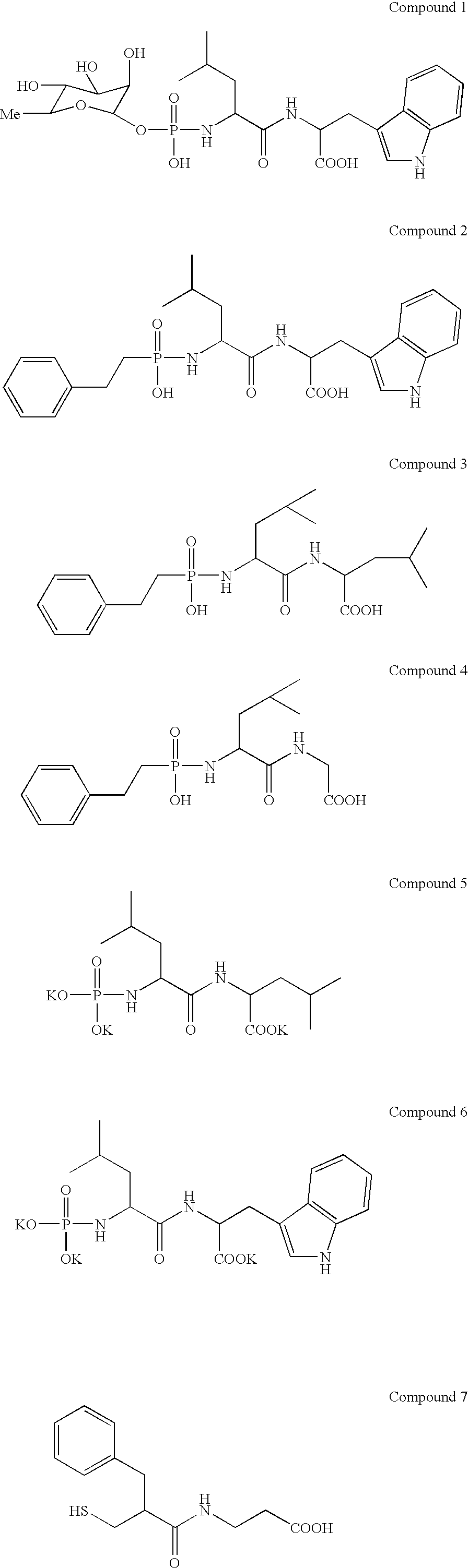
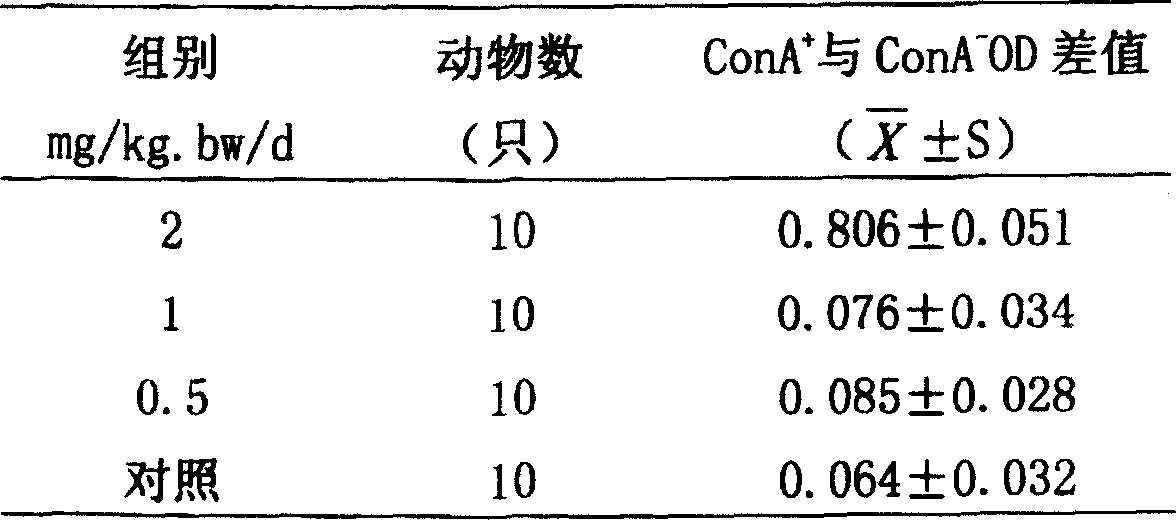
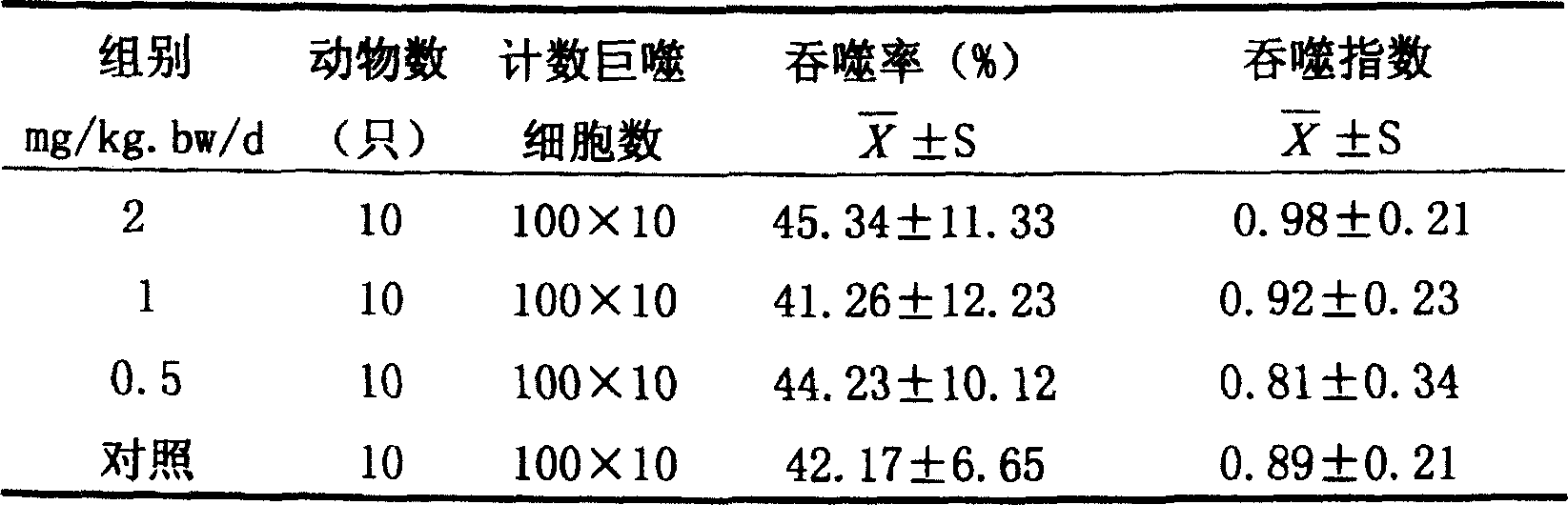
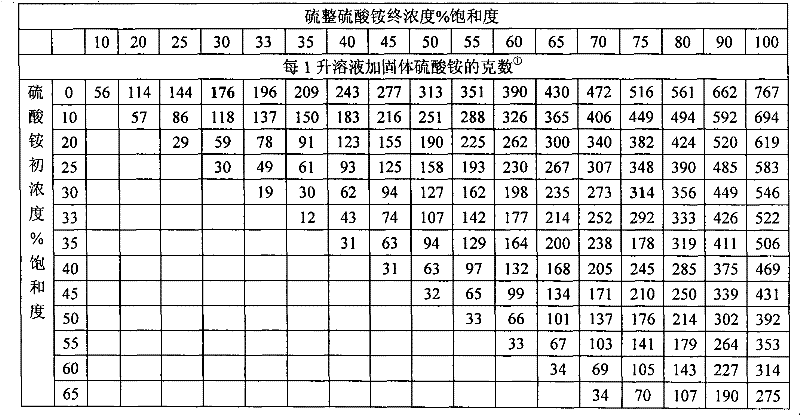
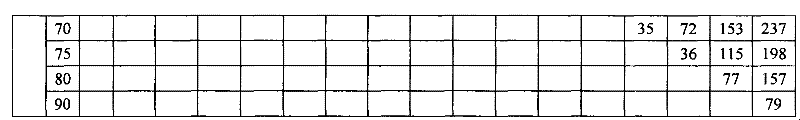
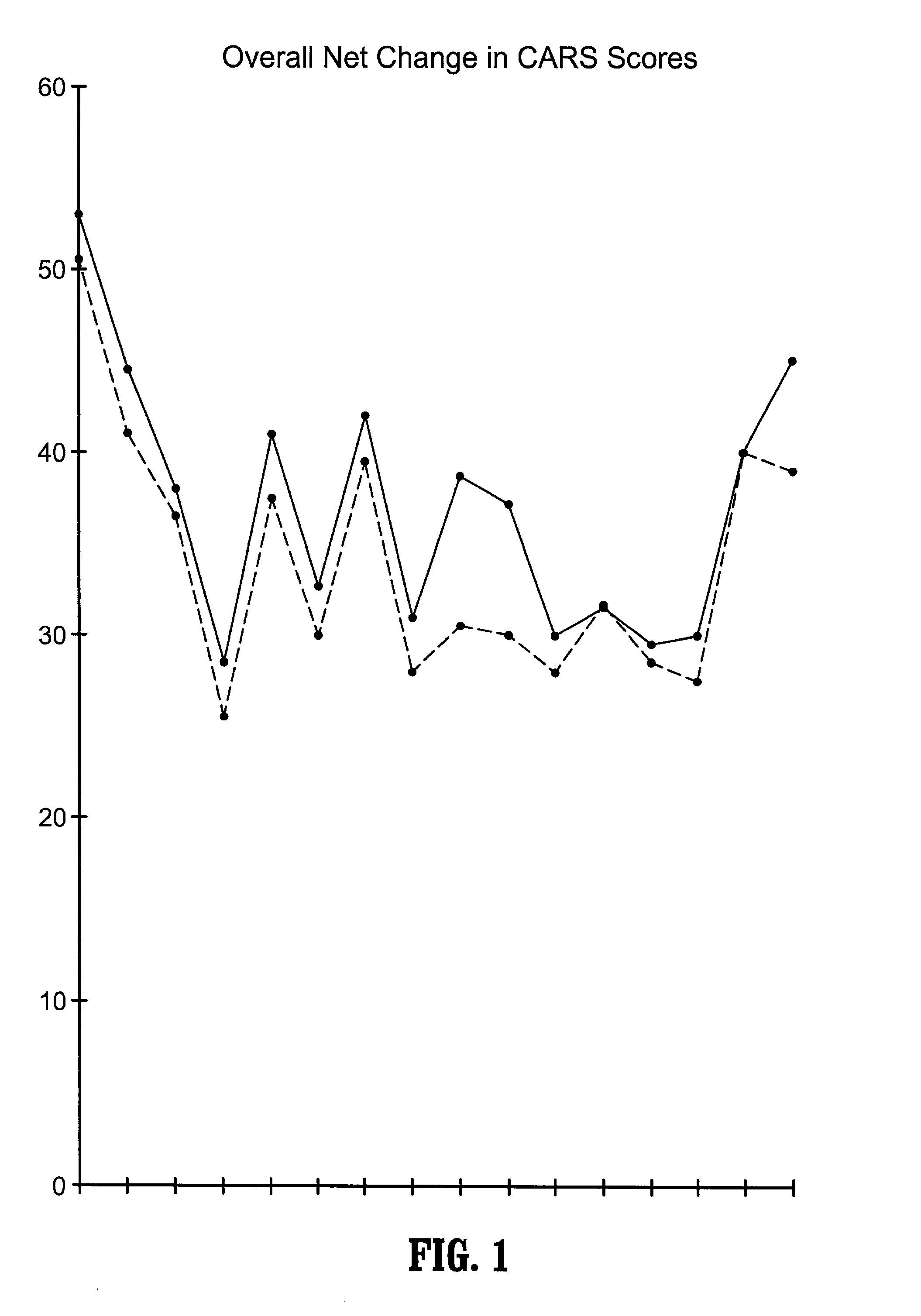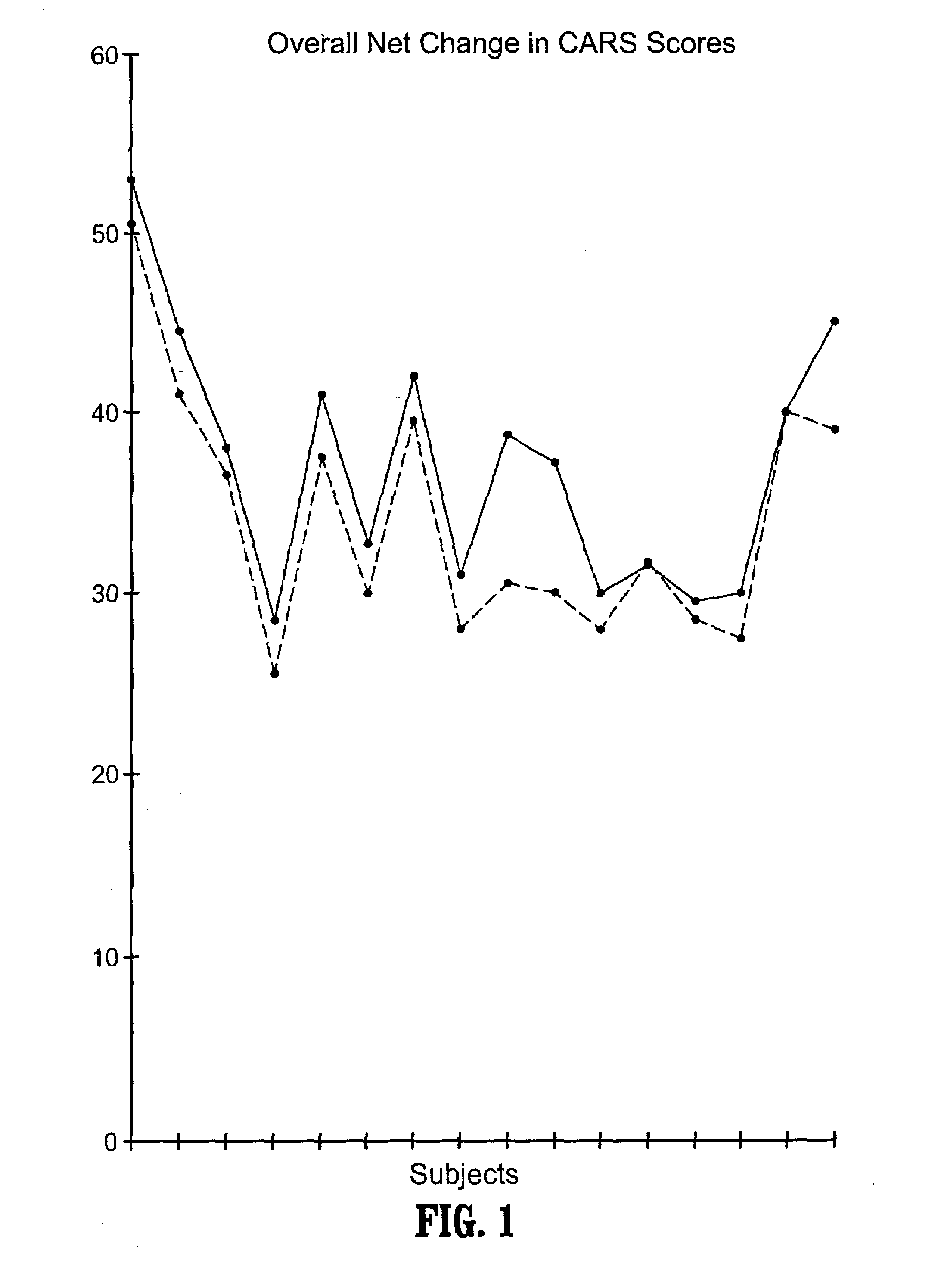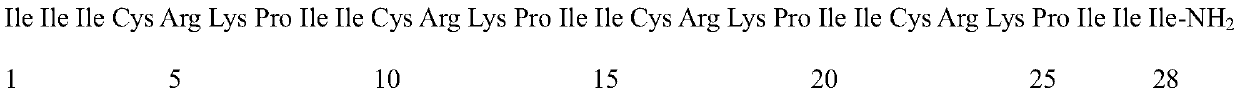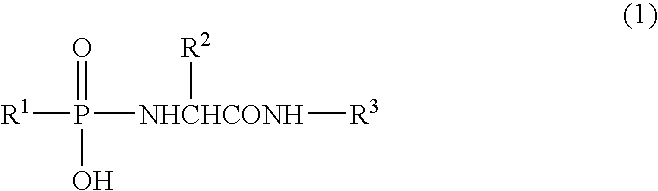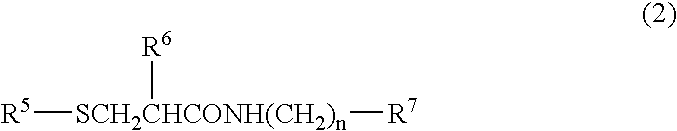Patents
Literature
266 results about "Chymotrypsin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
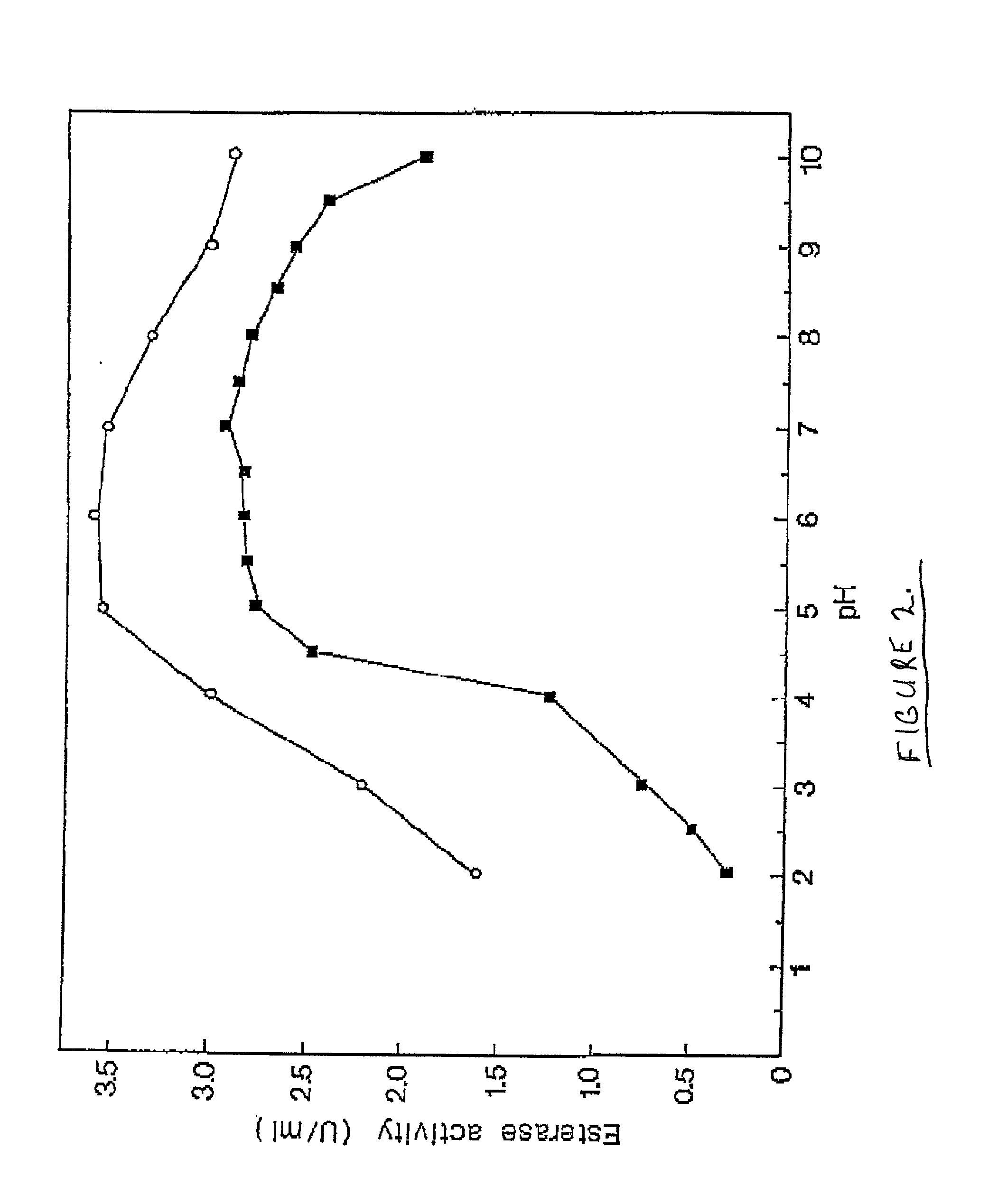
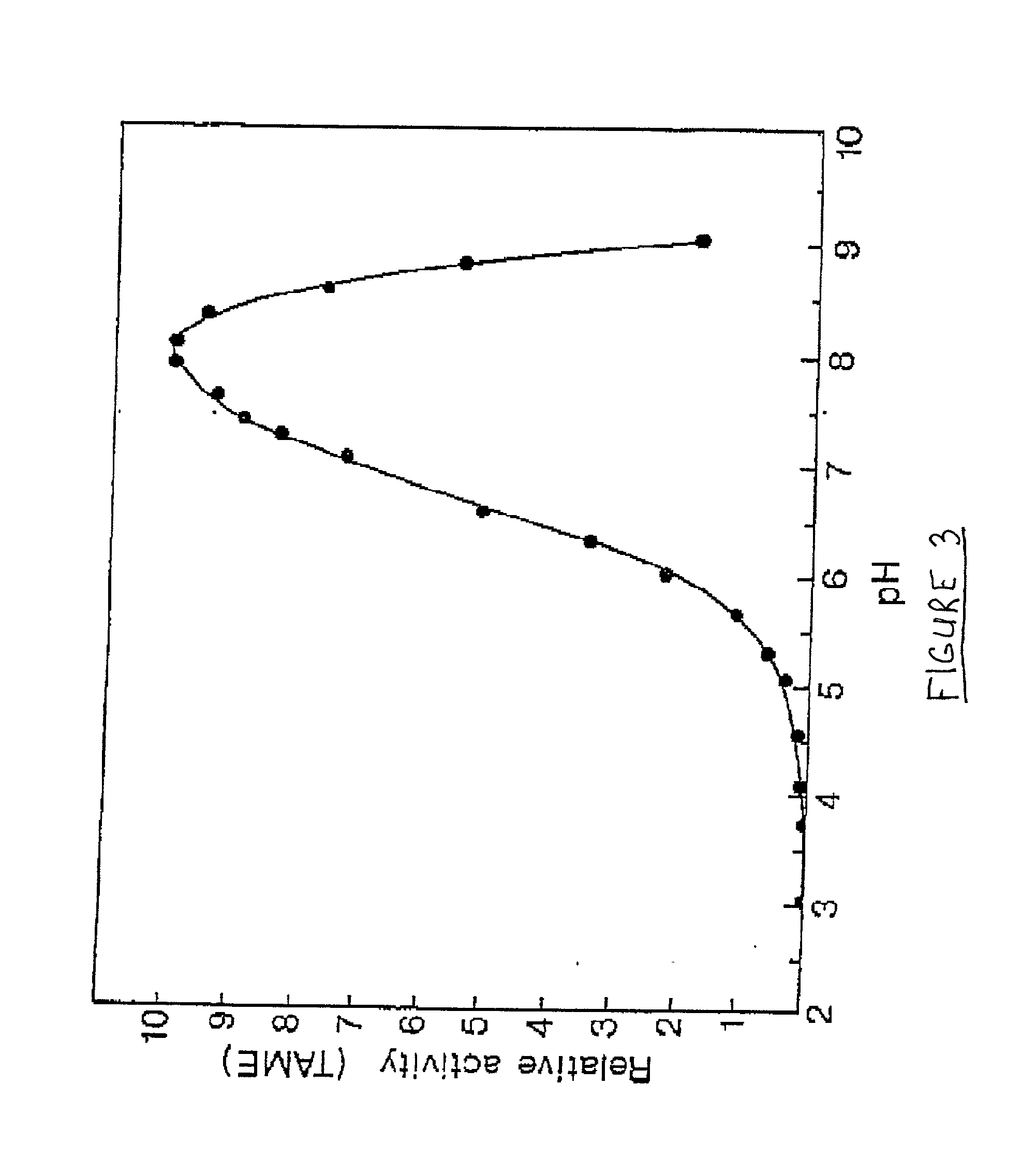
Chymotrypsin (EC 3.4.21.1, chymotrypsins A and B, alpha-chymar ophth, avazyme, chymar, chymotest, enzeon, quimar, quimotrase, alpha-chymar, alpha-chymotrypsin A, alpha-chymotrypsin) is a digestive enzyme component of pancreatic juice acting in the duodenum, where it performs proteolysis, the breakdown of proteins and polypeptides. Chymotrypsin preferentially cleaves peptide amide bonds where the side chain of the amino acid C-terminal to the scissile amide bond (the P₁ position) is a large hydrophobic amino acid (tyrosine, tryptophan, and phenylalanine). These amino acids contain an aromatic ring in their side chain that fits into a hydrophobic pocket (the S₁ position) of the enzyme. It is activated in the presence of trypsin. The hydrophobic and shape complementarity between the peptide substrate P₁ side chain and the enzyme S₁ binding cavity accounts for the substrate specificity of this enzyme. Chymotrypsin also hydrolyzes other amide bonds in peptides at slower rates, particularly those containing leucine and methionine at the P₁ position.
