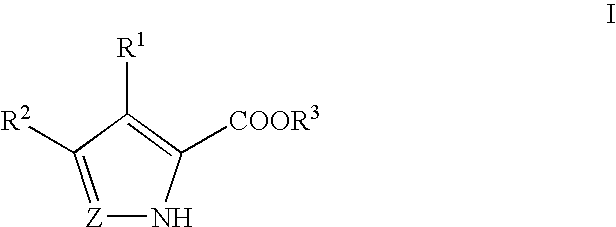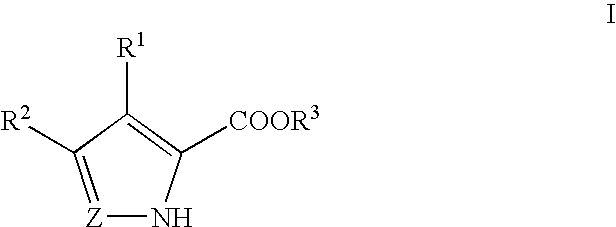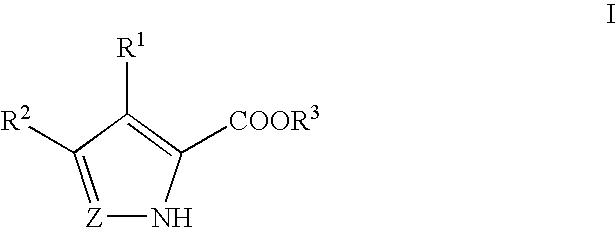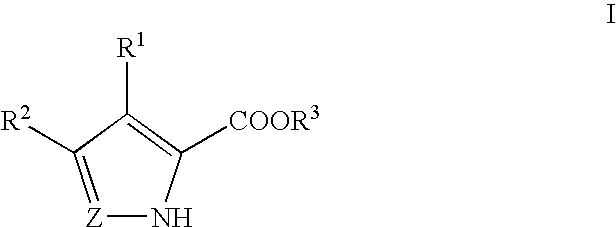Patents
Literature
70 results about "Cycloserine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used with other medications to treat tuberculosis (TB). In some cases, it may also be used to treat urinary tract infections (UTIs).
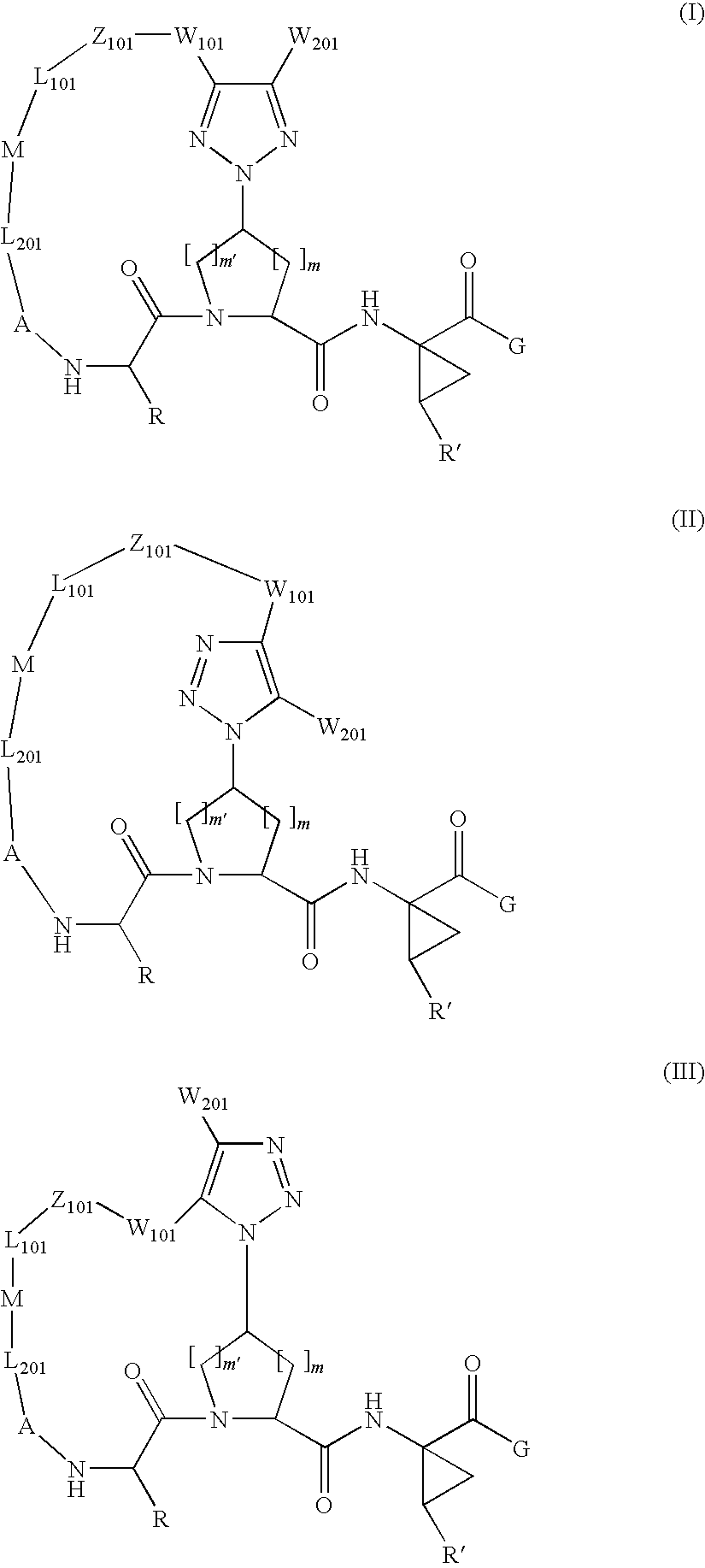
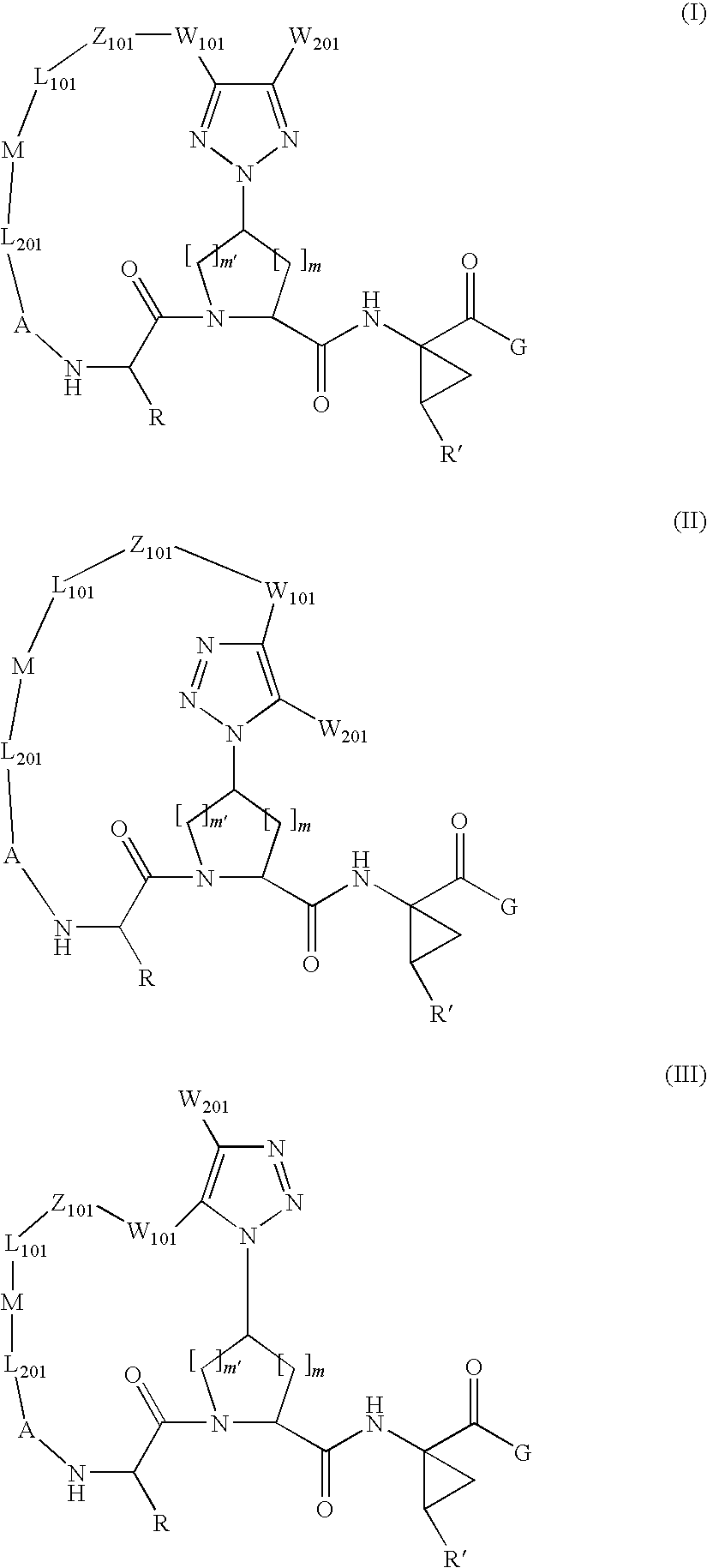
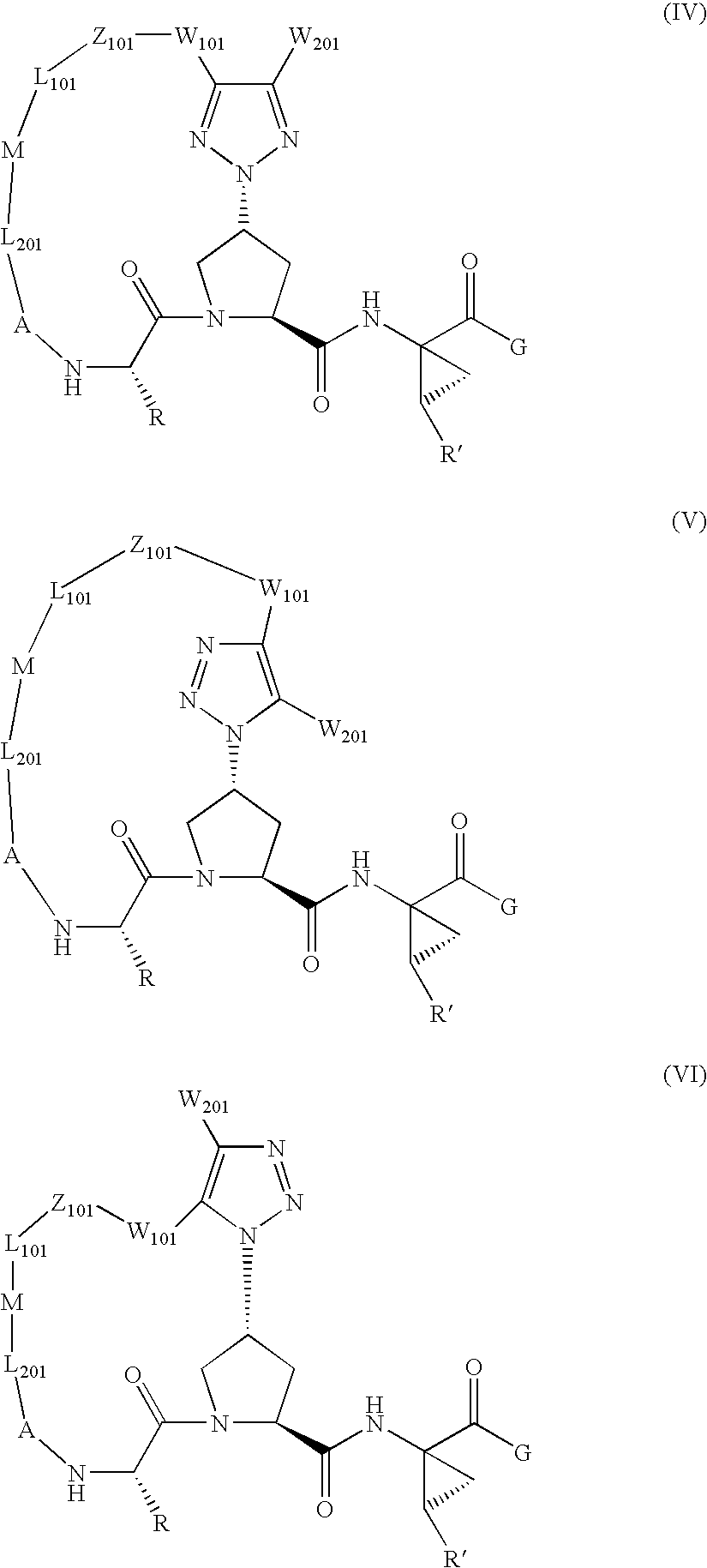
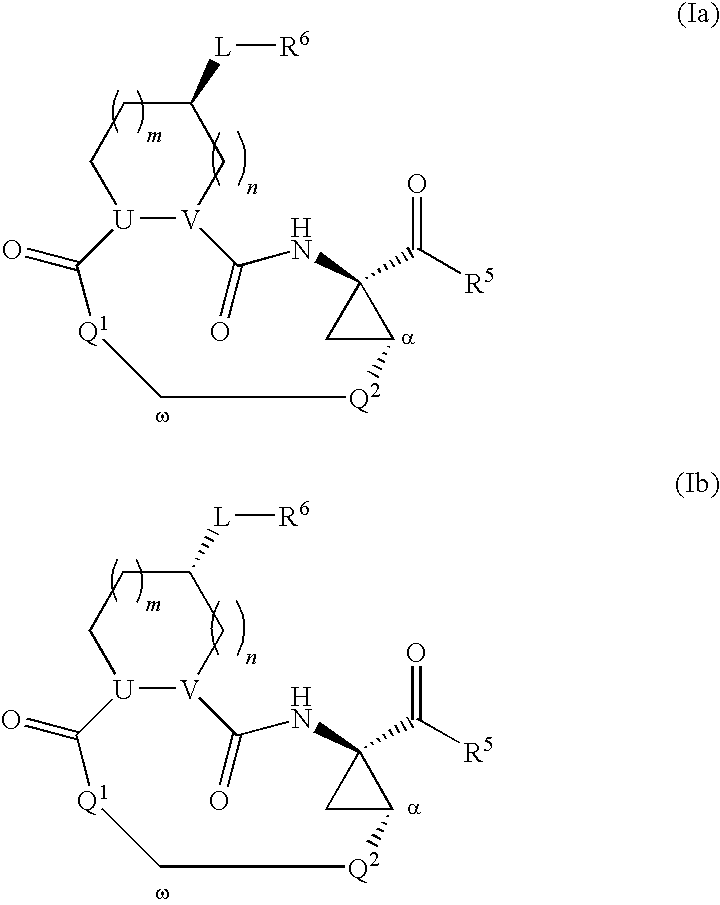
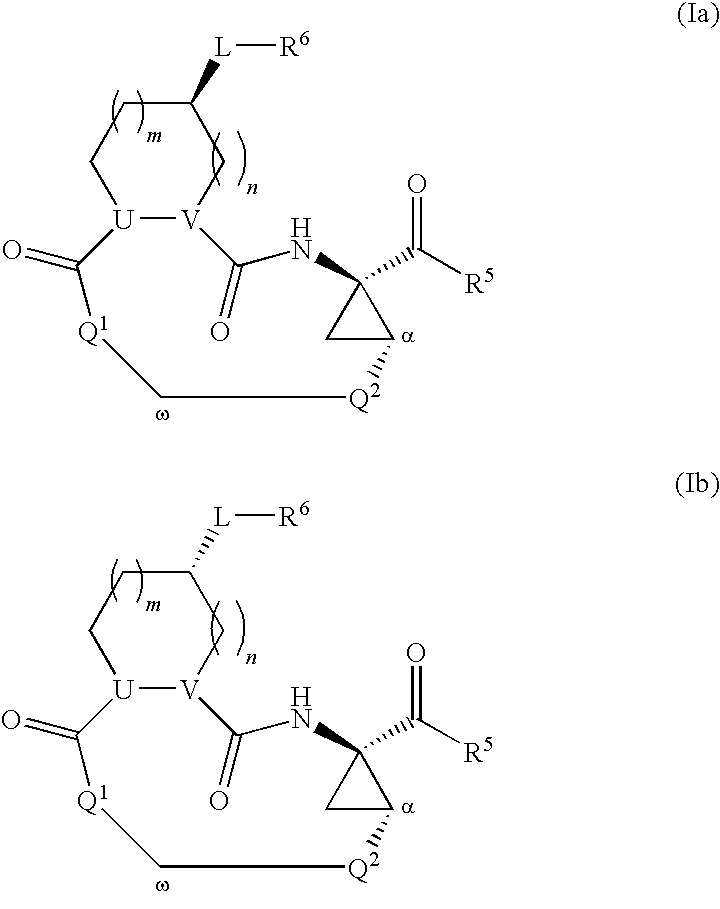
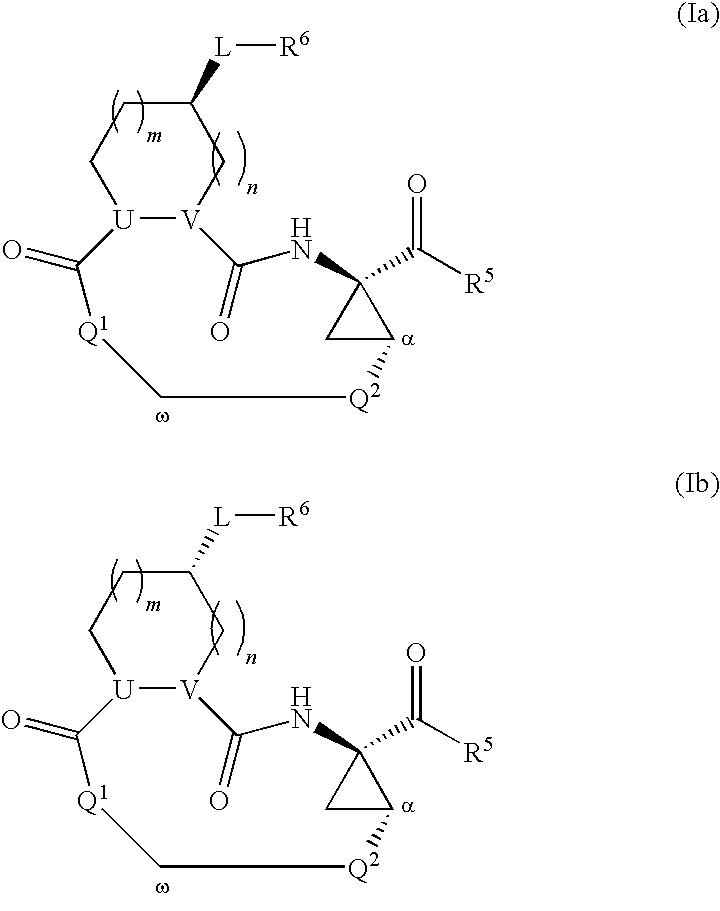
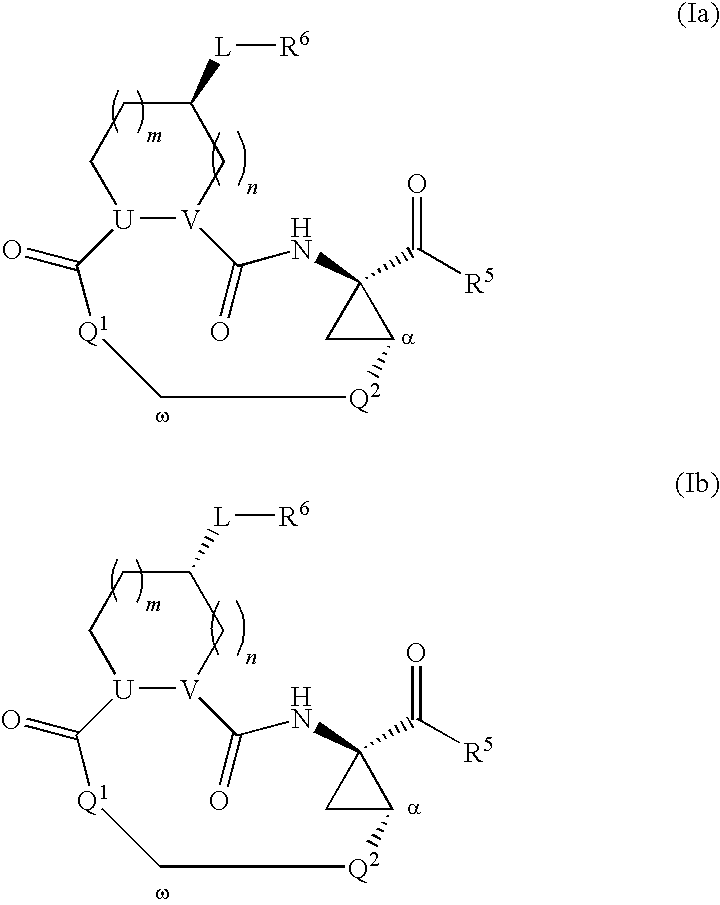
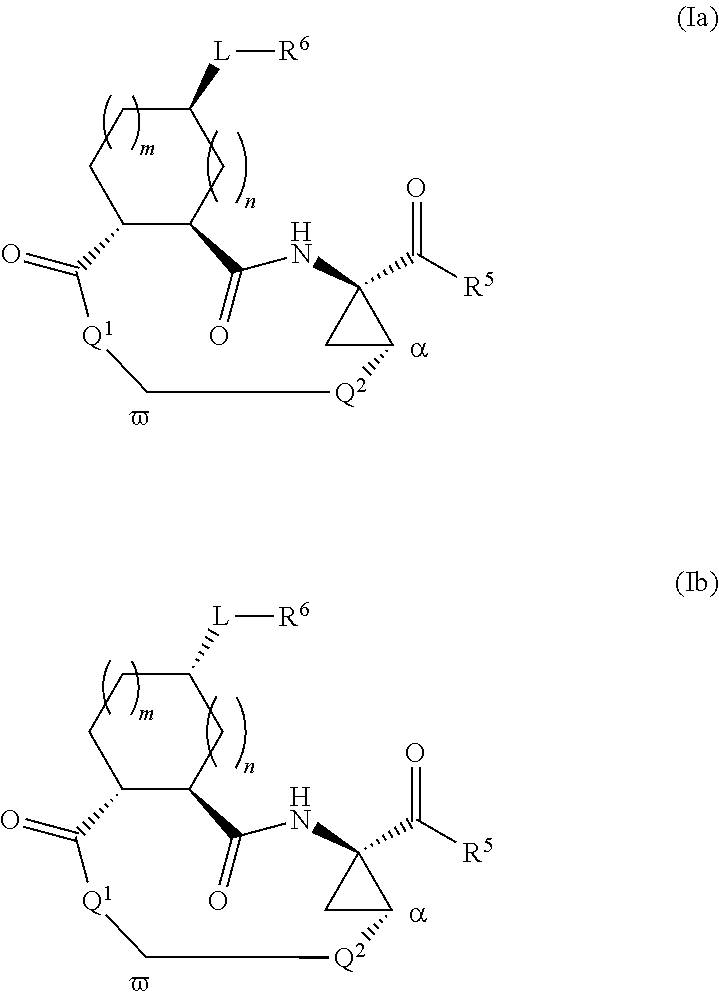
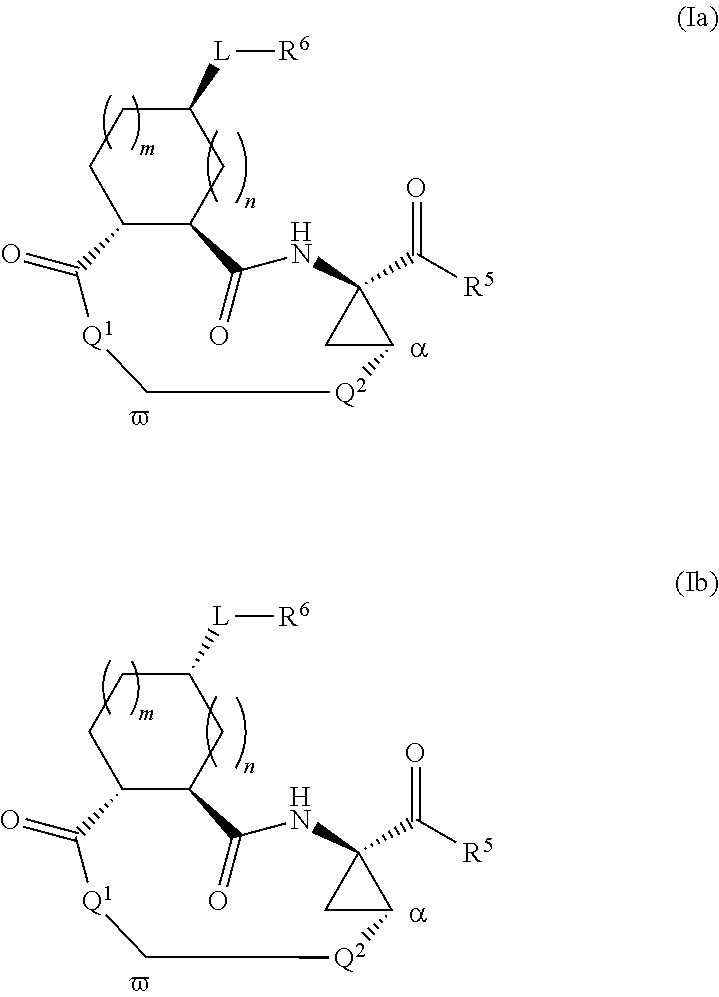
Triazole-containing macrocyclic hcv serine protease inhibitors
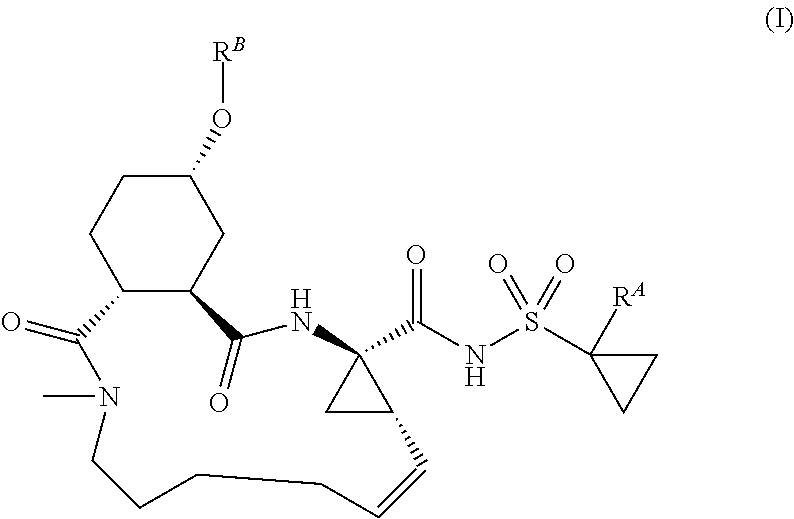
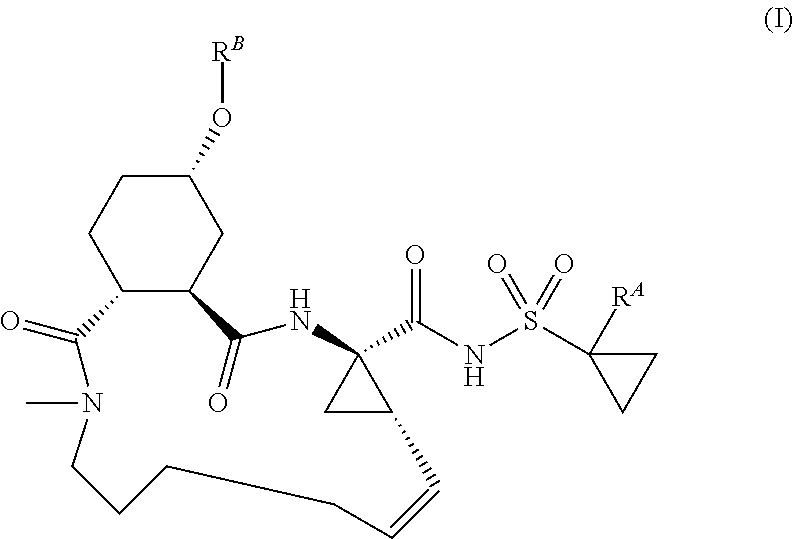
The present invention discloses compounds of formula I, II and III or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
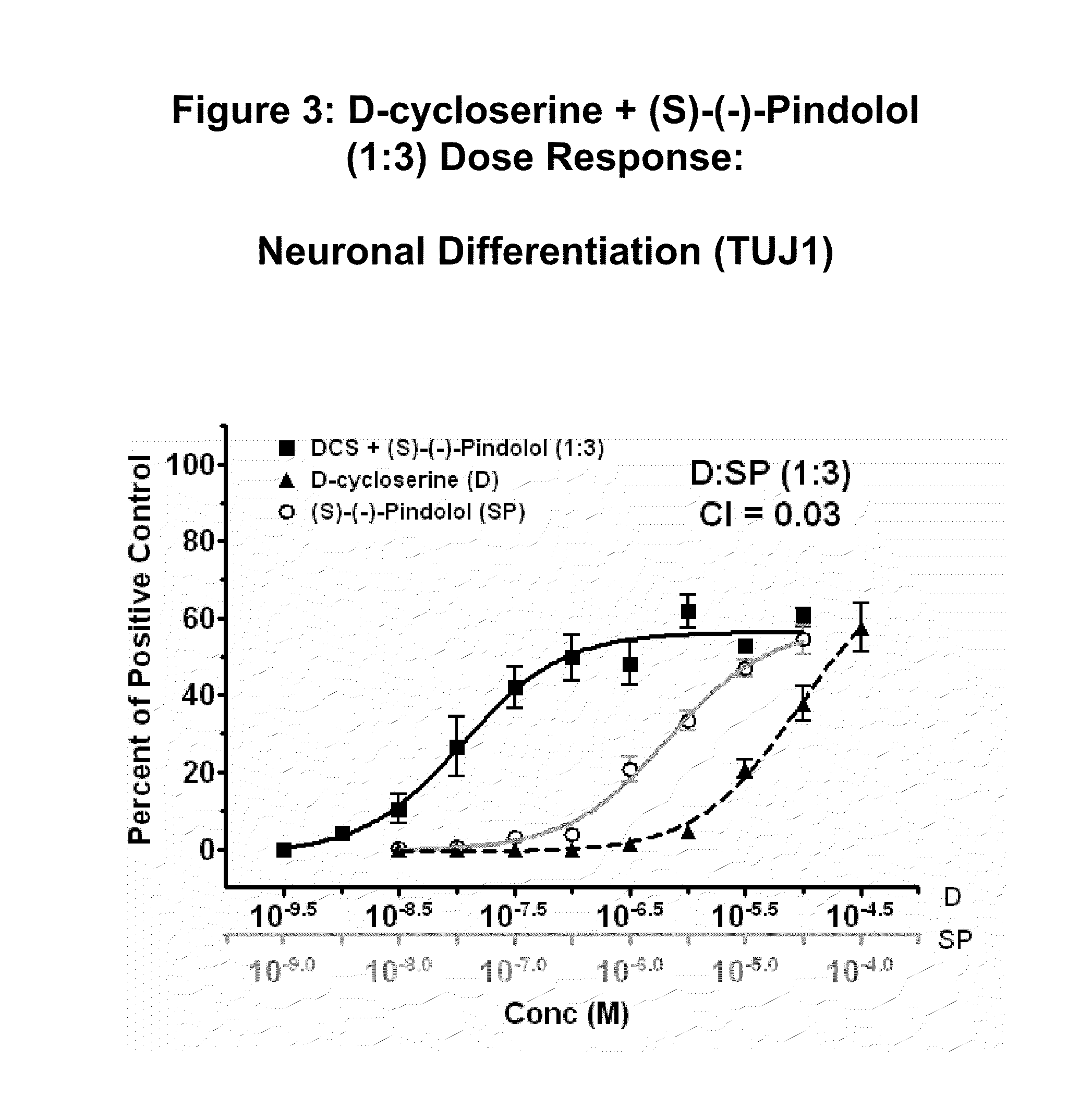
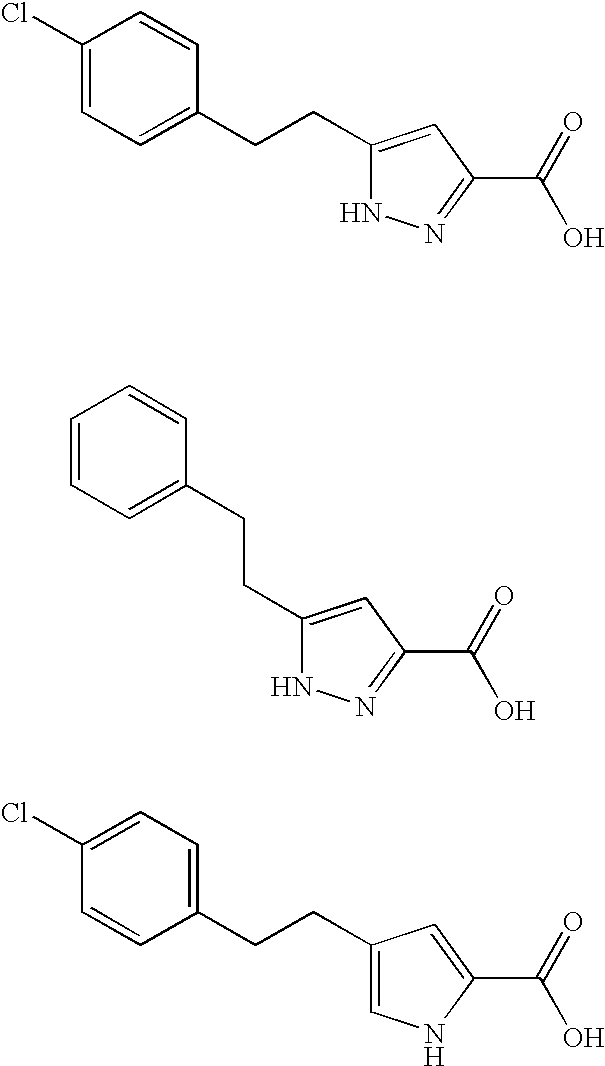
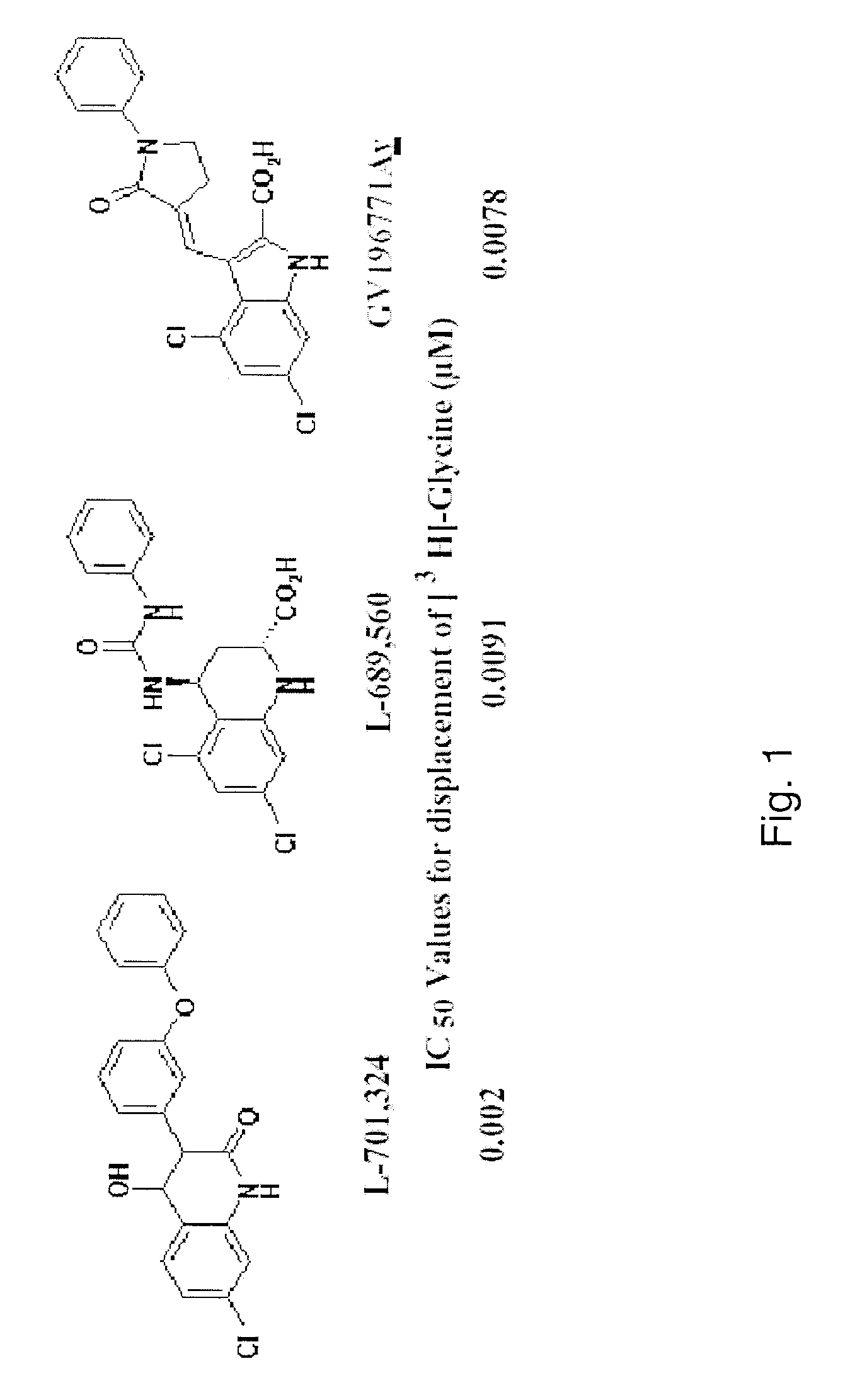
Pyrrole and pyrazole DAAO inhibitors
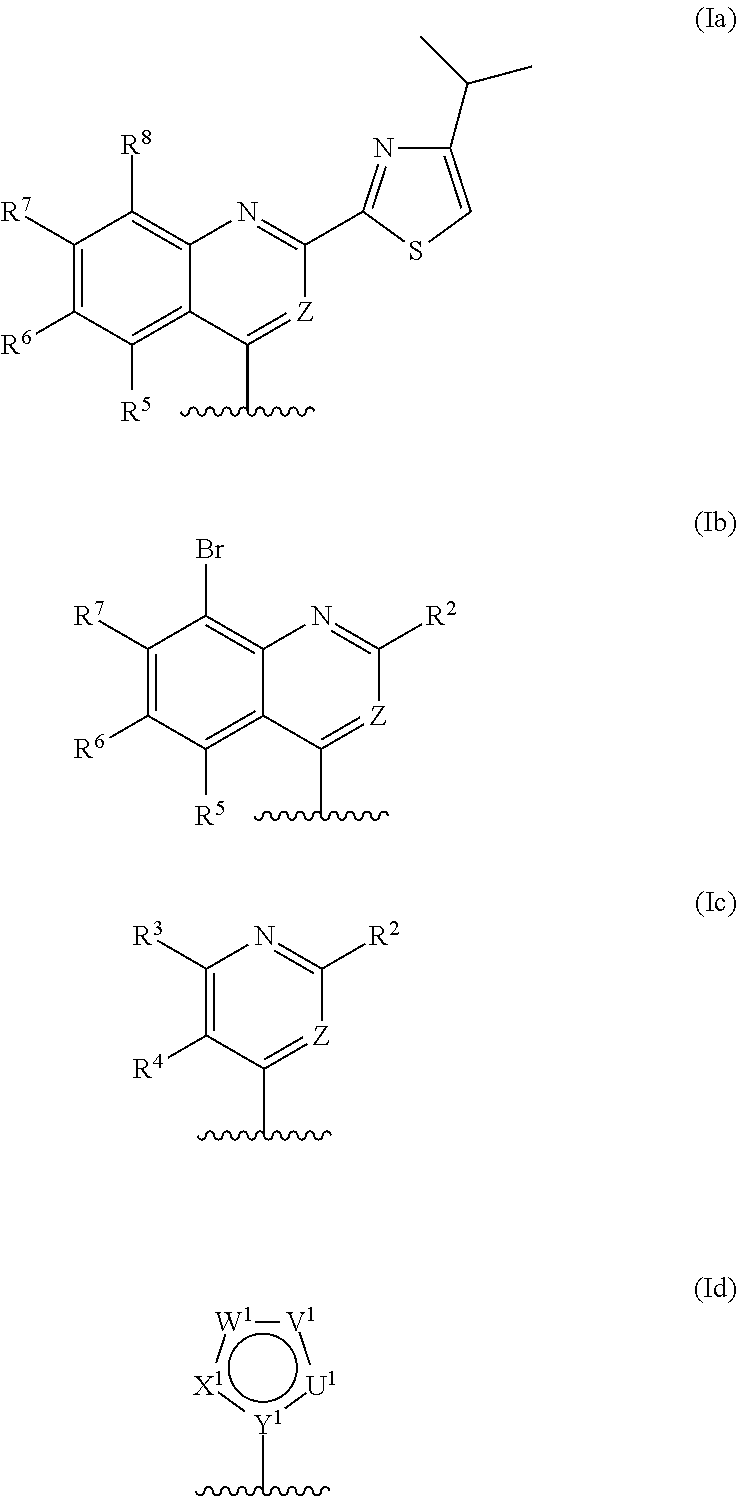
Methods for increasing D-Serine concentration and reducing concentration of the toxic products of D-Serine oxidation, for enhancing learning, memory and / or cognition, or for treating schizophrenia, Alzheimer's disease, ataxia or neuropathic pain, or preventing loss in neuronal function characteristic of neurodegenerative diseases involve