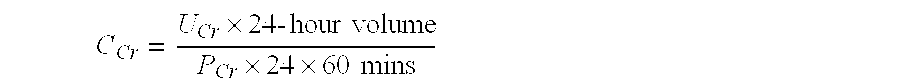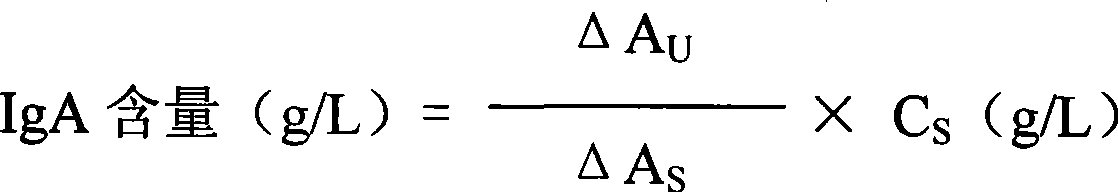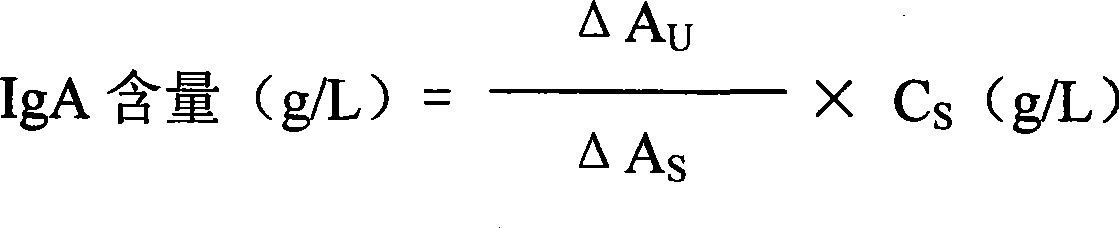Patents
Literature
110 results about "Immunoglobulin A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunoglobulin A (IgA, also referred to as sIgA in its secretory form) is an antibody that plays a crucial role in the immune function of mucous membranes. The amount of IgA produced in association with mucosal membranes is greater than all other types of antibody combined. In absolute terms, between three and five grams are secreted into the intestinal lumen each day. This represents up to 15% of total immunoglobulins produced throughout the body.
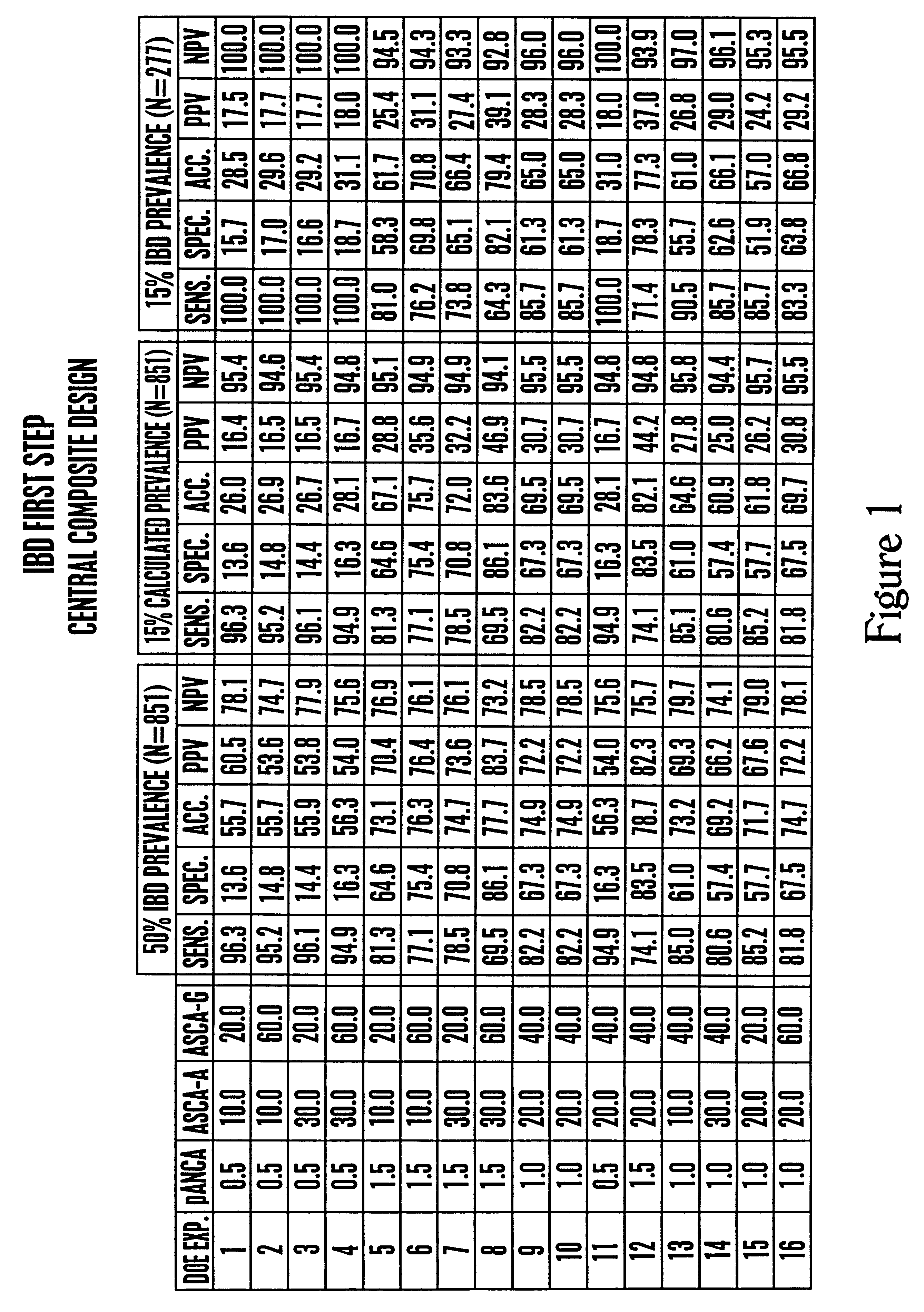
Inflammatory bowel disease first step assay system
The present invention provides a highly sensitive method of diagnosing inflammatory bowel disease (IBD) in an individual. The method includes the steps of isolating a sample from the individual; determining by non-histological means whether the sample is positive for anti-neutrophil cytoplasmic antibodies (ANCA); determining whether the sample is positive for anti-Saccharomyces cerevisiae immunoglobulin A (ASCA-IgA); determining whether the sample is positive for anti-Saccharomyces cerevisiae immunoglobulin G (ASCA-IgG); and diagnosing the individual as having IBD when the sample is positive for ANCA, ASCA-IgA or ASCA-IgG, and diagnosing the individual as not having IBD when the sample is negative for ANCA, ASCA-IgA and ASCA-IgG, provided that the method does not include histological analysis of neutrophils.
Owner:PROMETHEUS LAB +1
Stimulation of the immune system with polydextrose
InactiveUS20030157146A1Strengthening and improving health conditionImprove concentrationPowder deliveryBiocideImmunoglobulin APolyol
The invention relates to the use of polydextrose for stimulating the immune response (IgA) in the gastrointestinal tract of a mammal. Furthermore, the invention provides a method to potentiate the immunostimulative effect of polydextrose by mixing at least one polyol with polydextrose, said polyol being effective to synergistically increase the immunoglobulin A (IgA) concentration in the gut of a mammal. On the other hand, the invention provides also compositions containing polydextrose and polyols in which the laxative effects of polyols are reduced and which are effective to reduce the amount of biogenic amines in the gut of a mammal.
