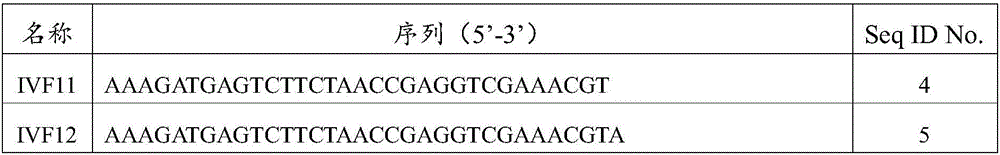Patents
Literature
313 results about "Influenza Viruses Type A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Influenza type A viruses can be further divided into subtypes based on two membrane proteins on the surface of the virus. These proteins are called hemagglutinin (HA) and neuraminidase (NA).
Adjuvanted influenza vaccines for pediatric use
ActiveUS8506966B2Enhance immune responseHigh seroprotection rateSsRNA viruses negative-senseViral antigen ingredientsAdjuvantSeroconversion
An influenza vaccine adjuvanted with a sub-micron oil-in-water emulsion elicits significantly higher immune responses in human pediatric populations. Compared to an existing unadjuvanted pediatric influenza vaccine, the adjuvanted vaccines provided herein can induce in children a longer persistence of high serum antibody titers and also longer seroconversion and seroprotection. The improvement in immune responses is seen for both influenza A virus and influenza B virus strains, but it is particularly marked for influenza B virus. Moreover, while the existing vaccine provides poor immunity in children after a single dose, the adjuvanted vaccine provides high seroprotection rates against the influenza A virus H3N2 subtype even after a single dose. Furthermore, the adjuvanted vaccine offers significantly better seroprotection against mismatched strains of influenza A virus.
Owner:SEQIRUS UK LTD
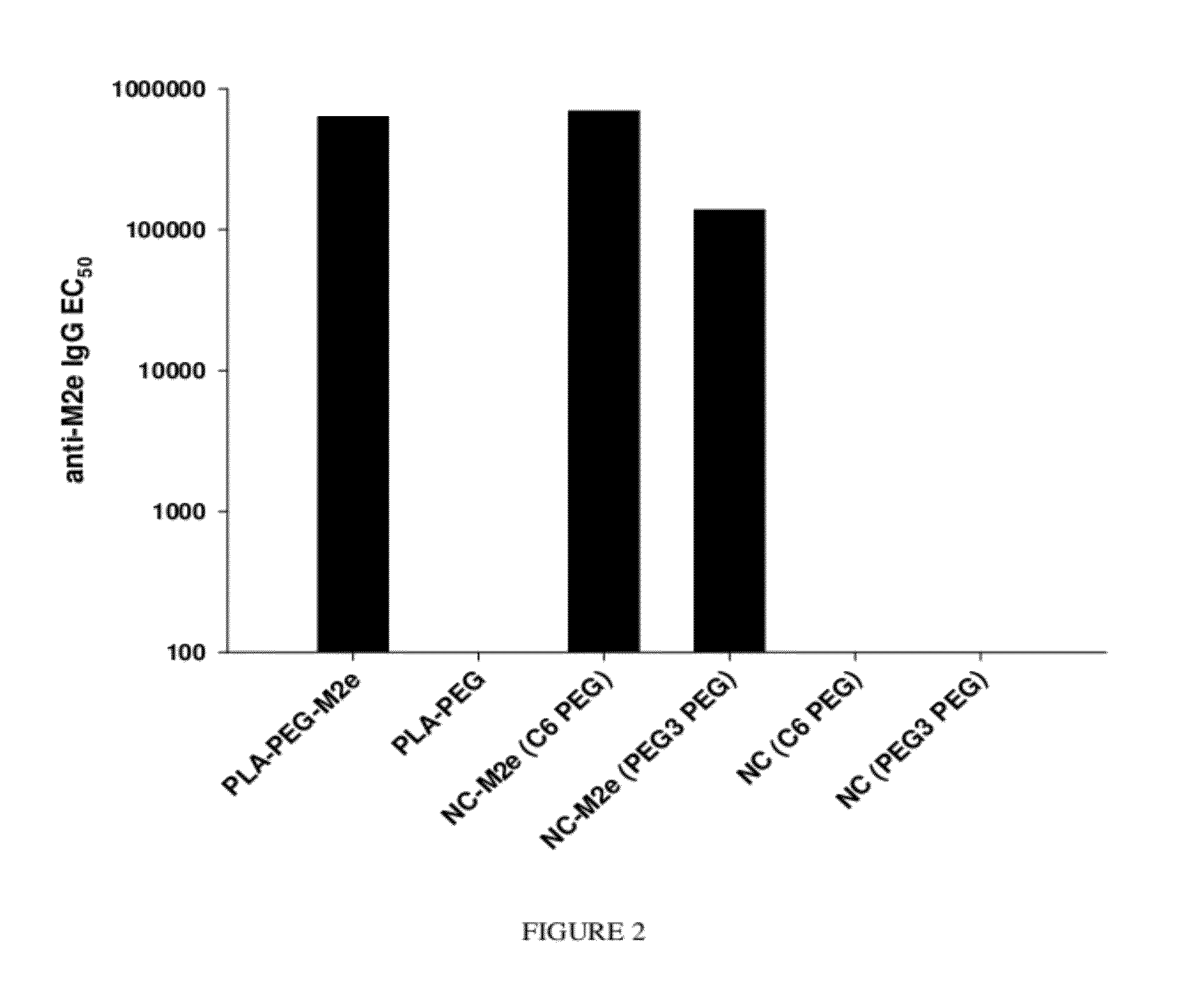
Synthetic nanocarrier vaccines comprising peptides obtained or derived from human influenza a virus m2e
InactiveUS20120058154A1Stimulate immune responseSsRNA viruses negative-sensePeptide/protein ingredientsHuman Influenza A VirusVector vaccine
This invention relates to compositions and methods that can be used immunize a subject against influenza. Generally, the compositions and methods include peptides obtained or derived from human influenza A virus M2 protein.
Owner:SELECTA BIOSCI
Vaccines and methods to treat canine influenza
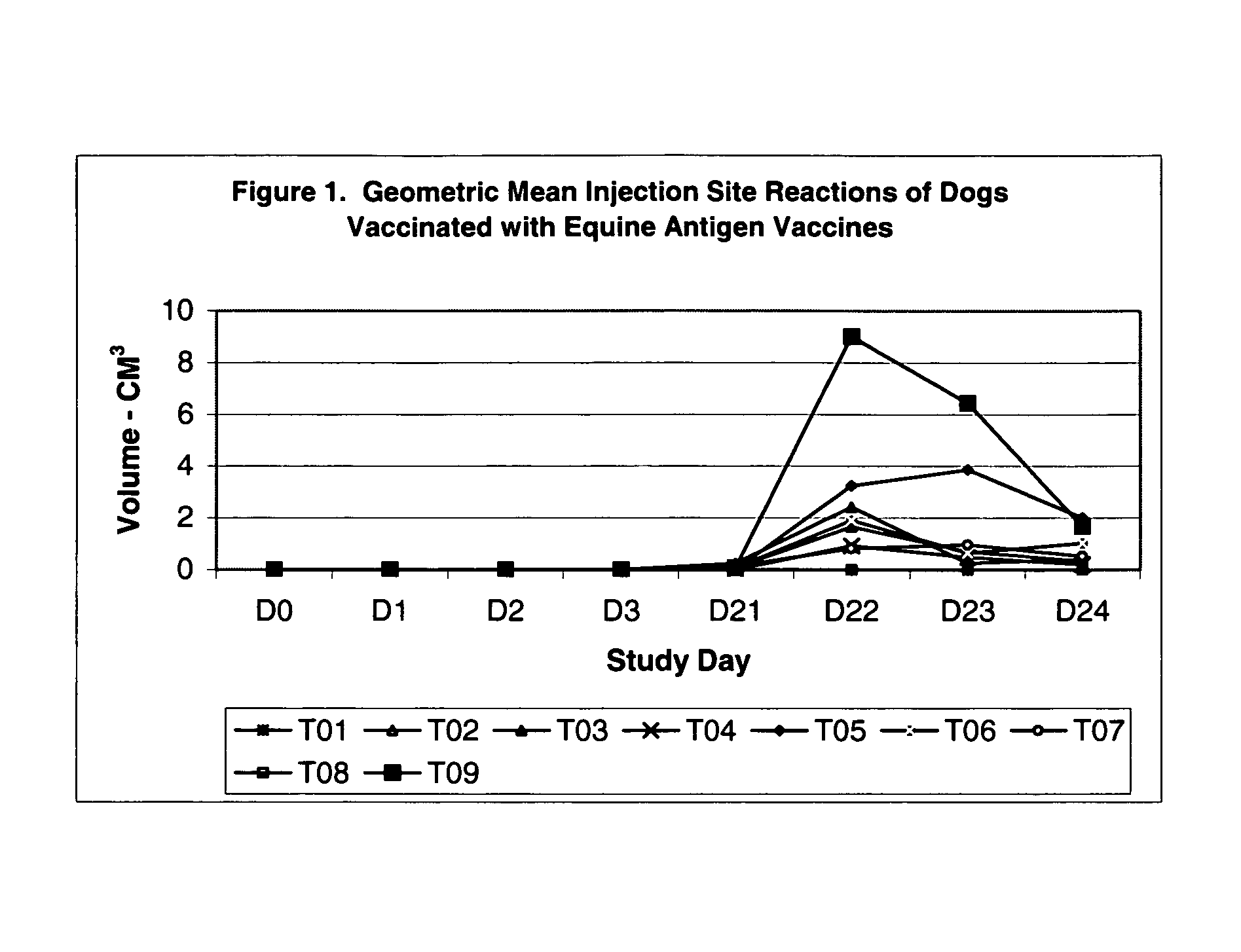
The present invention relates to providing new vaccines and treatments for the diseases related to canine influenza virus. It discloses influenza viral antigens, and methods of presenting these antigens to canines, especially dogs. It relates to attenuated and killed vaccines. The present invention relates to experimentally generated canine and equine influenza viruses. The invention also includes influenza A, including H3, N8, H3N8, H7N7 and viruses which contain at least one genome segment from an canine or equine influenza virus. The present invention also relates to the use of these viruses in therapeutic compositions to protect canines, dogs in particular, from diseases caused by influenza viruses.
Owner:ZOETIS SERVICE LLC
Vaccines Including Antigen From Four Strains of Influenza Virus
InactiveUS20140178429A1Reduce in quantityFacilitate matterSsRNA viruses negative-senseViral antigen ingredientsHemagglutininAdjuvant
Owner:SEQIRUS UK LTD
Cross-protective influenza vaccine
InactiveUS20120052082A1Broad and improved cross protectionSsRNA viruses negative-senseViral antigen ingredientsMultiple copyVirosome
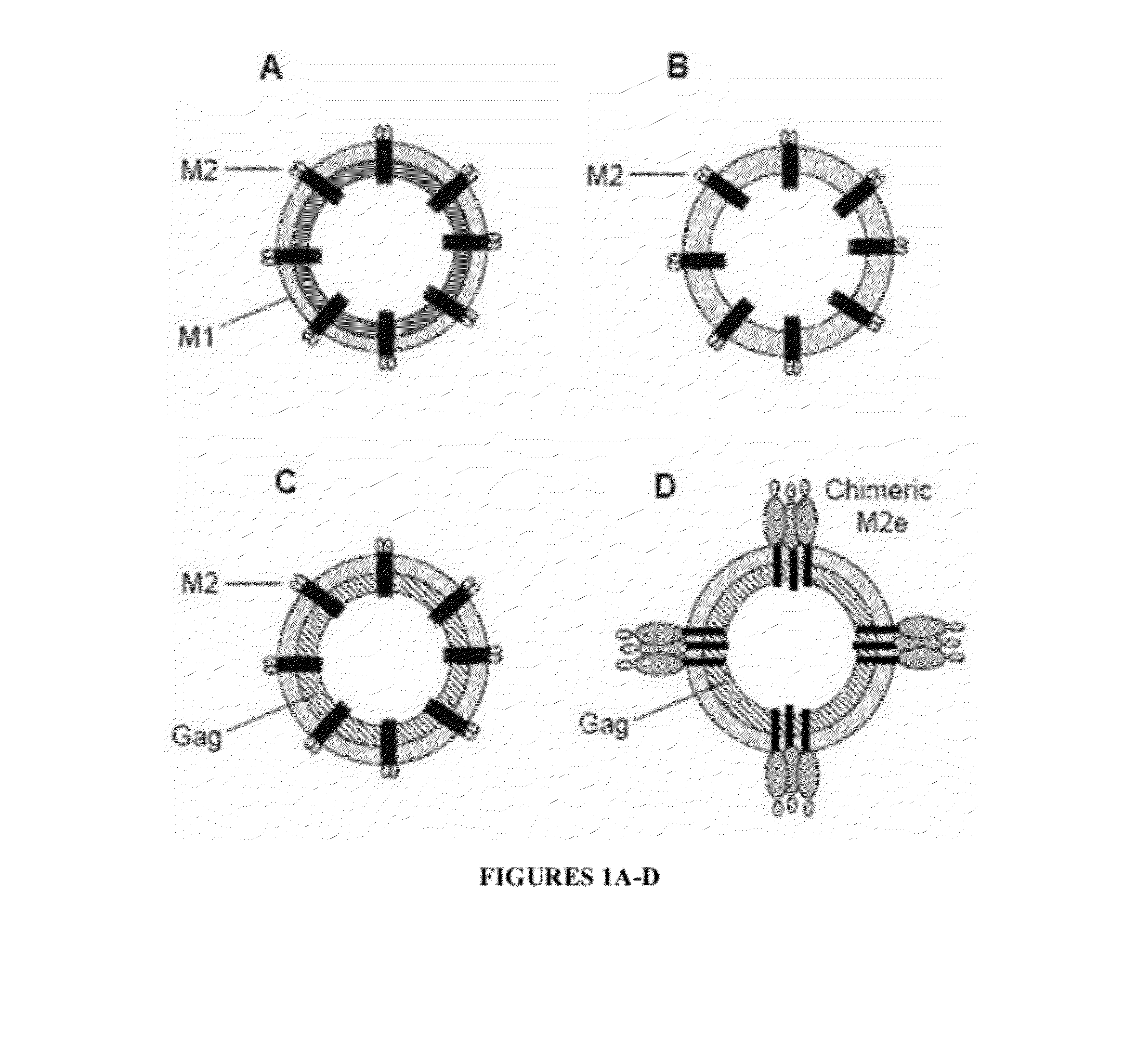
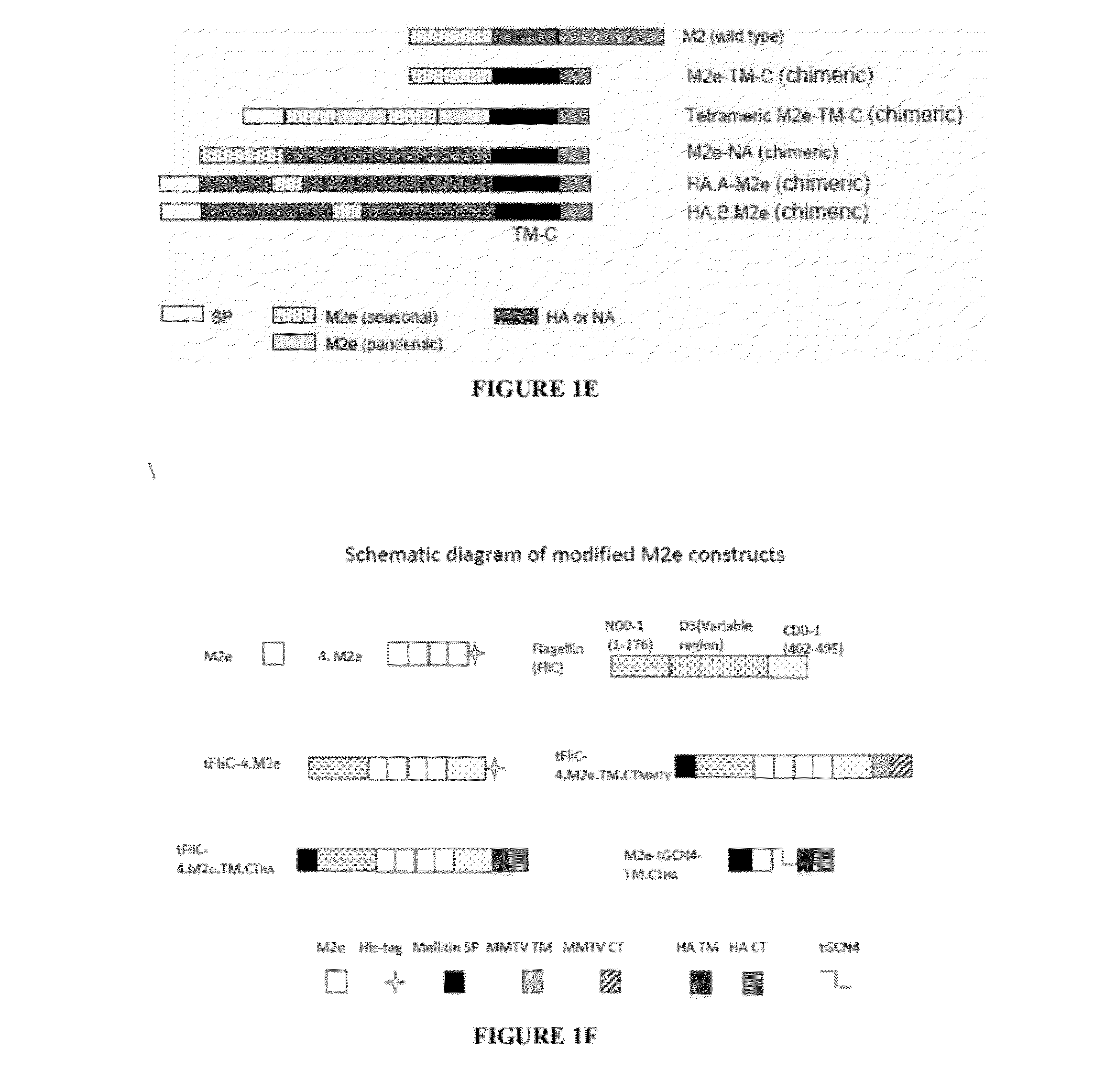
A cross-protective influenza virus vaccine has been designed based on the incorporation of the genetically engineered, highly conserved M2 influenza viral protein optionally in combination with an adjuvant such as a bacterial flagellin protein incorporated into the membrane of a virosome or virus-like particles. Immunogenicity and the breadth of cross protection efficacy are significantly enhanced using multiple copies of the influenza M2 protein as a membrane bound tetramer and / or in combination with a membrane bound adjuvant. A method for vaccinating a subject for influenza A has also been developed that results in broad and improved cross-protection against multiple subtypes of influenza A virus.
