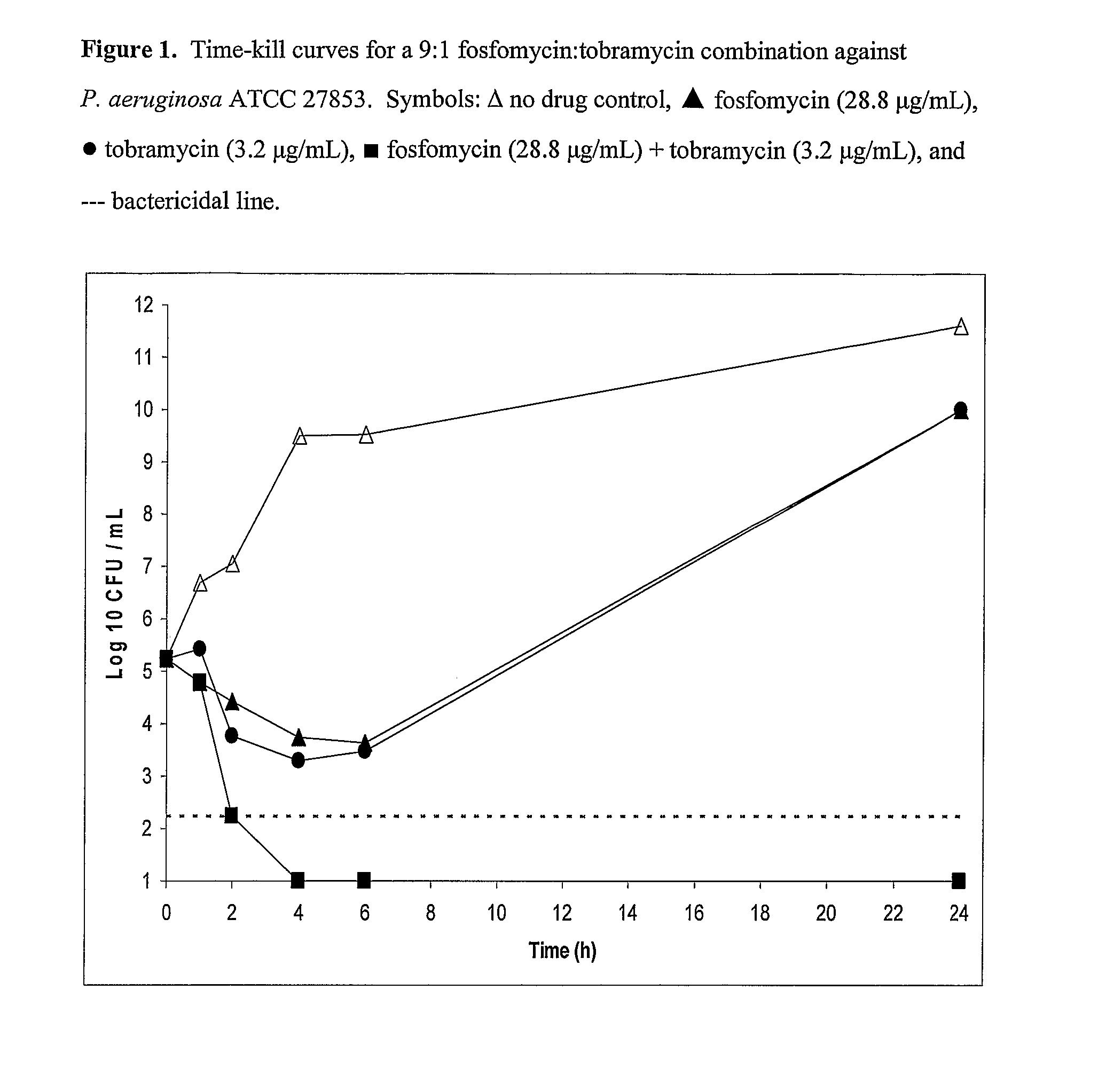
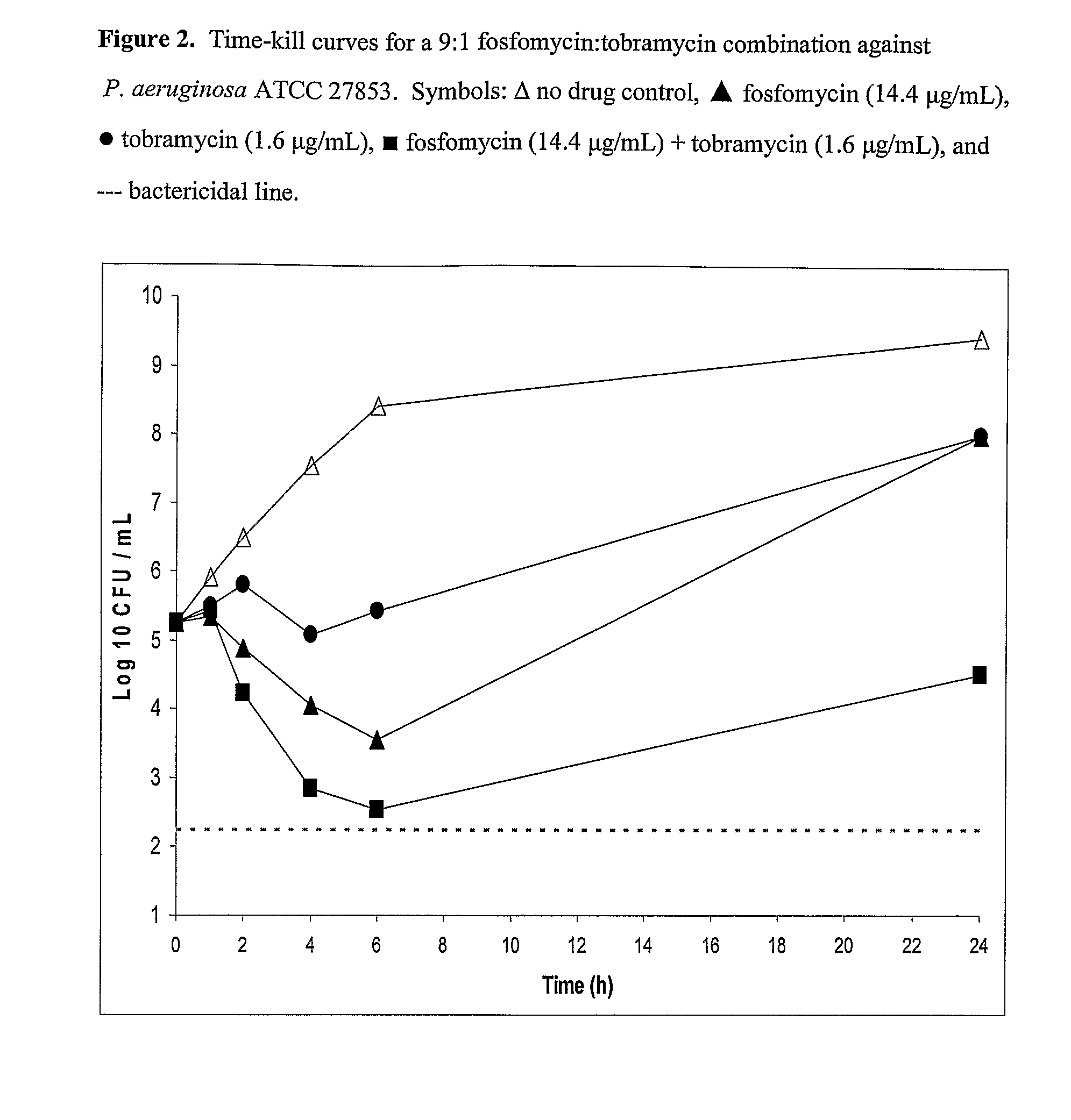
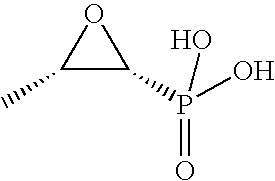
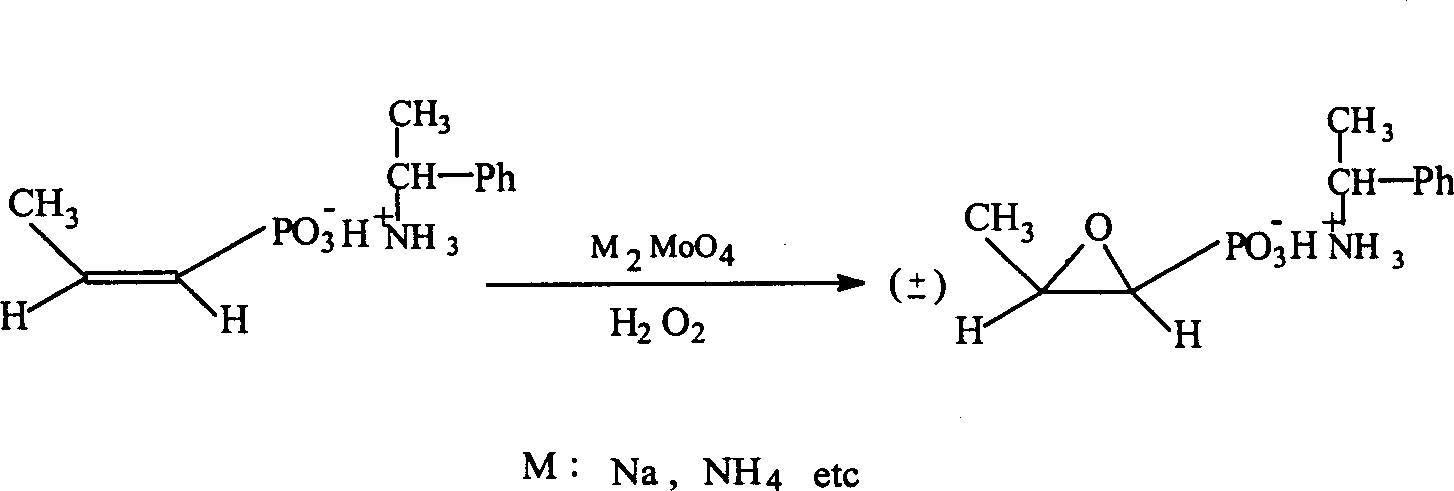
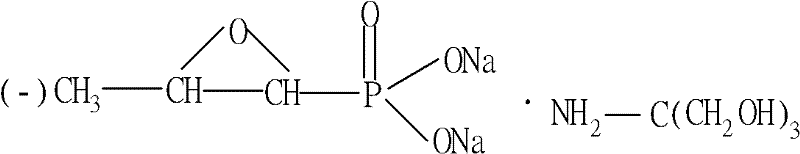
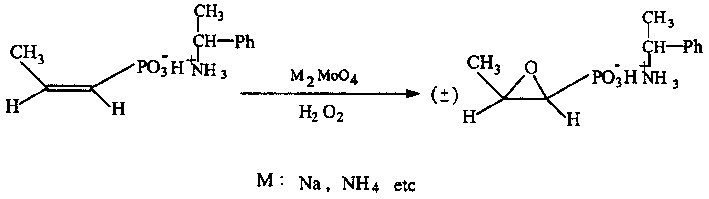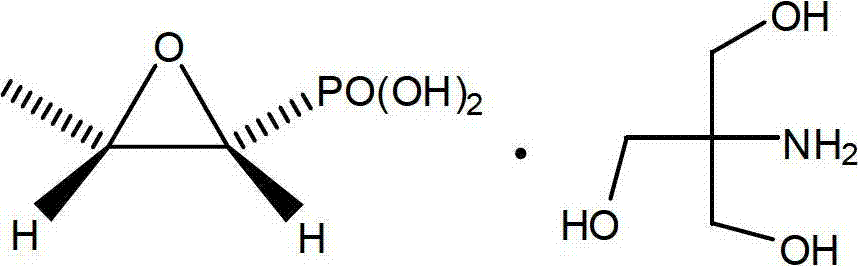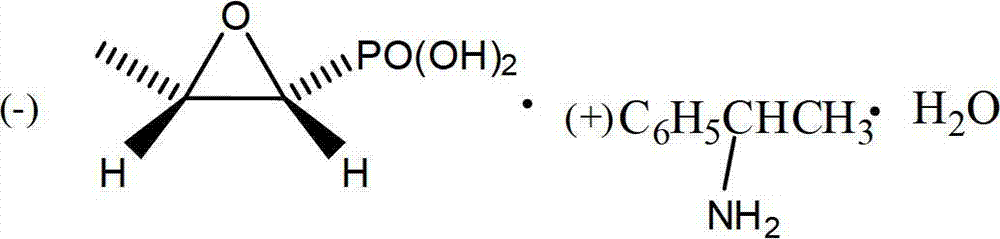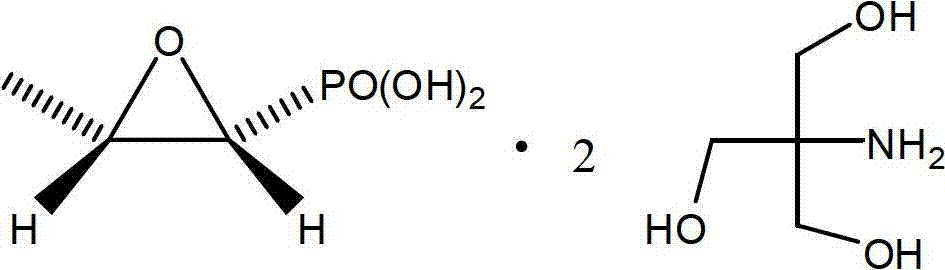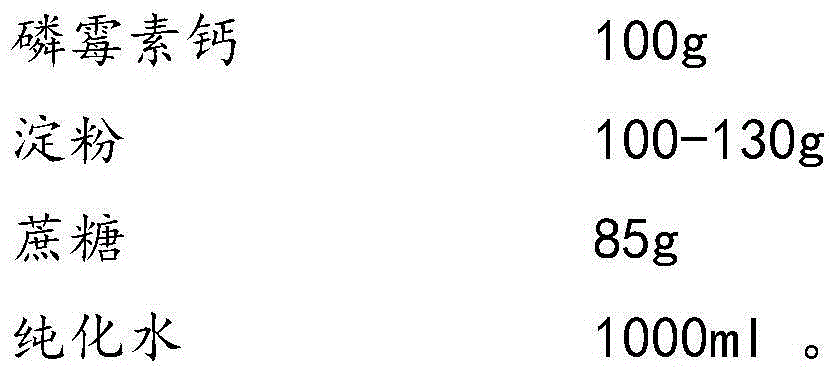Patents
Literature
98 results about "Fosfomycin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
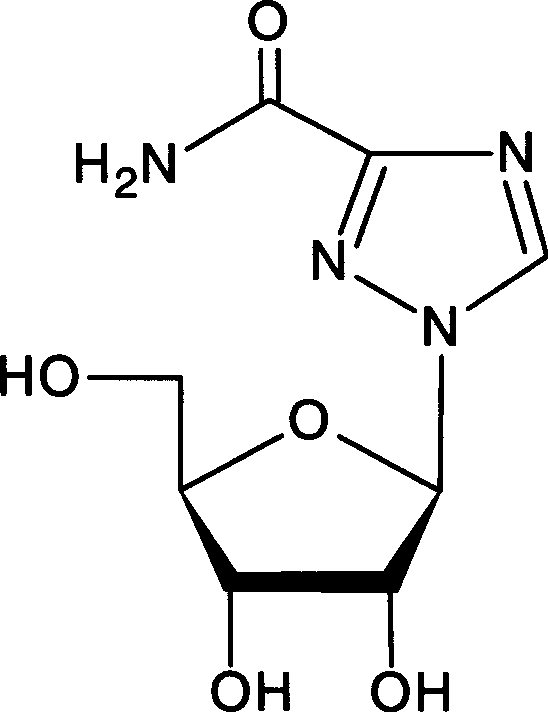
This medication is an antibiotic used to treat bladder infections (such as acute cystitis or lower urinary tract infections) in women.