administering to a subject in need of treatment a therapeutically effective amount of a compound of formula I, or a pharmaceutically acceptable salt or solvate thereof: wherein [0001]R1 and R2 are independently selected from hydrogen, halo, nitro, alkyl, acyl, alkylaryl, and XYR5; [0002]or R1 and R2, taken together, form a 5, 6, 7 or 8-membered substituted or unsubstituted carbocyclic or heterocyclic group; [0003]X and Y are independently selected from O, S, NH, and (CR6R7)n; [0004]R3 is hydrogen, alkyl or M+; M is aluminum, calcium, lithium, magnesium, potassium, sodium, zinc ion or a mixture thereof; [0005]Z is N or CR4; [0006]R4 is from selected from hydrogen, halo, nitro, alkyl, alkylaryl, and XYR5; [0007]R5 is selected from aryl, substituted aryl, heteroaryl and substituted heteroaryl; [0008]R6 and R7 are independently selected from hydrogen and alkyl; n is an integer from 1 to 6; [0009]at least one of R1, R2 and R4 is other than hydrogen; and [0010]at least one of X and Y is (CR6R7)n. D-serine or cycloserine may be coadministered along with the compound of formula I.
Owner:SEPACOR INC
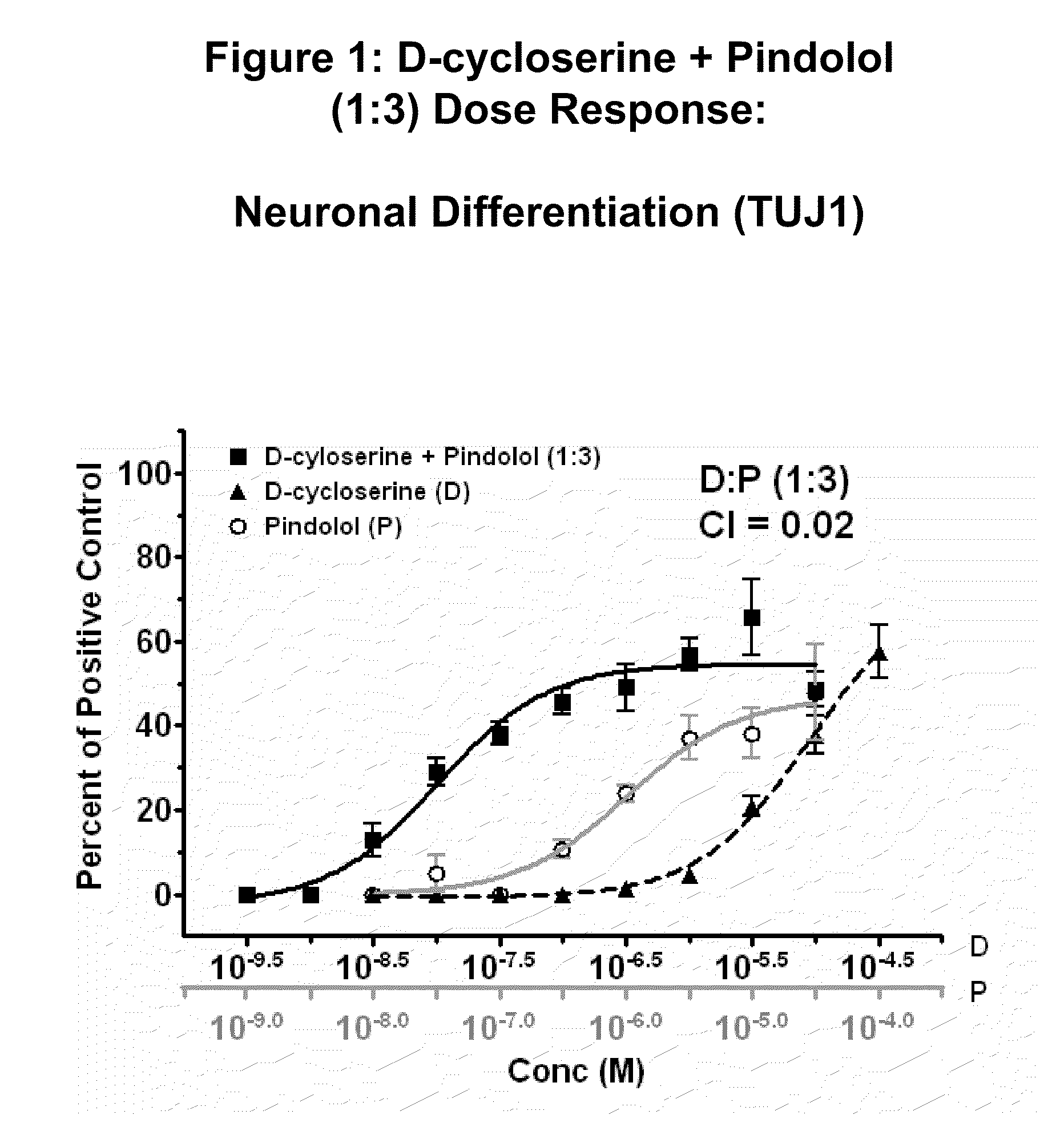
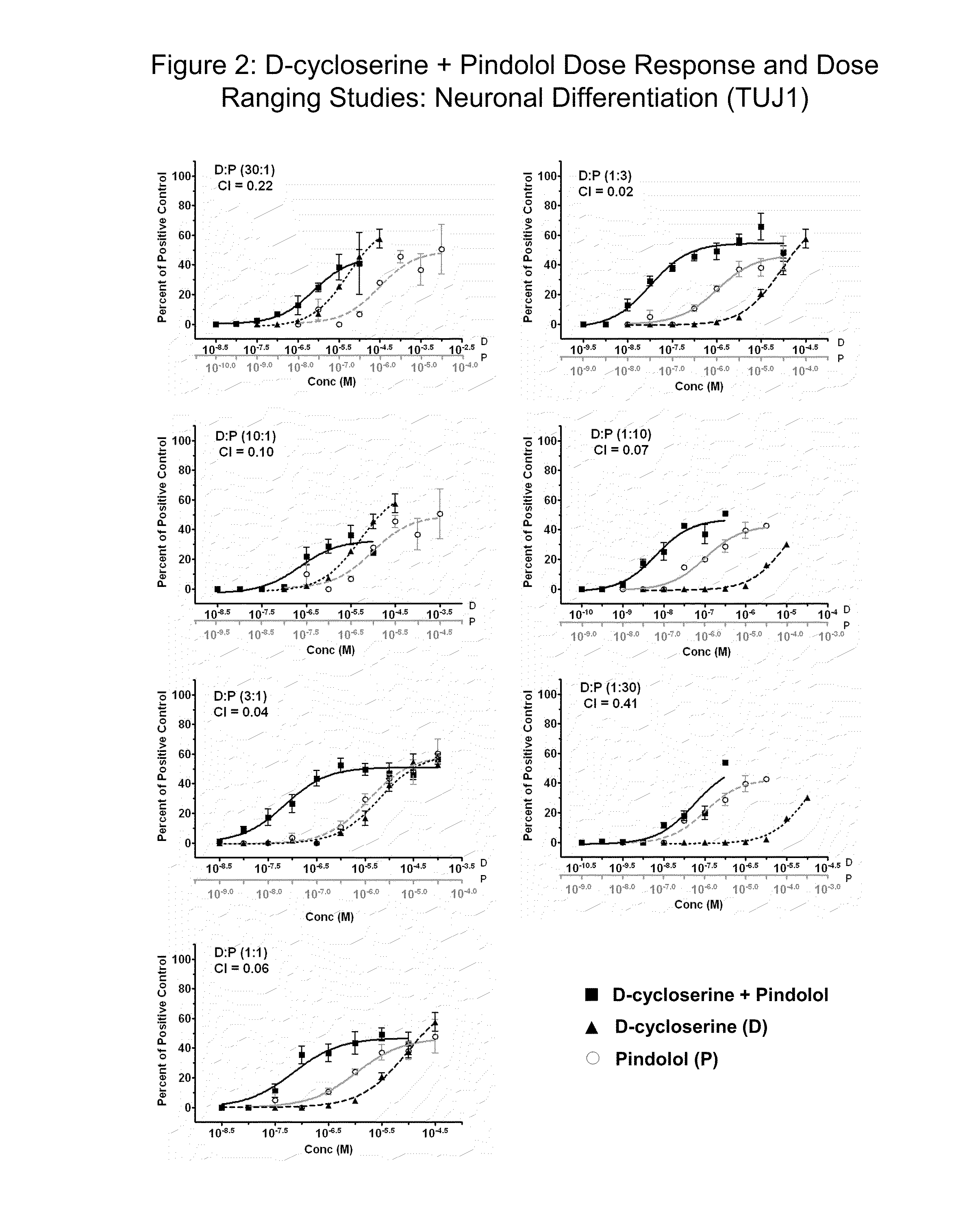
Modulation of neurogenesis using d-cycloserine combinations
InactiveUS20100216805A1Reduce decreaseLower Level RequirementsBiocideNervous disorderDiseaseNervous system
The disclosure provides compositions and methods for treating diseases and conditions of the central and peripheral nervous system by stimulating or increasing neurogenesis. The disclosure provides compositions and methods based on the use of D-cycloserine in combination with the neurogenic agent, which synergistically stimulates or activates the formation of new nerve cells.
