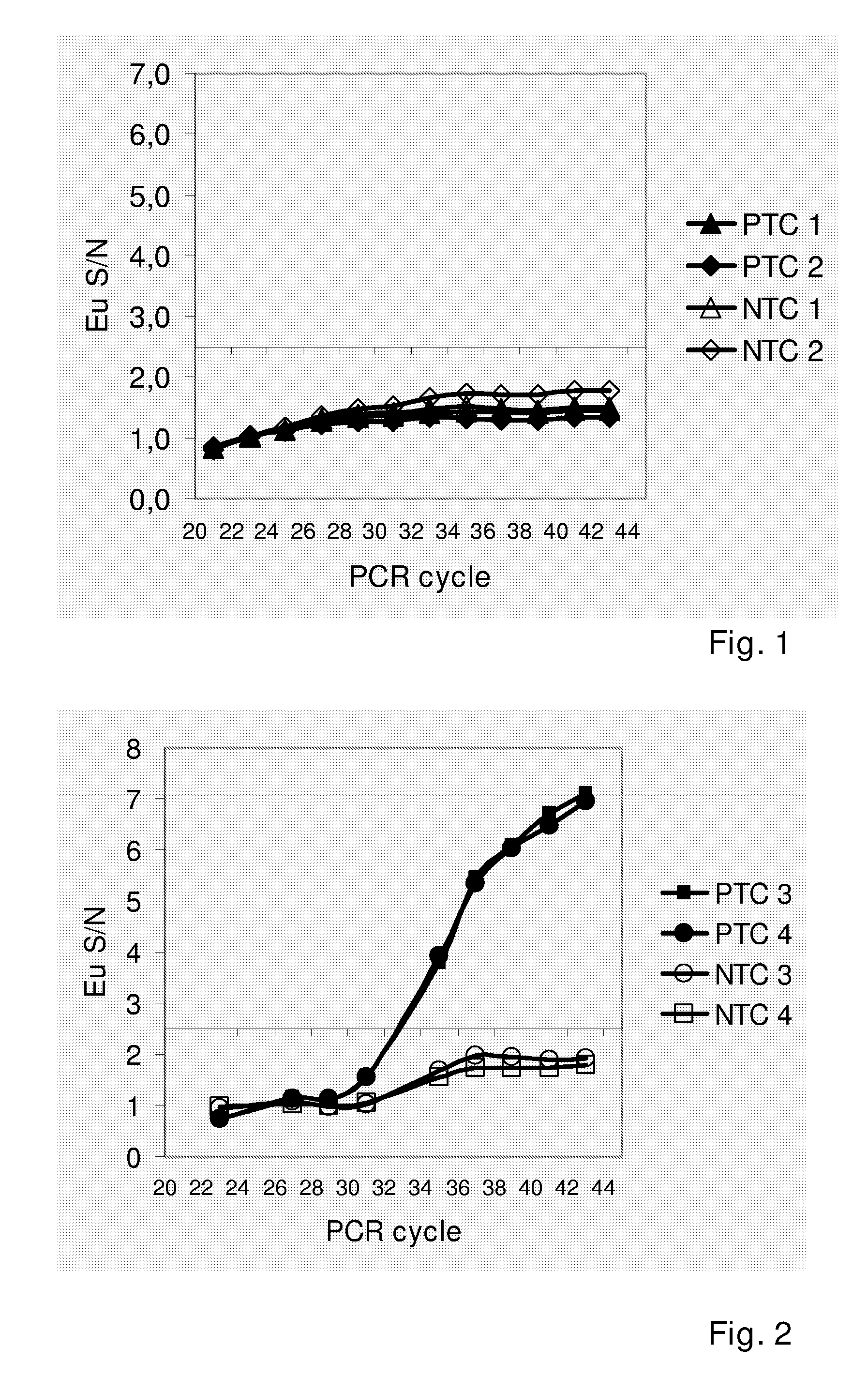
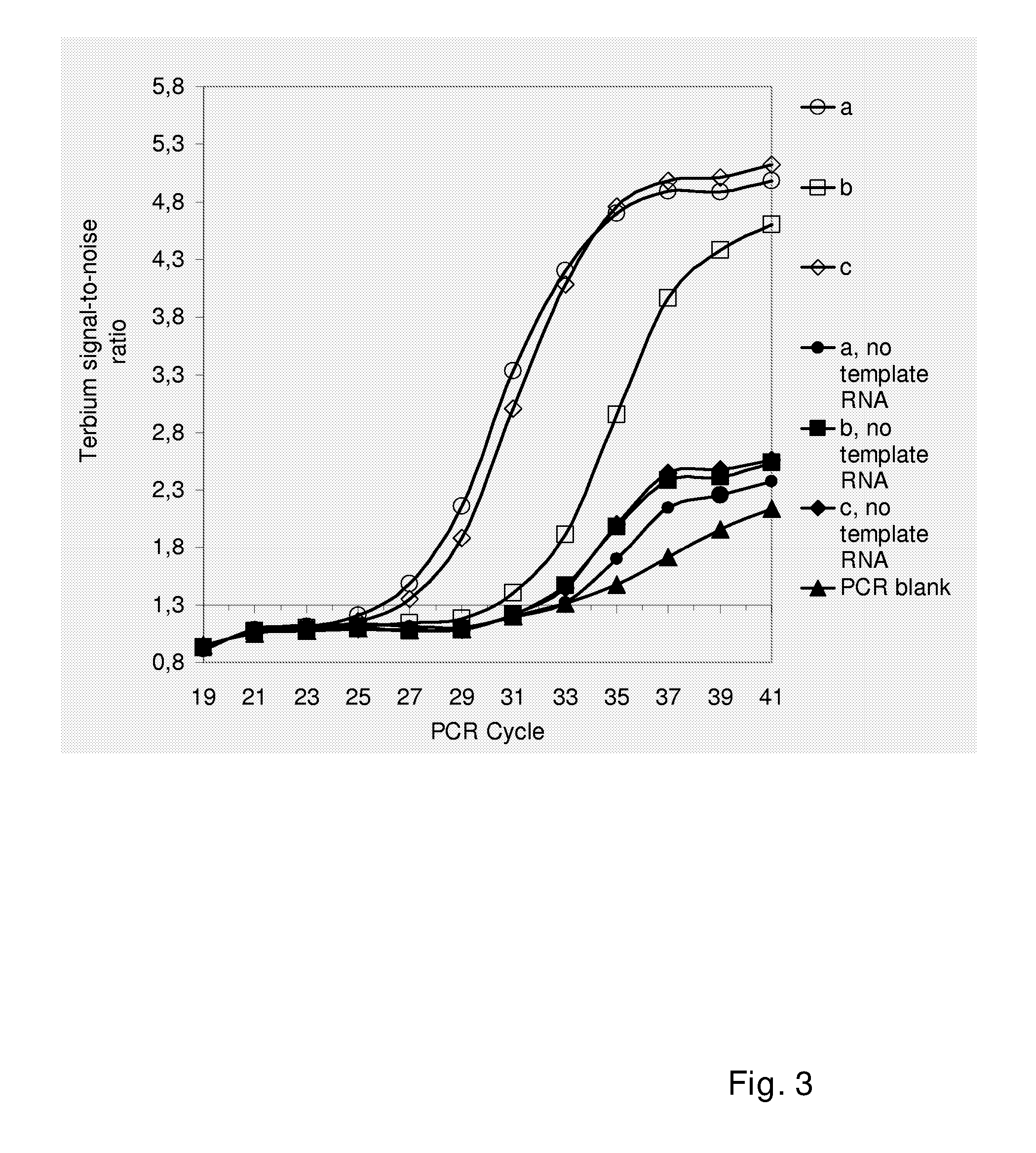
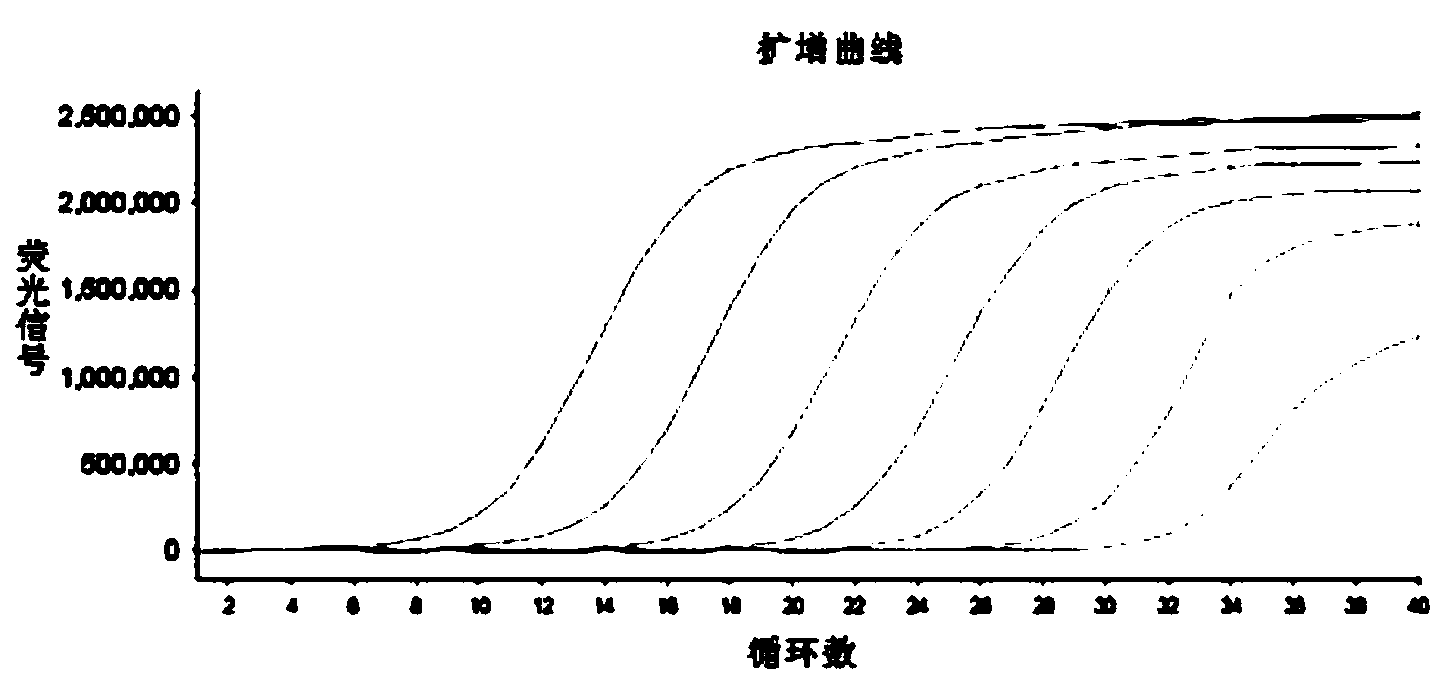
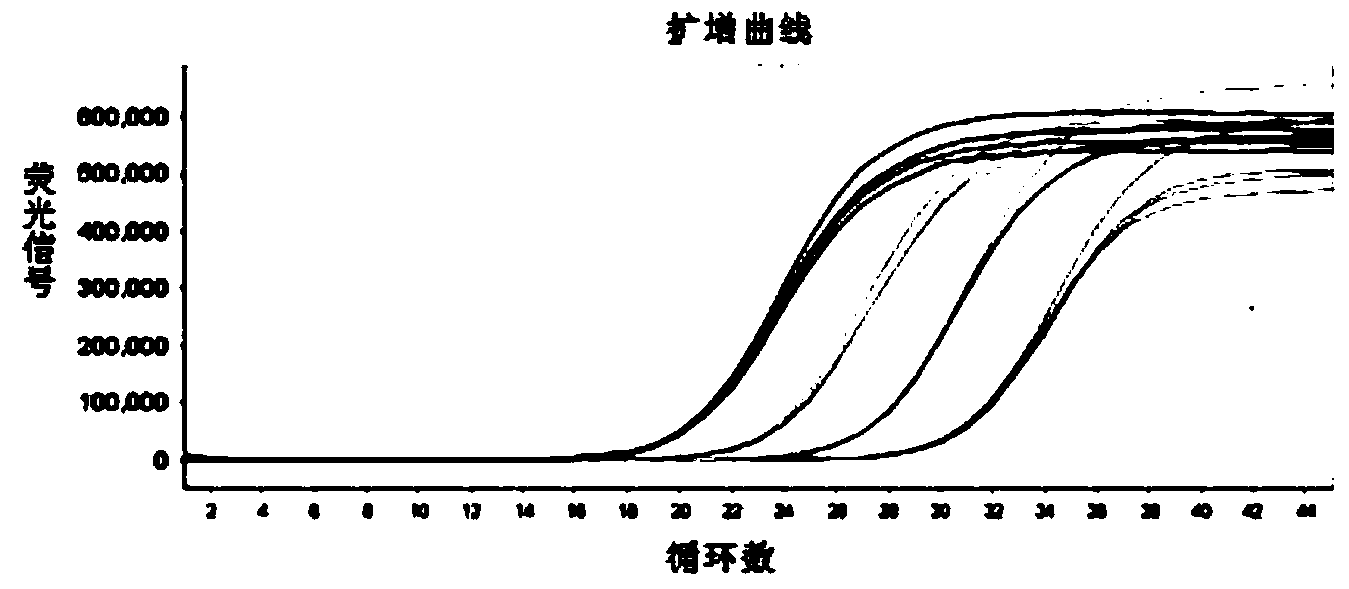
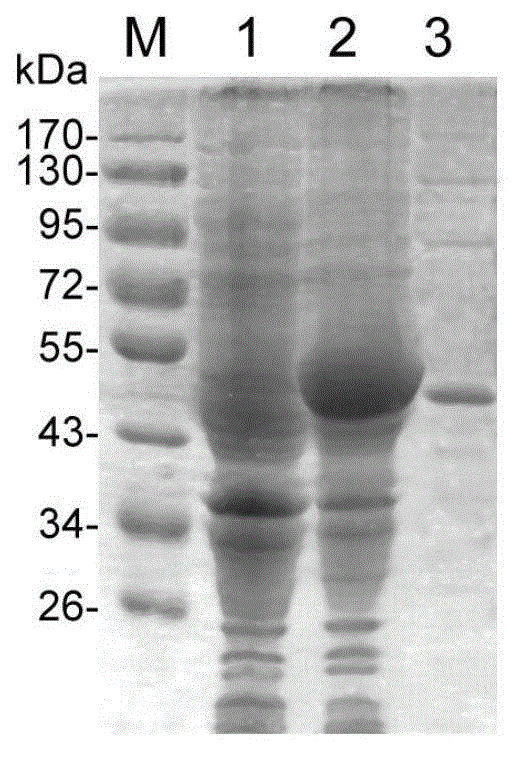
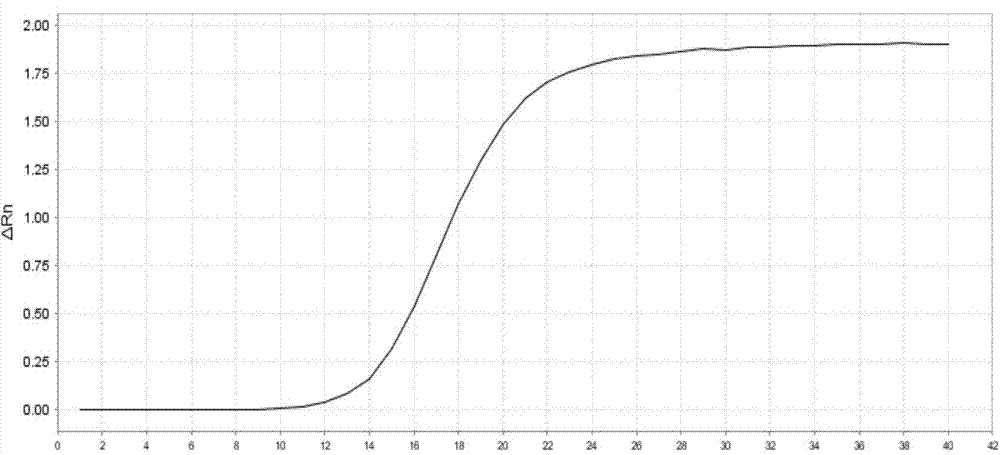
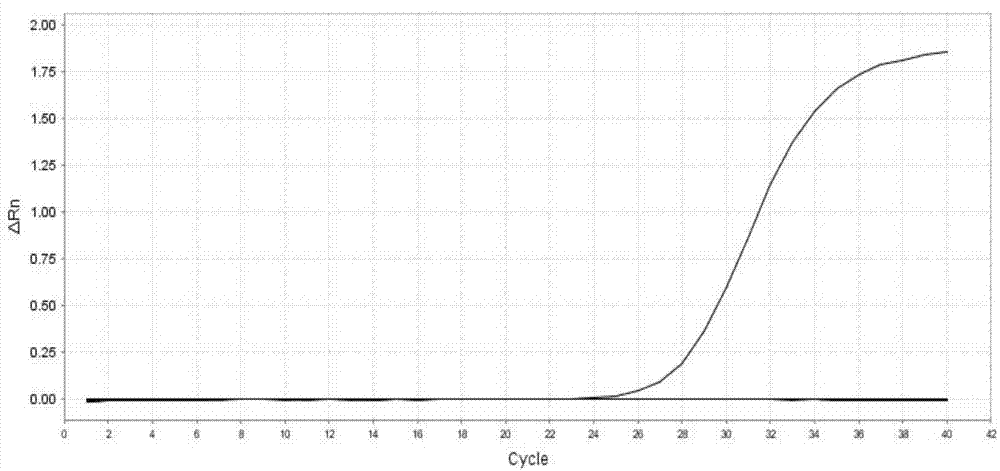
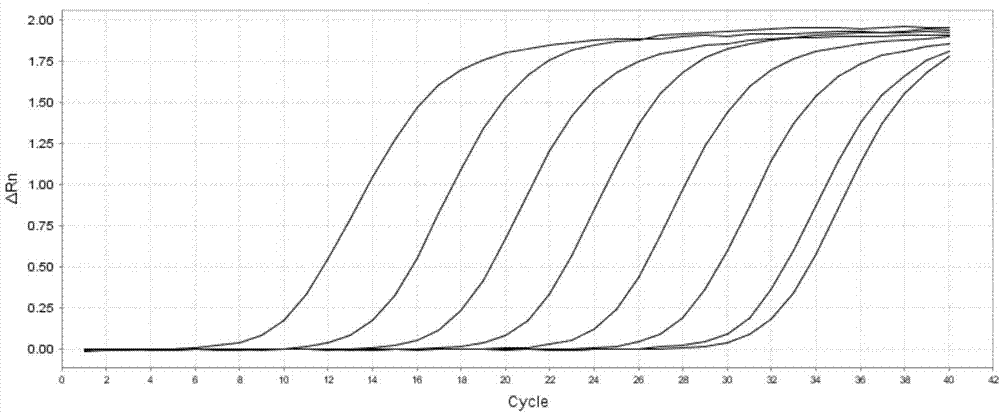
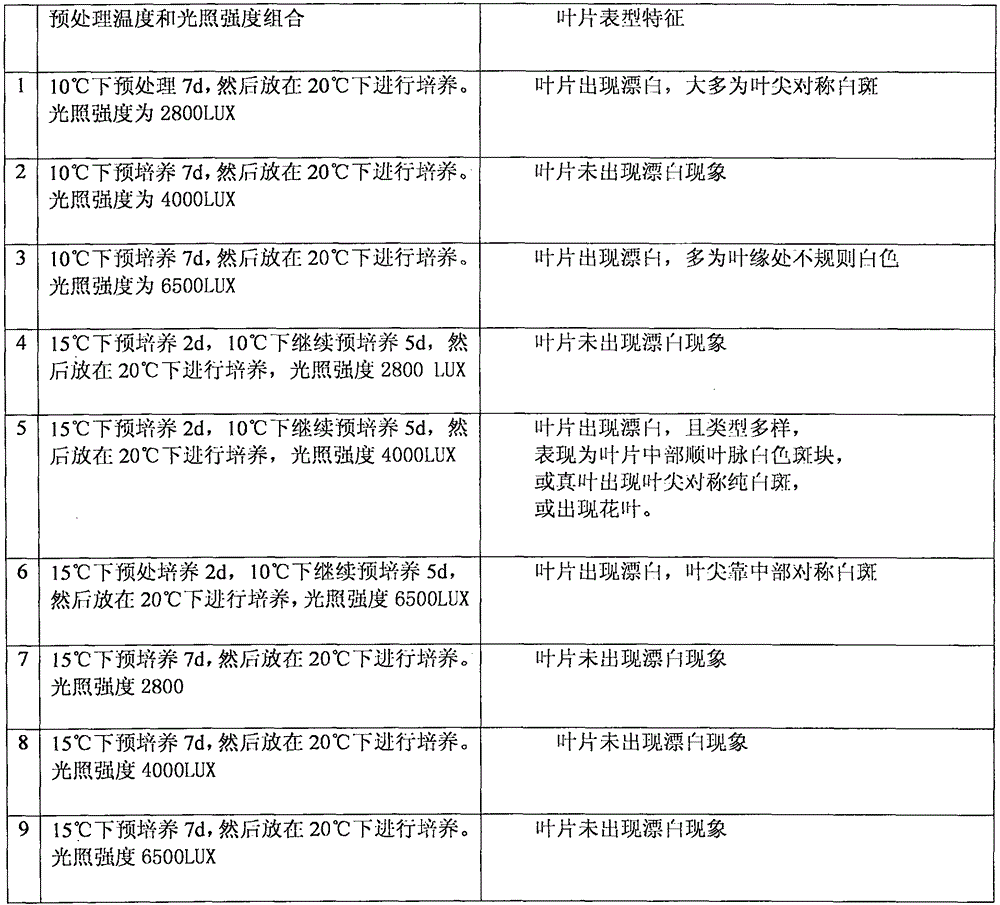
Patents
Literature
767 results about "Reverse transcription polymerase chain reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
<ul><li>A normal result is negative and a positive result indicates presence of a pathogen/an infection.</li></ul>
Method of preparing cell lysate
InactiveUS7258976B2Simple preparation processStable outputSugar derivativesMicrobiological testing/measurementTransfer cellCell layer
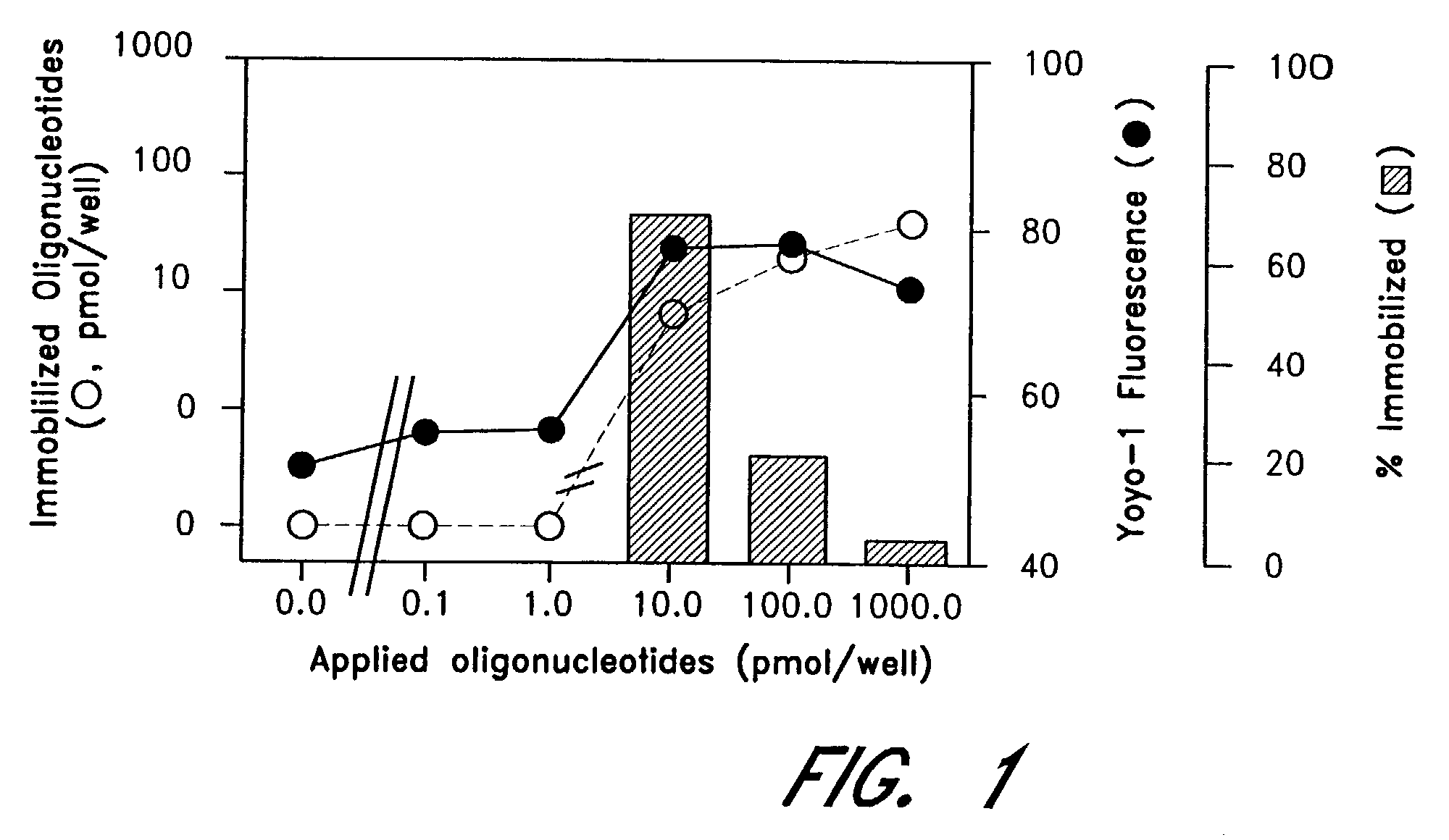
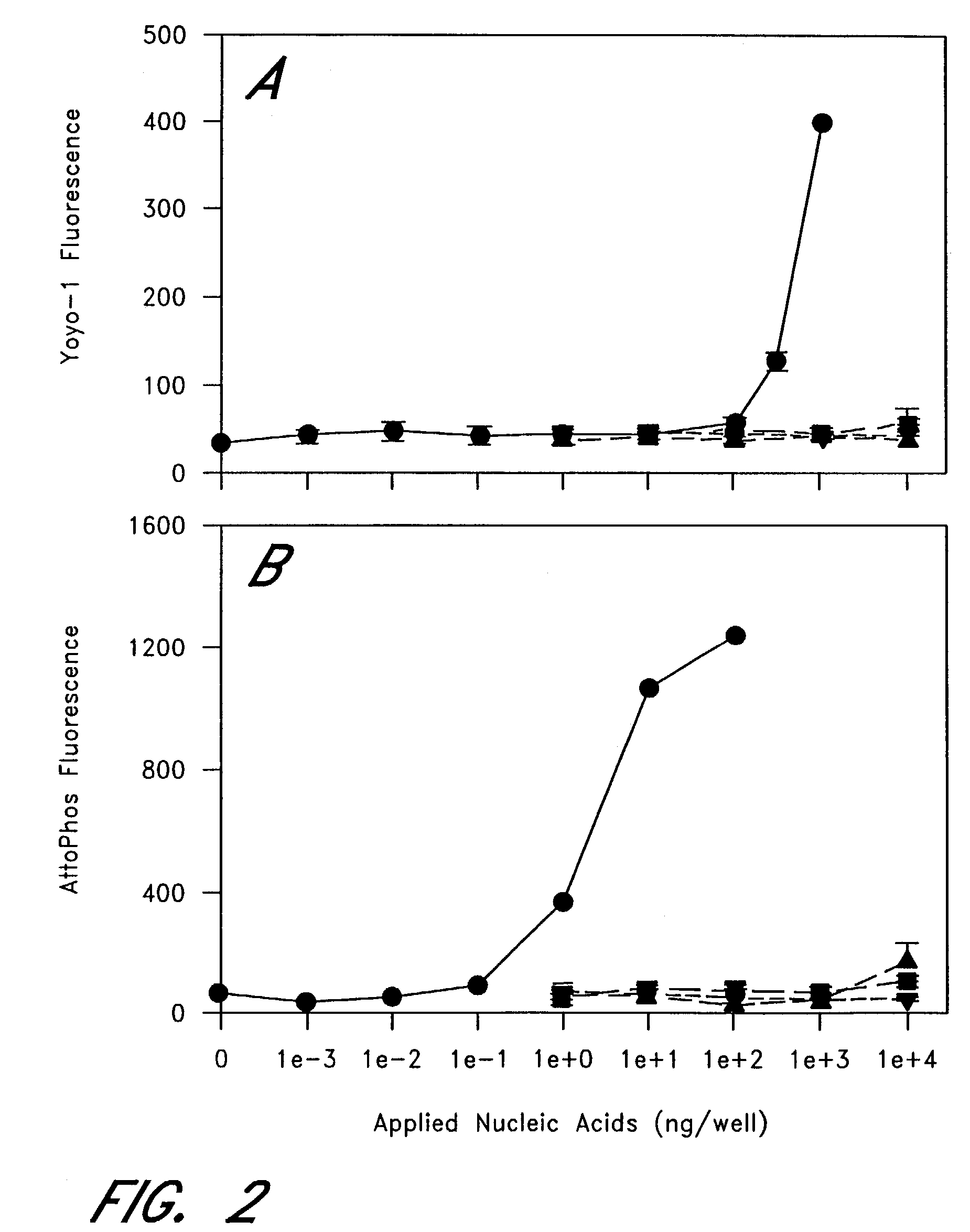
