Patents
Literature
157 results about "Amikacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to prevent or treat a wide variety of bacterial infections.
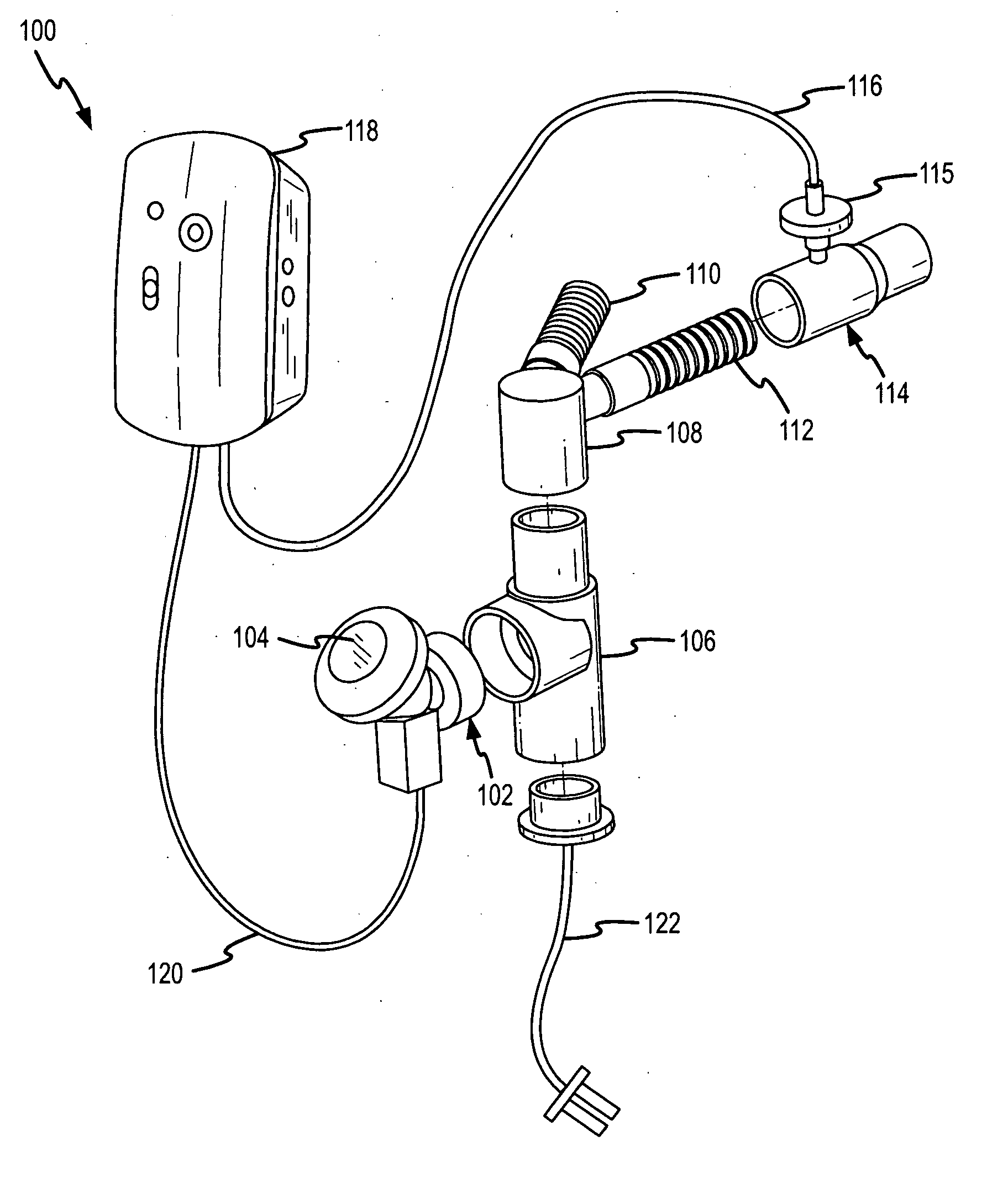
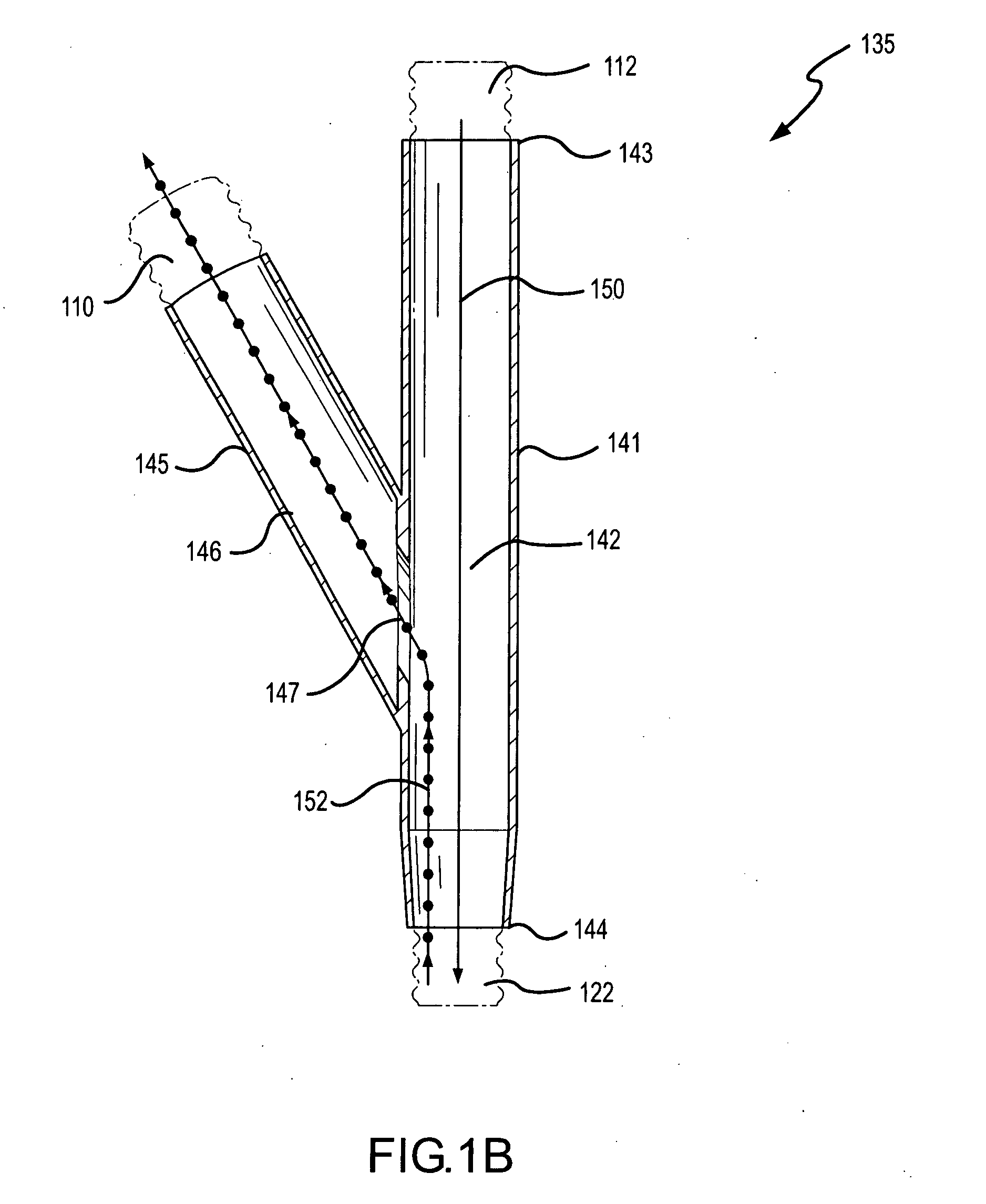
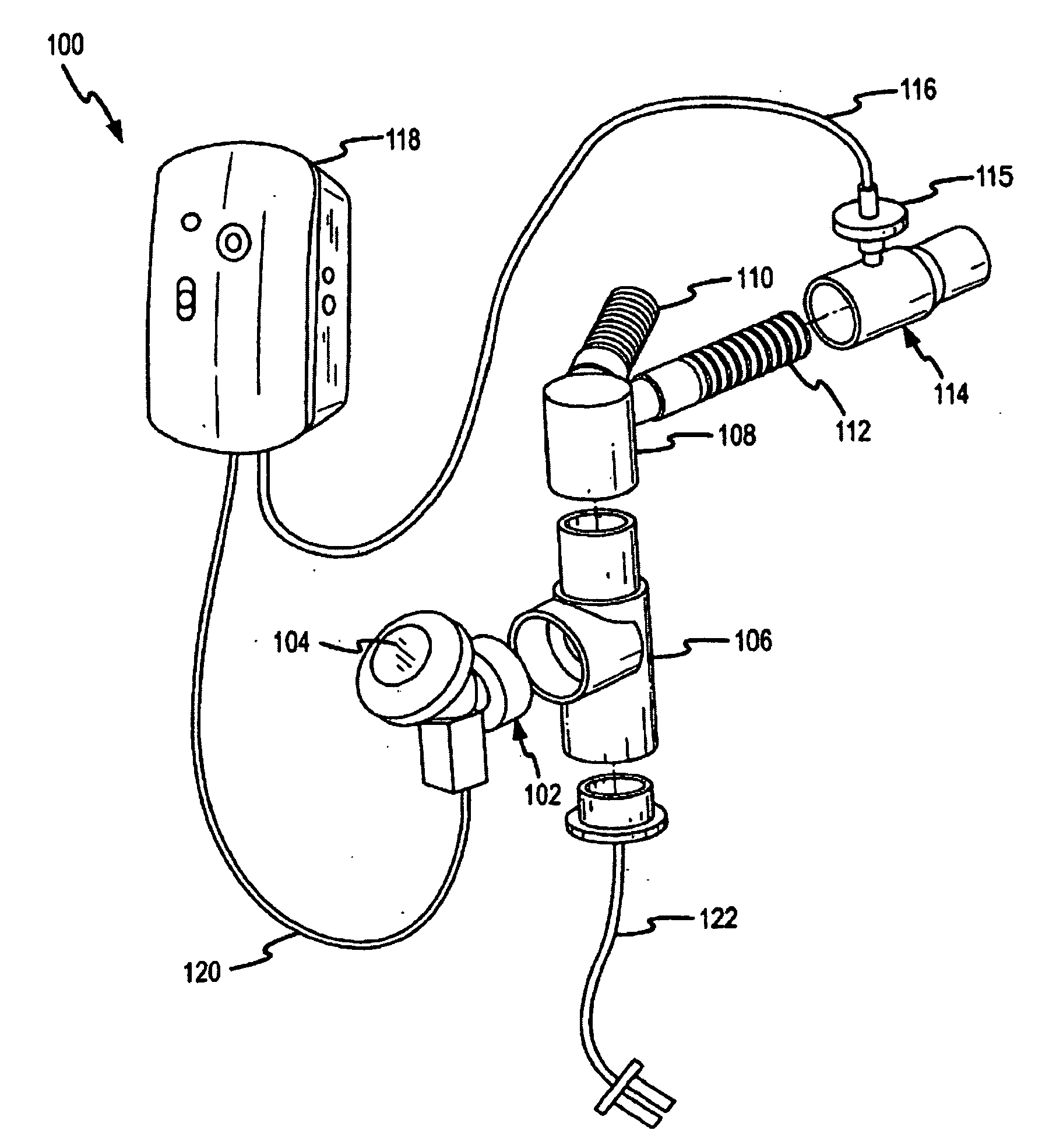
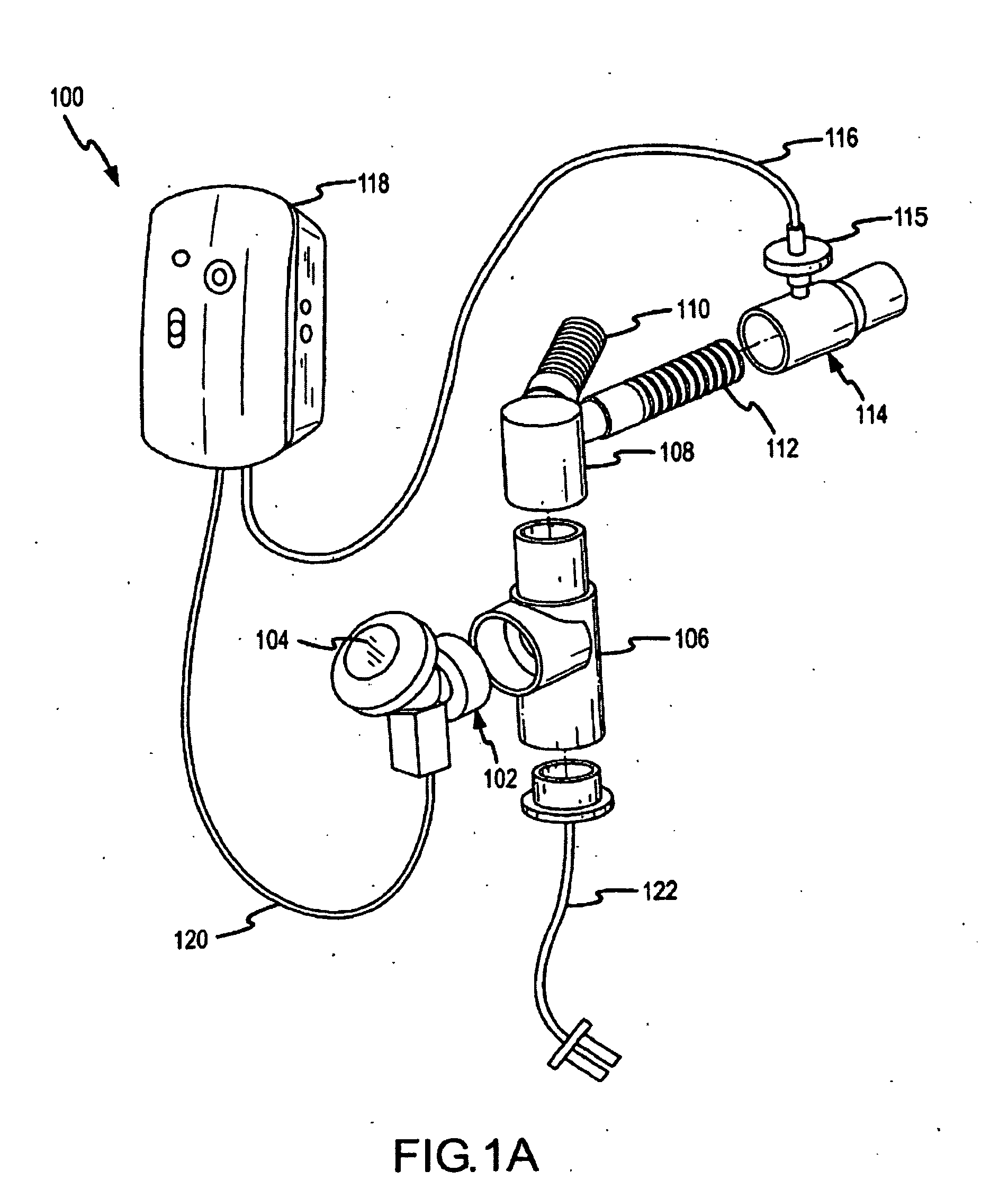
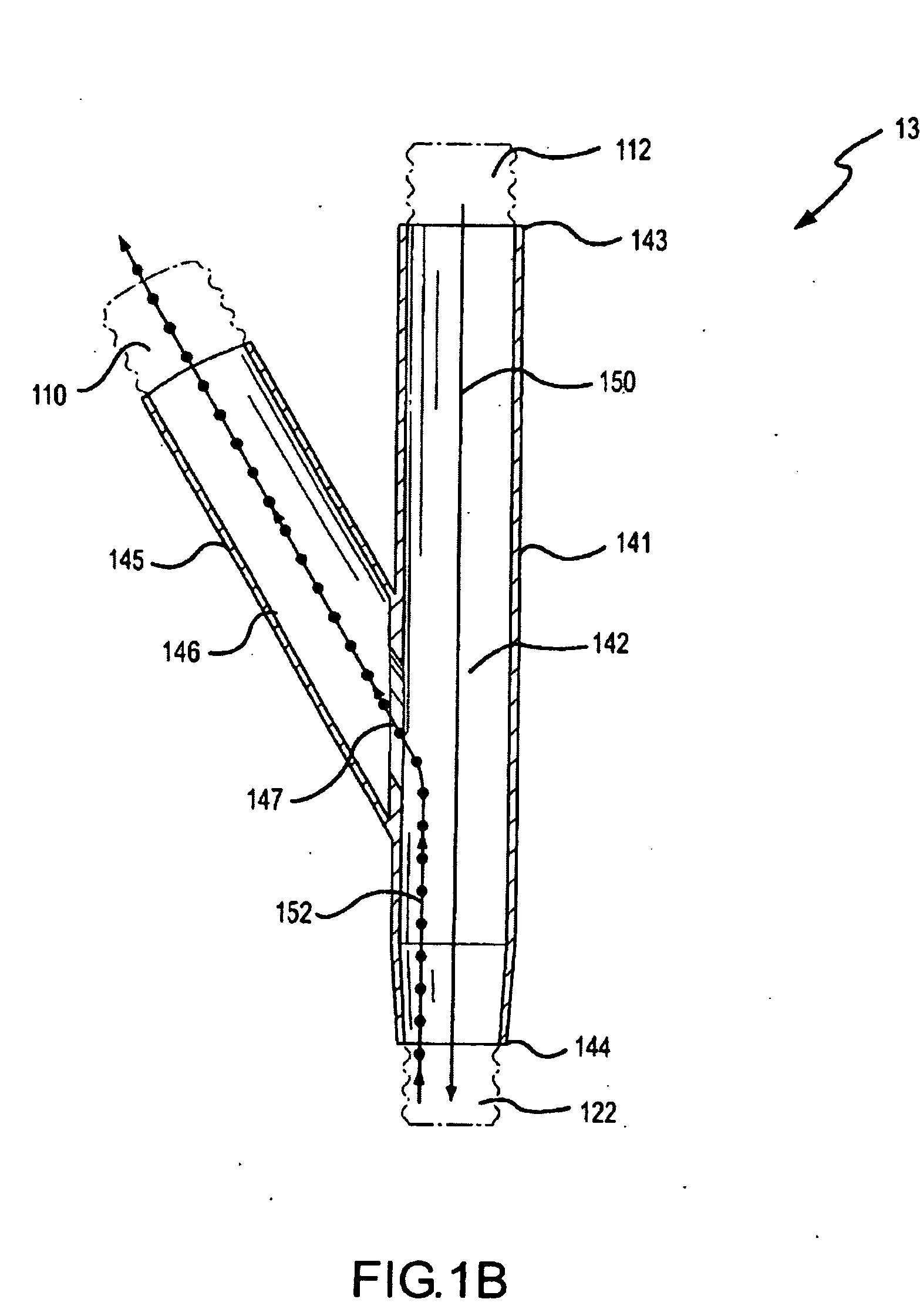
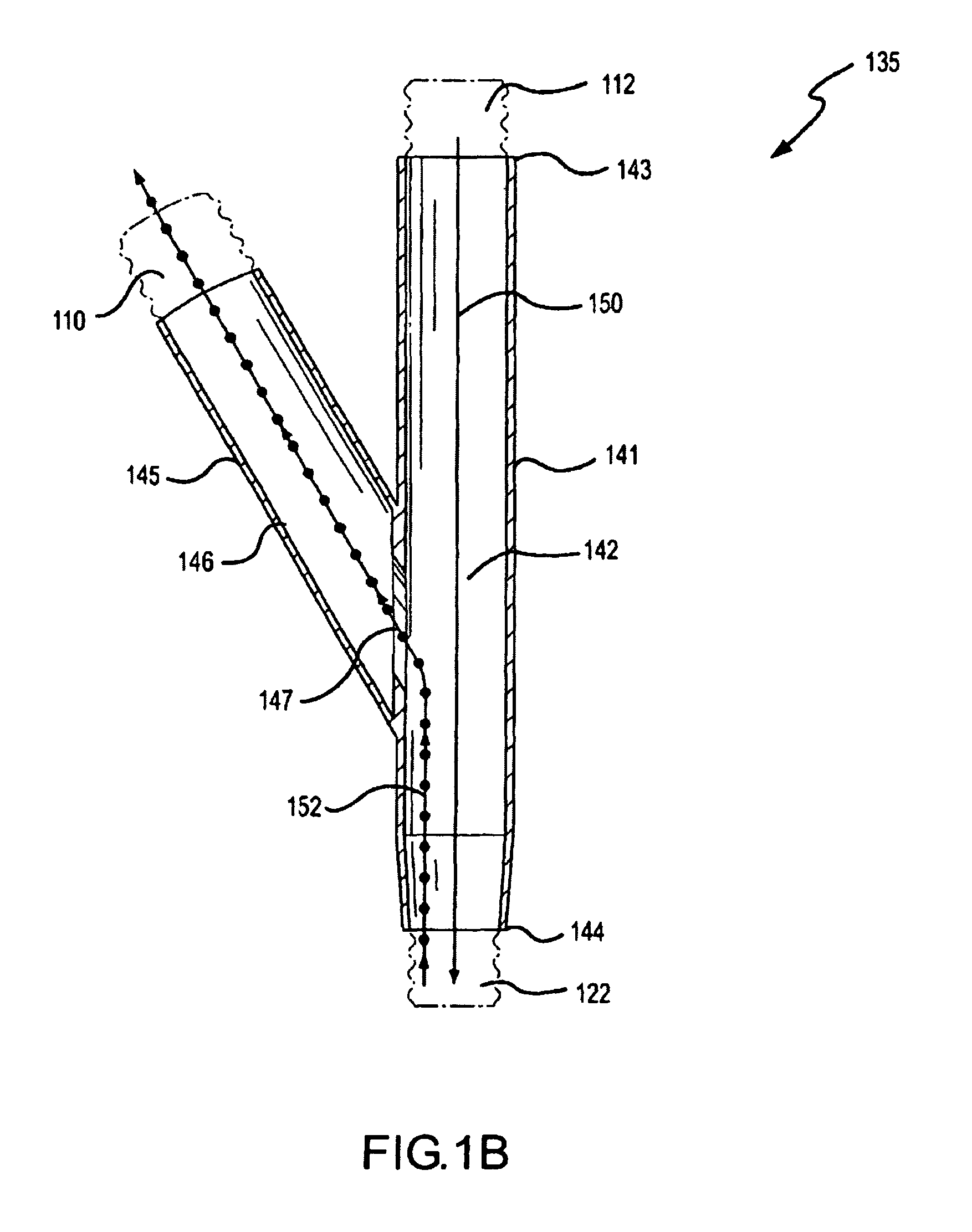
Methods and systems for operating an aerosol generator
InactiveUS20050217666A1Improve security levelImprove delivery efficiencyAntibacterial agentsOrganic active ingredientsDiseaseAmikacin
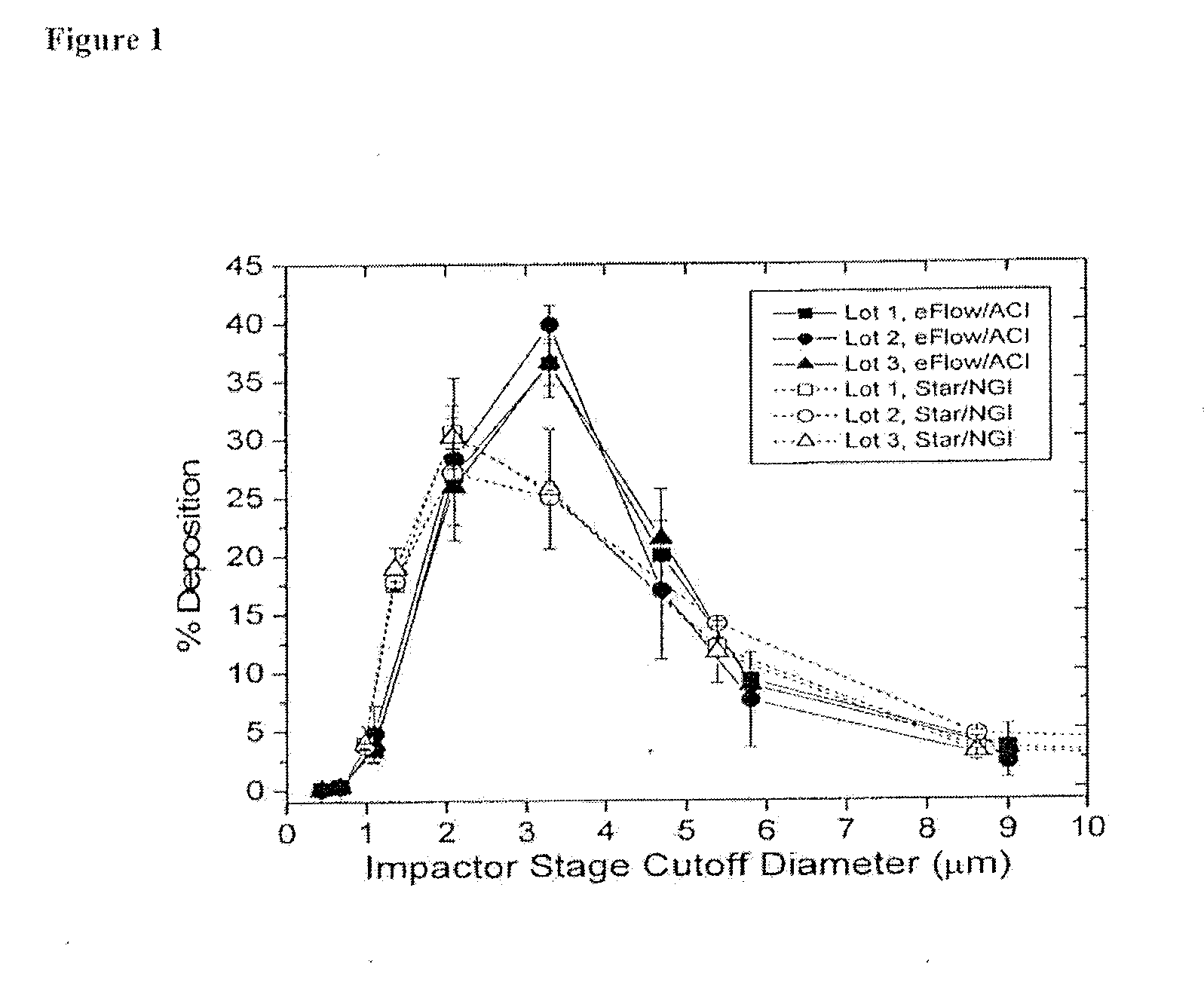
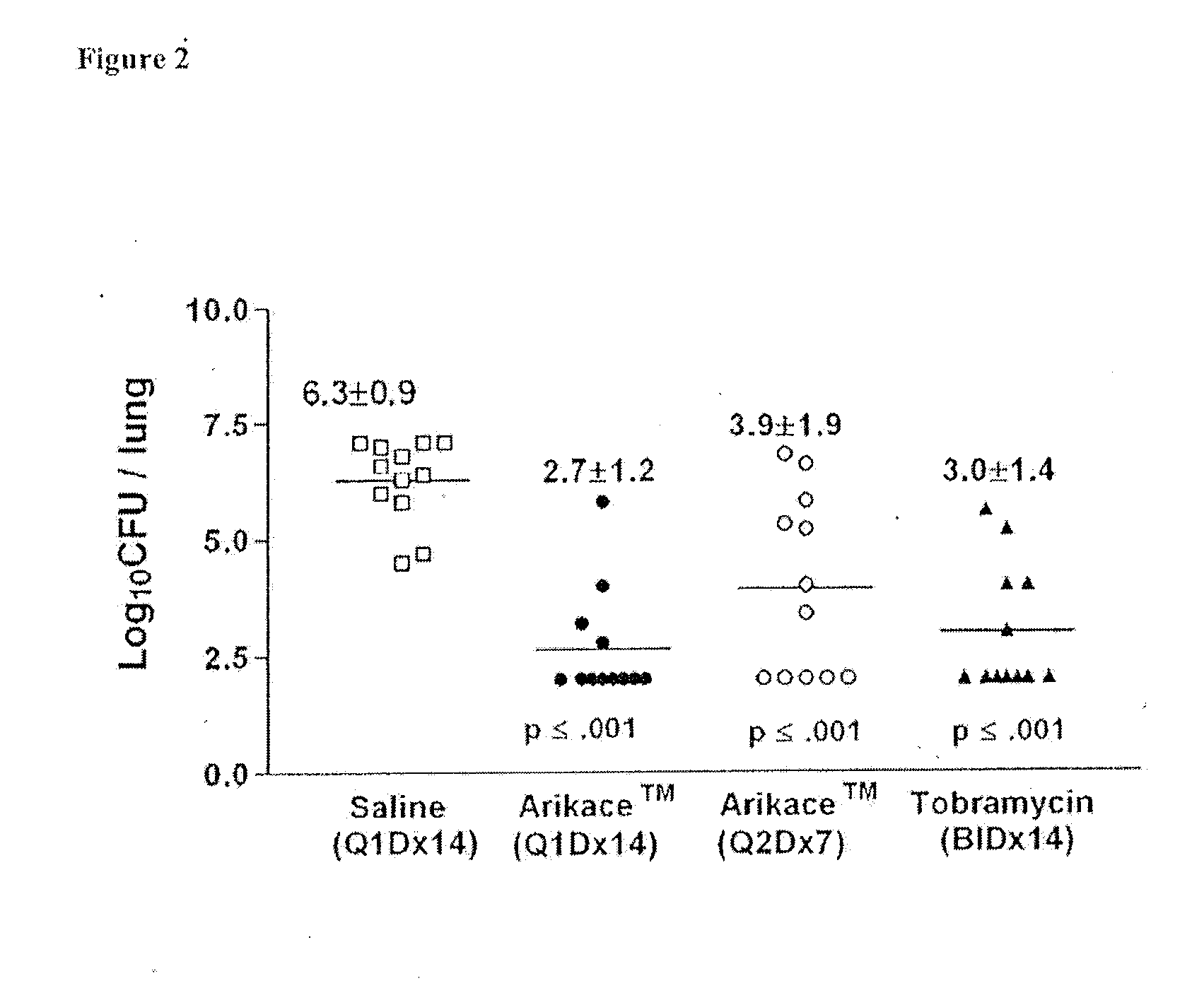
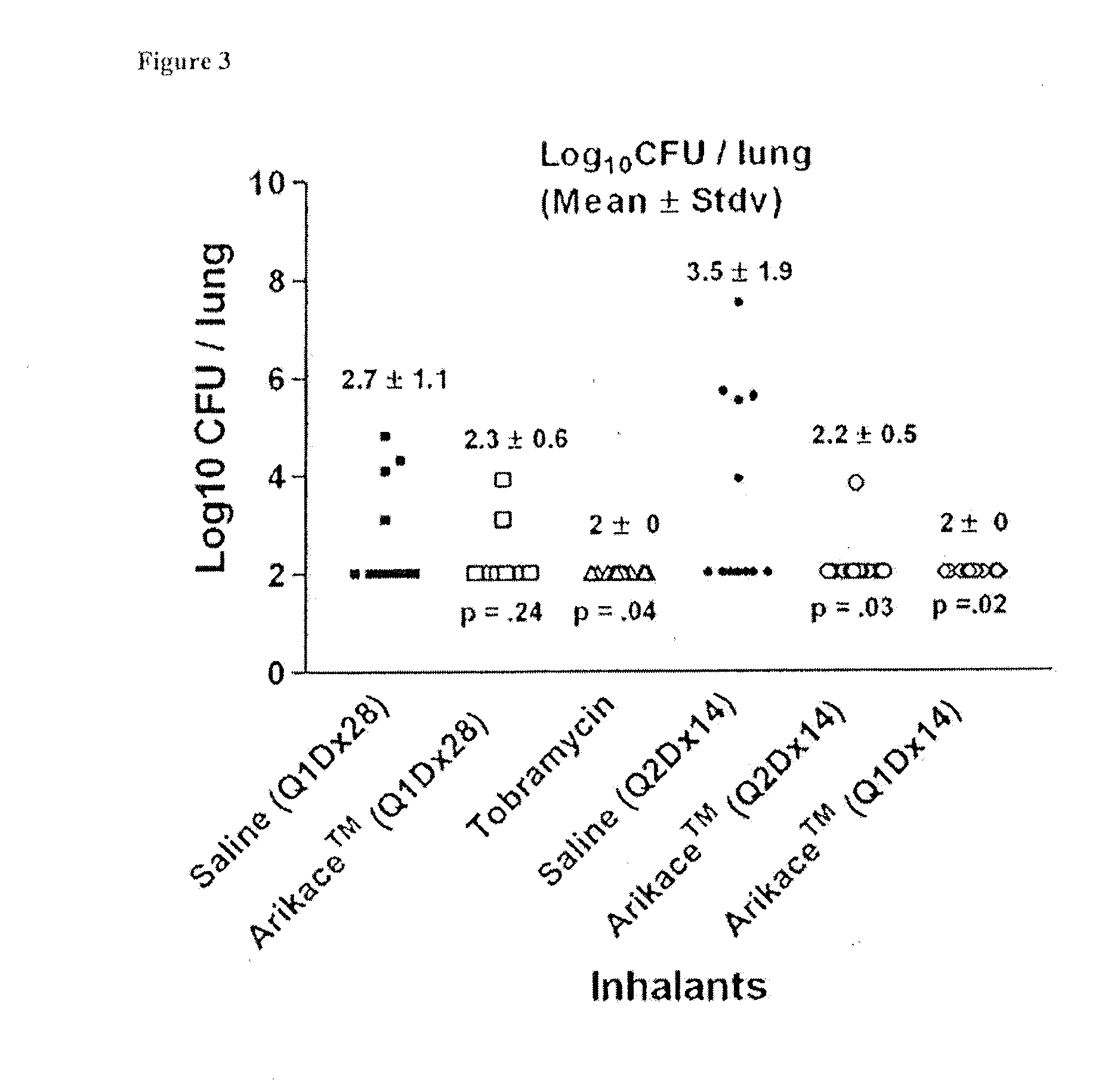
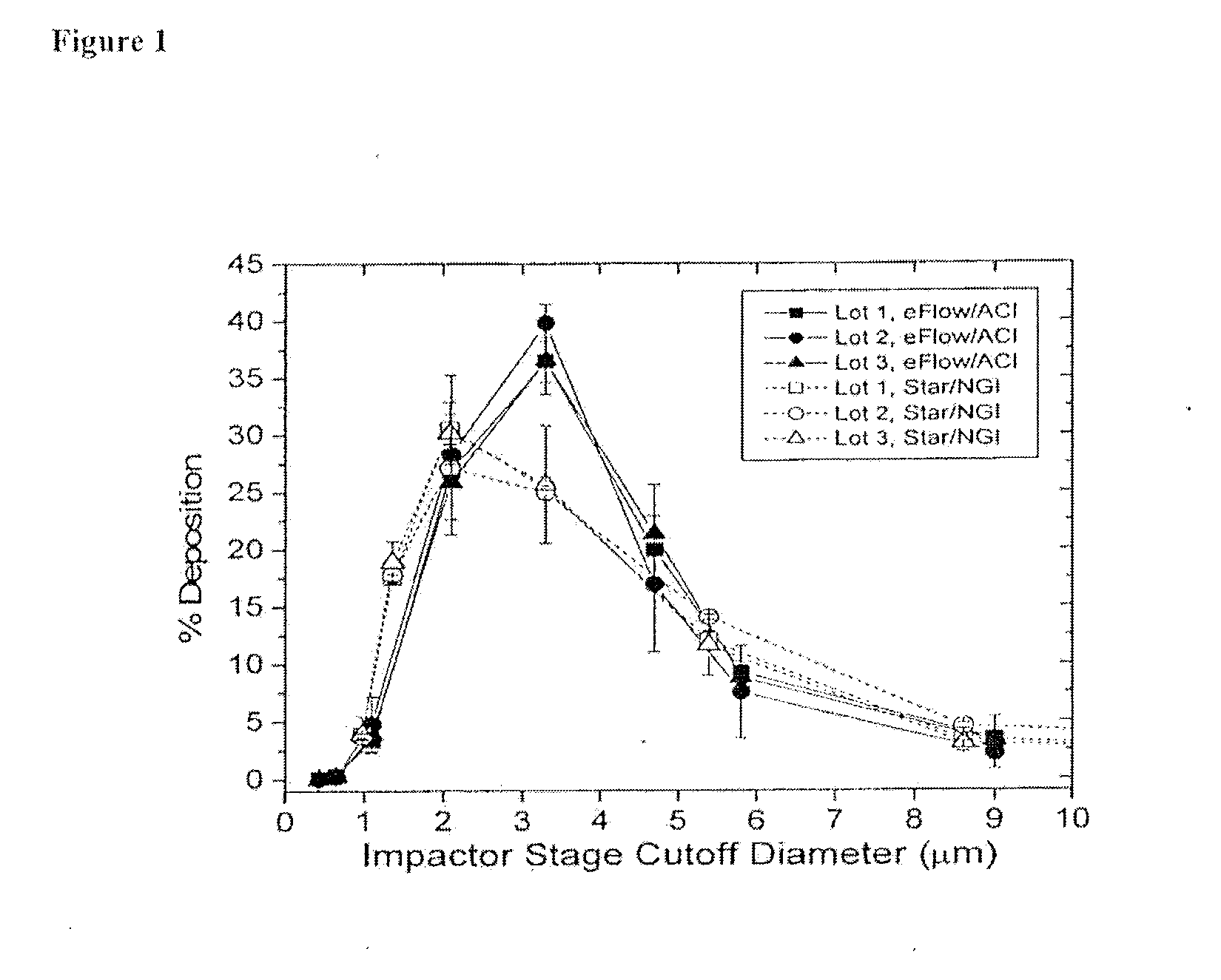
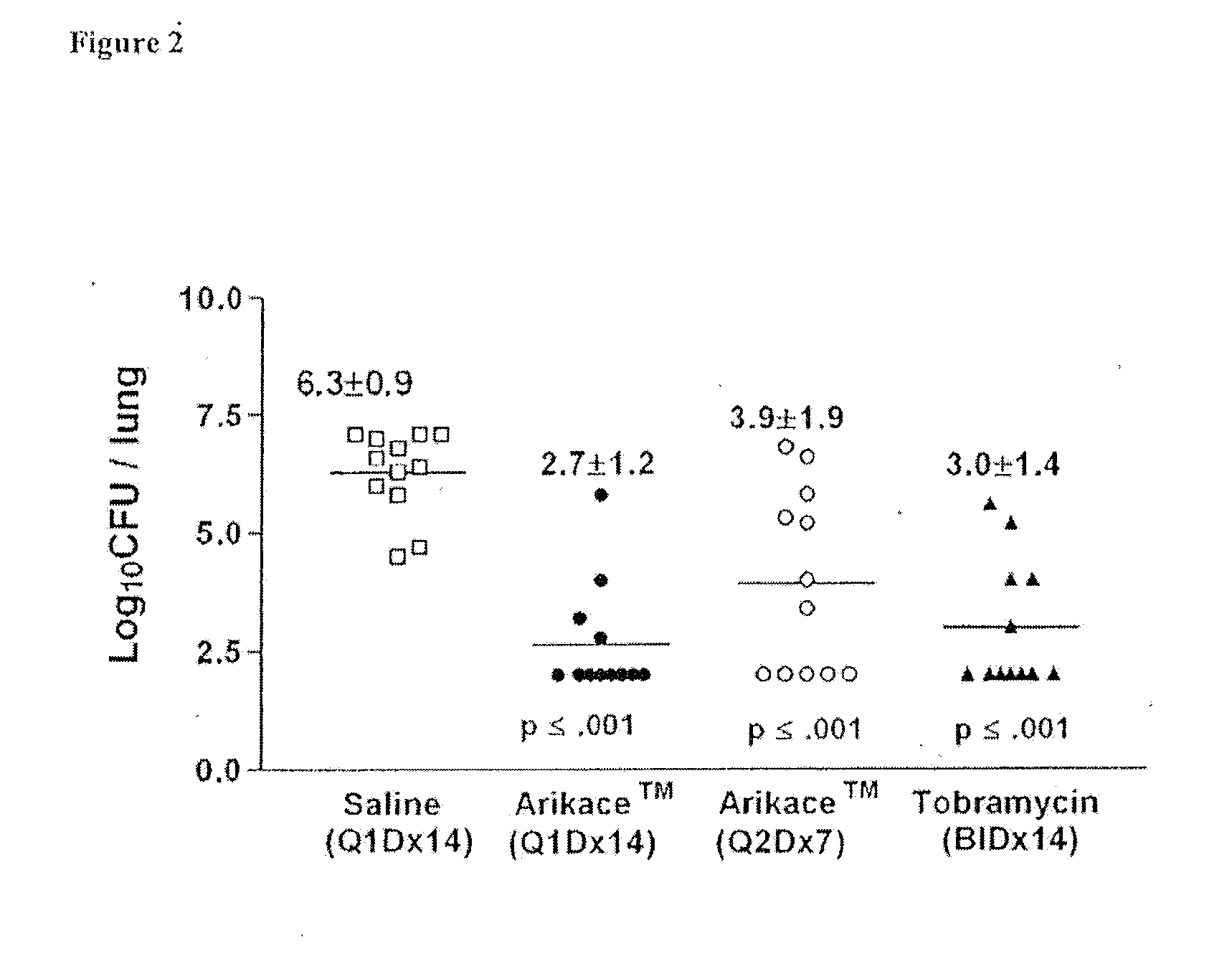
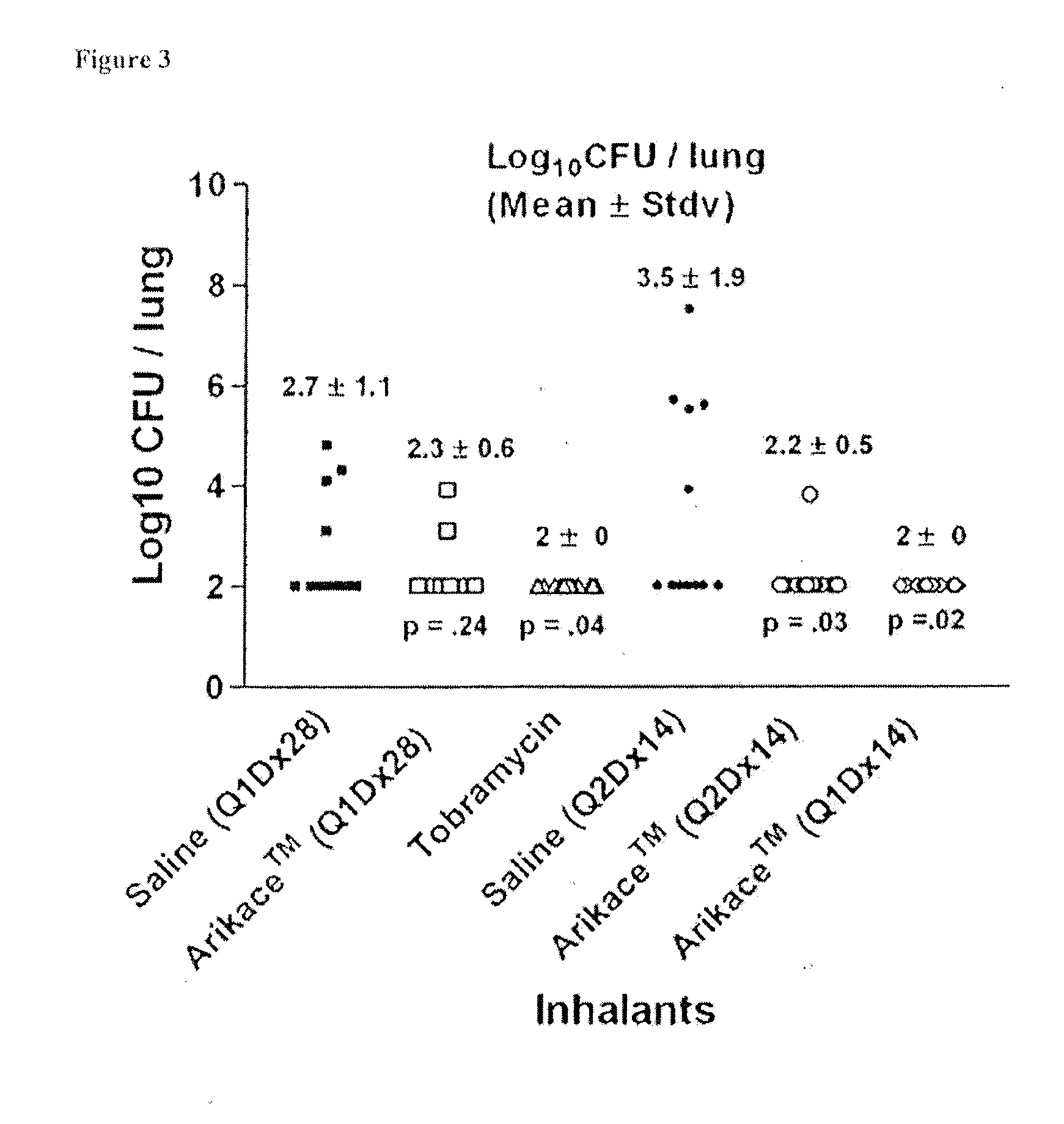
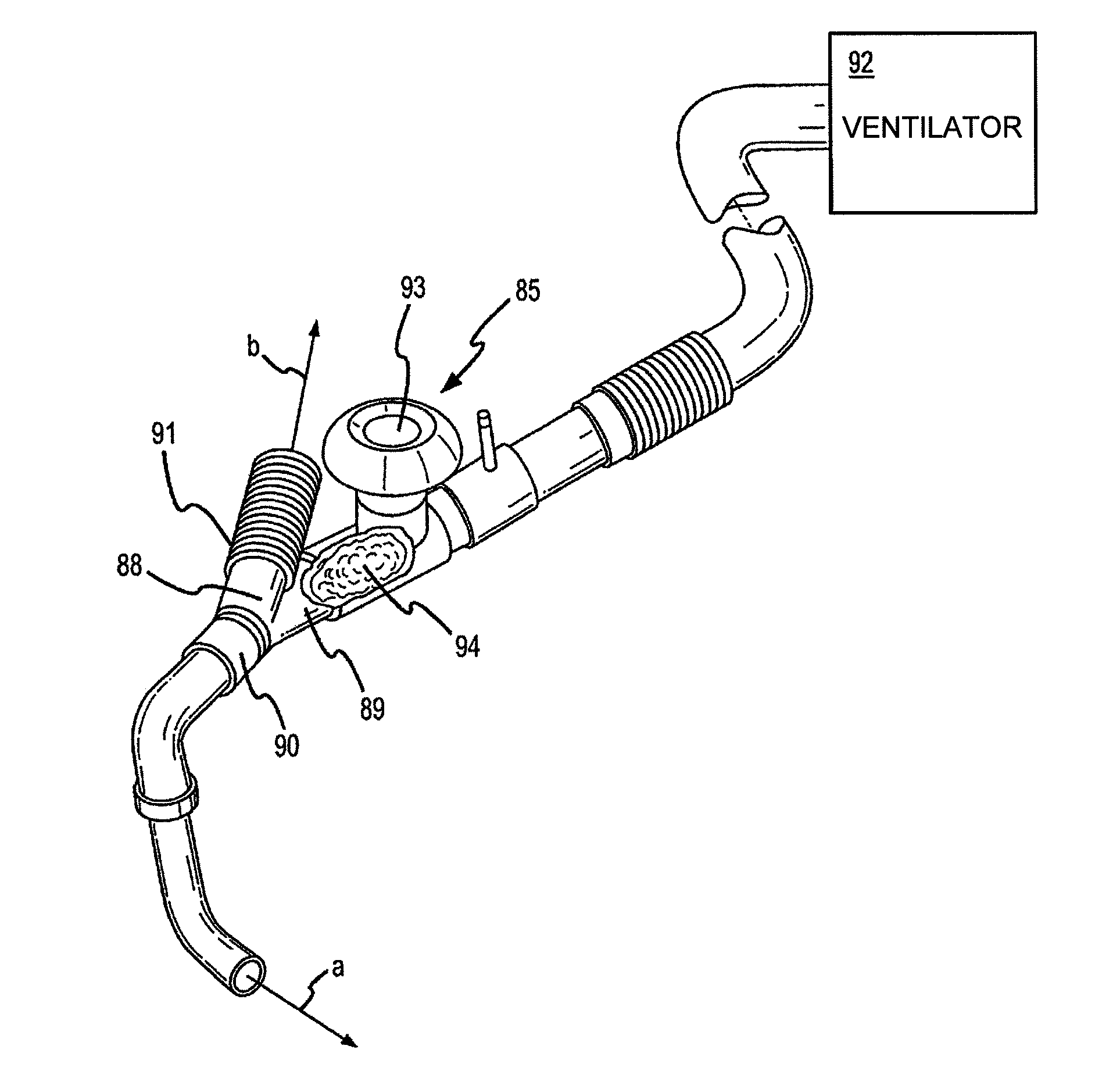
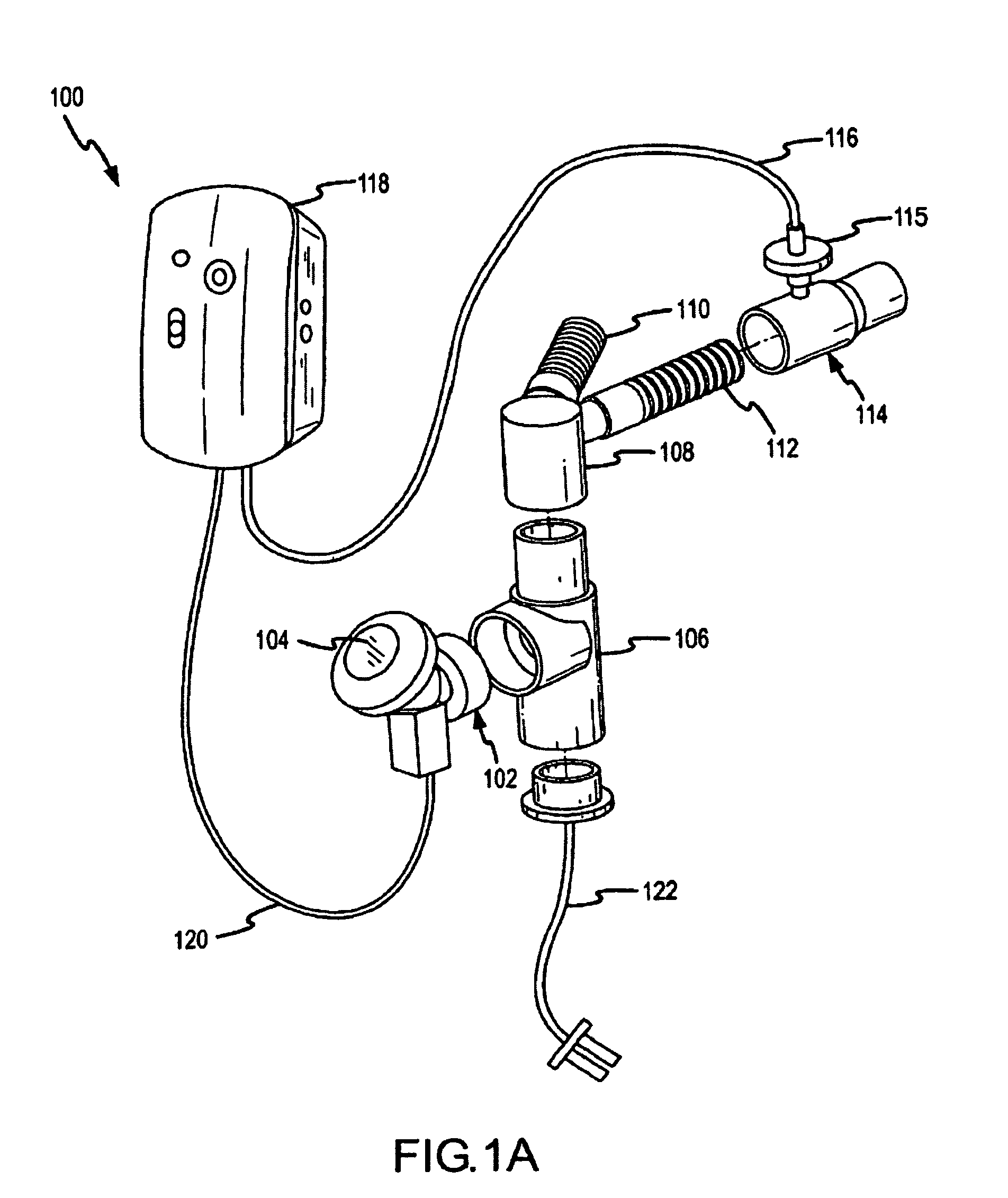
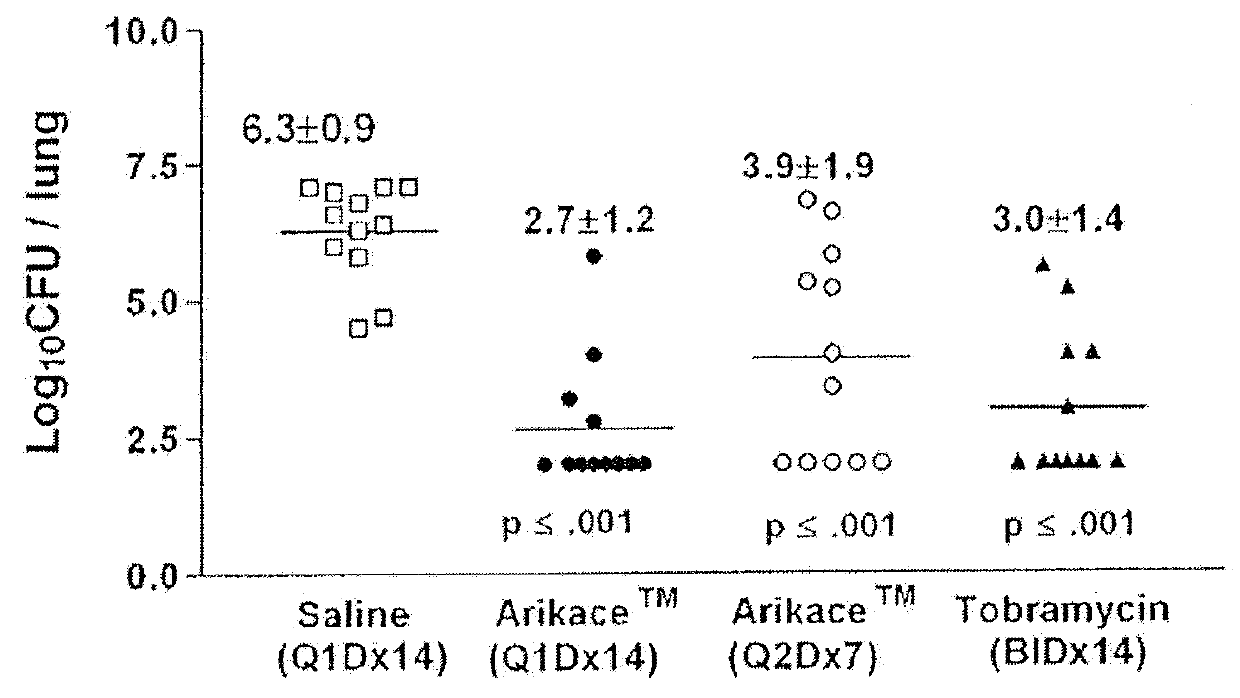
A method of treating a patient with a pulmonary disease, where the method includes delivering a dose of aerosolized medicament intermittently to a ventilator circuit coupled