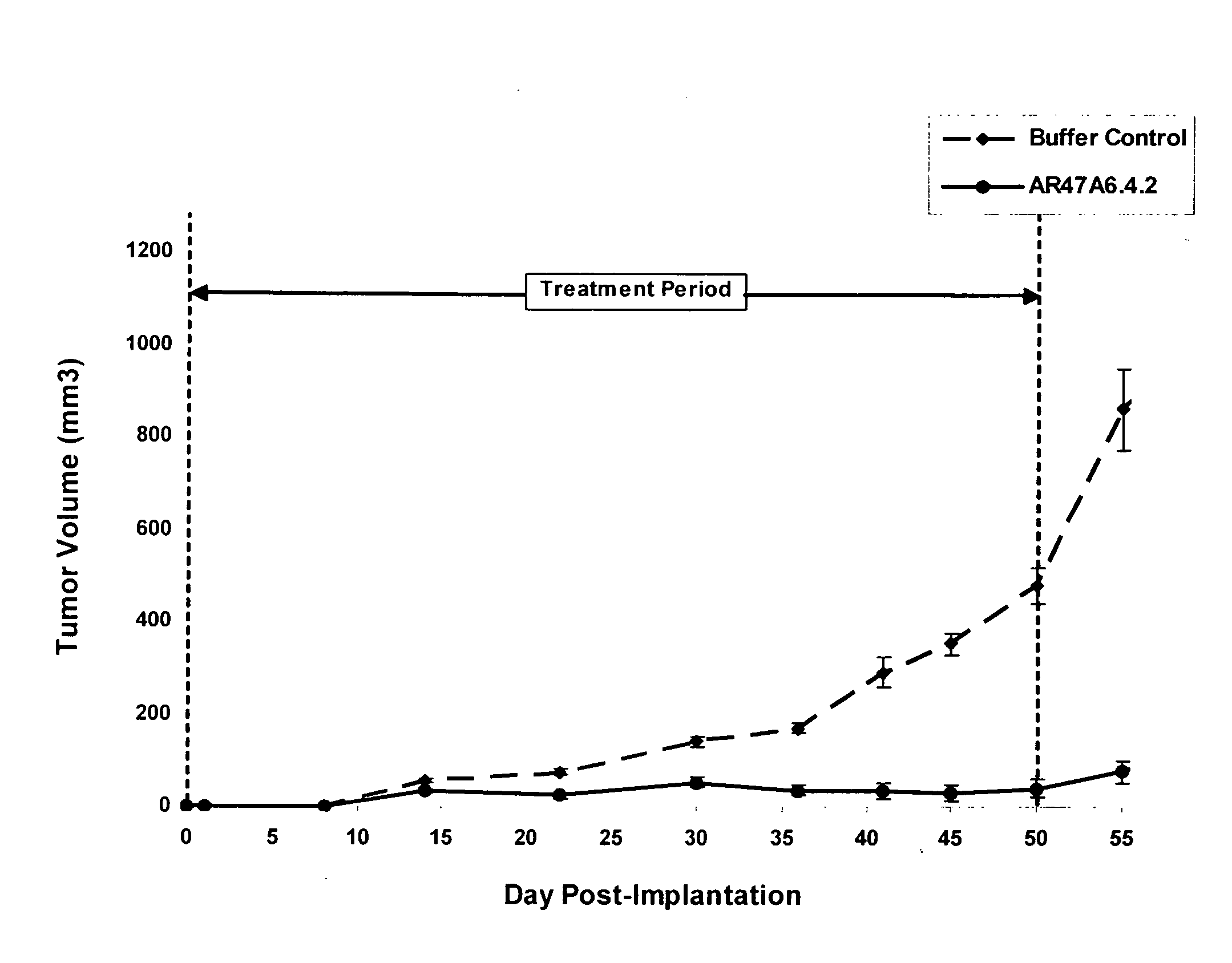
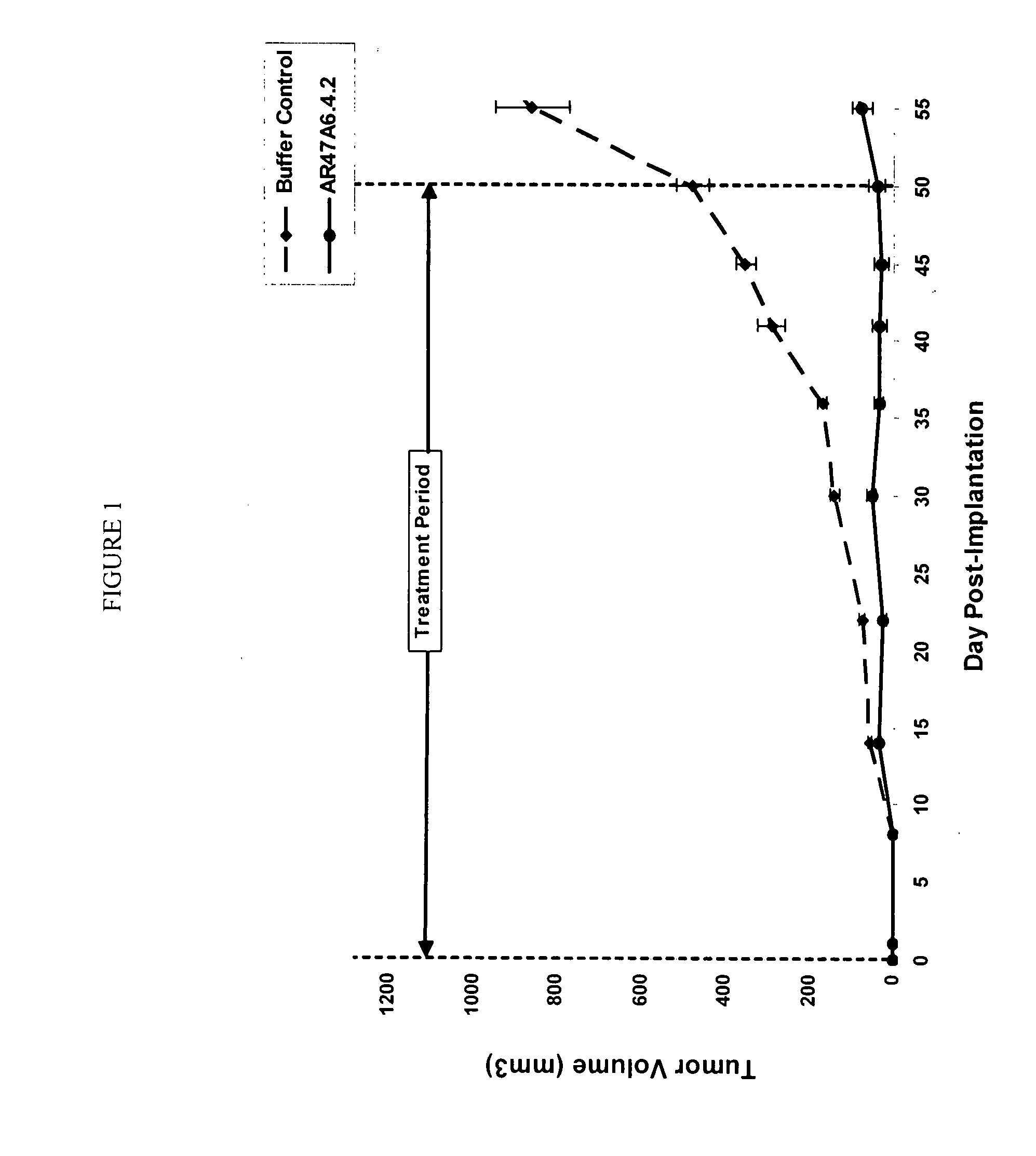
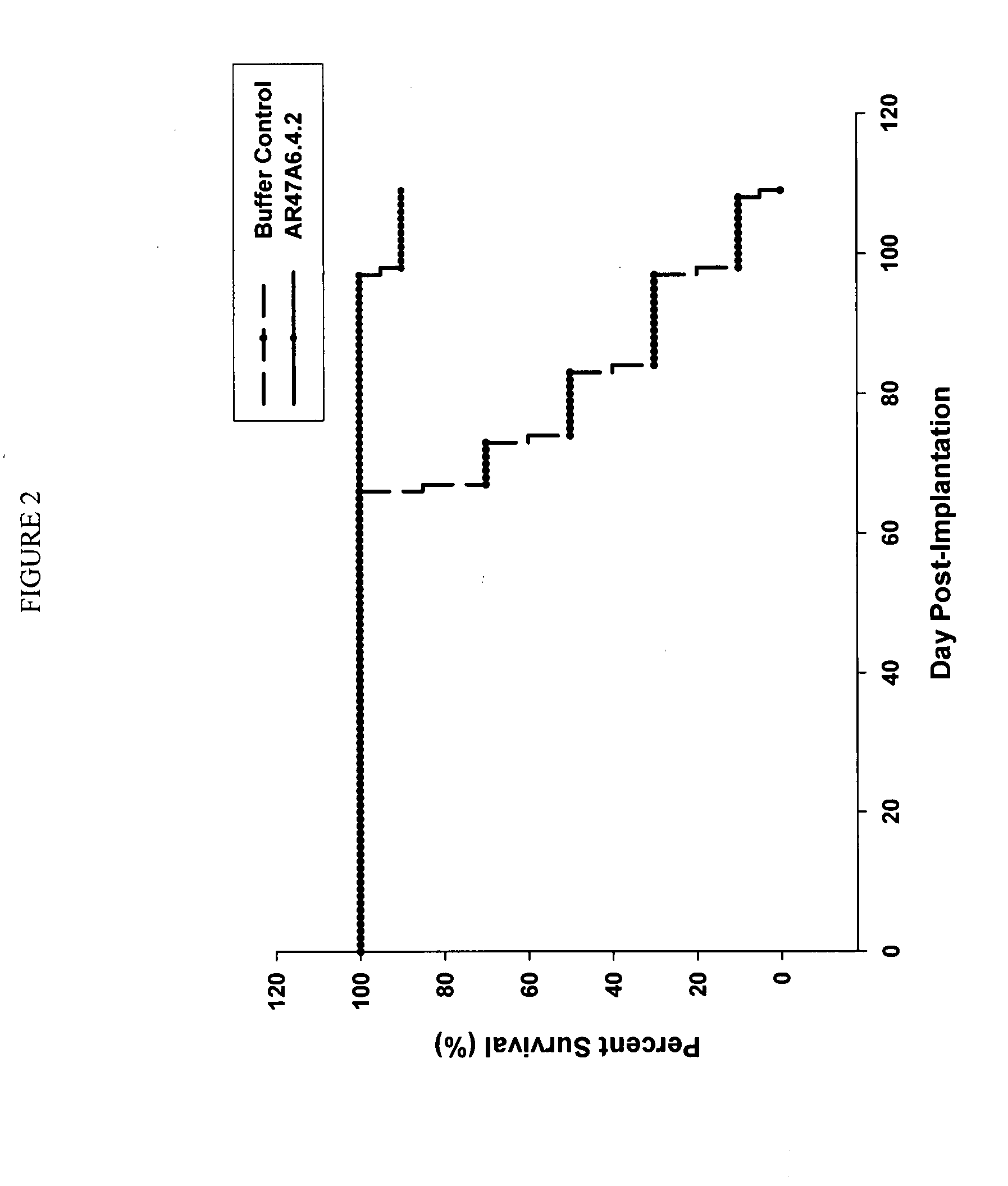
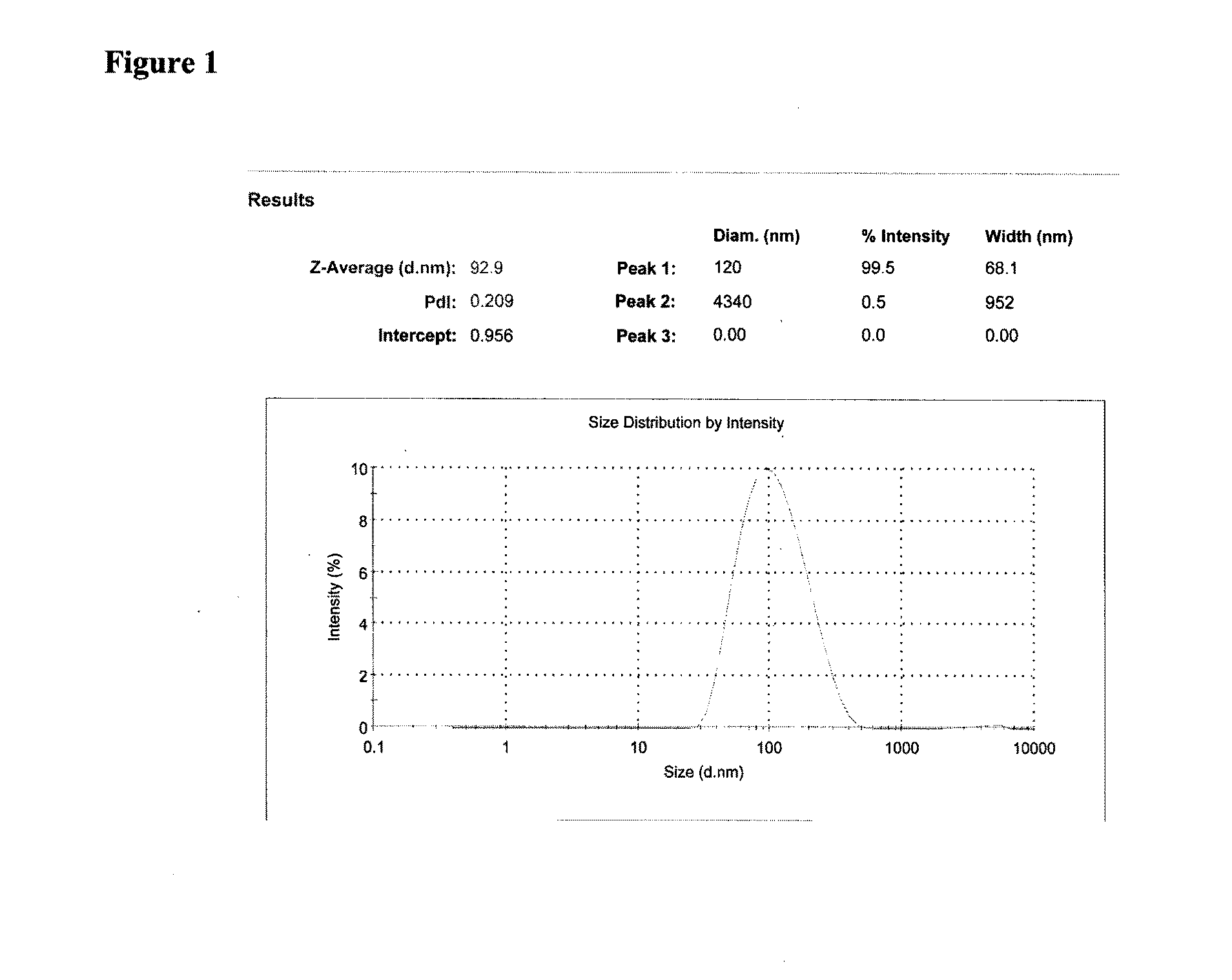
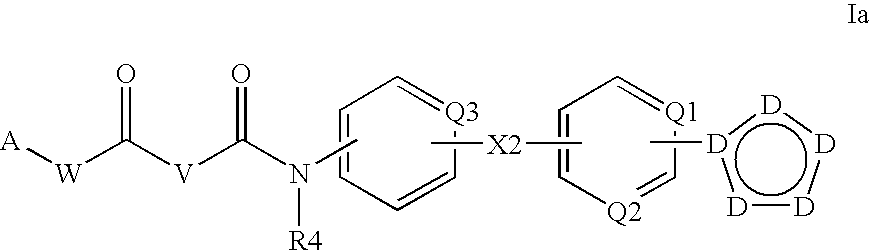
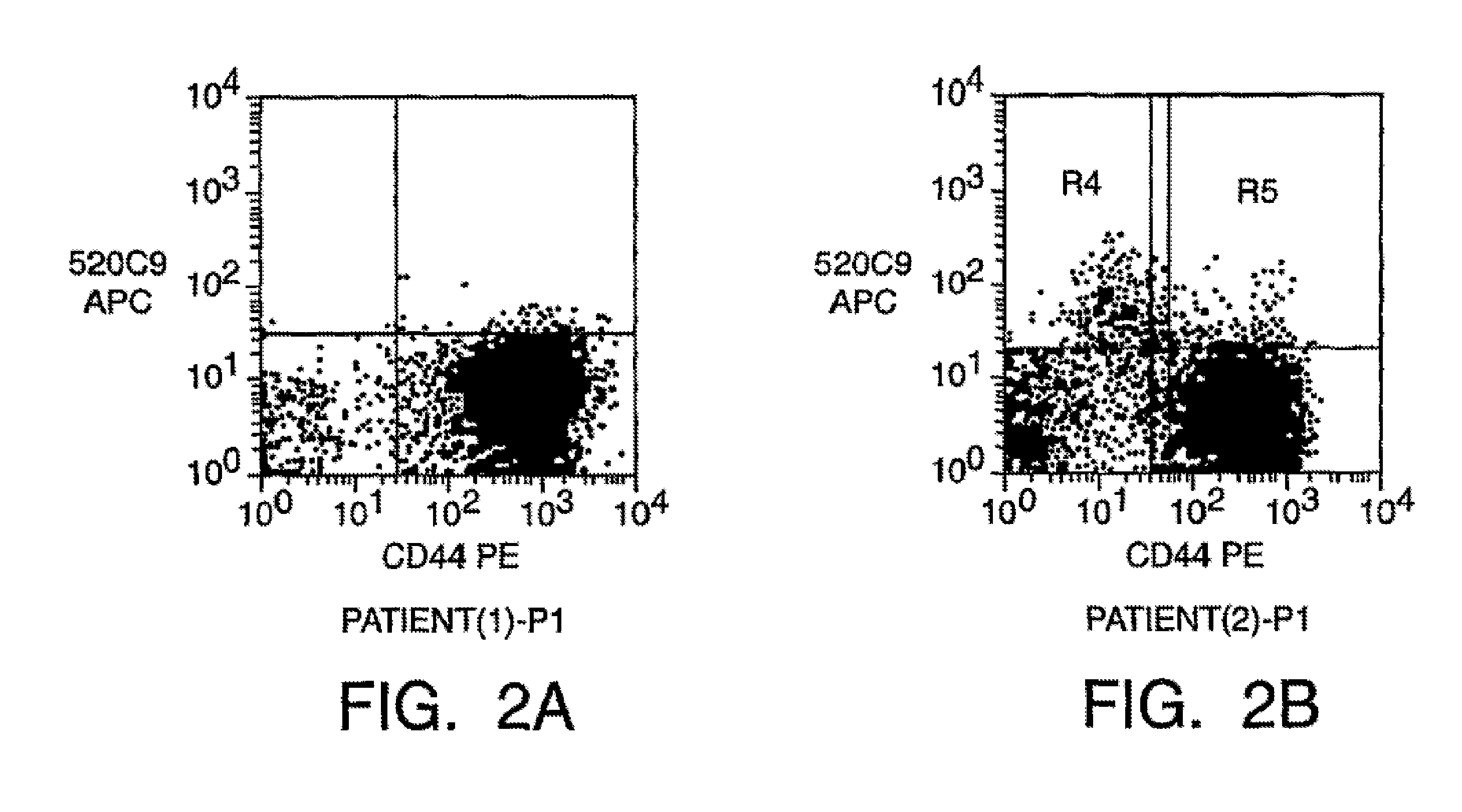
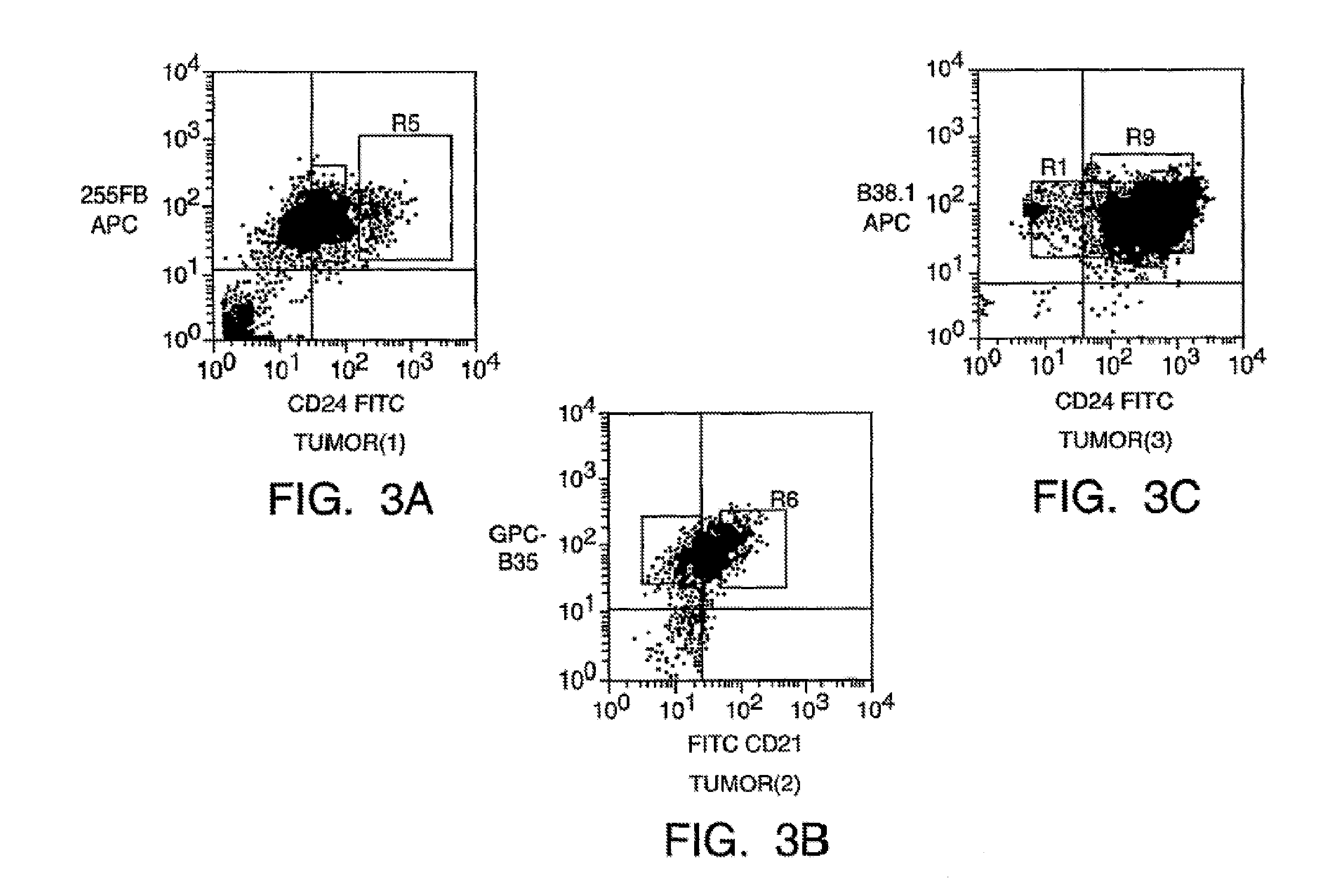
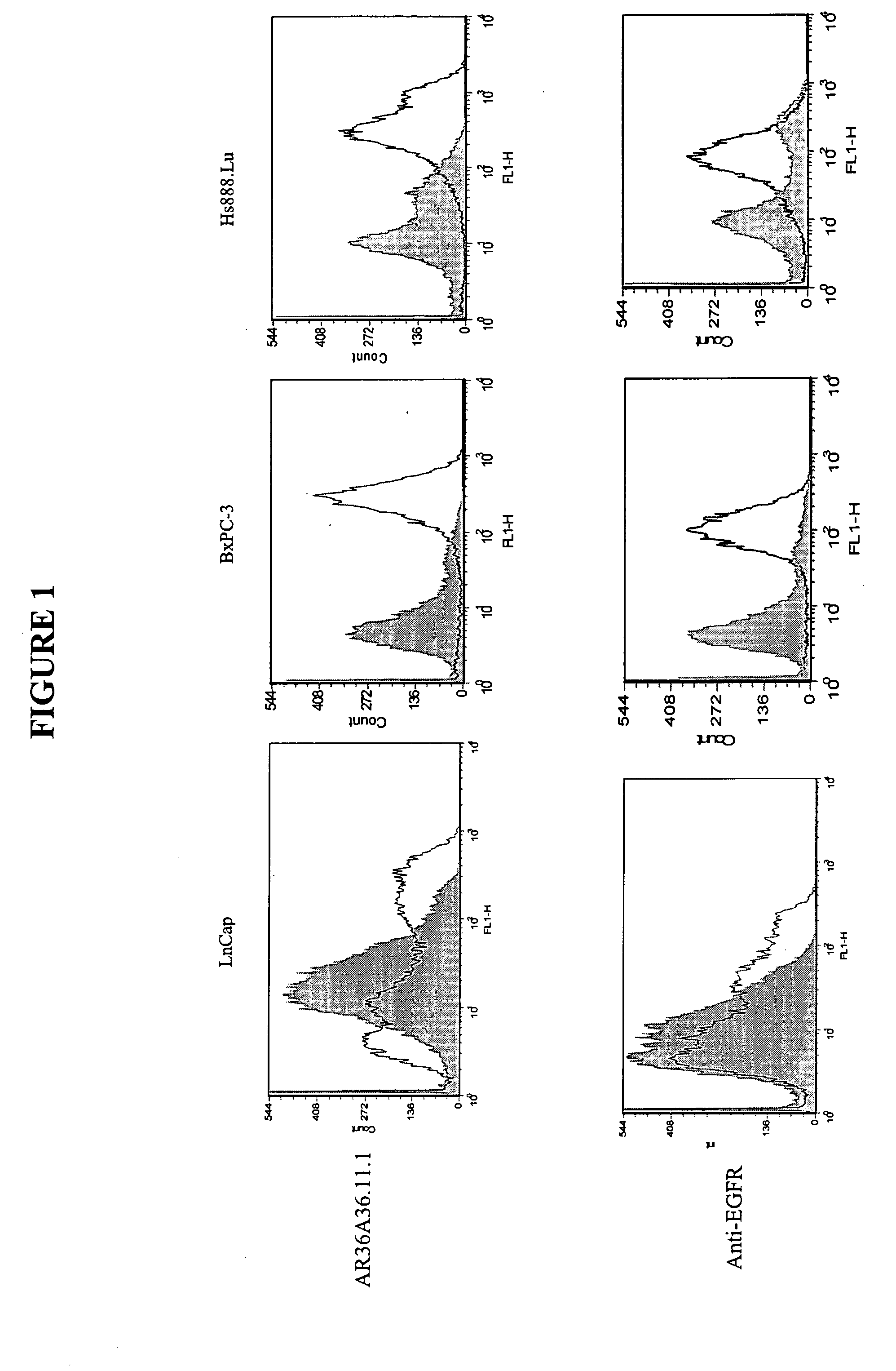
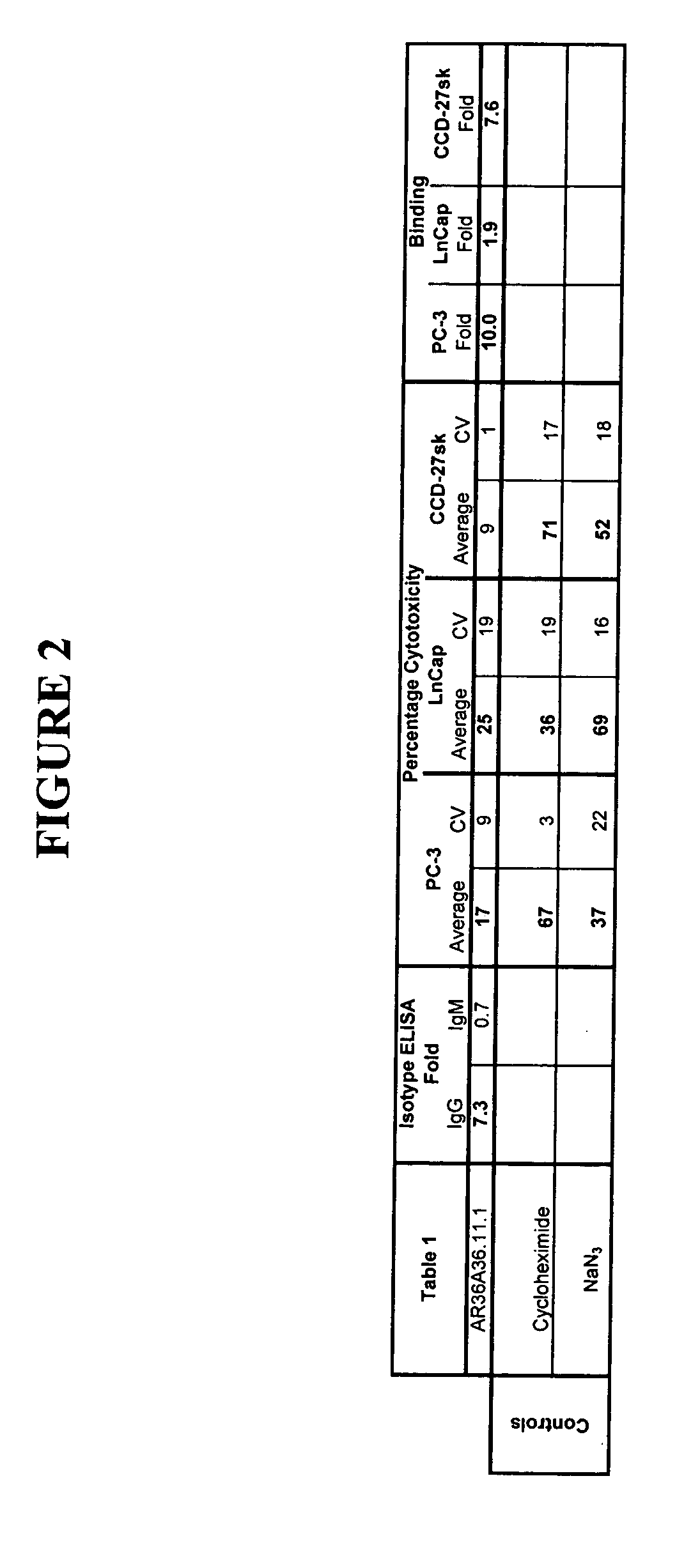
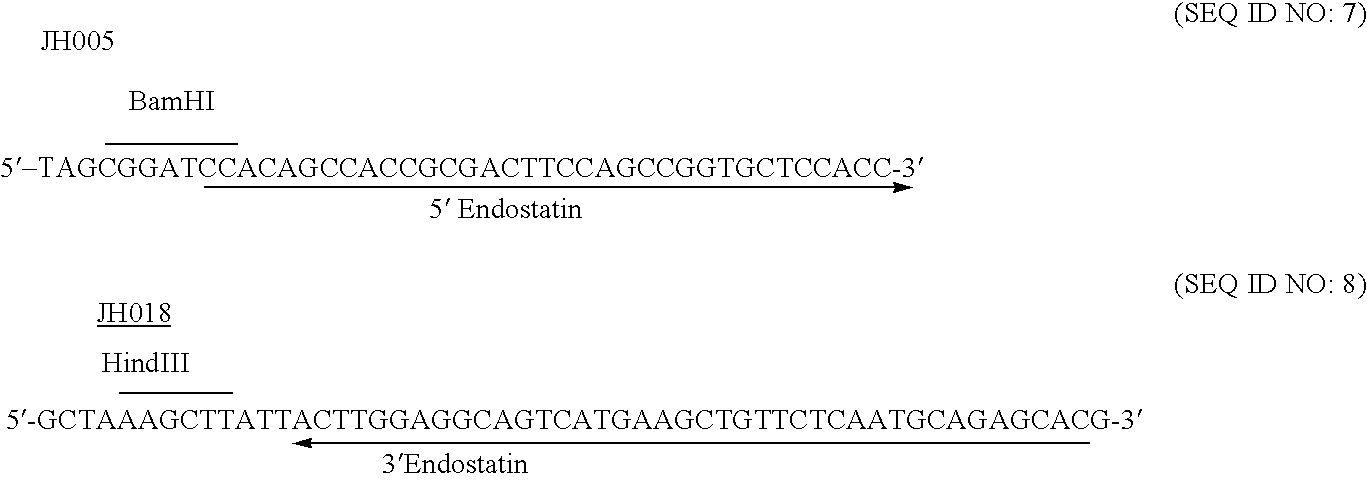Patents
