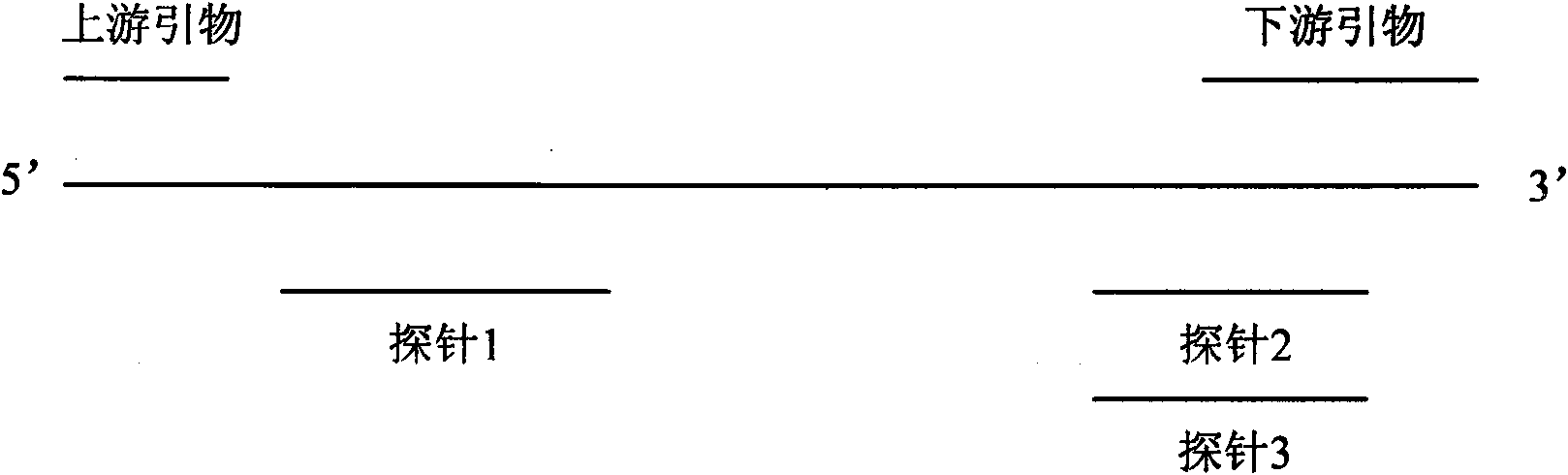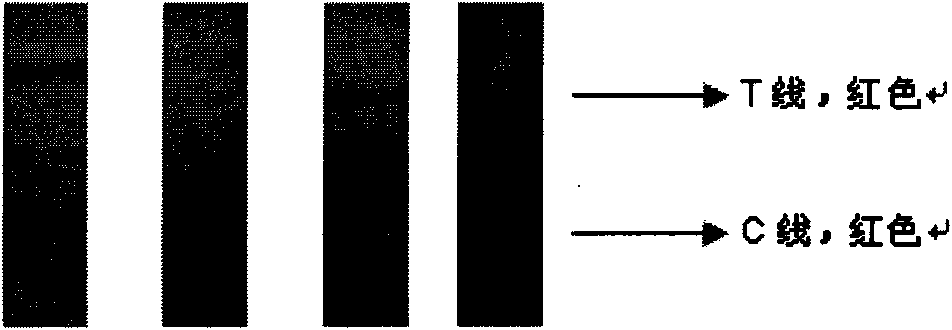Patents
Literature
242 results about "H1N1 influenza" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of nanometer cobweb/ nanometer fiber composite protective material
InactiveCN101564914AEffective protectionSimple processLaminationFilament/thread formingFiberBird flu
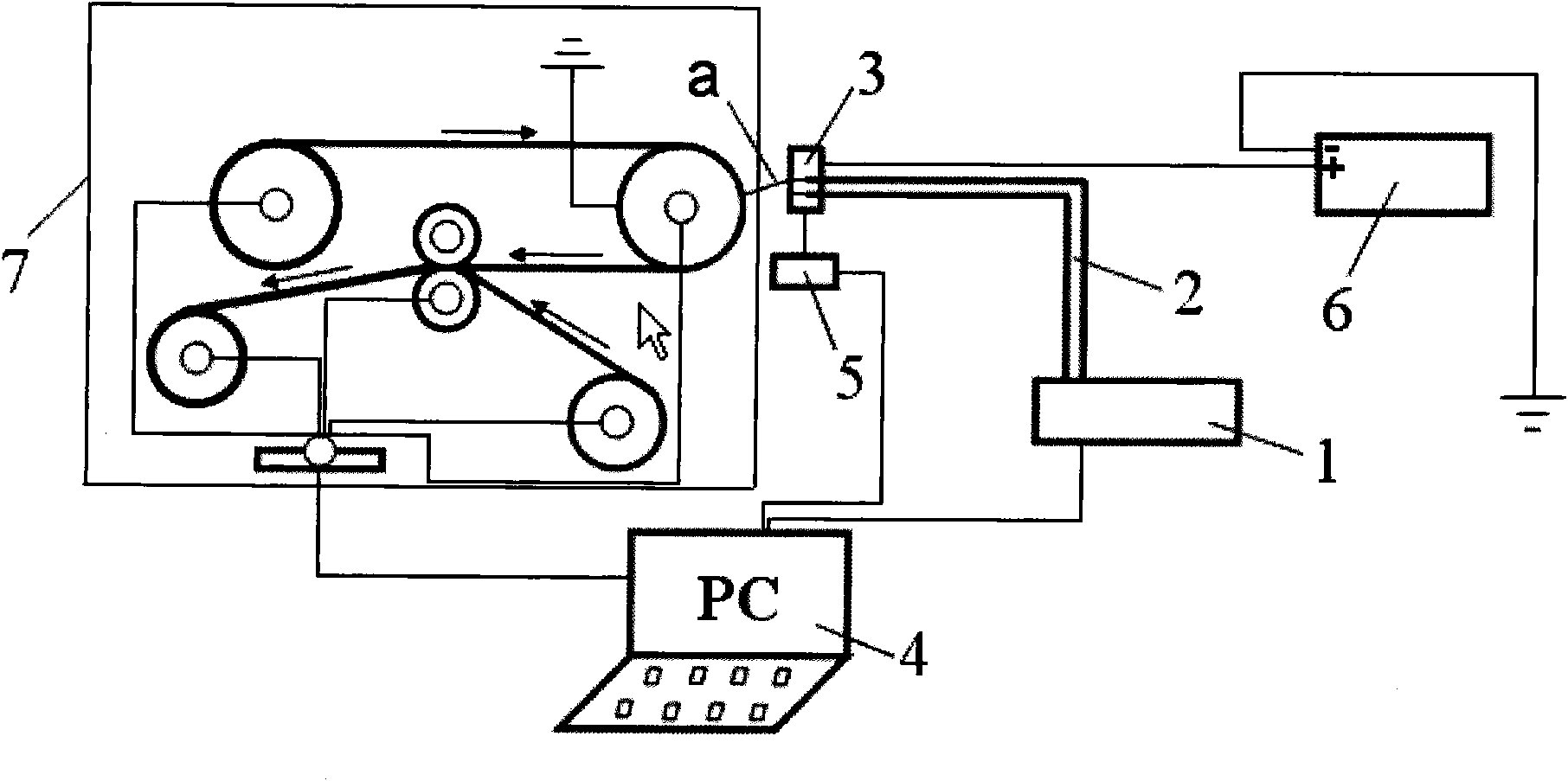
The invention provides a preparation method of nanometer cobweb / nanometer fiber composite protective material, which is characterized by comprising the specific steps of: I, using macromolecule for stirring and dissolving or dispersing spinning into a solvent to obtain an electric spinning material with a weight percentage of 5 to 30 percent; II, inputting the electric spinning material obtained in step I onto a spinneret at a flow speed of 0.1-10mL / h at the room temperature and humidity not more than 30 percent and simultaneously connecting the spinneret with a 10-80 kv power to conduct static spinning; using a layer of base materials to receive the spun threads, covering a layer of top materials on the threads, and then conducting hot-pressing on the threads to obtain the nanometer cobweb / nanometer fiber multi-layer composite protective material with an aperture of 10-80 nanometer and an average diameter of 15 nanometer. The material can be effectively used for preventing the viruses with diameters between 80 and 120 nanometers of H1N1 influenza A, bird flu, equine influenza, SARS pathogene and the like.
Owner:DONGHUA UNIV
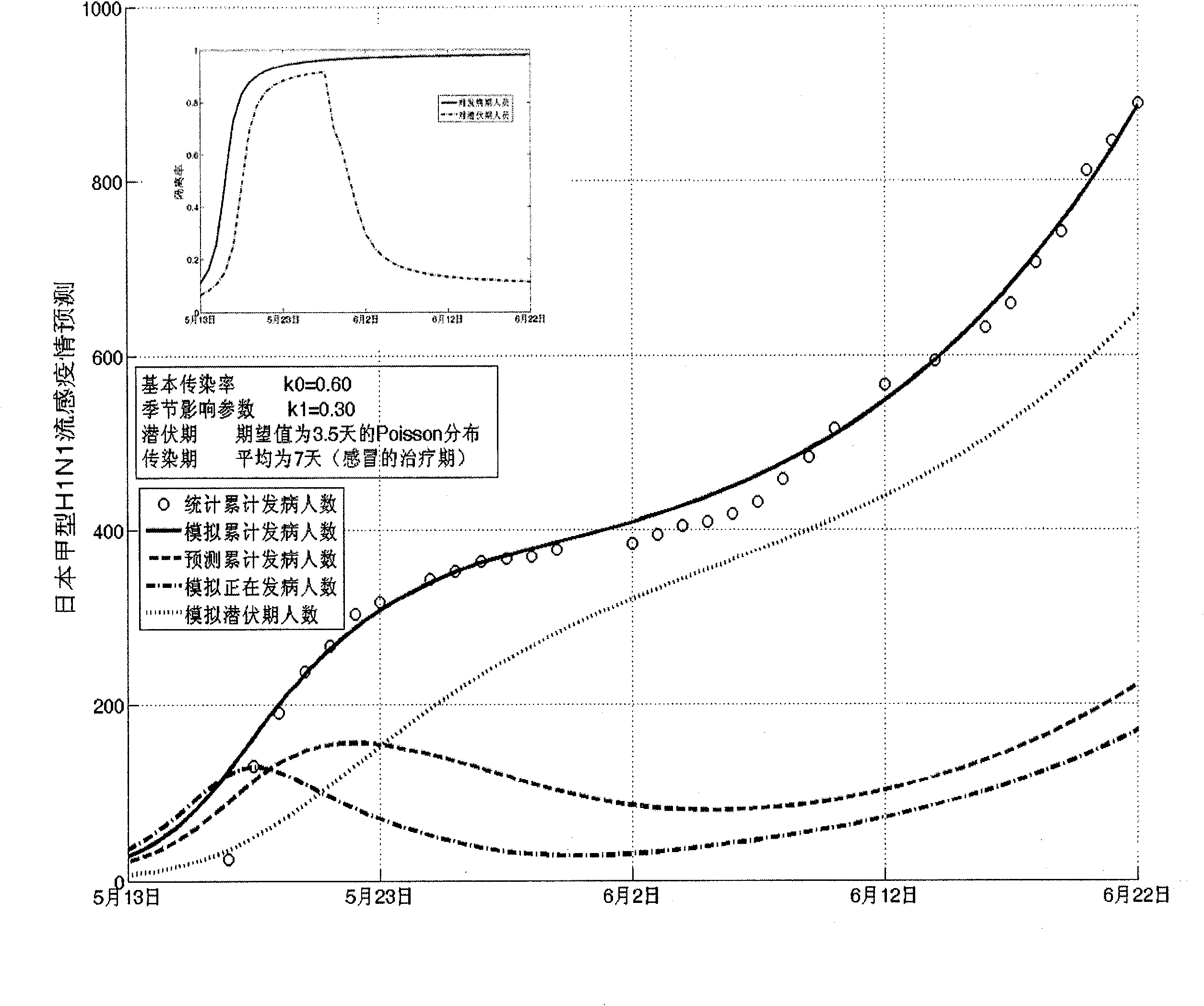
Infectious disease epidemic situation predicative analysis method based on nonlinear and coefficient variation predictive model
InactiveCN101794342ASpecial data processing applicationsSusceptible populationSevere acute respiratory syndrome
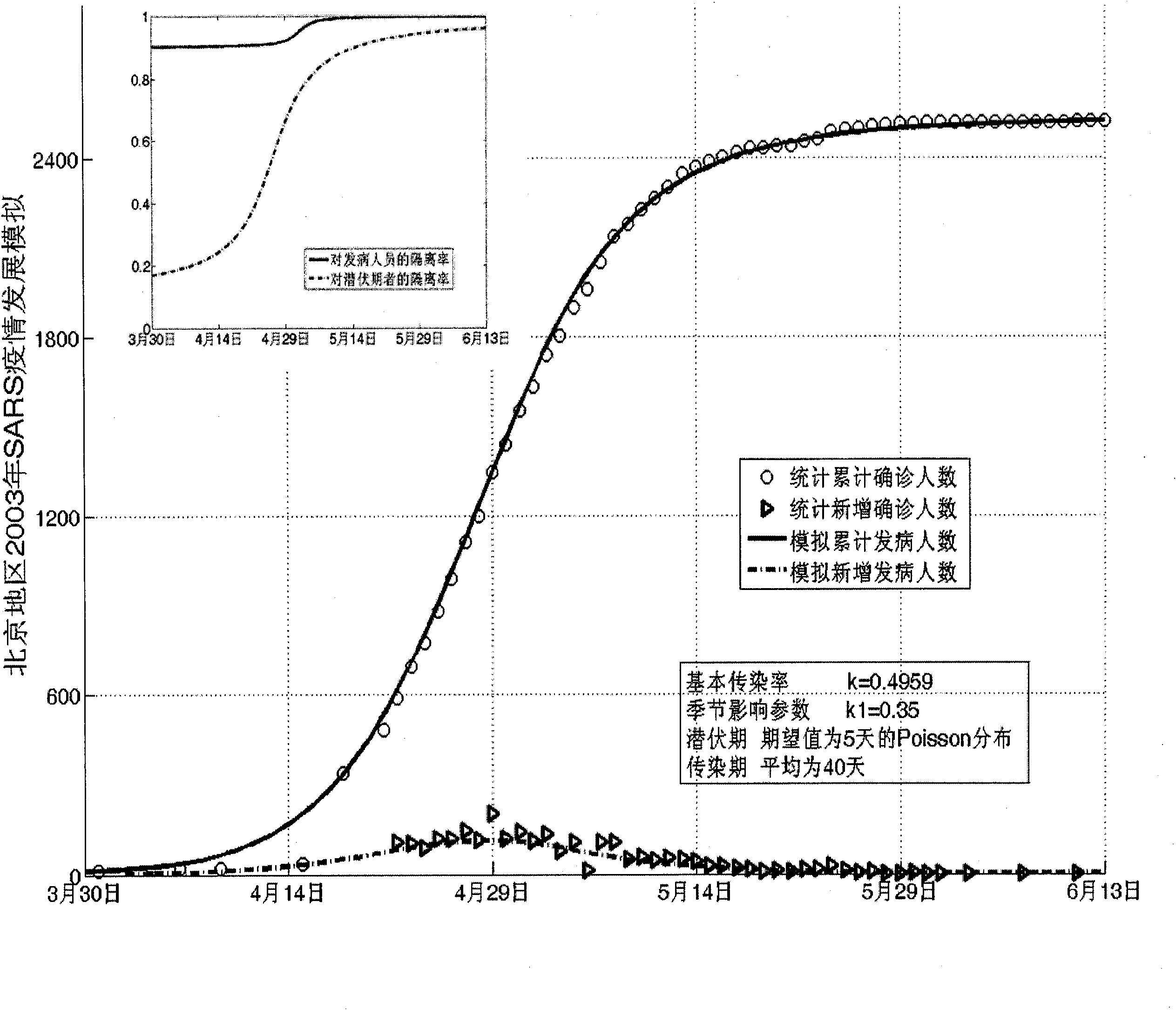
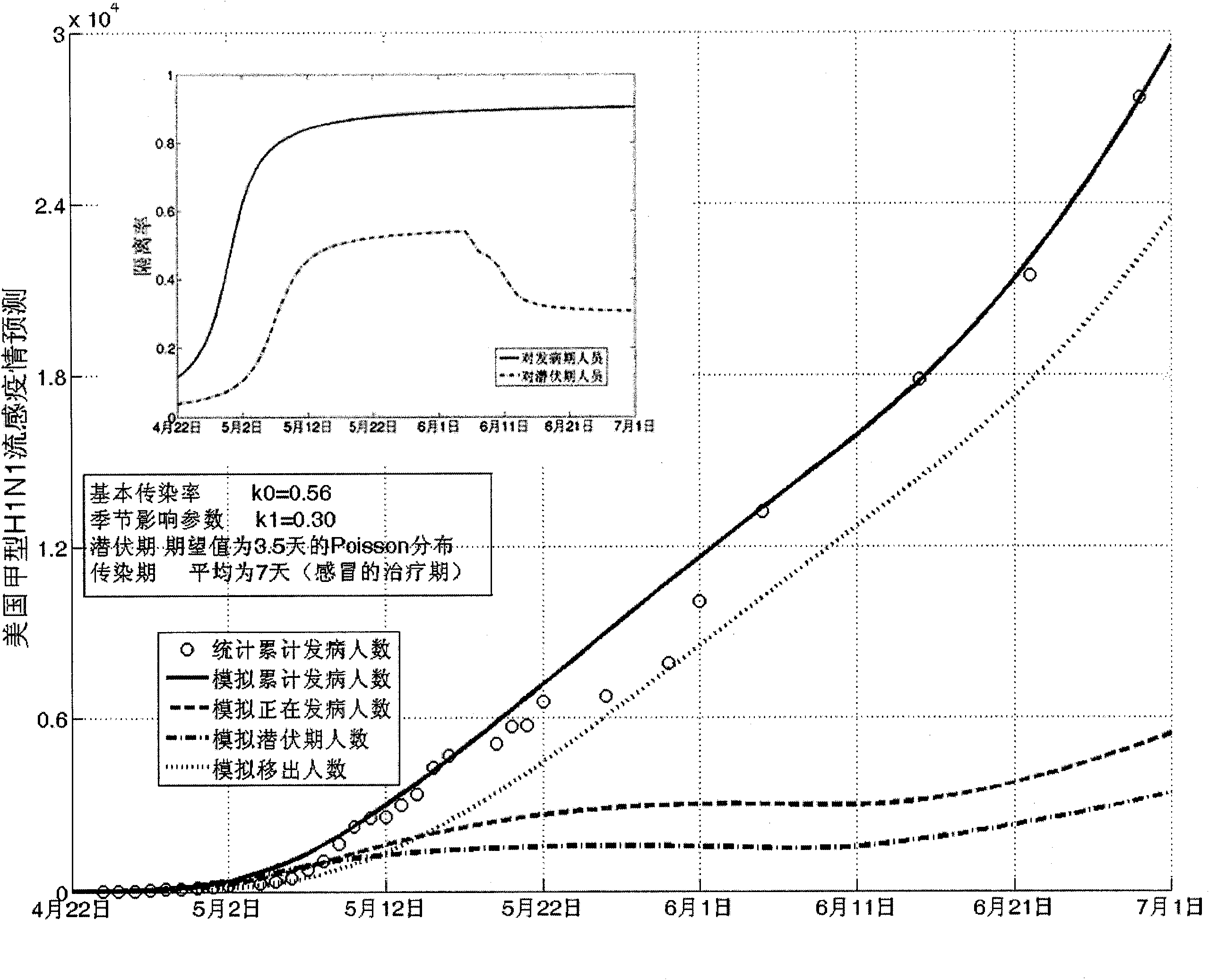
The invention establishes a nonlinear and coefficient variation infectious disease predictive model aiming at epidemic diseases with viruses which have infectivity at a latent period and a period of onset, provides an epidemic situation control function directly related to the model, simulating and predicting effects of different control measures and different control degrees on the basis of prediction in consideration of the control measures, considers the epidemic situation control as a continuous change process, integrally simulating and predicting development and control of the epidemic situation and provides crucial quantitative information for decision-making departments to optimally decide and control the epidemic situation with the smallest cost. By adopting the invention, a relative error for simulating SARS (Severe Acute Respiratory Syndromes) in Beijing areas in 2003 is 0.98% and predictive results for influenza A virus subtype H1N1 in US and Japan are well matched with the actual epidemic situation development, a quantified control factor for preventing the influenza A virus subtype H1N1 at an initial development stage and controlling the spread of the epidemic situation is obtained and epidemic situation development conditions of different control intensities and different susceptible people are predicted.