to the respiratory system of the patient. Also, a method of treating a patient with a pulmonary disease, where the method includes taking the patient off a ventilator, and administering to the patient, a nebulized aerosol comprising from about 100 μg to about 500 mg of a medicament. Additionally, an aerosolized medicament for the treatment of a pulmonary disease, where the medicament includes amikacin mixed with an aqueous solution having an adjusted pH from about 5.5 to about 6.3. The pH is adjusted by adding hydrochloric acid and sodium hydroxide to the aqueous solution.
Owner:NOVARTIS AG
Methods and systems for operating an aerosol generator
ActiveUS20070267010A1Improve security levelImprove delivery efficiencyRespiratorsBiocideDiseaseAmikacin
A method of treating a patient with a pulmonary disease, where the method includes delivering a dose of aerosolized medicament intermittently to a ventilator circuit coupled to the respiratory system of the patient. Also, a method of treating a patient with a pulmonary disease, where the method includes taking the patient off a ventilator, and administering to the patient, a nebulized aerosol comprising from about 100 μg to about 500 mg of a medicament. Also a method of treating a patient with a pulmonary disease, the method comprising administering an aerosolized first medicament comprising amikacin to the patient and administering, systemically a second medicament comprising an antibiotic to the patient that also treats the pulmonary disease, wherein a resulting amikacin concentration in the lung and / or pulmonary system is therapeutically-effective, and an amount of the systemically administered antibiotics is reduced.
Owner:NEKTAR THERAPEUTICS INC
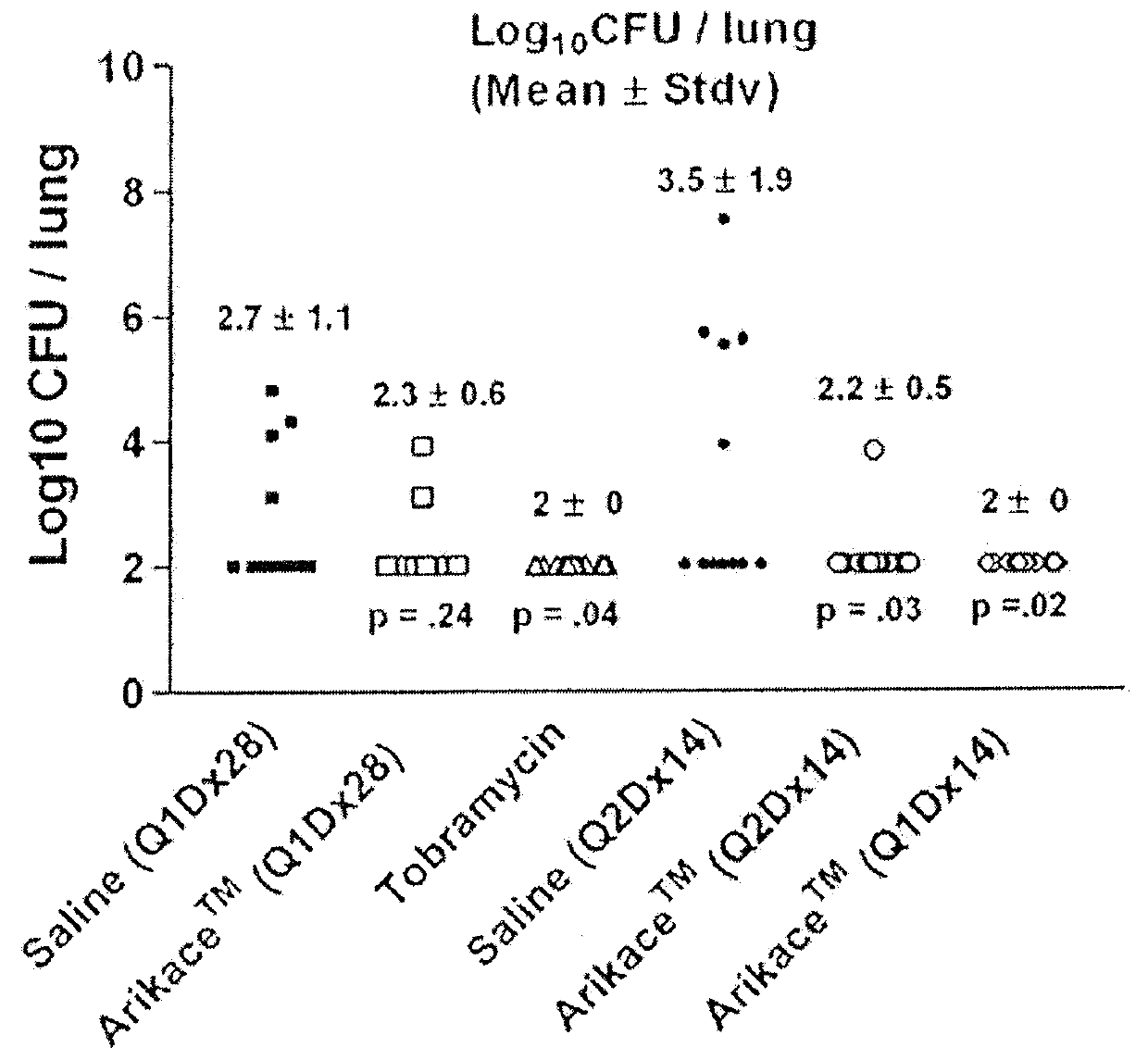
Methods of Treating Pulmonary Disorders with Liposomal Amikacin Formulations
Disclosed herein are methods of treating pulmonary disorders comprising administering to the patient an effective dose of a nebulized liposomal amikacin formulation for at least one treatment cycle, wherein: the treatment cycle comprises an administration period of 15 to 75 days, followed by an off period of 15 to 75 days; and the effective dose comprises 100 to 2500 mg of amikacin daily during the administration period.