Owner:BRAINCELLS INC
Pyrrole and pyrazole DAAO inhibitors
Methods for increasing D-Serine concentration and reducing concentration of the toxic products of D-Serine oxidation, for enhancing learning, memory and / or cognition, or for treating schizophrenia, Alzheimer's disease, ataxia or neuropathic pain, or preventing loss in neuronal function characteristic of neurodegenerative diseases involve administering to a subject in need of treatment a therapeutically effective amount of a compound of formula I, or a pharmaceutically acceptable salt or solvate thereof:whereinR1 and R2 are independently selected from hydrogen, halo, nitro, alkyl, acyl, alkylaryl, and XYR5;or R1 and R2, taken together, form a 5, 6, 7 or 8-membered substituted or unsubstituted carbocyclic or heterocyclic group;X and Y are independently selected from O, S, NH, and (CR6R7)n;R3 is hydrogen, alkyl or M+;M is aluminum, calcium, lithium, magnesium, potassium, sodium, zinc ion or a mixture thereof;Z is N or CR4;R4 is from selected from hydrogen, halo, nitro, alkyl, alkylaryl, and XYR5;R5 is selected from aryl, substituted aryl, heteroaryl and substituted heteroaryl;R6 and R7 are independently selected from hydrogen and alkyl;n is an integer from 1 to 6;at least one of R1, R2 and R4 is other than hydrogen; andat least one of X and Y is (CR6R7)n.D-serine or cycloserine may be coadministered along with the compound of formula I.