Owner:中国人民解放军防化指挥工程学院
Antibacterial antiviral treating agent, preparation method and application thereof
ActiveCN102669179AImprove the safety of useSimple production processBiocideDisinfectantsAvian influenza virusSilver colloid
The invention relates to an efficient antibacterial antiviral treating agent, a preparation method and an application thereof. The antibacterial antiviral treating agent comprises a nanometer silver colloid, an organic anti-bacterial agent, a cosolvent, a polymer binder and water. According to the antibacterial antiviral treating agent, the use security is high, the quick-acting antibacterial rate of processed medical masks can reach to above 99%, the quick-acting killing effect on H1N1 influenza a virus and H5N1 avian influenza virus is excellent, and the antibacterial antiviral treating agent is non-toxic and harmless to skin and has an excellent long lasting effect.
Owner:BEIJING CHAMGO NANO TECH
Respiratory pathogen multi-detection reagent kit
InactiveCN109355437AHigh detection sensitivityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoronavirus 229EFluorescence
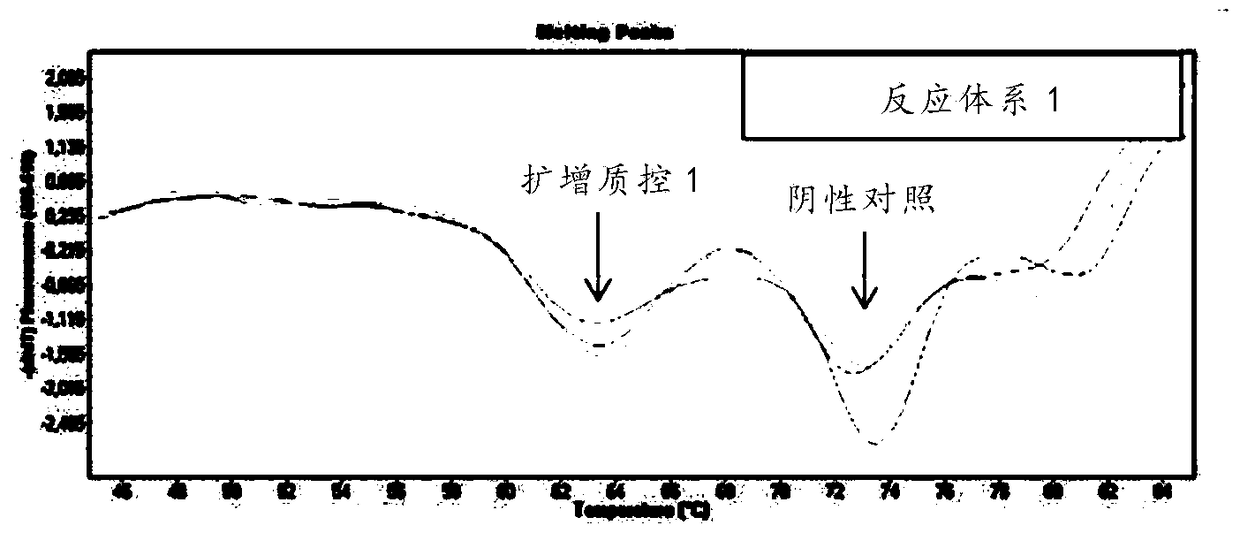
The invention discloses a respiratory pathogen multi-detection reagent kit. The respiratory pathogen multi-detection reagent kit has the advantages that the respiratory pathogen multi-detection reagent kit is based on multi-PCR (polymerase chain reaction) technologies, detection results can be determined by the aid of fluorescence resonance energy transfer via the melting temperature ranges, the respiratory pathogen multi-detection reagent kit can be used for qualitatively simultaneously detecting 16 types of respiratory pathogens, the 16 types of respiratory pathogens include 12 types of RNA(ribonucleic acid) viruses (influenza A viruses, influenza B viruses, H1N1 influenza A viruses, type A and type B respiratory syncytial viruses, type -1 / -2 / -3 parainfluenza viruses, type OC43 coronaviruses, type 229E coronaviruses, rhinoviruses and human metapneumovirus), 2 types of DNA (deoxyribonucleic acid) viruses (adenoviruses and bocavirus) and 2 types of bacteria (mycoplasma pneumoniae andbordetella pertussis), the respiratory pathogen multi-detection reagent kit is high in detection sensitivity, and the sensitivity even can reach 1 copy / reaction; the multi-detection reagent kit is good in specificity, and negative results of pathogens which have identical sampling sites and similar pathogenic mechanisms and are not in the detection range of the respiratory pathogen multi-detectionreagent kit can be obtained; the respiratory pathogen multi-detection reagent kit is short in operation time and easy to operate and can be used for quickly detecting the 16 types of respiratory pathogens in a single tube of a reaction system, the results are clear and are easy to interpret, and the like.