Owner:AS DE DANSKE SUKKERFABRIKKER
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20120283128A1Eliminate needEasy to adaptBioreactor/fermenter combinationsBiological substance pretreatmentsMatrilysinInterleukin-1beta
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a plurality of assays, one or more of which is configured to detect a kidney injury marker selected from the group consisting of Hyaluronic acid, Immunoglobulin A, Immunoglobulin G1, Immunoglobulin G2, Insulin-like growth factor-binding protein 7, Alpha-1 antitrypsin, Serum amyloid P component, Metalloproteinase inhibitor 2, Hepatocyte growth factor, Intercellular adhesion molecule 1, Beta-2-glycoprotein 1, Interleukin-1 beta, Neutrophil Elastase, Tumor necrosis factor receptor superfamily member 11B, Interleukin-11, Cathepsin D, C—C motif chemokine 24, C—X—C motif chemokine 6, C—C motif chemokine 13, C—X—C motif chemokines -1, -2, and -3, Matrilysin, Interleukin-2 receptor alpha chain, Insulin-like growth factor-binding protein 3, and Macrophage colony-stimulating factor 1 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
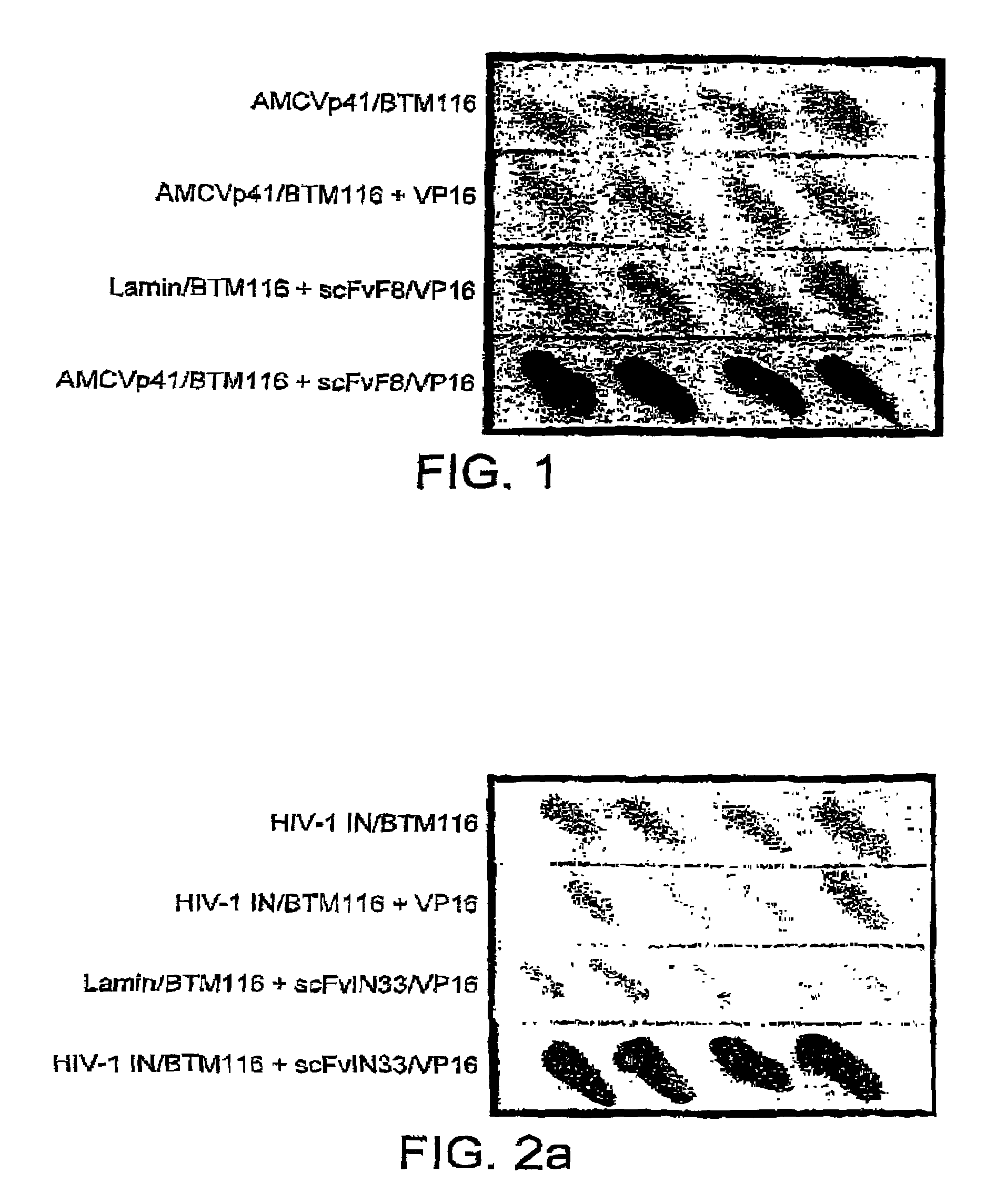
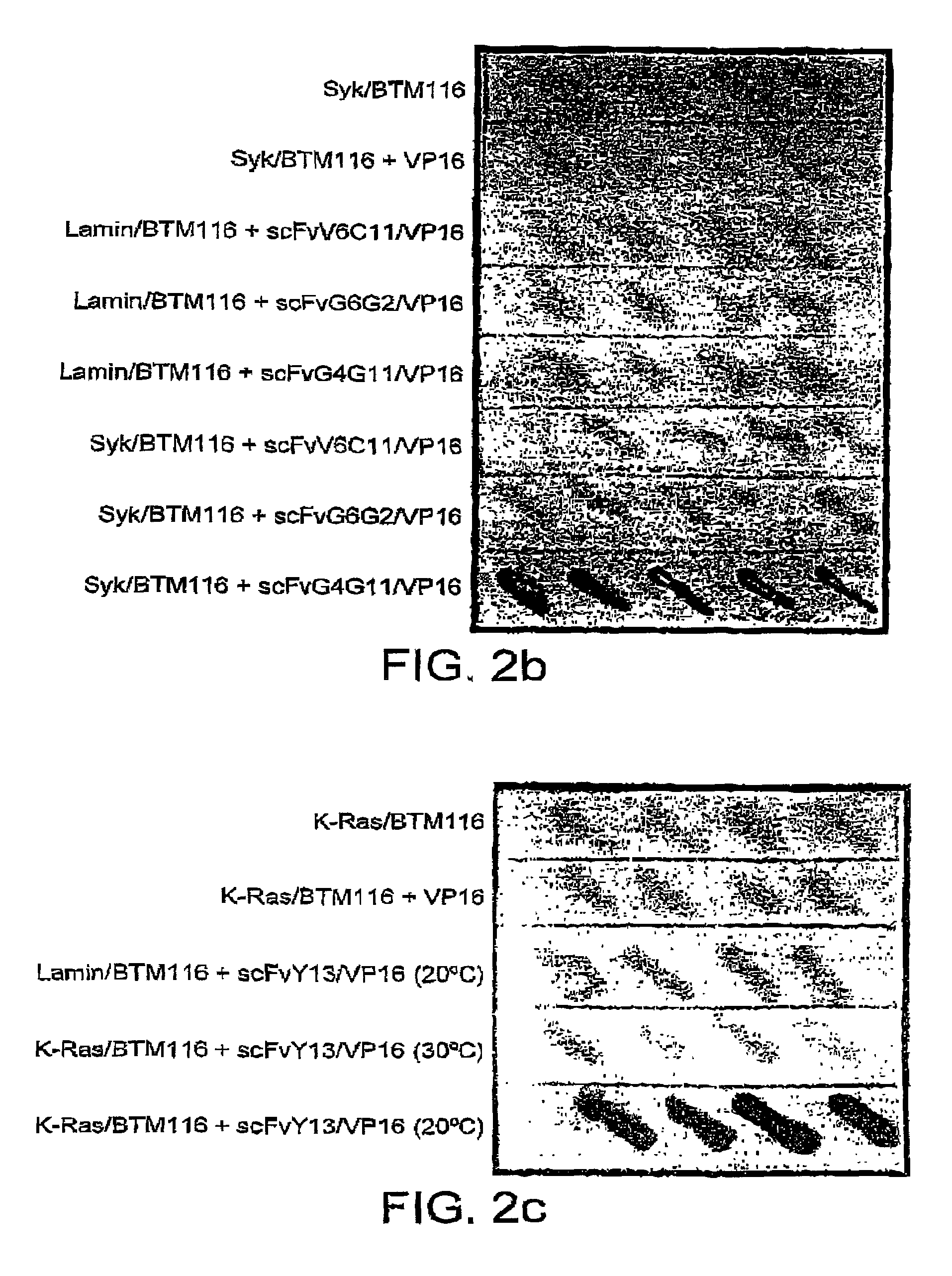
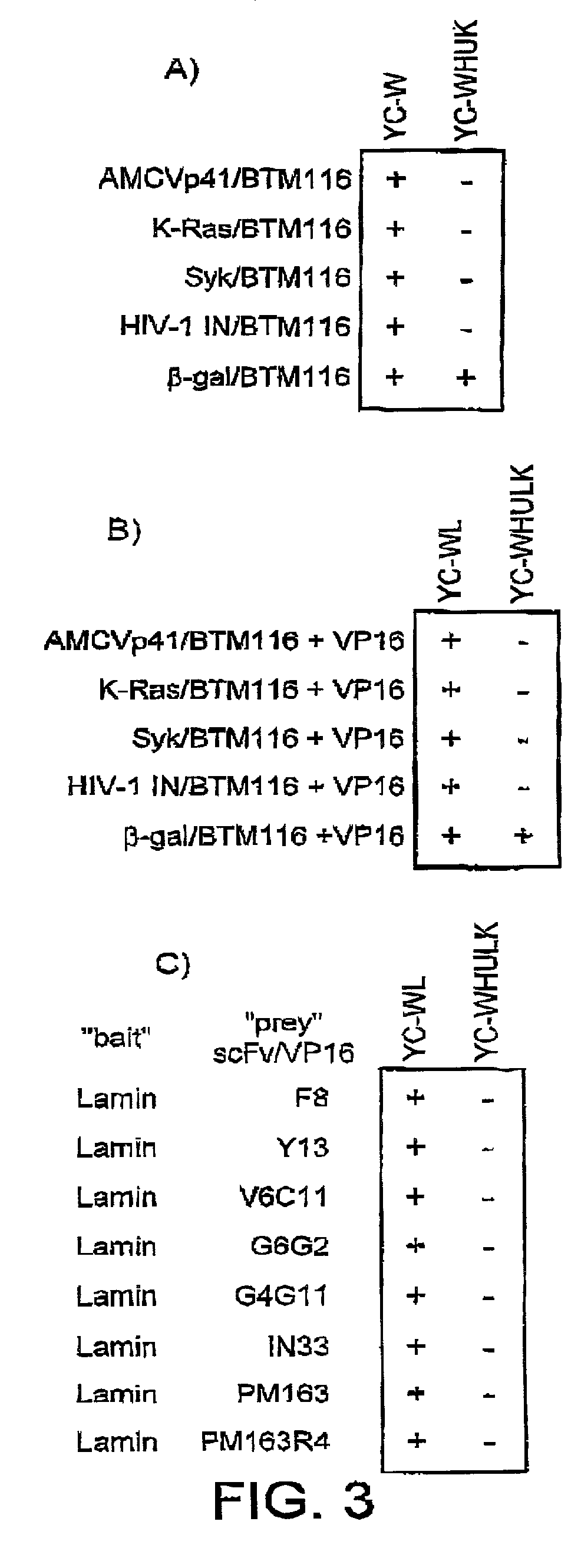
Selection of intracellular immunoglobulins
A general immunoglobulin-target assay system is provided, in which a positive outcome (the generation of a signal) depends only on the intracellular interaction of immunoglobulin with target. This can be accomplished for many immunoglobulins expressed in yeast and / or in mammalian cells and allows the selection of immunoglobulins which are capable of functioning in an intracellular environment.
Owner:UK RES & INNOVATION LTD +2
Pharmaceutical formulation containing immunoglobulin
InactiveUS20120237532A1High throughput generationImprove stabilityAntibody ingredientsImmunoglobulinsComplementarity determining regionImmunoglobulin A
A set of at least two different protein conjugate preparations, each protein conjugate preparation comprising histidine as a buffering agent and a protein conjugate comprising one or more immunoglobulin moieties conjugated to a carrier protein; wherein the immunoglobulin moieties of each element of said set of protein conjugate preparation have identical complementarity determining regions (CDRs); and wherein different protein conjugate preparations differ in that the immunoglobulin moieties of the protein conjugates have different CDRs.
