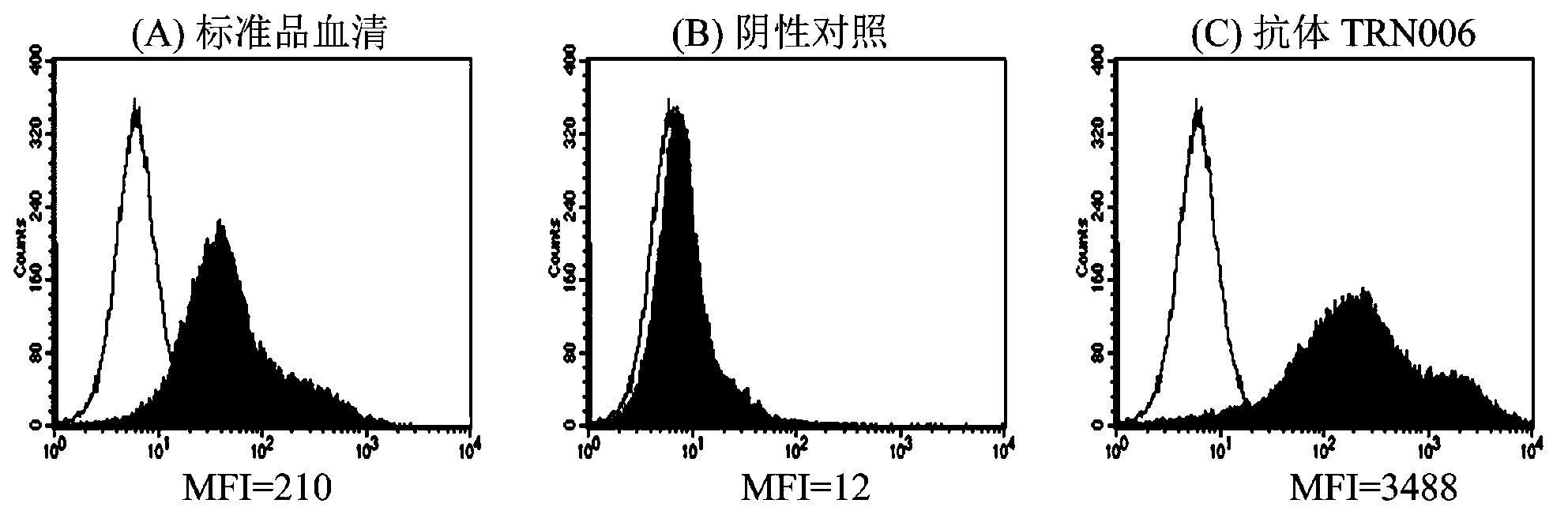
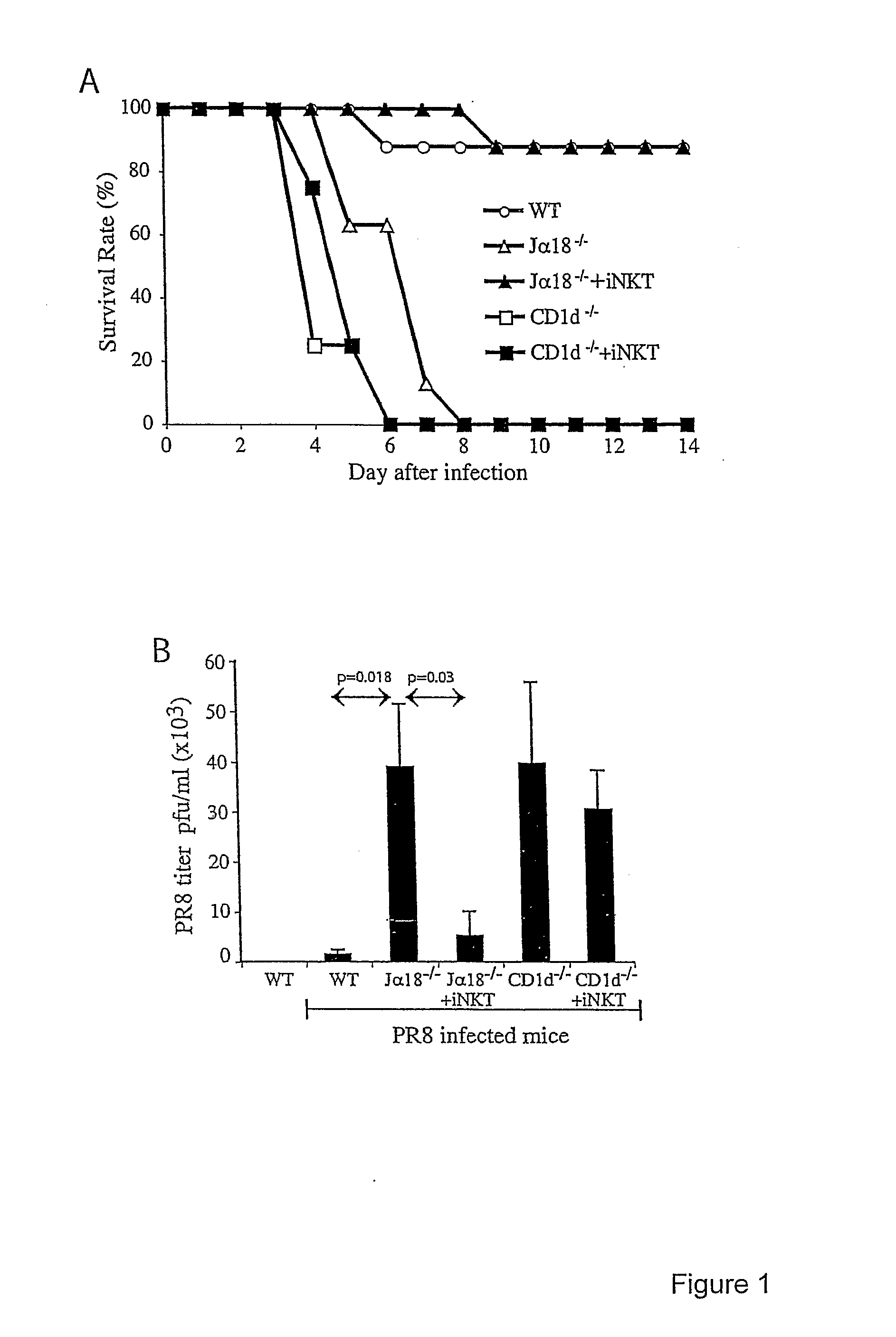
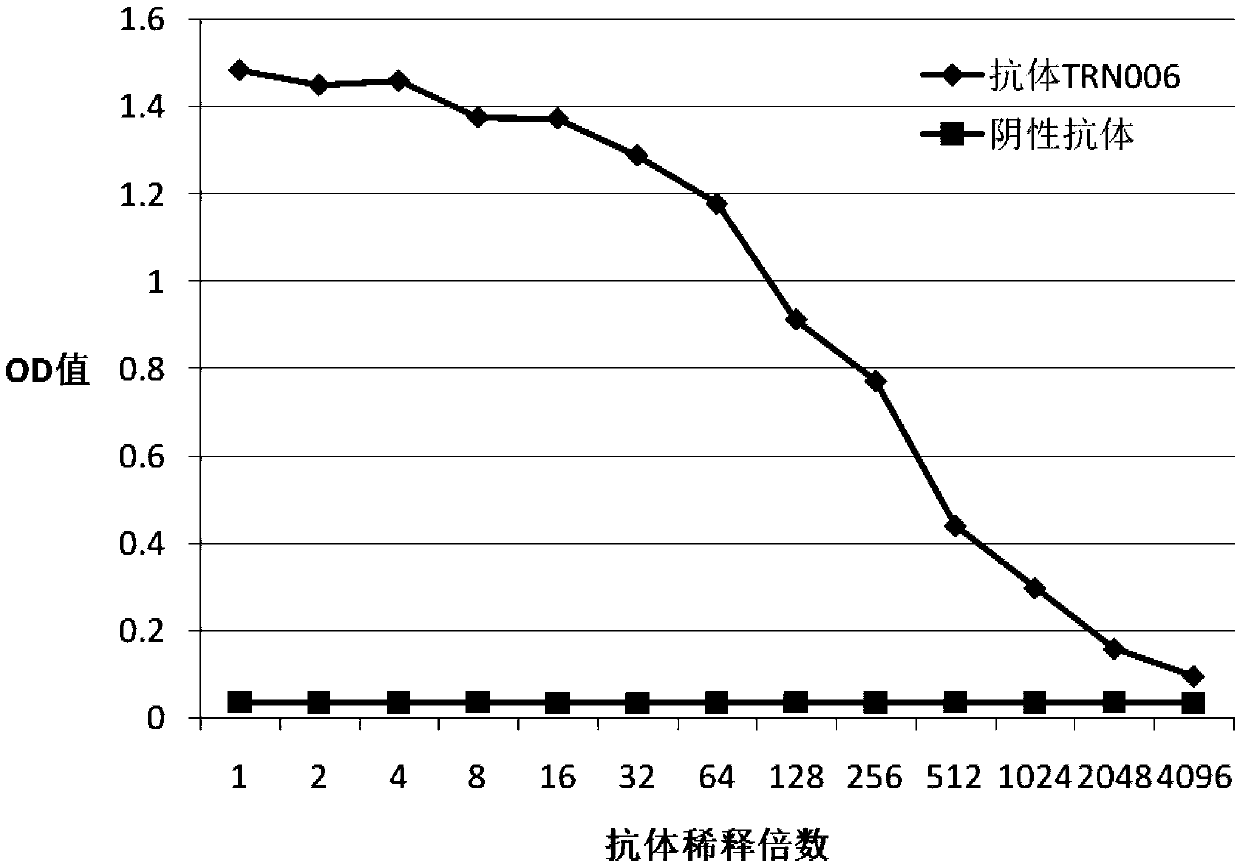
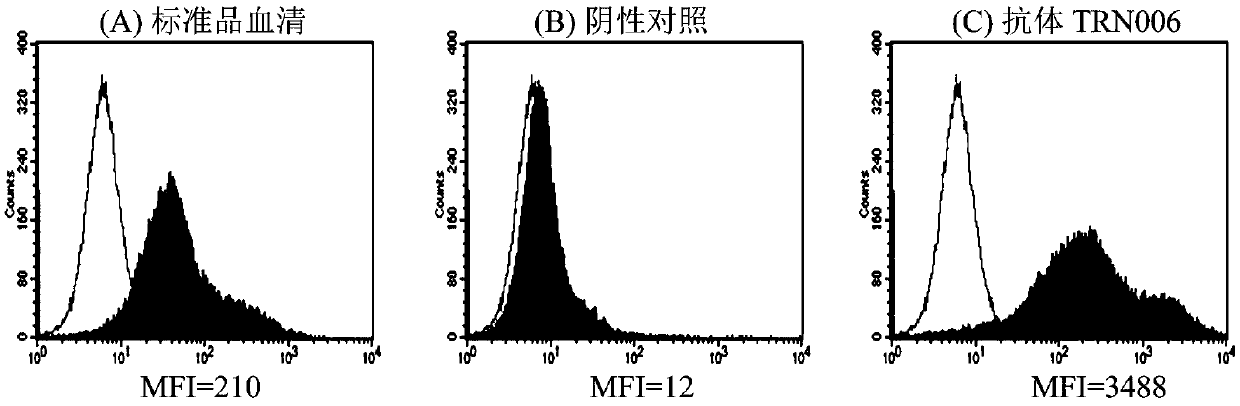
Patents
Literature
77 results about "Acute infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acute infection. An infection that appears suddenly and may be of brief or prolonged duration. infection. 1. invasion and multiplication of microorganisms in body tissues, especially that causing local cellular injury due to competitive metabolism, toxins, intracellular replication or antigen-antibody response.
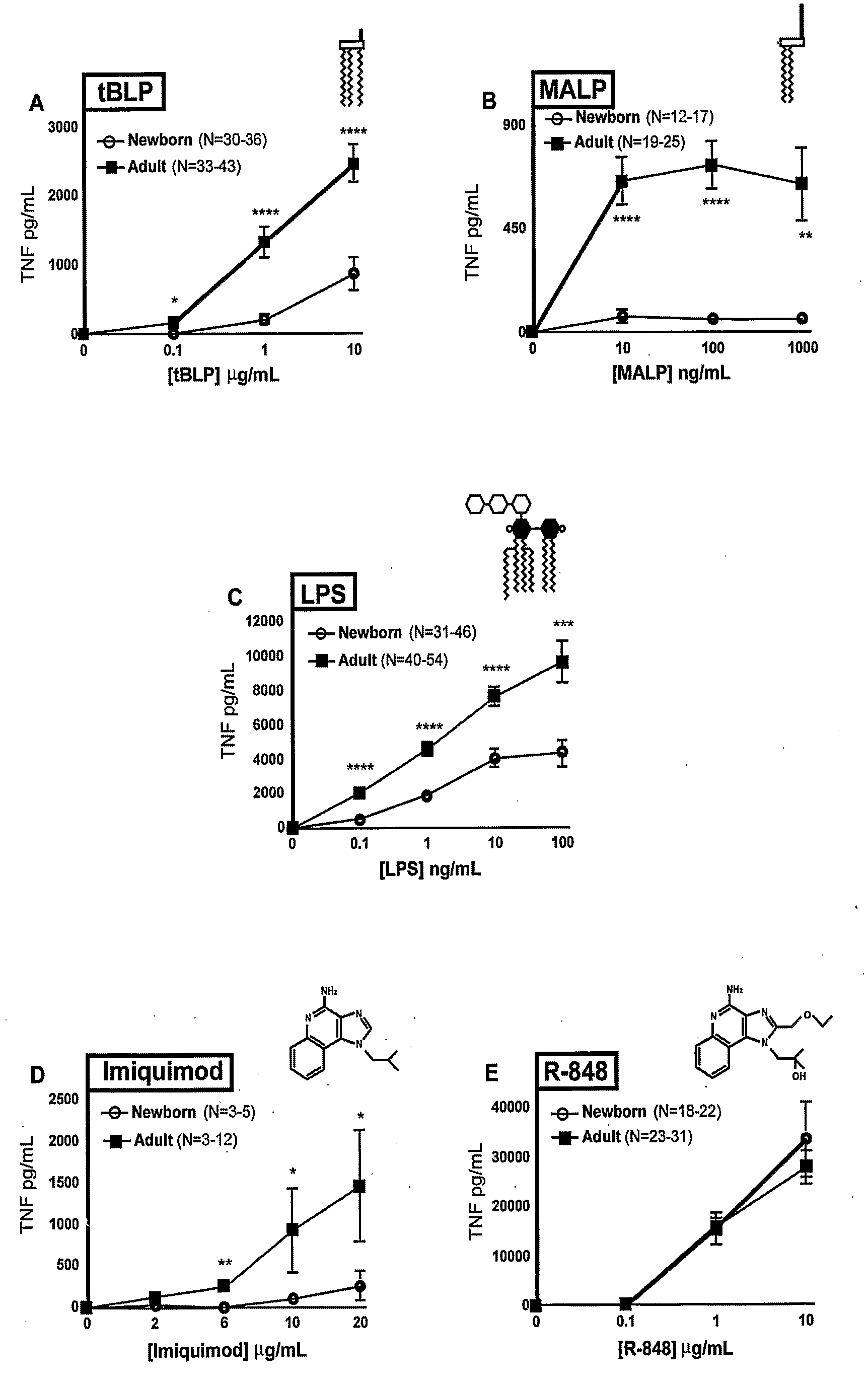
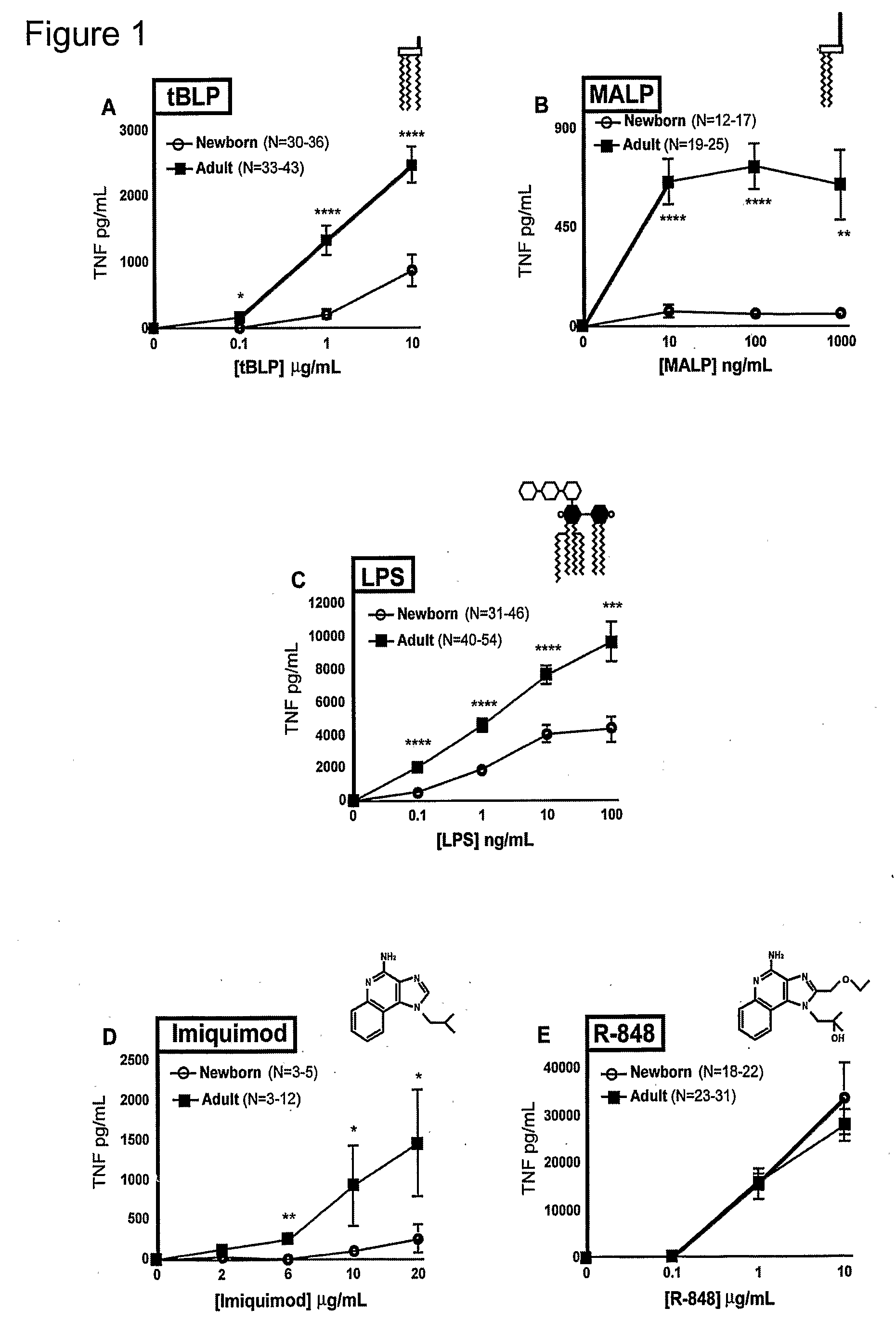
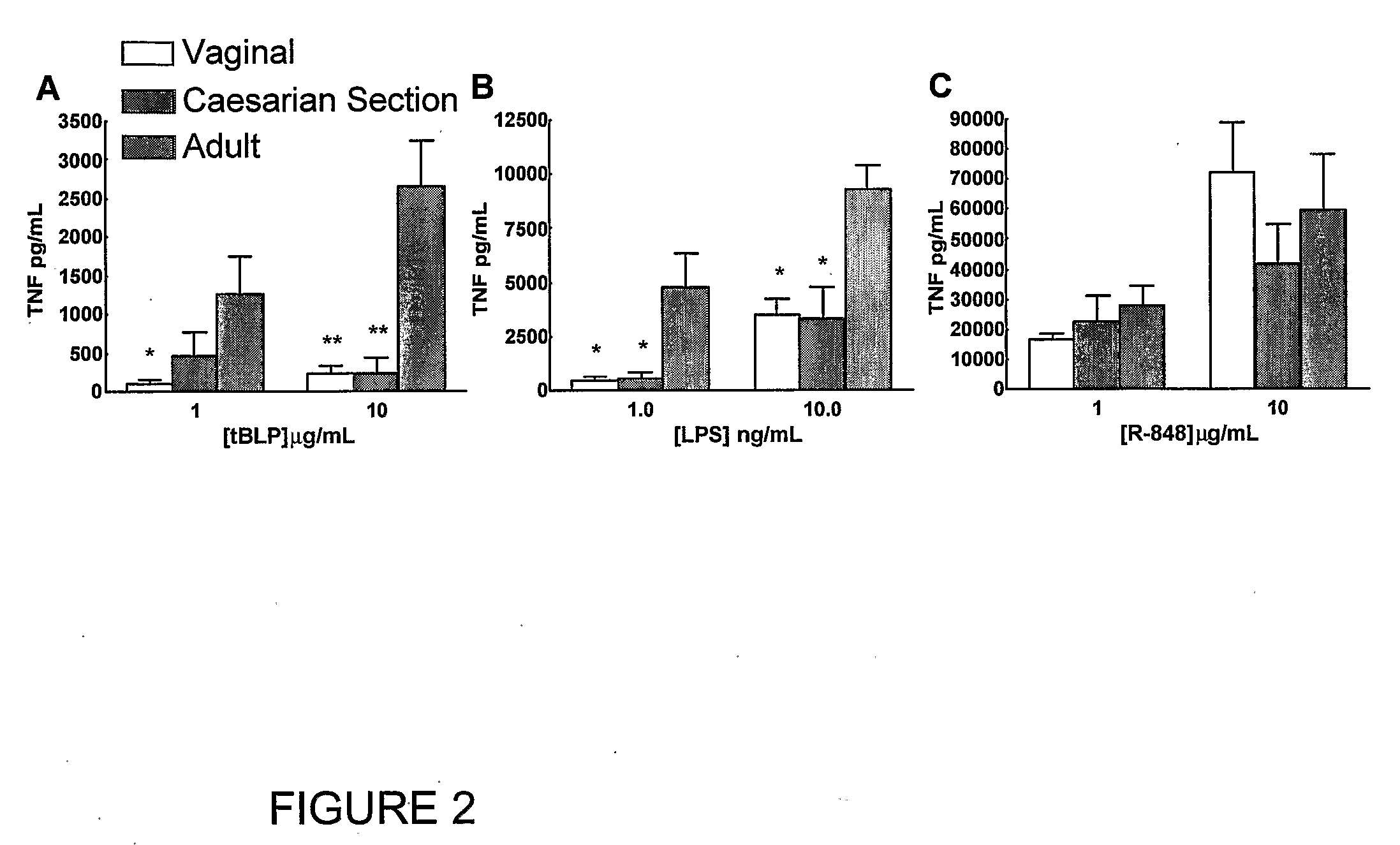
Method for Stimulating the Immune Response of Newborns
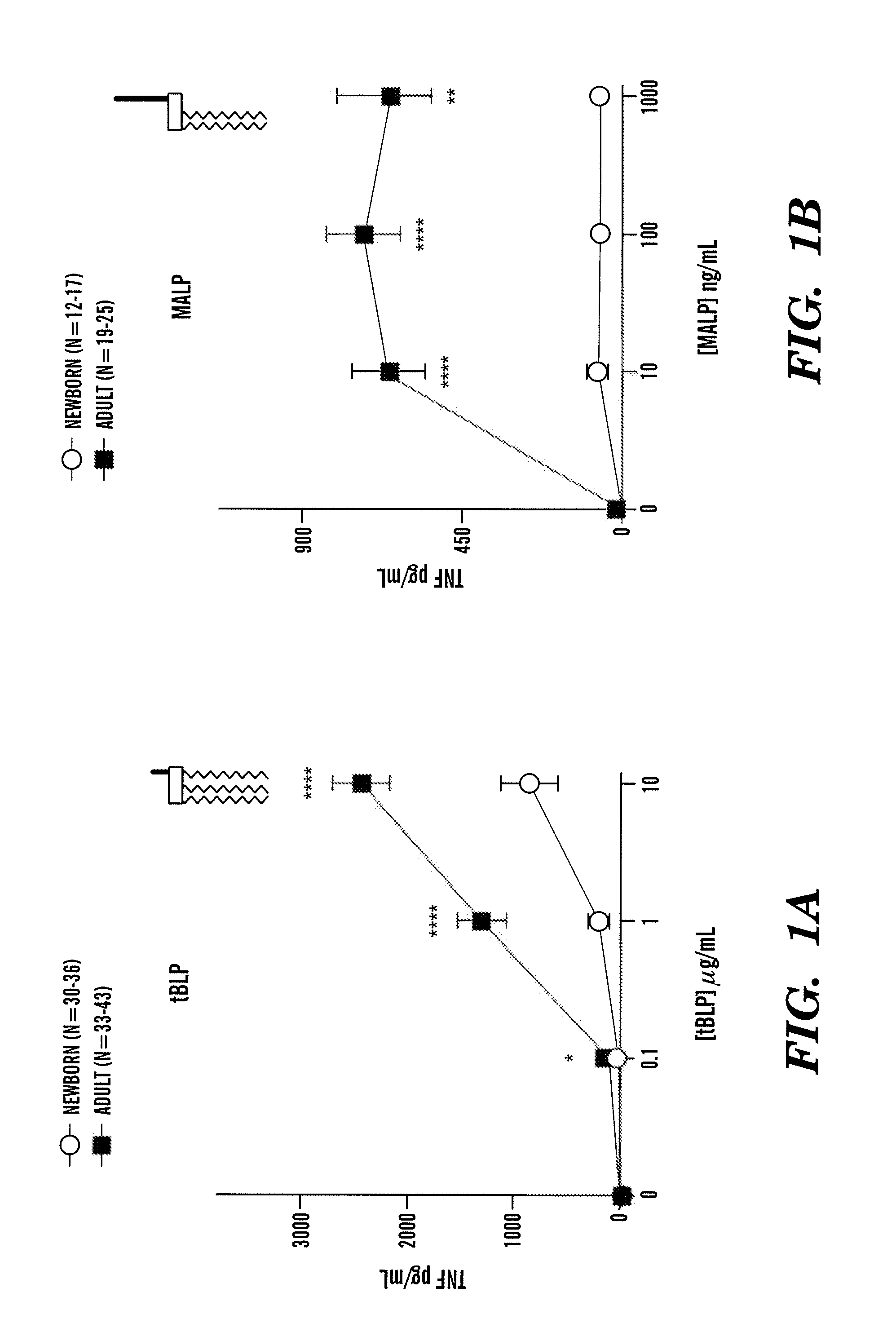
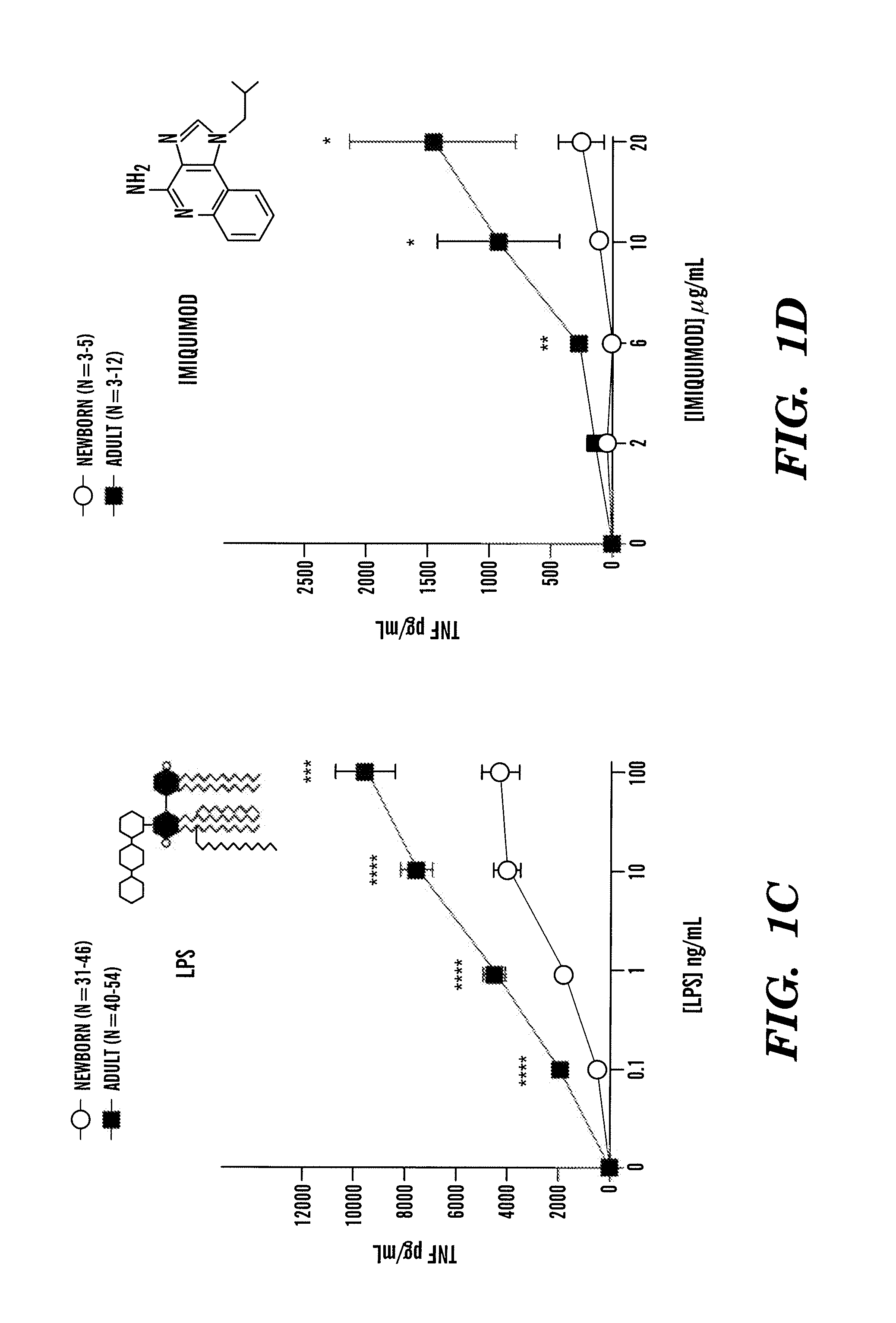
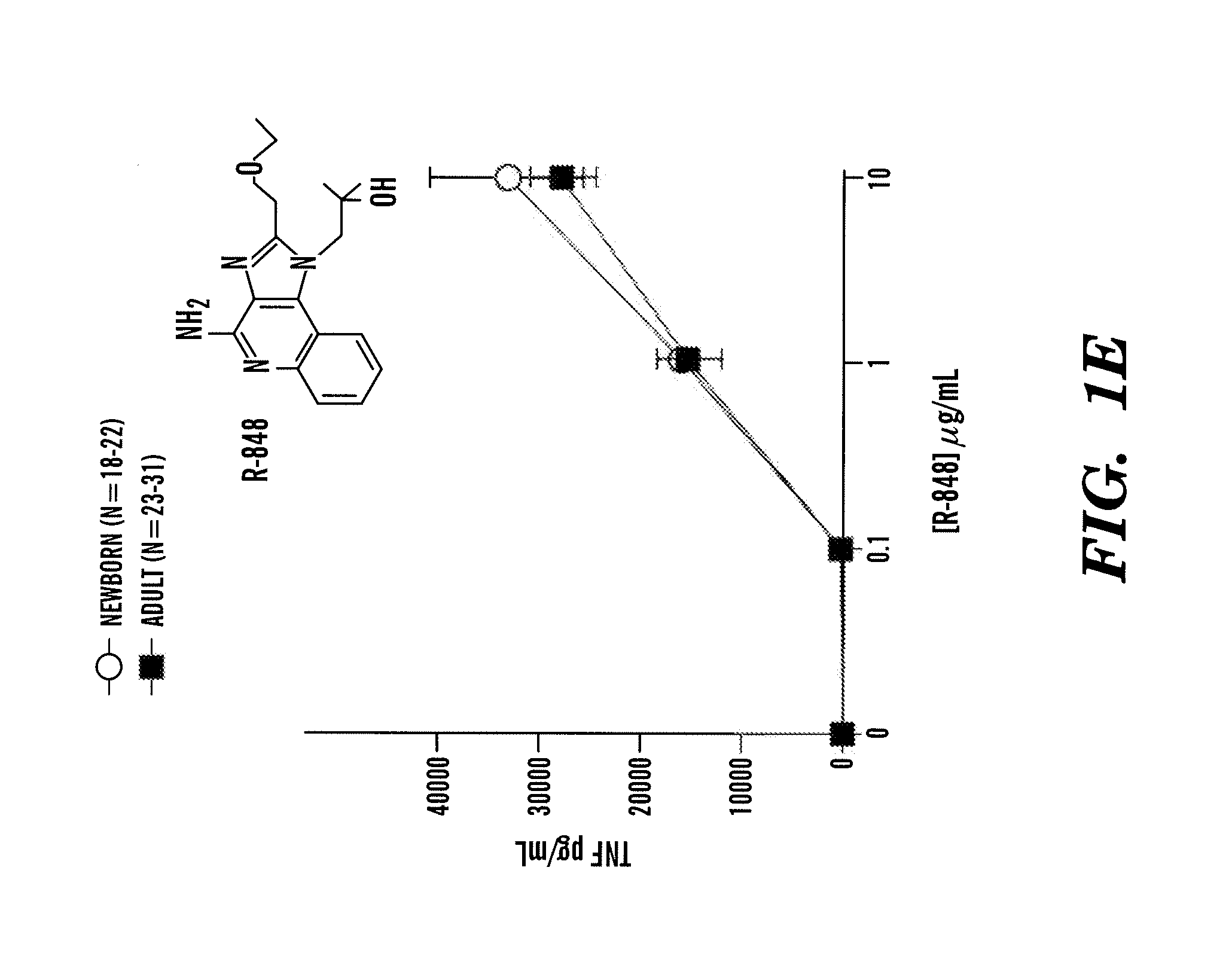
InactiveUS20080193468A1Enhance immune responsePreventing or treatingAntibacterial agentsBiocideTLR8Agonist
The present invention is based on the surprising discovery that agonists of TLR8 are uniquely efficacious in enhancing (e.g. inducing) the immune response of newborns. Thus, agonists of TLR8 serve as both vaccine adjuvants and as adjunctive therapies for acute infection in newborns, preferably the agonist is a TLR8-selective agonist. The immune response induced, or enhanced, in the neonatal host can be, for example, a cytokine immune response and / or a humoral immune response (e.g., antigen-specific).
Owner:3M INNOVATIVE PROPERTIES CO +1
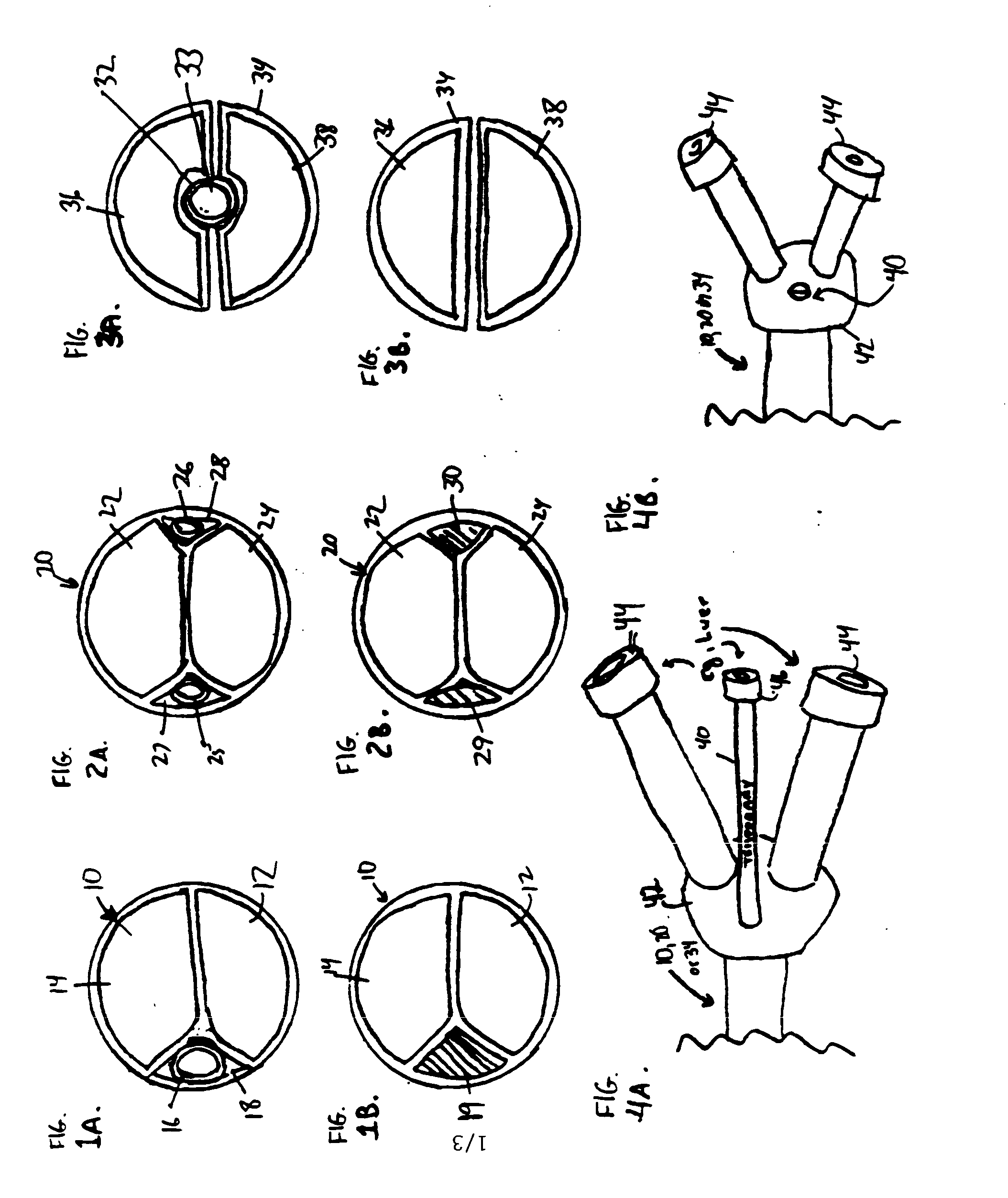
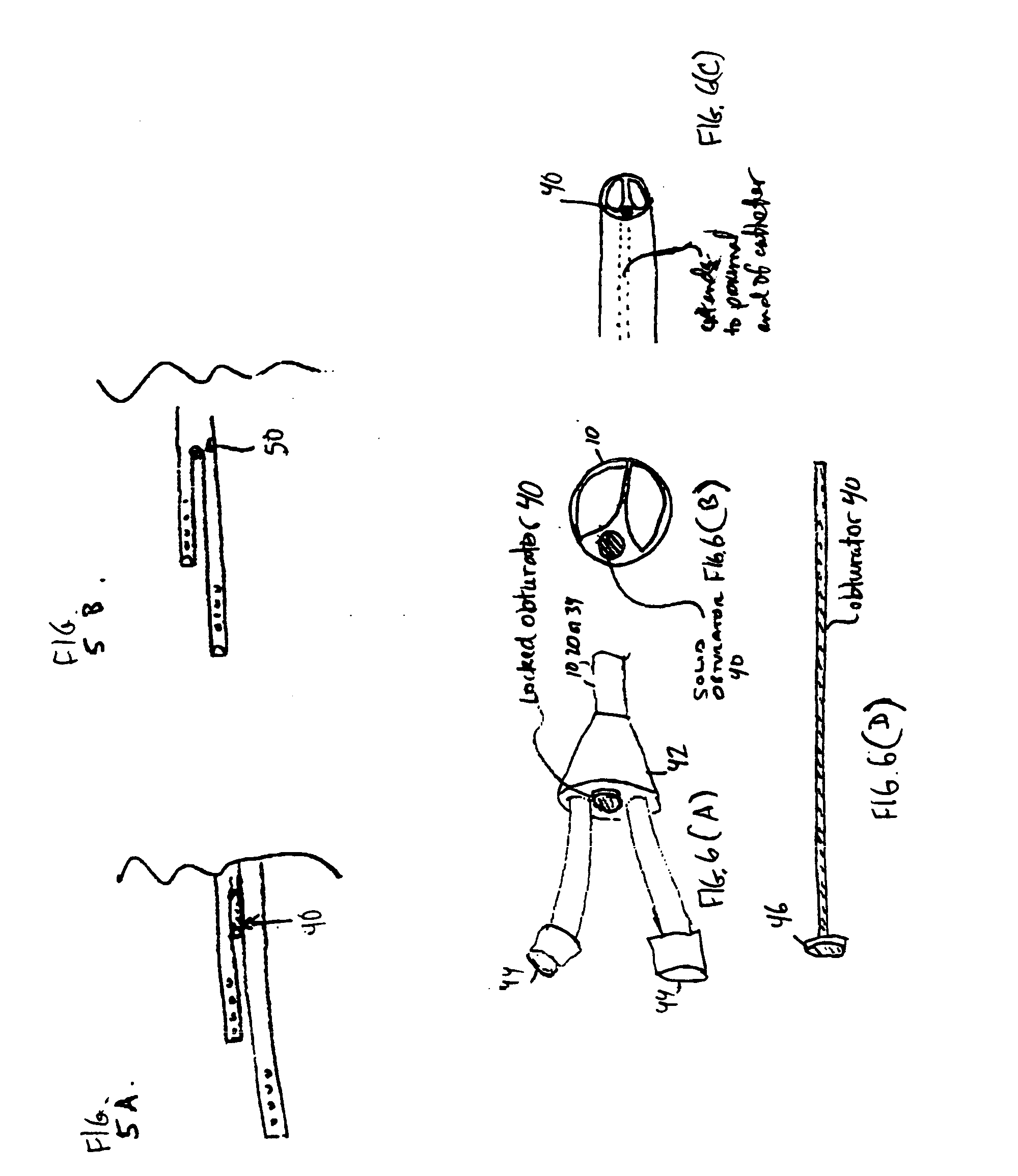
Convertible multi-lumen catheter
InactiveUS20050055012A1Minimize infection riskRigid enoughMulti-lumen catheterMedical devicesVeinHaemodialysis machine
A convertible multi-lumen catheter that may be used for hemodialysis or other indications involving infusion and / or withdrawal of fluids from the body. Unlike existing catheters having a set number of lumens which may limit their utility as both short and long-term venous vascular devices, the catheter of the invention allows one or more additional lumens during the acute phase of catheter use with removal of these lumens (i.e. conversion) for more permanent use. A typical example of this would be a triple lumen device for hemodialysis and antibiotic therapy during an acute infection with conversion to a chronic dual lumen hemodialysis catheter after successful treatment of the infection. The lumen is permanently or semi-permanently blocked using a biocompatible plastic obturator that is inserted into the unused lumen and locked and / or glued into place.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
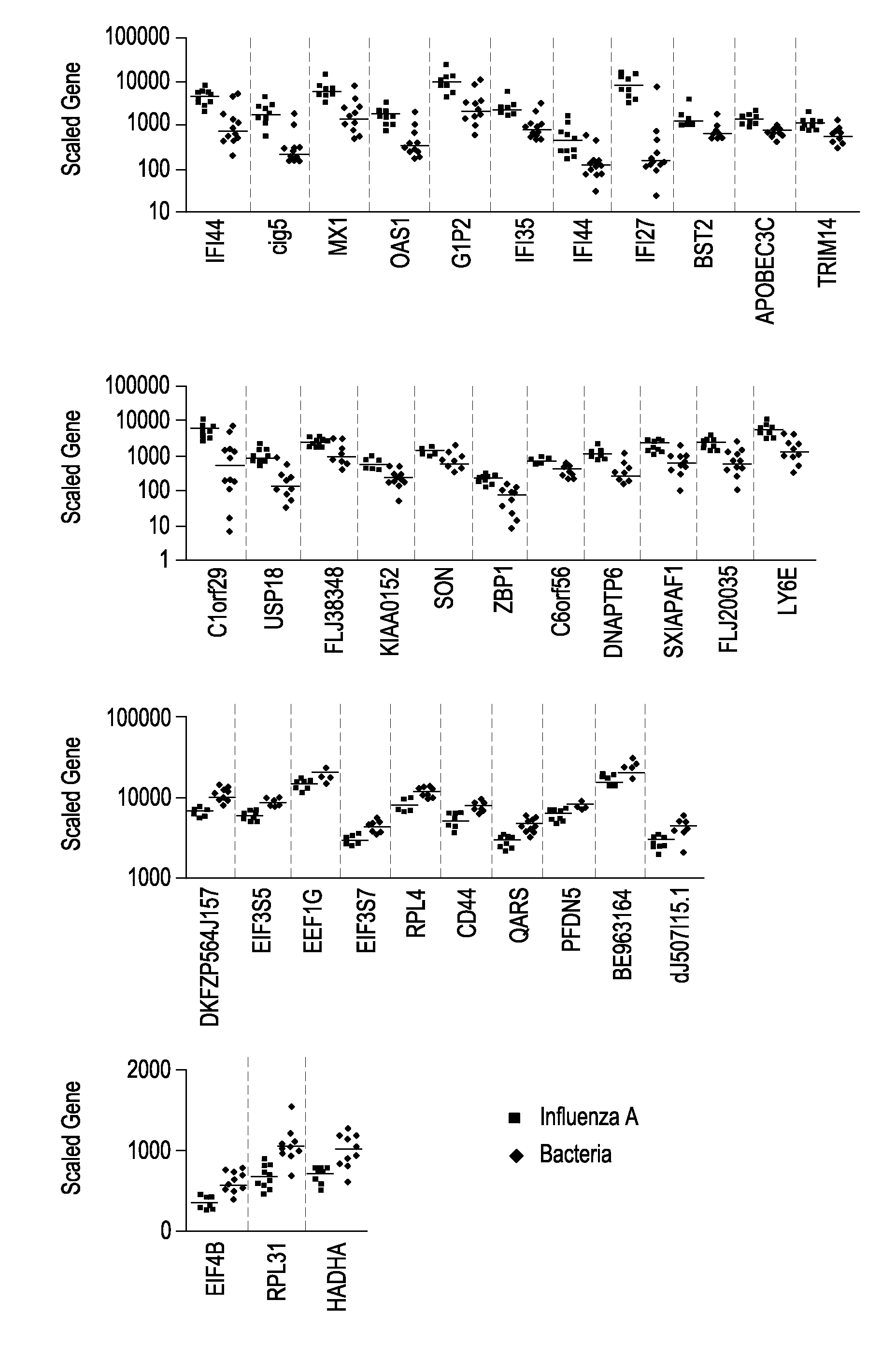
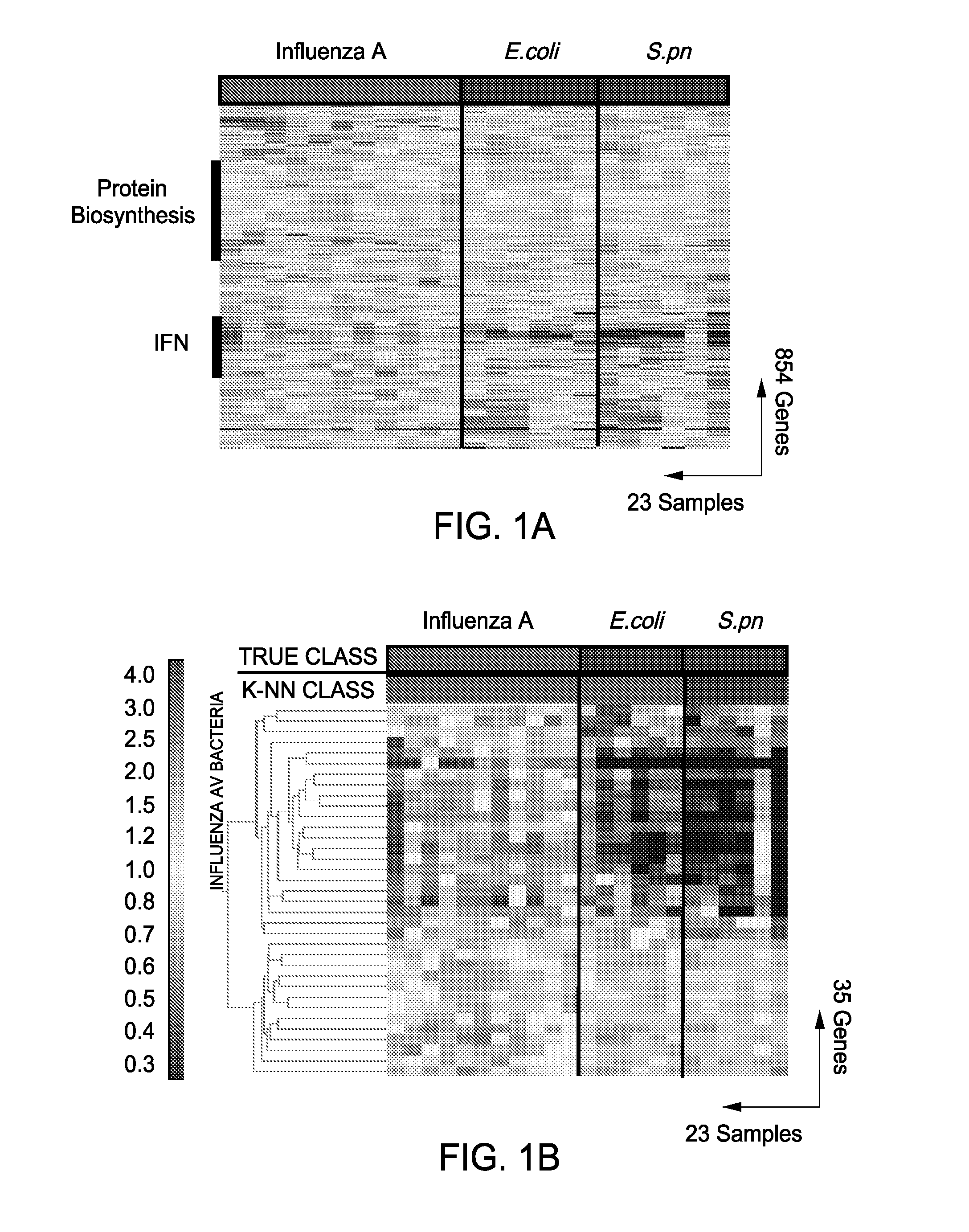
