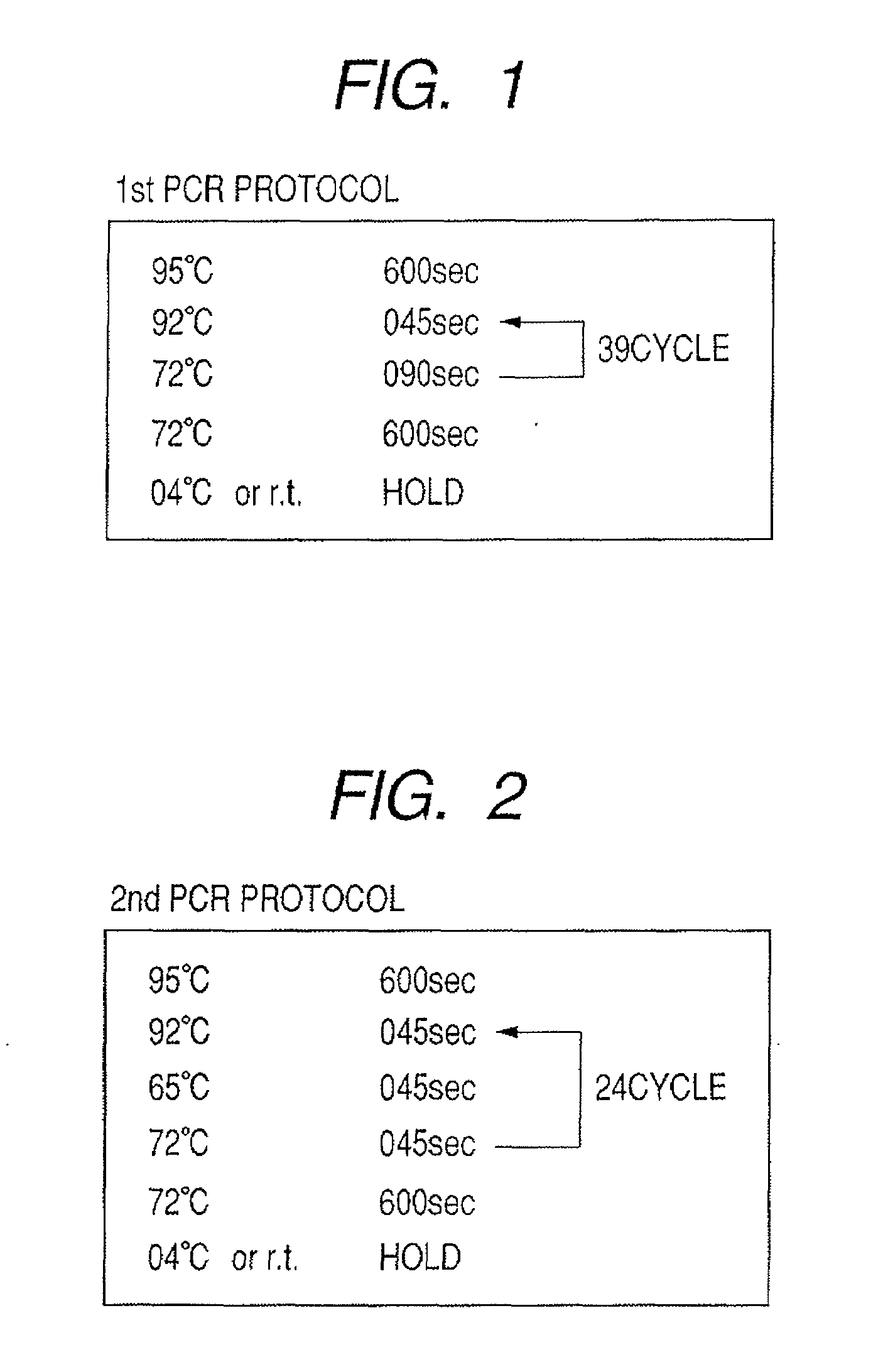
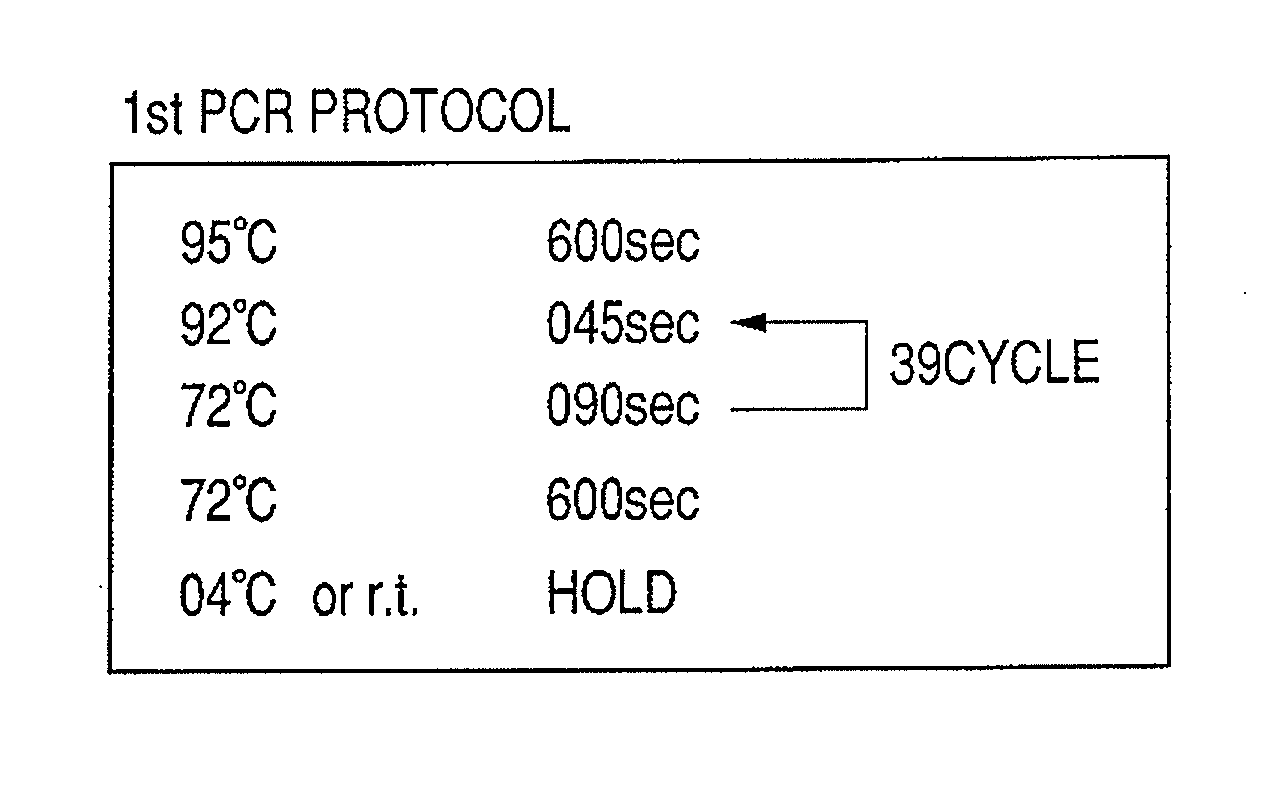
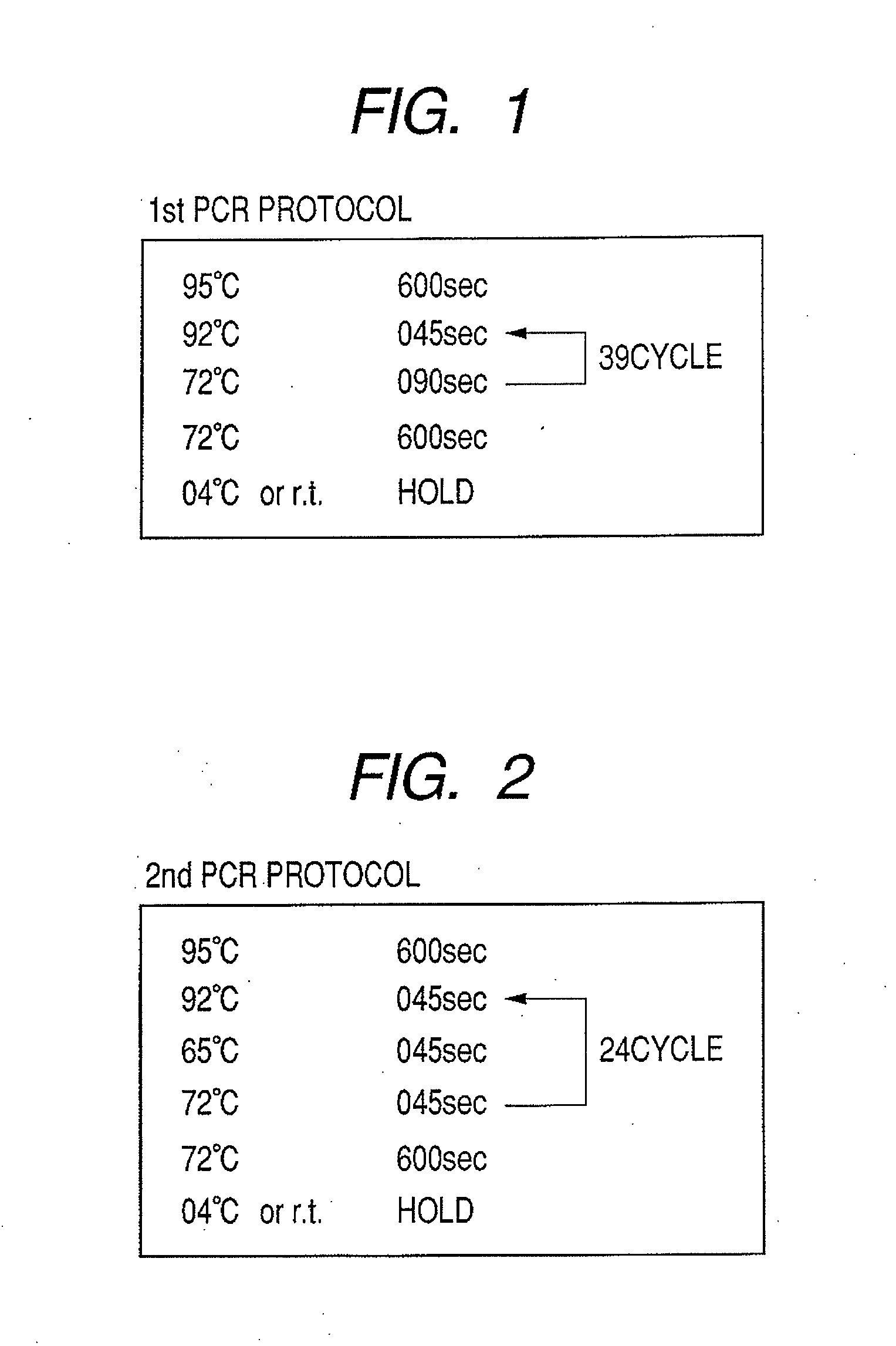
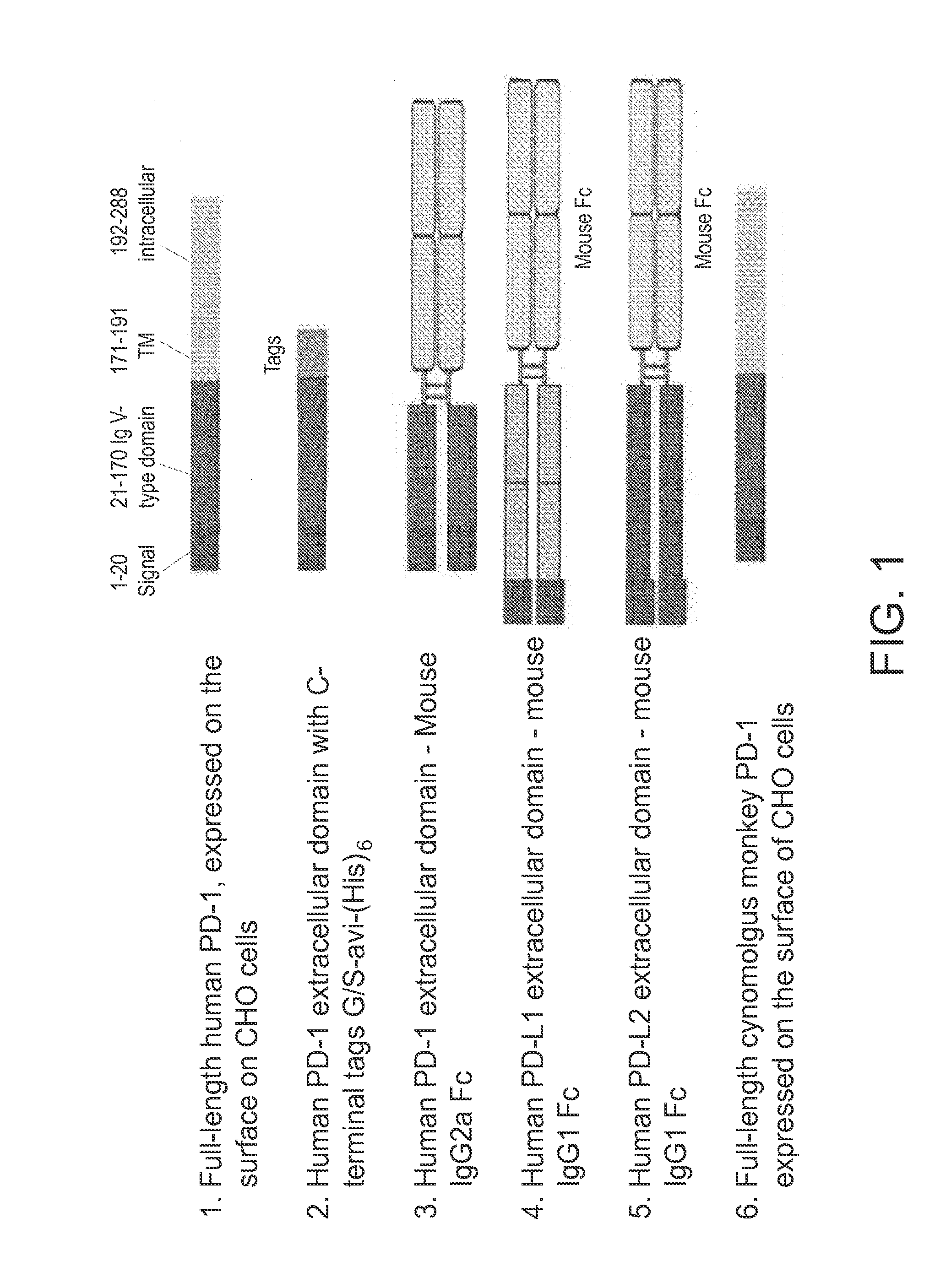
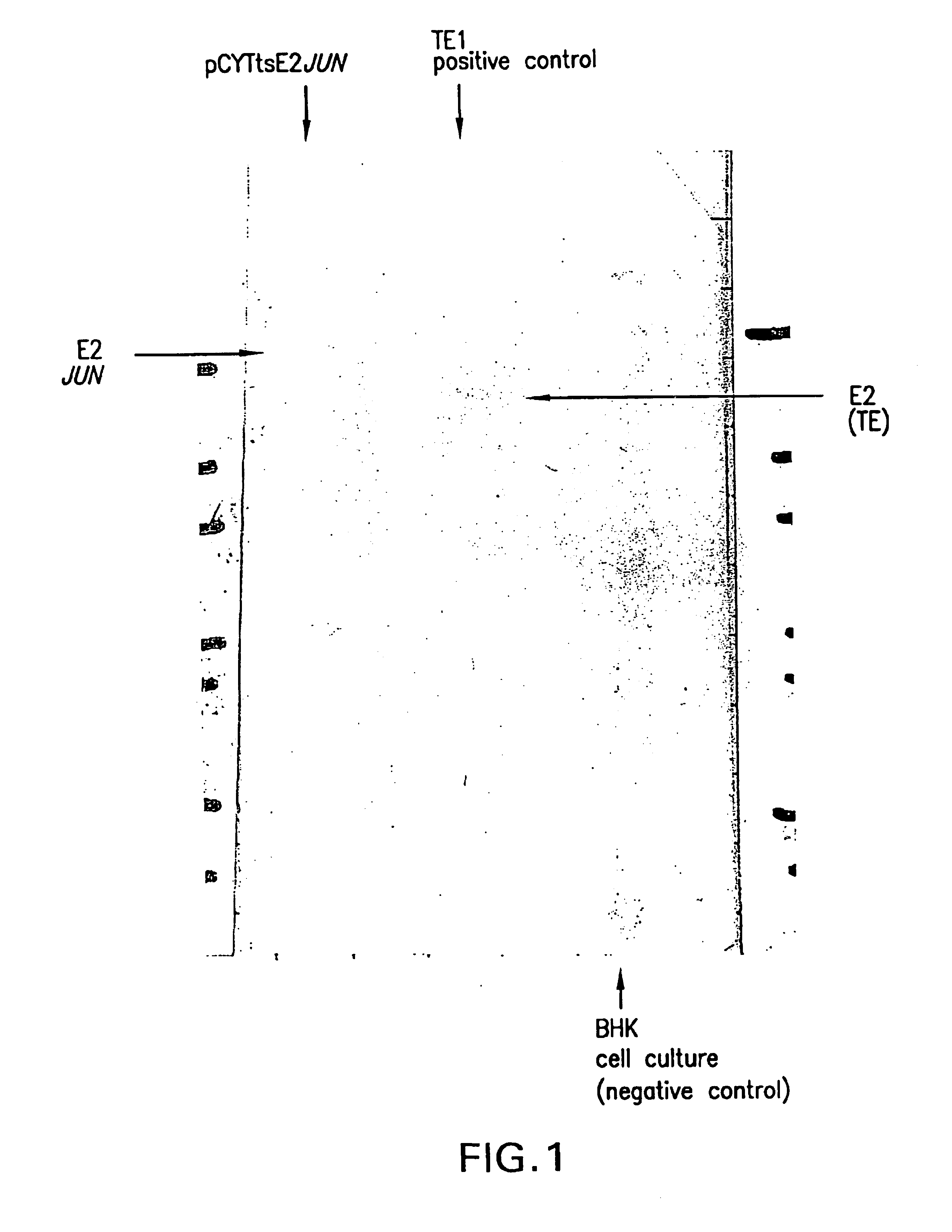
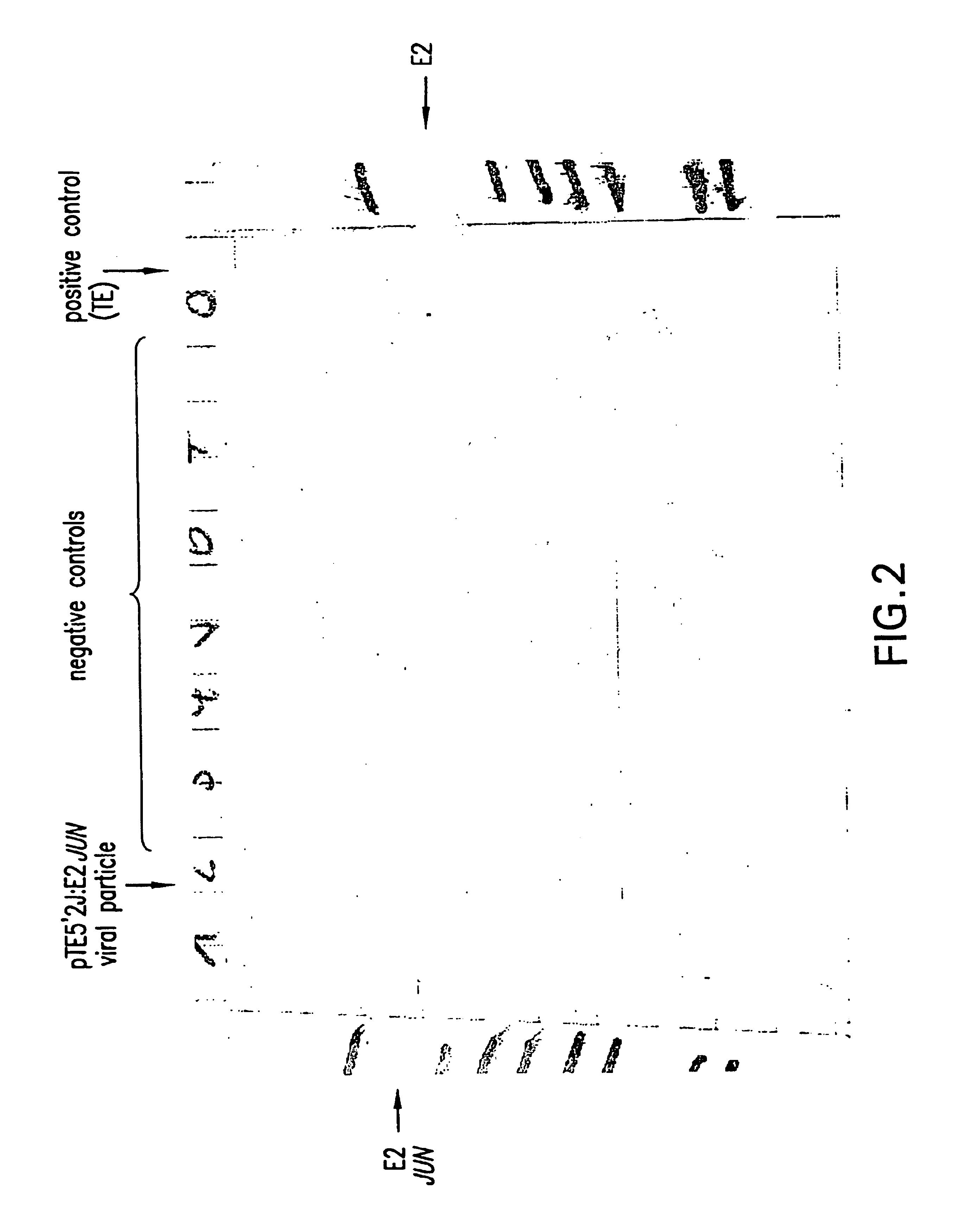
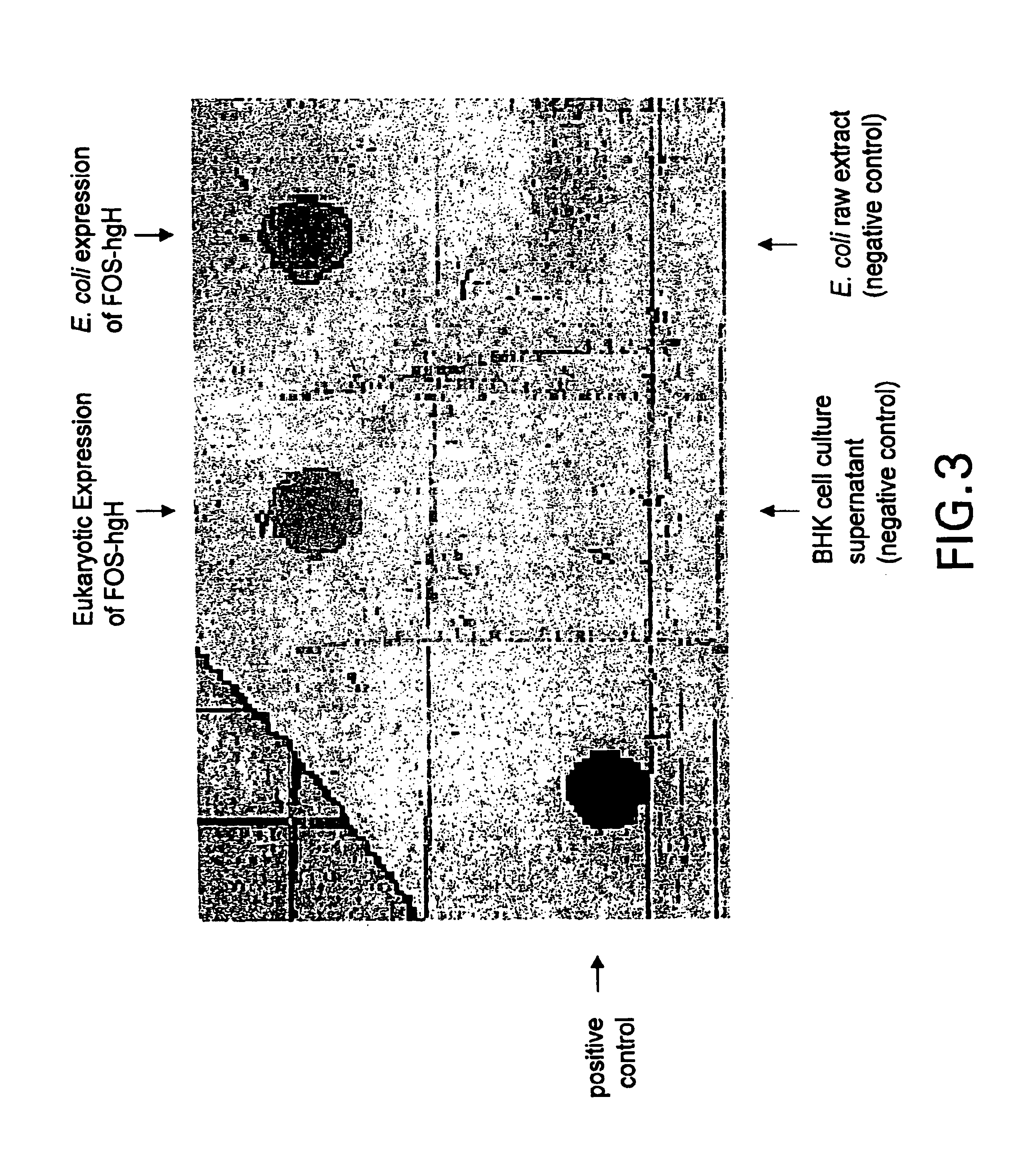
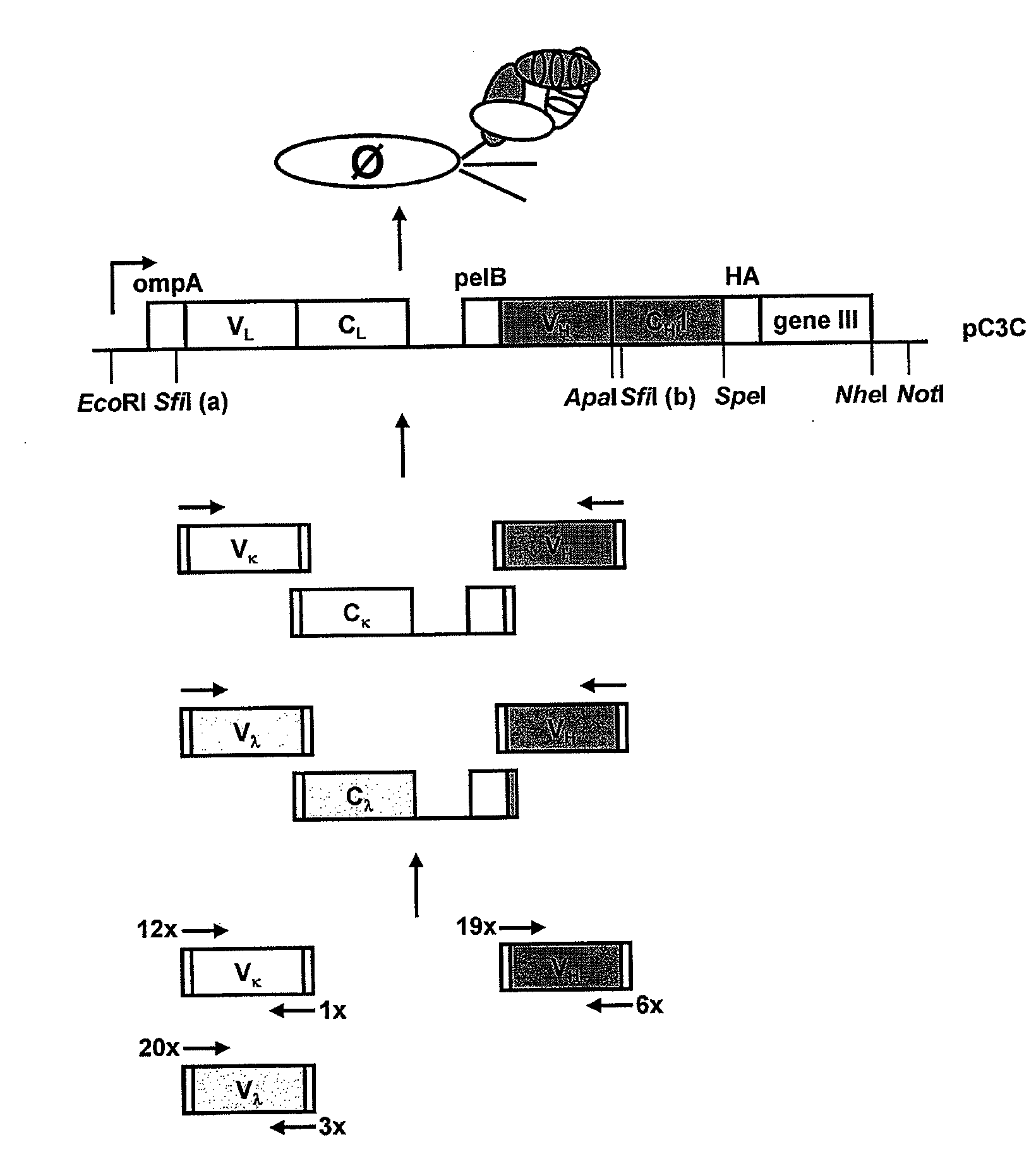
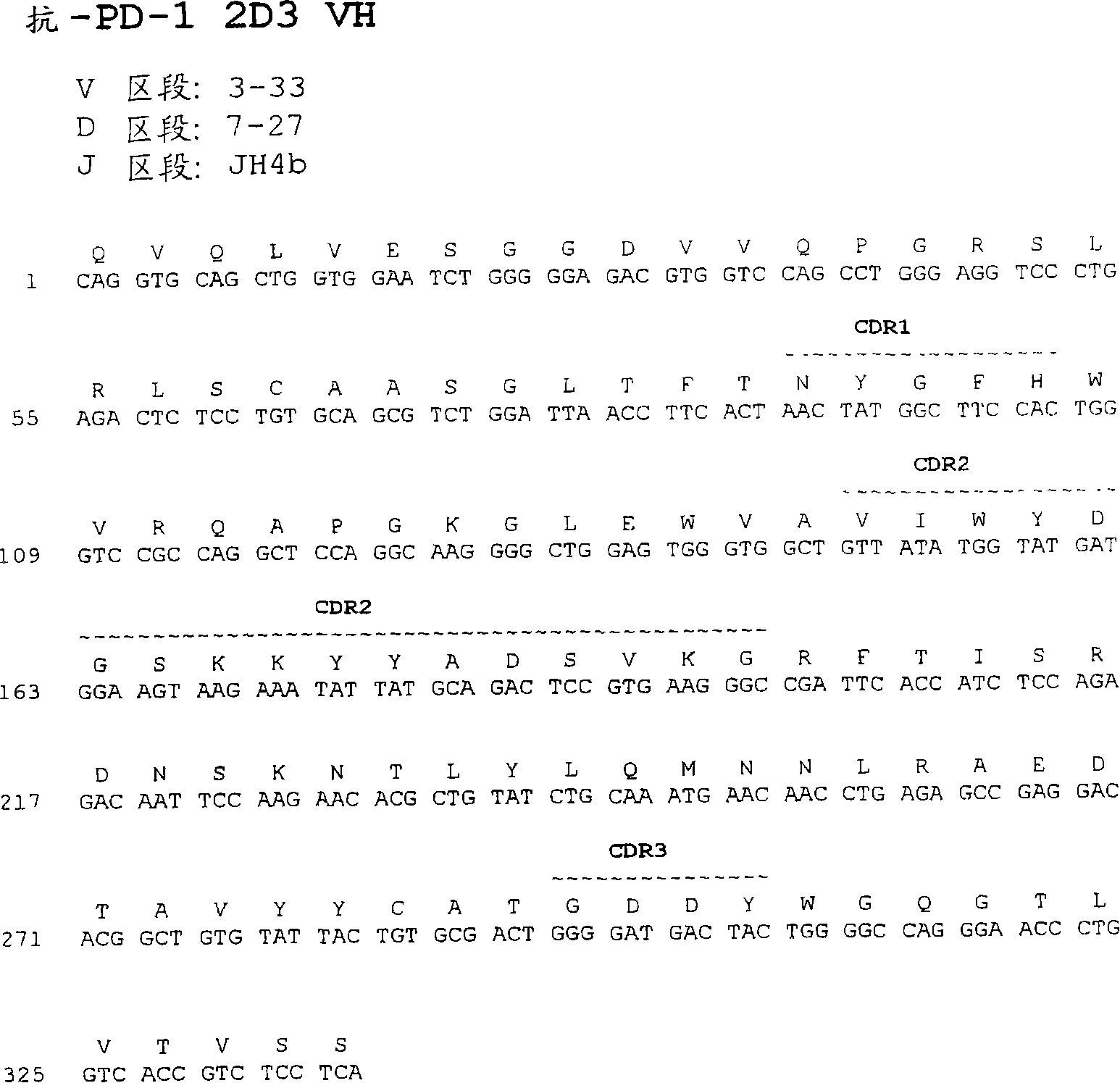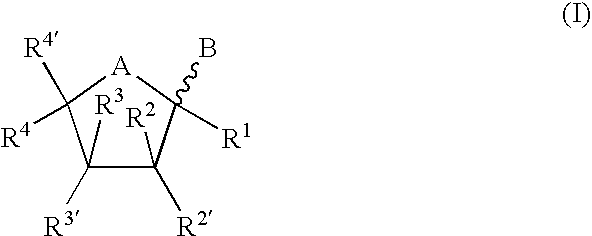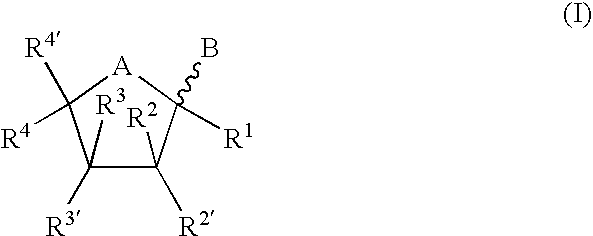Patents
Literature
930 results about "Infectious Disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infectious disorders alsoinfectious disease is a clinically evident condition resulting from the presence of pathogenic microbial agents, including viruses, bacteria, fungi, protozoa, multicellular