Three-in-one detection kit for cardiac troponin I, creatine kinase isoenzyme and myoglobin and preparation method of three-in-one detection kit
A cardiac troponin and detection kit technology, which is applied in biological tests, measuring devices, material inspection products, etc., can solve the problems of strong interference and high intensity, and achieve the effects of high sensitivity, fast response and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
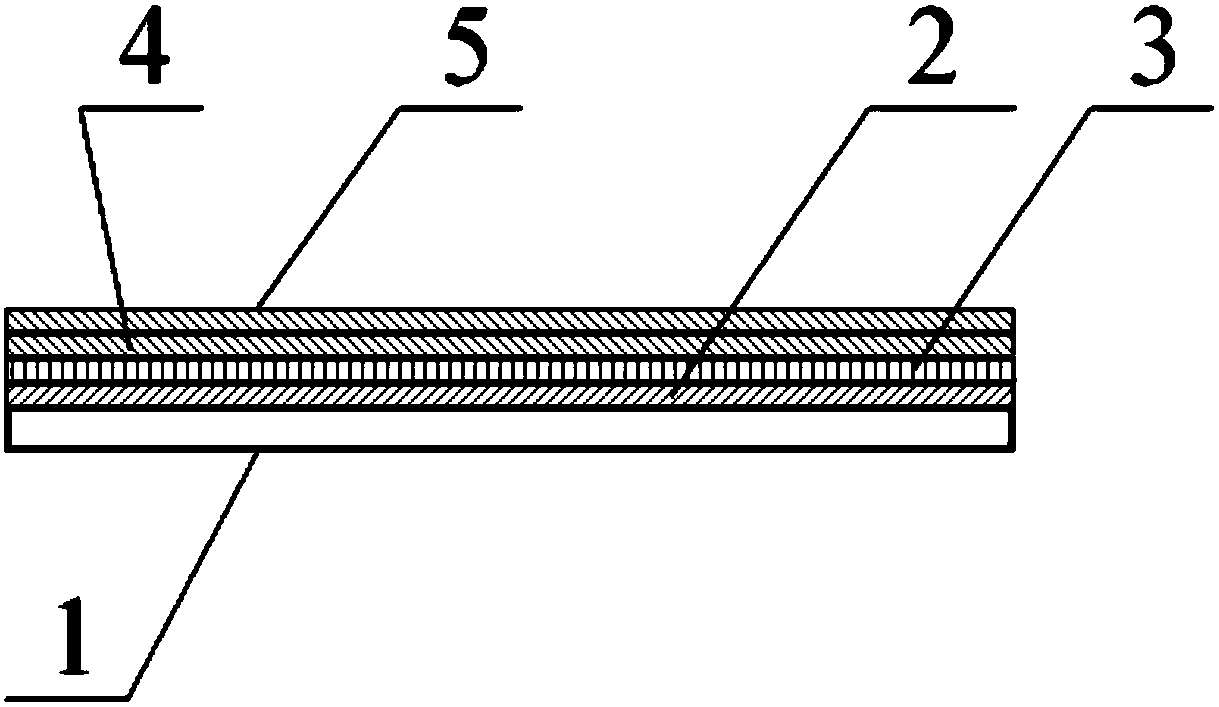
[0036] The various components of the test paper card in the cardiac troponin I, creatine kinase isoenzyme and myoglobin three-in-one detection kit can be prepared by the following measures:
[0037] 1. Preparation of sample pad 2:
[0038] Soak the glass fiber membrane in the treatment solution containing 2.0% Triton X-100, 2% BSA, 0.1M Tris buffer, pH7.5, soak at 4°C for 4 hours, then place it in an oven and dry it at 37°C 2 hours. 2. Preparation of binding pad 3 for absorbing fluorescent microsphere-labeled antibody:
[0039] Soak the glass fiber membrane in 200mM Tris-HCL treatment solution (containing 1.5% Triton X-100, 1.5% BSA, pH7.5), soak at 4°C for 4 hours, then take it out of a 37°C oven and dry it for 4 hours, and set it aside. Put the glass fiber membrane on the Bio-DotXYZ3050 three-dimensional spraying platform, and use the Bio-Jet Quanti300 non-contact micro-quantitative nozzle to combine rare earth fluorescent microspheres labeled cardiac troponin I, creatine ...