Owner:上海捷诺生物科技股份有限公司
Preparation of nose-spraying flu immunization pentavalent or multivalent inactivated vaccine and application thereof
InactiveCN101732711ANo side effectsAntiviralsViruses/bacteriophagesVirus-like particleMultivalent Vaccine
The invention discloses a nose-spraying flu immunization pentavalent or multivalent inactivated vaccine and preparation method thereof. The vaccine is inactivated vaccine antigen of totivirus, lytic virus, viron or virus-like particles, flue multivalent vaccine antigen is flue pentavalent, namely H1N1, H3N2, B, H5N1 and A (H1N1) or multivalent vaccine antigen combined on the basis at will, or flue multivalent vaccine antigen obtained by containing all the combination of the HA selecting from H1, H2, H3, H4, H5, H6, H7, H8, H9, H10, H11, H12, H13, H14, H15 and H16 and the NA selecting from N1, N2, N3, N4, N5, N6, N7, N8 and N9 subtypes on the basis. The content of flu multivalent inactivated vaccine antigen HA in the vaccine of the invention is 1.0-15.0 Mug / 0.2ml / per person, and the vaccine of the invention can effectively prevent routine human flue, high pathogenicity H5N1 avian-human flu, influenza A (H1N1) and infection of other subtype influenza viruses.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
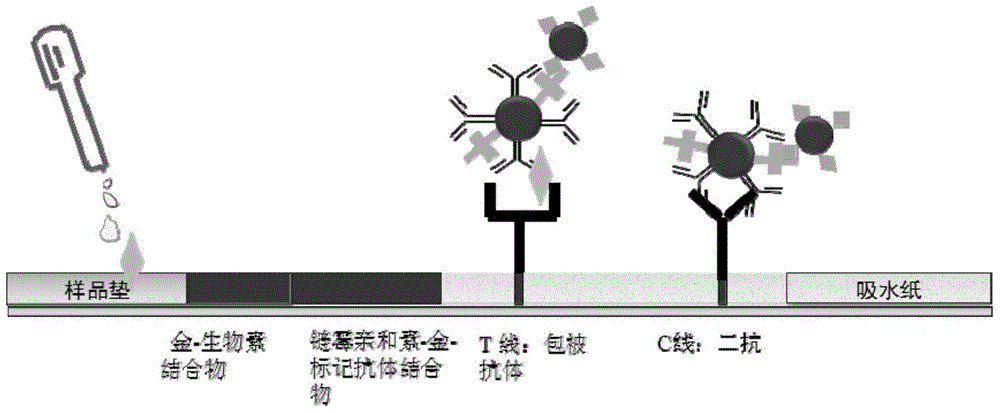
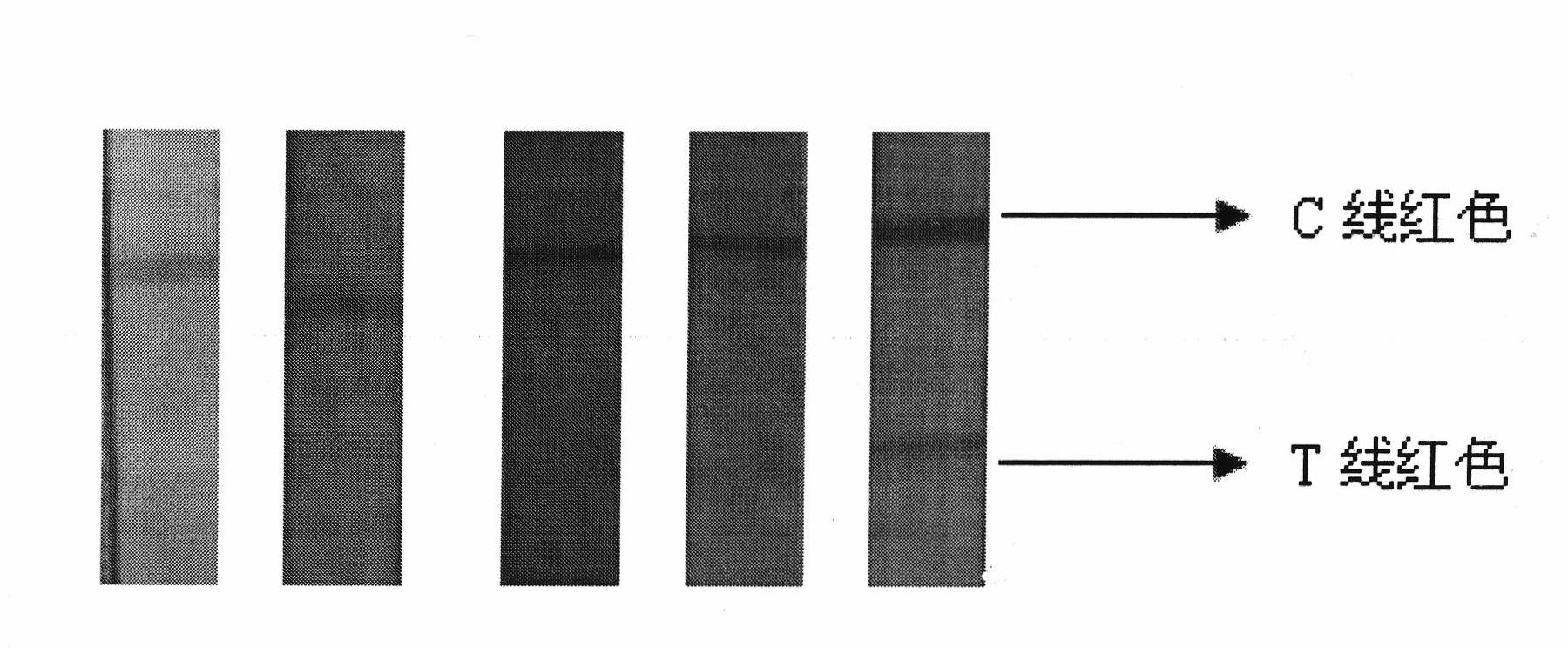
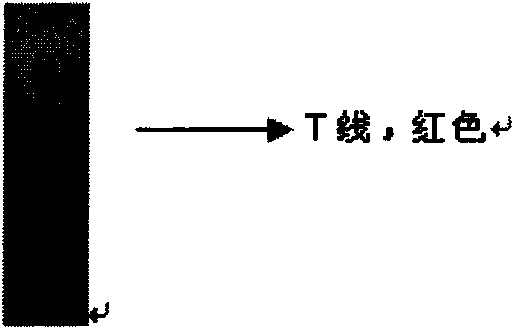
Colloidal gold immunochromatography test strip used for testing H1N1 influenza antigen and method for testing H1N1 influenza antigen
ActiveCN104101706ARead results quickly and intuitivelyGet rid of dependenceBiological material analysisAntibody conjugateTest strips
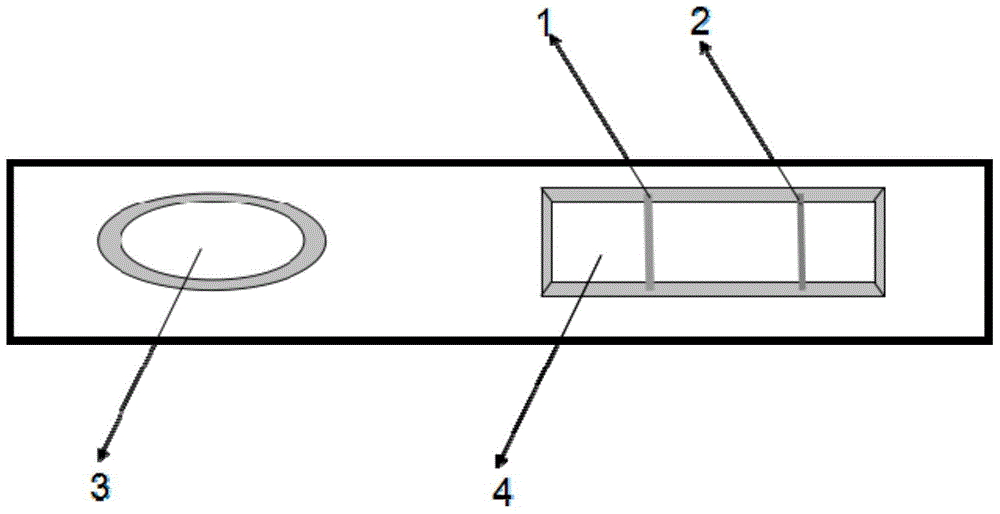
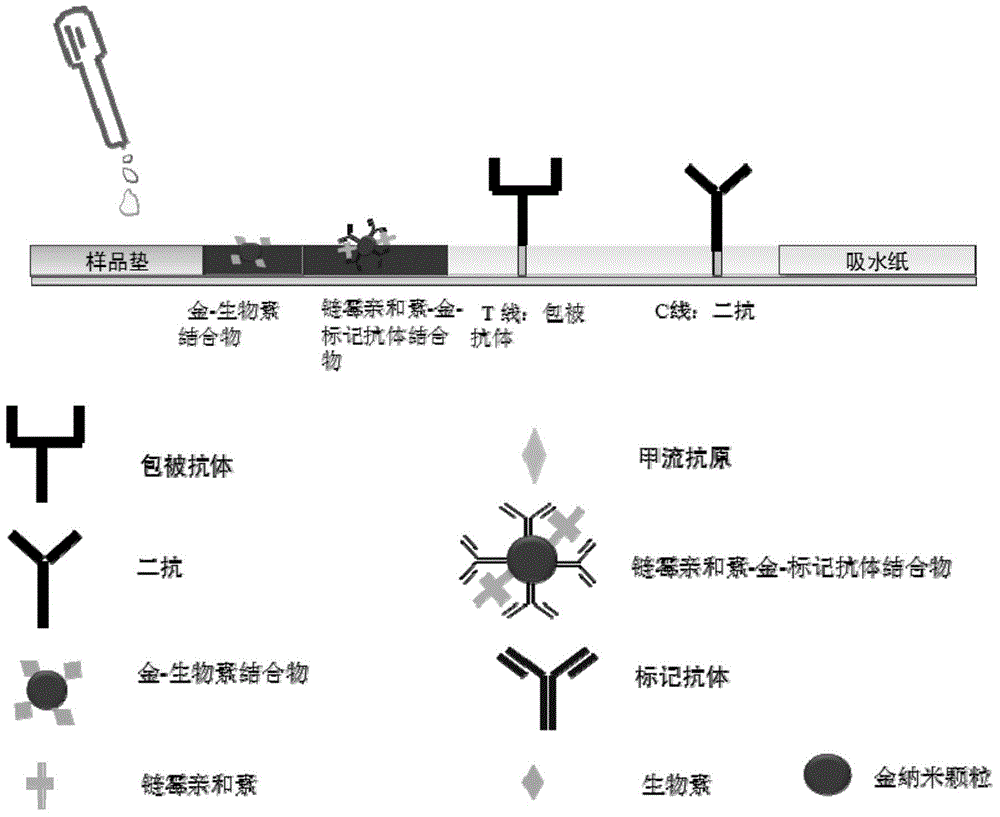
The invention relates to a colloidal gold immunochromatography test strip used for testing an H1N1 influenza antigen and a method for testing the H1N1 influenza antigen. The test strip comprises a base plate, a sample pad, a conjugate pad, a pyroxylin film and an absorbent paper, wherein the sample pad, the conjugate pad, the pyroxylin film and the absorbent paper are lapped and adhered on the base plate sequentially; the conjugate pad is a combination of a gold-label-biotin conjugate pad and a streptavidin-gold-antibody conjugate pad; according to the method for testing the H1N1 influenza antigen, a sample to be tested is dropwise added on the test strip; if a red band is displayed at a testing line (1), the sample to be tested contains the H1N1 influenza antigen. According to the advantages, the test strip provided by the invention is cheap in price, fast, simple, convenient, and greatly suitable for on-site testing; moreover, the test strip can meet the requirement of higher testing sensitivity, and has an important clinical diagnostic significance.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Recombinant baculovirus expressing manually modified and synthesized influenza A H1N1 virus HA-NA-M1 gene
InactiveCN101624580AImprove expression levelImprove screening efficiencyGenetic material ingredientsVirus peptidesInfluenza A (H1N1) virusSapovirus
The invention relates to the field of virology, in particular to a recombinant baculovirus which is manually modified and synthesized and contains a main immunogenic gene HA-NA-M1 of an influenza A H1N1 virus. The strain QP-Ac-HNM1 belongs to the baculovirus (Baculovirus) and is preserved in the China Center for Type Culture Collection (CCTCC) with the preserving number of CCTCC-V200912. The recombinant virus is capable of synchronously expressing the HA and NA of the influenza A H1N1 virus and M1 proteins to form virus particles which can be used for developing vaccines so as to prevent human beings and swine from being infected with the influenza A H1N1 virus.
