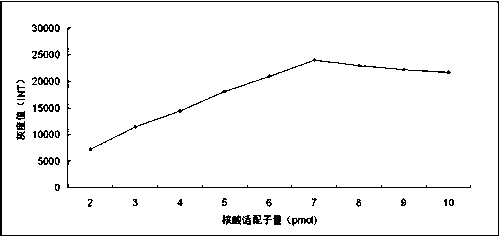
Patents
Literature
45results about How to "Excellent diagnostic value" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Recombinant antigenic protein for diagnosing echinococcosis granulosus, preparation method thereof and use thereof
ActiveCN101948521AIncreased sensitivityImprove featuresMicrobiological testing/measurementBiological testingEscherichia coliCystic echinococcosis
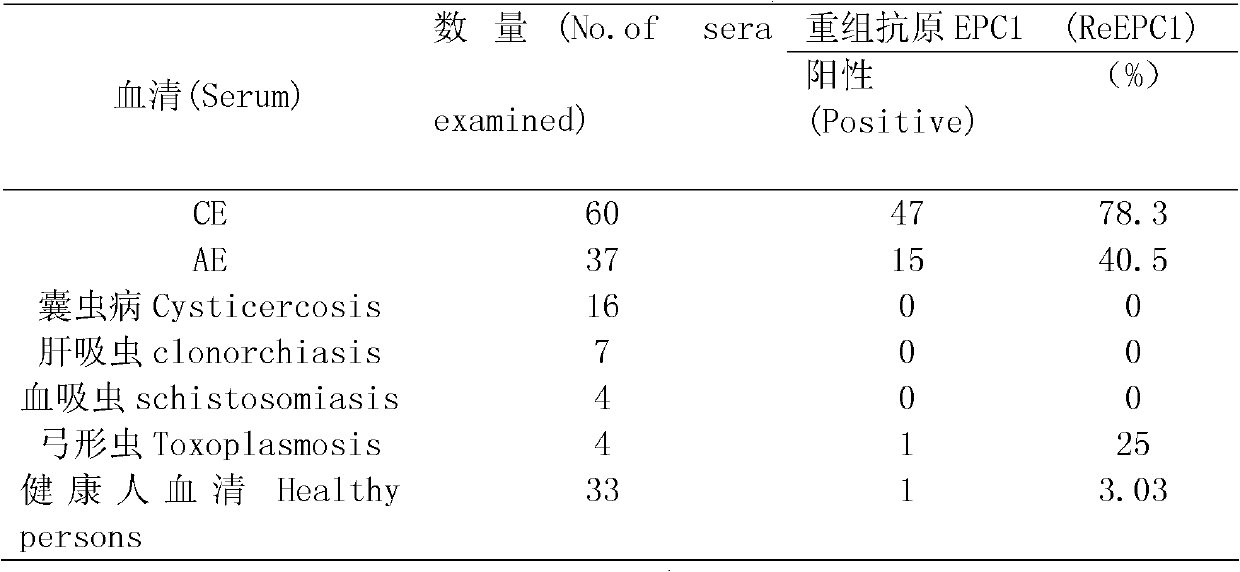
The invention discloses a recombinant antigenic protein for diagnosing echinococcosis granulosus (having an amino acid sequence represented by SEQIDNo.1). In addition, the invention also discloses the preparation method of the recombinant antigenic protein, which comprises: amplifying an EgEPC1 gene by using RT-PCR; cloning the EgEPC1 gene in a pGEM-T vector; connecting the EgEPC1 gene with an expression vector PET28a(+) to form a recombinant plasmid PET28a-EgEPC1; transforming the recombinant plasmid PET28a-EgEPC1 to Escherichia coli BL21(DE3) and expressing the recombinant protein through IPTG induction; and identifying a purified recombinant antigen by using SDS-PAGE and Western blotting. In addition, the invention also discloses the diagnosis use of the recombinant antigenic protein. Experiments show that the recombinant antigenic protein of the invention has the advantages of high sensibility and specificity for the diagnosis of echinococcosis granulosus, and has a promising application prospect in the diagnosis of the echinococcosis granulosus.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Cyclic chimeric citrullinated peptide antigen and application thereof
InactiveCN104262489AImprove stabilityIncrease exposureBiological testingHybrid peptidesPeptide antigenDisulfide bonding
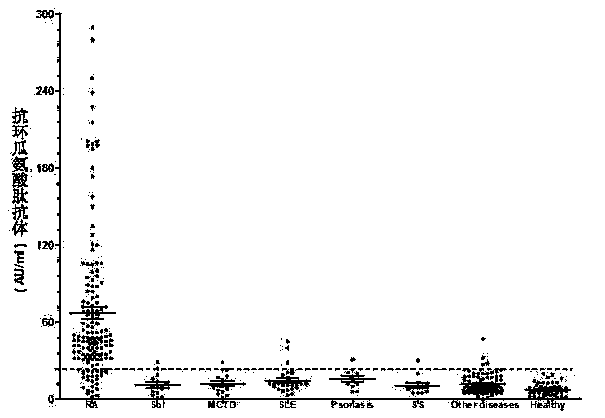
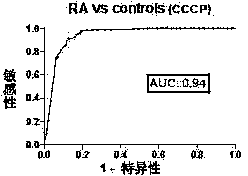
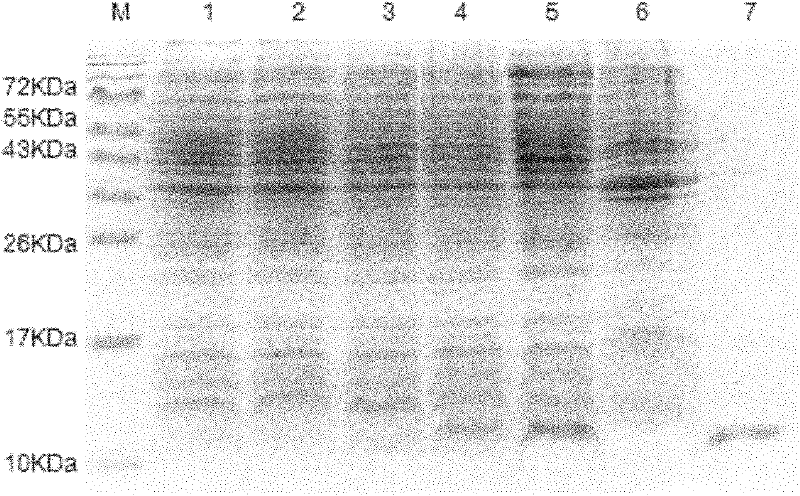
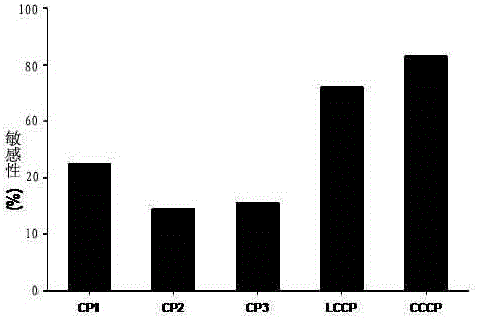
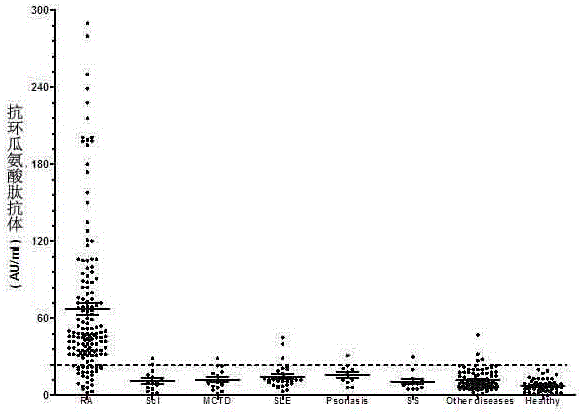
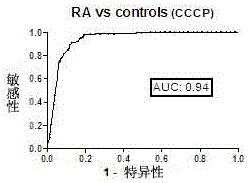
The invention discloses a cyclic chimeric citrullinated peptide antigen and an application thereof. The preparation of the cyclic chimeric citrullinated peptide antigen comprises the following steps: firstly connecting and jogging three small-molecular antigen peptides, namely a citrullinated peptide1, a citrullinated peptide 2 and a citrullinated peptide 3 derived from a silk polymerizing protein / an intermediate filament protein, and then synthetizing a cyclic polypeptide with a similar protein beta-corner structure by forming a disulfide bond through two cysteines inserted into the end N and the end C of a chimeric peptide. The cyclic chimeric citrullinated peptide antigen coats a solid-phase vector to prepare an indirect enzyme linked immunosorbent assay kit used for detecting the hypotype of multiple anti-citrullinated protein antibodies contained in RA (Rheumatoid Arthritis) serum. The cyclic chimeric citrullinated peptide antigen and the ELISA kit thereof which are disclosed by the invention have the advantages of simple preparation and experimental operation process, good result repeatability, qualification or quantification and wide clinical application and scientific research value and are outstandingly enhanced in detection sensibility and diagnosis value on RA compared with an international similar kit.
Owner:陈仁奋
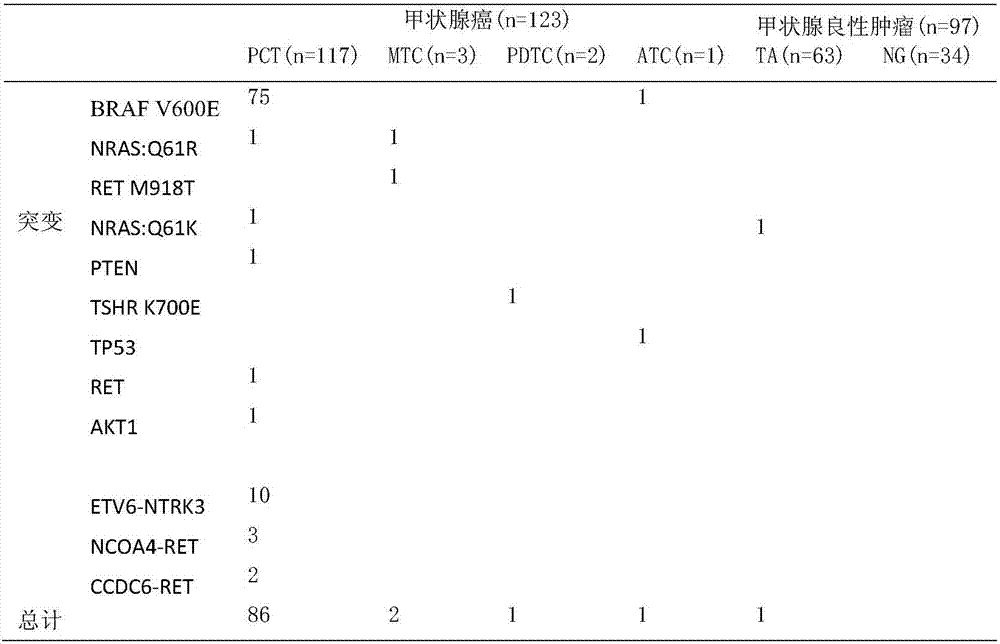
Gene polymorphism sites related to thyroid cancer and application thereof
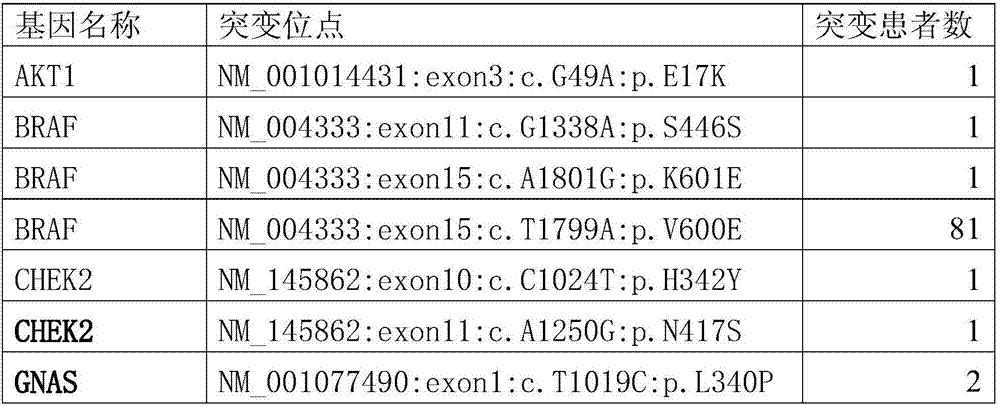
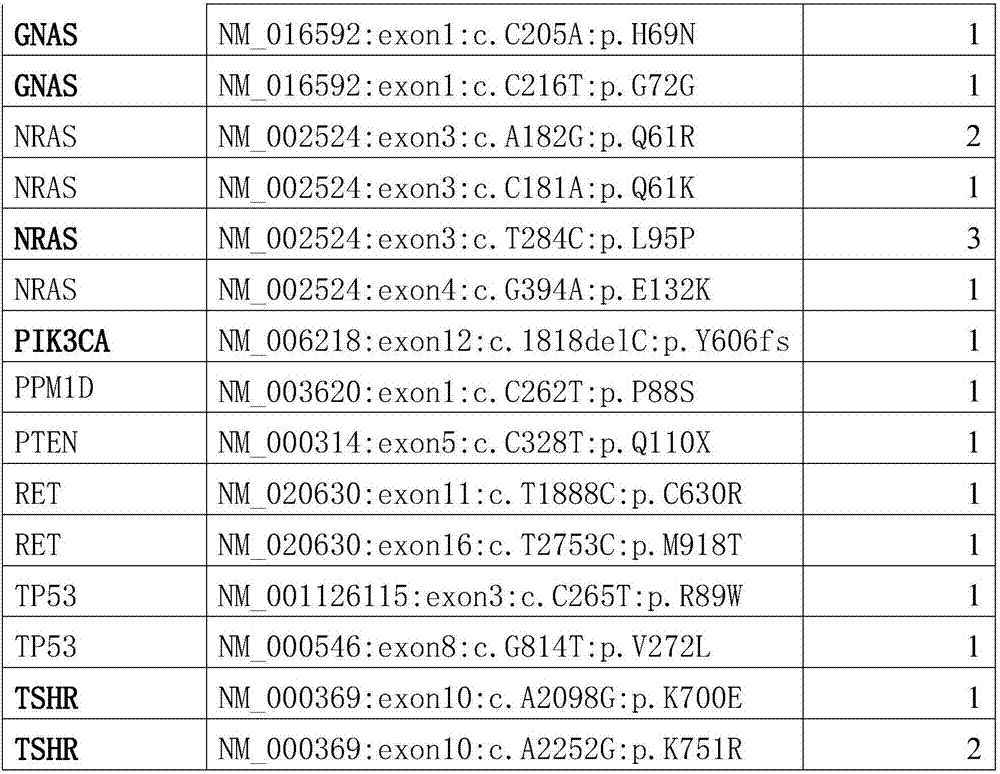
PendingCN107164496AStrong specificityExcellent diagnostic valueMicrobiological testing/measurementMaterial analysisGenes mutationSomatic cell
The invention provides somatic mutation sites of pathogenic genes of the thyroid cancer and application thereof, specifically to a group of mutation sites of the pathogenic genes of the thyroid cancer. The mutation sites are composed of a GNAS gene mutation site, a NRAS gene mutation site, a TSHR gene mutation site, etc. The gene mutation sites provided by the invention can be used as markers for identification of benign and malignant thyroid nodules.
