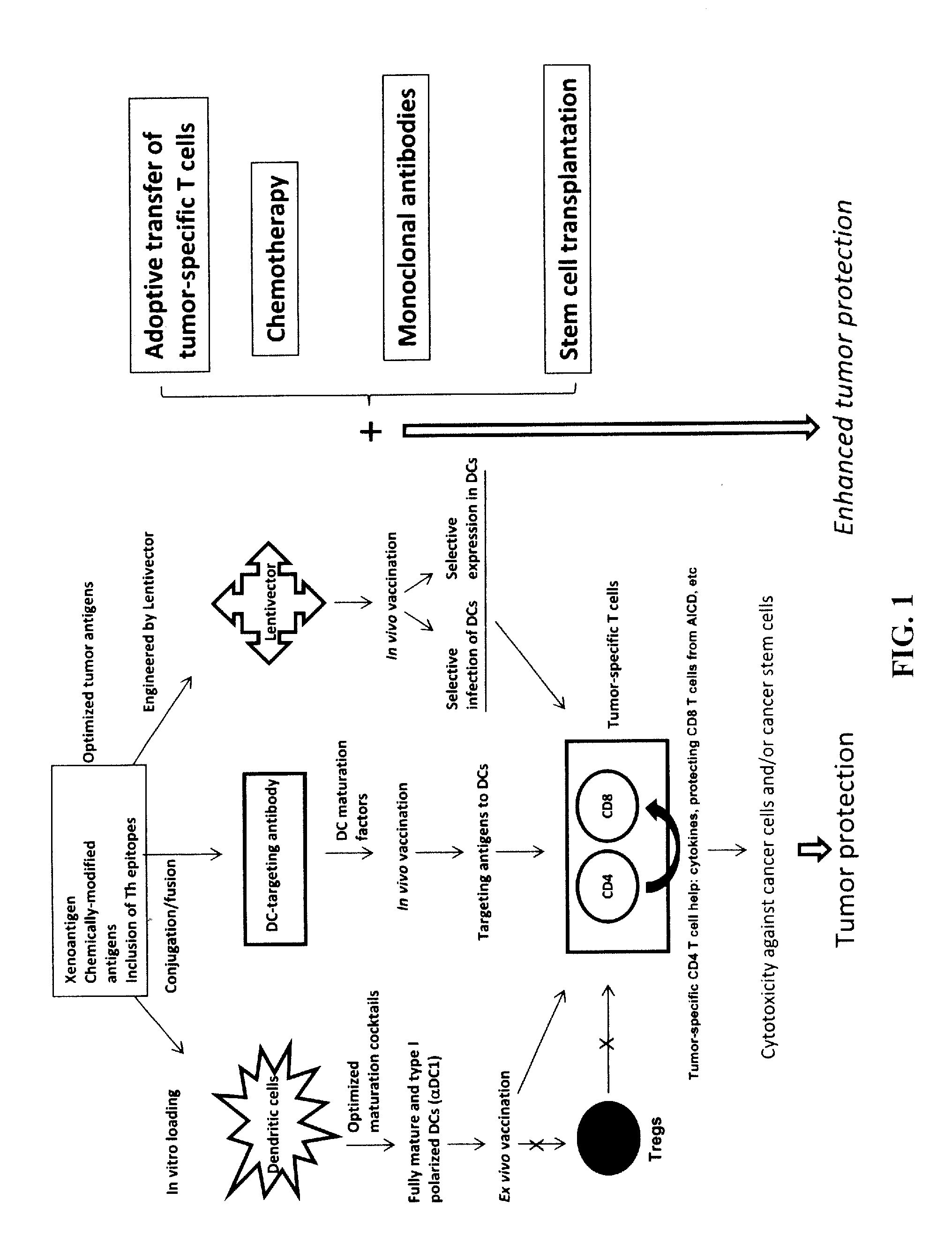
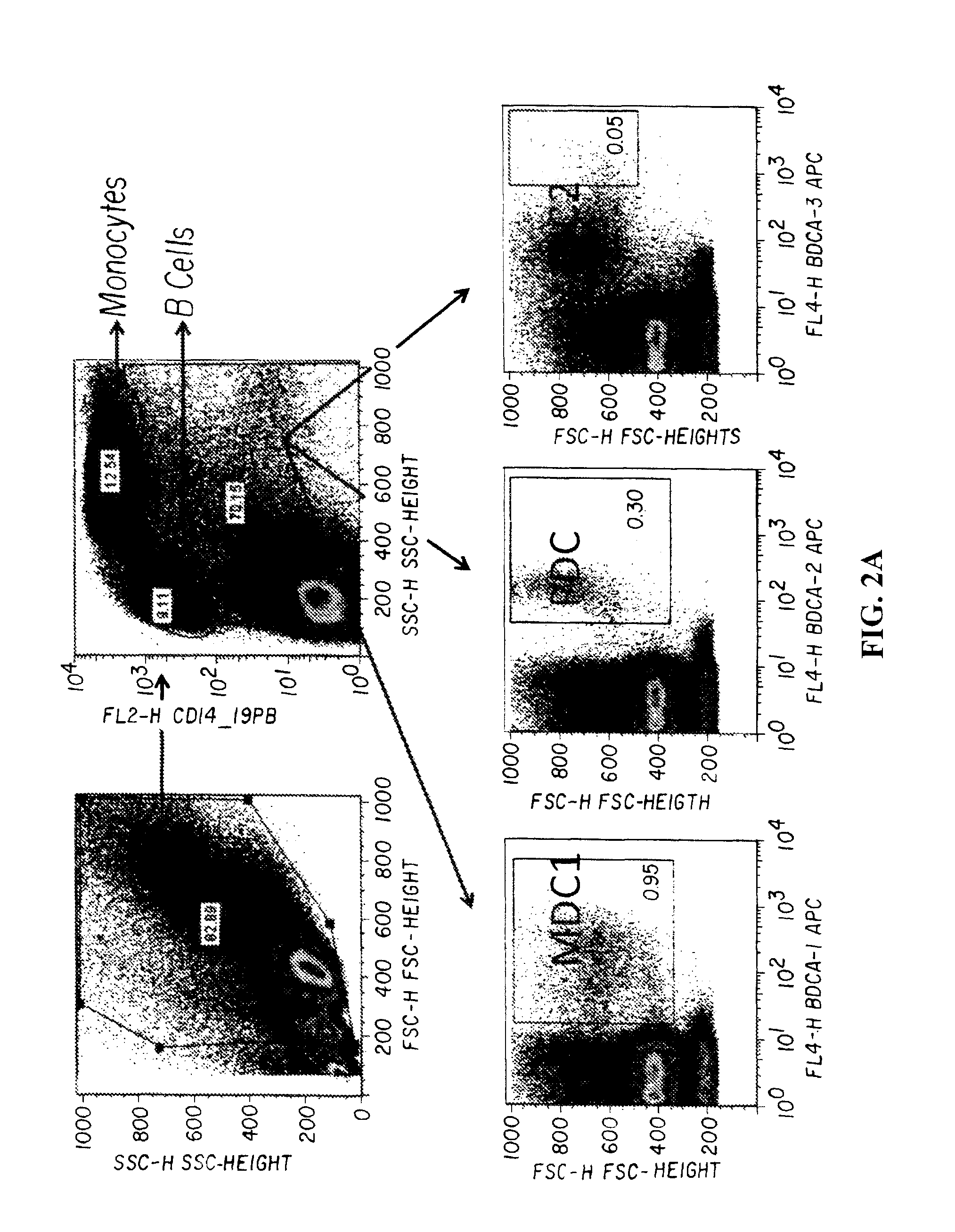
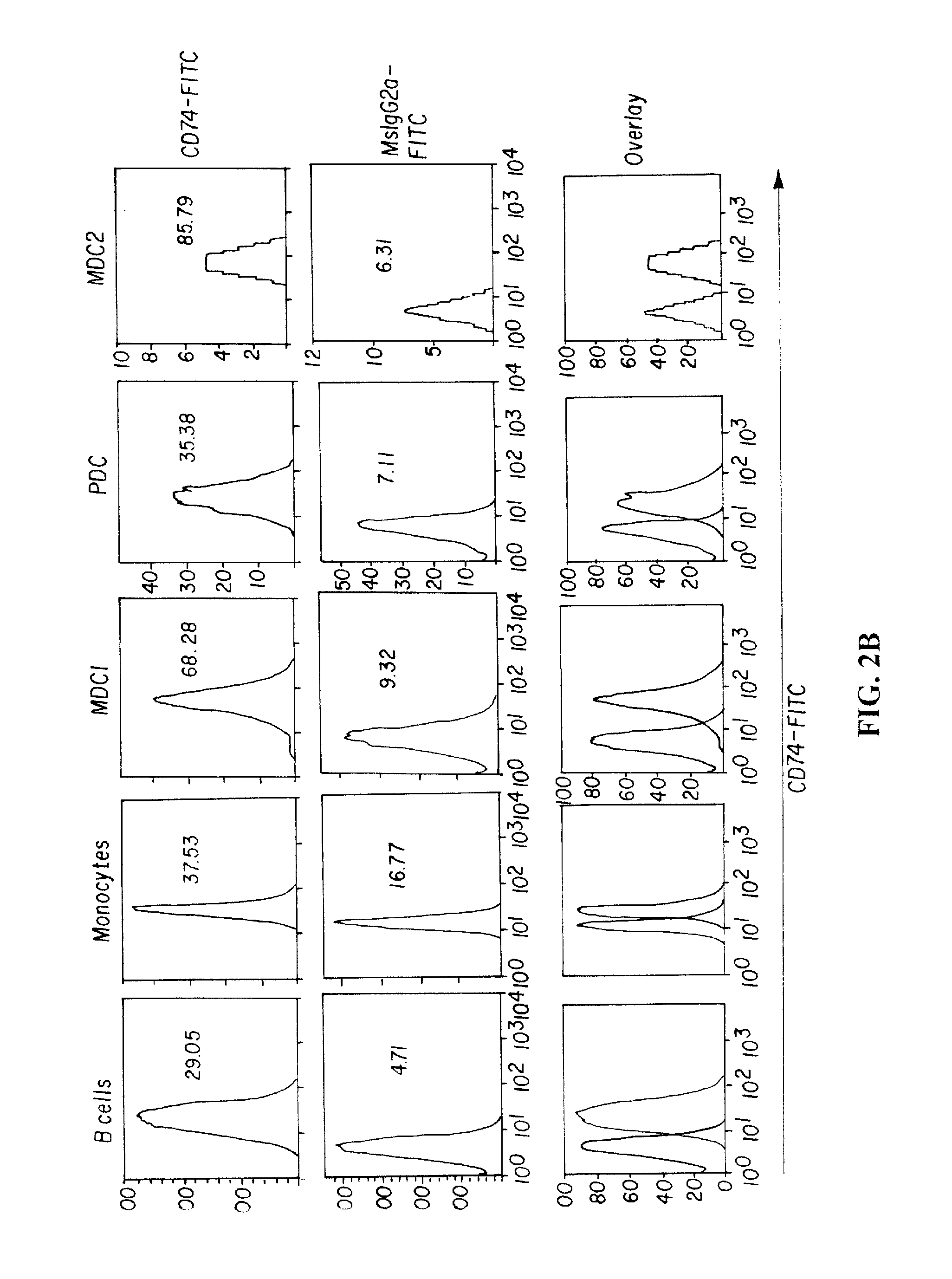
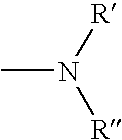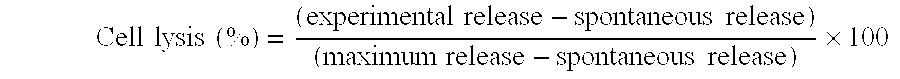Patents
Literature
431 results about "Cancer vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A cancer vaccine is a vaccine that either treats existing cancer or prevents development of a cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines. Some/many of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.
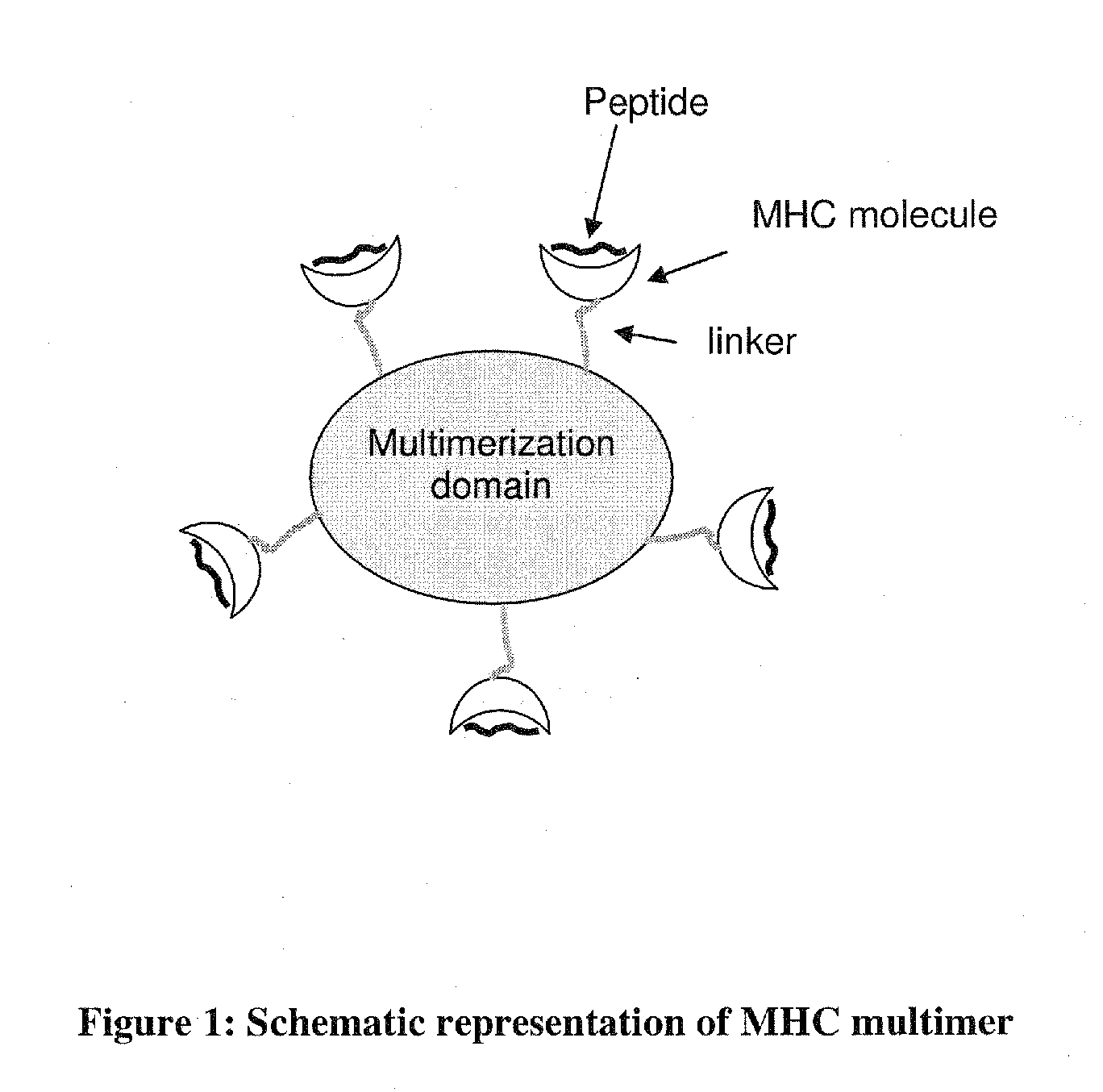
MHC Multimers in Cancer Vaccines and Immune Monitoring
InactiveUS20110318380A1Reduces infectious titerImprove efficacyPeptide/protein ingredientsImmunoglobulinsAntigenDisease
The present invention relates to MHC-peptide complexes and uses thereof in the diagnosis of, treatment of or vaccination against a disease in an individual. More specifically the invention discloses MHC complexes comprising cancer antigenic peptides and uses there of.
Owner:AGILENT TECH INC
Individualized vaccines for cancer
ActiveUS20140178438A1Reduces steric hindranceImprove translationVaccinesPharmaceutical delivery mechanismPrimary tumorTumour metastasis
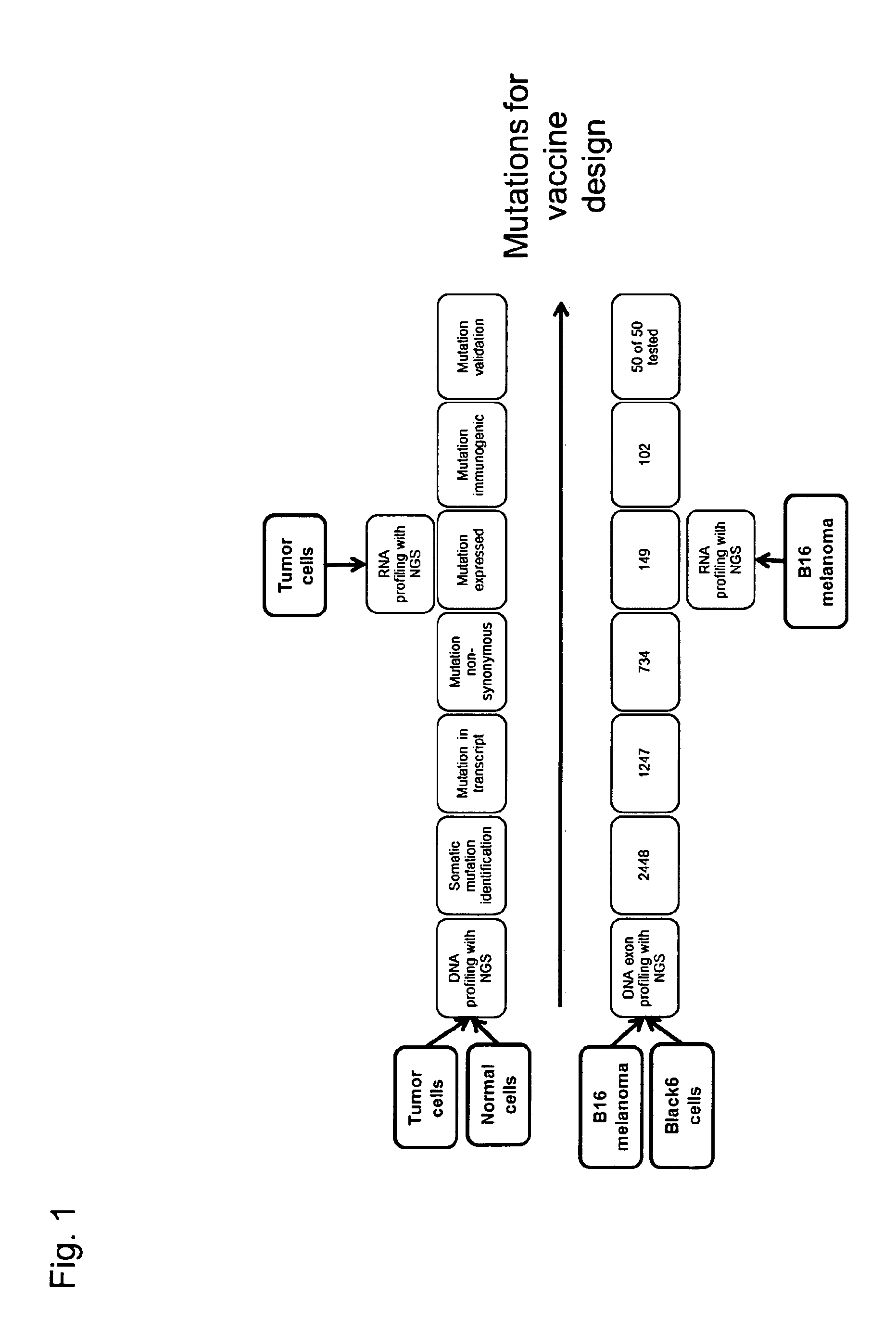
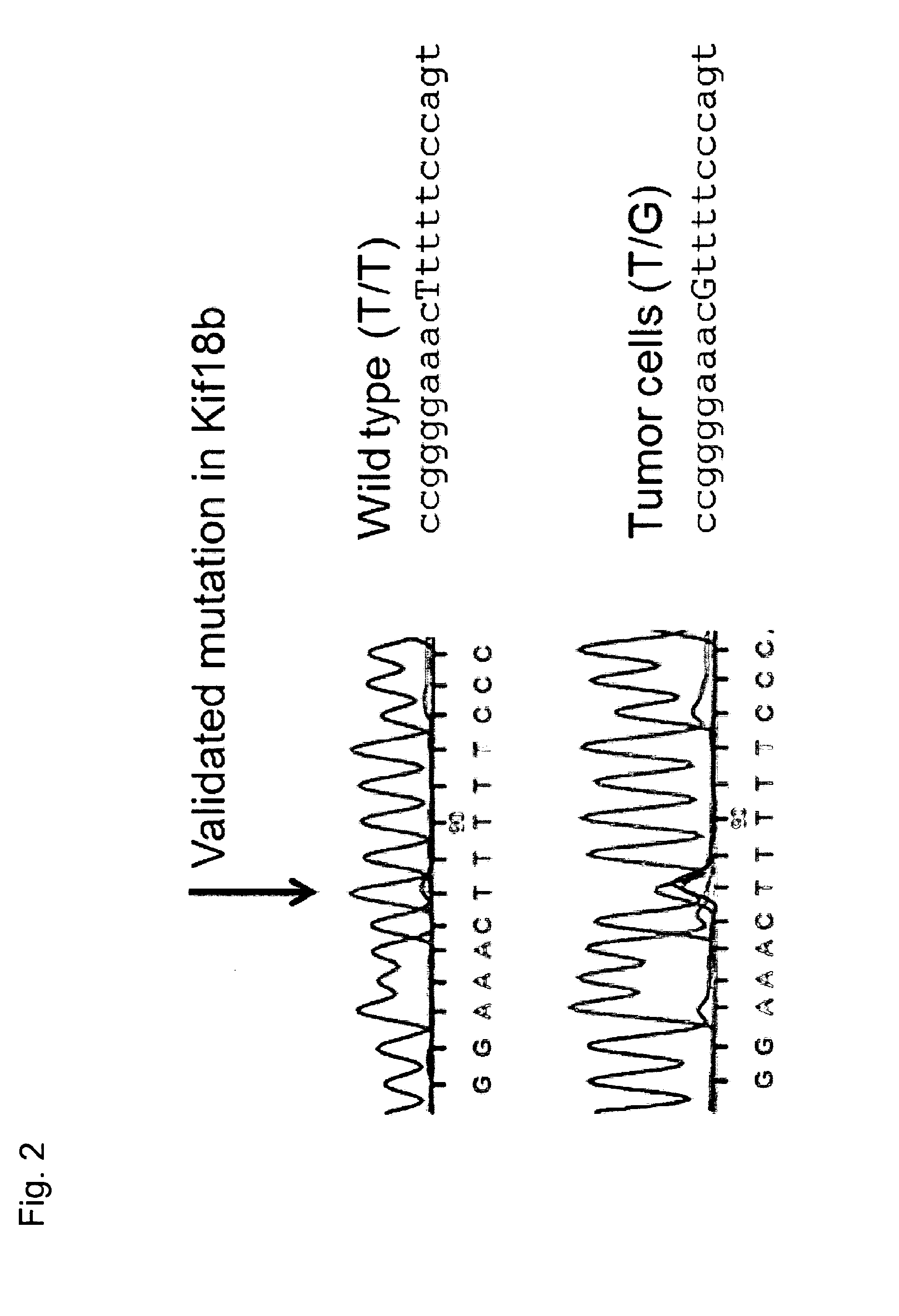
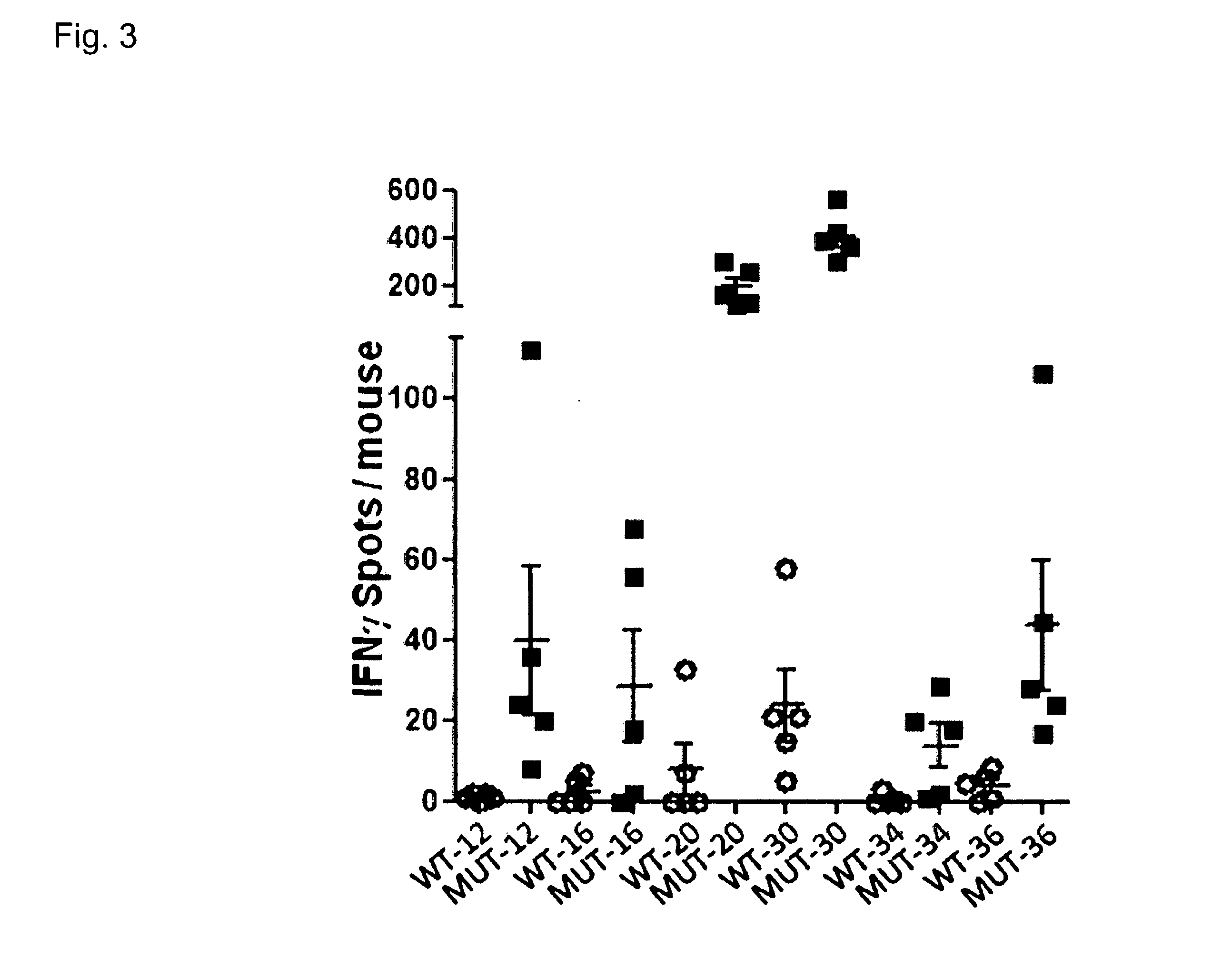
The present invention relates to the provision of vaccines which are specific for a patient's tumor and are potentially useful for immunotherapy of the primary tumor as well as tumor metastases. In one aspect, the present invention relates to a method for providing an individualized cancer vaccine comprising the steps: (a) identifying cancer specific somatic mutations in a tumor specimen of a cancer patient to provide a cancer mutation signature of the patient; and (b) providing a vaccine featuring the cancer mutation signature obtained in step (a). In a further aspect, the present invention relates to vaccines which are obtainable by said method.
