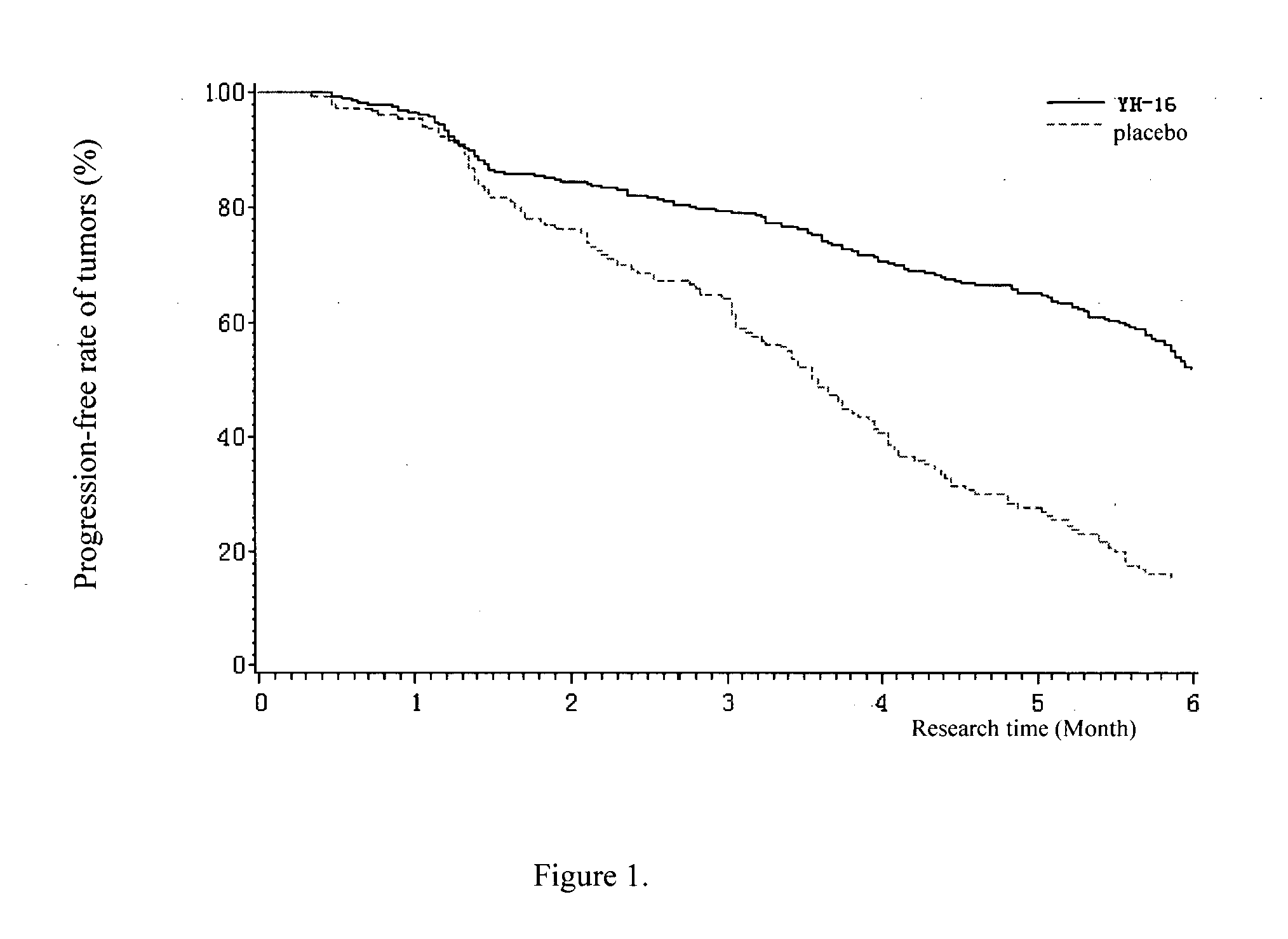
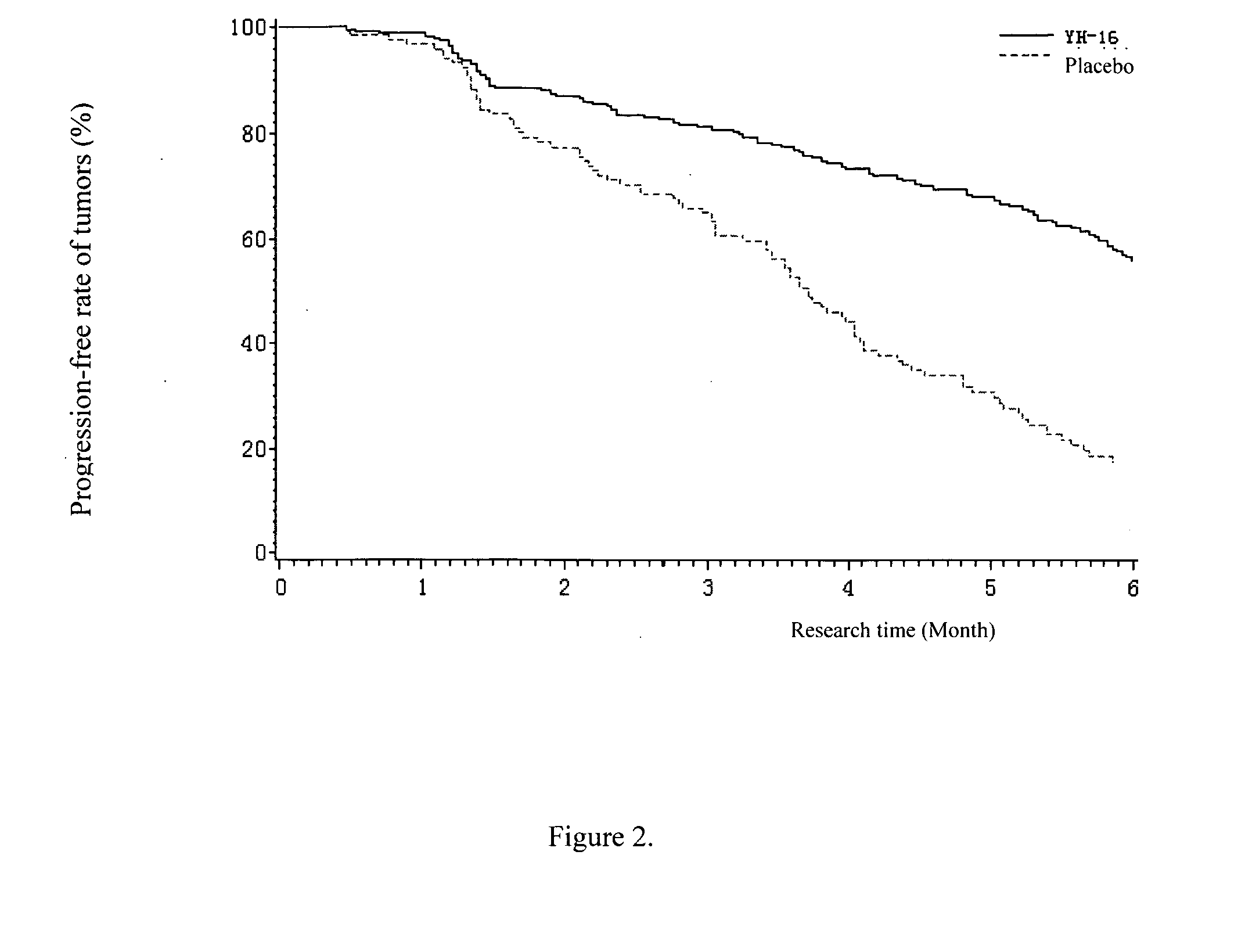
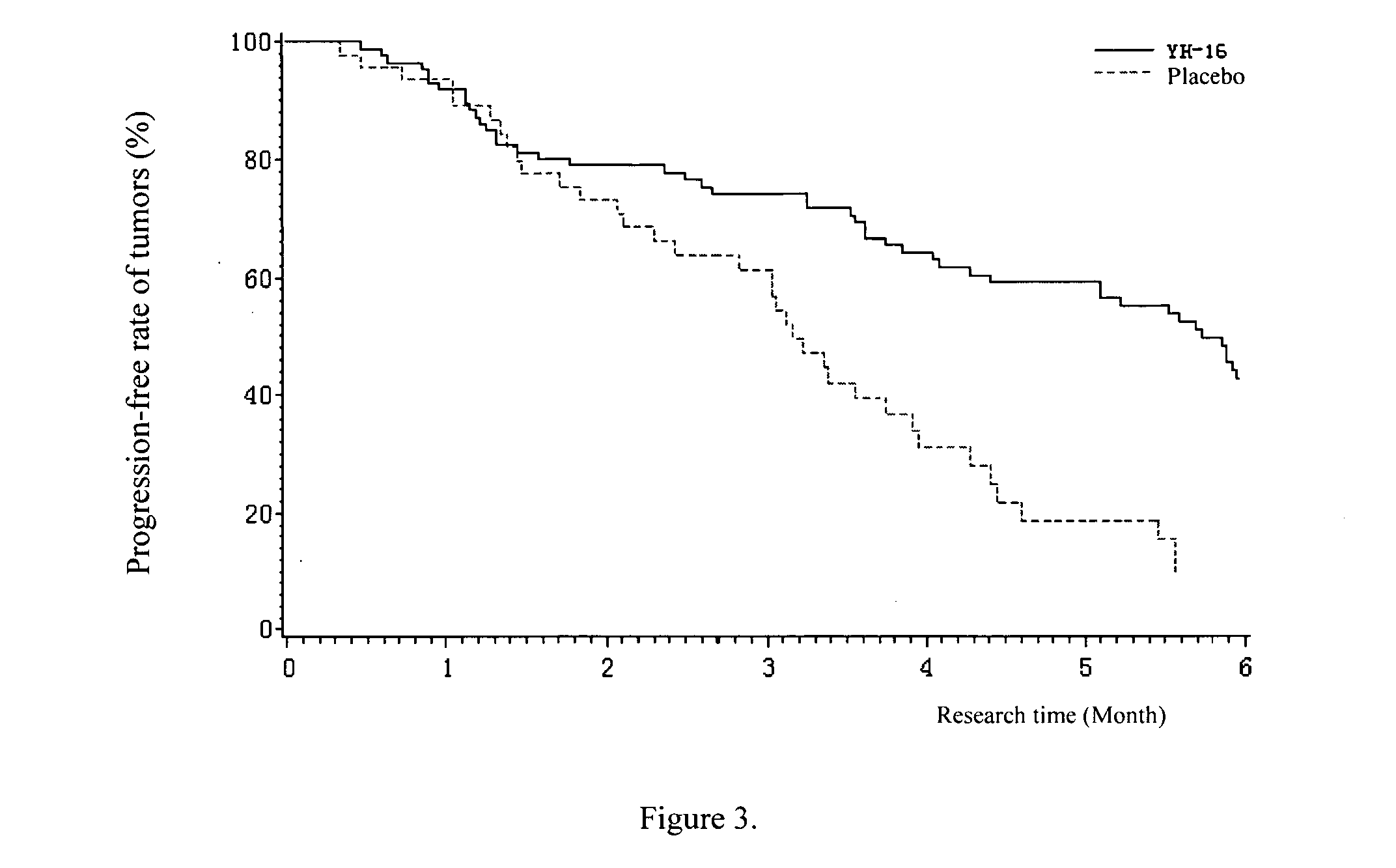
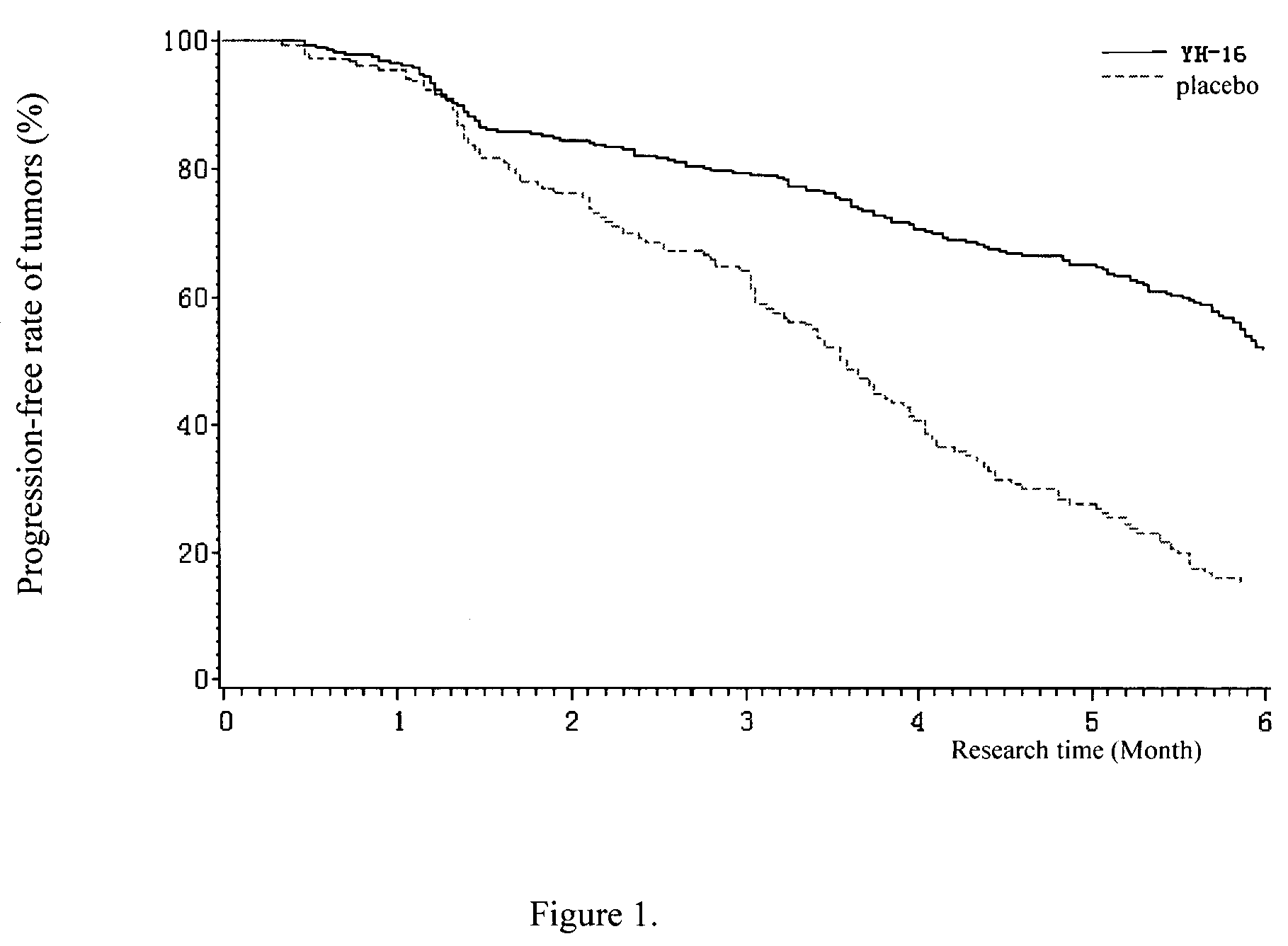
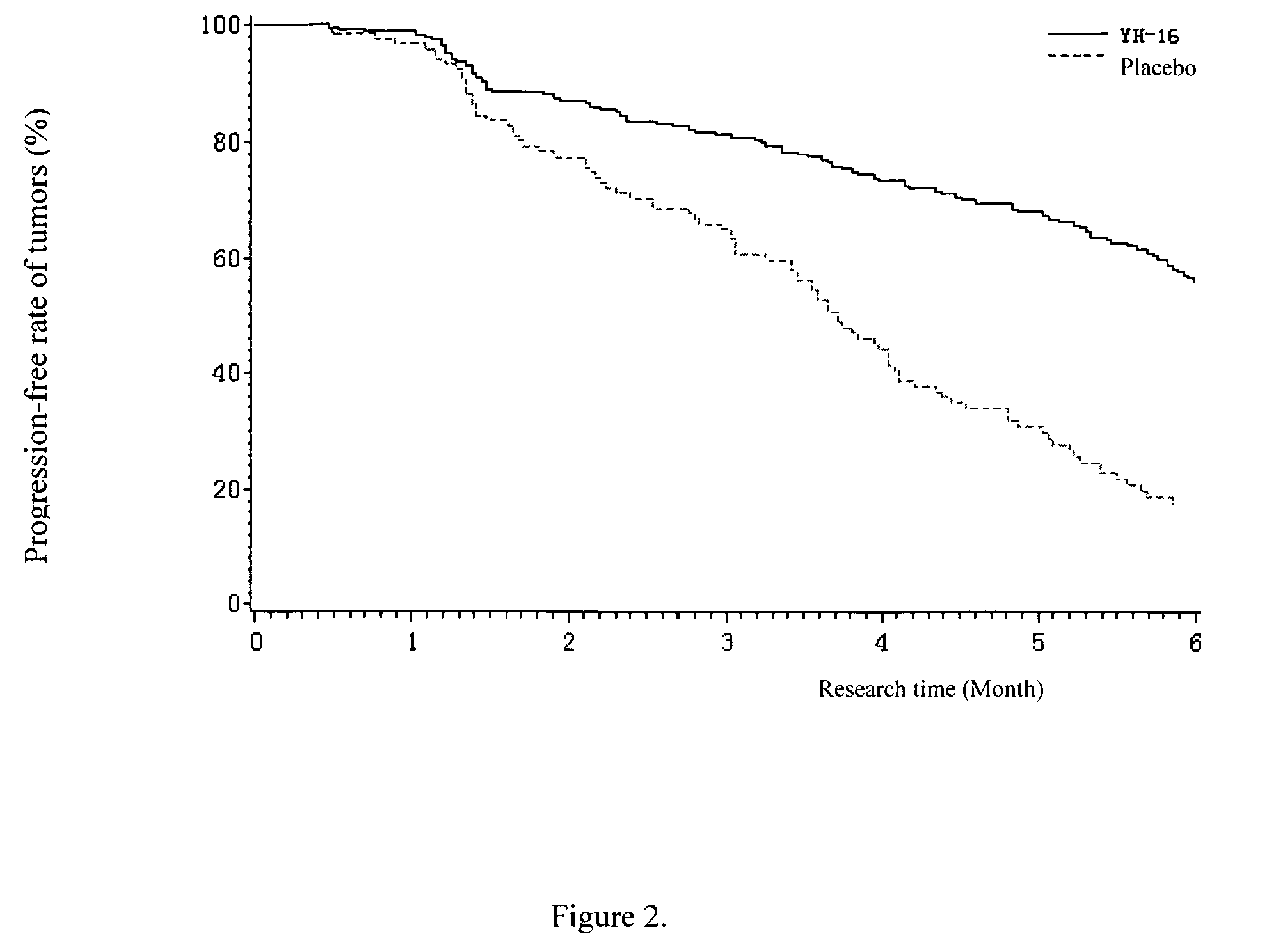
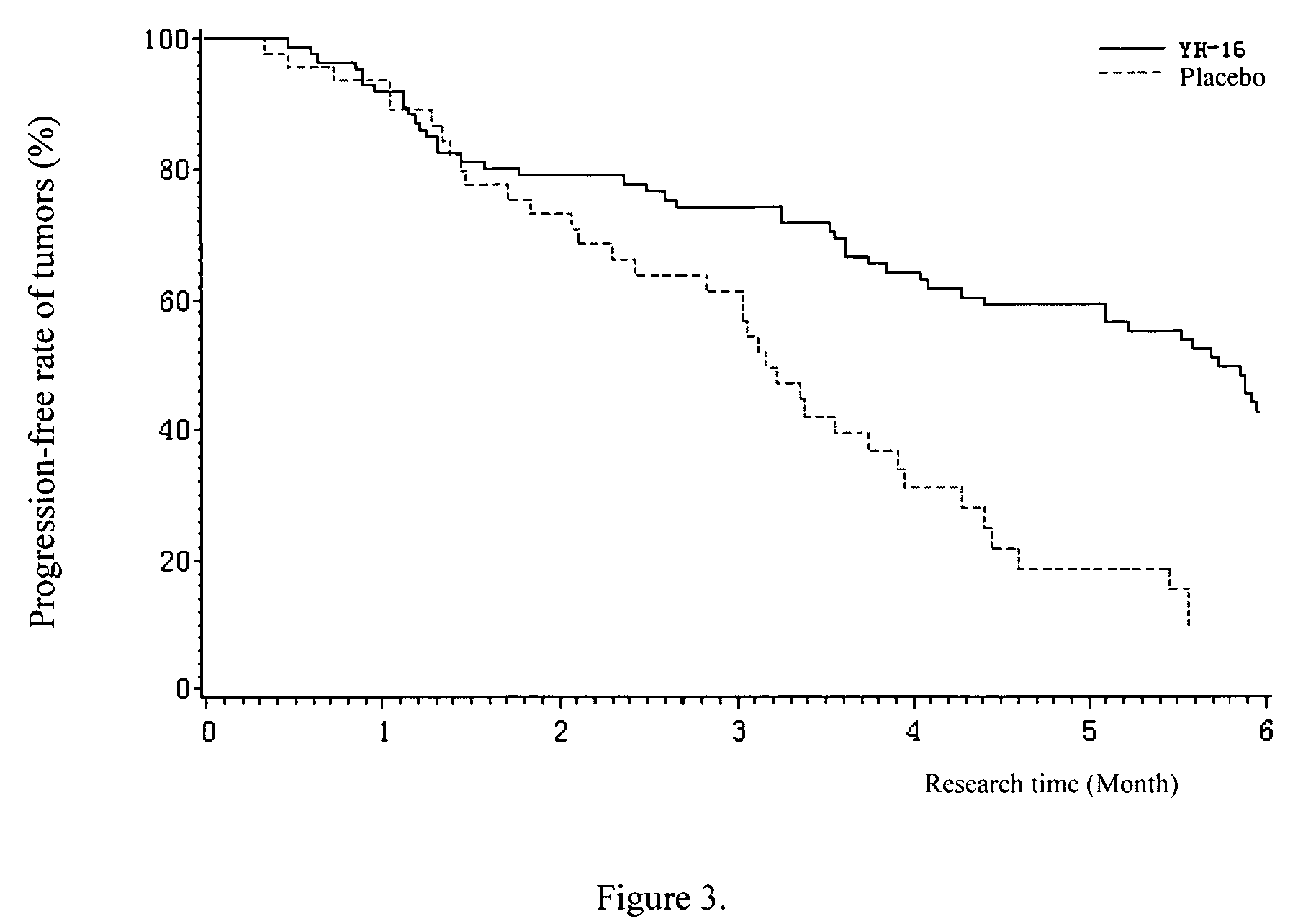
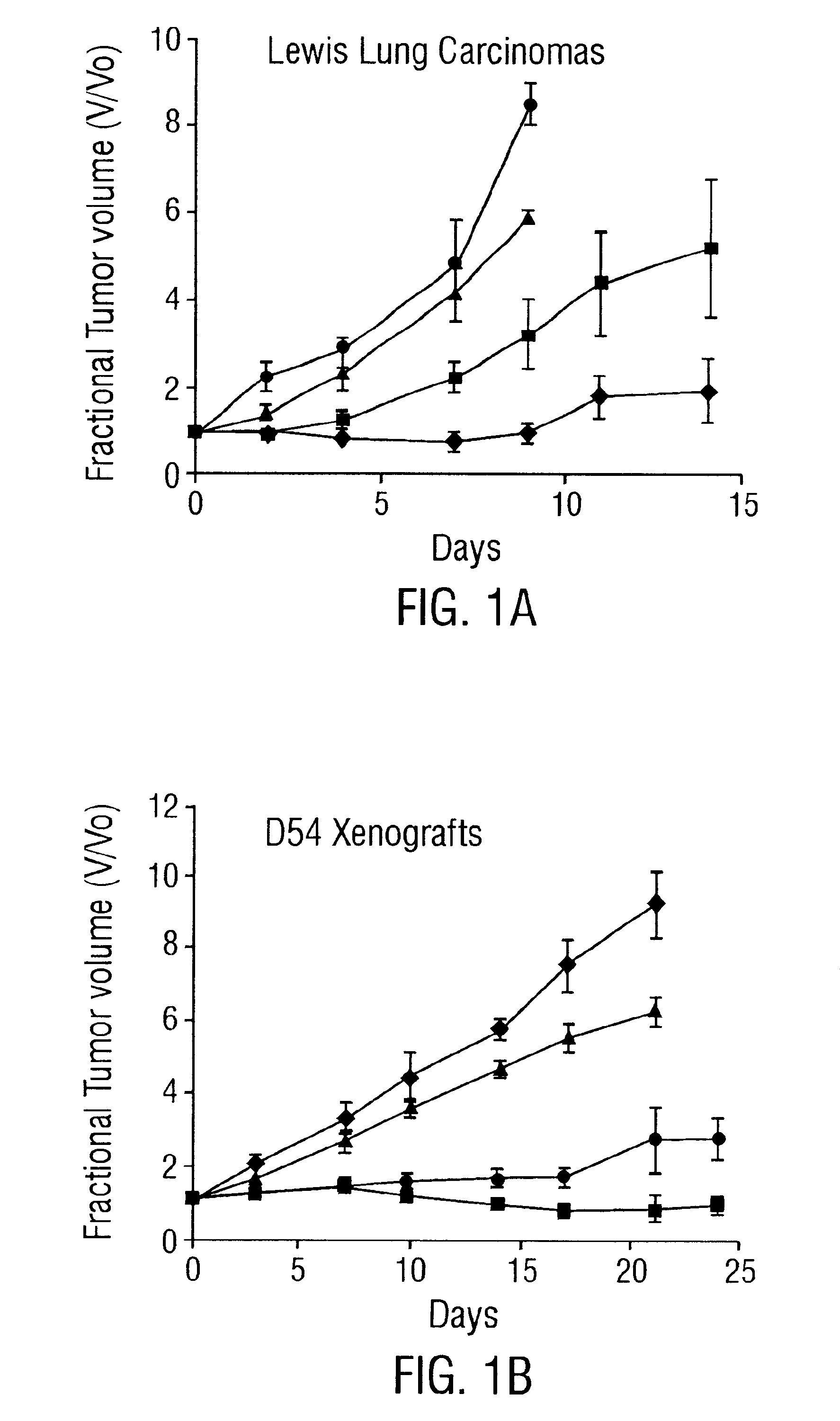
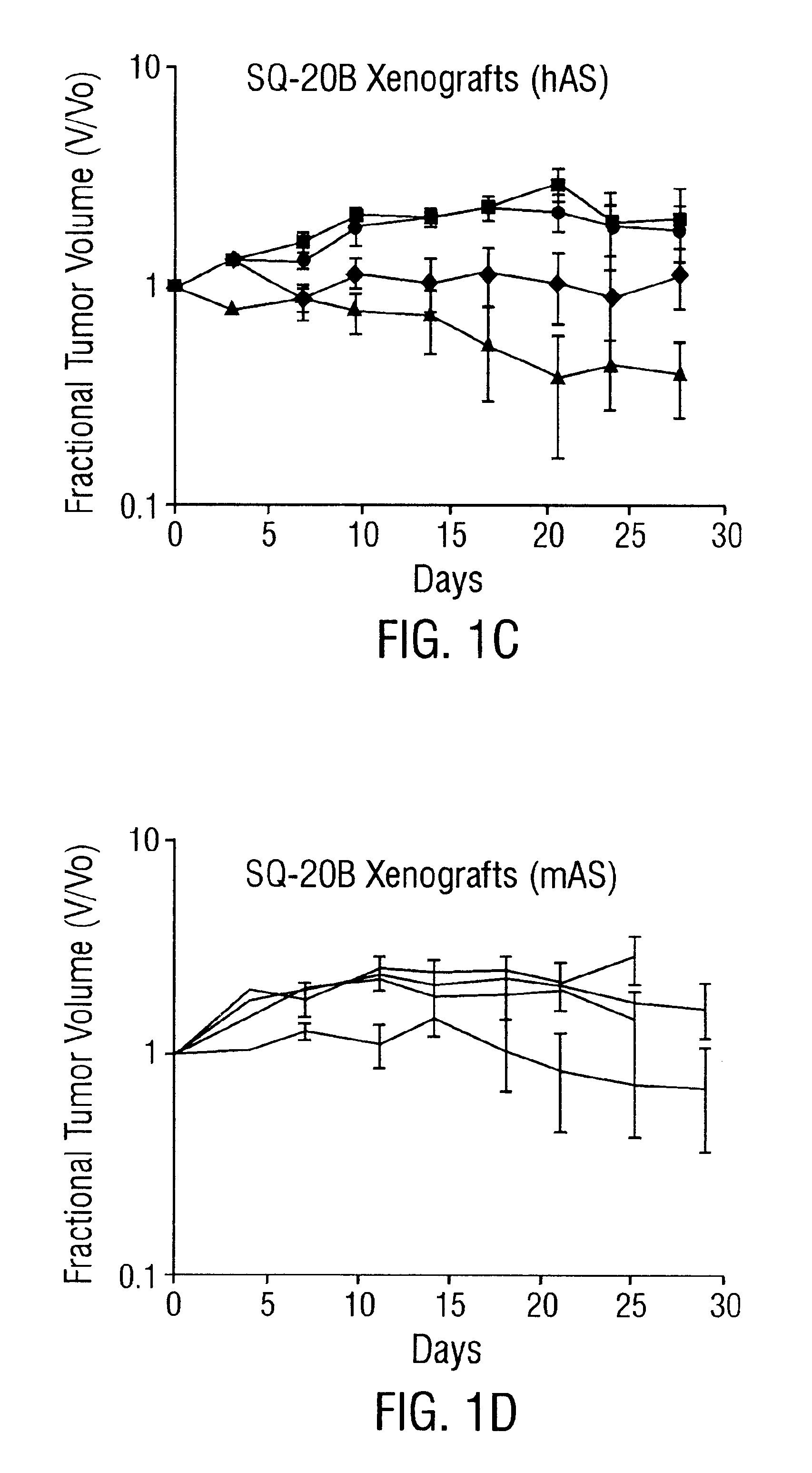
Patents
Literature
103 results about "Endostatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
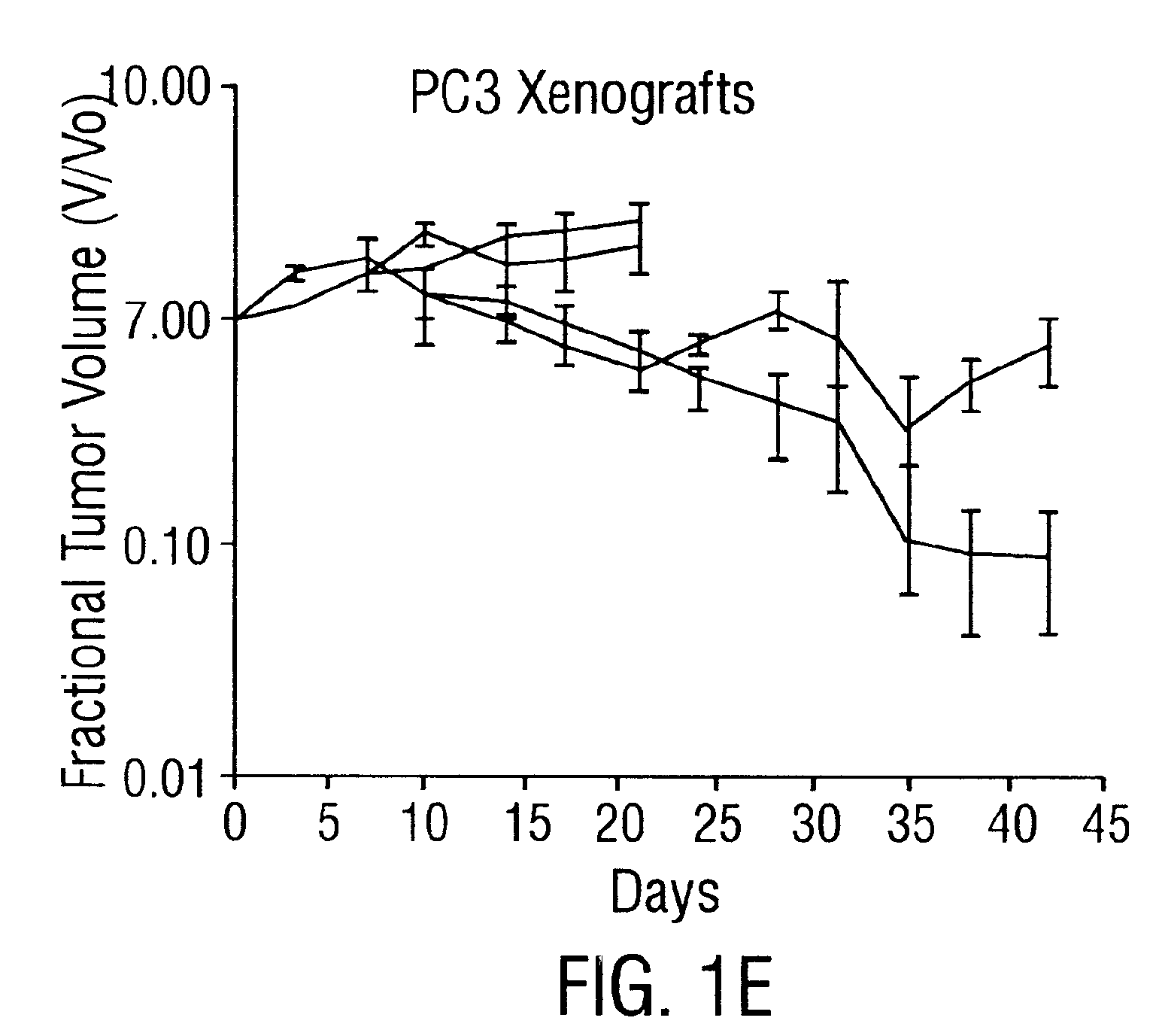
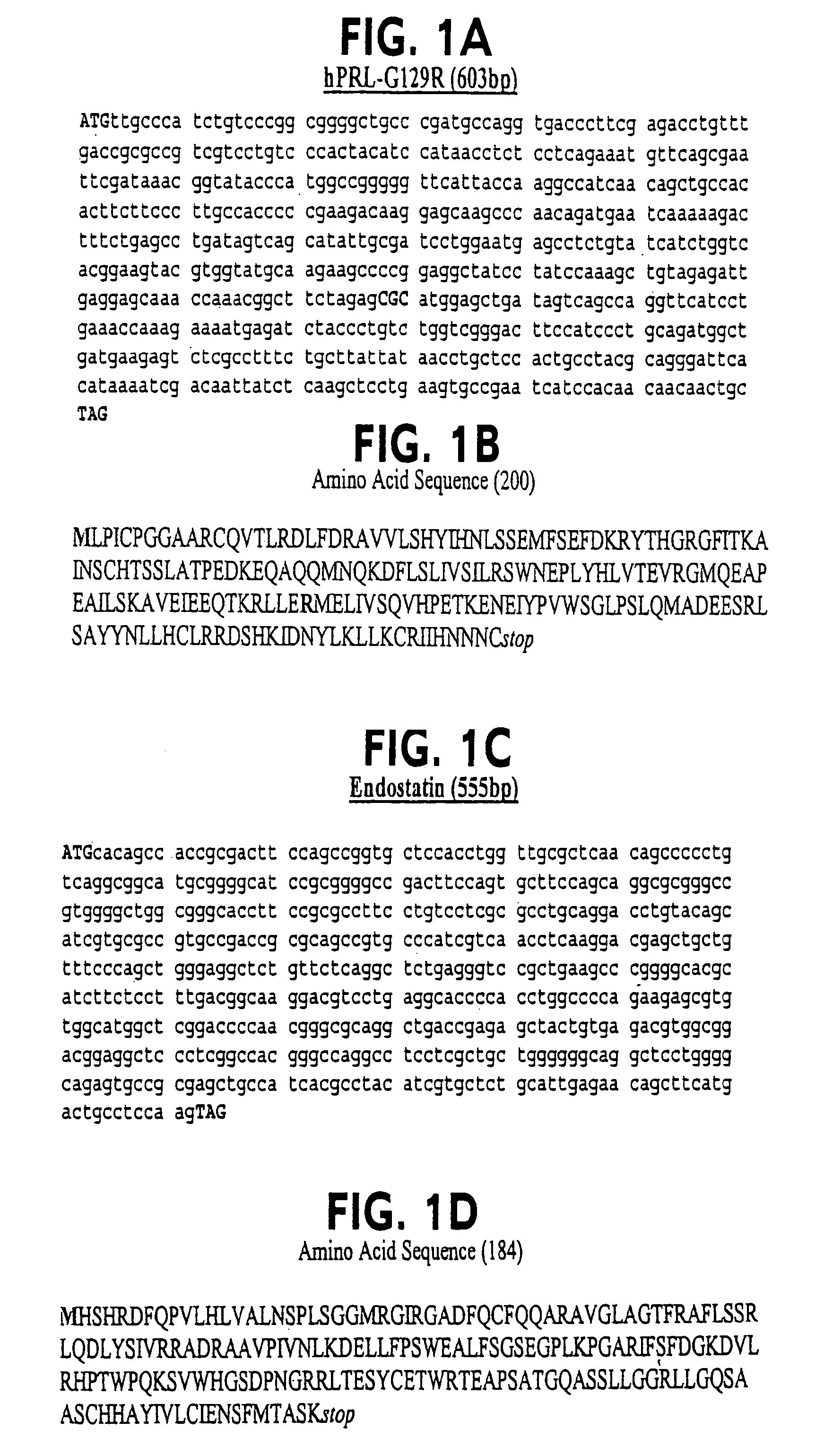
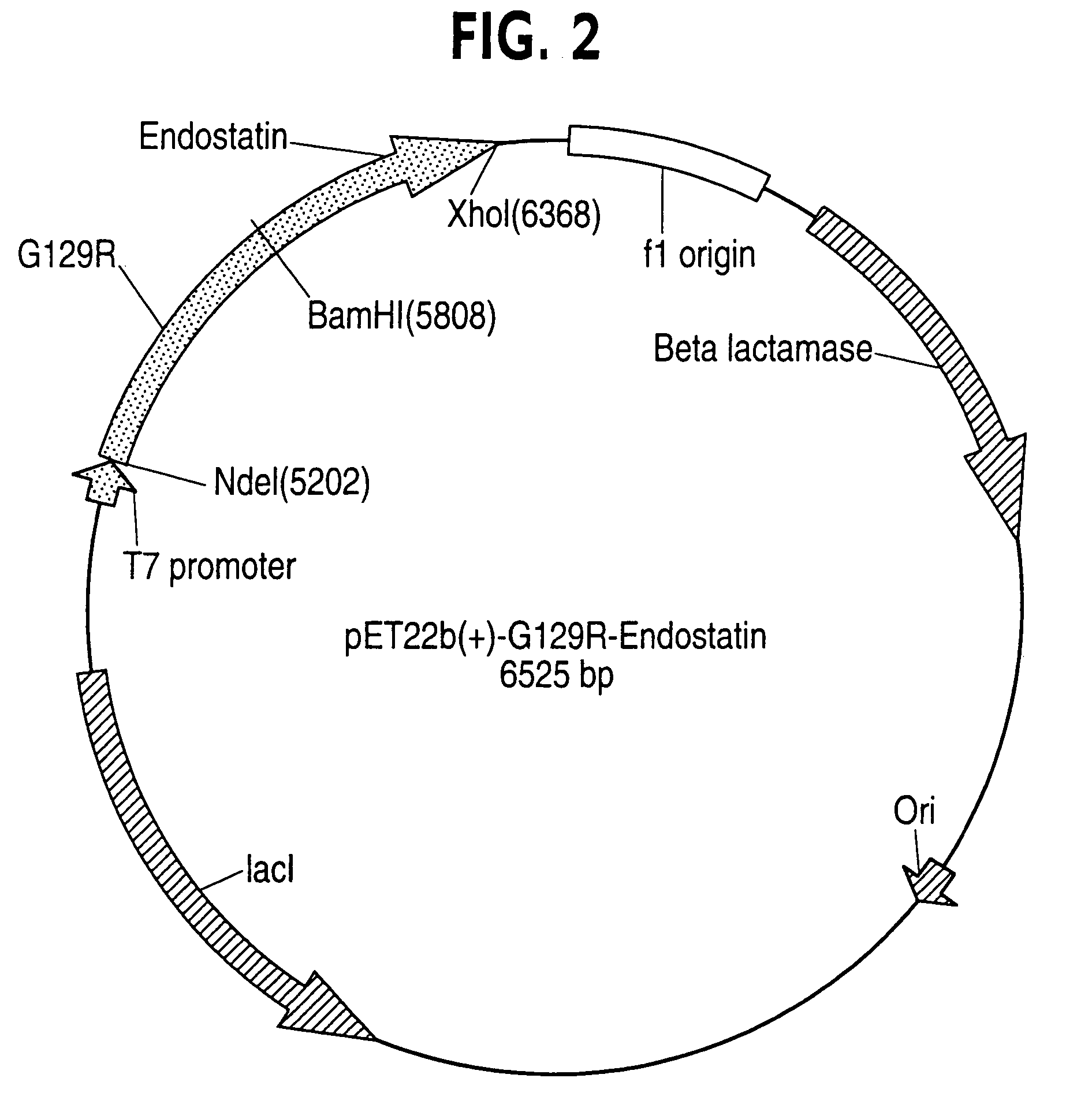
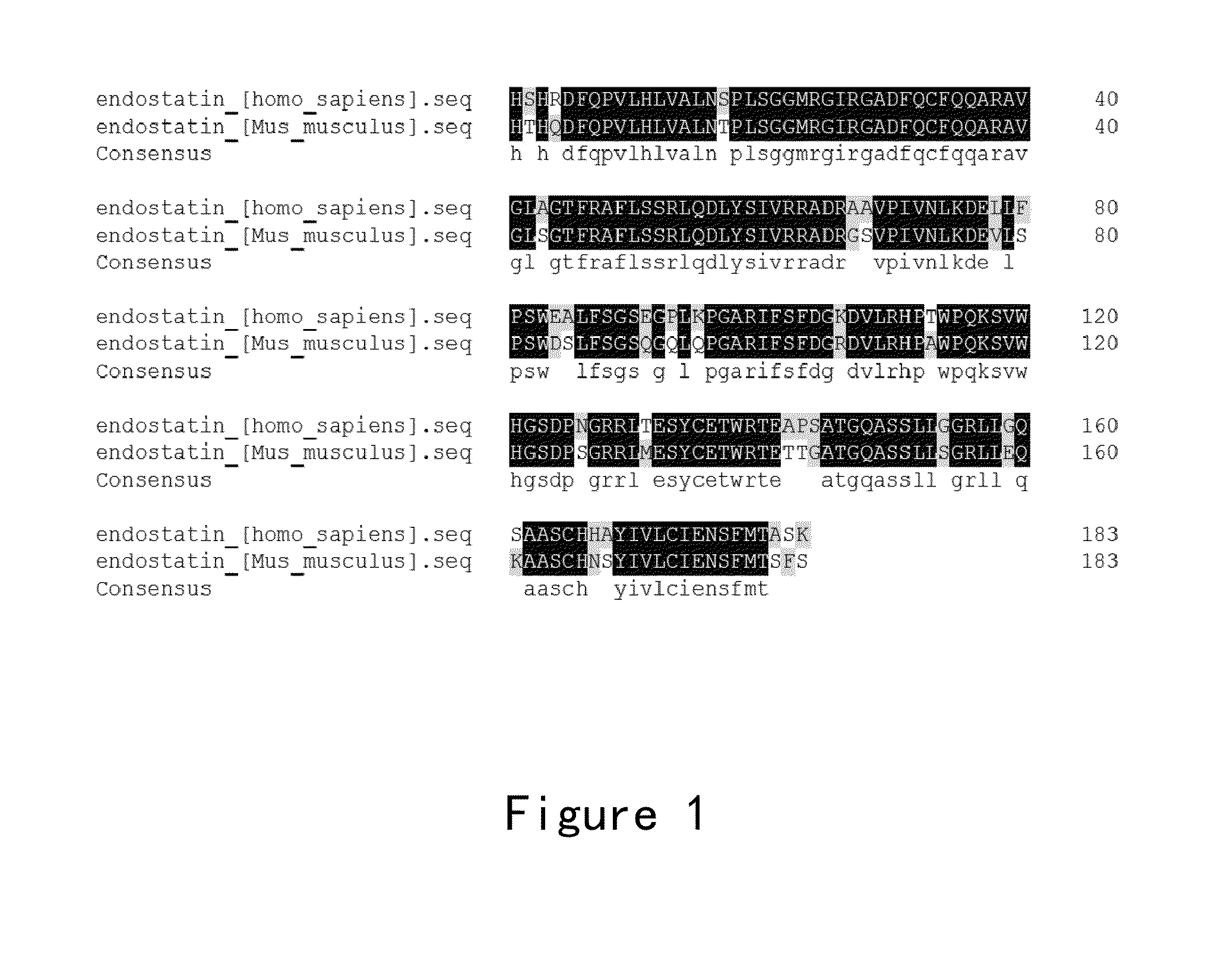
Endostatin is a naturally occurring, 20-kDa C-terminal fragment derived from type XVIII collagen. It is reported to serve as an anti-angiogenic agent, similar to angiostatin and thrombospondin. Endostatin