Patents
Literature
39 results about "Pneumovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A genus of the family PARAMYXOVIRIDAE (subfamily PNEUMOVIRINAE) where the human and bovine virions have neither hemagglutinin nor neuraminidase activity. RESPIRATORY SYNCYTIAL VIRUS, HUMAN is the type species.
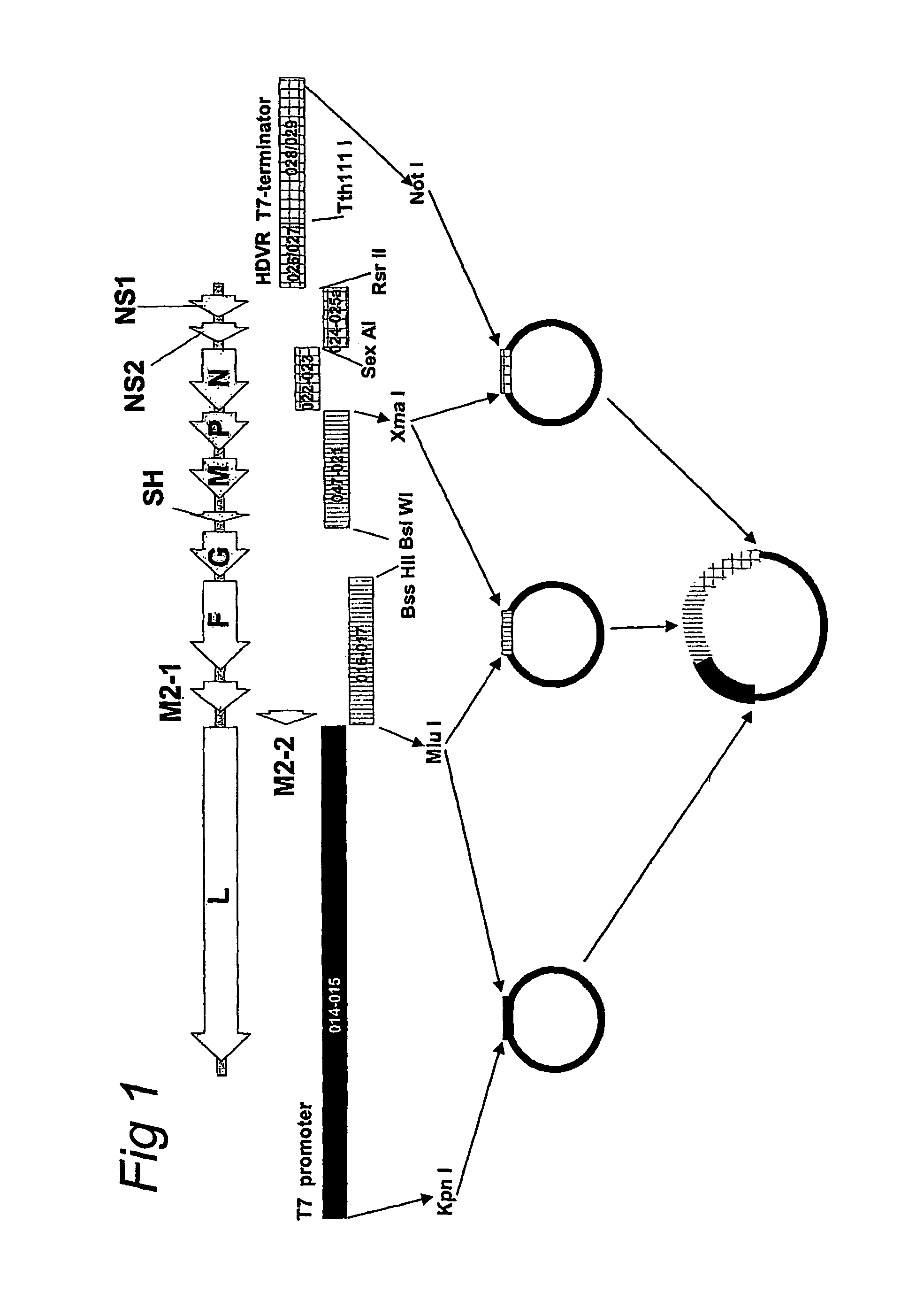
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
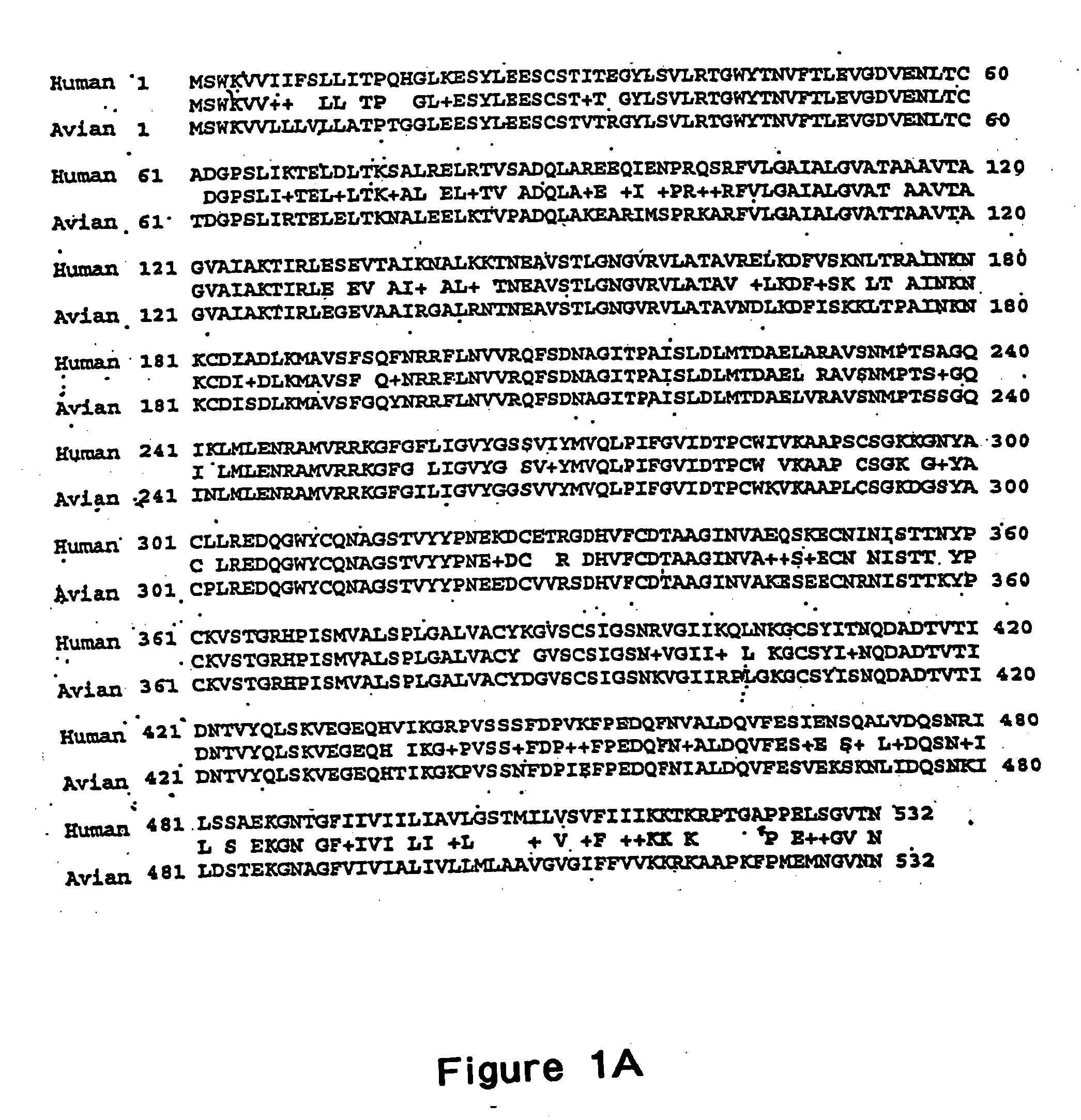
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
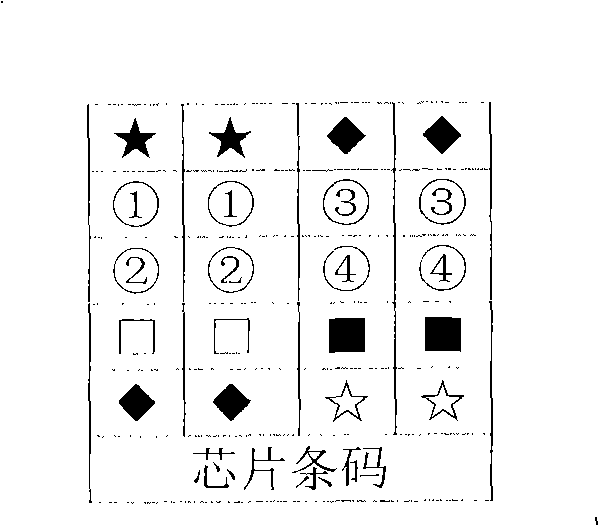
Gene chip for detecting horse infective virus, preparation, detecting method and reagent kit
InactiveCN101348838AAccurate acquisitionReasonableBioreactor/fermenter combinationsBiological substance pretreatmentsEquine infectious anemiaRabies virus
The invention relates to a gene chip for detecting pathogen of equine infectious virus. The gene chip comprises a solid phase carrier and an oligonucleotide probe fixed on the carrier, wherein the oligonucleotide probe comprises a detection probe and a quality control probe; and the detection probe is a nucleic acid fragment selected from the nucleotide sequences of rabies virus, equine infectious arteritis virus, equine infectious rhinopneumonitis virus and equine