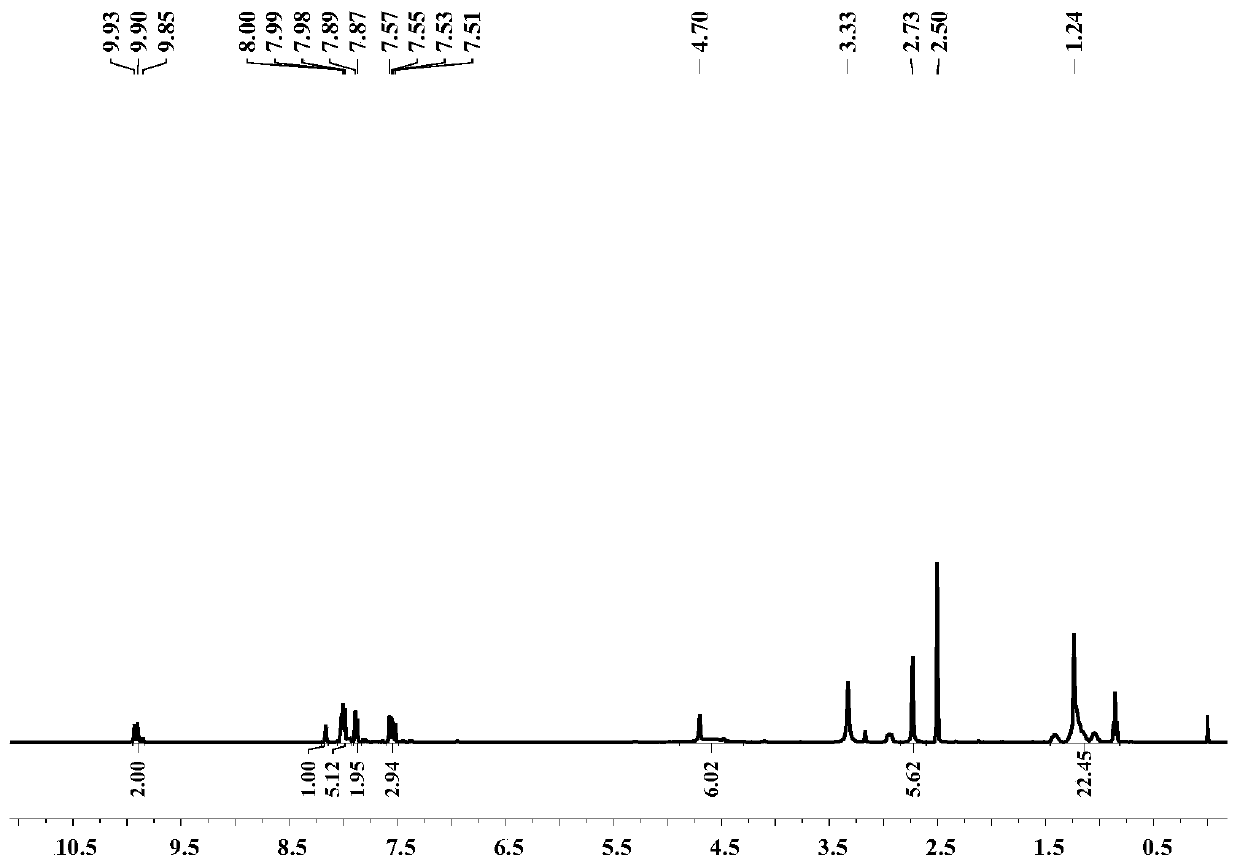
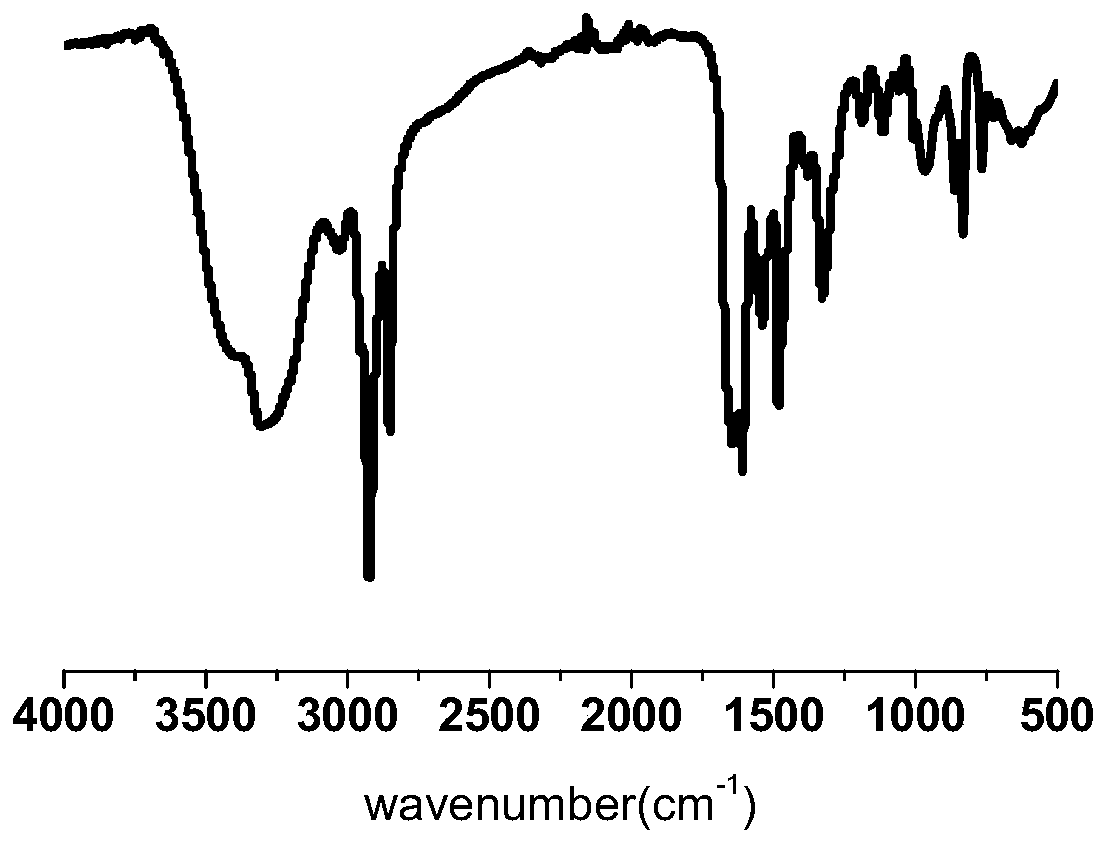
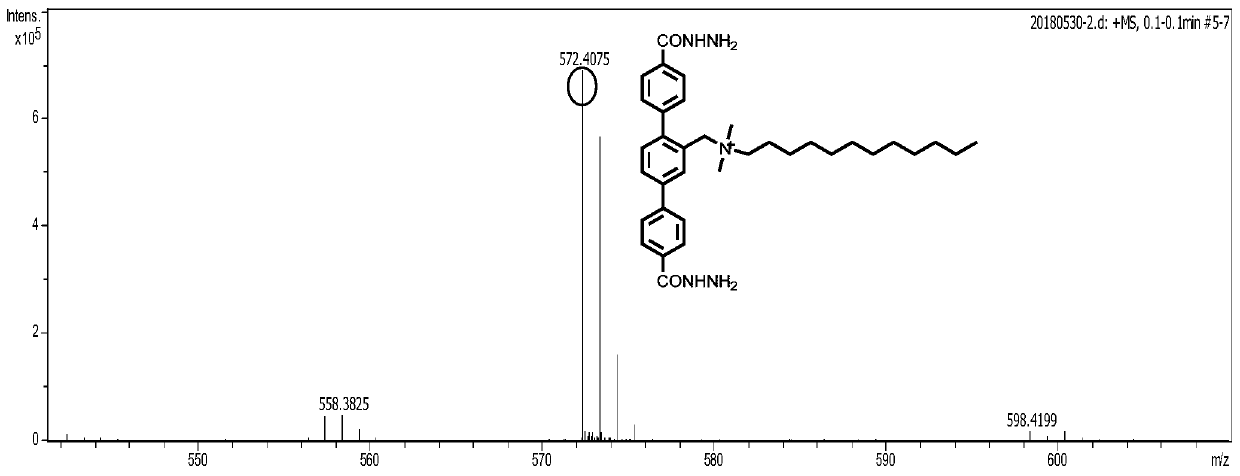
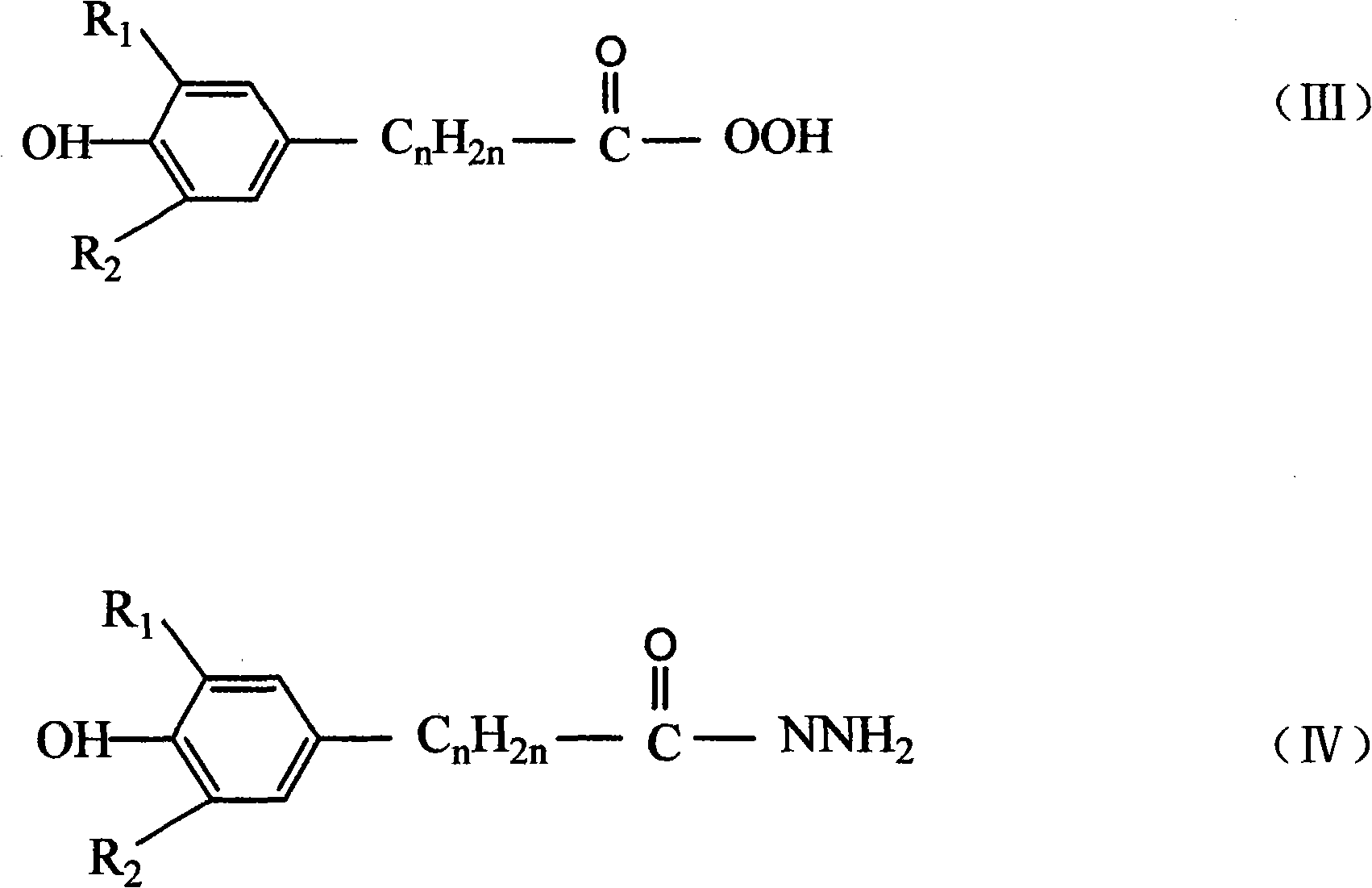
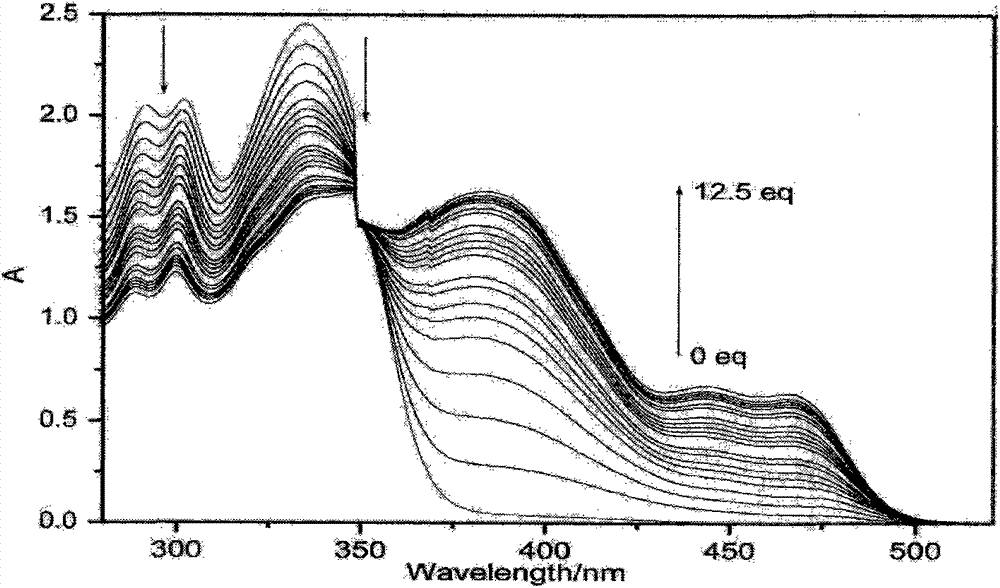
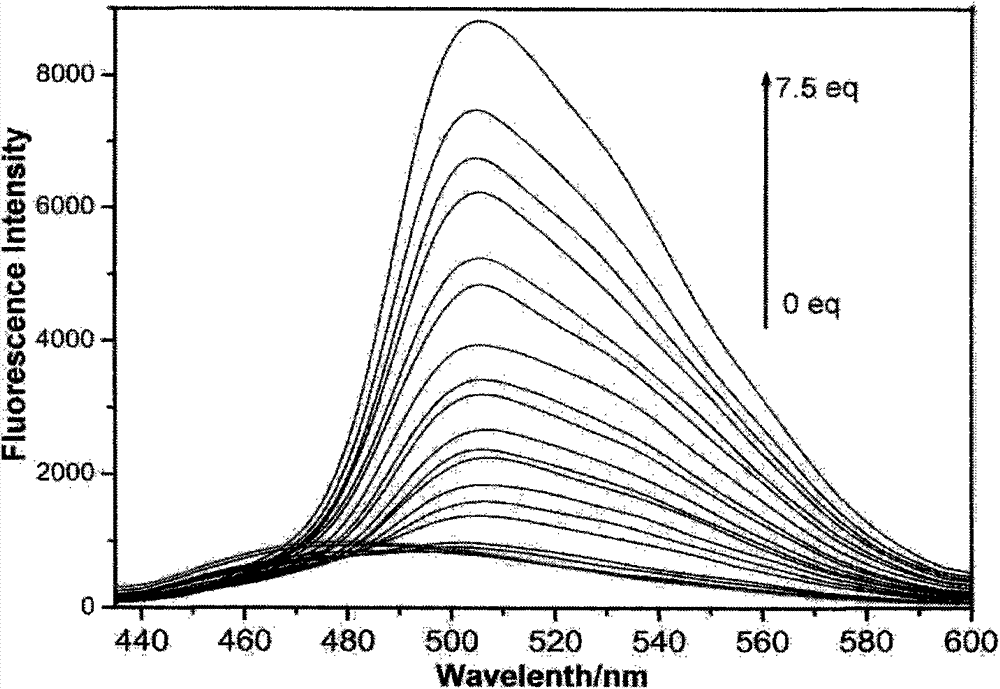
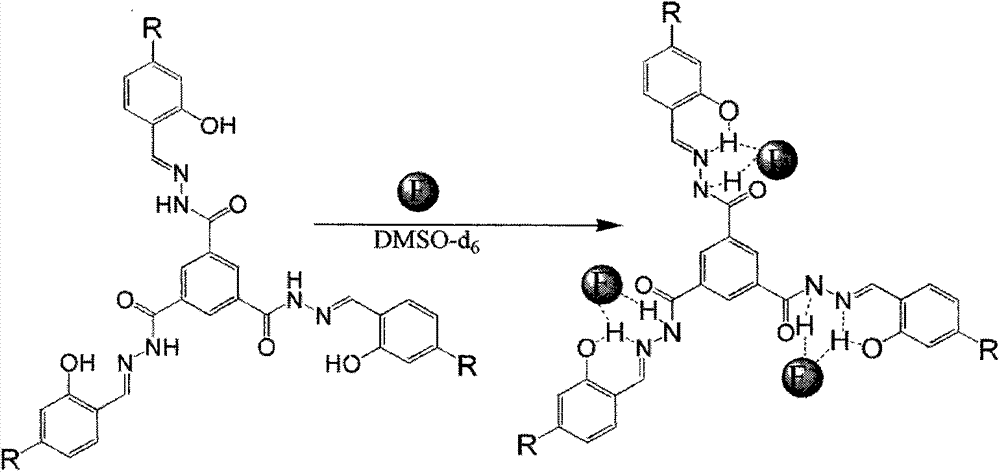
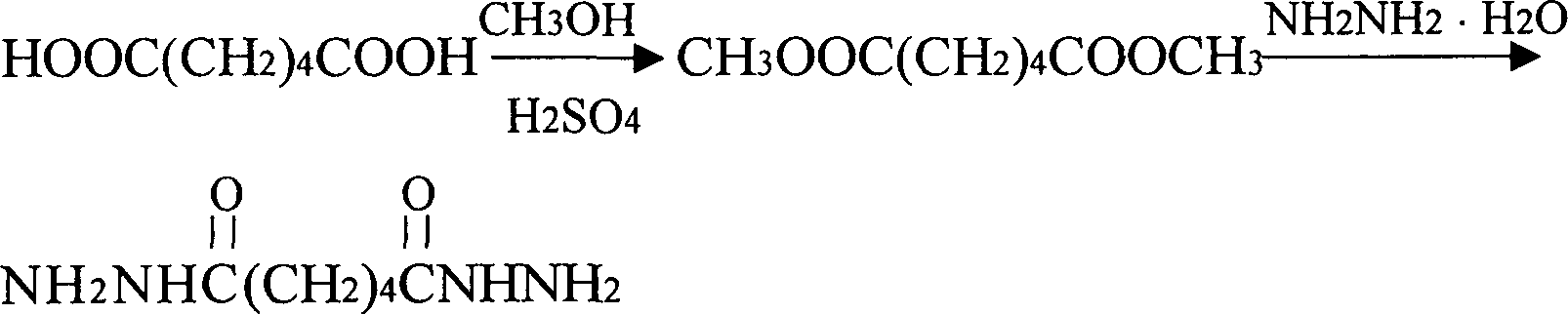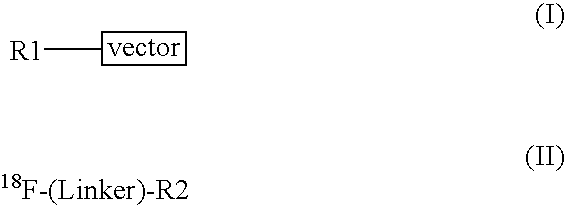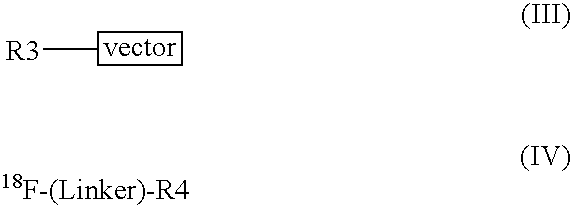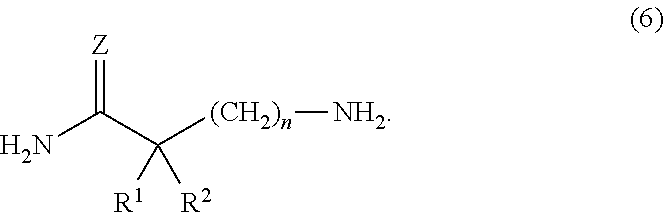Patents
Literature
274results about "Hydrazide preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
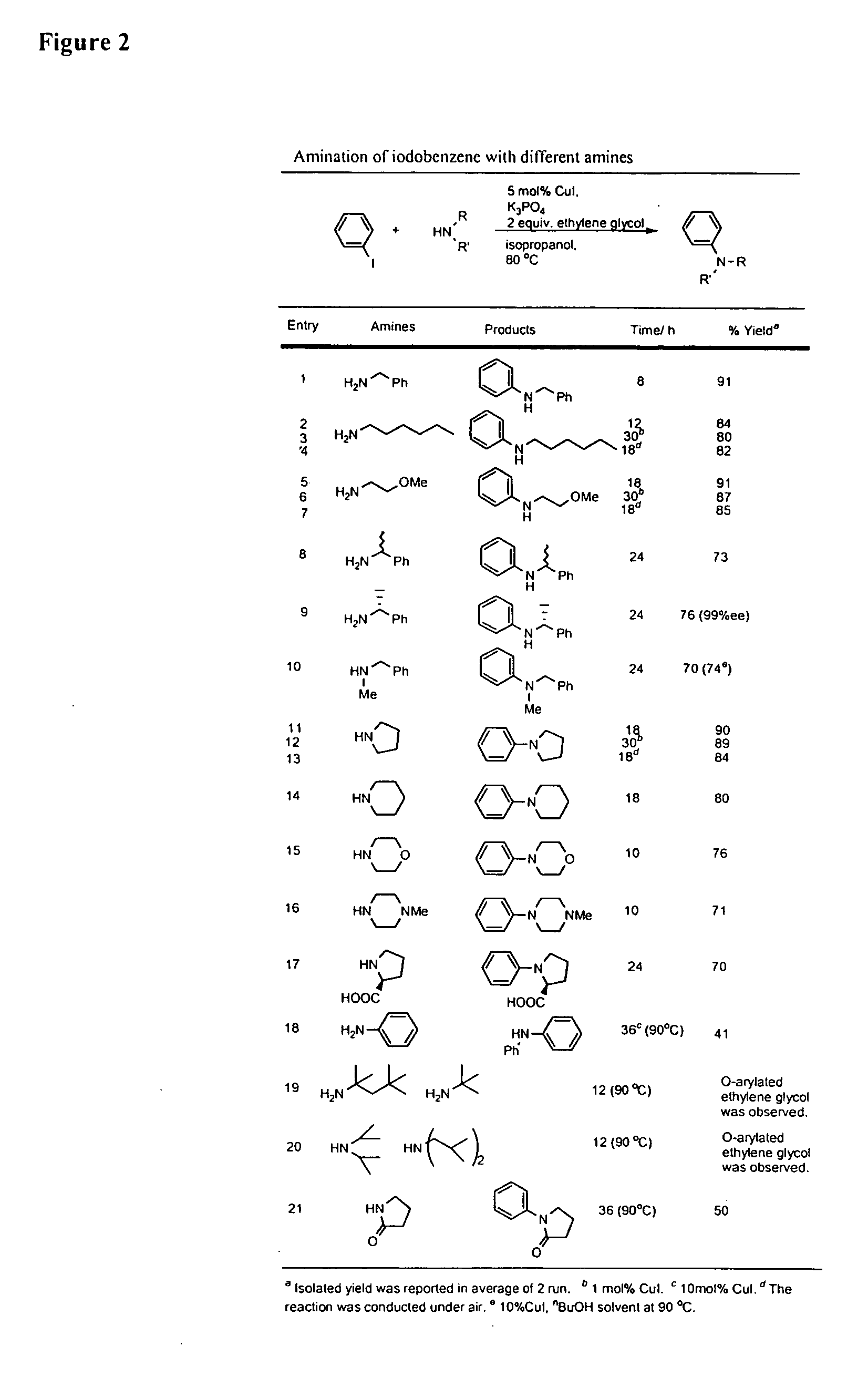
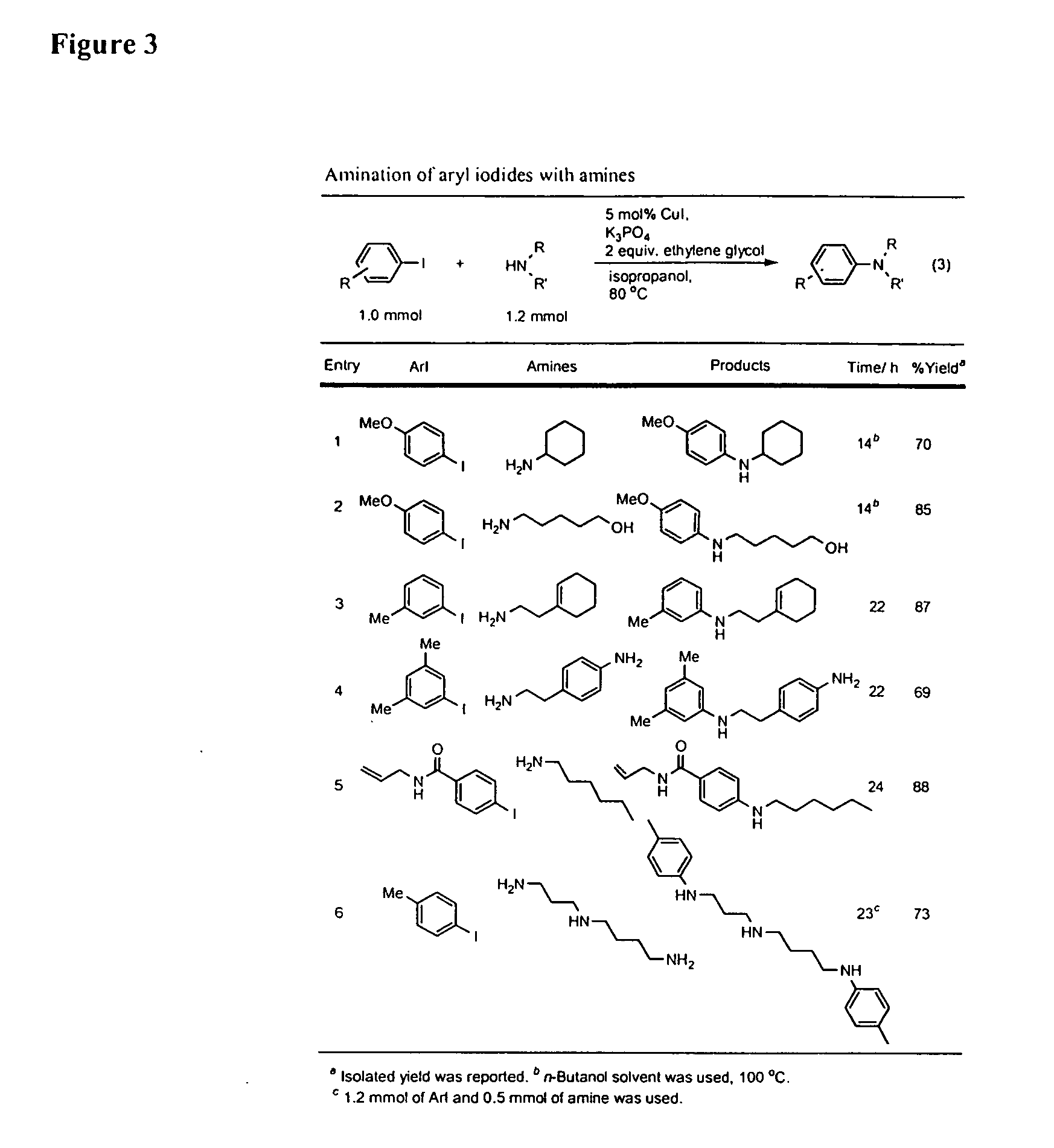
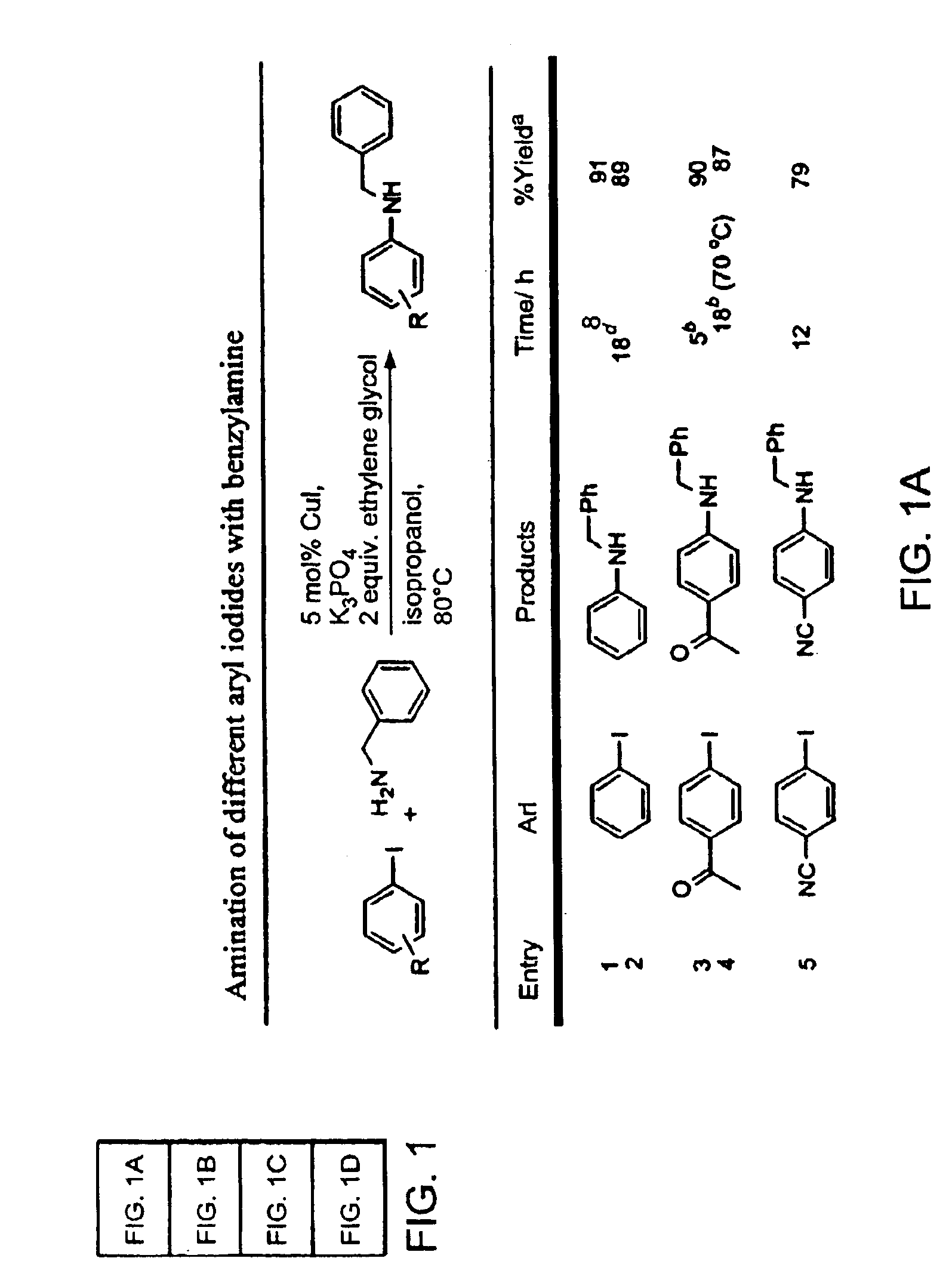
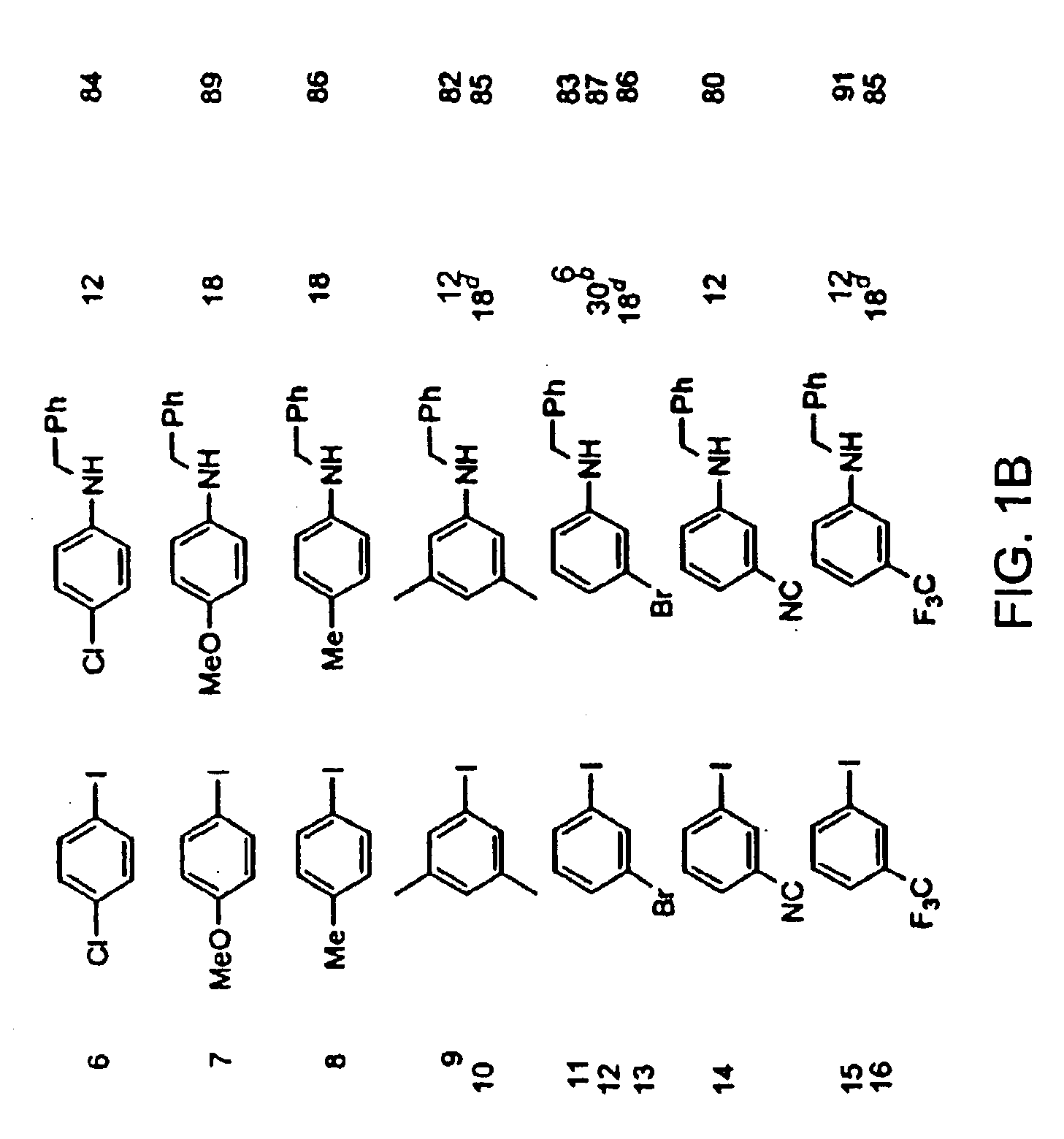
Copper-catalyzed formation of carbon-heteroatom and carbon-carbon bonds
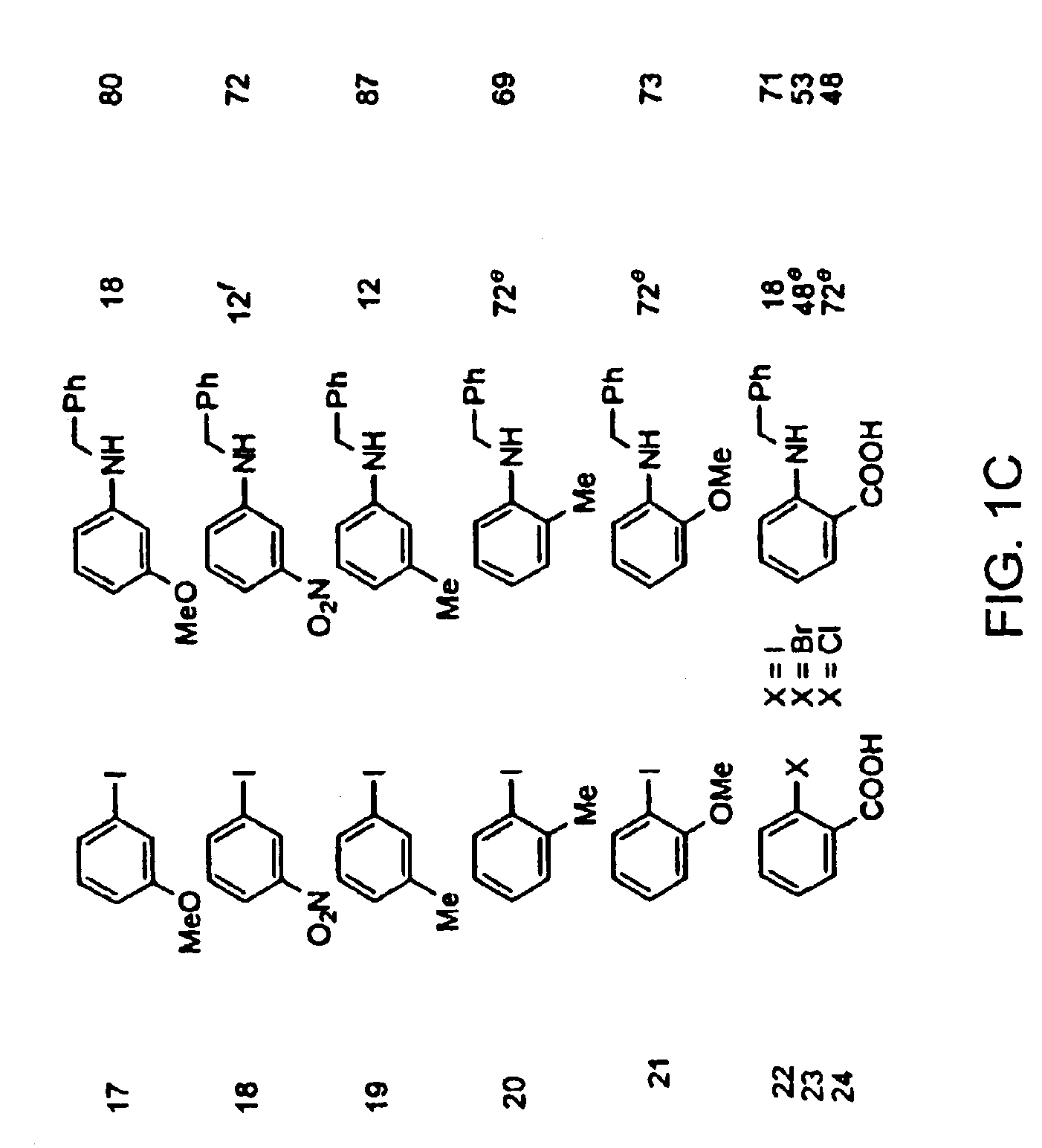
InactiveUS6867298B2Cheap and practicalLow costUrea derivatives preparationCarbamic acid derivatives preparationCarbon–oxygen bondHydrazine compound
The present invention relates to copper-catalyzed carbon-heteroatom and carbon-carbon bond-forming methods. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of an amide or amine moiety and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In additional embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between a nitrogen atom of an acyl hydrazine and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In other embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of a nitrogen-containing heteroaromatic, e.g., indole, pyrazole, and indazole, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-oxygen bond between the oxygen atom of an alcohol and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. The present invention also relates to copper-catalyzed methods of forming a carbon-carbon bond between a reactant comprising a nucleophilic carbon atom, e.g., an enolate or malonate anion, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. Importantly, all the methods of the present invention are relatively inexpensive to practice due to the low cost of the copper comprised by the catalysts.
