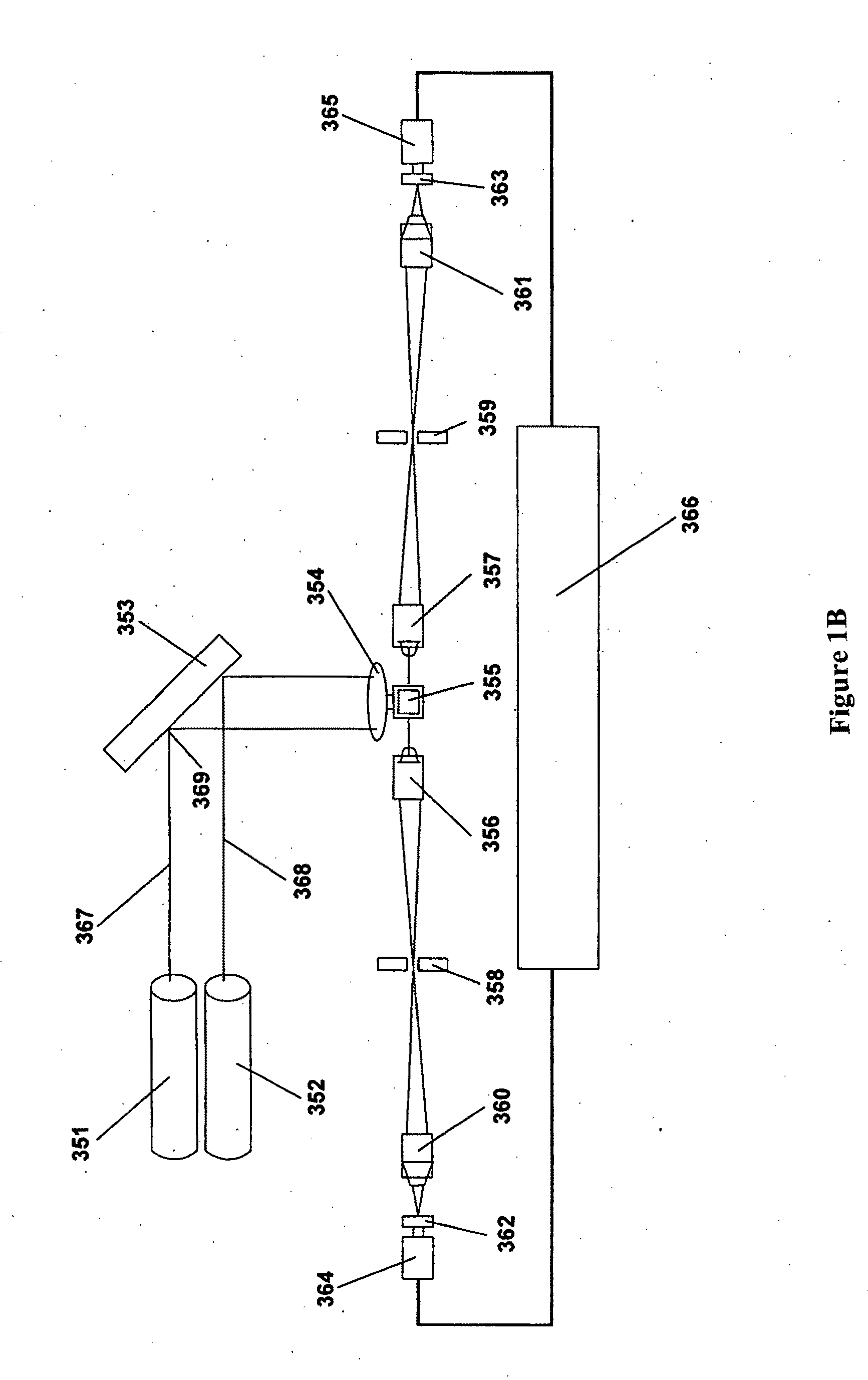
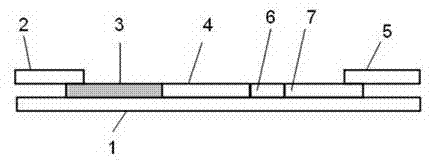
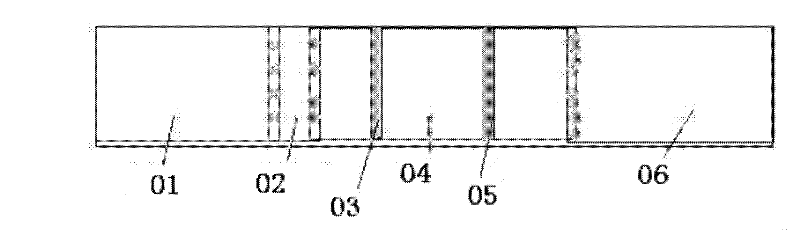
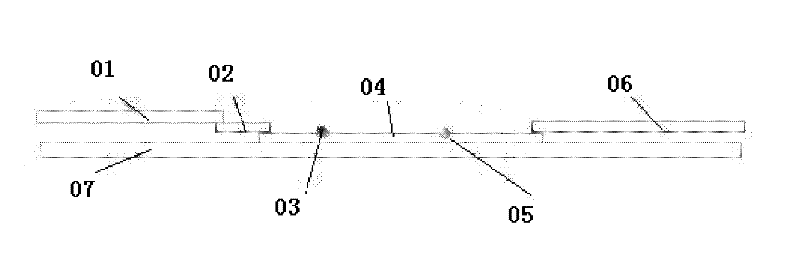
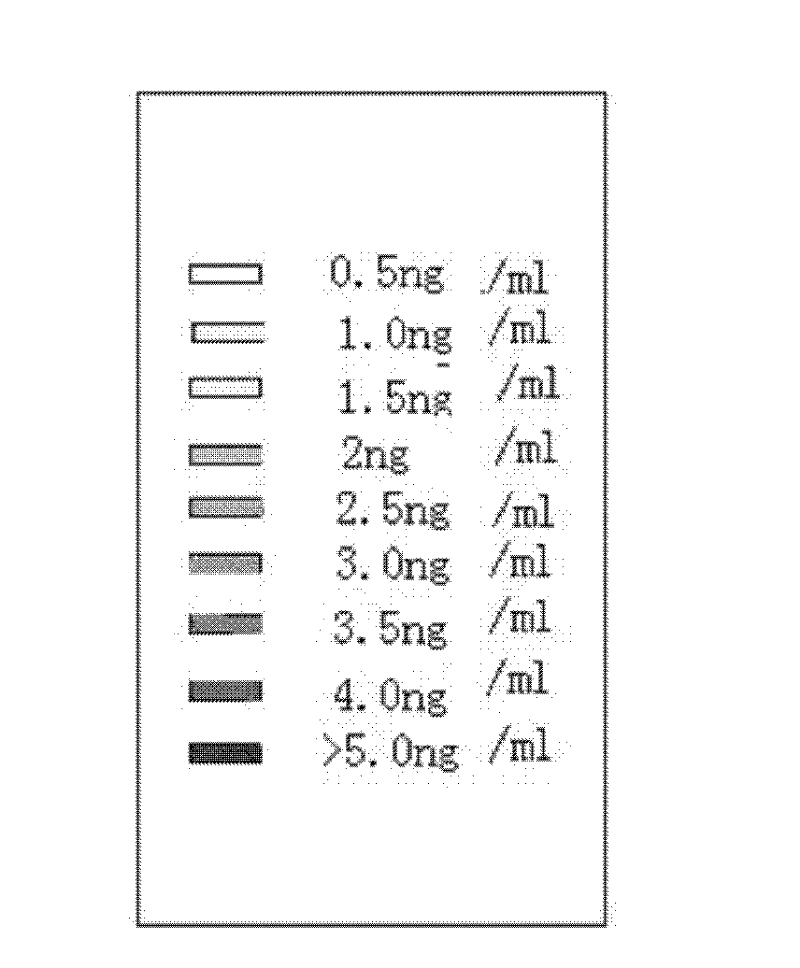
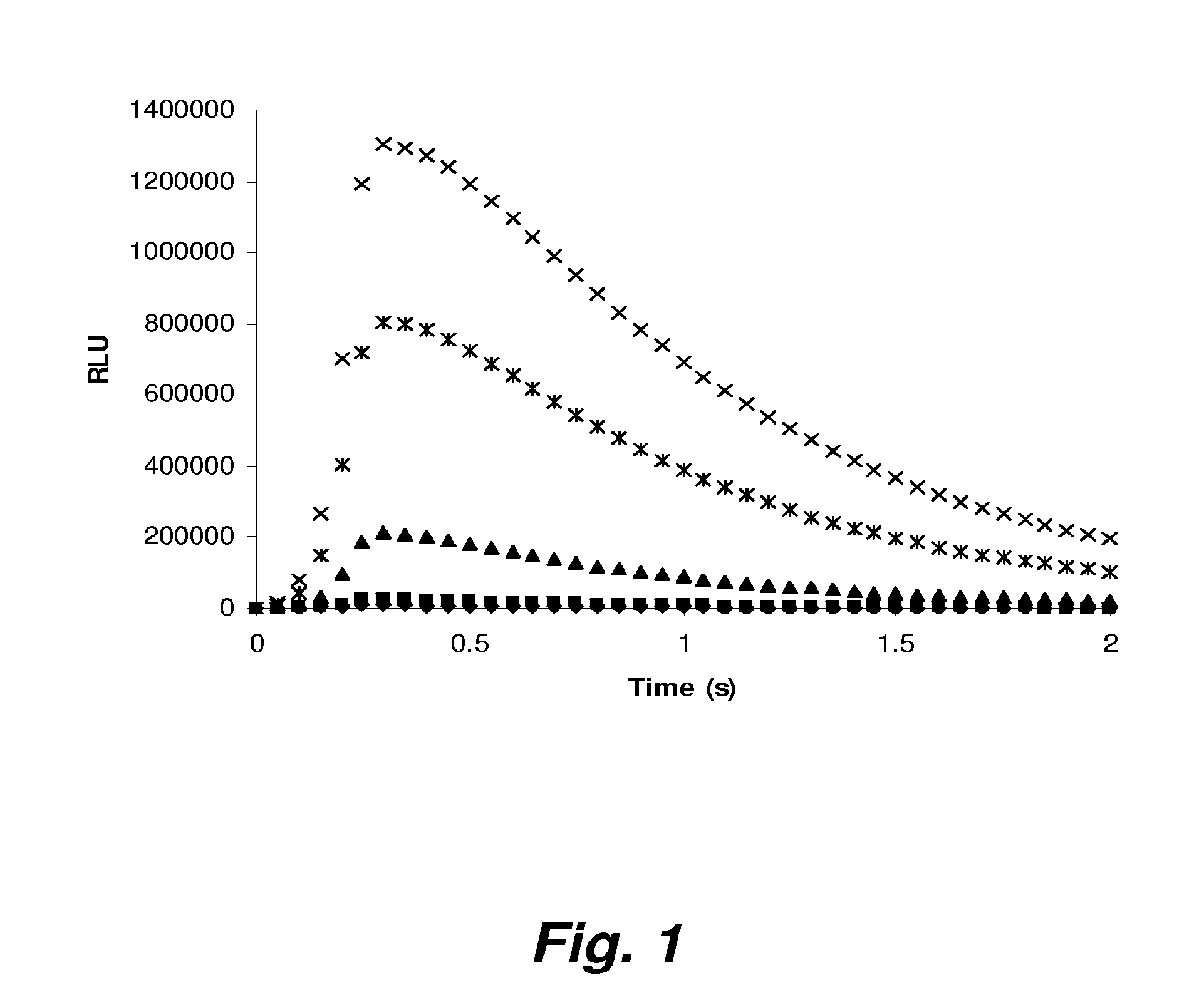
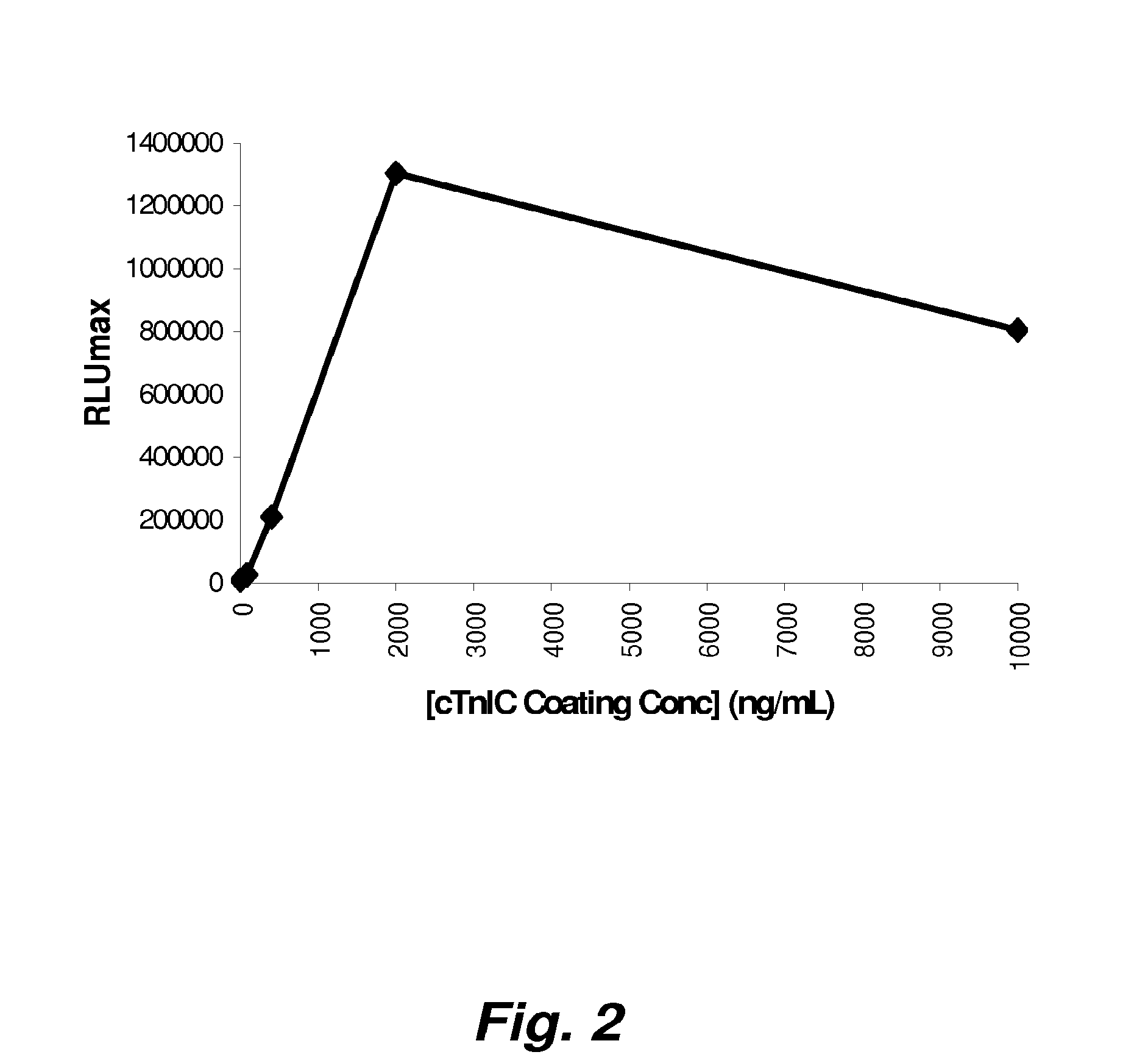
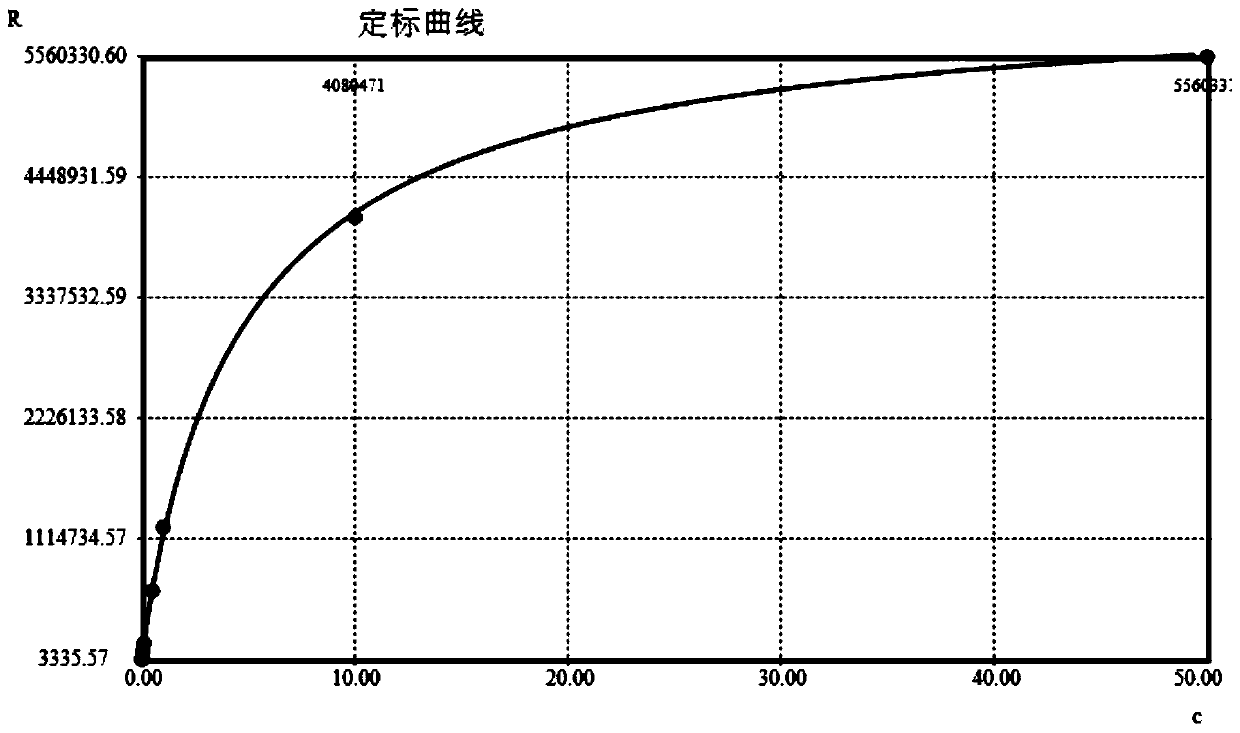
Patents
Literature
214 results about "Cardiac troponin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
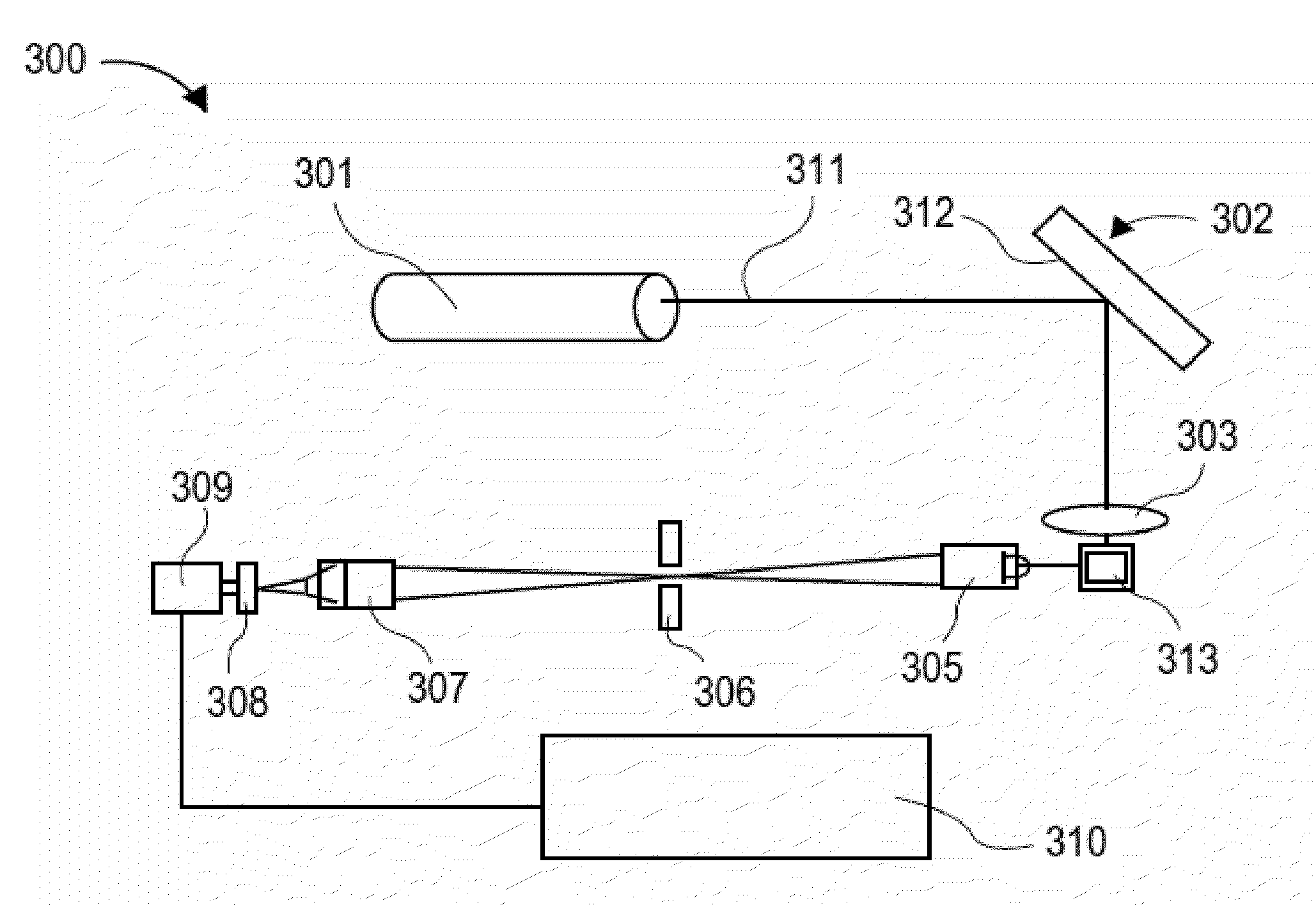
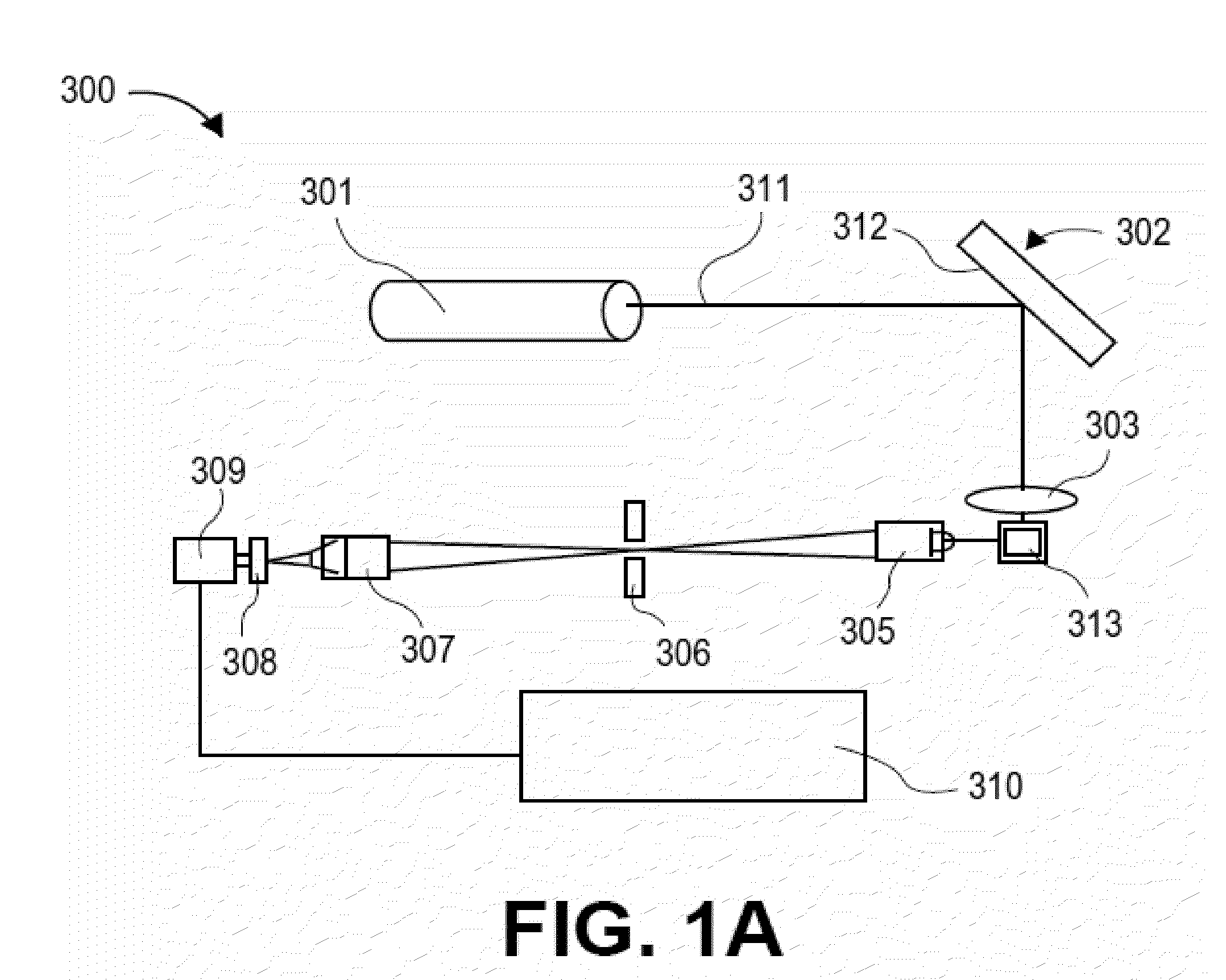
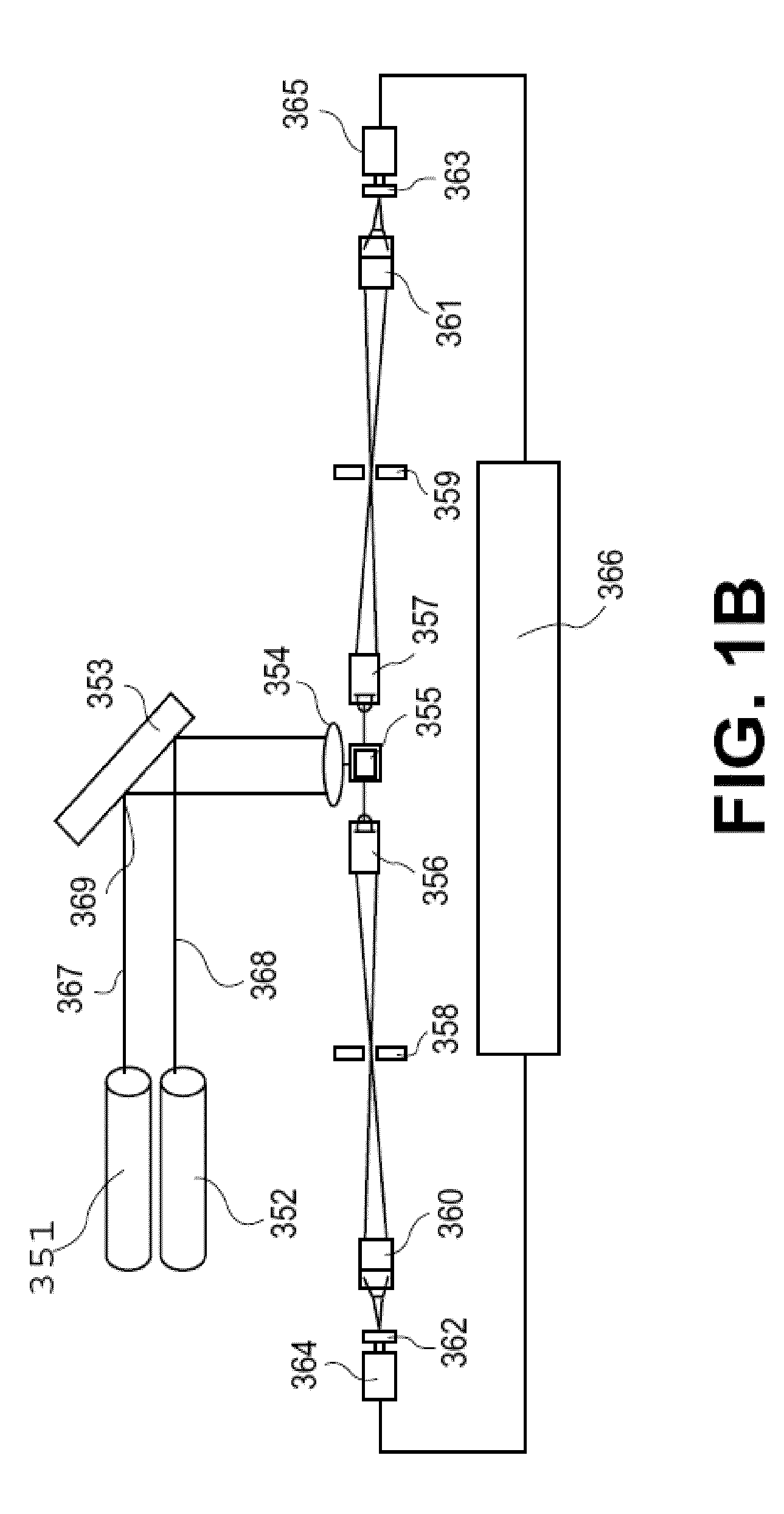
Conjunctive analysis of biological marker expression for diagnosing organ failure
InactiveUS6461828B1Easy to useHigh riskAnalysis using chemical indicatorsEnzymologyOrgan systemOrganism
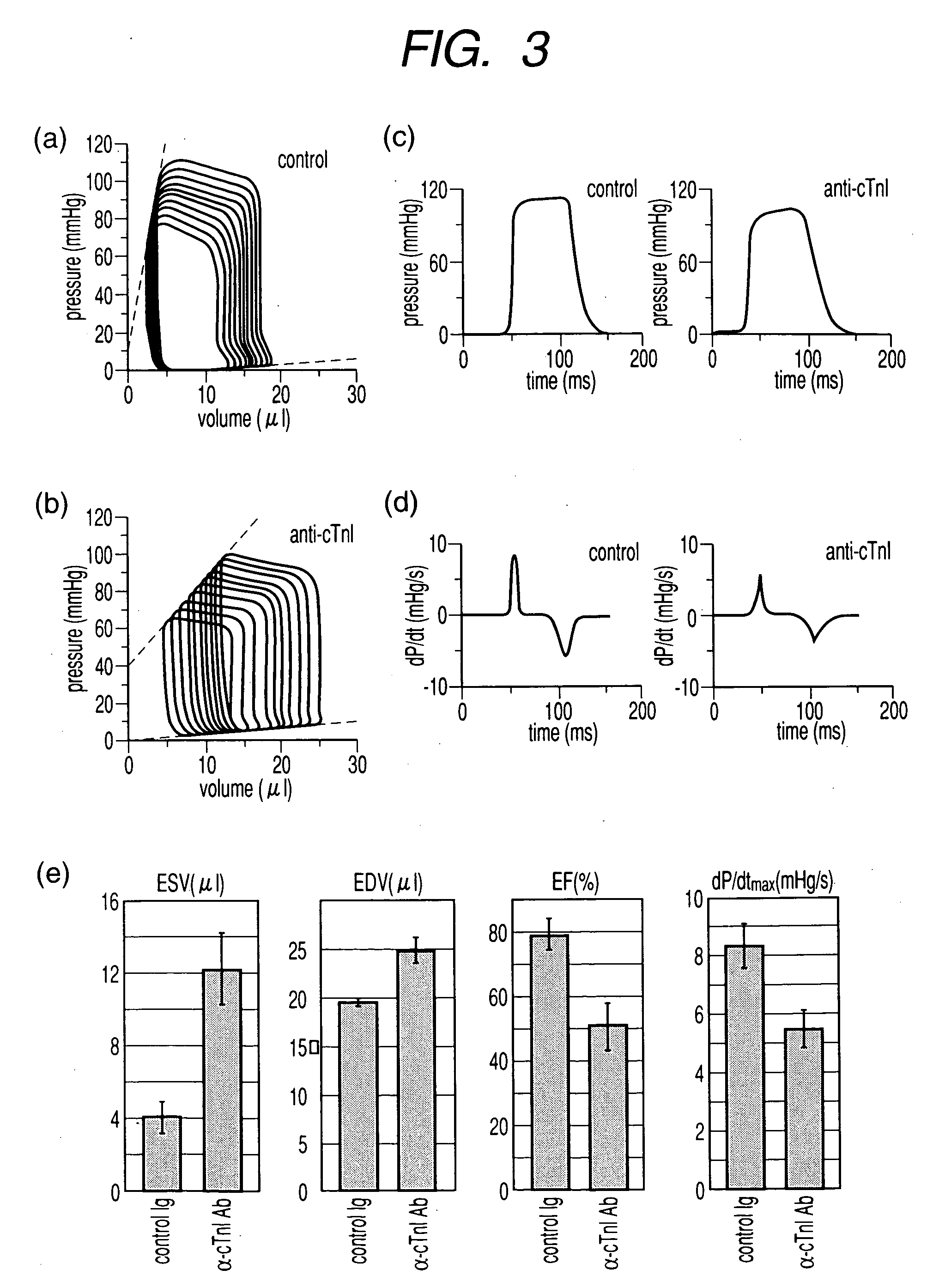
A diagnostic tool is disclosed for accurately and rapidly diagnosing the condition of an ailing organ. Although applicable to numerous organ and organ systems, this application particularly illustrates the concept of conjunctive marker utilization as it relates to diagnosing and distinguishing congestive heart failure. The invention particularly relates to the conjunctive utilization of cardiac Troponin I (cTn-I) and natriuretic peptide, e.g. ANP, pro-ANP, BNP, pro-BNP and CNP as a retrospective tool for diagnosing the underlying mechanism of heart failure and as a prospective analytical device for monitoring disease progression and efficacy of therapeutic agents.
Owner:SYN X PHARMA
Method and compositions for highly sensitive detection of molecules
InactiveUS20090234202A1Detection moreImprove the level ofElectrocardiographyMicrobiological testing/measurementVascular endothelial growth factorPhysiological markers
The present invention discloses methods for the detection and monitoring of a condition in a subject using highly sensitive detection of molecules. The invention provides a method for detecting or monitoring a condition in a subject, comprising detecting a first marker in a first sample from the subject and detecting a second marker, wherein the first marker comprises a biomarker, e.g., Cardiac Troponin-I (cTnI) or Vascular Endothelial Growth Factor (VEGF), and wherein the limit of detection of the first marker is less than about 10 pg / ml. The second marker can be a biomarker, physiological marker, a molecular marker or a genetic marker.
