Fosfomycin calcium and trimethoprim capsule used for gastrointestinal diseases
A technology of calcium trimethoprim and gastrointestinal diseases, applied in the field of compound preparations, can solve the problems of fast action, short course of treatment, low gastrointestinal absorption rate of oral fosfomycin, etc., and achieve good antibacterial effect and good sustained release The effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Sample preparation
[0021] According to the weight ratio of fosfomycin calcium, trimethoprim and glycyrrhizic acid is 8:3:1 to take raw materials as prescription 1, according to the weight ratio of fosfomycin calcium, trimethoprim and glycyrrhizic acid is 30:5: 3 Weigh the raw materials as prescription 2, and weigh the raw materials as prescription 3 according to the weight ratio of fosfomycin calcium, trimethoprim and glycyrrhizic acid as 5:5:3. According to the weight ratio of sodium carboxymethyl cellulose, stearic acid and sodium bicarbonate of 8:4:1, the raw materials are weighed, mixed and pulverized, and passed through a No. 5 sieve; Sodium cellulose, stearic acid, sodium bicarbonate, calcium mycin, trimethoprim and glycyrrhizinic acid were mixed and packed into empty capsules; the packed capsules were heat treated in a constant temperature heater at 60°C for 10 minutes; The heat-treated capsules are naturally cooled to room temperature to obtain the compoun...
