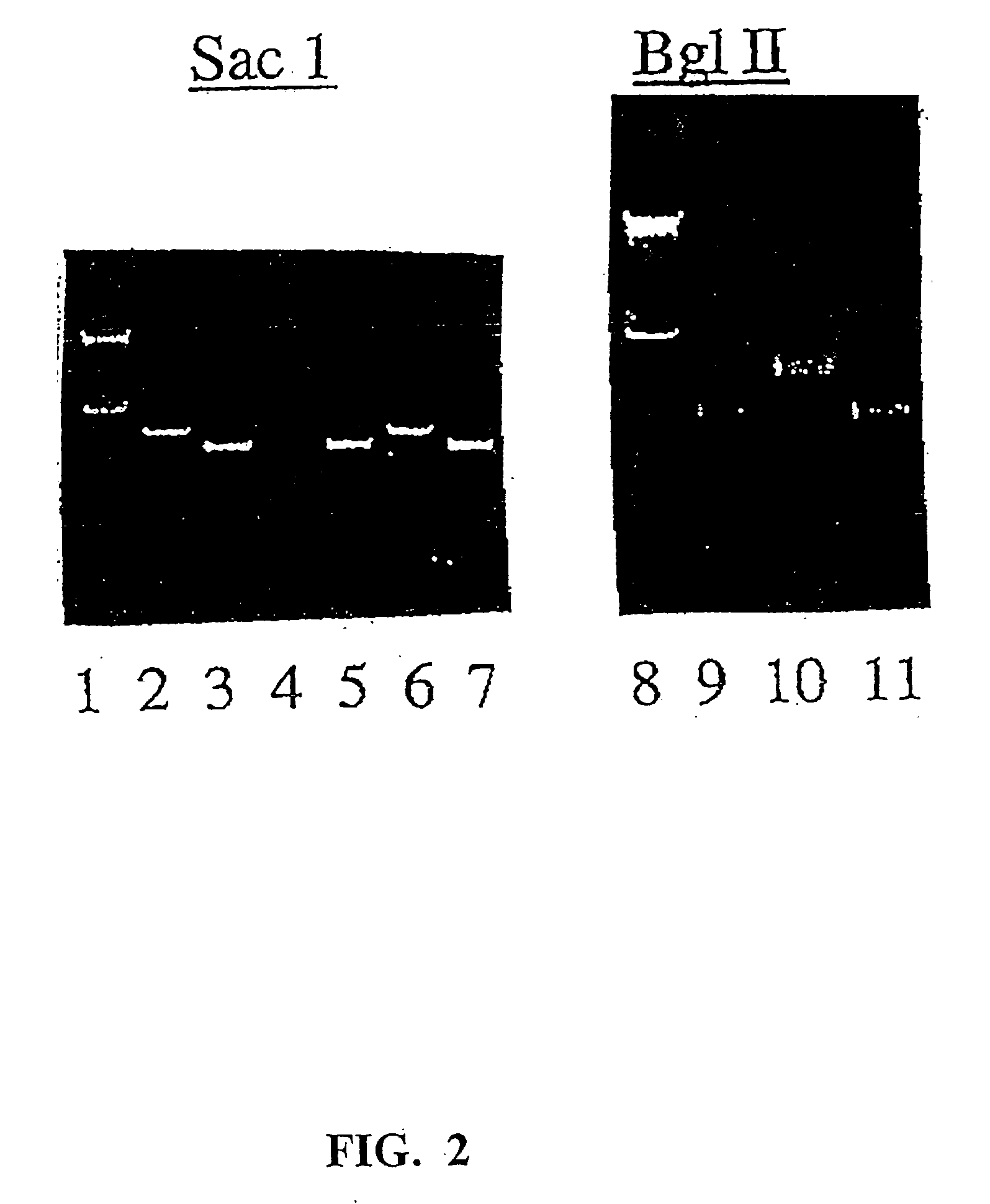
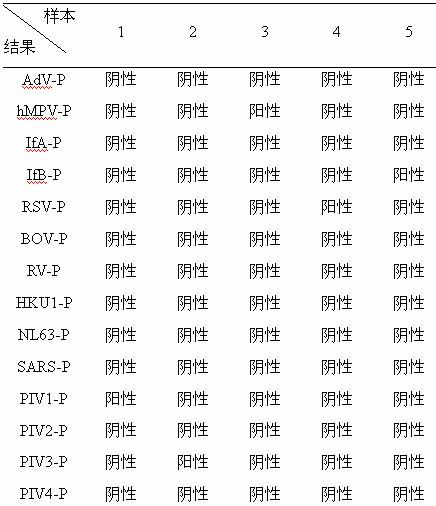
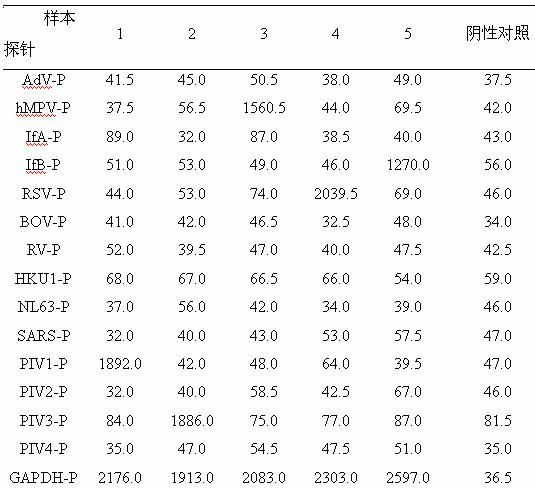
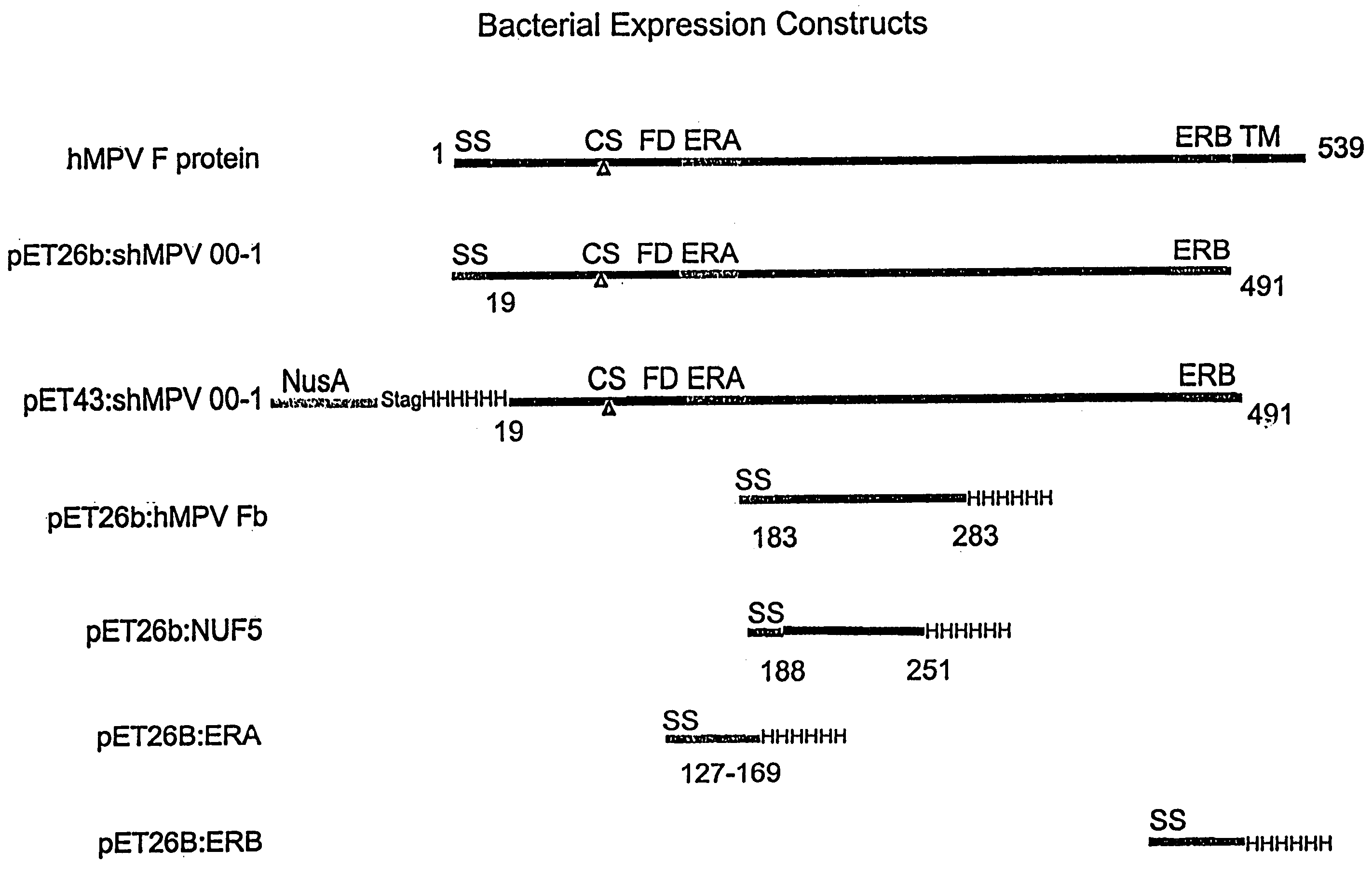
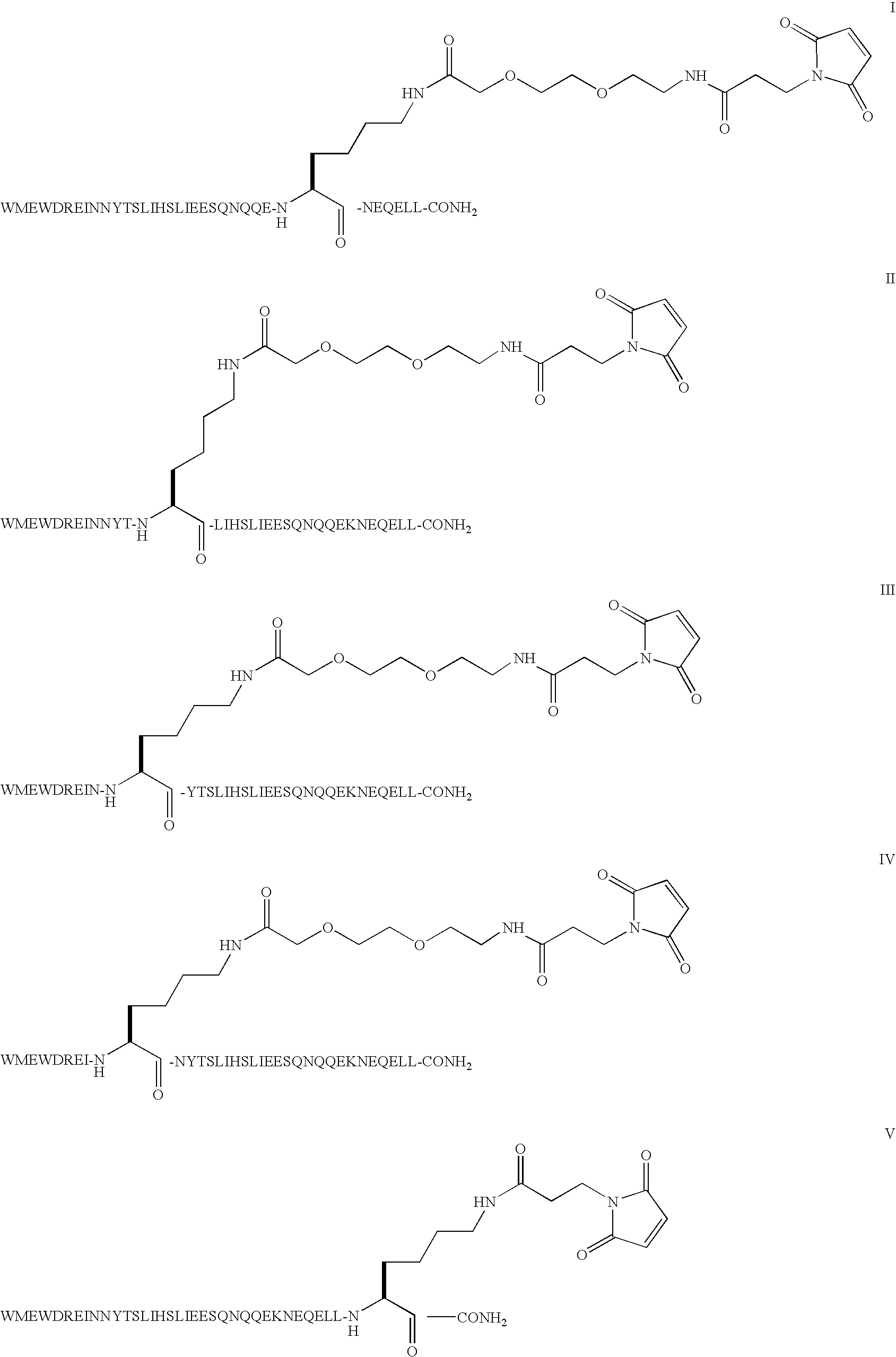
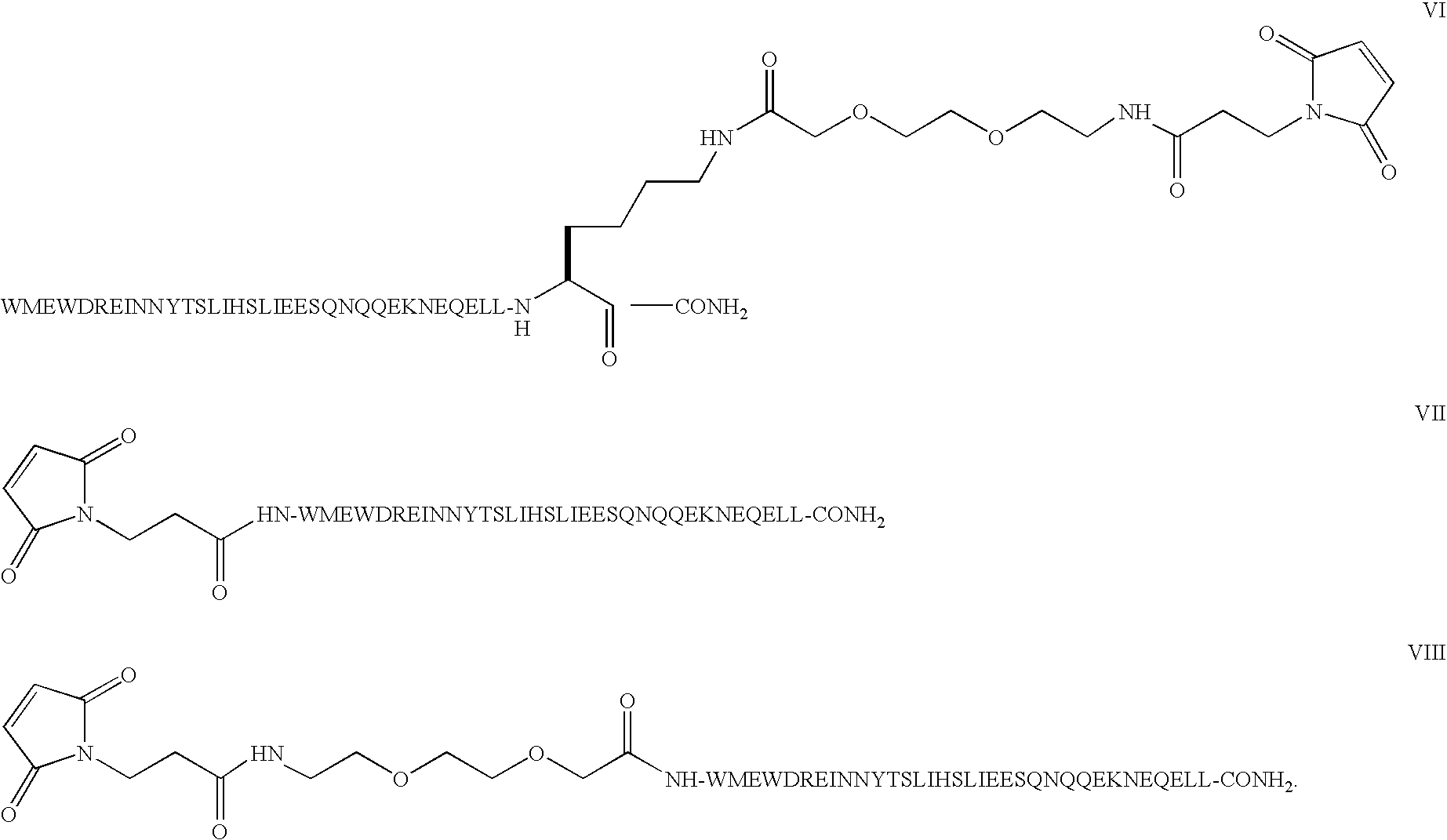
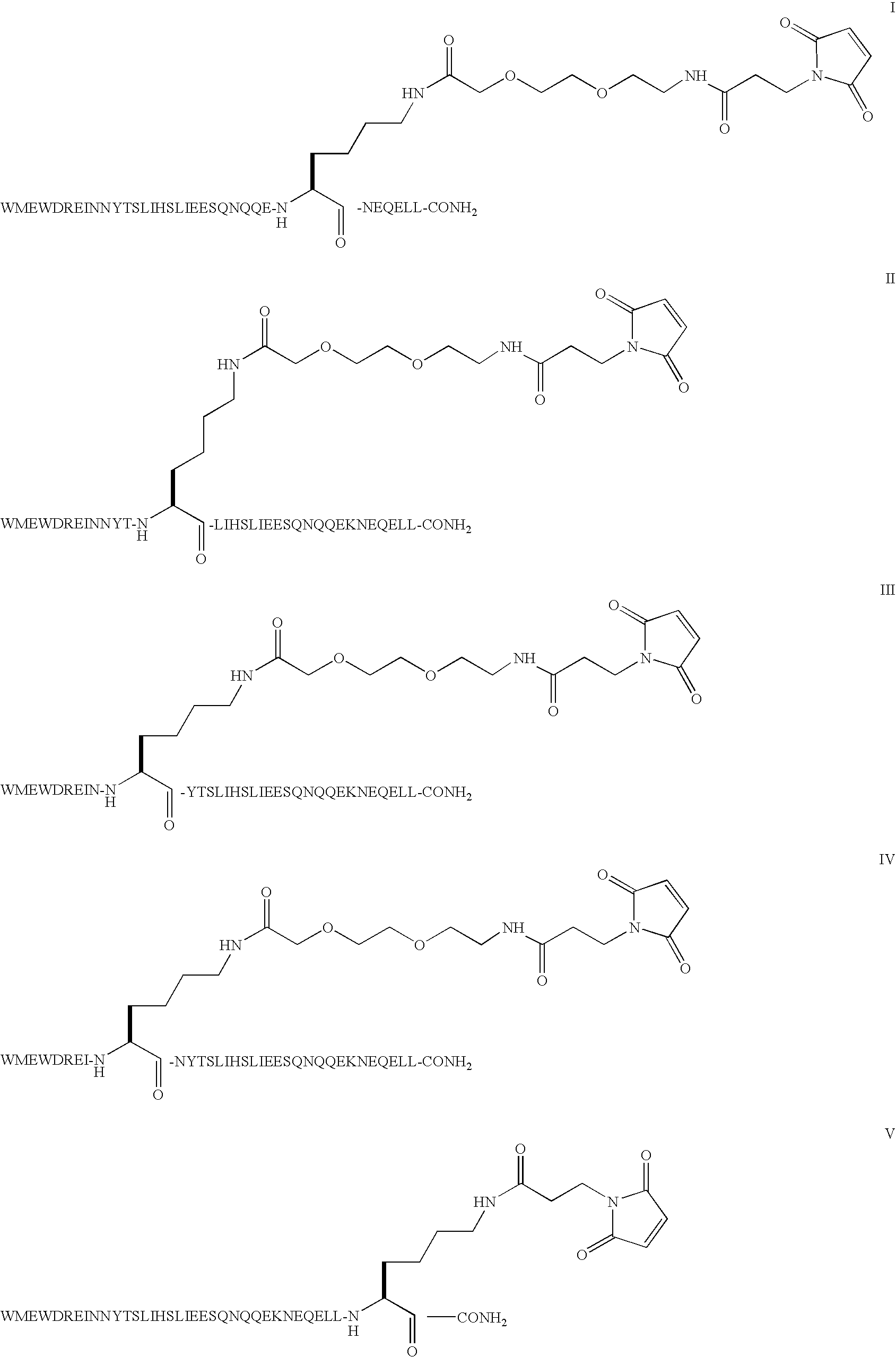
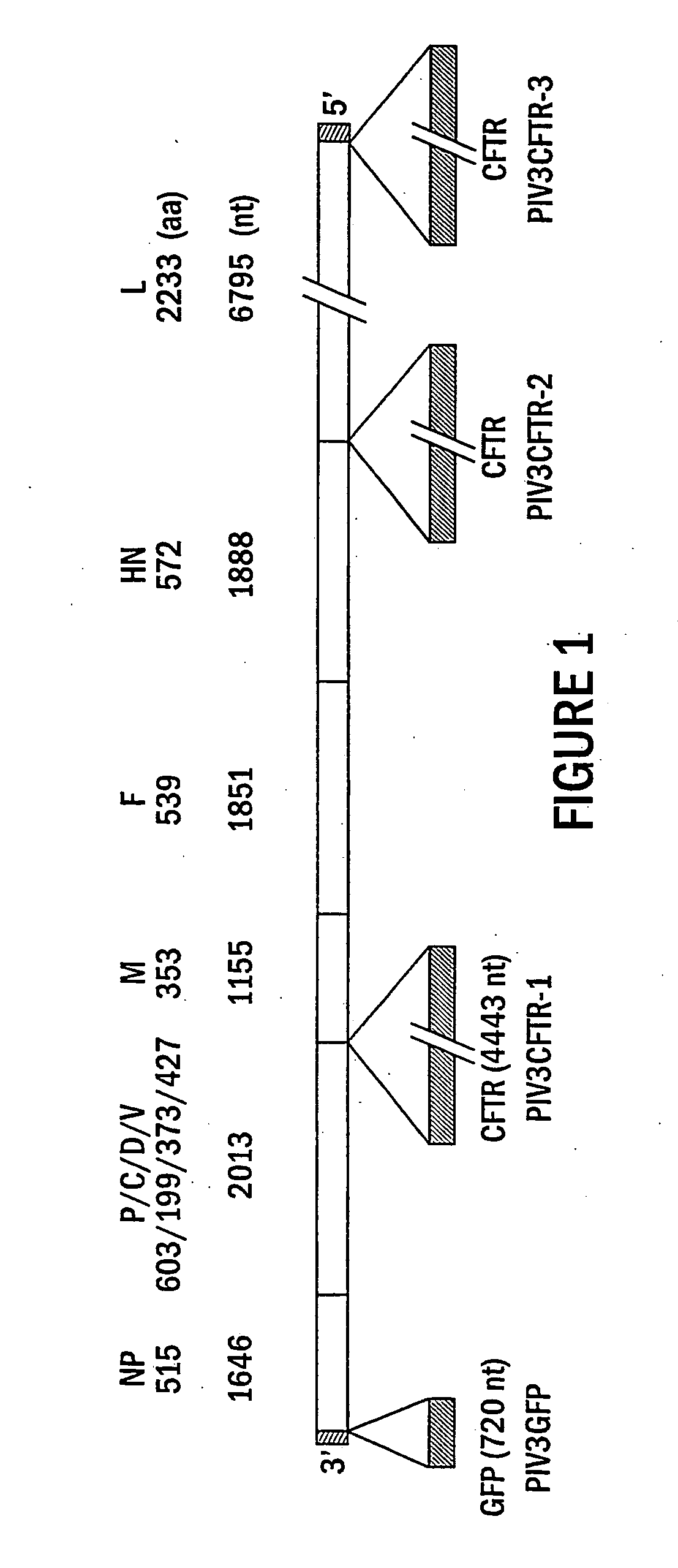
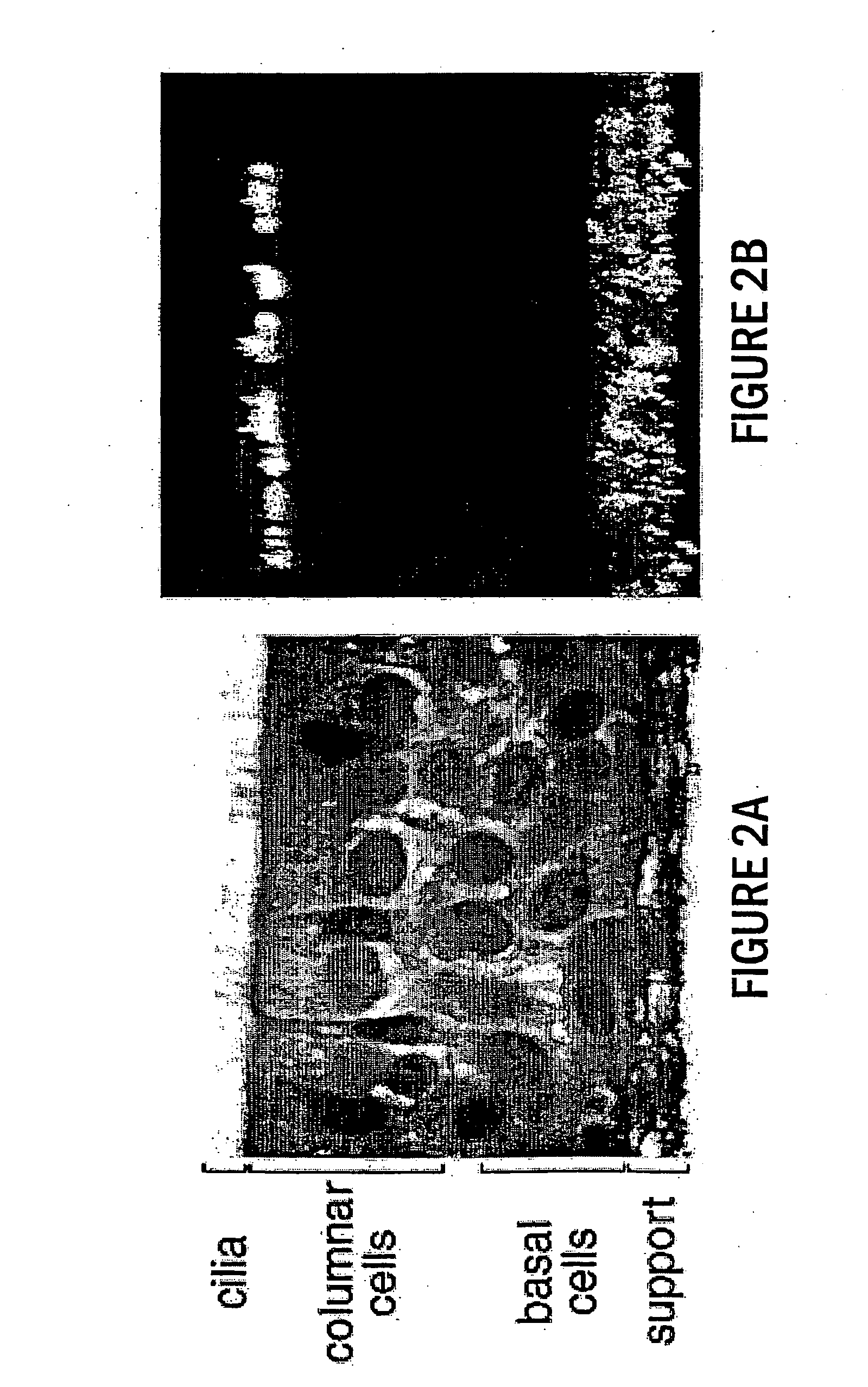
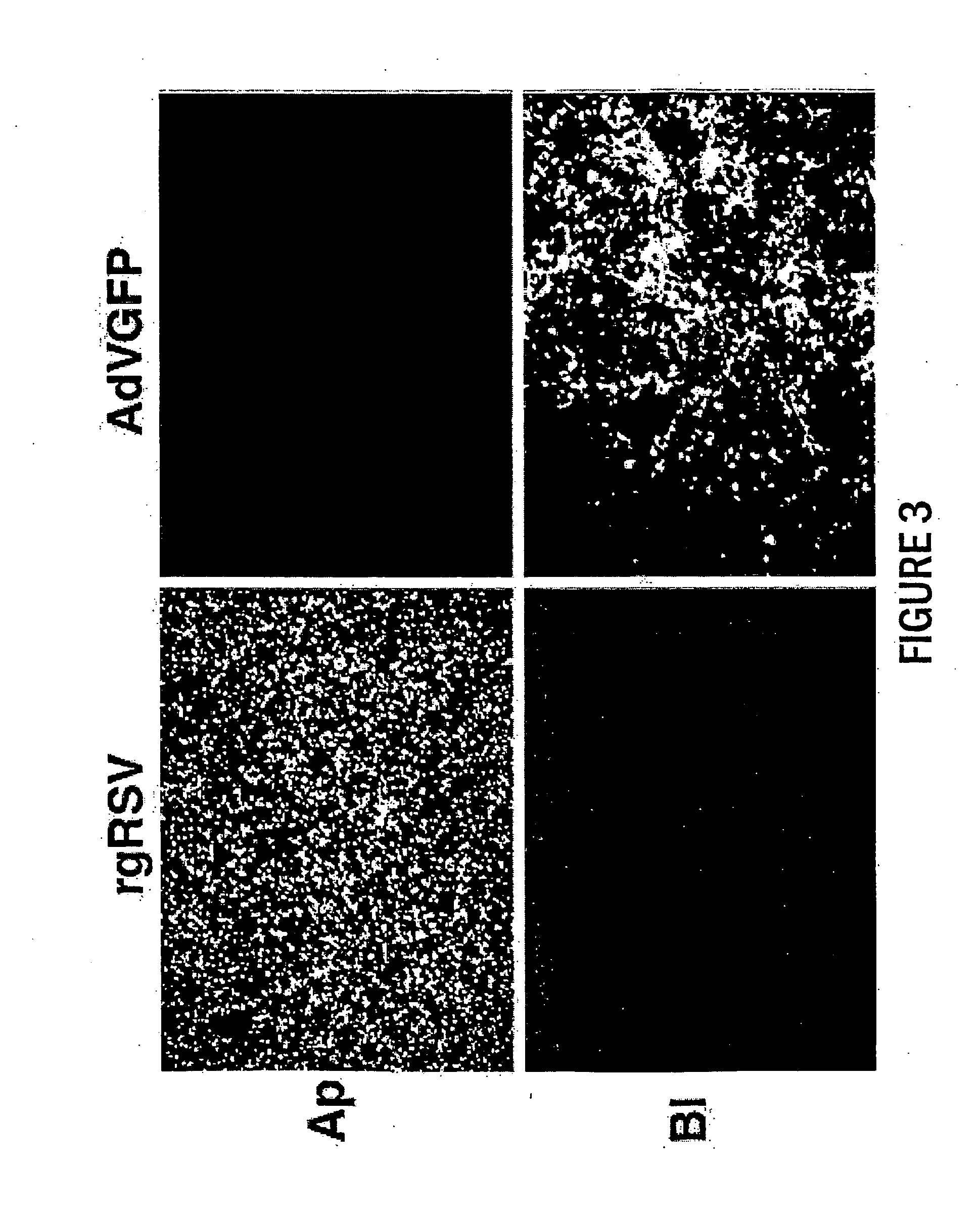
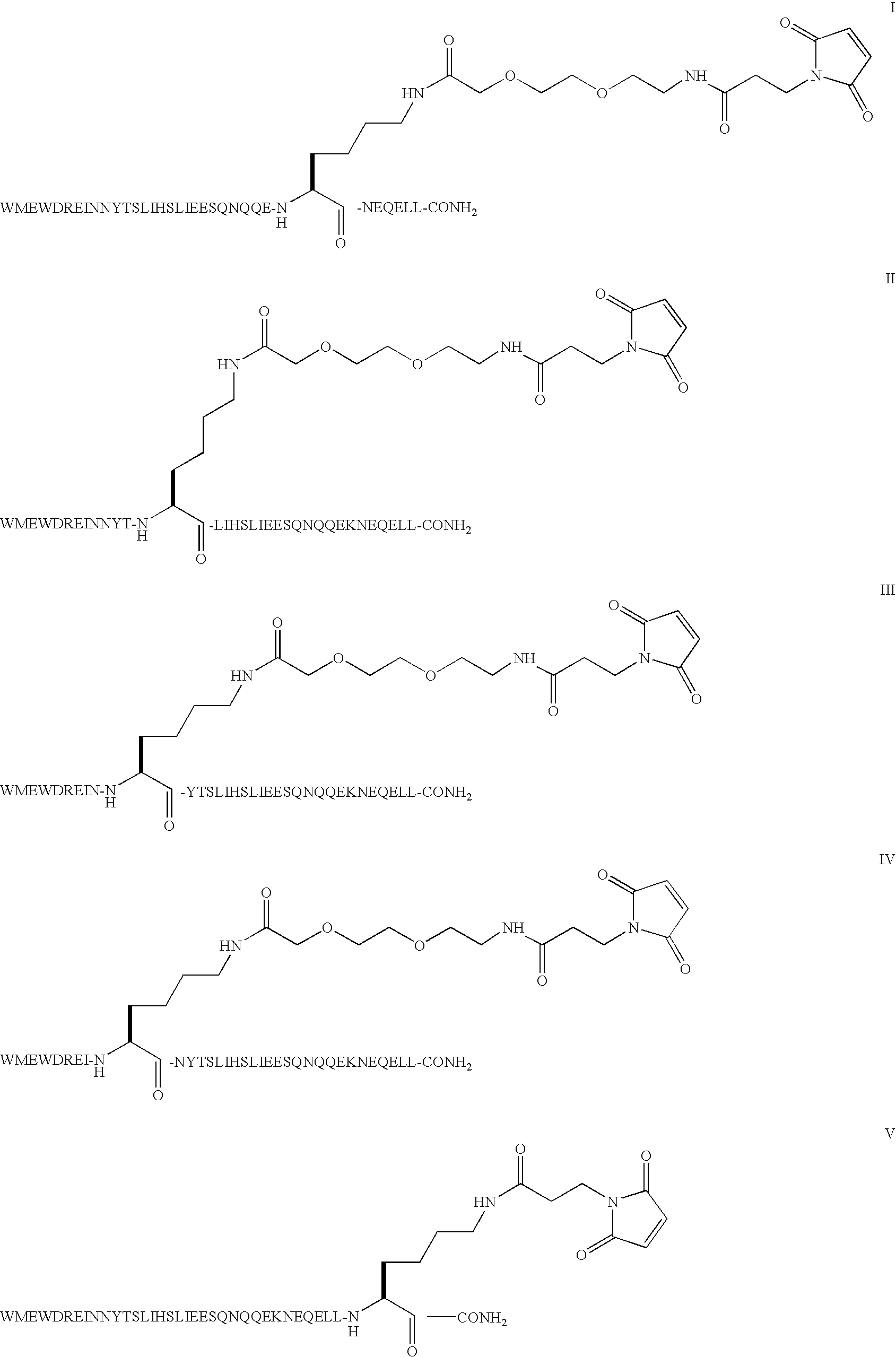
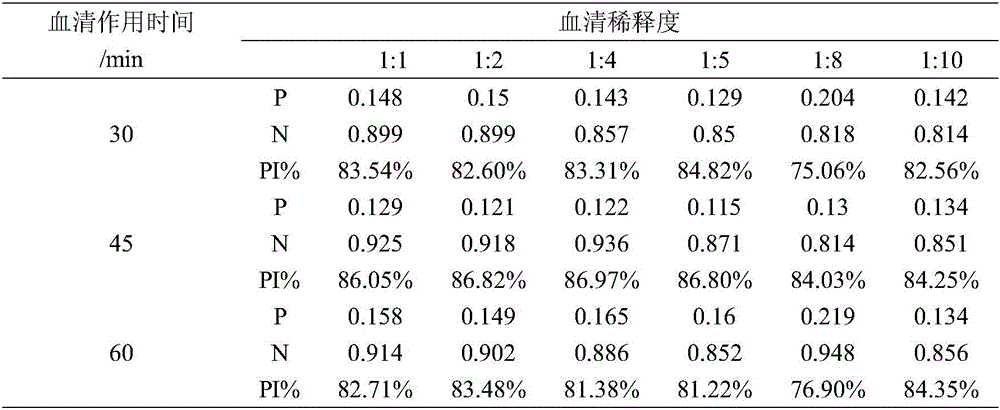
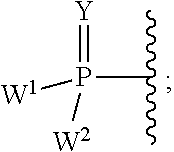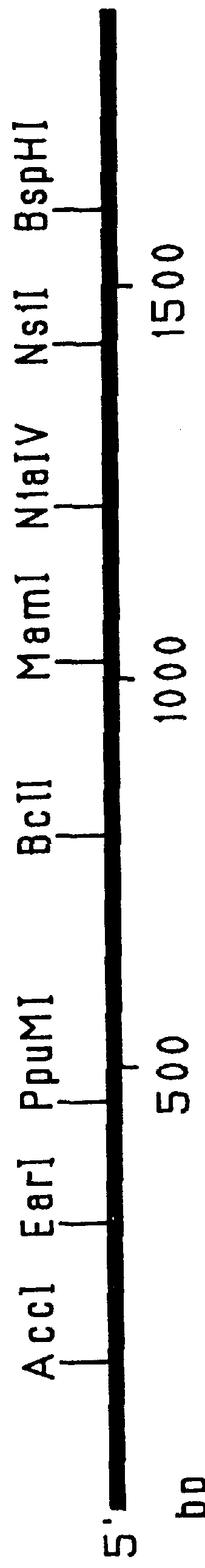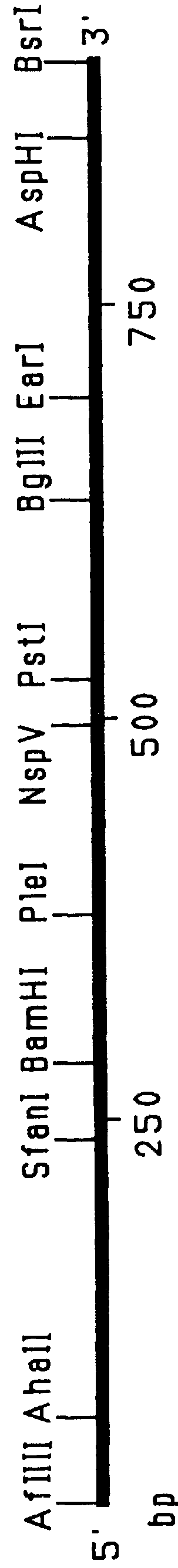Patents
Literature
201 results about "Parainfluenza virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
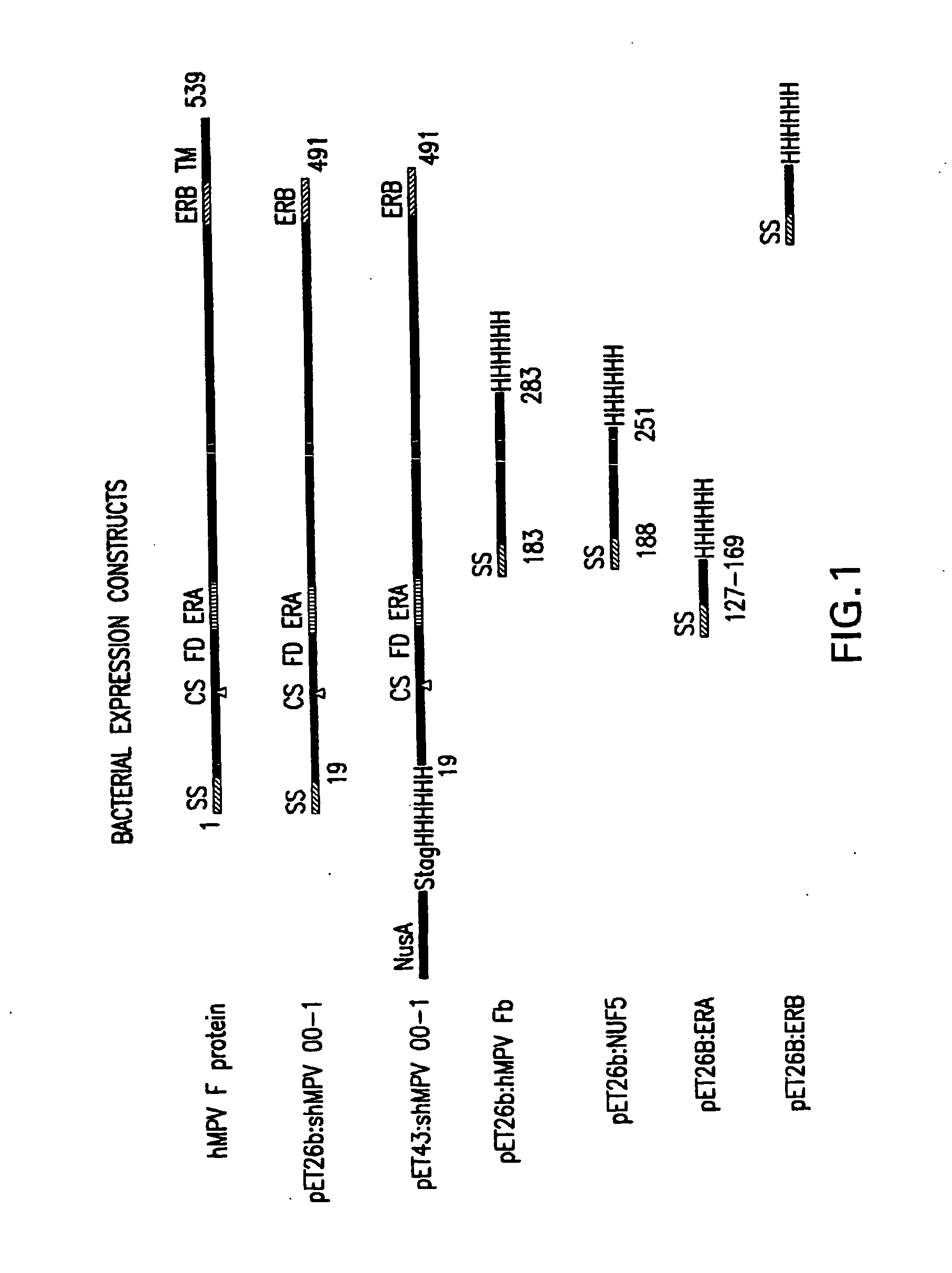
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
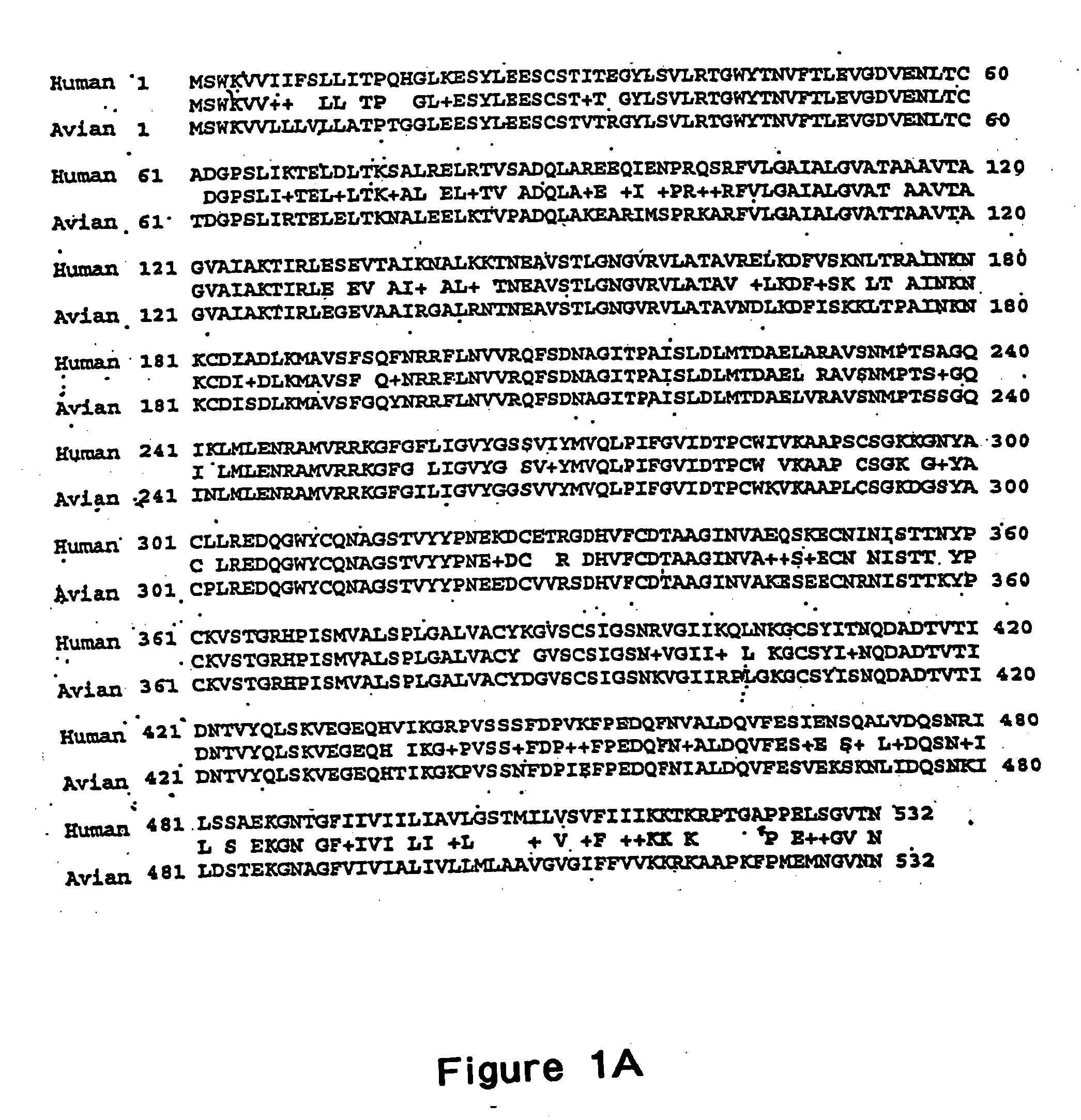
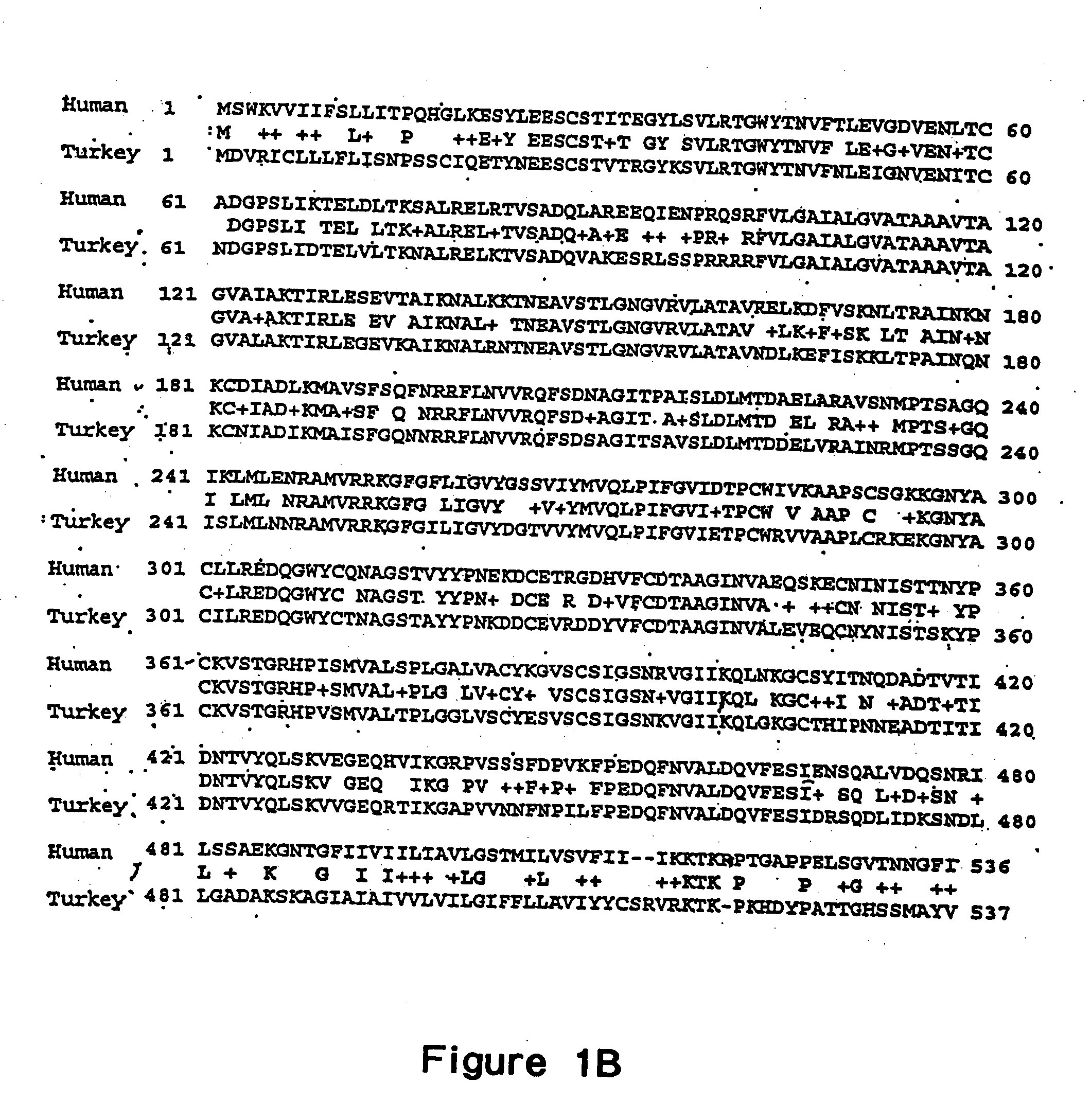
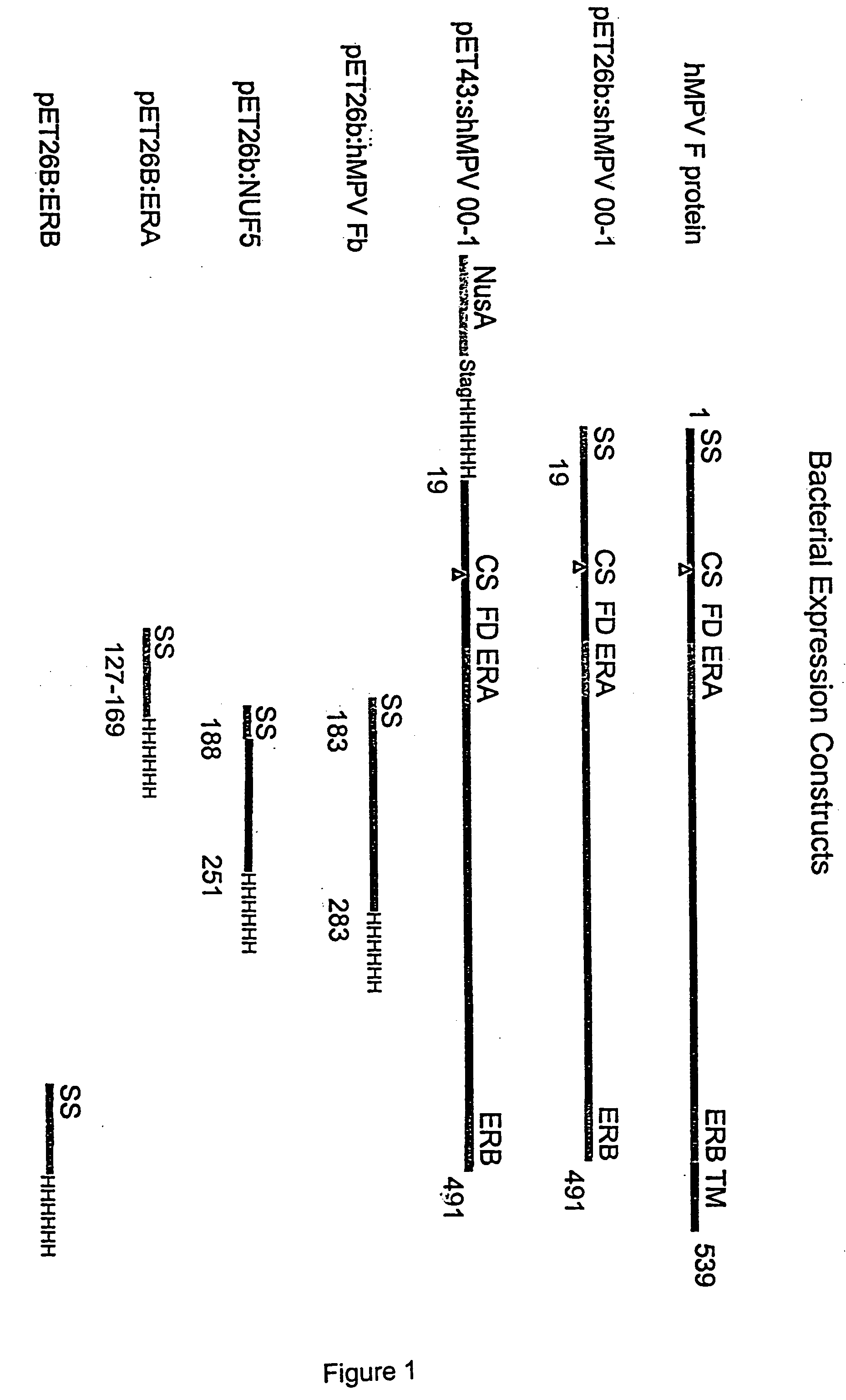
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
InactiveUS20030232061A1Optimization orderImprove scalabilitySsRNA viruses negative-senseVectorsNegative strandAntigen
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Method for detecting various respiratory viruses and primers and probes thereof
InactiveCN101985665AEasy to operateStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceMicrosphereNucleotide
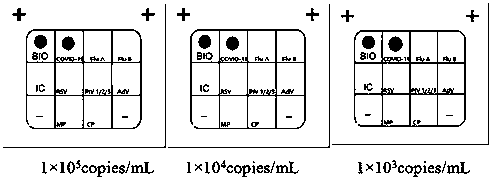
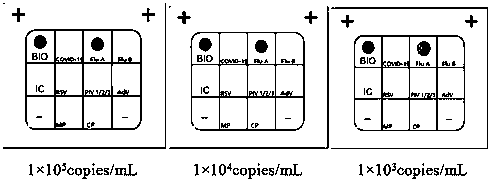