Owner:上海安甲生物科技有限公司
Recombinant antigen protein for diagnosing echinococcosis granulosa as well as preparation method and application thereof
InactiveCN102863524AIncreased sensitivityImprove featuresMicrobiological testing/measurementBiological testingAntigenThiogalactosides
The invention discloses a recombinant antigen protein (of which the amino acid sequence is shown as SEQ ID NO:1) for diagnosing echinococcosis granulose. Moreover, the invention further discloses a preparation method of the recombinant antigen protein. The method comprises the following steps of: amplifying an EgENO gene by adopting RT-PCR (Reverse Transcription-Polymerase Chain Reaction); cloning the EgENO gene into an expression vector PET28a (+) for constructing a recombined plasmid PET28a-EgENO; converting into escherichia coli BL21(DE3); inducing the expression of a recombinant protein through IPTG (Isopropyl-beta-d-Thiogalactoside); and identifying a purified recombinant antigen by using SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) and Western blotting. In addition, the invention further discloses a diagnosis application of the recombinant antigen protein. As proved by an experiment, the recombinant antigen protein has the advantages of high sensitivity, high specificity and the like for the diagnosis of the echinococcosis granulosa, and has a wide application prospect on the aspect of diagnosis of the echinococcosis granulosa.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Liver cancer diagnostic kit based on nucleic acid aptamers
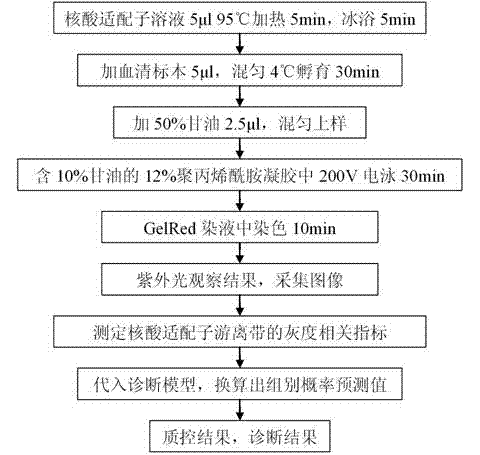
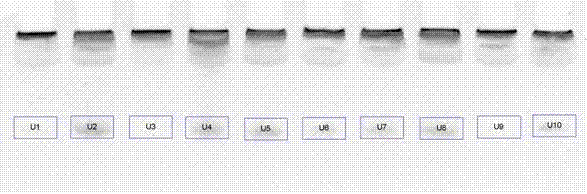
InactiveCN102732606AExcellent diagnostic valueHigh diagnostic valueMicrobiological testing/measurementImage analysisNegative control
The invention discloses a liver cancer diagnostic kit based on nucleic acid aptamers, comprises the following ingredients with independent packing: liver cancer nucleic acid aptamer solution, polyacrylamide gel mother liquor, ammonium persulfate, tetramethyl diethylamine, 50% glycerin solution II, 1.25M sodium chloride solution, GelRed dye solution, positive control serum, and negative control serum. The kit has the following characteristics: (1) high diagnostic value, sensitivity of about 85%, specificity of about 85%, and accuracy of about 85%; (2) simpleness and practicality, and only need of conventional vertical electrophoresis and a gel image analysis system to realize the diagnosis; and (3) low cost and high additional value. Liver cancer is one of common malignant tumors in clinic, thus by applying the kit of the invention in clinical application, the consumption is high, the diagnostic level of liver cancer can be effectively raised, good economic benefit and social benefit can be produced, and the application prospect is good.
Owner:NANCHANG UNIV
Plasmodium vivax PvMSP1 recombinant antigenic protein as well as preparation method and application thereof
ActiveCN103570817AIncreased sensitivityImprove featuresBiological material analysisMicroorganism based processesEpidemiologic surveyGlycosylphosphatidylinositol
The invention discloses a plasmodium vivax PvMSP1 recombinant antigenic protein, which is protein of which the amino acid sequence is shown in SEQ ID NO:1 which has glycophosphatidylinositol (GPI) anchor and epidermal growth factor-like (EGF-like) structure domain. Furthermore, the invention also discloses a preparation method of the recombinant antigenic protein, which comprises the steps of amplifying a plasmodium vivax PvMSP1 gene sequence, constructing and identifying recombinant plasmid, inducibly expressing and purifying recombinant protein and the like. Experiments prove that the PvMSP1 recombinant antigenic protein has the advantages of high sensitivity, strong specificity and the like to assay of the serum antibody of a plasmodium vivax infected patient, and has a wide application prospect in the aspect of plasmodium vivax epidemiological investigation.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心
Detection method and reagent kit of anti-keratin antibody
InactiveCN101320042AEasy to detectMany joints and strongBiological testingFluorescence/phosphorescenceAntigenPositive control
The invention discloses an anti-keratin antibody detection method and an anti-keratin antibody regent box, and belongs to the method for detecting the characteristics of blood in body. The method provided by the invention can be operated as follows: the esophagus of a bandicoot can be made into a biological thin slice which can be taken as an antigen and coated in the reaction region of a piece of slide glass; blood serum to-be-detected is added in the reaction region and then added with complement after the incubation, rinsing and drying in a spanning way, then added with the fluorescent markers of anti-complement antibodies after the incubation, rinsing and drying in a spanning way, and then observed under a fluorescence microscope after the incubation, rinsing and drying in a spanning way; if the horny layer appears typical and regular line-shaped or lamellar fluorescence, the detection result is positive. The regent box provided by the utility model is filled with regents, cover glass and slide glass with biological thin slices, such as negative control serum, positive control serum, condensed phosphate buffer solution and the application solution of the fluorescent markers of complement and anti-complement antibodies. The method provided by the invention is better than the prior method in indicators such as sensitivity, specificity, negative predictive value and positive predicative value, therefore, the method has higher diagnosis value in diagnosing rheumatoid diseases.
Owner:TIANJIN BAODI HOSPITAL
Gastrointestinal electronic pill
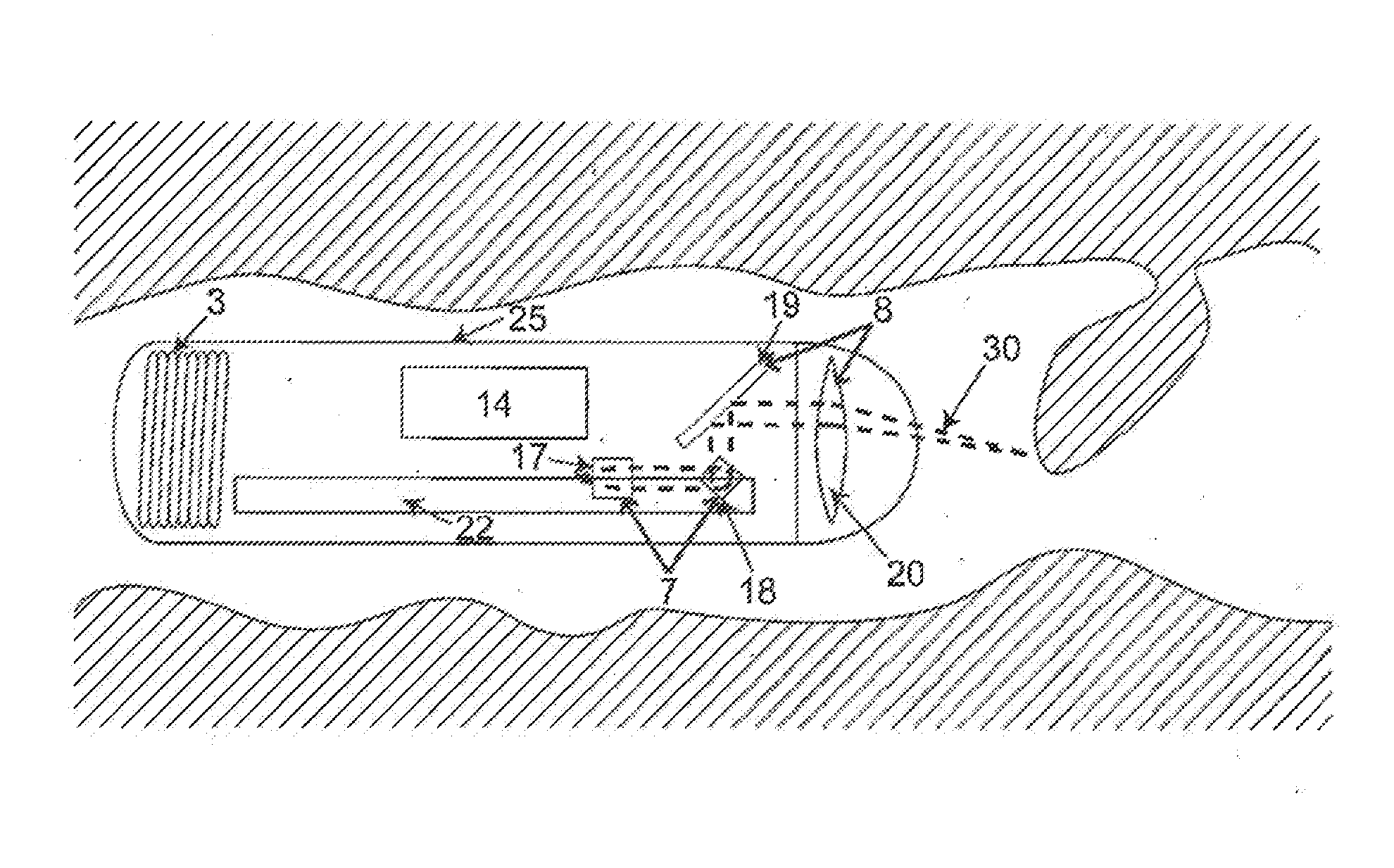
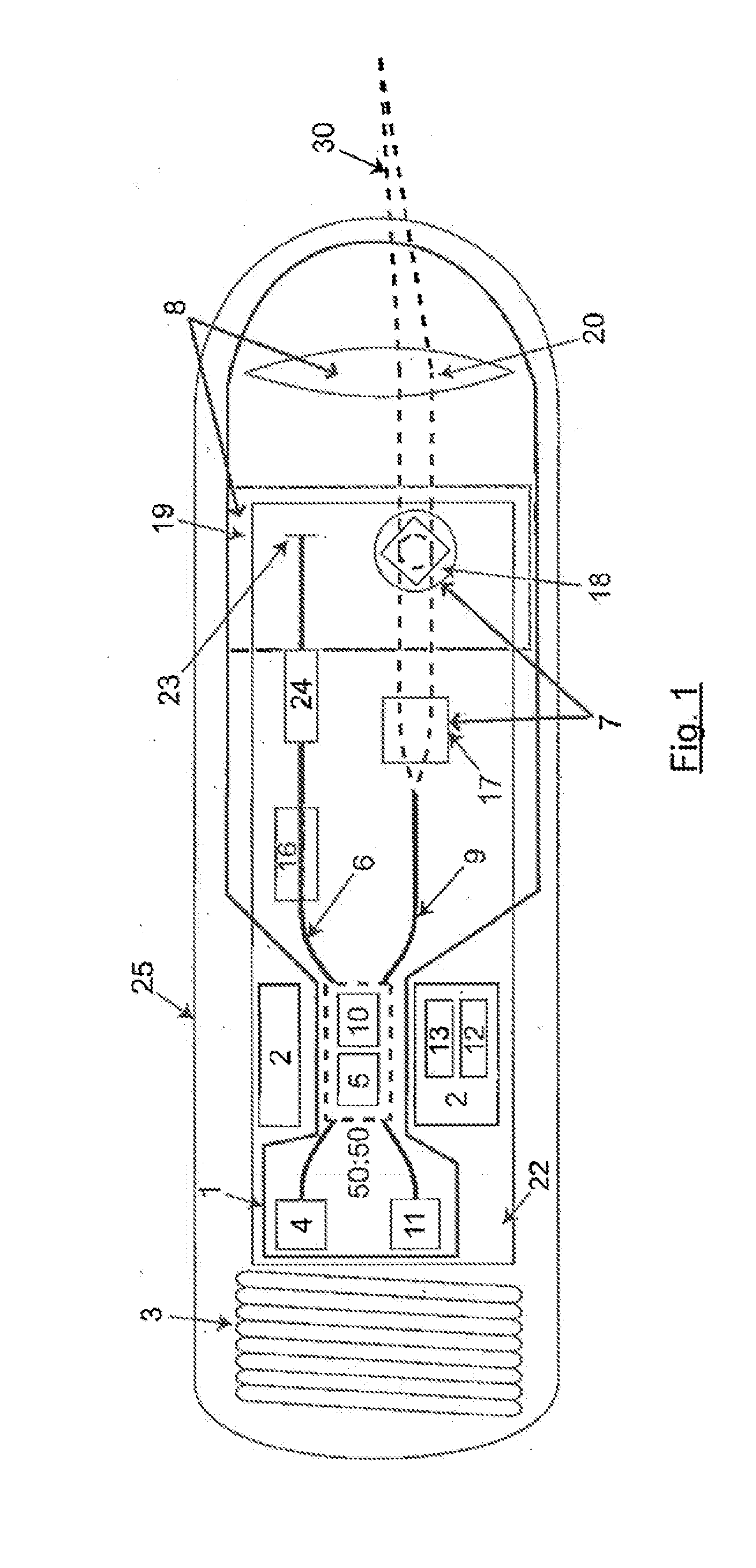
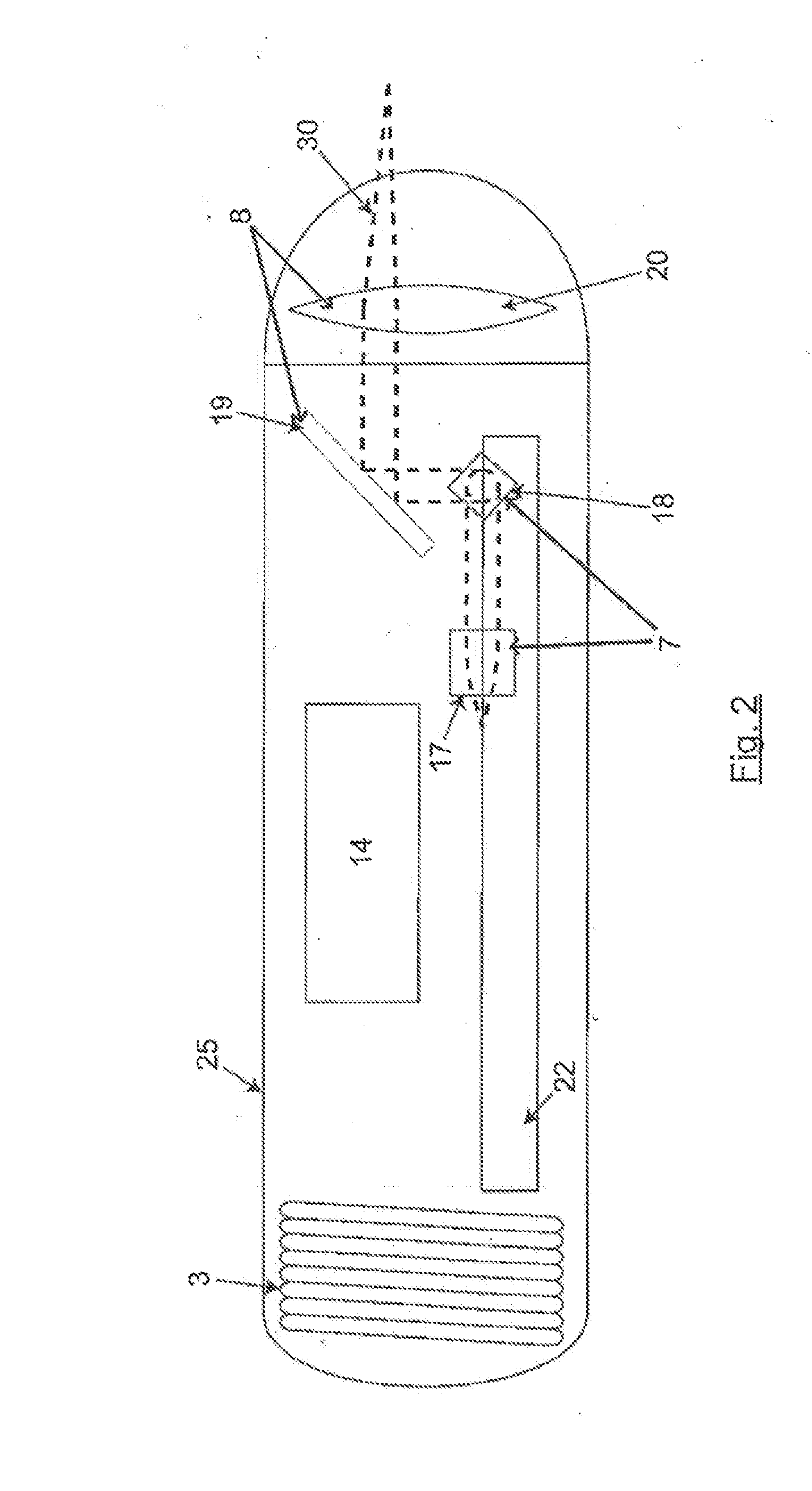
InactiveUS20140309526A1Minimize the numberExcellent diagnostic valueSurgeryVaccination/ovulation diagnosticsLight beamEngineering
The present invention relates to an electronic gastrointestinal capsule protected by an outer biocompatible shell resistant to the environment in the digestive system comprising a light source (4); splitting means (5) to split the light beam, directing it to the reference arm (6) and the sampling arm (9); an adjustable group delay element (24) depending on the distance to the tissue to analyse; optical moving means moving the intersection of the light beam (30) of the sampling arm (9) across the tissue; an optical system (8) focusing the light beam (30) from the sampling arm (9) on the tissue; interference means (10) producing interference between the reflected light; a detector (11) receiving said interference; processing means (2) processing the information acquired by the detector (11); and power supply means (3) supplying power to the capsule without a physical connection to the outside.
