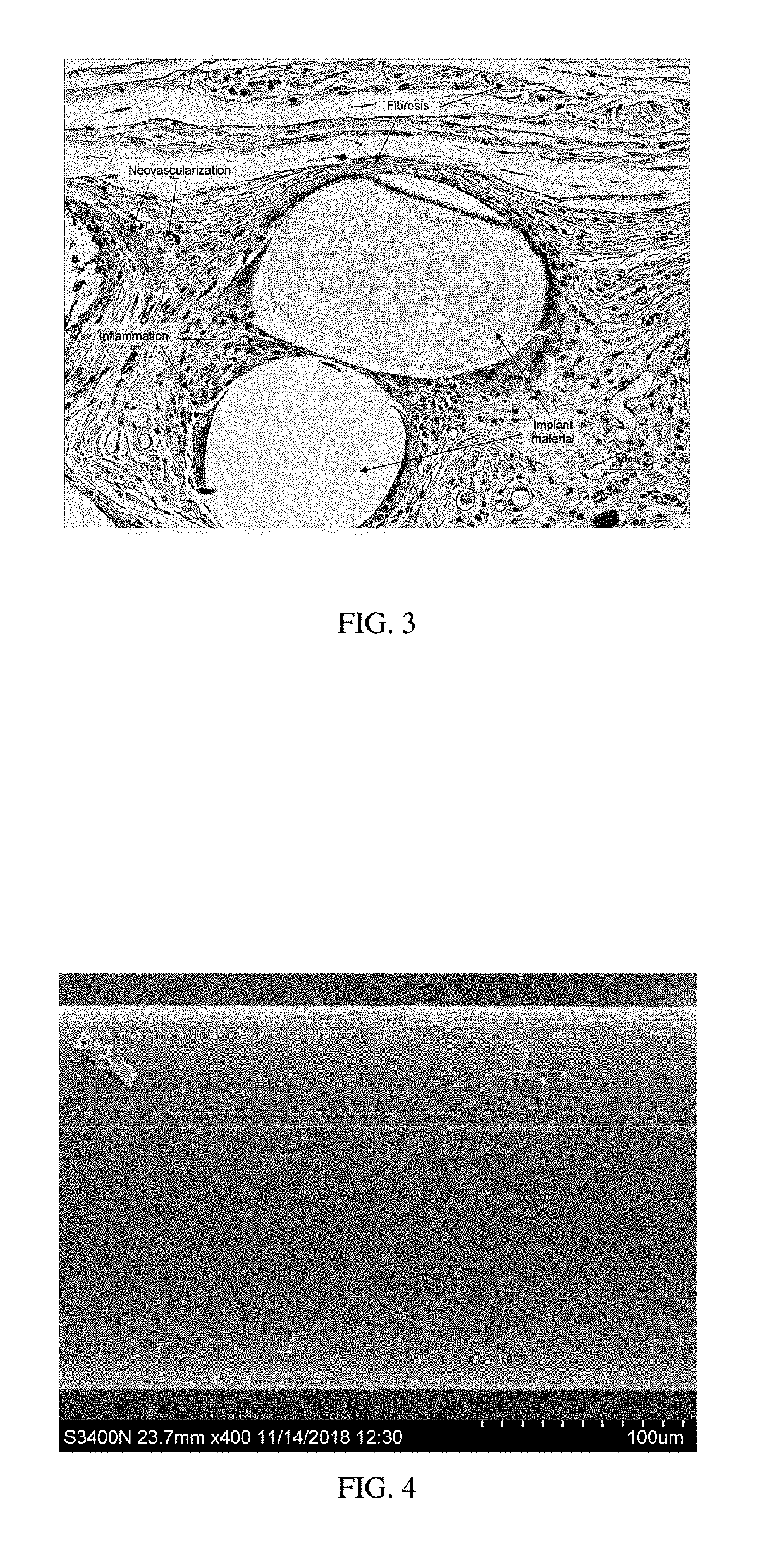
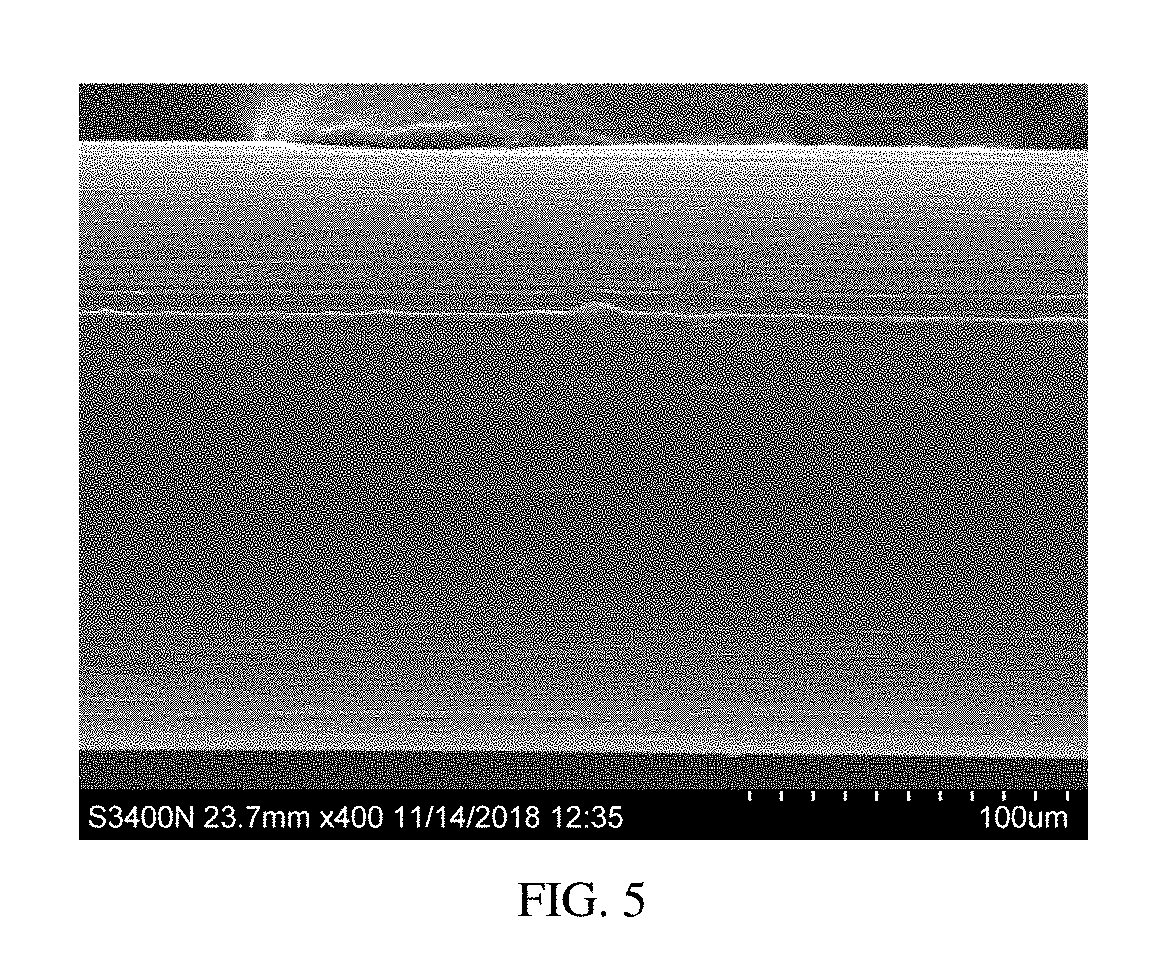
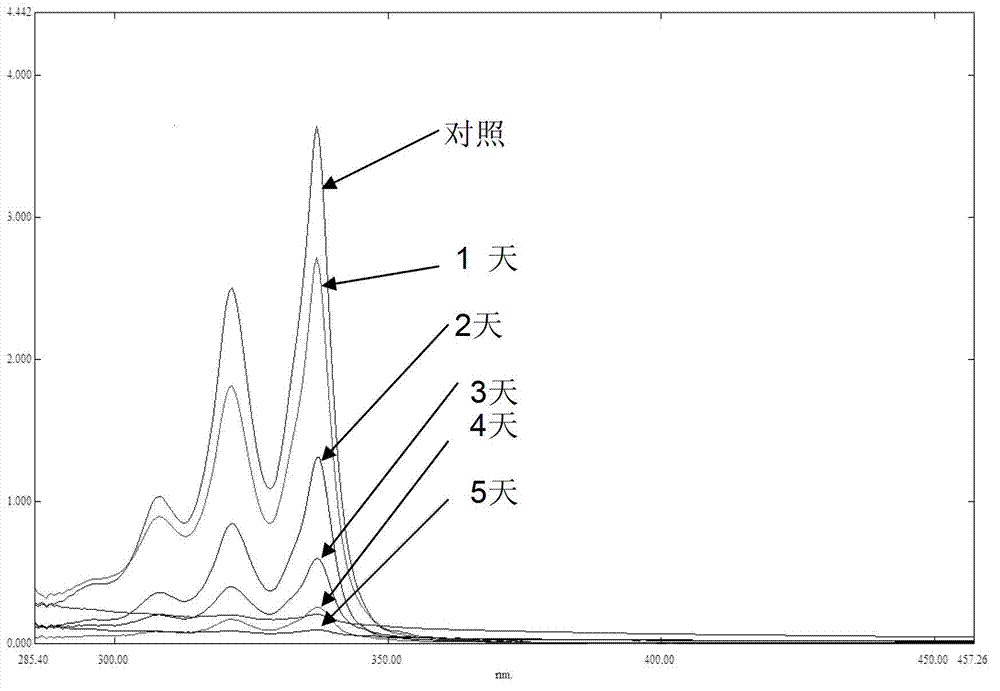
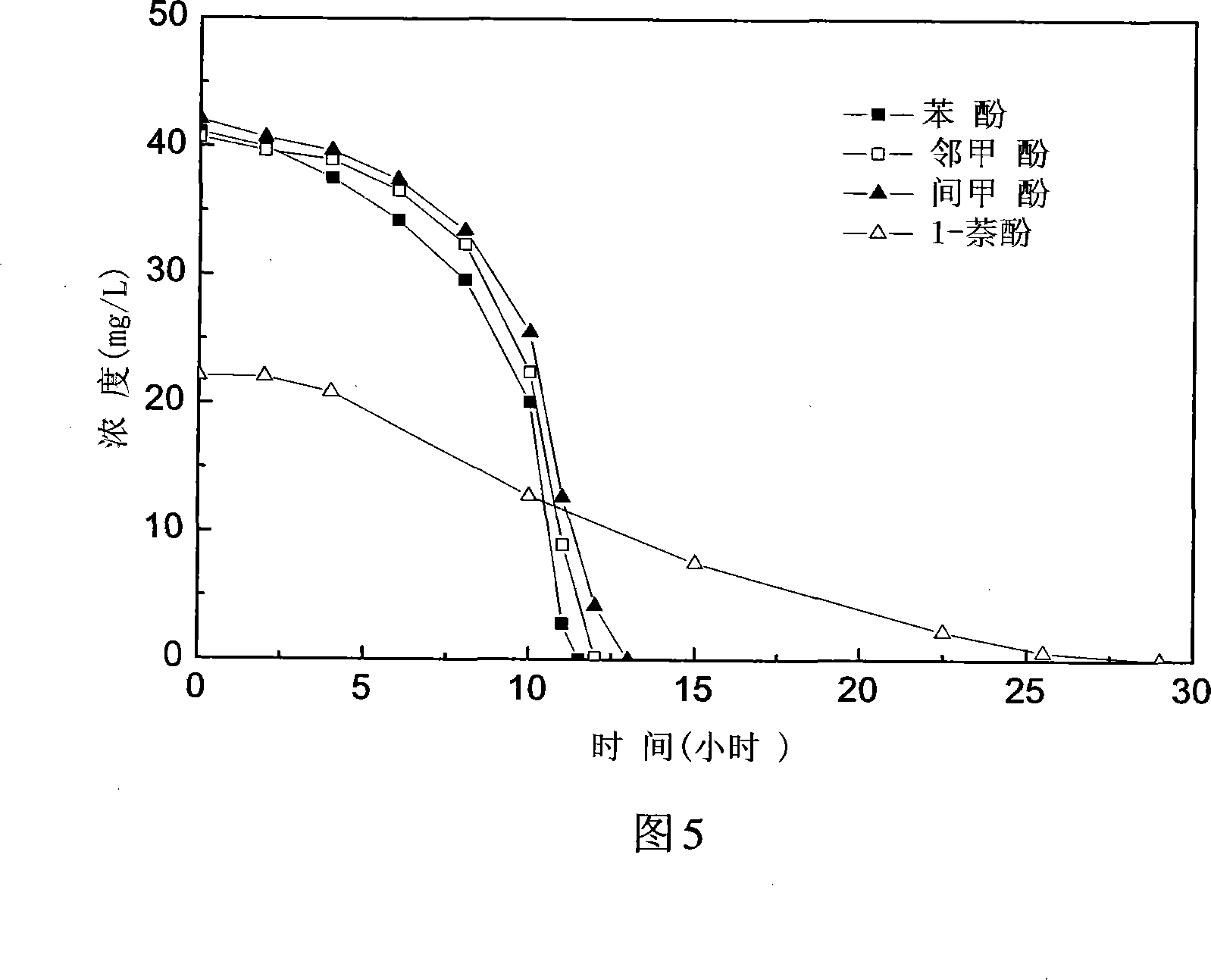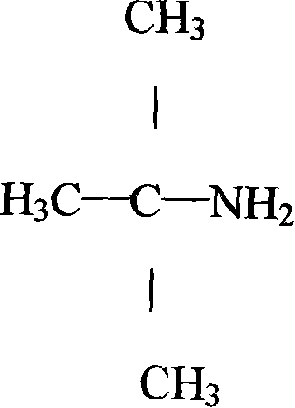Patents
Literature
283 results about "Rifampicin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
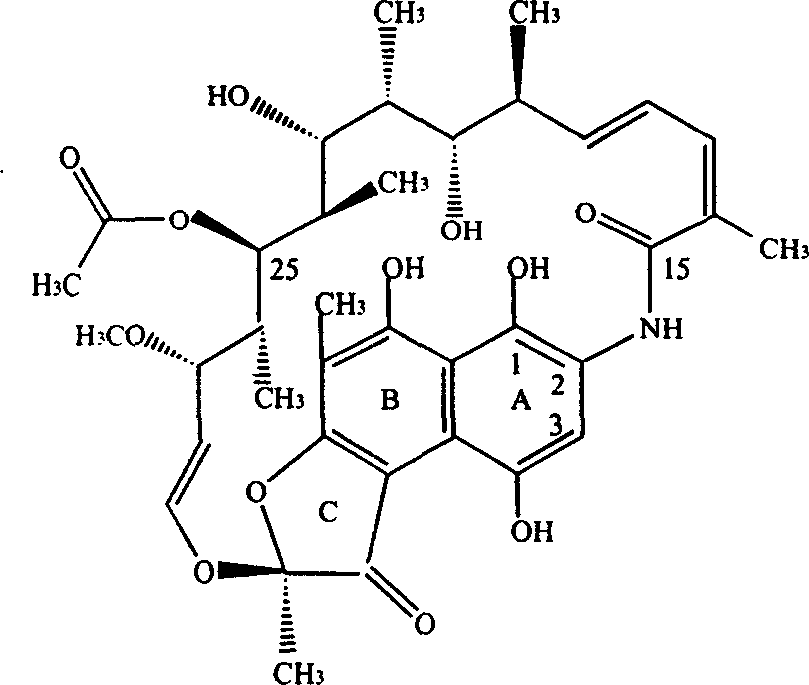
This medication is a rifamycin antibiotic used to prevent and treat tuberculosis and other infections.