Owner:HUAZHONG AGRI UNIV
Fast detection method of nucleic acid of A H1N1 influenza virus and kit thereof
InactiveCN101845517AIncreased sensitivitySolve pollutionMicrobiological testing/measurementFluorescence/phosphorescenceSingle-Stranded RNAReverse transcriptase
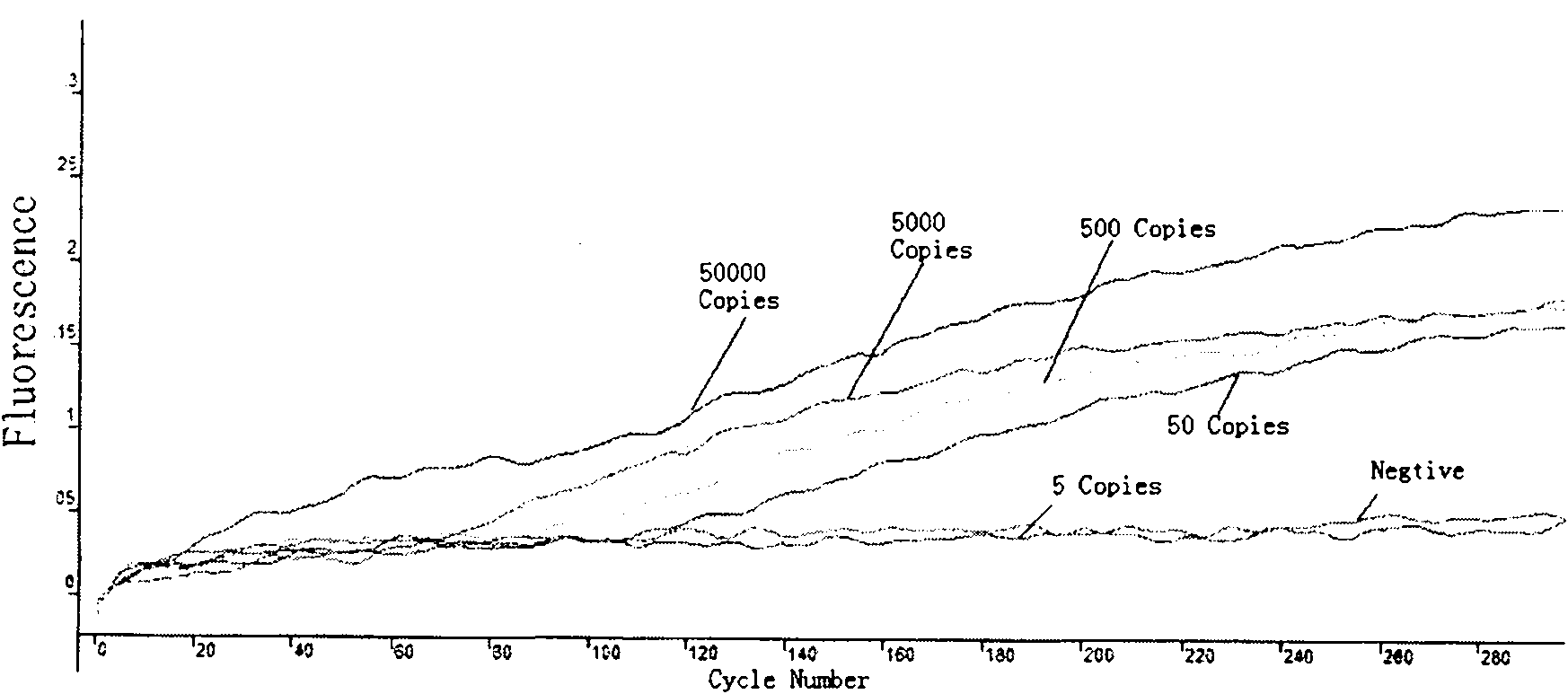
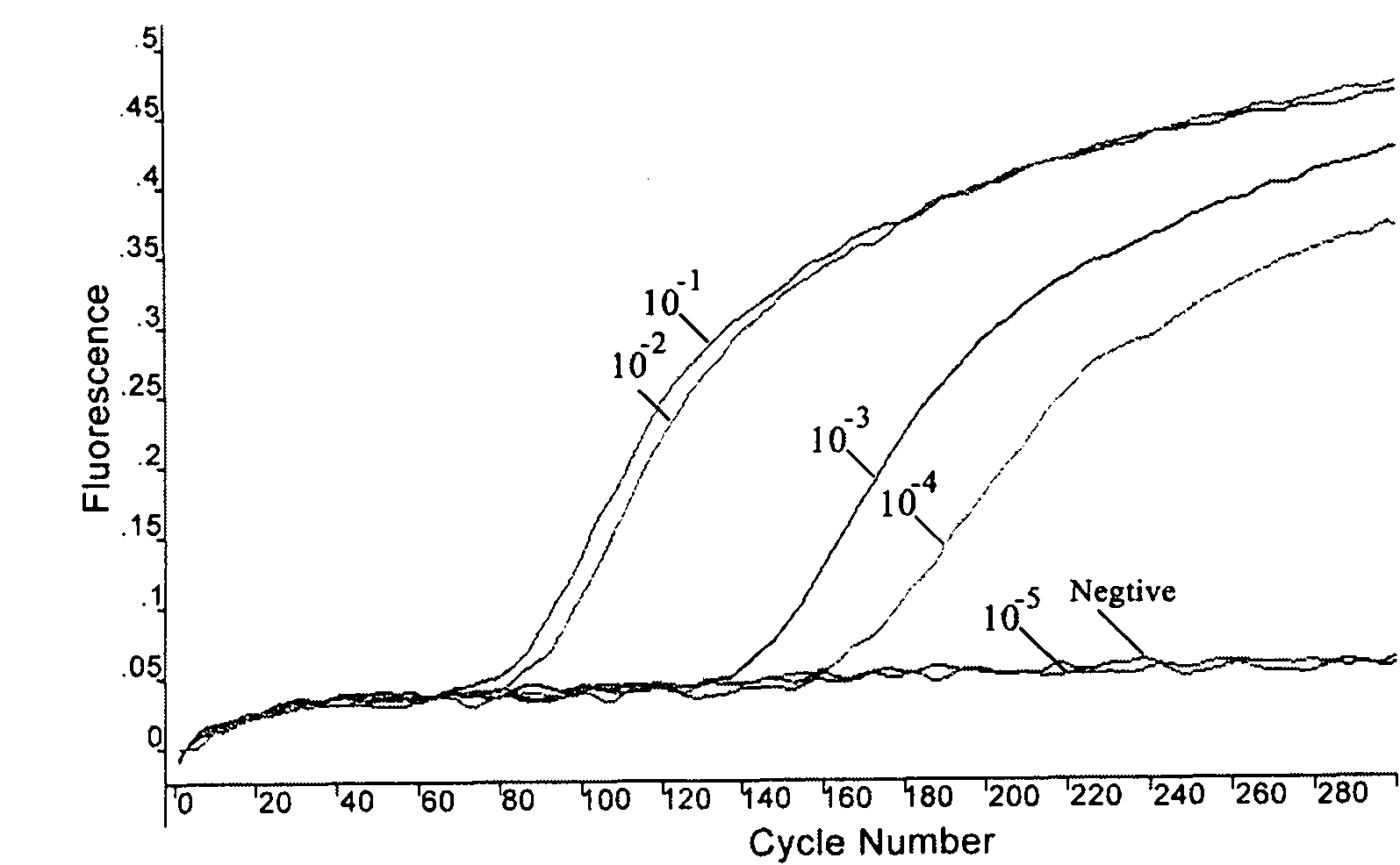
The invention relates to a fast detection method of nucleic acid of A H1N1 influenza virus, and a kit thereof, belonging to the technical field biotechnology. The invention provides a fast and synthermal RNA amplification method of A H1N1 influenza virus, and the accomplishment of the entire reaction depends on the cooperation of the AMV reverse transcriptase, the T7RNA polymerase and nuclease H (RNaseH), without the needs of the special instruments and the temperature cycling, the amplification efficiency is larger than or equal to that of the PCR, and the reaction product is single stranded RNA. The invention has the advantages of having equal or higher sensibility than that of the RT-PCR detection method, avoiding the pollution problem of the RT-PCR amplification product due to that the amplification product is RNA, finishing the reaction in one hour, and simple and convenient operation. The reaction can be monitored in real time and detected by the end-point method, can be used as the important tool in the detection of A H1N1 influenza virus.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Loop-mediated isothermal amplification detection method and kit for H1N1 influenza A viruses
InactiveCN102108418AStrong specificityRapid testing requirementsMicrobiological testing/measurementMicroorganism based processesNucleic acid amplification techniqueLoop-mediated isothermal amplification
The invention relates to a method for detecting H1N1 influenza A viruses based on the LAMP (loop-mediated isothermal amplification of nucleic acid) technology, which comprises the following steps: extracting virus RNA (ribonucleic acid); carrying out reverse transcription and LAMP amplification; converting the LAMP amplification product into single-chain substances; and detecting the LAMP amplification product by using a nucleic acid nano-gold biosensor. The invention also provides a kit for detecting H1N1 influenza A viruses. By using the specially-designed LAMP inner primer to introduce an enzyme cutting site sequence segment into the amplification product in the LAMP amplification process, using the restriction endoenzyme TspR I to digest the amplification product and acquiring a large number of single-chain DNA (deoxyribonucleic acid) products with the length of 140bp or so by physical shock cooling, the combination of the LAMP technology based on the specific primer and the nucleic acid nano-gold biosensor with a specific probe ensures that the detection method and kit has high specificity and high detection speed.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
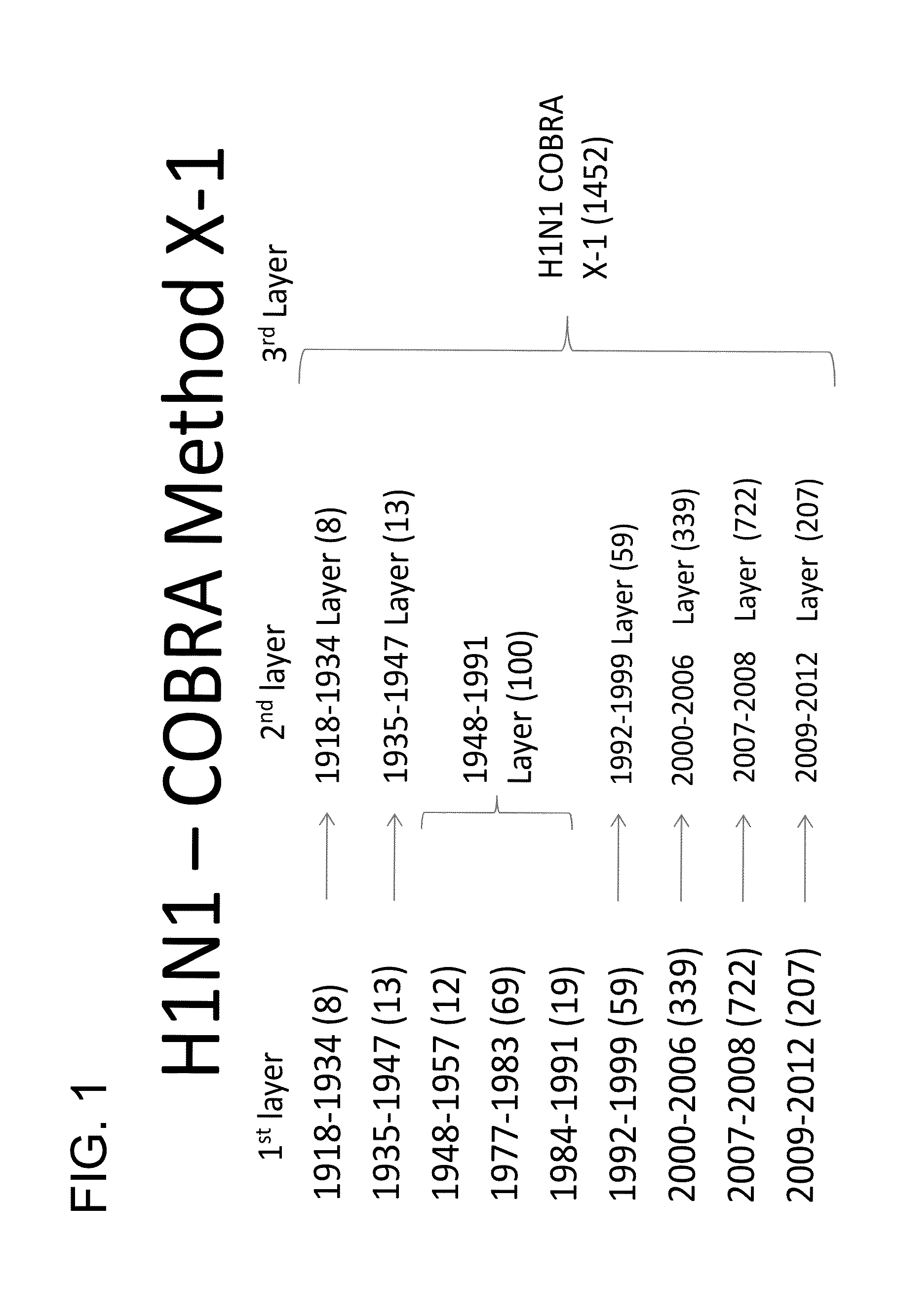
Computationally optimized broadly reactive antigens for h1n1 influenza
Described herein is the generation of optimized H1N1 influenza HA polypeptides for eliciting a broadly reactive immune response to H1N1 influenza virus isolates. The optimized HA polypeptides were developed through a series of HA protein alignments, and subsequent generation of consensus sequences, based on selected H1N1 viruses isolated from 1918-2012. Provided herein are optimized H1N1 HA polypeptides, and compositions, fusion proteins and VLPs comprising the HA polypeptides. Further provided are codon-optimized nucleic acid sequences encoding the HA polypeptides. Methods of eliciting an immune response against influenza virus in a subject are also provided by the present disclosure.
Owner:UNIVERSITY OF PITTSBURGH
Chinese medicinal composition for preventing and treating flu
InactiveCN101690745ANo side effectsShort course of treatmentAntiviralsRespiratory disorderSide effectMedicine
The invention relates to a Chinese medicinal composition for preventing and treating flu. The composition is characterized by being prepared from the following raw materials in part by weight: 12 to 15 parts of lonicera, 6 to 10 parts of weeping forsythia capsule, 3 to 6 parts of liquorice root, 12 to 15 parts of indigowoad root, 9 to 10 parts of baical skullcap root, 9 to 12 parts of mint, 6 to 9 parts of platycodon root, 9 to 12 parts of mulberry leaves, 12 to 15 parts of indigowoad leaves. The Chinese medicinal composition can be used for treating and preventing flu similar to symptom of A / H1N1 flu, and has remarkable curative effect, short curative period, low cost and no toxic or side effect.