Pharmaceutical preparations for attention deficit disorder, attention deficit hyperactivity disorder and other associated disorders
InactiveUS20070116695A1Well formedFormulation stabilityBiocidePeptide/protein ingredientsChymotrypsinAttention deficits
A pharmaceutical preparation for the treatment of attention deficit disorders combines a therapeutically effective amount of digestive enzymes, such as chymotrypsin, and medication used to treat attention deficit disorders, such as Ritalin®, Concert®, Adderall® and Strattera®. The preparation may be in the form of a tablet, capsule or time released formula in order to reduce the amount of pills per dosage. The pharmaceutical preparation ameliorates the symptoms of the attention deficit disorder. The preparation has a stabilizing matrix containing a solidified microcrystalline cellulose which captures and protects therapeutically effective amounts of digestive enzyme particles within the stabilizing matrix.
Owner:CUREMARK
Novel pharmaceutical preparation for preeclampsia, eclampsia, and toxemia, and their related symptoms and related disorders of pregnancy
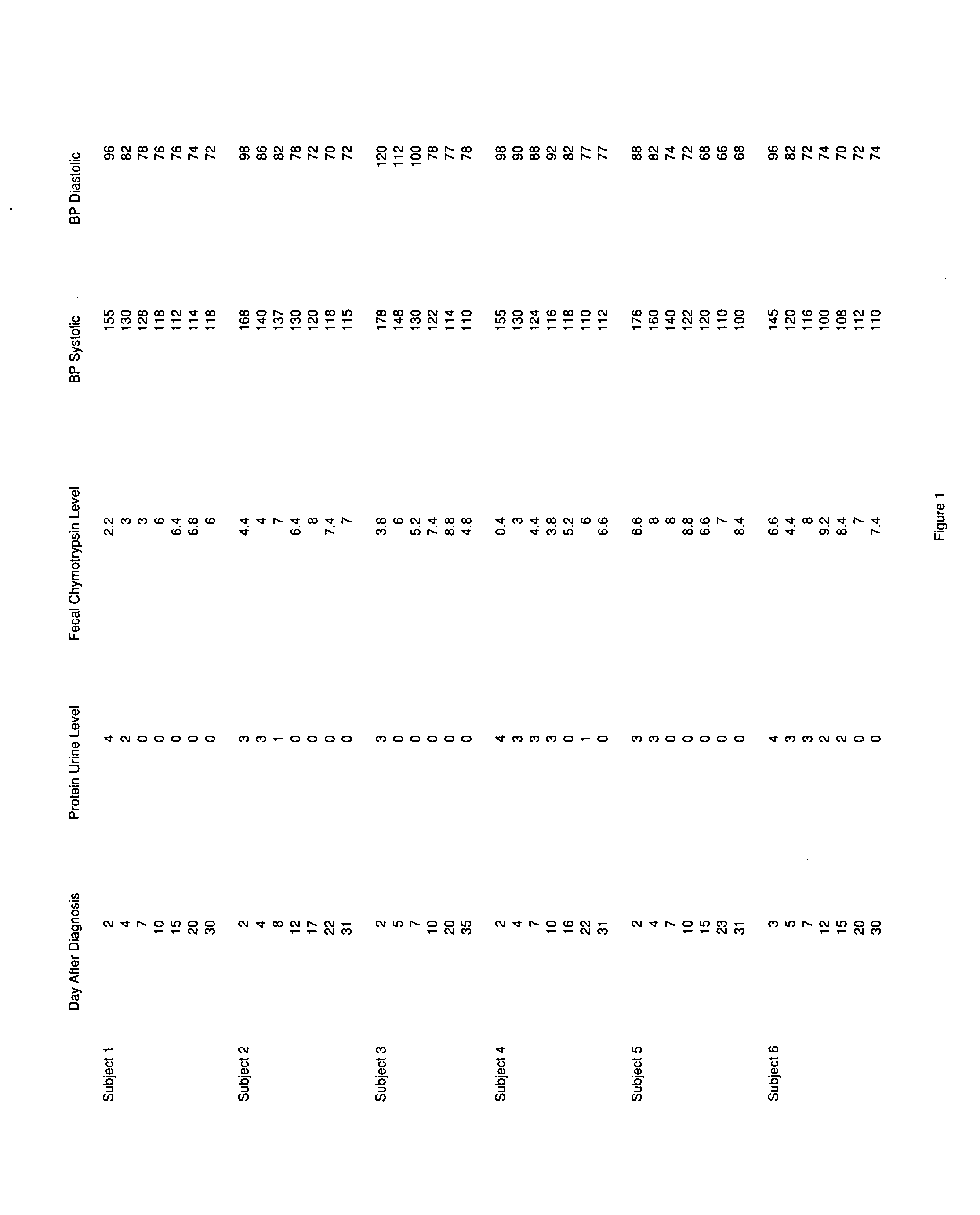
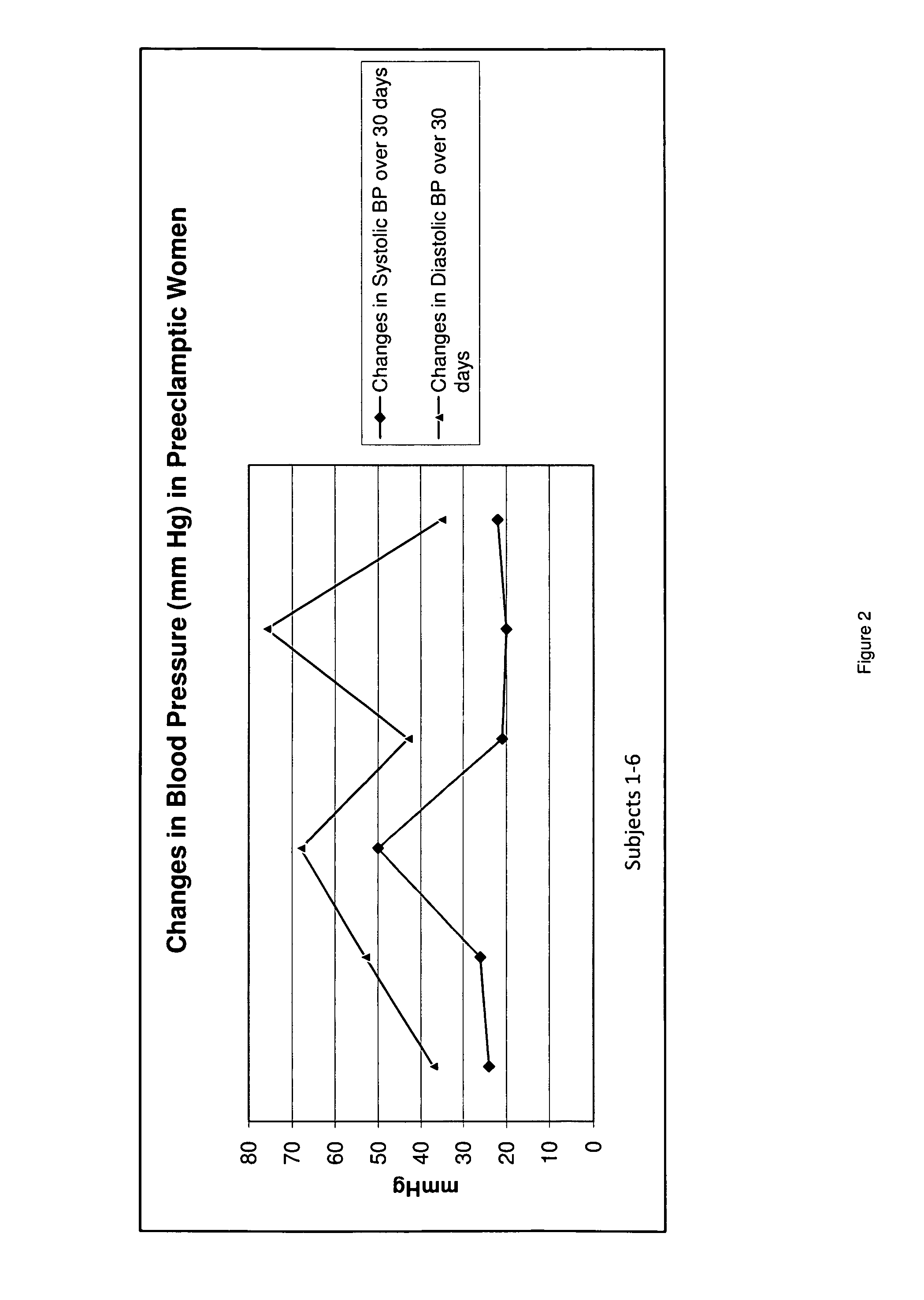
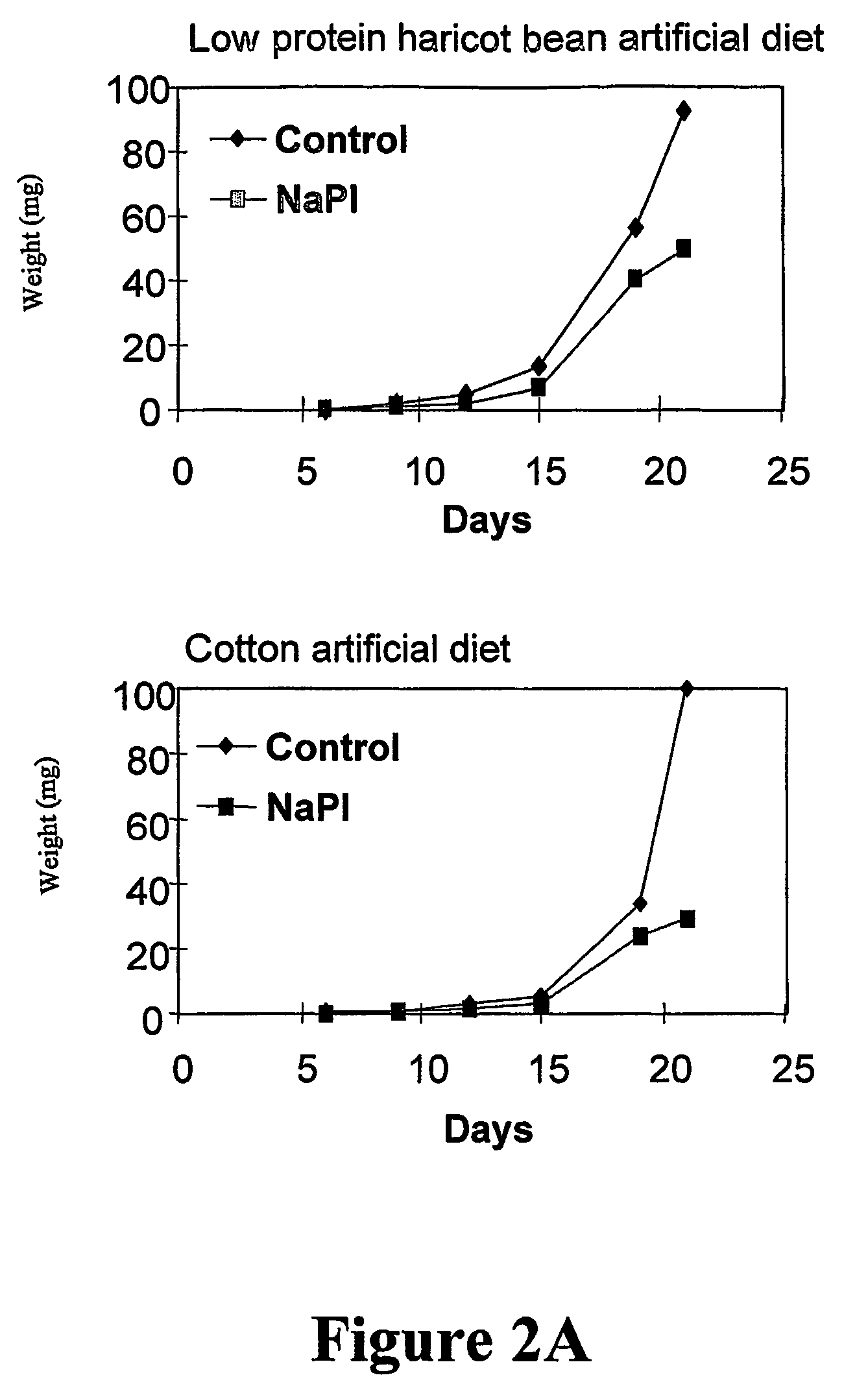
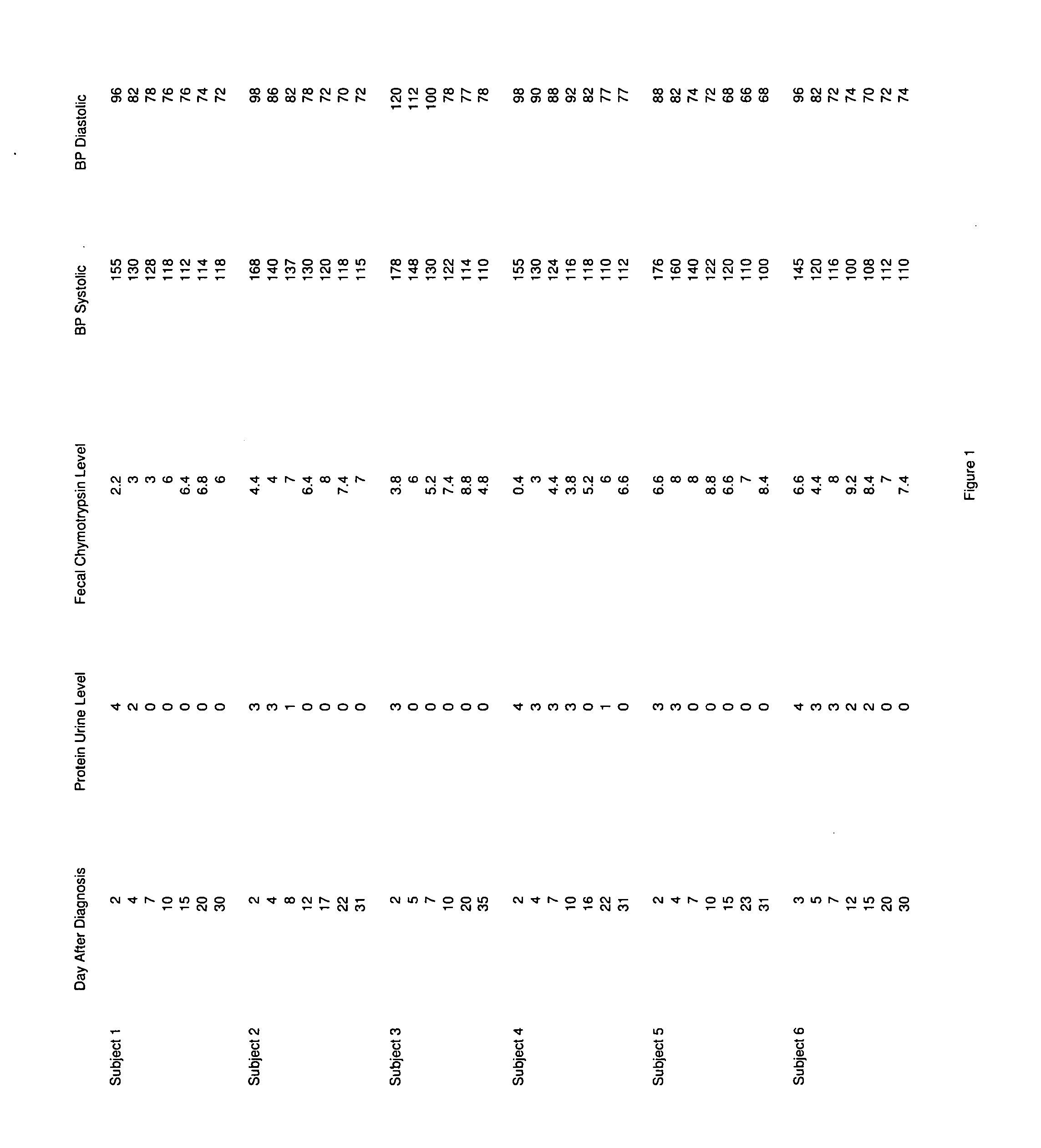
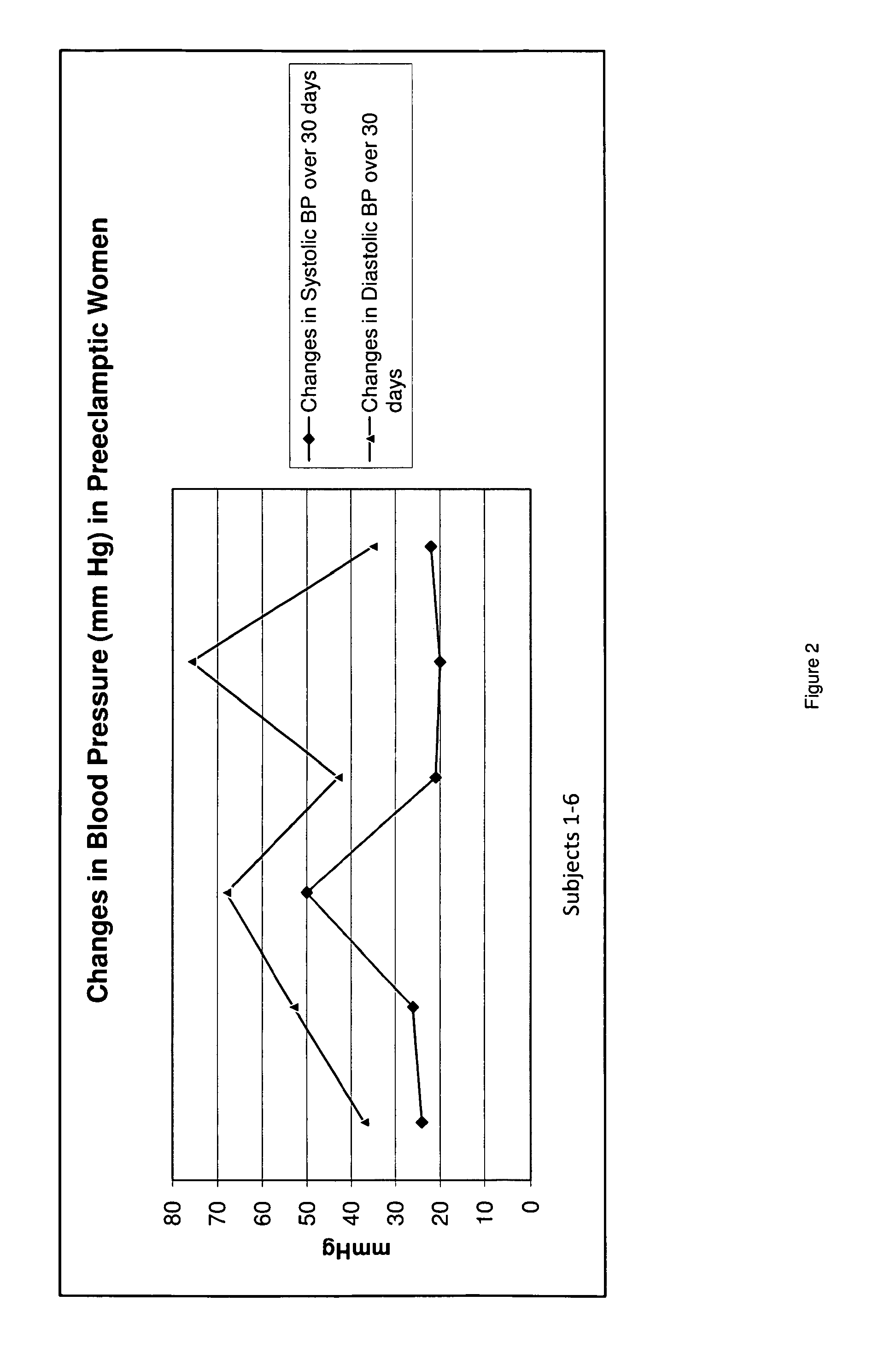
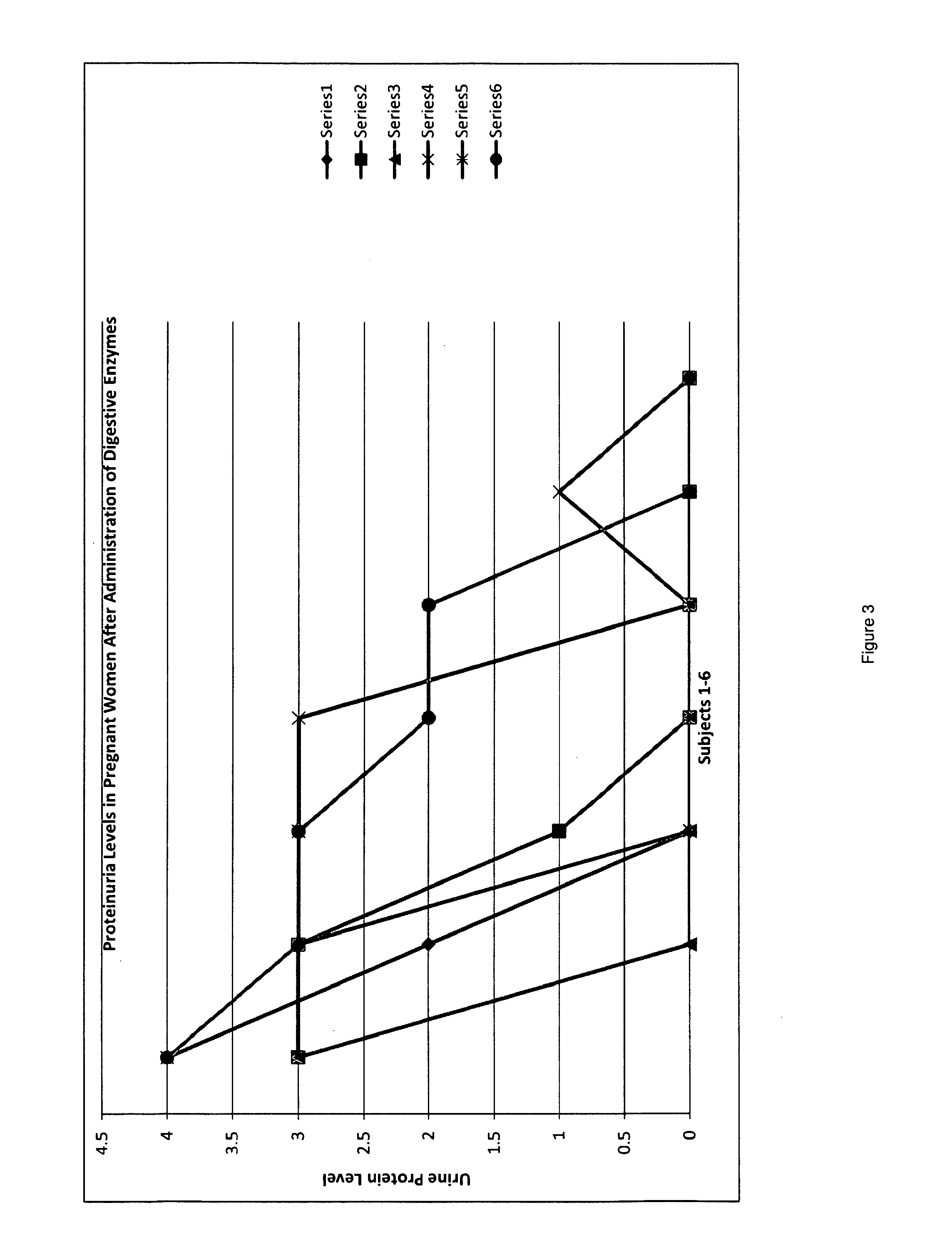
A therapeutic agent for the treatment of toxemia, preeclampsia and eclampsia and the method for preparing the therapeutic agents is disclosed. The therapeutic agent is a stable pharmaceutical preparation containing, but not limited to, digestive / pancreatic enzymes. The therapeutic agent may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic agent may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using of a biomarker, the presence of chymotrypsin in the maternal GI tract to determine the likelihood of developing preeclampsia, pregnancy induced hypertension, and eclampsia / toxemia is disclosed.
