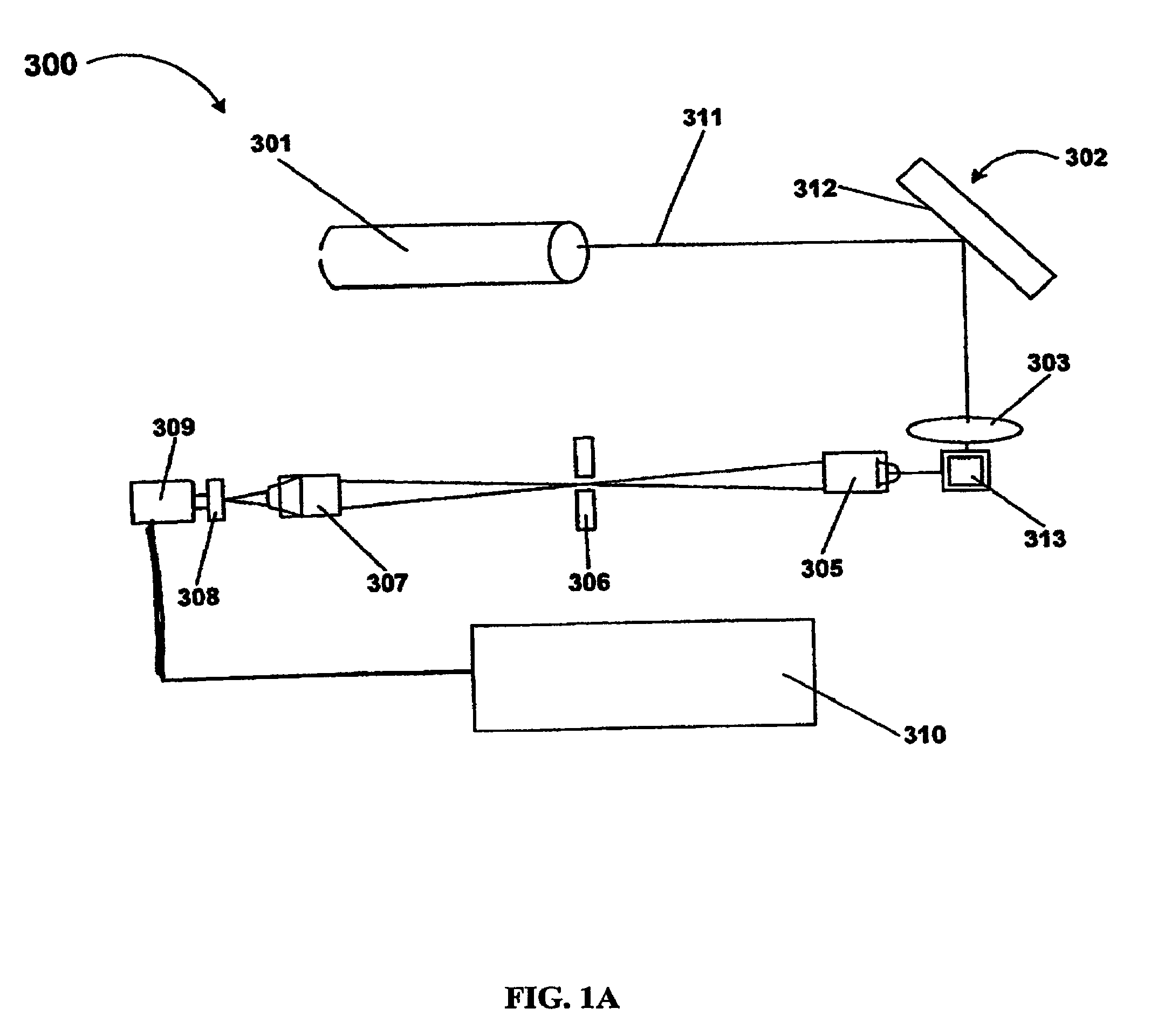
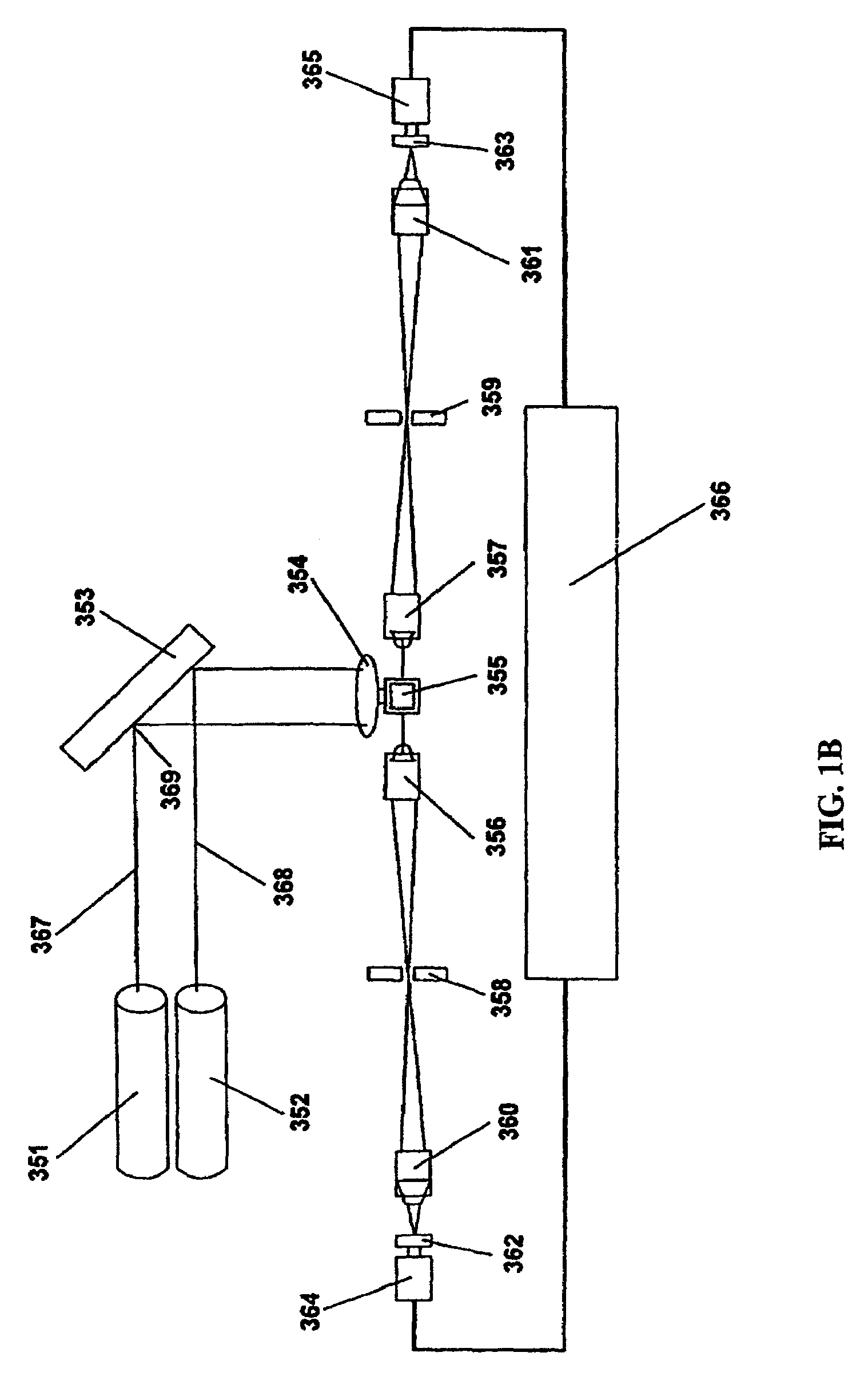
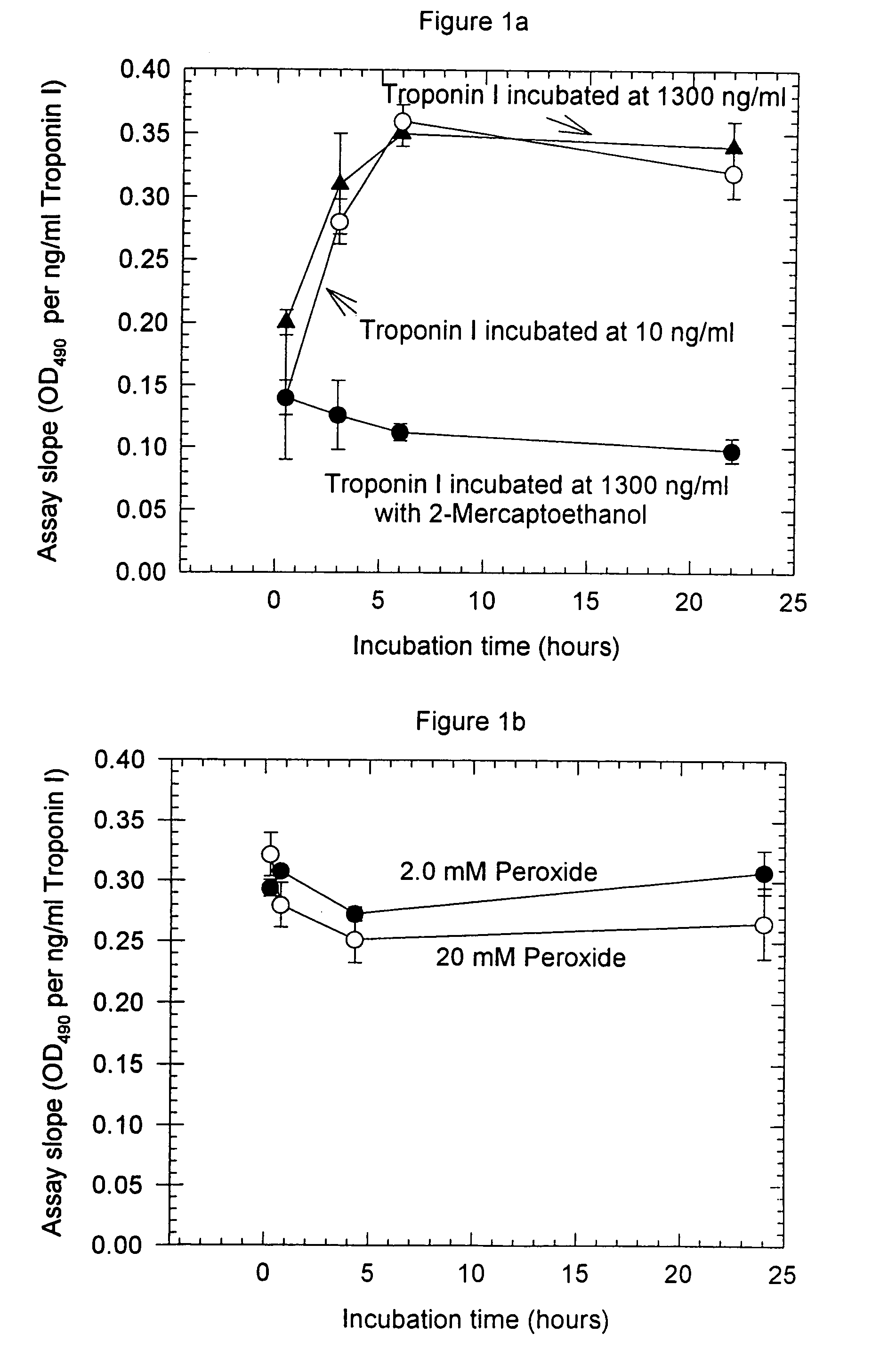
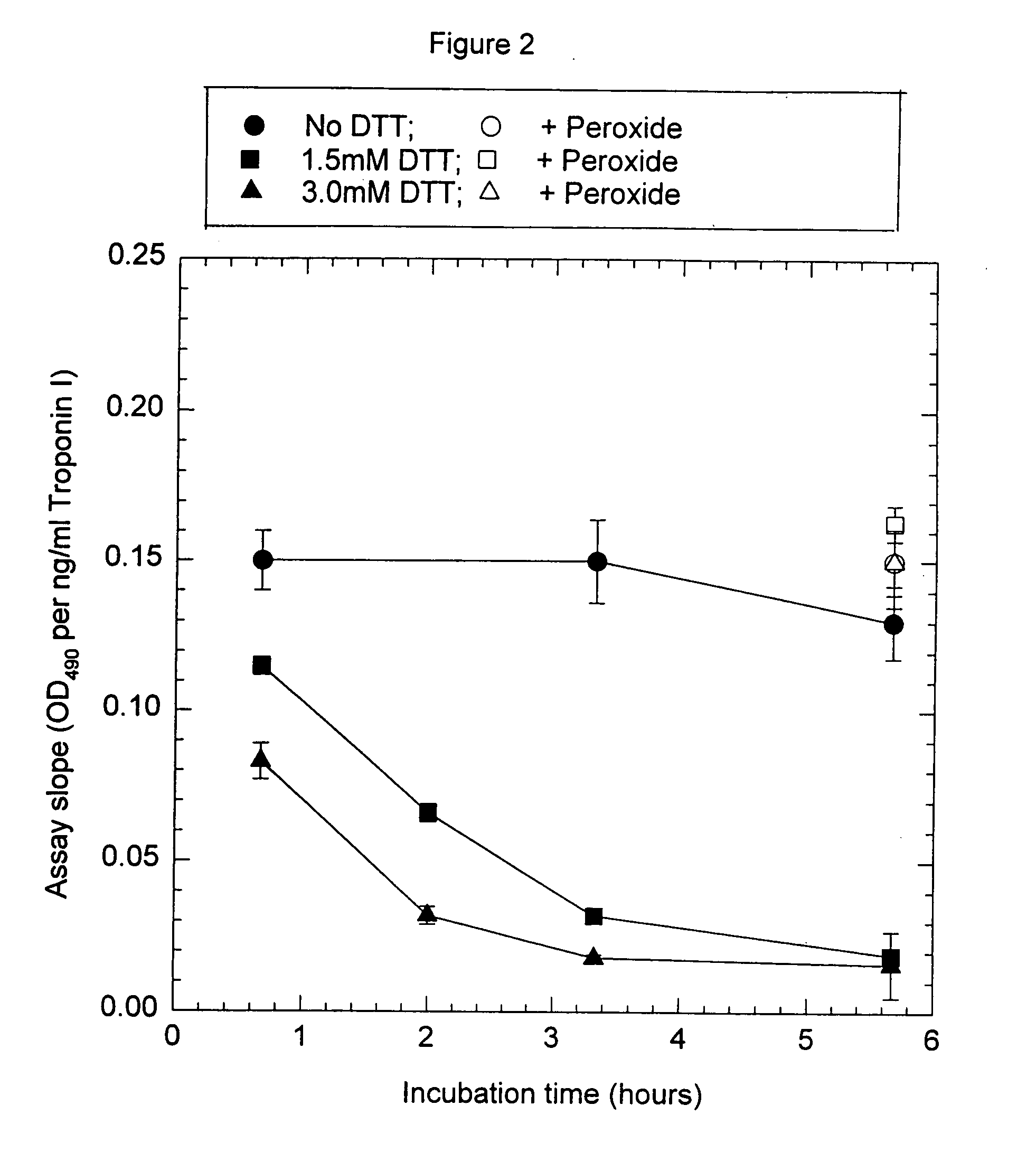
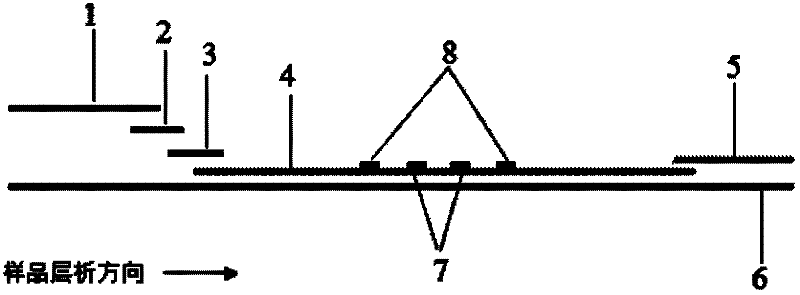
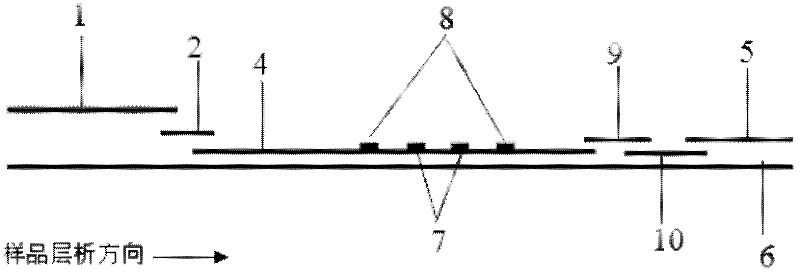
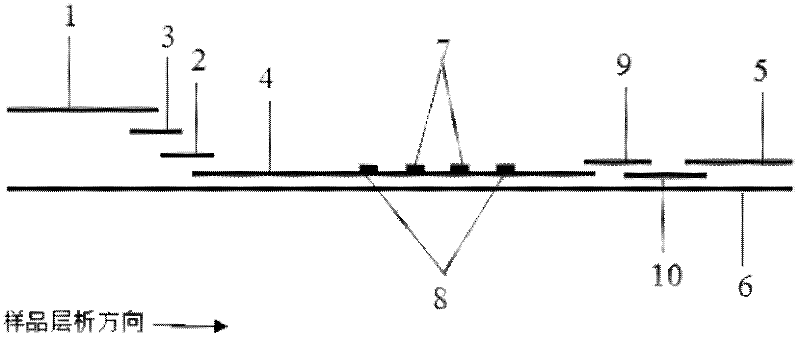
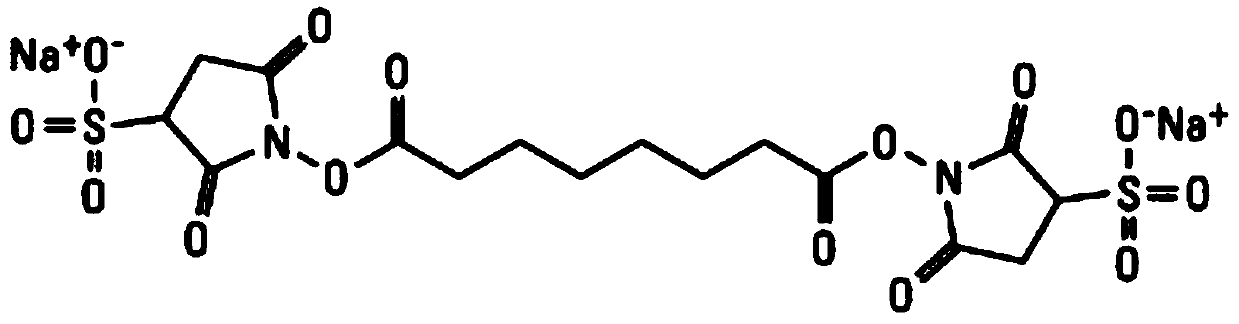
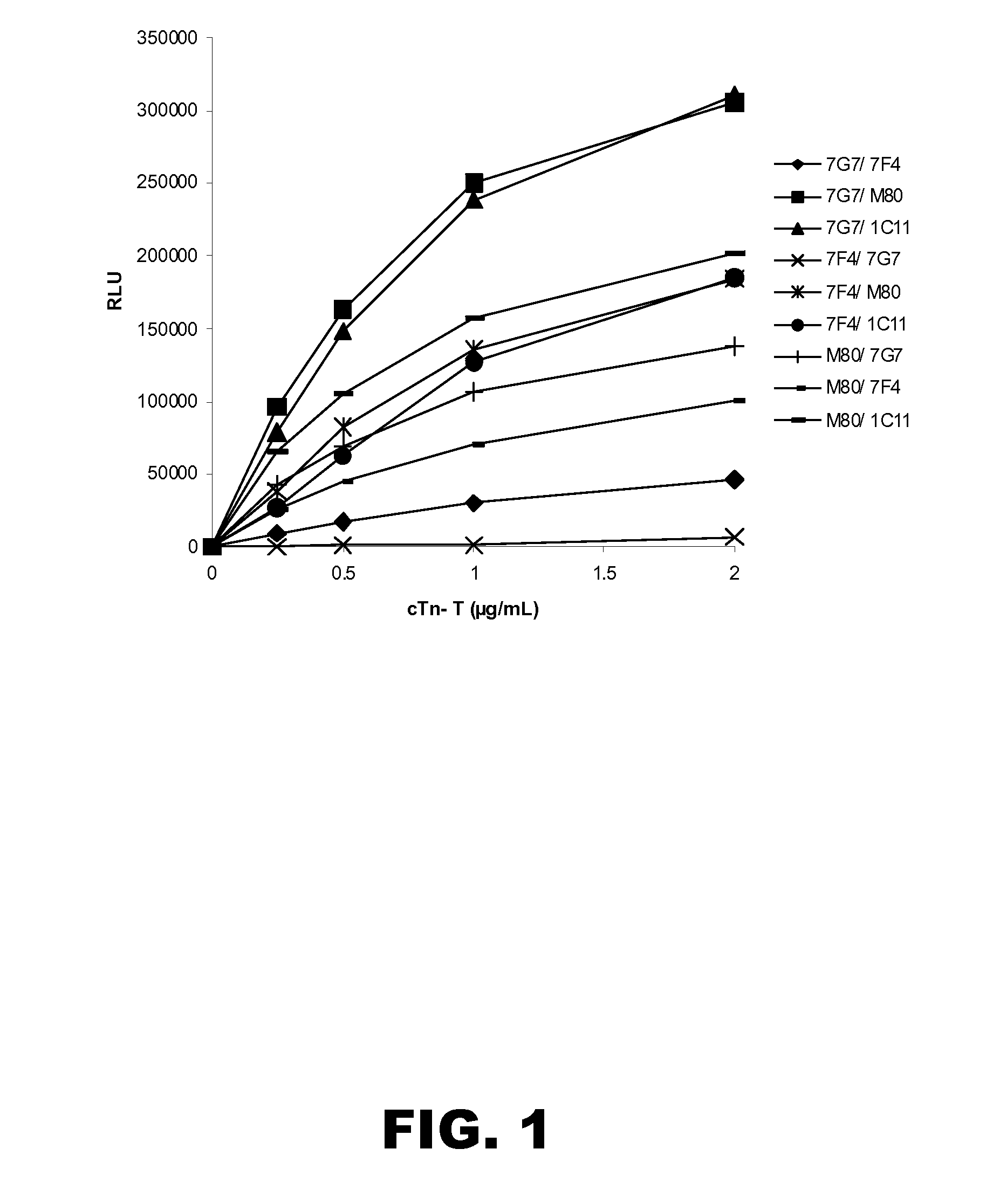
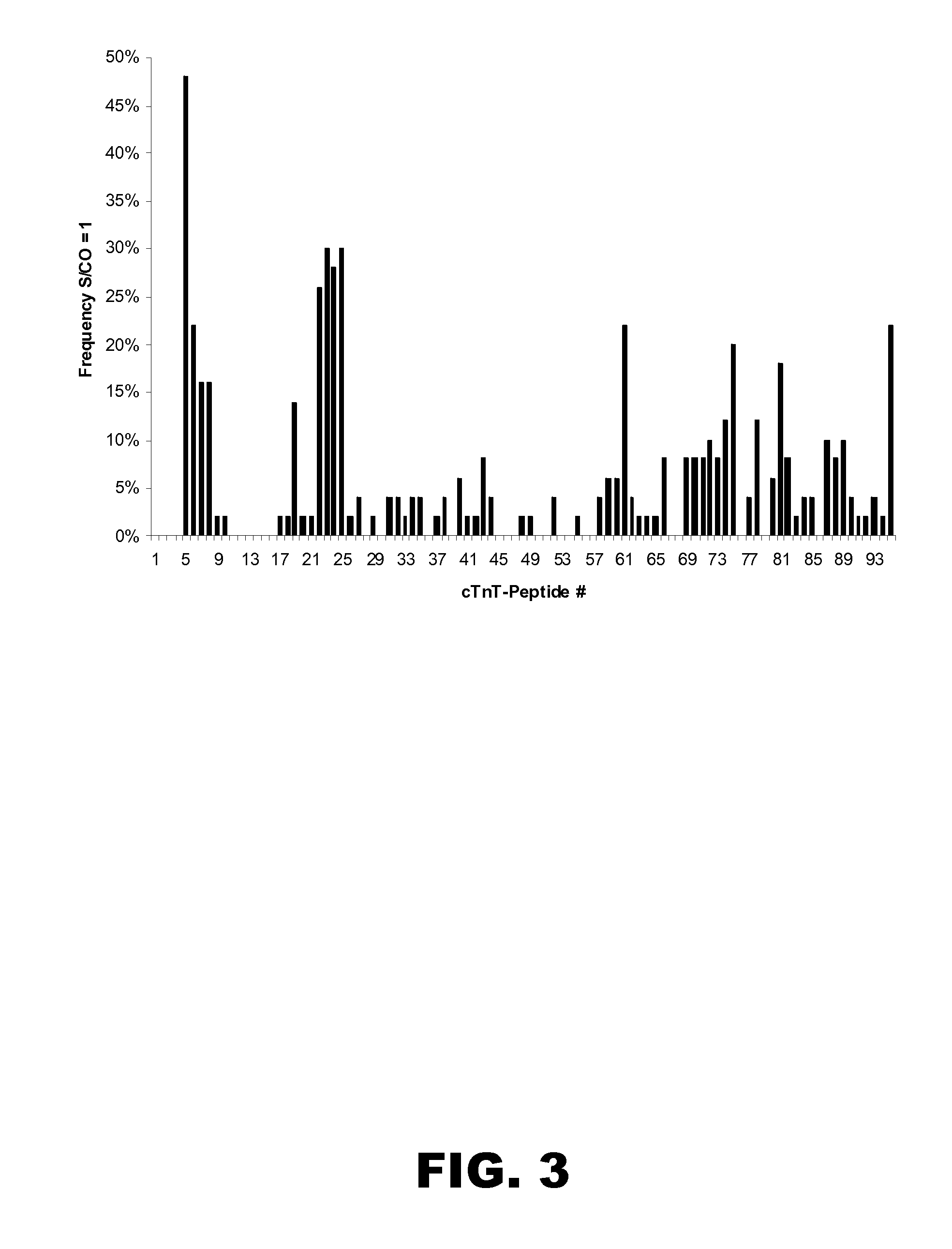
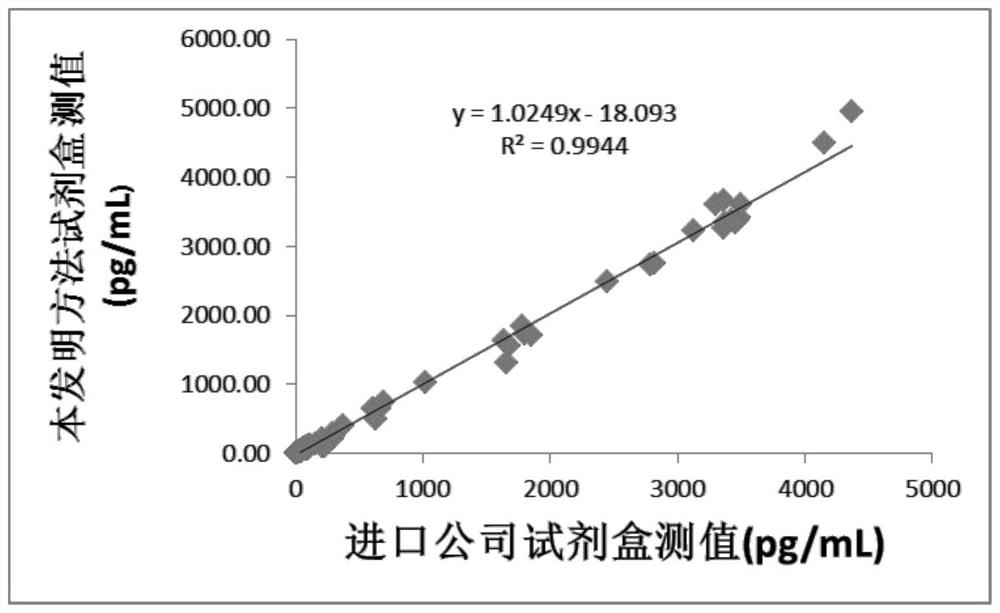
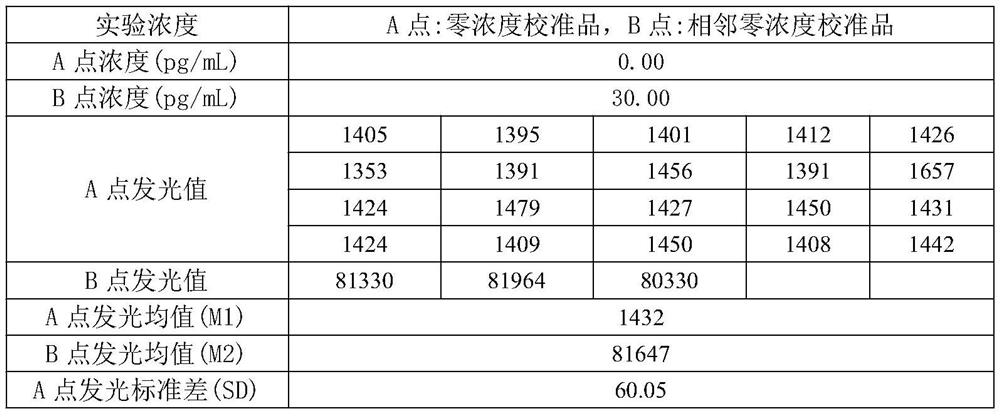
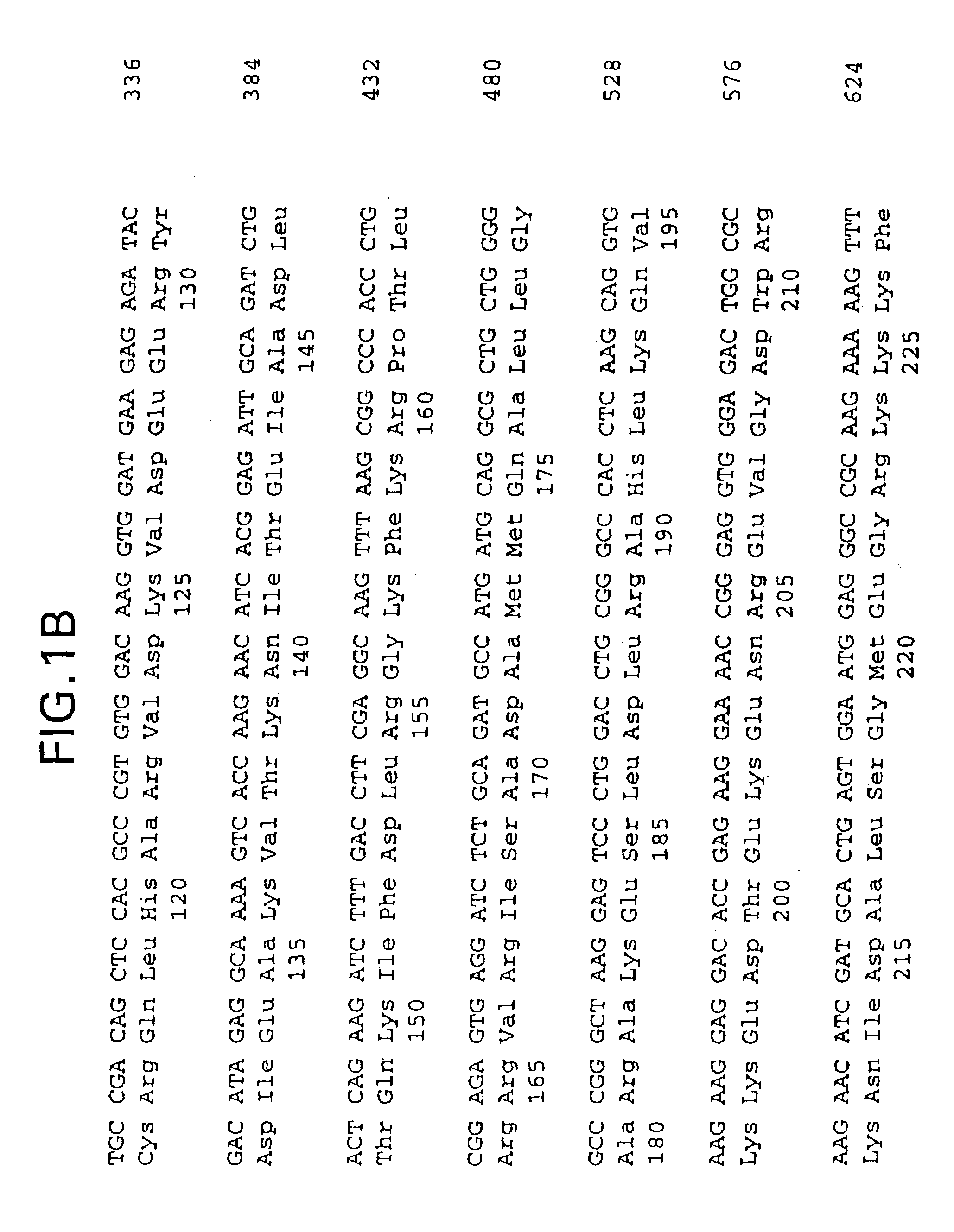
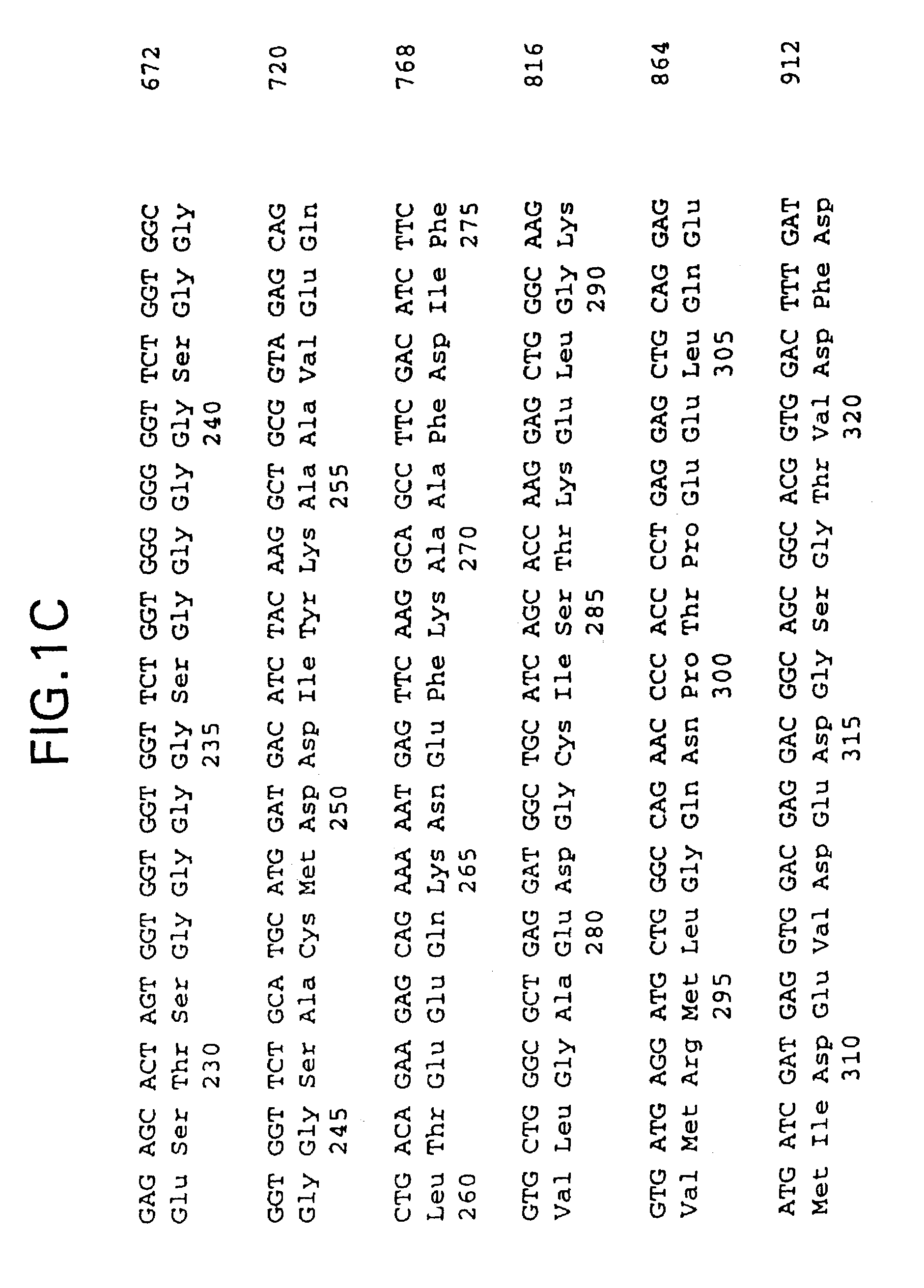
Patents
Literature
44 results about "Troponin T" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Troponin T is a part of the troponin complex, which are proteins integral to the contraction of skeletal and heart muscles. They are expressed in skeletal and cardiac myocytes. Troponin T binds to tropomyosin and helps position it on actin, and together with the rest of the troponin complex, modulates contraction of striated muscle. The cardiac subtype of troponin T is especially useful in the laboratory diagnosis of heart attack because it is released into the blood-stream when damage to heart muscle occurs. It was discovered by the German physician Hugo A. Katus at the University of Heidelberg, who also developed the troponin T assay.
Methods for the assay of troponin I and T and complexes of troponin I and T and selection of antibodies for use in immunoassays
InactiveUS6991907B1Promote recoveryChemiluminescene/bioluminescenceEnzymologyHeart diseaseClinical state
Antibodies and methods are described for the detection and quantitation of cardiac specific troponin I in samples. Cardiac-specific troponin isoforms exist in various forms in the blood, including free and complexed forms. By selecting antibodies that are insensitive and / or sensitive to these various forms, the present invention can provide immunoassays that more accurately reflect the clinical state of an individual. These described antibodies and methods can be used for providing indicators of myocardial infarction and other cardiac pathologies.
