Recombinant antigenic protein for diagnosing echinococcosis granulosus, preparation method thereof and use thereof
A technology for echinococcosis and recombinant antigenic protein, which is applied in the field of preparation of the above-mentioned recombinant antigenic protein, recombinant protein, and recombinant antigenic protein for the diagnosis of echinococcosis, which can solve the unsatisfactory treatment effect of echinococcosis, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of Echinococcus granulosus Recombinant Antigen Protein
[0032] 1 material
[0033] 1.1 Echinococcus granulosus
[0034] Echinococcus granulosus cysts in sheep liver were collected from the slaughterhouse in Qinghai Province, and the protoscole of Echinococcus granulosus from Qinghai (sheep source) was isolated and collected under aseptic conditions.
[0035] 1.2 Serum samples to be tested
[0036] Qinghai Provincial Institute of Endemic Disease Prevention and Control provided 60 sera from confirmed CE patients, 37 sera from alveolar echinococcosis (AE) patients, 11 sera from cysticercosis patients, and the Institute of Parasitic Diseases Prevention and Control of the Chinese Center for Disease Control and Prevention. Provide 33 serums of healthy people, 5 serums of cysticercosis patients, 7 serums of liver fluke patients, 4 serums of schistosomiasis patients, and 4 serums of toxoplasmosis patients.
[0037] 1.3 Main reagents and tool enzymes
...
Embodiment 2
[0058] Example 2 Western-blot identification of PET28a-EgEPC1 recombinant protein
[0059] 1. Western-blot identification method
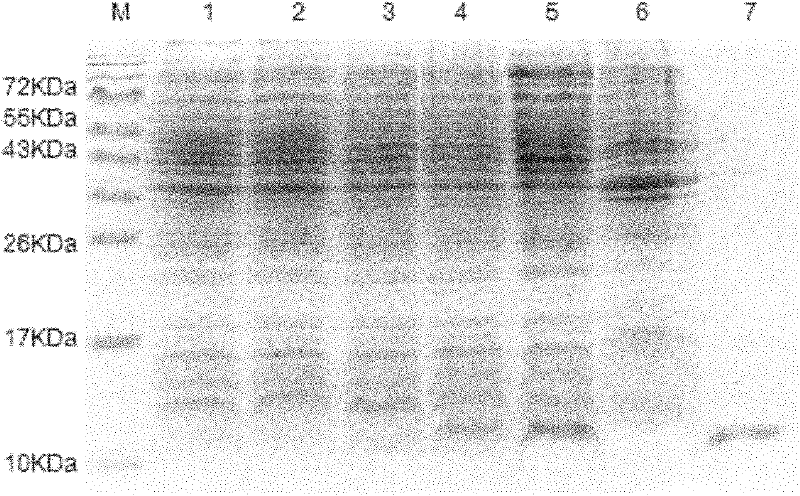
[0060] After the purified PET28a-EgEPC1 recombinant protein (produced by Example 1) was subjected to 15% SDS-PAGE electrophoresis, it was transferred to a nitrocellulose membrane; the membrane was cut into strips and placed in 5% skim milk powder solution to block overnight, Wash with PBS-T 3 times, 5 min each time; react with the mixed serum of normal healthy people, the mixed serum of CE patients, and the mixed serum of AE patients (1:100 dilution), shake gently at room temperature for 2 h, wash 3 times with PBS-T, each time 5min; add goat anti-human HRP-IgG (1:1000) and shake gently at room temperature for 2h, wash with PBS-T 3 times, 5min each time; add freshly prepared DAB combined chromogenic solution until the target band appears, stop with deionized water reaction.
[0061] 2. Western-blot identification and analysis results of recombinan...
Embodiment 3
[0063] Example 3 Diagnosis Effect Experiment of Echinococcus granulosus Recombinant Antigen Protein
[0064] 1. ELISA serological evaluation of recombinant antigens
[0065] Multiple parts of serum CE and healthy human serum were mixed in equal amounts to prepare positive and negative serum controls. The serum was diluted 1:100, and the working concentration of the recombinant antigen (prepared in Example 1) was determined to be 0.5 μg / ml by square matrix titration, and the working concentration of goat anti-human IgG horseradish peroxidase marker was 1:14000. O-phenylenediamine substrate (OPD) color development, A 490 . The mean absorbance of 33 cases of healthy human serum was 2 times SD as the positive judgment value.
[0066] 2. Statistical analysis of data
[0067] The experimental data were collated and statistically analyzed using Excel spreadsheet processing software.
[0068] 3. Analysis and evaluation of serological test results of recombinant antigens
[0069]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com