is a broad-spectrum angiogenesis inhibitor and may interfere with the pro-angiogenic action of growth factors such as basic fibroblast growth factor (bFGF/FGF-2) and vascular endothelial growth factor (VEGF).
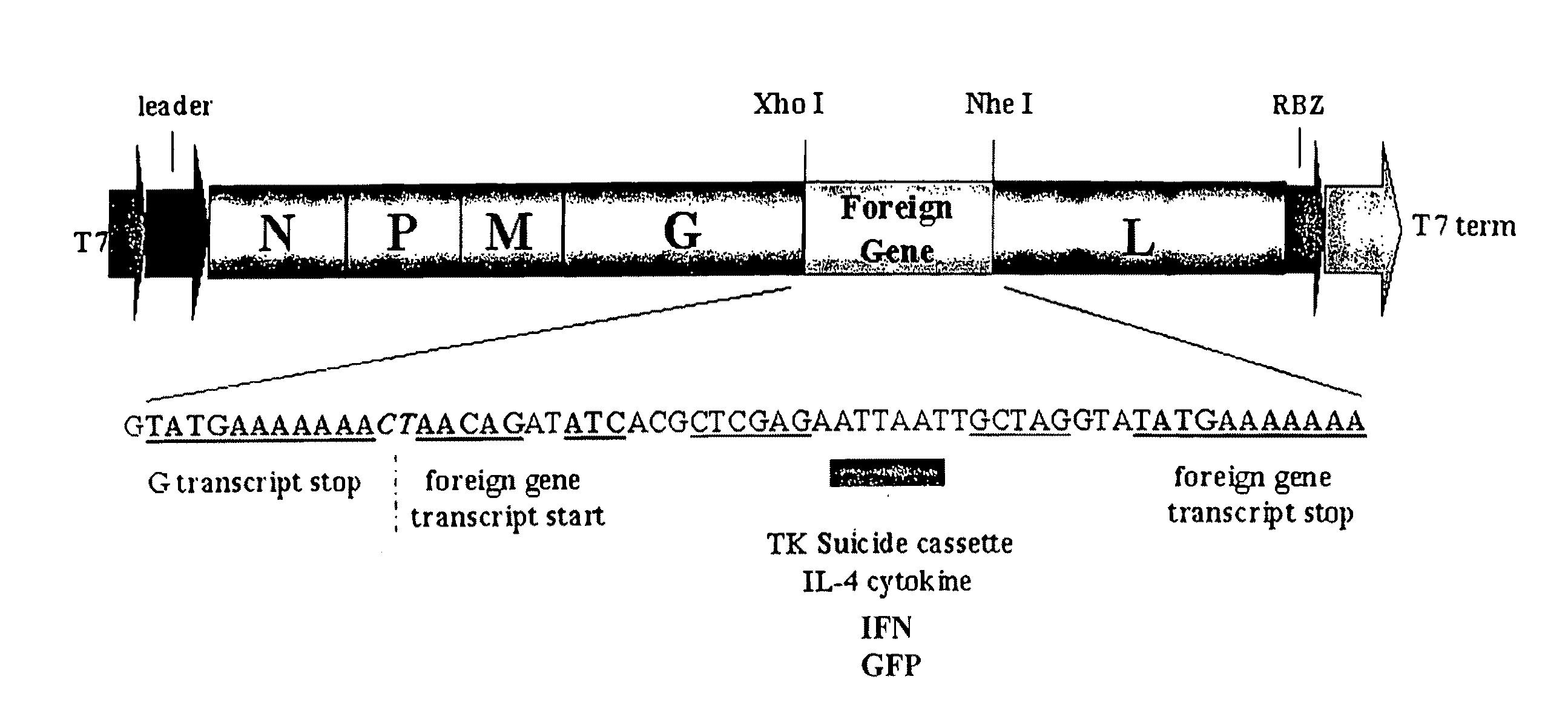
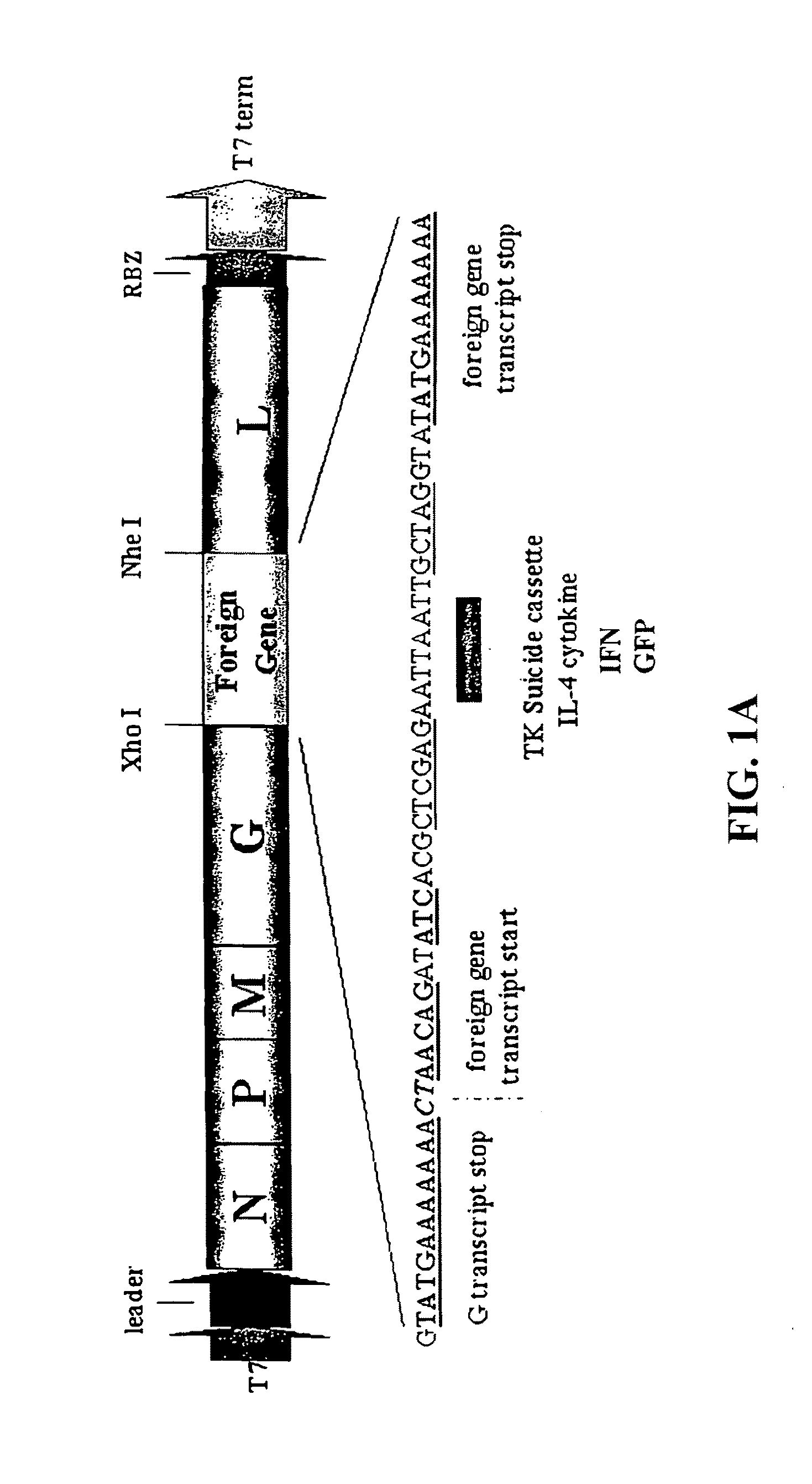
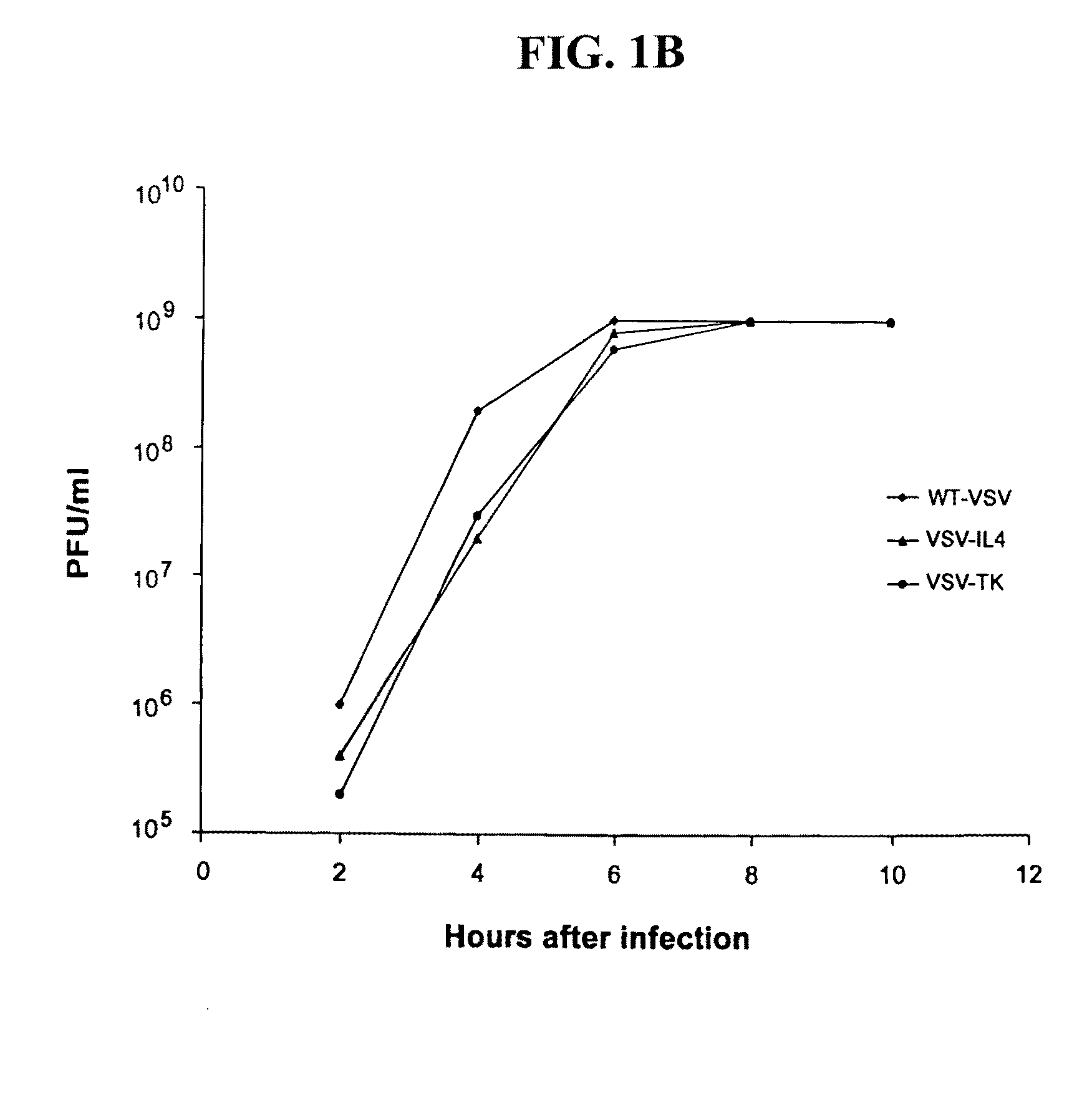
Recombinant VSV For The Treatment of Tumor Cells
The present invention relates to compositions and methods for the treatment of tumor and / or malignant and / or cancerous cells. The present invention provides VSV vectors comprising nucleic acid encoding a cytokine, such as interleukin or interferon, or a suicide gene, such as thymidine kinase, or other biological protein, such as heat shock protein gp96, or endostatin or angiostatin, wherein said VSV vectors exhibit greater oncolytic activity against the tumor and / or malignant and / or cancerous cell than a wild-type VSV vector. The present invention also provides methods of making such vectors, host cells, expression systems, and compositions comprising such VSV vectors, and viral particles comprising such VSV vectors. The present invention also provides methods for producing oncolytic activity in a tumor and / or malignant and / or cancerous cell comprising contacting said cell with a VSV vector of the present invention. The present invention also provides methods for suppressing tumor growth comprising contacting said tumor with a VSV vector of the present invention. The present invention also provides methods for eliciting an immune response to a tumor cell in an individual.
