, alkaline phosphatase, lipase and creatine kinase, uniformly mixing, and performing freeze drying. The method has the advantages that the source of the raw materials is sufficient, the raw materials are easyto get, possible matrix effect is furthest avoided, and precipitation of the raw materials is prevented.
Owner:NINGBO MEIKANG BAOSHENG BIOMEDICAL ENG
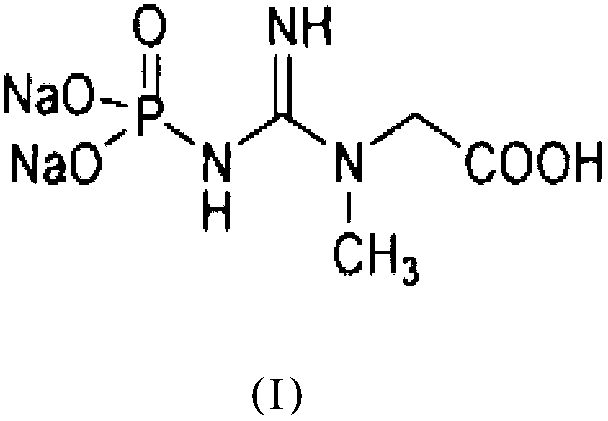
Bioengineering method for synthesizing sodium phosphocreatine
InactiveCN102533880AReduce processing costsNo biological toxicityBacteriaTransferasesEscherichia coliCreatine kinase
The invention provides a bioengineering method for synthesizing sodium phosphocreatine. The bioengineering method comprises the following steps of: recombining an escherichia coli engineering strain containing creatine kinase by using a genetic engineering method; carrying out large-scale high-density culture and separate purification by using the escherichia coli engineering strain to obtain high-activity creatine kinase; and carrying out enzyme-method catalytic synthesis by using the creatine kinase under the appropriate condition to obtain the phosphocreatine. The bioengineering method disclosed by the invention has the advantages of high substrate utilization rate, high product recovery rate, mild reaction conditions, short reaction time, little pollution to environment, low cost and the like and is suitable for industrial large-scale production.
Owner:BIOTRAND
Method of preparing creatine phosphate sodium
The invention relates to a method of preparing creatine phosphate sodium by utilizing a gene engineering technology. The method comprises the steps: respectively constructing creatine kinase and acetate kinase expression strains, removing the limit of creatine kinase-containing animal source, specially purifying the creatine kinase and acetate kinase by utilizing an affinity purification technology, co-immobilizing the creatine kinase and acetate kinase onto vector particles to form co-immobilized enzyme, continuously regenerating ATP from the acetate kinase for sustained reaction while the creatine kinase catalyzes and generates creatine phosphate sodium, thereby finally providing low-cost, green and pollution-free creatine phosphate sodium.
Owner:HUNAN BAOLISHI BIOTECH
Immunofluorescence dipstick component for quickly and quantitatively detecting protein of plurality of types and detection card component prepared from same and preparation method thereof
ActiveCN102662065AHigh dilution factorReduce matrix effectBiological testingCreatine kinaseCreatine kinase isoenzyme