Owner:CUREMARK
Methods for treating pervasive development disorders
InactiveUS20060183180A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsDiseasePervasive developmental disorder
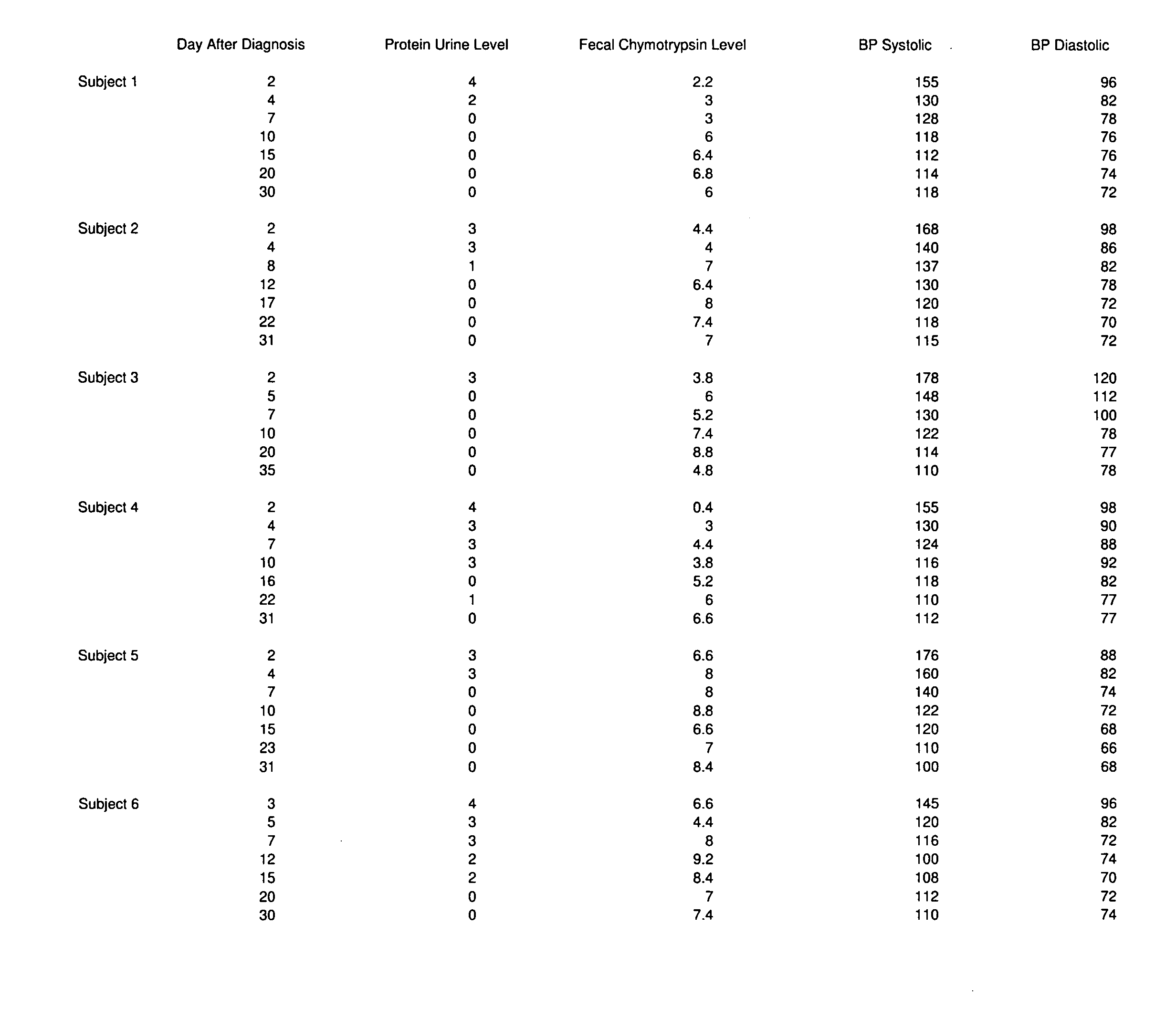
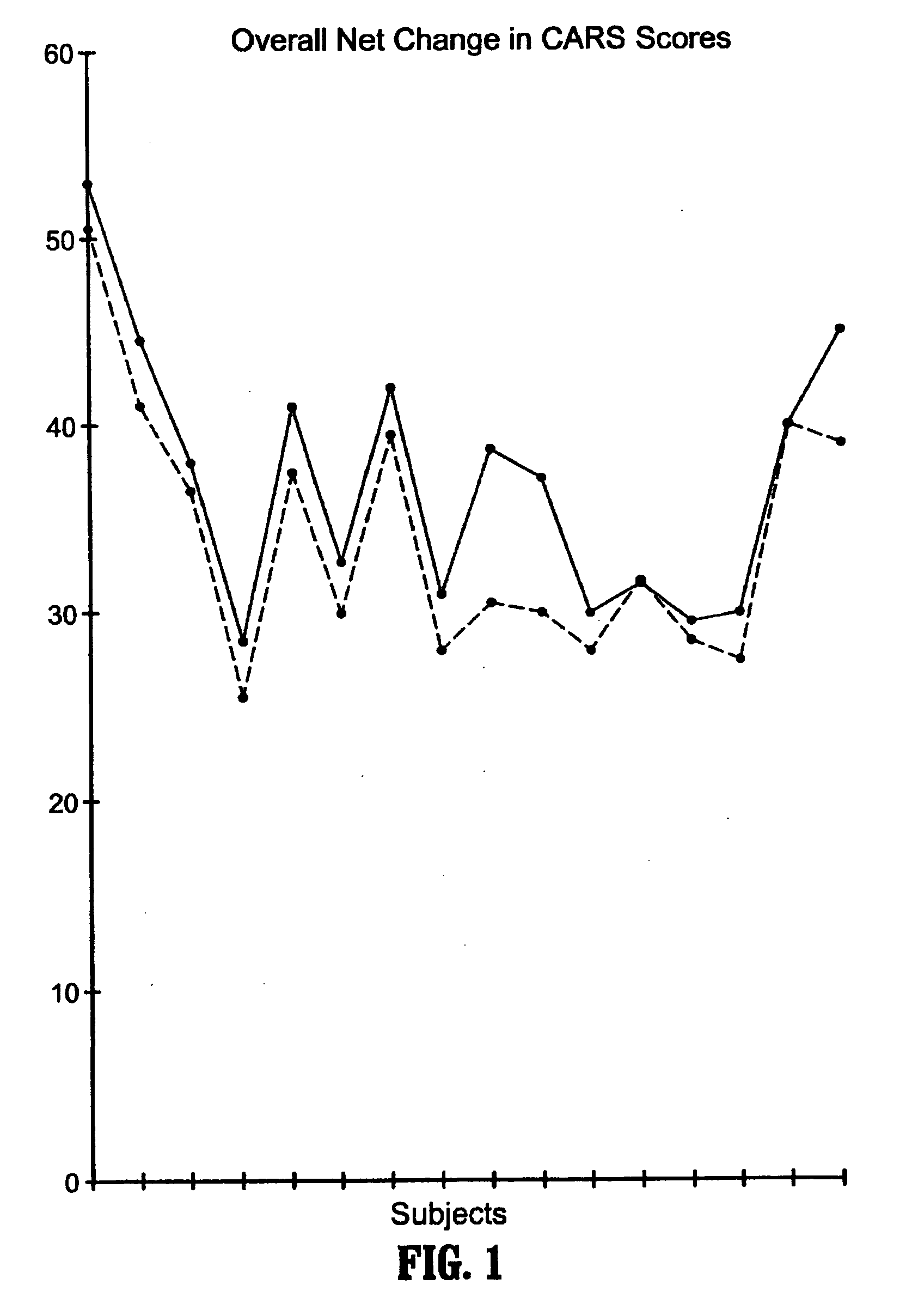
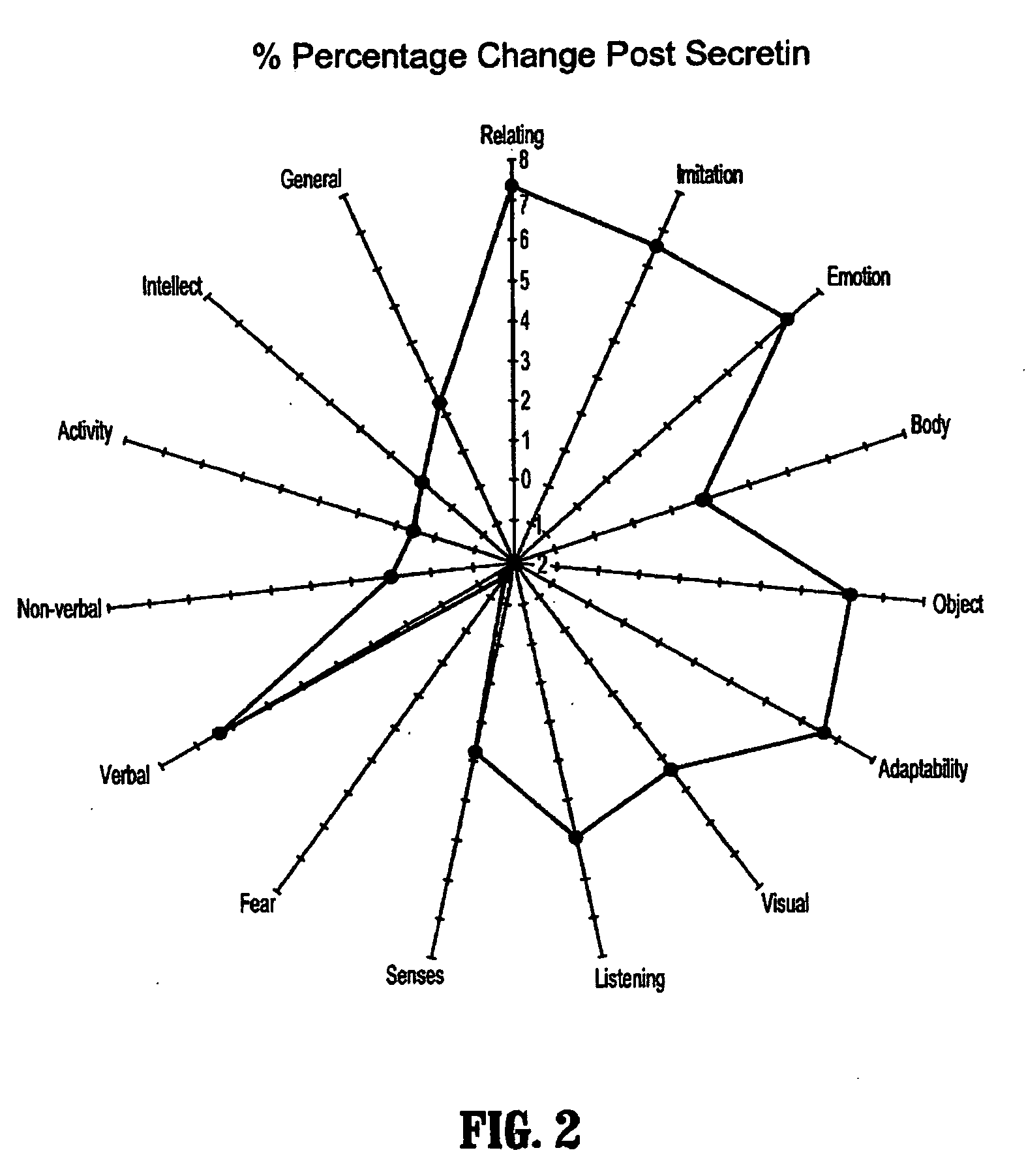
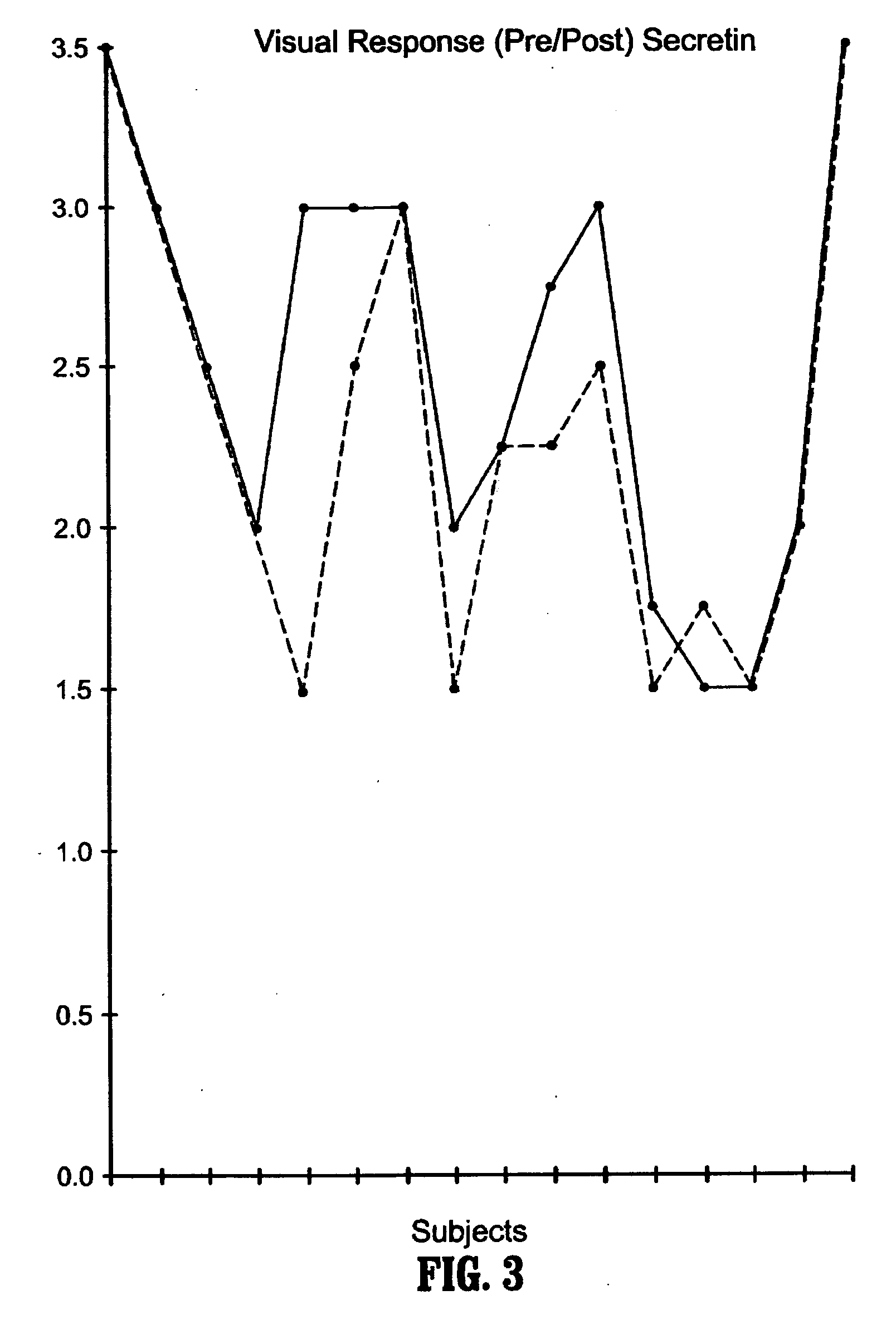
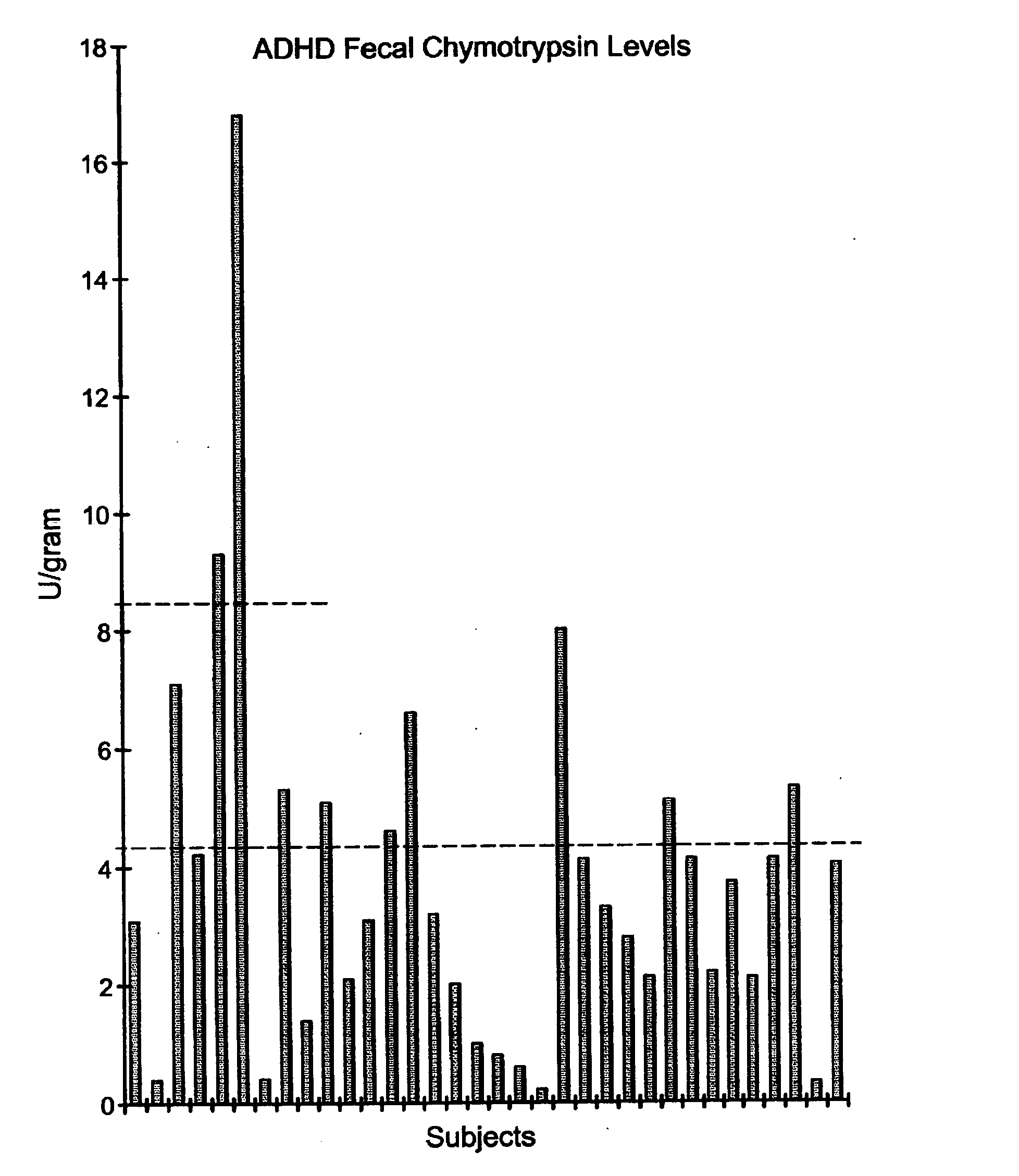
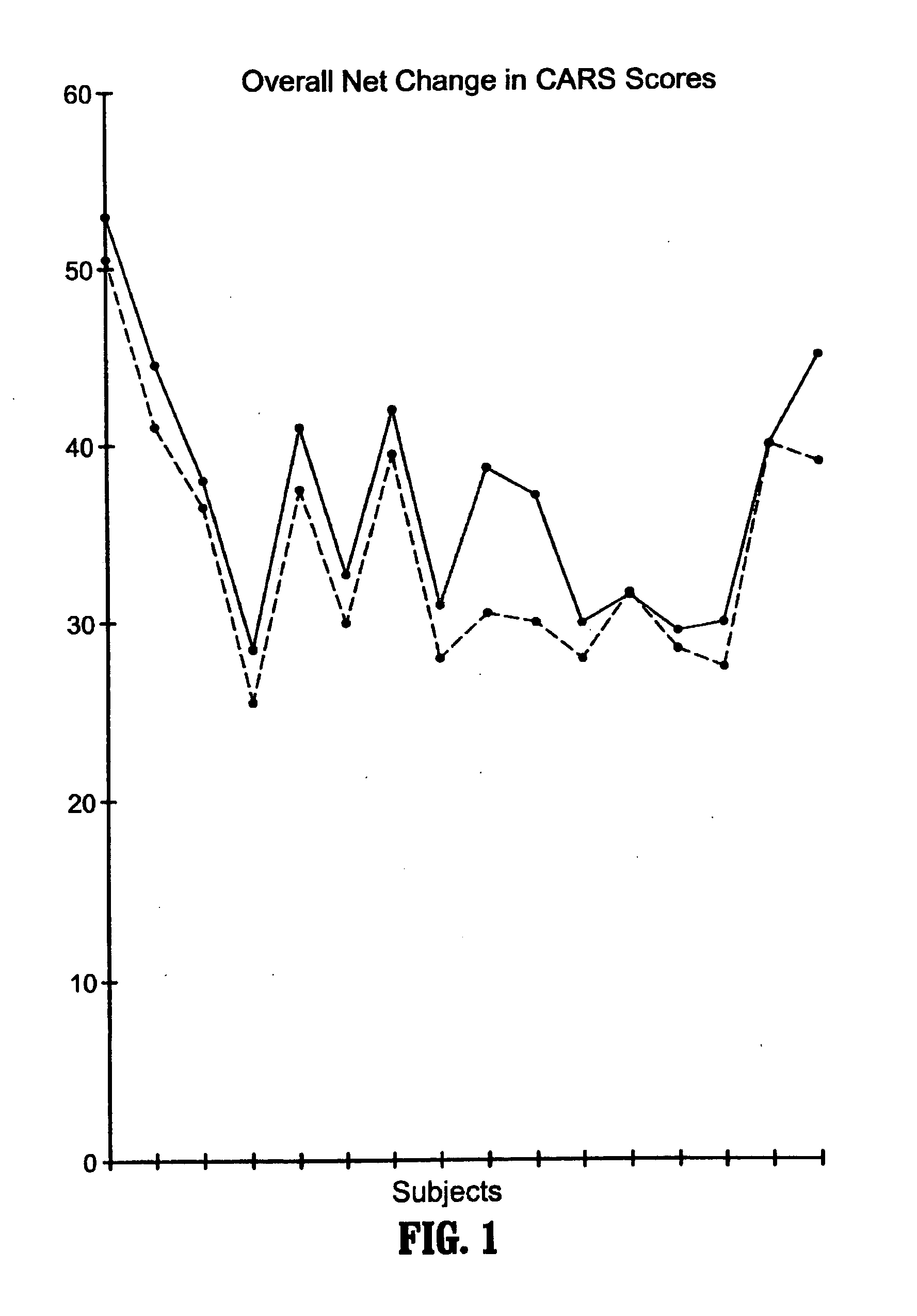
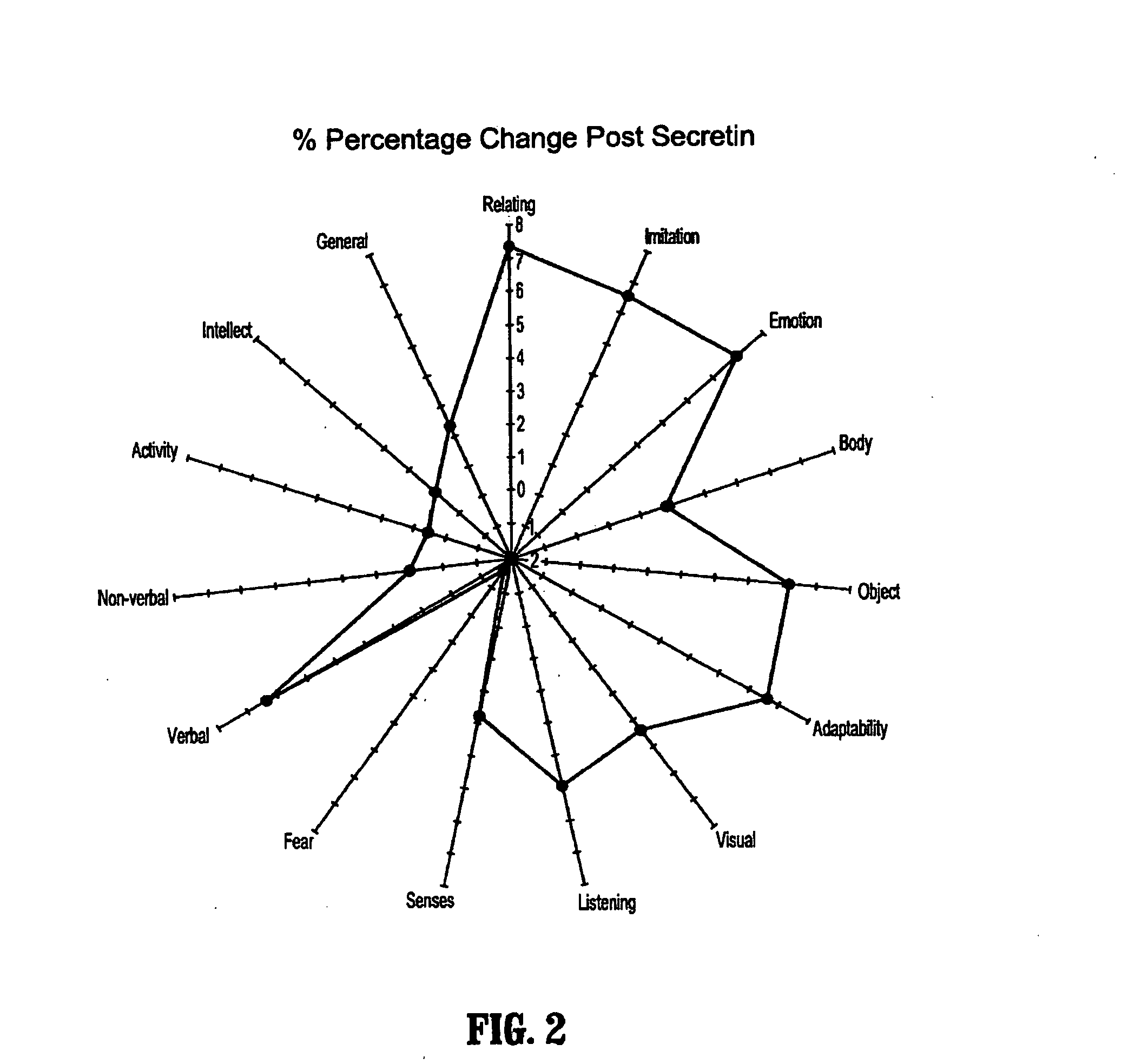
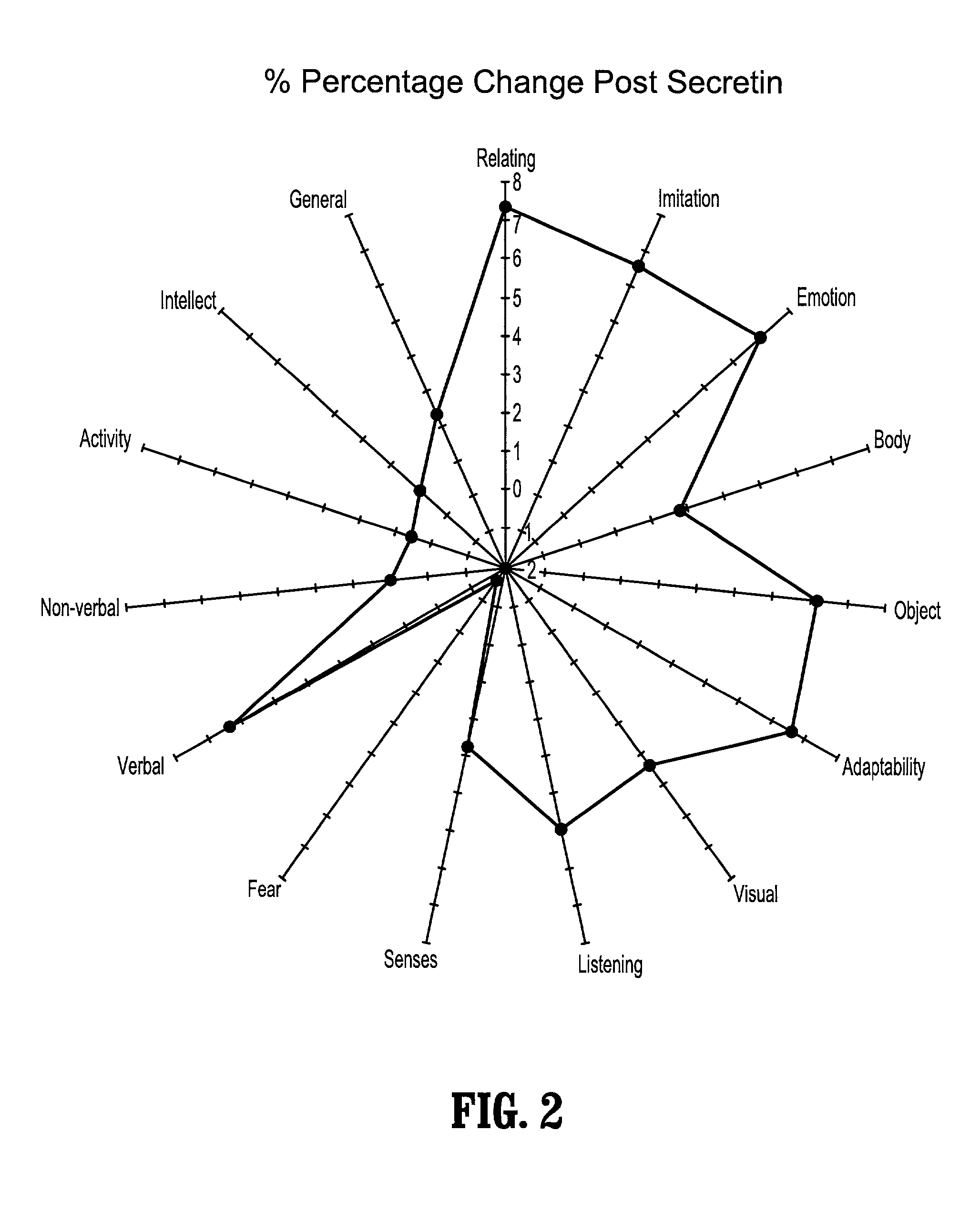
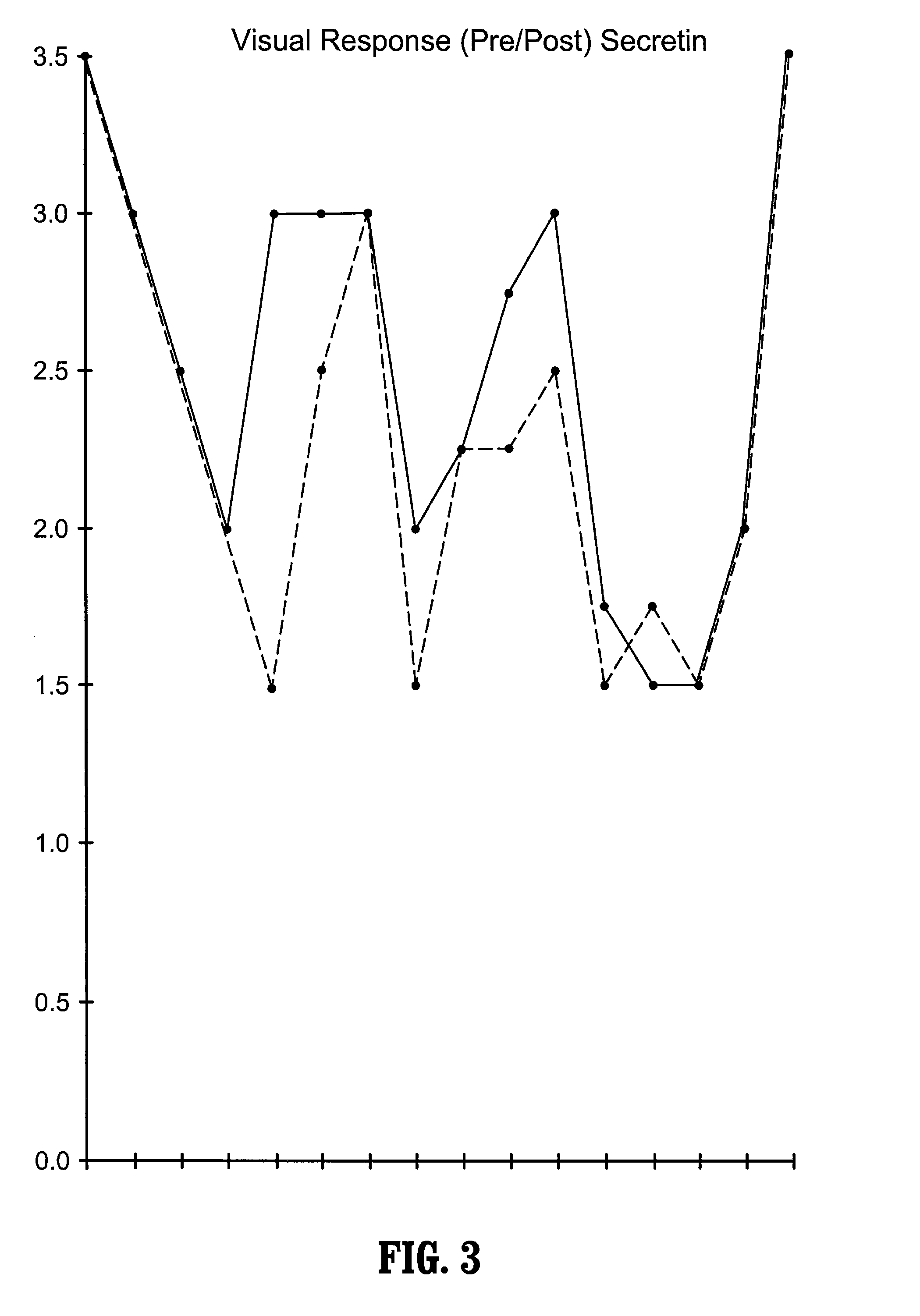
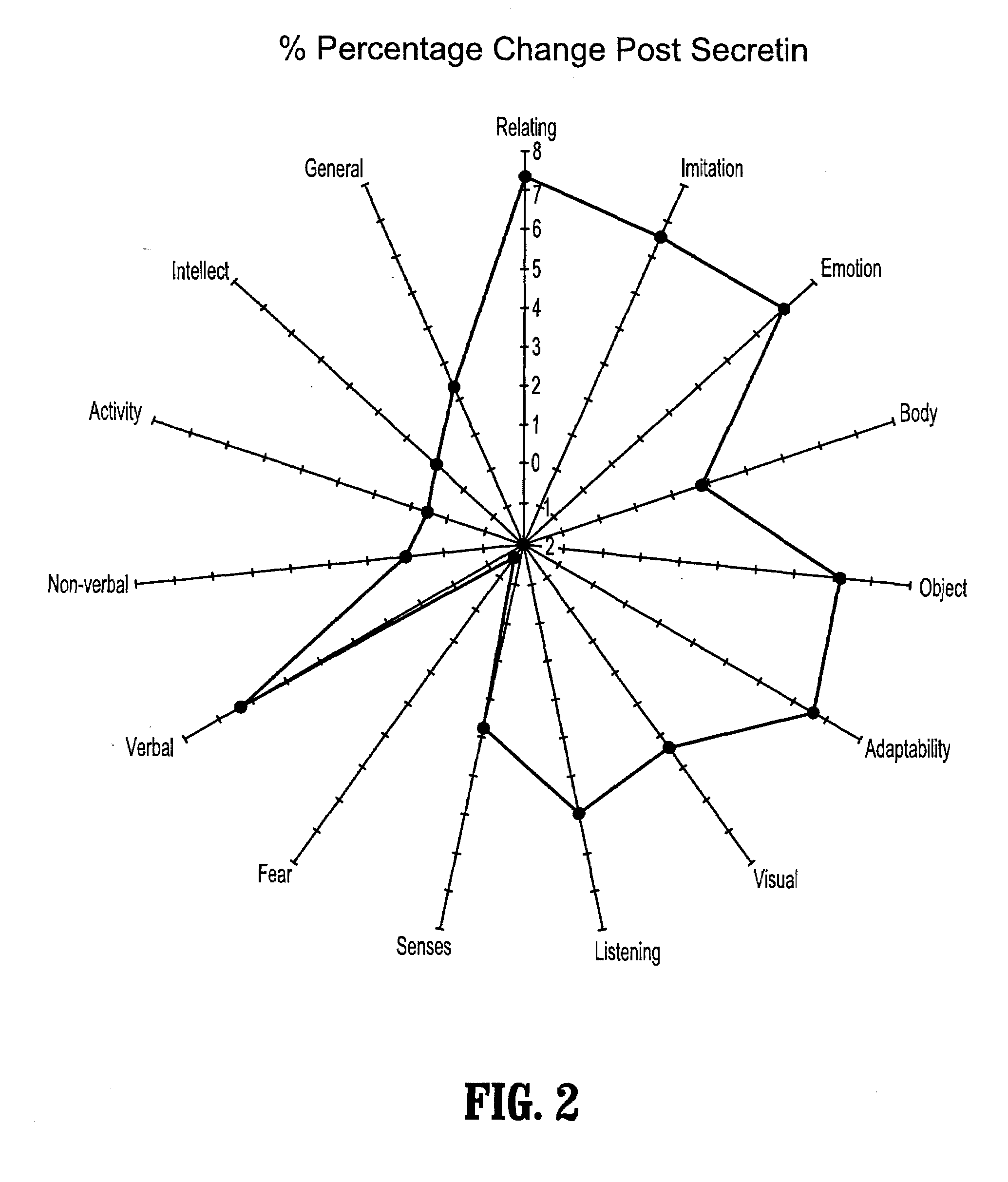
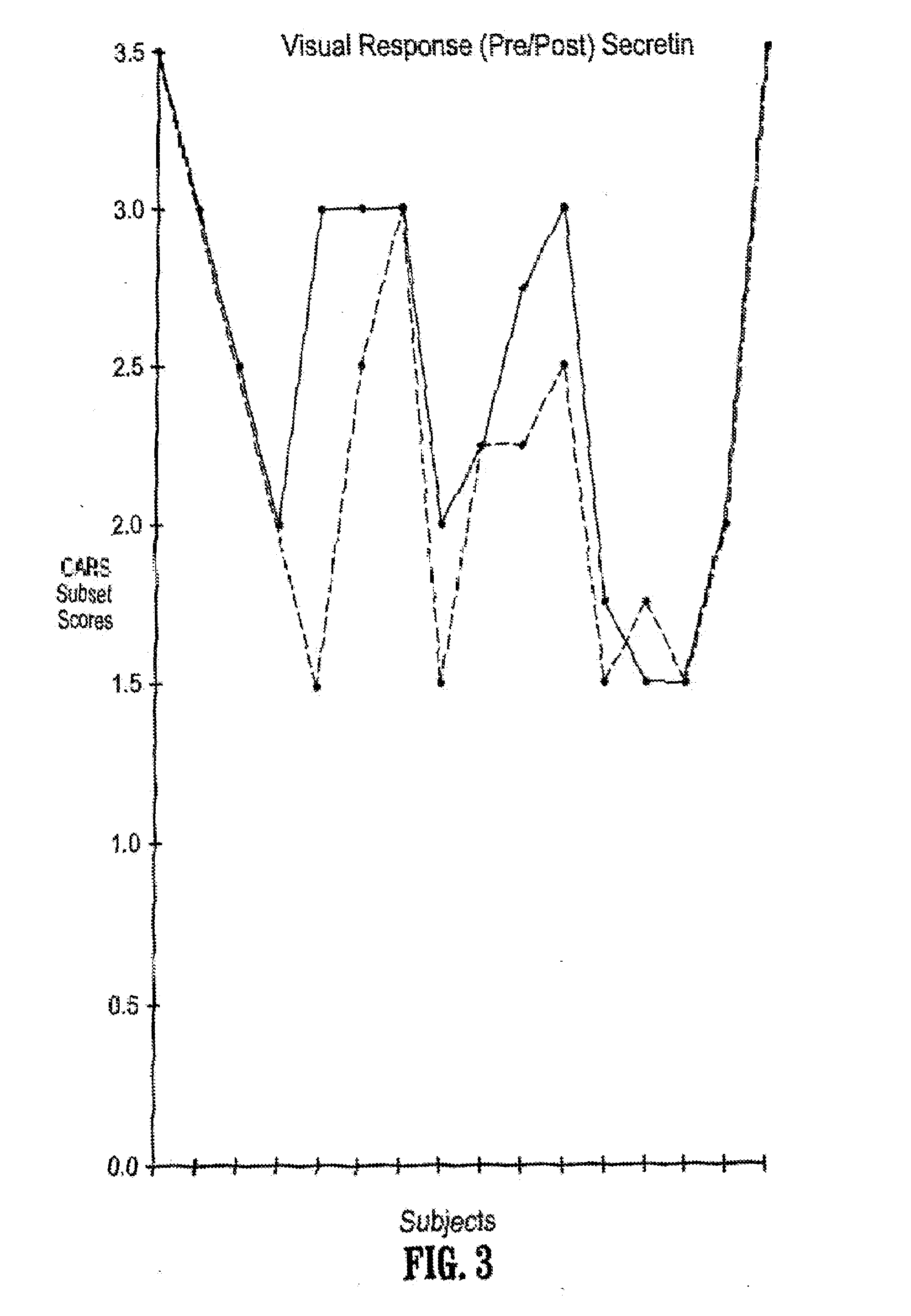
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with i PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Methods for treating pervasive development disorders
InactiveUS20060182728A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsDiseasePervasive developmental disorder
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with i PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Methods of treating pervasive development disorders
InactiveUS20020090653A1Symptoms improvedPromote digestionPeptide/protein ingredientsMicrobiological testing/measurementDiseaseFeces
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADD, ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with a PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Pharmaceutical preparation for the treatment of the symptoms of addiction and method of diagnosing same
ActiveUS20090263372A1Diminished pancreatic outputNervous disorderHydrolasesChymotrypsinPharmaceutical formulation
A therapeutic agent for the treatment of the symptoms of addiction and the method for preparing the therapeutic agent is disclosed. The therapeutic agent is a stable pharmaceutical preparation containing, but not limited to, digestive / pancreatic enzymes. The therapeutic agent may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic agent may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using of a biomarker, the presence of chymotrypsin in the gastrointestinal tract to determine the presence of symptoms of addiction, and the likelihood of relapsing into addiction is disclosed.
Owner:CUREMARK
Fish serine proteinases and their pharmaceutical and cosmetic use
InactiveUS20020141987A1Avoid injuryInfection-preventing effective amount of the enzymeBiocideCosmetic preparationsAtlantic codSerine proteinases
Fish derived serine proteinases including trypsins and chymotrypsin derived from cod such as Atlantic cod is used for treating and / or preventing a variety of diseases and disorders such as inflammatory diseases, infectious diseases caused by viruses, bacteria and fungal species and diseases where a receptor binding mechanism is involved in the pathogenesis. Pharmaceutical and cosmetic compositions comprising the proteinases are described.