The invention belongs to the technical fields of biochips and diagnostic reagents, and discloses a method for detecting various respiratory viruses, and primers and probes thereof. In the invention, nucleotide sequences of 14 respiratory viruses, namely adenovirus, human metapneumovirus, influenza virus A, influenza virus B, respiratory syncytial virus, bocavirus, rhinovirus, coronavirus (HKU1, NL63 and SARS), and parainfluenza virus (type I, type II, type III and type IV) are analyzed, and corresponding reverse transcription primers, PCR primers and specific probes are designed. Specific gene segments are amplified by reverse transcription and multiple asymmetric PCR methods; a fluorescence-coded microsphere group coupled with the virus specific probes and the PCR amplification product are incubated and hybridized by liquid phase chip technology; and finally the Bio-PlexTM200 is used for detection. The detection method has the advantages of high flux, high specificity and sensitivity, stable results and good repeatability, the detection method is easy to operate, and the detection speed is high.
Owner:FUDAN UNIV +1
Methods of treating and preventing RSV, hMPV, and PIV using anti-RSV, anti-hMPV, and anti-PIV antibodies
InactiveUS20040096451A1Effectively preventedEffectively treatedImmunoglobulins against virusesAntiviralsSerum igeAntigen Binding Fragment
The present invention relates to methods for broad spectrum prevention and treatment of viral respiratory infection. In particular, the present invention relates to methods for preventing, treating or ameliorating symptoms associated with respiratory syncytial virus (RSV), parainfluenza virus (PIV), and / or human metapneumovirus (hMPV) infection, the methods comprising administering to a subject an effective amount of one or more anti-RSV-antigen antibodies or antigen-binding fragments thereof, one or more anti-hMPV-antigen antibodies or antigen-binding fragments thereof, and / or one or more anti-PIV-antigen antibodies or antigen-binding fragments thereof. In certain embodiments, a certain serum titer of the anti-RSV-antigen antibodies, anti-PIV-antigen antibodies, and / or anti-hMPV-antigen antibodies or antigen-binding fragments thereof is achieved in said subject. In certain specific embodiments, the subject is human and, preferably, the anti-RSV-antigen antibody, anti-PIV-antigen antibody, and / or anti-hMPV-antigen antibodies are human or humanized. The present invention relates further to compositions comprising the anti-RSV-antigen antibodies, anti-PIV-antigen antibodies, and / or anti-hMPV-antigen antibodies or antigen-binding fragments thereof. The present invention also relates to detectable or diagnostic compositions comprising the one or more anti-RSV-antigen antibodies, anti-PIV-antigen antibodies, and / or anti-hMPV-antigen antibodies or antigen-binding fragments thereof and methods for detecting or diagnosing RSV, PIV and / or hMPV infection utilizing the compositions.
Owner:MEDIMMUNE LLC +1
Two-step immunization procedure against the pyramyxoviridae family of viruses using recombinant virus and subunit protein preparation
InactiveUS6180398B1Improve the level ofSlow and sustained releaseSsRNA viruses negative-senseViral antigen ingredientsDiseaseProtection sex
An immunization strategy to provide protection against disease caused by infection with a paramyxoviridae virus, specifically respiratory syncytial virus (RSV) and parainfluenza virus, is described. A priming intranasal administration of a recombinant virus expressing at least one RSV or PIV protein or immunogenic sequence there first is made to the host followed by a booster administration of at least one purified RSV or PIV protein or immunogenic fragment thereof, which may be adjuvanted with alum. This immunization strategy provides a safe and effective means of controlling RSV and PIV infections. The strategy leads to a stronger protective immune response than other strategies and to the induction of a more balanced Th-1 / Th-2 type response than previously attained. Novel recombinant poxviruses are provided containing nucleic acid encoding a paramyxovirus protein or immunogenic fragment thereof is a non-essential region of the poxvirus genome, specifically NYVAC-F and ALVAC-F, which produce the F glycoprotein of RSV.