Owner:SINGULEX
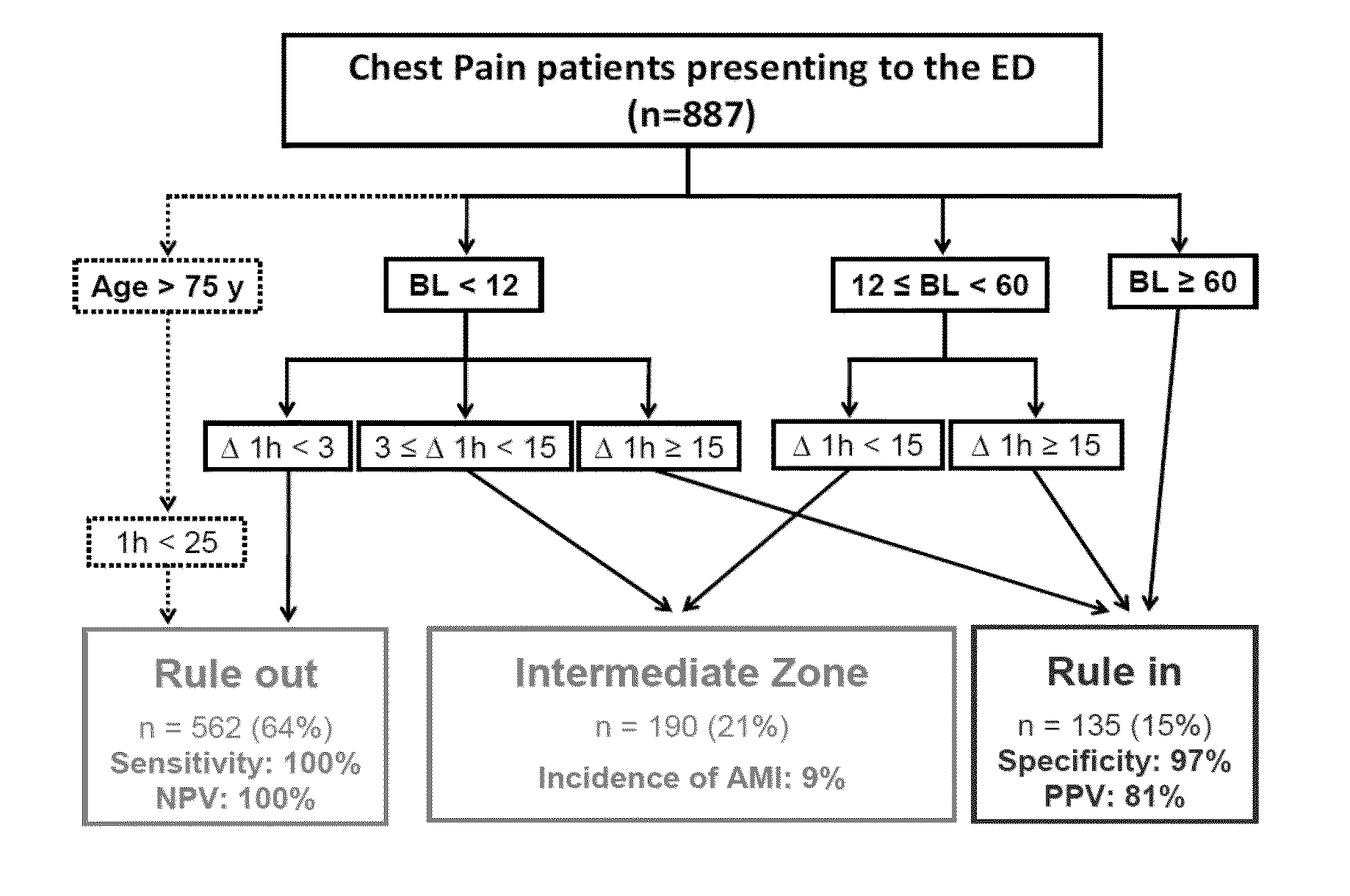
Troponin based rule-in and rule-out algorithm of myocardial infarction
InactiveUS20130035603A1Eliminate needPositive predictive valueCatheterDisease diagnosisChest painCardiac troponin
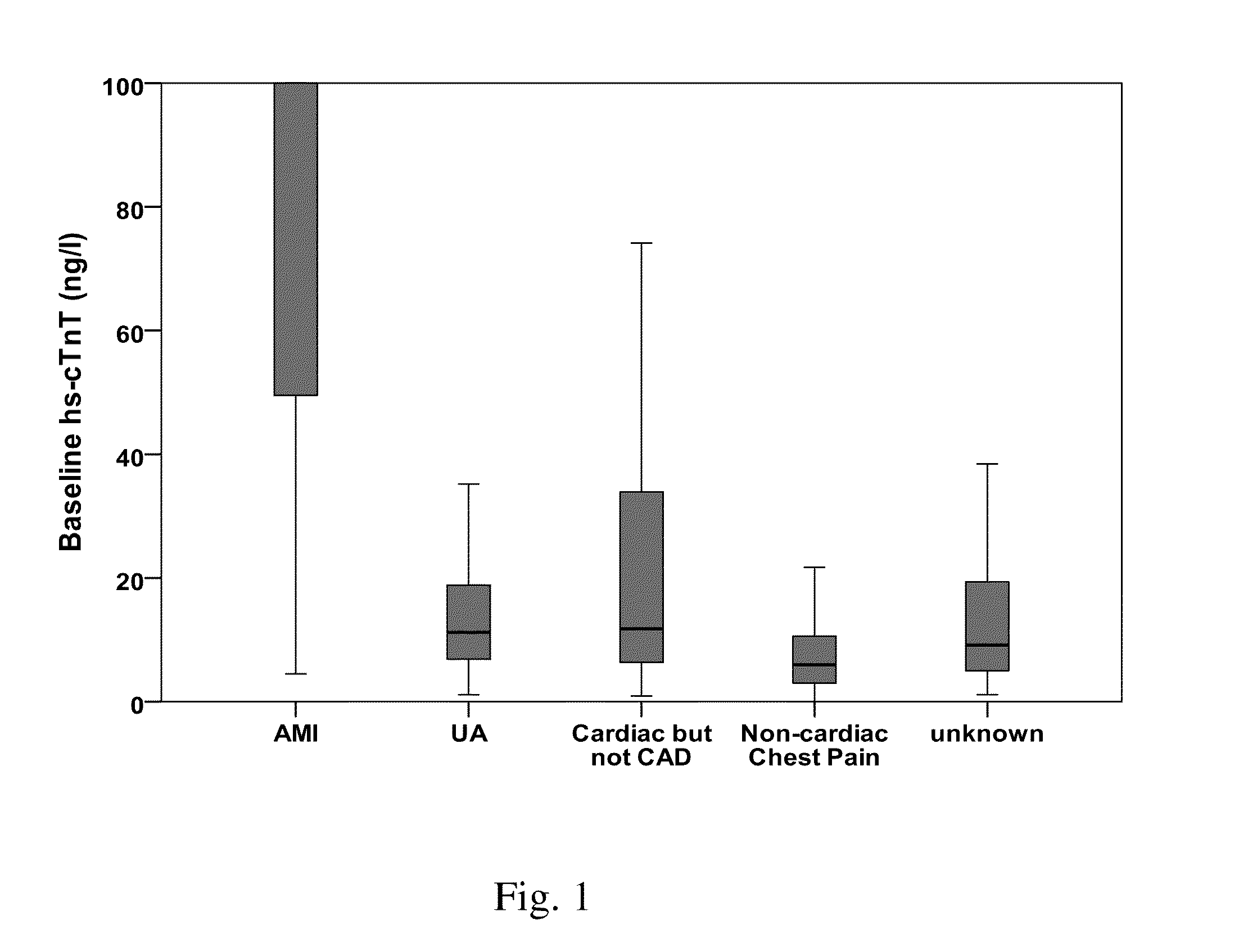
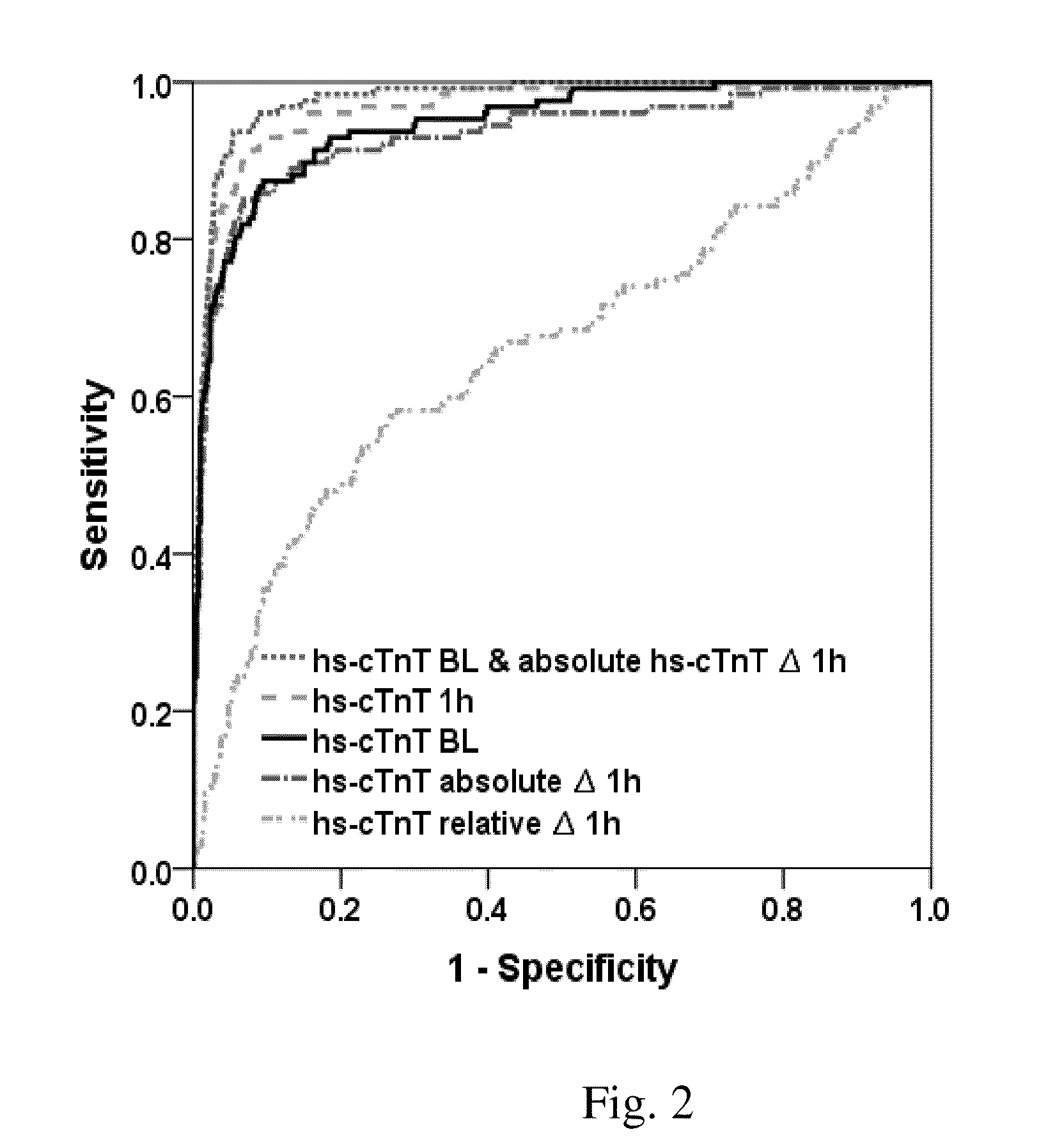
The present invention relates to a method for diagnosing myocardial infarction in a subject presenting with chest pain. The method is based on the determination of an amount of a cardiac troponin in a first sample from the subject obtained at presentation to a physician, and in a second sample obtained within one hour after the first sample. Moreover, the present invention envisages a method for ruling in myocardial infarction and a method for ruling out myocardial infarction. The said methods are also based on the determination of the amount of a cardiac troponin in a first sample from the subject obtained at presentation to a physician, and in a second sample obtained within one hour after the first sample.
Owner:JARAUSCH JOCHEN +6
Highly sensitive system and methods for analysis of troponin
The invention provides methods, compositions, kits, and systems for the sensitive detection of cardiac troponin. Such methods, compositions, kits, and systems are useful in diagnosis, prognosis, and determination of methods of treatment in conditions that involve release of cardiac troponin.
Owner:RGT UNIV OF CALIFORNIA +1
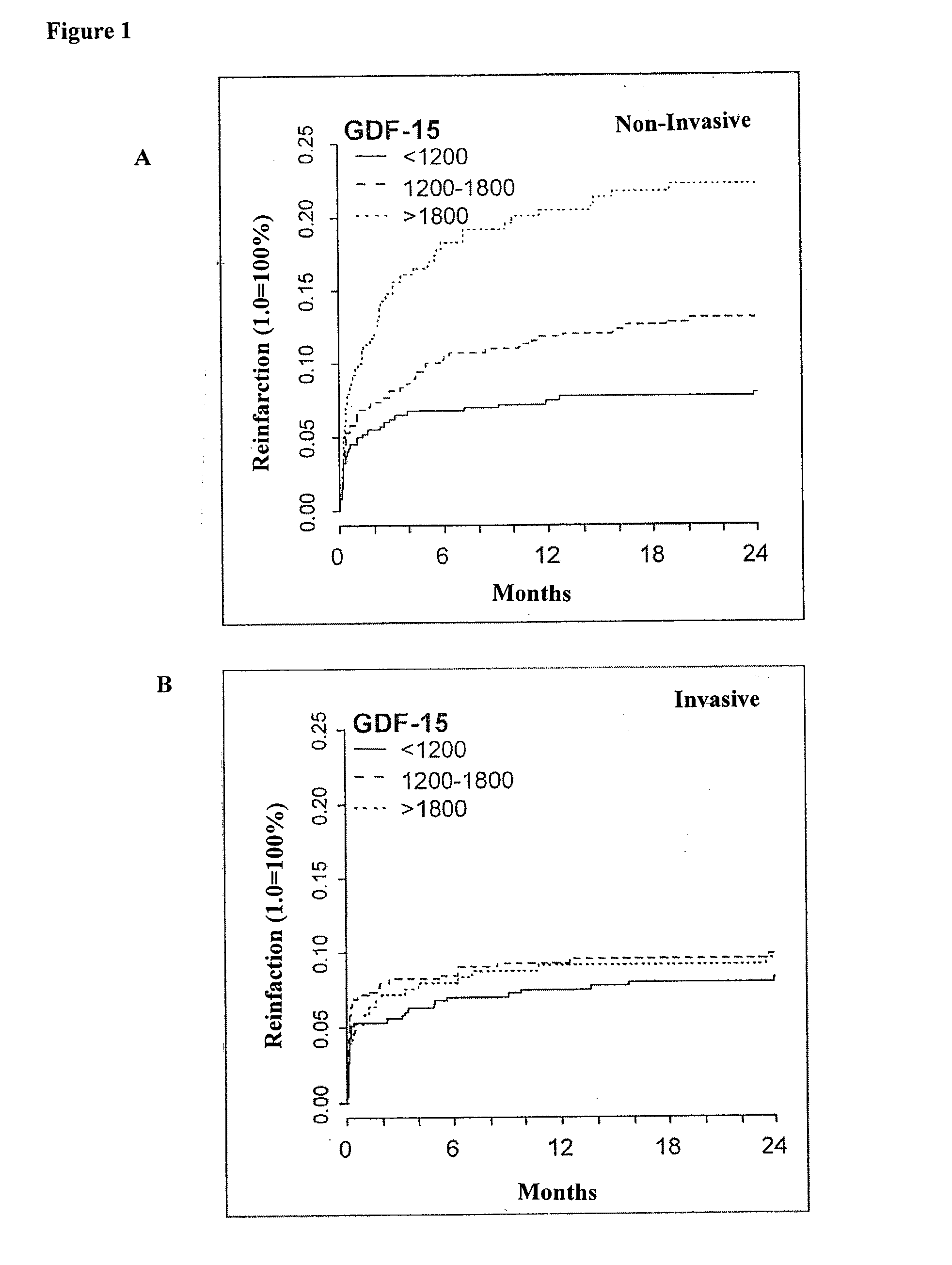
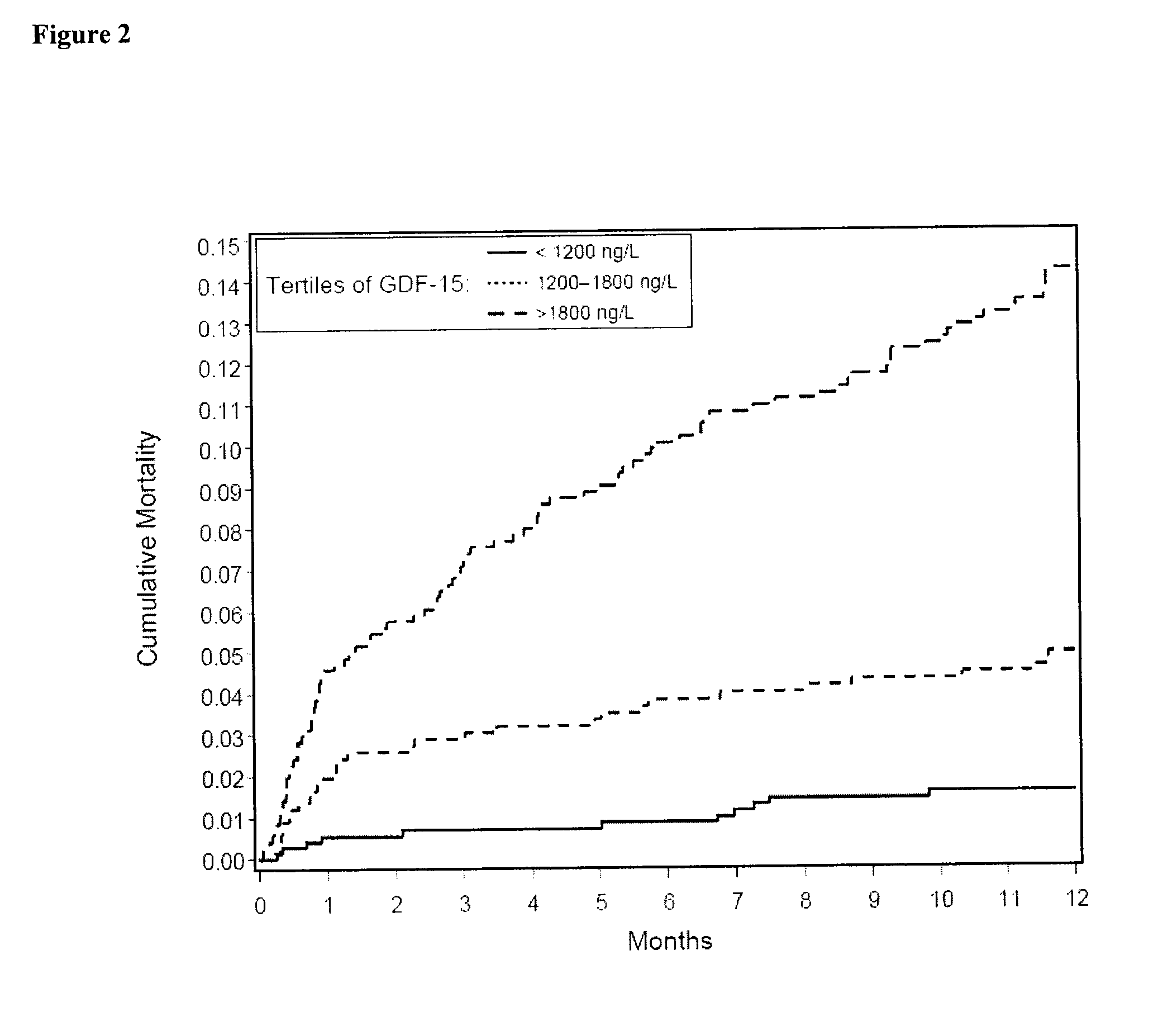
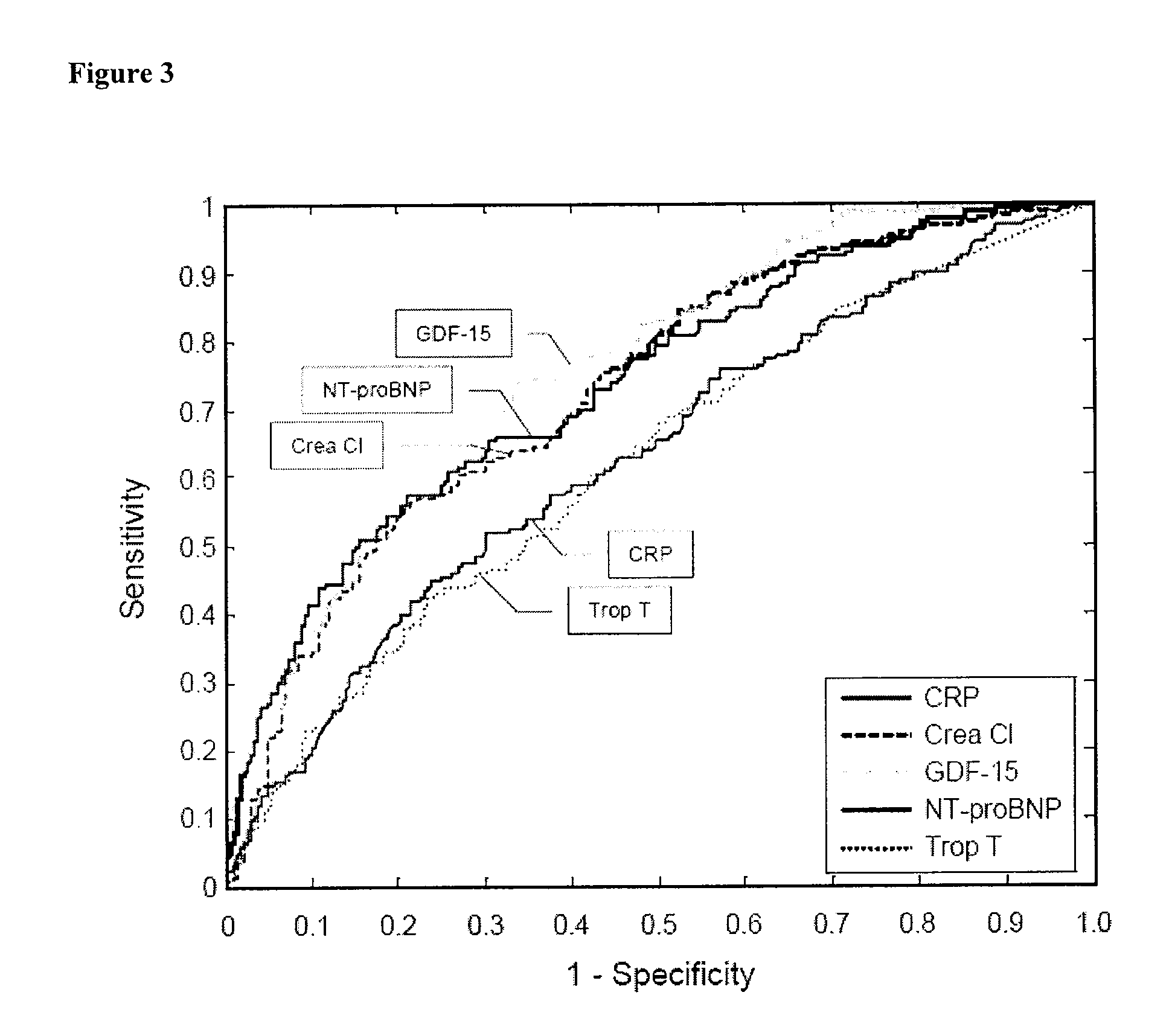
Assessing risk of cardiac intervention based on gdf-15
ActiveUS20110065204A1Analysis using chemical indicatorsComponent separationRisk of mortalityNatriuretic peptide
The present invention relates to a method of identifying a subject being susceptible to a cardiac intervention based on the determination of GDF-15 in a sample of a subject in need of a cardiac intervention. Moreover, the present invention pertains to a method for predicting the risk of mortality or a further acute cardiovascular event for a subject suffering from a cardiovascular complication based on the determination of GDF-15 and a natriuretic peptide and / or a cardiac troponin in a sample the said subject. Also encompassed by the present invention are devices and kits for carrying out the aforementioned methods.