Owner:TRANSLATIONALE ONKOLOGIE AN DER UNIVSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GGMBH +1
Anti-cancer vaccines
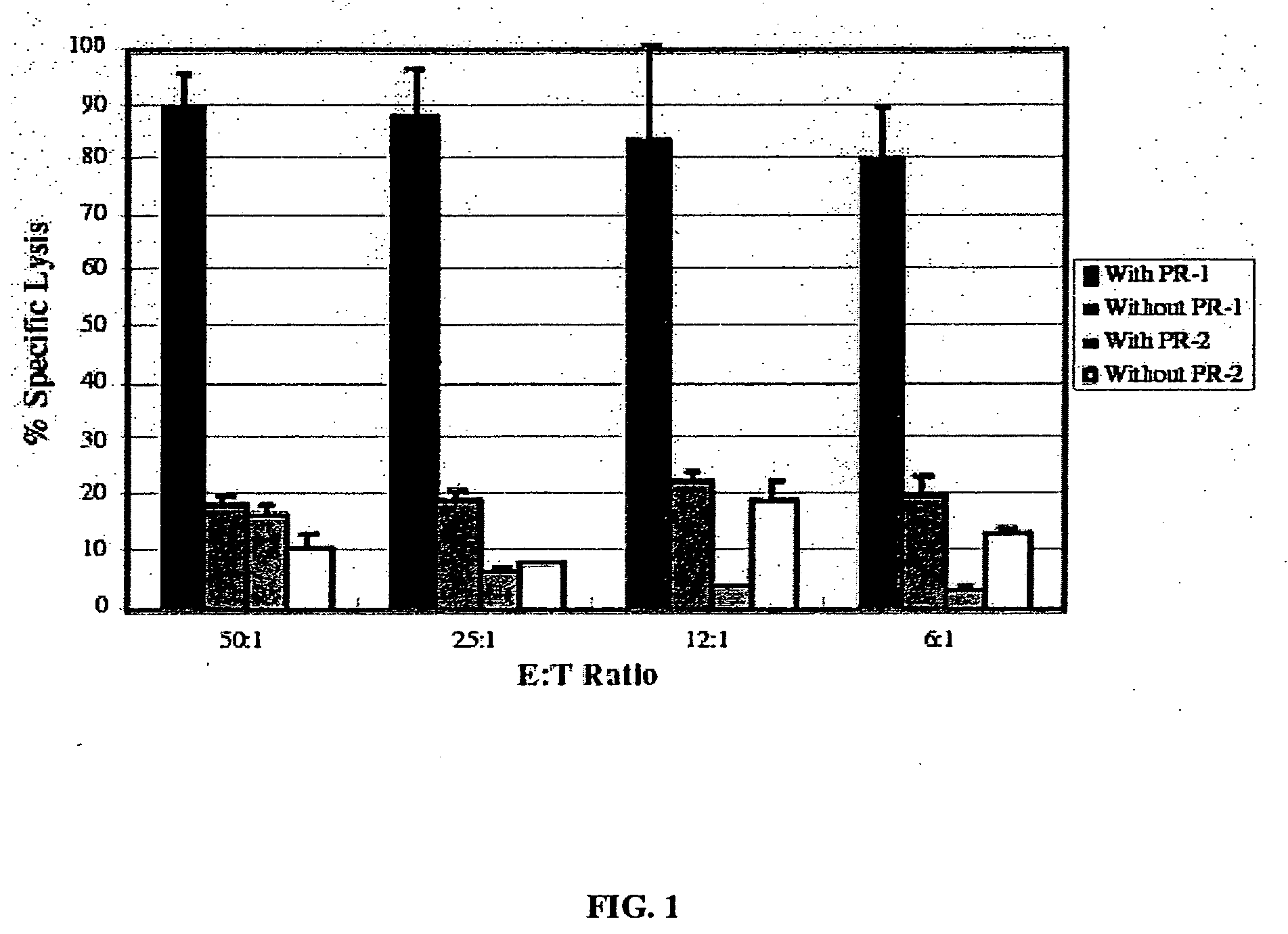
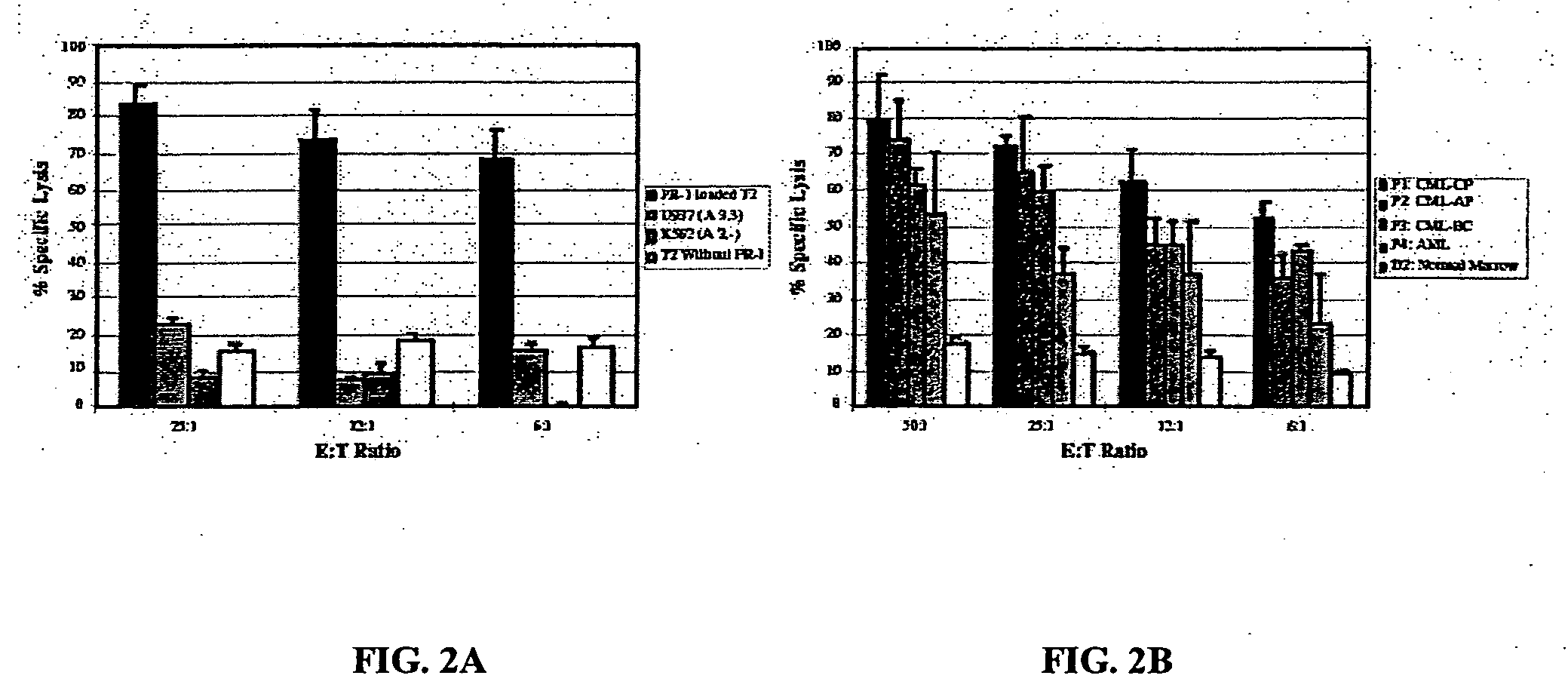
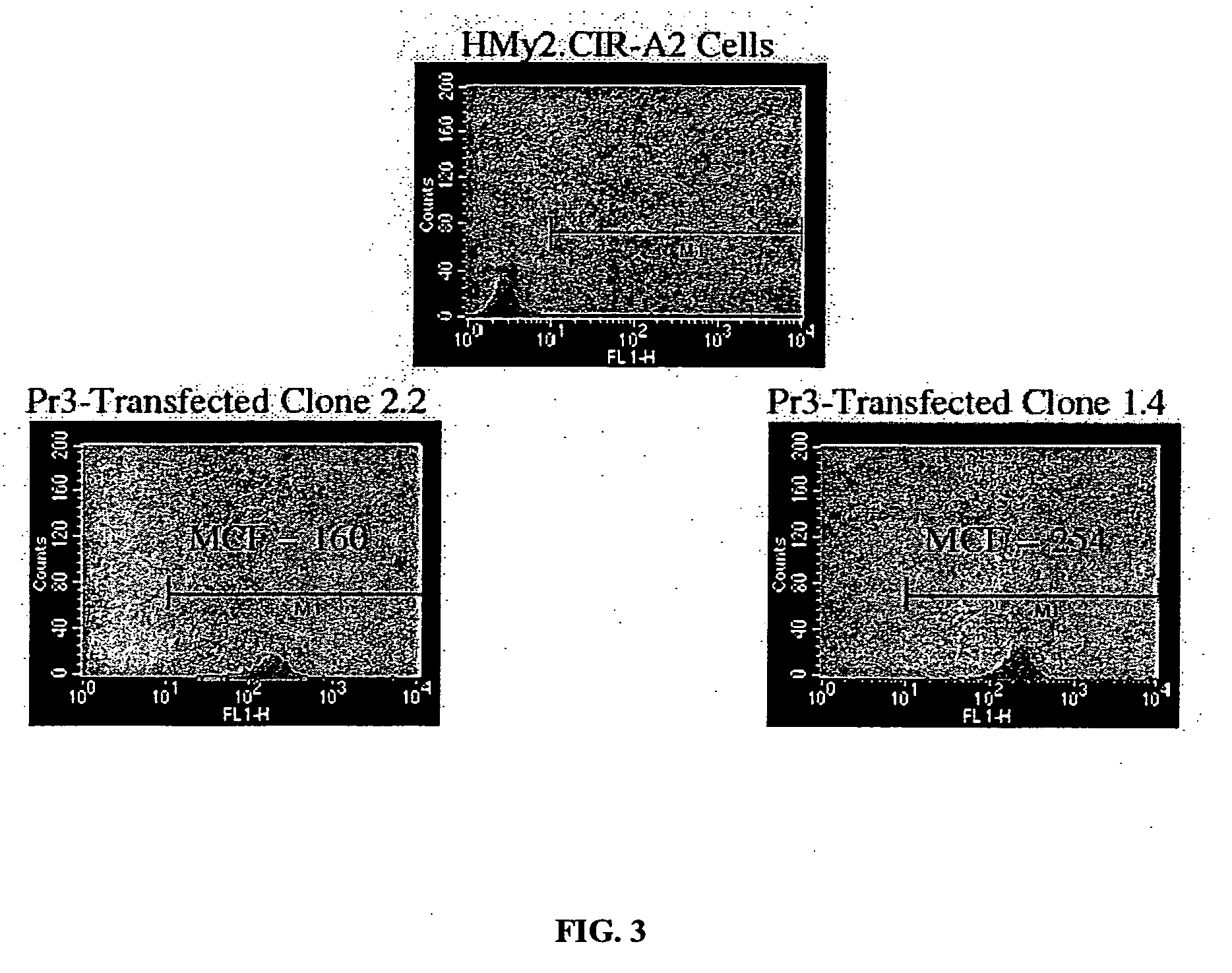
The present provides tumor-associated HLA-restricted antigens, and in particular HLA-A2 restricted antigens, as vaccines for treating or preventing cancers in a patient. In specific aspects, neutrophil elastase peptides other than PR1, cyclin E1 peptides, cyclin D peptides, or cyclin E2 peptides are provided. Such peptides can be used to elicit specific CTLs that preferentially attack tumor cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Dock-and-lock (DNL) vaccines for cancer therapy
Owner:IBC PHARMACEUTICALS INC
Tumour-associated peptides binding to human leukocyte antigen (HLA) class i or ii molecules and related Anti-cancer vaccine
InactiveUS20090274714A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to two novel peptide sequences derived from HLA class II molecules of human tumour cell lines, which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Generation of a cancer-specific immune response toward MUC1 and cancer specific MUC1 antibodies
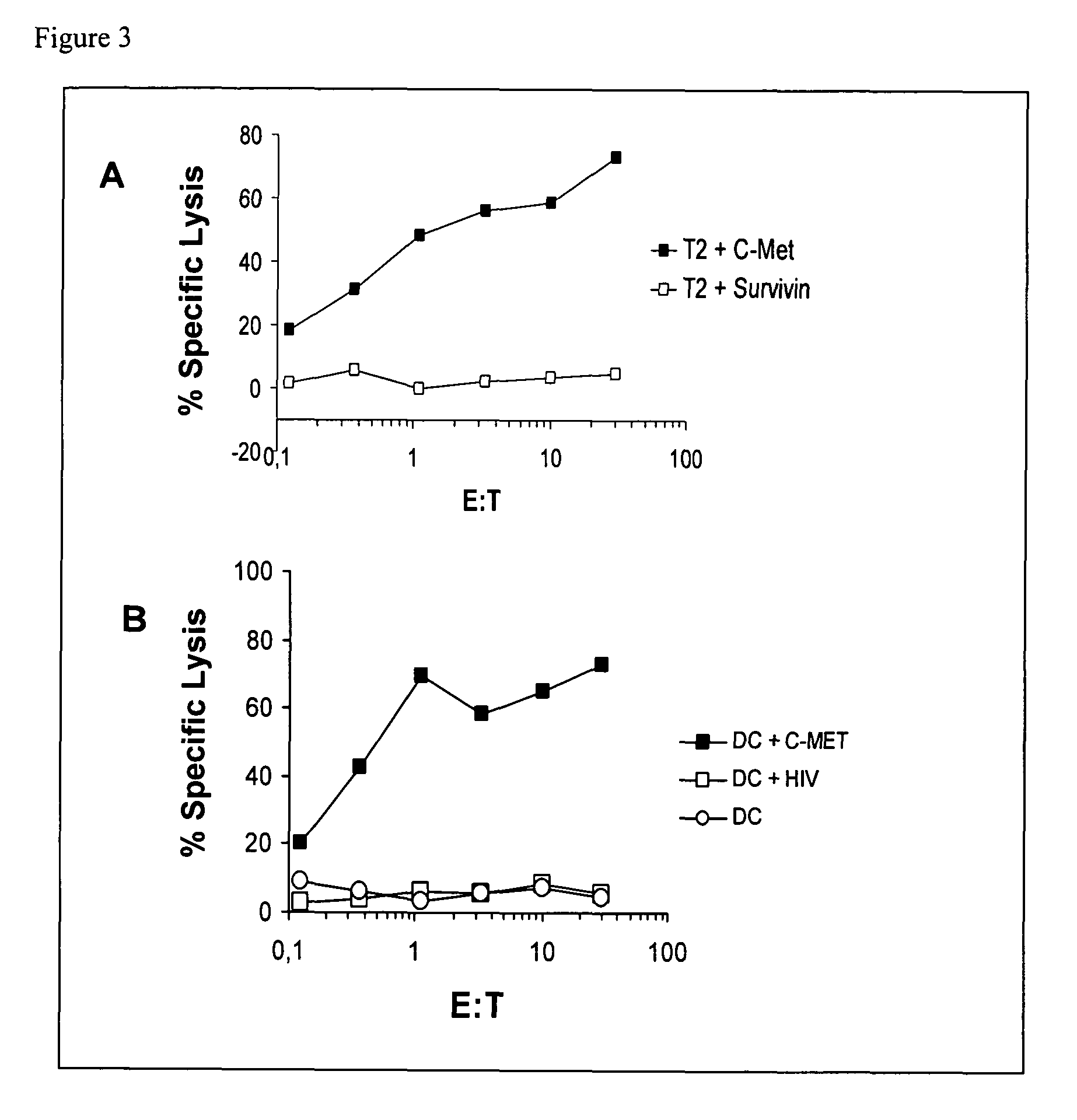
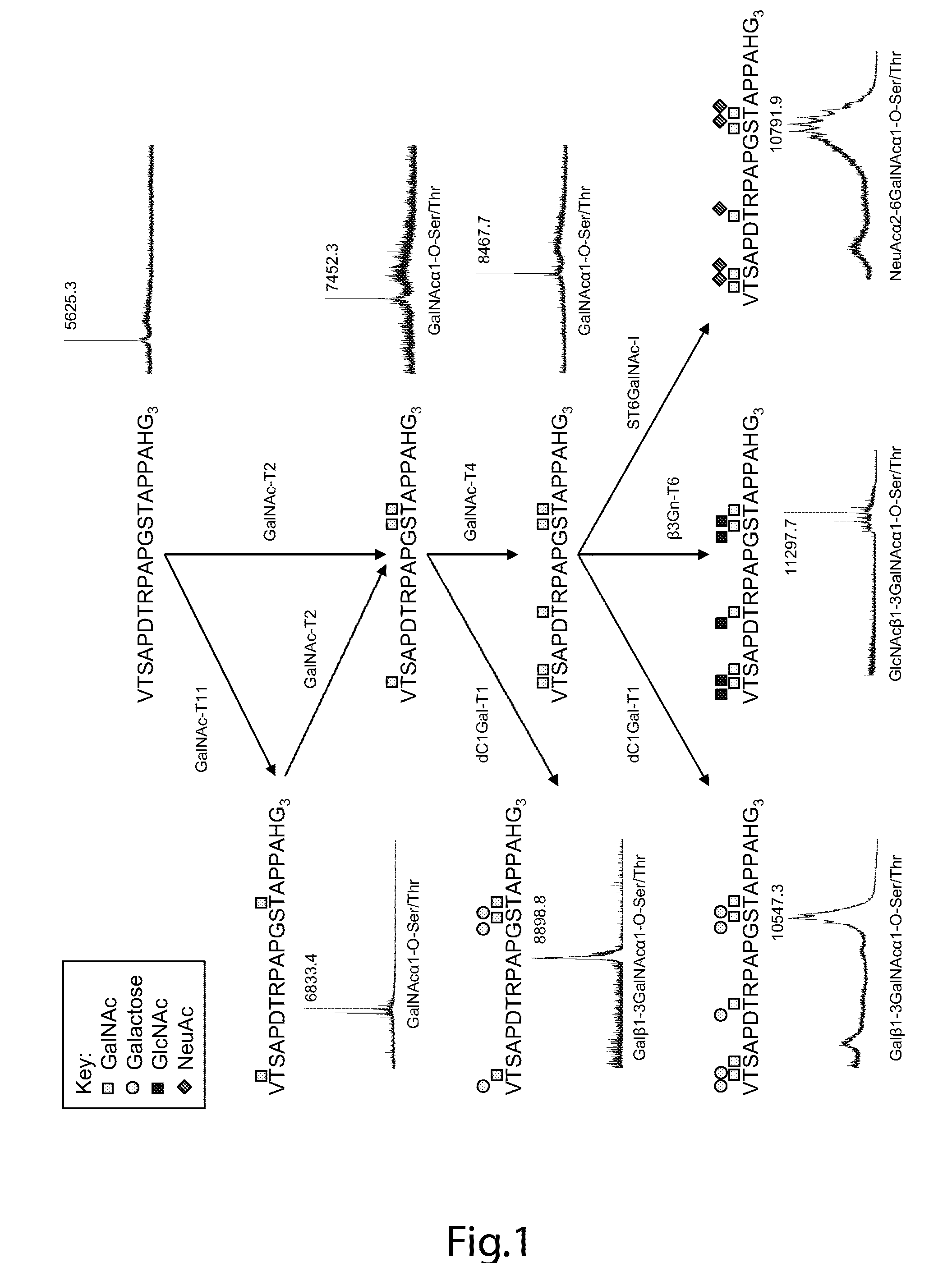
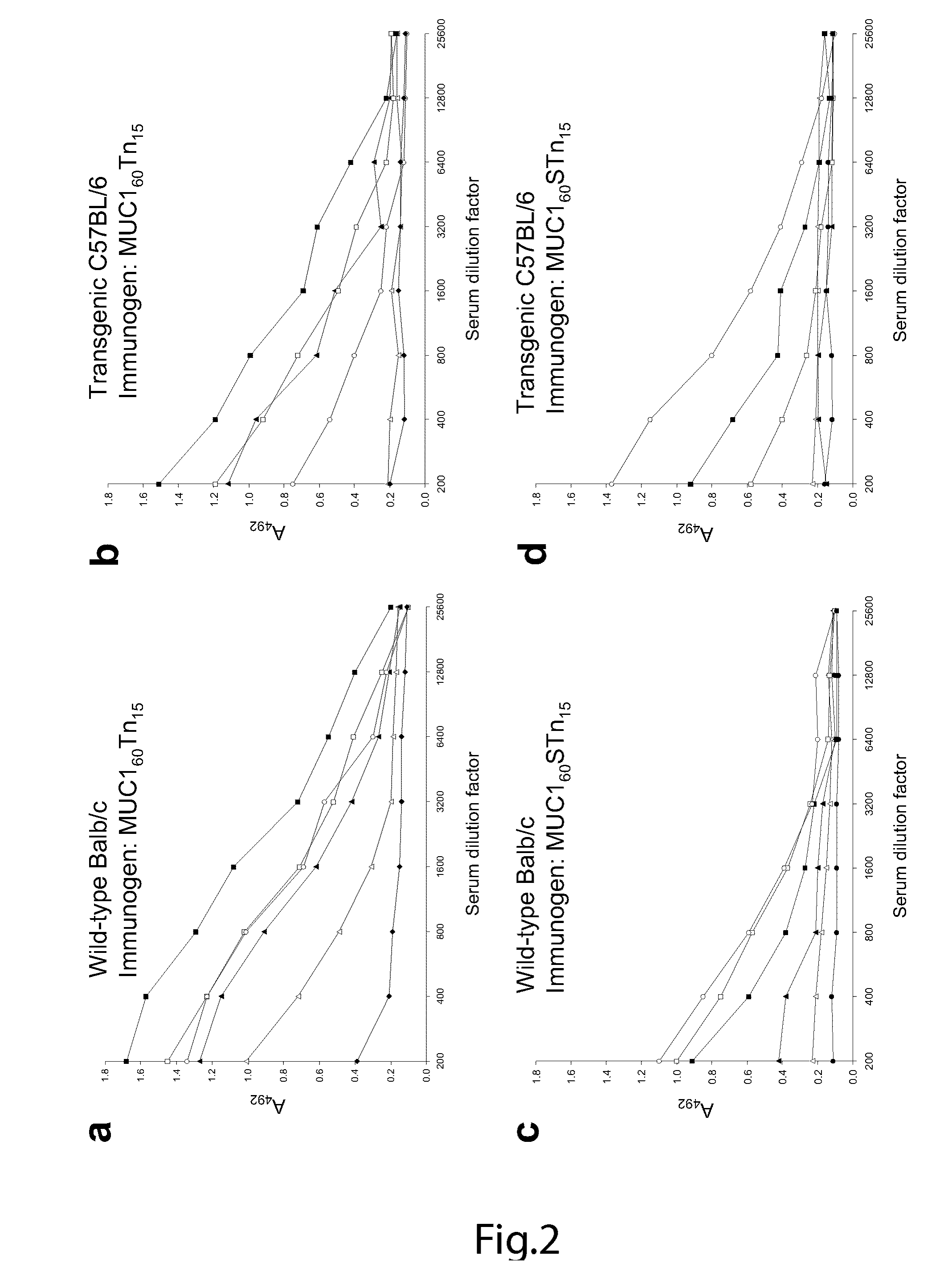
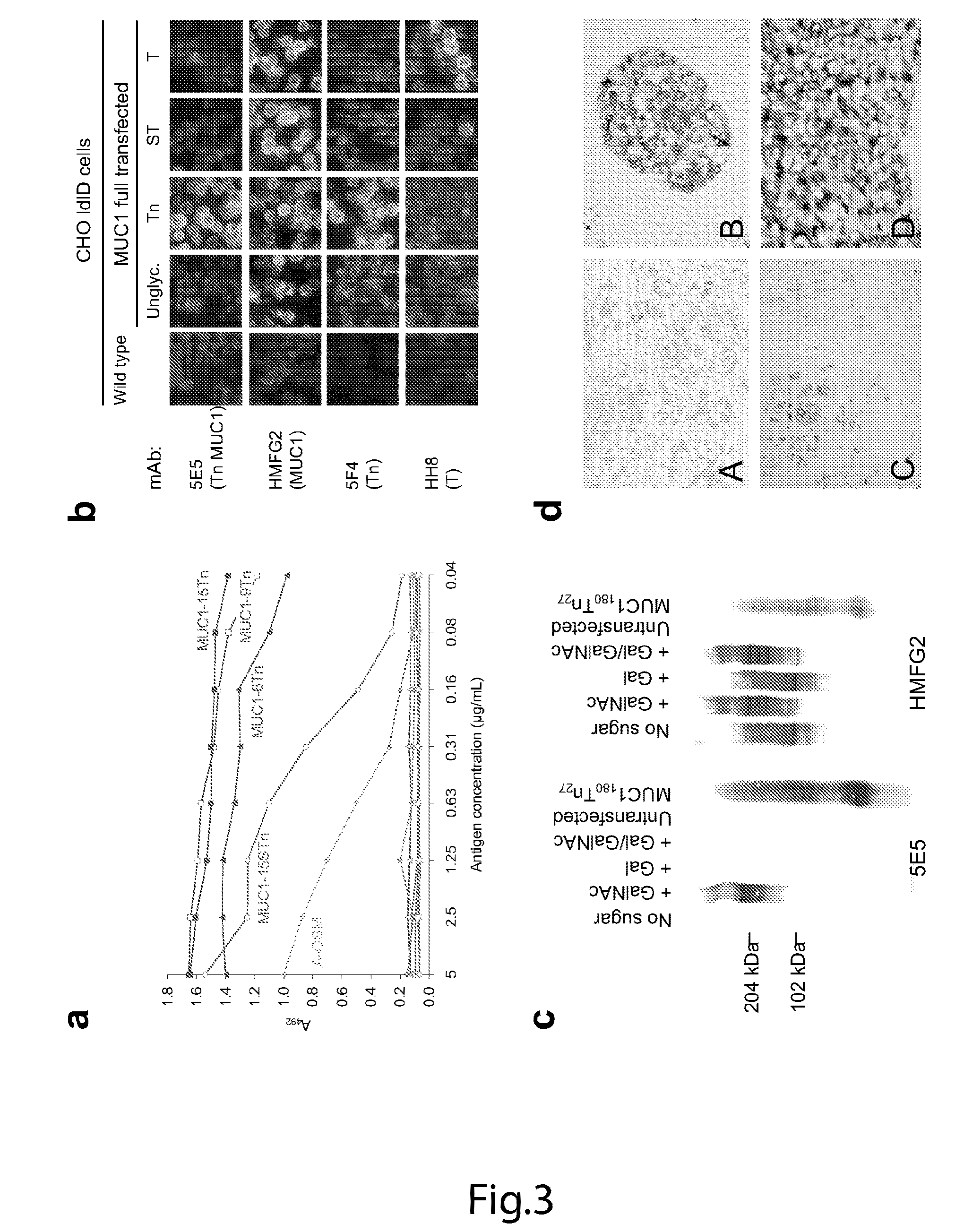
The present invention provides a method for inducing a cancer specific immune response against MUC1 using an immunogenic glycopeptide. Other aspects of the invention are a pharmaceutical composition comprising the immunogenic glycopeptide and a cancer vaccine comprising the immunogenic glycopeptide. Another aspect is an antibody generated using the immunogenic glycopeptide and the use of said antibody in therapy and diagnosis.