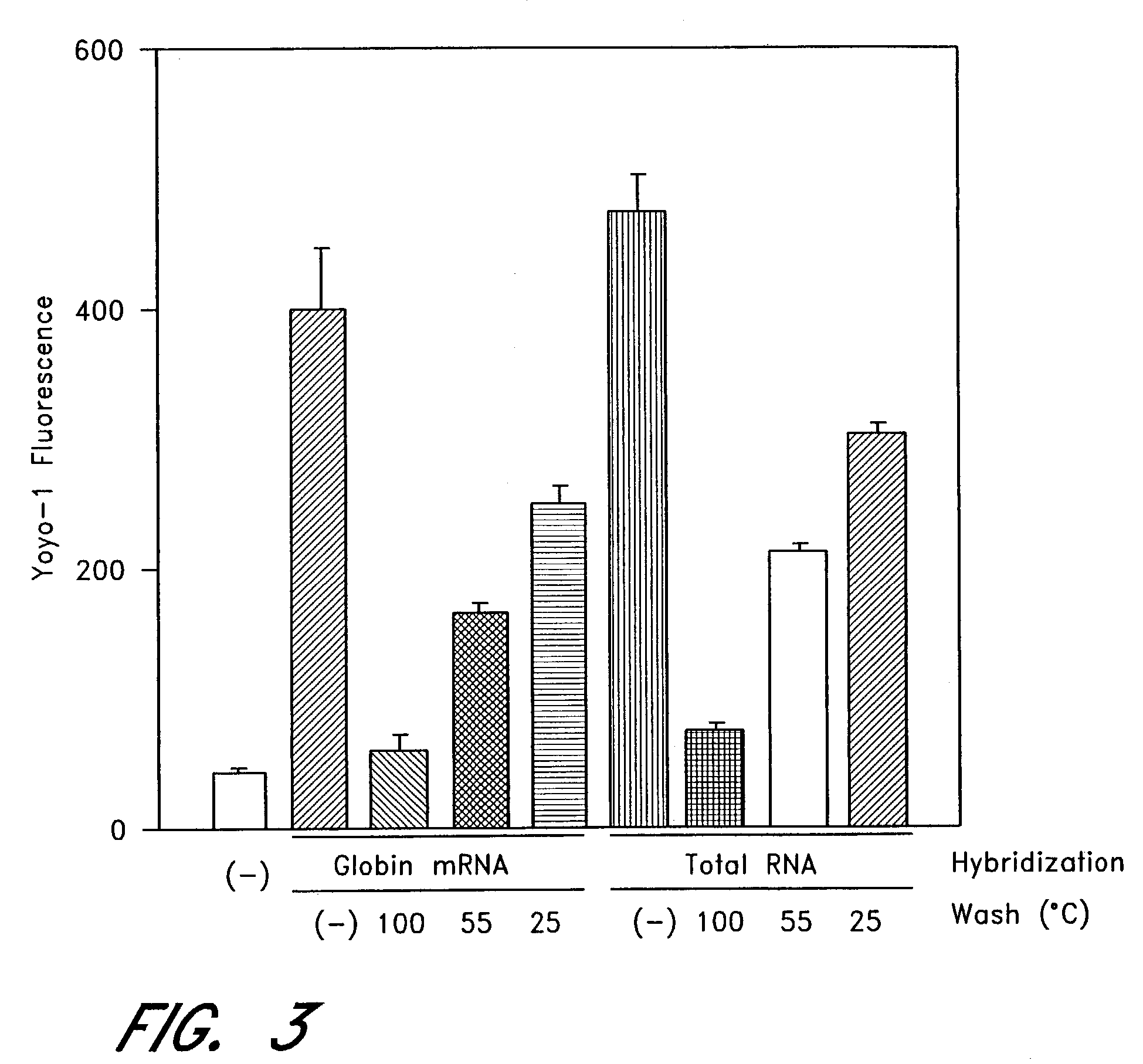
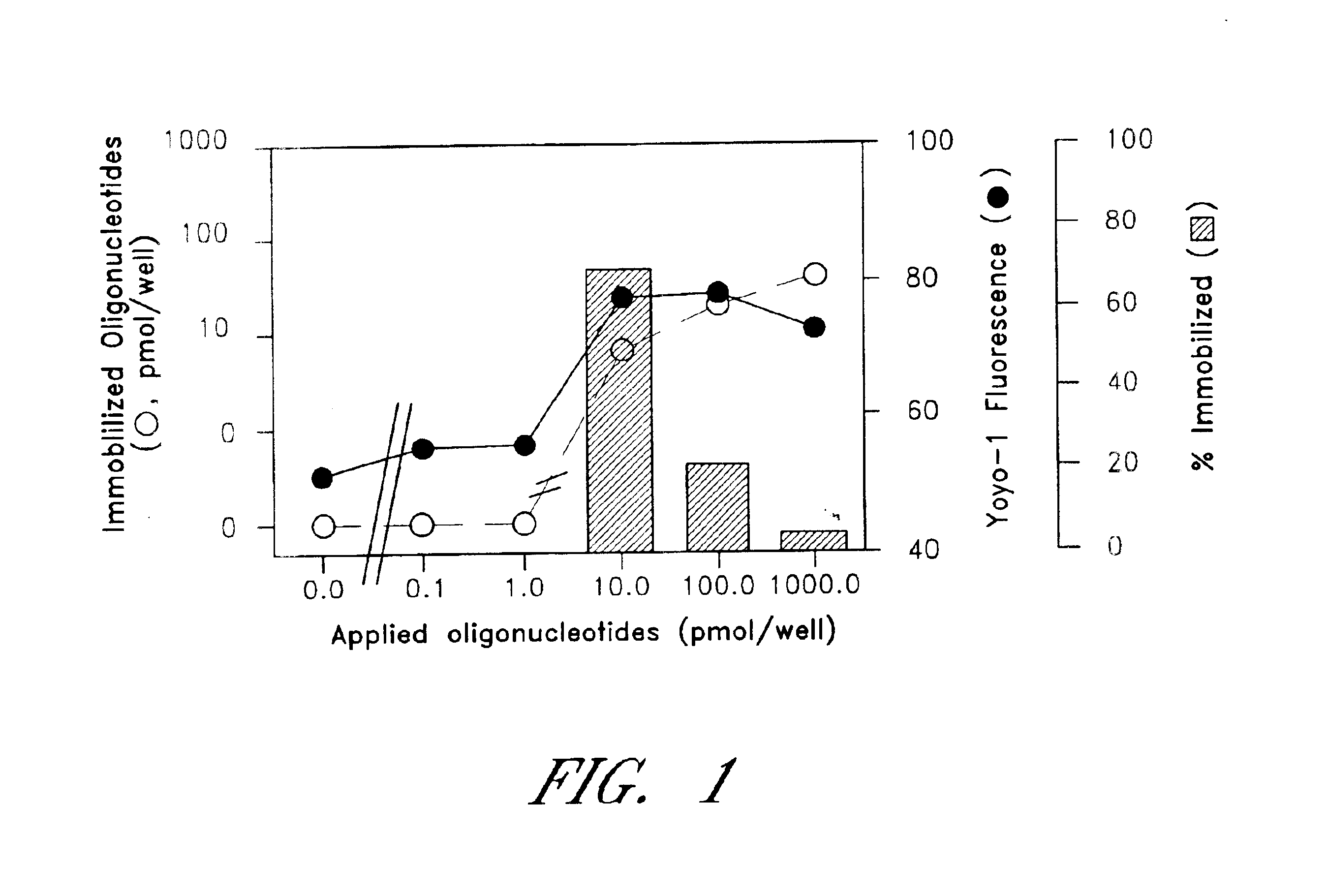
The entire process of reverse transcription-polymerase chain reaction (RT-PCR) is simplified by using oligonucleotide-immobilized microplates made of, e.g., polypropylene, to which oligonucleotides are securely immobilized and which can be subjected to thermal cycles of PCR. RT-PCR is preferably conducted in solid-phase. Capturing of mRNA and RT-PCR can be conducted in the same plates. The cDNA synthesized from the mRNA captured on the microplates can be used more than once. Further, in combination with the microplates, a filter plate is used for the preparation of cell lysates wherein target cells are placed on the filter plate, and a lysis buffer is passed through the cell layer on the filter to transfer cell lysate directly to the microplate via well-to-well communication.