Owner:MEDIZINISCHE HOCHSCHULE HANNOVER
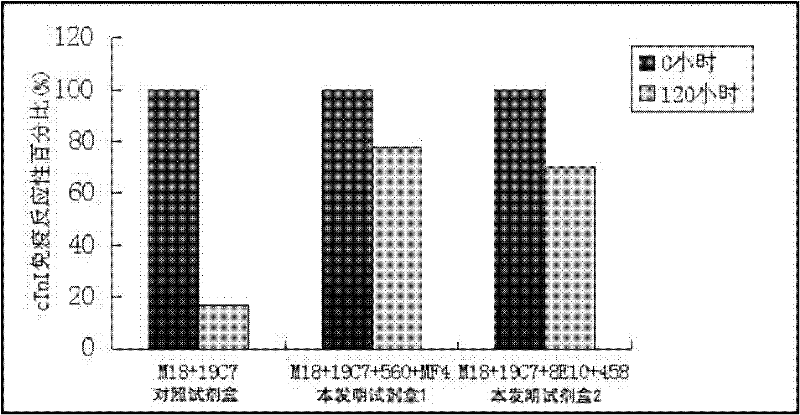
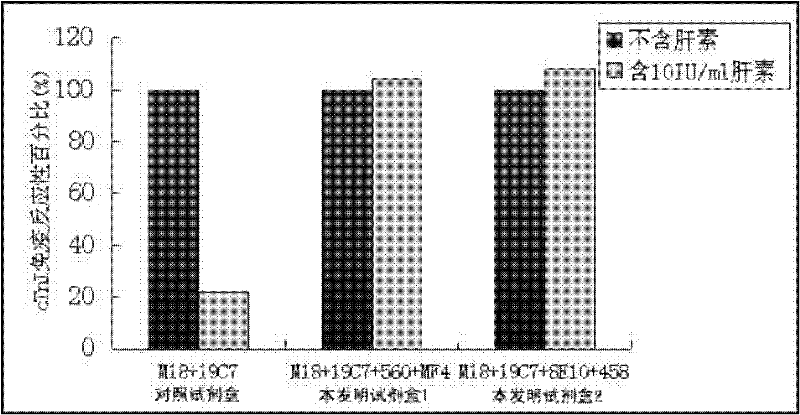
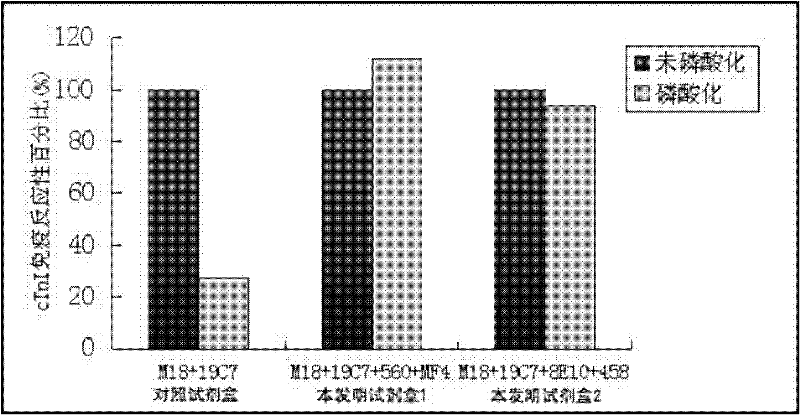
Cardiac troponin I (cTn I) hypersensitive detection kit and hypersensitive detection method
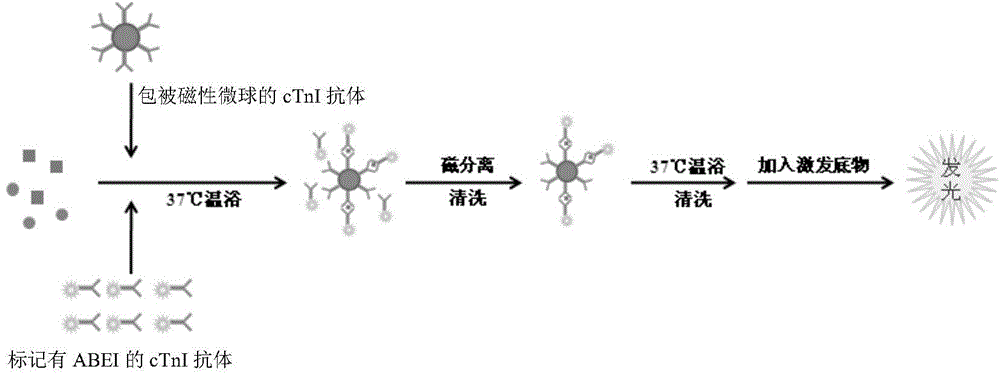
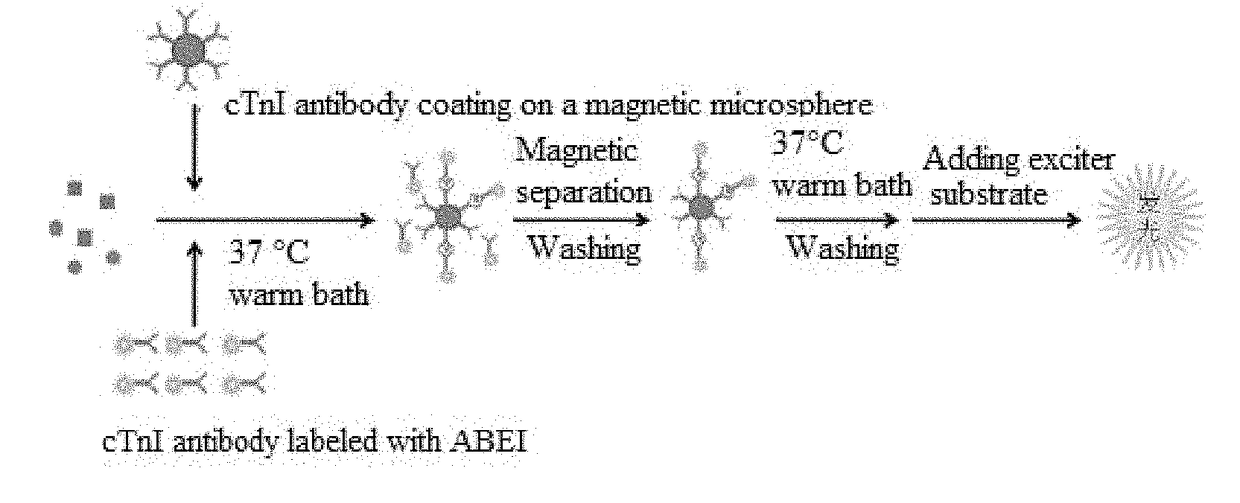
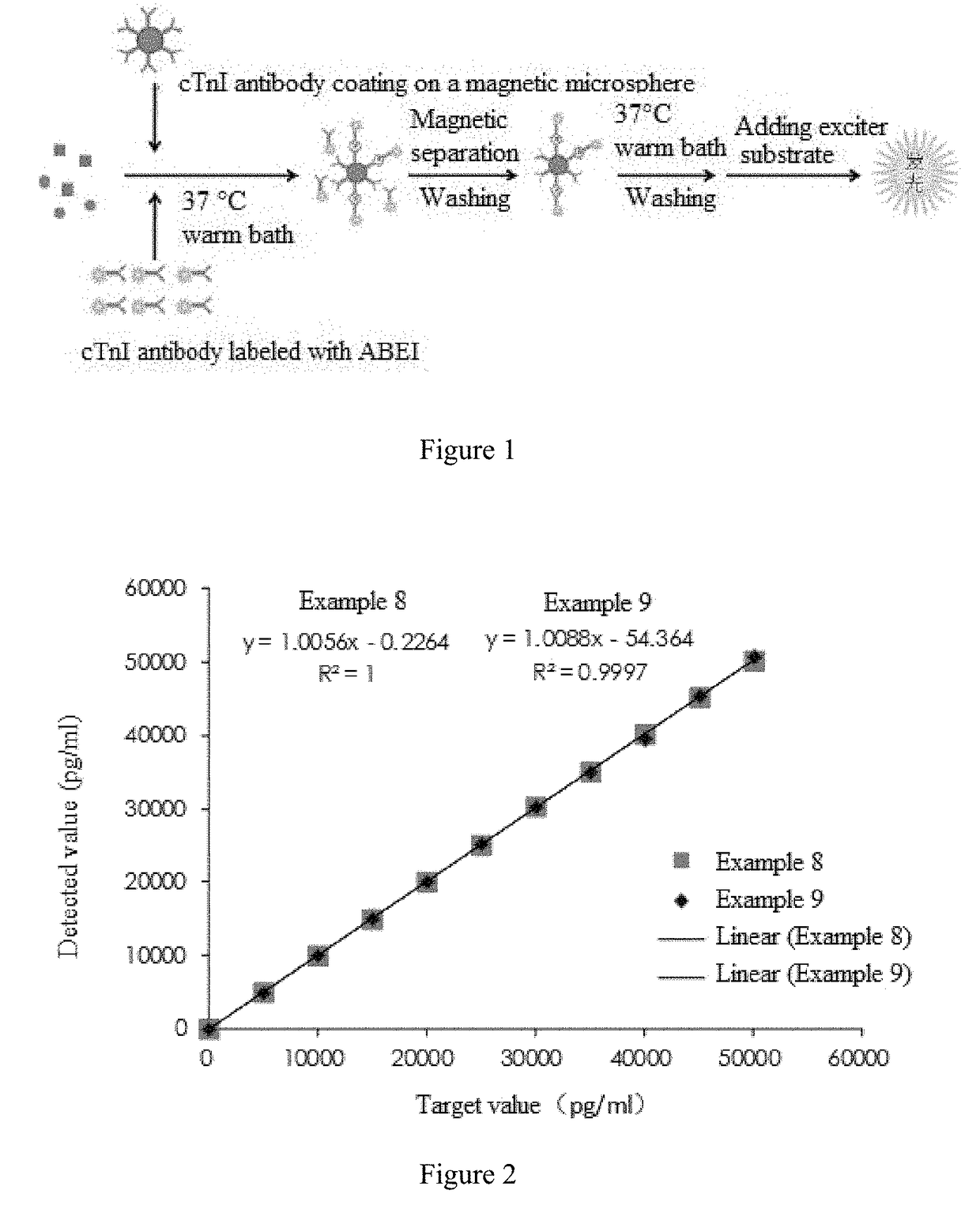
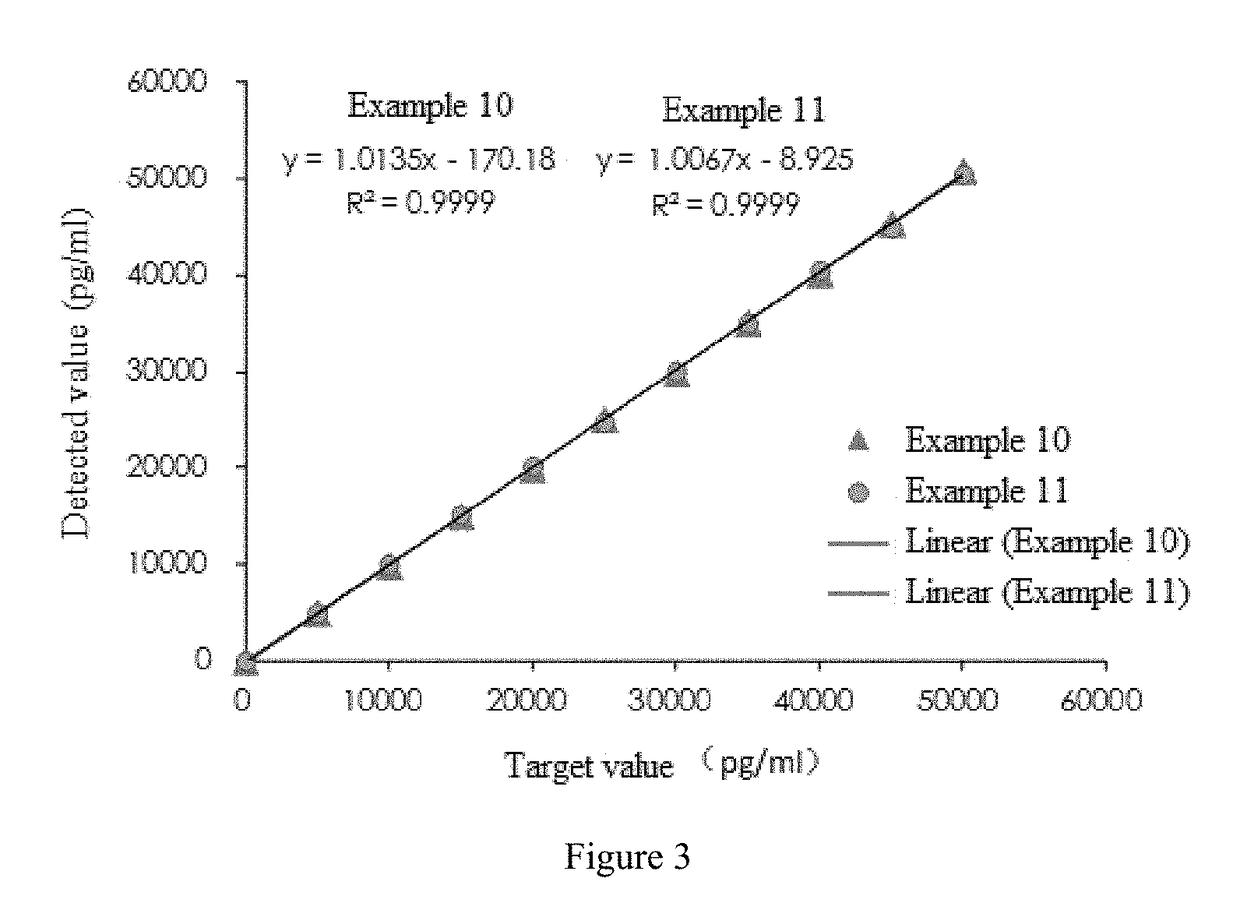
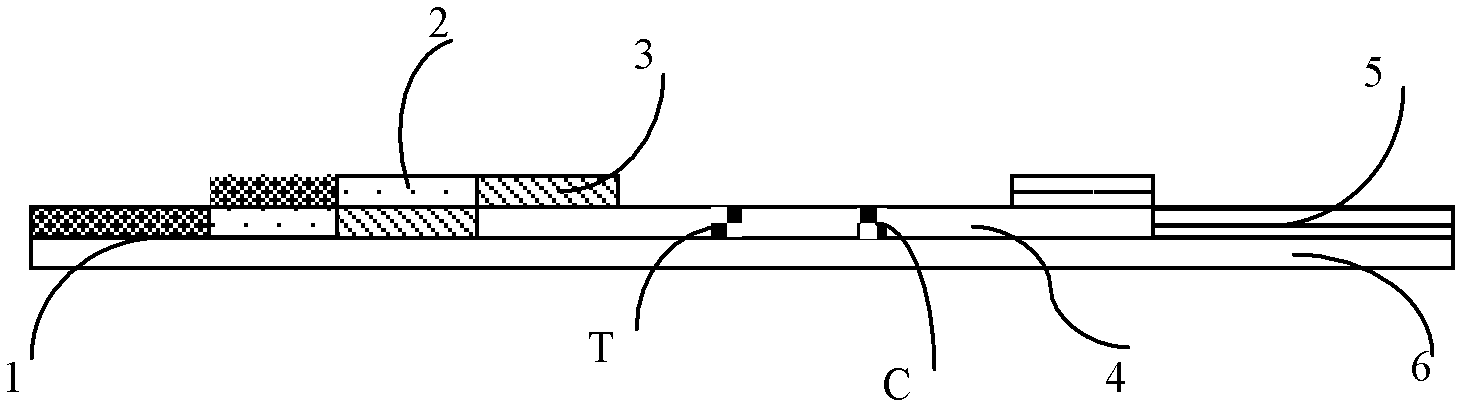
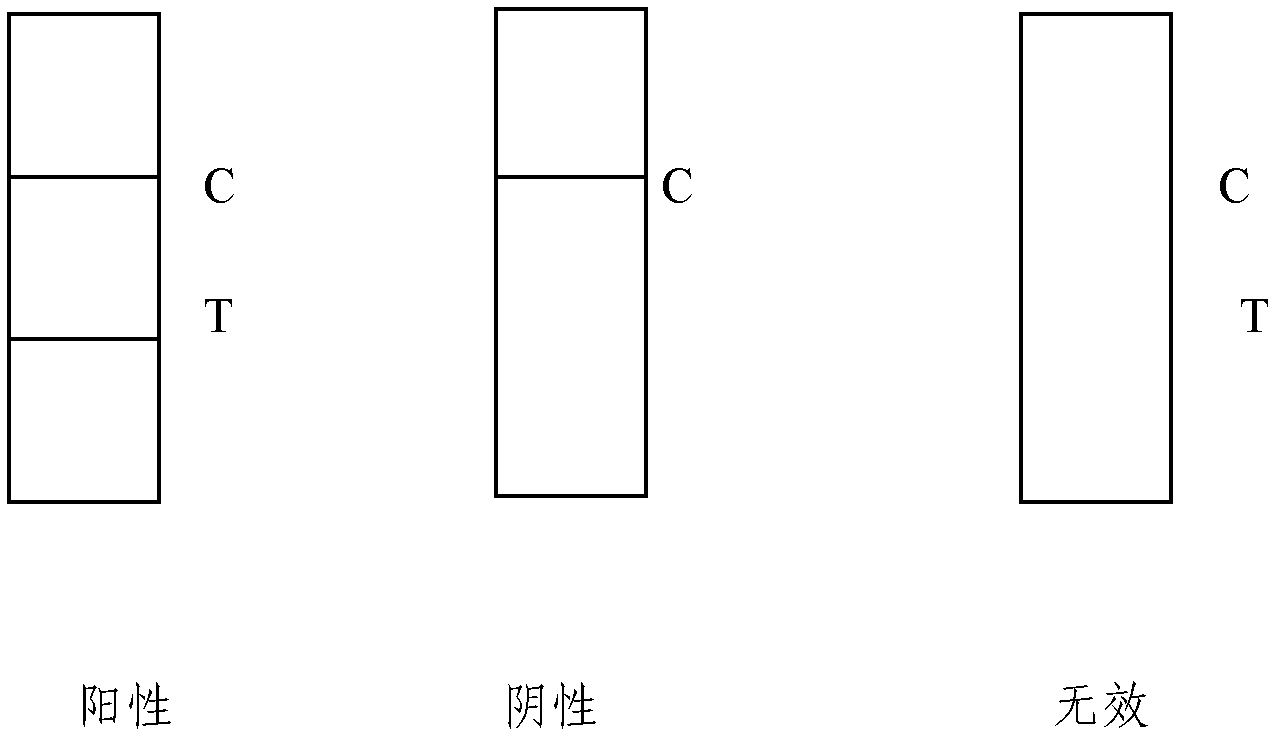
The invention provides a cTn I (cardiac troponin I) hypersensitive detection kit and a preparation method thereof. The kit comprises at least one strain of a first anti-cTn I antibody marked with a tracing marker and at least one strain of a second anti-cTn I antibody coated with magnetic micro-spheres, wherein a binding site between the first anti-cTn I antibody and cTn I is different from a binding site between the second anti-cTn I antibody and cTn I; and the kit can preferably further comprise a diluent, and by adopting the diluent, the non-specific binding in a detection process can be significantly reduced, and the detection accuracy and sensitivity can be further improved. The invention also provides a method for detecting cTn I by using the kit, and the method provided by the invention has very high sensitivity, can be used for sensitively and accurately detecting the cTn I content in a sample, and can provide more timely and reliable information for early diagnosis and treatment of AMI.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
Highly Sensitive System and Methods for Analysis of Troponin
The invention provides methods, compositions, kits, and systems for the sensitive detection of cardiac troponin. Such methods, compositions, kits, and systems are useful in diagnosis, prognosis, and determination of methods of treatment in conditions that involve release of cardiac troponin.