infectious equine infectious anemia virus. The invention also relates to a method for preparing the gene chip and a method for detecting four equine infectious diseases viruses by means of the chip, comprising the steps of probe synthesis, chip preparation and hybridization with a treated and labeled to-be-detected sample. The invention further relates to a reagent kit which is used for detecting pathogen of equine infectious virus and consists of the chip, a sample treatment reagent, a hybridization reagent, a colour-producing reagent and a specification. The gene chip can detect four viruses at the same time, and has the advantages of simple operation, high accuracy and strong repeatability, and the gene chip is of great significance to both laboratory research and production practice.
Owner:IPE BIOTECHNOLOGY CO LTD
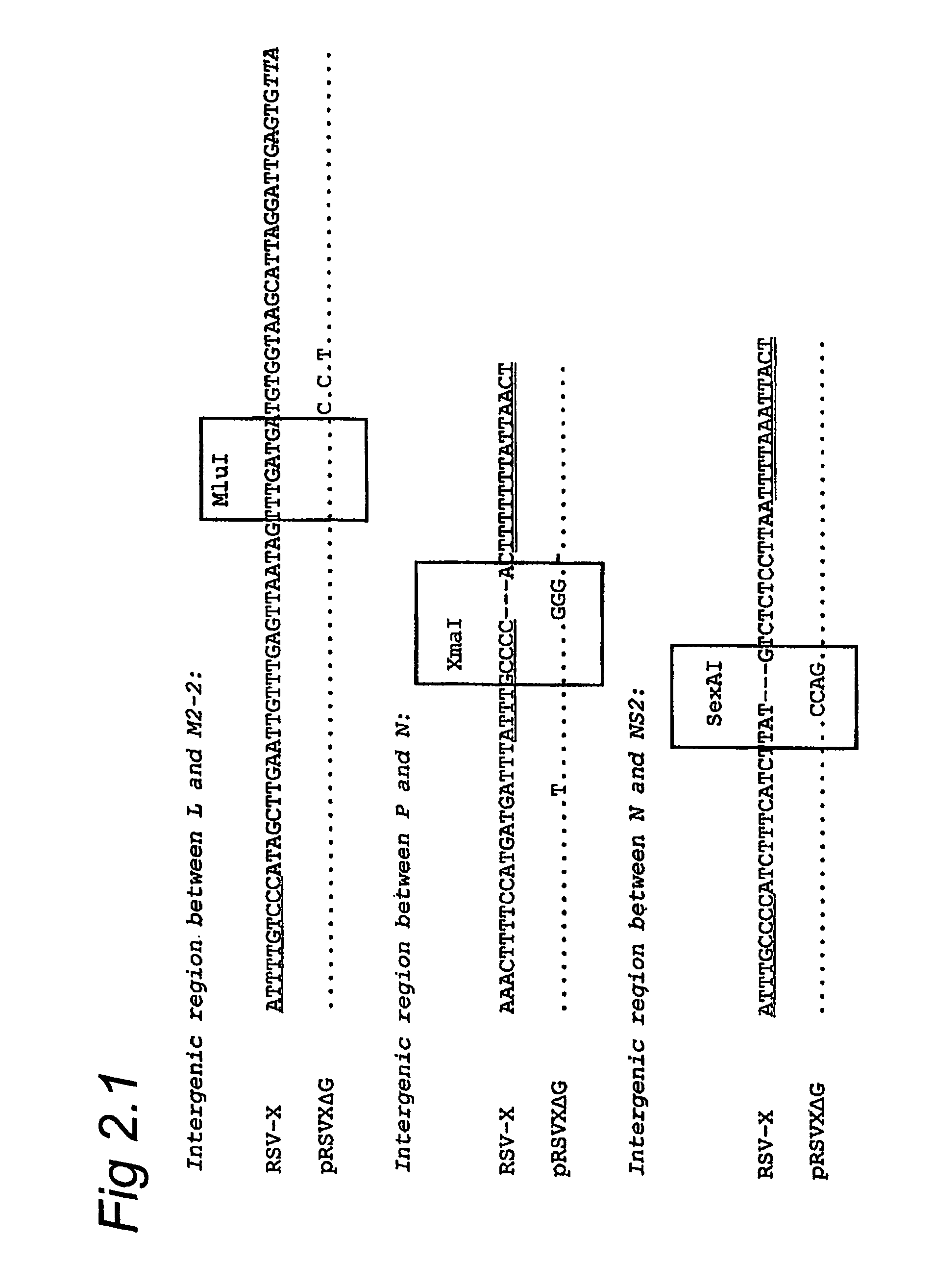
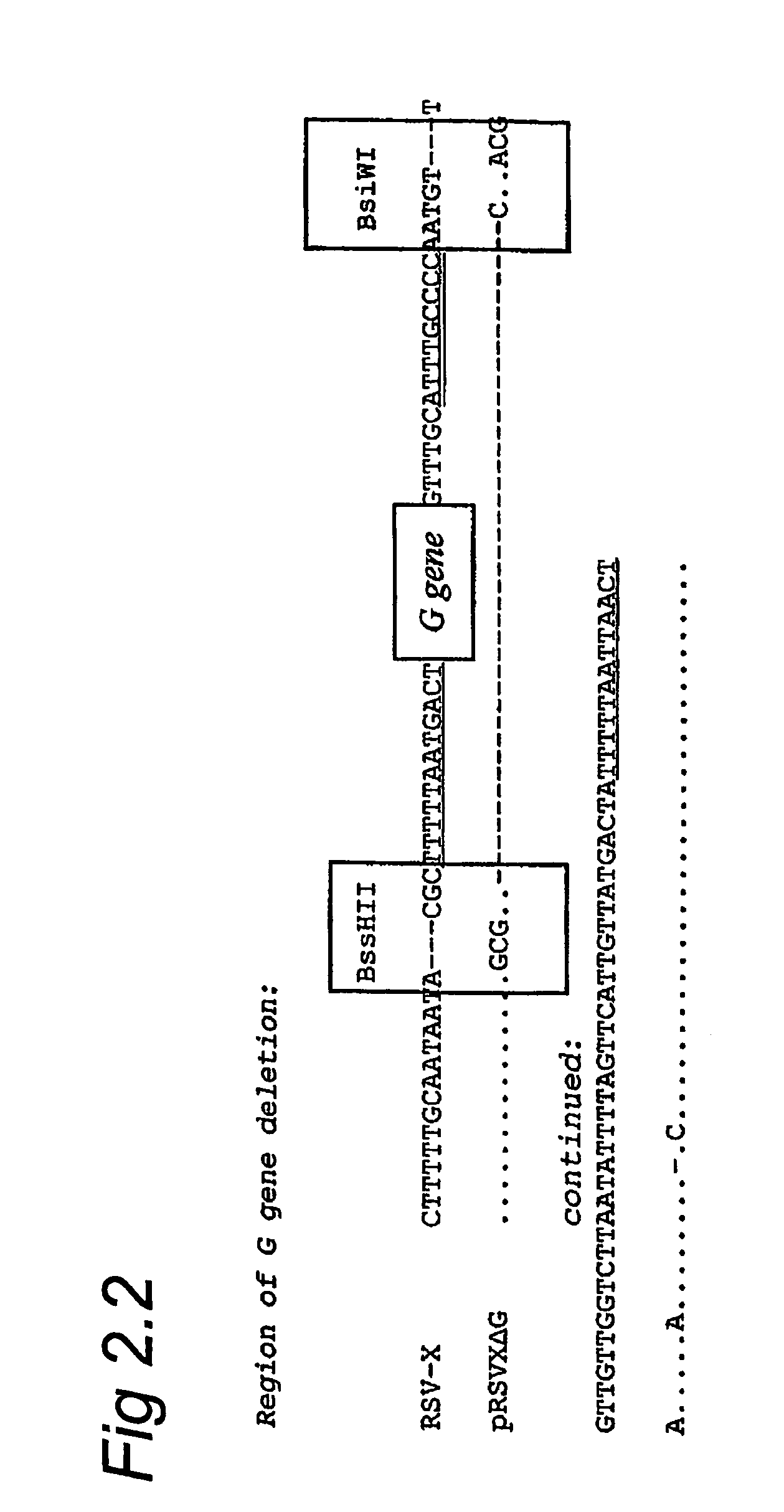
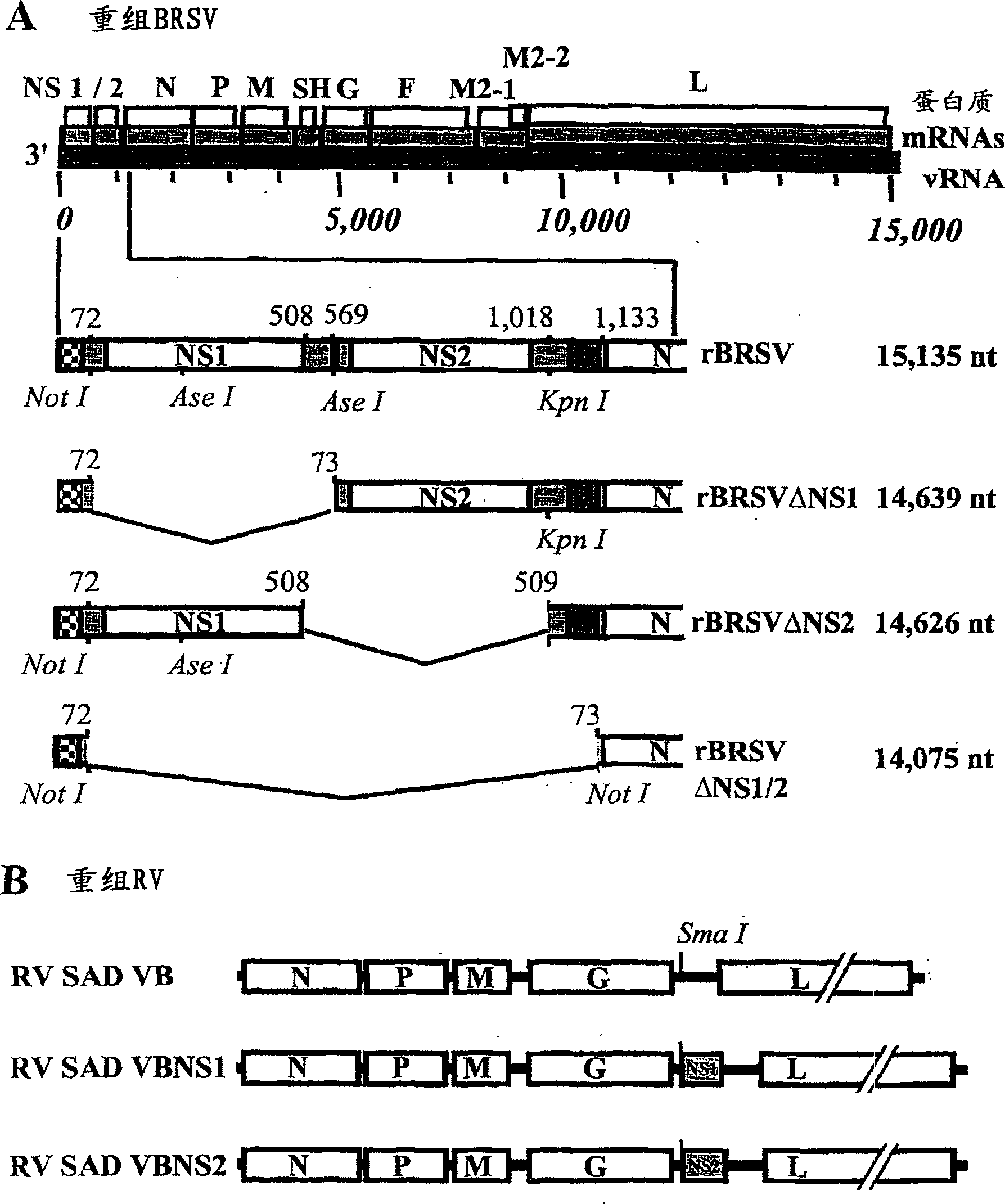
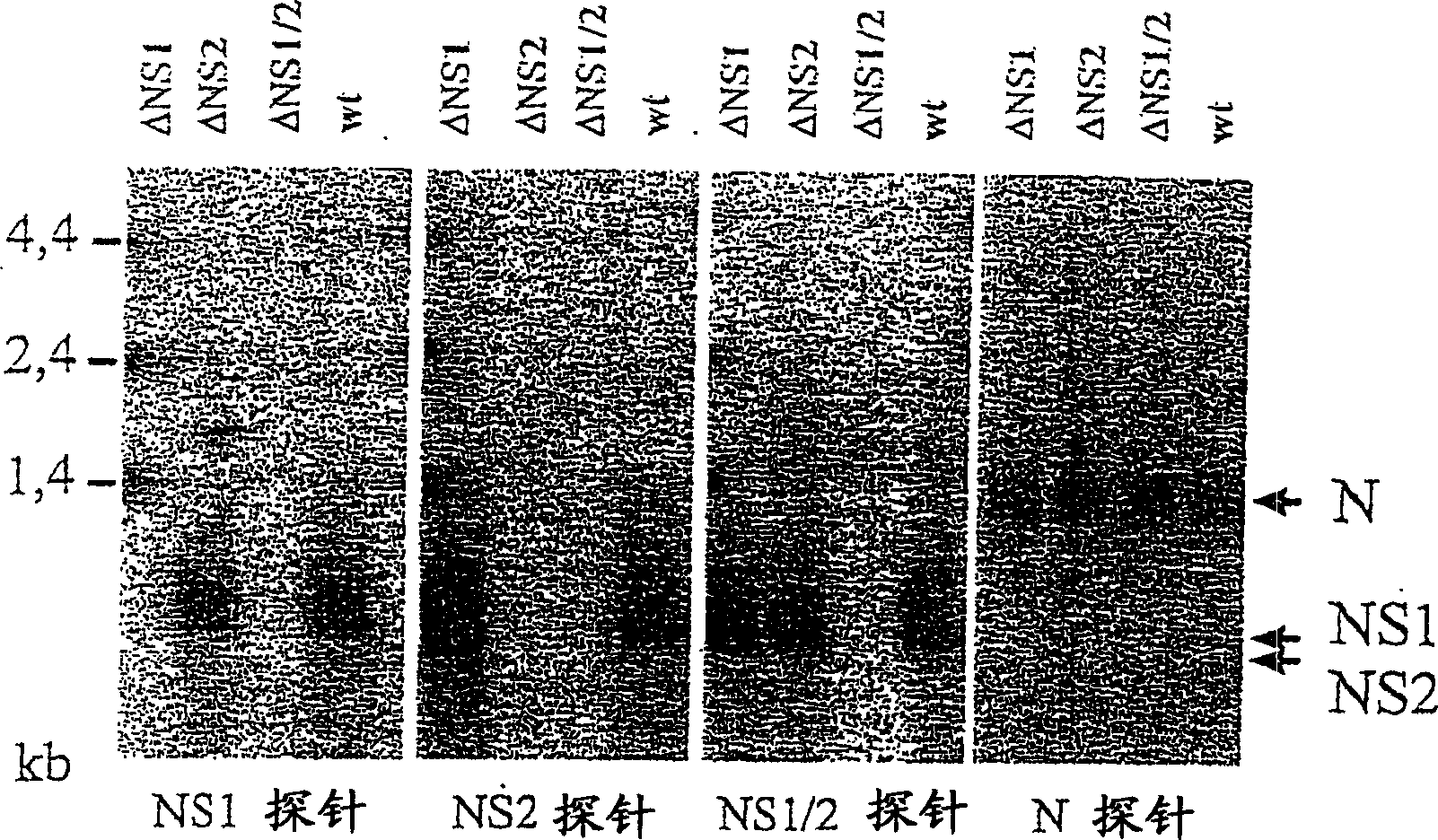
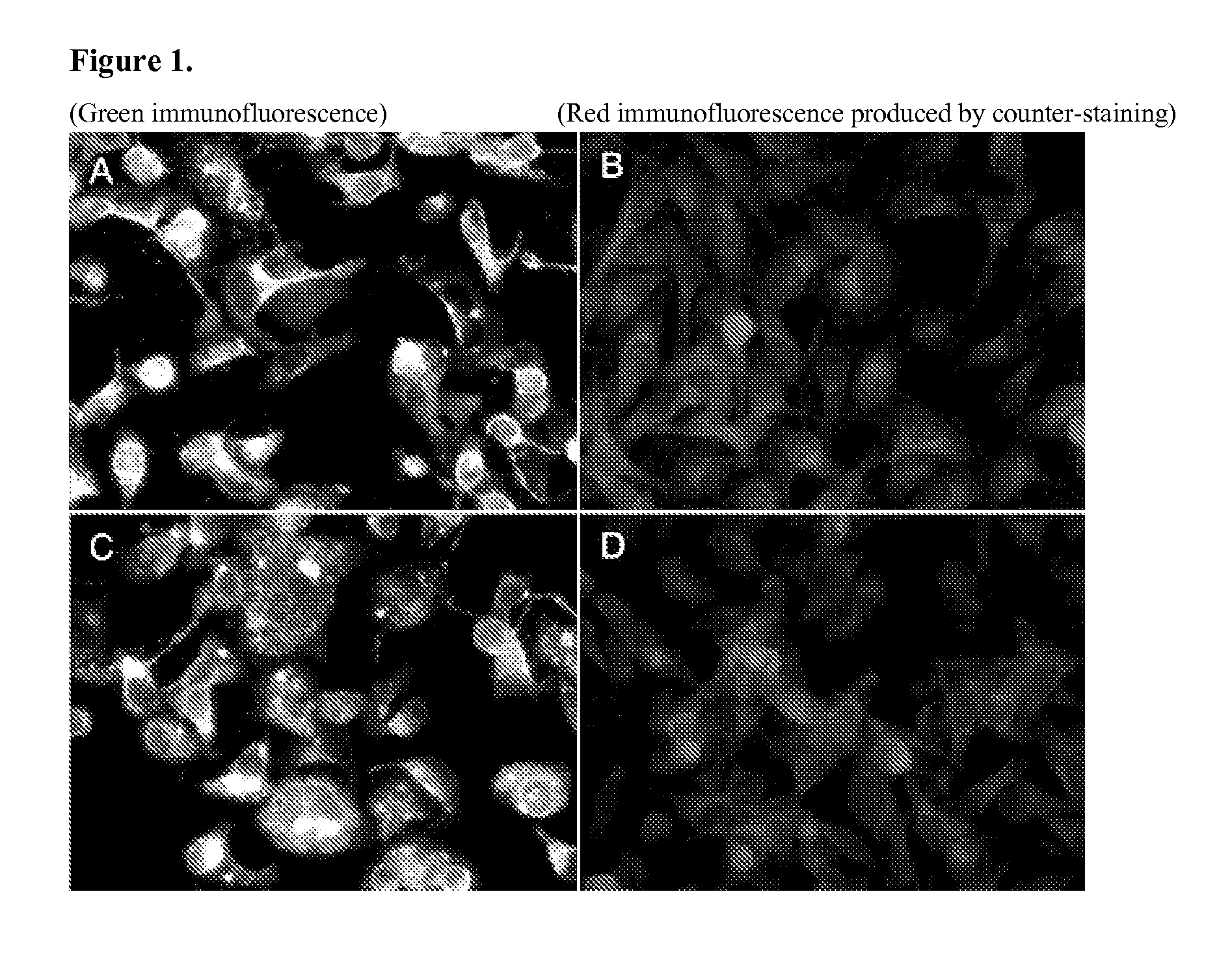
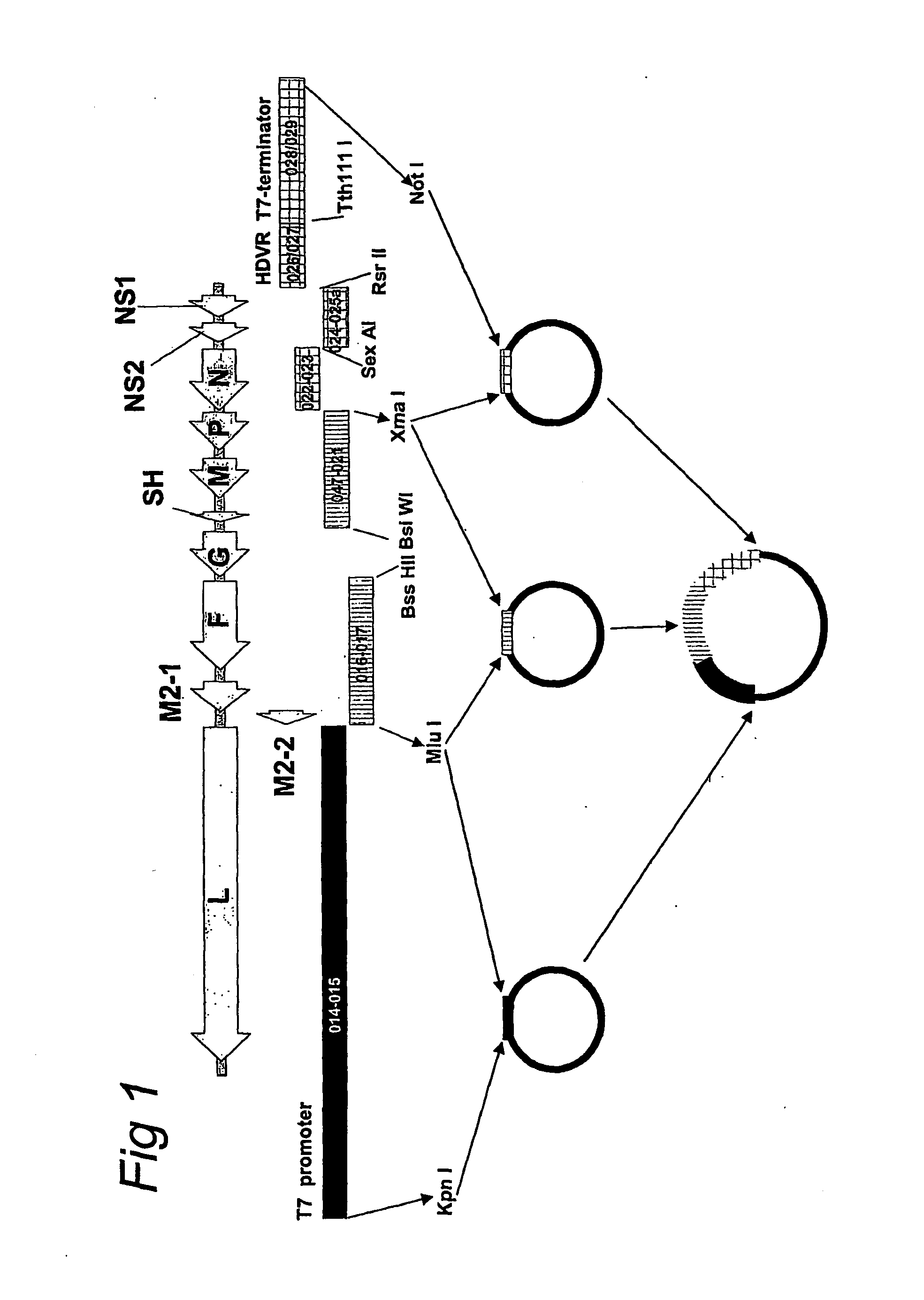
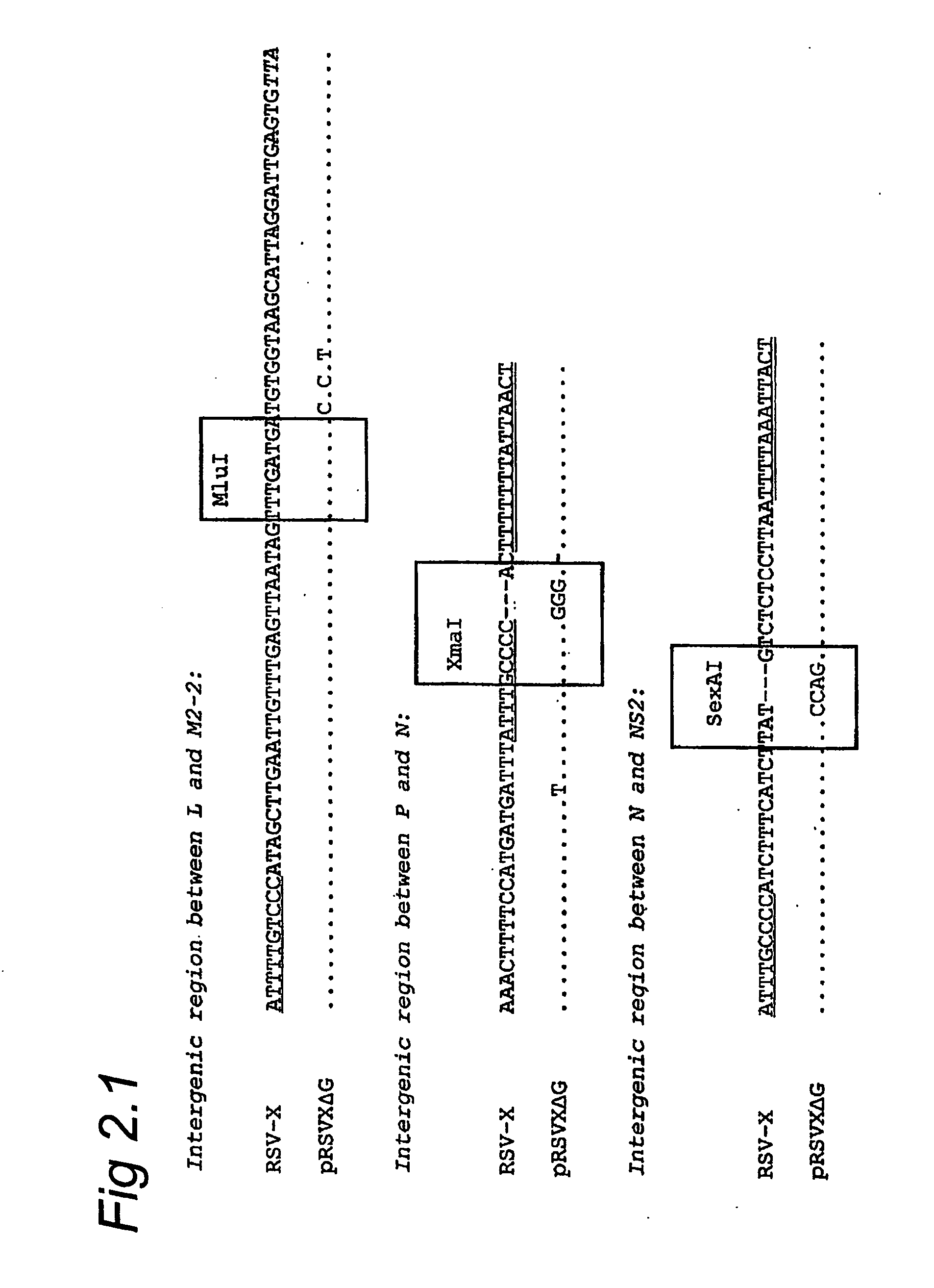
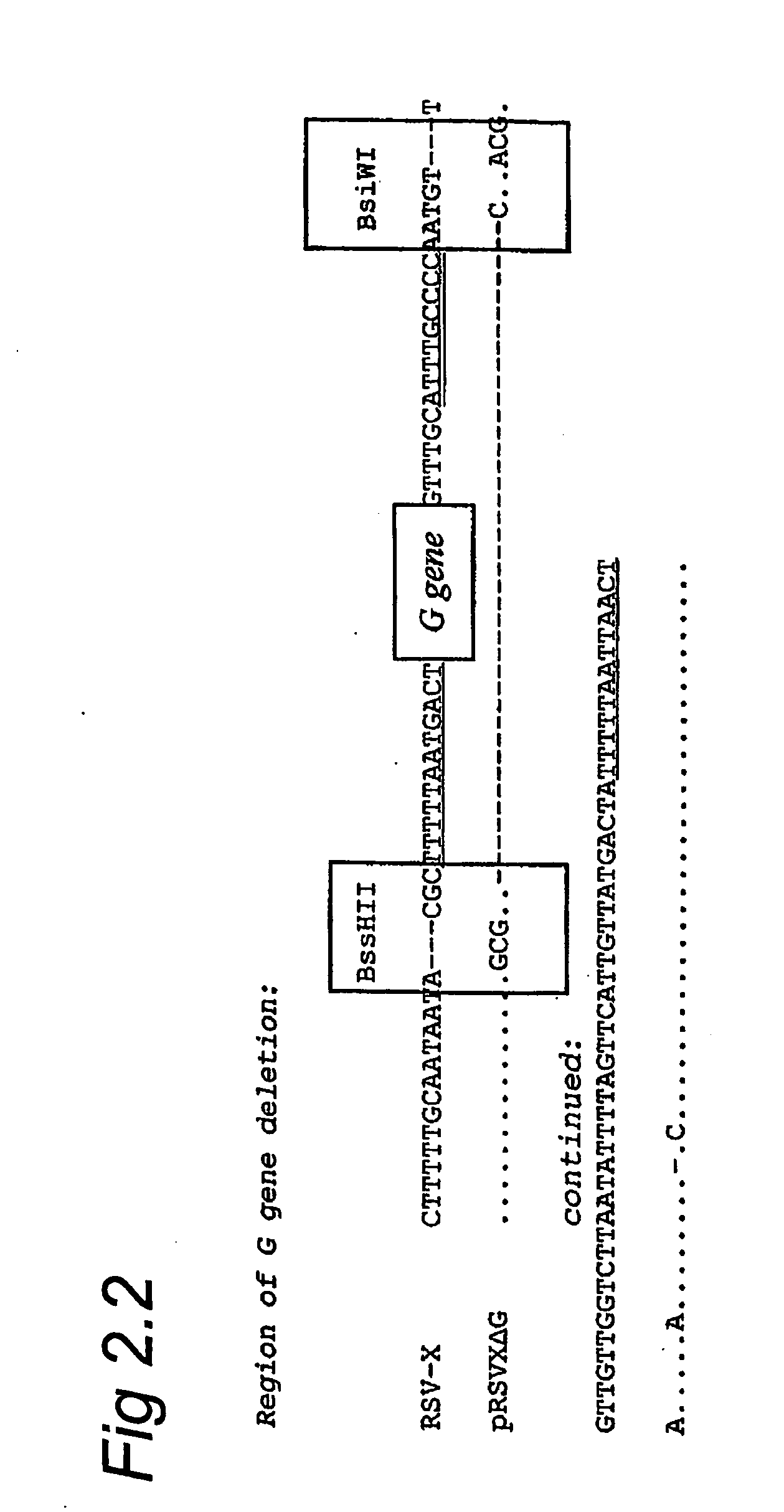
Respiratory syncytial virus with a genomic deficiency complemented in trans