Preparation of good quality benemicin
ActiveCN101486716AReduce dosageAvoid changeAntibacterial agentsOrganic chemistrySodium bicarbonateSide chain
The invention discloses a preparation method of high-quality rifampicin, solving the problem that the traditional preparation method has the defects such as unfavorable product quality and high cost; the preparation method comprises the operational steps: S-BA liquid is salified by alkalescence sodium bicarbonate during a salification reaction; S is separated out by acetate acid before a cyclization reaction; then, dihydroxy is added for a reaction to obtain oxazine; after the reaction is finished, DMF is recovered by a molecular distillation method and then dissolved by other hydrophilic solvents; oxazine is separated out by an elutriation method; after a hydrolysis reaction and a condensation reaction are finished, an azeotropic distillation method is adopted to recover un-reacted side chains; pH is adjusted and temperature is lowered so that crystals are separated to obtain the crude products of rifampicin; and then the crude products are refined to obtain the high-quality rifampicin. Compared with the existing preparation technique, the preparation method significantly improves the product purity, reduces the production cost, controls the overall quantity of impurities to be lower than 1.5 percent, reduces two thirds of the general consumption of raw materials and more than a half of the usage of 1-methyl-4-aminopyrazine which is a valuable raw material, and eliminates the environmental pollution caused by strong acid and alkali.
Owner:薛荔 +1
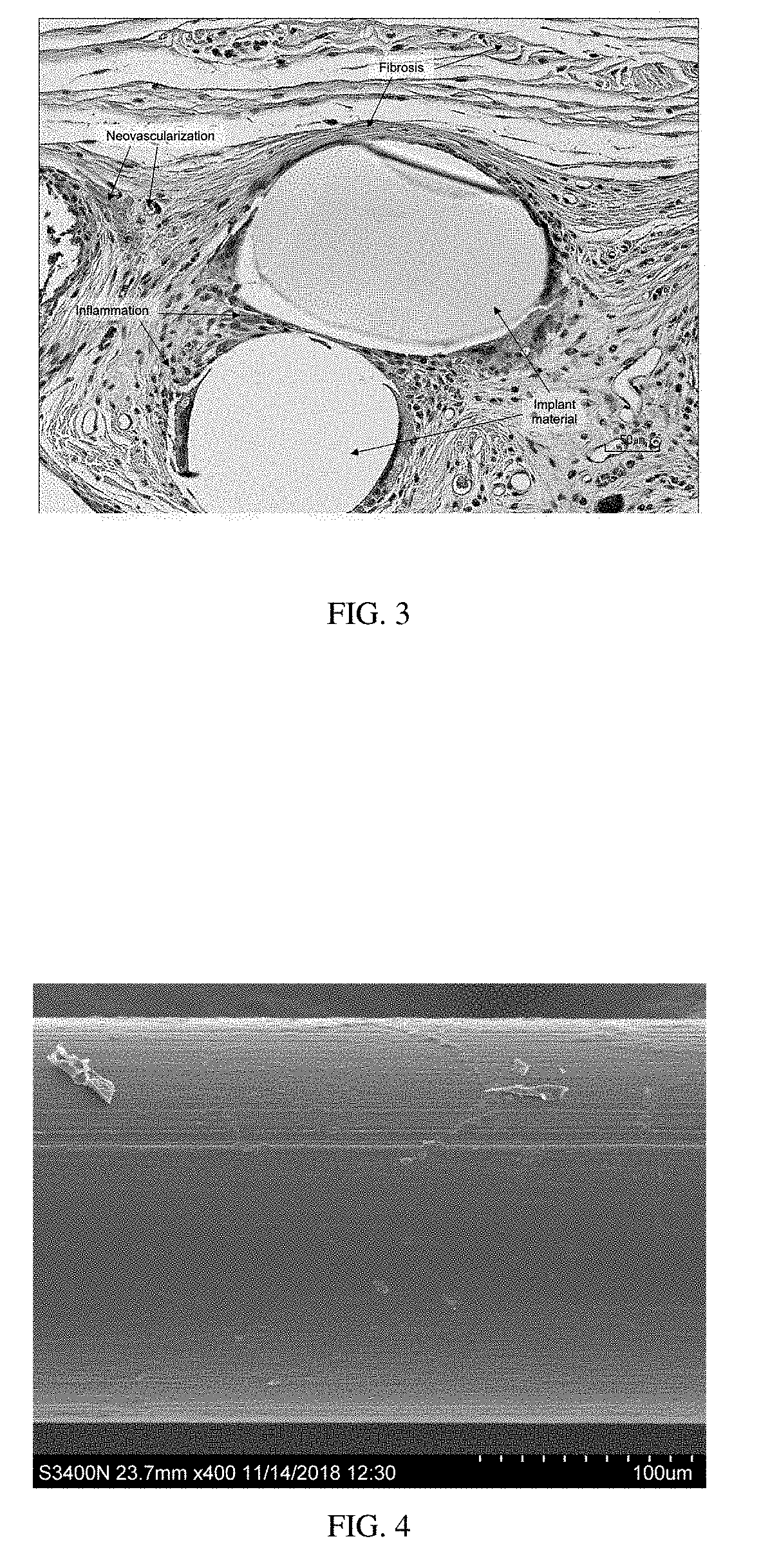
Surgial mesh implants containing poly(butylene succinate) and copolymers thereof
PendingUS20190269817A1Inhibition of colonizationReduce and prevent occurrenceSuture equipmentsMammary implantsFiberLimulus amebocyte lysate
Resorbable implants comprising poly(butylene succinate) and copolymers thereof have been developed. The implants implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing, and the fibers may be oriented. Coverings and receptacles made from forms of poly(butylene succinate) and copolymers thereof have also been developed for use with cardiac rhythm management devices and other implantable devices. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings and receptacles are made from meshes, webs, lattices, non-wovens, films, fibers, and foams, and contain antibiotics such as rifampin and minocycline.
Owner:TEPHA INC
Mycobacterium 16F for efficiently degrading polycyclic aromatic hydrocarbons and benzene organic matters and application thereof
ActiveCN102899271AEfficient degradationDegradation safetyBacteriaMicrobiological testing/measurementKanamycinM-Xylene
The invention provides a strain of Mycobacterium sp.16F for efficiently degrading polycyclic aromatic hydrocarbon and benzene organic matters, which has a preservation number of CGMCC No.6367. The mycobacterium 16F can efficiently, safely and rapidly degrade polycyclic aromatic hydrocarbons and benzene organic matters, can grow and degrade by using fluorene, naphthalene, anthracene, acenaphthene, phenanthrene, pyrene and benzopyrene as the sole carbon source and energy in aerobic condition, and can utilize benzene, m-xylene, toluene, salicylic acid, catechol and other multiple aromatic organic matters. The mycobacterium 16F is sensitive to streptomycin, rifampin, tetracycline, kanamycins and other antibiotics, has good degradation effects to mixed polycyclic aromatic hydrocarbons in aging soils and monocyclic benzene organic matters in water bodies, can be used for restoring and purifying the water-soil environment combinedly polluted by aromatic hydrocarbon organic matters, is important for promoting sustainable development, and has a wide application prospect.
Owner:ENVIRONMENTAL PROTECTION RES INST OF LIGHT IND