Owner:ZETRA BIOLOGICALS
Human Anti-Human Influenza Virus Antibody
InactiveUS20110319600A1Effect survival rateEffect weight lossSugar derivativesImmunoglobulins against virusesHemagglutininHuman Influenza A Virus
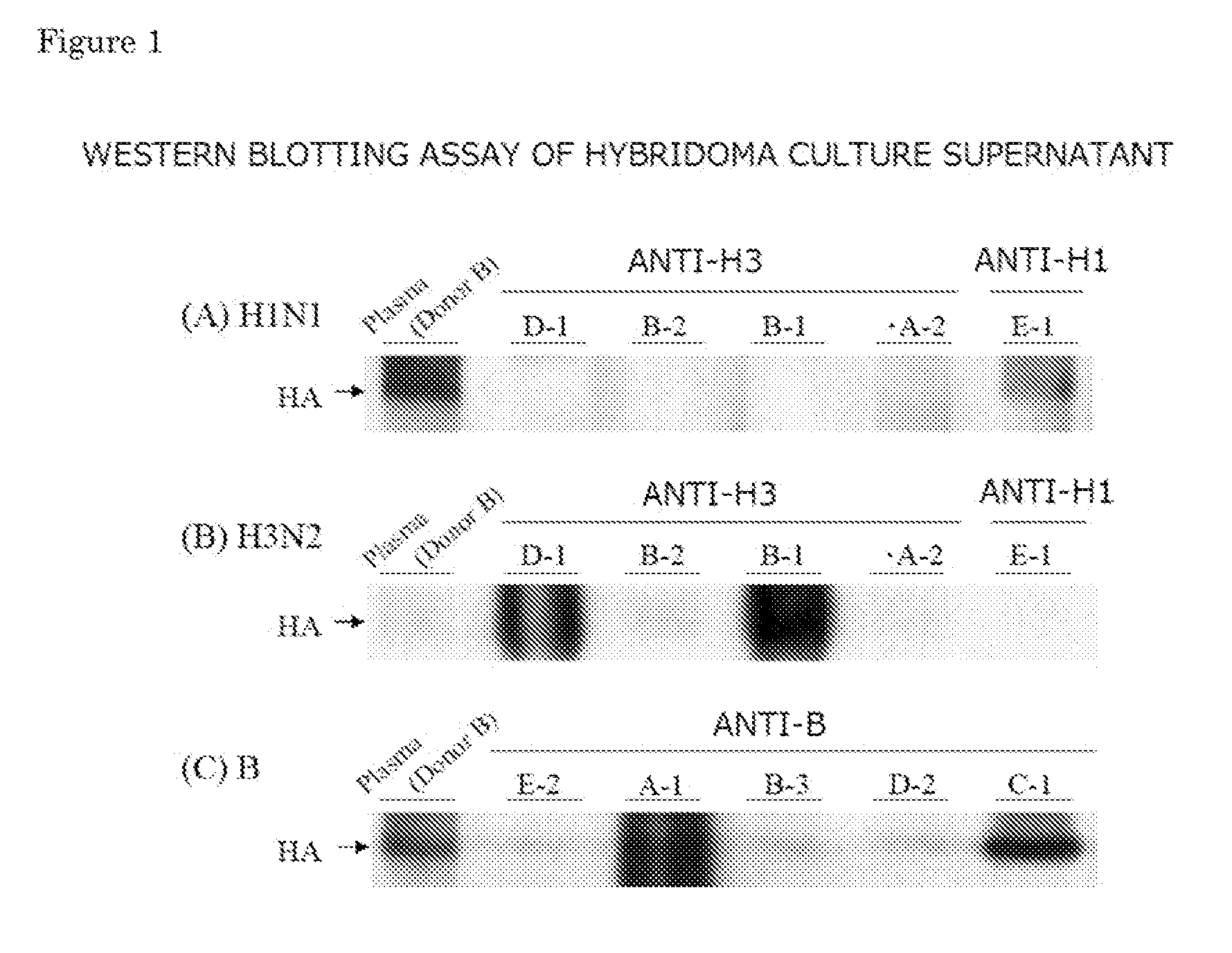
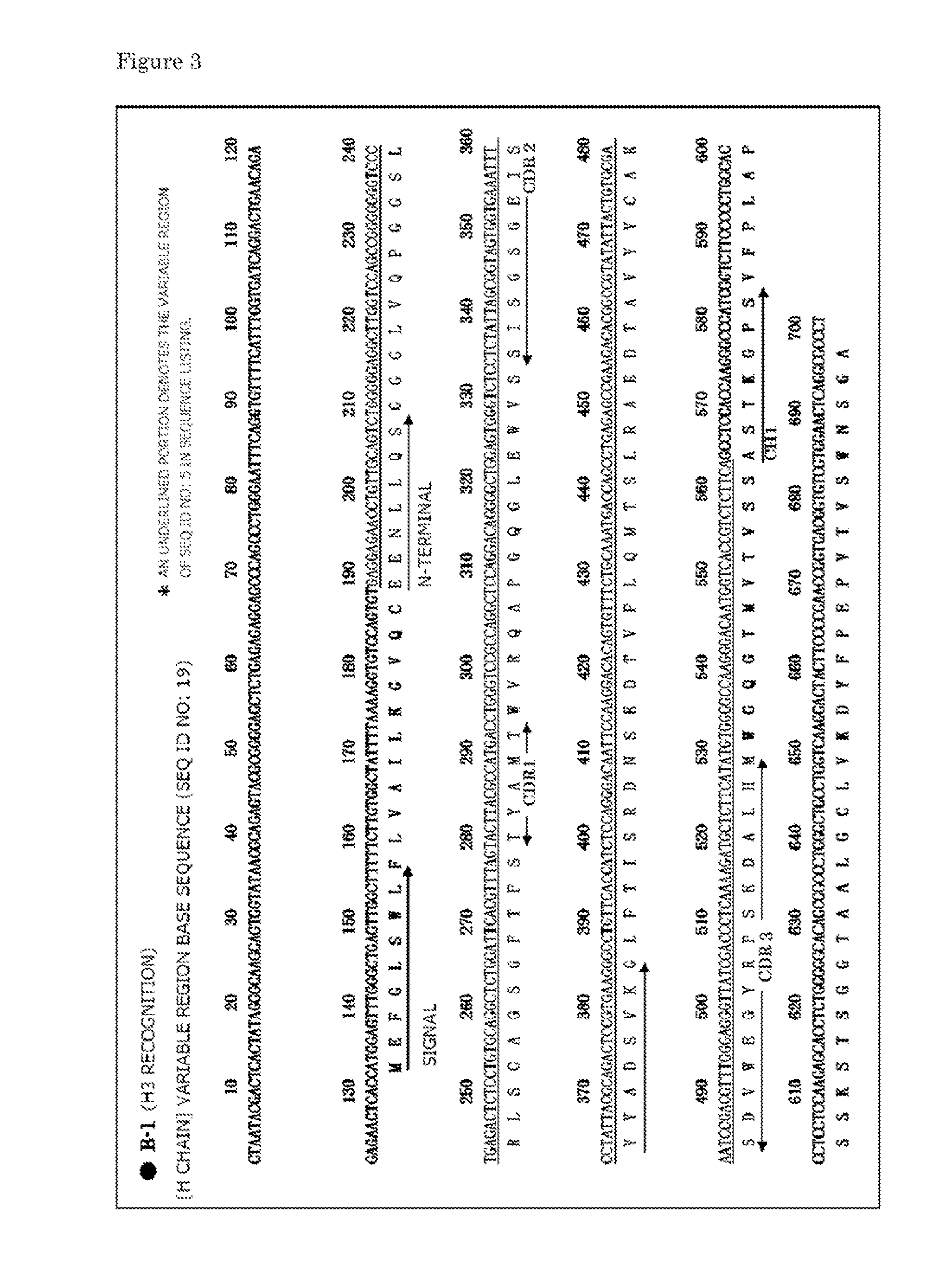
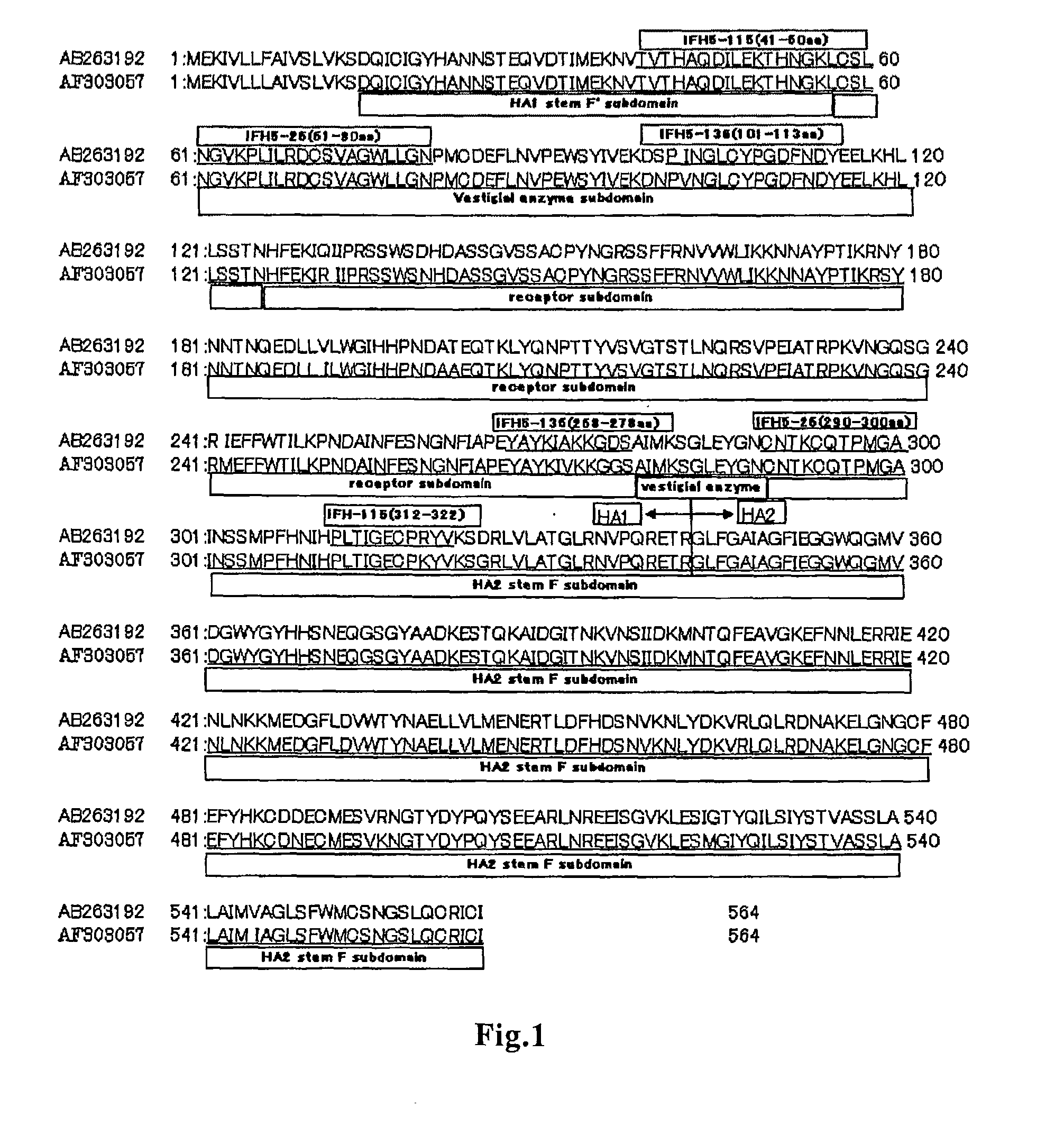
Provided is a human antibody having a neutralization activity against a human influenza virus. More specifically, provided is a human antibody which recognizes a highly conserved region in a human influenza A virus subtype H3N2 or a human influenza B virus and has a neutralization activity against the virus. The human antibody is a human anti-human influenza virus antibody, which has a neutralization activity against a human influenza A virus subtype H3N2 and binds to a hemagglutinin HA1 region of the human influenza A virus subtype H3N2, or which has a neutralization activity against a human influenza B virus, and includes, as a base sequence of a DNA encoding a variable region of the antibody, a sequence set forth in any one of SEQ ID NOS: 5 to 12.
