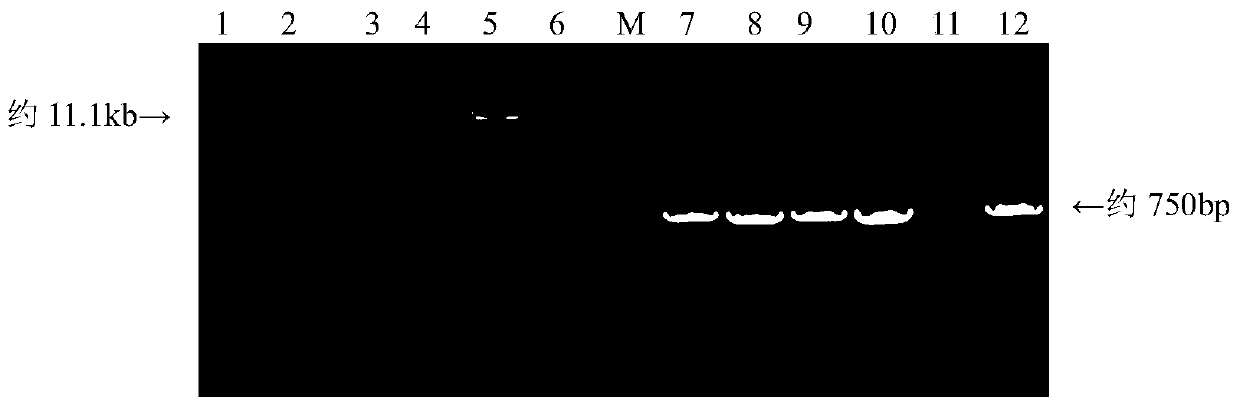
Patents
Literature
55 results about "Envelope Gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
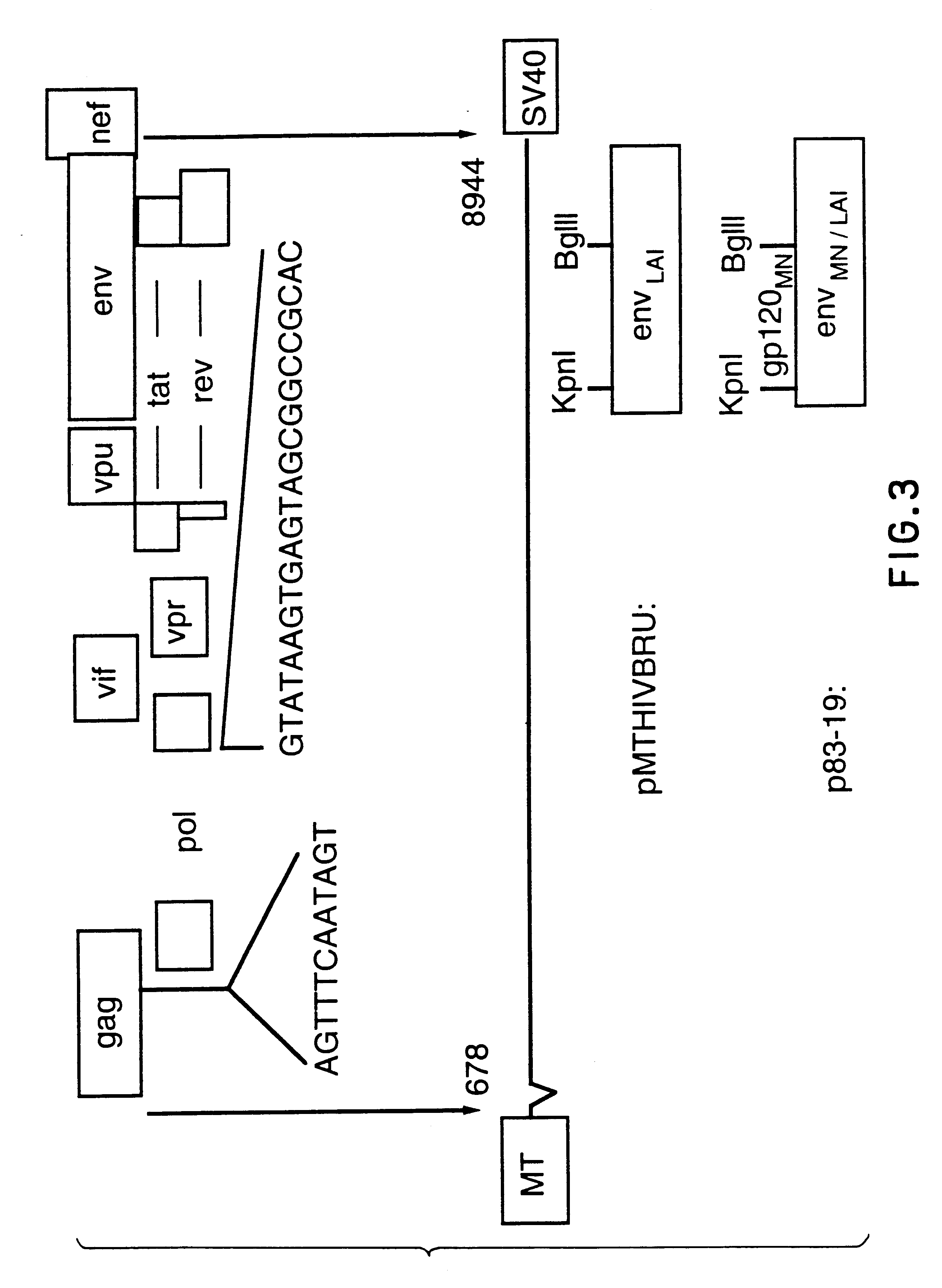
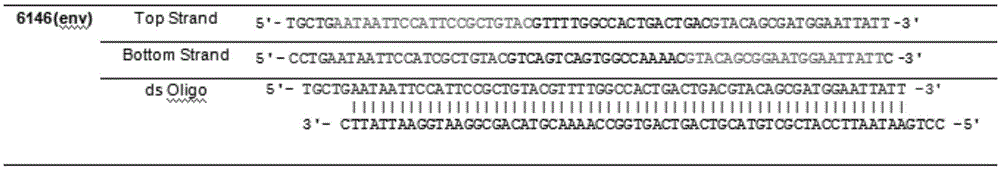
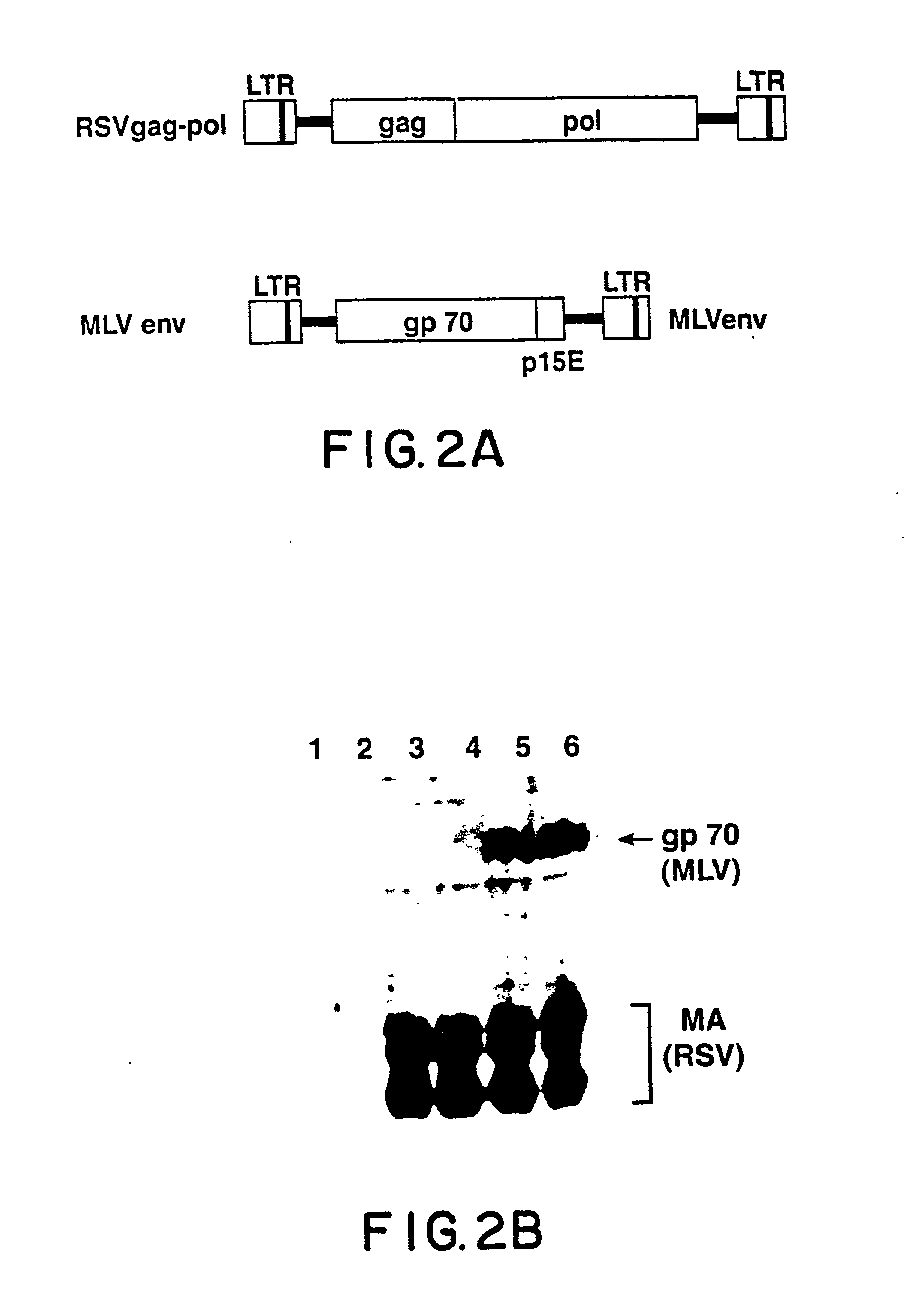
Env is a viral gene that encodes the protein forming the viral envelope. The expression of the env gene enables retroviruses to target and attach to specific cell types, and to infiltrate the target cell membrane.
Antigenically-marked non-infectious retrovirus-like particles
InactiveUS6291157B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesIn vivo
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:CONNAUGHT LAB
INDUCTION OF BROADLY REACTIVE NEUTRALIZING ANTIBODIES BY FOCUSING THE IMMUNE RESPONSE ON V3 EPITOPES OF THE HIV-1 gp120 ENVELOPE
InactiveUS20080279879A1Vigorous Ab responseViral antigen ingredientsAntibody mimetics/scaffoldsHeterologousNeutralizing antibody
Compositions, kits and methods for boosting, or for priming and boosting, high titer broadly neutralizing cross-clade antibody responses focused on single HIV-1 neutralizing epitopes are disclosed. gp120 DNA plasmids comprising HIV env genes are used to prime the antibody response. Primed subjects are immunized with recombinant fusion proteins that comprise a “carrier” protein fusion partner, preferably a truncated form of the MuLV gp70 Env protein, and a desired HIV neutralizing epitopes. Preferred epitopes are epitopes of V3 from one or more HIV clades. Immune sera from such immunized subjects neutralized primary isolates from virus strains heterologous to those from which the immunogens were constructed. Neutralizing activity was primarily due to V3-specific antibodies and cross-clade neutralizing Abs were present. This approach results in more potent and broader neutralizing antibody levels, a result of “immunofocusing” the humoral immune response on neutralizing epitopes such as V3.
Owner:NEW YORK UNIV
Molecular clones with mutated HIV gag/pol, SIV gag and SIV env genes
Nucleic acid constructs containing HIV-1 gag / pol and SIV gag or SIV env genes which have been mutated to remove or reduce inhibitory / instability sequences are disclosed. Viral particles and host cells containing these constructs and / or viral particles are also disclosed. The exemplified constructs and viral particles of the invention may be useful in gene therapy for numerous disorders, including HIV infection, or as a vaccine for HIV-1 immunotherapy and immunoprophylaxis.
Owner:UNITED STATES OF AMERICA
Diagnostic kits comprising genetically engineered human immunodeficiency virus-like particles containing heterologous antigenic markers
InactiveUS6342228B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesHeterologous
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:AVENTIS PASTEUR LTD
Trimer of HIV env gene expression product
InactiveUS6737067B1Peptide/protein ingredientsViral antigen ingredientsAdjuvantRecombinant glycoprotein
The invention concerns a purified recombinant glycoprotein having the following properties: a) an adherence capacity to CD4; b) an affinity with a anti-gp120 antibody capable of neutralizing in vitro HIV cell infection; c) an affinity with an anti-gp41 antibody; d) a timeric form free from inter-chain disulphide bonds. The invention also concerns a vaccine comprising said purified glycoprotein and an adjuvant. The invention further concerns a method for obtaining said glycoprotein, which consists in expressing, by means of genetic recombining techniques, a glycoprotein corresponding to the properties a), b) and c) mentioned above; purifying it, and subjecting it to steps involving at least a reducing agent, an ionic detergent and / or a neutral detergent in conditions leading to a glycoprotein having said properties.
Owner:AVENTIS PASTUER LTD
Antigentically-marked non-infectious retrovirus-like particles
InactiveUS6518030B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesIn vivo
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:AVENTIS PASTEUR LTD
Retrovirus vectors derived from avian sarcoma leukosis viruses permitting transfer of genes into mammalian cells
InactiveUS6096534AHigh titer stockEasy to usePolypeptide with localisation/targeting motifVectorsFowlAvian leukosis viruses
PCT No. PCT / US96 / 07370 Sec. 371 Date Nov. 28, 1997 Sec. 102(e) Date Nov. 28, 1997 PCT Filed May 22, 1996 PCT Pub. No. WO96 / 37625 PCT Pub. Date Nov. 28, 1996Recombinant avian sarcoma leukosis virus (ASLV)-derived retrovirus vectors having an expanded host range are described. The host range is expanded by the replacement of the ASLV envelope gene by an envelope gene from a virus capable of infecting both mammalian and avian cells. The resulting recombinant ASLV-derived retroviral vectors can replicate efficiently in avian cells, infect both avian and mammalian cells in high titer, and are replication-defective in mammalian cells. Thus, they are quite safe and advantageous for use in gene therapy and vaccines.
Owner:UNITED STATES OF AMERICA
Diagnosis method and therapeutic method for cancer
InactiveCN102215870AOrganic active ingredientsGenetic material ingredientsDiagnosis methodsEnvelope Gene
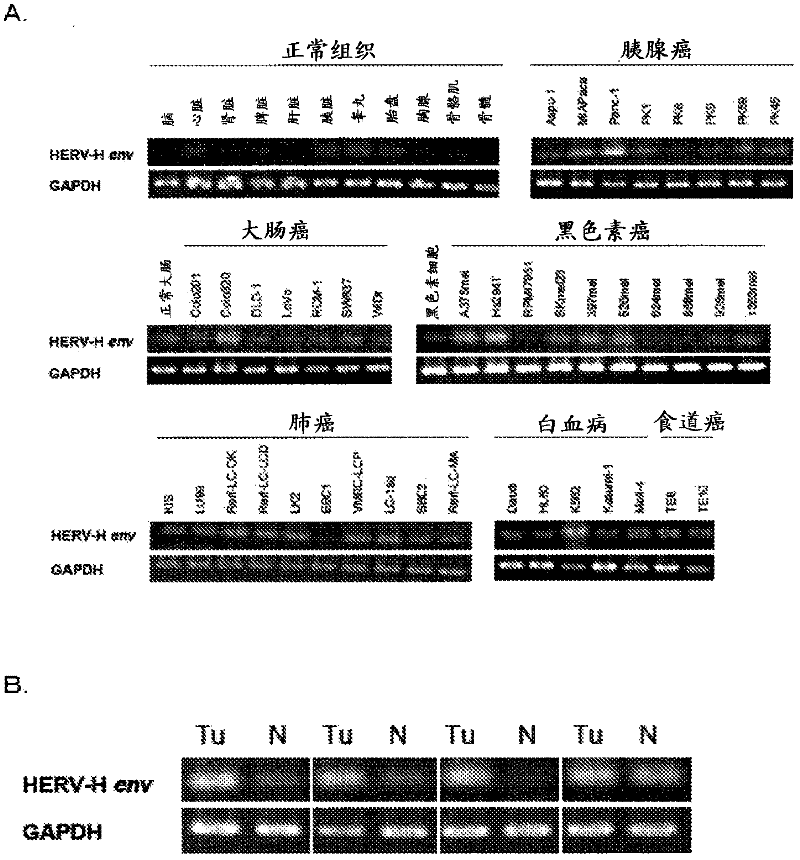
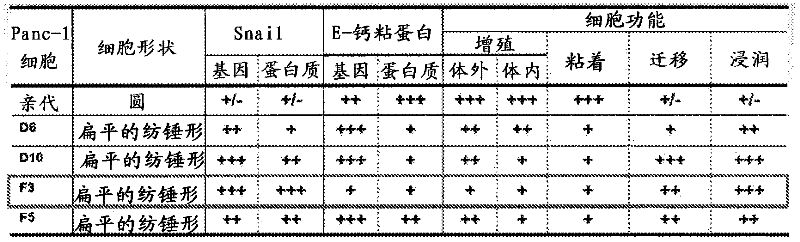
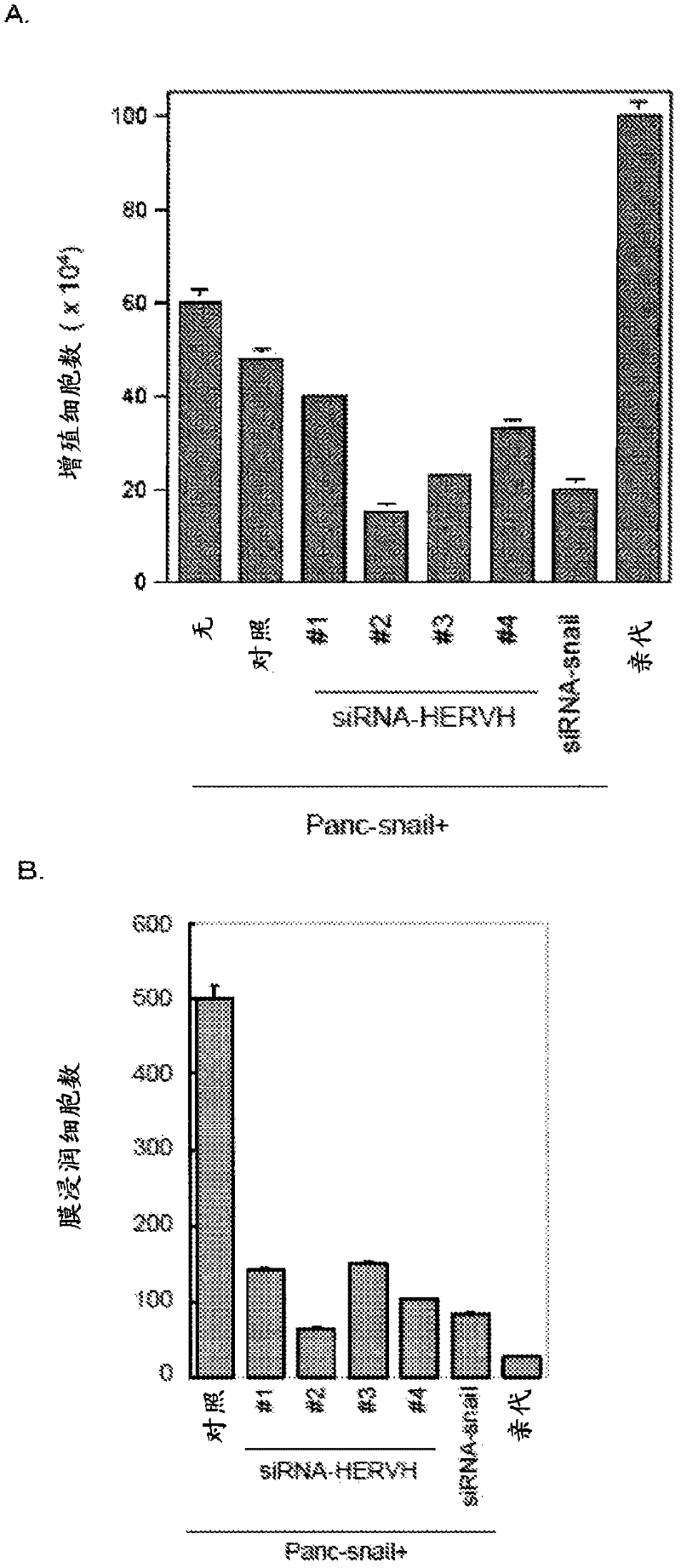
Disclosed are a diagnosis method and a therapeutic method for cancer, both of which utilize HERV-H env gene and protein. Particularly, cancer can be diagnosed by detecting the expression of HERV-H env gene, and a reagent used for the detection of the expression can be used as a diagnosing agent. Cancer can be treated by inhibiting the function of HERV-H env gene, and a reagent used for the inhibition of the function can be used as an anti-cancer agent. Alternatively, cancer can be treated by administering a peptide having a specific sequence contained in HERV-H env protein or the like, and the peptide or the like can be used as a cancer vaccine.