Owner:CONNAUGHT LAB +1
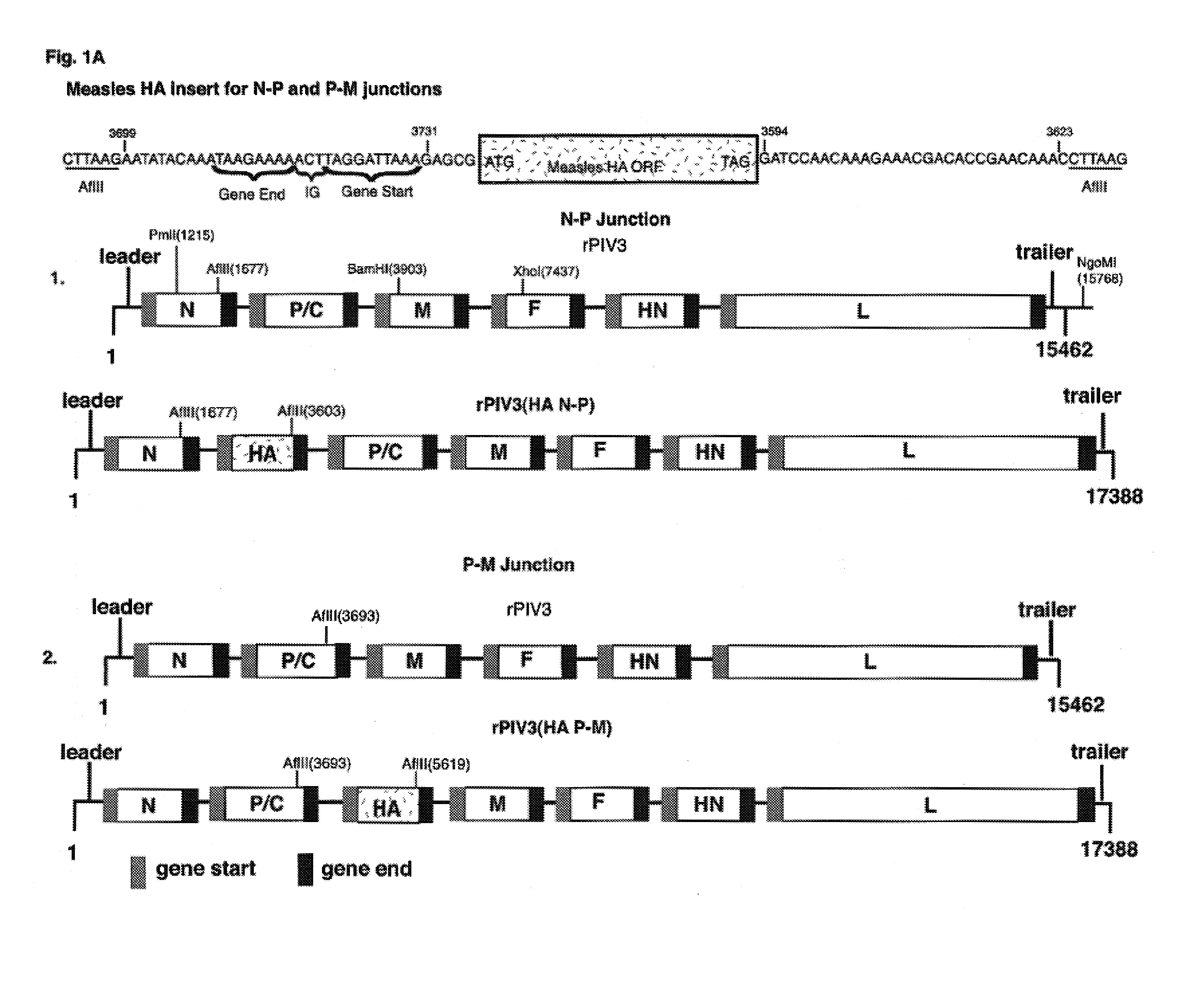
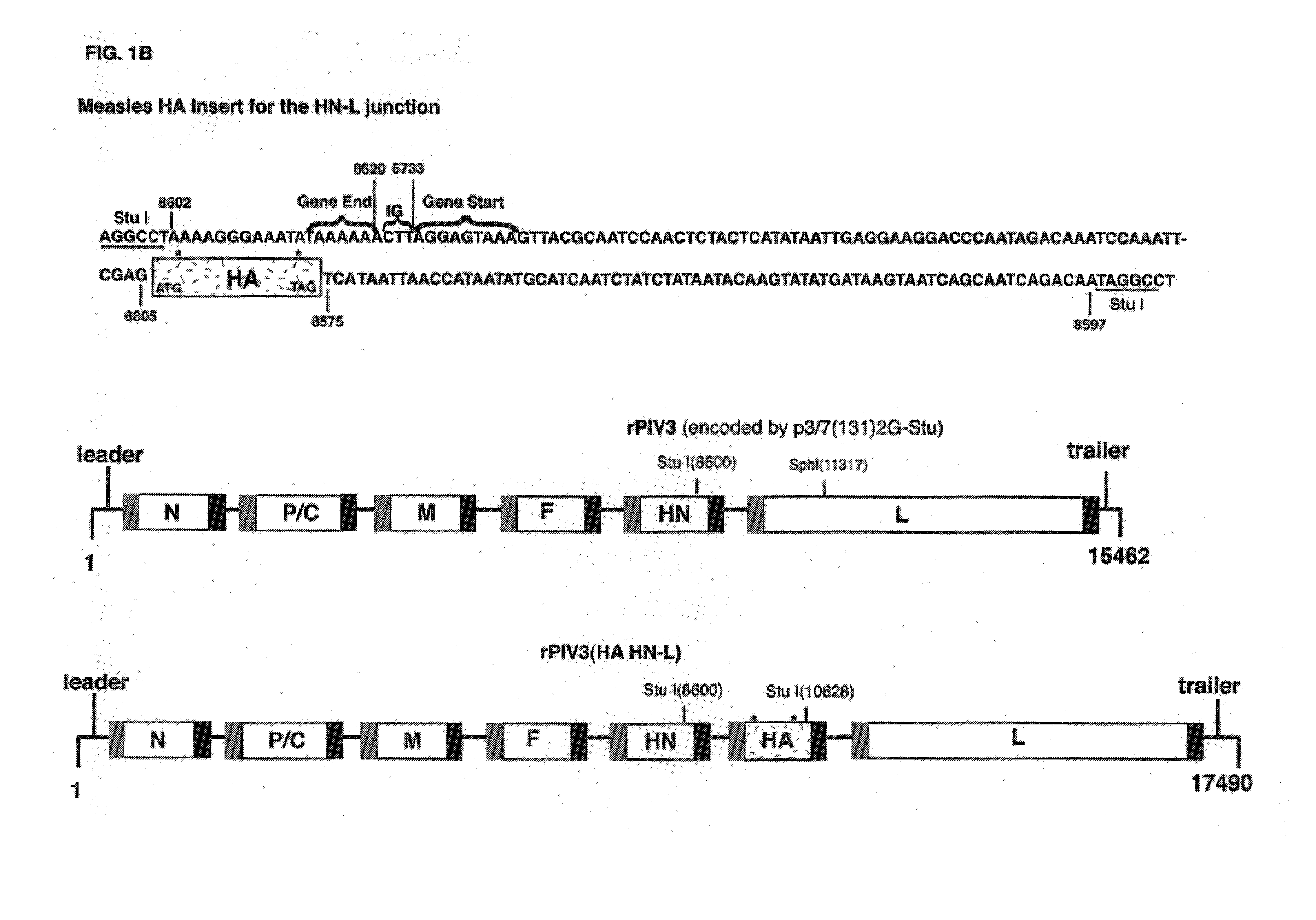
Construction and use of recombinant parainfluenza viruses expressing a chimeric glycoprotein
InactiveUS7250171B1Prone to infectionHigh titerSsRNA viruses negative-senseOrganic active ingredientsHeterologousEpitope
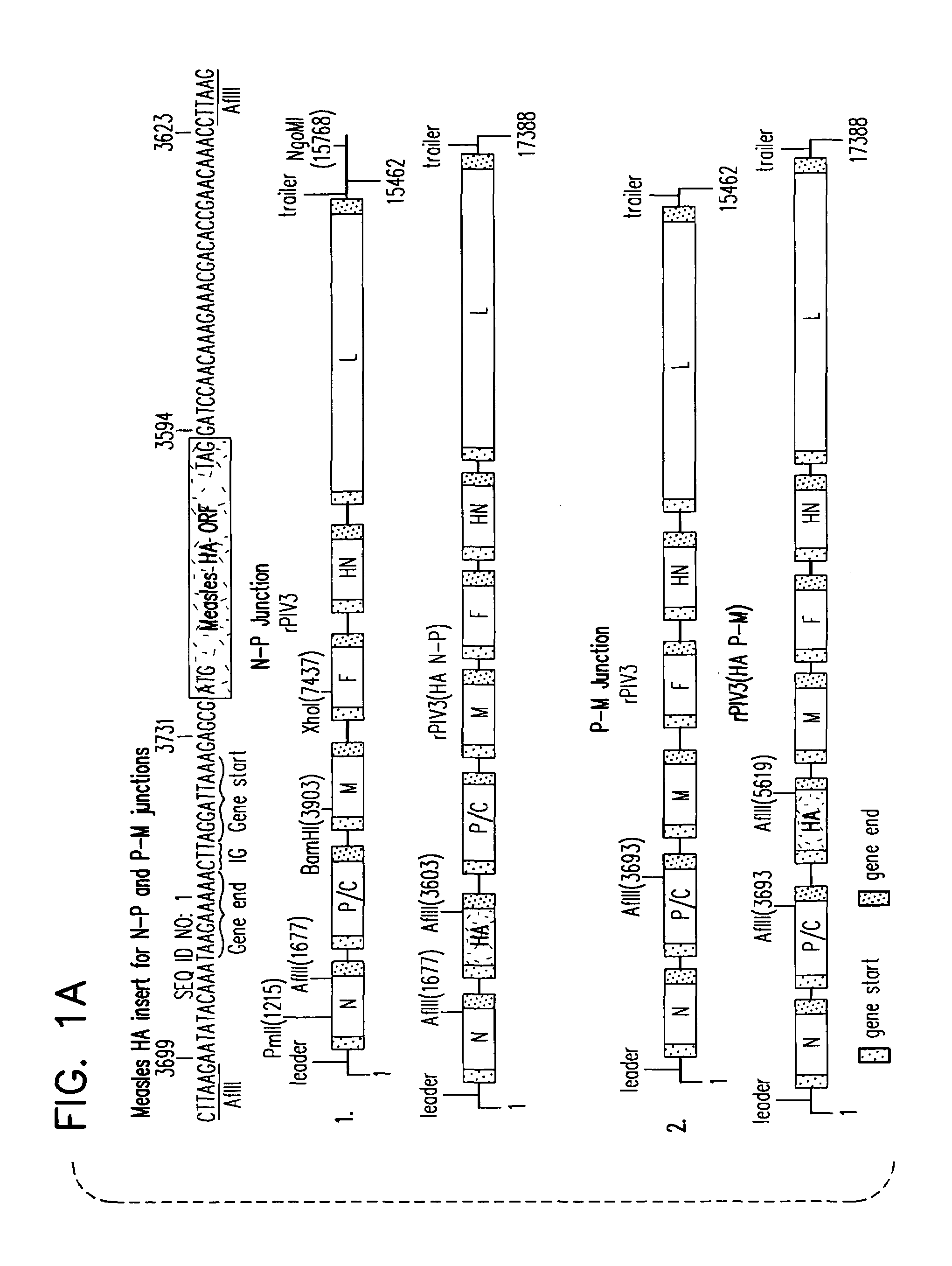
Chimeric parainfluenza viruses (PIVs) are provided that incorporate a PIV vector genome or antigenome modified to encode a chimeric glycoprotein incorporating one or more heterologous antigenic domains, fragments, or epitopes of a second, antigenically distinct HPIV. These chimeric viruses are infectious and attenuated in humans and other mammals and are useful in vaccine formulations for eliciting an immune responses against one or more PIVs, and, optionally against respiratory syncytial virus (RSV). Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric PIV genome or antigenome which includes a HPIV vector genome or antigenome combined or integrated with one or more heterologous genome segment(s) encoding one or more antigenic determinant(s) of a heterologous PIV to encode a chimeric glycoprotein. In preferred aspects of the invention, the chimeric virus is attenuated for use as a vaccine agent by additional mutations or nucleotide modifications introduced into the chimeric genome or antigenome.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE
Long lasting fusion peptide inhibitors for hiv infection
InactiveUS20050065075A1Prolong half-life in vivoBiocidePeptide/protein ingredientsImmunodeficiency virusHuman Parainfluenza Virus
The present invention relates to C34 peptide derivatives that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Respiratory pathogen multi-detection reagent kit
InactiveCN109355437AHigh detection sensitivityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoronavirus 229EFluorescence
The invention discloses a respiratory pathogen multi-detection reagent kit. The respiratory pathogen multi-detection reagent kit has the advantages that the respiratory pathogen multi-detection reagent kit is based on multi-PCR (polymerase chain reaction) technologies, detection results can be determined by the aid of fluorescence resonance energy transfer via the melting temperature ranges, the respiratory pathogen multi-detection reagent kit can be used for qualitatively simultaneously detecting 16 types of respiratory pathogens, the 16 types of respiratory pathogens include 12 types of RNA(ribonucleic acid) viruses (influenza A viruses, influenza B viruses, H1N1 influenza A viruses, type A and type B respiratory syncytial viruses, type -1 / -2 / -3 parainfluenza viruses, type OC43 coronaviruses, type 229E coronaviruses, rhinoviruses and human metapneumovirus), 2 types of DNA (deoxyribonucleic acid) viruses (adenoviruses and bocavirus) and 2 types of bacteria (mycoplasma pneumoniae andbordetella pertussis), the respiratory pathogen multi-detection reagent kit is high in detection sensitivity, and the sensitivity even can reach 1 copy / reaction; the multi-detection reagent kit is good in specificity, and negative results of pathogens which have identical sampling sites and similar pathogenic mechanisms and are not in the detection range of the respiratory pathogen multi-detectionreagent kit can be obtained; the respiratory pathogen multi-detection reagent kit is short in operation time and easy to operate and can be used for quickly detecting the 16 types of respiratory pathogens in a single tube of a reaction system, the results are clear and are easy to interpret, and the like.
Owner:上海捷诺生物科技股份有限公司
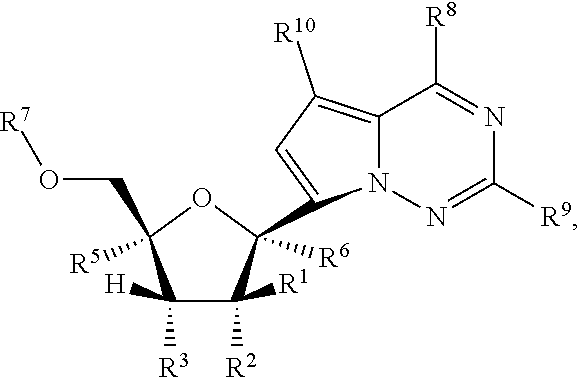
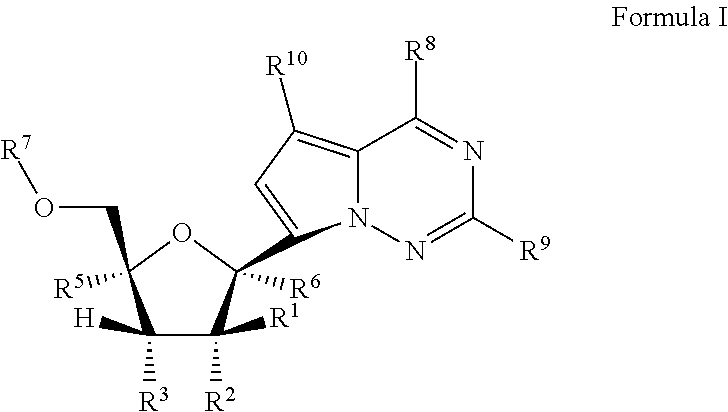
Methods and compounds for treating paramyxoviridae virus infections
Provided are methods for treating Paramyxoviridae virus infections by administering ribosides, riboside phosphates and prodrugs thereof, of Formula I:wherein the 1′ position of the nucleoside sugar is substituted. The compounds, compositions, and methods provided are particularly useful for the treatment of Human parainfluenza and Human respiratory syncytial virus infections.