Literature
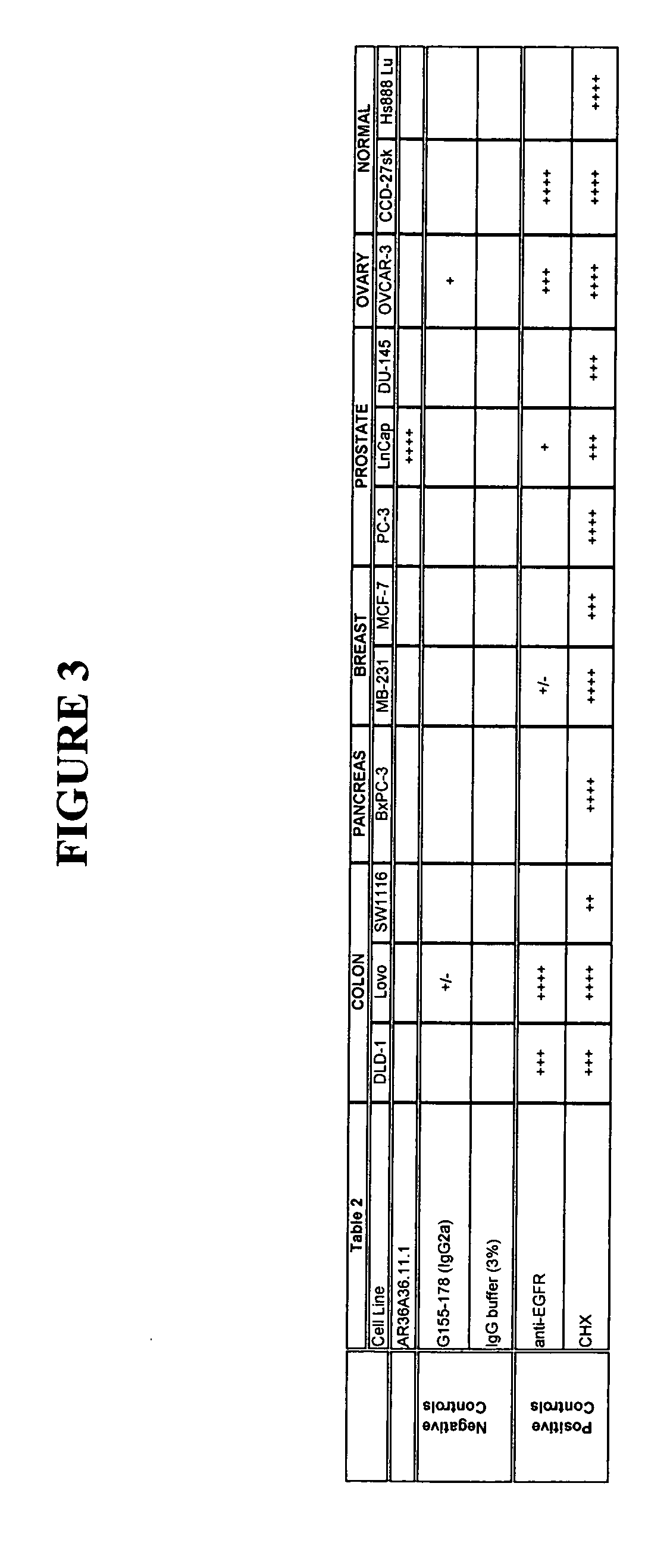
371 results about "Primary tumor site" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A primary tumor is a tumor growing at the anatomical site where tumor progression began and proceeded to yield a cancerous mass. Most cancers develop at their primary site but then go on to metastasize or spread to other parts of the body. These further tumors are secondary tumors.