Owner:UNIVERSITY OF COPENHAGEN +1
Cancer immunotherapy
InactiveUS7338929B2Increase killStimulate immune responseBiocideNervous disorderDiseaseImmuno therapy
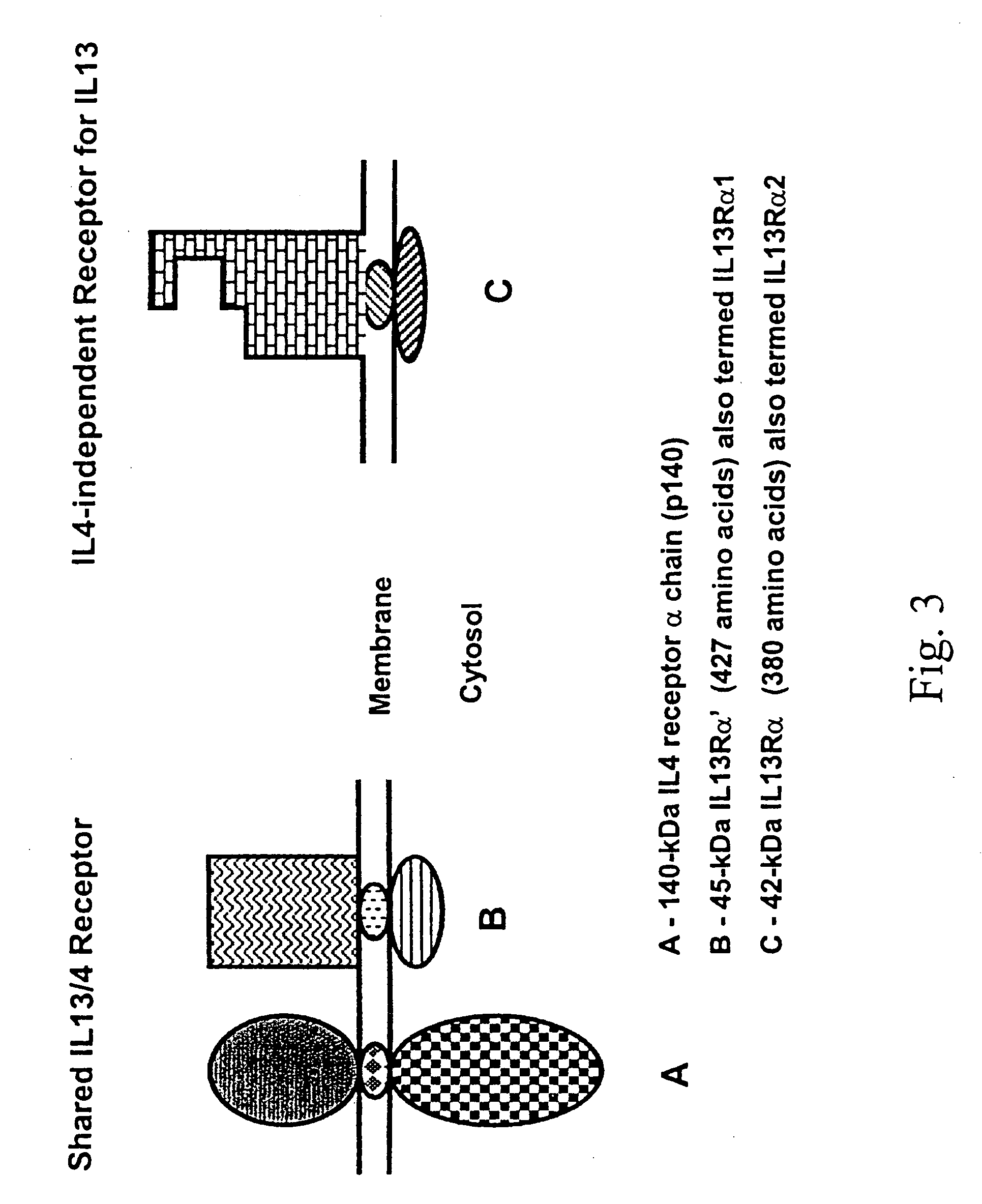
A method for stimulating a immune response against IL-13Rα2 in a subject having or at risk for developing a disease having cells expressing IL-13Rα2 includes the steps of formulating the anti-cancer vaccine outside of the subject and administering the vaccine to the subject in an amount sufficient to stimulate an immune response against IL-13Rα2 in the subject. A composition for stimulating a immune response against IL-13Rα2 in a subject having or at risk for developing a disease having cells expressing IL-13Rα2 includes an isolated agent that can stimulate immune response against IL-13α2.
Owner:PENN STATE RES FOUND
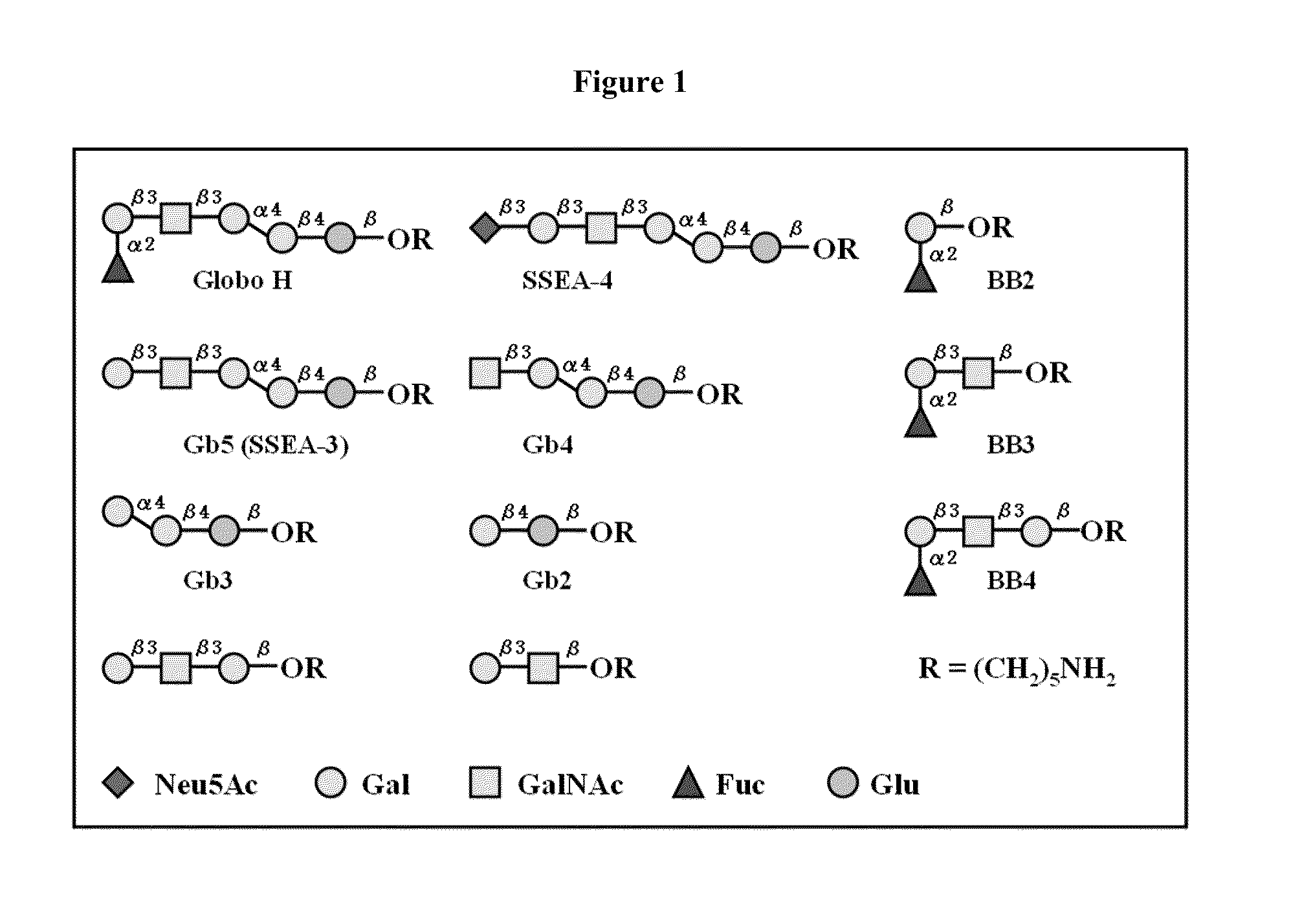
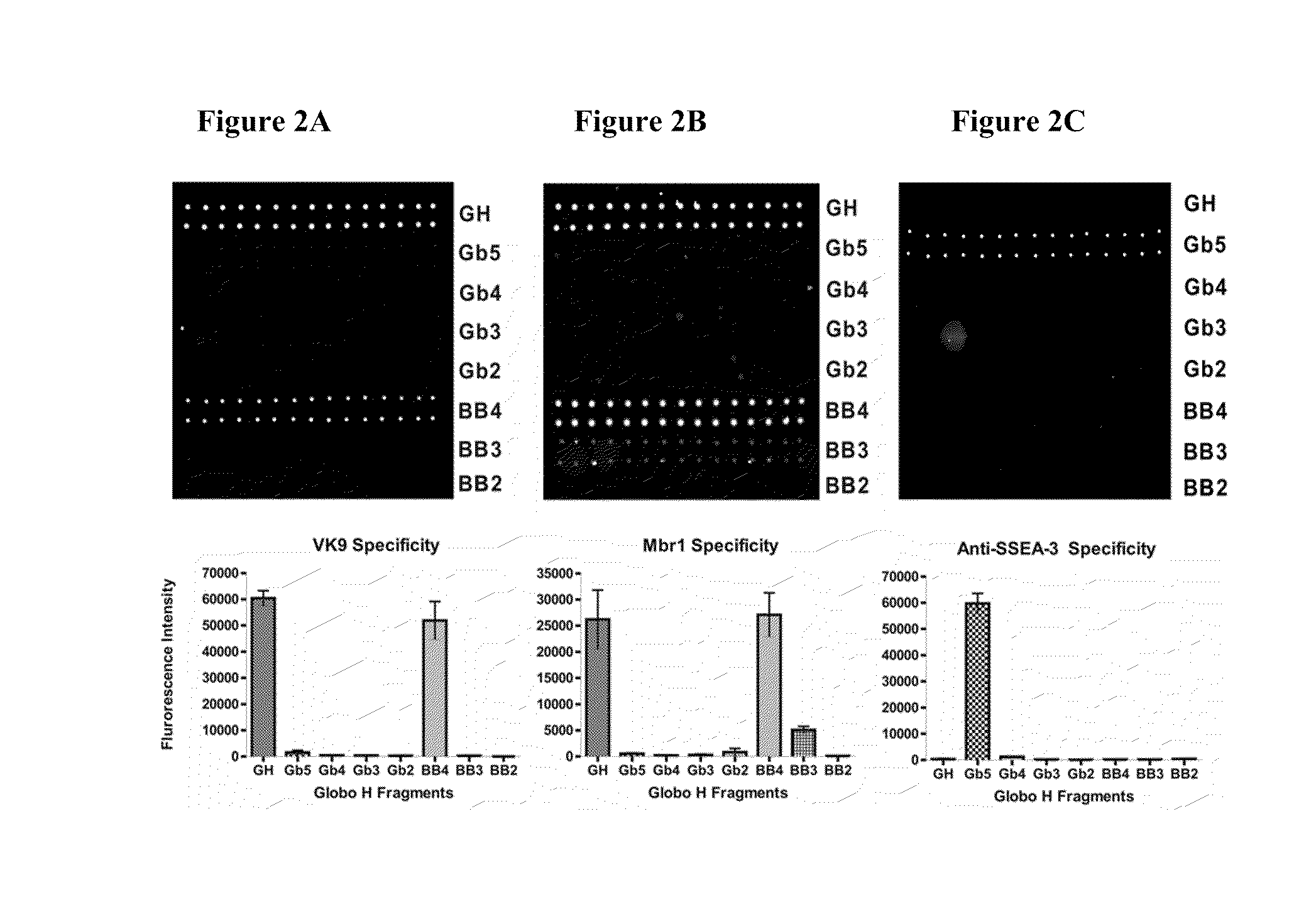
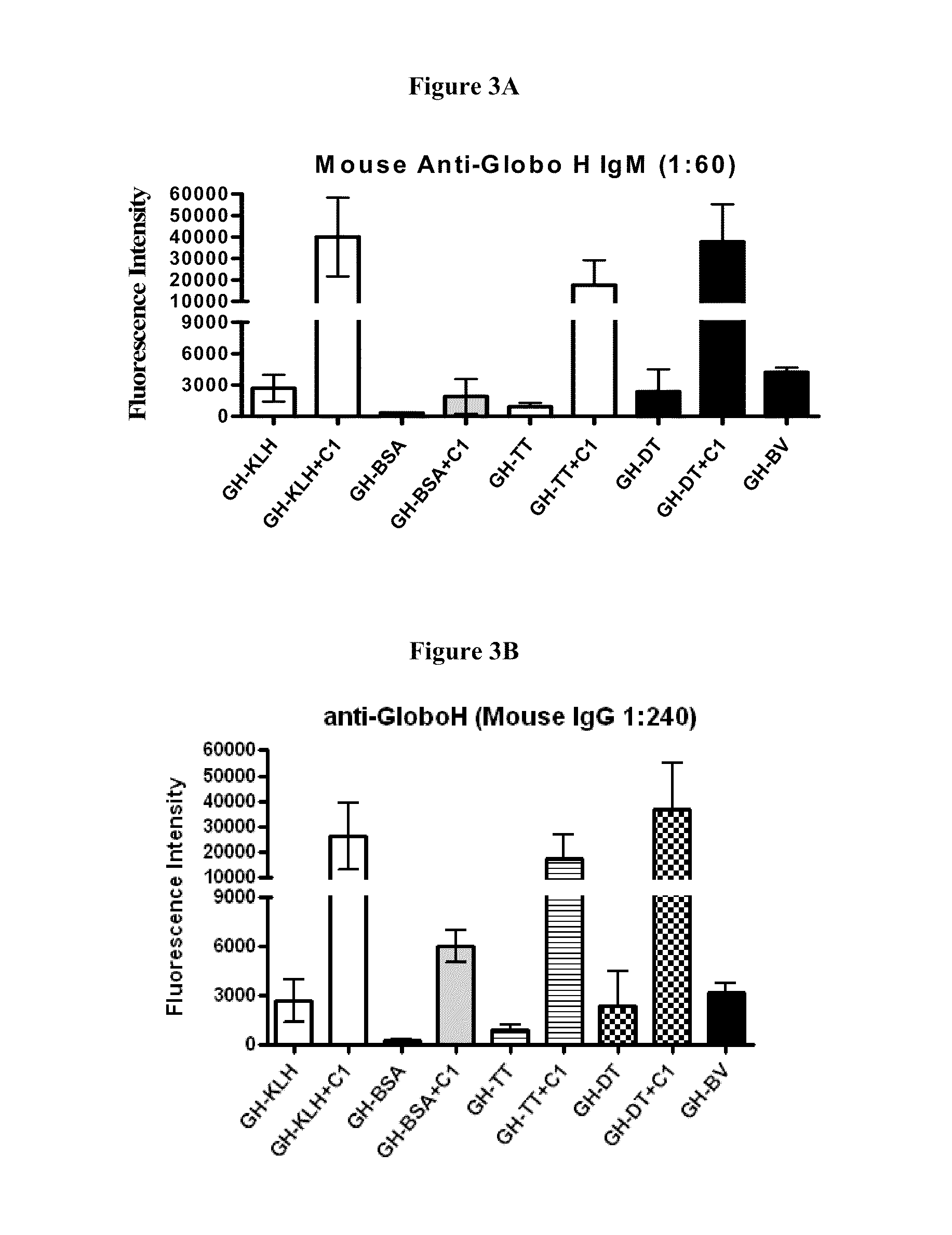
Globo h and related Anti-cancer vaccines with novel glycolipid adjuvants
ActiveUS20100136042A1Shrink tumorInhibit tumor growthOrganic active ingredientsSugar derivativesDendritic cellAdjuvant
Owner:ACAD SINIC
Novel Strategies for Improved Cancer Vaccines
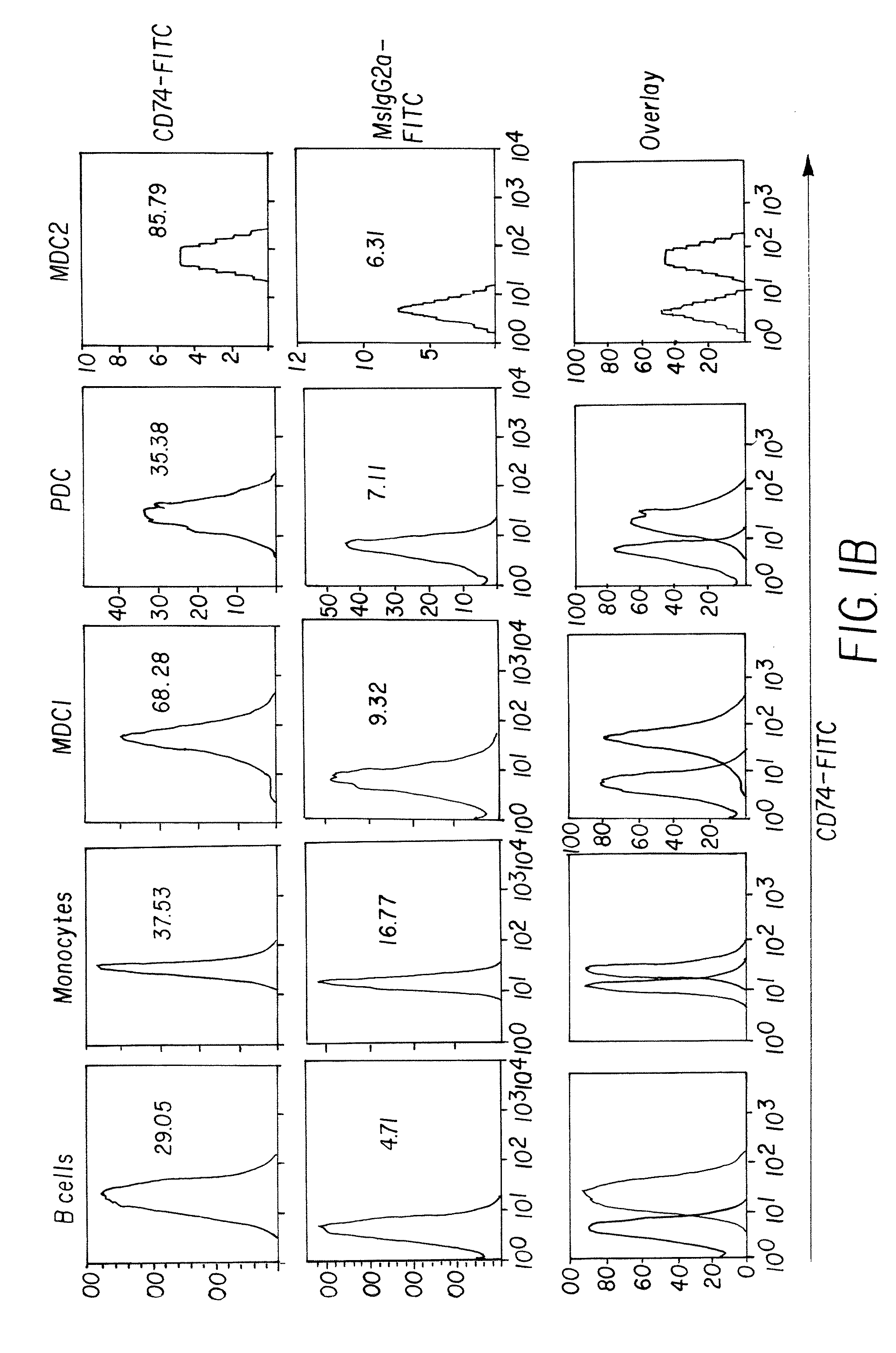
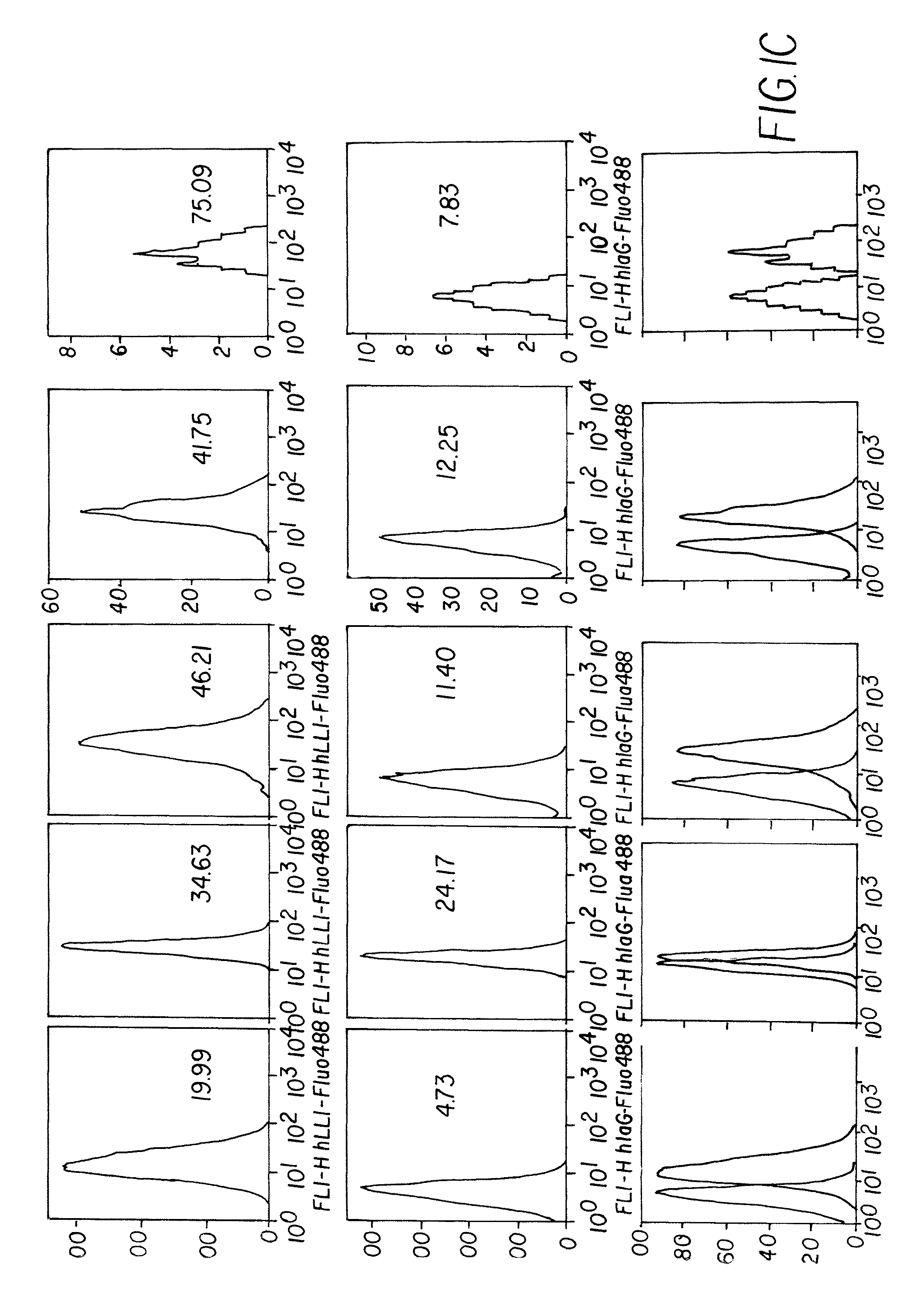
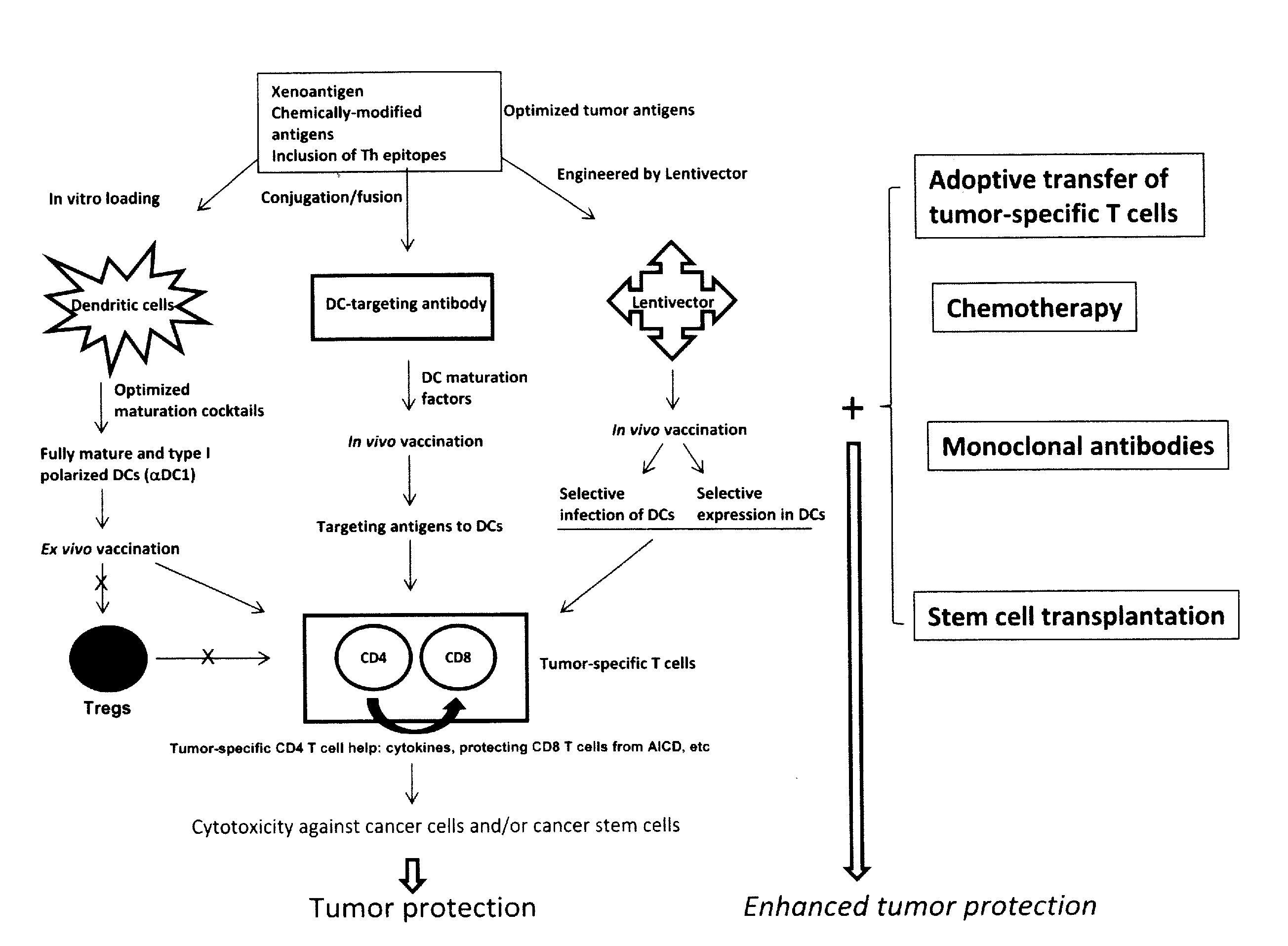
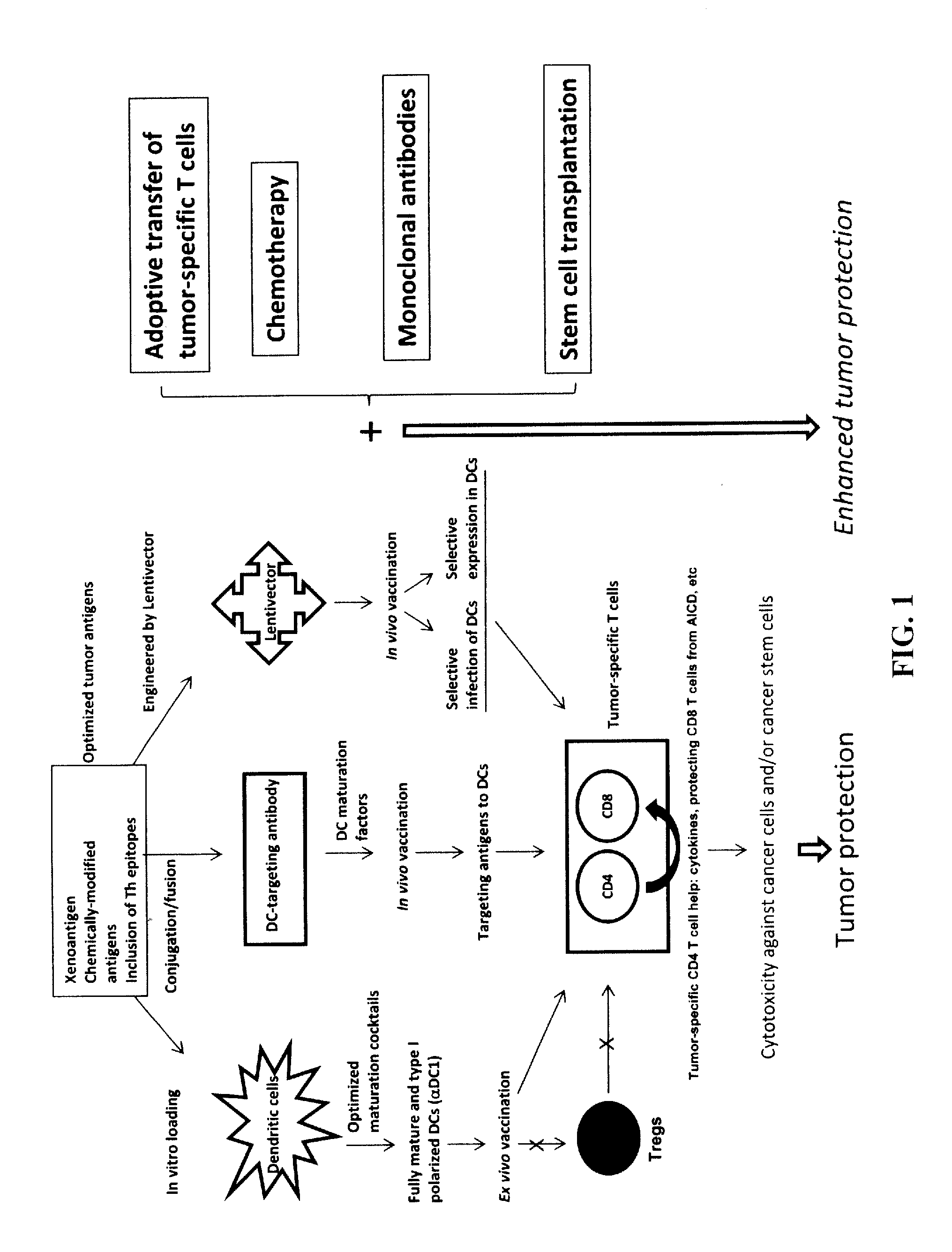
The present invention concerns methods and compositions for forming anti-cancer vaccine complexes. In preferred embodiments, the anti-cancer vaccine complex comprises an antibody moiety that binds to dendritic cells, such as an anti-CD74 antibody or antigen-binding fragment thereof, attached to an AD (anchoring domain) moiety and a xenoantigen, such as CD20, attached to a DDD (dimerization and docking domain) moiety, wherein two copies of the DDD moiety form a dimer that binds to the AD moiety, resulting in the formation of the vaccine complex. The anti-cancer vaccine complex is capable of inducing an immune response against xenoantigen expressing cancer cells, such as CD138negCD20+ MM stem cells, and inducing apoptosis of and inhibiting the growth of or eliminating the cancer cells.
Owner:IBC PHARMACEUTICALS INC
Genetically modified tumor cells as cancer vaccines
InactiveUS20060165668A1Convenient treatmentEasy to detectBiocidePeptide/protein ingredientsAbnormal tissue growthCancer cell