Owner:BIOSITE INC
Methods and compositions for the diagnosis of diseases of the aorta
InactiveUS20070224643A1Facilitate patient treatmentConvenient treatmentDiagnosticsSurgeryAortic dissectionSmooth Muscle Myosins
The present invention relates to methods and compositions for symptom-based differential diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to the use of biomarkers, either individually or in combinations with one another to rule in or out diseases of the aorta and its branches, most particularly aortic aneurysm and / or aortic dissection, and for risk stratification in such conditions. Preferred markers include one or more of creatine kinase-BB (CK-BB), creatine kinase-MB (CK-MB), acidic calponin, basic calponin, B-type natriuretic peptide (BNP), NT-proBNP, proBNP, BNP79-108, BNP3-108, caldesmon, caspase-3, D-dimer, soluble elastin fragments, endothelial cell-selective adhesion molecule (ESAM), fibrillin-1, heart-type fatty acid binding protein, MMP-9, myeloperoxidase, myoglobin, smooth muscle myosin, smooth muscle myosin heavy chain, TIMP-1, free cardiac troponin I, complexed cardiac troponin I, free and complexed cardiac troponin I, free cardiac troponin T, complexed cardiac troponin T, and free and complexed cardiac troponin T, and preferred assays are configured to detect these markers.
Owner:BIOSITE INC
Highly sensitive system and methods for analysis of troponin
The invention provides methods, compositions, kits, and systems for the sensitive detection of cardiac troponin. Such methods, compositions, kits, and systems are useful in diagnosis, prognosis, and determination of methods of treatment in conditions that involve release of cardiac troponin.
