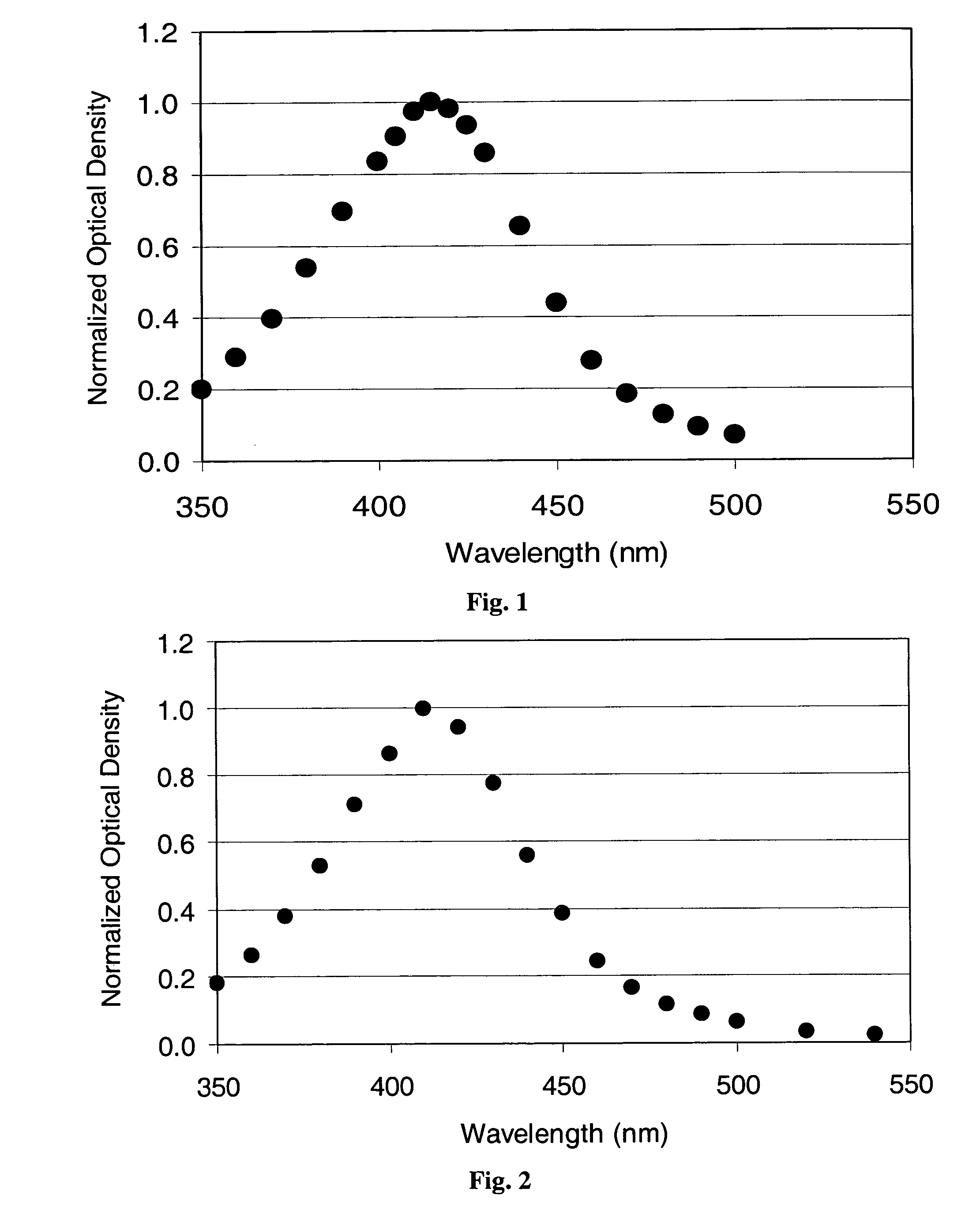
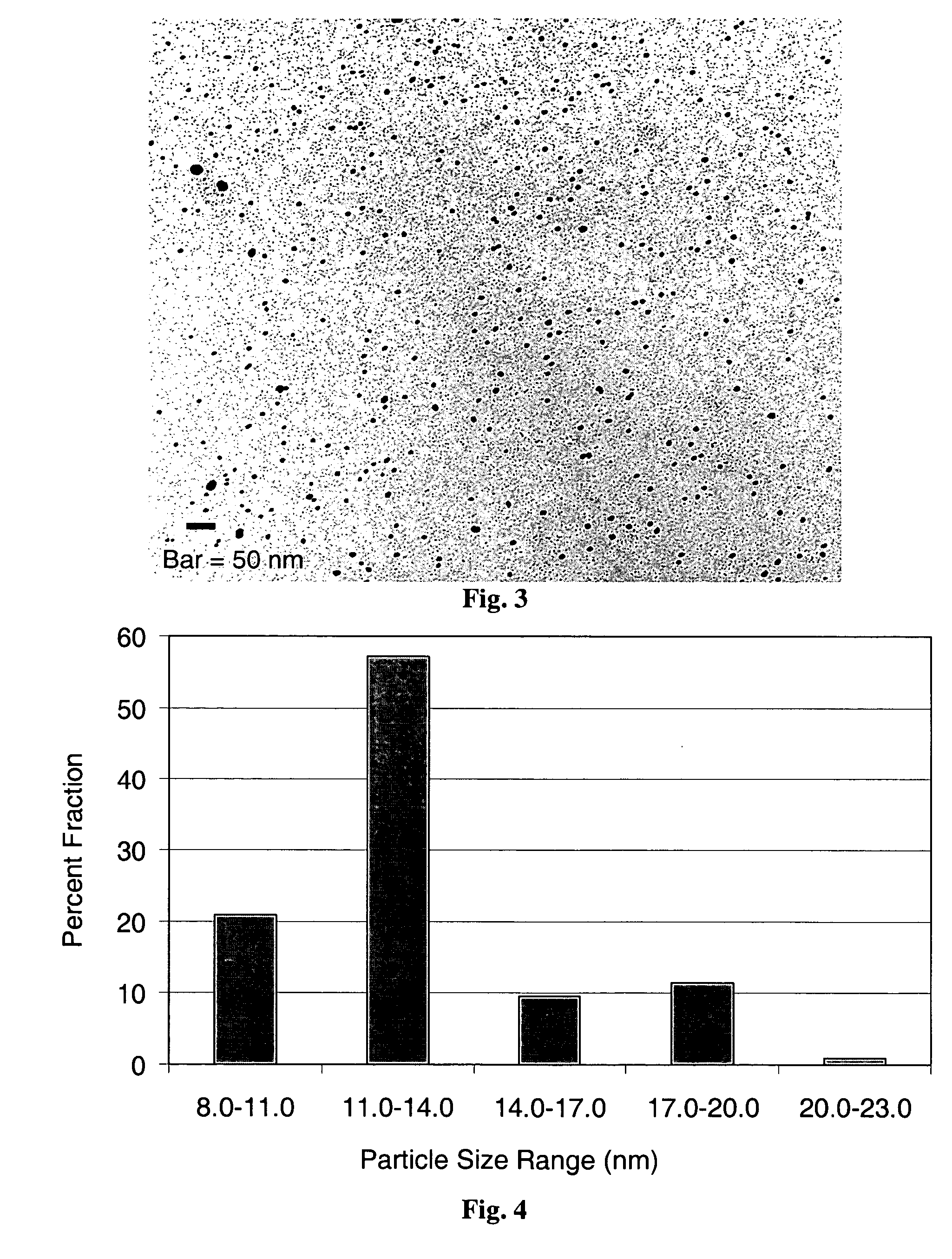
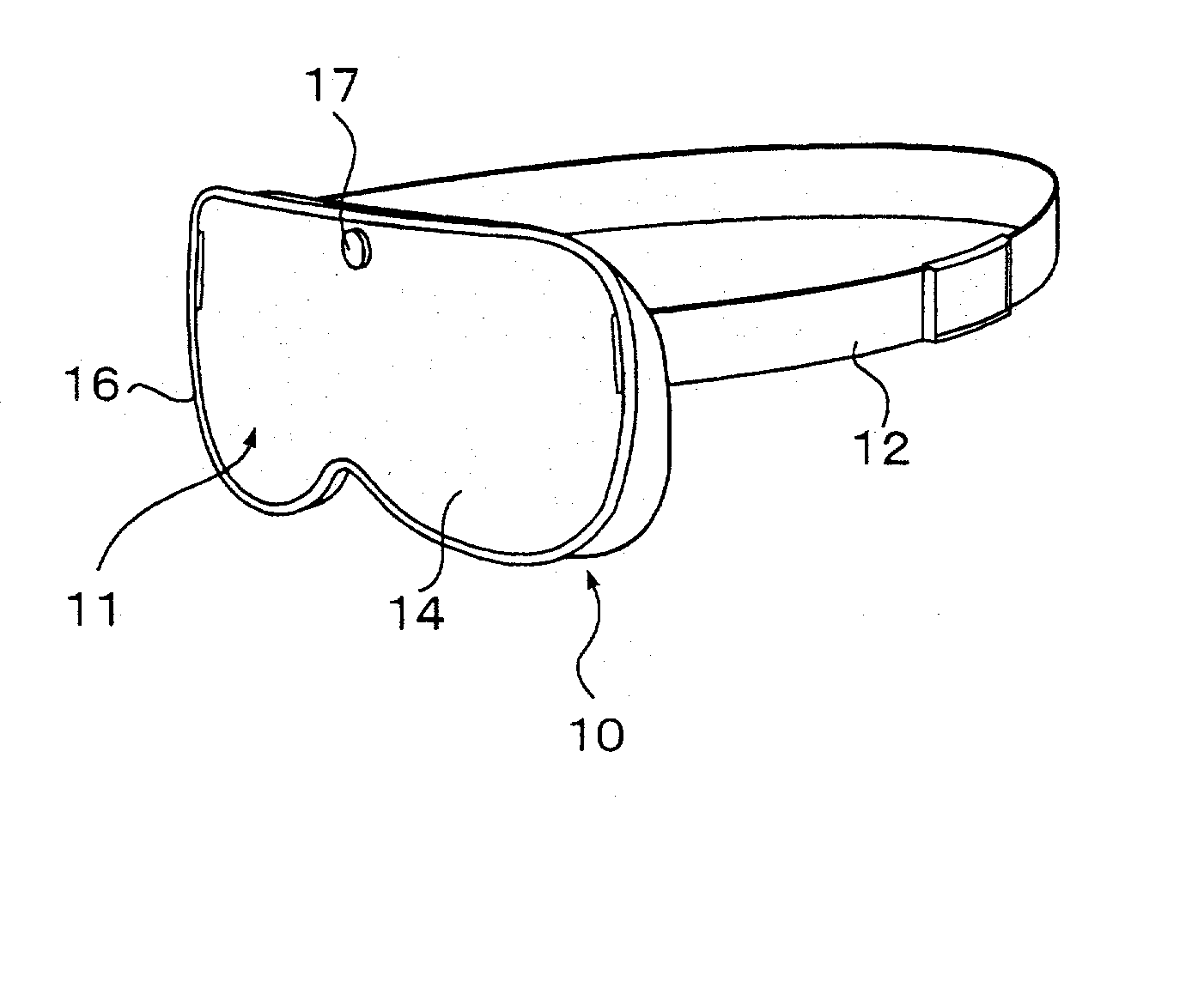
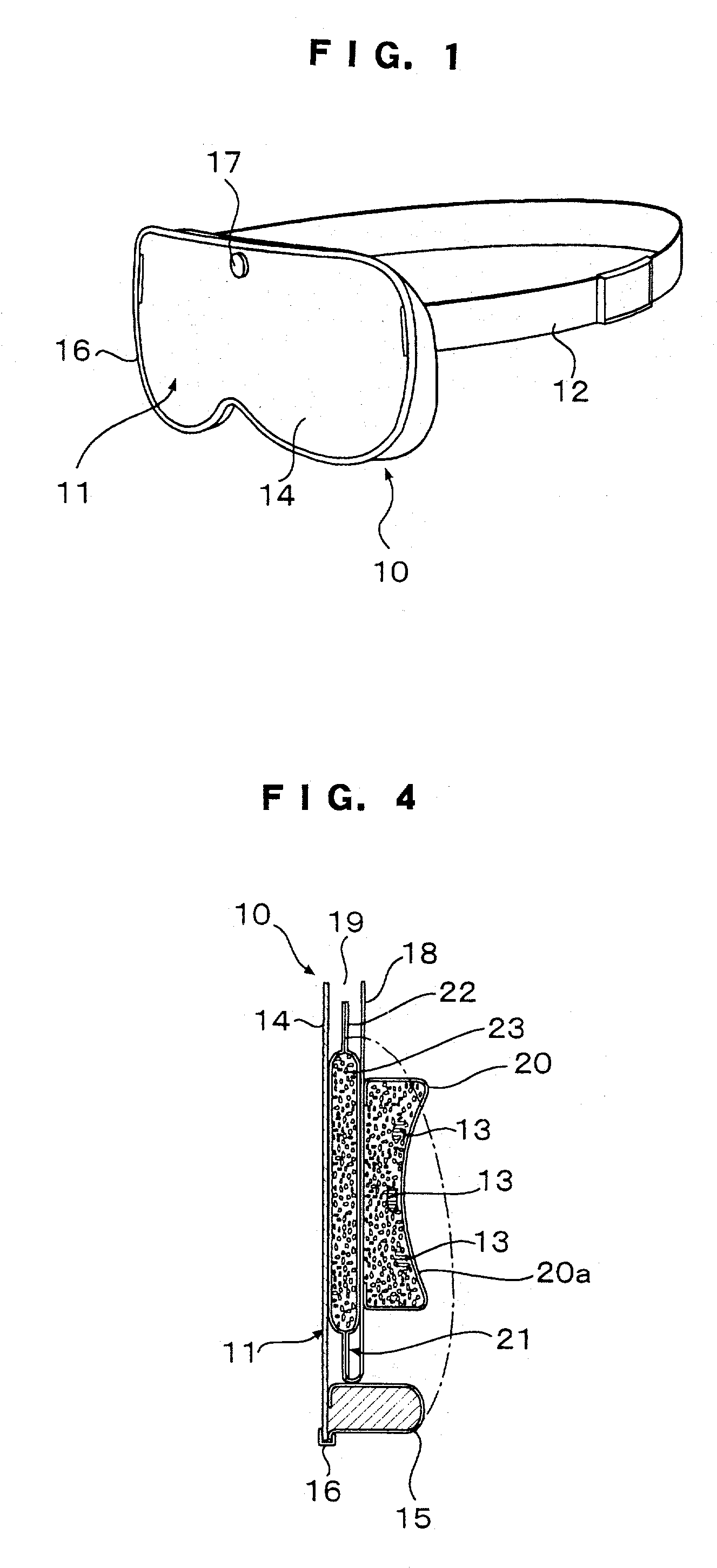
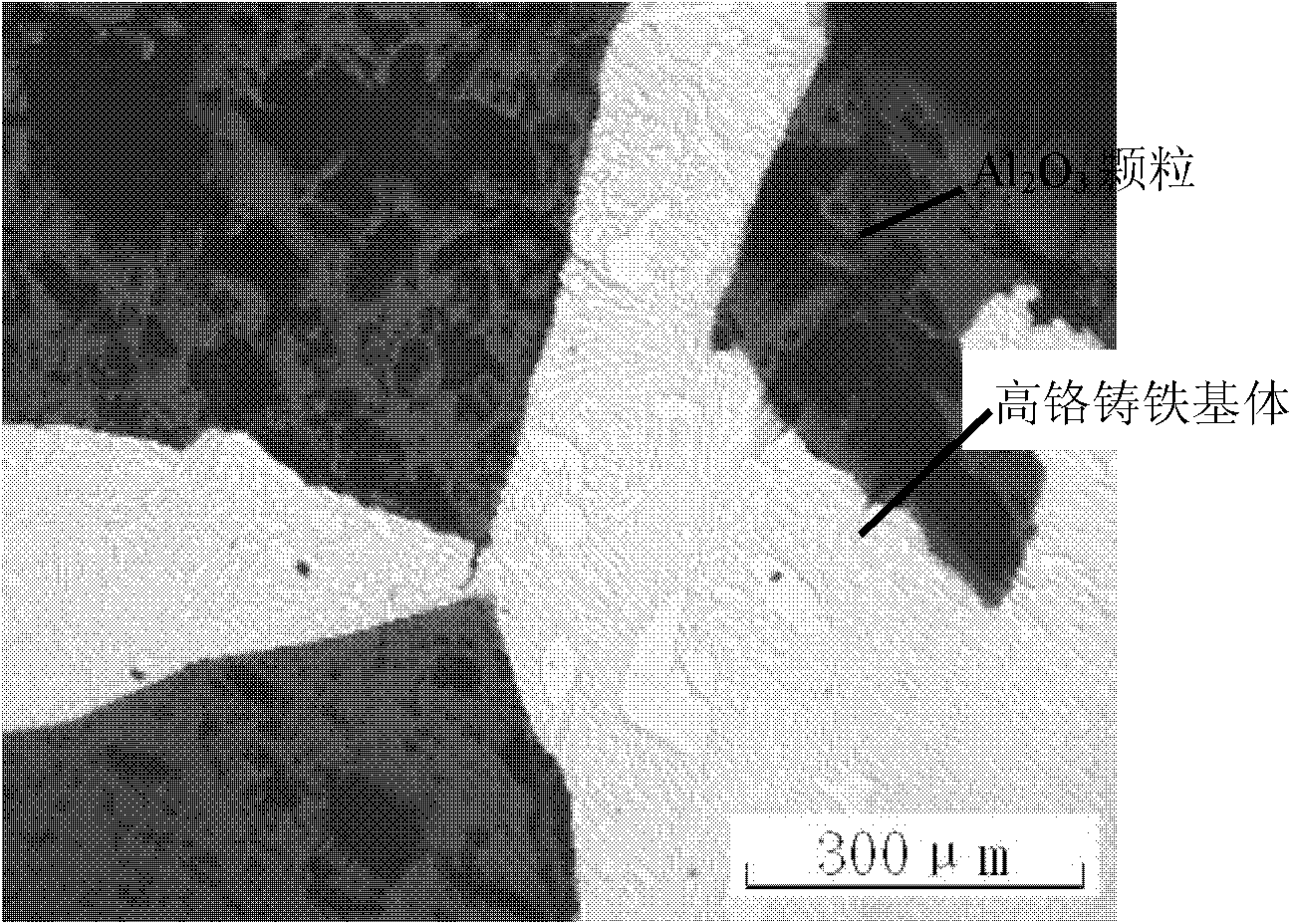Patents
Literature
1622results about How to "Mass production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
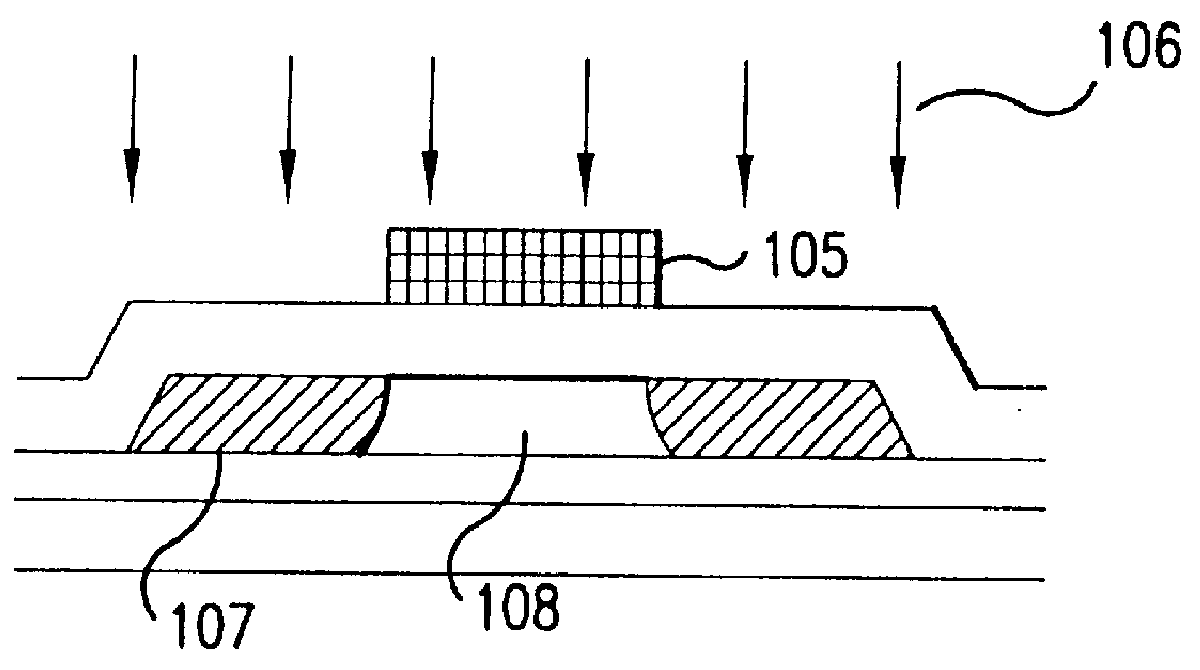
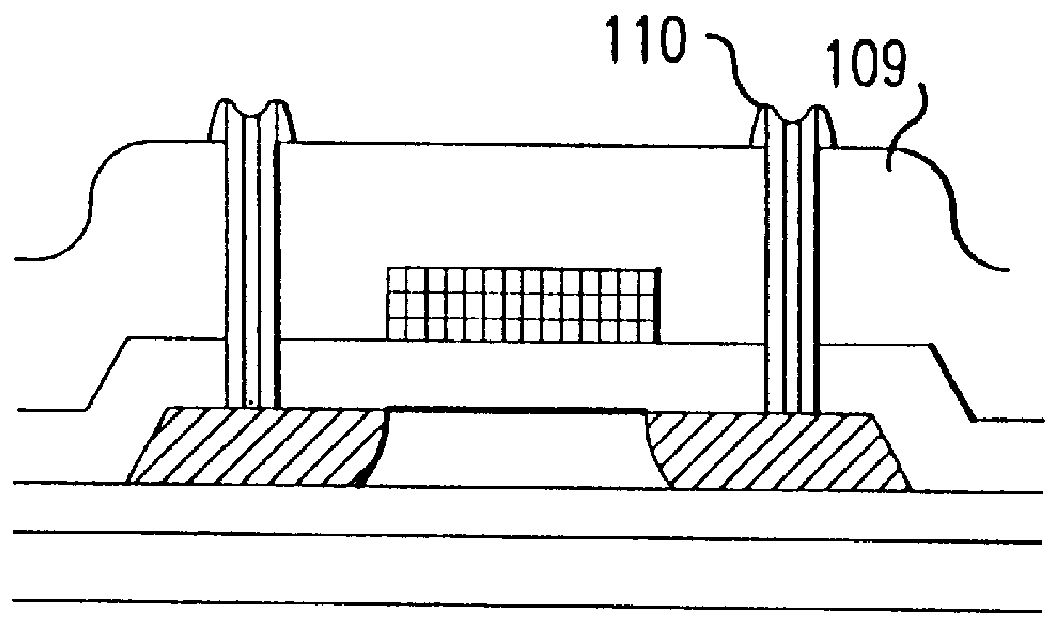
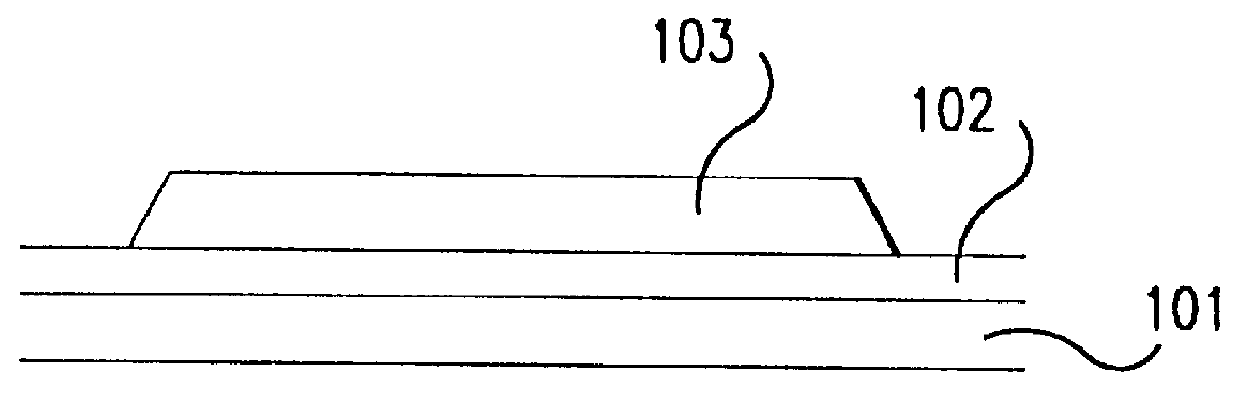
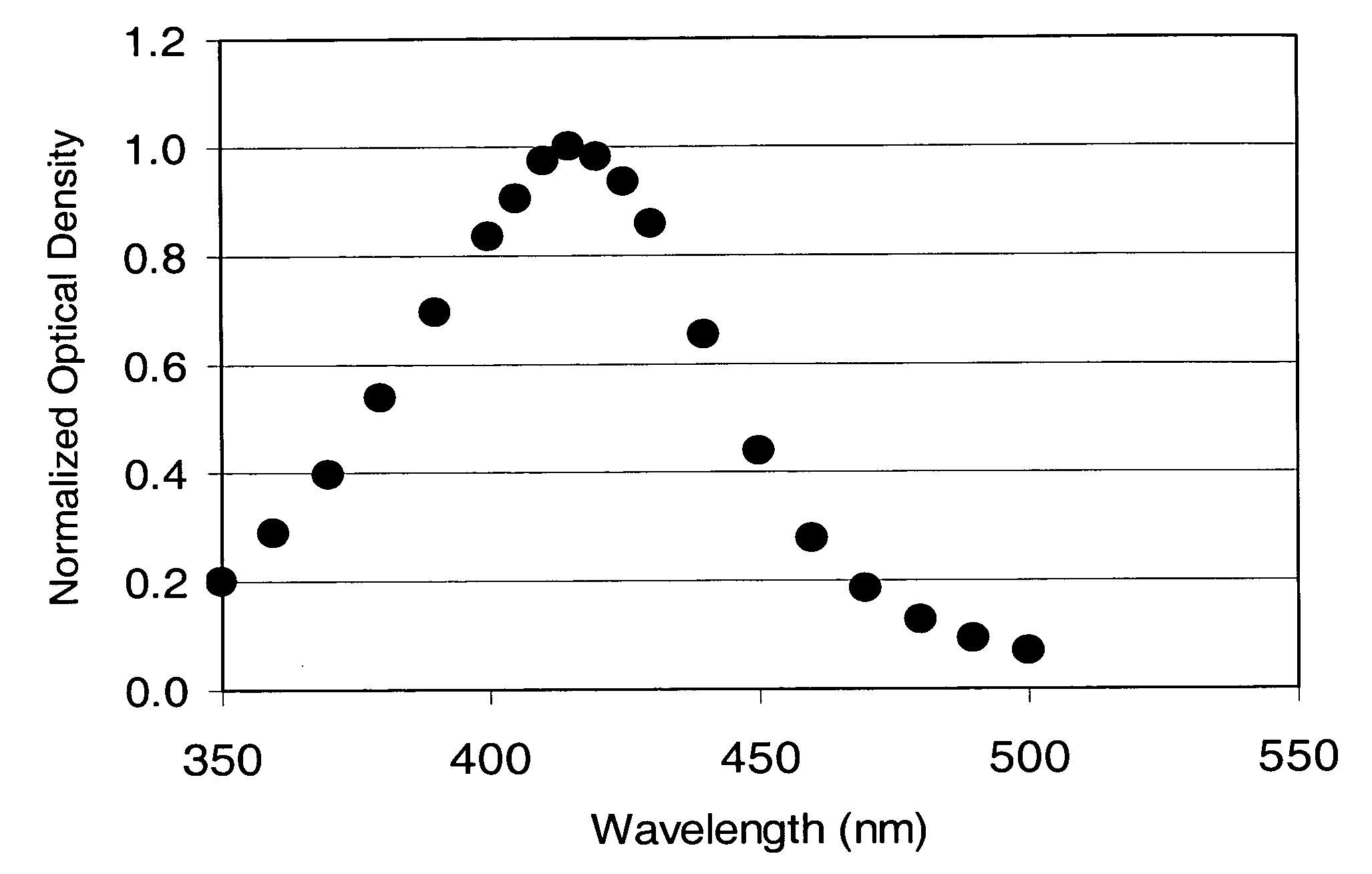
Fabrication method for a thin film semiconductor device, the thin film semiconductor device itself, liquid crystal display, and electronic device
InactiveUS6017779AImprove propertiesWell formedTransistorLinear bearingsElectronic circuitLiquid-crystal display
In order to fabricate a high performance thin film semiconductor device using a low temperature process in which it is possible to use low price glass substrates, a thin film semiconductor device has been fabricated by forming a silicon film at less than 450 DEG C., and, after crystallization, keeping the maximum processing temperature at or below 350 DEG C. In applying the present invention to the fabrication of an active matrix liquid crystal display, it is possible to both easily and reliably fabricate a large, high-quality liquid crystal display. Additionally, in applying the present invention to the fabrication of other electronic circuits as well, it is possible to both easily and reliably fabricate high-quality electronic circuits.