Owner:UNIV OF MIAMI
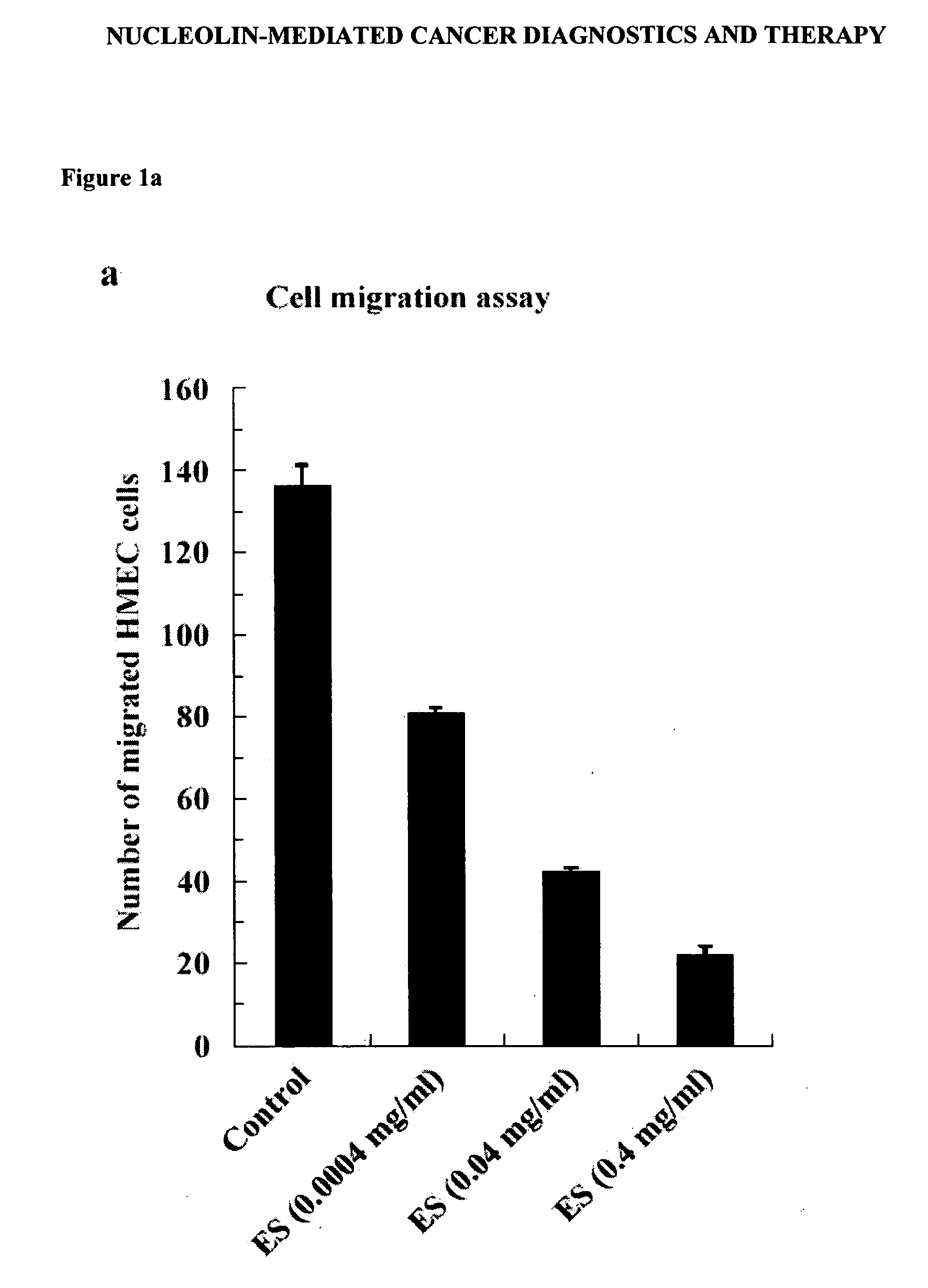
Nucleolin-mediated cancer diagnostics and therapy
InactiveUS20060258605A1High sensitivityEfficient killingGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthCancers diagnosis
The present invention provides for diagnostic kits for identifying cancer patients who are more susceptible to cancer therapies employing endostatin and other angiogenesis inhibitors, based upon the discovery that Nucleolin is a specific receptor for Endostatin. In particular, the diagnostic kits include antibody molecules against Nucleolin, DNA or RNA molecules that specifically bind to nucleic acid molecules encoding Nucleolin. The present invention also discloses methods of screening for angiogenesis inhibitors which specifically interact with Nucleolin, and act as angiogenesis inhibitors in an analogous manner as Endostatin. In addition, the present invention discloses methods of inhibiting the proliferation of endothelial cells or angiogenesis of tumor by administering an anti-nucleolin antibody linked to a cytotoxic agent such as tumor necrosis factor alpha to the endothelial cells.
Owner:TSINGHUA UNIV +1
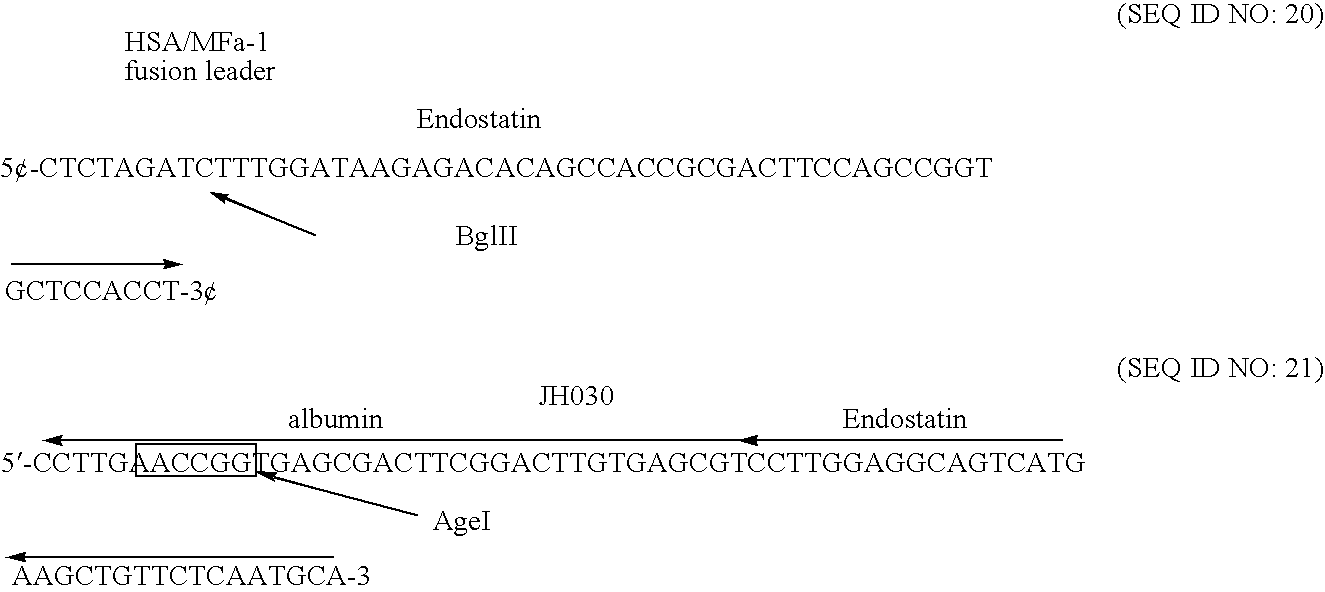
Albumin-fused anti-angiogenesis peptides
InactiveUS20060122374A1High activityExtended half-lifeOrganic active ingredientsFungiAbnormal tissue growthLymphatic Spread
The invention relates to proteins comprising angiogenesis inhibiting peptides, such as endostatin peptides (including, but not limited to, fragments and variants thereof), which exhibit anti-retroviral activity, fused or conjugated to albumin (including, but not limited to fragments or variants of albumin). These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins exhibit extended shelf-life and / or extended or therapeutic activity in solution. The invention encompasses therapeutic albumin fusion proteins, compositions, pharmaceutical compositions, formulations and kits. The invention also encompasses nucleic acid molecules encoding the albumin fusion proteins of the invention, as well as vectors containing these nucleic acuds, host cells transformed with these nucleic acids and vectors, and methods of making the albumin fusion proteins of the invention using these nucleic acids, vectors, and / or host cells. The invention also relates to compositions and methods for inhibiting proliferation of vascular endothelial cells and tumor aniogenesis induced cell fusion. The invention further relates to compositions and methods preventing growth of, or promoting regression of, primary tumors and metastases; and for treating cancer, diabetic retinophathy, progressive macular degeneration or rheumatoid arthritis.
Owner:NOVOZYMES BIOPHARMA DK AS
Polypeptide for inhibition of angiogenesis and method for preparing same and use thereof
ActiveCN1827640APrevent proliferationInhibition of anti-tumor effectPeptide/protein ingredientsDigestive systemTumor targetTumor targeting
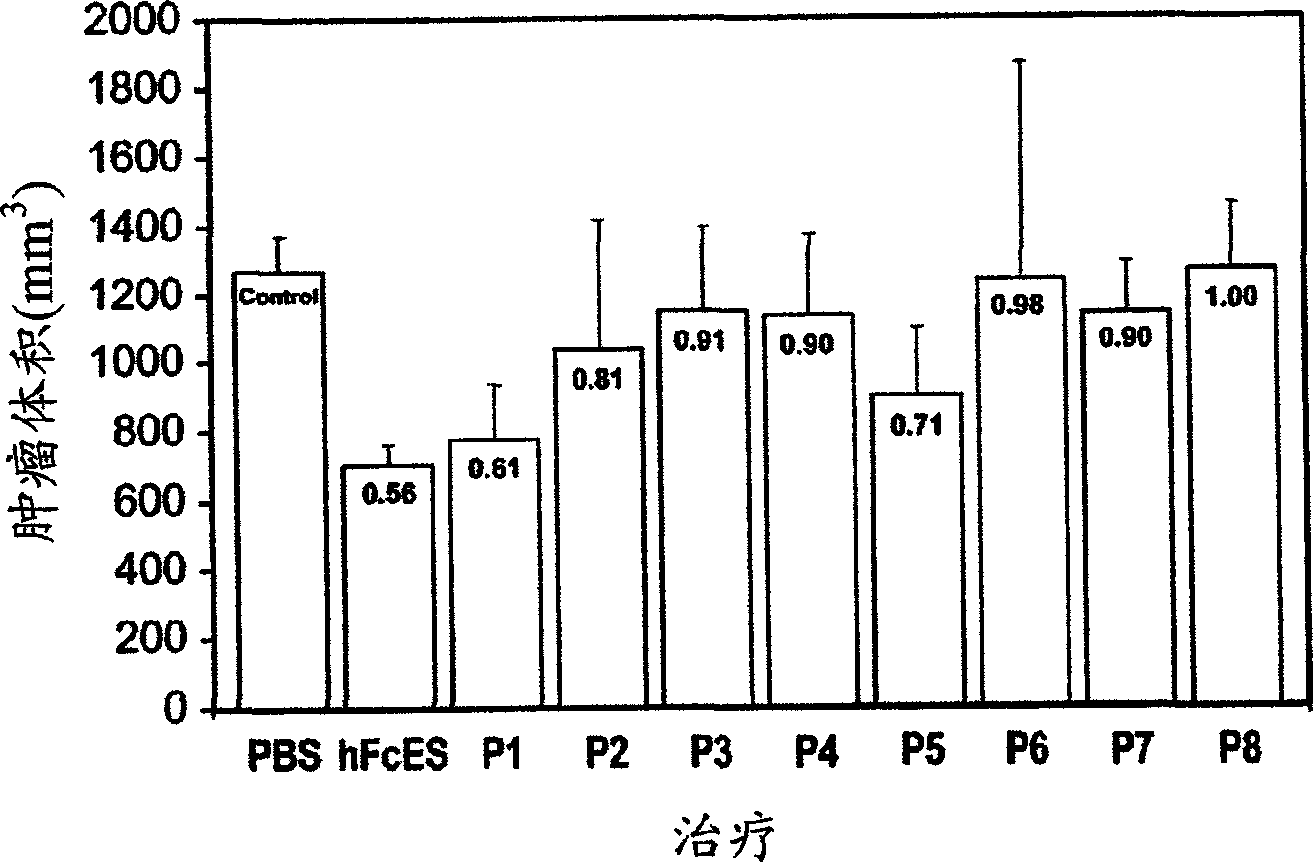
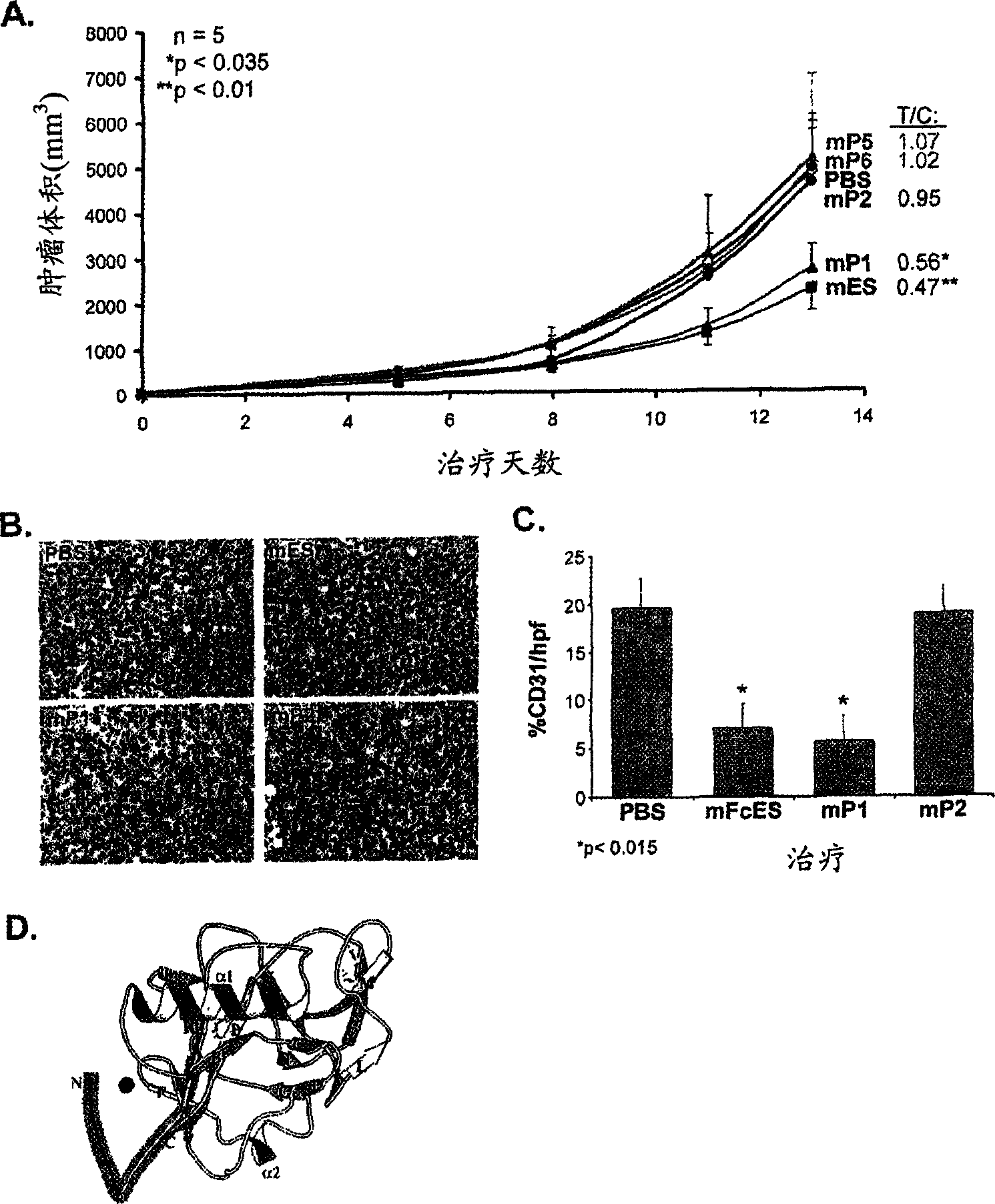
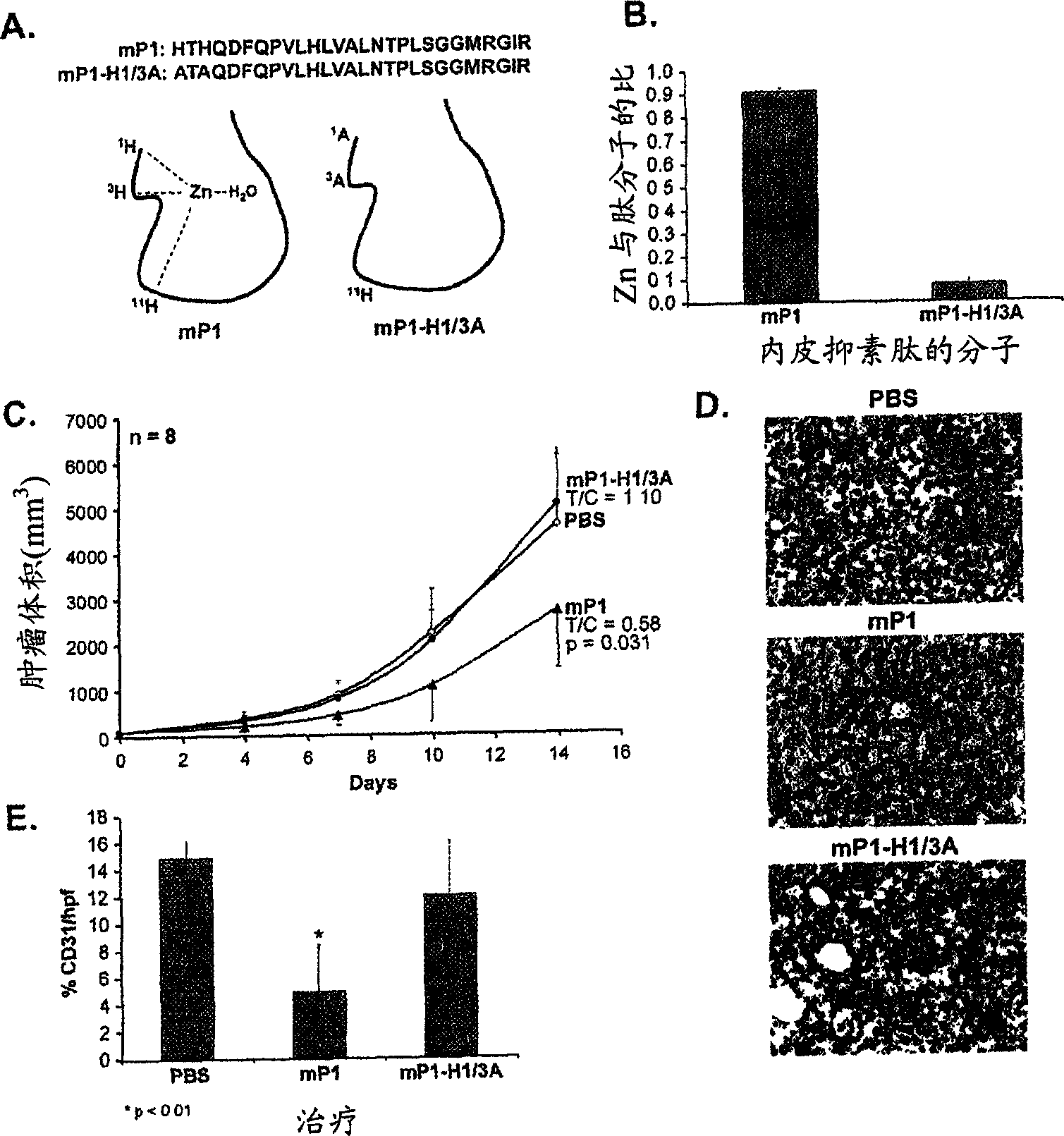
The invention relates to the field of biological engineering, more specifically to a group of polypeptides for the highly effective inhibition for blood vessel formation, its preparing process and use as anti-tumor medicament. By modification to No.6-49 amino acids of the esoderma chalone, the obtained polypeptides have stronger in vivo antineoplastic activity and tumor targeting property than esoderma chalone.
Owner:NANJING ANJI BIOLOGICAL TECH CO LTD
Methods of treating cancer using a modified endostatin protein
InactiveUS20060058232A1Produced efficiently and effectivelySlow tumor growthBiocideHeavy metal active ingredientsDiseaseEndostatin
The present invention provides methods of treating an angiogenesis-related disease of a subject using a therapeutically effective amount of a modified endostatin protein. In particular, the methods encompass treating an angiogenesis-related disease of a subject using a combination of the modified endostatin, and a known cancer therapy agent such as a chemotherapy agent, or a radiotherapy agent.
Owner:MEDGENN HONG KONG
Methods of treating cancer using a modified endostatin protein
InactiveUS7470667B2Produce efficientlySlow tumor growthBiocideHeavy metal active ingredientsEndostatinAngiogenesis growth factor
The present invention provides methods of treating an angiogenesis-related disease of a subject using a therapeutically effective amount of a modified endostatin protein. In particular, the methods encompass treating an angiogenesis-related disease of a subject using a combination of the modified endostatin, and a known cancer therapy agent such as a chemotherapy agent, or a radiotherapy agent.
Owner:MEDGENN HONG KONG
Endostatin conjugate and its preparation method
InactiveCN1876186ALow antigenicityPreserve proliferative effectSenses disorderPeptide/protein ingredientsNatural productHalf-life
The disclosed endotheliochalone compound is prepared as: the free amino with modified rate as 1.22~54.13% to combine 1-8 heparins and salt, and other amino with modified rate as 23.78~76.92% to combine 1-8 carbowaxs and derivative. Compared with natural product, this invention has low antigenicity, long half-life, and high stability, and fit to application in clinic.
