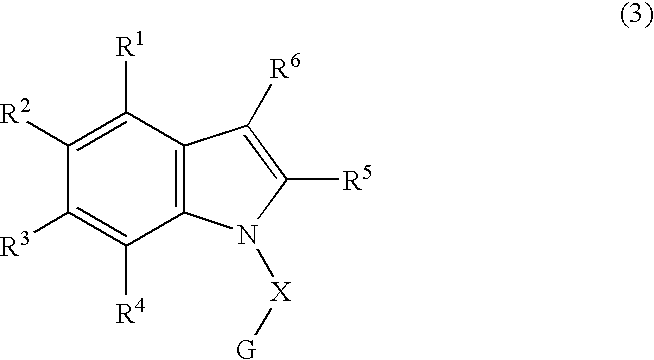
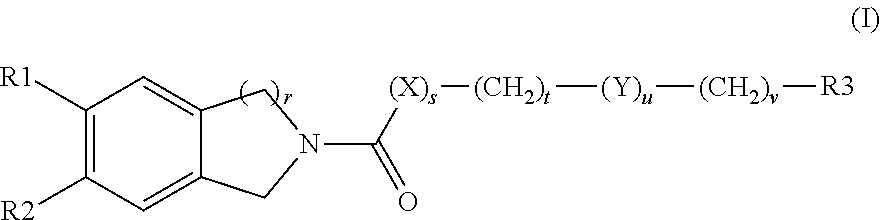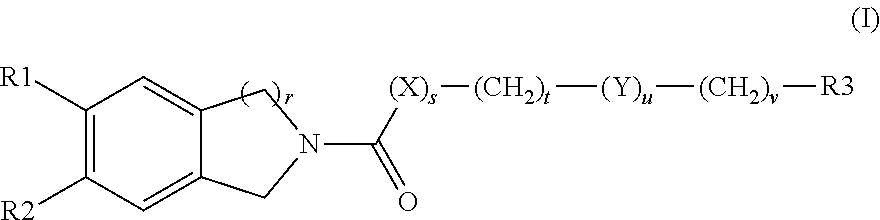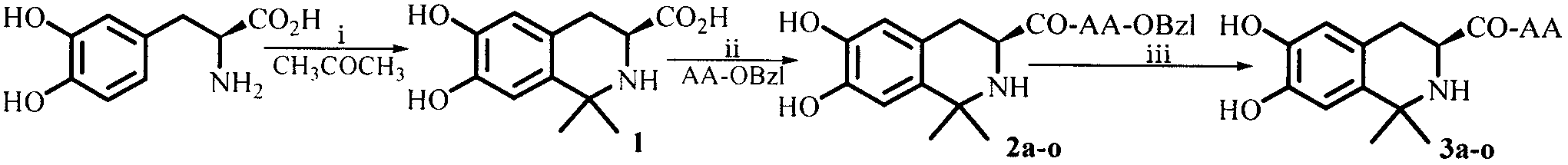Patents
Literature
524 results about "Tetrahydroisoquinoline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tetrahydroisoquinoline (TIQ or THIQ) is an organic compound with the chemical formula C₉H₁₁N. Classified as a secondary amine, it is derived from isoquinoline by hydrogenation. It is a colorless viscous liquid that is miscible with most organic solvents. The tetrahydroisoquinoline skeleton is encountered in a number of bioactive compounds and drugs.
Tetrahydroisoquinoline or isochroman compounds
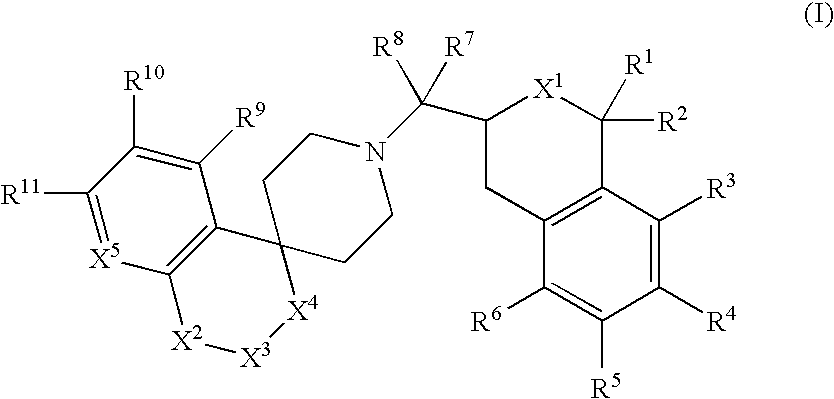
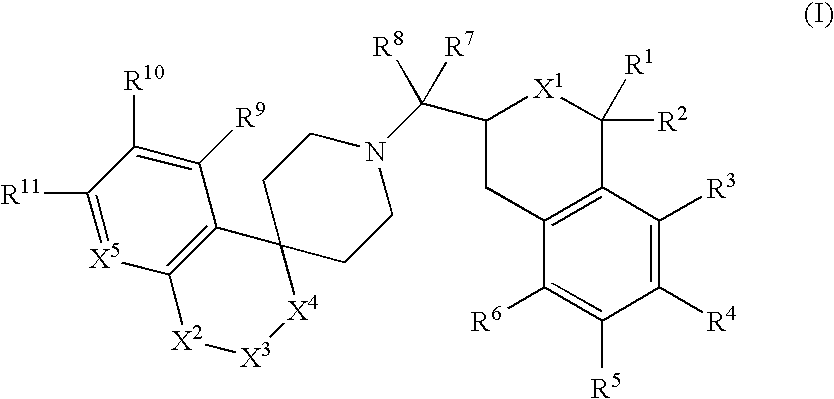
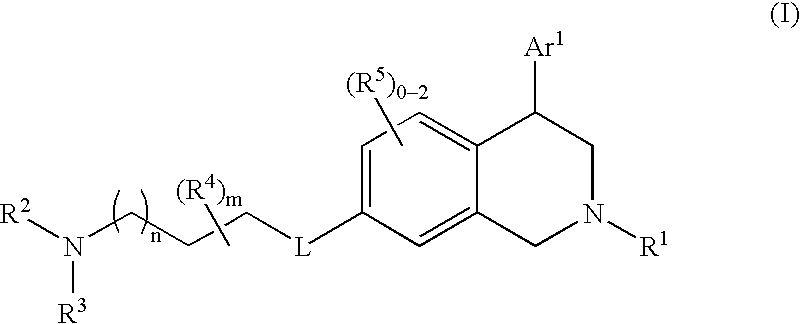
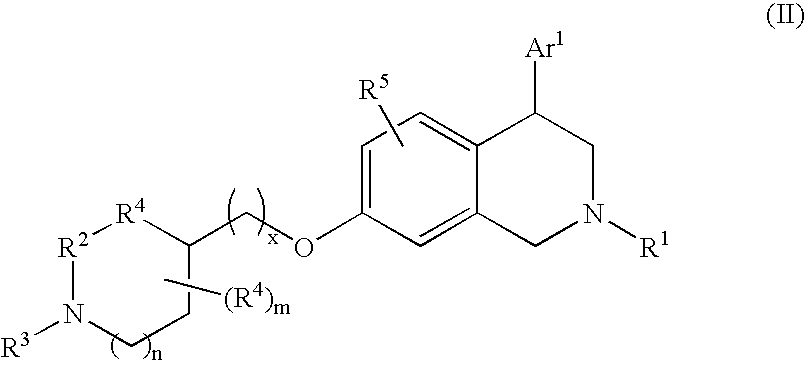
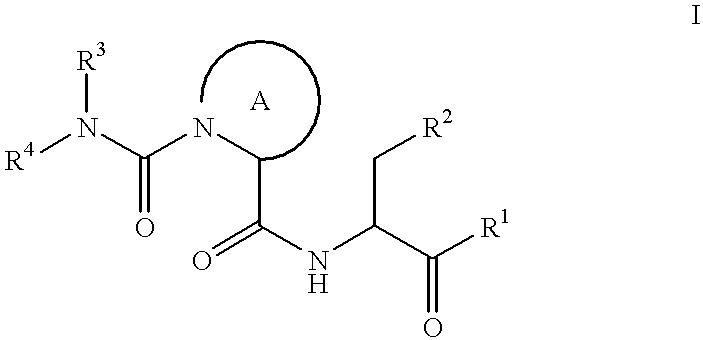
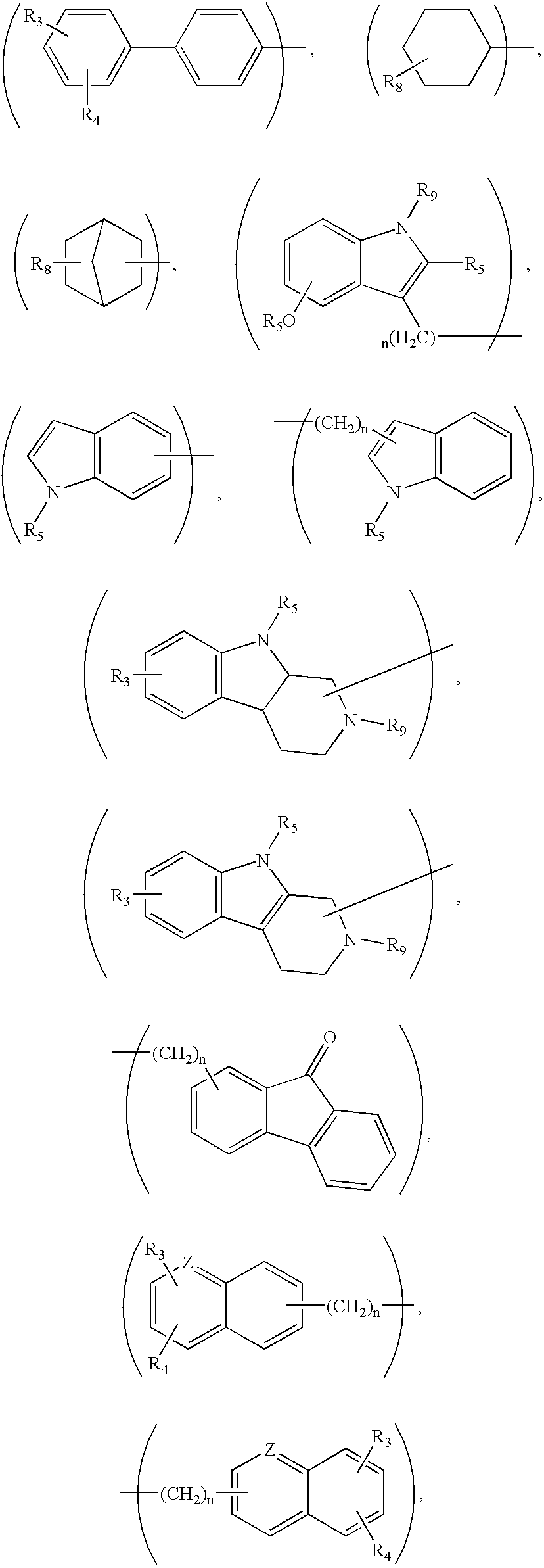
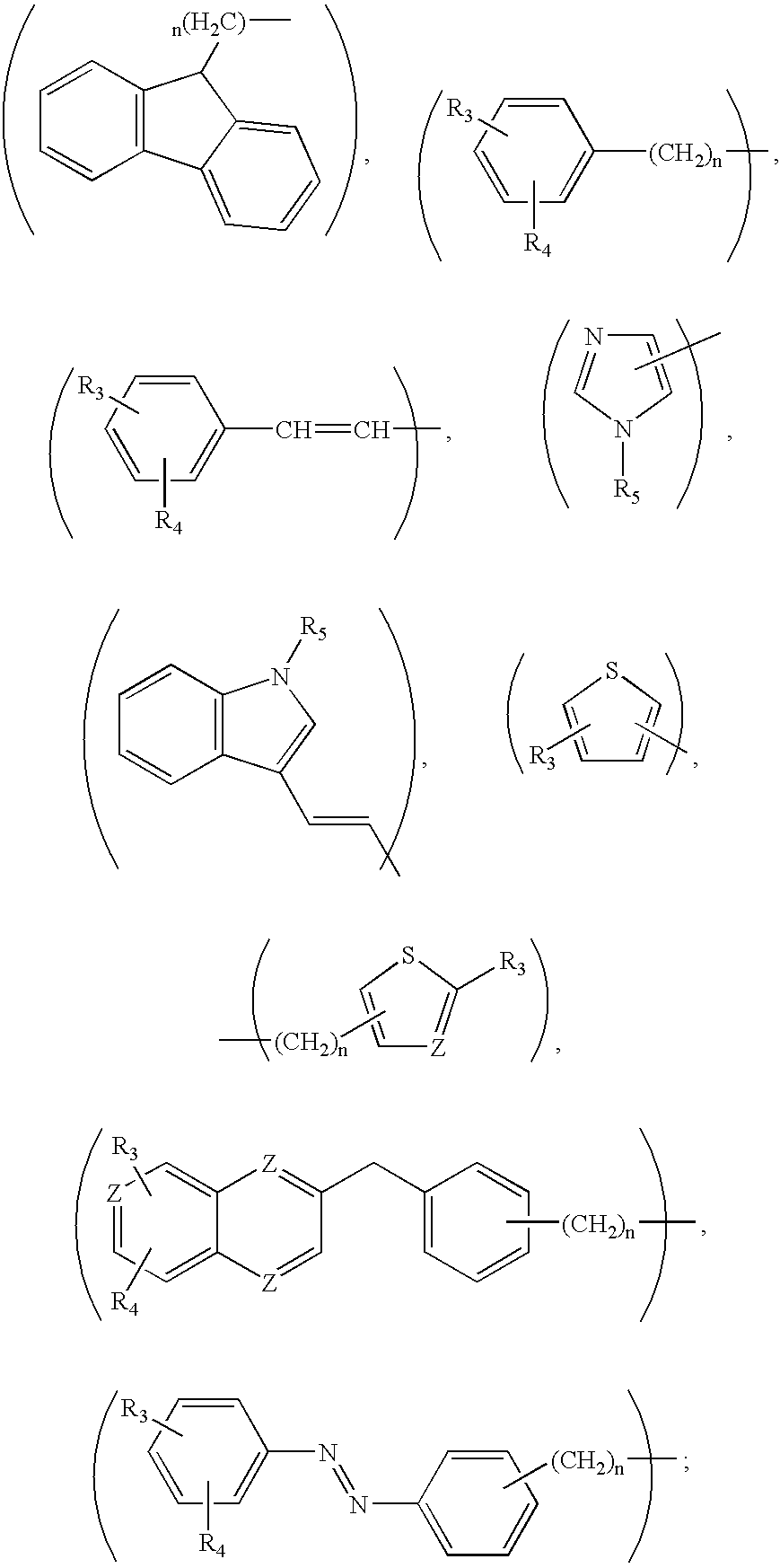
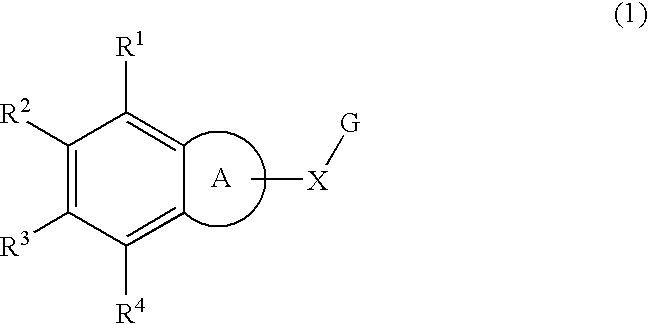
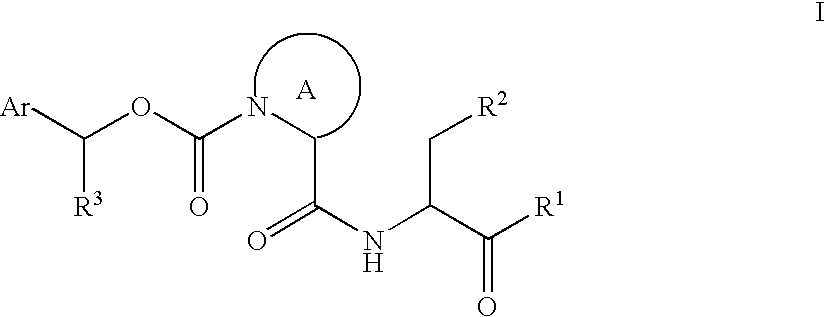
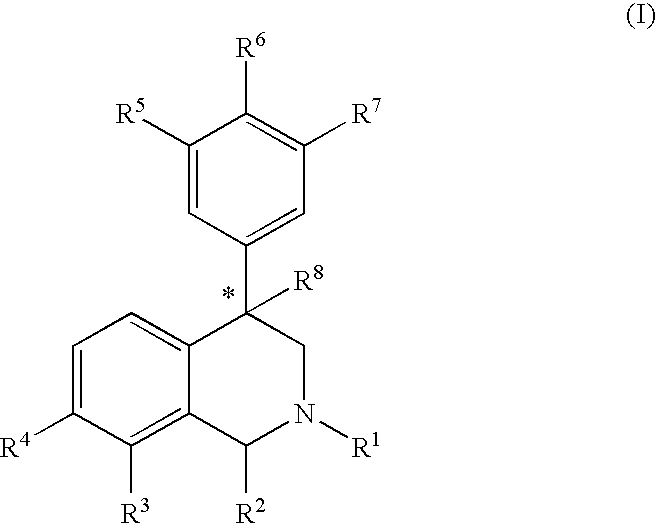
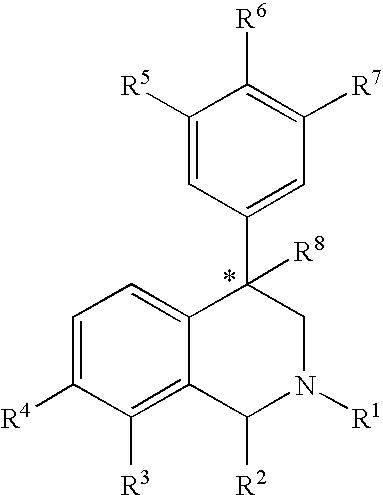
This invention provides the compounds of formula (I): or its a pharmaceutically acceptable ester or amide of such compound, or a pharmaceutically acceptable salt thereof, wherein X1 is NH; R1, R2, R4 through R6 and R7 through R11 are all hydrogen; R3 is hydroxy; X2 and X3 are methylene; X4 is a bond; and X5 is a carbon atom, and the like. These compounds have ORL1-receptor antagonist activity; and therefore, are useful to treat diseases or conditions such as pain, various CNS diseases etc.
Owner:ITO FUMITAKA +3
Isoquinoline compound melanocortin receptor ligands and methods of using same
The invention relates to melanocortin receptor ligands and methods of using the ligands to alter or regulate the activity of a melanocortin receptor. The invention further relates to tetrahydroisoquinoline aromatic amines that function as melanocortin receptor ligands and as agents for controlling cytokine-regulated physiologic processes and pathologies, and combinatorial libraries thereof.
Owner:LION BIOSCIENCE AG
Imine coupled covalent organic framework material and preparation method and application thereof