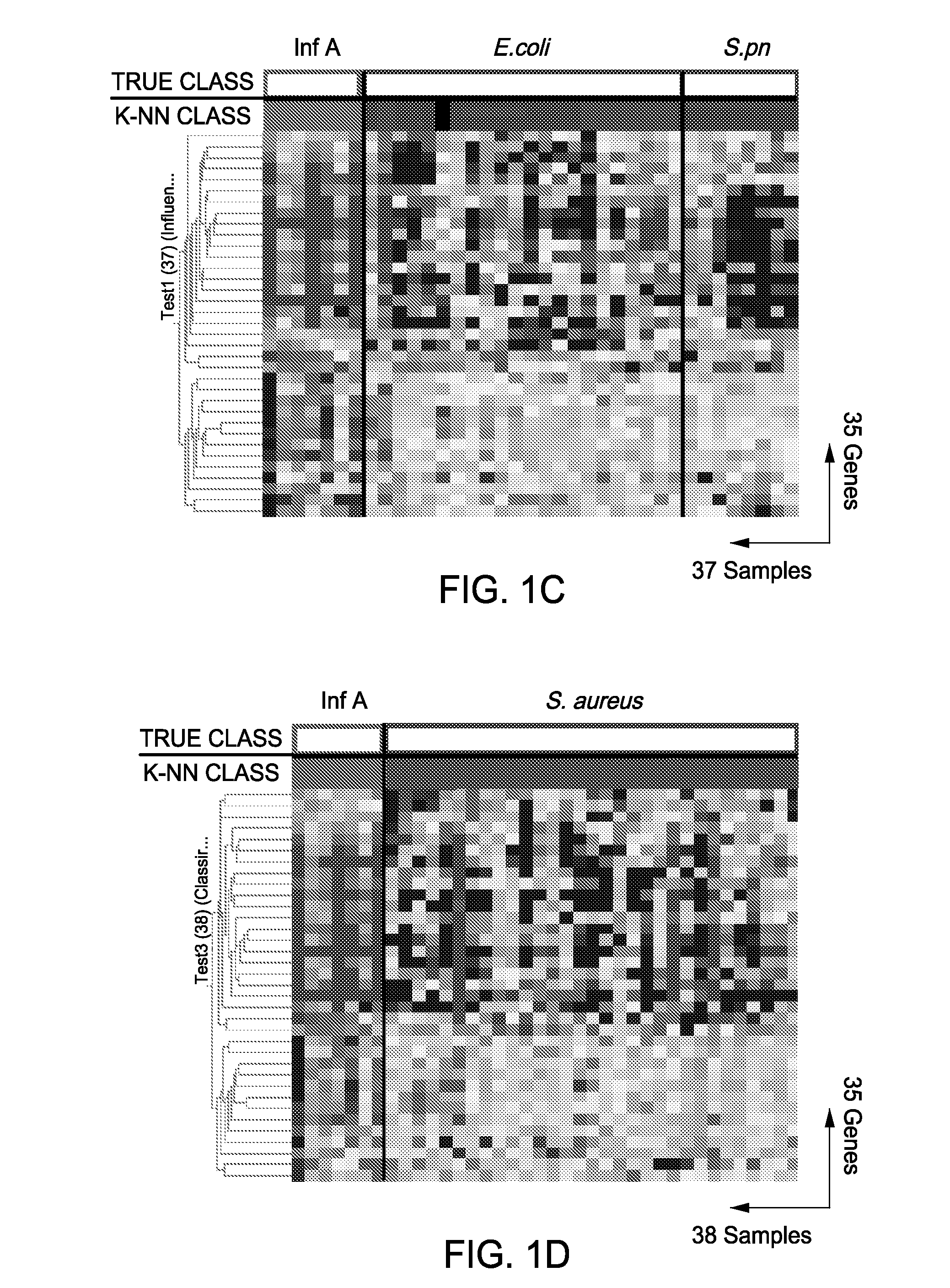
Gene Expression Signatures in Blood Leukocytes Permit Differential Diagnosis of Acute Infections
InactiveUS20080171323A1Improve trustMicrobiological testing/measurementLibrary screeningHost immunityWhite blood cell
The present invention includes compositions, systems and methods for the early detection and consistent determination of the extent, type and nature of a host immune response and the nature of the infectious disease using gene expression data.
Owner:BAYLOR RES INST +1
Dengue virus IgG/IgM antibody detection test strip, kit and preparation method thereof
InactiveCN110007096ASmall sample sizeHigh detection sensitivityBiological testingAgainst vector-borne diseasesIgm antibodyDisease course
The invention discloses a dengue virus IgG / IgM antibody detection test strip, kit and preparation method thereof, and relates to the technical field of dengue virus detection. The dengue virus IgG / IgMantibody detection test strip of the invention comprises a sample pad, an immune combination pad, a nitrocellulose membrane and absorbent paper, wherein the sample pad is sequentially stuck to the bottom of polyvinyl chloride; the immune combination pad is coated with dengue virus recombinant antigen marked by colloidal gold and chicken IgY polyclonal antibody; the nitrocellulose membrane is coated with dengue virus anti-human IgM antibody, a detection line of IgG antibody and a quality control line of anti-chicken IgY polyclonal antibody. The test strip can detect whether dengue virus IgG / IgM antibody exists in a sample to be detected through a method for detecting a marker. The test strip and the detection card comprising the test strip can be used as supplement for antigen detection onthe early acute infection of the dengue virus, covering the middle and later stages of the disease course and reducing the risk of missed detection.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Hepatitis C virus antigen-antibody combined detection kit and detection method
ActiveCN103630690AImprove blood consumptionImprove securityBiological material analysisAntigenTherapy Evaluation
The invention discloses a hepatitis C virus (HCV) antigen-antibody combined detection kit. The kit comprises a microwell plate coated with an HCV chimeric antigen and an HCV monoclonal antibody, a sample diluent, an HCV antigen-antibody combined detection enzyme working fluid, an HCV abzyme working fluid, an HCV antigen enzyme working fluid, a substance A fluid, a substance B fluid, a 20-times concentrated washing fluid and a stopping fluid. The invention also discloses preparation and usage of the key components of the kit, such as the microwell plate of the HCV chimeric antigen and the HCV monoclonal antibody, the