Owner:MASSACHUSETTS INST OF TECH
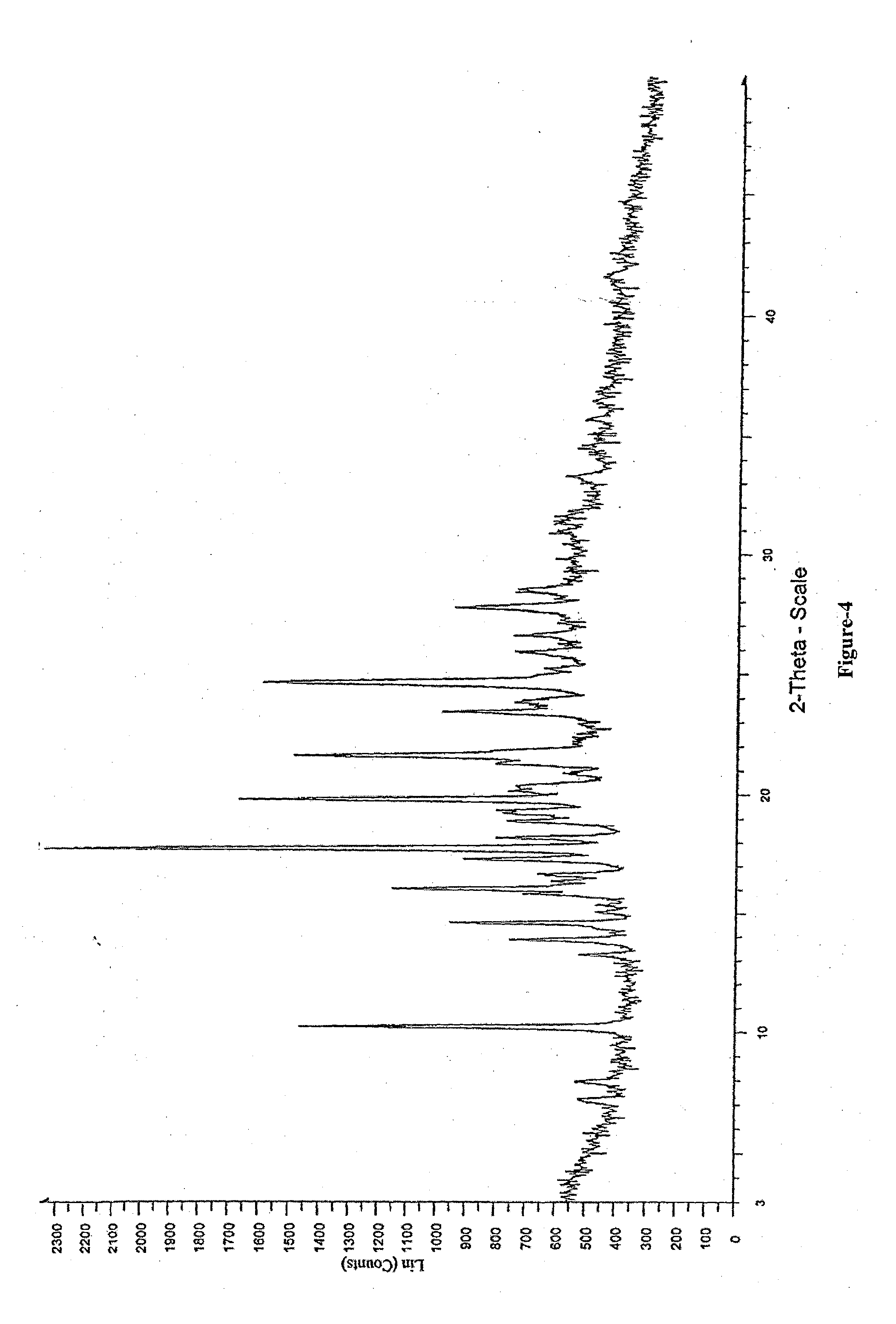
Synthetic method and application of covalent organic framework (COF) material
ActiveCN103755588AOrganic-compounds/hydrides/coordination-complexes catalystsHydrazide preparationMetal-organic frameworkNitromethane
The invention discloses a synthetic method of a covalent organic framework (COF) material. The method comprises the following steps: after mixing 1,3,5-benzenetricarboxaldehyde with 2,5-di(N,N-dimethyl)amino-1,4-benzdihydrazide uniformly in an organic solvent, reacting in the presence of a catalyst acetic acid to obtain the COF material, wherein the mole ratio of 1,3,5-benzenetricarboxaldehyde to 2,5-di(N,N-dimethyl)amino-1,4-benzdihydrazide is 1:(0.5-3). The COF material obtained by adopting the method has relatively large specific surface area and regular open framework structure with adjustable diameter, thus being beneficial for mass transfer of reactants and products in photoabsorption and catalytic processes; the material can serve as a photocatalyst and can increase the yield of the dehydrogenative coupling reaction between 2-phenyl-1,2,3,4-tetrahydroisoquinoline and nitromethane from 39% in the absence of catalysts to 89%.
Owner:LANZHOU UNIVERSITY
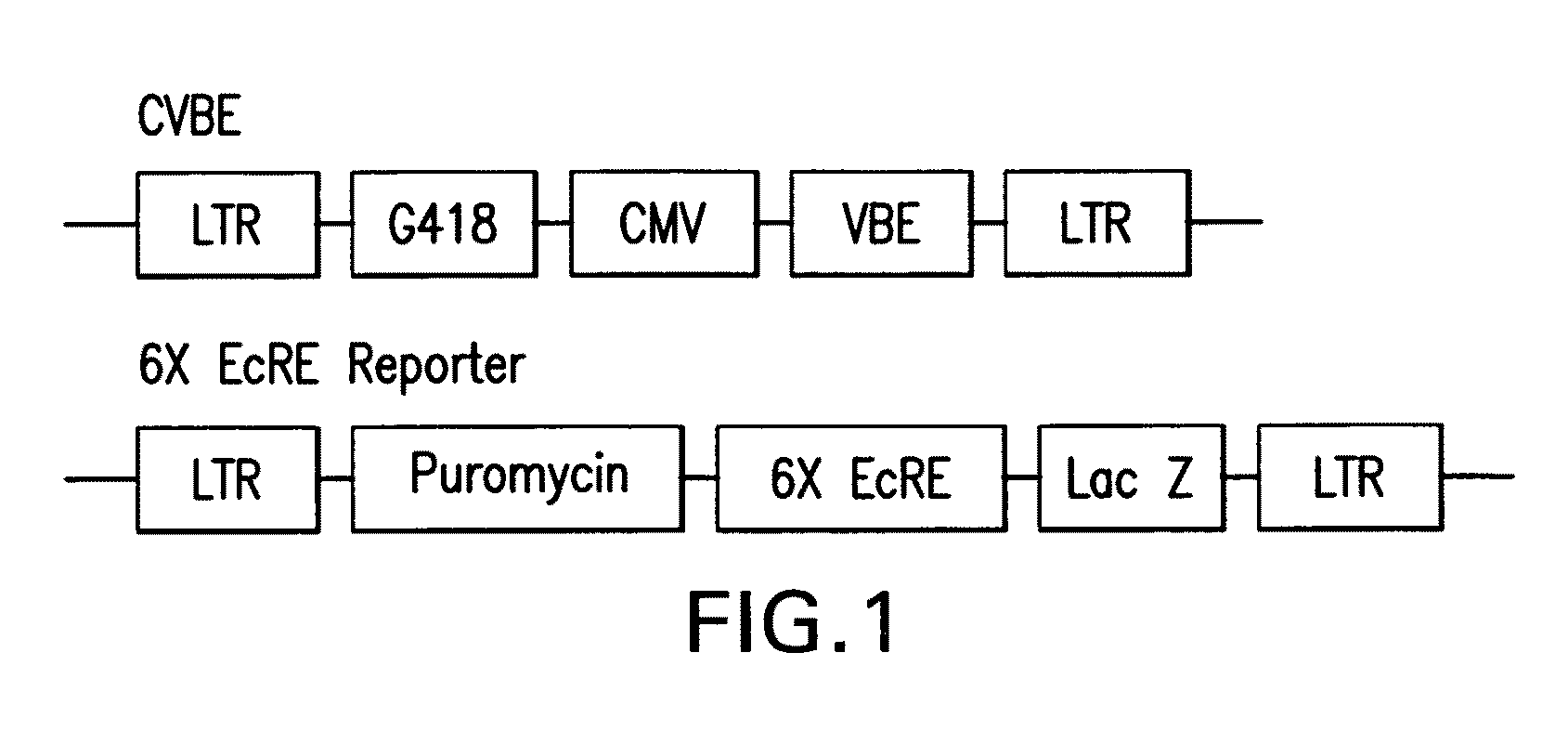
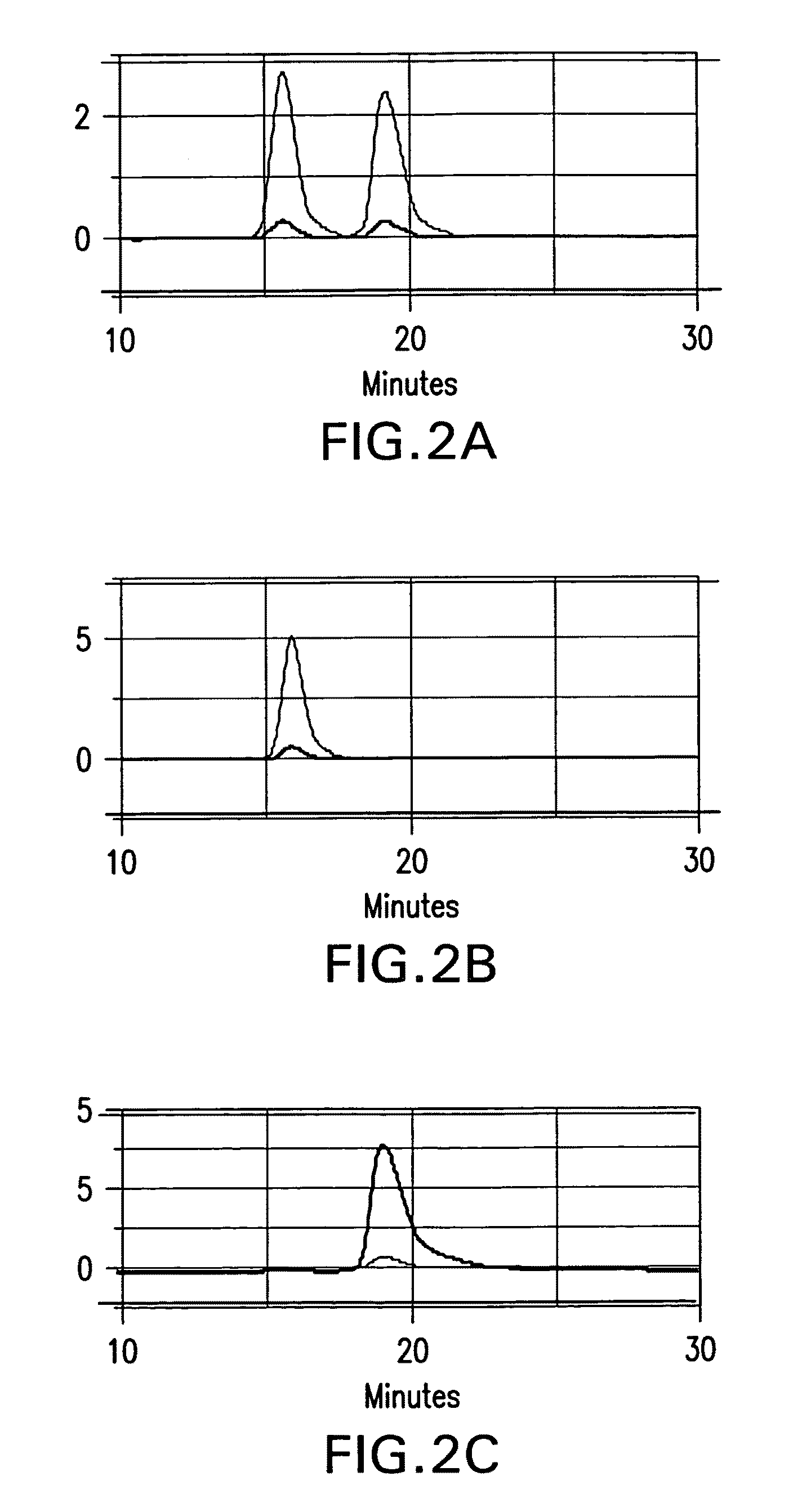
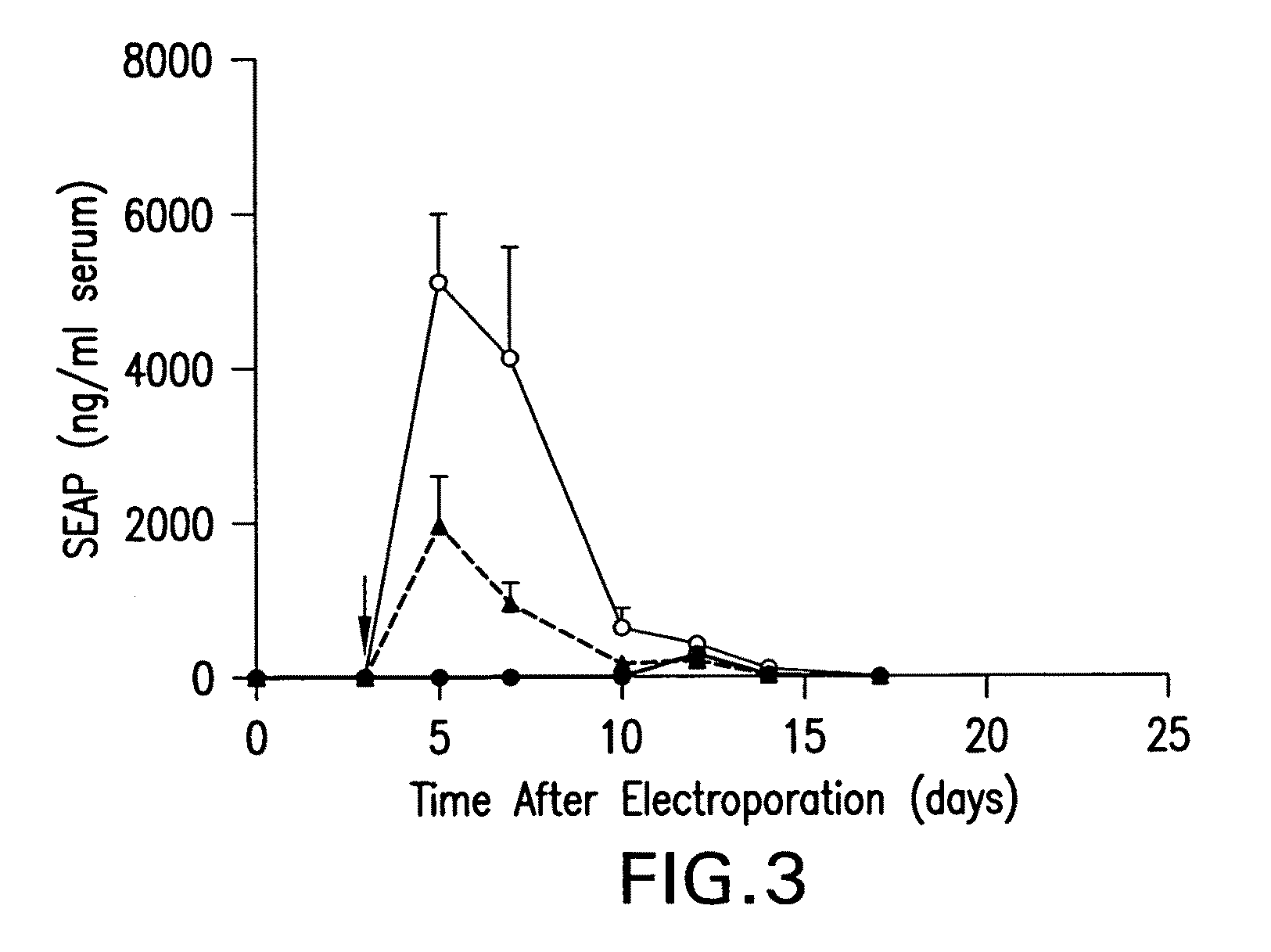
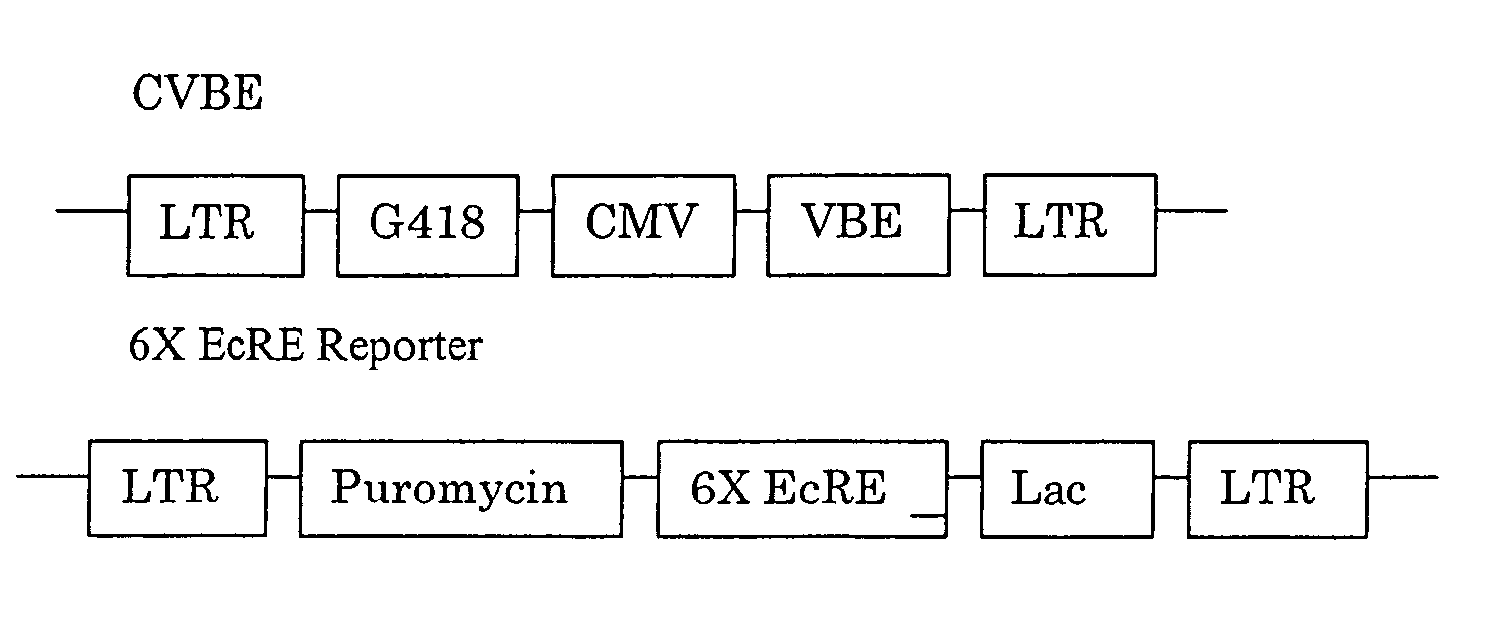
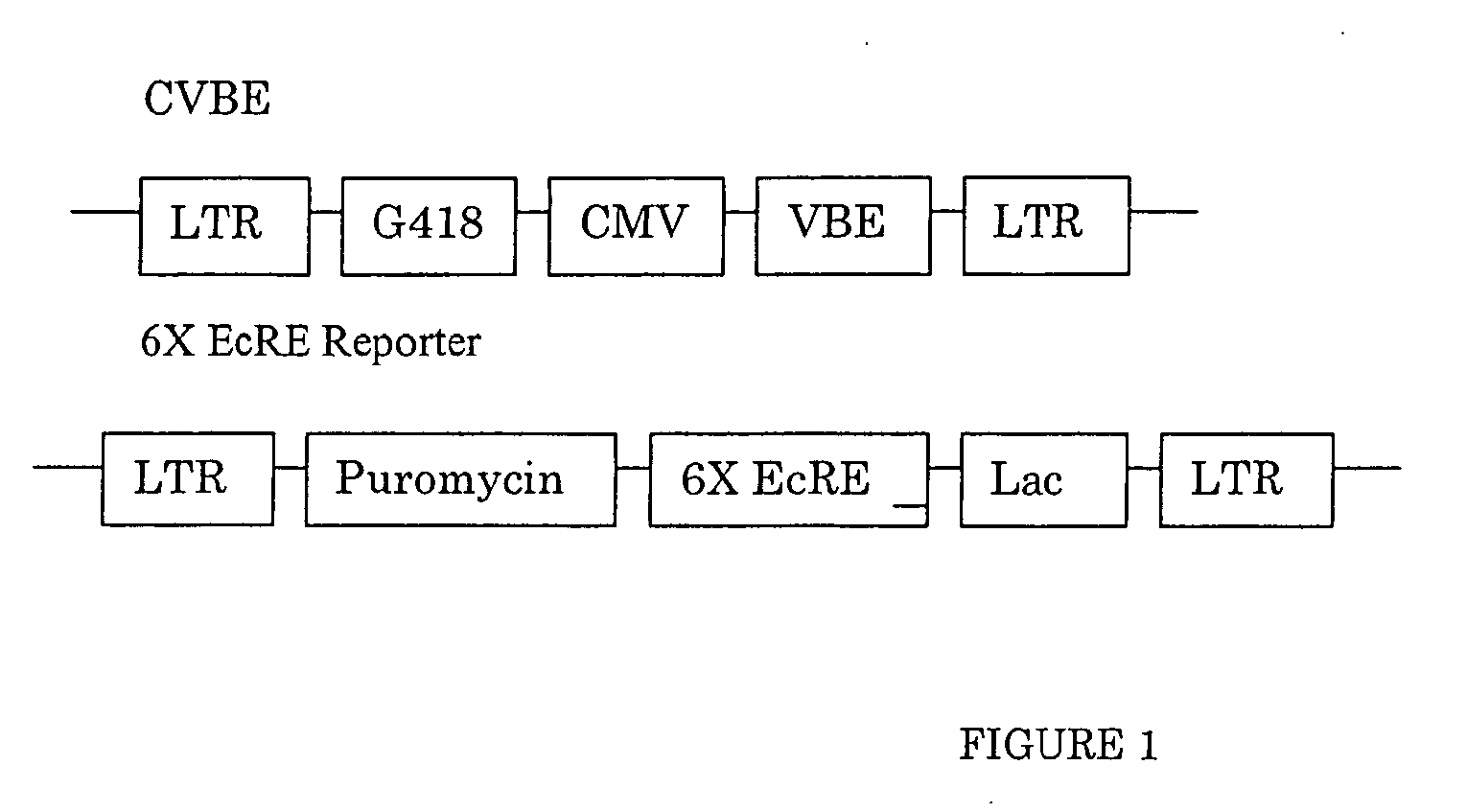
Chiral diacylhydrazine ligands for modulating the expression of exogenous genes via an ecdysone receptor complex
ActiveUS8076517B2Inhibit expressionUrea derivatives preparationOrganic active ingredientsGene expression levelOrganism
The present invention provides diacylhydrazine ligands and chiral diacylhydrazine ligands for use with ecdysone receptor-based inducible gene expression systems. Thus, the present invention is useful for applications such as gene therapy, large scale production of proteins and antibodies, cell-based screening assays, functional genomics, proteomics, metabolomics, and regulation of traits in transgenic organisms, where control of gene expression levels is desirable. An advantage of the present invention is that it provides a means to regulate gene expression and to tailor expression levels to suit the user's requirements.