Owner:INTELLECTUAL KEYSTONE TECH
Antimicrobial silver compositions
The present invention comprises methods and compositions for antimicrobial silver compositions comprising silver nanoparticles. The present invention further comprises compositions for preparing silver nanoparticles comprising at least one stabilizing agent, one or more silver compounds, at least one reducing agent and a solvent. In one aspect, the stabilizing agent comprises a surfactant or a polymer. The polymer may comprise polymers such as polyacrylamides, polyurethanes, and polyamides. In one aspect, the silver compound comprises a salt comprising a silver cation and an anion. The anion may comprise saccharinate derivatives, long chain fatty acids, and alkyl dicarboxylates. The methods of the present invention comprise treating devices with the silver nanoparticle compositions, including, but not limited to, such devices as woven wound care materials, catheters, patient care devices, and collagen matrices. The present invention further comprises treatment of humans and animals wacr6ith the antimicrobial devices described herein.
Owner:AVENT INC
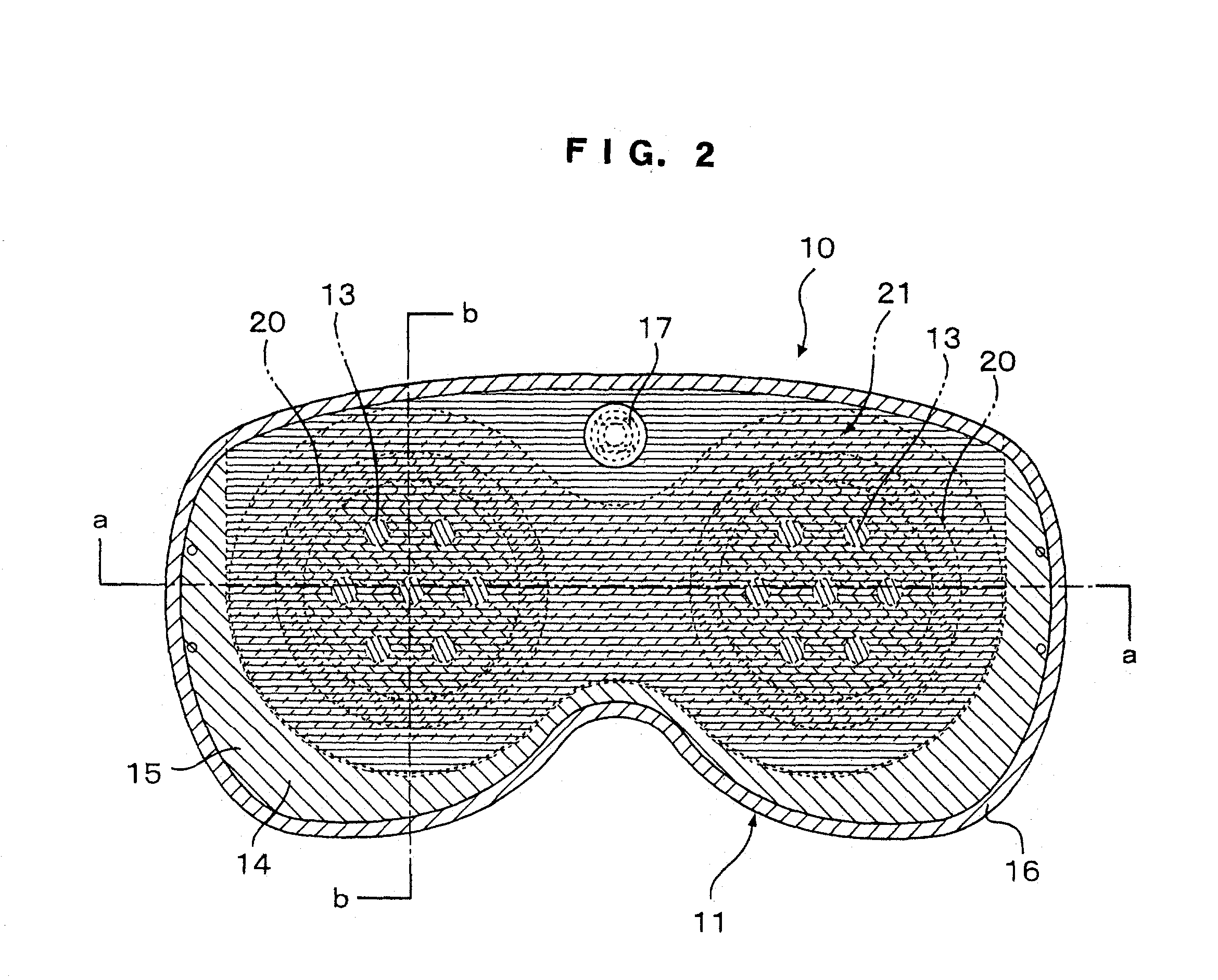
Eye mask
InactiveUS20030056281A1Recovering and restoringSimple structureElectrotherapyVibration massageDiseaseFarsightedness
An eye mask has magnetic bodies and self-heating warm members, which are inserted in eye pads on a mask member to be placed over eyeball parts. If required, vibrators and illumination bodies may be additionally placed in the eye pads. Thus, fatigue on the eyes and surroundings thereof can be relieved by the magnetic actions of the magnetic bodies and the warming effects of the warming member, in addition to expected effects of restoring ocular functions, recovering from various ocular diseases, and so on. Furthermore, the surface of each of the eye pads is gradually curved like the inner surface of a sphere. When the eye pads are press-contact to the eyeball parts at predetermined pressures for a long time, the cornea can be warmed by the warming members so that the shape of the cornea can be changed along the shape of the eye pad, resulting in the effects of recovering from eye sight disorder such as pseudo-myopia, moderate farsightedness, or moderate astigmatism.
Owner:HASEGAWA TOKUICHIRO
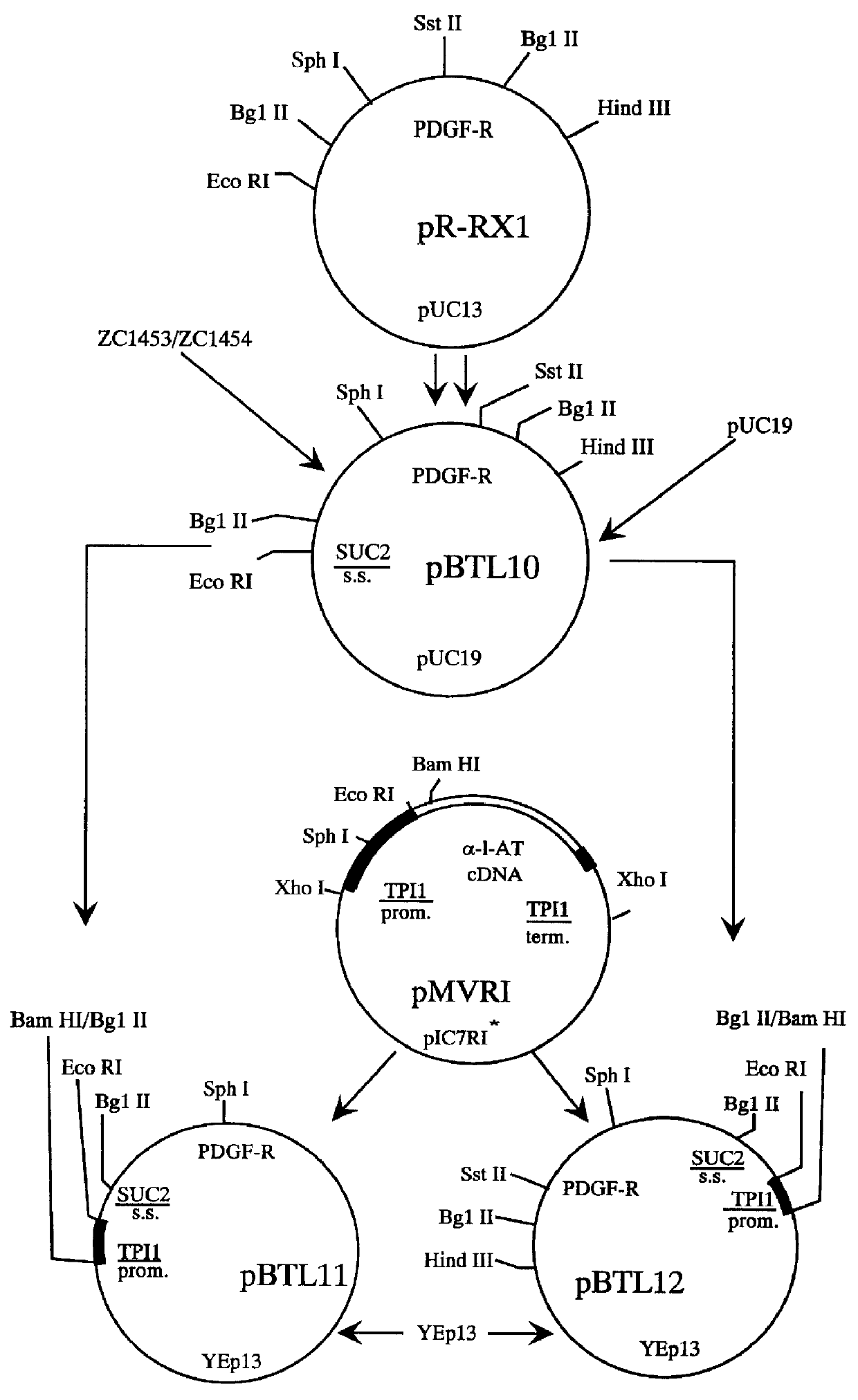
Biologically active dimerized and multimerized polypeptide fusions
InactiveUS6018026AImprove expression levelMass productionPeptide/protein ingredientsAntibody mimetics/scaffoldsExtracellular StructureA-DNA