Owner:KEIO UNIV
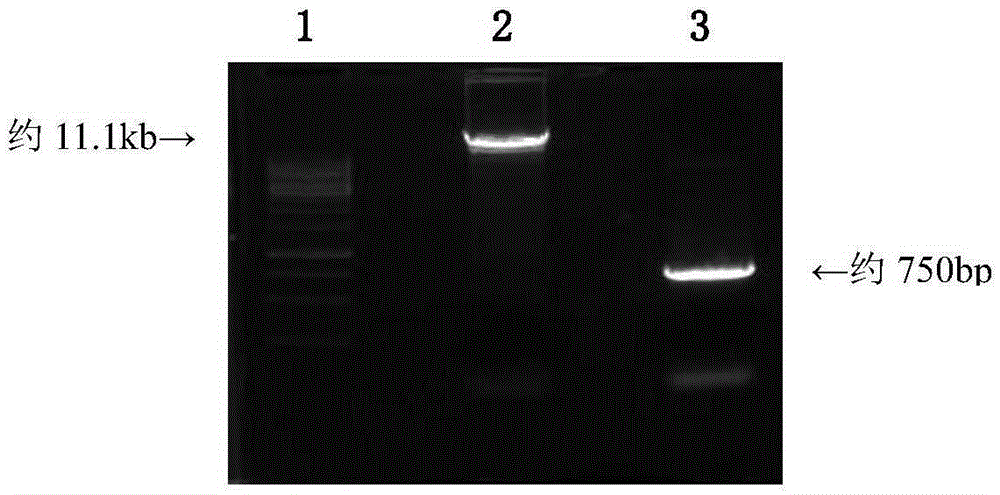
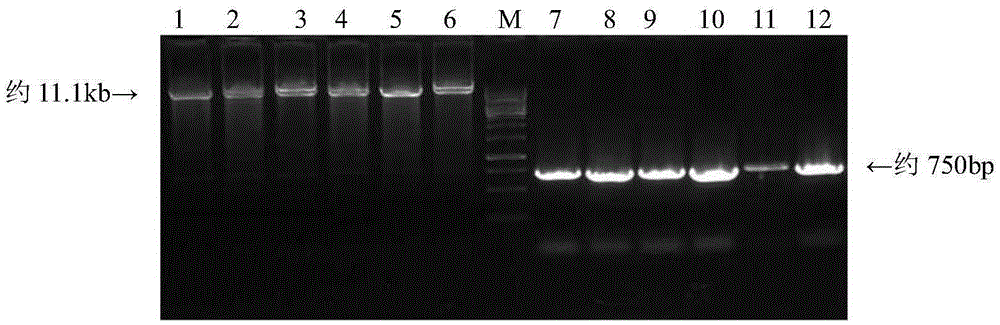
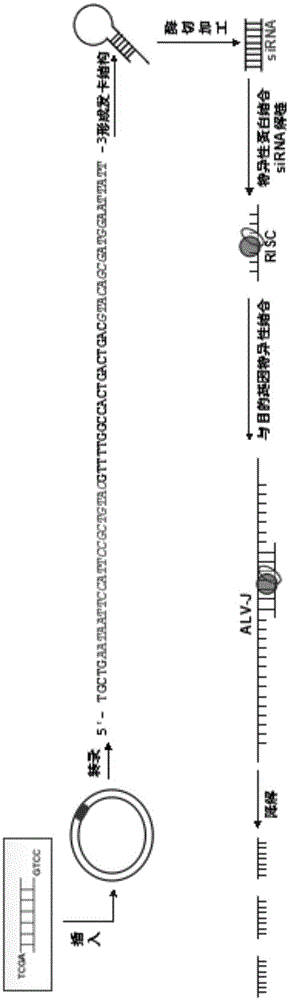
J avian leukosis virus subgroup env gene conserved sequence-based siRNA (Small Interfering RNA (Ribonucleic Acid)) recombinant interference carrier as well as preparation method and application thereof
InactiveCN103805635AEffective interferenceReduce economic lossGenetic material ingredientsAntiviralsConserved sequenceAvian leukosis viruses
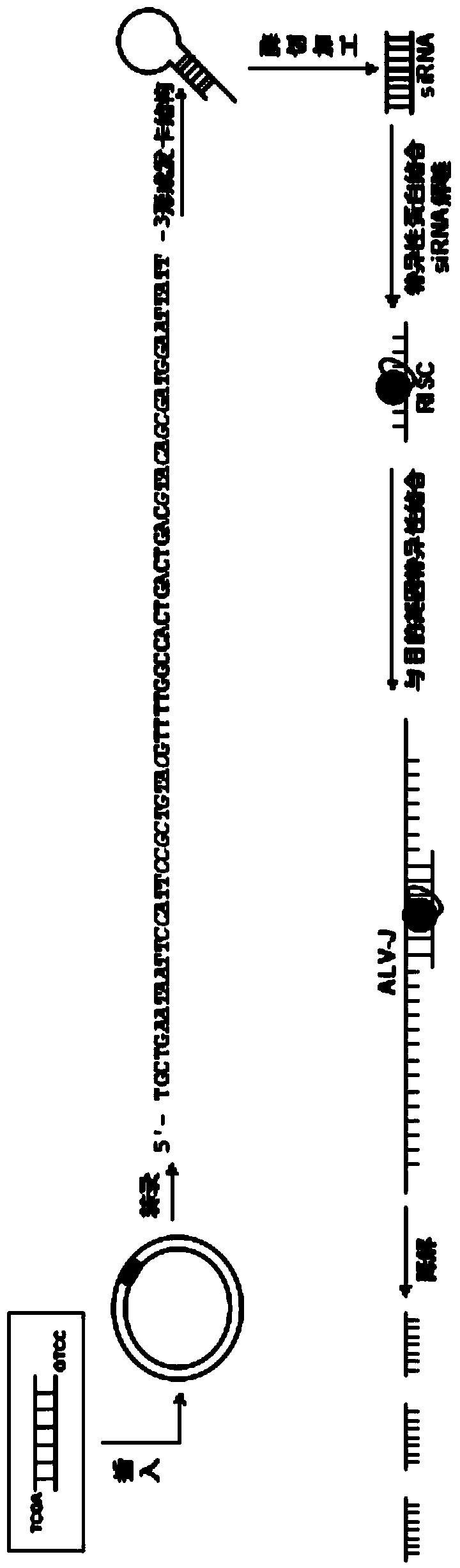
The invention relates to the technical field of genetic engineering, and provides a J avian leukosis virus subgroup env gene conserved sequence-based siRNA (Small Interfering RNA (Ribonucleic Acid)) recombinant interference carrier. The preparation method comprises the following steps: on the basis that the env gene has important meaning for ALV (avian leukosis virus), designing and synthesizing siRNA by the env gene, building to form a hairpin structure of siRNA, further obtaining annealing double-stranded DNA (Deoxyribonucleic Acid), and connecting the double-stranded DNA with the carrier to build the recombinant interference carrier. After the interference carrier and the virus co-transfect cells are adopted, the interference carrier provided by the invention can effectively interfere the transcription and the duplication of the J avian leukosis virus subgroup in the in-vitro cell and the live chicken, the scientific base and the technical support can be provided for preventing and curing the J avian leukosis virus subgroup, and the economic loss caused by the ALV-J infection in the poultry industry can be reduced.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Cell for Producing Retrovirus Vector
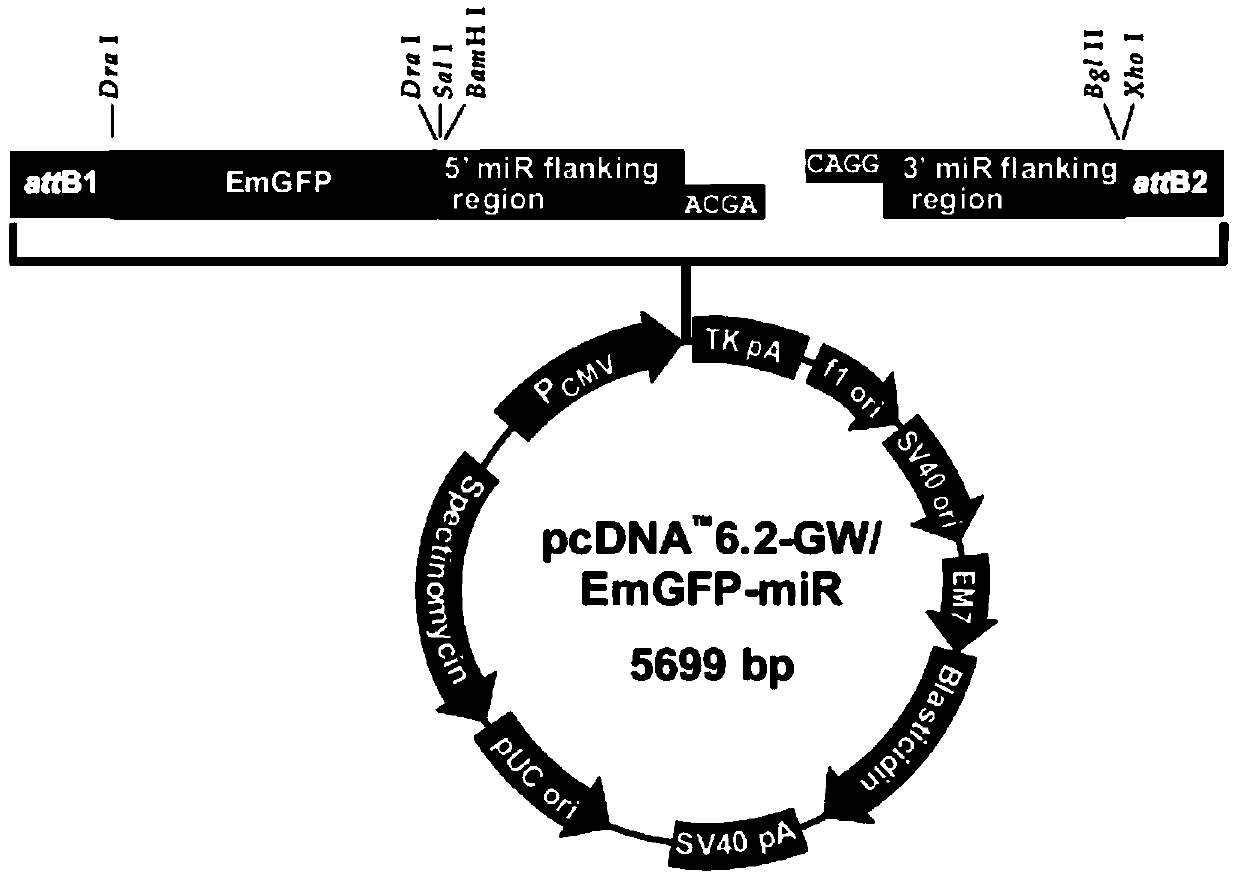
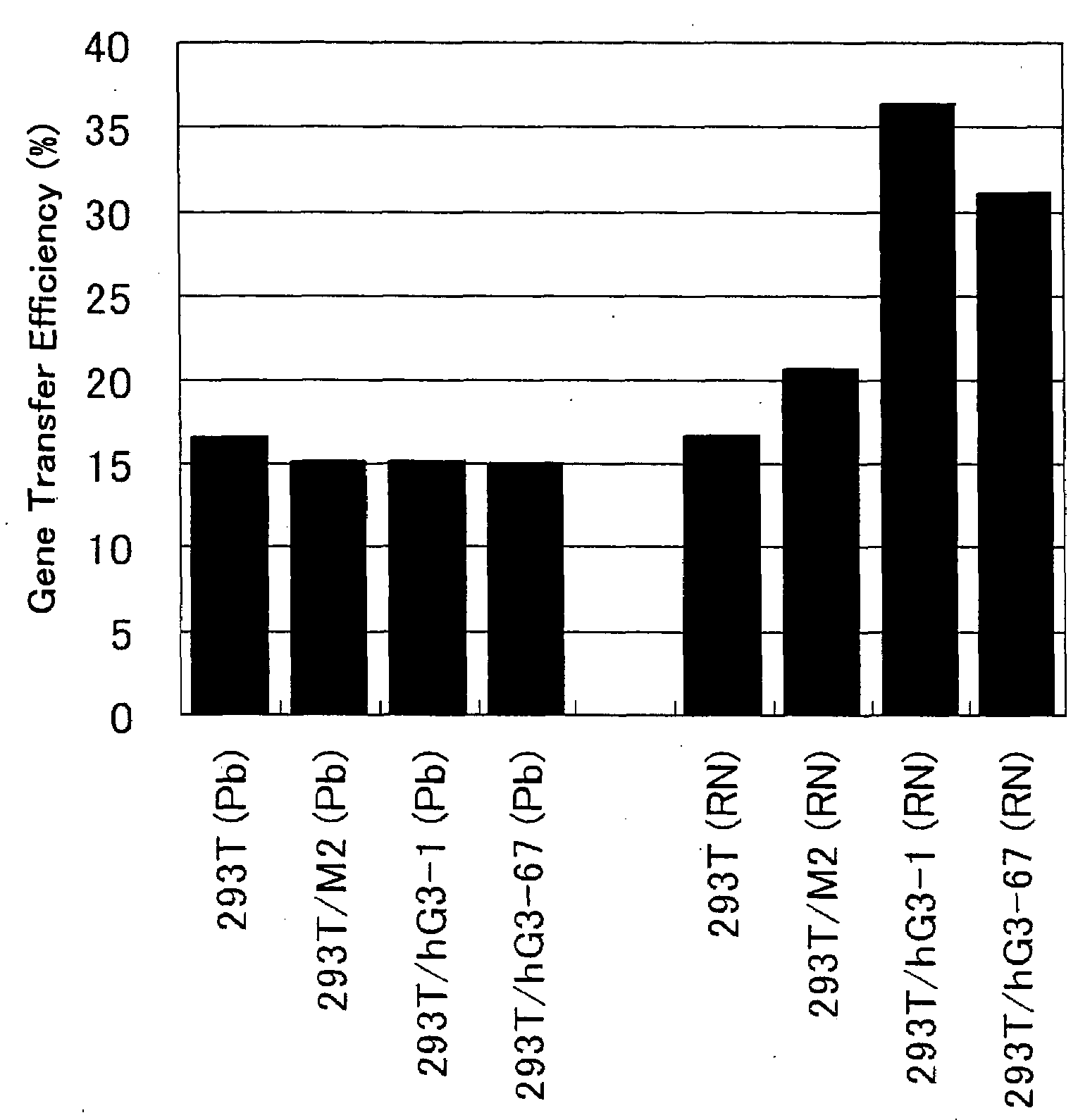
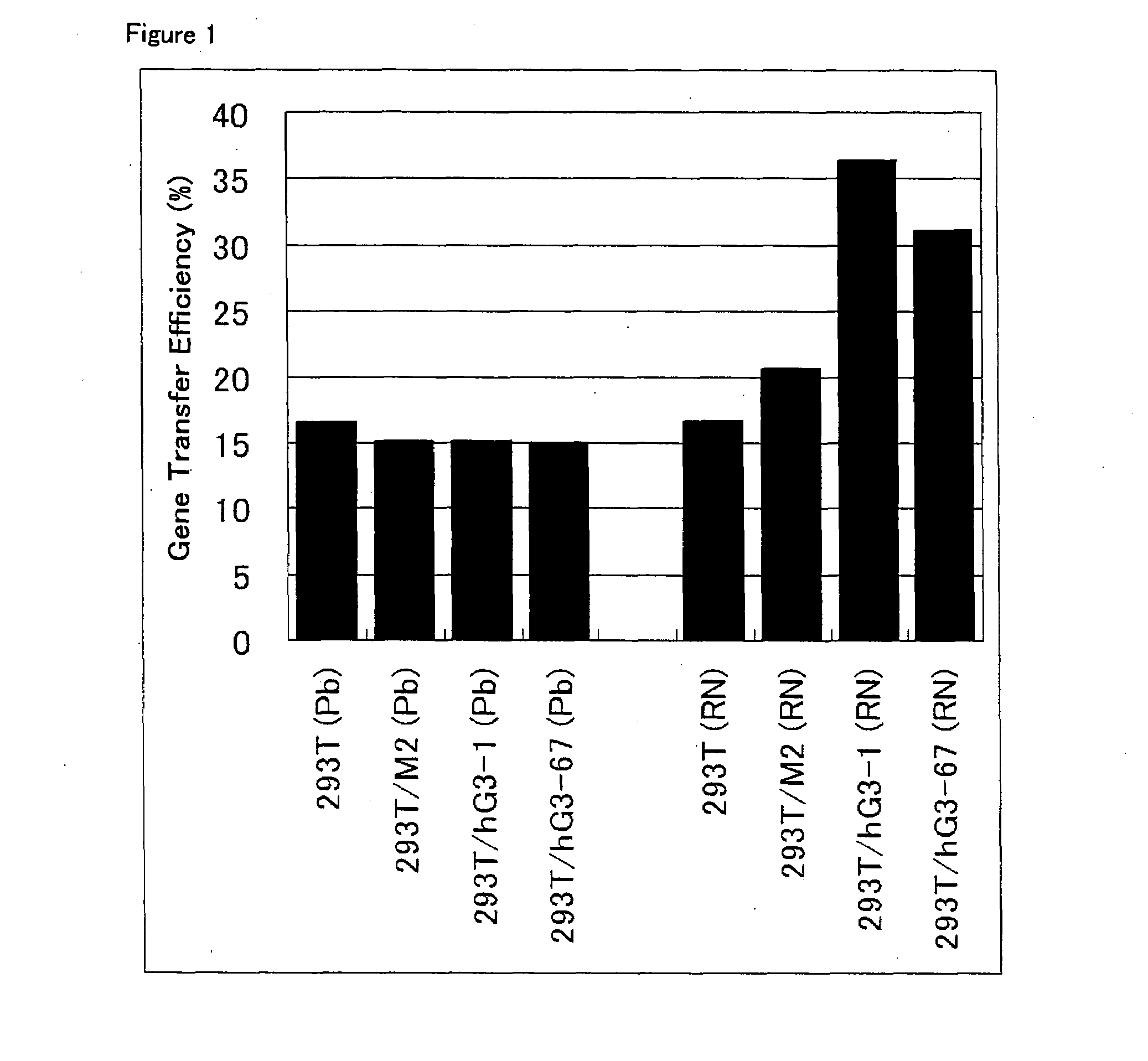
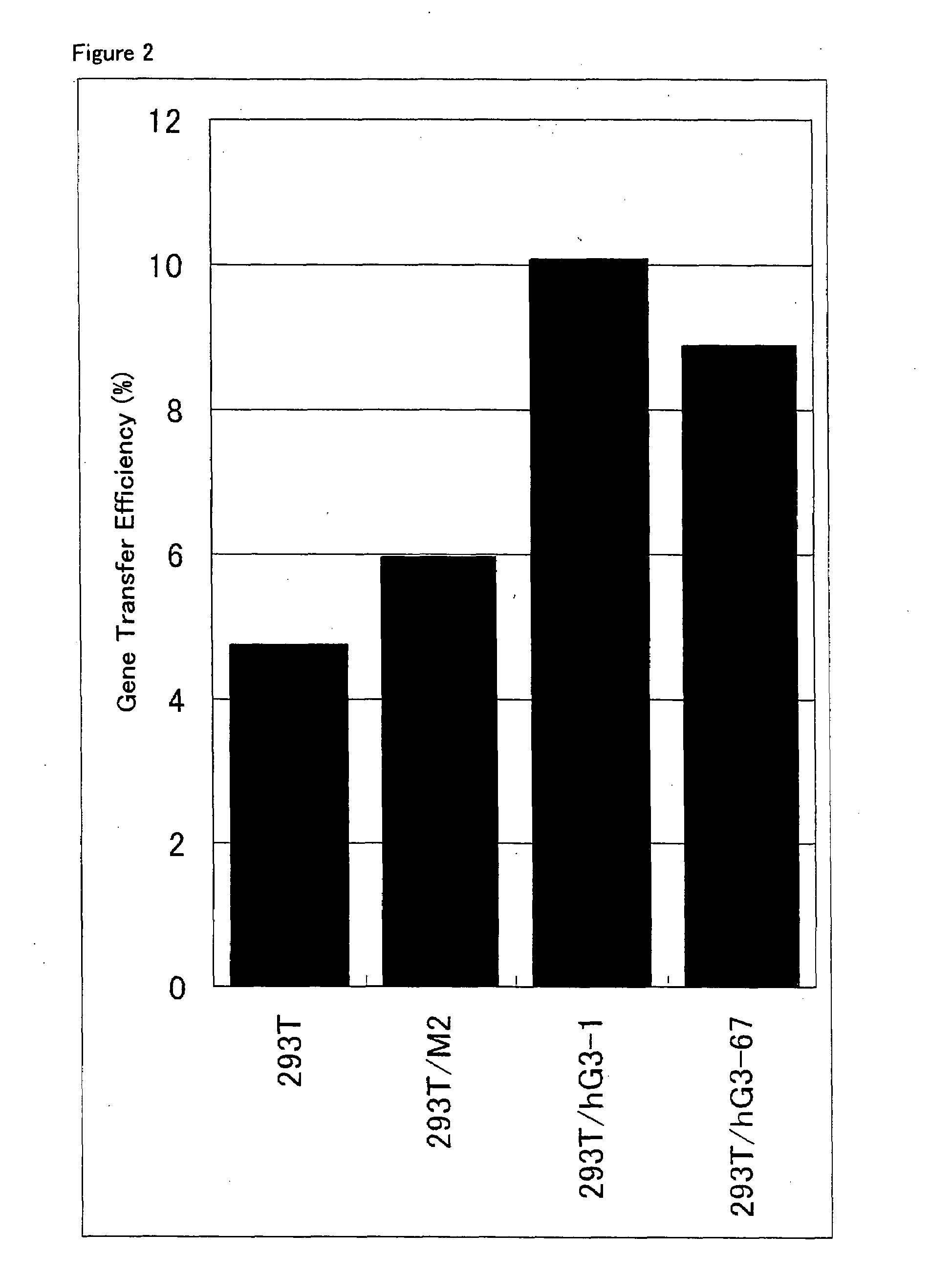
ActiveUS20080293141A1Improve gene transfer efficiencySmooth transferVectorsHybrid cell preparationPol genesEnvelope Gene
The N-acetylglucosaminyltransferase III activity is enhanced in a cell carrying retrovirus-origin gag-pol gene and env gene. By constructing a retrovirus vector with the use of the above cell, a retrovirus vector having a modified sugar chain structure can be obtained. The retrovirus vector constructed by this method shows a high infection efficiency particularly in the presence of a functional substance.
Owner:TAKARA HOLDINGS
Nucleotide and deduced amino acid sequences of the envelope 1 and core genes of isolates of hepatitis C virus and the use of reagents derived from these sequences in diagnostic methods and vaccines
The nucleotide and deduced amino acid sequences of cDNAs encoding the envelope 1 genes and core genes of isolates of hepatitis C virus (HCV) are disclosed. The invention relates to the oligonucleotides, peptides and recombinant envelope 1 and core proteins derived from these sequences and their use in diagnostic methods and vaccines.
Owner:UNITED STATES OF AMERICA
Monoclonal antibody of avian reticuloendotheliosis virus envelope protein and preparation method thereof