Owner:RESONAC CORPORATION +1
Adam12 as a biomarker for bladder cancer
InactiveUS20090029372A1Improve the level ofLower Level RequirementsMicrobiological testing/measurementMaterial analysisTissue ArraysAffymetrix genechip
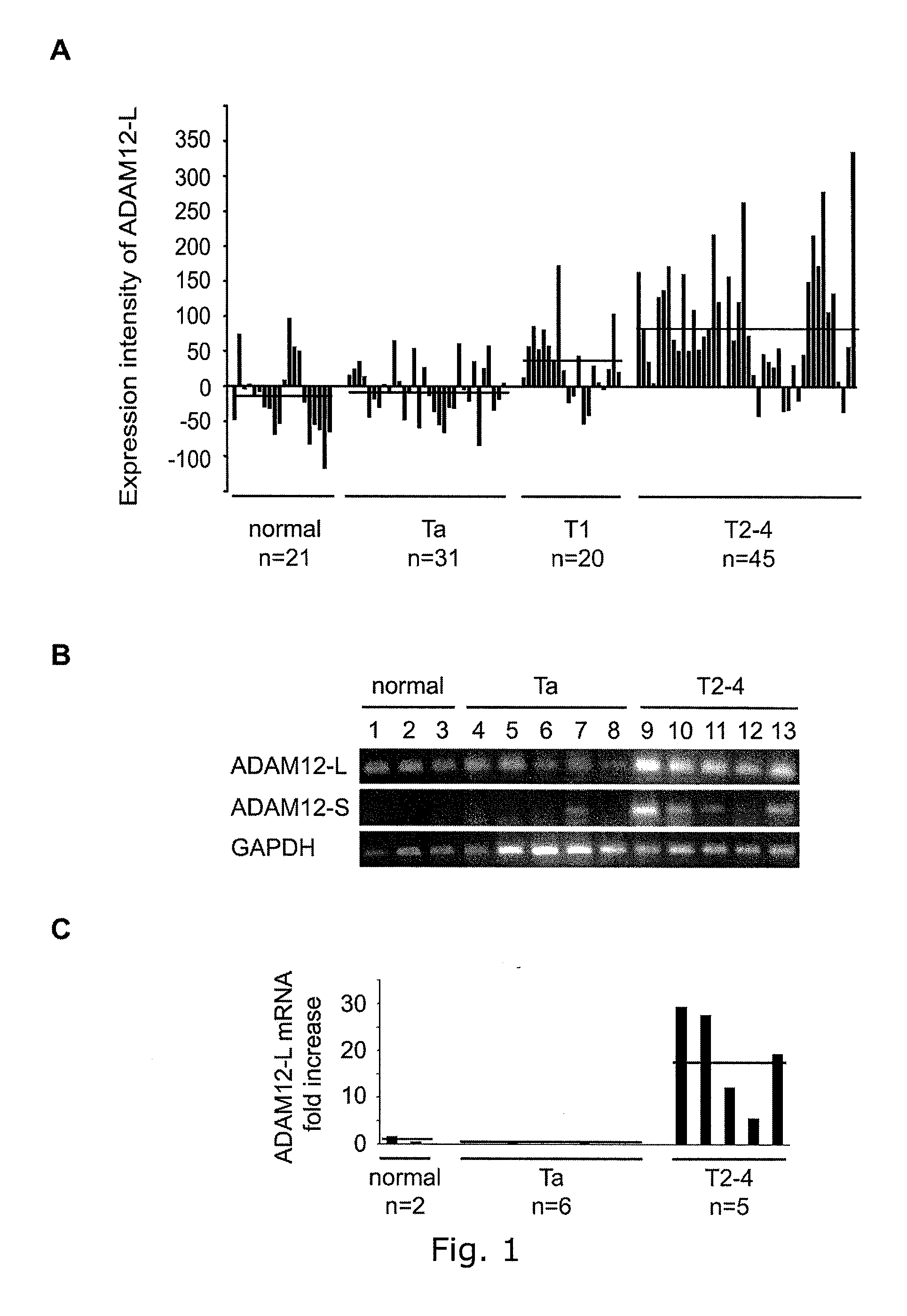
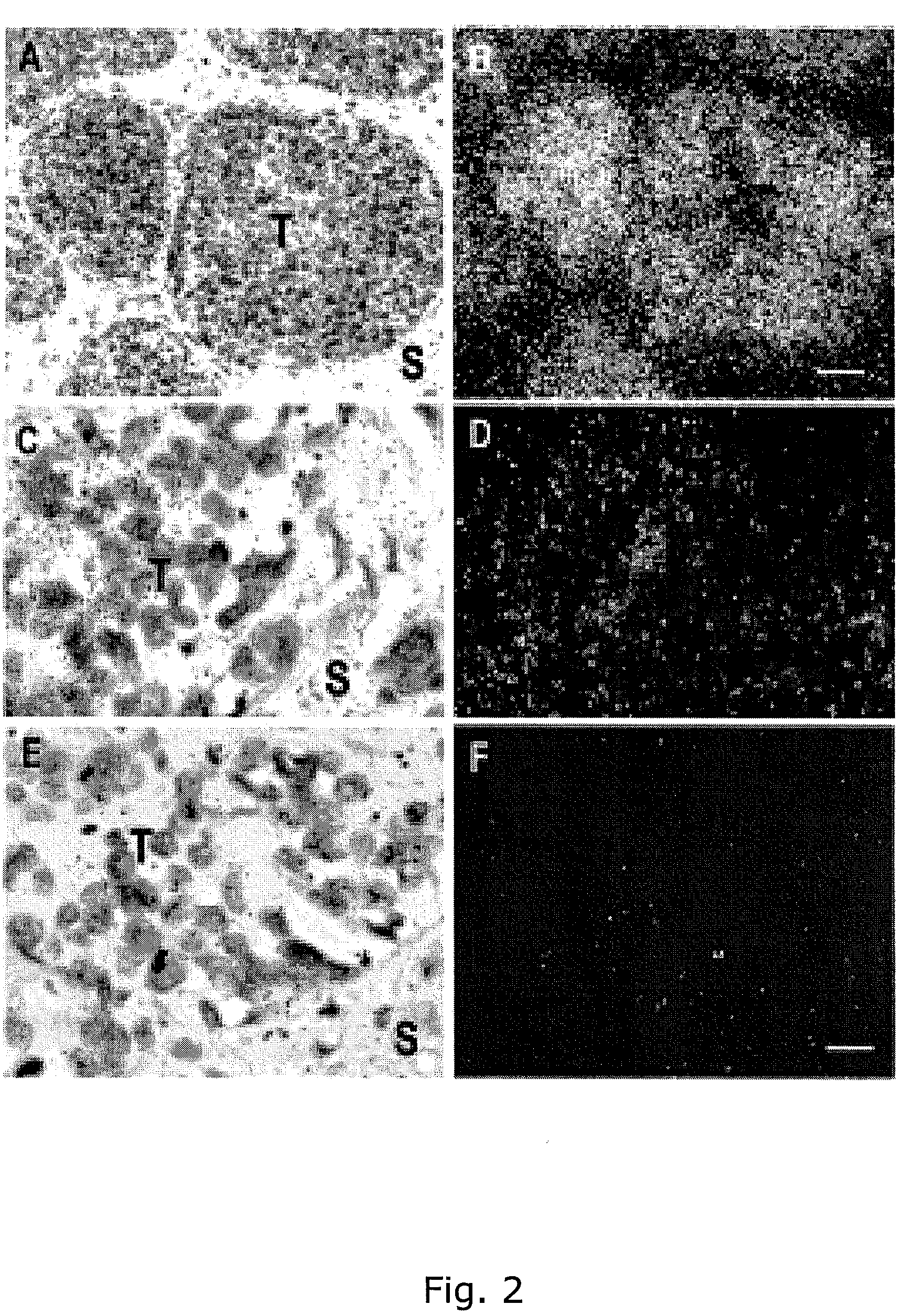
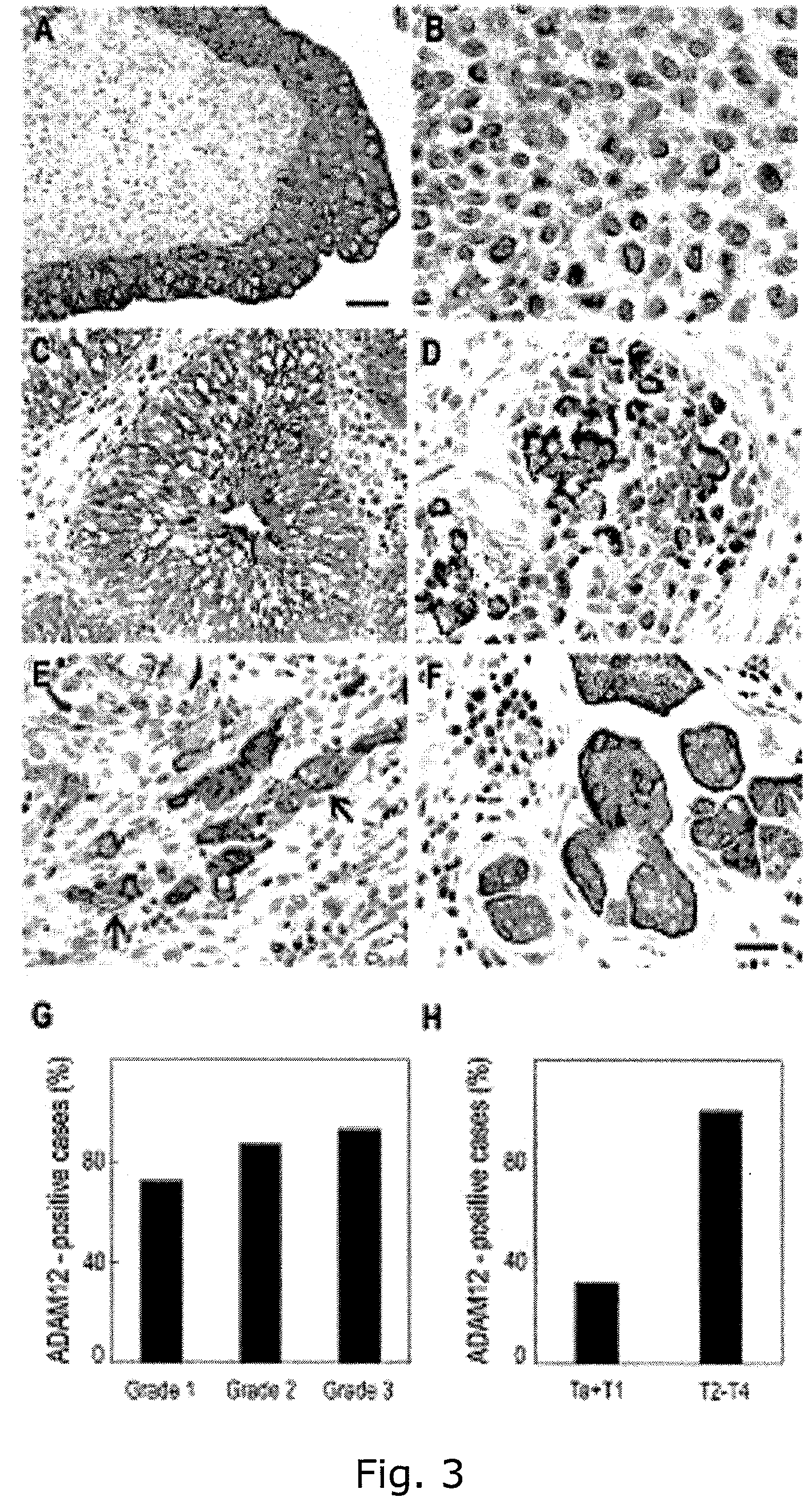
The present inventors have shown that the gene and protein expression profiles of ADAM8, ADAM10 and ADAM12 in different grades and stages of bladder cancer.ADAM12 gene expression was evaluated in tumors from 96 patients with bladder cancer using a customized Affymetrix GeneChip. Gene expression in bladder cancer was validated using reverse transcription-polymerase chain reaction (RT-PCR), quantitative PCR, and in situ hybridization. Protein expression was evaluated by immunohistochemical staining on tissue arrays of bladder cancers.The presence and relative amount of ADAM12 in the urine of cancer patients were determined by Western blotting and densitometric measurements, respectively.Particularly ADAM12 mRNA expression was significantly upregulated in bladder cancer, as determined by microarray analysis, and the level of ADAM12 mRNA correlated with disease stage. ADAM12 protein expression correlated with tumor stage and grade. ADAM12 was present in higher levels in the urine from bladder cancer patients than in urine from healthy individuals. Significantly, following removal of tumor by surgery, in most bladder cancer cases examined the level of ADAM12 in the urine decreased and, upon recurrence of tumor, increased.
Owner:PHYSICIANS CHOICE LAB SERVICES +1
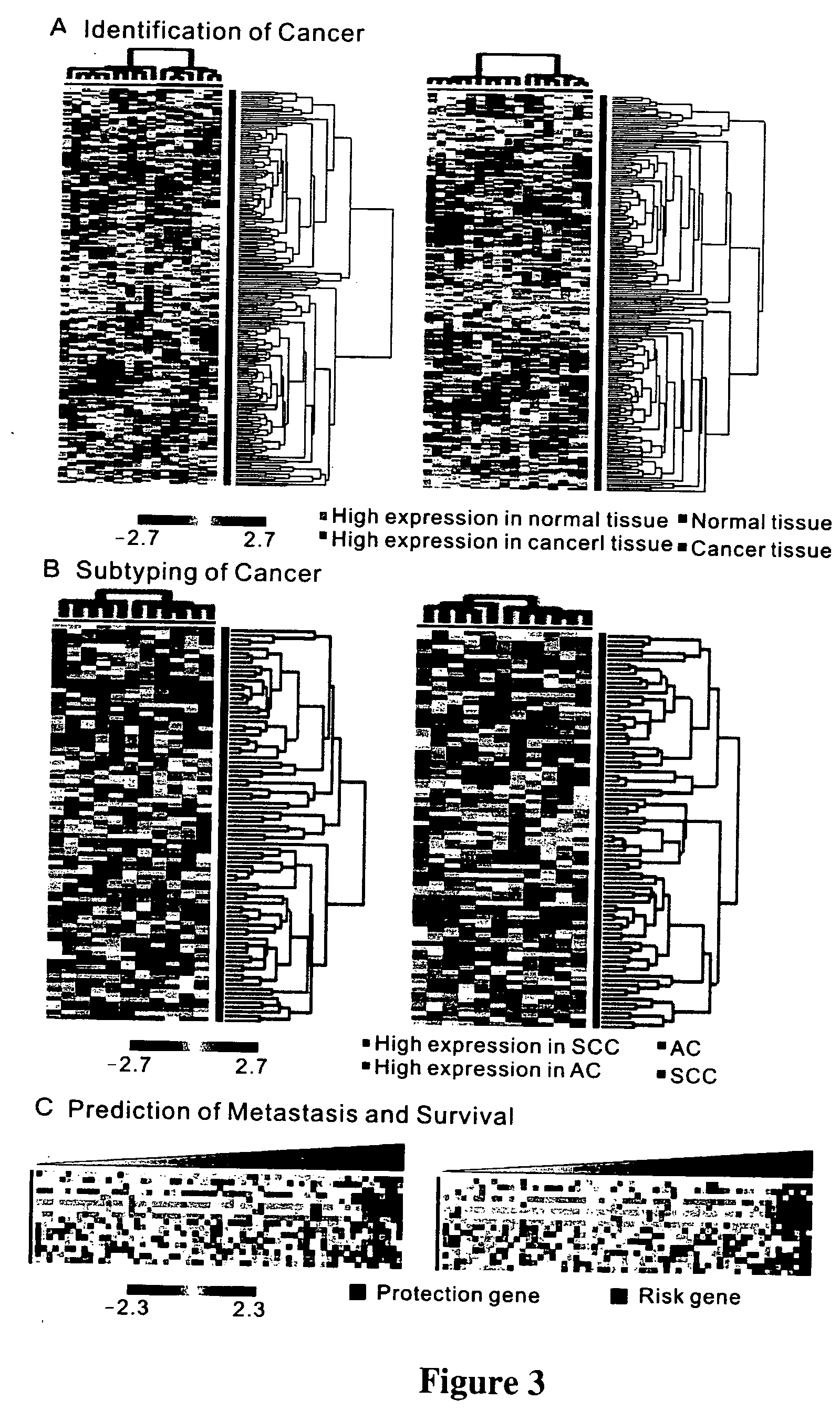
Metastasis-associated gene profiling for identification of tumor tissue, subtyping, and prediction of prognosis of patients
InactiveUS20060211036A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAbnormal tissue growthReal-Time PCRs
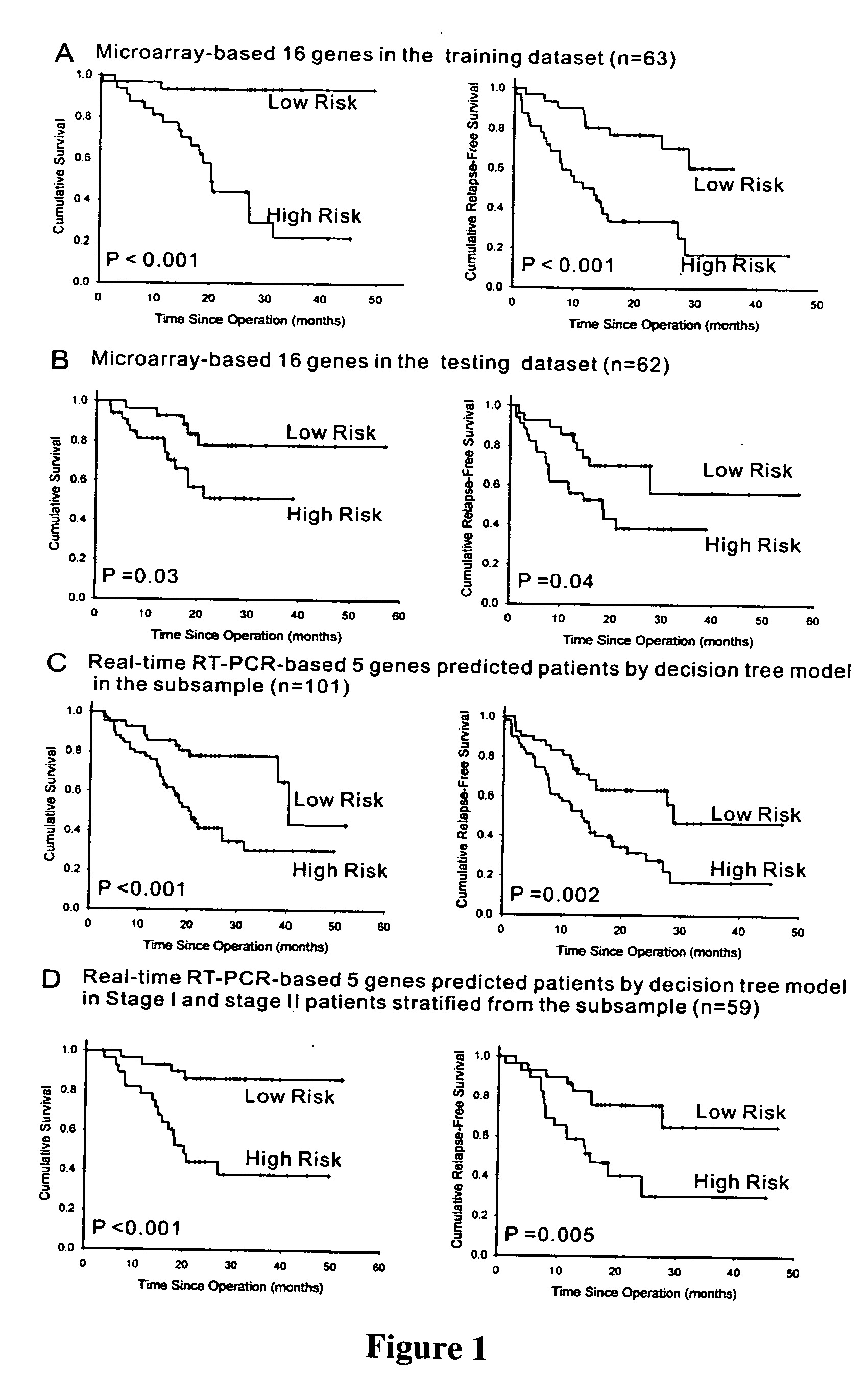
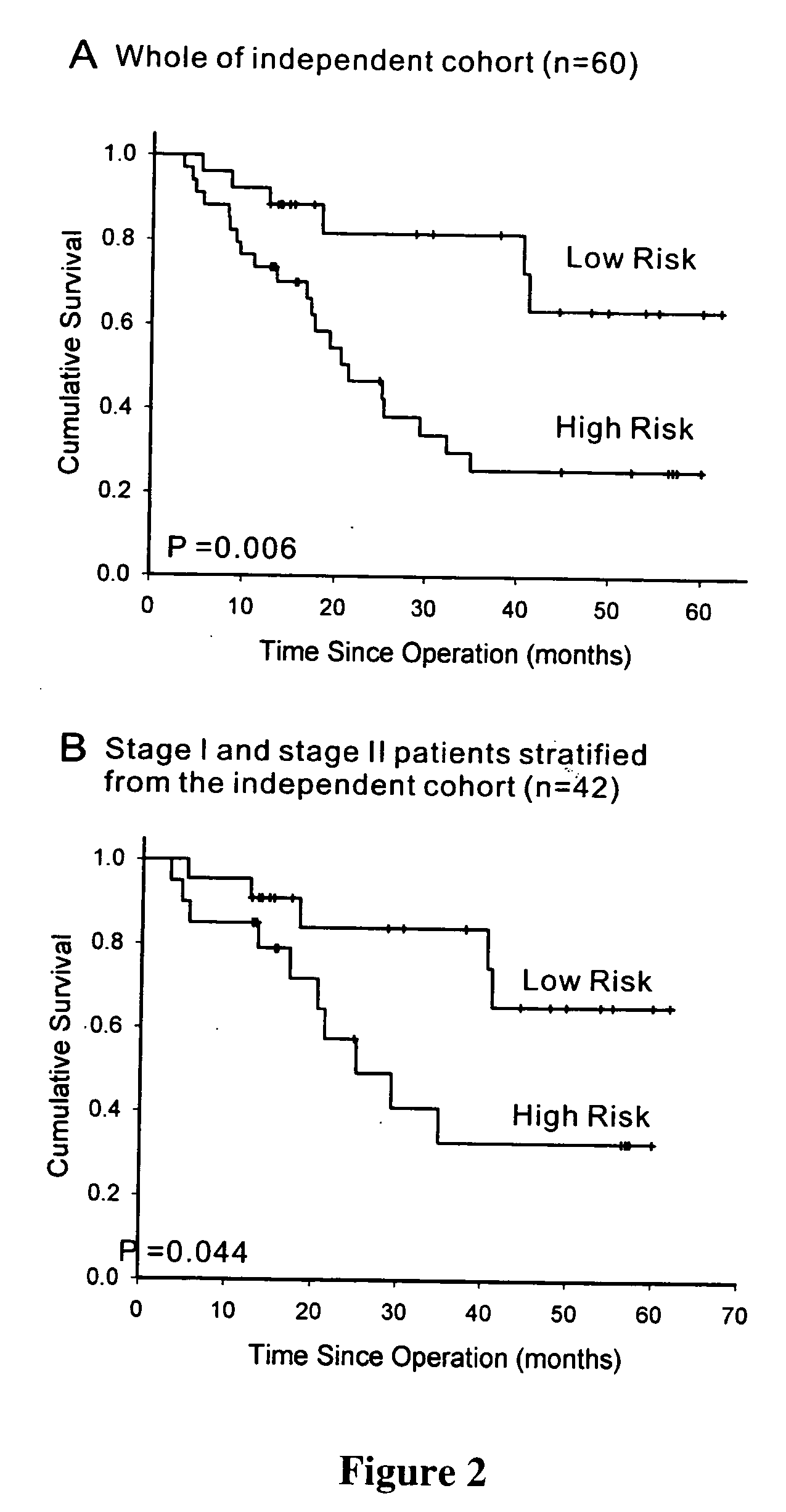
The present invention provides methods using a gene expression profiling analysis (1) to determine whether a human sample is a tumor using a gene set containing nucleic acid sequences of SEQ ID NOS: 1-7, 8-17 or 1-17; (2) to identify whether a tumor tissue is an adenocarcinoma (using a gene set containing nucleic acid sequences of SEQ ID NOS: 15, and 18-21) or a squamous cell carcinoma (using a gene set containing nucleic acid sequences of SEQ ID NOS: 22-27); and (3) to predict the prognosis of survival and metastasis in humans with tumor (using a gene set containing nucleic acid sequences of SEQ ID NOS:19, and 28-42 or SEQ ID NOS: 19, 29, 31, 40, and 42), particularly for those humans who are at the early stage of lung cancer. The gene expression profiling is preferably performed by cDNA microarray-based techniques and / or Real-Time Reverse Transcription-Polymerase Chain Reaction (Real-Time RT-PCR), and analyzed by statistical means.