Owner:ICON GENETICS
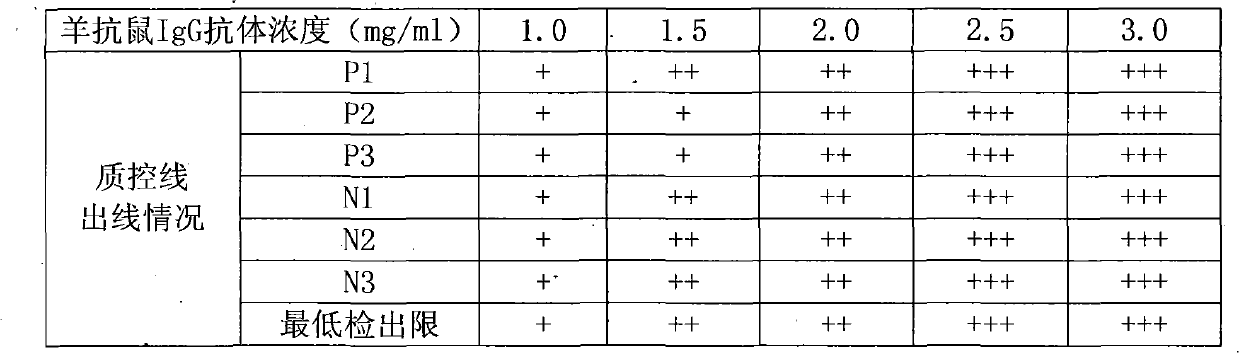
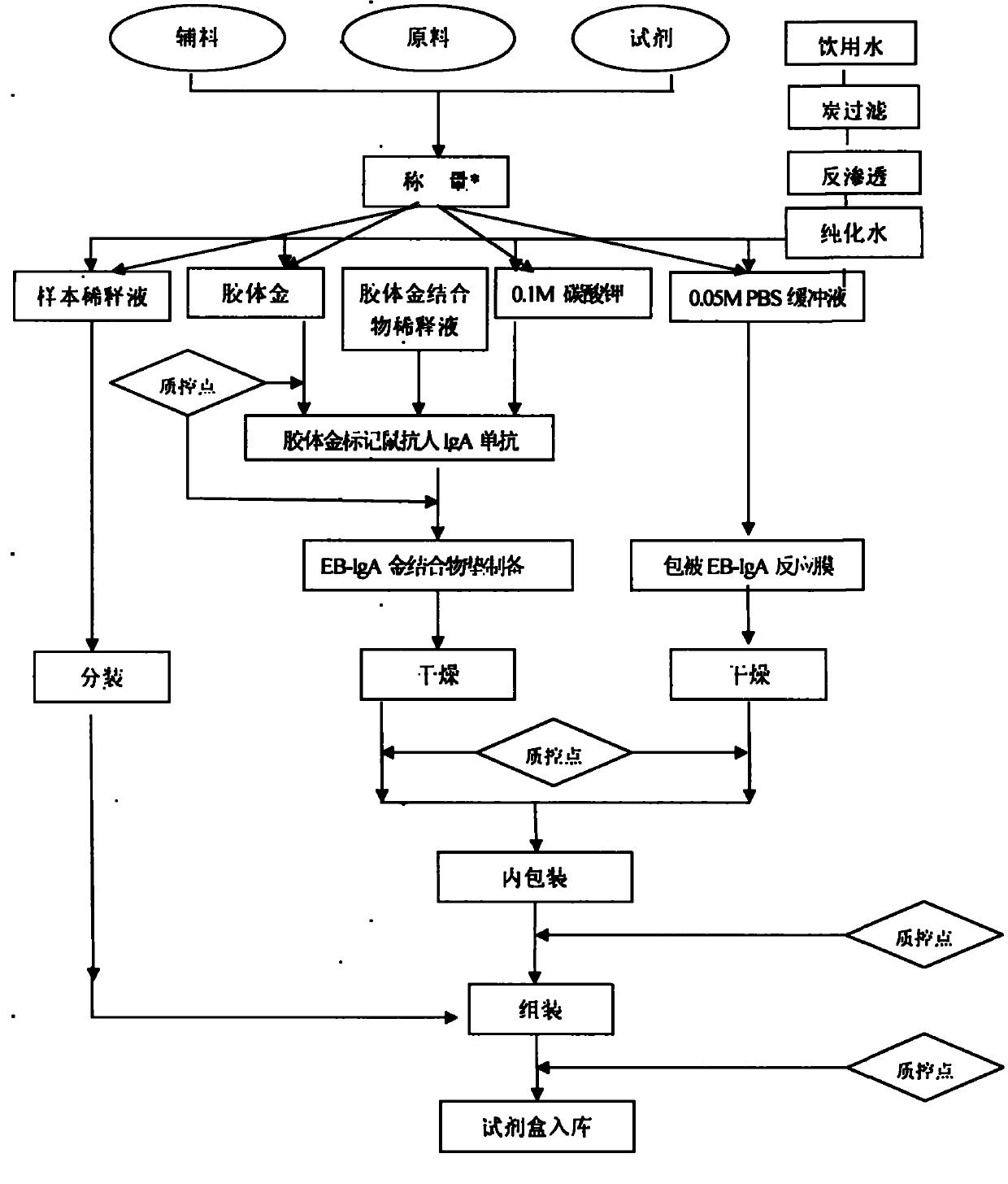
IgA (Immunoglobulin A) antibody detection reagent kit (colloidal gold method) for EB (Epstein-Barr) viruses and preparation method thereof
The invention relates to an IgA (Immunoglobulin A) antibody detection reagent kit (a colloidal gold method) for EB (Epstein-Barr) viruses and a preparation method thereof. The reagent kit comprises recombination antigen EB-NA1 coated by a nitrocellulose membrane detection line, a goat-anti-mouse IgG antibody coated on a quality control line and a mouse-anti-human IGA monoclonal antibody marked by colloidal gold and coated on a gold mark pad. The preparation method comprises the steps of: preparing a reaction membrane and a mouse-anti-human IGA monoclonal antibody gold combo pad, cutting and assembling to prepare the product. The invention has the advantages that: the IgA antibody detection reagent kit for the EB viruses has the characteristics of fast, simple and convenient detection, and high accuracy and sensitivity; the integrated operation time only requires 20 minutes to judge and read results; the colloidal gold is used for fast detecting test paper; a multi-epitope recombination antigen is used as a raw material; the method has the characteristics of simple and convenient operation, low cost, good specificity, high sensitivity, single portion detection and easy popularization; and the detection and control effect to the EB viruses is obvious.
Owner:北京中检安泰诊断科技有限公司
Human medical treatment by aerosol inhalation of immunoglobulin A
Pooled human plasma is processed by cold ethanol fractionation to produce purified immunoglobulin G antibodies for intravenous administration. Immunoglobulin A is an unwanted by-product since intravenous administration of immunoglobulin A-containing immunoglobulin G can cause life-threatening anaphylaxis in some people. The present invention is the topical application of immunoglobulin A coupled with J chain, and optionally coupled with secretory component in order to render the immunoglobulin A more physiologically active, for the prevention or treatment of ocular diseases including ocular immune deficiency and infections. Antigen-specific monoclonal immunoglobulin A may be used.
Owner:SIMON MICHAEL R
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in sepsis patients. In particular, the invention relates to using assays that detect one or more biomarkers selected from the group consisting of Insulin-like growth factor-binding protein 7, Beta-2-glycoprotein 1, Metalloproteinase inhibitor 2, Alpha-1 Antitrypsin, Leukocyte elastase, Serum Amyloid P Component, C-X-C motif chemokine 6, Immunoglobulin A, Immunoglobulin G subclass I, C-C motif chemokine 24, Neutrophil collagenase, Cathepsin D, C-X-C motif chemokine 13, Involucrin, Interleukin-6 receptor subunit beta, Hepatocyte Growth Factor, CXCL-1, -2, -3, Immunoglobulin G subclass II, Metalloproteinase inhibitor 4, C-C motif chemokine 18, Matrilysin, C-X-C motif chemokine 11, and Antileukoproteinase as diagnostic and prognostic biomarker assays of renal injury in the sepsis patient.
Owner:ASTUTE MEDICAL
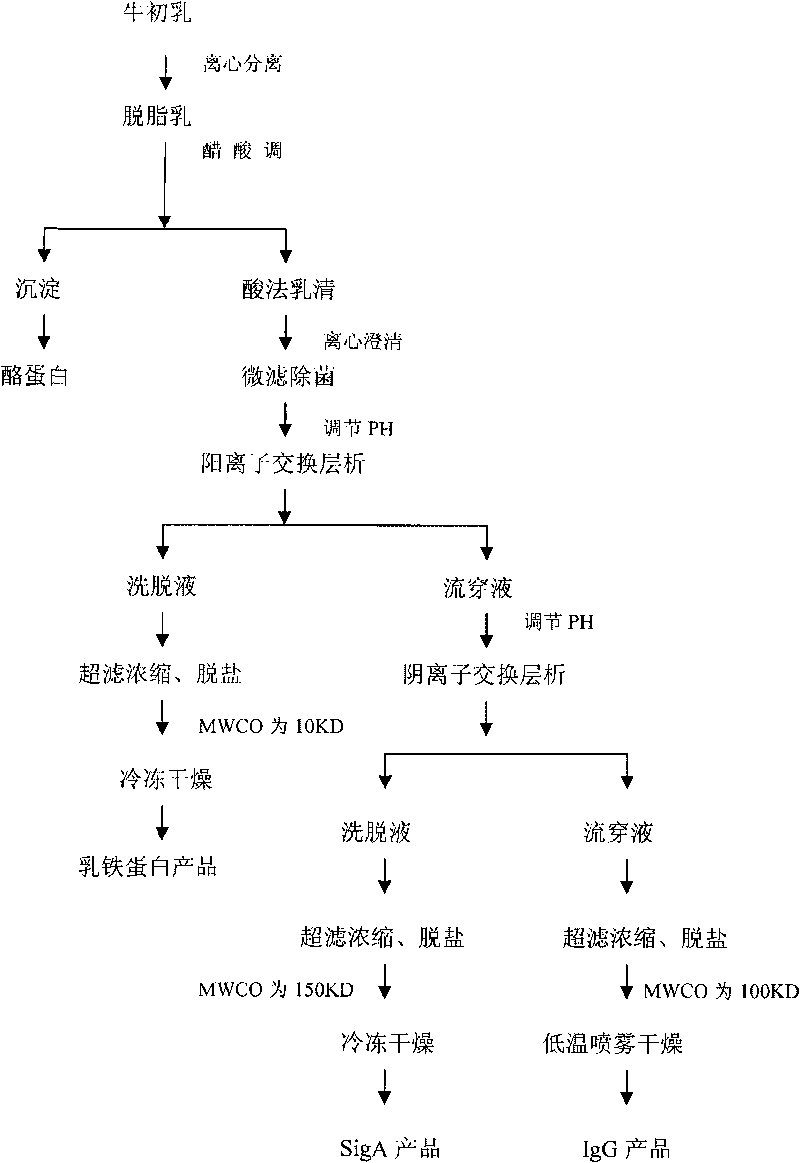
Method for separating and purifying immunoglobulin A, immunoglobulin G and lactoferrin from bovine colostrum in industrializing way
ActiveCN101724013AReduce pollutionIncrease productivityTransferrinsMilk immunoglobulinsAnion-exchange chromatographyLactoferrin
The invention provides a method for separating and purifying immunoglobulin A, immunoglobulin G and lactoferrin from bovine colostrum in an industrializing way, which comprises the following steps of: (1) degreasing the bovine colostrum and separating casein to prepare whey; (2) after micro-filtering the whey for degerming, carrying out cation exchange chromatography to obtain a first fast flow liquid, eluting a chromatographic column to obtain eluent, ultra-filtering, concentrating and desalting the eluent by using a first ceramic membrane, and freezing and drying to obtain the lactoferrin; (3) carrying out anion exchange chromatography on the first fast flow liquid to obtain a second fast flow liquid, eluting the chromatographic column to obtain eluent, ultra-filtering, concentrating and desalting the eluent by using a second ceramic membrane, and freezing and drying to obtain the immunoglobulin A; and (4) ultra-filtering, concentrating and desalting the second fast flow liquid by using a third ceramic membrane, and obtaining the immunoglobulin G by using low-temperature spray drying. By utilizing the method, the immunoglobulin A, the immunoglobulin G and the lactoferrin can be separated and purified from the bovine colostrumin efficiently and continuously in the industrializing way.