Owner:INSMED INC
Method for treating pulmonary disorders with liposomal amikacin formulations
Disclosed herein are methods of treating pulmonary disorders comprising administering to the patient an effective dose of a nebulized liposomal amikacin formulation for at least one treatment cycle, wherein: the treatment cycle comprises an administration period of 15 to 75 days, followed by an off period of 15 to 75 days; and the effective dose comprises 100 to 2500 mg of amikacin daily during the administration period.
Owner:INSMED INC
Method of treating pulmonary disorders with liposomal amikacin formulations
Disclosed herein are methods of treating pulmonary disorders comprising administering to the patient an effective dose of a nebulized liposomal amikacin formulation for at least one treatment cycle, wherein: the treatment cycle comprises an administration period of 15 to 75 days, followed by an off period of 15 to 75 days; and the effective dose comprises 100 to 2500 mg of amikacin daily during the administration period.
Owner:INSMED INC
Methods and systems for operating an aerosol generator
ActiveUS8336545B2Improve security levelImprove delivery efficiencyRespiratorsBiocideAmikacinWhole body
A method of treating a patient with a pulmonary disease, where the method includes delivering a dose of aerosolized medicament intermittently to a ventilator circuit coupled to the respiratory system of the patient. Also, a method of treating a patient with a pulmonary disease, where the method includes taking the patient off a ventilator, and administering to the patient, a nebulized aerosol comprising from about 100 μg to about 500 mg of a medicament. Also a method of treating a patient with a pulmonary disease, the method comprising administering an aerosolized first medicament comprising amikacin to the patient and administering, systemically a second medicament comprising an antibiotic to the patient that also treats the pulmonary disease, wherein a resulting amikacin concentration in the lung and / or pulmonary system is therapeutically-effective, and an amount of the systemically administered antibiotics is reduced.
Owner:NEKTAR THERAPEUTICS INC
Method of treating pulmonary disorders with liposomal amikacin formulations
Disclosed herein are methods of treating pulmonary disorders comprising administering to the patient an effective dose of a nebulized liposomal amikacin formulation for at least one treatment cycle, wherein: the treatment cycle comprises an administration period of 15 to 75 days, followed by an off period of 15 to 75 days; and the effective dose comprises 100 to 2500 mg of amikacin daily during the administration period.
Owner:INSMED INC
Method for treating pulmonary disorders with liposomal amikacin formulations
Disclosed herein are methods of treating pulmonary disorders comprising administering to the patient an effective dose of a nebulized liposomal amikacin formulation for at least one treatment cycle, wherein: the treatment cycle comprises an administration period of 15 to 75 days, followed by an off period of 15 to 75 days; and the effective dose comprises 100 to 2500 mg of amikacin daily during the administration period.
Owner:INSMED INC
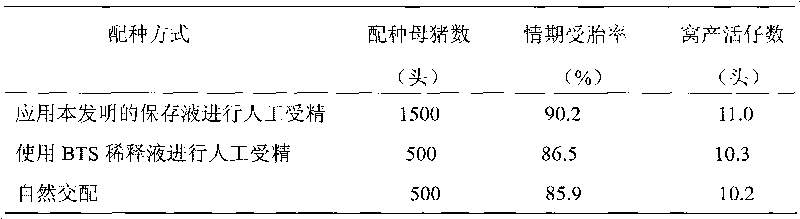
Preserving liquid for swine sperm
ActiveCN101731199AExceeding artificial insemination requirementsDead animal preservationSodium bicarbonateVitamin C
The invention relates to preserving liquid for sperm, in particular to the preserving liquid for swine sperm, and belongs to the technical field of animal reproduction. The preserving liquid for the swine sperm comprises the following components: 25.5 to 35.0g / l of anhydrous dextrose, 5.1 to 7.0 g / l of trisodium citrate, 1 to 10 g / l of sodium bicarbonate, 2 to 4g / l of EDTA, 1 to 3g / l of potassium chloride, 2.4 to 3.4g / l of citric acid, 8.5 to 9.5g / l of trihydroxymethylaminomethane, 0.26 to 1g / l of amikacin and 0.2 to 1g / l of vitamin C. Through detection, in the swine sperm preserved by the preserving liquid, the sperm viability reaches 0.60 to 0.85 and the integrity rate of sperm acrosome reaches 90 percent, which excess the requirement of the current artificial fertilization.
Owner:HANGZHOU DONGYUAN BIOLOGICAL TECH
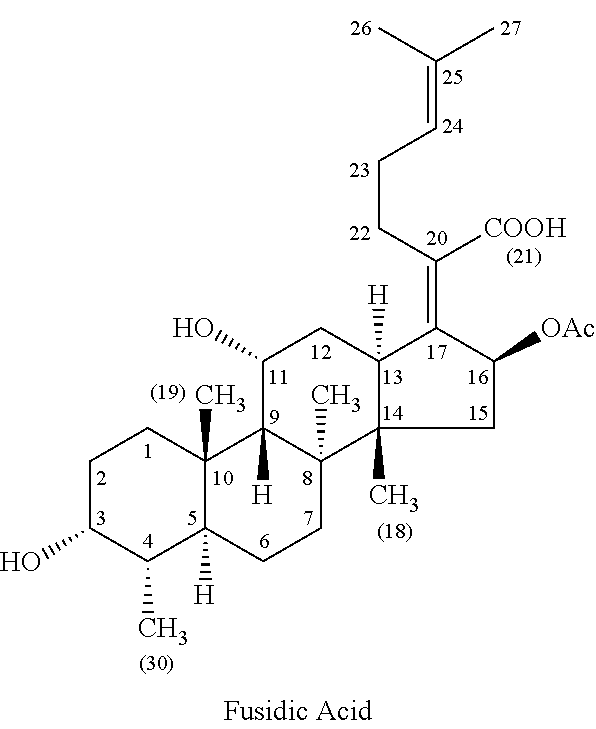
Methods of treating bacterial infections through pulmonary delivery of fusidic acid
Methods for the treatment of bacterial infections in the respiratory system of a subject, such as the lungs of a subject, using fusidic acid alone or in combination with a second bacterial agent such as tobramycin, amikacin, fosfomycin or levofloxacin are described.
Owner:CEMPRA PHARMA INC
Medical composite alginate dressing containing antibacterial drug and preparation method thereof
InactiveCN105999362AGood water vapor transmission ratePromote healingAbsorbent padsBandagesKanamycinWater vapor
The invention discloses a medical composite alginate dressing containing antibacterial drugs and a preparation method thereof. In parts by weight, the medical composite alginate dressing comprises the following components: 50-99.9 parts of alginate and 0.05-10 parts of antibacterial drugs, The antibacterial drug is selected from gentamicin or its salt, neomycin or its salt, amikacin or its salt, fusidic acid or its salt, kanamycin or its salt, polycresol , mupirocin, sulfamethonium or its salt and clindamycin or its salt at least one. The medical composite alginate dressing of the present invention has strong ability to absorb exudate, has good water vapor transmission rate, can be biodegraded and has good biocompatibility, does not adhere to the wound and can quickly form gel after absorbing wound exudate , has strong moisturizing properties, can effectively promote wound healing, shorten wound repair time, can effectively prevent or treat sensitive bacterial infections, and can be widely used in acute and chronic wounds with exudate.
Owner:SICHUAN KUIXING MEDICAL POLYMER PROD CO LTD
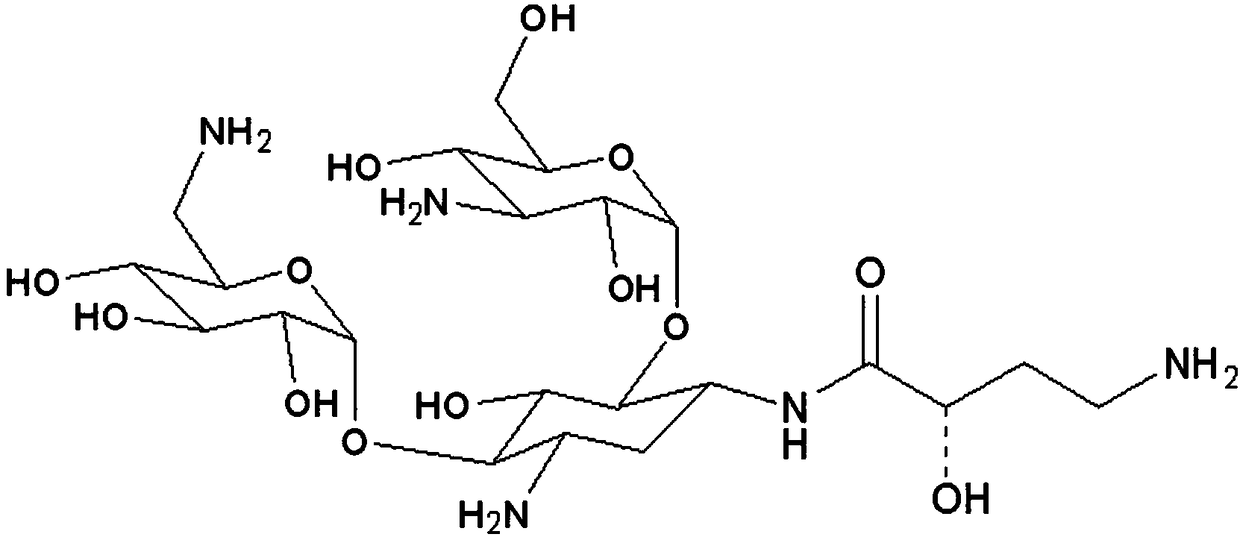
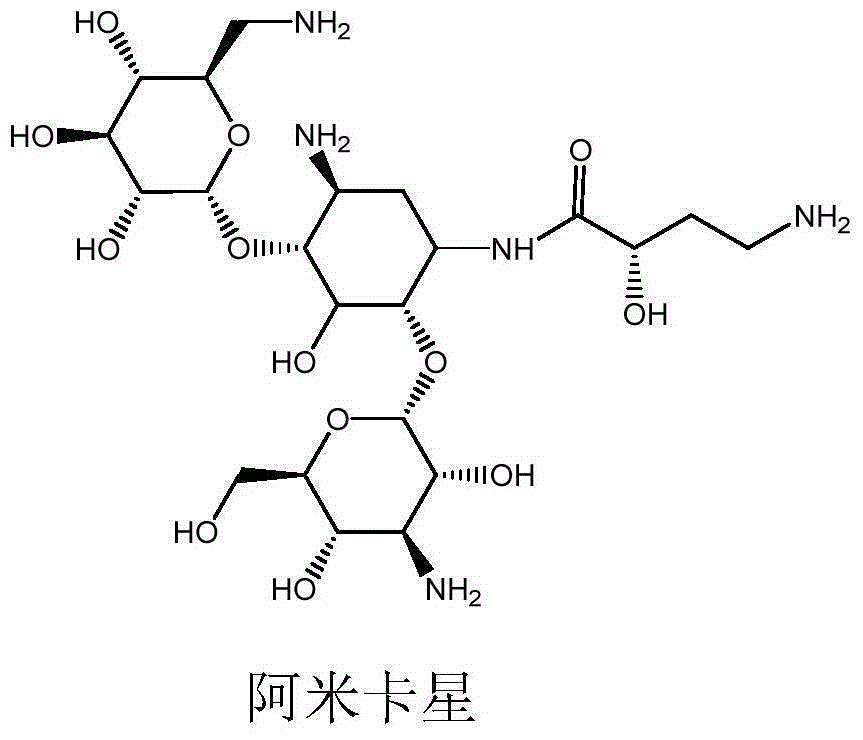
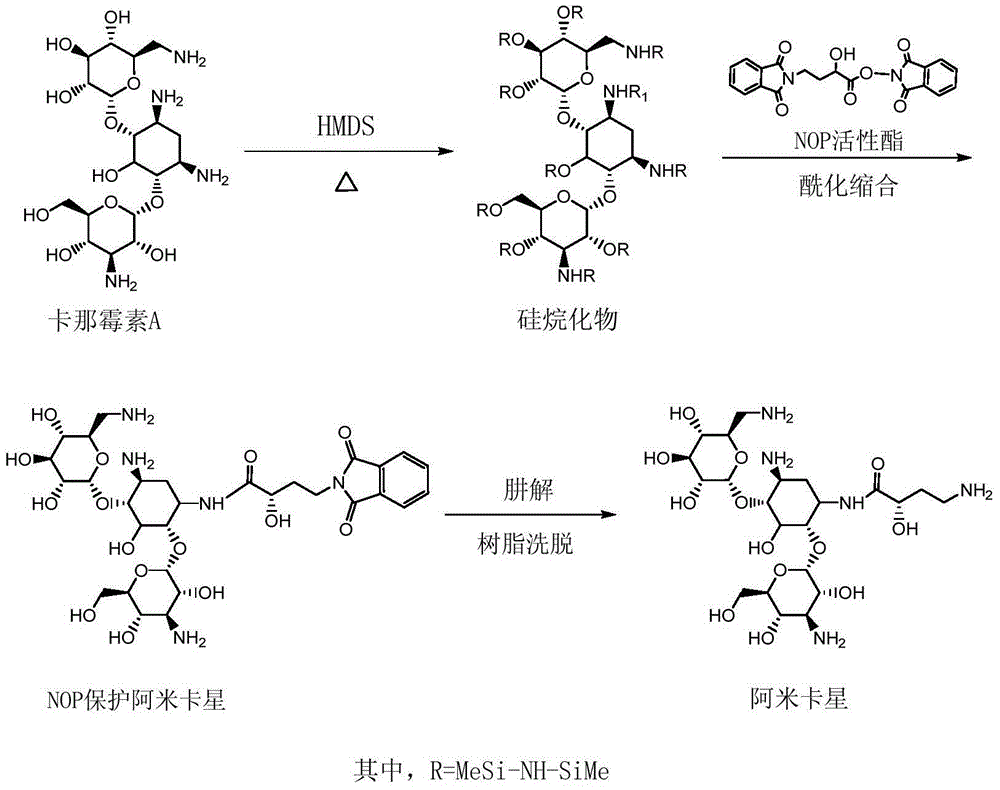
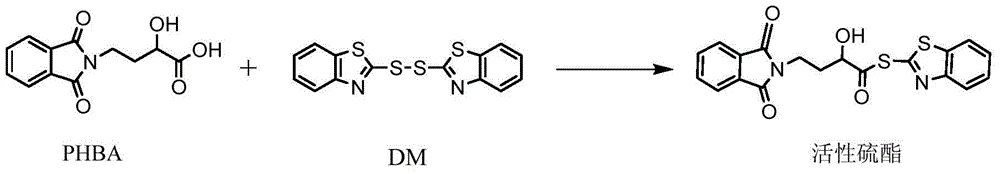
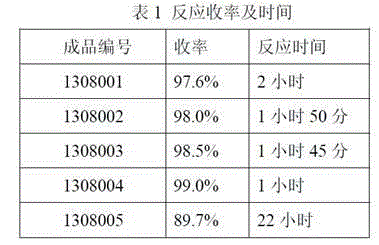
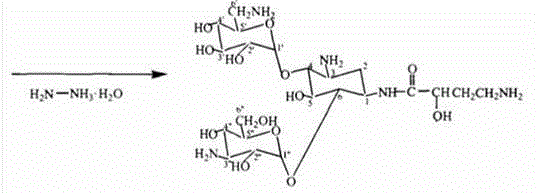
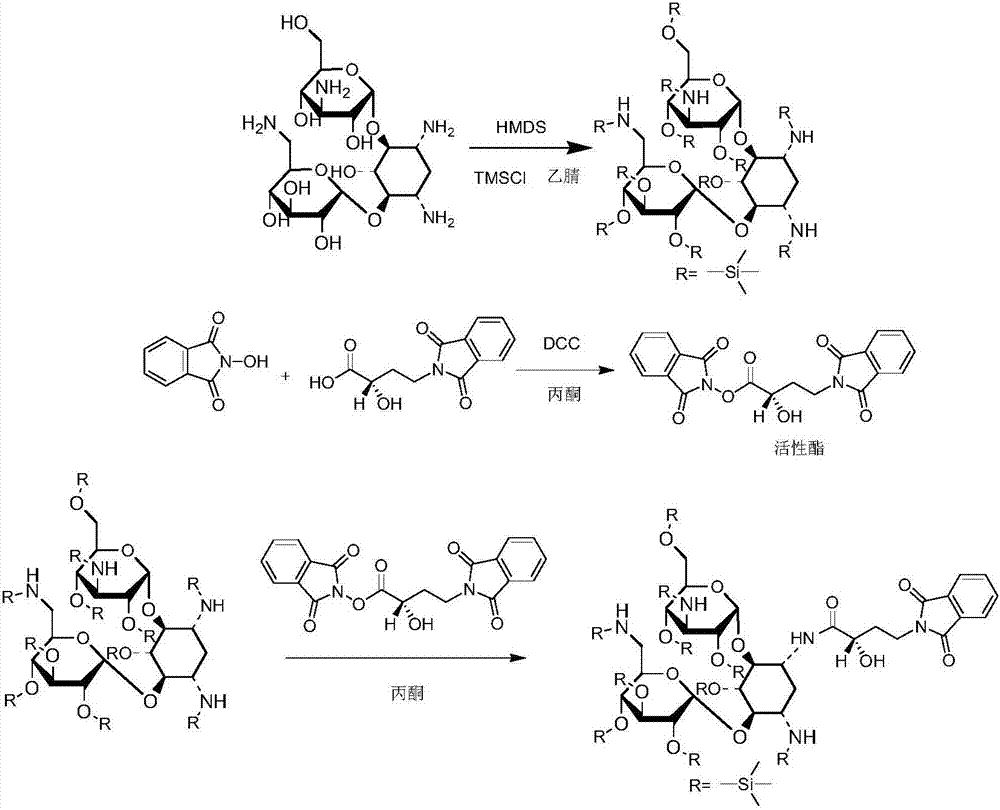
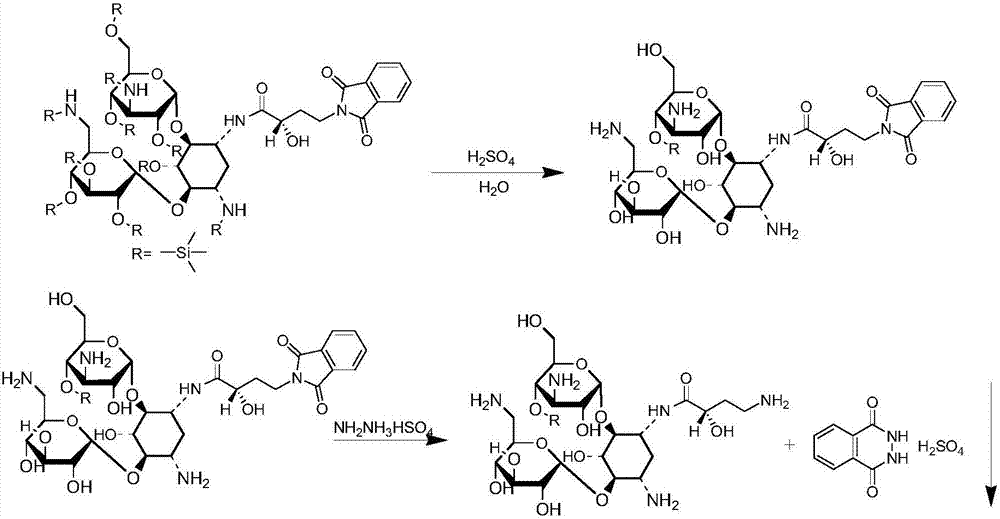
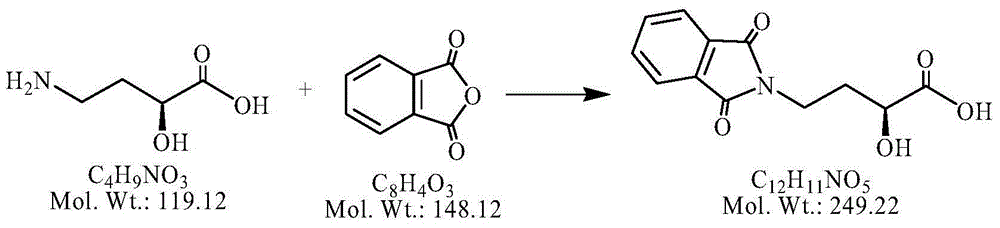
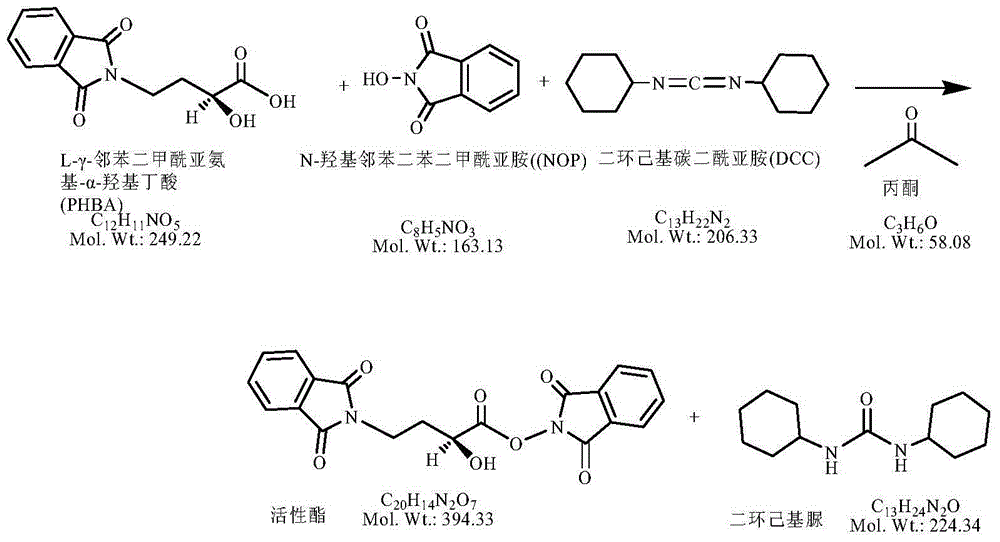
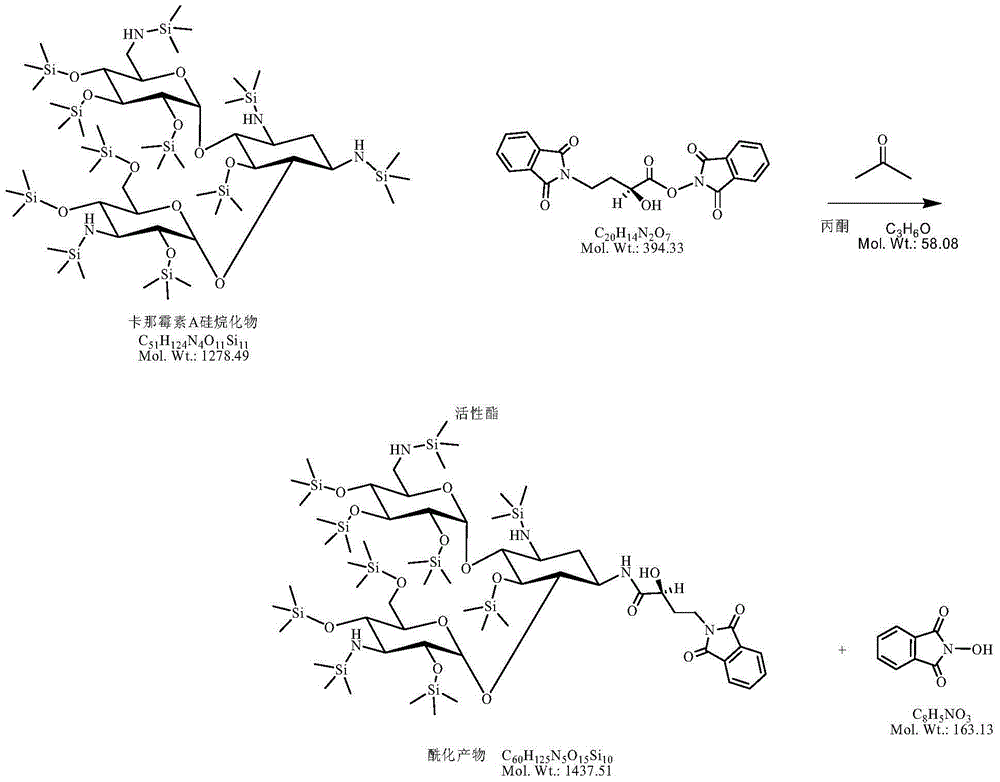
Preparation method of amikacin and intermediate activated thioester thereof
ActiveCN104447732AHigh selectivityLess side effectsSugar derivativesSugar derivatives preparationAmikacinAlcohol
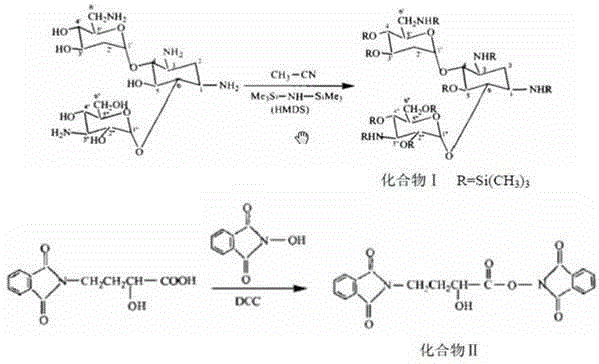
The invention discloses a preparation method of amikacin and an intermediate activated thioester thereof. The activated thioester has the structural formula (I). The preparation method comprises the following steps: using PHBA and DM as raw materials, under the condition that organic alkali is used as a catalyst, performing condensation reaction on the raw materials and a water absorbent triphenylphosphine or phosphite ester to obtain the activated thioester; using the activated thioester as the intermediate, performing acylation reaction on the activated thioester and kanamycin A silanized derivative, de-protecting by using an alcohol reagent, performing hydrazinolysis and crystallizing to obtain the amikacin. The preparation method is suitable for industrialized production; by the preparation method, the synthesis cost of the amikacin can be greatly reduced.