Owner:ADVPHARMA
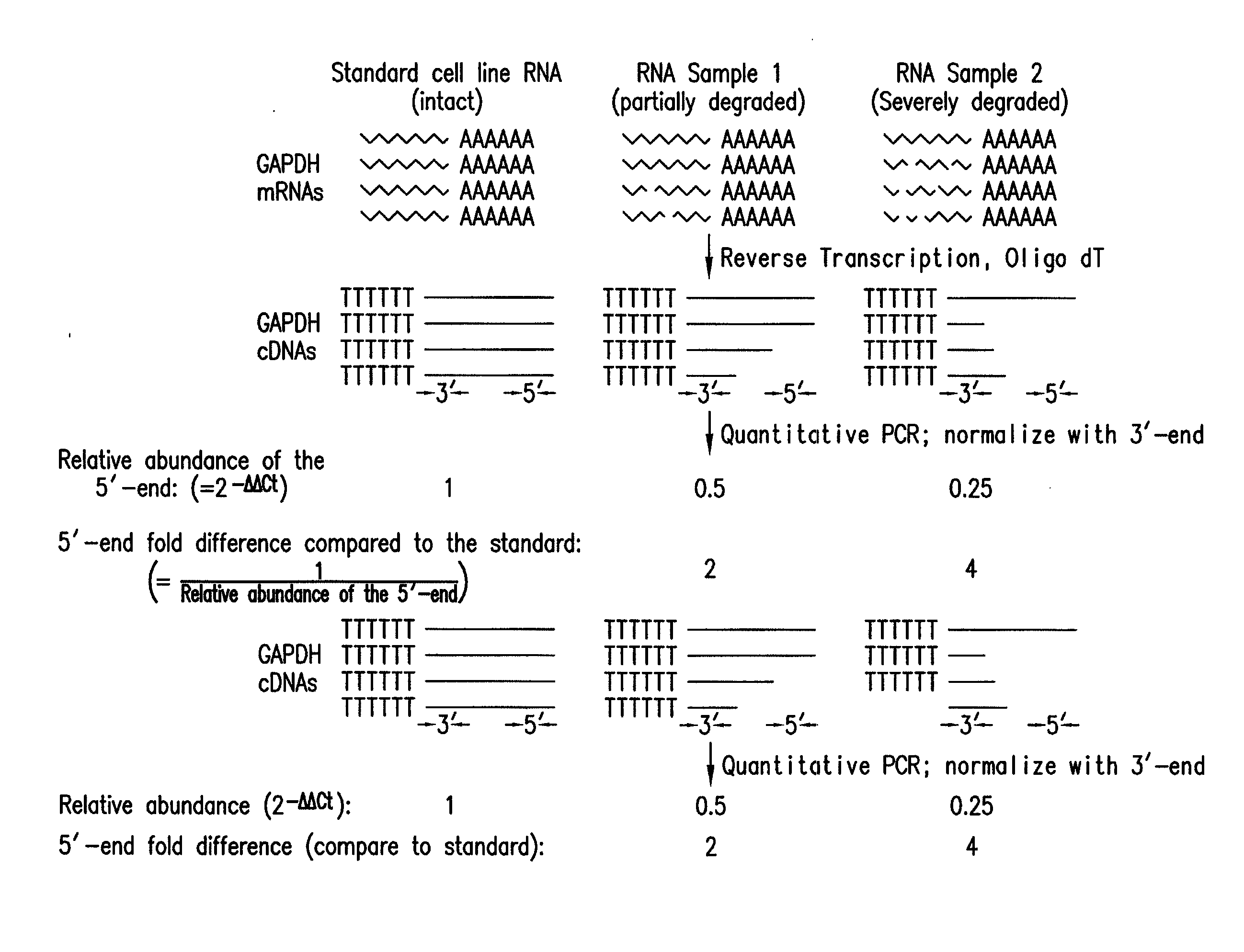
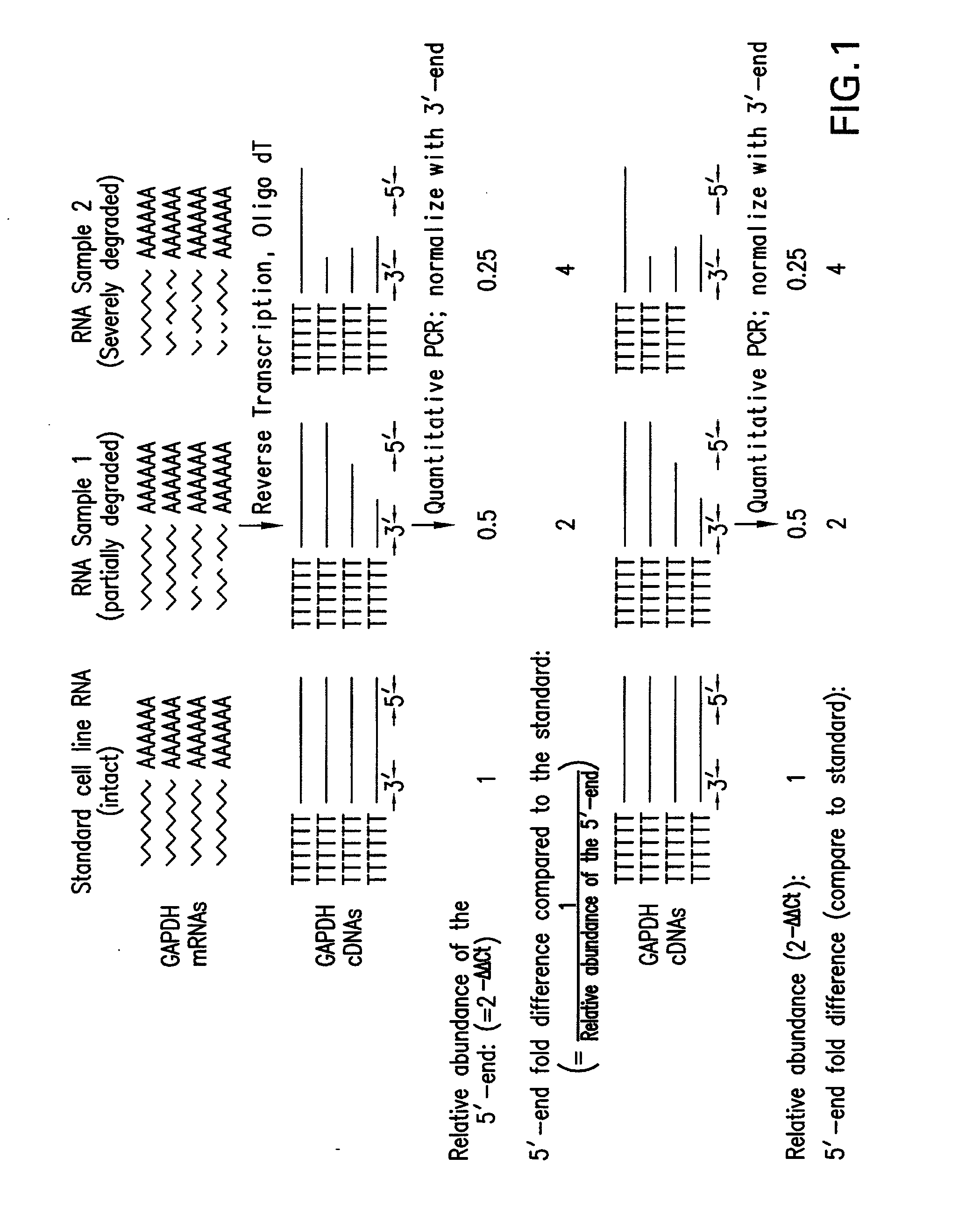
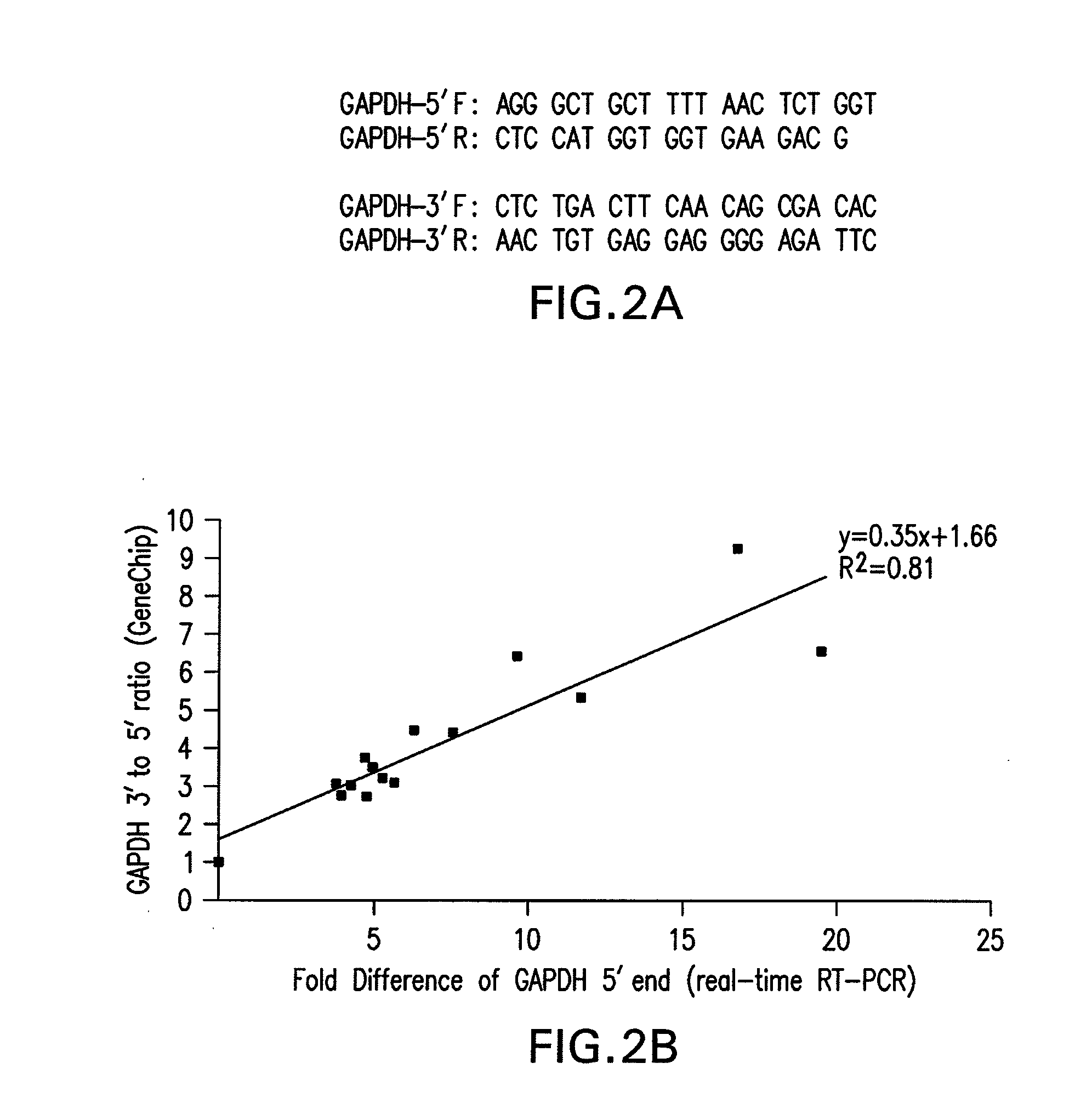
Method and kit for evaluating RNA quality
InactiveUS20080318801A1Microbiological testing/measurementLibrary screeningReverse transcription polymerase chain reactionBiology
The present invention relates to a method for analyzing and rapidly determining the quality of a test RNA of unknown quality utilizing a novel quantitative reverse transcription-polymerase chain reaction (RT-PCR) based method. The present invention is based on normalizing the 3′ end of the house-keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to the relative abundance of the 5′ end of GAPDH MRNA in the test RNA. The present invention is particularly useful in pre-screening postmortem tissue samples for microarray experiments and for evaluating large quantities of samples which would be time consuming and expensive to analyze by methods currently in use.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Direct RT-PCR on oligonucleotide-immobilized PCR microplates
InactiveUS6844158B1Simple preparation processStable outputSugar derivativesMicrobiological testing/measurementTransfer cellCell layer
The entire process of reverse transcription-polymerase chain reaction (RT-PCR) is simplified by using oligonucleotide-immobilized microplates made of, e.g., polypropylene, to which oligonucleotides are securely immobilized and which can be subjected to thermal cycles of PCR. RT-PCR is preferably conducted in solid-phase. Capturing of mRNA and RT-PCR can be conducted in the same plates. The cDNA synthesized from the mRNA captured on the microplates can be used more than once. Further, in combination with the microplates, a filter plate is used for the preparation of cell lysates wherein target cells are placed on the filter plate, and a lysis buffer is passed through the cell layer on the filter to transfer cell lysate directly to the microplate via well-to-well communication.
Owner:HITACHI CHEM CO LTD +1
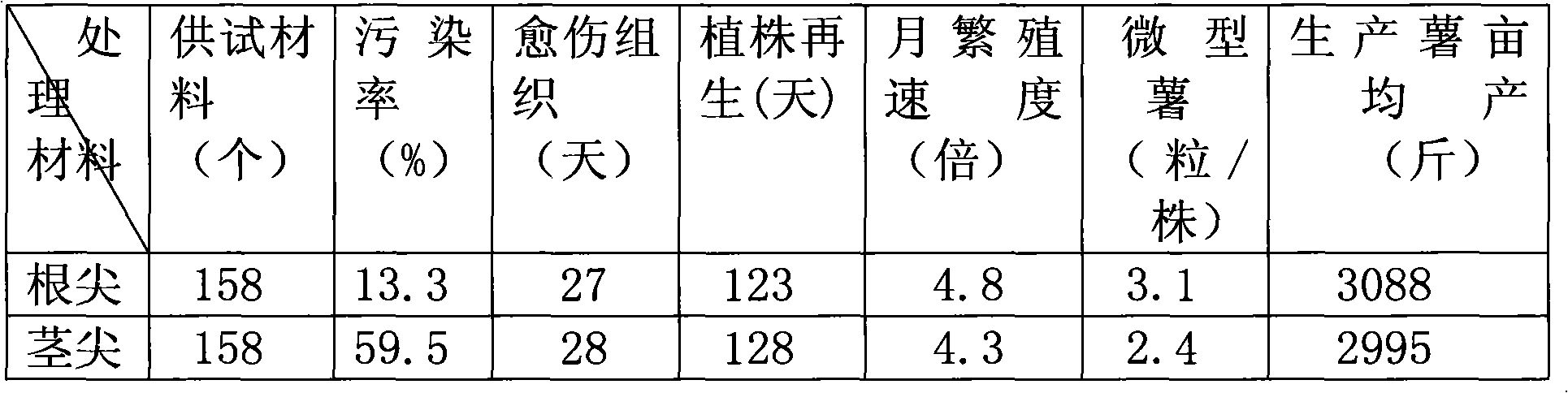
Root tip detoxification and rapid propagation technology for purple potatoes
InactiveCN101849509AEasy to operateImprove efficiencyHorticulture methodsPlant tissue cultureShoot apexPollution
The invention discloses root tip detoxification and rapid propagation technology for purple potatoes and belongs to the technical field of plant detoxification and tissue culture rapid propagation. The method comprises the following steps of: (1) preparing a culture medium; (2) performing root tip detoxification and rapid propagation; and (3) performing multi-reverse transcription-polymerase chain reaction (RT-PCR) synchronous and rapid detection on complex virus infection of a purple potato test tube plantlet or a test tube mini-tuber and the like. In the technology, each level of culture medium is adjusted according to the characteristic of the purple potato and conventional stem tip detoxification is changed into root tip detoxification so as to simplify operation process, avoid high pollution rate and improve working efficiency. Moreover, the technology has the advantages of good inoculant differentiation, high test tube plantlet propagation speed, complete detoxification, high mini-tuber growth vigor, monthly mini-tuber reproduction rate of 4 to 6 times and mini-tuber rooting rate and transplanting survival rate of over 98 percent. The technology can be popularized in regions which are suitable for the growth of purple potatoes.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Two-stage culture protocol for isolating mesenchymal stem cells from amniotic fluid
ActiveUS7101710B2Encourage illegal terminationRaise the possibilityArtificial cell constructsSkeletal/connective tissue cellsAmniotic fluid cellAmniotic fluid
A method of harvesting mesenchymal stem cells from human amniotic fluid uses a two-stage culture protocol comprising culturing human amniocytes and then culturing mesenchymal stem cells. For culturing human amniocytes, primary amniocyte cultures are set up using routine or standard culture protocol in a cytogenetic laboratory. Non-adherent human amniotic fluids cells in the supernatant medium are collected. For culturing mesenchymal stem cells (“MSC”), the non-adherent cells are centrifuged and then plated with an alpha-modified Minimum Essential Medium supplemented with fetal bovine serum. Incubate with humidified CO2 for MSC growth. Reverse transcription polymerase chain reaction (“RT-PCR”) and immunocytochemical analyses reveal that Oct-4 mRNA and OCT-4 protein expression is detectable in the cultured amniotic fluid mesenchymal stem cells (“AFMSCs”). Under differentiation culture conditions, the AFMSCs can be induced to develop into multi-lineage cells, such as adipocytes, osteocytes, neuronal cells, etc.