parasites, and aberrant proteins known as prions. These pathogens are able to cause disease in animals and/or plants.
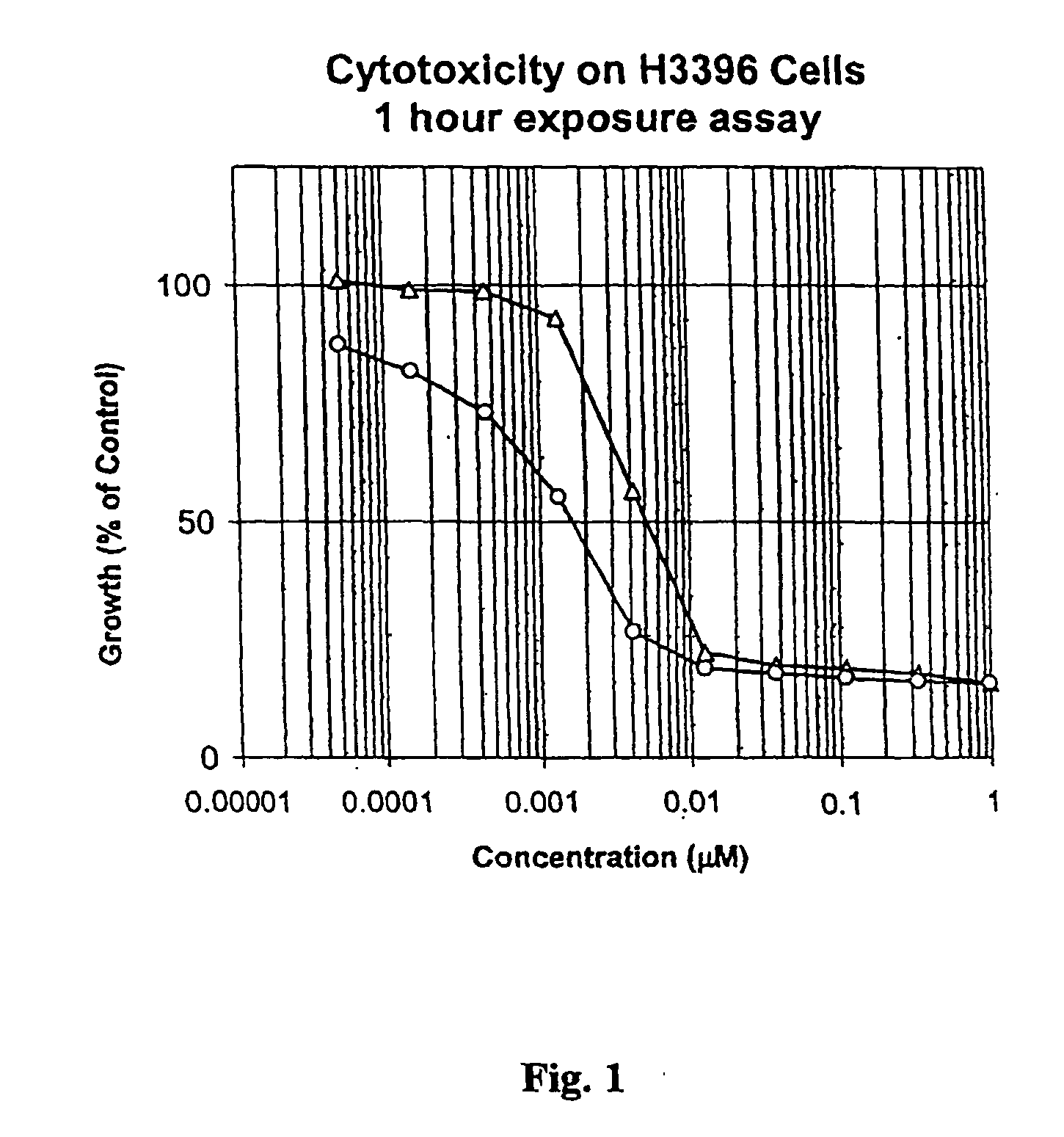
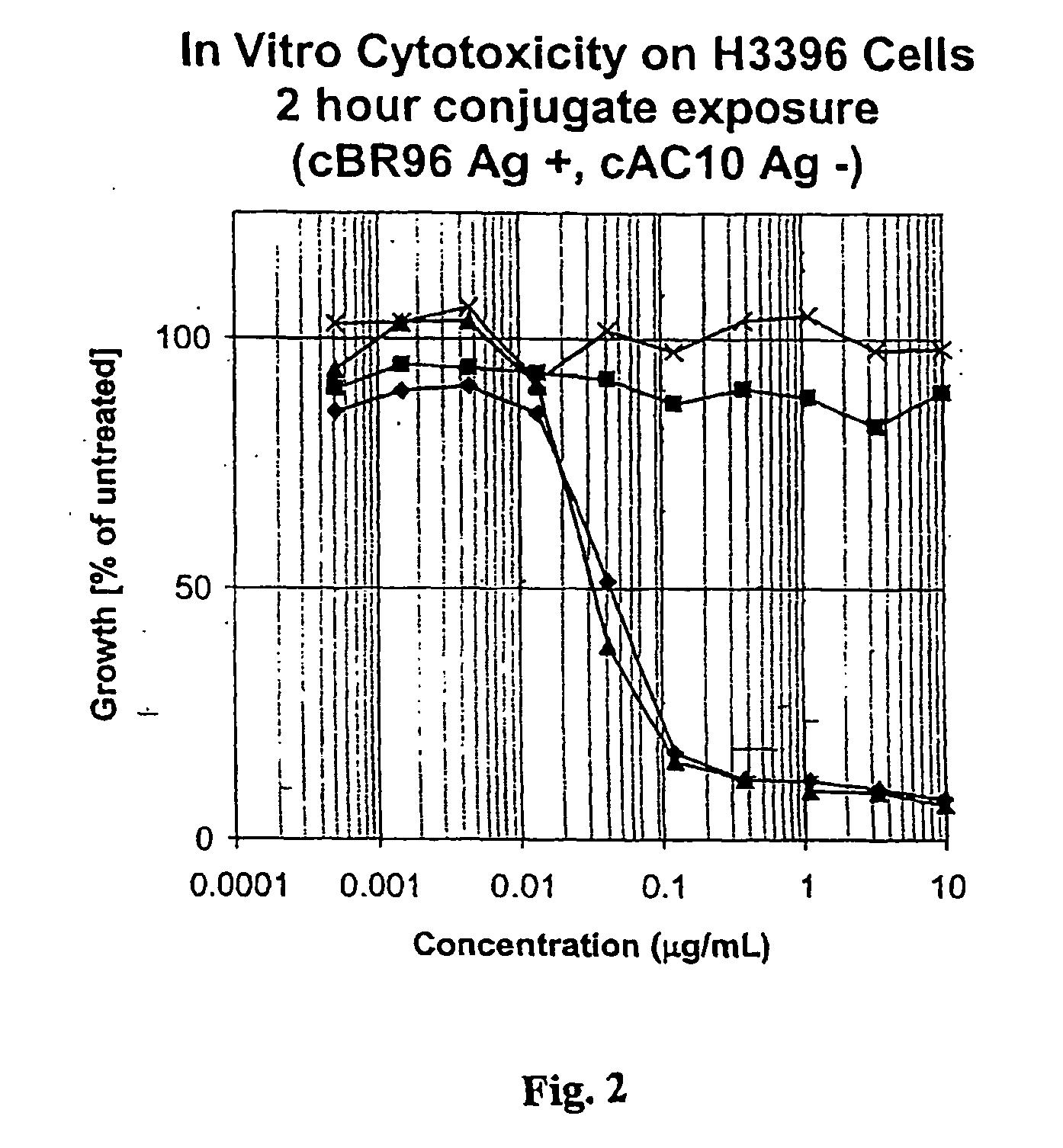
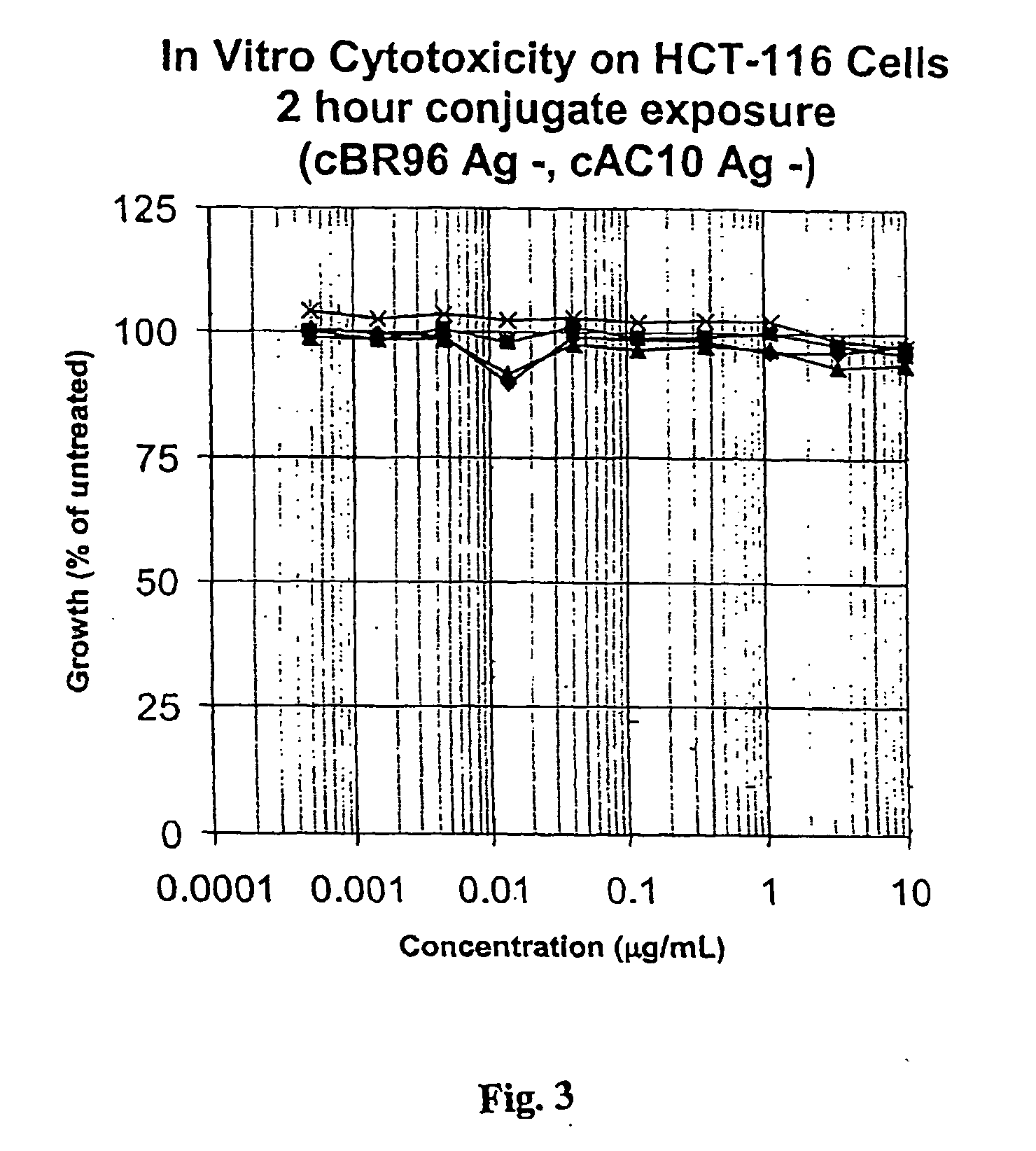
Drug conjugates and their use for treating cancer, an autoimmune disease or an infectious disease
Drug-Linker-Ligand Conjugates are disclosed in which a Drug is linked to a Ligand via a peptide-based Linker unit. In one embodiment, the Ligand is an Antibody. Drug-Linker compounds and Drug compounds are also disclosed. Methods for treating cancer, an autoimmune disease or an infectious disease using the compounds and compositions of the invention are also disclosed.