Owner:GILEAD SCI INC
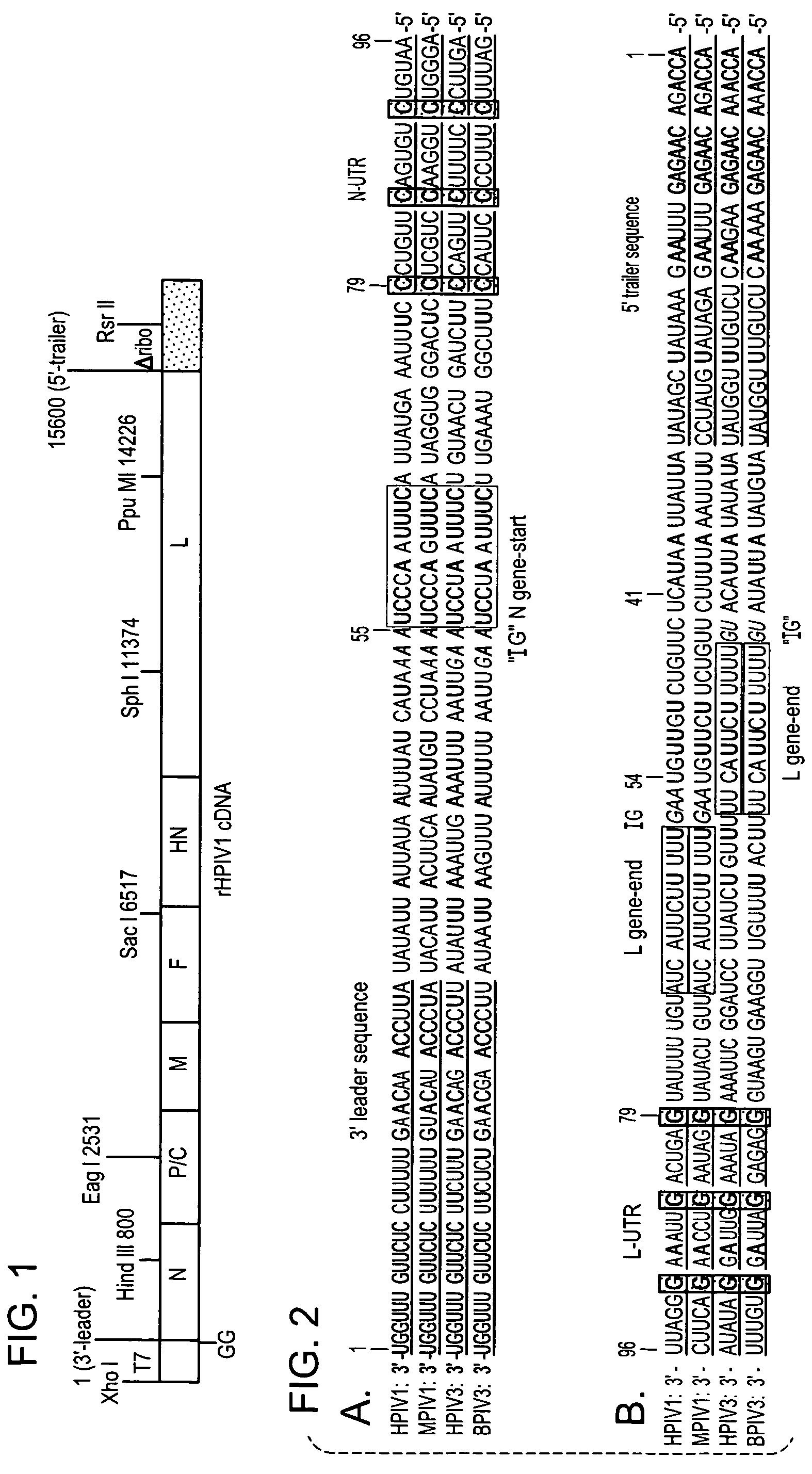
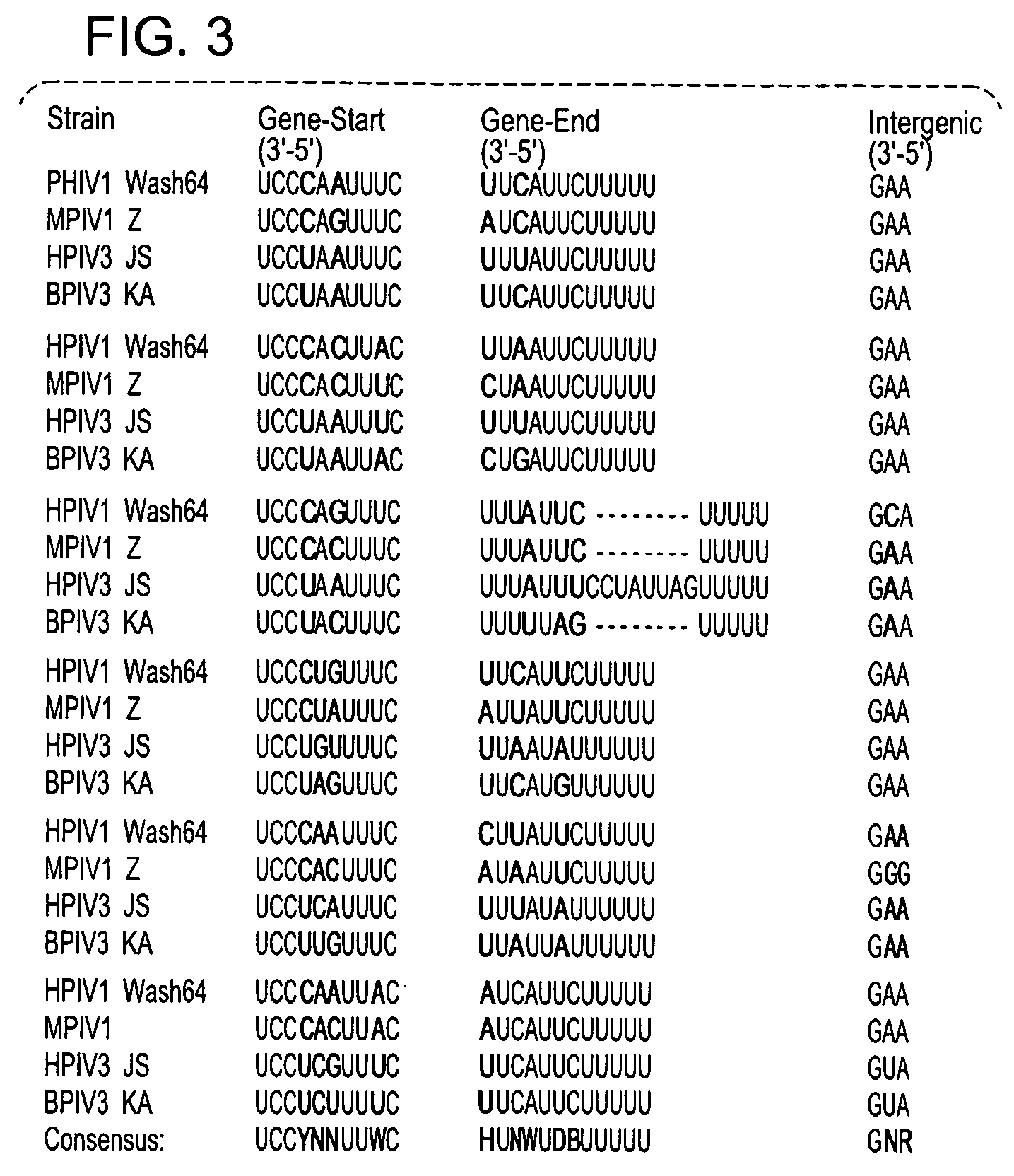
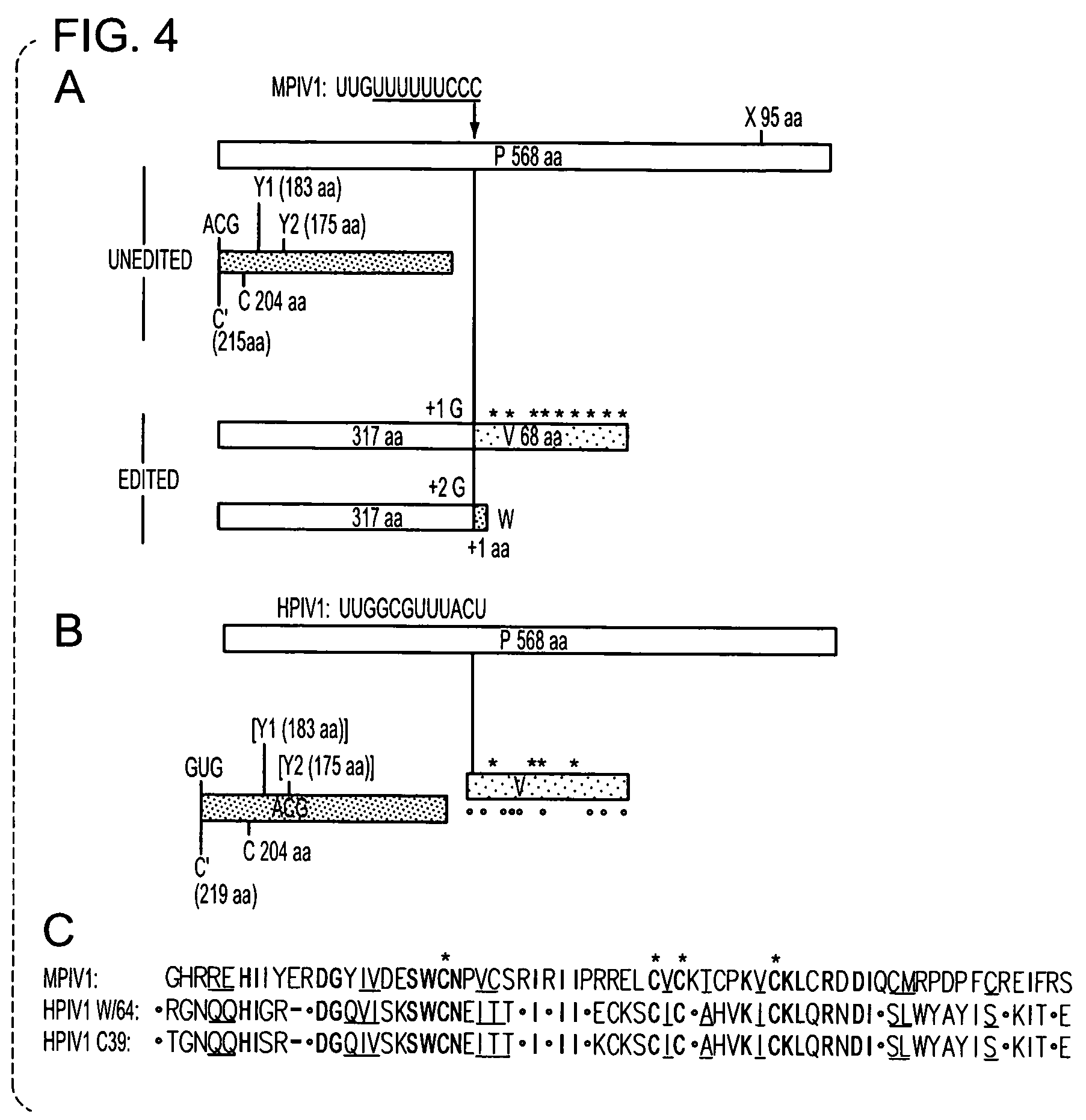
Recovery of recombinant human parainfluenza virus type 1 (HPIV1) from cDNA and use of recombinant HPIV1 in immunogenic compositions and as vectors to elicit immune responses against PIV and other human pathogens
InactiveUS7704509B2Mass productionBoost the response to both HPIVSsRNA viruses negative-senseViral antigen ingredientsHuman Parainfluenza VirusParainfluenza virus
Recombinant human parainfluenza virus type 1 (HPIV1) compostions, formulations and methods are provided. The recombinant HPIV1 viruses and HPIV1 chimeric and chimeric vector viruses provided according to the invention are infectious and attenuated in permissive mammalian subjects, including humans, and are useful in immunogenic composition s for eliciting an immune responses against one or more PIVs, against one or more non-PIV pathogens, or against a PIV and a non-PIV pathogen. Also provided are isolated polynucleotide molecules and vectors incorporating a recombinant HPIV1 genome or antigenome.
Owner:UNITED STATES OF AMERICA
Paramyxoviruses as gene transfer vectors to lung cells
The present invention provides infectious recombinant viral vectors (e.g., parainfluenza virus (PIV) and a respiratory syncytial virus (RSV) vectors) comprising a viral genome comprising a heterologous nucleic acid of interest. Also provided are pseudotyped recombinant viral vectors comprising (i) a viral envelope and (ii) a viral genome comprising heterologous nucleic acids of interest. The viral envelope comprises a structural protein selected from the group consisting of envelope proteins from PIV and / or RSV. Further provided are methods of delivering heterologous nucleic acids of interest into airway epithelial cells comprising introducing viral vectors of the present invention comprising nucleic acids of interest into airway epithelial cells so that the nucleic acids of interest are expressed therein.
Owner:RUSH PRESBYTERIAN ST LUKES MEDICAL CENT +2
Nano silver-selenium negative ion strongly-antibacterial aerosol for preventing and curing rhinitis and influenza
InactiveCN101947257ASmall doseLittle side effectsAntibacterial agentsHeavy metal active ingredientsPropolisBiology
The invention relates to a nano silver-selenium negative ion strongly-antibacterial aerosol for preventing and curing rhinitis and influenza, which can effectively achieve the purpose of killing bacteria and viruses. The rhinitis and the influenza are upper respiratory tract infection caused by multiple viruses, i.e. rhinoviruses, parainfluenza viruses, respiratory syncytial viruses, adenoviruses, and the like. No bacterium and virus can not be killed by silver within 6 minutes in a laboratory; the silver is a highly-effective broad-spectrum antimicrobial; selenium can eliminate internal free radicals, is anticancer and antiaging and can enhance immunity; tourmalines can generate air negative ions; and the aerosol prepared from nano micropowder obtained by mixing nano traditional Chinese medicine which has unique effect on the rhinitis and the influenza and propolis powder which has special effect on the influenza can effectively prevent and cure the rhinitis and the influenza.
Owner:许小丽
Nucleic acid combined testing kit of respiratory tract infection pathogens
InactiveCN111378789AHigh detection throughputAvoid false negative resultsMicrobiological testing/measurementMicroorganism based processesDiseaseNucleotide
The invention discloses a nucleic acid combined testing kit of respiratory tract infection pathogens. The invention develops a set of primer-probe combinations which can detect multiple types of respiratory tract infection pathogens such as novel coronavirus, influenza virus a, influenza virus b, respiratory syncytial virus, human parainfluenza virus, adenovirus, mycoplasma pneumonia and chlamydiapneumonia through combination of a multiple fluorescence quantitative PCR technology and a flow-through hybridization and gene chip technology, wherein nucleotide sequences thereof are shown by SEQ ID NO:1-36 respectively. The nucleic acid combined testing kit of the respiratory tract infection pathogens is established. The kid can realize synchronous combined testing of the 8 respiratory tract infection pathogens, is high in detection accuracy, specificity and sensitivity, good in repeatability, low in false negativity and false positivity, short in detection time and low in cost, can realize comprehensive detection of a patient, can locate a disease source accurately, can realize treatment in time or make corresponding quarantine measures and is of important significance to effective control of respiratory tract infection and subsequent prevention of outbreak of relevant contagion and infection.
Owner:GUANGZHOU HYBRIBIO MEDICINE TECH LTD +2
LAMP primer composite for detecting respiratory pathogens and kit of LAMP primer composite
InactiveCN107099619ADoes not affect amplificationMeet quality control requirementsMicrobiological testing/measurementMicroorganism based processesColor changesBiology
The invention relates to a primer composite for detecting respiratory pathogens. The primer composite comprises at least one group of a mycoplasma pneumoniae group, a chlamydia pneumoniae primer group, an influenza A / B virus primer group, a parainfluenza virus primer group, an adenovirus primer group and a respiratory syncytial virus primer group. The invention further relates to a kit comprising the primer composite. The kit further comprises a micro-fluidic chip wrapping primers, and a macroscopic indicator. The invention further relates to a detection method adopting the primer composite. The detection method comprises the steps of primer composite coating, to-be-detected sample nucleic acid extraction, LAMP reaction and visual result interpretation. The kit and the method are applied to the micro-fluidic chip for visual judgment, instant detection of the seven respiratory pathogens is rapidly and accurately realized, and a result is judged by a naked eye by color change, so that the kit and the method are simpler, more convenient and quicker in practical applications, are easy to operate, and are suitable for site operation.
Owner:SHANGHAI IGENETEC DIAGNOSTICS CO LTD
Use of recombinant parainfluenza viruses (PIVs) as vectors to protect against infection and disease caused by PIV and other human pathogens
InactiveUS7192593B2Prone to infectionHigh titerSsRNA viruses negative-senseSugar derivativesAntigenDisease
Chimeric parainfluenza viruses (PIVs) incorporate a PIV vector genome or antigenome and one or more antigenic determinant(s) of a heterologous PIV or non-PIV pathogen. These chimeric viruses are infectious and attenuated in humans and other mammals and are useful in vaccine formulations for eliciting an immune responses against one or more PIVs, or against a PIV and non-PIV pathogen. Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric Ply genome or antigenome which includes a partial or complete PIV vector genome or antigenome combined or integrated with one or more heterologous gene(s) or genome segment(s) encoding antigenic determinant(s) of a heterologous PIV or non-PIV pathogen.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE DEPT OF
Methods of treating and preventing RSV, hMPV, and PIV using anti-RSV, anti-hMPV, and anti-PIV antibodies
InactiveUS20100278813A1Broad preventionEffectively preventedImmunoglobulins against virusesAntiviralsSerum igeAntigen Binding Fragment
The present invention relates to methods for broad spectrum prevention and treatment of viral respiratory infection. In particular, the present invention relates to methods for preventing, treating or ameliorating symptoms associated with respiratory syncytial virus (RSV), parainfluenza virus (PIV), and / or human metapneumovirus (hMPV) infection, the methods comprising administering to a subject an effective amount of one or more anti-RSV-antigen antibodies or antigen-binding fragments thereof, one or more anti-hMPV-antigen antibodies or antigen-binding fragments thereof, and / or one or more anti-PIV-antigen antibodies or antigen-binding fragments thereof. In certain embodiments, a certain serum titer of the anti-RSV-antigen antibodies, anti-PIV-antigen antibodies, and / or anti-hMPV-antigen antibodies or antigen-binding fragments thereof is achieved in said subject. In certain specific embodiments, the subject is human and, preferably, the anti-RSV-antigen antibody, anti-PIV-antigen antibody, and / or anti-hMPV-antigen antibodies are human or humanized. The present invention relates further to compositions comprising the anti-RSV-antigen is antibodies, anti-PIV-antigen antibodies, and / or anti-hMPV-antigen antibodies or antigen-binding fragments thereof. The present invention also relates to detectable or diagnostic compositions comprising the one or more anti-RSV-antigen antibodies, anti-PIV-antigen antibodies, and / or anti-hMPV-antigen antibodies or antigen-binding fragments thereof and methods for detecting or diagnosing RSV, PIV and / or hMPV infection utilizing the compositions.