The present invention provides methods and compositions for electroporation-mediated gene transfer to cancer cells. The transfected cancer cells are genetically modified to express one or more therapeutic proteins. In certain embodiments, the cancer cells are modified to express one or more cytokines capable of enhancing the immunogenicity of the transfected cancer cell. Administering the transfected cancer cell to a subject will lead to enhanced immune-cell mediated killing of tumors. Accordingly, the present invention provides methods and compositions for improved treatment and prevention of cancer and other hyperproliferative diseases.
Owner:MAXCYTE
Antibodies against cancer
InactiveUS7318924B2Improve permeabilityPeptide/protein ingredientsGenetic material ingredientsAnticarcinogenCripto
An isolated binding partner of a Cripto-1 protein, Pim-1 protein or an antigen present in a colon cancer cell lysate is described. The binding partner inhibits growth of one or more cancer cell types and may be used in an anti-cancer agent for treating cancer in a subject. The binding partner may also be used in a method of inducing apoptosis in a cancer cell, as well as in a method of sensitizing a cancer cell to a cytotoxic compound. In addition, a cancer vaccine is described wherein the vaccine comprises a Cripto-1 protein (or an antigenic fragment thereof), Pim-1 protein (or an antigenic fragment thereof) or an antigen present in a colon cancer cell lysate or, alternatively, comprises an expressible DNA molecule encoding a Cripto-1 protein (or an antigenic fragment thereof), Pim-1 protein (or an antigenic fragment thereof) or an antigen present in a colon cancer cell lysate.
Owner:THE MACFARLANE BURNET INST FOR MEDICAL RES & PUBLIC HEALTH LTD
Tumor antigen useful in diagnosis and therapy of prostate and colon cancer
InactiveUS7037667B1Microbiological testing/measurementBiological material analysisCell membraneTumor antigen
Owner:AGENSYS
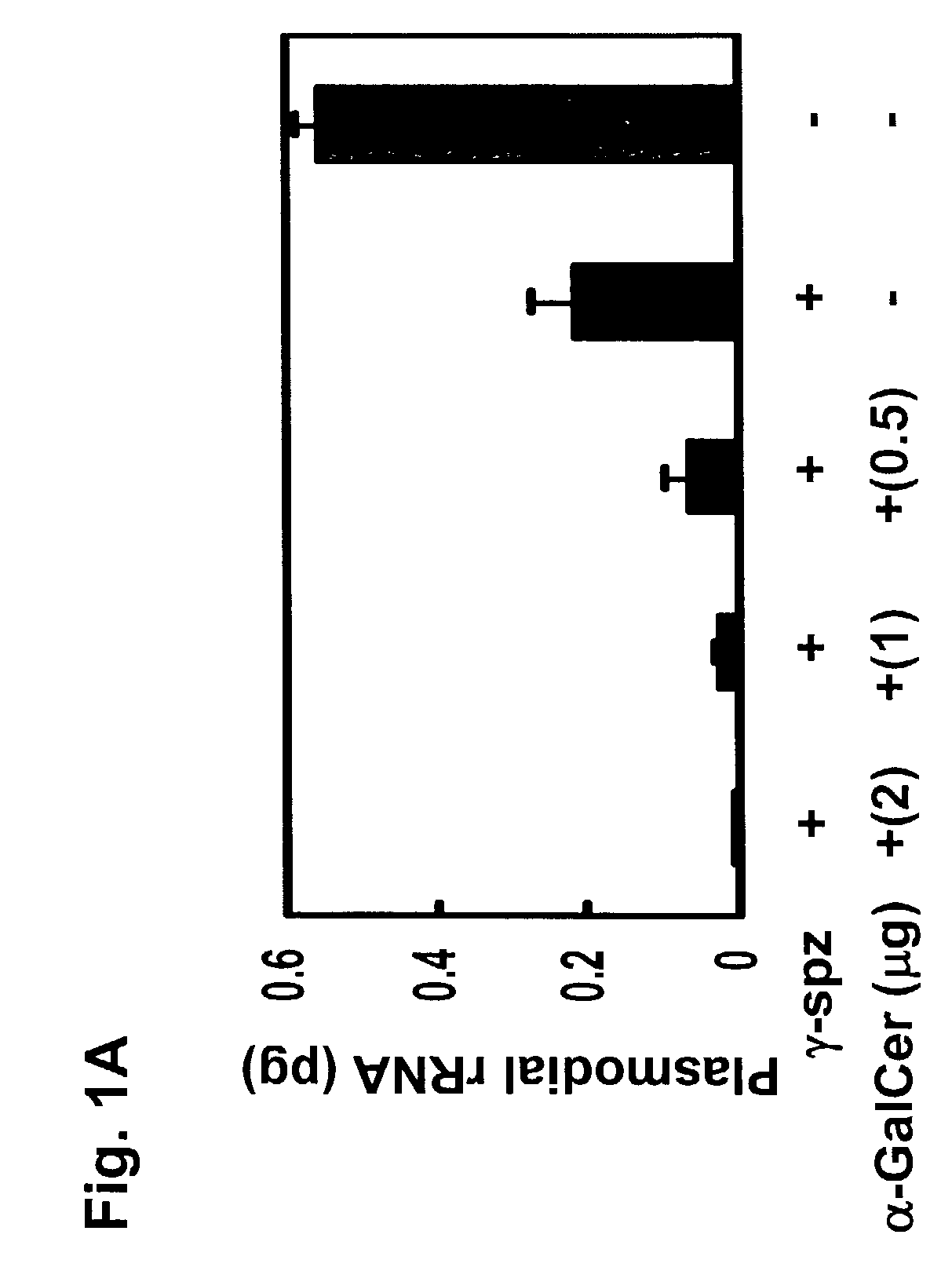
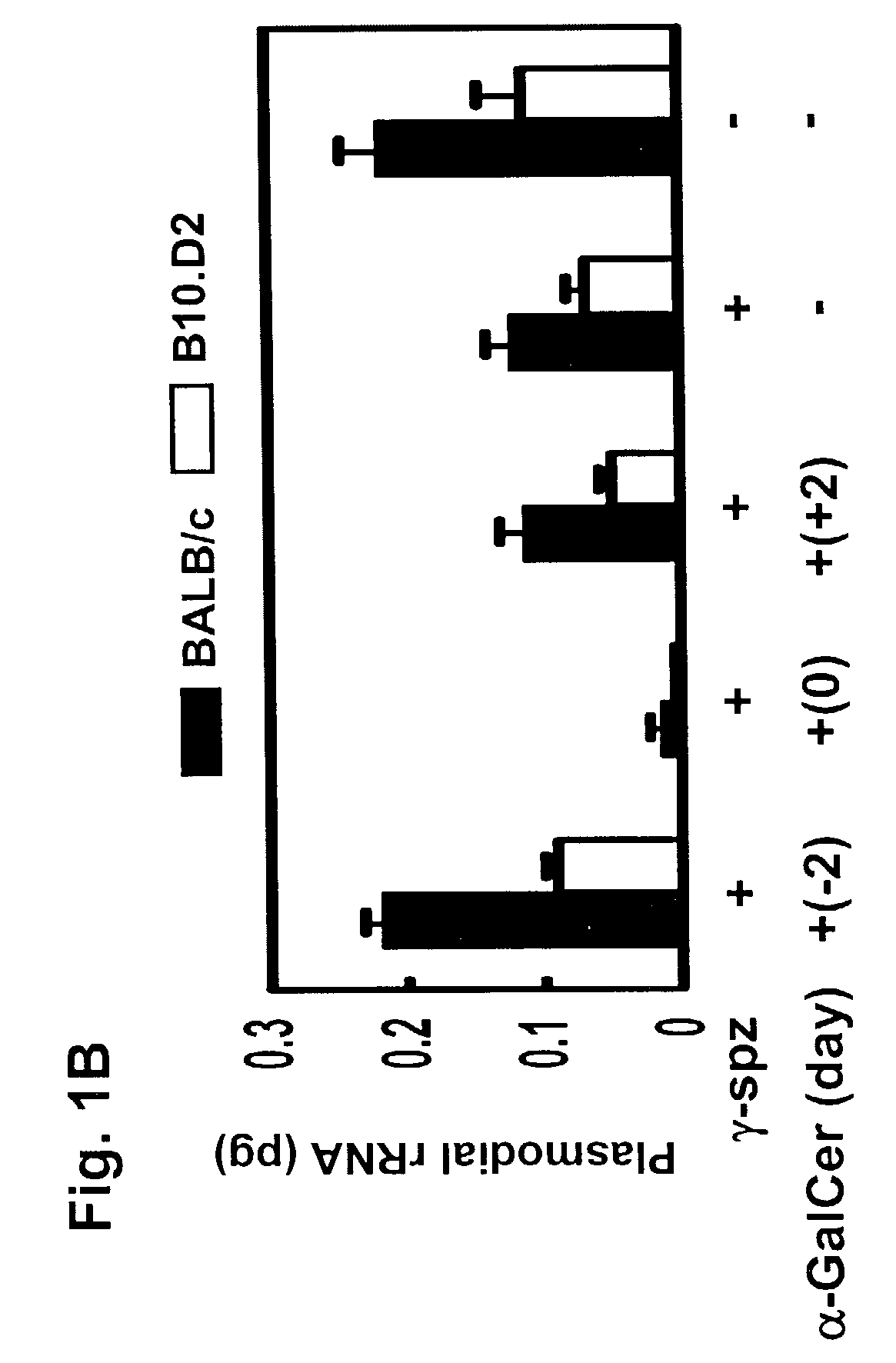
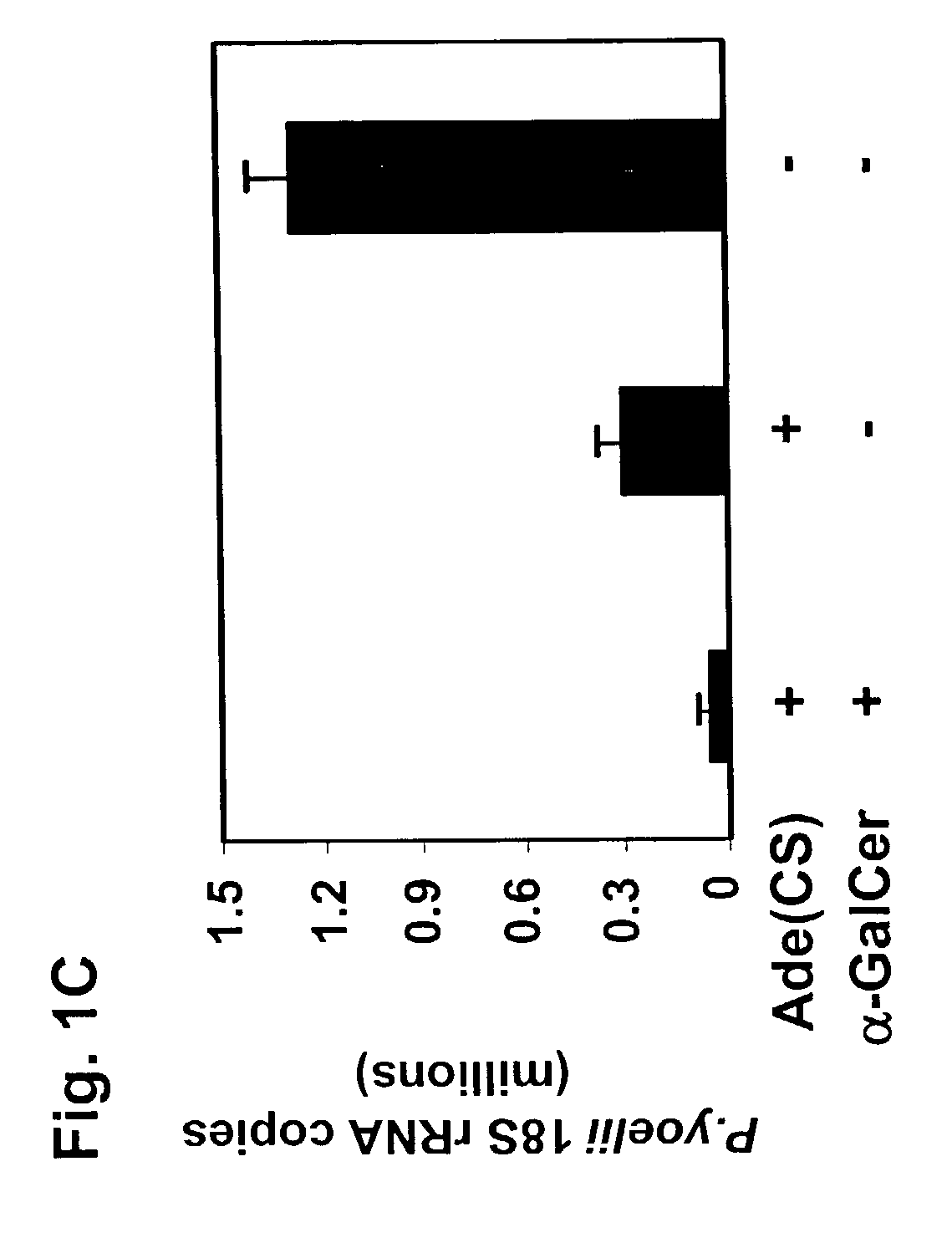
Use of glycosylceramides as adjuvants for vaccines against infections and cancer
InactiveUS7488491B2Enhancement and extension of durationAntibacterial agentsOrganic active ingredientsAdjuvantMammal
The present invention relates to methods and compositions for augmenting an immunogenicity of an antigen in a mammal, comprising administering said antigen together with an adjuvant composition that includes glycosylceramide, preferably α-galactosylceramide (α-GalCer). According to the present invention, the use of glycosylceramide as an adjuvant is attributed at least in part to the enhancement and / or extension of antigen-specific Th1-type responses, in particular, CD8+ T cell responses. The methods and compositions of the present invention can be useful for prophylaxis and treatment of various infectious and neoplastic diseases.