Owner:SEAGEN INC
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20050037000A1High affinityAltered affinityAntibacterial agentsSenses disorderTherapeutic antibodyWild type
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcgammaRIIA and / or FcgammaRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcgammaR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MARCOGENICS INC +1
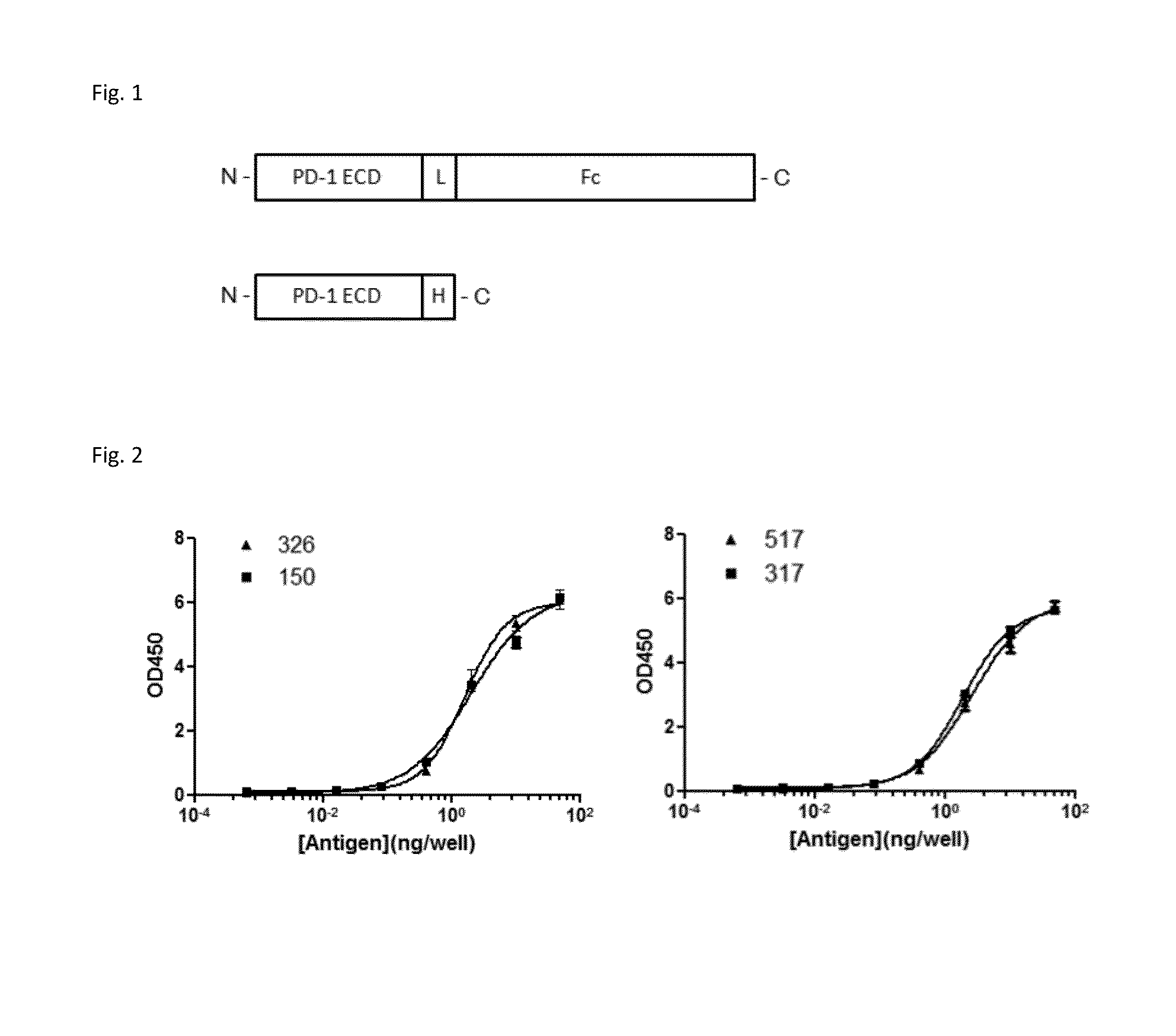
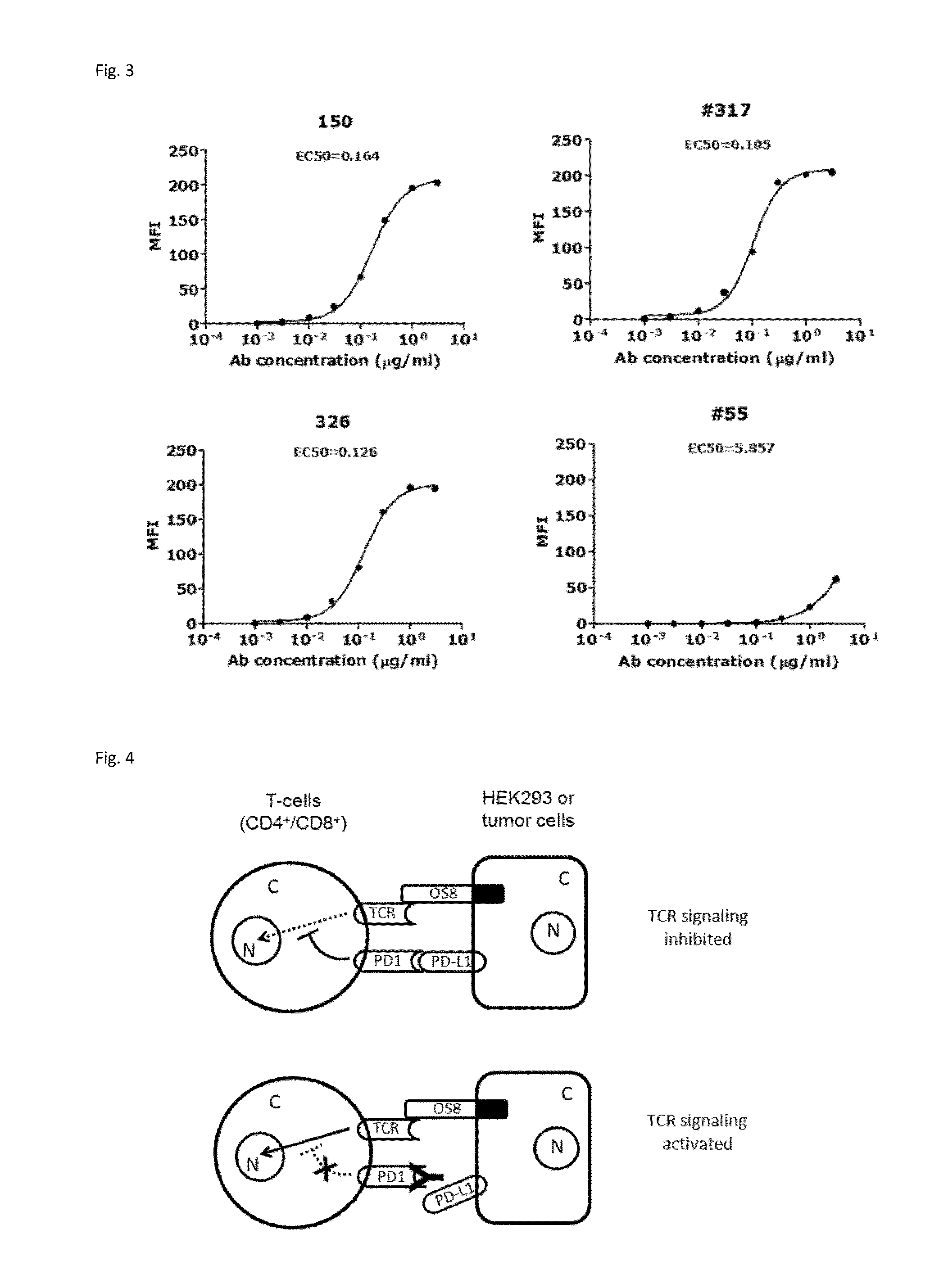
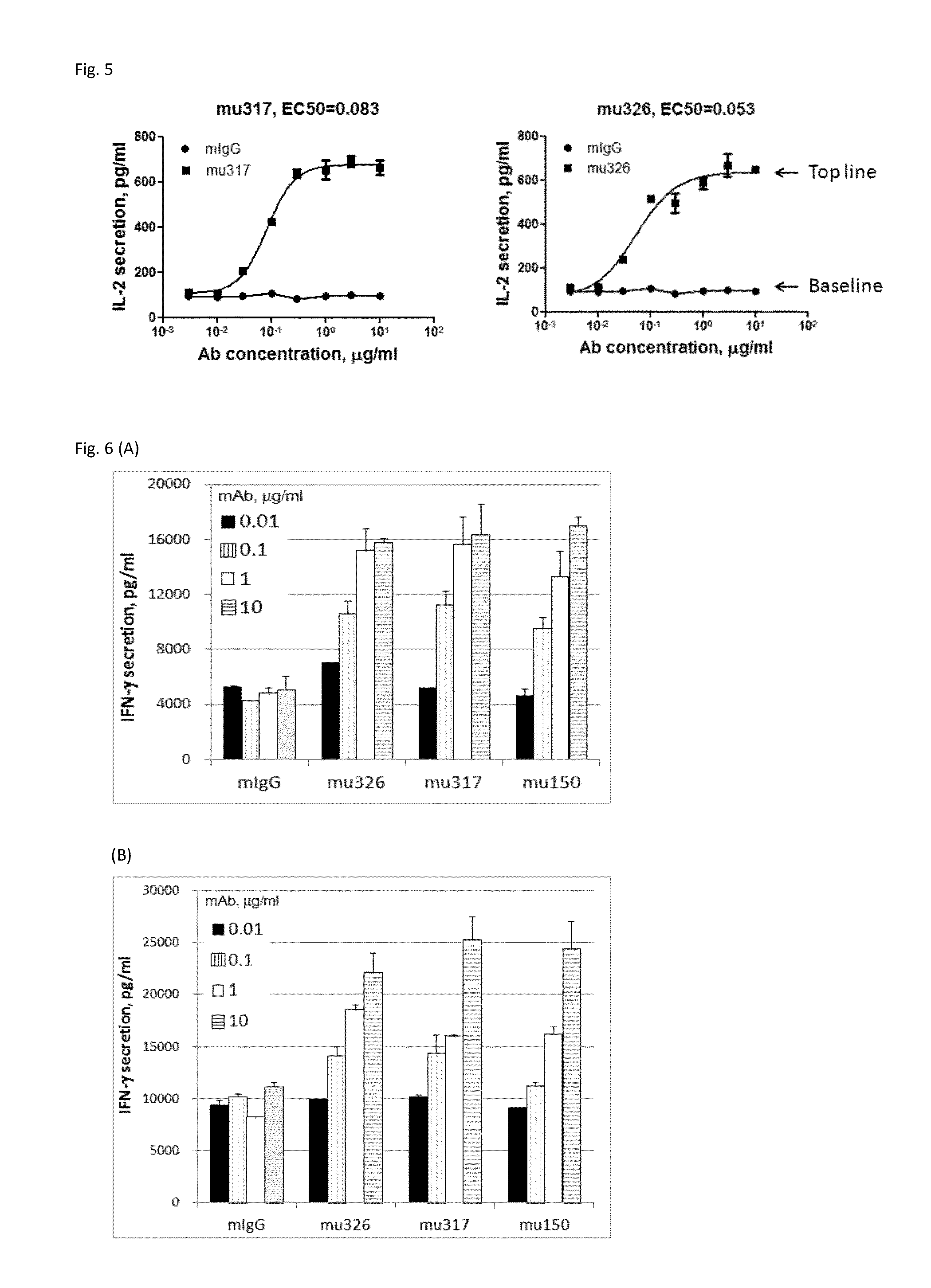
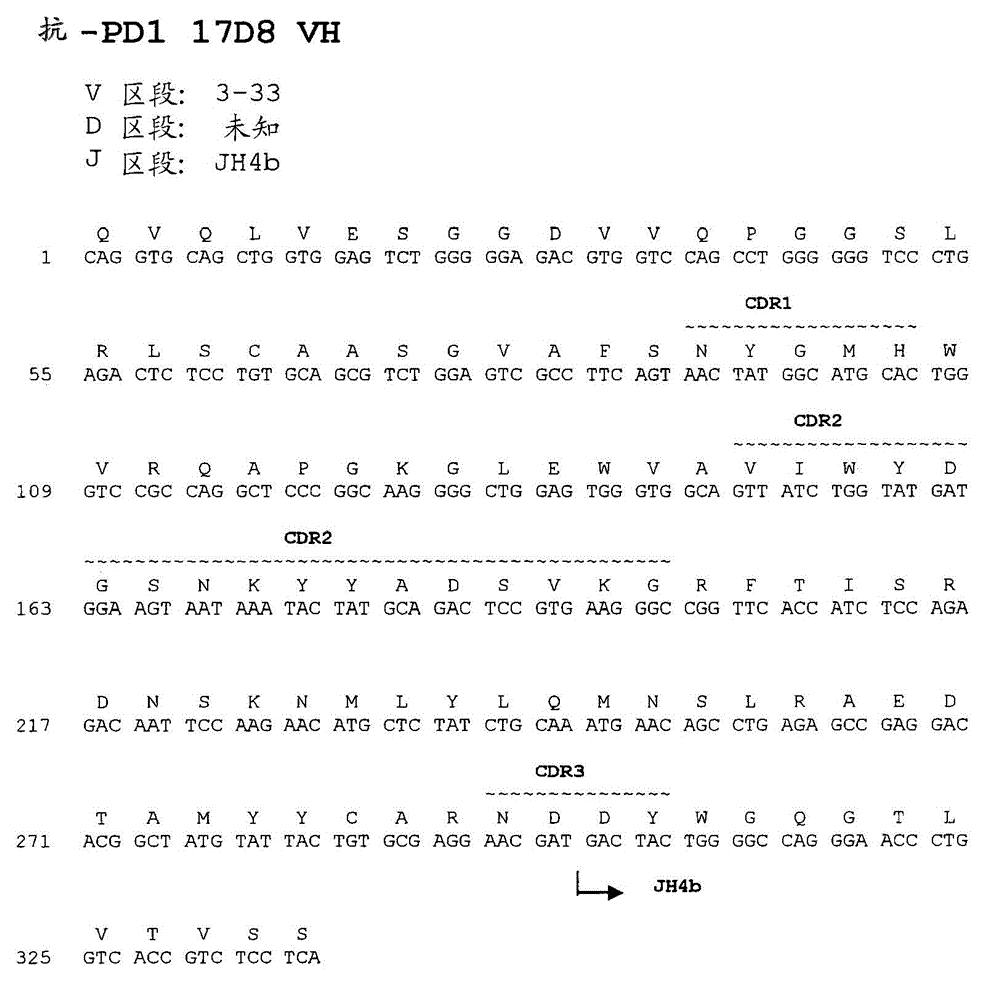
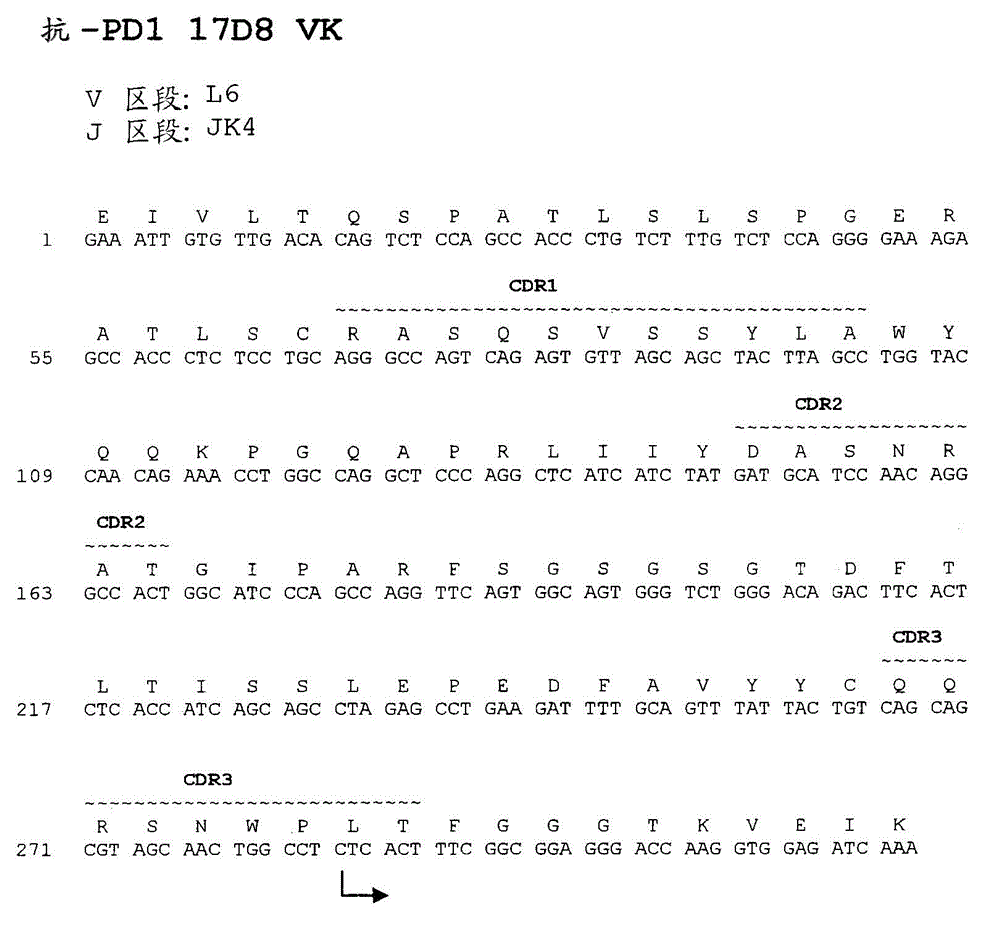
Anti-PD1 Antibodies and their Use as Therapeutics and Diagnostics
ActiveUS20150079109A1Improve the level ofHigh expressionNervous disorderAntiviralsAntiendomysial antibodiesInfectious Disorder
Provided are antibodies that specifically bind to Programmed Death-1 (PD1, Pdcd-1, or CD279) and inhibit PD1-mediated cellular signaling and activities in immune cells, antibodies binding to a set of amino acid residues required for its ligand binding, and uses of these antibodies to treat or diagnose cancer, infectious diseases or other pathological disorders modulated by PD1-mediated functions.
Owner:BEIGENE SWITZERLAND GMBH
Integrated health data capture and analysis system
ActiveUS20110093249A1Mitigate spreadReduce transmissionMedical simulationMedical data miningOutbreakChronic disease
The present invention provides an integrated health care surveillance and monitoring system that provides real-time sampling, modeling, analysis, and recommended interventions. The system can be used to monitor infectious and chronic diseases. When faced with outbreak of an infectious disease agent, e.g., influenza virus, the system can identify active cases through pro-active sampling in high risk locations, such as schools or crowded commercial areas. The system can notify appropriate entities, e.g., local, regional and national governments, when an event is detected, thereby allowing for proactive management of a possible outbreak. The system also predicts the best response for deployment of scarce resources.
