Tat PTD-Endostatin recombination protein, preparation method and application thereof
A recombinant protein and transduction protein technology, applied in the field of protein transduction, can solve the problems of increasing the economic burden of patients, large dosage, poor cell penetration ability, etc. over effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 adopts yeast expression system to express Tat PTD-ES fusion protein
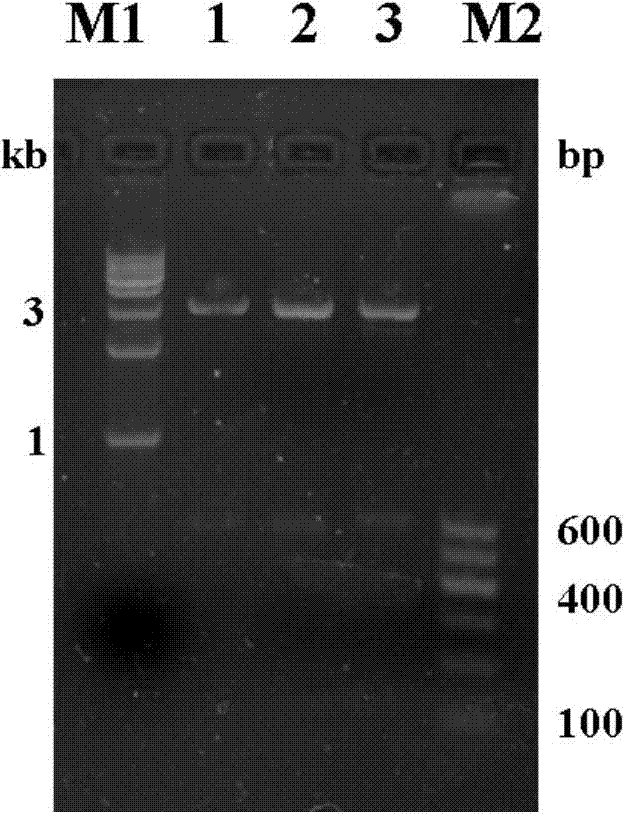
[0055] a. Amplification of Tat PTD-ES fusion gene: 33 codons encoding 11 amino acids of Tat-PTD and a selected enzyme cutting site (EcoR Ⅰ) were designed to the 5′ end of the upstream primer, and PCR technology was used to extract the endostatin-containing pGAPZ α The Tat PTD-ES fusion gene is amplified in a plasmid (owned by the inventor's laboratory and constructed by conventional methods), and the fusion gene fragments obtained by the amplification are about 600, and the PCR results are shown in figure 1 .
[0056] b. Construction of an expression plasmid containing the Tat PTD-ES fusion gene:
[0057] ① pGAPZ α A. Amplification and extraction of yeast expression plasmids: DH5α was routinely activated to prepare competent cells, and pGAPZ was taken α A. Plasmid DNA heat shock method was used to transform DH5α. After transformation, the bacterial solution was spread on a low-salt LB...
Embodiment 2
[0065] Embodiment 2 adopts Escherichia coli to express Tat PTD-ES fusion protein
[0066] a. Double digestion of fusion gene and empty plasmid: Nde Ⅰ and BamH Ⅰ were used to perform double digestion of Tat PTD-ES fusion gene (constructed by conventional methods) and pET28a expressed in the large intestine, and the fragments were recovered respectively.
[0067] b. Ligation of fusion gene and plasmid: Mix the recovered target gene and plasmid in the ligation system, incubate overnight at 4°C, and the ligation product is used for the next step of transformation.
[0068] c. Transformation of recombinant plasmids: Add 200 μl of E.coli JM109 competent cells to the above ligation reaction solution, mix well, ice-bath for 30 minutes, heat shock at 42°C for 90 seconds, ice-bath for 2 minutes, add 0.8ml LB medium, incubate at 37°C for 1 hour, Spread the incubation solution on the LK plate and incubate at 37°C for 12-16h.
[0069] d. Identification of positive recombinants: Randomly s...
Embodiment 3
[0074] Example 3 The technical process of the renaturation of the inclusion body protein expressed by Escherichia coli
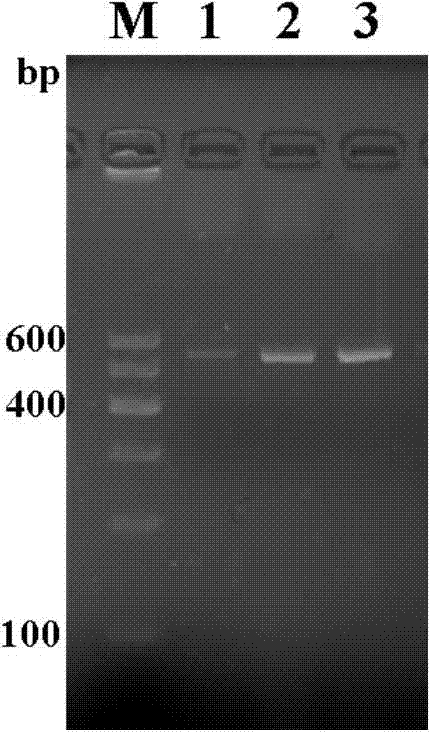
[0075] a. Inclusion body solubilization: after cell wall breaking and ultrasonication, centrifuge at 10,000g for 30 minutes, discard the supernatant to obtain inclusion bodies; wash with urea or guanidine hydrochloride solution containing detergent for several times to remove adherent impurities; then use denaturation The solution was used to dissolve the inclusion bodies, stirred at room temperature for 2 hours, centrifuged at 10000 g for 30 minutes, and the supernatant was the inclusion body solution. The detergent solution is urea with a concentration of 2mol / L, and the detergent solution also contains Triton x-100 at a concentration of 0.5% (v / v). Denaturation solution contains 20-100mmol / LTris / HCl, 8mol / L urea, 5mmol / LEDTA and 10mmol / LDTT. SDS-PAGE results such as Figure 7 shown.
[0076] b. Protein renaturation: After the renaturation buffer is fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com