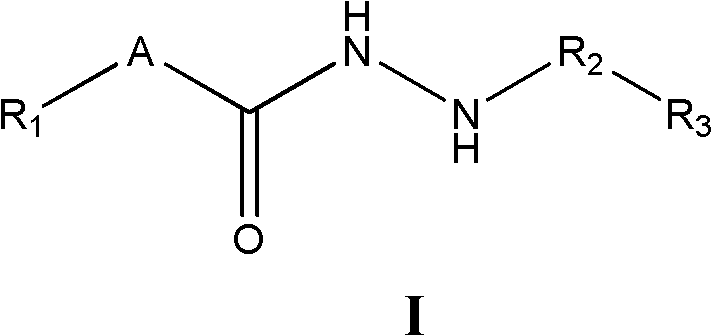
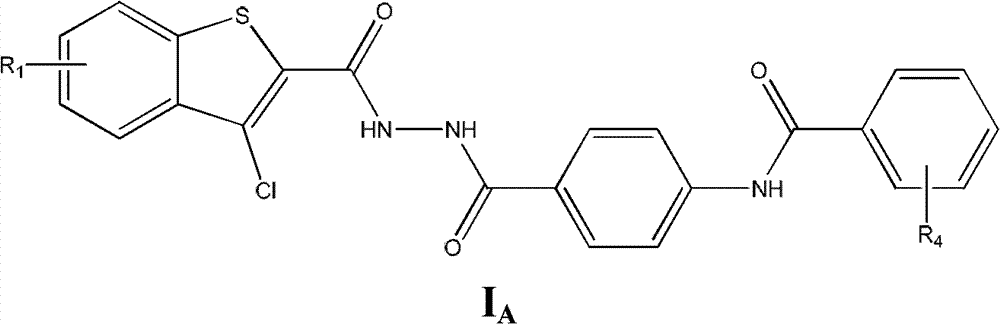
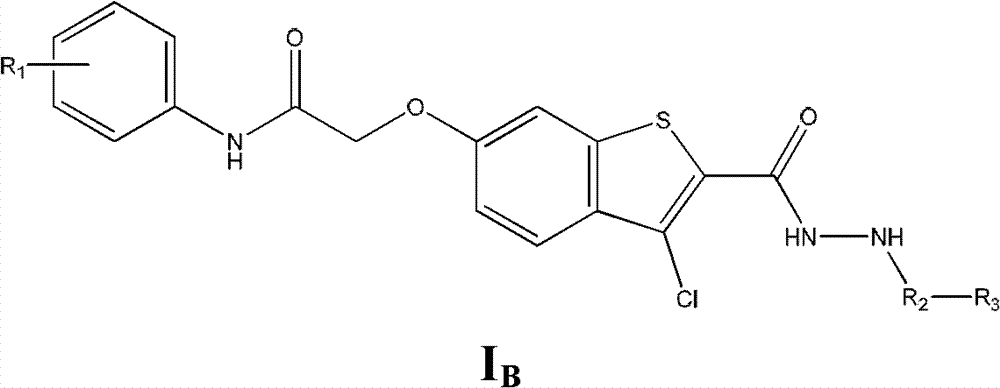
Substituted hydrazide compound, and its preparation method, medicinal compositions and application
A kind of compound, hydrazide technology, applied in substituted hydrazide compound and preparation method thereof, field of pharmaceutical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1 Preparation of p-methoxycinnamic acid (intermediate 2a)
[0083]
[0084] Dissolve 6.8 grams of p-methoxybenzaldehyde (Shanghai Pure Chemical Reagents) in anhydrous pyridine, then add 10.4 grams of malonic acid and 0.5 milliliters of piperidine in turn, heat and react at 100°C for 3 hours, and dissolve the Quickly pour the reaction liquid into the prepared cold dilute hydrochloric acid (30 ml concentrated hydrochloric acid + 200 ml ice water), stir, and precipitate a white solid, let it stand, filter, slowly wash the filter cake with ice water, and dry it at an appropriate temperature to obtain a powder Recrystallized with ethanol to obtain 7.8 g of p-methoxycinnamic acid (intermediate 2a), with a yield of 87%. 1 H NMR (400MHz, DMSO-d 6 )δ12.20(s, 1H), 7.63(d, J=8.4Hz, 2H), 7.54(d, J=16.0Hz, 1H), 6.96(d, J=8.8Hz, 2H), 6.37(d, J = 16.0 Hz, 1H), 3.79 (s, 3H).
Embodiment 2
[0085] Example 2 Preparation of 3-chloro-6-methoxybenzothiophene-2-formyl chloride (intermediate 3a)
[0086]
[0087] Under argon atmosphere at room temperature, add 3.56 g of compound intermediate 2a to 20 ml of chlorobenzene, then add 0.16 ml of pyridine and 7.3 ml of thionyl chloride, heat the reaction solution under reflux for 11 hours, then cool to about 40°C , filtered, and the solvent was evaporated under reduced pressure. The residue was heated and dissolved with 150 ml of 60-90°C petroleum ether, filtered while it was hot, and the filtrate was cooled in the refrigerator overnight, and a solid precipitated, filtered, the filter cake was washed with cold petroleum ether, and vacuum-dried to obtain 1.10 g of 3-chloro-6- Methoxybenzothiophene-2-carbonyl chloride (intermediate 3a), yield 21%.1 H NMR (400MHz, CDCl 3 )δ7.90(d, J=9.2Hz, 1H), 7.24(d, J=2.4Hz, 1H), 7.16(dd, J 1 =2.0Hz,J 2 =8.8Hz, 1H), 3.95(s, 3H).
Embodiment 3
[0088] Embodiment 3 Preparation of ethyl p-aminobenzoate (intermediate 4)
[0089]
[0090] Under ice-bath conditions, 11 milliliters of thionyl chloride was added dropwise to the ethanol suspension of 13.7 grams of p-aminobenzoic acid, the reaction solution was heated to reflux for 5.5 hours, cooled, and solids were precipitated, and the ethanol in the reaction solution was evaporated under reduced pressure , Dissolve the residue obtained with 100 ml of ethyl acetate, neutralize with saturated sodium bicarbonate solution, separate the layers, extract the aqueous phase with ethyl acetate several times, and combine the organic phases. The organic phase was washed successively with saturated brine, anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain a solid, which is recrystallized from ethyl acetate / petroleum ether to obtain 15.4 g of ethyl p-aminobenzoate (intermediate 4), with a yield of 93%. 1 H NMR (400MHz, CDCl 3 )δ7.87(d, J=8.4Hz, 2H), 6.65(d, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com