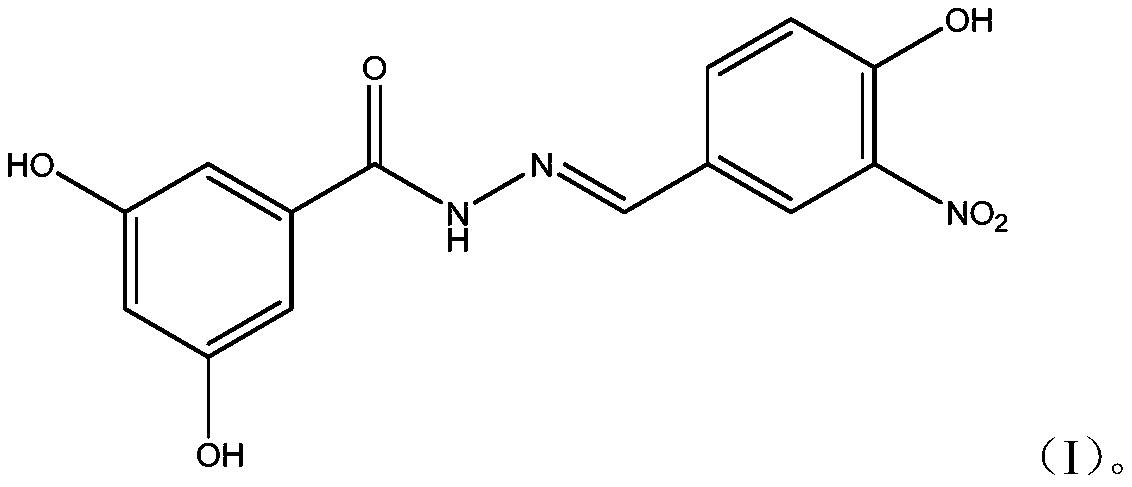
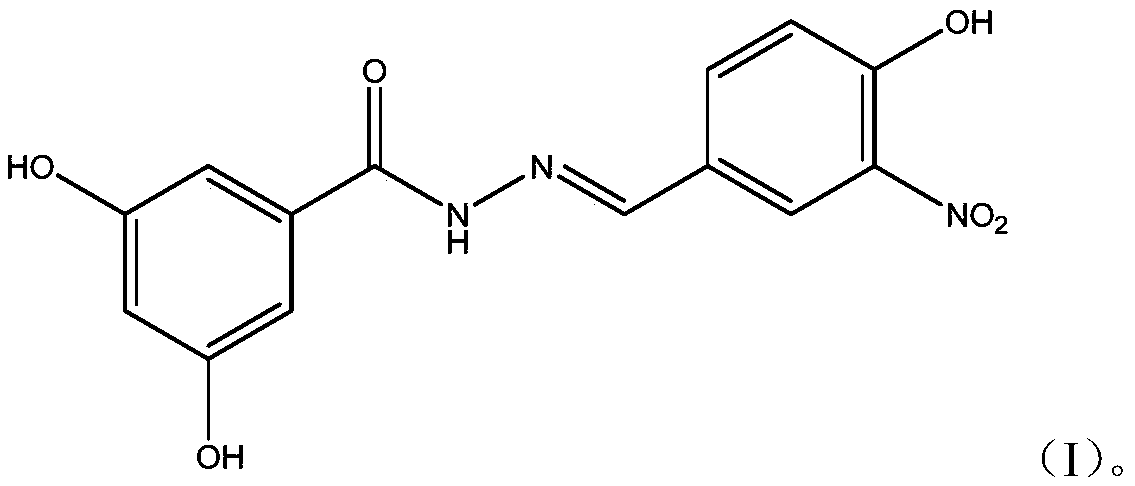
Dihydroxybenzoylhydrazone neuraminidase inhibitor and preparation and application thereof
A dihydroxybenzoyl hydrazone and neuraminidase technology, which is applied in the preparation of hydrazones, hydrazides, antiviral agents, etc., can solve the problems of single administration route, high price of Tamiflu, and low oral utilization rate , to achieve excellent anti-H1N1 influenza virus activity, good druggability, and good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] A preparation method of (E)-N-benzylidene-3,5-dihydroxybenzoylhydrazone neuraminidase inhibitor is as follows:
[0018]
[0019] The preparation method of (E)-N-benzylidene-3,5-dihydroxybenzoylhydrazone neuraminidase inhibitor is as follows:
[0020] (1) Accurately weigh 178.2mg (1.060mmol) of methyl 3,5-dihydroxybenzoate and 0.5mg (15.625mmol) of hydrazine hydrate into a round bottom flask, then add 5.0mL of absolute ethanol to dissolve and mix, and stir evenly , reacted at room temperature for 16 hours, concentrated, filtered, and dried to obtain the intermediate 3,5-dihydroxybenzoic acid hydrazide.
[0021] (2) Accurately weigh 160.0mg (0.952mmol) of 4-hydroxy-3-nitrobenzaldehyde, dissolve it in 5ml of ethanol, accurately weigh 160.0mg (0.952mmol) of the intermediate 3,5-dihydroxybenzoic acid hydrazide and add In the ethanol solution, 1-2 drops of acetic acid solution with a mass concentration of 99% was added dropwise, stirred evenly, and reacted at room tempera...
Embodiment 2
[0025] The present invention is further described in detail through examples below, and the technical solution of the present invention is not limited to the specific embodiments listed below.