ActiveCN106967216AGood chemical stabilityImprove thermal stabilityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsOrganic reactionStructural formula
The invention provides an imine coupled covalent organic framework material and a preparation method and application thereof and belongs to the technical field of porous materials. The structural formula of the imine coupled covalent organic framework material is shown in the description, and the material can be prepared by triazinyl aromatic polyamine A and alkoxy substituted aromatic aldehyde B through simple Schiff base condensation reaction. Under visible light excitation condition, the prepared imine coupled covalent organic framework material can catalyze multiple types of organic reactions, specifically for example reaction for photocatalysis of N-aryl tetrahydroisoquinoline and nucleophilic reagent. The prepared covalent organic framework material has very good chemical stability and heat stability, high specific surface area and high crystallinity, has strong visible light absorption properties, can serve as an excellent heterogeneous photocatalyst and has good industrial application prospect.
Owner:JILIN UNIV
Synthetic method and application of covalent organic framework (COF) material
ActiveCN103755588AOrganic-compounds/hydrides/coordination-complexes catalystsHydrazide preparationMetal-organic frameworkNitromethane
The invention discloses a synthetic method of a covalent organic framework (COF) material. The method comprises the following steps: after mixing 1,3,5-benzenetricarboxaldehyde with 2,5-di(N,N-dimethyl)amino-1,4-benzdihydrazide uniformly in an organic solvent, reacting in the presence of a catalyst acetic acid to obtain the COF material, wherein the mole ratio of 1,3,5-benzenetricarboxaldehyde to 2,5-di(N,N-dimethyl)amino-1,4-benzdihydrazide is 1:(0.5-3). The COF material obtained by adopting the method has relatively large specific surface area and regular open framework structure with adjustable diameter, thus being beneficial for mass transfer of reactants and products in photoabsorption and catalytic processes; the material can serve as a photocatalyst and can increase the yield of the dehydrogenative coupling reaction between 2-phenyl-1,2,3,4-tetrahydroisoquinoline and nitromethane from 39% in the absence of catalysts to 89%.