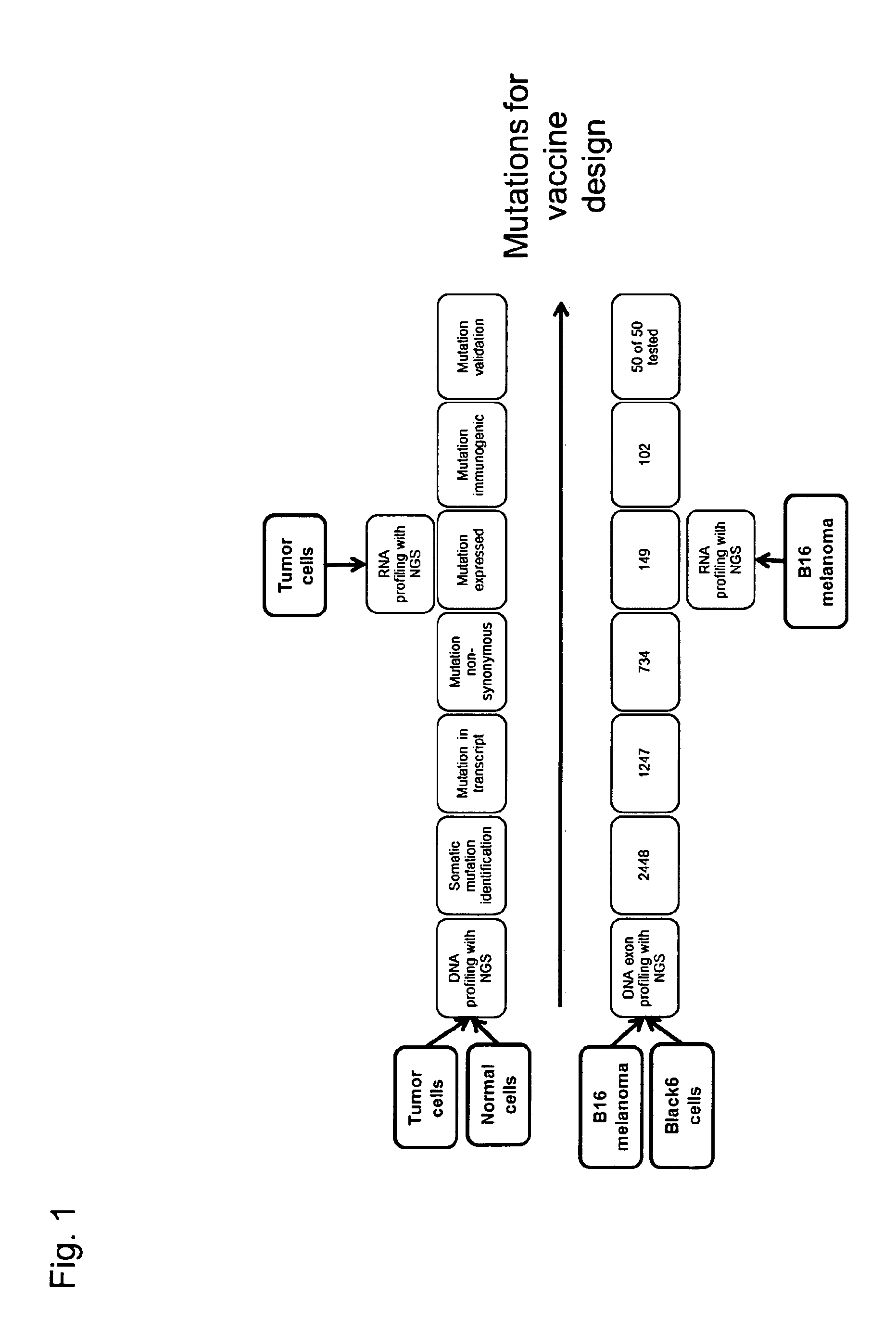
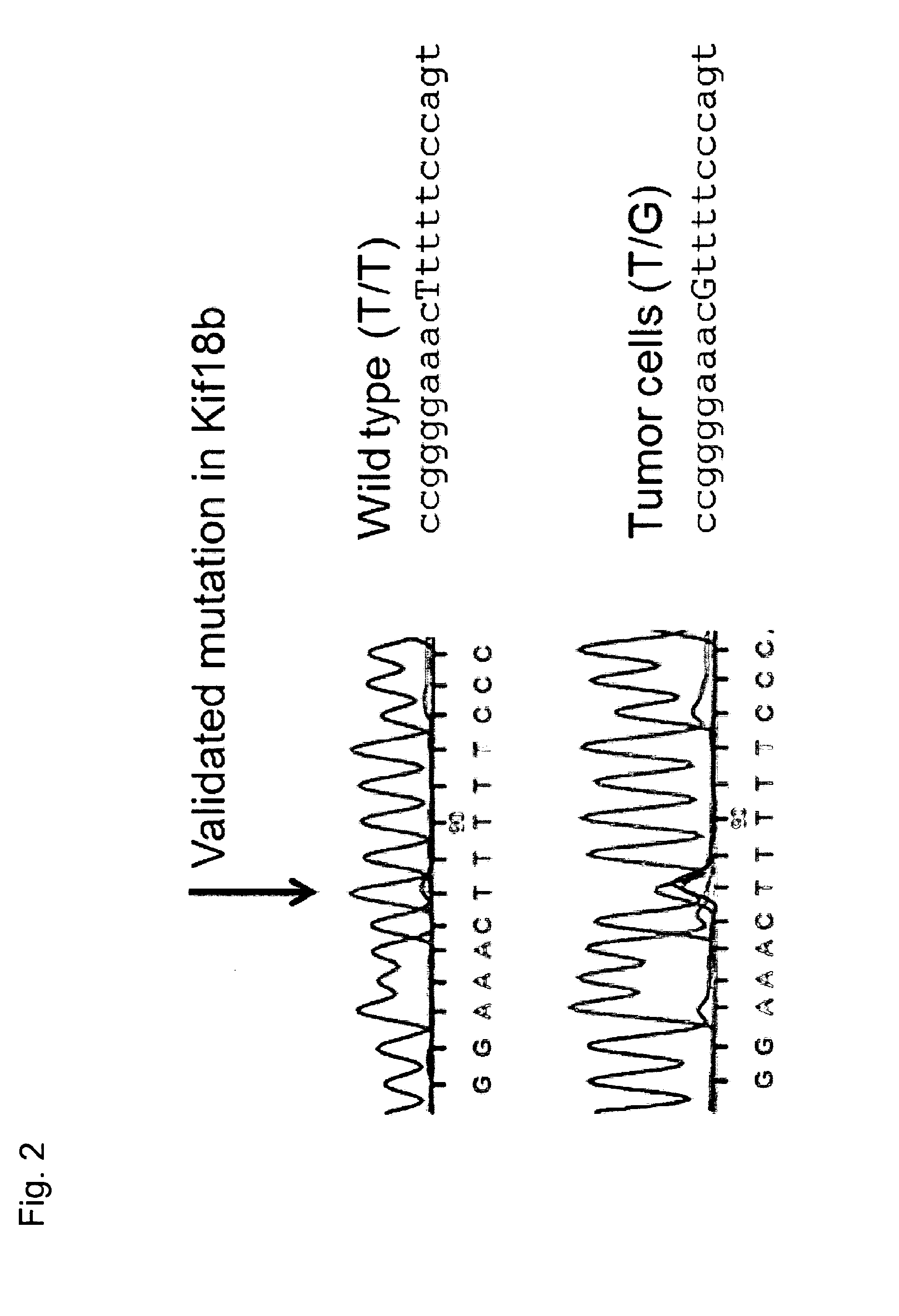
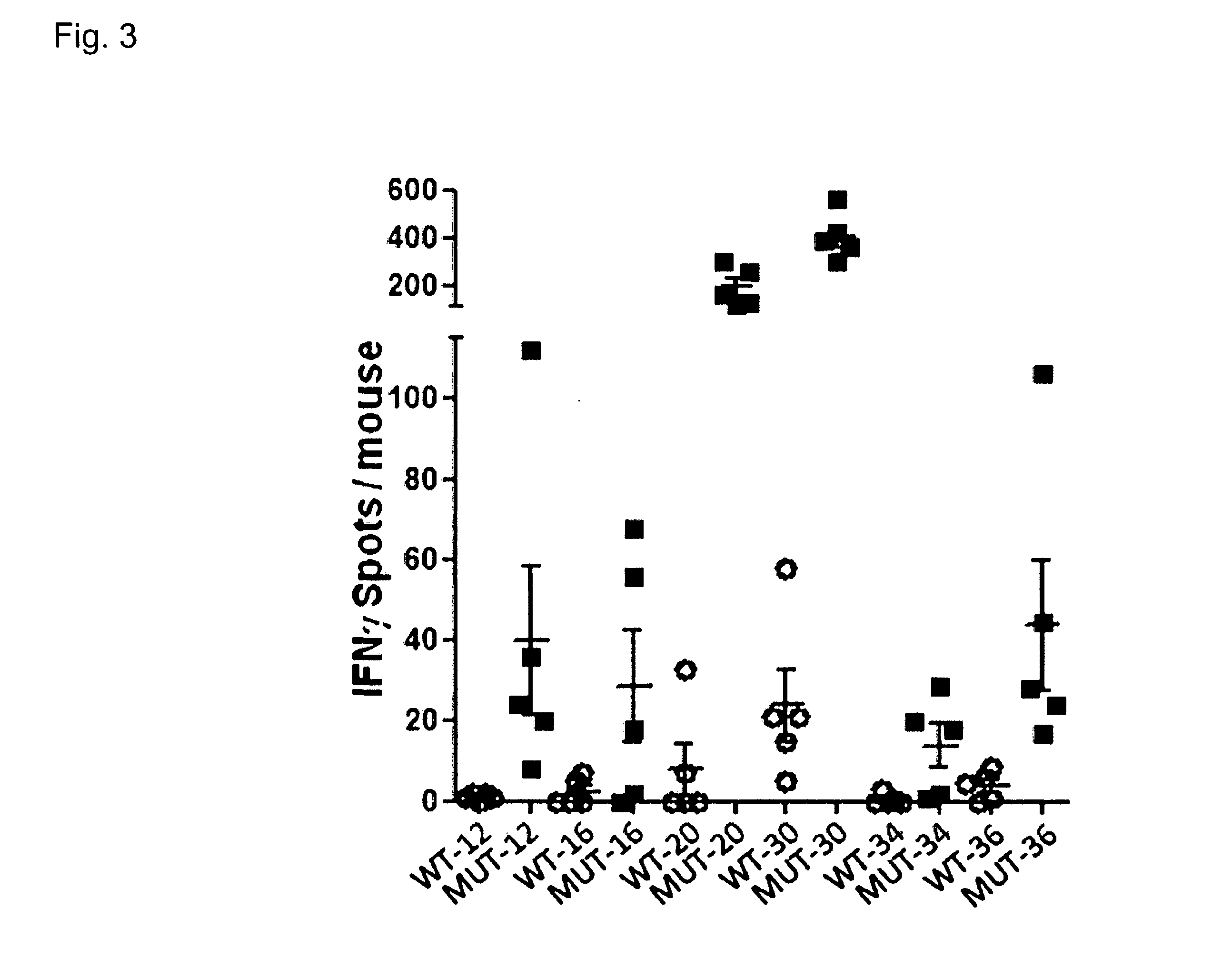
Individualized vaccines for cancer
ActiveUS20140178438A1Reduces steric hindranceImprove translationVaccinesPharmaceutical delivery mechanismPrimary tumorTumour metastasis
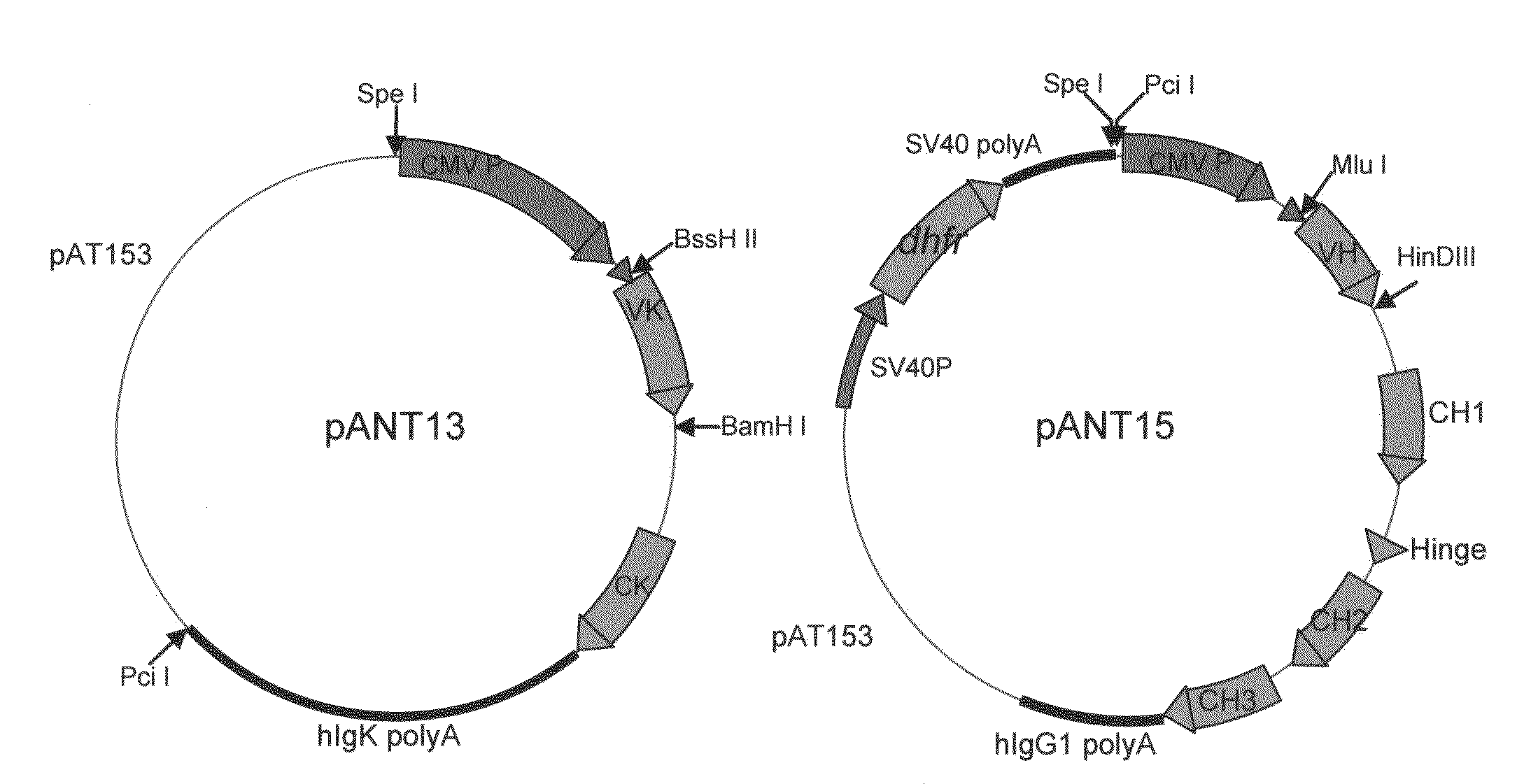
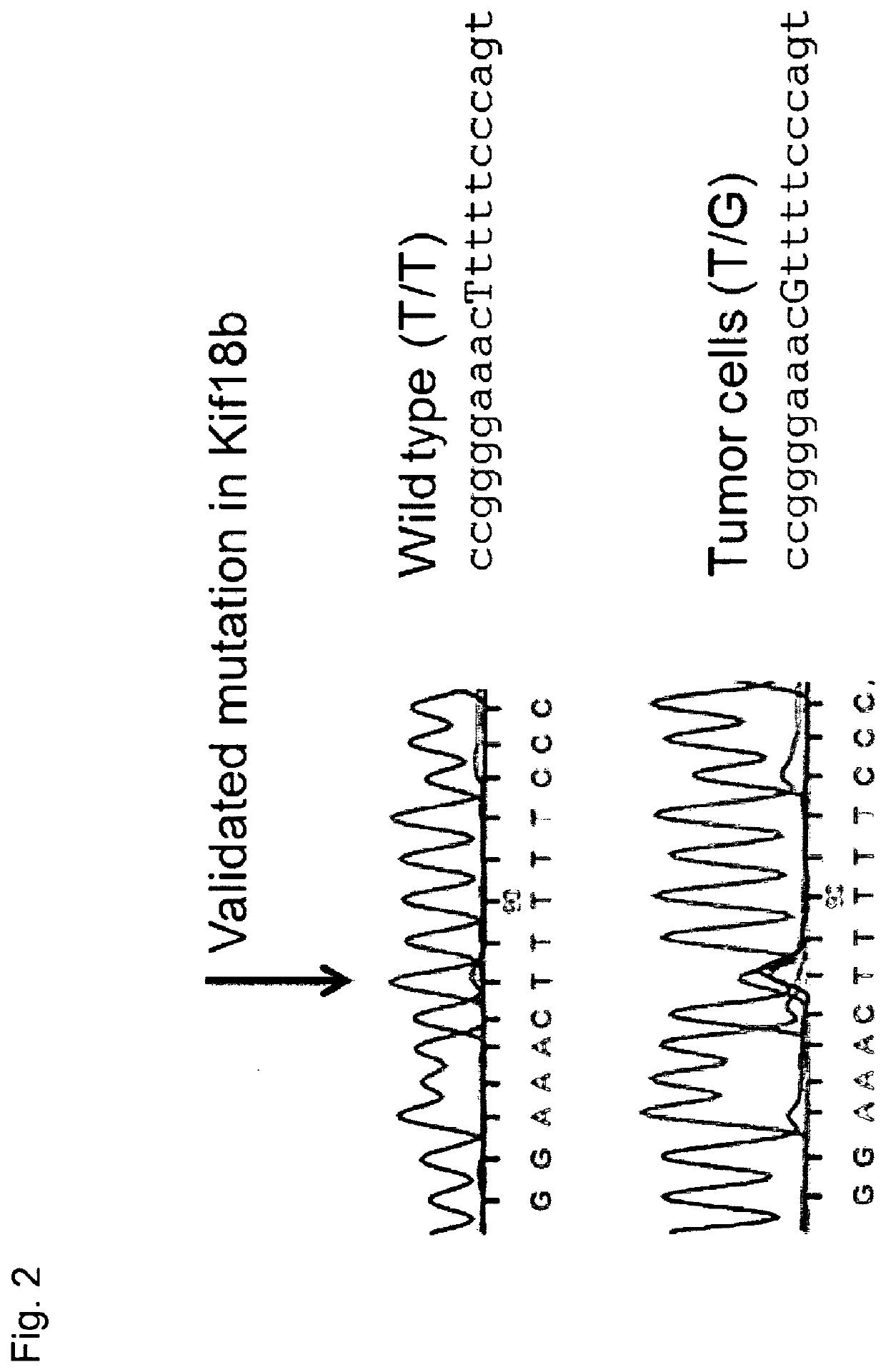
The present invention relates to the provision of vaccines which are specific for a patient's tumor and are potentially useful for immunotherapy of the primary tumor as well as tumor metastases. In one aspect, the present invention relates to a method for providing an individualized cancer vaccine comprising the steps: (a) identifying cancer specific somatic mutations in a tumor specimen of a cancer patient to provide a cancer mutation signature of the patient; and (b) providing a vaccine featuring the cancer mutation signature obtained in step (a). In a further aspect, the present invention relates to vaccines which are obtainable by said method.
Owner:TRANSLATIONALE ONKOLOGIE AN DER UNIVSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GGMBH +1
Isolation And Use Of Solid Tumor Stem Cells
InactiveUS20080178305A1Reduce spreadIncreased proliferationMaterial nanotechnologyMicrobiological testing/measurementAbnormal tissue growthMammary gland structure
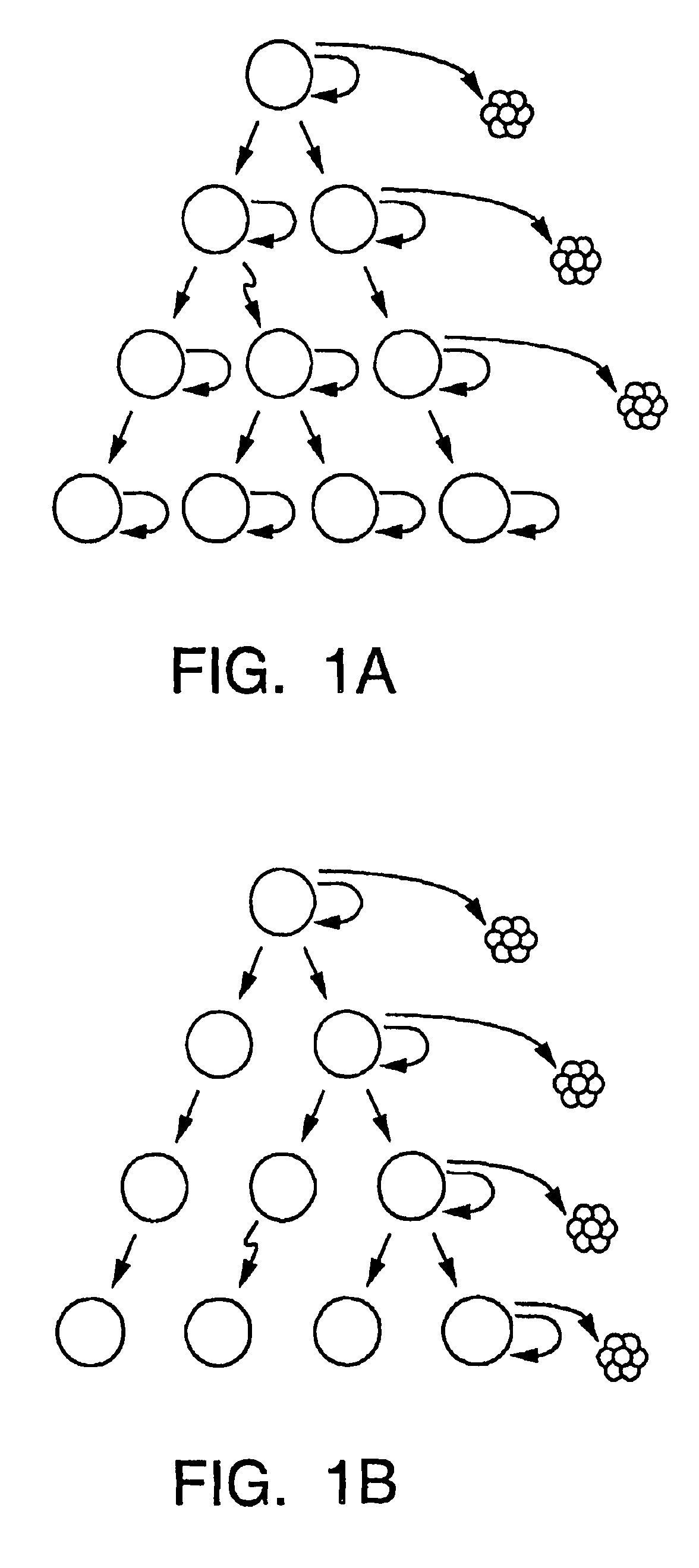
A small percentage of cells within an established solid tumor have the properties of stem cells. These solid tumor stem cells give rise both to more tumor stem cells and to the majority of cells in the tumor that have lost the capacity for extensive proliferation and the ability to give rise to new tumors. Thus, solid tumor heterogeneity reflects the presence of tumor cell progeny arising from a solid tumor stem cell. We have developed a xenograft model in which we have been able to establish tumors from primary tumors via injection of tumor cells in the mammary gland of severely immunodeficient mice. These xenograft assay have allowed us to do biological and molecular assays to characterize clonogenic solid tumor stem cells. We have also developed evidence that strongly implicates the Notch pathway, especially Notch 4, as playing a central pathway in carcinogenesis.