Owner:MEDLUMICS
Liver cancer serum nucleic acid aptamers
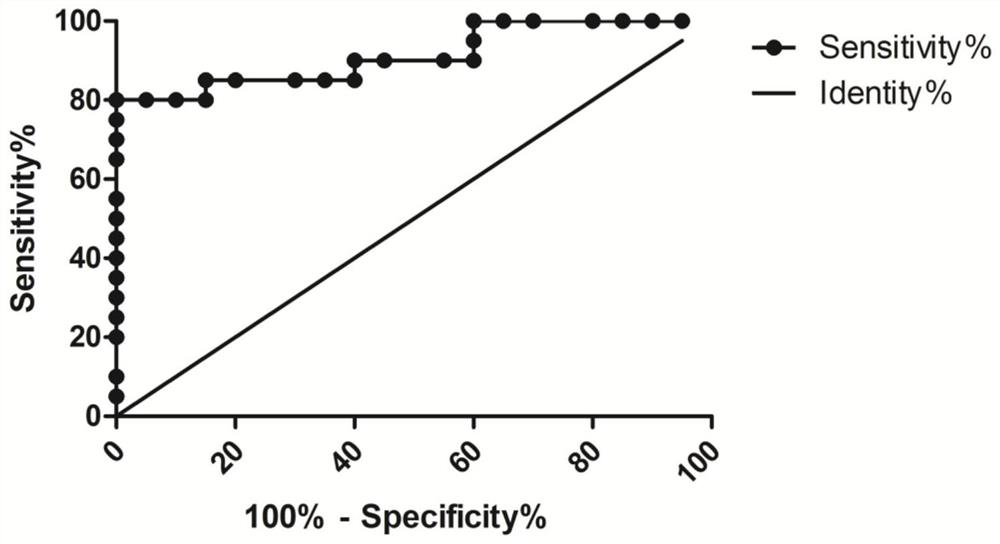
InactiveCN102732522AExcellent diagnostic valueMicrobiological testing/measurementDNA/RNA fragmentationSerum samplesReceiver operating characteristic
The invention discloses liver cancer serum nucleic acid aptamers, belonging to the technical field of biomedical detection and analysis. The invention discloses nine nucleic acid sequences of the liver cancer serum nucleic acid aptamers screened by using the liver cancer serum as a target, and the combination between the nucleic acid sequences and liver cancer and non-liver cancer serum samples is analyzed by polyacrylamide gel electrophoresis and gray level determination. According to the invention, each nucleic acid aptamer has good diagnostic value for liver cancer, the area under a receiver operating characteristic curve is 0.894-0.949, and the accuracy for identifying liver cancer patients and non-liver cancer patients is 84.4-94.4%. The nucleic acid aptamers can be directly applied in the analysis of the liver cancer serum sample or applied after modification, provides experimental basis for clinic diagnosis, treatment and prognosis of liver cancer, and can be applied in establishing new technology of liver cancer detection based on the nucleic acid aptamers. The invention has good application value.
Owner:NANCHANG UNIV
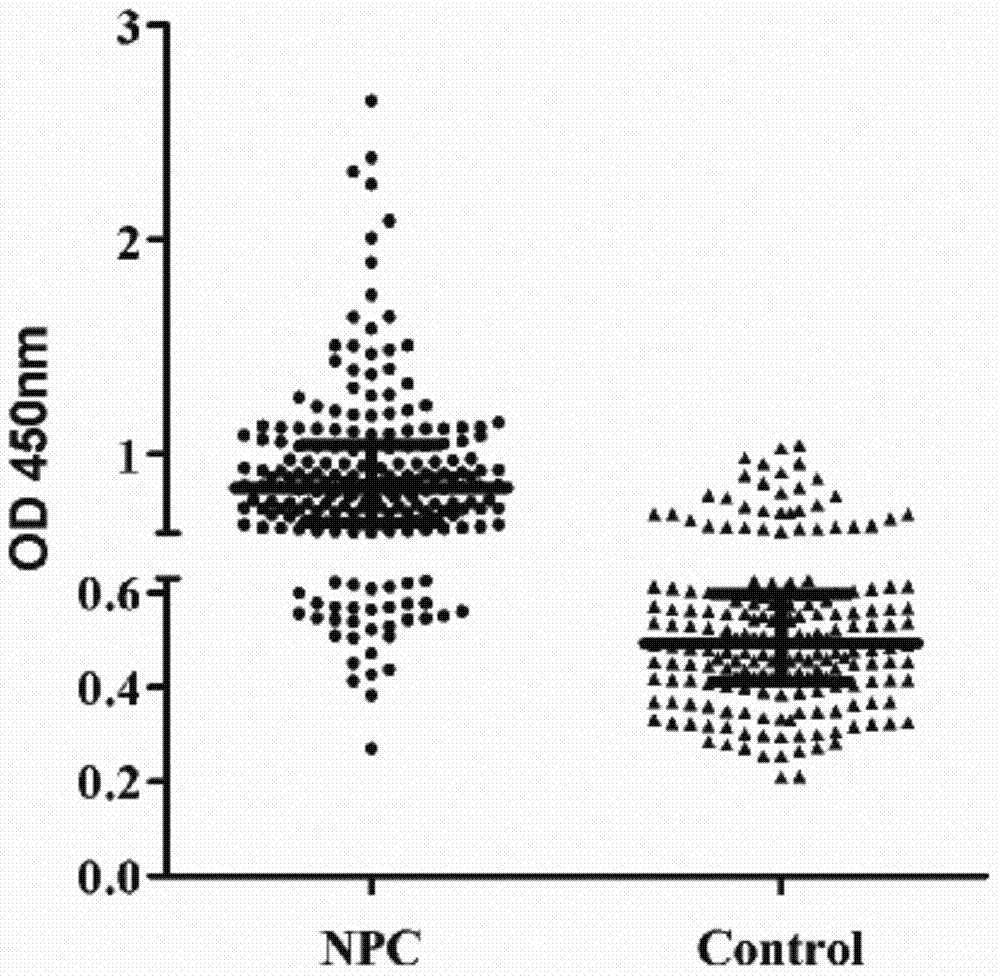
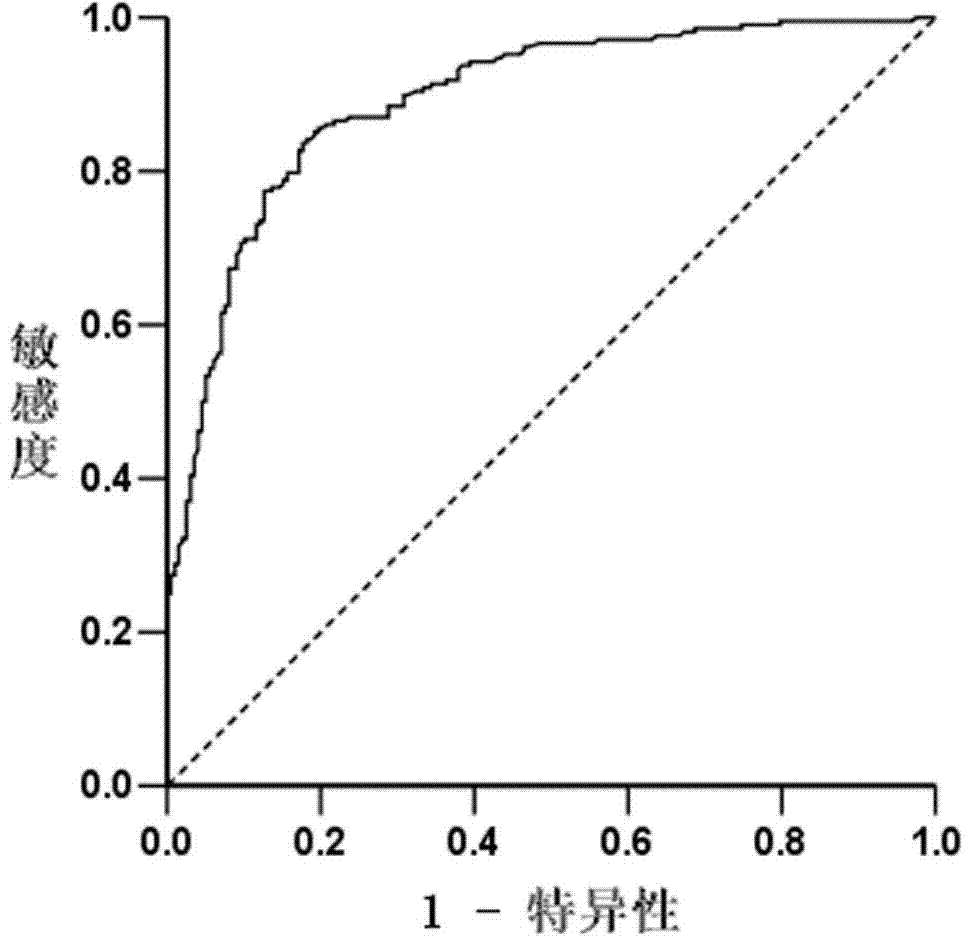
Kit for early diagnosis of nasal pharyngeal cancer (NPC)
The invention discloses a kit for early diagnosis of nasal pharyngeal cancer (NPC). The detection kit has the advantages of being simple to operate, good in repeatability, high in sensitivity and good in specificity; the experimental data proves that gH / gL-immunoglobulin A (IgA) takes OD value of 0.63 as critical value, and the sensitivity and the specificity are respectively 83.7% and 82.3%. The kit provides new detection indexes of the high sensitivity and specificity for the diagnosis of the NPC, thus being beneficial to early screening and diagnosis of the NPC. After being combined with the existing viral capsid antigen (VCA)-IgA detection result, the detection kit has better diagnostic value especially for the VCA-IgA negative patients; the detection kit is complementary with the VCA-IgA, so that missed diagnosis is reduced, and a new way is provided for the early screening and diagnosis of the NPC.
Owner:SUN YAT SEN UNIV +1
Serum protein marker for diagnosing depression and application thereof
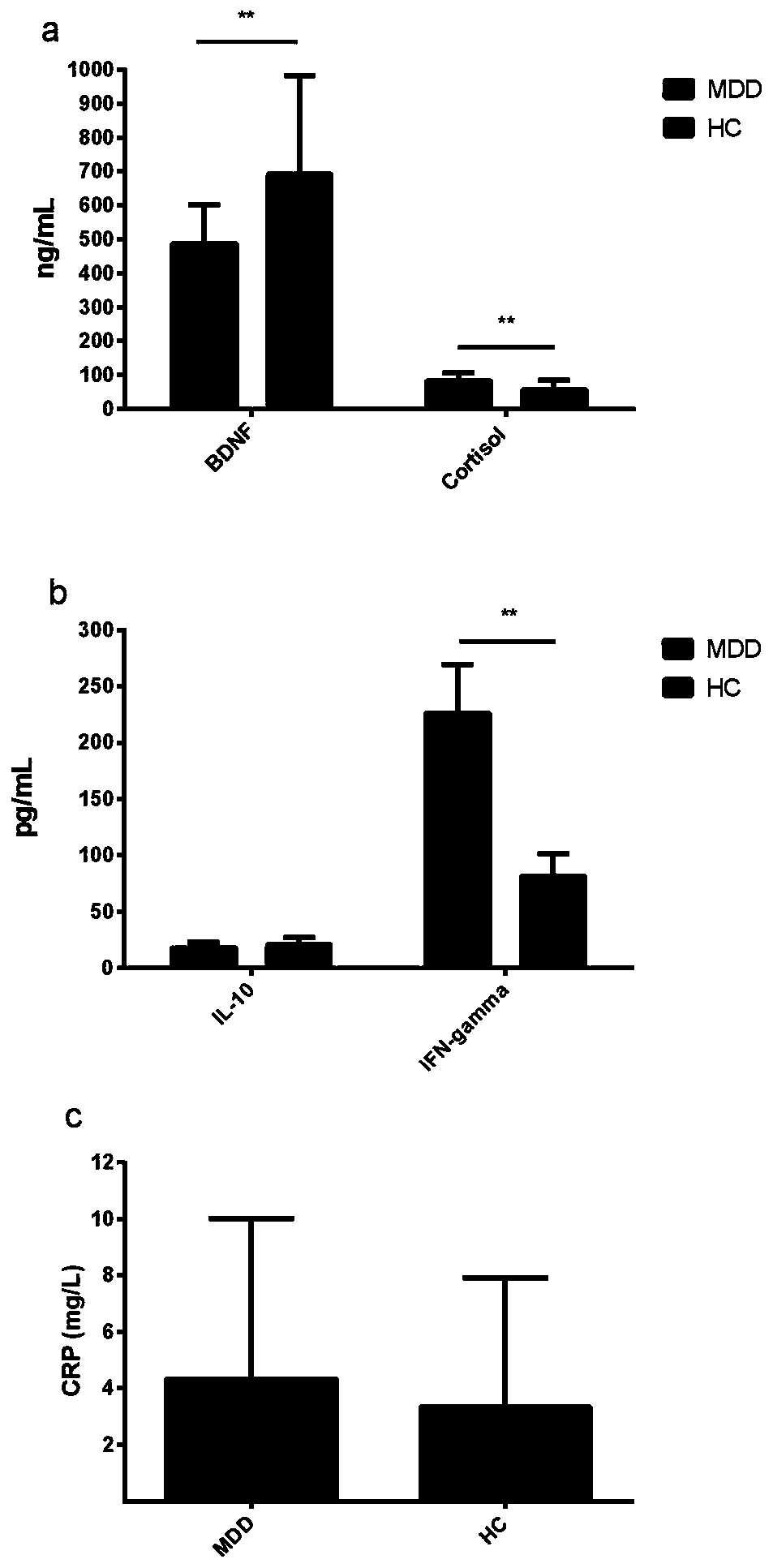
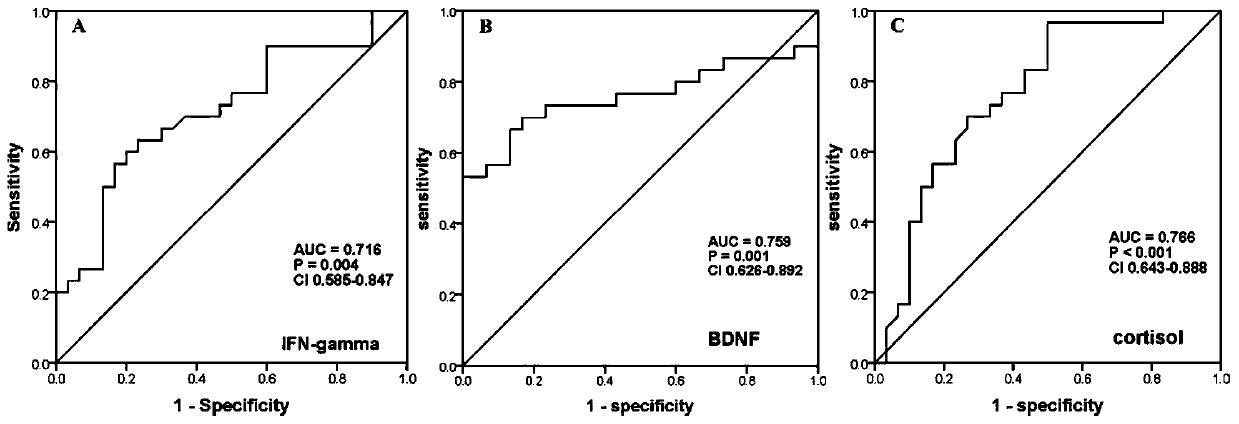
PendingCN111551751AMDD screening value is goodExcellent diagnostic valueDisease diagnosisBiological testingMental diseaseMolecular biology
The invention discloses a serum protein marker. The marker is one or more of BDNF, cortisol and / or IFN-gamma protein. The invention also discloses application of the reagent of the serum protein marker in preparation of a mental disease diagnosis product. The invention also discloses an enzyme-linked immunosorbent assay kit. When the three proteins are used together to distinguish MDD and HC, AUCunder an ROC curve is 0.884, the sensitivity and specificity are 86.7% and 83.3% respectively, and the kit has a very good MDD diagnosis value; when only BDNF and cortisol are used in a combined modeto distinguish MDD and HC, AUC is 0.841, specificity is 66.7%, but sensitivity is as high as 90%, and therefore the marker has a good MDD screening value.