Method for simultaneous detection of Mycobacterium tuberculosis complex and identification of mutations in mycobacterial DNA resulting in the resistance of microorganisms to rifampicin and isoniazid on biological microarrays, set of primers, biochip, and set of oligonucleotide probes used in the method
InactiveUS20100261163A1Low costShort timeBioreactor/fermenter combinationsBiological substance pretreatmentsIsoniazidNucleotide
The present invention relates to molecular biology, microbiology, and medicine and provides the method for detection of Mycobacterium tuberculosis complex with simultaneous evaluation of sensitivity of the strains to rifampicin and isoniazid in clinical sample on differentiating biochip. The method is based on two-stage multiplex PCR to obtain fluorescent DNA fragments followed by hybridization of these fragments on microarray containing the set of specific discriminating oligonucleotides. The determination of the resistance of Mycobacterium tuberculosis to rifampicin and isoniazid is carried out by evaluation of point nucleotide substitutions in DNA of microorganism. The present invention allows conduct analysis directly in clinical sample, to evaluate a number of mutations simultaneously, to decrease the cost price of analysis, and to reduce the time of its conducting. The present invention also relates to set of primers, biochip, and set of oligonucleotide probes used in realization of the method.
Owner:UCHREZHDENIE ROSSIISKOI AKADI NAUK INST MOLEKULYARNOI BLOLOGII IM V A ENGELGARDTA RAN IMB RAN
Sterilized minocycline and rifampin-containing medical device
InactiveUS20080075628A1Maximize recoveryGood curative effectSurgeryLavatory sanitoryMedical deviceBiomedical engineering
A sterilized medical device comprising minocycline and rifampin is described. The device is setrerilized by e-beam raditation such that degradation of minocycline and rifampin is minimized. Methods for sterilizing minocycline and rifampin-containing devices by e-beam radiation and methods of manufacturing devices, which methods include e-beam sterilization, are also described.
Owner:MEDTRONIC INC
Use of tigecycline, alone, or in combination with rifampin to treat osteomyelitis and/or septic arthritis
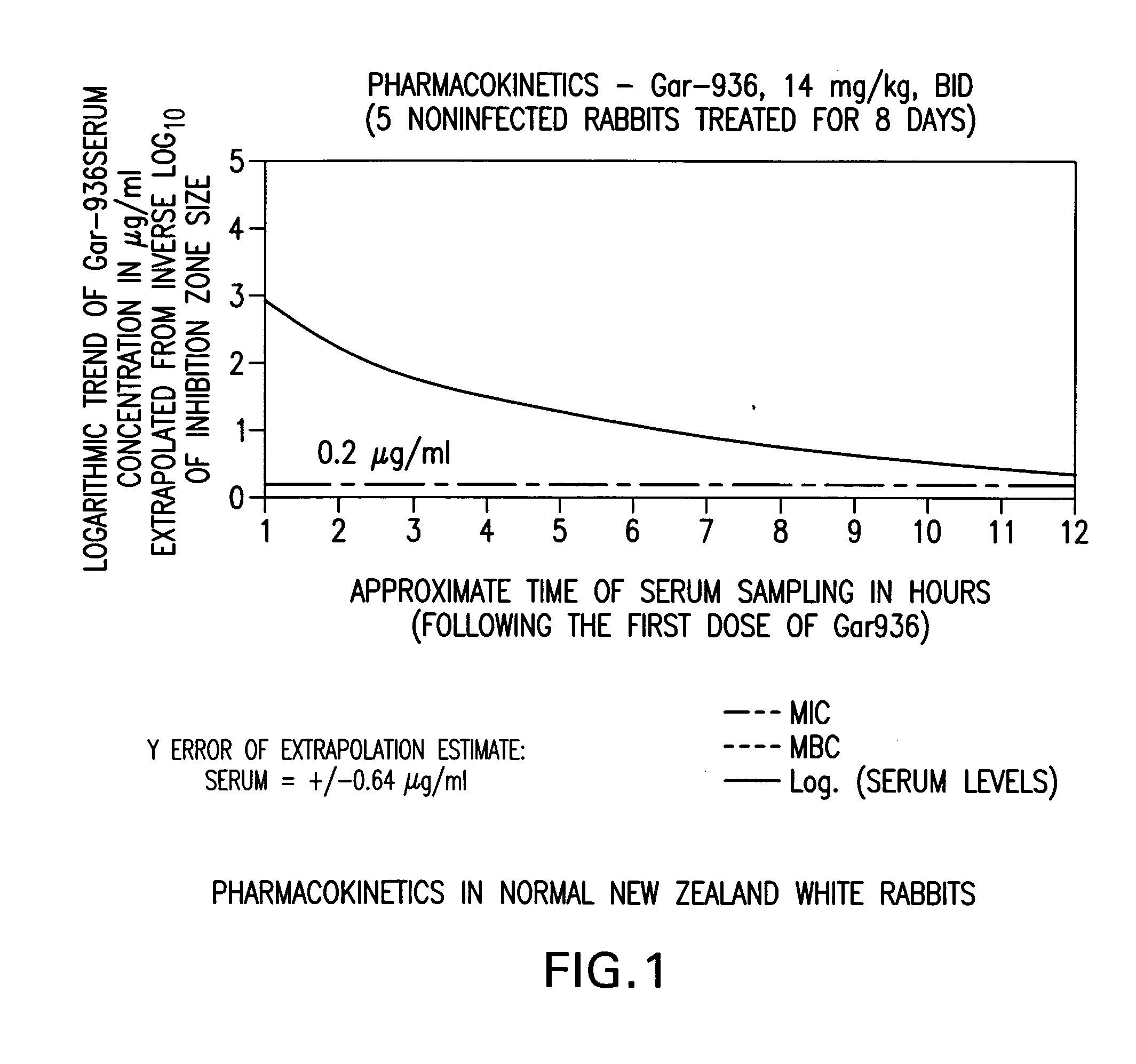
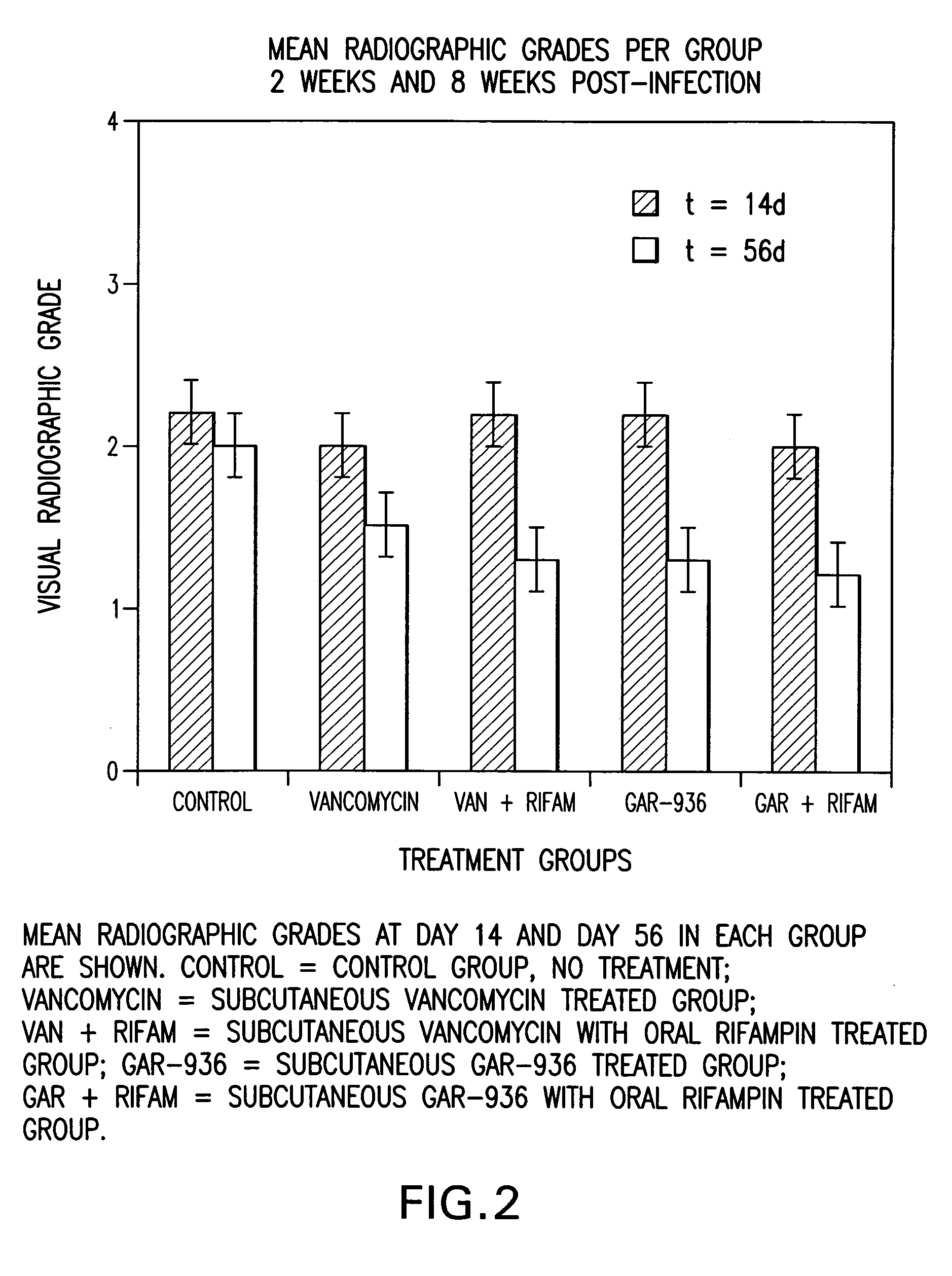
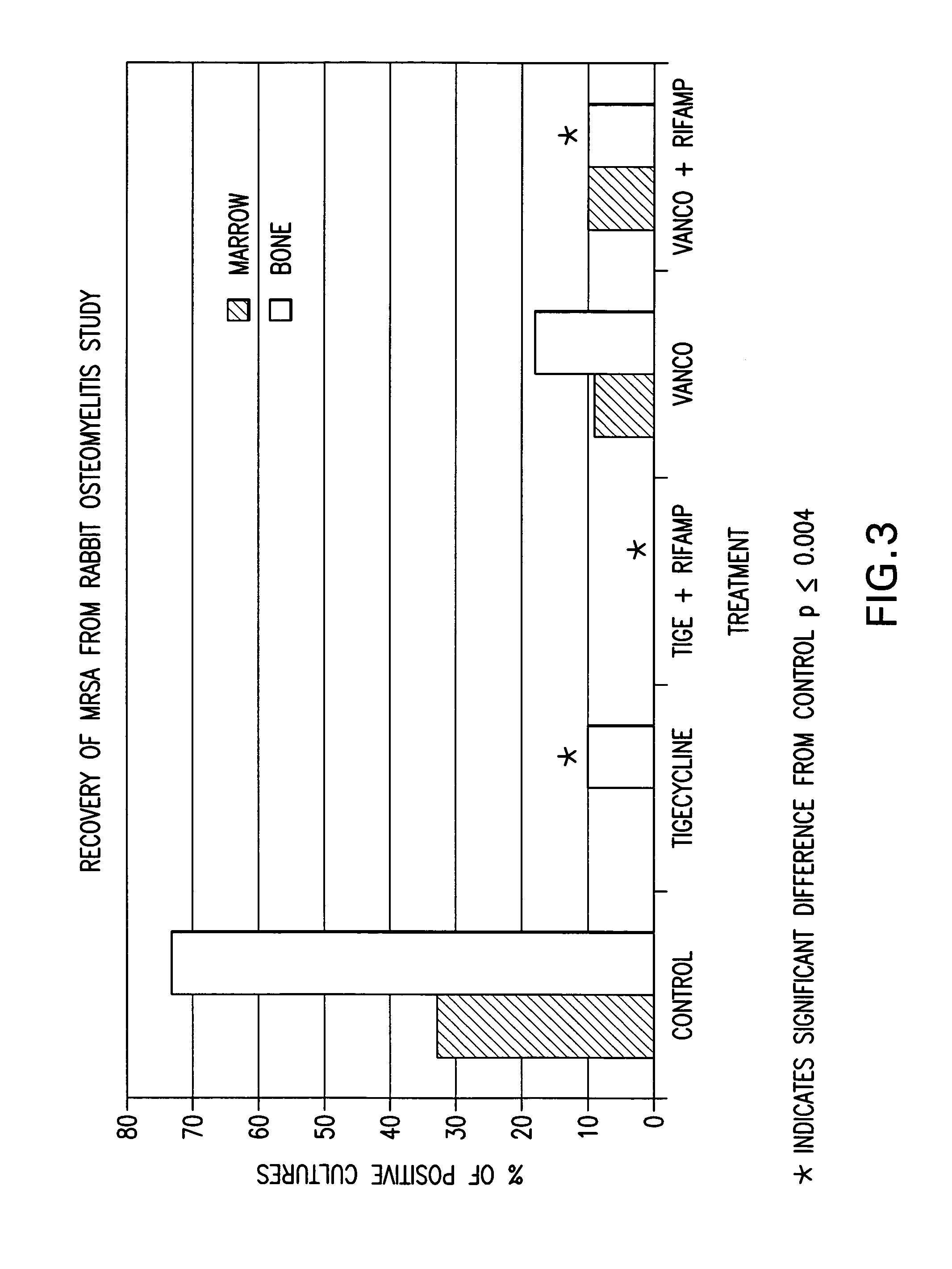
The present invention is directed to a method for treating bone or bone marrow infections, joint infection or infection of the tissues surrounding the joint by administration of the antibiotic tigecycline alone or in combination with a rifamycin antibiotic. In a preferred embodiment the bone or bone marrow infection causes osteomyelitis. In another embodiment the joint infection or infection of the tissues surrounding the joint causes septic arthritis. The invention is also directed to manufacture of a medicament for treatment of bone and / or bone marrow infections, or joint infections and / or infections in tissues surrounding the joint with tigecycline alone or in combination with rifampin.
Owner:WYETH +1
Polyamines and their use as antibacterial and sensitizing agents
InactiveUS20070197658A1High activityHigh propensityBiocideOrganic chemistryEscherichia coliChain length
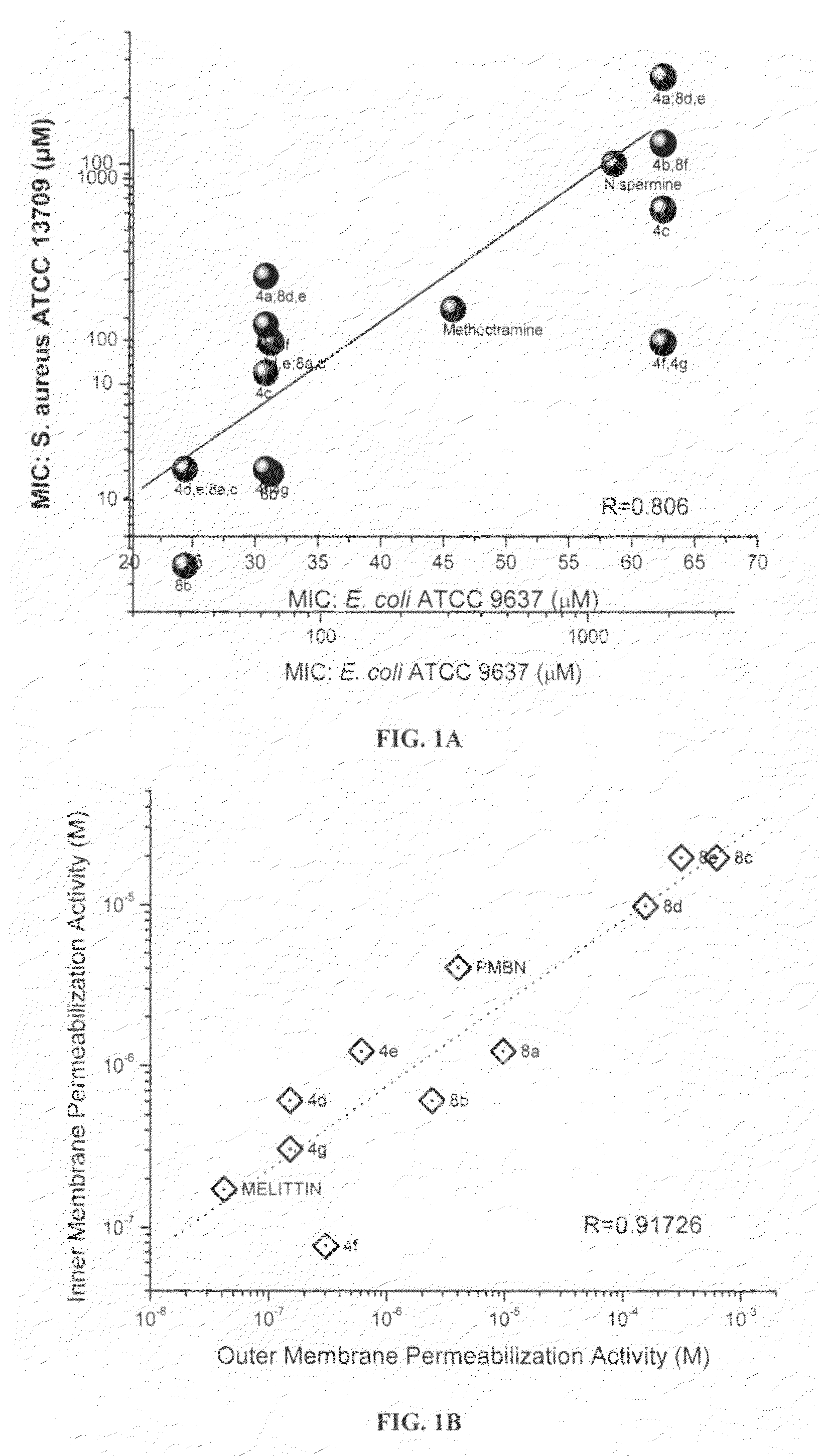
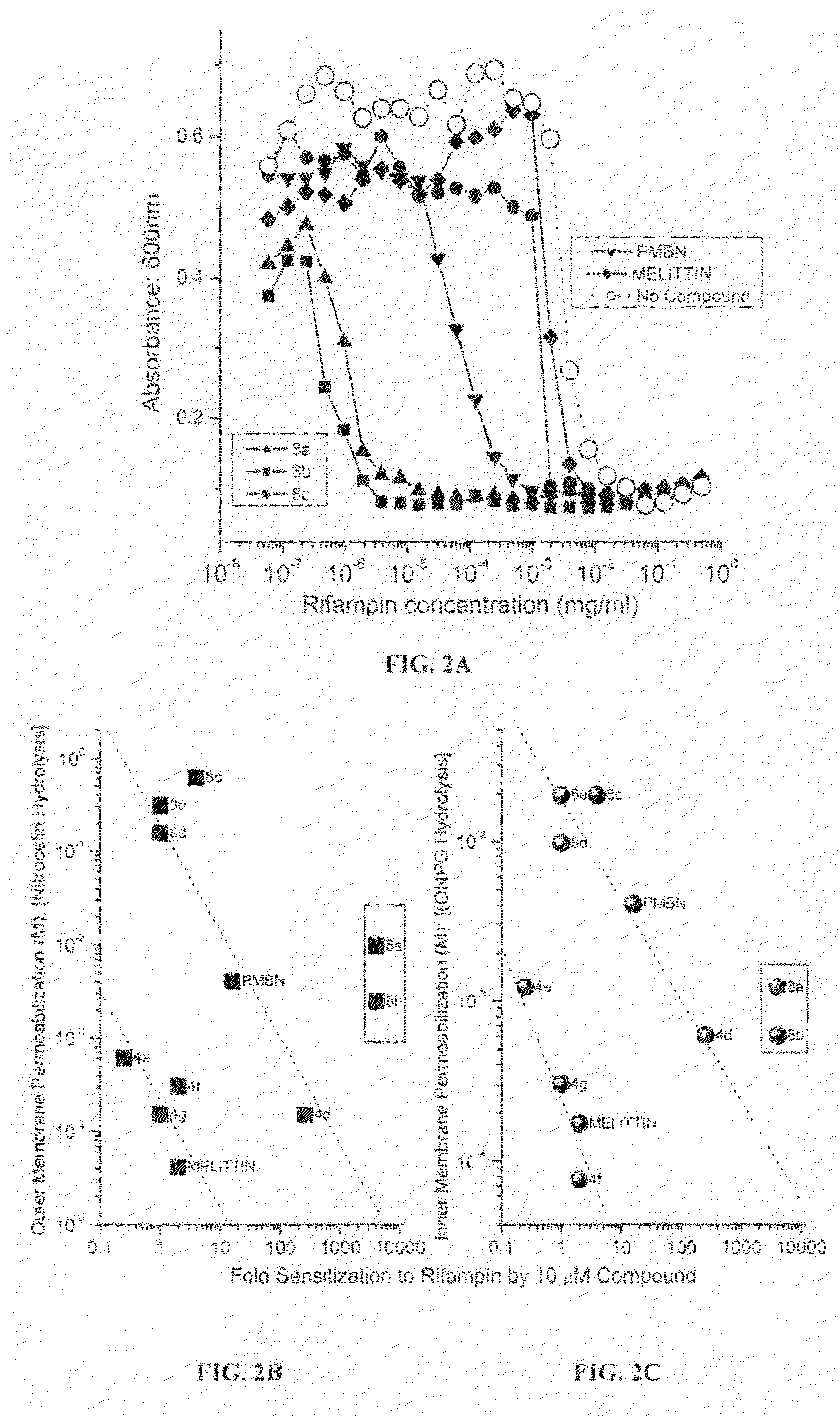
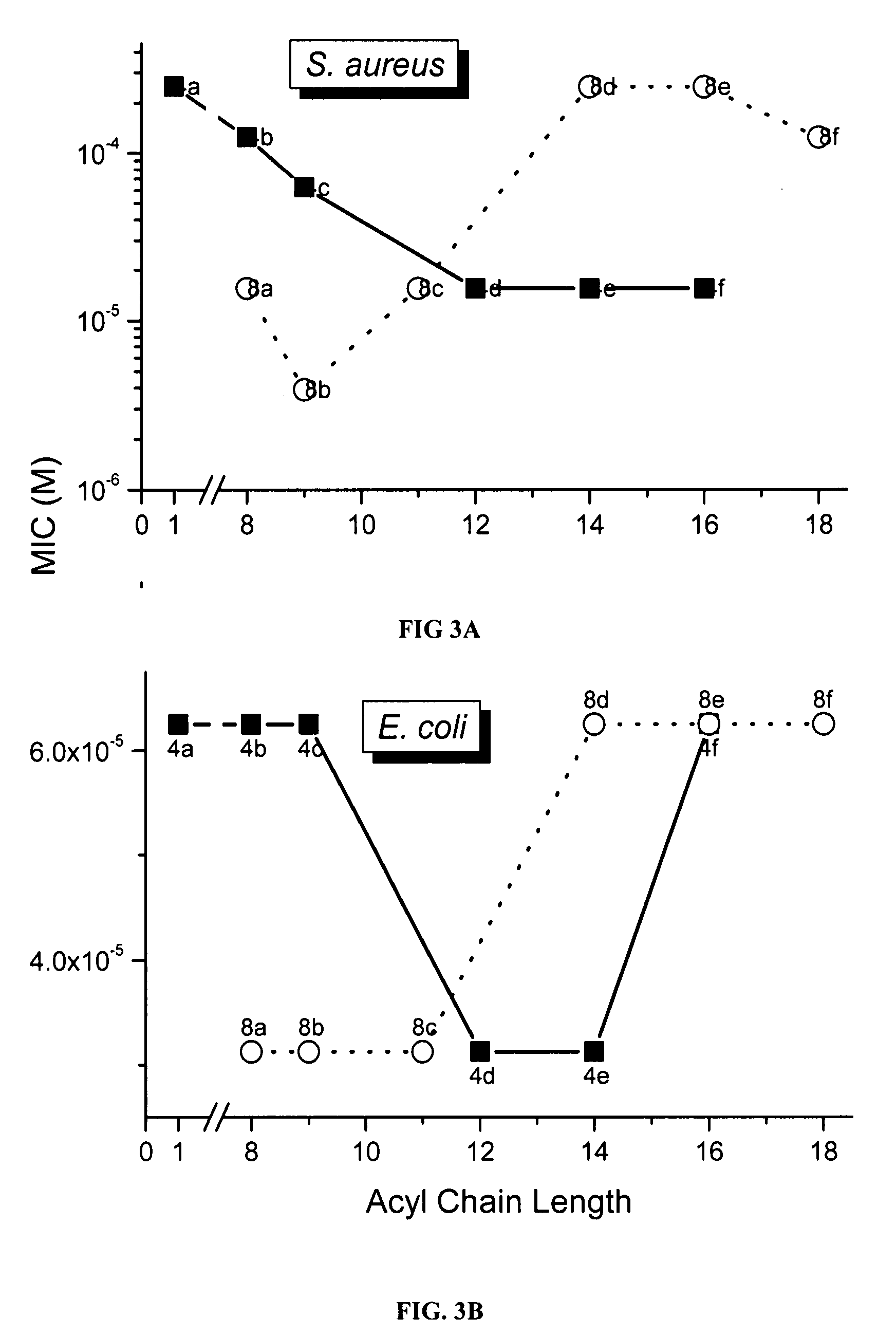
Polyamines with varying chain-lengths were evaluated for antimicrobial activity in order to test the hypothesis that these bis-cationic amphipathic compounds may also bind to and permeabilize intact Gram negative bacterial membranes. The compounds were found to possess significant antimicrobial activity and mediated via permeabilization of bacterial membranes. Homologated spermine, bis-acylated with C8 or C9 chains was found to profoundly sensitize E. coli to hydrophobic antibiotics such as rifampicin.