Methods for producing secreted receptor analogs and biologically active peptide dimers are disclosed. The methods for producing secreted receptor analogs and biologically active peptide dimers utilize a DNA sequence encoding a receptor analog or a peptide requiring dimerization for biological activity joined to a dimerizing protein. The receptor analog includes a ligand-binding domain. Polypeptides comprising essentially the extracellular domain of a human PDGF receptor fused to dimerizing proteins, the portion being capable of binding human PDGF or an isoform thereof, are also disclosed. The polypeptides may be used within methods for determining the presence of and for purifying human PDGF or isoforms thereof.
Owner:ZYMOGENETICS INC
Electric part and method of manufacturing the same
InactiveUS20040041154A1Simple structureIncrease productivityFixed microstructural devicesCell electrodesProduction rateCarbon nanotube
There are provided an electric part which can be produced through a production process which is excellent in an industrial productivity and a method of manufacturing the electric part, by including a matrix-shaped nonconductive base member, a carbon nanotube group that is sealed within the nonconductive base member and includes one carbon nanotube or plural carbon nanotubes which are electrically connected to each other, in which substantially only end portion of the one carbon nanotube or at least one carbon nanotube contained in the plural carbon nanotubes is exposed from one surface of the nonconductive base member, and an electrode that is connected to a side surface of the at least one carbon nanotube contained in the carbon nanotube group.
Owner:FUJIFILM BUSINESS INNOVATION CORP
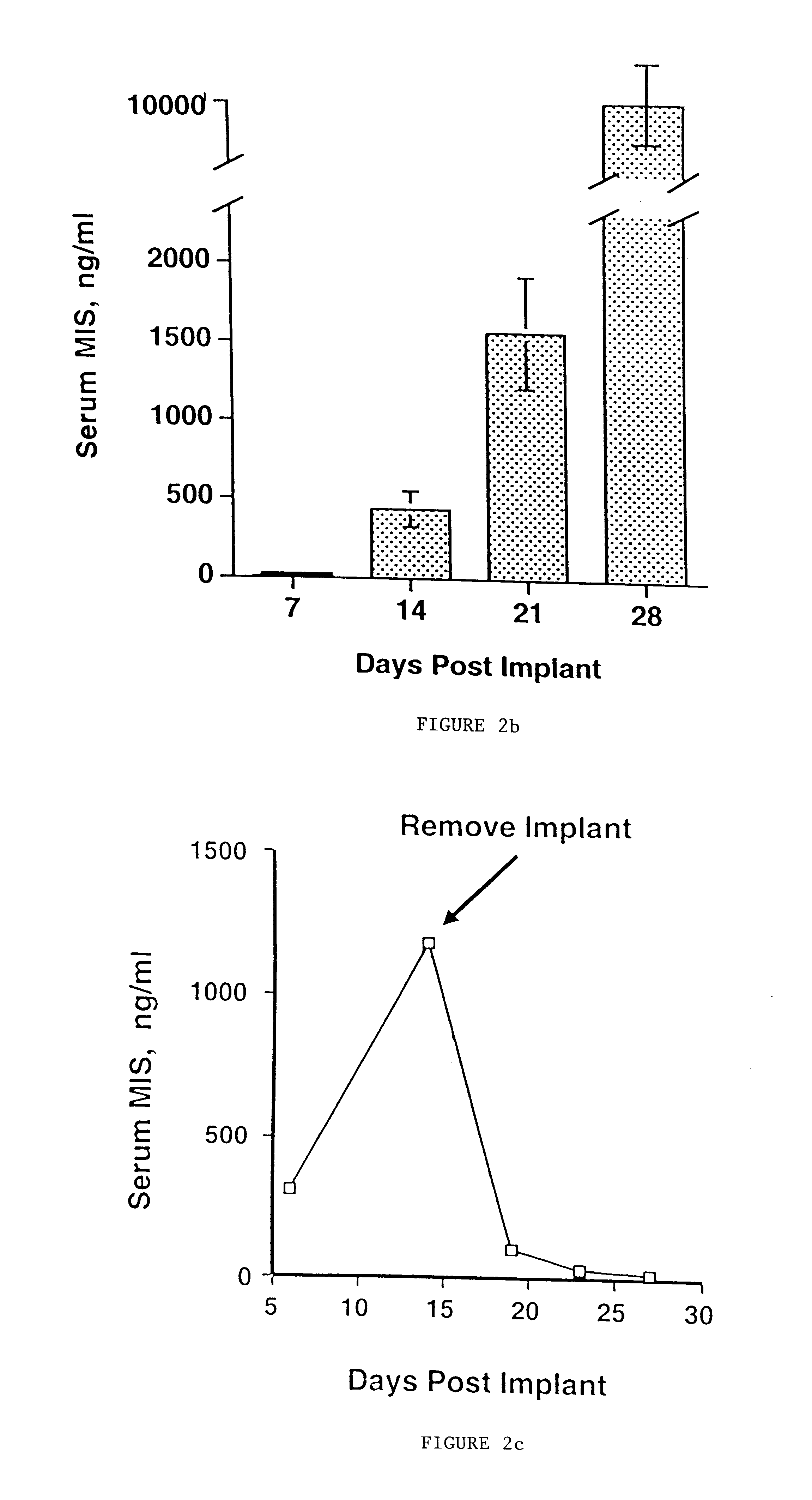
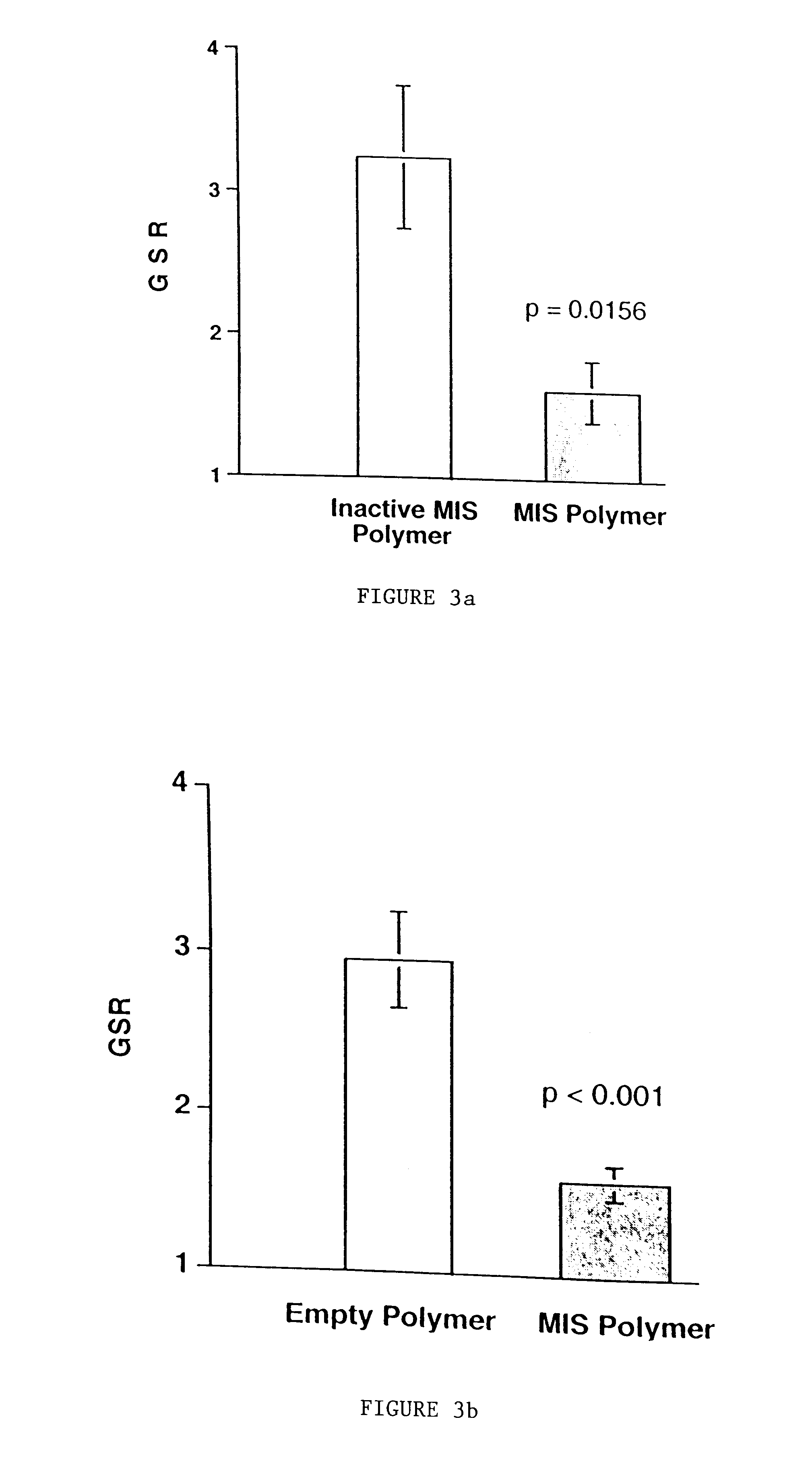
Delivery of therapeutic biologicals from implantable tissue matrices
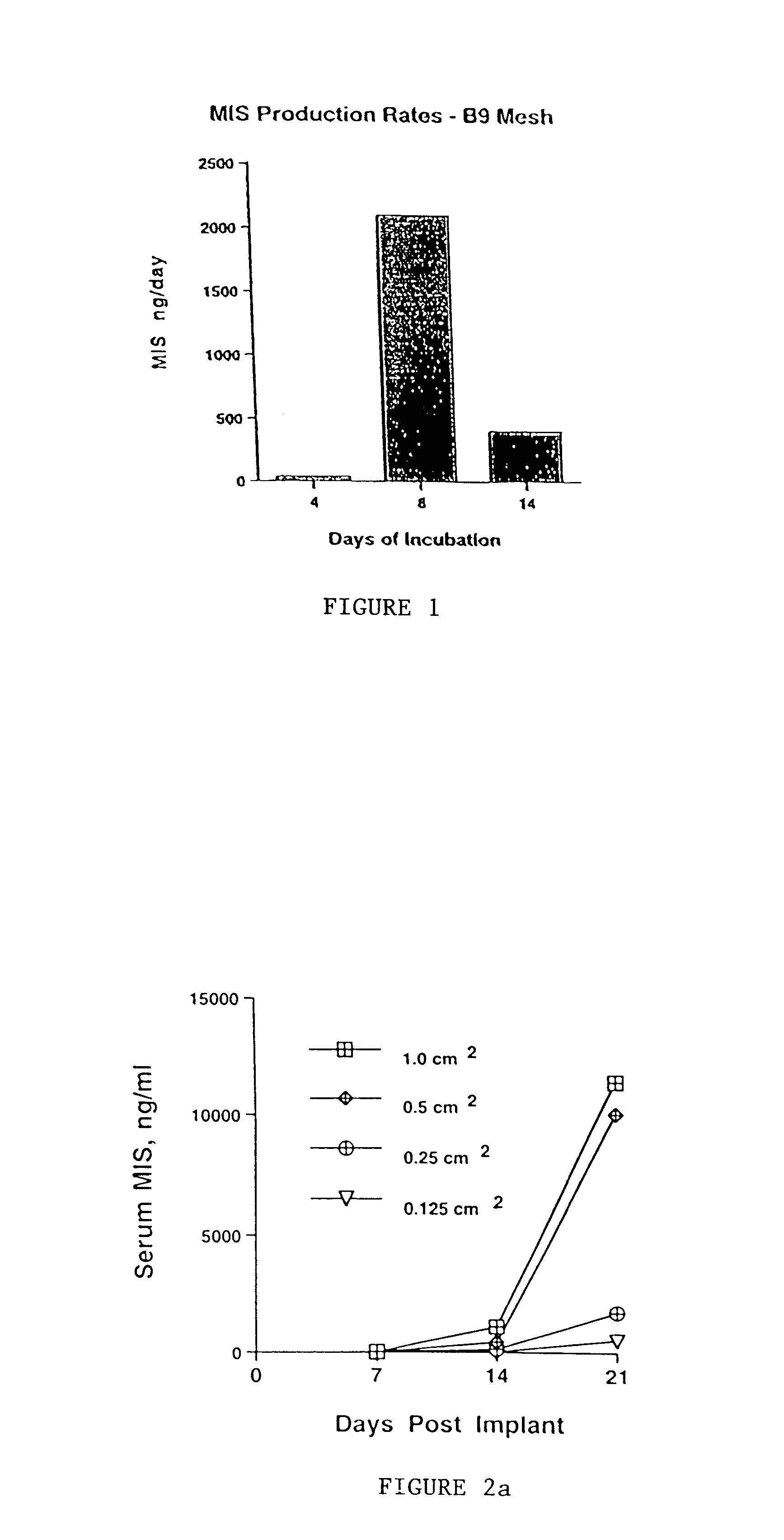
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
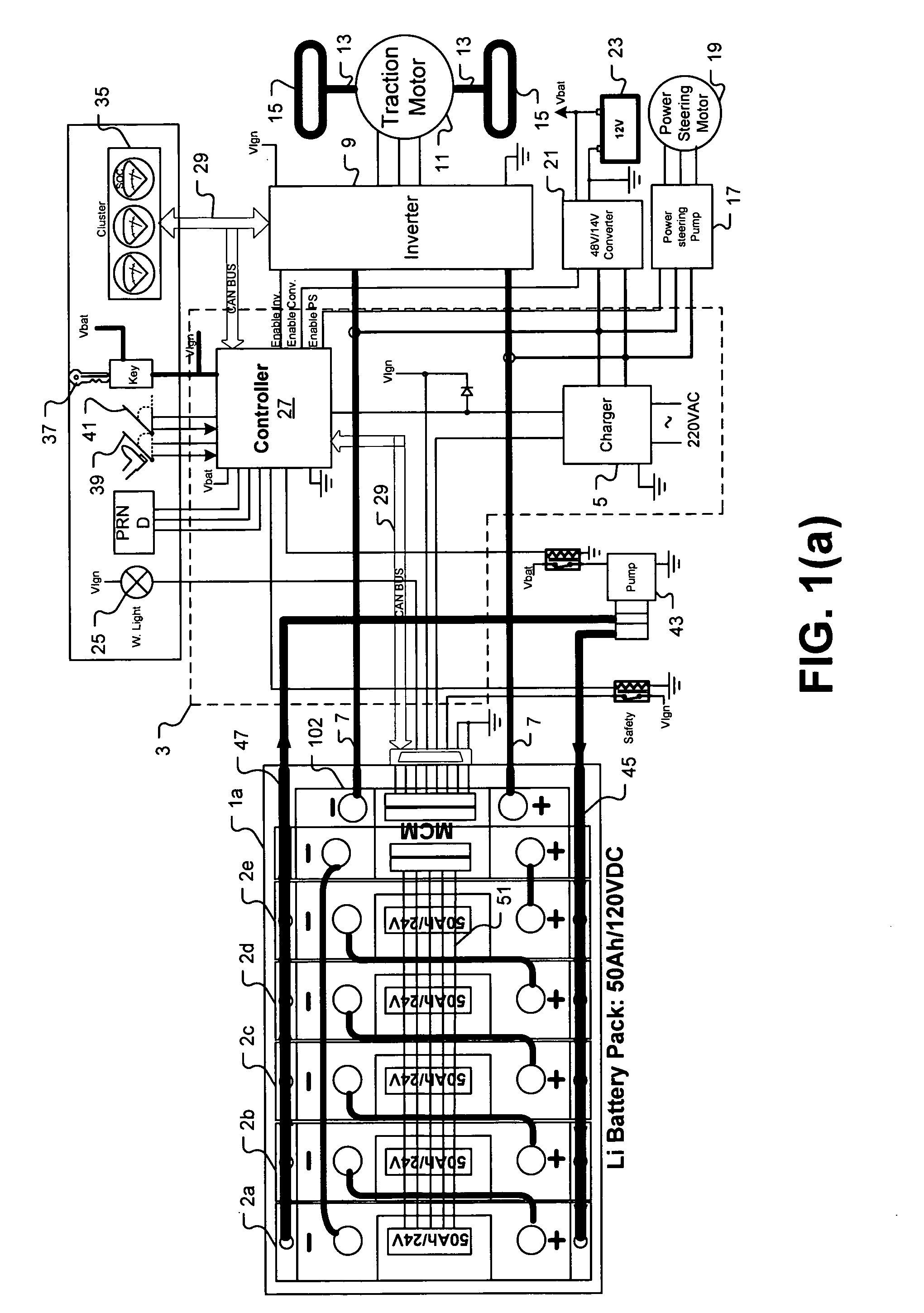
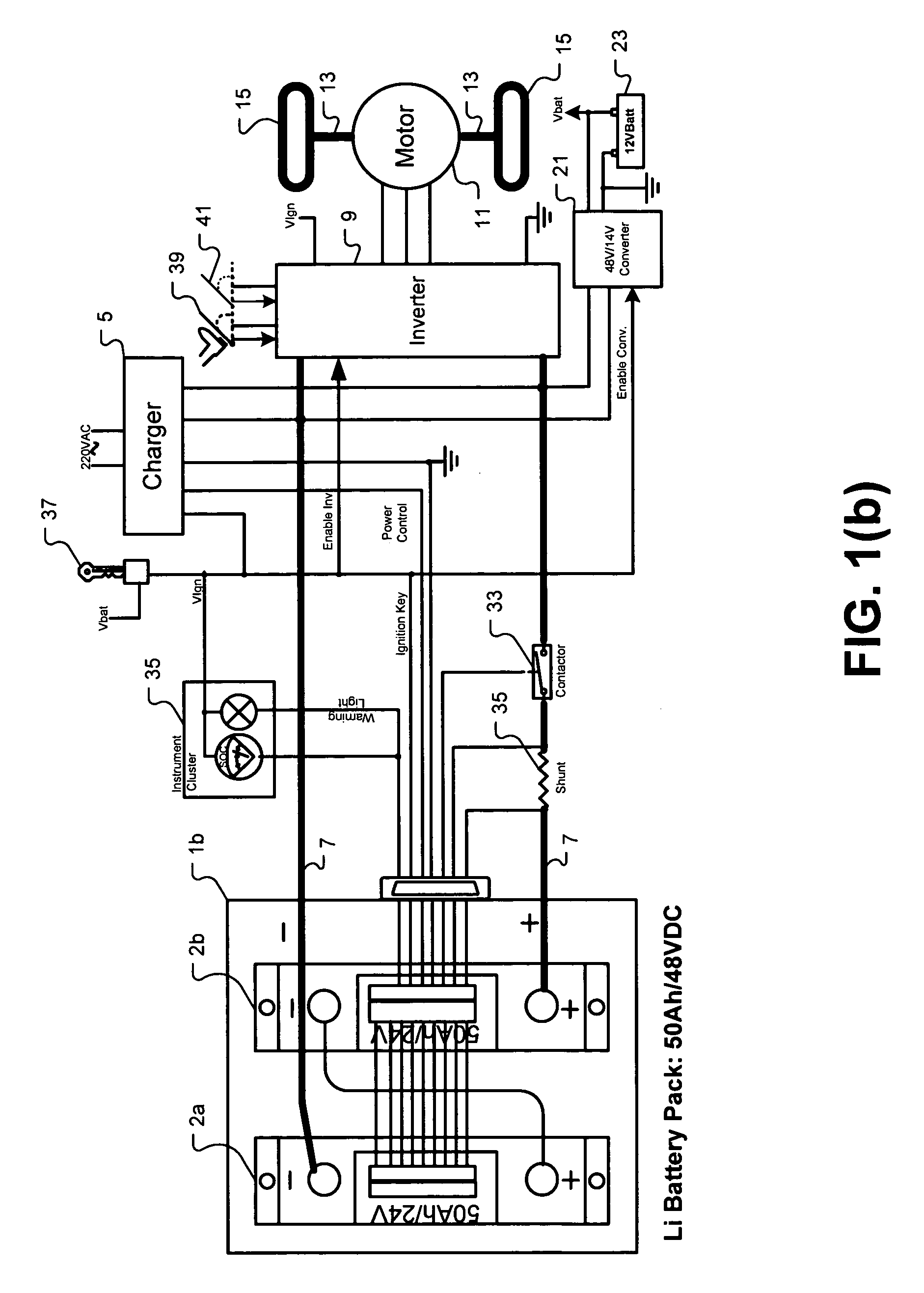
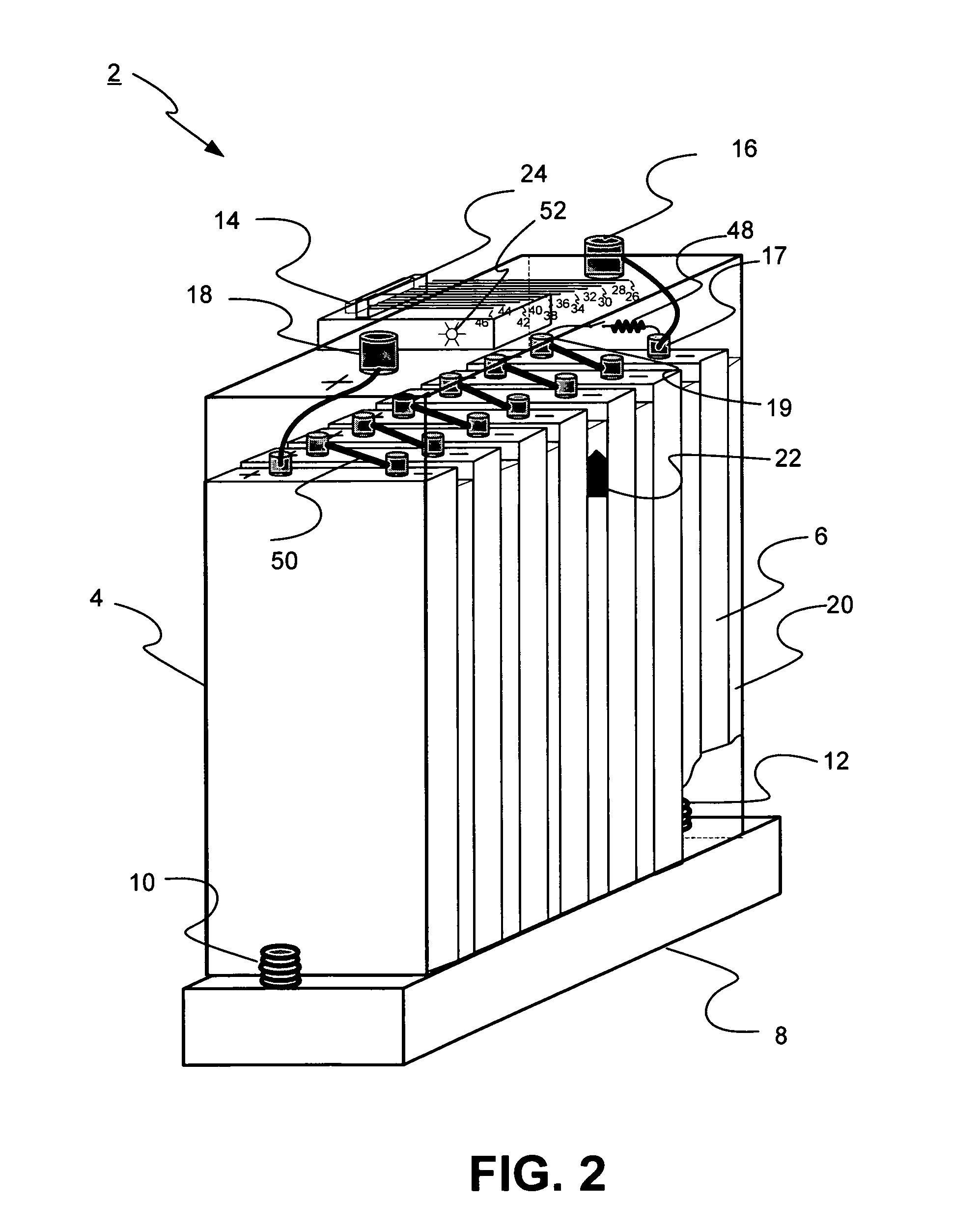
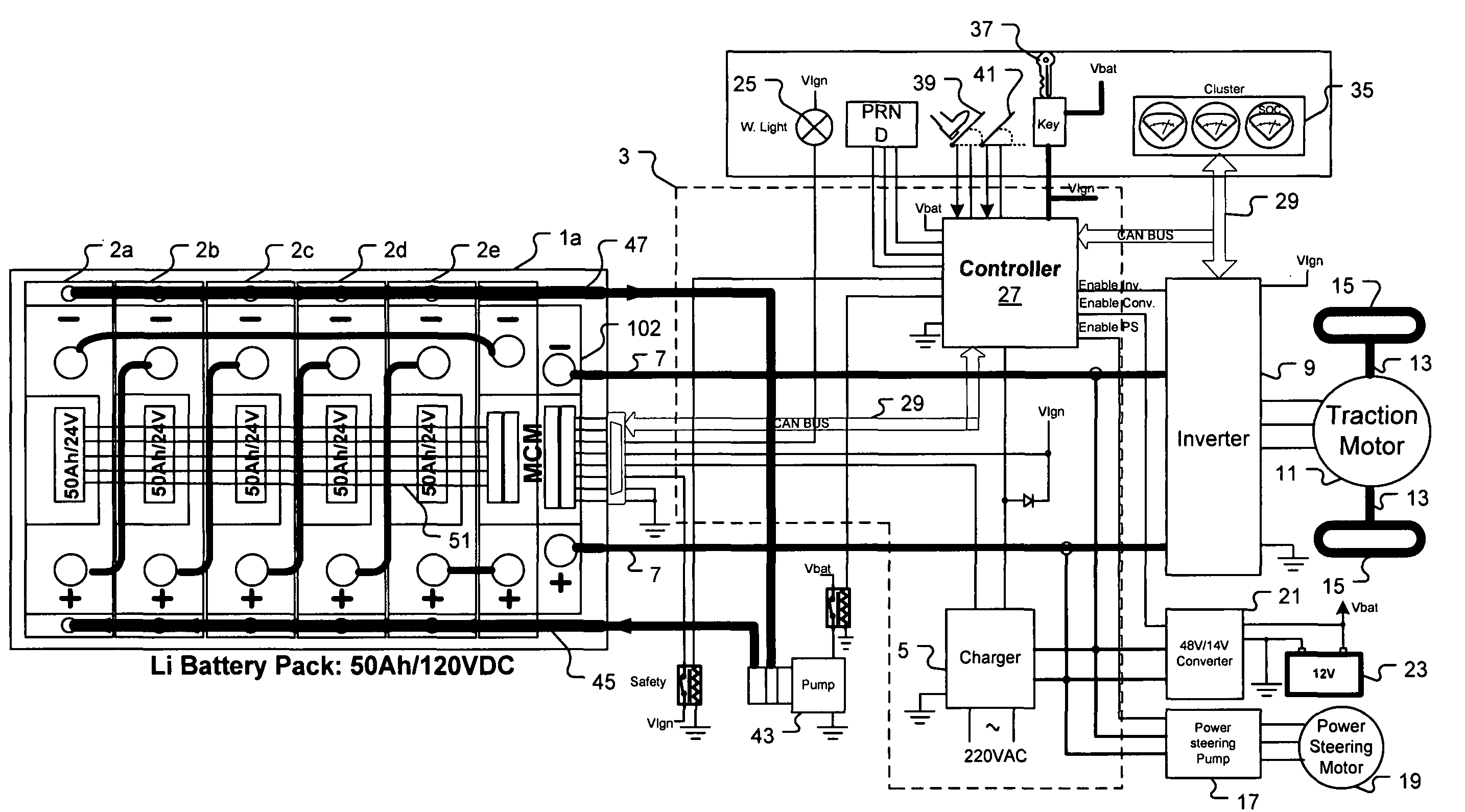
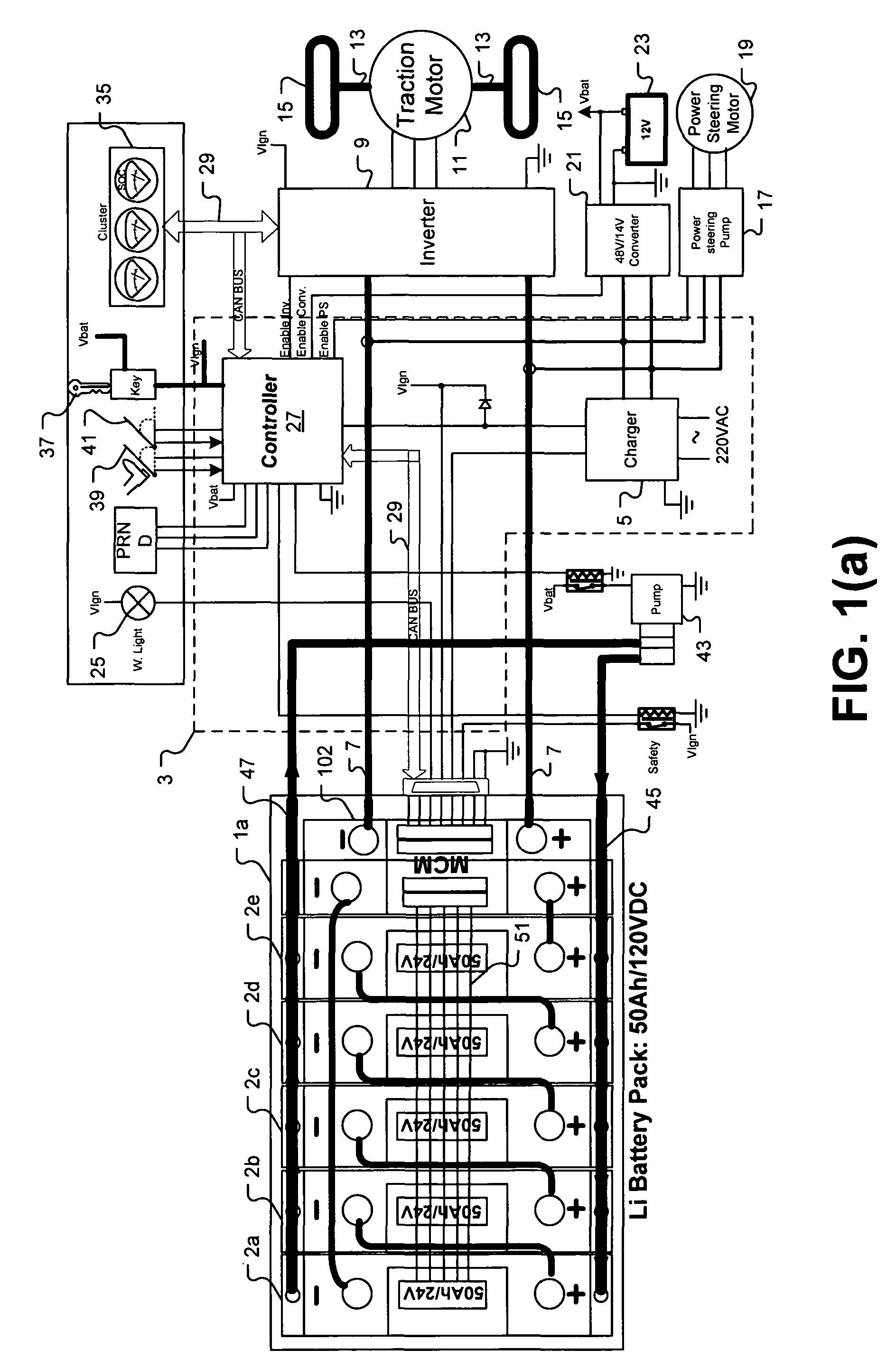
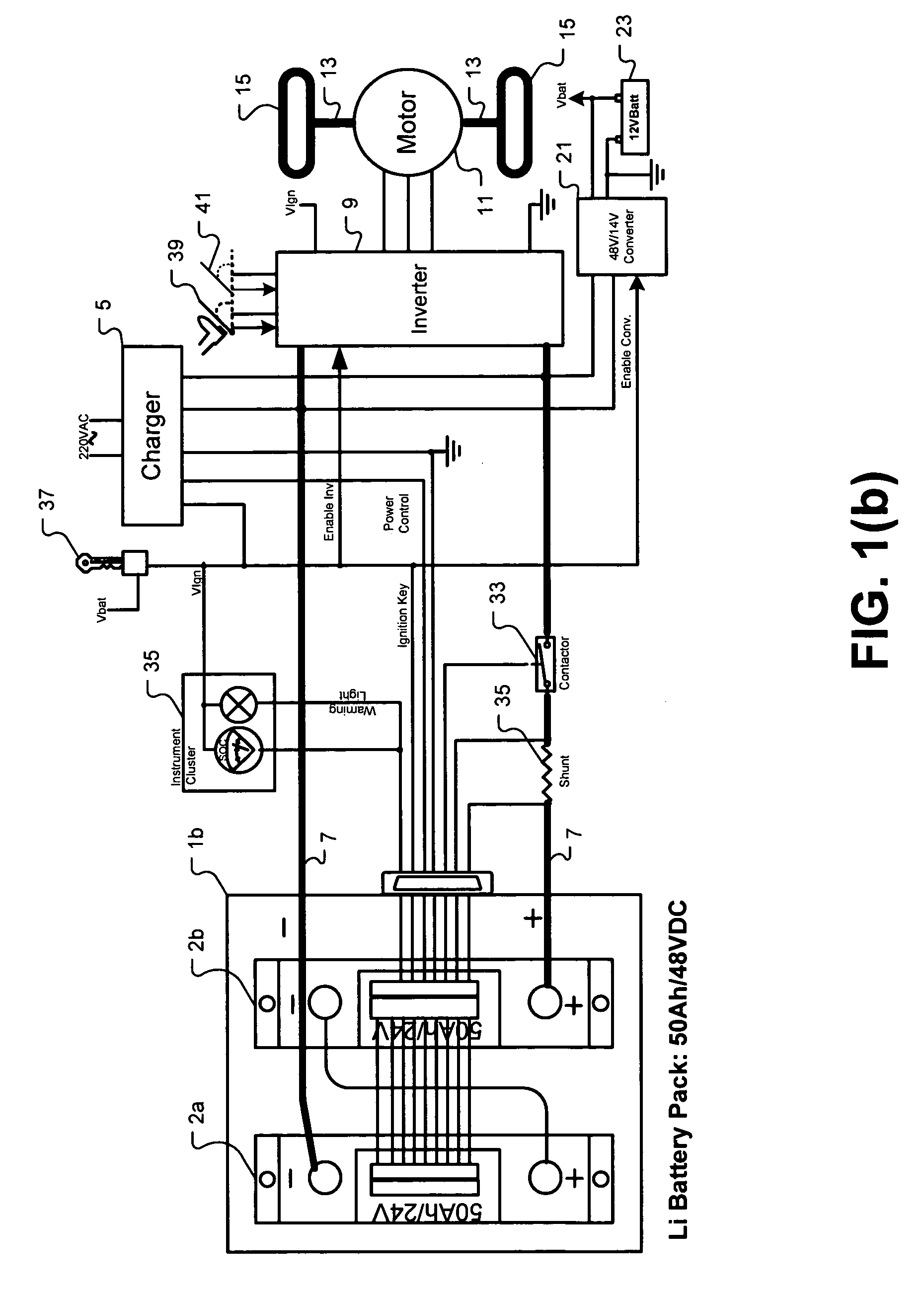
Universal battery module and controller therefor
InactiveUS20070080662A1Easy to integrateMass productionCircuit monitoring/indicationCharge equalisation circuitEmbedded systemBattery pack
A battery pack is provided including universal battery modules and a master control module. By selecting proper rated universal battery modules and connecting them either in series and / or parallel, a high performance and long life battery pack is assembled that is suitable for high power applications such as electrical vehicles whereby the master control module acts as the battery pack control and interface module.