sample diluent, and the diluent of enzyme working fluid. The kit and detection method, disclosed by the invention, are able to be used for detecting the HCV antigen and antibody at the same time, or individually detecting the situation of the HCV antigen during the early hepatitis c or the acute infection period, or before the antibody is produced, or when an antigen-antibody compound is produced, or individually detecting the situation of the HCV antibody after the antibody is produced; the kit and detection method can be applied to early HCV detection and therapy evaluation, so as to provide important detection evaluation index for clinical guideline.
Owner:山东莱博生物科技有限公司
Identification of pathogens in body fluids
ActiveUS20100255527A1Microbiological testing/measurementMaterial analysisMicroorganismProtein profiling
Identification of infectious pathogens, particularly viruses, bacteria and other microorganisms is effected with a method whereby pathogens of acute infections can be identified, without first culturing them in external nutrient media, by mass spectrometric measurement of their protein profiles obtained from pathogens directly precipitated from body fluid into pellets by centrifuging. With this method, pathogens which cause acute infections can be identified in less than one hour.
Owner:BRUKER DALTONIK GMBH & CO KG
Identification of pathogens in body fluids
ActiveUS8450081B2Microbiological testing/measurementOn/in organic carrierProtein profilingMicroorganism
Identification of infectious pathogens, particularly viruses, bacteria and other microorganisms is effected with a method whereby pathogens of acute infections can be identified, without first culturing them in external nutrient media, by mass spectrometric measurement of their protein profiles obtained from pathogens directly precipitated from body fluid into pellets by centrifuging. With this method, pathogens which cause acute infections can be identified in less than one hour.
Owner:BRUKER DALTONIK GMBH & CO KG
Anaphylatoxins for detecting clinical conditions
InactiveUS20090226374A1Compounds screening/testingMicrobiological testing/measurementVasculitisBasophilia
Non-allergic hypersensitivity reactions can be observed in a sample of cells from a subject in response to anaphylatoxins. Accordingly, methods are provided for detecting clinical conditions such as cellular hyper-reactivity, non-allergic hypersensitivity, asthma, inflammation, chronic or acute infection, bacterial infection, viral infection, parasite infection, adverse drug reaction, organ rejection, vasculitis, mastocytosis, eosinophilia, basophilia, leukemia, and / or C3a or C5a receptor defects in a subject. Also provided are kits for detecting such clinical conditions in a subject.