Owner:SEPACOR INC
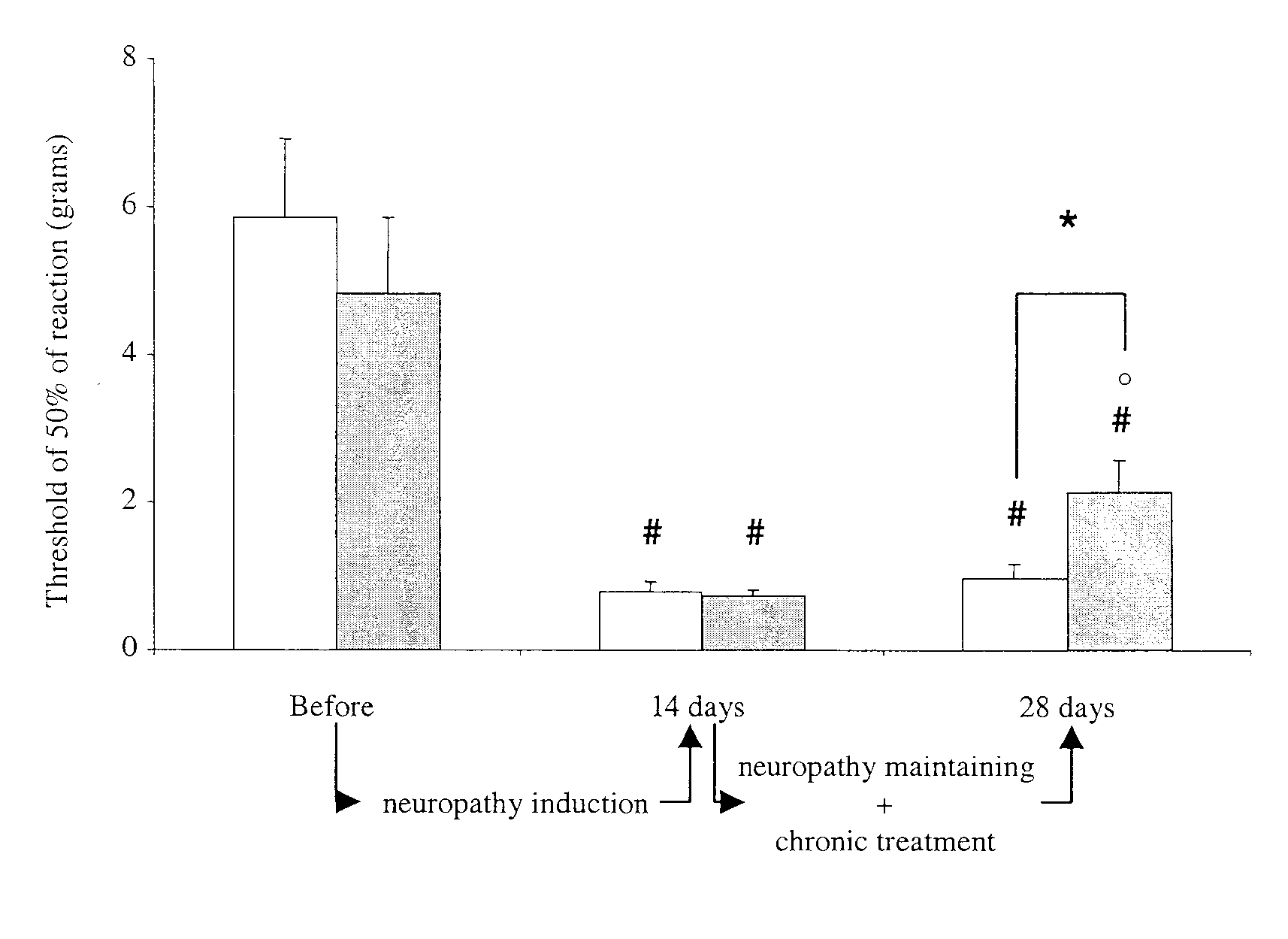
Method and compositions for treatment of chronic neuropathic pain
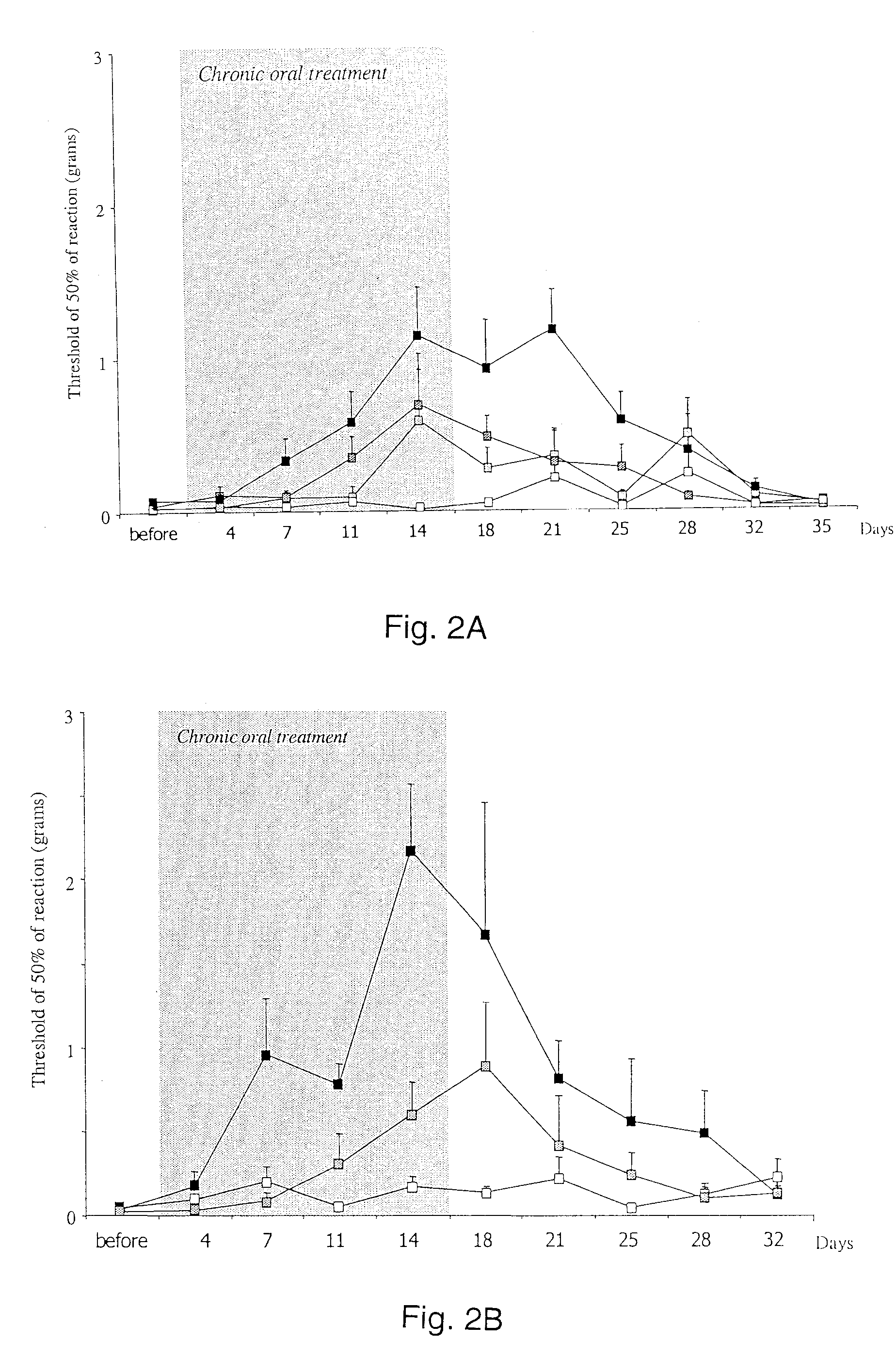
Chronic pain is treated in an individual suffering from chronic pain by administering to the individual an amount of a therapeutic containing a glycine receptor agonist such as D-cycloserine or a GlyT-1 glycine transporter antagonist such as sarcosine in an amount effective to treat the chronic pain. The therapeutic may also contain a secondary analgesic such as opiates, NSAIDs or cox-2 inhibitors. The analgesic can be formulated in a pharmaceutical composition in the form of an injectable solution that contains at least two different analgesics, at least one of the analgesics of which is a glycine receptor agonist or a GlyT-1 glycine transporter antagonist. Suitable pharmaceutical compositions contain D-cycloserine and / or sarcosine, optionally in combination with opiates, NSAIDs or cox-2 inhibitors.
Owner:APKARIAN TECH
Macrocyclic serine protease inhibitors, pharmaceutical compositions thereof, and their use for treating HCV infections
Owner:INDENIX PHARM LLC
Methods for treating neuropsychiatric disorders
InactiveUS20050250851A1High level of efficacyImprove usabilityBiocideNervous disorderAttention deficitsSerine
The invention provides methods for treating neuropsychiatric disorders such as schizophrenia, Alzheimer's Disease, autism, depression, benign forgetfulness, childhood learning disorders, closed head injury, and attention deficit disorder. The methods entail administering to a patient diagnosed as having a neuropsychiatric disorder a pharmaceutical composition containing (i) a therapeutically effective amount of D-alanine (or a modified form thereof), provided that the composition is substantially free of D-cycloserine, and / or (ii) D-serine (or a modified form thereof), and / or (iii) 105 to 500 mg of D-cycloserine (or a modified form thereof), and / or (iv) N-methylglycine (or a modified form thereof).
Owner:THE GENERAL HOSPITAL CORP
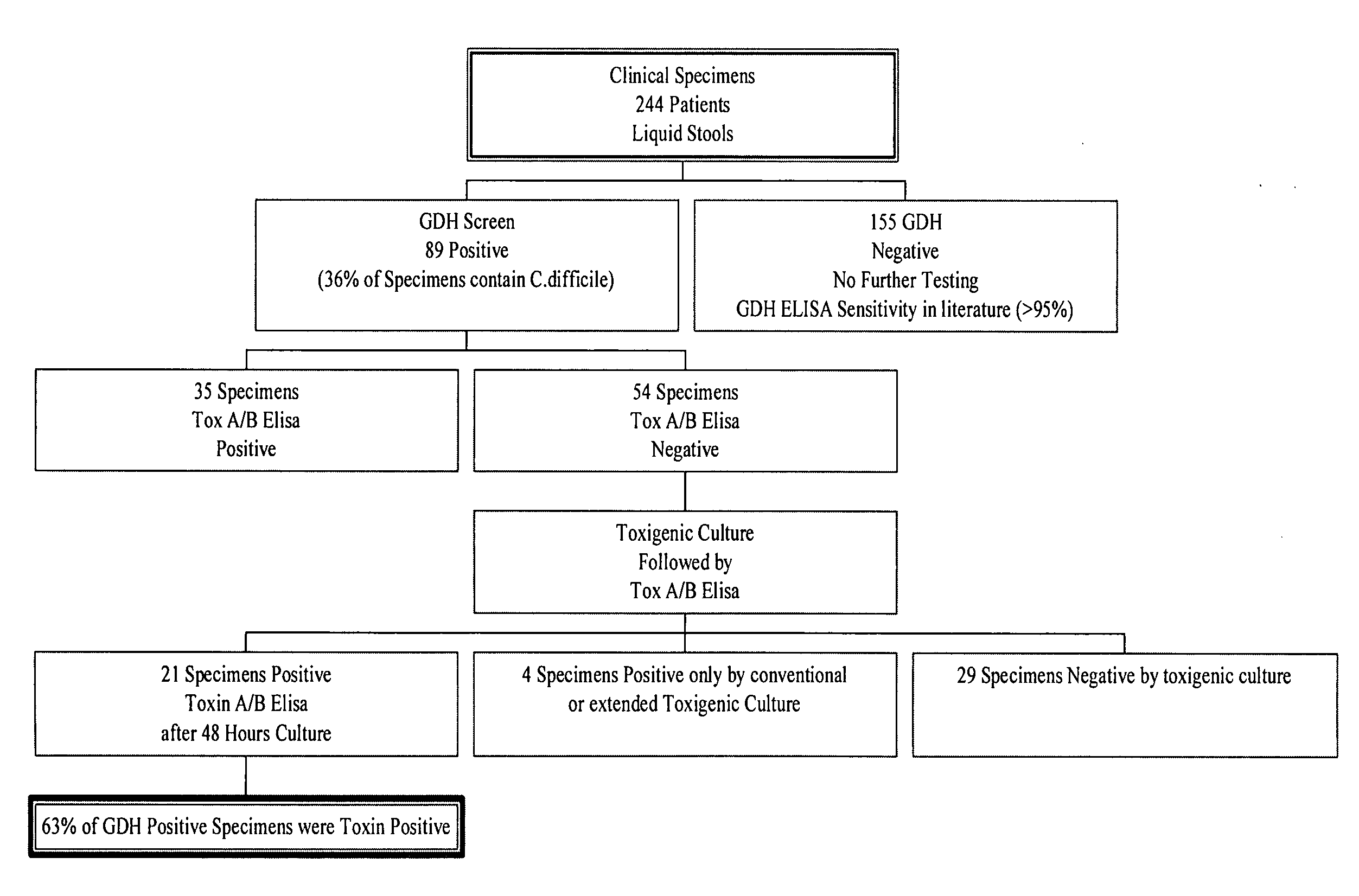
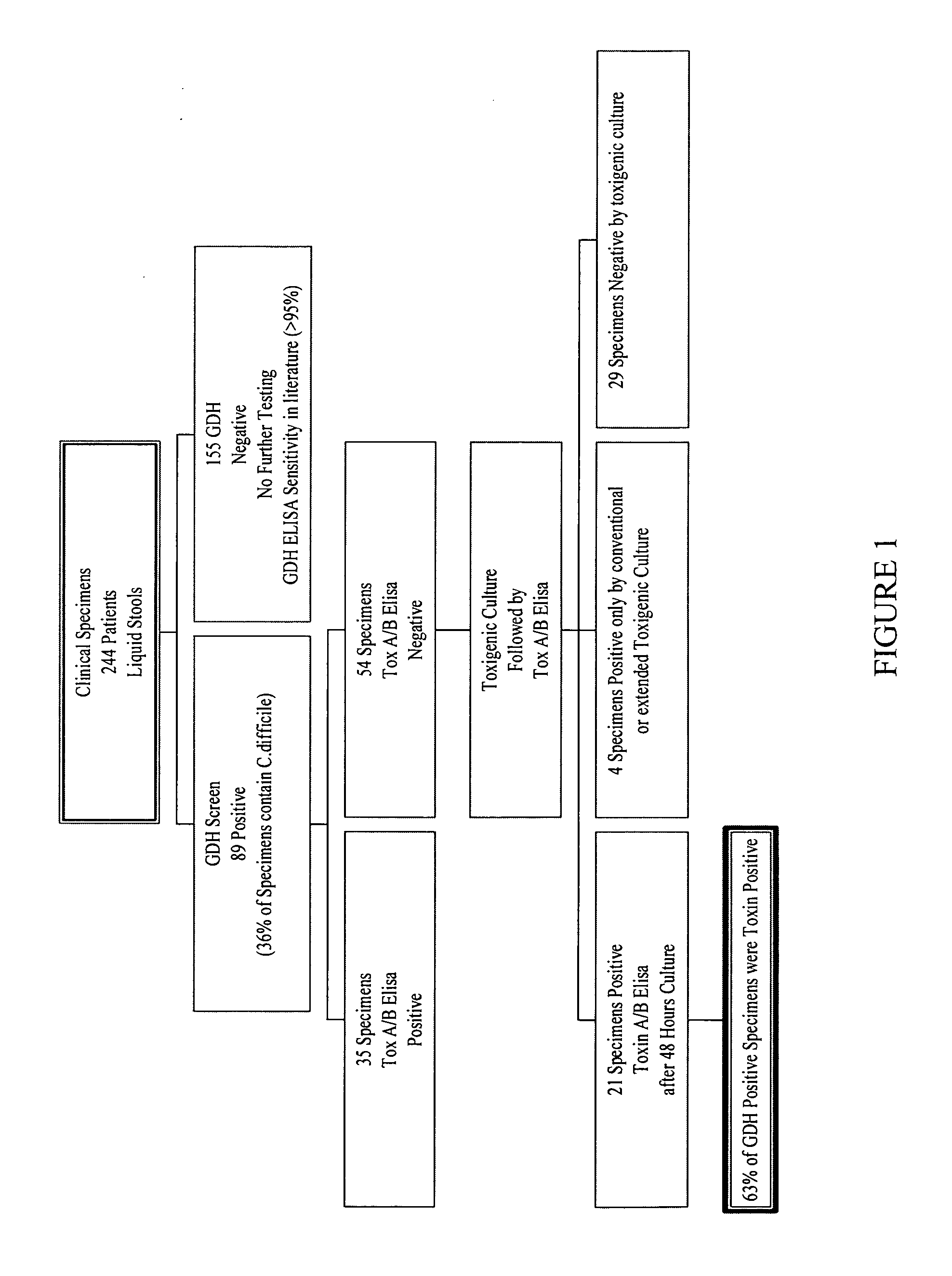
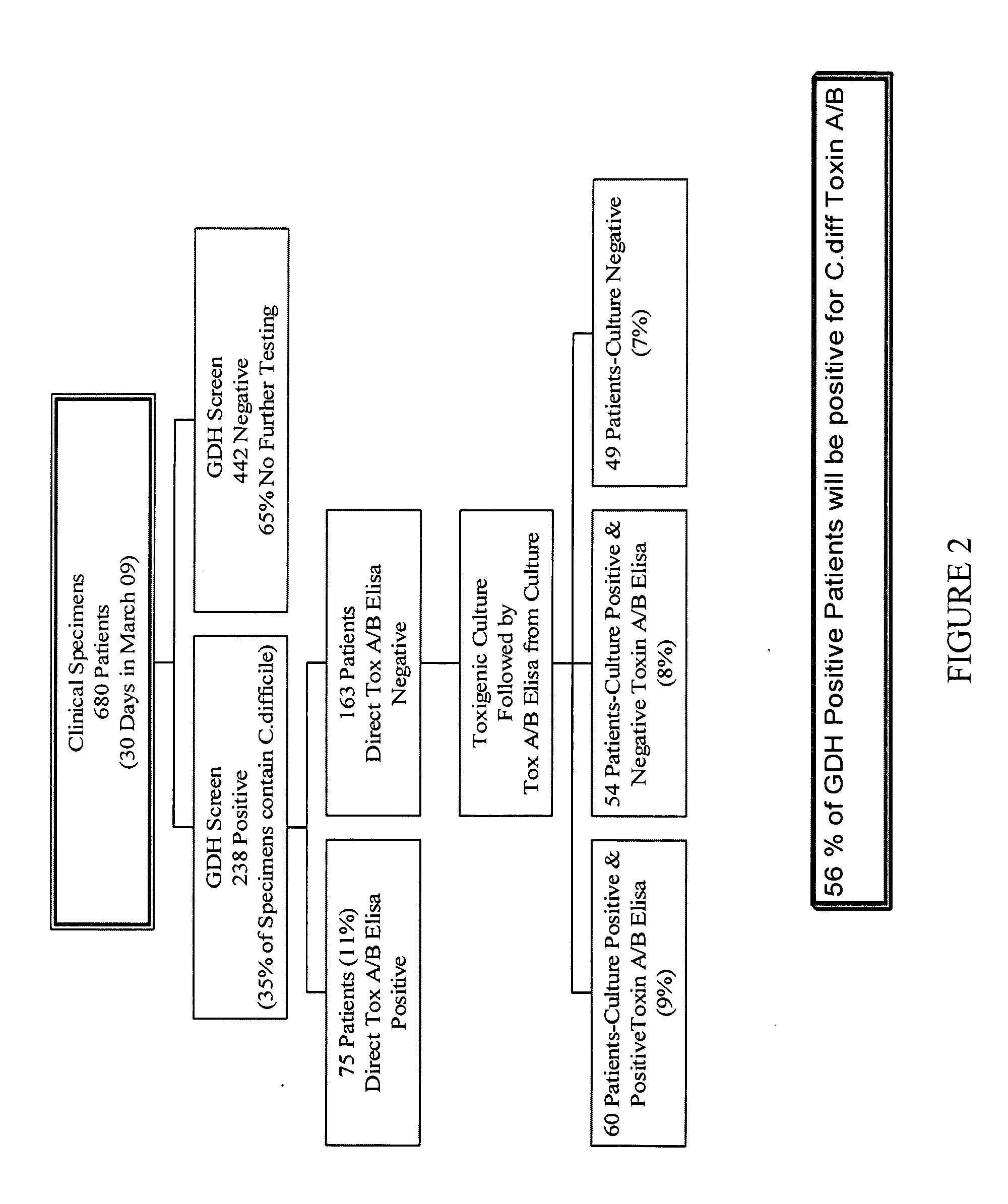
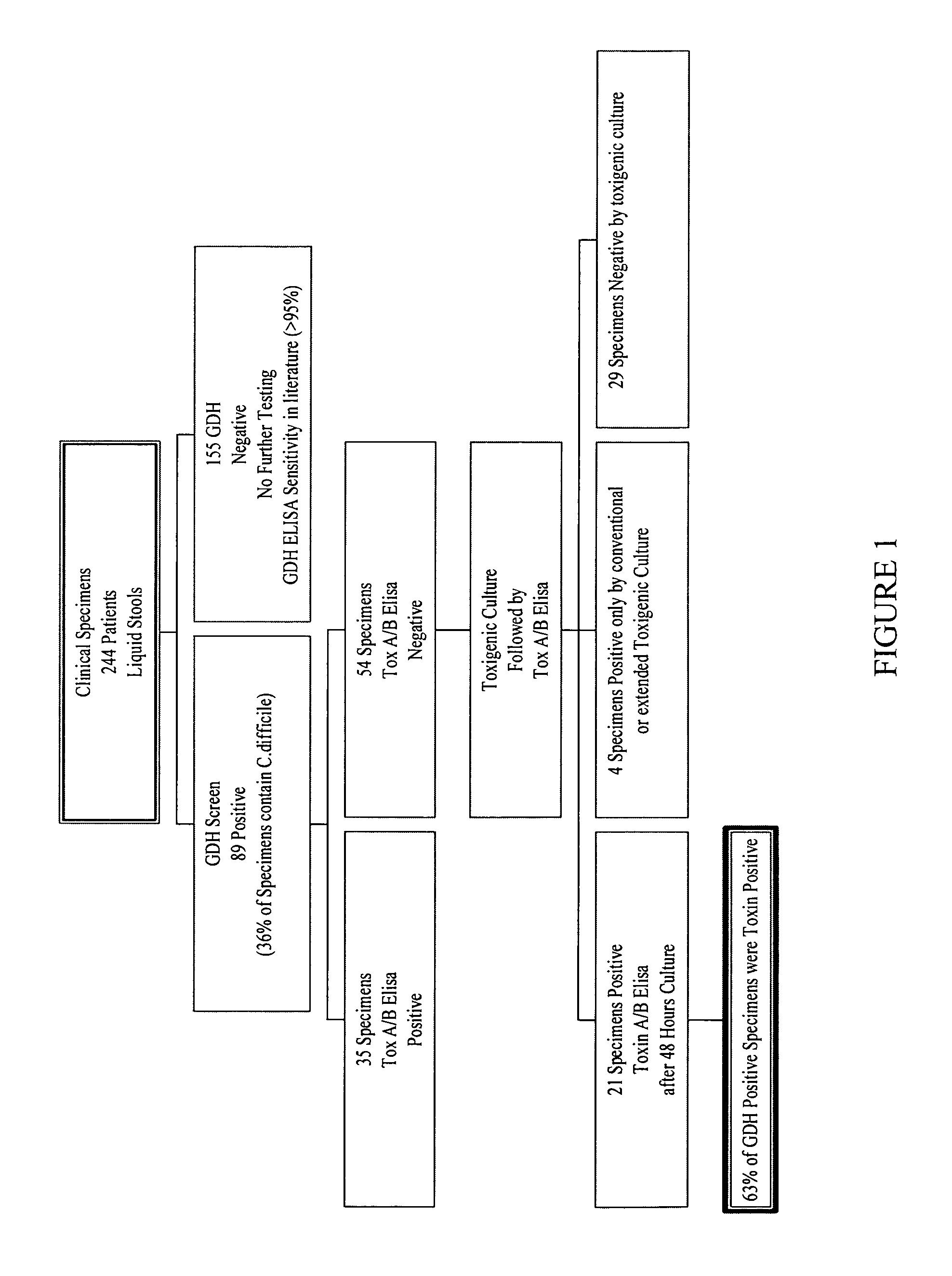
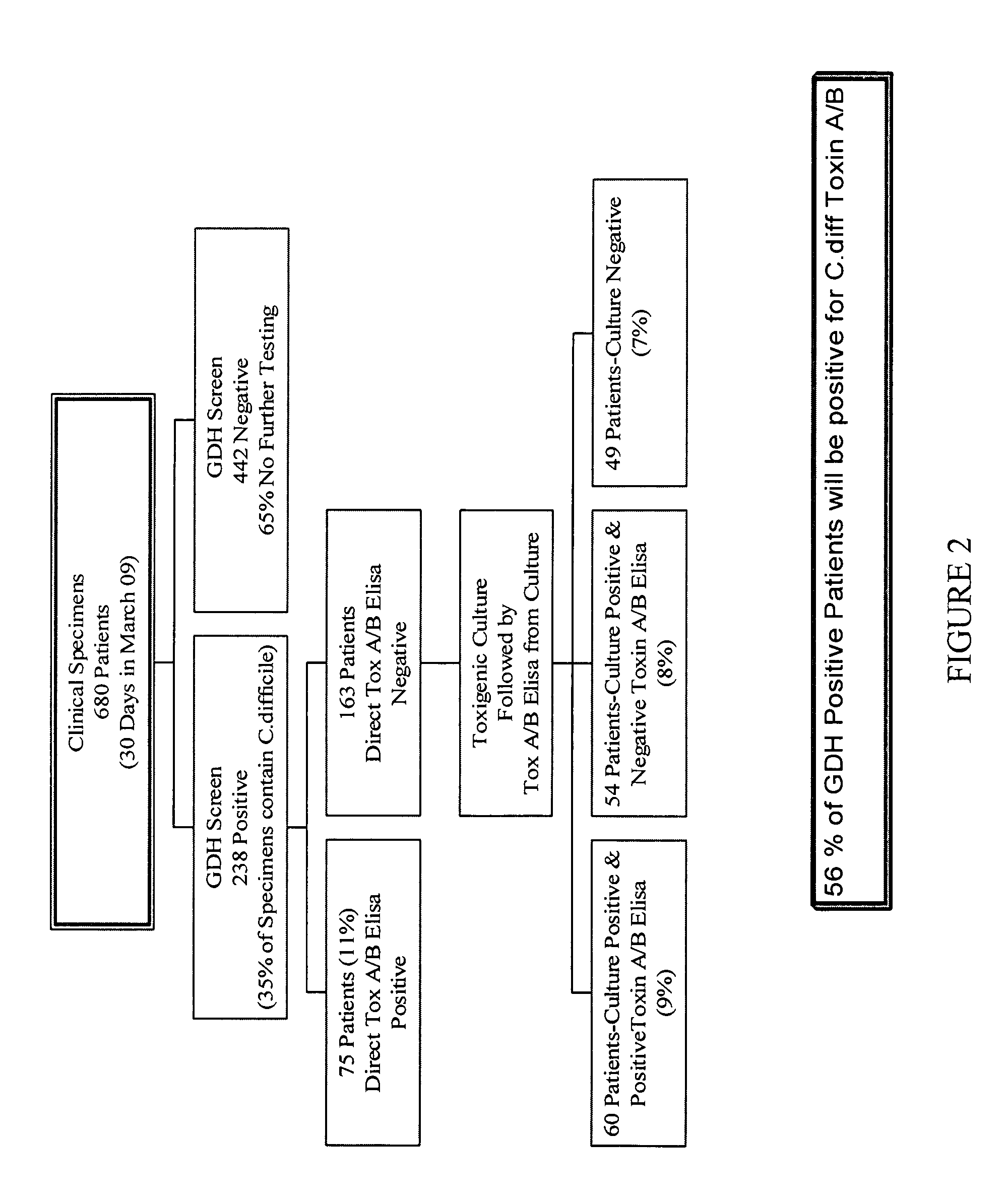
Cultures and protocols for diagnosis of toxigenic clostridium difficile
Disclosed are culture media, protocols and kits for diagnosis of toxigenic Clostridium difficile, where the culture medium comprises Cooked Meat Medium with glucose; yeast extract; taurocholate; cycloserine; and cefoxitin.
Owner:MONTEFIORE MEDICAL CENT INC
Process for producing D-serine
InactiveUS7186532B2Increase productionIncrease productivitySugar derivativesBacteriaEscherichia coliMicroorganism
The present invention relates to: a process for producing D-serine wherein a microbial cell which is modified to have a higher L-serine deaminase activity than Escherichia coli DH5α strain, a culture of said cell, or a processed product thereof is brought into contact with DL-serine in a DL-serine-containing medium to decompose L-serine, and the remaining D-serine is recovered from the medium; and a microorganism used for this production process. D-serine is a useful compound as a synthetic intermediate for useful medicaments such as D-cycloserine.
Owner:KYOWA HAKKO BIO CO LTD
Cultures and protocols for diagnosis of toxigenic Clostridium difficile
Disclosed are culture media, protocols and kits for diagnosis of toxigenic Clostridium difficile, where the culture medium comprises Cooked Meat Medium with glucose; yeast extract; taurocholate; cycloserine; and cefoxitin.
Owner:MONTEFIORE MEDICAL CENT INC
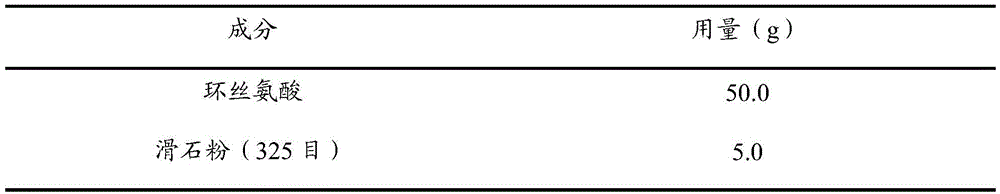
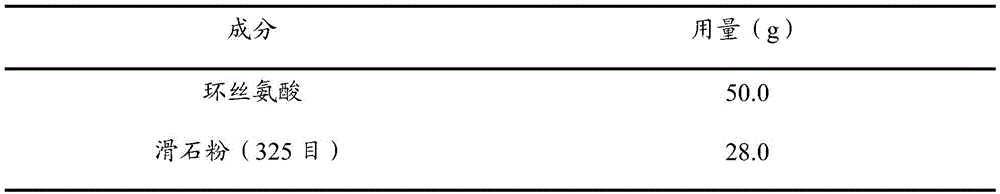
Medicine composition and preparing method and application thereof