Owner:HEILONGJIANG KANPURE BIOTECH
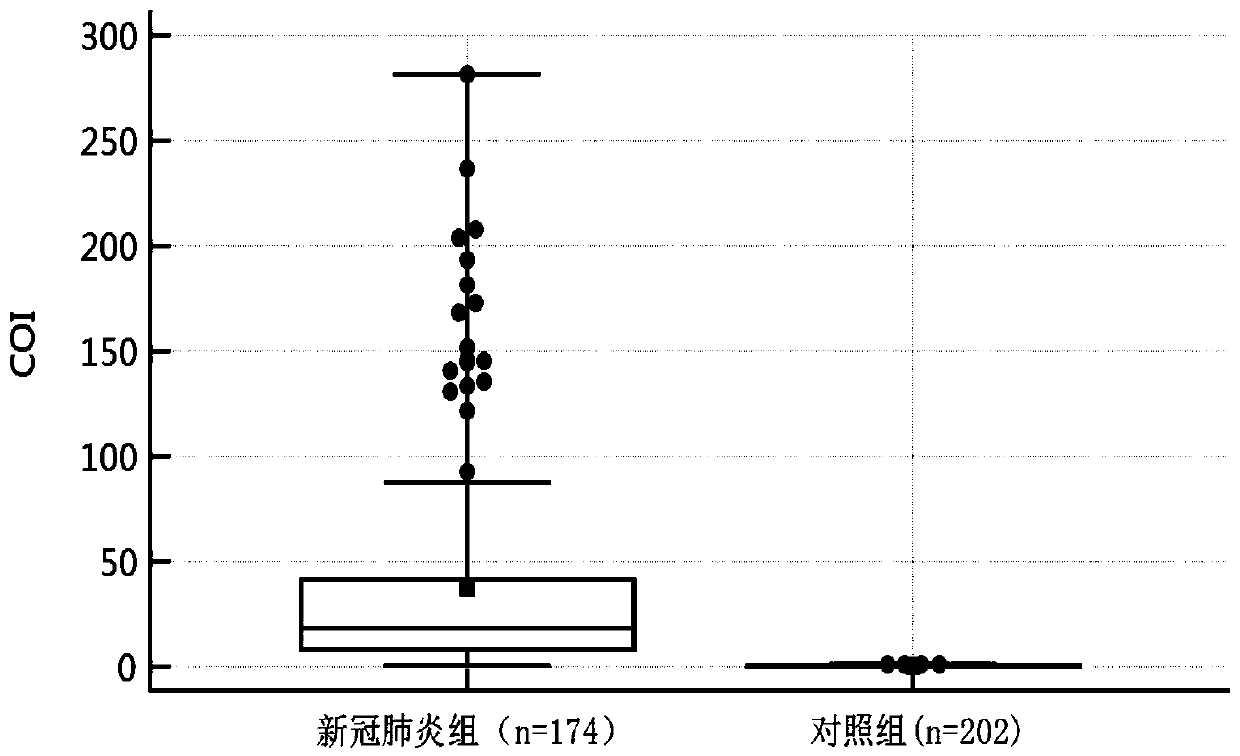
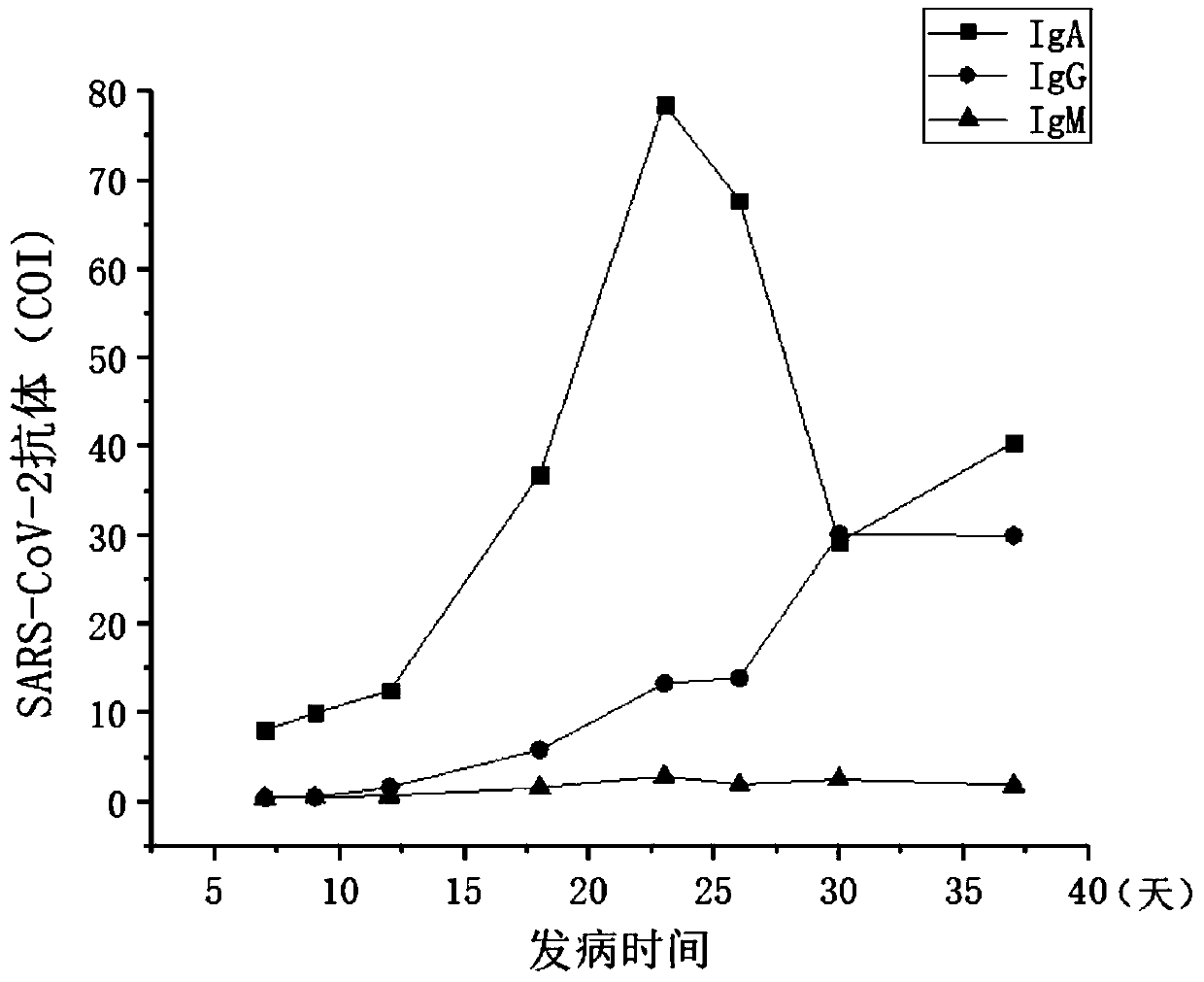
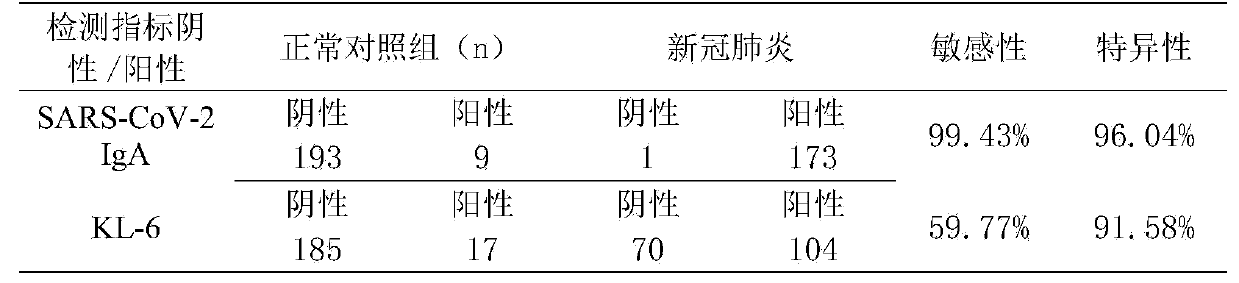
Method for screening 2019-coronavirus and pre-judging severe pneumonia by double indexes
Provided are a detection reagent and a kit. The reagent detects a 2019-coronavirus SARS-CoV-2 immunoglobulin A (SARS-CoV-2 IgA) antibody in blood and a salivary liquefied sugar chain antigen KL-6 at the same time, and is used for screening, especially for early screening of 2019-coronavirus infected pneumonia, and for pre-judging the detection reagent for severe patients. The kit comprises a testcard, and the test card is composed of an SARS-CoV-2 IgA test strip, a KL-6 test strip and a plastic box. The reagent and the kit disclosed by the invention can be used for diagnosing the new coronavirus pneumonia at an earlier stage and can be used for judging whether a patient suffering from the new coronavirus pneumonia has lung injury or not.
