Method for screening 2019-coronavirus and pre-judging severe pneumonia by double indexes
A coronavirus, a new type of technology, applied in the field of immune detection, can solve the problems of inability to effectively distinguish patients, no reports of successful development of IgA detection kits, increase in detection values, etc., and achieve the effect of solving insufficient medical conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
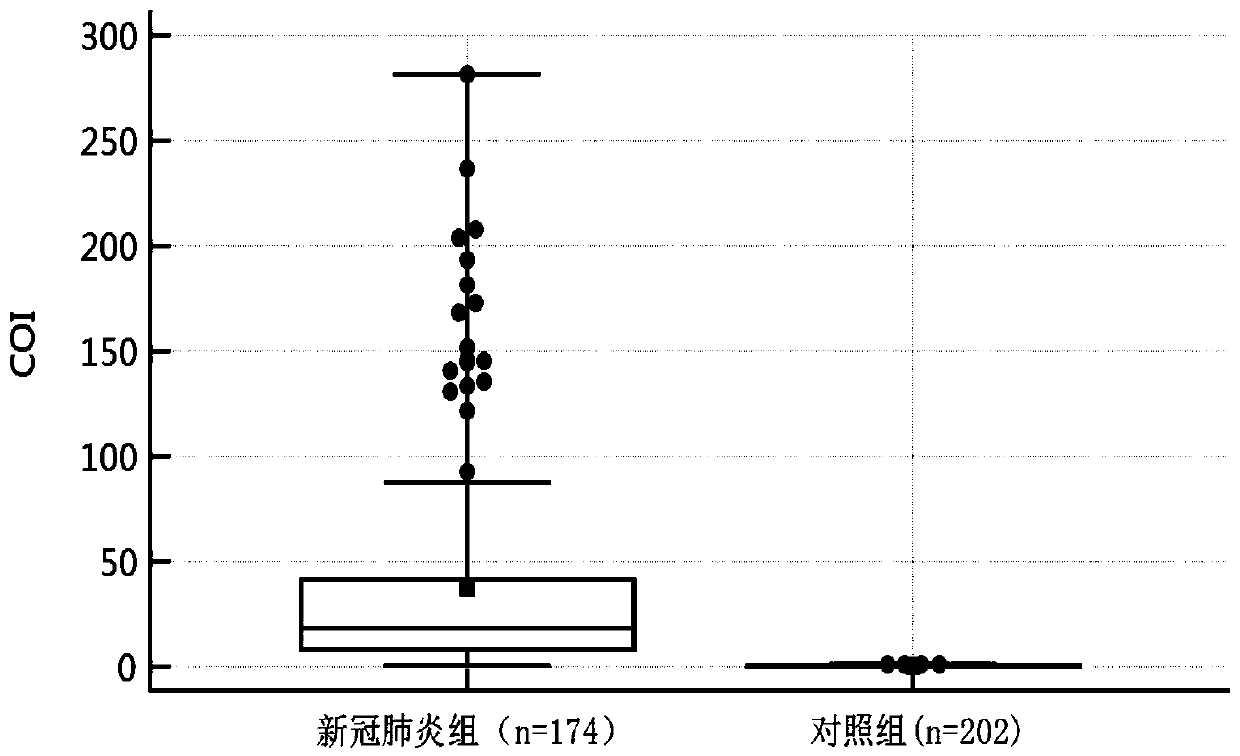
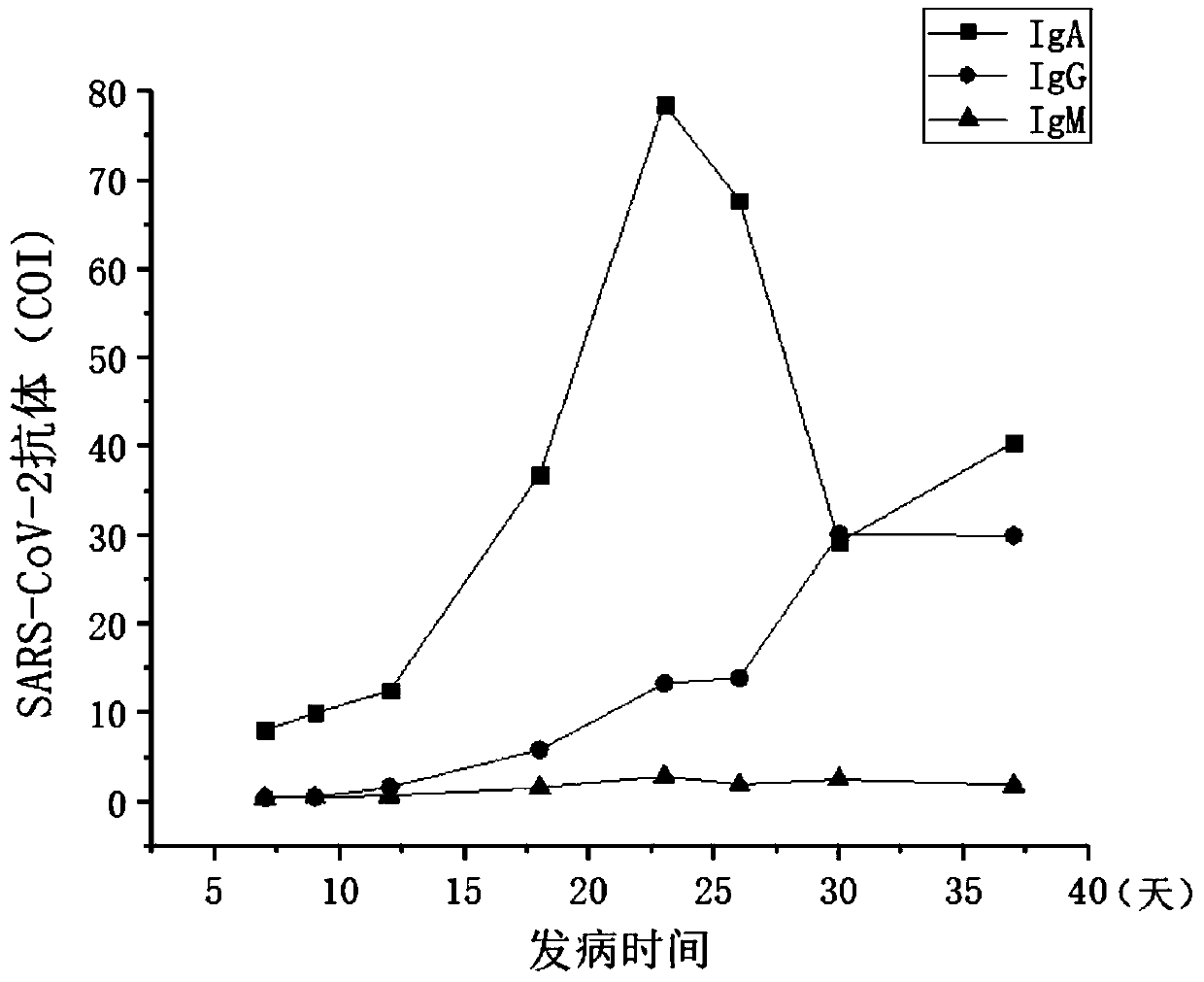
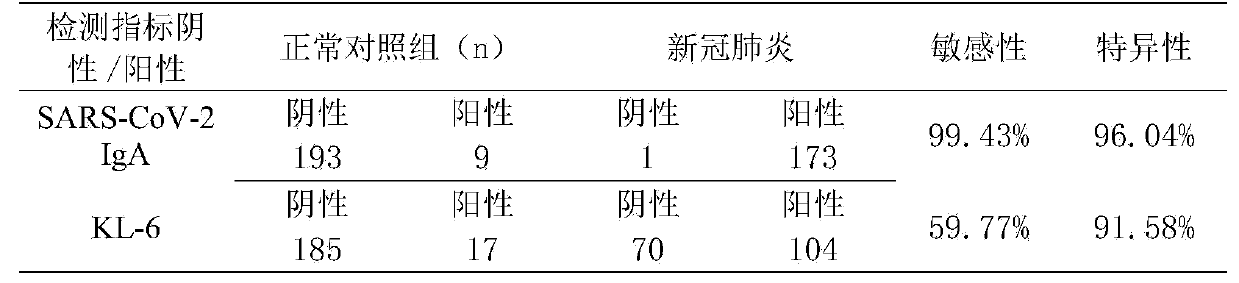
[0036] A detection reagent for detecting the novel coronavirus SARS-CoV-2 IgA antibody in the blood and detecting the sialoligated sugar chain antigen KL-6, which is used for screening novel coronavirus infection pneumonia, especially early screening novel coronavirus infection pneumonia, and The use of detection reagents for predicting severe patients.
[0037] Among them, severe patients are those with lung injury or fibrosis. When a severe patient is detected, it is generally reminded that the patient needs to seek medical treatment in a hospital, which provides the patient with an accurate basis for seeking medical treatment.
[0038] The detection reagent can detect the new coronavirus SARS-CoV-2 IgA antibody and sialoligated sugar chain antigen KL-6 in serum, plasma and whole blood.
[0039] The reagent of the present invention can simultaneously detect the novel coronavirus SARS-CoV-2 IgA antibody and the sialoglycosylated sugar chain antigen KL-6 in the blood, and can...
Embodiment 2