Owner:LABRADOR DIAGNOSTICS LLC
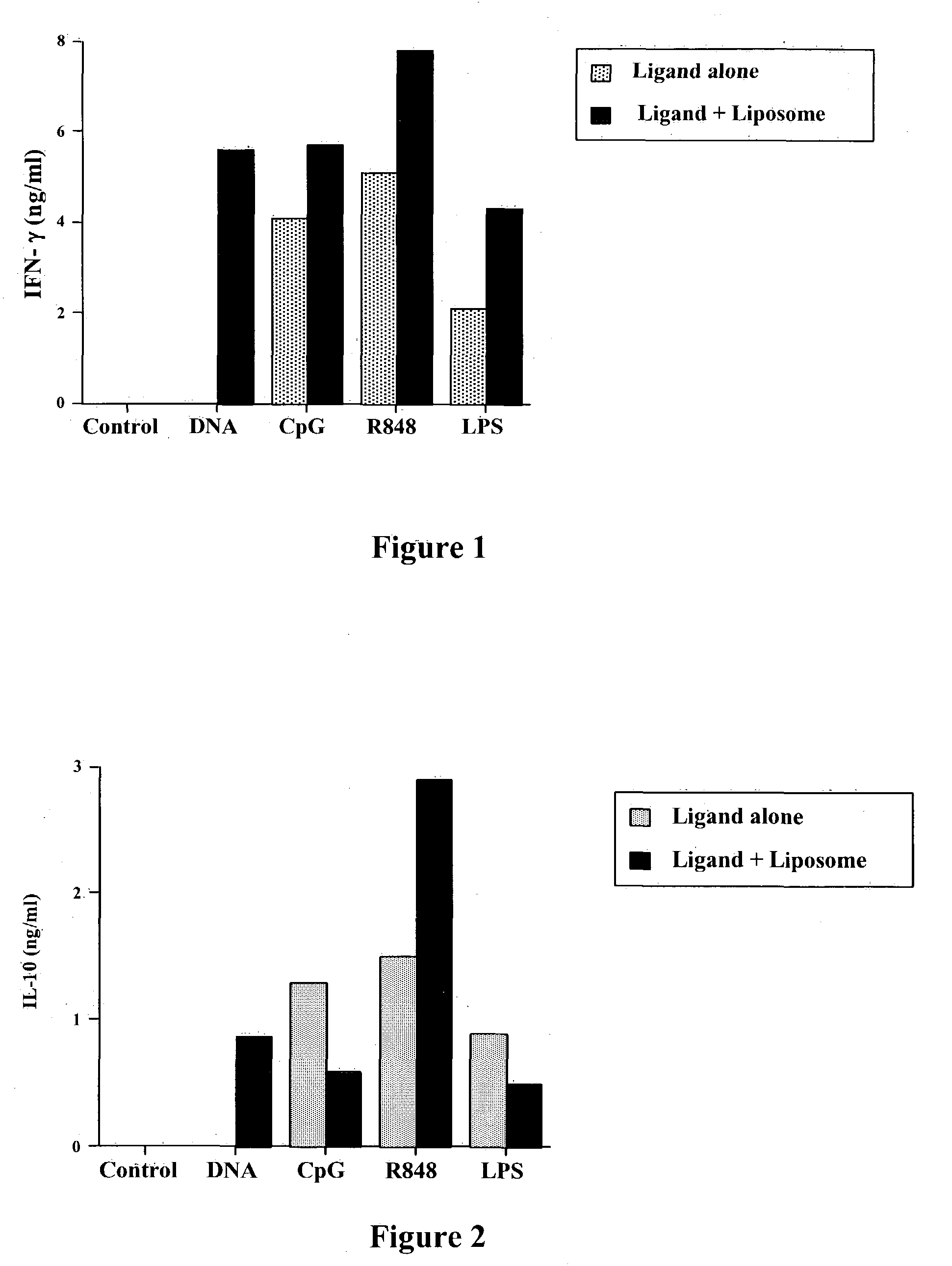
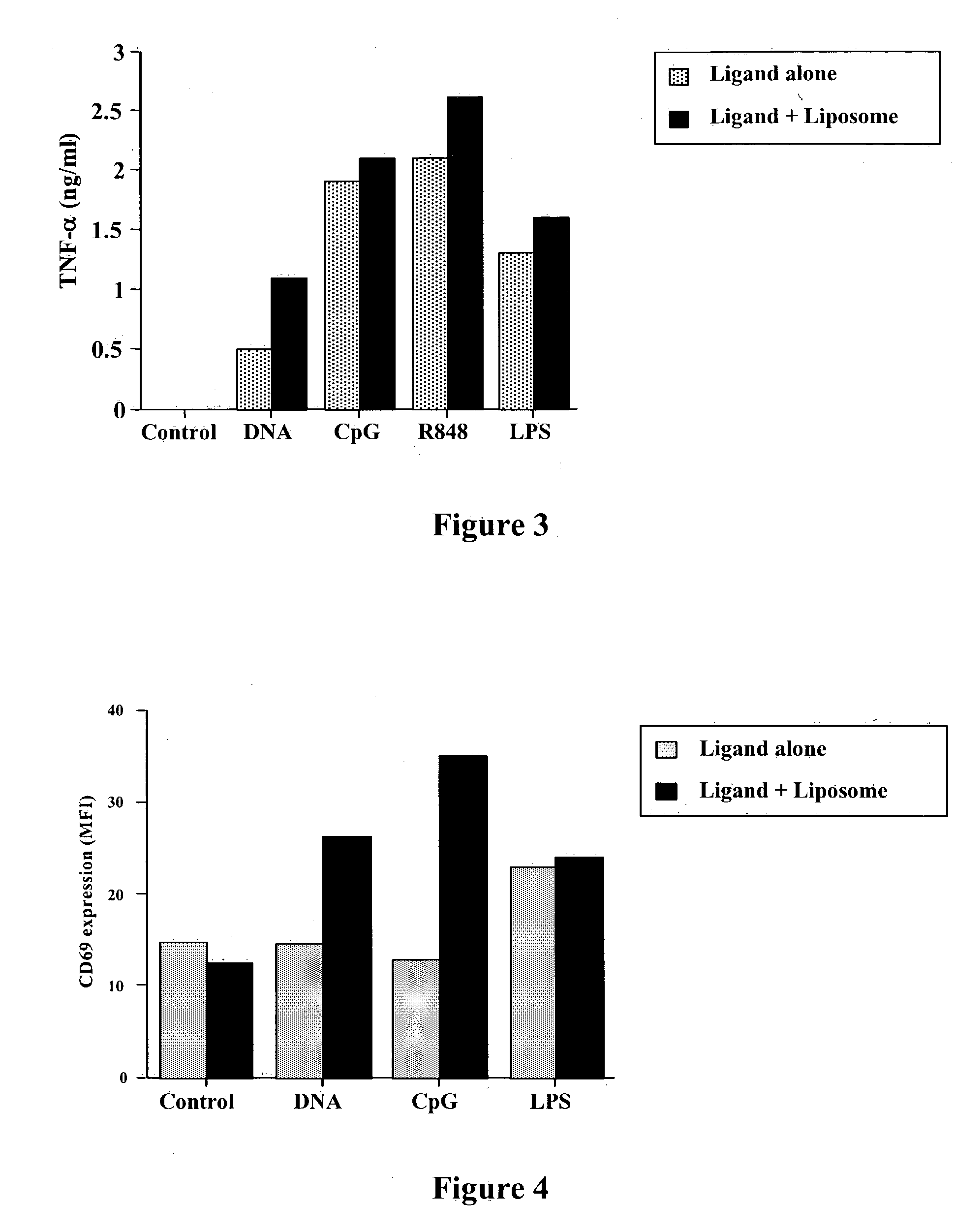
Vaccines using pattern recognition receptor-ligand:lipid complexes
InactiveUS20050013812A1Efficient activationEffective efficacyBacterial antigen ingredientsSkeletal disorderLipid formationInfectious Disorder
This invention relates to a vaccine and a method for immune activation which is effective for eliciting both a systemic, non-antigen specific immune response and a strong antigen-specific immune response in a mammal. The method is particularly effective for protecting a mammal from a disease including cancer, a disease associated with allergic inflammation, an infectious disease, or a condition associated with a deleterious activity of a self-antigen. Also disclosed are therapeutic compositions useful in such a method.
Owner:COLORADO STATE UNIVERSITY
Adjuvant in the form of a lipid-modified nucleic acid
The present invention relates to an immune-stimulating adjuvant in the form of a lipid-modified nucleic acid, optionally in combination with further adjuvants. The invention relates further to a pharmaceutical composition and to a vaccine, each containing an immune-stimulating adjuvant according to the invention, at least one active ingredient and optionally a pharmaceutically acceptable carrier and / or further auxiliary substances and additives and / or further adjuvants. The present invention relates likewise to the use of the pharmaceutical composition according to the invention and of the vaccine according to the invention for the treatment of infectious diseases or cancer diseases. Likewise, the present invention includes the use of the immune-stimulating adjuvant according to the invention in the preparation of a pharmaceutical composition for the treatment of cancer diseases or infectious diseases.
Owner:CUREVAC GMBH
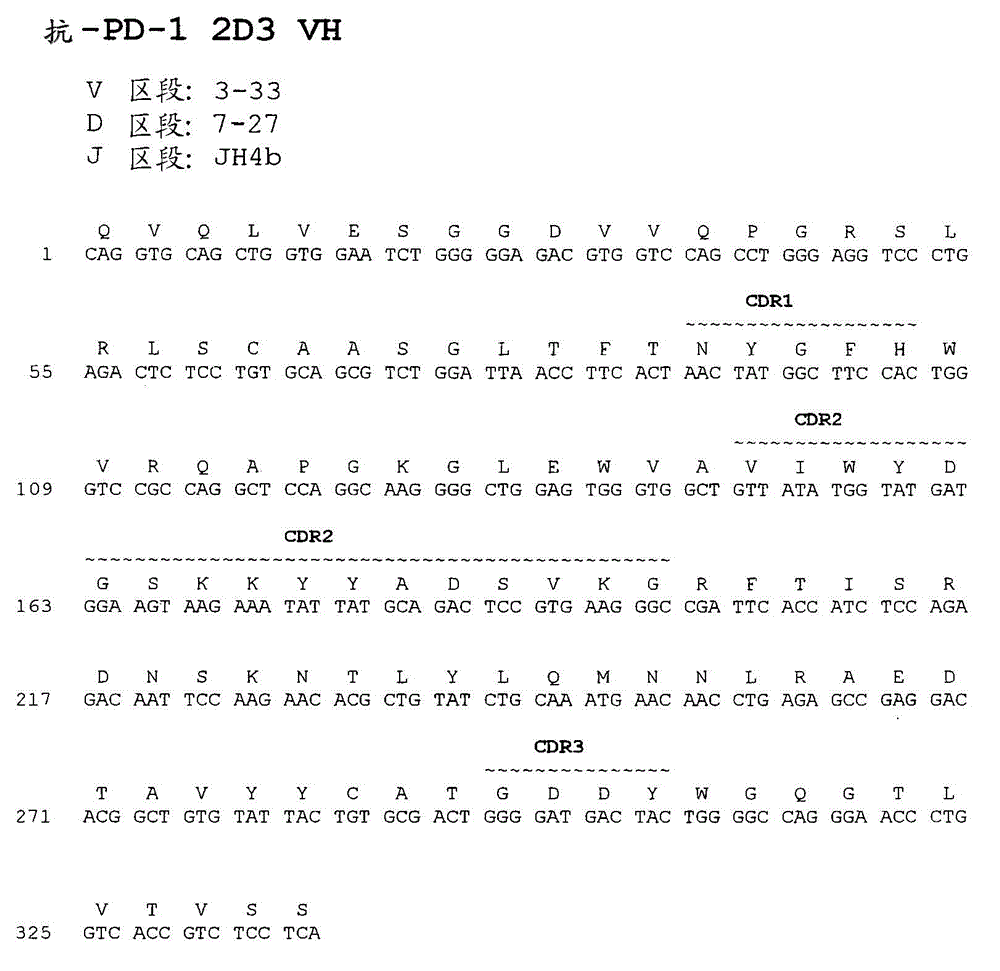
Human monoclonal antibodies to programmed death 1 (PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics
ActiveCN101213297AMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsImmunotherapeutic agentProgrammed death
The present invention provides isolated monoclonal antibodies, particularly human monoclonal antibodies, that specifically bind to PD-1 with high affinity. Nucleic acid molecules encoding the antibodies of the invention, expression vectors, host cells and methods for expressing the antibodies of the invention are also provided. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for detecting PD-1, as well as methods for treating various diseases, including cancer and infectious diseases, using anti-PD-1 antibodies. The present invention further provides methods for using a combination immunotherapy, such as the combination of anti-CTLA-4 and anti-PD-1 antibodies, to treat hyperproliferative disease, such as cancer. The invention also provides methods for altering adverse events related to treatment with such antibodies individually.
Owner:ONO PHARMA CO LTD +1
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS8217147B2High affinityLow affinityAntibody mimetics/scaffoldsImmunoglobulinsDiseaseTherapeutic antibody
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcγRIIIA and / or FcγRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcγR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MACROGENICS INC
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20080113365A1Quickly and precisely detectAccurate identificationBioreactor/fermenter combinationsBiological substance pretreatmentsInfectious DisorderNucleic Acid Probes
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 76 to 77 and complementary or modified sequences thereof or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20080113363A1Quickly and precisely identifiedAccurate distinctionSugar derivativesMicrobiological testing/measurementInfectious DisorderNucleic Acid Probes
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 70 to 72 and complementary or modified sequences thereof or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