Owner:SOUTHEAST UNIV
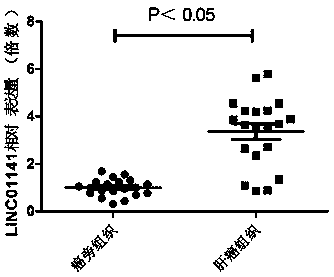
Application of long-chain non-coding RNA LINC01141 in preparation of pharmaceutical composition for treating liver cancer
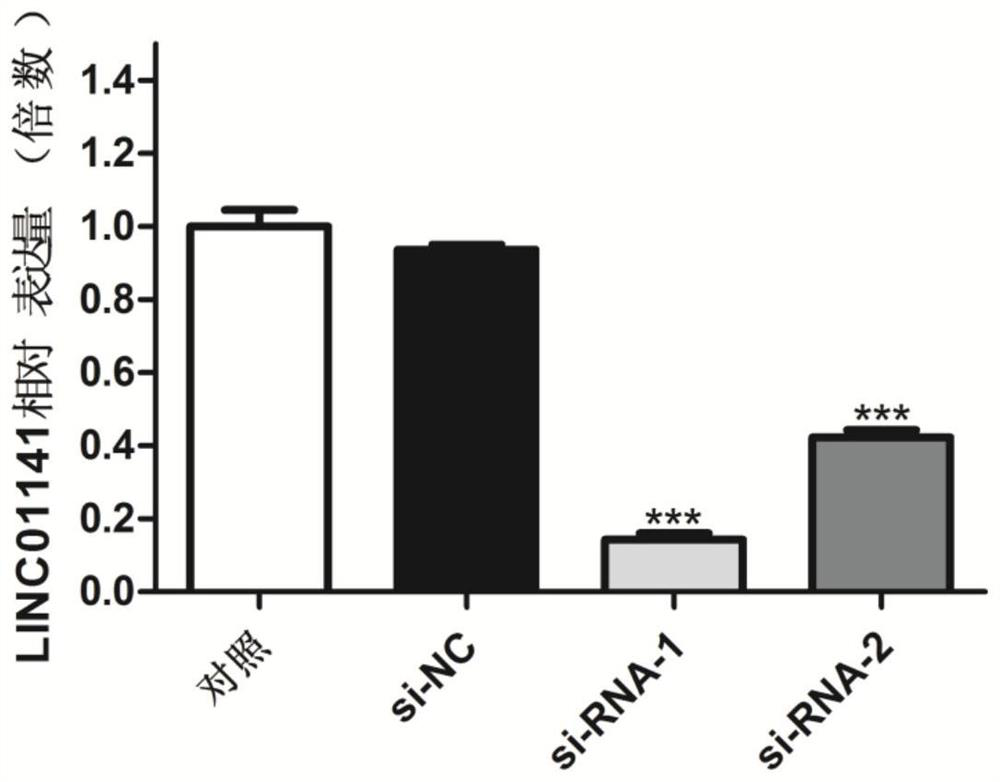
ActiveCN111893120AAchieve early diagnosisImprove survival rateOrganic active ingredientsMicrobiological testing/measurementCancer cellPharmaceutical drug
The invention provides application of long-chain non-coding RNA LINC01141 in preparation of a liver cancer treatment pharmaceutical composition, and belongs to the technical field of biological medicines. Experiments find that cell proliferation of liver cancer cells can be remarkably inhibited by inhibiting LINC01141 through siRNA, so that siRNA can be used for preparing a novel treatment medicine for treatment liver cancer patients and has important clinical value and application prospect.
Owner:CELLYAN THERAPEUTICS WUHAN CO LTD
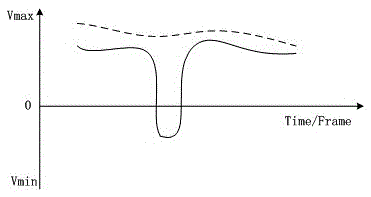
Correlation calculation method of colorful blood flow frame
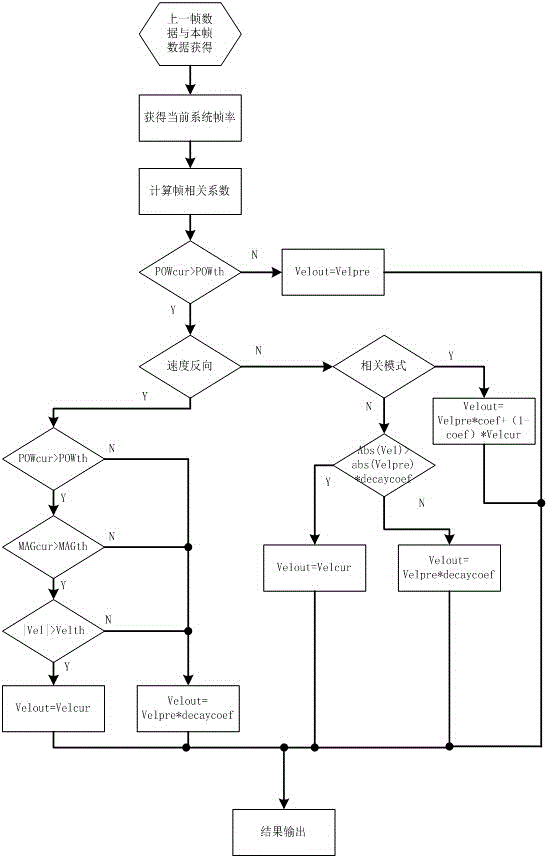
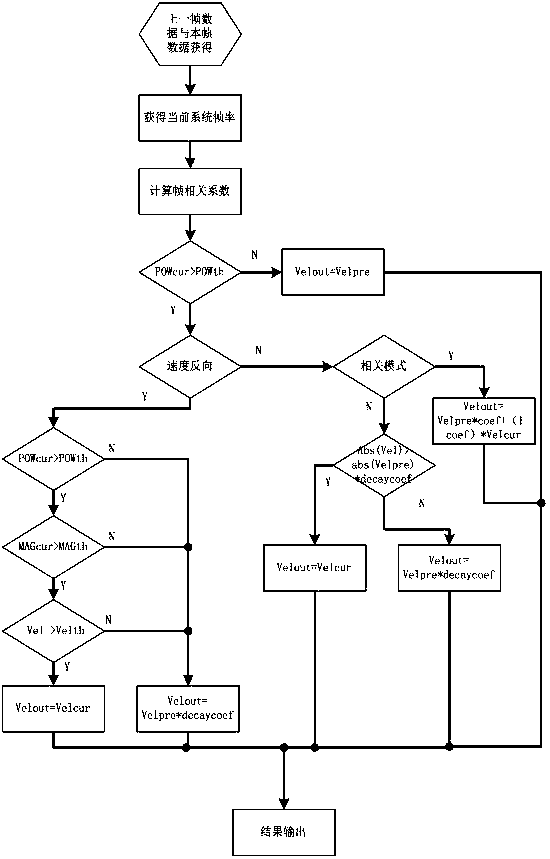
ActiveCN105640592AExcellent diagnostic valueOptimize the effect of clinical needsBlood flow measurement devicesInfrasonic diagnosticsCorrelation coefficientUltrasound attenuation
The invention relates to a correlation calculation method of a colorful blood flow frame. The correlation calculation method comprises the following steps: calculating a frame correlation coefficient Coef, reading an energy / amplitude / velocity threshold value defined by a system and an attenuation correlation coefficient decaycoef and enabling the energy threshold value to be used for overall control and judgment; judging whether current point blood flow energy reaches the energy threshold value 1 or not; if the current point blood flow energy does not reach the energy threshold value 1, directly using previous frame results for current point output; otherwise, entering the processing step of judging a blood flow velocity direction by using the energy threshold value 2; judging whether a current frame position and a previous identical position are in the same direction or not through front and rear frame point multiplication; if the current frame position and the previous same position are in the same direction, same-direction processing; otherwise, reverse-direction processing. According to the relevant calculation method provided by the invention, not only can a corresponding frame correlation effect be realized, but also the data chaos caused in the processing process can not be caused; different correlation modes can be selected, so that the best diagnostic value is realized; through selecting identical-direction processing modes listed in the correlation calculation method, clinically-required effects can be furthest optimized.
Owner:SHENZHEN ANASONIC BIO MEDICAL TECH CO LTD
Application of lipopolysaccharide extracted from brucella ovis vaccine strain M5 in preparation of product for diagnosing human brucellosis
ActiveCN111518225AEliminate infectivityNo infection hazardBiological testingImmunoassaysInfection diagnosisBrucella Vaccine
The invention discloses an application of lipopolysaccharide extracted from a brucella ovis vaccine strain M5 as a human brucellosis diagnosis antigen. According to the invention, the reactivity of anLPS antigen extracted from the brucella ovis vaccine strain M5 as a diagnosis antigen is obviously superior to that of an LPS antigen extracted from a brucella cattle strain S19 and a brucella pig strain S2 commonly used in a brucella antibody rapid detection kit on the market at present, therefore, the brucella ovis vaccine strain M5 can be used as a preferred source strain of a new brucella gold-labeled diagnosis LPS antigen, the extracted LPS has a good diagnosis value on human brucellosis, and a new way is provided for selection of a human brucellosis infection diagnosis antigen. The invention also finds that the antigenicity of the LPS antigen extracted from the brucella ovis vaccine strain M5 subjected to innocent treatment is not influenced.
Owner:新疆禹孚生物技术股份有限公司
Application of RSPH9 as diagnosis marker or treatment target for oligoasthenozoospermia
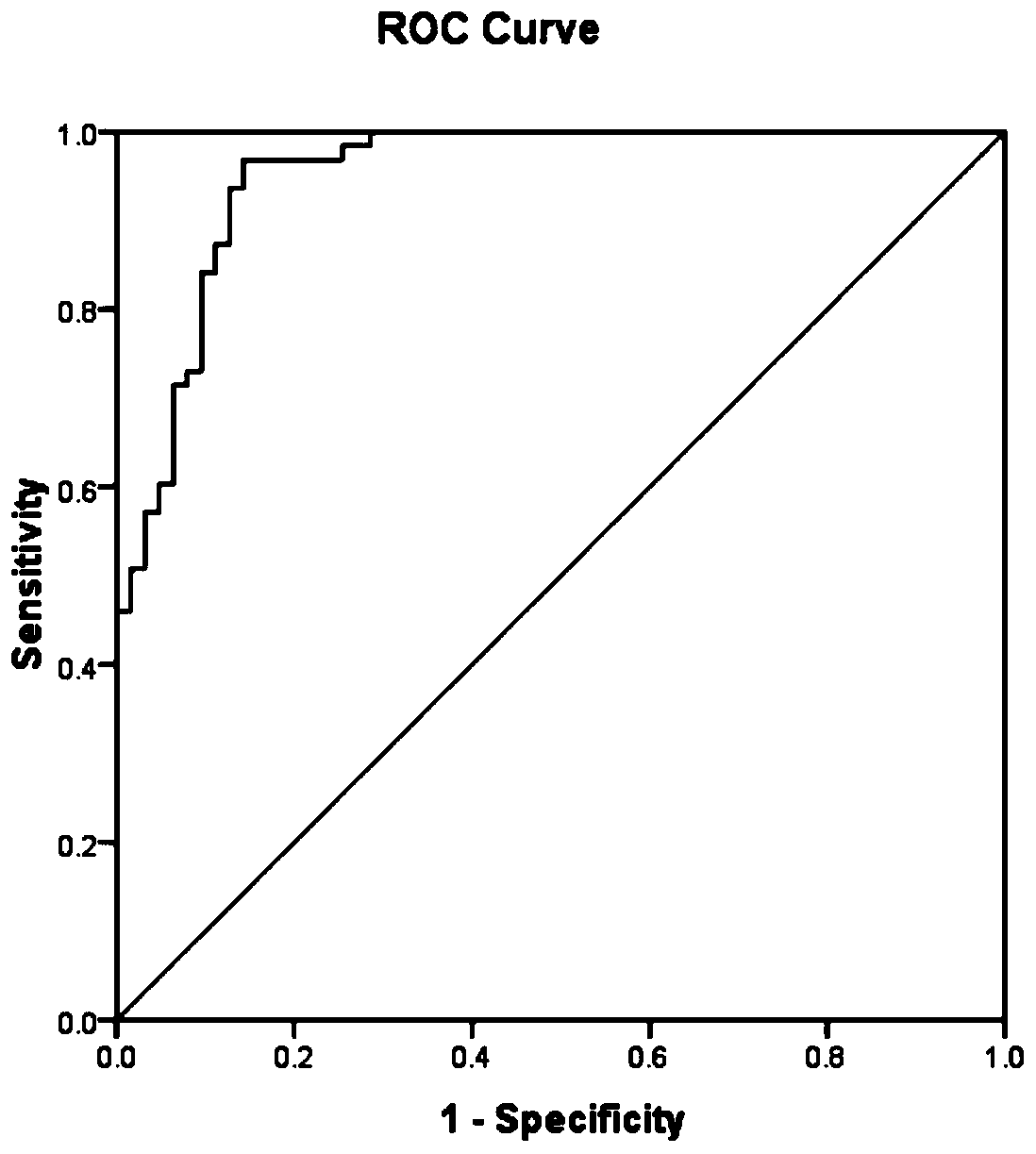
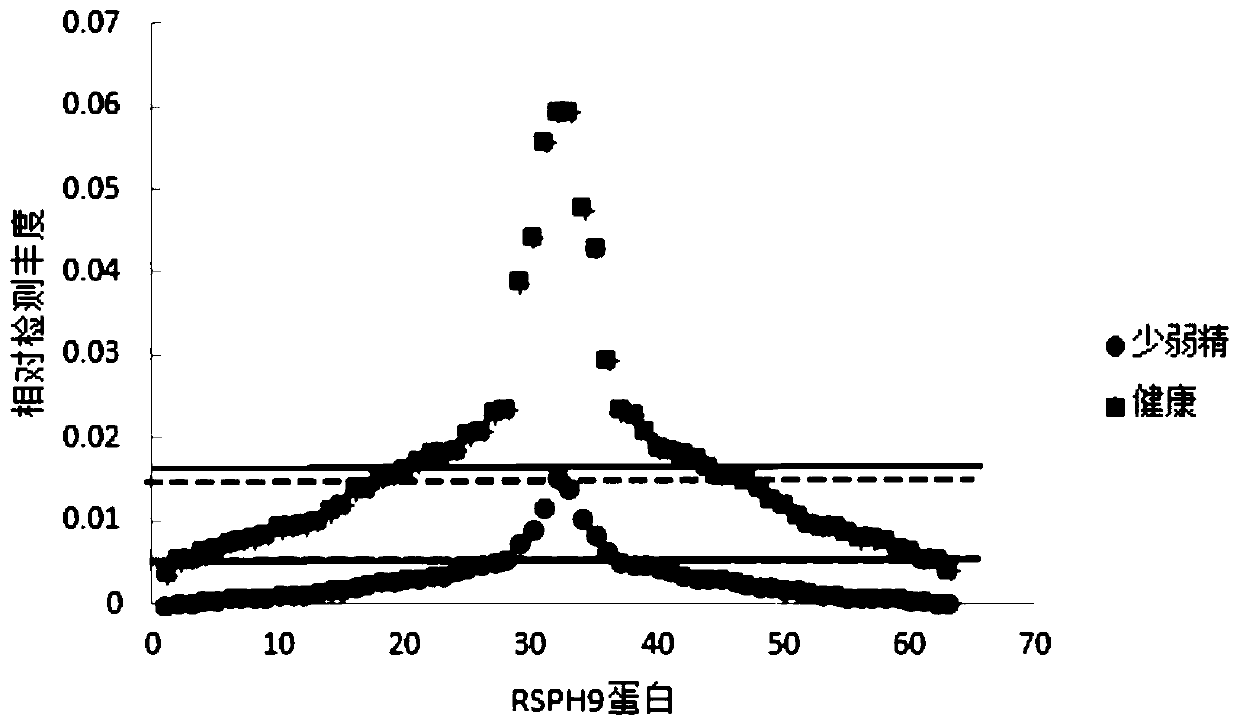
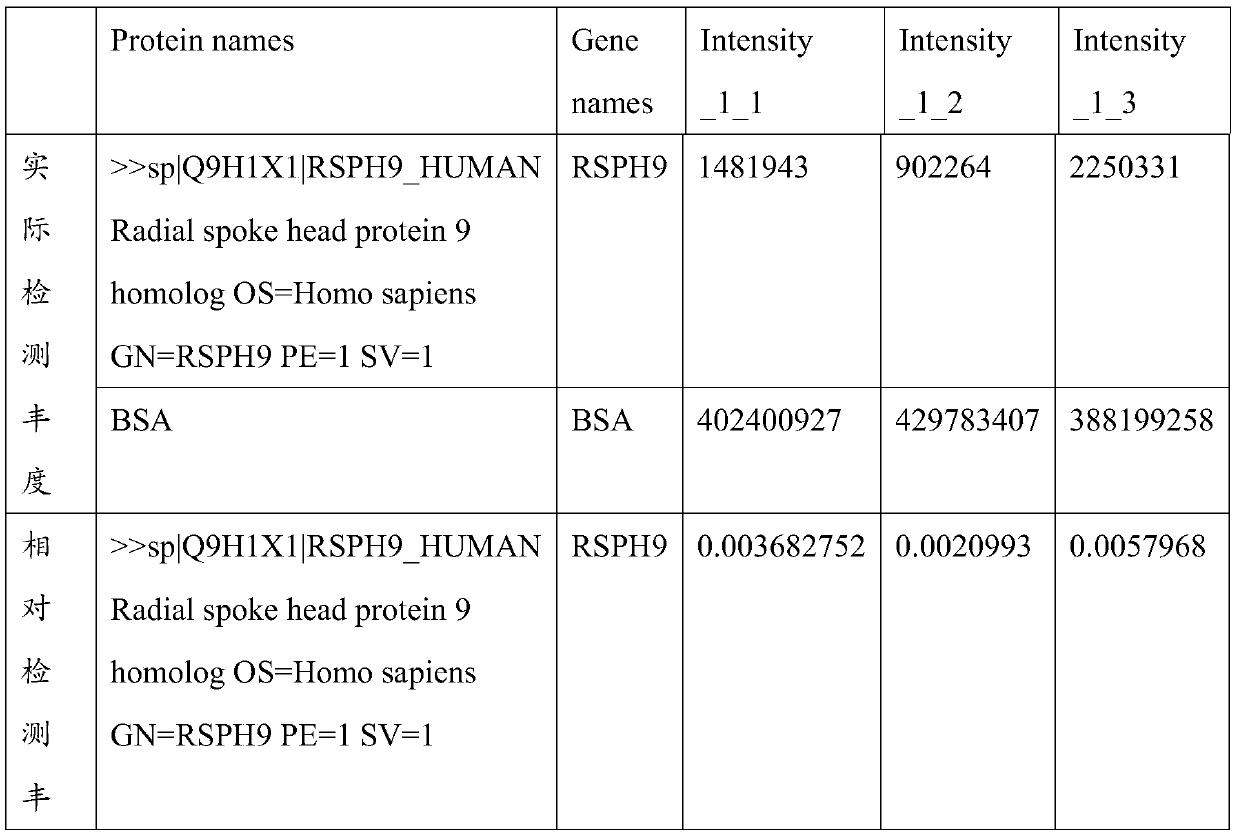
PendingCN111521828ASignificant decreaseHigh precisionDisease diagnosisBiological testingSperm proteinProteomics methods
The invention particularly relates to an application of RSPH9 as a diagnosis marker or a treatment target for oligoasthenozoospermia. Sperm proteins of normal and patients are extracted, bovine serumalbumin is used as a standard substance, and multiple groups of sperm proteins of severe application of RSPH9 as a diagnosis marker or a treatment target for oligoasthenozoospermia diseases are subjected to deep mass spectrometry by using high-resolution biomass spectrometry and a non-labeled quantitative proteomics method; the mass spectrum data are searched by using a protein quantitative analysis method, and the protein in the sperm is identified; and finally, normal and patient sperm proteomes are compared, and screening is conducted to obtain that the contents of RSPH9 in healthy and oligoasthenosperm samples are remarkably different. ROC analysis further verifies that RSPH9 has good specificity as a diagnostic marker, and is expected to be used as a diagnostic marker and a treatmenttarget for severe oligoasthenozoospermia.