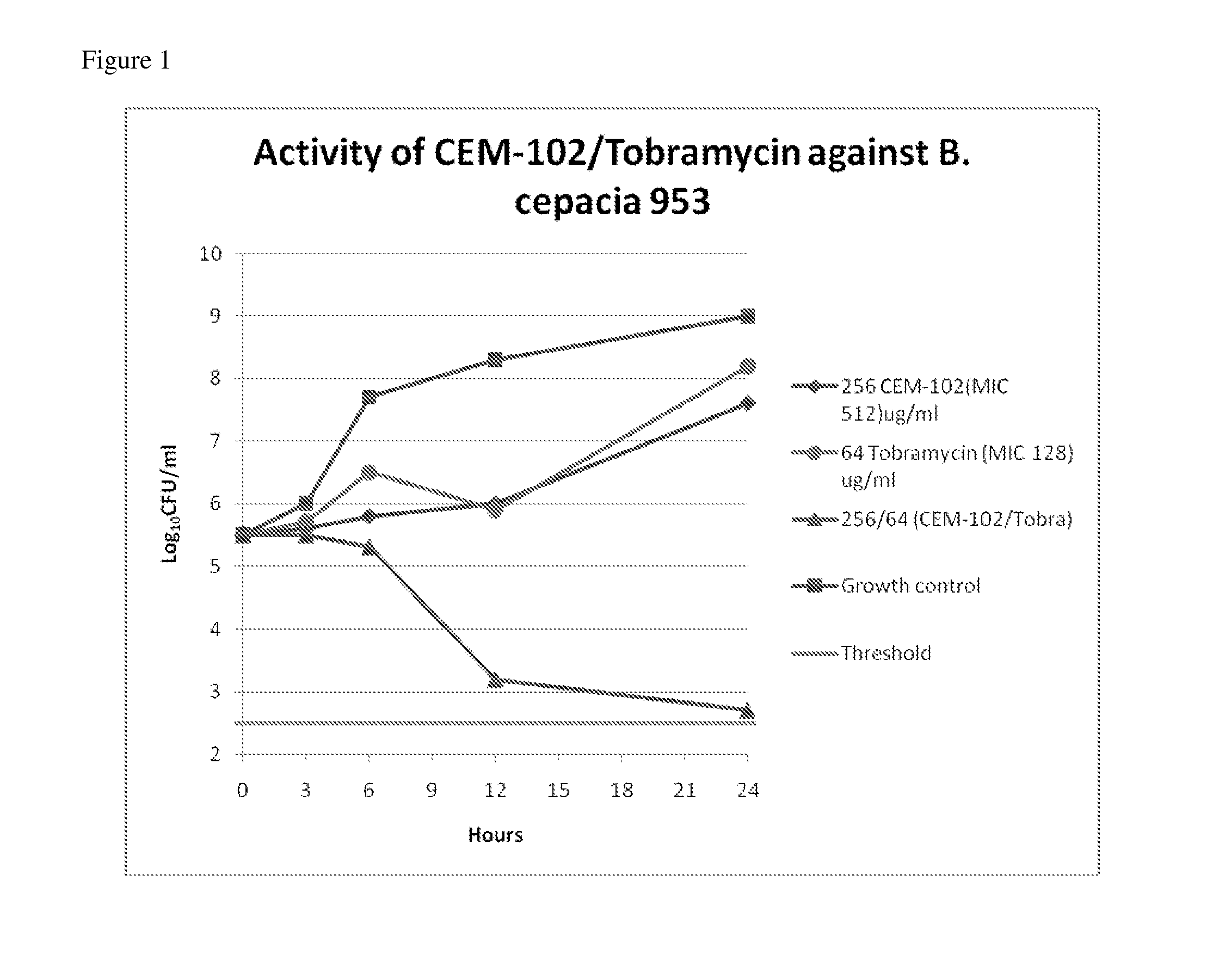
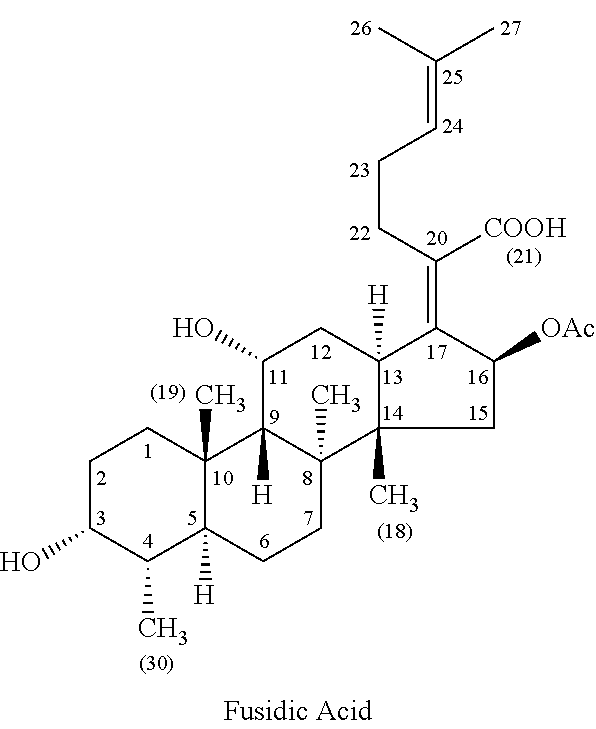
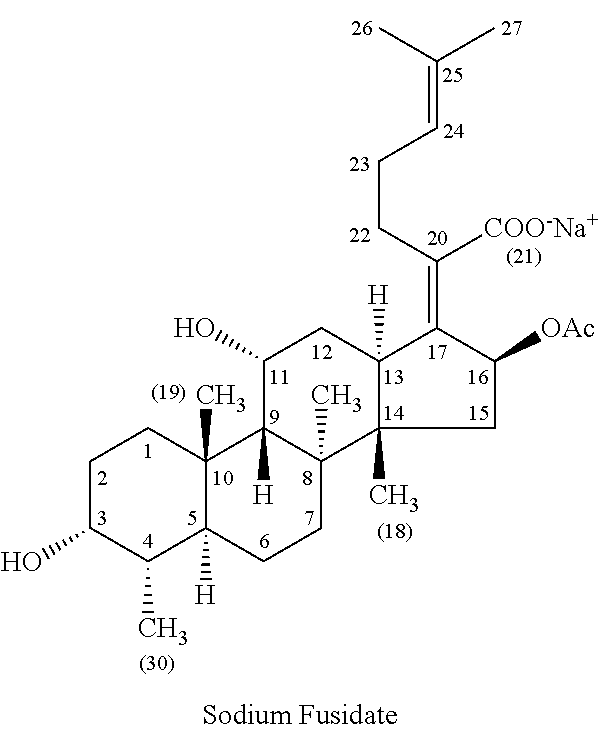
Methods of treating bacterial infections through pulmonary delivery of fusidic acid
Methods for the treatment of bacterial infections in the respiratory system of a subject, such as the lungs of a subject, using fusidic acid alone or in combination with a second bacterial agent such as tobramycin, amikacin, fosfomycin or levofloxacin are described.