Owner:PRECIGEN INC
Degradable hydrazide latent epoxy resin curing agent and application thereof
ActiveCN103193959AOptimize operating timeModerate viscosityHydrazide preparationPlastic recyclingEpoxyCrosslinked polymers
The invention provides a degradable hydrazide latent epoxy resin curing agent. The curing agent can be polymerized with epoxy resin to generate a degradable crosslinked polymer; and the degradable crosslinked polymer can be degraded in a mixed system of an acid and a solvent under the conditions of heating and stirring. The invention also provides a prepreg, consisting of the curing agent, the epoxy resin, an auxiliary material and a reinforcing material. The invention also provides a reinforcing composite material which is prepared from the curing agent, the epoxy resin, the auxiliary material and the reinforcing material. The reinforcing composite material can be prepared by a prepreg forming method. The reinforcing composite material can be degraded in the mixed system of the acid and the solvent under the conditions of heating and stirring, and can be recycled and reused after being neutralized. The reinforcing composite material has excellent mechanical property and is suitable for the field of application of different composite materials; and the degrading and recycling method of the reinforcing composite material is simple, economic, mild in condition and easy to control.
Owner:芜湖天道绿色新材料有限公司
Process for the Preparation of Triazole Antifungal Drug, Its Intermediates and Polymorphs Thereof
ActiveUS20140343285A1Extended reaction timeLow yieldOrganic compound preparationOrganic chemistry methodsTriazole antifungalsAntifungal
Owner:MSN LAB PTE LTD
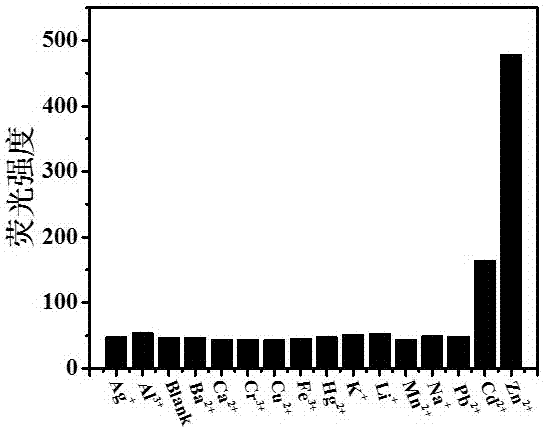
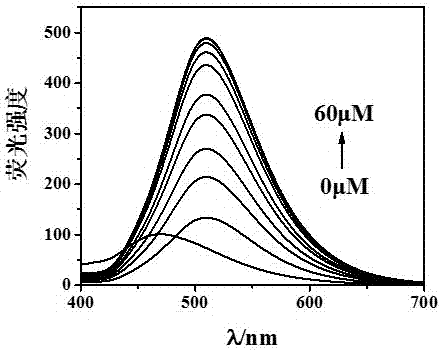
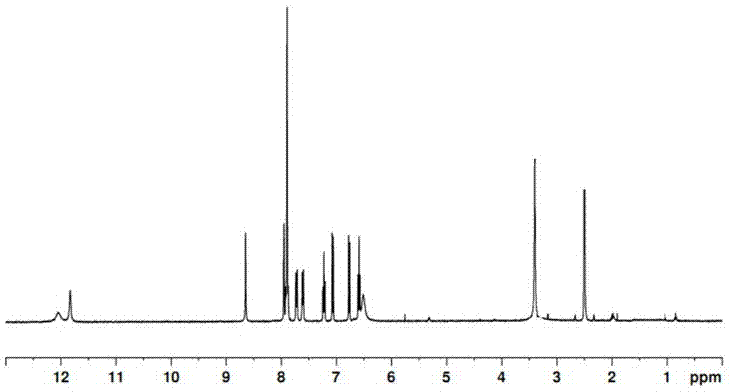
Preparation and application of zinc ion fluorescence probe based on Schiff base
InactiveCN106966924ALow detection limitHigh sensitivityCarboxylic acid nitrile preparationOrganic compound preparationFluorescenceHydrazine compound
The invention relates to preparation and application of a zinc ion fluorescence probe based on Schiff base. A zinc ion fluorescence probe compound has the structure shown by a formula I. The preparation method comprises the steps that 2-aminobenzoic acid (methyl anthranilate) and hydrazine hydrate are subjected to heating reflux in ethyl alcohol to obtain a white solid II, cyanophenyl phenol, anhydrous magnesium chloride and paraformaldehyde are subjected to heating reflux in a triethylamine-containing acetonitrile solution to obtain a white solid III, the white solid II and the white solid III are subjected to heating reflux in ethyl alcohol to a yellow solid I. The probe compound has very good selectivity and sensitivity on zinc ions, is low in detection limit and can be applied to determination of the content of zinc ions in a water body.
Owner:UNIV OF JINAN
Bioavailable diacylhydrazine ligands for modulating the expression of exogenous genes via an ecdysone receptor complex
The present invention relates to non-steroidal ligands for use in nuclear receptor-based inducible gene expression system, and a method to modulate exogenous gene expression in which an ecdysone receptor complex comprising: a DNA binding domain; a ligand binding domain; a transactivation domain; and a ligand is contacted with a DNA construct comprising: the exogenous gene and a response element; wherein the exogenous gene is under the control of the response element and binding of the DNA binding domain to the response element in the presence of the ligand results in activation or suppression of the gene.
Owner:RHEOGENE INC DE
Double-hindered phenol structure-contained hydrazides compound and preparation method thereof
ActiveCN102344389AHigh molecular weightEnhance thermal oxidation resistanceHydrazide preparationPtru catalystOrganosolv
The invention discloses a double-hindered phenol structure-contained hydrazides compound for a light stabilizer and a preparation method thereof. The double-hindered phenol structure-contained hydrazides compound has a double-hindered phenol and double-hydrazide structure, thus, the molecular weight is increased, and the migration resistance and thermal oxidation resistance capacity is strong. The preparation method comprises the following steps: performing addition reaction on hindered phenols and alpha, beta-acrylic ester under the action of a catalyst to obtain an addition product, wherein the molar ratio of the hindered phenols to the alpha, beta-acrylic ester is 1.0:(0.6-1.8), and the catalyst is alkali metal, an alkali metal hydroxide, an alkali metal hydride or an alkali metal alcoholate; then, performing carbonyl nucleophilic substitution reaction on the addition product and a hydrazine hydrate in an organic solvent A to obtain a hydrazides substitution product, wherein the molar ratio of the addition product to the hydrazine hydrate is 1.0:(0.8-5.0); performing the reaction between a diisocyanate compound and the hydrazides substitution product at the temperature of 0-10 DEG C; and carrying out recrystallization to obtain a goal product. By adopting the preparation method disclosed by the invention, the operation is simple, the raw materials are available, and the cost is low.
Owner:YANTAI RUILONG CHEM TECH CO LTD
Catalytic preparation method for hindered phenol derivative antioxygen
InactiveCN102050760AReduce usageReduce utilizationHydrazide preparationPolyolefinReaction temperature
The invention discloses a catalytic preparation method for hindered phenol derivative antioxygen. In the invention, a catalytic dehydration process is adopted to prepare the hindered phenol derivative antioxygen, the reaction raw materials are subjected to acidification and monohydrazidation respectively to form two intermediates, and thionyl chloride is not used so as to reduce pollution; and then a solvent and two intermediates are added into a reactor and heated and refluxed to undergo dehydration in the presence of a catalyst, and water is separated out by a water segregator. In the invention, due to the adoption of the catalyst, the reaction temperature is lower, the raw material utilization rate is high, the reaction time is short, and the product yield is high; therefore, the method can contribute to the reduction of production cost and is suitable for industrial production. Evaluation on the use of the antioxygen in polyolefin wire and cables shows that the antioxygen prepared by the method disclosed by the invention has high antioxygen performance and metal passivating capacity.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of trimesoyl hydrazone series derivatives and application of trimesoyl hydrazone series derivatives as probe molecules for identifying fluorine ions
InactiveCN103497121ASimple processHigh yieldHydrazide preparationColor/spectral properties measurementsSalicylaldehydeP-Toluenesulfonic acid
The invention relates to a preparation method of trimesoyl hydrazone series derivatives and application of the trimesoyl hydrazone series derivatives as probe molecules for identifying fluorine ions. The preparation method comprises the steps of dissolving trimesic acid, concentrated sulfuric acid and ethanol according to the mass ratio of 1:0.25:20 into ethanol, and refluxing for 48 hours to obtain a white product trimesic acid ethyl ester; dissolving trimesic acid ethyl ester, hydrazine hydrate and methanol according to the mass ratio of 1:0.5:5 into methanol, cooling reaction liquid to room temperature when points of raw materials do not exist on a thin-layer plate, and precipitating a large amount of white solid trimesoyl hydrazine; dissolving trimesoyl hydrazine and salicylaldehyde derivatives according to the molar ratio of 1:4.5 into a proper amount of methanol, adding p-toluenesulfonic acid serving as a catalyst, refluxing for 12 hours, and then cooling to room temperature to obtain fluorescent probe molecules.
Owner:HENAN POLYTECHNIC UNIV
Preparation of supermolecule gel factor based on three-column [5] aromatic hydrocarbon and organic gel thereof and application
InactiveCN108658804ATo achieve the recognition effectHydrazide preparationFluorescence/phosphorescenceOn columnCyclohexanol
The invention discloses preparation of a supermolecule gel factor based on three-column [5] aromatic hydrocarbon. The supermolecule gel factor is a complex organic gel factor which is synthesized fromamide modified column [5] aromatic hydrocarbon and trimesoyl chloride through a nucleophilic substitution reaction and is based on column aromatic hydrocarbon. According to the gel factor, blue organic gel with aggregation state induction florescence can be formed in cyclohexanol through C-H...pi,pi...pi accumulation and Van der Waals' force. In the organic gel, water solutions of Hg<2+>, Ca<2+>,Mg<2+>, Ni<2+>, Cr<3+>, Cd<2+>, Pb<2+>, Ag<+>, Zn<2+>, Ba<2+>, La<3+>, La<3+>, Eu<3+> and Tb<3+> are respectively added, only addition of Hg<2+> can quench blue florescence of the organic gel, and addition of other cationic ions does not remarkably influence fluorescence intensity of the organic gel, so that specific fluorescence detection on Hg<2+> can be achieved, and a detection limit is 1.02*10<-8>M.
Owner:NORTHWEST NORMAL UNIVERSITY
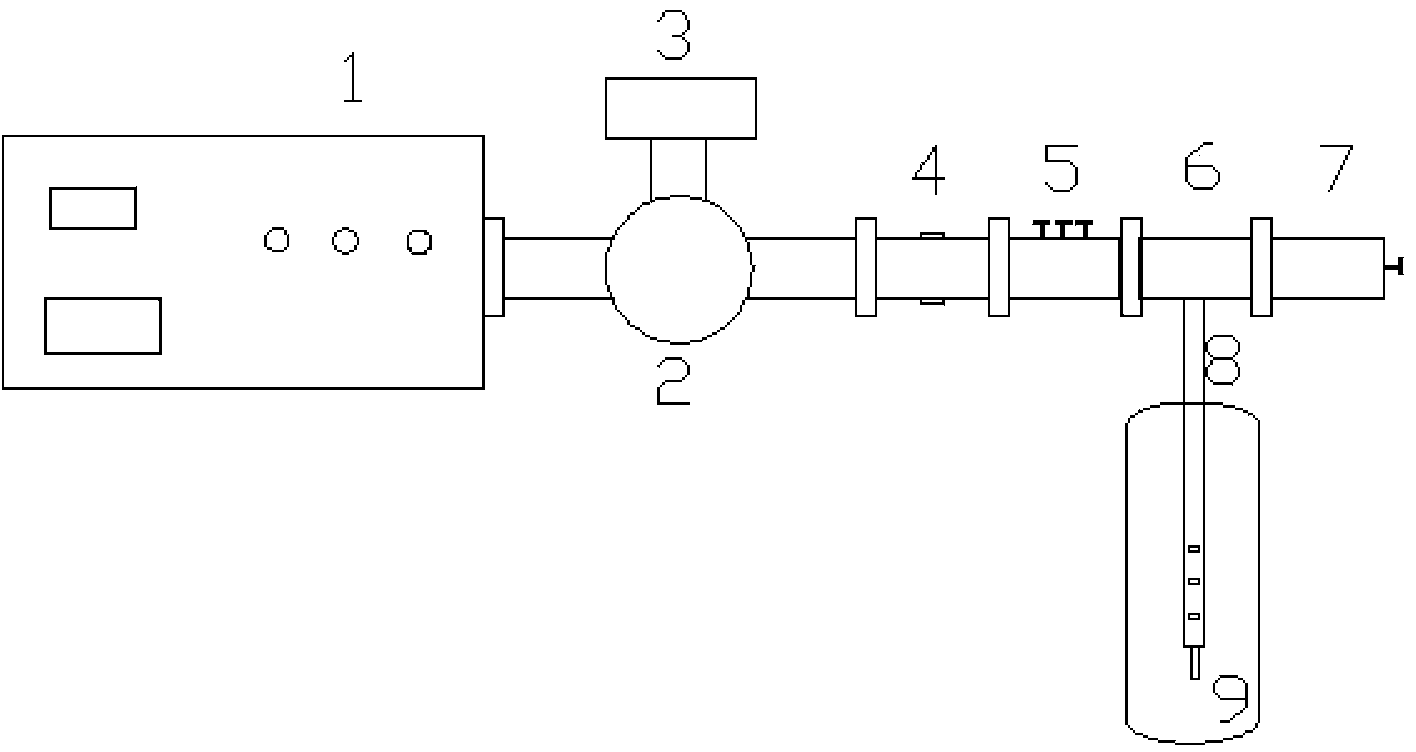
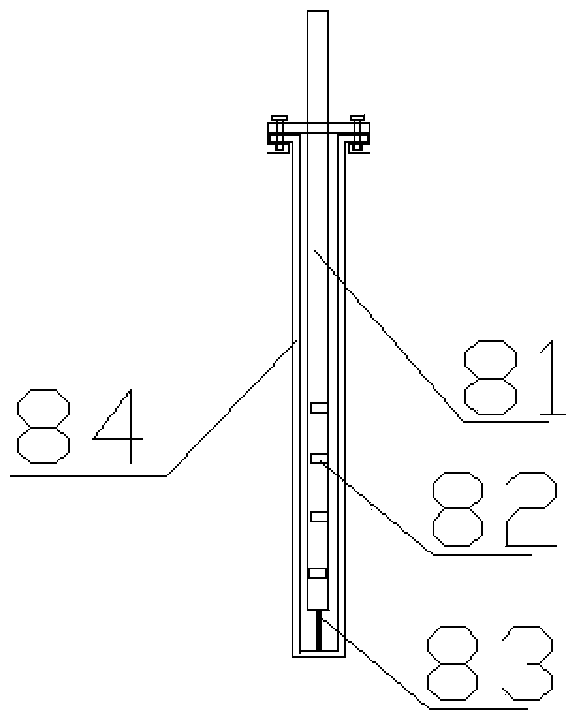
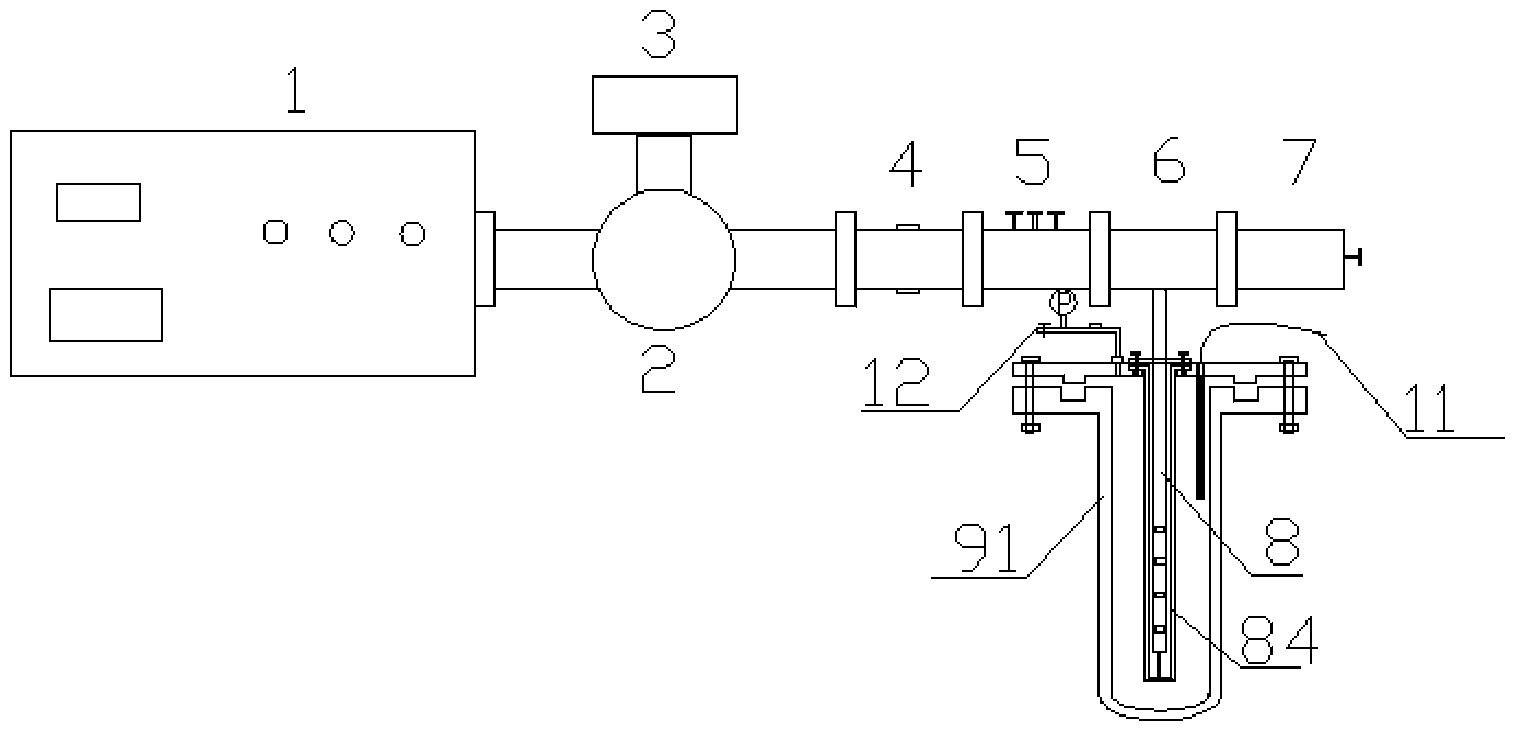
Microwave reaction device using coaxial leaky antenna and application of microwave reaction device using coaxial leaky antenna
InactiveCN104383866AUniform microwave radiationCarboxylic acid nitrile preparationOrganic compound preparationEngineeringMicrowave power
The invention provides a microwave reaction device using a coaxial leaky antenna and application of the microwave reaction device using the coaxial leaky antenna. The device comprises a microwave heating device and a reactor, wherein the microwave heating device comprises a microwave generation and transmission device and the coaxial leaky antenna; the microwave generation and transmission device comprises a microwave power source, an annular device, a false load, a microwave power detection device, a coaxial converter and a microwave tuning device; the coaxial leaky antenna is connected to the coaxial converter, and extends into from any end of the reactor, so that microwaves are fed back into the reactor. The device provided by the invention is high in microwave energy coaxial conversion efficiency, is uniform in microwave distribution in the reactor, can be applied to any reaction for heating by utilizing the microwaves, and is especially suitable for large-scale microwave reaction.