Owner:OSAKA UNIV +1
Influenza vaccine
InactiveUS20090304730A1Overcomes drawbackSsRNA viruses negative-senseViral antigen ingredientsEpitopeInfluenza vaccine
The present invention relates to influenza vaccines for human and veterinary use. In particular, the present invention provides a vaccine able to effect long term and cross-strain protection by including at least two influenza virus epitopes expressed as a chimeric polypeptide wherein at least one epitope is influenza A virus matrix protein epitope and the second epitope is a haemagglutinin peptide epitope.
Owner:YEDA RES & DEV CO LTD
Anti-(influenza a virus subtype h5 hemagglutinin) monoclonal antibody
InactiveUS20110065095A1Prevention of prevalenceFaster assayMicrobiological testing/measurementBiological material analysisHemagglutininEpitope
A method of immunoassay of H5 subtype influenza A virus by which the virus can be accurately assayed even in cases where a certain level of mutation has occurred in the H5 subtype influenza A virus, and a kit therefor, and a novel anti-H5 subtype influenza A virus monoclonal antibody which can be used for the immunoassay are disclosed. The antibody or an antigen-binding fragment thereof of the present invention undergoes antigen-antibody reaction with hemagglutinin of H5 subtype influenza A virus, and the corresponding epitope of the antibody or an antigen-binding fragment thereof is located in a region other than the receptor subdomain (excluding C-terminal region thereof consisting of 11 amino acids), which antibody or an antigen-binding fragment thereof does not have neutralizing activity against the influenza A virus.
Owner:FUJIREBIO CO LTD +1
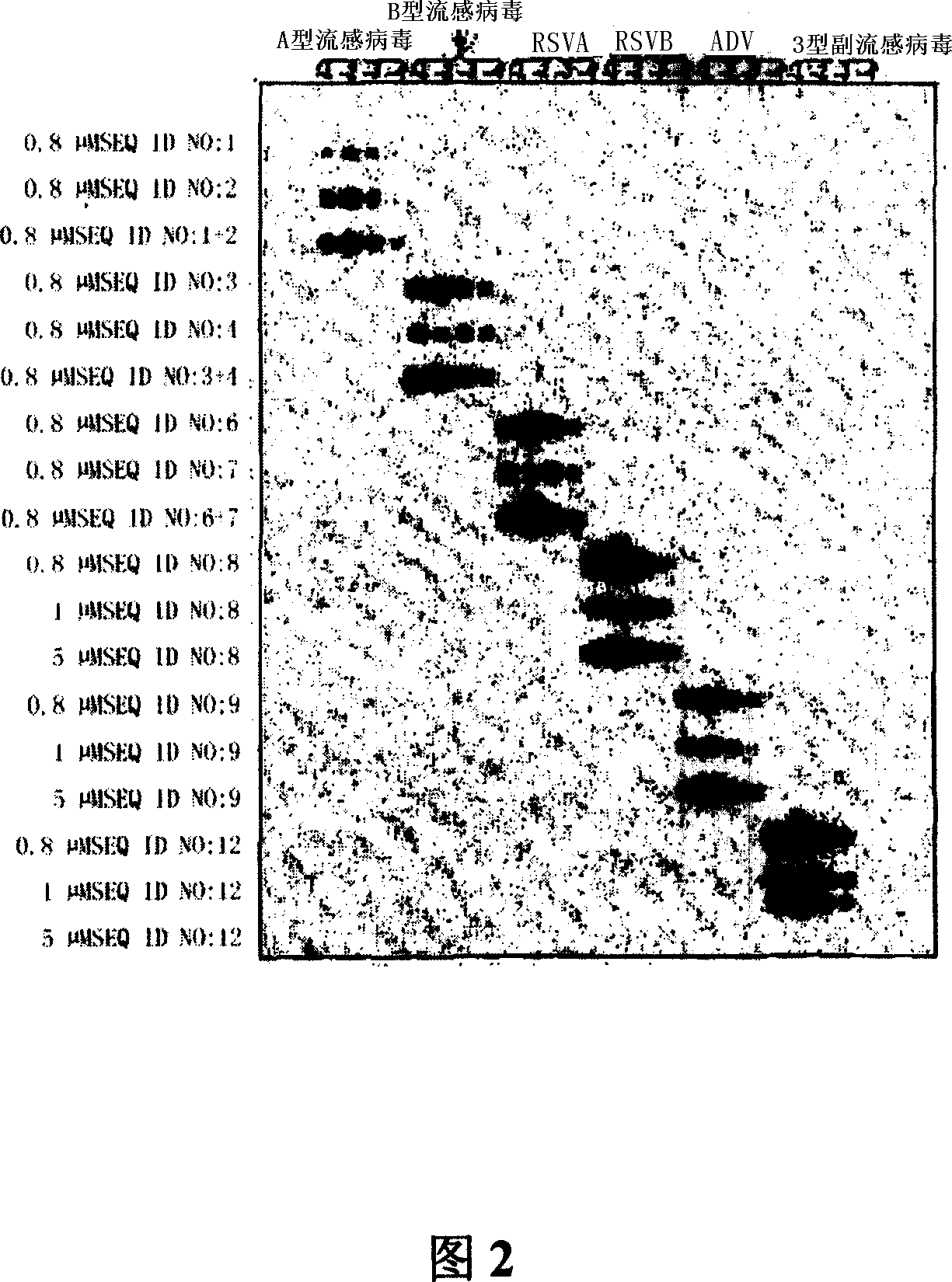
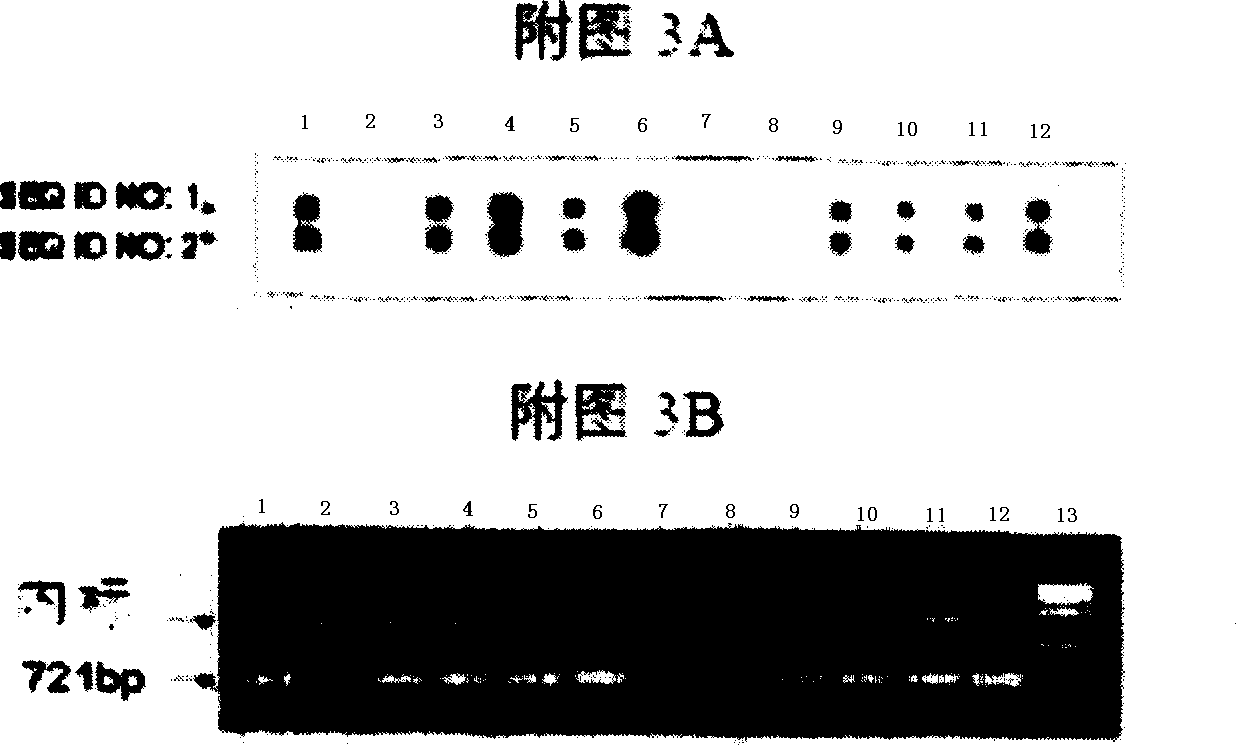
Kit for detecting 10 respiratory tract infection pathogens and application method thereof
ActiveCN107365876AStrong specificityReduce pollution interferenceMicrobiological testing/measurementAgainst vector-borne diseasesRespiratory tract infectionsPathogen
The invention provides a kit for detecting 10 respiratory tract infection pathogens and an application method thereof. The kit comprises primers and a TaqMan probe, wherein the primers are used for amplifying the 10 respiratory tract infection pathogens which include mycoplasma pneumoniae, chlamydia pneumoniae, legionella pneumophila, bordetella pertussis, rhinovirus, respiratory tract adenovirus, influenza A virus, influenza B virus, respiratory syncytial virus and parainfluenza virus. The application method includes: mixing 2 microliters of sample DNA / RNA co-extraction template, 20 microliters of RT-PCR buffer, 1 microliter of mixed enzyme liquid and 2 microliter of primer-probe mixed liquid to perform PCR amplification reaction. The kit uses the Taqman probe fluorescent PCR technology to detect the 10 common respiratory tract infection pathogens, a detection result can be obtained within 2 hours, the kit is high in specificity, and the sensitivity of the kit can reach 100copies / microliter.
Owner:NANJING LANSION BIOTECH CO LTD
Human binding molecules capable of binding to and neutralizing influenza b viruses and uses thereof
ActiveUS20130243792A1High economic impactSugar derivativesMicrobiological testing/measurementHemagglutininMonoclonal antibody
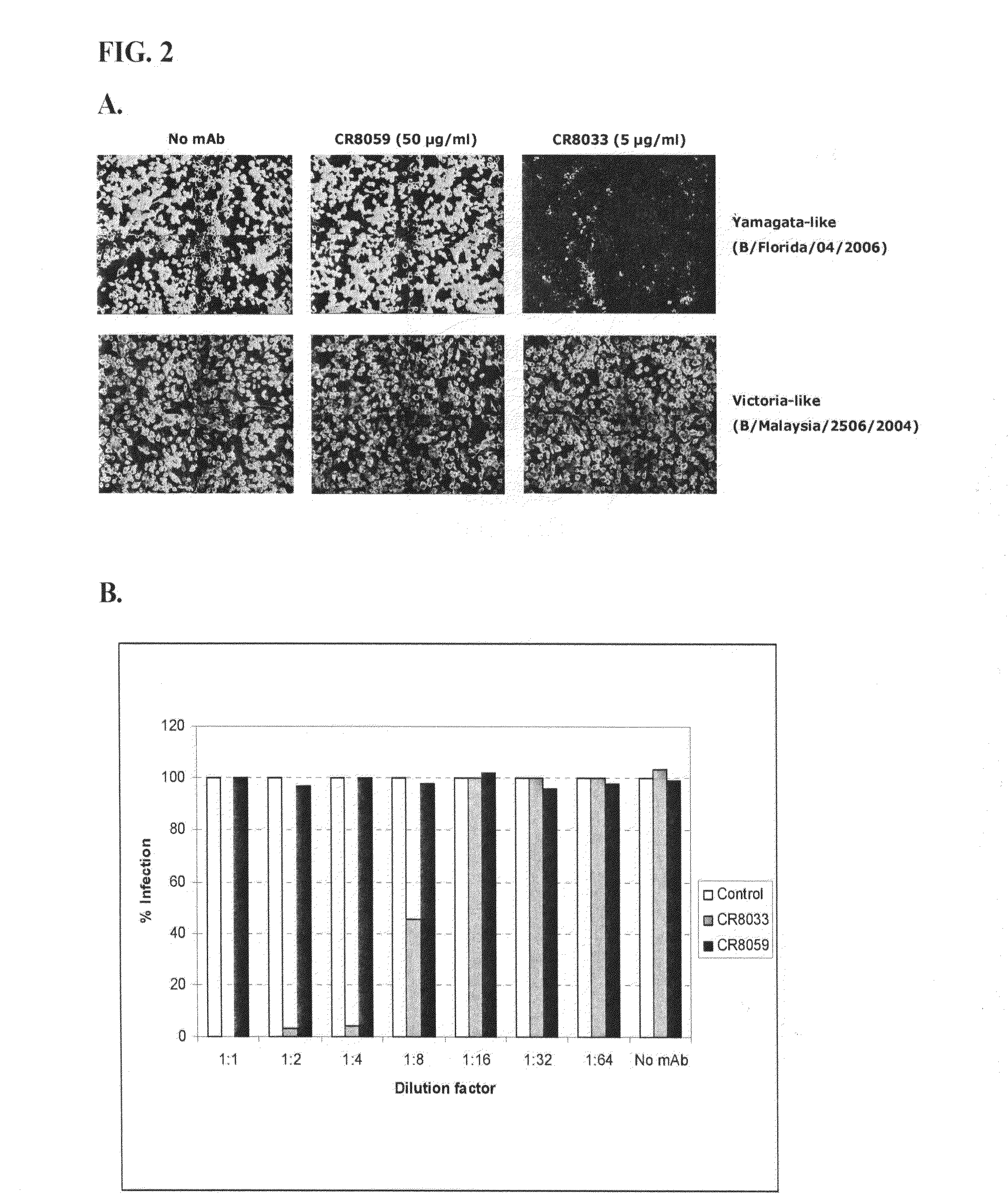
Described are binding molecules, such as human monoclonal antibodies, that bind to hemagglutinin of influenza B viruses, and have a broad neutralizing activity against such influenza viruses. These binding molecules do not bind to hemagglutinin of influenza A viruses. Further provided are nucleic acid molecules encoding the binding molecules, and compositions comprising the binding molecules. The binding molecules can be used in the diagnosis of, prophylaxis against, and / or treatment of influenza B virus infections.