Owner:NEW YORK UNIVERSITY
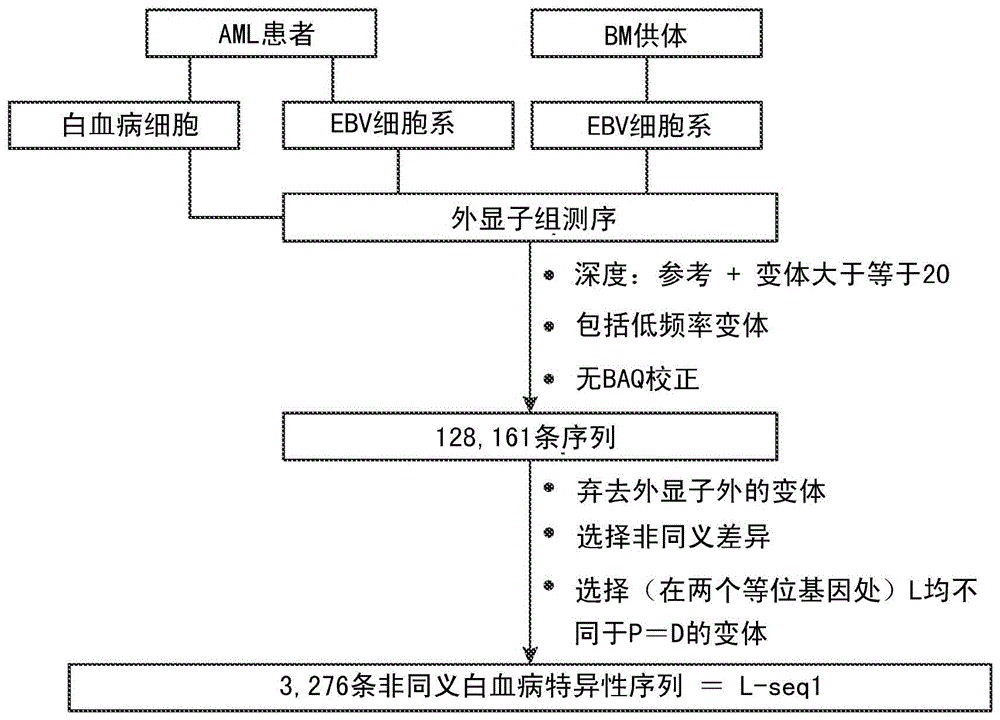
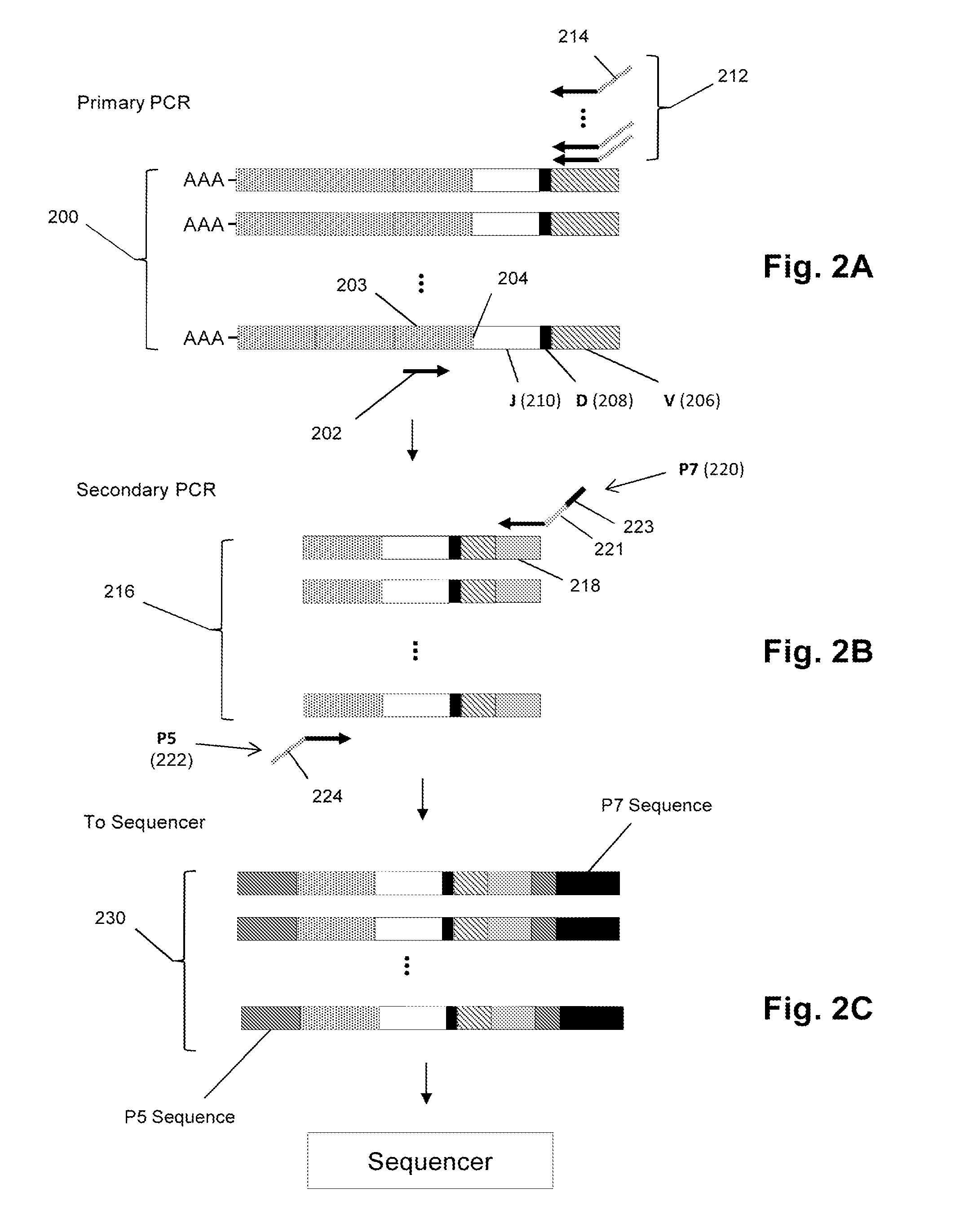
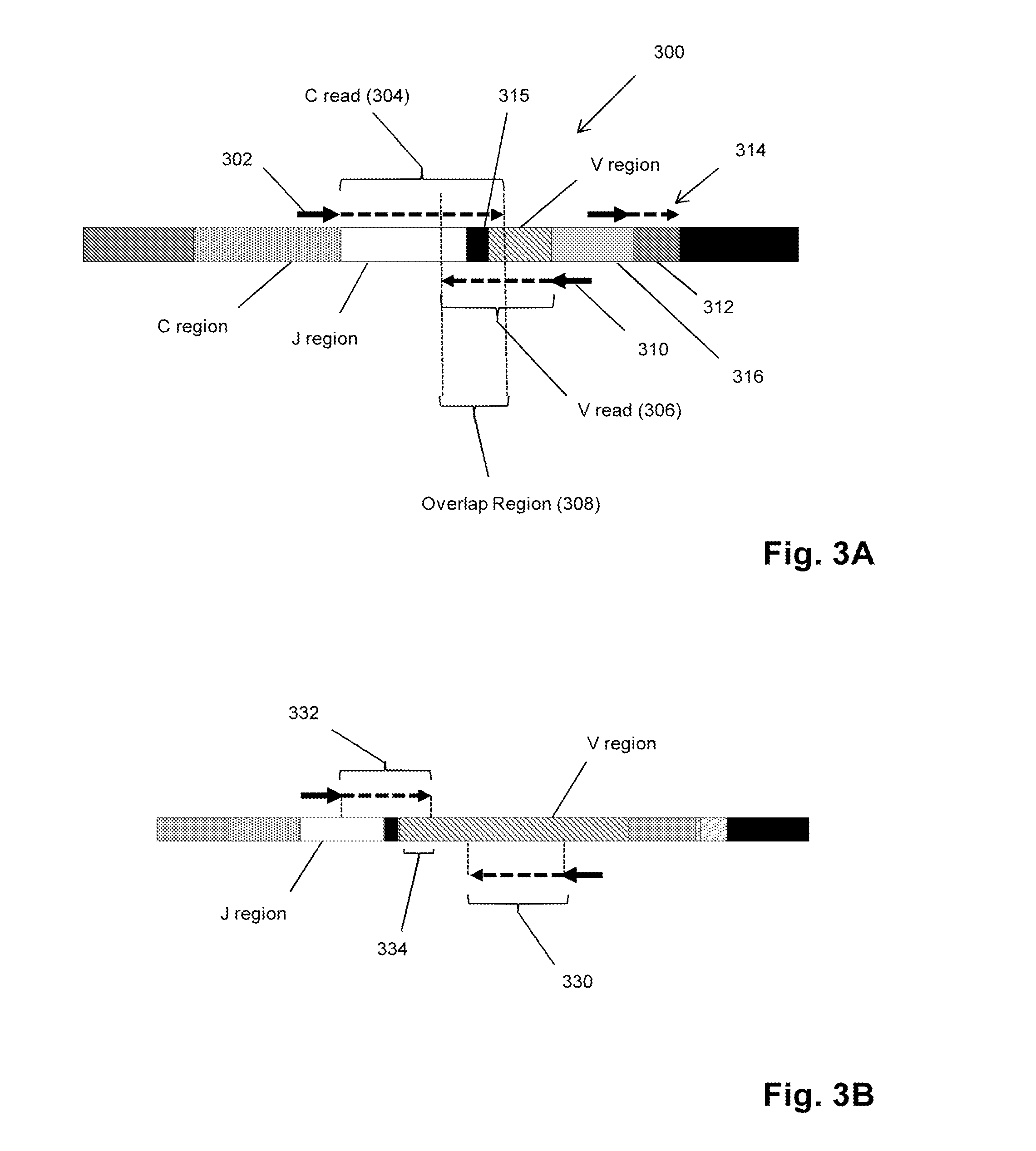
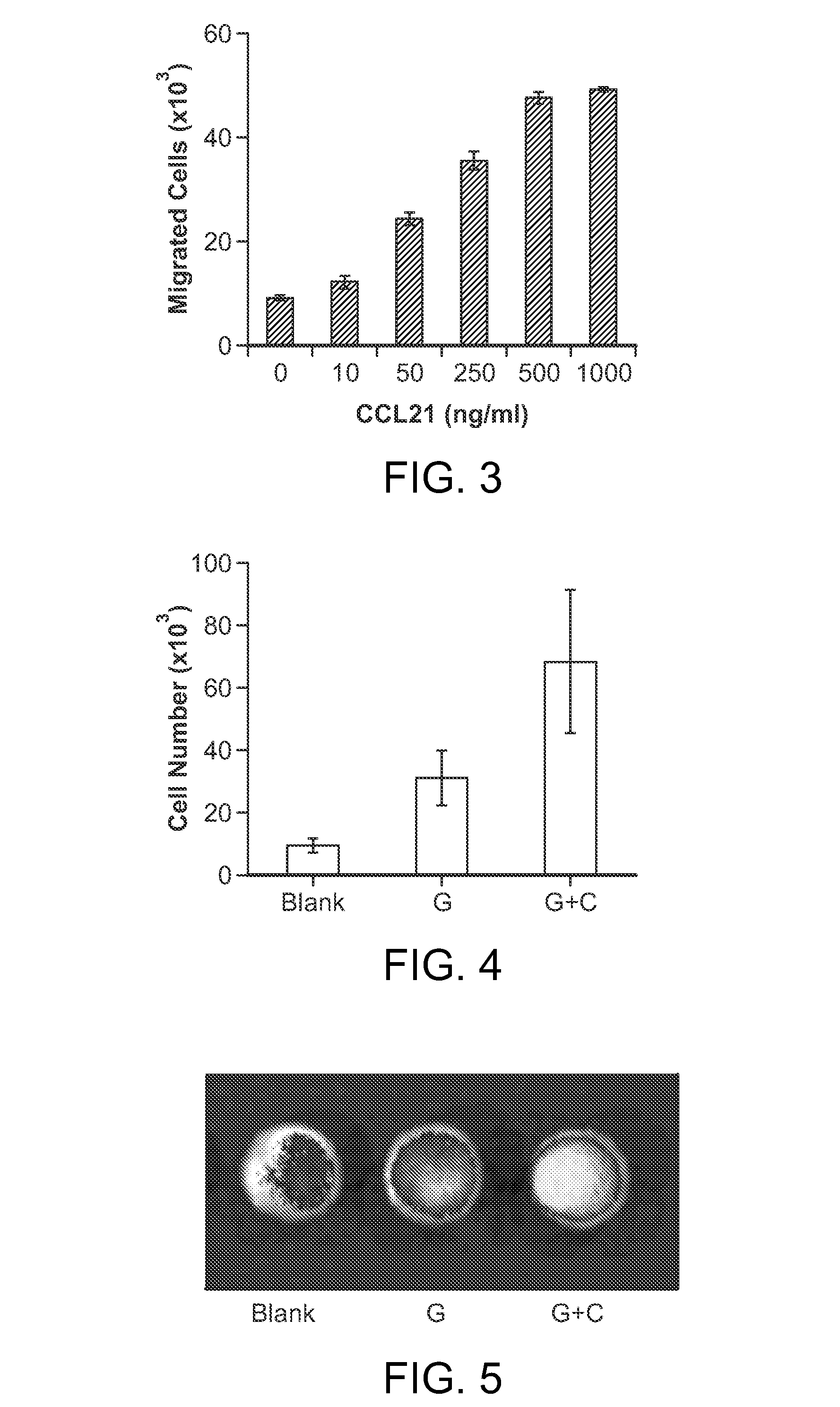
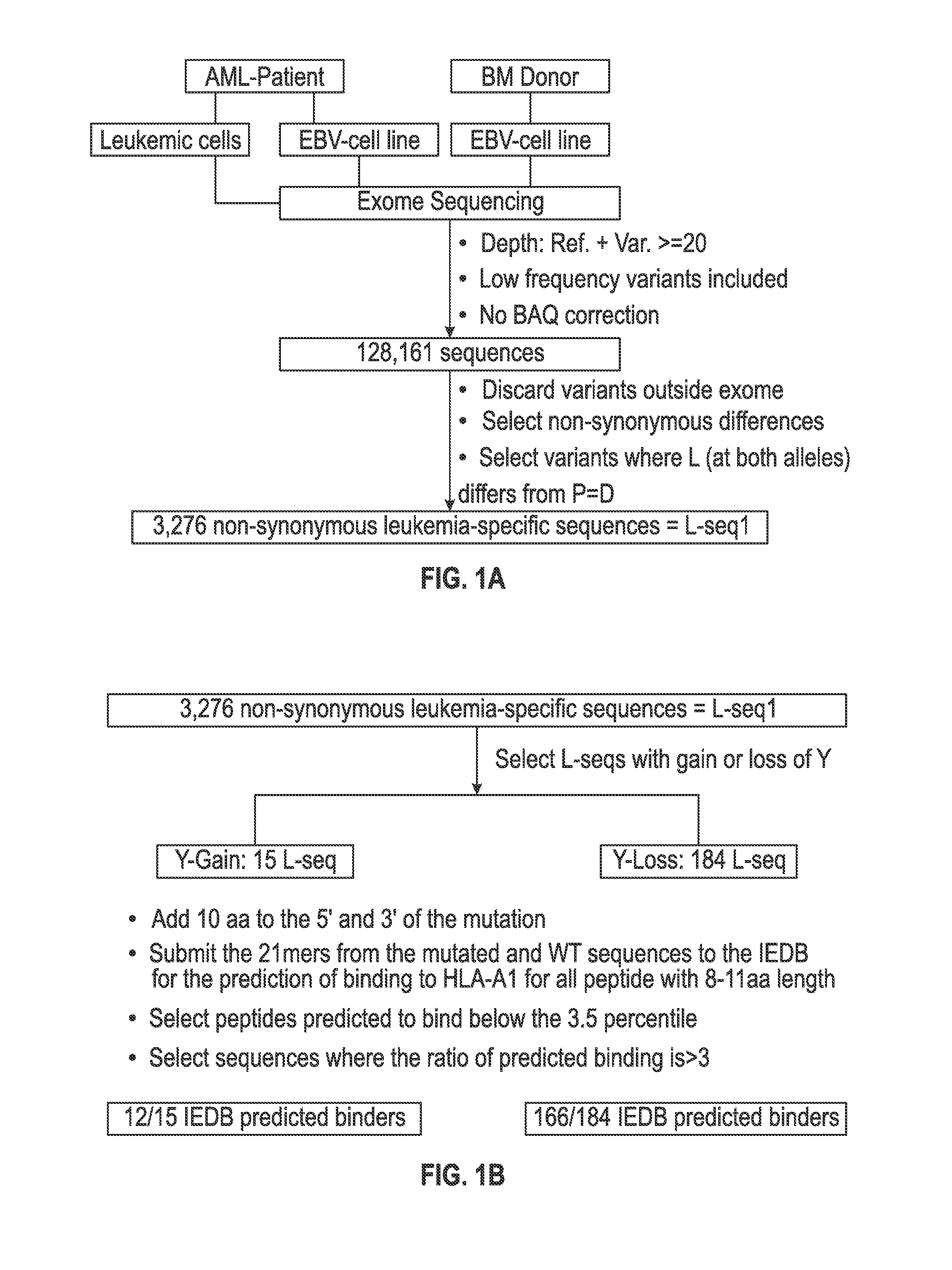
Personalized cancer vaccines and adoptive immune cell therapies
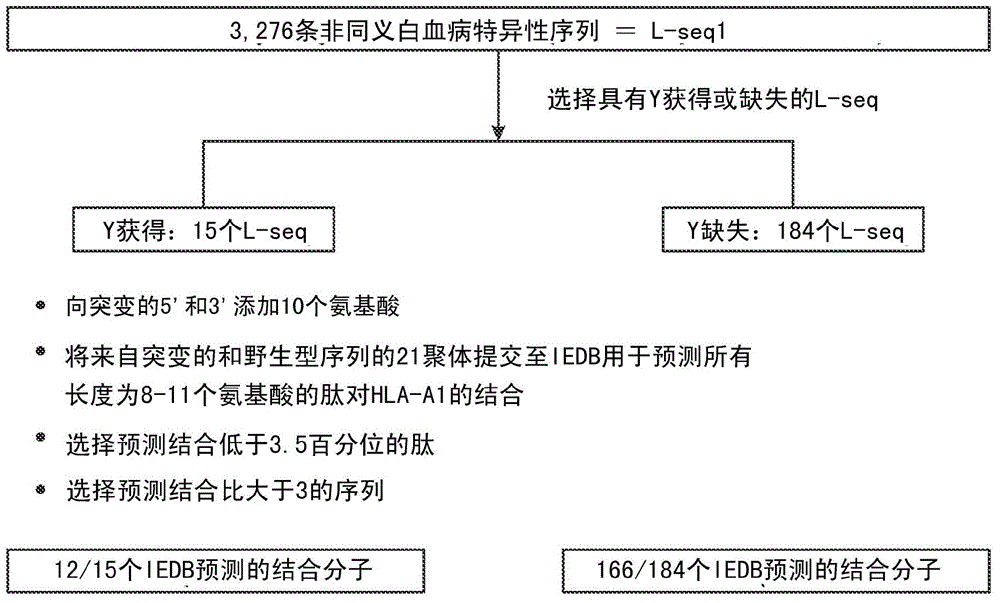
InactiveCN104662171AMicrobiological testing/measurementBiological material analysisCancer cellWild type
Cancer antigens containing mutations in an expressed gene of cancer cells from a cancer patient are identified. Sequences from cancer cells obtained using a parallel sequencing platform are selected by comparing to the patient's normal genes or to normal genes from an HLA-matched individual. Sequences are further selected by identifying an HLA supertype of the cancer patient and selecting for that HLA supertype, sequences that have a particular amino acid at the mutant position and / or corresponding wild-type position in the effected gene. Peptides containing cancer antigens (i.e., mutations—once a mutation is defined, what makes it an immunogen is its ability to induce an immune response) are optionally tested for binding to HLA antigens of the cancer patient. Peptides containing the cancer antigens are evaluated for activating T cells (e.g., helper T lymphocytes and cytotoxic T lymphocytes (CTL)) cell lines from the cancer patient or from an HLA-matched donor. The cancer antigen(s) identified for a cancer patient are used to prepare a cancer vaccine and to treat the cancer patient.