Owner:RGT UNIV OF CALIFORNIA +1
Reagent for detecting acute myocardial infarction by immunological method and test strip
ActiveCN101806804AHigh sensitivityImprove featuresImmunoglobulins against animals/humansTissue cultureSpecific antibodyHuman heart
The invention relates to a medical diagnostic reagent, in particular to a quick detection reagent for the early diagnosis of acute myocardial infarction and a test strip. The invention provides a double-index united detection reagent containing a specific antibody resisting a human heart-type fatty acid binding protein H-FABP and a human cardiac troponin cTnI. The invention also provides a colloidal gold labeling immunological chromatographic test strip containing the specific antibody resisting the H-FABP and the cTnI, which is used for quickly detecting the acute myocardial infarction. The double-index united detection reagent can carry out early diagnosis on a patient having the acute myocardial infarction, can also prevent the missed diagnosis of a patient having long-time chronic myocardial infarction with mild symptoms and better solves the influence caused by a difference existing in the detection time. The colloidal gold labeling immunological chromatographic test strip provides a quick, convenient, cheap and practical detection tool for the early diagnosis of the acute myocardial infarction, is hopefully used for hospitals at all levels and can also be used for the self monitoring of the patient.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Measuring troponin antibodies to assess cardiovascular risk
This invention relates to the field of myocardial disorders. It discloses that antibodies to a cardiac troponin found in a sample obtained from an individual can be used as a diagnostic marker, especially in the assessment of an individual's risk of developing a myocardial disorder. A method aiding in the assessment of an individual's risk of developing a myocardial disorder, comprising measuring in vitro antibodies to a cardiac troponin and optionally one or more other marker useful in assessing an individual's risk of developing a myocardial disorder, and correlating the value or the values obtained to the individual's risk of developing a myocardial disorder is decribed.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Anti-human cardiac troponin I specific monoclonal antibody and preparation method thereof
InactiveCN101942416AImmunoglobulins against animals/humansMicroorganism based processesSocial benefitsMortality rate
The invention relates to an anti-human cardiac troponin I hybridoma cell line, with the preservation number of CGMCCNO. 3951, and a monoclonal antibody secreted from the anti-human cardiac troponin I hybridoma cell line CGMCC3951. The class and subclass of immunoglobulin of the monoclonal antibody are respectively IgG and IgG3 and the monoclonal body is specifically combined with human cardiac troponin I; the potency is 1:16000 and the affinity constant is 1.08x10 to 9mol / L. By applying the monoclonal antibody of the invention to clinical diagnosis, the study on complete localization of diagnosis kit for cardiac troponin I is tremendously promoted, correctness of clinical diagnosis and prognosis for cardiovascular diseases is enhanced while suffering of patients and death rate are both reduced, thus prominent economical and social benefits are obtained.
Owner:INST OF RADIATION MEDICINE CHINESE ACADEMY OF MEDICAL SCI
Conjunctive analysis of biological marker expression for predicting cardiac mortality
InactiveUSRE39816E1Replace expensive and time-consumingEfficient use ofAnalysis using chemical indicatorsEnzymologyOrgan systemMortality rate
Owner:NEXUS DX
Preparing method of photoelectrochemical sensor based on sandwich cardiac troponin T marked by Ag2Se@CdSe and application
InactiveCN104849331AGood photoelectric conversion characteristicsImprove photoelectric signal responseBiological testingMaterial electrochemical variablesCardiac muscleEngineering
The invention relates to a preparing method of a photoelectrochemical sensor based on sandwich cardiac troponin T marked by Ag2Se@CdSe and application, and belongs to the technical field of novel functional materials and biosensing detection. According to concrete contents of the preparing method and the application, CeO2-TiO2 composite nanometer materials with photoelectrocatalytic activity are used as optical activity substrate materials; Ag2Se@CdSe is used as an antibody marker; the sandwich photoelectrochemical sensor is prepared through the signal amplification effect of the marker Ag2Se@CdSe on the substrate materials, and is used for the high-sensitivity detection of the cardiac troponin T of myocardial damage specific marker. The method has the important significance on the early diagnosis and treatment of acute myocardial infarction.
Owner:UNIV OF JINAN
Highly sensitive system and methods for analysis of troponin
InactiveUS20100255518A1Analysis using chemical indicatorsMicrobiological testing/measurementMedicineTroponin T
The invention provides methods, compositions, kits, and systems for the sensitive detection of cardiac troponin, Such methods, compositions, kits, and systems are useful in diagnosis, prognosis, and determination of methods of treatment in conditions that involve release of cardiac troponin.
Owner:RGT UNIV OF CALIFORNIA +1
Preparation of troponin I specific locus antibody and detection kit thereof
The invention relates to preparation of troponin I specific locus antibody and a detection kit thereof. A fixed point hydrolysis method is adopted to obtain the required three peptide sections of human cardiac troponin I (cTnI); the three peptide section are orderly subjected to immunogenicity modification and animal immunization so as to obtain high titer immune serum; the three peptide sections extracted in the hydrolysis are taken as the ligand and the polyacrylamide is taken as genin to prepare an affinity column; and finally the obtained high titer immune serum is subjected to immunoaffinity chromatography so as to obtain polyclonal antibodies having specific recognition on the three peptide sections. The antibodies have the advantages that: the affinity of the antibodies is higher than that of a corresponding monoclonal antibody, the specificity is equal to that of a corresponding monoclonal antibody, moreover, the preparation cost is lower, and the preparation process is simpler, compared to that of a monoclonal antibody. The obtained three polyclonal antibodies can be made into an immunity latex kit for a semi-automatic or automatic generation analysis instrument for detecting the content of cardiac troponin I (cTnI) in serum.
Owner:南陵县建设投资有限责任公司
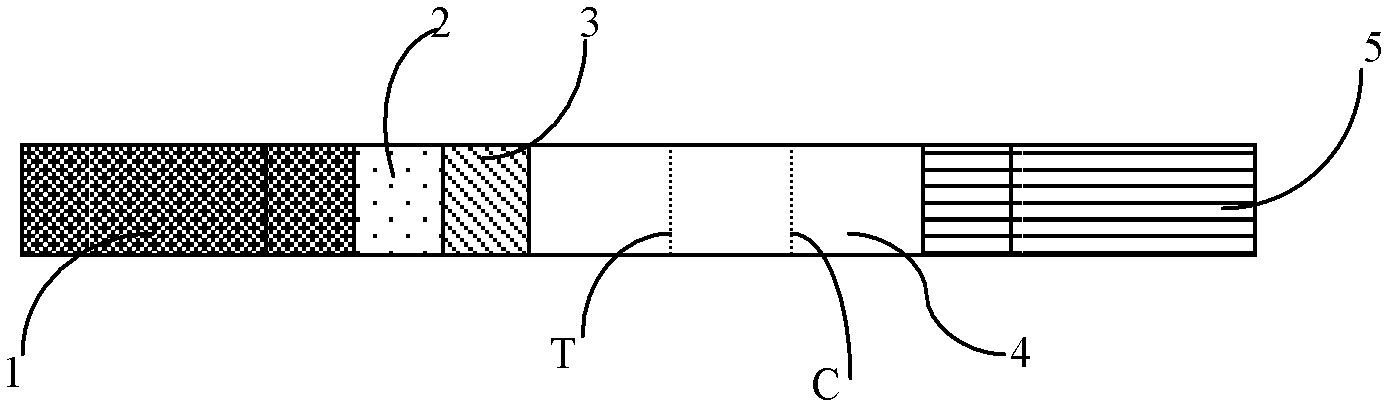
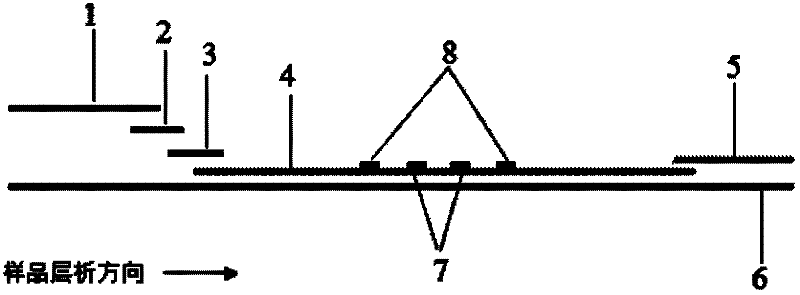
Immune chromatography fluorescence reagent strip for detecting cardiac troponin and preparation method thereof
InactiveCN104237537AShorten detection timeImprove featuresBiological testingReagent stripMonoclonal antibody
The invention discloses an immune chromatography fluorescence reagent strip for detecting cardiac troponin and a preparation method thereof, belonging to the field of clinical medicine detection. The reagent strip comprises a baseboard, a sample mat, a tracer particle combining mat, a nitrocellulose membrane and a water absorption mat; the sample mat, the tracer particle combining mat, the nitrocellulose membrane and the water absorption mat are orderly connected and fixed on the baseboard in the horizontal direction. The tracer particle combining mat is provided with the cTnI monoclonal antibody-fluorescence particle compound adsorbed layer covered on the surface of the nanocrystalline metal particle; the nitrocellulose membrane is provided with the detection line composed of the cTnI monoclonal antibody or polyclonal antibody capture molecule and the quality control line composed of the rabbit antimouse IgG antibody. The immune chromatography fluorescence reagent strip for detecting cardiac troponin is short in detection time, high in specificity and sensitivity and more accurate in detection result by improving the fluorescence intensity through the nanocrystalline metal particle.
Owner:GETEIN BIOTECH
Semi-quantitative detecting test paper of cardiac troponin and preparation method thereof
The invention relates to semi-quantitative detecting test paper of cardiac troponin, mainly comprising five parts: a sample pad, a combination pad, a water sucking pad, a soleplate and a nitric acid fibre film, wherein two parallel antibody capture lines are made on the nitric acid fibre film; one first anti-cardiac troponin I monoclonal antibody with the determined concentration close to the combination pad is used as a detecting line; another goat-anti-rabbit IgG antibody with the determined concentration is used as a standard concentration developing contrast line; the distance between the two lines is 4-7 mm; and the combination pad is sprayed with two substances of a second anti-cardiac troponin I monoclonal antibody and a gold rabbit IgG marked by colloidal gold or colour latex. The quantitative detecting test paper of cardiac troponin in the invention has the advantages of simple preparation method, low cost, convenience for detection and use, and capability of rapidly conveniently semi-quantitatively detecting the content of the cardiac troponin I in the human body blood sample, and is suitable for family application and popularization.
Owner:WUHAN J H BIO TECH
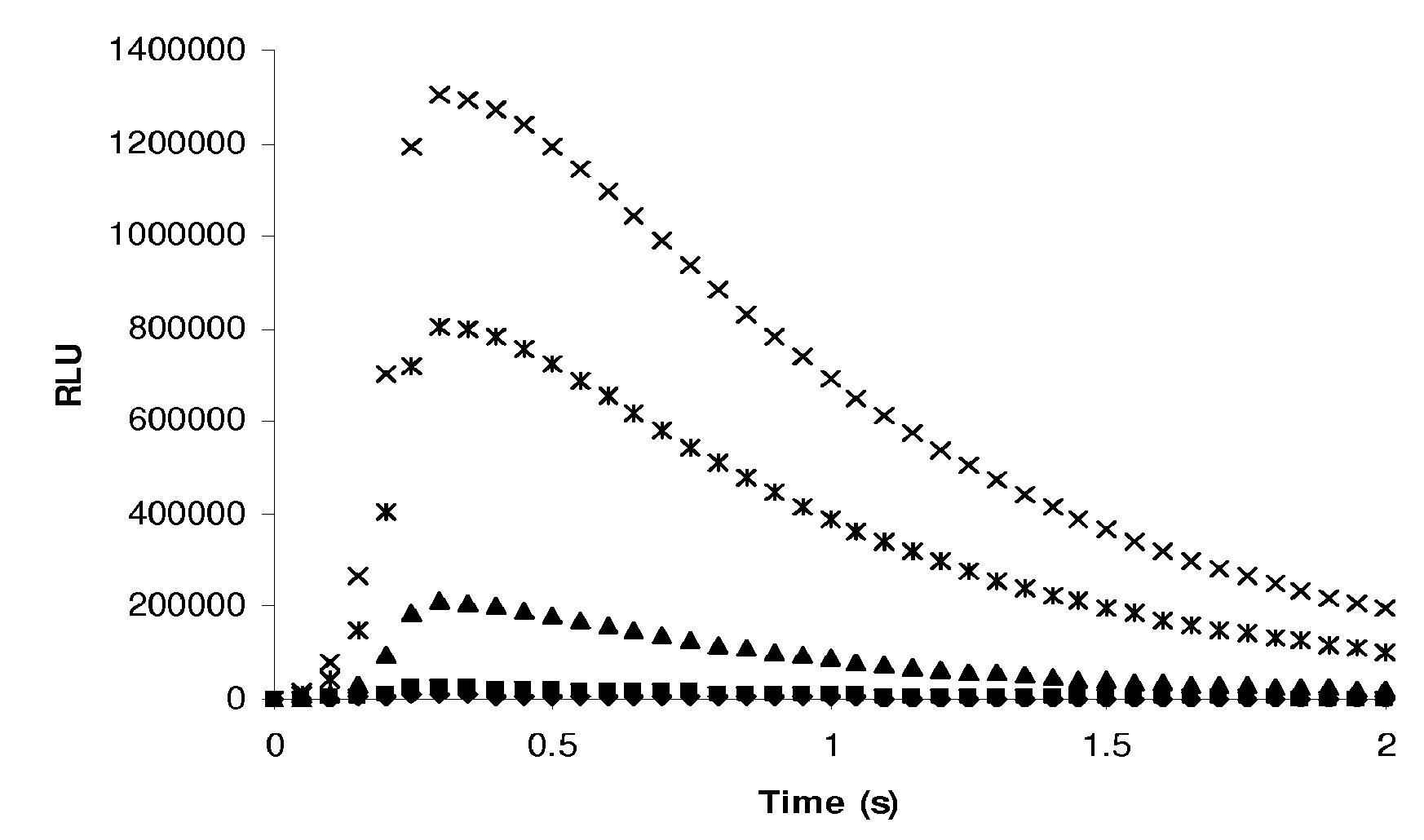
Cardiac troponin i ultra-sensitive detection reagent kit, and ultra-sensitive detection method therefor
ActiveUS20170305983A1The test result is accurateHigh sensitivityChemiluminescene/bioluminescenceAntibody medical ingredientsI antibodyBinding site
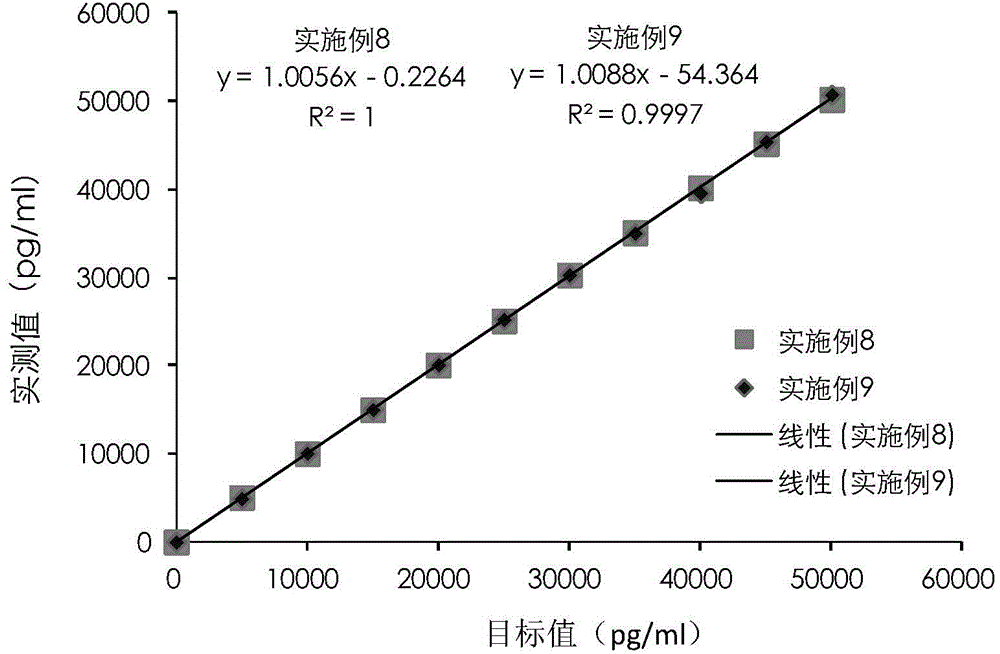
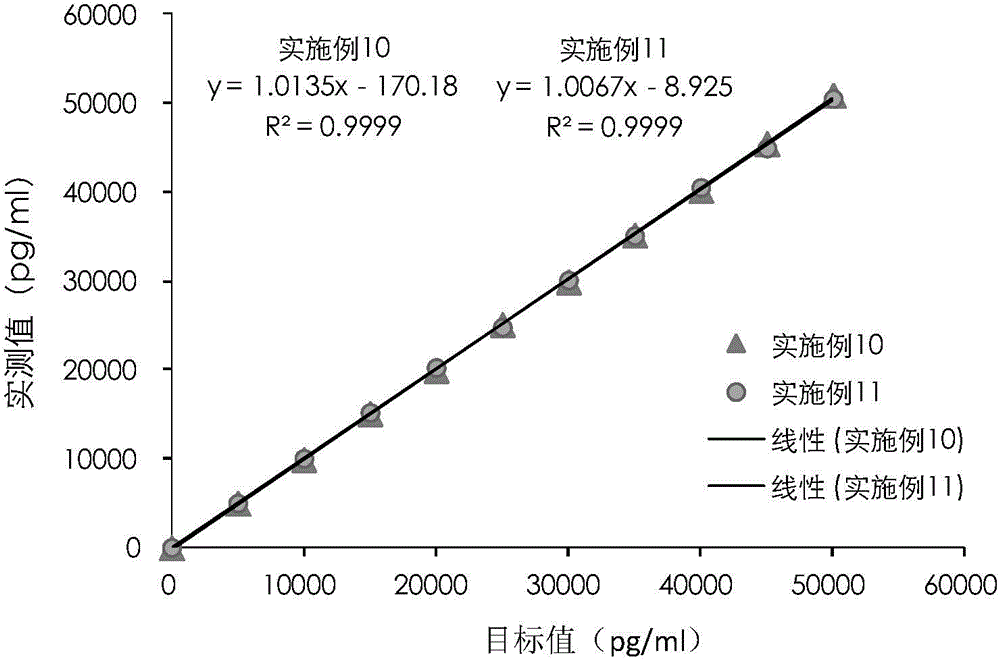
A cardiac troponin I ultra-sensitive detection reagent kit, a preparation method, and a detection method. The reagent kit comprises at least one first anti-cardiac troponin I antibody marked with a trace marker and at least one second anti-cardiac troponin I antibody coated on magnetic microspheres, the first anti-cardiac troponin I antibody and cardiac troponin I binding site being different from the second anti-cardiac troponin I antibody and cardiac troponin I binding site. The reagent kit may further comprise a diluent capable of significantly reducing non-specific binding in a detection process, so as to further increase the detection accuracy and sensitivity. The method using the reagent kit to detect cardiac troponin I sensitively and accurately detects the amount of cardiac troponin I in a sample, and provides more timely and reliable information for the early diagnosis and treatment of AMI.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
Colloidal gold test paper for quickly detecting troponin I and preparation method for colloidal gold test paper
ActiveCN102841208AHigh detection sensitivityExpand clinical applicationBiological testingClinical immunologyMedicine
The invention relates to the field of clinical immunology, particularly to colloidal gold test paper for quickly detecting troponin I and a preparation method for the colloidal gold test paper. The colloidal gold test paper comprises a coated film, a colloidal gold pad 1, a colloidal gold pad 2, a sample pad and a water absorbing pad which are mutually attached in a staggered way; the colloidal gold pad 1 is sprayed with a colloidal gold labeled cardiac troponin I resisting antibody; the colloidal gold pad 2 is sprayed with a colloidal gold labeled BSA (Bovine Serum Albumin)-resisting monoclonal antibody or PEG (Polyethylene Glycol)-resisting monoclonal antibody; and the coated film is coated with a cardiac troponin I resisting antibody detection line and a rabbit antimouse antibody quality control line. According to the improvement on the test paper strip, a PEG resisting technology and a BSA-resisting monoclonal antibody technology are introduced into the detection of the cardiac trooping I for the first time, so that the detection sensitivity of the cardiac trooping I is greatly improved and the clinical application of a traditional colloidal gold quick diagnosis test paper is enlarged.