Owner:DELAWARE POWER SYST CORP
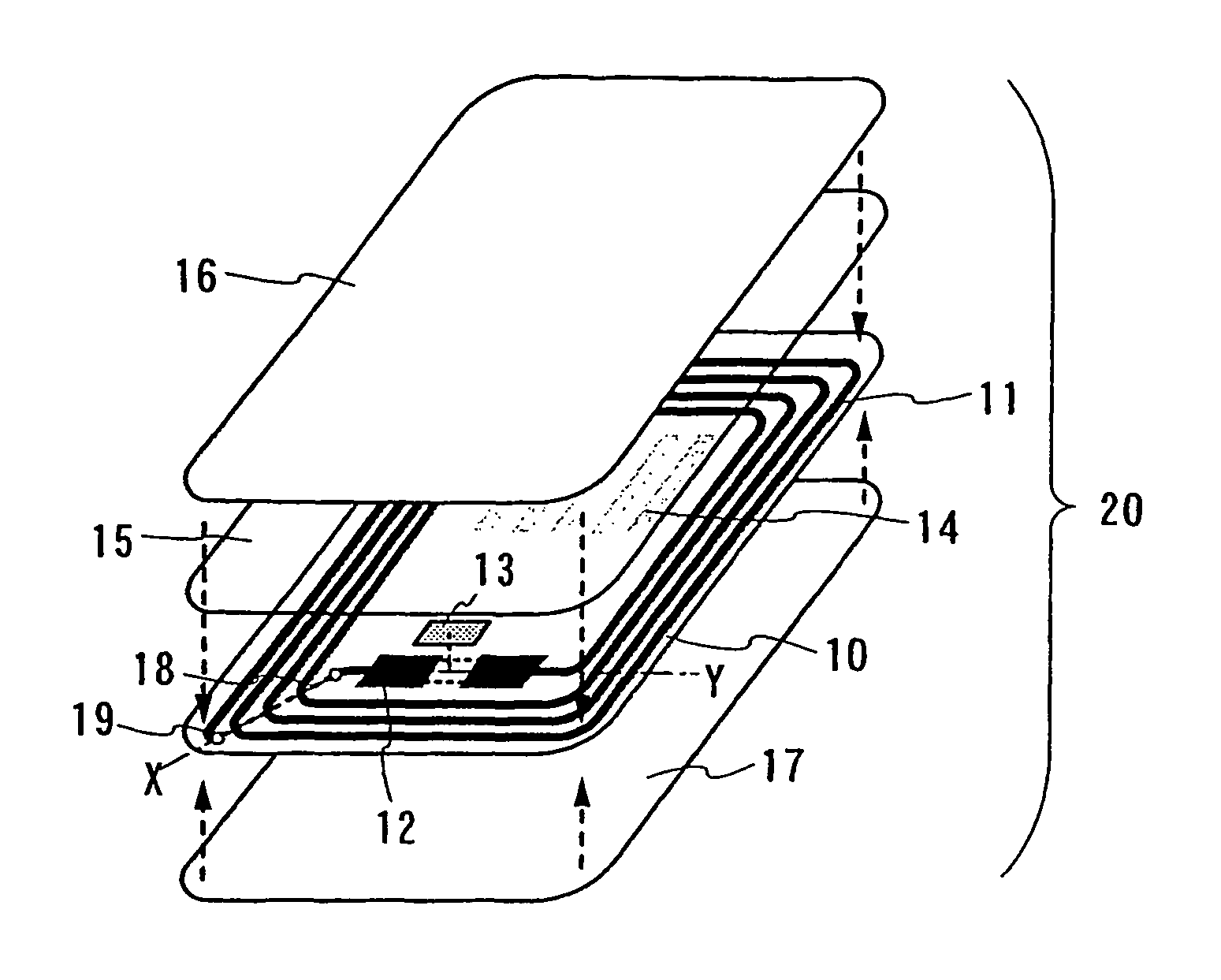
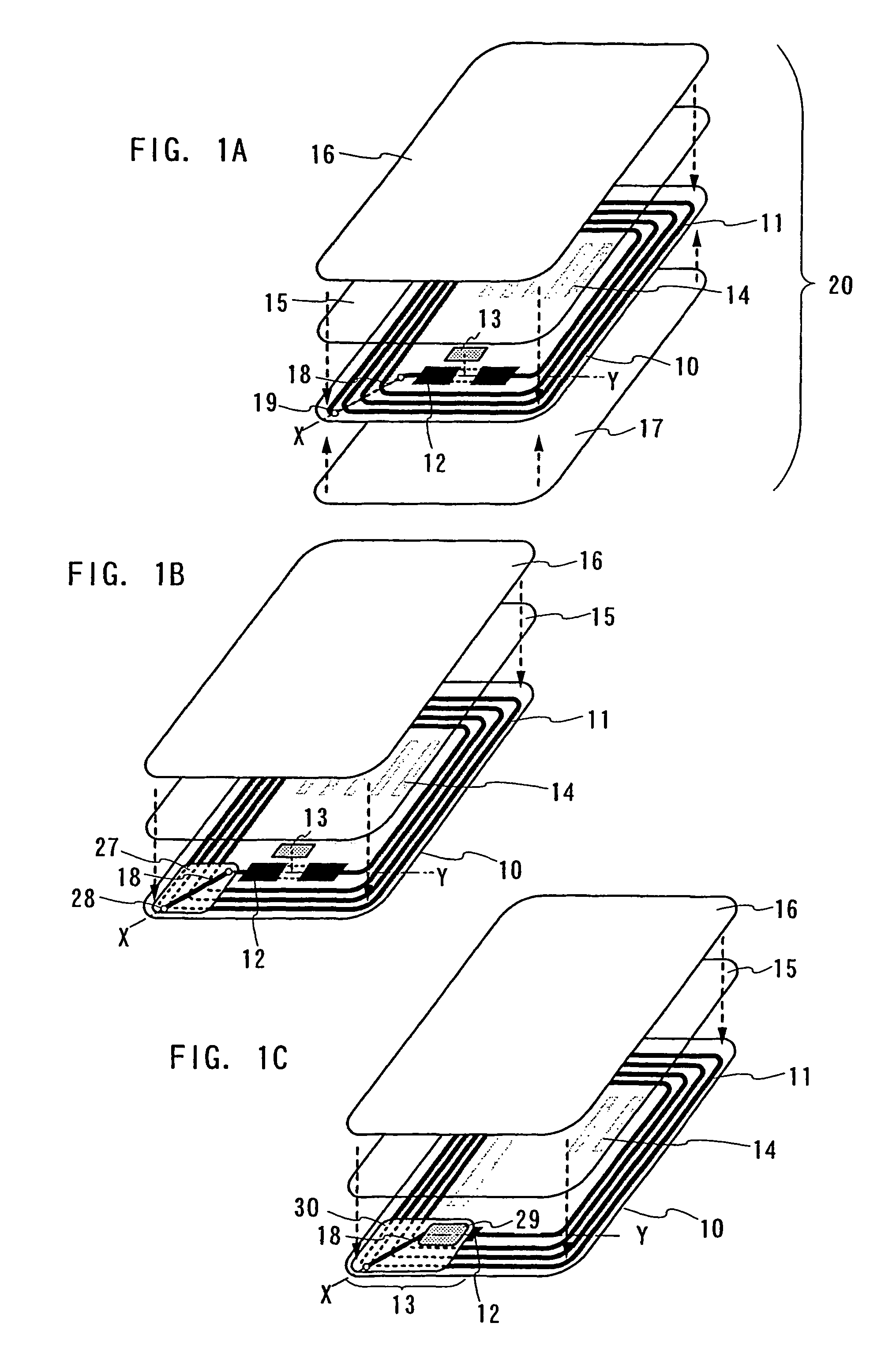
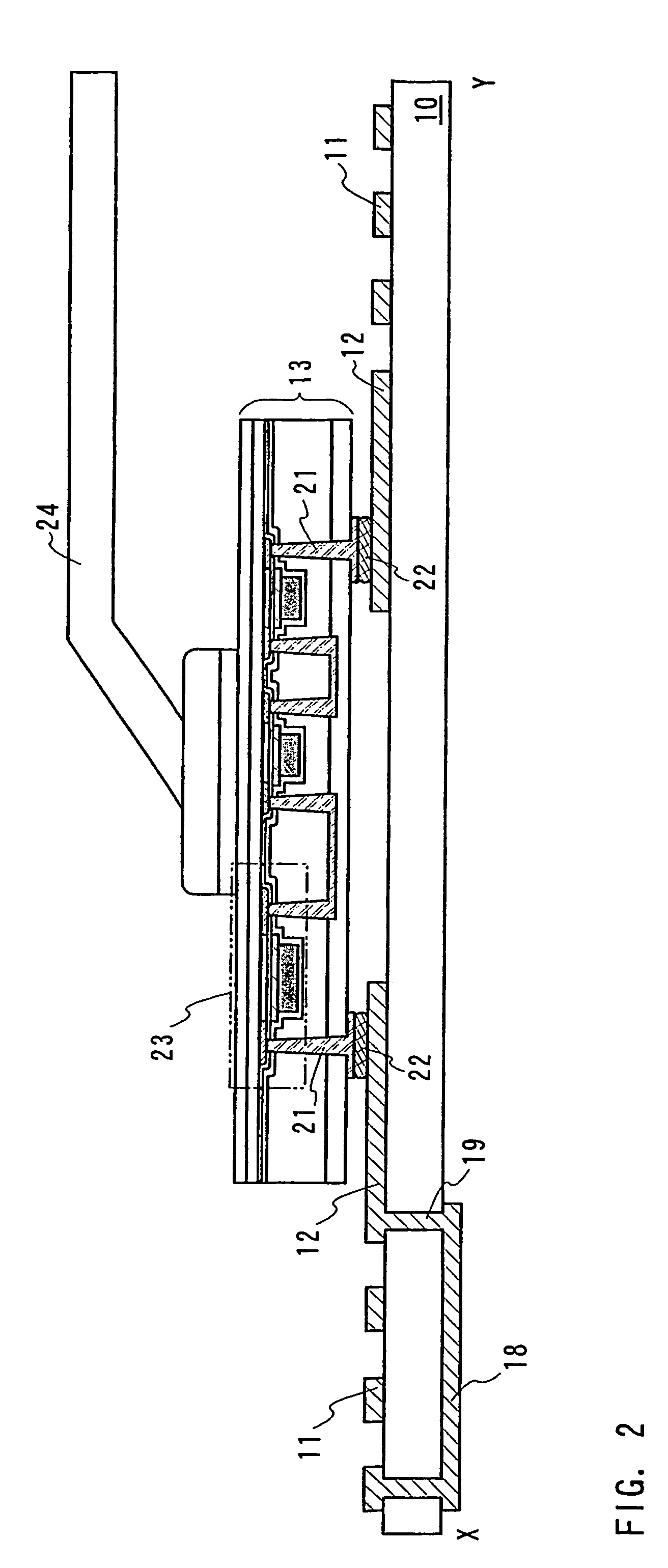
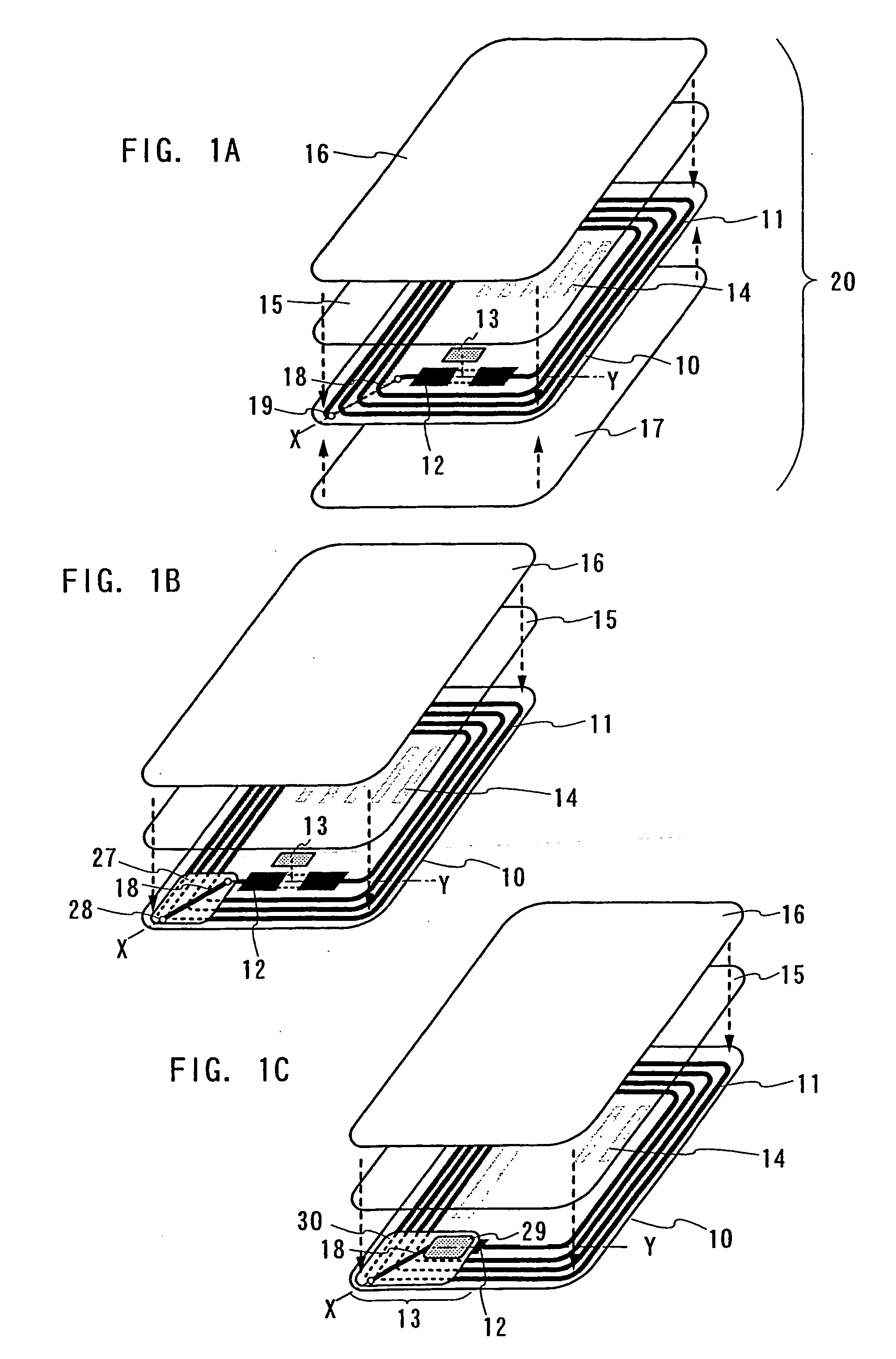
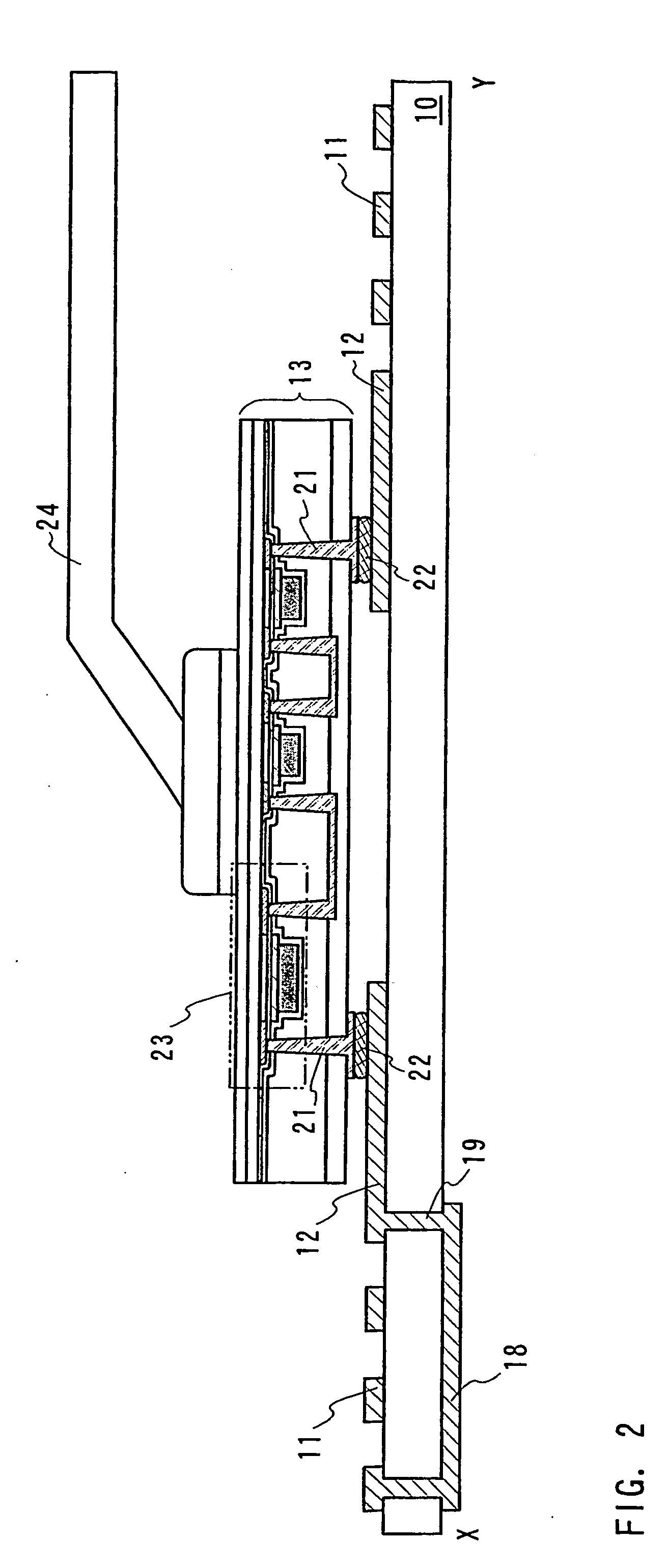
ID label, ID card, and ID tag
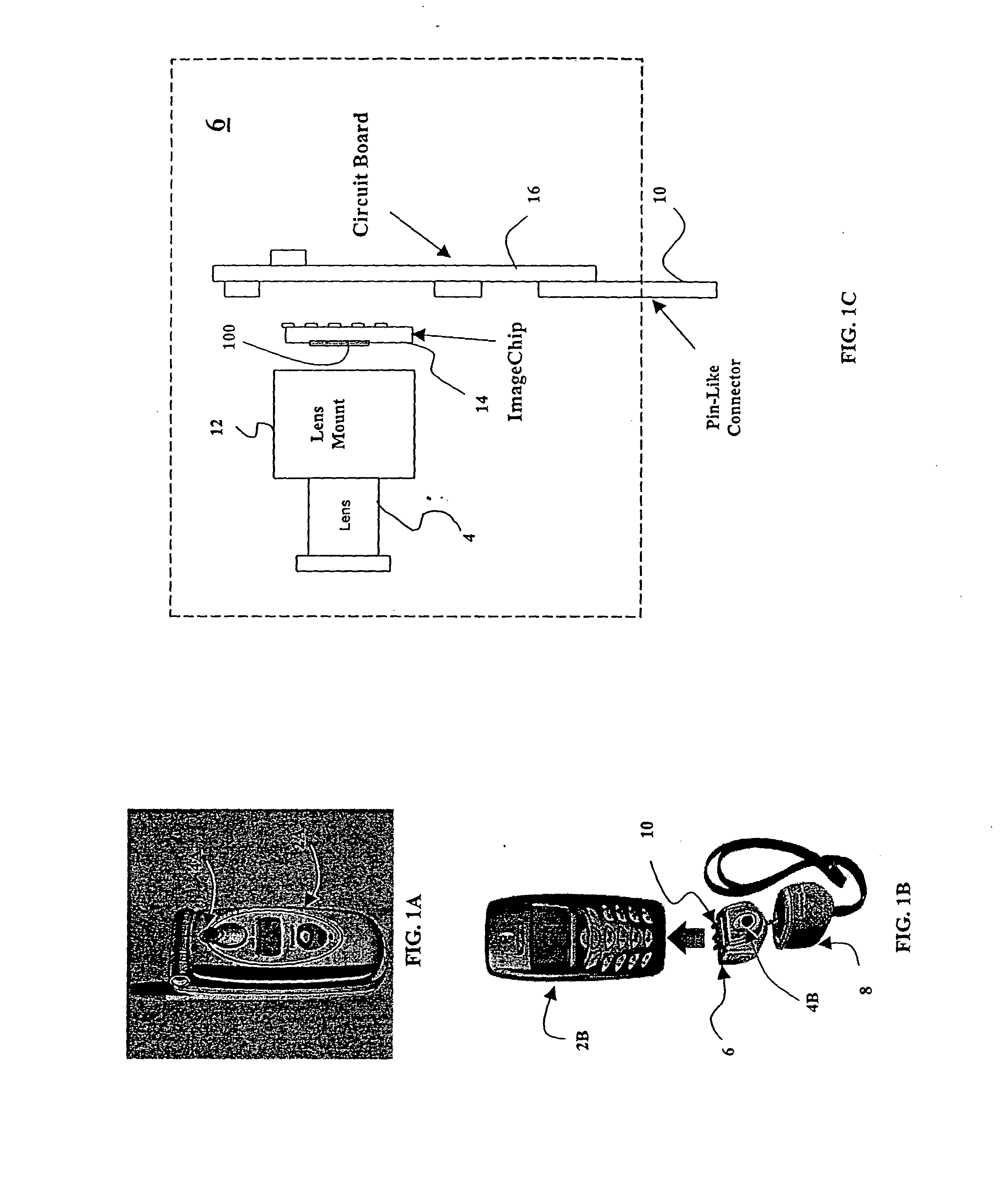
As a non-contact ID label, ID tag and the like being widespread, it is required to manufacture a considerable quantity of ID labels at quite a low cost. An ID label attached to a product is, for example, required to be manufactured at 1 to several yens each, or preferably less than one yen. Thus, such a structure and a process are demanded that an ID label can be manufactured in a large quantity at a low cost. A thin film integrated circuit device included in the ID label, the ID card, and the ID tag of the invention each includes a thin film active element such as a thin film transistor (TFT). Therefore, by peeling a substrate on which TFTs are formed for separating elements, the ID label and the like can be manufactured in a large quantity at a low cost.
Owner:SEMICON ENERGY LAB CO LTD
Dimerized polypeptide fusions
InactiveUS6291646B1Mass productionImprove expression levelPeptide/protein ingredientsAntibody mimetics/scaffoldsExtracellular StructureA-DNA
Methods for producing secreted receptor analogs and biologically active peptide dimers are disclosed. The methods for producing secreted receptor analogs and biologically active peptide dimers utilize a DNA sequence encoding a receptor analog or a peptide requiring dimerization for biological activity joined to a dimerizing protein. The receptor analog includes a ligand-binding domain. Polypeptides comprising essentially the extracellular domain of a human PDGF receptor fused to dimerizing proteins, the portion being capable of binding human PDGF or an isoform thereof, are also disclosed. The polypeptides may be used within methods for determining the presence of and for purifying human PDGF or isoforms thereof.
Owner:ZYMOGENETICS INC
Method and Apparatus For a Variable User Interface
ActiveUS20080194987A1Economy of scaleGrow with diabeticCatheterSensorsAnalyteGraphical user interface
An analyte monitoring system is provided. The system may include a housing (200) and a visual display (206) on the housing (200), the visual display (206) having at lease one visual indicator position next to a corresponding marking (208, 210, 212, 214 and 216) on the housing (200). The system may include a processor (60) driving the visual display (206), wherein the processor (60) runs software that is modifiable to provide a variable user interface on the visual display (206). The system may include a wireless communication to allow applets or programs to be down-loaded by the processor (60).
Owner:SANOFI AVENTIS DEUT GMBH
Light guide plate, lighting illuminating device using same, area light source and display
InactiveUS20060146573A1Small thicknessEasy to mass produceMechanical apparatusPlanar/plate-like light guidesLight guideRefractive index
A light guide plate includes (i) a first light guide layer made of a material having a refractive index n1, and (ii) a scattering light guide layer having a function of scattering light. A reflection means for irradiating the scattering light guide layer with the light having propagated in the first light guide layer is provided on a surface opposite to a light guide surface of the first light guide layer, the light guide surface on which the light is incident. The scattering light guide layer includes at least (i) a second light guide layer made of a material having a refractive index n2 (n2<n1), and (ii) a scattering layer for scattering the light propagating in the second light guide layer.
