Recombinant antigen protein for diagnosing echinococcosis granulosa as well as preparation method and application thereof
A technology for echinococcosis granulosus and recombinant antigenic protein, which is applied to the preparation of the above-mentioned recombinant antigenic protein, the recombinant antigenic protein for diagnosing echinococcosis granulosus, and the field of recombinant proteins, and can solve the therapeutic effect of echinococcosis granulosus. Not ideal, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
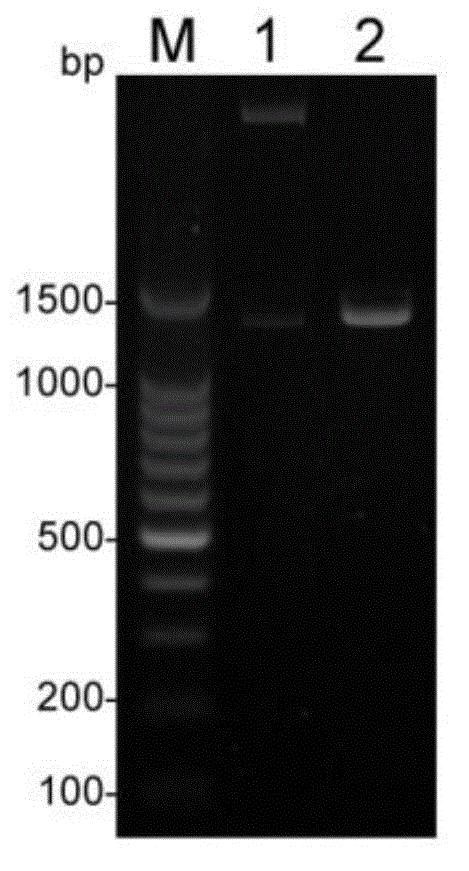
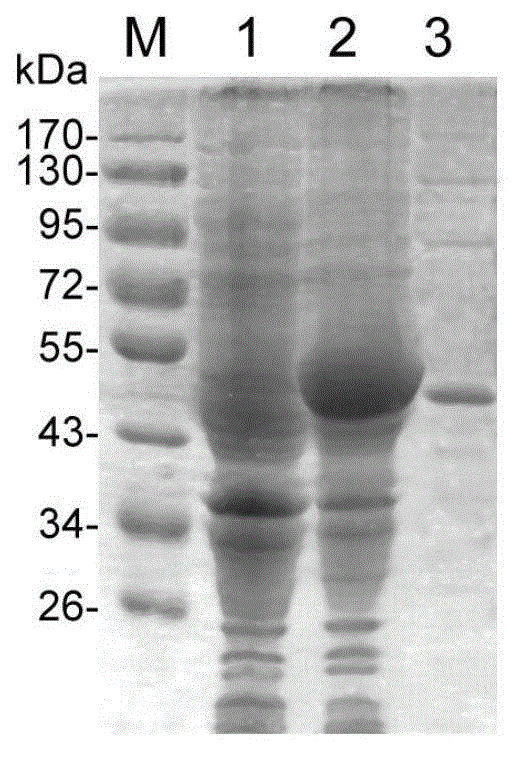
[0032] Example 1 Preparation of Echinococcus granulosus Recombinant Antigen Protein
[0033] 1 material
[0034] 1.1 Echinococcus granulosus
[0035] Echinococcus granulosus cysts from sheep liver were collected from slaughterhouses in Qinghai Province, and the protoscole of Echinococcus granulosus from Qinghai (sheep source) was isolated and collected under aseptic conditions.
[0036] 1.2 Serum samples to be tested
[0037]32 serums from patients with cystic echinococcosis (CE), 24 serums from patients with alveolar echinococcosis (AE), 5 serums from patients with Schistosoma japonicum, and serum from patients with Toxoplasma gondii provided by the Institute of Parasitic Disease Prevention and Control, Chinese Center for Disease Control and Prevention 5 samples, 5 serum samples from Clonorchis sinensis patients, 4 serum samples from cysticercosis patients, 2 serum samples from Paragonimia sinensis patients and 16 healthy human serum samples.
[0038] 1.3 Main reagents and...
Embodiment 2
[0059] Western-blot identification of embodiment 2PET28a-EgENO recombinant protein
[0060] 1. Western-blot identification method
[0061] The purified PET28a-EgENO recombinant protein (prepared in Example 1) was electrophoresed by 10% SDS-PAGE and transferred to PVDF membrane (polyvinylidene fluoride membrane, polyvinylidene fluoride); the membrane was placed in 5% degreased The milk powder solution was blocked for 1 hour, mixed with cystic echinococcosis patient serum (diluted 1:200) to react, overnight at 4°C, TBST (TBST contains Tris-Hcl, NaCl, and tween20, which are commonly used in Western-blot a buffer solution) for 3 times, 5 min each time; add donkey anti-human HRP-IgG (1:10000) and shake gently at room temperature for 1 h, wash 3 times with TBST, 5 min each time; add freshly prepared DAB (diaminobenzidine ) combined with the chromogenic solution until the target band appeared, and deionized water was used to terminate the reaction.
[0062] 2. Western-blot identifi...
Embodiment 3
[0064] Example 3 Diagnosis Effect Experiment of Echinococcus granulosus Recombinant Antigen Protein
[0065] 1. ELISA serological evaluation of recombinant antigens
[0066] A number of cystic echinococcosis patient sera and healthy human sera were mixed in equal amounts to prepare positive and negative serum controls. The serum was diluted 1:100, and the working concentration of the recombinant antigen (prepared in Example 1) was determined to be 10 μg / ml by square array titration, and the working concentration of donkey anti-human HRP-IgG was 1:10000. DAB color, test A 450 . The 2 times SD of the mean absorbance of 16 cases of healthy human serum was used as the positive judgment value.
[0067] 2. Statistical analysis of data
[0068] The experimental data were collated and statistically analyzed using Excel spreadsheet processing software.
[0069] 3. Analysis and evaluation of serological test results of recombinant antigens
[0070] Using the recombinant antigen Eg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com