[0026] A (E)-N-benzylidene-3,5-dihydroxybenzoylhydrazone neuraminidase inhibitor inhibits the neuraminidase activity test method as follows:
[0027] Reagents and experimental instruments used: neuraminidase inhibitor screening kit (purchased from Beyontian Company, product number: P0309), including: neuraminidase detection buffer; neuraminidase; neuraminidase fluorescent substrate ; Milli-Q water, black 96-well plate, microplate reader.
[0028] Neuraminidase inhibitors inhibit neuraminidase activity test steps are as follows:
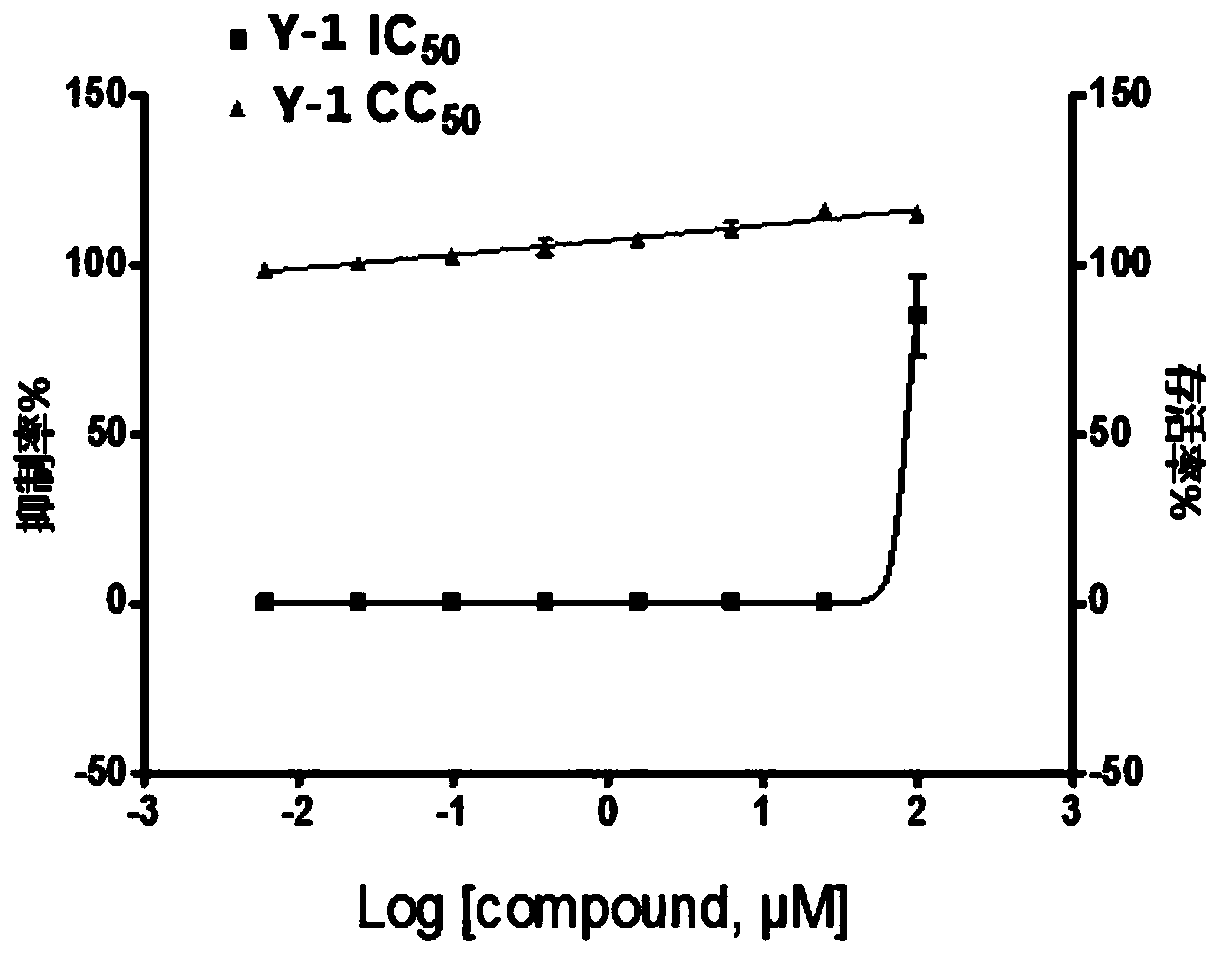
[0029] (1) The initial concentration of compound Y-1 was prepared to be 1000 μmol / L, and it was diluted into 7 concentration gradients according to the doubling ratio, which were 1000 μmol / L, 200 μmol / L, 40 μmol / L, 8 μmol / L, 1.6 μmol / L L, 0.32μmol / L, 0.064μm...
Embodiment 3
[0035] The following examples describe the present invention in further detail. The technical solutions of the present invention are not limited to the specific embodiments listed below. The anti-H1N1 influenza virus activity test in the present invention is entrusted to Shanghai WuXi AppTec New Drug Development Co., Ltd.
[0036] A (E)-N-benzylidene-3,5-dihydroxybenzoylhydrazone neuraminidase inhibitor anti-H1N1 influenza virus activity test method is as follows:
[0037] Reagents and experimental instruments used: cell maintenance solution: DMEM+10% phosphate buffered saline (PBS, pH=7.4)+1% penicillin and streptomycin double antibody solution (P / S); cell infection solution: DMEM+ 0.3%BSA+25mM HEPES+1mg / L trypsin; CellTiter- Luminesent Cell Viability Assay (promega); microplate reader.
[0038] TCID of influenza virus 50Obtained by the Reed Muench method.
[0039] The specific steps of testing the anti-H1N1 influenza virus activity of neuraminidase inhibitors are as foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com