Owner:山东安信制药有限公司
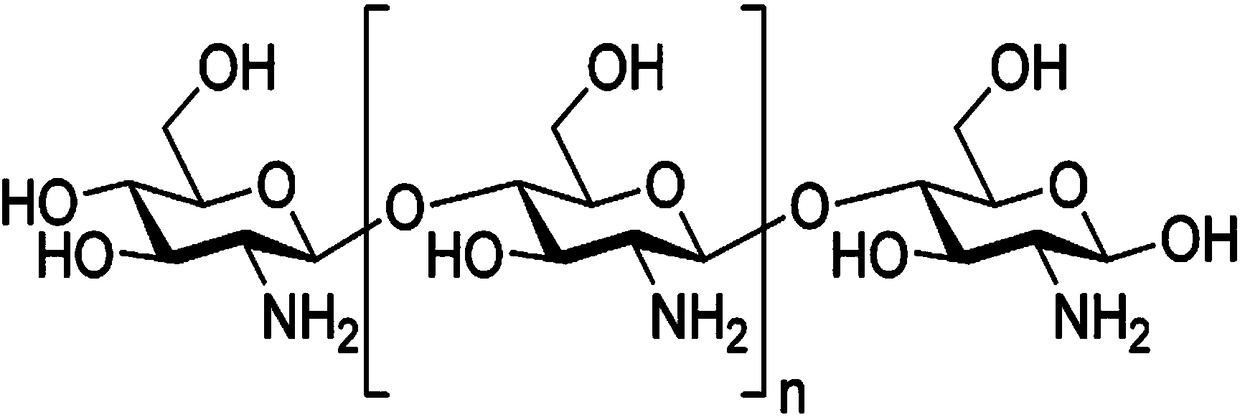
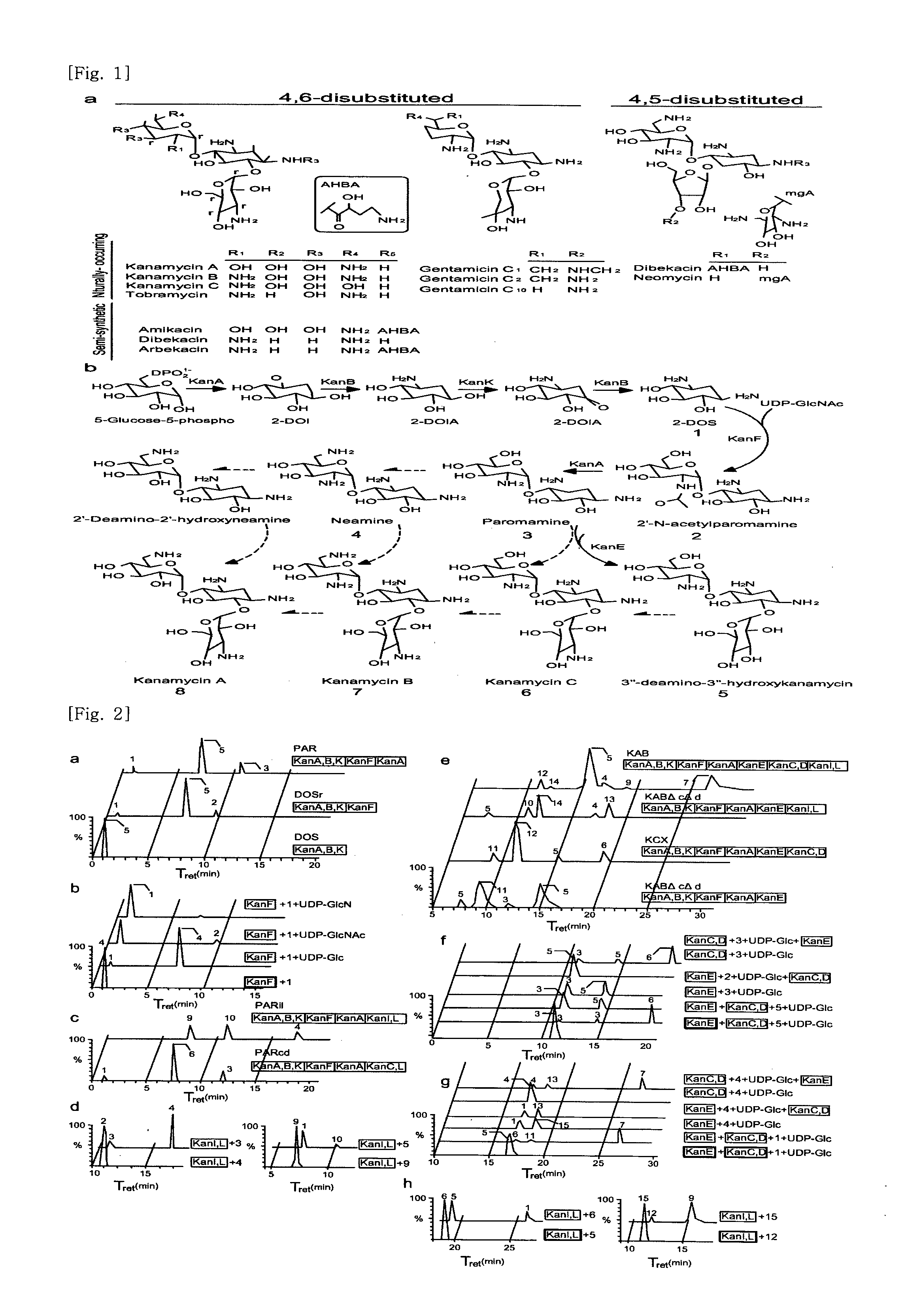
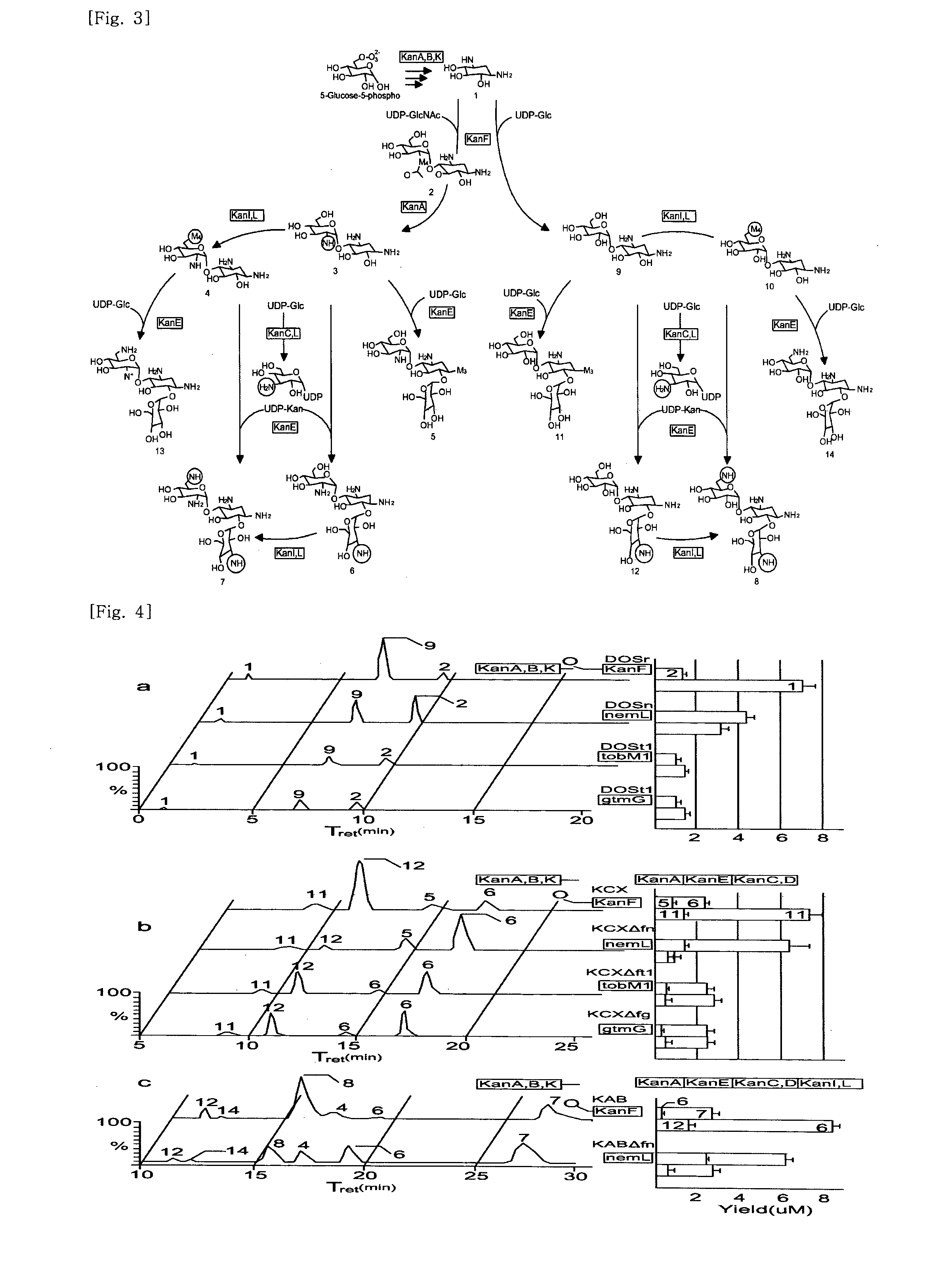
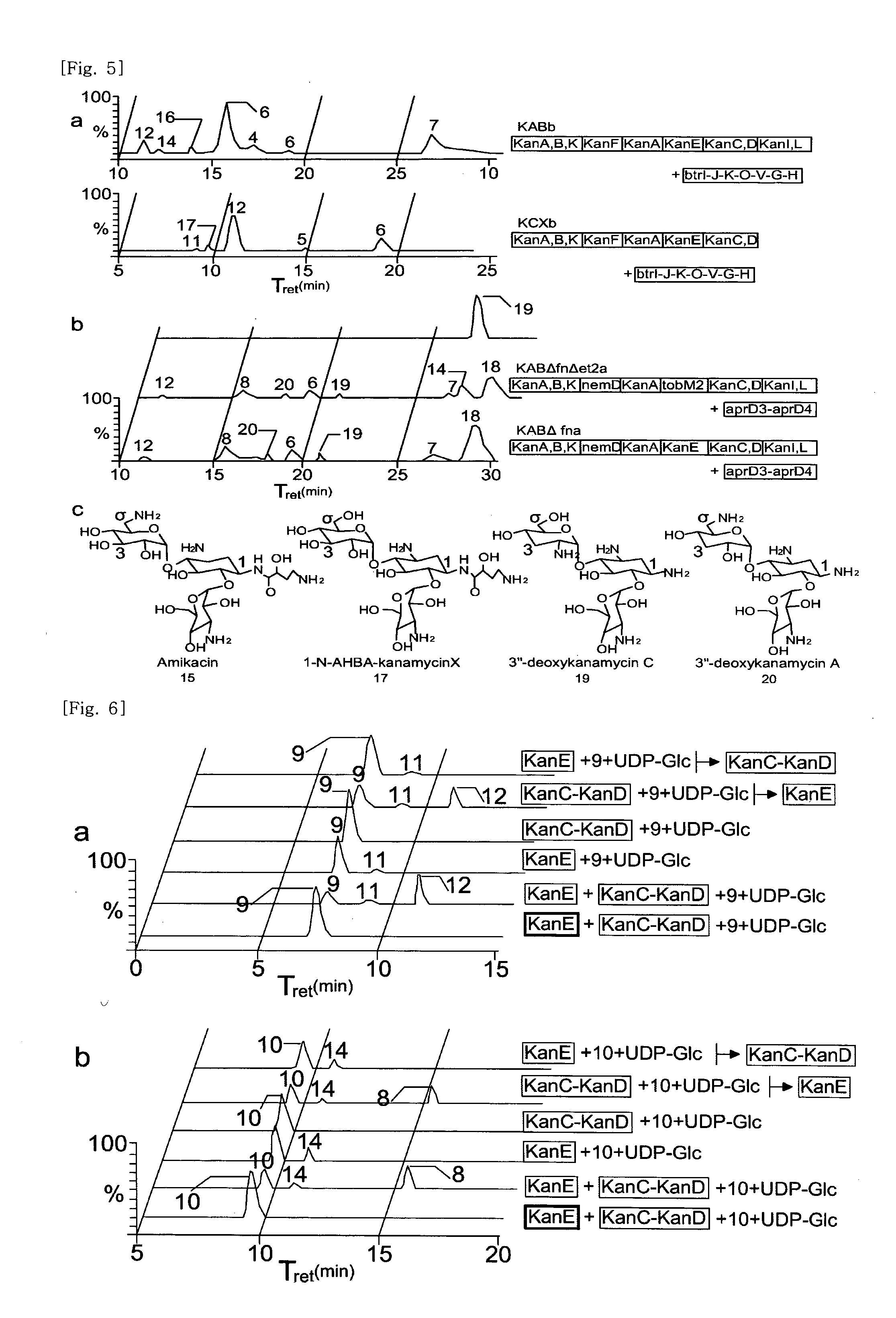
New kanamycin compound, kanamycin-producing streptomyces species bacterium, and method of producing kanamycin
Vectors expressing kanA-kanB-kanK and other kanamycin production-related genes, Streptomyces<i / > species recombinant bacteria transformed with the vectors, a method of producing kanamycin antibiotics by the bacteria, and a new kanamycin compound produced by the bacterium are provided. With the use of the recombinant bacteria of the present invention, the direct fermentative biosynthesis of amikacin and tobramycin as semi-synthetic kanamycins is possible, and the yield of kanamycin B as a precursor of the semi-synthetic kanamycin is improved.
Owner:INTRON BIOTECHNOLOGY INC
Slow released compound antituberculotic preparation
The slow released compound antituberculotic preparation contains at least one of rifampicin, pyrazinamide, kanamycin, isoniazide, rifapentine, etc. The slow released preparation is slow released injection or slow released implanting agent. The slow released injection consists of slow released microsphere and solvent, the slow released microsphere contains slow releasing supplementary material and antituberculotic, and the solvent is special solvent containing suspending agent carboxymethyl cellulose sodium and of viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is EVAc, PLA, PLGA, sebacic acid copolymer, etc. The slow released compound antituberculotic preparation is set or injected into local tuberulosis focus to treat various kinds of intractable tuberulosis, and has medicine releasing period up to 30-40 days, less systemic toxicity and unique curative effect.