InactiveCN102304180APreserve the antigenic siteEliminate distractionsMicroorganism based processesImmunoglobulins against virusesAntigenBALB/c
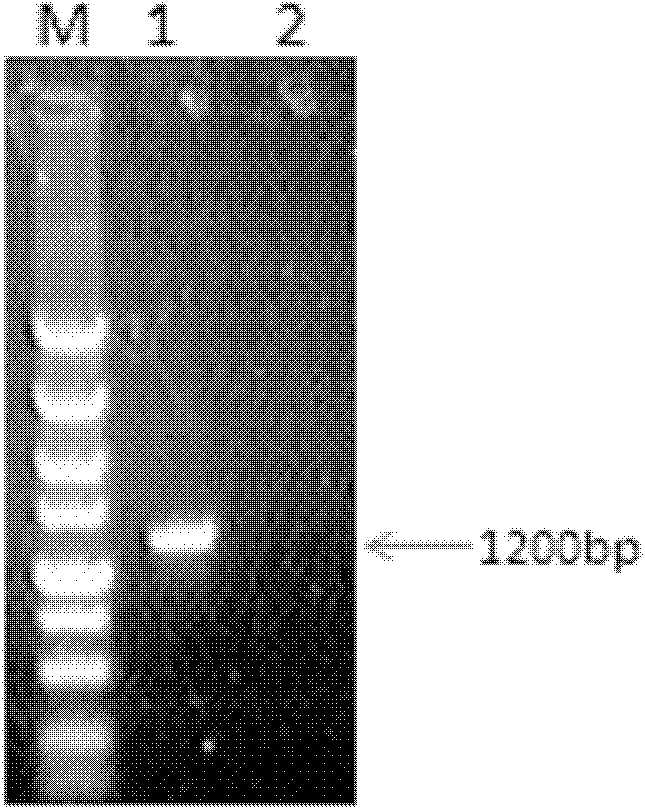
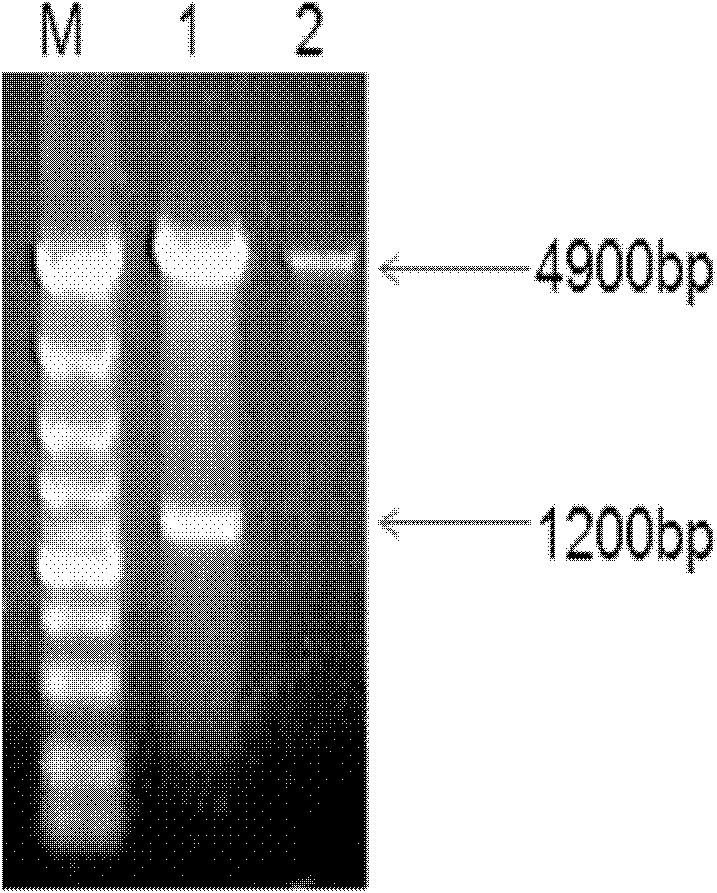
The invention belongs to the cross technical field of molecular immunology and virology, and particularly relates to a monoclonal antibody of avian reticuloendotheliosis virus envelope protein. The monoclonal antibody is characterized in that 1200bp of genetic fragment in a relative conservation region of an avian reticuloendotheliosis virus (REV) envelope (env) gene is selected, and 400 amino acids expressed by the fragment comprise more than 90% of antigen sites of the REV, so that most of the antigen sites of the virus are retained and the interference caused by genovariation is excluded, prokaryotic expression product His-env fusion protein is obtained by utilizing the env gene containing the conservation genetic fragment, the Balb / C mouse is immunized by the fusion protein, and the monoclonal antibody of the REV ENV (envelope) protein is obtained through fusion of oncocyte SP2 / 0 and splenocyte of the immunized mouse, screening and cloning. The monoclonal antibody provides a technical support for diagnosis and prevention as well as scientific research of avian reticuloendotheliosis.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Two-chain small-molecular interference ribonucleic acid and its compination for treating preventing and treating AIDS
The invention relates to double bond small molecule disturbance ribonucleic acid to prevent and cure AIDS. The double bond small molecule disturbance ribonucleic acid is RNA double bond molecule, which is called siRNA double bond molecule that has 19 base pairings. Two protrude basic groups dT are on the ends of 5' of positive bond and negative bond. GC content is 40-55%. Target sequence is gag gene, pol gene, vif gene, vpr gene, env gene, and nef gene selected from HIV gene. The positive bond sequence of siRNA double bond molecule is selected from SEQ ID NO.1-116, and the negative bond is corresponding to positive sequence. The invention conquers the problem of validity decreasing or losing effectiveness of siRNA because of the mutation of HIV-I virus.
Owner:李宝健
Primers and detection kit for avian leukosis J subgroup virus PCR detection
InactiveCN102943127AQuick checkAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationEnvelope GeneAvian leukosis viruses
The present invention provides primers and a detection kit for avian leukosis J subgroup virus PCR detection. According to the present invention, PCR detection primers are designed by analyzing avian leukosis J subgroup virus env gene, wherein nucleotide sequences of the primers are represented by sequence lists SEQ ID No.1 and SEQ ID No.2; and the primers have good specificity, and the detection method has characteristics of rapidness, simpleness, high accuracy and good sensitivity so as to provide an excellent detection method for avian leukosis J subgroup virus identification. The present invention further provides a detection kit for the avian leukosis virus, wherein the detection kit comprises a primer pair represented by the sequence lists SEQ ID No.1-2.