InactiveUS9107939B2Improve efficiencyPrevent lower respiratory tract infection—willSsRNA viruses negative-senseBiocideDiseaseAttachment protein
Owner:INTRAVACC BV
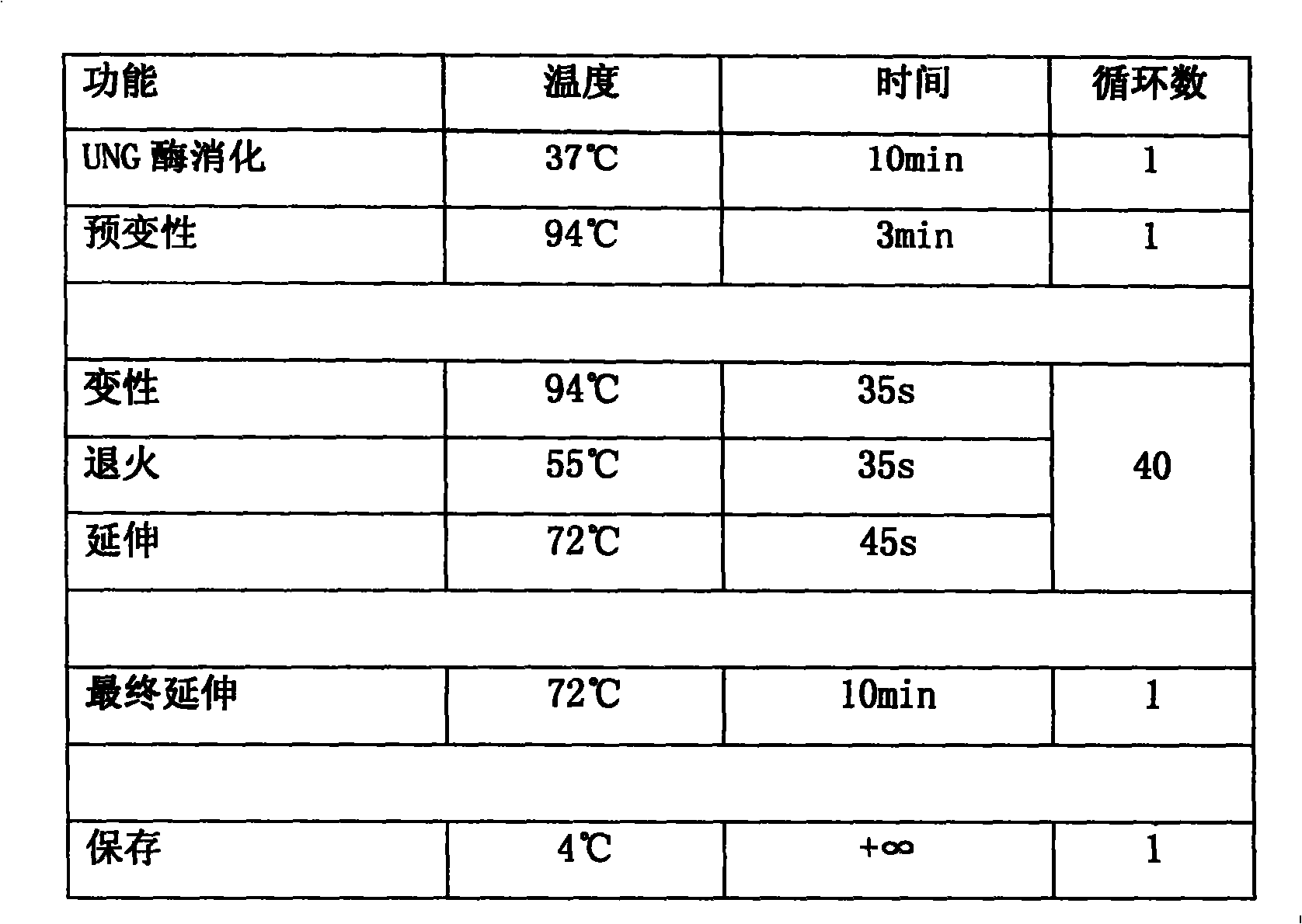
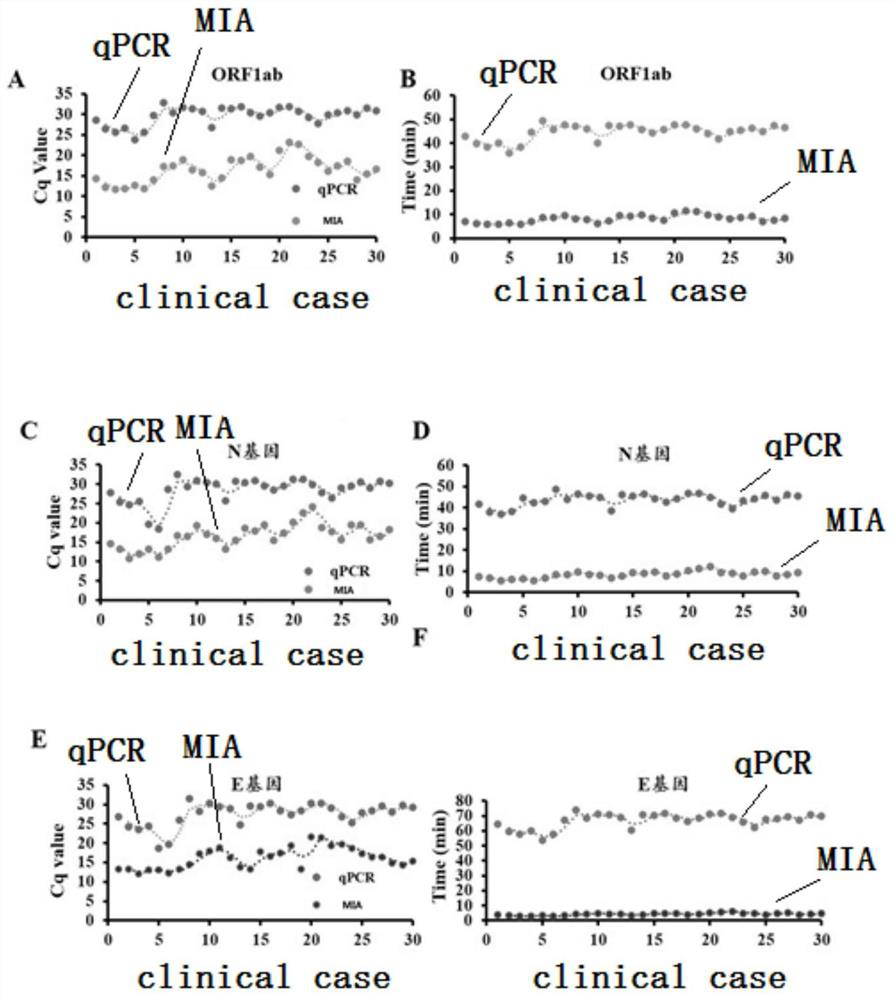
MIA primer, probe and kit for detecting COVID-19 and application of MIA primer and probe
PendingCN112094947AEasy to detectGuaranteed responseMicrobiological testing/measurementAgainst vector-borne diseasesPneumovirusEnvelope Gene
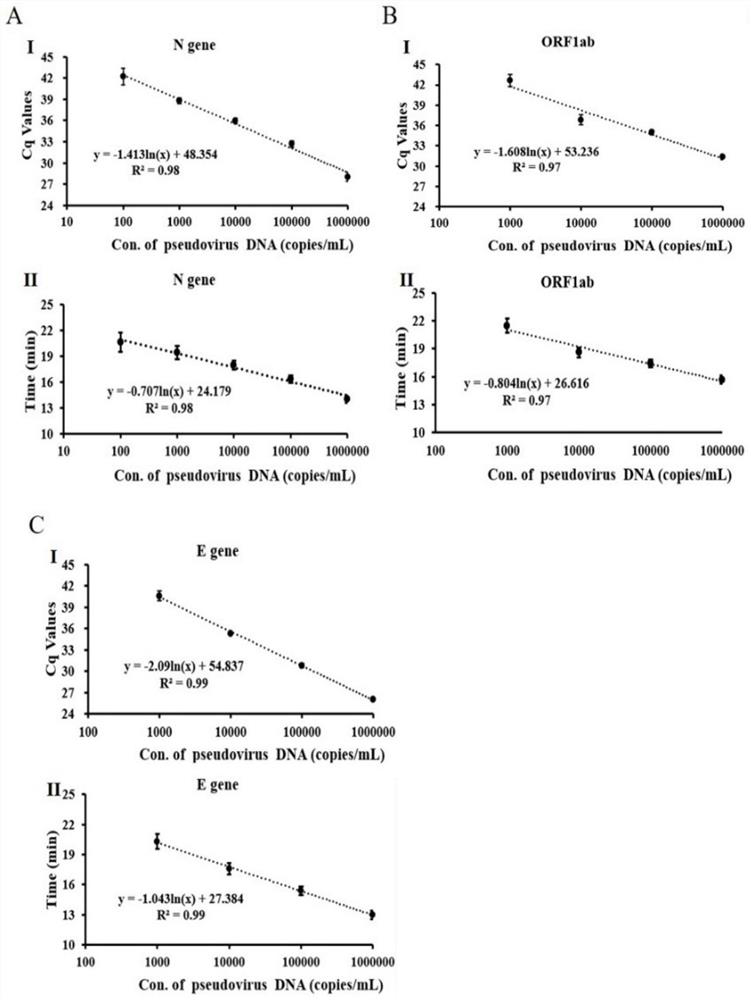
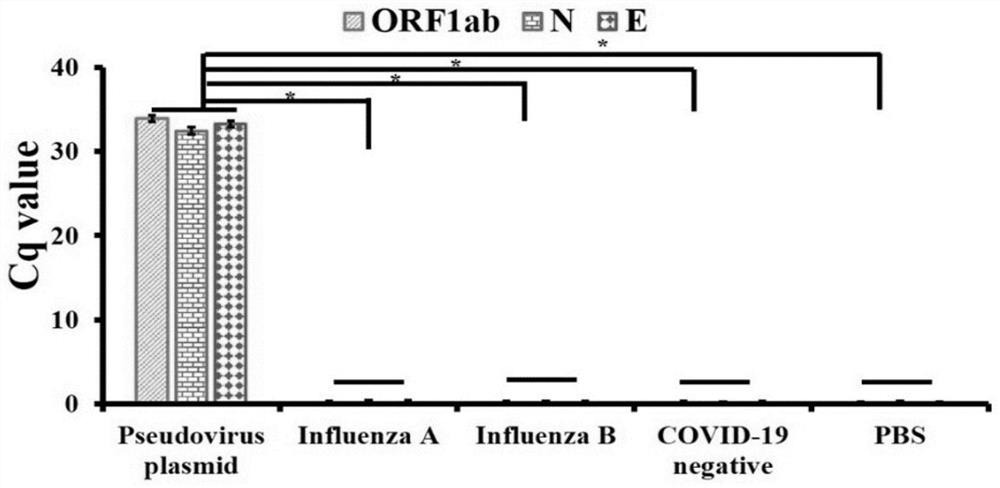
The invention discloses an MIA primer, a probe and a kit for detecting COVID-19 and an application of the MIA primer and the probe. According to the invention, the MIA primer and the probe are respectively designed based on a nucleocapsid N gene, an envelope E gene and an ORF1ab region of the COVID-19 publicized by NCBI. According to the MIA method disclosed by the invention, the detection of ORF1ab, E and N target genes of COVID-19 is realized in 3-15 minutes for the first time, and the detection sensitivity reaches 100 copies / ml (sample template concentration). The MIA method has no non-specific cross reaction with pathogen nucleic acid samples such as type A influenza, type B influenza and respiratory syncytial virus.