Owner:山东立菲生物产业有限公司
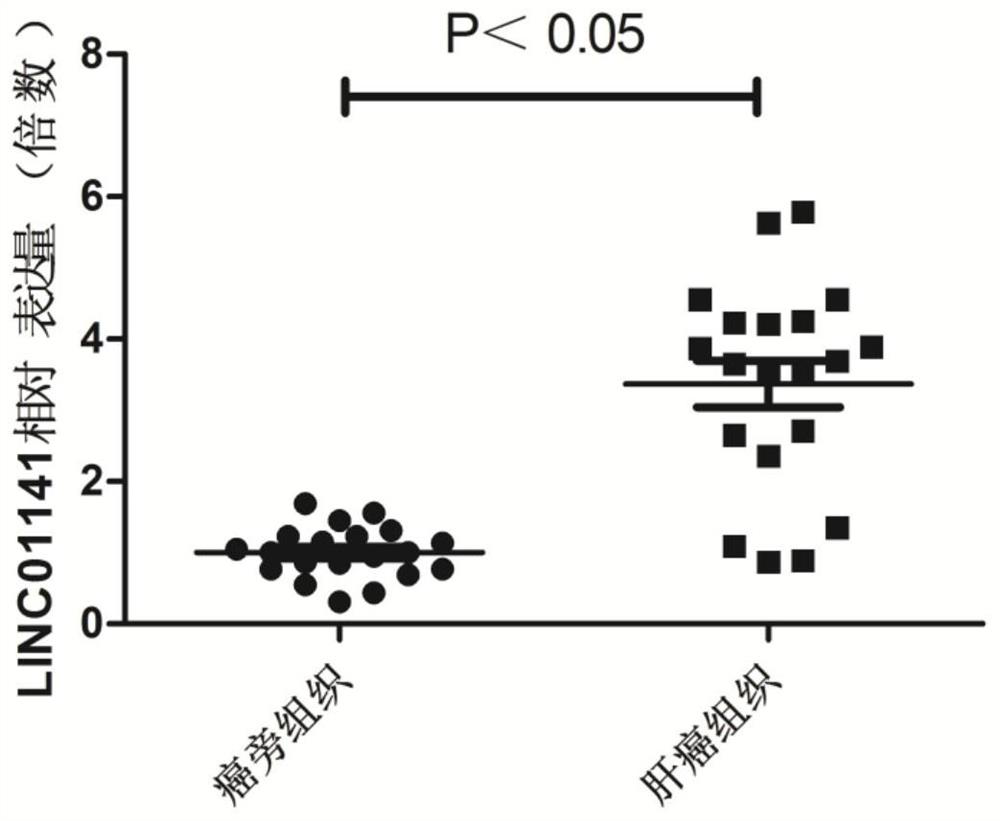
Long-chain non-coding RNA gene marker for detecting liver cancer and application of long-chain non-coding RNA gene marker for detecting liver cancer
ActiveCN111187773AAchieve early diagnosisImprove survival rateOrganic active ingredientsMicrobiological testing/measurementPharmaceutical drugLiver cancer
The invention relates to a long-chain non-coding RNA gene marker for detecting liver cancer and an application of the long-chain non-coding RNA gene marker for detecting liver cancer. The long-chain non-coding RNA is LINC01141. Through fluorescence quantitative PCR detection, the inventor of the invention finds that the expression of the LINC01141 in liver cancer patients is notably raised, whichillustrates that the LINC01141 can be used as a gene marker of the liver cancer and can be used for diagnosing and treating the liver cancer. The long-chain non-coding RNA gene marker for detecting liver cancer disclosed by the invention has the beneficial effects that early diagnosis of the liver cancer can be realized through detecting the expression level of the LINC01141 in the liver cancer patients, so that the survival rate of the liver cancer patients can be increased. Secondly, the invention provides an application of LINC01141 siRNA to preparation of a medical composition for liver cancer. The medical composition can be used as a new treatment medicine for treating the liver cancer patients, and has important clinical value and application prospects.
Owner:宜兴市拜奥精核生物科技有限公司
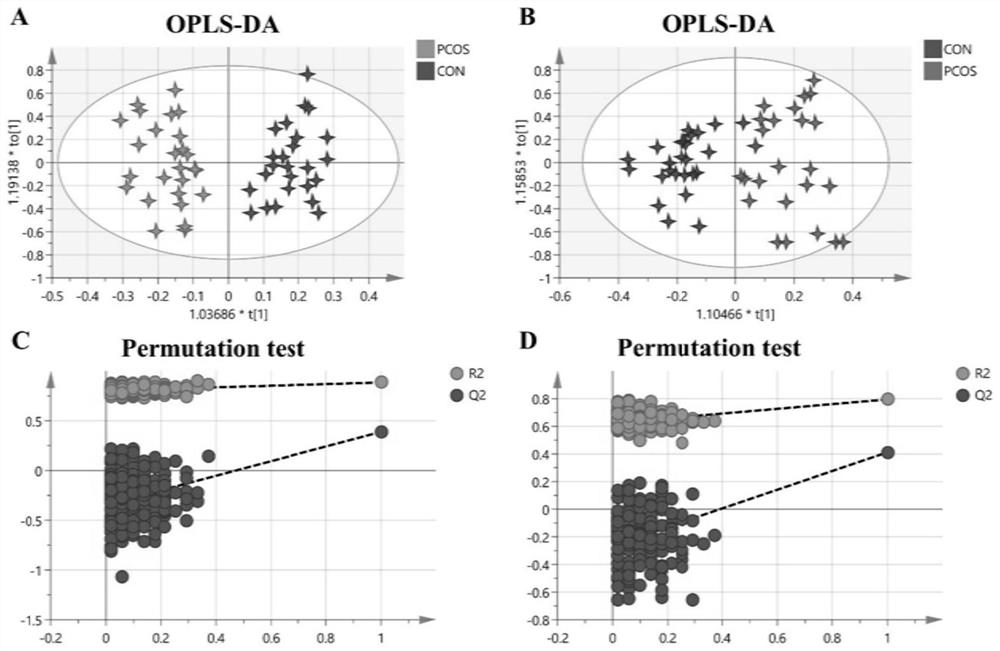
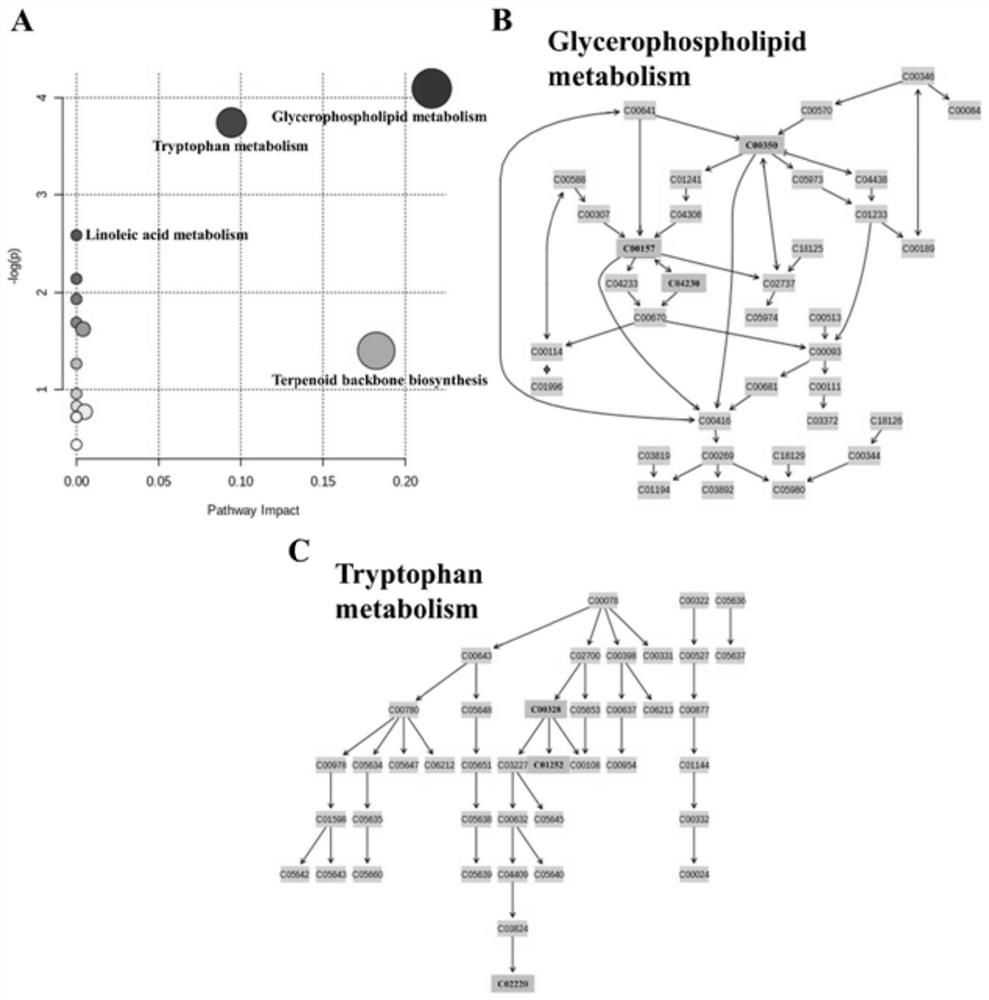
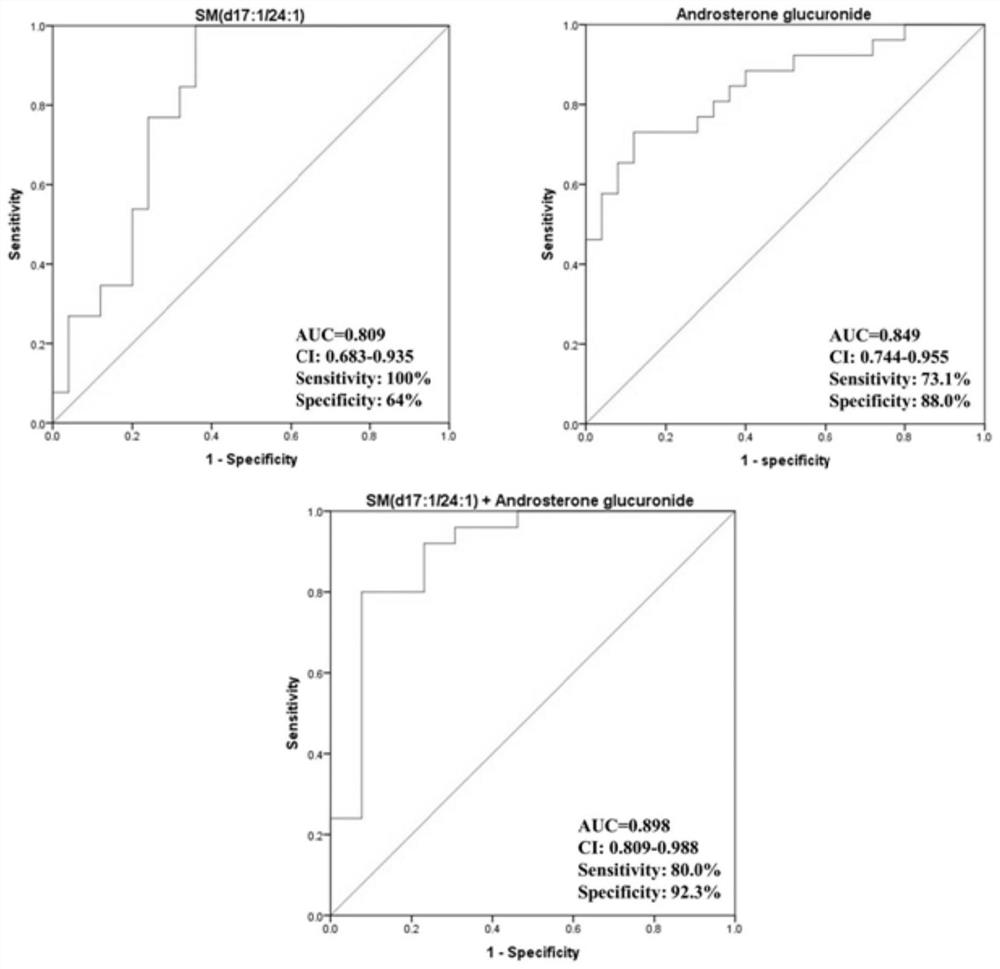
Compounds and applications for the diagnosis of polycystic ovary syndrome
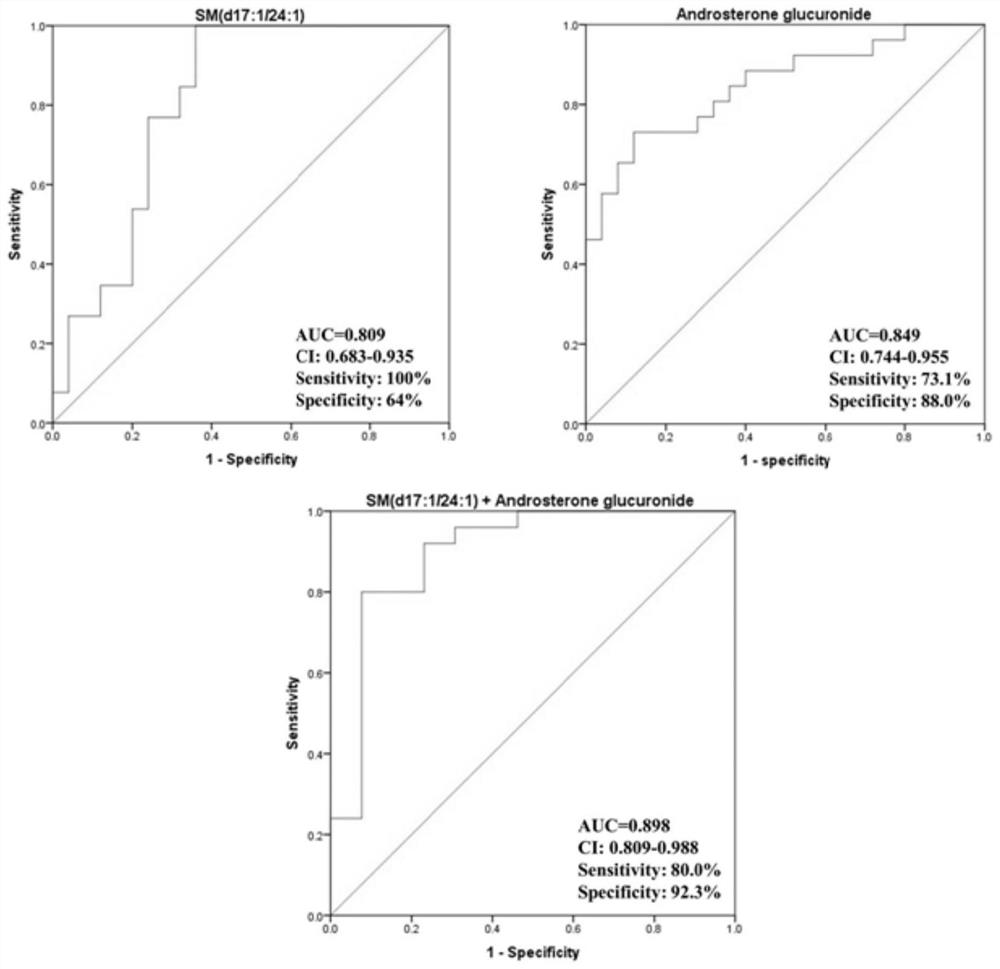
ActiveCN111830169BExcellent diagnostic valueEasy diagnosisComponent separationGroup 5/15 element organic compoundsAndrosterone glucuronideFluid phase
The invention discloses a compound used in the diagnosis of polycystic ovary syndrome. The compound is one of androsterone glucuronide or sphingomyelin (d17:1 / 24:1), or the compound is Combination of androstone glucuronide with sphingomyelin (d17:1 / 24:1). The beneficial effect of the present invention is that the non-target metabolomics research of polycystic ovary syndrome urine is carried out by ultra-high pressure liquid phase tandem time-of-flight mass spectrometry, and androsterone glucuronide, sphingomyelin lipid (d17:1 / 24: 1) and their combination have better diagnostic value for polycystic ovary syndrome. The purpose of simple and rapid diagnosis of polycystic ovary syndrome is achieved, and the detection cost is reduced at the same time.