Owner:RGT UNIV OF CALIFORNIA +1
Methods for the assay of troponin I and T and complexes of troponin I and T and selection of antibodies for use in immunoassays
InactiveUS6939678B1Promote recoveryChemiluminescene/bioluminescenceEnzymologyCysteine thiolateBlood plasma
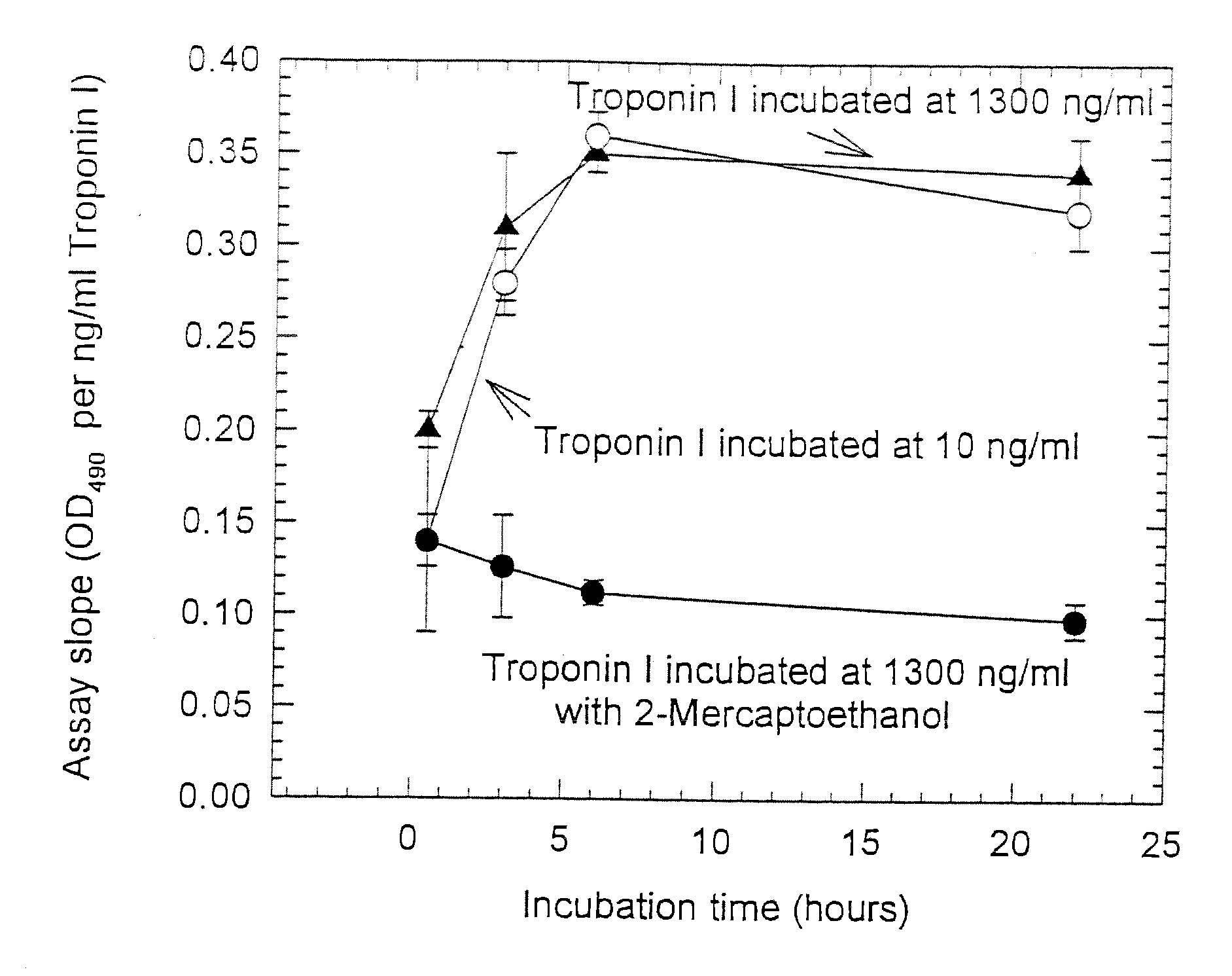
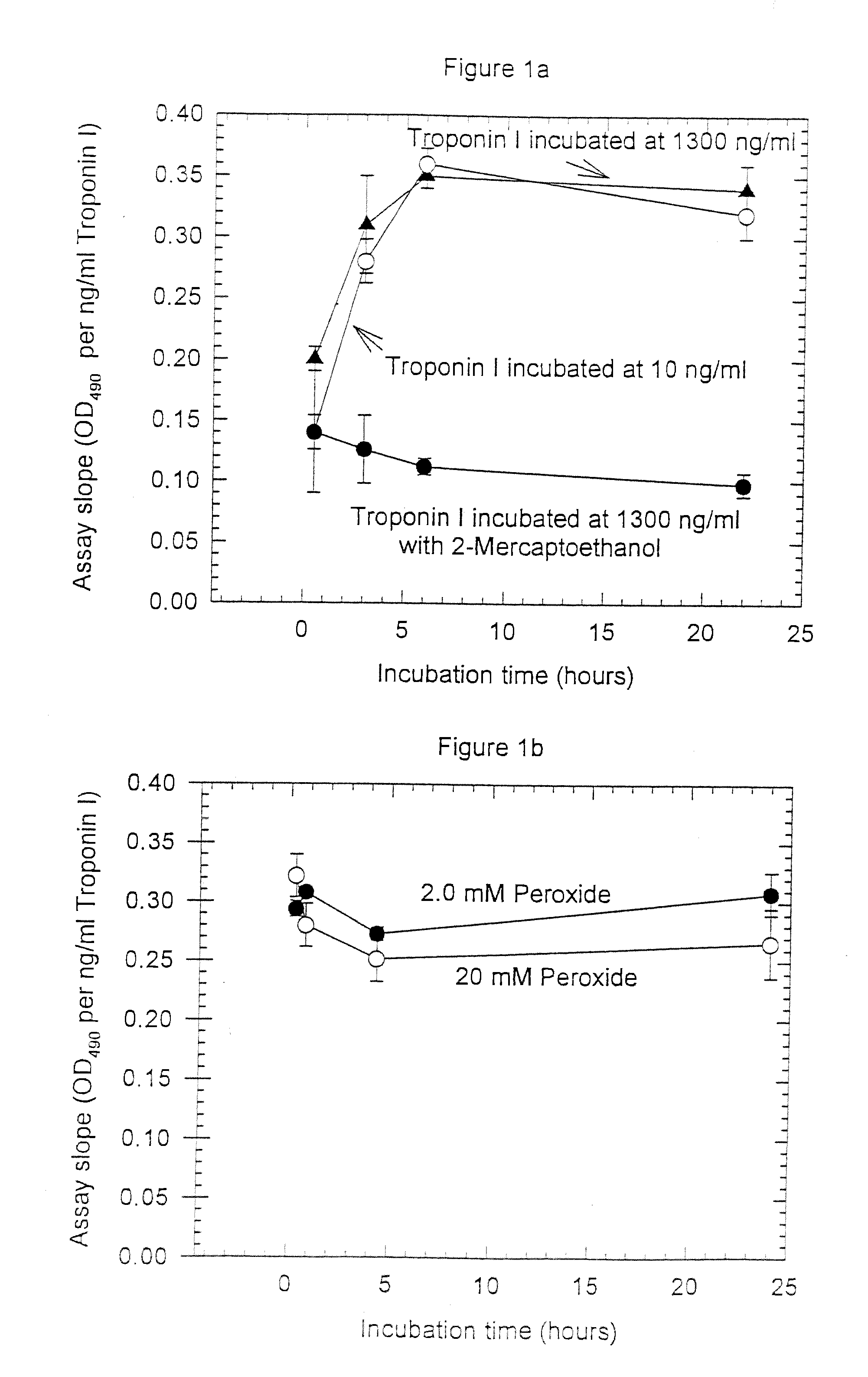
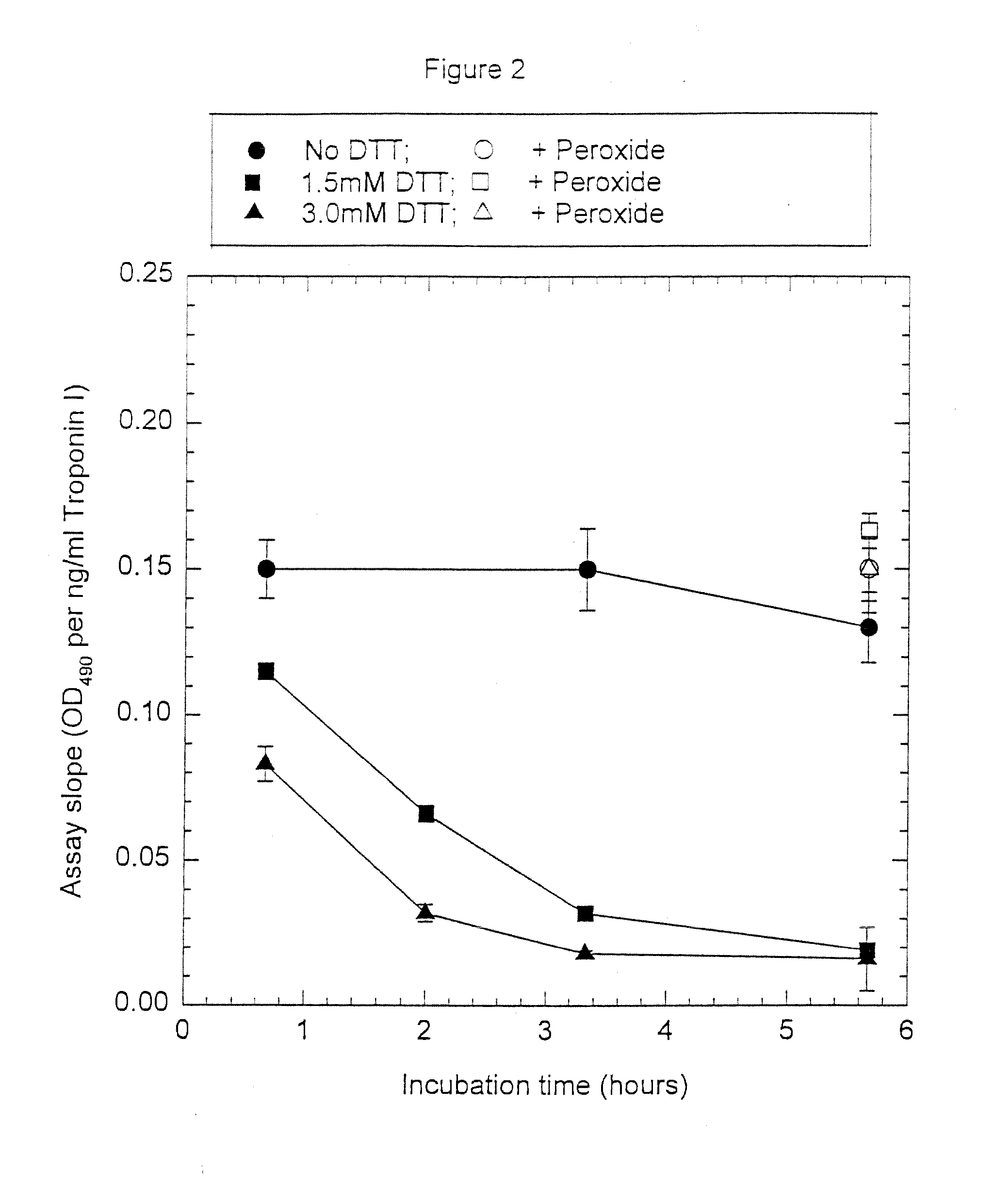
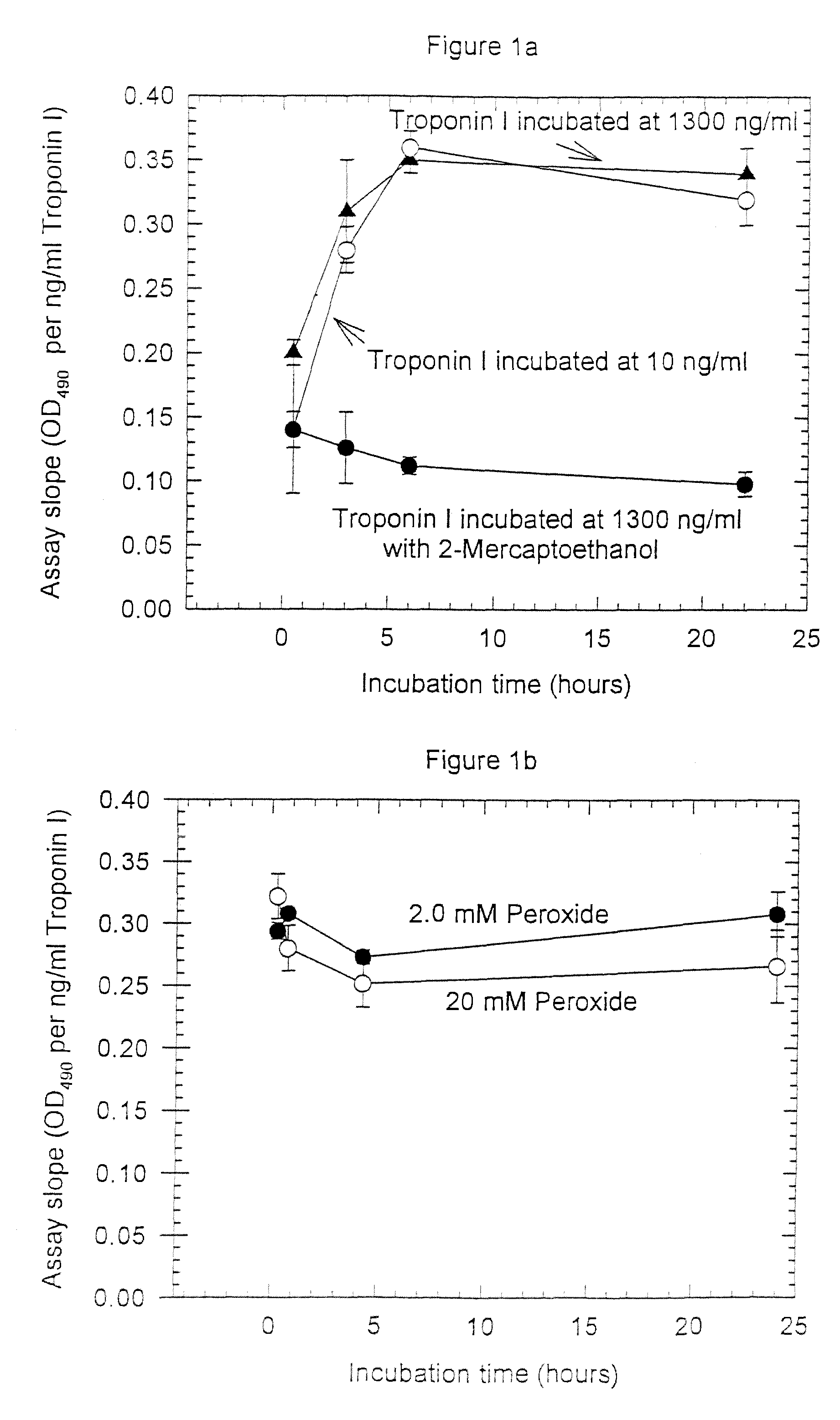
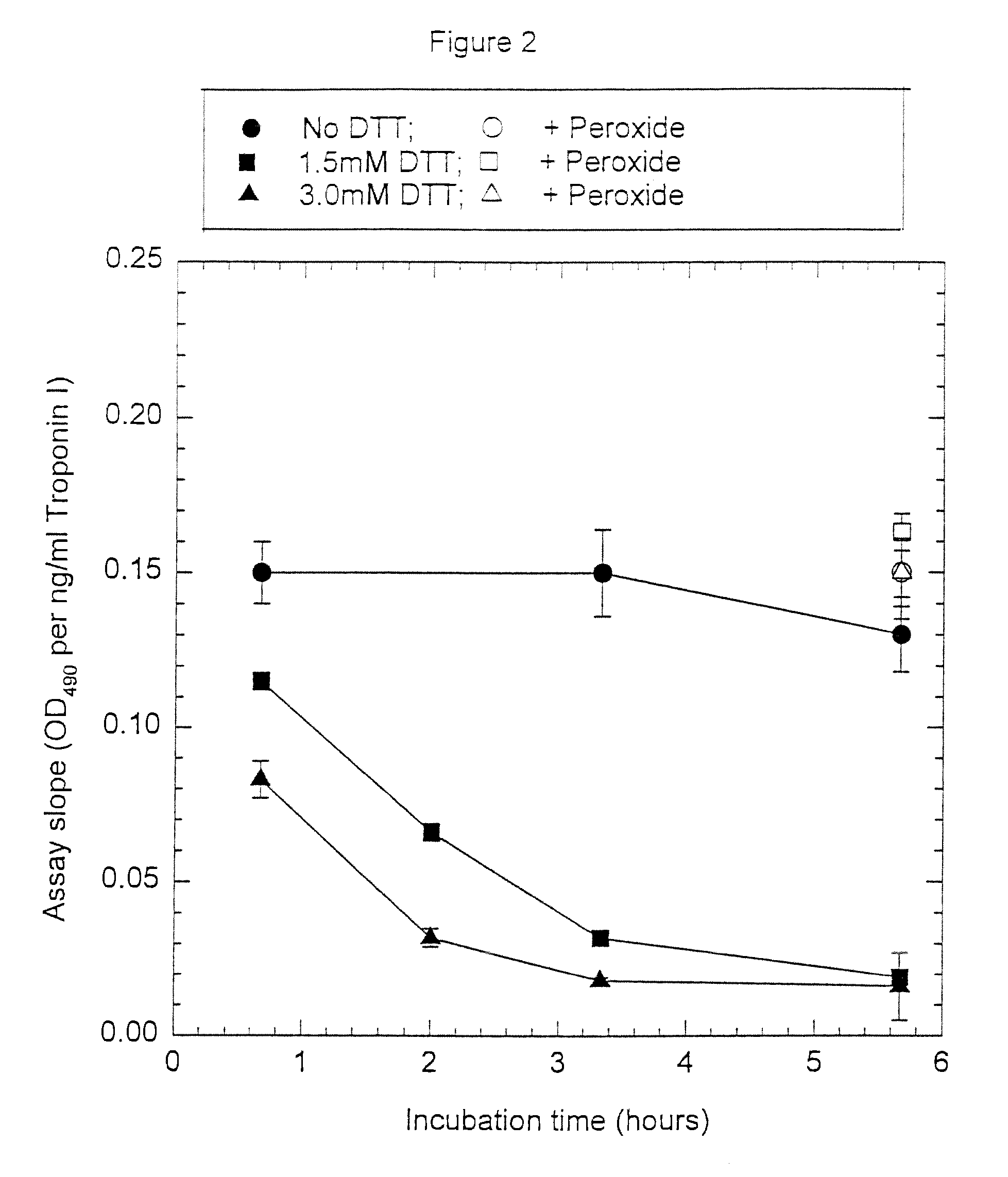
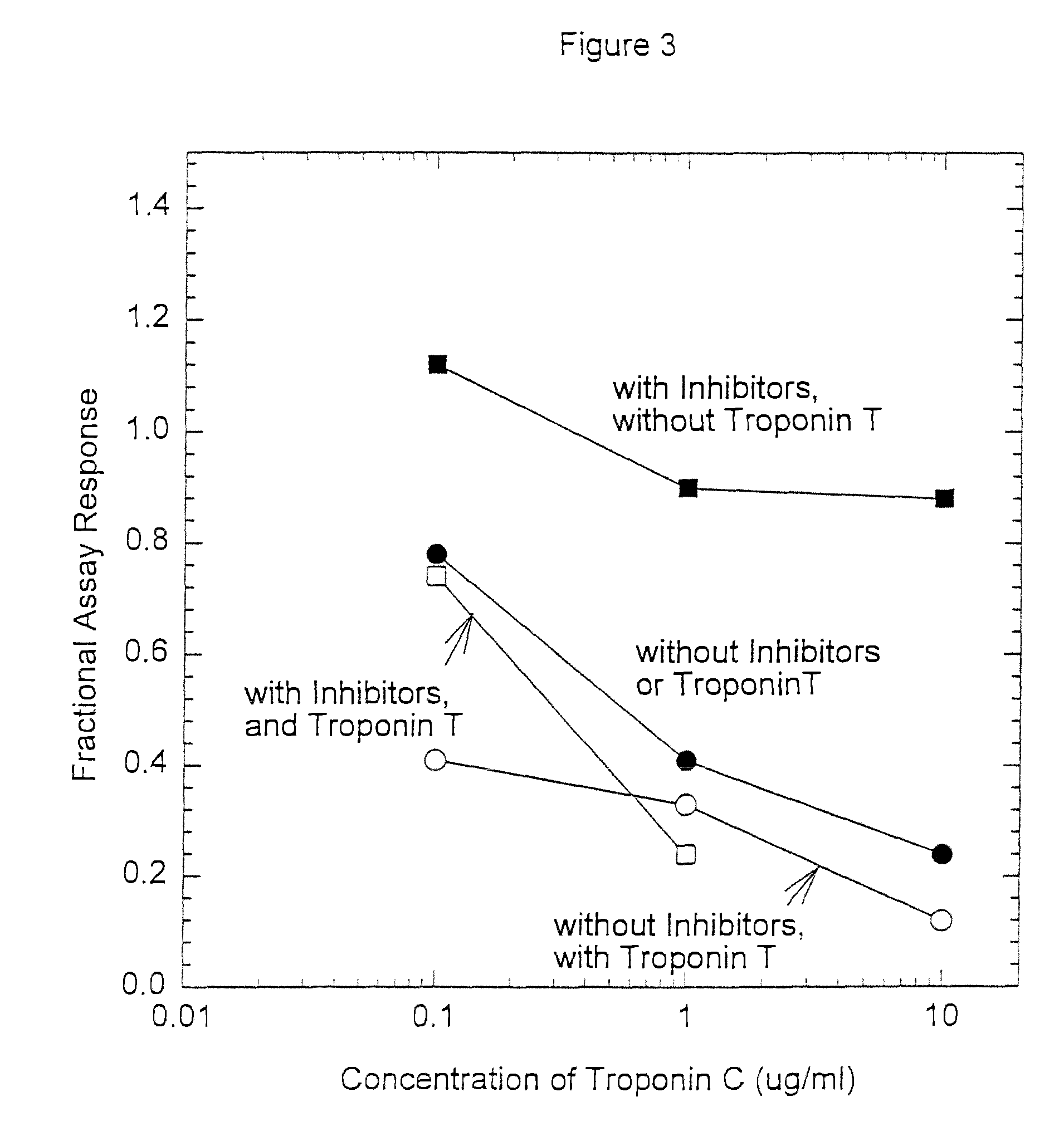
Assay systems and specialized antibodies for the detection and quantitation of troponin I and troponin T in body fluids as an indicator of myocardial infarction. Since troponin I and T exist in various conformations in the blood, the ratios of the monomeric troponin I an T and the binary and ternary complexes, as well as which form of troponin present in the blood, may be related to the metabolic state of the heart. Disclosed is a system to determine the presence of a troponin form or a group of troponin forms in a sample of whole blood, serum or plasma.Disclosed is a stabilized composition of troponin; the stabilized composition can comprise a stabilized composition of troponin I, wherein the troponin I is oxidized, the troponin I can be unbound or the troponin I can be in a complex.Disclosed is a method for improving the recovery of troponin I or T from a surface used in immunoassays.Also disclosed are antibodies which recognize, unbound troponin forms, the forms of troponin in binary complexes, the ternary complex of troponin I, T and C, and the conformations of troponin I having intramolecularly oxidized and reduced cysteines.
Owner:BIOSITE INC
Method and kit for the diagnosis of troponin I
InactiveUS7285418B2High affinityIncrease exposurePeptide/protein ingredientsChemiluminescene/bioluminescenceSerum igeTroponin I binding
The invention provides a method for assaying for troponin I in a sample such as serum, the method comprising a determination of the troponin I concentration in a sample using a standard preparation that comprises a complete native troponin complex. One exemplary assay for troponin I concentration according to the invention is an immunoassay. Further contemplated in the method is a step comprising contacting the sample with a Ca2+-binding agent. Another aspect of the invention is the standard preparation comprising a complete native troponin complex. Yet another aspect of the invention is a kit for use in the assay method comprising the standard preparation, a detectable label, and a troponin I binding partner, such as an anti-troponin I antibody. All aspects of the invention are usefully practiced using human materials, such as a human troponin complex, and / or human sources for samples.
Owner:HYTEST LTD
Methods for improving the recovery of troponin i and t in mebranes, filters and vessels
A method to facilitate recovery troponin I and / or troponin T from a sample comprising addition of troponin C to the sample or to a surface from which the troponin I and / or troponin T are recovered.
Owner:BIOSITE INC
Highly sensitive system and methods for analysis of troponin
InactiveUS20100255518A1Analysis using chemical indicatorsMicrobiological testing/measurementMedicineTroponin T
The invention provides methods, compositions, kits, and systems for the sensitive detection of cardiac troponin, Such methods, compositions, kits, and systems are useful in diagnosis, prognosis, and determination of methods of treatment in conditions that involve release of cardiac troponin.
Owner:RGT UNIV OF CALIFORNIA +1
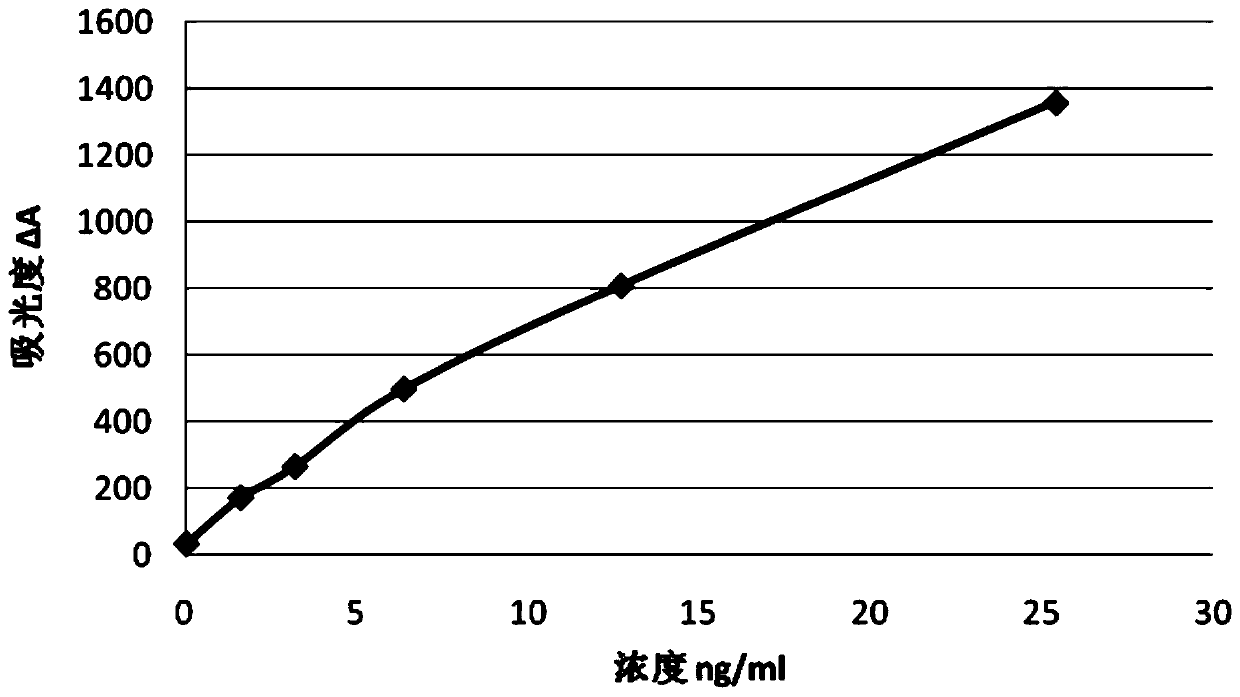
Troponin-T determination kit
ActiveCN102841206AHigh detection sensitivityEasy to operateMaterial analysis by observing effect on chemical indicatorBiological testingMicrosphereFully automatic
The invention relates to a kit for determining troponin-T (TNT) content of serum, aims to overcome the deficiencies of the above background technology, and provides a kit for detecting troponin-T by an immunoturbidimetry method, wherein the kit does not require sample dilution, has simple operation, high accuracy and good repeatability, and is suitable for various types of fully automatic biochemical analyzers and special protein instruments. A technical scheme is as below: the kit comprises: A. a reagent R1 containing a buffer solution, a preservative, an accelerating agent, inorganic salts, a surfactant and the balance of purified water; b. a reagent R2 containing a buffer solution, latex microspheres with diameter of 60-150 nm combined with anti-human troponin antibodies and an antiseptic; and c. a reference calibrator containing a buffer solution, a stabilizer, a preservative, a certain amount of recombinant human protein troponin-T pure product determined by concentration requirements and the balance of purified water.