Owner:GUANGZHOU KANGRUN BIOTECHNOLOGY CO LTD +1
Human medical treatment by aerosol inhalation of immunoglobulin A
InactiveUS6932967B2Physiologically effectiveDispersion deliverySerum immunoglobulinsDiseaseFractionation
Pooled human plasma is processed by cold ethanol fractionation to produce purified immunoglobulin G antibodies for intravenous administration. Immunoglobulin A is an unwanted by-product since intravenous administration of immunoglobulin A-containing immunoglobulin G can cause life-threatening anaphylaxis in some people. The present invention is the aerosol administration, by metered dose inhaler or nebulizer, of by-product immunoglobulin A for the prevention or treatment of diseases including immunodeficiencies and infections. Antigen-specific monoclonal immunoglobulin A may be used. Immunoglobulin A from any of the aforementioned sources may then be coupled with recombinant J chain, and may then be additionally coupled with recombinant secretory component in order to render the immunoglobulin A more physiologically active. Immunoglobulin A, with or without J chain and secretory component, is then administered by aerosol inhalation.
Owner:SIMON MICHAEL R
Traditional Chinese medicine scented sachet for preventing influenza
InactiveCN103007147AGood killing effectFlu preventionHydroxy compound active ingredientsAntiviralsBiotechnologyInfectious Disorder
The invention provides a traditional Chinese medicine scented sachet for preventing influenza. The scented sachet is characterized in that medicines in the scented sachet give off persistent aromatic odor, which stimulates the respiratory mucosa of the human body to generate secreted immunoglobulin A, and such an antibody has a strong action of killing virus and bacteria, so that these microorganisms can not survive on the upper respiratory mucosa, thus achieving the effect of preventing infectious diseases. The traditional Chinese medicine scented sachet can effectively prevent influenza.
Owner:徐曼丽
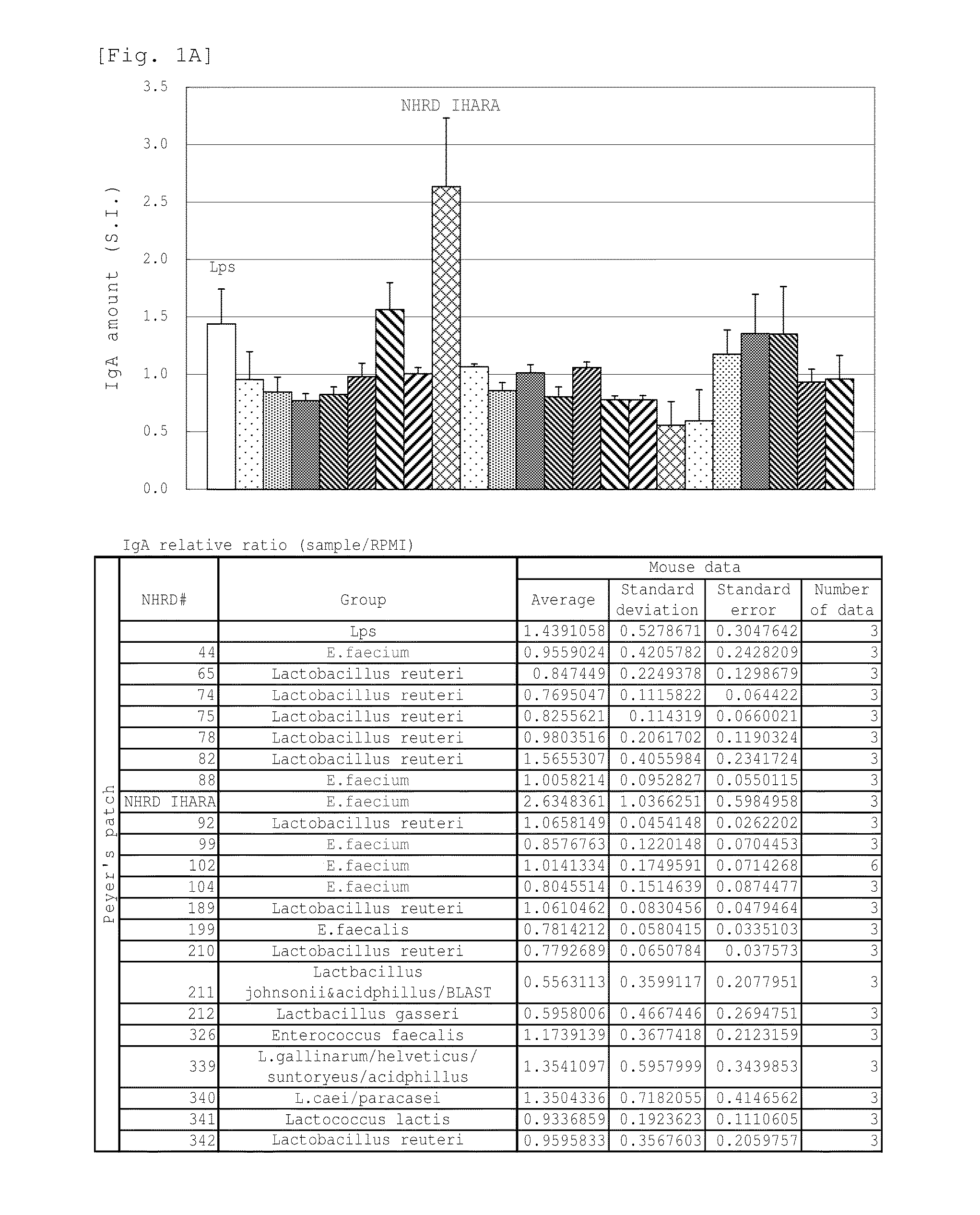
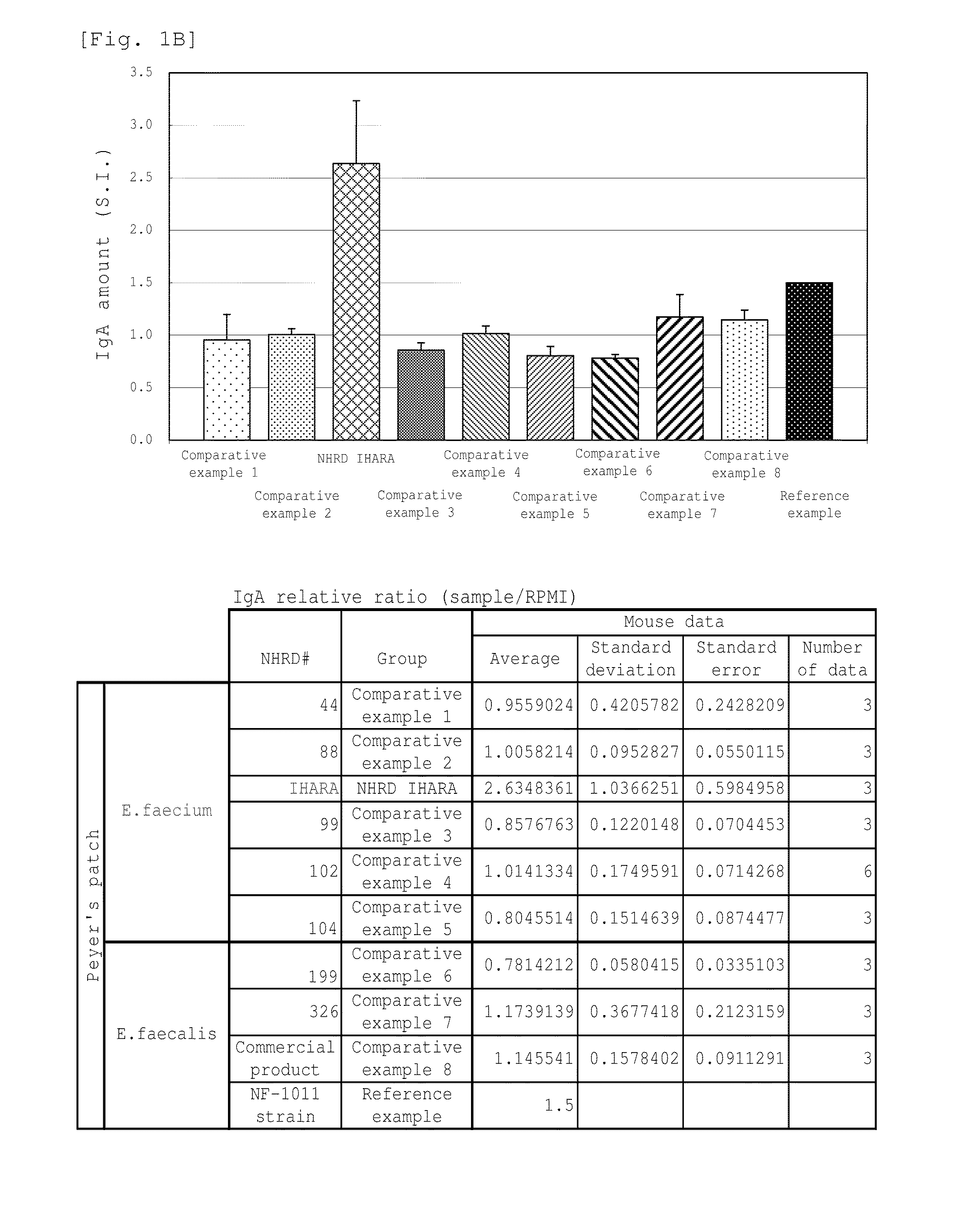
Novel lactic acid bacterium having high immunoglobulin-a-inducing ability
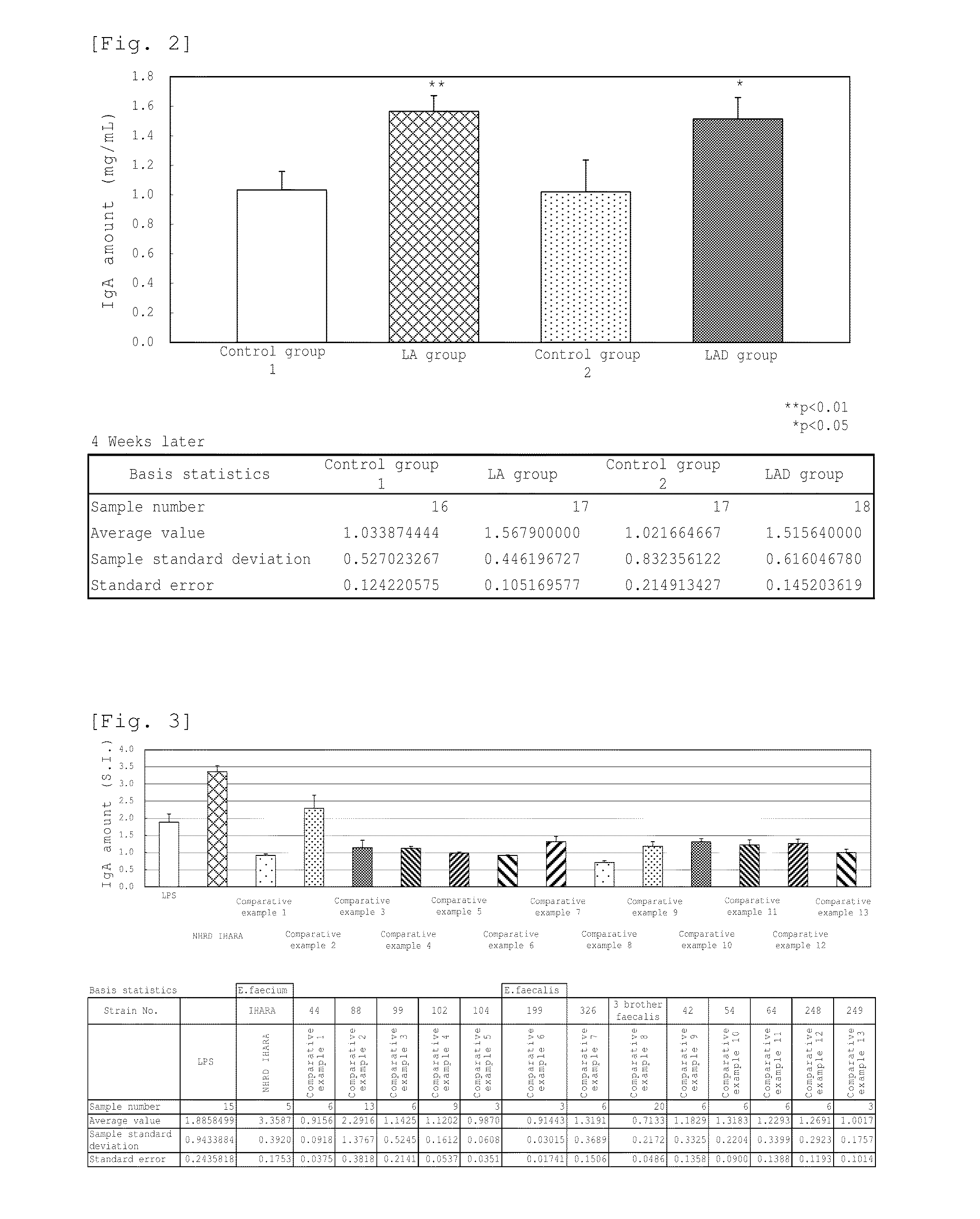
A novel lactic acid bacterium strain is found, which has a high IgA production-inducing ability and a high immunostimulating activity, and belongs to the genus Enterococcus. Thus, provided is an excellent lactic acid bacterium preparation for foods or feeds, which has a high enteric colonization rate. Specifically disclosed are: a novel lactic acid bacterium Enterococcus faecium NHRD IHARA (FERM BP-11090) which has a high IgA production-inducing ability and a high immunostimulating activity; and a lactic acid bacterium preparation for enhancing the production of immunoglobulin A, which comprises a culture of the bacterium strain, an extract of the bacterium strain, or a product of any treatment of cells of the bacterium strain.
Owner:NIPPON HAM
Health-care traditional Chinese medicine scented sachet
InactiveCN103007153AGood killing effectPlay a health effectAntibacterial agentsAntiviralsImmunoglobulin AVirus
The invention provides a health-care traditional Chinese medicine scented sachet, which is characterized in that medicines in the scented sachet do not have the function of directly killing virus and bacteria, but give off persistent aromatic odor, which stimulates the human body to generate secreted immunoglobulin A, and such an antibody has a strong action of killing virus and bacteria, thus achieving the health-care effect.
Owner:徐曼丽
Polypeptide, an affinity chromatography material, and a method for separating and/or purifying immunoglobulin
ActiveUS8198409B2Easy to collectTake advantage ofSugar derivativesMammal material medical ingredientsImmunoglobulin AMutated protein
A mutant of the polypeptide Protein A, wherein immunoglobulin binding properties can be altered by changing temperature under the conditions of pH 5-9, below 60° C. The use of the mutant Protein A include the use thereof as a ligand coupled to an affinity chromatography support for the purification of immunoglobulins by affinity chromatography, wherein the immunoglobulins is eluted by changing temperature and thereby the conformation of the mutant Protein A.
Owner:NOMADIC BIOSCI
Traditional Chinese medicine scented sachet for increasing resistance
InactiveCN103007168AGood killing effectImprove the immunityImmunological disordersJewelleryBacteroidesBacilli