Owner:JANSSEN VACCINES & PREVENTION BV
Monoclonal antibodies capable of reacting with a plurality of influenza virus a subtypes
ActiveUS20110014187A1EffectiveEffective preventionImmunoglobulins against virusesAntiviralsAnti idiotypeImmunogenicity
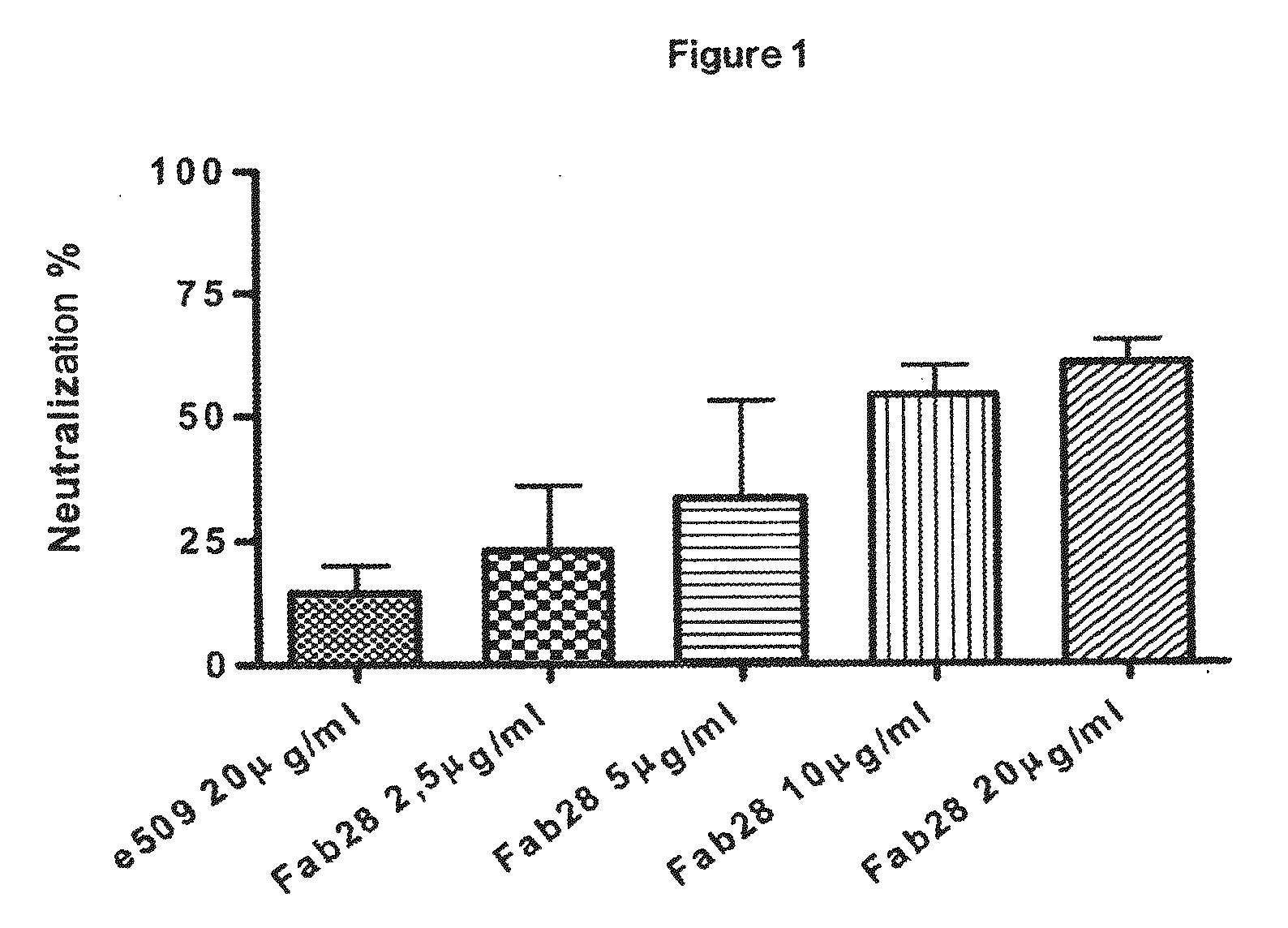
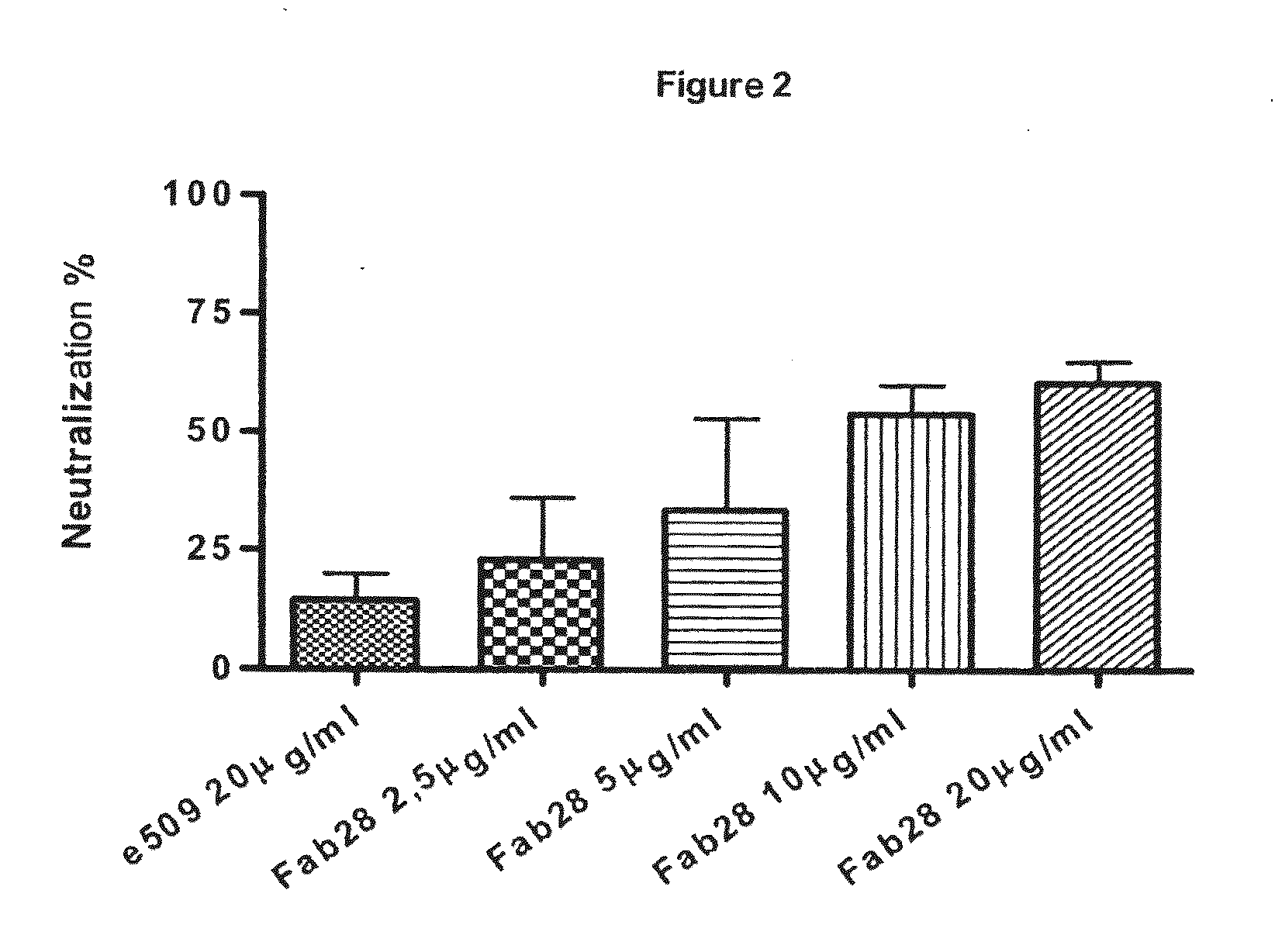
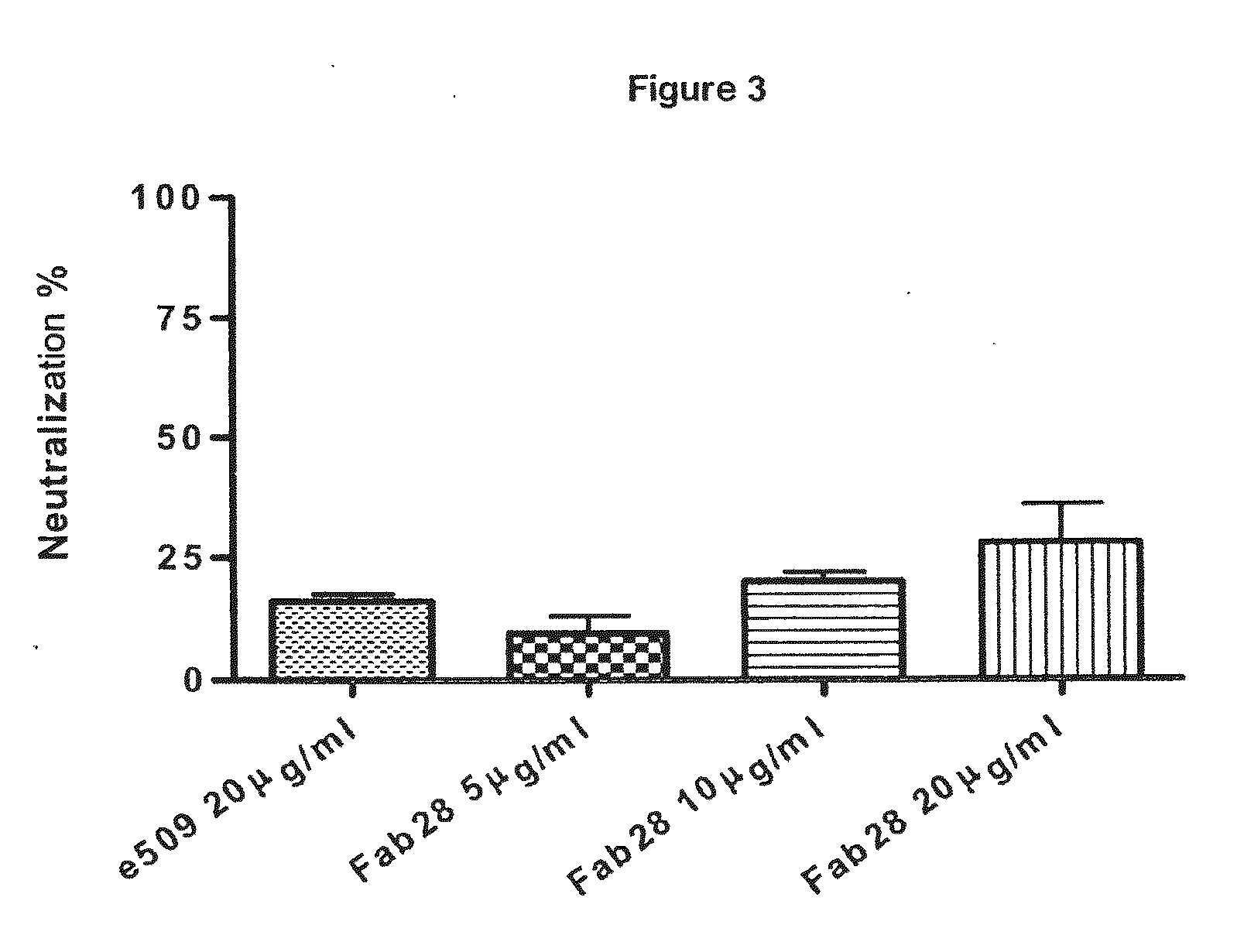
Monoclonal antibodies directed against the influenza A virus are described, which have the advantageous and unpredicted property of being able to bind a plurality of subtypes of the influenza A virus. One preferred embodiment is the antibody designated as Fab28, which displays a neutralizing activity against a plurality of subtypes of the influenza A virus. Anti-idiotype antibodies directed against the monoclonal antibodies of the invention, immunogenic or vaccine compositions comprising the monoclonal antibodies of the invention are also described, as well as therapeutic, prophylactic and diagnostic applications for the monoclonal antibodies of the invention. The monoclonal antibodies of the invention can also be used for testing antibody preparations to be used as vaccines.
Owner:POMONA RICERCA
Traditional Chinese medicine compound anti-coronavirus and anti-flu-virus composite antibacterial multifunctional fiber
ActiveCN111534877ASo as not to damageGood antiviral effectArtificial filaments from viscoseMonocomponent polyolefin artificial filamentBiotechnologyEngineering
The invention belongs to the field of functional fiber materials, and discloses a traditional Chinese medicine compound anti-coronavirus and anti-flu-virus composite antibacterial multifunctional fiber. The fiber comprises traditional Chinese medicine antiviral particles, inorganic antibacterial particles, health-care functional particles and a fiber matrix. The traditional Chinese medicine antiviral particles are silicon dioxide aerogel microspheres loaded with traditional Chinese medicine antiviral components, and the traditional Chinese medicine antiviral components comprise radix isatidis,dandelions, honeysuckle flowers, wild chrysanthemum flowers, folium isatidis, herba andrographitis, citrus chachiensis hortorum, ageratum and mint extracts. According to the traditional Chinese medicine compound anti-coronavirus and anti-flu-virus composite antibacterial multifunctional fiber, the silicon dioxide aerogel microspheres are innovatively adopted as carriers of the traditional Chinesemedicine antiviral components and are introduced into fiber materials, so that the traditional Chinese medicine antiviral functional components can be protected from being damaged in the fiber forming process, and a good antiviral effect is achieved. The antiviral activity rate of the fiber product obtained through testing against coronaviruses Hcov-229E and influenza A viruses H1N1 can reach 99%or above.