Owner:SICHUAN AGRI UNIV
MIA primer, probe and kit for detecting COVID-19 and application of MIA primer and probe
PendingCN112094947AEasy to detectGuaranteed responseMicrobiological testing/measurementAgainst vector-borne diseasesPneumovirusEnvelope Gene
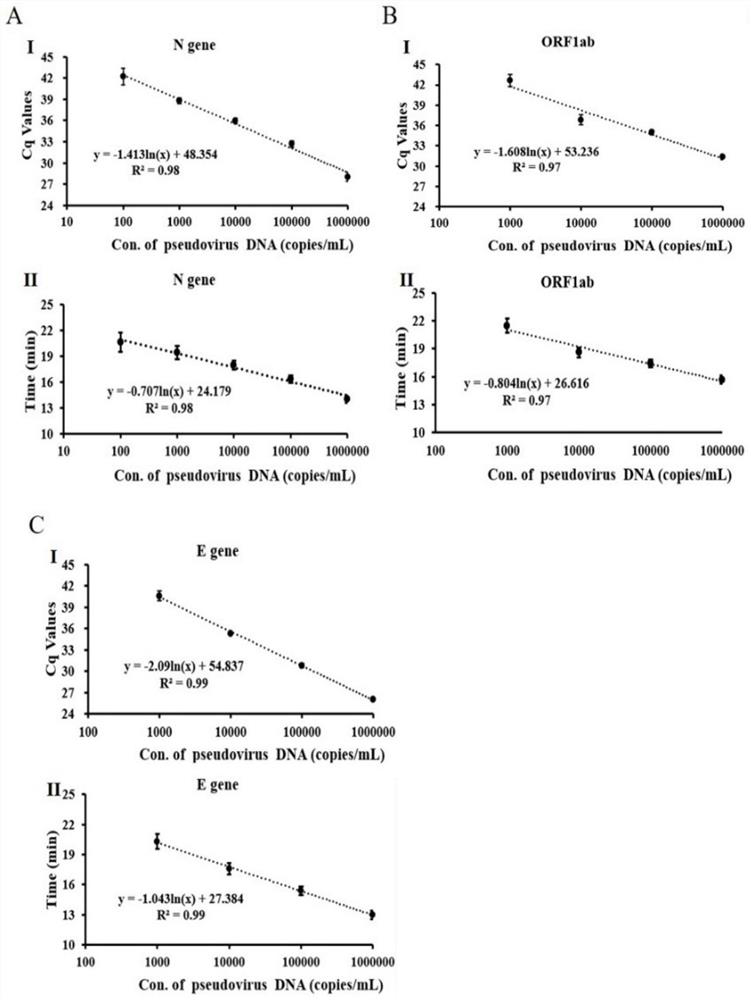
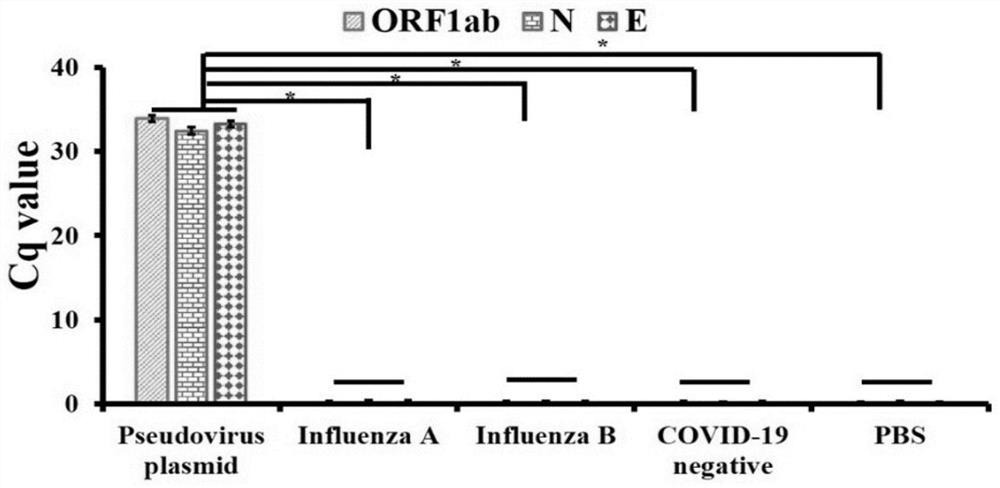
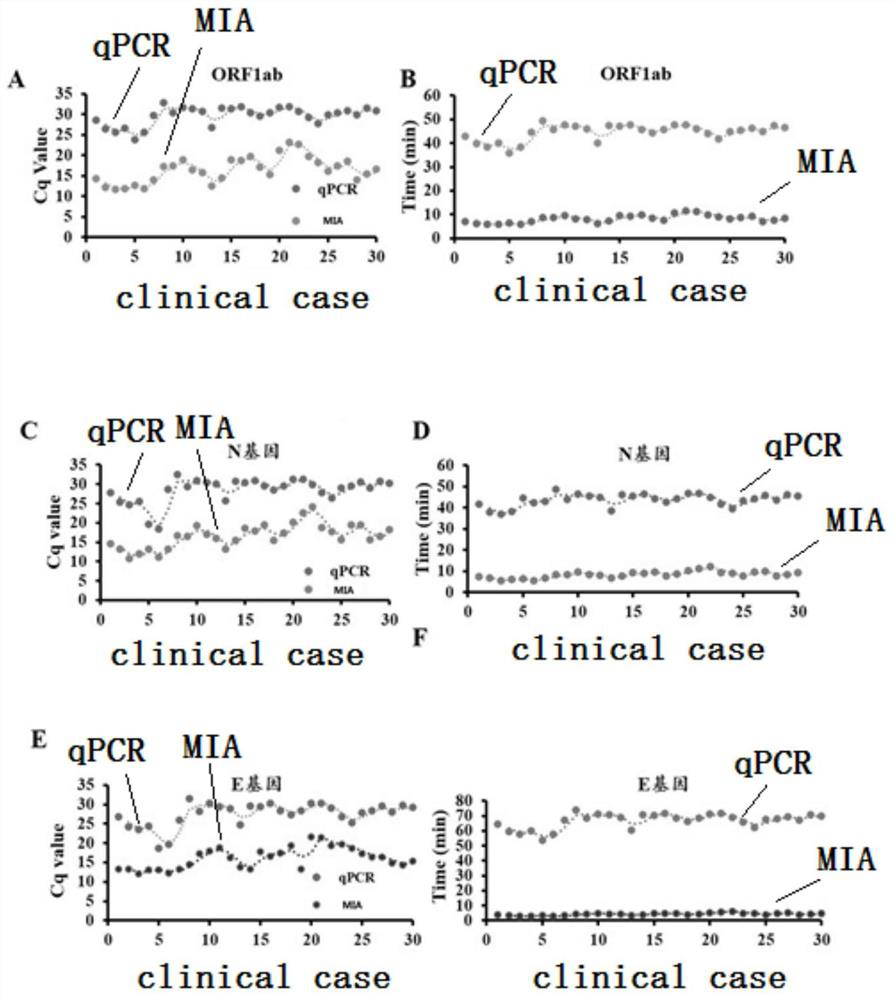
The invention discloses an MIA primer, a probe and a kit for detecting COVID-19 and an application of the MIA primer and the probe. According to the invention, the MIA primer and the probe are respectively designed based on a nucleocapsid N gene, an envelope E gene and an ORF1ab region of the COVID-19 publicized by NCBI. According to the MIA method disclosed by the invention, the detection of ORF1ab, E and N target genes of COVID-19 is realized in 3-15 minutes for the first time, and the detection sensitivity reaches 100 copies / ml (sample template concentration). The MIA method has no non-specific cross reaction with pathogen nucleic acid samples such as type A influenza, type B influenza and respiratory syncytial virus.
Owner:HANGZHOU BAOLIN BIOTECHNOLOGY CO LTD
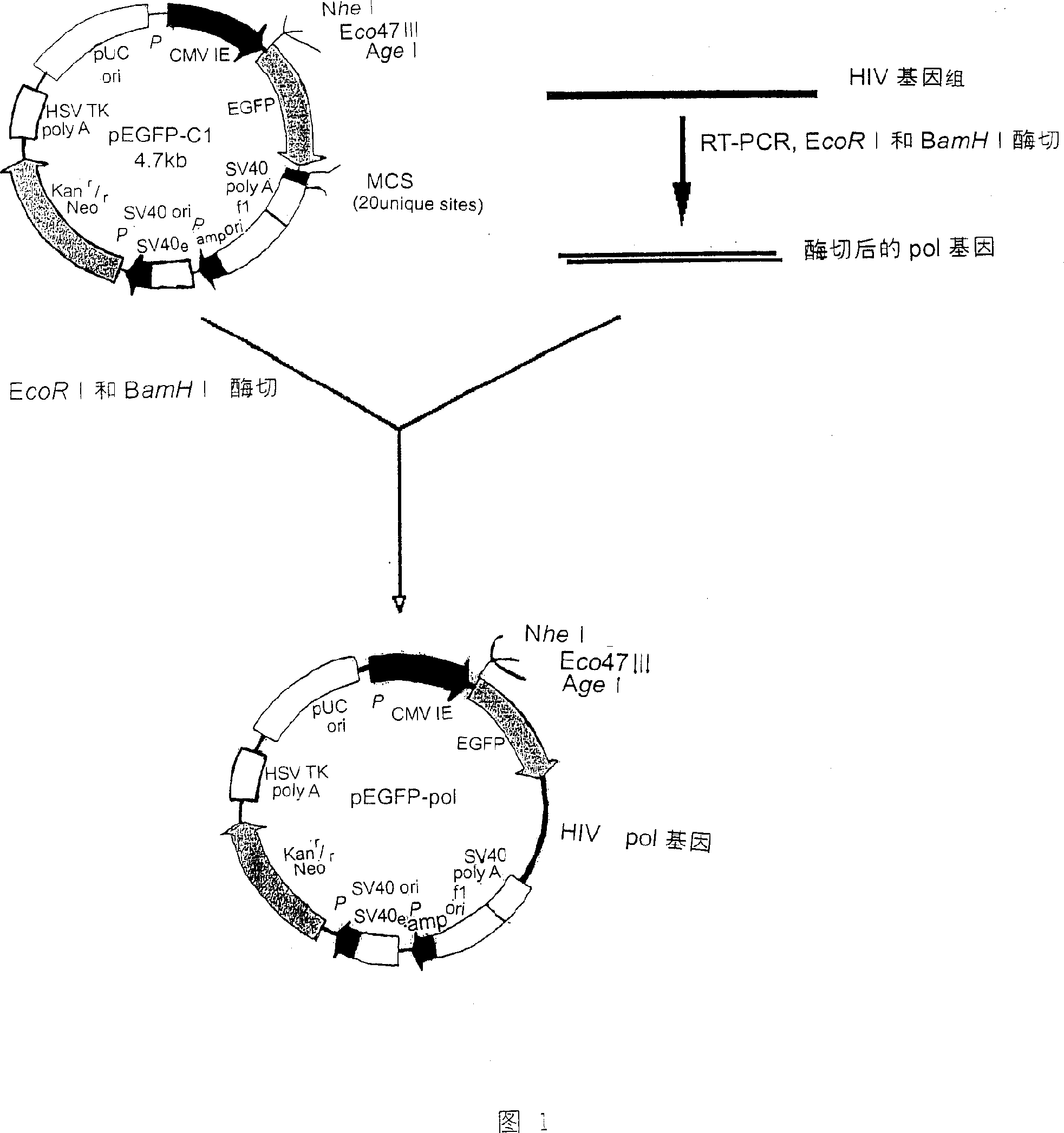
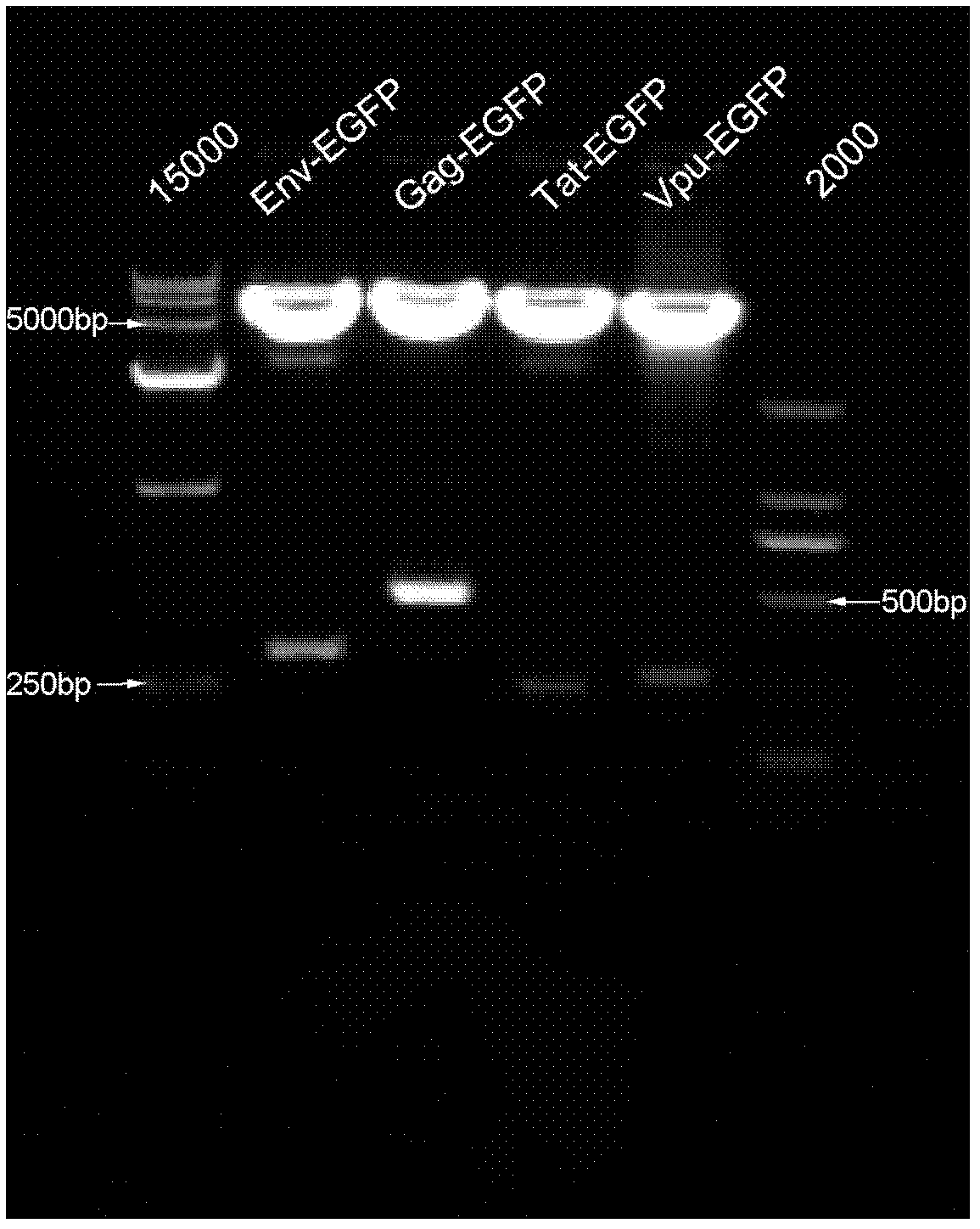
Construction method of REV virus infectious cloning for expressing green fluorescent envelope fusion protein
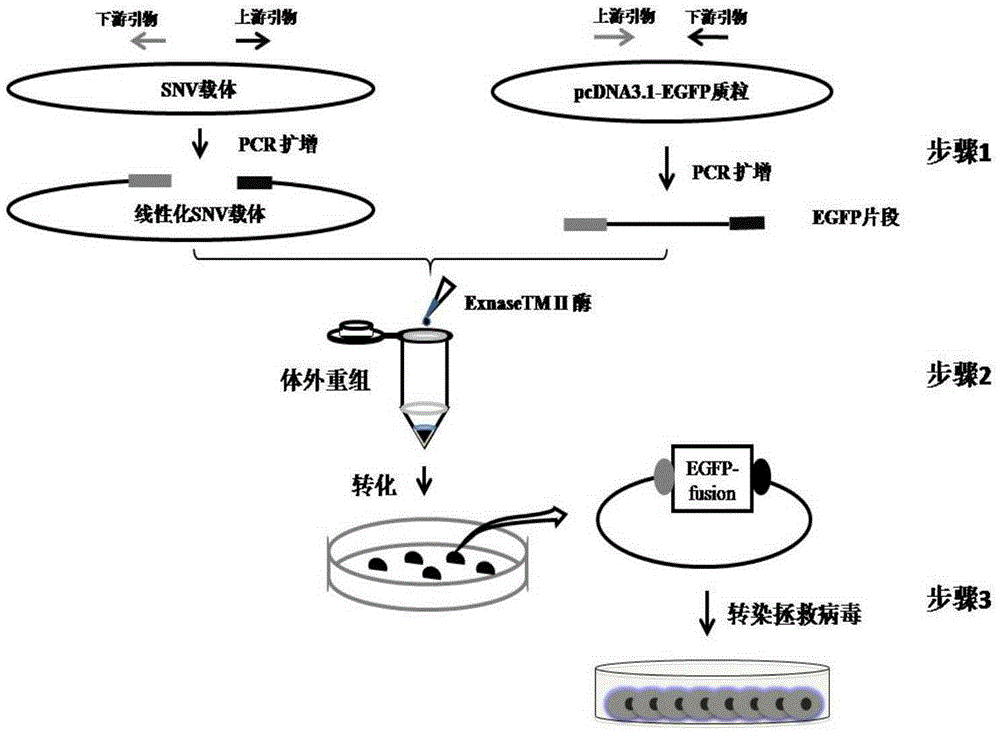
ActiveCN105274089ASimplify the build processImprove efficiencyVector-based foreign material introductionDNA preparationEscherichia coliEnvelope Gene
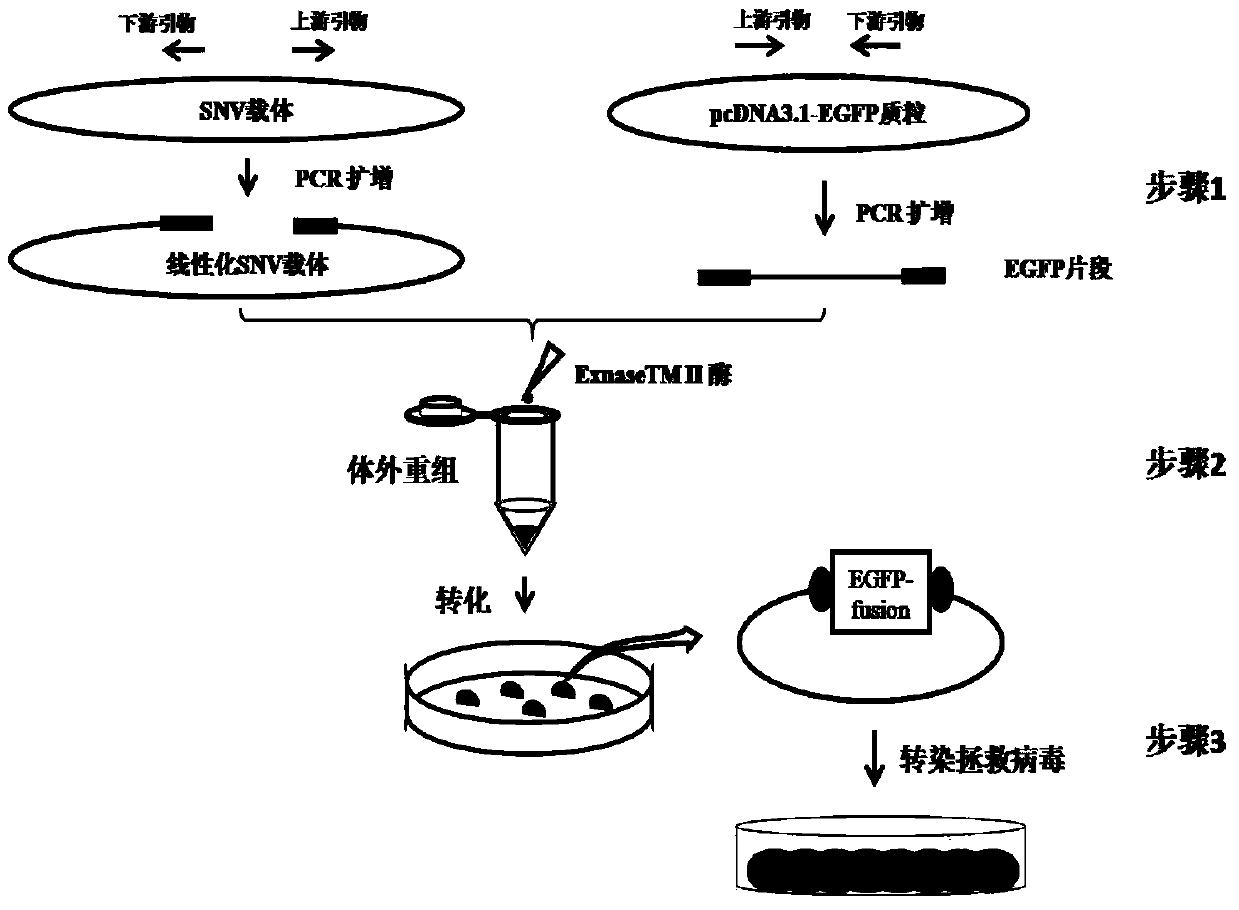
The invention relates to a construction method of REV virus infectious cloning for expressing green fluorescent envelope fusion protein. According to the construction method, green fluorescent EGFP gene segments amplified by the specific PCR primer are inserted into the linearized N end of Env genes of an REV viral genome, the commercial recombinase Exnase TM II is adopted for directly carrying out rapid recombinant cloning in vitro without the enzyme-cut and link up reaction, then the green fluorescent EGFP gene segments after the rapid recombinant cloning are transferred to the escherichia coli to be subjected to plasmid amplification and identification, and positive clone transfection DF1 cells are rescued to obtain REV viruses for expressing the green fluorescent envelope fusion protein.