Owner:SHANDONG UNIV
Combination of radiotherapy and anti-angiogenic factors
InactiveUS6420335B1Improve efficiencyBiocideOrganic active ingredientsAngiostatinAngiogenesis growth factor
The present invention relates generally to the fields of angiogenesis and cancer therapy. More particularly, it concerns the use of anti-angiogenic factors in cancer therapy. The present invention demonstrates that angiostatin or endostatin can sensitize a cell to radiation therapy. Methods and compositions for inhibiting growth, sensitizing a cell to radiotherapy and treating cancer growth by first inhibiting angiogenesis and then employing radiotherapy are described.
Owner:DANA FARBER CANCER INST INC +2
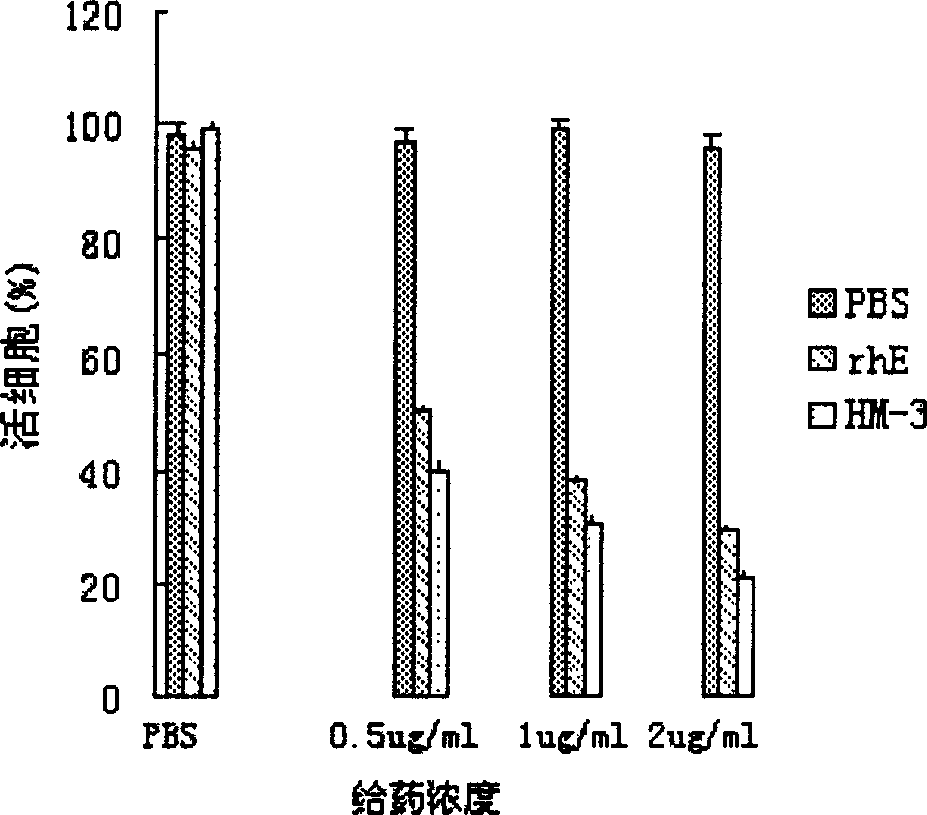
Blood vessel formation inhibitor IIM3 and its preparation method and application
InactiveCN1830487AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsAntineoplastic agentsEscherichia coliInclusion bodies
An efficient angiogenesis depressant HM-3 for treating the solid tumors including stomach cancer, lung cancer and liver cancer is prepared through expressing in colibacillus by genetic engineering method, separating the protein of inclusion body, dissolving, re-naturalizing, and separating-purifying by ion change and chromatography.
Owner:徐寒梅
TNF (Tumor Necrosis Factor)-related apoptosis-inducing ligand fusion protein and preparation method thereof
ActiveCN102775497AOvercomes the shortcoming of a half-life of only a few minutesMaintain apoptotic activityPeptide preparation methodsHybrid peptidesTumor cell apoptosisBlood vessel
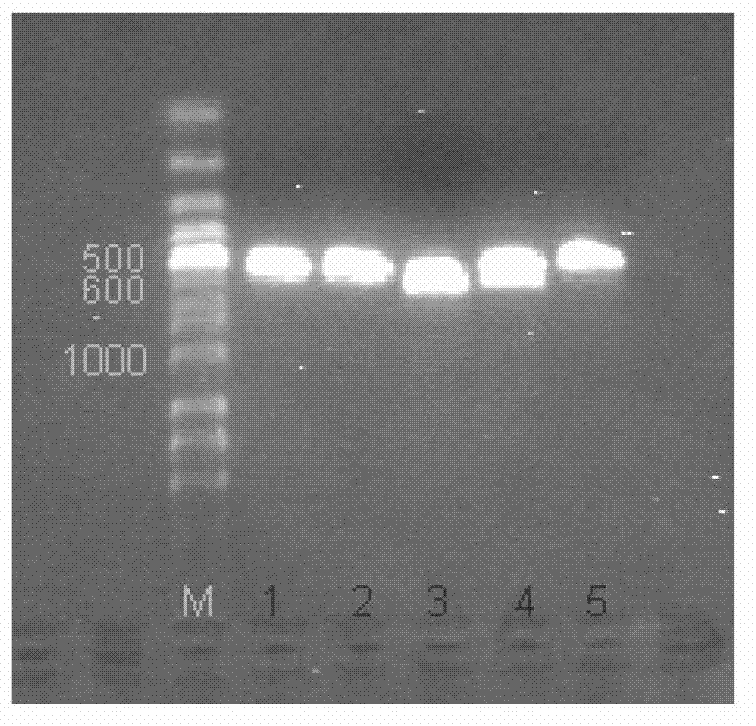
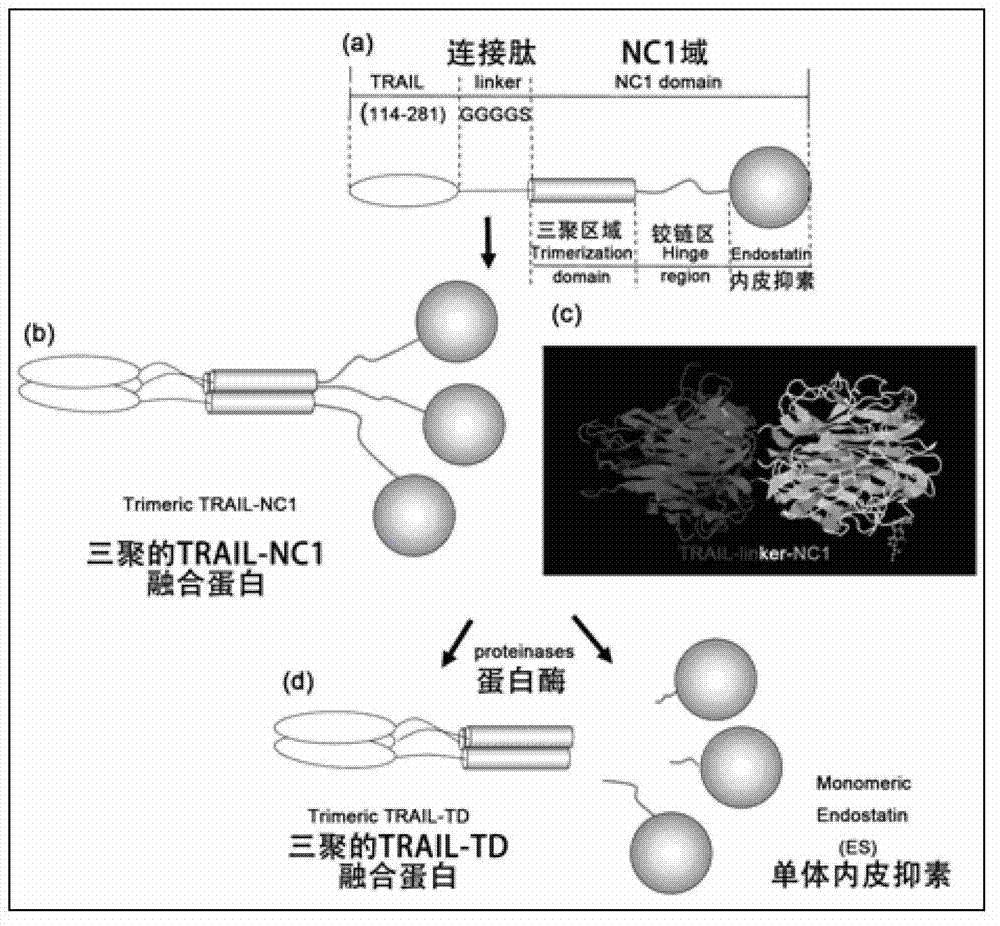
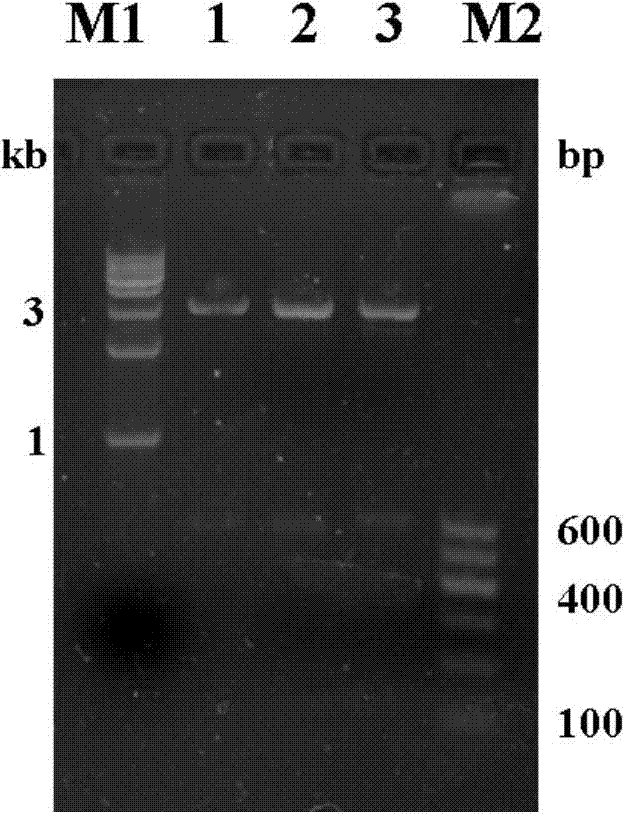
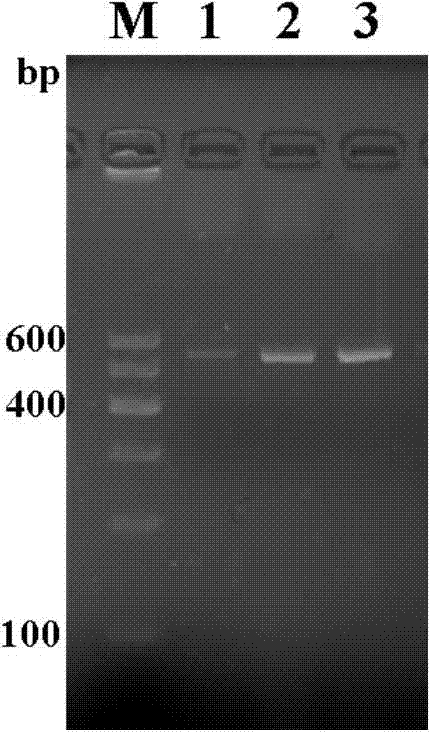
The invention provides a TNF (Tumor Necrosis Factor)-related apoptosis-inducing ligand fusion protein of extracellular parts and an XVIII type collagen NC1 domain or a trimerization domain, wherein the peptide adapter is [GlyGlyGlyGlySer]n, wherein the n is an integer of 0-4. The fusion protein is constructed and recombined; and the fusion protein is expressed for annealing, wherein the fusion protein after the annealing is expressed into relatively strong induced tumor cell apoptosis activity. According to the invention, the design of the fusion protein VXIII type collagen NC1 domain fusion TRAIL (Tnf Related Apoptosis Inducing Ligand) is adopted so that the half-life period of the related fusion protein in an experimental animal body can be prolonged and the defect that the half-life period of the TRAIL is just a little number of minutes can be overcome; the NC1 or trimerization domain has very strong trimerization characteristics and can stabilize a trimer thereof after the TRAIL is infused so as to keep to induce the tumor cell apoptosis activity; the endostatin domain contained in the NC1 domain has the effect of inhibiting the production of new vessels, and the effect can be cooperated with the apoptosis effect of the TRAIL so as to strengthen the anti-tumor effect of the infusion protein. The method disclosed by the invention can efficiently reduce the cost of the infusion protein production in a laboratory or in industry.
Owner:英百瑞(杭州)生物医药有限公司
Tat PTD-Endostatin recombination protein, preparation method and application thereof
ActiveCN102731658AOvercoming the limitations of poor film penetrationInhibition of productionPolypeptide with localisation/targeting motifSenses disorderDiseaseVascular proliferation
The present invention discloses a Tat PTD-Endostatin recombination protein, which is a fusion protein comprising a protein transduction domain of human immunodeficiency virus (HIV) transactivation transduction protein Tat and human endostatin, wherein the amino acid sequence of the protein transduction domain of the HIV transactivation transduction protein Tat is represented by SEQ ID NO.1, and the amino acid sequence of the human endostatin is represented by SEQ ID NO.2. The protein of the present invention has the following advantages that: the function of the endostatin can be maintained, wherein the function of the endostatin is that the endostatin can inhibit angiogenesis; the protein has advantages of high transduction efficiency, easy blood-brain barrier crossing, and easy blood-ocular barrier crossing; the protein can overcome the limitation of the poor membrane spanning effect of the endostatin and play the angiogenesis inhibition effect well; the protein can be used for treatments of various diseases caused by angiogenesis, including ocular vascular proliferative diseases and various tumors, such as diabetes retinopathy, non-small cell lung cancer, and the like.
Owner:SHANDONG UNIV
Anti-angiogenic peptides from the N-terminus of endostatin
Provided herein are anti-angiogenic comprising the N-terminal end of endostatin, nucleic acids encoding the same, pharmaceutical preparations comprising an effective amount of the peptide and nucleic acids and use of the pharmaceuticals in treating or preventing diseases or conditions associated with undesirable angiogenesis.
Owner:CHILDRENS MEDICAL CENT CORP
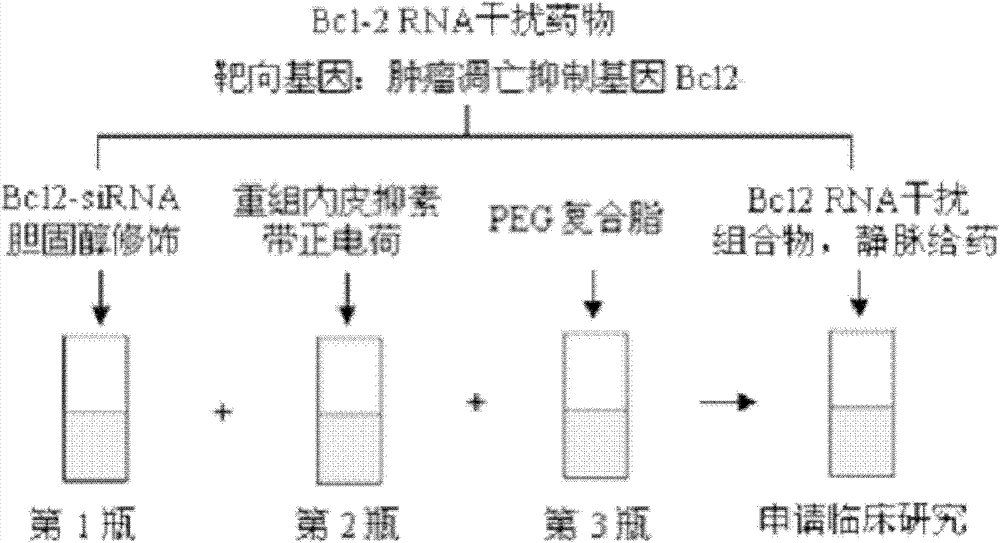
Composition comprising endostatin adopted as delivery system and chemically-synthesized RNA interference molecule, and application thereof
ActiveCN102784398AReduce the use effectReduce charge valueOrganic active ingredientsGenetic material ingredientsChemical synthesisSide effect
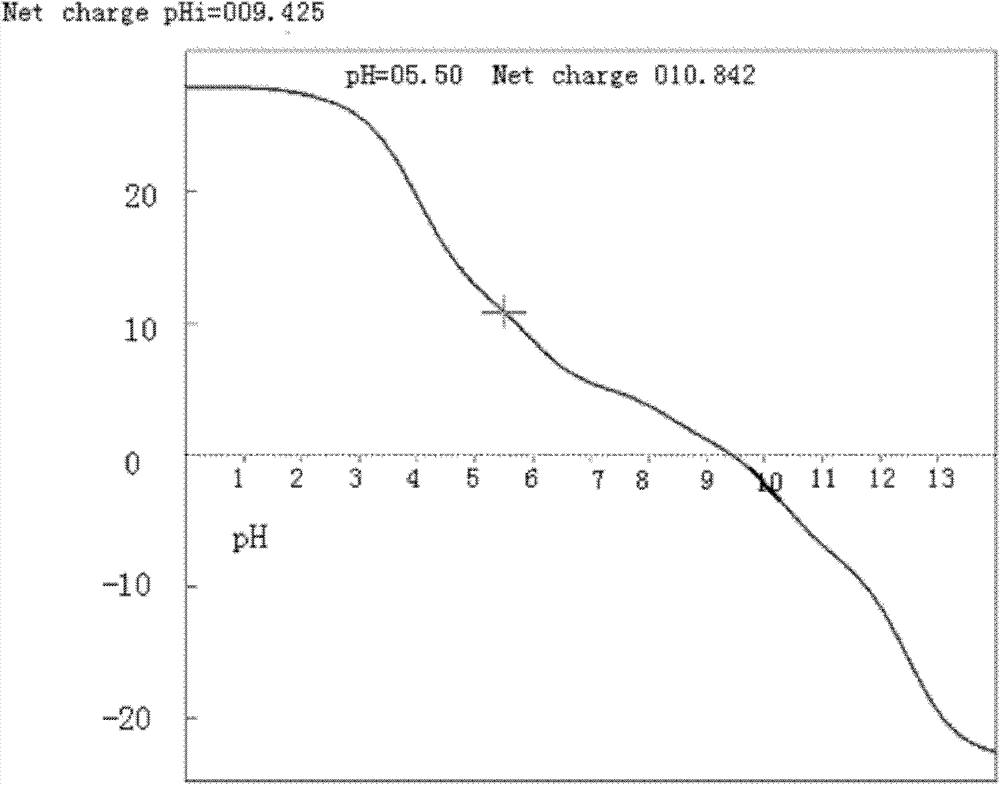
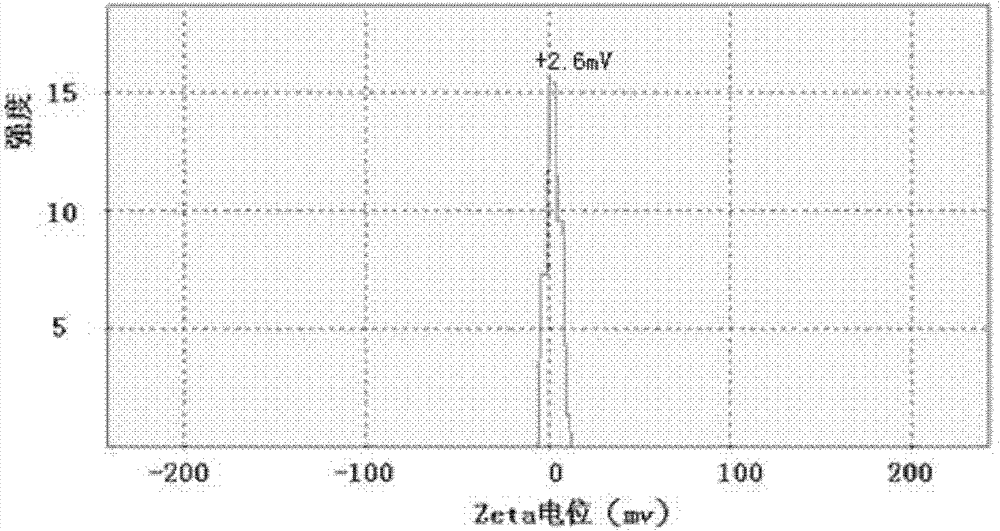
The present invention belongs to the field of biomedicine, and discloses a composition comprising endostatin adopted as a delivery system and a chemically-synthesized RNA interference molecule, and an application thereof. The composition contains endostatin recombination protein carrying a charge of +5 to +15, and a chemically-synthesized RNA interference molecule carrying a charge of -15 to -55, wherein the endostatin recombination protein and the chemically-synthesized RNA interference molecule are subjected to positive charge and negative charge combination to form a composition with a charge value ??of -20 to +20. With the composition, use amounts and charge values of the endostatin and the chemically-synthesized RNA interference molecule are greatly reduced; the endostatin providing a delivery effect has a special characteristic of lesion vascular endothelial cell targeting, such that the composition can target the target tissue and stably distribute after the composition is subjected to intravenous injection 1 hour or within 24 hours so as to provide advantages of good treatment effect, stable activity, long effective action time and low toxic and side-effect.
