Kit for detecting amikacin content in blood and preparation method thereof
A technology of amikacin and a kit is applied in the field of latex-enhanced immune turbidimetric reagent and its preparation, which can solve the problems of troublesome sample processing, long time-consuming detection process, expensive detection equipment and the like, so as to improve detection sensitivity and enhance detection. Signal, the effect of good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
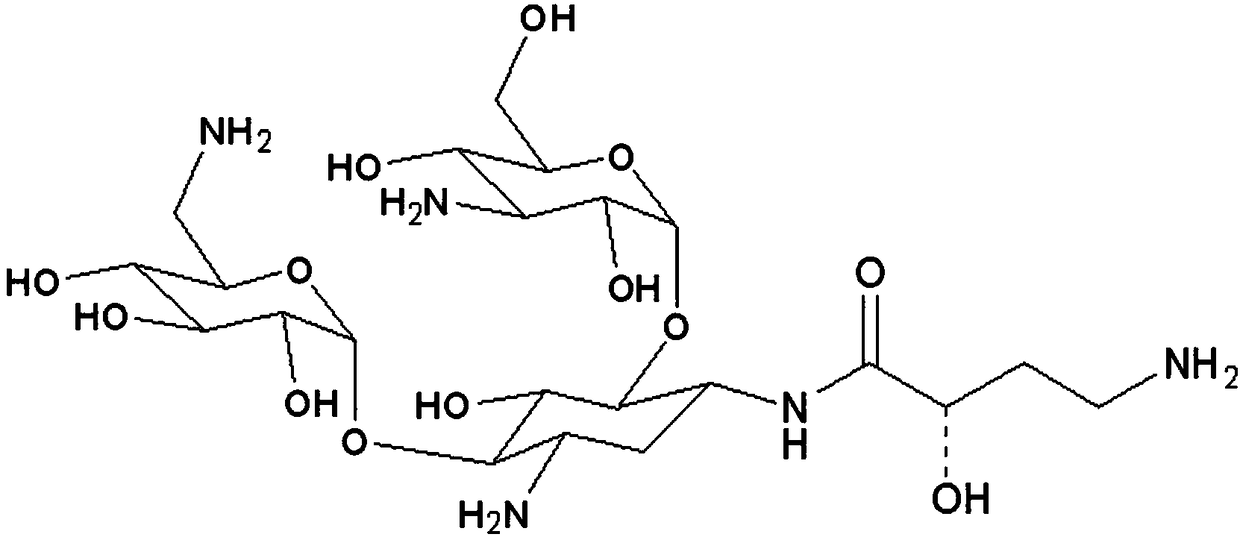
[0043] Example 1: Preparation of amikacin immunogen and anti-amikacin monoclonal antibody
[0044] Weigh 5.1 mg of amikacin and 55.0 mg of BSA and dissolve in 10 mL of PBS (phosphate buffer saline) (pH 7.2), stir well, add 20.0 mg of EDC dissolved in purified water dropwise, and stir at room temperature for reaction 6 Then the reaction solution was transferred to a dialysis bag, dialyzed in PBS (pH7.2) at 4°C for 4 days, centrifuged and then freeze-dried. Store at -20°C for later use. Referring to Yang Hanchun's "Animal Immunology" published in August 2003, Balb / C mice were immunized with amikacin-BSA (amikacin-bovine serum albumin) as an immunogen. According to the literature method (Zhu Liping, Chen Xueqing. Commonly used experimental methods in immunology. Beijing: People's Military Medical Publishing House, 2000), the monoclonal antibody was purified and obtained. The mouse Mab Isotyping Test Kit purchased from ROCKLAND Company was used to identify the subtype of the mon...
Embodiment 2
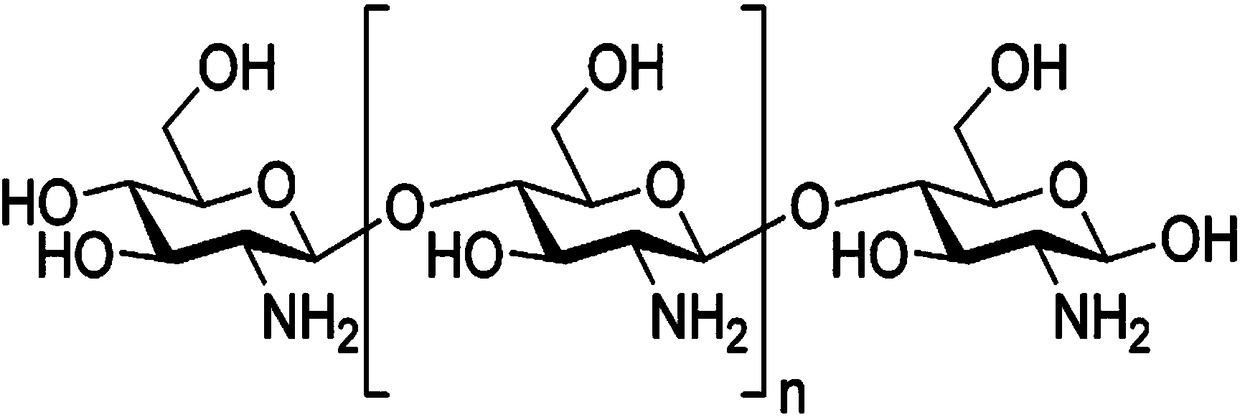
[0045] Embodiment 2: the preparation of chitosan and amikacin coupling conjugate
[0046] Dissolve 70mmol of chitosan in 10ml~30ml of water, then heat up the reaction system to 37~45°C; slowly add 70mmol of succinic acid dissolved in water dropwise, raise the temperature of the reaction system to 65°C, react for 4 hours, after the reaction, adjust the pH with to 7.0. Under the action of the activator EDC (carbodiimide), the above preparation is chemically coupled with the amikacin molecule to obtain the prepared conjugate;
Embodiment 3
[0047] Embodiment 3: the preparation of amikacin detection kit
[0048] 1. the kit of the present invention relates to the main raw material of reagent as follows:
[0049] anti-human amikacin antibody
[0050] Latex microspheres: use polystyrene latex particles with a diameter of 215nm and carboxyl groups (Agilent)
[0051] 2. the preparation of the main reagent of the present embodiment is as follows:
[0052] Reagent R1: 50mM PBS buffer, add 0.1% conjugate, 0.4% PEG6000 (polyethylene glycol 6000), 0.05% NaN 3 , 0.3% BSA, 0.8% NaCl, 0.2% Tween-20, pH 7.2, total preparation volume 1L, the reagent is a colorless or light yellow transparent solution.
[0053] Reagent R2: 50mM Tris buffer, latex microspheres coated with 0.2% amikacin antibody, 0.8% BSA, 0.9% NaCl, 0.05% NaN 3 , pH 7.4, the reagent is a milky white solution. Concrete preparation steps are as follows:
[0054] (1) 1.2g of 215nm latex microspheres, add 16ml of MES solution with pH 6.1, 2.5ml of freshly prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com