Owner:SUN YAT SEN UNIV
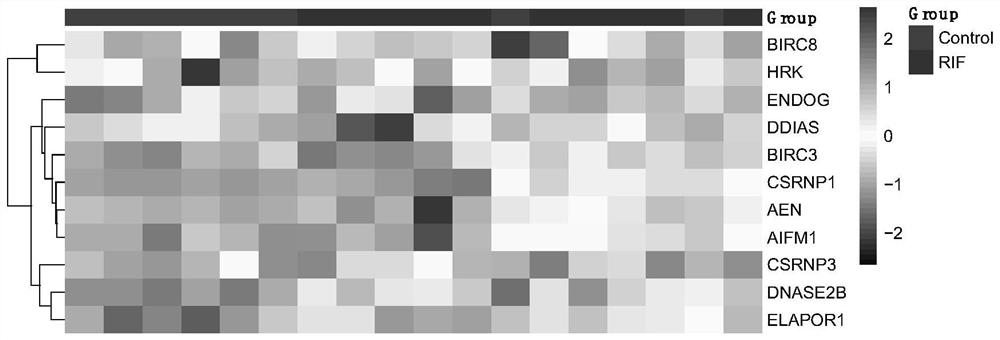
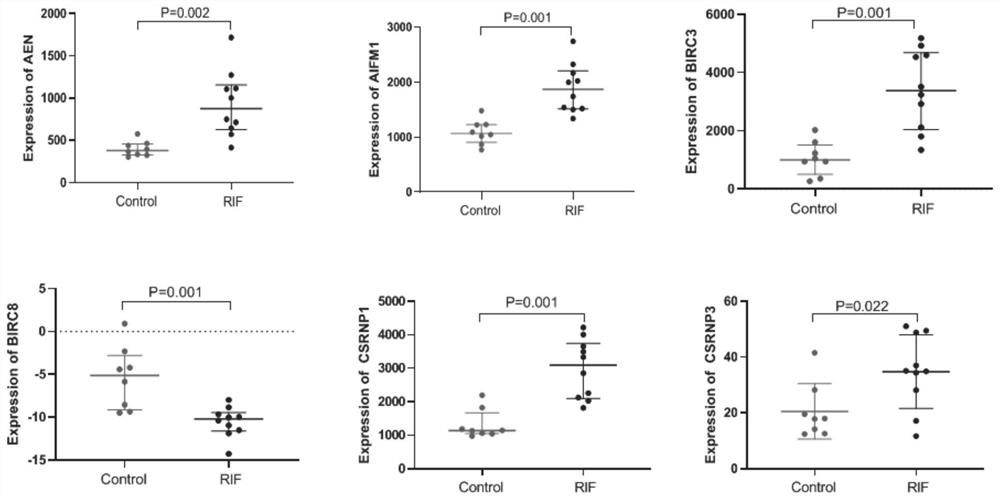
Application of apoptosis-related genes in repeated implantation failure
PendingCN113564242AExcellent diagnostic valueMicrobiological testing/measurementAbnormal expressionTreatment targets
The invention provides application of apoptosis-related genes in repeated implantation failure, and belongs to the technical field of biological medicine and molecular biology. According to the application of the apoptosis-related genes in repeated implantation failure, endometrial RNA-seq related data are called from a GEO database, a series of differential genes are screened out from a GSE92324 queue, finally, it is determined that abnormal expression of three apoptosis related genes, namely, CSRNP1, BIRC8 and ELAPOR1 is closely related to repeated implantation failure, verification is carried out through a verification queue (GSE71835), the apoptosis-related genes are proved to have a good diagnostic value for the repeated implantation failure, so that the apoptosis-related genes can be used as prediction and diagnosis markers and potential treatment targets of the repeated implantation failure, plays an important role in diagnosis and treatment of the repeated implantation failure, and has a good practical popularization and application value.
Owner:SHANDONG UNIV QILU HOSPITAL
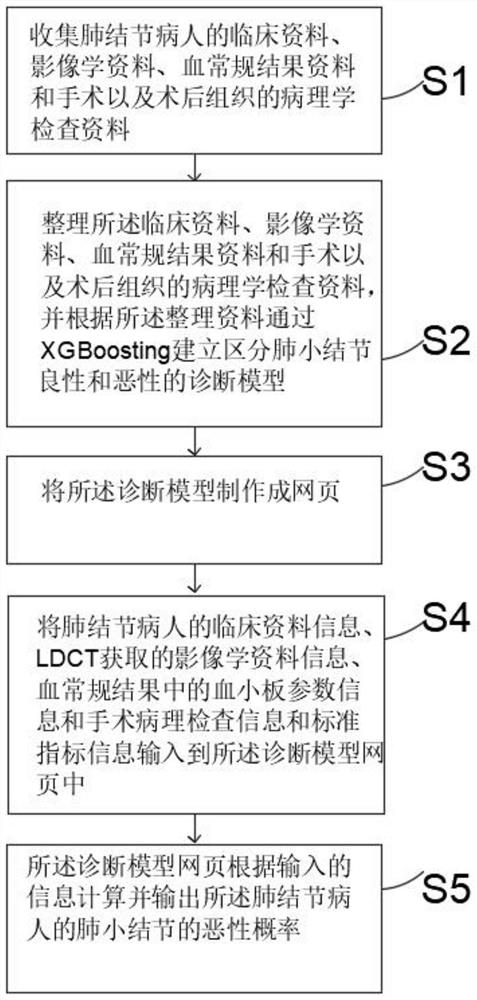
Model and system for predicting benign and malignant pulmonary nodules based on platelet parameters
PendingCN113288110ALow cost of treatmentImprove diagnostic efficiencyRespiratory organ evaluationSensorsPulmonary noduleMalignancy
The invention discloses a model for predicting benign and malignant pulmonary nodules based on platelet parameters. The model comprises the following steps: S1, collecting clinical data, imaging data, blood routine result data and pathological examination data of operations and postoperative tissues of pulmonary nodule patients; s2, sorting the clinical data, the imaging data, the blood routine result data and the pathological examination data of operation and postoperative tissues, and establishing a diagnosis model for distinguishing benign and malignant pulmonary nodules through XGBoost i ng according to the sorted data; s3, making the diagnosis model into a webpage; s4, inputting clinical data information of a pulmonary nodule patient, iconography data information obtained by LDCT, platelet parameter information in a blood routine result, surgical pathological examination information and standard index information into the diagnosis model webpage; and S5, the diagnosis model webpage calculates and outputs the malignant probability of the pulmonary nodules of the pulmonary nodule patient according to the input information.
Owner:SICHUAN CANCER HOSPITAL
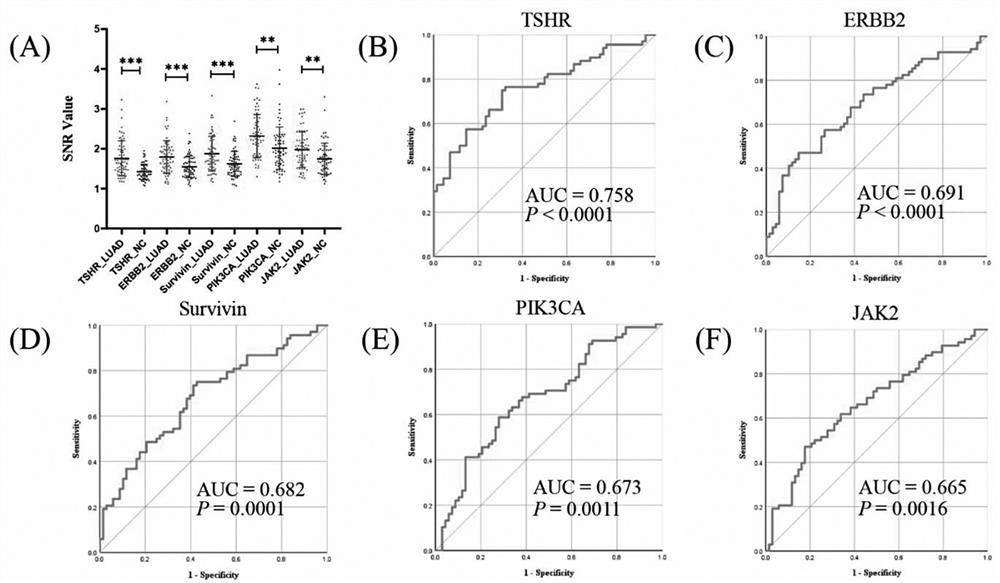
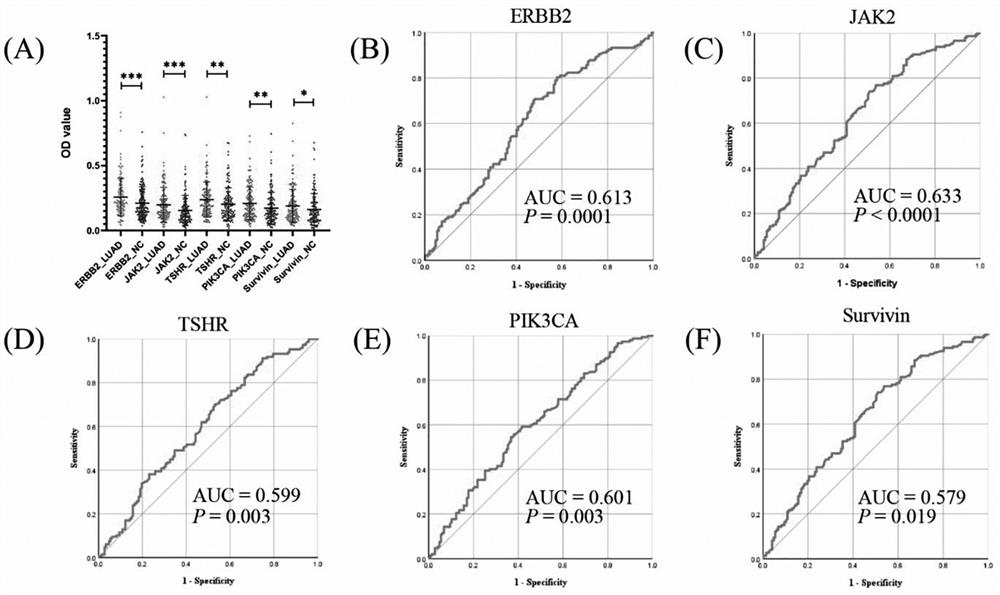
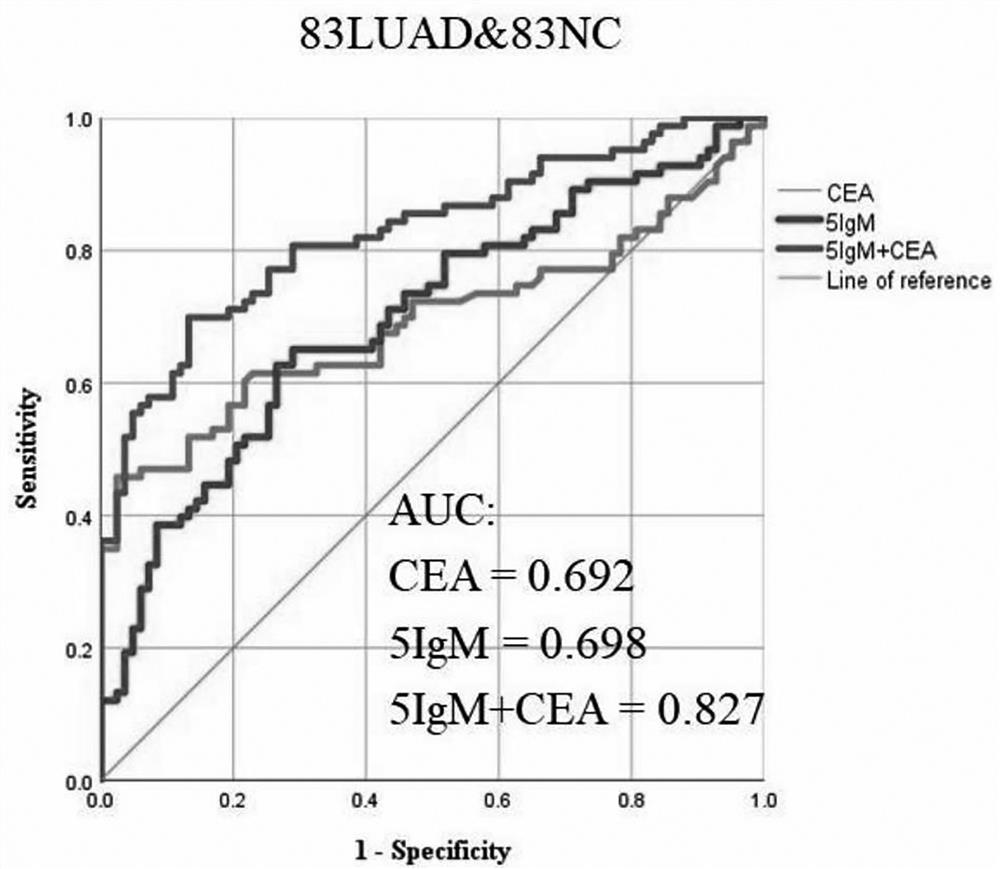
Joint detection serum marker for early diagnosis of lung adenocarcinoma and application of joint detection serum marker
The invention discloses a joint detection serum marker for early diagnosis of lung adenocarcinoma, comprising IgM autoantibodies of TSHR, ERBB2, Survivin, PIK3CA and JAK2. An ELISA serum detection kit is prepared by using a specific protein corresponding to the joint detection serum marker. The joint detection serum marker plays an important role in improving the sensitivity, specificity and accuracy of early diagnosis of lung adenocarcinoma. Meanwhile, the joint detection serum marker is combined with a CEA serum marker to construct a diagnosis model, and a serological detection method which is high in sensitivity, strong in specificity, low in cost and capable of assisting clinical diagnosis of lung adenocarcinoma is provided.