Owner:FUZHOU CANGSHAN JINDIANZI INFORMATION CONSULTING
Application of traditional Chinese medical composition in preparation of medicament for resisting influenza A H1N1 influenza viruses
The invention discloses the application of a traditional Chinese medical composition in the preparation of a medicament for resisting influenza A H1N1 influenza viruses. The traditional Chinese medical composition is prepared from the medical materials of forsythia, honeysuckle, ephedra, bitter almond, and the like, has broad-spectrum antiviral action, can effectively kill the viruses, reduce fever and diminish inflammation. Proved by experiments, the traditional Chinese medical composition can accurately resist the influenza A H1n1 influenza viruses.
Owner:BEIJING YILING PHARMA
Typing detection kit for influenza virus
ActiveCN102337351AEasy to operateShort timeMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceEnzyme
The invention relates to a typing detection kit for an influenza virus, especially to a kit for detecting influenza virus A, influenza virus B and influenza virus A (H1N1) with a multiple real-time fluorescent polymerase chain reaction technology. The kit mainly comprises an RT-PCR (real-time polymerase chain reaction) reaction solution, a mixed solution of primer and probe, RT-PCR reaction enzymes, DEPC H2O, and packing boxes for separation and centralized packaging of the reagent bottles or tubes. Able to distinguish influenza virus A, influenza virus B and influenza virus A (H1N1) accurately, the kit of the invention can be widely applied in multiple fields like differential diagnosis and epidemic prevention and control of influenza caused by the above influenza viruses.
Owner:DAAN GENE CO LTD +1
Traditional Chinese medicinal composition for preventing or treating common cold and/or flu, method for preparing same and application thereof
ActiveCN102085311AGive full play to the role of multi-channel treatmentAntipyreticAntipyreticAnalgesicsWhole body painHypericum perforatum
The invention discloses a traditional Chinese medicinal composition for preventing or treating common cold and / or flu. The Chinese medicinal composition is characterized by being prepared from the following raw materials: honeysuckle flower, weeping forsythia, mint, herba schizonepeta or schizonepetaspike, hypericum perforatum, burdock fruit, balloonflower, henon bamboo leaf and liquorice. The Chinese medicinal composition has the effects of dispelling wind, relieving exterior syndrome, clearing away heat and detoxicating, resisting H1N1 influenza virus and inhibiting avian influenza virus H9N2, and can be used for treating common cold and flu, particularly relieving the symptoms of fever headache, whole body pain, cough, throat pain, algidity, fatigue and the like. The preparation prepared by the method has obvious curative effect, is safety, non-toxic, convenient for taking and suitable for clinical application.
Owner:广州青蒿医药科技有限公司 +2
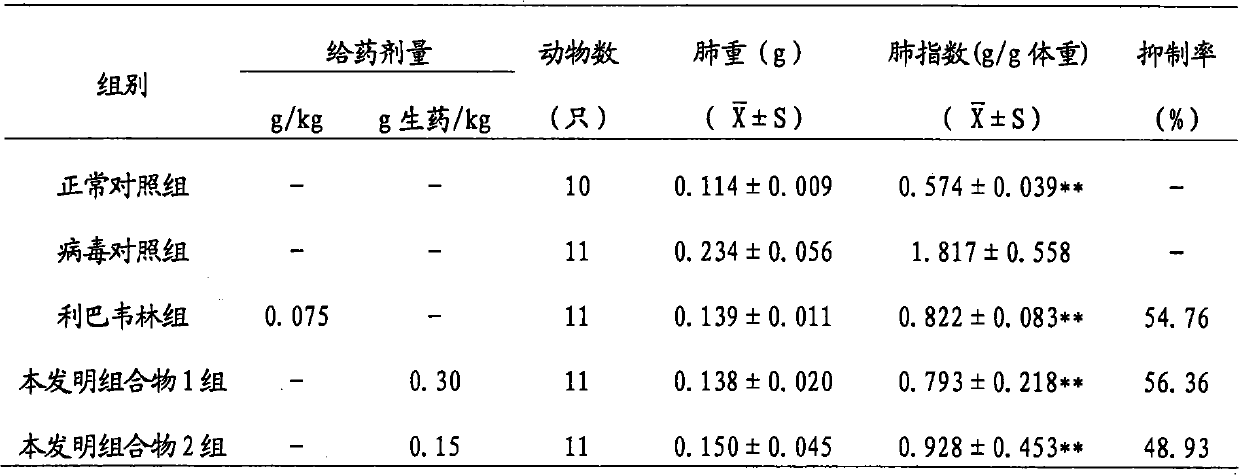
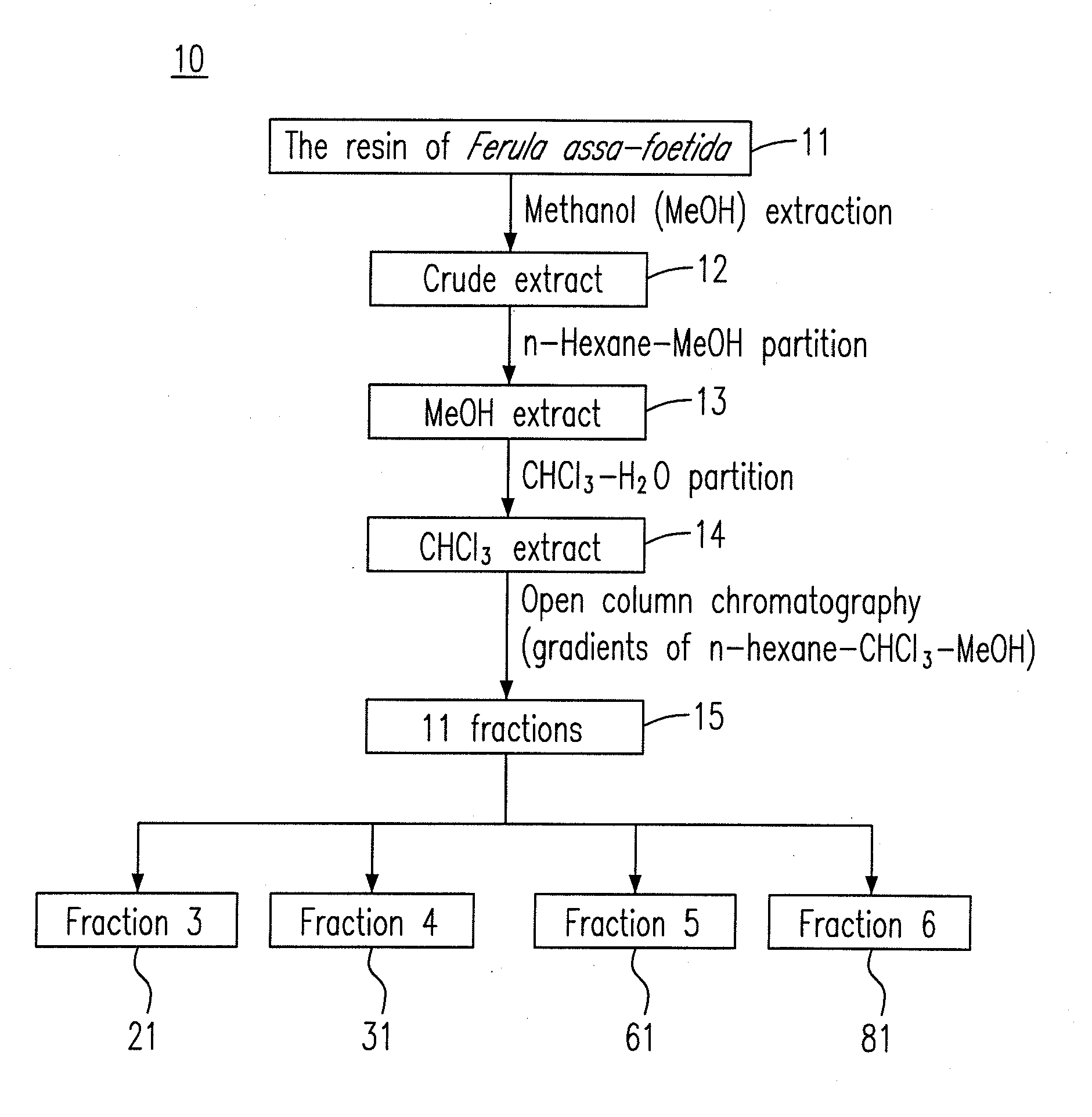
Composition for treating influenza a (H1N1) virus and a preparation method therefor
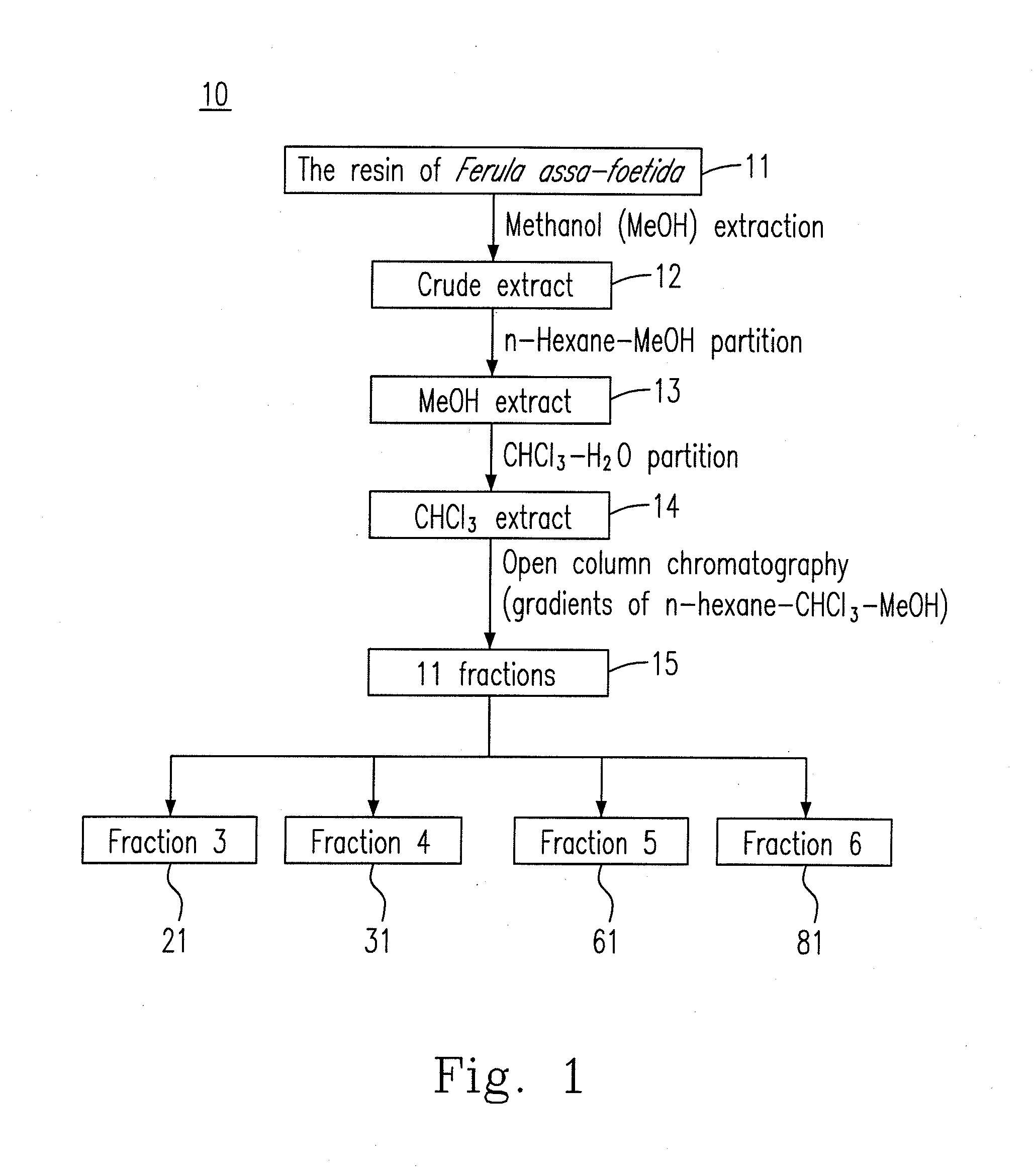
ActiveUS20110196029A1Inhibitory activityGrowth inhibitionBiocideOrganic chemistryChromatographic separationFerula
New pharmaceutical compositions extracted from Ferula assa-foetida are confirmed to effectively treat influenza A (H1N1) virus. The extraction method of the new pharmaceutical compositions mainly includes steps of (a) extracting F. assa-foetida with methanol to obtain a crude extract; (b) fractioning the crude extract with n-hexane-methanol to obtain a methanol extract; (c) fractioning the methanol extract with chloroform-water to obtain a chloroform extract; and (d) chromatographing the chloroform extract to obtain the pharmaceutical compositions, which can be further fractioned and chromatographed to obtain various sesquiterpene coumarins.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
Kit for dual detection of viruses by nucleic acid and antibodies and preparation method thereof
ActiveCN112063765AImprove accuracyLow costMicrobiological testing/measurementBiological material analysisNitrocelluloseVirus
The invention relates to a kit for dual detection of viruses by nucleic acid and antibodies and preparation method thereof. According to the invention, an RPA primer and a probe which specifically aimat H1N1 detection are obtained by analyzing an H1N1 sequence, and the RPA primer and the probe are prepared into a corresponding detection test strip. At the same time, monoclonal antibodies which have good effects are screened and obtained aiming at an H1N1 conserved region. An H1N1 influenza virus fluorescence quantum dot rapid detection test paper is prepared from a monoclonal antibody labeledquantum dot and other antibody labeled nitrocellulose membranes. The two detection methods are combined for use, so that the detection accuracy can be further improved, the cost is low, and the kit and method is suitable for large-scale popularization.