ActiveCN105476976ALarge particle sizeFast releaseAntibacterial agentsNervous disorderAcceleration UnitVeterinary medicine
The invention discloses a medicine composition and a preparing method and application thereof. The medicine composition is prepared from cycloserine and auxiliaries. A D90 particle size of the medicine composition is 75-380mum, and the dosage of the auxiliaries is 9-45% of the total mass of the medicine composition. The stability of the medicine composition is greatly improved compared with the prior art, in an optimal example, the medicine composition is hermetically stored for 180d in a normal temperature condition (temperature is 25 DEG C and humidity is 60%), a degrading content of a percentage content of a labeled amount of the preparation is smaller than 0.5%, and the medicine composition is hermetically stored for 180d in an acceleration condition (temperature is 40 DEG C and humidity is 75%), a degrading content of a percentage content of a labeled amount of the preparation is smaller than 4.0%.
Owner:LAKERSPHARMA CO LTD
Method for refining D-cycloserine
The invention discloses a method for refining D-cycloserine. The method comprises the following steps of: (1) preparing an ammonia water solution for later use; (2) cooling the ammonia water solution with brine ice; (3) adding D-cycloserine into the ammonia water solution and uniformly stirring till the D-cycloserine is fully dissolved to obtain a dissolved solution; (4) slowly adding alcohol into the solution obtained in the step (3) and stirring for 30 minutes; (5) adding 0.005-0.02 kilogram of 8-hydroxyquinoline into every 100 kilograms of D-cycloserine, adding 8-hydroxyquinoline into the alcohol-containing solution, preserving heat, stirring for 10 minutes, filtering with a film of 0.45 mum, and cooling the filtrate to -5 DEG C; and (6) adding acid into the filtrate obtained in the step (5), adjusting the pH value, stirring, centrifuging, and drying a solid under reduced pressure for 5 hours to obtain refined D-cycloserine. By adopting the method, D-cycloserine which is overdue clinically can be recovered and refined, so that a higher-purity qualified product can be recycled.
Owner:JINAN CHENGHUI SHUANGDA CHEM
Mediucm for detecting Van A and Van B vancomycin-resistant entercocci and method of using the same
InactiveUS7364874B2Reduce detectionEasy to useBacteriaMicrobiological testing/measurementSodium lactateMicroorganism
Owner:TOKYO WOMENS MEDICAL UNIV
Application of three compounds extracted from starfish to promotion of bone fracture healing, and preparation method for three compounds
ActiveCN102417509AConvenient treatmentQuick resultsOrganic active ingredientsSkeletal disorderMedicinal herbsDipeptide
The invention discloses application of three compounds extracted from starfish to the promotion of bone fracture healing, and a preparation method for the three compounds, and belongs to the field of methods for extracting Chinese medicines. The preparation method for the three compounds comprises the following steps of: crushing a starfish medicinal material, extracting by using a solvent, mixing extracting solutions, concentrating under reduced pressure, and drying to obtain a total extract; performing equal-volume extraction on the total extract by using ethyl acetate and n-butanol to obtain an n-butanol layer and an ethyl acetate layer; separating the ethyl acetate layer by a silicagel column, and purifying to obtain the compound 3, namely ergosta-7,22-diene-3beta,5,6-triol and the compound 1, namely cyclo(leucine-proline)dipeptide; and separating the n-butanol layer by the silicagel column to obtain the compound 2, namely cyclo(serine-leucine)dipeptide. The compounds have a good effect of promoting bone fracture healing, so that the application prospect of the three compounds used as novel medicines for promoting bone fracture healing is showed.