Owner:JINAN SHUAIHUA PHARMA TECH
Method for simultaneously analyzing various aminoglycoside compound residues in agricultural and animal products
InactiveCN108519456AEasy to keepThe result is accurateComponent separationAnimal productSolid phase extraction
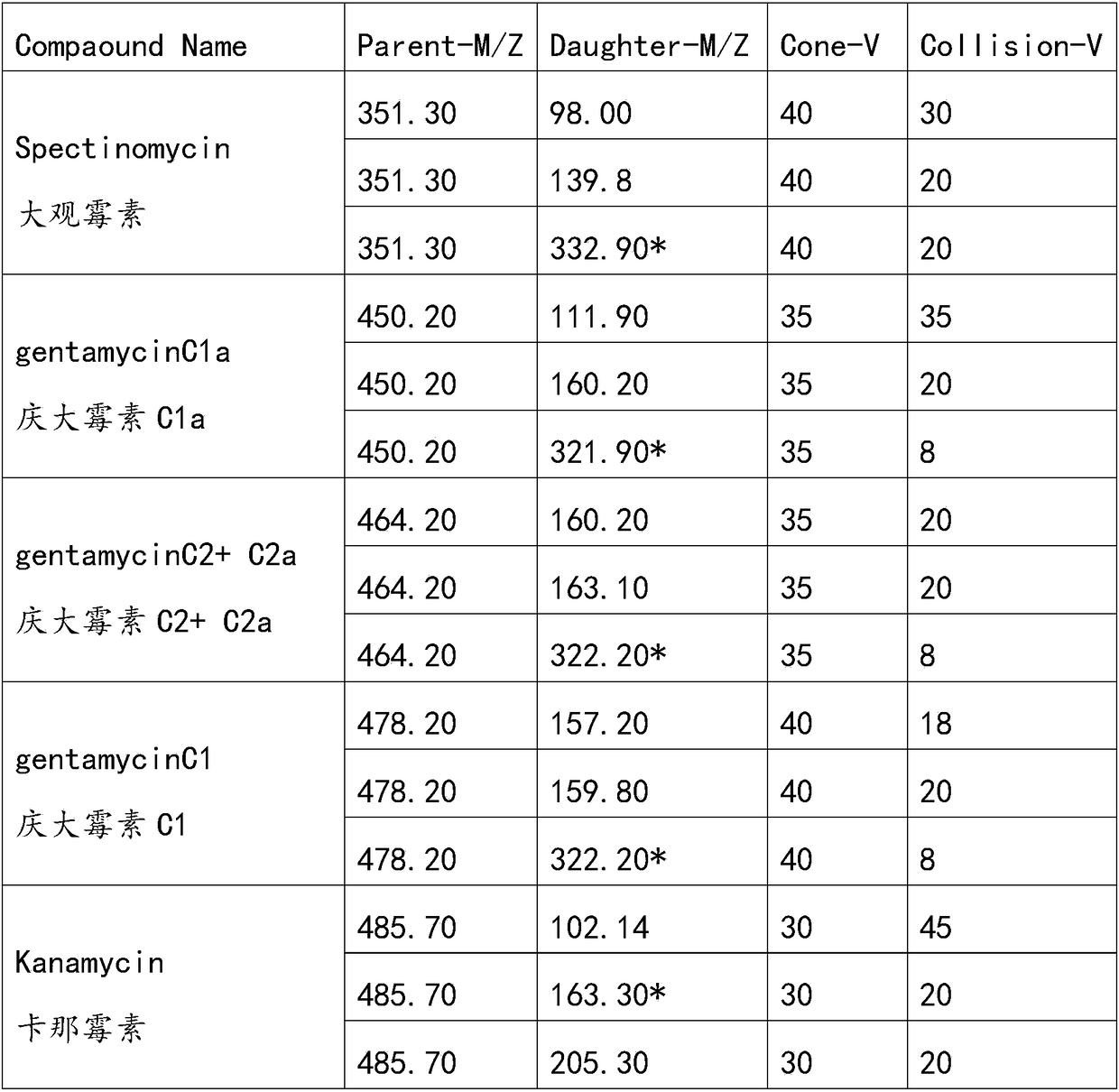
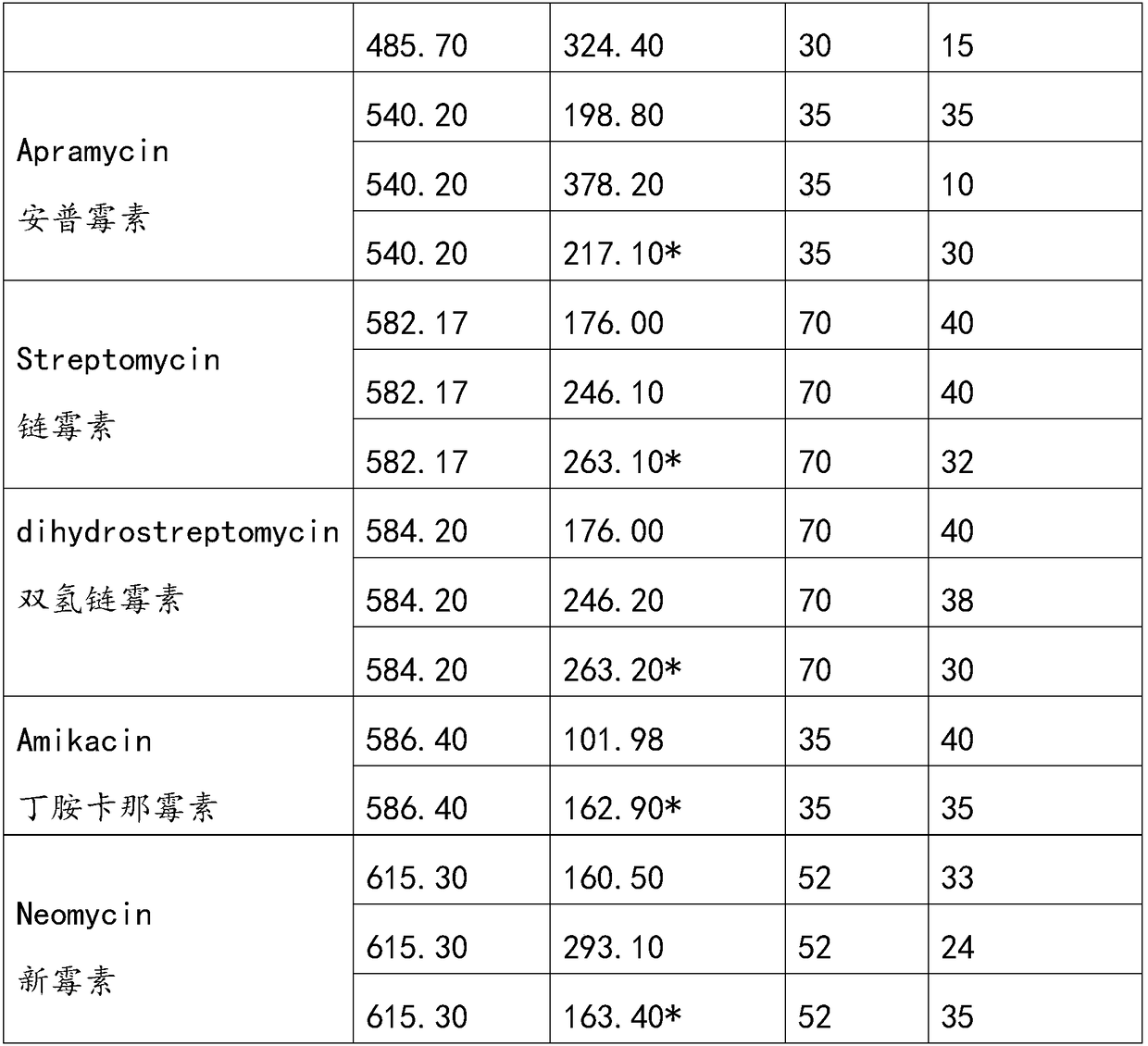
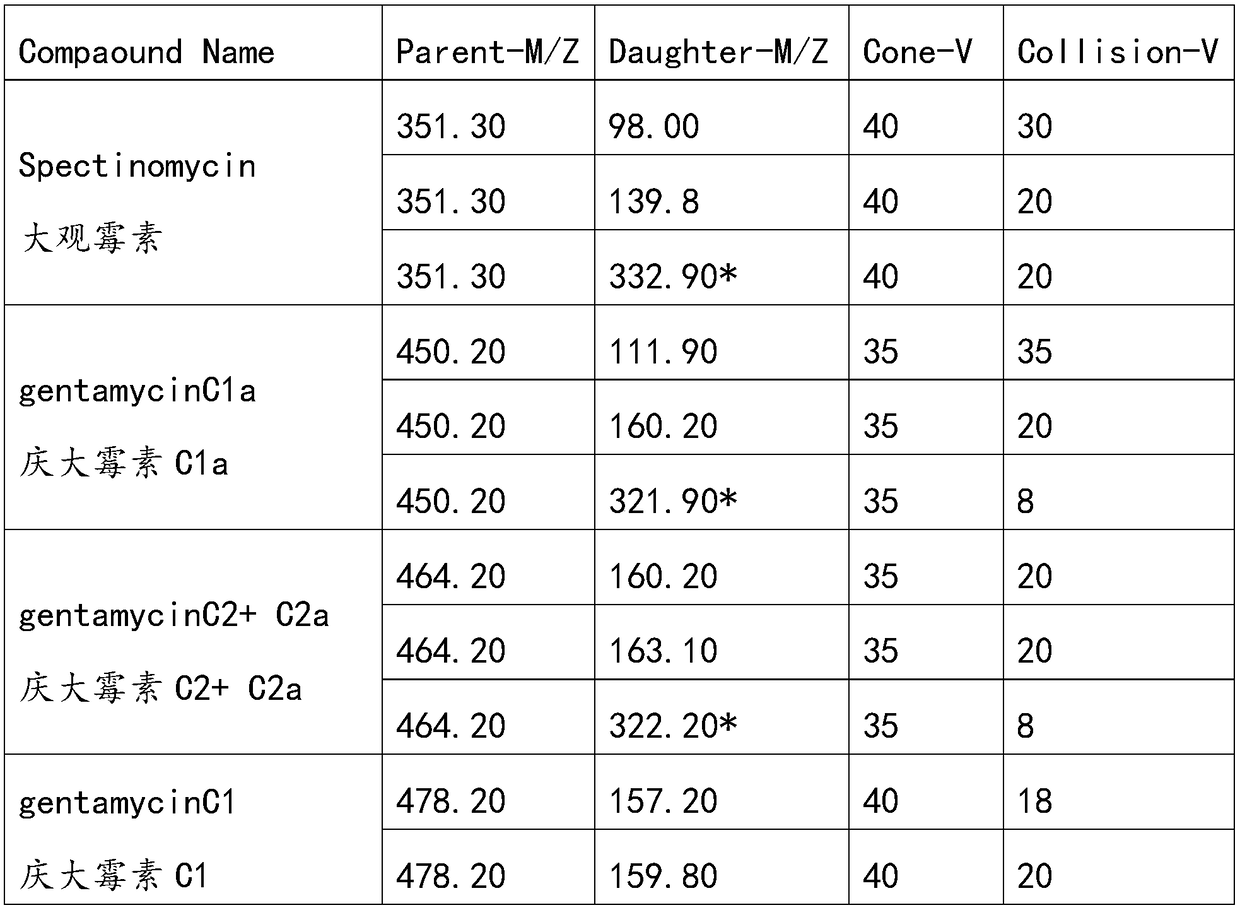
The invention discloses a method for simultaneously analyzing various aminoglycoside compound residues in agricultural and animal products. The method comprises the following steps: weighing a sample;adding an ammonium acetate buffering solution; after carrying out oscillation and extraction, carrying out centrifuging; separating an extracting solution obtained by centrifuging and regulating to asuitable range by utilizing a PH (Potential of Hydrogen) meter; purifying by adopting dispersed solid-phase extraction and a solid-phase extraction small column. Ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS / MS), electrospray ionization (ESI) and multi-reaction monitoring (MRM) scanning modes are adopted. Compared with other similar methods, the method disclosed by the invention has the advantages of simplicity in operation and high sample flux; eight types of aminoglycoside compounds including streptomycin, dihydrostreptomycin, spectinomycin, gentamicin, apramycin, kanamycin, amikacin and neomycin are detected at the same time; the detection efficiency is greatly improved, and rapid qualitative and quantitative detection can be rapidly carried out; the detection limit can completely meet domestic and overseas laws and regulations and limitation requirements, and powerful support is provided for food safety guarantees and import and export of the agricultural and animal products of China.
Owner:烟台杰科检测服务有限公司
Preparation method of amikacin
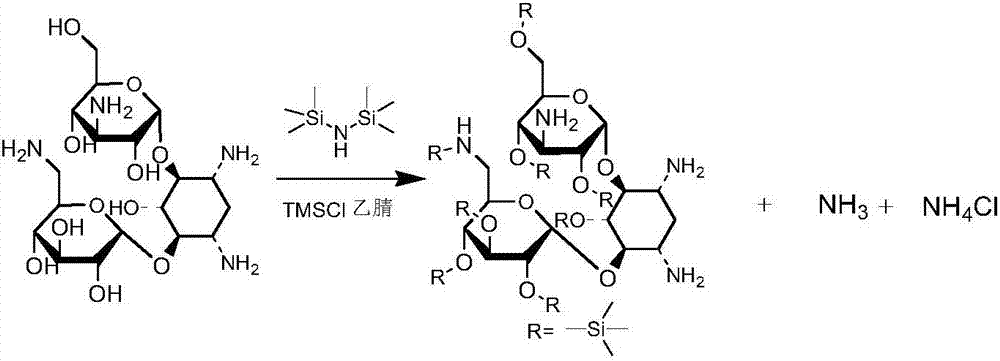
ActiveCN103601768ALarge specific surface areaAccelerate the speed of siliconization reactionPhysical/chemical process catalystsSugar derivativesAmikacinBis(trimethylsilyl)amine
The invention discloses a preparation method of amikacin, belonging to the technical field of medicines. In a silylation reaction process, ammonium sulfate is micronized to remove a commonly-used solvent, hexamethyl disilazane is not only used as a reactant, but also used as a solvent, reaction time is shortened to about 2 hours, materials are easy to operate, and the preparation method is environment-friendly. Besides, in the silylation reaction process, a divalent metal organic weak acid salt is used as a complexing agent, so that the silylation reaction time can be shorted to about 1 hour; and meanwhile, selectivity of subsequent acylation reaction is improved, and yield of synthesizing the amikacin is improved.
Owner:山东安信制药有限公司
Synthesis method of amikacin
ActiveCN106866755AReduce usageHigh activitySugar derivativesSugar derivatives preparationAmikacinKanamycin
The invention discloses a synthesis method of amikacin. The synthesis method comprises following steps: firstly, a partially silanization protection product of kanamycin A is prepared; dehydration of the partially silanization protection product with PHBA under the action of DCC is carried out so as to obtain a acylated product; the acylated product is subjected to hydrolysis, nitrilase, and column chromatography purification so as to obtain amikacin. According to the synthesis method, NOP is not used, synthesis of an active ester via esterification is avoided, damage on the health of workers is reduced greatly, solid waste generated in production process is reduced greatly, product yield is increased, and the synthesis method is especially suitable for industrialized production.
Owner:山东安信制药有限公司
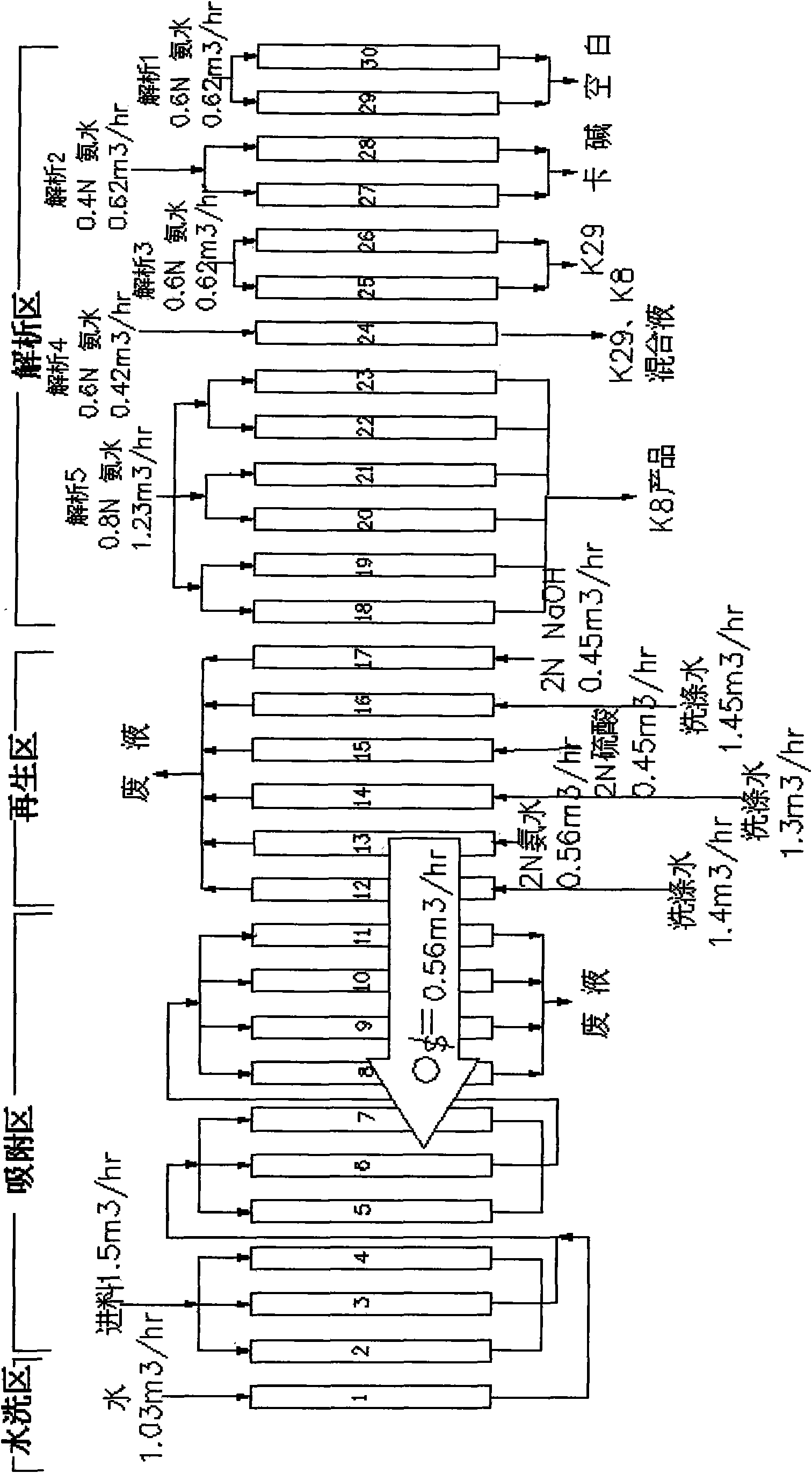
Method for separating and purifying amikacin
ActiveCN101643487AIncrease profitReduce consumptionSugar derivativesSolid sorbent liquid separationAmikacinChromatographic separation
The invention discloses a method for separating and purifying amikacin, which carries out continuous chromatographic resolution on amikacin material by using weak-acid cation resin and employing a continuous chromatographic resolution system technique. The method comprises the following steps: the material solution divides 30 separating units in the whole procedure into four regions comprising anadsorption region, a washing region, a separating region and a regenerating region through a continuous chromatographic resolution system, so as to replace the prior fixed-bed separation plant; and the original steps of absorption, washing, resolving, regenerating and the like in the fixed bed in the whole procedure are integrated into the disc-transmission type reverse-flow continuous chromatographic resolution system, so that the amikacin is effectively separated and purified, the production cost is reduced, the production technique is simplified, the production period is shortened, the pollution is reduced and the total yield is increased.
Owner:XIAMEN STARMEM TECH
Monoclonal antibody, enzyme-linked immunosorbent assay (ELISA) method and kit for detecting neomycin, amikacin and paromomycin
InactiveCN102585006ASimple processing methodNo health hazardMicroorganism based processesTissue cultureElisa methodImmuno detection
The invention discloses a specific monoclonal antibody capable of identifying neomycin, amikacin and paromomycin and an enzyme-linked immunosorbent assay (ELISA) method and kit for detecting neomycin, amikacin and paromomycin. The monoclonal antibody is secreted by a hybridoma cell EDC / 5G04 and the hybridoma cell is collected in the China Center for Type Culture Collection, with collection number being CCTCC NO:C201144. The ELISA method comprises the steps of preparation of immunogen, coating antigen and the antibody, treatment and detection of samples and the like. Compared with the prior art, the monoclonal antibody, the ELISA method and the kit have the following main advantages that the prepared monoclonal antibody can simultaneously identify neomycin, amikacin and paromomycin, thus improving the detection efficiency of the prior art; the animal tissue sample treatment method is simple and short in time; and the ELISA method and the kit also have the characteristics of high detection sensitivity, good precision and good accuracy.