Owner:EAST CHINA UNIV OF SCI & TECH
Copper-catalyzed formation of carbon heteroatom and carbon-carbon bonds
InactiveUS20050215794A1Cheap and practicalLow costUrea derivatives preparationCarbamic acid derivatives preparationCarbon–oxygen bondHydrazine compound
The present invention relates to copper-catalyzed carbon-heteroatom and carbon-carbon bond-forming methods. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of an amide or amine moiety and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In additional embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between a nitrogen atom of an acyl hydrazine and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In other embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of a nitrogen-containing heteroaromatic, e.g., indole, pyrazole, and indazole, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-oxygen bond between the oxygen atom of an alcohol and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. The present invention also relates to copper-catalyzed methods of forming a carbon-carbon bond between a reactant comprising a nucleophilic carbon atom, e.g., an enolate or malonate anion, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. Importantly, all the methods of the present invention are relatively inexpensive to practice due to the low cost of the copper comprised by the catalysts.
Owner:MASSACHUSETTS INST OF TECH
Aggregation luminescence enhancement ammonia sensitive metal-organic gel compound soft material and preparation thereof
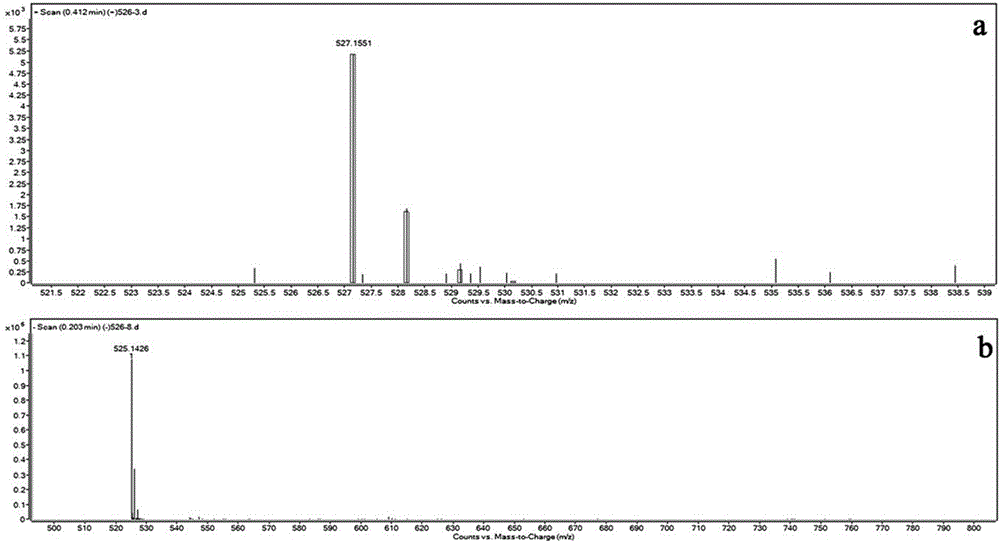
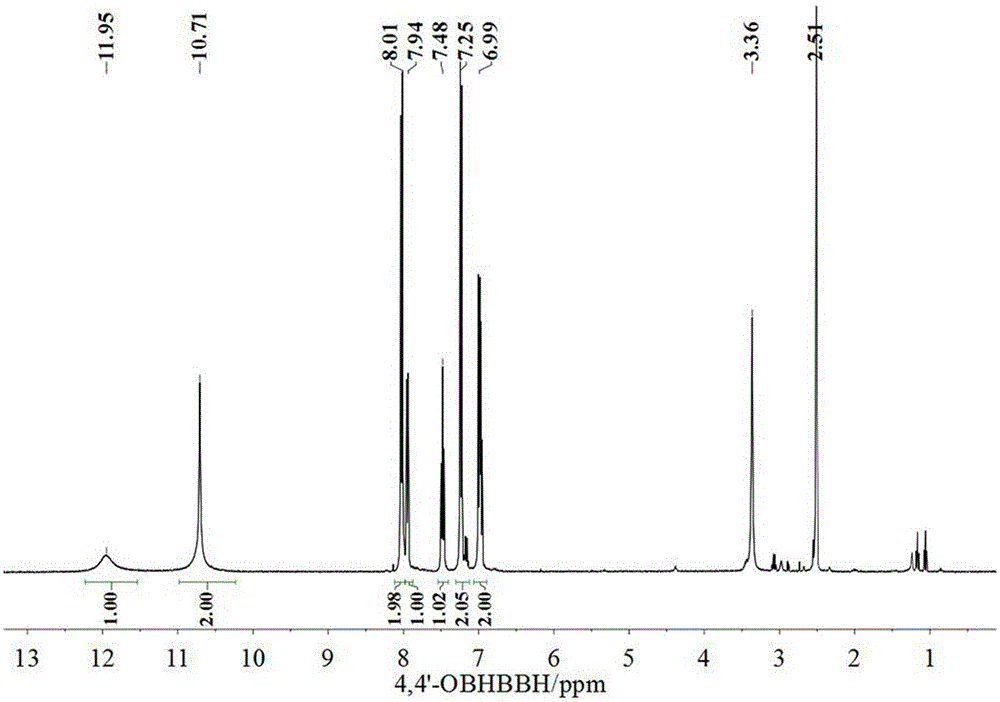
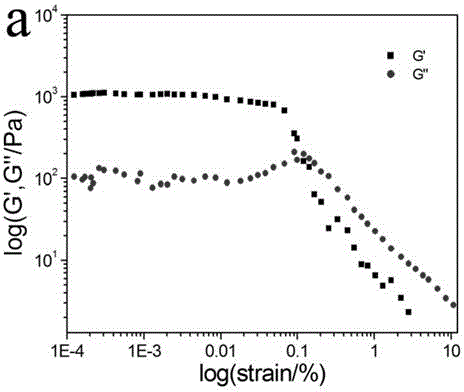
InactiveCN104829487AImprove stabilityReduce manufacturing difficultyOther chemical processesHydrazide preparationPhenacylOrganic solvent
The present invention discloses an aggregation luminescence enhancement ammonia sensitive metal-organic gel compound soft material and a preparation method thereof. According to the invention, the gel compound soft material is prepared from newly-synthesized 4, 4 '-oxybis-N '-(2-hydroxy benzoyl) benzoyl hydrazine as a ligand and zinc acetate hydrate in the presence of a polar organic solvent at room temperature, and the gel mass fraction is about 1.3 wt % (solute / solvent* 100%). The gel material has a significant aggregation luminescence enhancement effect and ammonia gas sensitive luminescence response function, also has the functions of selective adsorption of dyes and in-situ preparation of nano gold particles and the like. The preparation method is simple and easy to operate, the raw material price is moderate, and the production cost is low.
Owner:FUZHOU UNIV
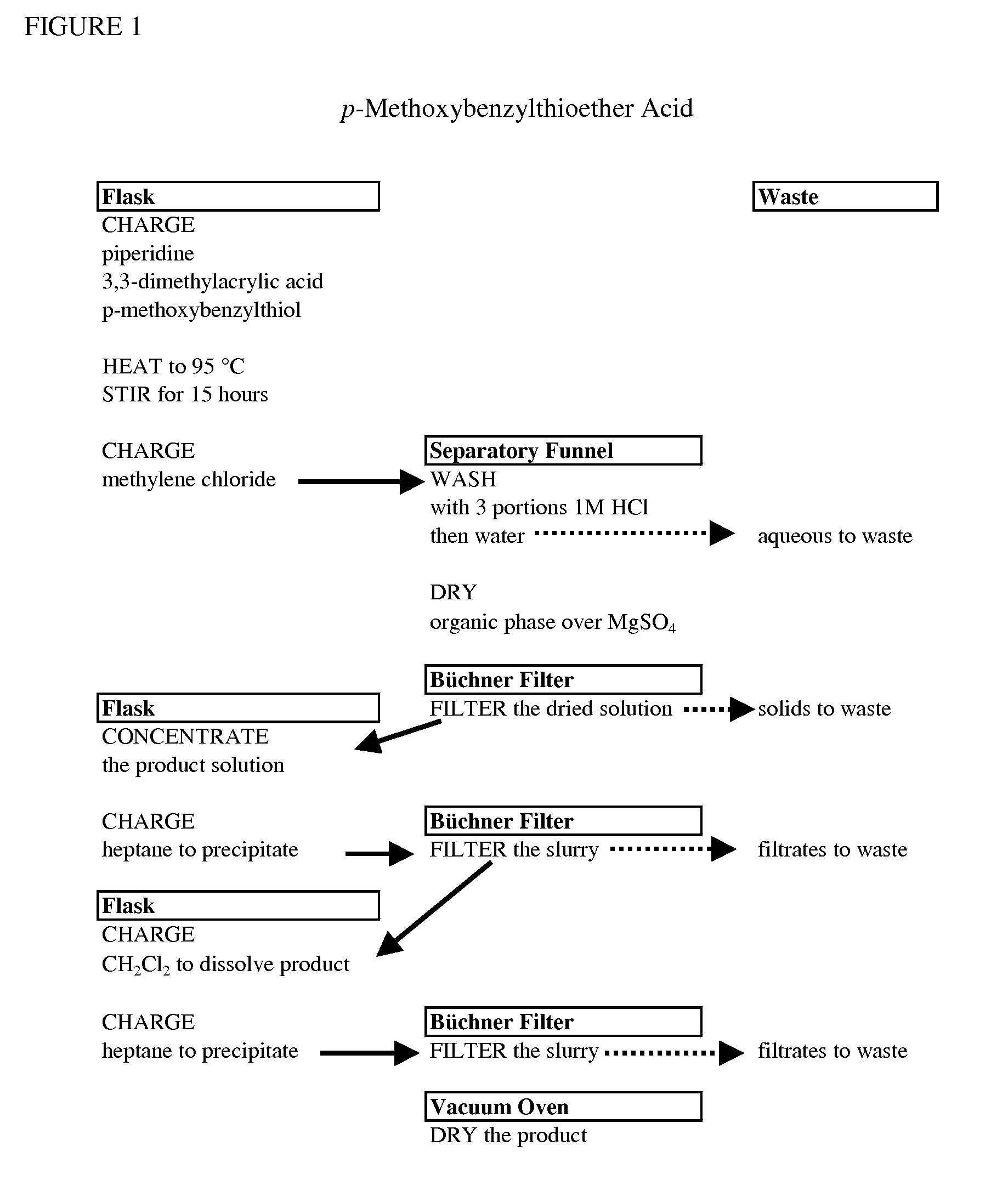
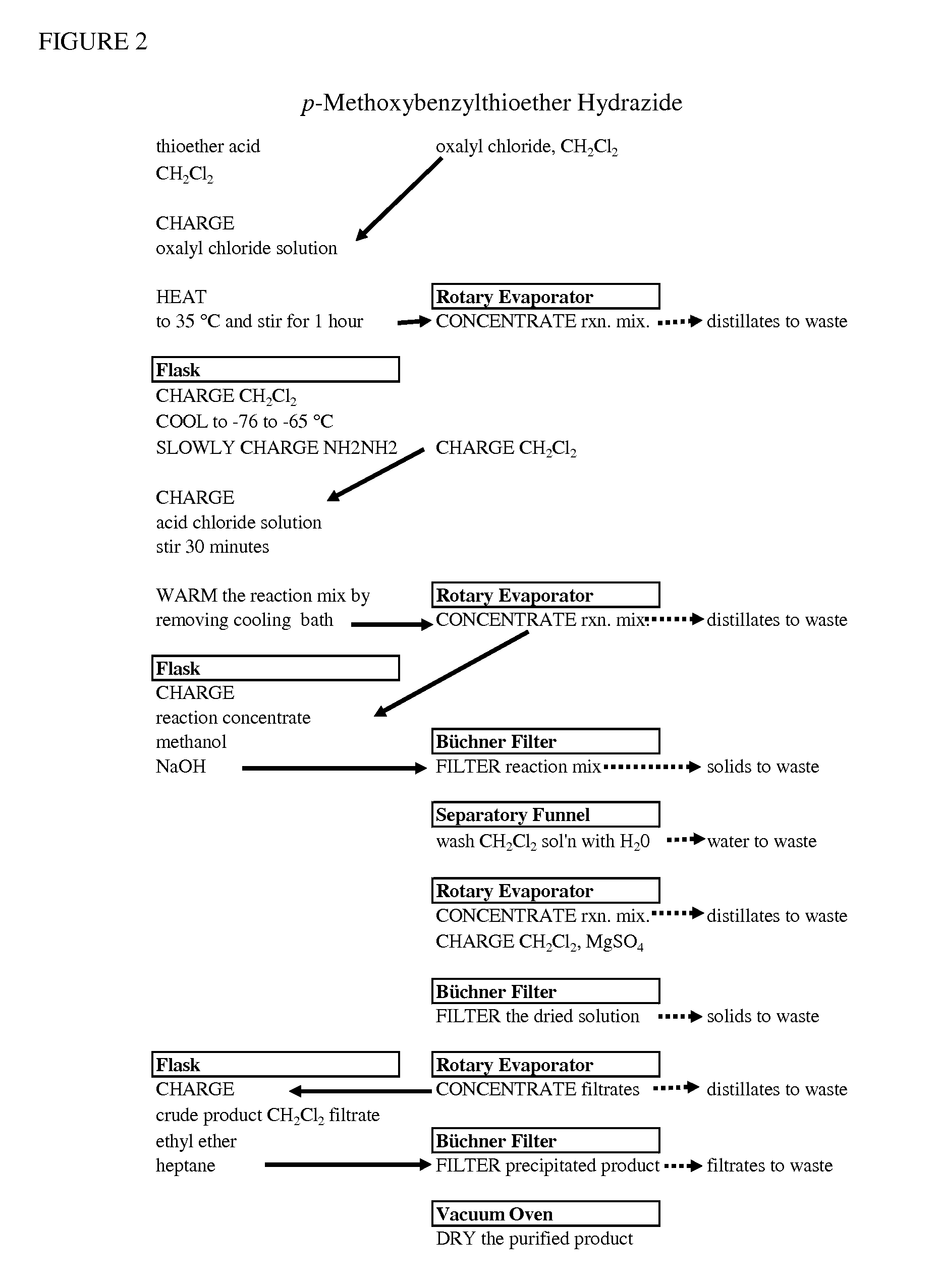
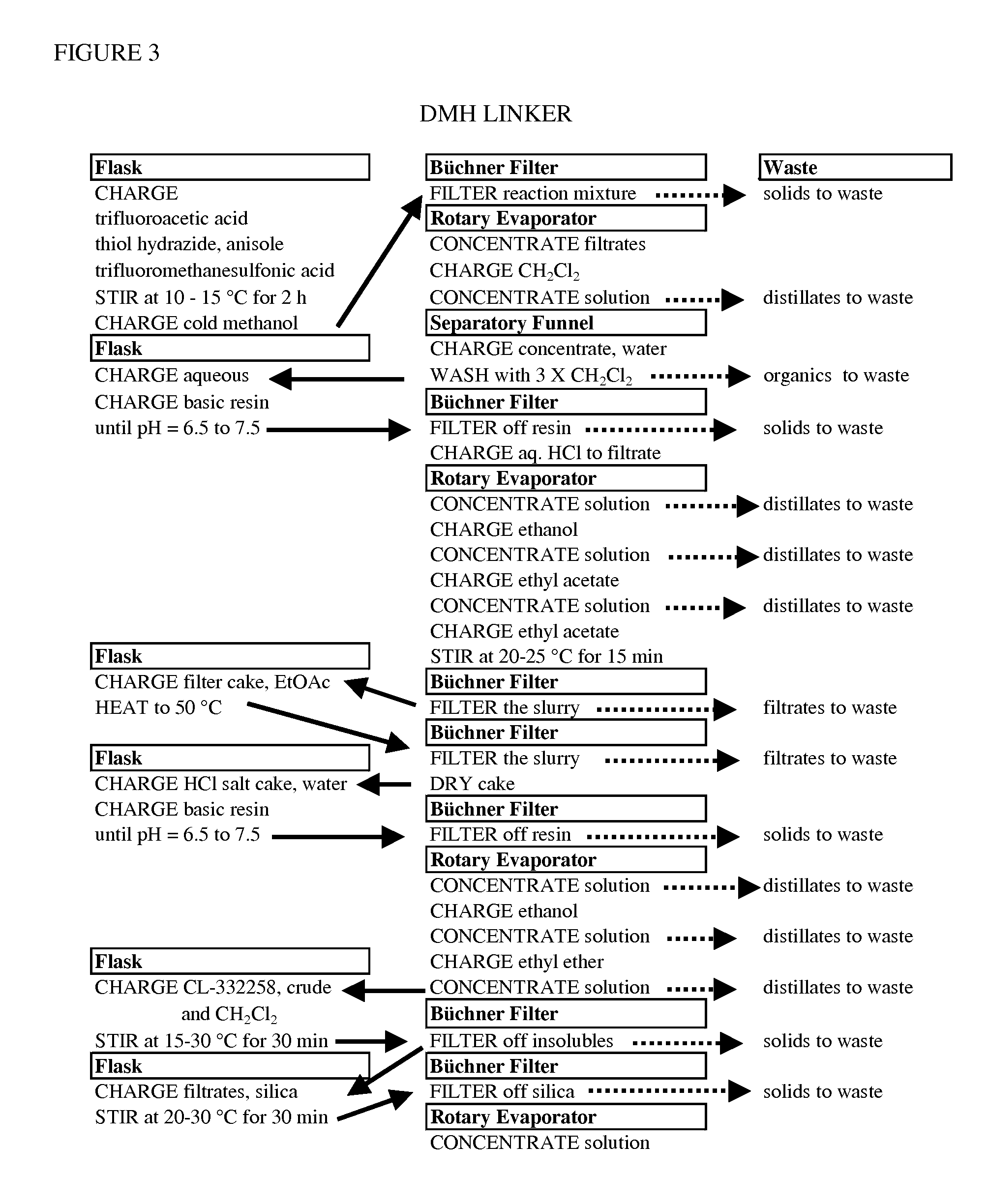
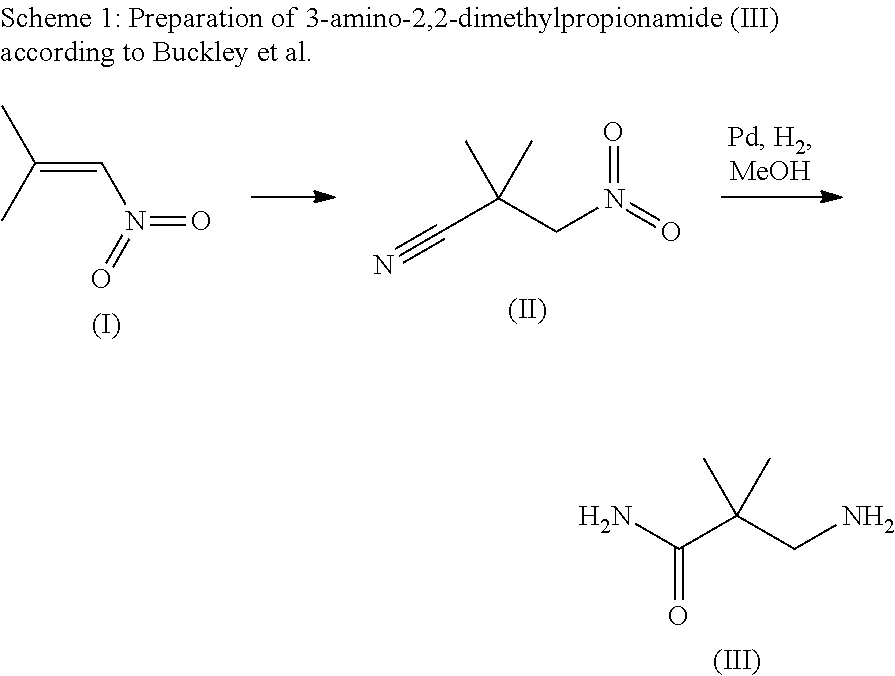
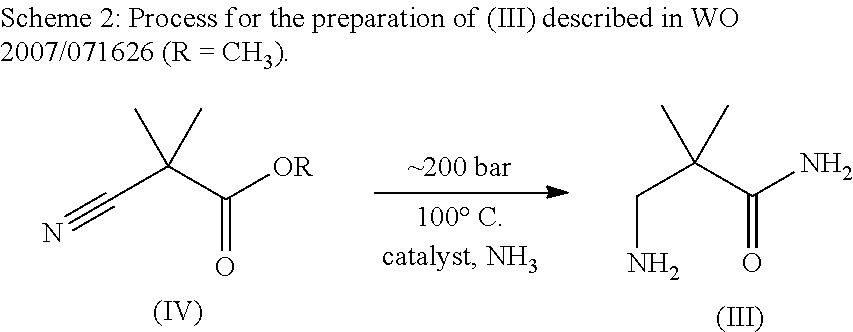
Processes for making hydrazides
ActiveUS8110705B2Minimize formationReduces and eliminates needOrganic compound preparationThiol preparationSlurrySolvent
A method is disclosed for preparing hydrazides from hydrazine and an acyl chloride which comprises the steps of (a) preparing a stirred substantially uniform slurry comprising hydrazine and an inert solvent at low temperature; and (b) adding an acyl chloride continuously to said slurry. The method avoids or limits production of undesired bis-hydrazide by-products. The method is used to prepare 3-methyl-3-mercaptobutanoic acid hydrazide, a molecule used to link calicheamicin to a monoclonal antibody.