Owner:HANGZHOU BAOLIN BIOTECHNOLOGY CO LTD
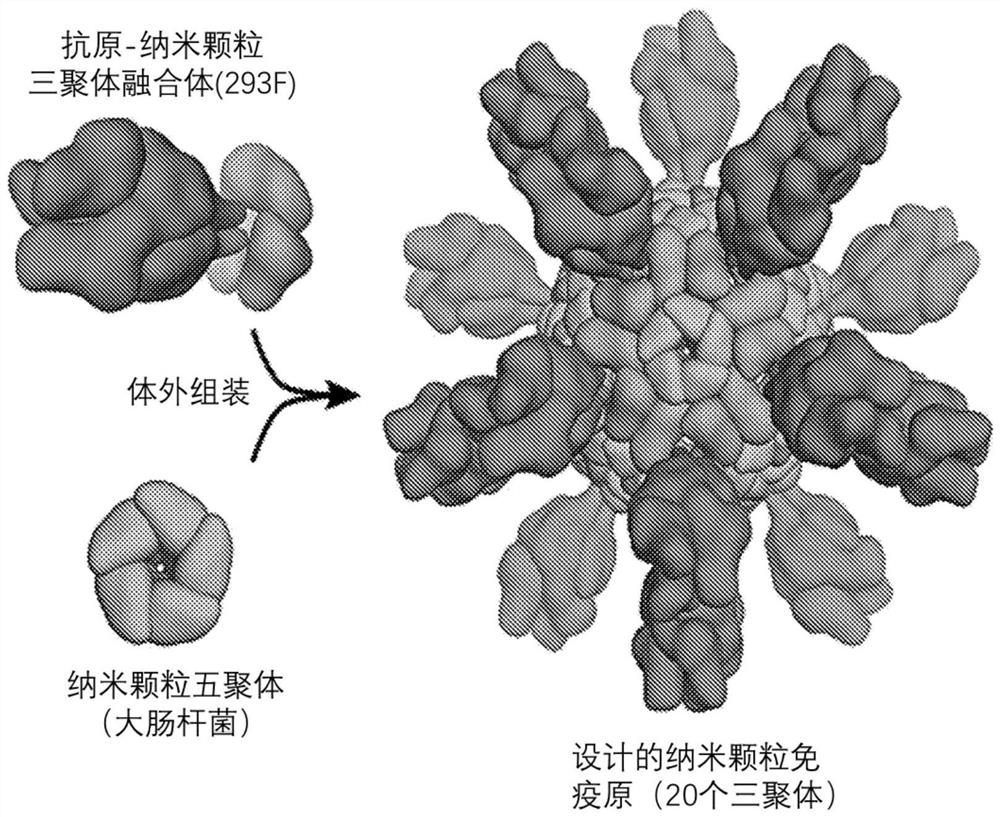
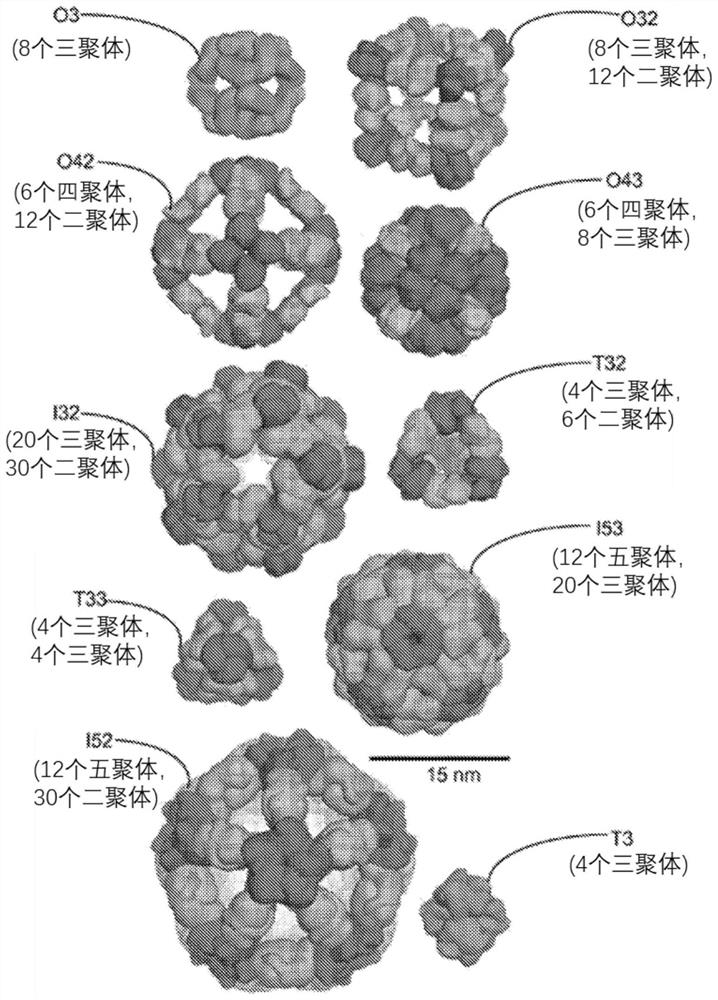
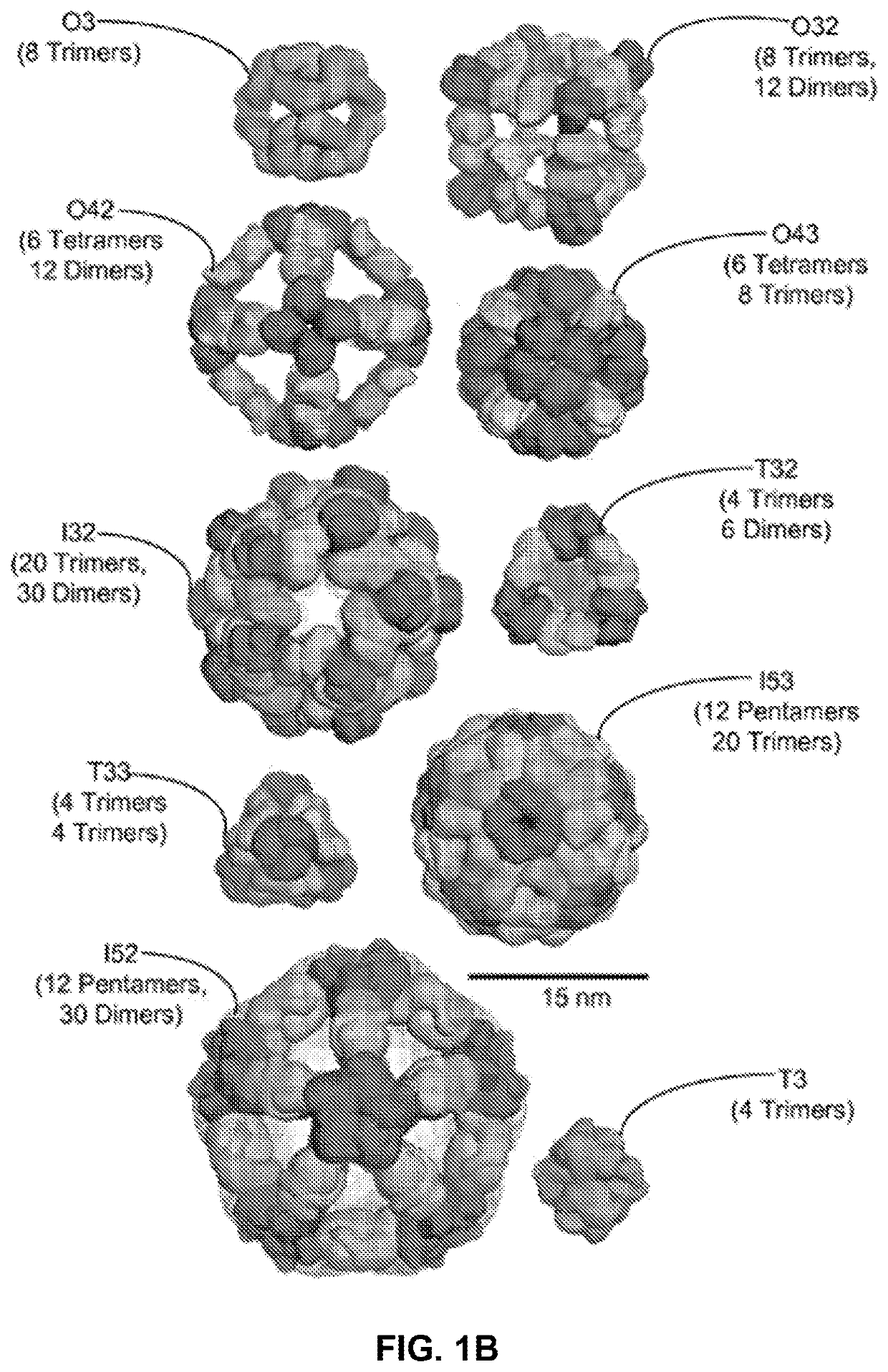
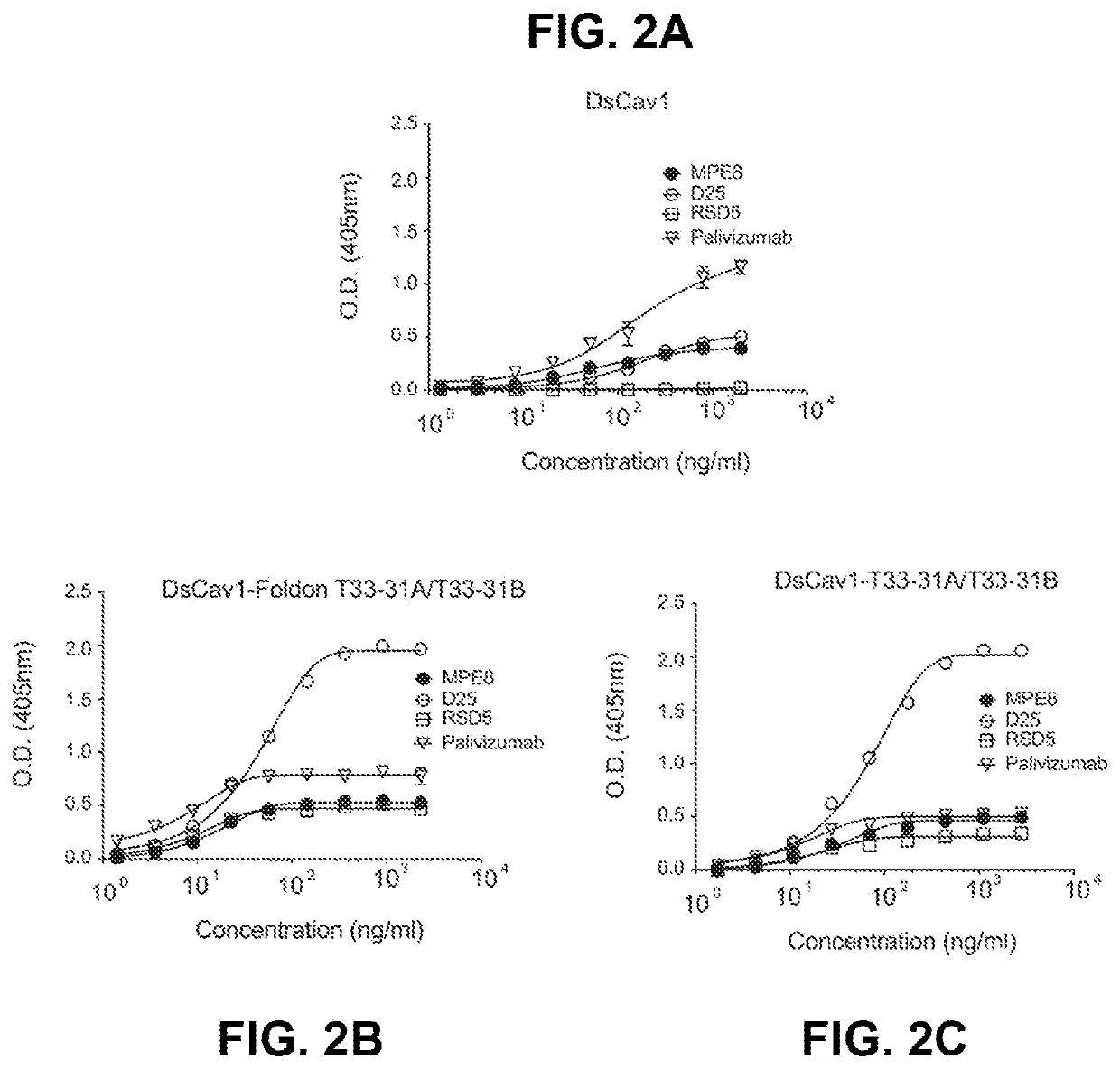
Self-asssembling nanostructure vaccines
The present disclosure provides nanostructures and nanostructure-based vaccines. Some nanostructures of the present disclosure display antigens capable of eliciting immune responses to infectious agents such as bacteria, viruses, and pathogens. Some vaccines of the present disclosure are useful for preventing or decreasing the severity of infection with an infectious agent, including, for exampleand without limitation, lyme disease, pertussis, herpes virus, orthomyxovirus, paramyxovirus, pneumovirus, filovirus, flavivirus, reovirus, retrovirus, meningococcus, or malaria. The antigens may be attached to the core of the nanostructure either non-covalently or covalently, including as a fusion protein or by other means disclosed herein. Multimeric antigens may optionally be displayed along asymmetry axis of the nanostructure. Also provided are proteins and nucleic acid molecules encoding such proteins, vaccine compositions, and methods of administration.