Owner:NANJING NORMAN BIOLOGICAL TECH
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Detection kit and preparation method thereof and detection method of troponin T
ActiveCN109596835AImprove coating efficiencyDoes not affect activityBiological testingBiotin-streptavidin complexAntigen
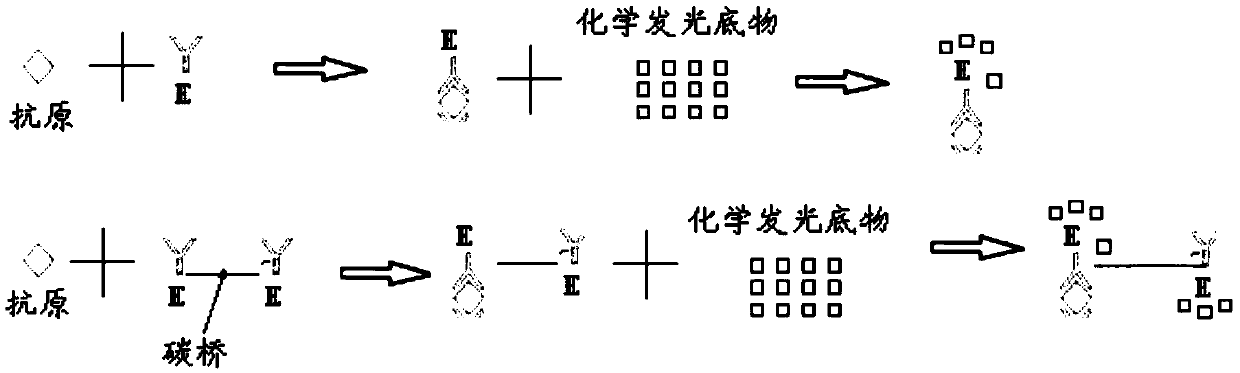
The invention belongs to the technical field of in-vitro detection, and particularly relates to a detection kit and a preparation method and application thereof. The detection kit comprises a magneticseparation reagent and an enzyme-labeled reagent; the magnetic separation reagent is prepared from troponin T antibody-coated gold magnetic particles or prepared from streptavidin-coated gold magnetic particles and a biotin-labeled troponin T antibody; and the enzyme-labeled reagent is prepared from an enzyme-labeled antibody polymer, wherein the enzyme-labeled antibody polymer is prepared from at least two enzyme-labeled antibodies and carbon bridges; the carbon bridges connect any two or more, adjacent or non-adjacent enzyme-labeled antibodies, and each carbon bridge has at least two connecting loci for connecting the enzyme-labeled antibodies; and each enzyme-labeled antibody is prepared from a detection antibody and a labeling enzyme coupled to the detection antibody, and the connecting loci are connected with the detection antibodies and / or the labeling enzymes. When chemiluminescence detection is conducted, a reaction signal value of a chemiluminescence method is amplified, thesensitivity of an antigen captured by the detection reagent can be enhanced, and the detection sensitivity is improved.
Owner:深圳天辰医疗科技有限公司
Method for chemiluminescence immunity analysis and detection myocardium calcium protein T
InactiveCN101206225AChemiluminescene/bioluminescenceBiological testingCalcium proteinAnalysis method
The invention relates to an analysis method for using the means of chemiluminescence immunoassay to detect cardiac troponins T(cTnT).
Owner:天津天美生物技术有限公司
Method for detecting human myocardial troponin T through cytometric bead array
The invention discloses a method for detecting human myocardial troponin T through cytometric bead array. The method comprises the following three steps of: activating a bead, coupling a monoclonal antibody and detecting the human myocardial troponin T, specifically, firstly, activating the beads; secondly, preparing the monoclonal antibody of the carboxylation bead coupled mouse anti human myocardial troponin T; thirdly, capturing the human myocardial troponin T in a specimen; and finally, adding multiple antigenic and fluorescein isothiocyanate labeled donkey anti goat intravenous gamma globulin G of goat anti human myocardial troponin T, and detecting the fluorescence intensity of fluorescein isothiocyanate (FITC) on the bead on a flow cytometry to indirectly detect the content of the human myocardial troponin T in the specimen. The monoclonal antibody coupled bead, the polyclonal antibody of the goat anti human myocardial troponin T, and the fluorescein isothiocyanate labeled donkey anti goat intravenous gamma globulin (IgG) can be prepared into a commodity kit, which can detect the human myocardial troponin T at any time. The method is simple and feasible; and by the method, the human myocardial troponin T can be quickly detected with convenience and the basis for disease diagnosis is provided.
Owner:贵州省临床检验中心
ASSAY FOR CARDIAC TROPONIN-T (cTnT)
InactiveUS20110129818A1Microbiological testing/measurementDisease diagnosisTest sampleAutoantibody production
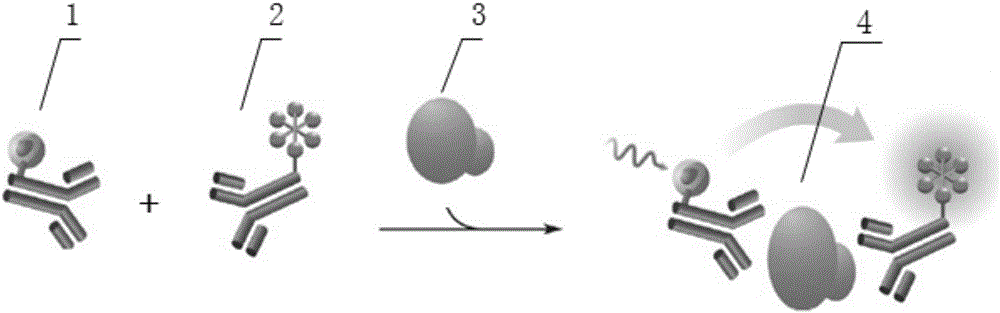
The present disclosure describes immunoassays for detecting cardiac troponin-T (cTnT) in a test sample, and in particular immunoassays and kits for detecting cTnT in a test sample suspected of containing substances that may interfere with the determination of cTnT, such as heterophilic endogenous antibodies and autoantibodies to cTnT. The methods use more than one capture phase antibody and more than one detection antibody to improve specificity, and provide for the use of humanized immunoreagents to overcome heterophilic antibody interferences.
Owner:ABBOTT LAB INC
Two-channel detection card of troponin I and troponin T
The invention relates to a two-channel detection card of troponin I and troponin T, which belongs to the technical field of blood detection. A test strip is arranged in a casing of the detection card and is stuck with a cellulose nitrate film by the middle part of a supporting back plate; a water absorption film and a sample pad are respectively stuck on the two end parts of the test strip; the inner end of the test strip is respectively in lap joint with one end of the cellulose nitrate film; two sections of colloidal gold films are clamped and stuck between the lap joint parts of the sample pad and the cellulose nitrate film and are respectively glass fibre films containing a monoclonal-antibody colloidal gold marker of the troponin I and a monoclonal-antibody colloidal gold marker of the troponin T; two detection strips are arranged on the cellulose nitrate film and contain an antibody resisting the troponin I and an antibody resisting the troponin T respectively, and another mass hole strip contains an anti-mouse antibody. The sample pad and the cellulose nitrate film are over against a sample loading hole and a detection window hole respectively. The invention has the advantage that the troponin I and the troponin T which are contained in the serum or the plasma of a patient can be detected simultaneously. The detection card is easy to prepare, saves the detection expense and has convenient use, quick detection, high sensitivity and accurate result.
Owner:WUXI ANKANG BIOLOGICAL TECH
Magnetic particle chemiluminiscence detection kit for determining content of hypersensitive troponin T
PendingCN112526140AImprove featuresImprove stabilityChemiluminescene/bioluminescenceBiological testingTroponin TMaterials science
The invention relates to a magnetic particle chemiluminiscence detection kit for determining the content of hypersensitive troponin T. The magnetic particle chemiluminiscence detection kit comprises areagent R1, a reagent R2, a magnetic separation reagent, a calibrator solution series and a chemiluminiscence substrate solution, wherein the R1 reagent is a buffer solution, and the magnetic separation reagent is a troponin T monoclonal antibody coated magnetic particle solution prepared by dissolving troponin T monoclonal antibody coated magnetic particles in a diluent of the magnetic separation reagent; the reagent R2 is an alkaline phosphatase-labeled troponin T monoclonal antibody solution prepared by dissolving an alkaline phosphatase-labeled troponin T monoclonal antibody in a diluentof the reagent R2, and the chemiluminescent substrate solution is a chemiluminescent substrate solution catalyzed by alkaline phosphatase to emit light. A more accurate, accurate, convenient, rapid and simple method is provided for clinically detecting troponin T in human serum.
Owner:BEIJING LEADMAN BIOCHEM
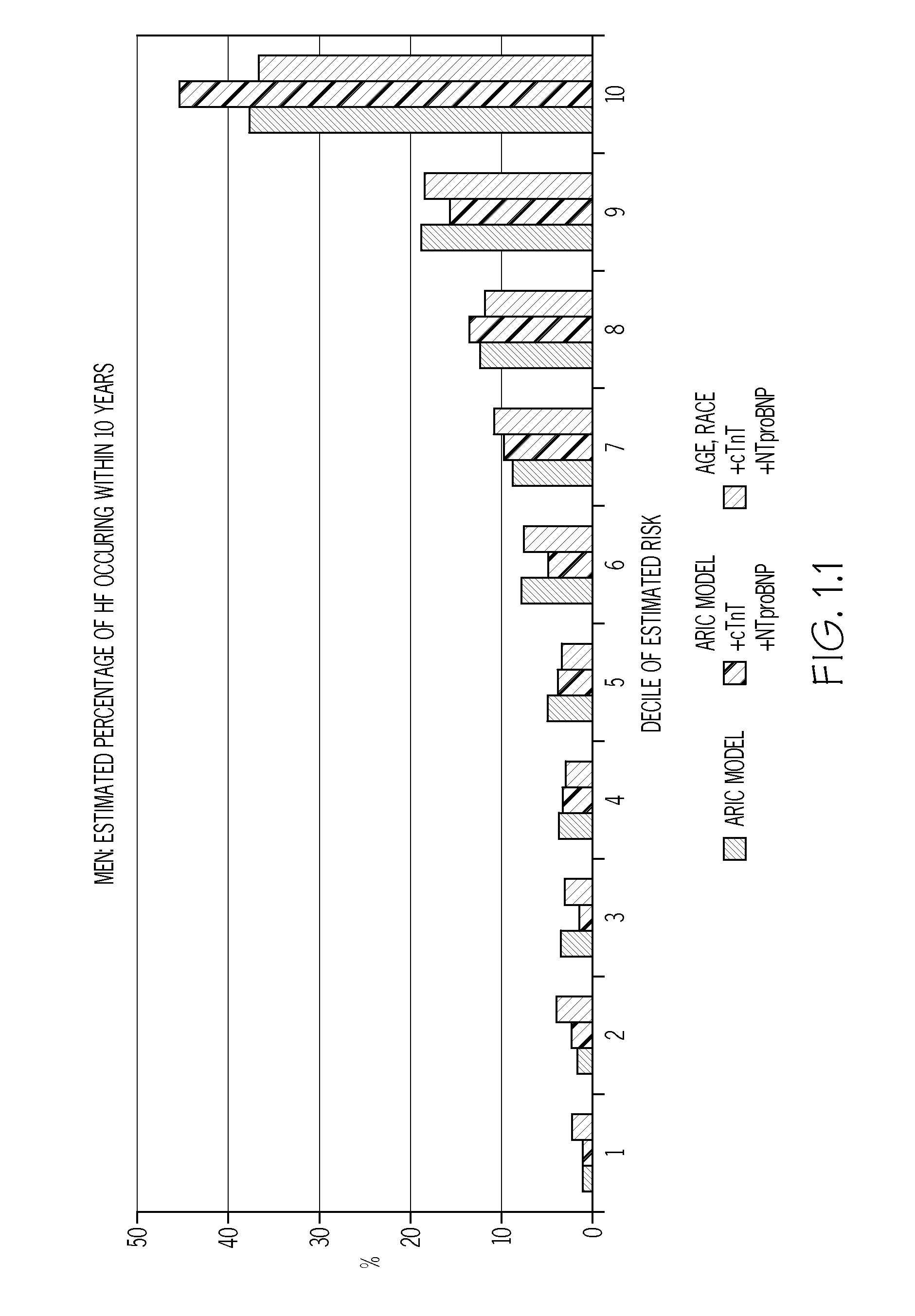
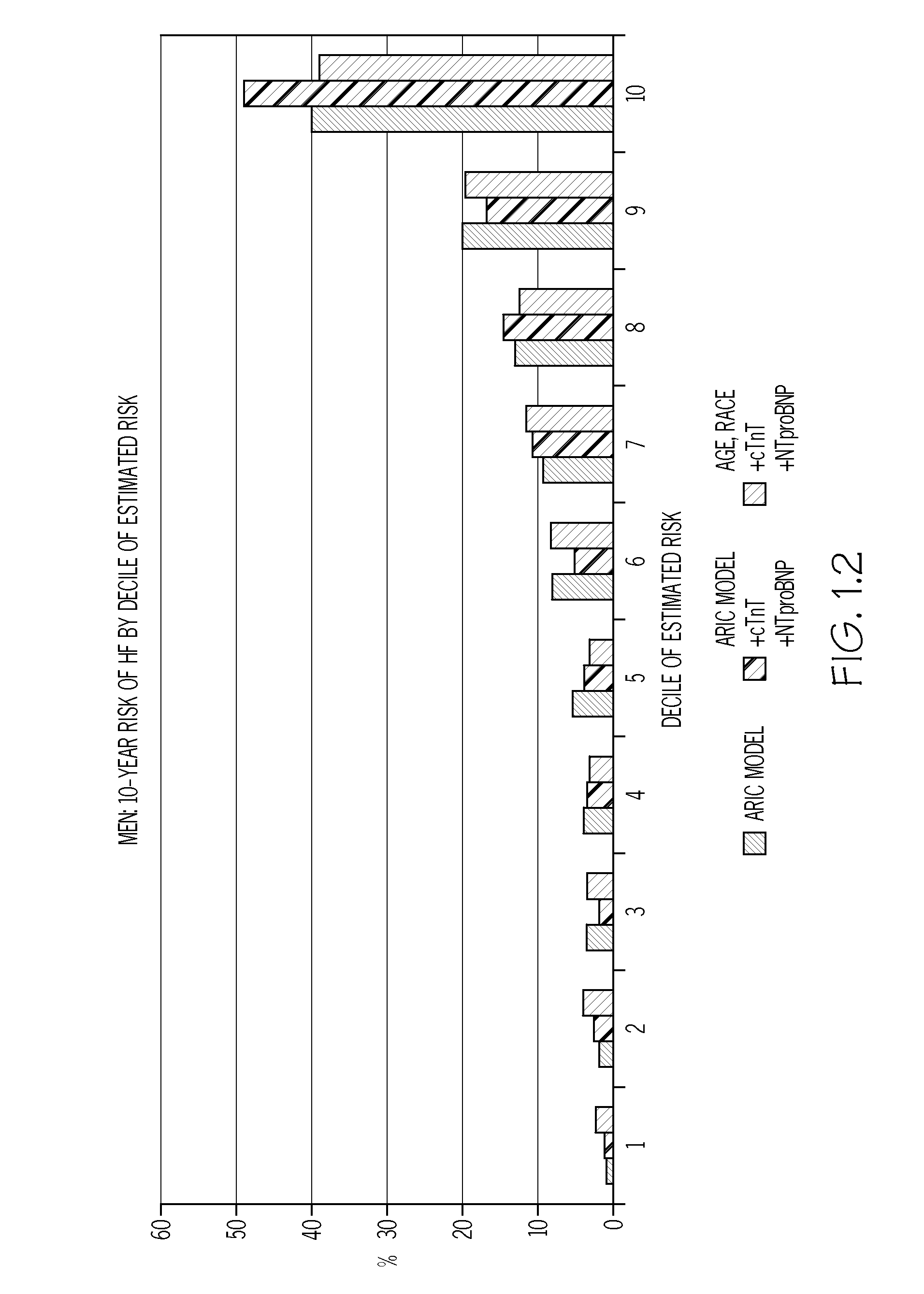
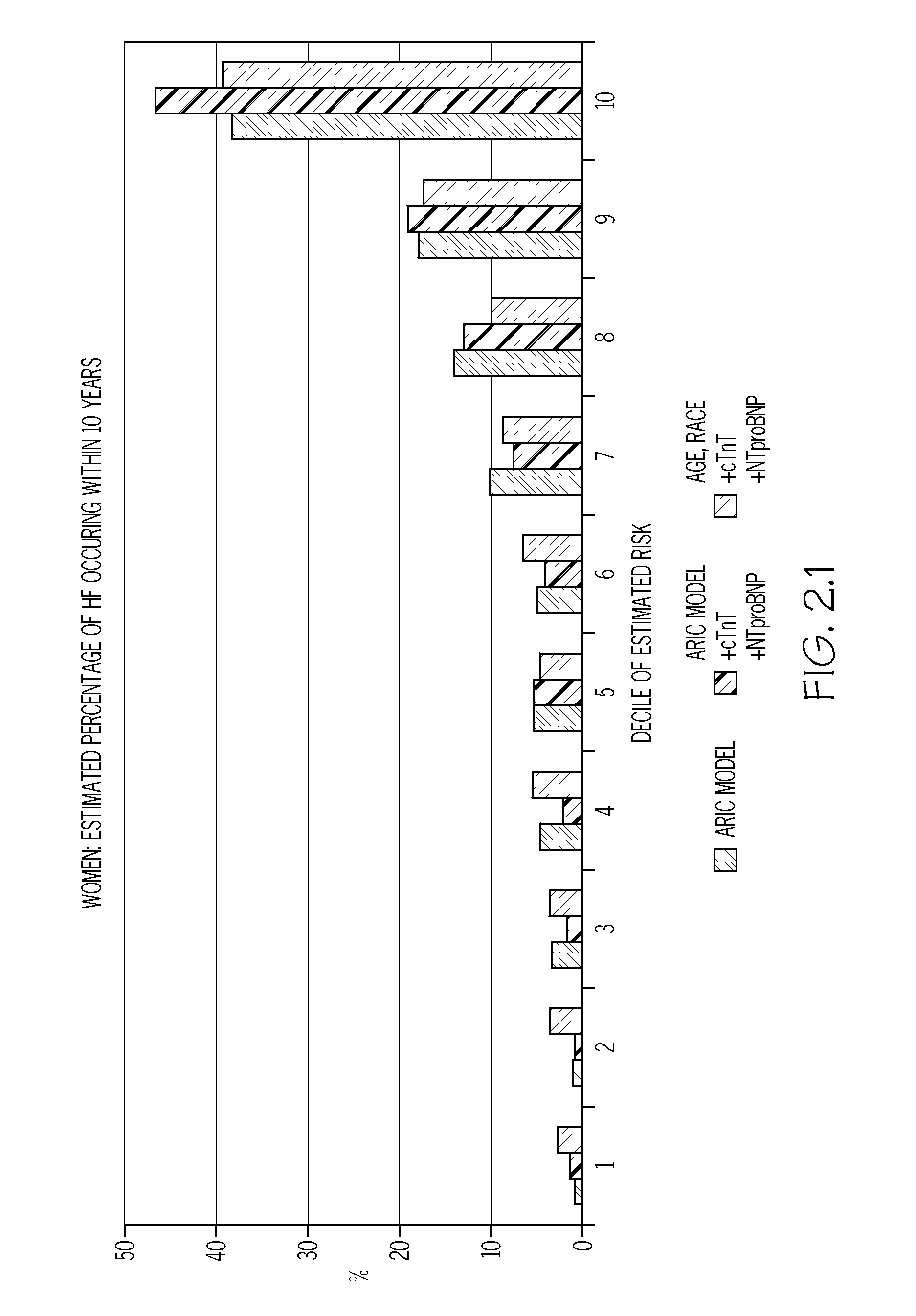
Biomarkers to improve prediction of heart failure risk
InactiveUS20140273273A1Easy diagnosisIncreased riskComponent separationHealth-index calculationRisk modelCvd risk
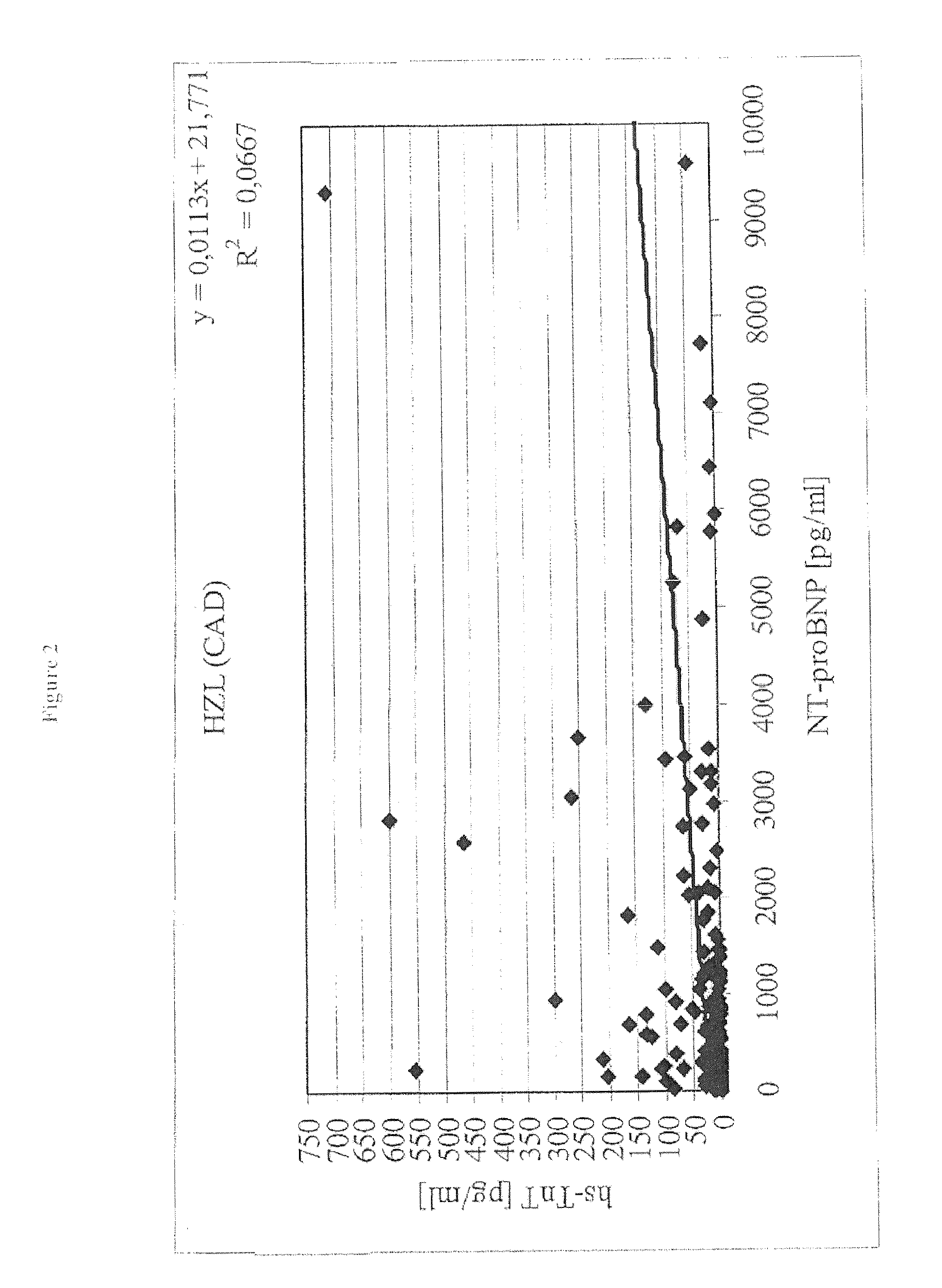
The present disclosure relates to the field of laboratory diagnostics. Specifically, methods are disclosed for determining a patient's risk of suffering from heart failure (HF) based on the detection of NT-proBNP, troponin T, and / or a natriuretic peptide. Also disclosed are methods for improving both the accuracy and speed of HF risk models by incorporating biomarker data from patient samples.