Owner:黄蕊烨
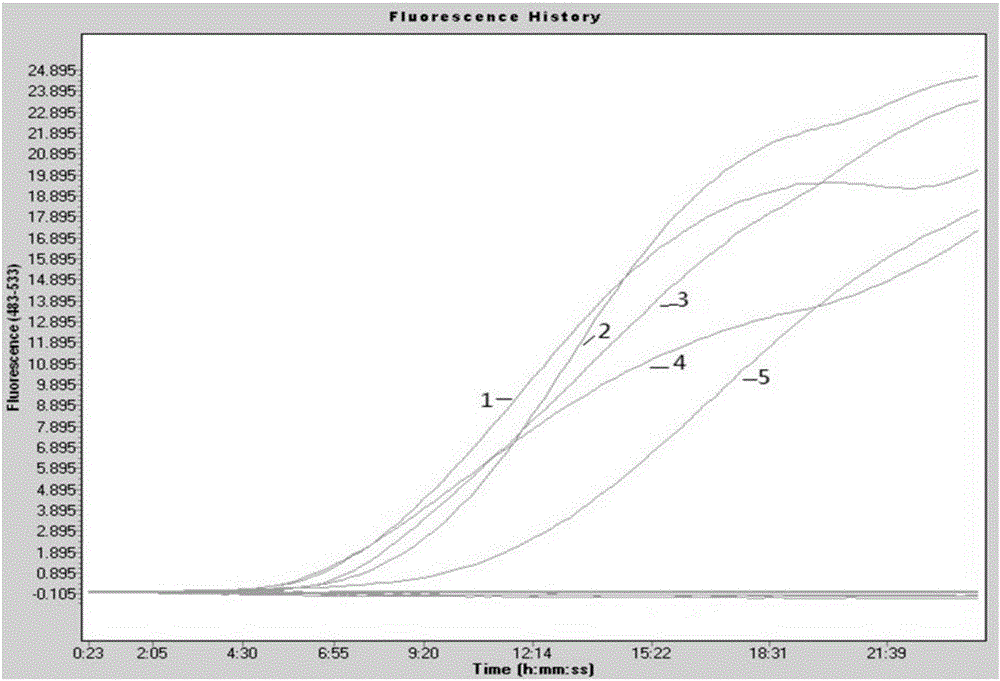
Fluorescent quantitative reverse transcription-polymerase chain reaction (RT-PCR) kit for detecting influenza A virus subtype H7N9
ActiveCN103275862AQuantitatively accurateLow costBioreactor/fermenter combinationsBiological substance pretreatmentsConserved sequenceInfluenza Viruses Type A
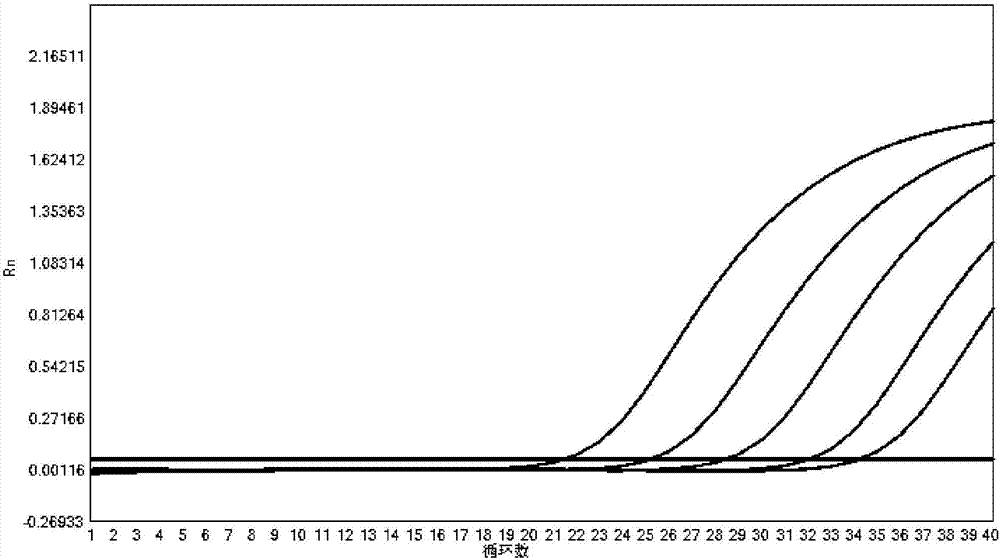
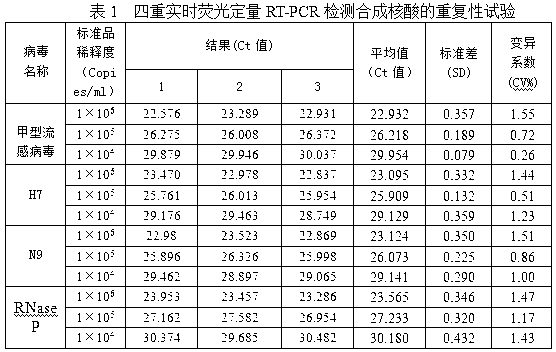
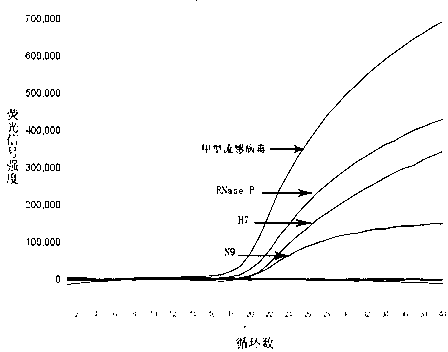
The invention provides a fluorescent quantitative reverse transcription-polymerase chain reaction (RT-PCR) kit for detecting an influenza A virus subtype H7N9. The fluorescent quantitative RT-PCR kit can be used for detection of influenza A viruses and the influenza A virus subtype H7N9. The fluorescent quantitative RT-PCR kit comprises a quantitative RT-PCR reaction solution, an enzyme mixed liquor, a primer and probe mixed liquor, standard substances of influenza A viruses, H7, N9 and RNaseP, positive reference substances of influenza A viruses, H7, N9 and RNaseP), and negative reference substances. Specific primers and probes are designed according to conserved sequences of influenza A viruses, H7 and N9. The RNaseP primers and probes are used as internal references. Through the one-step quadruple real-time fluorescent RT-PCR technology, the influenza A virus and the influenza A virus subtype H7N9 in the sample can be fast and accurately detected. The fluorescent quantitative RT-PCR kit has a reasonable design, very high singularity, sensitivity and repeatability, can be used for laboratory emergency diagnosis and fast screening of an epidemic disease caused by the influenza A virus subtype H7N9, and for an epidemiology study on the influenza A virus and the influenza A virus subtype H7N9 causing fever and respiratory tract syndrome.
Owner:ZHEJIANG UNIV
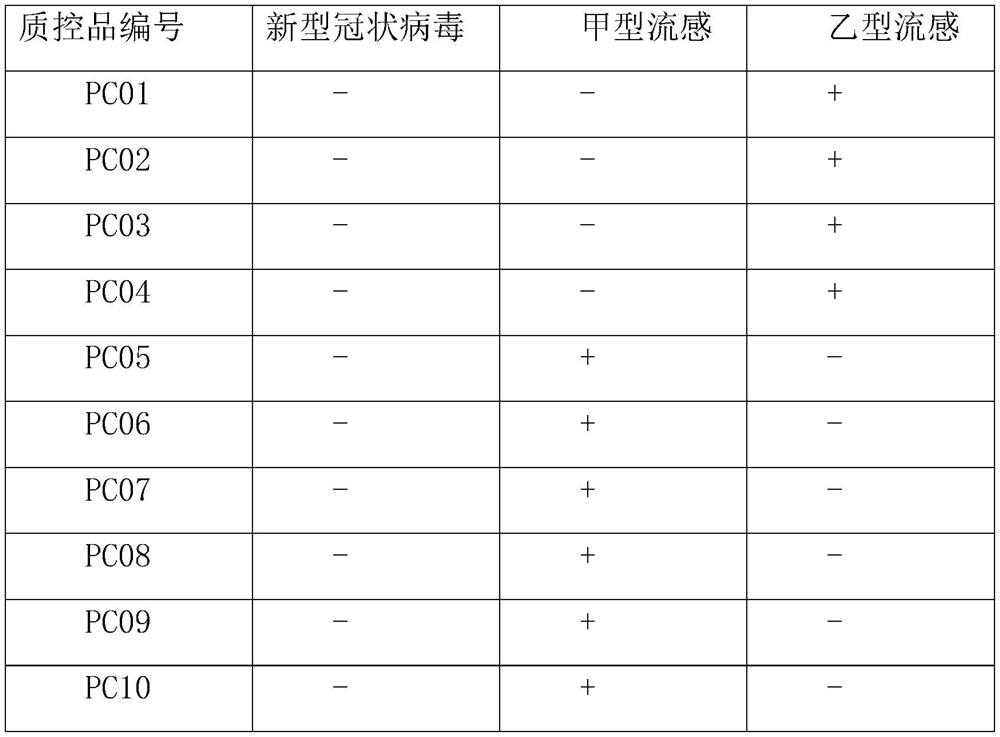
Kit for nucleic acid combined detection of influenza virus A, influenza virus B and respiratory syncytial virus
InactiveCN105400907AEasy to prepareAvoid pollutionMicrobiological testing/measurementMicroorganism based processesRespiratory virusRespiratory syncytial virus (RSV)
The invention provides a kit for nucleic acid combined detection of the influenza virus A, the influenza virus B and the respiratory syncytial virus. The kit comprises RT-PCR reaction liquid, an RT-PCR enzyme mixture, a respiratory virus primer probe, internal reference, negative contrast, clinical positive contrast and strong positive contrast. The one-step RT-PCR reaction can be directly conducted on the well-extracted respiratory virus nucleic acid, the influenza virus A, the influenza virus B and the respiratory syncytial virus in a sample can be detected in a classified and qualitative mode, the gene of the internal reference serves as the internal contrast, and contamination is prevented through UNG enzymes. The kit is simple in one-step amplification method, short in procedure, easy and convenient to operate, capable of preventing contamination, high in detection result specificity, high in sensitivity, clear in result, high in credibility, and capable of being used for qualitatively authenticating and detecting the influenza virus A, the influenza virus B and the respiratory syncytial virus in a human nose pharynx-mop sample.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD
Folium isatidis cellulose fiber having antiviral, antibacterial and skin-care functions and preparation method thereof
InactiveCN105603556AEasy to takeGood textile processabilityArtificial filaments from viscoseWet spinning methodsSodium CaseinateEngineering
The invention discloses a folium isatidis cellulose fiber having antiviral, antibacterial and skin-care functions and a preparation method thereof. The folium isatidis cellulose fiber contains, by weight, 2.0-8.0 parts of folium isatidis extract, 2.0-8.0 parts of sodium caseinate, 2-30 parts of porous starch and 2-50 parts of protein. The preparation method includes the following steps that 1, preparation of folium isatidis extract-sodium caseinate compound microcapsules, 2 preparation of a blended spinning stock solution and 3 spinning and post-processing. Compared with conventional viscose fibers, the influenza A virus inactivation rate of the fiber is 82.0% or above, the herpes virus inactivation rate is 84.0% or above, a bacteriostatic activity value is greater than or equal to 2.0, a bactericidal activity value is greater than or equal to 0.2, a variety of amino acids and trace elements are contained in the fiber, the fiber has good skin-care and health-care functions, the physical index of the product can meet the requirements of GB / T14463-2008 viscose staple fiber first-grade products, and the fiber has good wearability and textile processing performance.
Owner:单大伟
Neutralizing molecules to viral antigens
The present invention concerns methods and means for identifying, producing, and engineering neutralizing molecules against influenza A viruses, and to the neutralizing molecules produced. In particular, the invention concerns neutralizing molecules against various influenza A virus subtypes, including neutralizing antibodies against H5 and / or H3 and / or H1, such as, for example all of H1, H3, and H5 subtypes, and methods and means for making such molecules.
Owner:BIOASSETS LLC
Reagents and kits for detection of influenza virus and the like
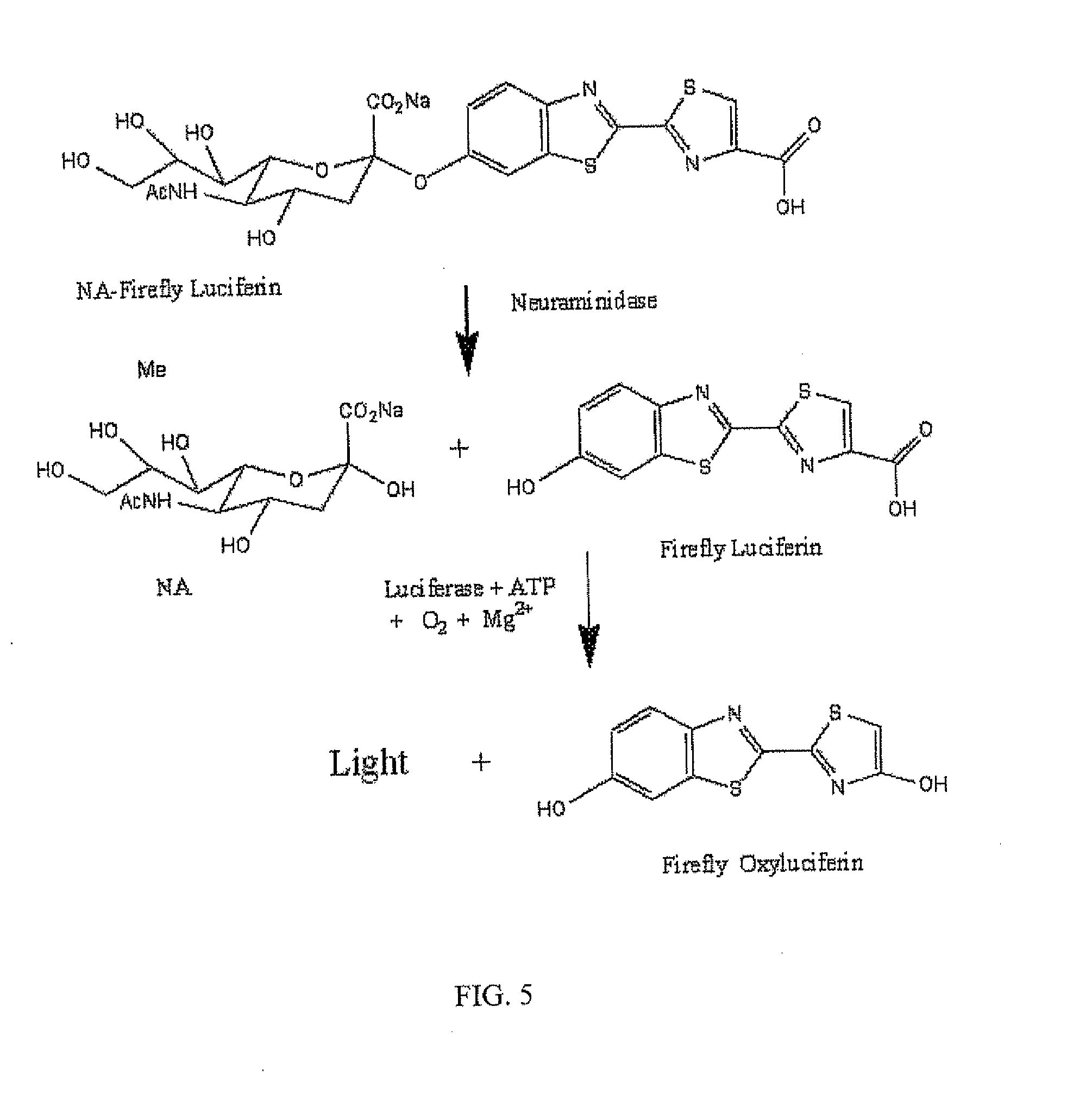
ActiveUS20110189655A1Simple and rapid and specific and sensitive detectionHigh detection sensitivitySugar derivativesMicrobiological testing/measurementNeuraminidaseFirefly luciferin
The present invention relates to reagents and methods for influenza virus detection. These reagents and methods disclosed in the present invention enable simple, rapid, specific and sensitive detection of influenza virus types A and B. These reagents are N-acetylneuraminic acid-firefly luciferin conjugates which can be cleaved by influenza virus neuraminidase.