Owner:WYETH LLC
Organogelator, organogel with double responses and preparation method of organogel
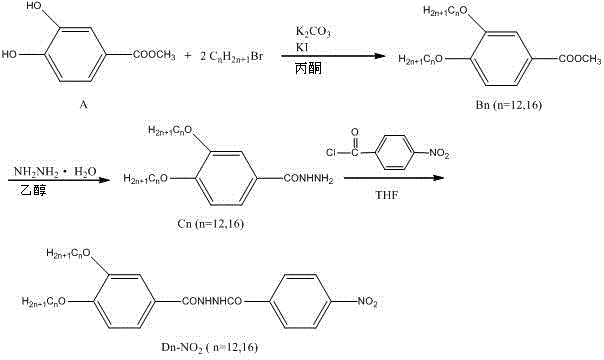
InactiveCN106565533AImprove gel performanceHydrazide preparationGel preparationBenzeneOrganic solvent
The invention discloses an organogelator, organogel with double responses and a preparation method of the organogel. The organogelator is N-(3,4-alkoxybenzoyl)-N'-(4'-nitrobenzoyl) hydrazine, and a structure formula of the organogelator is defined in the specification, wherein n is 12 or 16. The micromolecular organogelator, namely, N-(3,4-alkoxybenzoyl)-N'-(4'-nitrobenzoyl) hydrazine, has a negative ion identification function and has a good gel capability in various organic solvents such as methylbenzene, benzene, trichloroethylmethane and the like; in a gel state, molecules are aggregated into a fiber structure, the fiber diameter range is about 200nm, a main driving force of forming the organogel is an intermolecular hydrogen bond between hydrazide groups, and a Van der Waals force between alkyl chains also achieves a certain effect in the gel forming process; and macroscopic double responses, namely, a state response and a colorimetric response, are displayed for fluorine ions.
Owner:SHENZHEN UNIV
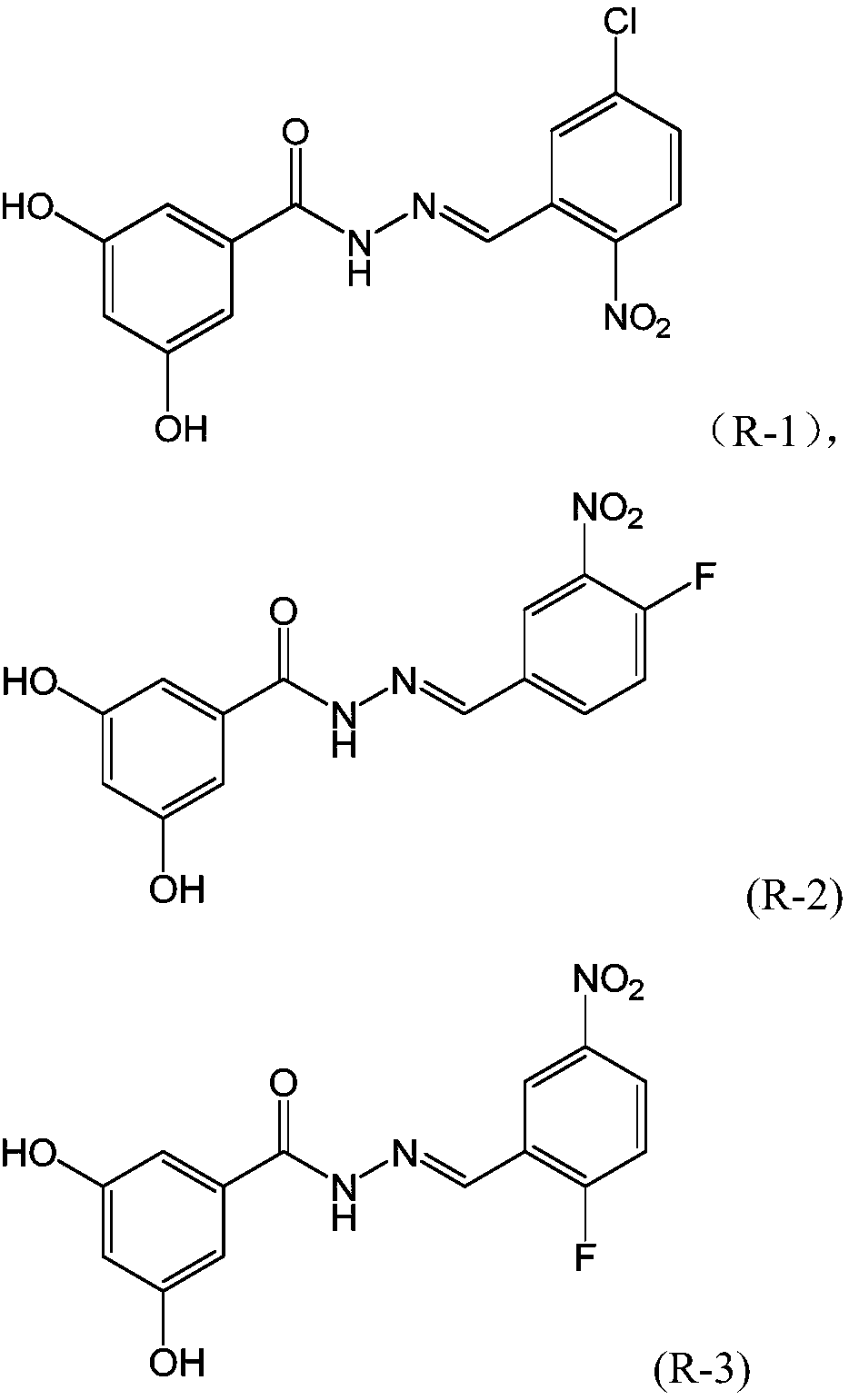
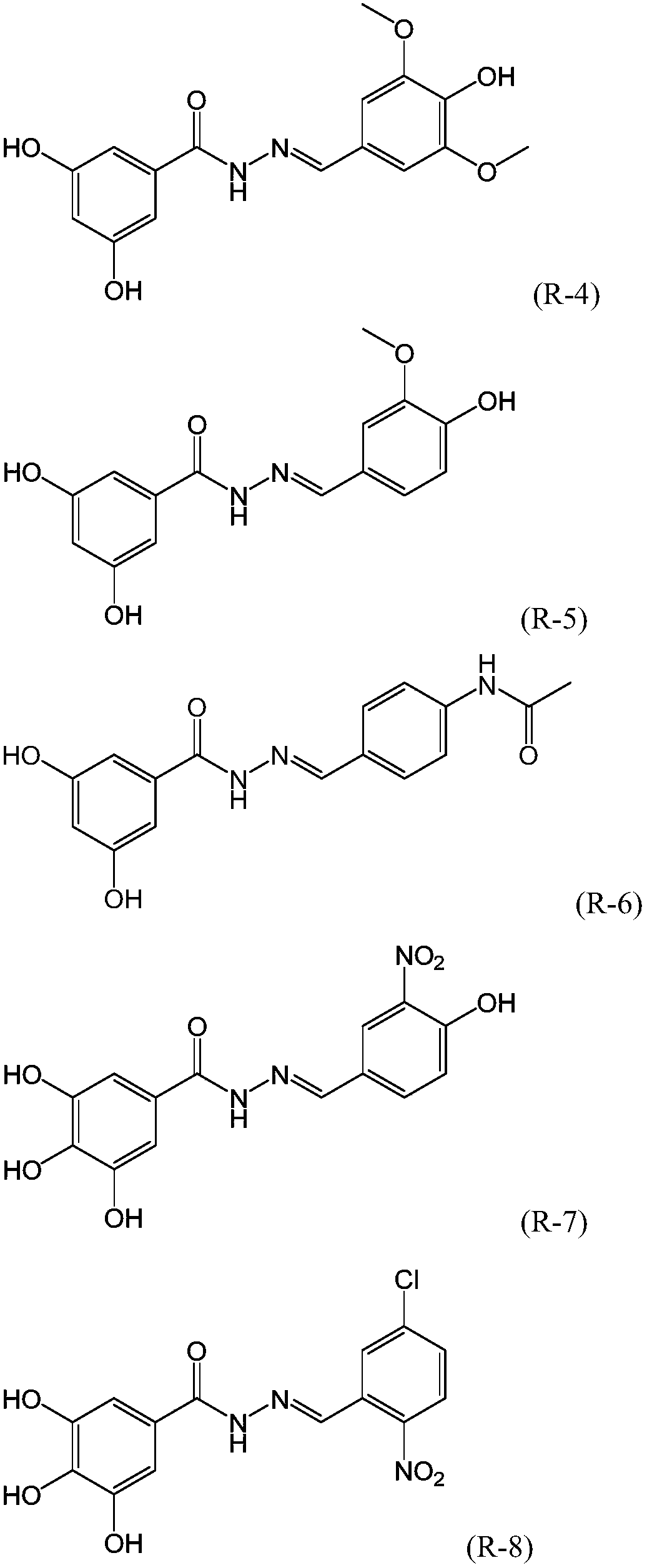
Dihydroxybenzoylhydrazone neuraminidase inhibitor and preparation and application thereof
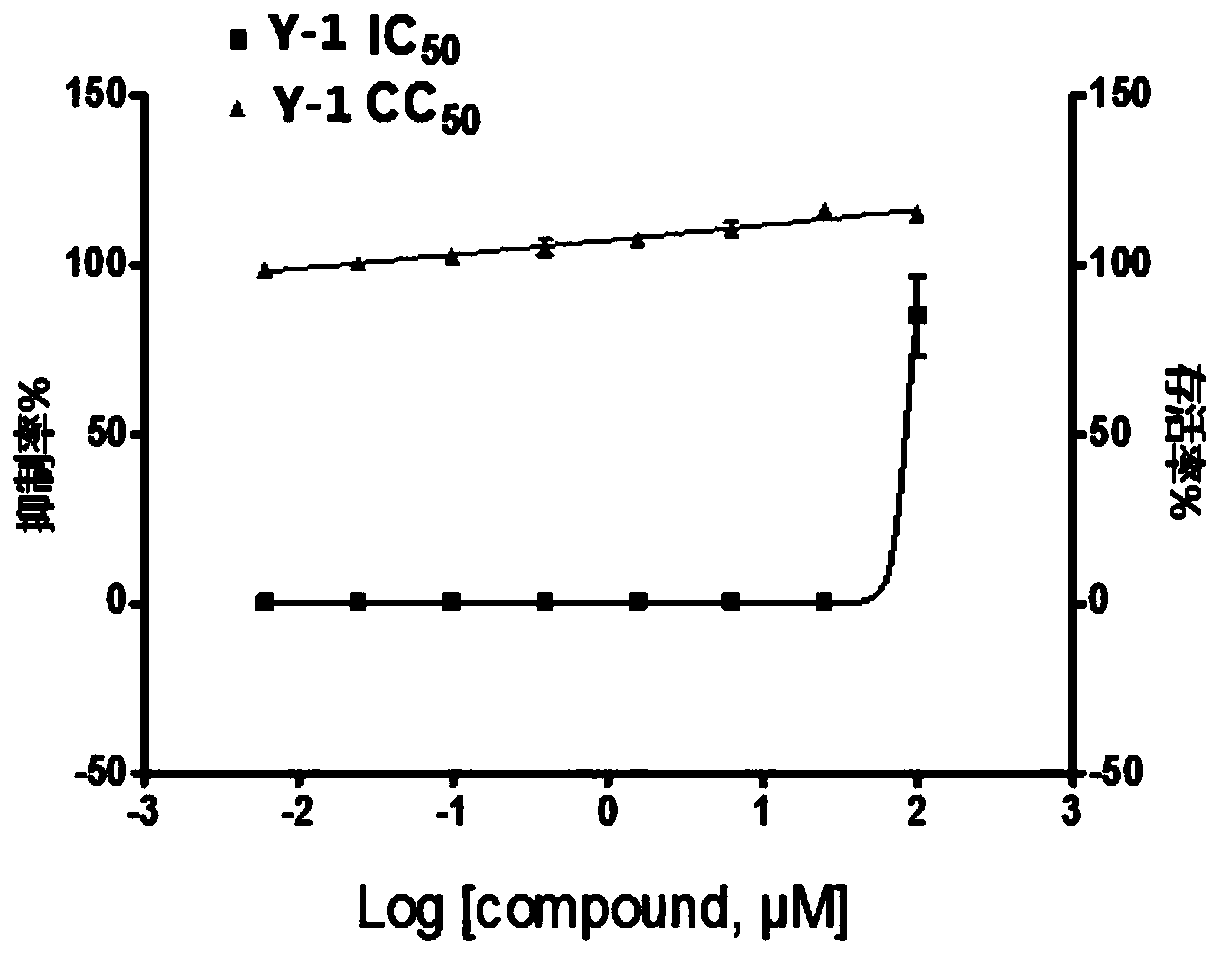
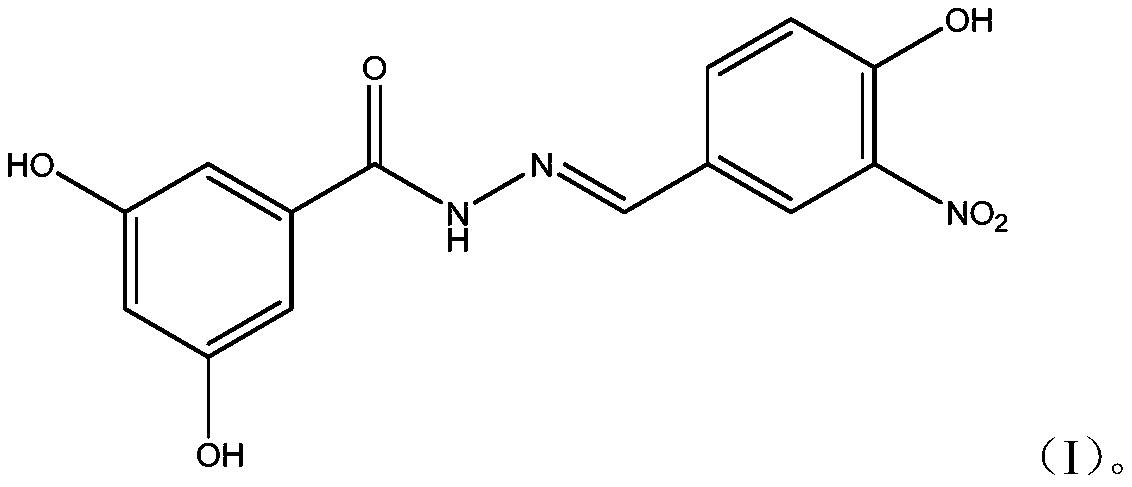
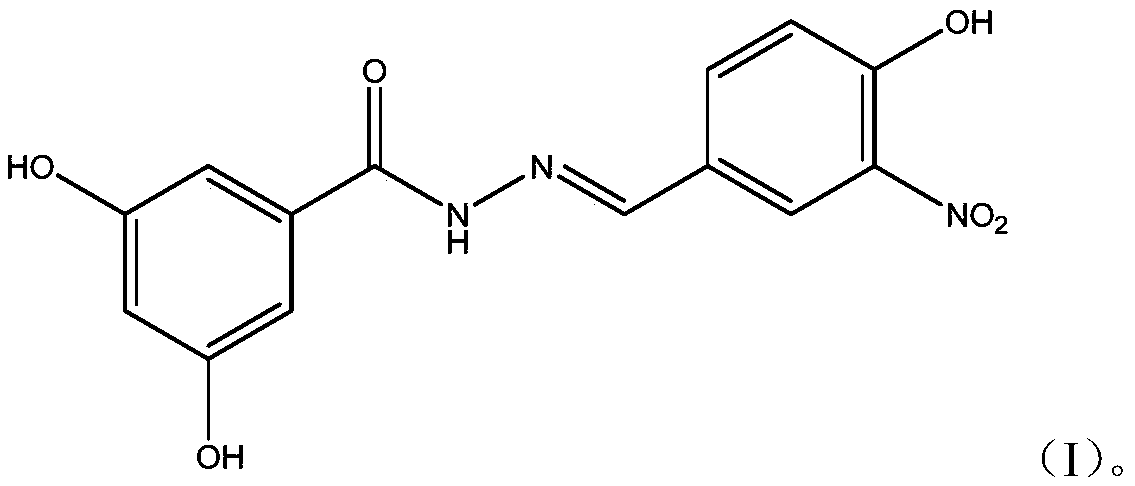
ActiveCN109776354AHigh activityEnhanced inhibitory effectHydrazide preparationAntiviralsNeuraminidaseStructural formula
The invention provides a dihydroxybenzoylhydrazone neuraminidase inhibitor and preparation and application thereof. The neuraminidase inhibitor is characterized by adopting a structural formula as shown in the description. The neuraminidase inhibitor has excellent anti-H1N1 influenza virus activity, adopts a structure completely different from that of previous neuraminidase, has a good effect of inhibiting A / WSN / 33H1N1 virus strain, is less in toxicity and has high druggability.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Benzoyl hydrazone neuraminidase inhibitor and preparation method and application thereof
ActiveCN109651189AGood neuraminidase inhibitory activityOrganic active ingredientsHydrazide preparationNeuraminidaseHydrazone
The invention provides a benzoyl hydrazone neuraminidase inhibitor. The inhibitor is characterized in that the structure of the inhibitor is shown in the formula I. The inhibitor has the advantage that according to the structural formula, the synthesized compound has good neuraminidase inhibiting activity.