Owner:LANZHOU UNIVERSITY
Tetrahydroisoquinoline compounds
Certain tetrahydroisoquinoline compounds are histamine H3 receptor and serotonin transporter modulators useful in the treatment of histamine H3 receptor- and serotonin-mediated diseases.
Owner:JANSSEN PHARMA NV
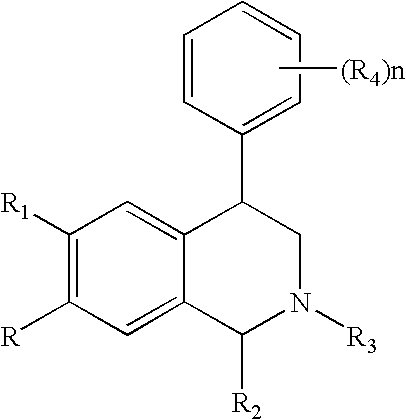
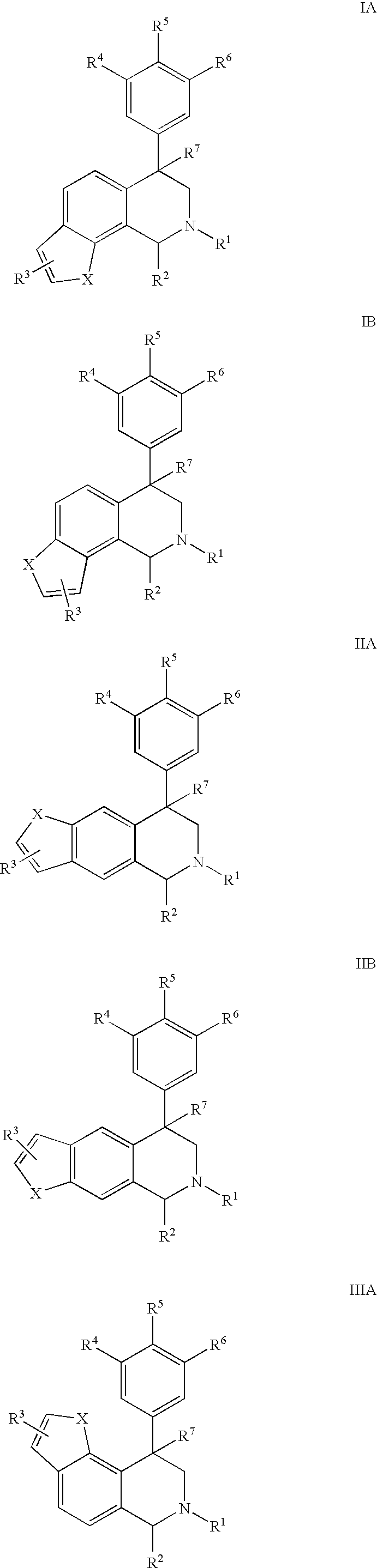
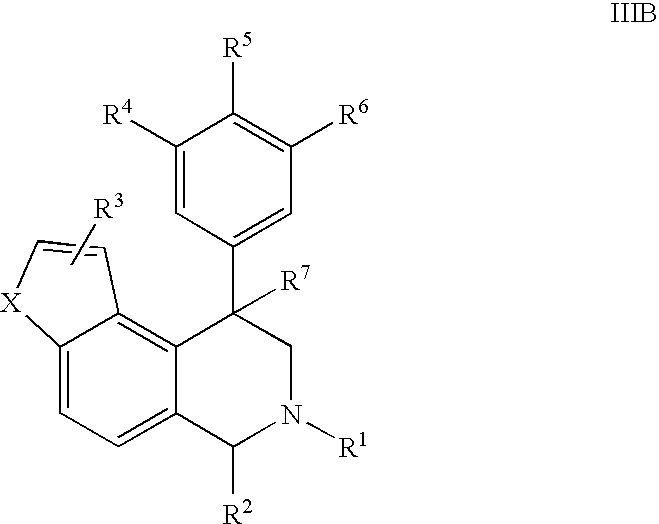
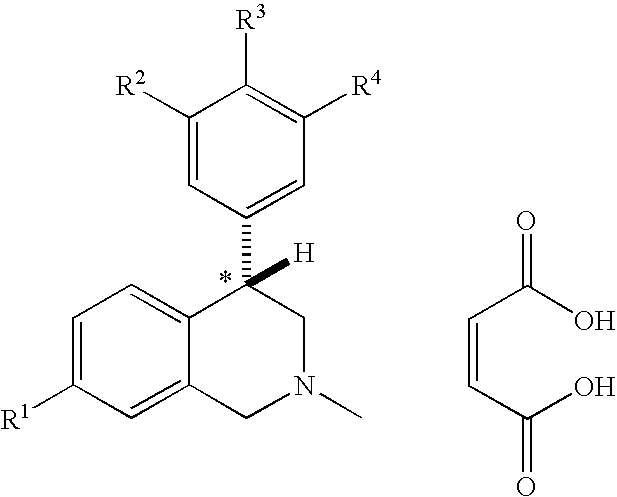
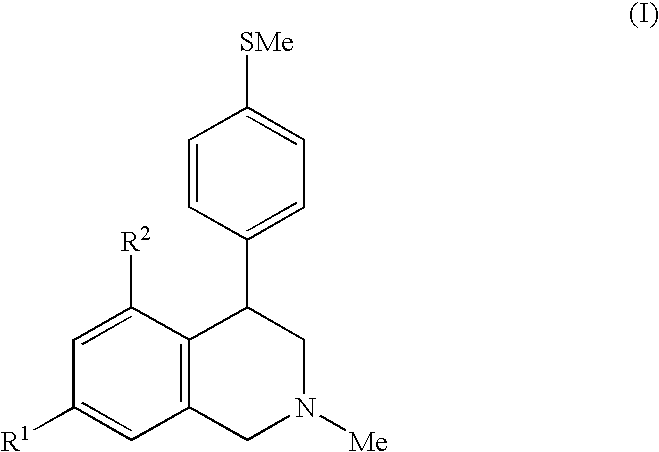
Novel 4-phenyl substituted tetrahydroisoquinolines and therapeutic use thereof
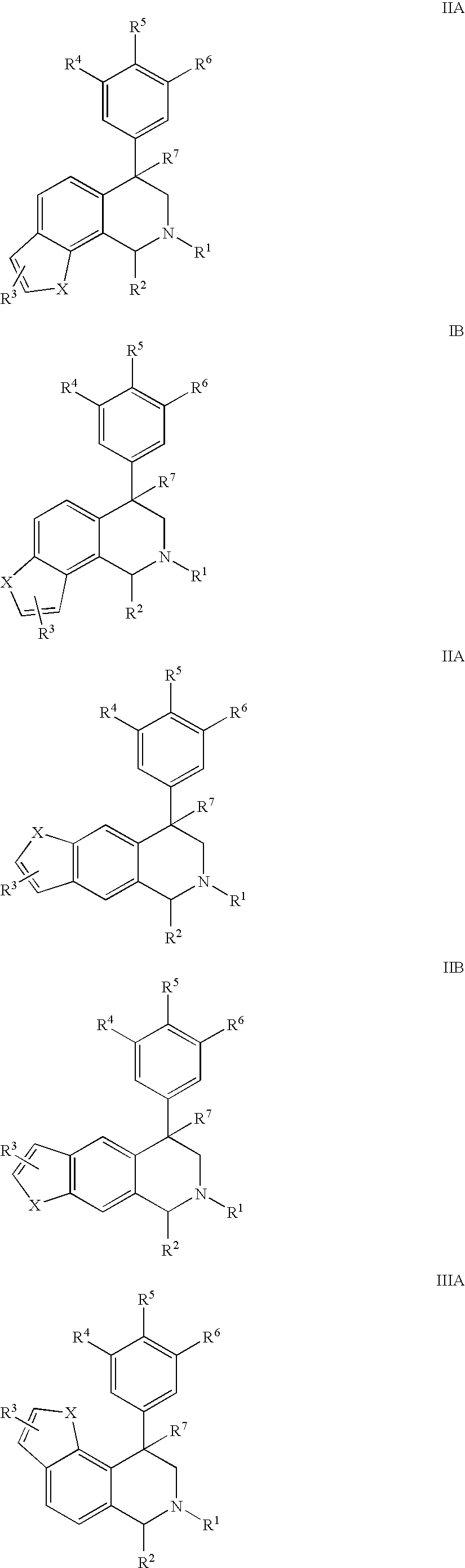
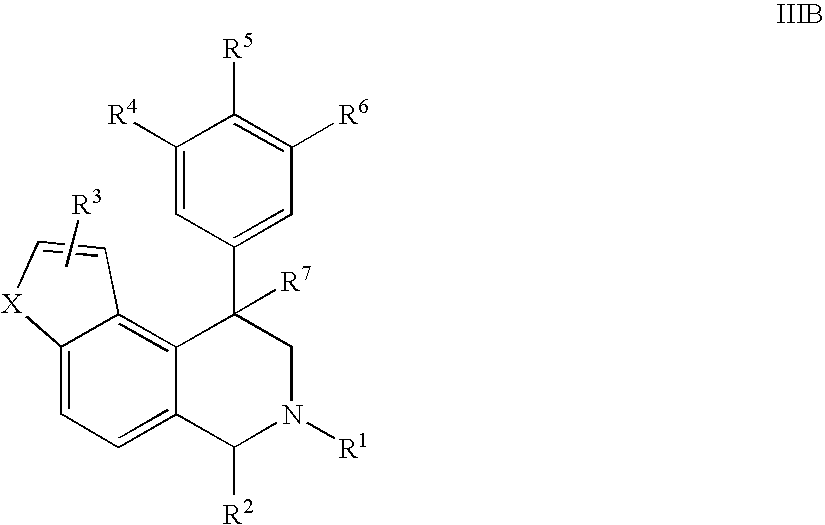
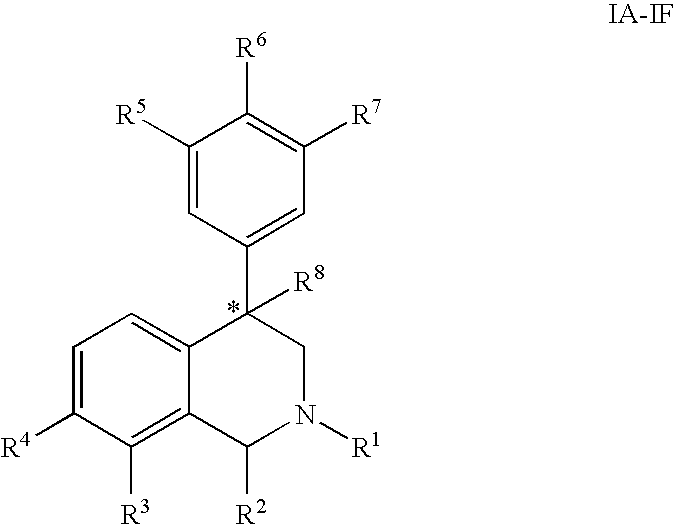
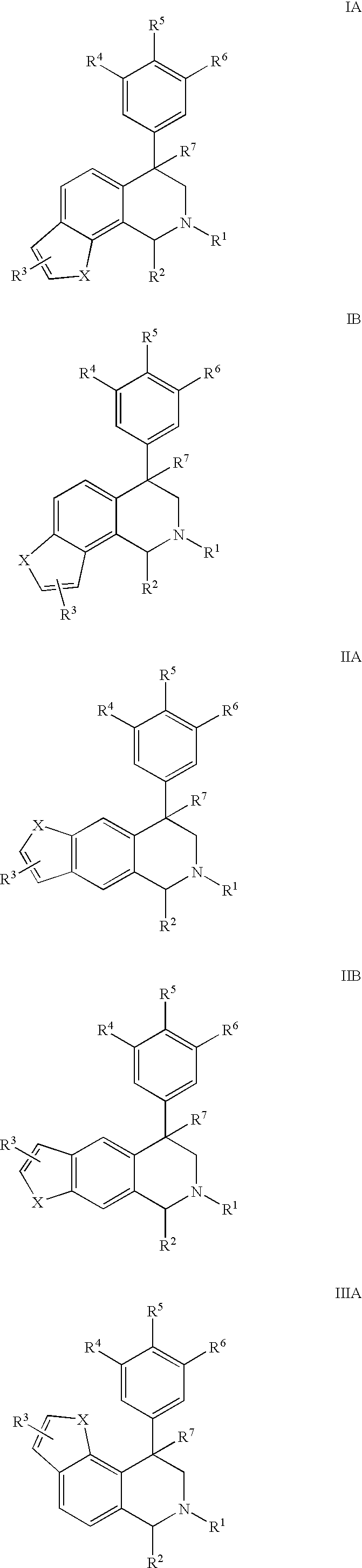
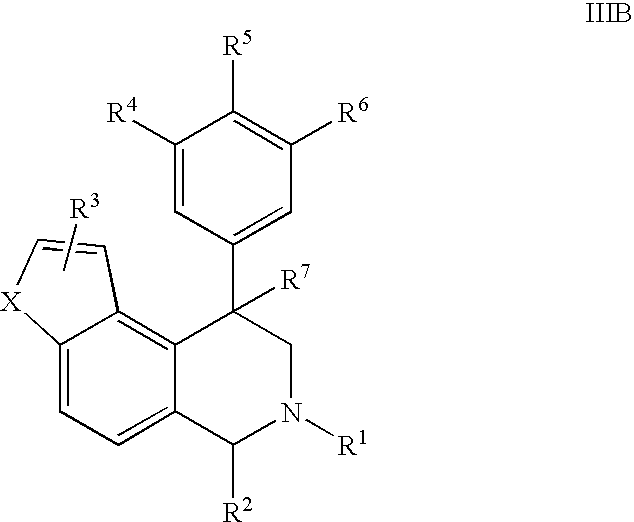
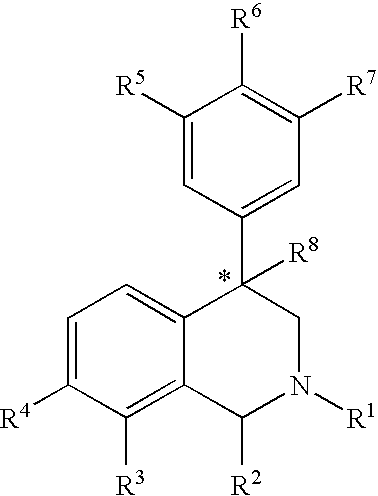
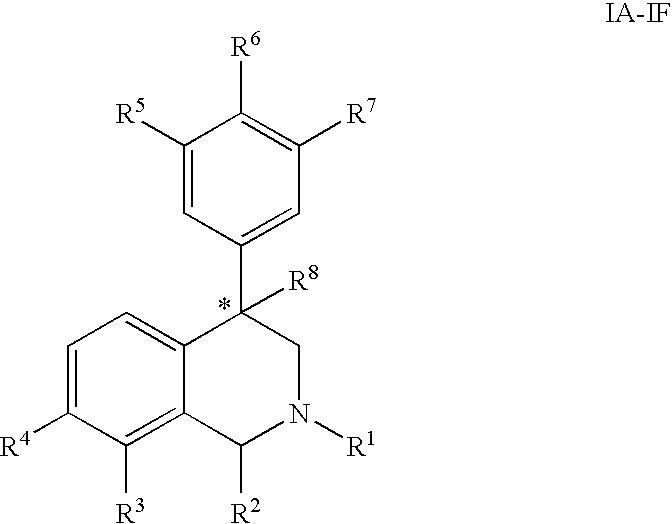
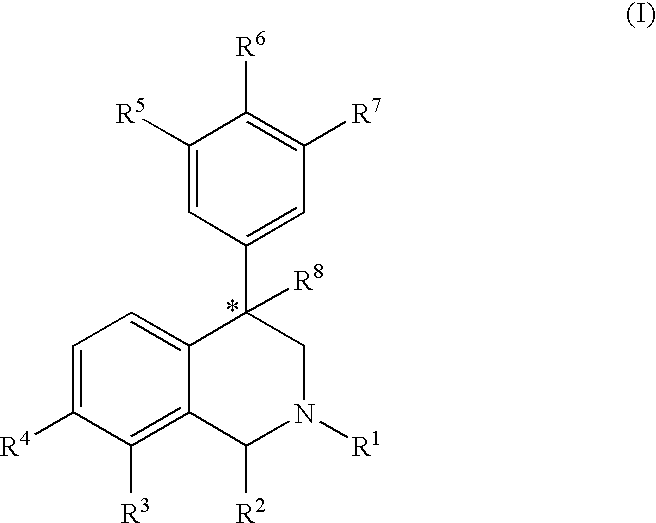
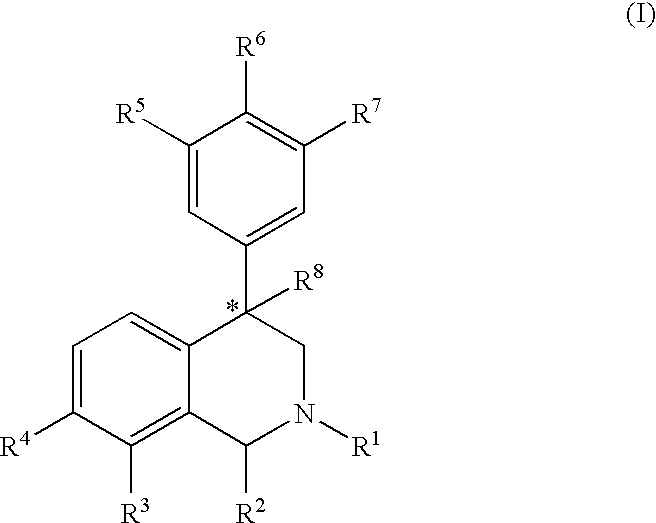
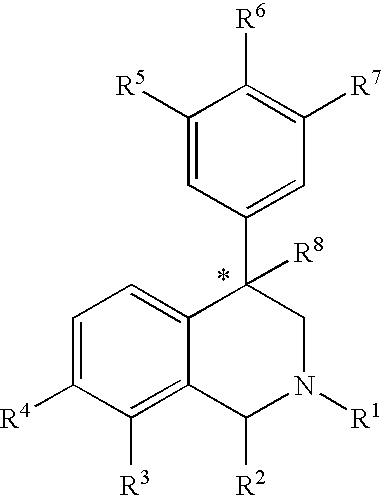
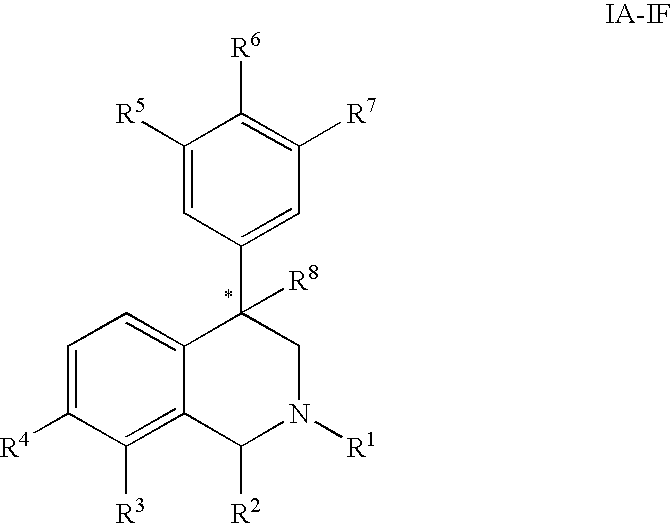
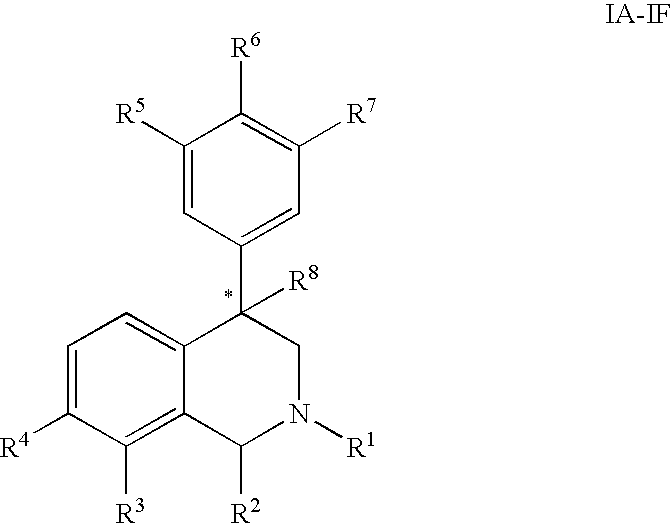
The present invention relates to a method of treating disorders including cognition impairment, generalized anxiety disorder, acute stress disorder, social phobia, simple phobias, pre-menstrual dysphoric disorder, social anxiety disorder, major depressive disorder, eating disorders, obesity, anorexia nervosa, bulimia nervosa, binge eating disorder, substance abuse disorders, chemical dependencies, nicotine addiction, cocaine addiction, alcohol addiction, amphetamine addiction, Lesch-Nyhan syndrome, neurodegenerative diseases, late luteal phase syndrome, narcolepsy, psychiatric symptoms anger, rejection sensitivity, movement disorders, extrapyramidal syndrome, Tic disorder, restless leg syndrome, tardive dyskinesia, sleep related eating disorder, night eating syndrome, stress urinary incontinence, migraine, neuropathic pain, diabetic neuropathy, fibromyalgia syndrome, chronic fatigue syndrome, sexual dysfunction, premature ejaculation, and male impotence. This method involves administering to a patient in need of such treatment a therapeutically effective amount of a disclosed compound. Such compounds are 4-phenyl substituted tetrahydroisoquinolines having the Formula IA, IB, IIA, IIB, IIIA or IIIC as set forth herein.