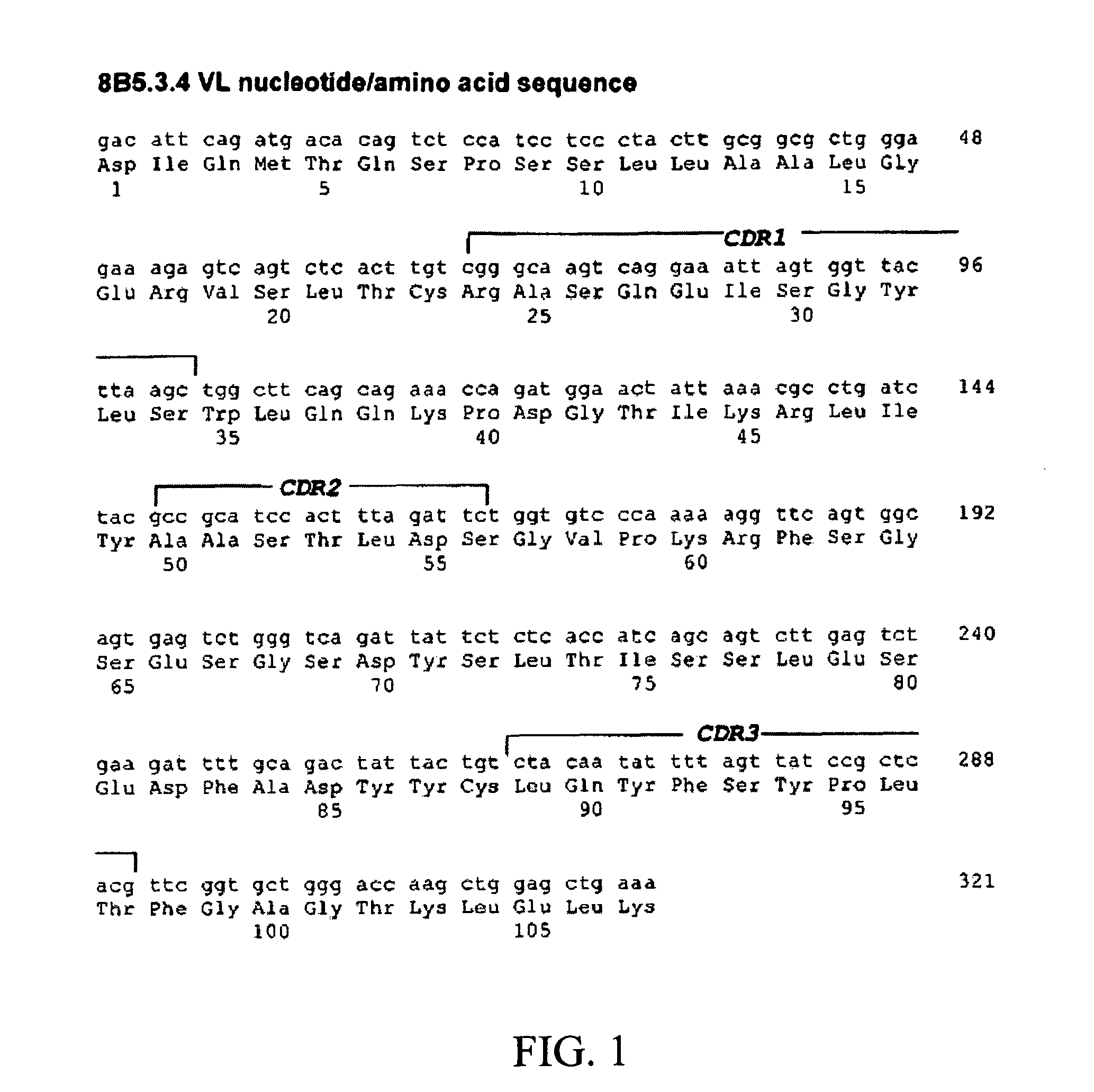
Antibodies directed against programmed death-1 (pd-1)
The invention relates to an isolated immunoglobulin heavy chain polypeptide and an isolated immunoglobulin light chain polypeptide that bind to a programmed death-1 (PD-1) protein. The invention provides a PD-1-binding agent that comprises the aforementioned immunoglobulin heavy chain polypeptide and immunoglobulin light chain polypeptide. The invention also provides related vectors, compositions, and methods of using the PD-1-binding agent to treat a cancer or an infectious disease.
Owner:ANAPTYSBIO INC
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20080113366A1Quickly and precisely detectAccurate identificationBioreactor/fermenter combinationsBiological substance pretreatmentsInfectious DisorderNucleic Acid Probes
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 68 to 69 and complementary or modified sequences thereof or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
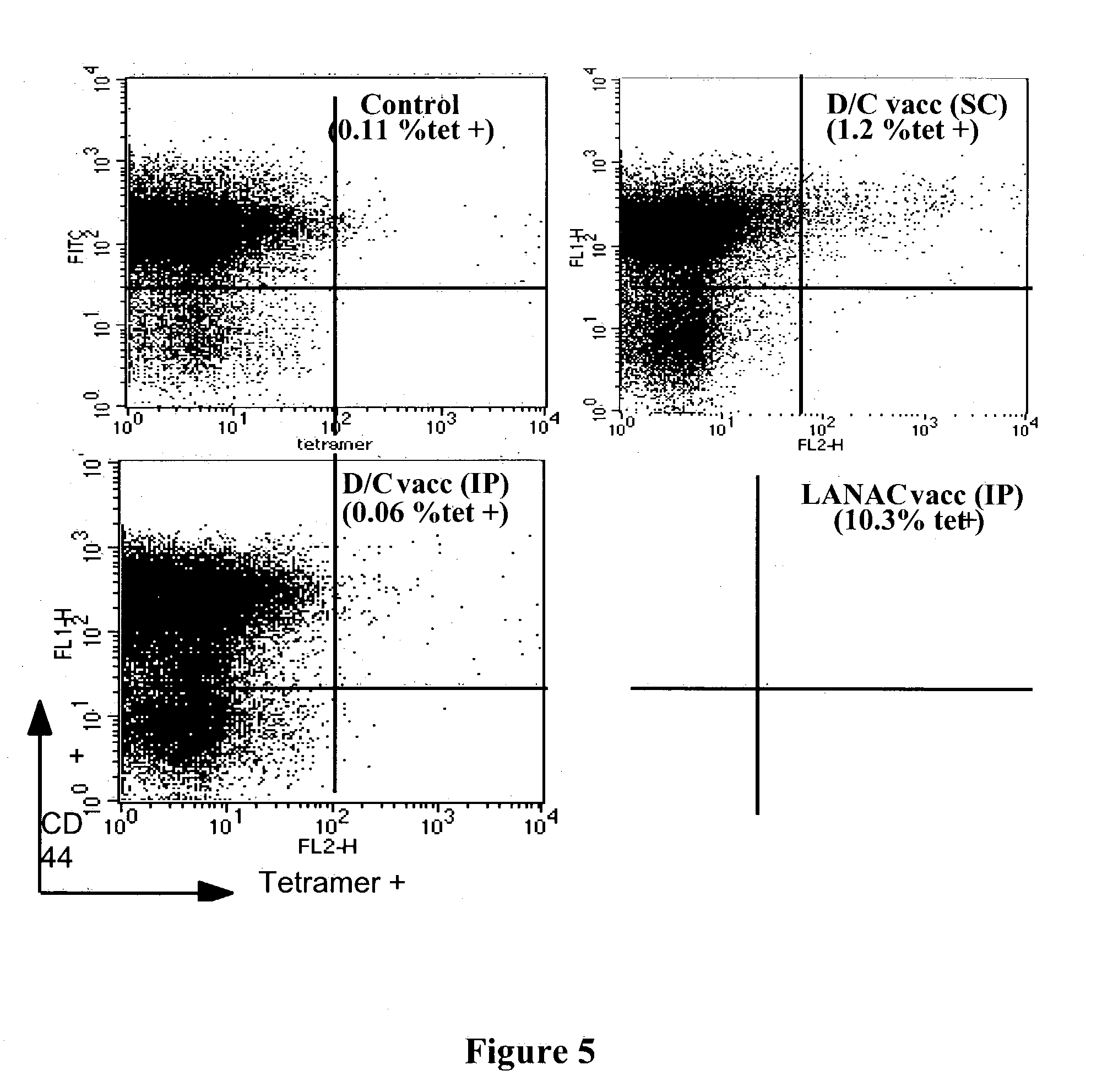
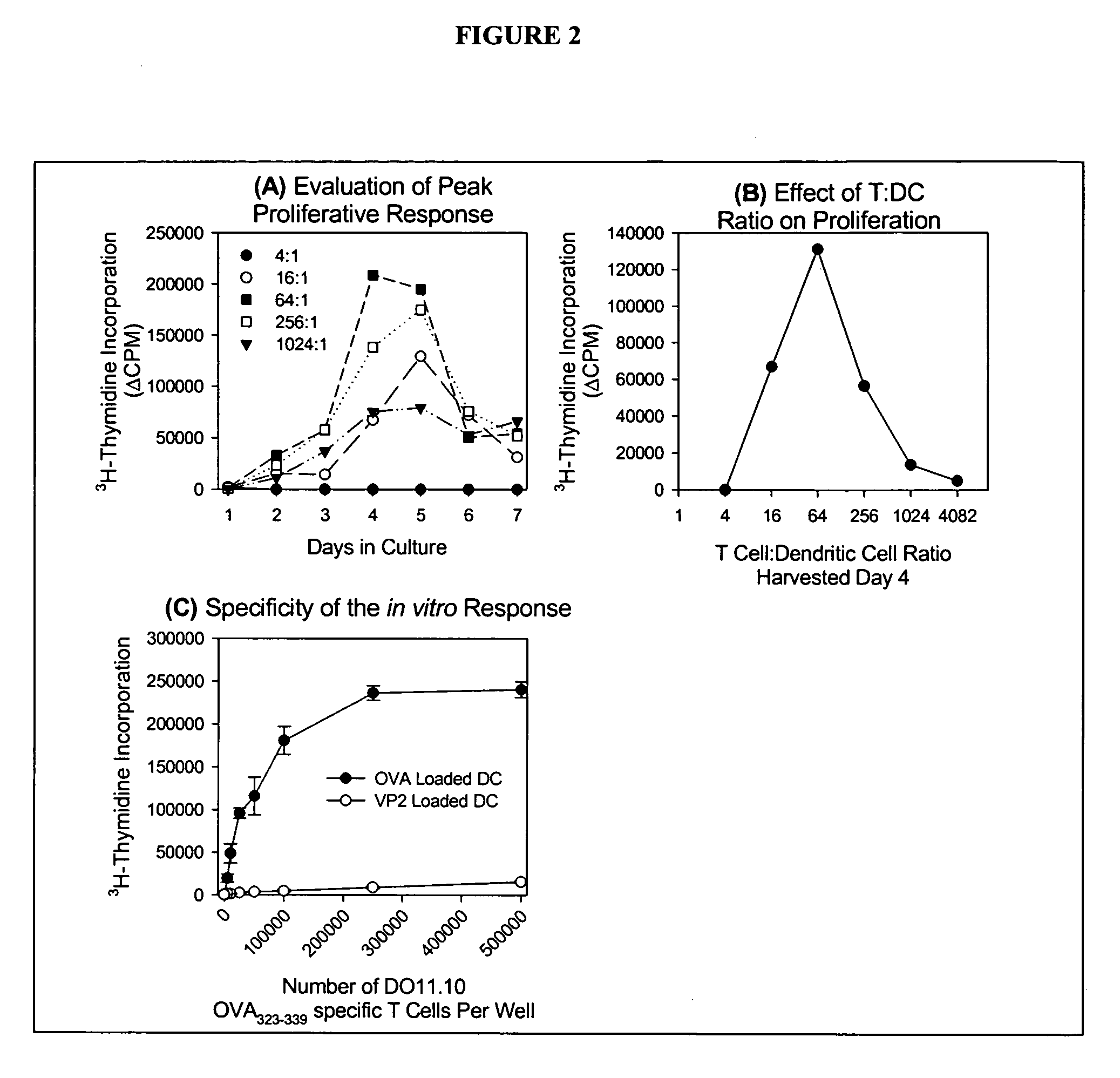
Programmed immune responses using a vaccination node
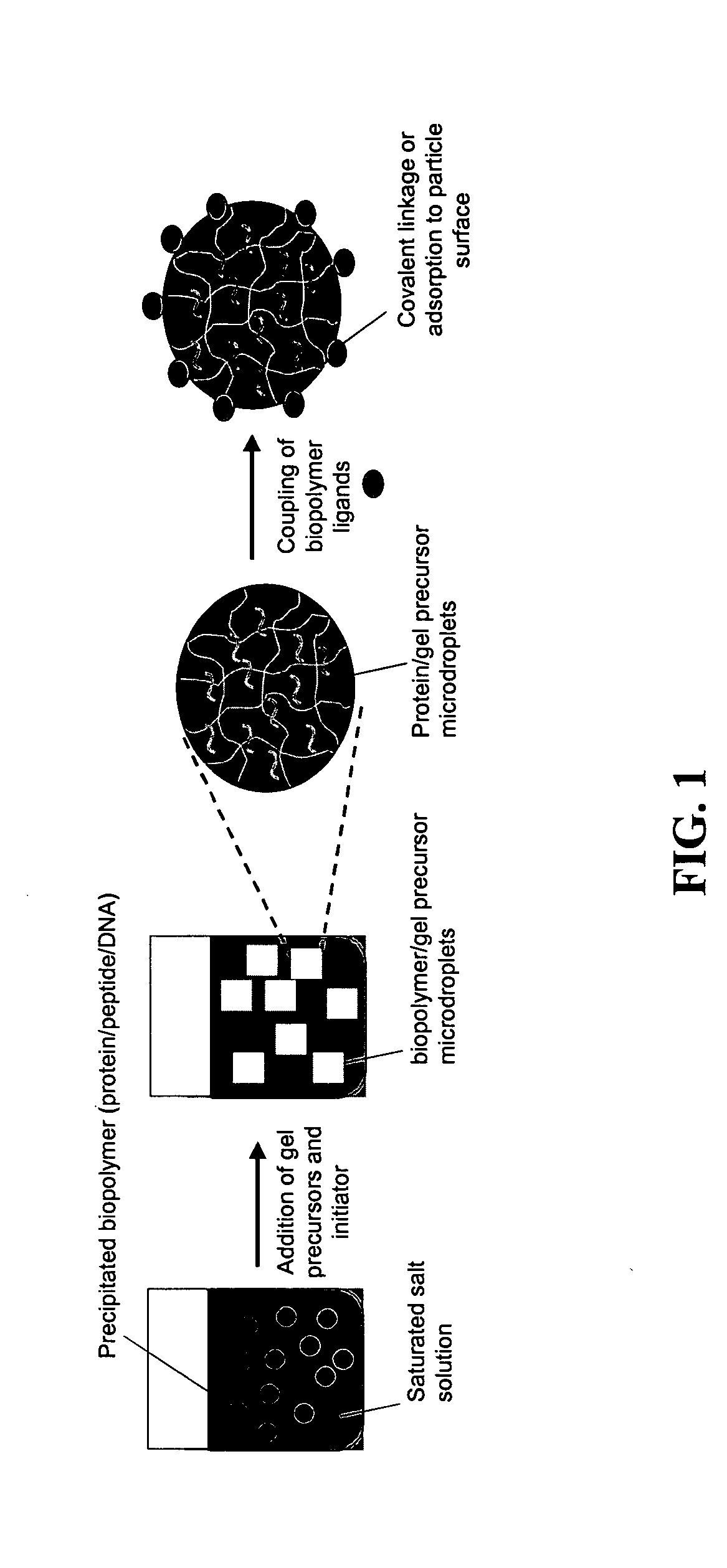
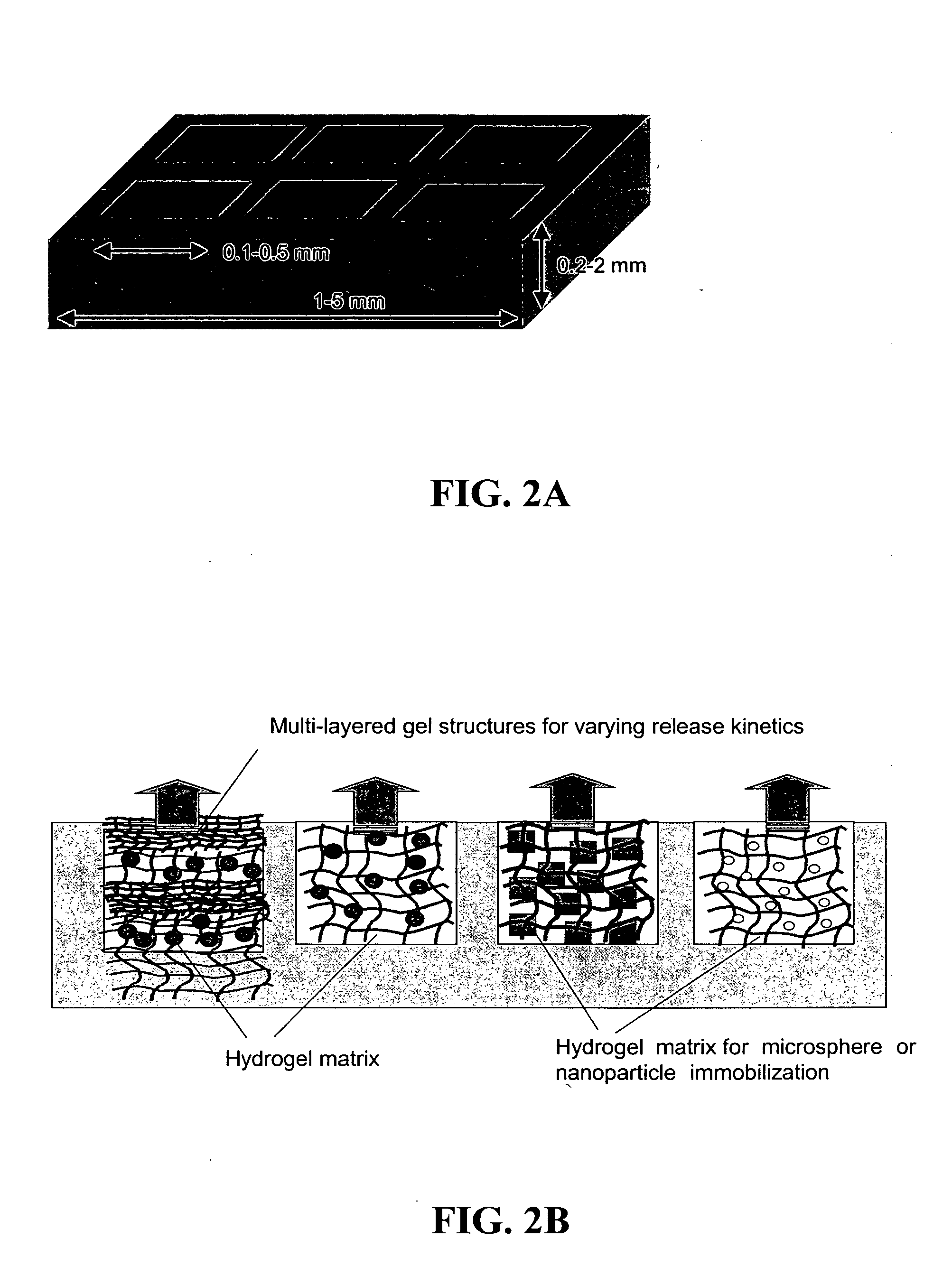
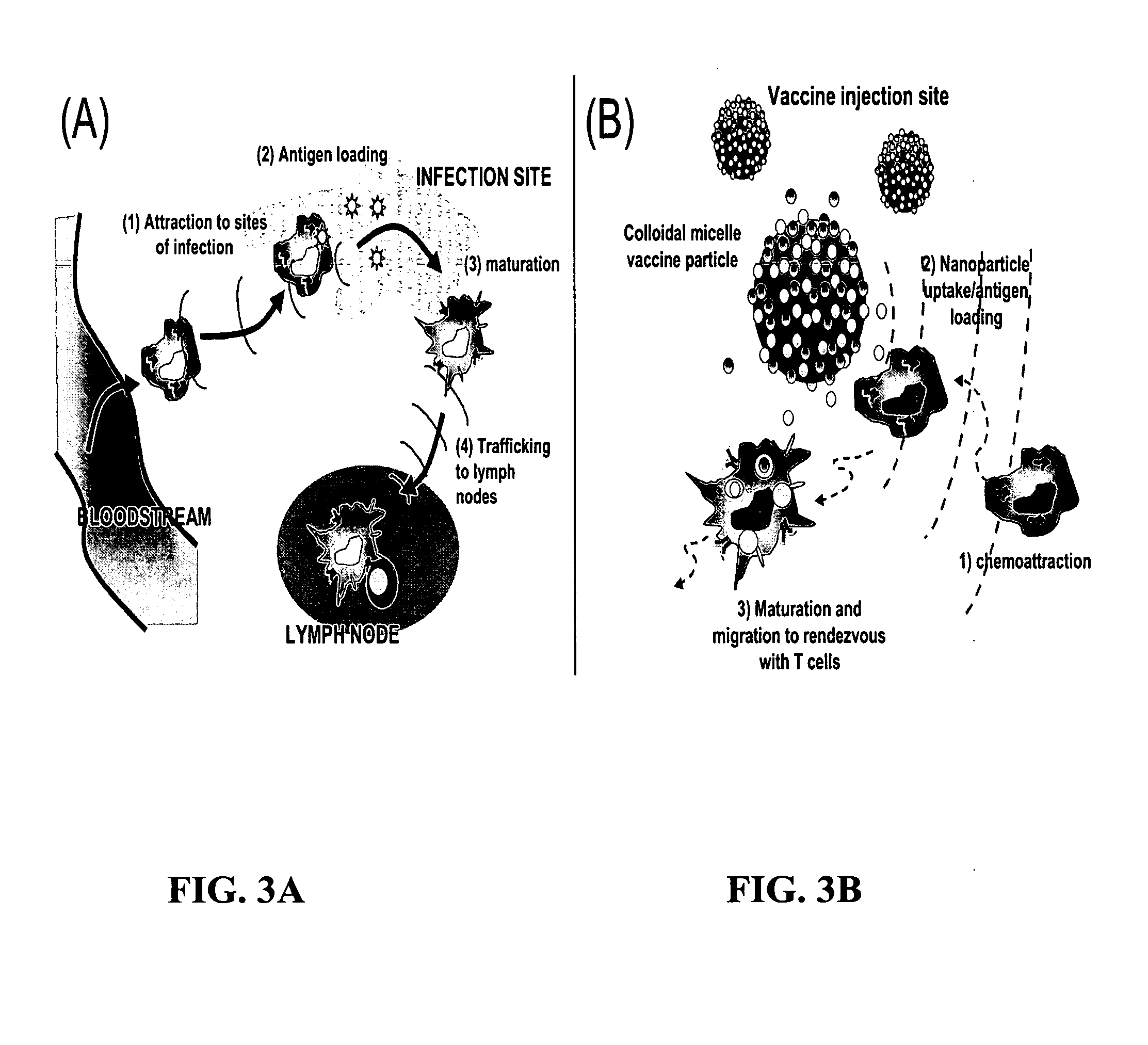
The present invention provides compositions and methods for modulating immune responses to antigens. One aspect of the present invention relates to a particle-based antigen delivery system (vaccination node) that comprises a hydrogel particle capable of both antigen presentation and DC activation. The VN may further comprise a chemoattractant-loaded microsphere capable of attracting DCs to the site of administration. Another aspect of the present invention relates to the use of the VN to modulate antigen presenting cells activation for the prevention and / treatment of various diseases, such as infectious diseases, cancers and autoimmune diseases.
Owner:VAXDESIGN
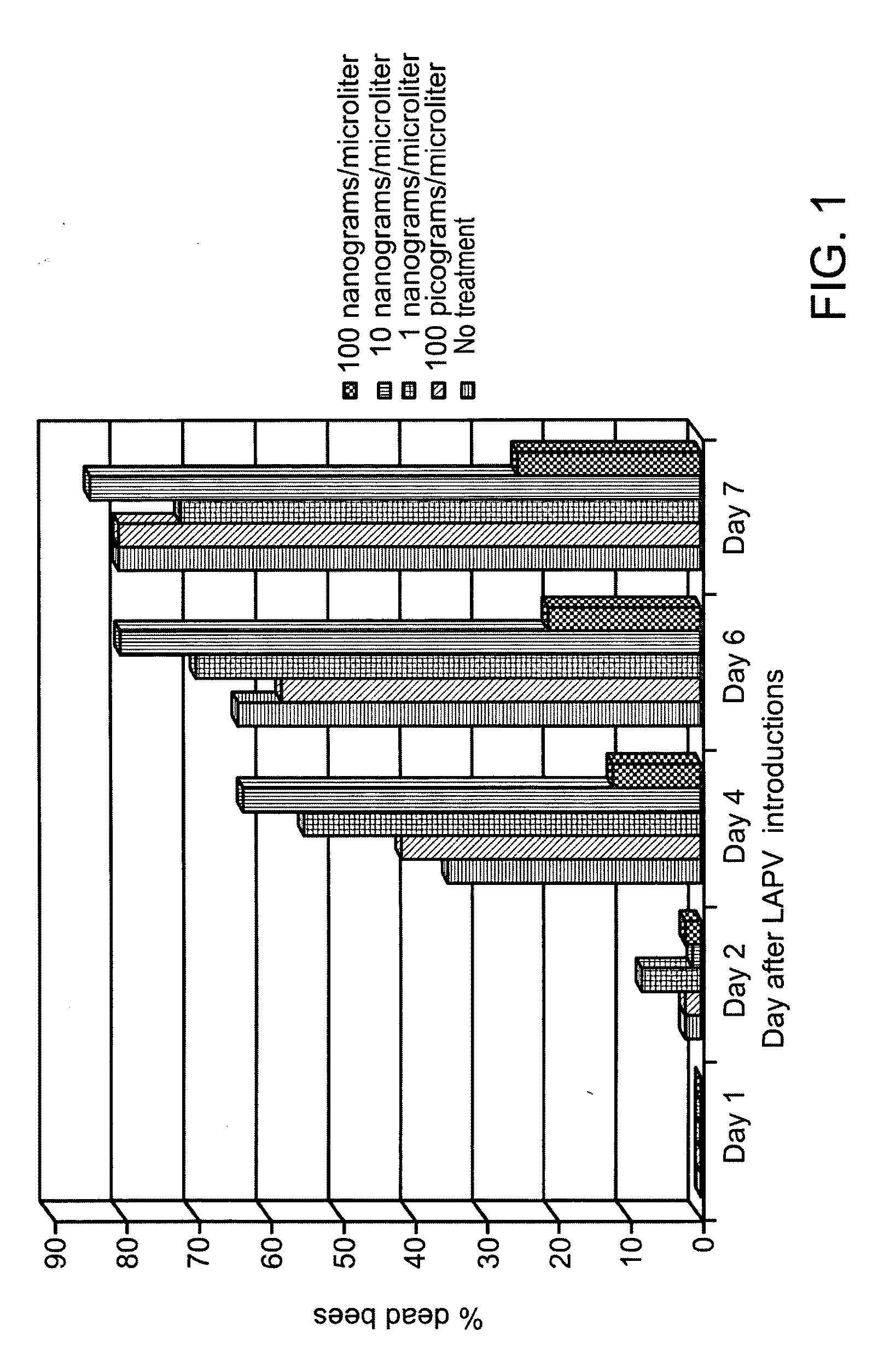
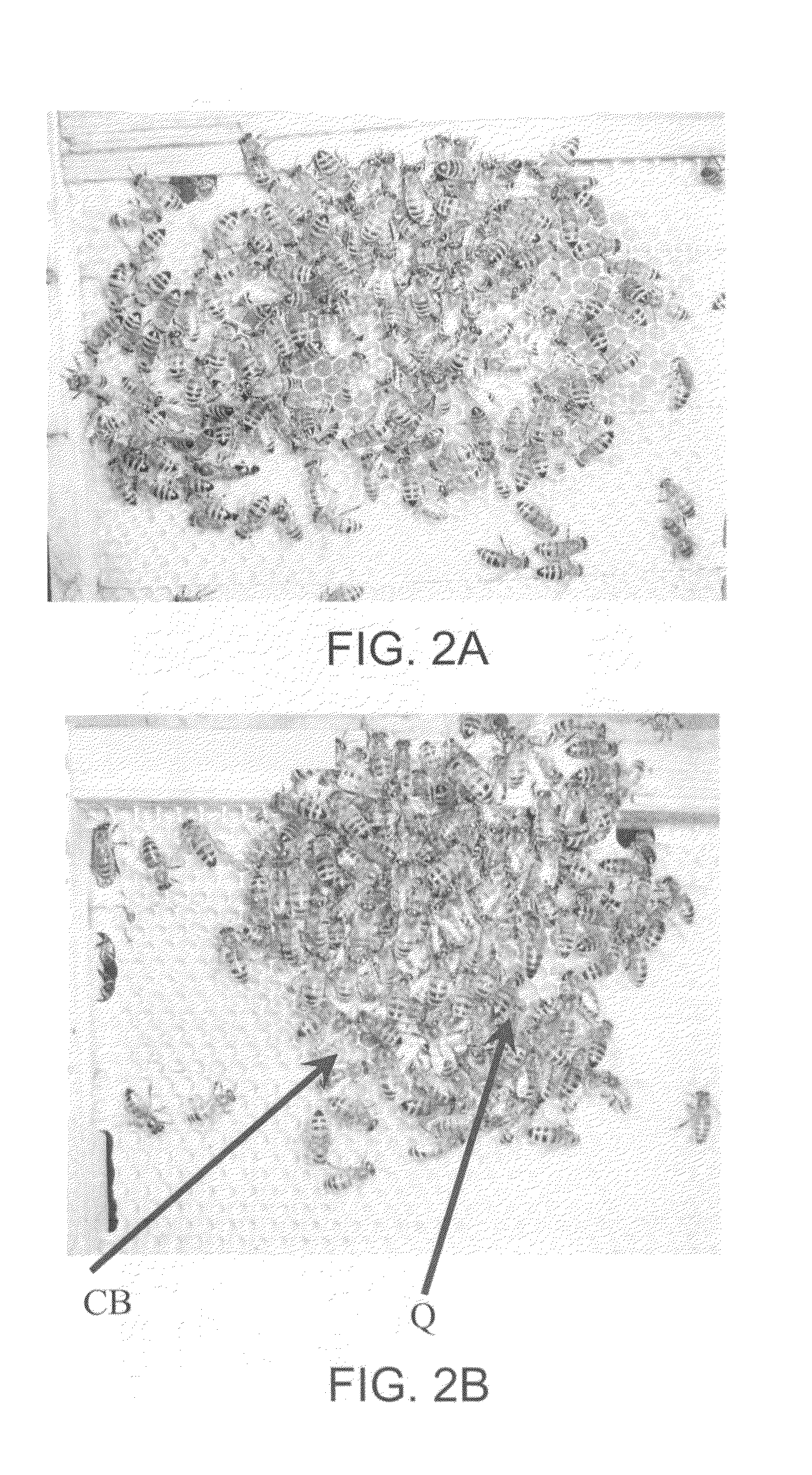
Compositions for conferring tolerance to viral disease in social insects, and the use thereof
ActiveUS20090118214A1Avoid spreadingImprove toleranceOrganic active ingredientsSsRNA viruses positive-senseInfectious DisorderViral disease
Compositions and methods for reducing susceptibility to infectious disease in bees using RNA interference technology, and more particularly, prevention and treatment of viral infections in honeybees such as Israel acute paralysis virus (IAPV) by feeding of pathogen-specific dsRNA. Further, multiple-pathogen specific dsRNA is disclosed.
Owner:GREENLIGHT BIOSCIENCES INC
Compositions and methods for altering immune function
InactiveUS7601355B2Overcome problemsGood curative effectSnake antigen ingredientsAntibody ingredientsImmune compromisedInfectious Disorder
The present invention relates to molecules that stabilize and / or enhance CD154 activity, compositions comprising these molecules, methods for using these molecules and compositions (e.g., pharmaceutical compositions) comprising them. Specifically, the molecules can be used for enhancing immune function (e.g., enhancing Th1 cytokine expression), stimulating immune responses, and / or treating certain disorders (e.g., cancer, infectious disease, and immune compromised states). The invention also relates to kits and compositions comprising the molecules.
Owner:NORTHWESTERN UNIV
Vaccines using nucleic acid-lipid complexes
InactiveUS20060223769A1SsRNA viruses negative-sensePeptide/protein ingredientsInfectious DisorderSelf-Antigens
This invention relates to a vaccine and a method for immune activation which is effective for eliciting both a systemic, non-antigen specific immune response and a strong antigen-specific immune response in a mammal. The method is particularly effective for protecting a mammal from a disease including cancer, a disease associated with allergic inflammation, an infectious disease, or a condition associated with a deleterious activity of a self-antigen. Also disclosed are therapeutic compositions useful in such a method.