The invention discloses an immunofluorescence dipstick component for quickly and quantitatively detecting protein of a plurality of types and a detection card component prepared from the same and a preparation method thereof, wherein the protein of a plurality of types comprises muscle hemoglobin / creatine kinase isoenzyme / troponin I; the dipstick component comprises a dipstick consisting of a bottom liner, a water-absorbing pad, a coated analysis membrane and a sample pad and independently packaged fluorescein mark specific antibodies; three detection lines and a quality control line are arranged on the coated analysis membrane; the detection line on the coated analysis membrane is respectively coated with an anti-myoglobin monoclonal antibody line, an anti-creatine kinase isozyme monoclonal antibody line and a troponin I monoclonal antibody line; the quality control line is coated with a rabbit IgG antibody; the fluorescein mark specific antibodies comprise the anti-myoglobin monoclonal antibody line, the anti-creatine kinase isozyme monoclonal antibody line, an troponin I monoclonal antibody and an anti-rabbit IgG antibody; and the detection card component comprises the dipstick, a card box consisting of a cover plate and a back plate and independently packaged platinum porphyrin mark specific antibodies and can synchronously detect the muscle hemoglobin / creatine kinase isoenzyme / troponin I, is simple to operate, is quick and sensitive, and has good specificity.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
Method, system and chip test paper for parallel detection on various cardiac markers
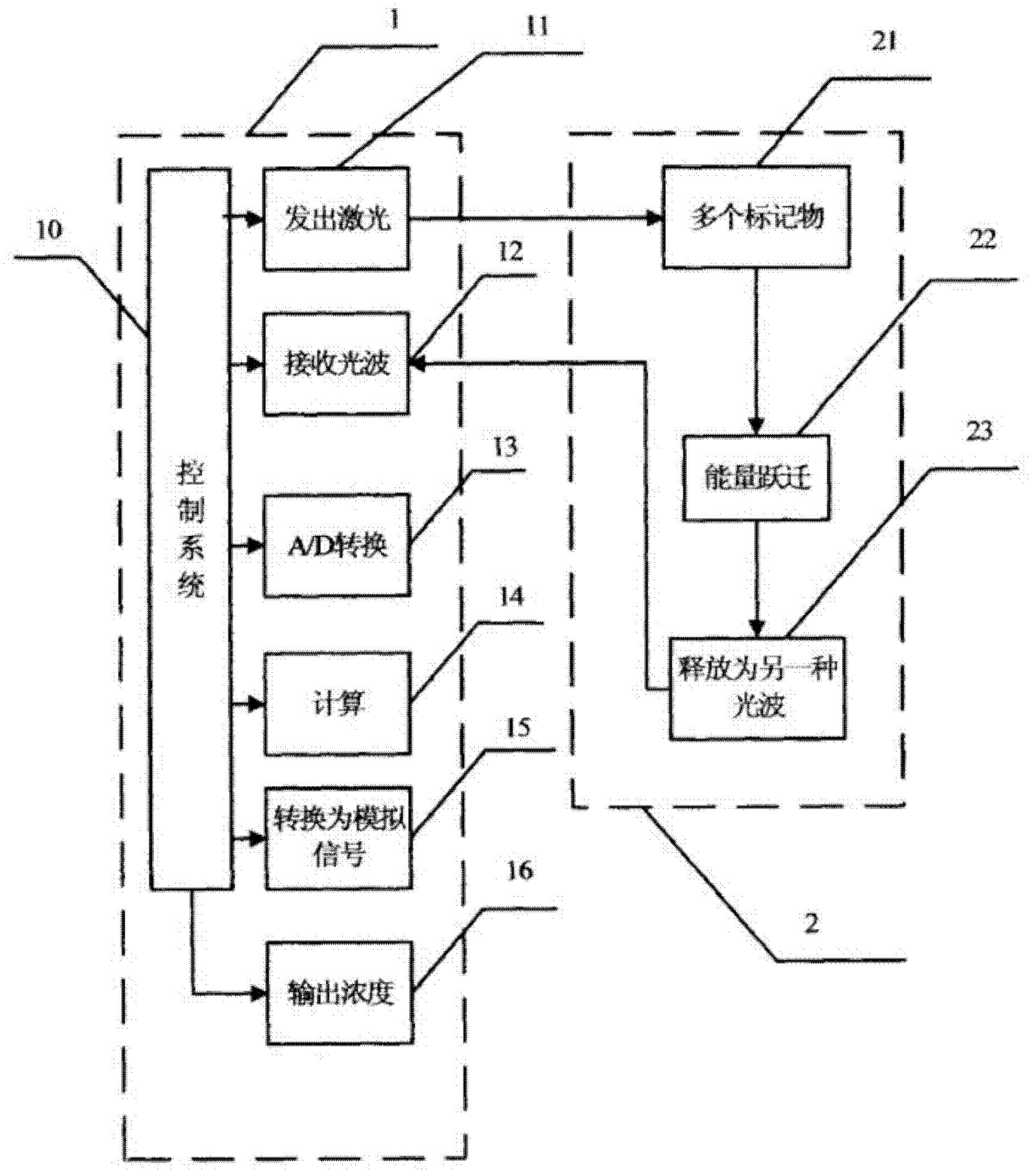
InactiveCN102636651AHigh sensitivityShorten detection timeAnalysis by material excitationBiological testingFiberCreatine kinase
The invention discloses a method, system and chip test paper for parallel detection on various cardiac markers. The chip test paper is used for detecting a part or all of the cardiac markers including cTNI (cardiac troponin I), cTNT (cardiac troponin T), MYO (myoglobin), CK-MB (isoenzymeof creatine kinase containing M and B subunits), BNP (B-type natriuretic peptide), CRP (C-reactive protein) and FABP (fatty acid-binding protein); the chip test paper comprises a first membrane and a second membrane; a ligand An of each marker is arranged on the first membrane, and a ligand Bn coupled with a signal marker is absorbed on the second membrane; and a detected material forming sandwich detection together with the ligands An and Bn is added from a sampling hole, then under the acting force of percolation or other factors, the detected material moves to be combined with the ligands An and Bn respectively to form a composite array of the first membrane-the ligand An-the detected material-the ligand Bn-the signal marker, which is fixed on the first membrane, the composite array and a capture fiber membrane are assembled simultaneously, and a detection hole is reserved. The method, the system and the chip test paper provided by the invention can be used for detecting the various cardiac markers simultaneously and quantitatively; moreover, the detection sensitivity can be improved, and the detection time is saved.