Owner:SHARP KK
Golf club
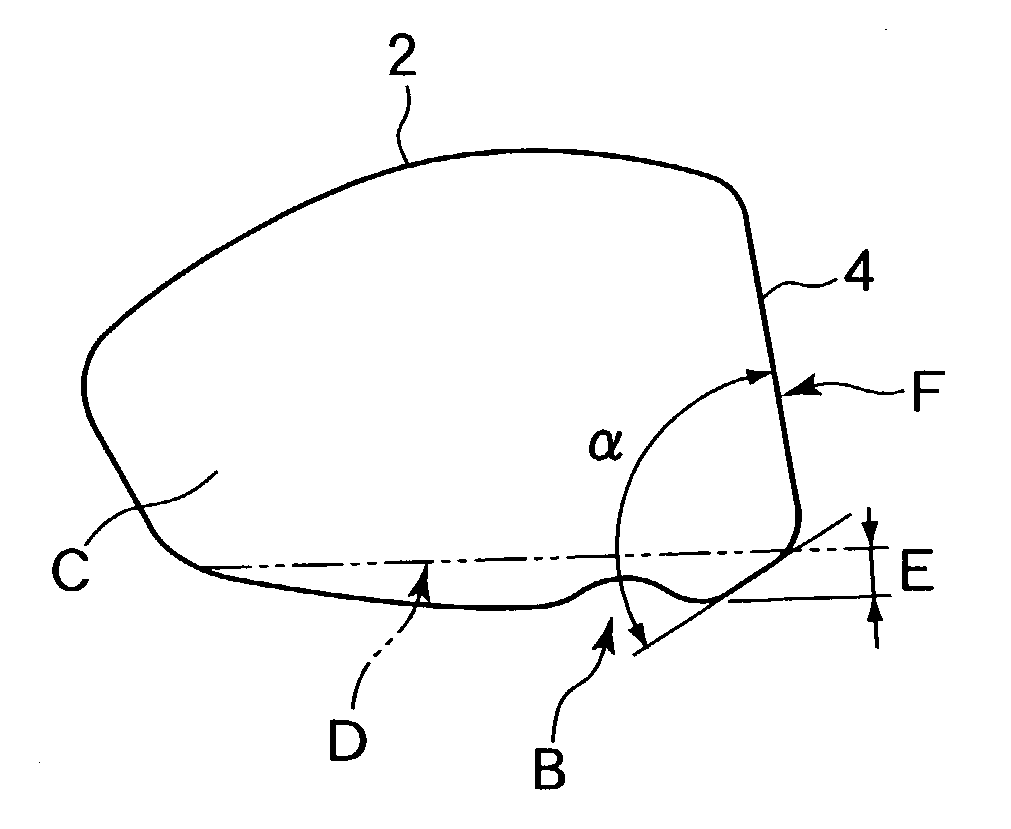
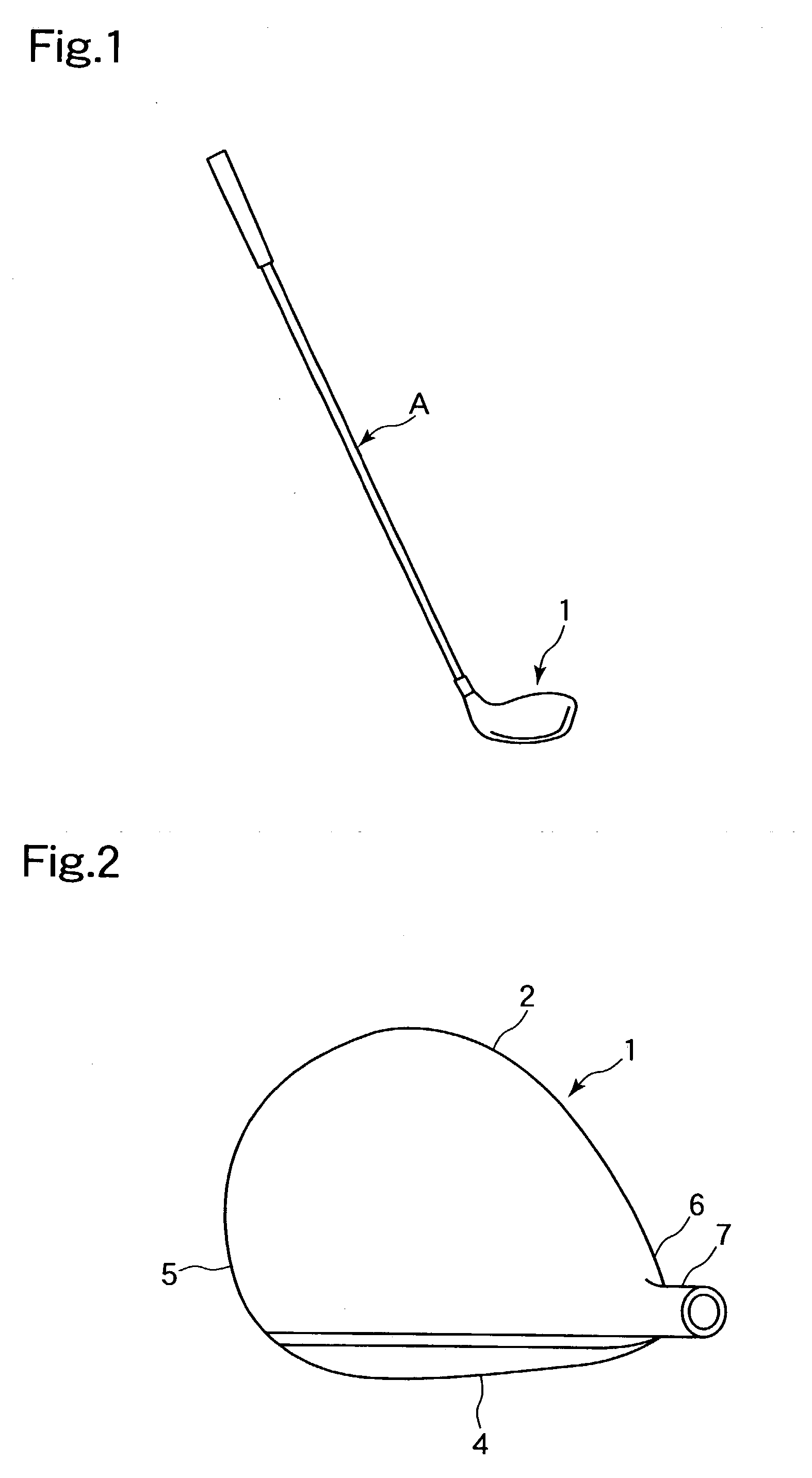
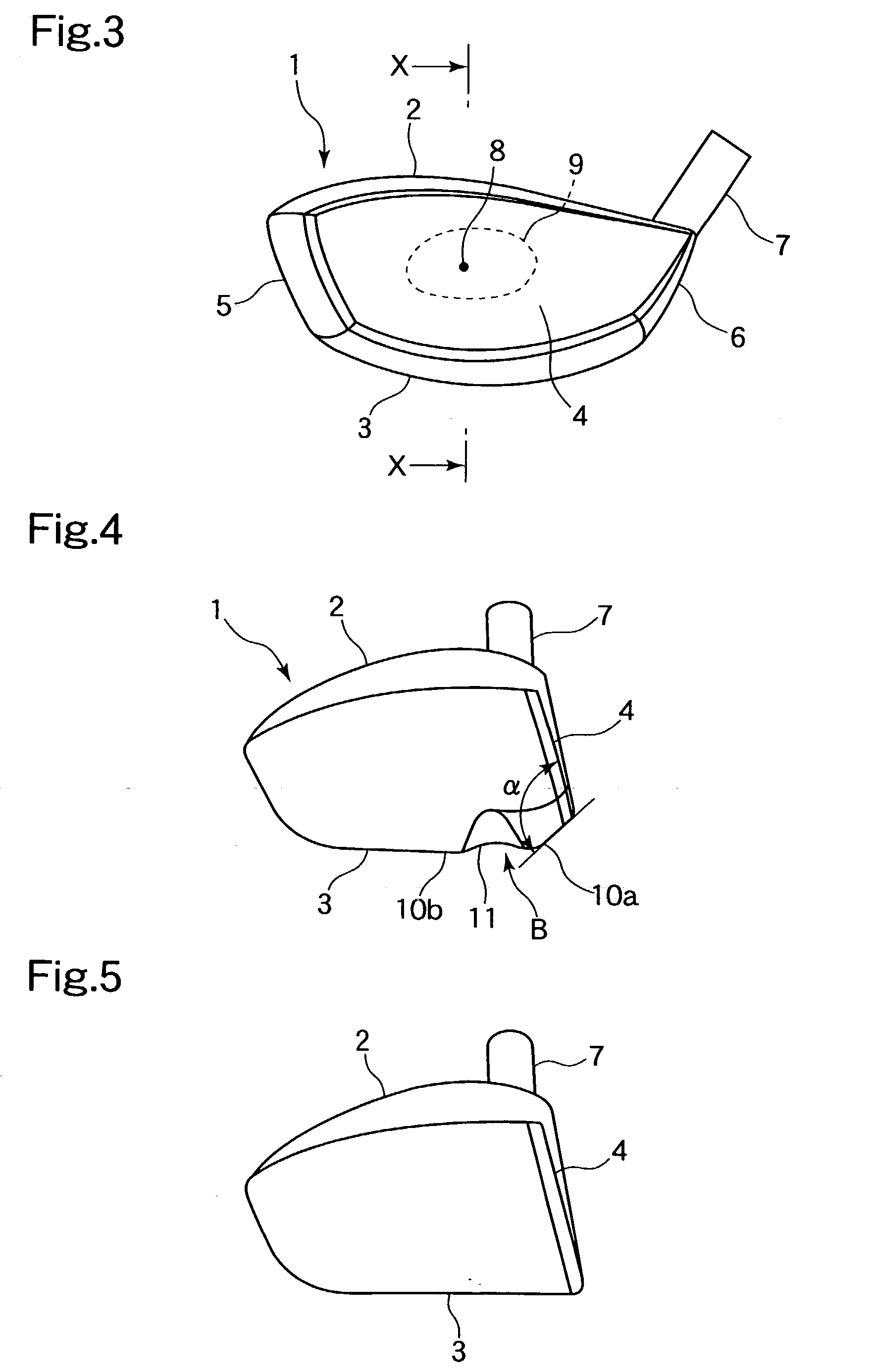
An object of the present invention is to provide a golf club in which carry does not deteriorate even when a golf ball is struck in a lower position on a face portion. In a golf club constituted by a face portion 4 having a hitting surface, a sole portion 3 which forms a lower portion, and a crown portion 5 which forms an upper portion, the sole portion 3 of a driver club head 1 is improved. A deformation portion B which is capable of elastic deformation is provided in concavoconvex form on the sole portion 3 in a position near the face portion 4. This deformation portion B is constituted by a protrusion 10 which is formed such that the sole portion 3 forms an obtuse angle alpha, and a concave groove 11 which is formed in a direction linking a toe portion and a heel portion by a protrusion protruding towards the crown portion 5. In order to increase hitting distance, restitution in a lower position on the face portion is increased.
Owner:ENDO MFG COMPANY
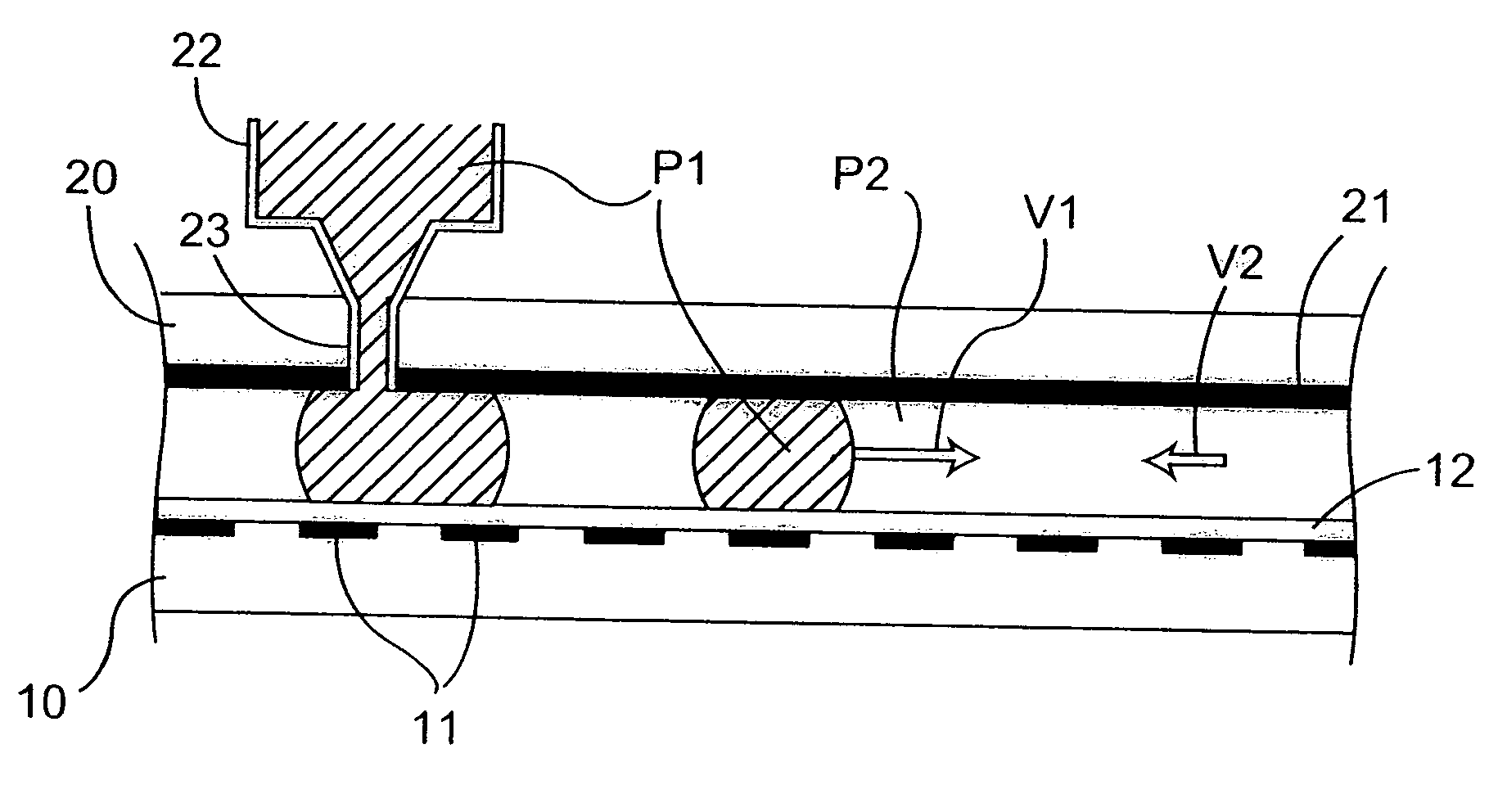
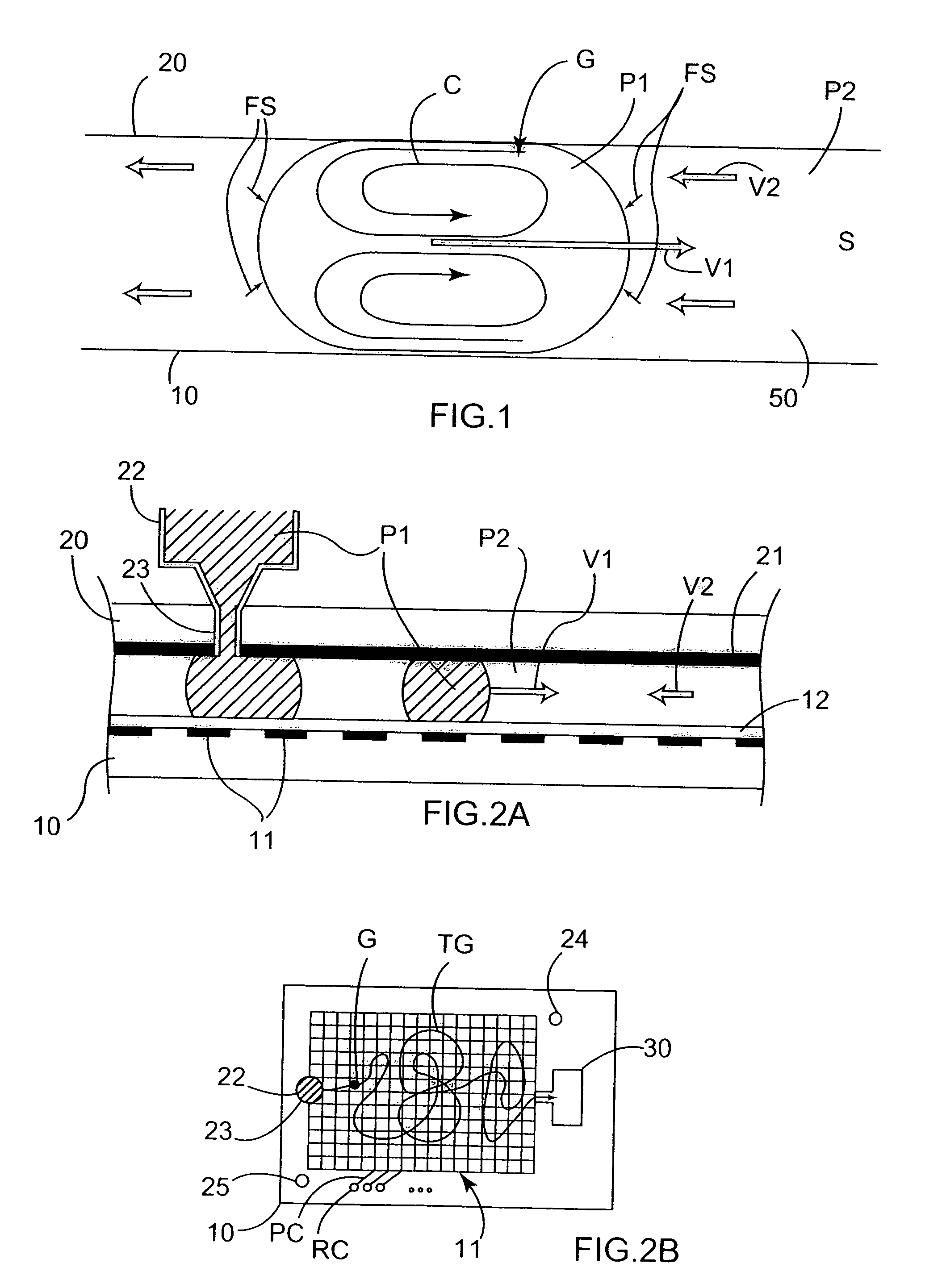
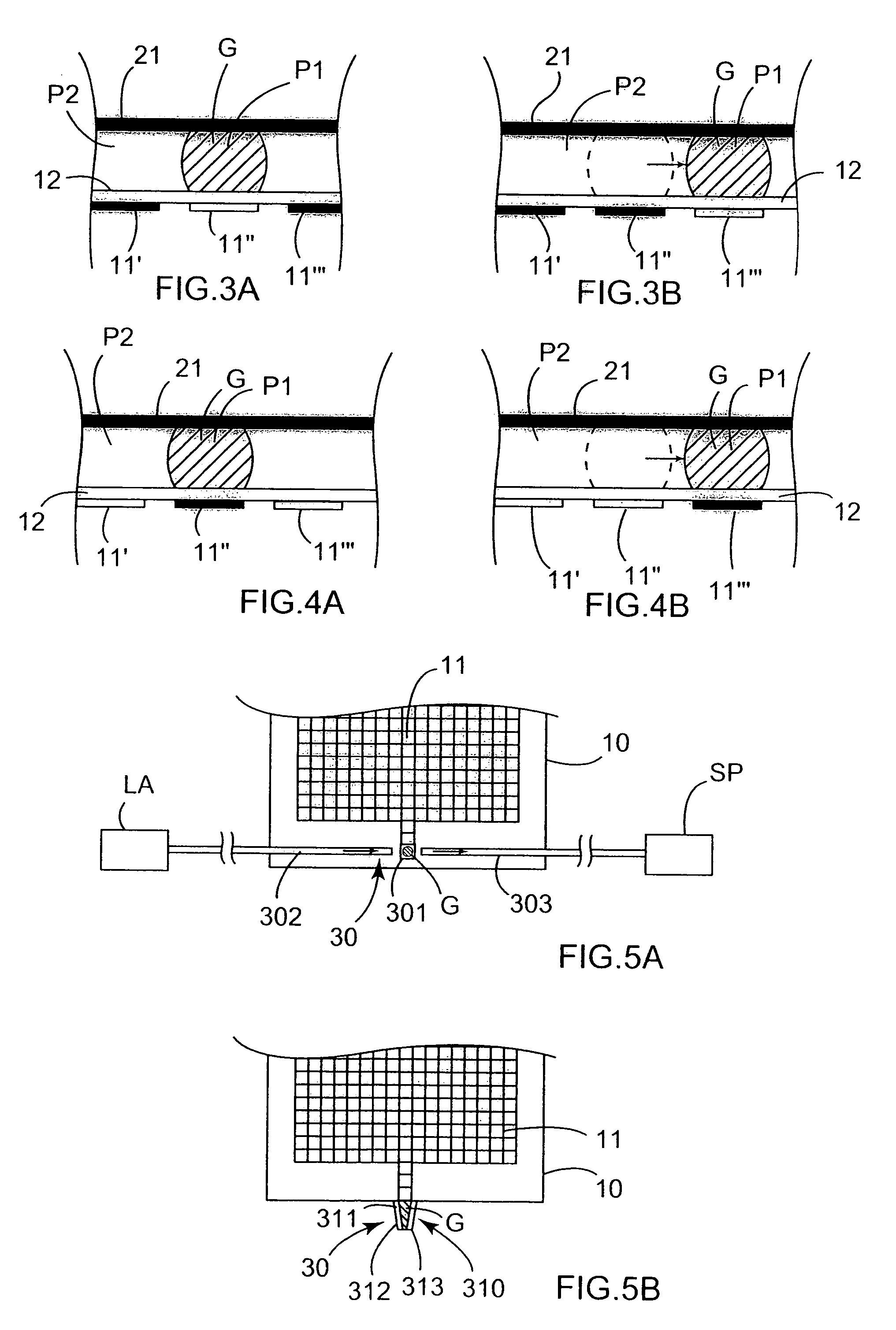
Microfluidic method and device for transferring mass between two immiscible phases
InactiveUS20060231398A1Mass productionLow costSludge treatmentVolume/mass flow measurementElectricityElectrophoresis
A method of transferring mass of at least one solute between a liquid first phase and a fluid second phase that is immiscible with the first phase, the method comprising moving at least one droplet of said liquid first phase in a microfluidic device by using electric-type forces (electrowetting or dielectrophoresis) within a space that is filled with said fluid second phase. Said droplet is preferably moved by said electric-type forces along a path between a point for injecting said droplet into said microfluidic device, and an extraction and / or analysis zone, said path being defined in such a manner that said droplet sweeps through a significant fraction of said space filled with said fluid second phase. The method may include a step of transferring said droplet using said electric-type forces to a chemical analysis device integrated in said microfluidic device, and a step of chemically analyzing said droplet. The invention also provides a device for implementing such a method.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
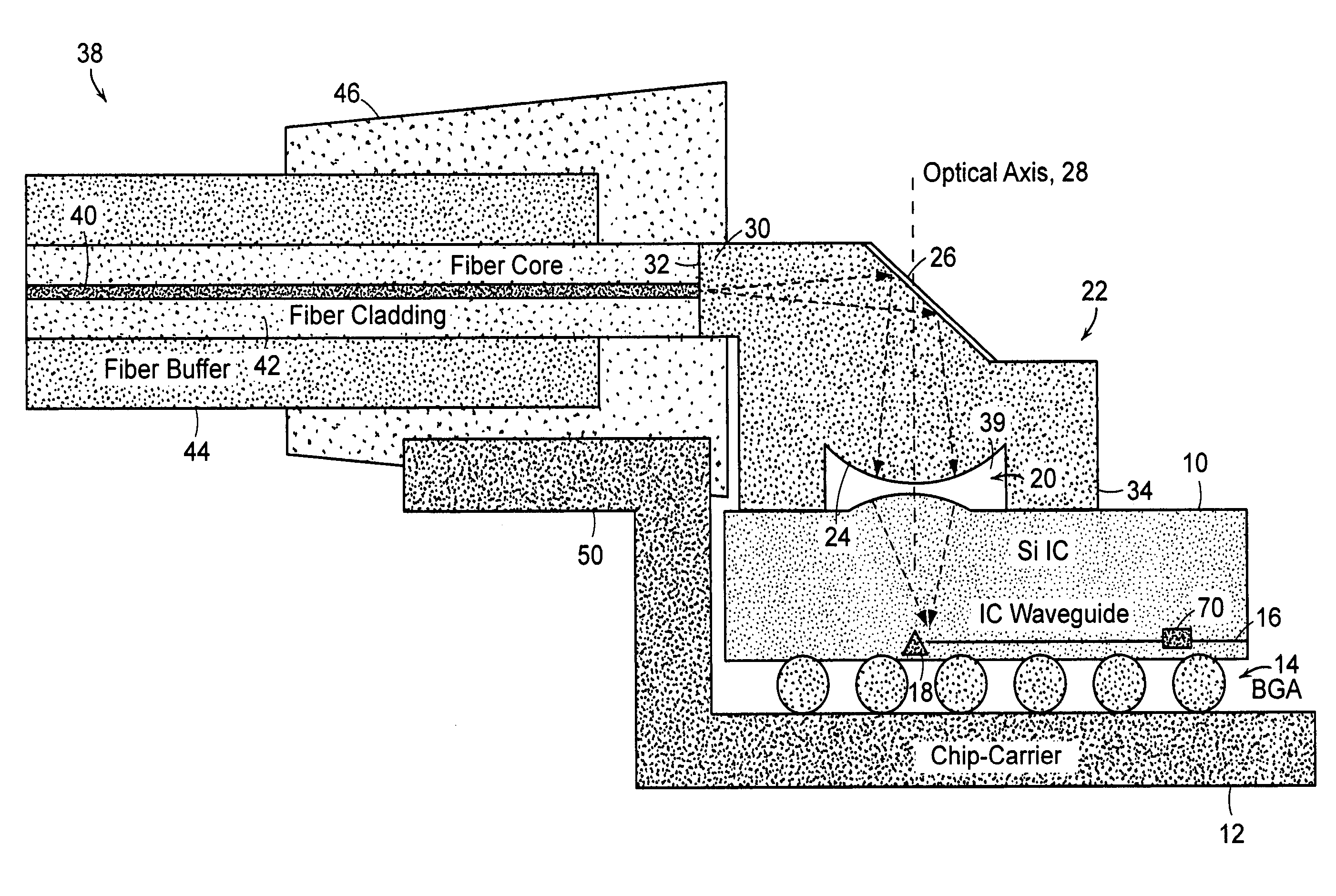
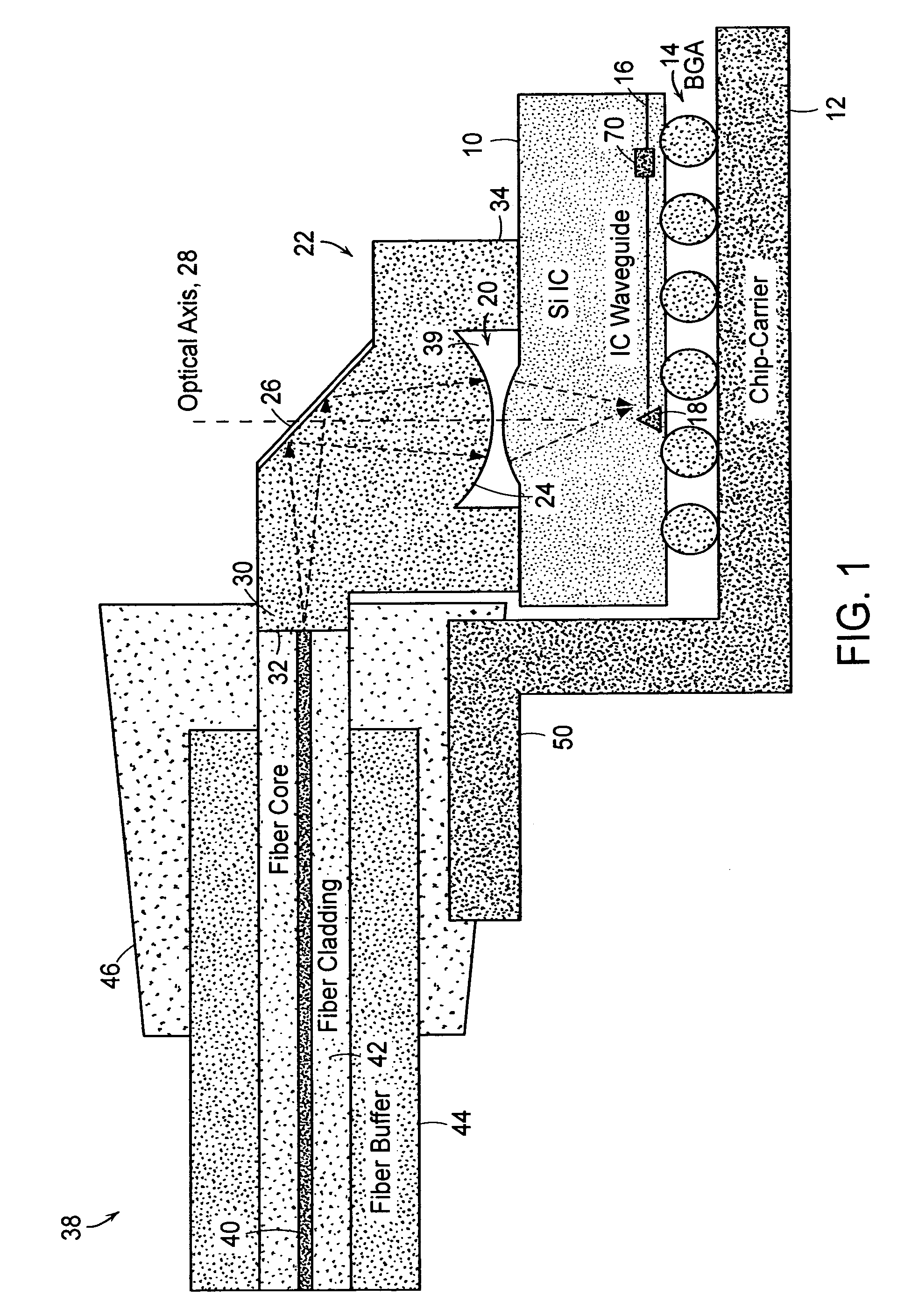
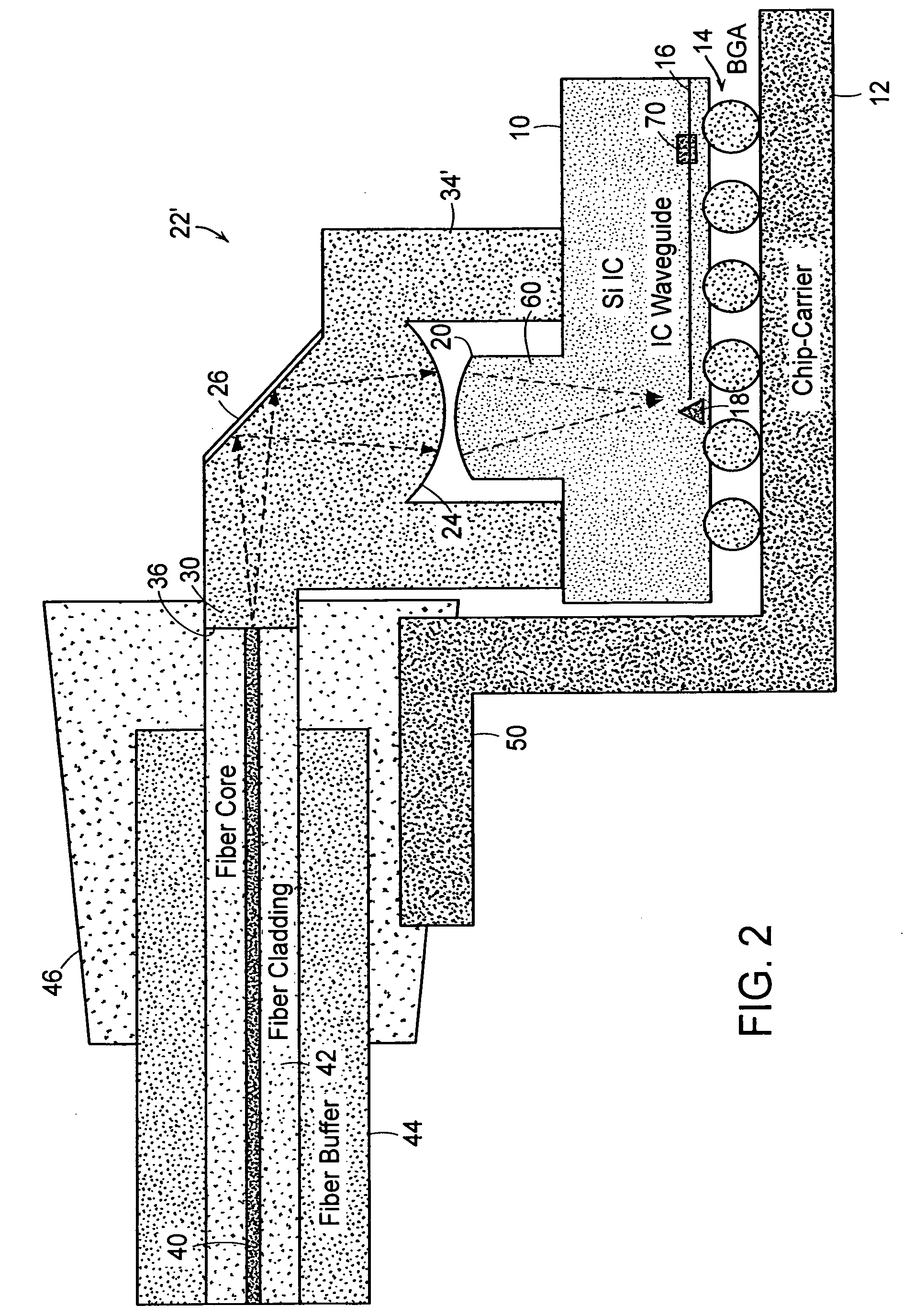
Optical coupling to IC chip
InactiveUS7298941B2Low costEnhanced couplingOptical articlesCoupling light guidesOptical couplerWaveguide
Owner:APPLIED MATERIALS INC
Device and method for enhancing transdermal flux of agents being delivered or sampled
A percutaneous agent delivery or sampling device comprising a sheet having a plurality of microblades for piercing and anchoring to the skin for increasing transdermal flux of an agent and for improving the attachment of the device to the skin.