Owner:ONCOMED PHARMA +1
Cytotoxicity mediation of cells evidencing surface expression of TROP-2
InactiveUS7420040B2Reduce the likelihood of problemsProlong survival timeImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationDiseaseHematopoietic cell
The present invention relates to a method for producing cancerous disease modifying antibodies using a novel paradigm of screening. By segregating the anti-cancer antibodies using cancer cell cytotoxicity as an end point, the process makes possible the production of anti-cancer antibodies for therapeutic and diagnostic purposes. The antibodies can be used in aid of staging and diagnosis of a cancer, and can be used to treat primary tumors and tumor metastases. The anti-cancer antibodies can be conjugated to toxins, enzymes, radioactive compounds, cytokines, interferons, target or reporter moieties and hematogenous cells.
Owner:F HOFFMANN LA ROCHE & CO AG
Cytotoxicity mediation of cells evidencing surface expression of TROP-2
InactiveUS7420041B2Reduce the likelihood of problemsProlong survival timePeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadCancer cell
The present invention relates to a method for producing cancerous disease modifying antibodies using a novel paradigm of screening. By segregating the anti-cancer antibodies using cancer cell cytotoxicity as an end point, the process makes possible the production of anti-cancer antibodies for therapeutic and diagnostic purposes. The antibodies can be used in aid of staging and diagnosis of a cancer, and can be used to treat primary tumors and tumor metastases. The anti-cancer antibodies can be conjugated to toxins, enzymes, radioactive compounds, cytokines, interferons, target or reporter moieties and hematogenous cells.
Owner:F HOFFMANN LA ROCHE & CO AG
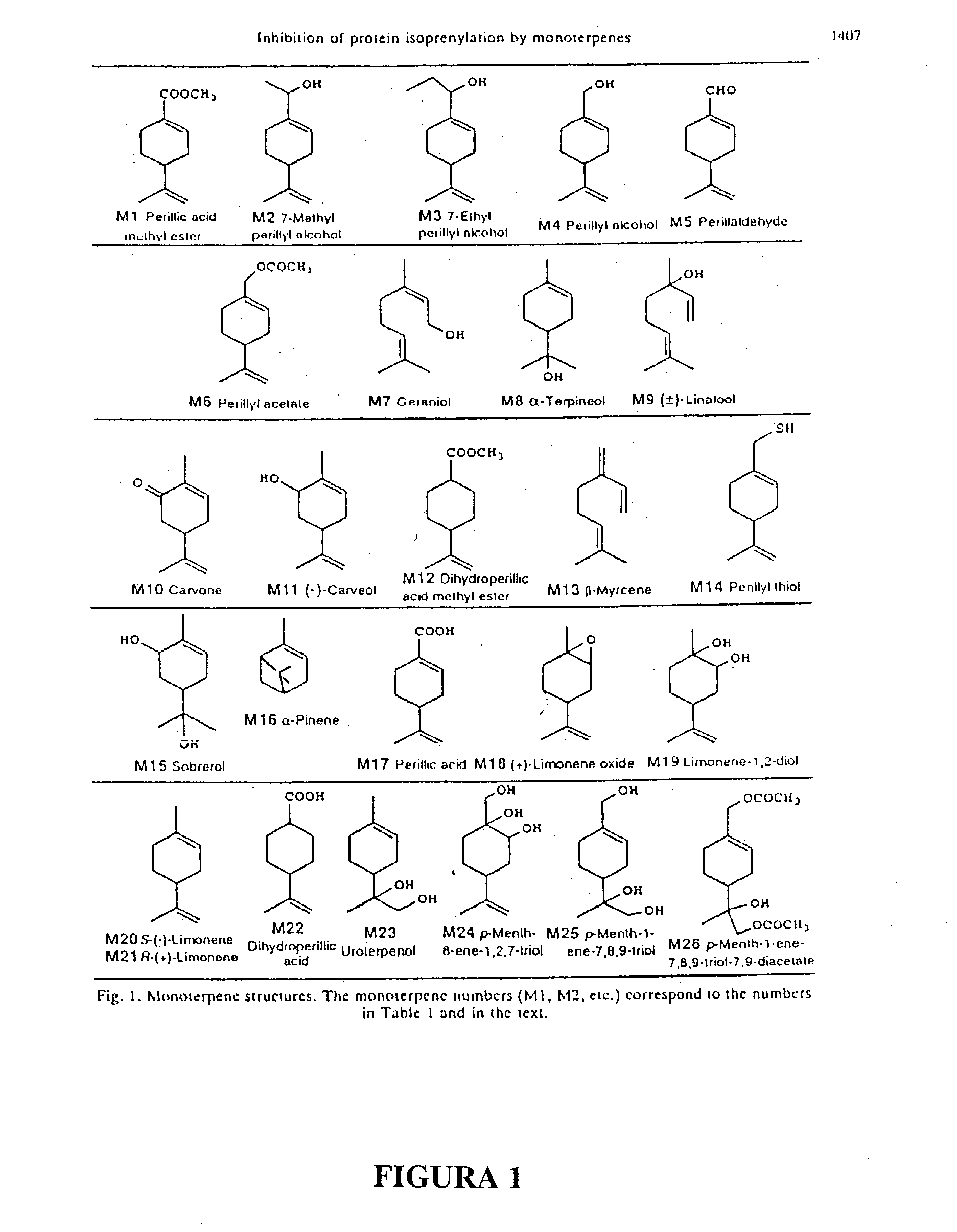
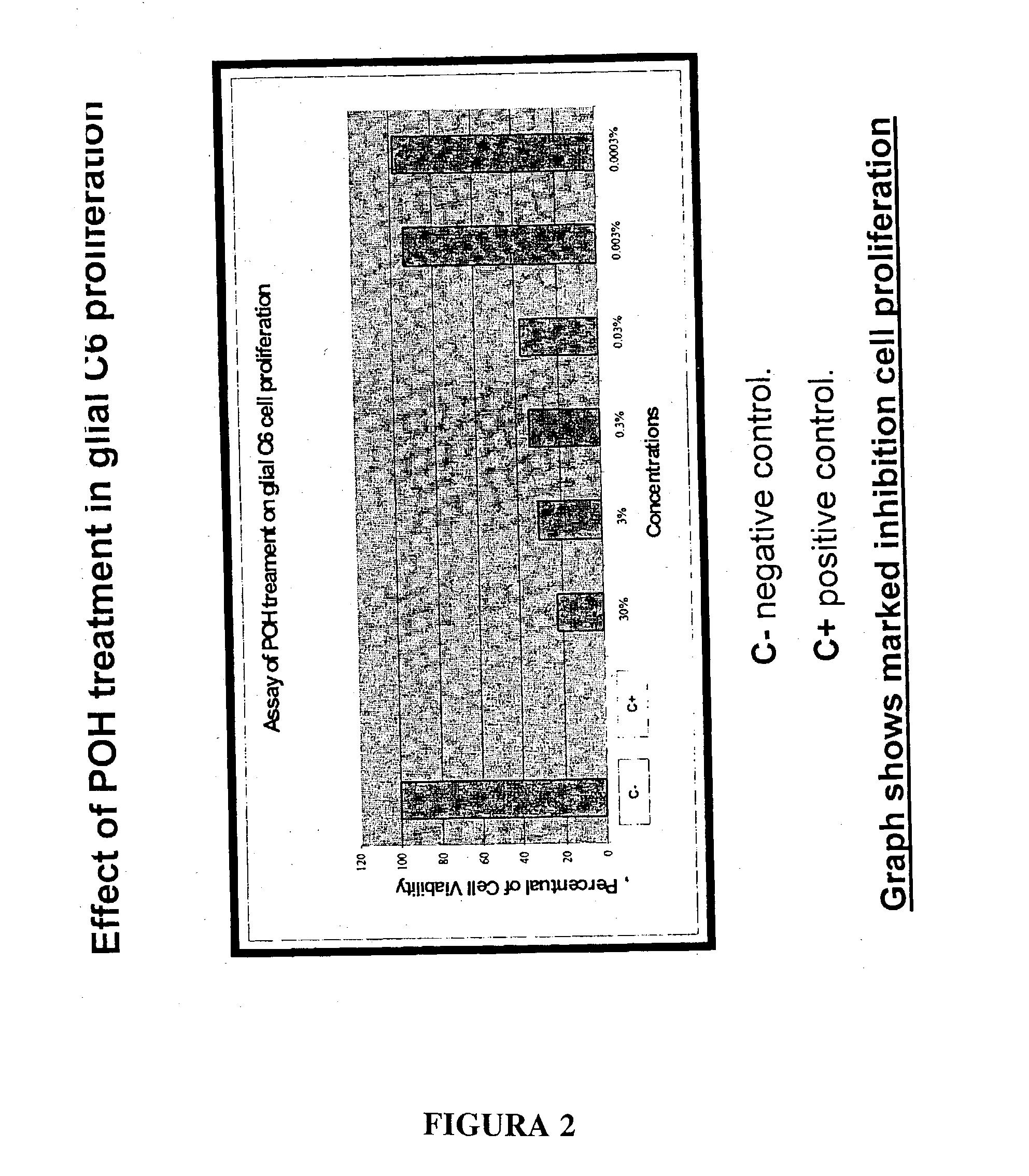
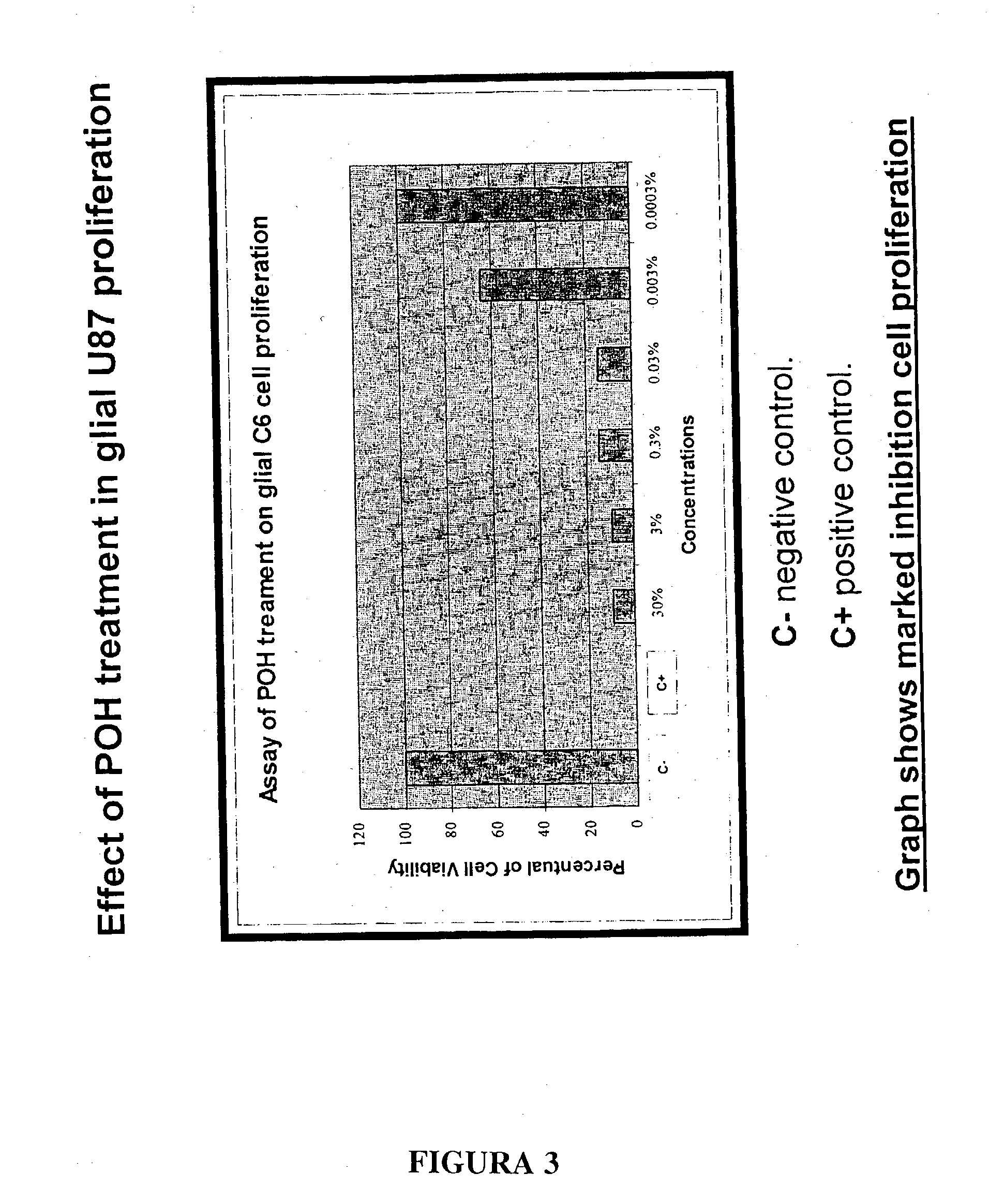
Monoterpene as a chemopreventive agent for regression of mammalian nervous system cell tumors, use of monoterpene for causing regression and inhibition of nervous system cell tumors, and method for administration of monoterpene perillyl alcohol
InactiveUS20040087651A1Prevent relapseBiocideHydroxy compound active ingredientsMetaboliteLymphatic Spread
The present invention refers to a composition based on monoterpenes with chemopreventive and chemotherapeutic effects in malignant neoplasias of humans and animals containing from 0,03% to 30% of monoterpenes and 99,97% of solvents. Another objective of the present invention is an application of monoterpenes in inhibition of cell growth and metastasis control of primary tumors being applied in vitro and in vivo gliomas cell lines C6 and U 87 and A172. Further another objective of the present invention refers to a specific methods for applying the composition with chemopreventive and chemotherapeutic effects in humans and animals showing malignant neoplasias by inhalation and nebulization treatment, oral and intratumoral, followed or not by radiotherapy with dilutions from 0,03% to 30% of the monoterpene perillyl or its derived metabolites diluted in the solvents specified by the usual techniques.
Owner:PEREIRA DA FONSECA CLOVIS ORLANDO +3
Multiple medical accelerators and a kV-CT incorporated radiation therapy device and semi-automated custom reshapeable blocks for all field synchronous image guided 3-D-conformal-intensity modulated radiation therapy
S-band, C-band or X-band microwave powered linear accelerators capable of delivering therapeutic photon and electron beams are mounted to a gantry with extensions to hold multiple accelerators and are combined with a kV CT for 3-D conformal—IMRT and IGRT to treat a patient by SSD or SAD methods and in a full circle. The invention's tertiary collimator system consists of semi-automated reusable custom field shaping with tungsten powder or melted Cerrobend blocks. The beam's intensity modulation is by means of simultaneous but independently operating multiple accelerators. This system's multiple accelerators enable to avoid interrupted subfractionated radiation therapy to each treatment fields. Hence its effective dose rate at the tumor site is high. The improved radiobiology reduces the total radiation dose to treat a tumor, reducing the incidence of developing second primary tumors is also minimized.
Owner:SAHADEVAN VELAYUDHAN
Blood test to monitor the genetic changes of progressive cancer using immunomagnetic enrichment and fluorescence in situ hybridization (FISH)
InactiveUS20080113350A1Accurate measurementEasy accessMicrobiological testing/measurementLymphatic SpreadGenetic Change
Amplification and overexpression of theHER-2 oncogene in breast cancer is felt to be stable over the course of disease and concordant between the primary tumor and metastases. Therefore, patients with HER-2 negative primary tumors will rarely receive anti-HER-2 antibody therapy. A very sensitive blood test is used to capture circulating tumor cells (CTC's) and evaluate their HER-2 gene status by FISH evaluation. The HER-2 status of the primary tumor and corresponding CTC's is used to assess the ratio of CTC's as a reliable surrogate marker. HER-2 expression of 10 CTC's is sufficient to make a definitive diagnosis of the HER-2 gene status for the whole population of CTC's in patients with recurrent breast cancer.
Owner:JANSSEN DIAGNOSTICS LLC
Kinase inhibitors useful for the treatment of myleoprolific diseases and other proliferative diseases
Compounds of the present invention find utility in the treatment of mammalian cancers and especially human cancers including but not limited to malignant, melanomas, glioblastomas, ovarian cancer, pancreatic cancer, prostate cancer, lung cancers, breast cancers, kidney cancers, cervical carcinomas, metastasis of primary tumor sites, myeloproliferative diseases, leukemias, papillary thyroid carcinoma, non small cell lung cancer, mesothelioma, hypereosinophilic syndrome, gastrointestinal stromal tumors, colonic cancers, ocular diseases characterized by hyperproliferation leading to blindness including various retinopathies, rheumatoid arthritis, asthma, chronic obstructive pulmonary disease, mastocyctosis, mast cell leukemia, a disease caused by c-Abl kinase, oncogenic forms thereof, aberrant fusion proteins thereof and polymorphs thereof, or a disease caused by c-Kit kinase, oncogenic forms thereof, aberrant fusion proteins thereof and polymorphs thereof.