Owner:SHANGHAI LINC BIO SCI
Three-in-one detection kit for cardiac troponin I, creatine kinase isoenzyme and myoglobin and preparation method of three-in-one detection kit
InactiveCN107664700ARapid responseClear clinical guidanceDisease diagnosisBiological testingCreatine kinaseCreatine kinase isoenzyme
The invention relates to the technical field of fluorescence immunochromatography in medical immunology and in particular relates to a three-in-one detection kit for cardiac troponin I, creatine kinase isoenzyme and myoglobin and a preparation method of the three-in-one detection kit. The three-in-one detection kit comprises a test paper card, and is characterized in that a PVC (Polyvinyl Chloride) panel, a sample pad, a conjugate pad, a nitrocellulose membrane and an absorbent pad are sequentially arranged on the test paper card from bottom to top, wherein a cardiac troponin I, creatine kinase isoenzyme and myoglobin three-in-one monoclonal antibody labeled with rare-earth fluorescence microspheres is adsorbed onto the conjugate pad; the diameter of the rare-earth fluorescence microspheres is 60-120nm; the rare-earth fluorescence microspheres are doped with rare-earth lanthanide elements, are stable in a ground state and can emit fluorescence in a wavelength range of 540-600nm under the action of an excitation light source of 340-380nm; and the monoclonal antibody is a purified and mixed monoclonal antibody and is derived from a monoclonal antibody cell strain targeted at 2-6 different cardiac troponin I, creatine kinase isoenzyme and myoglobin three-in-one antigen epitopes. The three-in-one detection kit disclosed by the invention has the advantages of being simple and convenient in operation, rapid in response, high in sensitivity, high in specificity and the like.
Owner:WEIHAI NEOPROBIO
Reagent for detecting myocardial infarction and application of reagent
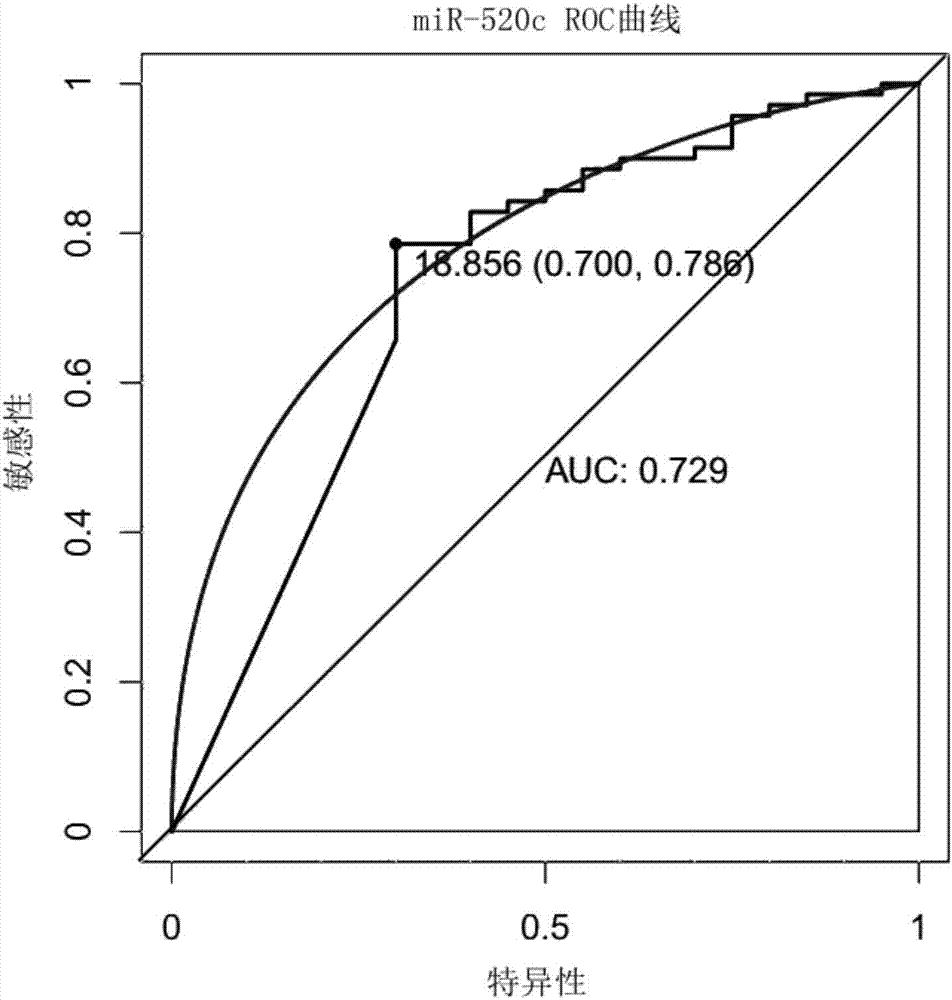
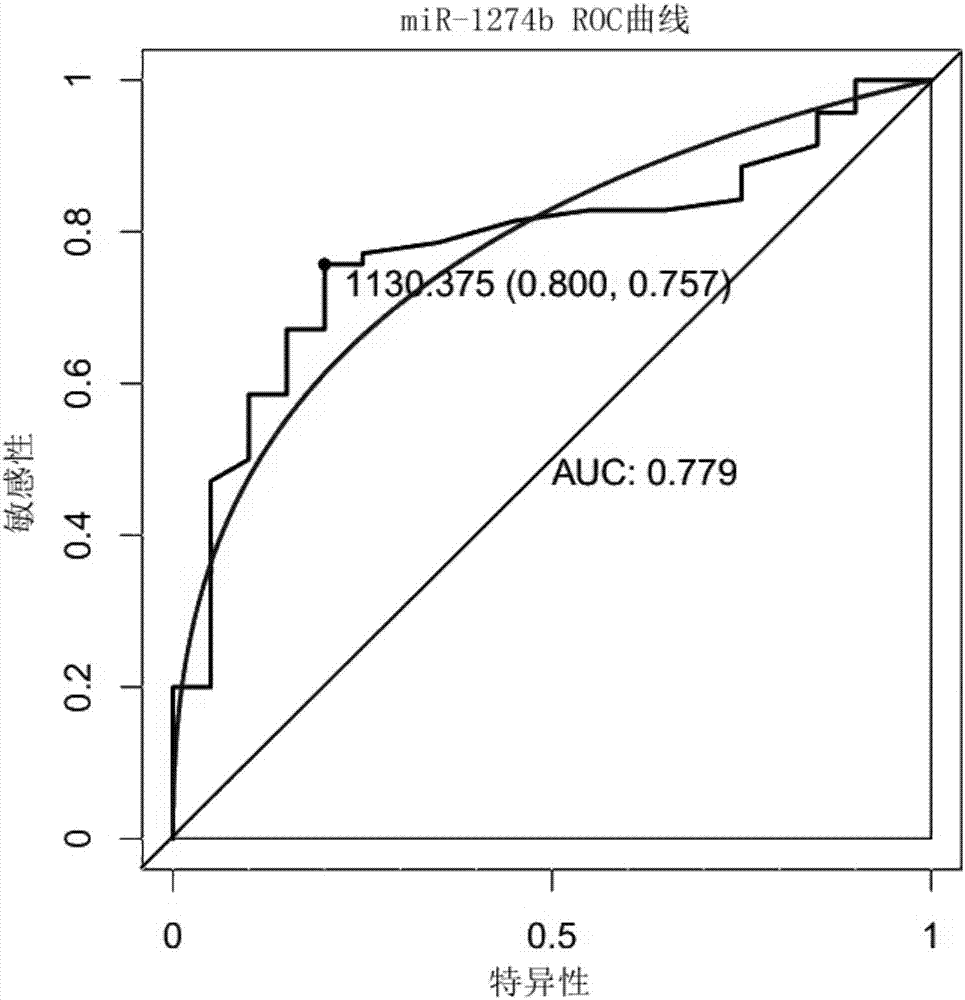
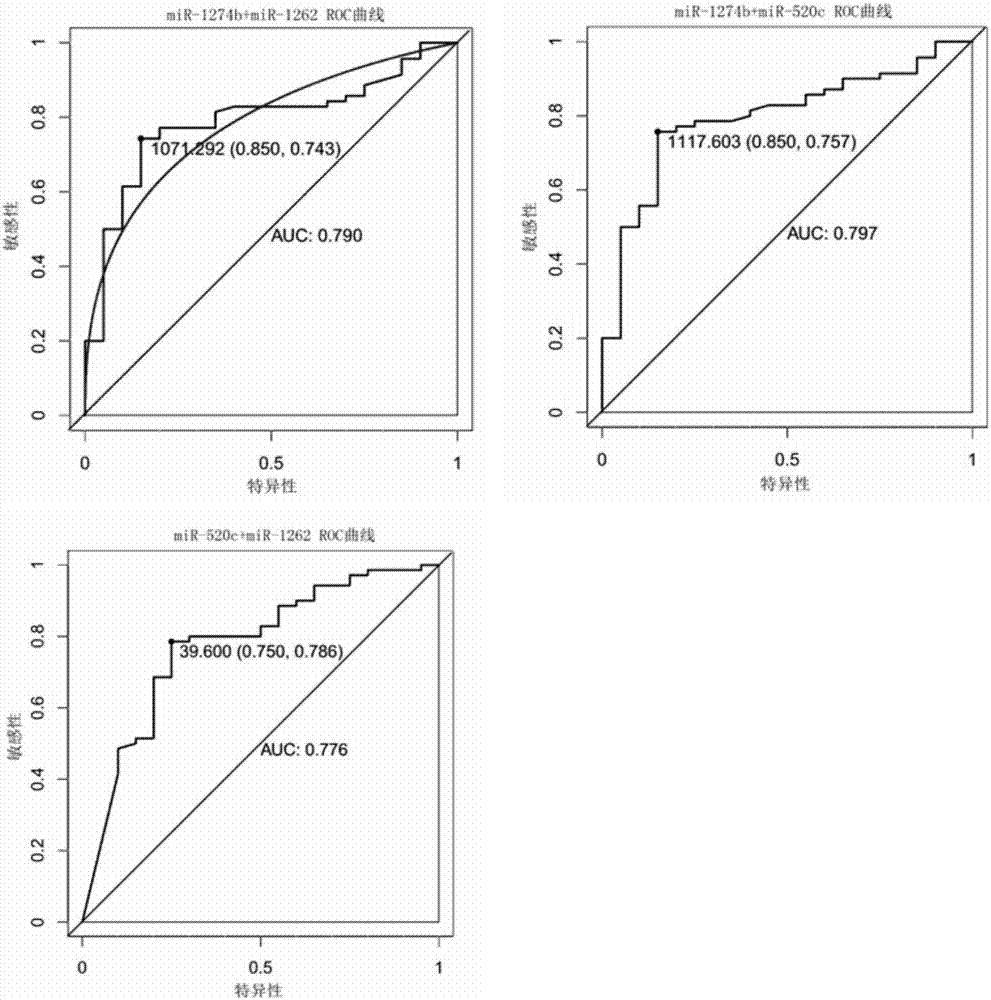
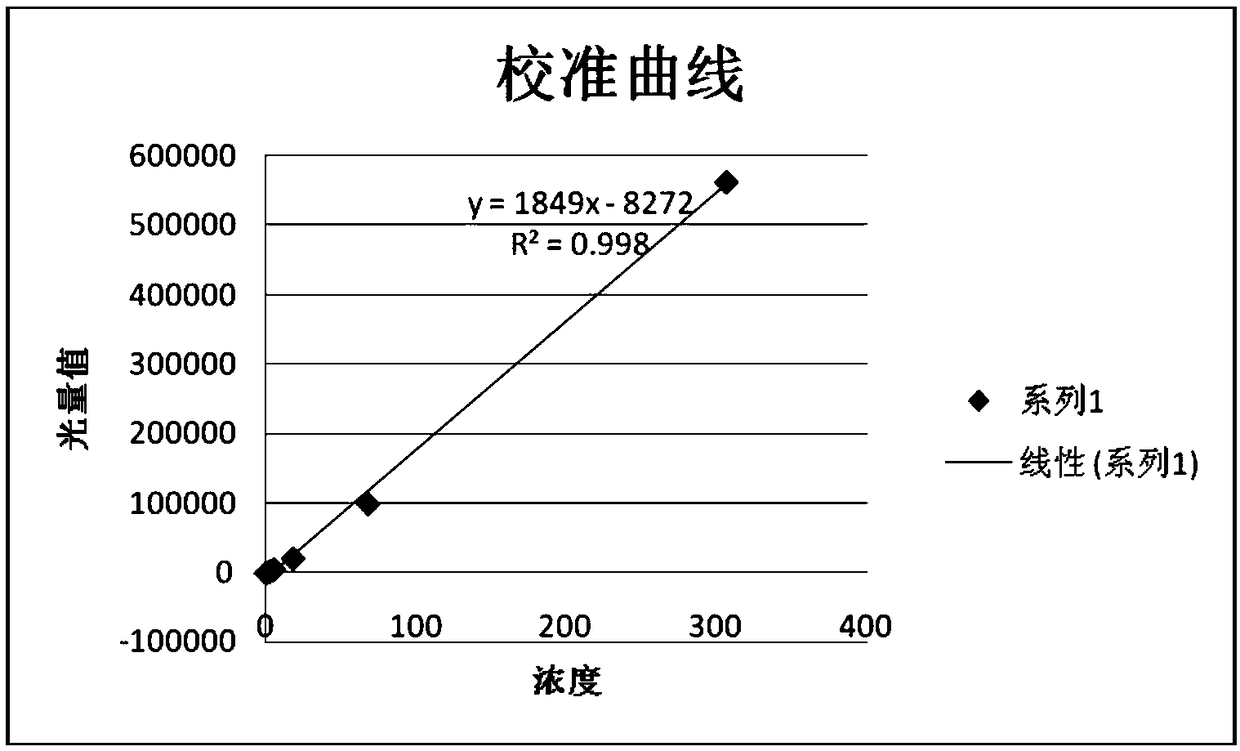