Owner:HEALTH AIRE
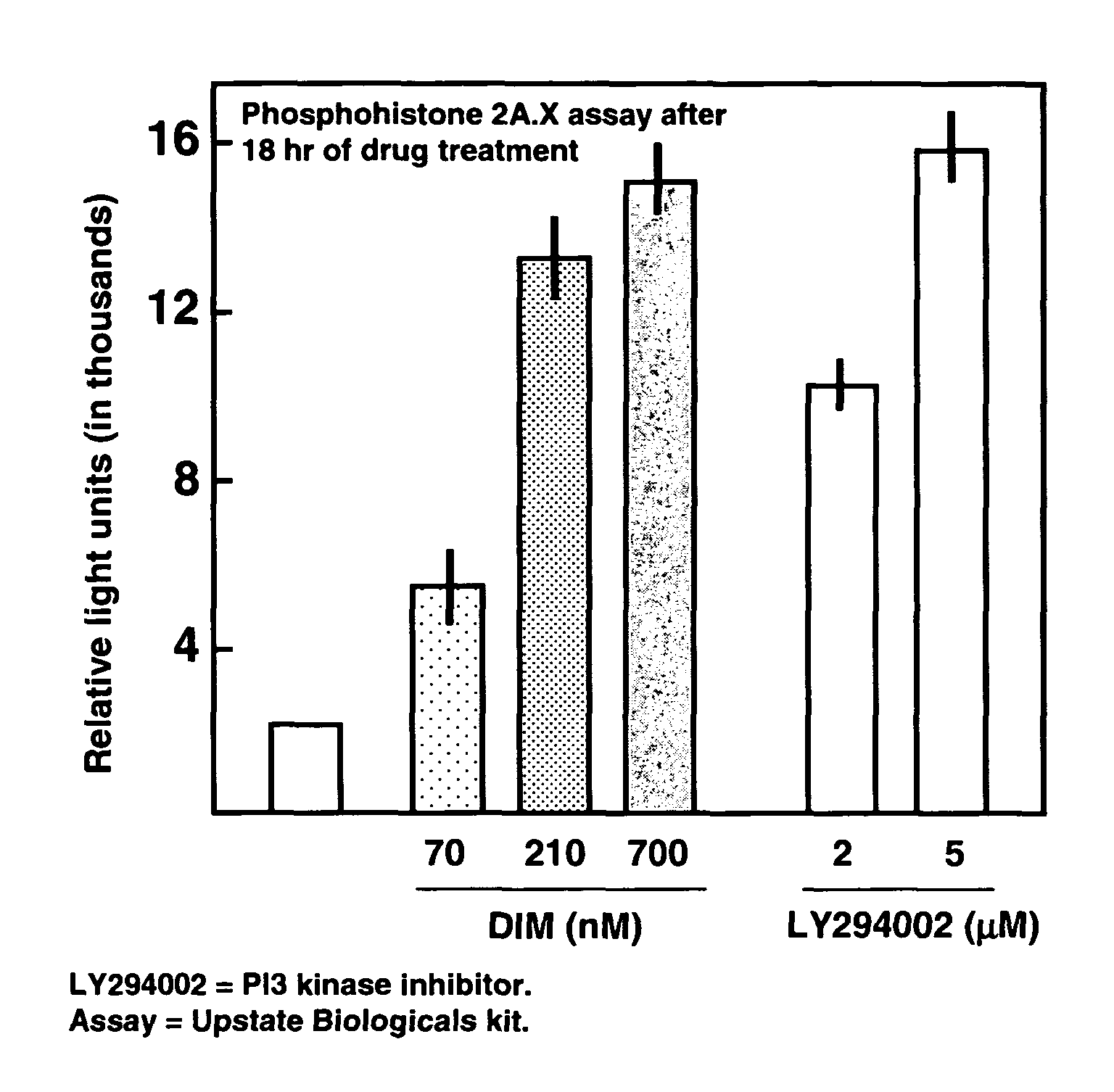
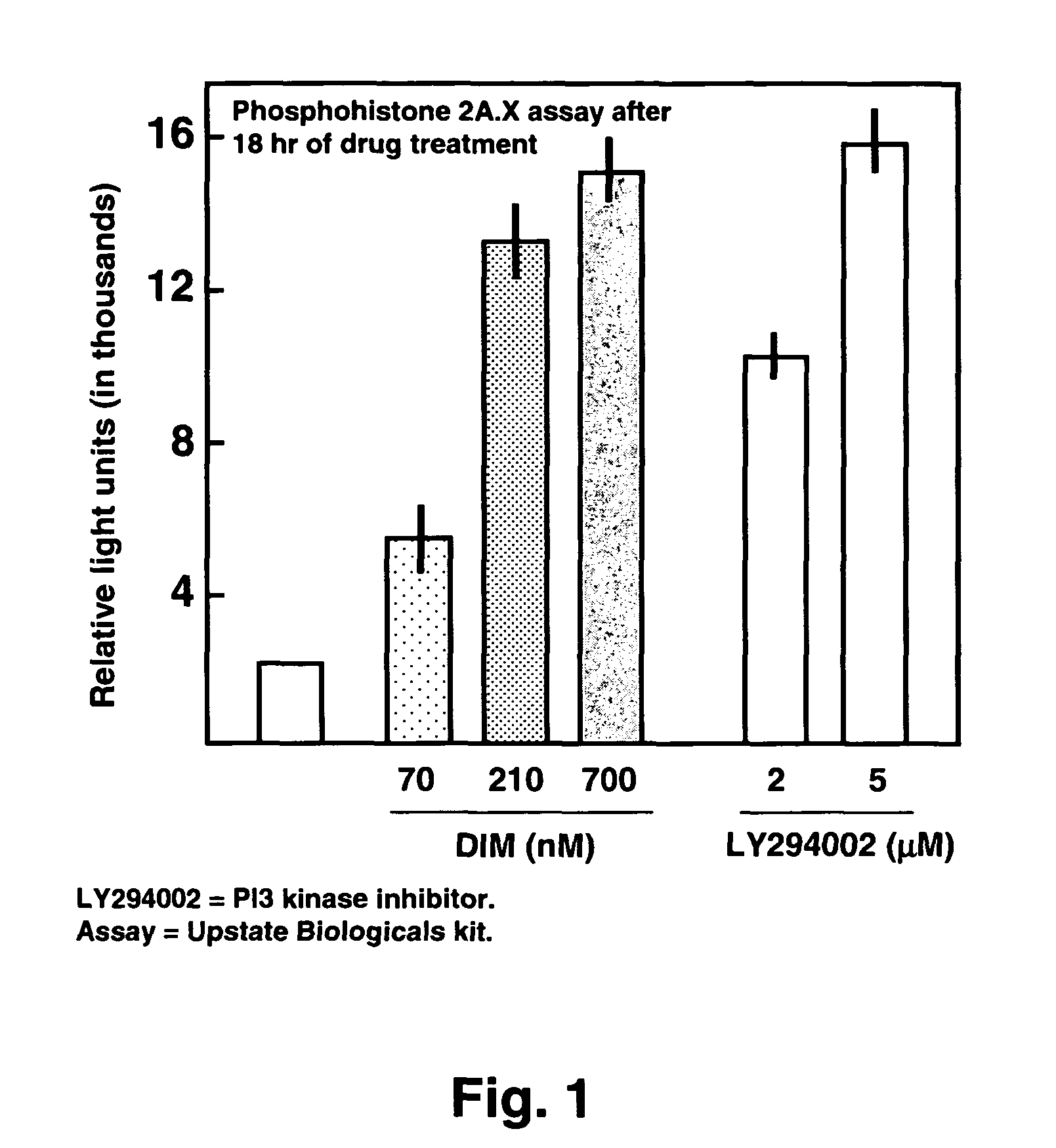
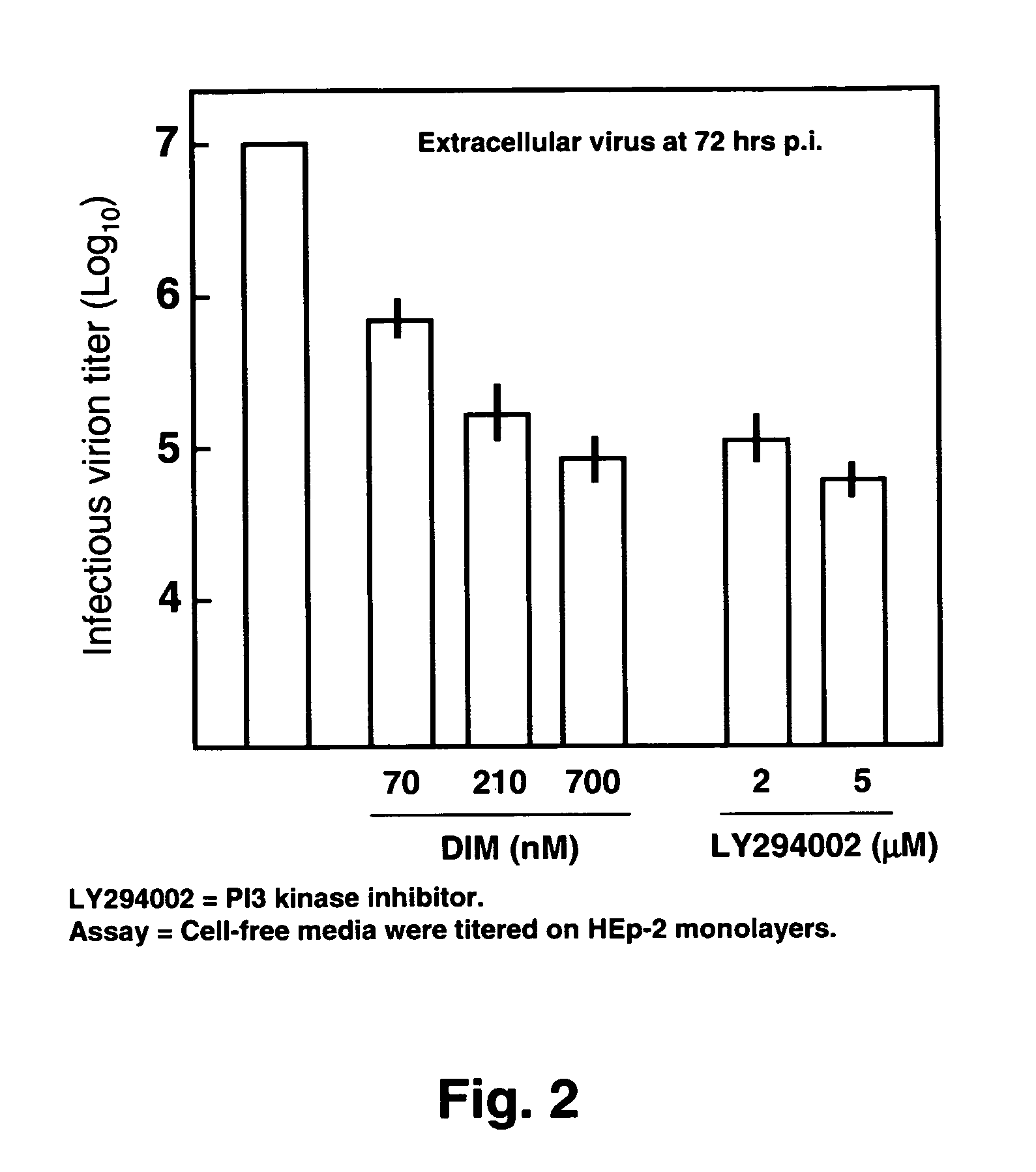
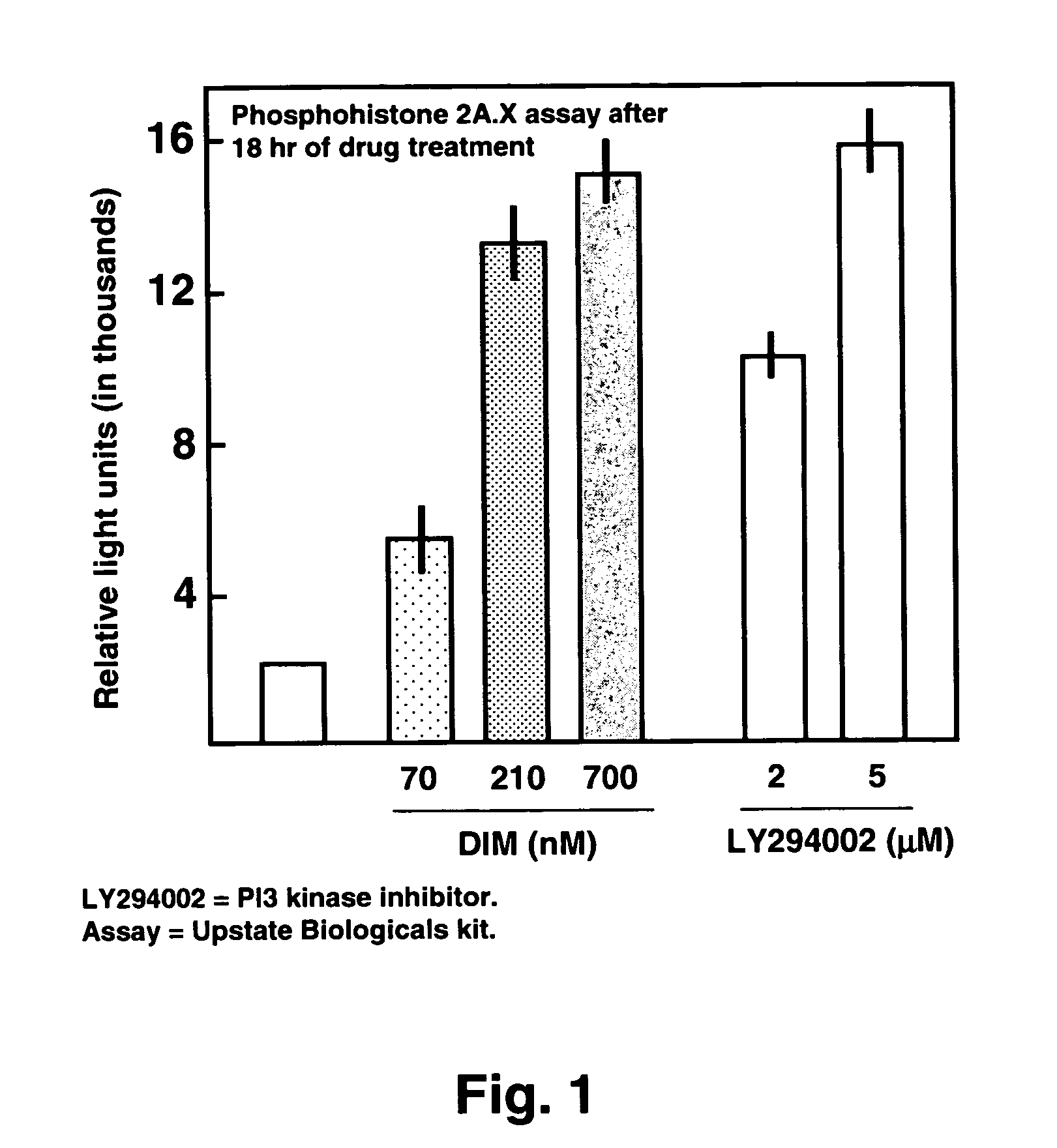
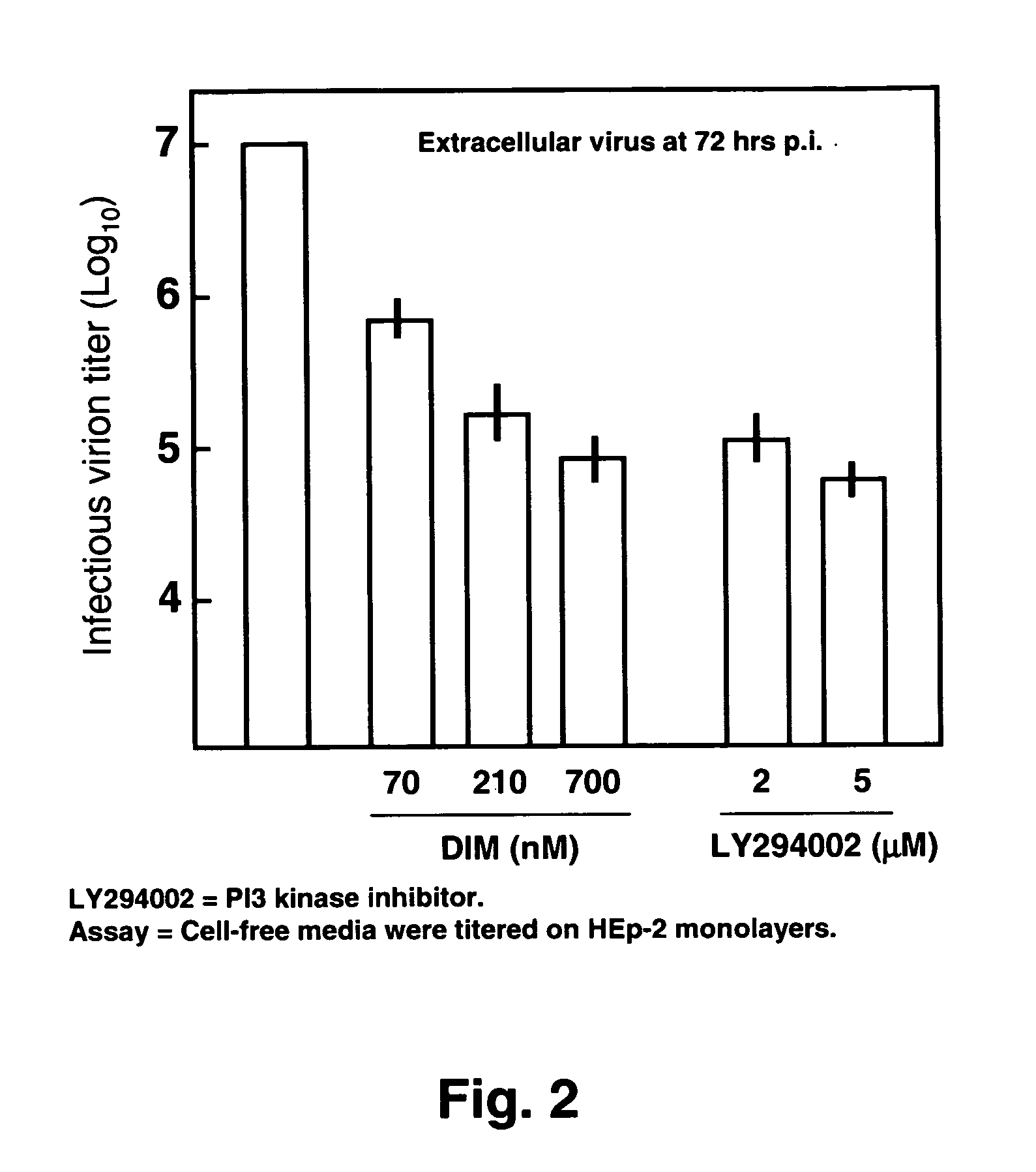
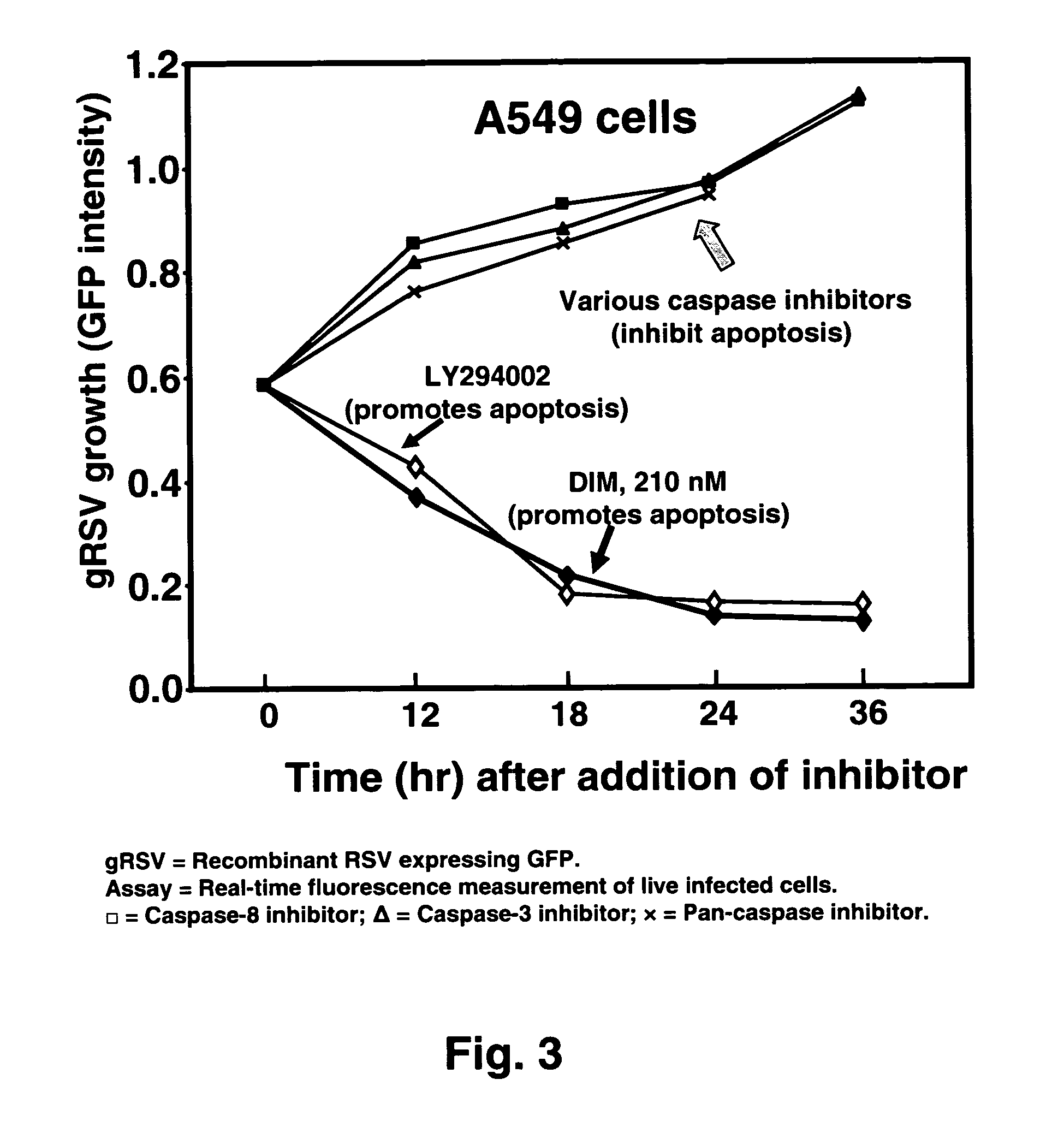
Use of diindolylmethane-related indoles for the treatment and prevention of respiratory syncytial virus associated conditions
The present invention includes compositions and methods for the treatment and prevention of conditions associated with Respiratory Syncytial Virus (RSV) infection. RSV-associated conditions include acute infections in mammals, typically bronchiolitis and pneumonia, and post-infectious chronic respiratory conditions. In particular, the present invention describes new therapeutic and preventative uses for 3,3′-diindolylmethane (DIM), or a DIM-related indole, alone or in combination with an inhibitor of a membrane bound Epidermal Growth Factor Receptor (EGFR) inhibitors, to treat conditions associated with exposure to RSV.