Owner:SHANGHAI INST OF TECH
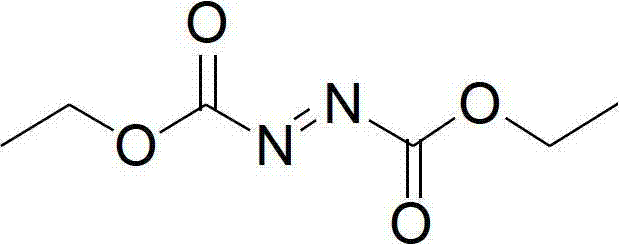
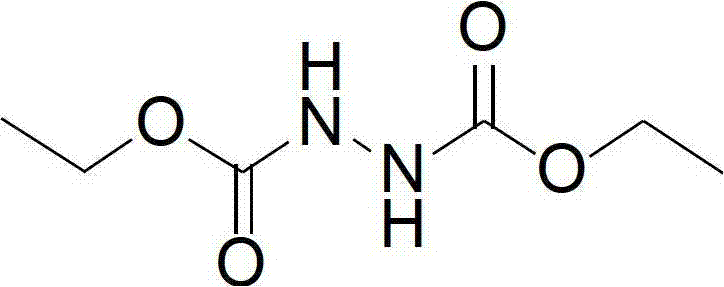
Synthesis method of diethyl azodicarboxylate and intermediate of diethyl azodicarboxylate
ActiveCN102898328ANo pollution in the processIn line with the concept of modern green chemistryHydrazide preparationDiisopropyl azodicarboxylateDistillation
The invention discloses a synthesis method of diethyl azodicarboxylate. The synthesis method comprises the following steps that (1) under the effect of sodium ethoxide, diethyl carbonate and ethyl carbazate are heated for reaction for 1 to 6 hours, the pH of solution is regulated to 3 to 8, white crystals are separated out, the recrystallization is carried out, and hydrogenated diethyl azodicarboxylate is obtained; and (2) the hydrogenated diethyl azodicarboxylate is added at minus 15 DEG C to 45 DEG C, in the acid solution, bromine or hydrobromic acid or sodium bromide and potassium bromide are used as catalysts, excessive hydrogen peroxide is dripped, the reaction is carried out for 1 to 10 hours, the extraction is carried out, organic solvents are removed through distillation, and saffron diethyl azodicarboxylate is obtained. The invention also provides a hydrogenated diethyl azodicarboxylate intermediate and a synthesis method of the hydrogenated diethyl azodicarboxylate intermediate. The synthesis method has the advantages that diethyl carbonate is used as raw materials, cleanness and environment protection are realized, no pollution exists, raw materials can be cyclically utilized, the economy is better, the operation is simple, the reaction temperature range is wide, the reaction is stable, the energy consumption is low, the yield is high, and the industrial production is favorably realized.
Owner:SHANDONG NORMAL UNIV
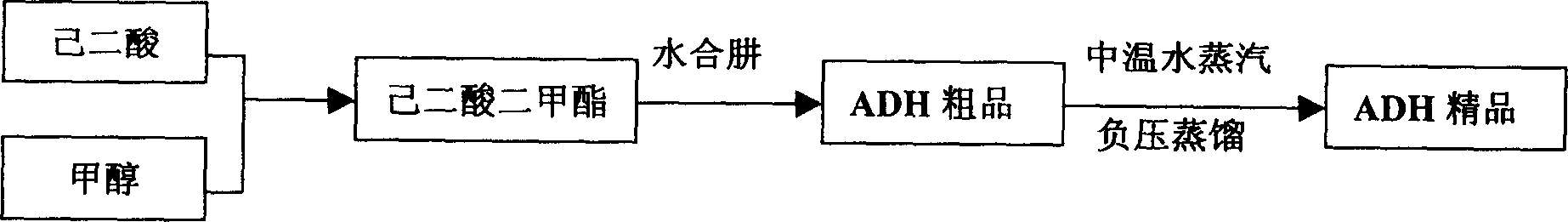
Prepn process of acyldhydrazino adipate with free hydrazine content lower than 10ppm
InactiveCN1810770AImprove performanceReduce pollutionHydrazide preparationWater vaporHydrazine compound
The present invention discloses the preparation process of dihydrazide adipate with free hydrazine content lower than 10 ppm. The preparation process includes synthesis of adipic acid and methanol to produce dimethyl adipate, the subsequent synthesis with hydrazine hydrate to obtain crude dihydrazide adipate product, and distilling the crude dihydrazide adipate product at negative pressure condition in medium temperature water vapor to obtain refined dihydrazide adipate product with free hydrazine content lower than 10 ppm. The refined dihydrazide adipate product has high performance, low pollution and environment friendship and is used in producing environment friendly water paint.
Owner:凌青云
Method for preparing adipic acid dihydrazide
InactiveCN105541657AHigh catalytic efficiencyNo pollution in the processHydrazide preparationChemical recyclingRefluxDistillation
The invention discloses a method for preparing adipic acid dihydrazide. The method comprises the following steps of (1) adding adipic acid and strong-acidic cation exchange resin taken as a catalyst into methanol, then heating a reaction system till a reflux state is formed, and performing an esterification reaction under the reflux state; after the completion of the esterification reaction, adding ammonia water into the reaction system at a temperature of 5 to 40 DEG C, then adding hydrazine hydrate, and enabling the reaction system to perform a hydrazination reaction at a temperature of 5 to 40 DEG C; after the completion of the hydrazination reaction, filtering out the catalyst, and preparing a reaction product; (2) obtaining an adipic acid dihydrazide crude product by means of removing methanol from a product obtained through a distillation reaction; obtaining the adipic acid dihydrazide through washing and drying the adipic acid dihydrazide crude product. The method disclosed by the invention has the advantages that the conversion rate can be improved, the side reaction is less, the catalyst can be recycled, and the production cost is low.
Owner:SHANDONG TIANYI CHEM
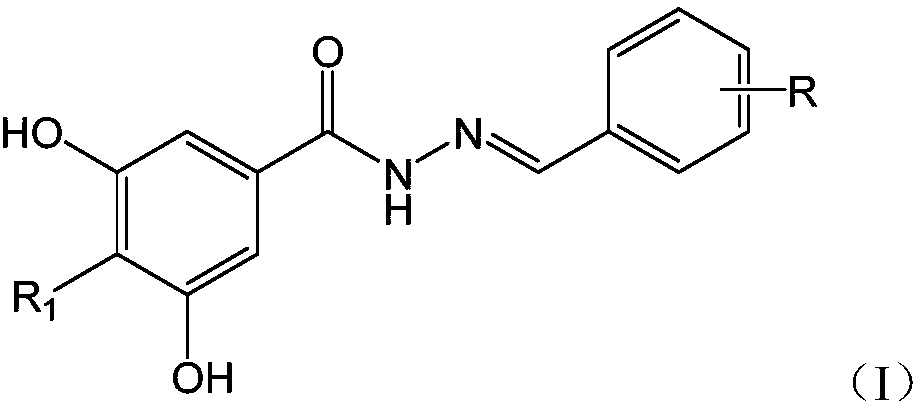
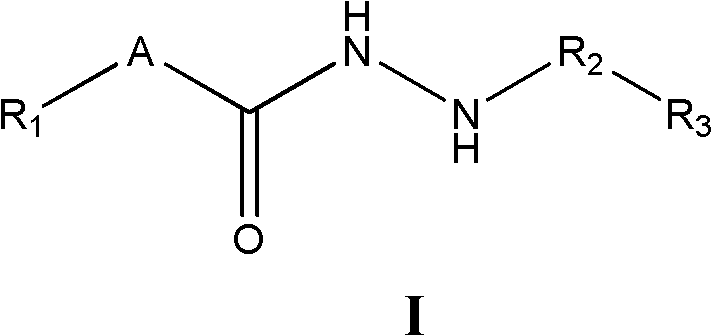
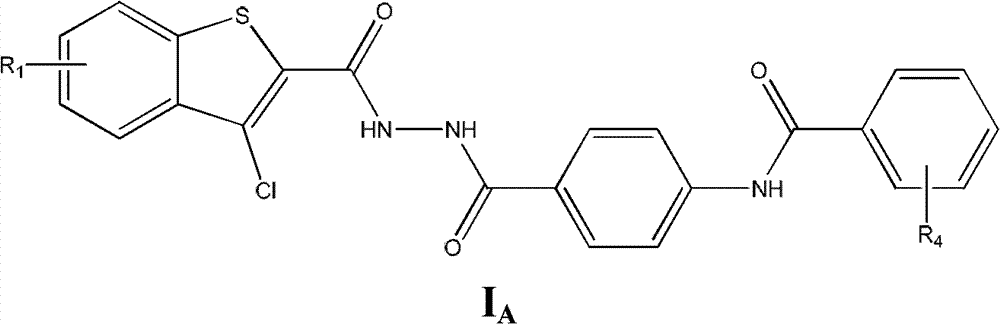
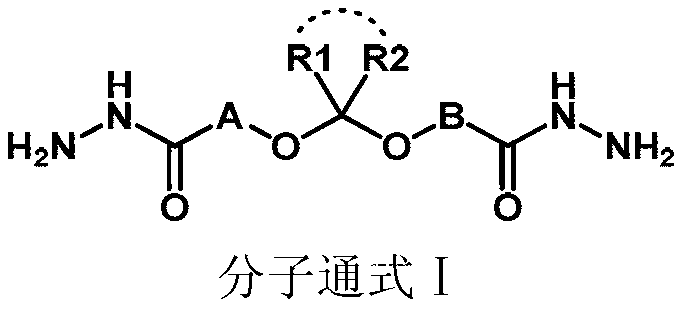
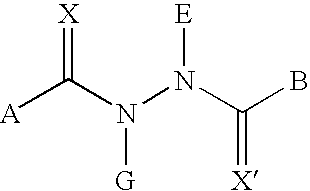
Substituted hydrazide compound, and its preparation method, medicinal compositions and application
InactiveCN102775381AGrowth inhibitionInhibitory activityOrganic active ingredientsHydrazide preparationDiseaseMutant
The invention relates to a substituted hydrazide compound with a structure shown in the following general formula I, its preparation method, medicinal compositions and application thereof. The compound can be used for preparing medicines treating diseases caused by B-Raf and its isoenzymes or mutants (such as B-RafV600E).
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +2
Diarylacetylene hydrazide containing tyrosine kinase inhibitors
Owner:SUN PHARMA INDS
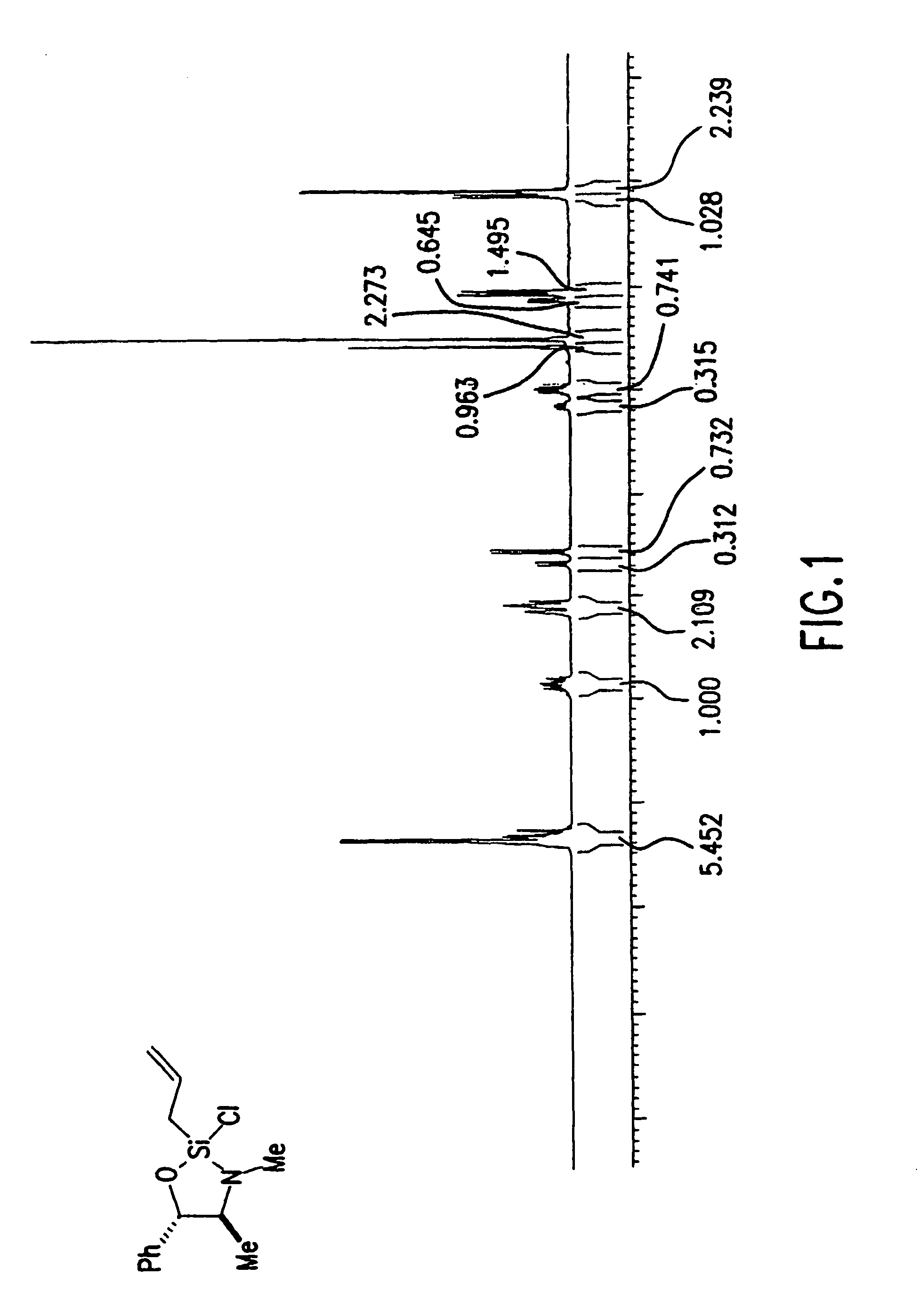
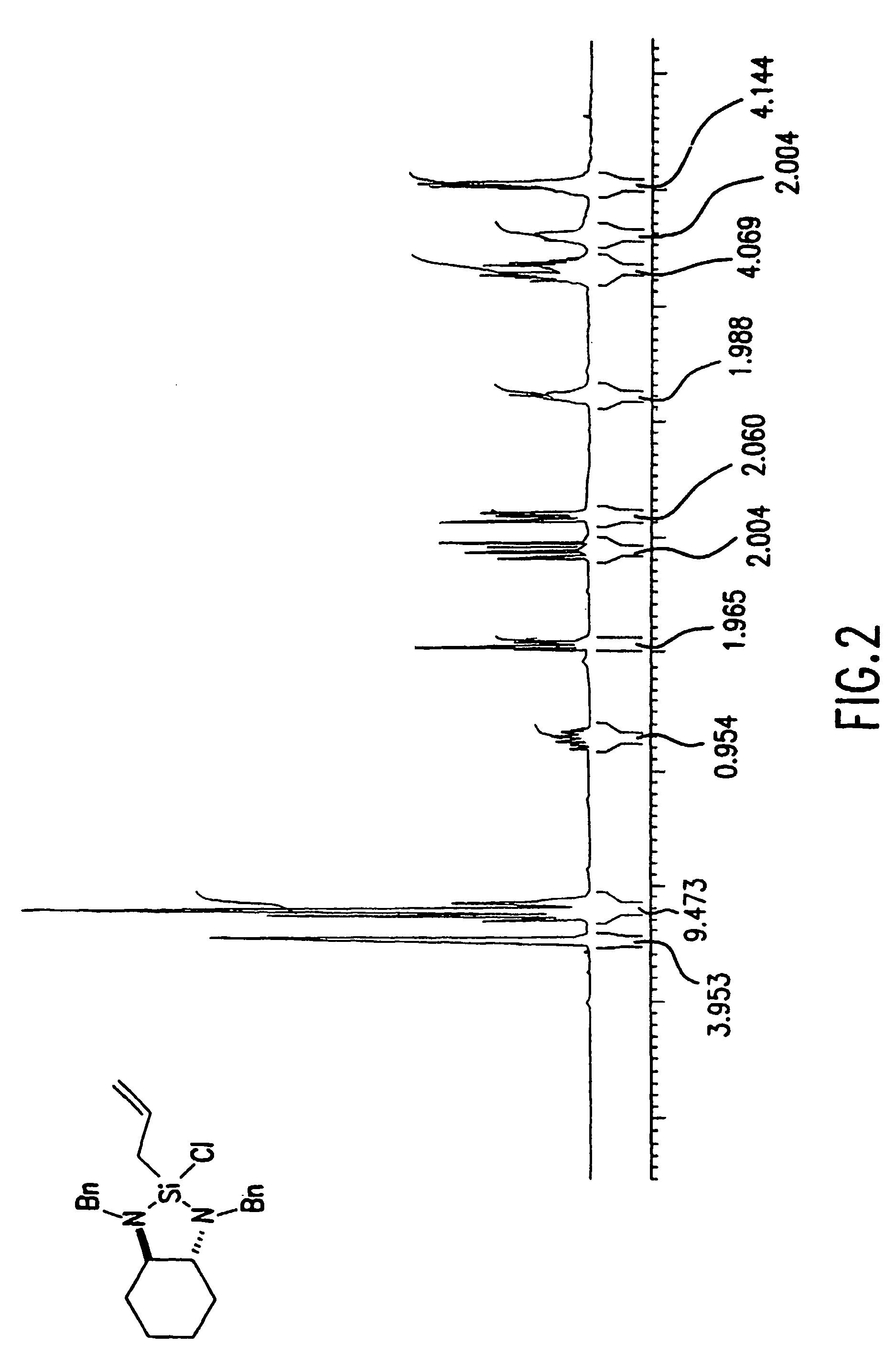
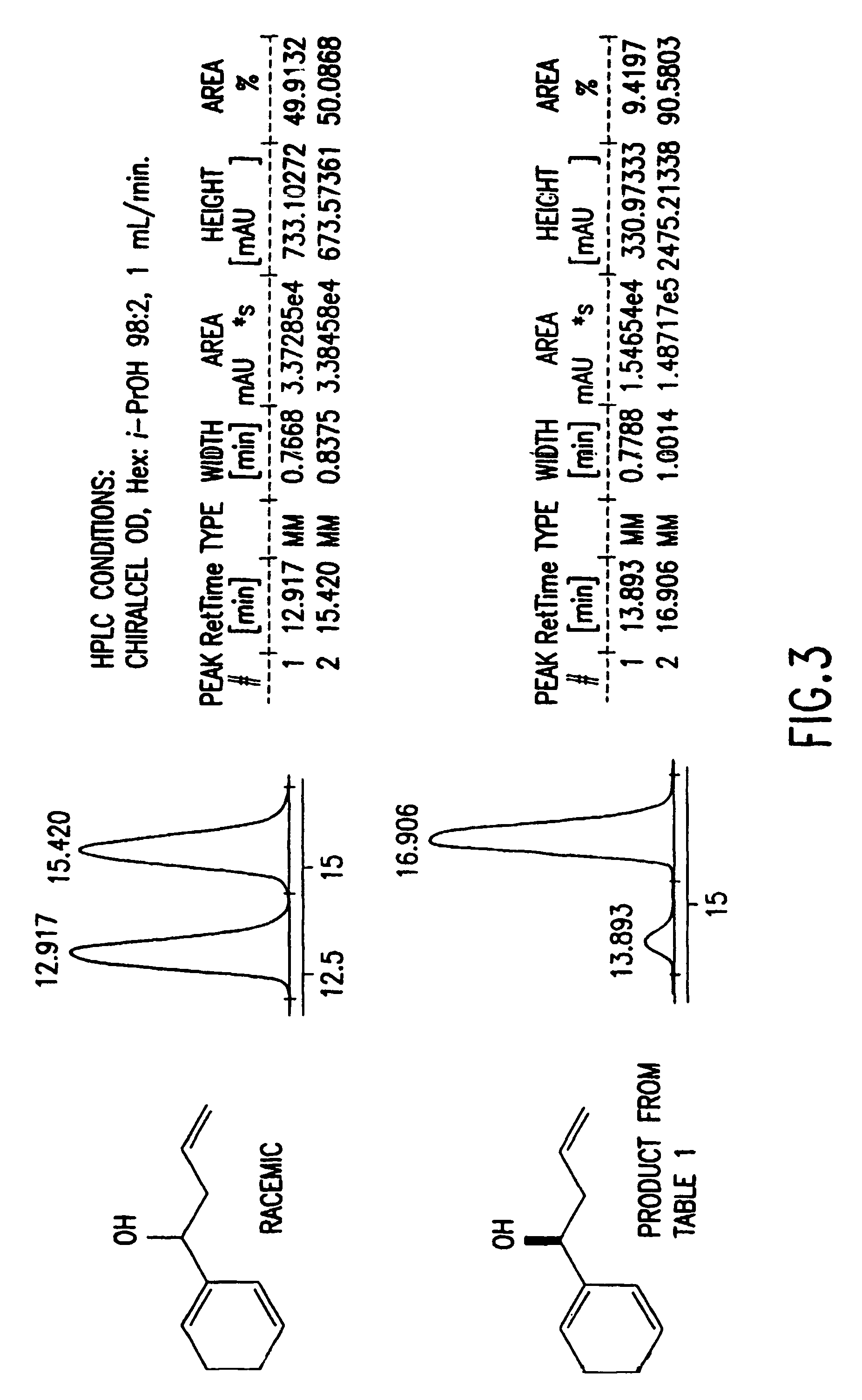
Reagents for asymmetric allylation, aldol, and tandem aldol and allyation reactions
A new class of reagents and method of use of the reagents in the reaction of the reagents with electrophilic compounds. The invention in one embodiment is directed to a method for the formation of an alcohol of the formula (I). The method includes reacting a reagent of the formula (II) with an aldehyde of the formula R10CHO to form the alcohol. X3 is one of O and C(R4)(R5). Each of X1 and X2 is independently O or N—R. Each of Ca and Cb is independently an achiral center, an (S) chiral center or an (R) chiral center. Ra and Rb are (i) each independently C1-10 alkyl, C6-10 aryl or C3-9 heteroaryl, or (ii) taken together to form a C3—C4 alkylene chain which together with Ca and Cb forms a 5-membered or 6-membered aliphatic ring. Rc and Rd are each independently hydrogen, C1-10 alkyl, C6-10 aryl or C3-9 heteroaryl. R is C1-10 alkyl, C6-10 aryl or C3-9 heteroaryl. Each of R1, R2, R3, R4, R5 is independently hydrogen, C1—C10 alkyl, C6-10 aryl, C3-9 heteroaryl, C1-10 alkoxy, C6-10 aryloxy, C1-10 dialkylamino, C1-10 alkyl-C6-10 arylamino, C1-10 diarylamino, or halogen. R6 is halogen, hydrogen, C1-10 alkyl, C6-10 aryl, C3-9 heteroaryl, C1-10 alkoxy, C6-10 aryloxy, C1-10 alkyl-C6-10 arylamino, C1-10 diarylamino, OSO2CF3 or SR. R10 may be C1-10 alkyl, C6-10 aryl, or C3-9 heteroaryl.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
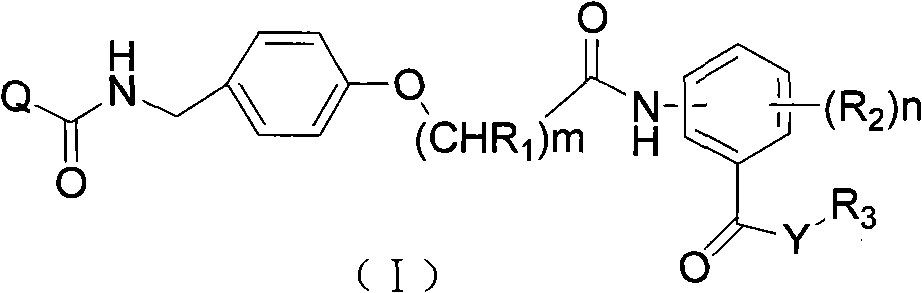
Acyl benzylamine compound and use thereof
The invention discloses an acyl benzylamine compound and a use thereof. The acyl benzylamine compound has wide-spectrum bactericidal activity and has a general structural formula shown in the formula I, wherein definitions of all substituent groups in the formula I are shown in the patent specification. The acyl benzylamine compound has wide-spectrum bactericidal activity, has effects of treating symptoms caused by cucumber downy mildew, corn rust and wheat powdery mildew, and especially, has excellent effects of preventing cucumber downy mildew and corn rust. The acyl benzylamine compound also has good effects of preventing and treating wheat powdery mildew. The acyl benzylamine compound has the advantages of high activity, low use amount and good safety.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
Preparation technology of methoxyfenozide
InactiveCN104803879AReduce processing difficultyHigh purityHydrazide preparationWastewaterCondensation reaction
The invention discloses a preparation technology of methoxyfenozide. The preparation technology comprises steps of a condensation reaction and an acylation reaction. The preparation technology has the advantages as follows: intermediate raw materials are recycled and reused, so that the production cost and the waste water treatment difficulty are reduced, the purity of finished products is high, the reaction process is convenient to control, and the requirement of modern pesticide preparation is met.