The invention provides a traditional Chinese medicine scented sachet for increasing resistance. The scented sachet is characterized in that medicines in the scented sachet do not have the function of directly killing virus and bacteria, but give off persistent aromatic odor, which stimulates the respiratory mucosa of the human body to generate secreted immunoglobulin A, and such an antibody has a strong action of killing virus and bacteria, so that these microorganisms can not survive on the upper respiratory mucosa, thus achieving the effect of increasing the resistance.
Owner:徐曼丽
Chinese medicinal compound preparation for treating IgA (Immunoglobulin A) nephropathy and preparation method thereof
InactiveCN102370798ASignificant effectEliminate side effectsAnthropod material medical ingredientsUrinary disorderSalvia miltiorrhizaSide effect
The invention relates to a compound Chinese medicinal preparation for treating IgA (Immunoglobulin A) nephropathy. The compound Chinese medicinal preparation comprises the following Chinese medicinal raw materials by weight: 13-17 grams of astragalus, 8-12 grams of rehmannia root, 8-12 grams of red-rooted salvia root, 8-12 grams of common bletilla pseudobulb, 2-4 grams of cicada shell, 8-12 grams of garden burnet root, 16-24 grams of cogongrass rhizome and 2-4 grams of Chinese caterpillar fungus (brewed). A preparation method of the compound Chinese medicinal preparation comprises the following steps of: cleaning the eight types of medicinal materials; mixing proportionally; adding water in an amount which is 6-8 times the total weight of the medicinal materials or 70 percent by volume of ethanol for decocting for three times, every time for 40 minutes; combining decoction solutions obtained in the three times; filtering; and concentrating to obtain a liquid extract of which the specific weight is 1.21 at 60 DEG C. The compound Chinese medicinal preparation can be prepared into any pharmaceutical dosage form, such as tablets, capsules, granules, oral liquid, pills and syrup with the conventional preparation process. The compound Chinese medicinal preparation prepared with the method has the advantages of great reduction in the concentrations of albuminuria and hematuria of patient suffering from IgA nephropathy, great improvement on the kidney function, remarkable curative effect and great reduction in side effects and untoward reactions.
Owner:INST OF BASIC RES & CLINICAL MEDICINE CHINA ACAD OF CHINESE MEDICAL SCI
Application of lactobacillus reuteri from breast milk to regulation of maternal and infant immune functions
ActiveCN111265553AImprove immunityPromote productionAntibacterial agentsDigestive systemBiotechnologyDisease
The invention discloses application of lactobacillus reuteri from breast milk to regulation of maternal and infant immune functions, and belongs to the field of microbial technology and food science.The invention provides a lactobacillus reuteri Fn041 for enhancing the immunity of females in the gestation period or the lactation period and infants, namely enhancing the mucosal barrier and promoting the production of intestinal antibacterial peptides and IgA (immunoglobulin A). The lactobacillus reuteri Fn041 has the effects of enhancing the development of an infant immune system, preventing pathogenic bacteria infection, and reducing the incidence of allergic diseases and the like.
Owner:FINE HUNAN BIOTECHNOLOGY CO LTD +2
Health-care traditional Chinese medicine scented sachet
InactiveCN103007137AGood killing effectPlay a health effectAntibacterial agentsAntiviralsImmunoglobulin AVirus
The invention provides a health-care traditional Chinese medicine scented sachet, which is characterized in that medicines in the scented sachet do not have the function of directly killing virus and bacteria, but give off persistent aromatic odor, which stimulates the human body to generate secreted immunoglobulin A, and such an antibody has a strong action of killing virus and bacteria, thus achieving the health-care effect.
Owner:徐曼丽
Traditional Chinese medicine sachet
InactiveCN102988859AGood killing effectHas a fragranceDigestive systemDeodrantsBacilliChinese herbology
The invention provides a traditional Chinese medicine sachet. Medicines emit sustained aromatic odor to stimulate the respiratory mucosa of a human body to generate secreted immunoglobulin A, and an antibody plays the great role of killing virus and bacterium, so that microorganisms can not survive on the respiratory mucosa, and further the sachet plays the role of preventing the communicable disease. The traditional Chinese medicine sachet provided by the invention has the functions of faint scent, parasite expelling, acute communicable diseases avoidance, and disease prevention.