Owner:北京乐普诊断科技股份有限公司
Human myocardial troponin I subunit nucleic acid adaptor and its application
InactiveCN1974594ASmall molecular weightLow costOrganic active ingredientsSugar derivativesChemical synthesisNucleotide
The present invention discloses one kind of human myocardial troponin I subunit nucleic acid adaptor and its application. The human myocardial troponin I subunit nucleic acid adaptor has the nucleotide sequence as shown and may be use in the early detection of myocardial troponin I, the clinical diagnosis of myocardial damage and the separation and purification of myocardial troponin I. The human myocardial troponin I subunit nucleic acid adaptor is oligonucleotide with relatively small molecular weight, may be synthesized chemically, and has affinity and specificity higher than that of antibody, easy labeling selectively in different parts, high repeatability and stability, easy preservation and excellent application foreground.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Method for detecting cardiac troponin I and application thereof
The invention provides a method for detecting cardiac troponin I (cTnI) and a cardiac troponin I detection kit prepared by applying the method. The method comprises the steps of coupling an antibody composition with polystyrene latex particles, then subjecting the coupled material to immunoreaction with corresponding antigen in a sample to be detected to form aggregated particles, and determining turbidity generated by the reactant under a wavelength of 400-700nm to obtain the content of cTnI in the sample to be detected. The antibody composition consists of at least three antibodies, and the antibodies are specific to cTnI without cross reaction with fast skeletal muscle troponin I and slow skeletal muscle troponin I. In addition, if one antibody in the antibody composition is sensitive to a certain factor influencing the accurate determination of cTnI in the sample to be detected, and then at least another antibody is not sensitive to the factor influencing the accurate determination of cTnI. The method of the invention is simple to operate, and has a wide scope of application, high precision, strong capability of resisting disturbance and low production cost.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
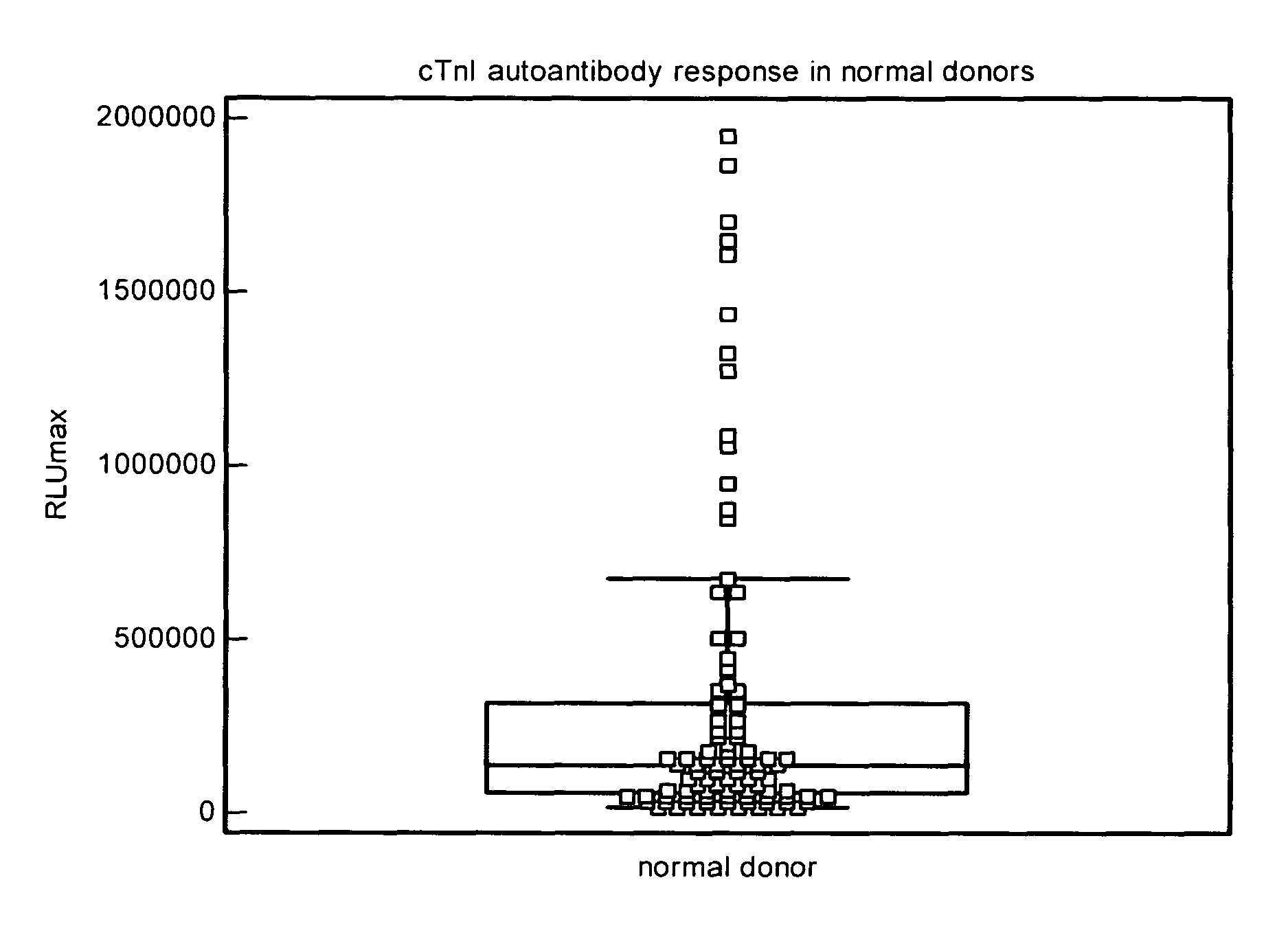
Assay for cardiac troponin autoantibodies
The invention provides among other things methods and kits based on assaying for cardiac troponin autoantibodies, either in conjunction with an assay for cardiac troponin and / or as an independent indicator of cardiac pathology, such as myocarditis, cardiomyopathy, and / or ischemic heart disease. Assay methods of the invention can be employed among other things to identify cardiac pathology, or risk thereof, in subjects who have an autoimmune disease or who are related to an individual with an autoimmune disease. In particular embodiments, the invention also provides a method of determining whether a subject having, or at risk for, a cardiac pathology is a candidate for immunosuppressive therapy or immunoabsorption therapy. The invention also provides kits and kit components that are useful for performing the methods of the invention.
Owner:ABBOTT LAB INC
High-sensitivity magnetic particle chemiluminescence immunoassay kit for cardiac troponin I (hs-cTnI) and preparation method and purpose thereof
InactiveCN109709323AAvoid defects that cannot be effectively recognized by antibodiesHigh analytical sensitivityMaterial analysisEpitopeMagnetic bead
The application provides a high-sensitivity magnetic particle chemiluminescence immunoassay kit for cardiac troponin I (hs-cTnI). The kit comprises a reagent R1, a reagent R2, a magnetic particle working solution and a cTnI antigen calibrator. The reagent R1 contains a biotin-labeled anti-cTnI polyclonal antibody; the reagent R2 contains two acridinium-ester-labeled anti-cTnI monoclonal antibodiesagainst different cTnI epitopes; the magnetic particle working solution includes streptavidin magnetic beads. In addition, the kit also can include a H2O2 solution and an alkaline solution. Besides,the application also provides a preparation method and purpose of the kit. The kit is capable of identifying multiple epitopes of the cTnI antigen, so that the analytical sensitivity and precision ofthe cardiac troponin I chemiluminescence immunoassay kit are improved.
