Organogelator, organogel with double responses and preparation method of organogel
A technology of organic gelling factors and organic gels, which is applied in the field of supramolecular chemistry, can solve the problems of lack of research on organic gelling factors, and achieve the effect of good gelling ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
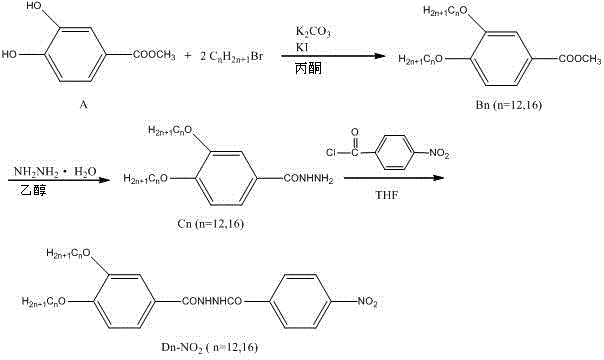
[0051] combine image 3 , image 3 It is the synthetic route diagram of N-(3,4-alkoxybenzoyl)-N'-(4'-nitrobenzoyl)hydrazine of this example. The specific synthesis and structural characterization are as follows:
[0052] 1), methyl 3,4-dialkoxybenzoate (B n , n=16 or 12) synthesis and structural characterization
[0053] Methyl 3,4-hexadecyloxybenzoate (B 16 ) preparation: Dissolve 3.36g (0.02mol) methyl 3,4-dihydroxybenzoate in an appropriate amount of acetone, add 12.5mL hexadecane bromide, 4g anhydrous potassium carbonate, 0.08g potassium iodide, stir and reflux for 35 hours . After the reaction was completed, filter while it was hot, discard the filter residue, put the filtrate into the refrigerator to cool for 5 hours, and then filter to obtain the crude product. The crude product was recrystallized with absolute ethanol and dried to obtain 10.6 g of white powder (methyl 3,4-hexadecyloxybenzoate), yield: 86%.
[0054] Methyl 3,4-hexadecyloxybenzoate (B 16 ) is cha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com