Owner:ALBANY MOLECULAR RESEARCH INC
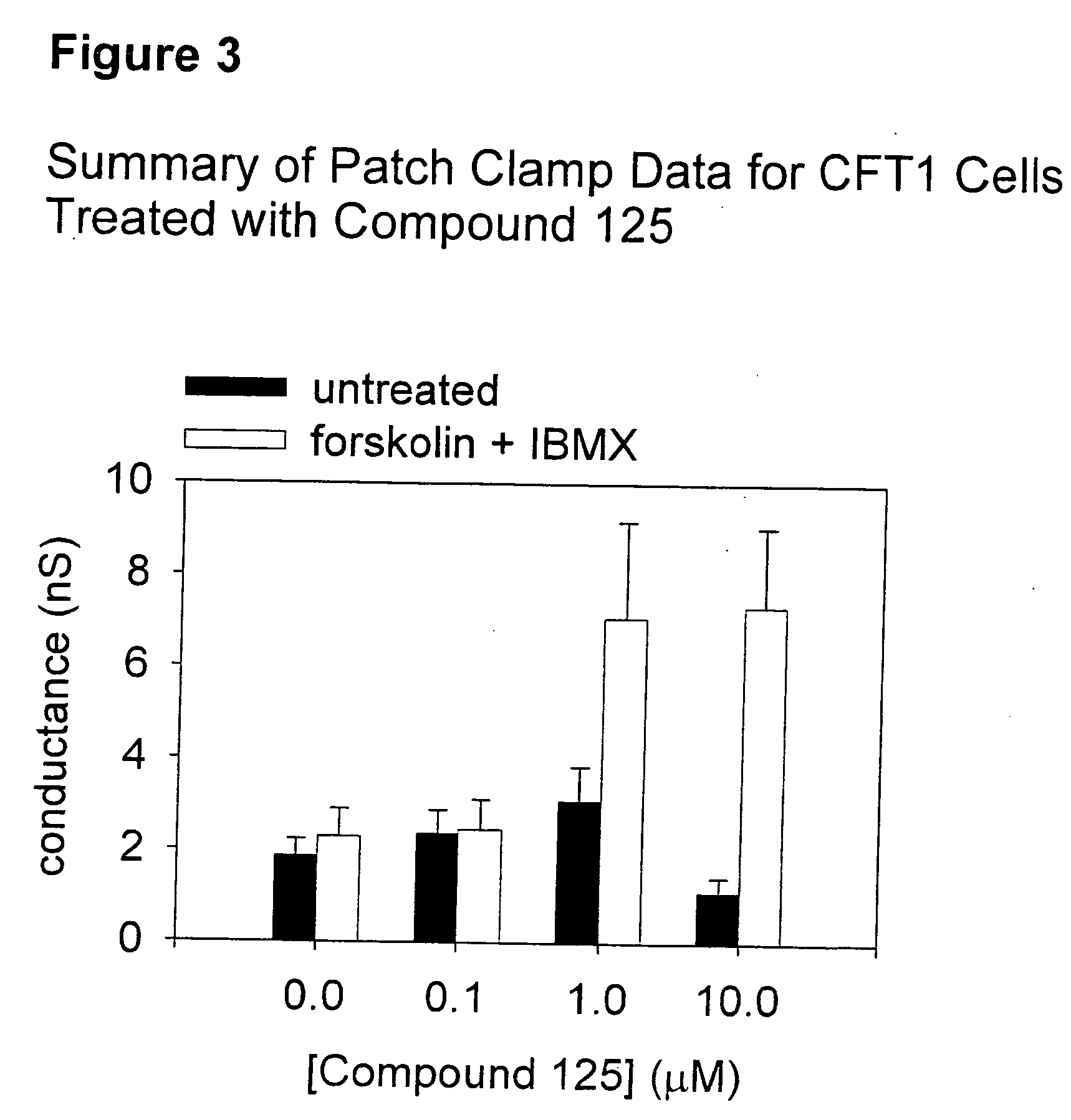
Tetrahydroisoquinoline derivatives for treating protein trafficking diseases
InactiveUS20050176761A1Useful in prevention of diseaseUseful in treatmentBiocideOrganic chemistryCystic fibrosisPneumatocele
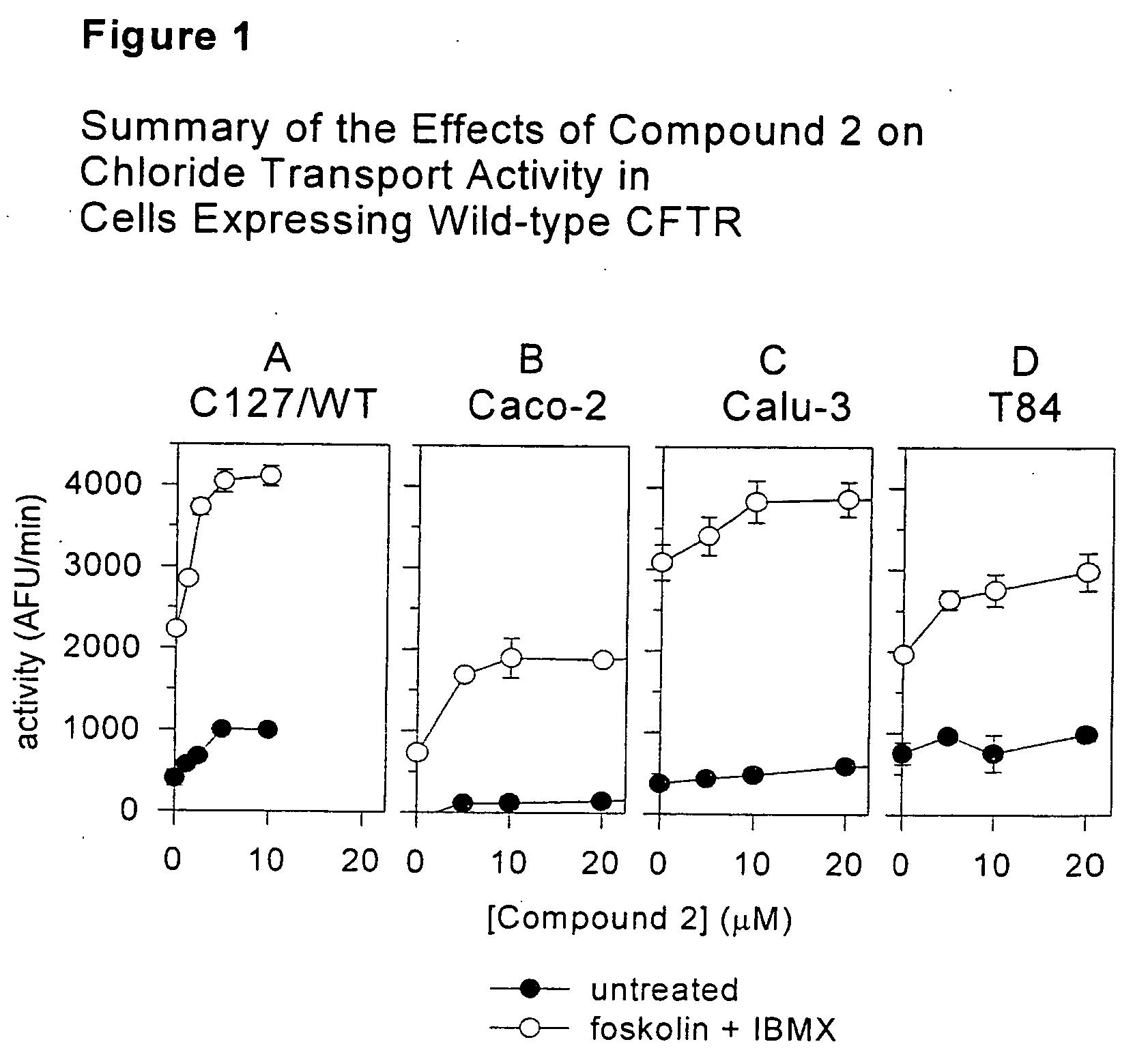
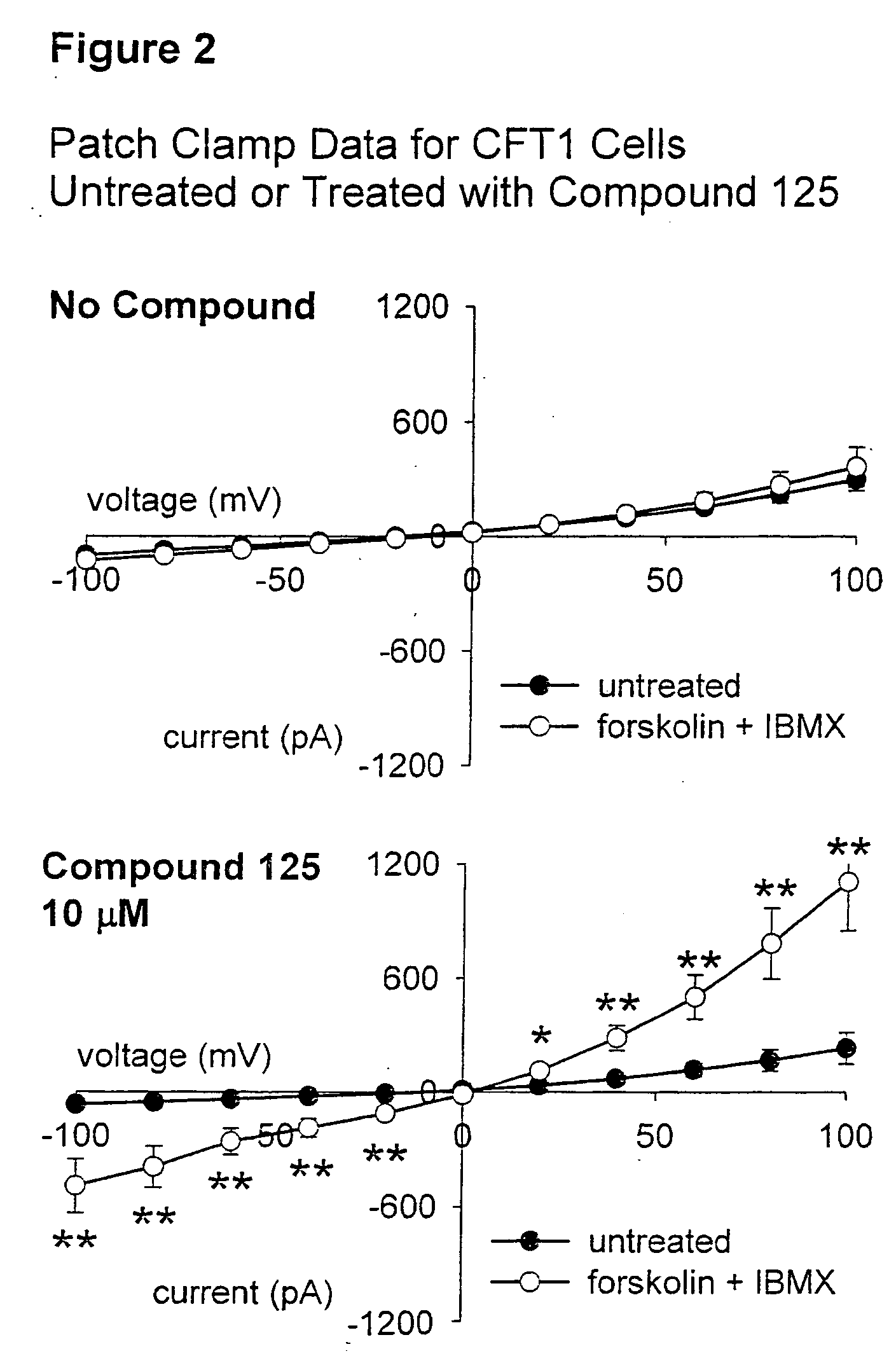
Tetrhydroisoquinoline derivatives, pharmaceutical compositions comprising them and methods of treating disease are disclosed herein. The disclosed compounds are useful in the treatment and prevention of diseases mediated by chloride channel activity and / or protein trafficking, including, but not limited to, diseases associated with impaired mucociliary clearance such as cystic fibrosis, bronchitis, emphysema, and the like.