Owner:BJARNASON JON BRAGI
Methods of treating pervasive development disorders
InactiveUS20080219966A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsPervasive developmental disorderMethylphenidate
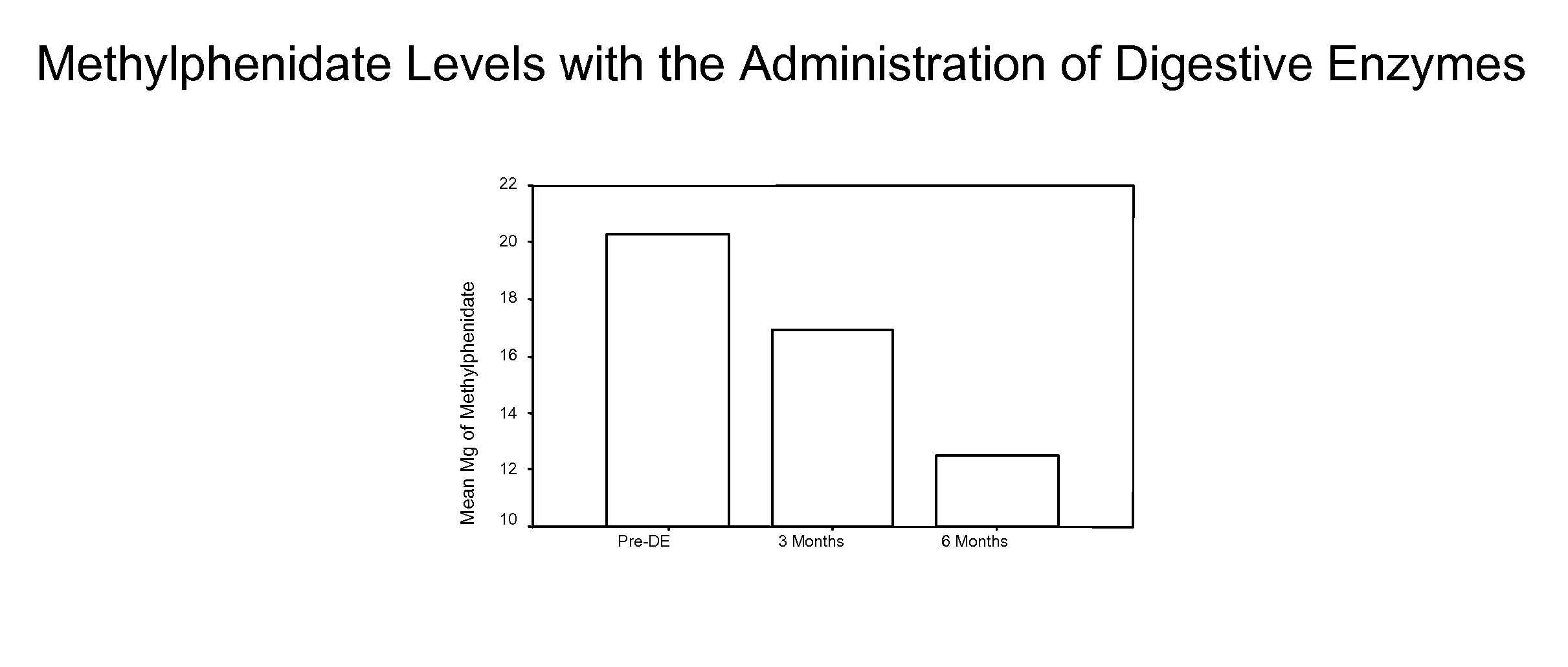
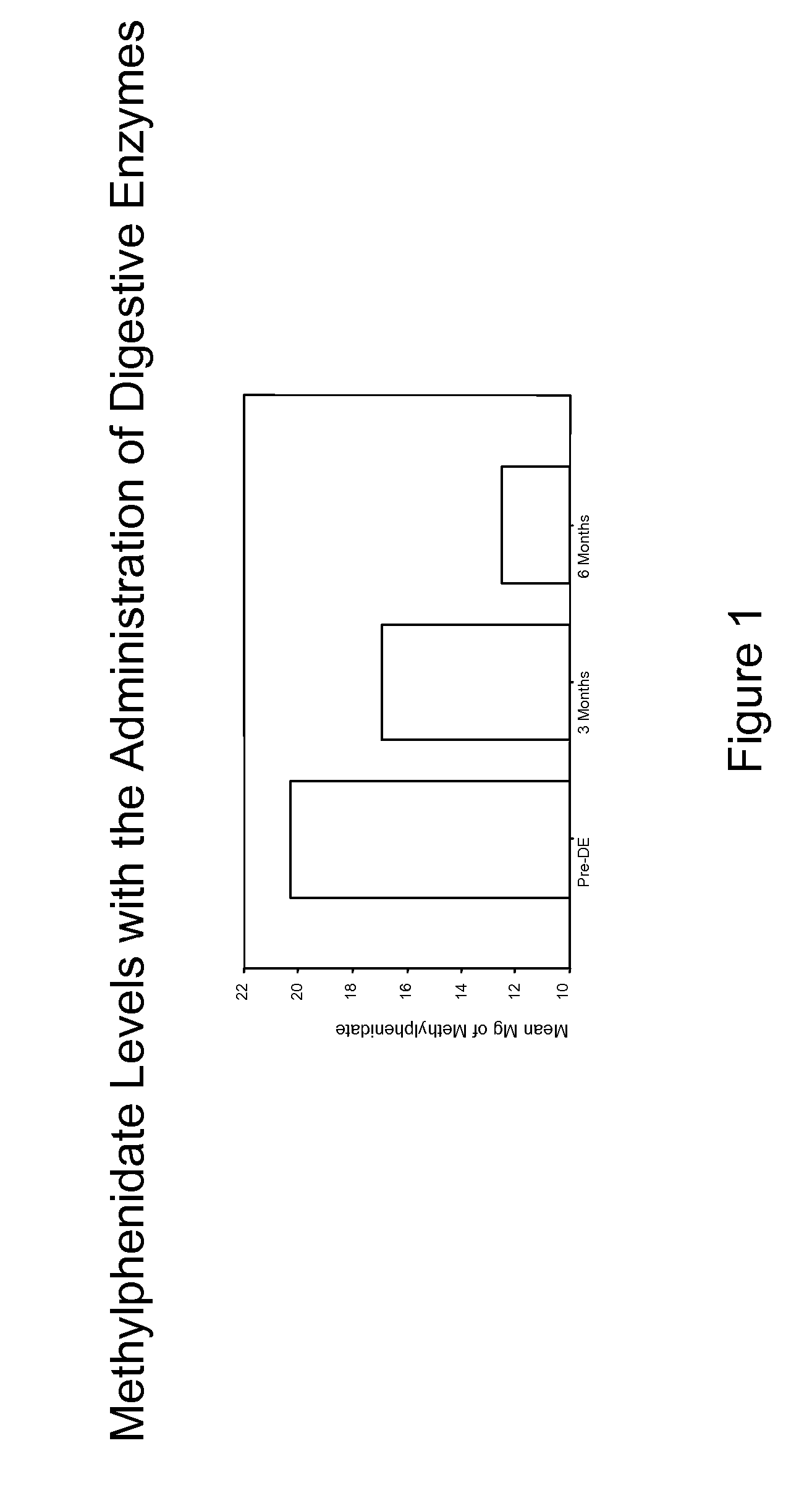
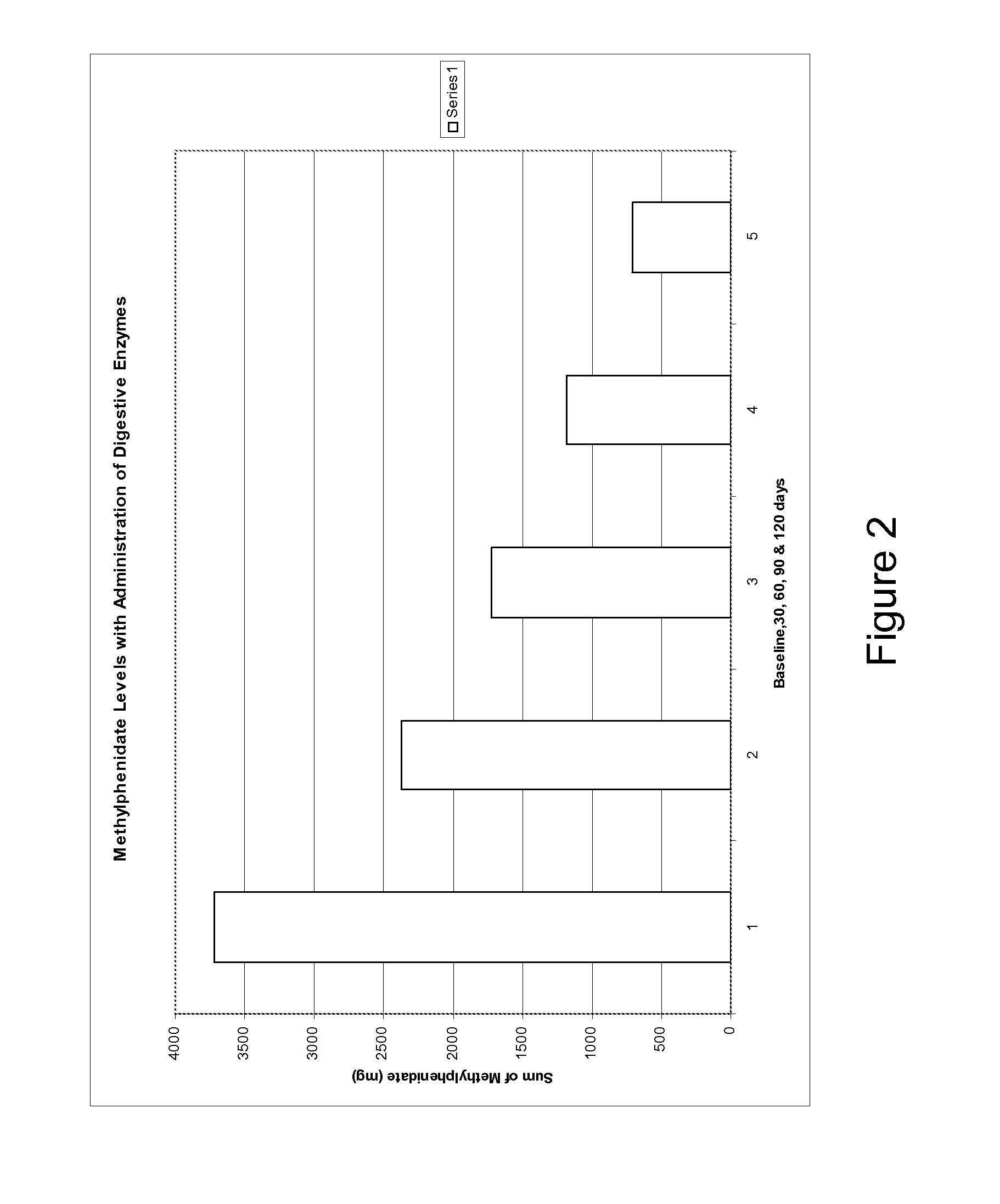
A therapeutic method for treating an individual diagnosed with PDD pervasive developmental disorder comprises determining the efficacy of digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level. A method for reducing the amount of methylphenidate (Ritalin) being taken by an individual with attention deficit disorder (ADD) or attention deficit hyperactivity disorder (ADHD) by administering a therapeutic amount of digestive enzymes is also provided.
Owner:CUREMARK
Method for the treatment of the symptoms of drug and alcohol addiction
A therapeutic agent for the treatment of the symptoms of addiction and the method for preparing the therapeutic agent is disclosed. The therapeutic agent is a stable pharmaceutical preparation containing, but not limited to, digestive / pancreatic enzymes. The therapeutic agent may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic agent may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using of a biomarker, the presence of chymotrypsin in the gastrointestinal tract to determine the presence of symptoms of addiction, and the likelihood of relapsing into addiction is disclosed.
Owner:CUREMARK
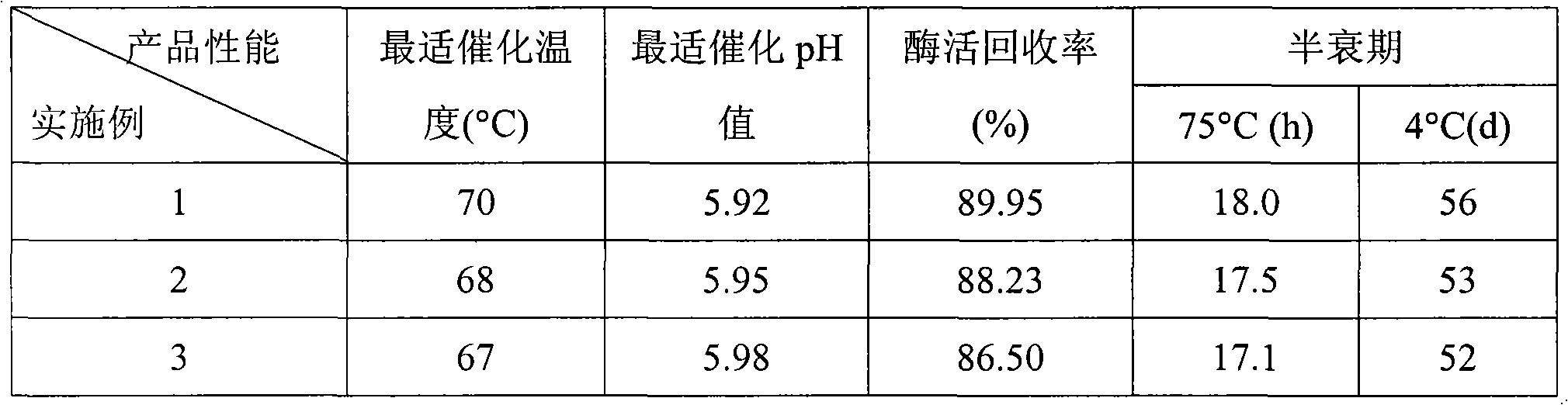
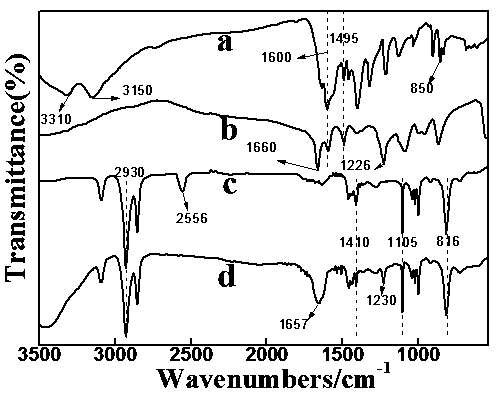
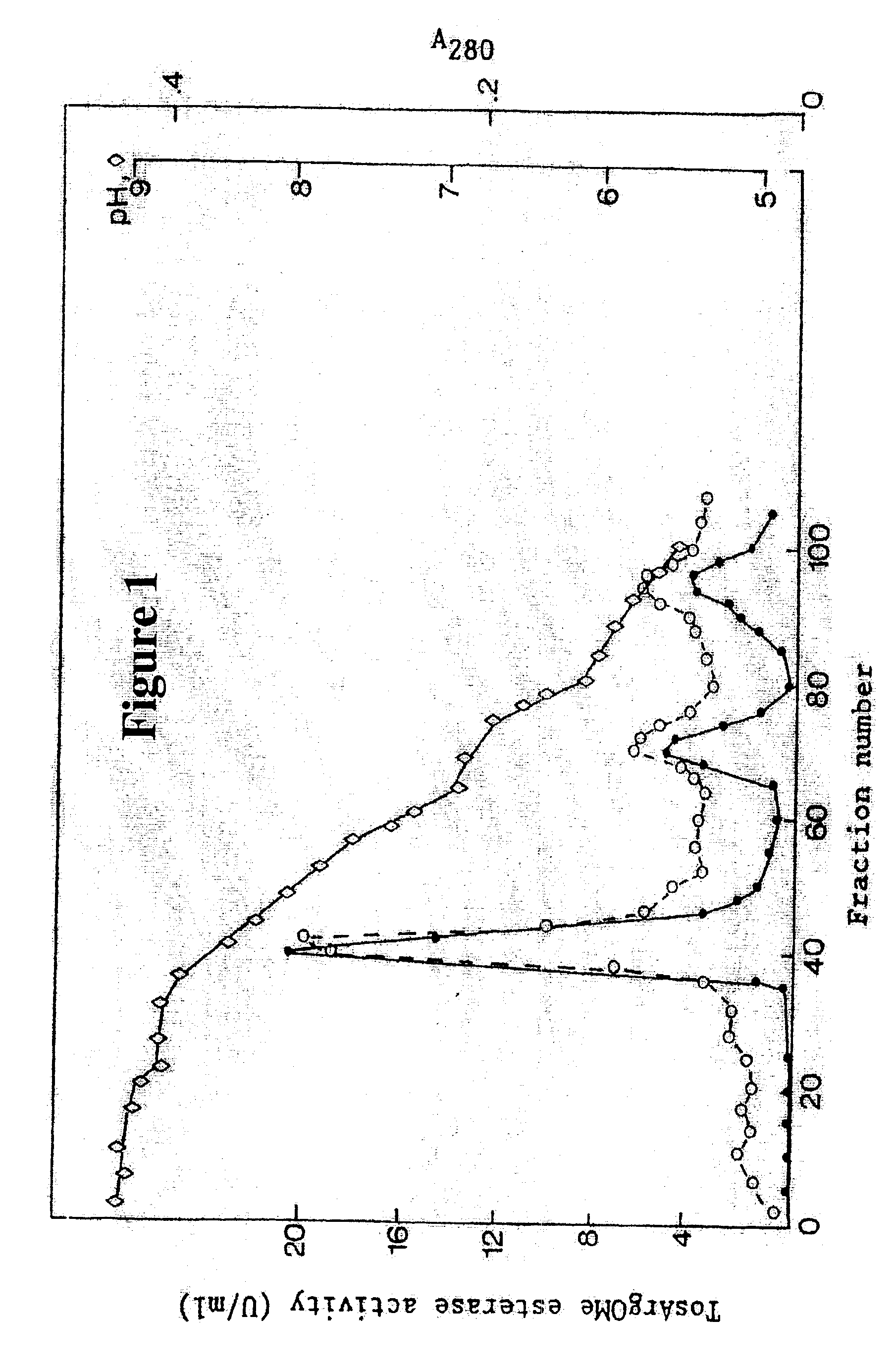
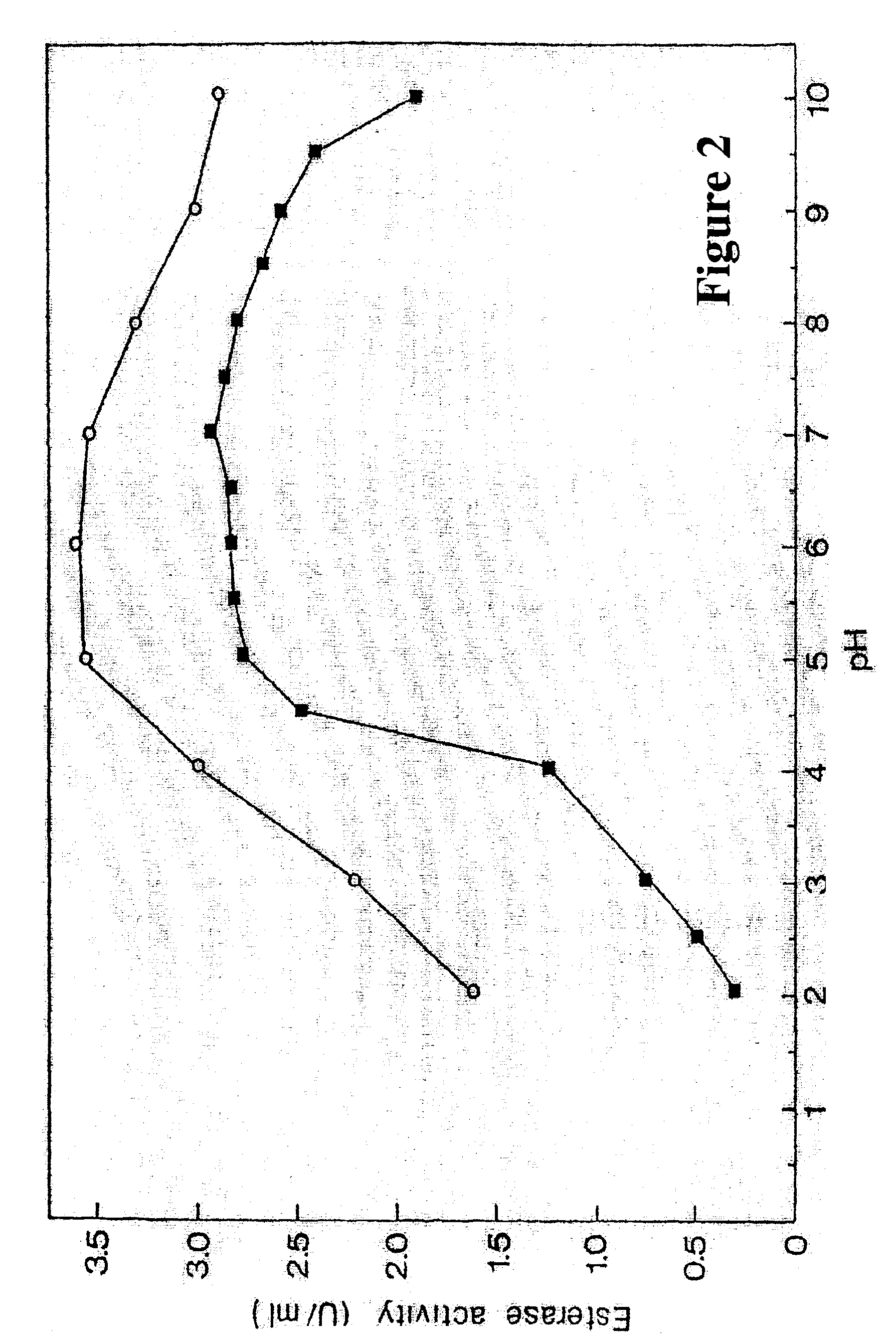
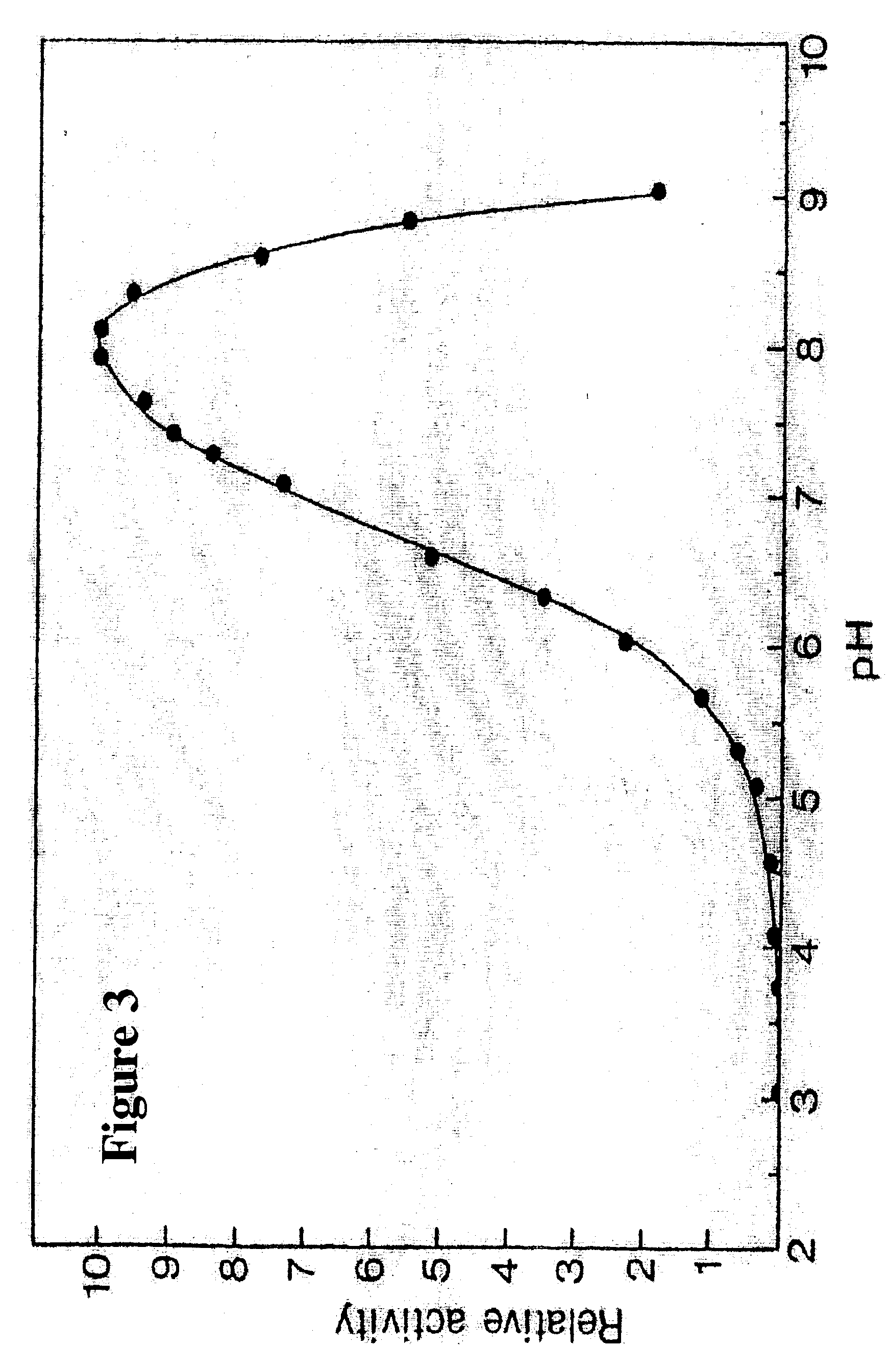
Method for preparing high purity chymotrypsin
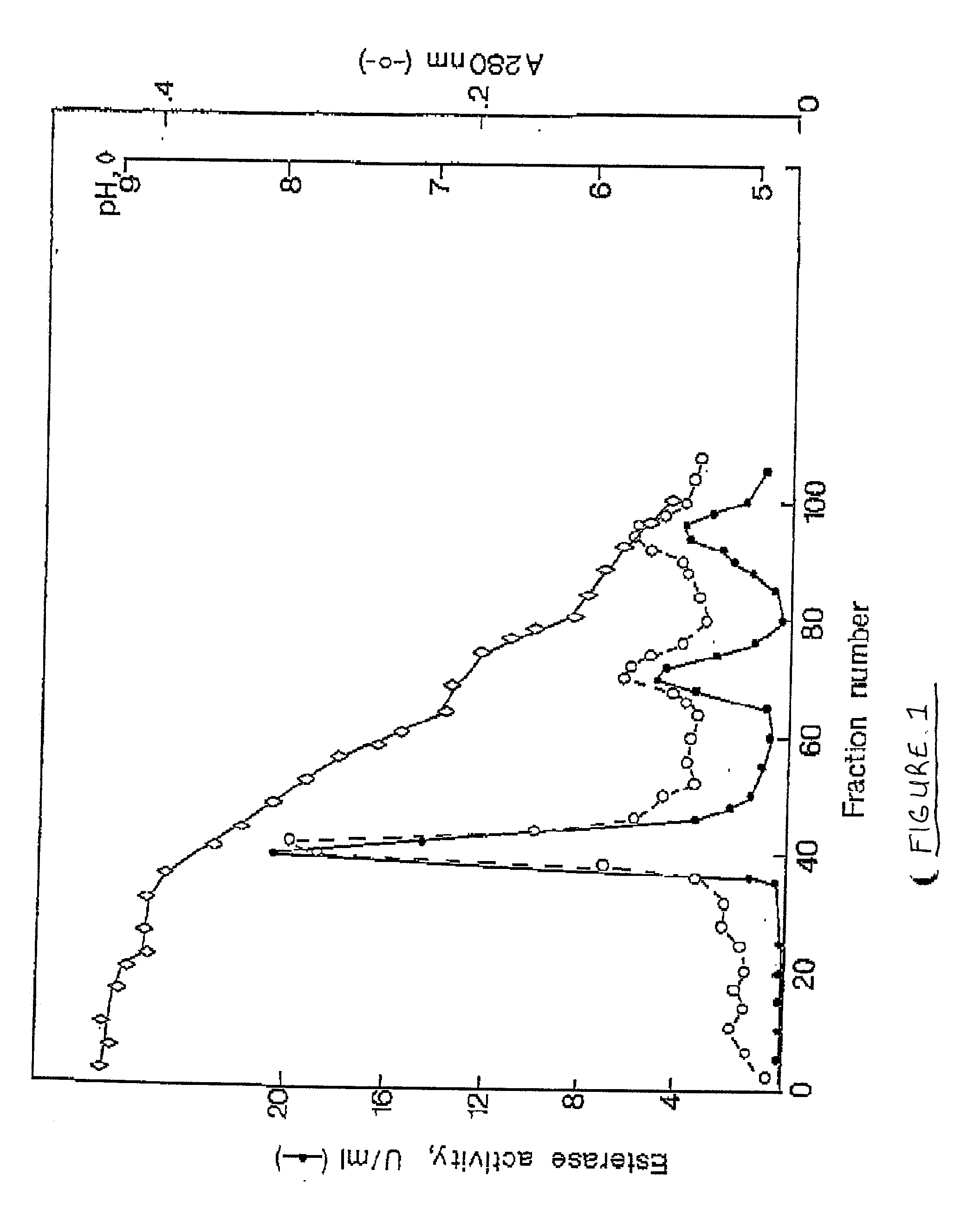
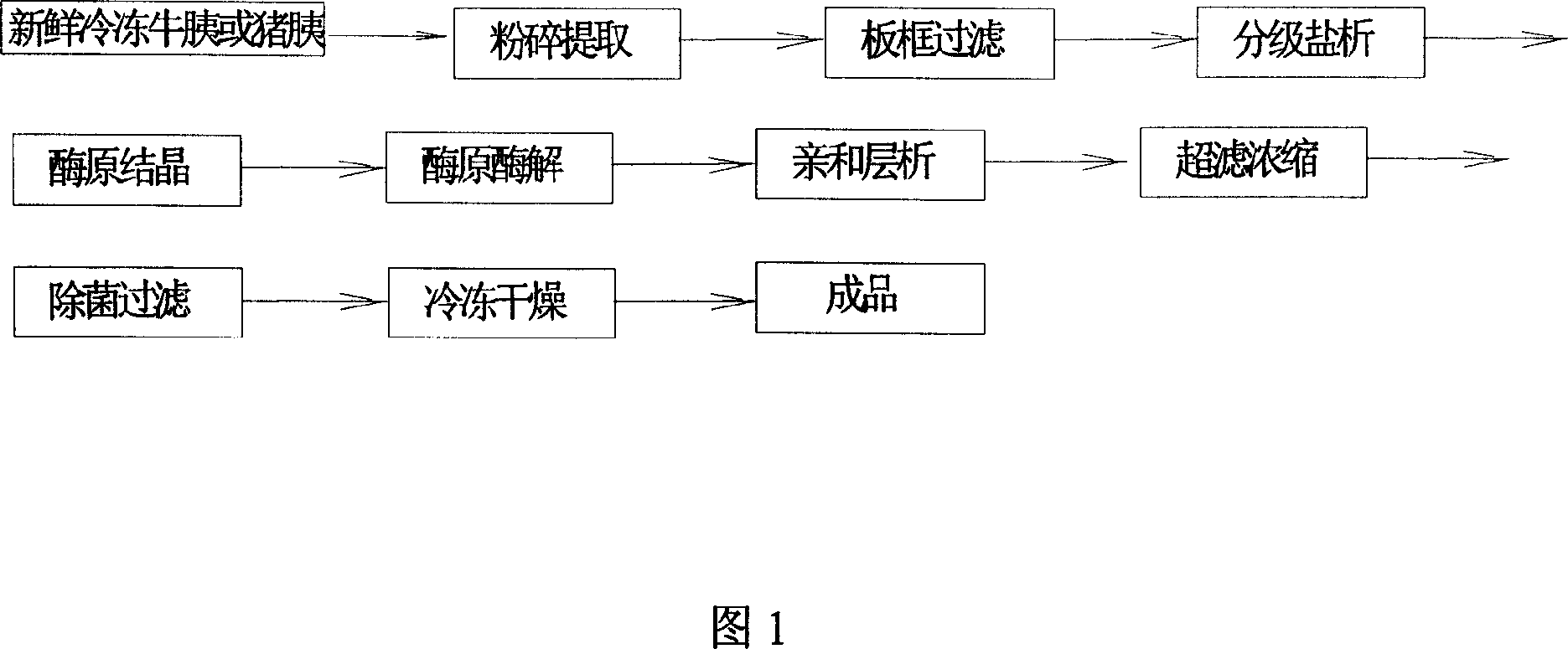
The affinity chromatographic process for producing high purity chymotrypsin includes the following technological steps: freezing fresh ox or pig pancreas as the material, crushing and extracting protein, stepped salting out and crystallizing zymogen, enzymolyzing to obtain coarse product, affinity chromatographic separating and purifying, ultrafiltering and concentrating sterilizing, and vacuum freeze drying to obtain product. Compared with available technology, the present invention has the advantages of suitability for large scale produce, high efficiency, specificity, low cost and high product quality.
Owner:宁波林叶生物科技有限公司
Insect chymotrypsin and inhibitors thereof
The present invention relates generally to a novel chymotrypsin that exhibits resistance to a plant serine proteinase inhibitor. More particularly, the present invention provides a chymotrypsin which is up-regulated in the gut of Helicoverpa armigera and Helicoverpa punctigera insect larvae when fed the serine proteinase inhibitors of Nicotiana alata. The novel chymotrypsin represents, therefore, a target for the identification of antagonists including inhibitors which are proposed to be useful in the control of Helicoverpa spp. populations that have become resistant to serine proteinase inhibitors produced in plants. The antagonists of the chymotrypsin may be topically applied to the plants or, when in proteinaceous form, may be produced by genetic means in plant cells. The antagonists may act at the level of gene expression or protein activity.
Owner:HEXIMA LTD
Novel Pharmaceutical Preparation for Preeclampsia, Eclampsia, and Toxemia and Their Related Symptoms and Related Disorders of Pregnancy