Owner:BIORESPONSE
Use of diindolylmethane-related indoles for the treatment and prevention of respiratory syncytial virus associated conditions
InactiveUS20080103114A1Improve developmentBiocideHydroxy compound active ingredientsMedicineBronchitis
The present invention includes compositions and methods for the treatment and prevention of conditions associated with Respiratory Syncytial Virus (RSV) infection. RSV-associated conditions include acute infections in mammals, typically bronchiolitis and pneumonia, and post-infectious chronic respiratory conditions. In particular, the present invention describes new therapeutic and preventative uses for 3,3′-diindolylmethane (DIM), or a DIM-related indole, alone or in combination with an inhibitor of a membrane bound Epidermal Growth Factor Receptor (EGFR) inhibitors, to treat conditions associated with exposure to RSV.
Owner:BIORESPONSE
Completely humanized neutralizing antibody for anti-rabies viruses
ActiveCN103910796ALow priceQuickly builds immune protectionHybrid immunoglobulinsImmunoglobulins against virusesAntigenAntigen epitope
The invention discloses a completely humanized neutralizing antibody for anti-rabies viruses. The amino acid sequence of a heavy chain of the antibody is shown in sequence table SEQ ID NO1, the amino acid sequence of a light chain of the antibody is shown in sequence table SEQ ID NO5, the complementary determining region (CDR) of a variable region of the heavy chain of the antibody is shown as follows: CDR1: SEQIDNO2, CDR2:SEQIDNO3 and CDR3: SEQIDNO4, and the complementary determining region (CDR) of a variable region of the light chain of the antibody is shown as follows: CDR1:SEQIDNO6, CDR2:SEQIDNO7, and CDR3: SEQIDNO8. The beneficial effects of the completely humanized neutralizing antibody are that: the completely humanized neutralizing antibody disclosed by the invention ahs the advantages of being completely humanized, good in specificity, high in affinity, good in neutralizing effect, and low in price, can be used as a biological engineering antibody drug to quickly establish immune protection force against rabies virus, can be used for passive immunotherapy of acute infection, and also can be used for preparing detection reagents for detecting the rabies virus, finding effective neutralization antigen epitope and developing recombinant proteins and subunit vaccines for the rabies virus.
Owner:CHANGCHUN BCHT BIOTECH
Novel slow-releasing ophthalmic compositions comprising povidone iodine
ActiveUS20140322345A1Increase the amount of lightBetter seeAntibacterial agentsSenses disorderClinical settingsOphthalmology
The present invention provides novel slow-releasing ophthalmic compositions containing Povidone Iodine (PVP-I) and uses thereof in the treatment of acute infections of at least one eye tissue from bacterial, mycobacterial, viral, fungal, or amoebic causes and for preventing such infections in appropriate clinical settings. Each of the ophthalmic compositions contains povidone iodine, osmotic pressure regulator, suspending agent and EDTA-Na, wherein povidone iodine exists as microsphere particles formed by PVP-I and sodium alginate.
Owner:IVIEW THERAPEUTICS
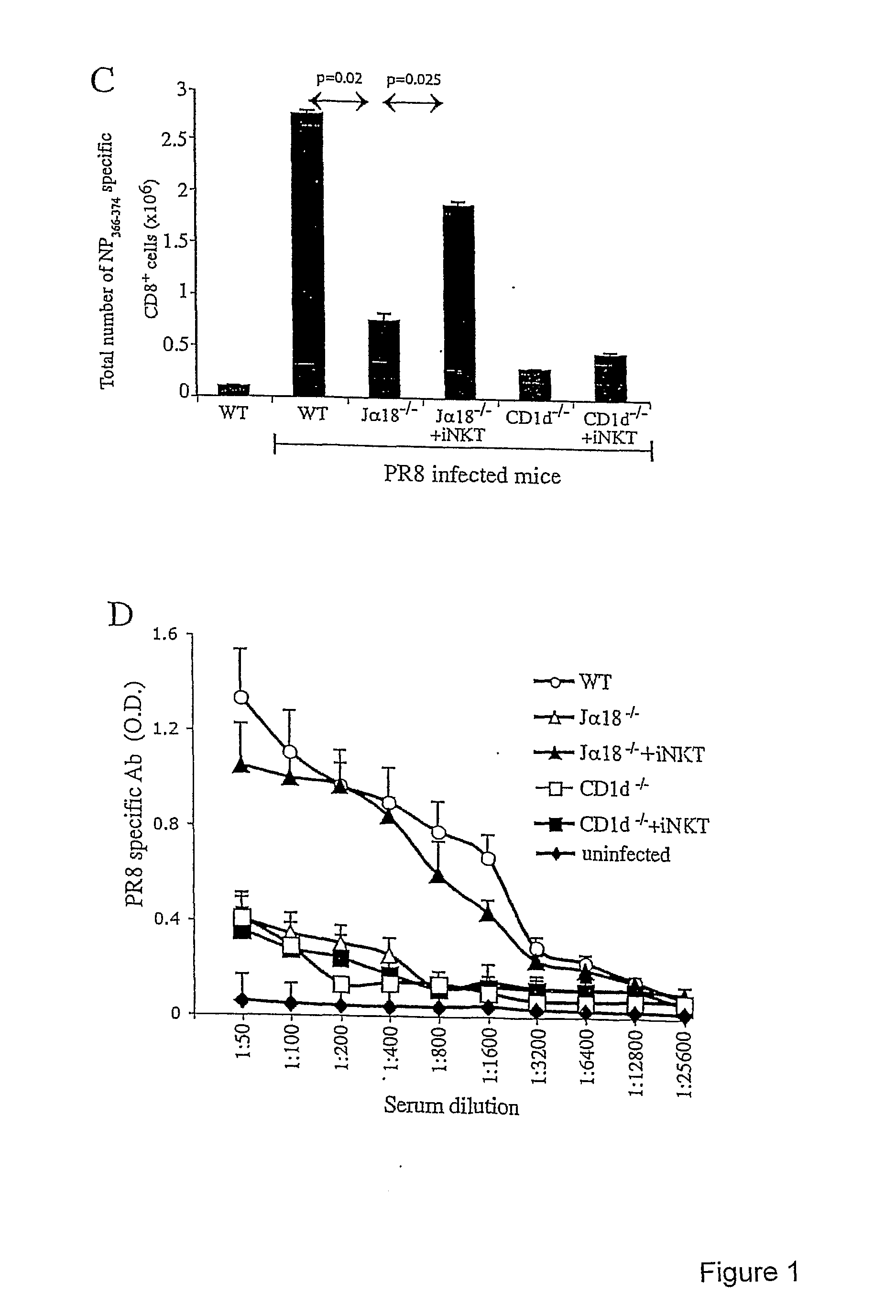
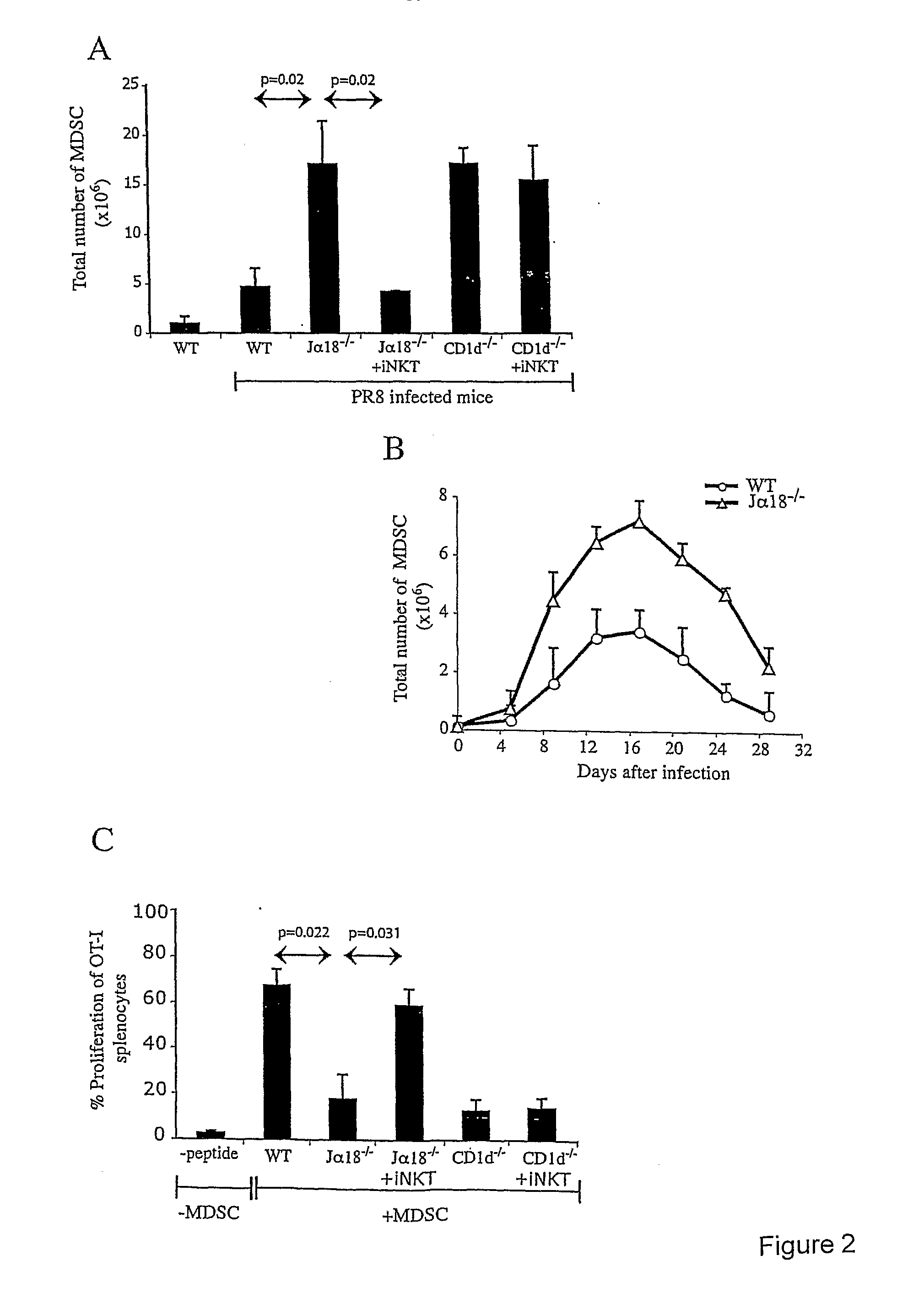
Use of inkt or tlr agonists for protecting against or treating a disease such as acute infection or cancer
InactiveUS20110293658A1Enhance MDSC suppressive activityIncrease the number ofAntibacterial agentsProtozoa antigen ingredientsAcute HIV infectionAcute infection
A method of protecting a mammalian subject against, or treating, a disease, wherein the mammalian subject has elevated numbers and / or activities of MDSC comprising administering to the subject a pharmaceutically acceptable amount of an iNKT agonist, such as alpha galactosylceramide or an analogue thereof, or a TLR agonist, or a combination thereof.
Owner:LUDWIG INST FOR CANCER RES
Diarrhea treating chinese medicine composition
ActiveCN100358549CInhibition of spontaneous movementNo obvious toxic reactionDigestive systemAgainst vector-borne diseasesInfective diarrhoeaToxic material
The diarrhea treating Chinese medicine composition is prepared with Chinese goldthread, skullcap root, white peony root, patchouly, guava leaf and tuckahoe as material. It has the functions of clearing away heat and toxic material, moisturizing and stopping diarrhea, and can kill diarrhea causing pathogenic bacteria and viruses to cure acute infectious diarrhea in high efficiency, quickly and safely.
Owner:惠州市九惠药业有限公司
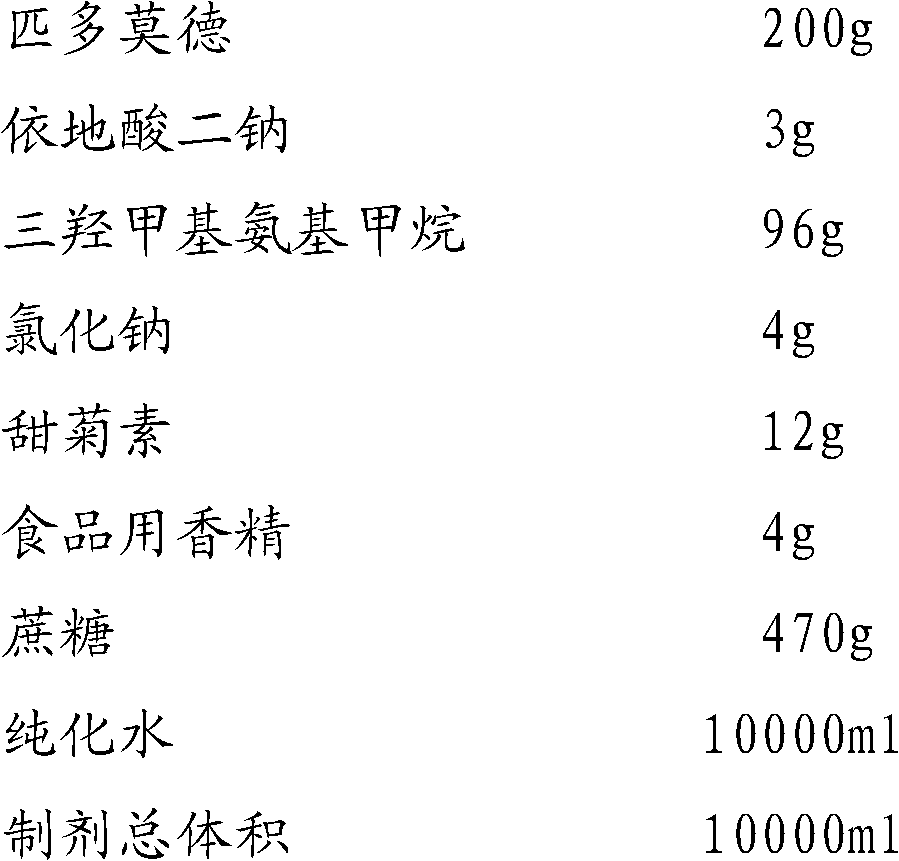
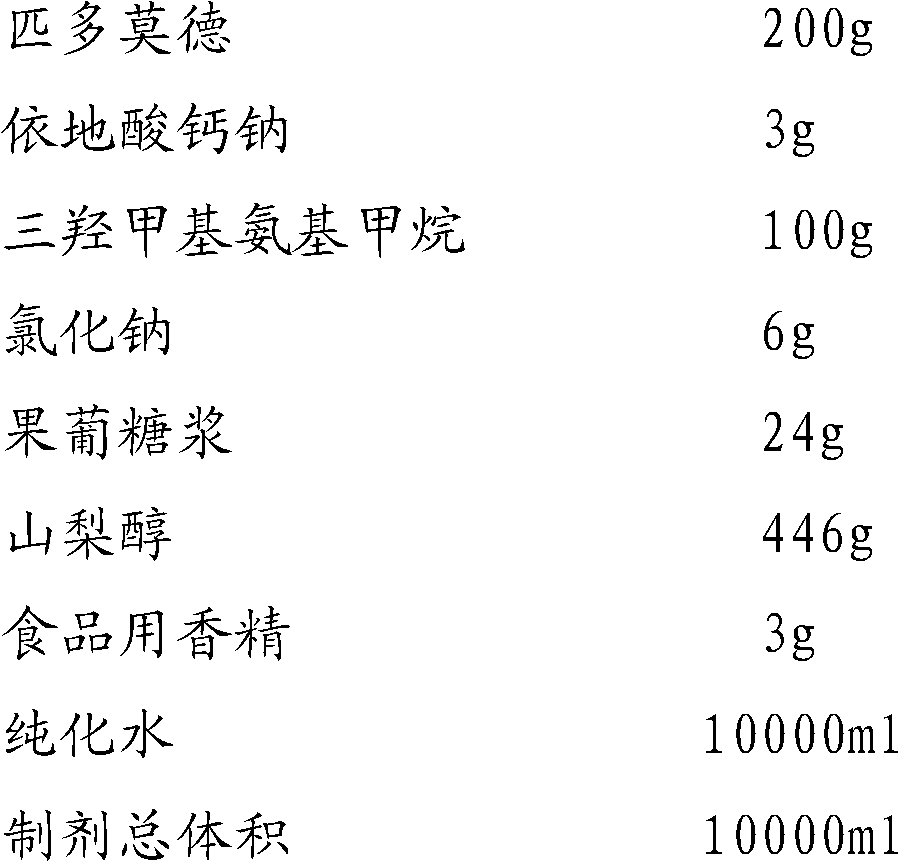
Oral liquid preparation of pidotimod
ActiveCN102525903AStable dissolutionStable storageDipeptide ingredientsPharmaceutical delivery mechanismDiseaseInduced infections
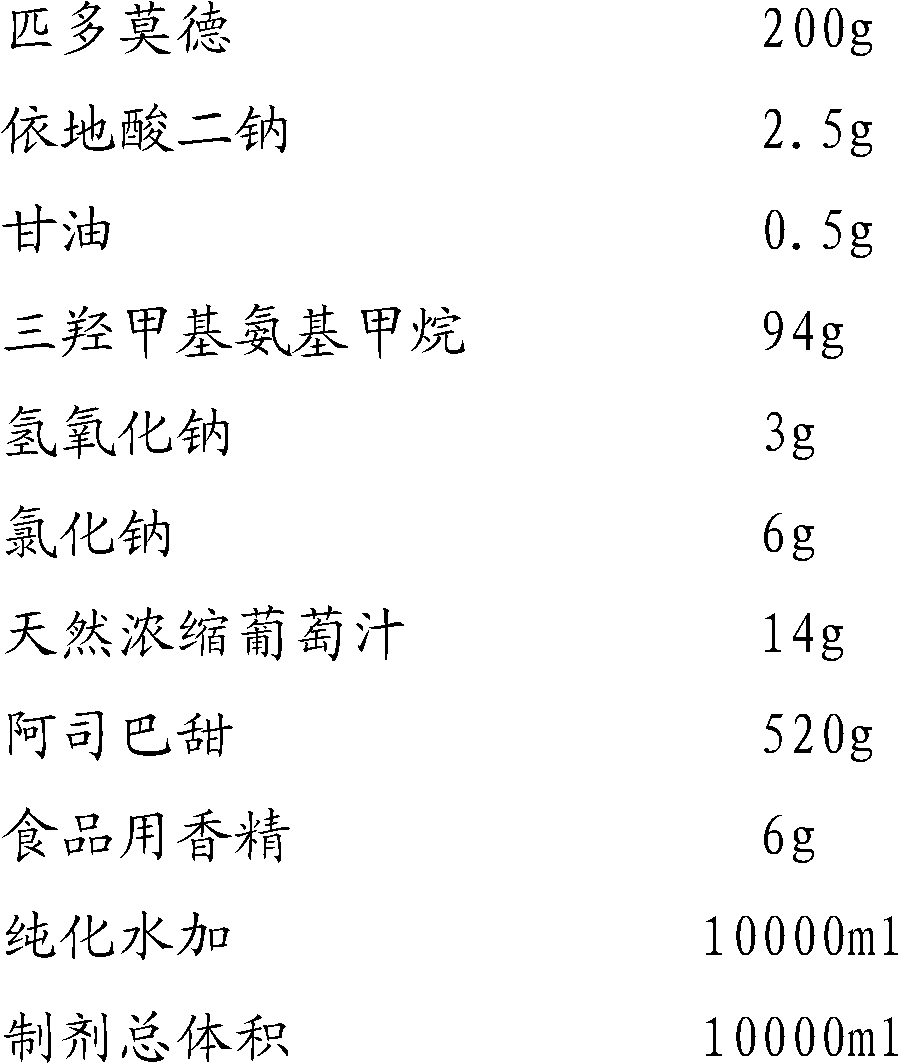
The invention provides an oral liquid preparation of pidotimod, which can promote immunoreaction of a human body, effectively reduces times and degree of treating acute attack of bacteria or virus induced infection, shortens the course of the disease, can serve as an auxiliary medicine of antibiotic during acute infection and belongs to the field of medicines. The oral liquid preparation comprises the pidotimod, a chemical stabilizer, a pH regulator, a flavoring agent and the like, wherein trihytdroxy methyl-aminomethane serves as the pH regulator; and stability of the solution can be effectively improved through quantitative proportion and synergistic effect of each ingredient. The oral liquid preparation has stable quality, is convenient to store for a long time and is particularly suitable for old people or children to take.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Human parainfluenza virus (HPIVs) IgM antibody detection test strip and preparation method thereof
The invention provides a human parainfluenza virus (HPIVs) IgM antibody detection test strip and a preparation method thereof. The human parainfluenza virus (HPIVs) IgM antibody detection test strip comprises a PVC bottom plate, a sample pad, a water absorption pad, a gold pad coated with an HPIVs specificity recombinant antigen, a detection line coated with a mouse anti-human IgM polyclonal antibody, and a nitrocellulose membrane (NC membrane) coated with a quality control line of a polyclonal antibody of an anti-HPIVs recombinant antigen. The human parainfluenza virus specific antibody IgM in serum of a human body is rapidly detected by applying an immunochromatographic method so that the acute infection of human parainfluenza viruses is conveniently subjected to differential diagnosis, and the certain significance is achieved for the clinical laboratory medicine.