Owner:大连美罗中药厂有限公司
Production technology of cycloserine
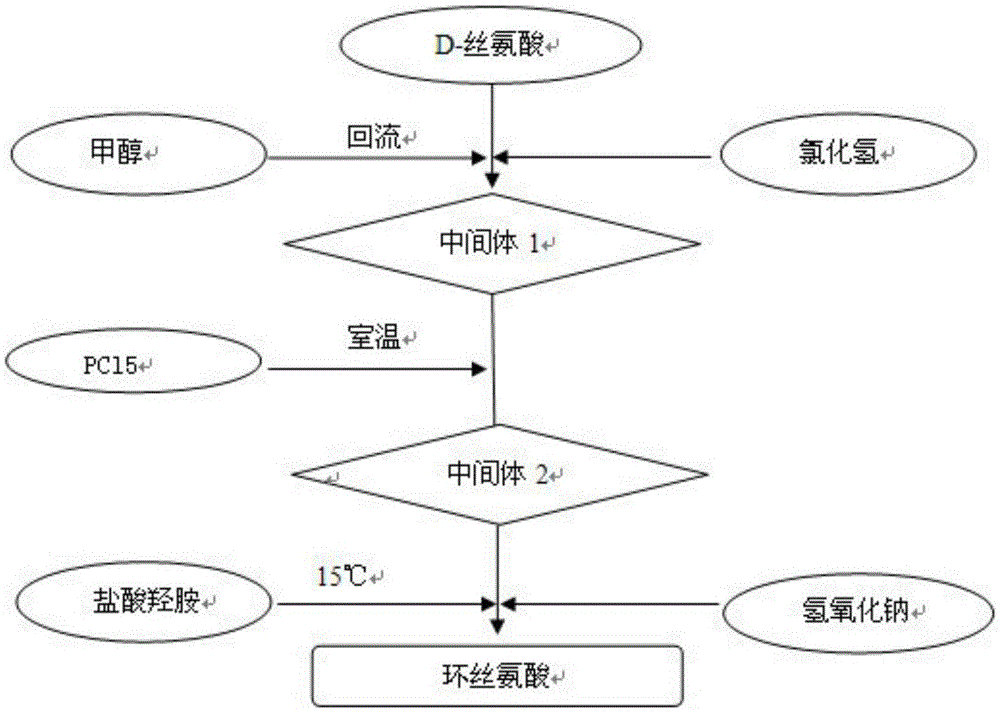
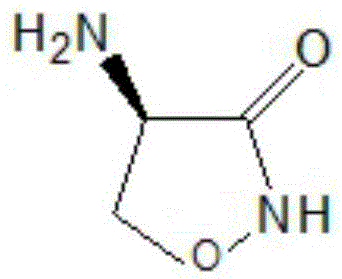
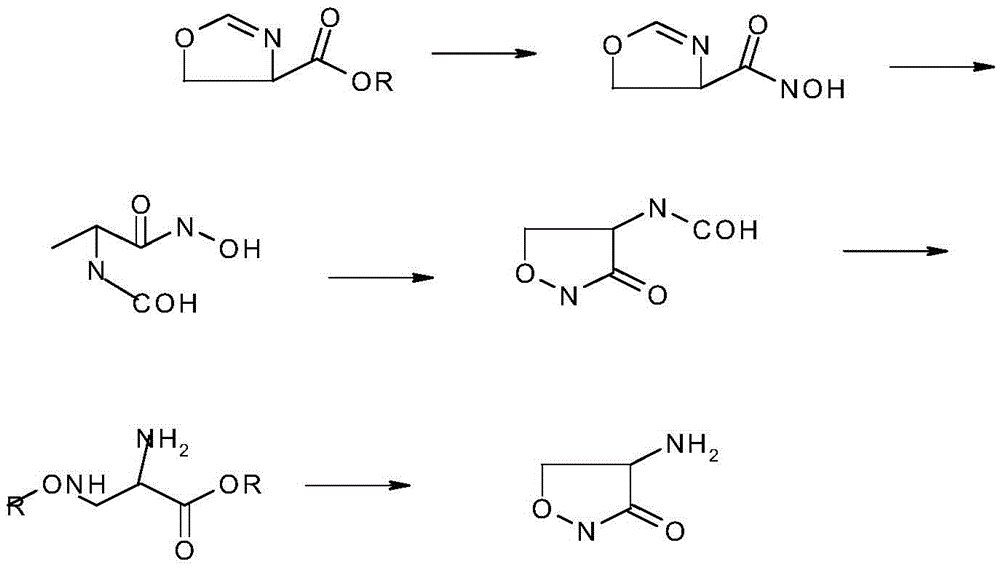
InactiveCN105646385AReduce usageShort synthetic routeOrganic chemistry methodsChemical synthesisAcetic acid
The invention belongs to the field of chemical synthesis, and particularly relates to a production technology of cycloserine. According to the synthesis technology, D-serine is used as a starting material, three reactions of esterification, chlorination and cyclization are performed, and the cycloserine is obtained. The technological process is simple, the yield is high, and the purity is high. During the esterification reaction, diethyl ether is replaced with ethyl acetate, no 8-hydroxyquinoline is added for crude product preparation after the cyclization reaction, and usage of reagent diethyl ether likely to produce toxicity and the dangerous product 8-hydroxyquinoline is avoided. Meanwhile, the synthesis route is short, the cost is low, and the obtained product is stable in yield and controllable in quality.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Slow released medicine containing antituberculotic
InactiveCN1857218AAntibacterial agentsOrganic active ingredientsCarboxymethyl celluloseTreatment effect
The slow released implanting agent and injection containing antituberculotic are set or injected to local tuberculosis focus for maintaining the local effective medicine concentration while lowing the systemic toxicity. The slow released injection consists of slow released microsphere and solvent. The slow released microsphere includes antituberculotic selected from Cycloserine, Ofloxacin, Ciprofloxacin and Sparfloxacin and slow releasing supplementary material, and the solvent is special solvent containing suspending agent. The suspending agent is carboxymethyl cellulose sodium, etc. and has viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is selected from EVAc, PLA, PLGA, etc. The slow released implanting agent may be prepared with slow released microsphere. The present invention has obvious and unique treating effect on various kinds of intractable tuberculosis.
Owner:JINAN KANGQUAN PHARMA TECH
Sublingual Formulations of D-Cycloserine and Methods of Using Same
The invention provides methods and compositions for treating anxiety-related disorders in a subject. The methods include sublingually administering D-cycloserine to a subject with the anxiety-related disorder, either alone or in combination with extinction training.
Owner:MCDEVITT JASON P +2
Mediucm for detecting vana and vanb vancomycin-resistant enterocci and method of using the same
Van A and Van B vancomycin resistant enterococci detection media as well as a method of selectively detecting Van A and Van B vancomycin resistant enterococci clinically important in vancomycin resistant enterococci from testing microorganisms or specimens using the media. The media for selectively detecting Van A and Van B VRE from testing microorganisms and specimens are media where enterococci can grow where vancomycin, D-cycloserine and D-lactate are added. Preferably 32-256 mug / ml of vancomycin, 1-64 mug / ml of D-cycloserine, and 0.025-0.8 mol / l of sodium lactate are added to culture medium where enterococci can grow.
Owner:TOKYO WOMENS MEDICAL UNIV
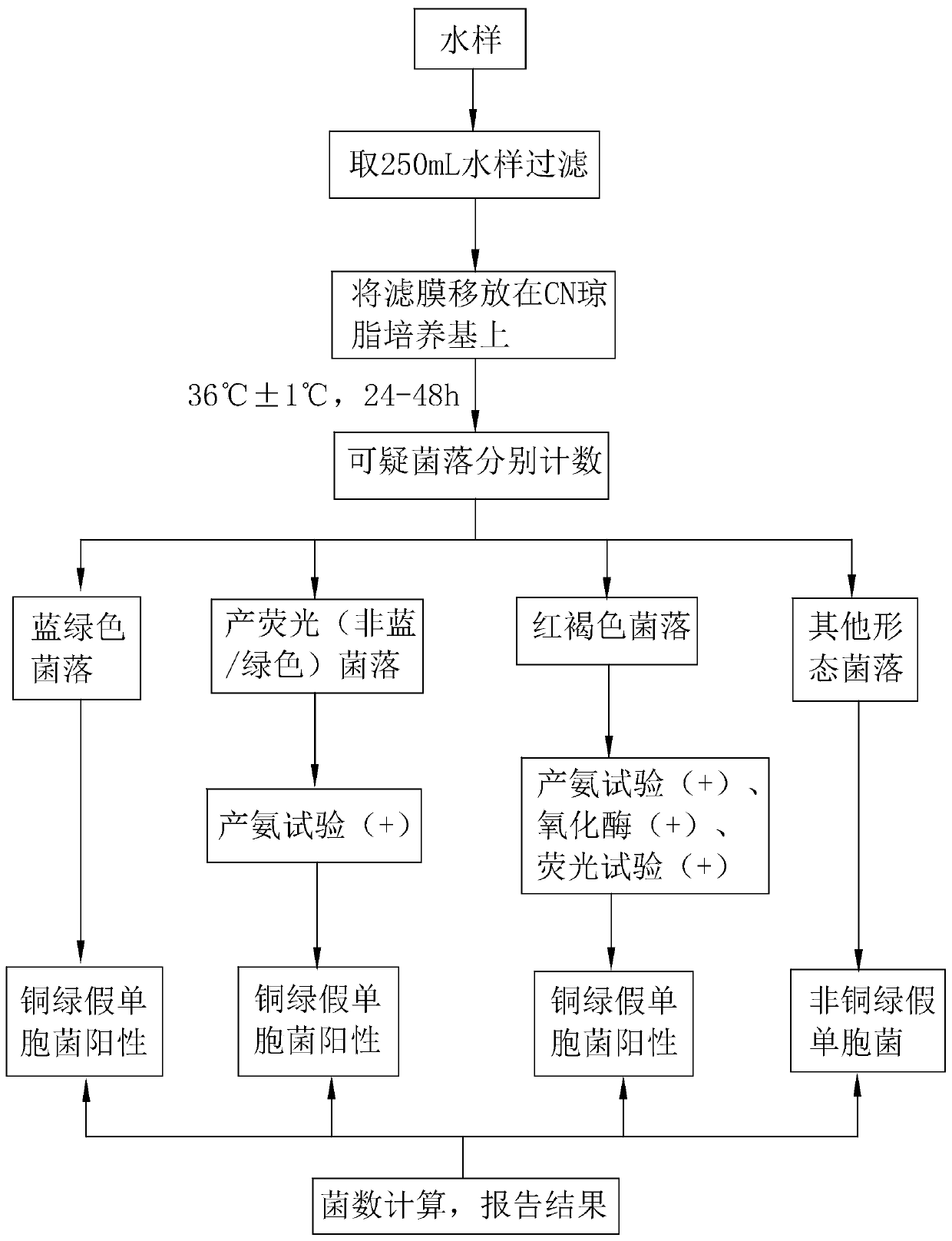
Pseudomonas aeruginosa chromogenic culture medium and method for quick detection by same
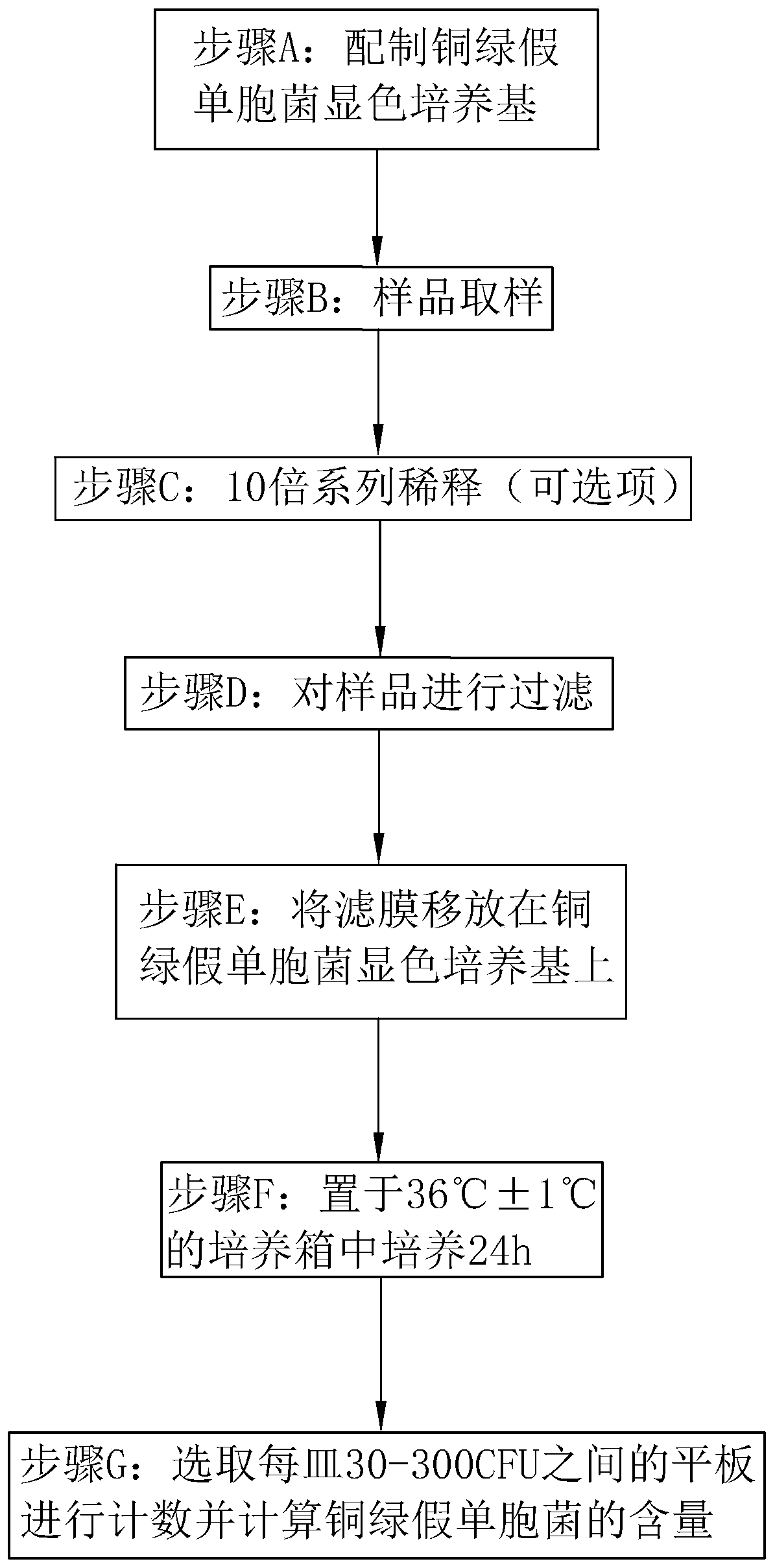
PendingCN110129406AThe difference is obviousStrong specificityMicrobiological testing/measurementBiological material analysisNalidixic acidUltraviolet
The invention relates to the technical field of pseudomonas aeruginosa detection and discloses a pseudomonas aeruginosa chromogenic culture medium and a method for quick detection by the same. The pseudomonas aeruginosa chromogenic culture medium comprises a basic medium, a chromogenic substrate and an antibacterial agent. The chromogenic substrate comprises 2,3,5-phenyl tetrazolium chloride, 4-methyl umbelliferone-D-glucuronide, and the antibacterial agent comprises nalidixic acid, cycloserine and bile salt. 2,3,5-phenyl tetrazolium chloride acts on a pseudomonas aeruginosa metabolite to showa red color; 4-methyl umbelliferone-D-glucuronide acts on pseudomonas aeruginosa to show fluorescence under a 366nm ultraviolet lamp; by joint action of 2,3,5-phenyl tetrazolium chloride and the antibacterial agent, growth of common disturbance bacteria can be inhibited, and false positive results are avoided. By adoption of the pseudomonas aeruginosa quick detection method, detection and count of pseudomonas aeruginosa can be finished in 24h, and time saving, labor saving, stability, reliability and high sensitivity are realized.
Owner:上海源本食品质量检验有限公司
Sustained-release agent containing tuberculosis-resistance medicament
The invention relates to a slow-release implant or a slow-release injection containing antituberculotic drug(s), which can be locally placed on or injected into tuberculosis foci to slowly release the antituberculotic drug(s), so as to effectively obtain and keep effective drug concentration at local foci and at the same time reduce the systemic toxicity of the drug. The slow-release injection comprises slow-release microspheres and a solvent, wherein the slow-release microspheres comprise slow-release adjuvants and antituberculotic drug(s) selected from cycloserine, ofloxacin, ciprofloxacin and / or sparfloxacin; the solvent is a special solvent containing a suspending agent; the suspending agent has viscosity of 100-3,000cp (at 20-30 DEG C) and is selected from sodium carboxymethyl cellulose, etc.; the slow release adjuvants are selected from EVAc, polifeprosan, polylactic acid (PLA), polys(lactic-co-glycolic acid) (PLGA), poly(erucic acid dimmer-sebacic acid or fumaric acid-sebacic acid) copolymers, etc.; and the slow-release implant can be made from the slow-release microspheres or by other methods. The inventive agent has distinct and unique therapeutic effect on refractory tuberculosis, such as multiple drugs resistant bacillus tuberculosis infection, tuberculoma, tuberculosis in lymph, joint, kidney, skin, intestine, mammary gland and genitalia, tuberculous osteomyelitis, fistulous tract, and cavermous pulmonary tuberculosis.