Owner:徐曼丽
Preparation method of APP (Actinobacillus Pleuropneumoniae) OMVs (Outer Membrane Vesicles) and vaccine of APP OMVs
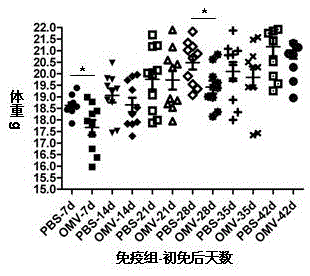
ActiveCN105420161AExperimental evaluation of immunostimulatory effectsAssessing immunostimulatory effectsAntibacterial agentsBacterial antigen ingredientsIMMUNE STIMULANTSFiltration
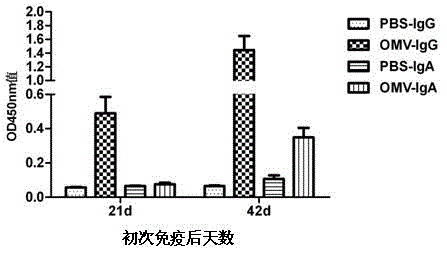
The invention relates to a preparation method of APP (Actinobacillus Pleuropneumoniae) OMVs (Outer Membrane Vesicles) and a vaccine of the APP OMVs, and belongs to the technical field of biology. The preparation method comprises the steps of culturing APP shope strains in vitro by using a culture medium which is in iron ion limit, obtaining acellular cultural supernatant after carrying out centrifugation and 0.22-[mu]m filtration treatment, and preparing the OMVs released by germs through ultracentrifugation, wherein the observation through a transmission electron microscope shows that the diameter of most OMVs is 50 to 100 nm, the OMVs are used as subunit vaccines to carry out secondary intranasal immunization on a mouse, the weight of the OMV immune mouse is increased for a long time, and the visual forms of lungs have no obvious difference from a PBS (Phosphate Buffer Saline) immune group. Meanwhile, an experiment shows that the OMVs are efficient immune stimulants, not only can high-level IgG (Immunoglobulin G) be stimulated to be generated by mouse sera, but also high-level IgA (Immunoglobulin A) can be generated in mouse lungs, mucosal immunity of the mouse lungs is effectively stimulated, and a better application prospect of using the OMVs as the subunit vaccines is expressed.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Novel polypeptide, an affinity chromatography material, and a method for separating and/or purifying immunoglobulin
ActiveUS20120184711A1Easy to collectTake advantage ofPeptide/protein ingredientsDepsipeptidesImmunoglobulin AMutated protein
Owner:NOMADIC BIOSCI
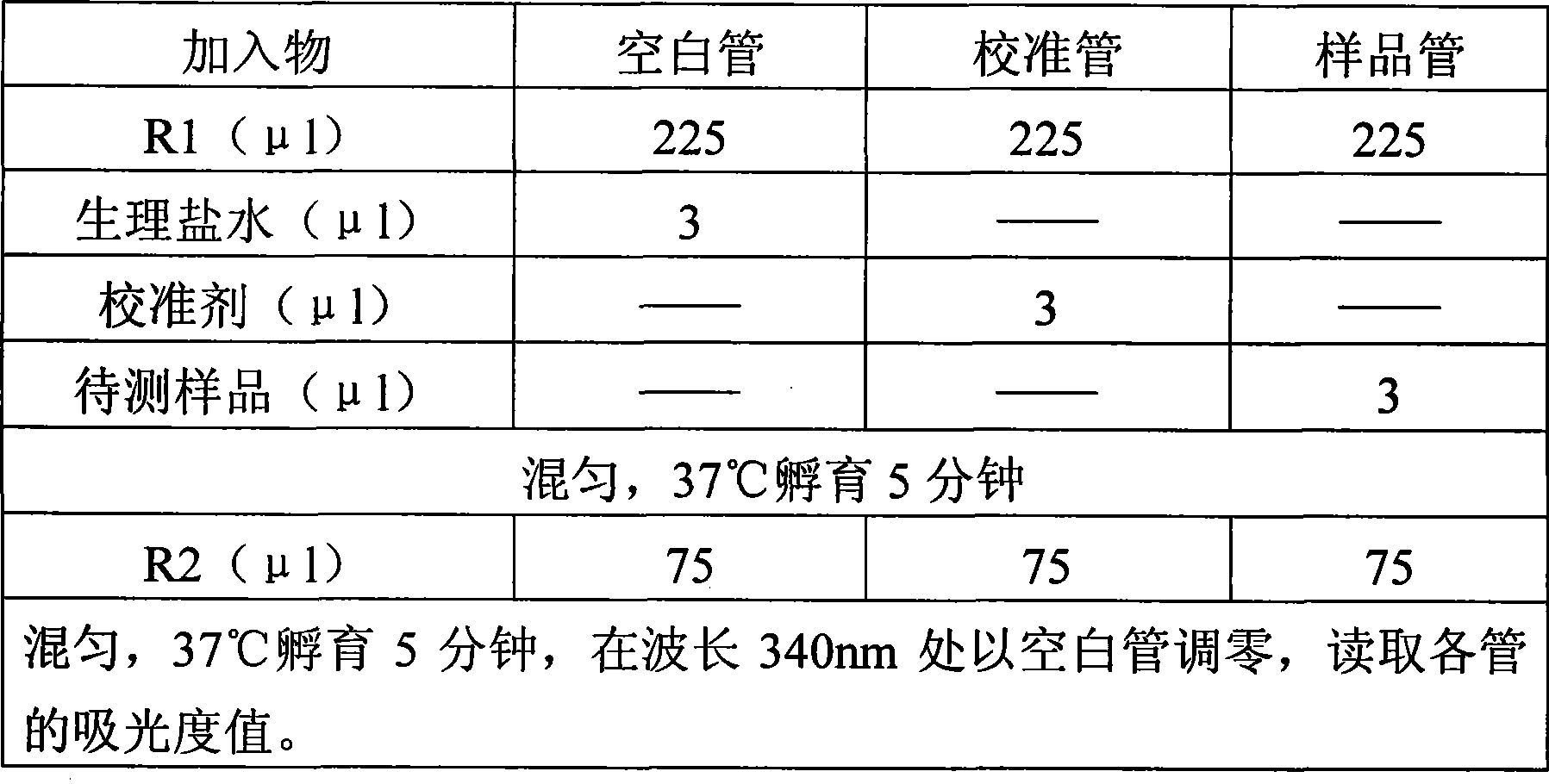
Immunoglobulin A detection kit and detection method thereof
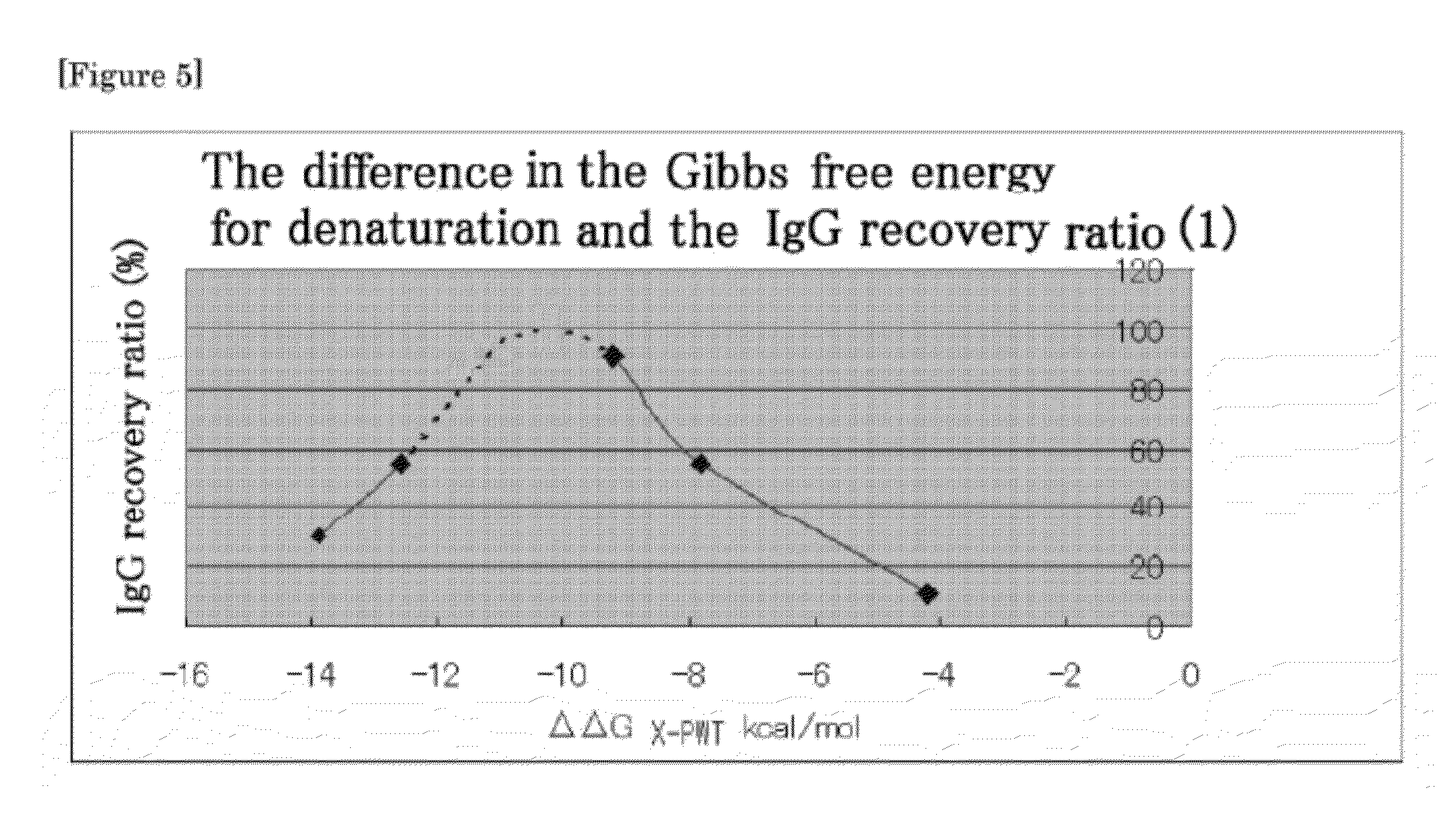
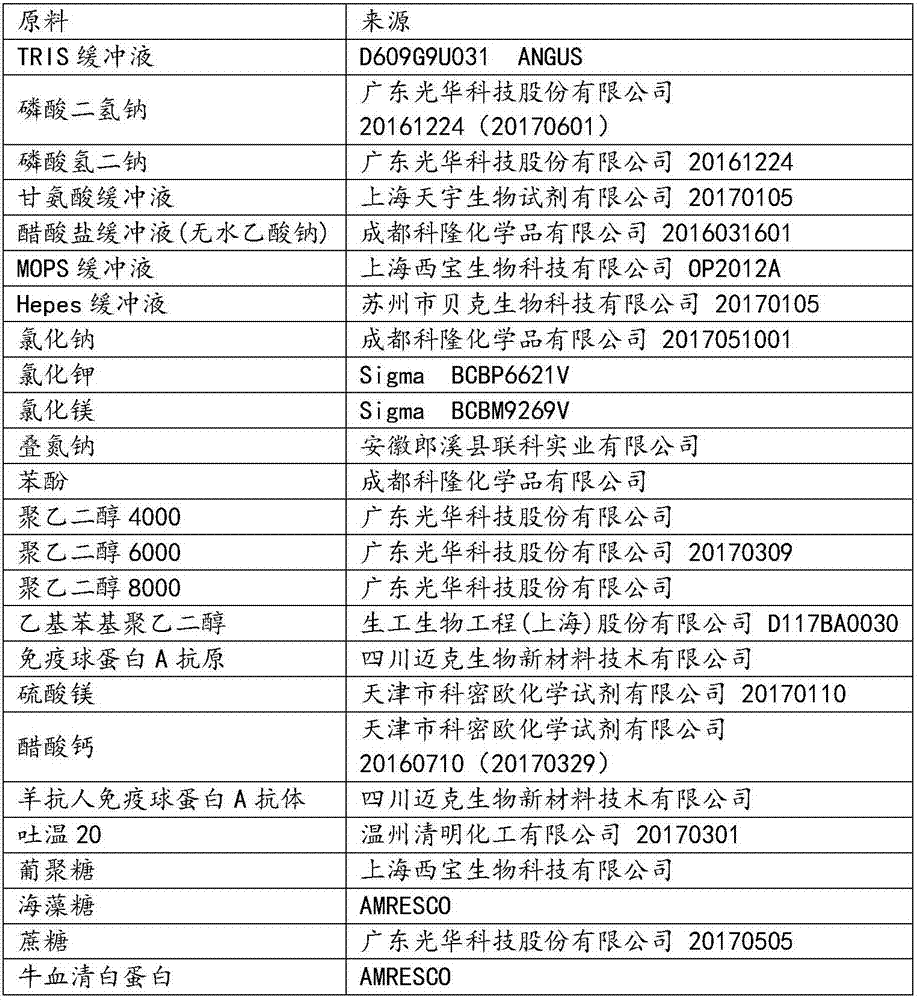
ActiveCN107462731AMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsImmunoglobulin AMagnesium salt
The invention provides an immune turbidity kit which contains a reagent R1 and a reagent R2, wherein the reagent R1 contains ethyl phenyl polyethylene glycol; the reagent R2 contains ethyl phenyl polyethylene glycol, magnesium salt, calcium acetate and an antibody. The kit provided by the invention is good in antibody performance, good in repeatability and accurate in reagent detection result and can meet use requirements.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Traditional Chinese medicine scented sachet for increasing resistance
InactiveCN103007140AGood killing effectImprove the immunityHydroxy compound active ingredientsImmunological disordersBacteroidesBacilli
The invention provides a traditional Chinese medicine scented sachet for increasing resistance. The scented sachet is characterized in that medicines in the scented sachet do not have the function of directly killing virus and bacteria, but give off persistent aromatic odor, which stimulates the respiratory mucosa of the human body to generate secreted immunoglobulin A, and such an antibody has a strong action of killing virus and bacteria, so that these microorganisms can not survive on the upper respiratory mucosa, thus achieving the effect of increasing the resistance.