Owner:JIANGSU CHANGQING AGROCHEM NANTONG
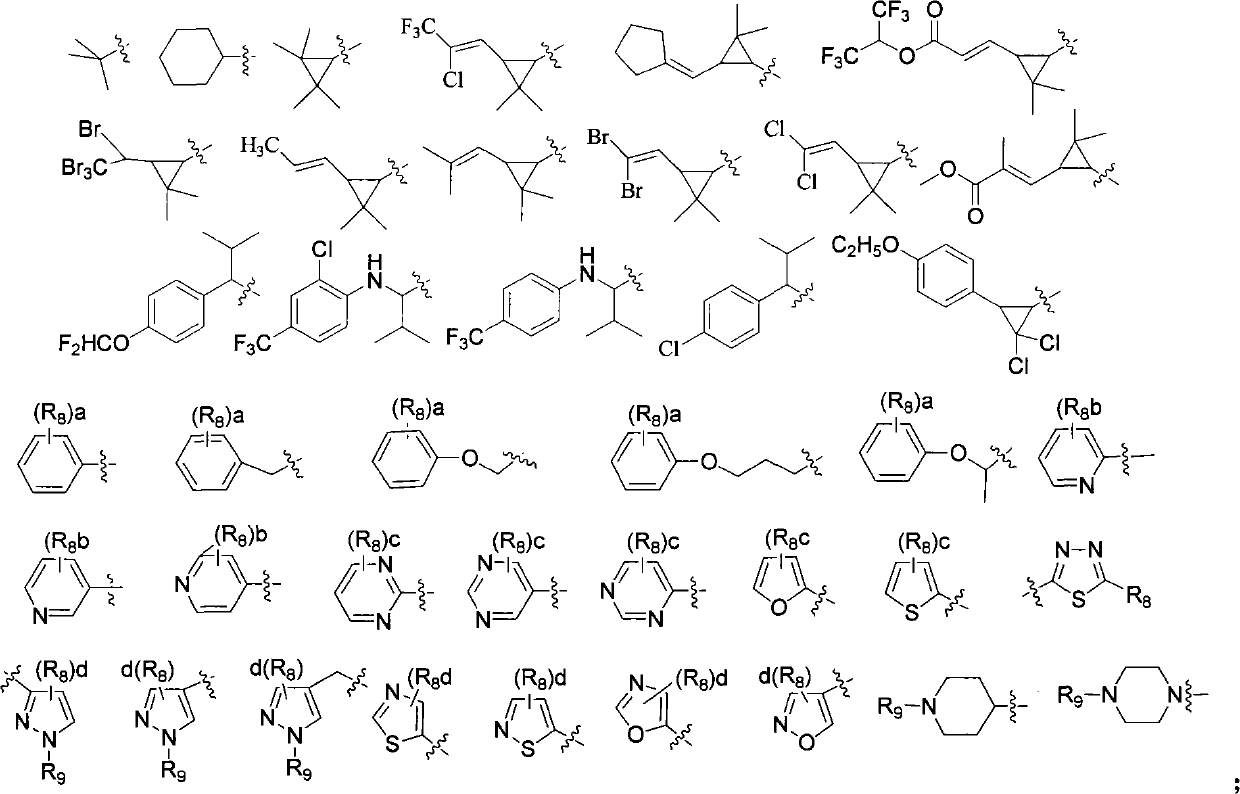
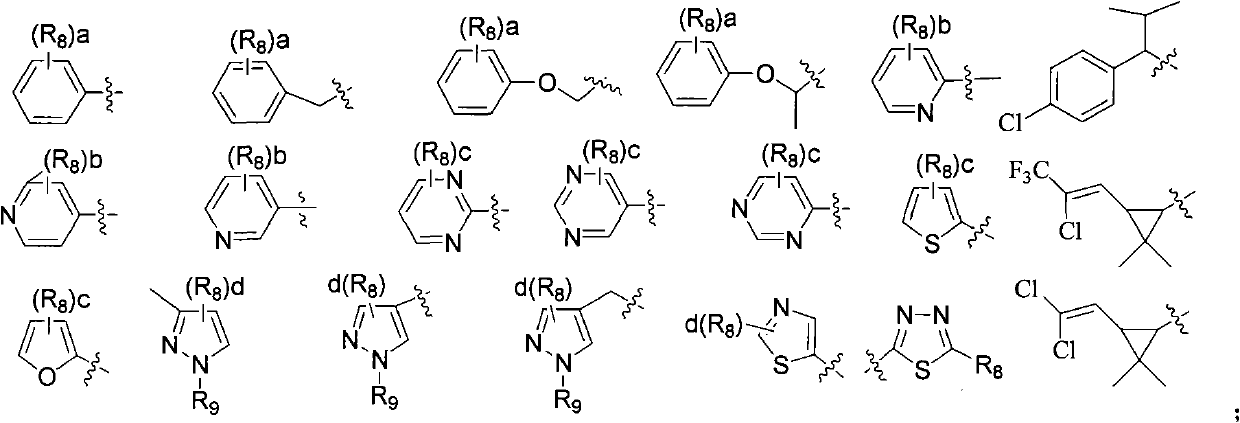
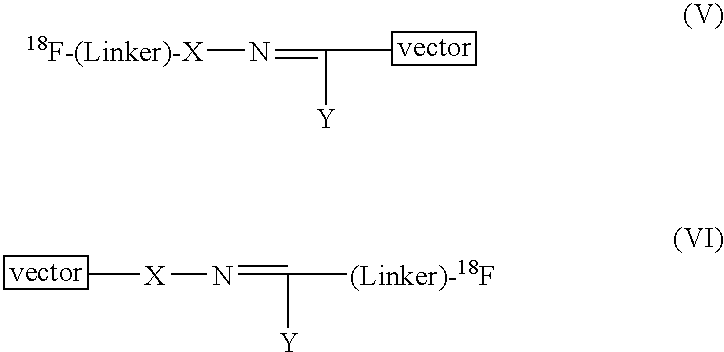
Methods of radiofluorination of biologically active vectors
ActiveUS8197793B2Hydrazine preparationOrganic compound preparationMedicinal chemistryBiological activity
Owner:GE HEALTHCARE AS
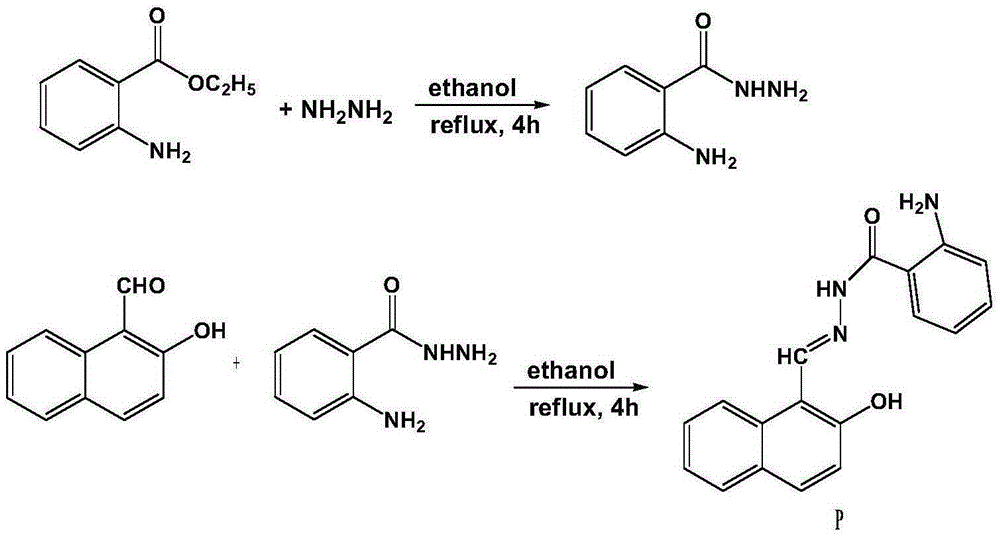
Preparation and application of benzoyl hydrazine derivative
InactiveCN105348141AGood choiceThe synthesis steps are simpleHydrazide preparationFluorescence/phosphorescenceHydrazine compoundFluorescence
The present invention relates to a fluorescent molecular probe, and particularly to preparation and application of a benzoyl hydrazine derivative . The structure of the benzoyl hydrazine derivative P is as shown in a formula 1. A preparation method comprises the following steps: heating ethyl anthranilate and hydrazine hydrate to reflux in anhydrous alcohol for 4-6 h, cooling down the mixture until the temperature reaches the room temperature, then precipitating and filtering a solid, and washing the solid with anhydrous alcohol , and drying to obtain an intermediate compound; and enabling the intermediate to react with 2-hydroxy-1-naphthaldehyde for 4-6 hours in anhydrous alcohol at a temperature of 70-80 DEG C, cooling down the mixture, then precipitating and leaching a solid, then washing the solid, and drying the solid to obtain a yellowish solid pure product P. The benzoyl hydrazine derivative P can be used as a fluorescent probe to implement identification and detection on Mg2+. The benzoyl hydrazine derivative P obtained by an effective synthesis means has relatively good selectivity on Mg2+, and on the basis of optimized experimental conditions, detection on Mg2+ can be implemented. The formula 1 is as shown in the specification.
Owner:HAINAN MEDICAL COLLEGE
Three-phase catalyst Pd@COF-QA as well as preparation method and application thereof
ActiveCN109988079AHarm reductionLow reaction temperatureAmino preparation from aminesCarboxylic acid nitrile preparationOrganic solventRoom temperature
The invention provides a three-phase catalyst Pd@covalent organic framework (COF)-QA as well as a preparation method thereof and an application of the catalyst in catalysis of a Suzuki-Miyaura reaction, and belongs to the technical field of catalyst preparation. The three-phase catalyst Pd@COF-QA provided by the invention is obtained by reacting covalent organic framework (COF)-QA with palladium acetate at room temperature, the preparation method is simple and convenient, through experimental verification, the three-phase catalyst has a high utilization rate, the reaction conditions are mild,the catalytic costs can be effectively reduced, high temperature and an organic solvent are not needed, the usage amount of the catalyst is less, and heterogeneous catalysis can be realized without other additives; and at the same time, the catalyst can be reused and easy to recover, the utilization rate of the catalyst is improved, the costs are reduced, and industrialized promotion application is facilitated.
Owner:SHANDONG NORMAL UNIV
Method for the preparation of w-amino- alkaneamides and w-amino-alkanethioamides as well as intermediates of this method
Owner:SANDOZ AG
Popular searches
Carboxylic acid esters preparation Carboxylic acid amides preparation Preparation by cyanide reaction Sulfonic acid amide preparation Group 3/13 element organic compounds Amino-carboxyl compound preparation Amino group formation/introduction Phosphorus organic compounds Organic substitution Amino-hyroxy compound preparation
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
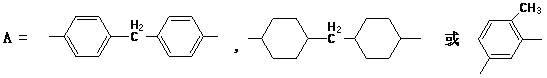
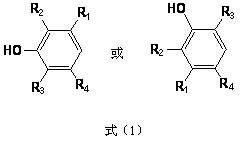
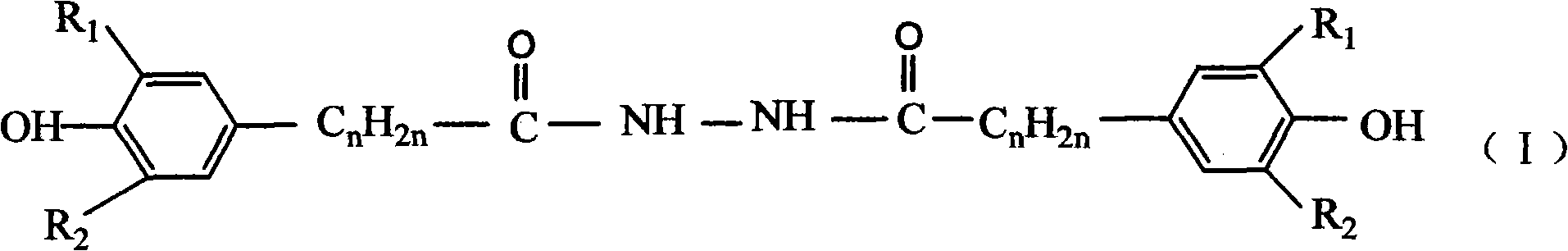
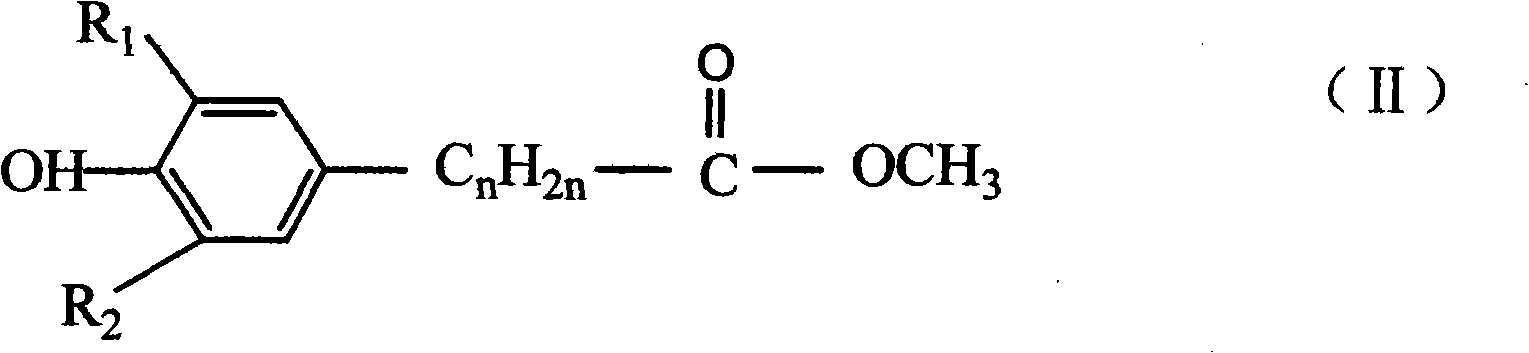
![Preparation of supermolecule gel factor based on three-column [5] aromatic hydrocarbon and organic gel thereof and application Preparation of supermolecule gel factor based on three-column [5] aromatic hydrocarbon and organic gel thereof and application](https://images-eureka.patsnap.com/patent_img/5aa28ea5-05e9-4dcc-b925-76cac88405be/180504163105.png)
![Preparation of supermolecule gel factor based on three-column [5] aromatic hydrocarbon and organic gel thereof and application Preparation of supermolecule gel factor based on three-column [5] aromatic hydrocarbon and organic gel thereof and application](https://images-eureka.patsnap.com/patent_img/5aa28ea5-05e9-4dcc-b925-76cac88405be/180504163109.png)
![Preparation of supermolecule gel factor based on three-column [5] aromatic hydrocarbon and organic gel thereof and application Preparation of supermolecule gel factor based on three-column [5] aromatic hydrocarbon and organic gel thereof and application](https://images-eureka.patsnap.com/patent_img/5aa28ea5-05e9-4dcc-b925-76cac88405be/180504163114.png)