Owner:JINAN KANGQUAN PHARMA TECH
Clostridium difficile chromogenic medium and application thereof
InactiveCN105838774AShort training periodThe culture cycle is shortened from the separation time of the existing technologyMicrobiological testing/measurementMicroorganism based processesSulfite saltSolvent
The invention discloses a Clostridium difficile chromogenic medium and an application thereof. The chromogenic medium consists of 1-20g / L of tryptone, 1-20g / L of digested animal tissue, and 0.1-5.5g / L of glucose. L, yeast extract 0.2‑5.5g / L, sodium chloride 0.5‑15g / L, sodium sulfite 0.05‑1g / L, escin 0.1‑20g / L, iron 0.1‑5g / L, amino acid 0.2‑25g / L , agar powder 5-20g / L, cycloserine 0.1-2.5g / L and cefoxitin sodium 0.01-0.15g / L, the solvent is deionized water, pH value 7.0~7.6; the medium culture period of the present invention is 24 hours, Colonies are specifically black, and C. difficile isolates can be accurately obtained from clinical stool samples by color when using this medium.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Texturizing lactic acid bacteria strains
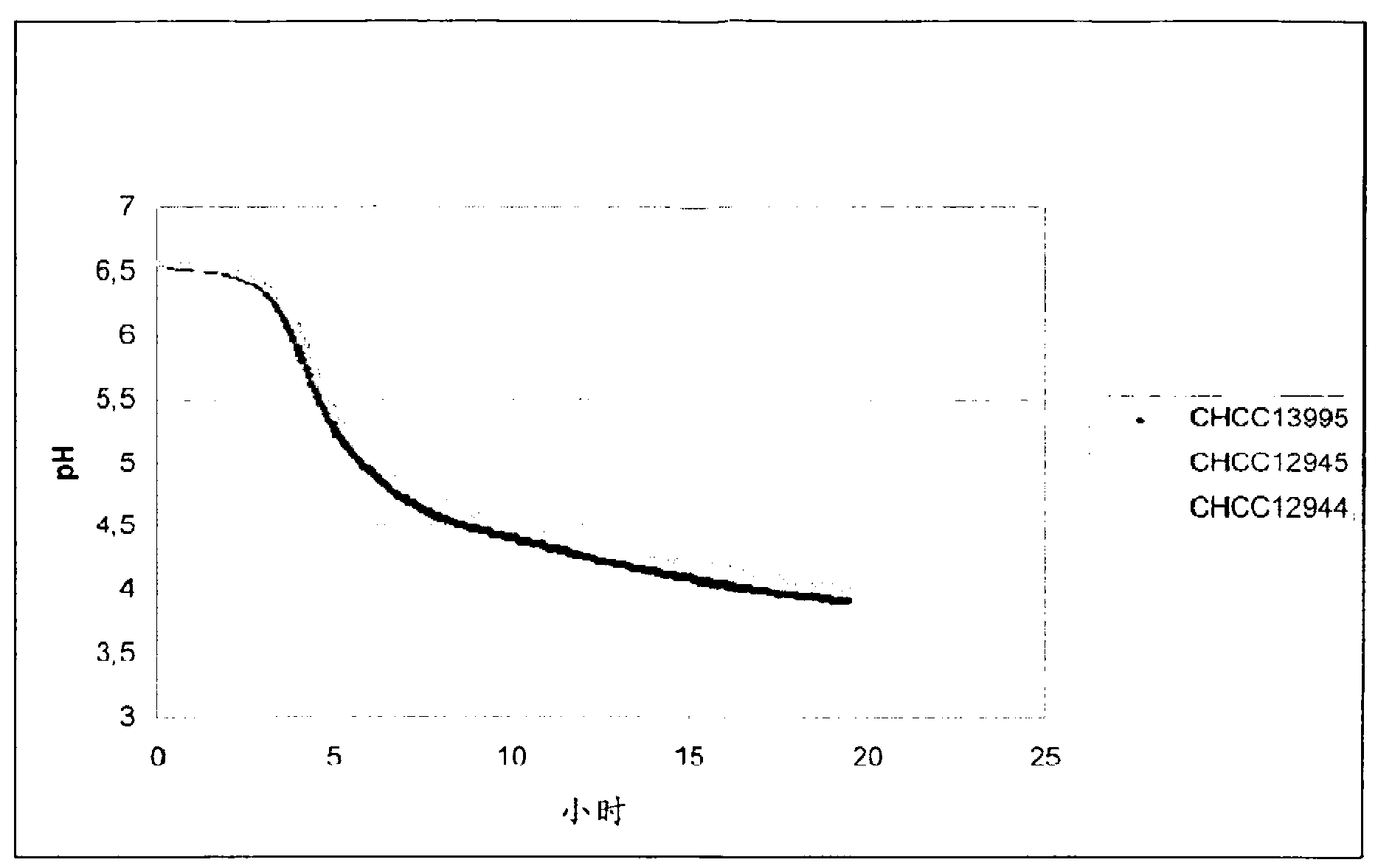
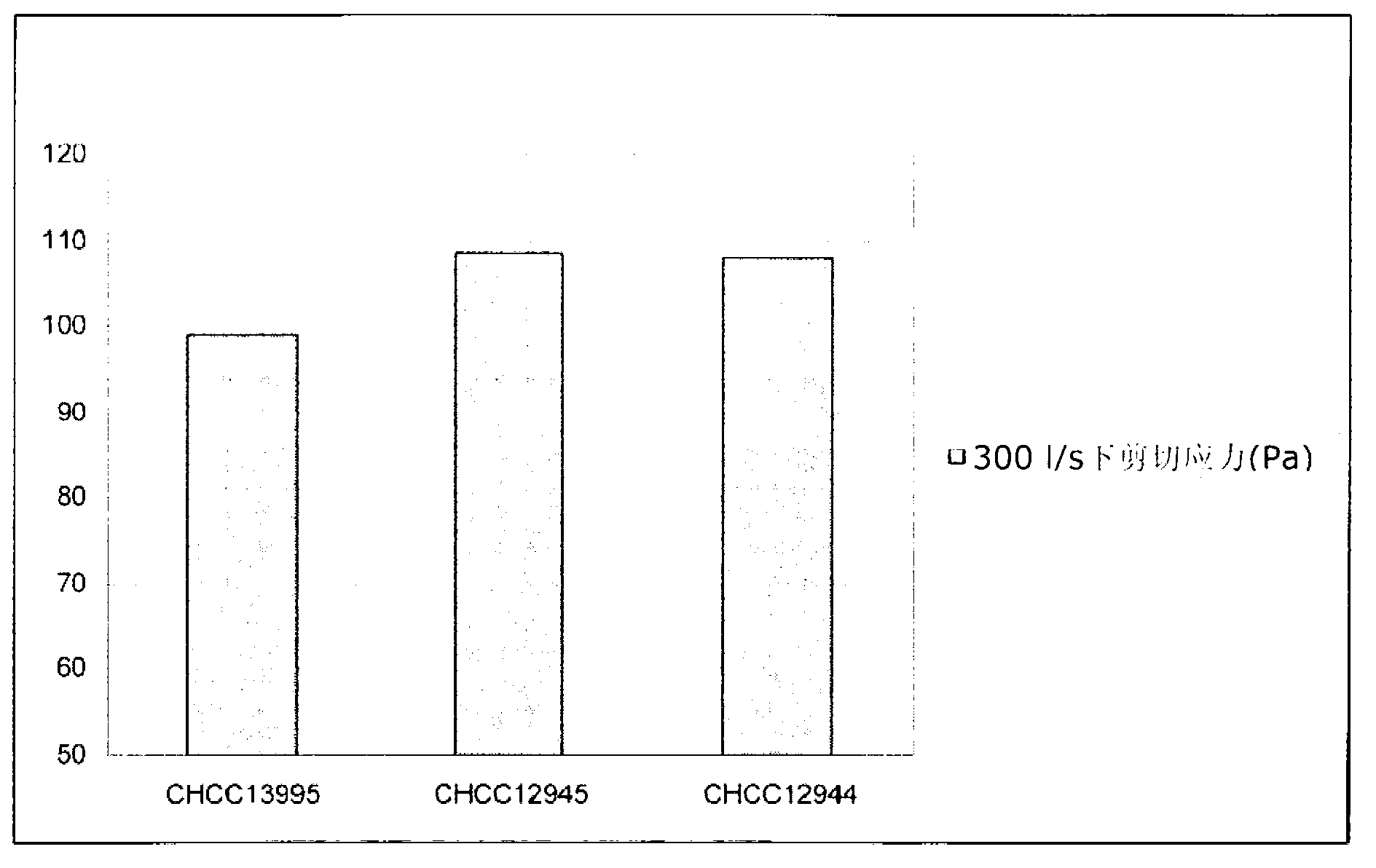
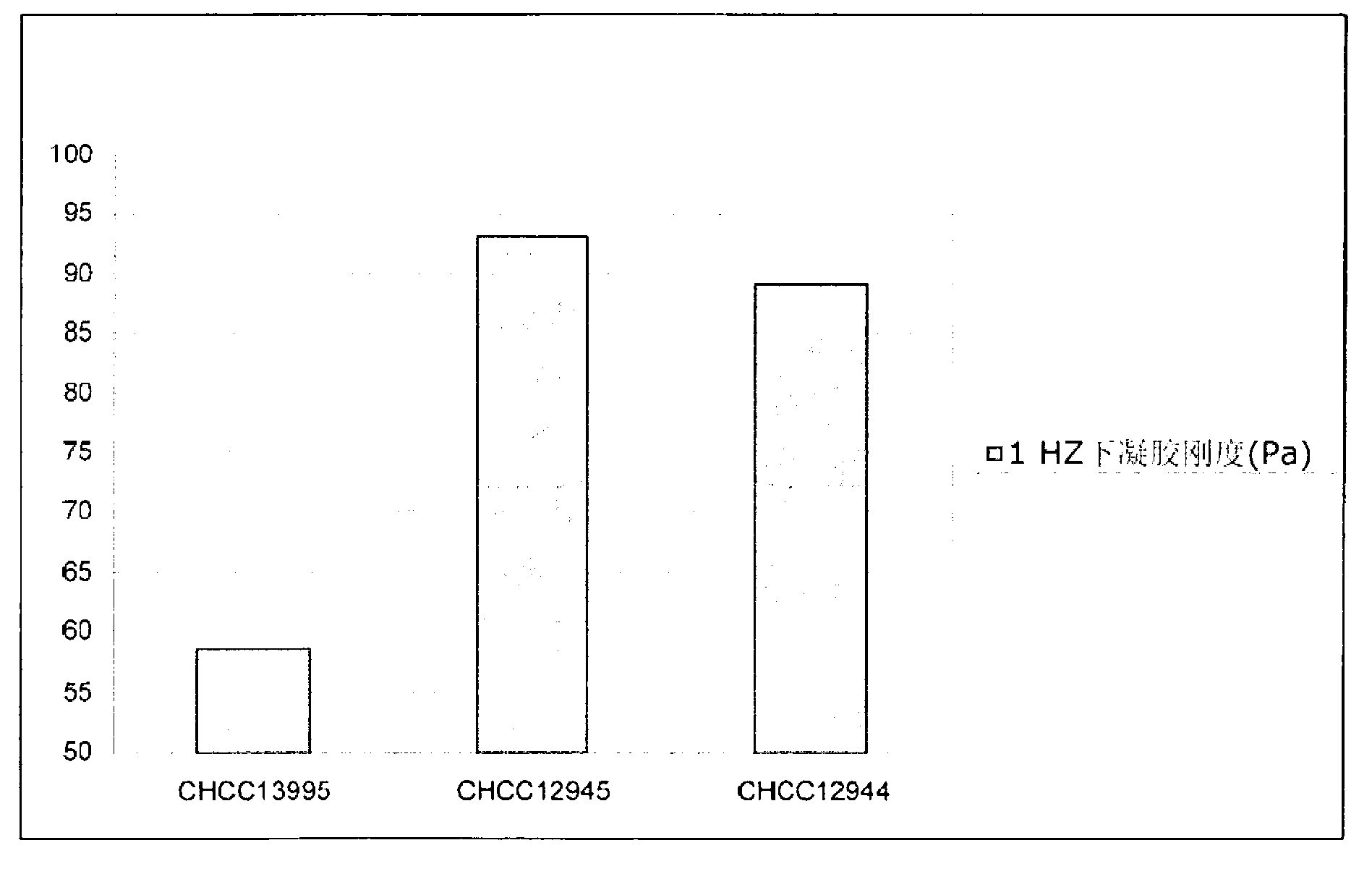
The present invention relates to mutants of lactic acid bacteria which are resistant towards the antibiotic D-cycloserine and / or functionally equivalent antibiotics and which were found to give an increased texture when grown in milk while maintaining the other growth properties of the parent strain. The present invention, furthermore, relates to compositions comprising such mutants, and to dairy products fermented with the lactic acid bacteria resistant towards D-cycloserine and / or functionally equivalent antibiotics.
Owner:CHR HANSEN AS
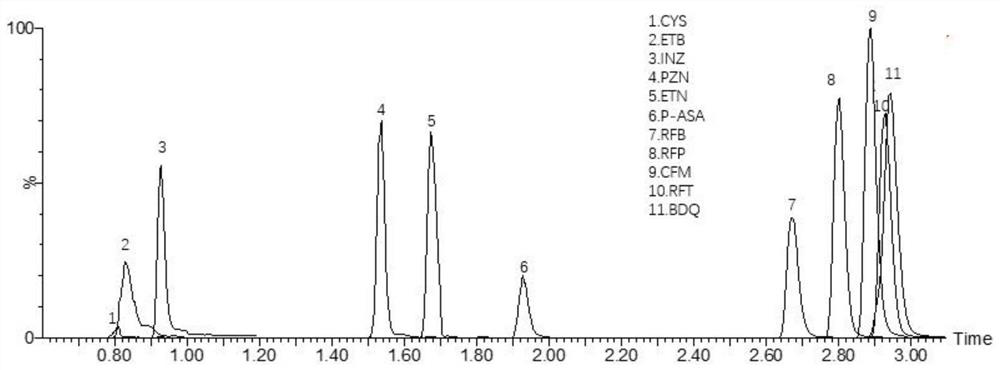
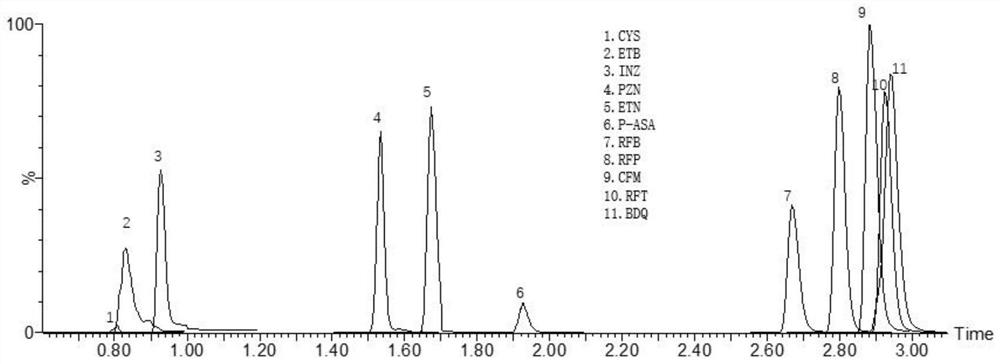
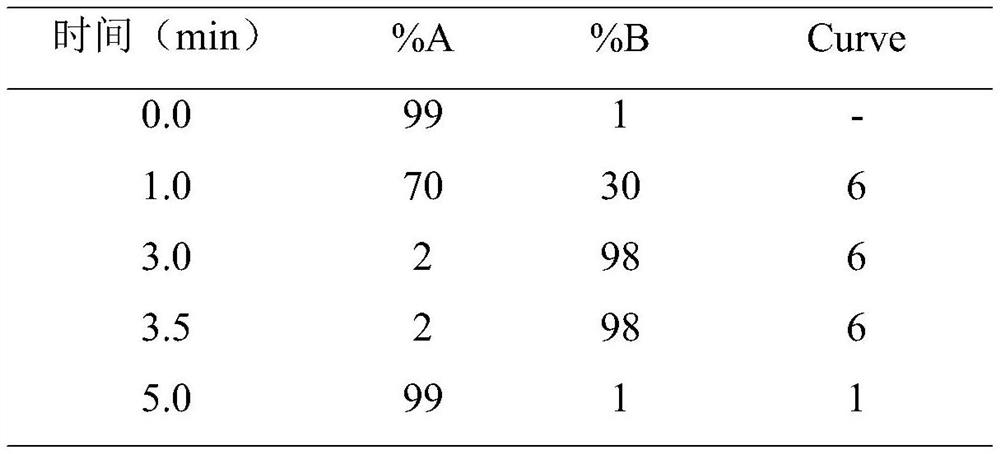
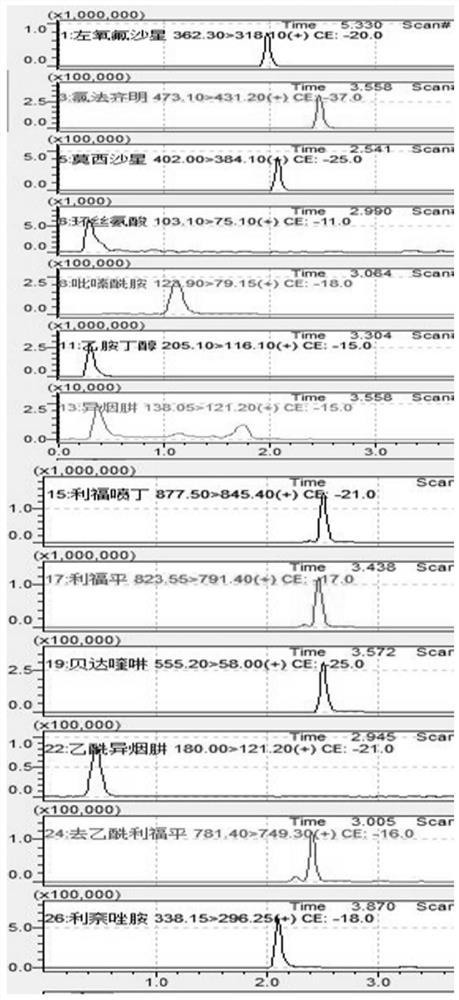
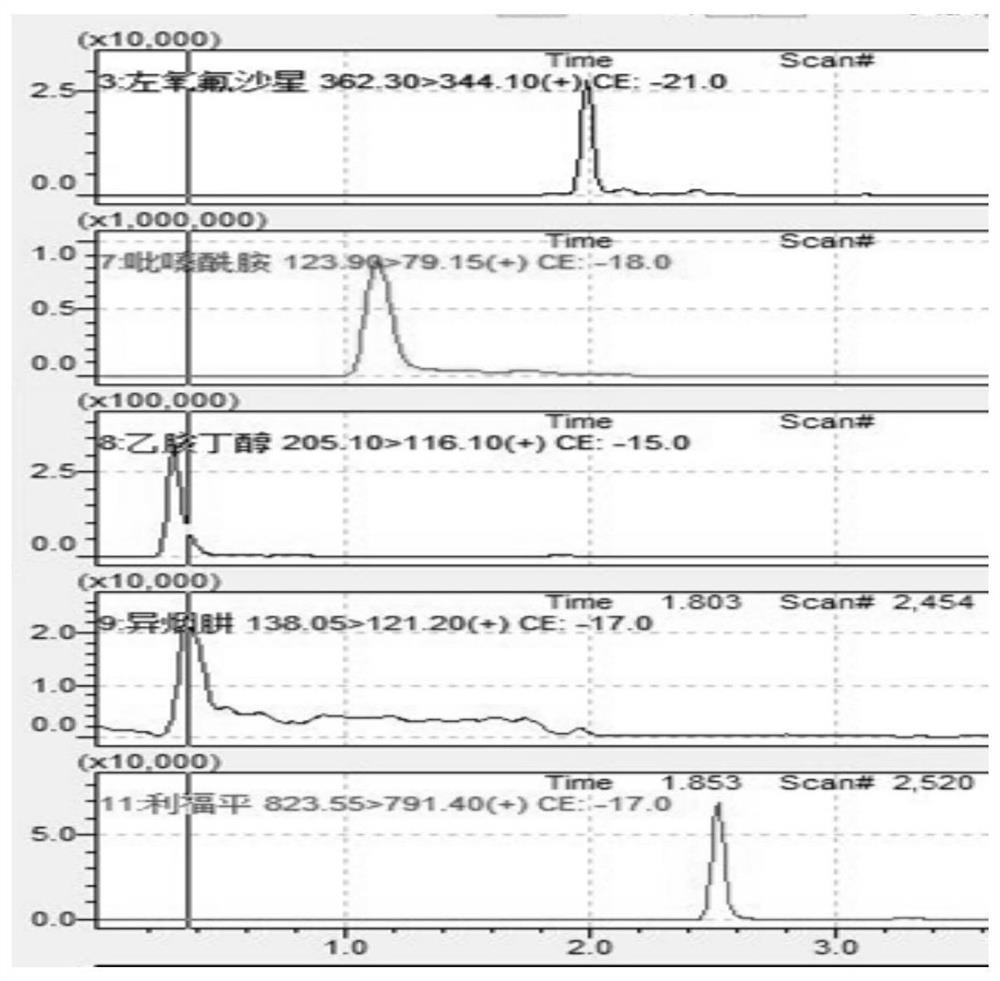
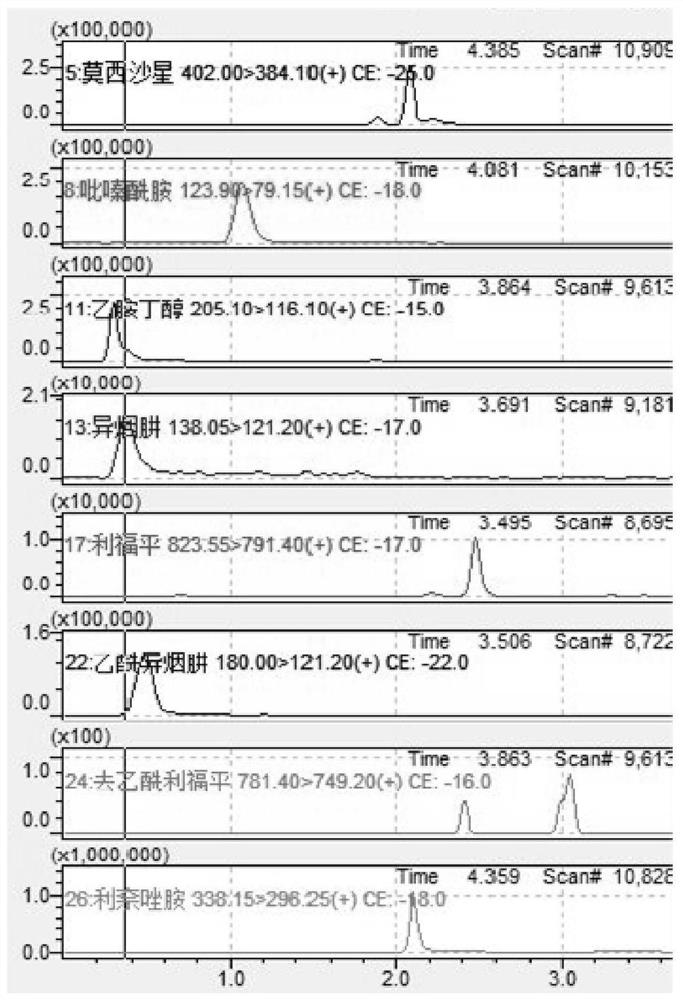
Method for detecting anti-tuberculosis drug in serum by ultra-high performance liquid chromatography-tandem mass spectrometry technology
InactiveCN111766311AHigh sensitivityStrong specificityComponent separationAntituberculosis drugPyrazine
The invention discloses a method for detecting an anti-tuberculosis drug in serum by an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The antitubercular drug comprises cycloserine (CYS), pyrazinamide (PZN), isoniazid (INZ), p-aminosalicylic acid (P-ASA), ethiisonicotinamide (ETN), ethambutol (ETB), clofazimine (CFM), bedaquiline (BDQ), rifampicin (RFP), rifbutine (RFB) and rifapentine (RFT). The method includes: detecting the content of the antituberculosis drug in the pretreated serum by adopting an ultra-high performance liquid chromatography-tandem mass spectrometry method, performing quantifying by utilizing a mass spectrometry isotope internal standard method, establishing a calibration curve by taking the concentration ratio of the standard substance to the internal standard substance as an X axis and the peak area ratio of the standard substance to the internal standard substance as a Y axis, and calculating the concentration of the target drug in the serum; the method is high in sensitivity, strong in specificity and simple in pretreatment process, separation and detection of the anti-tuberculosis drugs in serum are completed within 5 min, and a simple and rapid detection method is provided for clinical concentration monitoring of the anti-tuberculosis drugs.
Owner:南京品生医学检验实验室有限公司
Slow released compound antituberculotic preparation containing synergist
The slow released compound antituberculotic preparation containing synergist is implanting agent or slow released injection comprising slow released microsphere and solvent. The slow released microsphere consists of slow releasing supplementary material, at least one antituberculotic selected from rifampicin, isoniazid and pyrazinamide and at least one antituberculotic synergist selected from cycloserine, ofloxacin, ciprofloxacin, sparfloxacin and capreomycin. The solvent is special solvent containing suspending agent carboxymethyl cellulose, etc. and with viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is selected from EVAc, PLA, PLGA, etc. The slow released compound antituberculotic preparation can release antituberculotic in the local tubercolosis part for 30-40 days so as to maintain the local effective medicine concentration while lowering systemic toxicity. The present invention has obvious unique treating effect on various kinds of intractable tuberulosis.