Owner:CELLEX INC
Detection method and detection kit of influenza A virus, H1N1 and H3N2 subtype influenza virus
InactiveCN101818207AIncreased sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesReaction conditionsTaqMan
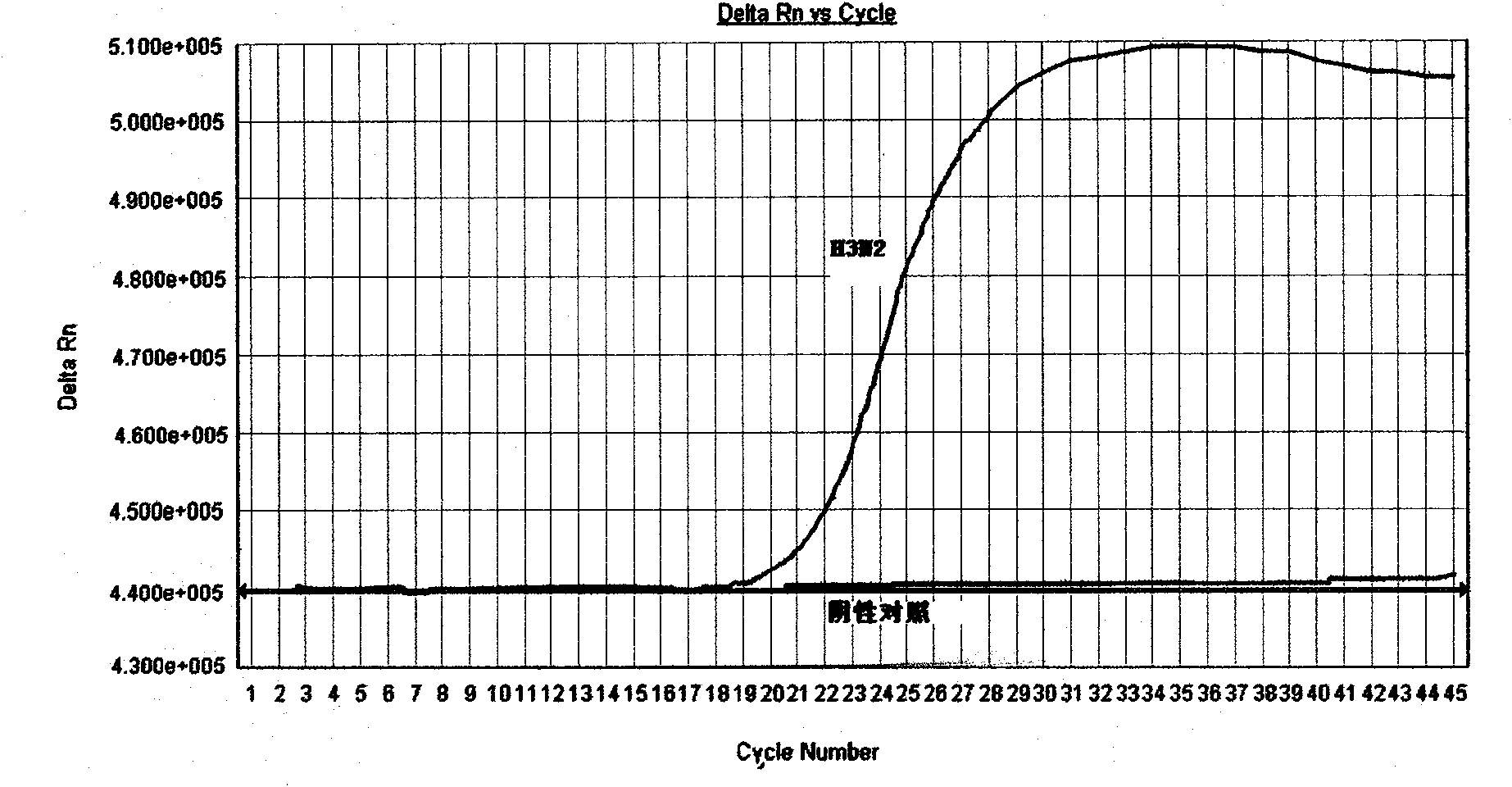
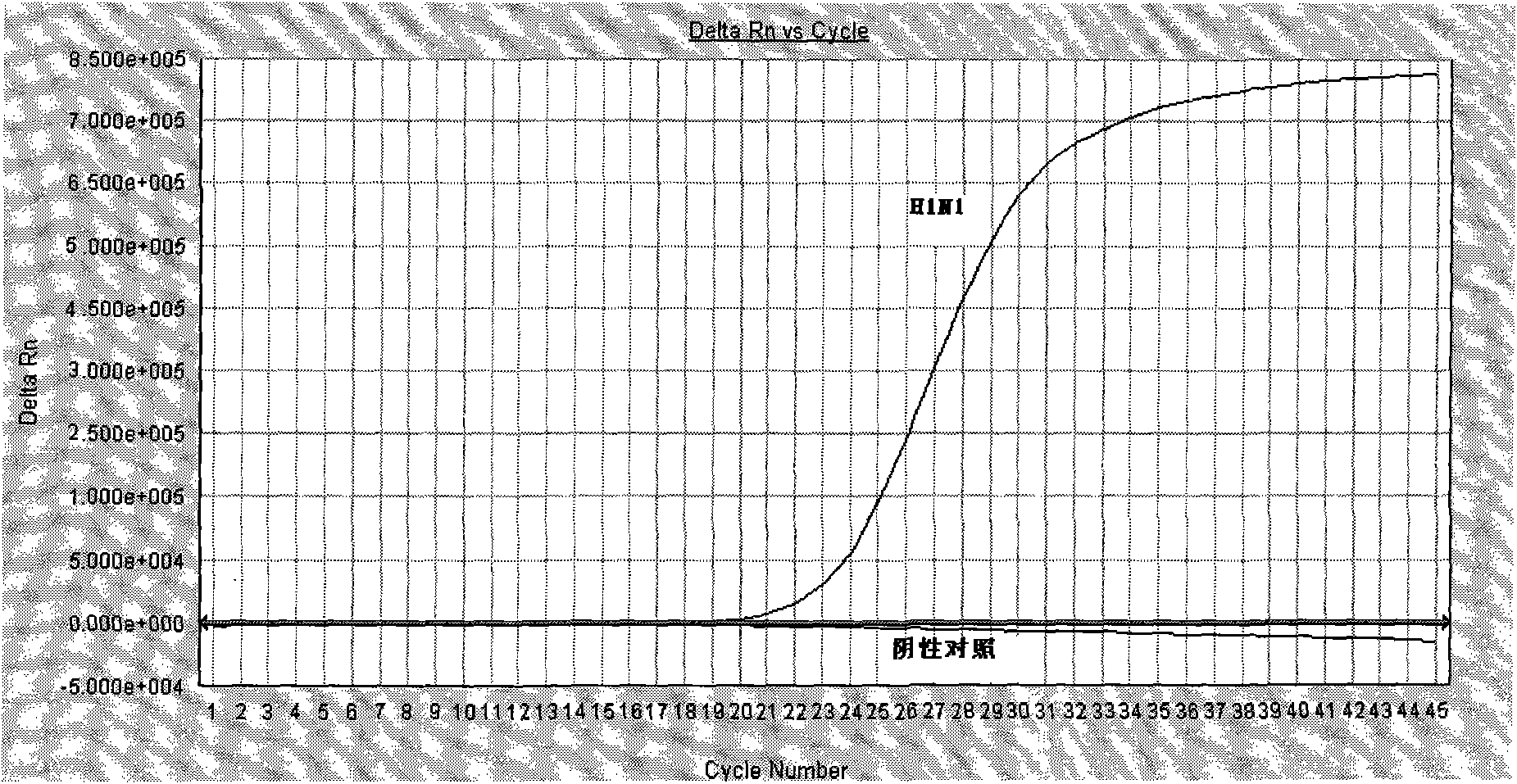
The invention discloses a detection method and a detection kit of influenza A virus, H1N1 and H3N2 subtype influenza virus, which has the advantages of specificity, sensitivity, good repeatability, fastness and low cost. A specific primer and a TaqMan probe are designed through sequence alignment for searching a highly conserved area according to a North American variant strain M gene of Influenza A Viruse(H1N1)virus in 2009, an HA gene sequence and the HA gene sequence of Influenza A Viruse(H3N2) epidemic strain, which are published in GeneBank; key reagents such as the probe, the primer and positive control (to structure the influenza A virus, the H1N1 and the H3N2 subtype influenza virus gene recombination clone plasmid) in the detection method are developed; and the influenza A virus, H1N1 and H3N2 subtype influenza virus real-time fluorescence RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection method is established through the optimization of various reaction conditions and the tests on the specificity, the sensitivity and the repeatability. The detection method can be used for simultaneously and specifically detecting the influenza A virus, the H1N1 subtype influenza virus and the H3N2 subtype influenza virus in one test.
Owner:中华人民共和国珠海出入境检验检疫局
Primer probe combination for detecting six respiratory viruses, kit and application
ActiveCN111041129AHigh clinical application valueRich varietyMicrobiological testing/measurementAgainst vector-borne diseasesRespiratory virusInfluenza Viruses Type A
The invention relates to the field of medical detection, in particular to a primer probe combination for detecting six respiratory viruses, a kit and application. According to the kit provided by theinvention, a method of combining isothermal amplification and a micro-fluidic chip is adopted; the kit can accurately detect novel coronavirus (2019-nCoV) S and N target genes and influenza A virus, novel influenza A H1N1 virus (2009), influenza A H3N2 virus, influenza B virus and respiratory syncytial virus at the same time, and the purpose of distinguishing the novel coronavirus from common influenza virus is achieved. The kit disclosed by the invention has the advantages of multiple detection indexes, simplicity and convenience in operation, short detection time and the like, six virus indexes can be simultaneously detected by one-time sample adding, the time from sample treatment to report is within 1.5 hours, and the kit is more time-saving and labor-saving than qPCR method.
Owner:CAPITALBIO CORP
Probes and methods for the simultaneous detection and identification of multiple viruses that cause respiratory infections in humans
InactiveCN101107366AShorten the lengthSynthetic economyMicrobiological testing/measurementAgainst vector-borne diseasesEnterovirusSevere acute respiratory syndrome
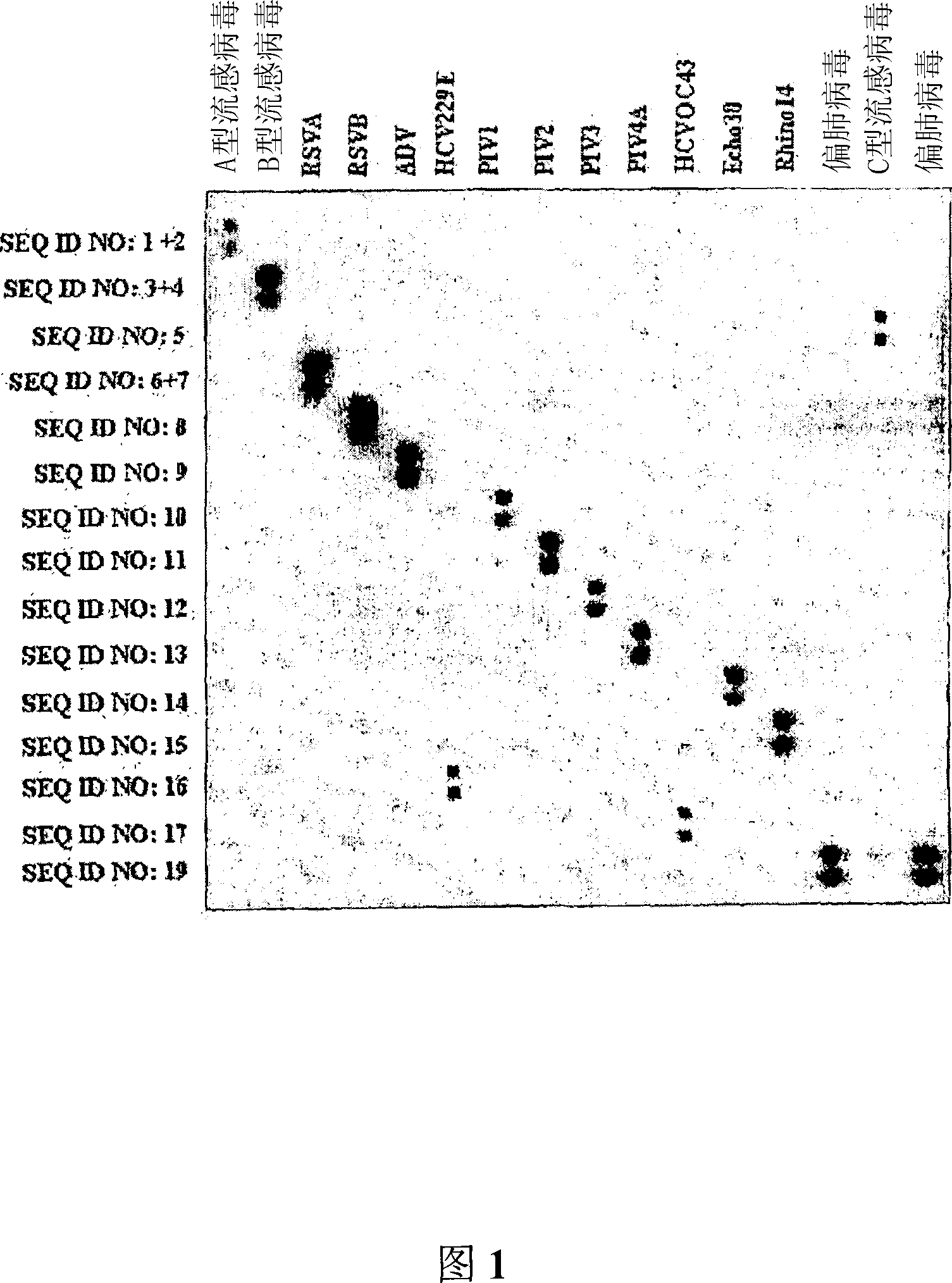
The invention relates to probes and assays which are used for the simultaneous detection, in a single assay sample, of a plurality of nucleic acid sequences of viruses that cause respiratory infections in humans, selected from among influenza virus type A, influenza virus type B, influenza virus type C, human respiratory syncytial virus type A, human respiratory syncytial virus type B, human adenovirus, human parainfluenza virus type 1, human parainfluenza virus type 2, human parainfluenza virus type 3, human parainfluenza virus types 4A and 4B, enterovirus, rhinovirus, human coronavirus type 229E, human coronavirus type OC43, coronavirus that causes severe acute respiratory syndrome (SARS), human metapneumovirus and combinations thereof.