Owner:CEMPRA PHARMA INC
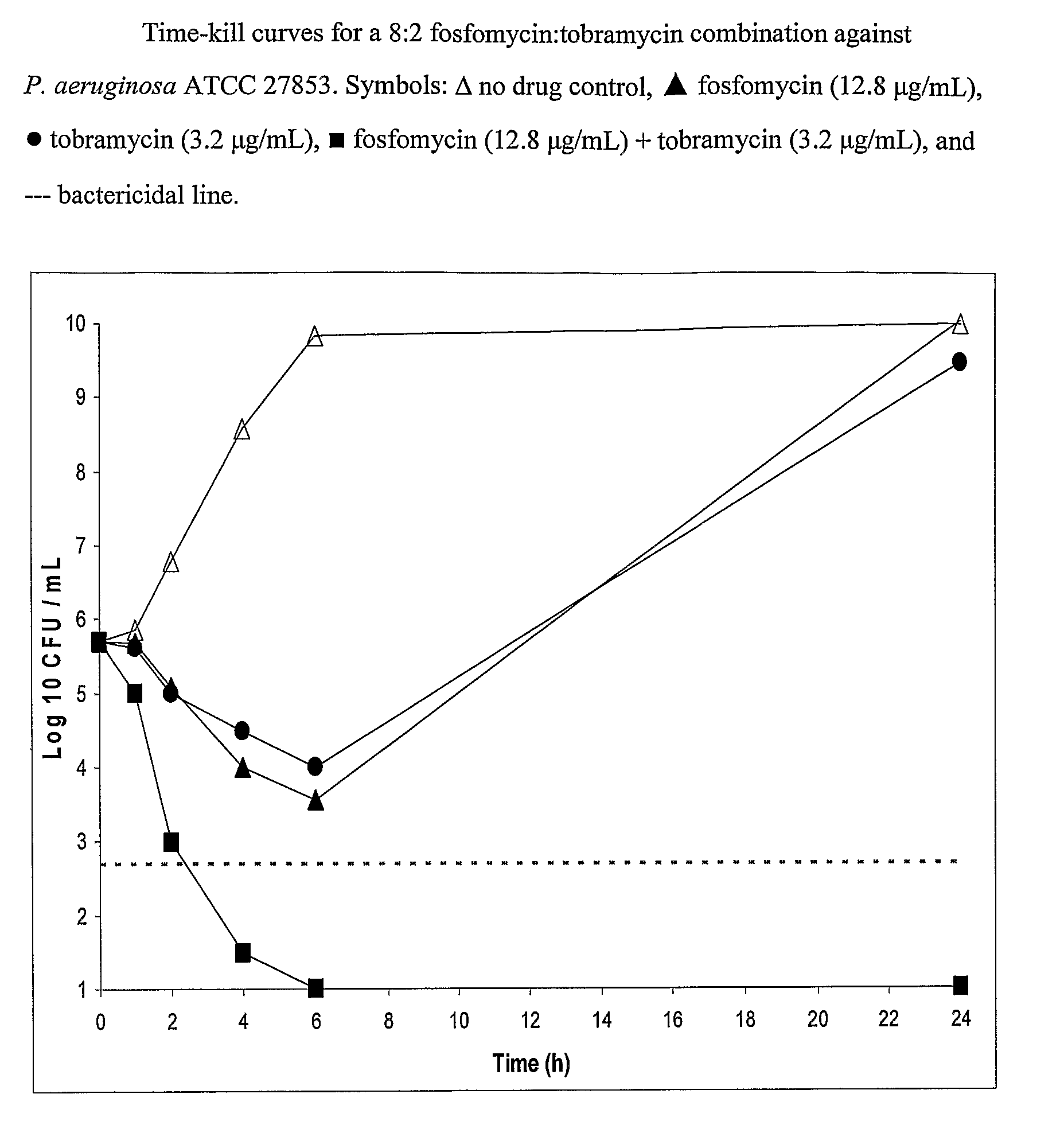
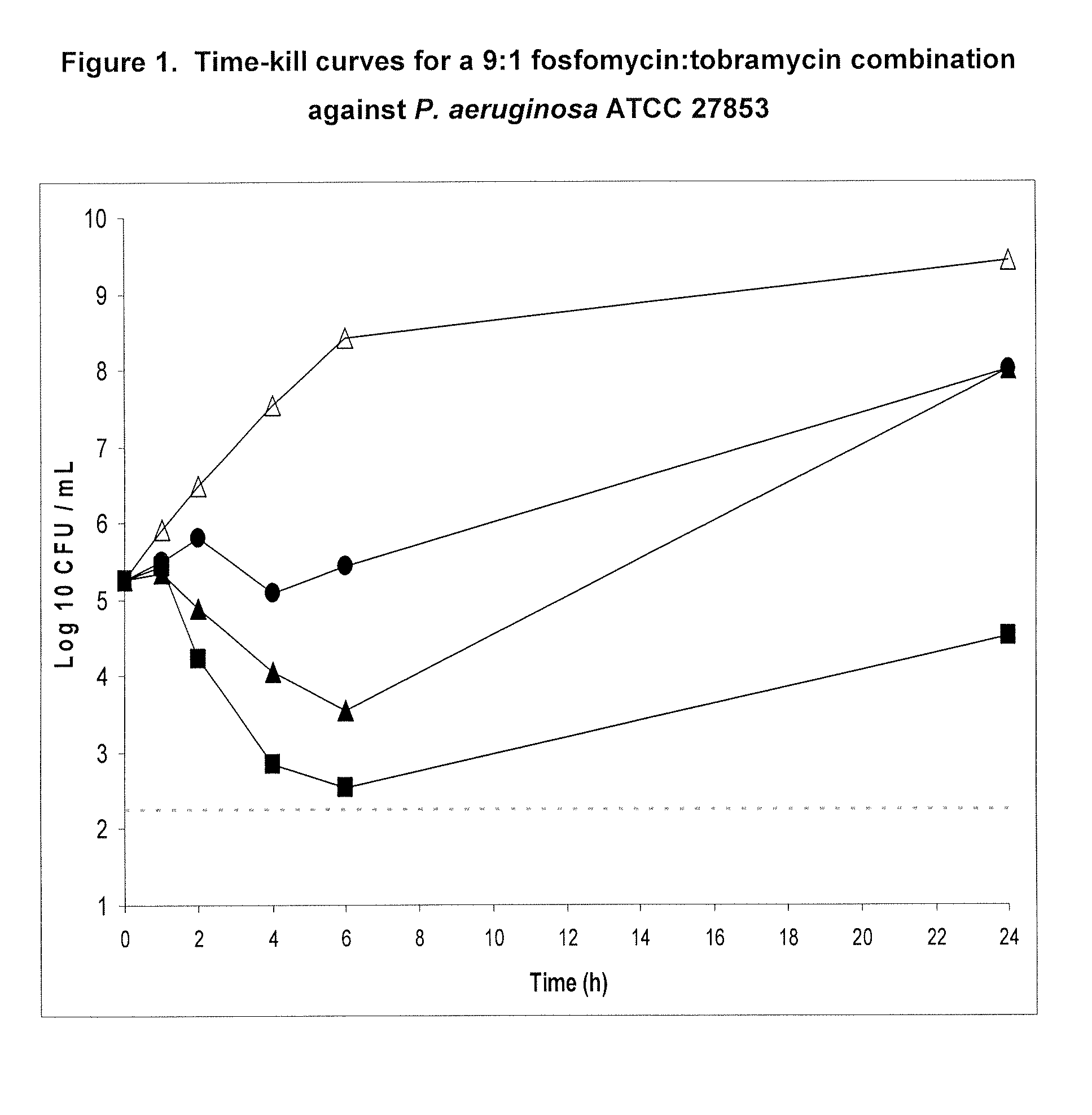
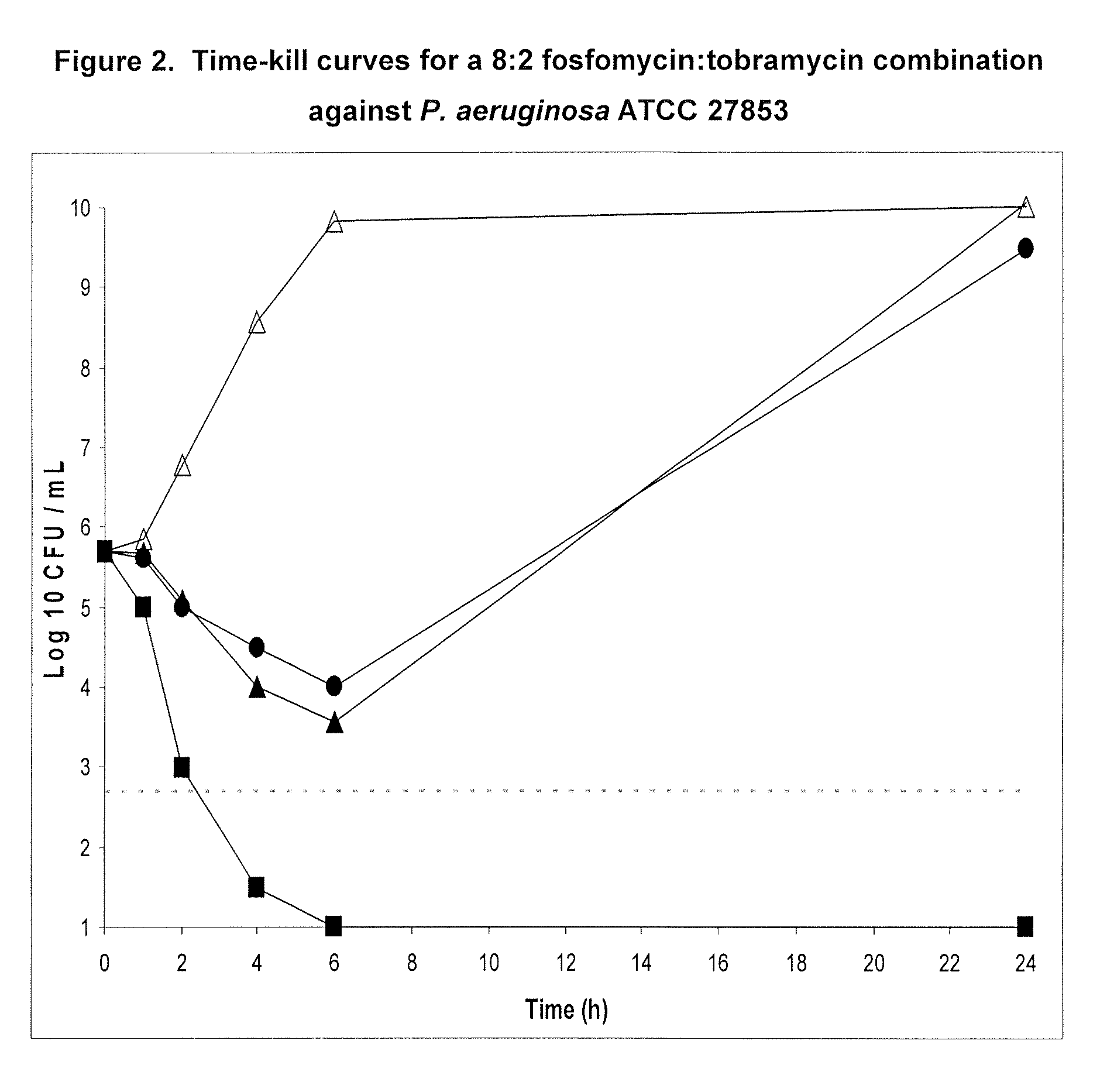
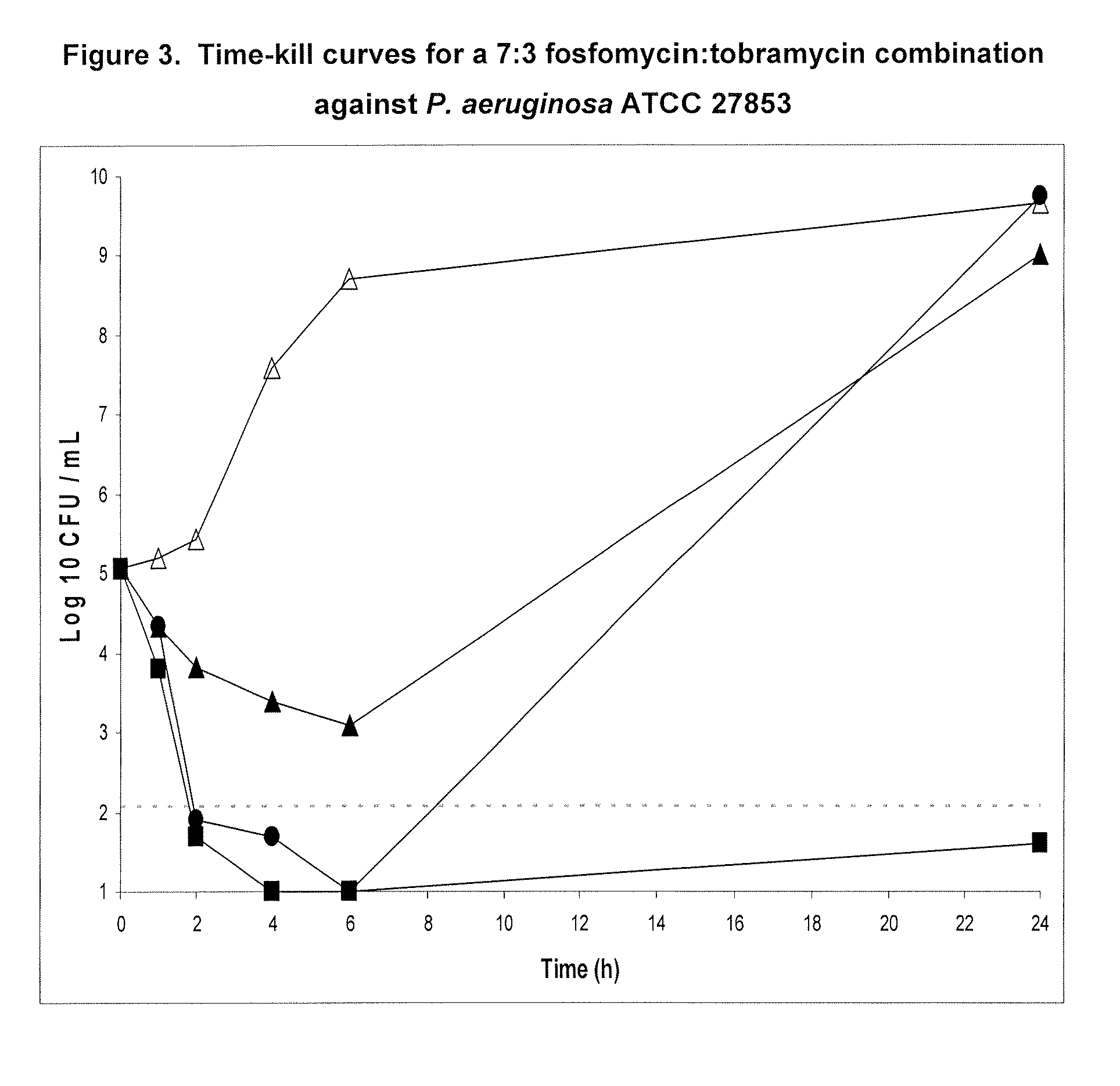
Aerosolized fosfomycin/aminoglycoside combination for the treatment of bacterial respiratory infections
InactiveUS7943118B2Reduce developmentIncreases the post antibiotic affect (PAE)Antibacterial agentsBiocideBacterial respiratory infectionTobramycin
Owner:GILEAD SCI INC
Methods for Treating Cystic Fibrosis or Pneumonia with Bacterial Infection via Pulmonary Administration of Fosfomycin
InactiveUS20100063005A1Minimizing systemic adverse reactionEffective treatmentAntibacterial agentsBiocideBacteroidesAdditive ingredient
The present invention provides methods for treating a bacterial infection and / or bacterial airway colonization in a subject by administering via pulmonary administration an effective amount of fosfomycin as the only active pharmaceutical ingredient. Methods of the present invention are useful in treating bacterial pneumonia infection of any type and / or airway colonization, cystic fibrosis with bacterial infection and / or bacterial lung and / or airway colonization.
Owner:HOIBERG
Process for synthesizing fosfomycin using cis-propenyl phosphonic acid as raw material
InactiveCN1385435AResidue reductionSmall toxicityGroup 5/15 element organic compoundsTungstateSolvent
The method for synthesizing phosphonomycin by using cis-propene phosphonic acid as raw material relates to an epoxidation eraction of cis-propene phosphonic acid by using molybdate which is sodium salt or ammonium salt generally as catalyst in the course of synthesizing phosphonomycin. According to identical process sand invention can prepare phosphonomycin disodium salt, in terminal phosphonomycin product the molbdenum content only is 1 / 10 (10-15 ppm) of tungsteon content, so that the use of molbdate as catalyst can reduce residual of heavy metal and can reduce toxic side effect of medicine.