Owner:BAYLOR COLLEGE OF MEDICINE +1
Latex enhanced immunoturbidimetric detection kit of cardiac troponin T
ActiveCN109765382AColor/spectral properties measurementsBiological testingPreservativeClinical tests
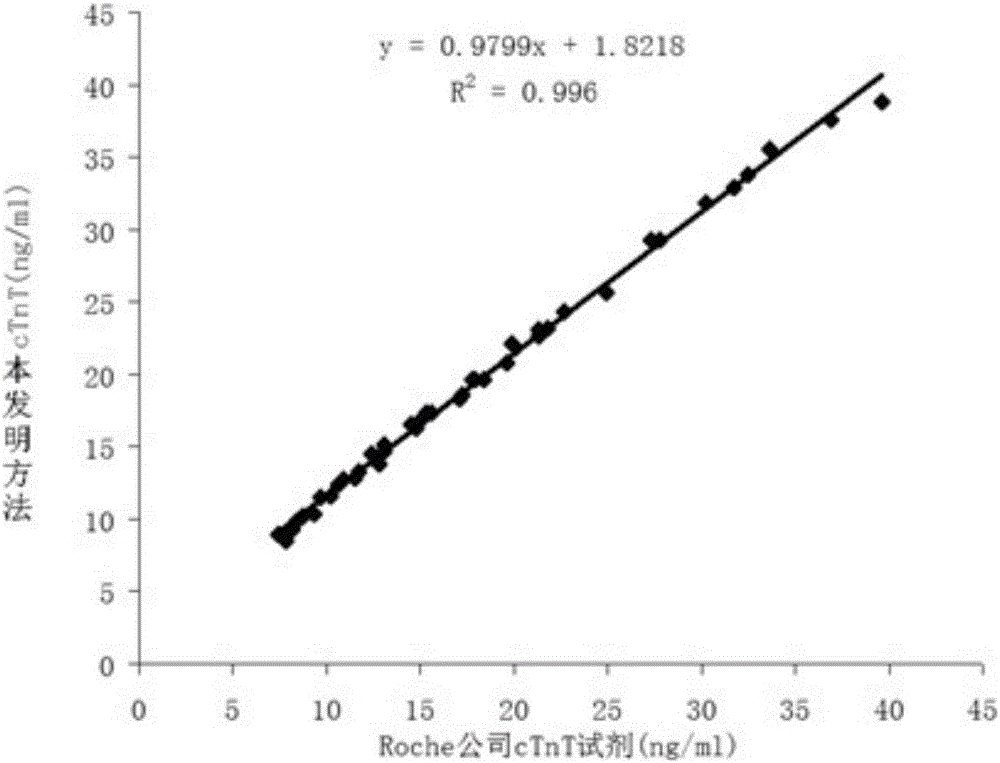
Theinvention relates to a latex enhanced immunoturbidimetric detection kit of cardiac troponin T.More precisely, the latex enhanced immunoturbidimetric detection kit comprises a first reagent and a second reagent, wherein the first reagent comprises a buffer solution, a stabilizer, a copolymer of 2-methyl allyl oxyethyl phosphonyl choline, and a preservative; and the second reagent comprises the buffer solution, latex granules bound with anti-human troponin T antibody, the stabilizer, and the preservative. The latex enhanced immunoturbidimetric detection kit of the cardiac troponin Thas a quantitative detection limit of about 0.1 ng / ml, the correlation with commercial chemiluminescence reagent is good, and can be applied to the clinical test of cTnT.
Owner:BEIJING STRONG BIOTECH INC
Immunofluorescence test strip component for quickly quantitatively testing troponin-T and test card component manufactured by same and production process of immunofluorescence test strip
The invention discloses an immunofluorescence test strip component for quickly quantitatively testing troponin-T and a test card component manufactured by the same. The test strip component comprises a test strip and a PT-porphyrin mark specific antibody packed individually. The test strip comprises a bottom liner, an envelope analysis membrane, a sample pad and an absorbent pad, wherein the envelope analysis membrane is attached on the bottom liner, the sample pad is attached on the bottom liner and jointed with one end of a nitrocellulose membrane, the absorbent pad is attached on the bottom liner and jointed with the other end of the nitrocellulose membrane, and the envelope membrane is provided with a test line and a quality control line. Further, the test line envelopes a troponin-T monoclonal antibody, the quality control line envelopes rabbit IgG antibody. The test card is formed by combining a card case with the test strip component. The test strip component and the test card consisting of the same have the advantages of operational simplicity, fastness, flexibility and good specificity and the like when used for testing troponin-T in human blood, serum or plasma, and have good clinical application prospects.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
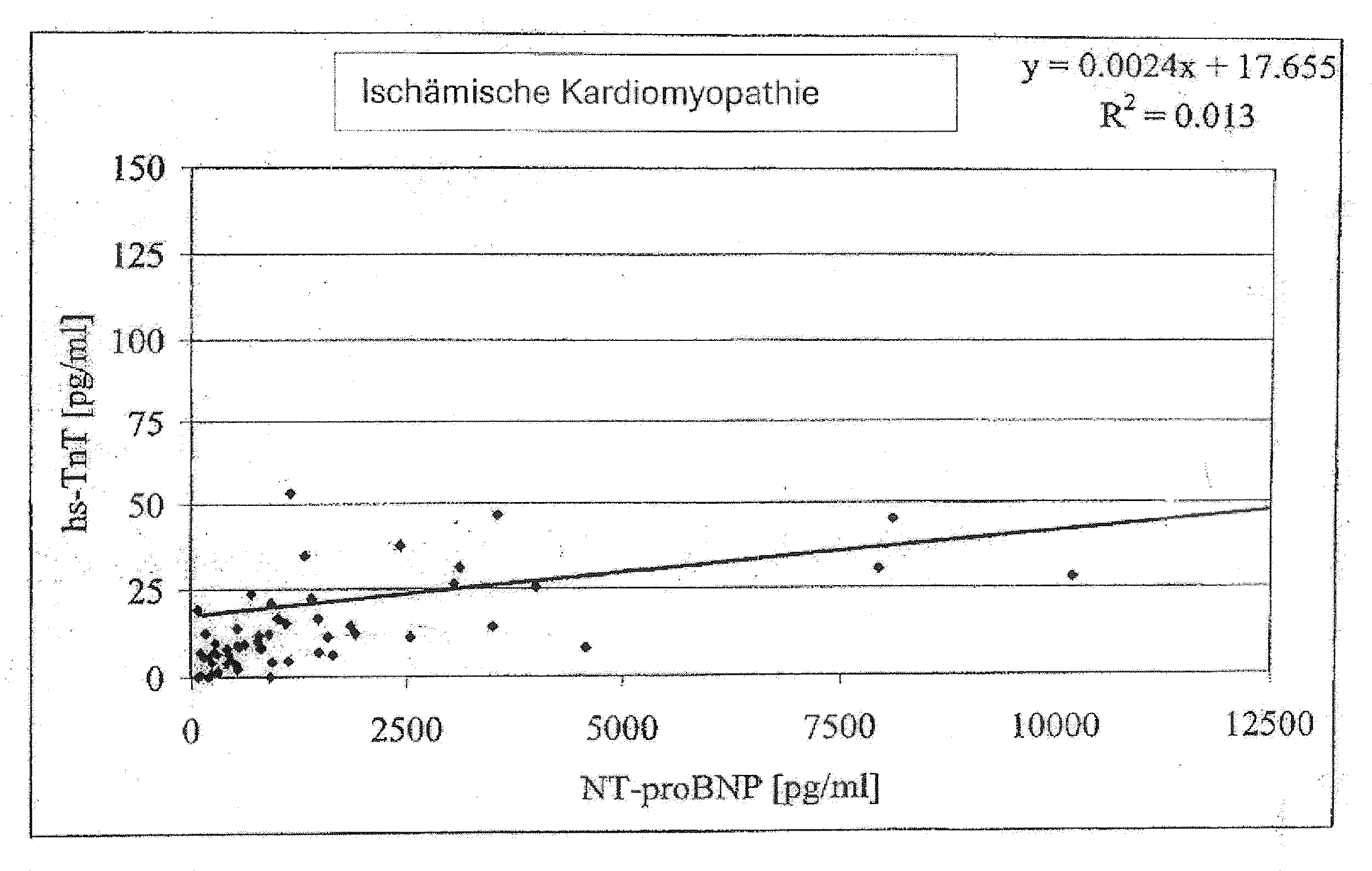
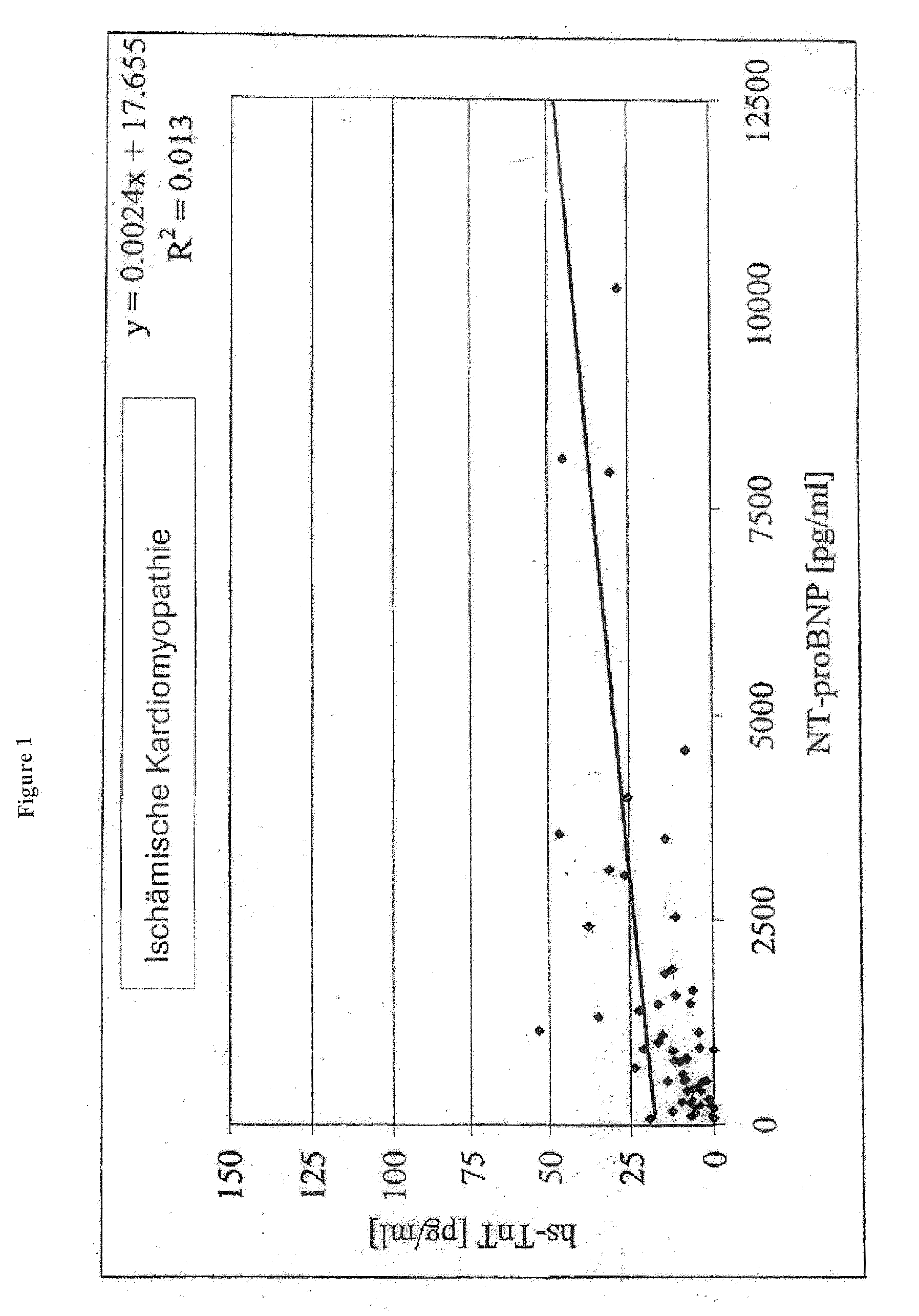
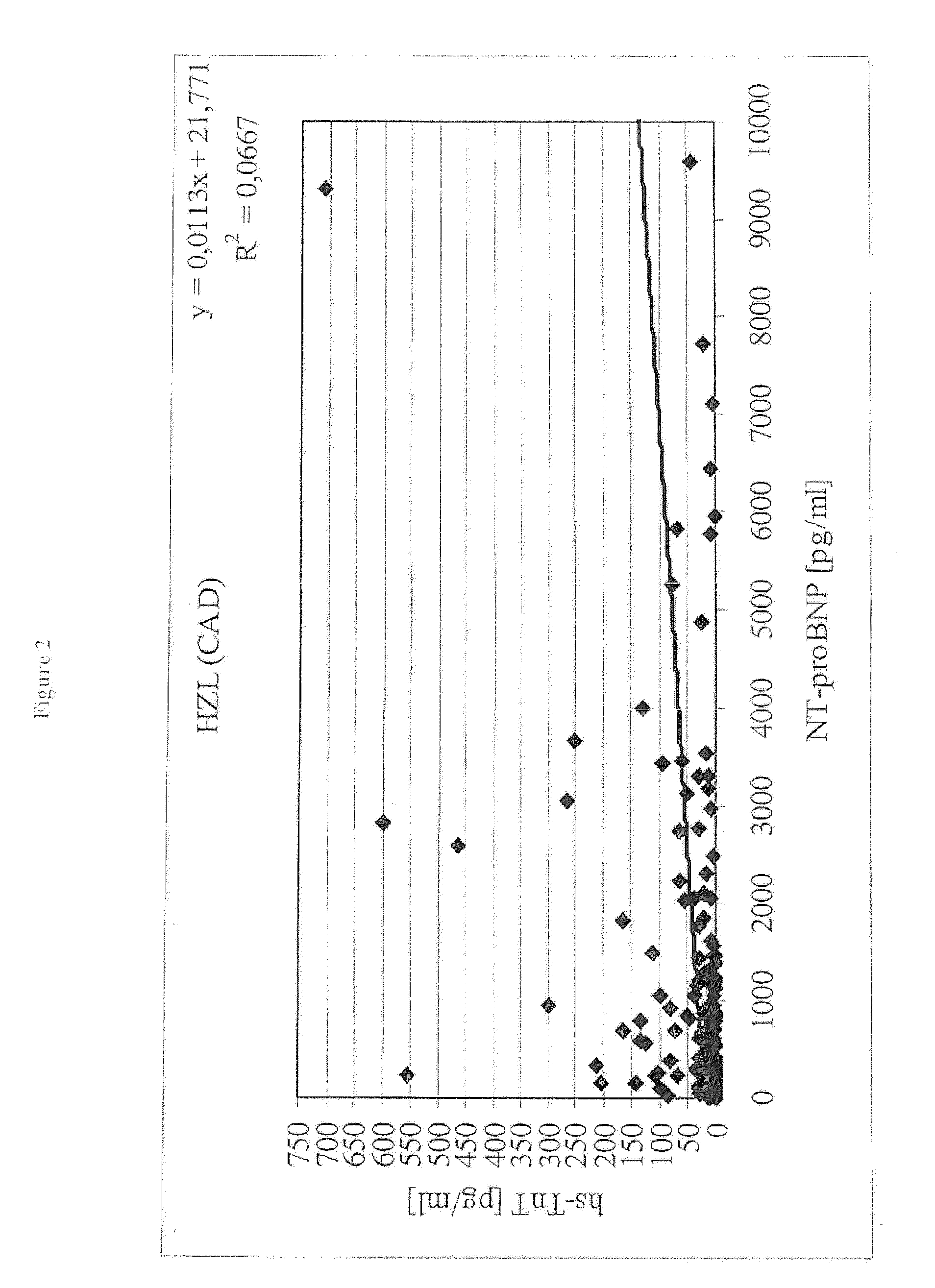
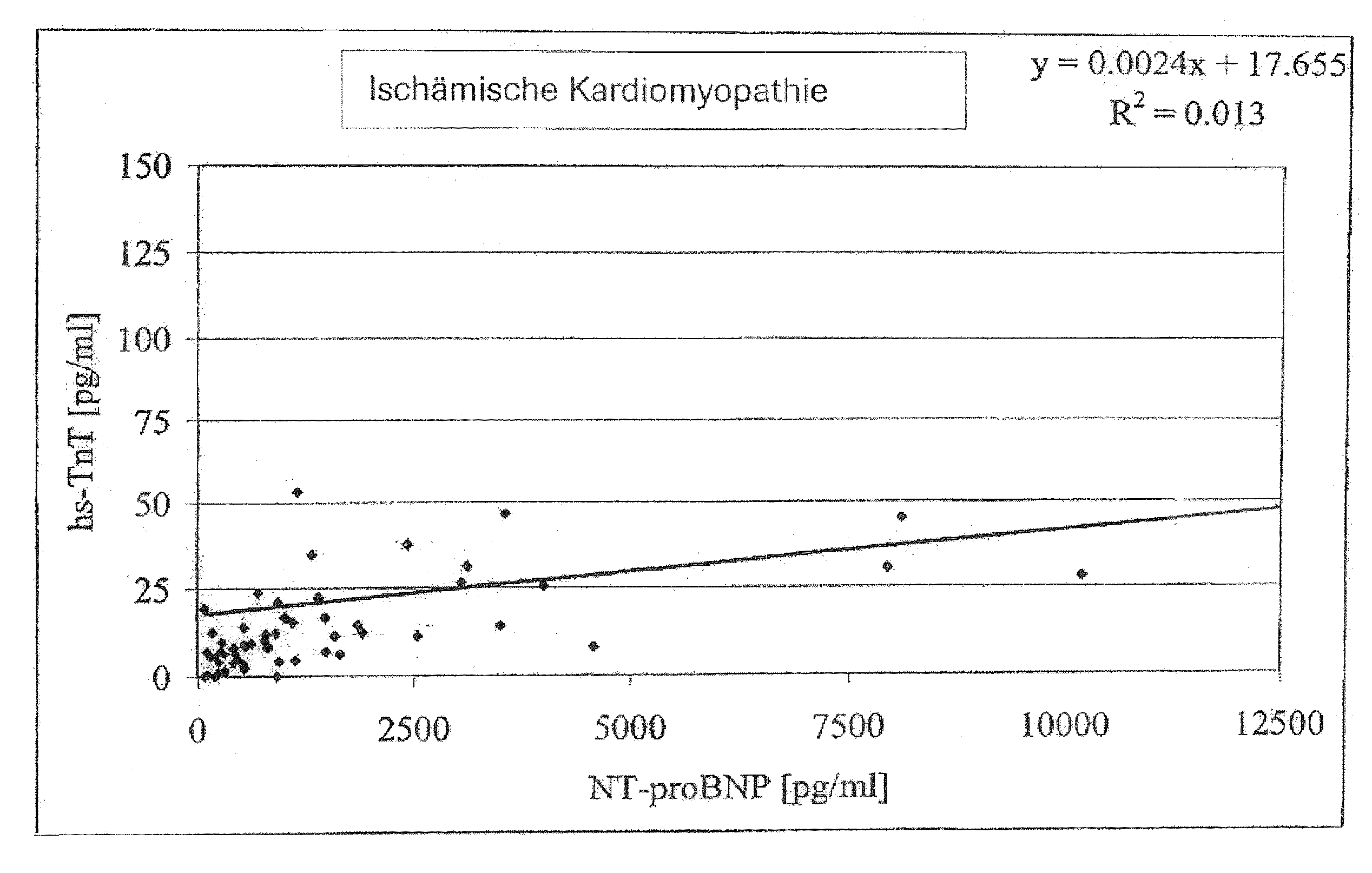
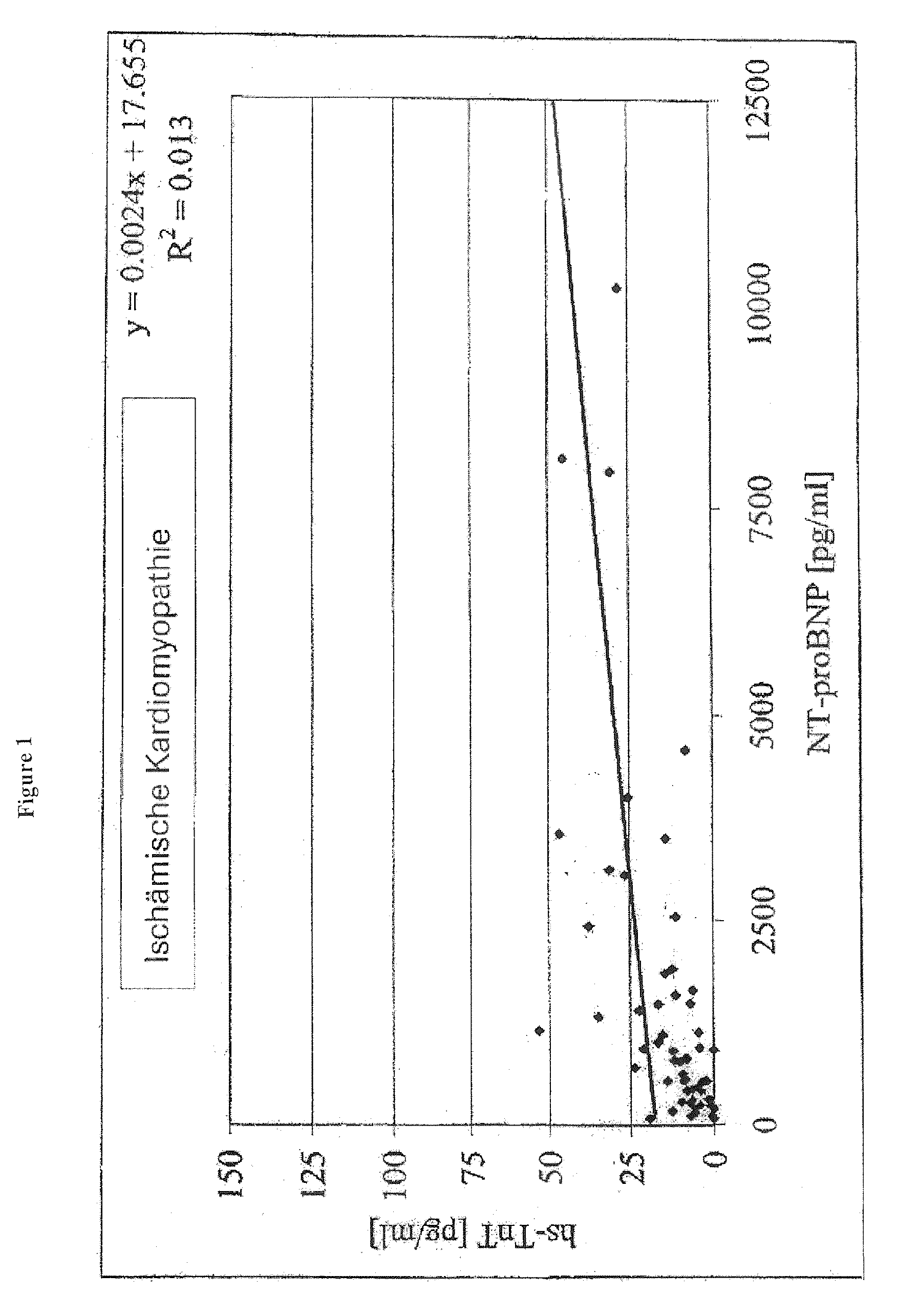
Diagnostic means and methods using troponin t and nt-probnp
ActiveUS20100075429A1Analysis using chemical indicatorsDisease diagnosisManagement of heart failureCoronary heart disease
The present invention relates to diagnostic means and methods. Specifically, the present invention encompasses a method of diagnosing the cause of cardiac necrosis in a subject comprising determining the amount of a cardiac troponin and the amount of a BNP-type peptide in a sample from a subject suffering from cardiac necrosis and comparing the amount of the cardiac troponin and the amount of the BNP-type peptide to reference amounts, whereby the cause of the cardiac necrosis is to be diagnosed. The present invention further relates to a method of determining whether a subject suffering from cardiac necrosis is susceptible for a therapy against initial heart failure and to a method for determining whether a subject suffering from cardiac necrosis is susceptible for a therapy against coronary heart disease. Also encompassed are diagnostic uses, devices, and kits.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
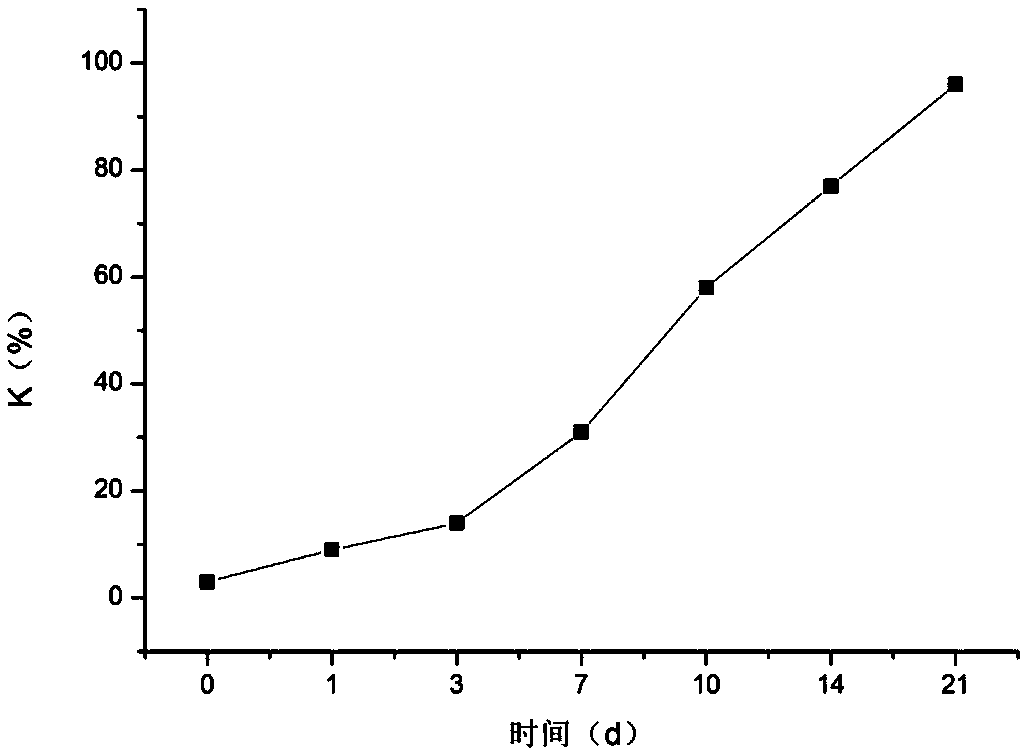
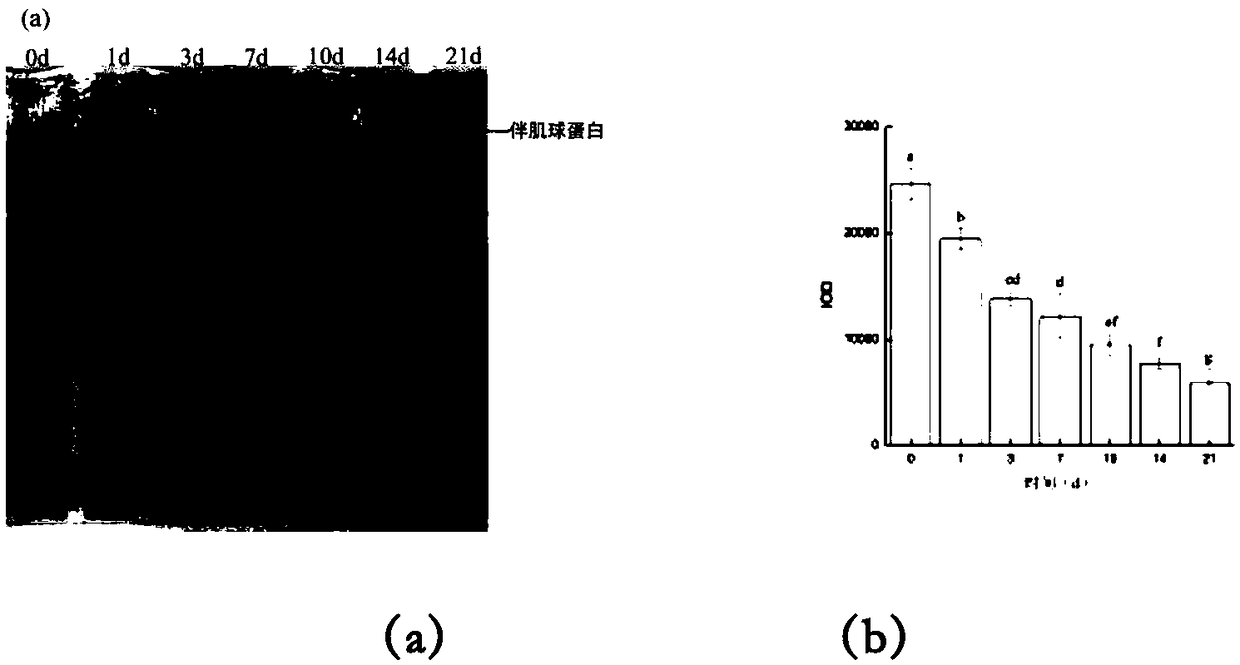
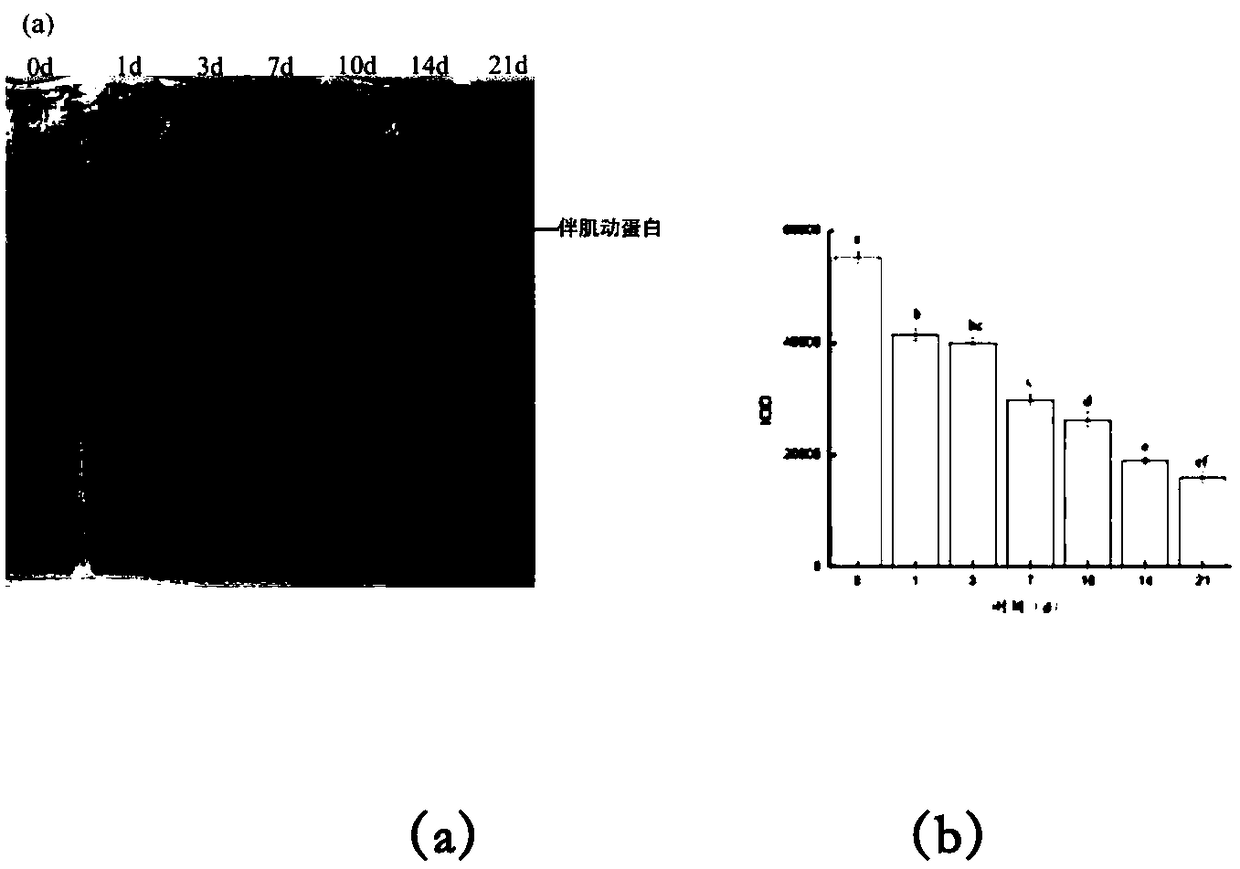
Method for evaluating fish meat freshness based on skeleton protein degradation
PendingCN109142495AAvoid damageSmall amount of sampleComponent separationMaterial analysis by electric/magnetic meansProtein DegradationsEcological environment