Owner:PERSIMMUNE
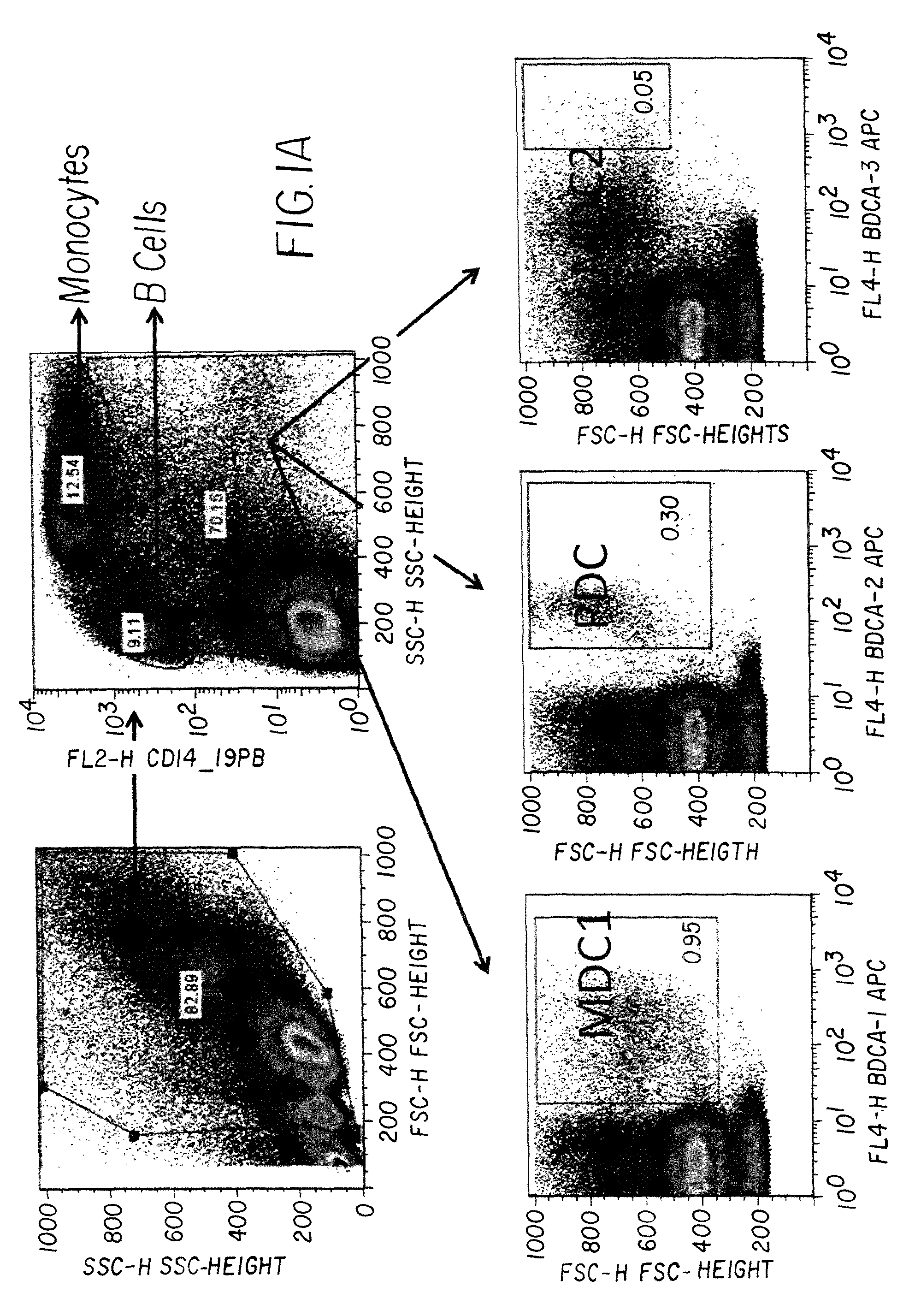
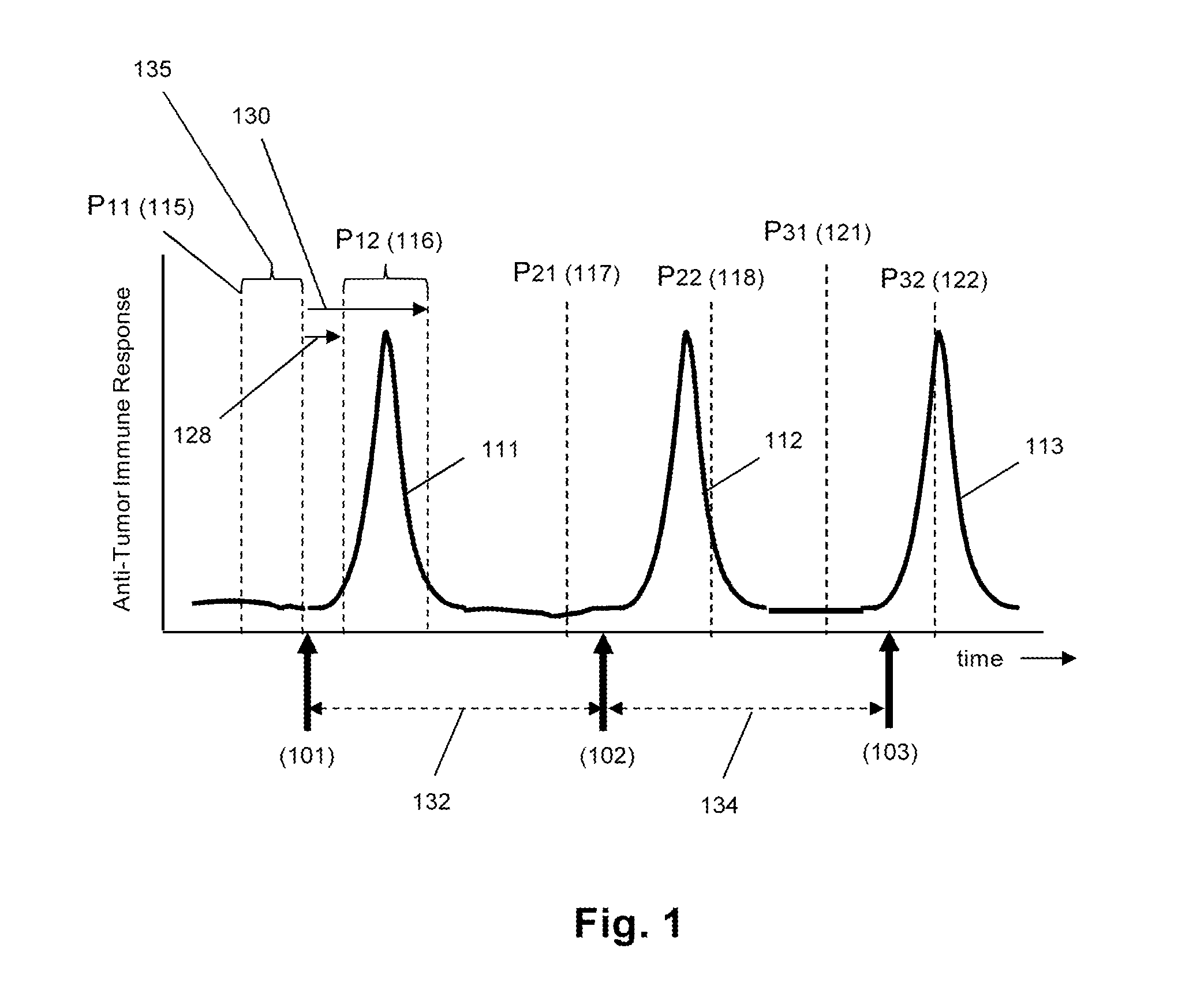
Monitoring immune responsiveness to cancer vaccination
InactiveUS20150038346A1Microbiological testing/measurementDisease diagnosisPost vaccinationMonitoring immune
The invention is direct to a method for determining a cancer patient's immune responsiveness to anti-cancer vaccination. In one aspect, for each of a plurality of vaccinations, pairs of clonotype profiles are obtained, one immediately prior to vaccination and one during the period of peak immune response, usually within two to twenty days after the vaccination. Responsiveness is correlated to successive increases in identical clonotypes within each pair of clonotype profiles in at least two successive vaccinations.
Owner:ADAPTIVE BIOTECH
Strategies for improved cancer vaccines
The present invention concerns methods and compositions for forming anti-cancer vaccine complexes. In preferred embodiments, the anti-cancer vaccine complex comprises an antibody moiety that binds to dendritic cells, such as an anti-CD74 antibody or antigen-binding fragment thereof, attached to an AD (anchoring domain) moiety and a xenoantigen, such as CD20, attached to a DDD (dimerization and docking domain) moiety, wherein two copies of the DDD moiety form a dimer that binds to the AD moiety, resulting in the formation of the vaccine complex. The anti-cancer vaccine complex is capable of inducing an immune response against xenoantigen expressing cancer cells, such as CD138negCD20+ MM stem cells, and inducing apoptosis of and inhibiting the growth of or eliminating the cancer cells.
Owner:IBC PHARMACEUTICALS INC
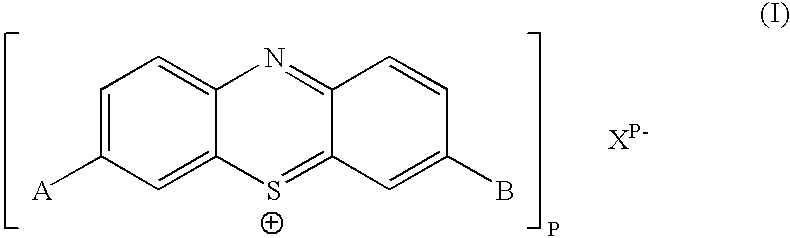
Biologically active methylene blue derivatives
InactiveUS20040147508A1Maintain long-termImprove stabilityAntibacterial agentsOrganic active ingredientsPhotodynamic therapyChemical compound
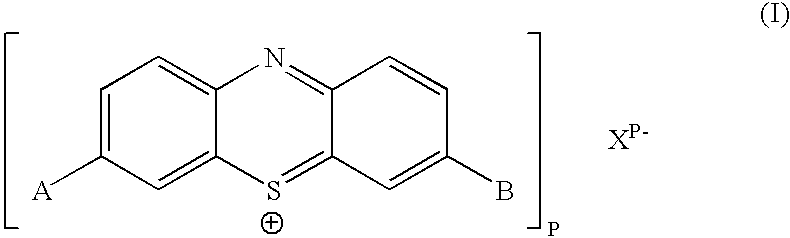
The present invention relates to a phenothiazinium compound of Formula (I): wherein: A and B each independently is in which R' and R'' each independently is a linear, branched or cyclic hydrocarbon group, or R' and R'' together with the N atom to which they are attached form an optionally substituted 5-, 6- or 7-membered ring; and where X<p-> is a counteranion and P is 1, 2 or 3; except for the compounds in which A and B are both either -N(CH3)2 or -N(CH2CH3)2 for use in a treatment that requires removal, deactivation or killing of unwanted tissues or cells. The invention also relates to compositions comprising the compounds of Formula I, to selected compounds of Formula I, use of the compounds of Formula I as medicaments and as a PDT agent or a photodiagnostic agent, a conjugate or composite formed between a compound of Formula I and a polymer; and to a method for sterilising fluids in which the fluid is passed over the conjugate or composite whilst it is illuminated. The compounds are biologically active photosensitisers which are strongly photocytotoxic and have application in the areas of photodynamic therapy (PDT), as well as for the diagnosis and detection of medical conditions and related uses in photochemical internalisation, in the production of cancer vaccines, in the treatment and prevention of microbial infections and in photodisinfection or photosterilisation.
Owner:PHOTOPHARMICA LTD
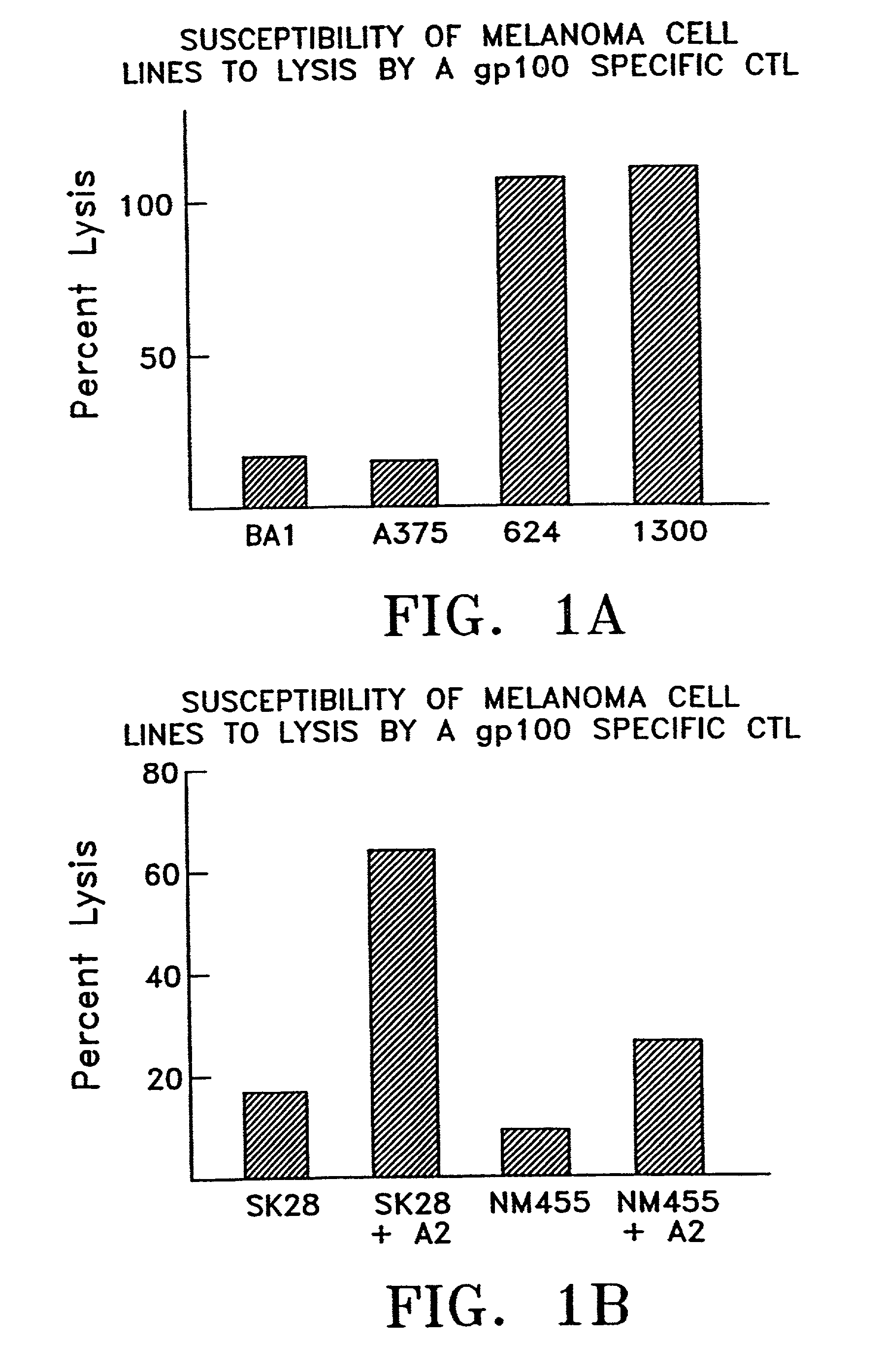
Hla-a24-restricted cancer antigen peptides
HLA-A24-restricted peptides derived from WT1 which have an activity to induce CTLs in vivo, polynucleotides encoding said peptides, cancer vaccines using those peptides or polynucleotides in vivo or in vitro, or the like are provided. The cancer vaccines of the present invention may be used to treat many cancer patients.
Owner:INT INST OF CANCER IMMUNOLOGY INC
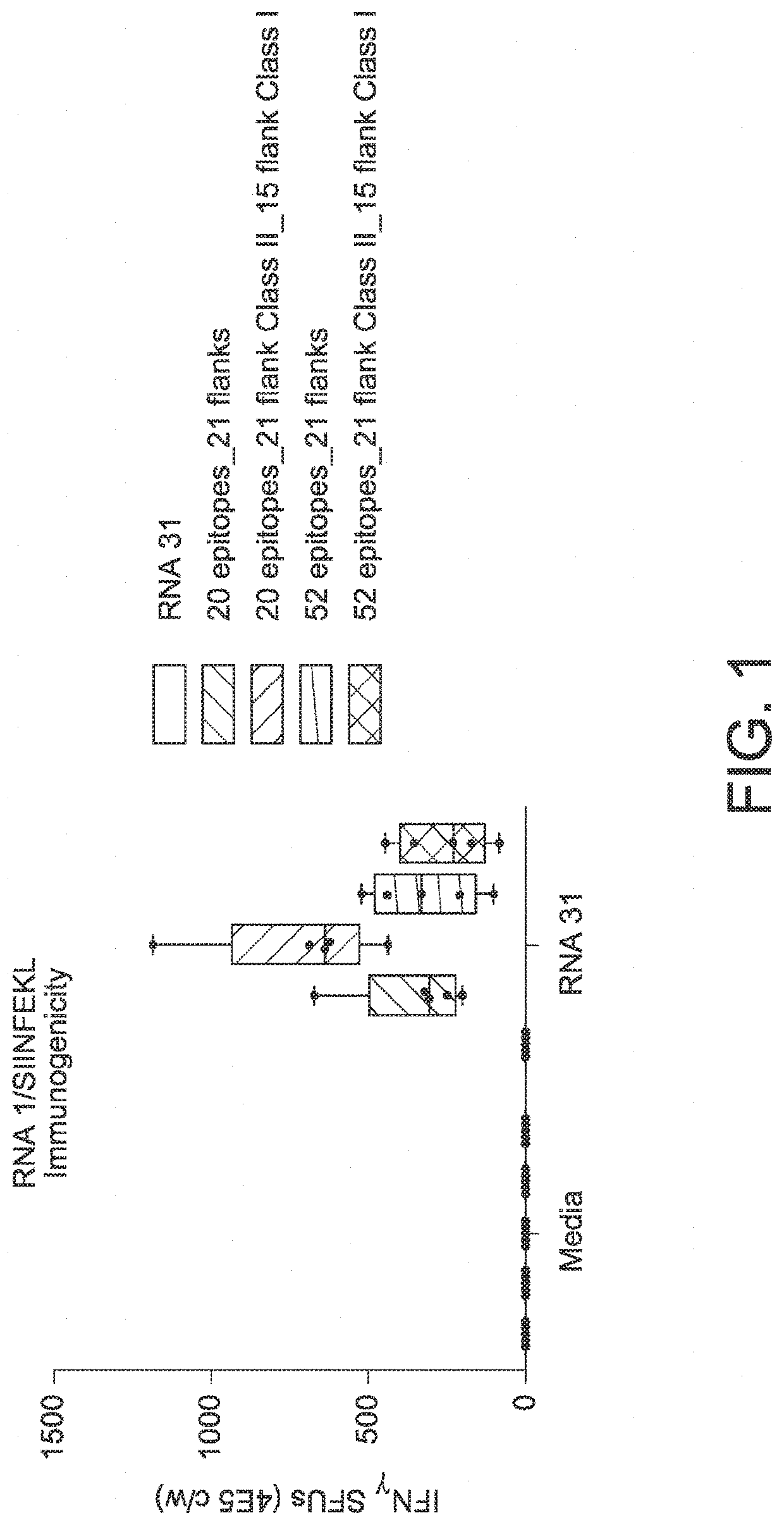
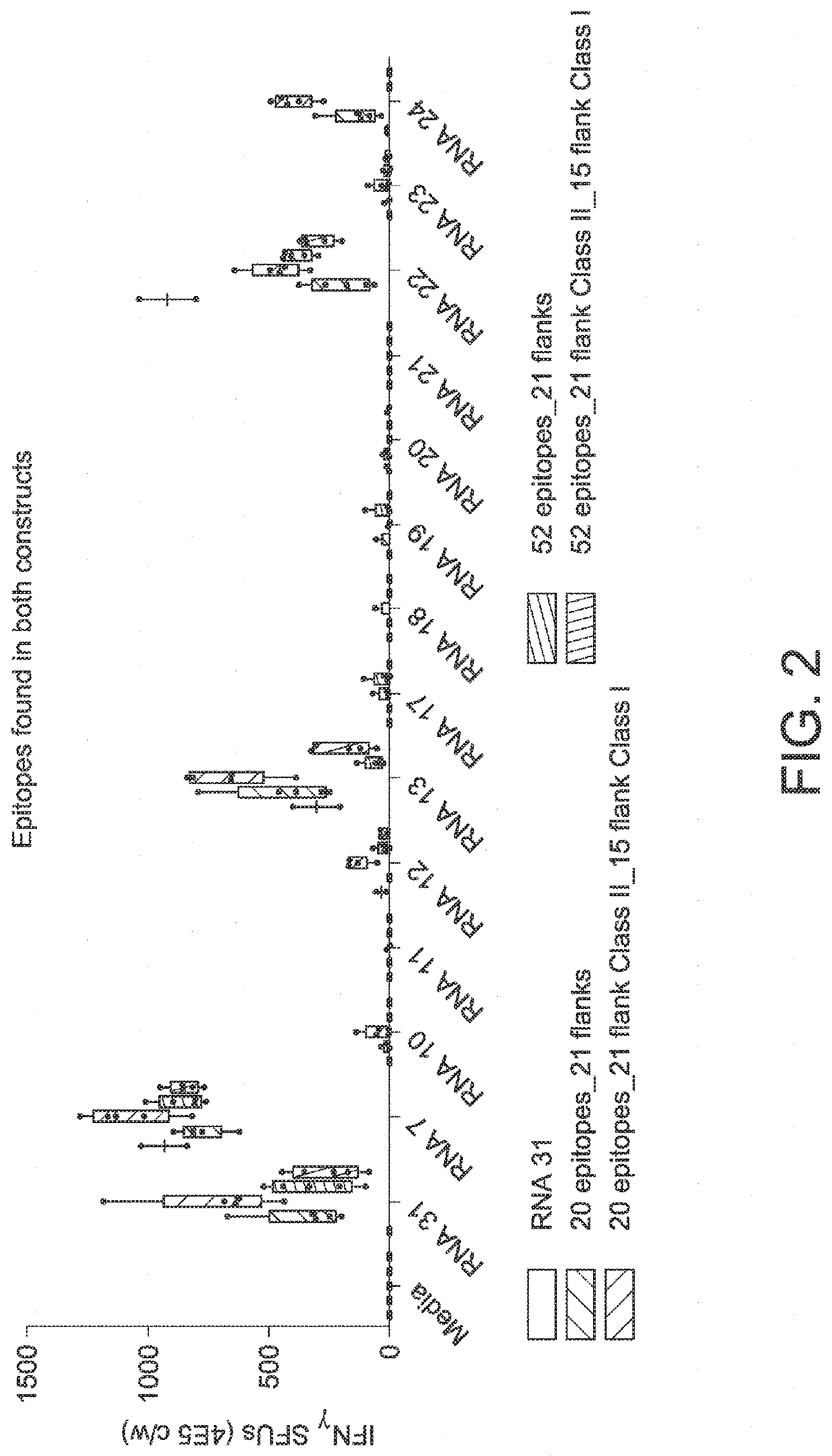
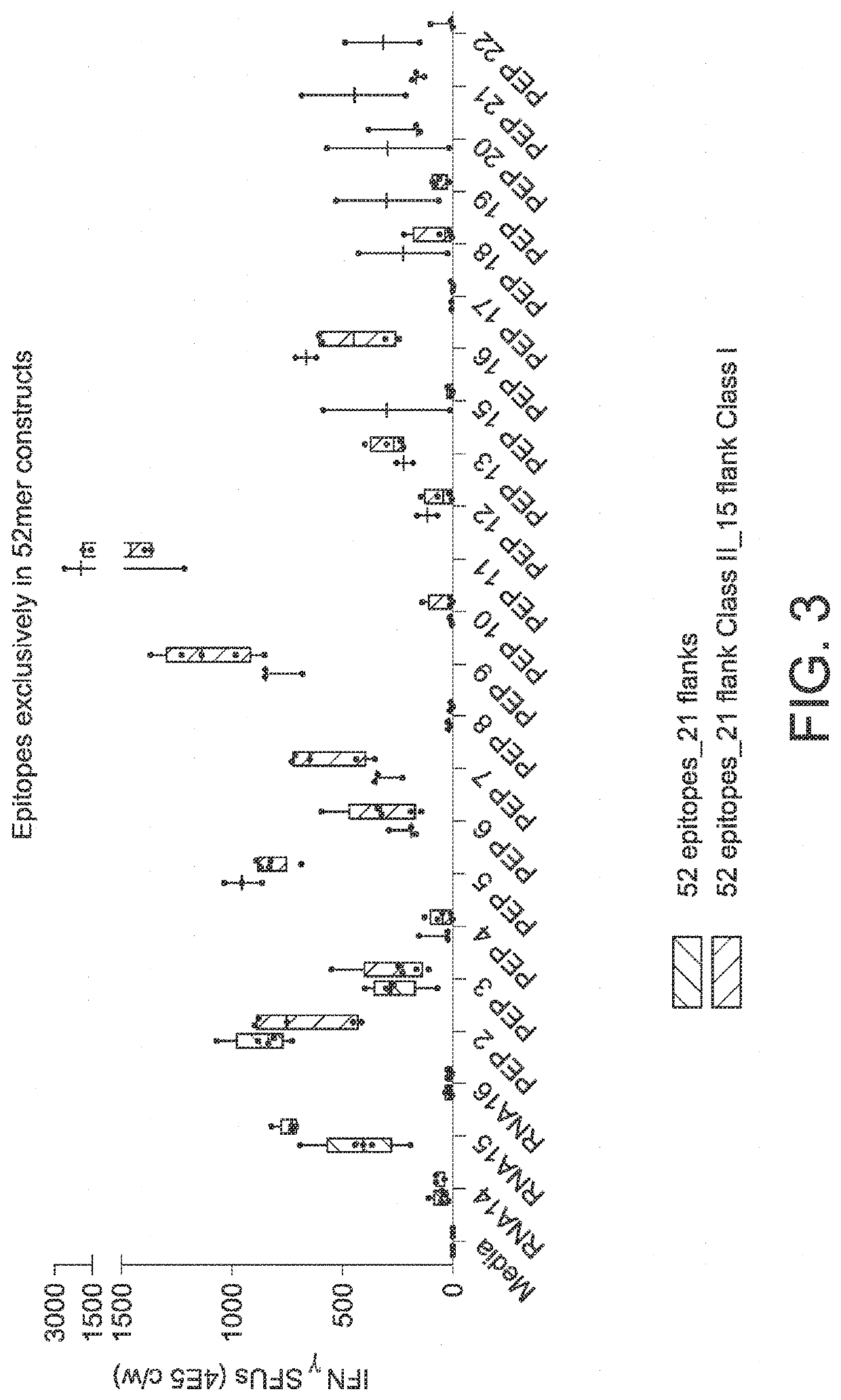
RNA cancer vaccines
PendingUS20190351040A1Balanced immune responseOrganic active ingredientsAntibody ingredientsTetanusAdjuvant
The disclosure relates to cancer ribonucleic acid (RNA) vaccines, as well as methods of using the vaccines and compositions comprising the vaccines. In particular, the disclosure relates to concatemeric mRNA cancer vaccines encoding several cancer epitopes on a single mRNA construct, i.e. poly-epitope mRNA constructs or poly-neo-epitope constructs. The disclosure further relates to p53 and KRAS mutations, as well as incorporation of immune enhancers such as STING, e.g. mRNA constructs further encoding an immune stimulator or adjuvant. The disclosure further relates to inclusion of universal T cell epitopes, such as tetanus or diphtheria toxins to elicit an enhanced immune response.