Owner:DECIPHERA PHARMA LLC
Cytotoxicity mediation of cells evidencing surface expression of TROP-2
InactiveUS20080131428A1Raise the possibilityEfficient targetingImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseLymphatic Spread
Owner:ARIUS RES +1
Screening assays and methods of tumor treatment
InactiveUS20060015952A1Growth inhibitionBiological material analysisSkeletal disorderAbnormal tissue growthPrimary tumor
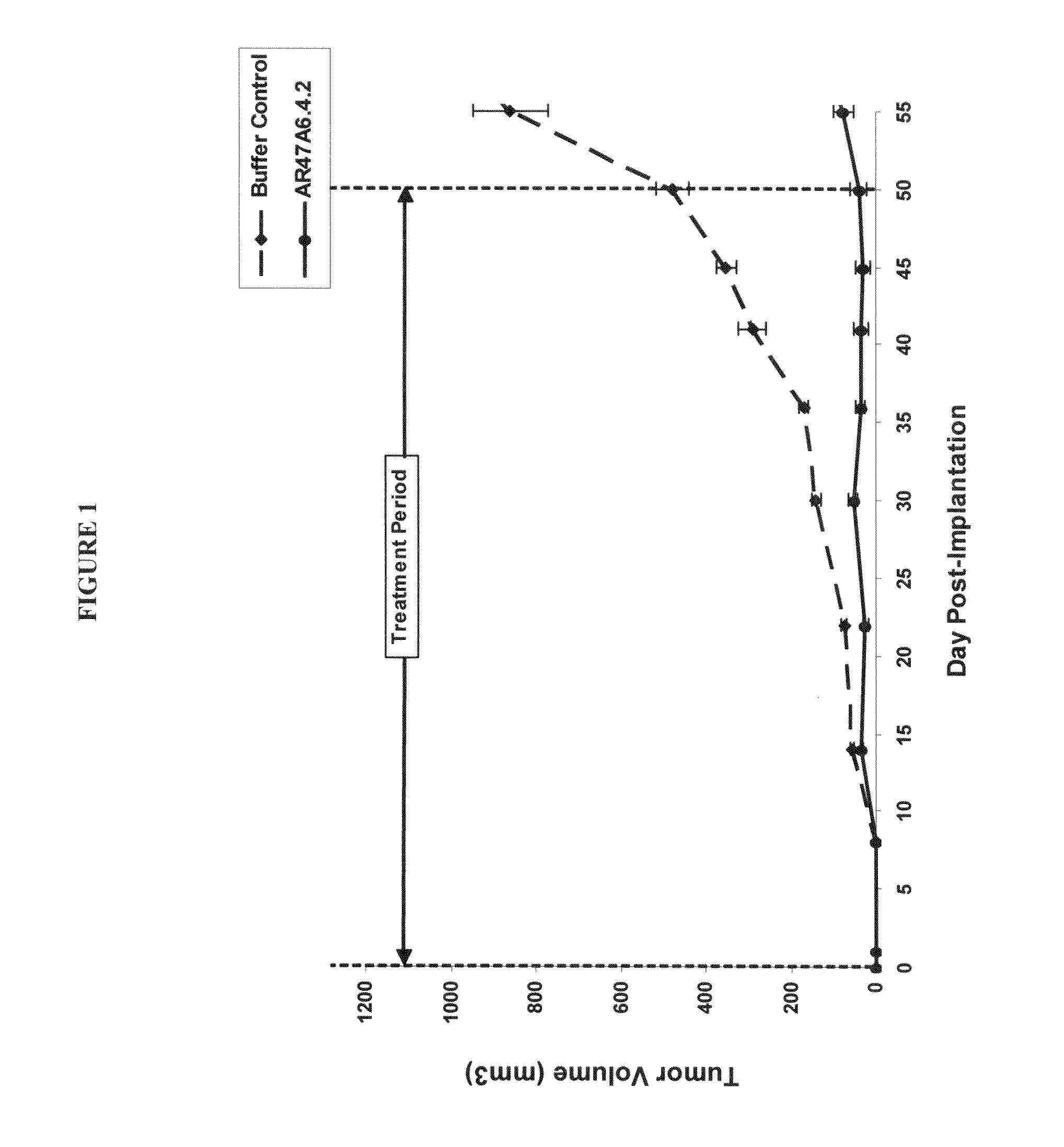
The invention relates generally to the screening of candidate molecules for the treatment of tumor metastasis, and treatment methods using such molecules. Thus, the invention includes a method of screening comprising the steps of: (1) administering a plurality of test substances to a non-human syngeneic immunocompetent animal model bearing at least one soft tissue or bone metastasis, in the presence or absence of a primary tumor; (2) determining the effects of the test substances on the soft tissue or bone metastasis and growth of the primary tumor, if present; and (3) identifying a test substance that inhibits the growth of a soft tissue or bone metastasis, without adverse effect on the status of the primary tumor, if present.
Owner:GENENTECH INC
Vascular endothelial growth factor fusion constructs and uses thereof
InactiveUS20050037967A1Inhibiting metastatic spreadInhibiting vascularizationPeptide/protein ingredientsAntibody mimetics/scaffoldsGeloninAbnormal tissue growth
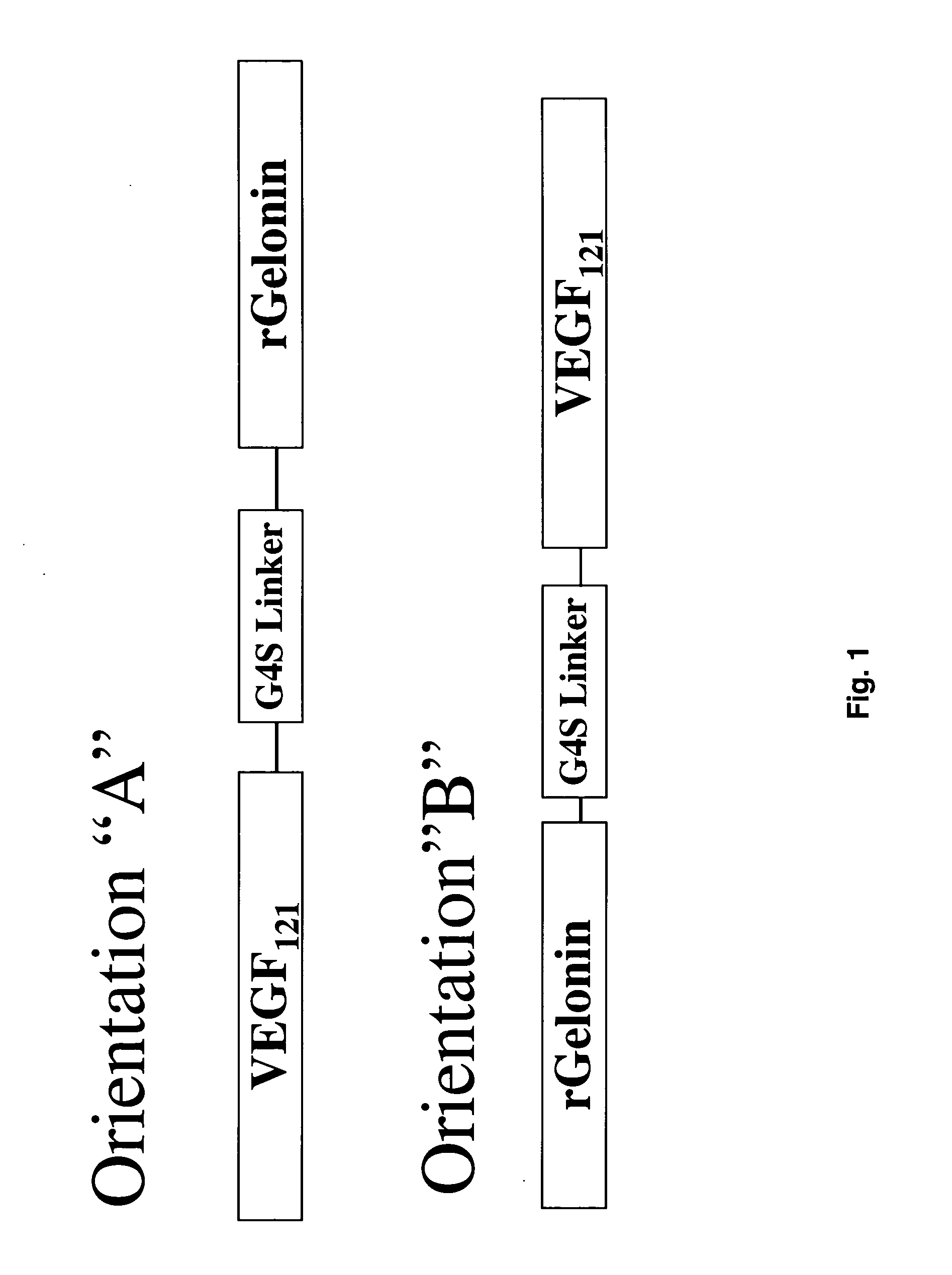
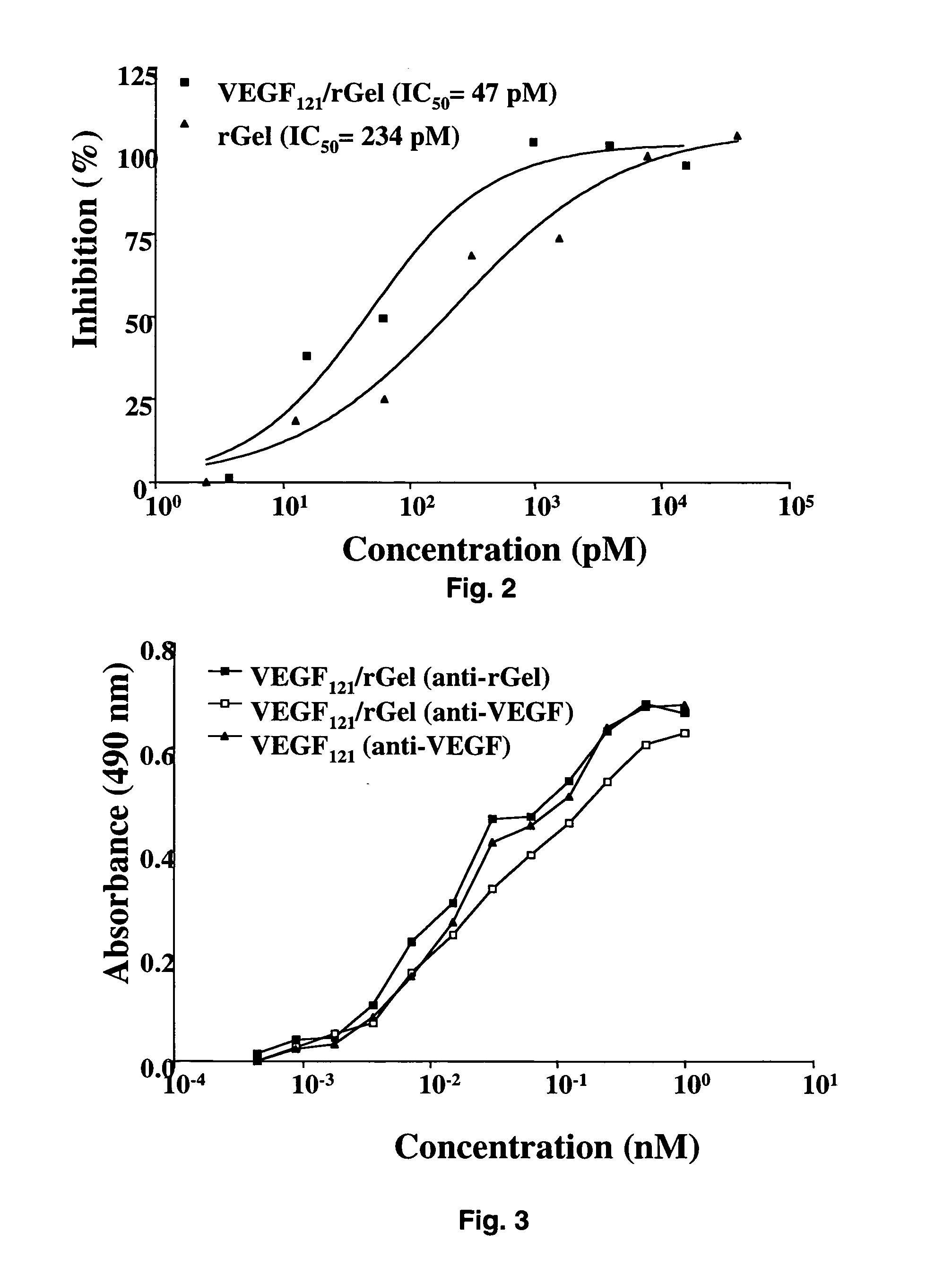
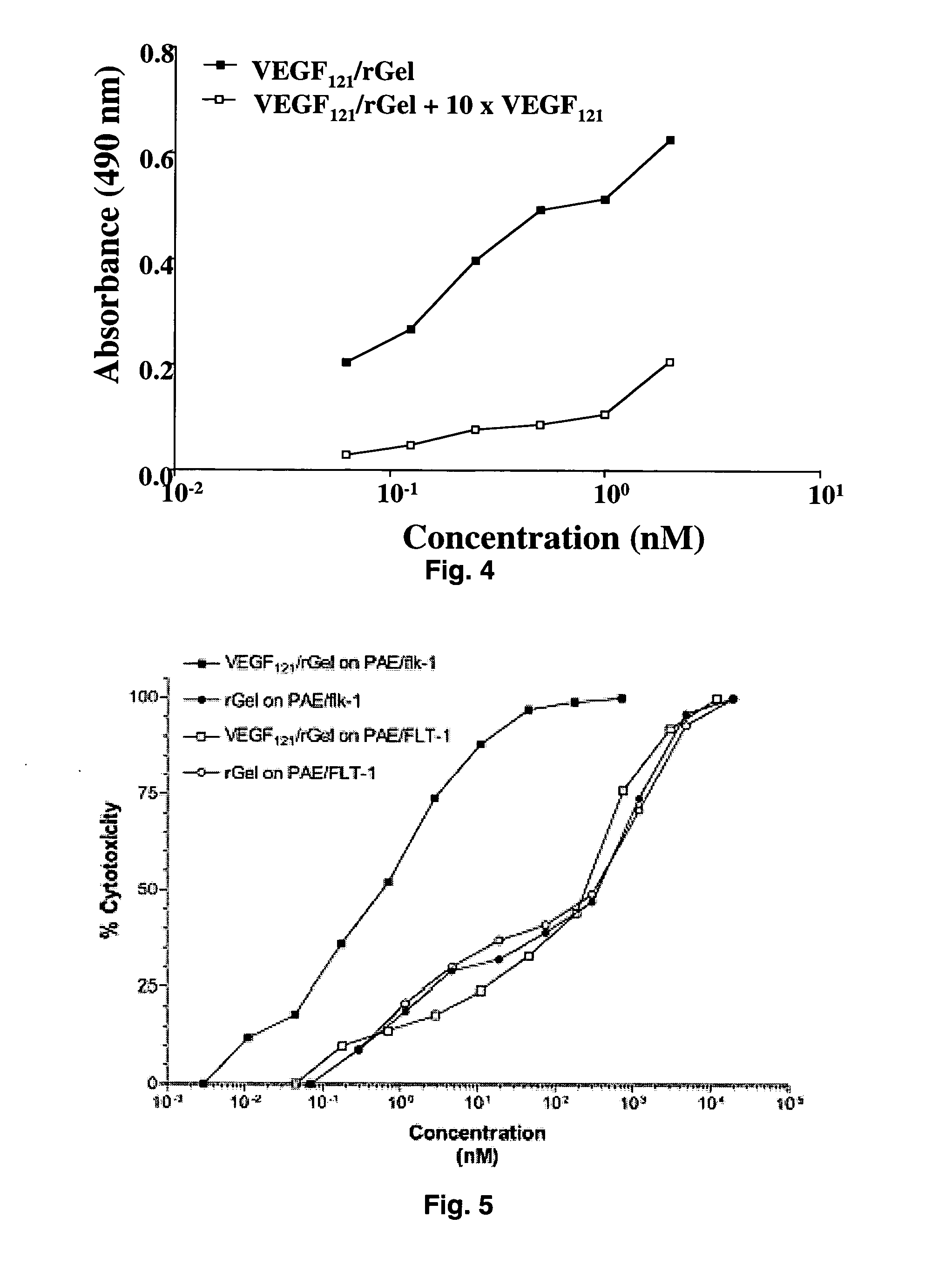
The 121-amino acid isoform of vascular endothelial growth factor (VEGF121) is linked by a flexible G4S tether to a cytotoxic molecule such as toxin gelonin or granzyme B and expressed as a soluble fusion protein. The VEGF12, fusion protein exhibits significant anti-tumor vascular-ablative effects that inhibit the growth of primary tumors and inhibit metastatic spread and vascularization of metastases. The VEGF121 fusion protein also target osteoclast precursor cells in vivo and inhibits osteoclastogenesis.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for the prognosis and treatment of cancer metastasis
InactiveUS20160032400A1Increased riskOrganic active ingredientsLibrary screeningPrimary NeoplasmCancer metastasis
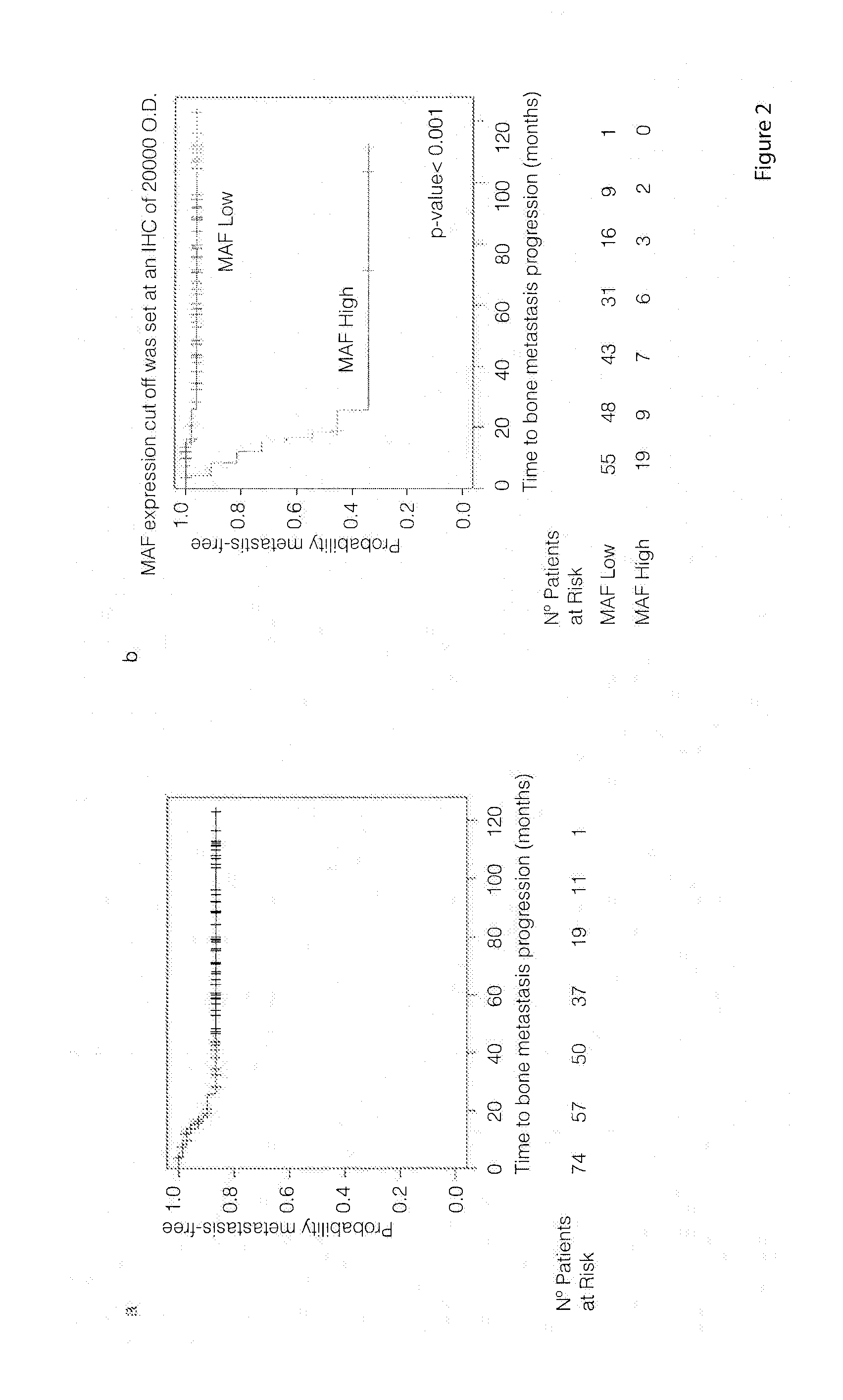
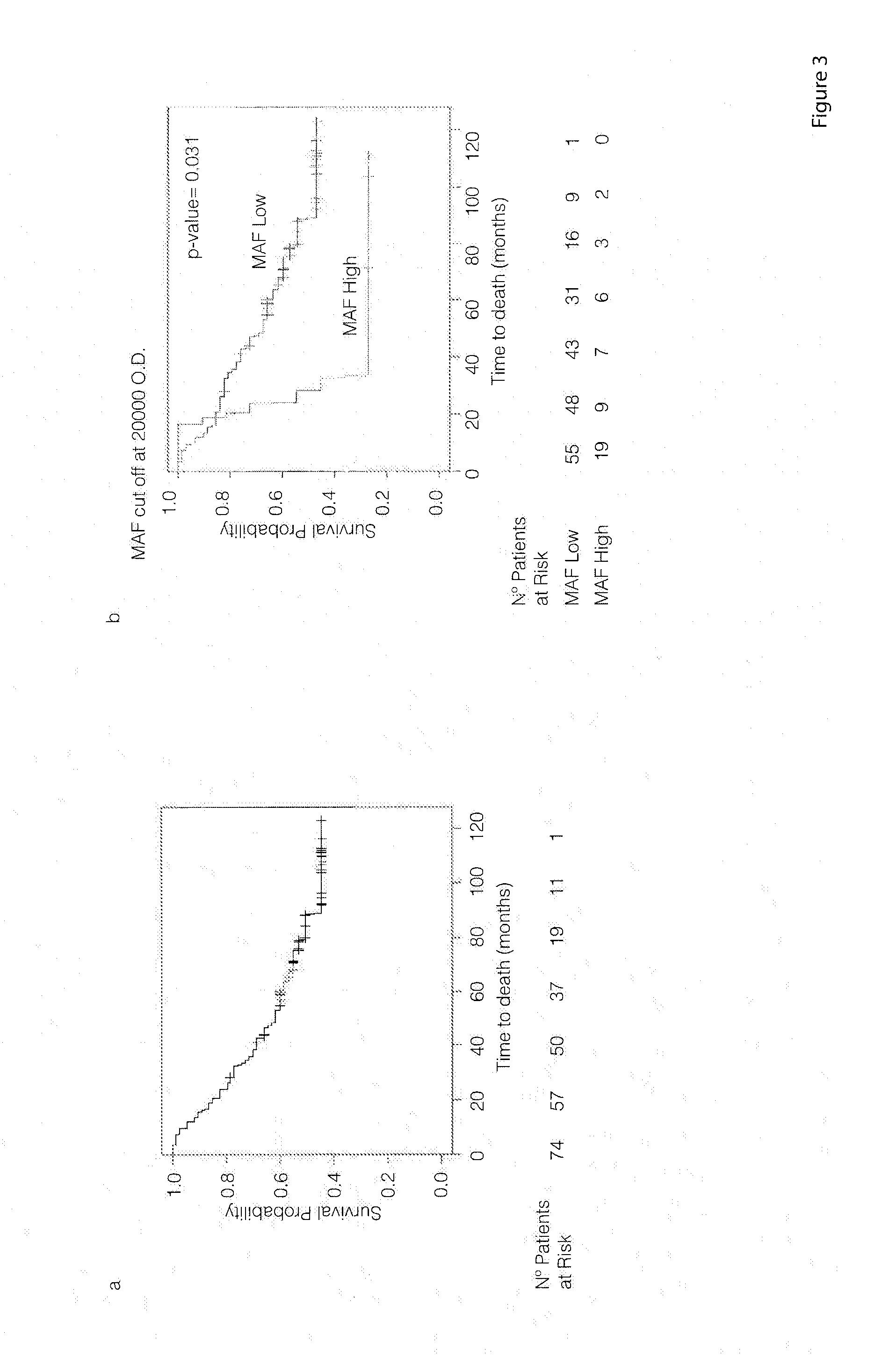
The present invention relates to a method for the prognosis of bone metastasis in cancer which comprises using a probe to determine if a gene of interest is amplified in a primary tumor sample. Likewise, the invention also relates to a method for determining the tendency to develop bone metastasis with respect to metastasis in other organs, which comprise using a probe to determine the expression level of a gene of interest, or the amplification or translocation of a gene of interest. The invention also relates to a method for predicting early bone metastasis in a subject suffering cancer. The invention also relates to a c-MAF inhibitor as therapeutic agent for use in the treatment of cancer metastasis. The invention relates to kits for predicting bone metastasis and predicting the clinical outcome of a subject suffering from bone metastasis. Finally, the invention relates to a method for typing of a subject suffering cancer and for classifying a subject from cancer into a cohort.