Owner:SHENZHEN AMTECH BIOENGINEERING LTD INC
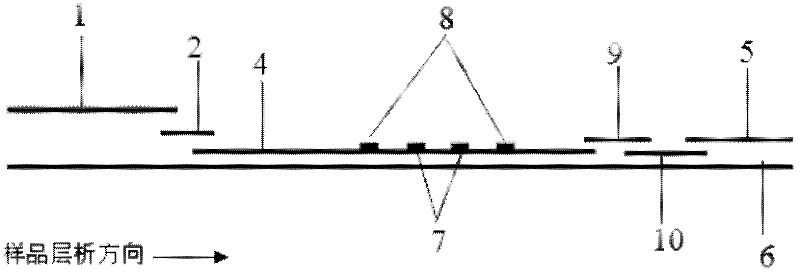
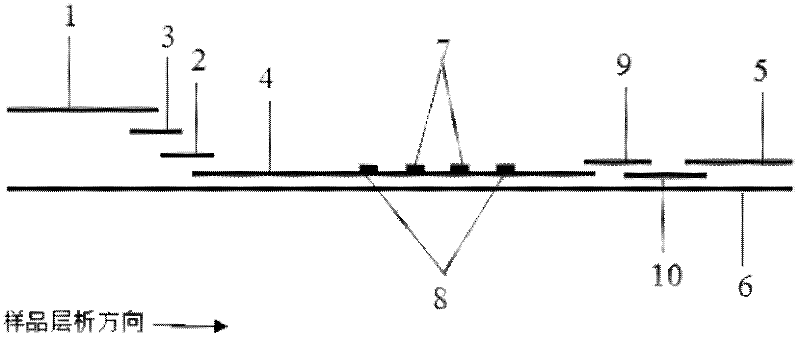
Preparation method of joint-inspection test paper for cardiac troponin I and heart-type fatty acid binding protein
InactiveCN104678098ACause some damagesQuick judgmentDisease diagnosisBiological testingNitrocelluloseControl line
The invention discloses a preparation method of joint-inspection test paper for cardiac troponin I (cTn I) and heart-type fatty acid binding protein (H-FABP). The preparation method comprises the following steps: using a membrane-spotting metal spraying device and a mixed liquid labeled by a cTn I labeled antibody and an H-FABP labeled antibody to spray metal to a pad, so as to obtain a gold pad; using a cTn I detection line coating buffer, an H-FABP detection line coating buffer and a control line coating buffer to respectively scribe on a polyvinyl chloride base plate adhered with nitrocellulose membrane, so as to obtain the polyvinyl chloride base plate spotted with the membrane; assembling a sample pad, the gold pad, the membrane spotted polyvinyl chloride base plate and absorbent paper together, and then cutting the assembled paper to obtain the detecting test paper. With adoption of the method, the detecting test paper obtained by the invention is based on the lateral flow immune chromatography technology, and can be used by manual operation and visible reading to judge whether the blood sample contains cardiac troponin I and heart-type fatty acid binding protein, so that the diagnosis is rapid and the result is accurate, and the body of the subject can be free of damage.
Owner:SUZHOU WANMUCHUN BIOLOGICAL TECH
Method of determining time of death
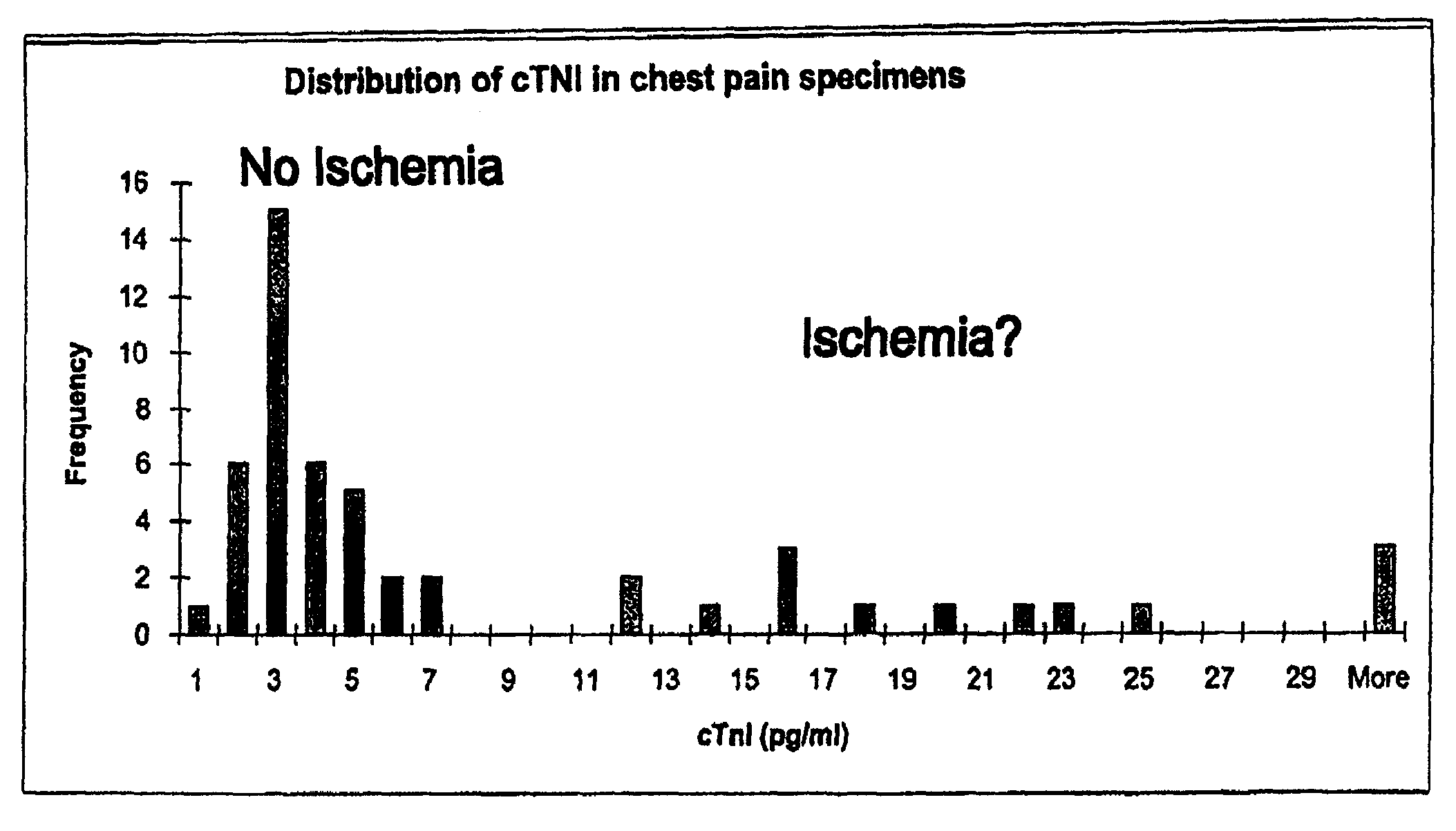
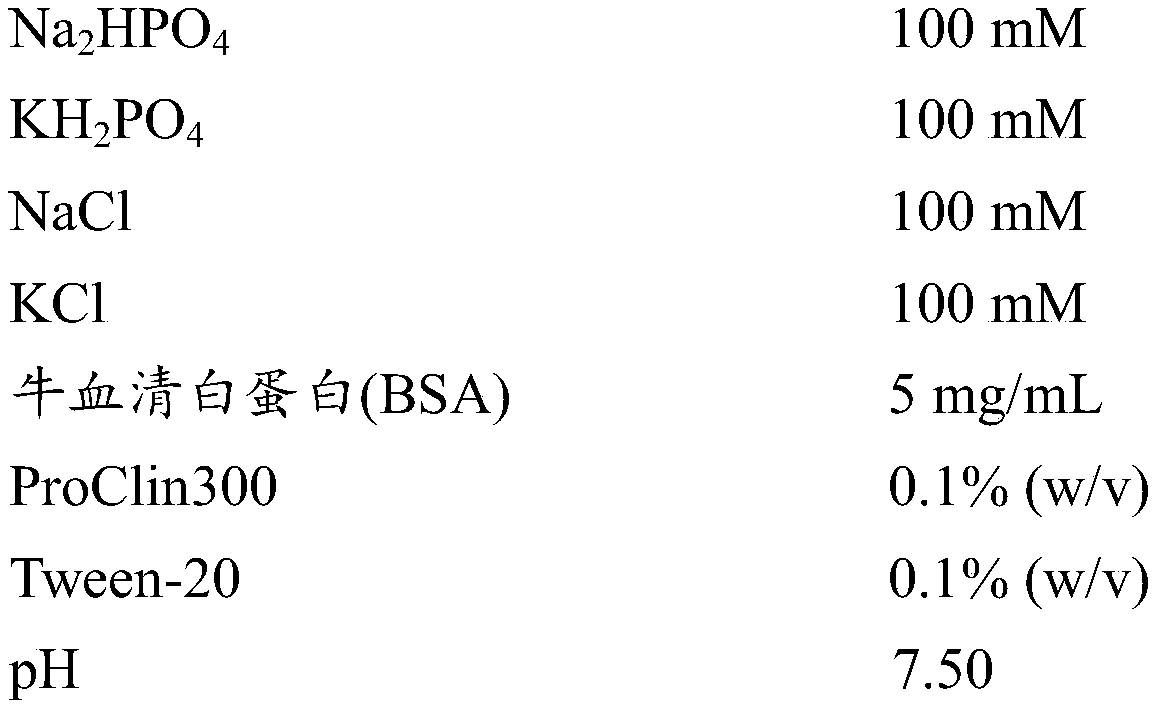
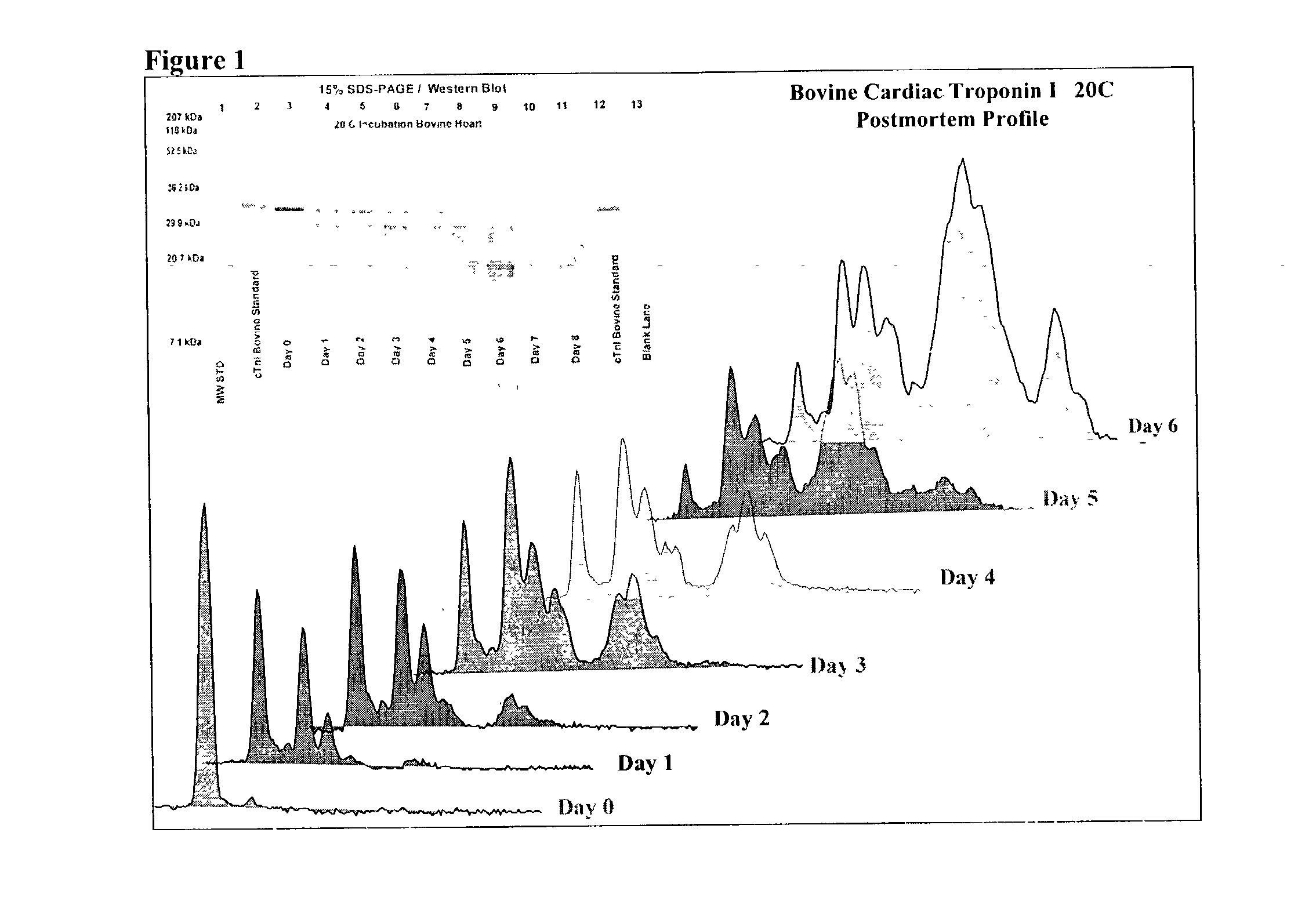
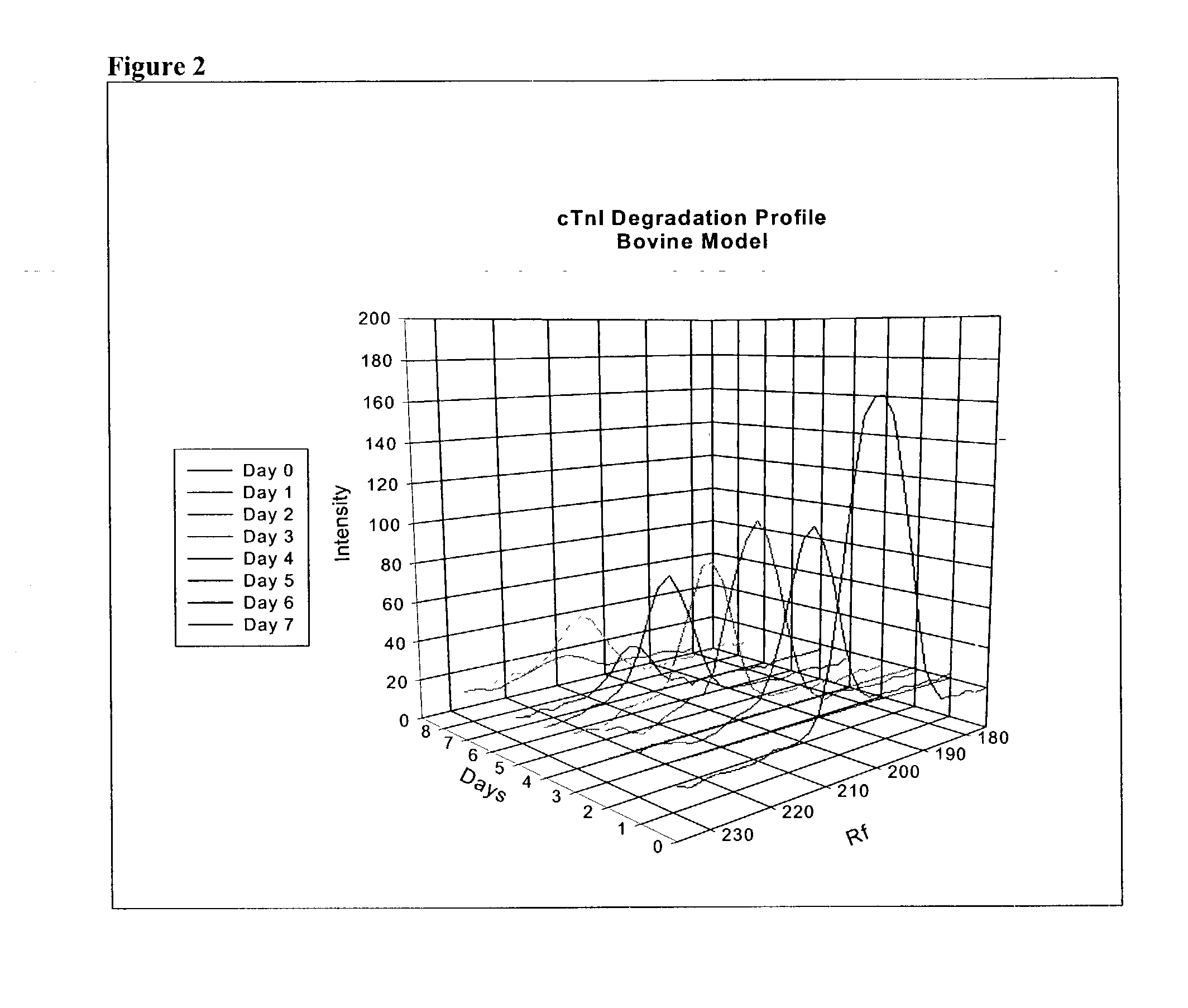
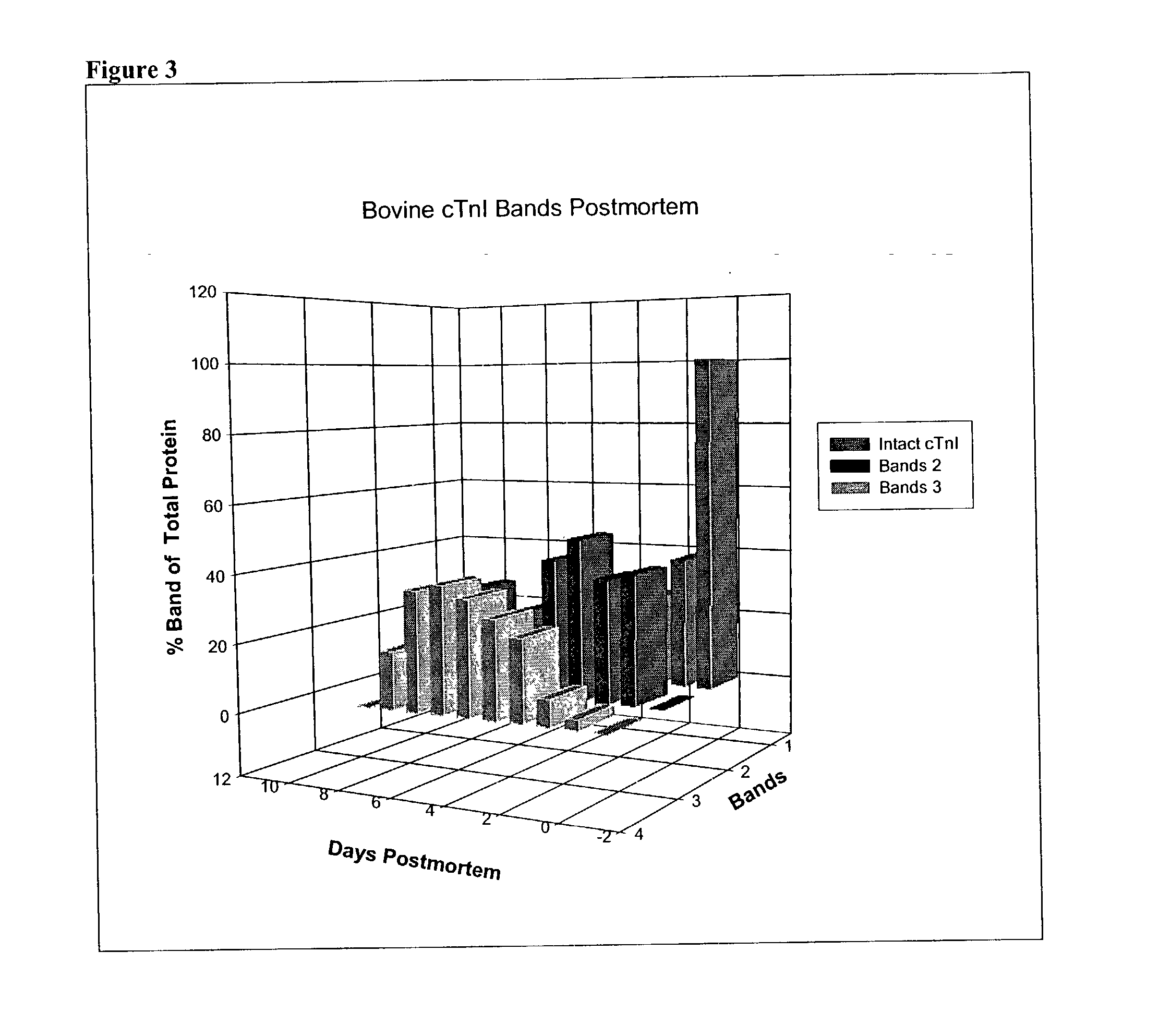
A method of determining time of death is disclosed. Cardiac troponin I (cTnI) is the most specific marker for the determination of myocardial infarction (MI). Cardiac troponin I has a distinctive temporal degradation profile after death, which is key to its use as a time of death marker in forensic medicine. The method consists of sampling cardiac tissue, extracting a cardiac protein from a homogenate via methods which may include the magnetic microparticles or magnetic microparticles linked to anti-cTnI antibodies, eluting the proteins, separating proteins by electrophoresis, transferring by western blot the different bands of proteins to paper. A comparison / analysis of the concentration and kinetics of degradation of the protein at a given temperature(s) against known standards after time of death provides an accurate measurement of the time of death. The present invention is more accurate and reliable than the rudimentary time of death techniques currently used by medical examiners.
Owner:SABUCEDO ALBERT J +1
Assay for cardiac troponin autoantibodies
The invention provides among other things methods and kits based on assaying for cardiac troponin autoantibodies, either in conjunction with an assay for cardiac troponin and / or as an independent indicator of cardiac pathology, such as myocarditis, cardiomyopathy, and / or ischemic heart disease. Assay methods of the invention can be employed among other things to identify cardiac pathology, or risk thereof, in subjects who have an autoimmune disease or who are related to an individual with an autoimmune disease. In particular embodiments, the invention also provides a method of determining whether a subject having, or at risk for, a cardiac pathology is a candidate for immunosuppressive therapy or immunoabsorption therapy. The invention also provides kits and kit components that are useful for performing the methods of the invention.
Owner:ABBOTT LAB INC
Immunoassay
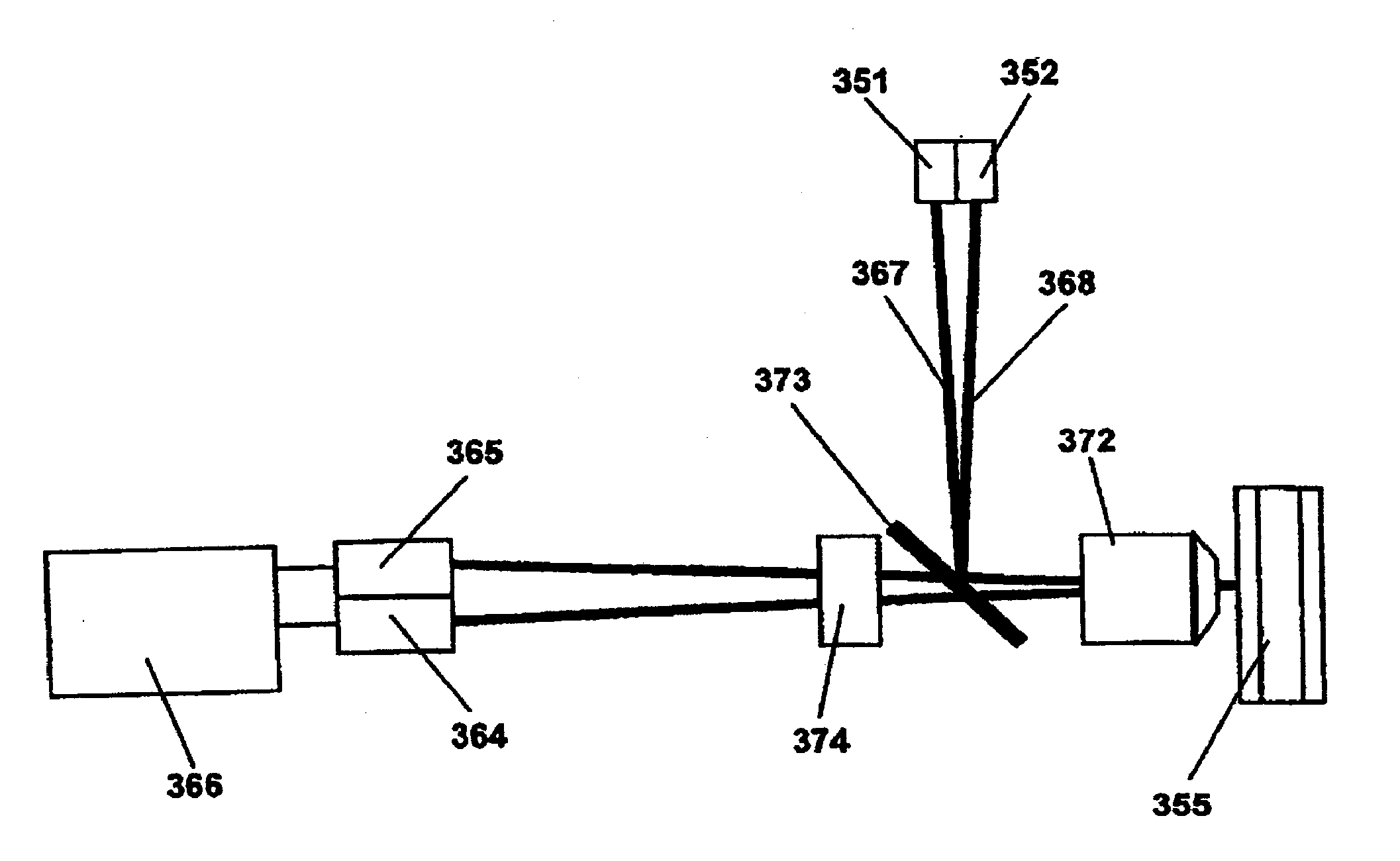
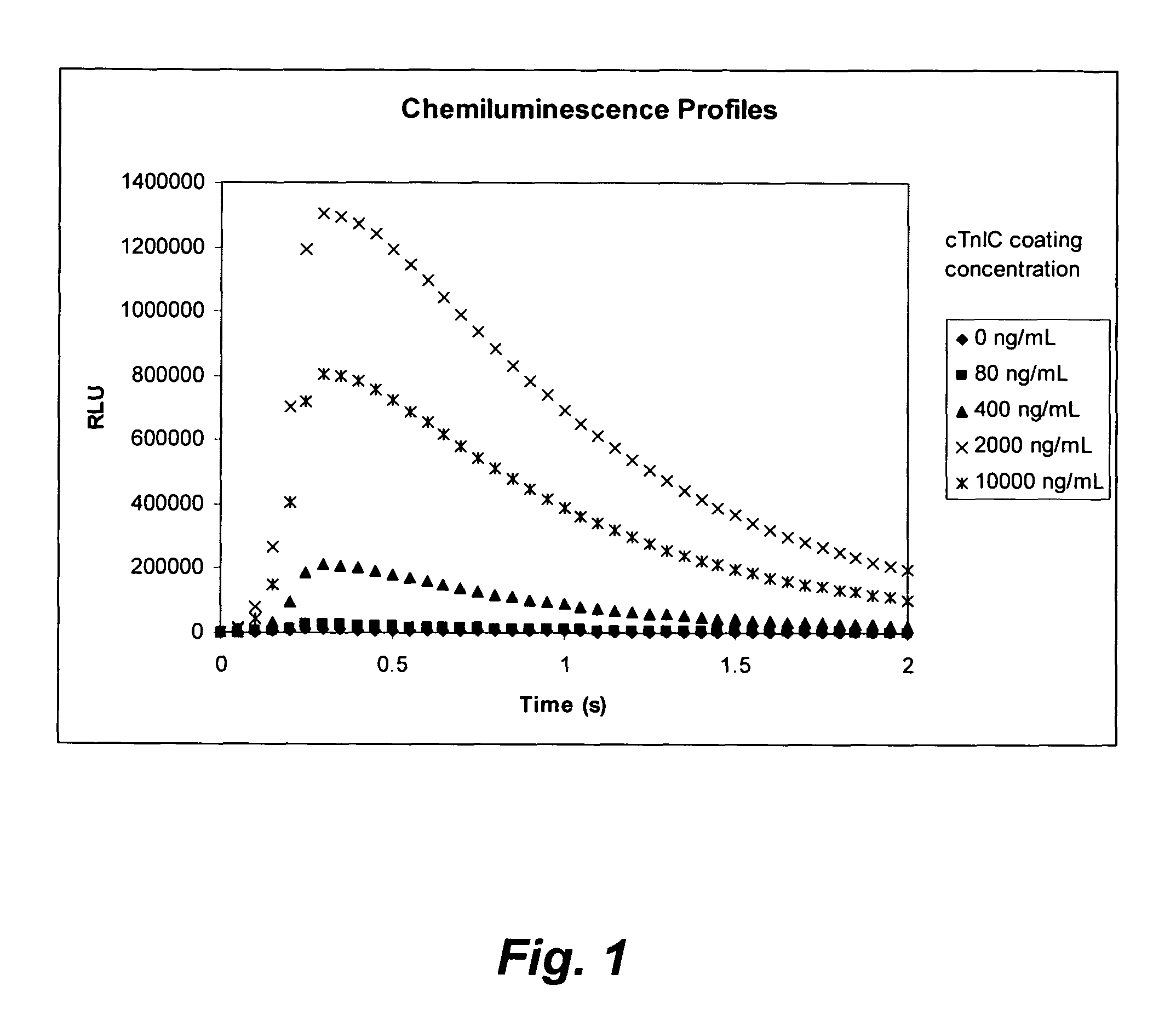
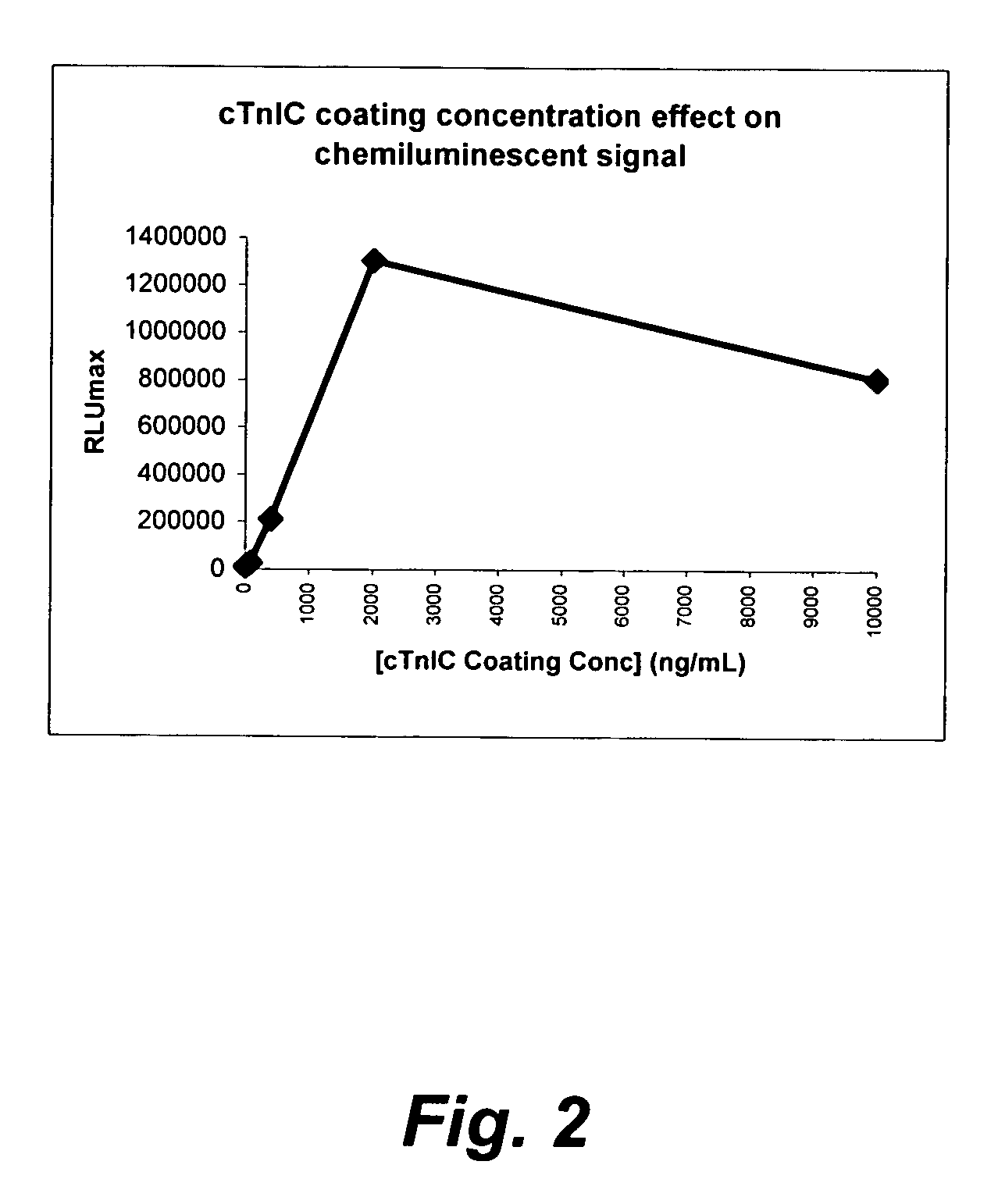
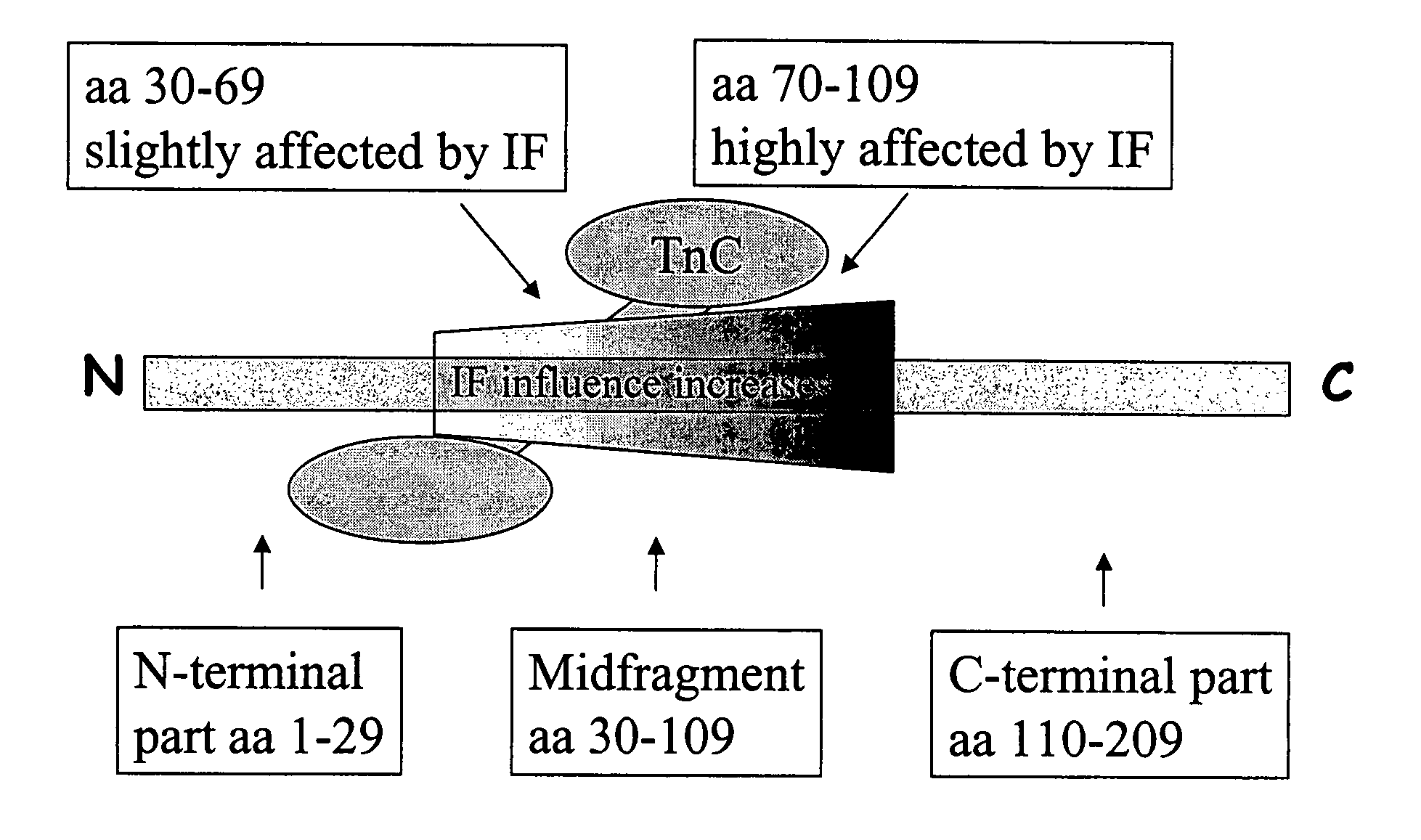