Owner:YANGZHOU UNIV
A kind of siRNA recombination interference vector based on the conserved sequence of J subgroup avian leukosis virus env gene and its preparation method and application
InactiveCN103805635BEffective interferenceReduce economic lossGenetic material ingredientsAntiviralsConserved sequenceAvian leukosis viruses
The invention relates to the technical field of genetic engineering, and provides a J avian leukosis virus subgroup env gene conserved sequence-based siRNA (Small Interfering RNA (Ribonucleic Acid)) recombinant interference carrier. The preparation method comprises the following steps: on the basis that the env gene has important meaning for ALV (avian leukosis virus), designing and synthesizing siRNA by the env gene, building to form a hairpin structure of siRNA, further obtaining annealing double-stranded DNA (Deoxyribonucleic Acid), and connecting the double-stranded DNA with the carrier to build the recombinant interference carrier. After the interference carrier and the virus co-transfect cells are adopted, the interference carrier provided by the invention can effectively interfere the transcription and the duplication of the J avian leukosis virus subgroup in the in-vitro cell and the live chicken, the scientific base and the technical support can be provided for preventing and curing the J avian leukosis virus subgroup, and the economic loss caused by the ALV-J infection in the poultry industry can be reduced.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
High-titer retroviral packaging cells
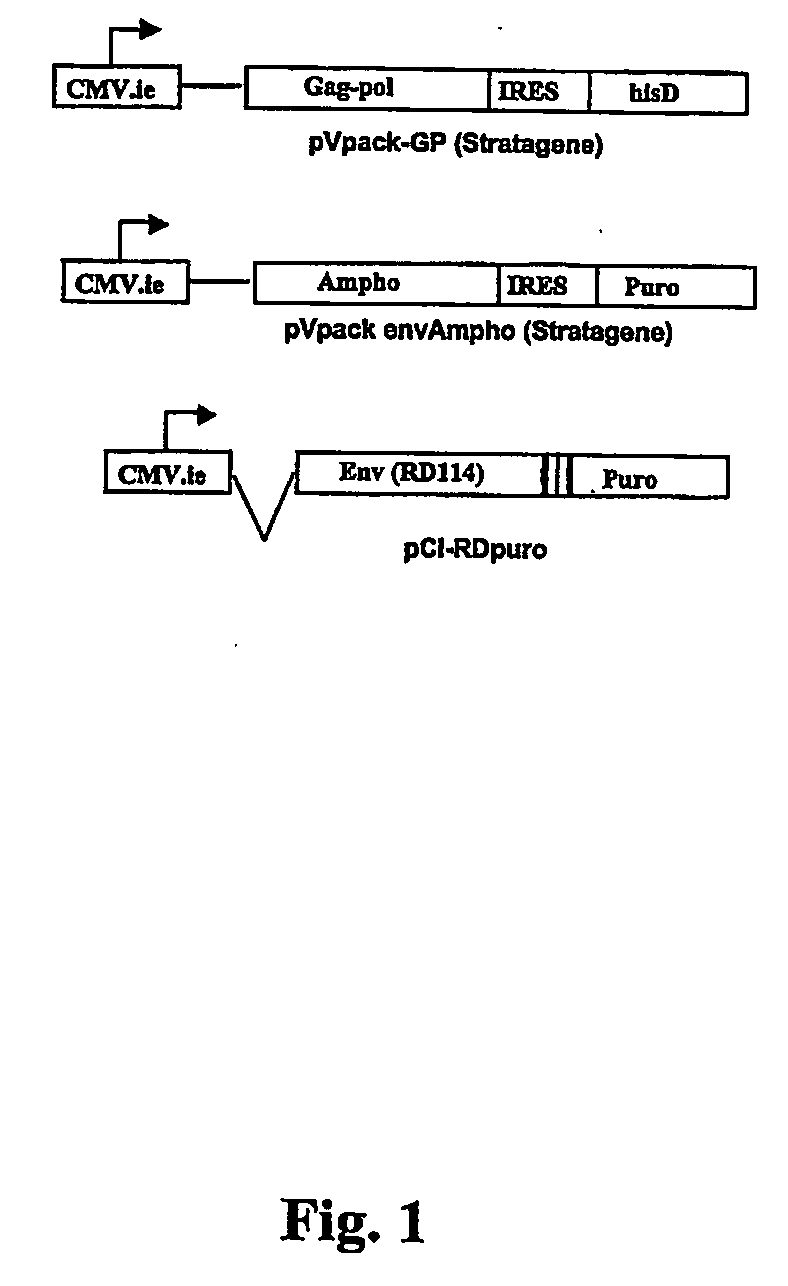
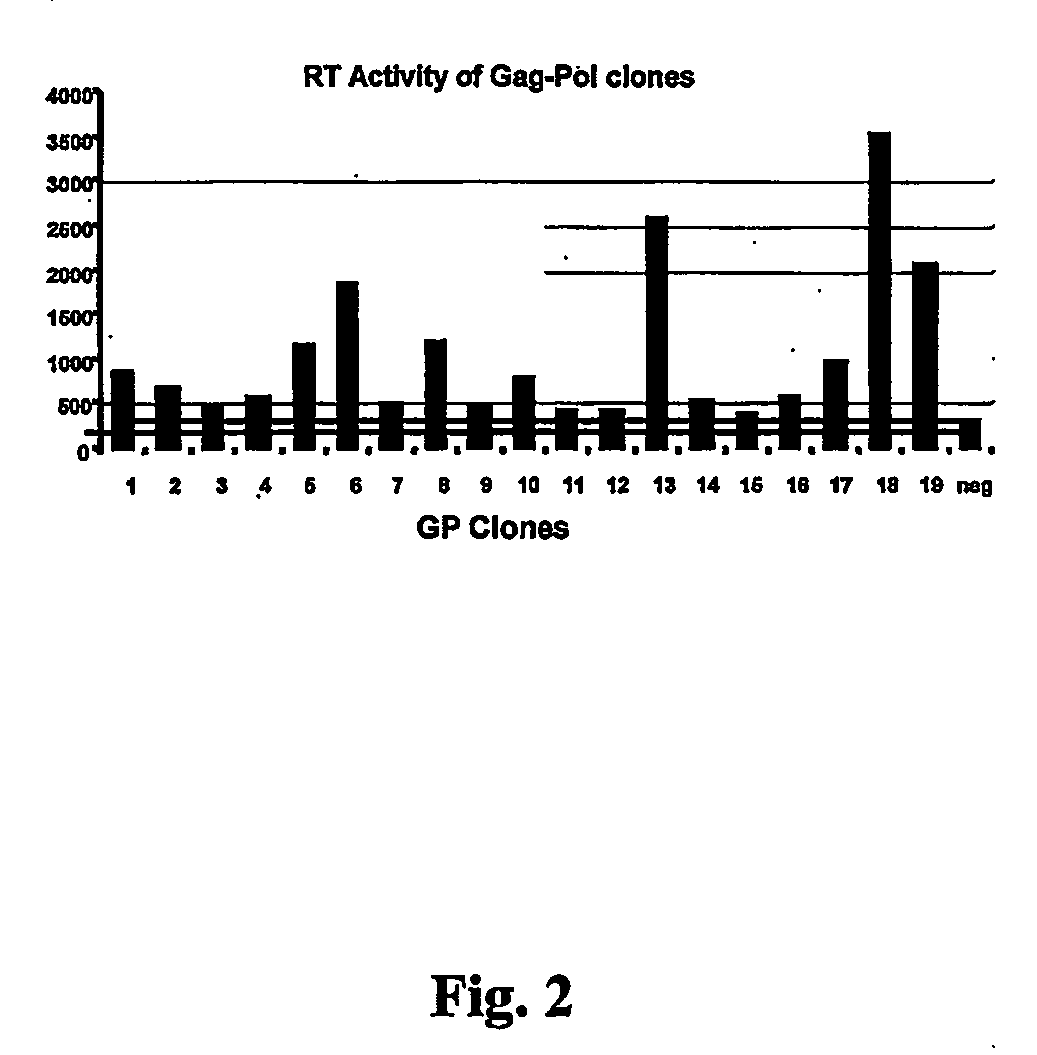
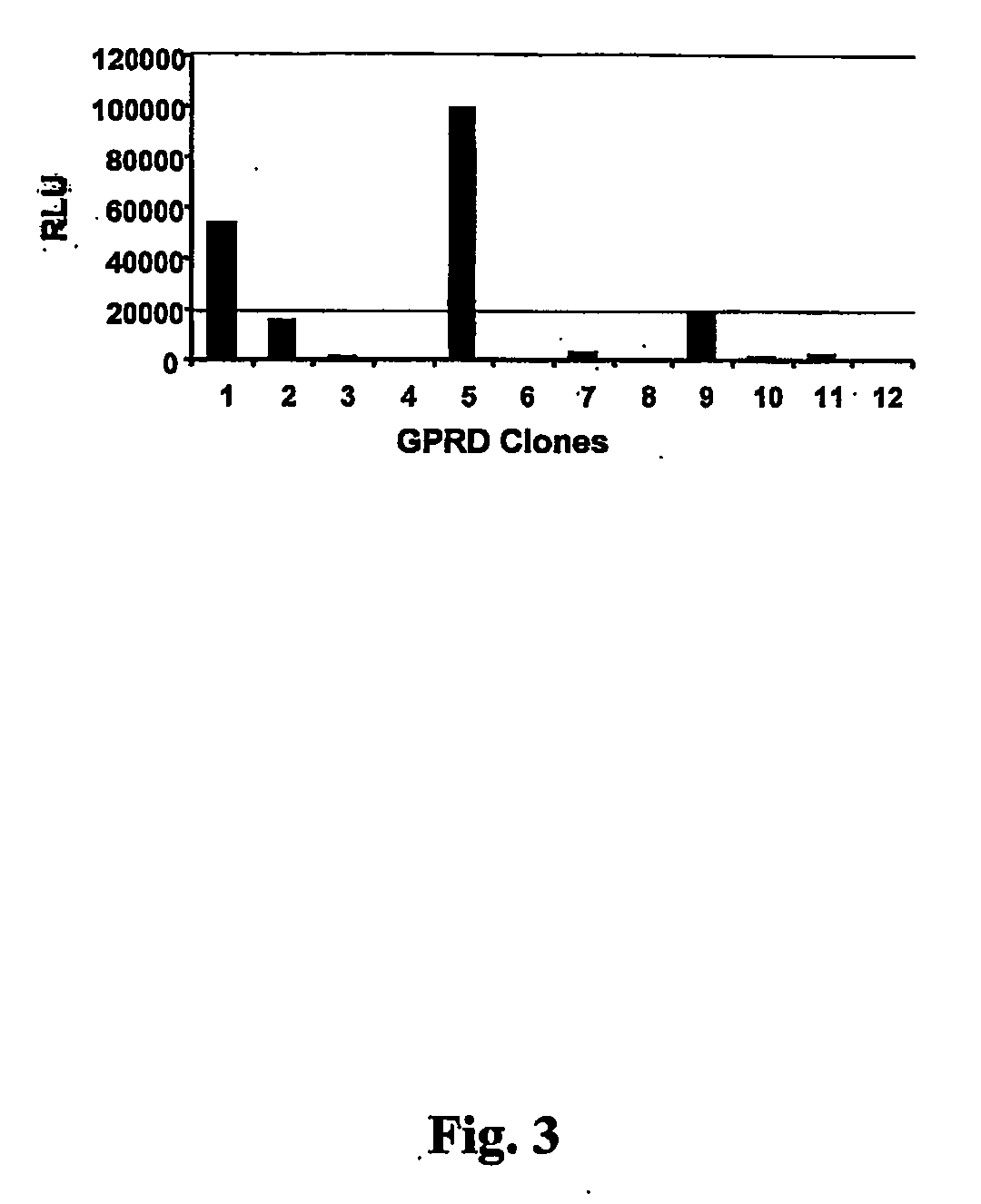
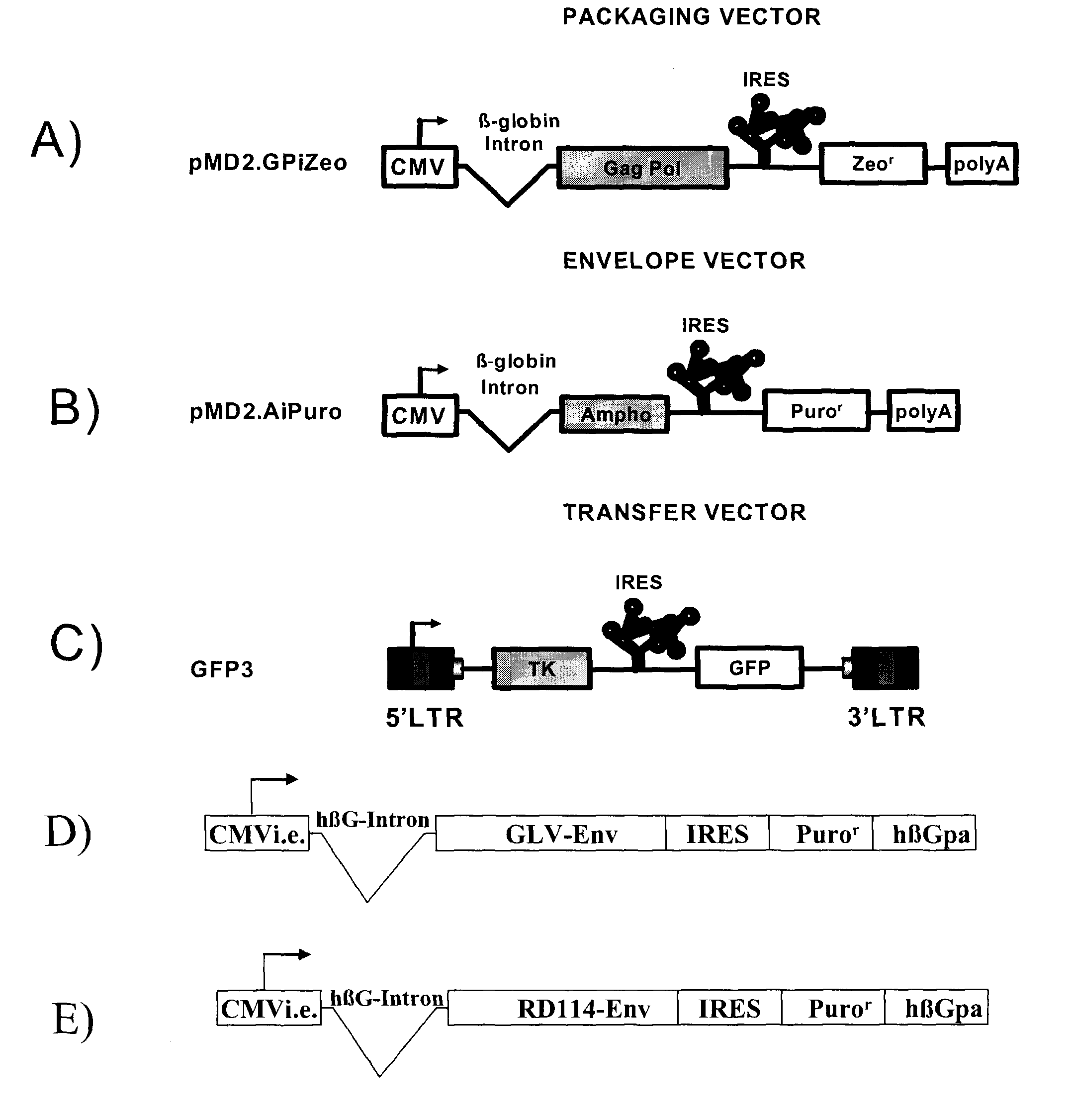
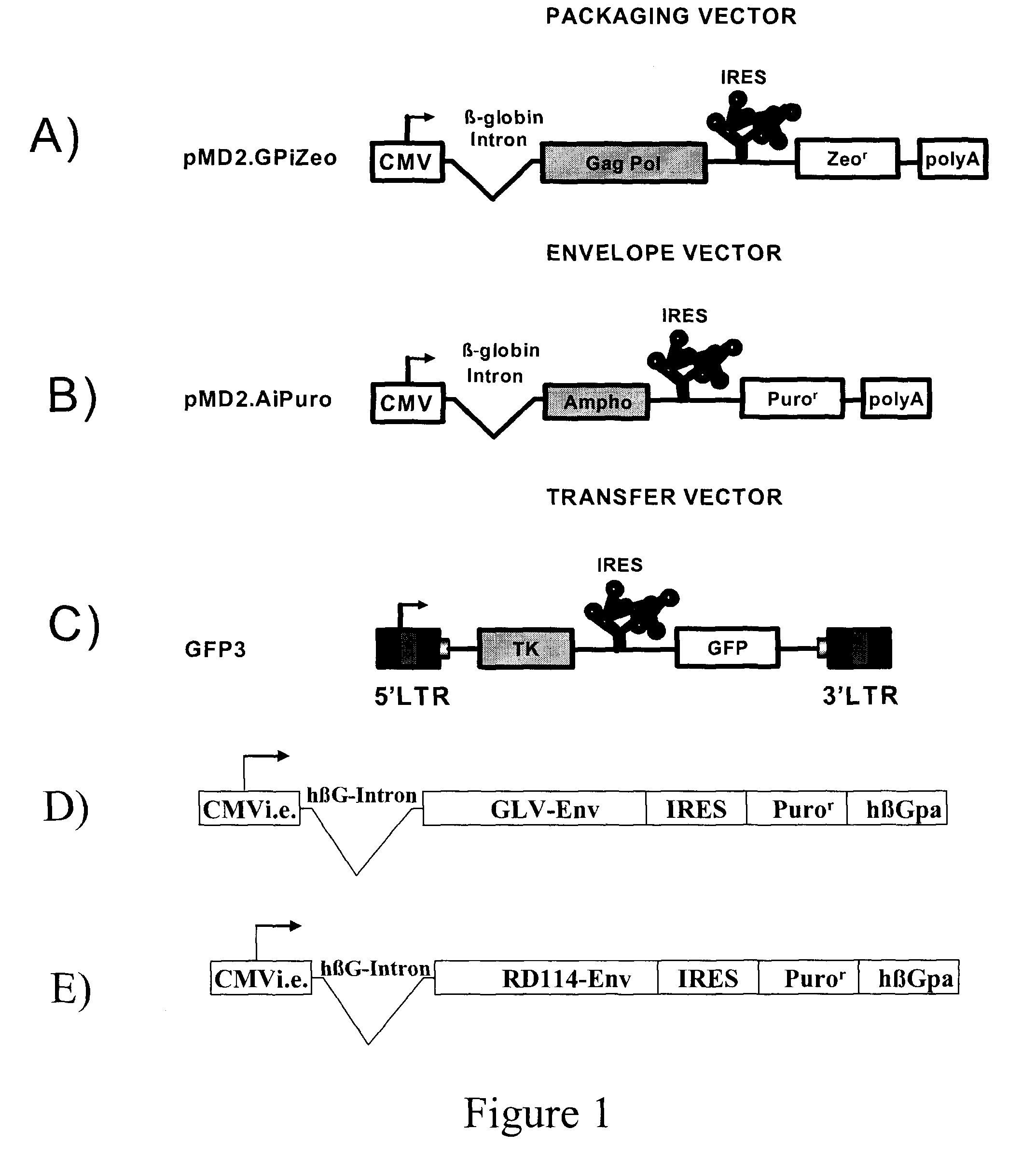
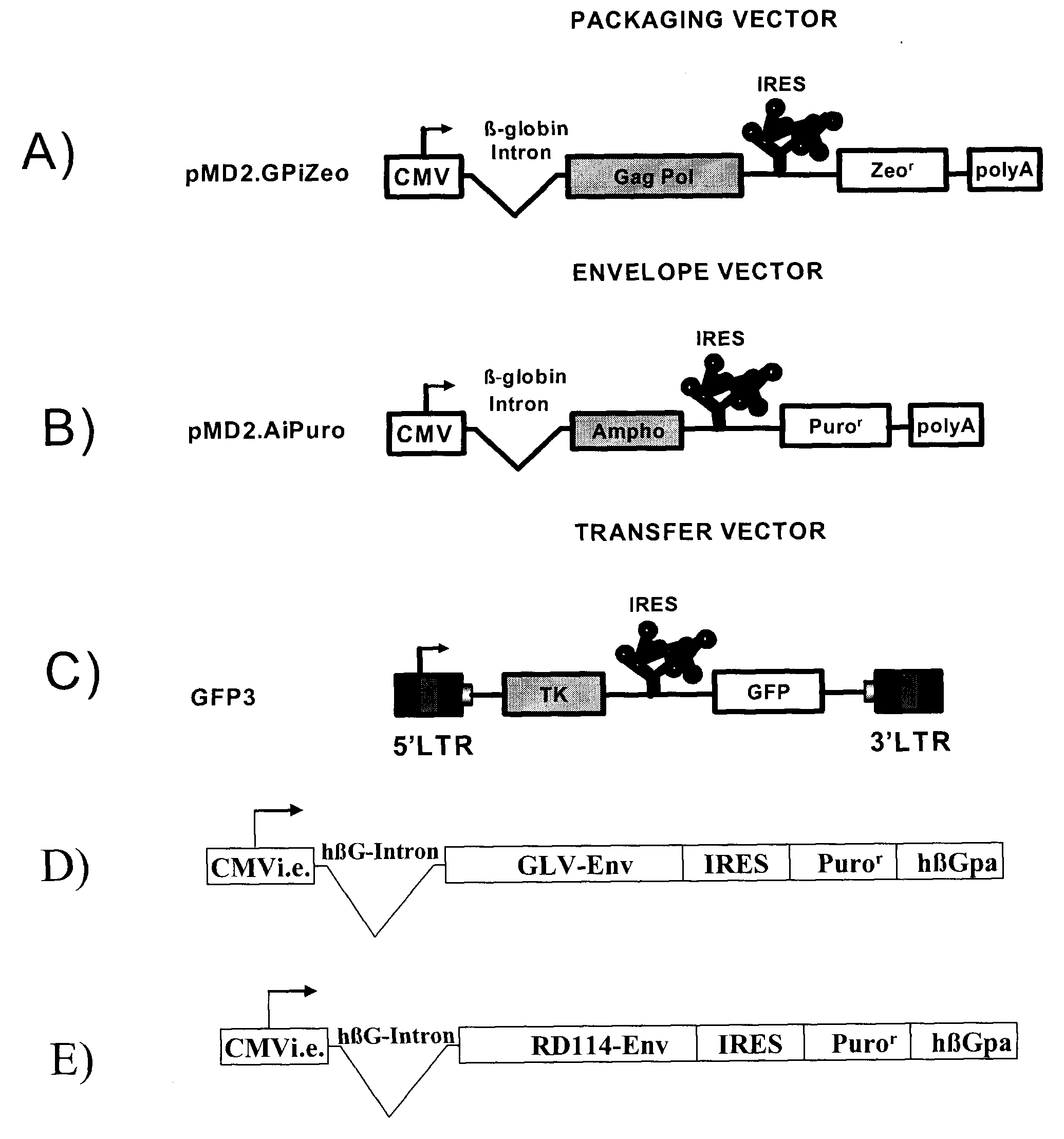
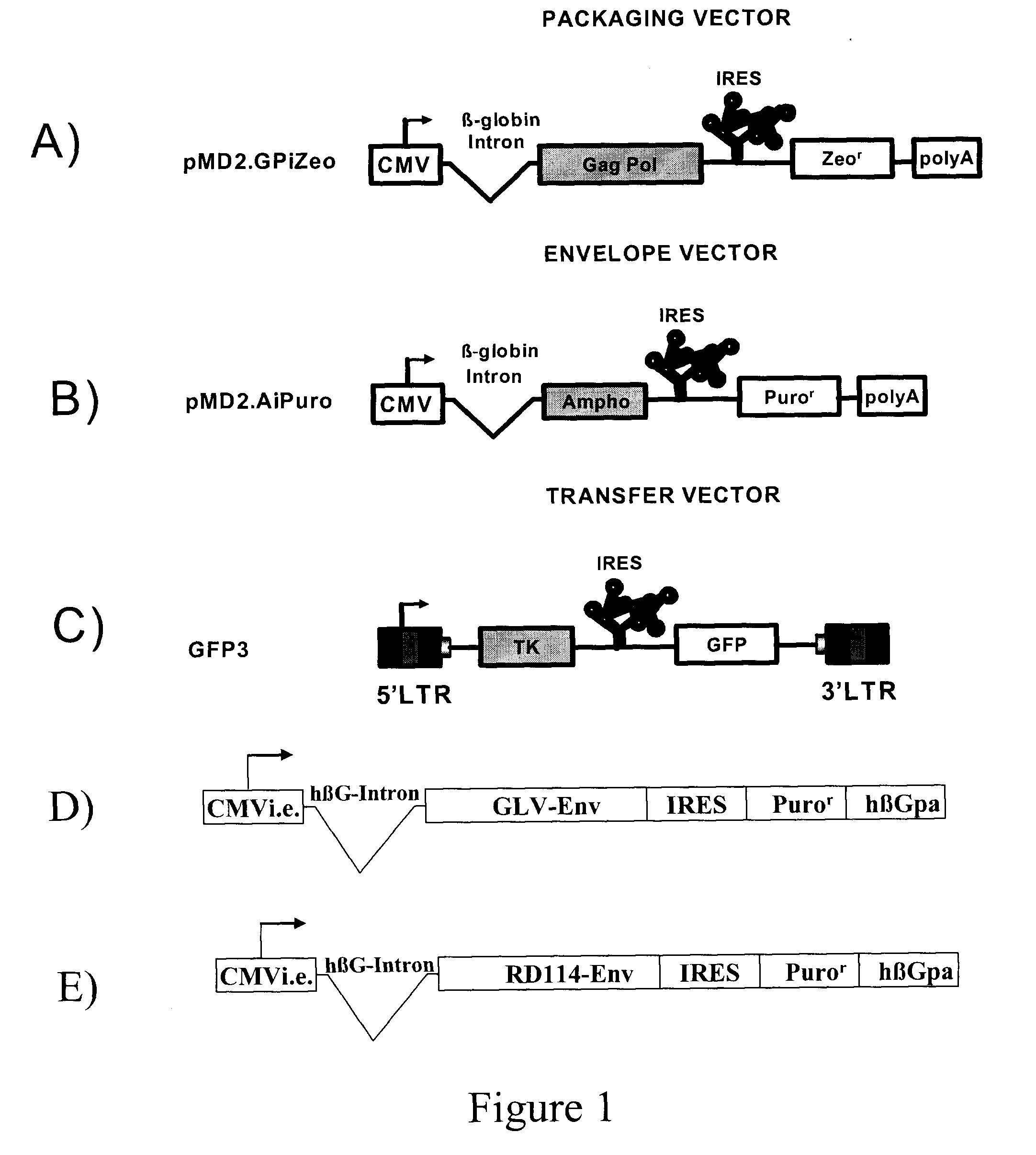
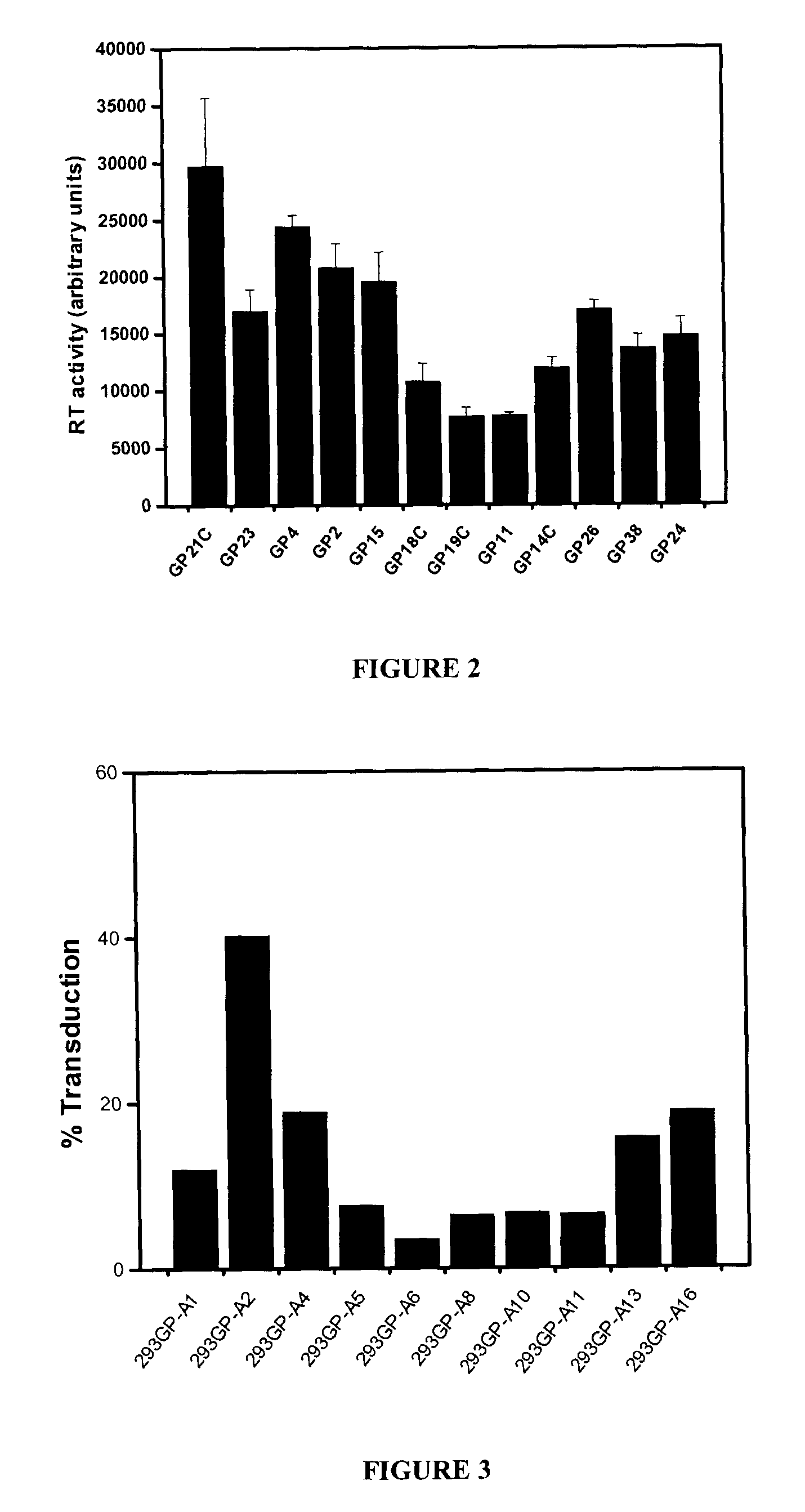
The present invention relates to non-replicative recombinant retrovirus packaging cells able to grow in suspension in a serum-free medium. In particular, the present invention relates to a human embryonic 293SF-based cell line stably expressing gag and pol gene products from the murine Moloney leukemia virus (MLV) and the feline RD114 env gene. This particular combination allows the production of high titer of non-replicative retrovirus pseudotyped and prevents the recombination of plasmids. The recombinant retroviruses produced from these cells are safer and easier to produce for clinical use in gene therapy.
Owner:UNIV LAVAL
High-titer retroviral packaging cells
The present invention relates to non-replicative recombinant retrovirus packaging cells able to grow in suspension in a serum-free medium. In particular, the present invention relates to a human embryonic 293SF-based cell line stably expressing gag and pol gene products from the murine Moloney leukemia virus (MLV) and either the feline RD114 env gene, the gibbon ape leukemia virus (GLV) env gene, or the amphotropic 4070Aenv gene. This particular combination allows the production of high titer of non-replicative retrovirus pseudotyped and prevents the recombination of plasmids. The recombinant retroviruses produced from these cells are safer and easier to produce for clinical use in gene therapy.