Owner:ALZA CORP
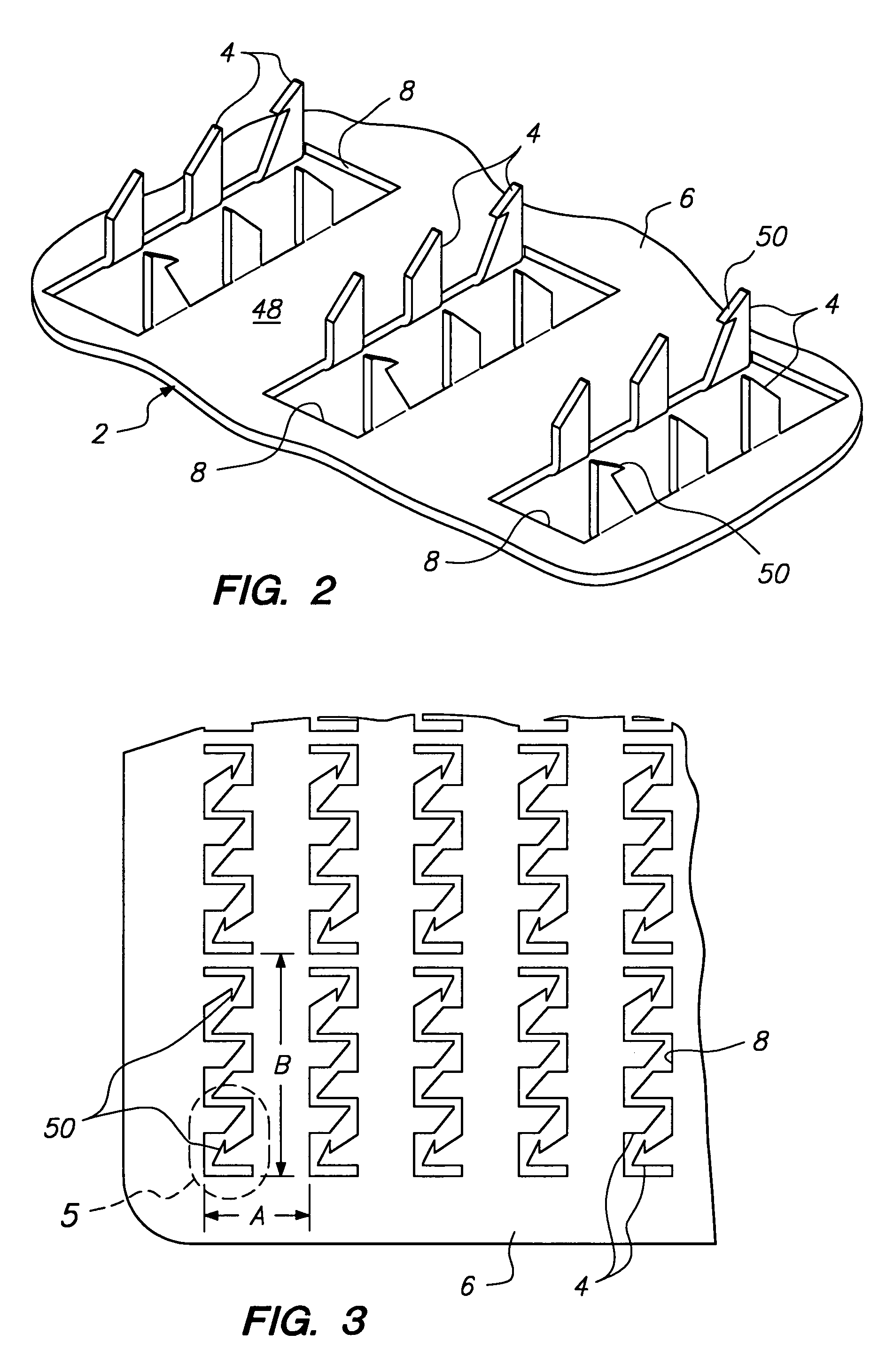
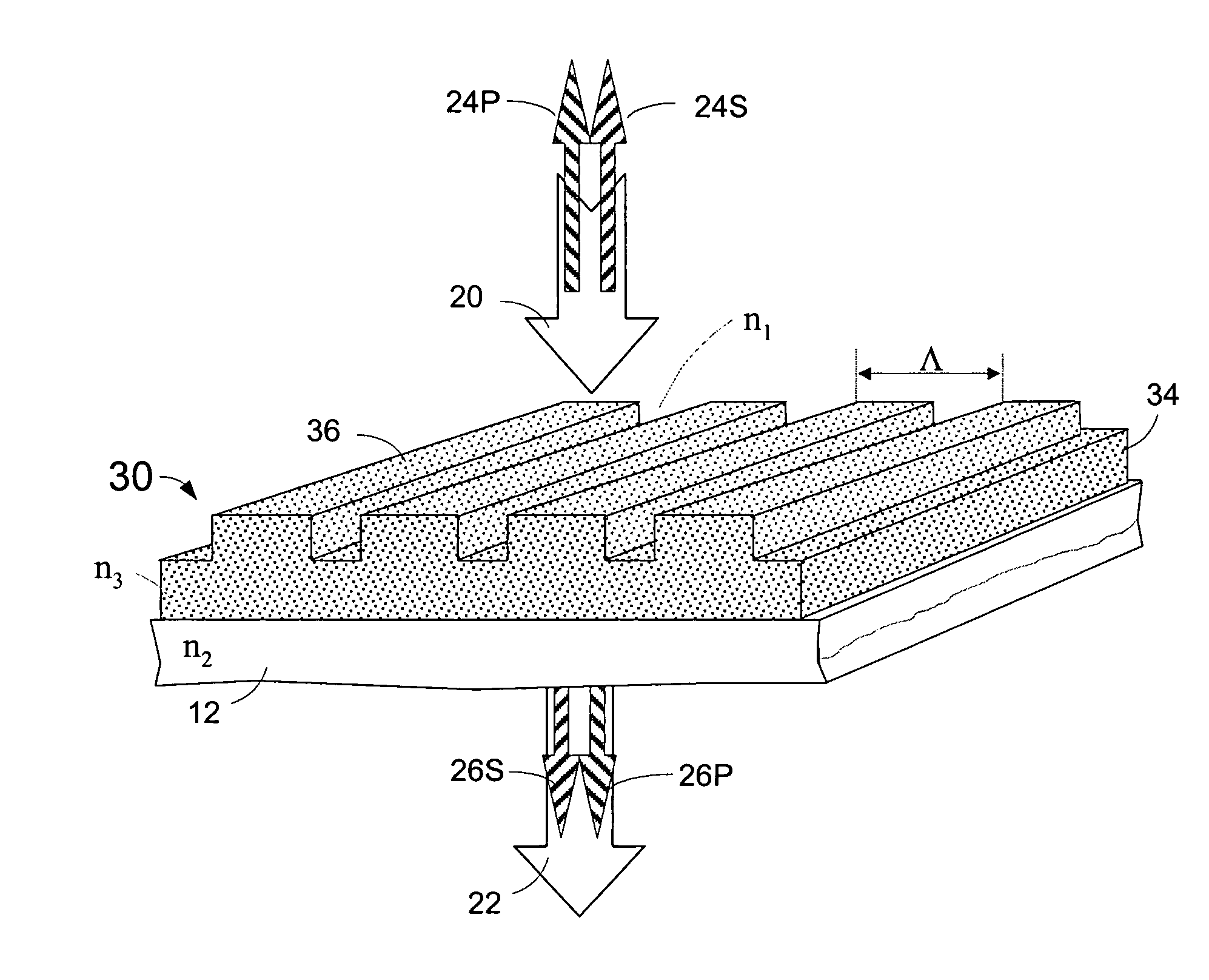
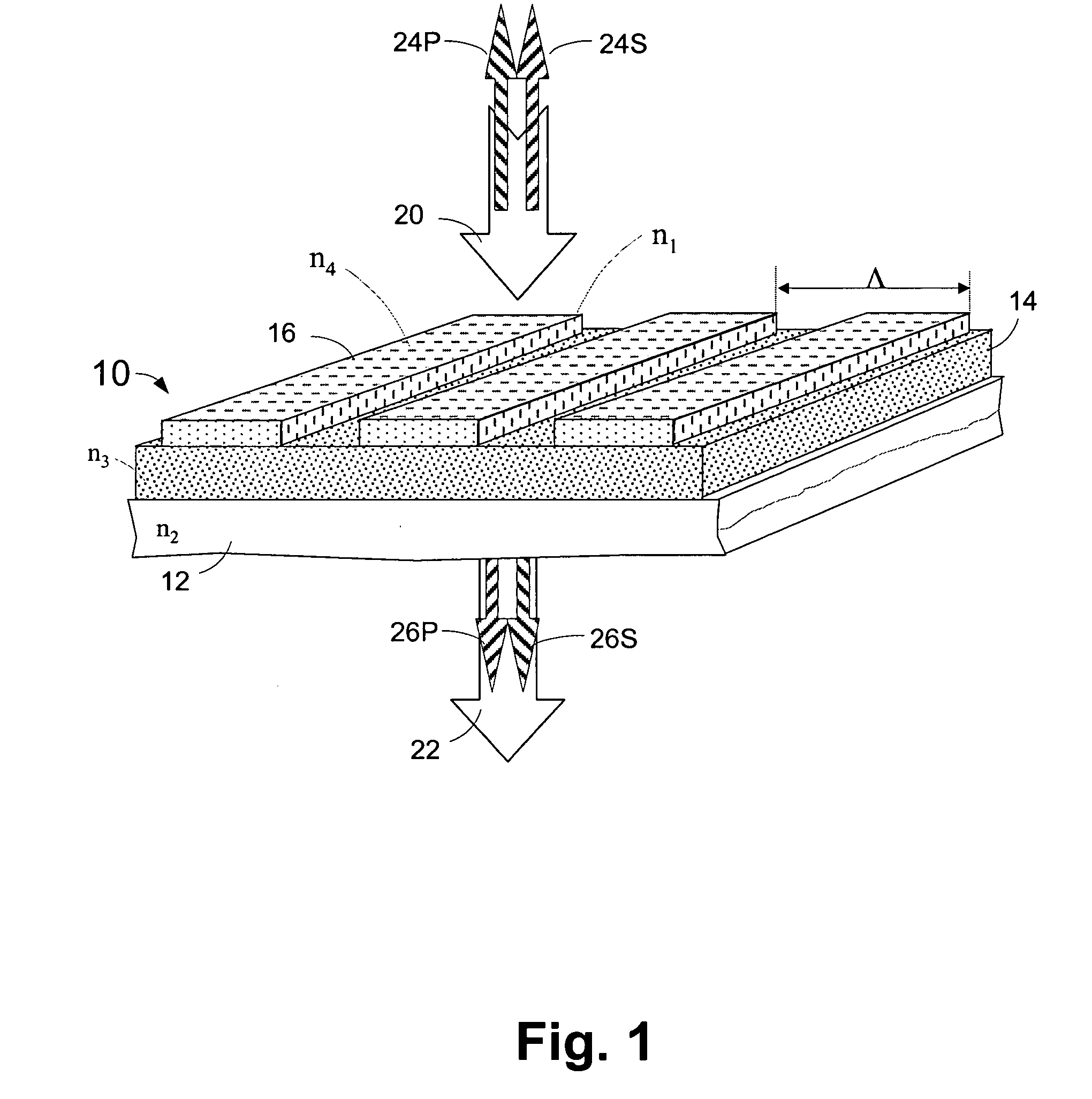
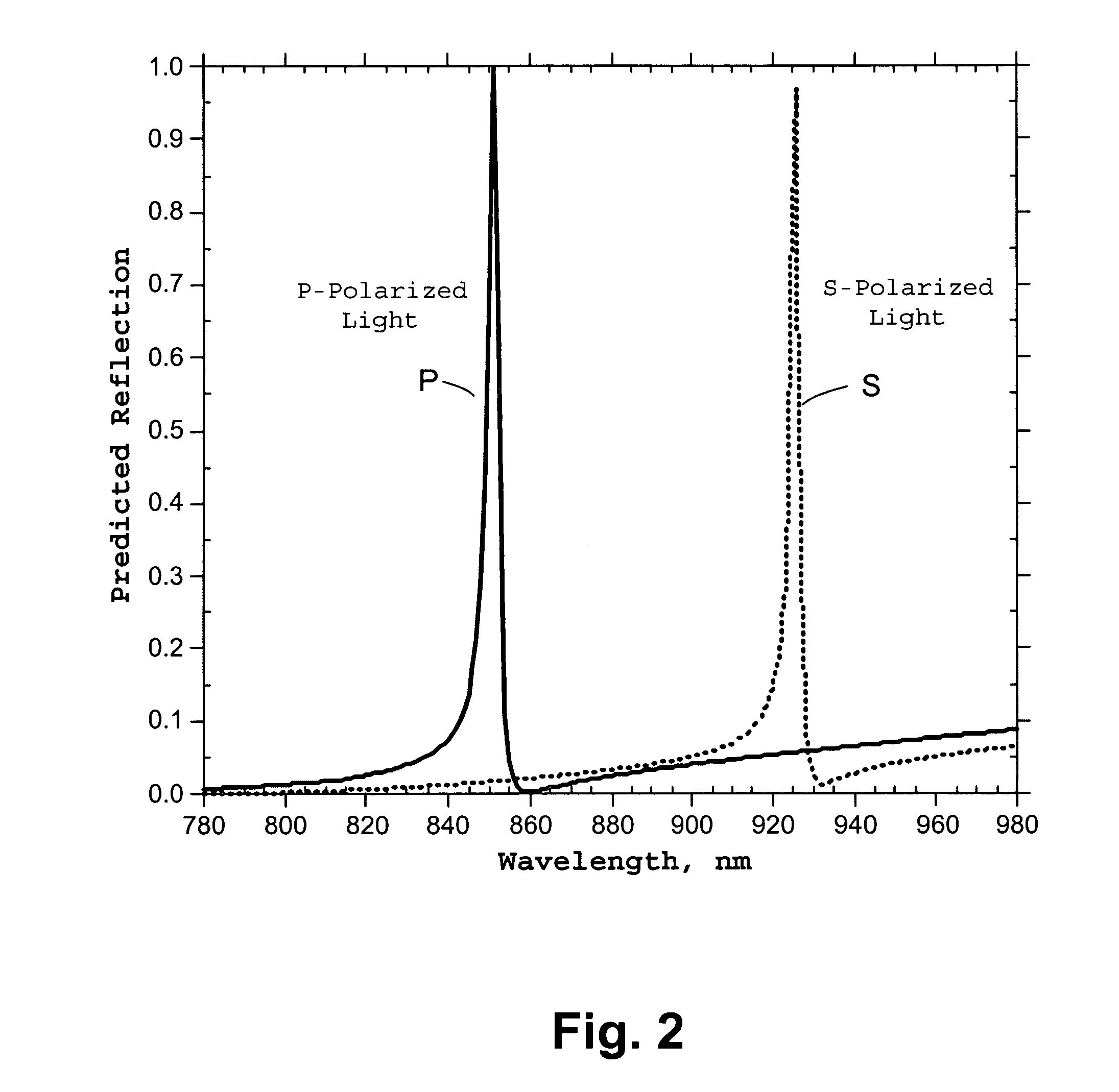
Microstructured optical device for polarization and wavelength filtering
InactiveUS20060262250A1Inexpensive to manufactureSimple and cheap to manufactureNon-linear opticsInformation cardsPolarizerSurface relief
A microstructure-based polarizer is described. The device acts as an electromagnetic wave filter in the optical region of the spectrum, filtering multiple wavelength bands and polarization states. The apparatus comprises a substrate having a surface relief structure containing dielectric bodies with physical dimensions smaller than the wavelength of the filtered electromagnetic waves, such structures repeated in an array covering at least a portion of the surface of the substrate. The disclosed structure is particularly useful as a reflective polarizer in a liquid crystal display, or as polarizing color filter elements at each pixel in a display. Other applications such as polarization encoded security labels, polarized room lighting, and color filter arrays for electronic imaging systems are made practical by the device.
Owner:HOBBS DOUGLAS S
Reconfigurable base station using mobile station RF ASIC
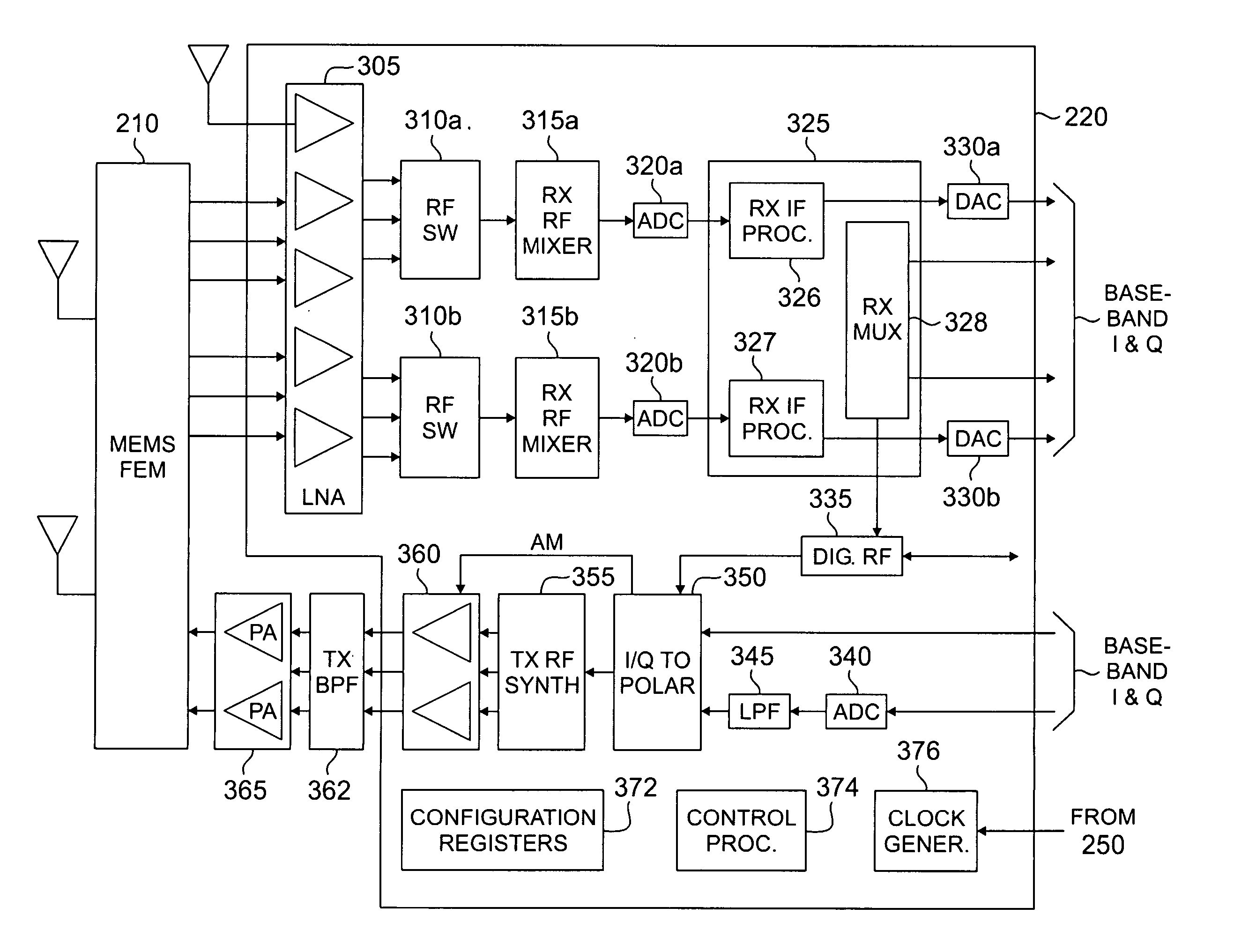
InactiveUS20060052124A1Low capacityHigh volume productionModulated-carrier systemsRadio/inductive link selection arrangementsFrequency bandTransceiver
A reconfigurable RF transceiver for use in either a base station or a mobile station Of a wireless network. The reconfigurable RF transceiver comprises a first receive path that down-converts an incoming RF signal to analog and digital baseband signals. The first receive path in a first mode down-converts the incoming RF signal in a receive frequency band for the base station and in a second mode down-converts the incoming RF signal in a receive frequency band for the mobile station. The reconfigurable RF transceiver further comprises a transmit path for up-converting an outgoing baseband signal to an outgoing RF signal. The transmit path in the first mode up-converts the outgoing baseband signal in a transmit frequency band for the base station and in the second mode up-converts the outgoing baseband signal in a transmit frequency band for the mobile station.
