Plasmodium vivax PvMSP1 recombinant antigenic protein as well as preparation method and application thereof
A technology for recombining antigenic proteins and recombinant proteins, which can be used in botany equipment and methods, biochemical equipment and methods, chemical instruments and methods, etc., and can solve the problems of missed detection, malaria parasite infection rate and infection degree, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
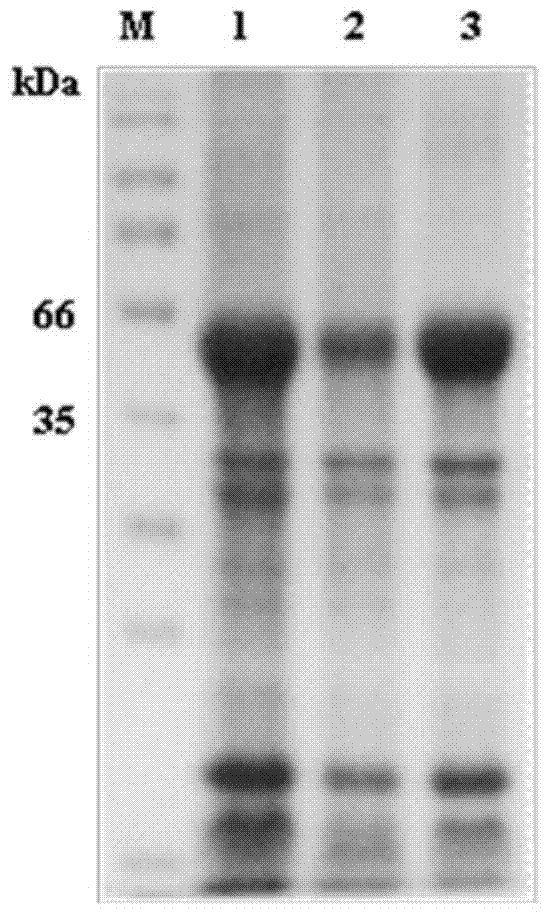
[0031]Example 1 Preparation of Plasmodium vivax PvMSP1 Recombinant Antigen Protein
[0032] 1 material
[0033] 1.1 Genomic DNA of Plasmodium vivax
[0034] Serum red blood cells from patients infected with Plasmodium vivax were collected from malaria-endemic areas in Yunnan Province.
[0035] 1.2 Host strains Escherichia coli DH5α, BL21(DE3), and plasmid pET-28a(+) were from Tiangen Biochemical Technology Co., Ltd. and Novagen respectively.
[0036] 1.3 Main reagents and tool enzymes
[0037]
[0038] 2 methods
[0039] 2.1 Amplification and cloning of Plasmodium vivax PvMSP1 gene
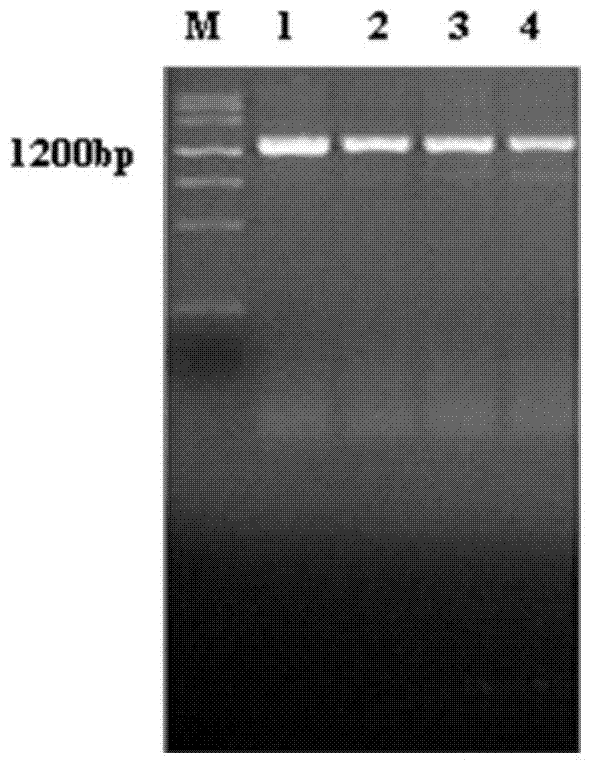
[0040] 2.1.1 PCR amplification of PvMSP1 gene
[0041] According to the reported sequence of the PvMSP1 gene (PVX_099980), a pair of specific primers were designed using the primer design software of Shanghai Yingjun Biotechnology Co., Ltd. The uppercase letters in the specific primers were 15 bases homologous to the end of the enzyme-cut vector, Wherein the single underline indicates the...
Embodiment 2
[0078] Example 2 Effect Experiment of Plasmodium vivax PvMSP1 Recombinant Antigen Protein Detection of Human Serum Antibody
[0079] 1 material
[0080] 1.1 Serum samples from vivax malaria patients and normal subjects were collected from malaria endemic areas in Yunnan Province.
[0081] 1.2 Goat anti-human whole molecule IgG, from SIGMA company.
[0082] 2 methods
[0083] A number of patient sera infected by Plasmodium vivax and normal human sera were mixed in equal amounts to prepare positive and negative serum controls. Specific steps are as follows:
[0084] 1) Dilute the antigen with coating solution to a final concentration of 4ng / μl, 100μl / well, incubate at 37°C for 2h, wash with PBS-T 3 times, 3min each time;
[0085] 2) Use 1% BSA (diluted in PBS), 100 μl / well, incubate at 37°C for 1 hour, wash with PBS-T 3 times, 3 minutes each time;
[0086] 3) Dilute the primary antibody (plasma vivax patient serum and normal human serum) with PBS-T (1:100), 100 μl / well, incub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com