Owner:UNIV LAVAL
Recombined chicken Marek's disease virus vaccine strain for expressing Gag and Env genes of avian leukosis virus subgroup J and construction method and application of recombined chicken Marek's disease virus vaccine strain
ActiveCN105695422AReduce distractionsHas a clearing effectViral antigen ingredientsVirus peptidesAvian leukosis virusesEnvelope Gene
The invention discloses a recombined chicken Marek's disease virus vaccine strain for expressing Gag and Env genes of an avian leukosis virus subgroup J (ALV-J) and a construction method and application of the recombined chicken Marek's disease virus vaccine strain, and belongs to the technical field of medicine or veterinary medicine. By means of the recombination and clone technology, a gene segment CAG-ALVGE containing the ALV-J Gag and Env genes and a CAG promoter sequence is inserted in a US2 gene of the strain 814 of the chicken Marek's disease virus, a recombined cosmid with a CAG-ALVGE expression frame inserted in a US2 gene is constructed, and the recombined chicken Marek's disease virus vaccine strain for expressing the Gag and Env genes is obtained through salvation of the recombined cosmid. Research shows that the obtained vaccine strain has the same in-vitro replication ability as a parent virulent vaccine strain 814 and good hereditary stability, and can resist attacks of a very virulent MDV strain and a very virulent ALV-J strain at the same time. It can be seen that the obtained recombined MDV vaccine strain can be used for preparing medicine for preventing or treating avian leukosis and the chicken Marek's disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Molecular clones with mutated HIV gag/pol, SIV gag and SIV env genes
Nucleic acid constructs containing HIV-1 gag / pol and SIV gag or SIV genes which have been mutated to remove or reduce inhibitory / instability sequences are disclosed. Viral particles and host cells containing these constructs and / or viral particles are also disclosed. The exemplified constructs and viral particles of the invention may be useful in gene therapy for numerous disorders, including HIV infection, or as a vaccine for HIV-1 immunotherapy and immunoprophylaxis.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
High-titer retroviral packaging cells
The present invention relates to non-replicative recombinant retrovirus packaging cells able to grow in suspension in a serum-free medium. In particular, the present invention relates to a human embryonic 293SF-based cell line stably expressing gag and pol gene products from the murine Moloney leukemia virus (MLV) and either the feline RD114 env gene, the gibbon ape leukemia virus (GLV) env gene, or the amphotropic 4070Aenv gene. This particular combination allows the production of high titer of non-replicative retrovirus pseudotyped and prevents the recombination of plasmids. The recombinant retroviruses produced from these cells are safer and easier to produce for clinical use in gene therapy.