Owner:KANSAS UNIV OF
Citrobacter farmeri Citrobacter farmeriSC01 and application thereof
InactiveCN101240256AHigh average degradation rateBacteriaMicroorganism based processesSludgeCitrobacter farmeri
The invention discloses a fashi citric acid rod Citrobacter farmeri SC01 and the application. The bacterium is obtained by artificial accumulation culture and separation cleansing from phenols cyanogen waste water treatment station one level aerobic pool sludge. The bacterium is preserved in china typical culture entreasure center of Wuhan University on January 7th 2008 year whose short form is CCTCC and preserving identification serial number is CCTCC NO: M208004. The fashi citric acid rod Citrobacter farmeri SC01 incorporating the invention can degrade phenyl hydrate, 2-methylphenol, m-cresol, 1-naphthyl hydroxide, 696mg / L m-cresol in 114 hours on aerobic condition, and the average degrading rate of speed ran up to 8.94mg / (L h) and the bacterium is sensitive to acheomycin, chloramphenicol, rifampicin and streptomycin, and the bacterium can be used safe and wide in harnessing and repairing wasted water and soil phenols pollution because the bacterium can not carryover drug-resistance factor spreading in aborigines bacterium when the bacterium is released into environment.
Owner:SOUTH CHINA UNIV OF TECH
Anti-infection venous catheter and preparation method thereof
ActiveCN102500033ALost fastImprove efficacyAntibacterial agentsTetracycline active ingredientsVeinIntravenous catheter
The invention relates to an anti-infection venous catheter and a preparation method thereof. The venous catheter comprises a conduit main body tube, a tip end and a tube seat, and preferably also comprises a connecting seat and a plurality of epitaxial tubes, wherein the parts retained in a human body are the conduit main body tube and the tip end; and an anti-infection medicament rifampicin, an anti-infection medicament minocycline or combination of the two are uniformly loaded on the conduit main body tube. The preparation method of the venous catheter comprises the following steps of: dissolving the anti-infection medicament or the medicament combination to form soak solution; soaking the conduit into the soak solution to fully soak medicaments into the conduit; and drying to remove solvent to prepare the anti-infection venous catheter. During use, the medicaments are slowly released to fulfill the anti-infection aim of the conduit and avoid infection of the conduit during retention in the human body in surgery.
Owner:BEIJING DEMAX MEDICAL TECH
Oriented implants containing poly(butylene succinate) and copolymer, and methods of use thereof
PendingUS20190269816A1Inhibition of colonizationReduce and prevent occurrenceSuture equipmentsMammary implantsFiberLimulus amebocyte lysate
Resorbable implants comprising poly(butylene succinate) and copolymers thereof have been developed. The implants implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing, and the fibers may be oriented. Coverings and receptacles made from forms of poly(butylene succinate) and copolymers thereof have also been developed for use with cardiac rhythm management devices and other implantable devices. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings and receptacles are made from meshes, webs, lattices, non-wovens, films, fibers, and foams, and contain antibiotics such as rifampin and minocycline.
Owner:TEPHA INC
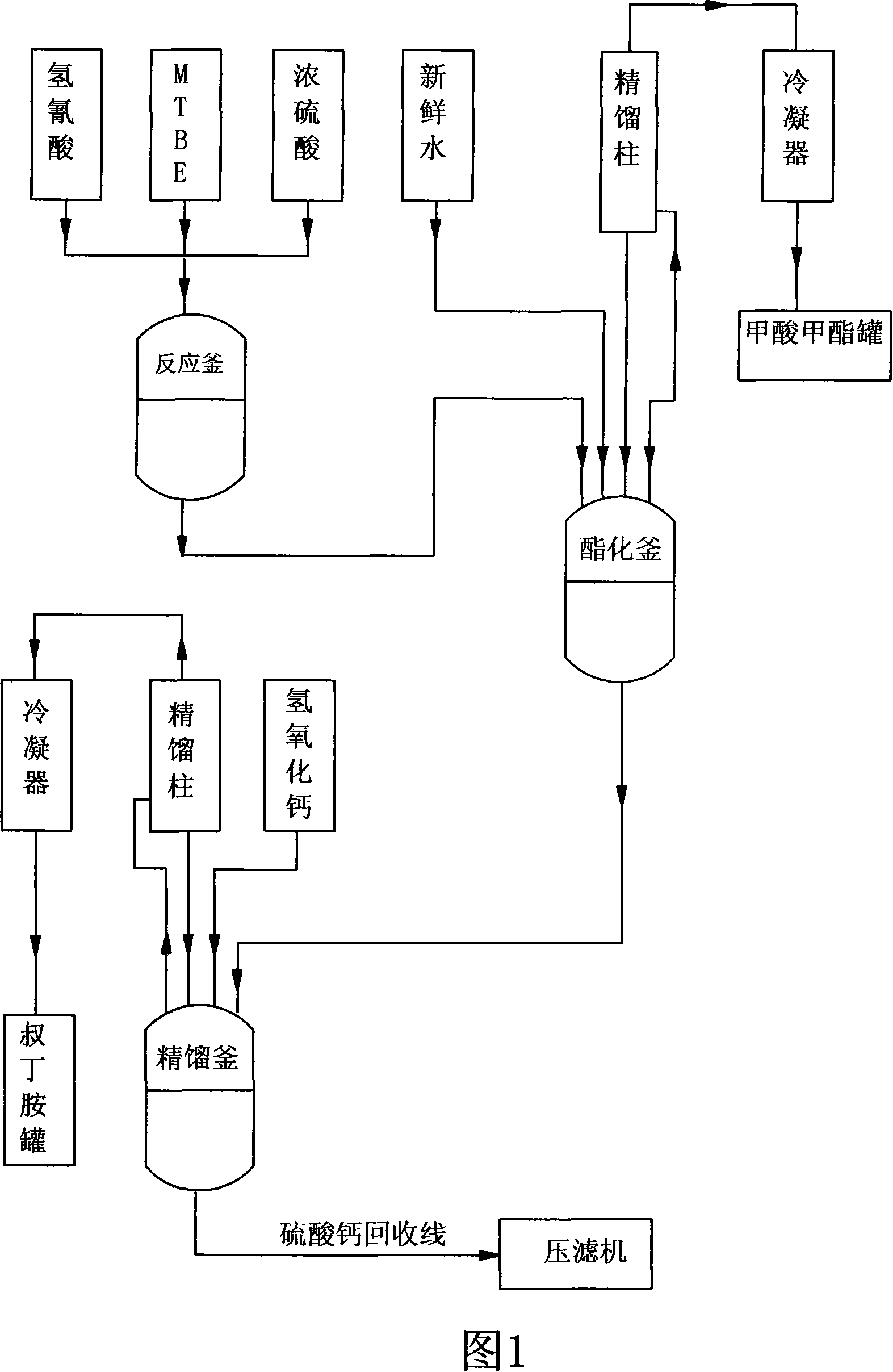
Technique for synthesizing tert-butylamine
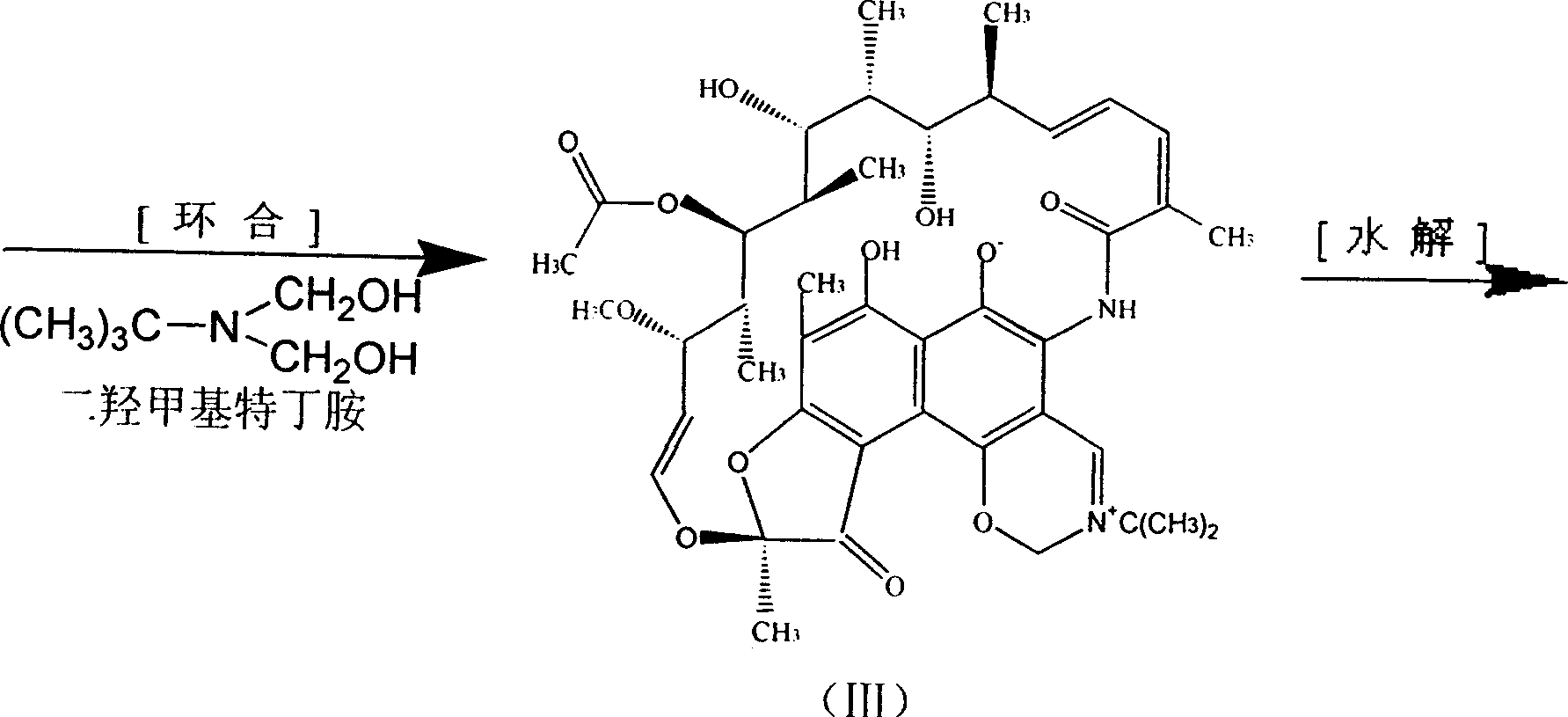
ActiveCN101108806APromote hydrolysisPromote esterification reactionOrganic compound preparationAmino compound preparationTert-ButylamineCALCIUM HYDROXIDE SOLUTION
The invention belongs to the fine chemical field, which is mainly used for the synthesis of rubber accelerator NS and the intermediate raw material of the rifampicin-tert-butylamine. The invention is characterized in that: the methyl tert-butyl ether-hydrocyanic acid method is adopted to produce tert-butylamine; under normal pressure, the methyl tert-butyl ether is added with hydrocyanic acid and is added with concentrated sulphuric acid to carry out fully catalytic reaction; the methanol produced in the catalytic reaction is fully adopted and is added with water to perform hydrolysis, deep hydrolysis and esterification, and methyl formate and methanol without react completely are distilled; sulphates in calcium hydroxide solution and the butylamine to replace the butylamine to gain the butylamine through rectification and produce the byproduct calcium sulfate. The invention adopts the water instead of the methanol to carry out the hydrolysis and esterification and neutralizes the raw materials and adopts the calcium hydroxide to produce butylamine, which has the advantages of simple process, short production period, high yield, stable product quality, low production cost and small environment pollution.