Owner:ZHENGZHOU UNIV
Compound for diagnosing polycystic ovarian syndrome and application
ActiveCN111830169AExcellent diagnostic valueEasy diagnosisComponent separationGroup 5/15 element organic compoundsFluid phaseMass Spectrometry-Mass Spectrometry
The invention discloses a compound for diagnosing polycystic ovarian syndrome. The compound is one of androsterone glucosidic acid or sphingomyelin (d17: 1 / 24: 1), or a combination of androsterone glucosidic acid and sphingomyelin (d17: 1 / 24: 1). The method has the beneficial effects that the non-target metabonomics research on the polycystic ovarian syndrome urine is carried out through ultrahighpressure liquid phase tandem time-of-flight mass spectrometry, and androsterone glucosidic acid, sphingomyelin (d17: 1 / 24: 1) and the combination thereof are obtained, so that the compound has a relatively good diagnosis value on the polycystic ovarian syndrome. The purpose of simply and rapidly diagnosing the polycystic ovarian syndrome is achieved, and meanwhile the detection cost is reduced.
Owner:SUN YAT SEN UNIV
Liver cancer serum nucleic acid aptamers
InactiveCN102732522BExcellent diagnostic valueMicrobiological testing/measurementDNA/RNA fragmentationSerum samplesReceiver operating characteristic
The invention discloses liver cancer serum nucleic acid aptamers, belonging to the technical field of biomedical detection and analysis. The invention discloses nine nucleic acid sequences of the liver cancer serum nucleic acid aptamers screened by using the liver cancer serum as a target, and the combination between the nucleic acid sequences and liver cancer and non-liver cancer serum samples is analyzed by polyacrylamide gel electrophoresis and gray level determination. According to the invention, each nucleic acid aptamer has good diagnostic value for liver cancer, the area under a receiver operating characteristic curve is 0.894-0.949, and the accuracy for identifying liver cancer patients and non-liver cancer patients is 84.4-94.4%. The nucleic acid aptamers can be directly applied in the analysis of the liver cancer serum sample or applied after modification, provides experimental basis for clinic diagnosis, treatment and prognosis of liver cancer, and can be applied in establishing new technology of liver cancer detection based on the nucleic acid aptamers. The invention has good application value.
Owner:NANCHANG UNIV
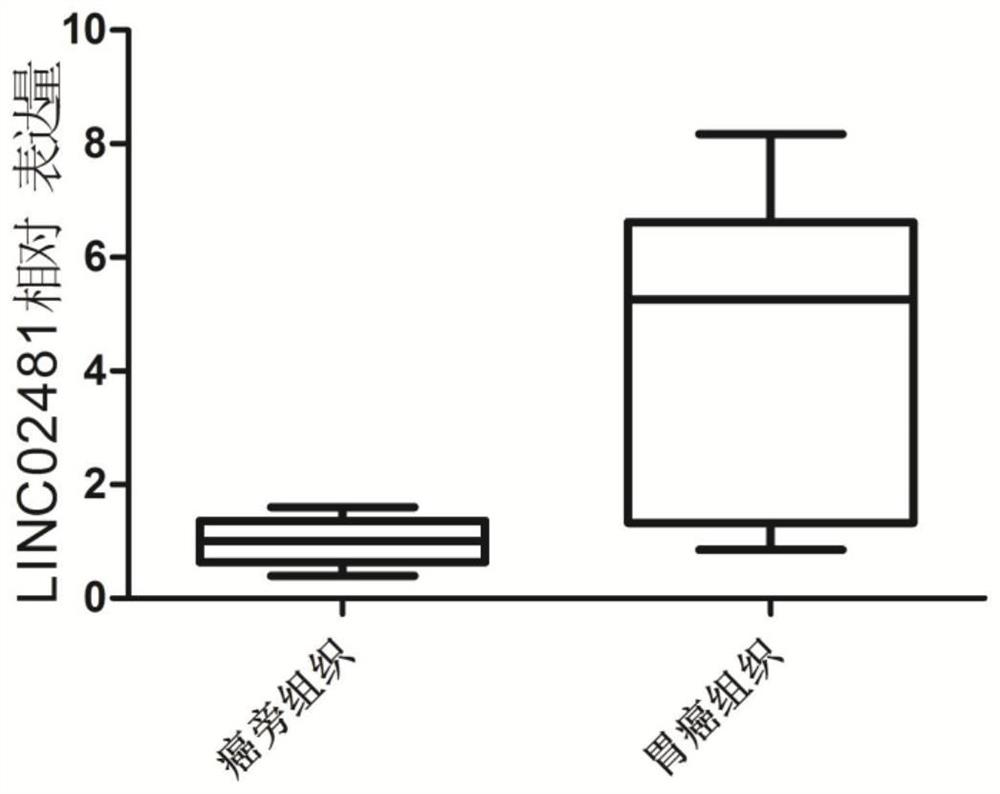
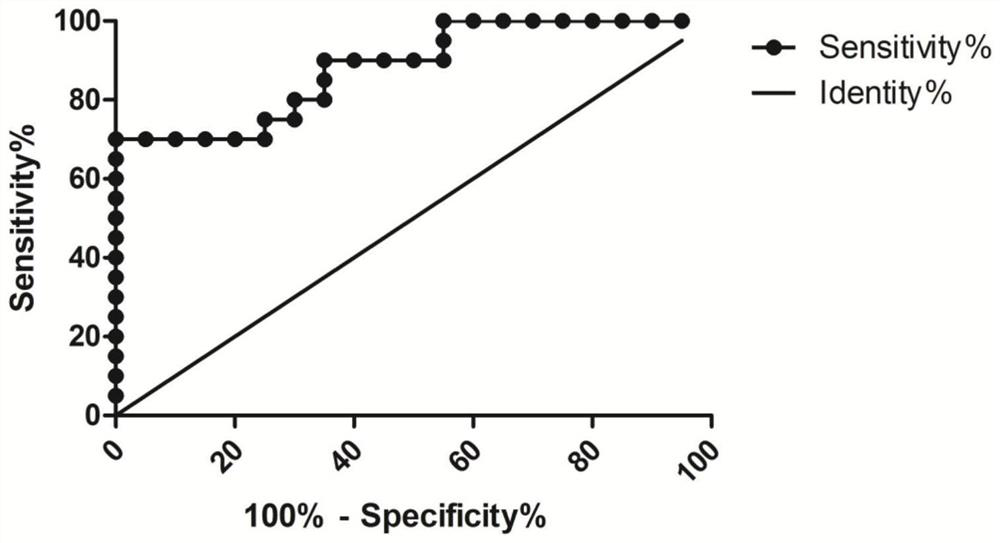
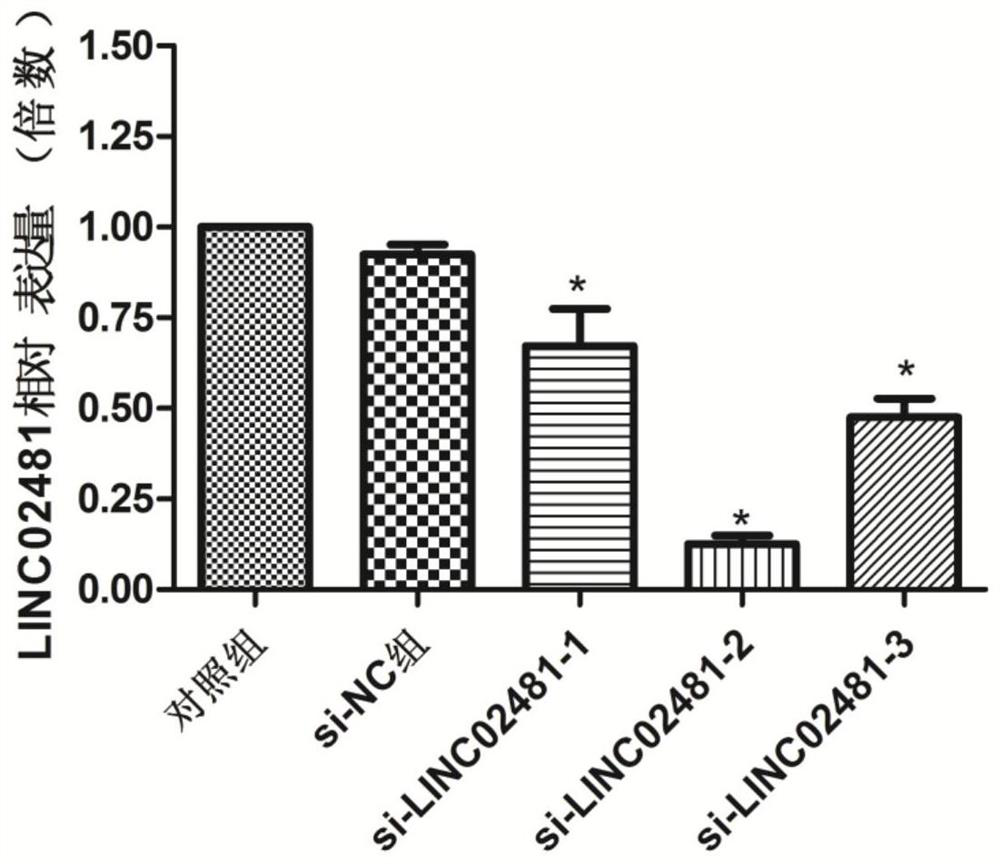
Application of long-chain non-coding RNA LINC02481 in inhibition of proliferation of gastric cancer cells and promotion of apoptosis of gastric cancer cells
ActiveCN111979246AExcellent diagnostic valueOrganic active ingredientsMicrobiological testing/measurementApoptosisGastric carcinoma
The invention aims to provide application of long-chain non-coding RNA LINC02481 in inhibition of proliferation of gastric cancer cells and promotion of apoptosis of gastric cancer cells. Because a siRNA inhibitor designed according to the LINC02481 can inhibit proliferation of gastric cancer cells and promote apoptosis of gastric cancer cells, the siRNA provided by the invention can be used for preparing a medicine for inhibiting proliferation of gastric cancer cells and promoting apoptosis of gastric cancer cells.
Owner:CELLYAN THERAPEUTICS WUHAN CO LTD
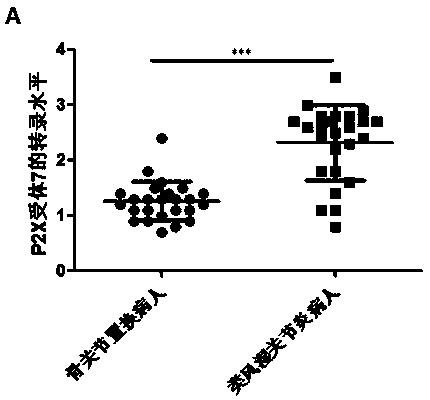
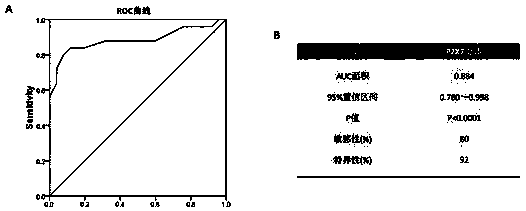
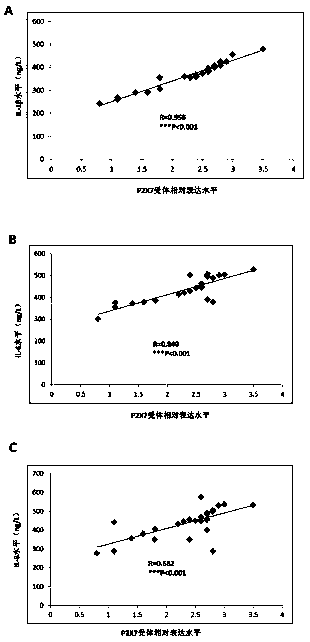
Application of P2X7 receptor in diagnosis and treatment of rheumatoid arthritis
InactiveCN109136361AStrong specificityExcellent diagnostic valueAntipyreticMicrobiological testing/measurementAdjuvantSeronegative rheumatoid arthritis
The invention belongs to the field of biomedical technology, and discloses an application of P2X7 receptor in diagnosis and treatment of rheumatoid arthritis. The P2X7 receptor is firstly identified to be abnormally high in the rheumatoid arthritis disease and is proportional to the inflammation factor level of the rheumatoid arthritis, the targeted inhibition of P2X7 receptor can inhibit the inflammatory response of the rheumatoid arthritis; therefore, the P2X7 receptor can be used for the diagnosis and treatment of rheumatoid arthritis, which overcomes the shortcomings of low specificity andinsufficient sensitivity of existing diagnostic markers of rheumatoid arthritis, which has a good diagnosis value for rheumatoid arthritis with rapid and convenient detection, high accuracy, strong specificity and high sensitivity. The accurate diagnosis of rheumatoid arthritis can be realized by applying of P2X7 receptor, which has a wide application prospect in the adjuvant diagnosis and treatment of rheumatoid arthritis in the medical industry.
Owner:THE THIRD AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIVERSITY
Recombinant antigenic protein for diagnosing echinococcosis granulosus, preparation method thereof and use thereof
ActiveCN101948521BIncreased sensitivityImprove featuresMicrobiological testing/measurementBiological testingEscherichia coliCystic echinococcosis
The invention discloses a recombinant antigenic protein for diagnosing echinococcosis granulosus (having an amino acid sequence represented by SEQIDNo.1). In addition, the invention also discloses the preparation method of the recombinant antigenic protein, which comprises: amplifying an EgEPC1 gene by using RT-PCR; cloning the EgEPC1 gene in a pGEM-T vector; connecting the EgEPC1 gene with an expression vector PET28a(+) to form a recombinant plasmid PET28a-EgEPC1; transforming the recombinant plasmid PET28a-EgEPC1 to Escherichia coli BL21(DE3) and expressing the recombinant protein through IPTG induction; and identifying a purified recombinant antigen by using SDS-PAGE and Western blotting. In addition, the invention also discloses the diagnosis use of the recombinant antigenic protein. Experiments show that the recombinant antigenic protein of the invention has the advantages of high sensibility and specificity for the diagnosis of echinococcosis granulosus, and has a promising application prospect in the diagnosis of the echinococcosis granulosus.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
A kind of cyclic citrulline chimeric peptide antigen and its application
InactiveCN104262489BImprove stabilityIncrease exposureBiological testingHybrid peptidesPeptide antigenElisa kit
The invention discloses a cyclic chimeric citrullinated peptide antigen and an application thereof. The preparation of the cyclic chimeric citrullinated peptide antigen comprises the following steps: firstly connecting and jogging three small-molecular antigen peptides, namely a citrullinated peptide1, a citrullinated peptide 2 and a citrullinated peptide 3 derived from a silk polymerizing protein / an intermediate filament protein, and then synthetizing a cyclic polypeptide with a similar protein beta-corner structure by forming a disulfide bond through two cysteines inserted into the end N and the end C of a chimeric peptide. The cyclic chimeric citrullinated peptide antigen coats a solid-phase vector to prepare an indirect enzyme linked immunosorbent assay kit used for detecting the hypotype of multiple anti-citrullinated protein antibodies contained in RA (Rheumatoid Arthritis) serum. The cyclic chimeric citrullinated peptide antigen and the ELISA kit thereof which are disclosed by the invention have the advantages of simple preparation and experimental operation process, good result repeatability, qualification or quantification and wide clinical application and scientific research value and are outstandingly enhanced in detection sensibility and diagnosis value on RA compared with an international similar kit.