Owner:GENZYME CORP
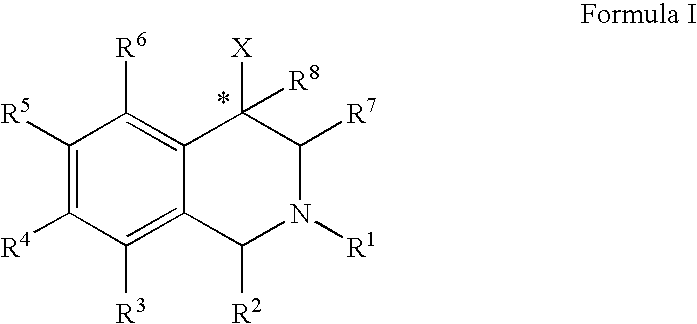
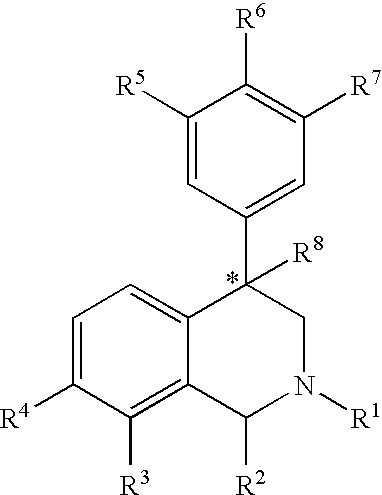
Aryl- and heteroaryl-substituted tetrahydroisoquinolines and use thereof to block reuptake of norepinephrine, dopamine, and serotonin
ActiveUS20060052378A1Good curative effectQuick effectBiocideNervous disorderBenzoxazoleChemical structure
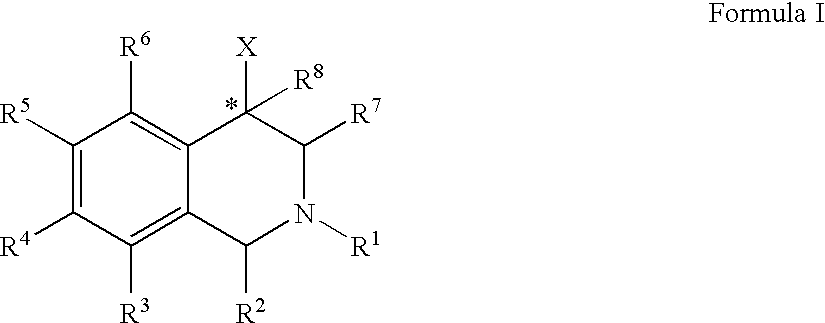
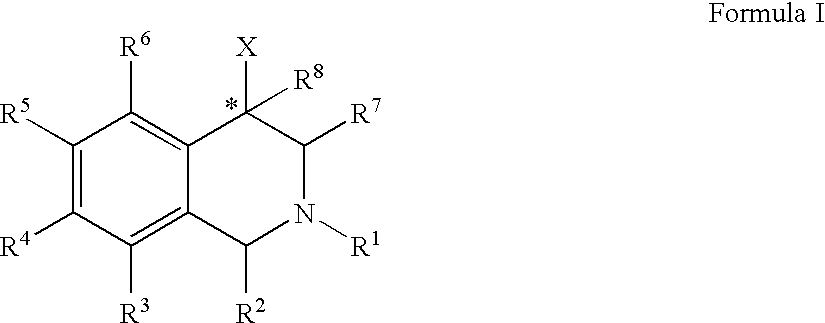
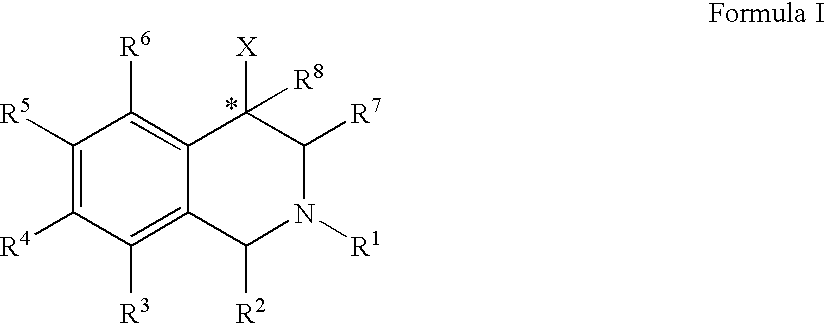
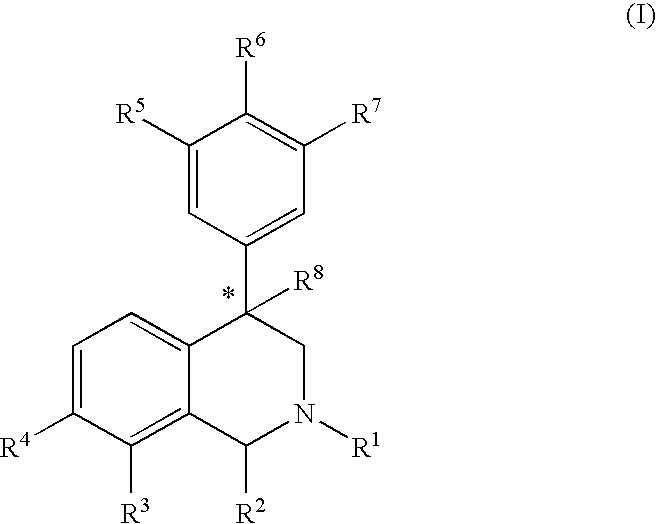
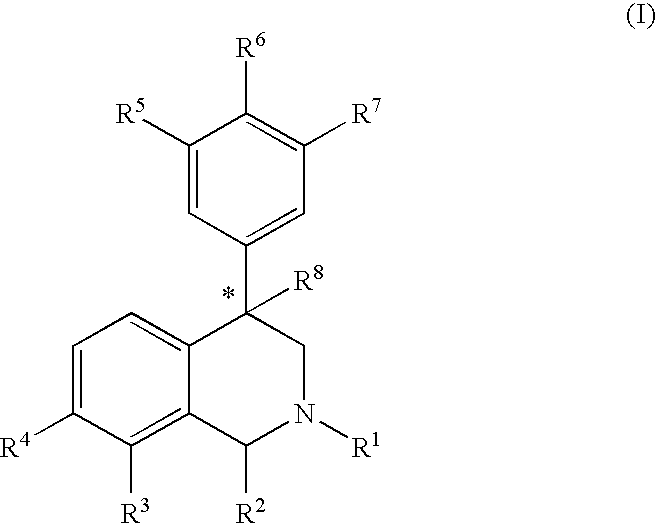
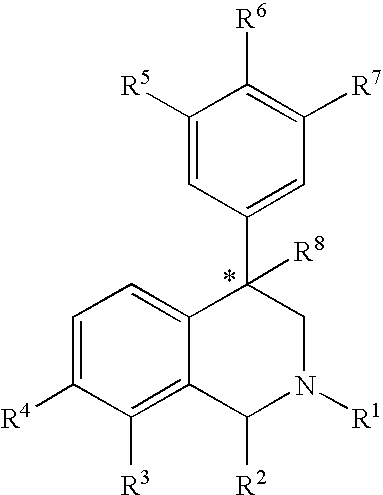
The compounds of the present invention are represented by the chemical structure found in Formula (I): wherein: the carbon atom designated * is in the R or S configuration; and X is a fused bicyclic carbocycle or heterocycle selected from the group consisting of benzofuranyl, benzo[b]thiophenyl, benzoisothiazolyl, benzoisoxazolyl, indazolyl, indolyl, isoindolyl, indolizinyl, benzoimidazolyl, benzooxazolyl, benzothiazolyl, benzotriazolyl, imidazo[1,2-a]pyridinyl, pyrazolo[1,5-a]pyridinyl, [1,2,4]triazolo[4,3-a]pyridinyl, thieno[2,3-b]pyridinyl, thieno[3,2-b]pyridinyl, 1H-pyrrolo[2,3-b]pyridinyl, indenyl, indanyl, dihydrobenzocycloheptenyl, tetrahydrobenzocycloheptenyl, dihydrobenzothiophenyl, dihydrobenzofuranyl, indolinyl, naphthyl, tetrahydronaphthyl, quinolinyl, isoquinolinyl, 4H-quinolizinyl, 9aH-quinolizinyl, quinazolinyl, cinnolinyl, phthalazinyl, quinoxalinyl, benzo[1,2,3]triazinyl, benzo[1,2,4]triazinyl, 2H-chromenyl, 4H-chromenyl, and a fused bicyclic carbocycle or fused bicyclic heterocycle optionally substituted with substituents (1 to 4 in number) as defined in R14; with R1, R2, R3, R4, R5, R6, R7, R8, and R14 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Novel 4-phenyl substituted tetrahydroisoquinolines and therapeutic use thereof
The present invention relates to a method of treating disorders including cognition impairment, generalized anxiety disorder, acute stress disorder, social phobia, simple phobias, pre-menstrual dysphoric disorder, social anxiety disorder, major depressive disorder, eating disorders, obesity, anorexia nervosa, bulimia nervosa, binge eating disorder, substance abuse disorders, chemical dependencies, nicotine addiction, cocaine addiction, alcohol addiction, amphetamine addiction, Lesch-Nyhan syndrome, neurodegenerative diseases, late luteal phase syndrome, narcolepsy, psychiatric symptoms anger, rejection sensitivity, movement disorders, extrapyramidal syndrome, Tic disorder, restless leg syndrome, tardive dyskinesia, sleep related eating disorder, night eating syndrome, stress urinary incontinence, migraine, neuropathic pain, diabetic neuropathy, fibromyalgia syndrome, chronic fatigue syndrome, sexual dysfunction, premature ejaculation, and male impotence. This method involves administering to a patient in need of such treatment a therapeutically effective amount of a disclosed compound. Such compounds are 4-phenyl substituted tetrahydroisoquinolines having the Formula IA, IB, IIA, IIB, IIIA or IIIC as set forth herein.
Owner:ALBANY MOLECULAR RESEARCH INC
4-Phenyl substituted tetrahydroisoquinolines and use thereof to block reuptake of norepinephrine, dopamine and serotonin
Owner:ALBANY MOLECULAR RESEARCH INC
4-phenyl substituted tetrahydroisoquinolines therapeutic use thereof
Compounds are provided that, by way of their selective neurotransmitter binding useful for the treatment of various neurological and psychological disorders, e.g., ADHD. Such compounds are 4-phenyl substituted tetrahydroisoquinolines having the Formula IA, IB, IIA, IIB, IIIA or IIIC as set forth herein.
Owner:ALBANY MOLECULAR RESEARCH INC
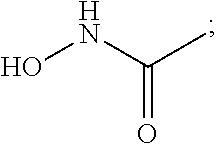
Compounds for treatment of neurodegenerative diseases
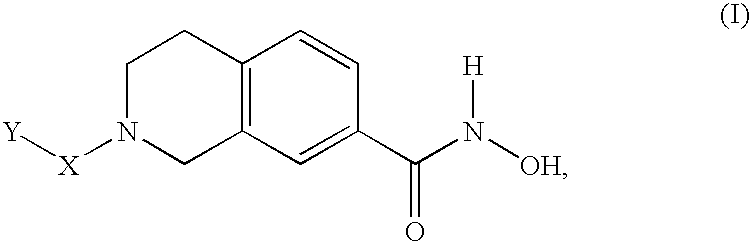
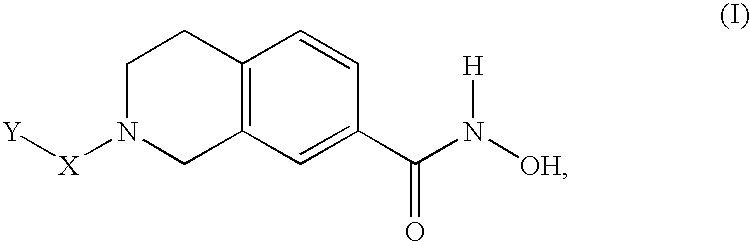
The present invention relates to a class of small molecule hydroxamic acid compounds capable of inhibiting histone deacetylases (HDACs). The present invention also relates to methods of preparation of hydroxamic acid HDAC inhibitor compounds of the invention, which are N-substituted-1,2,3,4-tetrahydroisoquinoline hydroxamic acid derivatives, and their incorporation into pharmaceutical compositions and methods of administration. The present invention also relates to N-substituted-1,2,3,4-tetrahydroisoquinoline hydroxamic acid derivatives, which may be prepared as a hydroxamic acid HDAC inhibitor compound library that can be utilized in screening methods known in the art.
Owner:FORUM PHARMA
Caspase inhibitors and uses thereof
InactiveUS20020058630A1Enhanced inhibitory effectGood effectAntibacterial agentsOrganic active ingredientsCaspase inhibitorsAryl
Described herein are compounds that are useful as caspase inhibitors having the formula: wherein Ring A is an optionally substituted piperidine, tetrahydroquinoline or tetrahydroisoquinoline ring; R1 is hydrogen, CN, CHN2, R, or CH2Y; R is an optionally substituted group selected from an aliphatic group, an aryl group, or an aralkyl group; Y is an electronegative leaving group; R2 is CO2H, CH2CO2H, or esters, amides or isosteres thereof; and R3 is hydrogen, an optionally substituted aryl group, an optionally substituted aralkyl group, or an optionally substituted C1-6 aliphatic group, R4 is an optionally substituted group selected from an aryl group or a heterocyclyl group, or R3 and R4 taken together with the nitrogen to which they are attached optionally form a substituted or unsubstituted monocyclic, bicyclic or tricyclic ring.
Owner:VERTEX PHARMA INC
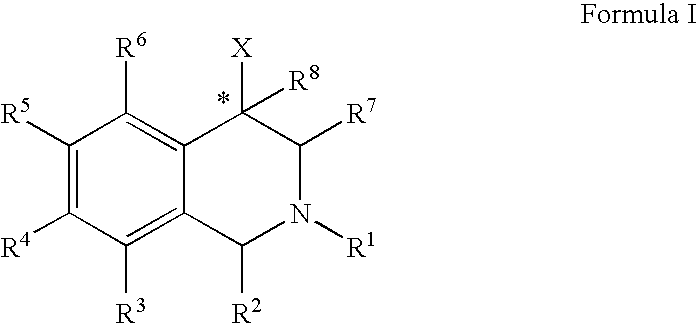
Use of aryl- and heteroaryl-substituted tetrahydroisoquinolines to block reuptake of norepinephrine, dopamine, and serotonin
InactiveUS20060063766A1Little and no activityMinimal potential for substance abuseBiocideNervous disorderBenzoxazoleBenzene
The compounds of the present invention are represented by the chemical structure found in Formula (I): wherein: the carbon atom designated * is in the R or S configuration; and X is a fused bicyclic carbocycle or heterocycle selected from the group consisting of benzofuranyl, benzo[b]thiophenyl, benzoisothiazolyl, benzoisoxazolyl, indazolyl, indolyl, isoindolyl, indolizinyl, benzoimidazolyl, benzooxazolyl, benzothiazolyl, benzotriazolyl, imidazo[1,2-a]pyridinyl, pyrazolo[1,5-a]pyridinyl, [1,2,4]triazolo[4,3-a]pyridinyl, thieno[2,3-b]pyridinyl, thieno[3,2-b]pyridinyl, 1H-pyrrolo[2,3-b]pyridinyl, indenyl, indanyl, dihydrobenzocycloheptenyl, tetrahydrobenzocycloheptenyl, dihydrobenzothiophenyl, dihydrobenzofuranyl, indolinyl, naphthyl, tetrahydronaphthyl, quinolinyl, isoquinolinyl, 4H-quinolizinyl, 9aH-quinolizinyl, quinazolinyl, cinnolinyl, phthalazinyl, quinoxalinyl, benzo[1,2,3]triazinyl, benzo[1,2,4]triazinyl, 2H-chromenyl, 4H-chromenyl, and a fused bicyclic carbocycle or fused bicyclic heterocycle optionally substituted with substituents (1 to 4 in number) as defined in R14; with R1, R2, R3, R4, R5, R6, R7, R8, and R14 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
4-Phenyl substituted tetrahydroisoquinolines and use thereof to block reuptake of norepinephrine, dopamine and serotonin
Owner:ALBANY MOLECULAR RESEARCH INC
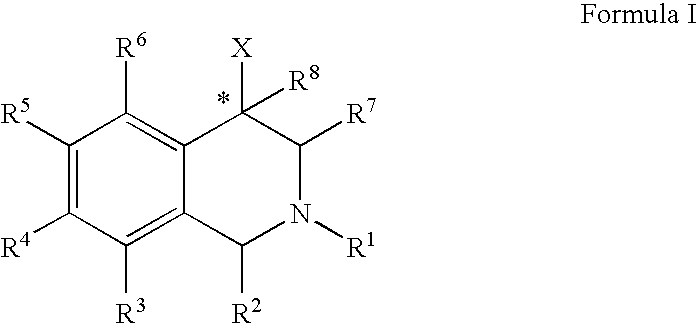
Aryl-and heteroaryl-substituted tetrahydroisoquinolines and use thereof to block reuptake of norepinephrine, dopamine and serotonin
The present invention relates to a method of treating disorders by administering to a patient in need of such treatment a therapeutically effective amount of a compound of formula (I): wherein R1—R8 are as described herein, R4 being aryl or heteroaryl.