Owner:ZIBO LUHUA HONGJIN NEW MATERIAL CO LTD
One-pot processing method for synthesizing rifampicin
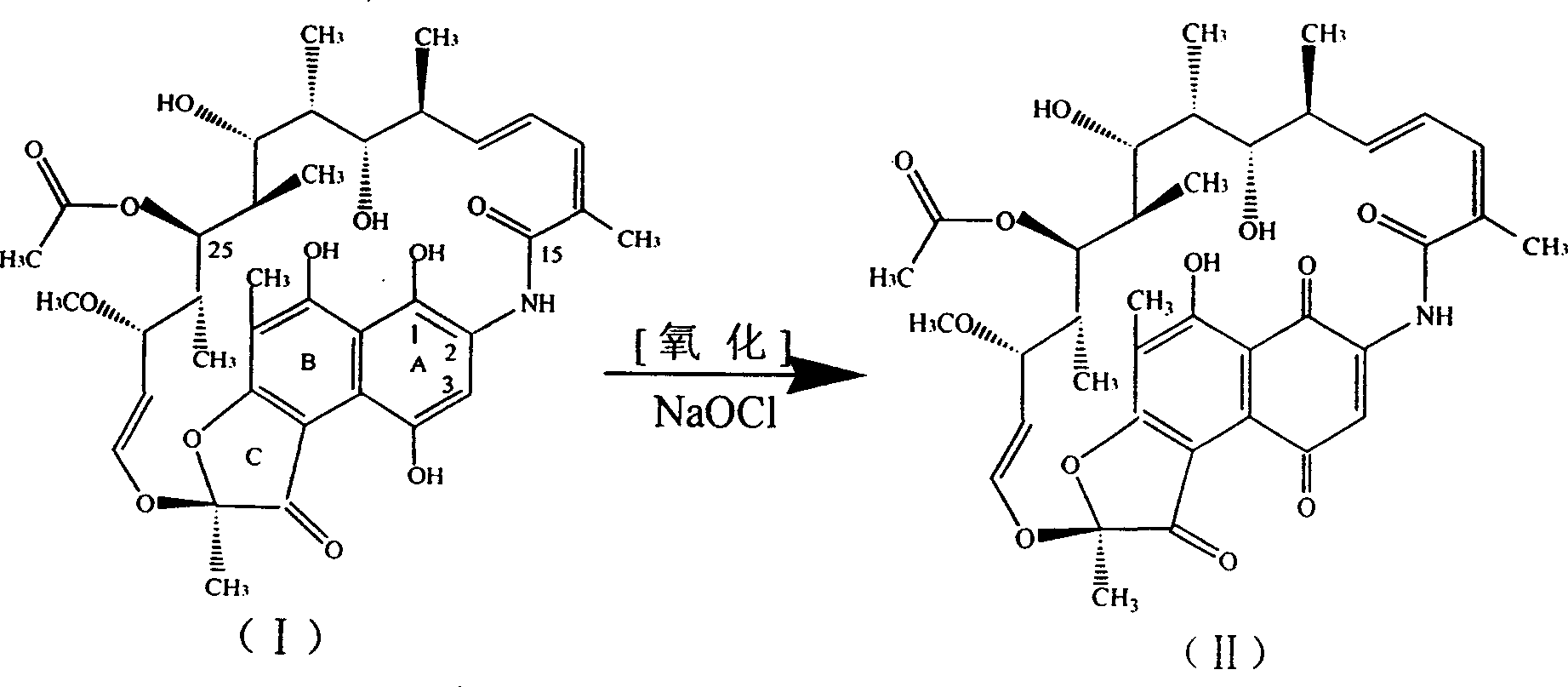
The invention synthetics benemicin by means of strewed in one pan, solving basically the side reaction formed by using much of inorganic acid and alkali of prior art, it's based on isolated purified nancimycin SV as raw material, to oxygenate to nancimycin S by using sodium hypochlorite as oxidant, without crystallization separating nancimycin S sodium salt and the acidifying process by concentrated sulfuric acid, with direct ring jointing, hydrolyzing and condensation reacting, the intermediate to synthetic benemicin by means of strewed in one pan free of separation. It simplifies the process, decreases mating equipment, and meanwhile well controls each side reaction, for removing the use of inorganic base employ and controlling the dosage of hydrogenous acid, and the quality of intermediate be basically guaranteed, so the quality of product is perfect, and the yield of benemicin is improved. Moreover, morpholine used in the condensation reaction lowering the dosage of valuable raw material 1-methyl-4-amidogen vermex, so plenty of chemical materials are saved, energy consumption is lowered, and the cost decreases dramatically.
Owner:薛荔 +1
Method for screening Mycobacterium tuberculosis drug-resistant protein
InactiveCN1945324AIncrease the burdenAccurate separation and identificationMicrobiological testing/measurementBiological testingVaccine antigenMicrobiology
The present invention belongs to the field of screening antituberculotic target, vaccine antigen and detecting target in molecular biological technology, and is especially process of screening protein resisting rifampicin as antituberculotic. The process includes the first culturing drug resistant strain, the subsequent separating protein of drug resistant strain from protein of sensitive strain, comparing protein of drug resistant strain and protein of sensitive strain to determine the drug resistant protein, and final separating and identifying drug resistant protein. The process of the present invention can separate and identify drug resistant protein of Mycobacterium tuberculosis to provide new way for further understanding the drug resisting mechanism of Mycobacterium tuberculosis, fast clinical detection of drug resistant strain, and developing vaccine and medicine.
Owner:FUDAN UNIV
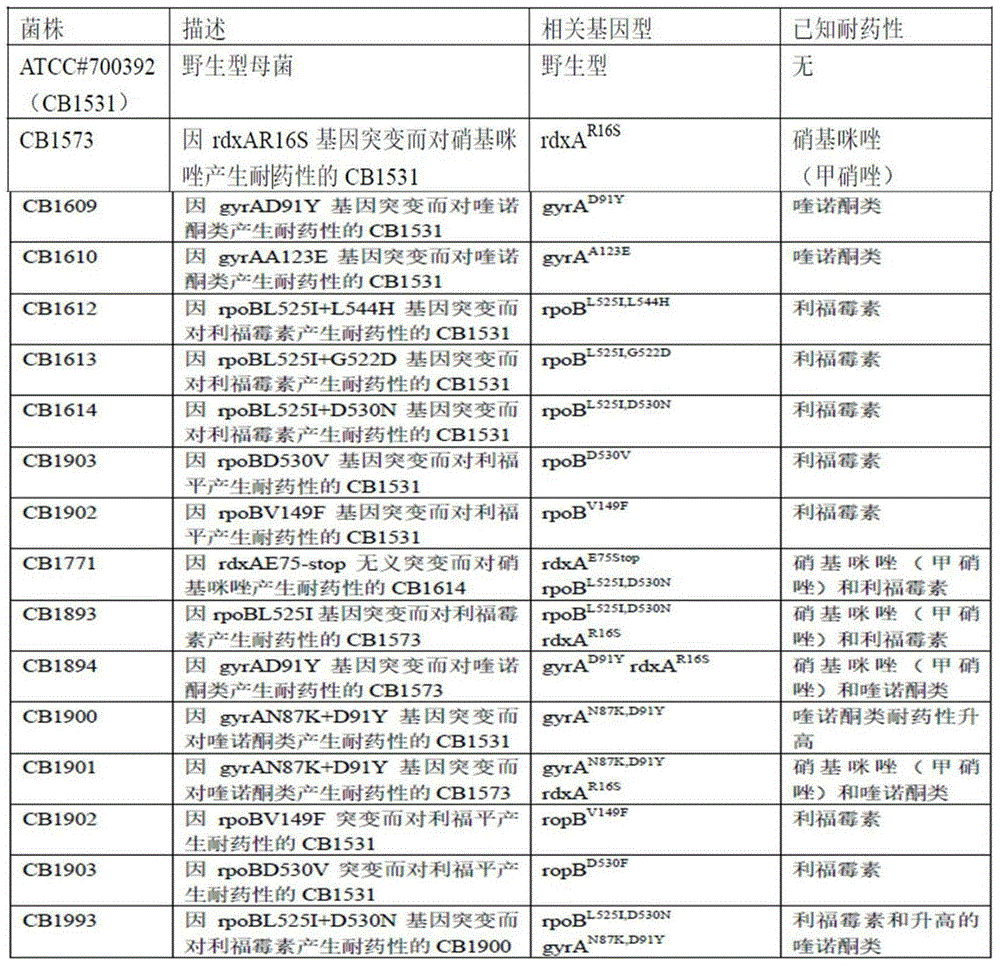
Method for detecting resistant mutation of mycobacterium tuberculosis to rifampin and kit thereof
InactiveCN102229988AImprove mutation detection rateHigh melting pointMicrobiological testing/measurementFluorescence/phosphorescencePositive controlDesign software
Relating to the detection technology of resistant mutation of mycobacterium tuberculosis, the invention aims to provide a rapid, sensitive and specific method for detecting the resistant mutation of mycobacterium tuberculosis to rifampin and a kit thereof. The method comprises the steps of: extracting the DNA of a mycobacterium tuberculosis sample; according to the rpoB gene sequence (GenBank: L27989) where an RRDR (rifampin resistance determining region) of mycobacterium tuberculosis is located, designing primers and probes by means of the primer design software Primer Premier 5; constructing a double-tubed and double-colored real-time PCR (polymerase chain reaction) system, detecting the mutation of the RRDR of mycobacterium tuberculosis, comparing the melting point (Tm value) differences of melting curves between the detected sample and a positive control, and determining whether the sample mutates. With the primers designed at two ends of the RRDR of the rpoB gene, the method of the invention is of high specificity.
Owner:XIAMEN UNIV +1
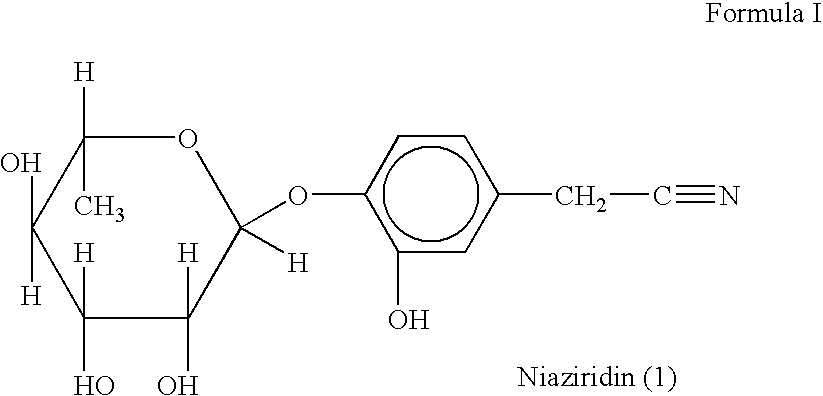
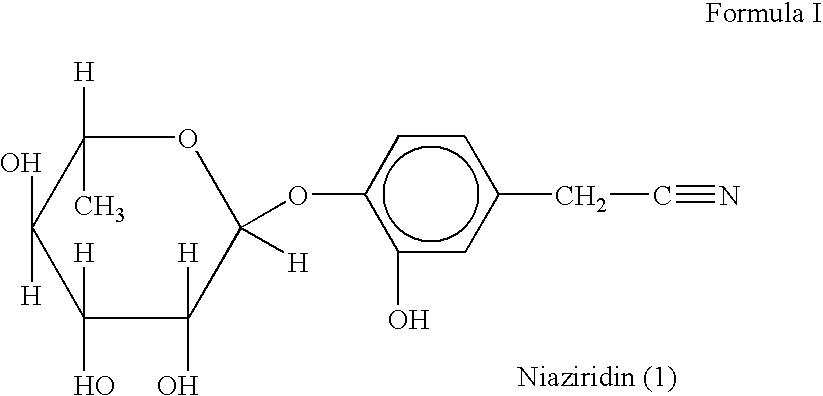
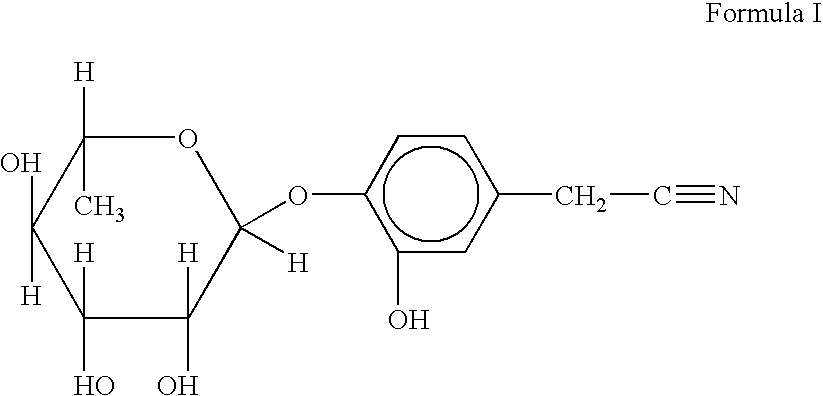
Novel nitrile glycoside useful as a bioenhancer of drugs and nutrients, process of its isolation from moringa oleifera
InactiveUS20040198669A1Improve biological activityEnhance bioavailability absorptionBiocideSugar derivativesAmpicillinBioactivity guided fractionation
The present invention relates to a novel nitrile glycoside of Formula I named NIAZIRIDIN and to analogues and derivatives thereof. The present invention also relates to a process for the isolation of a novel nitrile glycoside of Formula I below named NIAZIRIDIN and its derivatives and analogues by bioactivity-guided fractionation from the pods of Moringa oleifera. The present invention particularly relates to the bioenhancing activity of the novel nitrile glycoside of Formula I below named NIAZIRIDIN and its derivatives and analogues in enhancing bioactivity of commonly used antibiotics such as rifampicin, tetracycline and ampicillin against Gram (+) and (-) bacteria. The biomolecule also enhances the absorption of drugs, vitamins and nutrients through the gastro-intestinal membrane increasing their bio-availability. Therefore niaziridin can be used in combination therapy with drugs and nutrients resulting in reduced drug associated toxicity, reduced cost and duration of chemotherapy.
