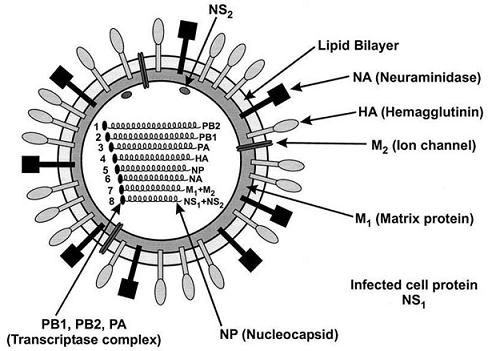
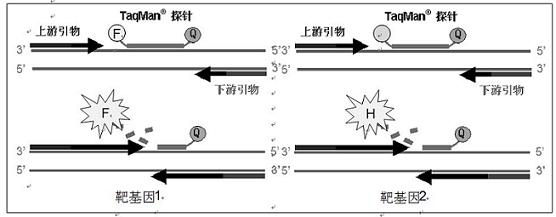
Influenza A H1N1/Influenza A Virus Nucleic Acid Dual Fluorescent PCR Detection Kit
A type of influenza A virus detection kit technology, applied in the direction of fluorescence/phosphorescence, microbial measurement/inspection, biochemical equipment and methods, etc., can solve the problem of increased detection cost and complicated operation, complex gene sequence determination methods, serum The hysteresis of diagnostic methods can be solved to achieve the effect of rapid and objective test results, strong repeatability, and reduced chances of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 A H1N1 / Influenza A virus nucleic acid detection kit
[0041] The influenza A H1N1 / influenza A virus nucleic acid double fluorescent quantitative PCR detection kit of the present embodiment includes RNase inhibitor, RT-PCR reaction solution, enzyme mixture, type A H1N1 / influenza A virus double reaction solution, positive control and negative controls,
[0042] Wherein, the RNase inhibitor is DEPC water; the RT-PCR reaction solution includes 10× buffer, MgCl2 and dNTPs;
[0043] Influenza A H1N1 / Influenza A double reaction solution includes the following components:
[0044]Component (1): consists of a pair of primers for detecting influenza A (H1N1) virus and a probe for detecting influenza A (H1N1) virus; wherein, the base sequences of the two primers are SEQ ID No.1 and SEQ ID No. Shown in .2; the base sequence of the probe is shown in SEQ ID No.3, the 5' end of the probe is marked with a fluorescent reporter group, and the 3' end is marked with a fluoresce...
Embodiment 2
[0068] Embodiment 2 Sensitivity test
[0069] The positive reference product is the inactivated virus culture solution, which comes from the Jiangsu Provincial Center for Disease Control and Prevention.
[0070] The negative reference product is RNase-free water. Weigh 1g of DEPC with an electronic balance, add purified water to 1000ml and mix well, then sterilize at 121°C / 20 minutes in a sterilizing pot, mark it, and store it at room temperature.
[0071] The kit of the present invention is used for detection.
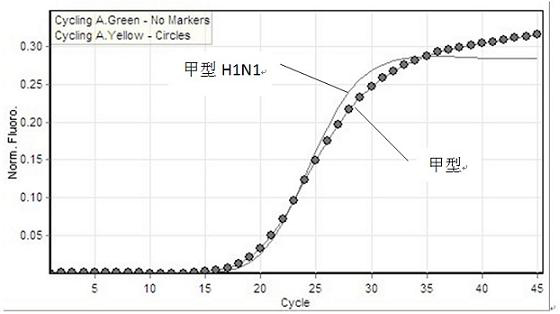
[0072] The test results show that the kit of the present invention has good sensitivity, and the CT value changes in a gradient as the concentration decreases ( Figure 4 , Figure 5 ). The test result shows that the kit of the present invention has high sensitivity for the diagnosis of type A H1N1 / type A influenza virus.
[0073]
Embodiment 3
[0074] Embodiment 3 specificity test
[0075] In order to detect the specificity of the A-H1N1 / influenza A virus detection kit of the present invention, the respiratory syncytial virus, human adenovirus, and human parainfluenza virus are detected with the A-H1N1 / influenza A virus detection kit of the present invention.
[0076] The test results show that: the FAM channel only amplifies the influenza A (H1N1) virus ( Figure 6 ), the HEX channel only amplifies influenza A virus ( Figure 7 ). It shows that the detection kit of the present invention can specifically amplify influenza virus without cross-reaction with other viral nucleic acids.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com