Owner:秦志浩
Combined test reagent card for cytomegalovirus and rubella virus
ActiveCN101738474AHigh compliance rateImprove accuracyMaterial analysisNitrocelluloseCytomegalovirus antigen
The invention discloses a combined test reagent card for cytomegalovirus and rubella virus, which comprises a card cover, a card bottom and a test strip. The test strip is provided with a test sample region, a gold label region, a nitrocellulose membrane region and a water absorption region sequentially; and the nitrocellulose membrane region is provided with a quality-controlling line coated with goat anti mouse IgG, a testing line coated with cytomegalovirus antigen and a testing line coated with rubella virus antigen sequentially. The technical scheme adopted by the invention can test cytomegalovirus IgG antibody and rubella virus IgG antibody in the sample simultaneously and the total accordance rate of the testing result is higher, so the application prospect is wide. Compared with the detection of IgM antibody, the combined test reagent card for the cytomegalovirus and the rubella virus tests the IgG antibody; the IgG antibody can be carried for the whole life and is only associated with acute infection; and the accuracy is high.
Owner:山东康华生物医疗科技股份有限公司
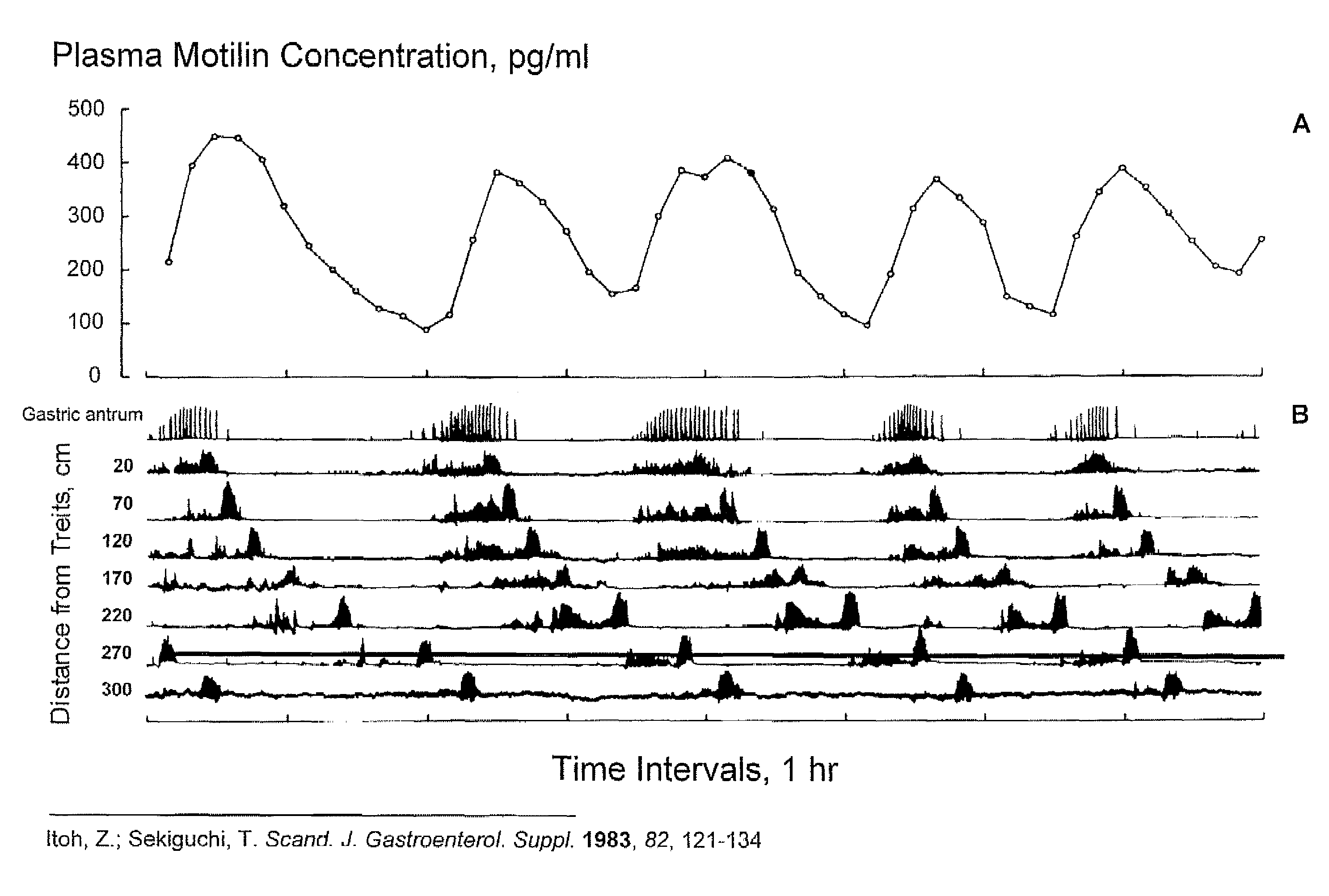
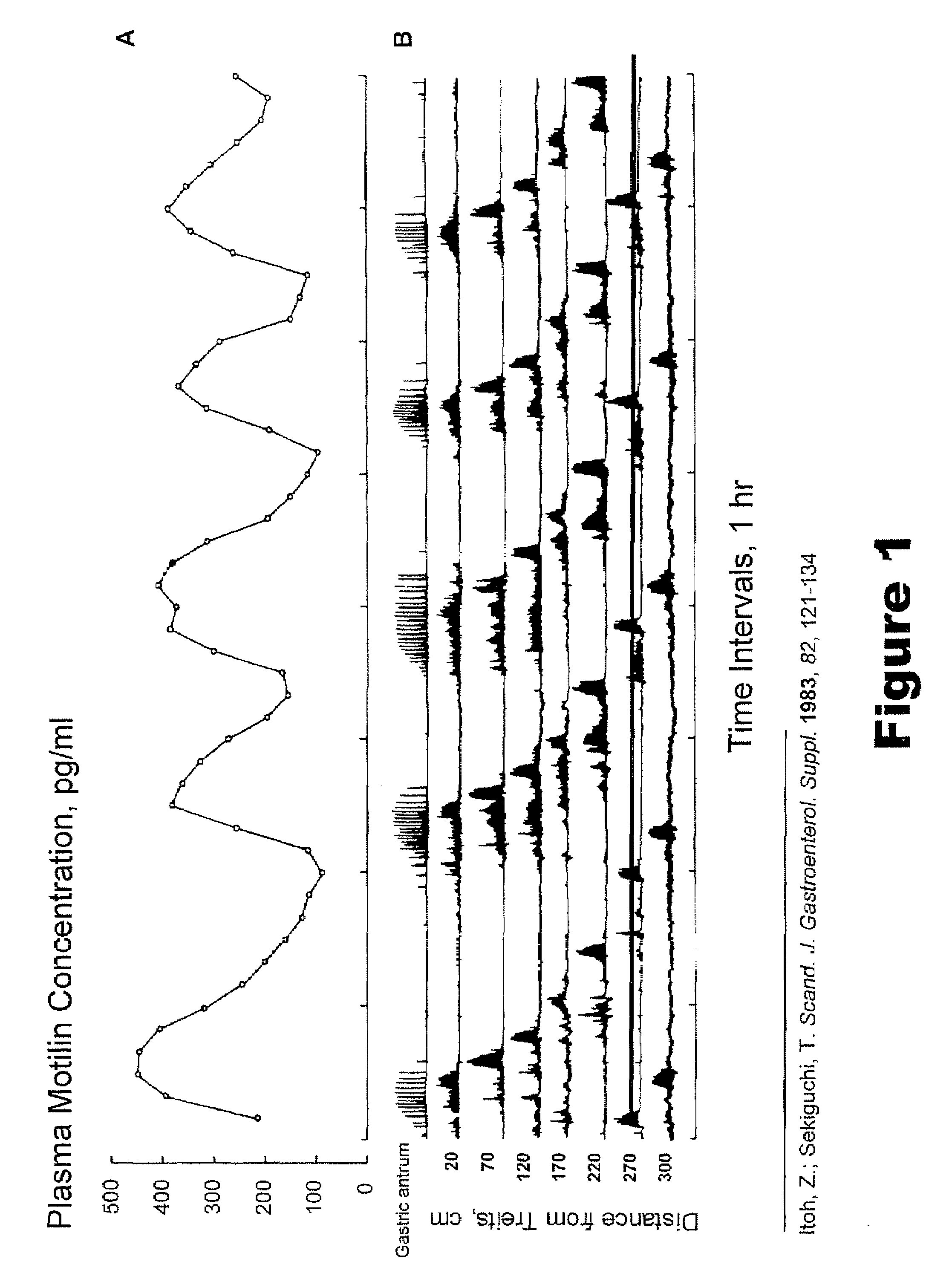
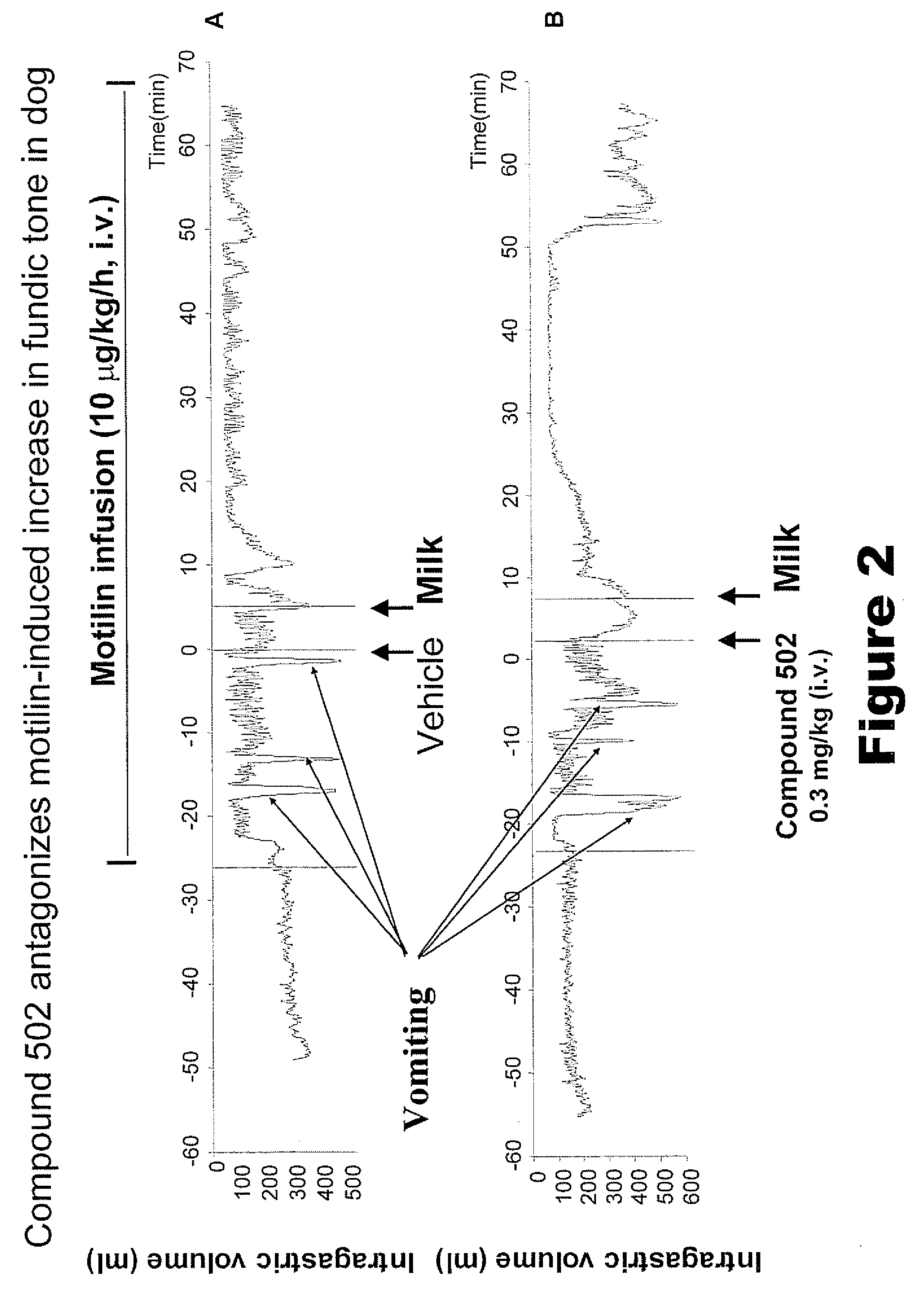
Macrocyclic antagonists of the motilin receptor for modulation of the migrating motor complex
InactiveUS20080287371A1Digestive systemTripeptide ingredientsStress inducedChemotherapy-induced nausea and vomiting
The present invention relates to novel conformationally-defined macrocyclic compounds that bind to and / or are functional modulators of the motilin receptor including subtypes, isoforms and / or variants thereof. These macrocyclic compounds are useful as therapeutics for a range of gastrointestinal disorders, in particular those in which suppression or inhibition of the migrating motor complex (MMC) is effective or malfunction of gastric motility or increased motilin secretion is observed, such as hypermotilinemia, imitable bowel syndrome, dyspepsia, including gallbladder dyspepsia, diarrhea, cancer treatment-related diarrhea, cancer-induced diarrhea, chemotherapy-induced diarrhea, radiation enteritis, radiation-induced diarrhea, stress-induced diarrhea, chronic diarrhea, AIDS-related diarrhea, C. difficile associated diarrhea, traveller's diarrhea, acute infectious diarrhea, diarrhea induced by graph versus host disease, other types of diarrhea, functional gastrointestinal disorders, chemotherapy-induced nausea and vomiting (emesis), post-operative nausea and vomiting, cyclic vomiting syndrome and functional vomiting. Accordingly, methods of treating such disorders with such macrocyclic compounds and pharmaceutical compositions thereof are also provided in addition to methods of modulating the migrating motor complex.
Owner:TRANZYME PHARMA INC
Injection containing pidotimod potassium salt and preparation method thereof
The invention discloses an injection containing a pidotimod potassium salt and a preparation method thereof. The preparation method comprises the following steps of: undergoing reaction between pidotimod and potassium hydroxide with equal mole in water or other solvent or a mixed solution of multiple solvents, preparing the pidotimod potassium salt and then preparing the injection. The injection containing the pidotimod potassium salt is characterized in that the effective component, the pidotimod, in the injection exists in the form of the pidotimod potassium salt, thus the solubility and the stability of the pidotimod in normal pH environment of a human body are improved. The injection containing the pidotimod potassium salt, disclosed by the invention, is used for treating the recurrent upper and lower respiratory tract infection (such as pharyngitis, tracheitis, bronchitis and tonsillitis), the recurrent infection (such as rhinitis, nasosinusitis and otitis), the urinary infection and gynecological infection, and can reduce the times of acute exacerbation, shorten the course of disease and relieve the degree of exacerbation; in addition, the injection can be also taken as the auxiliary treatment of antibiotics during the acute infection.
Owner:QINGDAO VLAND BIOTECH INC +1
Early detection method for arch insect infection
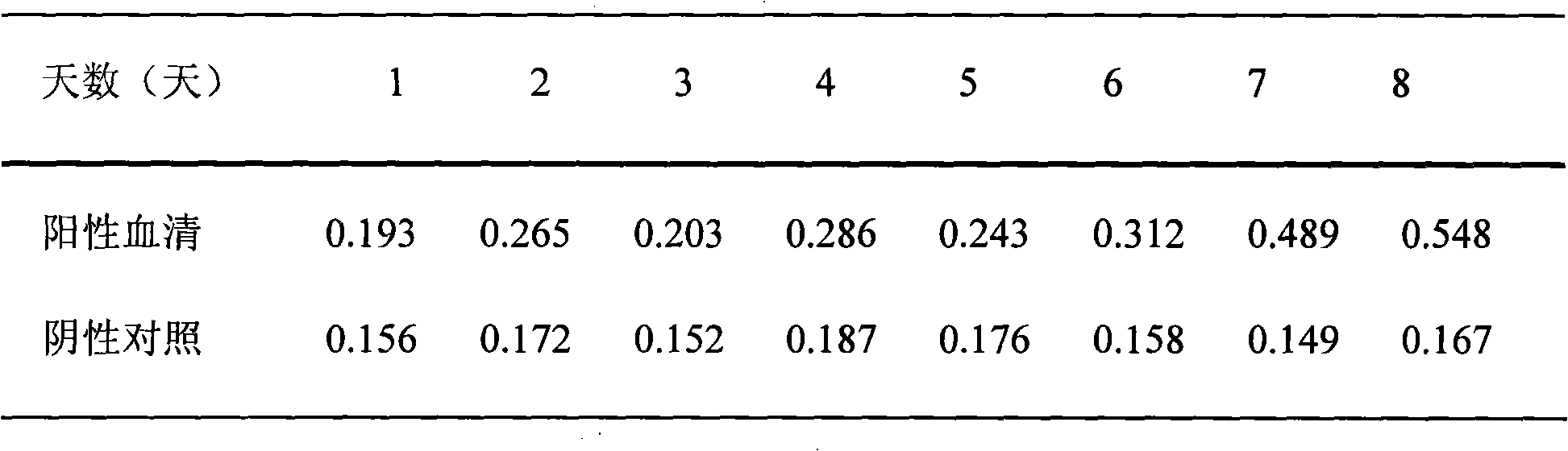
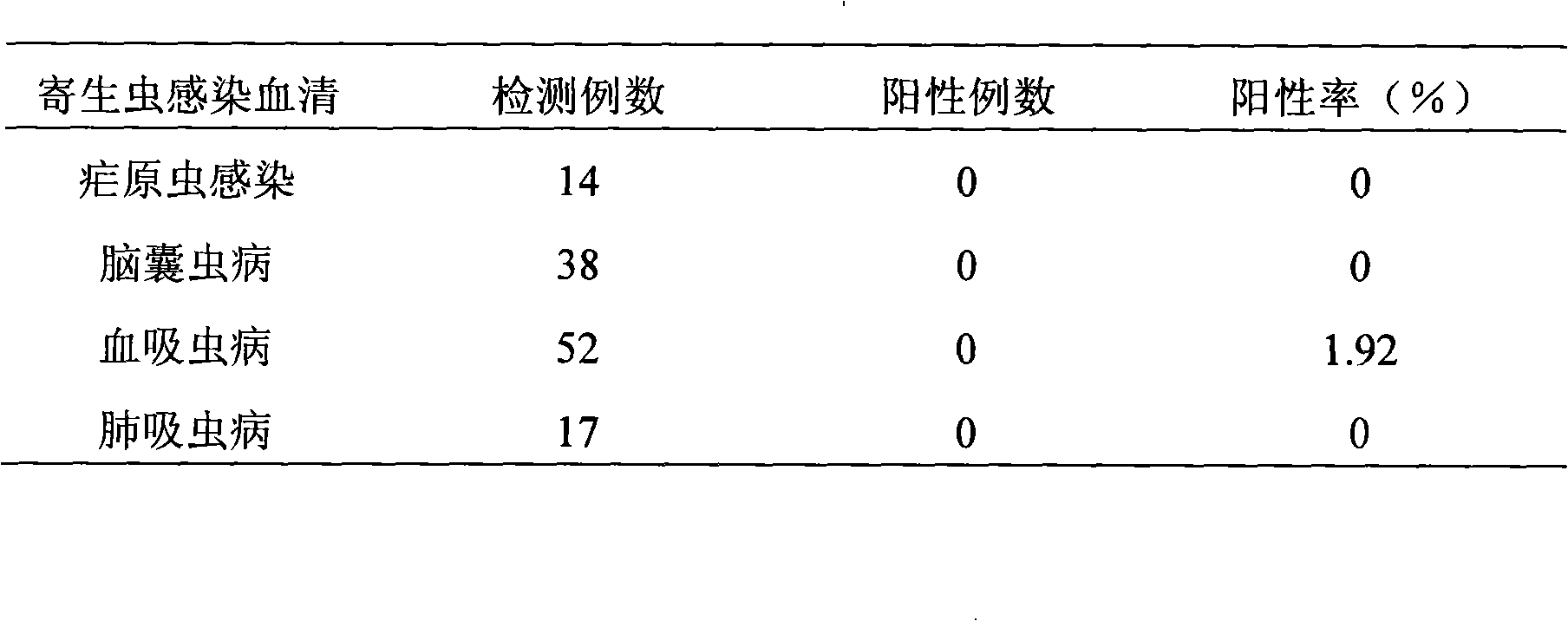
The invention relates to a method for detecting toxoplasma infection early. The nucleoside triphosphate hydrolase-II (NTPase-II) of toxoplasma is an indispensable key enzyme in the process that purine is remedied and synthesized by the toxoplasma by utilizing host cells, play an important part in parasitism and propagation of the toxoplasma, and is a major antigen for causing a body to generate immune reaction in the acute infection stage of the toxoplasma. The gene of the toxoplasma nucleoside triphosphate hydrolase-II is expanded by utilizing a polymerase chain reaction through the invention, expression plasmids are constructed and recombined in a expression vector, the recombined expression plasmids are transferred to procaryotic cells for expression, recombined fusion protein NTPase-II is obtained, the recombined fusion protein NTPase-II is used as an indirect ELISA method to establish a coating antigen, and is used as the method for detecting the toxoplasma infection early.