Owner:JINAN SHUAIHUA PHARMA TECH
Kit and method for detecting antituberculous drugs and metabolites thereof in sample
PendingCN114354804AExpand the types of testingIncrease varietyComponent separationMetaboliteAntituberculous drug
The invention particularly provides a kit and a method for detecting antituberculous drugs and metabolites thereof in a sample. The kit is used for detecting antituberculous drugs and metabolites thereof in a sample, and comprises a calibration product, a quality control product, an instrument quality control product and an isotope internal standard product, both the calibration material and the quality control material contain rifampicin, isoniazide, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazide, bedaquiline and deacetylrifampicin; the instrument quality control product comprises a methanol solution containing rifampicin, isoniazid, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazid, bedaquiline and deacetylrifampicin; the isotope internal standard substance contains an internal standard substance corresponding to a substance contained in the calibrator. The kit disclosed by the invention can be used for detecting the concentrations of the antituberculous drugs and metabolites thereof in various sample types.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
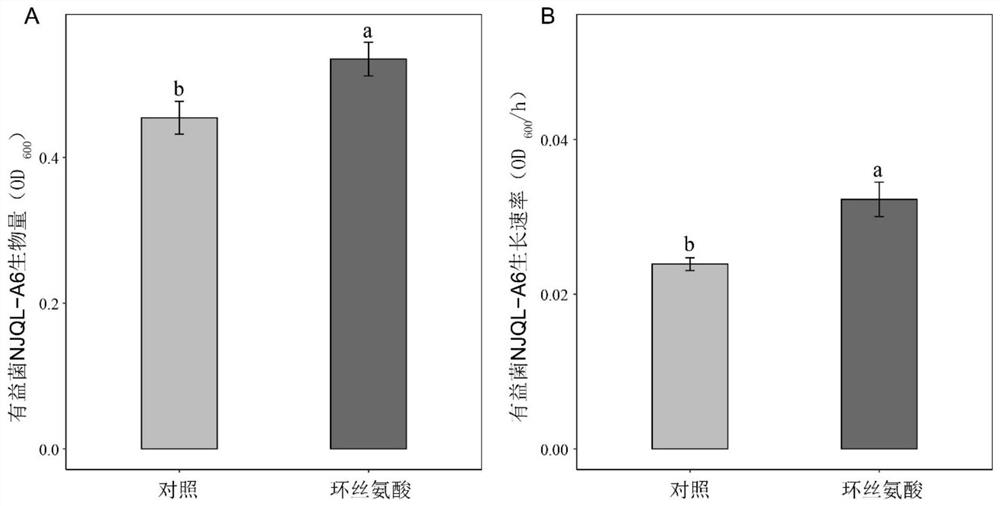
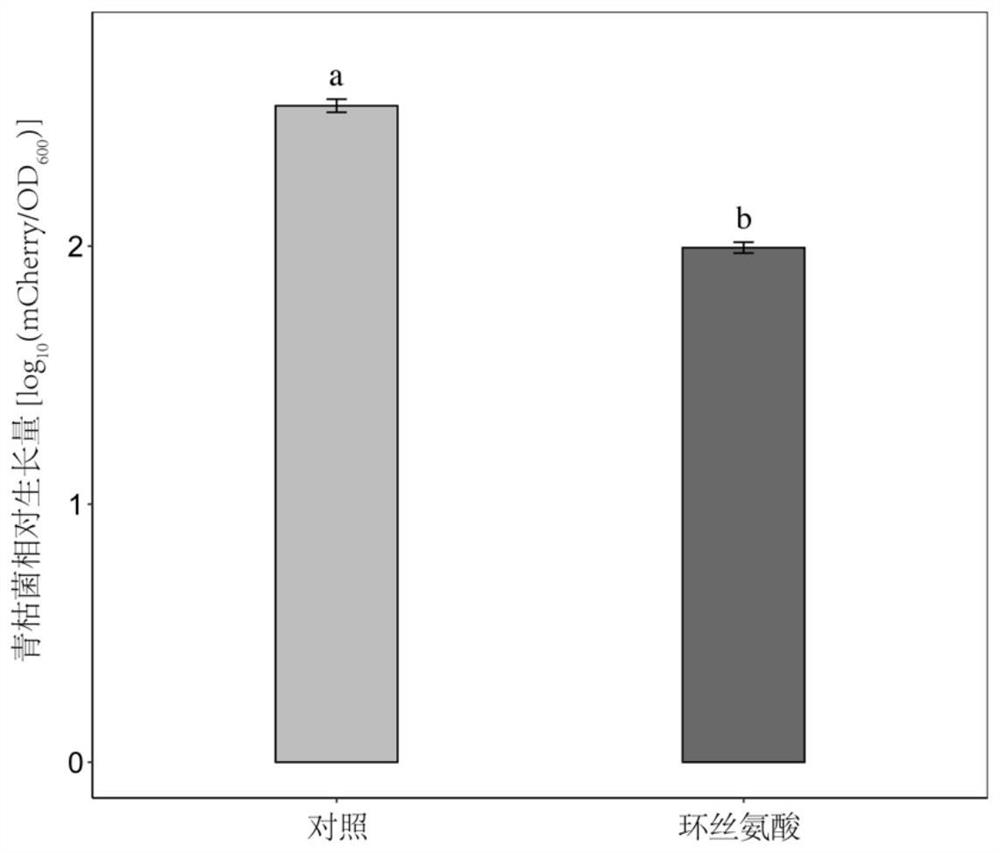
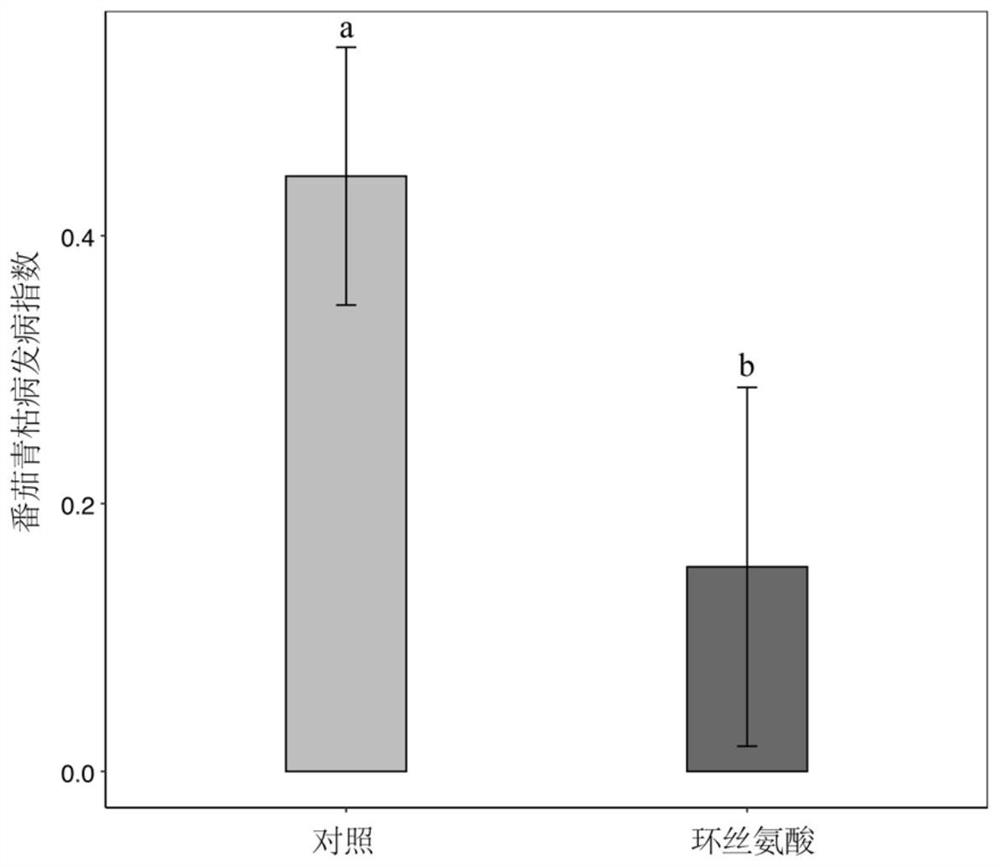
Application of cycloserine and beneficial bacteria in preventing and controlling tomato soil-borne ralstonia solanacearum
The invention discloses an application of cycloserine and beneficial bacteria in preventing and controlling tomato soil-borne ralstonia solanacearum. Cycloserine and beneficial bacteria are applied tosoil by adopting a root irrigation method, wherein the beneficial bacteria are bacterial strains NJQL-A6, the strain is named as Ralstonia pickettii, and is preserved in China General MicrobiologicalCulture Collection Center on September 26, 2012, the address of the preservation unit being Datsun Road, Chaoyang District, Beijing, and the preservation number of the strain being CGMCC No.6628. According to the invention, through a resource regulation and control mode, the soil-borne ralstonia solanacearum inhibition capability of beneficial bacteria can be enhanced; the beneficial bacteria andthe cycloserine are applied at the same time, and the cycloserine is used as a resource substance, so that the growth of the beneficial bacteria can be promoted, and the inhibition capability of thebeneficial bacteria on ralstonia solanacearum can be remarkably improved, the morbidity of tomato ralstonia solanacearum is reduced and tomato soil-borne diseases are prevented and controlled.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for treatment of chronic neuropathic pain
Chronic pain is treated in an individual suffering from chronic pain by administering to the individual an amount of a therapeutic containing a glycine receptor agonist such as D-cycloserine or a GlyT-1 glycine transporter antagonist such as sarcosine in an amount effective to treat the chronic pain. The therapeutic may also contain a secondary analgesic such as opiates, NSAIDs or cox-2 inhibitors. The analgesic can be formulated in a pharmaceutical composition in the form of an injectable solution that contains at least two different analgesics, at least one of the analgesics of which is a glycine receptor agonist or a GlyT-1 glycine transporter antagonist. Suitable pharmaceutical compositions contain D-cycloserine and / or sarcosine, optionally in combination with opiates, NSAIDs or cox-2 inhibitors.
Owner:APKARIAN TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com