Owner:NANJING UNIV +1
Human prolactin antagonist-angiogenesis inhibitor fusion proteins
ActiveUS7339027B2Peptide/protein ingredientsAntibody mimetics/scaffoldsAngiogenesis InhibitionIntracrine
A novel fusion protein, comprising a receptor-antagonizing domain and an angiogensis inhibiting domain, characterized, for example, by its ability to block apoptosis and / or inhibit endocrine response, is useful in treating cancer. For example, a human prolactin antagonist-endostatin fusion protein combines apoptosis induction and angiogenesis inhibition to combat cancer.
Owner:ONCOLIX
Endothelium chalone mutant containing non-natural amino acid and derivatives thereof
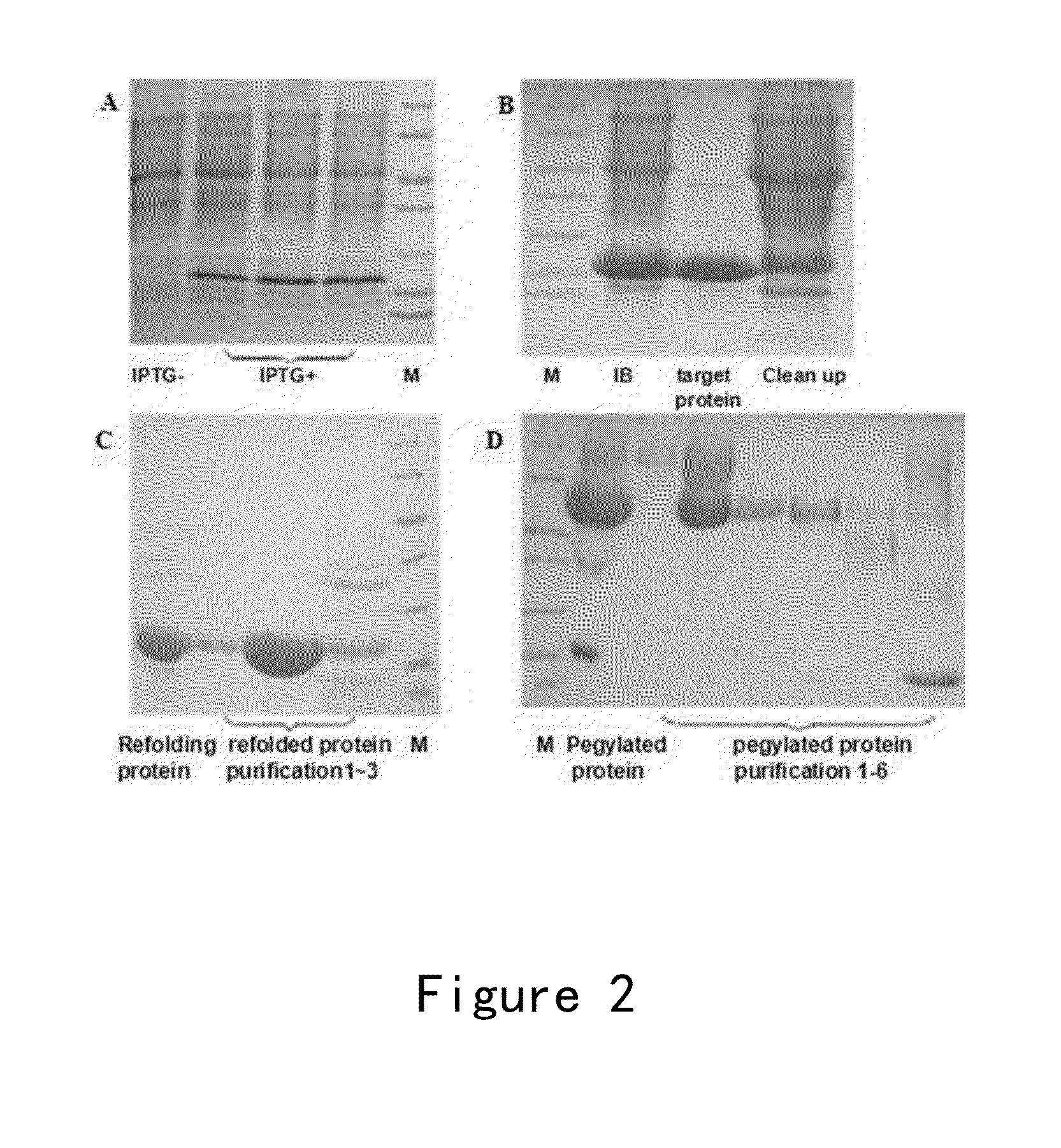
InactiveCN101265298AAchieving site-specific PEGylationSimplified renaturationPeptide/protein ingredientsAntineoplastic agentsSide chainProkaryotic expression
The invention relates to a non-natural amino acid-containing endostatin mutant and derivatives thereof, in particular to a non-natural amino acid-containing soluble endostatin expressed in prokaryotic expression system while the activity thereof is ensured. The endostatin mutant is endowed with new chemical property, and can specifically form the covalent linkage with other molecules through side chain of non-natural amino acid.
Owner:CHINA PHARM UNIV
Anti-cancer sustained-released agent
InactiveCN101396341AInhibition formationSpeed up entryPeptide/protein ingredientsSolution deliveryDepressantSuspending Agents
The invention relates to an anti-cancer sustained release injection, consisting of sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise sustained release auxiliary material, angiogenesis inhibitor and / or proteolytic enzyme; and the menstruum contains suspending agent. The angiogenesis inhibitor is selected from gefitinib, erlotinib, lapatinib, vatalanib, pelitinib, endostatin, imatinib, semaxanib, dasatinib, avastin, sorafenib, sunitinib, telcyta or panitumumab; the proteolytic enzyme is selected from one or the combination of collagenase, hyaluronidase, relaxin and plasmase; the sustained release auxiliary material is selected from polifeprosan, decanedioic acid copolymer, EVAc, polylactic acid, the mixture or the copolymer thereof, and the like; and the suspending agent is selected from carboxymethyl cellulose and the like with the viscosity of 100cp to 3000cp (under the temperature of 25 DEG C to 30 DEG C). The injection can also be made into a sustained release implant. The sustained release injection is injected or arranged in or around the tumour and can improve the local drug concentration selectively, reduce the general reaction of the drug, inhibit the growth of tumour cell and blood vessel and enhance the treatment effect of non-operative treatments, such as radiotherapy, chemotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Albumin-Fused Anti-Angiogenesis Peptides
InactiveUS20090175893A1High activityExtended half-lifeOrganic active ingredientsFungiDiabetic retinopathyLymphatic Spread
The invention relates to proteins comprising angiogenesis inhibiting peptides, such as endostatin peptides (including, but not limited to, fragments and variants thereof), which exhibit anti-retroviral activity, fused or conjugated to albumin (including, but not limited to fragments or variants of albumin). These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins exhibit extended shelf-life and / or extended or therapeutic activity in solution. The invention encompasses therapeutic albumin fusion proteins, compositions, pharmaceutical compositions, formulations and kits. The invention also encompasses nucleic acid molecules encoding the albumin fusion proteins of the invention, as well as vectors containing these nucleic acids, host cells transformed with these nucleic acids and vectors, and methods of making the albumin fusion proteins of the invention using these nucleic acids, vectors, and / or host cells. The invention also relates to compositions and methods for inhibiting proliferation of vascular endothelial cells and tumor angiogenesis induced cell fusion. The invention further relates to compositions and methods preventing growth of, or promoting regression of, primary tumors and metastases; and for treating cancer, diabetic retinopathy, progressive macular degeneration or rheumatoid arthritis.
Owner:NOVOZYMES BIOPHARMA DK AS
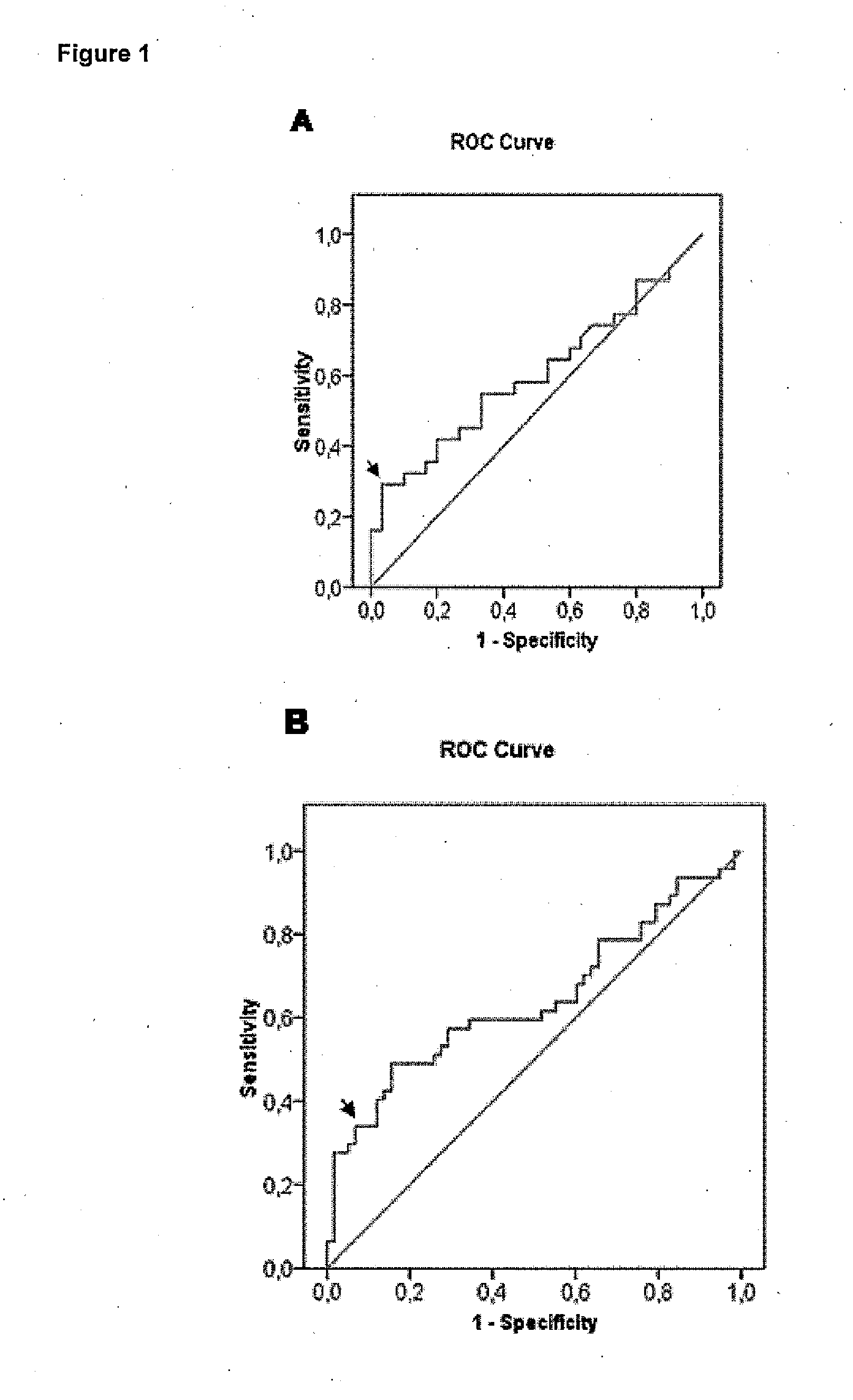
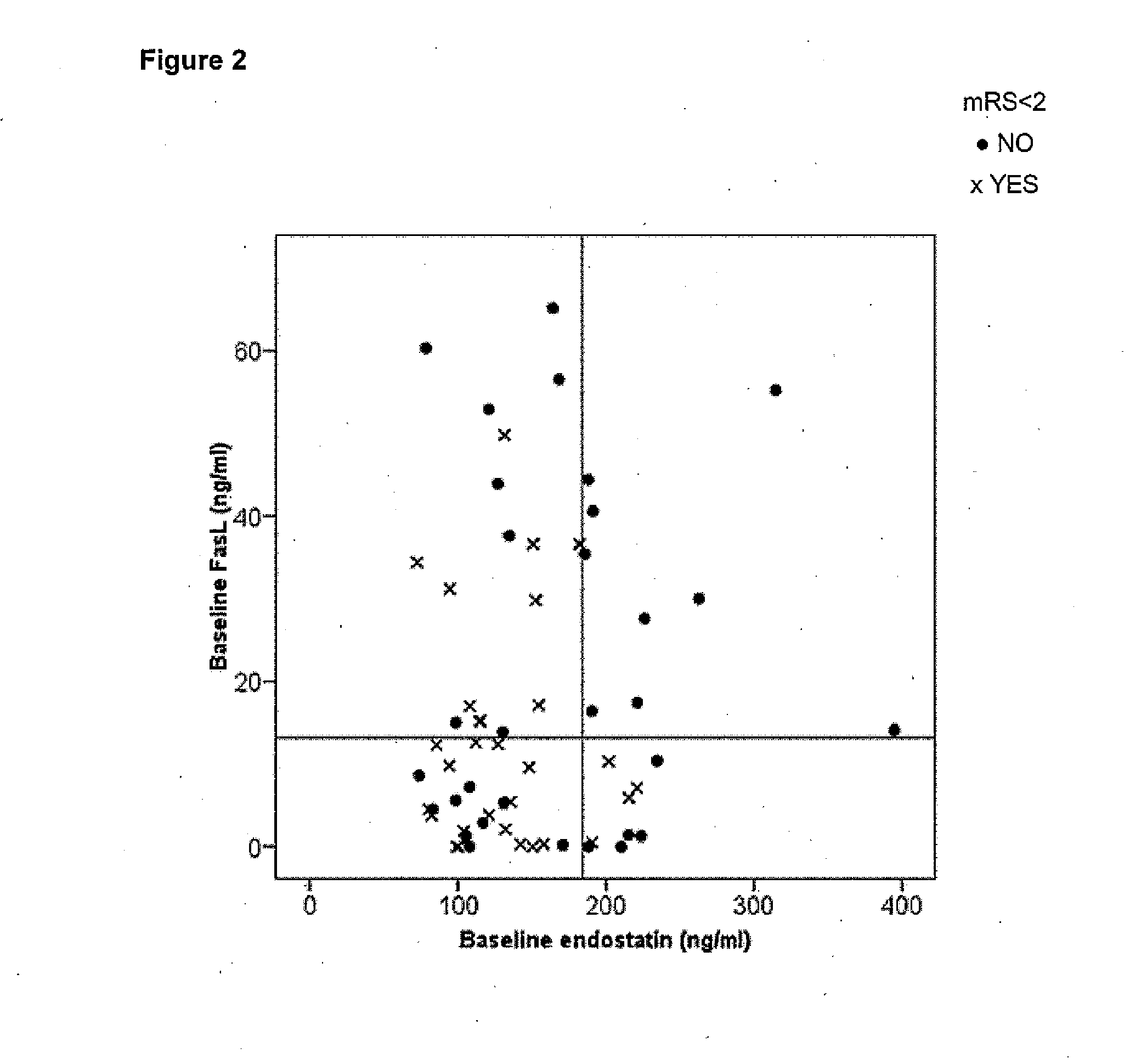
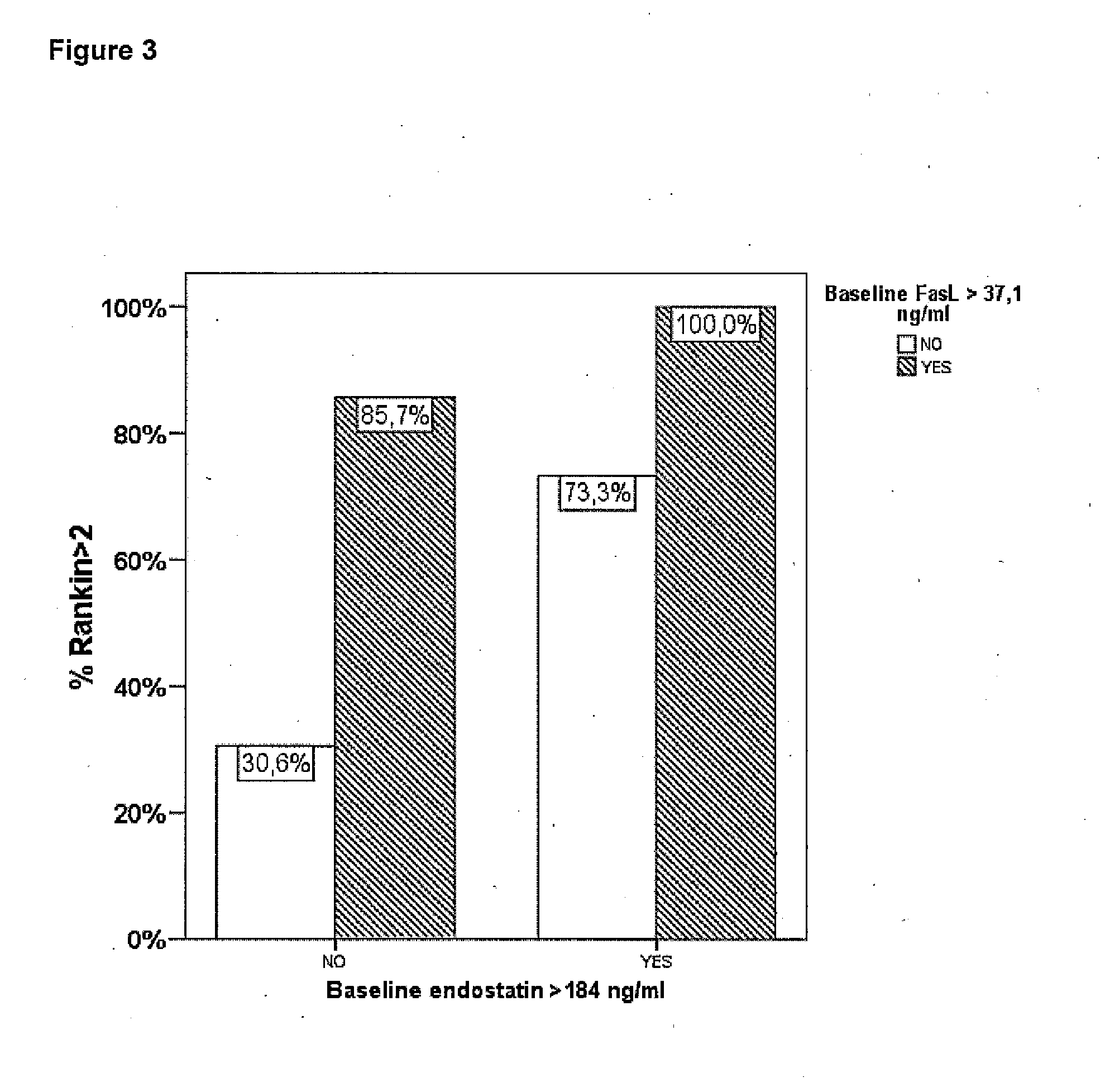
Method of predicting the evolution of a patient suffering of stroke
InactiveUS20140045198A1Improve the level ofPositive correlation of the systemic levelsBioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseClinical variables
The invention is a method of predicting the evolution of a patient suffering of a neurovascular disease, preferably stroke, comprising obtaining a biological sample of said patient, assessing in said biological sample the level of endostatin and FasL, determining whether said levels of endostatin and FasL together are above or below predetermined cut-off levels and predicting the functional outcome of said neurovascular disease on said patient evaluating the result of the previous step. The combination of endostatin and FasL is a more powerful predictor that any clinical prognostic tool and adds significant prognostic value to all other clinical variables of the art.