Owner:徐曼丽
Immune globulin A detection reagent
The invention discloses an immunoglobulin A detection reagent, comprising an IgA reagent, an anti-IgA antibody reagent and a liquid serotype constant value calibration agent; wherein, the IgA reagent enables the IgA antigenic sites in the sample to be fully exposed so as to facilitate the full combination with the anti-IgA antibody reagent; the anti-IgA antibody reagent has high idiosyncrasy with the IgA antigens in human serum; and the liquid serotype constant value calibration agent is compared with the sample for result calculation. The anti-IgA antibodies are from sheep, horses, rats or rabbits and other mammals. The IgA reagent and the anti-IgA antibody reagent can be used as two separate reagents to compose the double-reagent form of the product, and can also be mixed based on a certain percentage to constitute the single-reagent form of the product.
Owner:王贤理
Purification method of human immunoglobulin for intravenous injection
InactiveCN108623677AAccurately control pHGood removal effectPeptide preparation methodsImmunoglobulinsPurification methodsChromatography liquid
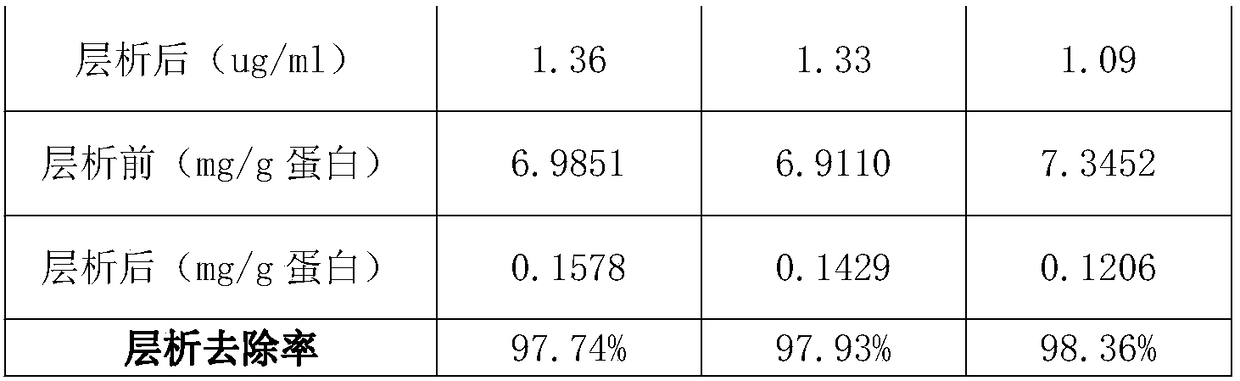
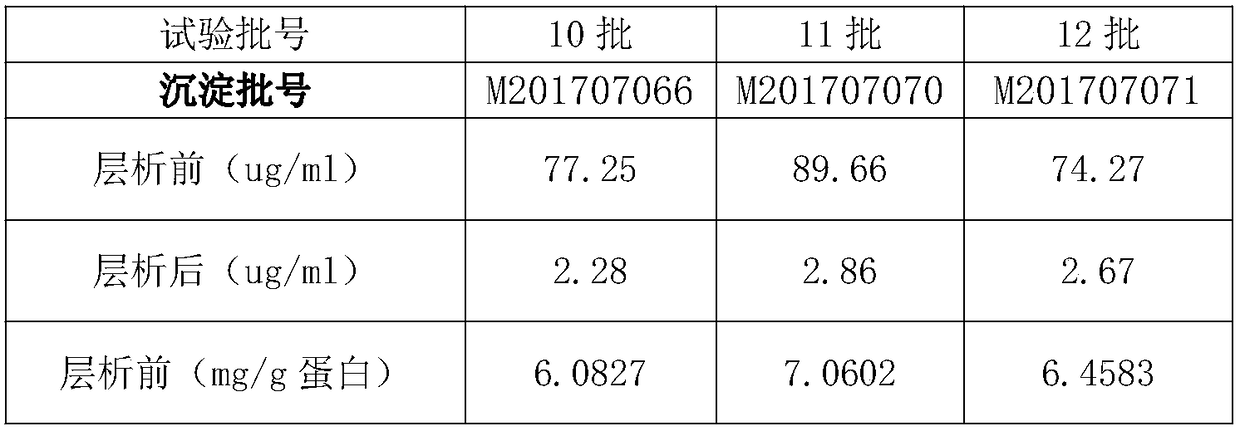
The invention discloses a purification method of human immunoglobulin for intravenous injection. The purification method is used for purification of a secondary sedimentation ingredient. The purification method of the human immunoglobulin for the intravenous injection is characterized by comprising the following steps of: S1, dissolve: dissolving the secondary sedimentation ingredient with water for injection, and stirring for 2-4h at 2.0-8.0 DEG C to form a dissolve liquid, S2, filtration: filtering the dissolve liquid with a 0.45 micrometers filter membrane and then with a 0.2 micrometers filter membrane to form a filtrate, S3: filtrate adjustment: adjusting a pH (potential of hydrogen) of the filtrate to 5.60-6.00, a protein concentration to 10-13g / L, and conductivity to 0.2-1.90ms / cm to form a pre-chromatography liquid, S4, chromatography: performing chromatography with strong anion exchange gel, flushing the gel before the chromatography for balancing to allow a difference betweena pH of the gel and a pH of the liquid before the chromatography to be from -0.10 to 0.10, performing gel chromatography sample loading at a linear flow rate of 0.5-1.5cm / min and chromatography loading capacity of not exceeding 600g / L, and collecting a liquid after the chromatography. The method is simple and controllable, and greatly reduces a content of IgA (immunoglobulin A) and IgM (immunoglobulin M) in the human immunoglobulin for the intravenous injection.
Owner:HUALAN BIOLOGICAL ENG CHONGQING +1
Health feed
InactiveUS20050107303A1Good for healthPromote and maintain good quality fecesAntibacterial agentsBiocideHuman animalImmunoglobulin A
The present invention relates to a method of promoting immunoglobulin A secretion in the mucosal membranes of non-human animals. The method comprises administering a foodstuff comprising glutamine to the non-human animal.
Owner:MARS UK
Health-care traditional Chinese medicine scented sachet
InactiveCN103007138AGood killing effectPlay a health effectAntibacterial agentsAntiviralsImmunoglobulin AVirus
The invention provides a health-care traditional Chinese medicine scented sachet, which is characterized in that medicines in the scented sachet do not have the function of directly killing virus and bacteria, but give off persistent aromatic odor, which stimulates the human body to generate secreted immunoglobulin A, and such an antibody has a strong action of killing virus and bacteria, thus achieving the health-care effect.
Owner:徐曼丽
Traditional Chinese medicine scented sachet for preventing influenza
InactiveCN103007162AGood killing effectFlu preventionAntibacterial agentsPharmaceutical delivery mechanismBacteroidesBacilli
The invention provides a traditional Chinese medicine scented sachet for preventing influenza. The scented sachet is characterized in that medicines in the scented sachet give off persistent aromatic odor, which stimulates the respiratory mucosa of the human body to generate secreted immunoglobulin A, and such an antibody has a strong action of killing virus and bacteria, so that these microorganisms can not survive on the upper respiratory mucosa, thus achieving the effect of preventing infectious diseases. The traditional Chinese medicine scented sachet can effectively prevent influenza.
Owner:徐曼丽
Traditional Chinese medicine scented sachet for preventing influenza
InactiveCN103007119AGood killing effectFlu preventionHydroxy compound active ingredientsAntiviralsMicroorganismImmunoglobulin A
The invention provides a traditional Chinese medicine scented sachet for preventing influenza. The scented sachet is characterized in that medicines in the scented sachet give off persistent aromatic odor, which stimulates the respiratory mucosa of the human body to generate secreted immunoglobulin A, and such an antibody has a strong action of killing virus and bacteria, so that these microorganisms can not survive on the upper respiratory mucosa, thus achieving the effect of preventing infectious diseases. The traditional Chinese medicine scented sachet can effectively prevent influenza.
Owner:徐曼丽
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com