Owner:WENZHOU MEDICAL UNIV
Attenuated live vaccine for preventing infection of toxoplasma gondii and application of attenuated live vaccine
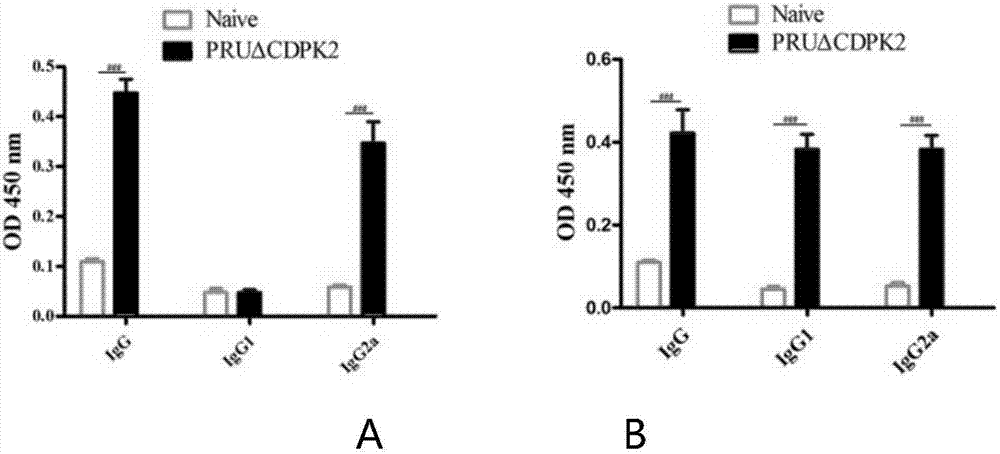
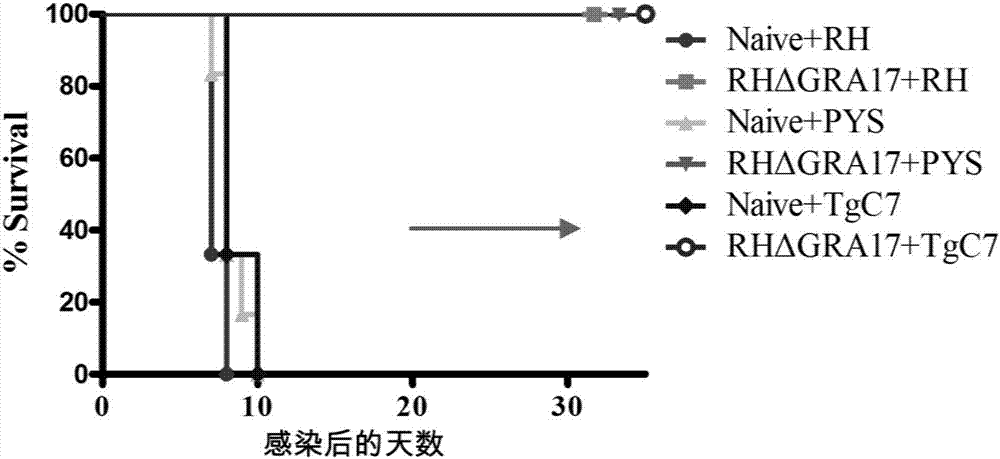
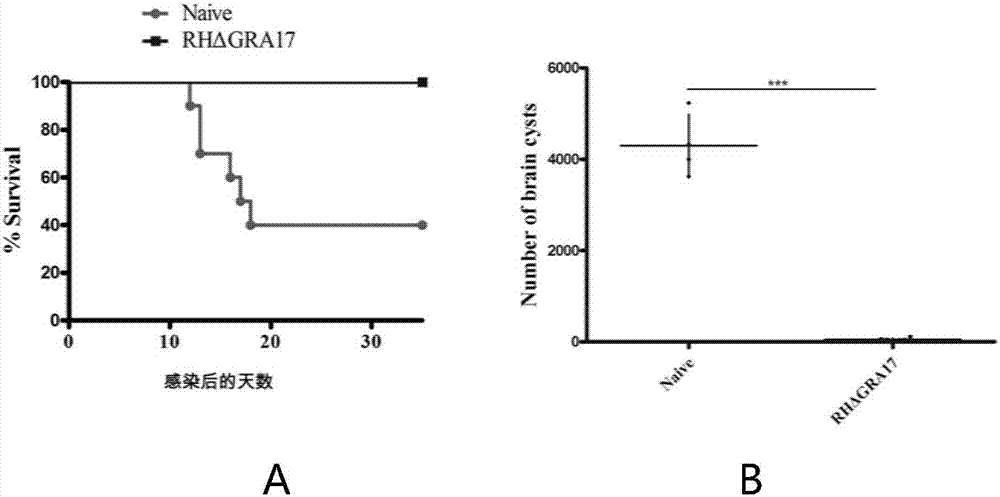
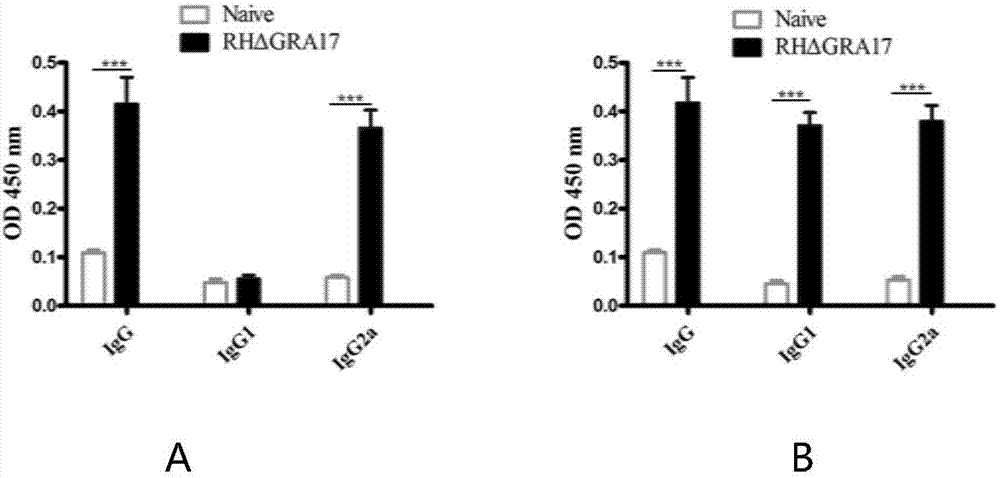
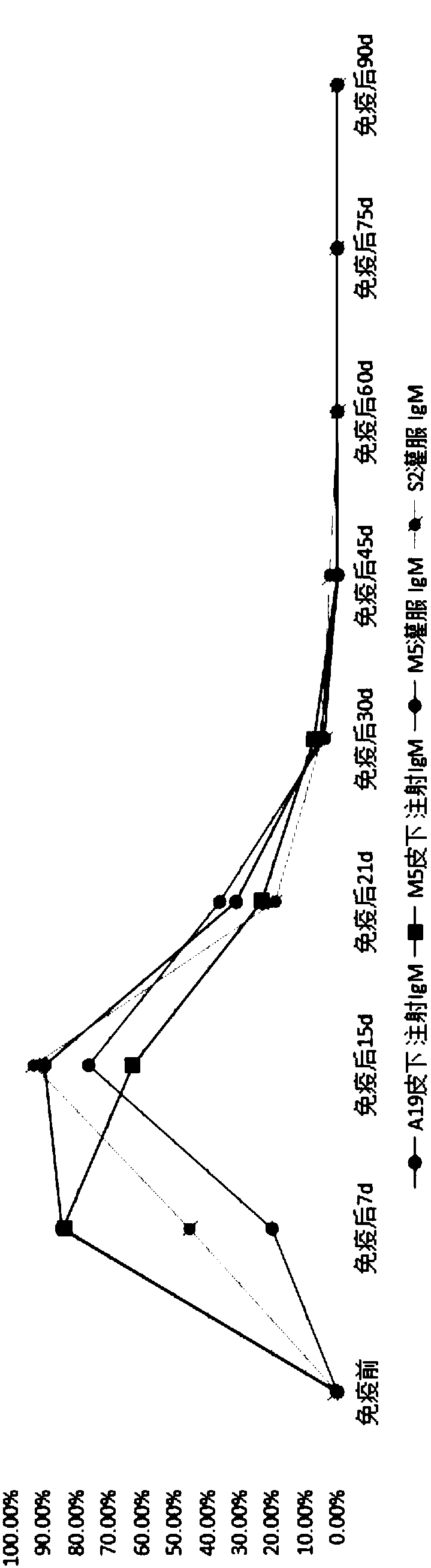
InactiveCN107281477AAvoid infectionPrevent acute infectionProtozoa antigen ingredientsSolution deliveryAcute infectionAttenuated Live Vaccine
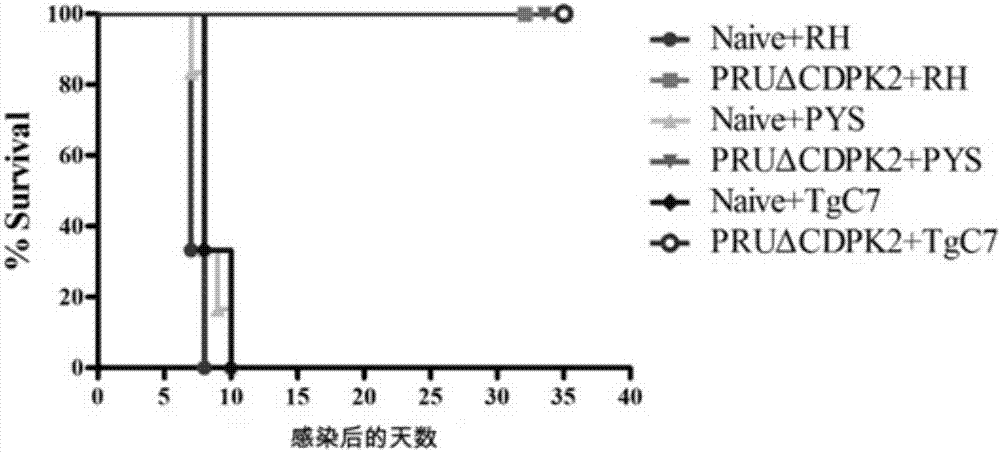
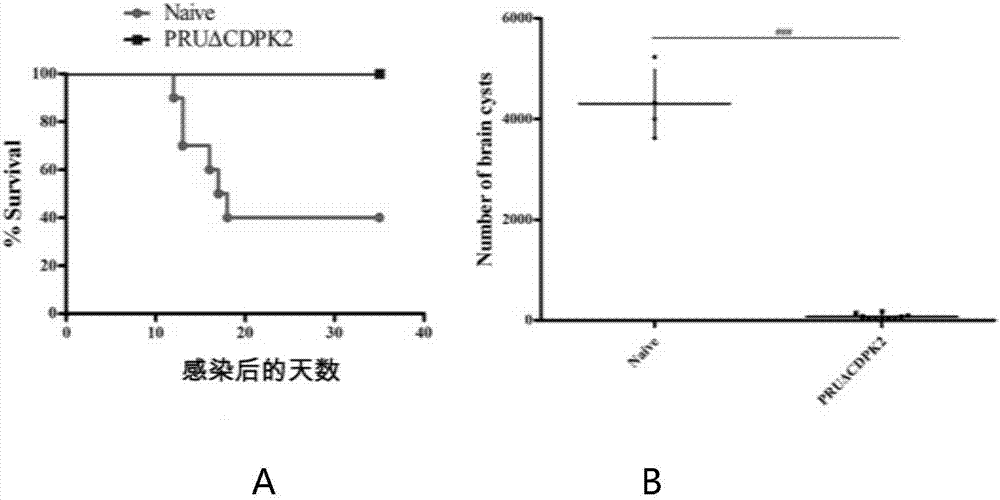
The invention discloses an attenuated live vaccine for preventing the infection of toxoplasma gondii and application of the attenuated live vaccine. The attenuated live vaccine is a toxoplasma gondii attenuated strain PRU delta CDPK2 tachyzoite suspension prepared by adopting PBS as a solvent. An immune dosage form of the attenuated live vaccine is determined, an immune inoculation program and an inoculation amount of the vaccine are ascertained, the attenuated live vaccine has higher immunity protection effect for the acute infection, chronic infection and congenital infection of the toxoplasma gondii by virtue of an animal experiment, not only can prevent the normal infection of the toxoplasma gondii, but also can effectively prevent the vertical propagation of the toxoplasma gondii by virtue of a mother-infant route, is attenuated live vaccine with great application value, can be used for preventing the chronic infection, acute infection and congenital infection of the toxoplasma gondii, and can effectively prevent the toxoplasma gondii.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Reagent case for diagnosing gene of pathogenic bacterial and para hematolysis vibrion of marine water product animal and hunman and testing method thereof
InactiveCN1560274AGuaranteed speedGuaranteed accuracyMicrobiological testing/measurementAgainst vector-borne diseasesAquatic animalVibrio parahaemolyticus
Owner:SUN YAT SEN UNIV +1
Method for stimulating the immune response of newborns
InactiveUS20120128715A1Enhance immune responsePreventing or treatingAntibacterial agentsOrganic active ingredientsAgonistAcute infection
The present invention is based on the surprising discovery that agonists of TLR8 are uniquely efficacious in enhancing (e.g. inducing) the immune response of newborns. Thus, agonists of TLR8 serve as both vaccine adjuvants and as adjunctive therapies for acute infection in newborns, preferably the agonist is a TLR8-selective agonist. The immune response induced, or enhanced, in the neonatal host can be, for example, a cytokine immune response and / or a humoral immune response (e.g., antigen-specific).
Owner:3M INNOVATIVE PROPERTIES CO +1
Kit for diagnosing gene of pathogenic bacterial and river vibrion of aquatic animal and human and testing method thereof
InactiveCN1560273AGuaranteed speedGuaranteed accuracyMicrobiological testing/measurementBacteroidesAquatic animal
The invention provides a gene diagnosis reagent box and its detecting method for aquatic animal (like cyprinoid, Penaeus monodon, Epinephelus awoara, ormer, etc) and human pathogens-Vibrio fluvialis, designed by using a pair of primers designed by the sequence in conservative region of Vibrio fluvialis gene to act as main body. It adopts polyase chain reaction (PCR) technique to qualitatively detect the specific DNA fragments of the Vibrio fluvialis, simply and conveniently, and rapidly, good-specificity, and high-sensitivity; can be used in bacteria tracking detection of aquatic animal pathogens in the course of breeding in each period, and also the clinic detection of human intestinal acute infections, as well as environmental monitoring, avoiding the pathogens infecting and popularizing and it has very great practical value.