Owner:INSTITUT DE RECERCA HOSPITAL UNIVRI VALL DHEBRON
Anti-angiogenic peptides for treating or preventing endometriosis
Provided herein are peptides from the N-terminal of endostatin proteins, including the first histidine of the protein, nucleic acids encoding the peptides, pharmaceutical compositions comprising the nucleic acids and proteins and methods for using the pharmaceutical compositions to treat or prevent endometriosis in a subject.
Owner:CHILDRENS MEDICAL CENT CORP
Biomarkers in the selection of therapy of heart failure
InactiveUS20150268251A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBeta blockerBiomarker (petroleum)
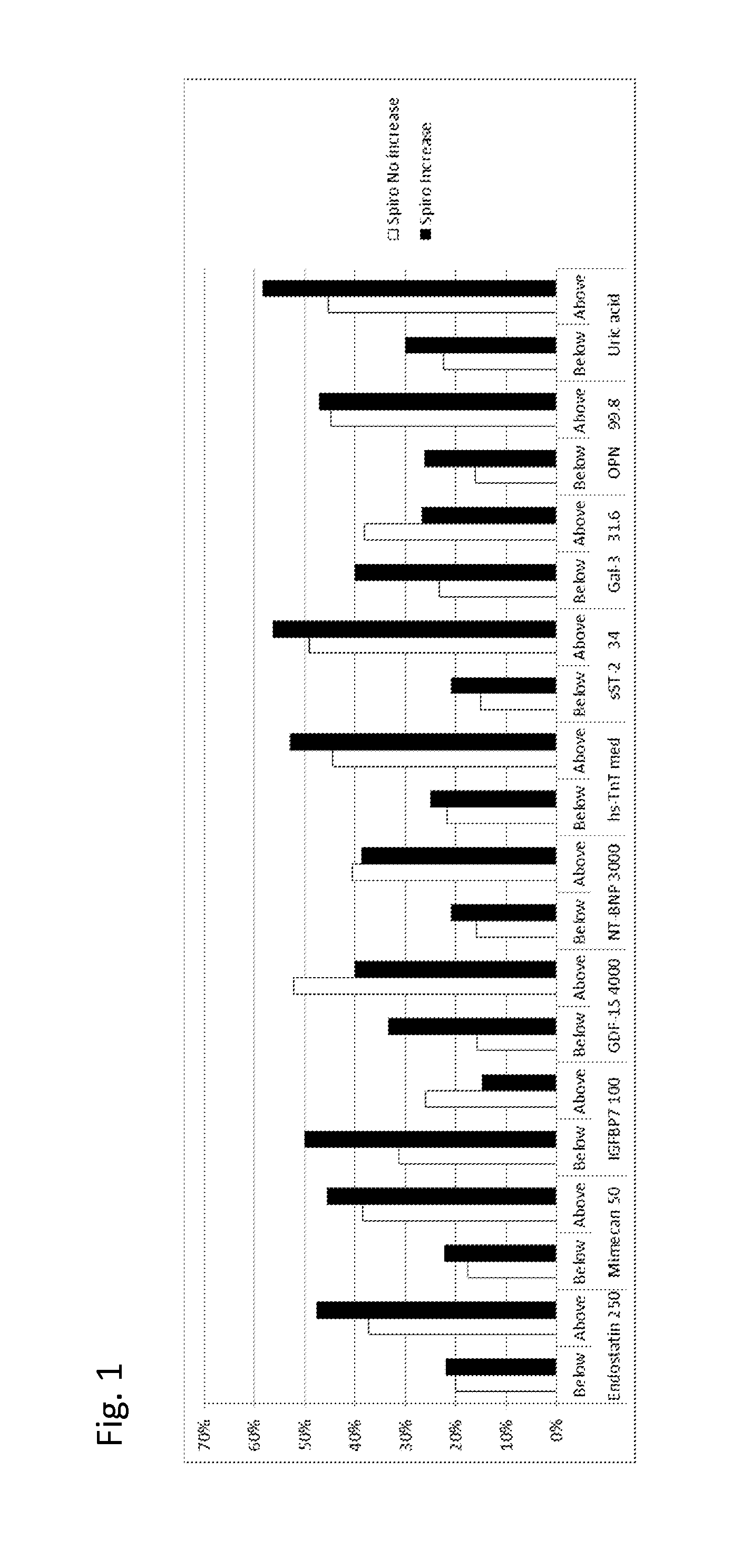
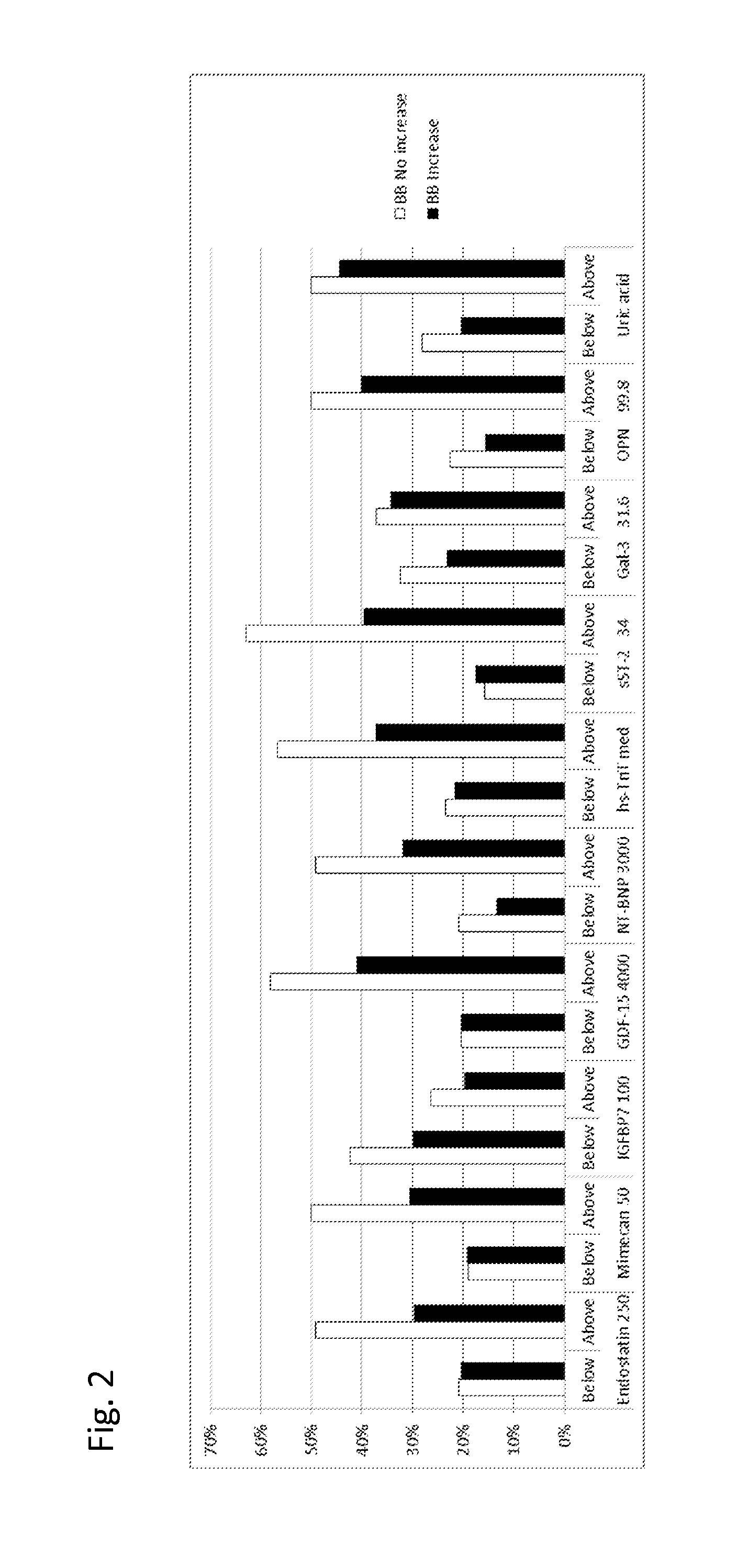
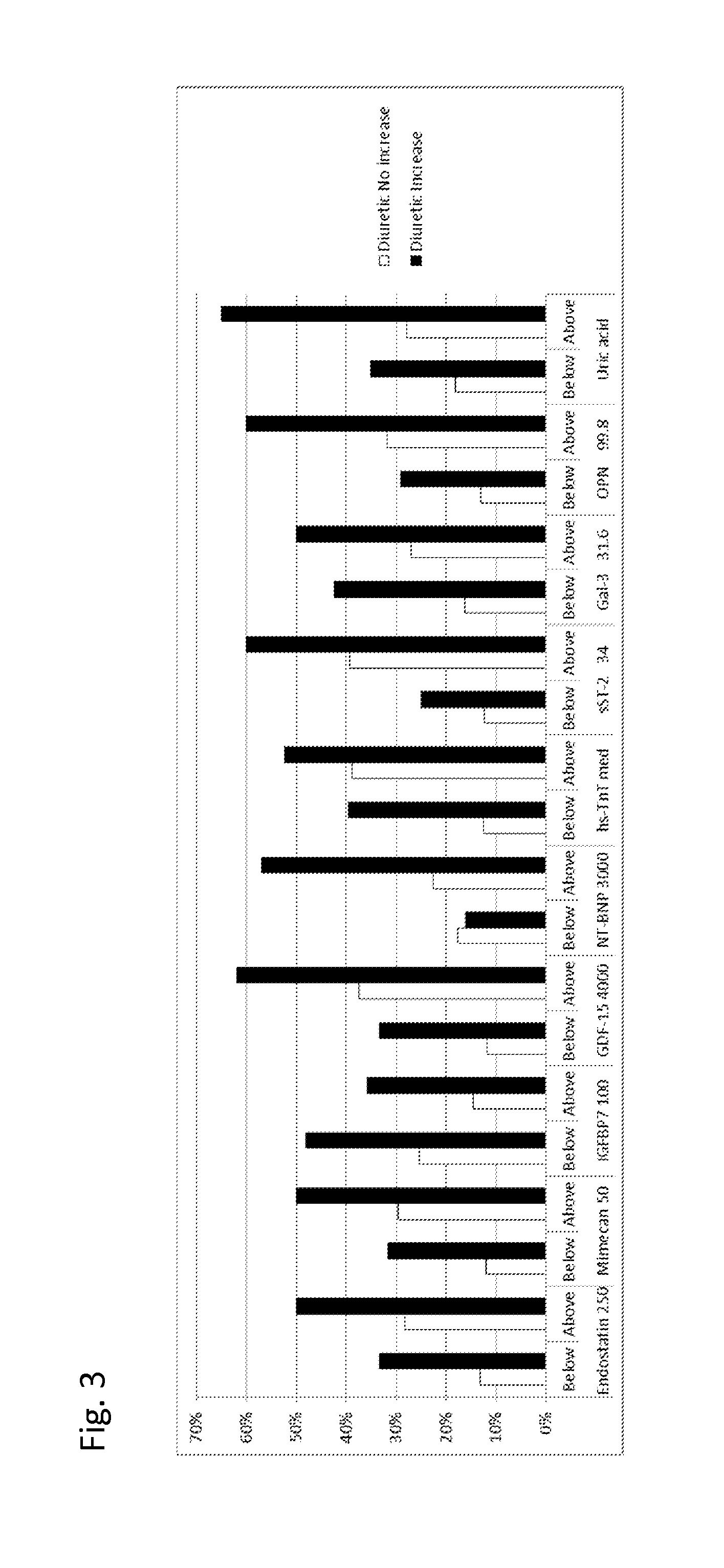
The present invention relates to a method for identifying a subject being eligible to the administration of at least one medicament selected from the group consisting of a beta blocker, an aldosterone antagonist, a diuretic, and an inhibitor of the renin-angiotensin system. The method is based on the determination of the amount of at least one biomarker selected from the group consisting of GDF-15 (Growth Differentiation Factor 15), endostatin, mimecan, IGFBP7 (IGF binding protein 7), a cardiac Troponin, a BNP-type peptide, uric acid, Gal3 (Galectin-3), osteopontin, sST2 (soluble ST2), PlGF, sFlt-1, P1NP, Cystatin C, Prealbumin, and Transferrin in a sample from a subject suffering from heart failure. Further, the method comprises the step of comparing the, thus, determined amount with a reference amount. Further envisaged by the present invention are kits and devices adapted to carry out the method of the present invention. The present invention also relates to a system for identifying a subject being eligible to the administration of at least one medicament as disclosed herein and to reagents and kits used in performing the methods as disclosed herein.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Polypeptide in small molecule of endothelium inhibin, nucleotide sequence for encoding the polypeptide and complementary strand
ActiveCN1916023ASmall molecular weightPromote absorptionPeptide/protein ingredientsGenetic engineeringNucleotideGenetic engineering
This invention relates to endostatin small molecular polypeptide, its coding nucleotide sequence and complementary strand. The endostatin small molecular polypeptide has 30 amino acid residues corresponding to a nucleotide sequence with a full length of 90 bp and a complementary strand with a length of 88 bp. The endostatin small molecular polypeptide avoids the defects of the current endostatin such as high molecular weight, limit to subcutaneous injection and intramuscular injection, large dosage and unsafety in intravenous injection. The endostatin small molecular polypeptide has such advantages as low molecular weight and no medicine accumulation, and is suitable for intravenous, intramuscular and subcutaneous injection. The coding nucleotide sequence and complementary strand can be synthesized by an automatic synthesizer, and used to manufacture endistatin in large scale by genetic engineering after recombination and transformation.