Owner:陈仁奋
Composition for detecting free DNA methylation of plasma based on digital PCR and application of composition
PendingCN113278699AHigh detection sensitivityThe result is accurateMicrobiological testing/measurementCancers diagnosisGastric carcinoma
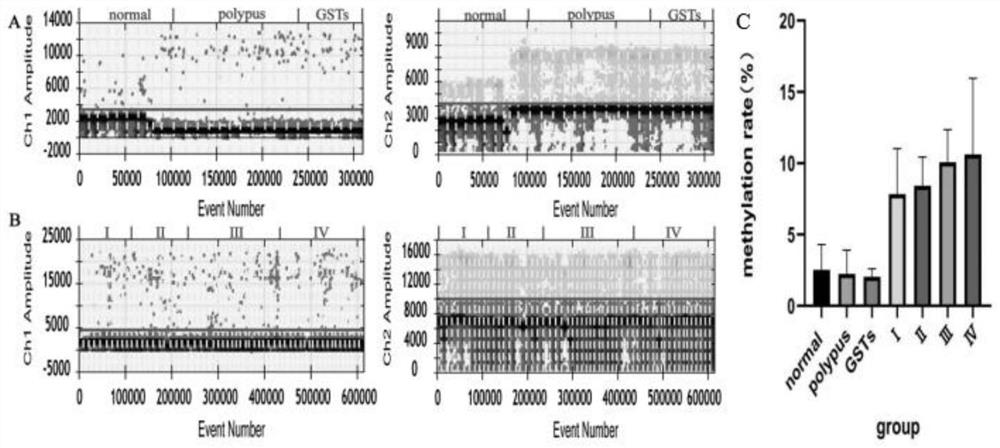
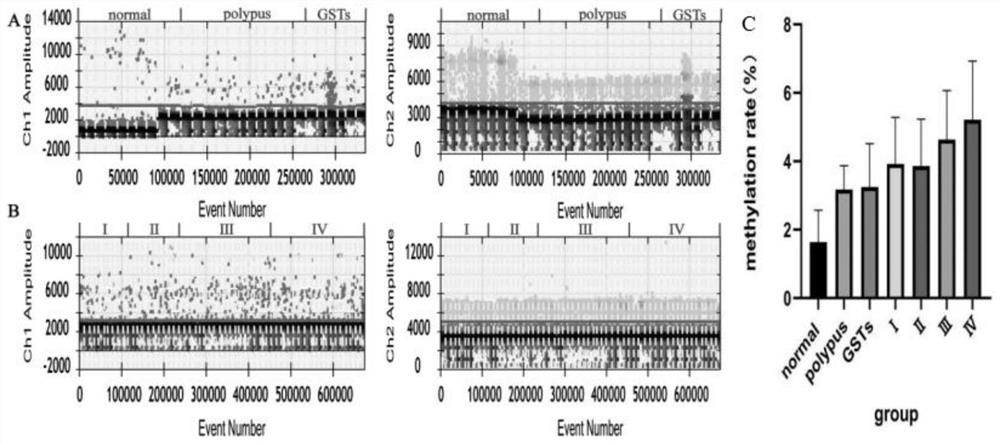
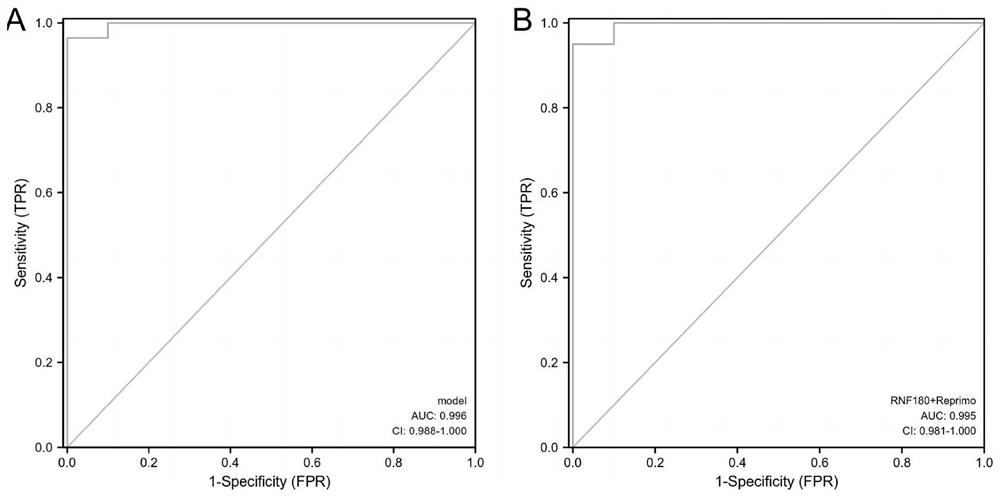
The invention discloses a composition, a kit and a detection method for detecting methylation of a gene Reprimo and / or a gene RNF180 in gastric cancer plasma cfDNA based on a digital PCR technology. The method for absolutely and quantitatively detecting the methylation of the Reprimo gene or the RNF180 gene in the gastric cancer plasma cfDNA based on the micro-droplet digital PCR technology is high in detection sensitivity, accurate in result and capable of absolutely quantifying, and has wide application prospects and industrialization prospects. More importantly, methylation of a gastric cancer marker Reprimo gene or RNF180 gene is subjected to joint detection, experiments prove that the diagnostic value of multi-parameter joint diagnosis analysis for distinguishing a healthy control group and a GC group as well as a benign group and the GC group is superior to that of single parameter detection, so that early discovery and early prevention of gastric cancer diagnosis can be realized, the important significance is achieved for treatment and prognosis of the gastric cancer.
Owner:GENERAL HOSPITAL OF PLA
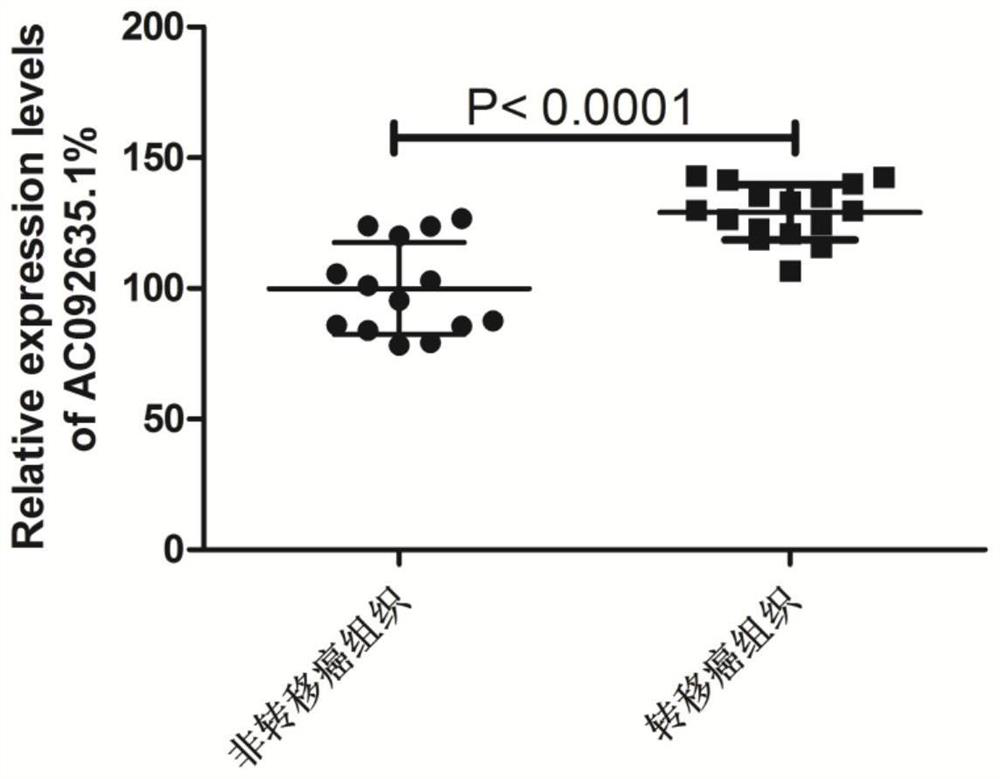
Application of gene preparation in preparation of colorectal cancer cell proliferation and metastasis inhibitor
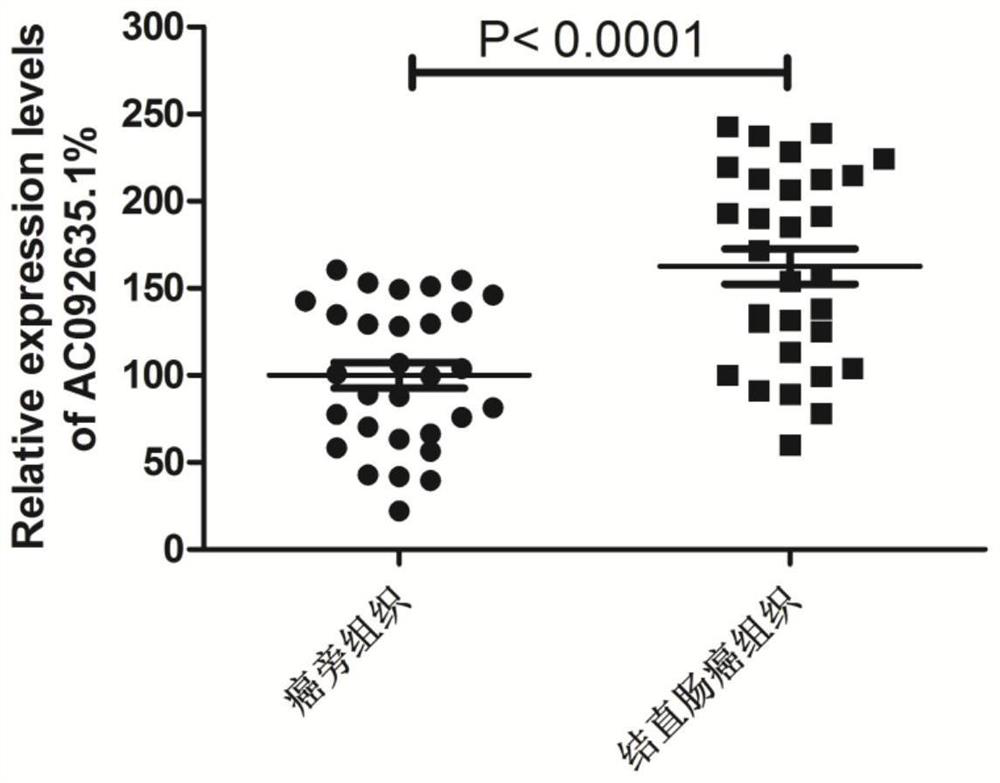
ActiveCN114522179AExcellent diagnostic valueHigh expressionOrganic active ingredientsAntineoplastic agentsOncologyCancer research
The invention provides application of a gene preparation in preparation of a colorectal cancer cell proliferation and metastasis inhibitor, and belongs to the technical field of tumor cell biology. The gene inhibitor provided by the invention is a gene inhibitor of an AC092635.1 gene, and the inhibitor can effectively inhibit proliferation, migration, invasion and EMT transformation of colorectal cancer cells, so that the inhibitor can be used for preparing a colorectal cancer cell proliferation and metastasis inhibitor.
Owner:广东齐美生命医学技术研究院 +3
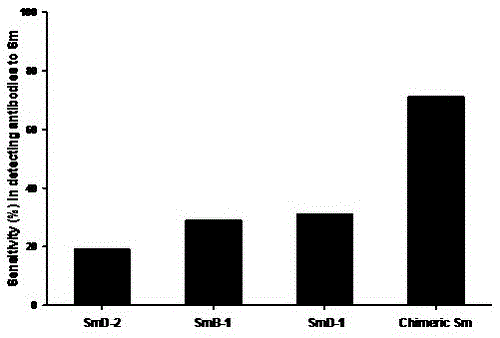
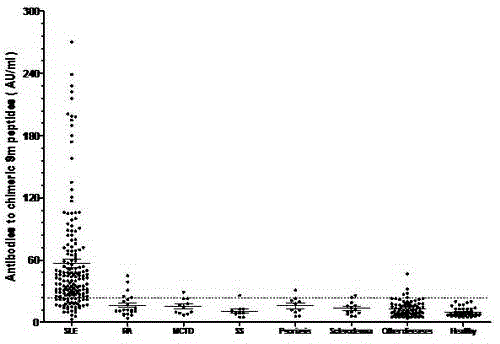
Novel Smith Chimeric Peptide Antigen and Its Application in Laboratory Diagnosis
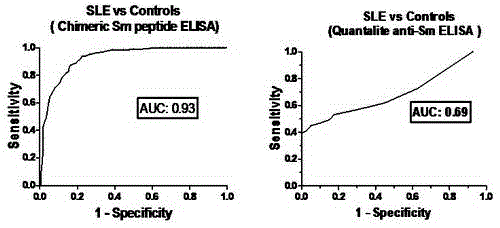
ActiveCN103833859BEasy to makeGood repeatabilityBiological testingHybrid peptidesPeptide antigenEpitope
The invention discloses novel Smith chimeric peptide antigen and application thereof in experimental diagnosis. The antigen is a Sm chimeric peptide containing a plurality of antigen epitopes including three synthetic peptides of SmD-1, SmD-2 and SmB-1, wherein the Sm chimeric peptide antigen is coated on a solid phase carrier and can be used for detecting an anti-Sm antibody. The detecting method comprises an indirect ELISA (Enzyme Linked Immunosorbent Assay) method which coats the Sm chimeric peptide antigen on an elisa plate to prepare an indirect enzyme-linked immunodetection kit. The novel Smith chimeric peptide antigen disclosed by the invention is simple in preparation process, high in sensibility and specificity of detecting the anti-Sm antibody in systemic lupus erythematosus blood serum, good in repeatability, simple to operate, capable of being widely applied to clinical normal inspection and scientific research in a qualitative or quantitative manner, and has a good application value.
Owner:陈仁奋
A Color Flow Frame Correlation Calculation Method
ActiveCN105640592BExcellent diagnostic valueOptimize the effect of clinical needsBlood flow measurement devicesInfrasonic diagnosticsUltrasound attenuationCorrelation coefficient
The invention relates to a correlation calculation method of a colorful blood flow frame. The correlation calculation method comprises the following steps: calculating a frame correlation coefficient Coef, reading an energy / amplitude / velocity threshold value defined by a system and an attenuation correlation coefficient decaycoef and enabling the energy threshold value to be used for overall control and judgment; judging whether current point blood flow energy reaches the energy threshold value 1 or not; if the current point blood flow energy does not reach the energy threshold value 1, directly using previous frame results for current point output; otherwise, entering the processing step of judging a blood flow velocity direction by using the energy threshold value 2; judging whether a current frame position and a previous identical position are in the same direction or not through front and rear frame point multiplication; if the current frame position and the previous same position are in the same direction, same-direction processing; otherwise, reverse-direction processing. According to the relevant calculation method provided by the invention, not only can a corresponding frame correlation effect be realized, but also the data chaos caused in the processing process can not be caused; different correlation modes can be selected, so that the best diagnostic value is realized; through selecting identical-direction processing modes listed in the correlation calculation method, clinically-required effects can be furthest optimized.
Owner:SHENZHEN ANASONIC BIO MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com