Owner:ALBANY MOLECULAR RESEARCH INC
Compounds for treatment of neurodegenerative diseases
InactiveUS20060004041A1Potential limitationInhibit deacetylationBiocideOrganic chemistryHydroxamic acidNeuro-degenerative disease
The present invention relates to a class of small molecule hydroxamic acid compounds capable of inhibiting histone deacetylases (HDACs). The present invention also relates to methods of preparation of hydroxamic acid HDAC inhibitor compounds of the invention, which are N-substituted-1,2,3,4-tetrahydroisoquinoline hydroxamic acid derivatives, and their incorporation into pharmaceutical compositions and methods of administration. The present invention also relates to N-substituted-1,2,3,4-tetrahydroisoquinoline hydroxamic acid derivatives, which may be prepared as a hydroxamic acid HDAC inhibitor compound library that can be utilized in screening methods known in the art.
Owner:FORUM PHARMA
Cell differntiation inhibiting agent, cell culture method using the same, culture medium, and cultured cell line
The object of the present invention is to provide a differentiation inhibiting agent which allows culture of a stem cell or an embryonic stem cell in an undifferentiated state without use of any feeder cell, a method for culturing using the same, a cell culture liquid using the same, and a cell prepared by culturing using this differentiation inhibiting agent. The present invention provides a differentiation inhibiting agent which comprises a low molecular weight compound, especially a tetrahydroisoquinoline derivative, as an active ingredient; a method for safely culturing a stem cell in large scale in undifferentiated state in the absence of feeder cell which comprises culturing a stem cell by using a tetrahydroisoquinoline derivative; a culture liquid for stem cells comprising a tetrahydroisoquinoline derivative; and a cell which is obtained by culture using a tetrahydroisoquinoline derivative as a differentiation inhibiting agent.
Owner:ASAHI KASEI KK
Aryl and heteroaryl substituted tetrahydroisoquinolines and use thereof
Provided herein are compounds of the formula (I): wherein R1-R8 are as described herein, R4 being phthalazinyl, pyrazinyl, pyridazinyl, or quinoxalinyl. Such compounds are particularly useful in the treatment of a disorder which is created by or is dependent upon decreased availability of serotonin, norepinephrine, or dopamine.
Owner:ALBANY MOLECULAR RESEARCH INC
Diaryl-substituted tetrahydroisoquinolines as histamine H3 receptor and serotonin transporter modulators
Certain diaryl-substituted tetrahydroisoquinoline compounds are histamine H3 receptor and / or serotonin transporter modulators useful in the treatment of histamine H3 receptor- and / or serotonin-mediated diseases.
Owner:JANSSEN PHARMA NV
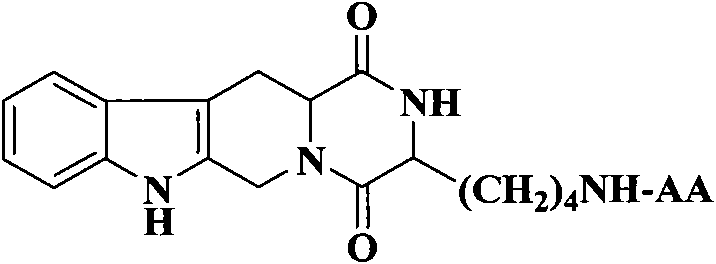
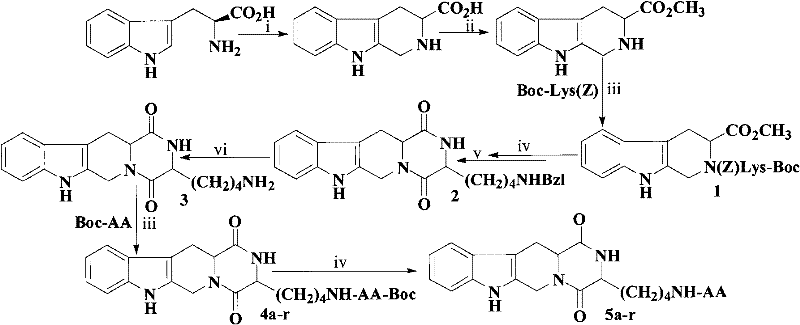
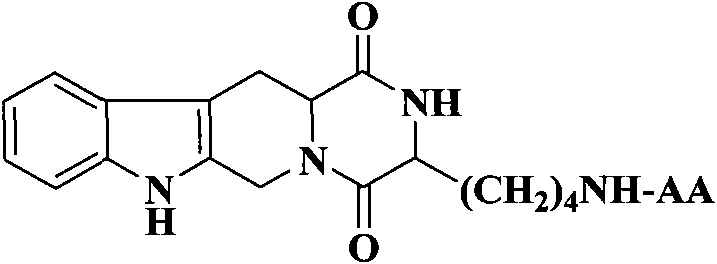
(3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid derivatives, and synthesis method and application thereof
The invention discloses (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid derivatives, and a synthesis method and application thereof. The structural formula of the compounds is shown as a general formula I. Hexa hydro-pyrazino-pyridino-indoldione compounds are structurally modified by natural amino acid, the water solubility and in-vivo absorption are improved and the anti-thrombotic activity is improved. In-vitro and in-vivo anti-thrombotic activity experiments indicate that the compounds in the general formula I have excellent anti-thrombotic activity, and can be prepared into anti-thrombotic medicines.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Caspase inhibitors and uses thereof
InactiveUS7053057B2Enhanced inhibitory effectGood effectAntibacterial agentsOrganic active ingredientsDiseaseAryl
This invention provides caspase inhibitors having the formula:wherein Ring A is an optionally substituted piperidine, tetrahydroquinoline or tetrahydroisoquinoline ring; R1 is hydrogen, CHN2, R, or —CH2Y; R is an optionally substituted group selected from an aliphatic group, an aryl group, an aralkyl group, a heterocyclic group, or an heterocyclylalkyl group; Y is an electronegative leaving group; R2 is CO2H, CH2CO2H, or esters, amides or isosteres thereof; Ar is an optionally substituted aryl group; and R3 is hydrogen, an optionally substituted C1-6 alkyl, F2, CN, aryl or R3 is attached to Ar to form an unsaturated or partially saturated five or six membered fused ring having 0–2 heteroatoms. The compounds are useful for treating caspase-mediated diseases in mammals.
Owner:VERTEX PHARMA INC
4-phenyl substituted tetrahydroisoquinolines and use thereof
Owner:ALBANY MOLECULAR RESEARCH INC
Synthesis method for benzothiazole unit-based covalent organic framework material
ActiveCN105440058AOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDehydrogenationQuinoline
The present invention discloses a synthesis method for a benzothiazole unit-based covalent organic framework material. The method comprises the following step of: in an organic solvent or an ionic liquid, performing a reaction on 2, 4, 6-trihydroxy benzenetricarboxaldehyde and 2, 6-diamino benzodithiazole to obtain the covalent organic framework material. The covalent organic framework material prepared by the method has a relatively large surface area and a regular channel structure, and is a good photocatalytic material; and the material can be used for cross-dehydrogenation coupling reaction for photocatalysis of N-aryl tetrahydroisoquinoline, and has good stability and an excellent catalytic effect.
Owner:LANZHOU UNIVERSITY
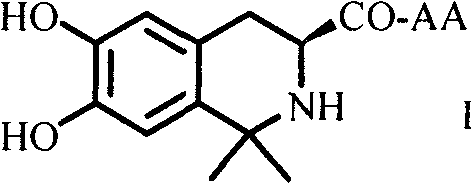
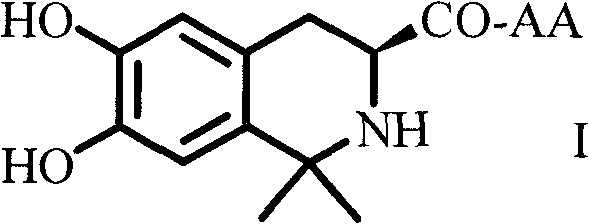
Dihydroxyltetrahydroisoquinoline-3-formyl amino acids as well as synthesis, antithrombotic effect and application thereof
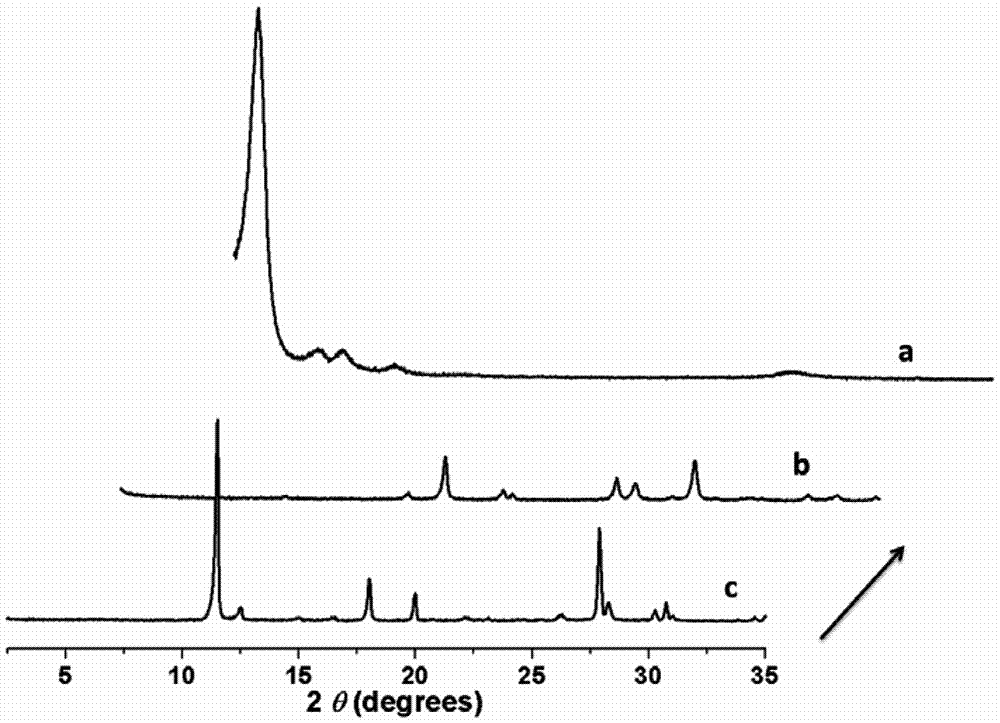
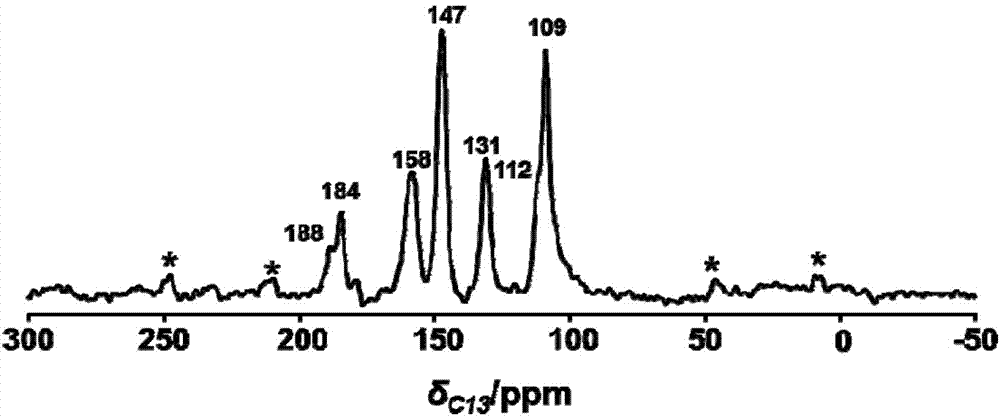
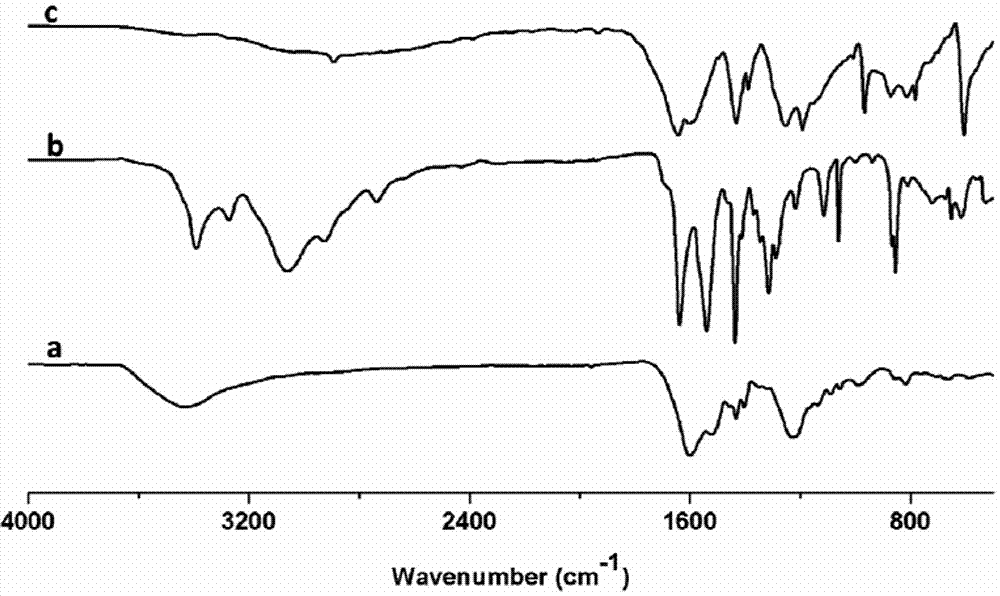
The invention provides 15 6,7-dibydroxyl-1,1-dibydroxyl-tetrahydroisoquinoline-3-formyl amino acids of a general formula I. In the general formula I, AA is selected from L-Ala, Gly, L-Phe, L-Val, L-Leu, L-Ile, L-Trp, L-Ser, L-Thr, L-Tyr, L-Lys, L-Pro, L-Asp, L-Arg and L-Glu residues. The invention provides preparation methods of amino acids, and further provides the antithrombotic effect thereof on a rat carotid artery / jugular vein bypass intubation antithrombotic model. Therefore, the 15 6,7-dibydroxyl-1,1-dibydroxyl-tetrahydroisoquinoline-3-formyl amino acids of the general formula I has application prospect in clinical antithrombotic agents.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Diaryl-substituted tetrahydroisoquinolines as histamine h3 receptor and serotonin transporter modulators
Certain diaryl-substituted tetrahydroisoquinoline compounds are histamine H3 receptor and / or serotonin transporter modulators useful in the treatment of histamine H3 receptor- and / or serotonin-mediated diseases.