The invention relates to a reagent for detecting myocardial infarction and the application of the reagent and particularly relates to a reagent for detecting miR-520c and application of the reagent in preparing reagents for detecting myocardial infarction. Because of specificity, sensitivity and the like, conventional myocardial infarction diagnosis biochemical indexes such as creatine kinase, lactic dehydrogenase and myoglobin are limited in clinical application. On the basis of a high-flux sequencing method, a molecular marker miR-520c tightly associated with myocardial infarction is obtained. The molecular marker and conventional biochemical indexes are supplemented by each other, defects of single detection methods are overcome, and bases are made for precise clinical diagnosis and treatment.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Chemiluminescent quantitative detection kit for detection of creatine kinase MB in serum/plasma and preparation method thereof
InactiveCN109490549ALow costHigh detection sensitivityChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexCreatine kinase
The invention belongs to the technical field of immunoassay and particularly relates to a chemiluminescent quantitative detection kit for detection of creatine kinase MB in serum / plasma and a preparation method thereof. The chemiluminescent quantitative detection kit for detection of creatine kinase MB in serum / plasma comprises: 1) magnetic particles coated with streptavidin; 2) biotin-labeled creatine kinase MB antibody; 3) acridinium ester-labeled creatine kinase MB antibody; 4) calibrator and quality control material. The calibrator and the quality control material contain high and low concentration points respectively. The chemiluminescent quantitative detection kit for detection of creatine kinase MB in serum / plasma provided herein employs the principle of double-antibody sandwich process as well as an avidin-biotin system and employs acridinium ester to chemically illuminate; detection sensitivity of the kit herein is greatly improved, reaction time is shortened, and agent cost is reduced.