Owner:INSTITUCIO CATALANA DE RECERCA I ESTUDIS AVANCATS +1
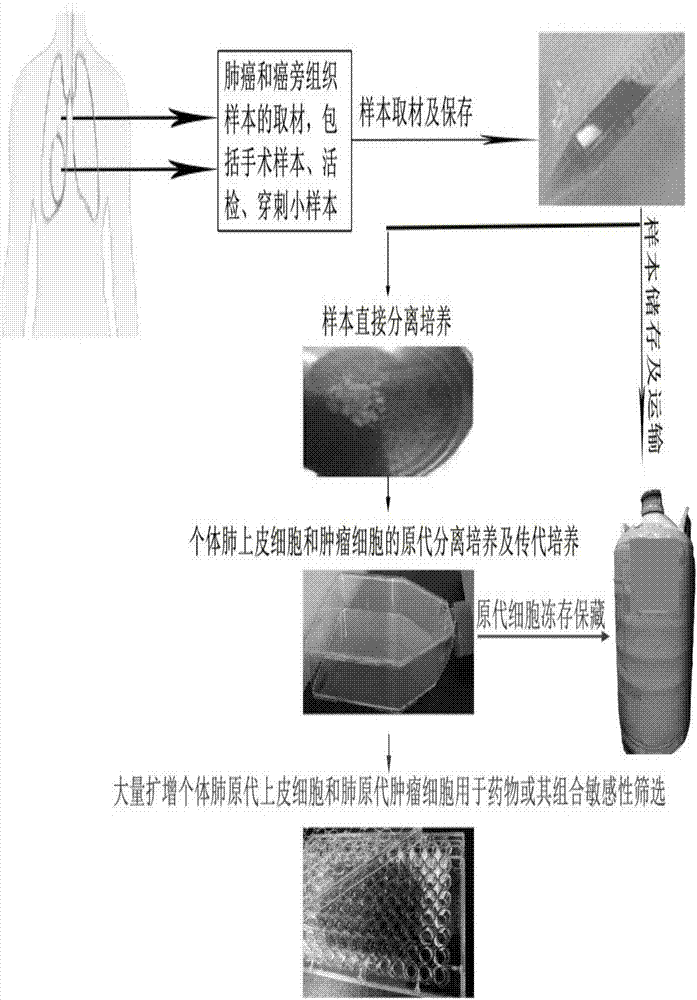
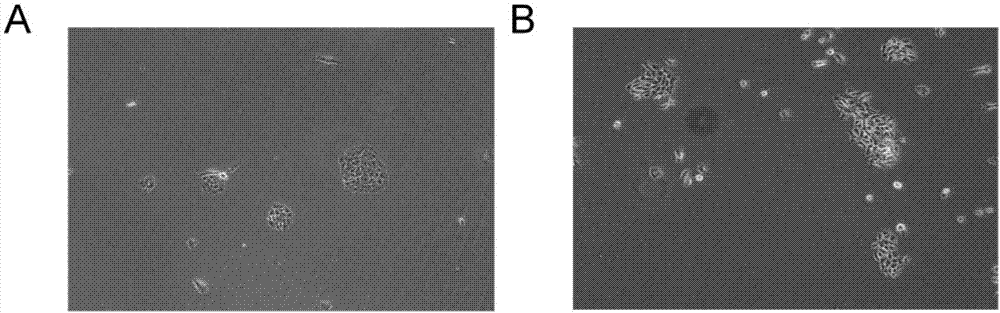
Primary tumor cell culture medium, culture method and application
ActiveCN108624561ASolve the problem of primary culture immortalizationEnable Personalized TreatmentMicrobiological testing/measurementSkeletal/connective tissue cellsAbnormal tissue growthCell culture media
The invention belongs to the technical field of medicine, and specifically relates to a primary tumor cell culture medium, culture method and application. The primary tumor cell culture method provided by the invention comprises the steps as follows: preparing a primary cell culture medium which comprises the following components: hydrocortisone, EGF, Insulin, a ROCK inhibitor, but not contains cholera toxin, and is an improvement of the existing primary cell culture medium; culturing tumor tissue epithelial cells on laid-out trophoblast cells by using the primary cell culture medium, and enabling the tumor tissue epithelial cells to proliferate rapidly under the combined action of growth factors secreted by the trophoblast cells and nutrient factors contained in the culture medium; and digesting and sub-culturing the tumor tissue epithelial cells when the tumor tissue epithelial cells grow to a cell density of about 80% to 90%. A convenient primary cell culture method is used to obtain immortalized cells possessing the biological characteristics of a patient's own tumor, and the problem of immortalization of the primary culture of tumor cells is solved, thereby realizing personalized treatment for the patient.
Owner:FUDAN UNIV
Cytotoxicity mediation of cells evidencing surface expression of TROP-2
InactiveUS20080213267A1Reduce the likelihood of problemsReduce the overall heightOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadCytokine
This invention relates to the staging, diagnosis and treatment of cancerous diseases (both primary tumors and tumor metastases), particularly to the mediation of cytotoxicity of tumor cells; and most particularly to the use of cancerous disease modifying antibodies (CDMAB), optionally in combination with one or more CDMAB / chemotherapeutic agents, as a means for initiating the cytotoxic response. The invention further relates to binding assays, which utilize the CDMAB of the instant invention. The anti-cancer antibodies can be conjugated to toxins, enzymes, radioactive compounds, cytokines, interferons, target or reporter moieties and hematogenous cells.
Owner:F HOFFMANN LA ROCHE INC
Microparticle compositions to modify cancer promoting cells
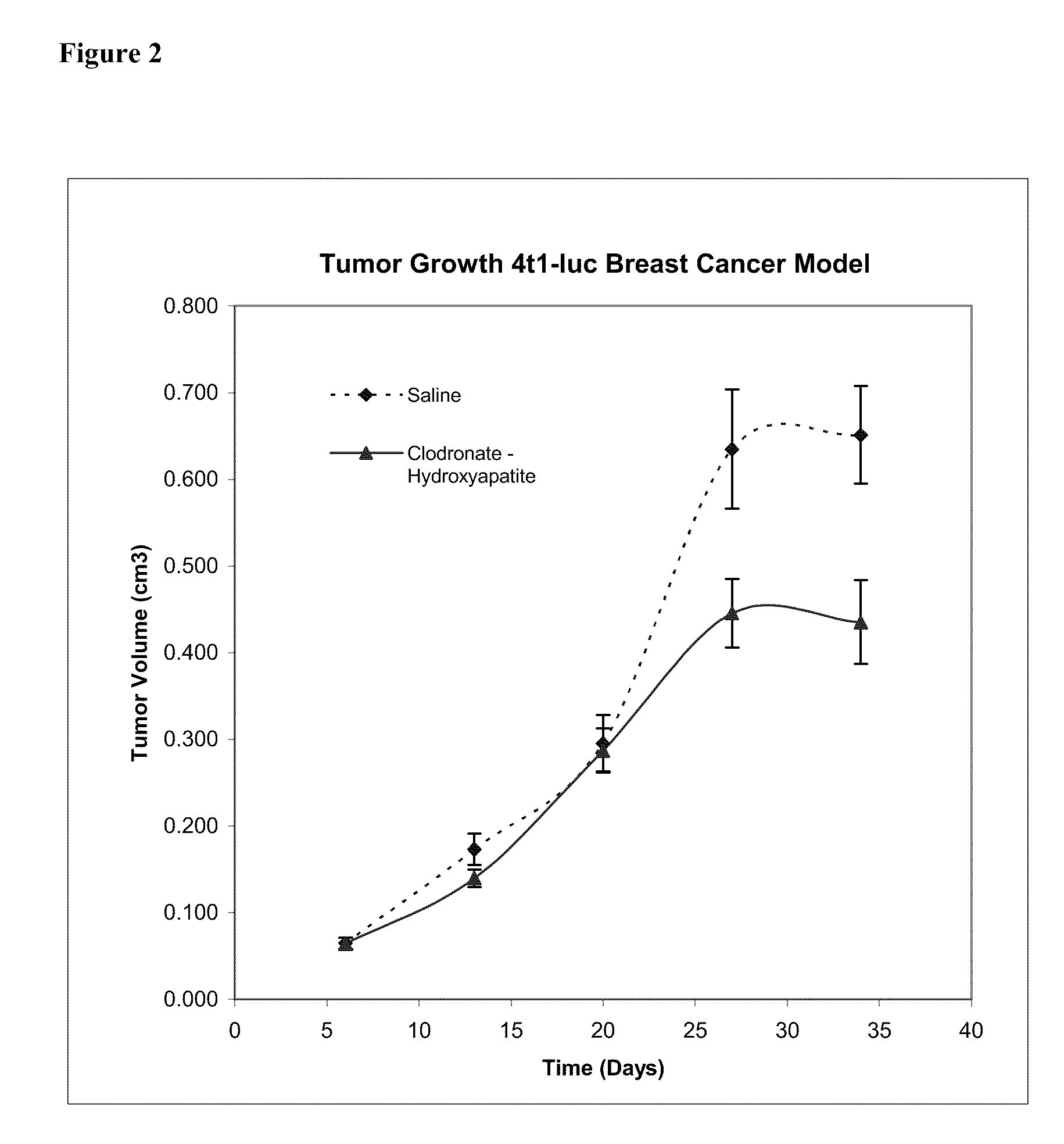
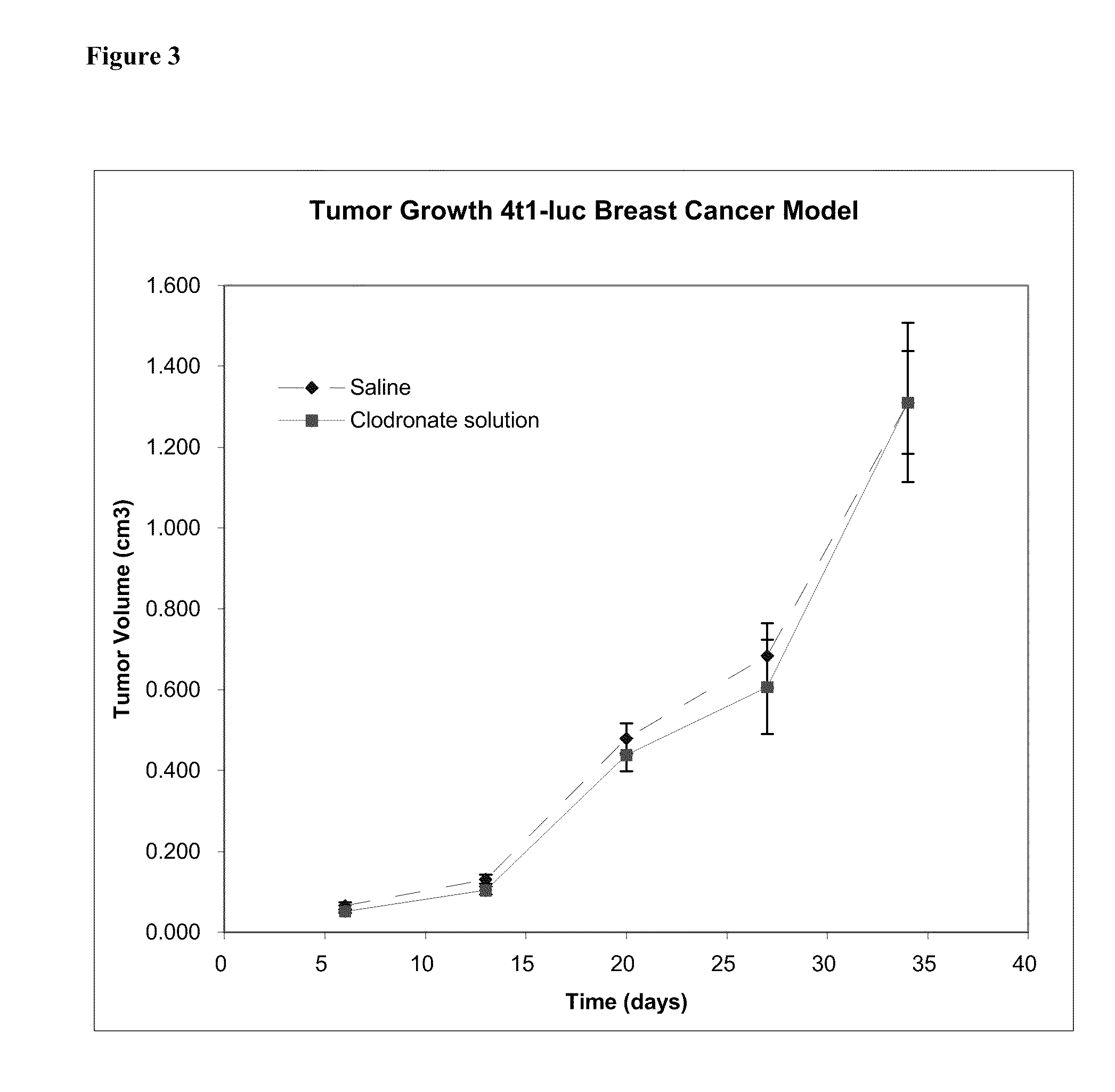
This invention provides pharmaceutical compositions and methods related to the prevention and treatment of primary tumors and metastatic, malignant or spreading cancers by selectively targeting cancer associated myeloid derived cells by the targeted delivery of a bisphosphonate formulated with a non-liposomal particle carrier. In some aspects, the bisphosphonate particles have one or more properties suitable for phagocytosis by cancer associated myeloid derived cells and release of the bisphosphonate within the macrophages. Advantageously, administering the particles to a subject reduces the level and / or activity of cancer associated myeloid derived cells in the subject.
Owner:JOVESIS
Cytotoxicity mediation of cells evidencing surface expression of TROP-2
InactiveUS20080305104A1Reduce the likelihood of problemsReduce the overall heightHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseLymphatic Spread
This invention relates to the staging, diagnosis and treatment of cancerous diseases (both primary tumors and tumor metastases), particularly to the mediation of cytotoxicity of tumor cells; and most particularly to the use of cancerous disease modifying antibodies (CDMAB), optionally in combination with one or more CDMAB / chemotherapeutic agents, as a means for initiating the cytotoxic response. The invention further relates to binding assays, which utilize the CDMAB of the instant invention. The anti-cancer antibodies can be conjugated to toxins, enzymes, radioactive compounds, cytokines, interferons, target or reporter moieties and hematogenous cells.
