Cardiac muscle control material, preparation method therefor, detection kit and detection device for cardiac muscle
A quality control product, cardiac troponin technology, which can be used in measurement devices, instruments, disease diagnosis, etc., and can solve problems such as unreasonable formula and poor stability of myocardial quality control products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
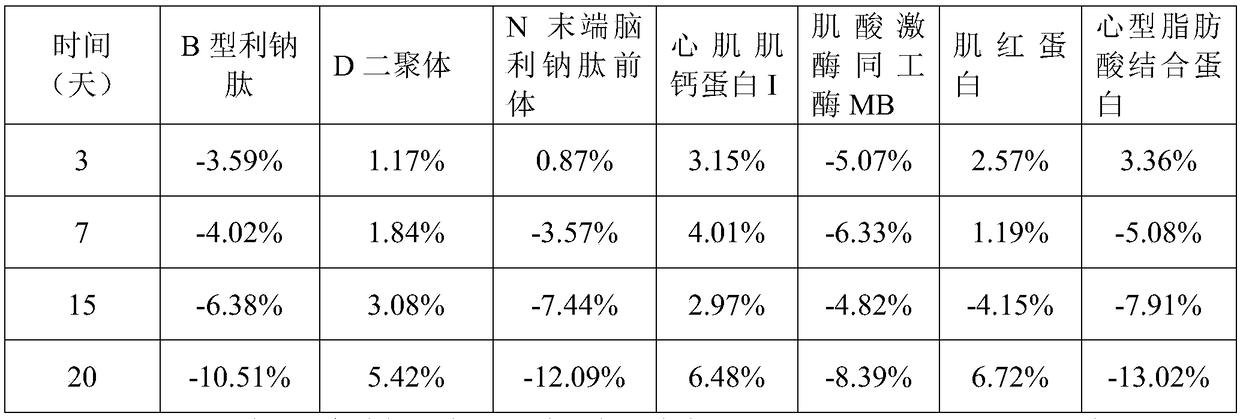
[0069] A method for preparing a myocardial quality control product in an embodiment includes the following operations S110-S130:
[0070] S110. Add the protection component to the water and mix well and adjust the pH to 6.0-8.0 to obtain a protection solution. The protection component includes 5mM-100mM buffer, 0.5%-15% by mass of excipient, 0.05mM -5mM antioxidant, 0.05mM-15mM protease inhibitor, 0.5%-10% stabilizer and 0.01-2% surfactant.
[0071] In one embodiment, the water is ultrapure water.
[0072] In one of the embodiments, HCl or NaOH is used to adjust the pH.
[0073] In one of the embodiments, the protection component is added to the water to mix well and the pH is adjusted to 7-7.5.
[0074]S120. Add quality control components to the protection solution and mix to obtain a mixed solution. The quality control components include B-type natriuretic peptide at 50 pg / mL-2000 pg / mL, and D-dimer at 0.2 μg / mL-5 μg / mL , 50pg / mL~10000pg / mL N-terminal brain natriuretic pe...
Embodiment 1
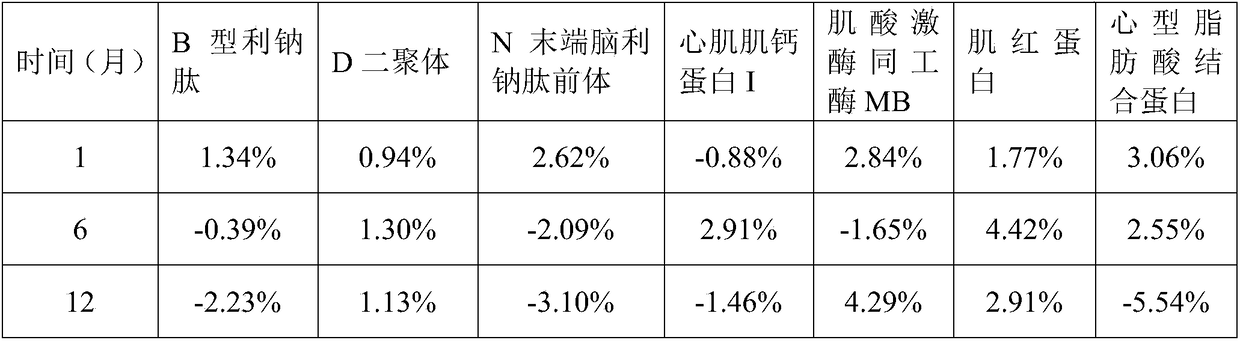
[0098] 1. The myocardial quality control product in this embodiment includes 25mM Tris buffer, 5% glycogen by mass percentage, 2% polyethylene glycol 8000 by mass percentage, 1mM Ascorbic acid, 5mM disodium edetate, bovine serum albumin with a mass percentage of 2%, sodium chloride with a mass percentage of 0.9%, arginine with a mass percentage of 0.5%, mass The percentage content is 0.2% Tween-20, the mass percentage content is 0.05% ProClin300, 100pg / mL B-type natriuretic peptide, 0.5μg / mL D dimer, 125pg / mL N-terminal natriuretic peptide Natriuretic peptide precursor, 0.5 ng / mL cardiac troponin I, 5 ng / mL myoglobin, 25 ng / mL creatine kinase isoenzyme MB, and 5 ng / mL heart fatty acid binding protein.