Owner:COUNCIL OF SCI & IND RES
Method and material for simultaneously detecting mycobacterium tuberculosis compound and identifying its medicament resistance
InactiveCN101210265AMicrobiological testing/measurementBiological material analysisIsoniazidFluorescence
The present invention relates to molecular biology, microbiology and medicine, and provides a method for detecting nodule offshoot bacilli complex body in clinic samples and evaluating the sensitivity of strain to rifampicin as well as isoniazid in distinguish biochip. The method obtains fluorescence DNA segments based on two-step multiple PCR, then to hybridize said segments on a micro-array containing differential distinguished oligonucleotide group. To determine the resistance of concretion offshoot bacilli to rifampicin and isoniazid by means of evaluating point nucleotide replacement in microbiology DNA. The present invention allows analyze directly clinic sample to evaluate mass mutation at one time, so as to reduce analysis cost and implement time. The present invention also relates to primer group, biochip and oligonucleotide probe group for implementing said method.
Owner:RUSN ACAD V A ENGERHARDT MOLECULAR BIOLOGY INST
Innovative methods of treatmenting tuberculosis
InactiveUS20160074480A1Good effectEradicating diseaseAntibacterial agentsBiocideVitamin CAutohemotherapy
A Safe and effective treatment methods to cure and curtail tuberculosis affliction described using high dose Vitamin C, and other known anti-mycobacterium tuberculosis drugs especially rifampicin administered intravenously for 6 weeks instead of 6 to 24 months of conventional treatment. Insulin is administered to induce moderate hypoglycemia to augment and add effectiveness of anti-tuberculosis drugs and Vitamin C. Invention also delivers the drugs directly to a tuberculin lesion through a catheter. An embodiment of the invention uses a nebulizer and other methods of administration of Vitamin C with anti-tuberculosis drugs, interferon Y−, Coley's vaccine, dinitrophenol hyperthermia, ozone therapy, Hydrogen peroxide therapy and artemisinin, combined with oxygen supplementation including autohemotherapy with ozone, hyperbaric therapy, and hypertheramia to increase the respiration of the M. tuberculosis bacteria which has a killing effect.
Owner:SHANTHA TOTADA R
Preparation method of medicated slow-release degradable bone scaffold
InactiveCN102188756AAvoid connectionImprove antibacterial propertiesSurgeryProsthesisPolyesterMicrosphere
The invention discloses a preparation method of a medicated slow-release degradable bone scaffold, which comprises the following steps: preparing a biological degradable polyester material (such as PLGA (poly(lactic-co-glycolic acid)) and a loaded antitubercular medicament (such as rifampicin or isoniazide) into PLGA microspheres containing the rifampicin or isoniazide, respectively mixing the PLGA microspheres containing the rifampicin or isoniazide with a biological medical adhesive, and molding with a mold, thus obtaining the medicated slow-release degradable bone scaffold. Having a certain porosity, the medicated slow-release degradable bone scaffold prepared by the preparation method is beneficial to the transportation and exchange of body water, inorganic salt and other nutrient substances and cell metabolism products, thereby being more beneficial to the normal growth and physiologic metabolism of bone cells, and providing an ideal place for the growth of bone tissues; and accompanied by the degradation of the PLGA, the medicament can be continuously released in the focal position and can be kept at a certain concentration, thereby inhibiting the growth of tubercle bacillus, and ultimately degrading the PLGA into carbon dioxide and water which are removed from the body through body metabolism.
Owner:TIANJIN HAIHE HOSPITAL
Mutant capable of raising cyclic lipopeptides antibiotic output by using RNA polymerase mutation, its preparation method and its application
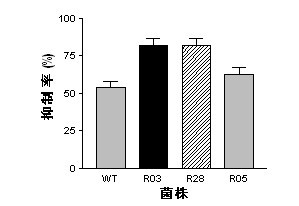
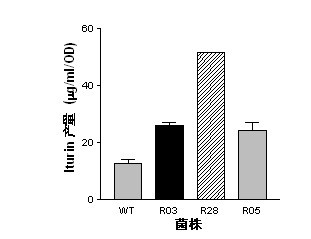
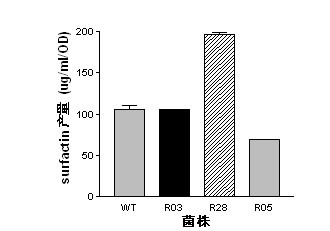
InactiveCN102492639AGenerated ahead of timeGood antifungal activityBiocideBacteriaWild typeAntibiotic Y
The present invention belongs to the field of a biological technique and relates to a RNA polymerase mutant of Bacillus spp CC09 bacterial strain of high-yield antimycotics cyclic lipopeptides substances screened by using a LB plate containing rifampin (50 mug / ml). The mutation site of the mutant is on any position of 485-490 in a RNA polymerase beta sub-unit amino acid sequence, Compared with wild type CC09 bacterial strain, the output of antimycotics cyclic lipopeptides antibiotics Iturin and Surfactin produced by the mutant can be increased by 100%-300%, the antifungal activity is increased by 20-60%, and the mutant can be used for preventing and treating a plurality of crop diseases.
Owner:NANJING UNIV
Oriented p4hb implants containing antimicrobial agents
ActiveUS20160082160A1Inhibition of colonizationReduce and prevent occurrenceSuture equipmentsPharmaceutical containersFiberMedicine
Oriented resorbable implants made from poly-4-hydroxybutyrate (P4HB) and copolymers thereof, have been developed that contain one or more antimicrobial agents to prevent colonization of the implants, and reduce or prevent the occurrence of infection following implantation in a patient. These oriented implants are particularly suitable for use in procedures where prolonged strength retention is necessary and there is a risk of infection. Coverings and receptacles made from poly-4-hydroxybutyrate and copolymers thereof, containing antimicrobial agents, have also been developed for use with implantable devices to prevent colonization of these devices, and to reduce or prevent the occurrence of infection following implantation of these devices in a patient. These coverings and receptacles may be used to hold, or partially or fully cover, devices such as pacemakers and neurostimulators. Preferably, the coverings and receptacles are made from meshes, non-wovens, films, fibers, and foams, and contain rifampin and minocycline.
Owner:TEPHA INC
Vaccines for diseases of fish
Fish are immunized by a mass vaccination method, such as by immersion in water containing an attenuated strain of a pathogenic bacterium that does not effectively cause disease in fish when the non-attenuated pathogenic bacterium is exposed to the fish by immersion. An illustrative example of the method is for immunizing against coldwater disease caused by Flavobacterium psychrophilum, which may be attenuated by serial passage in media containing increasing amounts of an antibiotic, such as rifampicin.
Owner:IDAHO UNIV OF
Method for transient expression of introducing exogenous gene into chrysanthemum or related species
ActiveCN103882054AResolution cycleSolve efficiency problemsGenetic engineeringFermentationKanamycinTransformation efficiency
The invention provides a method for transient expression of introducing an exogenous gene into chrysanthemum or related species. The exogenous gene is a GUS gene which is carried by an over-expression vector plasmid of smaller than 10 kb carrying a 35s promoter and a terminator, and the method mainly comprises the following steps: (1) obtaining of a plant; (2) building of a plant expression vector pORER1-2X35s; (3) preparation of an infection liquid; (4) preparation of chrysanthemum leaves; (5) transformation of leaves; (6) cultivation of leaves after transformation; and (7) detection of positive transformed leaves. According to the method, appropriate explants are selected, agrobacterium tumefaciens strains serve as the susceptible strains, and infection thalli are selected through rifampicin and kanamycin; simultaneously P19 and agrobacterium tumefaciens are mixed and cultivated to be prepared into the infection liquid, P19 can restrain the silence effect of plants to vector expression exogenously introduced, so that the expression level is improved, the co-culture conditions are reasonably optimized, transformation time based on the method is shortened to about 45 days, and the transformation rate reaches 60 percent above; the method of the invention provides possibility for effective screening and analysis of chrysanthemum gene function, protein positioning and further obtaining of stable transformants.
Owner:NANJING AGRICULTURAL UNIVERSITY
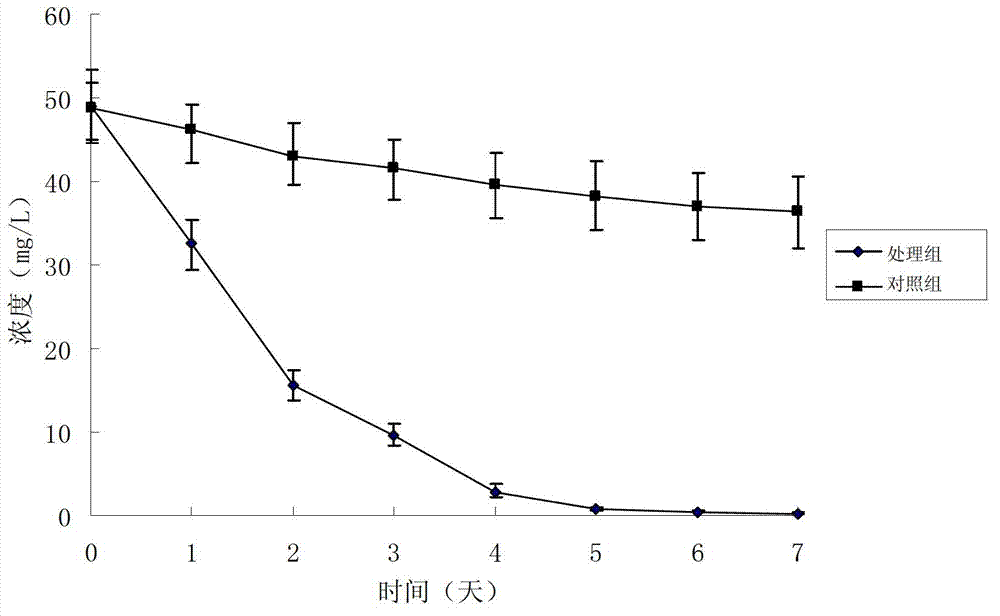
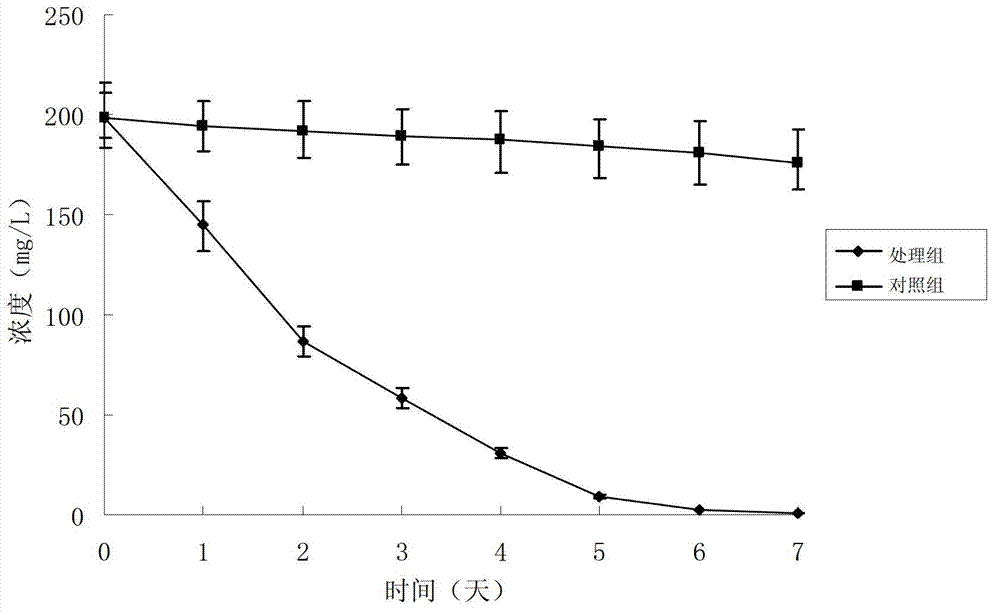
Method for preparing rifampicin I crystal form
The invention discloses a method for preparing a rifampicin I crystal form. The method comprises the following steps: under the action of stirring, adding rifampicin I crystal form crude products of which the purity is not less than 90% into a solution, wherein the concentration of the solutions is that the rifampicin : solvent equals to 0.16 to 0.30 g / mL; adding water of which the volume is 1 to 6% of the volume of the solvent, and rising temperature to 75 to 85 DEG C to dissolve the rifampicin crude products; performing vacuum evaporation crystallization, evaporating a rifampicin solution, stopping evaporation after crystals are separated out, growing the crystals for 20 to 40 minutes, then continuing evaporation, and when the volume of the distillate liquid is 20 to 60% of the volume of the solvent, stopping evaporation; performing cooling crystallization, reducing the temperature to 20 to 35 DEG C, and growing the crystals for 20 to 40 minutes; filtering magma to obtain products. The purity of the crystals is greater than 97%, the average granularity is larger than 170 microns, the bulk density is larger than 0.65 g / mL, and the yield is above 80%. The method has the characteristics that operation time is short, seed crystals are not required to be added, product appearances are regular and mobility is good, and the method is suitable for industrial application.
Owner:TIANJIN UNIV
Preparation of long-acting chitosan/apatite/rifampicin composite material by biomimetic mineralization method
The invention relates to a method for preparing a chitosan composite material, and particularly relates to the preparation of a long-acting chitosan / apatite / rifampicin composite material by biomimetic mineralization method. The invention aims to solve the problem that the physical adsorption manner of the existing chitosan / apatite composite material has short drug release time and the chemical adsorption manner can not ensure the efficacy when the existing composite material is used as the carrier of a local drug release system. The preparation method comprises the following steps: selecting a mold to prepare a capsule-shaped chitosan hydrogel film, injecting chitosan solution into the film, solidifying, washing and repeating the above steps multiple times to obtain chitosan hydrogel in a shape of concentric layer; loading rifampicin; soaking in a calcium salt solution, phosphate anion solution and physiological solution; freeze-drying the rifampicin-loaded chitosan / apatite composite hydrogel in the shape of concentric layer; soaking in a chitosan solution; taking out and fumigating with ammonia gas; and drying to obtain the composite material. The chitosan / apatite / rifampicin composite material has an acting duration equal to or larger than 400 hours, and can be used for the carrier material of sustained-release drug for repairing bone defect and lesions thereof.