Owner:TSINGHUA UNIV
Inhaled fosfomycin/tobramycin for the treatment of chronic obstructive pulmonary disease
InactiveUS20110124589A1Reduce frequencyReduce severityAntibacterial agentsPowder deliveryTobramycinObstructive Pulmonary Diseases
The present invention provides the use of an aerosol formulation comprising fosfomycin and tobramycin in the treatment of patients with chronic obstructive pulmonary disease (COPD) who are experiencing or are at risk of experiencing acute exacerbations of COPD. Formulations for such use and methods of treating humans with COPD are also provided.
Owner:GILEAD SCI INC
Preparation method of fosfomycin monoamine butantriol
InactiveCN102351902AImprove responseHigh product contentGroup 5/15 element organic compoundsOrganic solventMedicinal chemistry
The invention provides a preparation method of fosfomycin monoamine butantriol. The method comprises the following steps of: adding fosfomycin diamine butantriol salt into methanol, adding acid to adjust the pH to be between 4 and 7 at the temperature of between 5 DEG C below zero and 30 DEG C, filtering, removing filter residue, adding an organic solvent into the filtrate to separate out crystal, filtering, drying, and thus obtaining a fosfomycin monoamine butantriol finished product. The method is simple in reaction, the yield can reach 85 to 90 percent, the product content (the detection content can reach over 99 percent) is high, and the method is suitable for industrialized production and has great application value.
Owner:SHANXI C&Y PHARMACEUTICAL GROUP CO LTD
Preparation method of fosfomycin amine salt
ActiveCN102807586ALow costReduce energy consumptionGroup 5/15 element organic compoundsOrganic solventIon exchange
The invention discloses a preparation method of fosfomycin amine salt, which comprises the following steps of: in organic solvent, enabling fosfomycin phenylethylamine salt to react with isocyanate, isothiocyanate, ketene, dipolymer of isocyanate, dipolymer of isothiocyanate or dipolymer of ketene, and tromethamine; or in the organic solvent, enabling fosfomycin bistromethamine to react with isocyanate, isothiocyanate, ketene, dipolymer of isocyanate, dipolymer of isothiocyanate or dipolymer of ketene. The preparation method provided by the invention has the advantages that the fosfomycin amine salt can be prepared in one step only, a low-temperature ion exchange column is not required, the yield is high, the cost is low, the waste gas, waste water and waste residue are less, the reaction conditions are moderate, the energy consumption is low, the operation is convenient to conduct and the method is suitable for industrial production.
Owner:NORTHEAST PHARMA GRP
Compositions to promote the healing of skin ulcers and wounds
InactiveUS20160346354A1Promote defense mechanismPromote wound healingPeptide/protein ingredientsHydroxy compound active ingredientsSkin ulcerationsGranulocyte macrophage colony-stimulating factor
The present invention provides compositions comprising as an essential feature granulocyte-macrophage colony-stimulating factor (GM-CSF) together with fosfomycin for the treatment of wounds, ulcers, sores, burns and other injuries to the skin or mucous membranes of the body.
Owner:REPONEX PHARMA APS
Fosfomycin calcium composition freeze-dried orally disintegrating tablets and preparation method thereof
InactiveCN102784118ADisintegrates quicklyPromote dissolutionAntibacterial agentsOrganic active ingredientsFreeze-dryingOrally disintegrating tablet
The invention discloses fosfomycin calcium composition freeze-dried orally disintegrating tablets and a preparation method of the orally disintegrating tablets, relates to the field of a medicine and a medicine preparation method technology and mainly solves the problems in the prior art that the oral preparation made from fosfomycin calcium is poor in administration compliance for children and calcium salt orally taking absorption rate. The fosfomycin calcium composition freeze-drying orally disintegrating tablets are prepared from the following components by weight: 10-24% of fosfomycin calcium, 24-50% of mannitol, 2-4% of gelatin, 32-74% of hydroxypropyl-beta-cyclodextrin with medium substitution degree, 0.1-0.2% of trichlorosucrose and 50% of purified water. The fosfomycin calcium composition freeze-drying orally disintegrating tablets obtained by the above components are easy in preparation process, and simple in components, and mainly aim at the administration requirements of the children; moreover, the fosfomycin calcium composition freeze-drying orally disintegrating tablets have the advantages of being fast to disintegrate, convenient to take and good in taste; and the tablets take effect quickly and are fully absorbed, and the first pass effect of the liver is avoided.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Immobilized tungstophosphoric heteropoly acid catalyst and use thereof
ActiveCN105618138AEasy to recycleSolution to short lifeOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsSynthesis methodsHeteropoly acid
The invention relates to a synthesis method of an immobilized tungstophosphoric heteropoly acid catalyst and a use of the immobilized tungstophosphoric heteropoly acid catalyst in cis-propenylphosphonic acid epoxidation. The synthesis method of the immobilized tungstophosphoric heteropoly acid catalyst comprises 1, synthesizing low-carbon chain phosphotungstic heteropoly acid quaternary ammonium salt and 2, loading the phosphotungstic heteropoly acid quaternary ammonium salt on resin to obtain the immobilized tungstophosphoric heteropoly acid catalyst. The catalyst is prepared from easily available raw materials, effectively catalyzes cis-propenylphosphonic acid epoxidation to synthesize fosfomycin phenylethylamine salt, is convenient for recovery, has a long service life and can be recycled 5 times. The catalyst can reduce a fosfomycin phenylethylamine salt production cost and satisfies the current green chemical and atom economic requirements.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
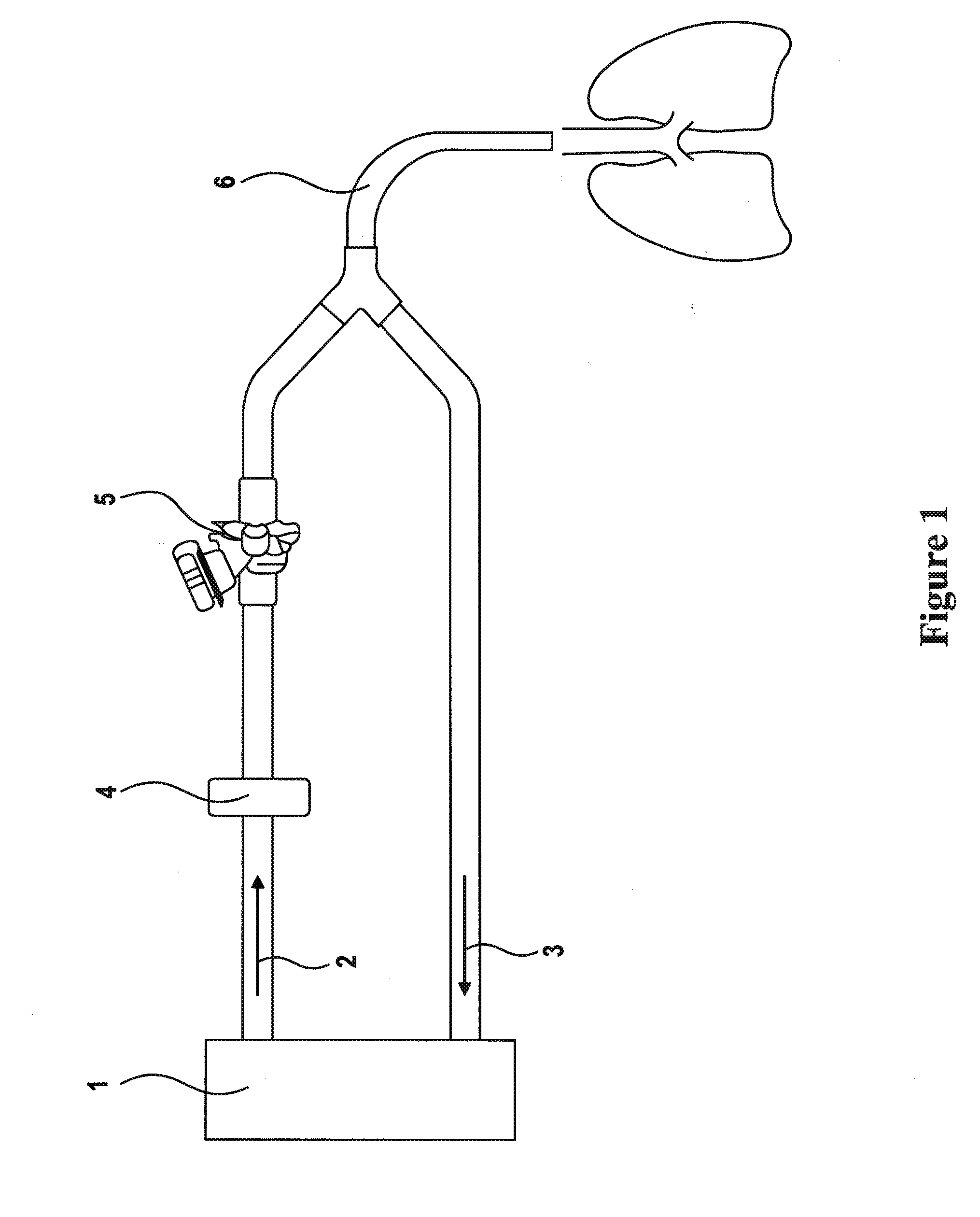
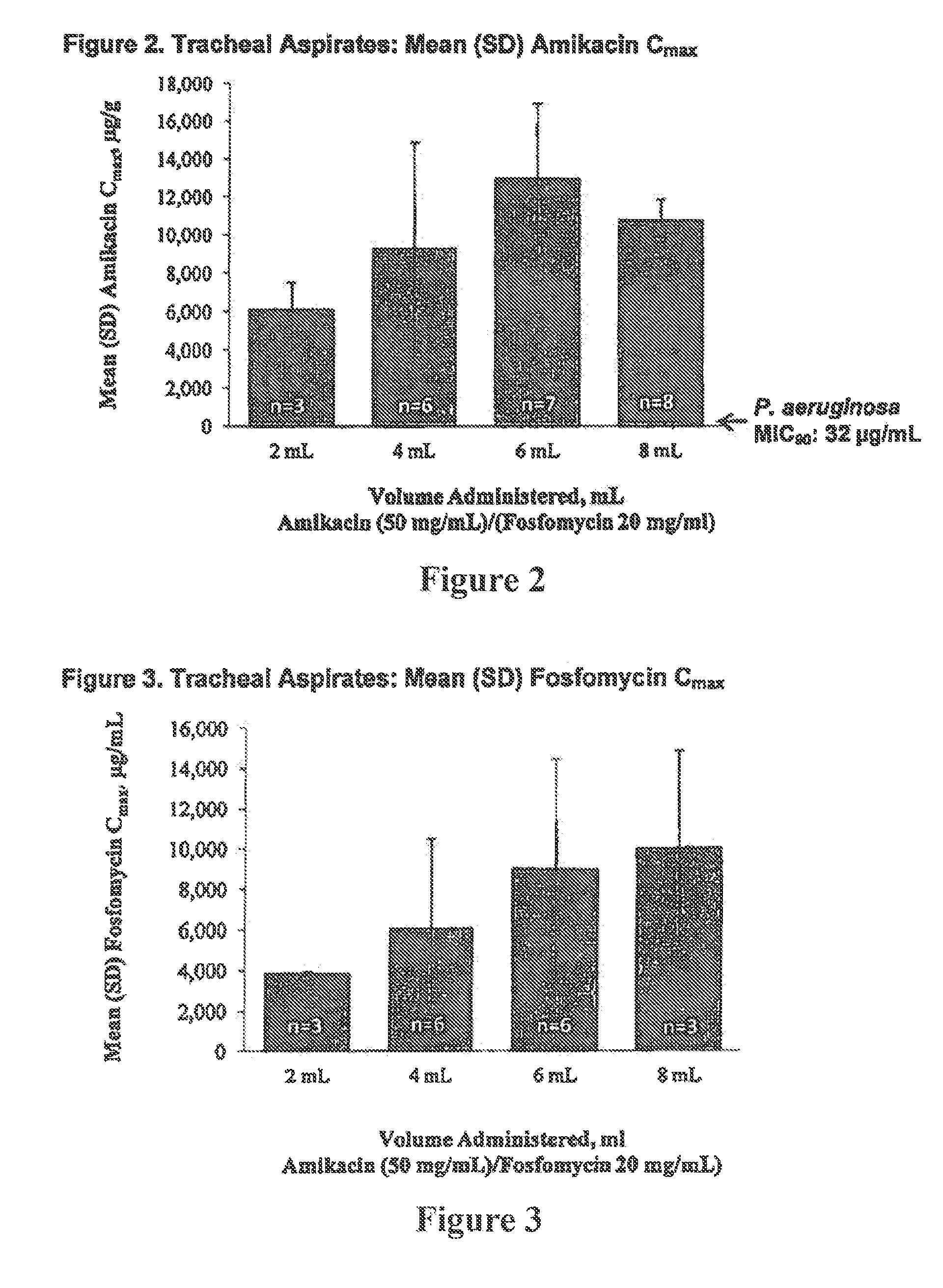
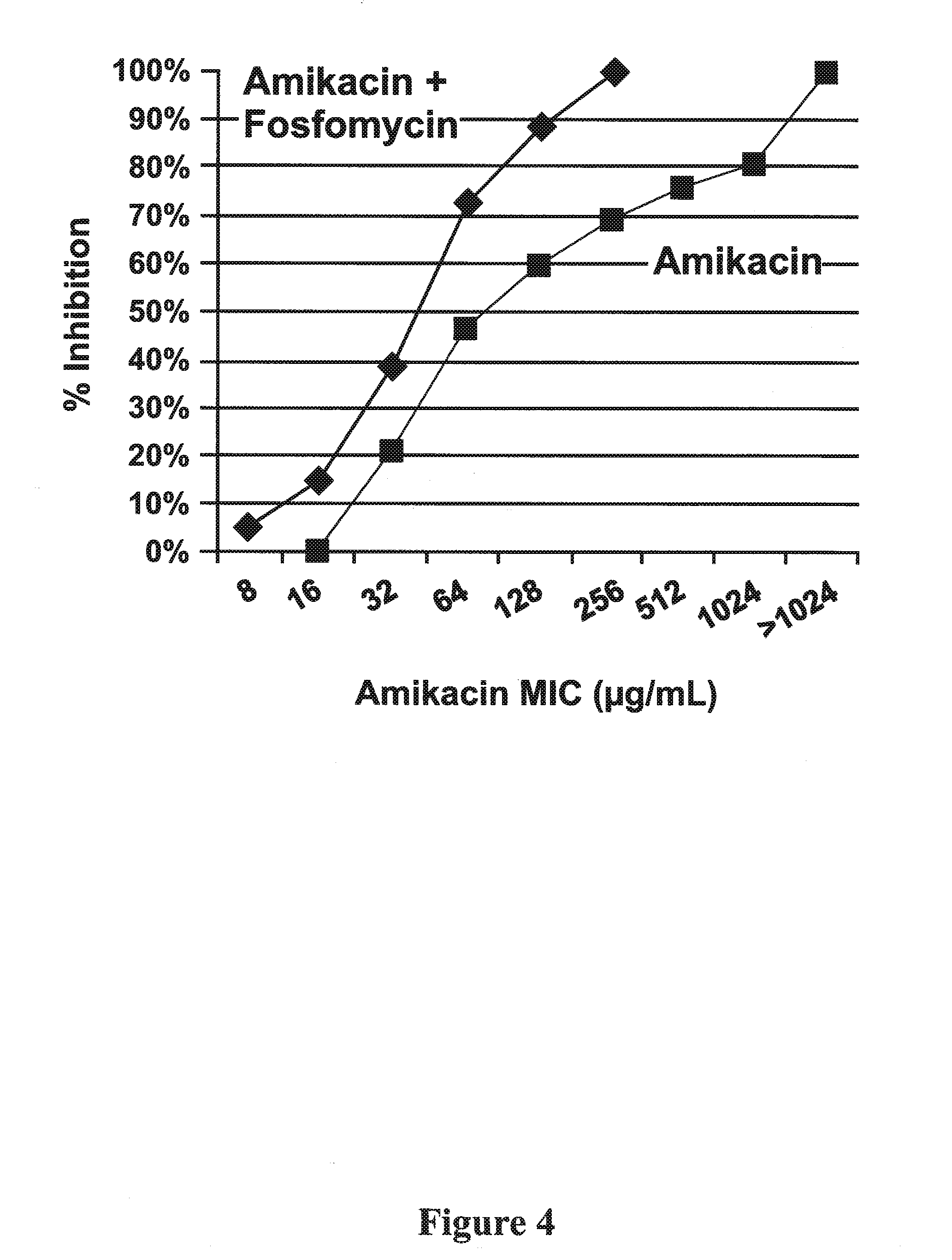
Formulations of aminoglycoside and fosfomycin combinations and methods and systems for treatment of ventilator associated pneumonia (VAP) and ventilator associated tracheal (VAT) bronchitis
InactiveUS20150057242A1Reduction in osmolalityReduce loadRespiratorsBiocidePhoslactomycin EAerosol Mist