A therapeutic agent for the treatment of toxemia, preeclampsia and eclampsia and the method for preparing the therapeutic agents is disclosed. The therapeutic agent is a stable pharmaceutical preparation containing, but not limited to, digestive / pancreatic enzymes. The therapeutic agent may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic agent may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using of a biomarker, the presence of chymotrypsin in the maternal GI tract to determine the likelihood of developing preeclampsia, pregnancy induced hypertension, and eclampsia / toxemia is disclosed.
Owner:CUREMARK
Procyanidins for treatment and prevention of enzymatic irritation to the skin
This invention relates to methods and compositions for preventing and treating skin rash, such as perineal dermatitis, associated with enzymatic dermatitis. More particularly, this invention relates to compounds containing procyanidins, which possess trypsin and / or chymotrypsin inhibitory activity and are suitable for use in compositions for preventing and treating skin irritation caused by protease exposure, such as perineal dermatitis.
Owner:JOHNSON & JOHNSON CONSUMMER COMPANY
Triple-enzyme hydrolysis preparation method of anti-tumor polypeptides of spirulina
ActiveCN104561208APromote development and utilizationHas inhibitory effectPeptide preparation methodsFermentationHydrolysateChymotrypsin
The invention provides a triple-enzyme hydrolysis preparation method of anti-tumor polypeptides of spirulina. The method comprises the following steps: preparing a solution having a concentration of 5 percent from spirulina powder with ultrapure water; repeatedly freezing and thawing, homogenizing and performing ultrasonic treatment, centrifuging to obtain supernate, freezing and drying for later use; adding the prepared protein fluid into pepsin for enzyme hydrolysis, controlling the pH value, adding trypsin for enzyme hydrolysis, adding chymotrypsin for enzyme hydrolysis, controlling the pH value, deactivating enzyme, cooling, and centrifuging to obtain supernate; sequentially filtering with ultrafiltration membranes having molecular weight cut-off of 10KD, 5KD and 3KD respectively to obtain three kinds of spirulina protein enzymatic hydrolysates; carrying out sephadex G15 column chromatography on 0-3K of enzymatic hydrolysate, eluting with water, and collecting four polypeptide ingredients, namely anti-tumor polypeptides of spirulina. The obtained spirulina polypeptide and monopeptide ingredients can be used for establishing a theoretical basis for development and utilization of anti-tumor medicines and health foods.
Owner:SOUTH CHINA UNIV OF TECH
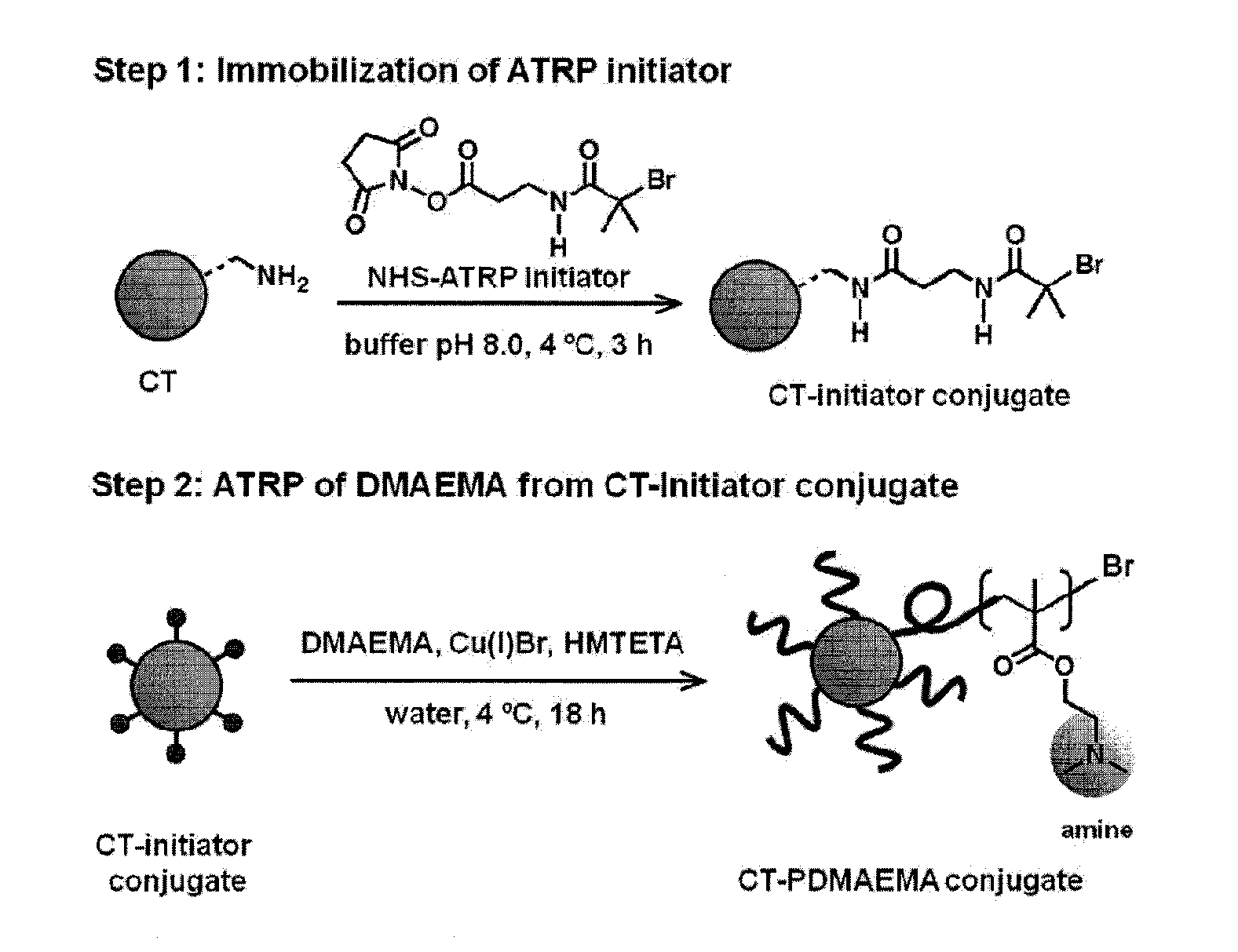
POLYMER-BASED PROTEIN ENGINEERING METHODS TO RATIONALLY TUNE ENZYME ACTIVITY, pH-DEPENDENCE AND STABILITY
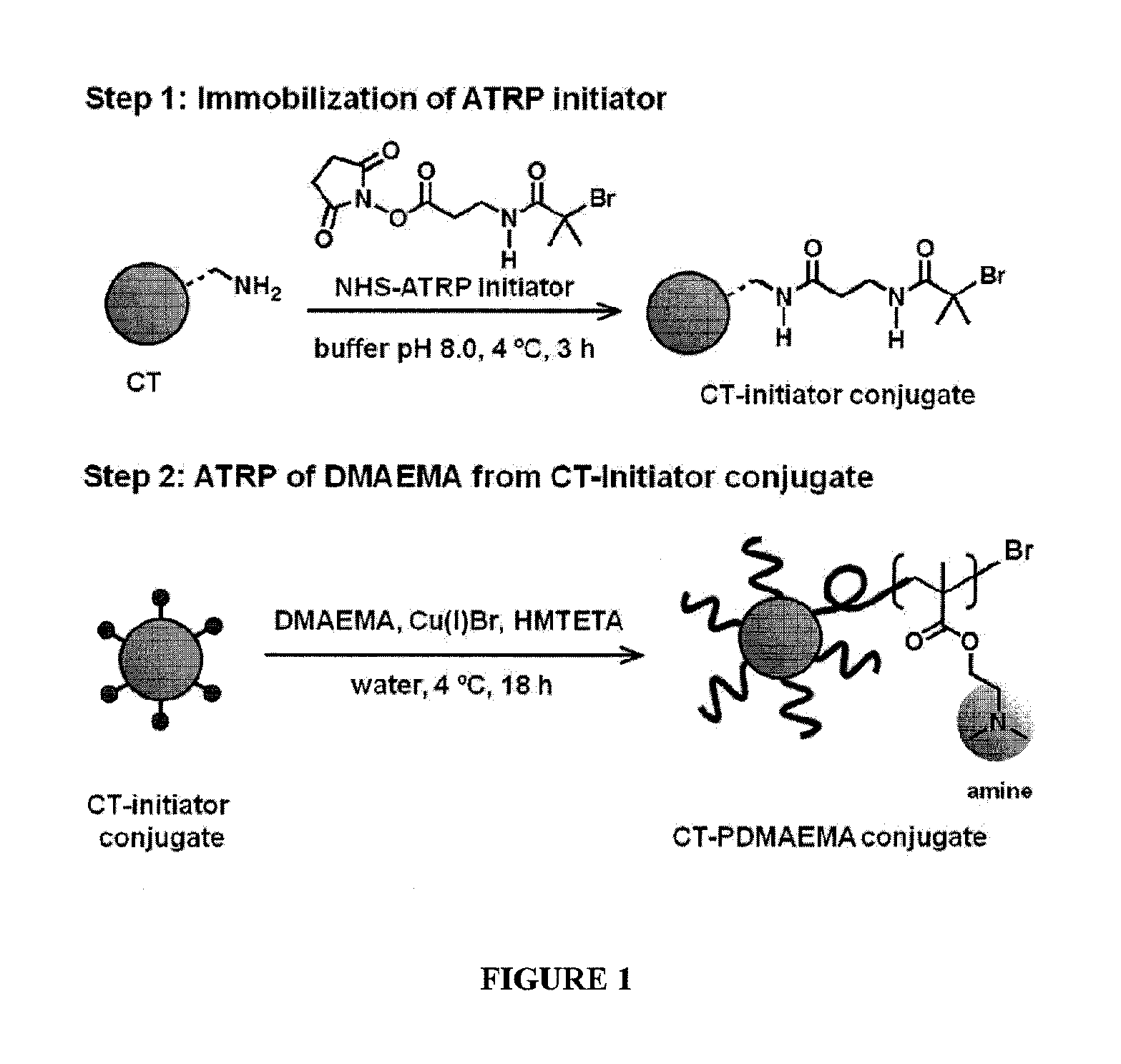
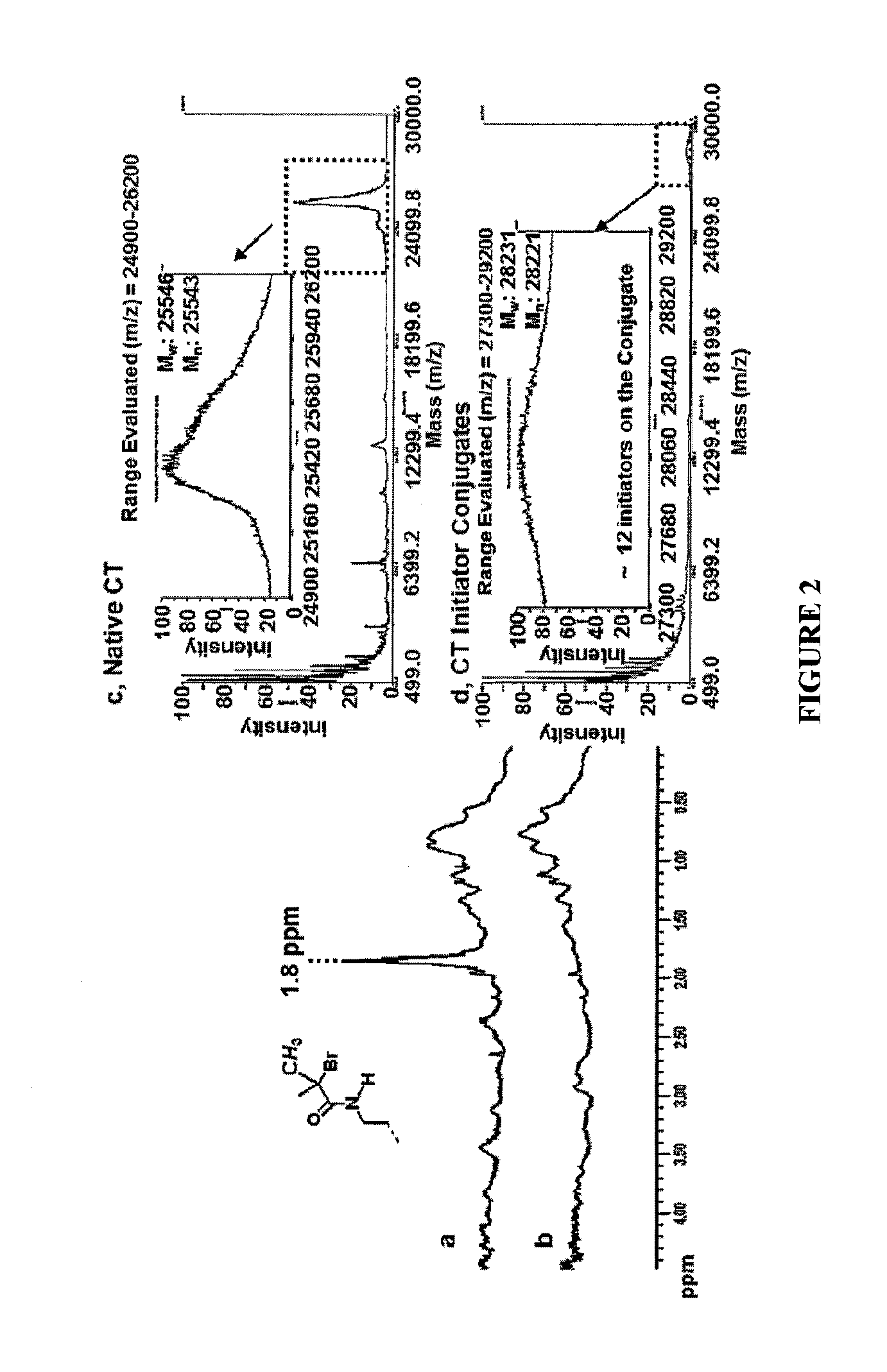
ActiveUS20160101190A1Improve biological activityHigh affinityPeptide/protein ingredientsEnzyme stabilisationStimuli responsiveChymotrypsin
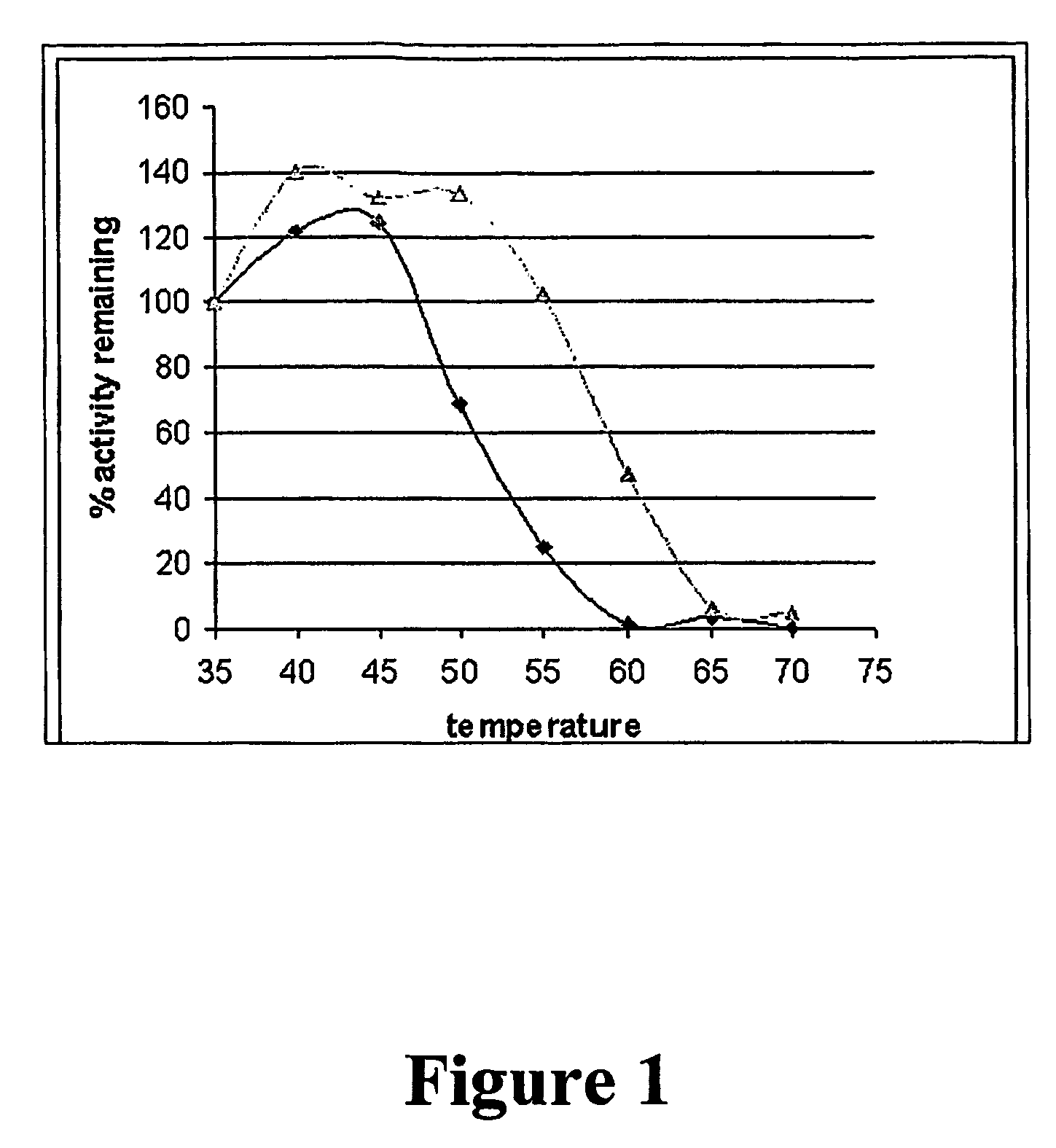
Using a novel water-soluble, active ester amide-containing functionalized controlled radical polymerization initiator, stimuli responsive polymers have been grown from the surface of a protein, exemplified by chymotrypsin or any protein having surface amino acids that will covalently bind to the active ester amide-containing functionalized initiator. It is shown that changes in temperature or pH can change the conformation of the polymer surrounding the enzyme, which in turn enabled the rational tailoring of enzyme activity and stability. This method has afforded an increase in the activity and stability of the enzyme by an order of magnitude at pH's where the enzyme is usually inactive or unstable. Multimodal temperature responsive protein-block copolymer conjugates are described.