Owner:VIRONOVATIVE
Use of recombinant live-attenuated parainfluenza virus (PIV) as a vector to protect against disease caused by PIV and respiratory syncytial virus (RSV)
InactiveUS7314631B1Prone to infectionHigh titerSsRNA viruses negative-senseSugar derivativesAntigenDisease
Chimeric parainfluenza viruses (PIVs) are provided that incorporate a PIV vector genome or antigenome and one or more antigenic determinant(s) of a heterologous PIV or non-PIV pathogen. These chimeric viruses are infectious and attenuated in humans and other mammals and are useful in vaccine formulations for eliciting and immune responses against one or more PIVs, or against a PIV and non-PIV pathogen. Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric PIV genome or antigenome which includes a partial or complete PIV vector genome or antigenome combined or integrated with one or more heterologous gene(s) or genome segment(s) encoding antigenic determinant(s) of a heterologous PIV or non-PIV pathogen. In preferred aspects of the invention, chimeric PIV incorporate a partial or complete human PIV vector genome or antigenome combined with one or more heterologous gene(s) or genome segment(s) from a heterologous PIV or non-PIV pathogen, wherein the chimeric virus is attenuated for use as a vaccine agent by any of a variety of mutations and nucleotide modifications introduced into the chimeric genome or antigenome.
Owner:DEPT OF HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE
Chimeric protein which confers protection against parainfluenza virus and respiratory syncytial virus
InactiveUS6017539AGood immune protectionVirusesAntibody mimetics/scaffoldsAntigenSusceptible individual
Multimeric hybrid genes encoding the corresponding chimeric protein comprise a gene sequence coding for an antigenic region of a protein from a first pathogen linked to a gene sequence coding for an antigenic region of a protein from a second pathogen. The pathogens particularly are parainfluenza virus (PIV) and respiratory syncytial virus (RSV). A single recombinant immunogen is capable of protecting infants and similar susceptible individuals against diseases caused by both PIV and RSV.
Owner:SANOFI PASTEUR LTD
Porous solid material for adsorbing and inactivating virus and application thereof
A porous solid material for adsorbing and deactivating the viruses, especially the parainfluenza virus, is prepared from one of TiO2, ZrO2, SiO2 and Al2O3 or P2O5 or their mixture. It can be modified by one or more elements in the families VIII, Ib, IIb, or IIa.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Chimeric protein which confers protection against parainfluenza virus and respiratory syncytial virus
Multimeric hybrid genes encoding the corresponding chimeric protein comprise a gene sequence coding for an antigenic region of a protein from a first pathogen linked to a gene sequence coding for an antigenic region of a protein from a second pathogen. The pathogens particularly are parainfluenza virus (PIV) and respiratory syncytial virus (RSV). A single recombinant immunogen is capable of protecting infants and similar susceptible individuals against diseases caused by both PIV and RSV.
Owner:SANOFI PASTEUR LTD
Long lasting fusion peptide inhibitors for HIV infection
InactiveUS7741453B2Prolong half-life in vivoPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunodeficiency virusHuman Parainfluenza Virus
The present invention relates to C34 peptide derivatives that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Fusogenic, self-propagating blebs as immunogenic compositions
InactiveUS20070128222A1Avoid infectionReduce severitySsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousCytopathic effect
Self-propagating, fusogenic blebs are produced from cells infected with a population of Venezuelan Equine Encephalitis virus replicon particles (VRP). The self-propagating, fusogenic nature of the blebs is derived from expression of heterologous genes encoding viral fusion proteins that are incorporated into the replication defective replicon particles. The resulting blebs can be harvested from supernatants of cells displaying severe cytopathic effects. The blebs are used to make immunogenic compositions and devise methods of immunizing mammals against paramyxoviruses such as parainfluenza virus type 3.
Owner:WYETH HOLDINGS CORP
Recombinant parainfluenza virus expression systems and vaccines
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
Real-time fluorescence multiplex PCR primer probe for seven common respiratory system influenza virus pathogens and kit
ActiveCN107937613AQuick checkAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceParainfluenza virus
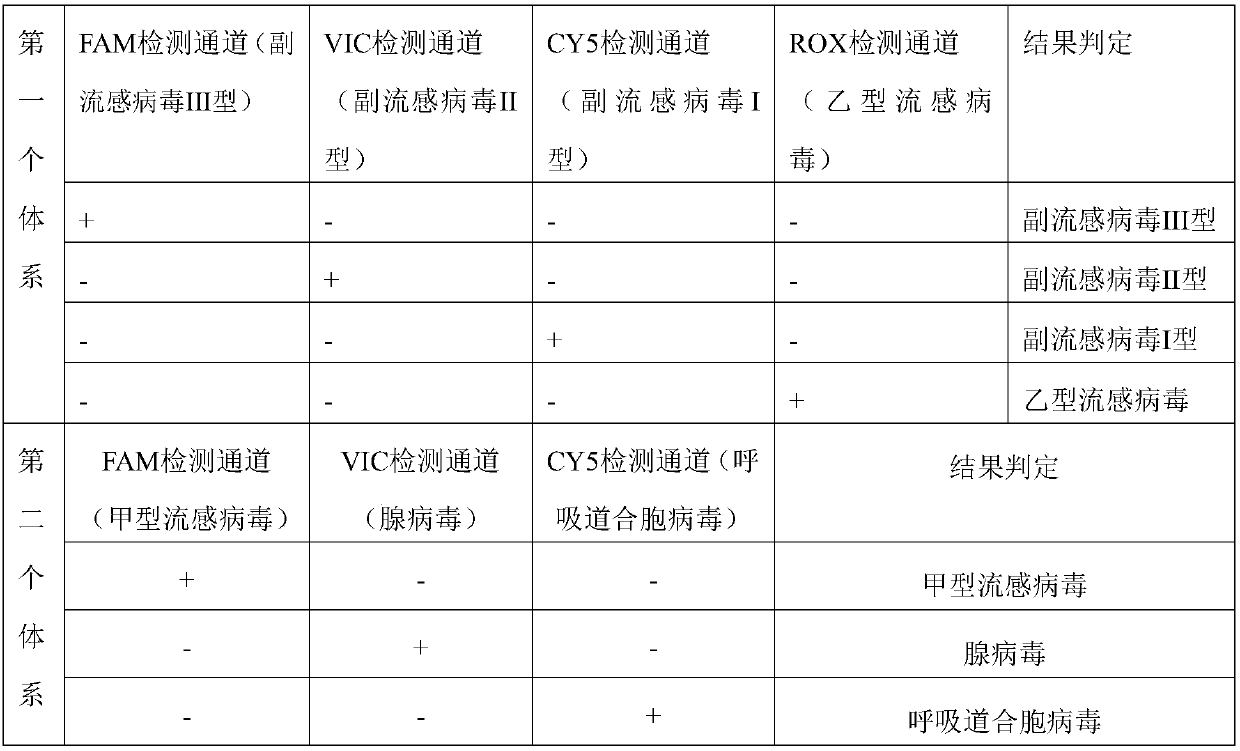
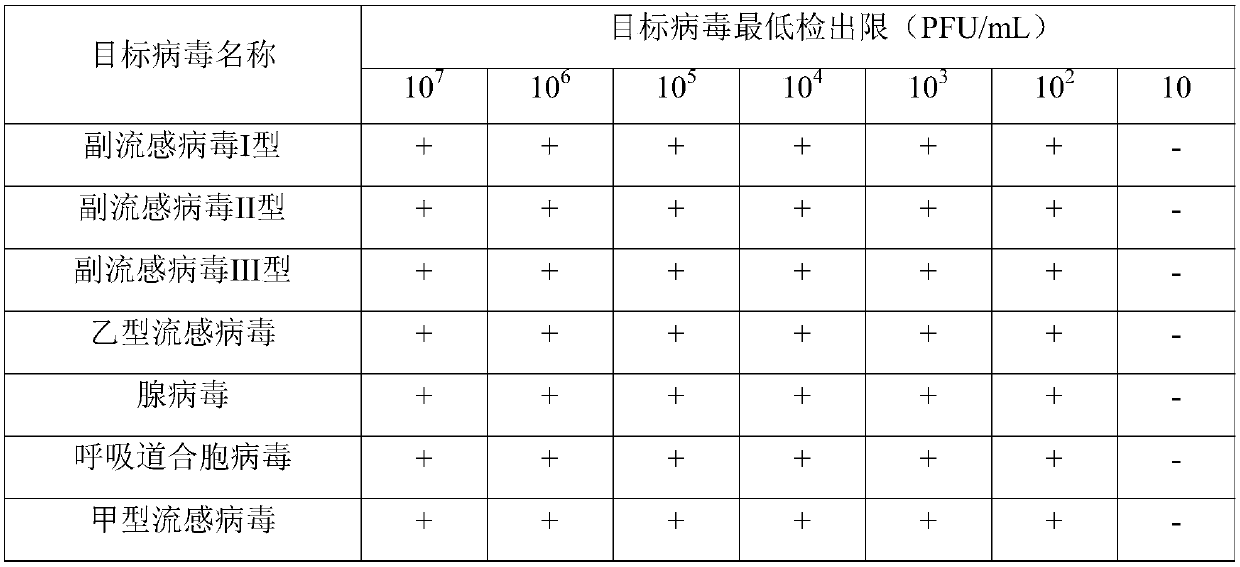
The present disclosure relates to a real-time fluorescence multiplex PCR primer probe for seven common respiratory system influenza virus pathogens and a kit. Seven influenza viruses are influenza A virus, influenza B virus, parainfluenza virus type I, parainfluenza virus type II, parainfluenza virus type III, respiratory syncytial virus and adenovirus. The present disclosure also provides a kit for multiplex fluorescence quantitative PCR detection of the seven influenza viruses, and the kit includes the primer probe set. The kit significantly improves the sensitivity, specificity, and simplicity of detection of the common respiratory system pathogens.
Owner:北京卓诚惠生生物科技股份有限公司
Hybridoma cell strain capable of secreting CPIV3 antibody and ELISA kit
ActiveCN105838678AHas a blocking effectMicroorganism based processesImmunoglobulins against virusesElisa kitSerum samples
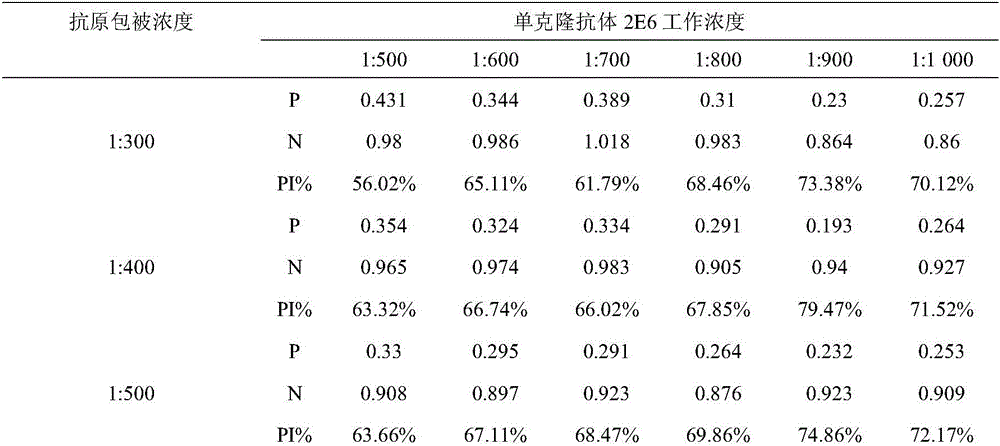
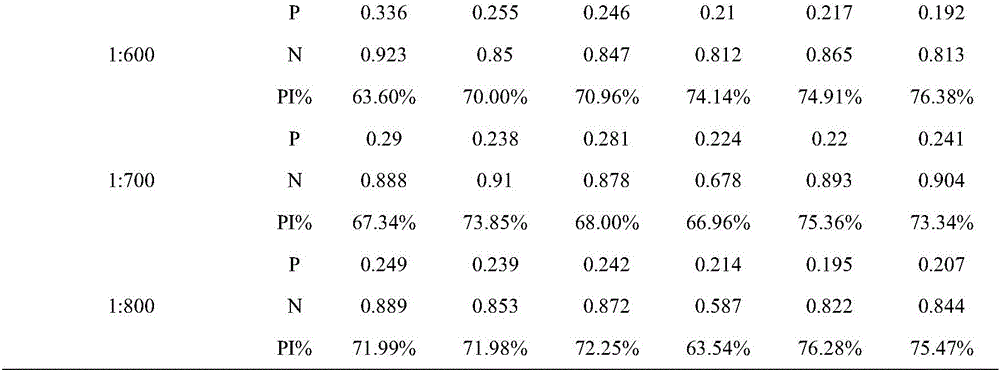
The invention belongs to the field of molecular biology, and relates to a hybridoma cell strain capable of secreting a CPIV3 antibody and an ELISA kit. A caprine parainfluenza virus 3 CPIV3 immunogen used in the invention is a CPIV3 JS2013 viral strain isolated clinically, and a hybridoma cell strain 2E6 is screened from an established hybridoma cell bank capable of secreting the CPIV3 antibody. The monoclonal antibody secreted by the hybridoma cell strain has the blocking effect, and is applied to the CPIV3 blocking ELISA kit. The CPIV3 blocking ELISA kit can specifically detect the antibody generated after the CPIV3 infection or caprine immunization. 212 caprine clinical serum samples are colleted, and the CPIV3 antibody in the serum is tested by the established blocking ELISA method and a neutralization test. Through the comparison between the result of the blocking ELISA method and the result of the neutralization test, the coincidence rate is 98.8%.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
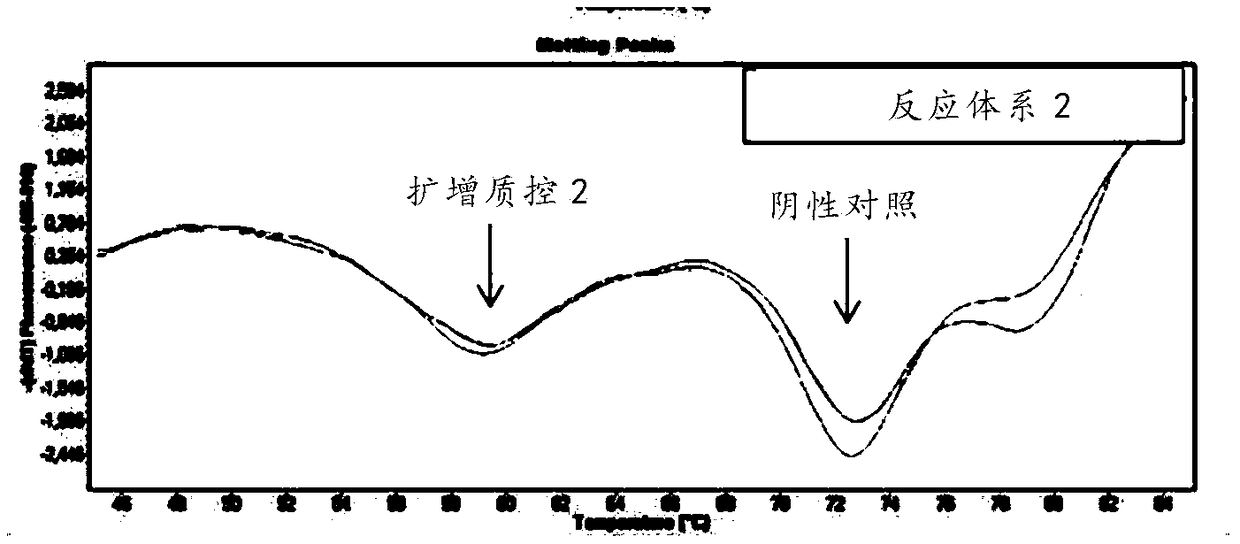
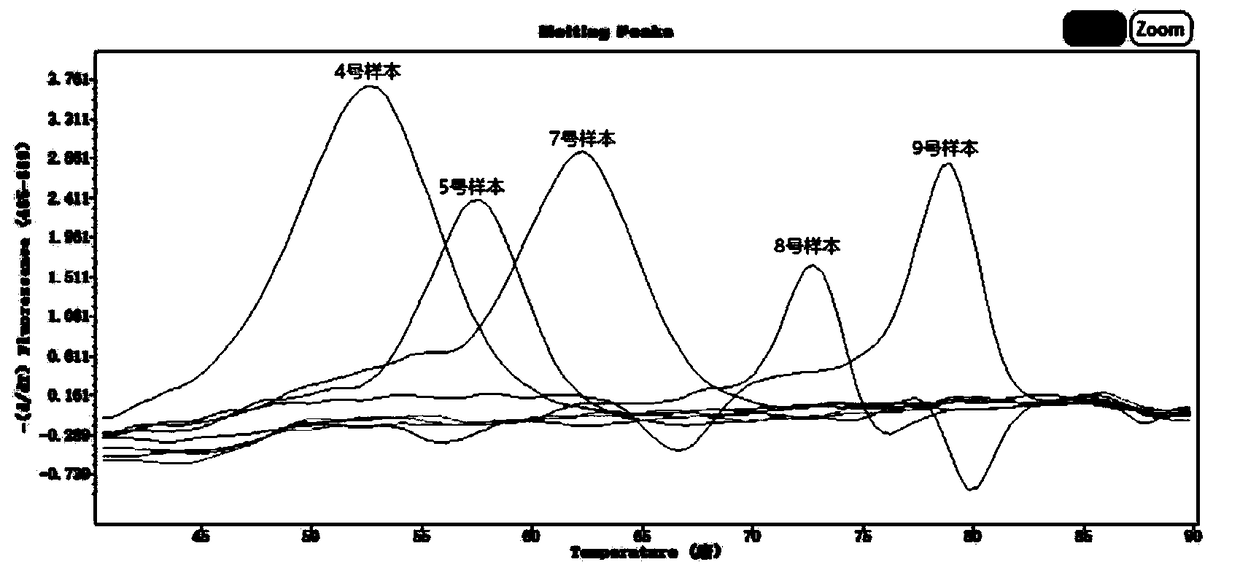
Human parainfluenza virus distinguishing and quantitative detection regent kit
ActiveCN101550455AAvoid the "plateau effect"Increased sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceHuman Parainfluenza VirusFluorescence
The invention relates to three reagent kits for detecting human parainfluenza viruses 1, 2, 3 type real-time fluorescence polymerase chain reaction, which respectively adopts a one-step method RT-PCR, a two-step method RT-PCR and a multicolor fluorescence method RT-PCR to distinguish types of the human parainfluenza viruses of multi-type specimens and fix an amount of the human parainfluenza viruses of the multi-type specimens. The detection methods of the reagent kits have simple operation, short consuming time and high sensitivity and specificity and can be extensively used in a plurality of fields, such as the auxiliary diagnosis of the infection of the human parainfluenza viruses, clinical medicine direction, epidemiology retrospective study, and the like.