Owner:UNIV OF WASHINGTON
Pneumovirus NS proteins antagonising interferon (IFN) response
InactiveCN1426462ASsRNA viruses negative-sensePeptide/protein ingredientsStructural proteinInterferon alpha
The present invention relates to the use of pneumonia virus NS1 protein and / or NS2 protein or nucleic acid encoding pneumonia virus NS1 protein and / or NS2 protein in the manufacture of pharmaceutical preparations for attenuating interferon (IFN)-mediated immune responses. The present invention also relates to recombinant pneumonia viruses, in particular respiratory syncytial virus (RSV) with enhanced, reduced resistance or lack of resistance to interferon (IFN)-mediated immune responses. The invention further relates to recombinant viruses with increased resistance to interferon (IFN)-mediated immune responses, and the use of said viruses in pharmaceutical formulations (eg, vaccines).
Owner:WYETH LLC
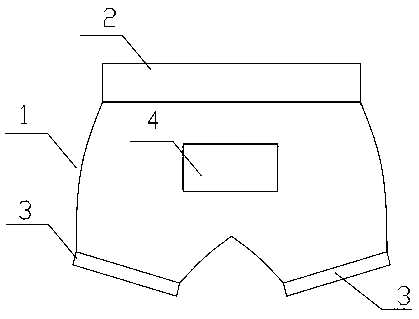
Isolation-level infectious disease protection underpants
The invention relates to isolation-level infectious disease protection underpants which comprise underpants, a trouser waist telescopic belt, trouser leg telescopic belts and an exhaust port and are made of rubber materials with good sealing performance. At present, viruses are found in excrement of most infectious disease virus carriers, such as SARS virus and new coronapneumonia virus. The excrement of a virus carrier is proved to be capable of transmitting viruses; a virus carrier can expel viruses from the anus, so that viruses can invade normal people from the anus to cause infection, theisolation-level infectious disease protection underpants capable of completely isolating the navel eyes, the anus and the external genitals are invented, a virus carrier can isolate viruses and can prevent the viruses from spreading outwards to infect others when wearing the isolation-level infectious disease protection underpants, and normal people can prevent the viruses from invading bodies from the navel eyes, the anus and the external genitals when wearing the isolation-level infectious disease protection underpants, thereby achieving infectious disease isolation and protection.
Owner:浙江豪能新能源有限公司
Self-asssembling nanostructure vaccines
The present disclosure provides nanostructures and nanostructure-based vaccines. Some nanostructures of the present disclosure display antigens capable of eliciting immune responses to infectious agents such as bacteria, viruses, and pathogens. Some vaccines of the present disclosure are useful for preventing or decreasing the severity of infection with an infectious agent, including, for example and without limitation, lyme disease, pertussis, herpes virus, orthomyxovirus, paramyxovirus, pneumovirus, filovirus, flavivirus, reovirus, retrovirus, meningococcus, or malaria. The antigens may be attached to the core of the nanostructure either non-covalently or covalently, including as a fusion protein or by other means disclosed herein. Multimeric antigens may optionally be displayed along a symmetry axis of the nanostructure. Also provided are proteins and nucleic acid molecules encoding such proteins, vaccine compositions, and methods of administration.
Owner:UNIV OF WASHINGTON
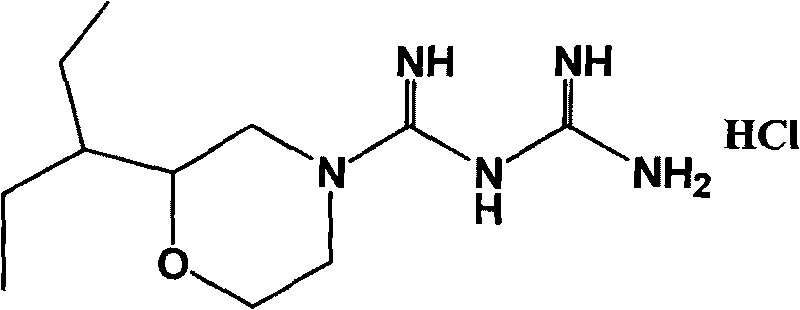
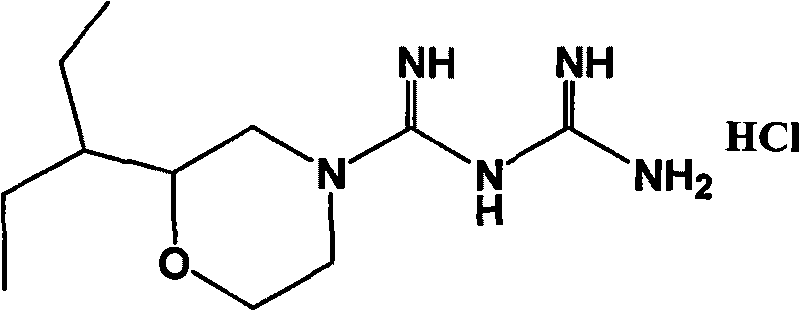
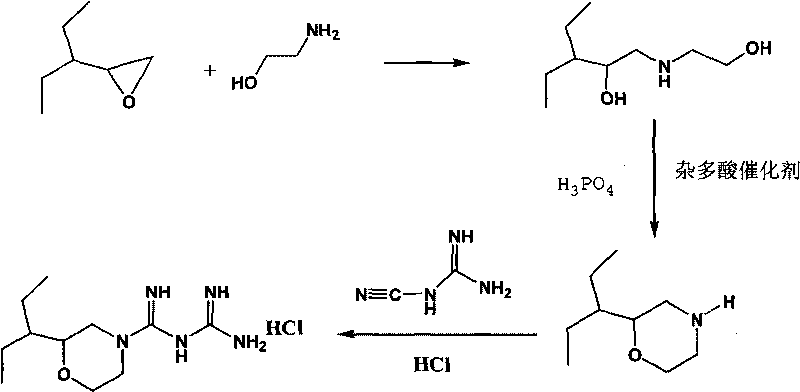
Hydrochloric acid 2-(1-ethyl propyl) moroxydine, preparation method and application thereof
InactiveCN101704795ALow toxicityStrong antiviral activityOrganic active ingredientsOrganic chemistryChemical synthesisMoroxydine
The invention provides a new hydrochloric acid 2-(1-ethyl propyl) moroxydine which can be obtained by chemical synthesis and has the advantages of low price of needed raw materials, easily obtained raw materials, simple and convenient preparation method, higher yield and suitability for industrialized production. The pharmacological activity experiment proves that compared with hydrochloric acid moroxydine, the hydrochloric acid 2-(1-ethyl propyl) moroxydine has the remarkably enhanced activity of in vivo resisting hepatitis B virus, hepatitis C virus, influenza virus, pneumovirus and herpes simplex virus, and the toxicity is one time lower than that of the hydrochloric acid moroxydine. Therefore, the new hydrochloric acid 2-(1-ethyl propyl) moroxydine can be applied to prepare antiviral drugs, especially anti-flu drugs, hepatitis C resisting drugs, hepatitis B resisting drugs, pneumovirus resisting drugs and herpes simplex viral keratitis resisting drugs.
Owner:上海双科医药科技有限公司
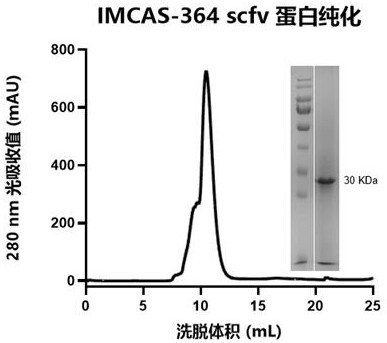
Humanized antibody of novel coronavirus rare broad-spectrum epitope and application thereof
ActiveCN114456264ASignificant application valueGenetically modified cellsImmunoglobulins against virusesEpitopePneumovirus
The invention relates to a novel human antibody of a coronavirus rare broad-spectrum epitope and application thereof. A model of successive screening of prototype strain RBD and Beta strain RBD antigens is utilized, conservative target sites are rapidly focused, and the fully human antibody IMCAS-364 which is combined with different epitopes of the RBD and can realize broad-spectrum neutralization of VOC is successfully separated. The affinity of the strain to a prototype strain, Alpha, Beta, Delta and Omicron reaches an nM level. A known epitope antibody competition experiment proves that IMCAS-364 is combined with a rare target position on the inner side surface of RBD and does not compete with a receptor, and the antibody combined with the target position has the characteristic that the antibody does not compete with ACE2 but can neutralize a new crown for the first time. The IMCAS-364 has the potential of being paired with an antibody which plays a neutralizing role through receptor blocking, has the capability affinity of being combined with atypical pneumovirus at nM level, and can be used for developing a neutralizing antibody of a general SARS coronavirus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Pneumovirus compositions and methods for using the same
Provided are newly identified pneumoviruses that can infect mammals, including dogs cats and potentially humans. Isolated polynucleotides and proteins of the viruses, as well as the isolated viruses themselves are provided. The invention includes compositions and methods for detecting the viruses, methods and compositions for prophylaxis and / or therapy of disease signs that are positively correlated with the presence of the viruses, and isolated cells comprising the viruses. Intact virions, viral proteins, and fragments thereof are also provided.
Owner:CORNELL UNIVERSITY
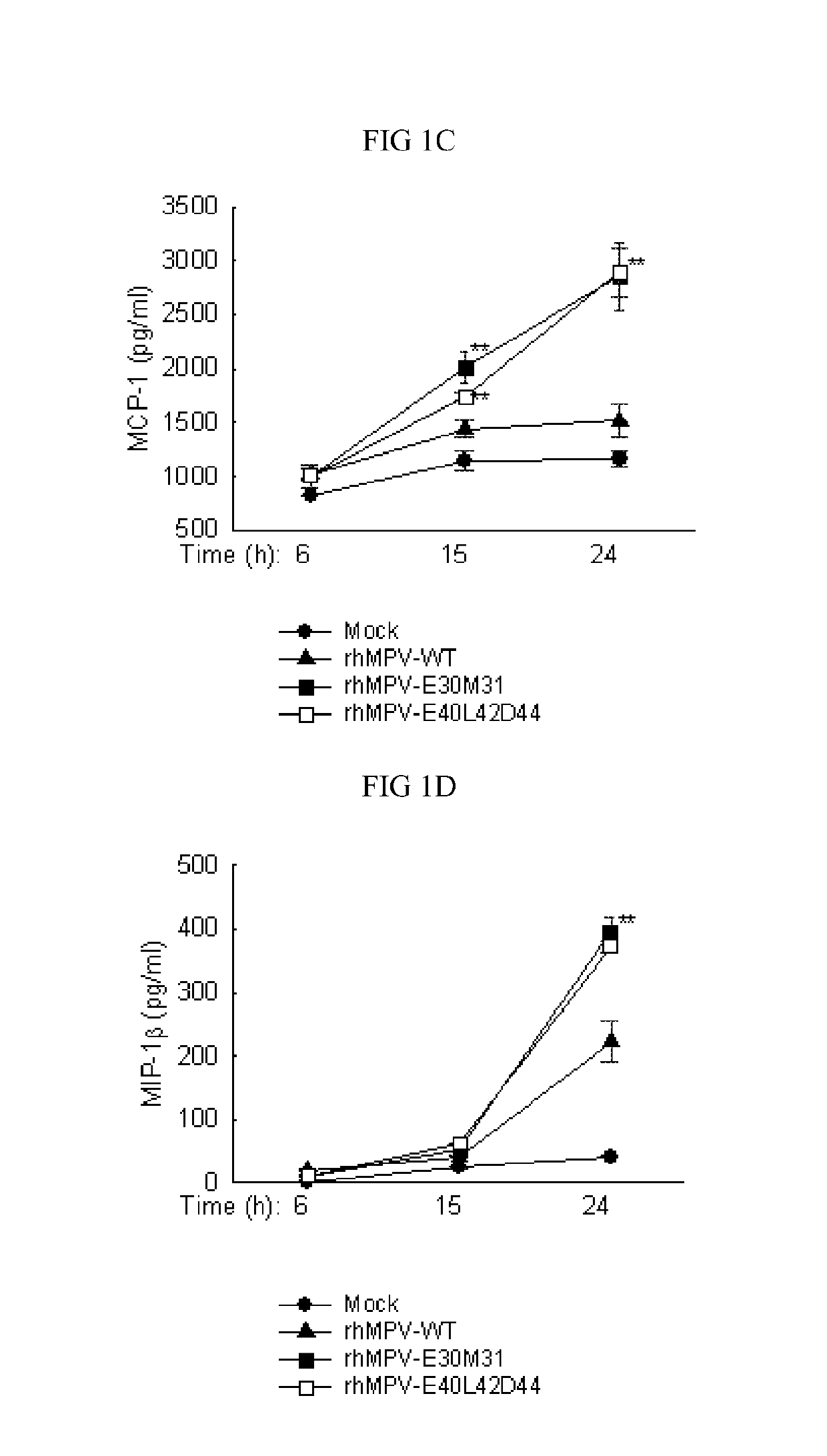
Live attenuated recombinant hmpv with mutations in pdz motifs of m2-2 protein, vaccine containing and use thereof
ActiveUS20190192592A1SsRNA viruses negative-senseViral antigen ingredientsPneumoviridaeImmunogenicity
The present application generally relates to the development of live attenuated Pneumoviridae strains suitable for use as a vaccine. Particularly, human metapneumovirus (hMPV) ΔM2-2 strains (rhMPV-E30M31 and rhMPV-E40L42D44) containing point mutations in a PDZ motif of M2-2, which results in a strain that is both attenuated and immunogenic and, notably, maintains the function of F and G proteins. These live attenuated hMPV strains should be suitable for use in a vaccine capable of providing protection against respiratory infection elicited by hMPV. Additionally, human respiratory syncytial virus (hRSV) strains containing point mutations in a PDZ motif of M2-2 should also be suitable for use as a vaccine capable of providing protection against respiratory infection elicited by hRSV. These Pneumoviridae strains should be useful in vaccines for use in humans and animals, e.g., companion animals and livestock, in treating or providing immunoprotection against respiratory infections.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
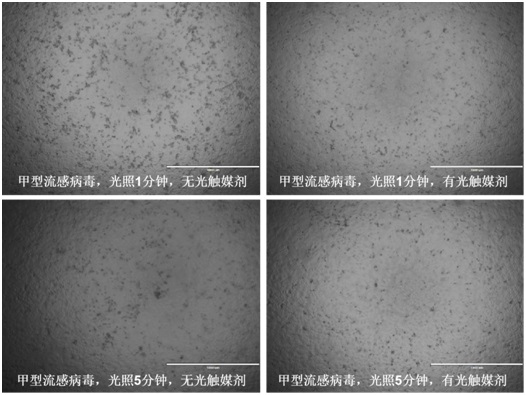
Photocatalyst sterilization disinfectant based on Ag and N co-doped titanium dioxide/silver nitrate as well as preparation method and application of photocatalyst sterilization disinfectant
InactiveCN112136830AHigh killing efficiencyBroaden the spectral response wavelengthBiocideCatalyst activation/preparationEscherichia coliDisinfectant
The invention provides a method for sterilizing and disinfecting by using an Ag and N co-doped titanium dioxide / silver nitrate photocatalyst, which comprises the following steps: mixing solid silver nitrate powder with P25 titanium dioxide nano powder, uniformly grinding, and calcining to prepare Ag and N co-doped titanium dioxide; dissolving the co-doped titanium dioxide, silver nitrate, a dispersing agent and plant essential oil in deionized water, and completely performing ultrasonic dispersion to obtain the photocatalyst sterilization disinfectant. The spectral absorption range of titaniumdioxide is widened to a visible light region through Ag and N co-doping, the utilization efficiency of sunlight and the concentration of photon-generated carriers are improved, meanwhile, the preparation method has the advantages of being simple, low in cost, good in sterilization and disinfection effect and the like, and coronapneumonia viruses, influenza viruses, escherichia coli and the like of the human body can be effectively killed.