Owner:NAT JEWISH MEDICAL & RES CENT
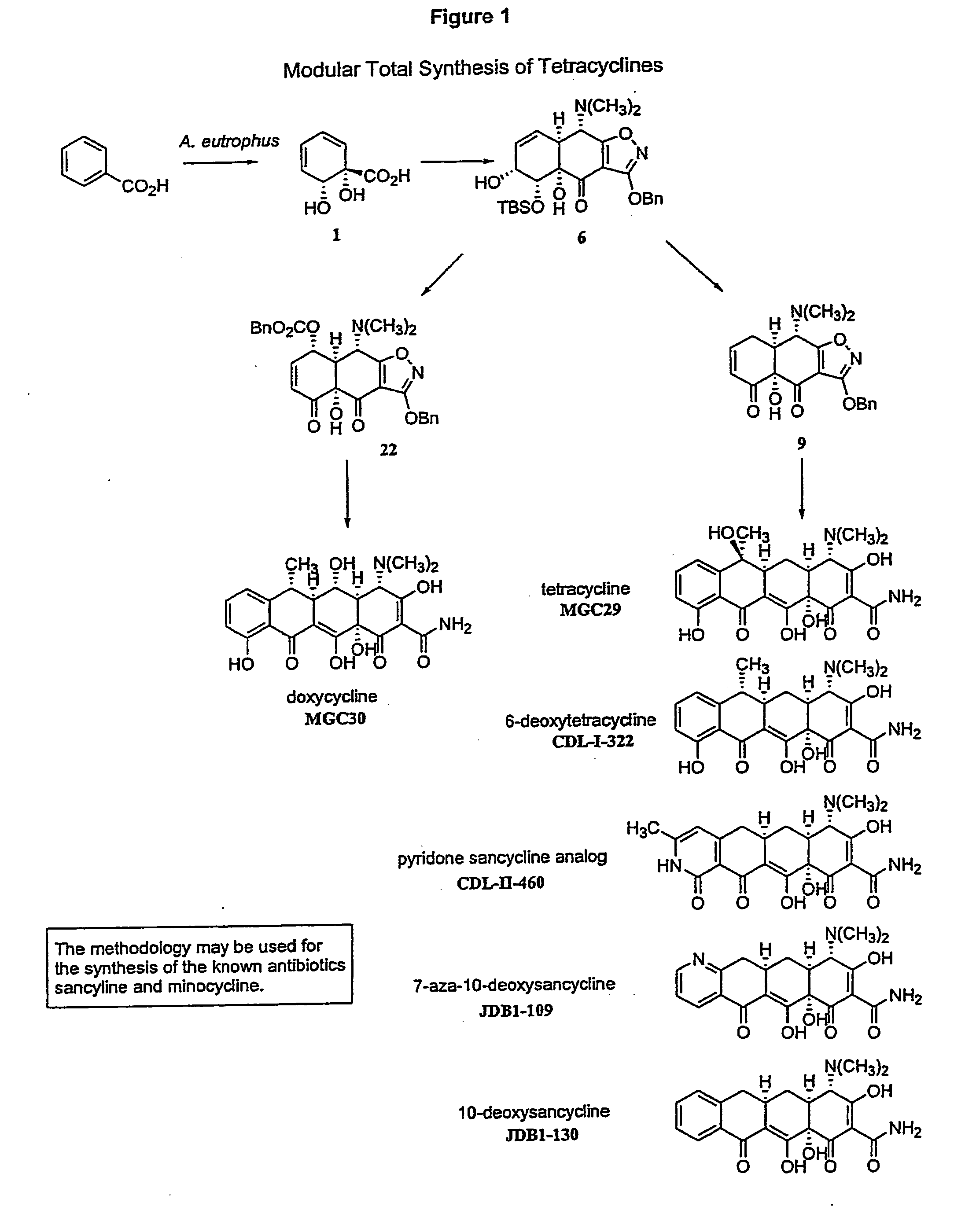
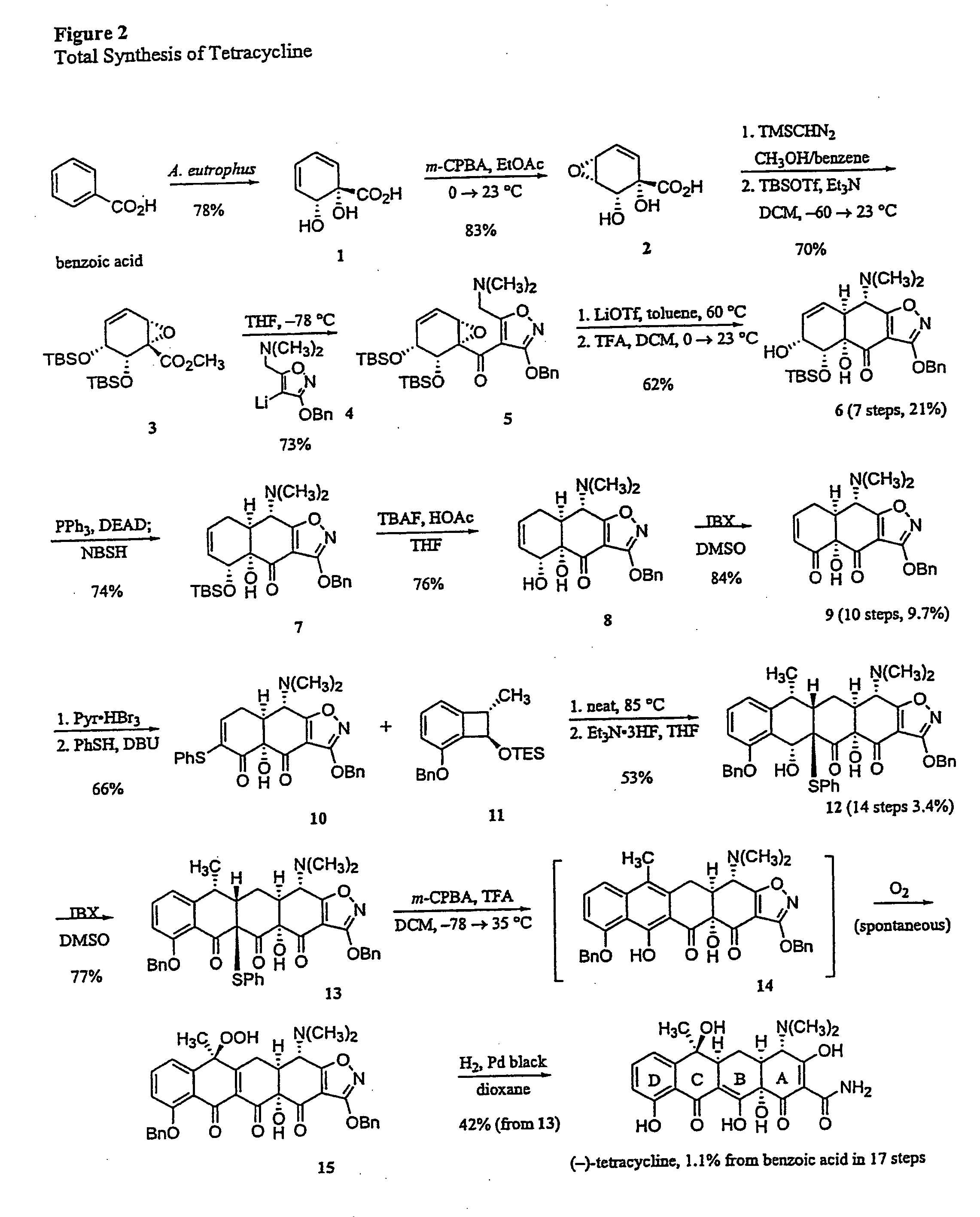
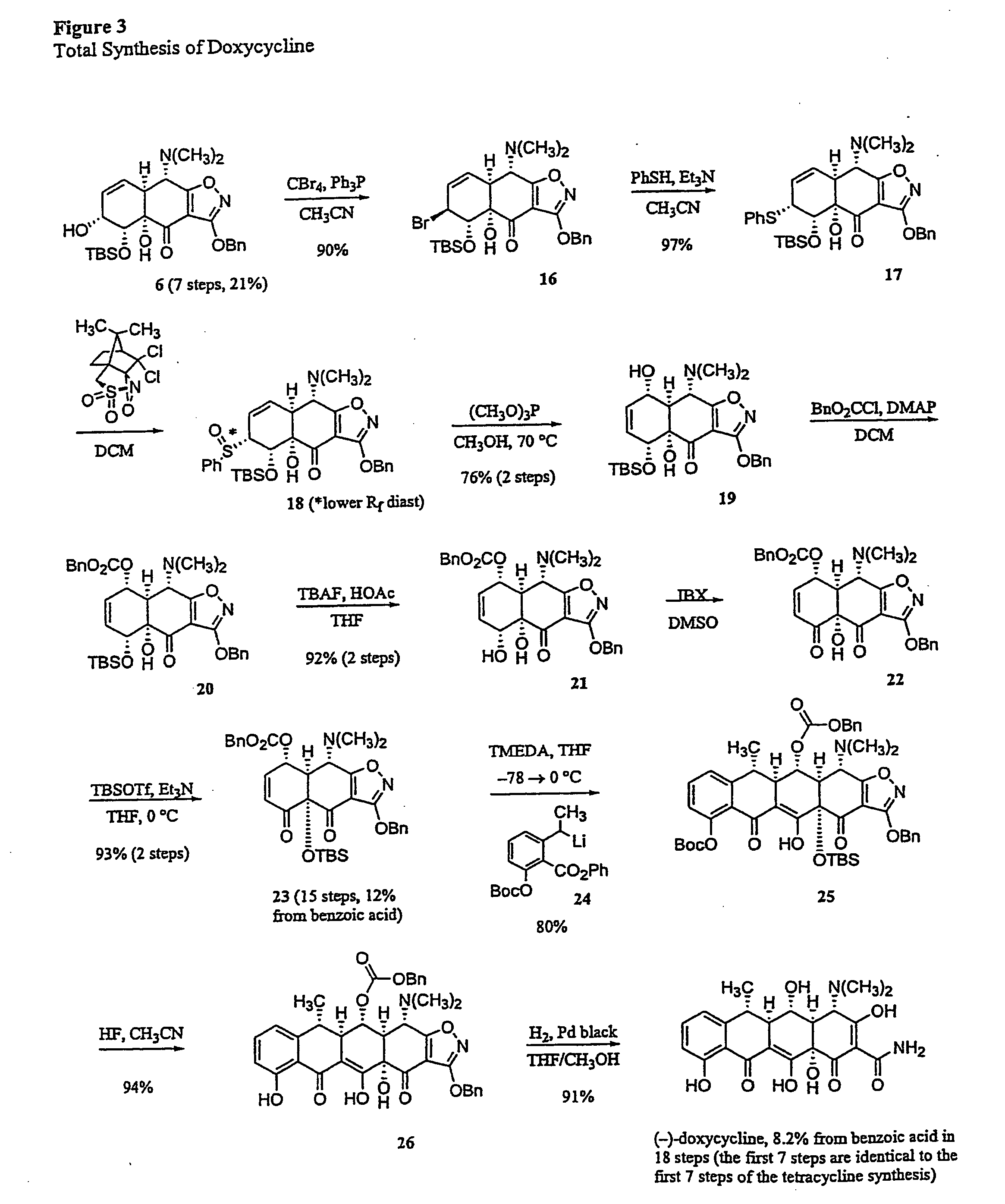
Synthesis of tetracyclines and analogues thereof
The tetracycline class of antibiotics has played a major role in the treatment of infectious diseases for the past 50 years. However, the increased use of the tetracyclines in human and veterinary medicine has led to resistance among many organisms previously susceptible to tetracycline antibiotics. The modular synthesis of tetracyclines and tetracycline analogs described provides an efficient and enantioselective route to a variety of tetracycline analogs and polycyclines previously inaccessible via earlier tetracycline syntheses and semi-synthetic methods. These analogs may be used as anti-microbial agents or anti-proliferative agents in the treatment of diseases of humans or other animals.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
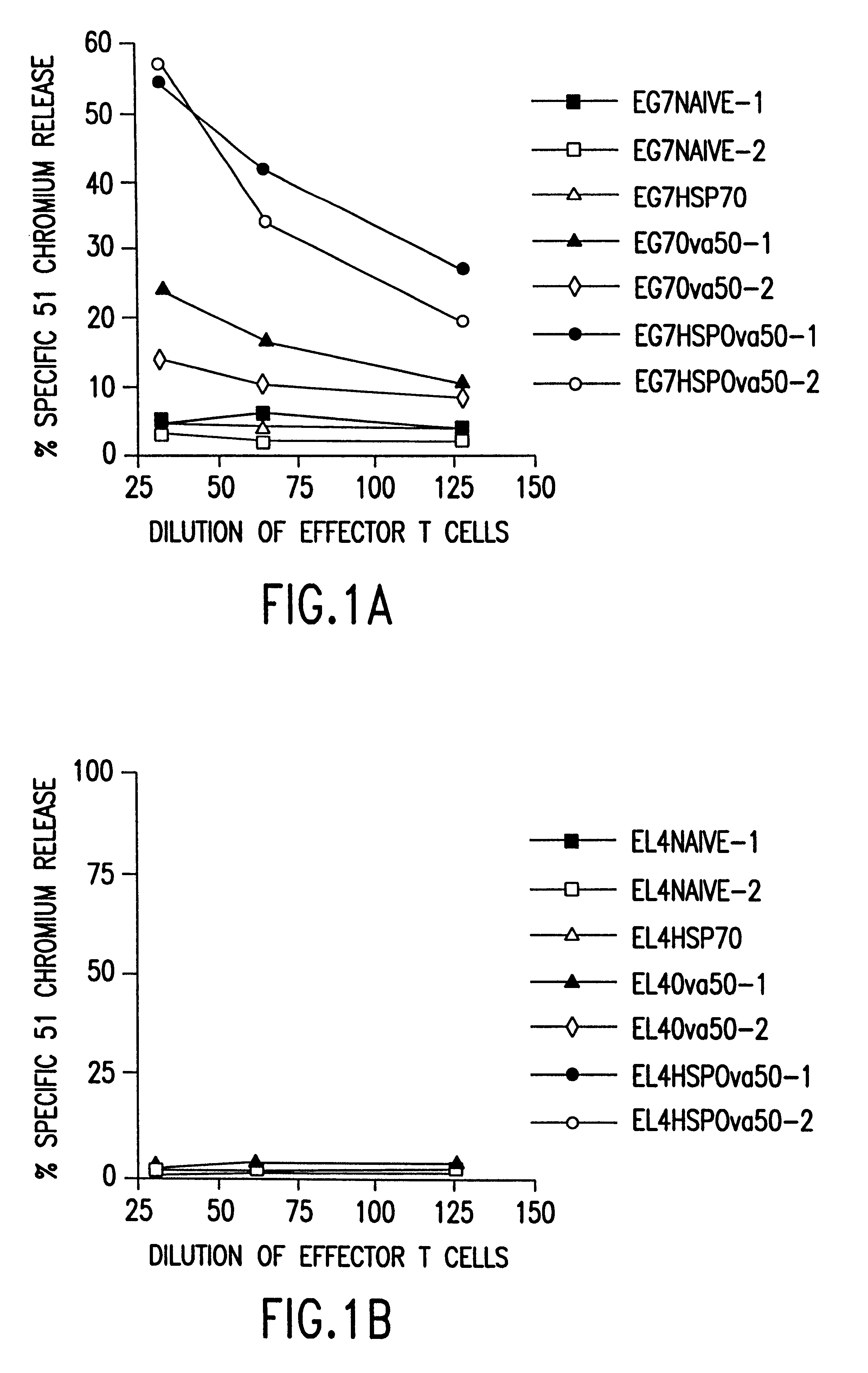
Therapeutic and prophylactic methods using heat shock proteins
The present invention relates to immunogenic complexes of heat shock proteins (hsp) noncovalently bound to exogenous antigenic molecules which when administered to an individual elicit specific immunological responses in the host. Methods of prevention and treatment of cancer and infectious disease are provided.
Owner:FORDHAM UNIVERSITY
Molecular antigen array
The invention provides compositions and processes for the production of ordered and repetitive antigen or antigenic determinant arrays. The compositions of the invention are useful for the production of vaccines for the prevention of infectious diseases, the treatment of allergies and the treatment of cancers. Various embodiments of the invention provide for a core particle that is coated with any desired antigen in a highly ordered and repetitive fashion as the result of specific interactions.
Owner:CYTOS BIOTECHNOLOGY AG
Fully human Anti-human nkg2d monoclonal antibodies
The invention relates to isolated fully human monoclonal antibodies having specificity for human NKG2D and compositions thereof. The invention further relates to methods for using such antibodies in treating diseases or conditions such as cancer, autoimmune disease, or infectious disease.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Fish serine proteinases and their pharmaceutical and cosmetic use
InactiveUS20020141987A1Avoid injuryInfection-preventing effective amount of the enzymeBiocideCosmetic preparationsAtlantic codSerine proteinases
Fish derived serine proteinases including trypsins and chymotrypsin derived from cod such as Atlantic cod is used for treating and / or preventing a variety of diseases and disorders such as inflammatory diseases, infectious diseases caused by viruses, bacteria and fungal species and diseases where a receptor binding mechanism is involved in the pathogenesis. Pharmaceutical and cosmetic compositions comprising the proteinases are described.
Owner:BJARNASON JON BRAGI
Identification and Engineering of Antibodies with Variant Fc Regions and Methods of Using Same