Owner:GUANGDONG CELL BIOTECHNOLOGY CO LTD
Method for preparing anti-H1N1 specific immunoglobulin of egg yolk
InactiveCN101787080AIncrease productionHigh activityEgg immunoglobulinsImmunoglobulins against virusesInfluenza aGene expression
The invention discloses methods for preparing and isolating anti-H1N1 specific immunoglobulin of egg yolk (IgY). The IgY is prepared by the following steps: 1. expressing HA segments of H1N1 by genes to obtain the antigen of H1N1; 2. carrying out injection immersion on the laying hens with the prepared H1N1 antigen to obtain anti-H1N1 immune eggs; and 3. extracting the crude products of the anti-H1N1 specific IgY and purifying and filtering the crude products, thus obtaining the finished anti-H1N1 IgY. The obtained anti-H1N1 IgY has low cost, high yield and high specificity and can be used for preparing various preparations having the functions of preventing and treating H1N1.
Owner:GUANGDONG UNIV OF TECH +1
Novel Kit for fluorescence quantitative PCR detection of influenza A H1N1 viruses and detecting method therefor
InactiveCN101665842AAccurate detectionHigh detection specificityMicrobiological testing/measurementMicroorganism based processesH1n1 virusInfluenza A (H1N1) virus
The invention discloses a novel kit for fluorescence quantitative PCR detection of influenza A H1N1 viruses, containing a primer, a probe, Taqman 2*universal PRC master mix, 40*multi scribe<TM> and RNase inhibitor mix and nuclease-free water. The primer and the probe have the advantages of good detecting specificity and high sensitivity so that the kit is particularly suitable for the influenza AH1N1 which breaks out at present and has no cross reaction with the Avian flu, swine flu and common seasonal flu and has lower cost.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Use of neuraminidase inhibitor and neuraminidase inhibitor prodrug
ActiveCN103845316AExtended half-lifeOrganic chemistryOrganic compound preparationPatient needViral infection
The invention provides use of a compound shown as formula Ia or a prodrug thereof in preparation of drugs for the prevention and / or treatment of Tamiflu resistant influenza viruses strain infection, a Tamiflu resistant influenza viruses strain is preferably H1N1 Swine-origin influenza A virus H274Y. The invention also provides a method for the treatment, prevention or delay of virus infection caused by the Tamiflu resistant influenza viruses strain, and the method comprises administering of a therapeutically effective amount of the compound or the prodrug thereof to patients needing the treatment.
Owner:BEIJING PRELUDE PHARM SCI & TECH
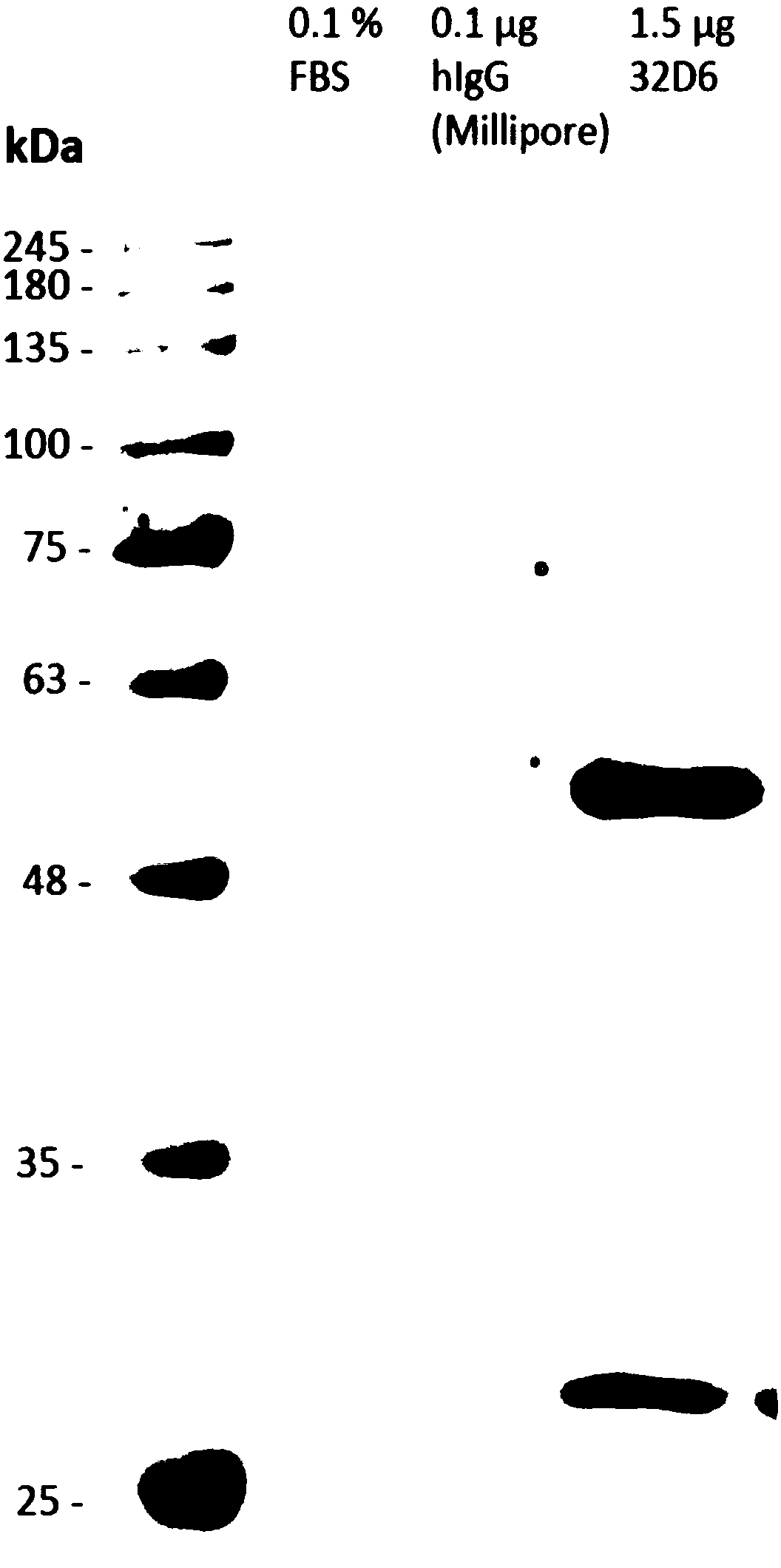
Monoclonal antibodies to influenza h1n1 virus uses thereof
InactiveUS20120100142A1Prevention and reduction of severitySugar derivativesMicrobiological testing/measurementH1n1 virusMonoclonal antibody
The present invention is directed to particular monoclonal antibodies and fragments thereof that find use in the detection, prevention and treatment of influenza virus infections. In particular, these antibodies may neutralize or limit the replication of H1N1 influenza virus. Also disclosed are improved methods for producing such monoclonal antibodies.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Application of poly mannuronic acid propyl ester sulfate in preparing anti- H1N1 influenza A virus medication
InactiveCN102743409AAvoid infectionPrevent proliferationOrganic active ingredientsAntiviralsCanine kidneyPolymannuronic acid
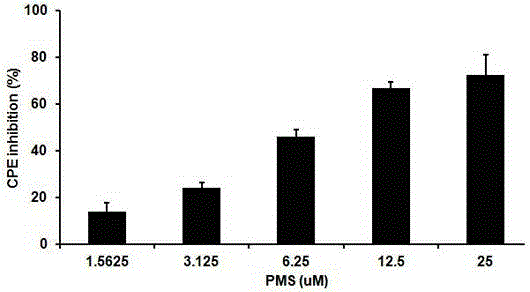
The invention provides applications of poly mannuronic acid propyl ester sulfate (PMS) in preparing anti- H1N1 influenza A virus medication. Experiments of the invention prove that PMS not only has great inhibition effect on the neuraminidase of the influenza A virus, but also has relatively good protection effect on canine kidney epithelial cells infected with the H1N1 influenza A virus, and can reduce the replication of the H1N1 virus with an effect that equal to the positive control medication ribavirin. Additionally, the PMS can effectively reduce the death rate of mice infected with the H1N1 influenza A virus and the survival time of the mice are prolonged. The poly mannuronic acid propyl ester sulfate provided by the invention has significant activity of inhibiting the neuraminidase of the H1N1 influenza A virus, and is proved to have good anti- H1N1 influenza A virus activity both in vivo and in vitro experiments, which shows the wide application prospect of the poly mannuronic acid propyl ester sulfate in preparing anti-H1N1 influenza A virus medication.
Owner:OCEAN UNIV OF CHINA
H1n1 flu virus neutralizing antibodies
ActiveCN107922481ABiological material analysisImmunoglobulins against virusesH1n1 virusComplementarity determining region
An antibody, or a binding fragment of the antibody, against H1N1 virus, includes a heavy chain variable region and a light chain variable region, wherein the heavy chain variable region contains complementarity determining regions (CDR) that have the amino acid sequences of SEQ ID NO: 5, SEQ ID NO: 6, and SEQ ID NO: 7; and wherein the light chain variable region contains complementarity determining regions that have the amino acid sequences of SEQ ID NO: 8, SEQ ID NO: 9, and SEQ ID NO: 10. A method for treating or preventing H1N1 infection in a subject includes administering to the subject theantibody or the binding fragment of the antibody.
Owner:MEDIGEN BIOTECH
Primers for detecting influenza virus by RT-PCR and method for detecting influenza virus
InactiveCN101560575AEasy to operateMicrobiological testing/measurementMicroorganism based processesInfluenza A (H1N1) virusQuarantine
The invention discloses primers for detecting influenza virus by RT-PCR and a method for detecting the influenza virus. The primers comprise three pairs of total six oligonucleotide primer sequences such as influenza A virus generality primer, influenza A H1N1 virus H1 generality primer, novel influenza A H1N1 virus H1 specificity primer and the like; and the invention simultaneously discloses treatment of a sample to be detected, an RT-PCR reaction system and reaction condition, and result analysis. The invention can quickly and effectively determine influenza A virus, influenza A H1N1 virus and novel influenza A H1N1 virus, and provide feasible technical support for influenza epidemic early warning mechanisms in the fields such as clinical diagnosis, inspection and quarantine and the like.
Owner:中国疾病预防控制中心病毒病预防控制所
Primers and probes for detecting influenza virus by rRT-PCR and method for detecting influenza virus
InactiveCN101560574AEasy to operateMicrobiological testing/measurementMicroorganism based processesOligonucleotide primersQuarantine
The invention discloses primers and probes for detecting influenza virus by rRT-PCR and a method for detecting the influenza virus. The primers and the probes comprise influenza A virus specificity primer and probe, two groups of novel influenza A H1N1 virus H1 specificity primers and probes, novel influenza A virus specificity primer and probe and the like, namely four kinds of total 12 oligonucleotide primer and probe sequences; and the invention simultaneously discloses treatment of a sample to be detected, a rRT-PCR reaction system and reaction condition, and result analysis. The invention can quickly and effectively determine influenza A virus, novel influenza A H1N1 virus and novel influenza A virus. The primers and the probes and the method have simple operation and convenient application, and provide feasible technical support for influenza epidemic early warning mechanisms in the fields such as clinical diagnosis, inspection and quarantine and the like.