Owner:HUAZHONG AGRI UNIV
Acalypha australis L. and amikacin containing compound medicine for livestock and poultry
InactiveCN103989725AReverse drug resistanceGood treatment effectAntibacterial agentsOrganic active ingredientsBacteroidesTreatment effect
Owner:GUANGXI UNIV
Creat/amikacin-containing compound medicine for livestock and fowl
InactiveCN103933086AReverse drug resistanceGood treatment effectAntibacterial agentsOrganic active ingredientsTreatment effectAntibacterial agent
Owner:GUANGXI UNIV
A synthetic method of amikacin
InactiveCN105440090AReduce manufacturing costEasy to operateSugar derivativesSugar derivatives preparationAmikacinSilylene
A synthetic method of amikacin is disclosed. Gamma-4-phthalimido-2-hydroxy butyric acid is adopted as a raw material for direct acylation. 4-dimethylaminopyridine (DMAP) or 1-hydroxybenzotriazole (HOBT) is adopted as a catalyst. N,N'-dicyclohexylcarbodiimide is adopted as a condensing agent. Silyl kanamycin A is directly acylated to obtain an acylation product and the acylation product is subjected to acidolysis and hydrazinolysis to obtain the amikacin. A production operation for specially preparing active ester for acylation is not needed in the method, thus simplifying operation steps and production equipment. N-hydroxyphthalimide for preparing the active ester is not used in the direct acylation, thus reducing the production cost. By optimization of acylation conditions, selectivity of the acylation is improved, the synthesis yield is ensured, contents of impurities is lowered, and beneficial conditions for subsequent purification of the amikacin are provided.
Owner:CHONGQING DAXIN PHARMA +2
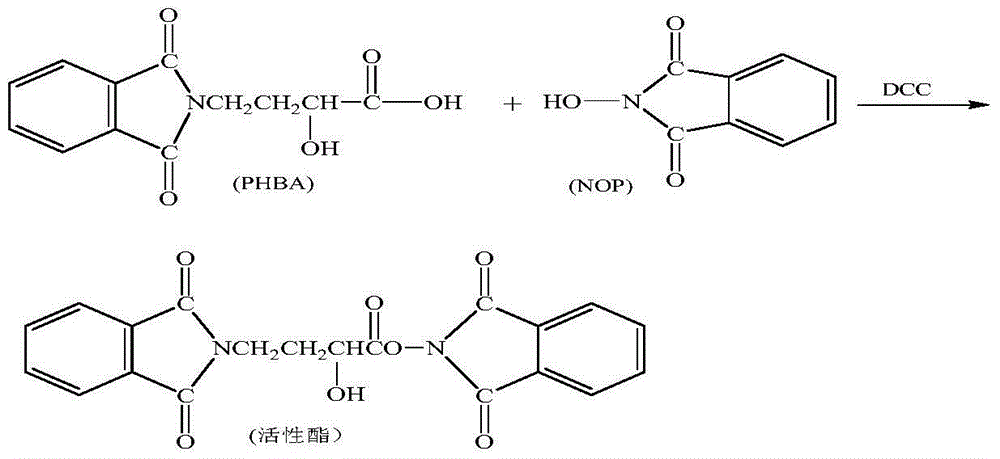
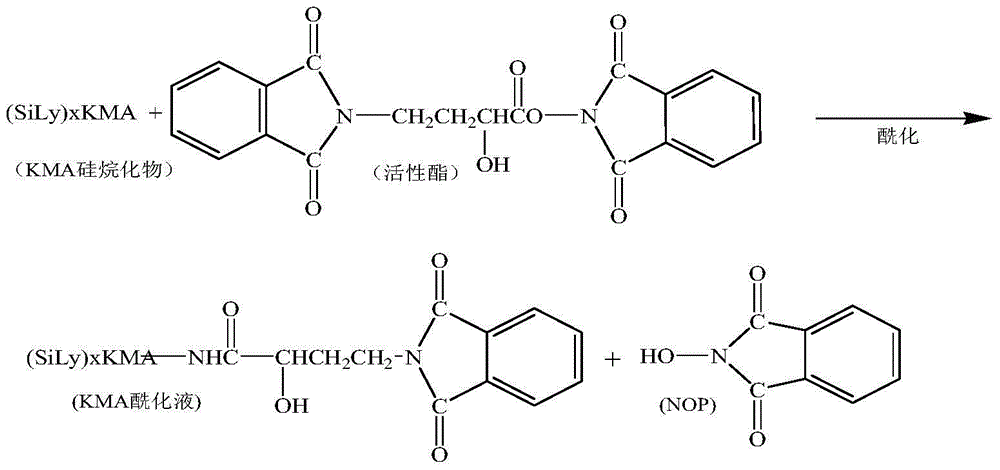
Method for increasing yield of amikacin
ActiveCN104356182AReduce lossesReduce degradationSugar derivativesSugar derivatives preparationHydroxybutyric acidKanamycin
The invention discloses a method for increasing yield of amikacin. The method for increasing yield of amikacin comprises the following steps: heating, refluxing and carrying out reaction on gamma-amino-alpha-hydroxybutyric acid and fluorenylmethyl chloroformate, so as to generate a reaction liquid containing gamma-amino-alpha-hydroxybutyric acid; directly adding N-hydroxyl phthalimide and N,N-dicyclohexylcarbodiimide into the reaction liquid, and carrying out reaction to generate a reaction liquid containing active ester; directly adding a kanamycin A silanization compound into the reaction liquid containing the active ester for carrying out acylation reaction, after complete reaction is finished, adding an HBr aqueous solution until the pH is adjusted to be 2-3, and carrying out suction filtration to remove solid impurities; and then carrying out reduced pressure distillation for removing the solvent, adjusting the pH to be 7-8 with concentrated liquor, purifying by virtue of a CD180 macroporous resin column, concentrating, and carrying out freeze drying, so that amikacin solid is obtained. The active ester causes selectivity of the acylation reaction to be greatly improved due to enlargement of a protective group, and a follow-up processing step is also greatly simplified, so that the amikacin synthesis yield is increased.
Owner:山东安信制药有限公司
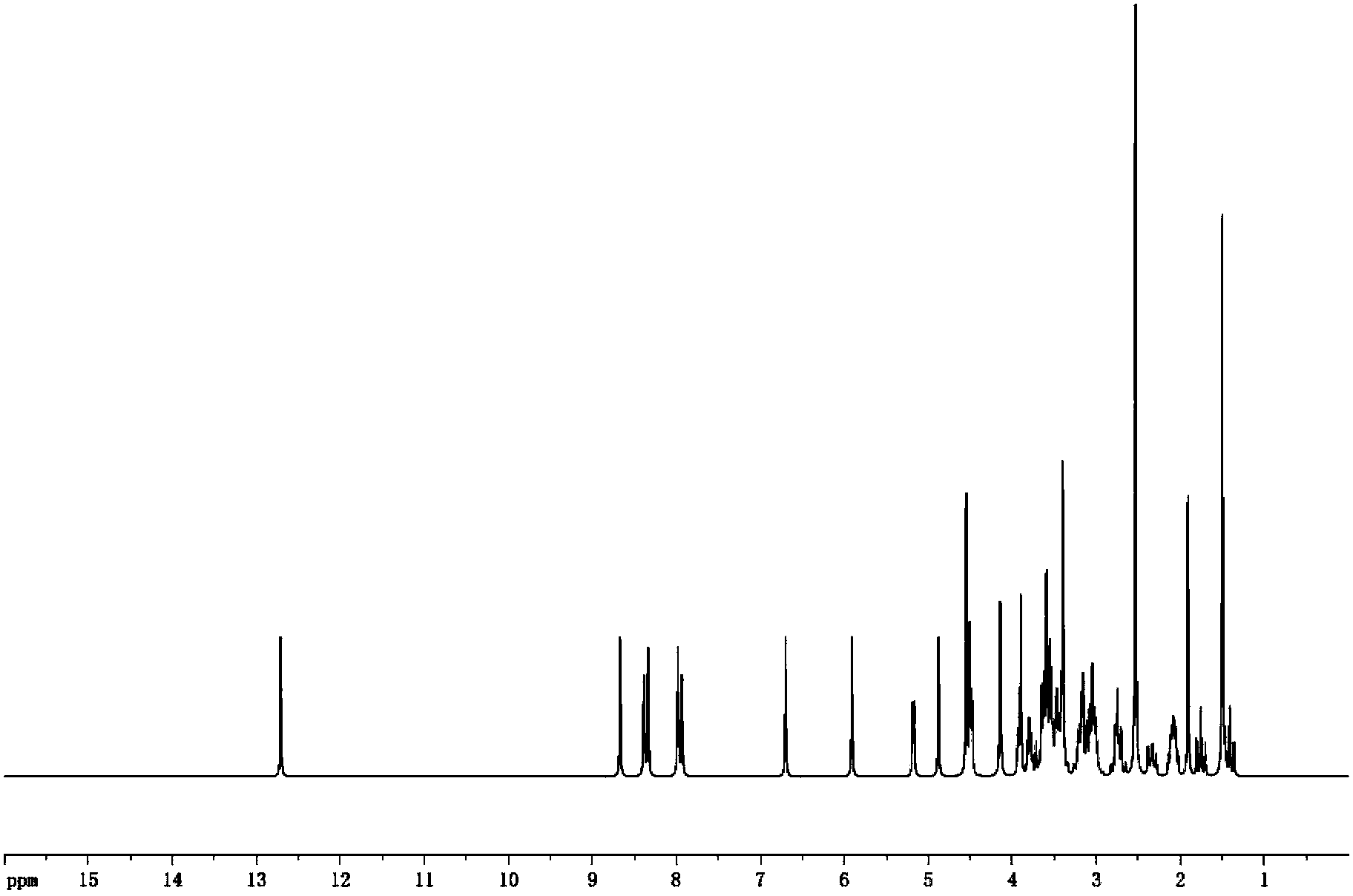
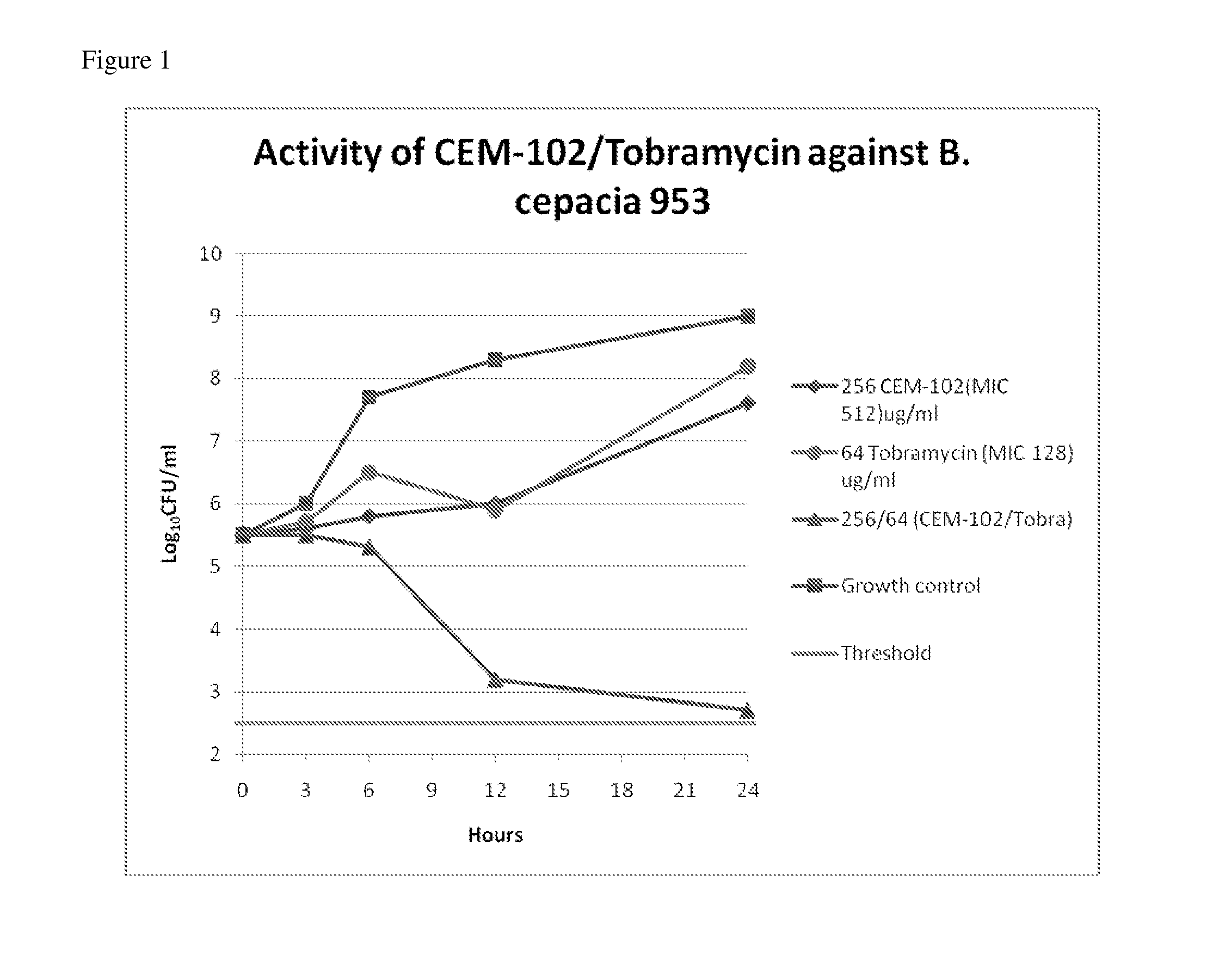
Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection
ActiveCN107243008AReduce healingLong treatment cycleAntibacterial agentsOrganic active ingredientsMycobacterium InfectionsMycobacterium abscessus Infections
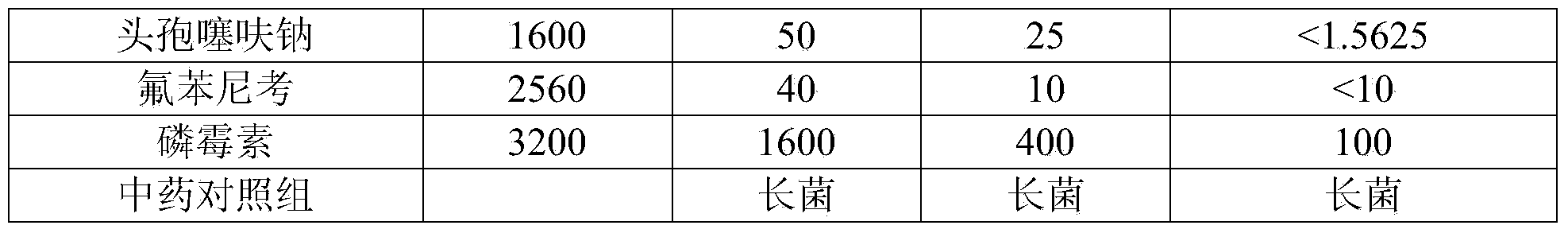
The invention discloses novel application of a pyrazolo[1, 5-a]pyridine compound and a composition for treatment of Mycobacterium abscessus infection. The pyrazolo[1, 5-a]pyridine compound can be used for preparation of an anti-Mycobacterium abscessus synergist of clofazimine, combined use of the two can reach a synergistic effect, and a low concentration pyrazolo[1, 5-a]pyridine compound can reinforce the anti-Mycobacterium abscessus effect of clofazimine. The active components of the composition for treatment of Mycobacterium abscessus infection provided by the invention contain clofazimine and / or macrolide antibiotic, and the pyrazolo[1, 5-a]pyridine compound serving as a synergist. The composition has the characteristics of rapid effect, no relapse after treatment, and efficacy significantly superior to the combined use of clarithromycin and amikacin, and overcomes the shortcomings of easy generation of induced drug-resistance and poor long-term treatment effect in existing macrolide antibiotic clinical treatment.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI +1
Antibiotic Combinations For Providing Total Solution to the Treatment of Infections
InactiveUS20090318378A1Improve efficacyImprove securityAntibacterial agentsBiocideHospitalized patientsNosocomial pneumonias
The invention relates to a new pharmaceutical composition, a method of treatment of infection and also a process to prepare the composition. The infectious complications are important causes of morbidity and mortality. Hospital acquired pneumonia (HAP) remains the most severe nosocomial infection in intensive care units. Beta-lactams alone are always considered inadequate when P. aeruginosa and / or methicillin-resistant S. aureus are implicated as pathogens or copathognes. The present invention provides the desired empirical therapy for control of all bacterial infections. The invention provides antibiotic combination products for delivering at least two different antibiotics, through parenteral dosage form comprising protein-synthesis-inhibiting antibiotic which is amikacin or its sulphate salt and non-protein-synthesis-inhibiting antibiotic which is cefepime or its hydrochloride salt. The invention provides a total solution, against multiresistant P. aeruginosa, or Acinetobacter spp. and / or methicillin-resistant S. aureus, and are useful for intramuscular or intravenous administration as antibiotics for hospitalized patients with acute or serious infections. The pharmaceutical compositions described here normally have the least nephrotoxicity and have better efficacy and safety of cefepime plus amikacin combination.
Owner:VENUS REMEDIES LTD
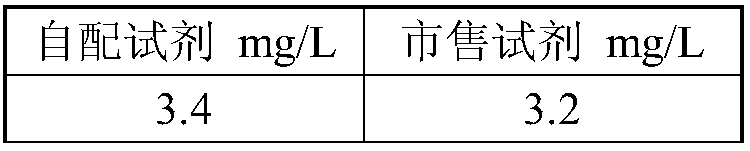
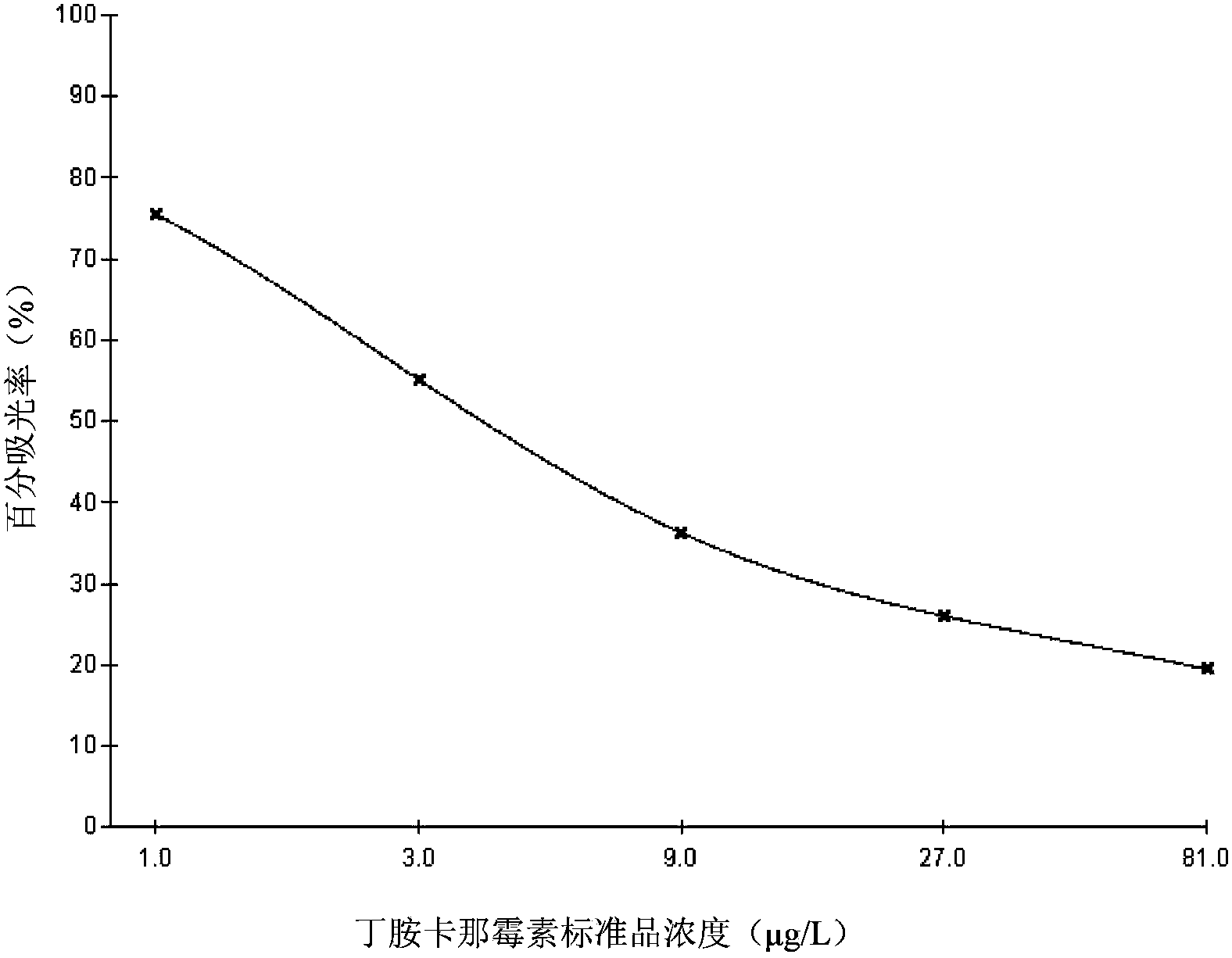
Kit for detecting amikacin content in blood and preparation method thereof
InactiveCN108614108AAccurate detectionQuick checkMaterial analysis by observing effect on chemical indicatorAmikacinPreservative
The invention provides a kit for detecting amikacin content in blood. The kit is composed of a reagent R1 and a reagent R2 in a volume ratio of 1 to 1. The reagent R1 is prepared from a 20 to 150 mmol / L buffer, a 3 to 20 g / L conjugate, a 3 to 20 g / L stabilizer, a 5 to 20 g / L electrolyte, a 3 to 20 g / L sensitizer, a 0.5 to 10 g / L surfactant, a 0.2 to 1 g / L preservative, and purified water, and thepH is 7.0 to 9.5. R2 is prepared from 1.5 g / L to 3.5 g / L latex microspheres conjugated to an anti-human amikacin monoclonal antibody, a 20 mmol / L to 150 mmol / L buffer, a 3 g / L to 20 g / L stabilizer, a5 g / L to 20 g / L electrolyte, a 0.5 g / L preservative, and purified water, and the pH is 7.0 to 9.5. The kit provided by the invention is accurate, rapid and has a good clinical application prospect.