ActiveUS7348157B2Accurate detectionSamplingChemiluminescene/bioluminescenceCapture antibodySandwich immunoassay
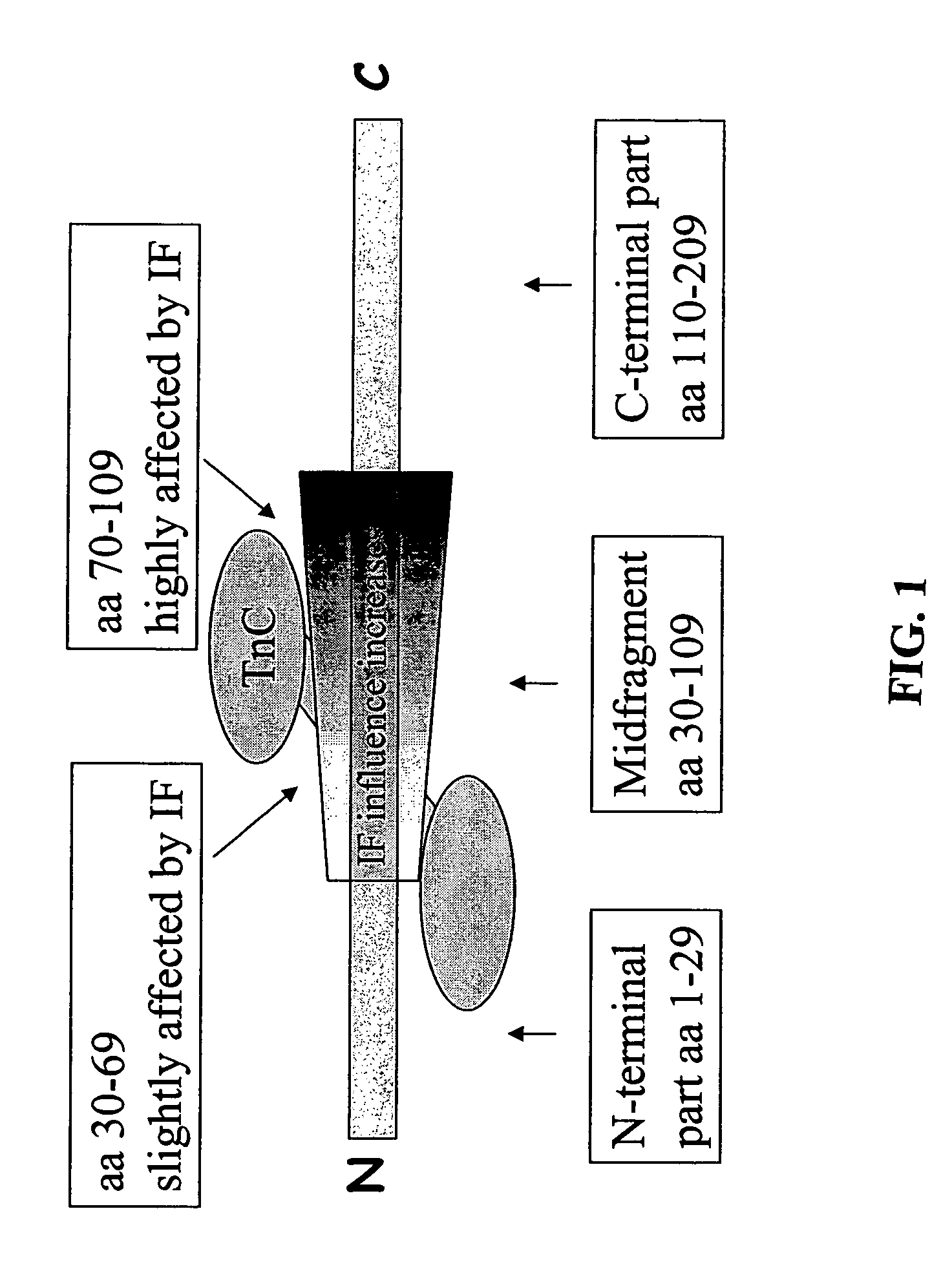
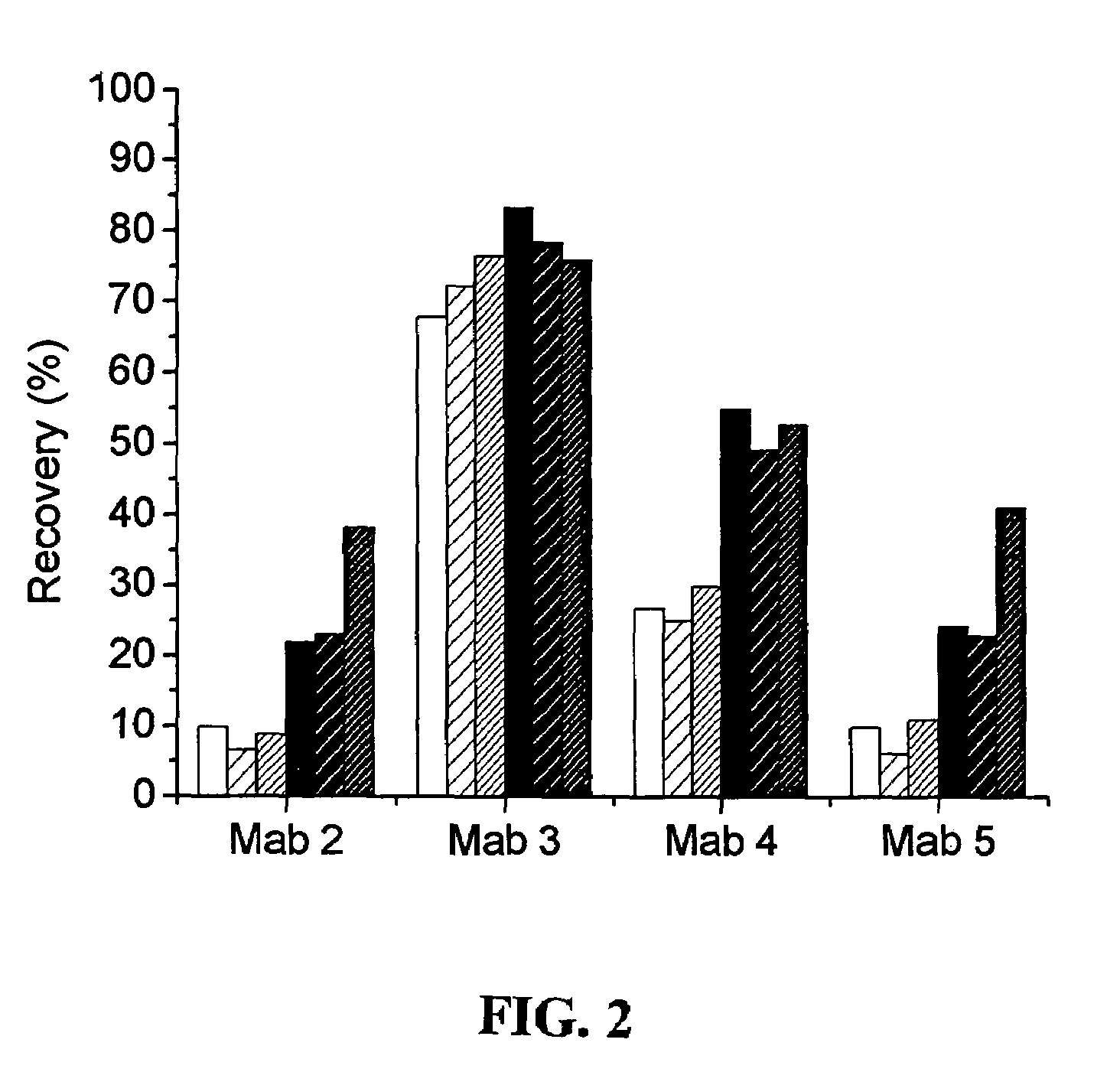
A method for detection and / or quantification of cardiac troponin I (cTnI) in a sample derived from an individual's blood, the method being based on a sandwich immunoassay employing at least two capture antibodies and at least one tracer antibody, in which a first capture antibody is directed to the N-terminal part of cTnI, to the C-terminal part of cTnI or to the part of the cTnI midfragment, which is slightly affected by the interfering factor, and a second capture antibody is directed to another of these parts, and a tracer antibody is directed to the N-terminal part of cTnI, to the C-terminal part of cTnI or to TnC, which is complexed with cTnI.
Owner:TURUN YLIOPISTO
Monoclone antibody against human cardiac troponin I and its use
InactiveCN1644685AImprove stabilityStrong specificityImmunoglobulins against animals/humansTissue cultureDiseaseAntigen
This invention relates to a cTnI monocloned antibody and its hybridoma cell system. The said monocloned antibody has high specificity sensitivity and is easily prepared. It can identify different antigenic determinants on amino acid peptide segments 28-42, so as to be used for preparation of kits for diagnosis of cardiac infarction, angina pectoris and myocarditis.
Owner:FUDAN UNIV
Method of screening remedy for heart disease and medicinal composition for treating heart disease
ActiveUS20060246525A1Improve solubilityImprove stabilityPeptide/protein ingredientsMicrobiological testing/measurementI antibodyHeart disease
A method for screening a substance that inhibits the onset of anti-cardiac troponin I autoantibody-related disease, a pharmaceutical composition and a base material for therapy of cardiac disease that contains the substance obtained by aforesaid method thereof, a therapeutic apparatus that removes anti-troponin I autoantibody for aforesaid antibody related disease, a method of making an animal model for evaluating cardiac disease characterized by administrating anti-cardiac troponin I antibody, a method of selection of a therapeutic substance for cardiac disease characterized by using aforesaid animals, and a diagnosis of dilated cardiomyopathy characterized by measuring anti-cardiac troponin I autoantibody. An apparatus of the present invention that removes anti-cardiac troponin I antibody and a pharmaceutical composition for therapy of the antibody related disease may be useful for a therapy and / or prevention of cardiac disease.
Owner:ONO PHARMA CO LTD +1
Preparation method and application of multilevel micro-meter cubic zinc stannate composite material based photoelectrochemical sensor to cardiac troponin I
ActiveCN107831198AHeavy loadImprove conductivityMaterial electrochemical variablesDoped graphenePolypyrrole
The invention relates to a preparation method and application of a multilevel micro-meter cubic zinc stannate composite material based photoelectrochemical sensor to cardiac troponin I and belongs tothe field of photoelectrochemical sensors. Polypyrrole / nitrogen subtracted graphite phase carbon nitride is taken as a template for synthesis of a multilevel structural Zn2SnO4 cube; nitrogen and sulfur doped graphene quantum dot N,S-GQDs is used for sensitizing Zn2SnO4 to improve its visible light absorption; the process of in-situ growth of CdS nanoparticles is carried out to obtain a multilevel micro-meter cubic zinc stannate composite material Zn2SnO4 / N,S-GQDs / Cds with photoelectric activity improved greatly; cardiac troponin I antibodies, bovine serum albumin and cardiac troponin I antigens are assembled to the composite material Zn2SnO4 / N,S-GQDs by means of the layer by layer self-assembly; thus, according to the excellent photoelectric activity of Zn2SnO4 / N,S-GQDs and binding of specificity of cardiac troponin I antigens and cardiac troponin I antibodies, ultra-sensitive detection to cardiac troponin I is achieved, which has a great significance in analysis and detection of cardiac troponin I.
Owner:UNIV OF JINAN
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com