Owner:青岛亿恩方能源环保科技有限公司
Unmanned system for killing new coronal pneumovirus of cold-chain food and wrappage
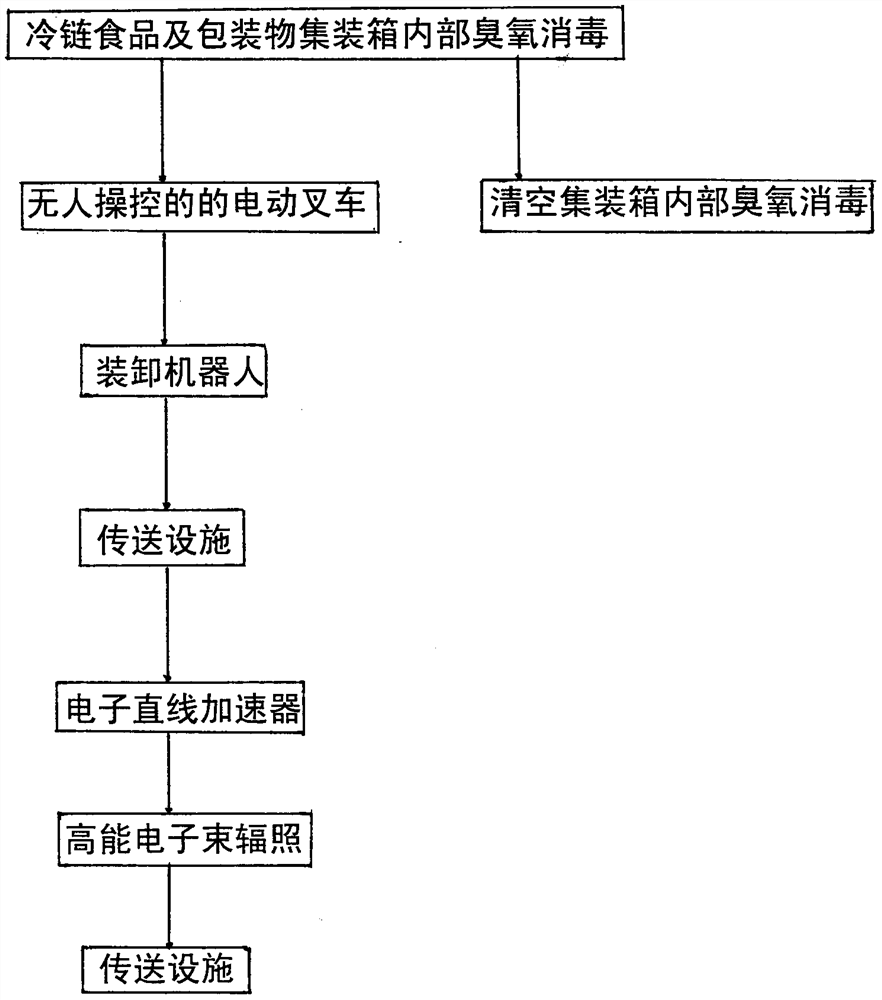
PendingCN114652876AAvoid person-to-personSignificant comprehensive benefitsGaseous substancesChemicalsCold chainEngineering
The invention relates to an unmanned control system for killing new coronal pneumovirus of cold-chain food and wrappage. The device consists of an ozone disinfection and unmanned electric forklift, a loading and unloading robot, an electron linear accelerator, a high-energy electron beam irradiation device and a transmission facility, and is characterized by comprising the following steps of: firstly, carrying out ozone disinfection on a container of cold-chain food and packaging materials with new coronal pneumovirus; taking out the tray loaded with the cold-chain food and the packaging material by using an unmanned electric forklift and transferring the tray to a loading and unloading robot near an electron linear accelerator conveying facility; the loading and unloading robot gripper transfers the cold-chain food and the packaging material single piece to the conveying facility; conveying to a position below the electron linear accelerator through the conveying facility; staying; after the viruses are killed through irradiation of high-energy electron beams of the electron linear accelerator, the viruses are conveyed out through the conveying facility; and the interior of the emptied container is disinfected again with ozone and the like. The method is safe and reliable, and has remarkable social benefits and economic benefits.
Owner:胡晓平
Bicyclic fused pyrazole derivatives for the treatment of rsv
Disclosed herein are compounds and compositions for treating or inhibiting RSV and related members of the pneumovirus and paramyxovirus families such as human metapneumovirus, mumps virus, human parainfluenzaviruses, and Nipah and hendra virus, and methods of treatment or prevention thereof.
Owner:GEORGIA STATE UNIV RES FOUND INC
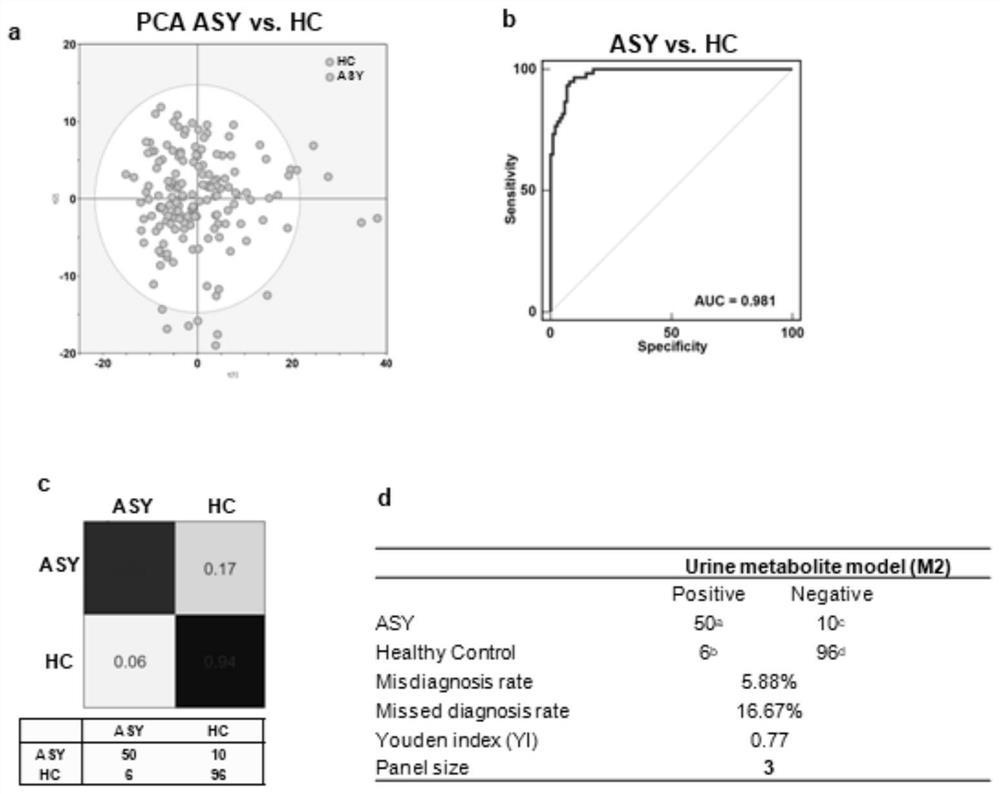
Application of reagent for detecting biomarkers in urine in preparation of kit for screening new coronal pneumovirus asymptomatic infected persons
PendingCN114705800AAvoid harmMeet clinical screening requirementsComponent separationAntigenNon invasive