The invention discloses a method for evaluating the fish meat freshness based on skeleton protein degradation. The method comprises the steps that polyacrylamide gel electrophoresis is adopted for determining light density values IOD of skeleton proteins of fish meat samples under different storage days; the efficient liquid chromatography is adopted for determining triphosadenine degradation product concentrations of the fish meat samples under different storage days, and a freshness index K value is calculated; the light density values IOD and the K value are combined, a least square supportvector machine is utilized, and a prediction model for the fish meat freshness index K value is built. By adopting combination of titin, nebula and TnT representation skeleton protein as the fish meat freshness evaluation index, the sampling amount is small, and damage to detection samples is small; no other chemical index needs to be combined, and damage to ecological environment is small; the detection steps are simple, the detection time is saved, and online real-time monitoring can be achieved; the detection level reaches the molecular level, and the accuracy is high.
Owner:JIANGNAN UNIV
Single-chain polypeptides comprising troponin I and troponin C
InactiveUS7078486B2Confers conformational stabilityConfers immunostabilityBacteriaFermentationAntigenTroponin measurement
This invention relates to single-chain polypeptides and their genetic sequences comprising human cardiac troponin I and troponin C. The single-chain polypeptide may be expressed recombinantly, and a linker peptide may be interposed between the troponin sequences. A linker peptide of about 6 to about 30 amino acids is preferred. The single-chain polypeptide has utility as a control or calibrator for troponin assays, for the purification of troponin subunits and as an antigen for the preparation of antibodies.
Owner:SPECTRAL DIAGNOSTICS
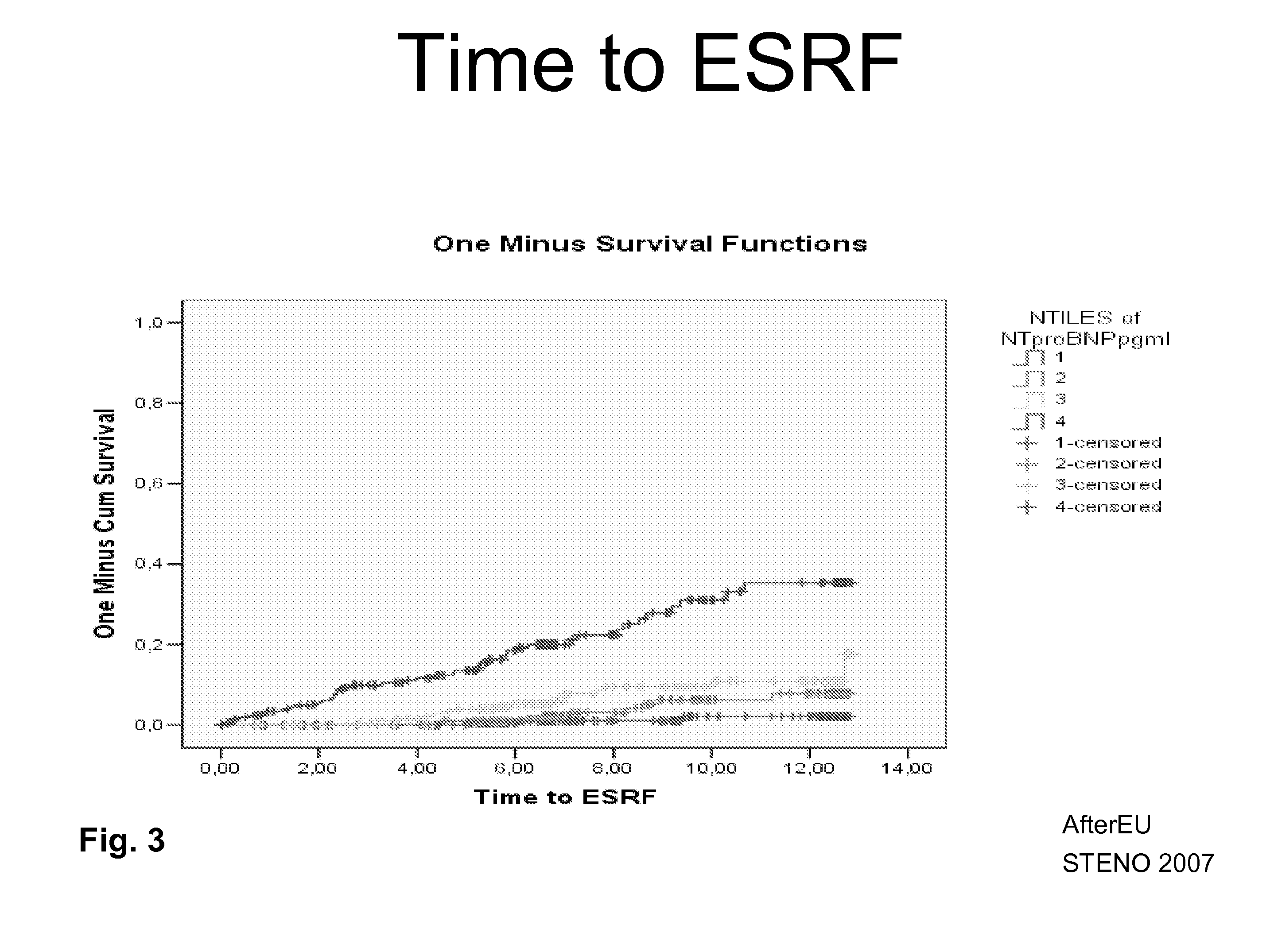
GDF-15 and/or Troponin T for Predicting Kidney Failure in Heart Surgery Patients
The present disclosure relates to the field of laboratory diagnostics. Specifically, means and methods are disclosed for determining a patient's risk of suffering from acute kidney injury after a surgical procedure based on the detection of GDF-15, troponin T and / or a natriuretic peptide.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Diagnostic means and methods using troponin T and NT-proBNP
ActiveUS8361800B2Disease diagnosisBiological testingManagement of heart failureCoronary heart disease
The present invention relates to diagnostic means and methods. Specifically, the present invention encompasses a method of diagnosing the cause of cardiac necrosis in a subject comprising determining the amount of a cardiac troponin and the amount of a BNP-type peptide in a sample from a subject suffering from cardiac necrosis and comparing the amount of the cardiac troponin and the amount of the BNP-type peptide to reference amounts, whereby the cause of the cardiac necrosis is to be diagnosed. The present invention further relates to a method of determining whether a subject suffering from cardiac necrosis is susceptible for a therapy against initial heart failure and to a method for determining whether a subject suffering from cardiac necrosis is susceptible for a therapy against coronary heart disease. Also encompassed are diagnostic uses, devices, and kits.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Homogeneous fluorescence immunoassay reagent for rapid and quantitative detection of troponin T and preparation and detection methods thereof
InactiveCN106645744ASensitive and goodImprove featuresDisease diagnosisBiological testingRare-earth elementFluorescence
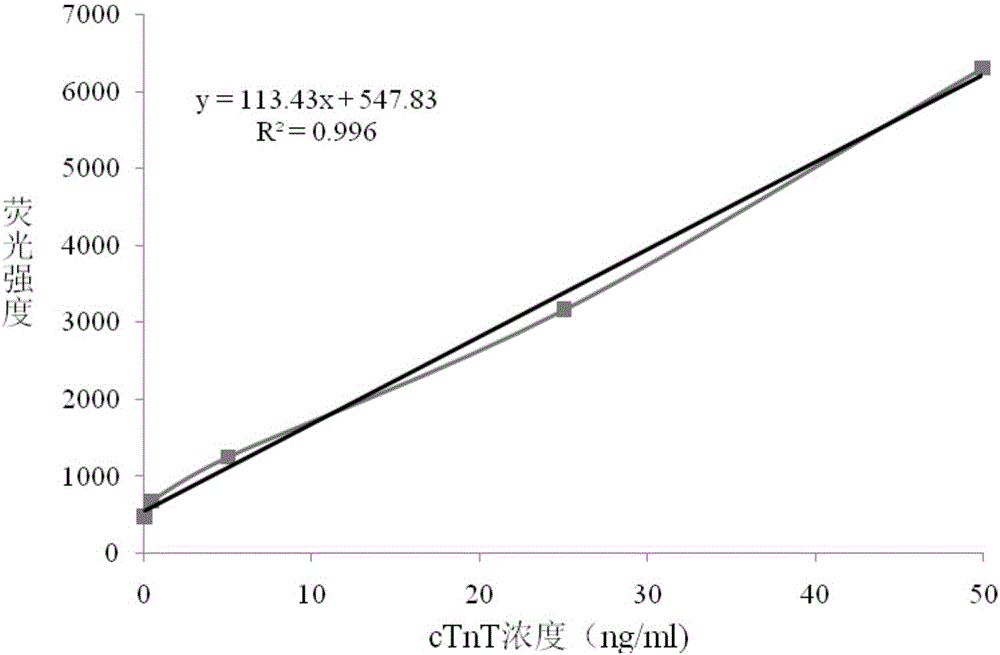
The invention discloses a homogeneous fluorescence immunoassay reagent for rapid and quantitative detection of troponin T and preparation and detection methods thereof. The homogeneous fluorescence immunoassay reagent for rapid and quantitative detection of troponin T comprises anti-cTnT (cardiac troponin) labeled with rare earth element chelate, another anti-cTnT labeled with a near infrared fluorescent compound and cTnT calibrators with a series concentrations. With the adoption of a rapid homogeneous fluorescence detection technology as well as the high sensitivity characteristic of fluorescence, adverse effects on detection accuracy and repeatability due to the non-uniform characteristics of pores of a nitrocellulose membrane in colloidal gold or fluorescent cTnT dry immune test paper are also prevented, and the detection sensitivity and the linear range can be greatly improved. The reagent has the characteristics of being easy to operate, rapid, sensitive, good in specificity and the like when applied to detection of cTnT in human blood, serum or plasma, and has good clinical application prospect.