InactiveUS20110243941A1High affinityLow affinitySenses disorderNervous disorderTherapeutic antibodyInfectious Disorder
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcγRIIIA and / or FcγRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcγR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MACROGENICS INC
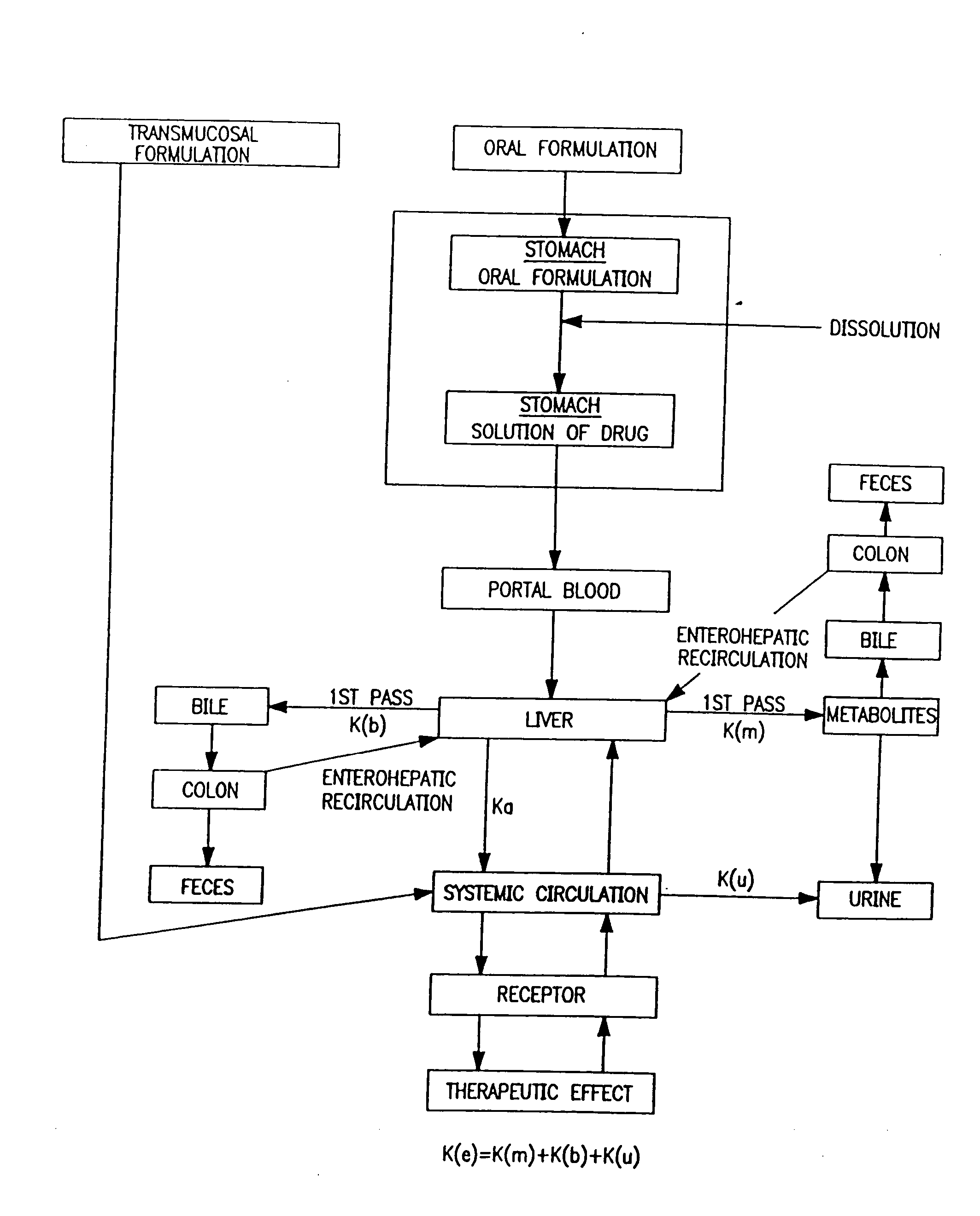
Buccal, polar and non-polar spray or capsule containing drugs for treating an infectious disease or cancer
InactiveUS20050142069A1Fast absorptionRapid onsetGenetic material ingredientsAerosol deliverySolventBioactive compound
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
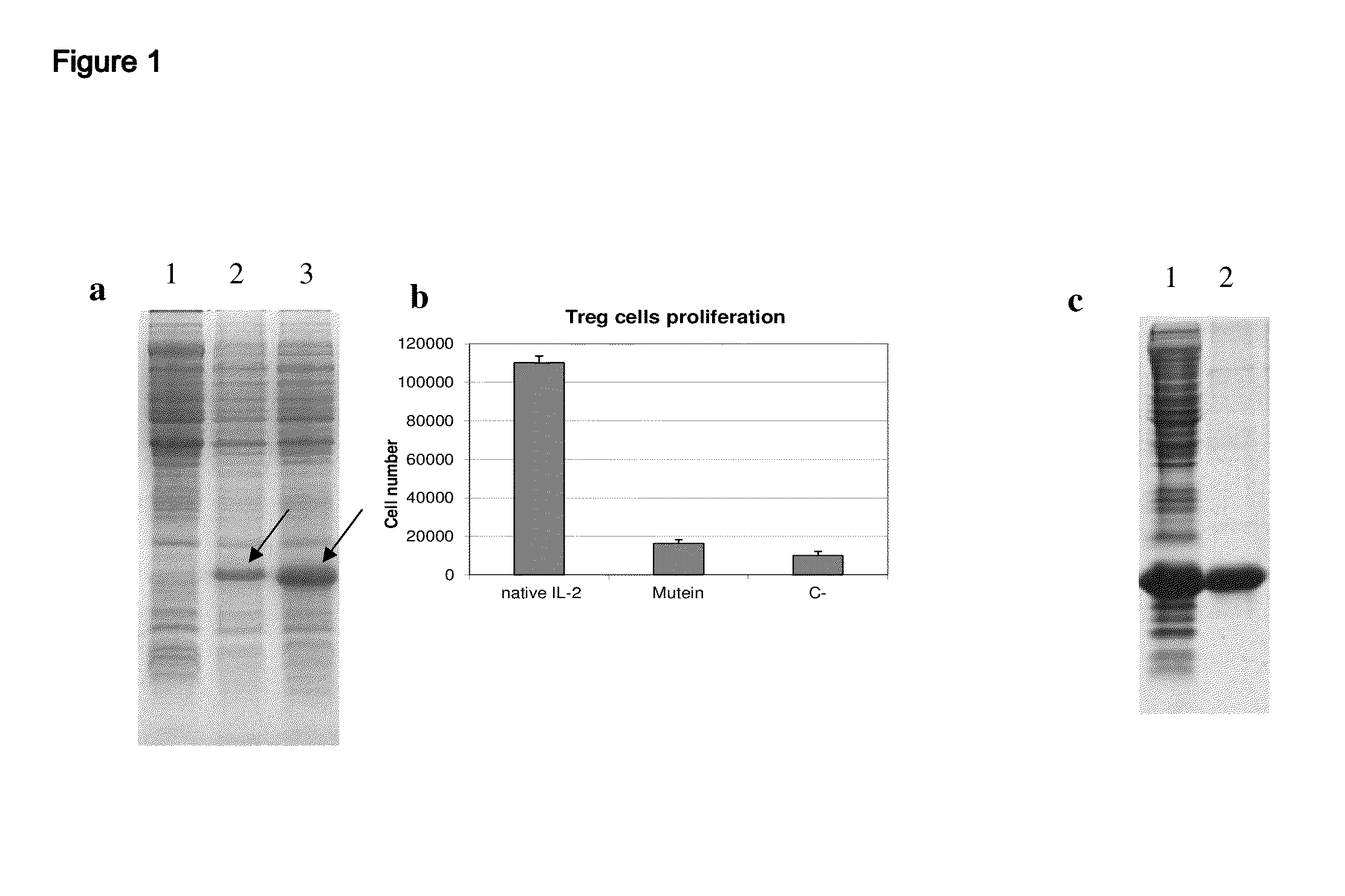
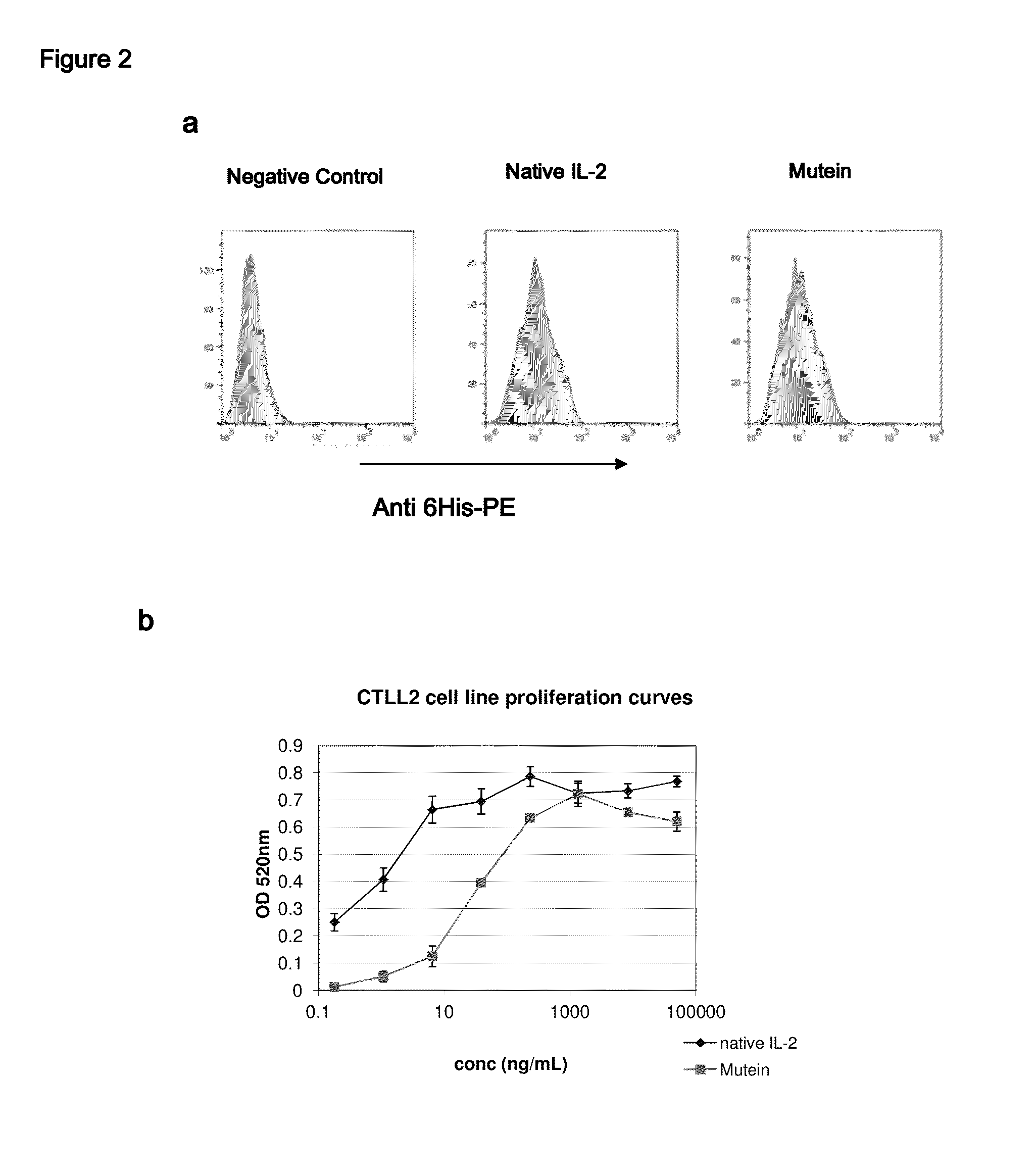
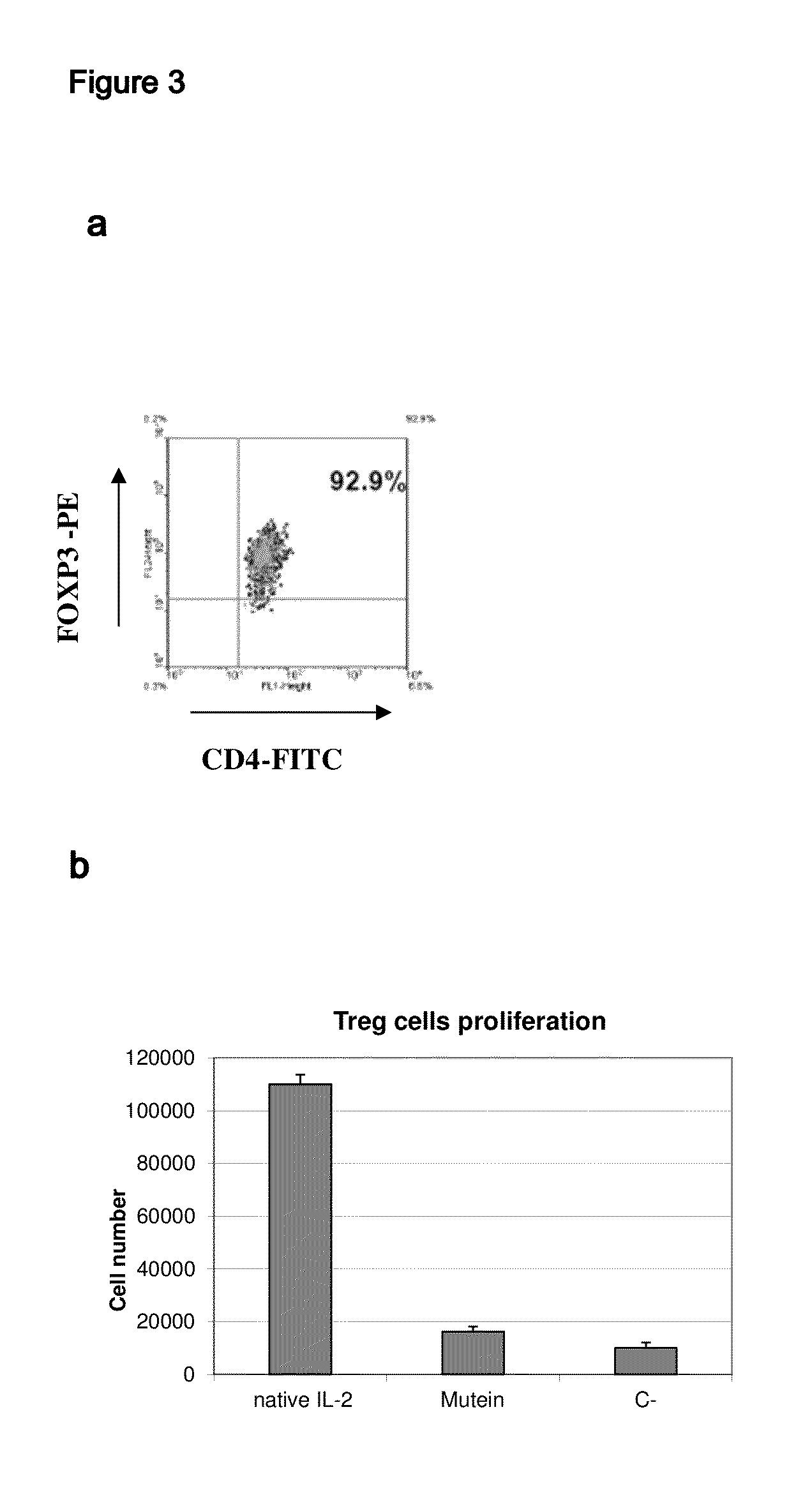
IL-2 derivative polypeptides
ActiveUS9206243B2Good treatment effectLow ability to stimulateAntibacterial agentsPeptide/protein ingredientsRegulatory T cellInfectious Disorder
The present invention relates to polypeptides which share primary sequence with human IL-2, except for several amino acids that have been mutated. The mutations introduced substantially reduce the ability of these polypeptides to stimulate in vitro and in vivo regulatory T cells (T CD4+CD25+FoxP3+) and make them more effective in the therapy of murine transplantable tumors. Also includes therapeutic uses of these mutated variants, used alone or in combination with vaccines for the therapy of diseases such as cancer or infections where the activity of regulatory T cells (Tregs) is relevant. In another aspect the present invention relates to pharmaceutical compositions comprising as active principle the polypeptides disclosed. Finally, the present invention relates to the therapeutic use of the polypeptides and pharmaceutical compositions disclosed due to their modulating effect of the immune system on diseases like cancer and chronic infectious diseases.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
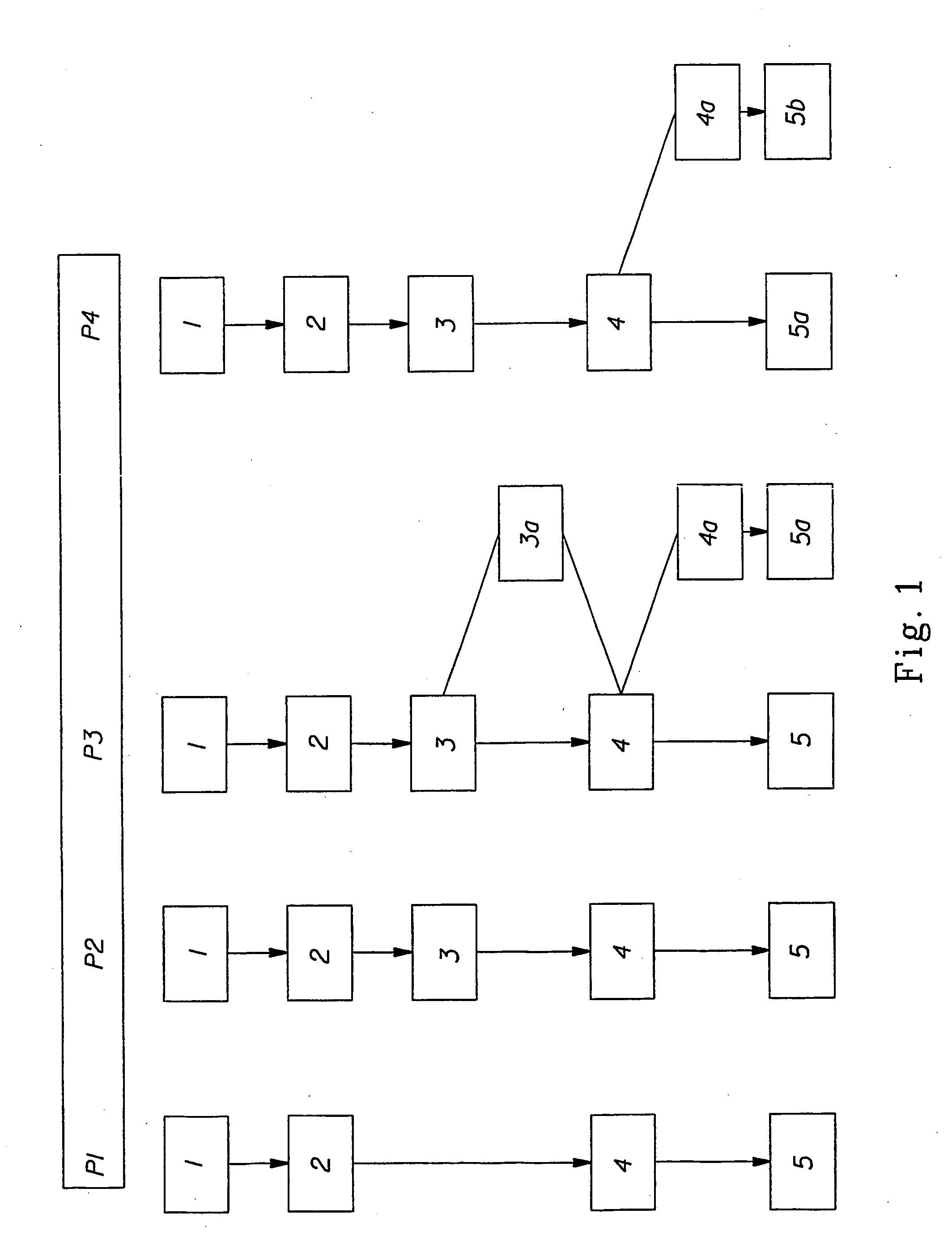
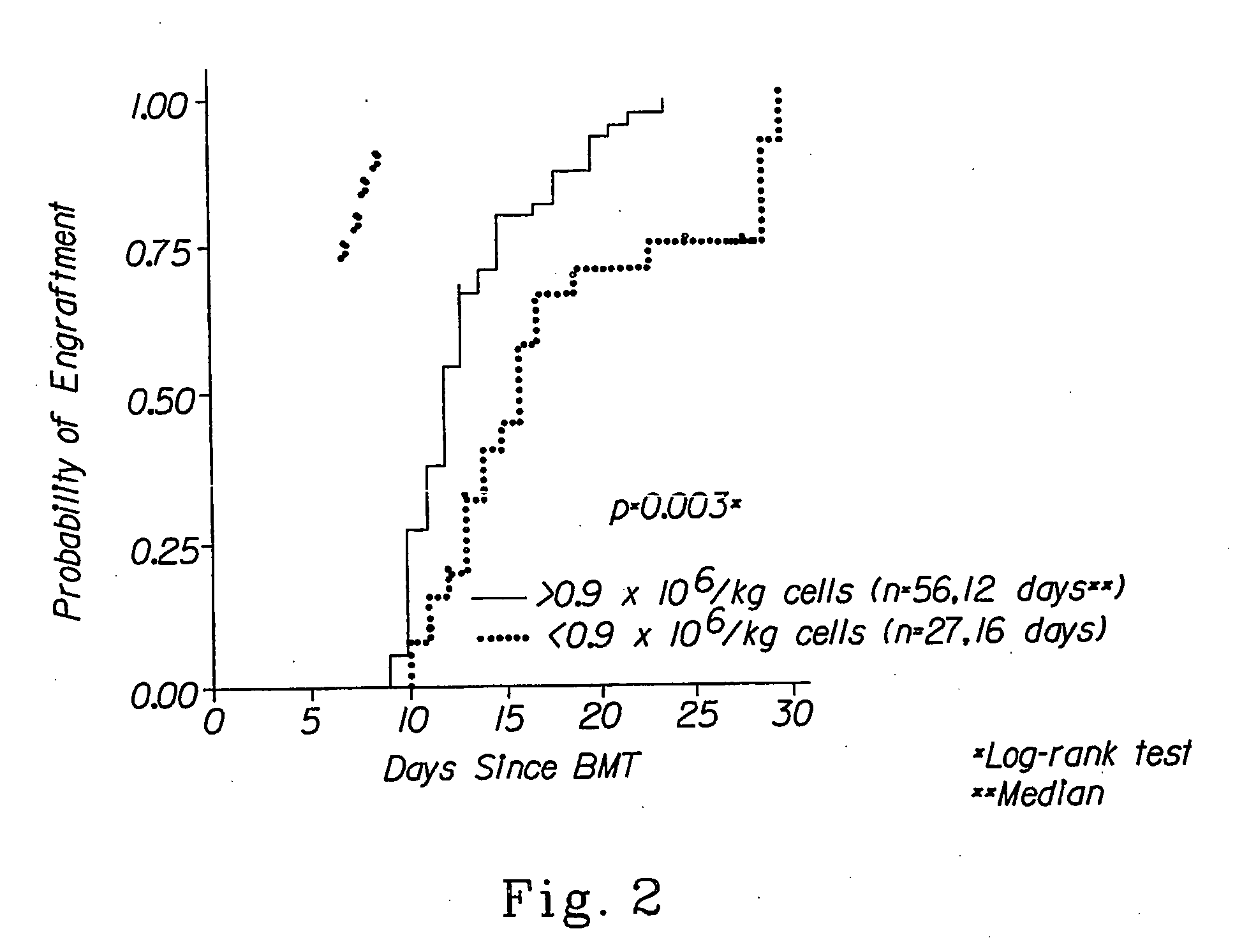
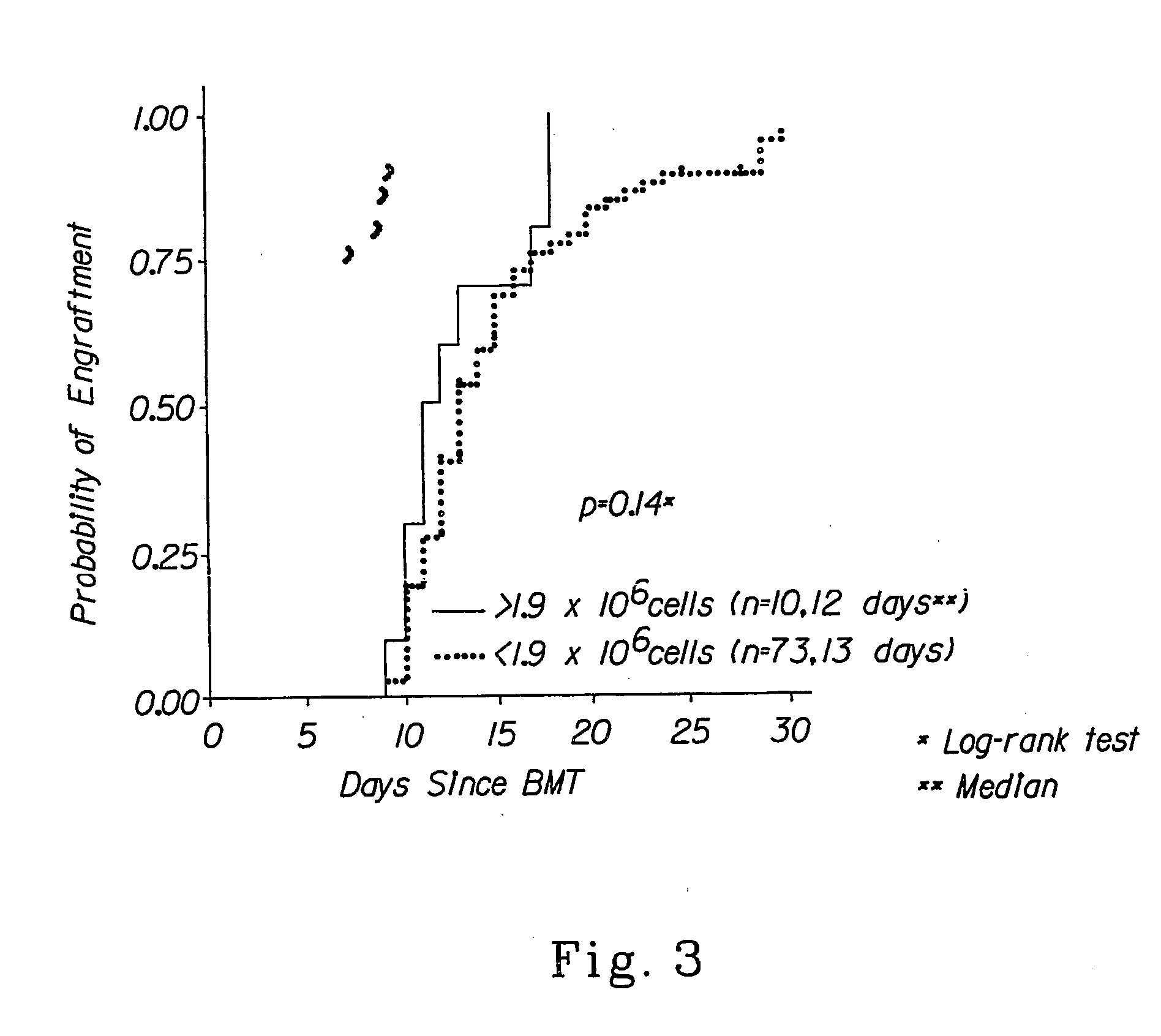
Cellular compositions which facilitate engraftment of hematopoietic stem cells while minimizing the risk of gvhd
The present invention provides for cellular compositions which facilitate engraftment of hematopoietic stem cells from a syngeneic, allogeneic or xenogeneic donor. The cellular compositions of the invention facilitate engraftment while minimizing the risk of graft versus host disease in the graft recipient. According to a preferred embodiment of the invention, a cell composition is provided, which cell composition comprises hematopoietic stem cells, such as CD34+ cells and / or facilitating cells, in combination with αβ TCR+ T cells. The invention also relates to methods of using the cellular compositions of the invention to induce donor specific tolerance in a recipient, thus allowing the transplantation of donor organs, cells and tissues. Also disclosed are methods of treating leukemia and cancer as well as infectious diseases caused by viruses.
