Medicine composition and preparing method and application thereof
A technology of composition and medicine, which is applied in the field of pharmaceutical composition and its preparation, can solve the problems of not finding the stability of cycloserine preparations, poor variety stability, discoloration of contents, etc., and achieve good fluidity, lubricity, and stability Improvement of sex and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
[0076] Cycloserine accounts for 64.1% of the total, the content of cycloserine is 99.713%, and the talc powder is 200 mesh (75μm in diameter).
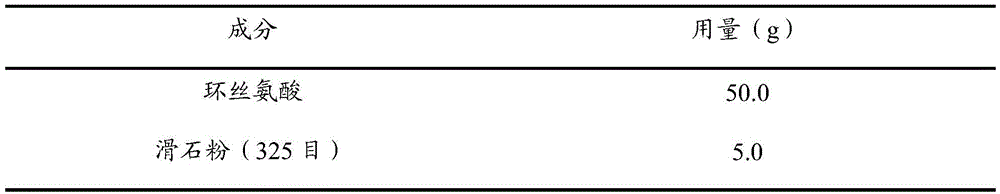
[0077] prescription:
[0078]
[0079] Preparation:
[0080] Take the prescription amount of cycloserine through a 200-mesh sieve, then mix it with the prescription amount of talc, and fill the No. 1 hollow gelatin capsule with 250 mg of cycloserine per capsule.
[0081] The capsule prepared by the above method has a loss on drying of 0.496%, a disintegration time in water of 5 minutes, and a 15-minute dissolution rate of greater than 90% at pH 1.2, pH 4.0, pH 6.8 and water.
[0082] The stability data is shown in Table 2.
Embodiment 6
[0084] Cycloserine accounts for 64.1% of the total, the content of cycloserine is 99.713%, and the talc powder is 200 mesh.
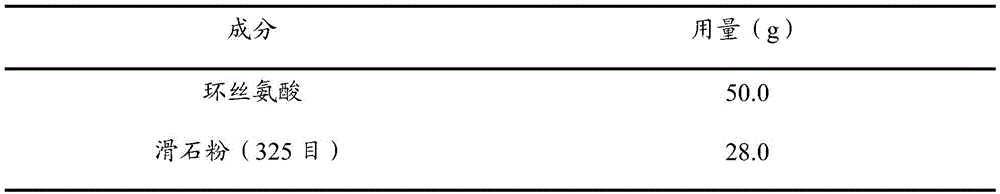
[0085] prescription:
[0086]
[0087] Preparation:
[0088] Take the prescription amount of cycloserine through a 100-mesh sieve, then mix it with the prescription amount of talc, and fill the No. 1 hollow gelatin capsule with 250 mg of cycloserine per capsule.
[0089] The capsule prepared by the above method has a loss on drying of 0.583%, a disintegration time in water of 5 minutes, and a dissolution rate of more than 90% at pH 1.2, pH 4.0, pH 6.8 and 15 minutes in water.
[0090] The stability data is shown in Table 2.
Embodiment 7
[0092] Cycloserine accounts for 64.1% of the total, the content of cycloserine is 99.713%, and the talc powder is 100 mesh (particle size 150μm).
[0093] prescription:
[0094]
[0095] Preparation:
[0096] Take the prescription amount of cycloserine through a 100-mesh sieve, then mix it with the prescription amount of talc, and fill the No. 1 hollow gelatin capsule with 250 mg of cycloserine per capsule.
[0097] The capsule prepared by the above method has a loss on drying of 0.578%, a disintegration time in water of 4 minutes, and a dissolution rate of more than 90% at pH 1.2, pH 4.0, pH 6.8 and 15 minutes in water.
[0098] The stability data is shown in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com