The present invention is antibiotic compositions, ventilator-based systems and methods relating to ventilator-associated pneumonia (VAP) and ventilator-associated tracheal (VAT) bronchitis. Antibiotic combinations of fosfomycin and an aminoglycoside, preferably amikacin, are administered via an inline nebulizer within the airway of the ventilator. Humidified conditions create an improved aerosol mist to treat VAP and VAT.
Owner:SAVARA
Acalypha australis L. and fosfomycin containing compound medicine for livestock and poultry
InactiveCN103989726AReverse drug resistanceGood treatment effectAntibacterial agentsOrganic active ingredientsBacteroidesTreatment effect
Owner:GUANGXI UNIV
Method for conversion from right-handed phosphonomycin to left-handed phosphonomycin
ActiveCN101153302ABiocatalysis possibleGet reusedBacteriaMicroorganism based processesCatalytic methodRotation velocity
The invention relates to the biological medicine and biological catalytic field, in particular to a biological catalytic method for transforming the D-fosfomycin into the L-fosfomycin through a biological catalytic process, aiming to solve the puzzle to transform the D-fosfomycin into the L-fosfomycin. The strain with catalytic ability is proliferated, centrifuged, harvested and inoculated into a cultivation substrate, which comprises the components by weight percentage of: 0.5-3 percent of D-fosfomycin, 0.5-1.0 percent of (NH4)2SO4, 0.2 percent of NaCl, 0.1 percent of KCl, 0.01-0.02 percet of MgSO4.7H2O, 0.01-0.03 percent of FeSO4.7H2O,0.05-0.15 percent of ,KH2PO4; the left volume is water, and the pH is 7.5. The sterilization is carried out at 121 DEG C for 20 minutes, and cultivation is made for 3-7 days under the temperature of 28-37 DEG C, and the rotation velocity of 150-250 rpm of the rocking bed. The qualitative and quantitative analysis on the L-fosfomycin in the fermentation liquid is carried out respectively through thin-layer chromatography and the method of bioassay disc. The invention provides a novel catalytic method for transforming the D-fosfomycin into the L-fosfomycin, and accomplishes the operable bio-catalysis of the D-fosfomycin.
Owner:SHENYANG INST OF APPL ECOLOGY CHINESE ACAD OF SCI +1
Aerosolized Fosfomycin/Aminoglycoside Combination for the Treatment of Bacterial Respiratory Infections
InactiveUS20110117030A1Reduce developmentIncreases the post antibiotic affect (PAE)Antibacterial agentsBiocideBacterial respiratory infectionTobramycin
A fosfomycin plus tobramycin combination formulation for delivery by aerosolization is described. The concentrated fosfomycin tobramycin combination formulation containing an efficacious amount of fosfomycin plus tobramycin is able to inhibit susceptible bacteria. Fosfomycin and tobramycin are formulated separately in a dual ampoule such that when reconstituted, the pH is between 4.5 and 8.0 or as a dry powder. The method for treatment of respiratory tract infections by a formulation delivered as an aerosol having mass median aerodynamic diameter predominantly between 1 to 5μ, produced by a jet or ultrasonic nebulizer (or equivalent) or dry powder inhaler.
Owner:GILEAD SCI INC
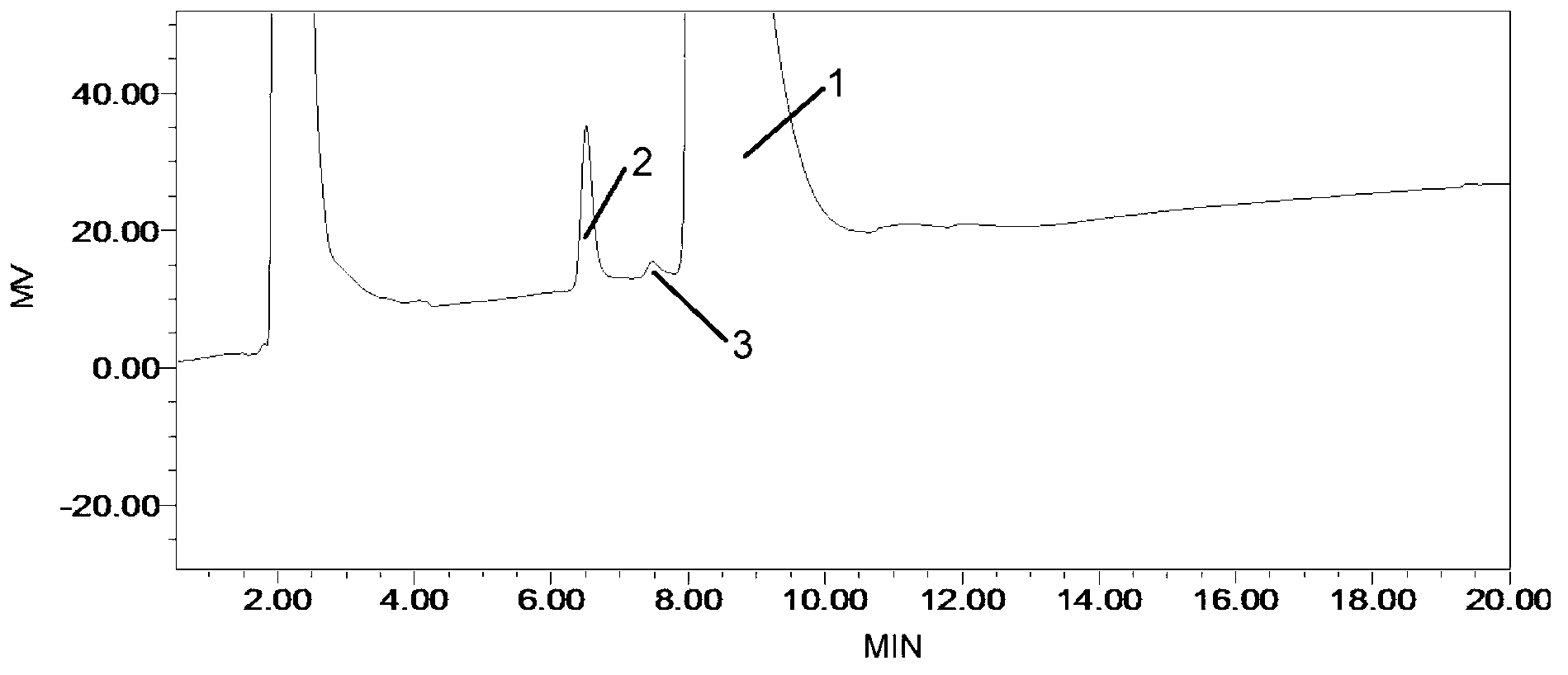
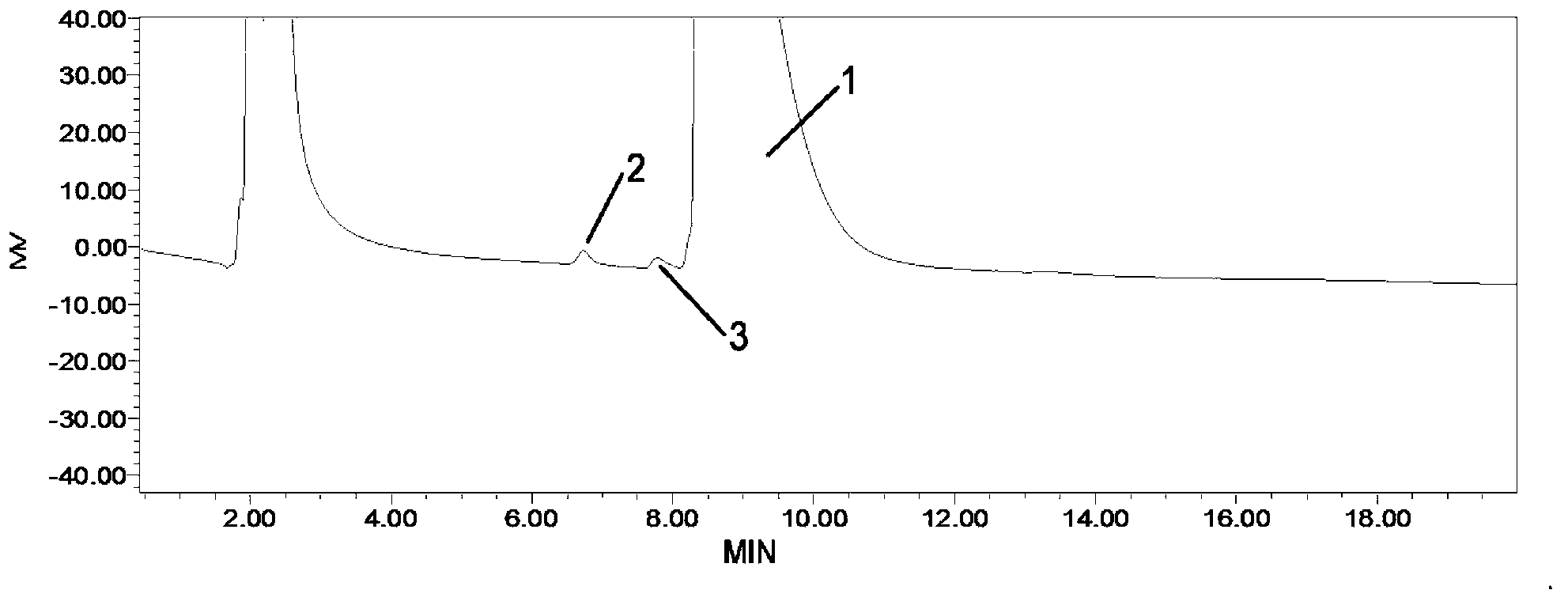
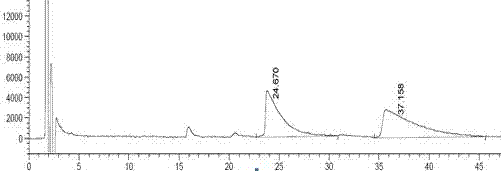
Method for detecting related substances in fosfomycin sodium by high-performance liquid chromatography
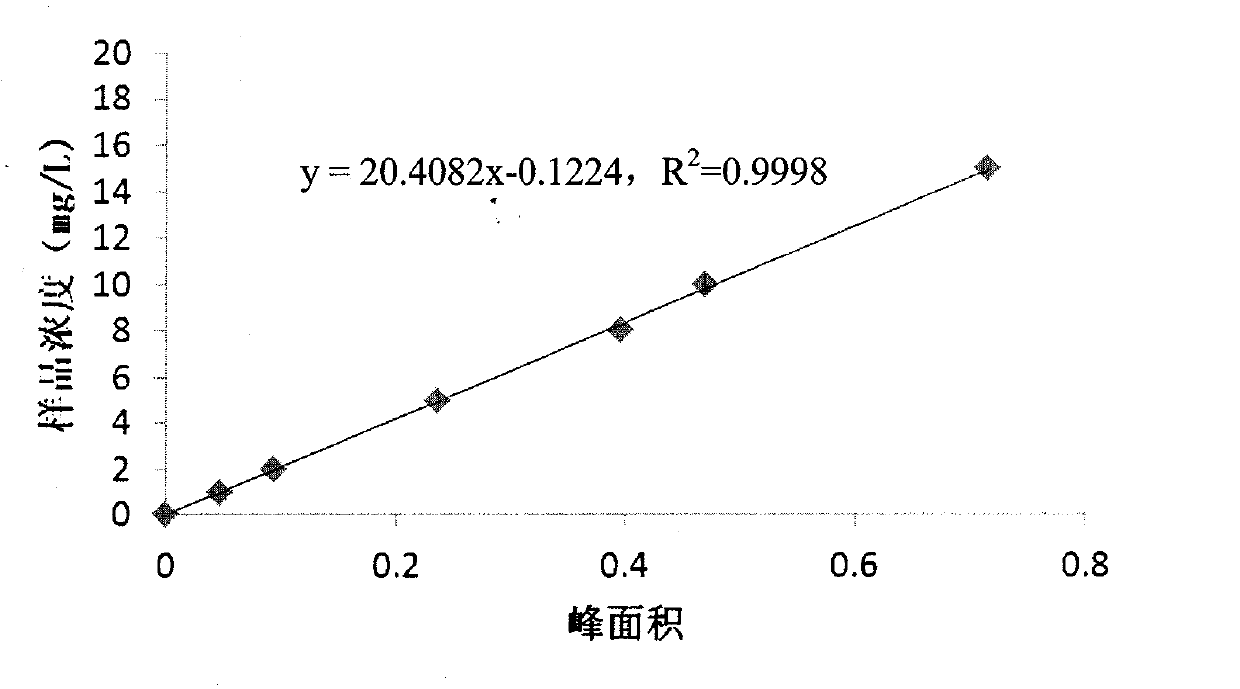
ActiveCN102841155AGood reproducibilityWide variety of sourcesComponent separationRelative standard deviationPeak area
The invention discloses a method for detecting related substances in fosfomycin sodium by a high-performance liquid chromatography applied to the field of substance detection of a compound. The method comprises the following steps: taking a reference solution 1 to inject into a high performance liquid chromatograph; recording a spectrogram, and computing the separation degree of a fosfomycin peak and an impurity A peak, wherein the separation degree is greater than or equal to 1.5; taking the reference solution, sampling in parallel, and injecting into the high performance liquid chromatograph; recording the spectrogram, and computing relative standard deviation of the fosfomycin peak area, wherein the standard deviation is smaller than or equal to 0.85%; taking a test solution and the reference solution 2 to respectively inject into the high performance liquid chromatograph; recording the spectrogram; and computing the peak area of the impurities in the test solution. The method is good in reproducibility of related substance detection in the fosfomycin sodium, high in sensitivity, strong in specificity, accurate in detection, simple to operate, mild in detection condition, wide in application of detection device and apparatus, wide in source of a mobile phase orthophosphate aqueous solution and simple to prepare.
Owner:NORTHEAST PHARMA GRP
Ribavirin soluble powder
InactiveCN101390835AGood curative effectStable storagePowder deliveryOrganic active ingredientsSolubilityAdditive ingredient
The invention relates ribavirin soluble powder and the preparation method thereof. Ribavirin, and fosfomycin sodium are used as main ingredients, together with proper amount of perfume, soluble flavoring agent and soluble filler; the ribavirin soluble powder is prepared through the following conventional methods, drying, weighing, crushing, stirring, homogenized mixing, inspection, measuring and packing. The ribavirin soluble powder has the advantages of simple application, low cost, complete aroma, stable storage, convenient transportation and good water solubility; the ribavirin soluble powder can be prepared into drinking agent to improve the treatment effect of ribavirin for the non-food animals so that the ribavirin soluble powder is special non-food animal powder which is novel, broad-spectrum and efficient.
Owner:上海恒丰强生物技术有限公司
Levo-phosphonomycin biotransformation strain screening method using dextro-phosphonomycin as substrate
ActiveCN1876830AImprove efficiencyEasy to operateMicrobiological testing/measurementMicroorganismScreening method
The invention relates to biological transformation / biocatalytic technology microbial bacterial strain sieving domain, especially to a sieving policy and method for left-handed fosfomycin biological converting bacterial strain with right-handed fosfomycin as substrate. The said sieving policy and specific method of operation consist of two big steps including bacterial strain culture and conversion product identification in screening process, in which the essential point includes preliminary enrichement of laboratory bacterial and product calibration policy which combines biological calibration with thin-layered chromatography (TLC). Because the bacterial which can be changed from right-handed fosfomycin to left-handed fosfomycin in nature is rare and the lab available bacterial grows up slowly, the preliminary enrichement of laboratory bacterial is very important. Moreover, adopting product calibration policy which combines biological calibration with thin-layered chromatography (TLC), whether there is left-handed fosfomycin in product can be exactly determined.