Owner:INST DE SALUD CARLOS III
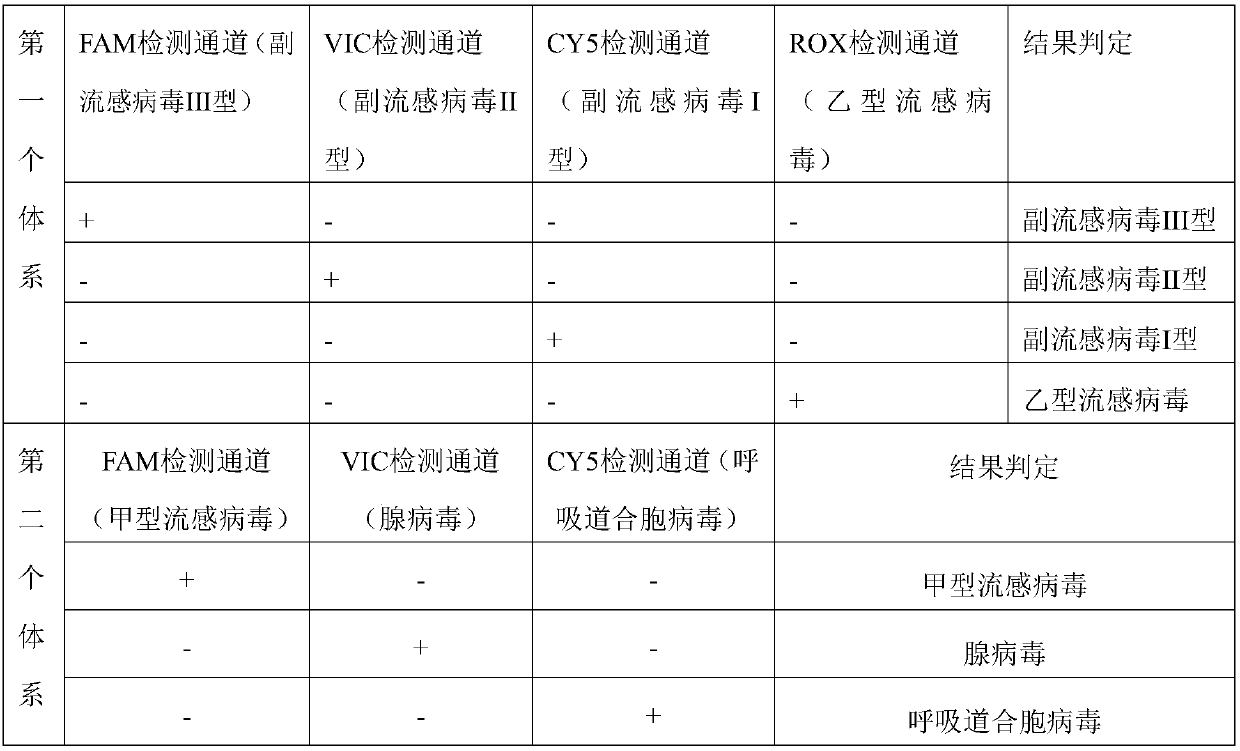
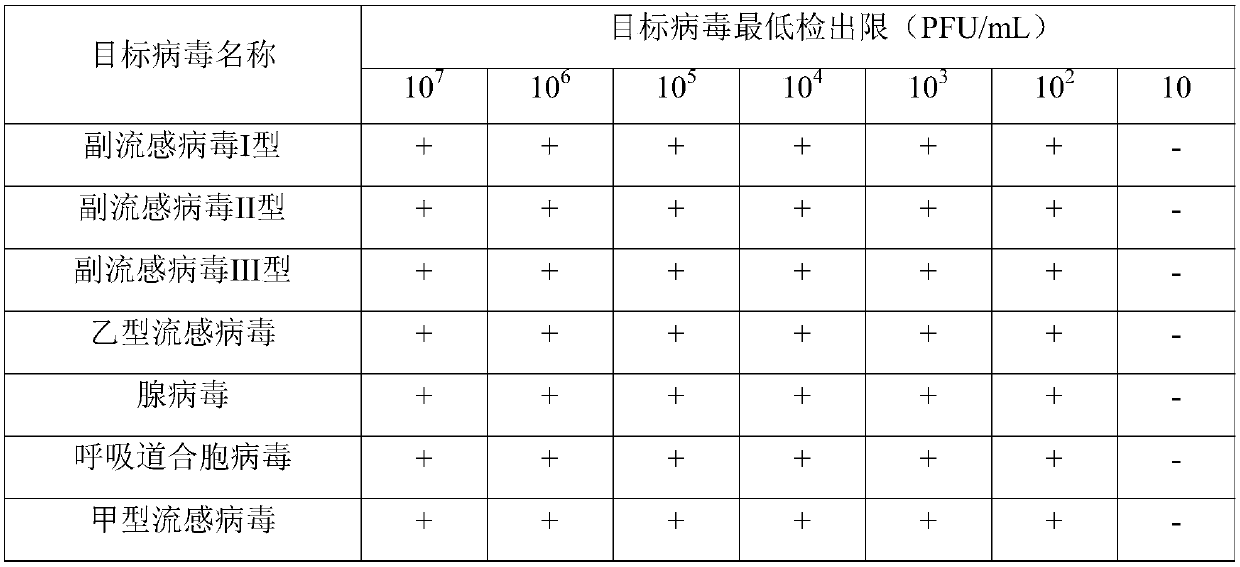
Real-time fluorescence multiplex PCR primer probe for seven common respiratory system influenza virus pathogens and kit
ActiveCN107937613AQuick checkAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceParainfluenza virus
The present disclosure relates to a real-time fluorescence multiplex PCR primer probe for seven common respiratory system influenza virus pathogens and a kit. Seven influenza viruses are influenza A virus, influenza B virus, parainfluenza virus type I, parainfluenza virus type II, parainfluenza virus type III, respiratory syncytial virus and adenovirus. The present disclosure also provides a kit for multiplex fluorescence quantitative PCR detection of the seven influenza viruses, and the kit includes the primer probe set. The kit significantly improves the sensitivity, specificity, and simplicity of detection of the common respiratory system pathogens.
Owner:北京卓诚惠生生物科技股份有限公司
Application of traditional Chinese medical composition in preparation of medicament for resisting influenza A H1N1 influenza viruses
The invention discloses the application of a traditional Chinese medical composition in the preparation of a medicament for resisting influenza A H1N1 influenza viruses. The traditional Chinese medical composition is prepared from the medical materials of forsythia, honeysuckle, ephedra, bitter almond, and the like, has broad-spectrum antiviral action, can effectively kill the viruses, reduce fever and diminish inflammation. Proved by experiments, the traditional Chinese medical composition can accurately resist the influenza A H1n1 influenza viruses.
Owner:BEIJING YILING PHARMA
Multiple RT-RPA primer combination for influenza A virus detecting and H1 and H3 typing and application thereof
ActiveCN108192996AEasy to detectReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationTypingTrue positive rate
The invention belongs to the field of molecular biological detection, and relates to a multiple RT-RPA primer combination for influenza A virus detecting and H1 and H3 typing and application thereof.The multiple RT-RPA primer combination for influenza A virus detecting and H1 and H3 typing comprises an RT-RPA primer for detecting an influenza A virus Matrix gene, an H1 subtype HA gene and an H3 subtype HA gene, and the sequences are sequentially shown in SEQ ID NO.1-NO.6. The multiple RT-RPA primer combination can be used for detecting an influenza A virus and verifying H1 and H3 subtypes. According to the multiple RT-RPA primer combination, a multiple RT-RPA method is adopted for detecting the influenza A virus and verifying the H1 and H3 subtypes for the first time, and the combinationis short in consumed time, high in sensitivity and specificity and capable of being quickly and effectively used for influenza A virus detecting and H1 and H3 typing.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A
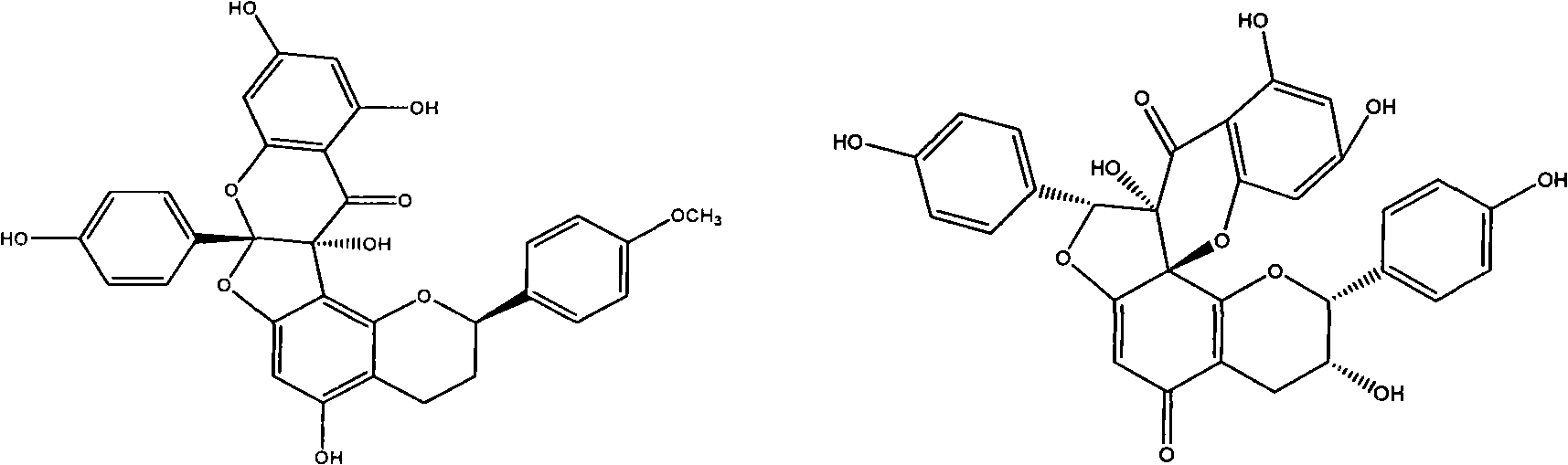
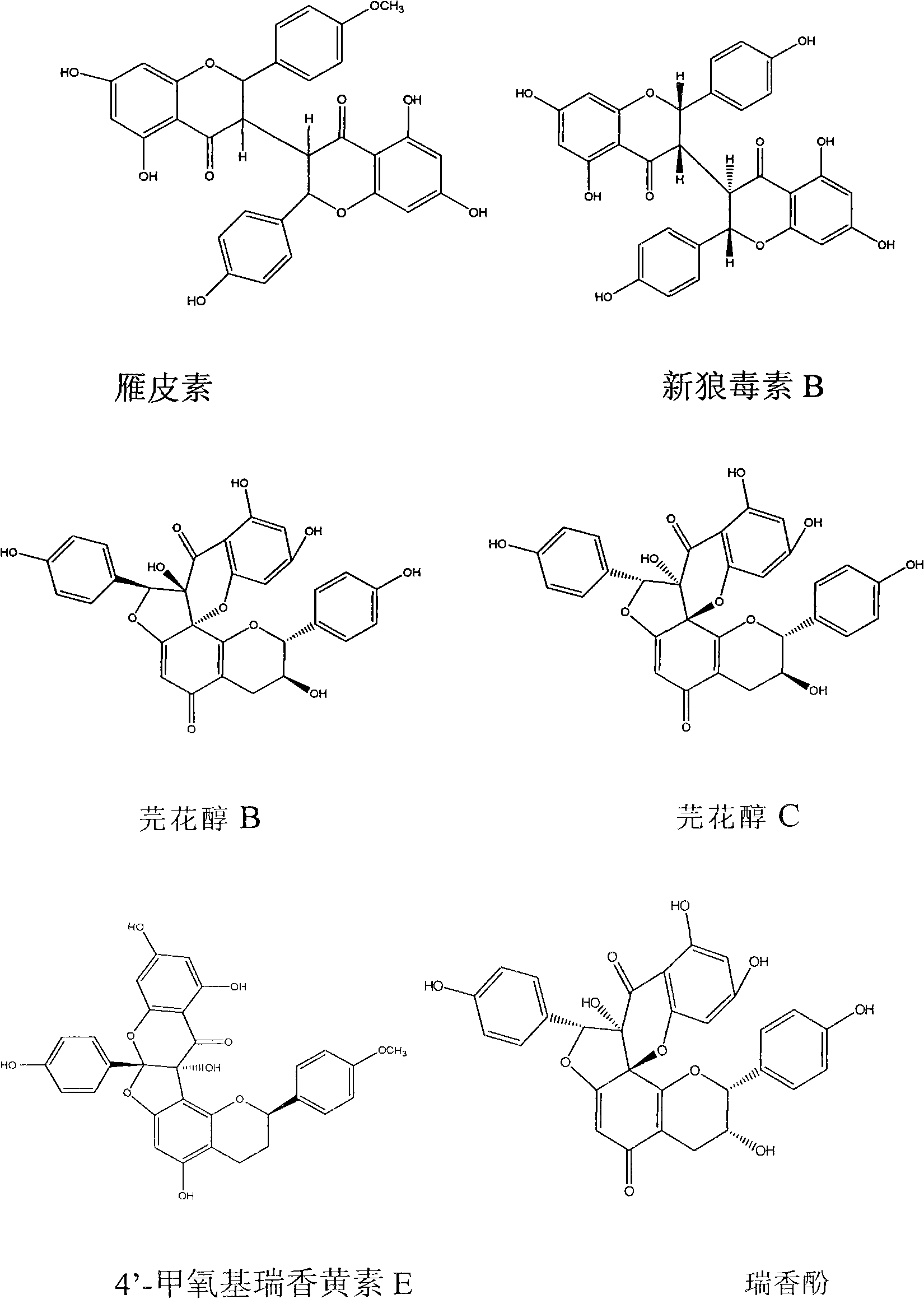
Radix wikstroemae extractive and preparation method and application thereof
InactiveCN101601666AGood anti-respiratory virus activityGood active ingredientOrganic active ingredientsOrganic chemistryMedicinePolyamide
The invention discloses a radix wikstroemae extractive and a preparation method and an application thereof. The radix wikstroemae extractive comprises 4'-methoxyl daphne odora E and thymol. The preparation method of the radix wikstroemae extractive has the following steps: dried root and root bark of the radix wikstroemae is extracted by ethanol water, then the extractive is enriched by macroporous absorption resin column or polyamide column. The extractive can be used for preparing medicine or health-care products for resisting respiratory syncytial virus, parainfluenza type III virus and influenza A virus.