Owner:CARNEGIE MELLON UNIV +1
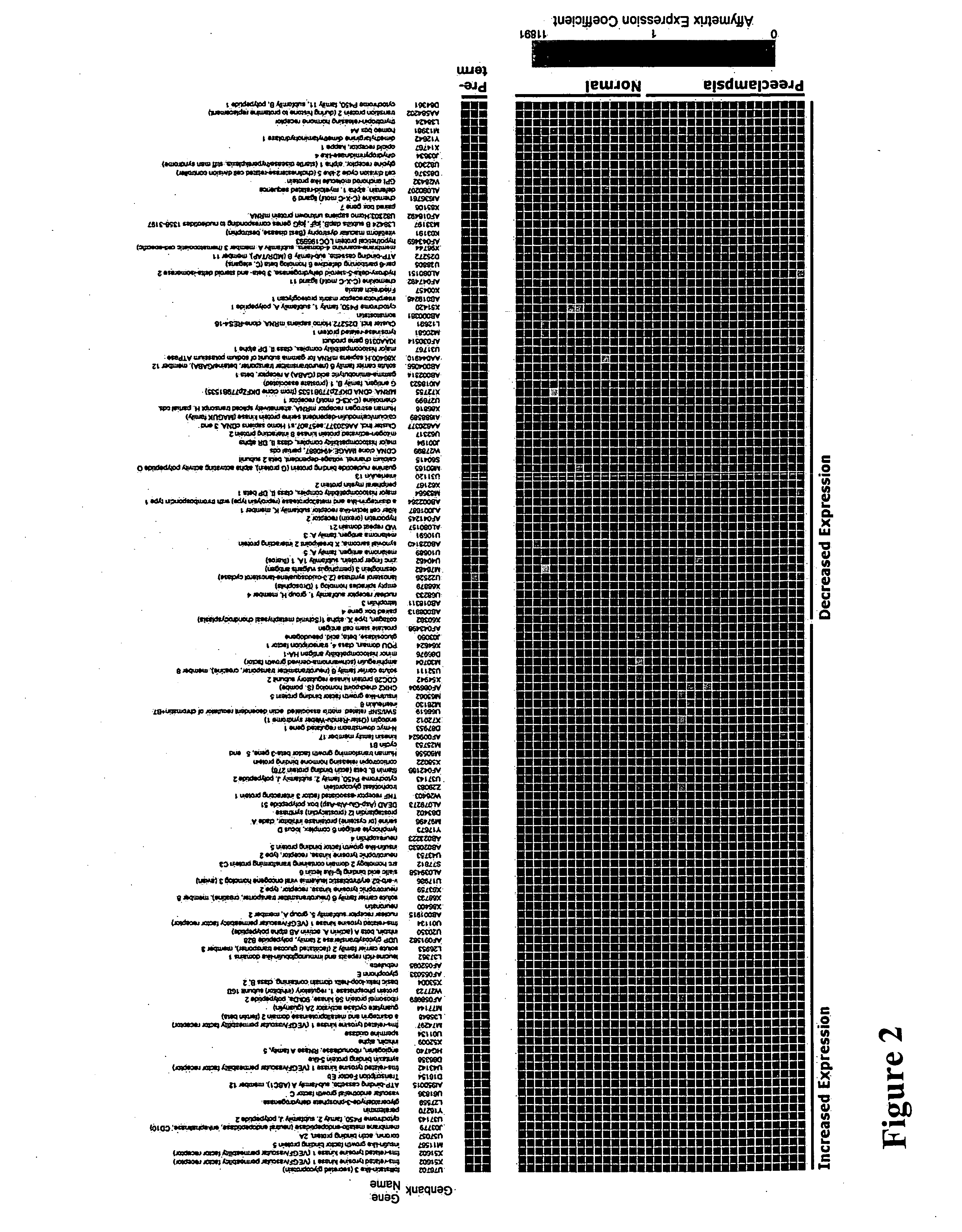
Nucleic acids and polypeptides useful for diagnosing and treating complications of pregnancy
ActiveUS20060166277A1Diagnosing and effectively treatingSave maternalMicrobiological testing/measurementDisease diagnosisPregnancyUdp glycosyltransferase
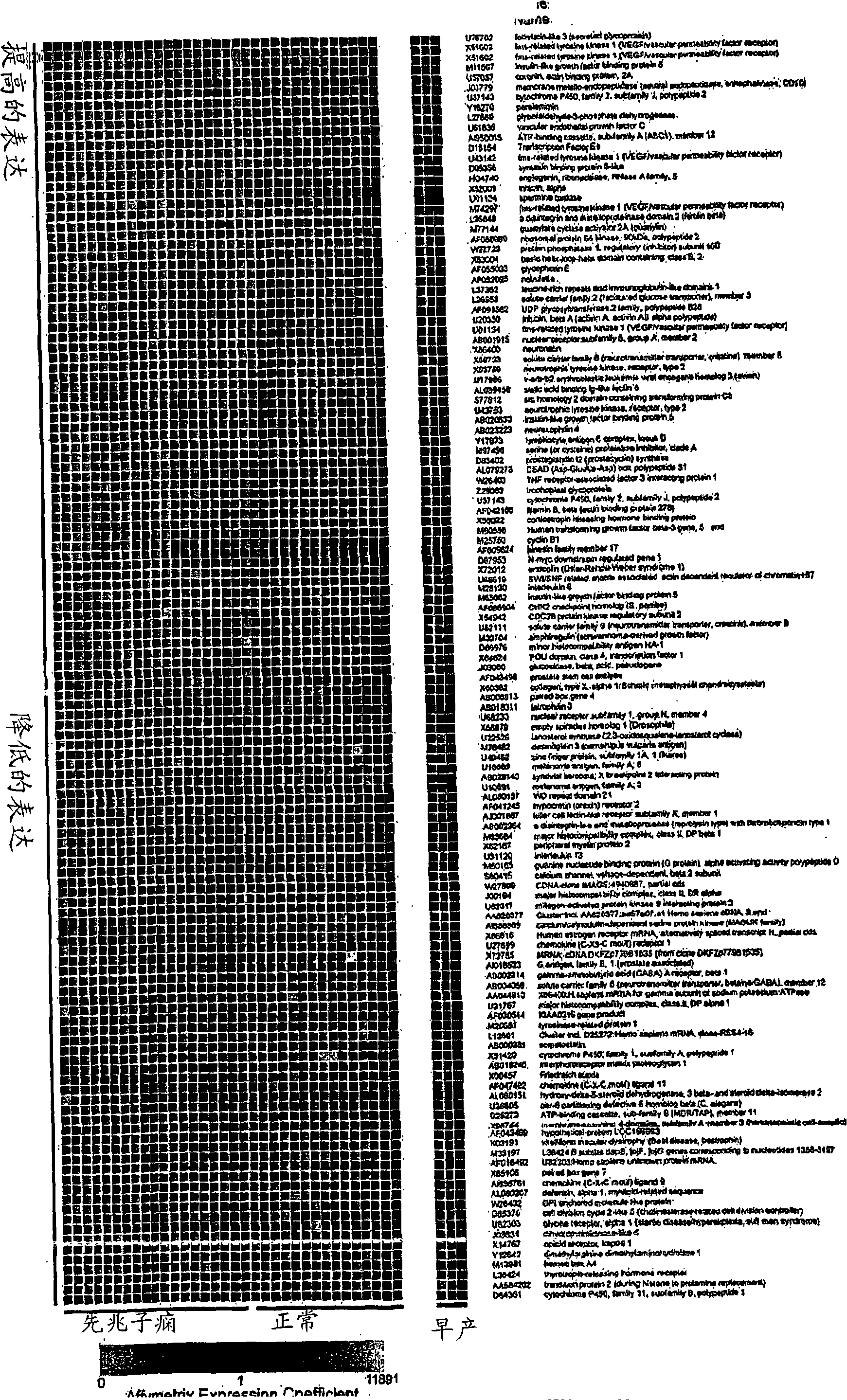
Disclosed herein are methods for diagnosing or treating pregnancy related hypertensive disorders that include the use of a polypeptide or a nucleic acid encoding a polypeptide selected from the following: follistatin related protein, interleukin 8, inhibin A, VEGF-C, angiogenin, beta fertilin, hypothetical protein, leukocyte associated Ig-like receptor secreted protein, erythroid differentiation protein, adipogenesis inhibitory factor, corticotropin releasing factor binding protein, alpha-1 anti-chymotrypsin, insulin-like growth factor binding protein-5, CD33L, cytokine receptor like factor 1, platelet derived endothelial growth factor, lysyl hydroxylase isoform 2, stanniocalcin precursor, secreted frizzled related protein, galectin-3, alpha defensin, ADAM-TS3, cholecystokinin precursor, interferon stimulated T-cell alpha chemoattractant precursor, azurocidin, sperminine oxidase, UDP glycosyltransferase 2 family polypeptide B28, neurotrophic tyrosine kinase receptor 2, neutral endopeptidase, CDC28 protein kinase regulatory subunit 2, beta glucosidase, lanosterol synthase, calcium / calmodulin-dependent serine protein kinase, estrogen receptor-alternatively spliced transcript H, chemokine (CX3C motif) receptor 1, tyrosinase-related protein 1, hydoxy-delta-5-steroid dehyrogenase, dihydropyramidinase-like-4, and cytochrome P450-family 11.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Process of chitosan-arginine resin anion immobilizing chymotrypsin
Owner:CHONGQING UNIV
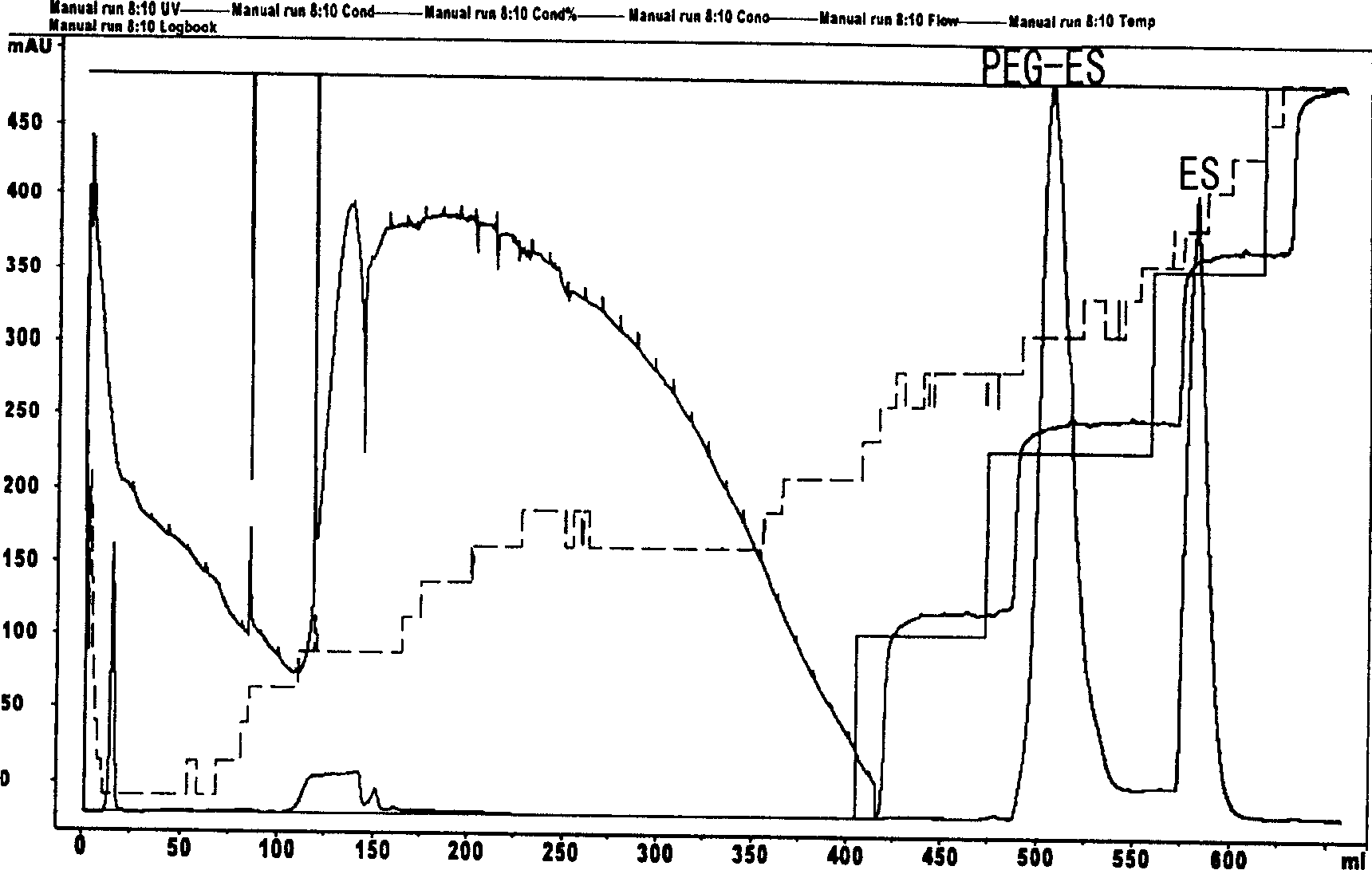
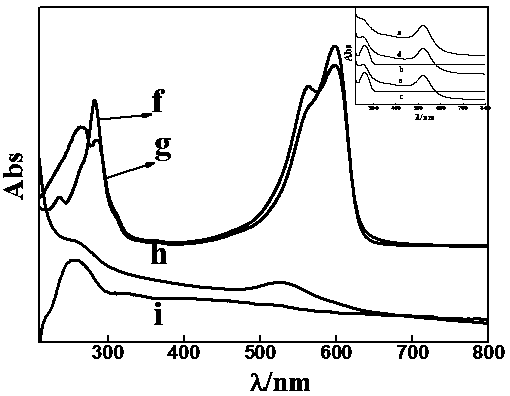
Chemical modification method of endostatin and its use
InactiveCN1891717ANot easy to hydrolyzeStable pHPeptide/protein ingredientsPeptide preparation methodsMonomethoxypolyethylene glycolChymotrypsin
The invention belongs to the field of bio-pharmaceutical products, which provides a chemical modification method for recombinate human rh-Endostatin ('rh-ES' is called for short) through the way of using mPEG-propionaldehyde to be combined on the fixed point of free amino of N-terminal amino acid of rh-ES. Mono-PEG-rhES is the output material, its purity meet the requirements of biological products. It plays a key role in the hydrolysis resistance of trypsin and chymotrypsin, it can be used as long-acting formulations for its stability for pH and temperature and there is a great perspective in the clinical treatment of cancer.
Owner:NANJING UNIV +1
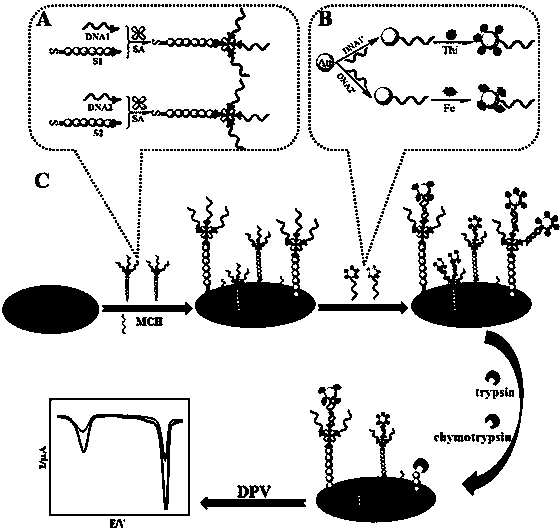
Trypsin-chymotrypsin electrochemical synchronous detection method based on enzyme digestion
InactiveCN104049007APeak current dropMaterial electrochemical variablesEnzyme digestionCarboxyl radical
The invention discloses a trypsin-chymotrypsin electrochemical synchronous detection method based on enzyme digestion and belongs to the technical field of electrochemical sensing. Through gold-mercapto bond action, a DNA-polypeptide compound is fixed to the surface of a gold electrode, and through DNA hybridization reaction, a DNA-gold nanoparticle-electronic mediator nanometer signal probe is captured. When target protease exists, an arginine carboxyl site of one of polypeptides is cut by trypsin and a tyrosine carboxyl site of the other one of the polypeptides is cut by chymotrypsin so that the corresponding nanometer signal probes connected to the polypeptides are separated from the surface of the electrode and thus peak current of the corresponding electronic mediator is reduced. Therefore, the trypsin-chymotrypsin electrochemical synchronous detection method can realize synchronous detection of sensitivity and selectivity of trypsin and chymotrypsin.
