A class of benzylamino flurbiprofen amide compounds, its preparation method and use
A technology of benzylamino flurbiprofen amide and arylamide amino flurbiprofen amide, which is applied to the preparation of organic compounds, carboxylic acid amide preparation, chemical instruments and methods, etc., and can solve the problem of single action mechanism and inability to Improve cognitive decline, daily behavior, and low aggregation activity in patients with moderate AD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
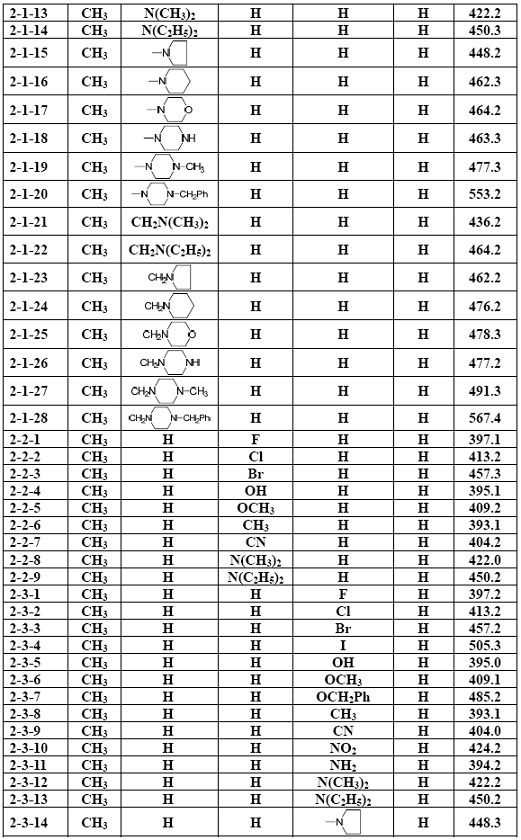
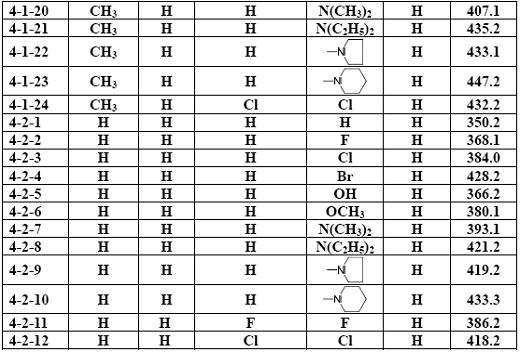
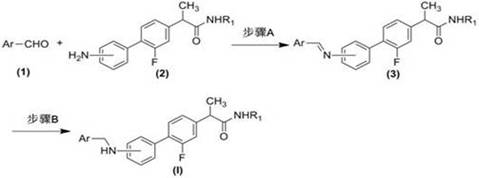
[0038] Example 1 The general method for the preparation of arylmethylaminoflurbiprofen amide compounds (3)
[0039] Add 2.3 mmol of the corresponding aromatic formaldehyde compound (1), 2.0 mmol of the corresponding aminoflurbiprofen amide compound (2) and 20 ml of ethanol into the reaction flask, stir evenly, then raise the temperature and reflux and stir for 2.0 to 12.0 hours ( The reaction process was monitored by TLC); after the reaction was completed, it was cooled to room temperature, the solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography to obtain the corresponding arylideneaminoflurbiprofen amide compound (3), with a yield of 52.6 %-88.2%, its structure is 1 Confirmed by H-NMR.
Embodiment 2
[0040] Example 2 General method for the preparation of benzylaminoflurbiprofen amide compounds (I)
[0041] Add 1.0 mmol of the corresponding arylmethylaminoflurbiprofen amide compound (3) prepared in Example 1 and 20 ml of tetrahydrofuran into the reaction flask, stir at room temperature, add 2.5 mmol of sodium borohydride, and continue stirring at room temperature React for 1.0 to 15.0 hours (the reaction process is monitored by TLC); after the reaction, adjust the pH of the reaction solution to strong acidity with 10% hydrochloric acid aqueous solution, then adjust the pH of the reaction solution to neutrality with saturated aqueous sodium bicarbonate solution, and distill off tetrahydrofuran under reduced pressure , add 20mL deionized water to the residual liquid, extract three times with 90 mL dichloromethane, wash the organic layer with saturated aqueous sodium chloride solution after combining, filter after drying over anhydrous sodium sulfate, distill off the solvent un...
Embodiment 3
[0061] Example 3 General method for the preparation of benzylaminoflurbiprofen amides (I) and acid salt formation
[0062] Add 1.0 mmol of the benzylaminoflurbiprofen amide compound (I) obtained in Example 2 above and 20 ml of acetone into the reaction flask, stir evenly, add 3.0 mmol of the corresponding acid, heat and reflux and stir for 20 minutes, the reaction After cooling to room temperature, the solvent was evaporated under reduced pressure, and the residue was recrystallized by conventional methods to obtain the corresponding salt of benzylamino flurbiprofen amide compounds (I), whose chemical structure was 1 Confirmed by H NMR and ESI-MS.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com