Owner:JINAN UNIVERSITY
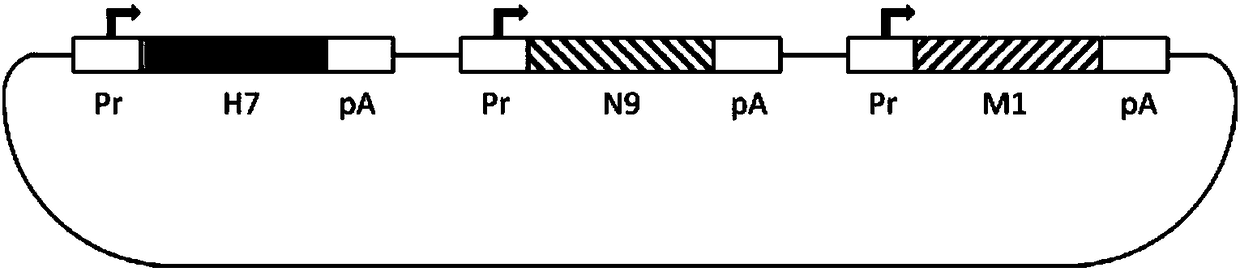
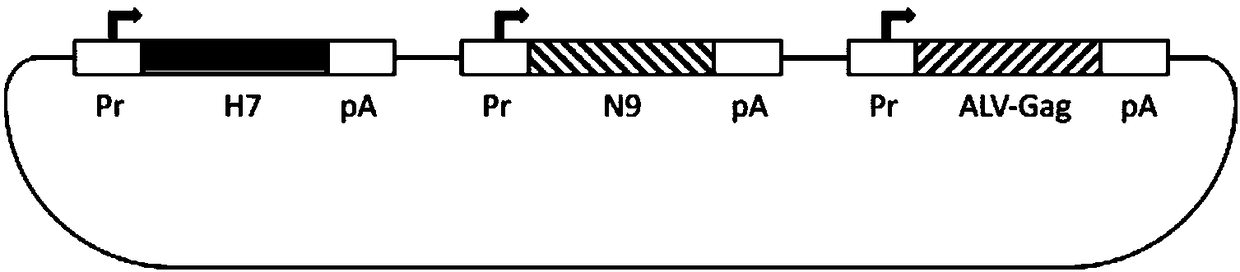
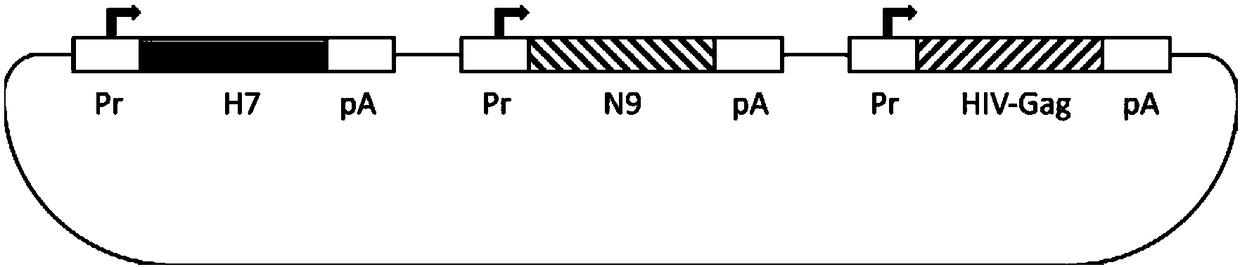
Ordinary/chimeric virus-like particle of H7 subtype influenza virus H7N9, preparation method and application of ordinary/chimeric virus-like particle, and vaccine
ActiveCN108329379AGood effectFix security issuesSsRNA viruses negative-senseViral antigen ingredientsVirus-like particleViral Vaccine
The invention provides an ordinary / chimeric virus-like particle of an H7 subtype influenza virus H7N9, a preparation method and application of the ordinary / chimeric virus-like particle, and a vaccine,and belongs to the technical field of bioengineering and viral vaccines. The ordinary / chimeric virus-like particle of the H7 subtype influenza virus is used for preparing the vaccine, and accordingly, the safety problems of existing vaccines are well solved; the obtained vaccine has high antibody valence and obvious effect; the ordinary / chimeric virus-like particle has wide application range andhigh actual application value.
Owner:NOVARTIS BIOTECH WUHAN +1
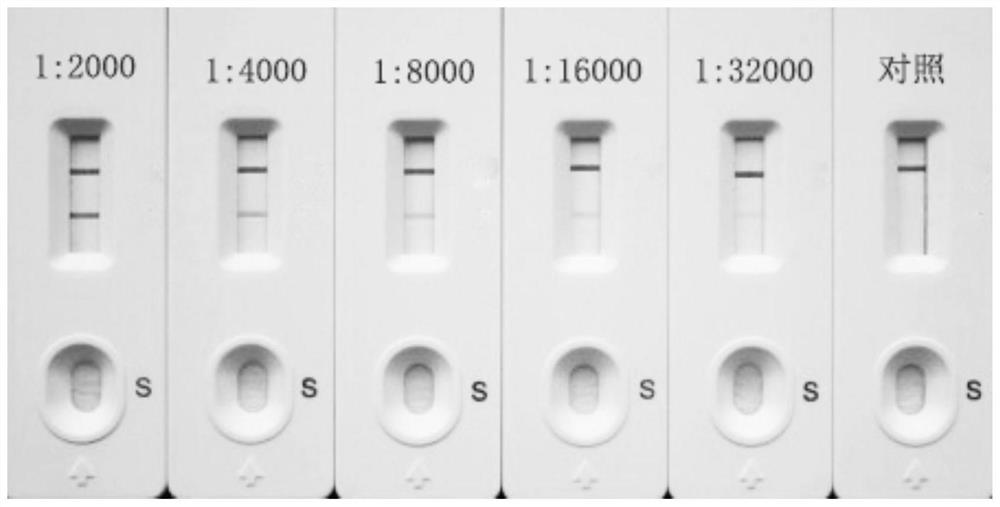
Influenza Virus A colloidal gold quick detection test paper
The present invention discloses hybridoma cell strain whose conservative number is CGMCC 0987, anti-influenza A virus nucleoprotein monoclonal antibody produced by said hybridoma cell strain and influenza A virus colloidal gold fast detection testing paper containing said monoclonal antibody. Said detection testing paper can be used for quickly detecting influenza A virus, its specificity, sensitivity and accuracy are high, and its storage and transportation are convenient.
Owner:北京阿斯可来生物工程有限公司
Novel coronavirus antigen and influenza virus antigen combined detection reagent strip and preparation method thereof
PendingCN112198312AReduce false positive interferenceHigh sensitivityBiological testingImmunoassaysReagent stripCoronavirus antibody
The invention discloses a novel coronavirus antigen and influenza virus antigen combined detection reagent strip which comprises a bottom plate. A sample pad, a gold-labeled pad, a nitrocellulose membrane and absorbent paper are sequentially pasted on the bottom plate, and the surface of the nitrocellulose membrane is sequentially coated with an anti-novel coronavirus antibody, an anti-influenza Avirus antibody and an anti-influenza B virus antibody. A rabbit anti-mouse IgG antibody coats the end close to the absorbent paper. A colloidal gold labeled anti-novel coronavirus antibody, an anti-influenza A virus antibody and an anti-influenza B virus antibody are sprayed on the gold-labeled pad. The binding protein A / G is marked on the surface of the colloidal gold, the protein A / G is specifically bound with the Fc end of the antibody, so that the ideal conformation of Fab end abduction is constructed, and meanwhile, the binding of the protein A / G with the Fc can reduce the false positiveinterference of rheumatoid factors on a detection structure. The reagent strip can simultaneously realize qualitative detection of the novel coronavirus antigen and the influenza antigen in one test,and is convenient to use, good in sensitivity, high in specificity and short in detection time.
Owner:南京佰抗生物科技有限公司
Reagent for detection of animal influenza virus type A, detection method and application thereof
InactiveCN106555012ALower requirementGuarantee production safetyMicrobiological testing/measurementMicroorganism based processesForward primerQuarantine
The application discloses a reagent for detection of animal influenza virus type A, a detection method and an application thereof. The reagent includes a primer pair and a probe used for recombinase polymerase amplification of the animal influenza virus type A, wherein a forward primer of the primer pair is represented as the SEQ ID No.1, a reverse primer of the primer pair is represented as the SEQ ID No.2, and the probe is represented as the SEQ ID No.3; the 29th basic group in the probe is marked by a FAM group, the 31st basic group is replaced by dSpacer, the 34th basic group is marked by a BHQ1 group, and a 3' terminal is modified by C3Spacer. The reagent provides a novel detection scheme and approach for the detection of animal influenza virus type A. The animal influenza virus type A RPA detection method based on the reagent is quick and accurate, is low in demand on hardware equipment, is suitable for on-site quick detection and is especially suitable for departments such as animal quarantine and the like.
Owner:SHENZHEN AUDAQUE DATA TECH
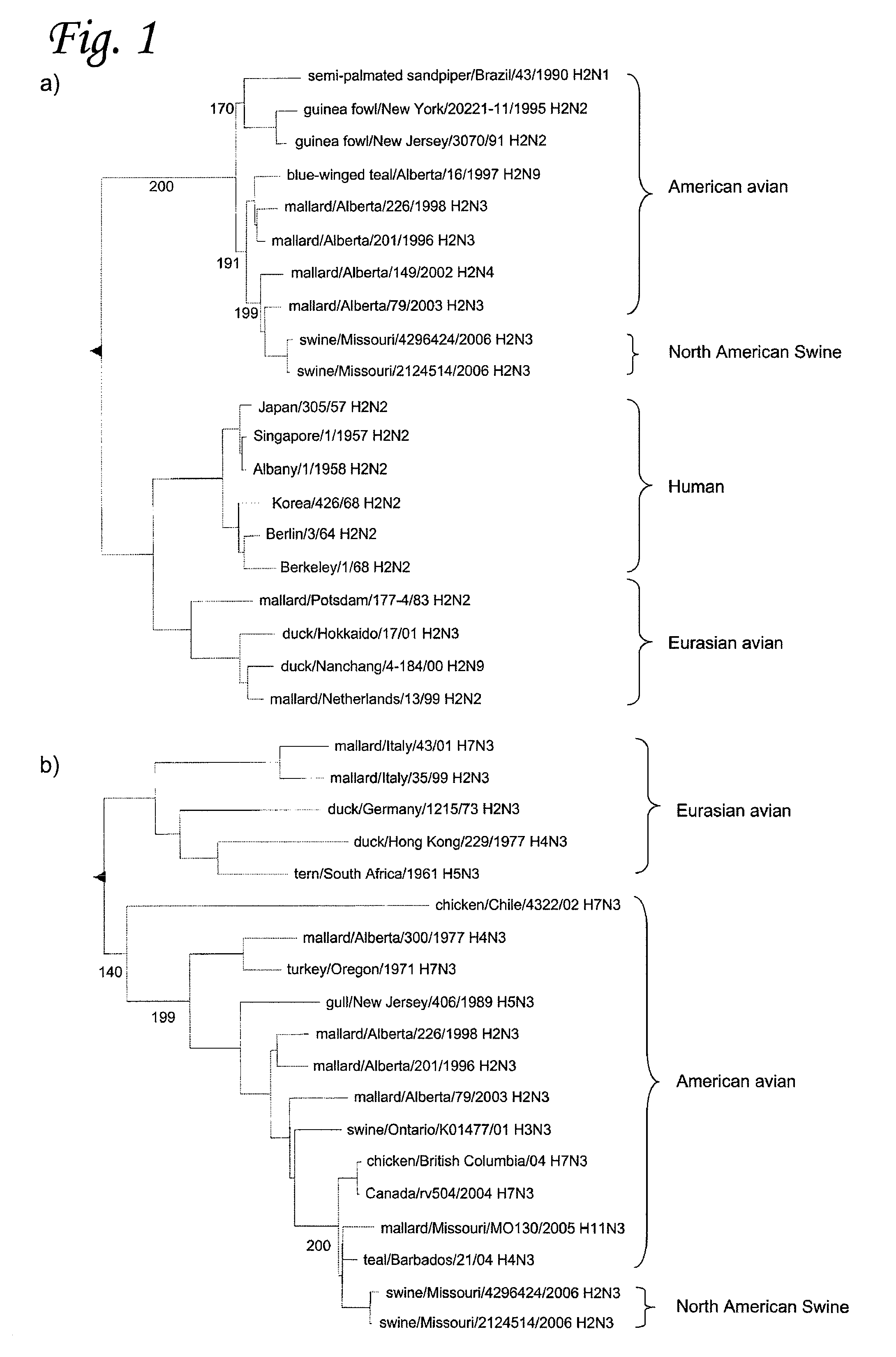
H2N3 influenza A viruses and methods of use
Owner:RGT UNIV OF MINNESOTA +2
Cold-adapted equine influenza viruses
InactiveUS20060121521A1Antibacterial agentsSsRNA viruses negative-senseVirus influenzaRespiratory disease
The present invention provides experimentally-generated cold-adapted equine influenza viruses, and reassortant influenza A viruses comprising at least one genome segment of such an equine influenza virus, wherein the equine influenza virus genome segment confers at least one identifying phenotype of the cold-adapted equine influenza virus, such as cold-adaptation, temperature sensitivity, dominant interference, or attenuation. Such viruses are formulated into therapeutic compositions to protect animals from diseases caused by influenza A viruses, and in particular, to protect horses from disease caused by equine influenza virus. The present invention also includes methods to protect animals from diseases caused by influenza A virus or other infectious agents utilizing the claimed therapeutic compositions. Such methods include using a therapeutic composition as a vaccine to generate a protective immune response in an animal prior to exposure to an infectious agent, as well as using a therapeutic composition as a treatment for an animal that has been recently infected with an infectious agent leading to respiratory disease, or is likely to be subsequently exposed to such an agent in a few days whereby the therapeutic composition reduces such respiratory disease, even in the absence of antibody-mediated immunity. The present invention also provides methods to produce cold-adapted equine influenza viruses, and reassortant influenza A viruses having at least one genome segment of an equine influenza virus generated by cold-adaptation.
Owner:DOWLING PATRICIA W +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com