Owner:JEWISH HOSPITAL HEALTHCARE SERVICES
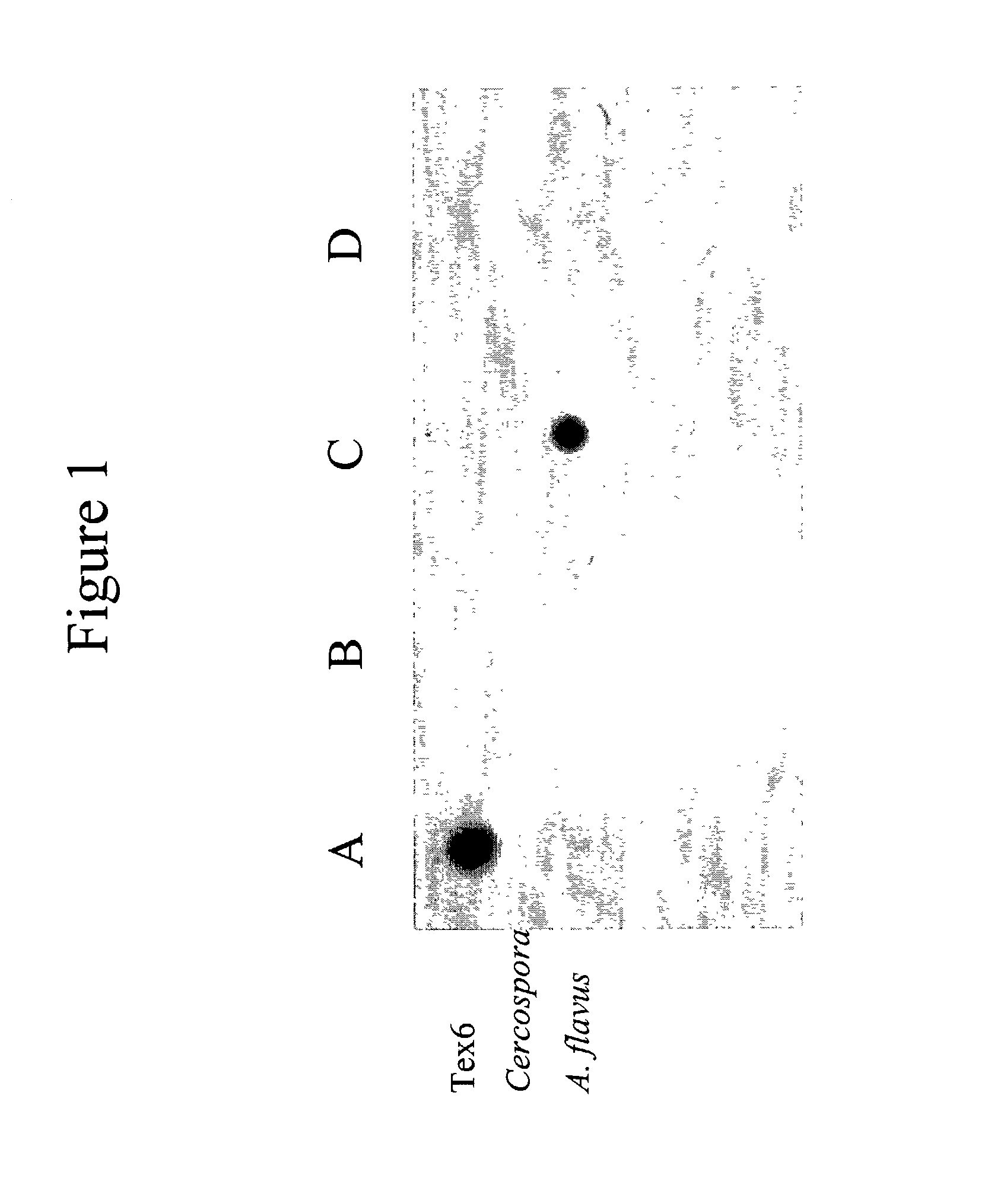
Infectious disease microarray
InactiveUS20030143571A1High sensitivityImprove automationMicrobiological testing/measurementInfectious DisorderBioinformatics
A method for detecting one or more pathogens in a subject. The method includes the steps of: (a) procuring a biological sample, wherein the biological sample comprises nucleic acid material; (b) amplifying the nucleic acid material using random primers to produce a set of random amplicons; (c) providing one or more pathogen-specific probes or probe sets; (d) hybridizing the set of random amplicons with the one or more pathogen-specific probes or probe sets; and (e) determining selective hybridization between a random amplicon and a pathogen-specific probe or probe set, whereby the presence of a pathogen in a biological sample is detected.
Owner:NORTH CAROLINA STATE UNIV
Immunization and/or treatment of parasites and infectious agents by live bacteria
ActiveUS8771669B1Reducing eliminatingReducing or eliminating the targeted parasite, infectious diseaseVirusesBacteriaLytic peptideHuntingtons chorea
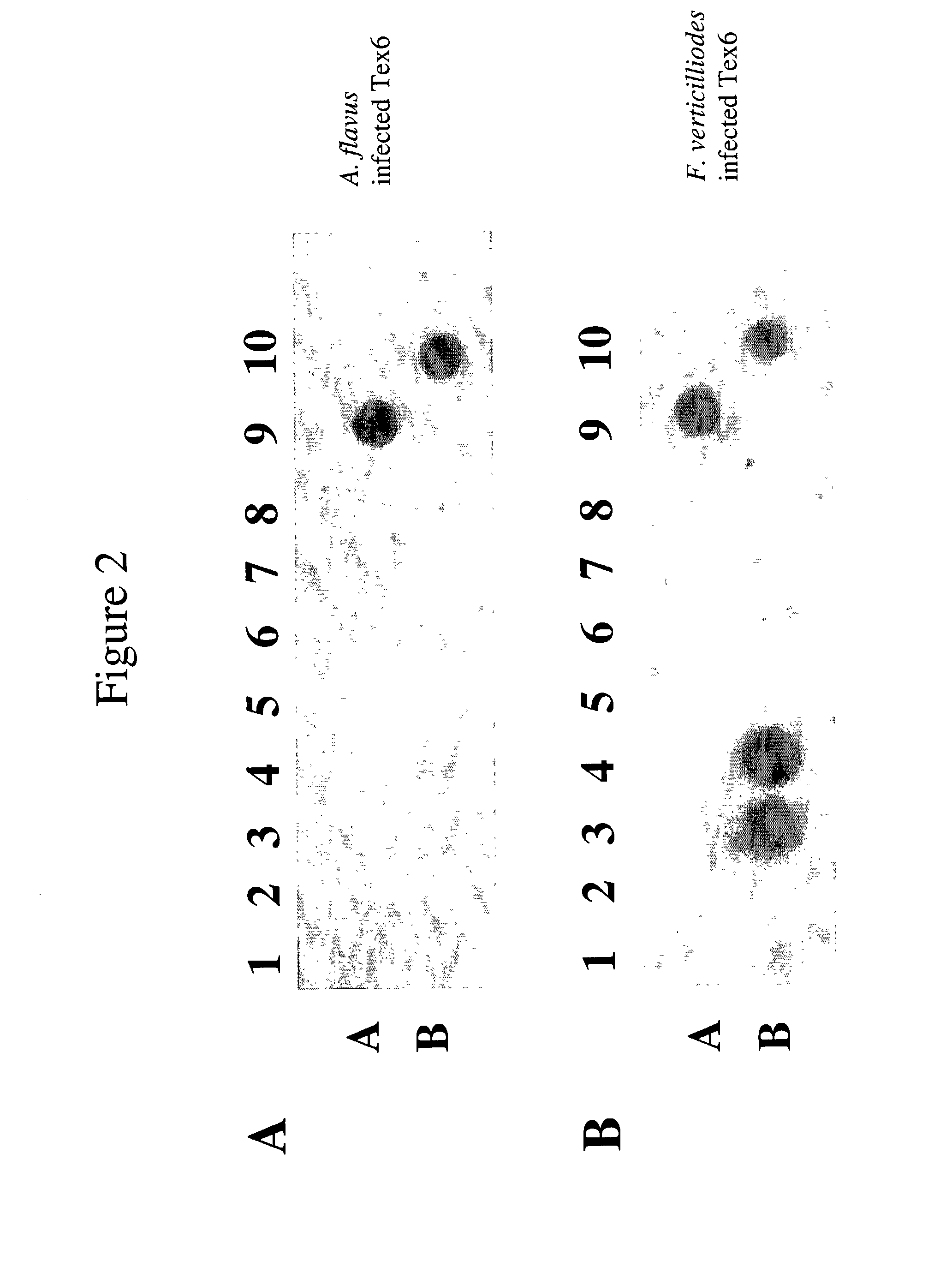
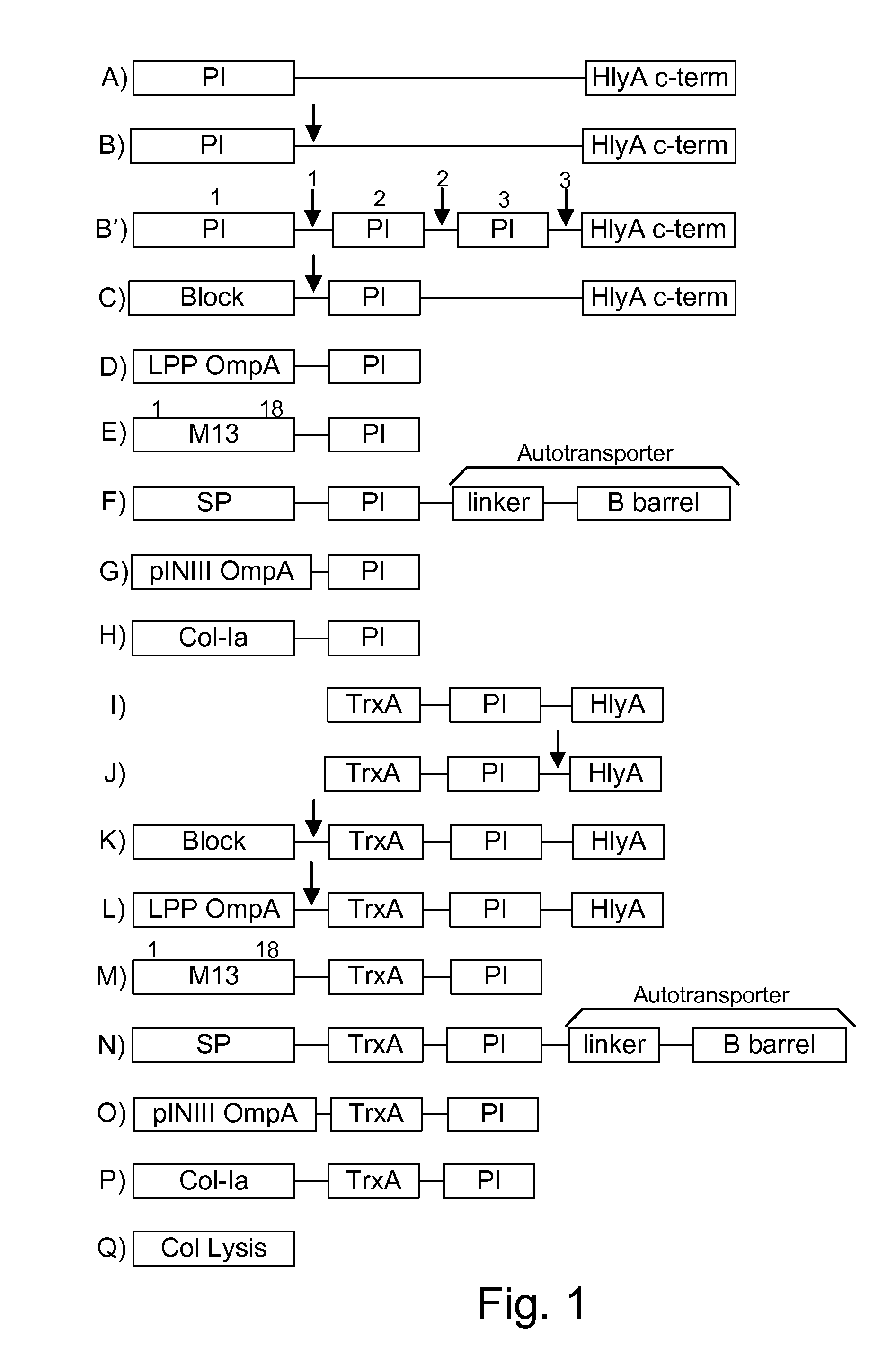
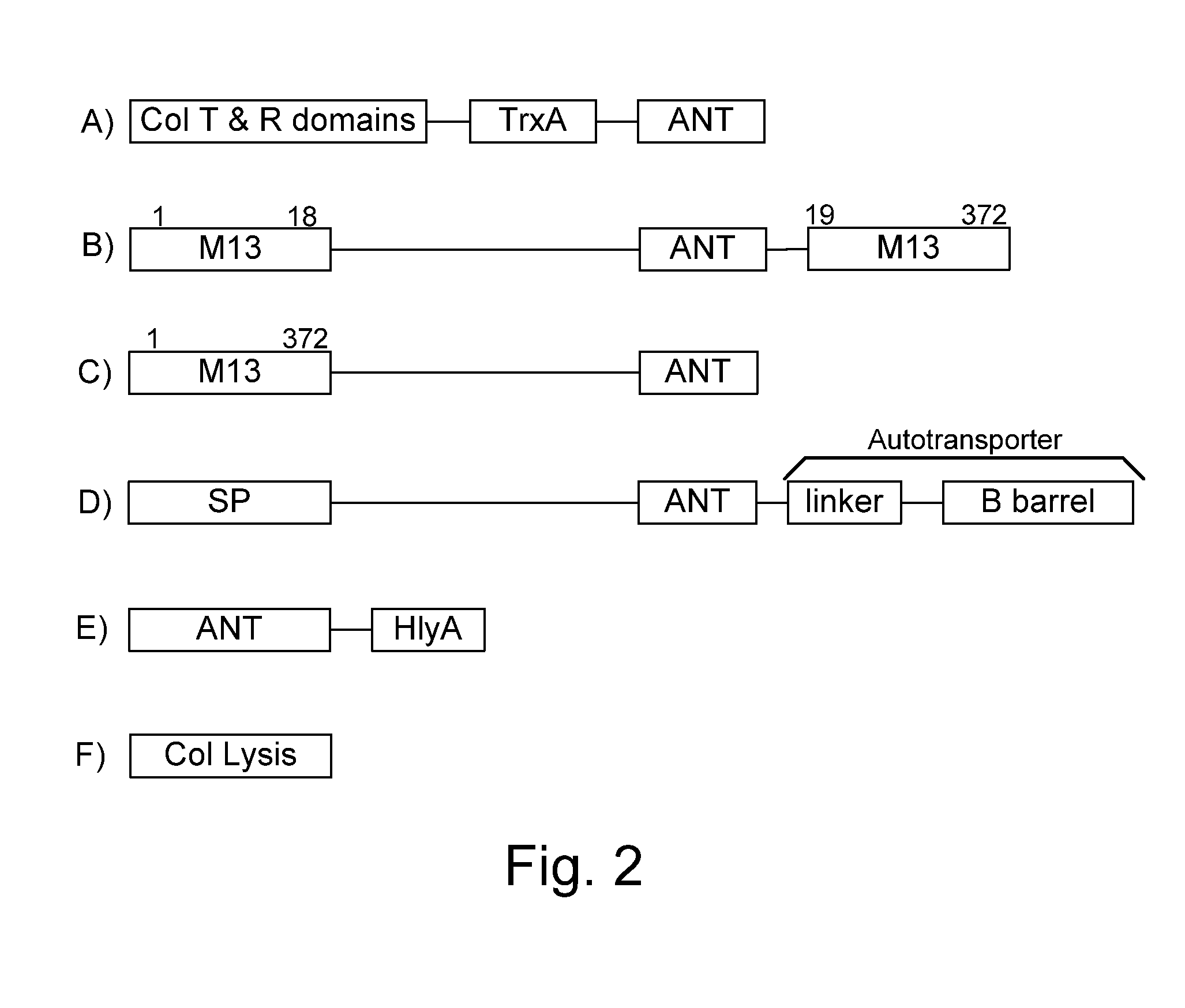
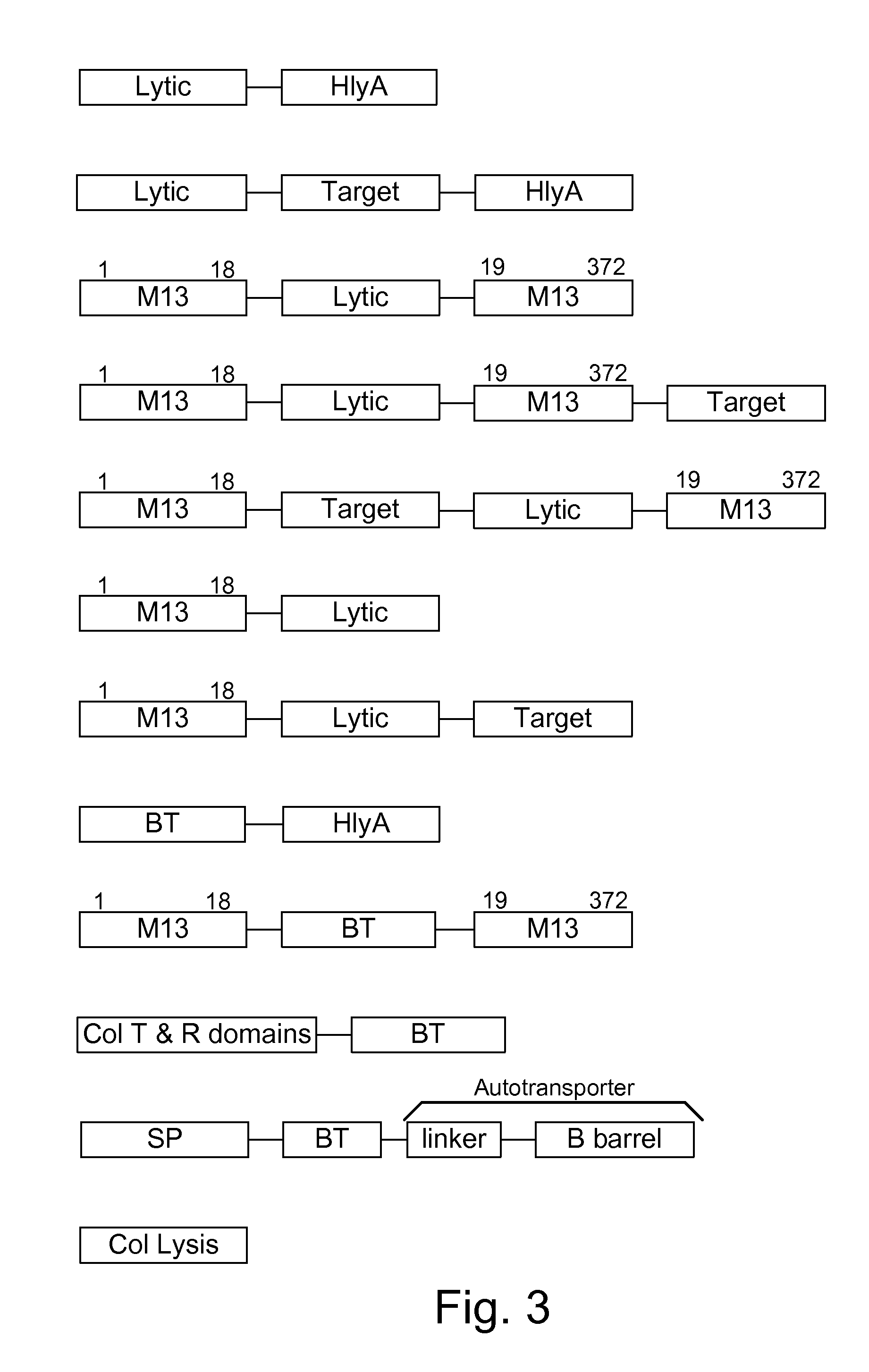
Chimeric proteins are expressed, secreted or released by a bacterium to immunize against or treat a parasite, infectious disease or malignancy. The delivery vector may also be attenuated, non-pathogenic, low pathogenic, or a probiotic bacterium. The chimeric proteins include chimeras of, e.g., phage coat and / or colicin proteins, bacterial toxins and / or enzymes, autotransporter peptides, lytic peptides, multimerization domains, and / or membrane transducing (ferry) peptides. The active portion of the immunogenic chimeric proteins can include antigens against a wide range of parasites and infectious agents, cancers, Alzheimer's and Huntington's diseases, and have enhanced activity when secreted or released by the bacteria, and / or have direct anti-parasite or infectious agent activity. The activity of the secreted proteins is further increased by co-expression of a protease inhibitor that prevents degradation of the effector peptides. Addition of an antibody binding or antibody-degrading protein further prevents the premature elimination of the vector and enhances the immune response.
Owner:BERMUDES DAVID G DR
Bicyclic Nucleosides and Nucleotides as Therapeutic Agents
Owner:BIOTA SCI MANAGEMENT PTI LTD
Human monoclonal antibodies to programmed death 1 (pd-1) and methods for treating cancer using anti-pd-1 antibodies alone or in combination with other immunotherapeutics
ActiveCN103059138ARadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesInfectious Disorder
The present invention provides isolated monoclonal antibodies, particularly human monoclonal antibodies, that specifically bind to PD-1 with high affinity. Nucleic acid molecules encoding the antibodies of the invention, expression vectors, host cells and methods for expressing the antibodies of the invention are also provided. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for detecting PD-1, as well as methods for treating various diseases, including cancer and infectious diseases, using anti-PD-1 antibodies. The present invention further provides methods for using a combination immunotherapy, such as the combination of anti-CTLA-4 and anti-PD-1 antibodies, to treat hyperproliferative disease, such as cancer. The invention also provides methods for altering adverse events related to treatment with such antibodies individually.
Owner:ONO PHARMA CO LTD +1
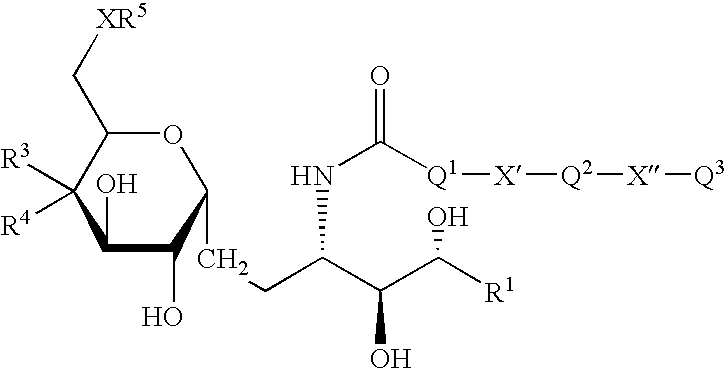
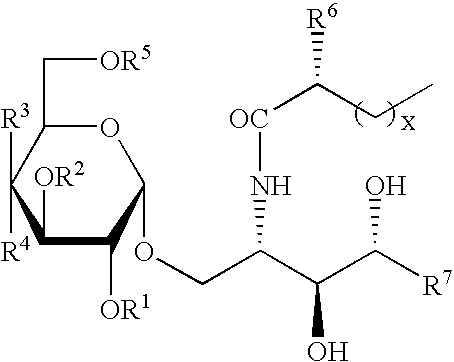
Synthetic C-glycolipid and its use for treating cancer, infectious diseases, and autoimmune diseases
InactiveUS20060019246A1Improve in vivo stabilityBiocideAntiviralsAutoimmune conditionInfectious Disorder
The invention is directed to compounds of formula (I) wherein X is O or NH; R′ is a hydrocarbon chain; R3 and R4 are hydrogen, OH or a monosaccharide; R5 is hydrogen or a monosaccharide; Q′ is optionally present and may be a C1-10 hydrocarbon; X′ is optionally present and may be O, S or NR8; and Q3 may be a hydrocarbon or hydrogen. The invention is also directed to the use of the compounds for treating cancer, infectious diseases and autoimmune diseases. The invention is also directed to syntheses of the compounds of formula (I).
Owner:NEW YORK UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com