Owner:SHENYANG INST OF APPLIED ECOLOGY - CHINESE ACAD OF SCI +1
Antimicrobial and Anti-inflammatory action pharmaceutical composition for parenteral administration and its production process
InactiveUS20130164336A1Improve effectivenessQuality improvementAntibacterial agentsPowder deliveryDiseaseParenteral nutrition
There is proposed herein a process for production of composite antimicrobial preparations for parenteral administration, featuring a higher therapeutic efficiency in case of grave infection and inflammatory diseases. The proposed compositions include an active agent being fosfomycin and finely dispersed nanostructured silica dioxide, with a weight ratio from 10:1 to 75:1 respectively. The mentioned production process includes mixing fosfomycin with finely dispersed nanostructured silica dioxide. Its main difference is that the mixture of aforementioned substances with the mentioned weight ratio is exposed to mechanical processing by blow impact and abrasive actions until a portion of the fine powder fraction with particles smaller than 5 micrometers, contained in the mixture, increases to at least 25%. The so obtained mixture is used for injection preparations.
Owner:LIMONOV VIKTOR LVOVICH +2
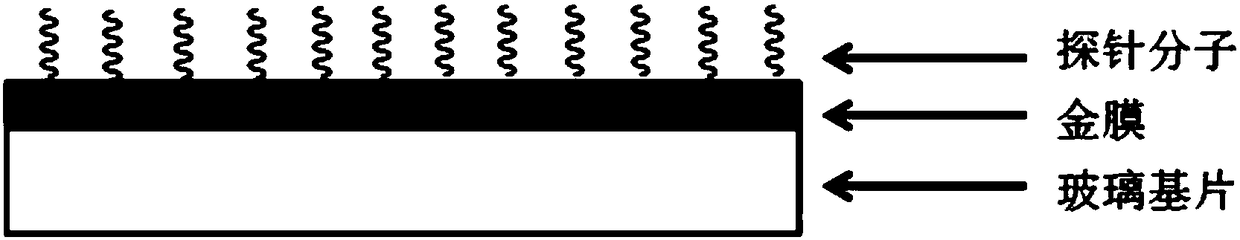
Surface plasmon resonance sensor chip for detecting gram-negative bacteria and preparation method and application thereof
ActiveCN108169182AHigh sensitivityGood choiceMaterial analysis by optical meansMicronomicinSurface plasmonic resonance
The invention discloses a surface plasmon resonance sensor chip for detecting gram-negative bacteria and a preparation method and application thereof. The chip comprises a glass substrate layer, a gold film layer and a probe molecular layer. The gold film layer is disposed on the glass substrate layer. The probe molecular layer is disposed on the gold film layer. A probe molecule of the probe molecule layer contains at least one of drug molecules of streptomycin sulfate, kanamycin sulfate, gentamicin sulfate, gentamycin-micronomicin, vistamycin sulfate, neomycin, polymyxin B, rifampicin, chloramphenicol and fosfomycin. The invention also discloses a preparation method and application of the chip. The surface plasmon resonance sensor chip creatively realizes detection of gram-negative bacteria based on surface plasmon resonance, has high sensitivity and good selectivity, has a gram-negative bacterium concentration detection linear range of 10-10<6> CFU / mL and can be used for detecting gram-negative bacteria in solutions such as detection water, drinking water or human body fluids.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Drying method of fosfomycin calcium
ActiveCN106949710ADrying solid materials without heatPreliminary solid treatment for dryingCalcium crystalsDrying time
A drying method of fosfomycin calcium comprises the following steps: step 1, crushing a fosfomycin calcium monohydrate wet product; step 2, feeding the crushed fosfomycin calcium monohydrate wet product into a double-cone dryer, and then controlling a vacuum degree in the double-cone dryer to be 20-30Kpa; step 3, controlling the vacuum degree in the double-cone dryer to be 20-30Kpa and temperature to be 40-50DEG C, enabling the double-cone dryer to rotate at intervals, and carrying out drying for 16h; step 4, controlling the vacuum degree in the double-cone dryer to be smaller than or equal to 30Kpa, temperature to be 55-65DEG C and drying time to be 8-16h so as to enable fosfomycin calcium crystals to form lumps with 2-5cm diameters; and step 5, controlling the vacuum degree in the double-cone dryer to be smaller than or equal to 5Kpa, temperature to be 55-65DEG C and drying time to be longer than or equal to 8h so as to enable the water content of the dried fosfomycin calcium crystals to be 8.7-12.0%. In the invention, the double-cone dryer is used for drying, and spinning extrusion is carried out on the fosfomycin calcium crystals in the drying process so as to obtain fine lump solids.
Owner:千辉药业(安徽)有限责任公司
Levo-fosfomycin dextro-phenethylamine salt and detection method for enantiomer thereof
The invention discloses a levo-fosfomycin dextro-phenethylamine salt and a detection method for an enantiomer thereof, and relates to the field of pharmaceutical analysis. The levo-fosfomycin dextro-phenethylamine salt and the enantiomer thereof are analyzed by using a high performance liquid chromatograph, and sample analysis is performed by adopting a negative ion exchange chiral column and a flowing phase of specific components and proportion. The method can be used for easily, rapidly and accurately analyzing and separating the levo-fosfomycin dextro-phenethylamine salt and the enantiomer thereof. The method has the advantages of high reproducibility, high separation degree, stability in detecting samples, and the like.
Owner:JIANGSU SKYRUN PHARMA CO LTD
Seedless medicament of grape
InactiveCN101297651ANo particle sizeUniform fruitingBiocidePlant growth regulatorsVitamin CKanamycin
The invention relates to a grape seedless agent, in particular to a regulating agent which can lead the grape to be seedless, and aims at solving the problems that after the existing grape seedless agent is applied, the seedless rate of the grape and the percentage of fertile fruit of the flower are not ideal, the grape with different sizes occurred and the sweetness level is not increased obviously. The regulating agent is prepared by the following materials by weight ratio: 20 to 60g of phytokinin or cytokinin, 20 to 50g of gibberellin, 2 to 5g of fosfomycin sodium, 3 to 5g of florel, 1 to 2g of cycocel, 10 to 15g of organic nitrogen, 3 to 5g of paclobutrazol, 20 to 40g of vitamin E, vitamin B and vitamin C, 10 to 15g of kanamycin, 10 to 30g of 2-4-D and 3 to 5g of borax. The regulating agent has the advantages that the seedless rate of the grape can be up to 100 percent, the percentage of fertile fruit of the flower can be up to 90 percent, the fruits of medium and late maturing varieties are ripe in advance of 20 to 30 days, the sweetness level of the grape is increased by about 2 degrees, the content of dry matters are high and the storage is easy.