Owner:U NEURON BIOMEDICAL INC
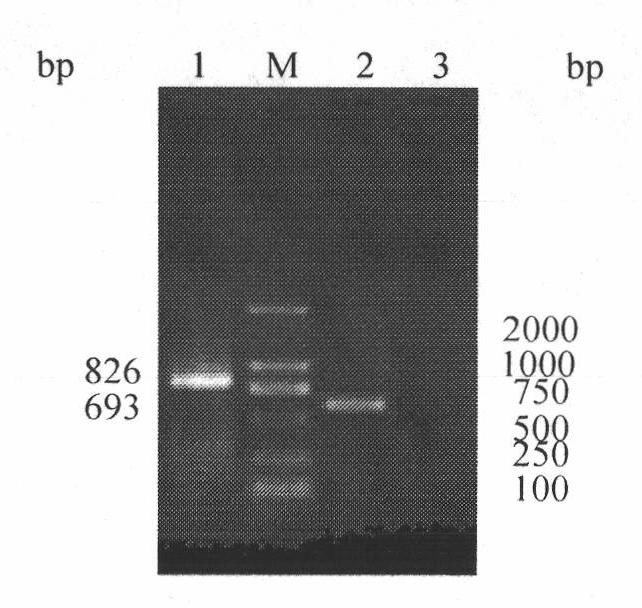
h5, h7, h9 subtype avian influenza virus detection kit
InactiveCN102260749AMicrobiological testing/measurementAvian influenza virusComplementary deoxyribonucleic acid
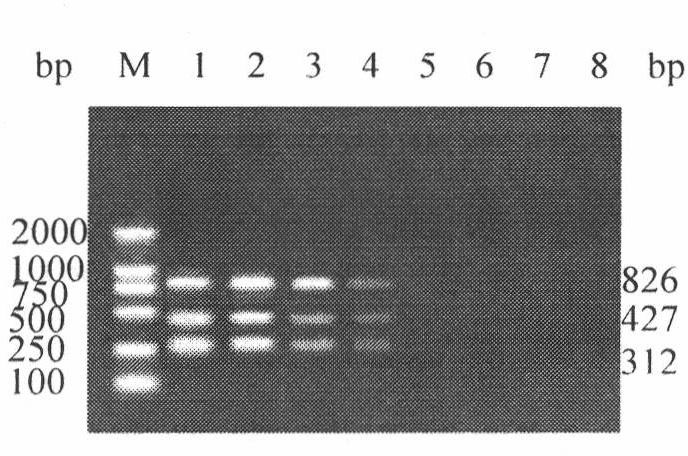
The invention discloses an H5, H7 and H9 subtype avian influenza virus detection kit. In the GenBank, three kinds of subtype HA gene sequences are searched, and specific primers are designed. By many tests and actual detections, three pairs of primers and reaction systems thereof are screened out and optimized, and the sizes of the amplified cDNA (complementary deoxyribonucleic acid) fragments are respectively 427bp, 312bp and 826bp. The result shows that by optimizing multiple RT-PCR (reverse transcription-polymerase chain reaction) amplification conditions, a multiple RT-PCR detection kit capable of detecting three kinds of subtype avian influenza viruses at the same time is prepared, and the minimum detectable quantity is 10pg. The detection kit does not have cross reaction to various kinds of chicken infectious diseases and normal structures, has strong specificity, and has consistent sensibility to three kinds of subtype detections. The avian influenza can be diagnosed in a shorttime in a primary laboratory and can be positioned in a subtype so as to gain the time for preventing and controlling the avian influenza, thus the spread of the avian influenza can be controlled in a small range as much as possible by adopting measures in time.
Owner:JILIN AGRICULTURAL UNIV
Three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as kit thereof
InactiveCN101886138AEasy to detectHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16Reverse transcription polymerase chain reaction
The invention provides a three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as a kit thereof. The method can rapidly and accurately detect the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of enterovirus in a sample. The method comprises the following steps of: (1) acquiring and conveying a sample of an infected patient or a suspected patient; (2) preprocessing the sample and extracting RNA; (3) detecting the sample by adopting a one-step PCR-three-color fluorescent probe in-vitro amplification method; and (4) analyzing the corresponding sample according to the fluorescence intensity of each amplification reaction after the amplification reaction is finished, thereby judging the existence of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus in the acquired sample and being capable of carrying out accurate quantitation (a figure 3) on the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus. The invention realizes the aim of carrying out rapid and accurate combined detection of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus.
Owner:BEIJING SUOAO BIOTECH
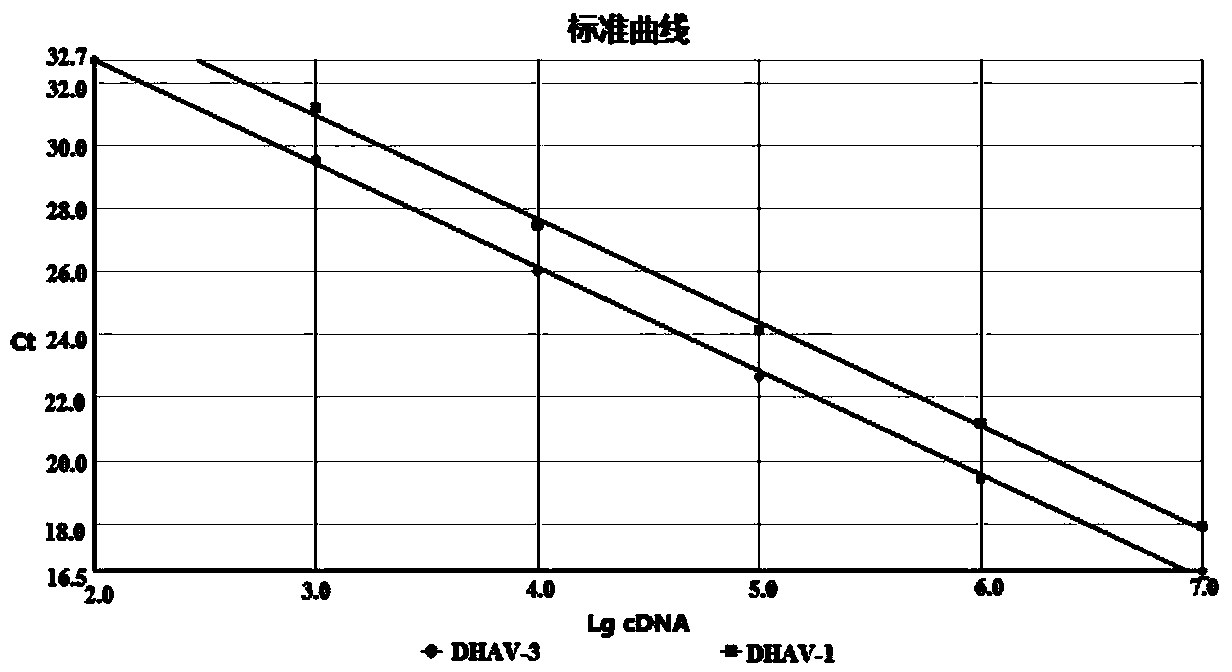
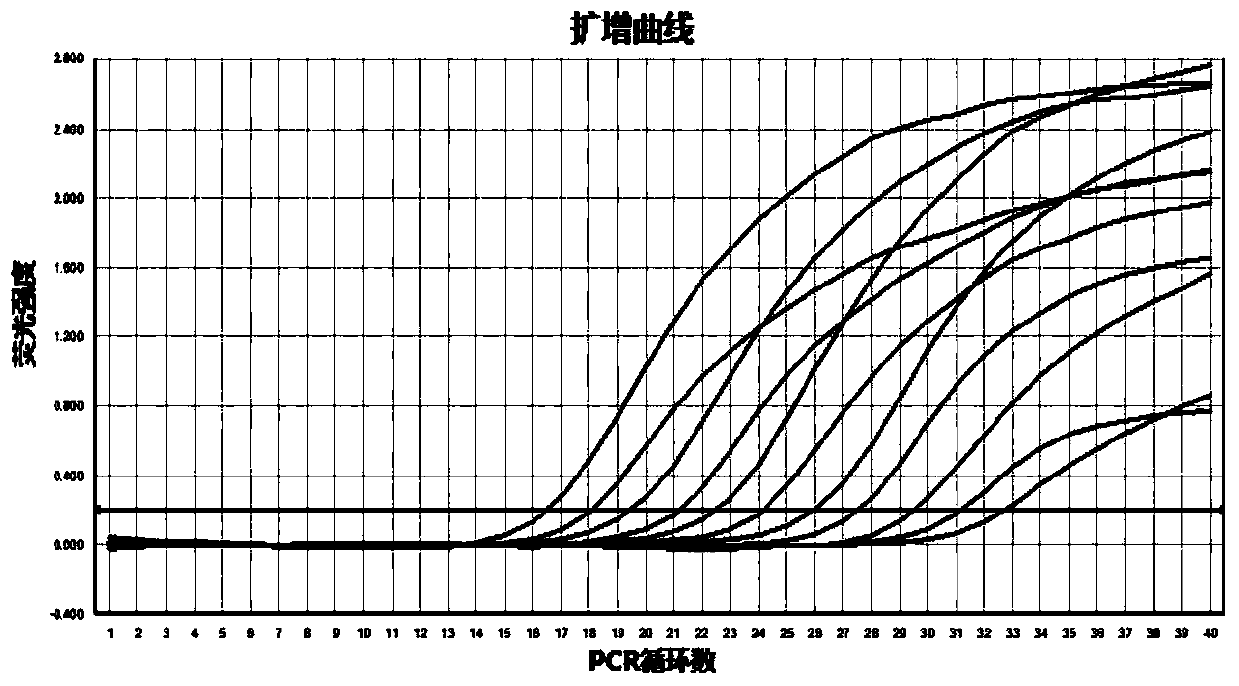
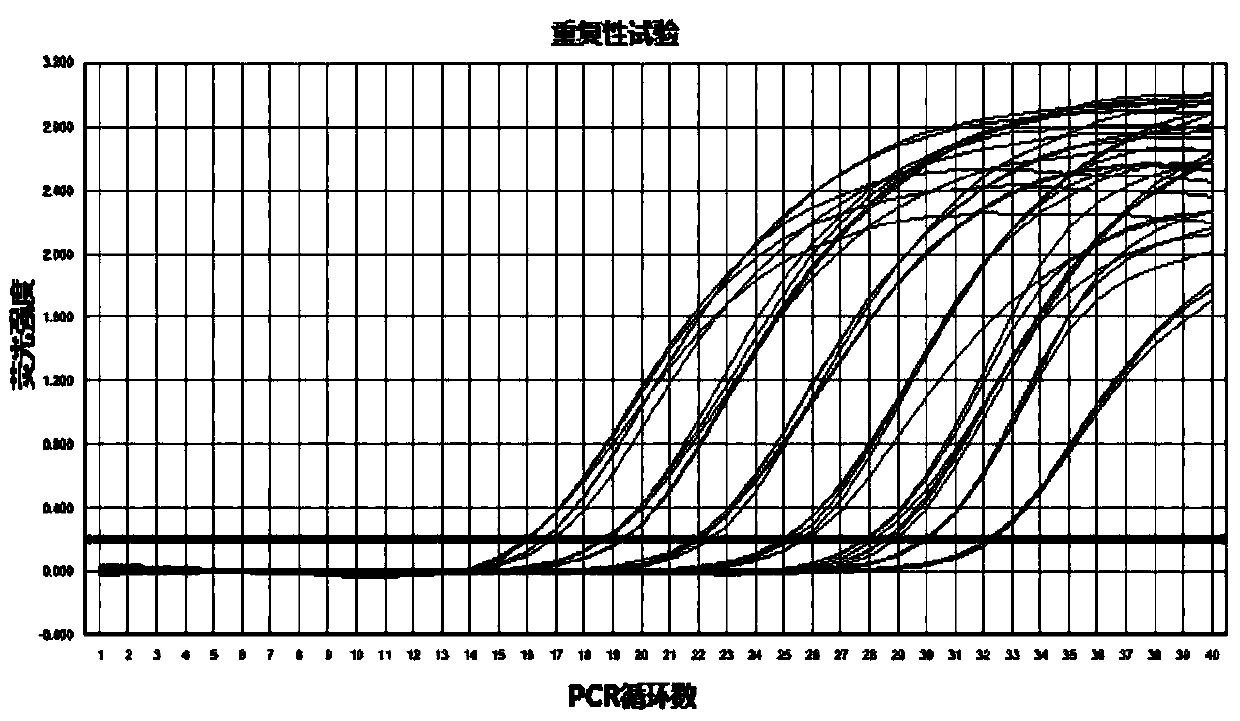
Dual fluorescence quantitative method for quickly identifying type 1 and type 3 duck hepatitis A viruses
InactiveCN104046704ARapid identificationOptimization parametersMicrobiological testing/measurementAgainst vector-borne diseasesDuck hepatitis A virusSpecific detection
The invention provides a dual fluorescence quantitative RT-PCR (reverse transcription-polymerase chain reaction) specific primer for detecting type 1 and type 3 duck hepatitis A viruses. A dual fluorescence quantitative method comprises the following steps: comparing sequences of DHAV-1 and DHAV-3 published on GenBank, and designing a pair of specific detection primers SEQ1 and SEQ2 aiming at DHAV-1 and a Taqman probe (probe1) as well as a pair of specific detection primers SEQ3 and SEQ4 aiming at DHAV-3 and a Taqman probe (probe2) respectively in a region with conservation and relatively large difference between gene sequences of the two viruses; confirming the concentration of the dual fluorescence quantitative RT-PCR specific primer and the Taqman probe aiming at DHAV-1 and DHAV-3. The dual fluorescence quantitative method built by virtue of the group of primers is good in specificity and high in sensitivity, and can be used for quick serum type identification and real-time quantitative analysis of the type 1 and type 3 duck hepatitis A viruses.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Reagent kit for quantitatively assessing long-term recurrence risks of breast cancer
InactiveCN102586410AEasy to operateTest results are stableMicrobiological testing/measurementFluorescence/phosphorescenceGenomicsBreast cancer metastasis
The invention relates to the functional genomic and gene expression detection technology and the analysis technology, and discloses a reagent kit for quantitatively assessing long-term recurrence risks of breast cancer. Particularly, a type of genes capable of being used for breast cancer metastasis and prognostic molecular classification is screened within a human functional genome expression profile range, the detection technology is created, and the reagent kit is prepared and applied to breast cancer metastasis and prognostic assessment for a patient. The reagent kit for quantitatively assessing long-term recurrence risks of breast cancer comprises 21 pairs of primers, 21 specific taqman fluorescent probes, 10XRT-PCR (reverse transcription-polymerase chain reaction) buffer solution, 2.5mM of dNTP (diethyl-nitrophenyl thiophosphate) mixed liquor, reverse transcriptase, DNA (deoxyribose nucleic acid) polymerase, 10XPCR buffer solution and RNA (ribonucleic acid) enzyme inhibitor. The reverse transcription PCR technology is combined with the taqman fluorescent quantitative PCR technology, reverse transcription primers, real-time PCR primers and the taqman fluorescent probes are self-designed and optimized, reverse transcription PCR reagent and taqman fluorescent quantitative PCR reagent are integrated to prepare the detection reagent kit, operation is simple and fast, detection results are more stable, and detection cost is lower.
Owner:苏州科贝生物技术有限公司
Quantitative real-time assay for noroviruses and enteroviruses with built in quality control standard
InactiveUS20060110724A1Efficient RT-PCRMicrobiological testing/measurementFermentationEnterovirusQuality control
A method is provided for reverse transcription-polymerase chain reaction (RT-PCR) comprising a) amplifying a reverse transcribed cDNA in a mixture comprising Norovirus Genogroup I and Norovirus Genogroup II primers and probes, wherein said Norovirus primers and probes distinguish between Genogroup I and Genogroup II viruses; b) quantifying virus; and c) normalizing data based on a universal internal RNA control. Optionally, the method may also include primers and probes for Enteroviruses. A reaction mixture comprising Norovirus Genogroup I and Norovirus Genogroup II primers and probes, wherein said Norovirus primers and probes distinguish between Genogroup I and Genogroup II viruses and universal internal RNA control primers and probes are also included.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE US SEC DEPT OF
Real time fluorescence reverse transcription polyase chain reaction solid phase reagent kit with one-step method of citrus decline virus and testing method thereof
InactiveCN101270395ASignificant technological progressOvercoming defects such as low detection sensitivityMicrobiological testing/measurementDNA preparationFluorescenceDiagnosis methods
The invention relates to a solidification examination reagent box of one-step real-time fluorescence quantitative reverse transcription polymerase chain reaction for Citrus Tristeza Virus and a method of utilizing the reagent box to detect Citrus Tristeza Virus, which is a fluorescence quantitative detection method and product of especially establishing for Citrus Tristeza Virus. The detection time only need 4 hours and the method has the high specificity, the high sensitivity and the stability and reliability. The method overcomes the shortcomings of the high erroneous recognition and the low sensitivity of serological method for Citrus Tristeza Virus of the conventional diagnosis method according to symptomatology, is suitable for the rapid diagnosis of the viral existence of Citrus seedling and viruliferous insect sector, and can be used in quality inspection of citrus seedling and identification of Citrus Tristeza Virus.
Owner:CHONGQING UNIV
Method for Stabilizing Assay Reagents, Reagent Container with Stabilized Assay Reagents and Use Thereof
ActiveUS20070243601A1Bioreactor/fermenter combinationsBiological substance pretreatmentsReverse transcriptasePolymerase L
This invention relates to a reagent container for detection and / or quantitation of at least one biological or chemical analyte from a sample, said reagent container comprising an inner surface enclosing a volume, wherein volume analytical reactions of at least one analysis for detection and / or quantitation of at least one analyte take place, and at least two reagents of an analysis of an analyte have been dried onto said inner surface and at least a first said reagent has been dried onto a first area of the inner surface distinctly separate, i.e. without any overlap, from a second area of the inner surface onto which at least a second said reagent has been dried. Characteristic for the reagent container is that the first reagent and the second reagent form a pair wherein the first reagent is an enzyme and the second reagent is a substrate of said enzyme, and said pair consists of a nucleic acid polymerase and substrate thereof. This invention also relates to a method for stabilising onto an inner surface of a reagent container dried assay reagents for the detection and / or quantitation of a biological or chemical analyte from a sample, said method comprising the steps of dispensing at least two reagents, a first reagent and a second reagent, needed in the detection of the analyte onto the inner surface of the reagent container; and removing excess water from the reagents, wherein first reagent is dispensed onto a first area of the inner surface distinctly separate, i.e. without any overlap, from a second area of the inner surface onto which the second reagent is dispensed. Characteristic for the method is that the first reagent and the second reagent form a pair wherein the first reagent is an enzyme and the second reagent is a substrate of said enzyme, and said pair consist of a nucleic acid polymerase and substrate thereof. This invention further relates to the use of the reagent container to perform an assay selected from the group consisting of a polymerase chain reaction (PCR) assay, an assay that utilizes a reverse transcriptase, a reverse transriptase polymerase chain reaction, an immuno-PCR assay, a nucleic acid sequence based assay (NASBA), a proximity ligation assay, a ligase chain reaction (LCR) assay, a rolling circle amplification (RCA) assay, and a strand displacement amplification (SDA) assay.
Owner:UNIOGEN OY
High-precision nucleic acid quantitative detection kit for hepatitis C virus (HCV)
ActiveCN103642942AAvoid PCR false negativesIncrease coverageMicrobiological testing/measurementMagnetic beadGenotype
The invention relates to a high-precision nucleic acid quantitative detection kit for hepatitis C virus (HCV) applied to the field of biomedical clinical diagnosis. The kit comprises a nucleic acid extraction kit based on a paramagnetic particle method and an HCV nucleic acid amplification box, wherein the nucleic acid extraction kit based on the paramagnetic particle method comprises a splitting combining liquid, a rinsing liquid A, a rinsing liquid B, a rinsing liquid C, an eluting liquid and a magnetic bead liquid. The HCV nucleic acid amplification box comprises an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) reaction liquid, enzyme mixed liquor, an HCV-interior label, an HCV quantitative reference 1-4, a negative quality control product, a critical positive quality control product and a positive quality control product. The splitting combining liquid in the nucleic acid extraction agent based on the paramagnetic particle method comprises 0.5-2.5% of lauryl sodium sulfate, 0.5-2.0ml / 100ml of TritonX-100, 2-6mol / L of guanidinium isothiocyanate and 1-10mM of EDTA (Ethylene Diamine Tetraacetic Acid) (PH 7.5). The kit provided by the invention is simple and fast to operate, low in cost, high in coverage of primer and probe genotype, high in conservative property, strong in specificity, high in detection sensitivity and good in repeatability, and can better simulate the extraction state of real viruses.