Owner:DIRUI MEDICAL TECH CO LTD
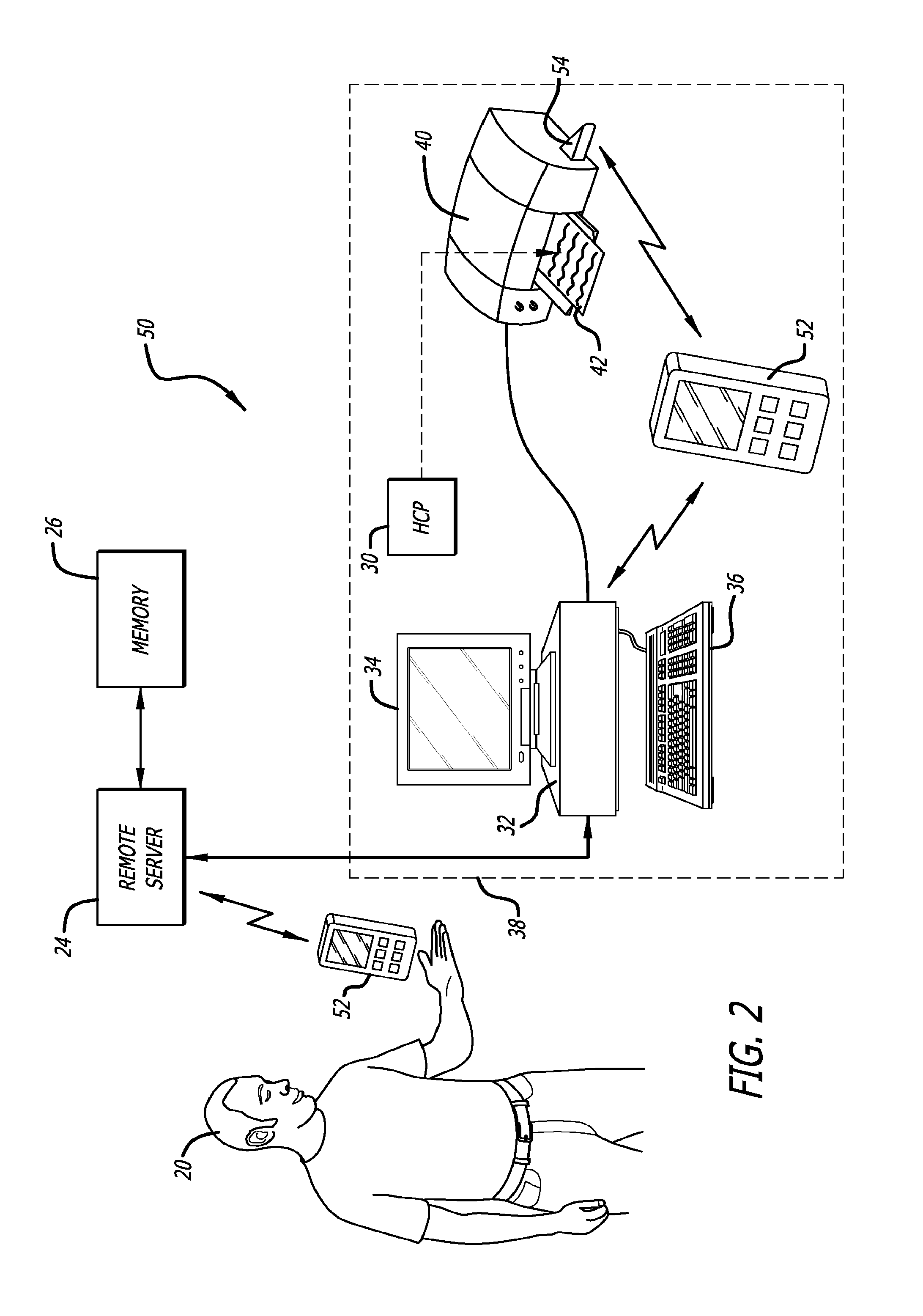
Infusion of insulin into a patient and diabetes mellitus medical methods based on the patients monitored analyte concentration
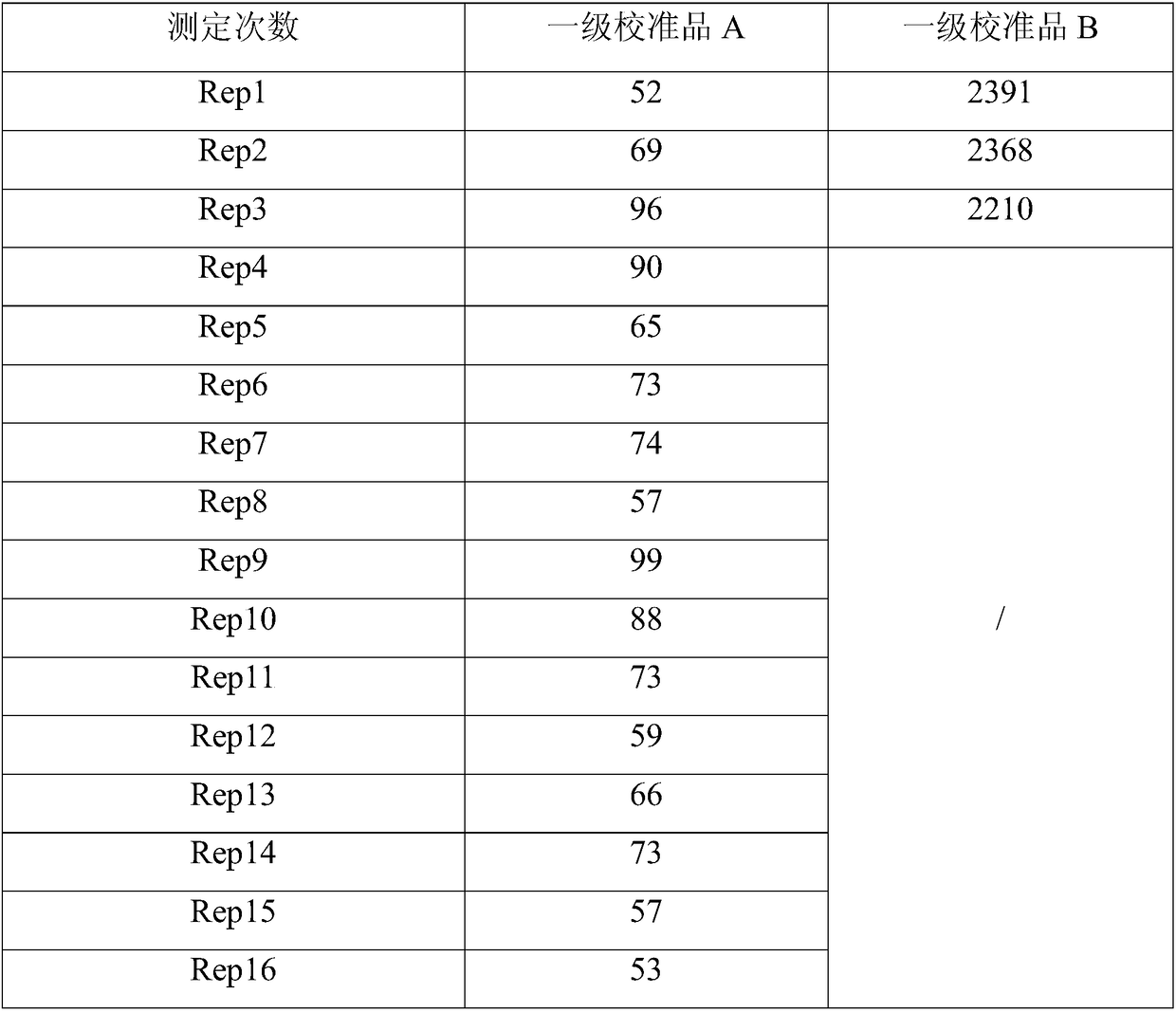
InactiveUS20170007762A1Reduce data transfer costsDrug and medicationsMedical devicesAmylaseCreatine kinase
Medical data provided by a physiological parameter sensor is used for management of the patient's medical condition. Analytes that may be monitored and managed include, but are not limited to, acetyl choline, amylase, bilirubin, cholesterol, chorionic gonadotropin, creatine kinase (e.g., CK-MB), creatine, glucose, glutamine, growth hormones, hormones, ketones, lactate, oxygen, peroxide, prostate-specific antigen, prothrombin, thyroid stimulating hormone, and troponin.
Owner:ABBOTT DIABETES CARE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com