Owner:中国疾病预防控制中心病毒病预防控制所
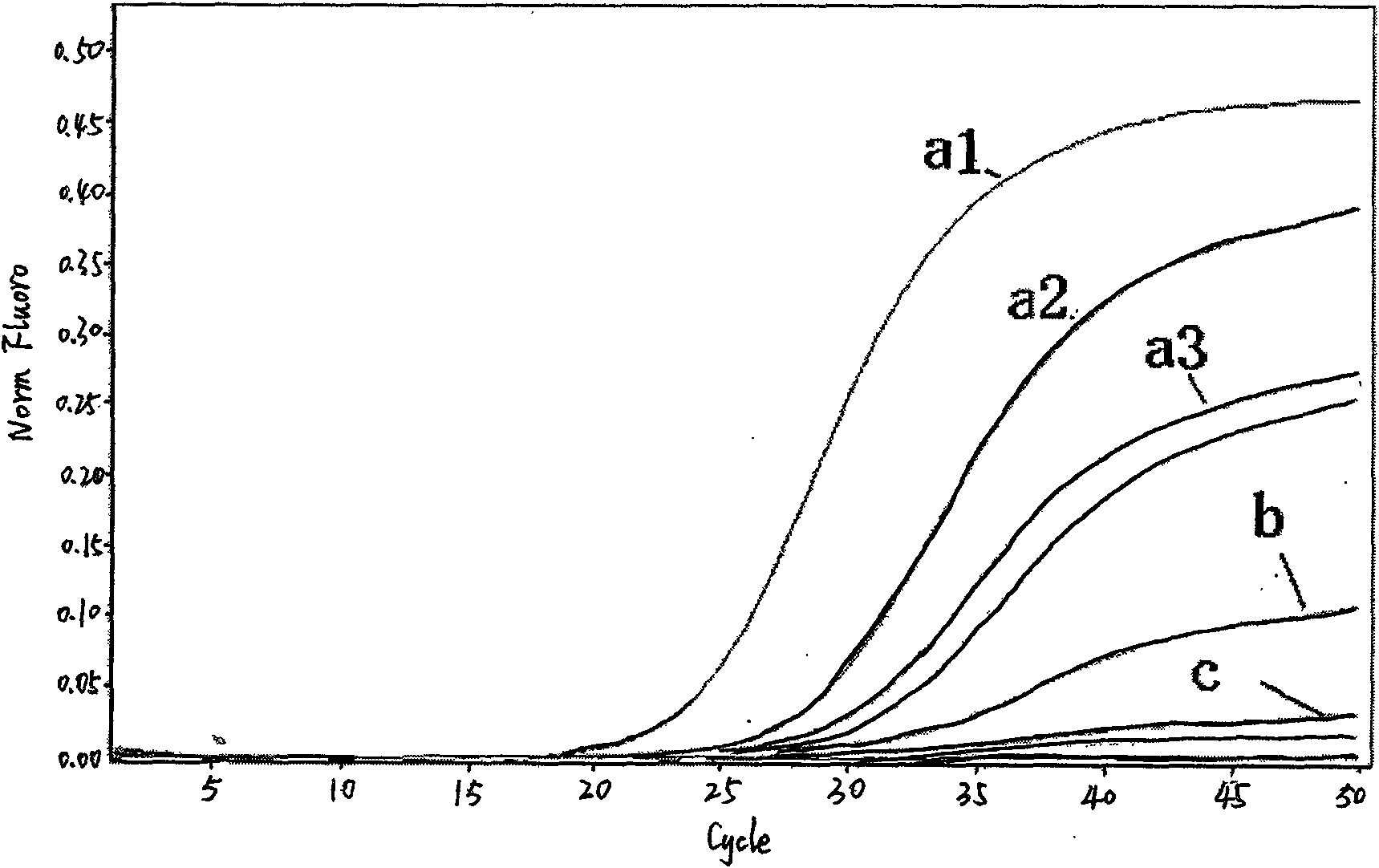
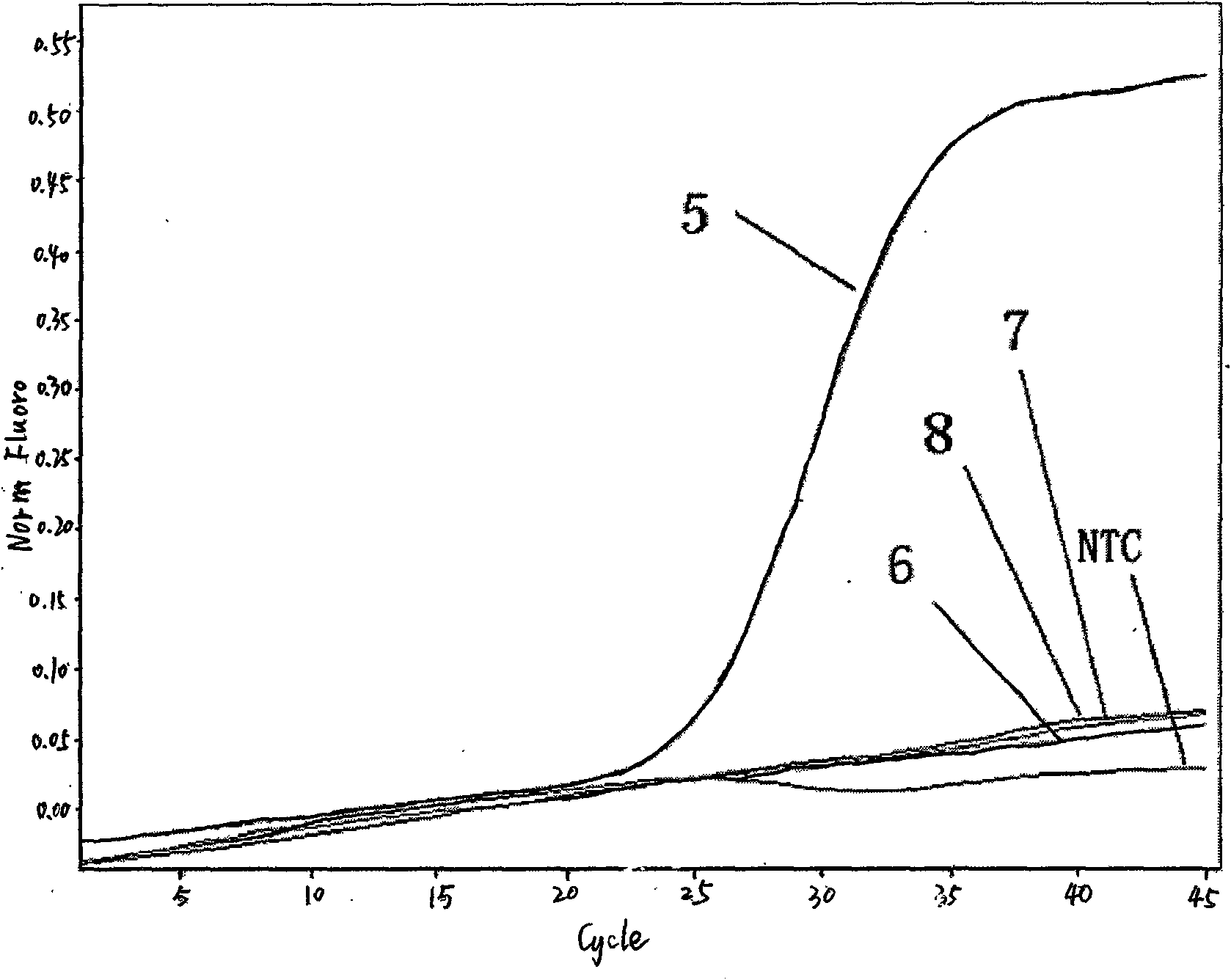
Influenza A H1N1/Influenza A Virus Nucleic Acid Dual Fluorescent PCR Detection Kit
InactiveCN102286639ASimple and fast operationAvoid pollutionMicrobiological testing/measurementFluorescence/phosphorescenceReverse transcriptaseFluorescent pcr
The invention provides a double fluorescent quantitative PCR detection kit of type A H1N1 / type A influenza virus nucleic acid, comprising RNase inhibitor, RT-PCR reaction solution, enzyme mixed solution, type A H1N1 / type A influenza virus double reaction solution , a positive control and a negative control, wherein said A / H1N1 / influenza A double reaction solution includes the following components: Component (1): a pair of primers for detecting A / H1N1 influenza virus and a primer for detecting A Type H1N1 influenza virus probe composition; component (2): composed of a pair of primers for detecting influenza A virus and a probe for detecting influenza A virus; said enzyme mixture includes Taq enzyme and reverse transcriptase . The invention can detect influenza A H1N1 and influenza A virus at the same time, and solves the problem that the existing products can only detect one type of influenza virus in one tube reaction. The invention has the advantages of simple operation, strong repeatability, rapid and objective detection results, etc., and has great application prospects in the field of in vitro diagnosis of influenza virus.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Nucleic acid nano-gold biosensor for detecting influenza A viruses and influenza A (H1N1) viruses
InactiveCN101942387AEasy to manufactureQuick checkBioreactor/fermenter combinationsBiological substance pretreatmentsNucleotideOligonucleotide
The invention relates to a nucleic acid nano-gold biosensor for detecting influenza A viruses and influenza A (H1N1) viruses. The nucleic acid nano-gold biosensor comprises a sample pad, glass fiber, a nitrocellulose membrane and absorbent paper fixed on a rubber plate in turn from left to right; the glass fiber is coated with nano-gold marked oligonucleotide probes which have a nucleotide sequence shown by SEQ ID No.2 or 7; two kinds of oligonucleotide probes are immobilized on the nitrocellulose membrane; the nitrocellulose membrane probes immobilized close to one end of the absorbent paper form a quality control line and have a nucleotide sequence shown by SEQ ID No.3 or 8; and the nitrocellulose membrane probes immobilized close to one end of the glass fiber form a detection line and have a nucleotide sequence shown by SEQ ID No.1 or 6. The nucleic acid nano-gold biosensor has the advantages of simple manufacturing and fast detection without needing professional technicians and expensive instrument and equipment.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Fluorescent quantitative detection kit of H1N1 influenza virus A and detection method thereof
ActiveCN101649356AStrong specificitySensitive detectionMicrobiological testing/measurementMicroorganism based processesReverse transcriptasePolymerase L
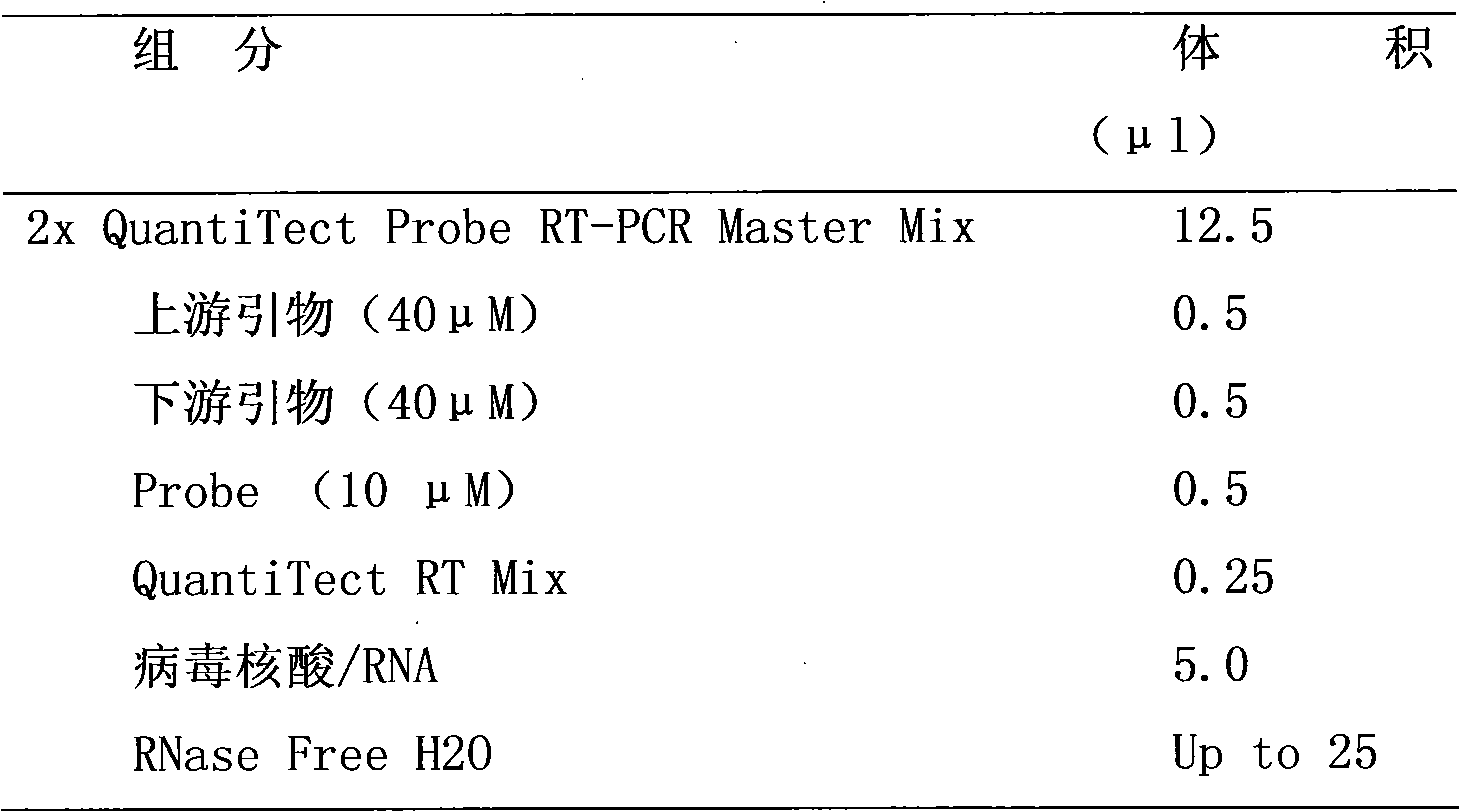
The invention provides a nucleic-acid fluorescent quantitative RT-PCR detection kit of H1N1 influenza virus A and a detection method thereof. The detection kit mainly comprises a specific primer, a fluorescent probe, a PCR buffer solution, a deoxidized nucleoside triphosphate compound, a DNA polymerase, a reverse transcriptase and an RNA enzyme inhibitor, wherein the specific primer and the fluorescent probe have the following sequences: an upstream primer H1N1 A-FP:5'-AGGTTTGAGATATTCCCCAAGACA-3'; a downstream primer H1N1 A-RP:5'-AATTTTTGTAGAAGCTTTTTGCTCC-3'; and a specific probe H1N1 A-P:5'-F-CATGGCCCAATCATGACTCGAACA-Q-3', wherein F is a fluorescent reporter group; and Q is a fluorescent quenching group. The invention has the advantages that the nucleic-acid fluorescent quantitative RT-PCR detection kit of the H1N1 influenza virus A and the detection method thereof can be applied to the emergent detection and the monitoring of a lab where the H1N1 influenza virus A causes an outbreakepidemic.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
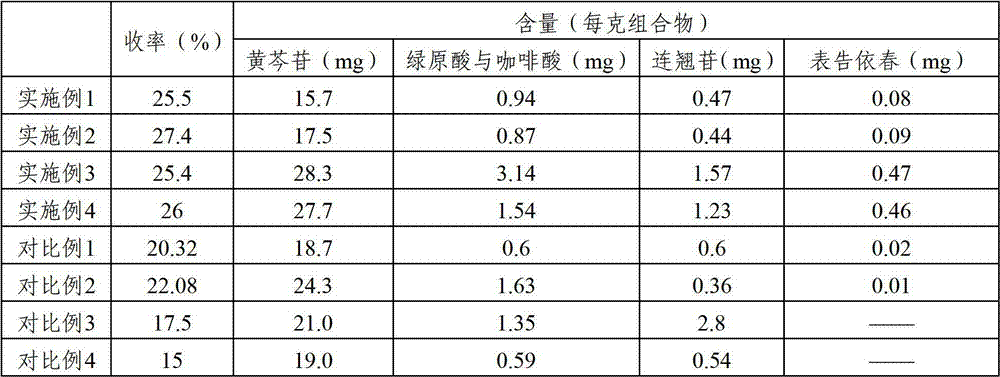
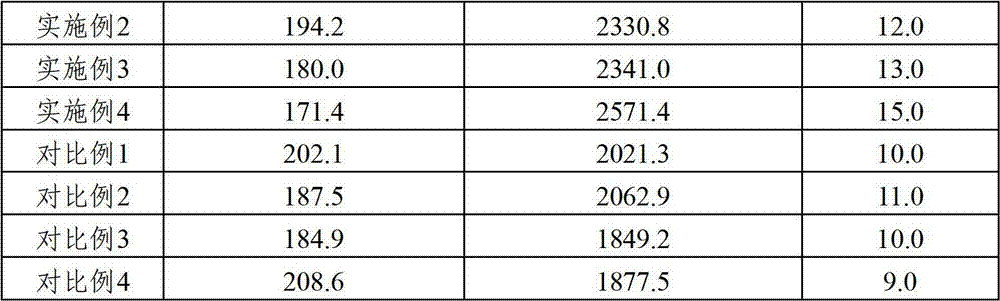
Antivirulent drug composition, and preparation and application thereof
ActiveCN102755386AIncrease contentEnhanced inhibitory effectOrganic active ingredientsAntiviralsChlorogenic acidAvian influenza virus
The invention relates to an antivirulent drug composition, and a preparation and a preparation method thereof. The drug composition comprises scutelloside, chlorogenic acid and caffeic acid, and phillyrin, wherein the weight ratio of scutelloside to chlorogenic acid to caffeic acid to phillyrin is (10-18):(0.6-2):(0.3-1). The drug composition and the preparation provided by the invention have a better inhibition effect on avian influenza virus and influenza A H1N1, with the effect being superior to that in the prior art.