Owner:SAMSUNG ELECTRONICS CO LTD
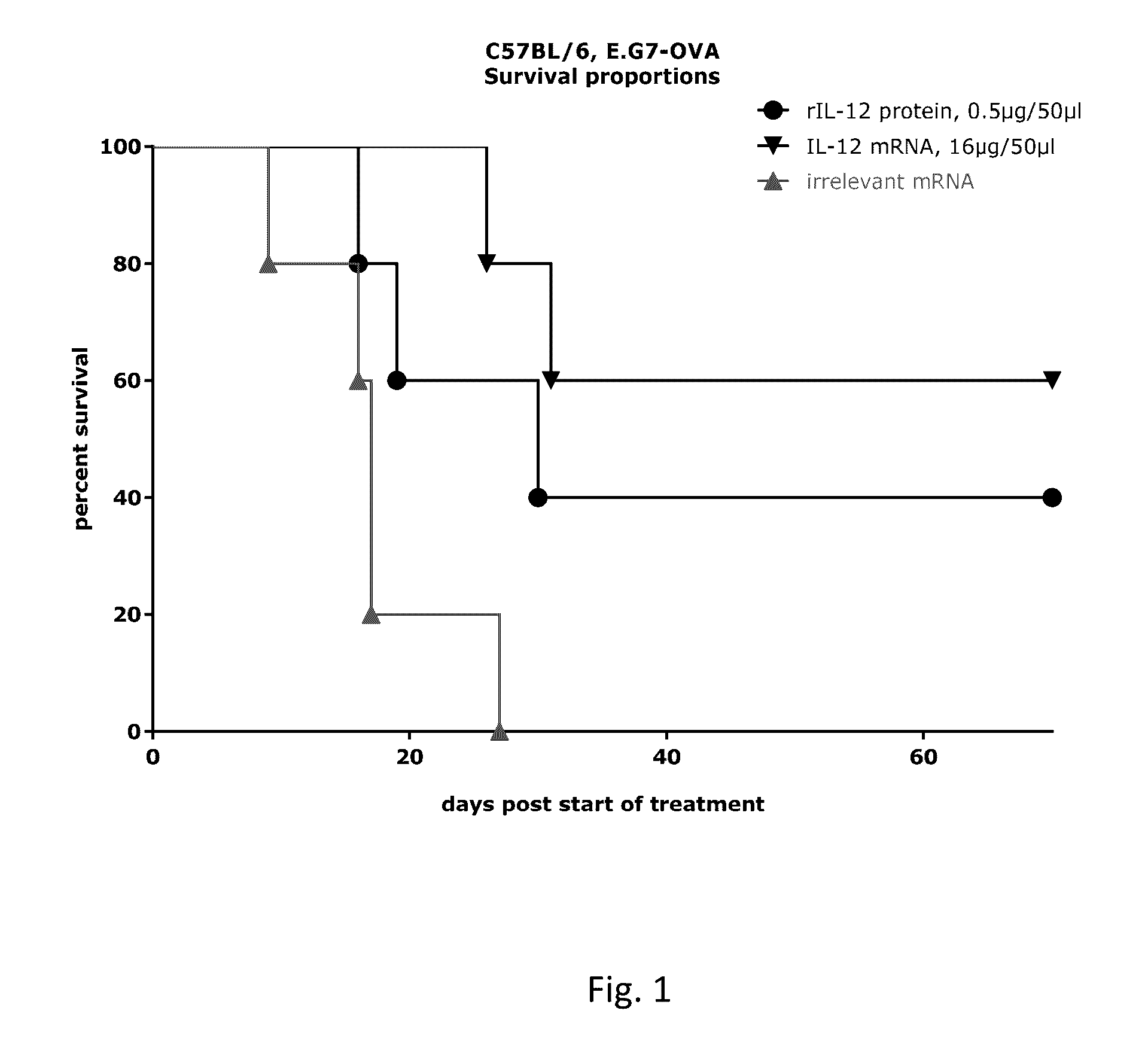
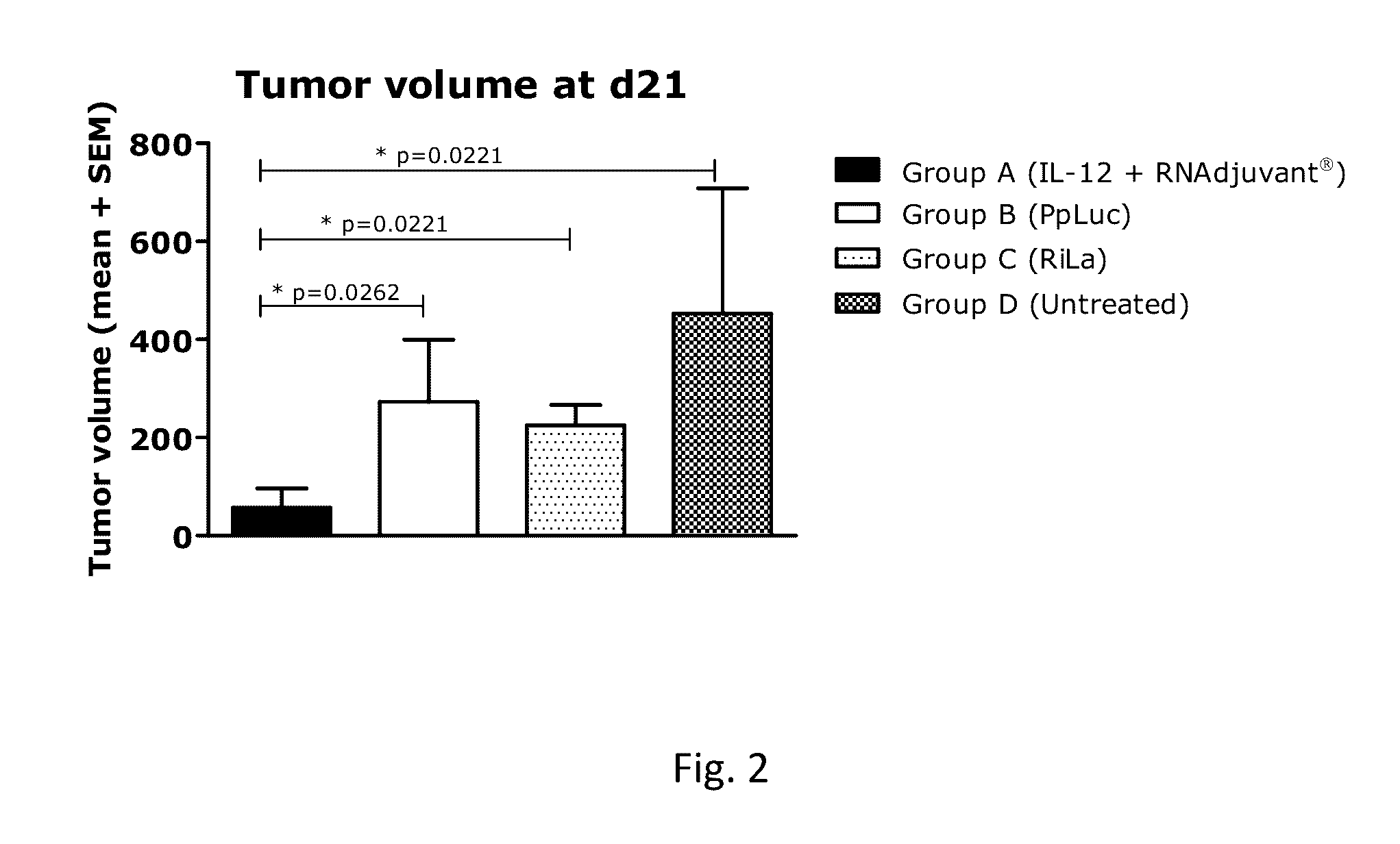
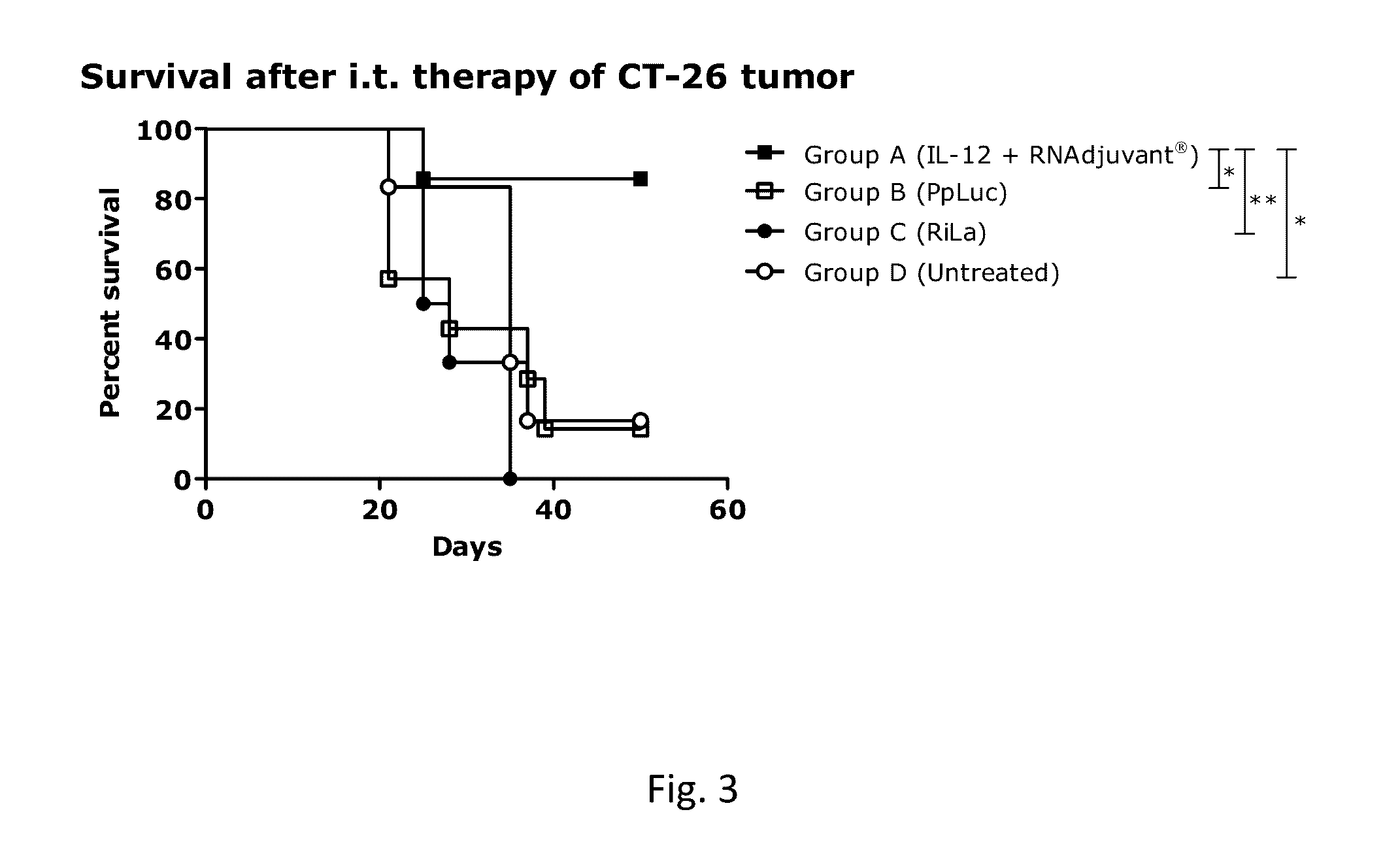
RNA containing composition for treatment of tumor diseases
ActiveUS20160331844A1Improve survivalEffectively treating tumor and/or cancer diseasesSsRNA viruses negative-senseOrganic active ingredientsDiseasePharmaceutical drug
Owner:CUREVAC SE
Universal battery module and controller therefor
InactiveUS7772799B2Easy to integrateMass productionCharge equalisation circuitCircuit monitoring/indicationEngineeringEmbedded system
A battery pack is provided including universal battery modules and a master control module. By selecting proper rated universal battery modules and connecting them either in series and / or parallel, a high performance and long life battery pack is assembled that is suitable for high power applications such as electrical vehicles whereby the master control module acts as the battery pack control and interface module.
Owner:DELAWARE POWER SYST CORP
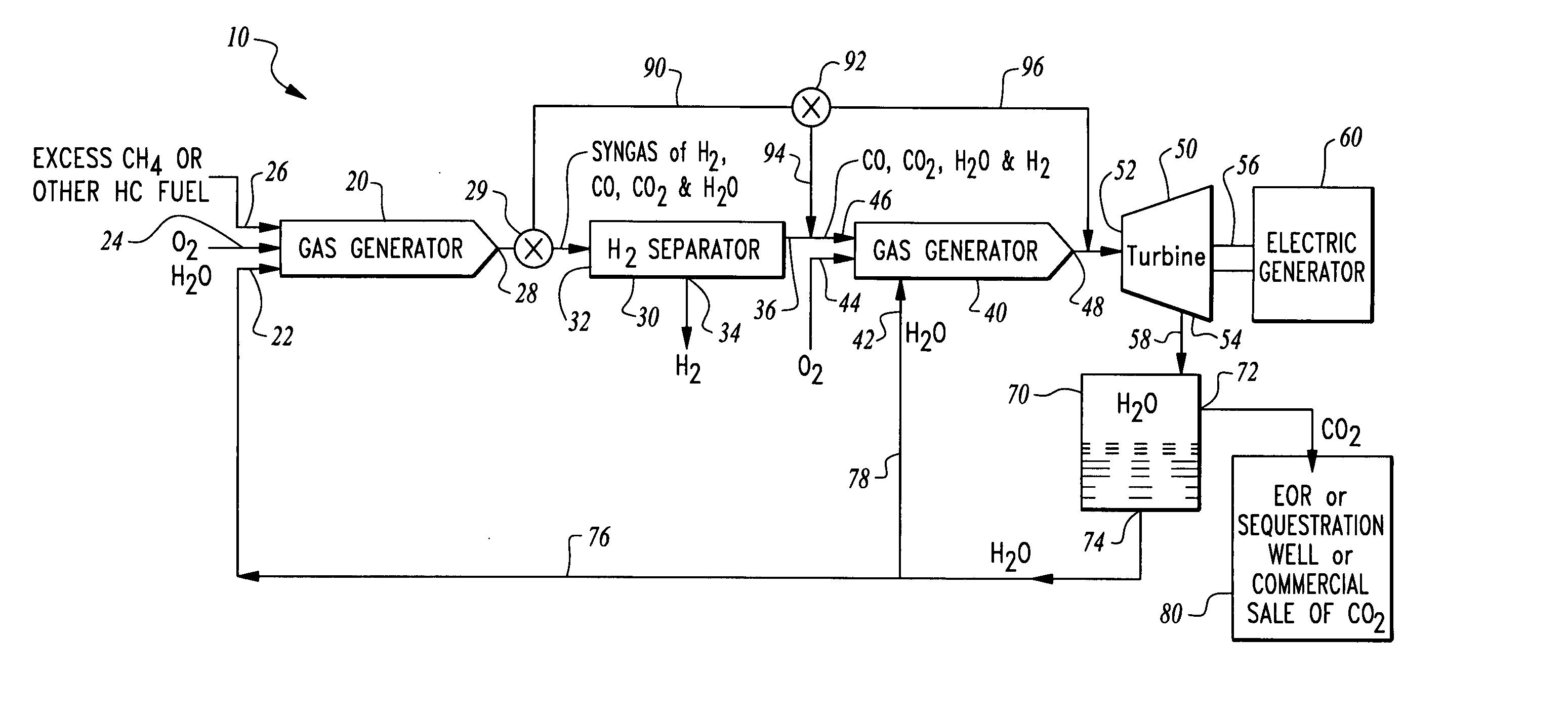
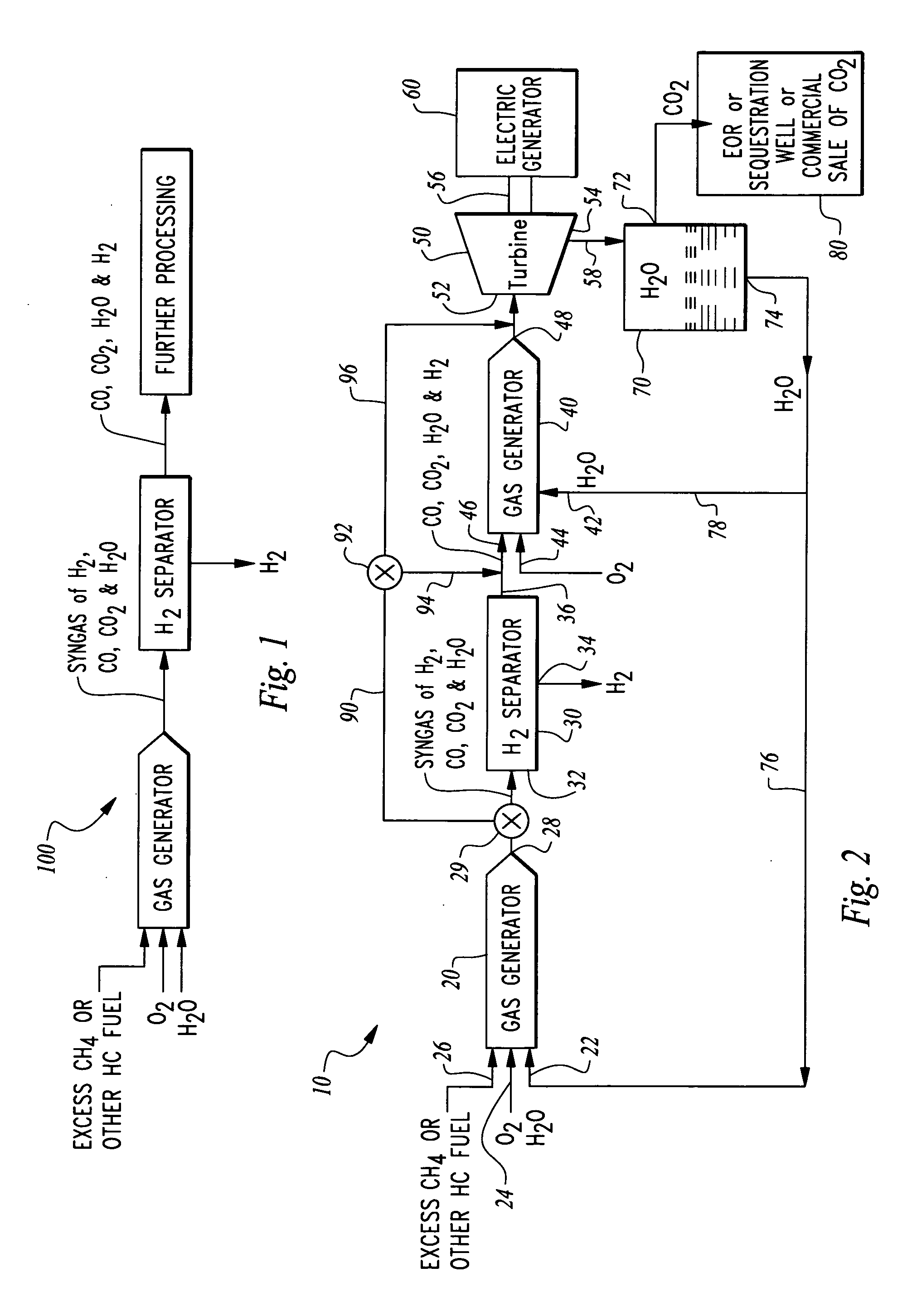
Hydrogen production from an oxyfuel combustor
InactiveUS20070044479A1Low costDemanded electric powerHydrogenGas turbine plantsChemistryCarbon dioxide
A system is provided for hydrogen production from a hydrogen and carbon containing fuel combusted within an oxyfuel combustor. The oxyfuel combustor combusts hydrogen and carbon containing fuel with oxygen at a non-stoichiometric ratio, typically fuel rich. In such an operating mode, products of combustion include steam, carbon dioxide, carbon monoxide and hydrogen. These products of combustion are then passed through a hydrogen separator where hydrogen is separated. Remaining products of combustion can be optionally combusted at a stoichiometric ratio with oxygen in a second oxyfuel combustor discharging substantially only steam and carbon dioxide. A turbine can be provided downstream from the gas generator to produce power and eliminate carbon monoxide from the system. The system can be operated in a second mode where the gas generator combusts the fuel with oxygen at a stoichiometric ratio to maximize electric power generation without hydrogen production at periods of peak electric power demand.