Owner:HARBIN INST OF TECH
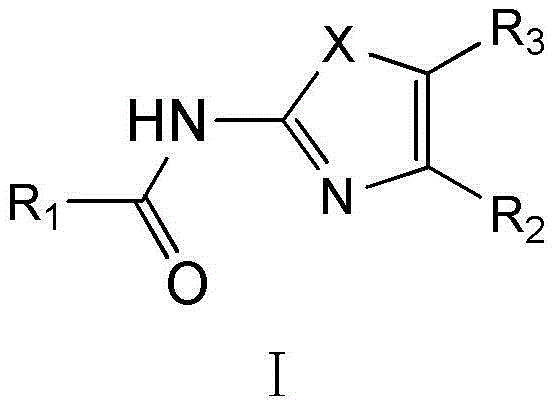
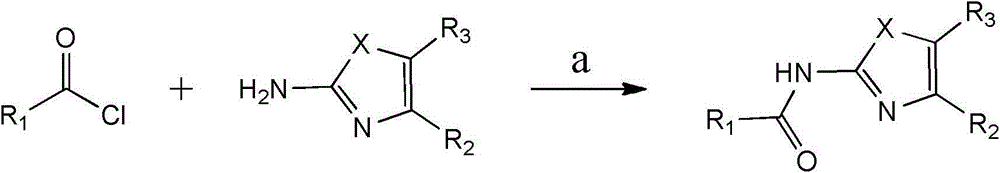
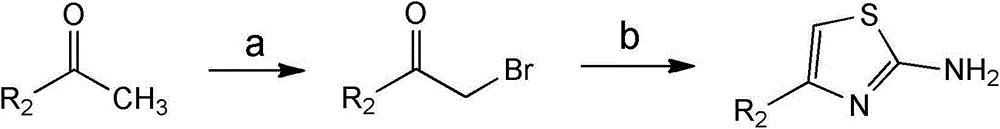
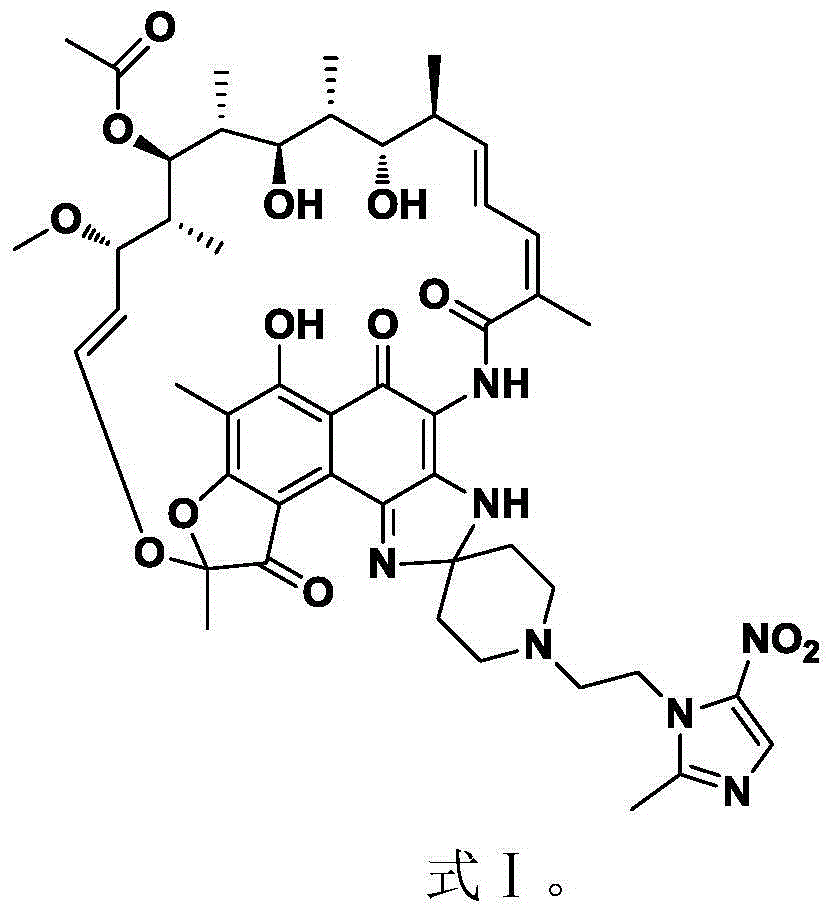
Substituted 1,3-miscellaneous azole compound, preparation method thereof, pharmaceutical composition containing substituted 1,3-miscellaneous azole compound and purpose thereof
ActiveCN105820163AGood choiceAntibacterial agentsOrganic active ingredientsIsoniazidAntituberculous drug
The invention relates to a substituted 1,3-miscellaneous azole compound, a preparation method thereof, a pharmaceutical composition containing the substituted 1,3-miscellaneous azole compound and a purpose thereof. The invention relates to the compound in a formula I, or its pharmaceutically acceptable salt, a stereisomer or a solvate, wherein R1, R2, R3 and X are defined as a specification; and the invention also relates to the preparation method, the pharmaceutical composition containing the substituted 1,3-miscellaneous azole compound and the purpose thereof. The compound of the present invention is the novel antituberculous compound, is effective to mycobacterium tuberculosis-susceptible strains, also possesses activity on strains with tolerance on traditional first-line antituberculous drugs such as isoniazide and rifampicin, and has good selectivity to mycobacterium tuberculosis.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
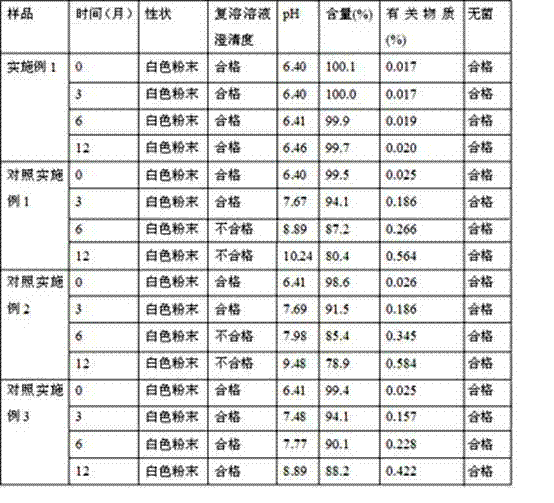
Rifampin lyophilized powder and preparation method thereof
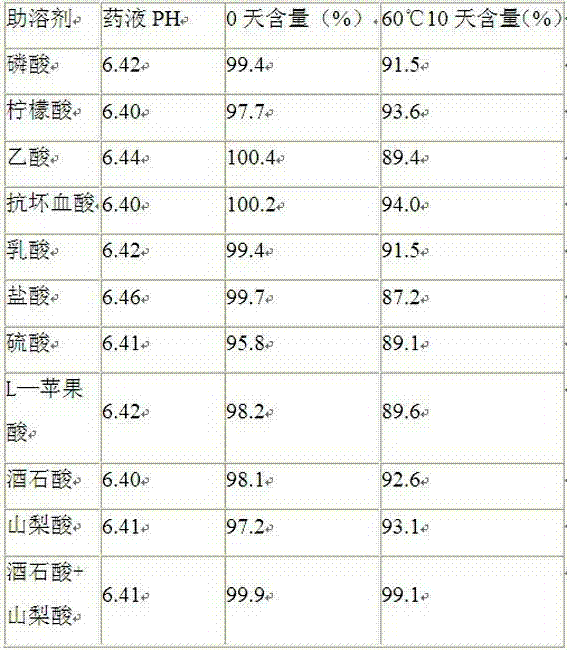
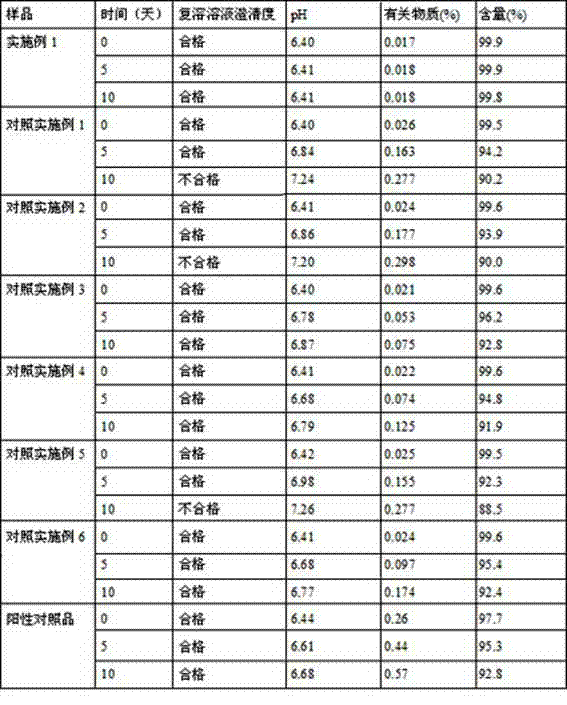
The invention relates to rifampin lyophilized powder. The rifampin lyophilized powder is prepared from the following raw materials: 20g of rifampin, 12g of tartaric acid, 10g of sorbic acid, 55g of polyethylene glycol 400 and 2000ml of water for injection; and the pH is adjusted to 6.3-6.5.
Owner:南通丝乡丝绸有限公司
Novel application of rifamycin-nitroimidazole coupling molecule
ActiveCN104971061AHigh antibacterial activityImprove antibacterial propertiesAntibacterial agentsOrganic active ingredientsNitroimidazoleAntibacterial activity
The present invention discloses a novel application of rifamycin-nitroimidazole coupling molecule and belongs to the field of medicinal chemistry. The rifamycin-nitroimidazole coupling molecule shows high activity to drug-resistant strains in the treatment of undistinguishable Clostridium, Helicobacter pylori infection related diseases, not only has antibacterial resistant activity to the rifamycin single drug-resistance bacteria and metronidazole single drug-resistance bacteria, but also has antibacterial activity against rifamycin resistant and metronidazole double drug-resistance bacteria; and the activity of the coupling molecule is superior to that of the composition of rifampicin and metronidazole in the molar ratio of 1:1.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
Primers, probes and method for detecting Mycobacterium tuberculosis drug-resistant gene mutation sites
ActiveCN105603084AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesPectobacteriumGene mutation
The invention relates to primers, probes and a method for liquid-phase chip detection of Mycobacterium tuberculosis drug-resistant gene mutation sites. The primers, probes and method are used for detecting drug-resistant gene mutation sites in Mycobacterium tuberculosis for drugs isoniazide, rifampicin and fonoquantel. The isoniazide drug-resistant mutation sites are positioned in katG gene and inhA gene; the rifampicin drug-resistant mutation sites are positioned in rpoB gene; and the fonoquantel drug-resistant mutation sites are positioned in gyrA gene. The method comprises the following steps: respectively carrying out homology analysis according to the nucleotide sequences of the four drug-resistance related genes in the gene bank, designing the primers and probes, carrying out PCR (polymerase chain reaction) twice, carrying out molecular hybridization, and carrying out detection by using a Luminex200 system, thereby determining whether the sample contains the drug-resistant mutation sites. The detection of drug-resistant gene mutation sites is of crucial importance for treating Mycobacterium tuberculosis infection by adopting correct therapeutic schedules. The primers, probes and method have the advantages of high detection speed, high sensitivity, high specificity and the like, are simple to operate, and are beneficial to popularization and application.