Owner:东北制药集团辽宁生物医药有限公司
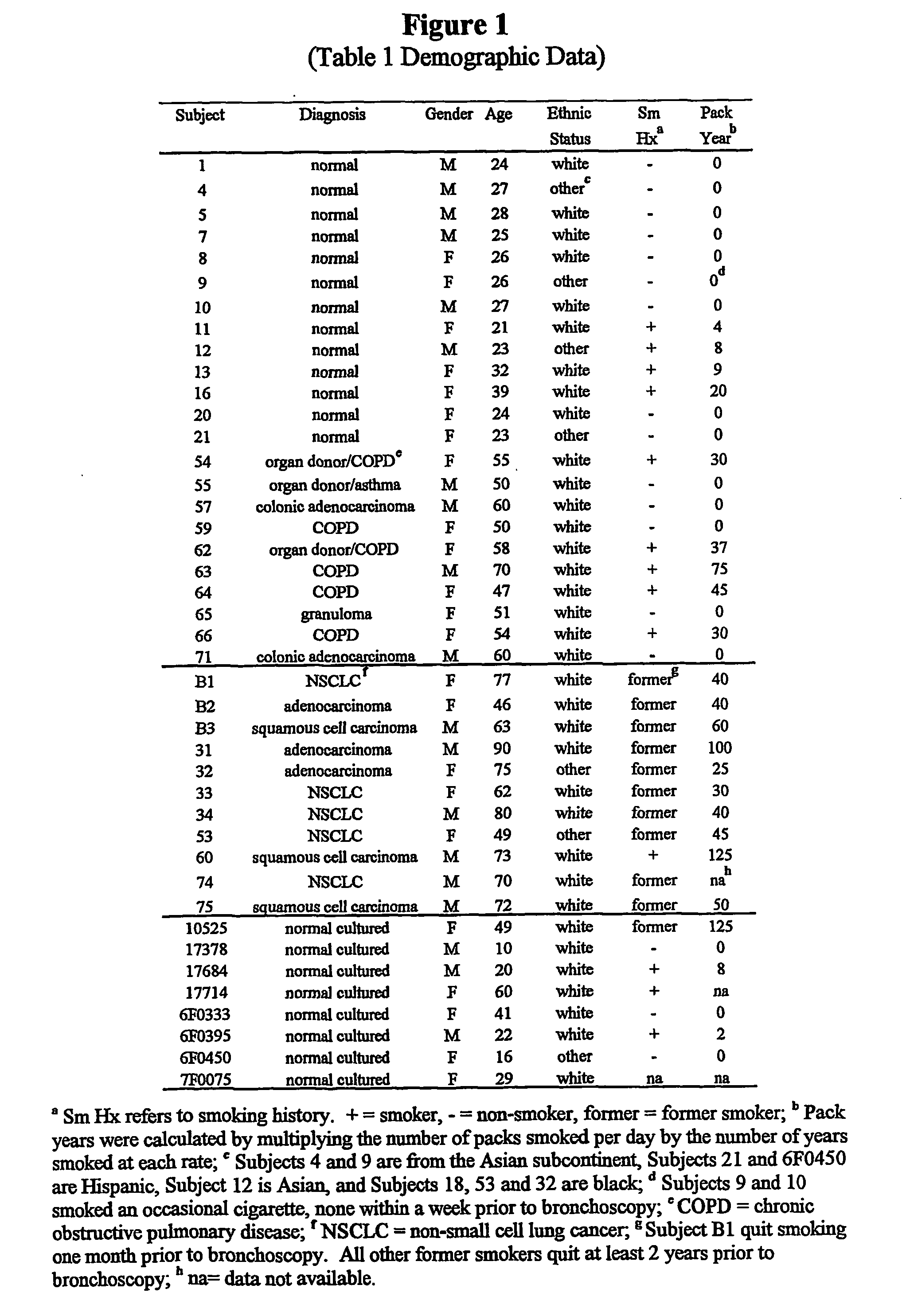
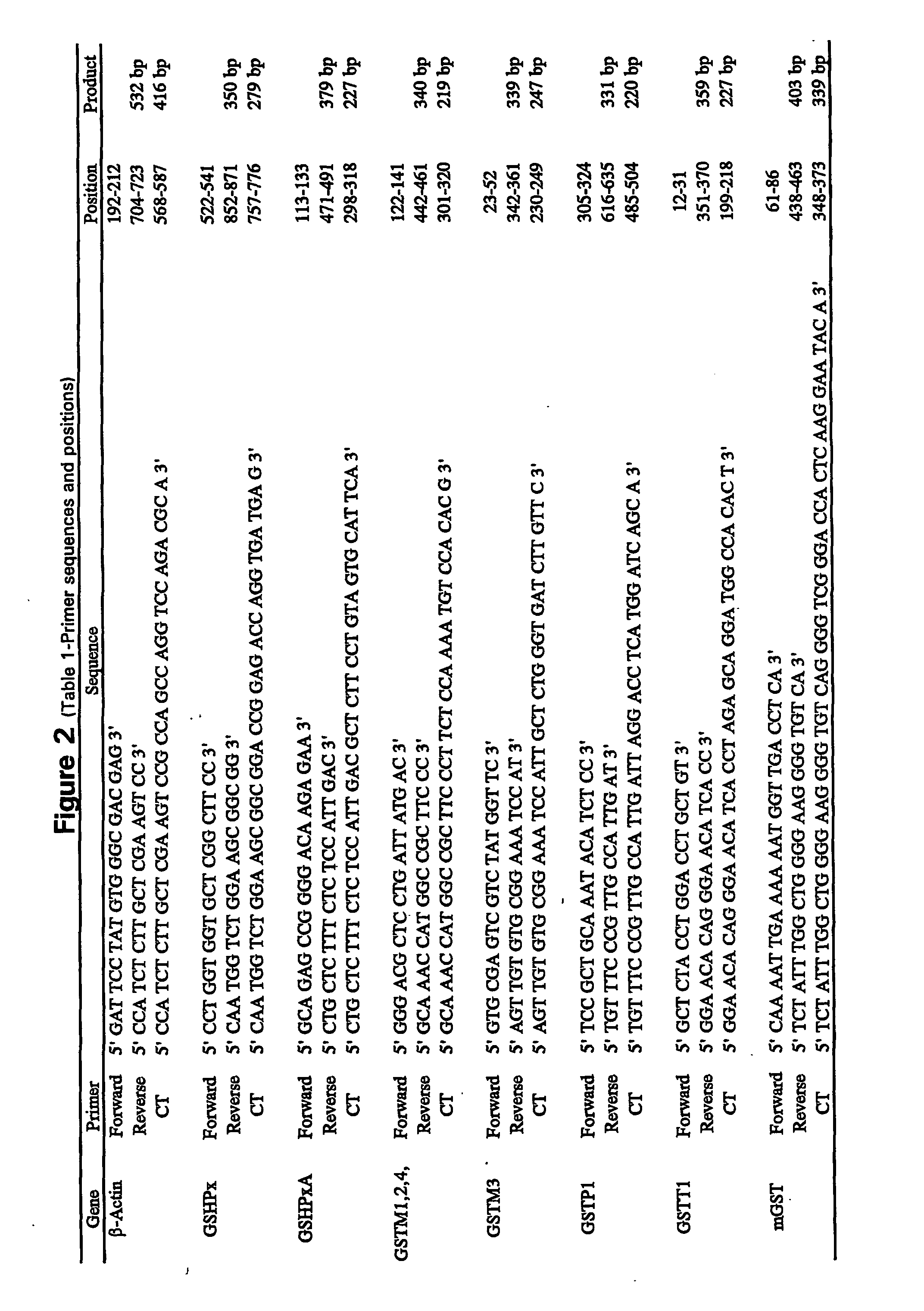
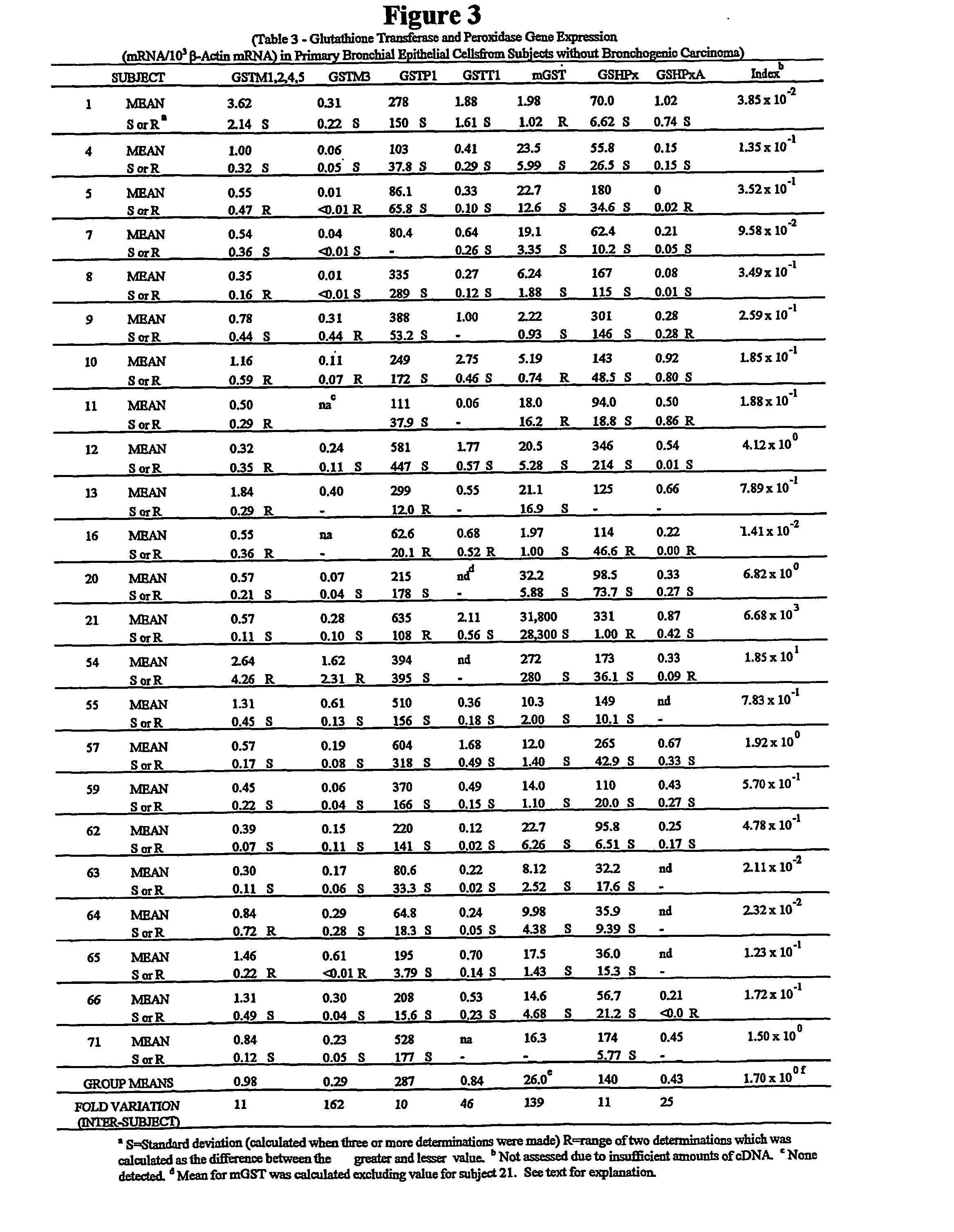
Method for quantitative measurement of gene expression for indentifying individuals at risk for bronchogenic carcinoma
InactiveUS20040197785A1Microbiological testing/measurementFermentationExpression MeasurementReverse transcription polymerase chain reaction
A method measure expression of multiple target genes in a progenitor cell for bronchogenic carcinoma comprising the use of reverse transcription-polymerase chain reaction (RT-PCR) to allow simultaneous expression measurement of the multiple target genes is disclosed.
Owner:MEDICAL COLLEGE OF OHIO
Method for obtaining sogatella furcifera carrying SRBSDV (southern rice black-streaked dwarf virus) and application of sogatella furcifera
InactiveCN102630640AOvercome technical bottlenecks that are difficult to preserve for a long timeRapid identificationHorticulture methodsRice cultivationDiseaseDiseased plant
The invention discloses a method for obtaining sogatella furcifera carrying SRBSDV (southern rice black-streaked dwarf virus) and an application sogatella furcifera. The method for obtaining the sogatella furcifera carrying the SRBSDV, comprises the following steps of: feeding the sogatella furcifera by utilizing rice infecting the SRBSDV or cryopreserved rice diseased plant so that the sogatella furcifera obtains the SRBSDV, transferring the infected sogatella furcifera to a healthy rice to be fed till passing through a circulation period of virus, and detecting the sogatella furcifera by utilizing an RT-PCR (reverse transcription-polymerase chain reaction) technology and a serology method to obtain the sogatella furcifera carrying the SRBSDV, wherein the carrier rate of the sogatella furcifera can reach more than 50% through a manual feeding manner based on the experiment. Through utilizing the method for obtaining the sogatella furcifera carrying the SRBSDV, the obtained sogatella furcifera carrying the SRBSDV can be applied to screening of SRBSDV-resisting monoclonal antibody to carry out a rice inoculation experiment to identify the resistance of the rice, screen disease-resistant variety and provide a service of the breeding for disease resistance. The invention can be further applied to researching mutual relation between the SRBSDV and a vector insect sogatella furcifera and provides powerful theoretical evidence for prevention and treatment on the viruses.
Owner:ZHEJIANG UNIV
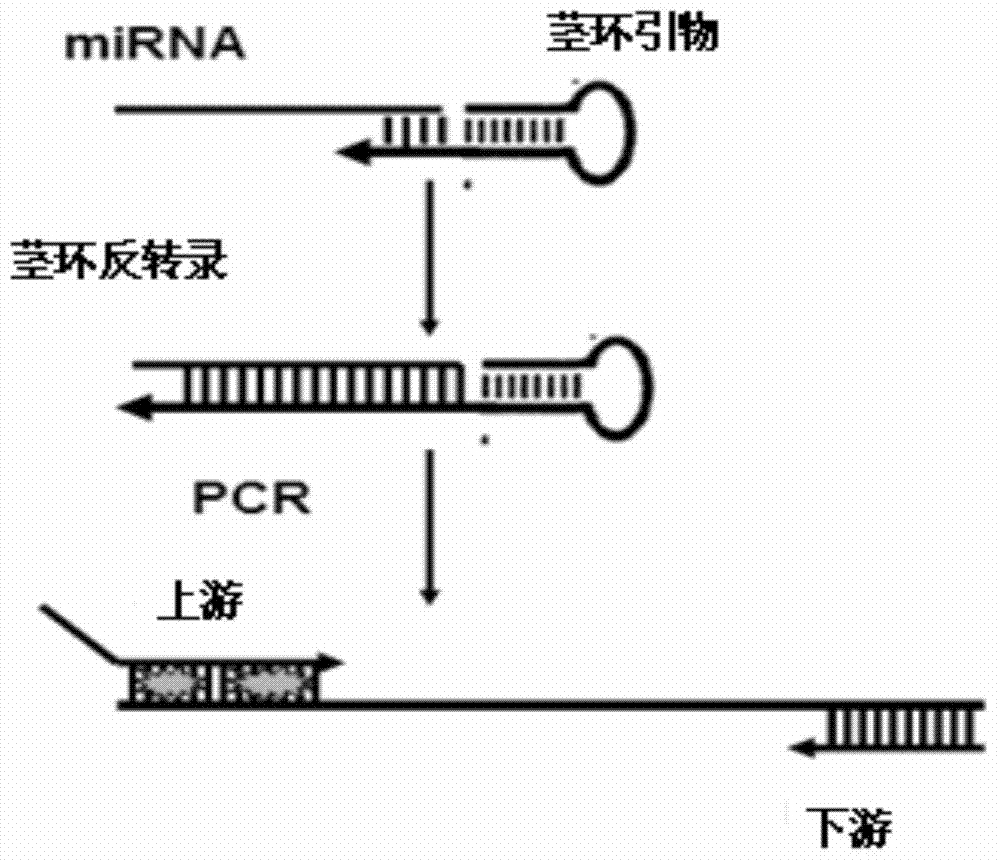
Method for detecting miRNA (micro Ribose Nucleic Acid) by improved stem-lop primer qRT-PCR (Quantitative Reverse Transcription Polymerase Chain Reaction)
InactiveCN103866009ALow technical costStrong specificityMicrobiological testing/measurementFluorescenceComplementary pair
The invention relates to a method for detecting miRNA (micro Ribose Nucleic Acid) by an improved stem-lop primer qRT-PCR (Quantitative Reverse Transcription Polymerase Chain Reaction). The method comprises the steps: (1), designing a miRNA detection primer, wherein a stem-loop primer comprises two parts of a general stem loop sequence and a specific primer sequence which is in complementary pairing with a 3' end of a target miRNA, a base number which is in complementary pairing with the target miRNA is 11bp, a downstream primer needle of qPCR is specific to a general stem loop sequence, an upstream primer needle is specific to a 5' end of the target miRNA, a GC tail is added in the 5' end of the upstream primer so that the annealing temperatures of the upstream primer and the downstream primer are close; (2), performing inverse transcription on the target miRNA into cDNA (complementary Desoxvribose Nucleic Acid); and (3), performing a quantitative PCR amplification and detecting by adopting an SYBR Green qPCR detection method. The method disclosed by the invention is good in specificity, and high in sensitivity; and one downstream primer is only synthesized, and an SYBR Green I fluorochrome method is adopted, and thus the cost is greatly saved.
Owner:DONGHUA UNIV
Recombinant antigen protein for diagnosing echinococcosis granulosa as well as preparation method and application thereof
InactiveCN102863524AIncreased sensitivityImprove featuresMicrobiological testing/measurementBiological testingAntigenThiogalactosides
The invention discloses a recombinant antigen protein (of which the amino acid sequence is shown as SEQ ID NO:1) for diagnosing echinococcosis granulose. Moreover, the invention further discloses a preparation method of the recombinant antigen protein. The method comprises the following steps of: amplifying an EgENO gene by adopting RT-PCR (Reverse Transcription-Polymerase Chain Reaction); cloning the EgENO gene into an expression vector PET28a (+) for constructing a recombined plasmid PET28a-EgENO; converting into escherichia coli BL21(DE3); inducing the expression of a recombinant protein through IPTG (Isopropyl-beta-d-Thiogalactoside); and identifying a purified recombinant antigen by using SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) and Western blotting. In addition, the invention further discloses a diagnosis application of the recombinant antigen protein. As proved by an experiment, the recombinant antigen protein has the advantages of high sensitivity, high specificity and the like for the diagnosis of the echinococcosis granulosa, and has a wide application prospect on the aspect of diagnosis of the echinococcosis granulosa.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and preparation method and application thereof
InactiveCN104745731AMicrobiological testing/measurementMicroorganism based processesPositive controlAfrican swine fever
The invention discloses a triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and a preparation method and application thereof. Three sets of specific primers and Taqman probes as well as positive controls specific to African swine fever virus CP530R genes, swine fever virus 5 minute-UTR genes and swine respiratory syndrome virus NSP2 genes respectively are designed and synthesized, and a rapid, easy and convenient triple fluorescent RT-PCR detection system with high specificity and high sensitivity is established by using the three sets of primers and probes, so that nucleic acids of the African swine fever viruses, the swine fever viruses and the respiratory syndrome viruses can be detected synchronously from a detected sample within 3-4 hours in a rapid, accurate, specific, safe, easy and convenient way. The detection reagent can be applied to synchronous detection of the nucleic acids of trace African swine fever viruses, swine fever viruses and respiratory syndrome viruses in hogs and relevant samples thereof.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Quick method for testing chain reaction of multiple reverse transcription polymerase of lily virus
InactiveCN1873019ADetection speedReduce testing costsMicrobiological testing/measurementPolymerase LReverse transcription polymerase chain reaction
This invention relates to a method for rapidly detecting lily virus by multiplex RT-PCR. The method comprises: (1) synthesizing three pairs of primers of lily symptomless virus (LSV), lily mottle virus (LMoV) and Cucumber mosaic virus (CMV) sequences, and optimizing the concentrations, amplification temperatures, amplification time and cycle time; (2) introducing the three pairs of primers to a same reaction system, and performing PCR reaction. The method can detect CMV, LSV and LmoV simultaneously, and has such advantages as rapid detection, high accuracy, high efficiency, high sensitivity, high specificity.