Owner:杨秀文
Fosfomycin sodium composition lyophilized powder for injection
InactiveCN103550176AImprove antibacterial propertiesImprove stabilityAntibacterial agentsOrganic active ingredientsChitosan nanoparticlesBiological activation
The invention provides a fosfomycin sodium composition lyophilized powder for injection, and relates to the technical field of medicines and medicine preparation. The fosfomycin sodium composition lyophilized powder for injection comprises the following raw materials in parts by weight: 7.26-9.17 parts of fosfomycin sodium, 7.02-8.97 parts of chitosan nanoparticle, and 81.38-87.10 parts of injection water. The fosfomycin sodium composition lyophilized powder for injection has the following advantages that (1) the antibacterial effect on klebsiella pneumoniae is good, the antibiotic sensitive rate is 89.9%, and the advantage in klebsiella pneumoniae producing ESBLs (Extended Spectyum beta Lactamase) is obvious; (2) the stability on beta-lactamase produced by gram-negative bacterium is increased, and the in-vivo distribution is good; (3) as the activity is improved, the medication period of a patient is shortened, and the possibility of untoward effect caused by the accumulation of fosfomycin sodium is reduced; and (4) the chitosan nanoparticle can replace mannitol to function as the lyophilization skeleton agent of the lyophilized powder, and the activation effect on a human body by the mannitol is eliminated.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Method for synthesizing fosfomycin phenylethylamine calt
InactiveCN101759719AEliminate potential safety hazardsIncrease the number of applicationsGroup 5/15 element organic compoundsSodium bicarbonateMass content
The invention discloses a method for synthesizing fosfomycin phenylethylamine calt. The invention relates to the fine chemical field. The method of the invention comprises the following steps: under the condition of stirring, after the hydrogenation reaction is finished, adding 95% mass-content ethanol into the hydrogenation solution in which palladium-carbon catalyst is filtrated; adding sodium bicarbonate saturated solution while stirring; controlling temperature within the range of 30-40 DEG C, then adding DL-alpha-phenylethylamine or D-alpha-phenylethylamine; stirring continuously and adding the aqueous solution of sodium tungstate and EDTA disodium salt; heating the reaction system to 40-50 DEG C and adding hydrogen peroxide, and then heating again till the temperature reaches 50-55 DEG C and maintaining the temperature; cooling to 5 below-10 below DEG C and maintaining the temperature; and washing filter cake with ethanol after filtration and separation, thus obtaining the levorotary-dextrorotatory mixed salts or the crude levorotary salts. The obtained levorotary-dextrorotatory mixed salts are separated to yield crude levorotary salts, and further the crude levorotary salts are refined to form fine levorotary salts. The method has the advantages of simple process, convenient operation, high safety, low material consumption, less pollution discharge, low cost, and short production cycle.
Owner:NORTHEAST PHARMA GRP
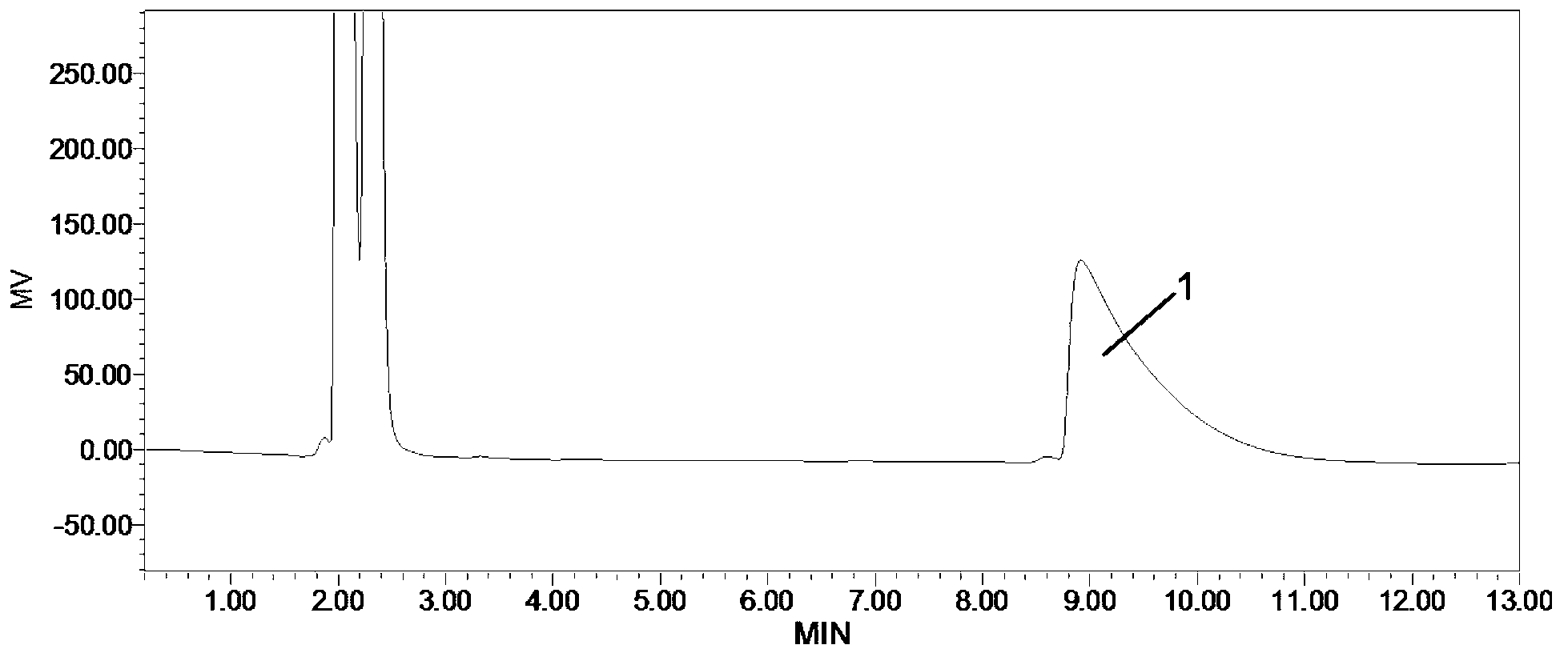
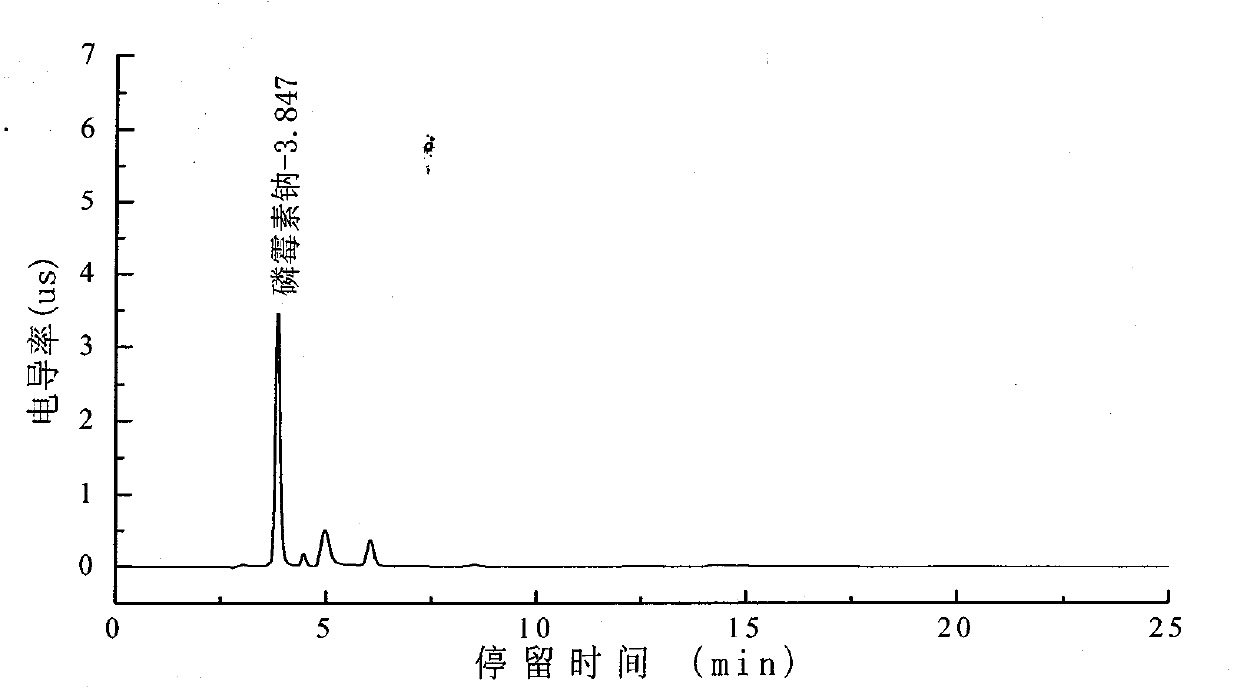
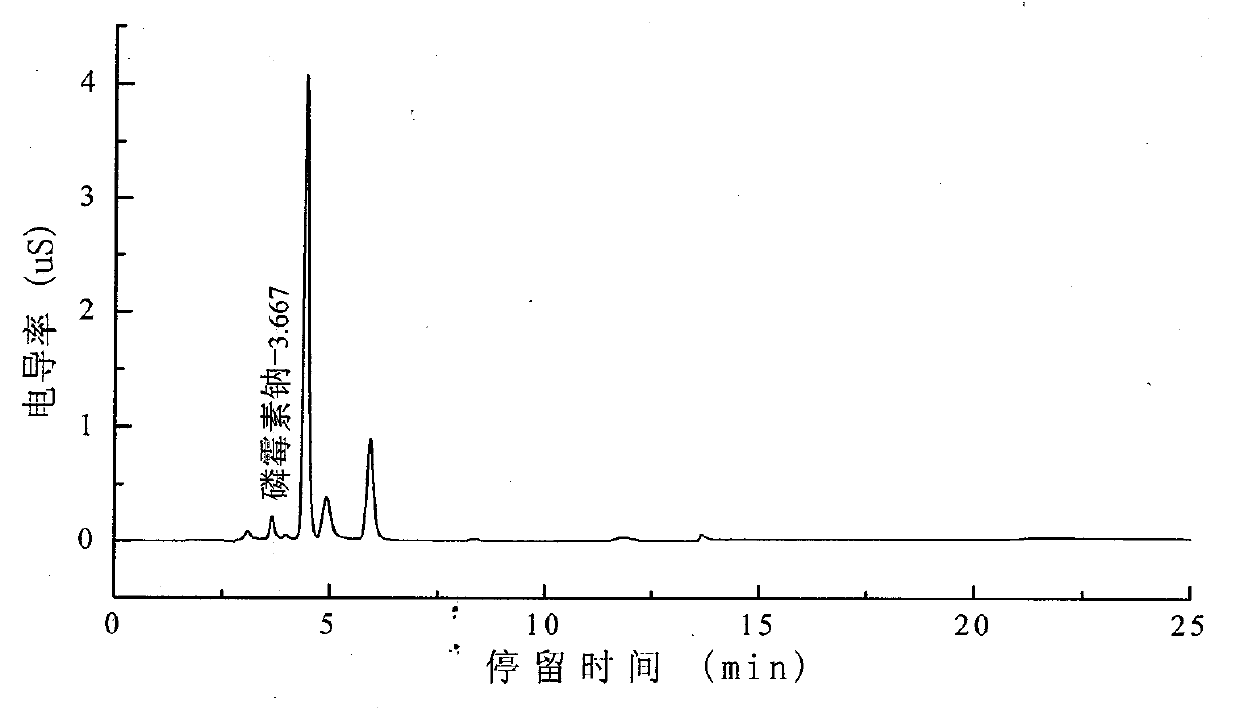
Method for detecting fosfomycin sodium in pharmaceutical wastewater by adopting ion chromatography
ActiveCN103728385AGood reproducibilityLow detection limitComponent separationIon chromatographyLow volume
The invention relates to a method for detecting fosfomycin sodium in pharmaceutical wastewater by adopting an ion chromatography. The method comprises the following steps that (1) chromatographic condition: chromatographic columns include a low-volume hydroxyl system cathodic protection column and a high-volume anion exchange separation column, a suppressor is an electrolytic film suppressor, an electrical conductivity detector is used, and leacheate is a sylvite solution; (2) the preparation of a standard solution: a standard product of the fosfomycin sodium is mixed with ultrapure water to obtain into the standard solution; (3) the pretreatment of a sample: a sample to be detected is filtered by using a 0.45mum filtering film and then processed by a C18 colonnette so that organic matters in the water sample are removed; (4) a detecting method: the standard solution is added in an ion chromatograph, then a spectrogram is recorded, the position of a standard peak is determined, the area of a fosfomycin sodium peak is calculated, and a standard curve is drawn according to the calculated area of the peak; a testing sample is added in the ion chromatograph, then a spectrogram is recorded, and the concentration of the sample is calculated according to the area of the fosfomycin sodium peak. The method provided by the invention has the advantages that the detection repeatability of the fosfomycin sodium in the pharmaceutical wastewater is good, the detection limit is low, the sensitivity is high, the operation is simple, the detecting condition is gentle, the detection equipment and instrument are widely applied, the source of a leacheate sylvite solution is wide, and the configuration is simple, and the method is widely used for detecting fosfomycin sodium in the industrial wastewater.