The invention provides a kit and a device for screening a new coronal pneumovirus asymptomatic infected person. According to the kit and the device for screening the new coronal pneumovirus asymptomatic infected person, in the screening process, the accuracy is good, the Youden index is 0.82, and the clinical requirements on a screening method can be met. Compared with a throat swab nucleic acid detection reagent and a serological antigen-antibody reagent in the prior art, the throat swab nucleic acid detection reagent can effectively overcome the defect that the risk of virus propagation is increased due to missed diagnosis caused by the reason that an asymptomatic infection patient has no obvious symptoms, viruses are not easy to fall off, false negative is easy to appear in nucleic acid detection and the like; secondly, by collecting the urine sample in a non-invasive mode and detecting and screening hypoxanthine, uric acid and dihydro-5-pentyl-2 (3H)-furanone in the urine sample, the method is convenient and rapid, low in infection risk and harmless to patients, and has clinical application and popularization value.
Owner:重庆市公共卫生医疗救治中心
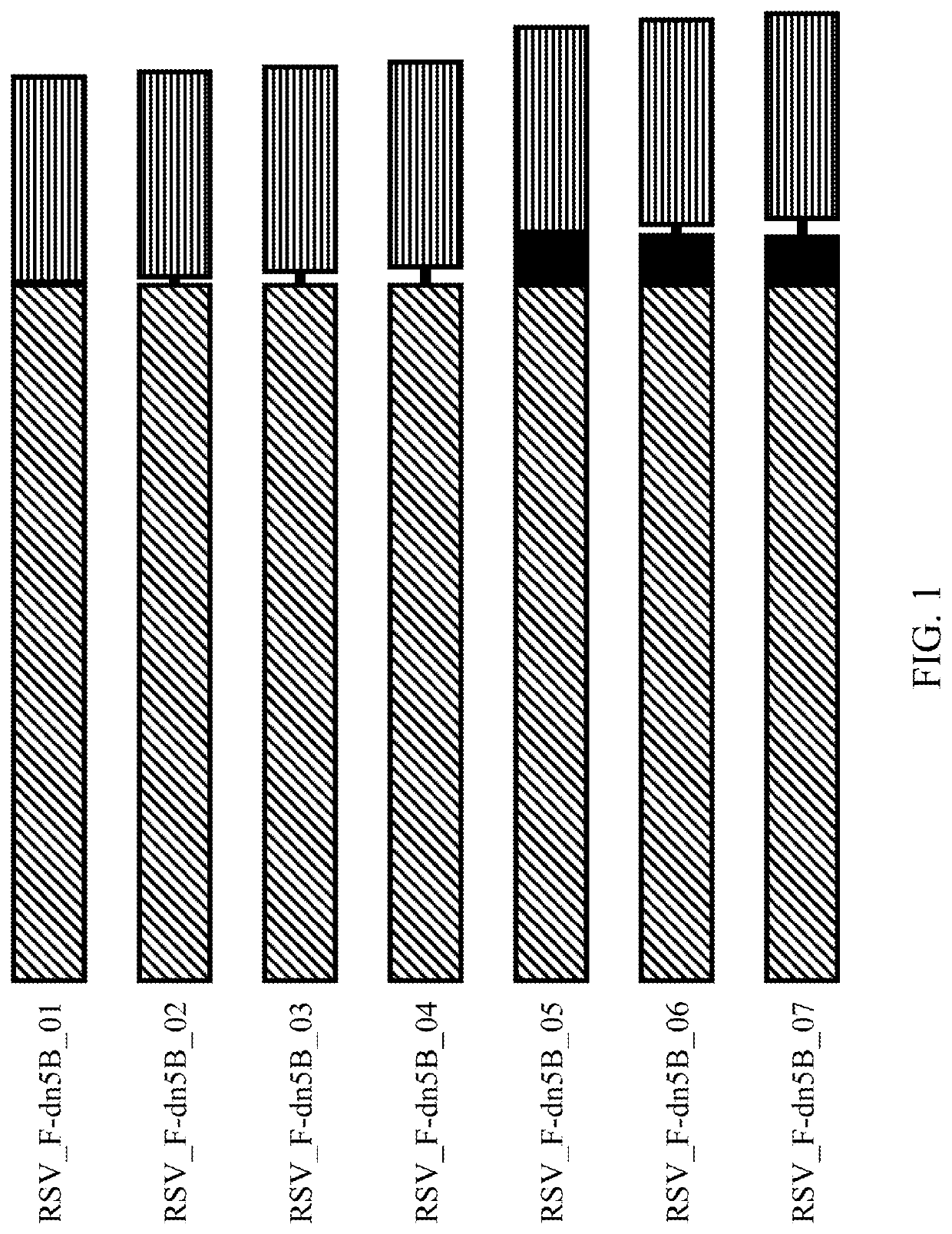
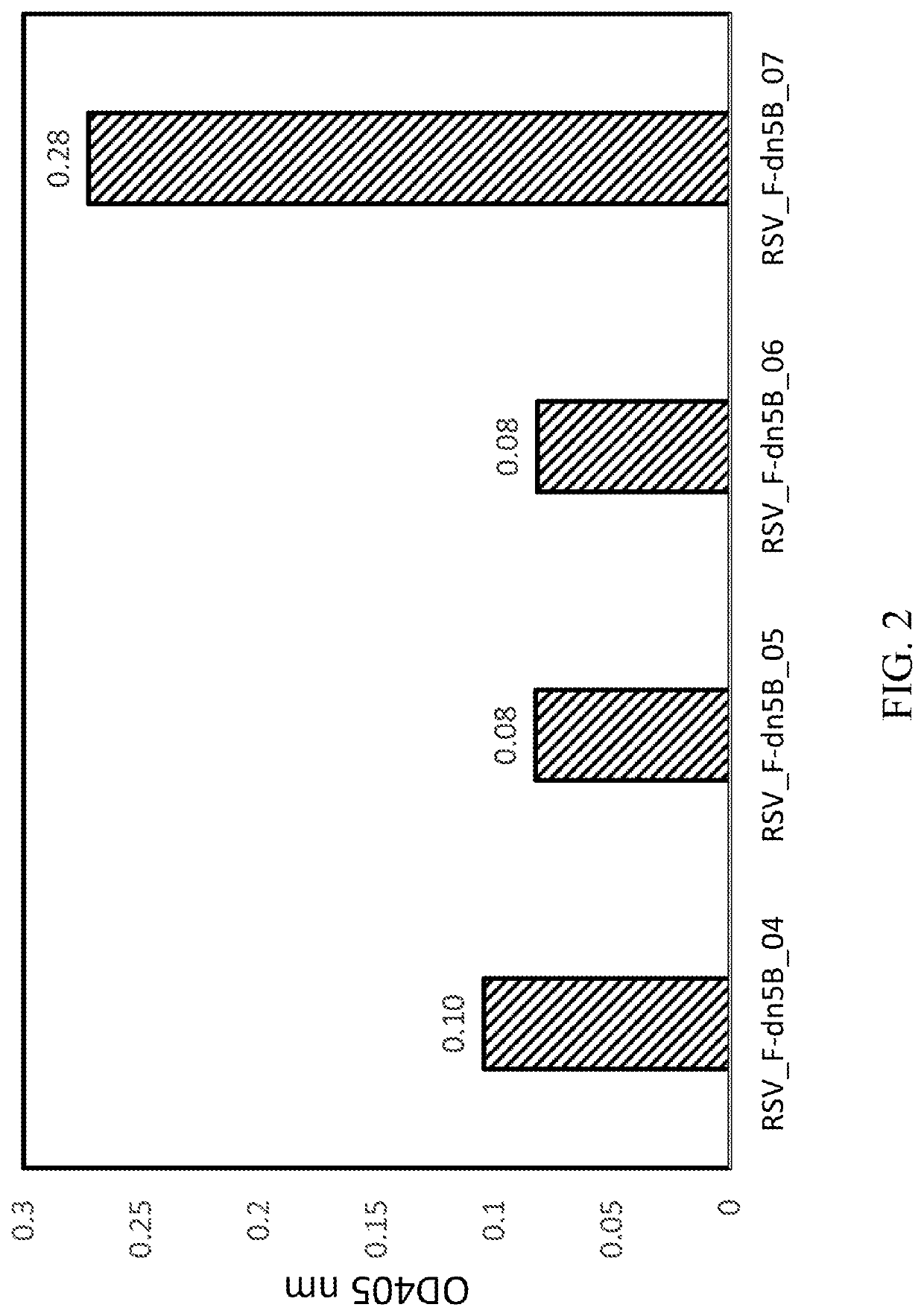
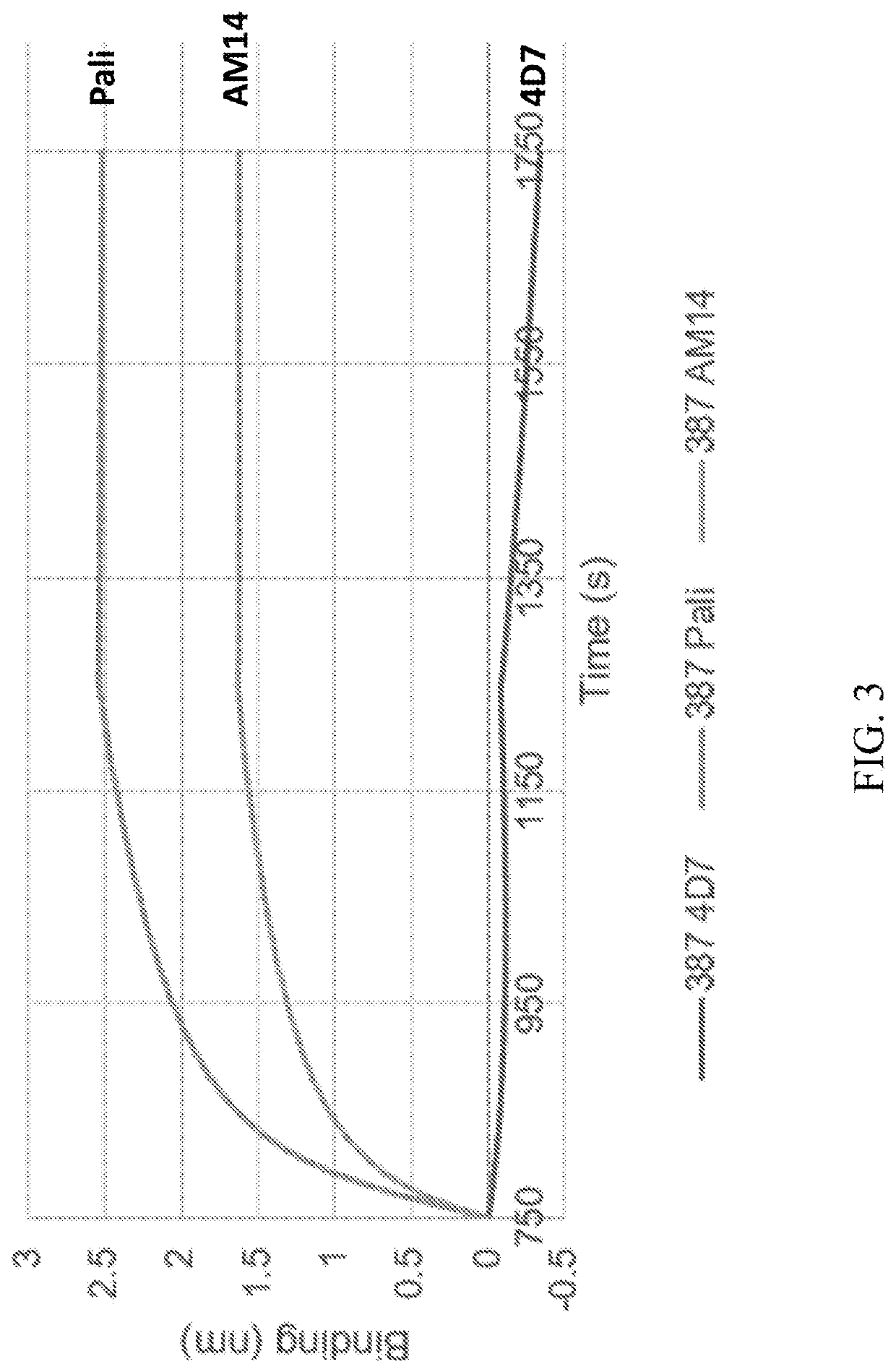
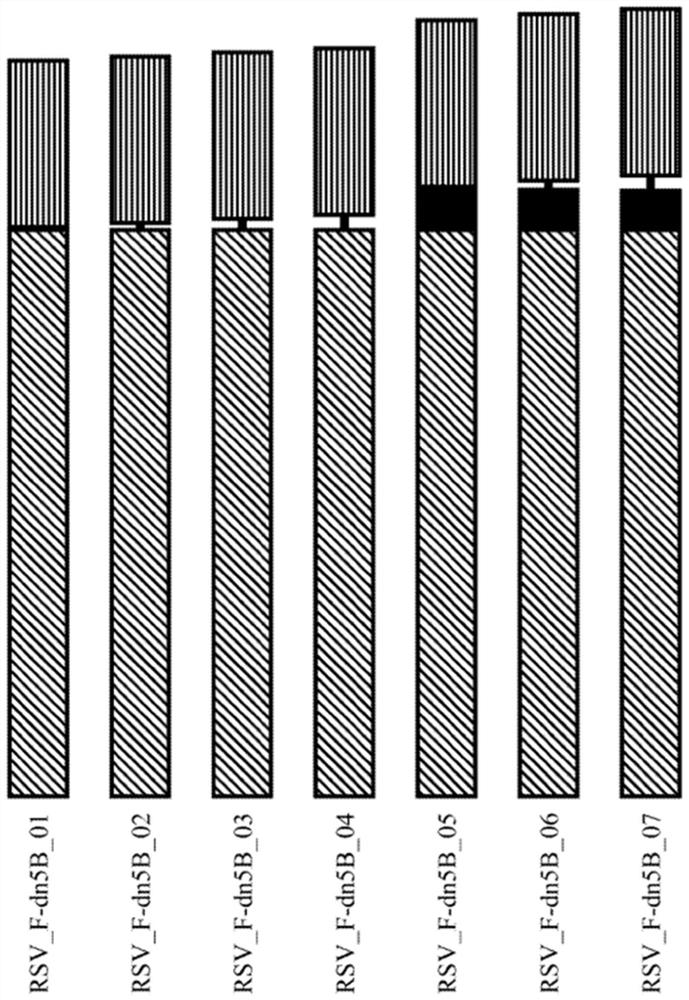
Self-Assembling Protein Nanostructures Displaying Paramyxovirus and/or Pneumovirus F Proteins and Their Use
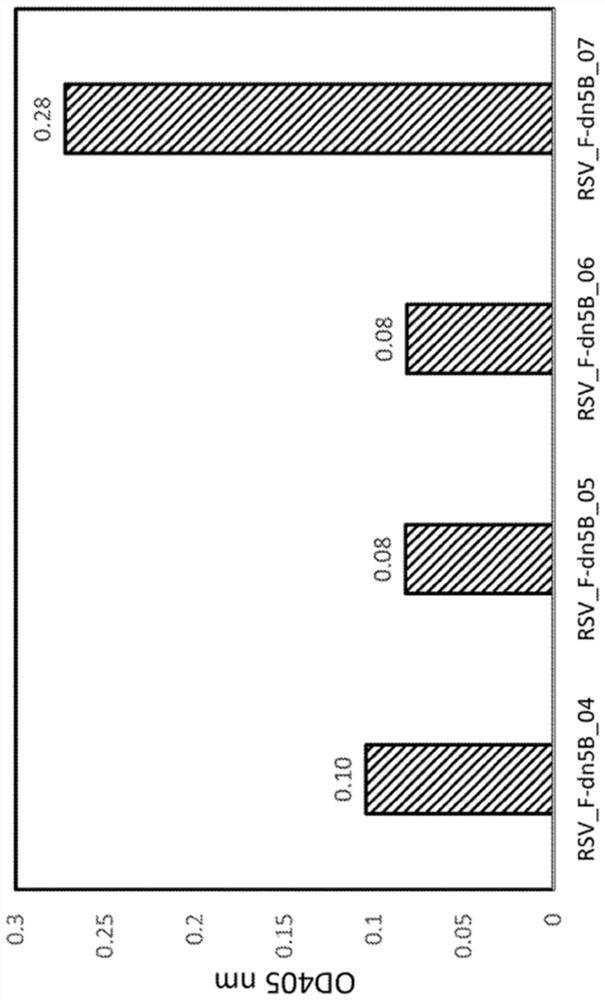
Disclosed herein are nanostructures and their use, where the nanostructures include a plurality of first assemblies, each first assembly comprising a plurality of identical first polypeptides selected from 153_dn5A, 153_dn5A.1 and I53_dn5A.2, or variants thereof; and a plurality of second assemblies, each second assembly comprising a plurality of identical second polypeptides being 153 dn5B or a variant thereof, wherein the plurality of first assemblies non-covalently interact with the plurality of second assemblies to form a nanostructure; and wherein the nanostructure displays multiple copies of one or more paramyxovirus and / or pneumovirus F proteins, or antigenic fragments thereof.
Owner:UNIV OF WASHINGTON
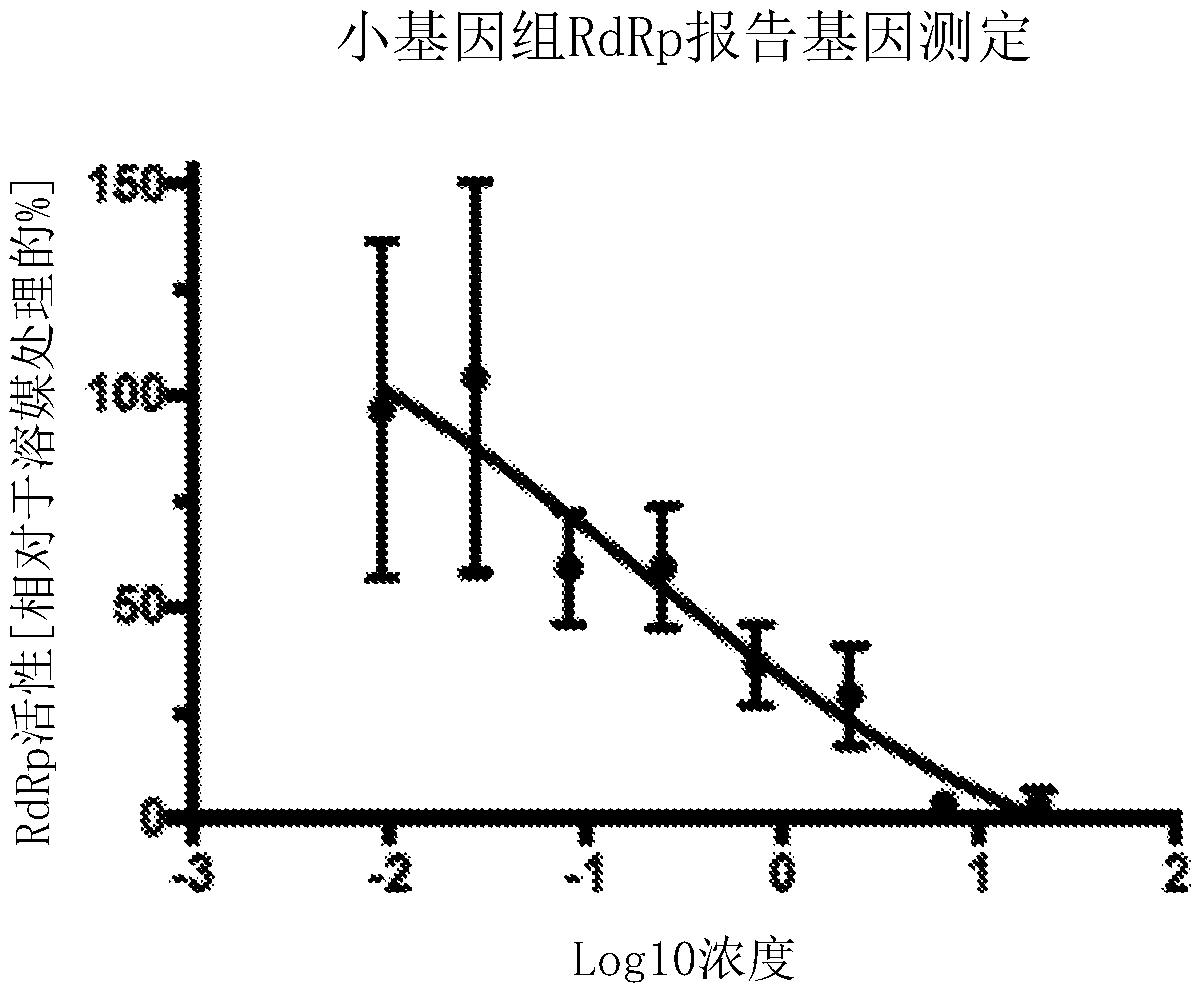
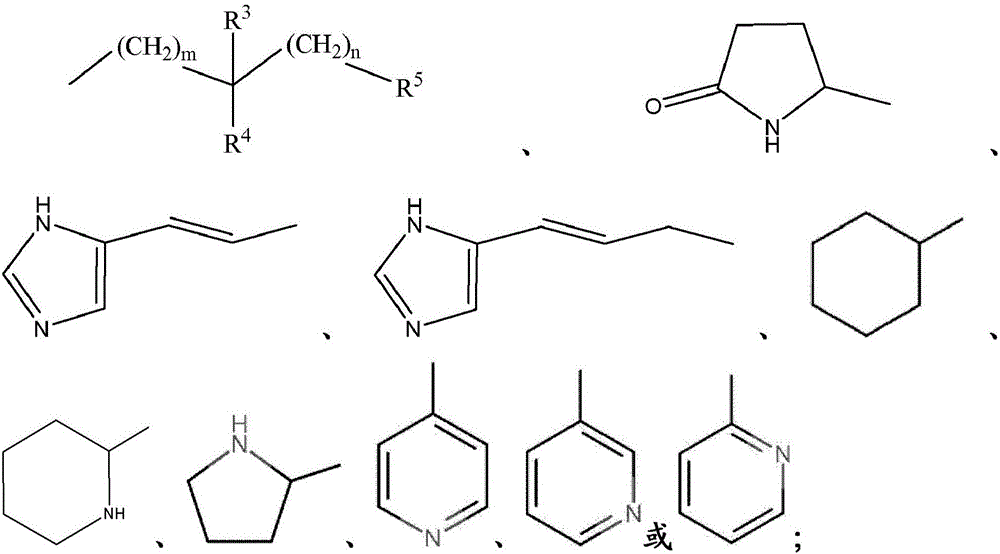
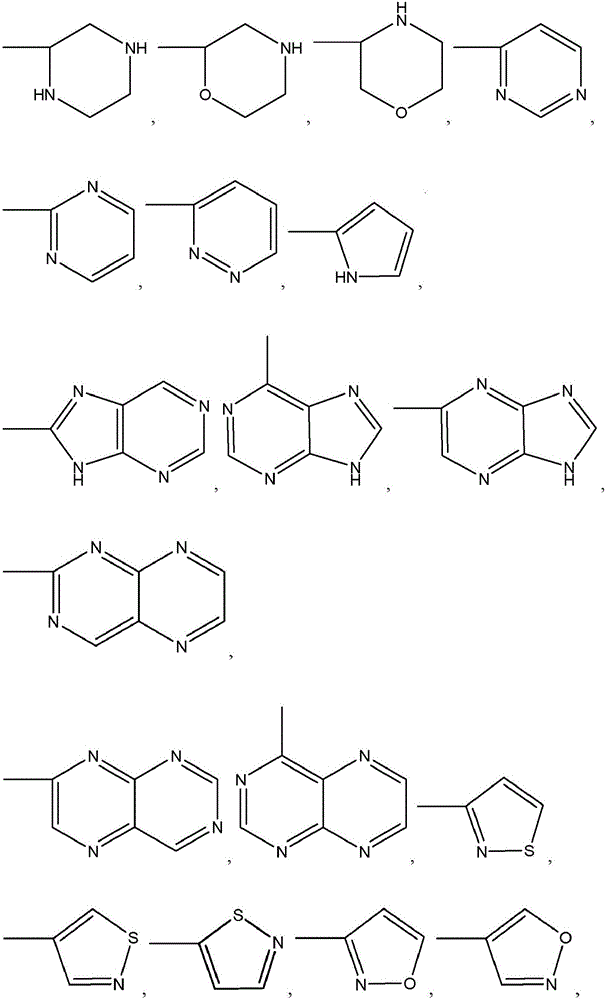
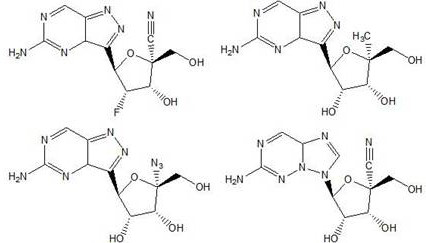
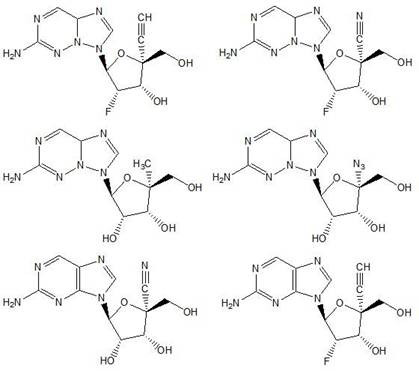
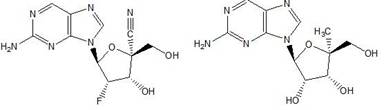
Thieno[3,2‑d]pyrimidines, furo[3,2‑d]pyrimidines, and pyrrolo[3,2‑d]pyrimidines for the treatment of respiratory syncytial virus infection
ActiveCN106573939AEsterified saccharide compoundsSaccharide with heterocyclic radicalsViral infectionPyrrole
The application provides preparations, methods and substituted thieno[3,2-d]pyrimidines, furo[3,2-d]pyrimidines and pyrrolo[ 3,2‑d]pyrimidine compounds, wherein the pneumovirinae virus infection includes respiratory syncytial virus infection, and thieno[3,2‑d]pyrimidine, furo[3,2 Processes and intermediates for ‑d]pyrimidine and pyrrolo[3,2‑d]pyrimidine compounds.
