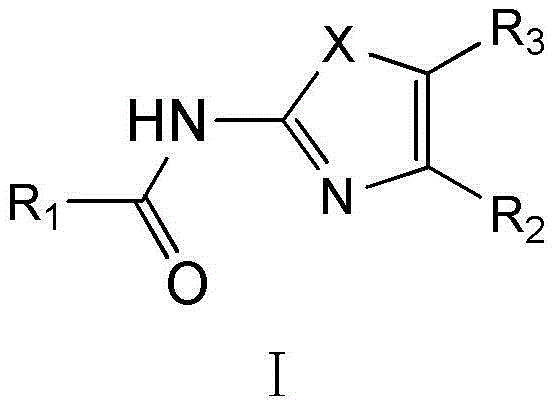
Substituted 1,3-miscellaneous azole compound, preparation method thereof, pharmaceutical composition containing substituted 1,3-miscellaneous azole compound and purpose thereof
A compound and drug technology, applied in the direction of medical preparations containing active ingredients, organic chemistry, antibacterial drugs, etc., can solve the problems of high treatment costs, patients can not follow the doctor's treatment guidelines well, and achieve good selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
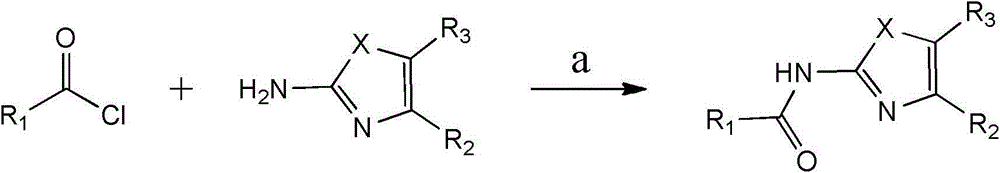
[0054] The present invention also relates to the preparation method of the compound of the present invention, it comprises the following steps:
[0055] a) make formula compound and formula The compound undergoes acylation reaction in dry tetrahydrofuran solution to generate the formula compound of;
[0056]
[0057] or
[0058] b) make formula compound and formula The compound is condensed in DMF in the presence of HATU and DIPEA to obtain the formula compound of,
[0059] where R 1 , R 2 , R 3 and X are as defined above.
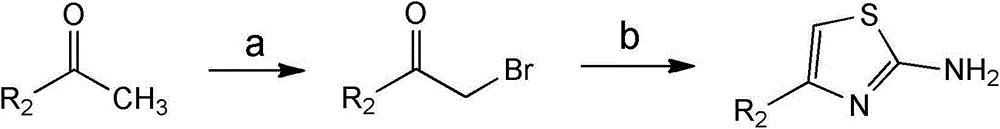
[0060] In a preferred embodiment, the formula The compound is synthesized by the following synthetic route:
[0061] When R 3 When is hydrogen and X is S, the formula The compound is synthesized by the following synthetic route:
[0062]
[0063] a) make formula The compound reacts with liquid bromine in dichloromethane compounds; and
[0064] b) make formula The compound of the formula is prepared by reacting with thiou...
Embodiment 1
[0118] Embodiment 1: Synthesis of 3-(methoxycarbonylhydrazone)-ethyl butyrate
[0119]
[0120] Take 7.28g (0.055mol) of ethyl acetoacetate in a 100mL three-neck flask, add 35mL of ethanol to dissolve, and add dropwise 15mL of an ethanol solution of 5.02g (0.055mol) of methyl carbazate at room temperature. After the addition was completed, the mixture was stirred at room temperature for 6 hours, and the solvent was evaporated under reduced pressure to obtain the title compound as a light yellow thick oily liquid, which turned into yellow crystals upon standing and cooling, 10.51 g, yield 94.5%.
Embodiment 2
[0121] Example 2: Synthesis of ethyl 4-methyl-1,2,3-thiadiazole-5-carboxylate
[0122]
[0123] Add 13 mL (0.18 mol) of thionyl chloride to a 100 mL three-neck flask equipped with a drying tube and an exhaust gas absorption device, and slowly add 11.80 g of 3-(methoxycarbonylhydrazone)-butyric acid ethyl ester dropwise under ice-salt bath conditions (0.06mol) of dichloromethane solution 50mL. After 24 hours of reaction, excess thionyl chloride and dichloromethane were distilled off under reduced pressure to obtain a brown solid. Column separation, eluting with petroleum ether-ethyl acetate: 10:1 (V / V), gave the title compound as a yellow oil, 6.72 g, yield 65.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com