Owner:CHINESE RES ACAD OF ENVIRONMENTAL SCI
Compound medicine containing bracken and fosfomycin for livestock
InactiveCN104337847AReverse drug resistanceGood treatment effectAntibacterial agentsPowder deliveryTreatment effectLivestock
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Formulations of aminoglycosides and fosfomycin in a combination having improved chemical properties
ActiveUS20150057241A1Component safetyImprove propertiesAntibacterial agentsBiocidePhoslactomycin ECvd risk
The present invention is synergistic antibiotic compositions having pH adjusted profiles for manufacturing combination, and administration, particularly for patients at risk or suffering from ventilator-associated pneumonia (VAP) and ventilator associated tracheal (VAT) bronchitis. Antibiotic compositions containing fosfomycin and aminoglycosides having individually predetermined and selected pH ranges are manufactured and stored for in combination prior to aerosolization, preferably with a specially designed in-line nebulizer attached to a ventilator.
Owner:SAVARA
Fosfomycin calcium composition freeze-dried tablet and preparation method thereof
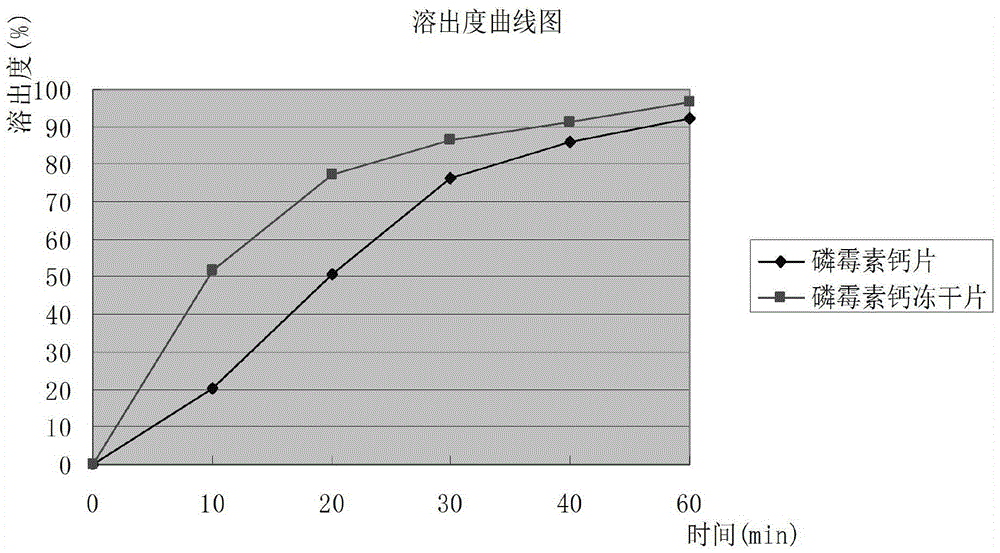
InactiveCN104546767AGood molding effectHigh dissolution rateAntibacterial agentsOrganic active ingredientsSucroseFreeze-drying
The invention provides a fosfomycin calcium composition freeze-dried tablet and a preparation method thereof, and relates to the technical field of medicines and medicine production. The fosfomycin calcium composition freeze-dried tablet comprises fosfomycin calcium, starch and cane sugar, wherein the starch and the cane sugar are used as auxiliary materials, and heating process processing is performed on common corn starch, so that the bonding and disintegrating functions of the starch in the tablet can be improved, and the formability of the tablet is improved; the fosfomycin calcium composition freeze-dried tablet only needs the starch and the cane sugar which are used as the auxiliary materials. The fosfomycin calcium composition freeze-dried tablet adopts a freeze-drying process that temperature is reduced and increased for two times, and the process that the temperature is reduced and increased for two times enables the formability of the tablet to be better, and increases the dissolution rate of the tablet, so that the bioavailability of the tablet is improved. The tablet disclosed by the invention overcomes the defects of a common fosfomycin calcium tablet, and has the characteristics that the types and the dosage of the auxiliary materials in the fosfomycin calcium tablet are reduced, the tablet has a high dissolution rate and high bioavailability, and the curative effect and the safety of clinical medication are guaranteed.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Fosfomycin calcium and trimethoprim capsule used for gastrointestinal diseases
InactiveCN106491563AGood sustained release effectImprove antibacterial propertiesAntibacterial agentsOrganic active ingredientsDiseaseTrimethoprim
The invention provides a compound fosfomycin calcium drug. The active component of the compound fosfomycin calcium drug is composed of fosfomycin calcium, trimethoprim and glycyrrhizic acid in a weight ratio of (10-30): (5-10): (3-6). The invention provides a capsule of the compound fosfomycin calcium drug, and the capsules has good slow-release effect; and the compound fosfomycin calcium drug provided by the invention has better antibacterial effect compared with commercially-available related products.
Owner:重庆希尔安药业有限公司
Composition of fosfomycin or fosfomycin arginine salt and arginine
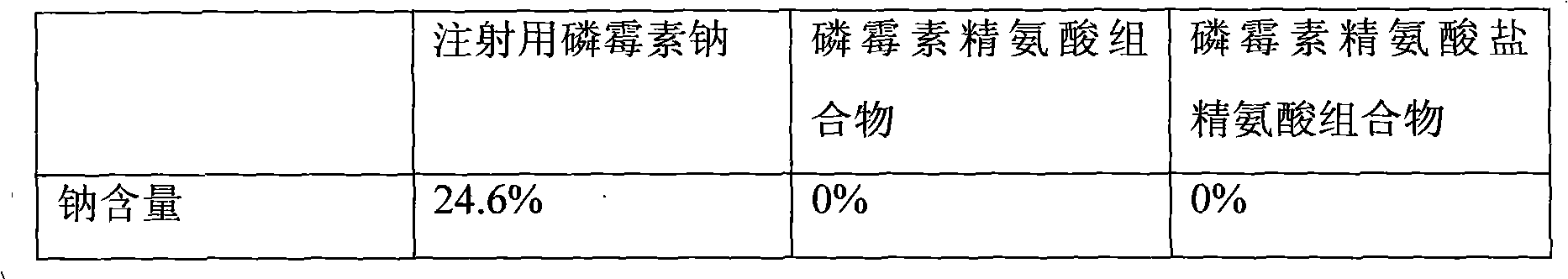
InactiveCN102579466AImprove liquidityImprove stabilityOrganic active ingredientsAntiinfectivesHypernatremiaArginine
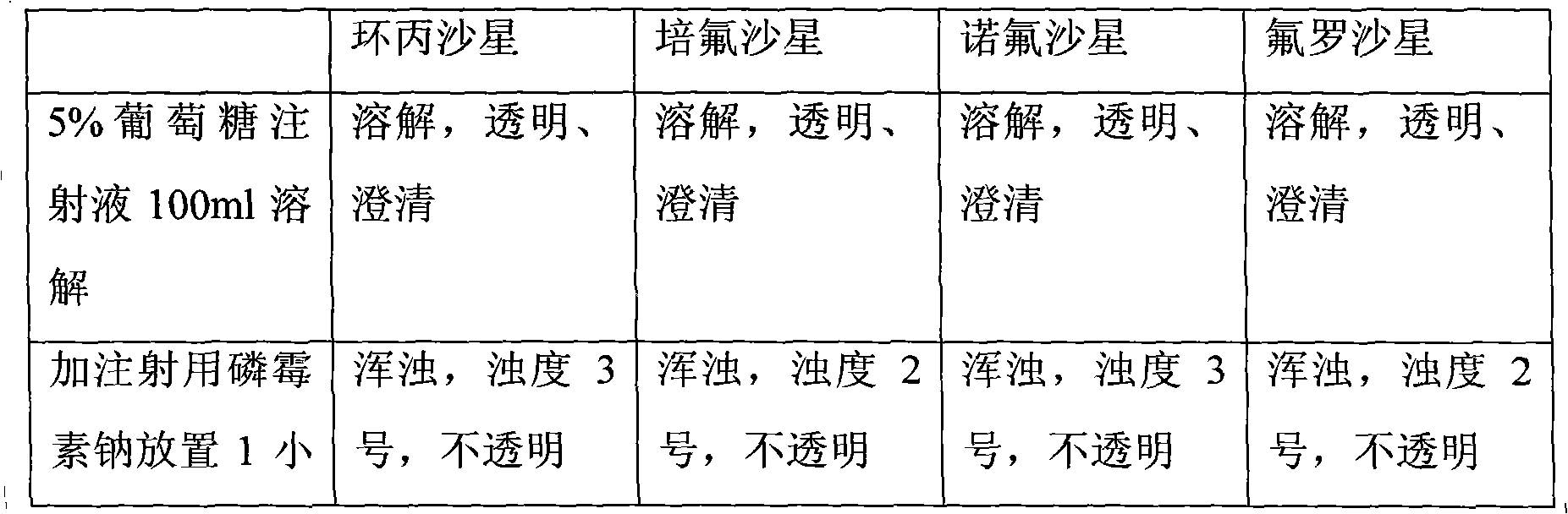
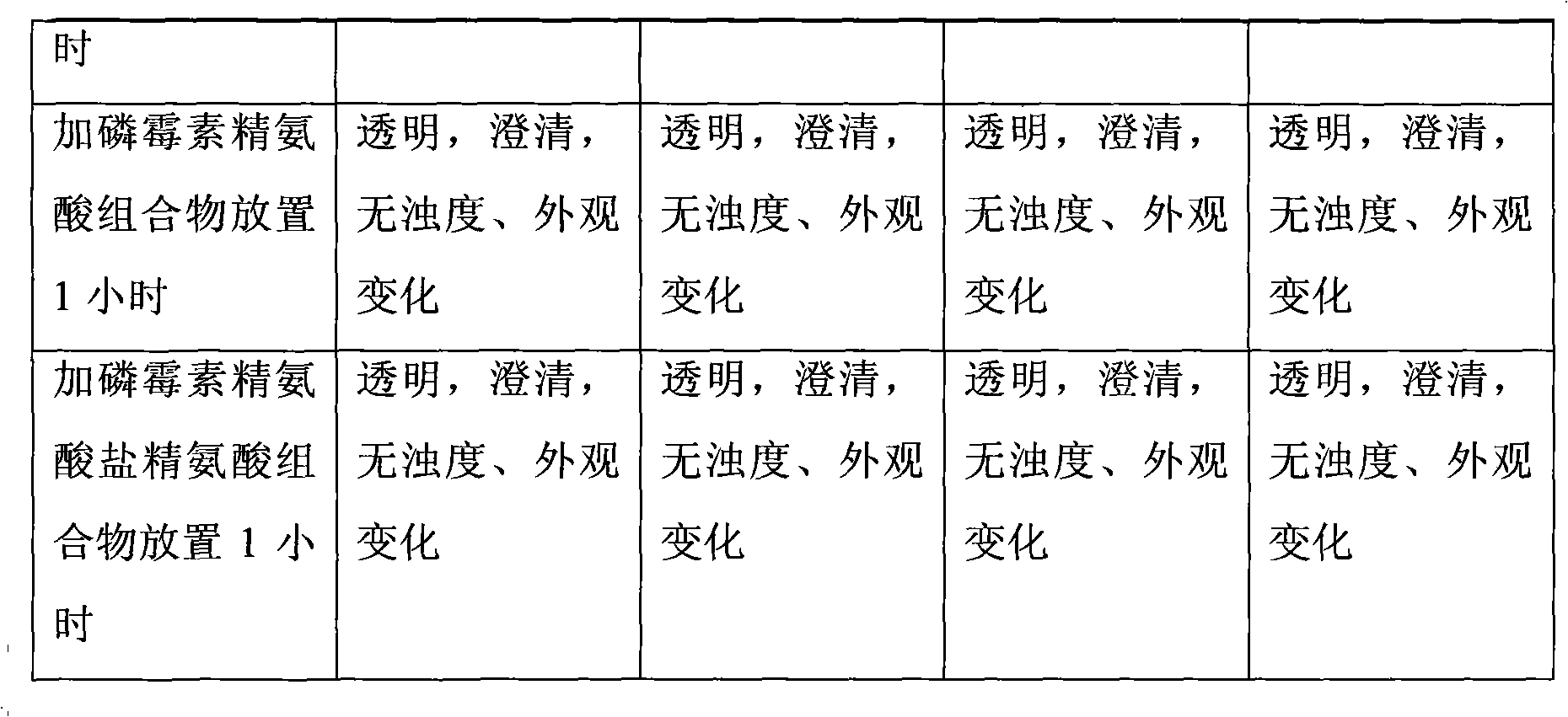
The invention relates to a composition of two antibiotics, in particular to a composition of fosfomycin and arginine and another composition of fosfomycin arginine salt and arginine. The composition of fosfomycin and arginine consists of the fosfomycin and the arginine, wherein the optimal weight ratio of the fosfomycin to the arginine is 1:2.5. The composition of fosfomycin arginine salt and arginine consists of the fosfomycin arginine salt and the arginine, wherein the optimal weight ratio of the fosfomycin arginine salt to the arginine is 1.6:1. Both compositions can be prepared into powder injection for injection; neither compositions contains sodium ions, so that hypernatremia is not caused; and after being dissolved in 5 percent glucose or 0.9 percent sodium chloride solution, the composition can be compatible with a quinolone antibiotic for use. Thus, the composition has a wider and safer application range and is more stable and convenient to use.
Owner:王乐
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com