Owner:GILEAD SCI INC
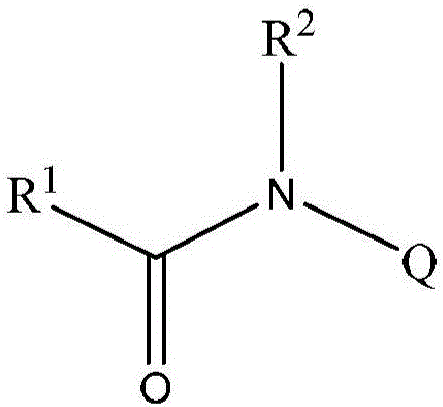
Amide compounds and methods for the production and use thereof
This invention relates to the field of medicine and concerns a method for the prophylaxis or treatment of diseases caused by RNA- and DNA-containing viruses, and concomitant diseases, which envisages the use of an effective amount of a compound of general formula I or a pharmaceutically acceptable salt thereof. The invention also relates to methods for producing the aforesaid compounds, and to pharmaceutical compositions for the prophylaxis or treatment of diseases caused by RNA- and DNA-containing viruses, which contain an effective amount of a compound of general formula I or a pharmaceutically acceptable salt thereof. The invention solves the problem of providing a novel agent effective in the treatment of diseases caused by RNA-containing viruses belonging to the enterovirus genus, the metapneumovirus genus, the pneumovirus genus, the respirovirus genus or the alphacoronavirus genus, and DNA-containing viruses belonging to the adenovirus family and the herpesvirus family, and also effective in the prophylaxis and treatment of asthma exacerbations, chronic obstructive pulmonary disease, cystic fibrosis, conjunctivitis, gastroenteritis, hepatitis and myocarditis, and in the prophylaxis and treatment of rhinorrhea, acute and infectious rhinitis, pharyngitis, nasopharyngitis, tonsillitis, laryngitis, laryngotracheitis, laryngotracheobronchitis, bronchitis, bronchiolitis, pneumonia or obstructive airways syndrome.
Owner:OBSCHESTVO S OGRANICHENNOI OTVETABTVENNOSTIYU PHARMENTERPRISES
Compound for treating viral infection and preparation method and application of compound
The invention provides a preparation for treating viral infection and pneumovirus subfamily viral infection, a method, a compound as shown in a formula (I) and a method and intermediate for synthesis of the compound as shown in the formula (I).
Owner:HC SYNTHETIC PHARMA CO LTD
Respiratory syncytial virus with a genomic deficiency complemented in trans
ActiveUS20100291035A1Improve efficiencyPrevent lower respiratory tract infection—willSsRNA viruses negative-senseBiocideDiseaseAttachment protein
The invention relates to pneumoviral virions comprising a viral genome that has a mutation in a gene coding for a protein that is essential for infectivity of the pneumovirus, whereby the mutation causes a virus produced from only the viral genome to lack infectivity, and whereby the virion comprises the protein in a form and in an amount that is required for infectivity of the virion. The invention also relates to methods for producing the pneumoviral virions and for using the virions in the treatment or prevention of pneumoviral infection and disease. A preferred pneumoviral virion is a virion of Respiratory Syncytial Virus in which preferably the gene for the G attachment protein is inactivated and complemented in trans.
Owner:INTRAVACC BV
Mucosal adjuvant composition
ActiveUS20130302360A1Antibacterial agentsSsRNA viruses negative-senseCulture fluidCanine distemper virus CDV
Problem to be Solved: The present invention provides a novel mucosal adjuvant.Solution: The present invention provides a mucosal adjuvant composition containing at least a composition comprising molecules having a molecular weight in a range of 100 to 300 kDa obtained from cells or culture fluid of Bordetella bronchiseptica. Administration of the mucosal adjuvant composition to a non-human animal at a surface of the mucous membrane can enhance immunity at the surface of mucous membrane. Therefore preventive effects against trans-mucosal infection can be increased by administering an inactivated vaccine against trans-mucosal infection and the mucosal adjuvant composition to a non-human animal at a surface of mucous membrane. The present invention is effective for preventing trans-mucosal infections, including one or more infections of e.g., canine parainfluenza, canine adenovirus, canine coronavirus, canine parvovirus, canine distemper virus, canine herpesvirus, reovirus and pneumovirus.
Owner:KYORITSU SEIYAKU
Live attenuated vaccines for pneumoviruses and related methods and materials
Described herein are mutant pneumoviruses comprising a nucleotide sequence which encodes a mutated zinc binding motif in an M2-1 protein of the pneumovirus, wherein the zinc binding motif is mutated relative to wild-type pneumovirus. The mutant pneumoviruses described herein grow to high titer in cell culture, are genetically stable, are attenuated in vitro and in vivo, and are highly immunogenic. Also described herein are vaccines and vaccine compositions comprising the live attenuated mutant pneumoviruses. Vaccine compositions can further comprise a pharmaceutically acceptable carrier, vehicle, excipient, and / or adjuvant. Methods for inducing a protective immune response in a subject against a pneumovirus infection are also described and disclosed. The vaccine compositions and methods described herein can be used to prevent metapneumovirus and respiratory syncytial virus infection in humans, respiratory syncytial virus infection in cattle, avian metapneumovirus infection in various avian species, and pneumonia virus of mice in rodents.
Owner:RES INST AT NATIONWIDE CHILDRENS HOSPITAL +1
SELF-ASSEMBLY PROTEIN NANOSTRUCTURES DISPLAYING PARAmyxoVIRUS AND/OR
Owner:UNIV OF WASHINGTON
LAMP (Loop-medicated isothermal amplification) detection kit for metapneumovirus
ActiveCN102943130BQuick checkLow costMicrobiological testing/measurementMicroorganism based processesPneumovirusNucleotide
The invention discloses an LAMP (loop-medicated isothermal amplification) detection kit for metapneumovirus. The kit internally comprises a primer set formed by six primers designed aiming at eight regions of a metapneumovirus F gene conserved region, wherein the nucleotide sequence of the primer set is as shown in a sequence table from sequence 1 to sequence 6. The detection kit disclosed by the invention can be used for specifically detecting the metapneumovirus, wherein the minimum detection limit of the detection kit is 100fg / muL, and the sensitivity of the detection kit is 100 times as high as that of the normal RT-PCR (reverse transcription-polymerase chain reaction). The LAMP detection kit is quick to detect, low in cost, free from expensive apparatuses, simpler in operation method, and suitable for the quick detection in the clinic.
Owner:GUANGXI VETERINARY RES INST
Multifunctional sterilizing and disinfecting incense as well as preparation method and application thereof
PendingCN114668026AHas a bactericidal and detoxifying effectComply with the regulations and standardsBiocideDisinfectantsBiotechnologyControl mosquito
The invention discloses multifunctional sterilization and disinfection incense as well as a preparation method and application thereof. The multifunctional sterilization and disinfection incense is mainly prepared from folium artemisiae argyi stems, rhizoma atractylodis rhizomes, agastache rugosus leaf stems, wild chrysanthemum flowers, asarum, rhizoma nardostachyos, acorus calamus and spices. The traditional Chinese medicine composition has sterilization and disinfection effects, mosquito and fly killing effects and effects of pre-controlling and inhibiting viruses and germs such as new coronal pneumovirus and diplococcus pneumoniae in a human body, the pre-controlling rate reaches 35% or above through smoking contrast practice tests, and the immune effect rate is increased by 25% or above.
Owner:葛加君
Novel Pneumovirus Compositions and Methods For Using the Same
InactiveUS20120288522A1SsRNA viruses negative-senseChemiluminescene/bioluminescenceDiseasePneumovirus
Provided are newly identified pneumoviruses that can infect mammals, including dogs cats and potentially humans. Isolated polynucleotides and proteins of the viruses, as well as the isolated viruses themselves are provided. The invention includes compositions and methods for detecting the viruses, methods and compositions for prophylaxis and / or therapy of disease signs that are positively correlated with the presence of the viruses, and isolated cells comprising the viruses. Intact virions, viral proteins, and fragments thereof are also provided.
Owner:CORNELL UNIVERSITY
Pneumovirus NS proteins antagonising interferon (IFN) response
InactiveCN100473721CSsRNA viruses negative-sensePeptide/protein ingredientsPneumovirusStructural protein
The present invention relates to the use of a pneumovirus NS1 protein and / or NS2 protein or a nucleic acid encoding pneumovirus NS1 protein and / or NS2 protein for the preparation of a pharmaceutical formulation for reducing the immune response mediated by interferon (IFN). The invention further relates to recombinant pneumoviruses, in particular respiratory syncytial viruses (RSV), having an increased, reduced, or lacking a resistance to the interferon (IFN) mediated immune response, recombinant viruses having an increased resistance to the interferon (IFN) mediated immune response, and the use of the viruses in pharmaceutical applications, e.g. as vaccines.
Owner:WYETH LLC
Thieno[3,2-d]pyrimidines, furo[3,2-d]pyrimidines, and pyrrolo[3,2-d]pyrimidines for the treatment of respiratory syncytial virus infection
ActiveCN106573939BEsterified saccharide compoundsOrganic active ingredientsPneumovirusViral infection
The application provides preparations, methods and substituted thieno[3,2-d]pyrimidines, furo[3,2-d]pyrimidines and pyrrolo[ 3,2‑d]pyrimidine compounds, wherein the pneumovirinae virus infection includes respiratory syncytial virus infection, and thieno[3,2‑d]pyrimidine, furo[3,2 Processes and intermediates for ‑d]pyrimidine and pyrrolo[3,2‑d]pyrimidine compounds.
Owner:GILEAD SCI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Thieno[3,2‑d]pyrimidines, furo[3,2‑d]pyrimidines, and pyrrolo[3,2‑d]pyrimidines for the treatment of respiratory syncytial virus infection Thieno[3,2‑d]pyrimidines, furo[3,2‑d]pyrimidines, and pyrrolo[3,2‑d]pyrimidines for the treatment of respiratory syncytial virus infection](https://images-eureka.patsnap.com/patent_img/11446f67-1c8d-45b3-9d3b-290a894bde03/BDA0001219126510000021.png)
![Thieno[3,2‑d]pyrimidines, furo[3,2‑d]pyrimidines, and pyrrolo[3,2‑d]pyrimidines for the treatment of respiratory syncytial virus infection Thieno[3,2‑d]pyrimidines, furo[3,2‑d]pyrimidines, and pyrrolo[3,2‑d]pyrimidines for the treatment of respiratory syncytial virus infection](https://images-eureka.patsnap.com/patent_img/11446f67-1c8d-45b3-9d3b-290a894bde03/BDA0001219126510000031.png)
![Thieno[3,2‑d]pyrimidines, furo[3,2‑d]pyrimidines, and pyrrolo[3,2‑d]pyrimidines for the treatment of respiratory syncytial virus infection Thieno[3,2‑d]pyrimidines, furo[3,2‑d]pyrimidines, and pyrrolo[3,2‑d]pyrimidines for the treatment of respiratory syncytial virus infection](https://images-eureka.patsnap.com/patent_img/11446f67-1c8d-45b3-9d3b-290a894bde03/BDA0001219126510000032.png)

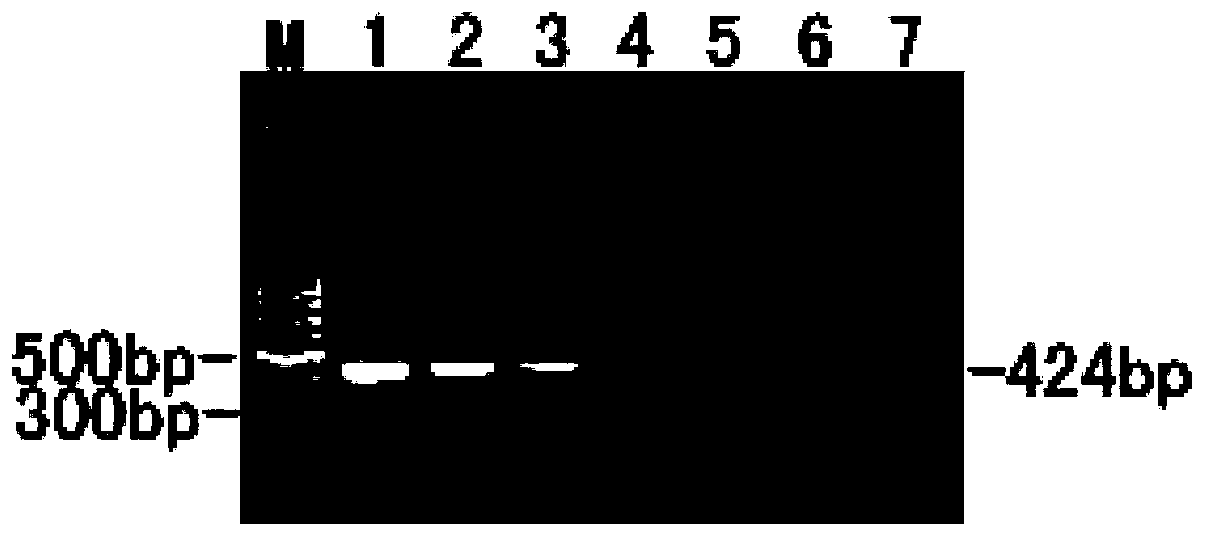

![Thieno[3,2-d]pyrimidines, furo[3,2-d]pyrimidines, and pyrrolo[3,2-d]pyrimidines for the treatment of respiratory syncytial virus infection Thieno[3,2-d]pyrimidines, furo[3,2-d]pyrimidines, and pyrrolo[3,2-d]pyrimidines for the treatment of respiratory syncytial virus infection](https://images-eureka.patsnap.com/patent_img/8cdb5eaa-5bf6-4583-b1f0-942a437a708f/BDA0001219126510000021.png)
![Thieno[3,2-d]pyrimidines, furo[3,2-d]pyrimidines, and pyrrolo[3,2-d]pyrimidines for the treatment of respiratory syncytial virus infection Thieno[3,2-d]pyrimidines, furo[3,2-d]pyrimidines, and pyrrolo[3,2-d]pyrimidines for the treatment of respiratory syncytial virus infection](https://images-eureka.patsnap.com/patent_img/8cdb5eaa-5bf6-4583-b1f0-942a437a708f/BDA0001219126510000031.png)
![Thieno[3,2-d]pyrimidines, furo[3,2-d]pyrimidines, and pyrrolo[3,2-d]pyrimidines for the treatment of respiratory syncytial virus infection Thieno[3,2-d]pyrimidines, furo[3,2-d]pyrimidines, and pyrrolo[3,2-d]pyrimidines for the treatment of respiratory syncytial virus infection](https://images-eureka.patsnap.com/patent_img/8cdb5eaa-5bf6-4583-b1f0-942a437a708f/BDA0001219126510000032.png)