Owner:NANCHANG UNIV
Fish serine proteinase and their pharmaceutical and cosmetic use
InactiveUS6846485B2Avoid injuryInfection-preventing effective amount of the enzymeBiocideCosmetic preparationsAtlantic codDisease
Fish derived serine proteinases including trypsins and chymotrypsin derived from cod such as Atlantic cod is used for treating and / or preventing a variety of diseases and disorders such as inflammatory diseases, infectious diseases caused by viruses, bacteria and fungal species and diseases where a receptor binding mechanism is involved in the pathogenesis. Pharmaceutical and cosmetic compositions comprising the proteinases are described.
Owner:BJARNASON JON BRAGI
Epitope composition
A pharmaceutical composition for sublingual, buccal or enteric administration comprising at least one substance obtainable by hydrolysis with chymotrypsin of an antigenic structure which induces graft rejection, allergic reaction or autoimmune disease.
Owner:BIOTECH TOOLS
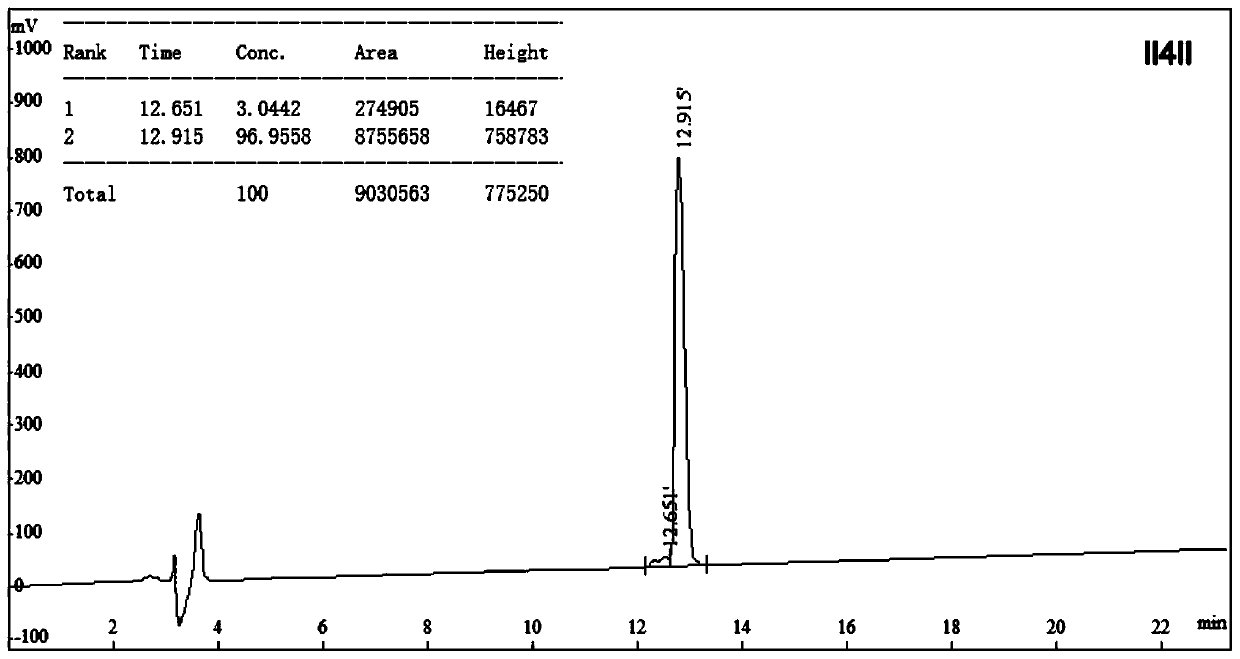
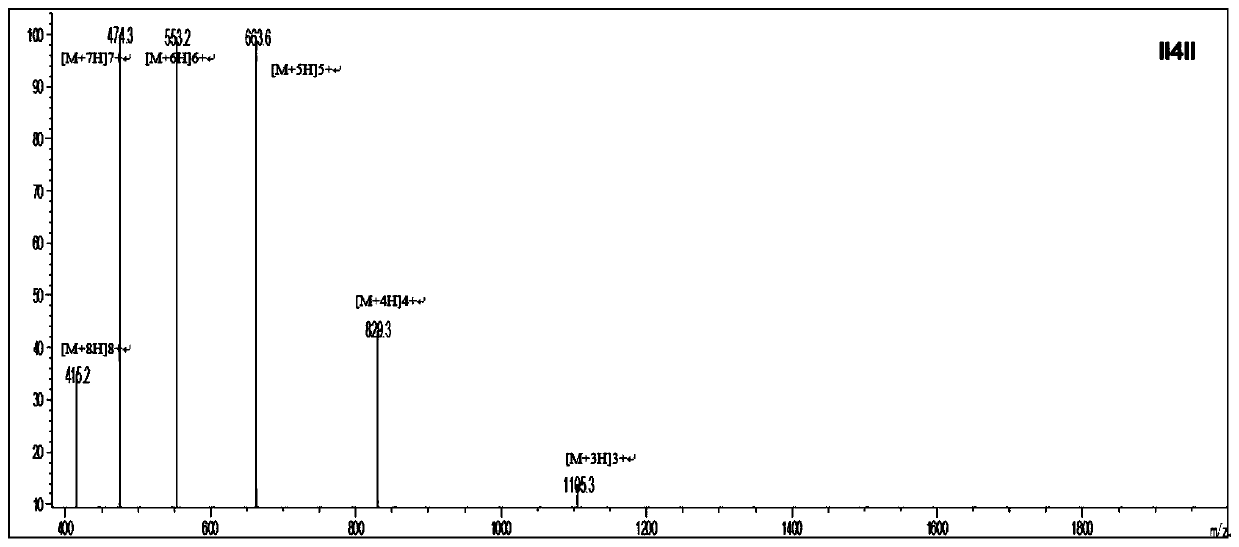
Anti-enzymolysis antibacterial peptide II4II and preparation method and application thereof
ActiveCN111454334AThe experimental technical route is simpleAvoid restriction sitesAntibacterial agentsPeptide/protein ingredientsDiseaseChymase
The invention belongs to the technical field of biology and provides an anti-enzymolysis antibacterial peptide II4II and a preparation method and application thereof. The amino acid sequence of the antibacterial peptide II4II is shown in SEQ ID No. 1. The preparation method comprises the following steps of reasonably arranging amino acids in the sequence based on specific restriction enzyme cutting sites of trypsin, chymotrypsin and pepsin and basic characteristics of the antibacterial peptide, and selecting Ile as a hydrophobic amino acid to obtain the antibacterial peptide II4II. The invention further provides application of the antibacterial peptide II4II in preparation of medicines for treating gastrointestinal tract infection diseases caused by gram-negative bacteria or / and gram-positive bacteria. The antibacterial peptide II4II has a relatively good inhibition effect on multiple bacteria, is relatively low in hemolytic activity, has a treatment index of 80.63, has relatively goodanti-enzymolysis property, is kept unchanged in antibacterial activity after being treated by trypsin, chymotrypsin or pepsin, and has application potential as an antibiotic substitute.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
D-type non-natural amino acid containing antimicrobial peptide analog, synthesis therefor and application of D-type non-natural amino acid containing antimicrobial peptide analog
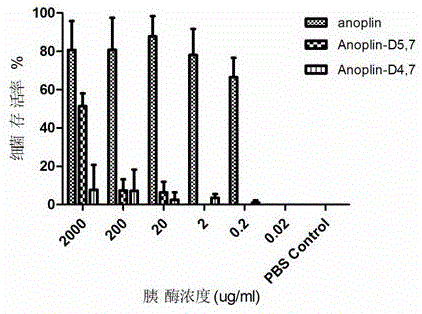
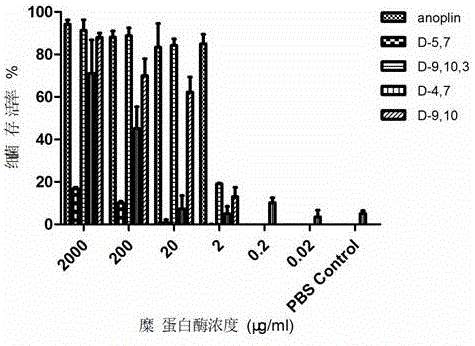
ActiveCN106632600AInhibition formationInhibition formation rateAntibacterial agentsPeptide/protein ingredientsAntimicrobial peptidesAntibacterial activity
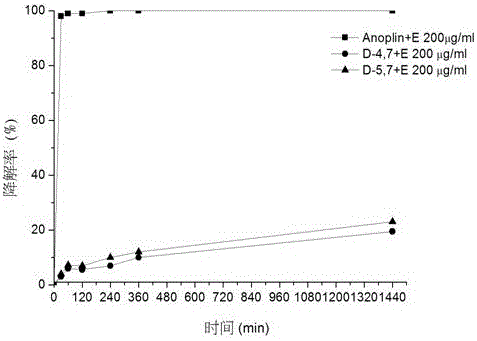
The invention discloses a D-type amino acid containing antimicrobial peptide analog. The D-type amino acid containing antimicrobial peptide analog is obtained through separately introducing D-type amino acids to a hydrophilic surface and a hydrophobic surface of a natural antimicrobial peptide Anoplin and modifying the hydrophilic surface and the hydrophobic surface. Shown by determination on minimal inhibitory concentration to common standard bacteria, biomembrane formation inhibiting tests and enzymolysis stability tests, synthesized hydrophilic-surface D-type peptide analogs all reserve the antimicrobial activity of the original stock peptide and meanwhile represent relatively high bacterial biomembrane formation inhibiting capability. The chymotrypsin tolerance is enhanced by 10 times compared with that of the stock peptide Anoplin. Although the antimicrobial activity of synthesized hydrophobic-surface D-type substituted analogs is lowered to some extent compared with that of the stock peptide, the stability of the synthesized hydrophobic-surface D-type substituted analogs is improved remarkably; and compared with the stock peptide, the trypsin tolerance is improved by 10<4> to 10<5> times, and the chymotrypsin tolerance is improved by 10<2> times. Therefore, the synthesized D-type amino acid containing analog has a very good application prospect in the aspect of preparation of long-acting clinical antibacterials.
Owner:倪京满
Novel boric acid derivative and medicinal composition
ActiveCN108440583ABoron compound active ingredientsGroup 3/13 element organic compoundsProteinase activityChymotrypsin
The invention discloses a novel boric acid derivative and a medicinal composition thereof, and belongs to the field of pharmaceutical chemistry. The boric acid derivative is a compound represented byformula (I). The invention also provides uses of the boric acid derivative in the preparation of an antitumor medicine or a proteasome inhibitor medicine. The uses concretely comprise a use of the boric acid derivative in the preparation of proteasome and chymotrypsin protease inhibitors, and a use in the preparation of medicines for preventing and / or treating multiple myeloma, colorectal carcinoma and other cancers. The novel boric acid derivative provides a new choice for clinically screening and / or preparing proteasome inhibitor medicines and multiple myeloma and colorectal carcinoma medicines, and has a wide application prospect.
Owner:CHENGDU ORIGIN BIOTECH LTD
Treating method for suppressing hair growth
InactiveUS7211278B2Excellent hair growth inhibitory effectStrong growth inhibitory effectCosmetic preparationsBiocideMalt GrainChymotrypsin
treating method for hair growth inhibition, which comprises administering (A) the extract of a plant of the family Juniperus or a malt. In addition, the present invention relates to a dermatologic composition for external use, which comprises (B) an elastase inhibitor or neutral endopeptidase inhibitor, and the above-described component (A) and / or (C) at least one proteolytic enzyme selected from the group consisting of papain, trypsin, chymotrypsin, pepsin, bromelain, ficin and pancreatin.
Owner:KAO CORP
Bacteroides signal molecule microecological preparation and its preparation method
InactiveCN101125151ANumber of Spontaneous Activities NoneBlood pressure noBacteria material medical ingredientsPill deliveryAcute toxicity testingCulture fluid
The present invention relates to a b.fragilis signal molecule microbial preparation, the b.fragilis bacterium are inoculated in a culture medium for anaerobic fermentation at 35 to 38 DEG C, after that, the b.fragilis bacteria concentrate is obtained by centrifugal concentration; the bacteria cell walls are broken by high pressure; further, the bacteria concentrate is treated with enzyme hydrolysis by trypsin, chymotrypsin and pepsin, and then the preparation raw powder is obtained by solvent extraction and decompression drying. The preparation raw powder is taken as the main ingredient, and other excipients are added to prepare the oral capsules, tablets, granules, oral liquor and other preparations. The b.fragilis signal molecule product of the present invention is proven to have no acute toxicity, no influence on the times of spontaneous activity, blood pressure and respiration of the animals and can not cause the allergic reactions of the animals by animal trials. The animal trials further prove that: the product has the effects of regulating blood-lipid metabolism, reducing fat accumulation and slimming; at the same time, the present invention can improve the immunity and the anti-tumor effect by adjusting the gene expression and regulating the immune functions.
Owner:SENBAIAO SCI & TECH UNIV DALIAN
Method for preparing active polypeptides from deep-sea fish meat
InactiveCN107604029AImprove stabilityIncrease enzyme activityMicroorganism based processesPeptide preparation methodsFreeze-dryingUltrafiltration
The invention discloses a method for preparing active polypeptides from deep-sea fish meat, and the method includes degreasing, first deodorization, enzymolysis, second deodorization, ultrafiltration,decolorization, desalting and freeze-drying. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide is used to activate chitosan, and coupled with glutaraldehyde to form a lipase immobilized carrier, an active short peptide is used to improve the physical and chemical adsorption force between a carrier and lipase, the lipase stability, activity, heat resistance and use frequency are improved; through useof the complementary effect and synergistic effect of thermolysin and chymotrypsin hydrolysis sites, the protein hydrolysis degree and enzymatic hydrolysis speed of minced deep-sea fish meat can be improved, terminal group type can be changed, and the active polypeptides with antitumour activity can be obtained by hydrolysis.
Owner:兰溪市哥特生物技术有限公司
Method for extracting chymocotrypsin
InactiveCN102174495AThe extraction and separation method is simple and feasibleSuitable for industrial productionEnzymesZymogenFreeze-drying
The invention relates to the field of bioengineering, in particular relating to a method for extracting chymocotrypsin. In the method, CaCl2 is used for activating chymocotrypsin zymogen into chymocotrypsin while saturated (NH4)2SO4 is added, thus CaSO4 is generated to adsorb the chymocotrypsin. The complicated steps that a salting-out multiple crystallization method combined with dialysis and an organic solvent method using ethanol, acetone and the like are adopted to prepare crude chymocotrypsin and then CM-cellulose column chromatography and affinity chromatography are adopted, are not adopted for purification and preparation; and the steps of elution, salting-out, ultrafiltration and freeze-drying are directly performed to obtain white freeze-dried powder. The extraction method is simple and feasible and is suitable for industrial production; and by adopting the method, the cost can be saved and the time can be shortened from about 7-8 days to 3-4 days. 4.0-4.5g of freeze-dried chymocotrypsin powder can be prepared from one kilogram of pancreas, wherein the specific activity of the chymotrypsin is 360U. / mg protein (nitrogen) and the specific activity of trypsin is 1380U. / mg protein (nitrogen).
Owner:马忠仁 +1
Nucleic acids and polypeptides useful for diagnosing and treating complications of pregnancy
InactiveCN101299962AMicrobiological testing/measurementDisease diagnosisAlpha defensinInsulin-like growth factor-binding protein
Disclosed herein are methods for diagnosing or treating pregnancy related hypertensive disorders that include the use of a polypeptide or a nucleic acid encoding a polypeptide selected from the following: follistatin related protein, interleukin 8, inhibin A, VEGF-C, angiogenin, beta fertilin, hypothetical protein, leukocyte associated Ig-like receptor secreted protein, erythroid differentiation protein, adipogenesis inhibitory factor, corticotropin releasing factor binding protein, alpha-1- anti-chymotrypsin, insulin-like growth factor binding protein-5, CD33L, cytokine receptor like factor 1, platelet derived endothelial growth factor, lysyl hydroxylase isoform 2, stanniocalcin precursor, secreted frizzled related protein, galectin-3, alpha defensin, ADAM-TS3, cholecystokinin precursor, interferon stimulated T-cell alpha chemoattractant precursor, azurocidin, sperminine oxidase, UDP glycosyltransferase 2 family polypeptide B28, neurotrophic tyrosine kinase receptor 2, neutral endopeptidase, CDC28 protein kinase regulatory subunit 2, beta glucosidase, lanosterol synthase, calcium / calmodulin-dependent serine protein kinase, estrogen receptor-alternatively spliced transcript H, chemokine (CX3C motif) receptor 1, tyrosinase-related protein 1, hydoxy-delta-5-steroid dehyrogenase, dihydropyramidinase-like-4, and cytochrome P450-family 11.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
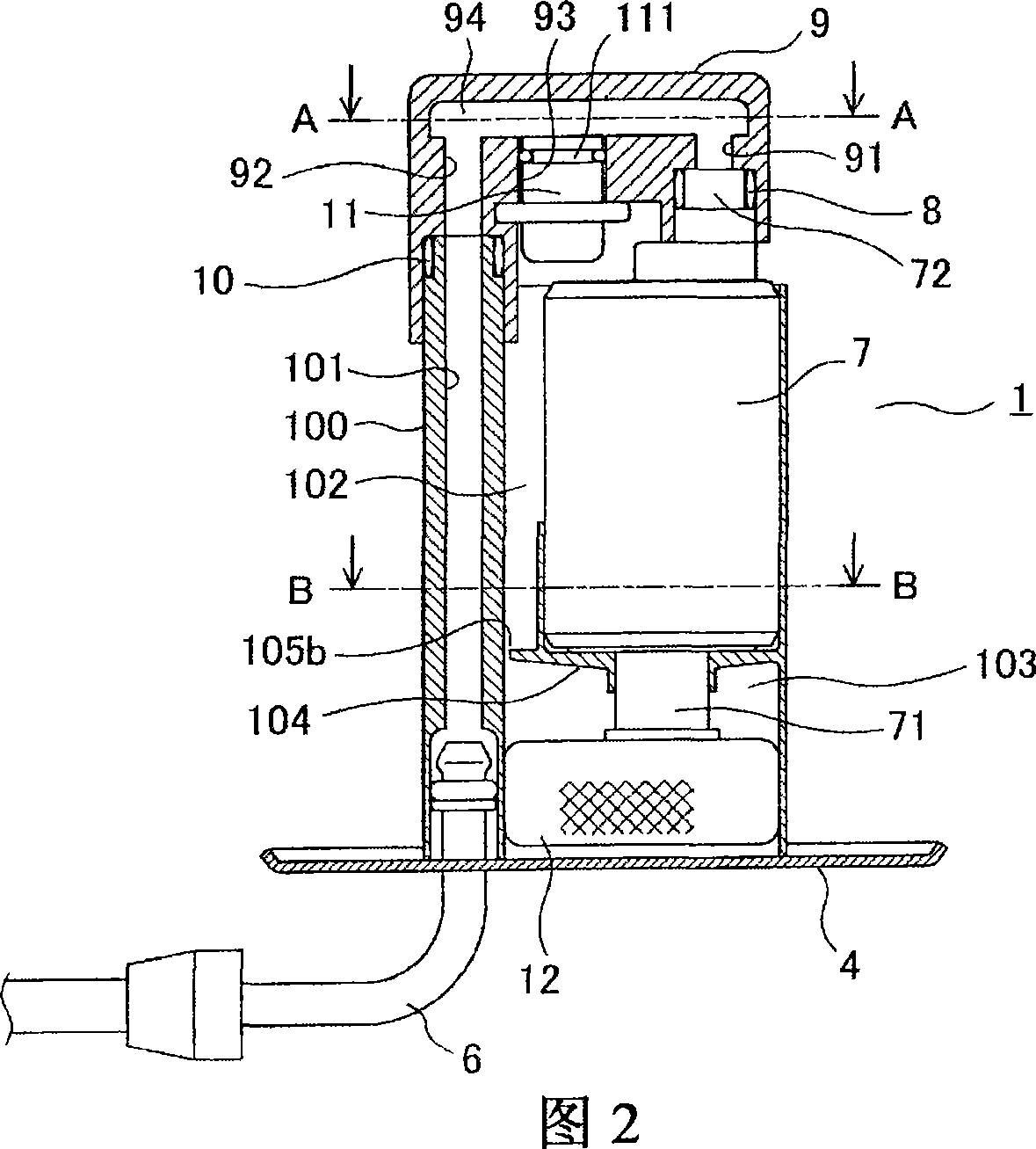
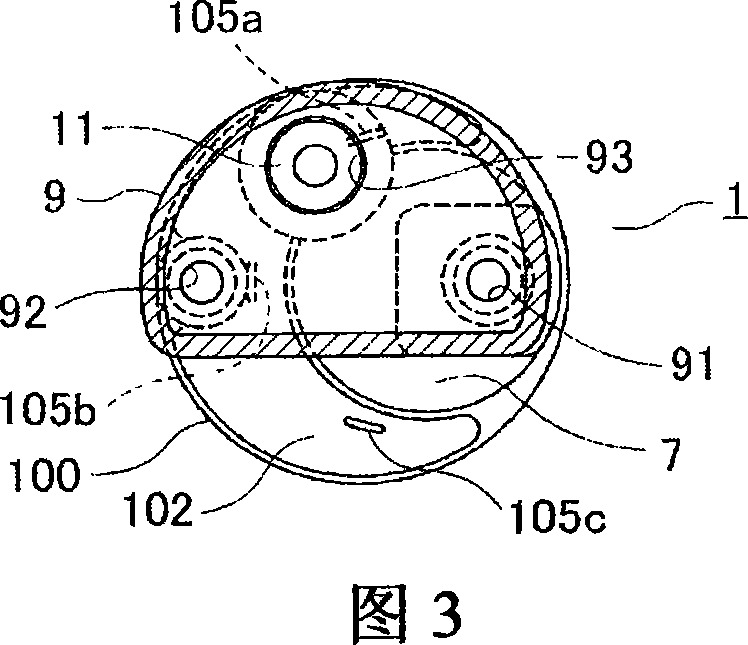
Fuel supply device for vehicle
The invention relates to a multifunctional enzyme that can be derived from crustaceans or fish. The enzyme has at least one of a chymotrypsin, trypsin, elastase, collagenase and exo peptidase activity, and a molecular weight between about 20 kd and about 40 kd as determined by SDS PAGE. Preferably, the multifunctional enzyme has substantial anti cell-cell adhesion activity. Preferably, the multifunctional enzyme has substantial homology with the krill multifunctional enzyme. These enzymes are useful for treating viral infections such as herpes outbreaks, fungal, bacterial or parasitic infections, including the primary and secondary infections of leprosy, colitis, ulcers, hemorrhoids, corneal scarring, dental plaque, acne, cystic fibrosis, blood clots, wounds, immune disorders including autoimmune disease and cancer. Additionally, the invention relates to a method of purifying the multifunctional enzyme, and to a preparation of essentially purified multifunctional enzyme.
Owner:MITSUBISHI ELECTRIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com