Owner:MODERNATX INC
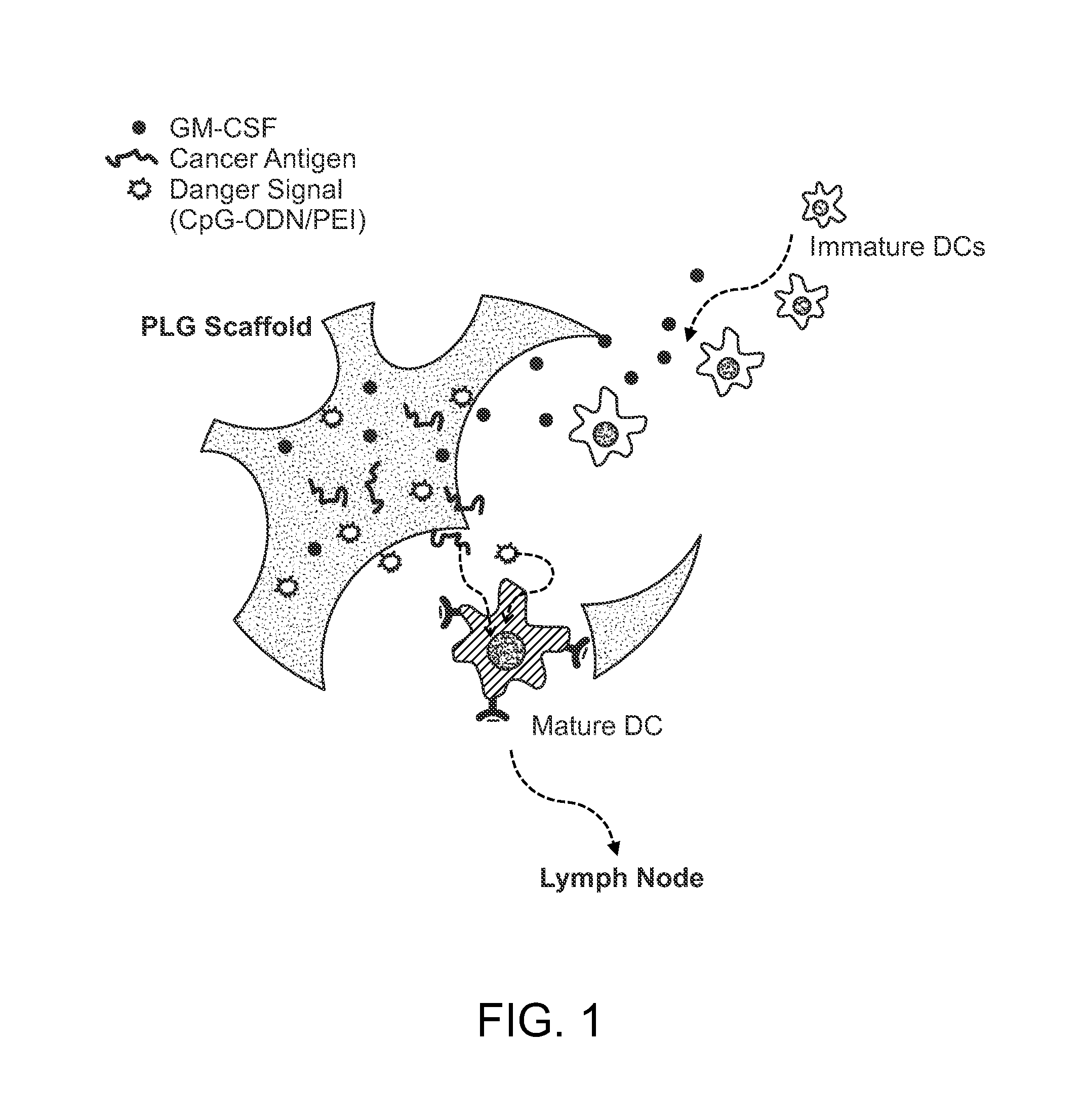
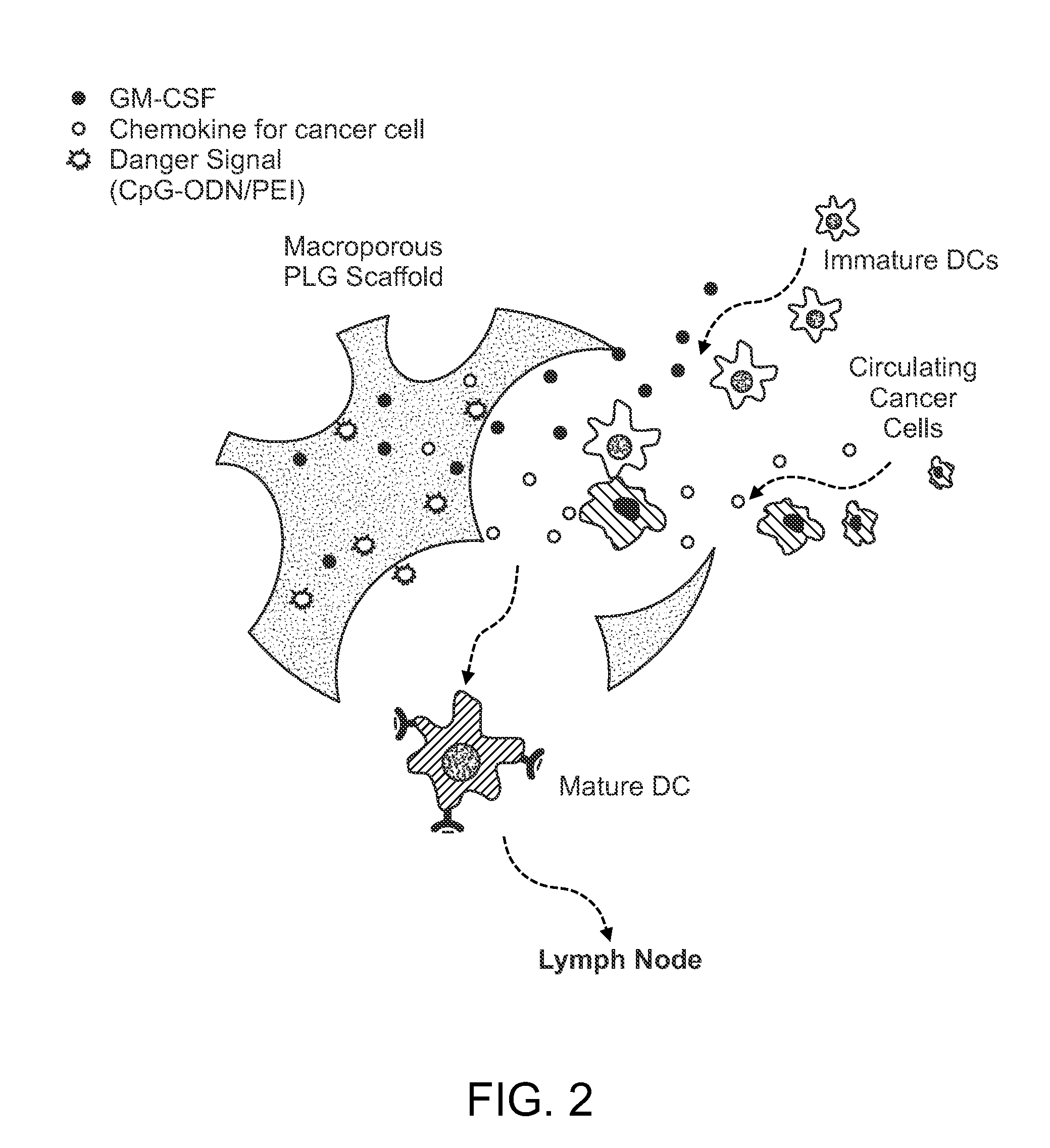
In Situ Antigen-Generating Cancer Vaccine
ActiveUS20140193488A1Reduced responsivenessActivated cellsPeptide/protein ingredientsEnergy modified materialsAntigenCancer research
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
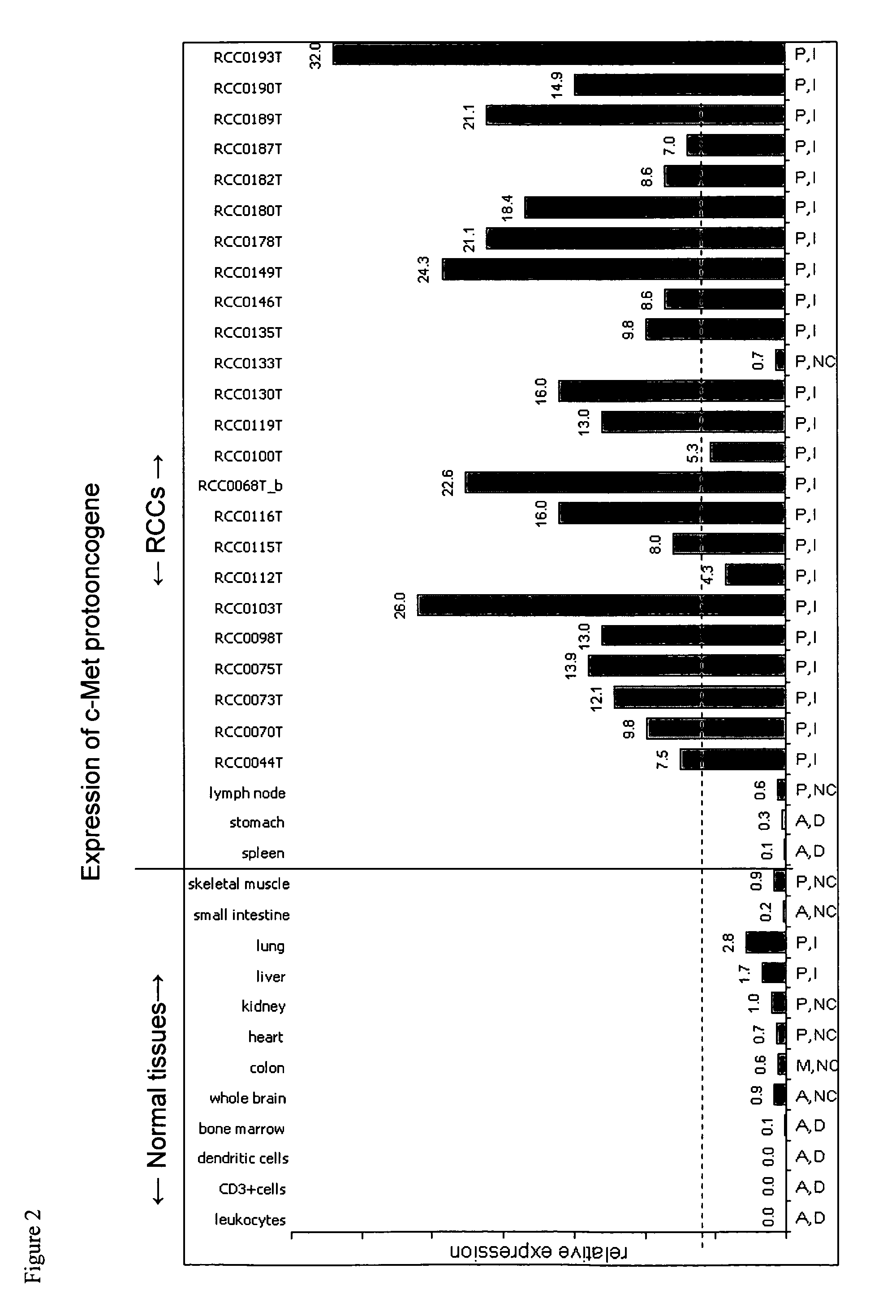
Genes differentially expressed in cancer cells to design cancer vaccines
Owner:GENZYME CORP
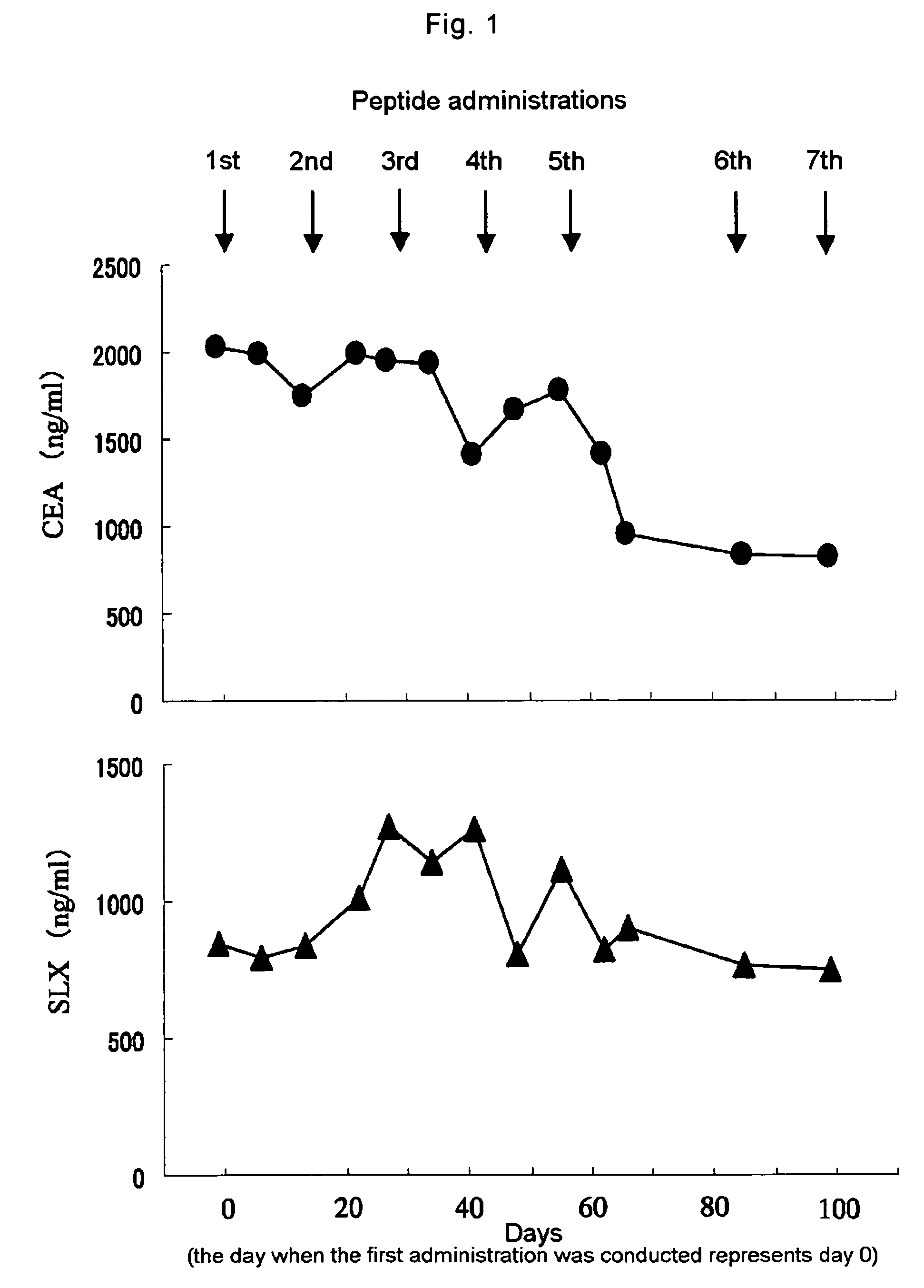
Cancer antigen peptide formulations
Cancer antigen peptides derived from WT1 which have an in vivo efficacy, particularly a clinical usefulness, and cancer vaccines as dosage forms suitable for said cancer antigen peptides, are provided.The invention relates to water-in-oil emulsions which comprise as an effective ingredient either one or both of peptides selected from the group consisting of a peptide having an amino acid sequence of Cys Met Thr Trp Asn Gln Met Asn Leu (SEQ ID NO: 2), and a peptide having an amino acid sequence of Cys Tyr Thr Trp Asn Gln Met Asn Leu (SEQ ID NO: 3), as well as processes for preparation of said emulsion.
Owner:INT INST OF CANCER IMMUNOLOGY INC
System and method for the treatment of cancer, including cancers of the central nervous system
The invention relates to the treatment of cancer, and particularly to the treatment of cancers of the central nervous system, such as glioblastoma multiforme. A dual therapeutic approach is provided, including the administration of a dendritic cell-based cancer vaccine and a regimen of chemotherapy. The two therapies may be administered concurrently with one another and / or with an initial vaccination preceding chemotherapy. In various embodiments, the dendritic cell-based cancer vaccine includes either primed or unprimed dendritic cells; for instance, the dendritic cells may be autologous tumor antigen-presented dendritic cells. The dual therapeutic approach of the instant invention beneficially influences the chemosensitivity of a mammal with cancer.
Owner:CEDARS SINAI MEDICAL CENT
HLA-A24-restricted cancer antigen peptides
HLA-A24-restricted peptides derived from WT1 which have an activity to induce CTLs in vivo, polynucleotides encoding said peptides, cancer vaccines using those peptides or polynucleotides in vivo or in vitro, or the like are provided. The cancer vaccines of the present invention may be used to treat many cancer patients.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Auto-stimulating cells and methods for making and using the same
Methods for transferring one or more proteins to a cell are disclosed. The protein or proteins to be transferred are in the form of a fusion protein, and contain at least one domain encoding for a protein or peptide having trans signaling and / or adhesion function. The fusion protein is transferred to a cell by binding to a lipidated protein, which has been incorporated into the cell membrane. In an additional aspect of the invention, methods of making fusion proteins having cis signaling capabilities, as well as the ability to bind with receptors on the cell's own surface, are provided. Fusion proteins incorporating GPI or a homing element, and a costimulator or inhibitor domain can also be directly transferred to the cell surface. Methods for using cells which have undergone protein transfer according to the present methods are also disclosed. This includes use in a cancer vaccine, use for treatment of cancer or autoimmune disease, and use in determining costimulator threshold levels.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Antigen specific multi epitope vaccines
ActiveUS20100074925A1Strong and comprehensive responseEffective immune responseTumor rejection antigen precursorsSugar derivativesMHC class IProtein target
The present invention relates to cancer vaccines composed of the signal peptide domain of tumor associated antigens or proteins. The peptide vaccines of the invention are characterized by having multiple MHC class I and class II epitopes which are highly abundant in the population. Therefore, these vaccines are likely to induce a strong, comprehensive immune response against the target proteins in the majority of the vaccinated population, and thereby induce an immune reaction against tumors expressing such target proteins. Specifically, the invention relates to peptide vaccines composed of the signal peptide domain of Mucin (MUC1), BAGE-1 or ARMET, and their use for the treatment of cancers which express Mucin (MUC1), BAGE-1 or ARMET.