Owner:CLEAN ENERGY SYST
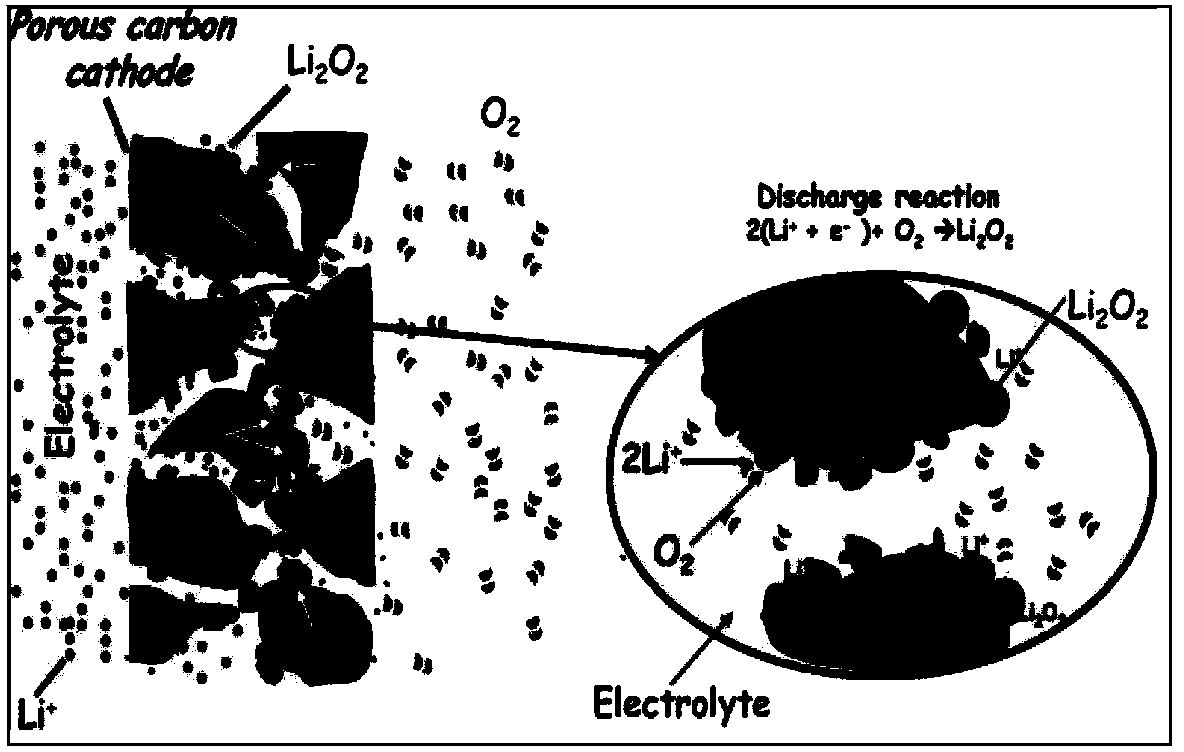
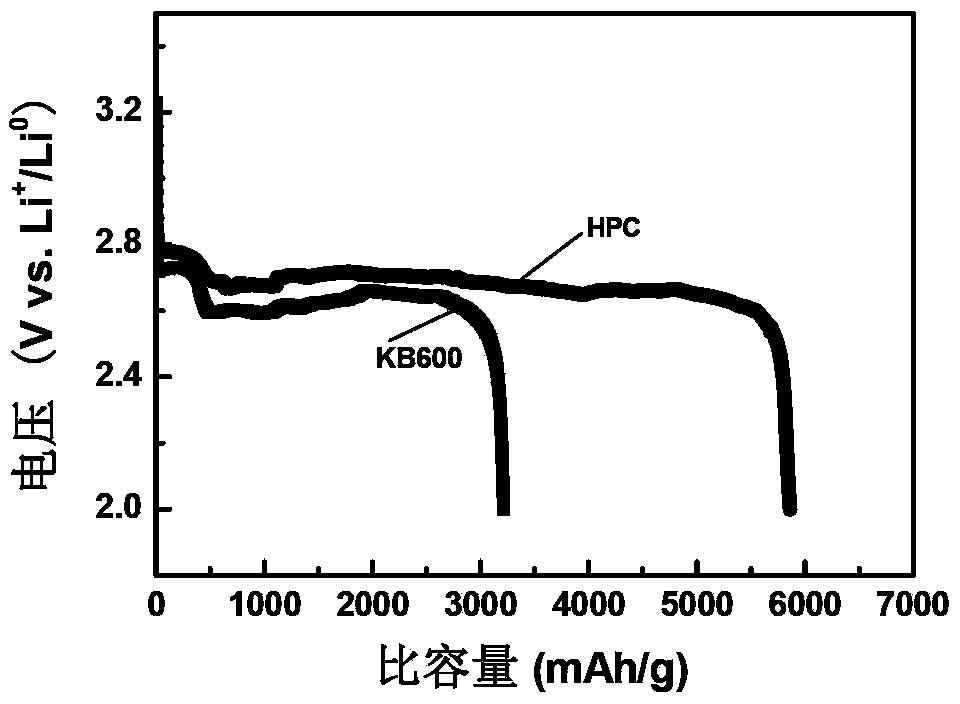
Nitrogen-doped porous carbon material for lithium-air battery positive electrode
ActiveCN103855366AHigh discharge specific capacityHigh voltage platformFuel and secondary cellsCell electrodesPorous carbonCharge discharge
The present invention relates to a nitrogen-doped porous carbon material for a lithium-air battery positive electrode, wherein the nitrogen-doped porous carbon material has an interconnected graded pore structure, N is uniformly doped in the C skeleton, N accounts for 0.2-15% of the carbon material atomic ratio, the graded pores comprise mass transfer pores and deposition holes, the deposition holes account for 40-95% of the total pore volume, and the mass transfer pores account for 4-55% of the total pore volume. According to the present invention, with application of the carbon material as the lithium-air battery electrode material, the space utilization rate of the carbon material during the charge-discharge process can be increased at a maximum, and the energy density and the power density of the lithium-air battery can be effectively increased; and the preparation process is simple, the material source is wide, the pore structure of the graded pore carbon material can be regulated, the regulation manner is diverse, and the nitrogen doping manner is easily achieved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Microfluidic method and device for transferring mass between two immiscible phases
InactiveUS8236156B2Mass productionLow costSludge treatmentVolume/mass flow measurementEngineeringPartial filling
A method of transferring mass of at least one solute between a liquid first phase and a fluid second phase that is immiscible with the first phase, the method comprising moving at least one droplet of said liquid first phase in a microfluidic device by using electric-type forces (electrowetting or dielectrophoresis) within a space that is filled with said fluid second phase. Said droplet is preferably moved by said electric-type forces along a path between a point for injecting said droplet into said microfluidic device, and an extraction and / or analysis zone, said path being defined in such a manner that said droplet sweeps through a significant fraction of said space filled with said fluid second phase. The method may include a step of transferring said droplet using said electric-type forces to a chemical analysis device integrated in said microfluidic device, and a step of chemically analyzing said droplet. The invention also provides a device for implementing such a method.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Protective shield for aircraft cockpit crew
InactiveUS20030052227A1Light weightEasy to installAir-treatment apparatus arrangementsFuselagesAircrewFlight vehicle
This invention relates generally to the field of aircraft technology and systems utilized for protection of aircraft, occupants, and operators thereof from hijackers, terrorists, and other anomalous problems while in flight. More particularly, the present invention relates to a device used in conjunction with walls, floor, and ceiling of a cockpit's entry / exit passageway for protecting the cockpit crew from weapons, hostility, decompression, and / or physical intrusions. A light weight protective shield for the cockpit of an aircraft (i.e. bullet-proof door) along with a internal locking and release device, none of which is provided by prior art. The protective shield can absorb repeated blows, provide pressure relief in the event of aircraft decompression, resist penetration of firearms, knives, and explosive devices, and is compliant with FAA regulations. The protective shield provides a new level of protection for the crew of a passenger or cargo airplane and protects the cockpit of an aircraft from hijacking attempts as well as other anomalous events and is comprised of a light weight weapon-proof protective shield that when closed fits snugly with the flight deck's adjoining floor, walls, and ceiling. This protective shield system is intended to readily install inside existing aircraft (e.g. over-night retrofit installation) or be installed into new aircraft as they are assembled.
Owner:PITTMAN DONALD MERVE
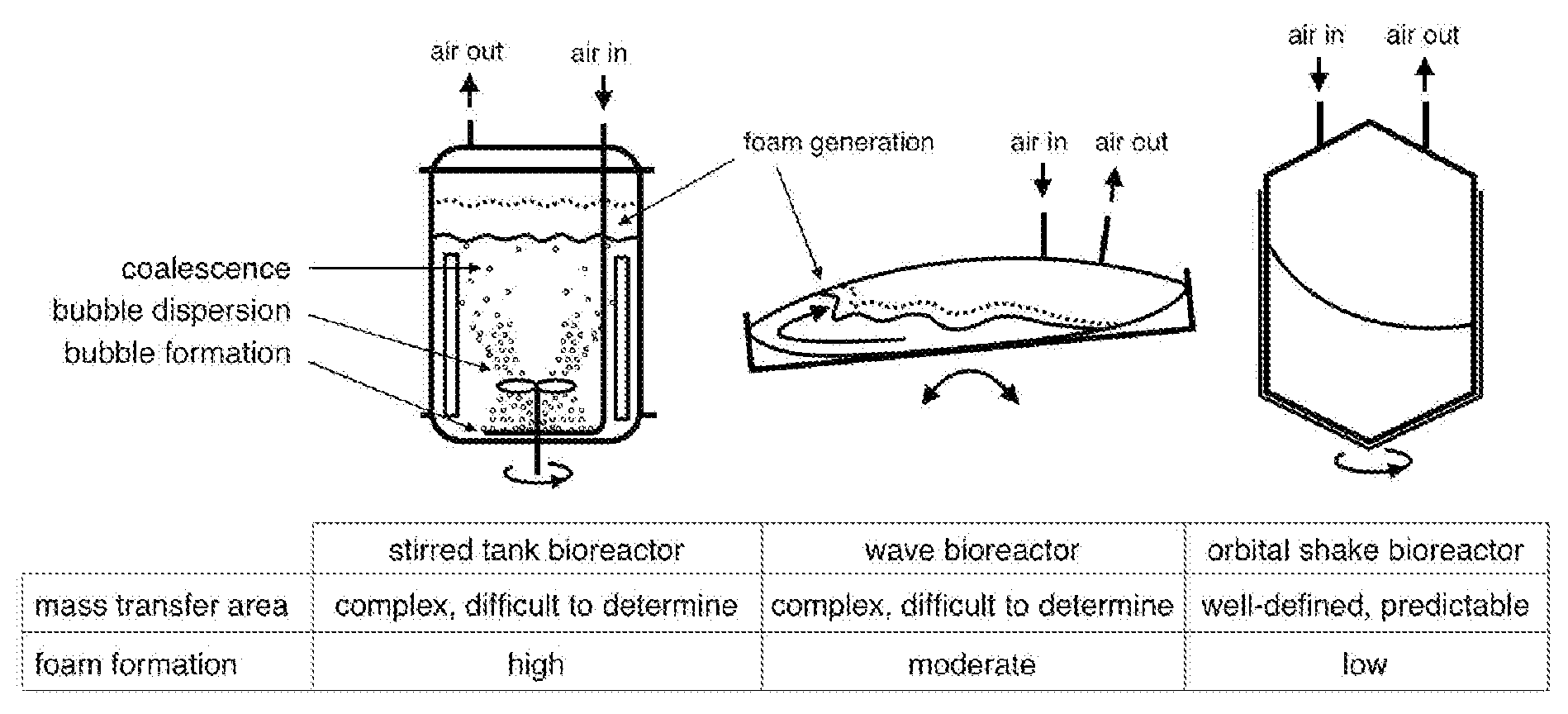
Cell cultivation and production of recombinant proteins by means of an orbital shake bioreactor system with disposable bags at the 1,500 liter scale
InactiveUS20090233334A1High degreeEasy to handleMicroorganismsBiochemistry apparatusCulture cellBiology
The present invention provides a novel method for culturing cells as well as a novel method for producing a recombinant protein by culturing cells at large scale (up to 1,500 L nominal volume and 750 L working volume), whereby an inflated bag provides a sterile, disposable cultivation chamber. The inflated bag is partially filled with liquid cultivation media and cells, and placed into a containment vessel. The containment vessel is positioned onto an orbitally shaken platform. The orbital shaking moves the containment vessel and thus the bag and induces thereby motion to the liquid contained therein (“shake mixing”). This motion (caused by orbital shaking) induces a dynamic force field that ensures cell suspension, bulk mixing, and oxygen transfer from the liquid surface to the respiring cells without damaging shear or foam generation.
Owner:EXCELLGENE SA
Photoconductor on active pixel image sensor
InactiveUS20040036010A1Low costMass productionTelevision system detailsTelevision system scanning detailsSemiconductor materialsSpectral response
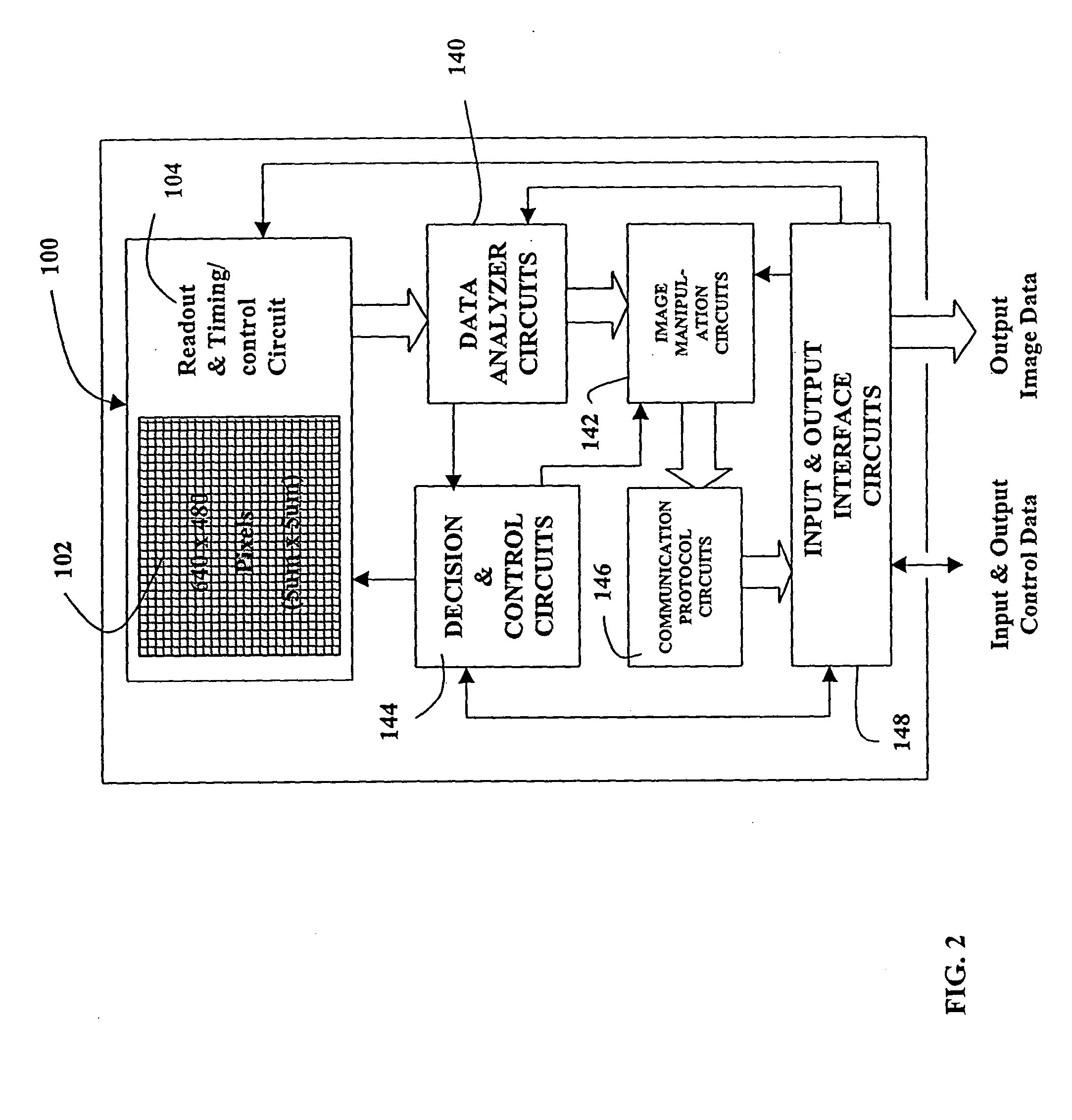
A MOS or CMOS based photoconductor on active pixel image sensor. Thin layers of semi-conductor material, doped to PIN or NIP photoconducting layers, located above MOS and / or CMOS pixel circuits produce an array of layered photodiodes. Positive and negative charges produced in the layered photodiodes are collected and stored as electrical charges in the MOS and / or CMOS pixel circuits. The present invention also provides additional MOS or CMOS circuits for reading out the charges and for converting the charges into images. With the layered photodiode of each pixel fabricated as continuous layers of charge generating material on top of the MOS and / or CMOS pixel circuits, extremely small pixels are possible with almost 100 percent packing factors. MOS and CMOS fabrication techniques permit sensor fabrication at very low costs. In preferred embodiments all of the sensor circuits are incorporated on or in a single crystalline substrate along with the sensor pixel circuits. Techniques are disclosed for tailoring the spectral response of the sensor for particular applications.
Owner:E PHOCUS
Method of manufacturing devices to protect election components
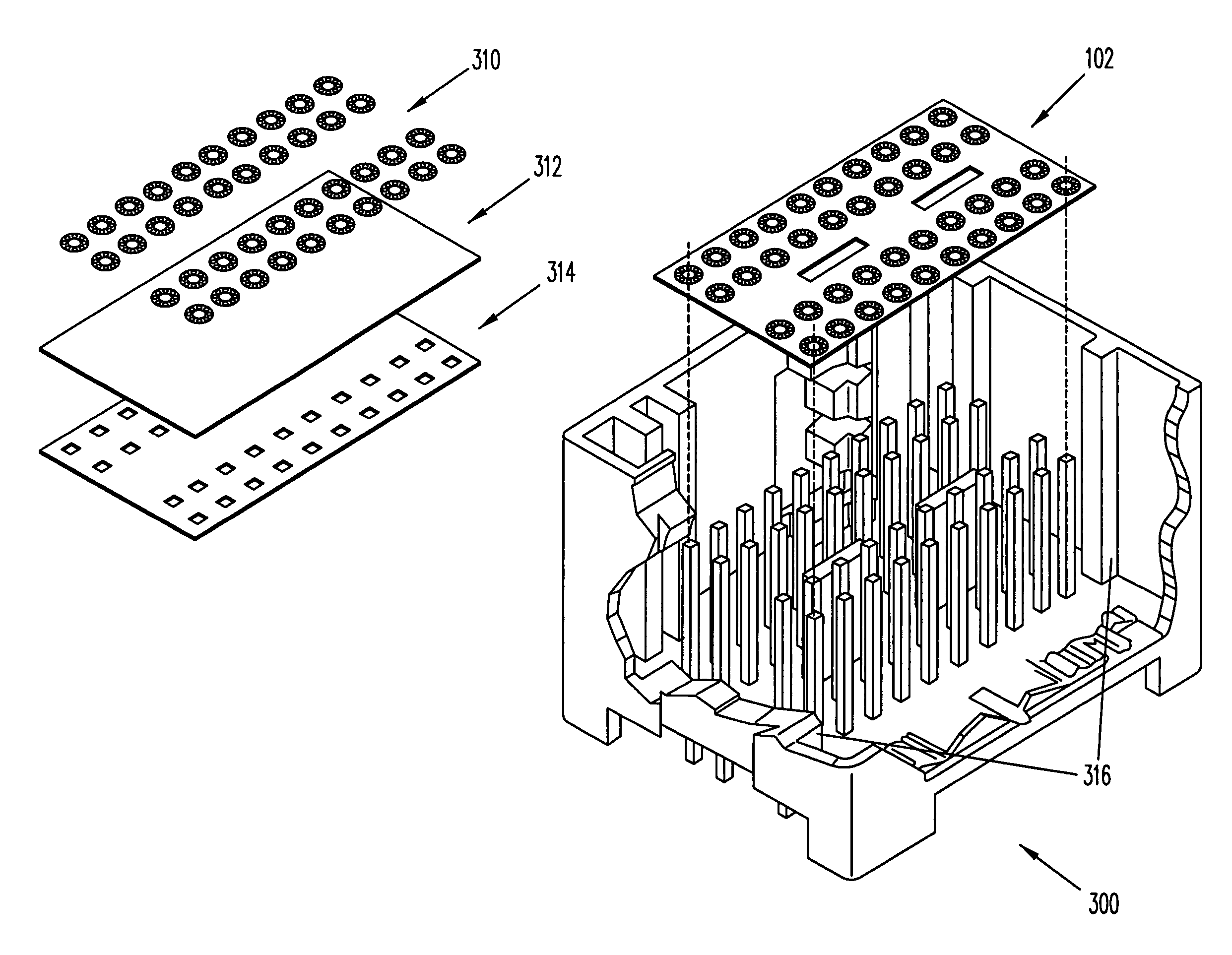
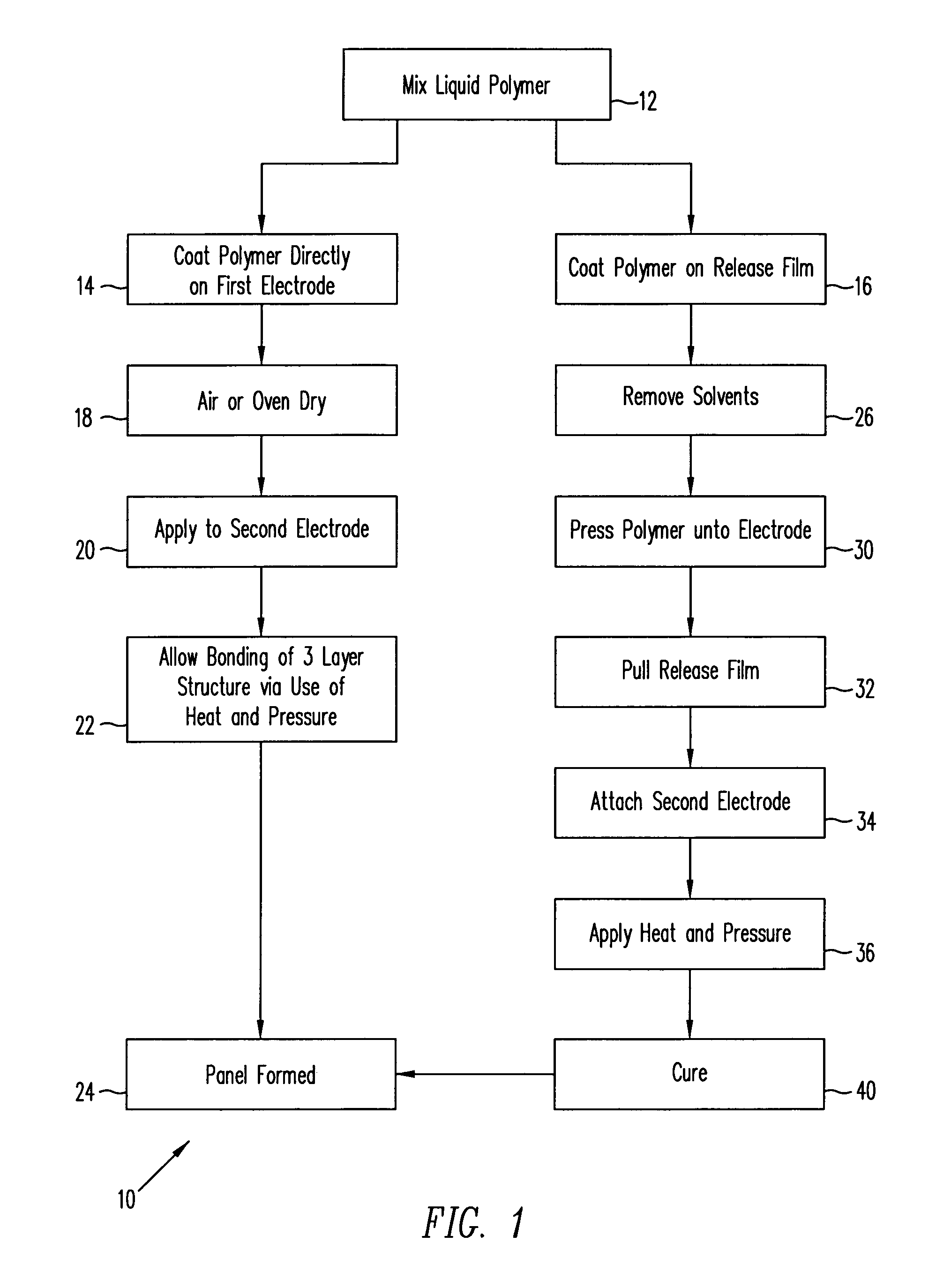
InactiveUS6981319B2Easy to customizeMake fastFuse device manufactureInsulating layers/substrates workingManufacturing technologyElectronic component
Devices capable of protecting electronic components during the occurrence of a disturbance event using printed circuit board manufacturing techniques. A three (3) layer structure is formed comprising a polymer-based formulation sandwiched between two electrode layers. The devices can be manufactured in panel form providing high quantities of devices which can be removed from the panel and applied directly to the component to be protected. Desired patterns can be formed on either one of the electrode layers by photo-etch techniques thereby providing a process that can be tailored to a large number of applications.
Owner:ELECTRONICS POLYMERS NEWCO
Ceramic particle reinforced composite wear-resistant part and preparation method thereof
InactiveCN101898238ASolve the problem of difficult penetrationImprove wear resistanceCeramic particleWear resistant
The invention relates to a ceramic particle reinforced composite wear-resistant part and a preparation method thereof. The method comprises the following steps: ceramic particles and metal powder are mixed evenly to fill in a special mould; then the mixture along with the mould is placed in a vacuum sintering furnace to sinter, wherein metal powder and ceramic particles are bound together to form a perform; after cooled, the mould is opened to take the perform out and place the perform on the side of the end face of a casting mold cavity; parent metal material is smelted by a medium-frequency induction furnace to form molten metal; during the casting, the molten metal is poured, metal powder in the perform is molten to form a cast-penetration path under the heat of molten metal, thus the molten metal can easily penetrate ceramic particles to form particle reinforced composite material in situ; and the surface layer of the obtained wear-resistant part consists of parent metal and composite material. The wear resistance of the composite material prepared by the method of the invention is ensured and the composite material has high impact resistance.
Owner:XI AN JIAOTONG UNIV +1
Hydrogen production from hydro power
InactiveUS6841893B2Economic valueMass productionLevel controlFinal product manufactureWater flowEngineering
A method is provided for operating a hydroelectric power generating facility configured for operating in first and second operating modes. The facility includes a turbine driven power generating unit receiving a flow of water through an upstream conduit to generate electrical power. The method comprises computing a first economic value for the generated electrical power when operating in the first operating mode, and computing a second economic value for the generated electrical power when operating in the second operating mode. The method further comprises comparing the first economic value with the second economic value to identify the operating mode that provides the higher economic value, and operating the turbine facility in the identified operating mode.
Owner:VOITH SIEMENS HYDRO POWER GENERATION
Id Label, Id Card, and Id Tag
As a non-contact ID label, ID tag and the like being widespread, it is required to manufacture a considerable quantity of ID labels at quite a low cost. An ID label attached to a product is, for example, required to be manufactured at 1 to several yens each, or preferably less than one yen. Thus, such a structure and a process are demanded that an ID label can be manufactured in a large quantity at a low cost. A thin film integrated circuit device included in the ID label, the ID card, and the ID tag of the invention each includes a thin film active element such as a thin film transistor (TFT). Therefore, by peeling a substrate on which TFTs are formed for separating elements, the ID label and the like can be manufactured in a large quantity at a low cost.
Owner:SEMICON ENERGY LAB CO LTD
Semiconductor light emitting device
ActiveUS20080191215A1Minimizes reflectionMinimizes absorptionSemiconductor devicesActive layerLight emitting device
There is provided a semiconductor light emitting device that minimizes reflection or absorption of emitted light, maximizes luminous efficiency with the maximum light emitting area, enables uniform current spreading with a small area electrode, and enables mass production with high reliability and high quality. A semiconductor light emitting device according to an aspect of the invention includes first and second conductivity type semiconductor layers, an active layer formed therebetween, first electrode layer, and a second electrode part electrically connecting the semiconductor layers. The second electrode part includes an electrode pad unit, an electrode extending unit, and an electrode connecting unit connecting the electrode pad unit and electrode extending unit.
Owner:SAMSUNG ELECTRONICS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com