Owner:UNIV LAVAL
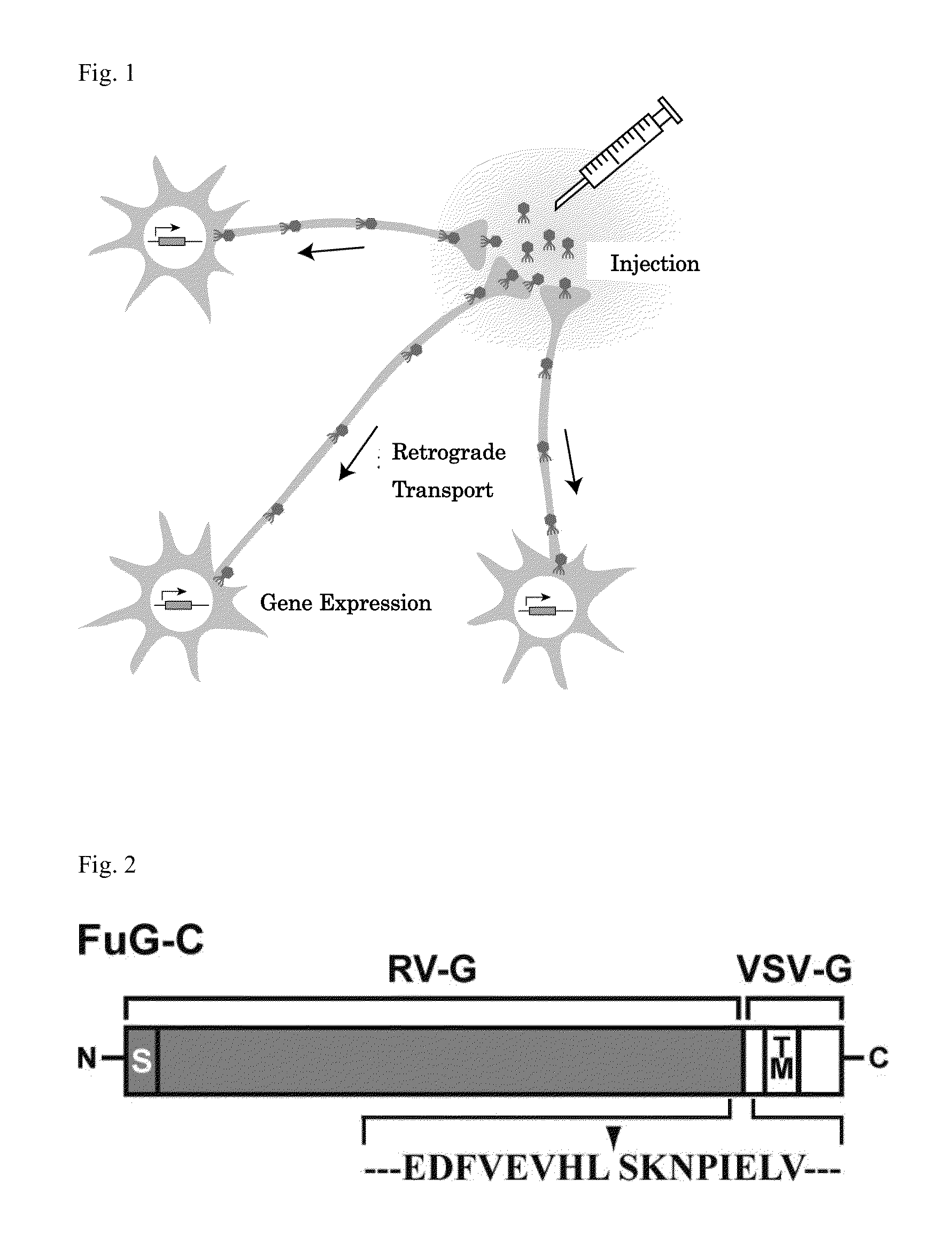
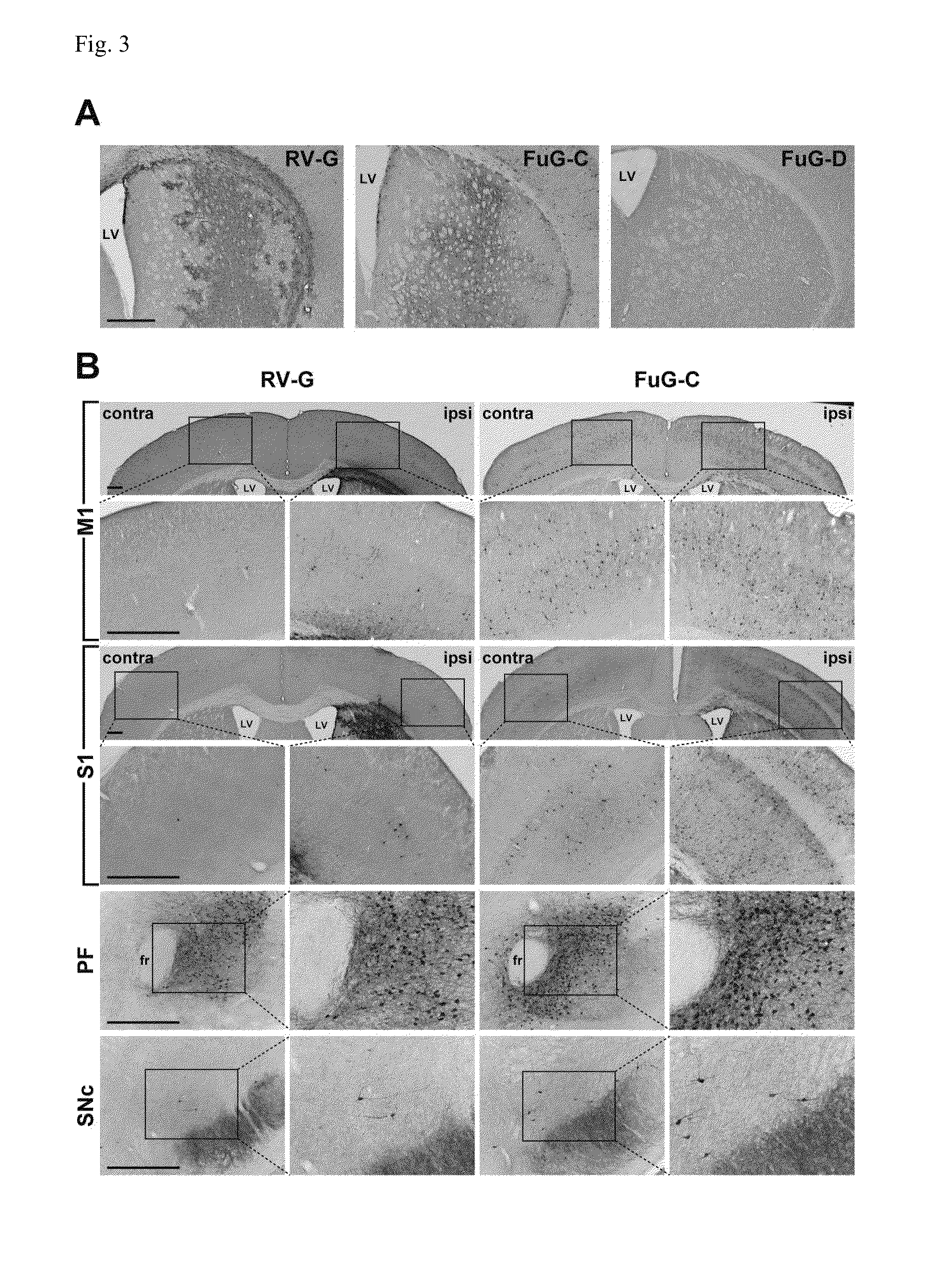
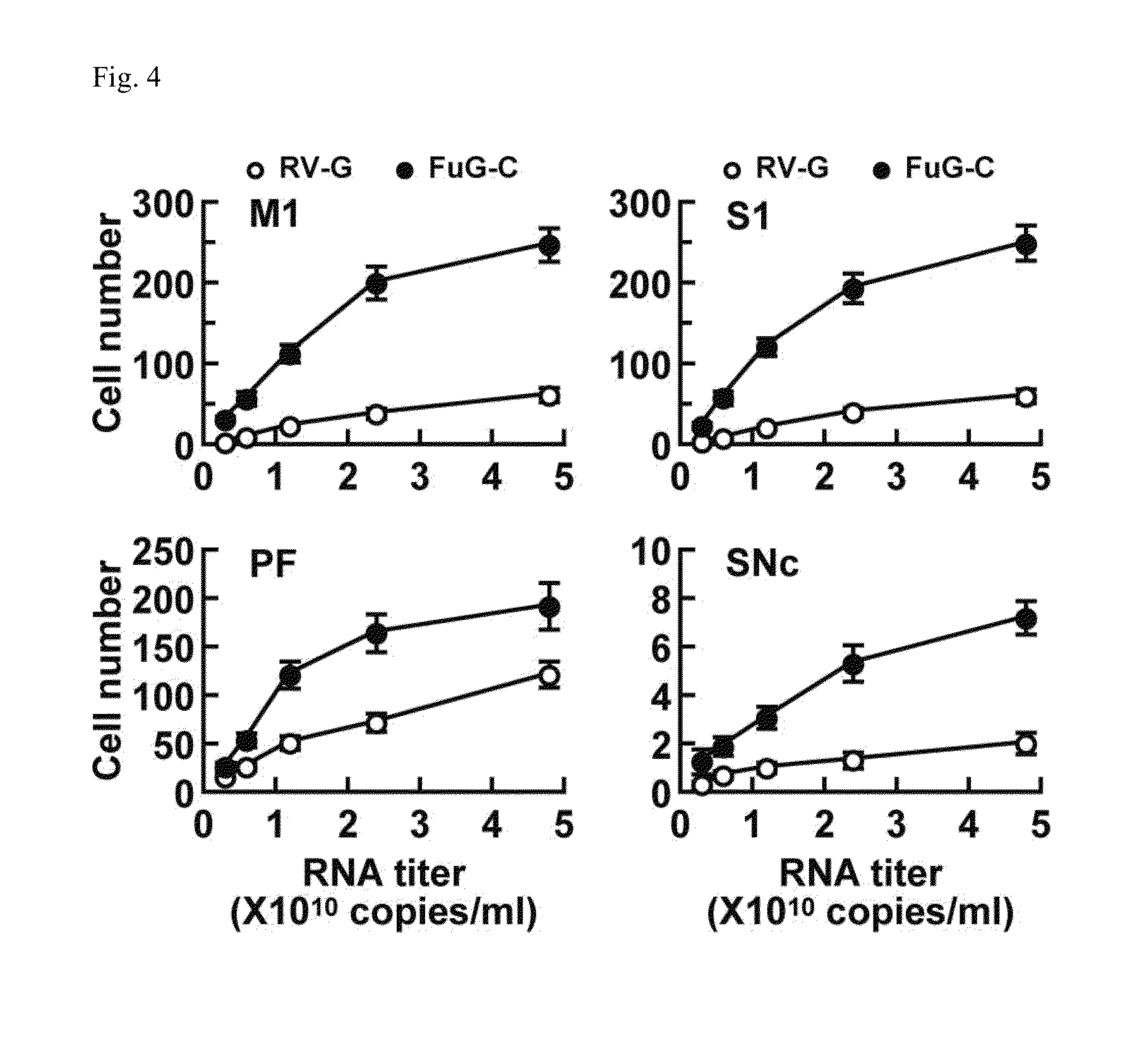
Neuron-specific retrograde transport vector
The present invention provides a lentiviral vector system having a higher titer, while sustaining an excellent retrograde transport ability, particularly, in the brain.The present invention also provides a kit for preparing a retrograde transport viral vector comprising:(1) a packaging plasmid containing the gag gene and the pol gene of HIV-1;(2) a packaging plasmid containing an accessory gene of HIV-1;(3) a transfer plasmid containing an target gene (a transgene); and(4) an envelope plasmid containing, as an envelope gene, a gene encoding a fused polypeptide comprising a fused extracellular domain consisting of the N-terminal region of an extracellular domain of rabies virus glycoprotein (RV-G) and the C-terminal region of an extracellular domain of vesicular stomatitis virus glycoprotein (VSV-G), a transmembrane domain of RV-G or VSV-G, and an intracellular domain of VSV-G, and the like.
Owner:JAPAN SCI & TECH CORP
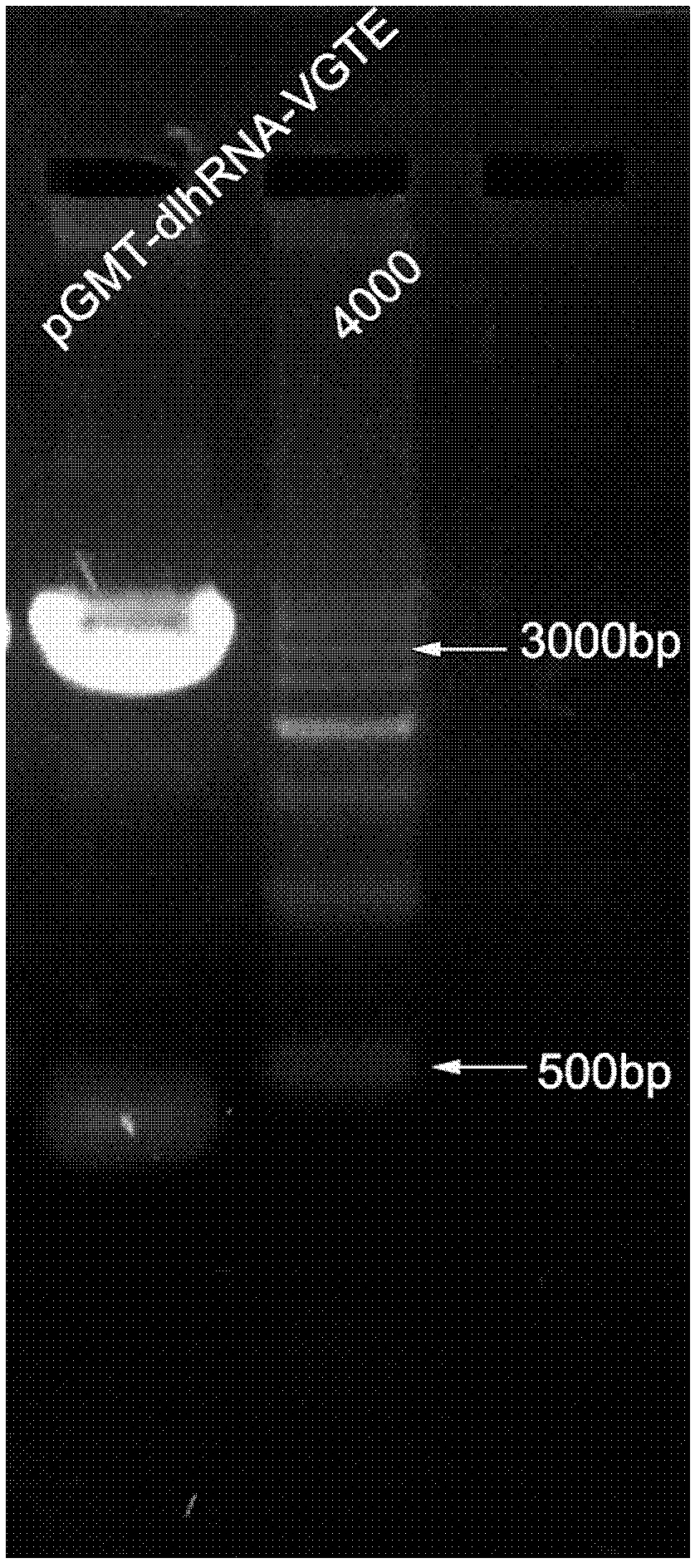
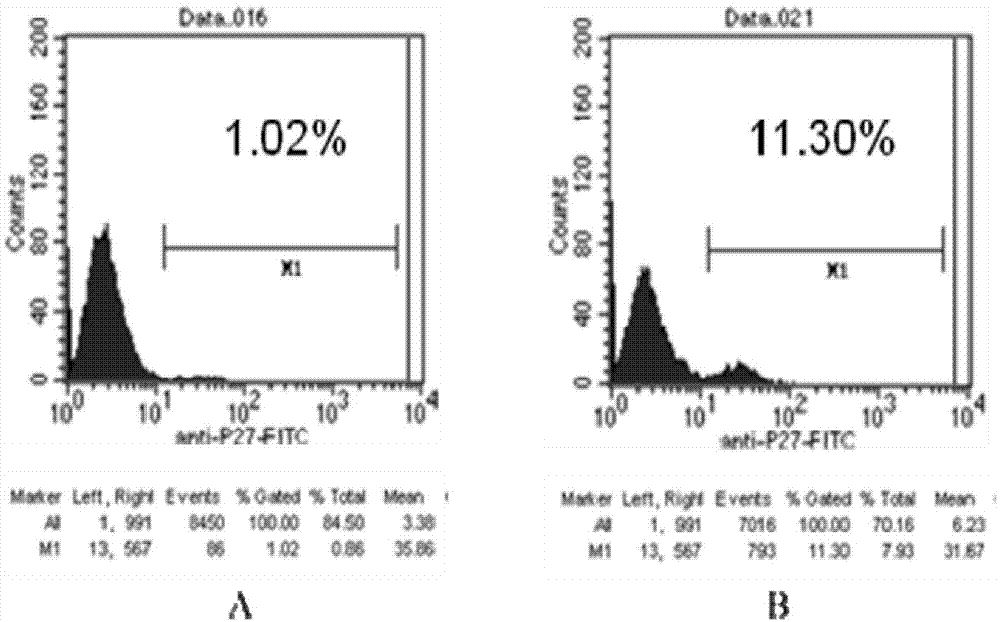
Construction and function test of double-long-hairpin RNA (Ribonucleic Acid) expression element for inhibiting HIV-1 (Human Immunodeficiency Virus-1)
InactiveCN102352360ARealize multi-target RNAiEasy to operateMicrobiological testing/measurementVector-based foreign material introductionFluorescenceHuman immunodeficiency virus 1
The invention relates to construction and function test of a double-long-hairpin RNA (Ribonucleic Acid) expression element for inhibiting HIV-1 (Human Immunodeficiency Virus-1), belonging to the technical field of gene engineering. In accordance with the design principle of siRNA and the conservative analysis of the HIV-1 sequence, an HIV-1 capsid protein gag gene (532-552), an envelope protein env gene (1428-1448), a non-structural protein tat gene (131-151) and a vpu gene (143-161) are selected and designed into a corresponding siRNA sequence; through a two-step PCR (Polymerase Chain Reaction) method, a double-long-hairpin RNA expression element (the sequence list is shown as SEQ ID No.1) is finally constructed; and through microscopic fluorescent observation and flow cell analysis quantitative test, the inhibition effect of the double-long-hairpin RNA expression element on the eukaryotic expression of an HIV gene EGFP (Enhanced Green Fluorescent Protein) fusion protein is tested. Experiments prove that the dlh RNA expression element can effectively inhibit the expression of multiple HIV genes.