Owner:JANSSEN PHARMA NV
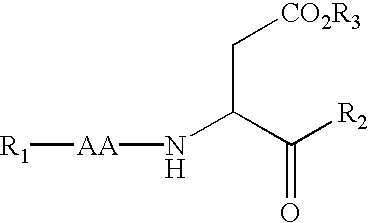
(3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid kyrine conjugate, preparation method and application thereof
InactiveCN101899084ATripeptide ingredientsPeptide preparation methodsAntithrombotic AgentNanoparticle
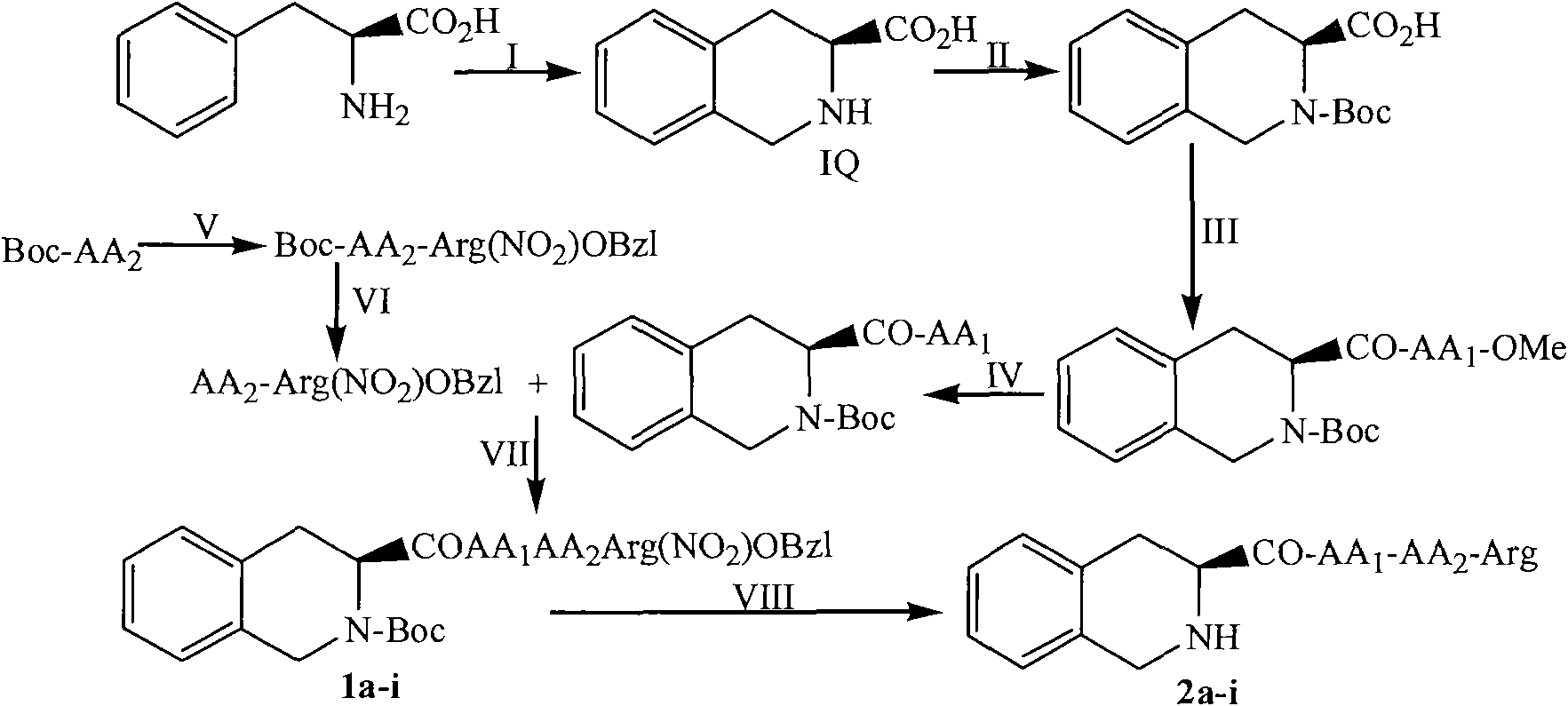
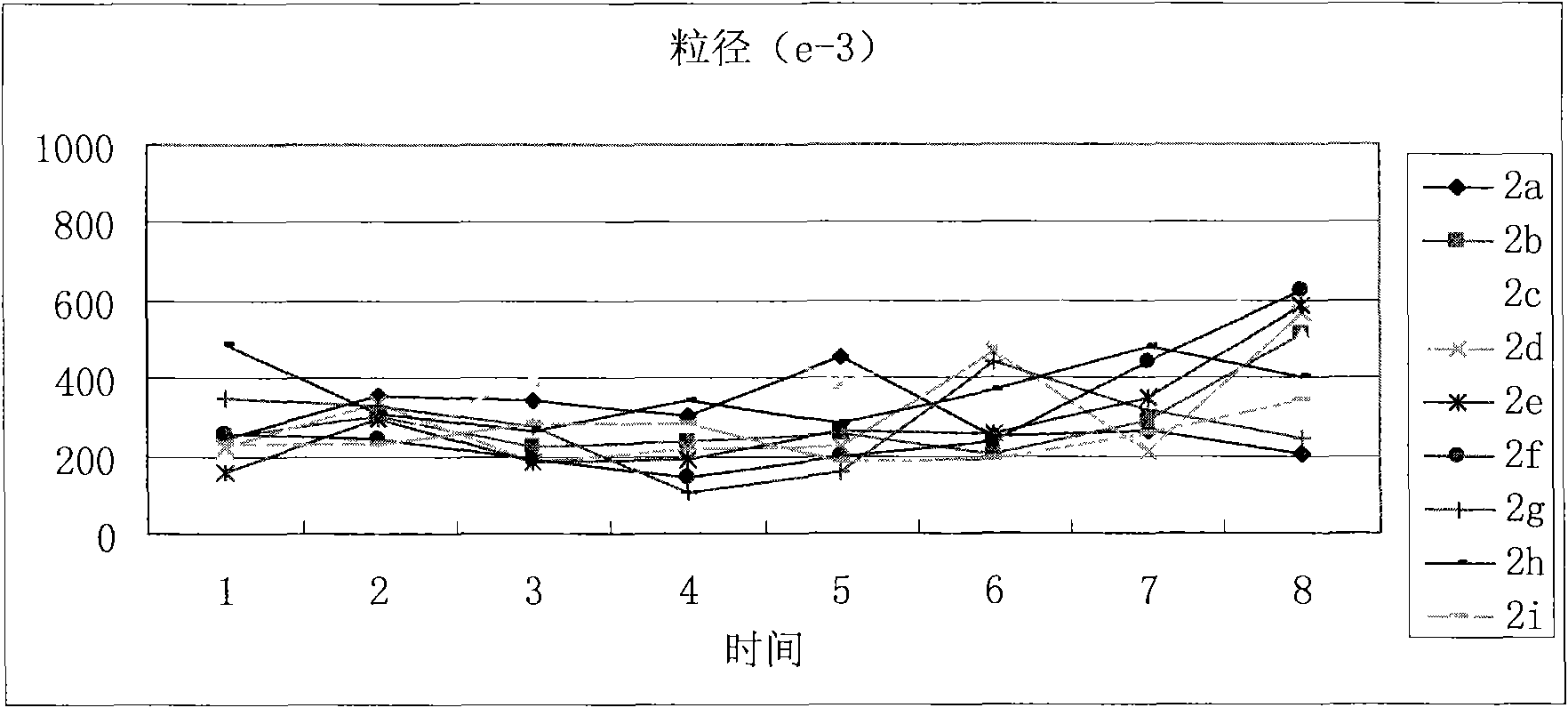
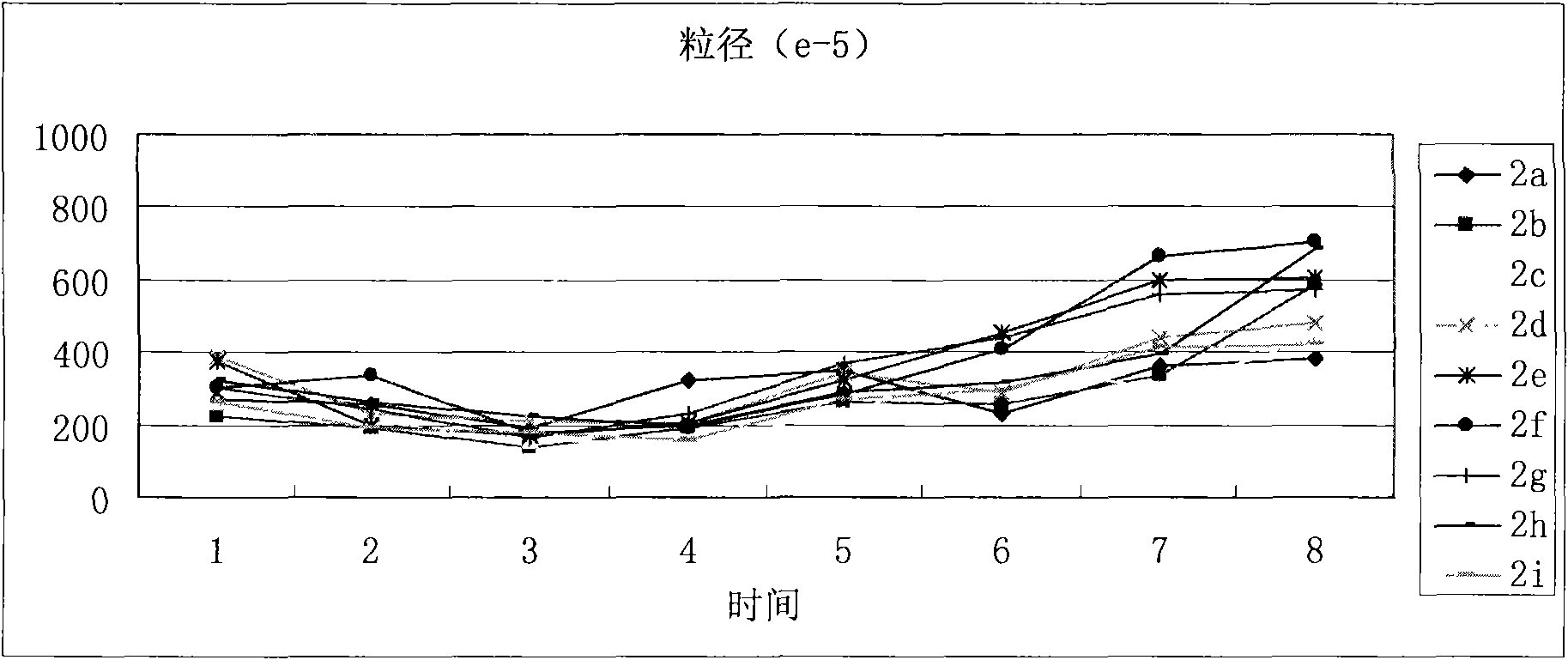
The invention discloses a (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid kyrine conjugate, a preparation method and application thereof. The preparation method of the conjugate comprises the following steps of: preparing N-[(3S)-N-Boc-1,2,3,4- tetrahydroisoquinoline-3-formoxyl]-AA1-AA2-Arg (NO2)-OBzl by coupling N-[(3S)-N-Boc-1,2,3,4-tetrahydroisoquinoline-3-formoxyl]-AA1 and AA2-Arg (NO2)-OBzl; and detracting a protecting group, wherein AAl is selected from Gly, Asp or Gln, and AA2 is selected from Gly, Asp or Gln. The conjugate has excellent antithrombotic activity, can be clinically used as an antithrombotic agent, can be automatically assembled into nano particles in a water solution and is stable in the water solution; and the particle diameter is mostly from 200nm to 600nm.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Tetrahydro isoquinoline derivatives, preparation methods and medicinal uses thereof