Owner:SHANGHAI FOSUN LONG MARCH MEDICAL SCI CO LTD
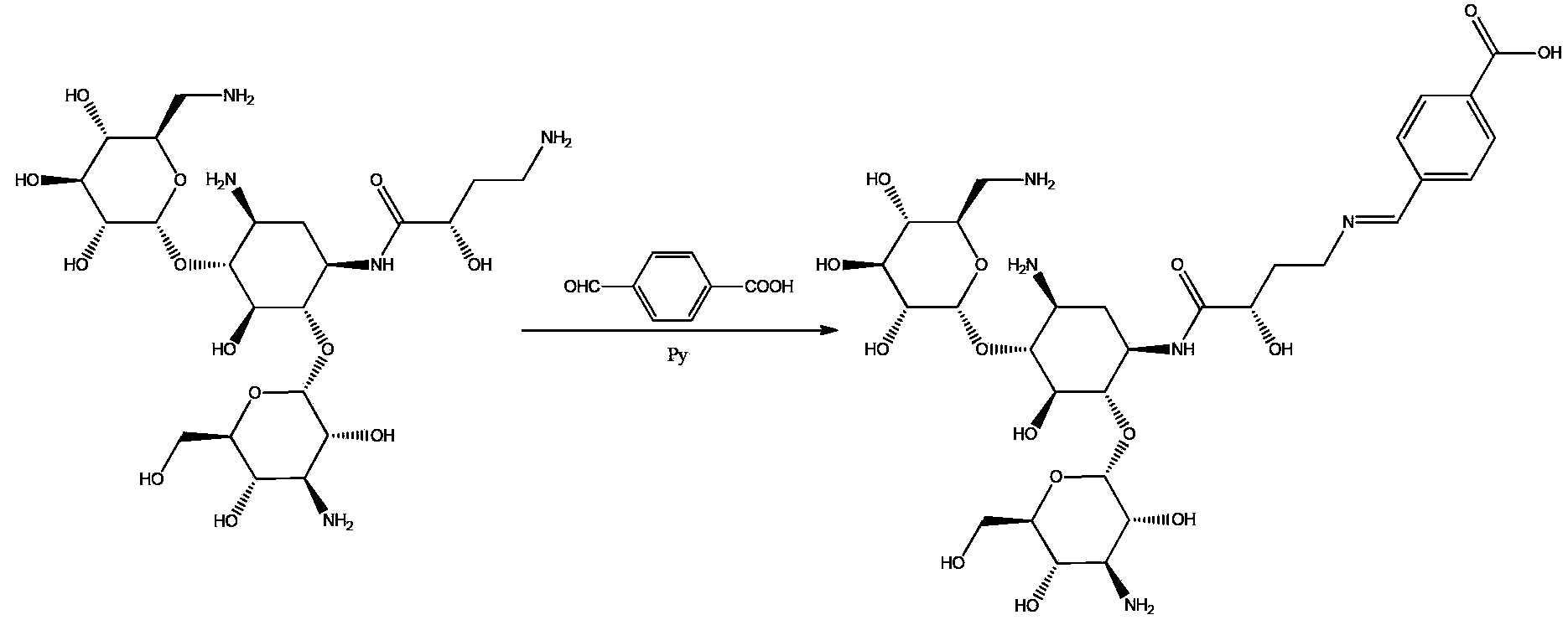
Amikacin semi-antigen, and preparation method and application thereof
ActiveCN103509068ARetain chemical structureGood potencyTesting eggsFibrinogenAmikacinSite monitoring
The invention discloses a semi-antigen and a preparation method and application thereof and specifically relates to an amikacin semi-antigen. The invention further discloses a preparation method and application of the amikacin semi-antigen. A rapid detection kit product established on the basis of the amikacin semi-antigen has the advantages of convenient usage, low detection cost, usage of highly efficient, accurate and rapid detection methods and capacity of simultaneous on-site monitoring of amikacin residue and screening of considerable samples.
Owner:BEIJING KWINBON BIOTECH
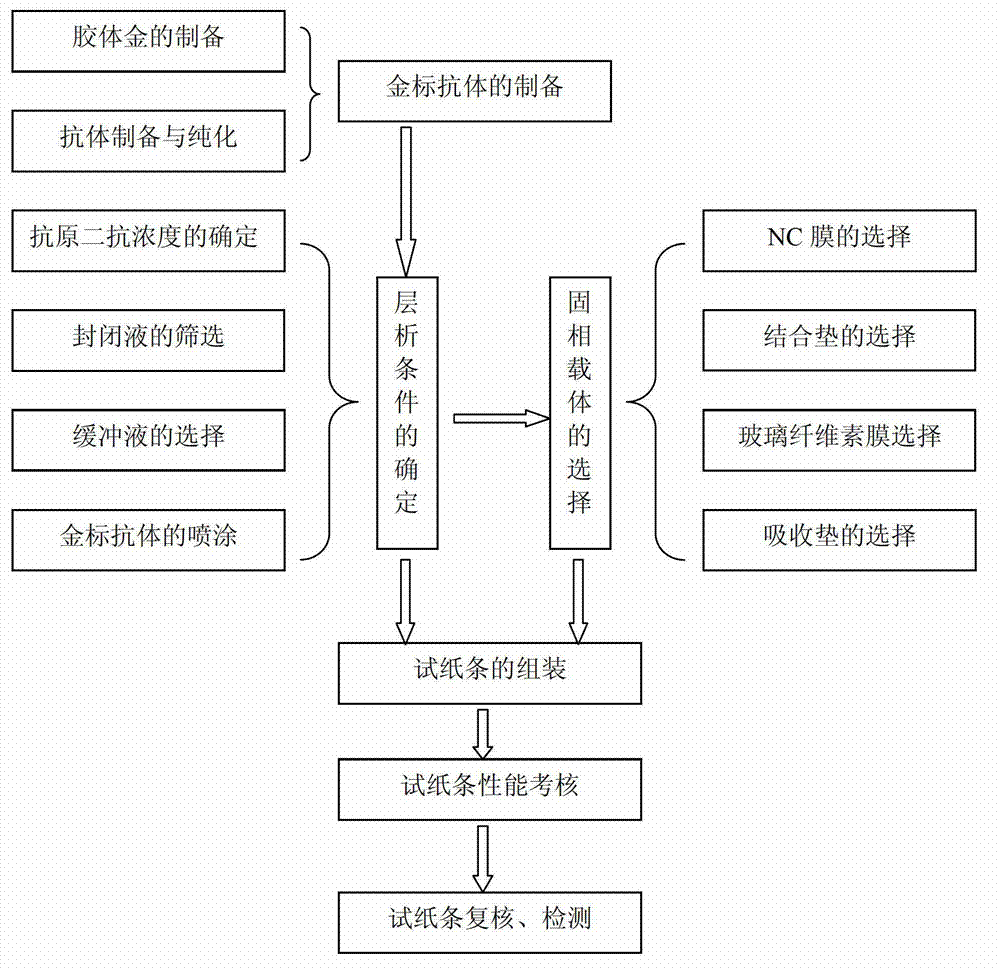
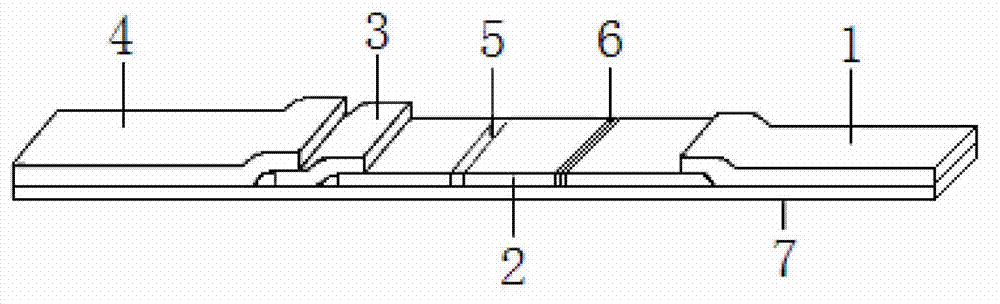
Colloidal gold test strip for detecting neomycin residual and use method and application thereof
Owner:HUAZHONG AGRI UNIV
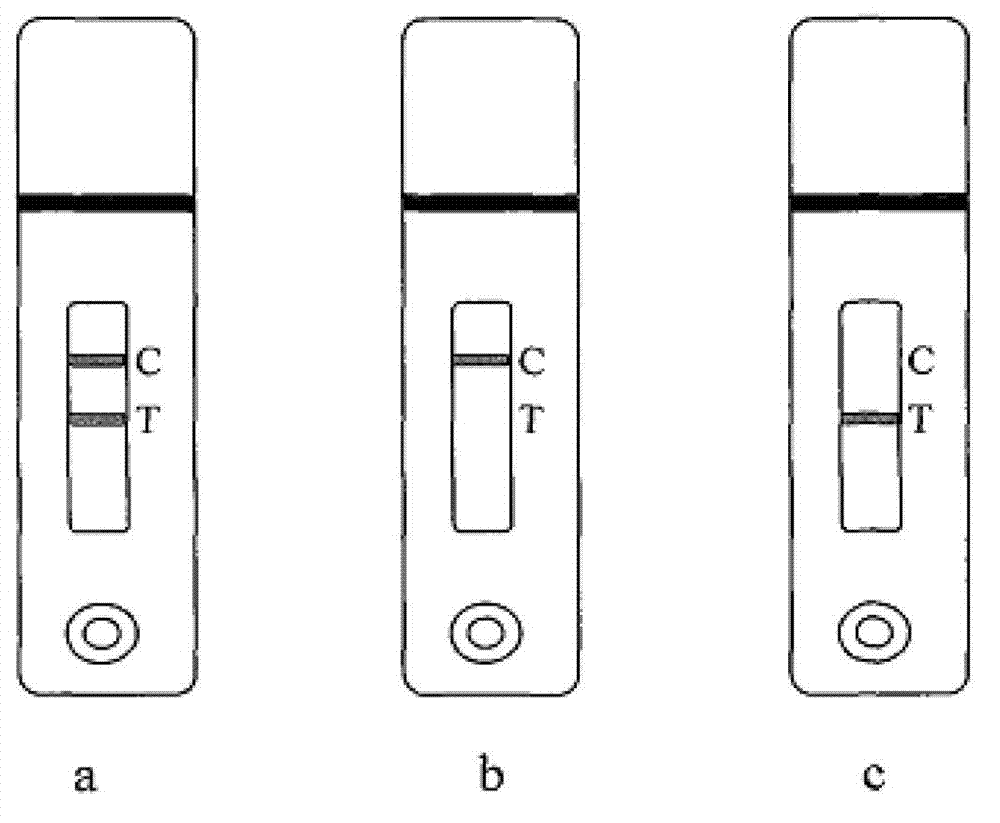
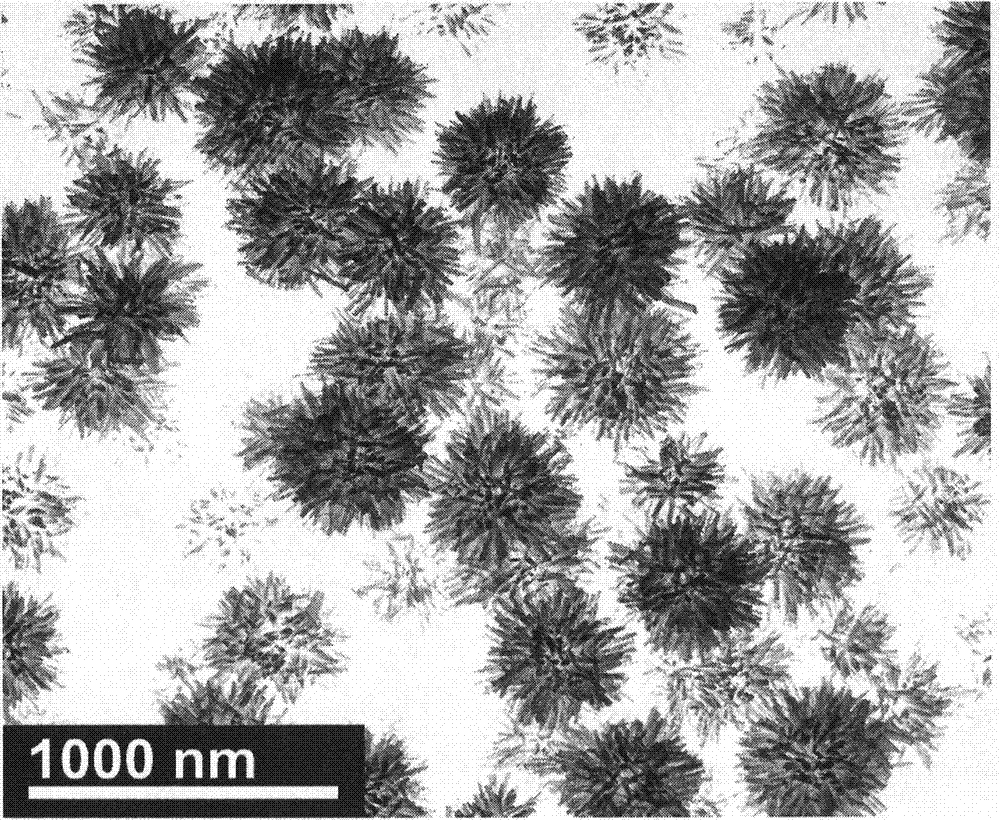
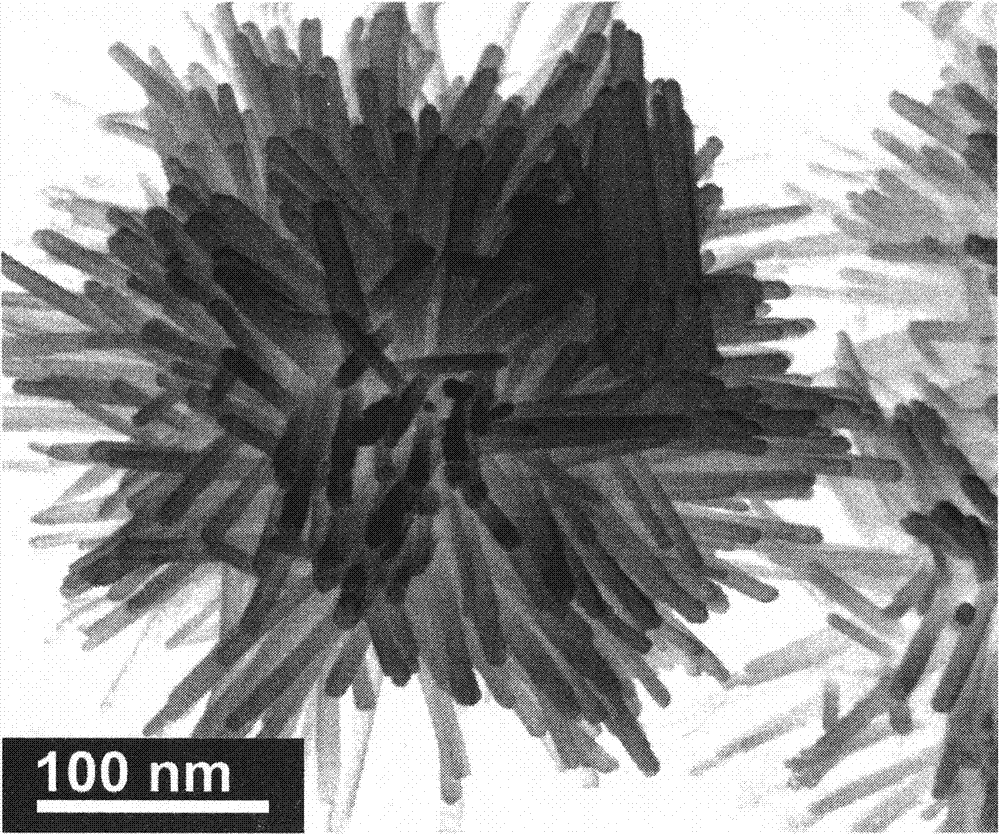
Preparing method of sea-urchin-shaped amorphous Ni-B alloy nanometer materials
InactiveCN104289724AHigh selectivityLarge specific surface areaNanotechnologyKanamycinPlasma technology
The invention discloses a preparing method of sea-urchin-shaped amorphous Ni-B alloy nanometer materials. A liquid phase plasma technology is used for synthesizing sea-urchin-shaped Ni-B particles with mesoporous structures successfully. Compared with amikacin and kanamycin, high antimicrobial activity on pseudomonas aeruginosa is especially achieved. Compared with conventional Ni-B, sea-urchin-shaped Ni-B is higher in magnetism. Meanwhile, compared with Raney Ni and traditional Ni-B, the sea-urchin-shaped Ni-B is higher in N2H4 dehydrogenation catalyzing performance. The catalyzing performance is enhanced due to the high specific surface area and the enhanced inherent activity determined by the unique structure. In the current research results, the sea-urchin-shaped Ni-B has antibacterial effect, magnetism and catalytic dehydrogenation performance, and the application range can be widened from medical instrument coating to direct wound or burn injury treating and the field of magnetic equipment and catalyzing.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

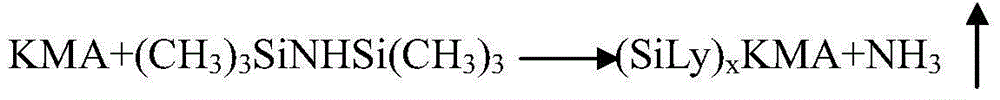
![Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection](https://images-eureka.patsnap.com/patent_img/6dedd4ce-d271-4b08-8f49-f5b5c4194c5a/HDA0001269169880000011.png)
![Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection](https://images-eureka.patsnap.com/patent_img/6dedd4ce-d271-4b08-8f49-f5b5c4194c5a/HDA0001269169880000012.png)
![Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection](https://images-eureka.patsnap.com/patent_img/6dedd4ce-d271-4b08-8f49-f5b5c4194c5a/HDA0001269169880000021.png)