[0041] A detection reagent, which contains a detection reagent for detecting the new coronavirus SARS-CoV-2 IgA antibody in blood and a detection reagent for detecting the sialoligated sugar chain antigen KL-6, is used for early screening of new coronavirus infection pneumonia, and A kit for predicting severe patients. Among them, severe patients are those with lung injury or fibrosis. When a severe patient is detected, it is generally reminded that the patient needs to seek medical treatment in a hospital, which provides the patient with an accurate basis for seeking medical treatment. Among them, the detection reagent can detect the new coronavirus SARS-CoV-2 IgA antibody and the sialoligated sugar chain antigen KL-6 in serum, plasma and whole blood.
[0042] The kit contains test card, sample diluent, pipette, etc.
[0043] Test card, the test card is composed of SARS-CoV-2 IgA test strips, KL-6 test strips and plastic box, each test strip contains nitrocellulose membrane...
Embodiment 3
[0080] Using N protein as a kit for magnetic bead-coated antigens to detect 12 normal human sera, 5 mild cases and 5 severe cases of new coronary pneumonia patients with onset of 2-8 days as an example, the detection operation process is as follows:
[0081] 1. Equilibrate the samples to be tested, test reagents and other test materials to room temperature before testing, and the test should be carried out at room temperature. Note: Room temperature refers to a temperature of 10°C to 30°C and a humidity of 45% to 75%.
[0082] 2. Unscrew the dropper of the dropper, draw the sample to be tested with a straw, add a drop of the sample to be tested into the dropper for dilution (1:10 dilution), tighten the dripper, shake up and down, and mix well.
[0083] 3. Tear open along the incision of the aluminum foil bag, take out the test card and place it flat on the table (note: do not touch the surface of the interlayer film of the test strip with your fingers).
[0084] 4. Open the b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com