Owner:HEILONGJIANG ZBD PHARMA
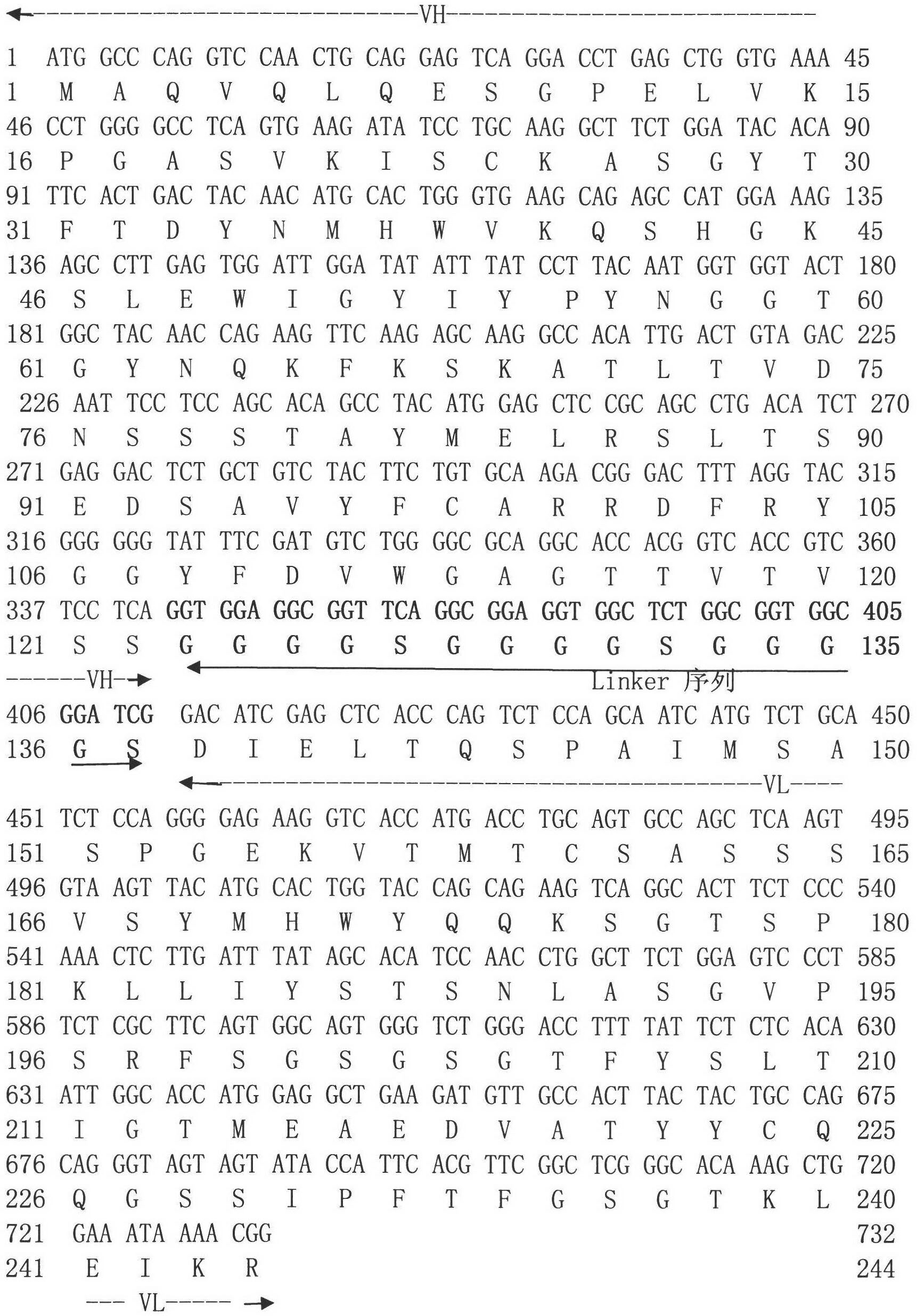
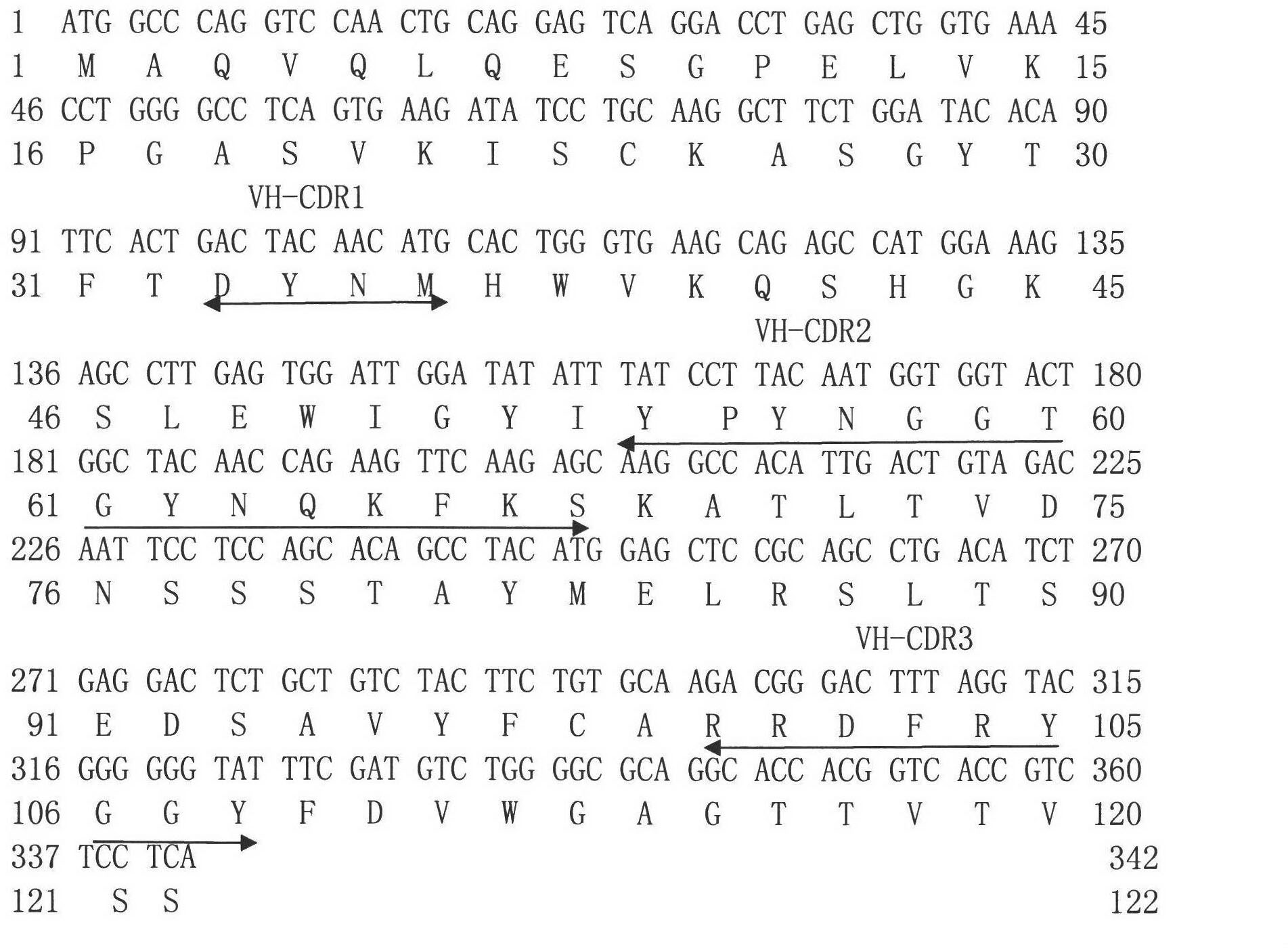
Single-chain antibody for resisting influenza viruses, preparation method for single-chain antibody, application of single-chain antibody
InactiveCN102558348ABlock bindingNeutralizing activityBacteriaMicroorganism based processesSerum igeSingle-Chain Antibodies
The invention discloses a single-chain antibody ScFv for resisting influenza viruses, a preparation method for the single-chain antibody ScFv, application of the single-chain antibody ScFv, a gene for encoding the single-chain antibody ScFv, a carrier containing the gene, a host cell and the like. The single-chain antibody ScFv for resisting the influenza viruses is one of the following proteins: 1) a single-chain antibody formed by connecting a heavy chain variable region and a light chain variable region of the antibody through a linker peptide, wherein an amino acid sequence of the light chain variable region and an amino acid sequence of the heavy chain variable region are shown as SEQ ID NO.1 and SEQ ID NO.2 in a sequence table respectively; and 2) a derived antibody obtained by improving the single-chain antibody in step 1), wherein the improvement comprises the deletion, substitution or insertion of amino acid, and the derived antibody has the antibody activity of resisting H1N1 influenza viruses. The molecular weight of the single-chain antibody is about 27kD; and the single-chain antibody can specifically identify the H1N1 influenza viruses and block the combination of the viruses and natural serum. The single-chain antibody can be used for diagnosing, preventing, controlling and treating the infection of the H1N1 influenza viruses, or is used for antiviral breeding of transgenic animals.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Recombinant homologous avian H1N1 influenza virus inactivated vaccine strain (JS40/PR8) as well as preparation method and application of inactivated vaccine strain
ActiveCN104232594AQuality improvementReduce manufacturing costMicroorganism based processesAntiviralsHemagglutininNeuraminidase
The invention discloses a recombinant homologous avian H1N1 influenza virus inactivated vaccine strain (JS40 / PR8) as well as a preparation method and an application of the inactivated vaccine strain. The recombinant avian H1N1 influenza virus inactivated vaccine strain takes A / swine / Jiangsu / 40 / 2011 (H1N1) as genetic donors of surface hemagglutinin (HA) and neuraminidase (NA), and influenza virus A / PR / 8 / 34(H1N1) as donors of six internal genes of a polymerase PB2 subunit, a polymerase PB1 subunit, a polymerase PA subunit, nucleoprotein (NP), a matrix protein (M) and non-structural protein (NS); the vaccine strain is obtained by natural recombination; and the preservation number of the vaccine strain is CCTCC NO:V201419. The invention also discloses a method for building the recombinant homologous avian H1N1 influenza virus inactivated vaccine strain, and an application of the homologous recombinant avian H1N1 influenza virus inactivated vaccine strain for preventing homologous avian H1N1 influenza of animals or people.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com