Owner:F HOFFMANN LA ROCHE & CO AG
Method for the diagnosis, prognosis and treatment of prostate cancer metastasis
InactiveUS20140105918A1Prevents and reduces bone metastasisPrevent and reduce riskOrganic active ingredientsSugar derivativesPrimary tumorLymphatic Spread
The present invention relates to a method for the diagnosis or the prognosis of metastasis in prostate cancer which comprises determining if the c-MAF gene is amplified in a primary tumor sample. Likewise, the invention also relates to a method for the diagnosis or the prognosis of metastasis in prostate cancer, as well as to a method for determining the tendency to develop bone metastasis with respect to metastasis in other organs, which comprise determining the c-MAF expression level. Finally, the invention relates to the use of a c-MAF inhibitor as therapeutic target for treating the prostate cancer.
Owner:INBIOMOTION
N-acyl ureas exhibiting anti-cancer and anti-proliferative activities
Compounds of the present invention find utility in the treatment of mammalian cancers and especially human cancers including, but not limited to, malignant melanomas, solid tumors, glioblastomas, ovarian cancer, pancreatic cancer, prostate cancer, lung cancers, breast cancers, kidney cancers, hepatic cancers, cervical carcinomas, metastasis of primary tumor sites, myeloproliferative diseases, chronic myelogenous leukemia, leukemias, papillary thyroid carcinoma, non-small cell lung cancer, mesothelioma, hypereosinophilic syndrome, gastrointestinal stromal tumors, colonic cancers, ocular diseases characterized by hyperproliferation leading to blindness including various retinopathies, diabetic retinopathy, rheumatoid arthritis, asthma, chronic obstructive pulmonary disease, mastocytosis, mast cell leukemia, and diseases caused by PDGFR-α kinase, PDGFR-β kinase, c-KIT kinase, cFMS kinase, c-MET kinase, and oncogenic forms, aberrant fusion proteins and polymorphs of any of the foregoing kinases.
Owner:DECIPHERA PHARMA LLC
Isolation and use of solid tumor stem cells
InactiveUS20110092378A1Promote resultsPromotes significant proliferationDiagnosticsMicrobiological testing/measurementPrimary tumorImmunodeficient Mouse
A small percentage of cells within an established solid tumor have the properties of stem cells. These solid tumor stem cells give rise to both more tumor stem cells and to the majority of cells in the tumor that have lost the capacity for extensive proliferation and the ability to give rise to new tumors. Thus, solid tumor heterogeneity reflects the presence of tumor cell progeny arising from a solid tumor stem cell.We have developed a xenograft model in which we have been able to establish tumors from primary tumors via injection of tumors in the mammary gland of severely immunodeficient mice. These xenograft assay have allowed us to do biological and molecular assays to characterize clonogenic solid tumor stem cells.We have also developed evidence that strongly implicates the Notch pathway, especially Notch 4, as playing a central pathway in carcinogenesis.
Owner:RGT UNIV OF MICHIGAN
Cancerous disease modifying antibodies
InactiveUS20050191305A1Reduce the likelihood of problemsMicrobiological testing/measurementRadioactive preparation carriersAbnormal tissue growthCancer cell
The present invention relates to a method for producing patient cancerous disease modifying antibodies using a novel paradigm of screening. By segregating the anti-cancer antibodies using cancer cell cytotoxicity as an end point, the process makes possible the production of anti-cancer antibodies for therapeutic and diagnostic purposes. The antibodies can be used in aid of staging and diagnosis of a cancer, and can be used to treat primary tumors and tumor metastases. The anti-cancer antibodies can be conjugated to toxins, enzymes, radioactive compounds, and hematogenous cells.
Owner:F HOFFMANN LA ROCHE & CO AG
Method for the diagnosis, prognosis and treatment of prostate cancer metastasis
ActiveUS20150362495A1Prevent and reduce riskReduce and preventBiocideLibrary screeningPrimary tumorLymphatic Spread
The present invention relates to a method for the diagnosis or the prognosis of metastasis in prostate cancer which comprises determining if the c-MAF gene is amplified in a primary tumor sample. Likewise, the invention also relates to a method for the diagnosis or the prognosis of metastasis in prostate cancer, as well as to a method for determining the tendency to develop bone metastasis with respect to metastasis in other organs, which, comprise determining the c-MAF expression level. Finally, the invention relates to the use of a c-MAF inhibitor as therapeutic target for treating the prostate cancer.
Owner:INBIOMOTION
Isolation and use of solid tumor stem cells
InactiveUS20080194022A1Promote resultsPromotes significant proliferationDiagnosticsBiological testingPrimary tumorImmunodeficient Mouse
A small percentage of cells within an established solid tumor have the properties of stem cells. These solid tumor stem cells give rise both to more tumor stem cells and to the majority of cells in the tumor that have lost the capacity for extensive proliferation and the ability to give rise to new tumors. Thus, solid tumor heterogeneity reflects the presence of tumor cell progeny arising from a solid tumor stem cell.We have developed a xenograft model in which we have been able to establish tumors from primary tumors via injection of tumors in the mammary gland of severely immunodeficient mice. These xenograft assay have allowed us to do biological and molecular assays to characterize clonogenic solid tumor stem cells.We have also developed evidence that strongly implicates the Notch pathway, especially Notch 4, as playing a central pathway in carcinogenesis.
Owner:RGT UNIV OF MICHIGAN
Method for the diagnosis, prognosis and treatment of lung cancer metastasis
ActiveUS20150152506A1Valuable informationOrganic active ingredientsLibrary screeningPrimary tumorLymphatic Spread
The present invention relates to a method for the diagnosis or the prognosis of metastasis in lung cancer which comprises determining if the c-MAF gene is amplified in a primary tumor sample. Likewise, the invention also relates to a method for the diagnosis or the prognosis of metastasis in lung cancer, as well as to a method for determining the tendency to develop bone metastasis with respect to metastasis in other organs, which comprise determining the c-MAF gene expression level. Finally, the invention relates to the use of a c-MAF inhibitor as therapeutic target for treating the lung cancer.
Owner:INSTITUCIO CATALANA DE RECERCA I ESTUDIS AVANCATS +1
Method for the Prognosis and Treatment of Renal Cell Carcinoma Metastasis
The present invention relates to a method for the prognosis of bone metastasis in renal cell carcinoma which comprises determining if the c-MAF gene is amplified in a primary tumor sample. Likewise, the invention also relates to a method for determining the tendency to develop bone metastasis with respect to metastasis in other organs, which comprise determining the c-MAF gene expression level, amplification or translocation. The invention also relates to a method for predicting early bone metastasis in a subject suffering renal cell carcinoma. The invention also relates to a c-MAF inhibitor as therapeutic agent for use in the treatment of renal cell carcinoma metastasis. The invention relates to kits for predicting bone metastasis and predicting the clinical outcome of a subject suffering from bone metastasis. Finally, the invention relates to a method for typing of a subject suffering renal cell carcinoma and for classifying a subject from renal cell carcinoma into a cohort.
Owner:INBIOMOTION
Individualized vaccines for cancer
ActiveUS10738355B2Reduces steric hindranceImprove translationVaccinesPharmaceutical delivery mechanismPrimary tumorTumour metastasis
The present invention relates to the provision of vaccines which are specific for a patient's tumor and are potentially useful for immunotherapy of the primary tumor as well as tumor metastases. In one aspect, the present invention relates to a method for providing an individualized cancer vaccine comprising the steps: (a) identifying cancer specific somatic mutations in a tumor specimen of a cancer patient to provide a cancer mutation signature of the patient; and (b) providing a vaccine featuring the cancer mutation signature obtained in step (a). In a further aspect, the present invention relates to vaccines which are obtainable by said method.
Owner:TRANSLATIONALE ONKOLOGIE AN DER UNIVSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GGMBH +1
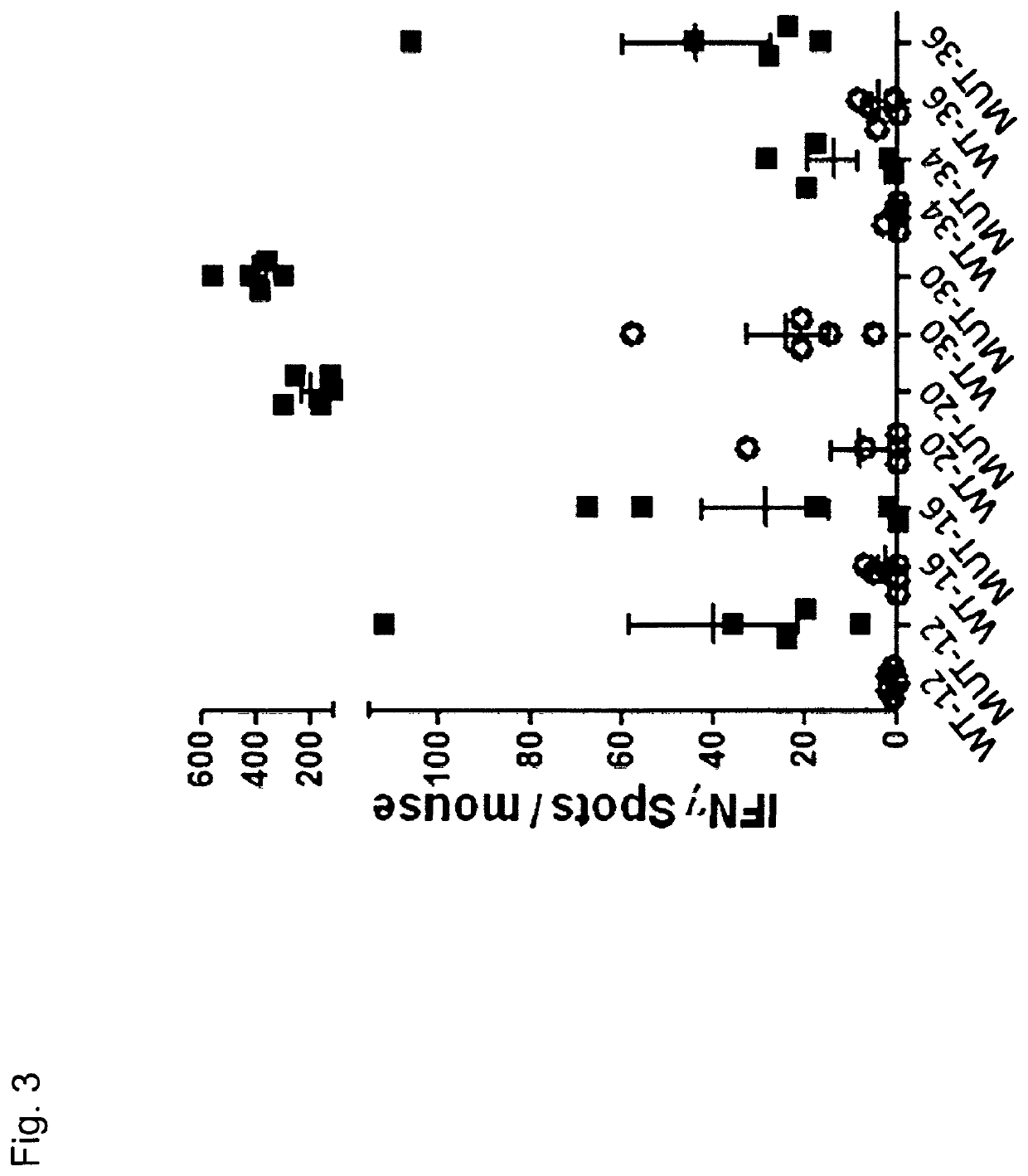
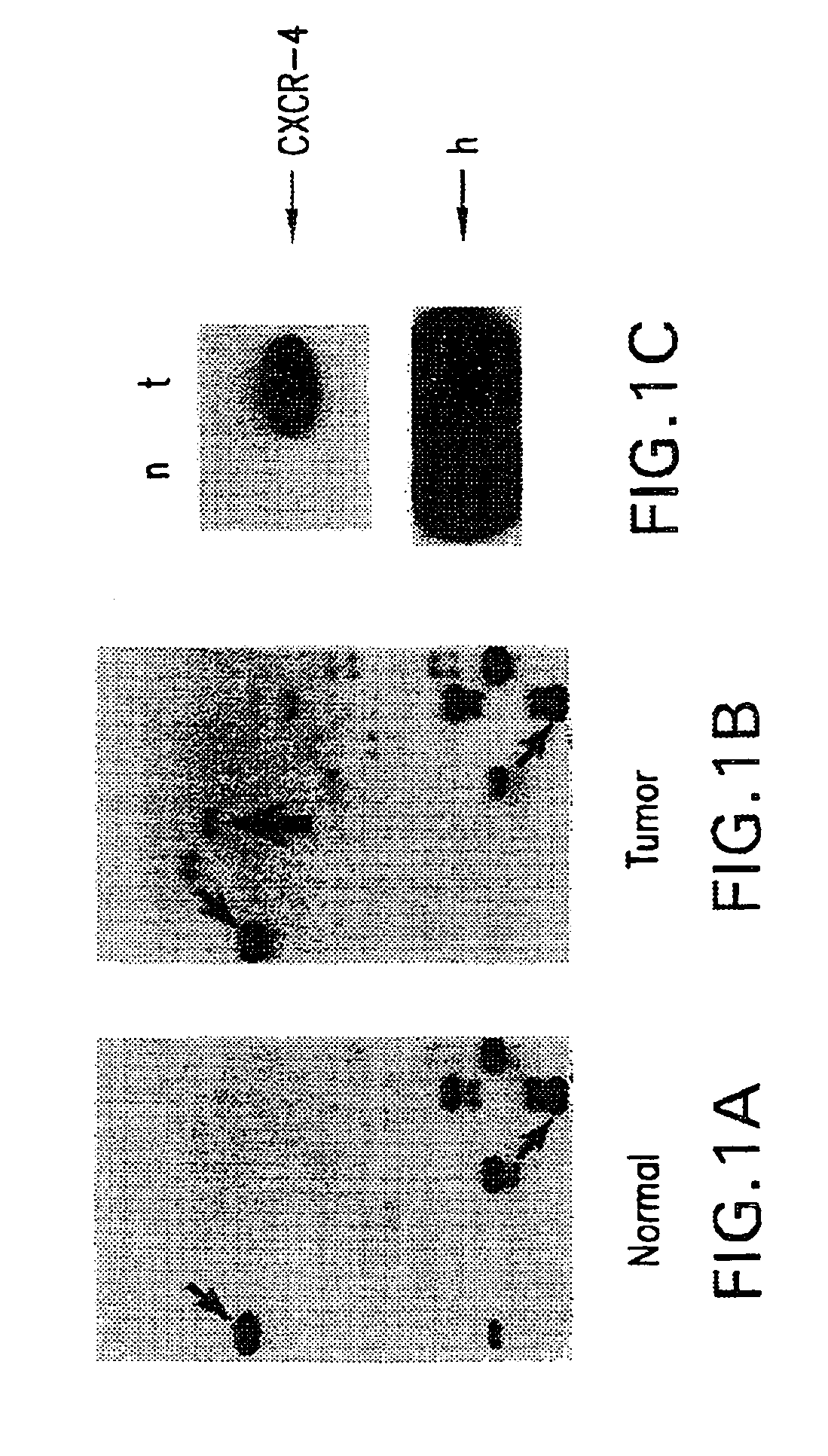
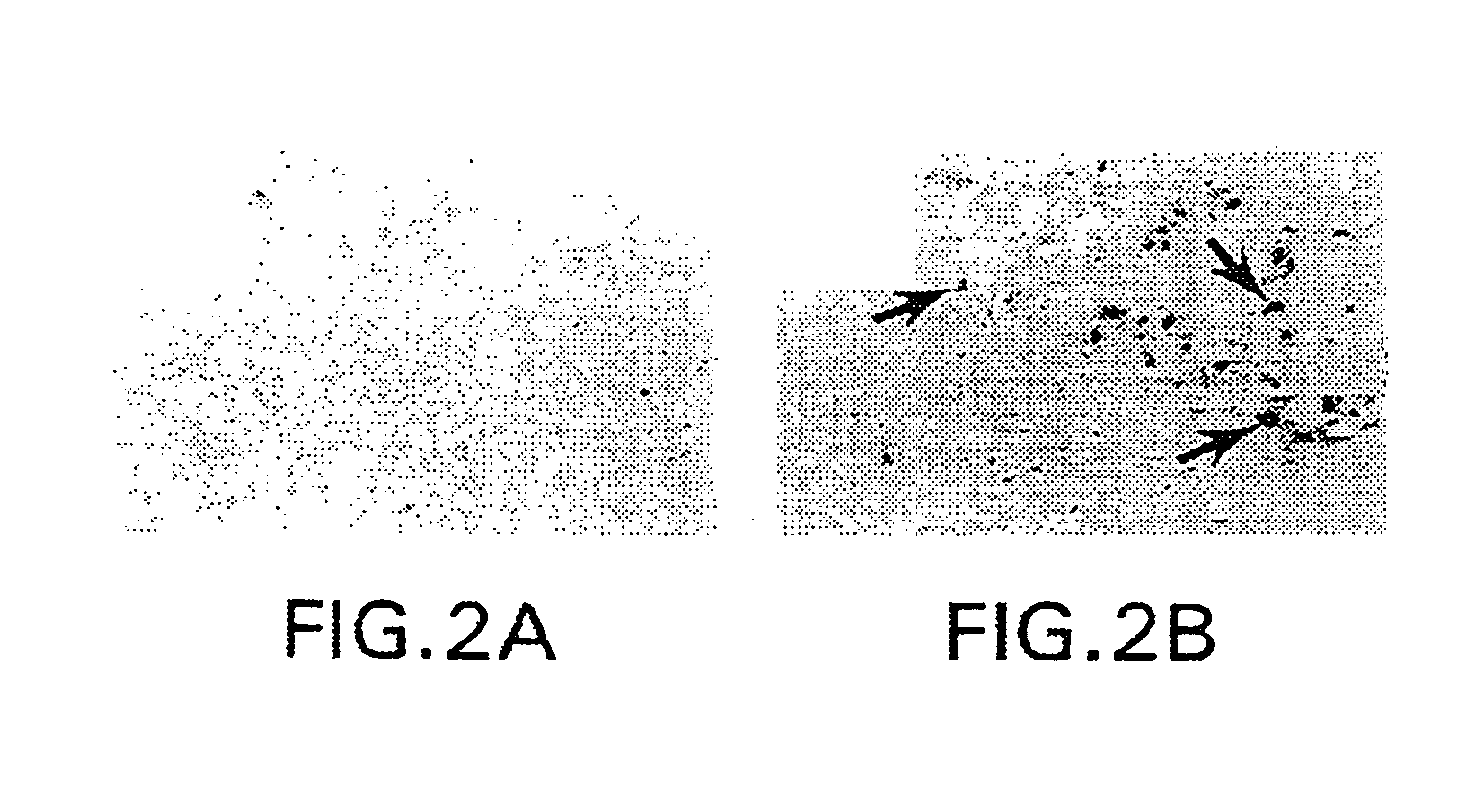
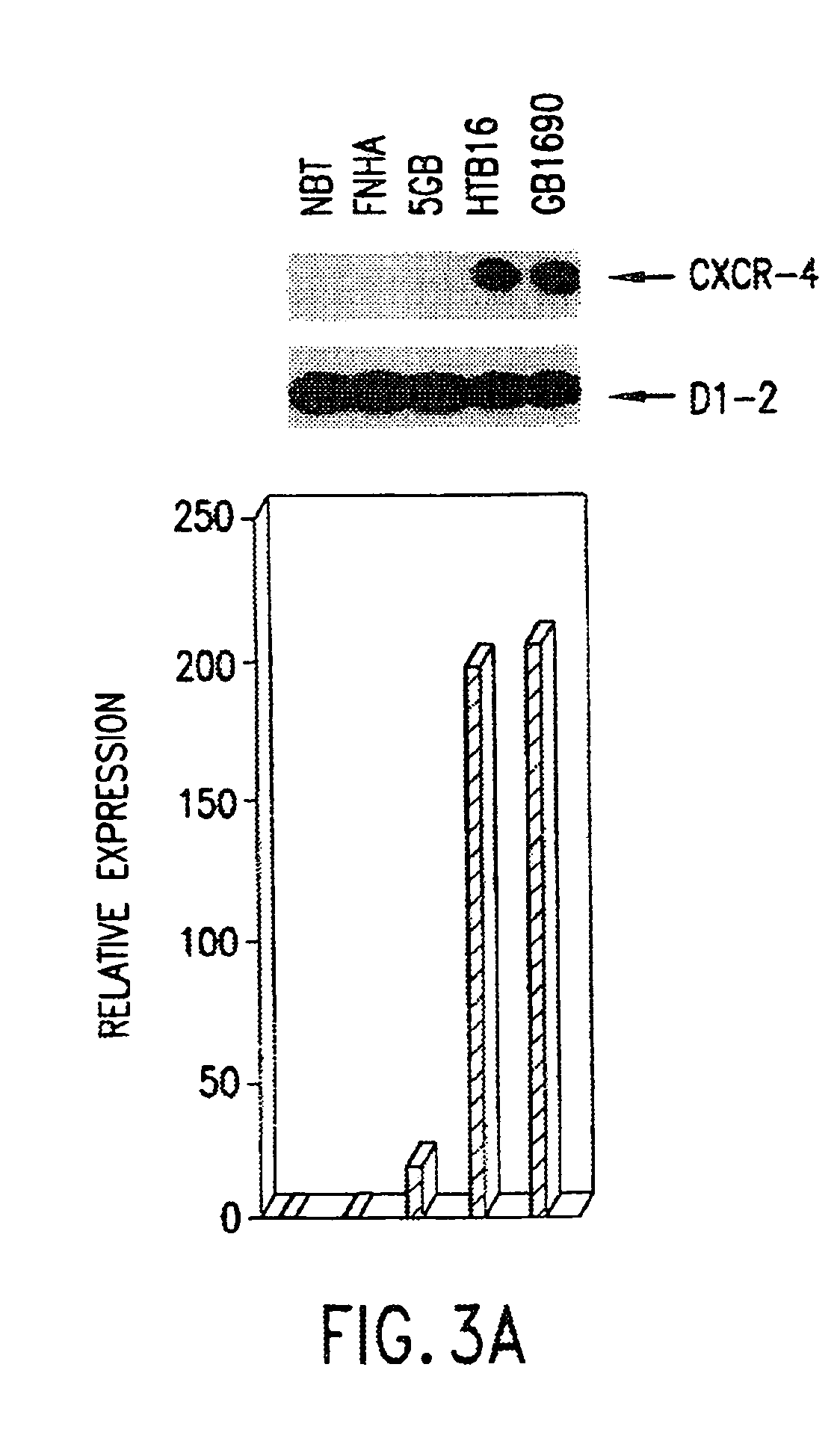
Therapeutic and diagnostic applications based on the role of the CXCR-4 gene in tumorigenesis
InactiveUS6863887B1Peptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsPrimary tumorPrimary tumor site
The present invention relates to the identification of a novel role of CXCR-4 in cell transformation and aberrant cellular proliferation. In particular, the present invention relates to the altered gene expression of CXCR-4 in a number of primary tumors and cell lines derived from tumors, in addition to, the altered gene expression of ligands for CXCR-4. Further, the present invention relates, in part, to the Applicants' surprising discovery that the inhibition of CXCR-4 gene expression or the inhibition of CXCR-4 activity in transformed cells reverses the transformed phenotype.