Embodiment 2
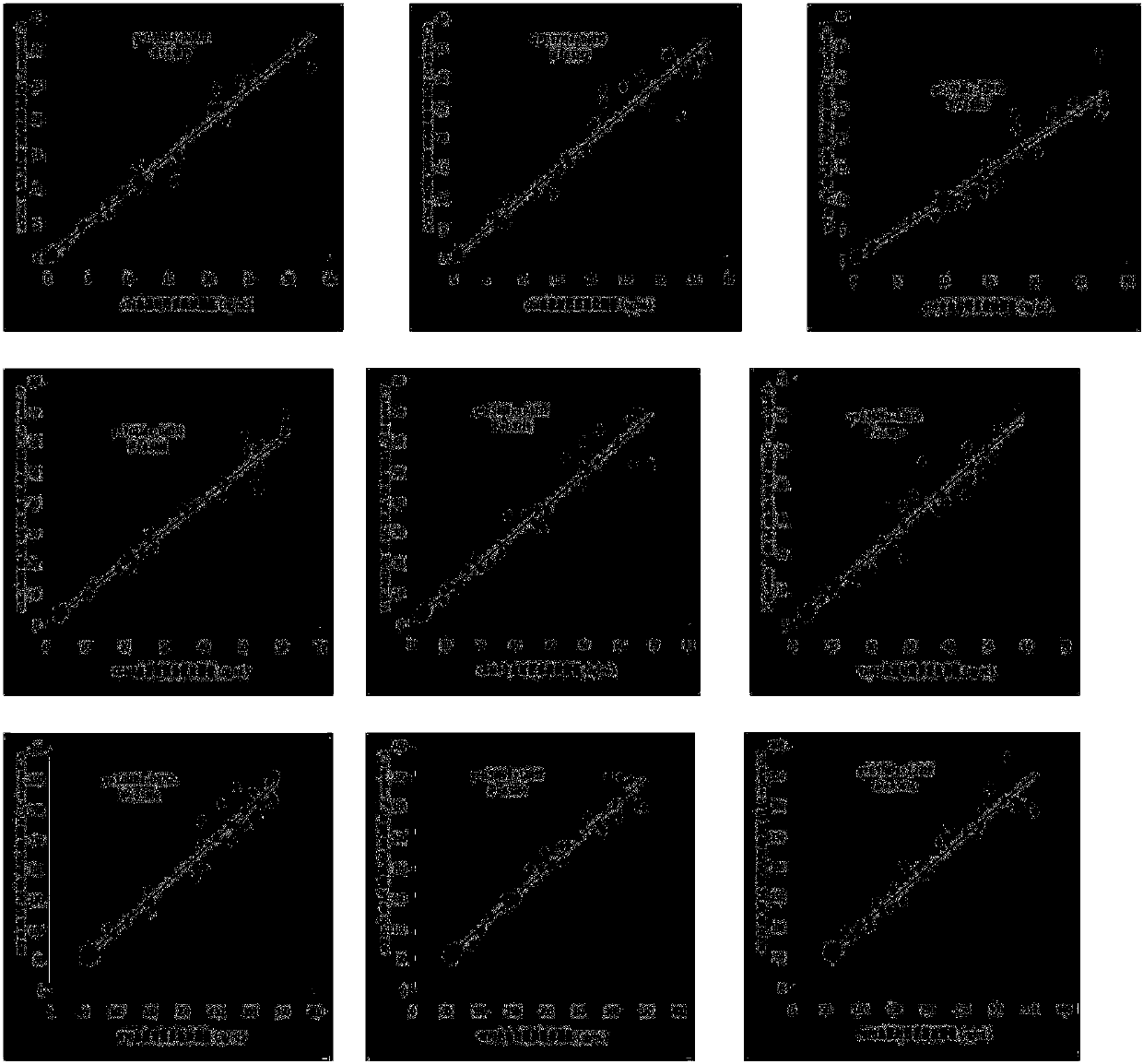
[0048] Embodiment 2: accuracy test
[0049] Select the above test paper card and fluorescence immunochromatography analyzer (model: NEO-007),
[0050] Setting of the parameters of the fluorescence immunoassay analyzer: After setting the process parameters of the test paper card on the fluorescence immunoassay analyzer, take the above-mentioned assembled test paper card, and use 0.2, 0.5, 1, 2, 5, 20 ng / ml of myocardial muscle Calpain I, creatine kinase isozyme and myoglobin three-in-one calibrator are measured with a test paper card to obtain the fluorescence intensity value of each calibrator, and the results are input into the parameters of the analyzer to complete the parameters of the analyzer. settings.
[0051] Main testing materials: clinical samples obtained from relevant hospitals, a total of 200 Roche electrochemiluminescence immunoassay value samples, including 100 serum samples, 100 whole blood samples, cardiac troponin I, creatine kinase isoenzyme and myoglobin ...
Embodiment 3
[0060] Embodiment 3: precision test
[0061] Using the test paper card and measuring system of Example 2, the test paper card and the fluorescent immunochromatographic analyzer of the present invention were tested for precision.
[0062] Main testing materials: clinical samples were obtained from relevant hospitals, a total of 2 serum samples with chemiluminescence immunoassay value, among which the clinical measurement value of the low value fixed value sample was 0.24ng / ml, and the clinical measurement value of the high value fixed value sample was 1.78ng / ml .
[0063] Preparation:
[0064] Using the test paper card and measuring system of Example 2, each of the 2 fixed-value samples was repeatedly measured 20 times.
[0065] Analysis of test results:
[0066] After the clinical sample testing reagents are prepared, the clinical samples are tested according to the preparation method, and the test results are analyzed.
[0067] test results:
[0068] As shown in Table 1, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com