Owner:SHANDONG UNIV QILU HOSPITAL
Biomarker for cardiac disorders
InactiveUS20160299154A1Improve performanceHigh sensitivityImmunoglobulins against animals/humansDisease diagnosisDiseaseCardiac disorders
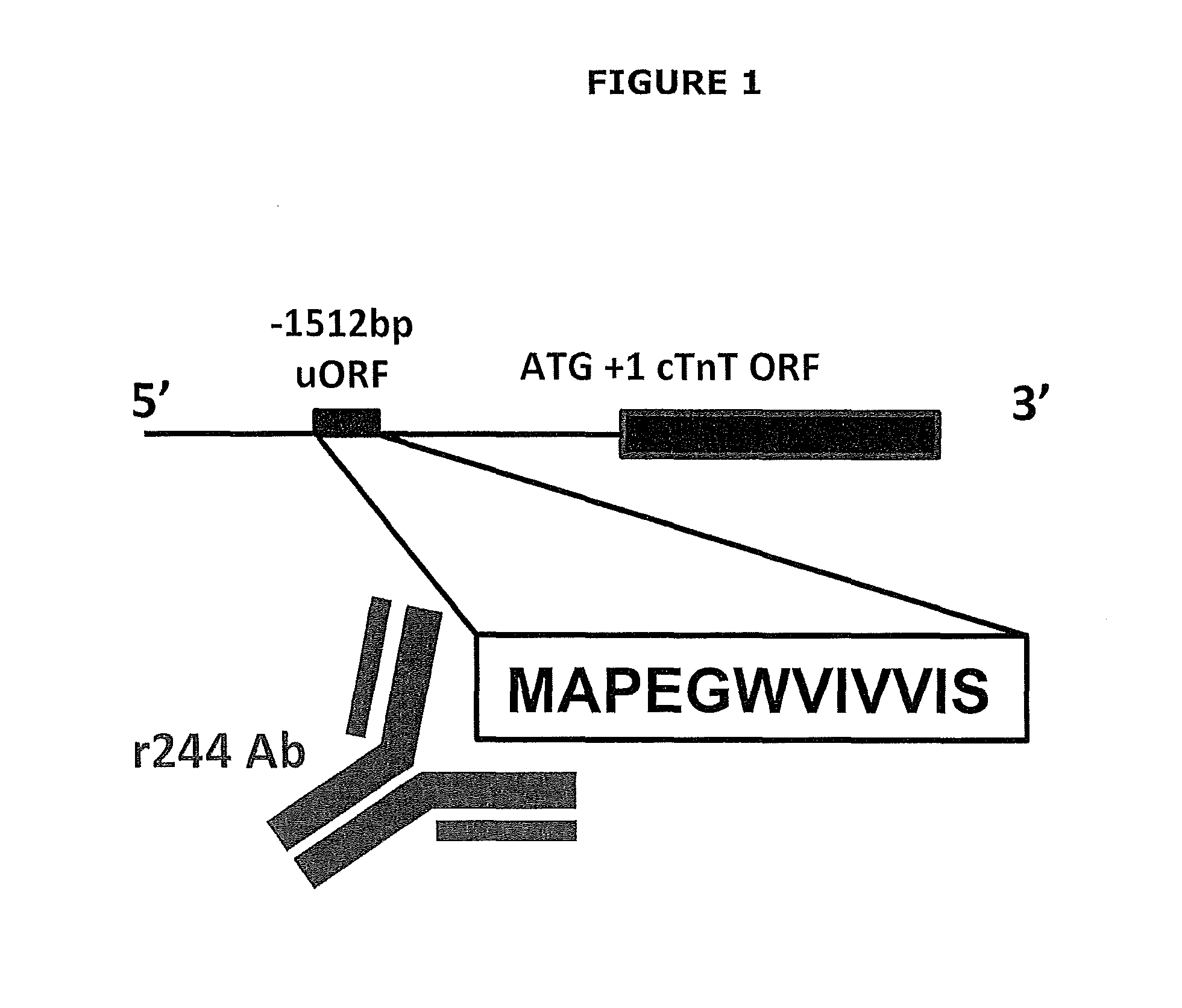
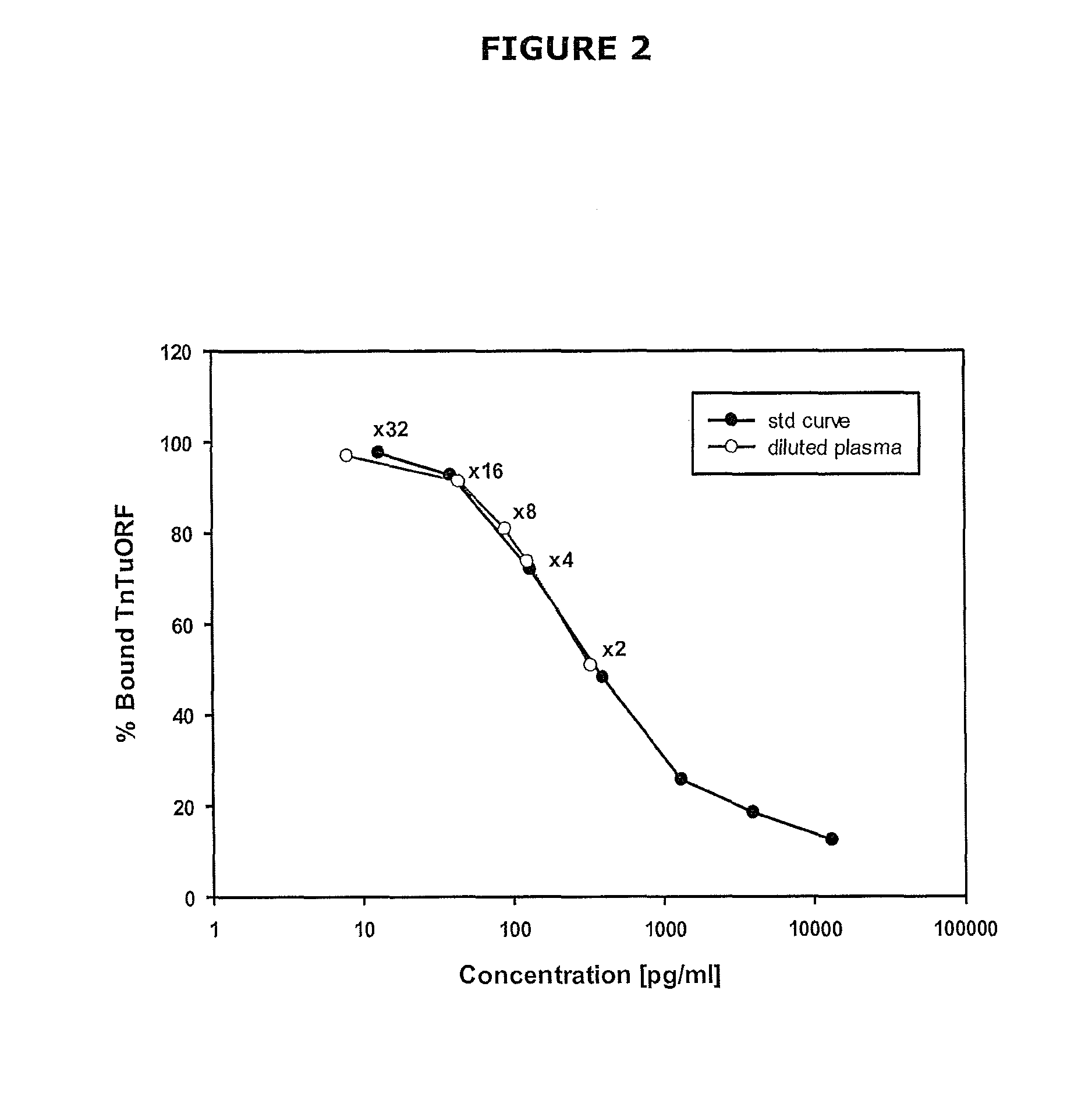
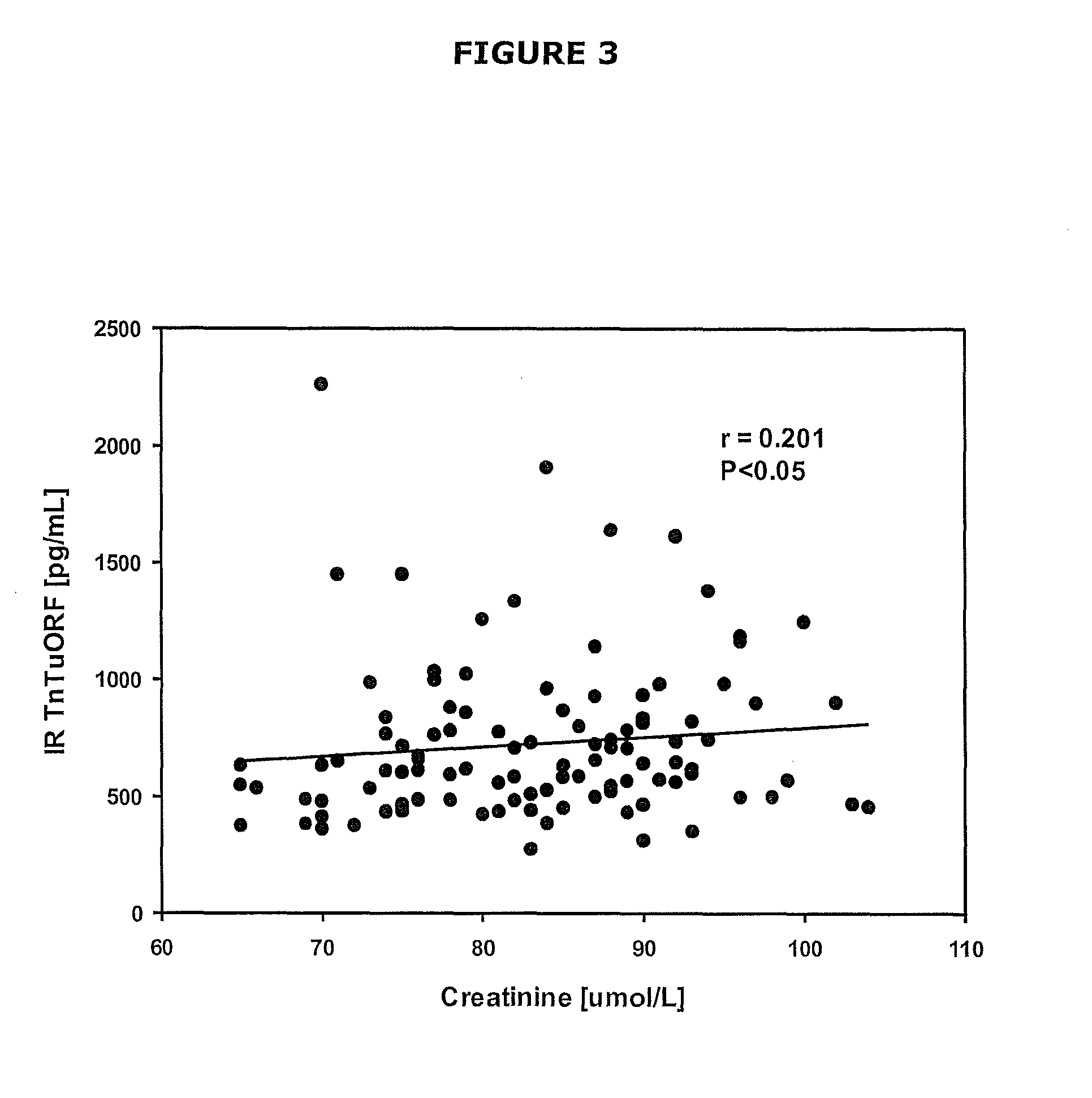
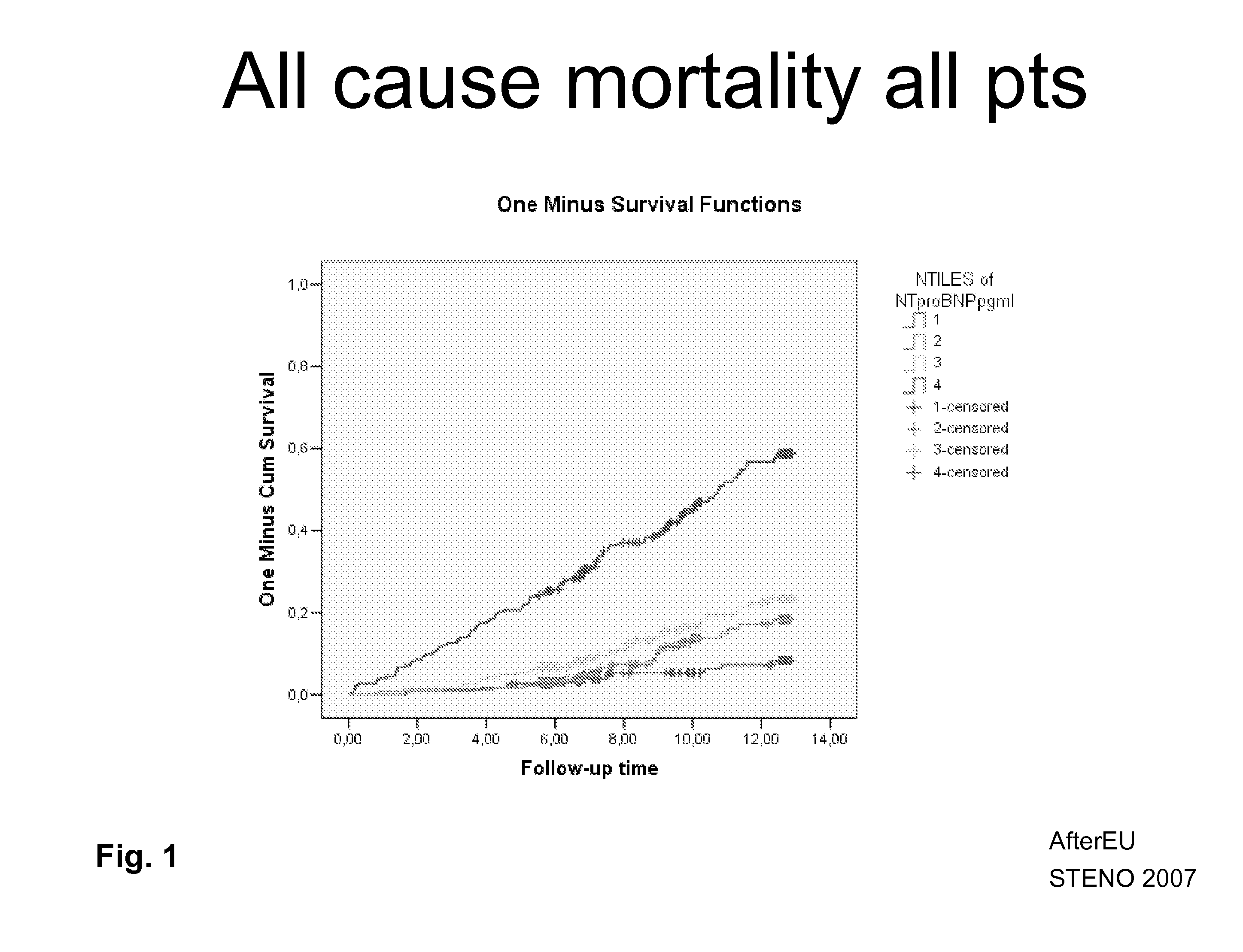
The present invention relates generally to assays, methods and kits for the prognosis and / or diagnosis of cardiac disorders. The present invention also provides, for example, binding agents to a novel class of circulating cardiac troponin T upstream open reading frame (TnTuORF) peptide biomarkers for use in predicting or diagnosing a cardiac disorder other than myocardial infarction or unstable angina. In addition, the binding agents of the present invention may be used to enhance the sensitivity and false positive performance of cardiac troponins in the prognosis and diagnosis of myocardial infarction.
Owner:UPSTREAM MEDICAL TECH LTD
Assessment of complications of patients with type 1 diabetes
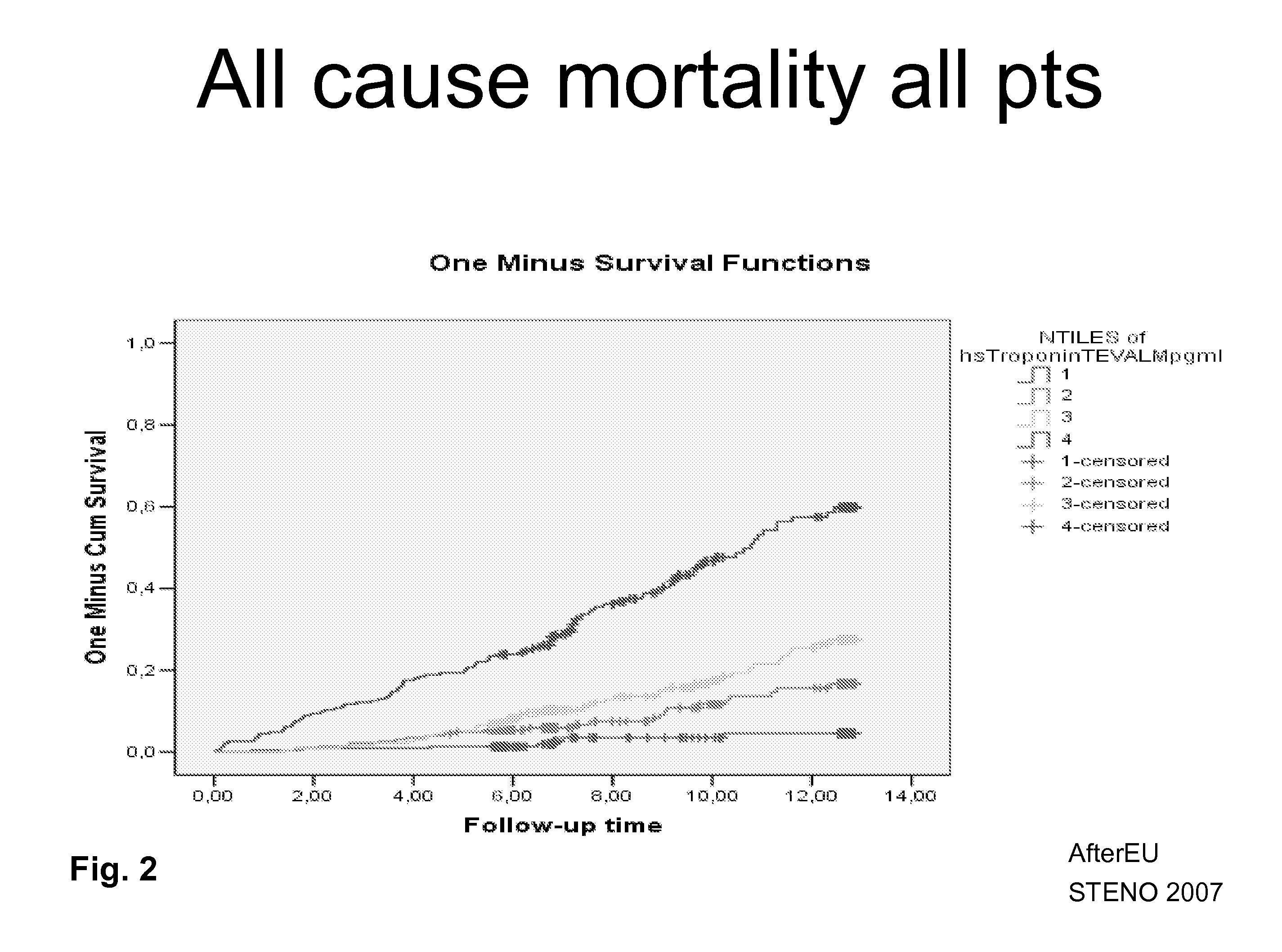
InactiveUS20110081725A1Analysis using chemical indicatorsComponent separationRenal FailuresKidney Failures
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Methods for improving the recovery of troponin I and T in membranes, filters and vessels
InactiveUS7723059B2Promote recoveryPeptide/protein ingredientsEnzymologyBiologyBiomedical engineering
A method to facilitate recovery troponin I and / or troponin T from a sample comprising addition of troponin C to the sample or to a surface from which the troponin I and / or troponin T are recovered.