Owner:广州达安临床检验中心有限公司
Primer set for respiratory tract infection pathogen detection, rapid diagnostic kit and detection method
InactiveCN108300803AImmediate rapid diagnosisMultiplexingMicrobiological testing/measurementAgainst vector-borne diseasesPolymerase LRecombinase
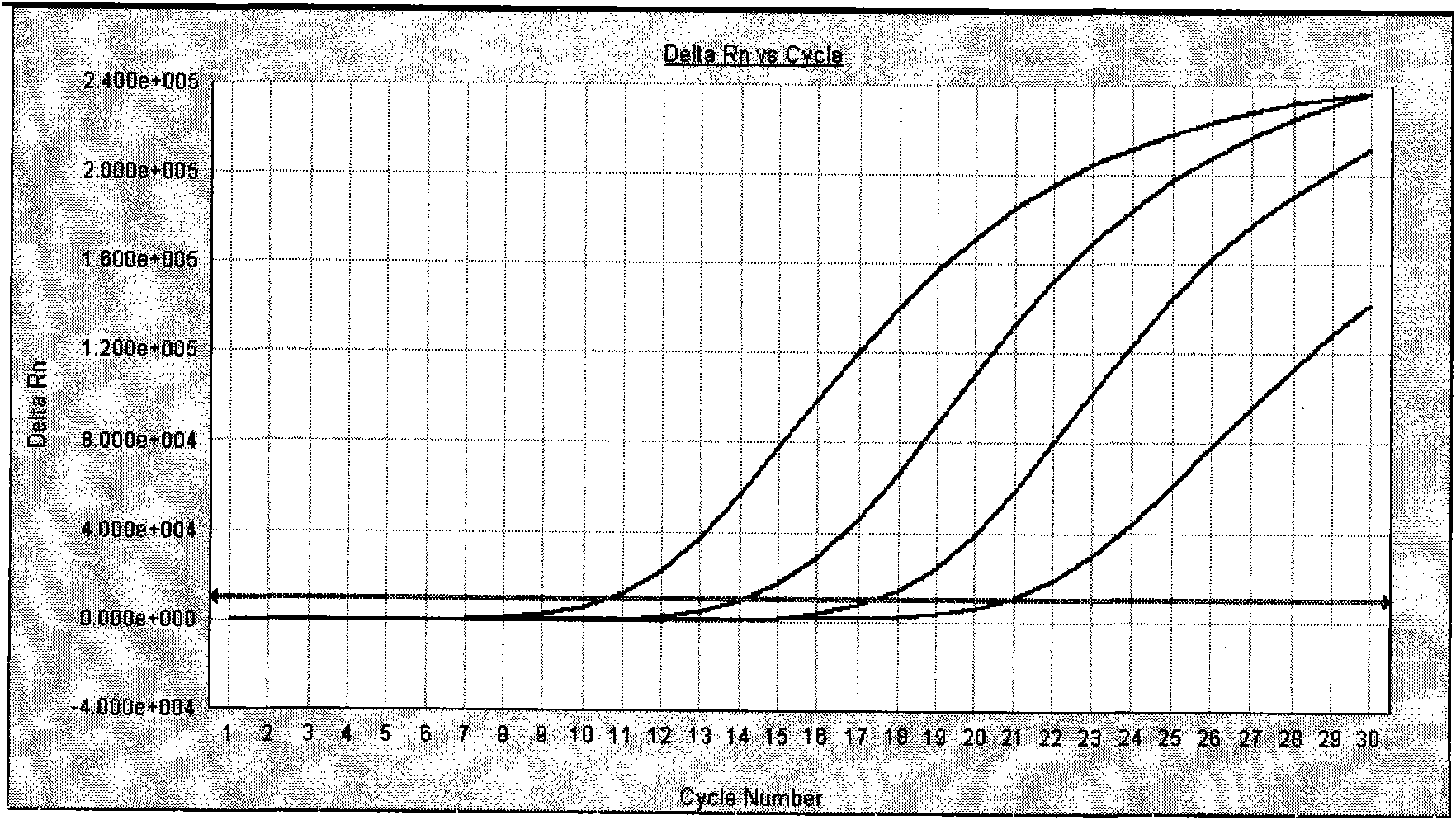
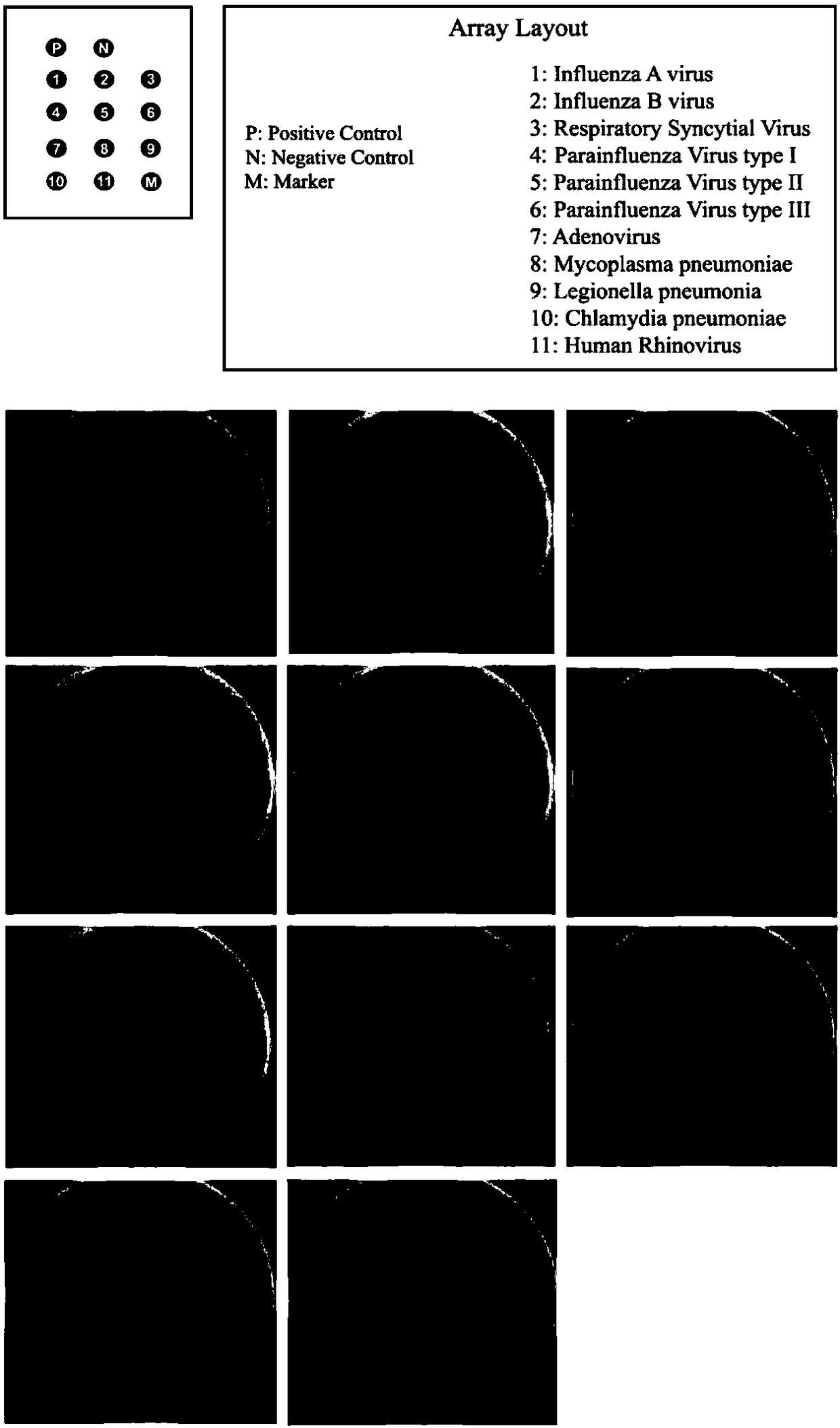
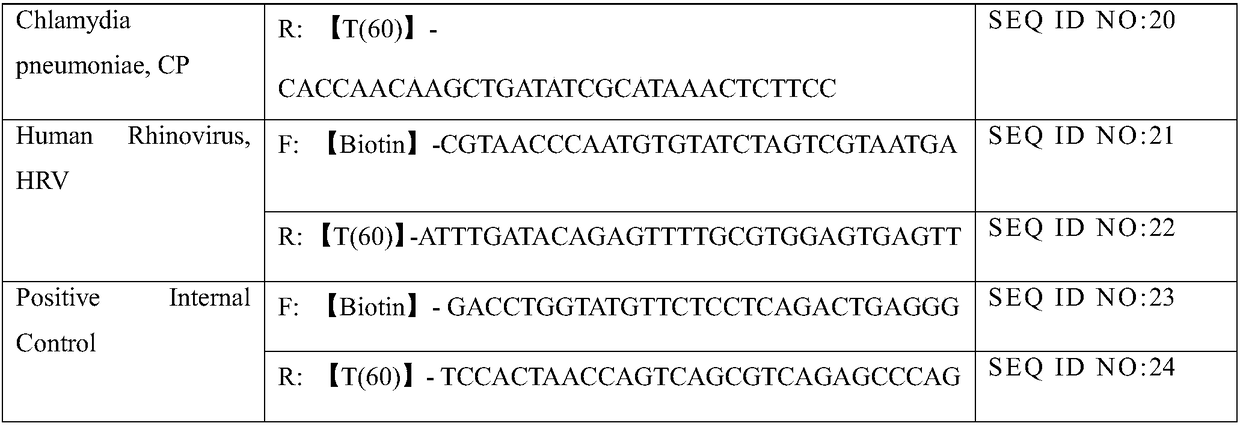
Belonging to the molecular biology field, the invention relates to a primer set for respiratory tract infection pathogen detection, a rapid diagnostic kit and a detection method. The primer set can achieve one-time detection of the following 9 respiratory tract infection pathogens: influenza A virus, influenza B virus, respiratory syncytial virus, parainfluenza virus type I, parainfluenza virus type II and parainfluenza virus type III, adenovirus, mycoplasma pneumonia, legionella pneumonia, chlamydia pneumonia and human rhinovirus. The invention adopts solid phase recombinase-polymerase constant temperature gene amplification method to detect respiratory multiple infection pathogens. The primer set adopted by the invention for respiratory tract infection pathogen detection has strong specificity and high amplification efficiency, can effectively and rapidly detect the 9 pathogens simultaneously, and realizes multiplex detection.
Owner:博迪泰(厦门)生物科技有限公司
Double-fluorescent PCR detection primer, probe, reaction liquid and kit capable of detecting pathogens of respiratory tract
InactiveCN105463129ASave testing timeSave testing costMicrobiological testing/measurementMicroorganism based processesEnterovirusCoronavirus 229E
The invention discloses a double-fluorescent PCR detection primer, a probe, reaction liquid and a kit capable of rapidly detecting 16 pathogens of the respiratory tract. The kit comprises pre-subpackaged PCR reaction liquid, RT-PCR enzyme, a positive quality control product and a negative quality control product, wherein the pre-subpackaged PCR reaction liquid is provided with a primer and a TaqMan probe for detecting at least two of the following pathogens: respiratory syncytial virus, enterovirus, coronavirus NL63, coronavirus HKU1, coronavirus 229E, coronavirus OC43, parainfluenza virus type I, parainfluenza virus type II, rhinovirus, parainfluenza virus type III, human bocavirus, human metapneumovirus, mycoplasma pneumoniae, chlamydia pneumoniae, adenovirus and legionella pneumophila. The kit is convenient to operate, and can be used for at most screening 16 syndrome pathogens of the respiratory tract within 2 hours, so that the detection time and cost are greatly saved. The kit has the greatest advantages of simplicity in operation and strong practicability, and can be easily popularized in laboratories of the ports.
Owner:SHENZHEN INT TRAVEL HEALTHCARE CENT +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com