[0099] The preparation process of the quality control product of the present embodiment is as follows:
[0100] Add protective components to ultrapure water and mix well and adjust the pH to 7.0 to obtain a protective solution; add quality control components to the protective ...
Embodiment 2
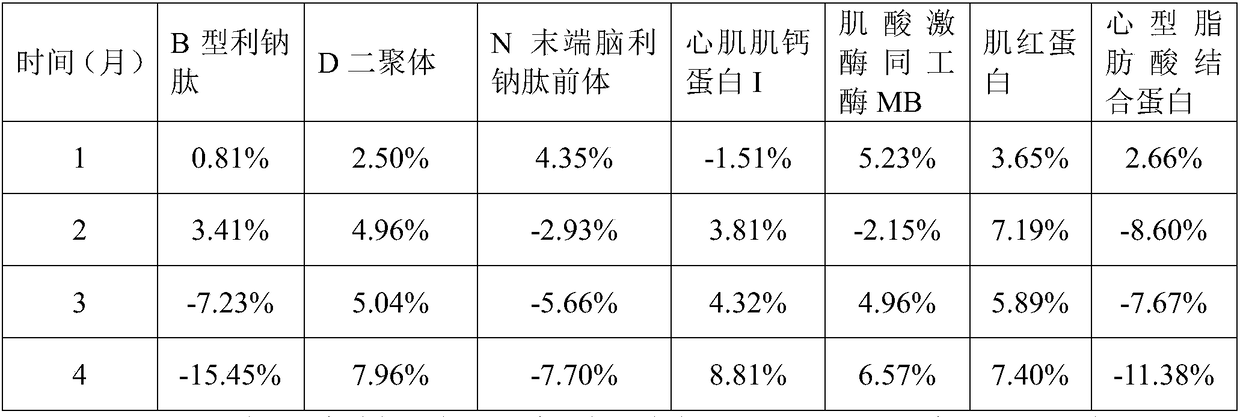
[0127] 1. The myocardial quality control product of the present embodiment includes 25mM phosphate buffer, 5% sucrose by mass, 3% polyvinylpyrrolidone by mass, and 1% sorbic acid by mass Alcohol, 1mM ascorbic acid, 1mM phenylmethylsulfonyl fluoride, 2% casein by mass, 0.9% sodium chloride by mass, 0.5% histidine by mass, The mass percent content is 0.2% Tween-20, the mass percent content is 0.05% ProClin300, the B-type natriuretic peptide of 1000pg / mL, the D dimer of 2.5μg / mL, the N-terminal brain of 5000pg / mL Pronatriuretic peptide, 5 ng / mL cardiac troponin I, 100 ng / mL myoglobin, 500 ng / mL creatine kinase isoenzyme MB, and 50 ng / mL heart fatty acid binding protein.
[0128] The preparation process of the quality control product of the present embodiment is as follows:
[0129] Add protective components to ultrapure water and mix well and adjust the pH to 7.5 to obtain a protective solution; add quality control components to the protective solution to obtain a mixed solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com