Owner:HARBIN MEDICAL UNIVERSITY
An anticancer sustained releasing agent
InactiveCN1961861AInhibition formationSpeed up entryPeptide/protein ingredientsPharmaceutical delivery mechanismDepressantHyaluronidase
Disclosed is an anti-cancer slow release agent comprising slow release microspheres and dissolvent, wherein the slow release microspheres include slow release auxiliary materials, anti-angiogenesis agent and / or proteolytic enzyme, the dissolvent comprises suspension adjuvant. The anti-angiogenesis agent is selected from gefinitib, erlotinib, lapatinib, pelitinib, endostatin, imatinib, smasnib, avastin, sorafenib, sunitinib, oersted or panitoma, the proteolytic enzyme is selected from collagenase, hyaluronidase, relaxin and thrombase or the combination of them, the slow release auxiliary materials are selected from Polifeprosan, sebacylic acid copolymer, EVAc, polylactic acid and their mixture of copolymer, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The agent can also be prepared into implanting agent for injection or placement in or around tumor with the effects of selectively increasing local concentration, lowering general reaction of the drugs, suppressing growth of tumor cells and blood vessel, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:SHANDONG LANJIN PHARMA +1
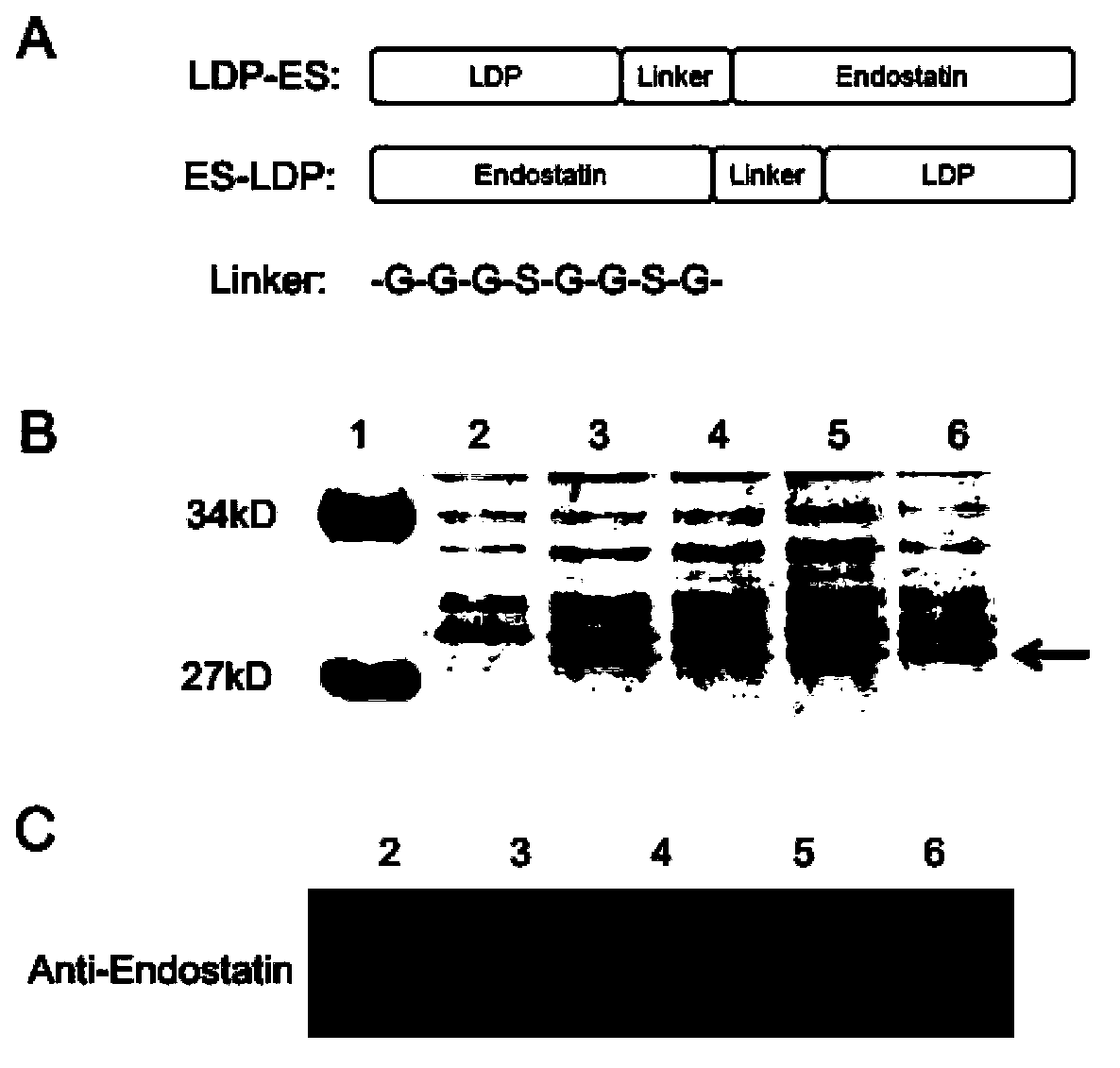
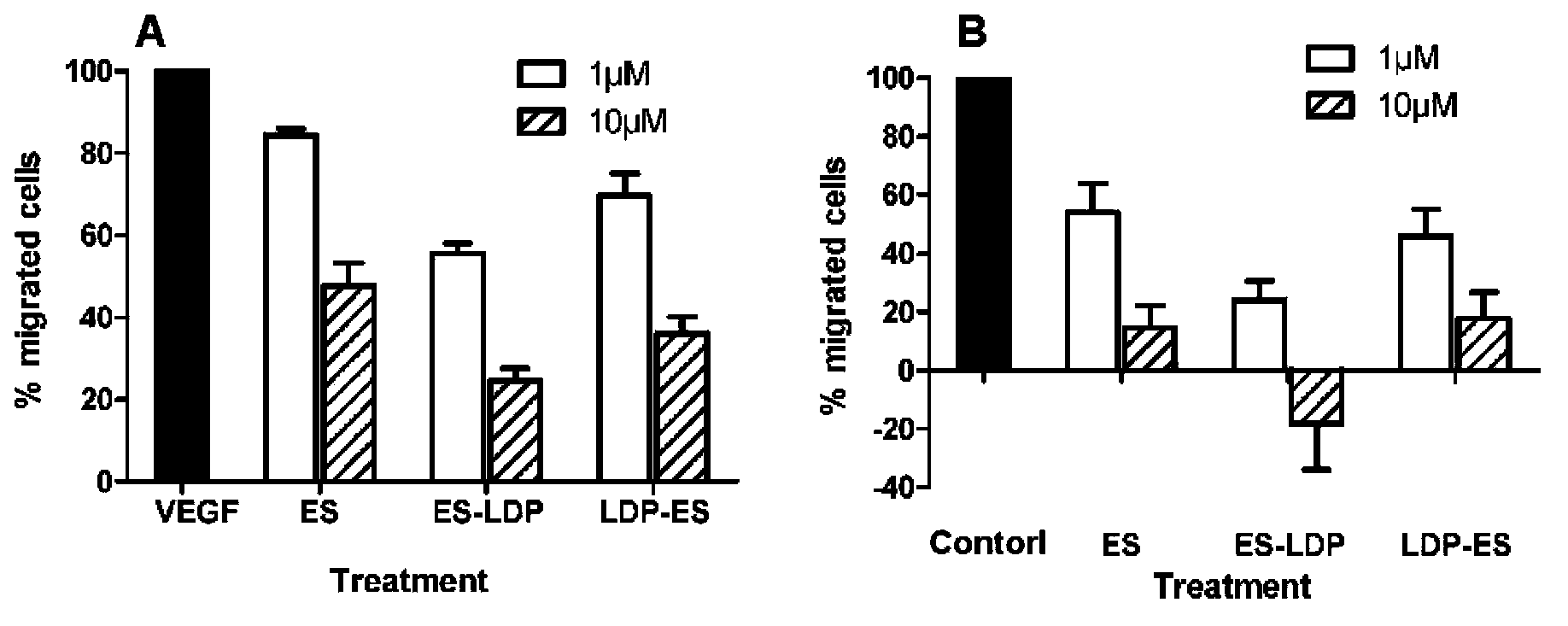
Anti-tumor fusion protein, and preparation method and application thereof
The invention discloses anti-tumor fusion protein, and a preparation method and application thereof. Concretely, the invention discloses fusion protein containing lidamycin apoprotein and endostatin, and the fusion protein can be reinforced by using lidamycin chromophore. The invention also discloses a method of generating the fusion protein through recombination and application of the fusion protein to treat tumor.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI +2
Endostatin-like angiogenesis inhibition
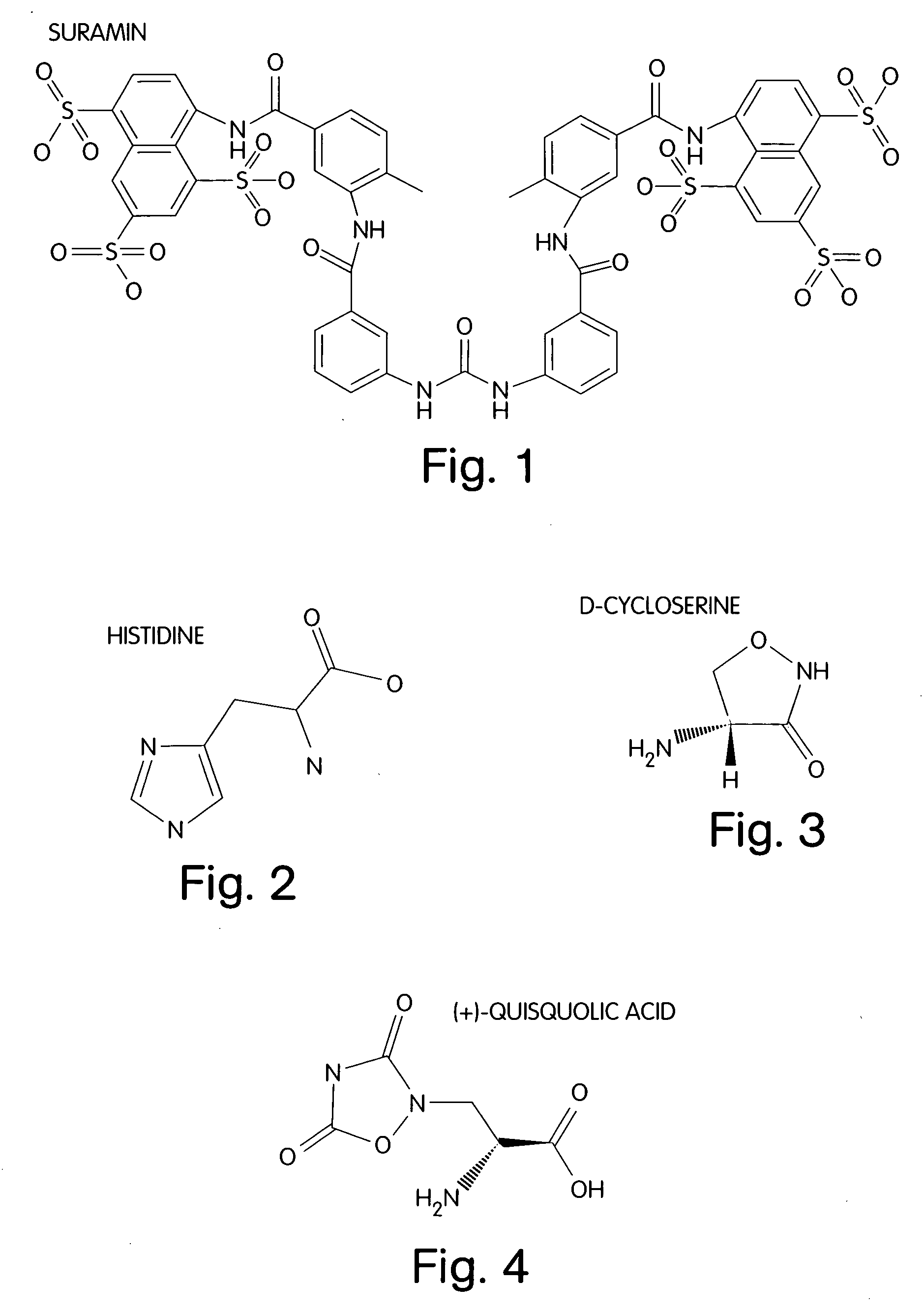
InactiveUS20050014784A1Accelerate emissionsAvoid quenchingBiocideMaterial nanotechnologyAngiogenesis InhibitionEndostatin
A treatment for cancer is provided. The treatment may include administering a therapeutic amount of L-histidine, D-cycloserine, quisqualic acid or suramin or analogs thereof.
Owner:MINERVA BIOTECH
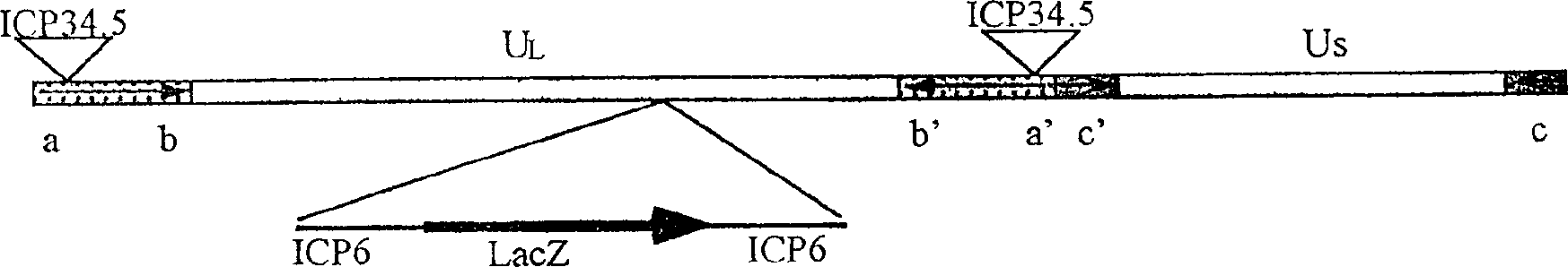
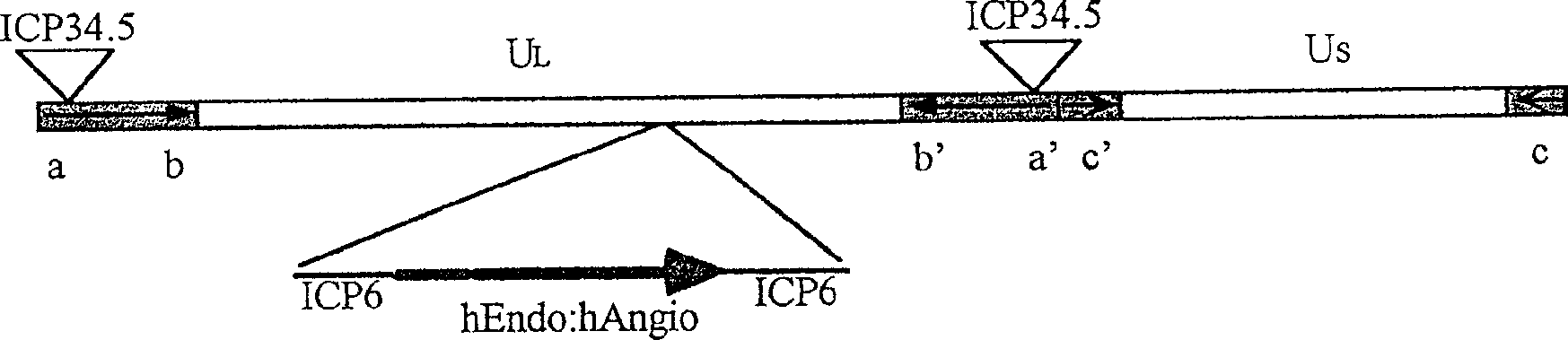
Recombinant herpesvirus carrying antivascular and antiendothelial cell factors fusion gene
InactiveCN1397642AGenerate blockEnsure safetyViruses/bacteriophagesDNA/RNA fragmentationAngiostatinEndostatin
A recombinant herpesvirus carrying the endostatin and angiostatin fused gene for effectively treating colloima features that its original virus HSV-1 contains a long nonrepetitive region (UL) and a short one (US). After the ICP6 gene in the UL is partly removed, the human endostatin and angiostatin is inserted to obtain the fused gene.
Owner:罗益(无锡)生物制药有限公司
Nucleolin-Mediated Cancer Diagnostics and Therapy
ActiveUS20090191224A1High sensitivityEfficient killingPeptide/protein ingredientsMicrobiological testing/measurementCancers diagnosisTumor necrosis factor alpha
The present invention provides for diagnostic kits for identifying cancer patients who are more susceptible to cancer therapies employing endostatin and other angiogenesis inhibitors, based upon the discovery that Nucleolin is a specific receptor for Endostatin. In particular, the diagnostic kits include antibody molecules against Nucleolin, DNA or RNA molecules that specifically bind to nucleic acid molecules encoding Nucleolin. The present invention also discloses methods of screening for angiogenesis inhibitors which specifically interact with Nucleolin, and act as angiogenesis inhibitors in an analogous manner as Endostatin. In addition, the present invention discloses methods of inhibiting the proliferation of endothelial cells or angiogenesis of tumor by administering an anti-nucleolin antibody linked to a cytotoxic agent such as tumor necrosis factor alpha to the endothelial cells.
Owner:TSINGHUA UNIV +1
Endostatin mutants with mutations at atp binding sites
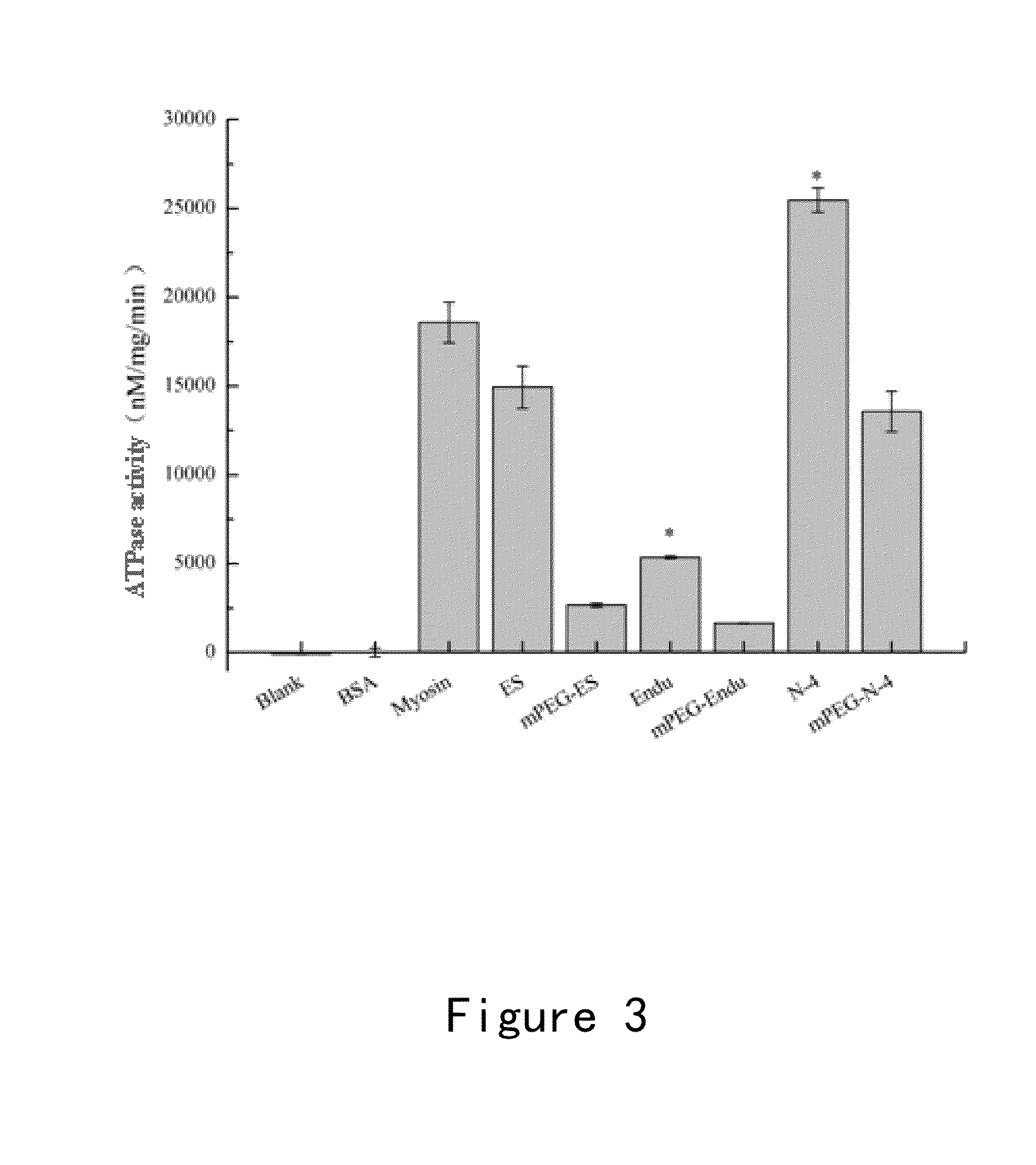
ActiveUS20140308263A1Easy to operateGood reproducibilityHydrolasesPeptide/protein ingredientsBinding siteEndostatin
The present invention discloses a new anti-tumor medicament comprising a mutant of endostatin. The mutant comprises a mutation in the ATP-binding site of endostatin and has a decreased ATPase activity and an increased anti-angiogenesis activity.
Owner:BEIJING PROTGEN +1
Tat PTD-Endostatin-RGD recombinant protein and preparation method and application thereof
ActiveCN105218683AImprove targetingExtended stayPeptide/protein ingredientsPharmaceutical non-active ingredientsDiseaseTat PTD-endostatin
The invention discloses Tat PTD-Endostatin-RGD recombinant protein which is composed of the protein transduction domain of transactivation transduction protein Tat of human immunodeficiency viruses, the human endostatin and RGD tripeptide coming from the fiber cell attachment domain. The invention further discloses a preparation method of the Tat PTD-Endostatin-RGD recombinant protein, and application of the Tat PTD-Endostatin-RGD recombinant protein to medicine for treating diseases caused by new vessels and medicine for restraining endothelial cell migration. The Tat PTD-Endostatin-RGD recombinant protein reserves the activity of endostatin for restraining new vessel proliferation, and can penetrate through blood brain barriers or eyeball barriers to be combined with integrin specificity over-expressed at new vessels, the targeting performance of the fused protein is greatly improved, and the retention time of the fused protein is greatly prolonged.
Owner:SHANDONG UNIV
Anticancer sustained-released formulation loaded with blood vessel inhibitor and synergist thereof
InactiveCN101380304AIncreased sensitivityGrowth inhibitionOrganic active ingredientsPharmaceutical delivery mechanismAngiostatinDepressant
An anticarcinogenic slow release injection carrying an angiogenesis inhibitor and a synergist thereof is made from slow release microspheres and a dissolvant. The slow release microspheres comprise anticancer active components and a slow release adjuvant, and the dissolvant is a special dissolvant containing a suspending agent. The anticancer active components are angiogenesis inhibitors such as gefitinib, erlotinib, lapatinib, vatalanib, pelitinib, thalidomide, ranolamine, angiostatin, endostatin, imatinib, thalidomide, ranolamine, simatinib, dasatinib, avastin, kanatini, sorafenib, sunitinib, telstar or panitoma, and the like, and / or cytotoxic drugs selected from a phosphoinositide-3-kinase inhibitor, pyrimidine analogue and / or a DNA repair enzyme inhibitor; the slow release adjuvant is biocompatible macromolecule; the viscosity of the suspending agent is 100cp-3,000cp (at the temperature of 20-30 DEG C), and the suspending agent is selected from sodium carboxymethyl cellulose, and the like. The slow release microspheres can be also made into a slow release implant, and the curative effects of non-operative therapies such as radiotherapy, chemotherapy, and the like, can be improved when the slow release injection is injected or placed in tumors or around the tumors.
Owner:SHANDONG LANJIN PHARMA +1
Recombinant herpes simplex virus capable of excreting angiostatin and endostatin protein and its application in treating lung cancer
InactiveCN1702171AIncrease volumeDoes not affect assemblyGenetic material ingredientsViruses/bacteriophagesAngiostatinEndostatin
The present invention relates to a recombinant herpes simplex virus and a method of gene treat of human tumour by using the recombinant virus, particularly relates to a recombinant herpes simplex virus with blood vessel chalone and endodermis chalone albumen, and its application in treating lung cancer. The invention also relates to medicine compound containing the recombinant virus. The recombinant herpes simplex virus of the invention can represent blood vessel chalone and endodermis chalone amalgamation albumen having effect of restraining the generation of blood vessel in the tumour cell, and the virus disintegratively increases in the tumour, such that the purpose of treating lung cancer by restraining the generation of blood vessel and disintegratively decomposing tumour cell is achieved.
Owner:罗益(无锡)生物制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com