Owner:FLOWER RES INST OF YUNNAN ACAD OF AGRI SCI
Citrus fruit fly odorant binding protein-based attractant screening method
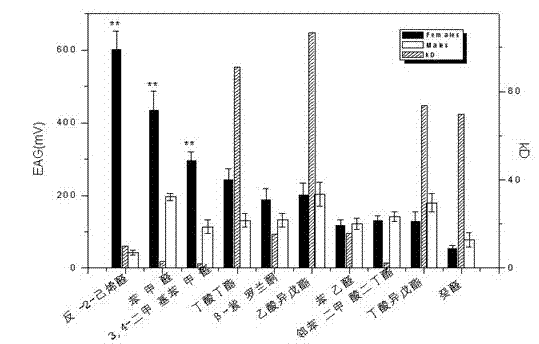
The invention discloses a citrus fruit fly odorant binding protein-based attractant screening method belonging to the technical field of bioengineering. The citrus fruit fly odorant binding protein-based attractant screening method comprises the following steps of: collecting the total RNA (Ribonucleic Acid) of antennae of a citrus fruit fly; obtaining the overall length of a citrus fruit fly odorant binding protein through RT-PCR (Reverse Transcription-Polymerase Chain Reaction); constructing a prokaryotic expression vector of the citrus fruit fly odorant binding protein; inducing the expression of a citrus fruit fly recombinant odorant binding protein through IPTG (isopropyl-beta-d-thiogalactoside), and purifying the citrus fruit fly recombinant odorant binding protein through a nickel sepharose gel affinity column; obtaining a conjugation reaction spectrum of the citrus fruit fly recombinant odorant binding protein and a host fruit odor volatile matter through a competitive fluorescence combing method, wherein a dissociation constant (KD) is lower than below 10 mu mol / L host fruit smell; and the IC 50 value of fluorescence competition is less than 30 mu mol / L, determining a host fruit smell attractant suitable for the citrus fruit fly. The invention provides a new strategy for screening and designing a citrus fruit fly odorant host fruit smell odor information attractant formula.
Owner:CHINA JILIANG UNIV
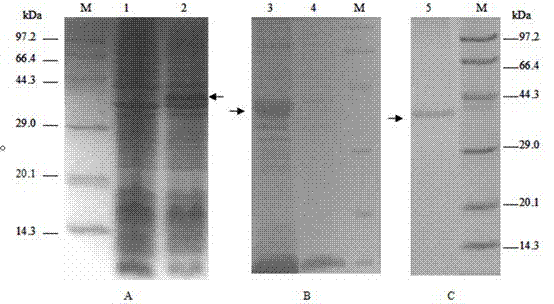
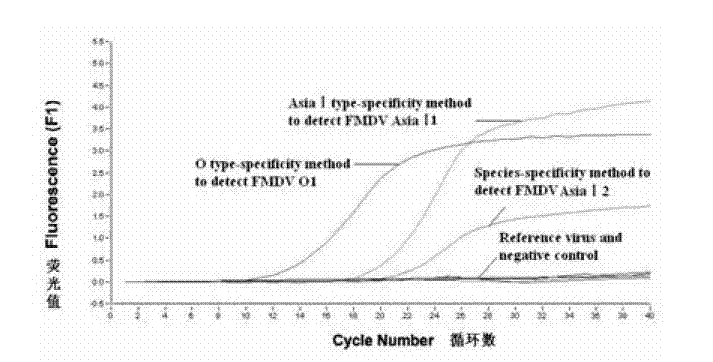
Method for detecting FMDV (Foot and Mouth Disease Virus) through real-time fluorescent quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction)
InactiveCN102220436AReduce pollutionGuaranteed inspection resultsMicrobiological testing/measurementMicroorganism based processesInfectious virusReverse transcription polymerase chain reaction
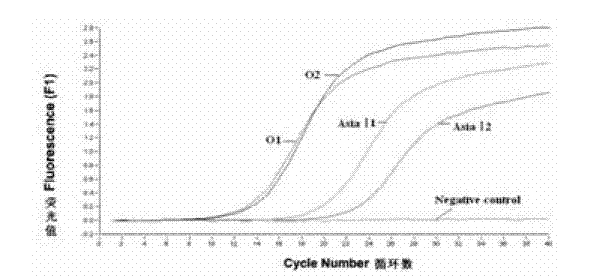
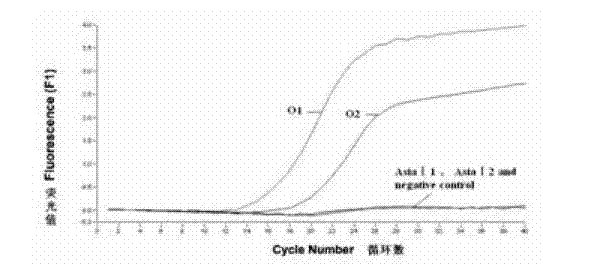
The invention discloses a method for detecting an FMDV (Foot and Mouth Disease Virus) group and O type and AsiaI type thereof through real-time fluorescent quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction). By carrying out specificity and sensitivity verification on the method established by the invention, the result shows that the FMDV can be well set apart from other Artiodactyla infectious viruses as well as O type and AsiaI type, and the sensitivity can be up to 10 copies of a template. The method established by the invention is characterized in that the whole reaction process is a closed pipe operation, thereby maximally reducing the pollution to the system. The whole process sequentially including the acquisition of a sample to be detected, the extraction of nucleic acid, the preparation of a fluorescent quantitative RT-PCR system and the representation of a reaction result can be completed within 2.5 hours, and the time required for detection is greatly shortened on the premise of ensuring the detection result.
Owner:珠海出入境检验检疫局检验检疫技术中心
miRNA (microribonucleic acid) biomarkers and detection kit for ovarian cancer diagnosis
InactiveCN105177173AImprove consistencyHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMir 145 5pMir 193b
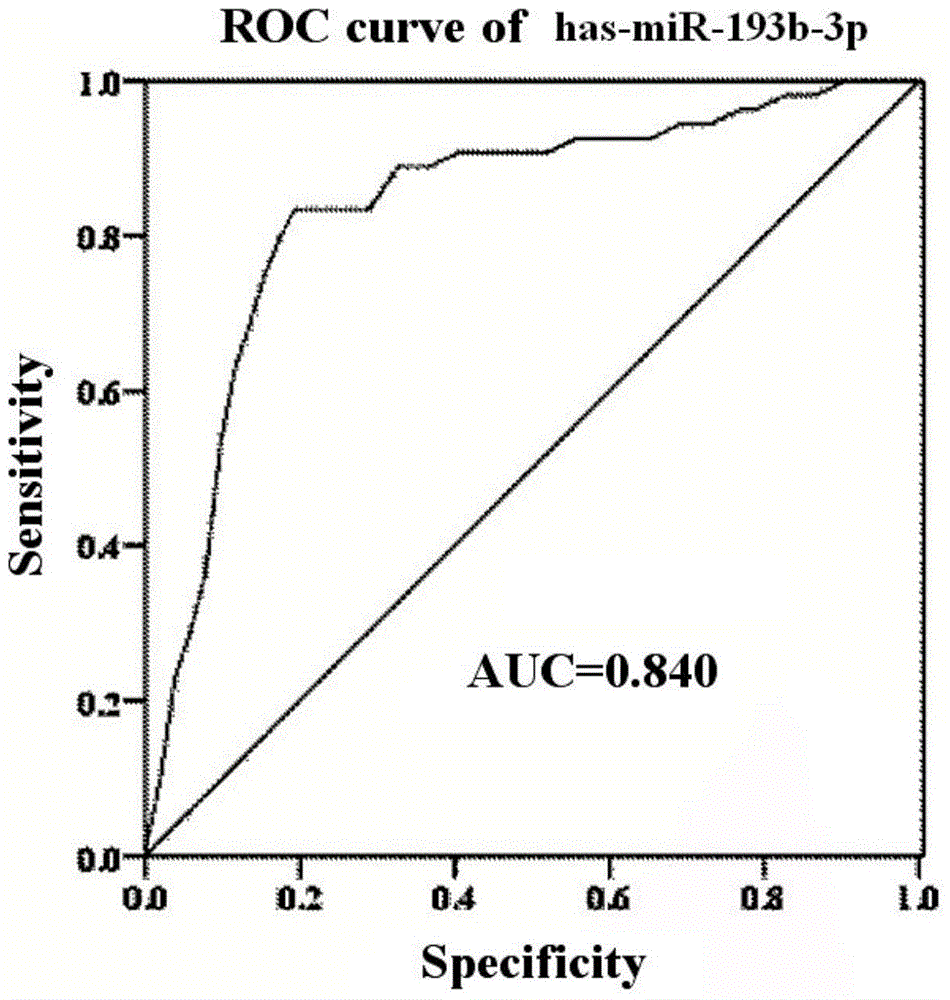
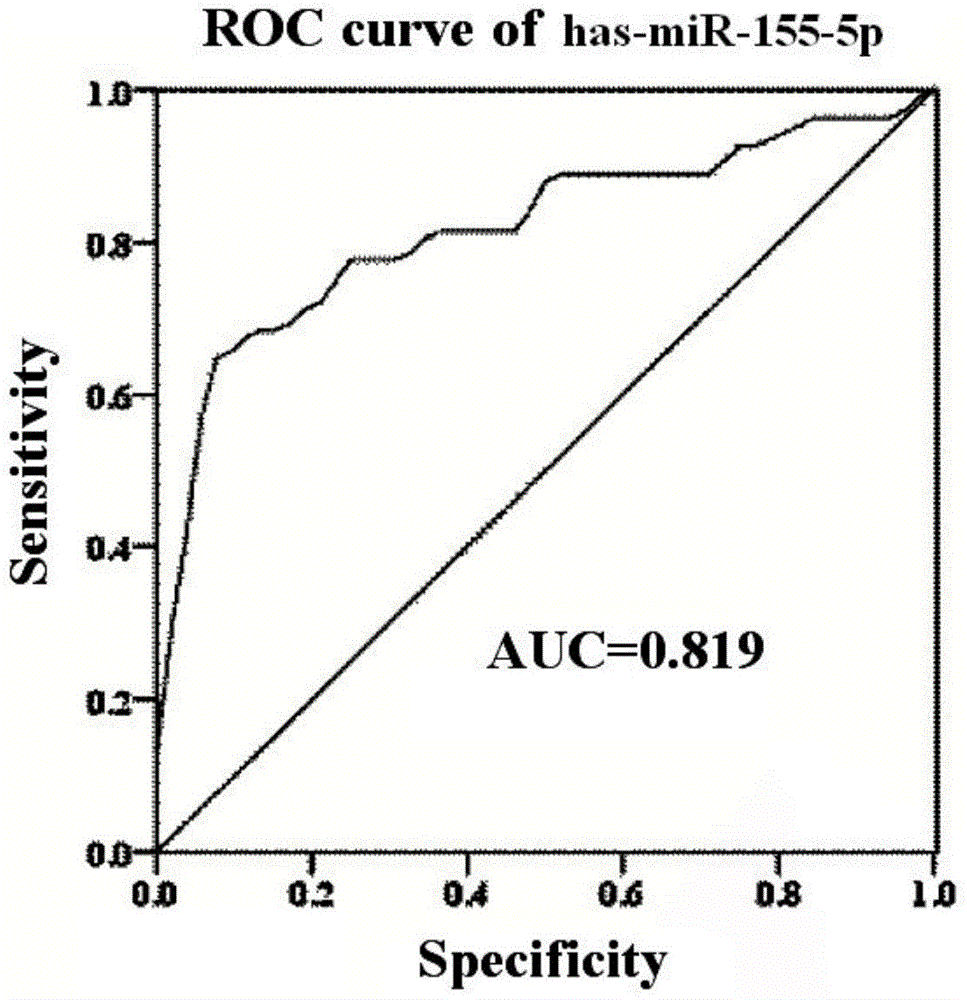
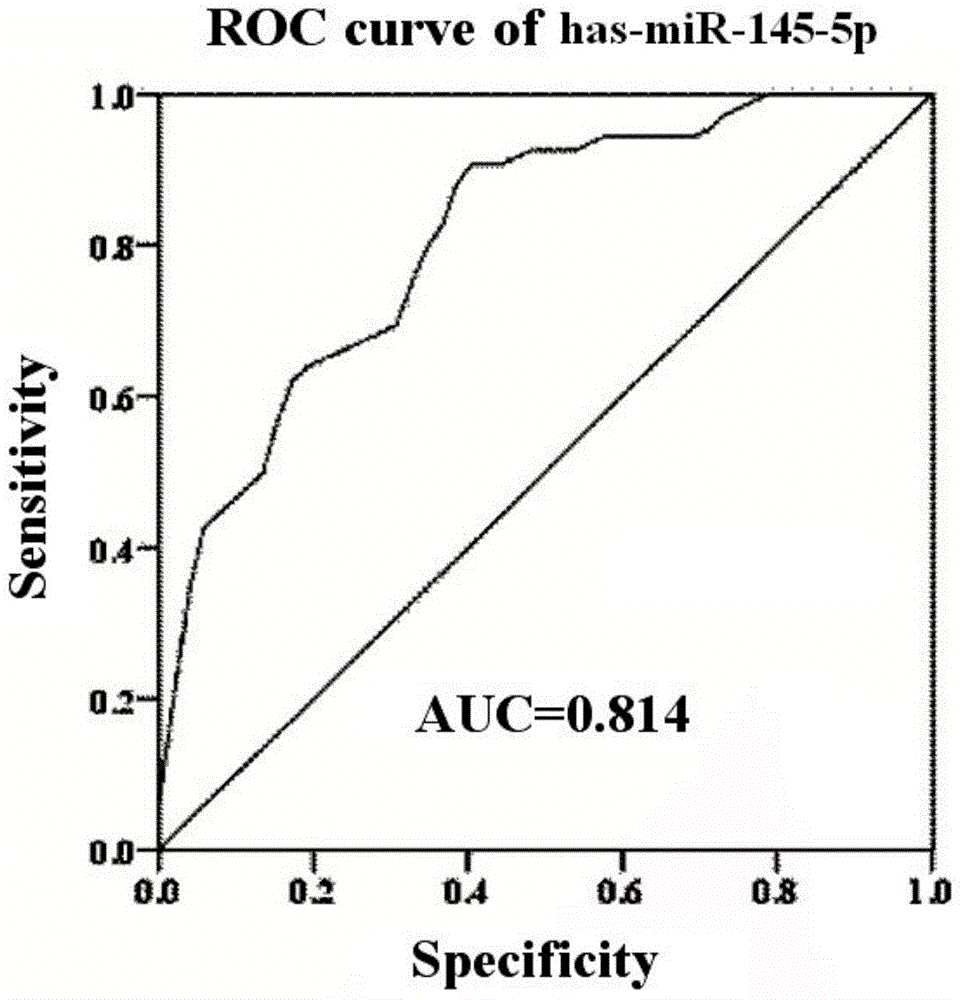
The invention discloses miRNA (microribonucleic acid) biomarkers and a detection kit for ovarian cancer diagnosis. The microRNA biomarkers are composed of the following microRNAs: has-miR-193b-3p, has-miR-155-5p, has-miR-145-5p, has-miR-132-3p and has-miR-143-3p. The serological expression analysis on the five miRNAs has-miR-193b-3p, has-miR-155-5p, has-miR-145-5p, has-miR-132-3p and has-miR-143-3p of ovarian cancer with obvious para-carcinoma tissue expression differences (the differential expression level is greater than 2 folds, and the CT value in RT-PCR (reverse transcription-polymerase chain reaction) is less than 30) indicates that the five miRNAs have stable expression in serum, the serum miRNA expression has favorable consistency with the tissue, the expressions of the has-miR-193b-3p, has-miR-155-5p and has-miR-145-5p are enhanced, and the expressions of the has-miR-132-3p and has-miR-143-3p are lowered. The five miRNAs can be used as biomarkers for ovarian cancer diagnosis, and the sensitivity and specificity of combined diagnosis are obviously higher than the sensitivity and specificity of single-miRNA diagnosis.
Owner:崔长友
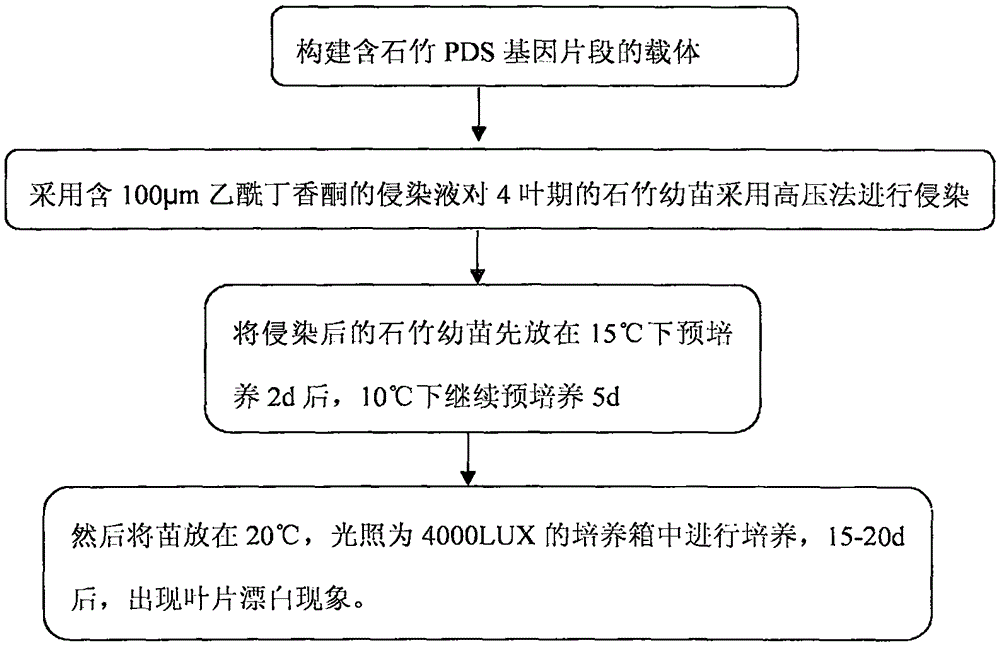
Efficient virus-induced phytoene desaturase gene silence system for Chinese pink
InactiveCN105296535ASolving questions about gene functionGenetic engineeringFermentationComplementary deoxyribonucleic acidMultiplex polymerase chain reaction
The invention discloses an efficient virus-induced phytoene desaturase gene silence system for Chinese pink. A specific primer is designed according to a coding gene sequence of PDS (phytoene desaturase), and about 500bp cDNA (complementary deoxyribonucleic acid) fragments are amplified in the Chinese pink through RT-PCR (reverse transcription-polymerase chain reaction). Obtained PDS cDNA fragments are connected to TRV2, and acetosyringone concentration, seedling age and preculture temperature after infection as well as light conditions for plants after preculture are integrated in an assorted manner by optimizing an agrobacterium-mediated virus-induced gene silence system according to photobleaching frequency, bleached leaf area and bleached conditions of the plants, so that the rapid efficient virus-induced silence system is established, virus-induced gene silence character can be obtained, and further, the problem that Chinese pink gene functions are verified effectively is solved.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
Recombinant oral protein TAT-GH of tilapia, preparation method for recombinant oral protein TAT-GH and application of recombinant oral protein TAT-GH
ActiveCN102703483AGuaranteed accuracyIntegrity guaranteedBacteriaAnimal feeding stuffBiotechnologyEscherichia coli
The invention discloses recombinant oral protein TAT-GH of tilapia, a preparation method for the recombinant oral protein TAT-GH and application of the recombinant oral protein TAT-GH. The method comprises the following steps of: extracting total messenger ribonucleic acid (mRNA) from a pituitary gland of male tilapia, performing reverse transcription-polymerase chain reaction (RT-PCR) to obtain complementary deoxyribonucleic acid (cDNA), performing PCR amplification to obtain a growth hormone (GH) gene of the tilapia, and amplifying a TAT-GH gene by a PCR overlap extension method; constructing a recombinant plasmid pET-32a(+)-TAT-GH; and transforming Escherichia coli BL21 (DE3) by using the recombinant plasmid pET-32a(+)-TAT-GH, efficiently expressing fusion protein TAT-GH in the form of an inclusion body under the induction of isopropyl thiogalactoside (IPTG), and purifying and renaturing to obtain active protein. The protein can effectively promote the growth and development of fries of the tilapia after being fed to the fries of the tilapia.