Owner:VAXIL BIOTHERAPEUTICS
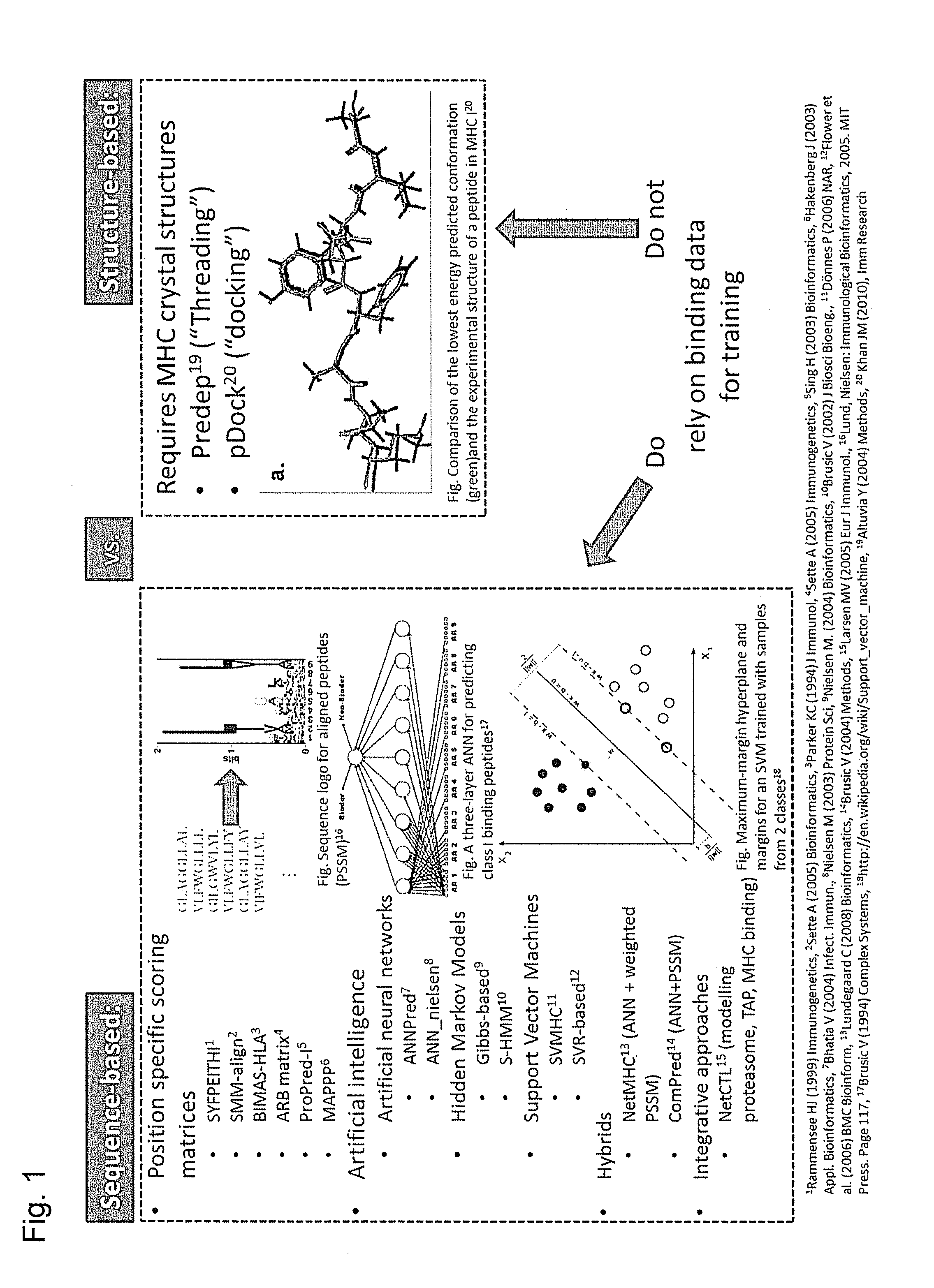
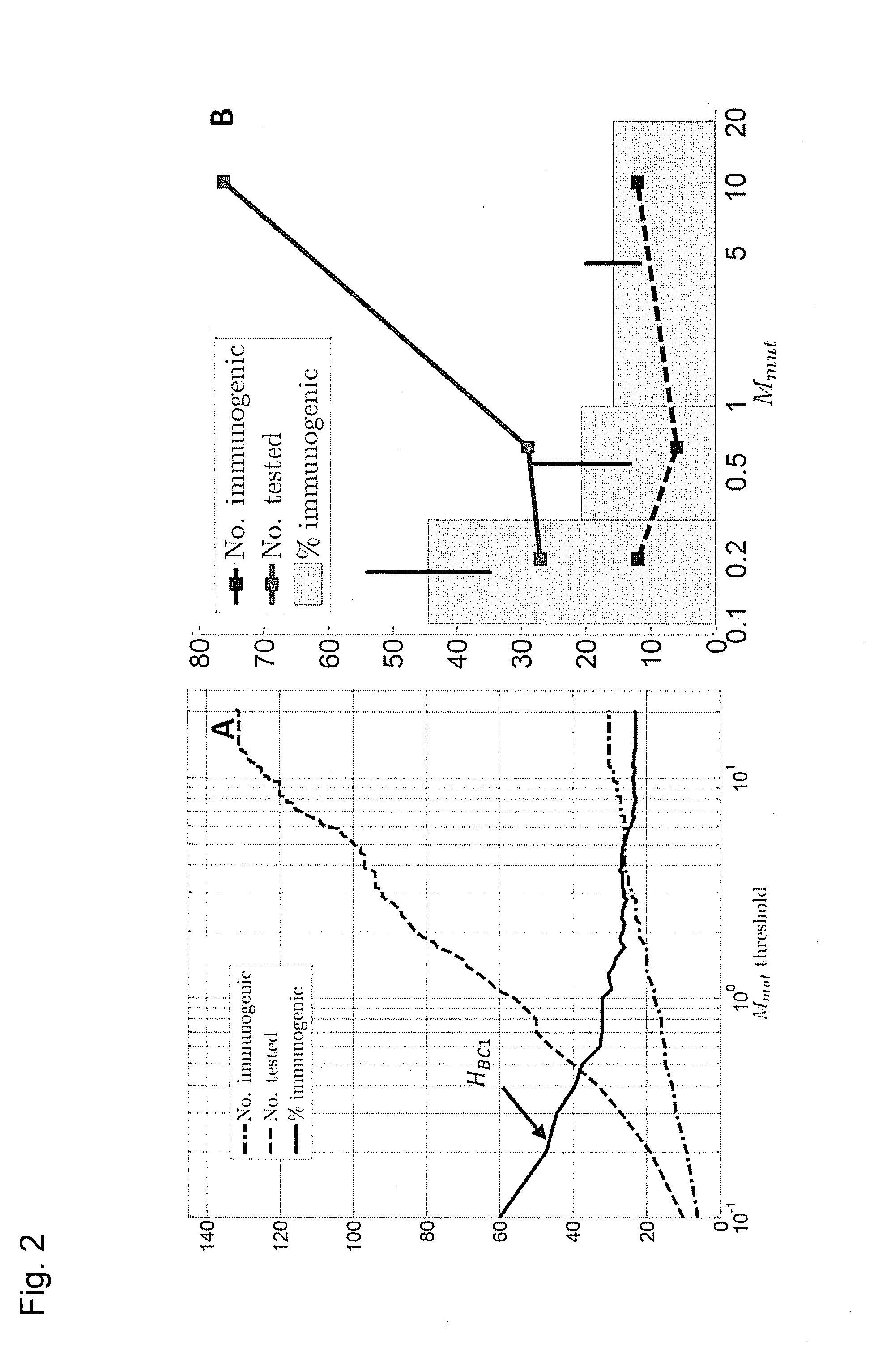
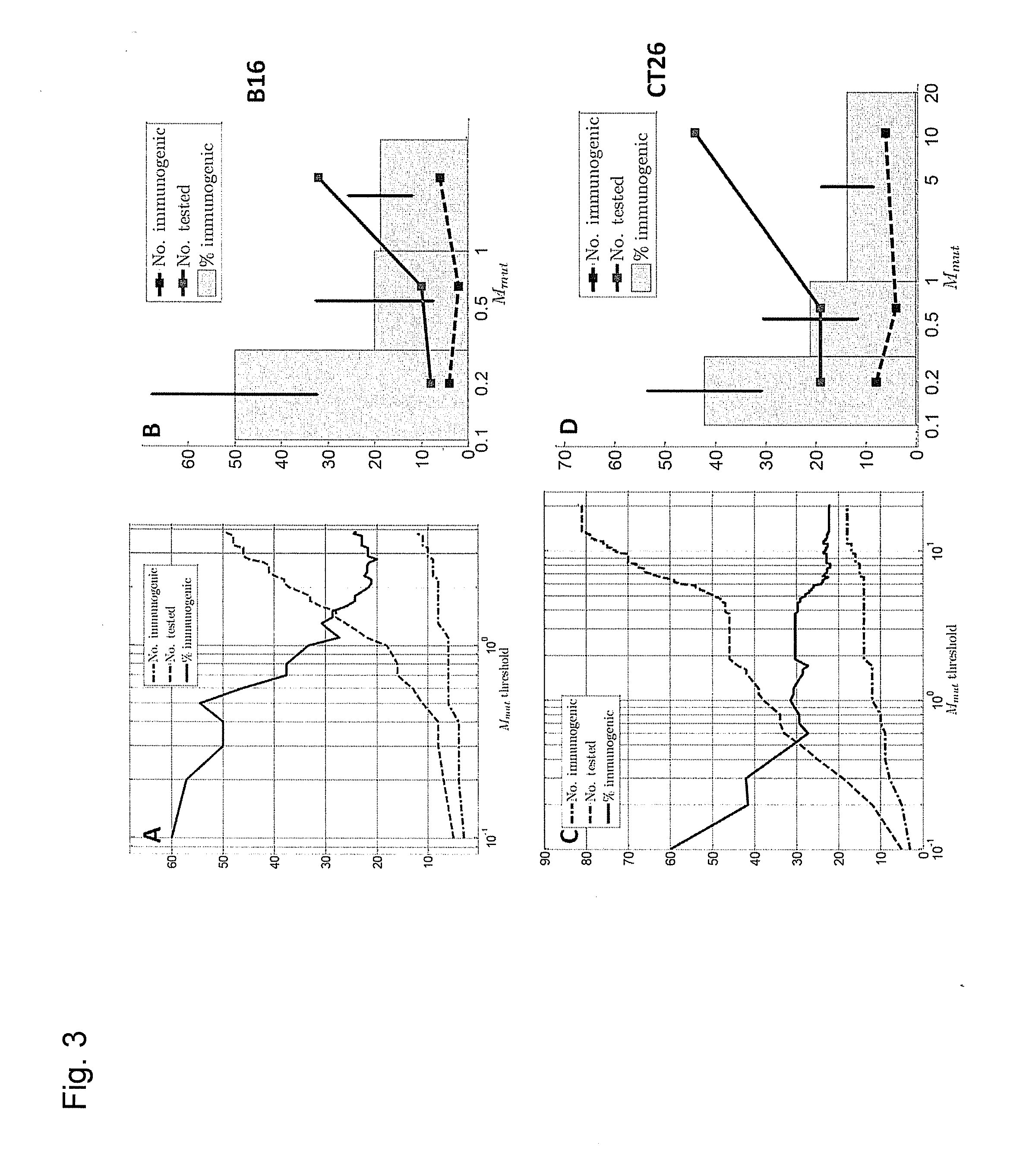
Predicting immunogenicity of t cell epitopes
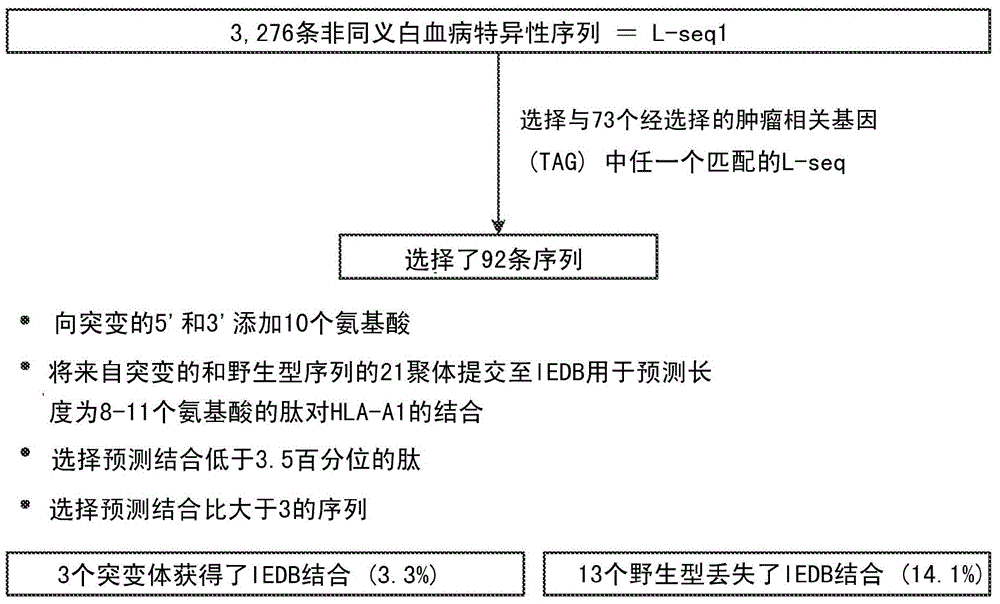
ActiveUS20160125129A1Minimizing chanceDevelopmental delayMicrobiological testing/measurementVaccinesOncologyImmunogenicity
The present invention relates to methods for predicting T cell epitopes. In particular, the present invention relates to methods for predicting whether modifications in peptides or polypeptides such as tumor-associated neoantigens are immunogenic or not. The methods of the invention are useful, in particular, for the provision of vaccines which are specific for a patient's tumor and, thus, in the context of personalized cancer vaccines.
Owner:BIONTECH SE +1
Personalized cancer vaccines and adoptive immune cell therapies
Cancer antigens containing mutations in an expressed gene of cancer cells from a cancer patient are identified. Sequences from cancer cells obtained using a parallel sequencing platform are selected by comparing to the patient's normal genes or to normal genes from an HLA-matched individual. Sequences are further selected by identifying an HLA supertype of the cancer patient and selecting for that HLA supertype, sequences that have a particular amino acid at the mutant position and / or corresponding wild-type position in the effected gene. Peptides containing cancer antigens (i.e., mutations—once a mutation is defined, what makes it an immunogen is its ability to induce an immune response) are optionally tested for binding to HLA antigens of the cancer patient. Peptides containing the cancer antigens are evaluated for activating T cells (e.g., helper T lymphocytes and cytotoxic T lymphocytes (CTL)) cell lines from the cancer patient or from an HLA-matched donor. The cancer antigen(s) identified for a cancer patient are used to prepare a cancer vaccine and to treat the cancer patient.
Owner:PERSIMMUNE
Cancer Vaccines And Methods Of Treatment Using The Same
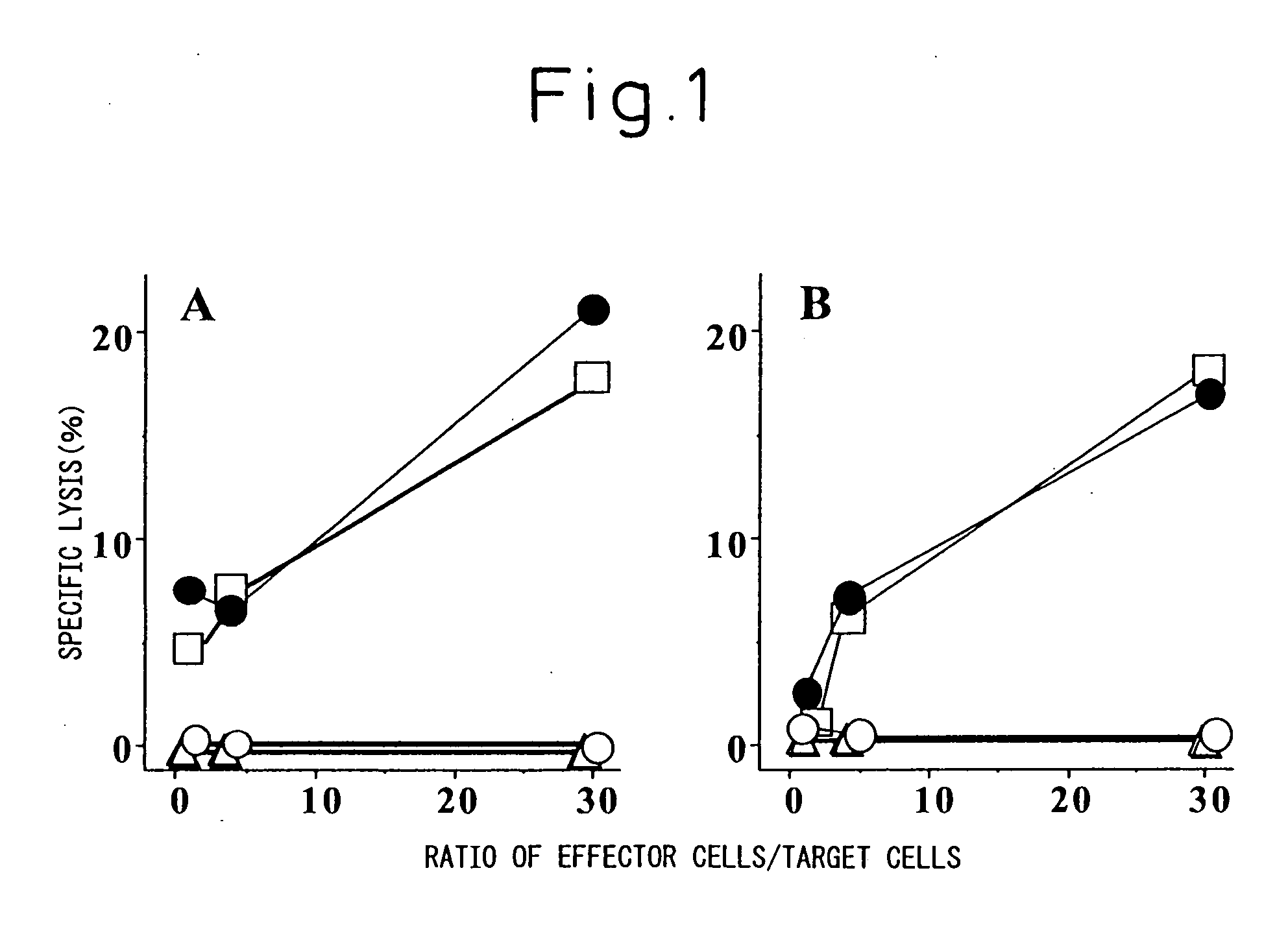
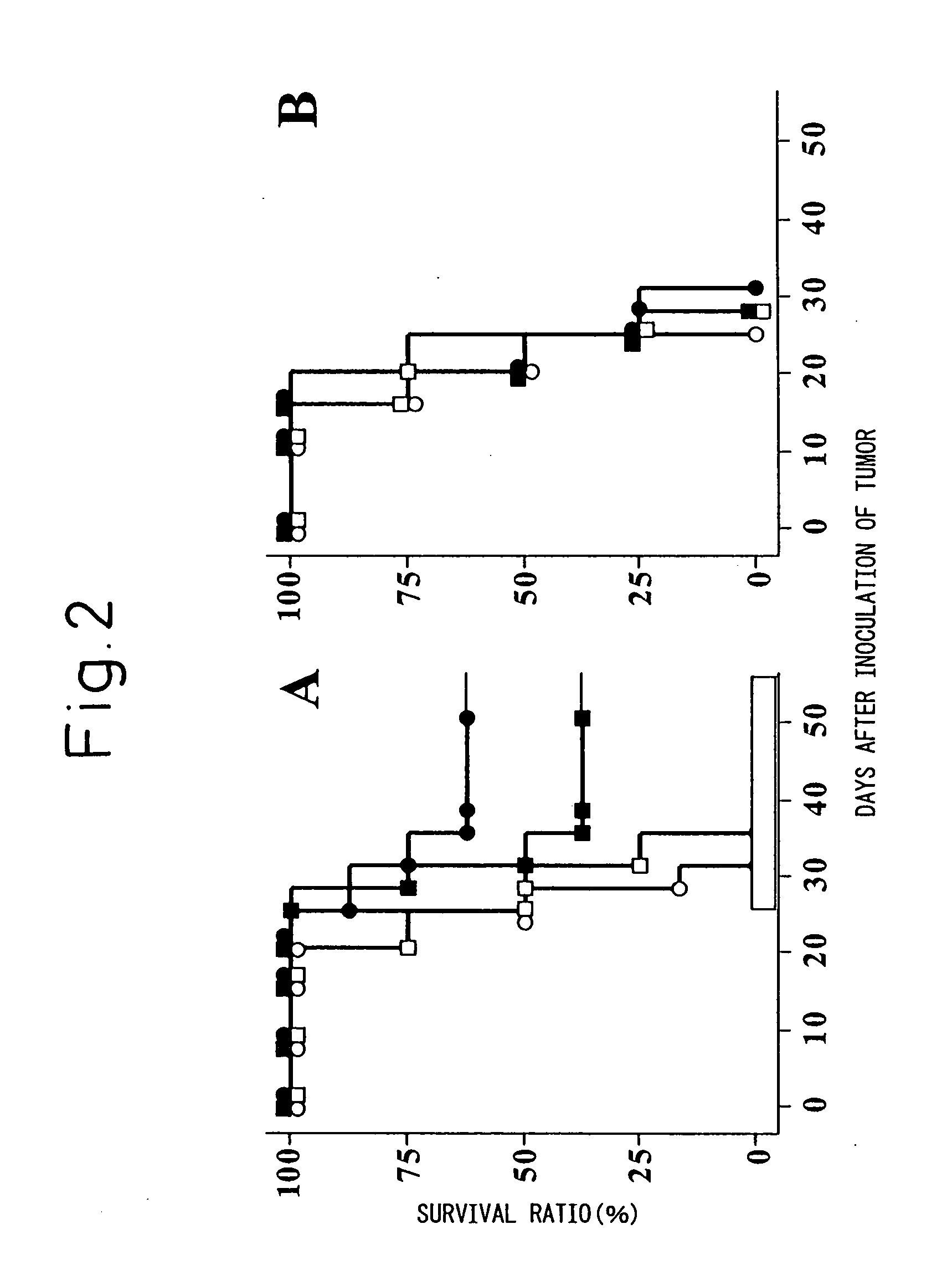
Disclosed herein are compositions and methods for treating cancer and in particular vaccines that treat and provide protection against tumor growth.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Cancer vaccine containing cancer antigen based on tumor suppressor gene wt1 product and cationic liposomes
InactiveUS20060165708A1Tumor rejection antigen precursorsTumor specific antigensAntigenCancer antigen
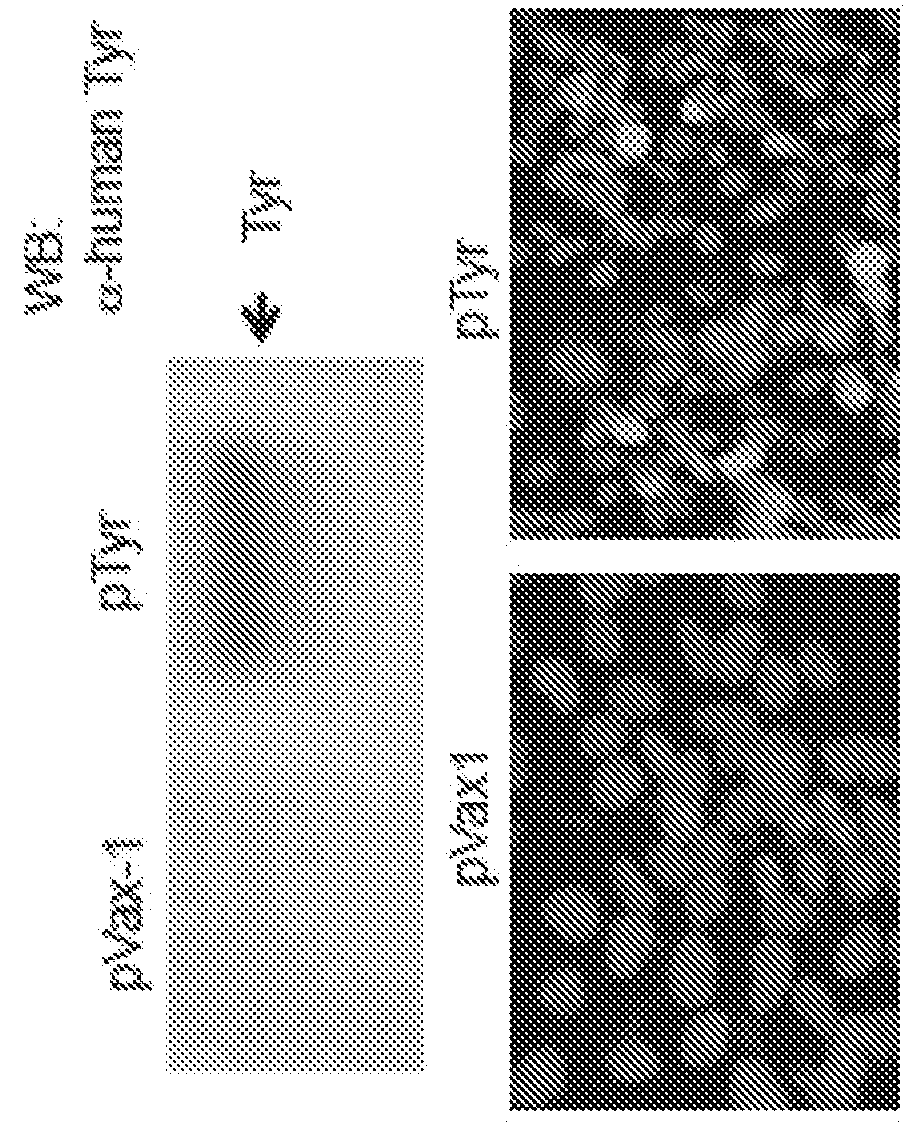
A cancer vaccine comprising a cancer antigen which comprises, as an active ingredient, the product of a tumor suppressor gene WT1, a partial peptide or a modified version thereof, and a cationic liposome.
Owner:MAYUMI TADANORI +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com