Owner:NORTHWEST BIOTHERAPEUTICS INC
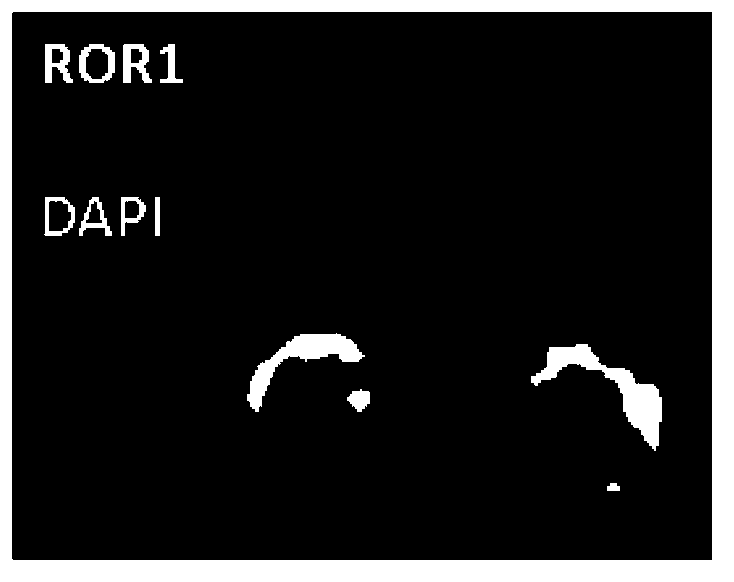
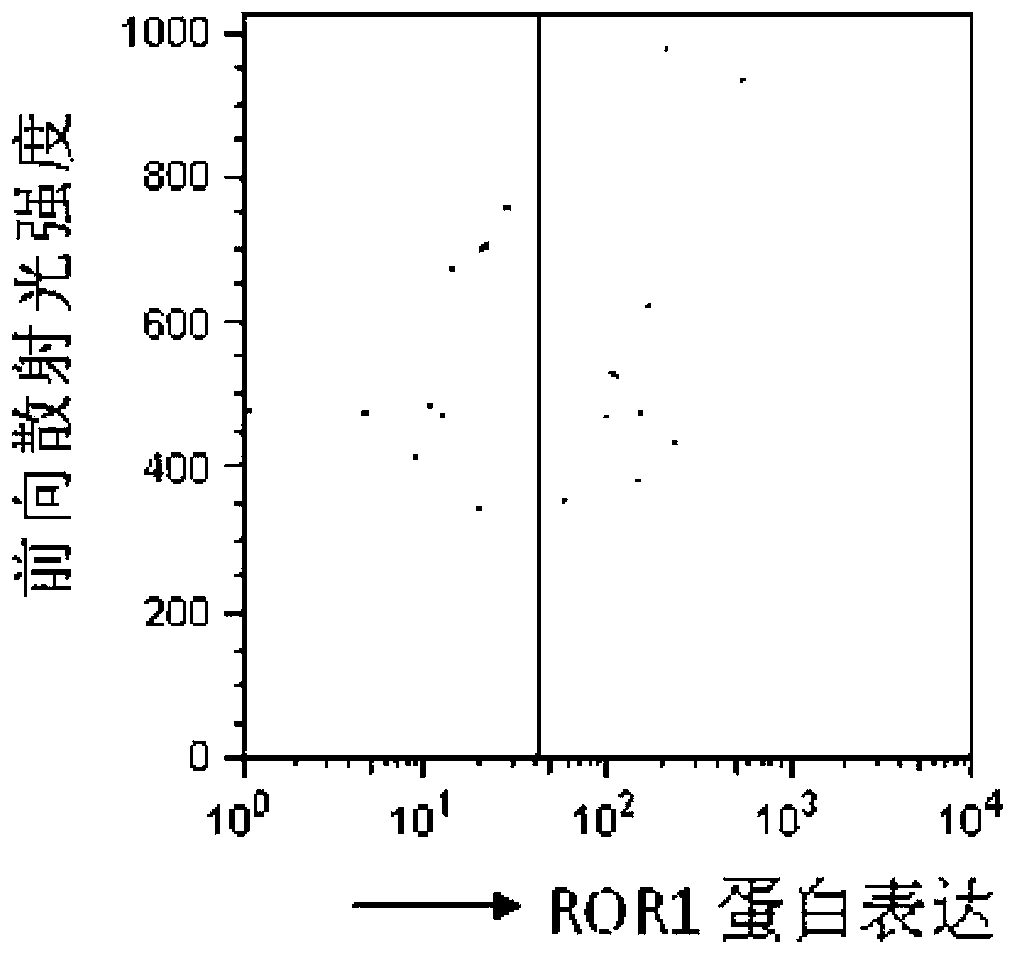
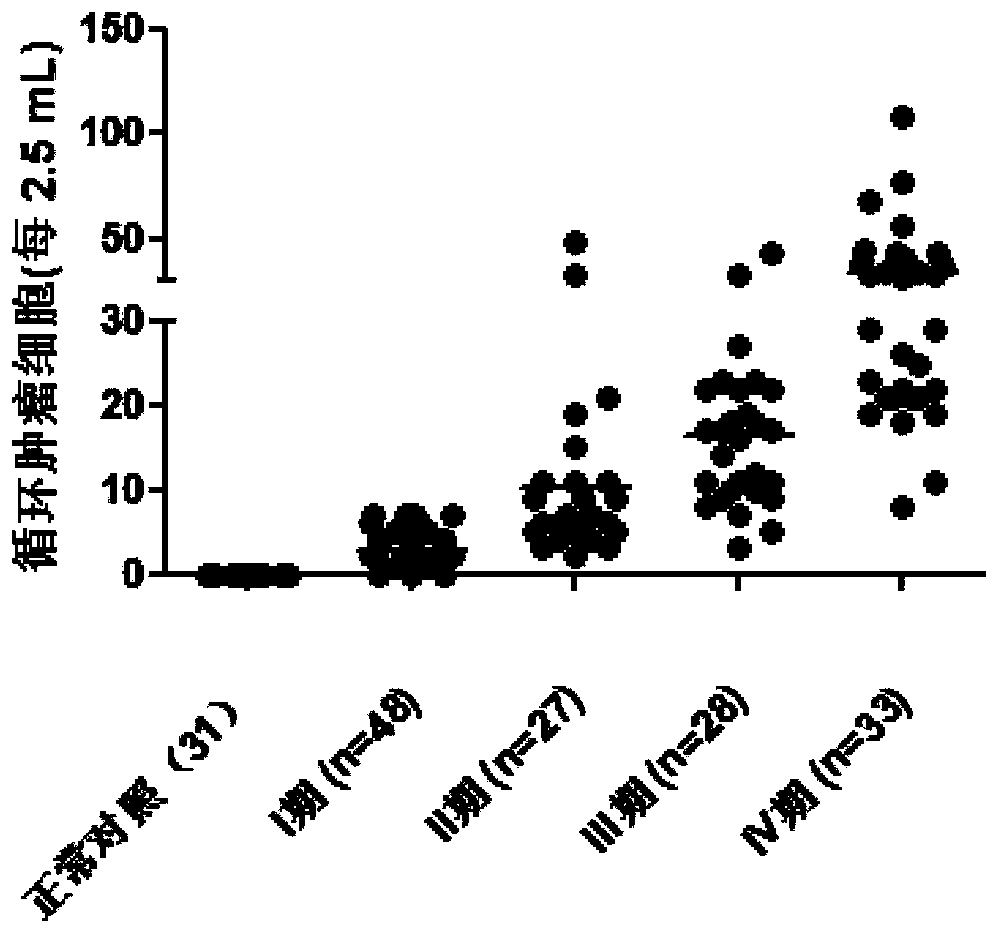
Reagent for detection of ROR1 protein of circulating tumor cells in peripheral blood and application thereof
The invention discloses a reagent for detection of an ROR1 protein in circulating blood and an application thereof, and particularly discloses the reagent for the detection of the ROR1 protein of circulating tumor cells in peripheral blood. The reagent includes an ROR1 antibody, a buffer solution, a marker, a nuclear dye and a lymphocyte identification antibody. A characteristic that ROR1 is only expressed in cancer cells and is not expressed in normal adult tissue cells is utilized, and the reagent is applied to detect the expressed ROR1 protein of the circulating tumor cells or tumor stem cells in the circulating blood, wherein the circulating tumor cells or the tumor stem cells fall off from a primary tumor nidus into the blood; limitations of a traditional method are overcome, and the accuracy and the reliability of the detection of the circulating tumor cells are greatly improved. The reagent can be used for helping detect the tumor cells in the circulating blood of a tumor patient, and is a potential breakthrough point for tumor disease diagnosis, evaluation of therapeutic effects and cancer metastasis monitoring.
Owner:益杰立科(上海)生物科技有限公司
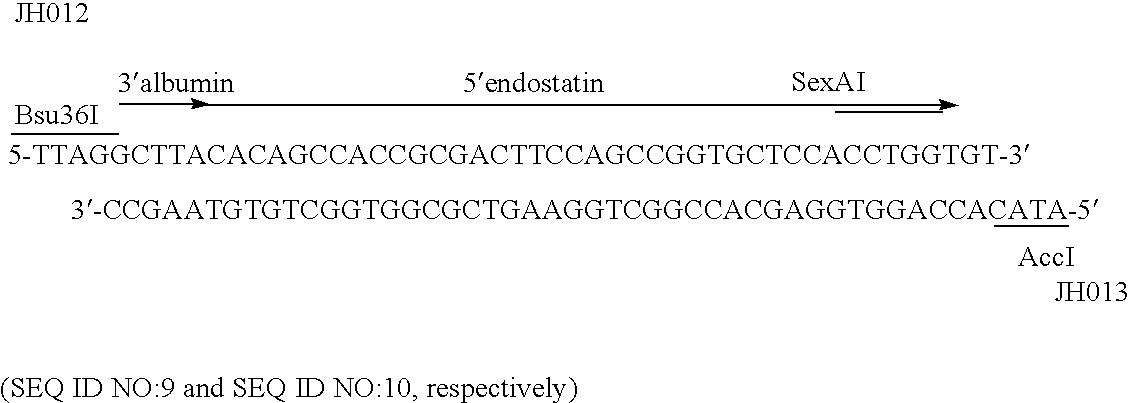
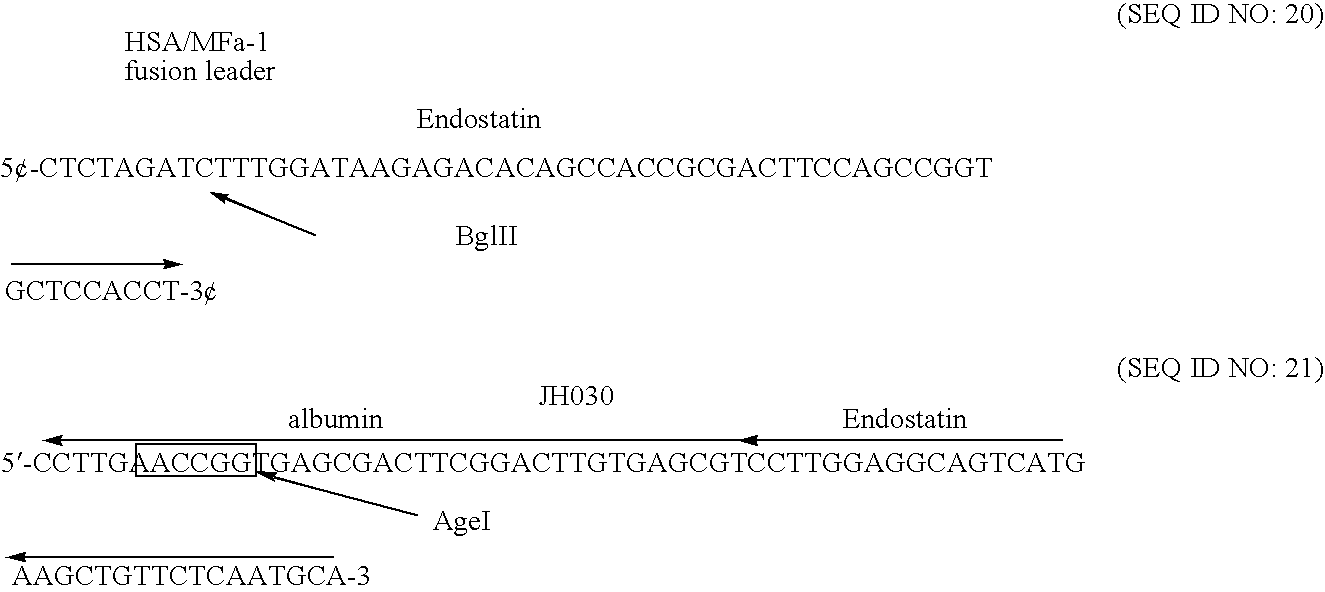
Albumin-fused anti-angiogenesis peptides
InactiveUS20060122374A1High activityExtended half-lifeOrganic active ingredientsFungiAbnormal tissue growthLymphatic Spread
The invention relates to proteins comprising angiogenesis inhibiting peptides, such as endostatin peptides (including, but not limited to, fragments and variants thereof), which exhibit anti-retroviral activity, fused or conjugated to albumin (including, but not limited to fragments or variants of albumin). These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins exhibit extended shelf-life and / or extended or therapeutic activity in solution. The invention encompasses therapeutic albumin fusion proteins, compositions, pharmaceutical compositions, formulations and kits. The invention also encompasses nucleic acid molecules encoding the albumin fusion proteins of the invention, as well as vectors containing these nucleic acuds, host cells transformed with these nucleic acids and vectors, and methods of making the albumin fusion proteins of the invention using these nucleic acids, vectors, and / or host cells. The invention also relates to compositions and methods for inhibiting proliferation of vascular endothelial cells and tumor aniogenesis induced cell fusion. The invention further relates to compositions and methods preventing growth of, or promoting regression of, primary tumors and metastases; and for treating cancer, diabetic retinophathy, progressive macular degeneration or rheumatoid arthritis.
Owner:NOVOZYMES BIOPHARMA DK AS
In-vitro individualized medicine test method for lung cancer and culture medium
InactiveCN107151645AProlong survival timeLow toxicityCell dissociation methodsCompound screeningProcess systemsIndividualized treatment
The invention discloses an in-vitro individualized medicine test method for lung cancer and a culture medium, and belongs to the field of cell biology and pharmacology. The in-vitro individualized medicine test method and the culture medium have the advantages that the culture medium M can be applied to normal epithelial cells and primary tumor cells of human or mammals, accordingly, in-vitro culture systems for tumor cells and para-carcinoma cells of lung cancer patients can be established, and primary cells, with continuous passage and quick proliferation functions, of patient individuals can be obtained; the culture systems can be used for detecting the sensitivity and the safety of medicines or combination groups of the medicines, and accordingly stable, accurate and reliable lung cancer individualized treatment-medicine sensitivity test standard detection process systems can be established; individualized sensitive chemotherapy medicines or compositions screened by the aid of the lung cancer individualized treatment-medicine sensitivity test standard detection process systems can be combined with clinical medicine or relevant subjects, accordingly, clinical individualized treatment schemes can be formulated, and the in-vitro individualized medicine test method and the culture medium can ultimately serve for clinical application.
Owner:SHENZHEN RES INST OF WUHAN UNIVERISTY
Cancerous disease modifying antibodies
InactiveUS7009040B2Reduce the likelihood of problemsHybrid cell preparationImmunoglobulins against cell receptors/antigens/surface-determinantsPrimary tumorLymphatic Spread
The present invention relates to a method for producing patient cancerous disease modifying antibodies using a novel paradigm of screening. By segregating the anti-cancer antibodies using cancer cell cytotoxicity as an end point, the process makes possible the production of anti-cancer antibodies for therapeutic and diagnostic purposes. The antibodies can be used in aid of staging and diagnosis of a cancer, and can be used to treat primary tumors and tumor metastases. The anti-cancer antibodies can be conjugated to toxins, enzymes, radioactive compounds, and hematogenous cells.
Owner:F HOFFMANN LA ROCHE & CO AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com