Owner:BIOSITE INC
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422BHigh luminous intensityWide excitation spectrumBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
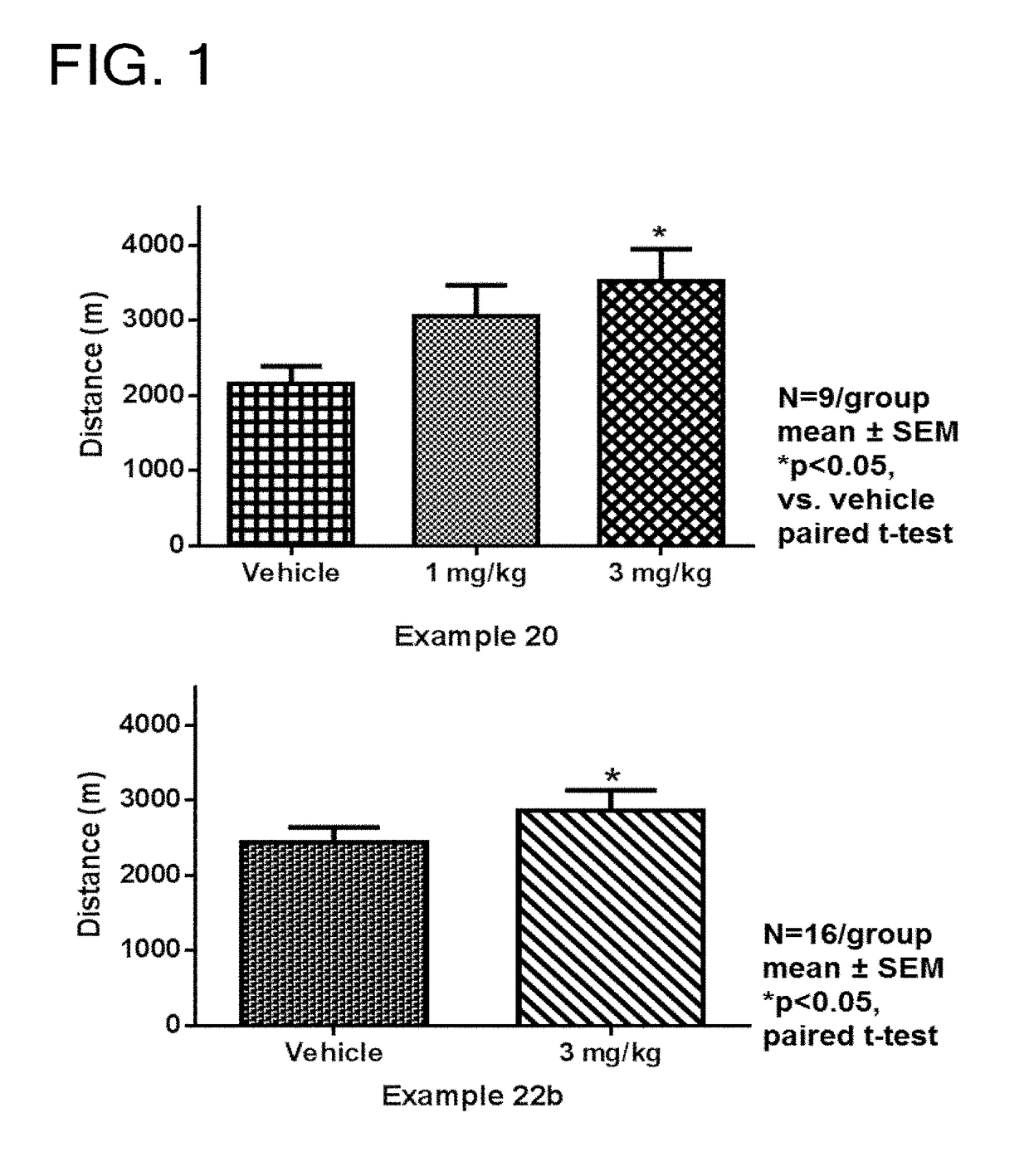
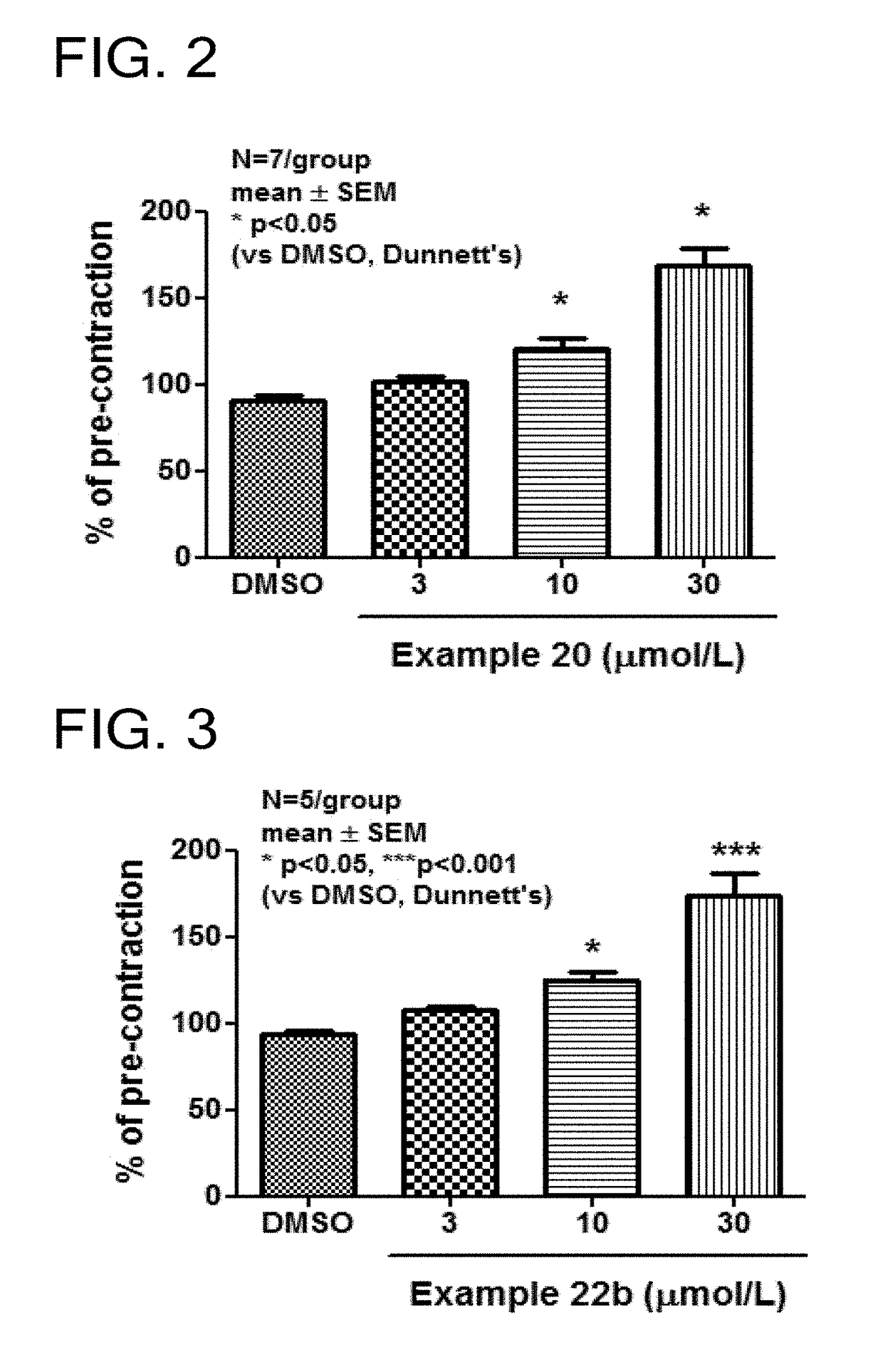
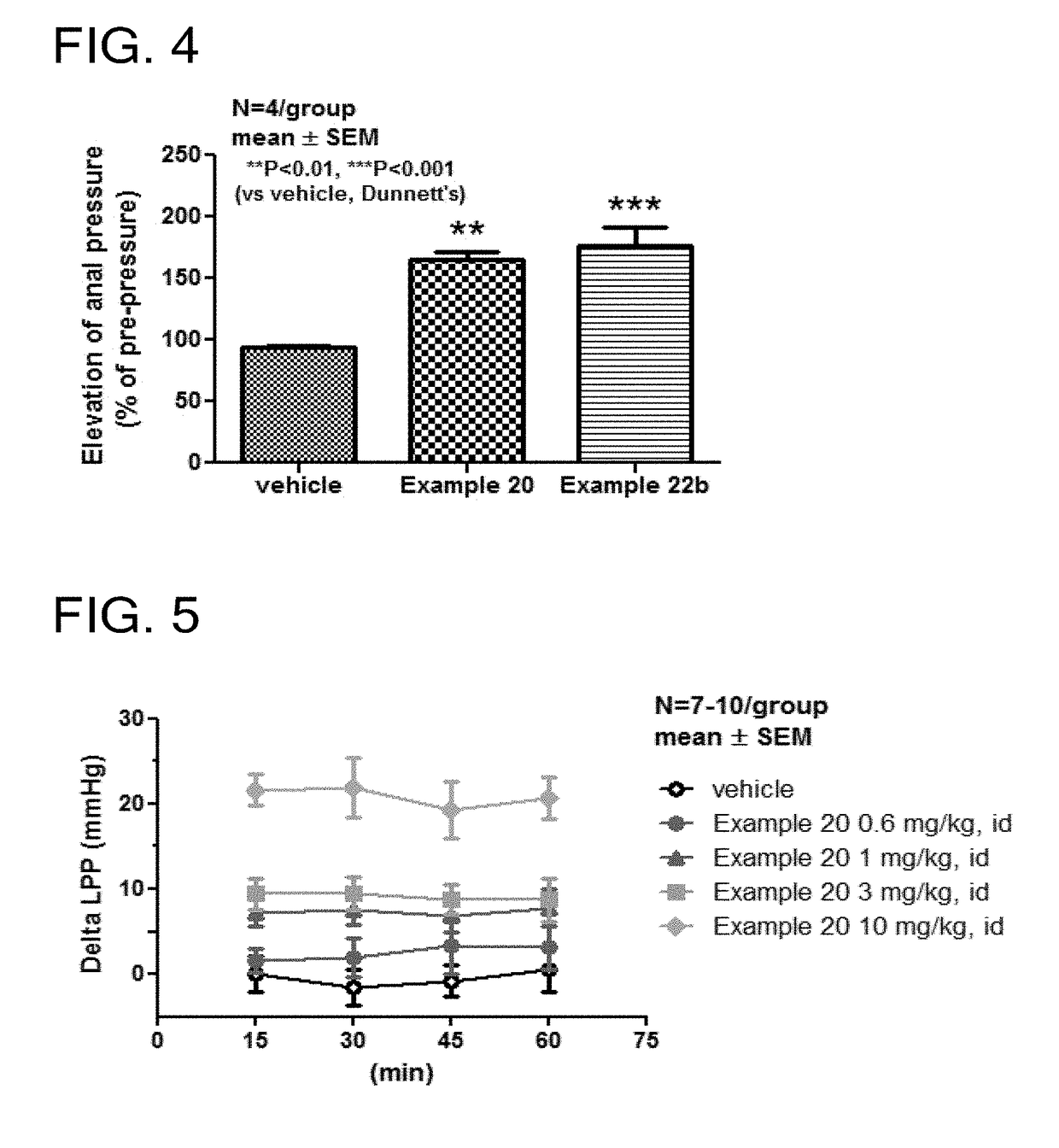
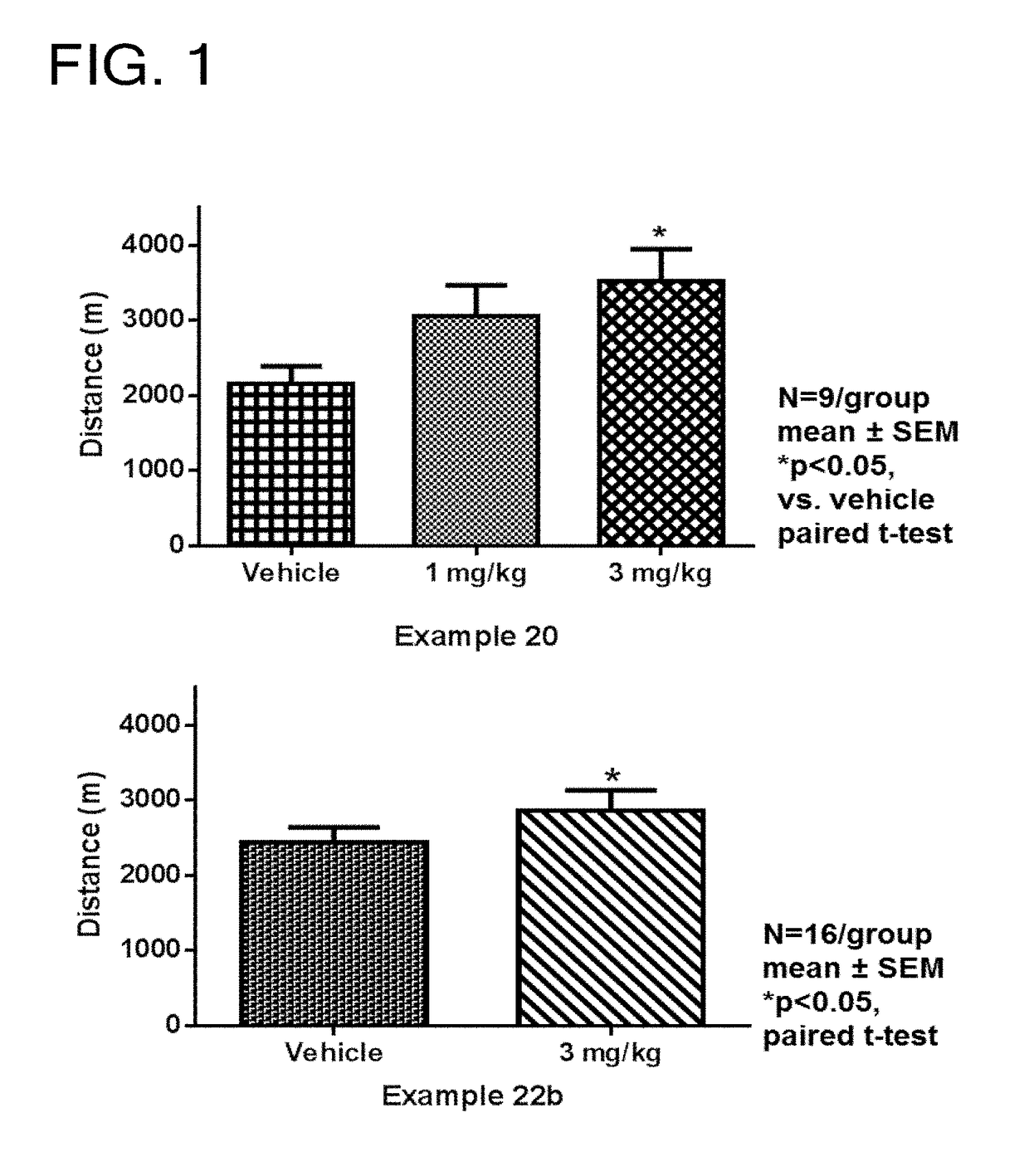
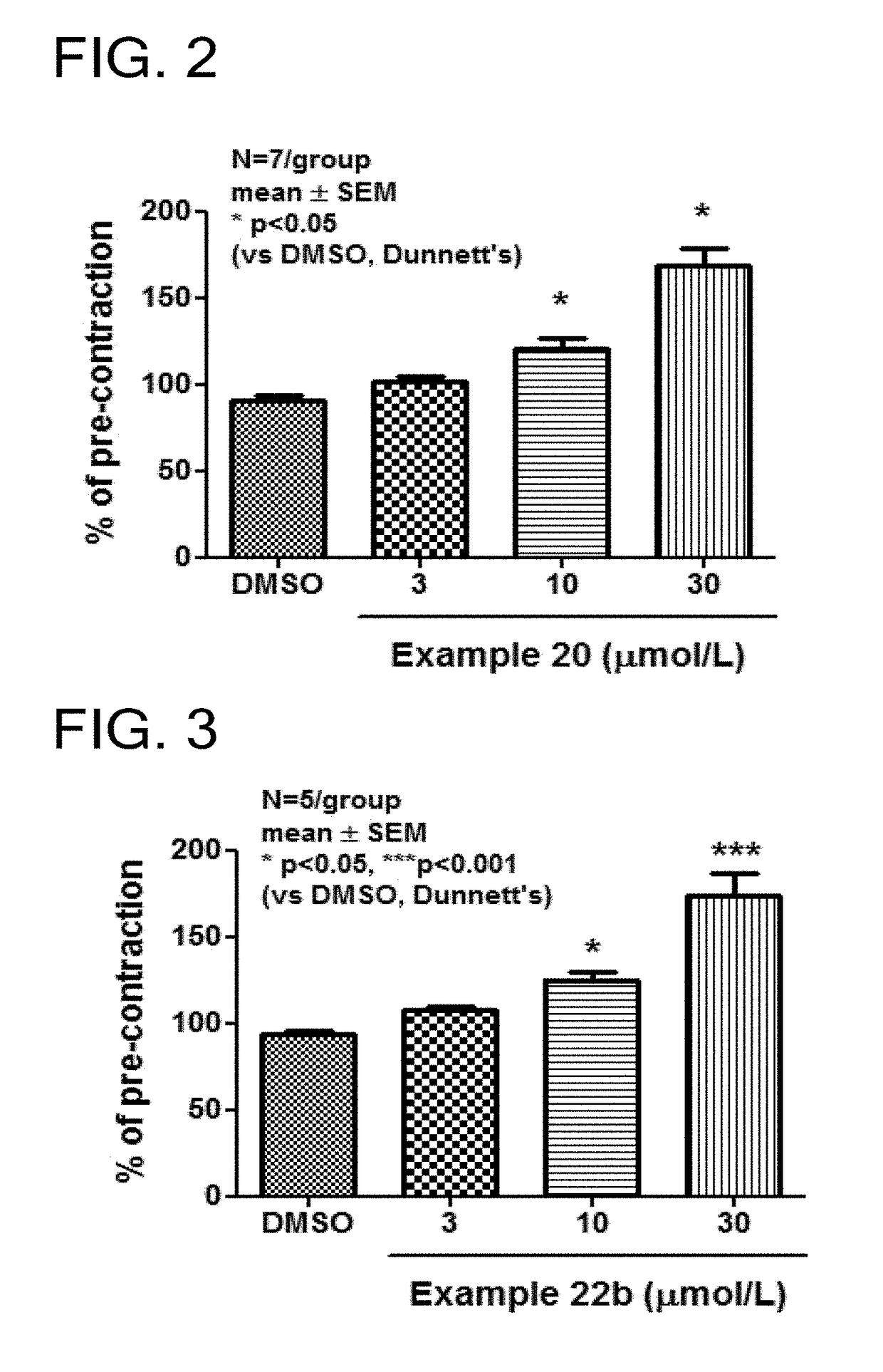
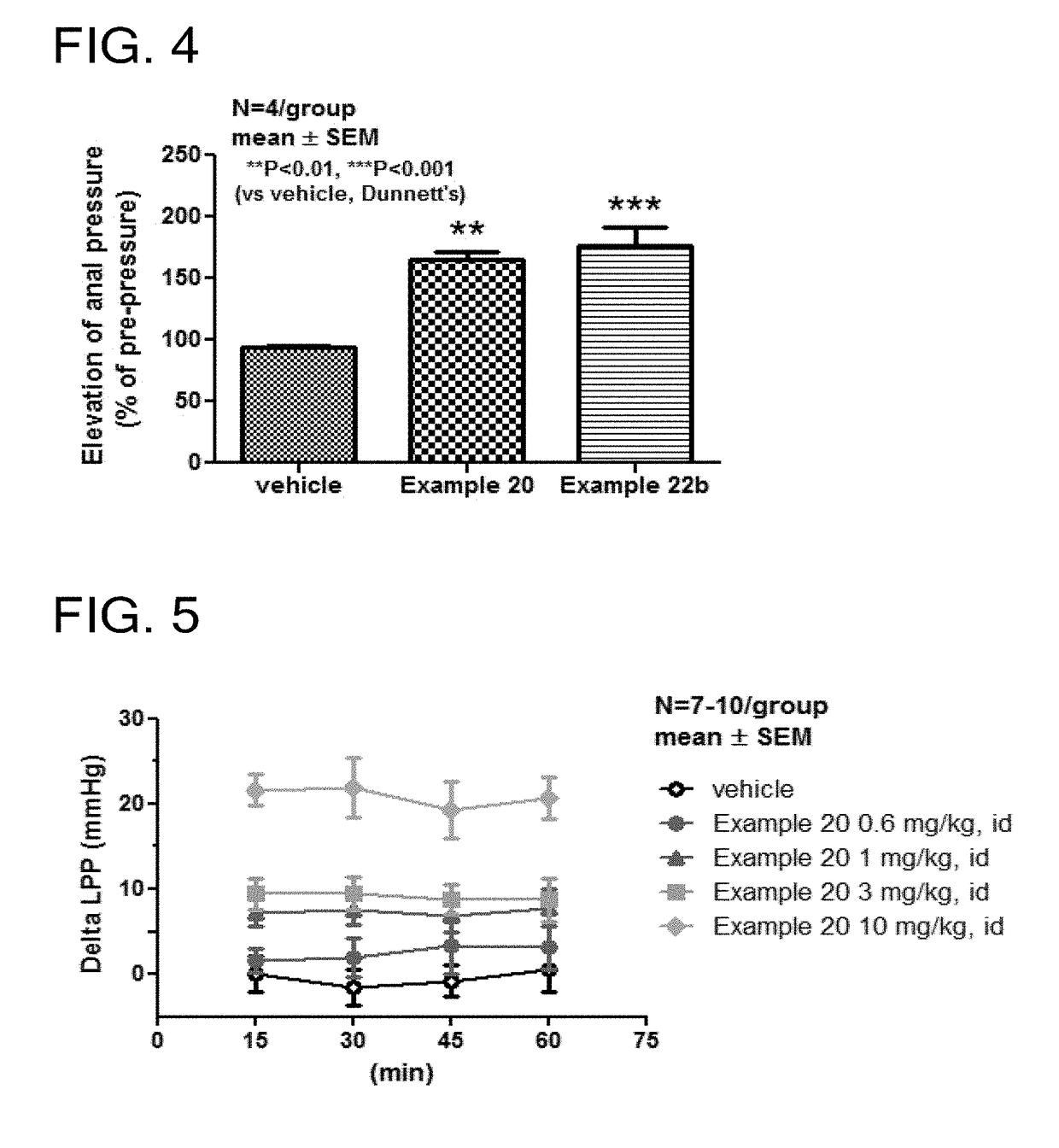
Tetrahydroisoquinoline derivatives
Novel tetrahydroisoquinoline derivative compounds are disclosed herein that may be used as an active ingredient for a pharmaceutical composition, and in particular, for a pharmaceutical composition useful for preventing or treating a disease or condition responsive to modulation of the contractility of the skeletal sarcomere. This may be accomplished, for example, by modulation of the troponin complex of the fast skeletal muscle sarcomere through one or more of fast skeletal myosin, actin, tropomyosin, troponin C, troponin I, and troponin T, and fragments and isoforms thereof. The tetrahydroisoquinoline derivative compounds can thus be used as an agent for preventing or treating 1) neuromuscular disorders, 2) disorders of voluntary muscle, 3) CNS disorders in which muscle weakness, atrophy, and fatigue are prominent symptoms, 4) muscle symptoms stemming from systemic disorders, and 5) dysfunctions of pelvic floor and urethral / anal sphincter muscle.
Owner:CYTOKINETICS INC
Tetrahydroisoquinoline derivatives
Novel tetrahydroisoquinoline derivative compounds are disclosed herein that may be used as an active ingredient for a pharmaceutical composition, and in particular, for a pharmaceutical composition useful for preventing or treating a disease or condition responsive to modulation of the contractility of the skeletal sarcomere. This may be accomplished, for example, by modulation of the troponin complex of the fast skeletal muscle sarcomere through one or more of fast skeletal myosin, actin, tropomyosin, troponin C, troponin I, and troponin T, and fragments and isoforms thereof. The tetrahydroisoquinoline derivative compounds can thus be used as an agent for preventing or treating 1) neuromuscular disorders, 2) disorders of voluntary muscle, 3) CNS disorders in which muscle weakness, atrophy, and fatigue are prominent symptoms, 4) muscle symptoms stemming from systemic disorders, and 5) dysfunctions of pelvic floor and urethral / anal sphincter muscle.
Owner:CYTOKINETICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com