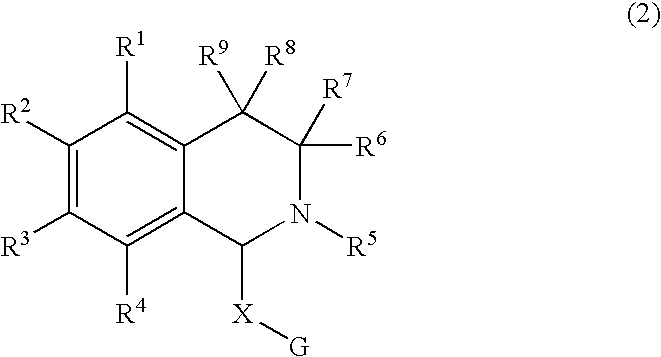
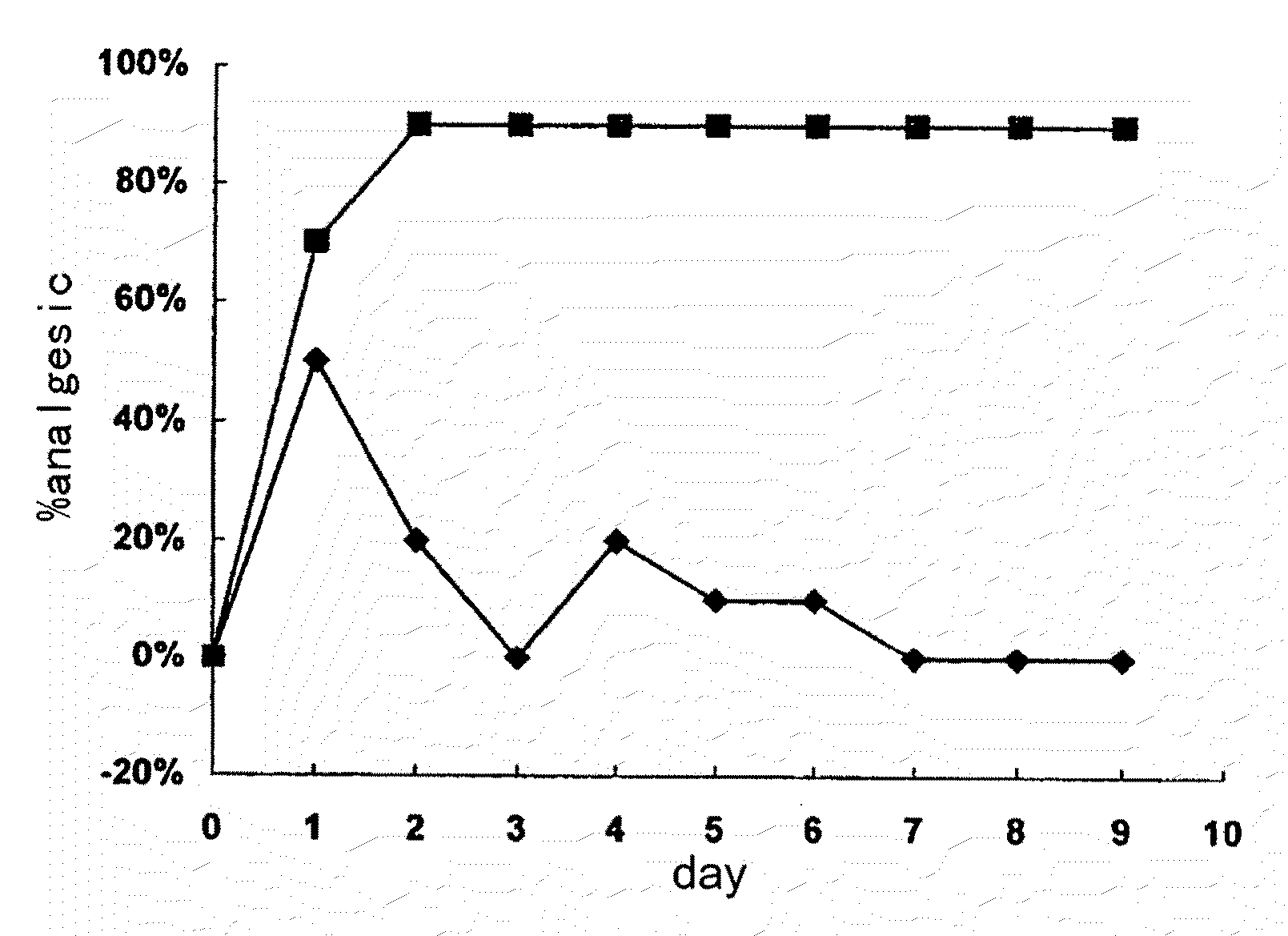
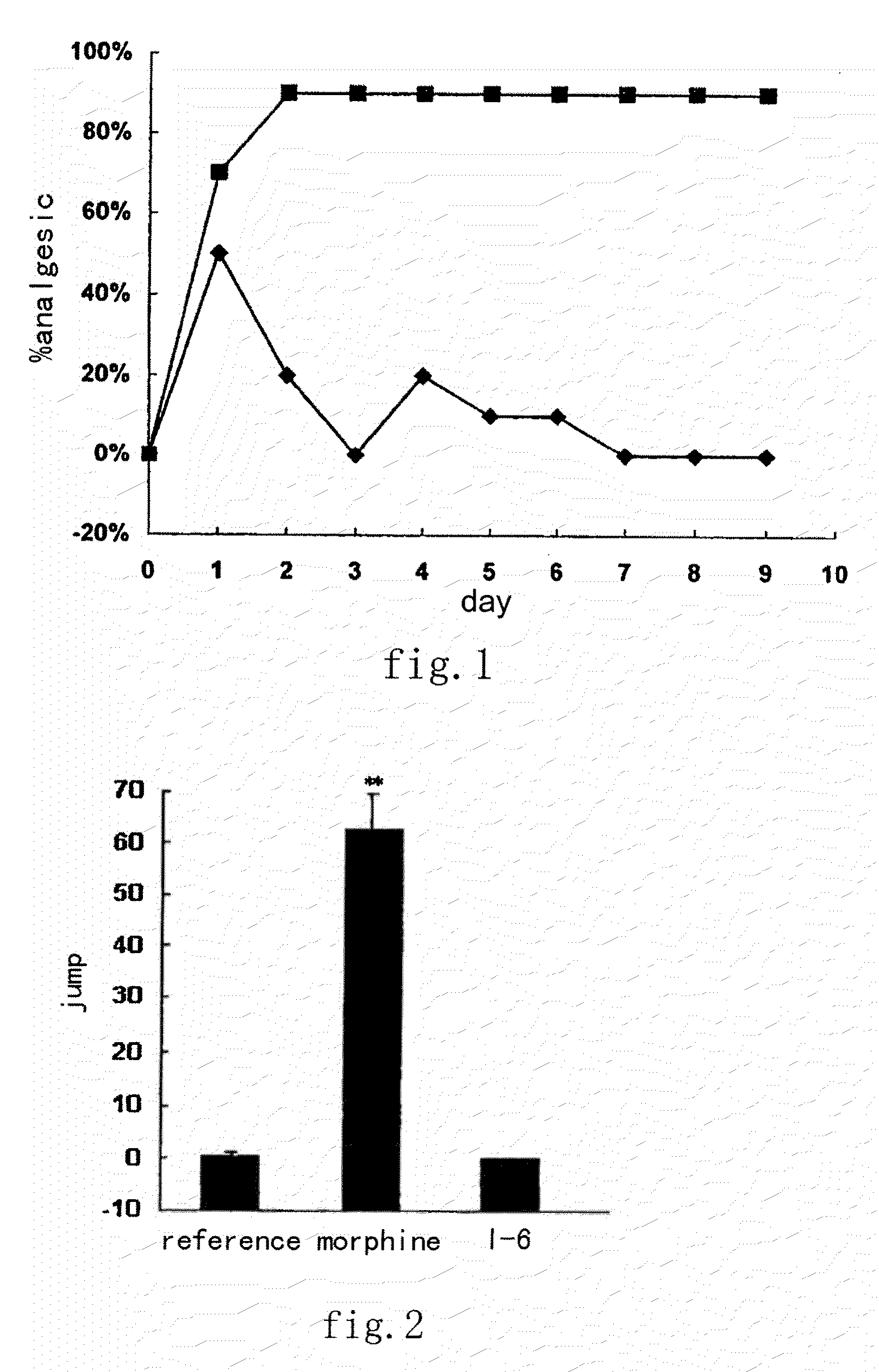
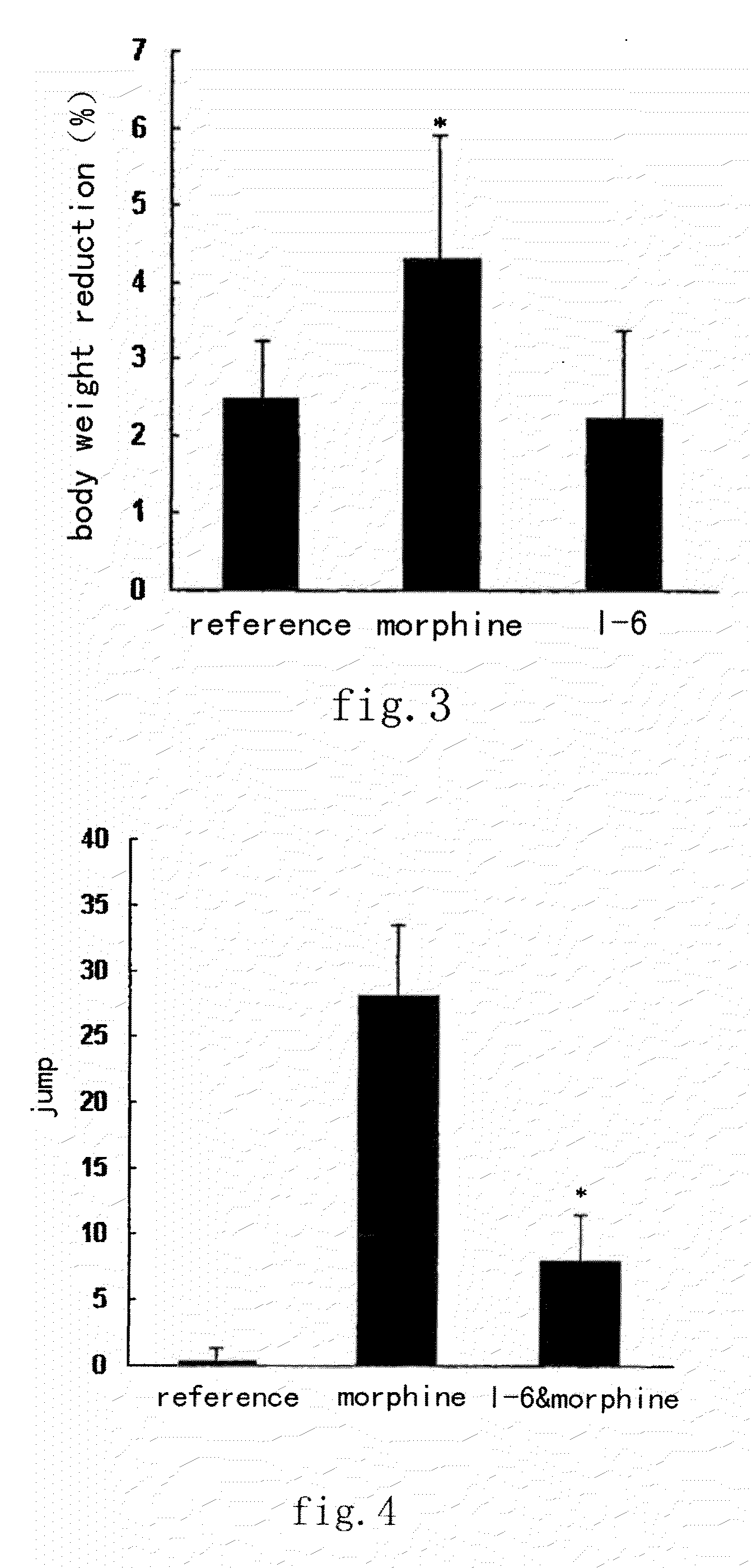
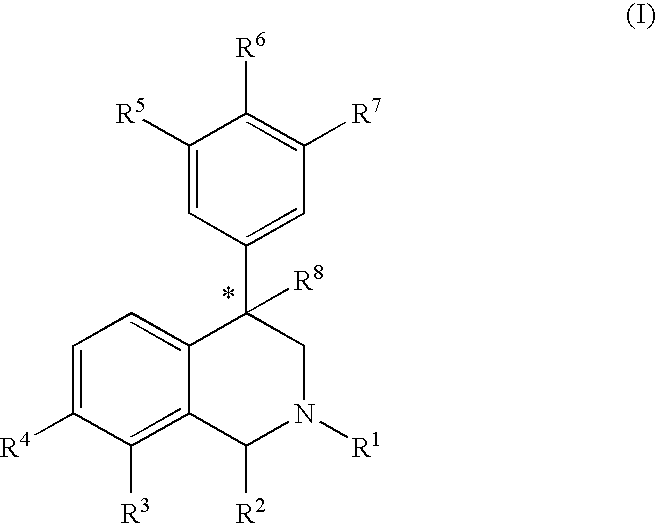
A kind of tetrahydro isoquinoline derivatives (I), their preparation methods, medicine compositions and medicinal uses thereof, especially their uses as κ-opioid receptor excitant in pain relieving, which belongs to the medicine chemistry. The substituents R1, R2, R3, R4 of general formula (I) are defined as the description.
Owner:CHINA PHARM UNIV +2
Aryl and heteroaryl substituted tetrahydroisoquinolines and use thereof
Provided herein are compounds of the formula (I):wherein R1-R8 are as described herein, R4 being phthalazinyl, pyrazinyl, pyridazinyl, or quinoxalinyl. Such compounds are particularly useful in the treatment of a disorder which is created by or is dependent upon decreased availability of serotonin, norepinephrine, or dopamine.
Owner:ALBANY MOLECULAR RESEARCH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com