Owner:SUN YAT SEN UNIV +1
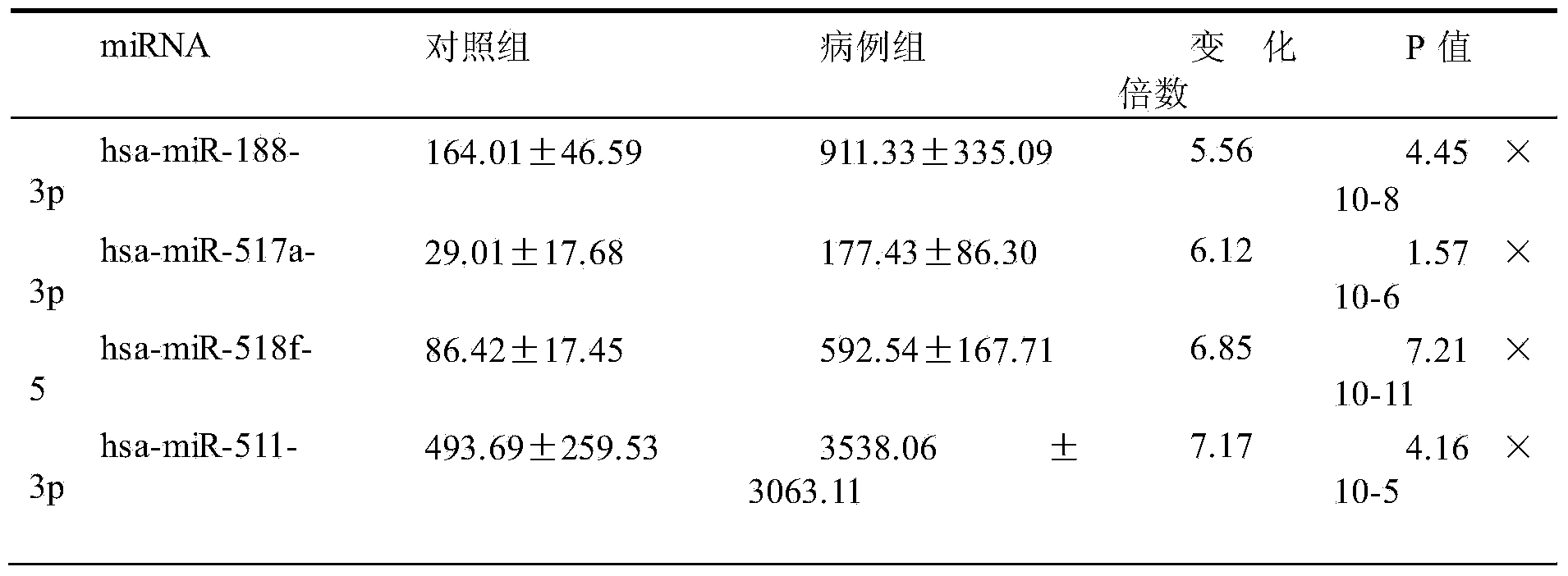
Kit based on serum miRNA as well as use method and application of kit
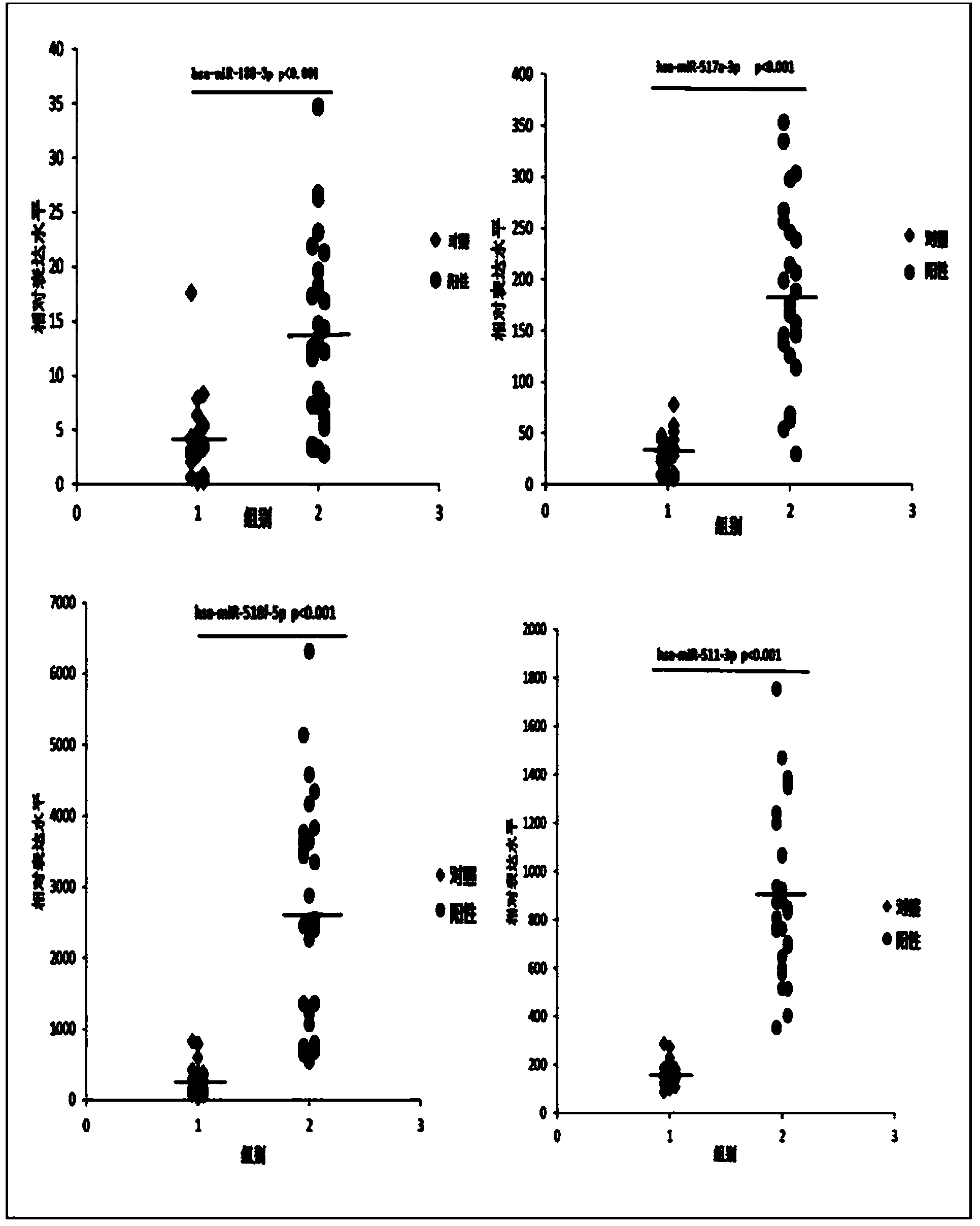
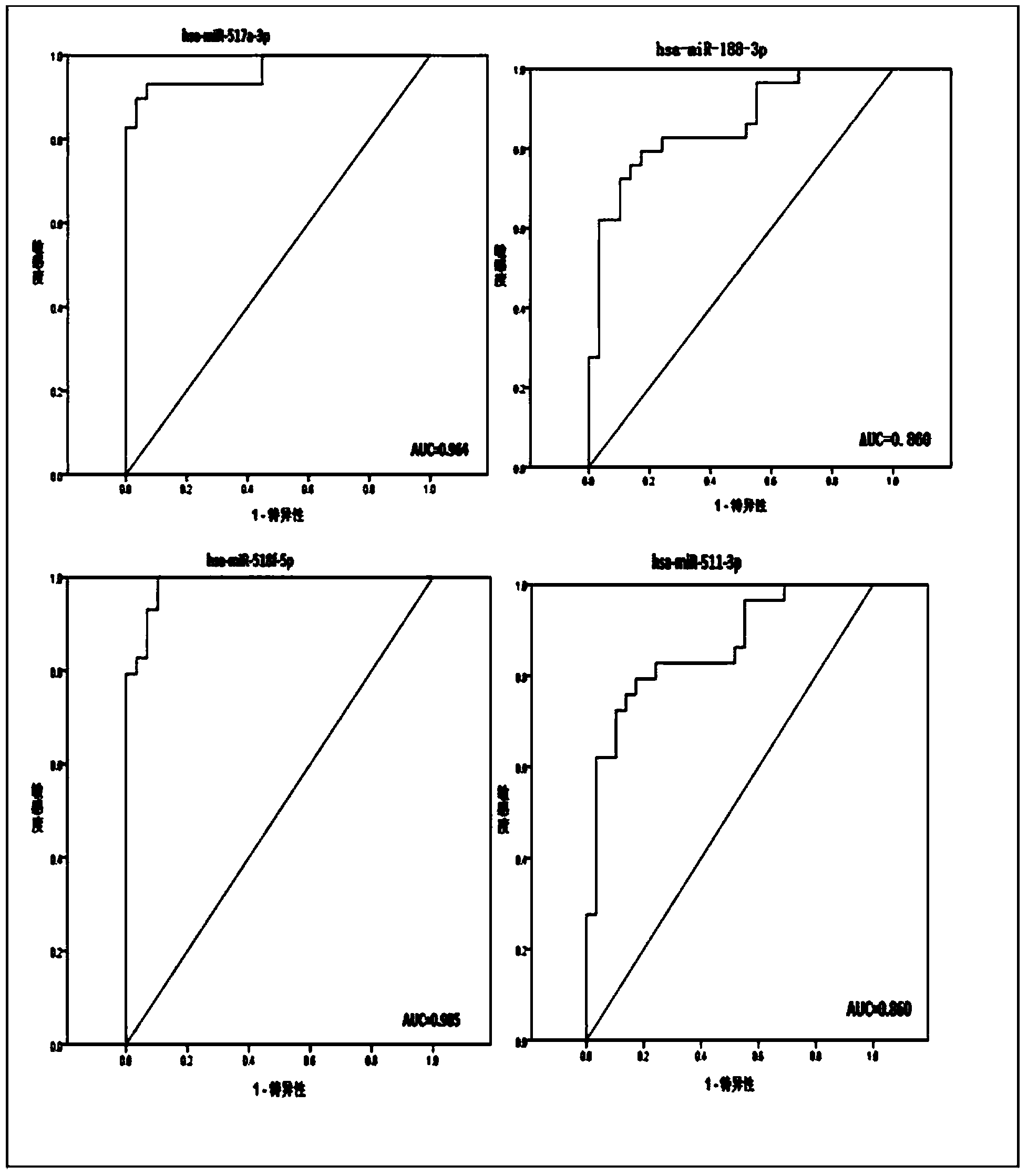
The invention belongs to the technical field of biology and in particular relates to a kit based on serum miRNA as well as a use method of the kit and application of the kit to early infection detection of fever with thrombocytopenia syndrome viruses. MiRNA molecules comprise hsa-miR-188-3p, hsa-miR-517a-3p, hsa-miR-518f-5p and hsa-miR-511-3p. The expression of the four miRNAs in early attack of the fever with thrombocytopenia syndrome viruses is specifically and remarkably increased through identification by adopting a Taqman low-density chip and a qRT-PCR (quantitative reverse transcription polymerase chain reaction) method. The four serum miRNAs are used as molecular targets, and a quick, sensitive and specific detection method for acute infection of the fever with thrombocytopenia syndrome viruses is built. The hsa-miR-188-3p, the hsa-miR-517a-3p, the hsa-miR-518f-5p and the hsa-miR-511-3p are applied to early diagnosis of fever with thrombocytopenia syndrome virus infection, and the kit is used for detecting the hsa-miR-188-3p, the hsa-miR-517a-3p, the hsa-miR-518f-5p and the hsa-miR-511-3p.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
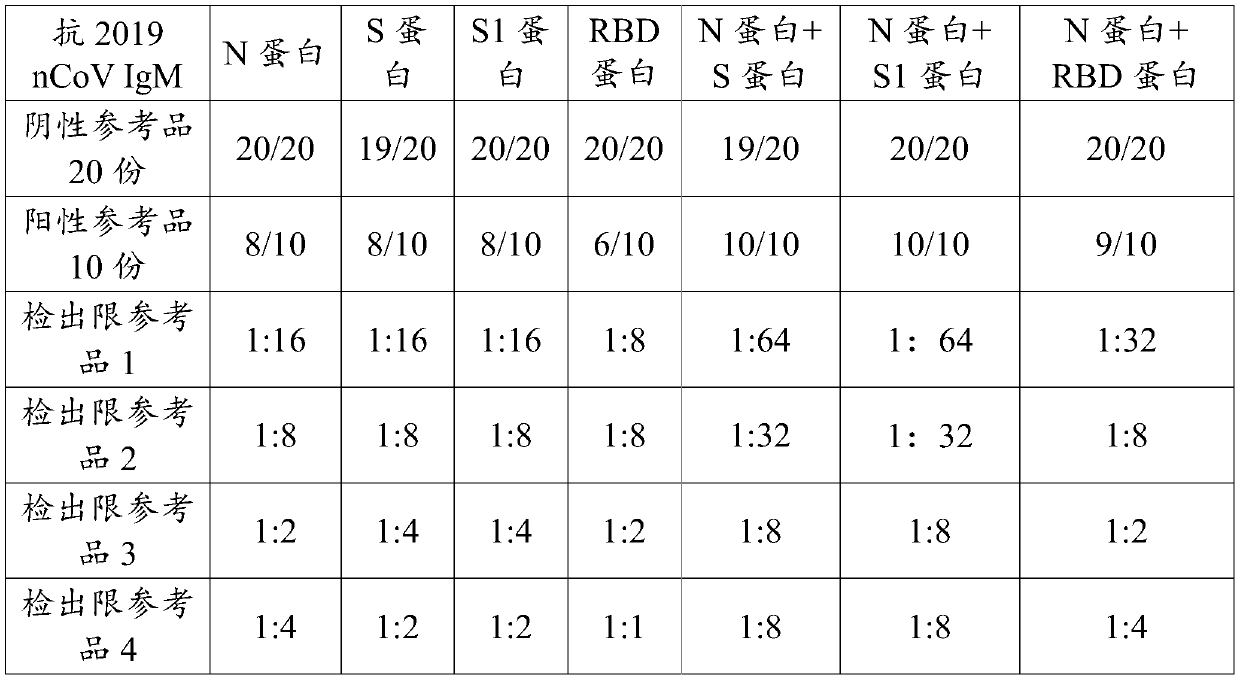
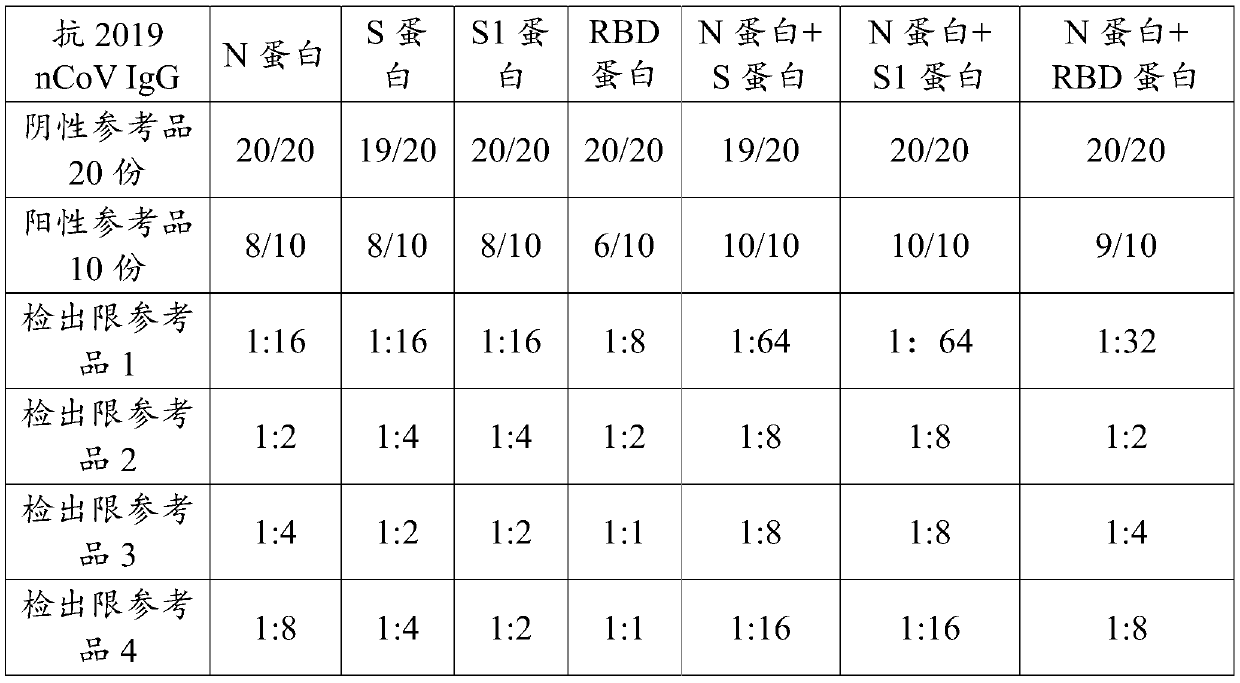
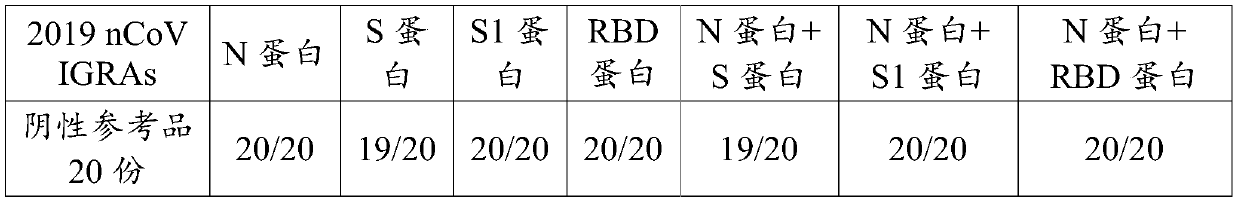
Novel coronavirus detection kit and detection method
ActiveCN111474348AImprove the detection rateEasy to trackBiological material analysisAgainst vector-borne diseasesInapparent InfectionAsymptomatic
The invention discloses a detection kit for detecting a novel coronavirus. The kit comprises an anti-2019-nCoV IgM reaction conjugate, an anti-2019-nCoV IgG reaction conjugate, a 2019-nCoV IGRAs reaction conjugate, an anti-2019-nCoV IgM marker, an anti-2019-nCoV IgG marker, a 2019-nCoV IGRAs marker and the like. The kit disclosed by the invention has the advantages that the detection results of the anti-2019-nCoV IgM, the anti-2019-nCoV IgG and the 2019-nCoV IGRAs are combined; the kit is helpful for early auxiliary diagnosis of the 2019-nCoV in the acute infection period and the recurrence period, judgment of recent infection and long-term infection of the 2019-nCoV, and auxiliary diagnosis of dominant infectors and recessive infectors when antibodies of the 2019-nCoV infectors do not appear. Positive infected people can be detected and confirmed more simply, conveniently, rapidly and accurately, the detection rate of novel coronavirus nucleic acid negative suspected patients is increased, and light or asymptomatic novel coronavirus pneumonia restorers are tracked.
Owner:GUANGZHOU FENGHUA BIOENG
Attenuated live vaccine used for preventing toxoplasma infection and application of attenuated live vaccine
InactiveCN107308444AAvoid infectionPrevent acute infectionProtozoa antigen ingredientsAntiparasitic agentsAcute infectionSolvent
The invention discloses an attenuated live vaccine used for preventing toxoplasma infection and application of the attenuated live vaccine. The attenuated live vaccine takes PBS as a solvent to prepare a tachyzoite suspension of a toxoplasmosis attenuative strain RHdetltaGRA17. An immune preparation for the attenuated live vaccine is established, the immunization procedure and the inoculum size of the vaccine are explored, and the animal experiment shows that the attenuated live vaccine has a stronger immunoprotection function for the acute infection, the chronic infection and the congenital infection of toxoplasmosis, the normal infection of the toxoplasmosis is prevented, meanwhile, the mother-to-fetus vertical propagation of the toxoplasmosis is effectively stopped, and the vaccine has a huge application value, and can be used for preventing the acute infection, the chronic infection and the congenital infection of toxoplasmosis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
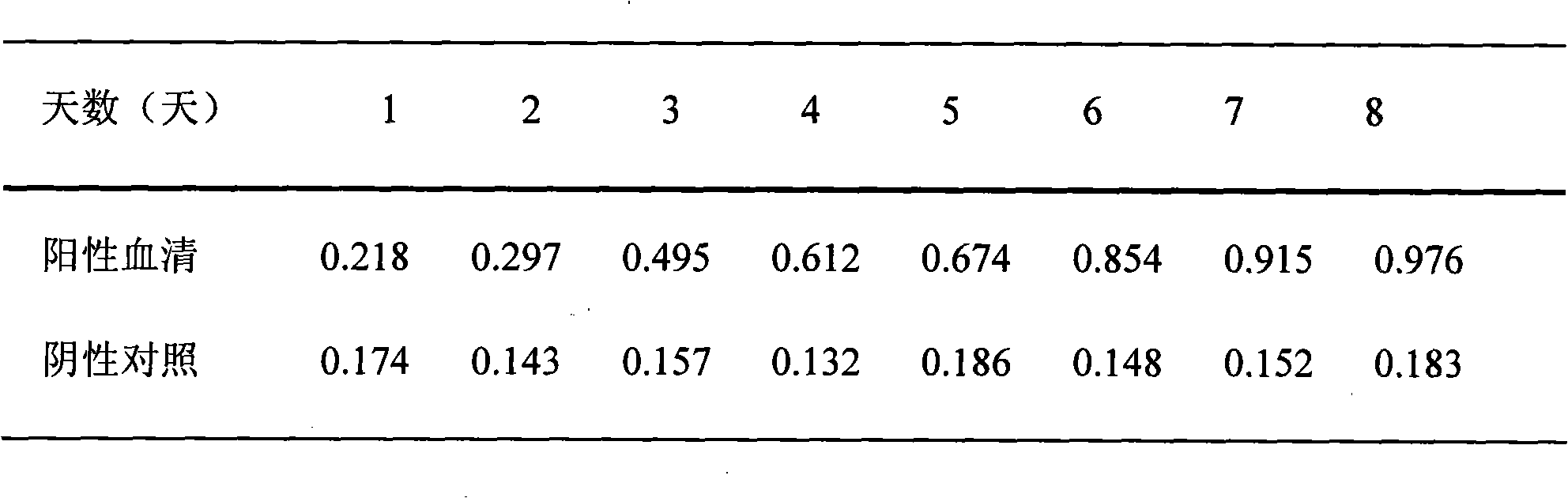
ELISA (enzyme-linked immunosorbent assay) reagent kit for detecting IgM antibodies of brucellosis and application of ELISA reagent kit
The invention discloses an ELISA (enzyme-linked immunosorbent assay) reagent kit for detecting IgM antibodies of brucellosis and application of the ELISA reagent kit. The ELISA reagent kit comprises brucella antigen coated microporous plates, HRP (horseradish peroxide) labeled brucella antigens, negative reference substances, positive reference substances, color developing agents A, color developing agents B, stop solution and concentrated cleaning solution. The application of the ELISA reagent kit on the basis of double-antigen sandwich immunoassay principles includes adding to-be-detected samples into the microporous plates, binding unknown antibodies in the samples with solid-phase antigens, carrying out washing to form stable unknown antibody-antigen complexes, adding labeled antigensinto the unknown antibody-antigen complexes to bind the labeled antigens with blank sites of IgM antibodies so as to form labeled antigen-IgM antibody-antigen complexes, washing the labeled antigen-IgM antibody-antigen complexes, and then adding the color developing agents into the labeled antigen-IgM antibody-antigen complexes; measuring absorbance OD (optical density) values under the conditionof detection wavelengths of 450 nm and then judging the to-be-detected samples according to critical values. The shade of colors and the degrees of concentration are in positive correlation function relationships. The ELISA reagent kit and the application have the advantage that the ELISA reagent kit can be used for monitoring early acute infection to detect the brucellosis of pigs, cattle and sheep.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Slow-releasing ophthalmic compositions comprising povidone iodine
ActiveUS9308173B2Increase the amount of lightBetter seeAntibacterial agentsSenses disorderClinical settingsOphthalmology
Owner:IVIEW THERAPEUTICS
A fully human neutralizing antibody against rabies virus
ActiveCN103910796BLow priceQuickly builds immune protectionHybrid immunoglobulinsImmunoglobulins against virusesPassive ImmunizationsAntigen epitope
The invention discloses a completely humanized neutralizing antibody for anti-rabies viruses. The amino acid sequence of a heavy chain of the antibody is shown in sequence table SEQ ID NO1, the amino acid sequence of a light chain of the antibody is shown in sequence table SEQ ID NO5, the complementary determining region (CDR) of a variable region of the heavy chain of the antibody is shown as follows: CDR1: SEQIDNO2, CDR2:SEQIDNO3 and CDR3: SEQIDNO4, and the complementary determining region (CDR) of a variable region of the light chain of the antibody is shown as follows: CDR1:SEQIDNO6, CDR2:SEQIDNO7, and CDR3: SEQIDNO8. The beneficial effects of the completely humanized neutralizing antibody are that: the completely humanized neutralizing antibody disclosed by the invention ahs the advantages of being completely humanized, good in specificity, high in affinity, good in neutralizing effect, and low in price, can be used as a biological engineering antibody drug to quickly establish immune protection force against rabies virus, can be used for passive immunotherapy of acute infection, and also can be used for preparing detection reagents for detecting the rabies virus, finding effective neutralization antigen epitope and developing recombinant proteins and subunit vaccines for the rabies virus.
Owner:CHANGCHUN BCHT BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com