Owner:NANKAI UNIV
Retrovirus vectors derived from avian sarcoma leukosis viruses permitting transfer of genes into mammalian cells and therapeutic uses thereof
InactiveUS20020110896A1High titer stockEasy to usePolypeptide with localisation/targeting motifVectorsLeucosisAvian leukosis viruses
Recombinant avian sarcoma leukosis virus (ASLV)-derived retrovirus vectors having an expanded host range are described. The host range is expanded by the replacement of the ASLV envelope gene by an envelope gene from a virus capable of infecting both mammalian and avian cells. The resulting recombinant ASLV-derived retroviral vectors can replicate efficiently in avian cells, infect both avian and mammalian cells in high titer, and are replication-defective in mammalian cells. Thus, they are quite safe and advantageous for use in gene therapy and vaccines.
Owner:UNITED STATES OF AMERICA
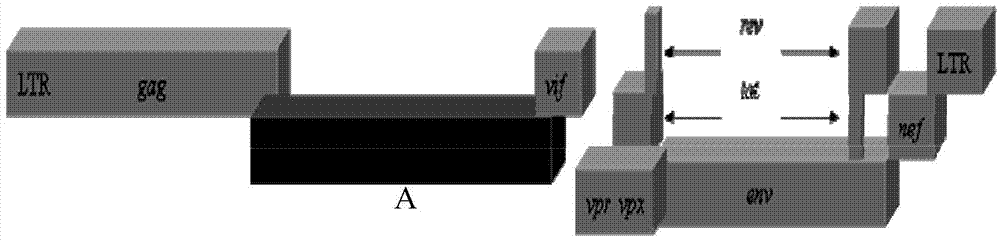
Recombinant immunodeficiency plasmid and virus and application thereof
ActiveCN104263745AEfficient replication capabilityViruses/bacteriophagesVector-based foreign material introductionFeline immunodeficiency virusImmunodeficiency virus
The invention relates to the technical field of biology, and particularly discloses a recombinant immunodeficiency plasmid and virus and application thereof. The immunodeficiency plasmid comprises LTR, gag gene, nucleotide sequence disclosed as SEQ ID No.1, vif gene, vpr gene, vpx gene, tat gene, rev gene, env gene and nef gene. The nucleotide sequence disclosed as SEQ ID NO.1 is introduced to the pathogenic SIVmac239 full-length plasmid frame to construct the immunodeficiency plasmid comprising a plurality of drug target spots, and packaging is performed in the 293T cell to generate immunodeficiency virus. The virus has the capacity for infecting target cells and the capacity of efficient replication, and is applicable to researching human immunodeficiency virus (HIV) in-vivo infection process, pathogeny characteristic, pathogenesis and immunoreaction and screening anti-HIV drugs and anti-HIV drug application strategies.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Modification of HIV-1 (Human immunodeficiency virus type one) Chinese epidemic strain CRF01_AE env gene
The invention provides a modified CRF01_AE env gene. According to the modified CRF01_AE env gene, the GC content is increased, and an internal inhibitory sequence (INS) with higher AU content is damaged, so that the gene is more suitable for being expressed in a eukaryotic cell. The gene can be expressed efficiently in the eukaryotic cell without depending on regulatory protein Rev and a response element of the regulatory protein Rev; compared with the gene before modification, the expression quantity is remarkably increased; the modified mod.AE env gene is selected as a vaccine gene; and if HIV-1 vaccines are restructured, the immunogenicity of the vaccines can be improved possibly.
Owner:中国疾病预防控制中心病毒病预防控制所 +2
Construction and application of SHIV (simian/human immunodeficiency virus) infectious clone
InactiveCN103374550AGood replicationStable productive infectionMicroorganism based processesViruses/bacteriophagesNucleotideEnvelope Gene
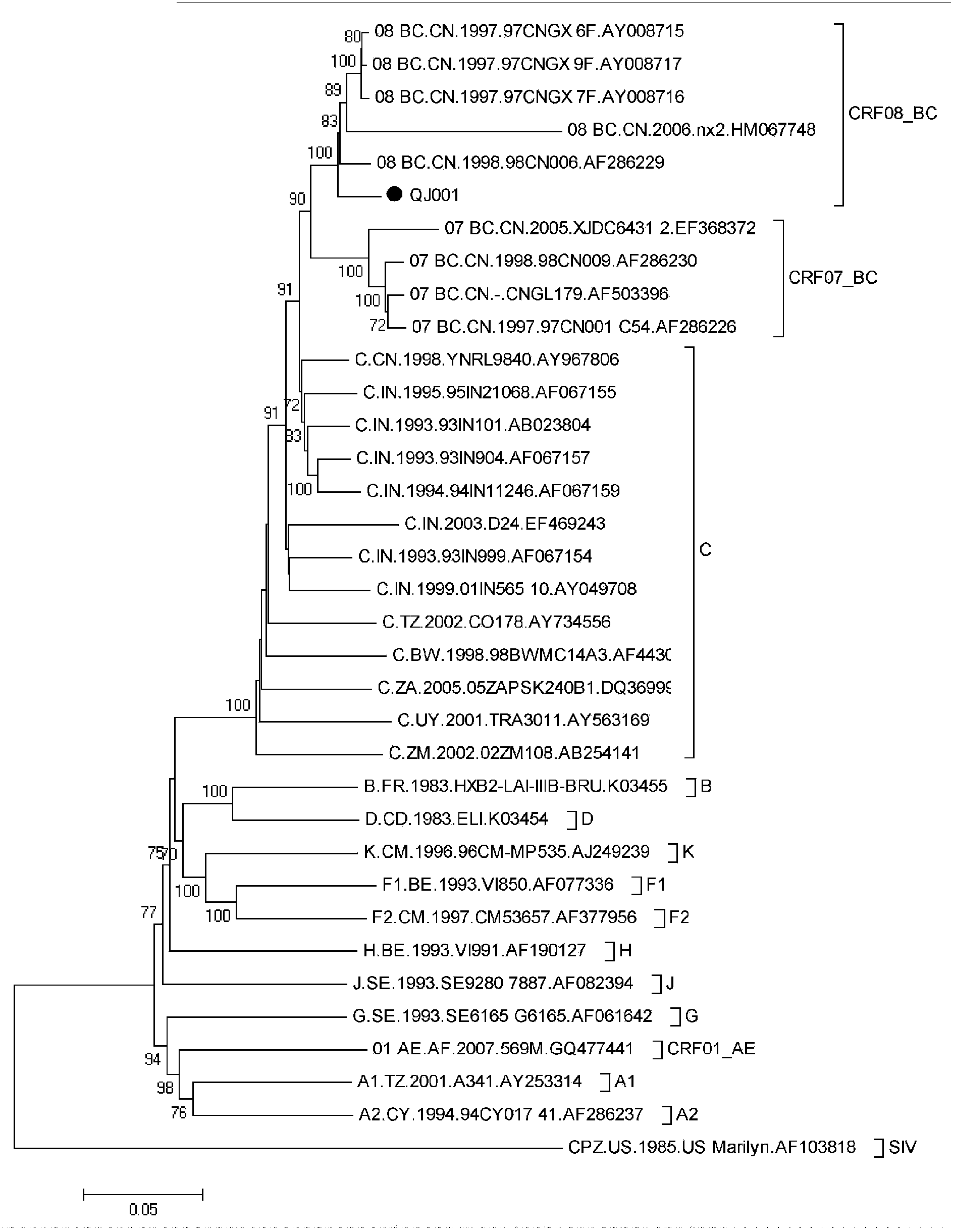
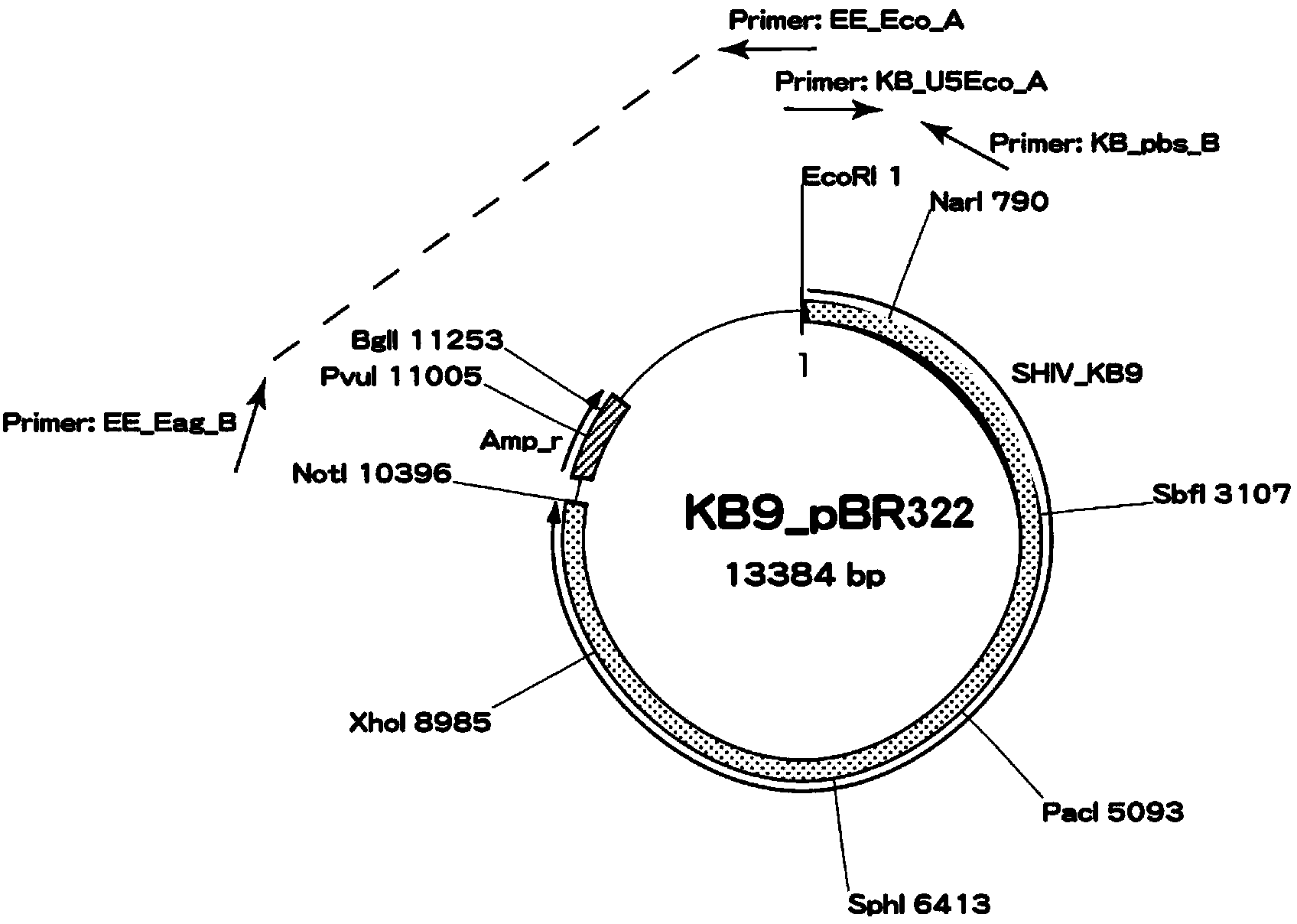
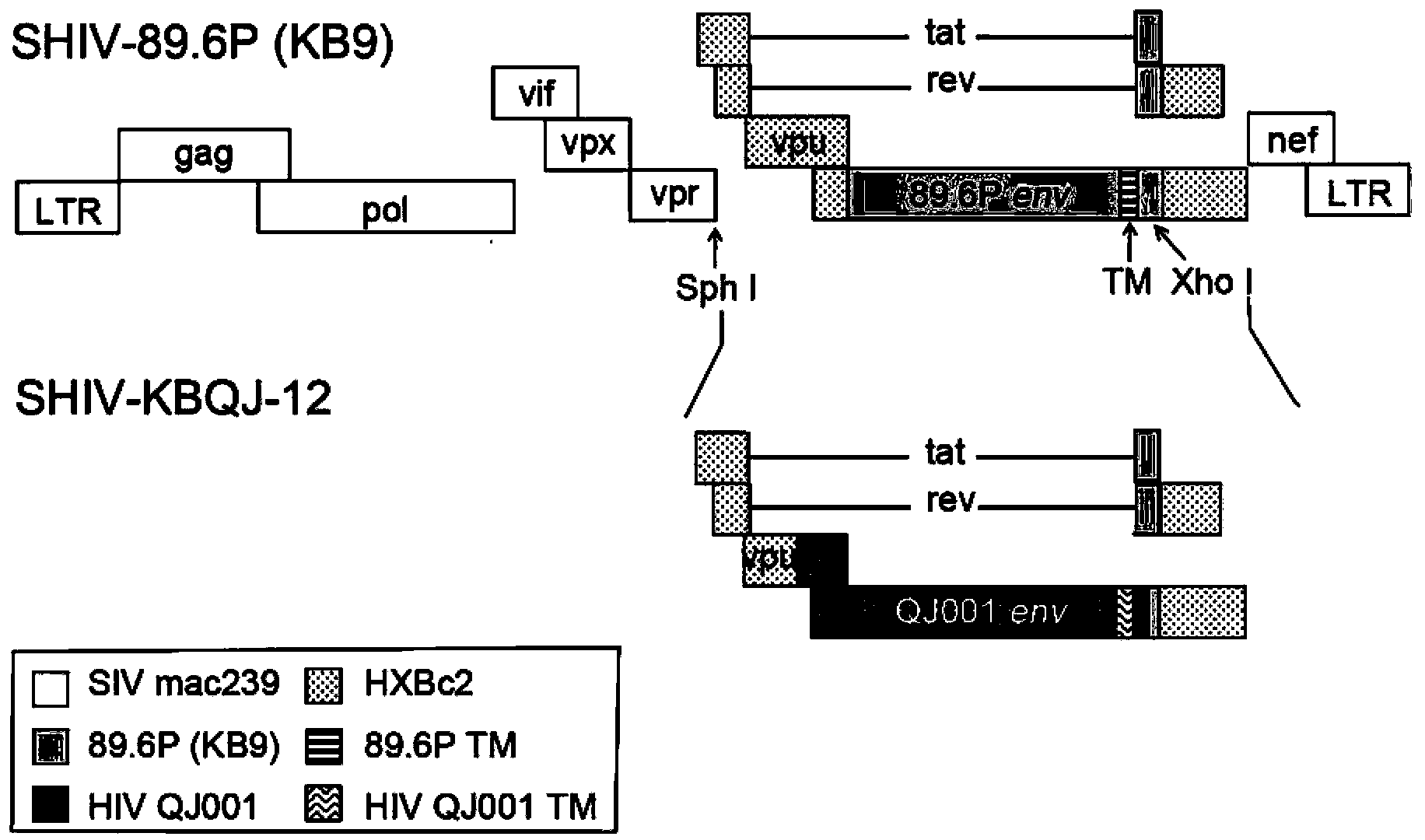
The invention relates to construction and application of SHIV (simian / human immunodeficiency virus) infectious clone. Firstly, the overall length genome of a virulent strain SHIV-KB9 embedded with a B subtype env gene is cloned to a low copy number carrier pBR322, the obtained plasmid SHIV-KB9 / pBR322 is taken as a genome skeleton, the env gene of Chinese main prevalent strain CRF08_BC of HIV-1 is used for substituting for corresponding gene segments so as to obtain the infectious clone SHIV-KBQJ-12, the replacing env gene is from the infectious clone strain QJ001 of HIV-1CRF08_BC and comprises the whole intracellular region, a transmembrane region and part of an extracellular region of gp120 and gp41, and the position of the replacing env gene, corresponding to the position of the nucleotide of a standard reference strain HXB2, is 6103-8249. The construction is simple and convenient to operate, has high success rate, can obtain the infectious clone in a short time, and can be used for establishing an SHIV / Chinese rhesus infection model.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
A construction method of rev virus infectious clone expressing green fluorescent envelope fusion protein
ActiveCN105274089BSimplify the build processImprove efficiencyVector-based foreign material introductionDNA preparationEscherichia coliDigestion
The invention relates to a method for constructing an infectious clone of REV virus expressing green fluorescent capsule membrane fusion protein, which is to insert the green fluorescent EGFP gene fragment amplified by specific PCR primers into the N-terminal of the linearized REV virus genome Env gene , using the commercialized recombinase ExnaseTM II without enzyme digestion and ligation reaction, rapid recombination clones in vitro, and then transformed into Escherichia coli for plasmid amplification and identification; and the positive clones were transfected into DF1 cells to rescue and obtain the expression of green fluorescent capsules Membrane fusion protein of REV virus.
Owner:YANGZHOU UNIV
Method for constructing recombinant virus for expressing ALV-K envelope protein
The invention discloses a method for constructing a recombinant virus for expressing an ALV-K envelope protein, specifically comprising the following steps: designing a PCR amplification ALV-J infectious clone linear expression vector primer and a PCR amplification ALV-K-env gene primer sequence; carrying out PCR amplification on an ALV-K envelope protein gene fragment and the ALV-J infectious clone linear vector, constructing a recombinant infectious cloning plasmid, and transfecting the recombinant plasmid into DF-1 cells to rescue the recombinant virus for expression of the ALV-K envelope protein. By ALV-J infectious cloning, the recombinant virus for efficient replication and expression of the ALV-K envelope protein is constructed. The acquisition of the recombinant virus not only laysa foundation for studying functional receptors of the ALV-K envelope protein and its pathogenicity, but also provides a target virus for the clinical detection of neutralizing antibodies of the ALV-K.
Owner:YANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com