Owner:HAINAN MEDICAL COLLEGE +1
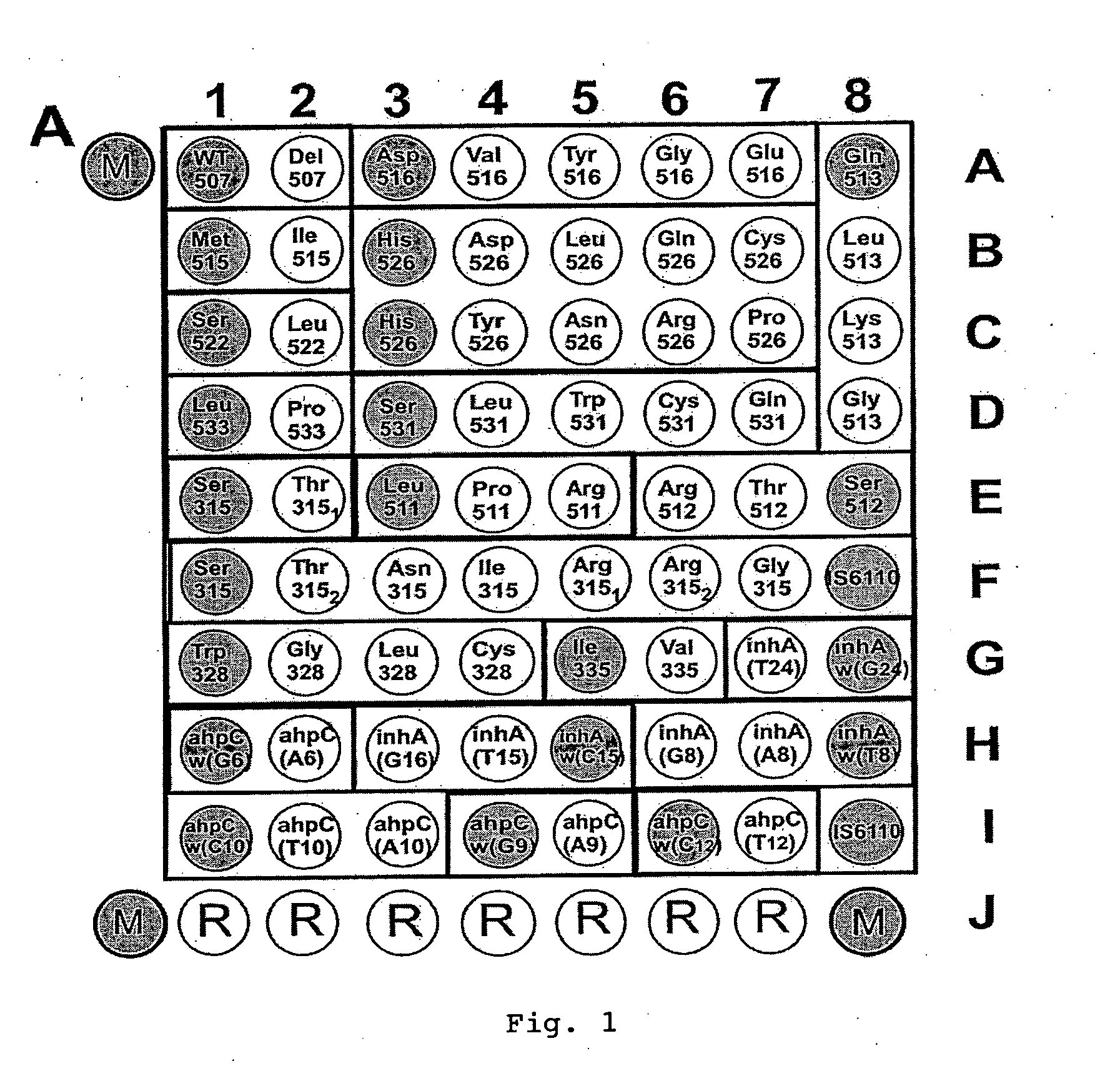
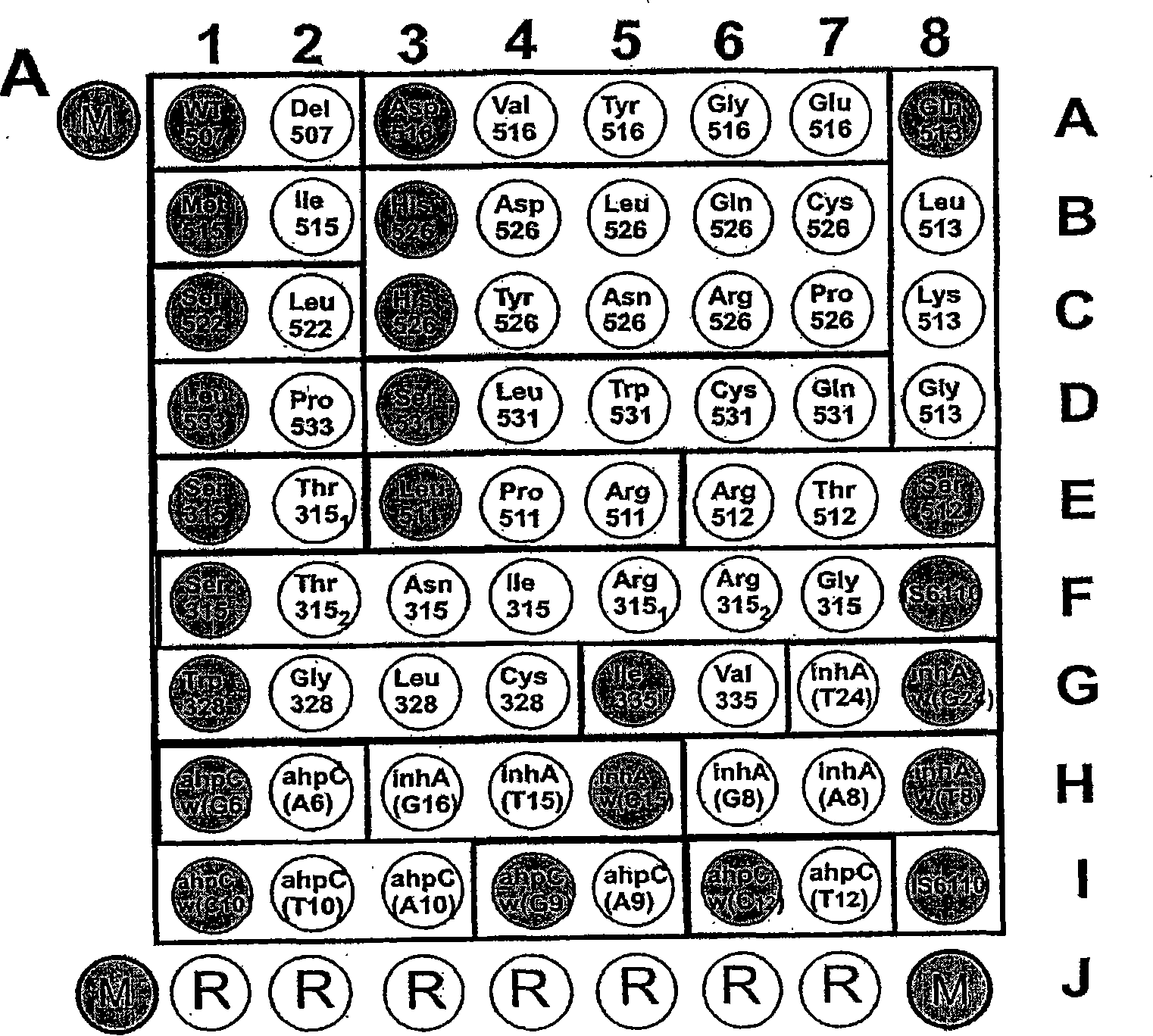
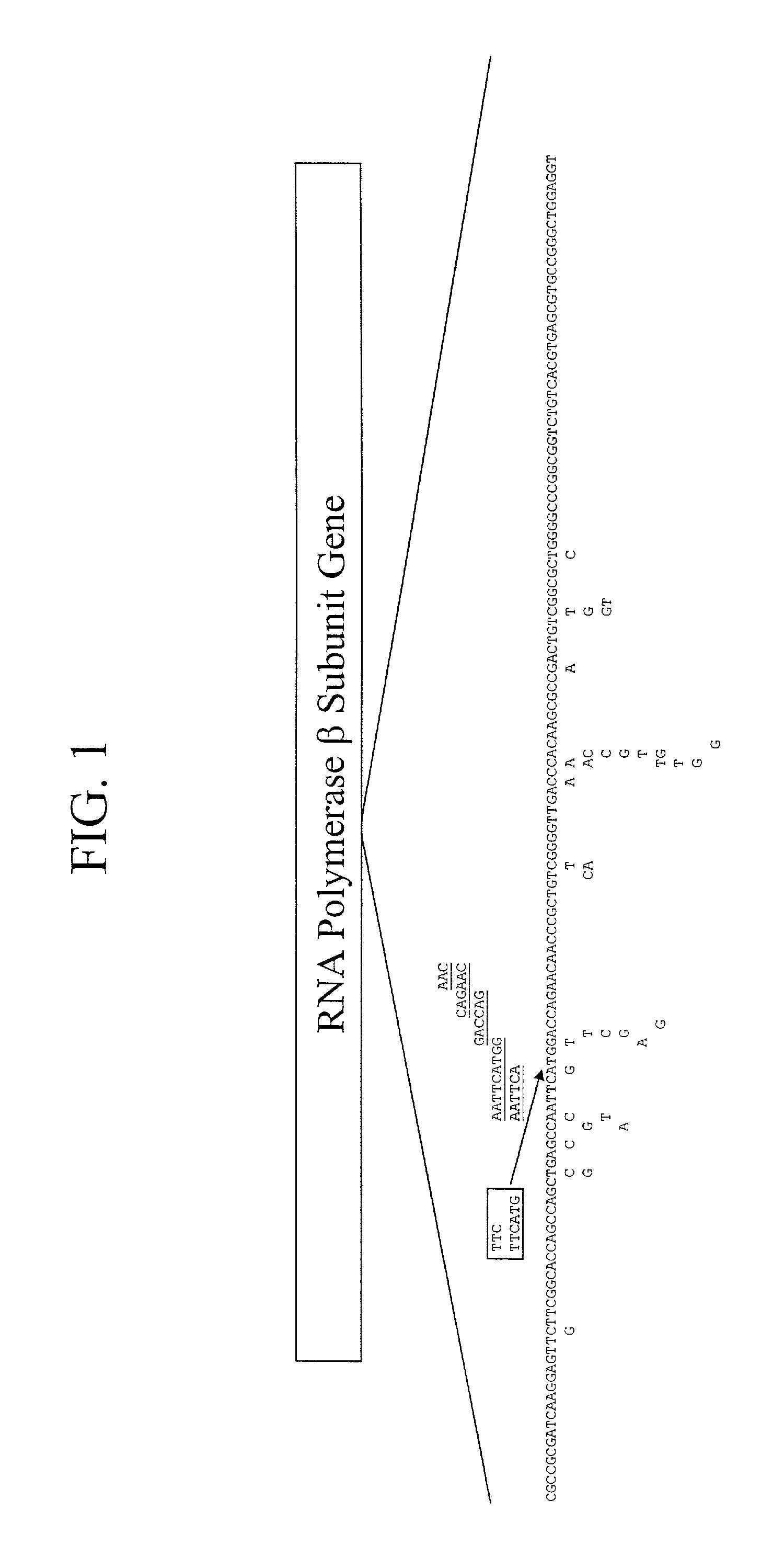
Mutation detection on RNA polmerase beta subunit gene having rifampin resistance
InactiveUS20030104387A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiagnostic testNucleotide
The present invention provides a diagnostic test method for detecting a tendency to rifampin resistance caused by mutations in a rpoB gene of M. tuberculosis, comprising the steps of (i) extracting genomic DNA from a biological sample containing M. tuberculosis cells; (ii) amplifying from the extracted genomic DNA the rpoB gene coding sequence or at least one distinct fragment thereof containing nucleotides encoding at least one test amino acid of the group consisting of amino acid numbers 511, 512, 513, 514, 515, 516, 517, 518, 522, 526, 529, 531, 533 to produce fluorescently labeled amplification product; (iii) contacting said fluorescently labeled amplification product with a first control array of oligonucleotide probes having DNA sequences specific to the wildtype M. tuberculosis rpoB gene coding sequence, including the nucleotides encoding the at least one test amino acid, and with a second test array of oligonucleotide probes having DNA sequences specific to the M. tuberculosis rpoB gene coding sequence, including nucleotides encoding mutations in the at least one test amino acid, wherein at least 3 mutations of the rpoB gene are probed for by the second test array of oligonucleotide probes; detecting any fluorescent hybridization signal of said purified fluorescently labeled amplification product which hybridized with the first and second arrays of oligonucleotide probes; (iv) correlating said detected hybridization with a tendency to rifampin resistance; and (v) correlating the detected hybridization to a tendency to rifampcin resistance and MDR.
Owner:GENETEL PHARMA
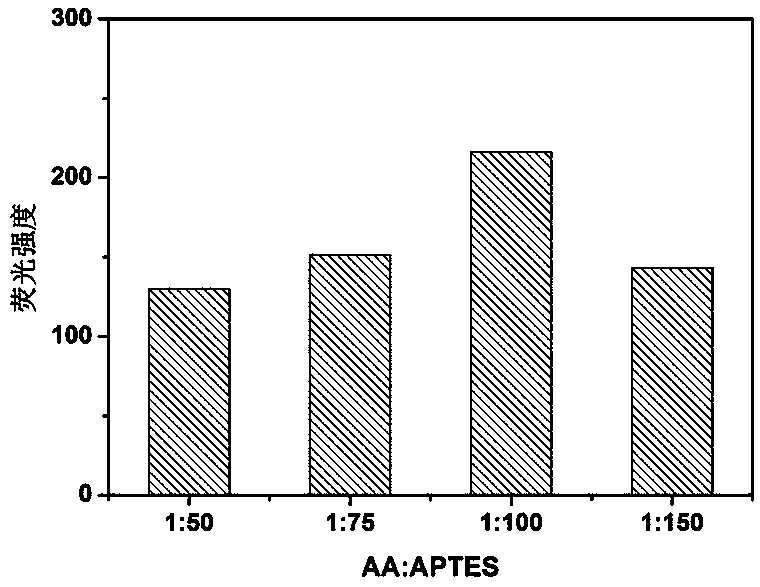
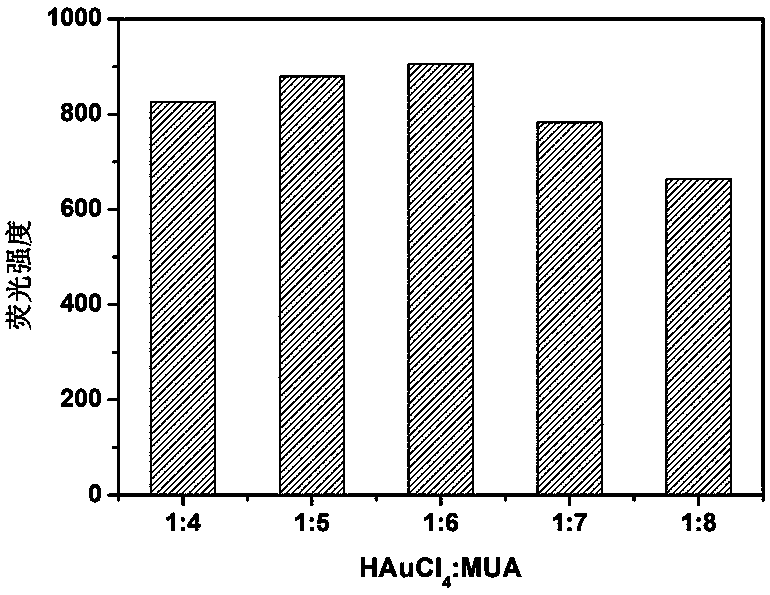
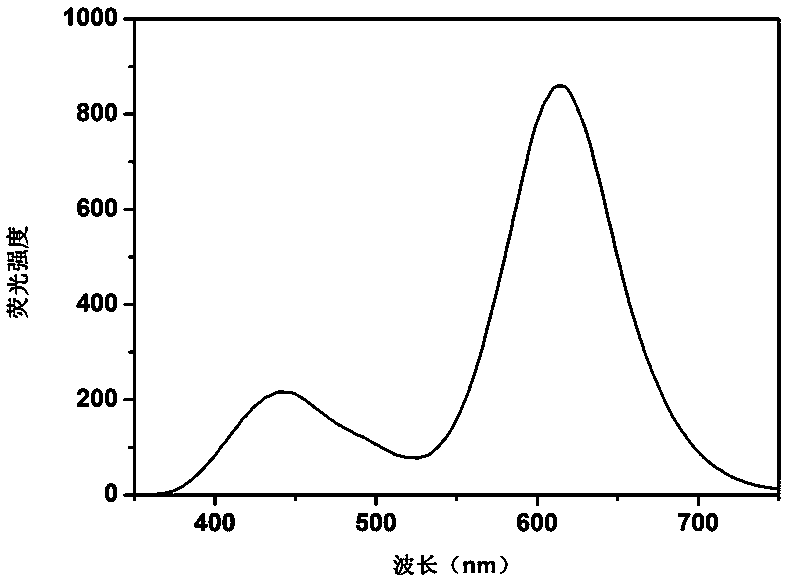
Synthesis of silicon nanoparticle/gold nano-cluster ratio fluorescence probe and application thereof to fluorescence detection of rifampicin ratio
ActiveCN109097029ARatiometric fluorescence detection implementationEasy to detectMaterial nanotechnologyFluorescence/phosphorescenceFluorescenceRelative standard deviation
The invention provides a synthesis method of a silicon nanoparticle / gold nano-cluster ratio fluorescence probe. The synthesis method comprises the following steps: firstly, preparing amino protected silicon nanoparticles by adopting a hydrothermal reduction method; then preparing a carboxyl protected gold nano-cluster by adopting a stirring reduction method; finally, carrying out self-assembly onthe silicon nanoparticles and the gold nano-cluster and synthesizing the ratio fluorescence probe. The synthesis method of the silicon nanoparticle / gold nano-cluster ratio fluorescence probe self-assembled at room temperature, provided by the invention, has the advantages of simple process, easiness in operation, mild conditions and short consumed time. The silicon nanoparticle / gold nano-cluster ratio fluorescence probe, provided by the invention, can accurately detect rifampicin by adopting a ratio fluorescence method; when the silicon nanoparticle / gold nano-cluster ratio fluorescence probe is applied to detect trace rifampicin in human serum; the result shows that the recovery rate of the silicon nanoparticle / gold nano-cluster ratio fluorescence probe is 97.0 to 103.5 percent and the relative standard deviation (RSDs) is lower than 4 percent.
Owner:JILIN INST OF CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com