Owner:HUBEI TAIYANGHONG BIOLOGICAL TECH CO LTD
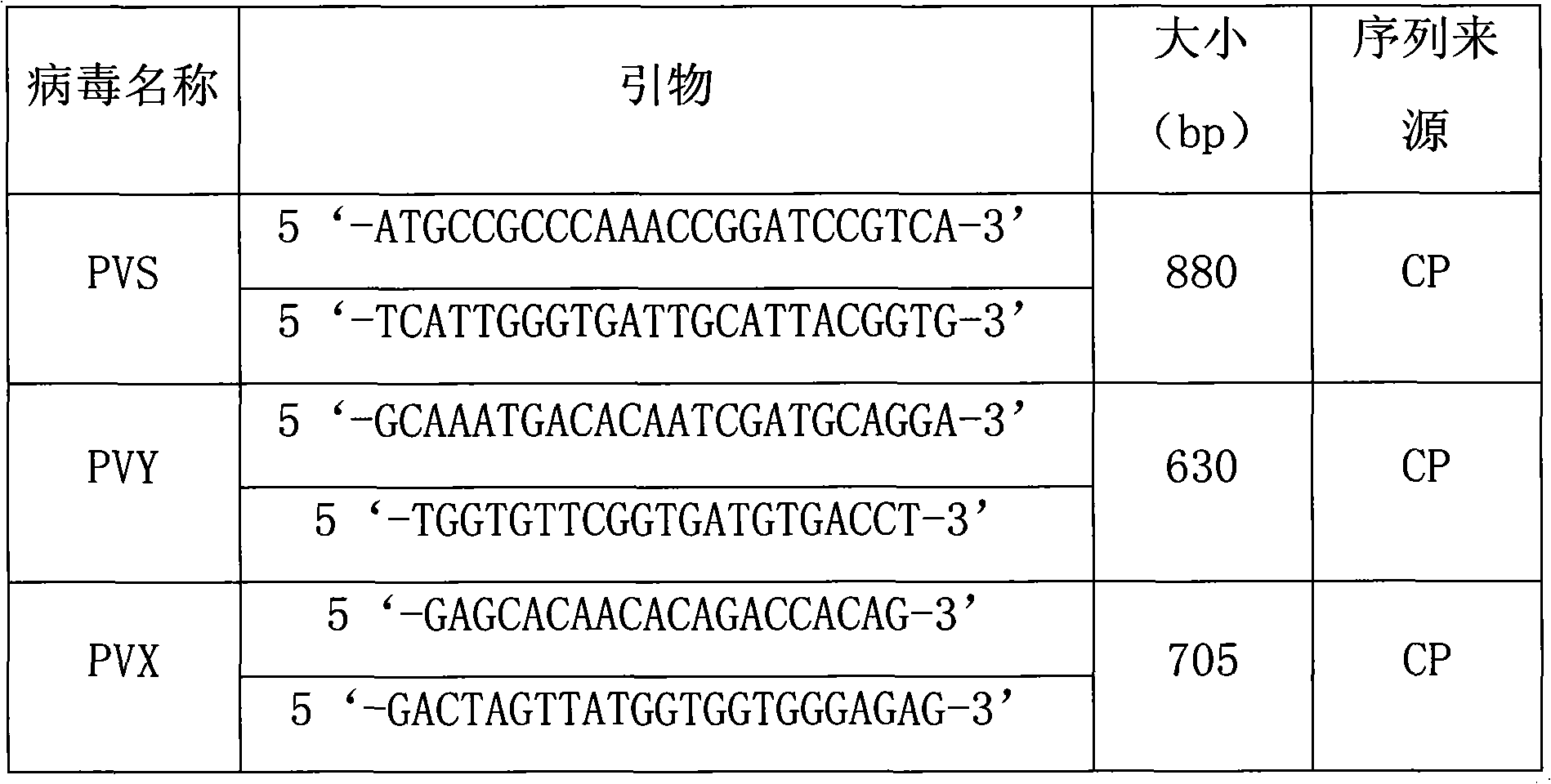
Quadruple RT-PCR (reverse transcription-polymerase chain reaction) detection primers and method capable of simultaneously detecting multiple chili viruses
InactiveCN105177188AIncrease varietyImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseBroad bean wilt virus
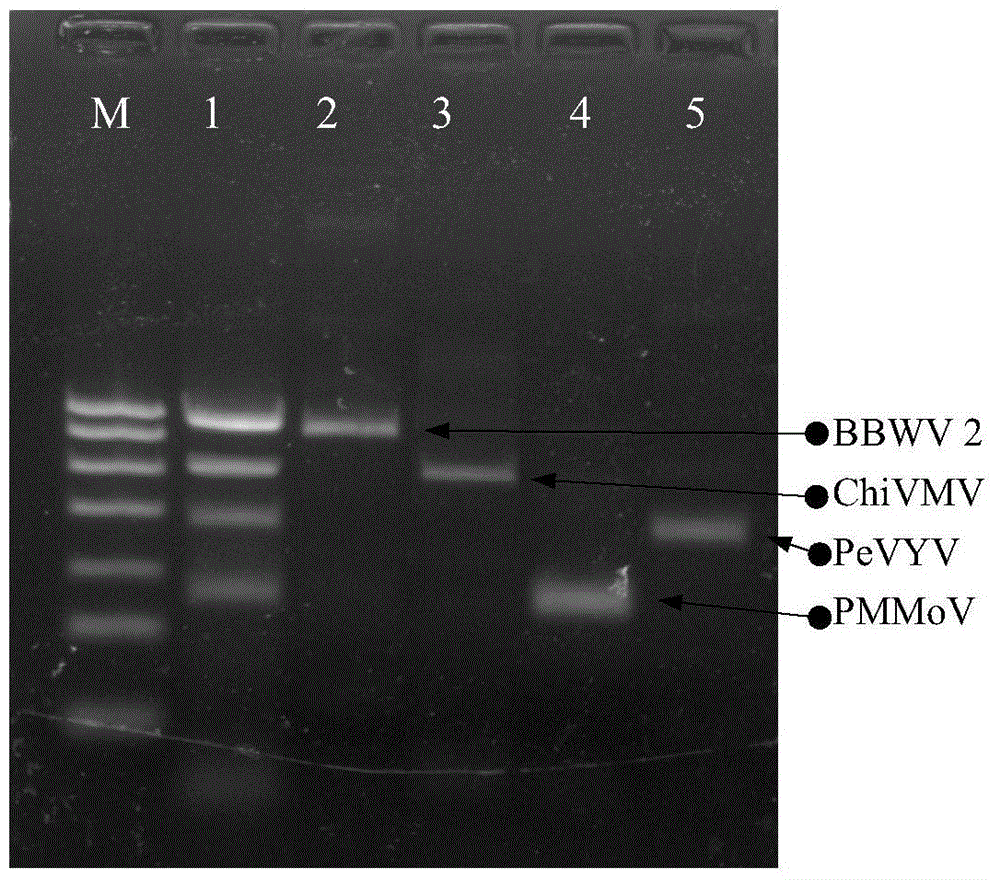
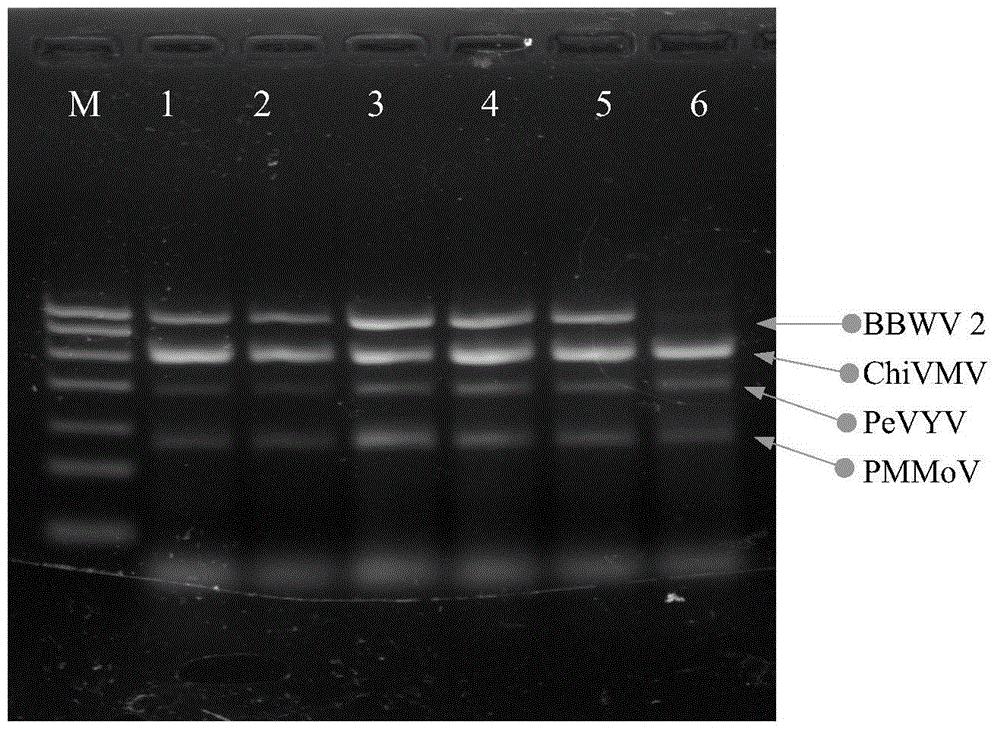
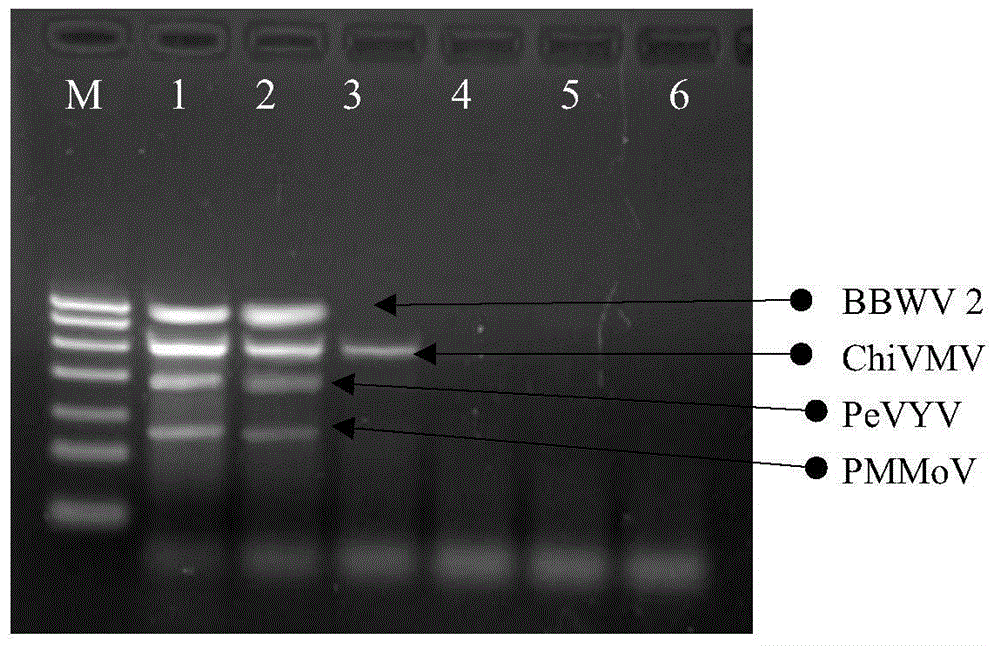
The invention discloses quadruple RT-PCR (reverse transcription-polymerase chain reaction) detection primers and a method capable of simultaneously detecting multiple chili viruses. The method adopts four pairs of primers disclosed as SEQ ID NO:1-SEQ ID NO:8; and one RT-PCR process can simultaneously detect four chili viruses: Broad bean wilt virus 2 (BBWV 2), Pepper vein yellows virus (PeVYV), Chilli veinal mottle virus (ChiVMV) and Pepper mildmottle virus (PMMoV). Compared with the existing single RT-PCR and duplex RT-PCR detection methods, the method disclosed by the invention can simultaneously detect the four chili viruses by one RT-PCR process, and is capable of detecting more virus varieties, greatly enhancing the detection efficiency and obviously lowering the detection cost.
Owner:HUNAN AGRICULTURAL UNIV
Primer and method for detecting GI type and GII type norovirus by utilizing primer
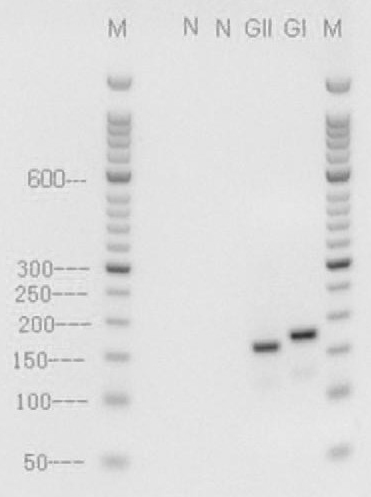
InactiveCN102304514AEasy to operateShorten detection timeMicrobiological testing/measurementMicroorganism based processesReverse transcription polymerase chain reactionBiology
The invention relates to a primer and a method for detecting GI type and GII type norovirus by utilizing the primer, belonging to the technical field of biological detection. The sequence of the primer is as follows: Nov-F: 5-GTGAATGAWGATGGCGTCKA-3, Nov-R: 5-GTHAGWATCCAGGGGTCAAT-3. The invention focuses on the design of the sequence of the primer, the composition of an RT-PCR (reverse transcription-polymerase chain reaction) system, the selection of reaction conditions and the judgment of reaction results can be performed according to the conventional methods in the field. By adopting the primer and the method provided by the invention, a pair of primers can simultaneously detect and differentiate the GI type and the GII type norovirus in a same reaction tube, thereby not only simplifying the operation steps, but also shortening the detection time.
Owner:湖州市疾病预防控制中心
Method for detecting swine epidemic diarrhea by reverse transcription-loop-mediated isothermal amplification
InactiveCN102021249AQuick checkSensitive detectionMicrobiological testing/measurementReverse transcriptaseBiology
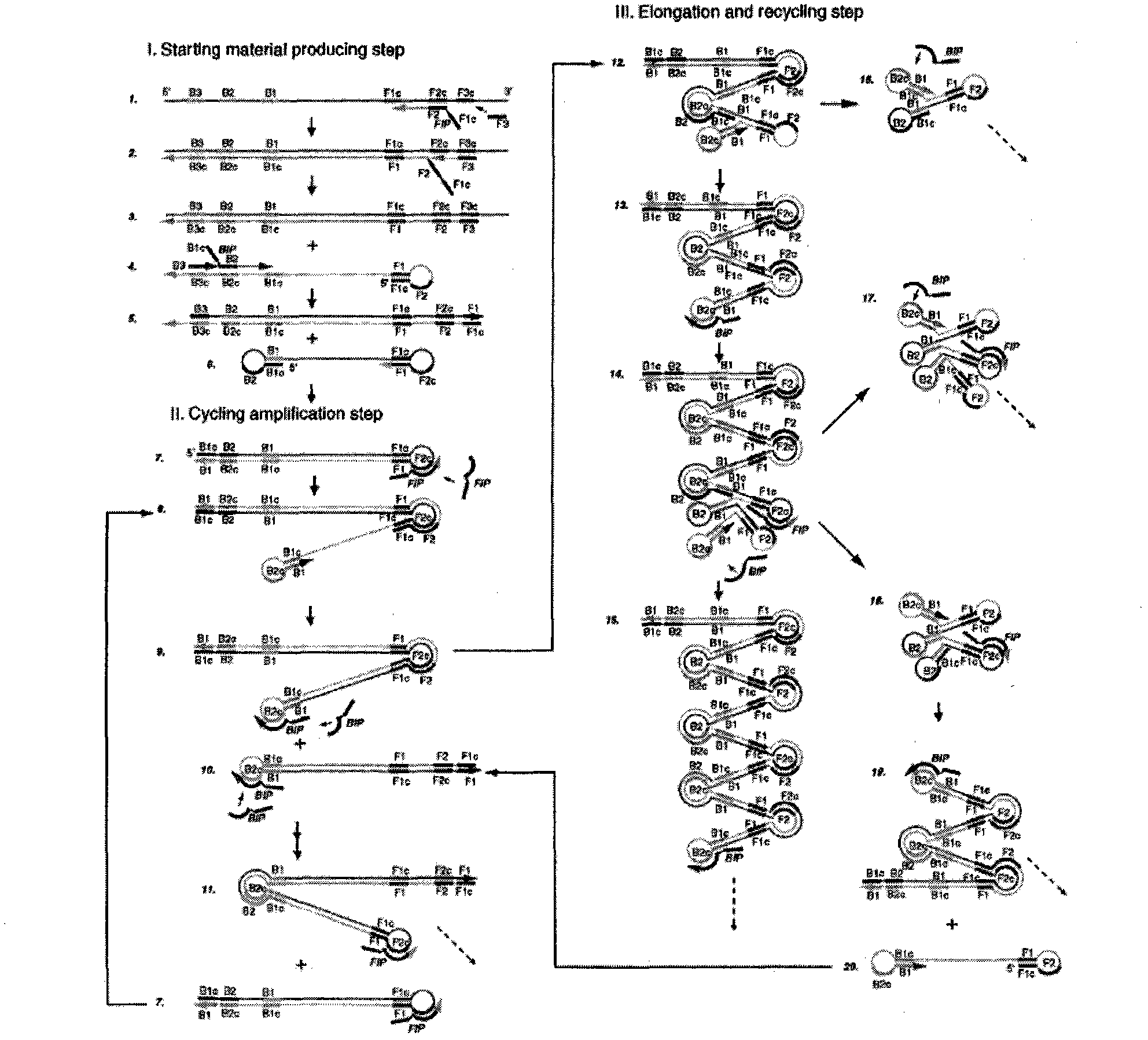
The invention provides a method for detecting swine epidemic diarrhea by reverse transcription-loop-mediated isothermal amplification. According to the conserved domain gene of PEDV (Porcine Epidemic Diarrhea Virus) N protein, three pairs of primers aiming at six areas on a target gene are designed, two RT-PCR (Reverse Transcription-Polymerase Chain Reaction) primers are combined, and PED (Porcine Epidemic Diarrhea) viruses are identified by the RT-LAMP (Reverse Transcription-Loop-Mediated Isothermal Amplification) technology; RT-LAMP reacts; reverse transcription is performed on obtained RNA(Ribose Nucleic Acid); and the obtained cDNA is used for PCR reaction. The method provided by the invention ensures amplification specificity, has high amplification speed and can obtain a result within 30-60 minutes, and people can observe amplification effect with eyes without electrophoresis. Reverse transcription and nucleic acid amplification can be realized in 1 hour at the constant temperature of 65 DEG C by using enzyme mixture; RT-LAMP detection is carried out on the basis of LAMD amplification DNA; a reverse transcription enzyme is added to realize amplified detection of RNA; and reverse transcription and amplification are finished in one step so as to omit the reverse transcription step which is firstly carried out by the traditional RT-PCR.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Pseudovirion containing hepatitis c virus RNA (Ribonucleic Acid) fragment and preparation method thereof
InactiveCN104845993ANo dangerImprove the simulation effectMicrobiological testing/measurementInactivation/attenuationEscherichia coliPHA granule
The invention provides a pseudovirion containing hepatitis c virus (HCV) RNA (Ribonucleic Acid) fragment and preparation method thereof which are applied in the field of biomedical clinical vitro diagnostic. The pseudovirion is a RNA-protein complexes that MS2 bacteriophage capsid protein wraps the hepatitis c virus, and the complexes is icosahedral; the method comprises the following steps of designing and synthesizing primer to obtain target gene MS2 by overlapping and splicing PCR (Polymerase Chain Reaction) method; connecting the target gene MS2 to plasmid pET-32a (+) to obtain recombinant plasmid pET-MS2; connecting the recombinant plasmid pET-MS2 and the HCV fragment to obtain pET-MS2-HCV recombinant plasmid; the obtained pET-MS2-HCV recombinant plasmid is introduced into escherichia coli for prokaryotic expression; releasing virus-like particles by adopting ultrasonication and the obtained virus-like particles are the pseudovirion containing the hepatitis c virus RNA fragment. The pseudovirion preparation method provided by the invention has the advantages that the preparation method is simple, the operation is easy, the obtained pseudovirus is high in purity, and the pseudovirion can be used as standard and quality control material of detection of RT-PCR (Reverse Transcription-Polymerase Chain Reaction), is good in stability, has no infectivity, has RNase-resistant, and the like.
Owner:宝瑞源生物技术(北京)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com