Embodiment 2
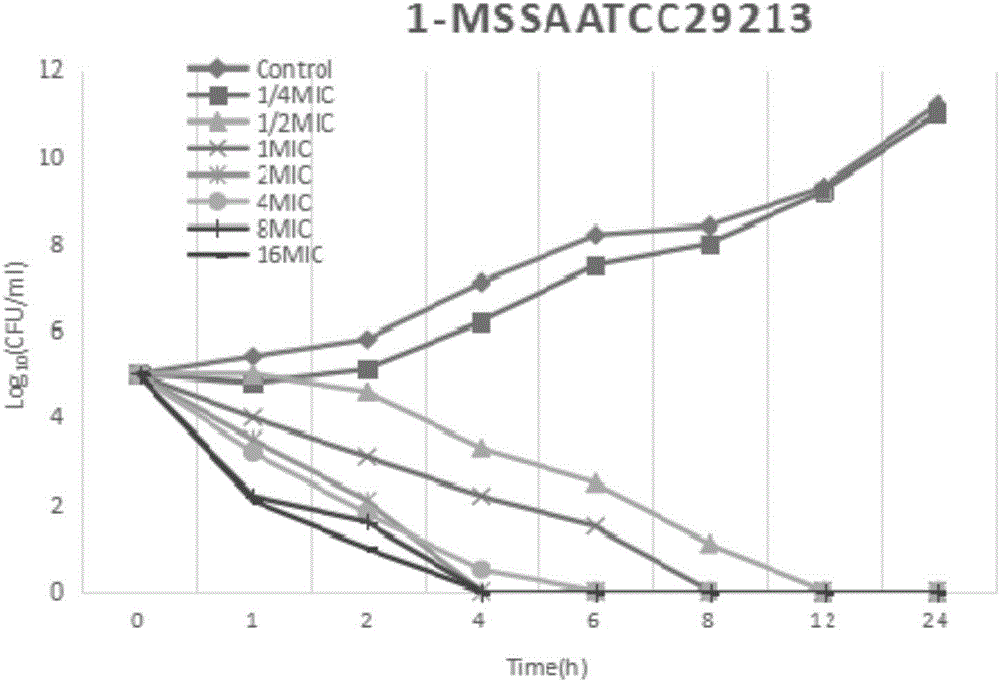
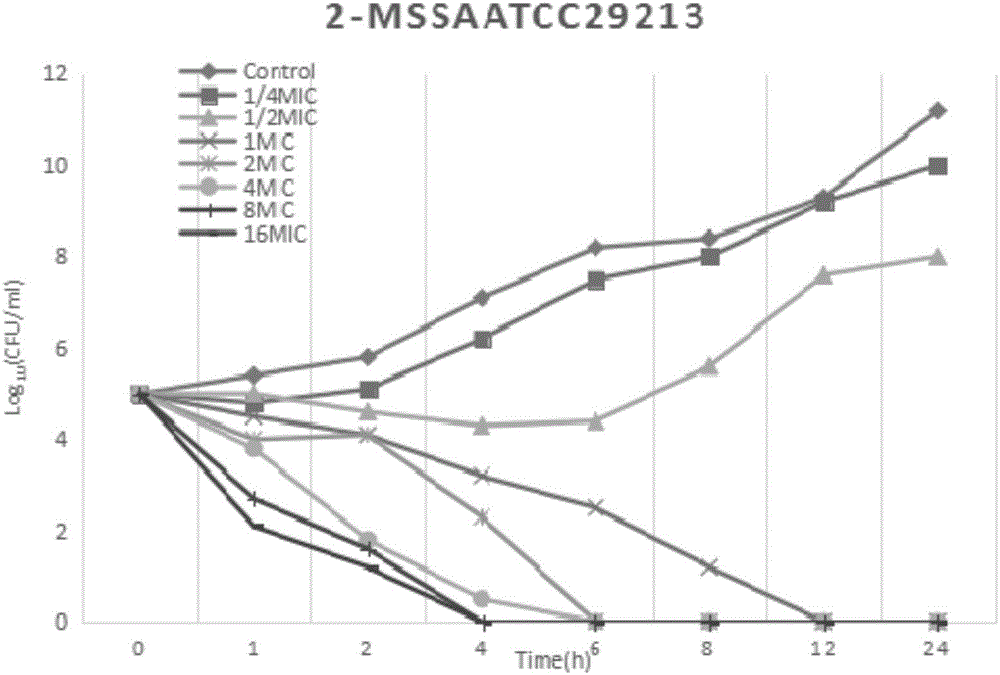
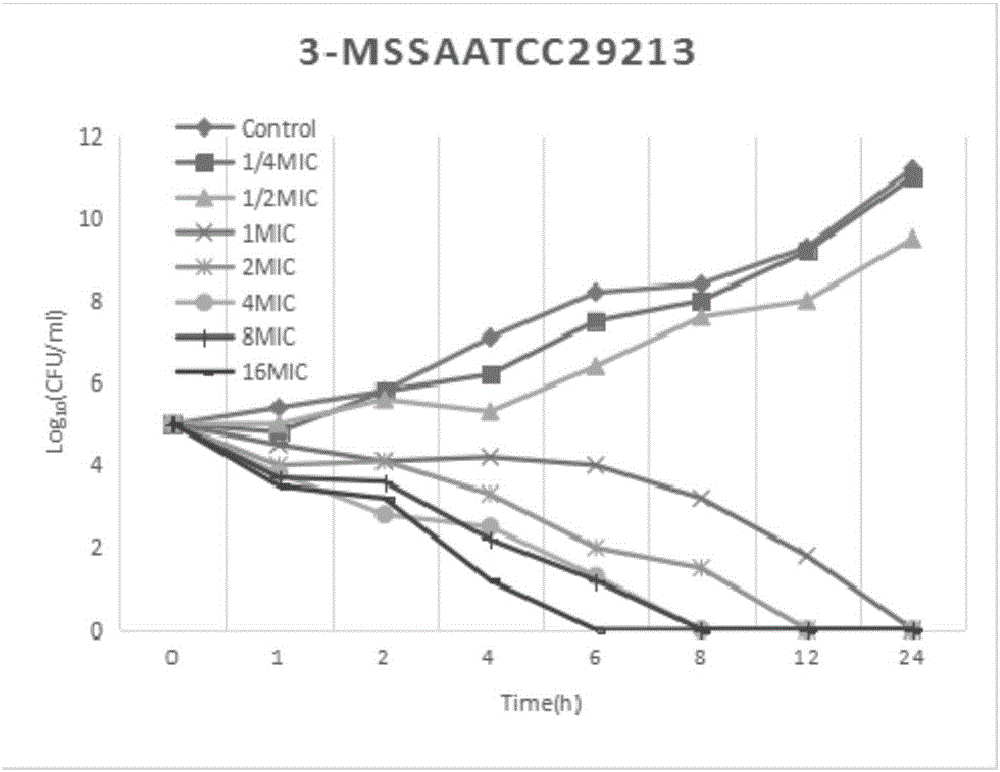
[0027] Example 2 Research on the in vitro bactericidal activity of Staphylococcus aureus
[0028] The test bacteria is the standard strain MSSA-ATCC29213 of Staphylococcus aureus. The minimum inhibitory concentration (MIC) was determined by the agar plate double dilution method, and the MIC of fosfomycin was determined by adding 25 μg / mL of 6-phosphate-glucose according to the requirements of CLSI.
[0029] According to the MIC test results, the antibacterial drug preparation method will be the experimental group A (the mass ratio of fosfomycin calcium, trimethoprim and glycyrrhizic acid is 8:3:1) and the experimental group B ( The mass ratio of fosfomycin calcium, trimethoprim and glycyrrhizic acid is 30:5:3), experimental group C (the mass ratio of fosfomycin calcium, trimethoprim and glycyrrhizic acid is 5:5:3) and The stock solution of the control group (the mass ratio of fosfomycin calcium and trimethoprim is 5:1) was diluted to 7 serial concentrations of 1 / 4MIC, 1 / 2MIC,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com